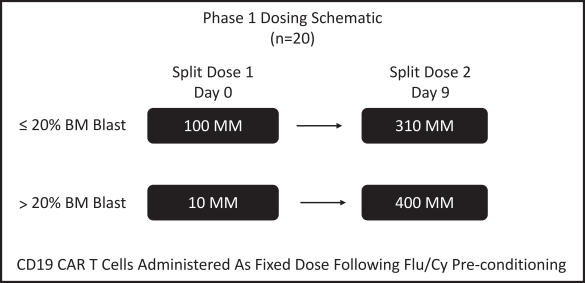
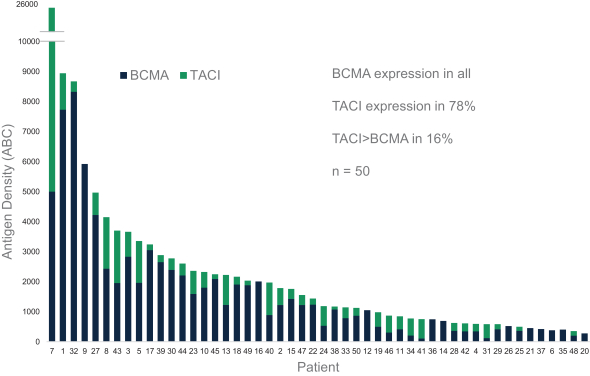
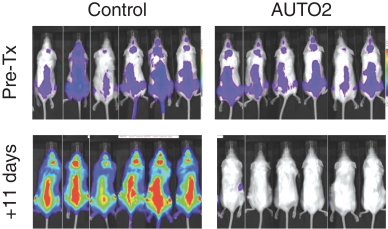
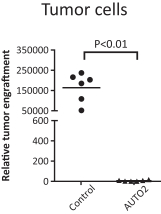
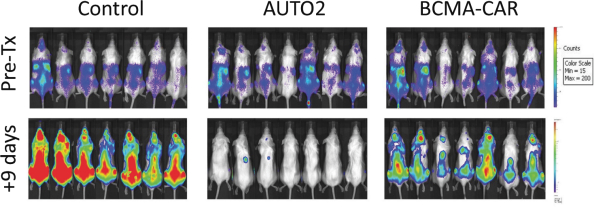
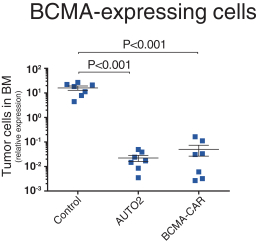
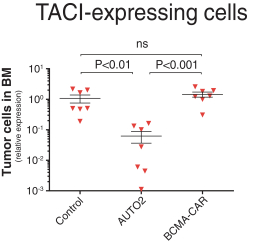
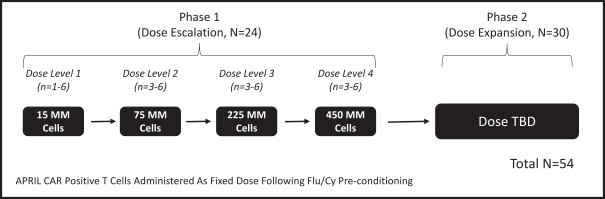
Our employees, independent contractors, principal investigators, consultants, commercial partners and vendors may engage in misconduct or other improper activities, includingnon-compliance with regulatory standards and requirements.
We are exposed to the risk of employee fraud or other misconduct or failure to comply with applicable regulatory requirements. Misconduct by employees and independent contractors, such as principal investigators, consultants, commercial partners, and vendors, could include failures to comply with regulations of the FDA, the EMA and other comparable regulatory authorities, to provide accurate information to such regulators, to comply with manufacturing standards we have established, to comply with healthcare fraud and abuse laws, to report financial information or data accurately or to disclose unauthorized activities to us. In particular, sales, marketing and other business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of business activities, including, but not limited to, research, manufacturing, distribution, pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Employee and independent contractor misconduct could also involve the improper use of individually identifiable information, including, without limitation, information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation.
In addition, federal procurement laws impose substantial penalties for misconduct in connection with government contracts and require certain contractors to maintain a code of business ethics and conduct. It is not always possible to identify and deter employee and independent contractor misconduct, and any precautions we take to detect and prevent improper activities may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws. If any such actions are instituted against us, those actions could have a significant impact on our business, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, disgorgement of profits, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, National Health Service in the United Kingdom, or other government supported healthcare in other jurisdictions, contractual damages, reputational harm, diminished profits and future earnings, additional reporting or oversight obligations if we become subject to a corporate integrity agreement or other agreement to resolve allegations ofnon-compliance with the law and curtailment or restructuring of our operations, any of which could adversely affect our ability to operate.
Our business operations and current and future relationships with healthcare professionals, principal investigators, consultants, customers and third-party payors in the United States and elsewhere may be subject, directly or indirectly, to applicable anti-kickback, fraud and abuse, false claims, physician payment transparency, health information privacy and security and other healthcare laws and regulations, which could expose us to substantial penalties.
Healthcare providers, physicians and third-party payors in the United States and elsewhere will play a primary role in the recommendation and prescription of any product candidates for which we obtain marketing approval. Our current and future arrangements with healthcare professionals, principal investigators, consultants, customers and third-party payors may expose us to broadly applicable fraud and abuse and other healthcare laws, including, without limitation, the U.S. federal Anti-Kickback Statute and the U.S. federal False Claims Act, that may constrain the business or financial arrangements and relationships through which we sell, market and distribute any product candidates for which we obtain marketing approval. In addition, we may be subject to physician payment transparency laws and patient privacy and security regulation by the U.S. federal government
47