
Full Year 2023 Financial Results and Business Updates March 14, 2024 EX-99.2

Disclaimer 2Developing Next Generation Programmed T Cell Therapies These slides contain forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements are statements that are not historical facts, and in some cases can be identified by terms such as "may," "will," "could," "expects," "plans," "anticipates," and "believes." These statements include, but are not limited to, statements regarding Autolus’ development of its product candidates, including the obe-cel program; the profile and potential application of obe-cel in additional disease settings; the future clinical development, efficacy, safety and therapeutic potential of the Company's product candidates, including progress, expectations as to the reporting of data, conduct and timing and potential future clinical and preclinical activity and milestones; expectations regarding the initiation, design and reporting of data from clinical trials and preclinical studies; the extension of the pipeline beyond obe-cel; expectations regarding the regulatory approval process for any product candidates; the benefits of the collaboration between Autolus and BioNTech, including the potential and timing of milestone payments and royalties under the terms of the strategic collaboration; the Company’s current and future manufacturing capabilities; and the Company’s anticipated cash runway. Any forward-looking statements are based on management's current views and assumptions and involve risks and uncertainties that could cause actual results, performance, or events to differ materially from those expressed or implied in such statements. These risks and uncertainties include, but are not limited to, the risks that Autolus’ preclinical or clinical programs do not advance or result in approved products on a timely or cost effective basis or at all; the results of early clinical trials are not always being predictive of future results; the cost, timing and results of clinical trials; that many product candidates do not become approved drugs on a timely or cost effective basis or at all; the ability to enroll patients in clinical trials; and possible safety and efficacy concerns. For a discussion of other risks and uncertainties, and other important factors, any of which could cause Autolus’ actual results to differ from those contained in the forward-looking statements, see the section titled "Risk Factors" in Autolus' Annual Report on Form 20-F filed with the Securities and Exchange Commission, or the SEC, on March 7, 2023 and in Autolus' Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 9, 2023, as well as discussions of potential risks, uncertainties, and other important factors in Autolus' subsequent filings with the Securities and Exchange Commission. All information in this presentation is as of the date of the presentation, and Autolus undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events, or otherwise, except as required by law. You should, therefore, not rely on these forward-looking statements as representing the Company’s views as of any date subsequent to the date of this presentation.
Agenda • Welcome and Introduction: Olivia Manser, Director, Investor Relations • Operational Highlights: Dr. Christian Itin, CEO • Financial Results: Rob Dolski, CFO • Upcoming Milestones and Conclusion: Dr. Christian Itin, CEO • Q&A: Dr. Christian Itin and Rob Dolski 3Developing Next Generation Programmed T Cell Therapies

Strategic updates Developing Next Generation Programmed T Cell Therapies 4 Strong cash position: Year-end 2023 cash of $240M & gross proceeds of $600M from activities in February 2024 • In February 2024 completed an underwritten registered direct equity financing – Gross proceeds of $350M • In parallel established strategic collaboration with BioNTech aimed at advancing both companies’ autologous CAR T programs – $200M equity, $50M cash upfront – Up to $582 million in further option exercise and milestones payments – BioNTech to support launch and expansion of obe-cel in adult ALL for royalty on net sales – BioNTech has option to use Autolus’ manufacturing capacity for BNT211 – BioNTech has co-commercialization options for Autolus’ AUTO1/22 and AUTO6NG programs and option on Autolus target binders and cell programming technologies Financing BioNTech collaboration

Obe-cel highlights in r/r B-ALL Developing Next Generation Programmed T Cell Therapies 5 FELIX data, regulatory review and preparation for launch • Obe-cel in relapsed / refractory (r/r) adult ALL - pooled analysis presented at ASH in December 2023 – FELIX Phase 1b/2 study - prolonged event free survival and low overall immunotoxicity across all cohorts particularly in patients with low leukemic burden at lymphodepletion – ALLCAR19 study and FELIX Phase 1b - durable remissions with obe-cel as a stand-alone therapy in a subset of patients after a median follow up of >3 years • Robust manufacturing process and state of the art commercial manufacturing – Completed first facility inspection in February 2024 – the Nucleus manufacturing facility in Stevenage has obtained a Manufacturer’s Importation Authorization (MIA) together with accompanying GMP certificate – Poster presentation at ASH on obe-cel manufacturing performance • Biologics License Application (BLA) accepted by US Food and Drug Administration (FDA) – PDUFA target action date of November 16, 2024 • Marketing Authorization Application (MAA) recently submitted to EMA • Commercial capability and infrastructure preparation on track – Commercial systems setup on track – Focus on clinical center onboarding and medical affairs Clinical Manufacturing Regulatory Commercial Readiness

Other pipeline highlights Developing Next Generation Programmed T Cell Therapies 6 Expanding opportunity beyond ALL • Obe-cel in B-cell mediated autoimmune diseases – Phase 1 CARLYSLE Study – Phase 1 dose confirmation study in refractory SLE patients – first trial site opened for enrollment in Q1 2024 – Potential best-in-class risk/benefit profile, based on data from pivotal FELIX trial in adult ALL 8 in Multiple Myeloma – Phase 1 MCARTY Study Obe-cel in Autoimmune Diseases AUTO8 AUTO6NG • AUTO8 in Multiple Myeloma – Phase 1 MCARTY Study – Initial data in multiple myeloma presented at ASH in December 2023 demonstrated AUTO8 was well tolerated, with responses observed in all patients • AUTO6NG in Neuroblastoma – Phase 1 MAGNETO Study – A Phase 1 clinical study in children with r/r neuroblastoma was opened for enrollment in Q4 2023

Organizational changes • Promotions of Dr Chris Williams to Chief Business Officer and Alex Driggs to Senior Vice President, Legal Affairs and General Counsel • Dr. Edgar Braendle to step down as Chief Development Officer to pursue other opportunities. Edgar will continue to advise the Company through the BLA and MAA review process • Miranda Neville, Senior Vice President and obe-cel Program Leader, to run Development team • Strengthening of the Board with the appointments of Dr. Elisabeth (Lis) Leiderman and Robert W. Azelby Developing Next Generation Programmed T Cell Therapies 7

Obe-cel LEAD CLINICAL PROGRAM A standalone, potentially best-in-class CD19 CAR T cell therapy candidate

We believe obe-cel has a unique mechanism of action • Avoided over-activation of CAR T cells • Increased CAR T peak expansion • Avoided exhaustion of CAR T-cells 9 Enhanced cytotoxicity and proliferation Off Rate: Kd [S-1] ]1-S 1 - [M aK: e Ra t - On Other CD19 Binders Obe-cel Binder Off Rate: Kd [S-1] O n- Ra te : K a [M -1 S- 1 ] Shorter half-life of interaction compared to binders used in approved products • obe-cel = 9.8 seconds • Kymriah® = 21 minutes % T um or C el l K ill in g Pr ol ife ra tio n Ghorashian et al. Nature Medicine 2019 Fast off-rate CD19 binder with fast off-rate Designed for increased activity and reduced toxicity Developing Next Generation Programmed T Cell Therapies Differentiated CD19 binder Potential for improved potency, reduced toxicity Reduced toxicities Improved persistence Improved engraftment Improved persistence

Obe-cel pooled analysis ASH 2023 FELIX Phase 1b/2 trial

FELIX Phase 1b/2 pooled analysis: patient disposition Developing Next Generation Programmed T Cell Therapies 11 127/153 (83%) enrolled patients received obe-cel* Discontinued n (%) 26 (17) Death 15 (10) Manufacturing-related 7 (5) Adverse event 2 (1) Physician decision 1 (0.7) Progressive disease 1 (0.7) *Seven patients received Dose 1 only; **All eligibility criteria met and the leukapheresate accepted for manufacturing; obe-cel, obecabtagene autoleucel; Roddie et al., ASH 2023, Data cut-off date: September 13, 2023 Infused N = 127 (83%) Enrolled** N = 153 Cohort A n = 107 (84%) ≥5% BM blasts at screening Cohort B n = 13 (10%) MRD-positive at screening Cohort C n = 7 (6%) Isolated EMD at screening Morphologic disease*** (n = 98) • 74% of patients had CR/CRi (n = 73) • 95% of evaluated responders were MRD-negative‡ No morphologic disease (n = 29) • 100% of evaluable patients were MRD-negative§ ***Morphologic disease defined as ≥5% BM blasts or presence of EMD regardless of BM blast status; ‡MRD status available for 64/73 patients, as assessed by NGS or flow cytometry; §MRD status available for 27/29 patients, as assessed by NGS or flow cytometry; BM, bone marrow; CR, complete remission; CRi, CR with incomplete hematologic recovery; EMD, extramedullary disease; MRD, measurable residual disease; NGS, next-generation sequencing; obe-cel, obecabtagene autoleucel

FELIX Phase 1b/2 pooled analysis: leukemic burden in all treated patients Developing Next Generation Programmed T Cell Therapies 12 Leukemic burden at screening is not predictive of leukemic burden prior to lymphodepletion *Bridging therapy per physician’s choice, including inotuzumab ozogamicin; BM, bone marrow; Roddie et al., ASH 2023 16% 28% 53% 40% 31% 31% BM blasts % at screening BM blasts % prior to lymphodepletion 118/127 (93%) patients received bridging therapy* <5% ≥5−≤75% >75% 53% 31% 31% <5% ≥5−≤75% >75% 28% 41% 16%
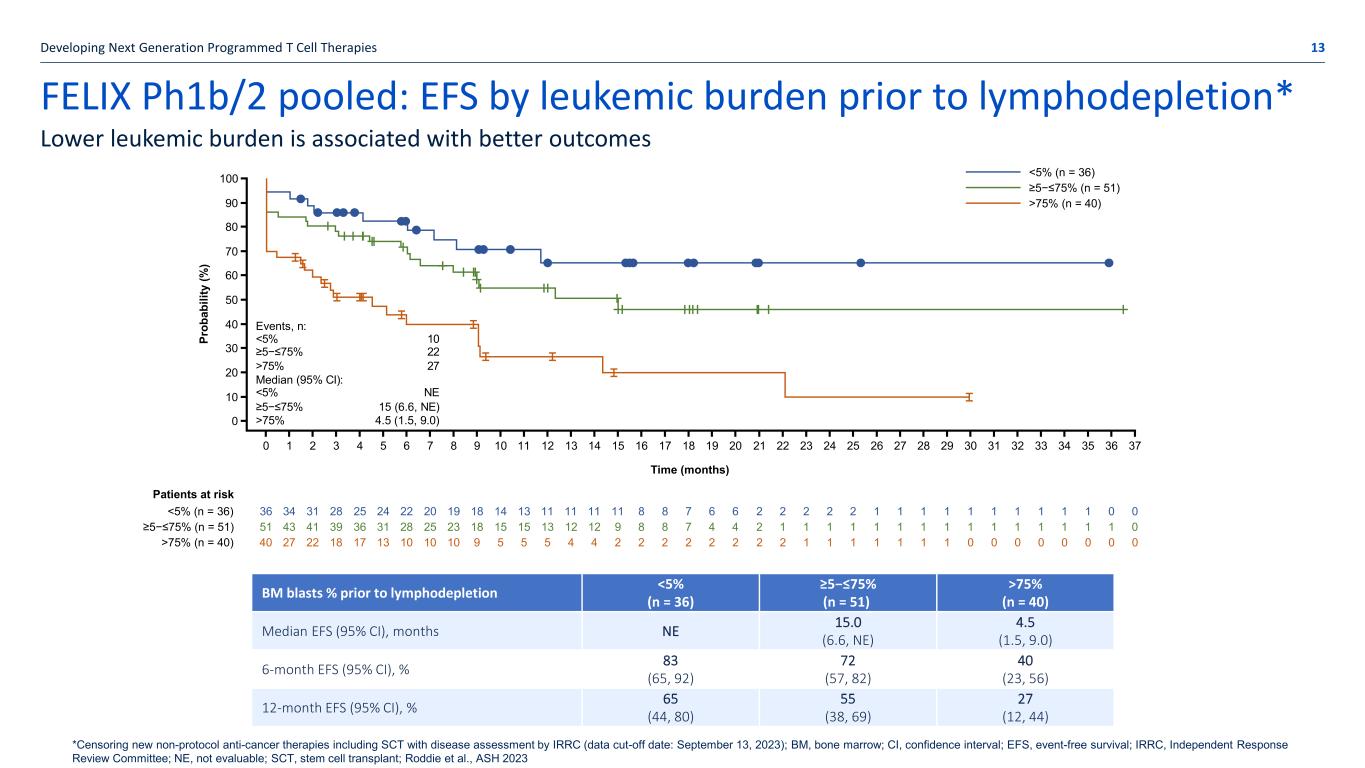
FELIX Ph1b/2 pooled: EFS by leukemic burden prior to lymphodepletion* Developing Next Generation Programmed T Cell Therapies 13 Lower leukemic burden is associated with better outcomes BM blasts % prior to lymphodepletion <5% (n = 36) ≥5−≤75% (n = 51) >75% (n = 40) Median EFS (95% CI), months NE 15.0 (6.6, NE) 4.5 (1.5, 9.0) 6-month EFS (95% CI), % 83 (65, 92) 72 (57, 82) 40 (23, 56) 12-month EFS (95% CI), % 65 (44, 80) 55 (38, 69) 27 (12, 44) Events, n: <5% 10 ≥5−≤75% 22 >75% 27 Median (95% CI): <5% NE ≥5−≤75% 15 (6.6, NE) >75% 4.5 (1.5, 9.0) Patients at risk <5% (n = 36) ≥5−≤75% (n = 51) >75% (n = 40) <5% (n = 36) ≥5−≤75% (n = 51) >75% (n = 40) 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 36 34 31 28 25 24 22 20 19 18 14 13 11 11 11 11 8 8 7 6 6 2 2 2 2 2 1 1 1 1 1 1 1 1 1 1 0 0 51 43 41 39 36 31 28 25 23 18 15 15 13 12 12 9 8 8 7 4 4 2 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 0 40 27 22 18 17 13 10 10 10 9 5 5 5 4 4 2 2 2 2 2 2 2 2 1 1 1 1 1 1 1 0 0 0 0 0 0 0 0 Time (months) 0 10 20 30 40 50 60 70 80 90 100 Pr ob ab ili ty (% ) *Censoring new non-protocol anti-cancer therapies including SCT with disease assessment by IRRC (data cut-off date: September 13, 2023); BM, bone marrow; CI, confidence interval; EFS, event-free survival; IRRC, Independent Response Review Committee; NE, not evaluable; SCT, stem cell transplant; Roddie et al., ASH 2023

FELIX Phase 1b/2 pooled analysis: CRS and ICANS Developing Next Generation Programmed T Cell Therapies 14 Low rates of Grade ≥3 CRS and/or ICANS were observed BM blasts % at lymphodepletion 2% 7% 20 0 40 60 80 100 In ci de nc e, % CRS ICANS 0% 4% 3% 20 0 40 60 80 100 In ci de nc e, % <5% ≥5−≤75% >75% 0% 6% 15% 20 0 40 60 80 100 In ci de nc e, % <5% ≥5−≤75% >75% CRS and ICANS in all patients CRS by % BM blasts ICANS by % BM blasts Light colors = grade ≤2 Dark colors = grade ≥3 69% 23% 47% 69% 88% 8% 18% 43% • No grade ≥3 CRS and/or ICANS were observed in patients with <5% BM blasts at lymphodepletion • Vasopressors were used to treat CRS in 2.4% of patients • The treatment was generally well tolerated • Two deaths were considered treatment-related per investigator assessment: neutropenic sepsis (n = 1); acute respiratory distress syndrome and ICANS (n = 1) BM, bone marrow; CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; ICU, intensive care unit; Roddie et al., ASH 2023

Obe-cel pooled analysis ASH 2023 ALLCAR19 Phase 1b /FELIX Ph 1b

Long-term follow up in R/R B-ALL demonstrates favorable EFS and OS Developing Next Generation Programmed T Cell Therapies 16 Median follow up 36.5 months; pooled analysis Phase 1b ALLCAR19/Phase 1b FELIX *Censored for allo-HSCT and other anti-cancer treatment. Investigator-assessed disease evaluations were performed locally by CT and BM biopsy for B-ALL. Allo-HSCT, allogeneic hematopoietic stem cell transplant; B-ALL, B-cell acute lymphoblastic leukemia; BM, bone marrow; CI, confidence interval; CT, computed tomography; EFS, event-free survival; N/A, not available; obe-cel, obecabtagene autoleucel; OS, overall survival; R/R, relapsed/refractory. Roddie et al, ASH 2023, Poster 2114. Median OS: 16.4 months (95% CI: 7.1–N/A) 36-month OS rate: 41% (95% CI: 24–56) With censoring*: • Median EFS: 9.0 months (95% CI: 5.1–N/A) • 36-month EFS rate: 45% (95% CI: 27–62) Without censoring: • Median EFS: 9.6 months (95% CI: 5.1–N/A) • 36-month EFS rate: 36% (95% CI: 21–51)
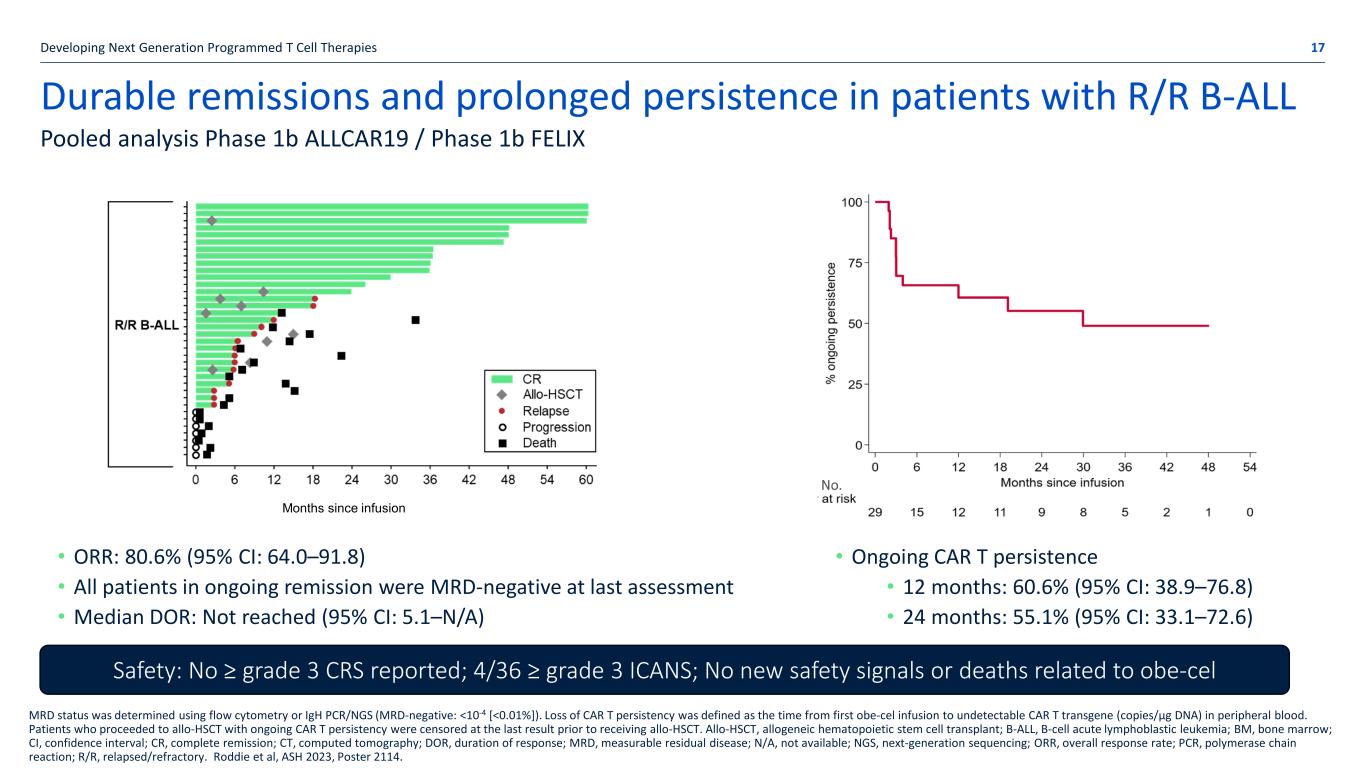
Durable remissions and prolonged persistence in patients with R/R B-ALL Developing Next Generation Programmed T Cell Therapies 17 Pooled analysis Phase 1b ALLCAR19 / Phase 1b FELIX MRD status was determined using flow cytometry or IgH PCR/NGS (MRD-negative: <10-4 [<0.01%]). Loss of CAR T persistency was defined as the time from first obe-cel infusion to undetectable CAR T transgene (copies/µg DNA) in peripheral blood. Patients who proceeded to allo-HSCT with ongoing CAR T persistency were censored at the last result prior to receiving allo-HSCT. Allo-HSCT, allogeneic hematopoietic stem cell transplant; B-ALL, B-cell acute lymphoblastic leukemia; BM, bone marrow; CI, confidence interval; CR, complete remission; CT, computed tomography; DOR, duration of response; MRD, measurable residual disease; N/A, not available; NGS, next-generation sequencing; ORR, overall response rate; PCR, polymerase chain reaction; R/R, relapsed/refractory. Roddie et al, ASH 2023, Poster 2114. Months since infusion • ORR: 80.6% (95% CI: 64.0–91.8) • All patients in ongoing remission were MRD-negative at last assessment • Median DOR: Not reached (95% CI: 5.1–N/A) Safety: No ≥ grade 3 CRS reported; 4/36 ≥ grade 3 ICANS; No new safety signals or deaths related to obe-cel • Ongoing CAR T persistence • 12 months: 60.6% (95% CI: 38.9–76.8) • 24 months: 55.1% (95% CI: 33.1–72.6) No.

Commercial Launch Readiness

Obe-cel steps to commercialization in r/r adult B-ALL 19 Roadmap to a 2024 commercial launch Obe-cel BLA accepted by FDA Obe-cel EU authorization application MHRA Nucleus inspection & approval Medical affairs engagement, value and HEOR evidence generation and center onboarding US launch preparation and execution Developing Next Generation Programmed T Cell Therapies FDA PDUFA Target FDA Action Date November 16 Biologics License Application (BLA) to U.S. Food and Drug Administration (FDA) accepted Obe-cel MHRA authorization application Filing of MHRA marketing authorization application (MAA) planned for H2 2024 • Obtained MIA together with accompanying GMP certificate • Filed EU marketing authorization application (MAA) Q1 Q2 Q3 Q4

Expanding the obe-cel opportunity Deep value program with potentially broad applicability
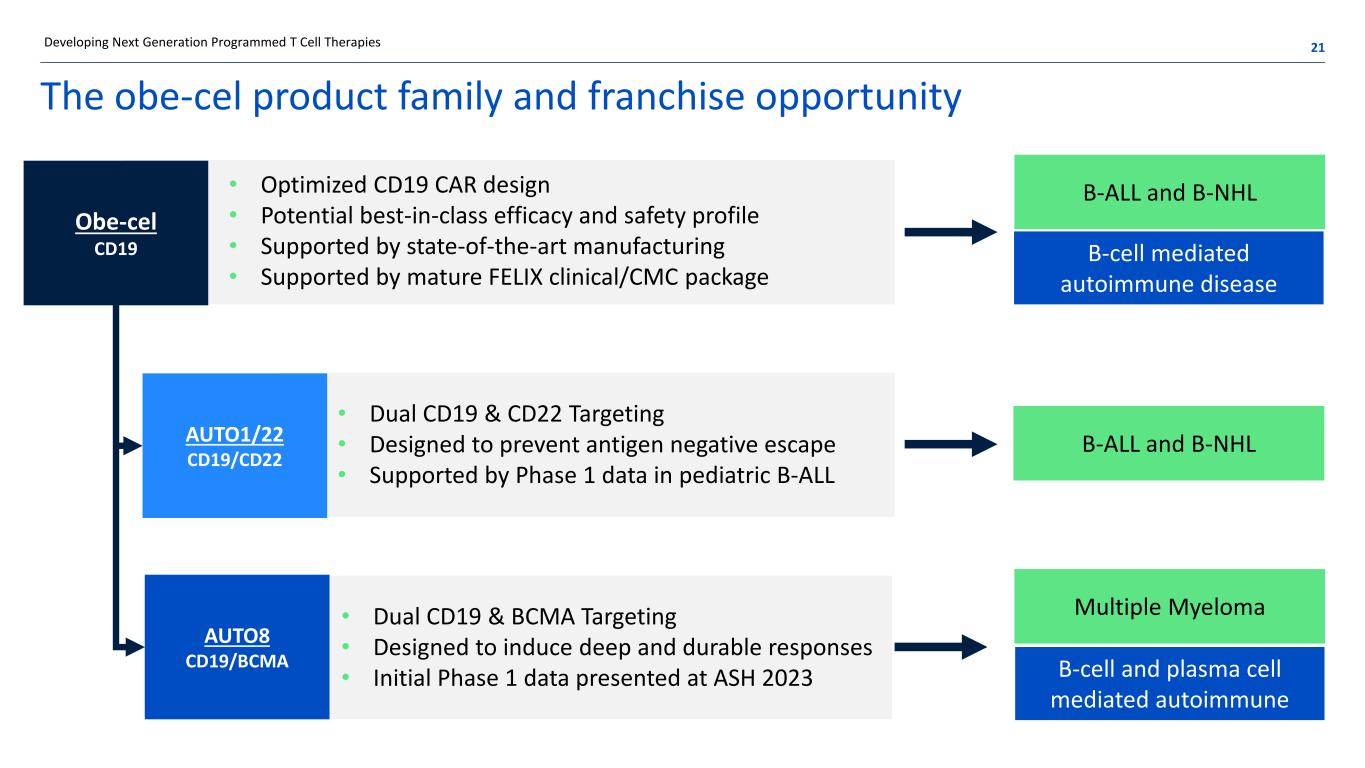
The obe-cel product family and franchise opportunity • Optimized CD19 CAR design • Potential best-in-class efficacy and safety profile • Supported by state-of-the-art manufacturing • Supported by mature FELIX clinical/CMC package • Dual CD19 & CD22 Targeting • Designed to prevent antigen negative escape • Supported by Phase 1 data in pediatric B-ALL • Dual CD19 & BCMA Targeting • Designed to induce deep and durable responses • Initial Phase 1 data presented at ASH 2023 B-ALL and B-NHL B-cell mediated autoimmune disease B-ALL and B-NHL Multiple Myeloma B-cell and plasma cell mediated autoimmune Developing Next Generation Programmed T Cell Therapies 21 Obe-cel CD19 AUTO1/22 CD19/CD22 AUTO8 CD19/BCMA

Phase 1 SLE study – CARLYSLE trial Developing Next Generation Programmed T Cell Therapies 22 A Single-Arm, Open-Label, Phase I Study to Determine the Safety, Tolerability and Preliminary Efficacy of Obecabtagene Autoleucel in Patients with Severe, Refractory Systemic Lupus Erythematosus (SLE) Placeholder • Study details – Number of patients: 6 - (option to add further cohort of 6 patients) – Primary endpoint: to establish the tolerability and safety of obe-cel in patients with severe, refractory SLE – Secondary endpoints: to evaluate the preliminary efficacy of obe-cel using measures of SLE disease activity – Dosing: 50 x 106 (± 20%) CD19 CAR-positive T cells – Follow up: up to 12 months 8 in Multiple Myeloma – Phase 1 MCARTY Study

Other pipeline programs and technologies A broad portfolio of potential next generation modular T cell therapies
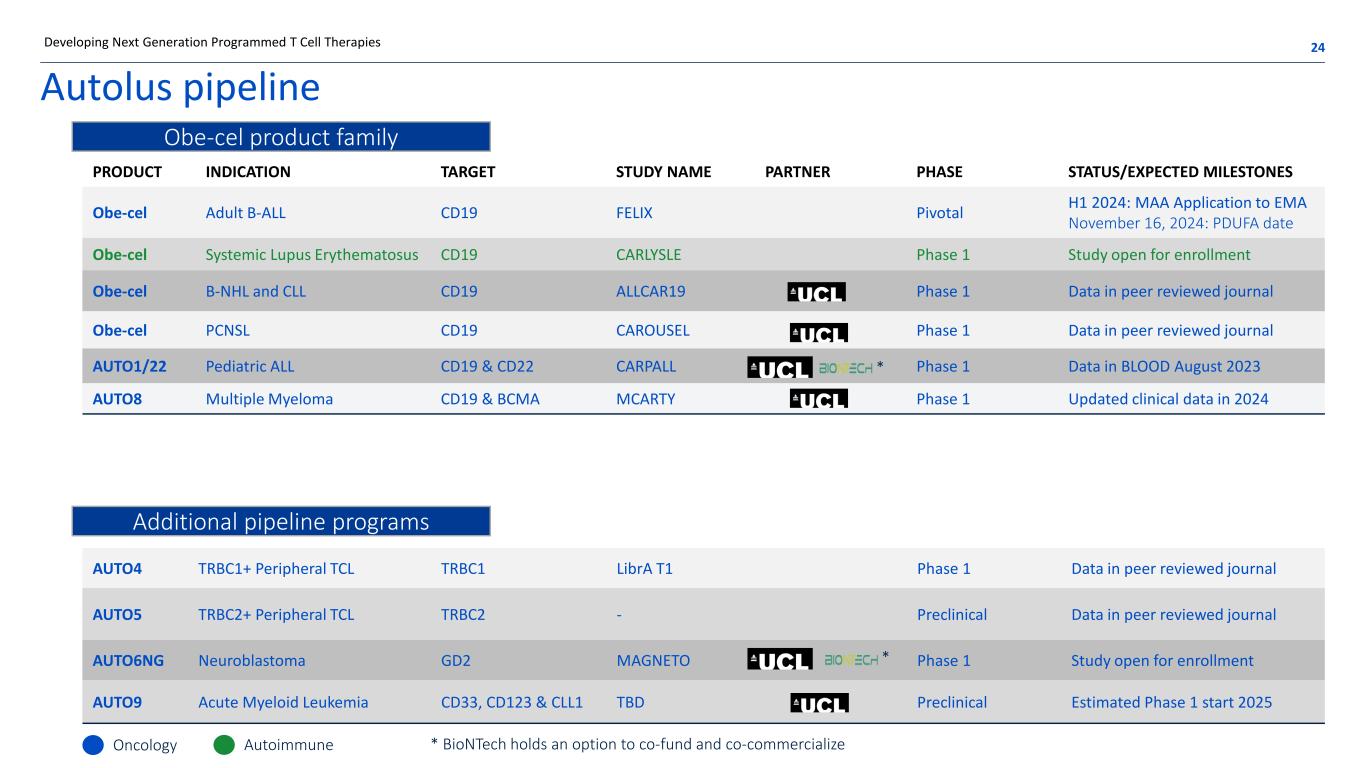
PRODUCT INDICATION TARGET STUDY NAME PARTNER PHASE STATUS/EXPECTED MILESTONES Obe-cel Adult B-ALL CD19 FELIX Pivotal H1 2024: MAA Application to EMA November 16, 2024: PDUFA date Obe-cel Systemic Lupus Erythematosus CD19 CARLYSLE Phase 1 Study open for enrollment Obe-cel B-NHL and CLL CD19 ALLCAR19 Phase 1 Data in peer reviewed journal Obe-cel PCNSL CD19 CAROUSEL Phase 1 Data in peer reviewed journal AUTO1/22 Pediatric ALL CD19 & CD22 CARPALL Phase 1 Data in BLOOD August 2023 AUTO8 Multiple Myeloma CD19 & BCMA MCARTY Phase 1 Updated clinical data in 2024 Autolus pipeline 24Developing Next Generation Programmed T Cell Therapies AUTO4 TRBC1+ Peripheral TCL TRBC1 LibrA T1 Phase 1 Data in peer reviewed journal AUTO5 TRBC2+ Peripheral TCL TRBC2 - Preclinical Data in peer reviewed journal AUTO6NG Neuroblastoma GD2 MAGNETO Phase 1 Study open for enrollment AUTO9 Acute Myeloid Leukemia CD33, CD123 & CLL1 TBD Preclinical Estimated Phase 1 start 2025 Oncology Autoimmune Obe-cel product family Additional pipeline programs * BioNTech holds an option to co-fund and co-commercialize * *

Financial Results

Financial summary (unaudited) USD Q4 2023 YTD ($ '000) Q4 2022 YTD ($ '000) Variance ($ '000) Grant Income - 166 (166) License revenues 1,698 6,194 (4,496) R&D1 (130,481) (117,354) (13,127) G&A (46,745) (31,899) (14,846) Loss on disposal of property and equipment (3,791) (515) (3,276) Impairment of right-of-use and related assets (382) - (382) Total operating expense, net (179,701) (143,408) (36,293) Other income (expense), net 2,861 2,038 823 Interest Income 13,505 1,708 11,797 Interest expense (45,067) (8,905) (36,162) Income tax benefit (expense)1 19 (272) 291 Net loss after tax (208,383) (148,839) (59,544) USD Q4 2023 ($ '000) Q4 2022 ($ '000) Variance ($ '000) Cash and cash equivalents 239,566 382,436 (142,870) Building a fully integrated, next-generation CAR T company 26 1Includes the presentation of our U.K. SME R&D Tax Credit with Income tax benefit as contra research and development expense in the amounts of $19.5 million and $24.6 million for the years ended December 31, 2023 and 2022, respectively.

Upcoming news flow

Autolus planned news flow 28Developing Next Generation Programmed T Cell Therapies Anticipated Milestone or Data Catalysts Anticipated Timing Obe-cel FELIX data update at ASCO, EHA & ASH 2024 June & December 2024 Obe-cel Marketing Authorization Application to MHRA Second half 2024 Obe-cel U.S. FDA PDUFA target action date November 16, 2024 Obe-cel in autoimmune disease – initial data from SLE Phase 1 study End 2024

Summary

Building a leading CAR T company developing therapies for cancer and autoimmune diseases 30 • FELIX pivotal trial showed high ORR, encouraging EFS and favorable tolerability with low levels of high-grade CRS and ICANS • PDUFA date 16 Nov 2024 • EMA filing submitted • Strategic multi-platform R&D collaboration with BioNTech • Established technology collaborations with Moderna, BMS and Cabaletta • Long-standing academic collaboration with University College London • Expand obe-cel opportunity in B cell malignancies, autoimmune diseases & life cycle strategy – SLE – B-NHL indications – Bi-specific therapies (CD19 /CD22; CD19/BCMA) • Expand to additional indications with novel CAR T therapies, alone or with partners • Demonstrated reliable clinical trial supply (96% target dose reached in FELIX pivotal study) • New commercial cell manufacturing facility in qualification stage; planned annual capacity 2,000+ batches • Expected vein-to- delivery time at launch of ~16 days Obe-cel potentially best in class CAR T for r/r adult ALL Strategic collaborations Pipeline expansion strategy Scalable manufacturing and in-house facility • Cash $240M (Q4 2023) and gross proceeds of $600m from financing and BioNTech transaction • Fully funds obe-cel launch and allows for autoimmune program acceleration Strong cash position Scaling company toward commercialization $ Developing Next Generation Programmed T Cell Therapies Abbreviations and notes: r/r ALL - relapsed/refractory acute lymphoblastic leukemia; B-NHL – B-cell non-Hodgkin’s lymphoma; SLE – systemic lupus erythematosus.

autolus.com Thank you






























