
Q3 2024 Financial Results and Business Updates 12 November 2024 Autolus.com For Investor communication only. Not for use in product promotion. Not for further distribution. EX-99.2

Disclaimer These slides contain forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements are statements that are not historical facts, and in some cases can be identified by terms such as “may,” “will,” “could,” “expects,” “plans,” “anticipates,” and “believes.” These statements include, but are not limited to: statements regarding Autolus’ development and commercialization of its product candidates; Autolus' manufacturing, sales and marketing plans for AUCATZYL, including expectations regarding the timing of commercial launch in the United States and the ability to reach patients in a timely manner; the amount and timing of milestone payments under Autolus' collaboration and license agreements; and future development plans of obe-cel, including the timing or likelihood of expansion into additional markets or geographies and related regulatory approvals. Any forward-looking statements are based on management’s current views and assumptions and involve risks and uncertainties that could cause actual results, performance, or events to differ materially from those expressed or implied in such statements. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation: Autolus' ability to maintain regulatory approval of AUCATZYL; its ability to execute its commercialization strategy for AUCATZYL; its ability to develop, manufacture and commercialize its other product candidates and the timing or likelihood of expansion of AUCATZYL into additional markets or geographies; Autolus' ability to establish and expand a commercial infrastructure and to successfully launch, market and sell AUCATZYL; actions of regulatory agencies, which may affect the initiation, timing and progress of clinical trials or future regulatory approval; the labelling for AUCATZYL/obe-cel in any future indication or patient population, if approved; the potential for payors to delay, limit or deny coverage for AUCATZYL; Autolus' ability to obtain, maintain and enforce intellectual property protection for AUCATZYL or any product candidates it is developing; the results of clinical trials are not always being predictive of future results; the cost, timing and results of clinical trials; that many product candidates do not become approved drugs on a timely or cost effective basis or at all; the ability to enroll patients in clinical trials; and possible safety and efficacy concerns. For a discussion of other risks and uncertainties, and other important factors, any of which could cause Autolus’ actual results to differ from those contained in the forward-looking statements, see the section titled “Risk Factors” in Autolus' Annual Report on Form 10-K filed with the Securities and Exchange Commission, or the SEC, on March 21, 2024, as well as discussions of potential risks, uncertainties, and other important factors in Autolus’ subsequent filings with the Securities and Exchange Commission. All information in this presentation is as of the date of the presentation, and Autolus undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events, or otherwise, except as required by law. You should, therefore, not rely on these forward-looking statements as representing the Company’s views as of any date subsequent to the date of this presentation. 2

Agenda 3 • Welcome and Introduction: Olivia Manser, Director, Investor Relations • Operational Highlights: Dr. Christian Itin, CEO • Financial Results: Rob Dolski, CFO • Upcoming Milestones and Conclusion: Dr. Christian Itin, CEO • Q&A: Dr. Christian Itin and Rob Dolski

AUCATZYL® now FDA approved 4 ✓ AUCATZYL indicated for the treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (B-ALL) ✓ First chimeric antigen receptor T-cell (CAR T) therapy approved by the FDA with no requirement for a REMS program (Risk Evaluation Mitigation Strategy) ✓ Novel and differentiated mechanism of action: first and currently only approved CD19 CAR T with a fast off-rate ✓ First and currently only approved CAR T therapy with customized, tumor-burden guided dosing Please see full prescribing information Prescribing information including BOXED WARNING Post-period event

Pillars to drive launch success 5 30 key centers primed for activation covering ~ 60% of r/r B-ALL target population with ~30 additional centers to follow by end 2025 Prioritizing activation of centers Post-approval Pricing reflects clinical evidence, differentiated safety profile, economic value $525,000 WAC1 Pricing strategy focused on delivering value to customers and achieving broad coverage Robust and reliable supply Team dedicated to successful commercial efforts Experienced team with multiple CAR T launches Strong scientific communication and physician engagement within medical affairs Dedicated single point-of-contact for every center 1Wholesale acquisition cost, or WAC, before any discounts, rebates or other price concessions The Nucleus: Autolus’ state-of-the-art, dedicated purpose-built facility Target vein-to-release time of ~16 days

Autolus executed to plan in Q3 2024 6 Clinical Operational • Obe-cel progressing according to plan – AUCATZYL approved by FDA ahead of PDUFA target action date – Under review by both the EMA and MHRA and submitted to NICE in the UK – Additional data from the FELIX study – Society of Hematologic Oncology meeting in August 2024 demonstrating the rationale for tumor burden (TB)-guided dosing – Post period – Lymphoma Leukemia & Myeloma Congress in October 2024 which suggested that patients who underwent Stem Cell Transplant had poorer outcomes and reducing tumor burden prior to lymphodepletion is crucial for improved outcomes • Appointed Matthias Will, MD as Chief Development Officer • Continued expansion of commercial team and onboarding of treatment centers

Upcoming data at ASH 7 Clinical • Deep Molecular Remission May Predict Better Outcomes – Abstract 194508 - Oral presentation – Dr. Elias Jabbour - Monday, December 9, 2024; 4:30 PM - 6:00 PM PT • The Impact of Bridging Therapies on CAR T-Cell Expansion and Persistence – Abstract 201514 – Poster presentation – Dr. Jae Park – Sunday, December 8, 2024; 6:00 PM - 8:00 PM PT • Healthcare Resource Utilization and Costs Associated with Managing CRS and ICANS – Abstract 205694 – Poster presentation – Dr. Bijal D Shah – Monday, December 9, 2024; 6:00 PM - 8:00 PM PT • What We Have Learned from the FELIX Trial – Abstract 208028 – Poster presentation – Dr. Claire Roddie - Monday, December 9, 2024; 6:00 PM - 8:00 PM PT

Expanding the obe-cel opportunity Deep value program with potentially broad applicability
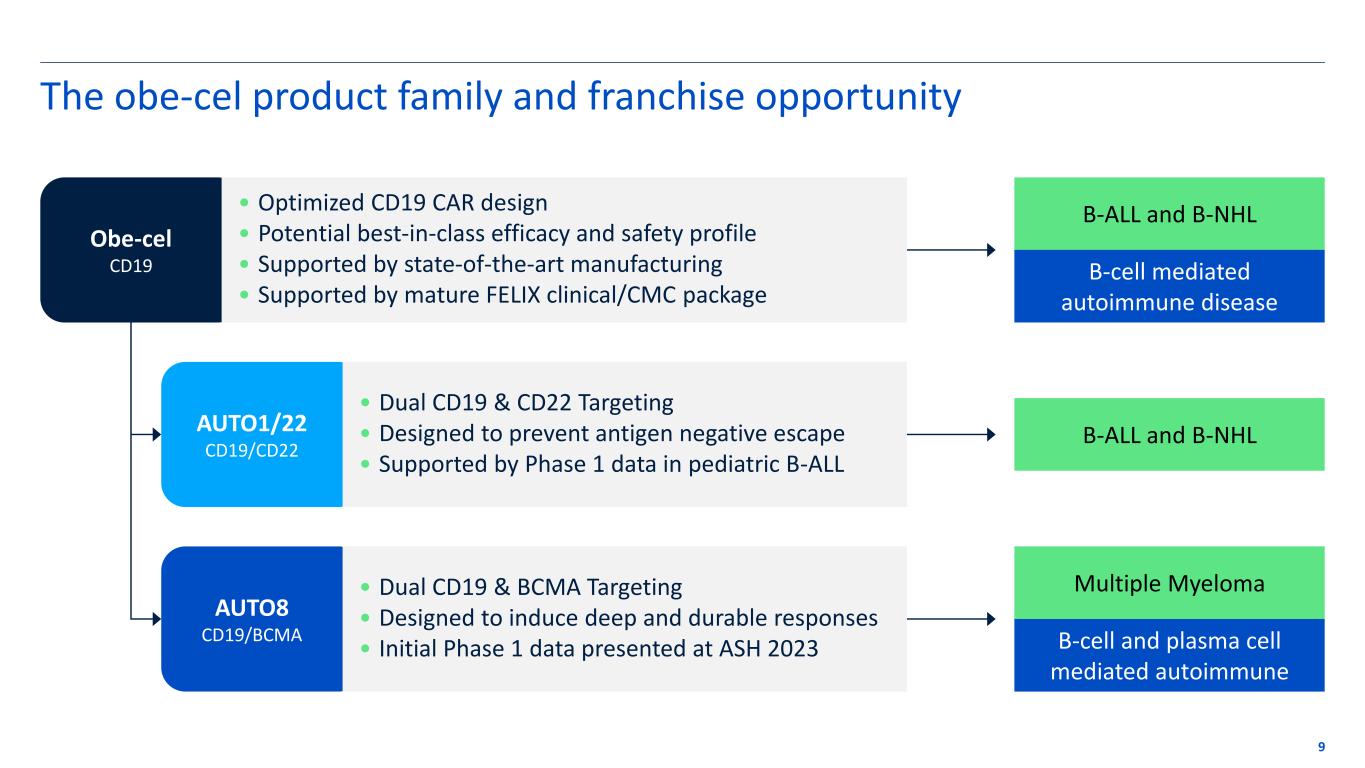
The obe-cel product family and franchise opportunity 9 Obe-cel CD19 AUTO1/22 CD19/CD22 AUTO8 CD19/BCMA B-ALL and B-NHL B-cell mediated autoimmune disease B-ALL and B-NHL Multiple Myeloma B-cell and plasma cell mediated autoimmune • Optimized CD19 CAR design • Potential best-in-class efficacy and safety profile • Supported by state-of-the-art manufacturing • Supported by mature FELIX clinical/CMC package • Dual CD19 & CD22 Targeting • Designed to prevent antigen negative escape • Supported by Phase 1 data in pediatric B-ALL • Dual CD19 & BCMA Targeting • Designed to induce deep and durable responses • Initial Phase 1 data presented at ASH 2023

Dynamic environment in cell therapy for autoimmune patients 10 EULAR updates and abstracts for ACR2024 continue to support overall proof of concept/biology in autoimmune disease • Available clinical data is largely based on compassionate use experience with more clinical trial data emerging • A Kymriah-like autologous CAR T program showed transformational clinical outcomes in refractory autoimmune patients – To date a single myositis patient relapsed after 18 months (compassionate use cohort) – Response rate expectations for the field were set high ~100% • Some variability in clinical outcomes is beginning to emerge – All CD19 CAR Ts may not be alike; different design and manufacturing process may contribute – Patient populations vary across data sets – All autoimmune indications may not be alike – inflammatory process versus structural damage • Obe-cel has shown profound removal of the B cell compartment, indicated by the long-term outcomes in ALL without subsequent therapy while showing a favorable safety profile in this challenging patient population. • Obe-cel is well positioned for autoimmune disease – Phase 1 CARLYSLE study in advanced SLE ongoing – Enrolment due to complete in Q1 25 and initial data in Q1 25 Autoimmune

Financial Results

Financial summary (unaudited) USD ($’ 000) Q3 2024 Q4 2023 Variance Cash and cash equivalents 657,067 239,566 417,501 USD ($’ 000) Q3 2024 Q3 2023 Variance License revenue - 406 (406) Operating expenses: R&D (40,323) (32,318) (8,005) G&A (27,330) (10,611) (16,719) Loss on disposal of property and equipment (223) - (223) Impairment of operating lease right-of-use assets and related property and equipment - (382) 382 Total operating expense, net (67,876) (42,905) (24,971) Other income, net 54 136 (82) Foreign exchange losses (11,884) (1,733) (10,151) Interest Income 8,320 3,646 4,674 Interest expense (10,686) (5,014) (5,672) Income tax (expense) benefit (22) 21 (43) Net loss (82,094) (45,849) (36,245) 12

Upcoming news flow

Autolus planned news flow 14 Anticipated Milestone or Data Catalysts Anticipated Timing Obe-cel FELIX data update at ASH 2024 December 2024 Initial data from SLE Phase 1 trial Q1 2025 Initial data from PY01 trial in pediatric ALL 2H 2025 SLE Phase 1 trial presentation at medical conference 2H 2025 Oncology Autoimmune

Autolus.com Thank you














