
Developing Next Generation Programmed T Cell Therapies January 2025 Autolus.com

Disclaimer These slides contain forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements are statements that are not historical facts, and in some cases can be identified by terms such as “may,” “will,” “could,” “expects,” “plans,” “anticipates,” and “believes.” These statements include, but are not limited to, statements regarding Autolus’ development of its product candidates, including the obe-cel program; the profile and potential application of obe- cel in additional disease settings; the future clinical development, efficacy, safety and therapeutic potential of the Company’s product candidates, including progress, expectations as to the reporting of data, conduct and timing and potential future clinical and preclinical activity and milestones; expectations regarding the initiation, design and reporting of data from clinical trials and preclinical studies; the extension of the pipeline beyond obe-cel; expectations regarding the regulatory approval process for any product candidates; the benefits of the collaboration between Autolus and BioNTech, including the potential and timing of milestone payments and royalties under the terms of the strategic collaboration; the Company’s current and future manufacturing capabilities; and the Company’s anticipated cash runway. Any forward-looking statements are based on management’s current views and assumptions and involve risks and uncertainties that could cause actual results, performance, or events to differ materially from those expressed or implied in such statements. These risks and uncertainties include, but are not limited to, the risks that Autolus’ preclinical or clinical programs do not advance or result in approved products on a timely or cost effective basis or at all; the results of early clinical trials are not always being predictive of future results; the cost, timing and results of clinical trials; that many product candidates do not become approved drugs on a timely or cost effective basis or at all; the ability to enroll patients in clinical trials; and possible safety and efficacy concerns. For a discussion of other risks and uncertainties, and other important factors, any of which could cause Autolus’ actual results to differ from those contained in the forward-looking statements, see the section titled “Risk Factors” in Autolus' Annual Report on Form 10-Q filed with the Securities and Exchange Commission, or the SEC, on November 12, 2024, as well as discussions of potential risks, uncertainties, and other important factors in Autolus’ subsequent filings with the Securities and Exchange Commission. All information in this presentation is as of the date of the presentation, and Autolus undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events, or otherwise, except as required by law. You should, therefore, not rely on these forward-looking statements as representing the Company’s views as of any date subsequent to the date of this presentation. Developing Next Generation Programmed T Cell Therapies 2

Autolus is positioned for commercial execution and market expansion Obe-cel product franchise supports multiple growth opportunities • EU/UK approvals expected 2H 2025 • Expanding obe-cel opportunity in hem-oncology and autoimmune diseases • Developing early-stage pipeline of novel CAR-T therapies Commercial execution and market expansion supported by: Developing Next Generation Programmed T Cell Therapies 3 Design Build Operations In-house, purpose-built manufacturing facility Strategic collaborations and strong cash position $657M as of Q3 2024

AUCATZYL® AUTOLUS’ FIRST APPROVED PRODUCT A potentially best-in-class, standalone CD19 CAR T cell therapy

AUCATZYL® now FDA approved 5 AUCATZYL indicated for the treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (B-ALL) First chimeric antigen receptor T-cell (CAR T) therapy approved by the FDA with no requirement for a REMS program (Risk Evaluation Mitigation Strategy) Novel and differentiated mechanism of action: first and currently only approved CD19 CAR T with a fast off-rate First and currently only approved CAR T therapy with customized, tumor-burden guided dosingPlease see full prescribing information Prescribing information

Important Safety Information 6 • The safety of AUCATZYL includes a boxed warning for CRS, neurologic toxicities, and secondary hematological malignancies. ICANS, including fatal or life-threatening reactions, occurred in patients receiving AUCATZYL. T-cell malignancies have occurred following treatment of hematologic malignancies with BCMA- and CD19-directed genetically modified autologous T-cell immunotherapies. • In the FELIX trial, severe, including life-threatening and fatal infections occurred in patients after AUCATZYL infusion. The non-COVID-19 infections of all grades occurred in 67% (67/100) of patients. Grade 3 or higher non-COVID-19 infections occurred in 41% (41/100) of patients. • Please see full Prescribing Information, including BOXED WARNING and Medication Guide.
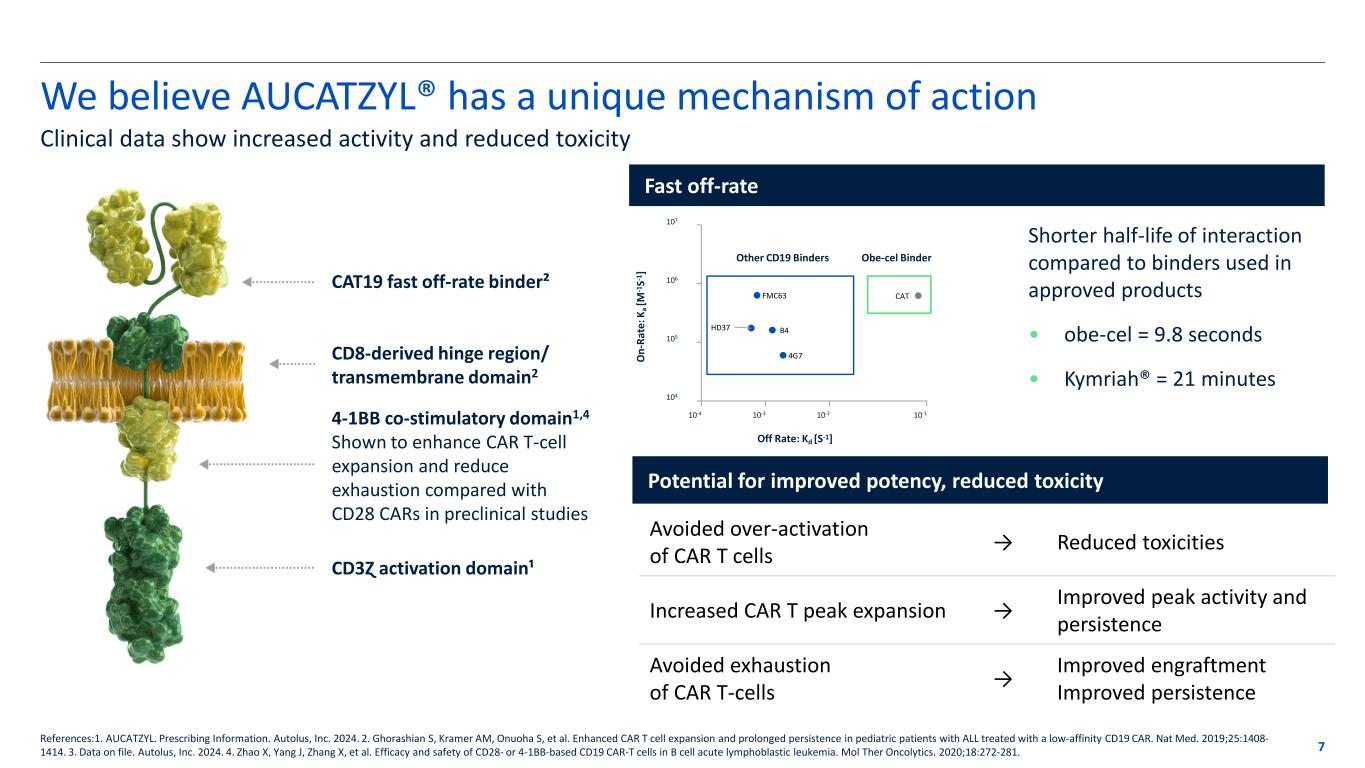
Potential for improved potency, reduced toxicity We believe AUCATZYL® has a unique mechanism of action 7 Clinical data show increased activity and reduced toxicity References:1. AUCATZYL. Prescribing Information. Autolus, Inc. 2024. 2. Ghorashian S, Kramer AM, Onuoha S, et al. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat Med. 2019;25:1408- 1414. 3. Data on file. Autolus, Inc. 2024. 4. Zhao X, Yang J, Zhang X, et al. Efficacy and safety of CD28- or 4-1BB-based CD19 CAR-T cells in B cell acute lymphoblastic leukemia. Mol Ther Oncolytics. 2020;18:272-281. Fast off-rate Avoided over-activation of CAR T cells → Reduced toxicities Increased CAR T peak expansion → Improved peak activity and persistence Avoided exhaustion of CAR T-cells → Improved engraftment Improved persistence CAT19 fast off-rate binder² CD8-derived hinge region/ transmembrane domain2 4-1BB co-stimulatory domain1,4 Shown to enhance CAR T-cell expansion and reduce exhaustion compared with CD28 CARs in preclinical studies CD3Ɀ activation domain¹ HD37 FMC63 B4 4G7 CAT Off Rate: Kd [S-1] O n- Ra te : K a [M -1 S- 1 ] Other CD19 Binders Obe-cel Binder 10-4 10-3 10-2 10-1 107 106 105 104 Shorter half-life of interaction compared to binders used in approved products • obe-cel = 9.8 seconds • Kymriah® = 21 minutes

AUCATZYL was approved based on results from the FELIX trial 8 FELIX Phase 1b/2 Cohort IA ≥5% BM blast 1,2 Roddie C, et al "Obecabtagene autoleucel in B-cell acute lymphoblastic leukemia" N Engl J Med 2024; DOI: 10.1056/NEJMoa2406526 Cohort IB <5% BM blast MRD+ Cohort IIA ≥5% BM blast Cohort IIB <5% BM blast MRD+ Cohort IIC Isolated EMD at screening Patients (N) Ph1b/2 pooled1 Enrolled 153 Infused 127 Background • Open-label, multinational, single-arm Phase 1b/2 trial in adult patients with R/R B-ALL1-2; largest CAR T cell therapy trial in R/R B-ALL to date (N=153 enrolled) • Conducted during COVID-19 pandemic with highly immune compromised patients Summary of Trial Experience • High ORR, encouraging EFS/OS and favorable tolerability with low levels of high- grade CRS and ICANS • Timely and reliable clinical product supply and logistics despite COVID-19 pandemic restrictions • Across all Phase 1b/2 cohorts, 40% of responders in ongoing remission without subsequent stem cell transplant/other therapy1 • Survival outcomes suggesting potential of long-term plateau1
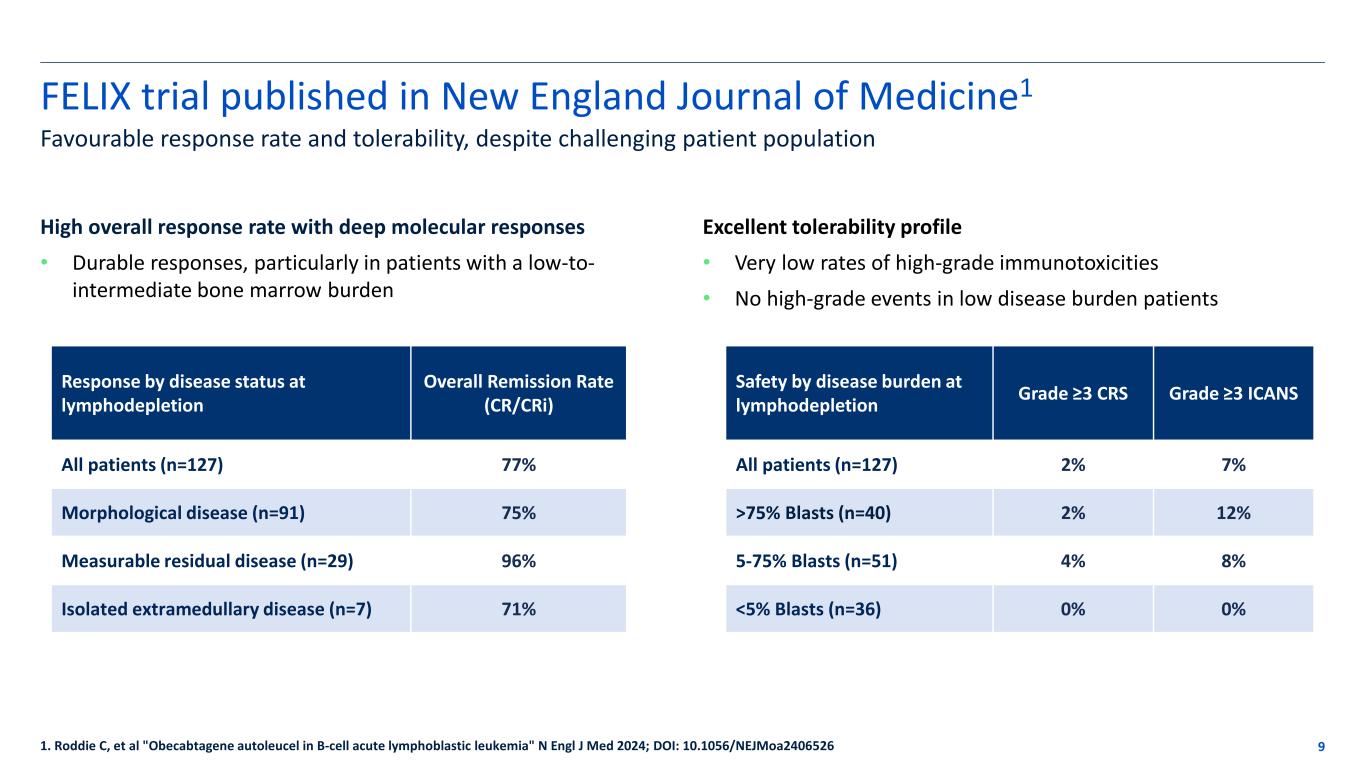
FELIX trial published in New England Journal of Medicine1 9 Favourable response rate and tolerability, despite challenging patient population 1. Roddie C, et al "Obecabtagene autoleucel in B-cell acute lymphoblastic leukemia" N Engl J Med 2024; DOI: 10.1056/NEJMoa2406526 High overall response rate with deep molecular responses • Durable responses, particularly in patients with a low-to- intermediate bone marrow burden Response by disease status at lymphodepletion Overall Remission Rate (CR/CRi) All patients (n=127) 77% Morphological disease (n=91) 75% Measurable residual disease (n=29) 96% Isolated extramedullary disease (n=7) 71% Excellent tolerability profile • Very low rates of high-grade immunotoxicities • No high-grade events in low disease burden patients Safety by disease burden at lymphodepletion Grade ≥3 CRS Grade ≥3 ICANS All patients (n=127) 2% 7% >75% Blasts (n=40) 2% 12% 5-75% Blasts (n=51) 4% 8% <5% Blasts (n=36) 0% 0%

Deep molecular responses result in long term remissions in adult ALL Survival outcomes show potential of long-term plateau with 12-month EFS rates 49.5% • In all patients, the median EFS was 11.9 months • Lower disease burden at lymphodepletion was associated with better outcomes 1. Roddie C, et al "Obecabtagene autoleucel in B-cell acute lymphoblastic leukemia" N Engl J Med 2024; DOI: 10.1056/NEJMoa2406526

Deep molecular responses result in long term remissions in adult ALL • In all patients, the median OFS was 15.6 months • Lower disease burden at lymphodepletion was associated with better outcomes 1. Roddie C, et al "Obecabtagene autoleucel in B-cell acute lymphoblastic leukemia" N Engl J Med 2024; DOI: 10.1056/NEJMoa2406526 Estimated 6- and 12-month overall survival rates were 80.3% and 61.1%, respectively

AUCATZYL® is poised to fill the unmet need for r/r ALL patients •We believe AUCATZYL is a transformative product in the r/r ALL space •Unique MOA designed to deliver potency and persistency that results in deep and durable efficacy •Favorable tolerability profile •Customized tumor-burden guided dosing •Well-positioned to deliver therapy globally with Autolus’ proven reliable manufacturing 12

Commercial Launch

Pillars to drive commercial success 14 30 key centers primed for activation covering ~ 60% of r/r B-ALL target population with ~30 additional centers to follow by end 2025 Prioritizing authorization of centers Post-approval Pricing reflects clinical evidence, differentiated safety profile, economic value $525,000 WAC1 Pricing strategy focused on delivering value to customers and achieving broad coverage Scalable, efficient and reliable supply Team dedicated to successful commercial efforts Experienced team with multiple CAR T launches Strong scientific communication and physician engagement within medical affairs Dedicated single point-of-contact for every center 1Wholesale acquisition cost, or WAC, before any discounts, rebates or other price concessions The Nucleus: Autolus’ state-of-the-art, dedicated purpose-built facility Target vein-to-release time of ~16 days

AUCATZYL® Authorized Treatment Centers 15 30 centers covering 60% of target population ~60 centers covering 90% of population Near-Term Plan: End of 2025: https://www.autolusassist.com/find-a-treatment-center/ 24 Centers Authorized as of January 10, 2025
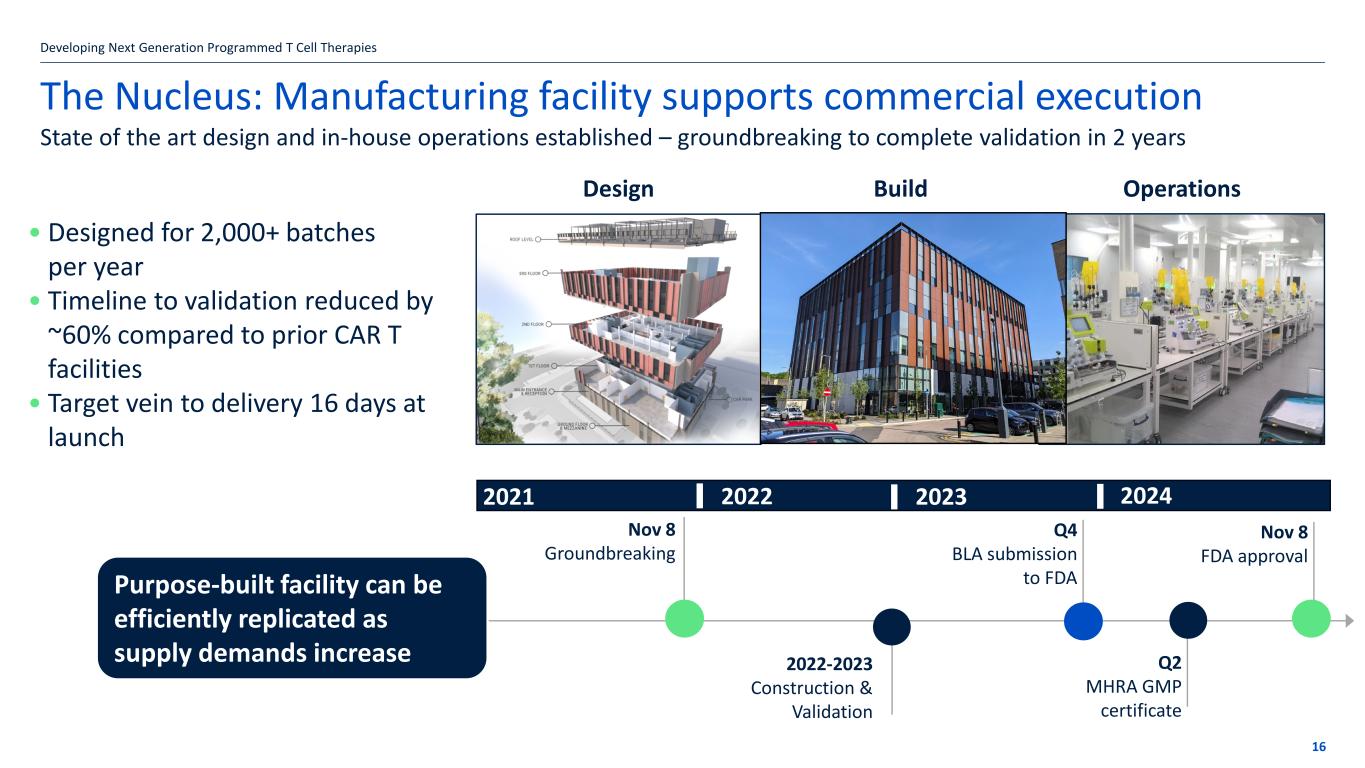
The Nucleus: Manufacturing facility supports commercial execution • Designed for 2,000+ batches per year • Timeline to validation reduced by ~60% compared to prior CAR T facilities • Target vein to delivery 16 days at launch Developing Next Generation Programmed T Cell Therapies 16 State of the art design and in-house operations established – groundbreaking to complete validation in 2 years Design Build Operations Nov 8 Groundbreaking 2021 2024 Q2 MHRA GMP certificate Q4 BLA submission to FDA Nov 8 FDA approval 2022-2023 Construction & Validation 2022 2023 Purpose-built facility can be efficiently replicated as supply demands increase

ALL: unmet need and market overview
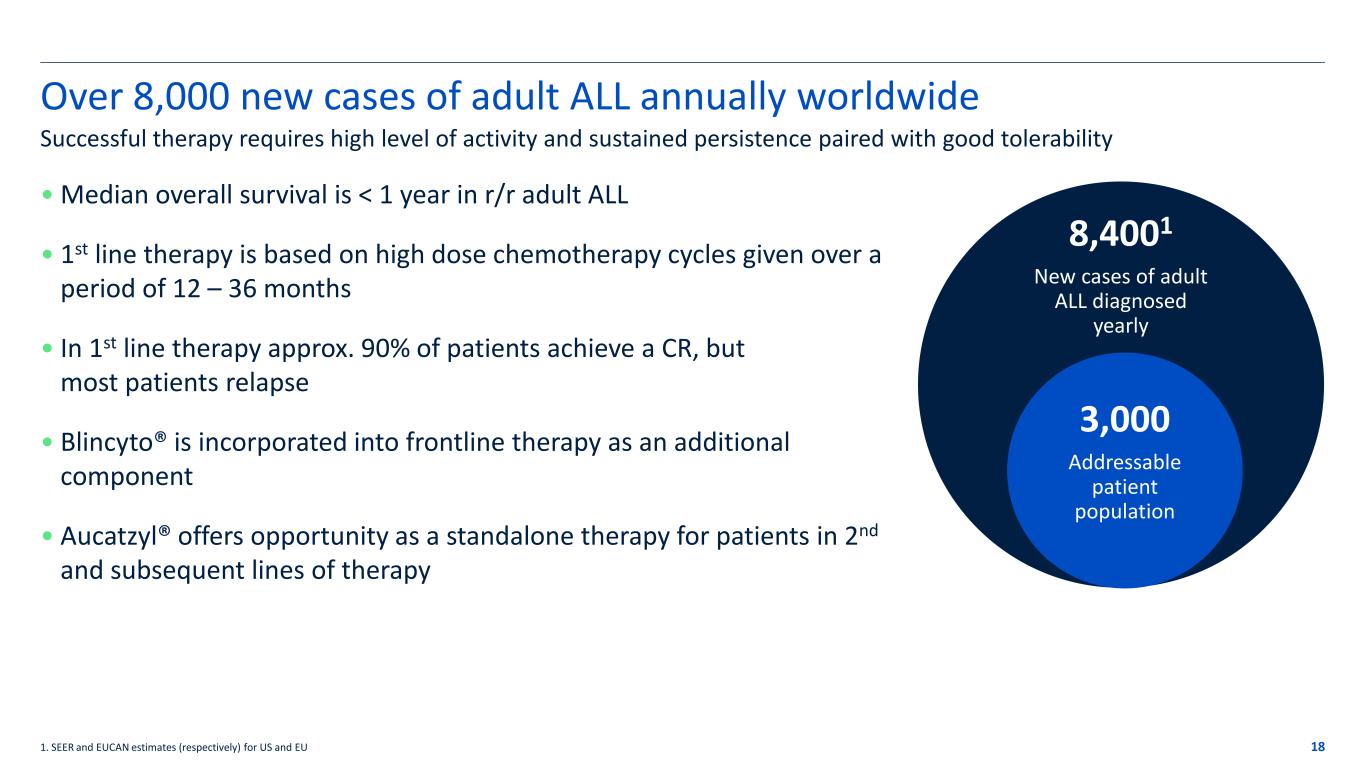
Over 8,000 new cases of adult ALL annually worldwide • Median overall survival is < 1 year in r/r adult ALL • 1st line therapy is based on high dose chemotherapy cycles given over a period of 12 – 36 months • In 1st line therapy approx. 90% of patients achieve a CR, but most patients relapse • Blincyto® is incorporated into frontline therapy as an additional component • Aucatzyl® offers opportunity as a standalone therapy for patients in 2nd and subsequent lines of therapy 18 Successful therapy requires high level of activity and sustained persistence paired with good tolerability 1. SEER and EUCAN estimates (respectively) for US and EU 8,4001 New cases of adult ALL diagnosed yearly 3,000 Addressable patient population

Expanding the obe-cel opportunity Deep value program with potentially broad applicability
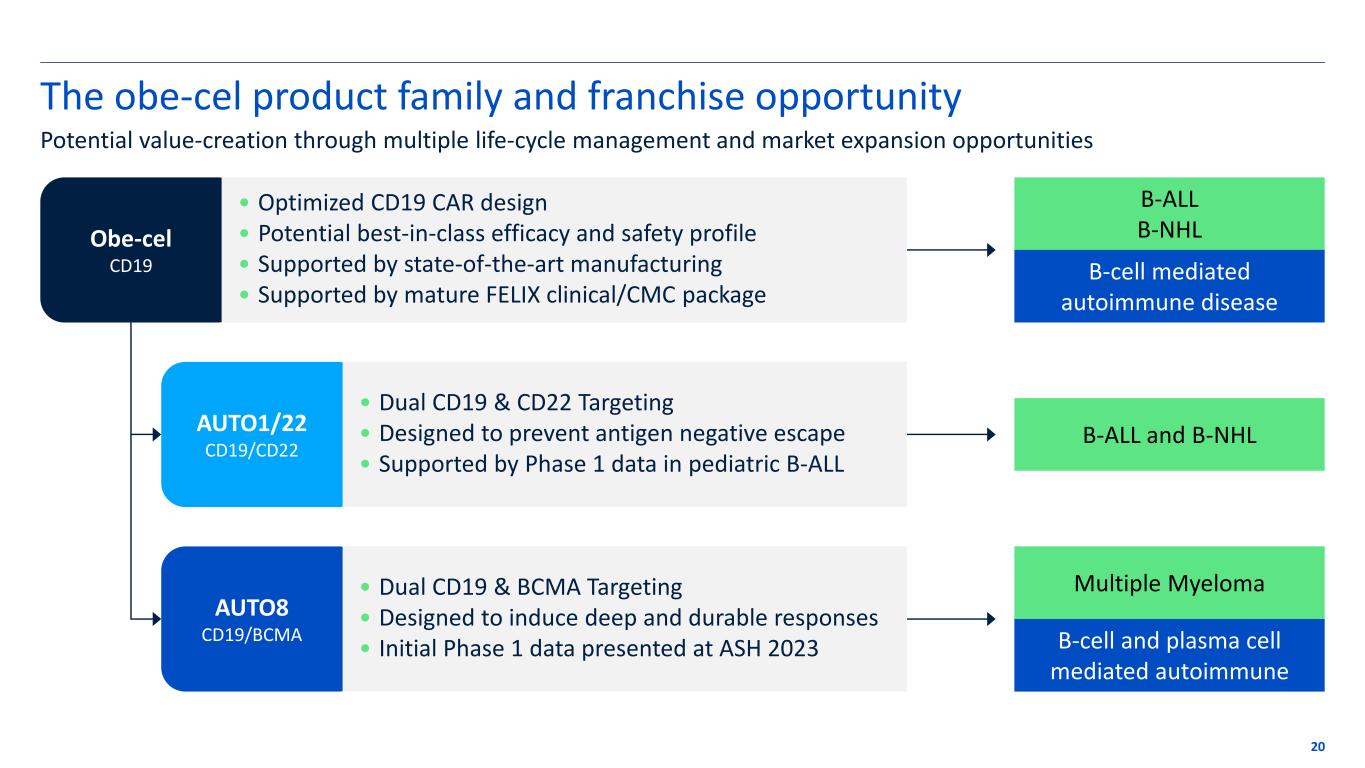
The obe-cel product family and franchise opportunity 20 Potential value-creation through multiple life-cycle management and market expansion opportunities Obe-cel CD19 AUTO1/22 CD19/CD22 AUTO8 CD19/BCMA B-ALL B-NHL B-cell mediated autoimmune disease B-ALL and B-NHL Multiple Myeloma B-cell and plasma cell mediated autoimmune • Optimized CD19 CAR design • Potential best-in-class efficacy and safety profile • Supported by state-of-the-art manufacturing • Supported by mature FELIX clinical/CMC package • Dual CD19 & CD22 Targeting • Designed to prevent antigen negative escape • Supported by Phase 1 data in pediatric B-ALL • Dual CD19 & BCMA Targeting • Designed to induce deep and durable responses • Initial Phase 1 data presented at ASH 2023
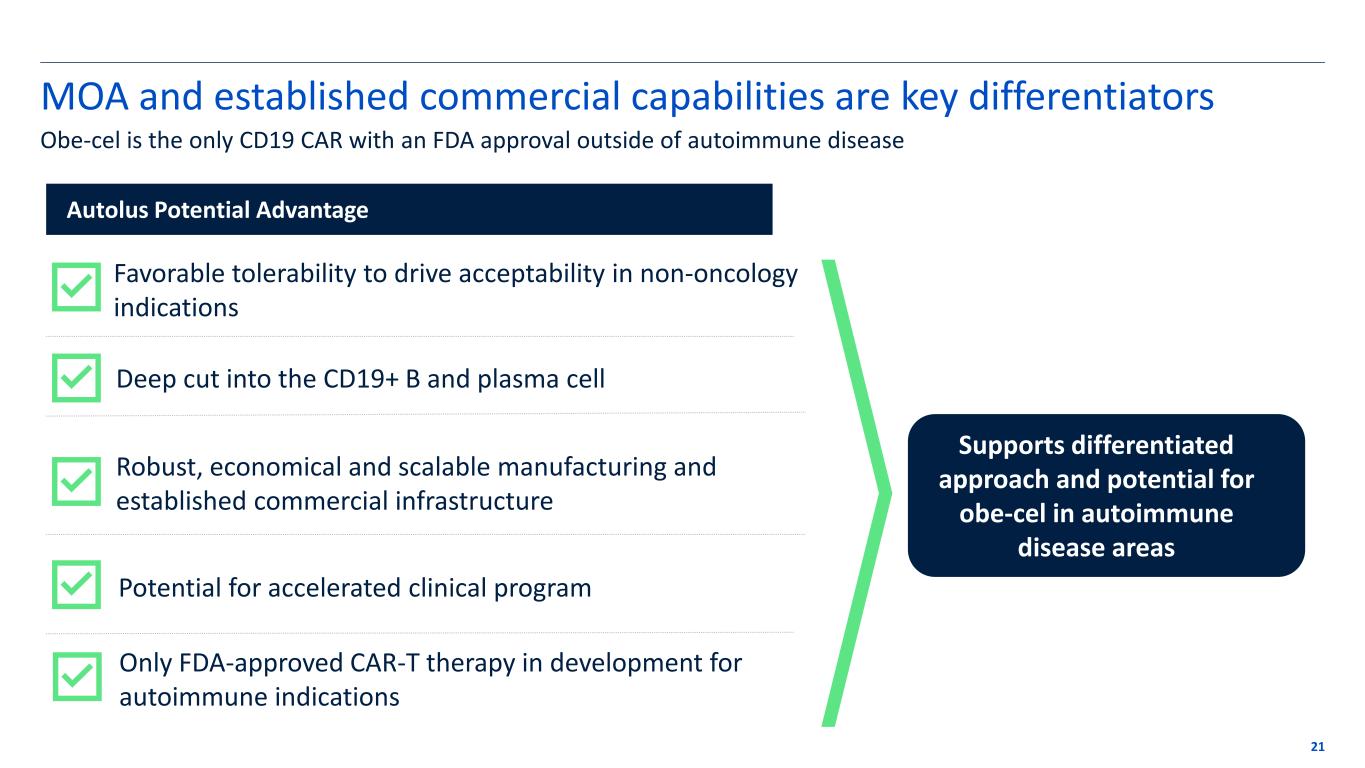
MOA and established commercial capabilities are key differentiators 21 Autolus Potential Advantage Favorable tolerability to drive acceptability in non-oncology indications Deep cut into the CD19+ B and plasma cell Robust, economical and scalable manufacturing and established commercial infrastructure Potential for accelerated clinical program Supports differentiated approach and potential for obe-cel in autoimmune disease areas Only FDA-approved CAR-T therapy in development for autoimmune indications Obe-cel is the only CD19 CAR with an FDA approval outside of autoimmune disease
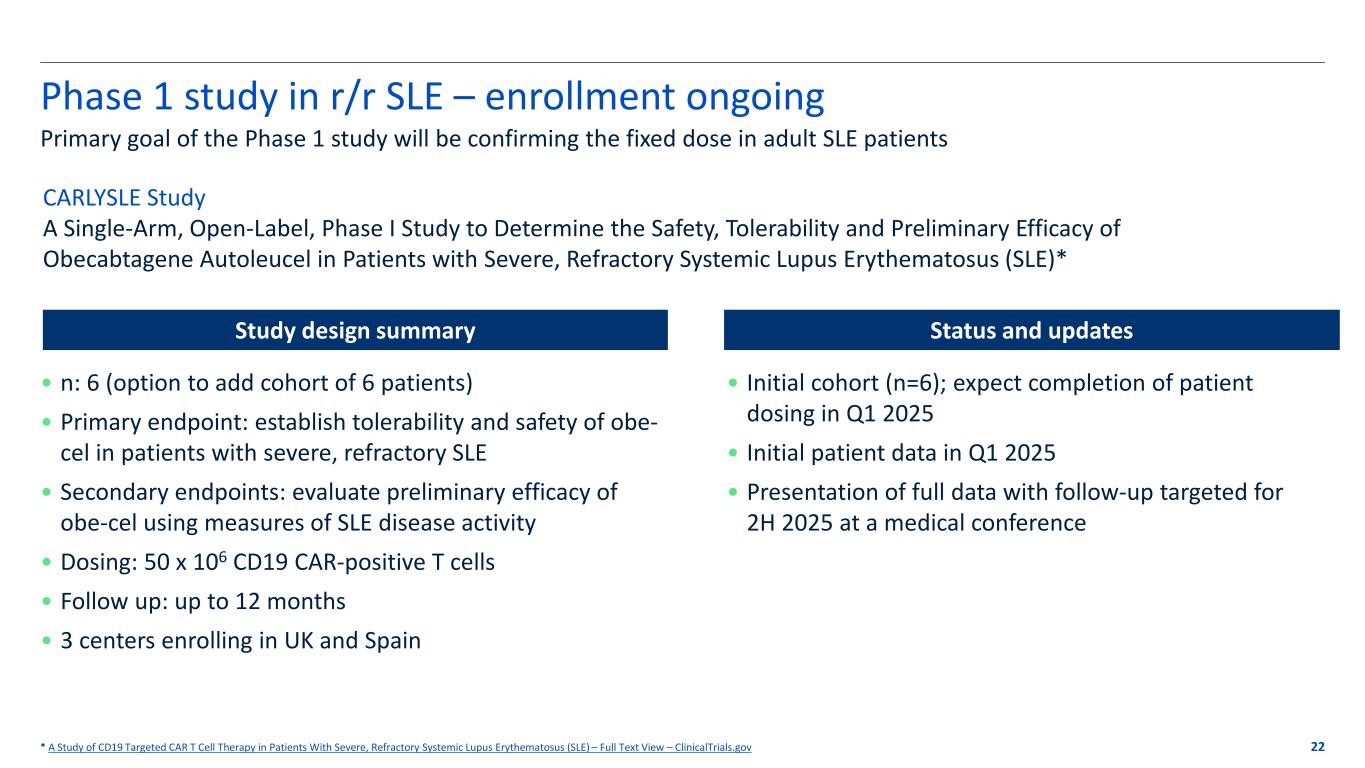
Phase 1 study in r/r SLE – enrollment ongoing 22 Primary goal of the Phase 1 study will be confirming the fixed dose in adult SLE patients * A Study of CD19 Targeted CAR T Cell Therapy in Patients With Severe, Refractory Systemic Lupus Erythematosus (SLE) – Full Text View – ClinicalTrials.gov CARLYSLE Study A Single-Arm, Open-Label, Phase I Study to Determine the Safety, Tolerability and Preliminary Efficacy of Obecabtagene Autoleucel in Patients with Severe, Refractory Systemic Lupus Erythematosus (SLE)* • n: 6 (option to add cohort of 6 patients) • Primary endpoint: establish tolerability and safety of obe- cel in patients with severe, refractory SLE • Secondary endpoints: evaluate preliminary efficacy of obe-cel using measures of SLE disease activity • Dosing: 50 x 106 CD19 CAR-positive T cells • Follow up: up to 12 months • 3 centers enrolling in UK and Spain • Initial cohort (n=6); expect completion of patient dosing in Q1 2025 • Initial patient data in Q1 2025 • Presentation of full data with follow-up targeted for 2H 2025 at a medical conference Study design summary Status and updates

Partnerships, pipeline programs and technologies A broad portfolio of potential next generation modular T cell therapies

Pipeline supports growth with multiple development opportunities 24 Product Indication Target Preclinical Phase 1 Phase 2/Pivotal Approved Status AUCATZYL® Adult ALL CD19 MAA review EMA & MHRA obe-cel Systemic Lupus Erythematosus CD19 Initial data Q1 2025 obe-cel Pediatric ALL CD19 Initial data H2 2025 obe-cel* B-NHL & CLL CD19 Data in peer reviewed journal obe-cel* Primary CNS Lymphoma CD19 Data in peer reviewed journal AUTO1/22*§ Pediatric ALL CD19 & CD22 AUTO8* Multiple Myeloma BCMA & CD19 *UCL Collaboration § BioNTech holds option to co-fund and co-commercialise Product Indication Target Preclinical Phase 1 Phase 2/Pivotal Approved Status AUTO4 TRBC1+ Peripheral TCL TRBC1 Data in peer reviewed journal AUTO5 TRBC2+ Peripheral TCL TRBC2 Data in peer reviewed journal AUTO6NG*§ Neuroblastoma GD2 Open and recruiting AUTO9* Acute Myeloid Leukemia CD33,123,CLL1 Initiate Ph1 2025 Oncology Autoimmune O be -c el p ro du ct fa m ily Ad di tio na l p ro gr am s

Leveraging our industry leading technology platform via partnerships Leveraging technology platform for BioNTech’s programs 25 Technology partnerships Access to the RQR8 safety switch for selected cell therapy programs for the treatment of cancer Leveraging our modular programming technology to generate safer and more effective therapies Tumor targeting, pharmacological control and activity enhancement for cellular therapies Validating collaborations with leading pharma and biotech companies Access to proprietary binders for the development of mRNA-based therapeutics for the treatment of cancer Potential for value creation through near term option exercise fees, milestone payments and royalties from net sales

Upcoming news flow

Autolus planned news flow 27 Anticipated Milestone or Data Catalysts Anticipated Timing Obe-cel FELIX data update at ASH 2024 December 2024 Initial data from SLE Phase 1 trial Q1 2025 Obe-cel UK and EU approvals 2H 2025 Initial data from PY01 trial in pediatric ALL 2H 2025 SLE Phase 1 trial presentation at medical conference 2H 2025 Oncology Autoimmune

Summary

Autolus is positioned for commercial execution and market expansion Obe-cel product franchise supports multiple growth opportunities • EU/UK approvals expected 2H 2025 • Expanding opportunity for obe-cel in hem-oncology and autoimmune diseases • Developing early-stage pipeline of novel CAR-T therapies Commercial execution and market expansion supported by: Developing Next Generation Programmed T Cell Therapies 29 Design Build Operations In-house, purpose-built manufacturing facility Strategic collaborations and strong cash position $657M as of Q3 2024

Autolus.com Thank you

Autolus.com Appendix
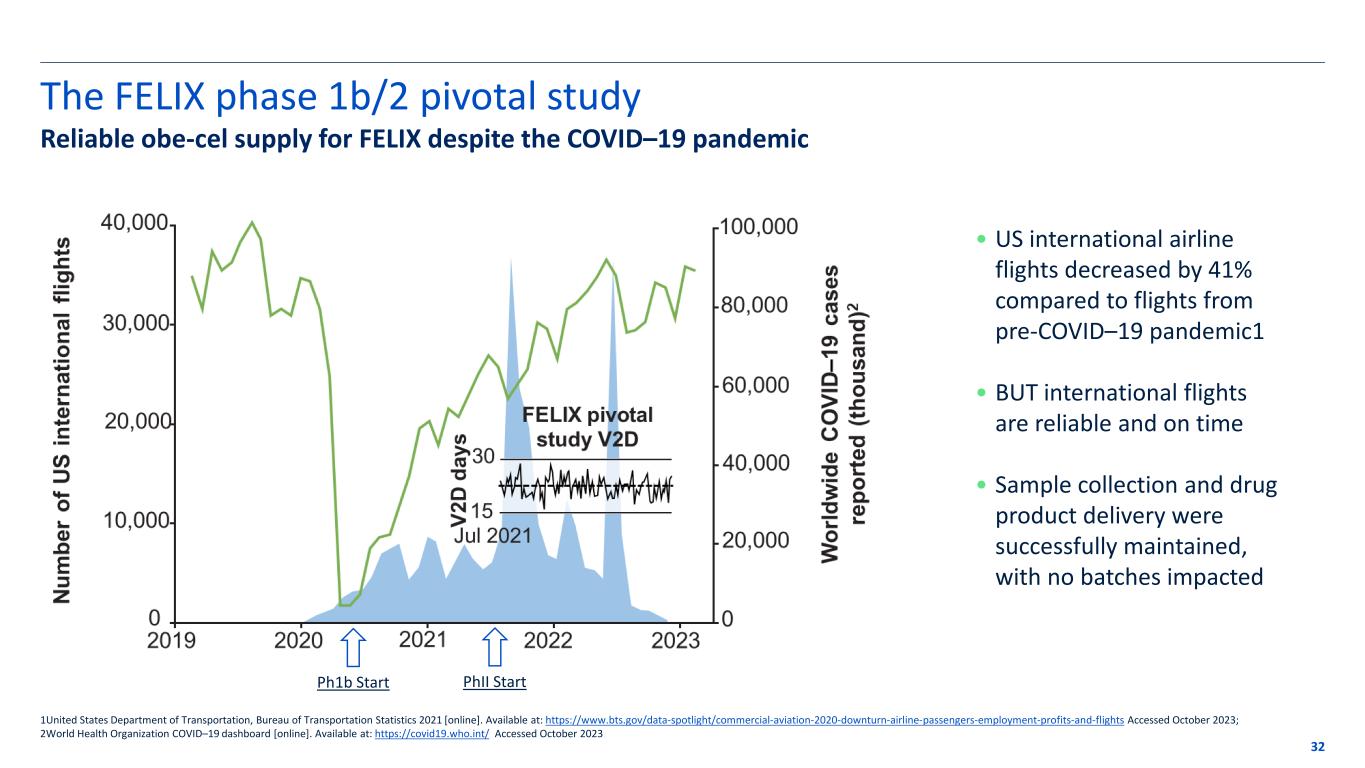
The FELIX phase 1b/2 pivotal study 32 1United States Department of Transportation, Bureau of Transportation Statistics 2021 [online]. Available at: https://www.bts.gov/data-spotlight/commercial-aviation-2020-downturn-airline-passengers-employment-profits-and-flights Accessed October 2023; 2World Health Organization COVID–19 dashboard [online]. Available at: https://covid19.who.int/ Accessed October 2023 Ph1b Start PhII Start Reliable obe-cel supply for FELIX despite the COVID–19 pandemic • US international airline flights decreased by 41% compared to flights from pre-COVID–19 pandemic1 • BUT international flights are reliable and on time • Sample collection and drug product delivery were successfully maintained, with no batches impacted
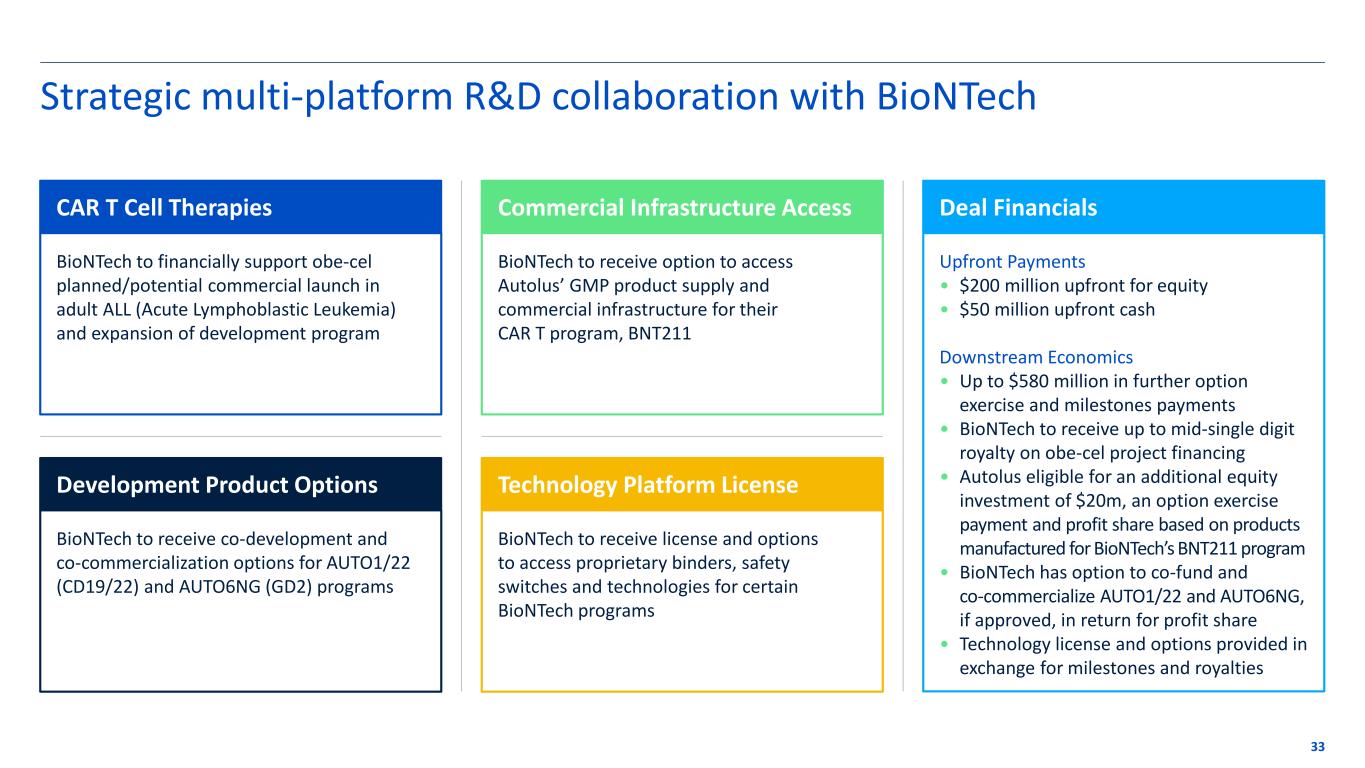
Strategic multi-platform R&D collaboration with BioNTech BioNTech to financially support obe-cel planned/potential commercial launch in adult ALL (Acute Lymphoblastic Leukemia) and expansion of development program 33 BioNTech to receive option to access Autolus’ GMP product supply and commercial infrastructure for their CAR T program, BNT211 Upfront Payments • $200 million upfront for equity • $50 million upfront cash Downstream Economics • Up to $580 million in further option exercise and milestones payments • BioNTech to receive up to mid-single digit royalty on obe-cel project financing • Autolus eligible for an additional equity investment of $20m, an option exercise payment and profit share based on products manufactured for BioNTech’s BNT211 program • BioNTech has option to co-fund and co-commercialize AUTO1/22 and AUTO6NG, if approved, in return for profit share • Technology license and options provided in exchange for milestones and royalties CAR T Cell Therapies Commercial Infrastructure Access Deal Financials BioNTech to receive co-development and co-commercialization options for AUTO1/22 (CD19/22) and AUTO6NG (GD2) programs BioNTech to receive license and options to access proprietary binders, safety switches and technologies for certain BioNTech programs Development Product Options Technology Platform License
































