Exhibit 99.1

1 NASDAQ: TFFP Corporate Investor Presentation February 2023 BETTER DELIVERY, BETTER THERAPY | Powerful Drug Delivery Solutions

2 Safe Harbor Statement SPECIAL NOTE REGARDING FORWARD - LOOKING STATEMENTS This document contains forward - looking statements concerning TFF Pharmaceuticals, Inc . (“TFF”, the “Company,” “we,” “us,” and “our”) . The words “believe,” “may,” “will,” “potentially,” “estimate,” “continue,” “anticipate,” “intend,” “could,” “would,” “project,” “plan,” “expect” and similar expressions that convey uncertainty of future events or outcomes are intended to identify forward - looking statements . These forward - looking statements include, but are not limited to, statements concerning the following : • our future financial and operating results ; • our intentions, expectations and beliefs regarding anticipated growth, market penetration and trends in our business ; • the timing and success of our plan of commercialization ; • our ability to successfully develop and clinically test our product candidates ; and • our ability to fi le for FDA approval of our product candidates through the 505 (b)( 2 ) regulatory pathway . These forward - looking statements are subject to a number of risks, uncertainties and assumptions that could cause actual results to differ materially . Among those factors are : (i) no drug product incorporating the TFF platform has received FDA pre - market approval or otherwise been incorporated into a commercial drug product, (ii) the Company has no current agreements or understandings with any large pharmaceutical companies for the development of a drug product incorporating the TFF Platform , (iii) success in early phases of pre - clinical and clinicals trials do not ensure later clinical trials will be successful, and ( iv ) those other risks disclosed in the section “Risk Factors” included in the Company’s prospectus supplement filed November 18 , 202 2 with the SEC . TFF Pharmaceuticals cautions readers not to place undue reliance on any forward - looking statements . TFF Pharmaceuticals does not undertake, and specifically disclaims, any obligation to update or revise such statements to reflect new circumstances or unanticipated events as they occur, except as required by law . This document contains only basic information concerning TFF . Because it is a summary it does not contain all of the information you should consider before investing . Please refer to our reports and registration statements on file with the SEC for more comprehensive information concerning TFF Pharmaceuticals . 2
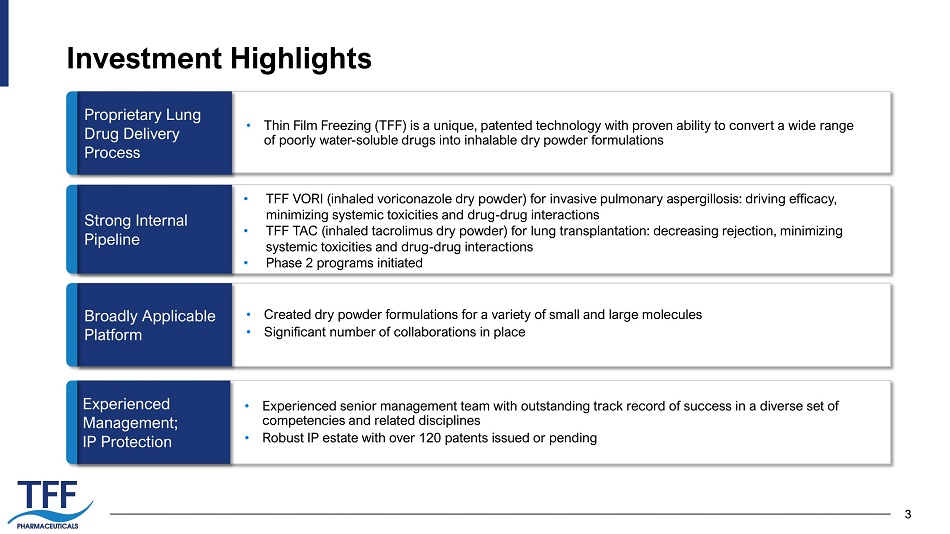
3 Investment Highlights 3 Experienced Management; IP Protection Strong Internal Pipeline Broadly Applicable Platform Proprietary Lung Drug Delivery Process • Experienced senior management team with outstanding track record of success in a diverse set of competencies and related disciplines • Robust IP estate with over 120 patents issued or pending • TFF VORI (inhaled voriconazole dry powder) for invasive pulmonary aspergillosis: driving efficacy, minimizing systemic toxicities and drug - drug interactions • TFF TAC (inhaled tacrolimus dry powder) for lung transplantation: decreasing rejection, minimizing systemic toxicities and drug - drug interactions • Phase 2 programs initiated • Created dry powder formulations for a variety of small and large molecules • Significant number of collaborations in place • Thin Film Freezing (TFF) is a u nique, patented technology with proven ability to convert a wide range of poorly water - soluble drugs into inhalable dry powder formulations
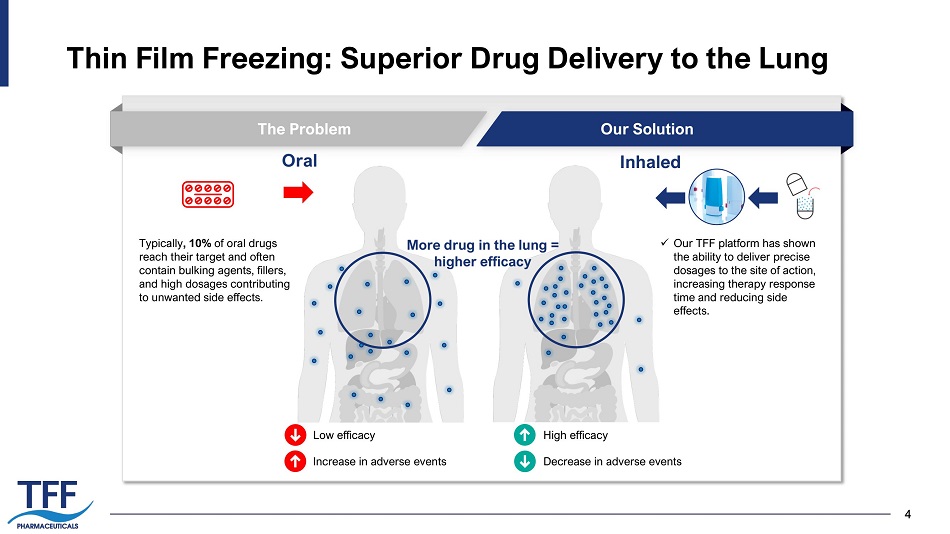
4 Thin Film Freezing: Superior Drug Delivery to the Lung 4 The Problem Our Solution Oral Inhaled Typically , 10% of oral drugs reach their target and often contain bulking agents, fillers, and high dosages contributing to unwanted side effects. x Our TFF platform has shown the ability to deliver precise dosages to the site of action, increasing therapy response time and reducing side effects. High efficacy Decrease in adverse events Low efficacy Increase in adverse events More drug in the lung = higher efficacy
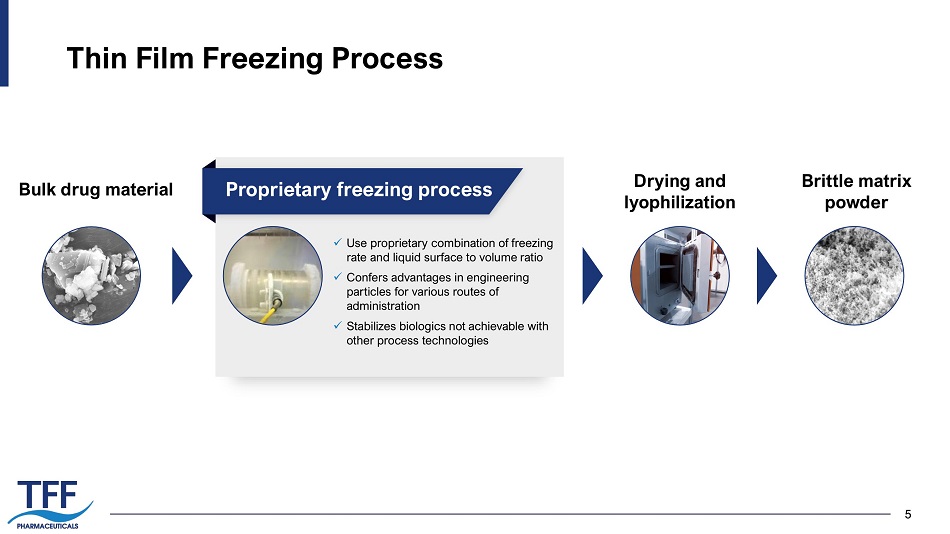
5 Thin Film Freezing Process 5 Bulk drug material x Use proprietary combination of freezing rate and liquid surface to volume ratio x Confers advantages in engineering particles for various routes of administration x Stabilizes biologics not achievable with other process technologies Drying and lyophilization Brittle matrix powder Proprietary freezing process
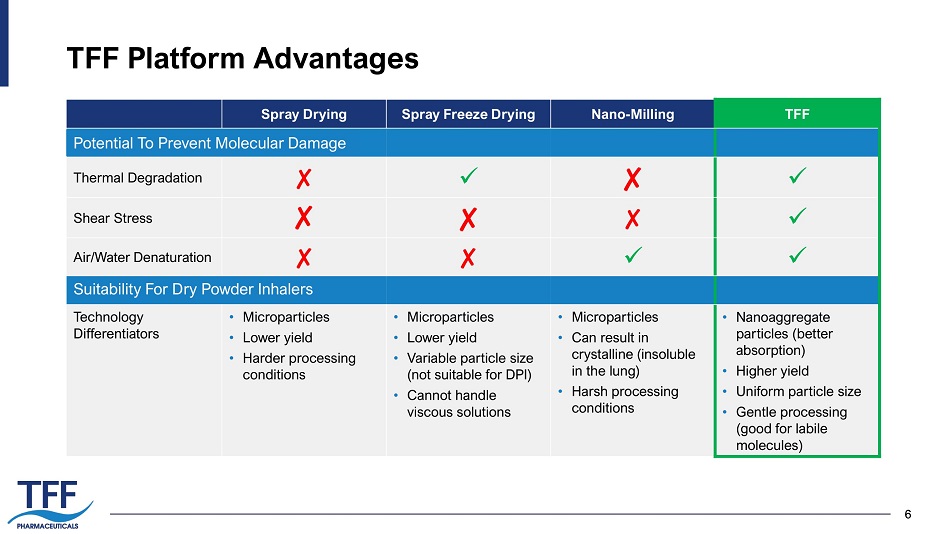
6 TFF Platform Advantages 6 Spray Drying Spray Freeze Drying Nano - Milling TFF Thermal Degradation ✘ x ✘ x Shear Stress ✘ ✘ ✘ x Air/Water Denaturation ✘ ✘ x x Technology Differentiators • Microparticles • Lower yield • Harder processing conditions • Microparticles • Lower yield • Variable particle size (not suitable for DPI) • Cannot handle viscous solutions • Microparticles • Can result in crystalline (insoluble in the lung) • Harsh processing conditions • Nanoaggregate particles (better absorption) • Higher yield • Uniform particle size • Gentle processing (good for labile molecules) Potential To Prevent Molecular Damage Suitability For Dry Powder Inhalers
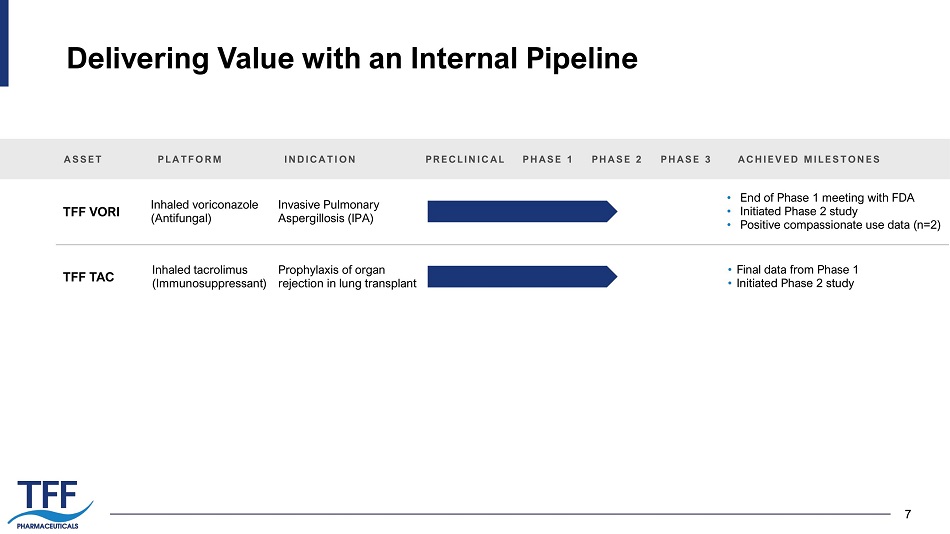
7 Delivering Value with an Internal Pipeline 7 ASSET TFF VORI TFF TAC PLATFORM INDICATION Inhaled voriconazole (Antifungal) Invasive Pulmonary Aspergillosis (IPA) PRECLINICAL PHASE 1 PHASE 2 PHASE 3 • End of Phase 1 meeting with FDA • Initiated Phase 2 study • Positive compassionate use data (n=2) ACHIEVED MILESTONES Inhaled tacrolimus (Immunosuppressant) Prophylaxis of organ rejection in lung transplant • Final data from Phase 1 • Initiated Phase 2 study

8 8 TFF VORI Voriconazole Inhalation Powder in Phase 2 for Invasive Pulmonary Aspergillosis
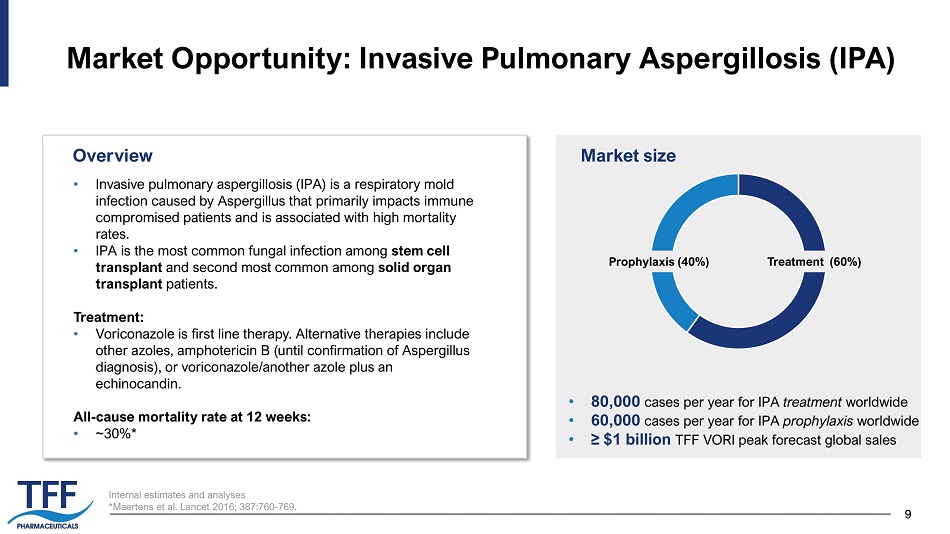
9 9 Internal estimates and analyses * Maertens et al. Lancet 2016; 387:760 - 769. Market Opportunity: Invasive Pulmonary Aspergillosis (IPA) • Invasive pulmonary aspergillosis (IPA) is a respiratory mold infection caused by Aspergillus that primarily impacts immune compromised patients and is associated with high mortality rates. • IPA is the most common fungal infection among stem cell transplant and second most common among solid organ transplant patients. Treatment: • Voriconazole is first line therapy. Alternative therapies include other azoles, amphotericin B (until confirmation of Aspergillus diagnosis), or voriconazole/another azole plus an echinocandin. All - cause mortality rate at 12 weeks: • ~30%* Market size • 80,000 cases per year for IPA treatment worldwide • 60,000 cases per year for IPA prophylaxis worldwide • ≥ $1 billion TFF VORI peak forecast global sales Overview Prophylaxis (40%) Treatment (60%)
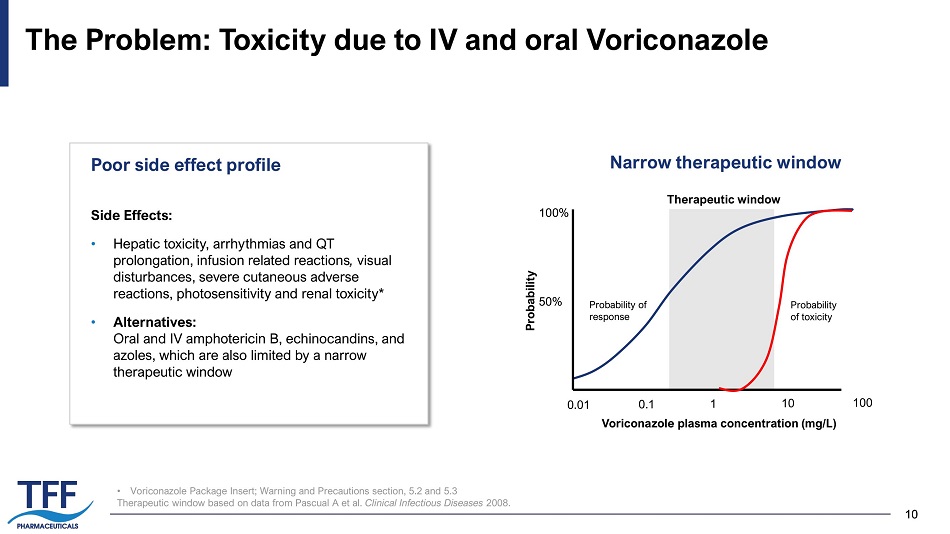
10 10 • Voriconazole Package Insert; Warning and Precautions section, 5.2 and 5.3 Therapeutic window based on data from Pascual A et al. Clinical Infectious Diseases 2008. The Problem: Toxicity due to IV and oral Voriconazole Probability of toxicity 100% 50% 0.01 0.1 1 10 100 Voriconazole plasma concentration (mg/L) Probability Probability of response Therapeutic window Side Effects: • H epatic toxicity, arrhythmias and QT prolongation, infusion related reactions , visual disturbances, severe cutaneous adverse reactions, photosensitivity and renal toxicity* • Alternatives: Oral and IV amphotericin B, echinocandins, and azoles, which are also limited by a narrow therapeutic window Poor side effect profile Narrow therapeutic window
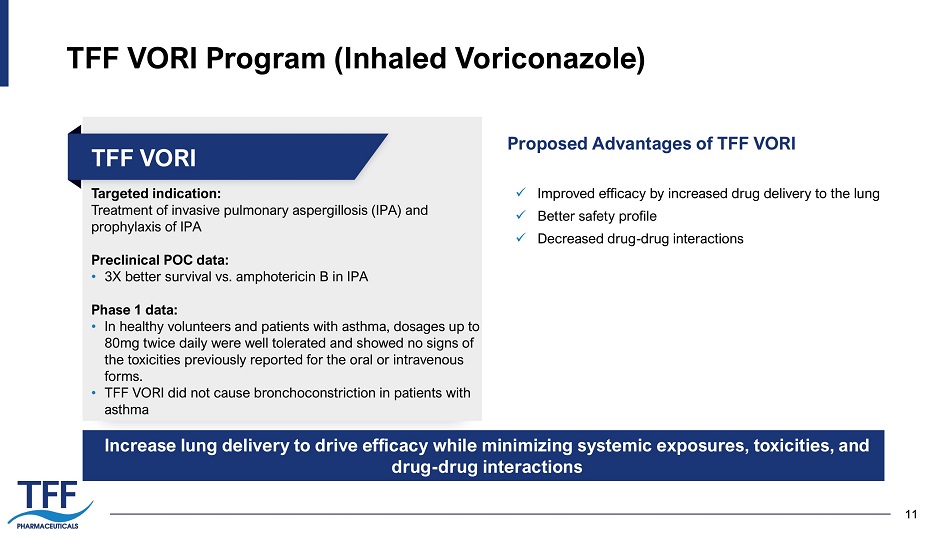
11 11 TFF VORI Program (Inhaled Voriconazole) Targeted indication: Treatment of invasive pulmonary aspergillosis (IPA) and prophylaxis of IPA Preclinical POC data: • 3X better survival vs. amphotericin B in IPA Phase 1 data: • In healthy volunteers and patients with asthma, dosages up to 80mg twice daily were well tolerated and showed no signs of the toxicities previously reported for the oral or intravenous forms. • TFF VORI did not cause bronchoconstriction in patients with asthma TFF VORI Proposed Advantages of TFF VORI Increase lung delivery to drive efficacy while minimizing systemic exposures, toxicities, and drug - drug interactions x Improved efficacy by increased drug delivery to the lung x Better safety profile x Decreased drug - drug interactions

12 12 Preclinical Data of Inhaled Voriconazole Control Inhaled voriconazole Amphotericin B Control Inhaled voriconazole Amphotericin B *p<0.01 Increased survival Decreased necrotic lesions in the lung ~3x higher survival rate with inhaled VORI vs. AMB Tolman JA et al. Antimicrobial Agents and Chemotherapy . 2009.
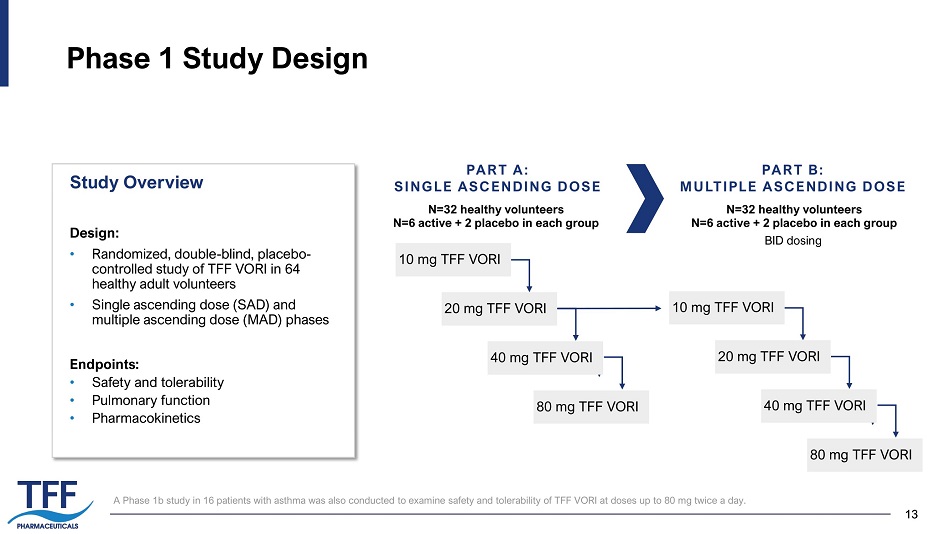
13 13 Phase 1 Study Design PART A: SINGLE ASCENDING DOSE 10 mg TFF VORI PART B: MULTIPLE ASCENDING DOSE N=32 healthy volunteers N=6 active + 2 placebo in each group N=32 healthy volunteers N=6 active + 2 placebo in each group BID dosing Design: • Randomized, double - blind, placebo - controlled study of TFF VORI in 64 healthy adult volunteers • Single ascending dose (SAD) and multiple ascending dose (MAD) phases Endpoints: • Safety and tolerability • Pulmonary function • Pharmacokinetics Study Overview 2 0 mg TFF VORI 40 mg TFF VORI 8 0 mg TFF VORI 10 mg TFF VORI 2 0 mg TFF VORI 40 mg TFF VORI 8 0 mg TFF VORI A Phase 1b study in 16 patients with asthma was also conducted to examine safety and tolerability of TFF VORI at doses up to 80 mg twice a day.
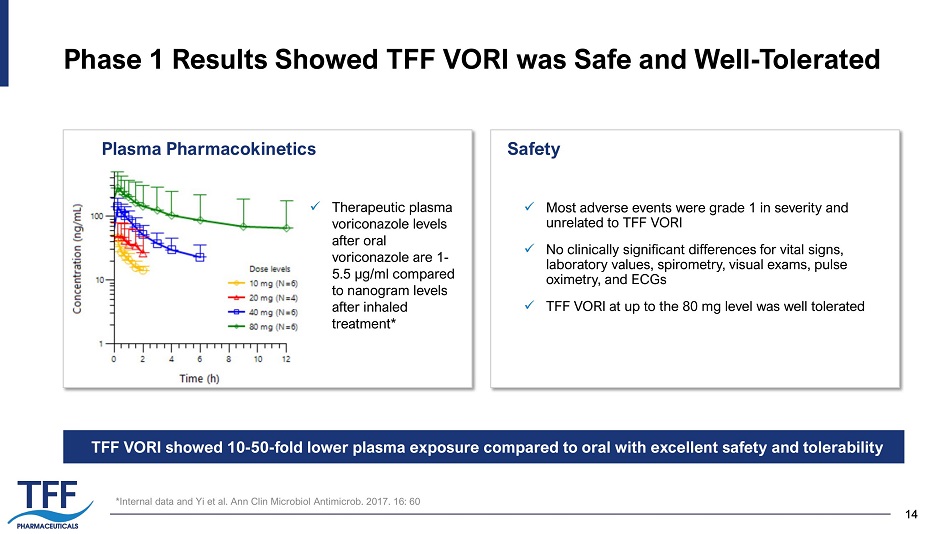
14 14 Phase 1 Results Showed TFF VORI was Safe and Well - Tolerated x Most adverse events were grade 1 in severity and unrelated to TFF VORI x No clinically significant differences for vital signs, laboratory values, spirometry, visual exams, pulse oximetry, and ECGs x TFF VORI at up to the 80 mg level was well tolerated Plasma Pharmacokinetics Safety *Internal data and Yi et al. Ann Clin Microbiol Antimicrob . 2017. 16: 60 TFF VORI showed 10 - 50 - fold lower plasma exposure compared to oral with excellent safety and tolerability x Therapeutic plasma voriconazole levels after oral voriconazole are 1 - 5.5 µg/ml compared to nanogram levels after inhaled treatment*
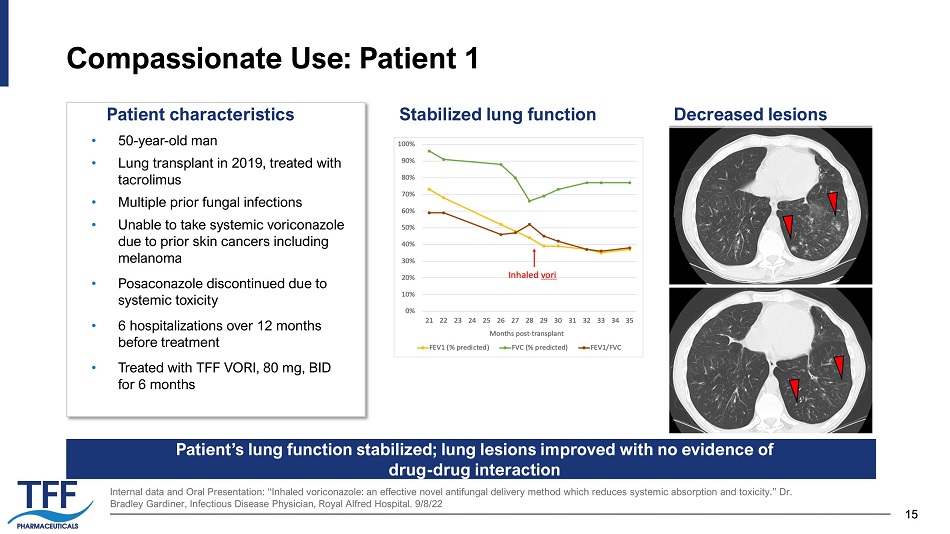
15 15 Compassionate Use: Patient 1 Internal data and Oral Presentation: “Inhaled voriconazole: an effective novel antifungal delivery method which reduces syste mic absorption and toxicity.” Dr. Bradley Gardiner, Infectious Disease Physician, Royal Alfred Hospital. 9/8/22 • 50 - year - old man • Lung transplant in 2019, treated with tacrolimus • Multiple prior fungal infections • Unable to take systemic voriconazole due to prior skin cancers including melanoma • Posaconazole discontinued due to systemic toxicity • 6 hospitalizations over 12 months before treatment • Treated with TFF VORI, 80 mg, BID for 6 months Stabilized lung function Decreased lesions Patient’s lung function stabilized; lung lesions improved with no evidence of drug - drug interaction Patient characteristics
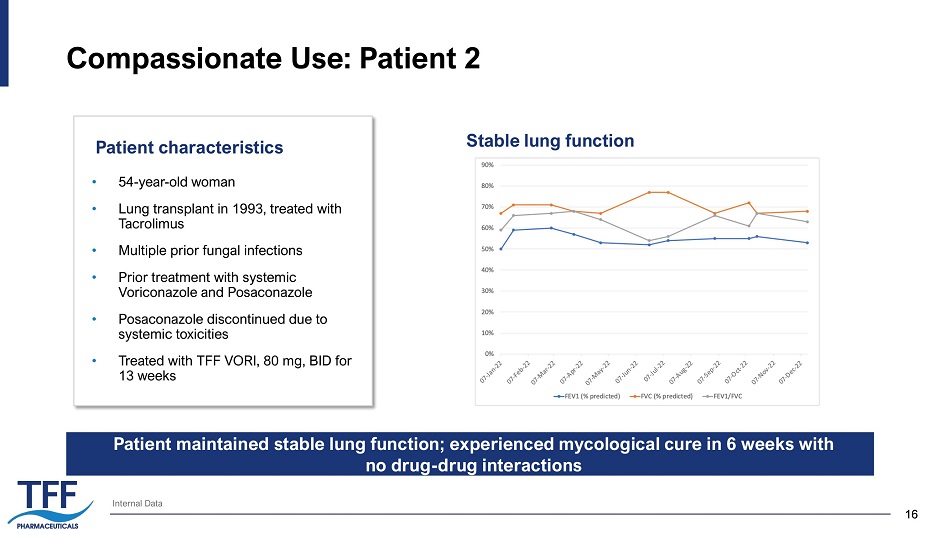
16 16 Compassionate Use: Patient 2 Patient characteristics Internal Data • 54 - year - old woman • Lung transplant in 1993, treated with Tacrolimus • Multiple prior fungal infections • Prior treatment with systemic Voriconazole and Posaconazole • Posaconazole discontinued due to systemic toxicities • Treated with TFF VORI, 80 mg, BID for 13 weeks Stable lung function Patient maintained stable lung function; experienced mycological cure in 6 weeks with no drug - drug interactions
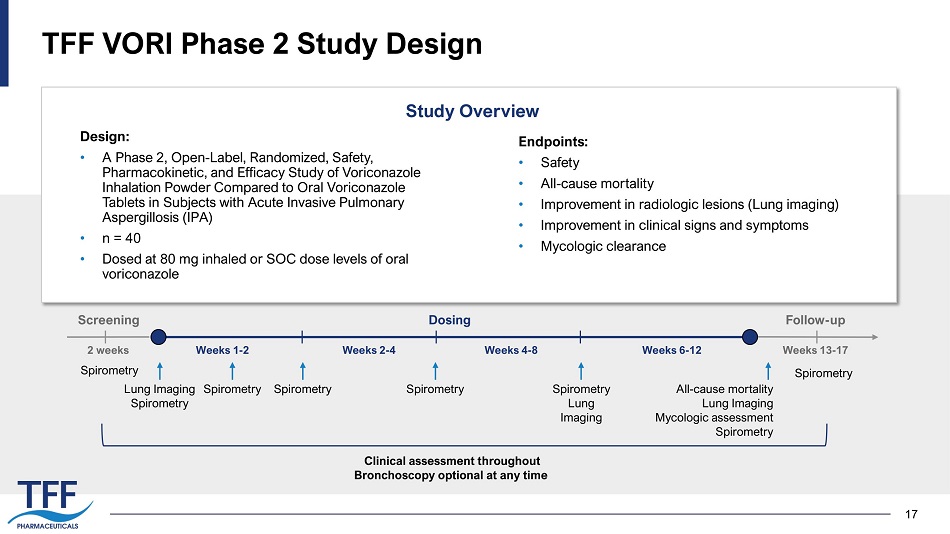
17 TFF VORI Phase 2 Study Design Design: • A Phase 2, Open - Label, Randomized, Safety, Pharmacokinetic, and Efficacy Study of Voriconazole Inhalation Powder Compared to Oral Voriconazole Tablets in Subjects with Acute Invasive Pulmonary Aspergillosis (IPA) • n = 40 • Dosed at 80 mg inhaled or SOC dose levels of oral voriconazole Study Overview Endpoints: • Safety • All - cause mortality • Improvement in radiologic lesions (Lung imaging) • Improvement in clinical signs and symptoms • Mycologic clearance Weeks 1 - 2 Weeks 4 - 8 Weeks 6 - 12 Screening All - cause mortality Lung Imaging Mycologic assessment Spirometry Weeks 2 - 4 Weeks 13 - 17 2 weeks Dosing Follow - up Spirometry Spirometry Spirometry Spirometry Lung Imaging Spirometry Spirometry Lung Imaging Spirometry Clinical assessment throughout Bronchoscopy optional at any time
18 18 TFF TAC Tacrolimus Inhalation Powder in Phase 2 for Lung Transplant Rejection
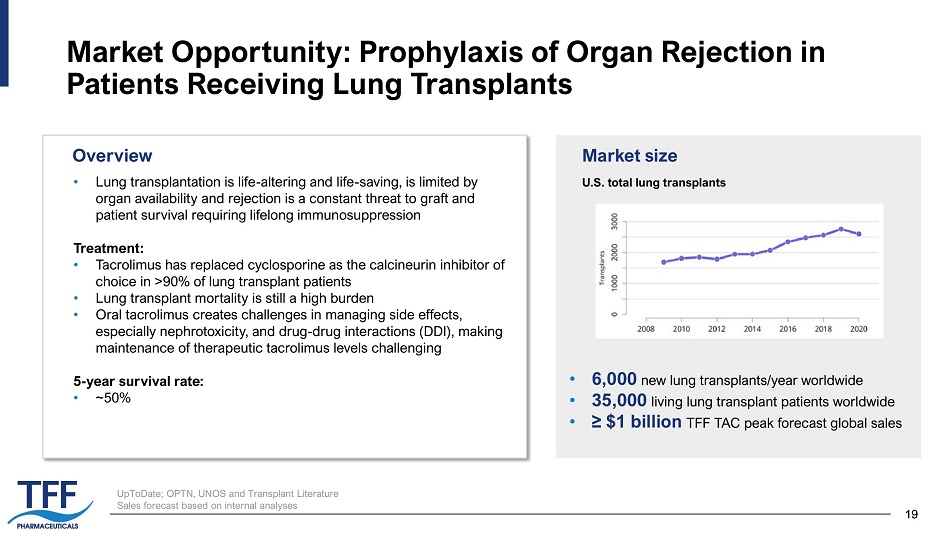
19 19 Market Opportunity: Prophylaxis of Organ Rejection in Patients Receiving Lung Transplants • Lung transplantation is life - altering and life - saving, is limited by organ availability and rejection is a constant threat to graft and patient survival requiring lifelong immunosuppression Treatment: • Tacrolimus has replaced cyclosporine as the calcineurin inhibitor of choice in >90% of lung transplant patients • Lung transplant mortality is still a high burden • Oral tacrolimus creates challenges in managing side effects, especially nephrotoxicity, and drug - drug interactions (DDI), making maintenance of therapeutic tacrolimus levels challenging 5 - year survival rate: �� ~50% U.S. total lung transplants Market size • 6,000 new lung transplants/year worldwide • 35,000 living lung transplant patients worldwide • ≥ $1 billion TFF TAC peak forecast global sales Overview UpToDate; OPTN, UNOS and Transplant Literature Sales forecast based on internal analyses
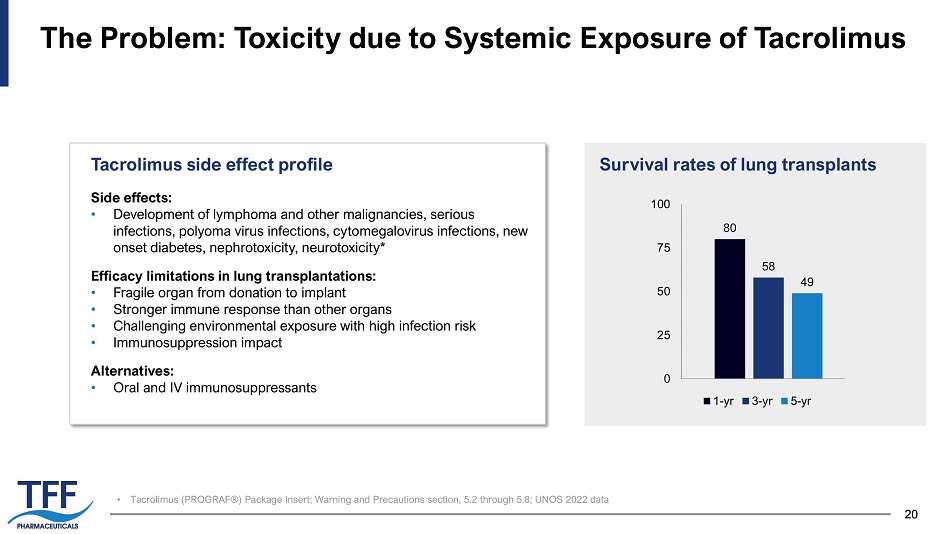
20 20 • Tacrolimus (PROGRAF®) Package Insert; Warning and Precautions section, 5.2 through 5.8; UNOS 2022 data The Problem: Toxicity due to Systemic Exposure of Tacrolimus Side effects: • Development of lymphoma and other malignancies, serious infections, polyoma virus infections, cytomegalovirus infections, new onset diabetes, nephrotoxicity, neurotoxicity* Efficacy limitations in lung transplantations: • Fragile organ from donation to implant • Stronger immune response than other organs • Challenging environmental exposure with high infection risk • Immunosuppression impact Alternatives: • Oral and IV immunosuppressants Tacrolimus side effect profile 80 58 49 0 25 50 75 100 1-yr 3-yr 5-yr Survival rates of lung transplants
21 21 TFF TAC Program (Inhaled Tacrolimus) Targeted Indication: • Prophylaxis of organ rejection in patients receiving lung transplants Preclinical POC Data: • Inhaled tacrolimus had similar efficacy compared to intramuscular tacrolimus in a rat lung transplant model Phase 1 data: • Successfully completed single and multiple ascending dosing of TFF TAC in healthy subjects in Phase 1 trial • Doses up to 5 mg (single dose) and 1.5 mg (repeated dose) daily were generally well tolerated TFF TAC Increase lung delivery to drive efficacy while minimizing systemic exposures, toxicities, and drug - drug interactions x Improved efficacy by increased drug delivery to the lung x Better safet y profile x Decreased drug - drug interactions Proposed Advantages of TFF TAC
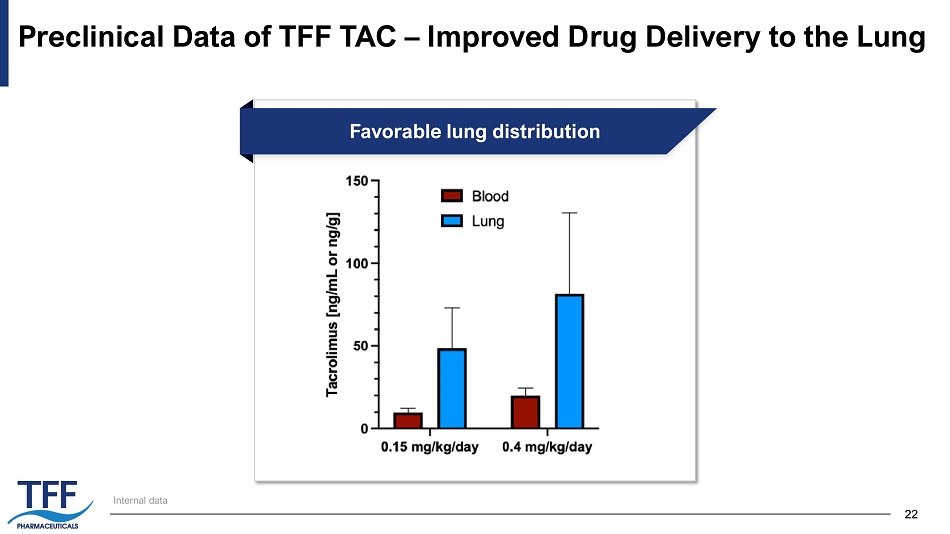
22 22 Preclinical Data of TFF TAC – Improved Drug Delivery to the Lung Internal data Favorable lung distribution
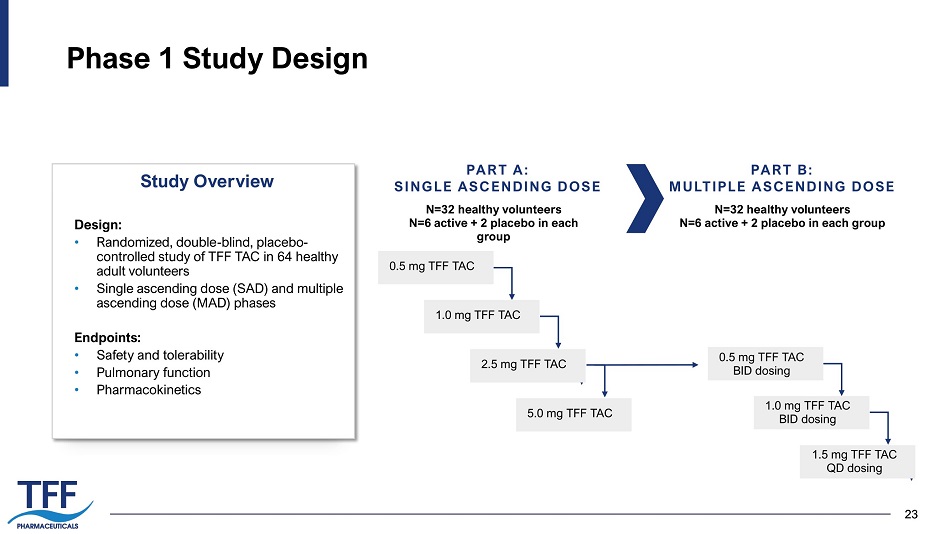
23 23 Phase 1 Study Design PART A: SINGLE ASCENDING DOSE PART B: MULTIPLE ASCENDING DOSE N=32 healthy volunteers N=6 active + 2 placebo in each group N=32 healthy volunteers N=6 active + 2 placebo in each group Design: • Randomized, double - blind, placebo - controlled study of TFF TAC in 64 healthy adult volunteers • Single ascending dose (SAD) and multiple ascending dose (MAD) phases Endpoints: • Safety and tolerability • Pulmonary function • Pharmacokinetics Study Overview 0.5 mg TFF TAC 1.0 mg TFF TAC 2.5 mg TFF TAC 5.0 mg TFF TAC 0.5 mg TFF TAC BID dosing 1.0 mg TFF TAC BID dosing 1.5 mg TFF TAC QD dosing
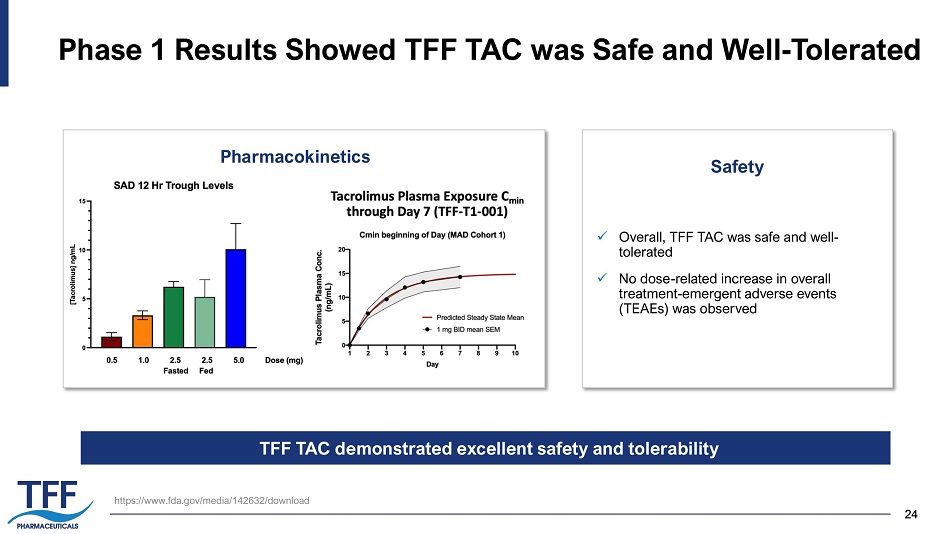
24 24 Phase 1 Results Showed TFF TAC was Safe and Well - Tolerated x Overall, TFF TAC was safe and well - tolerated x No dose - related increase in overall treatment - emergent adverse events (TEAEs) was observed Pharmacokinetics Safety https:// www.fda.gov /media/142632/download TFF TAC demonstrated excellent safety and tolerability
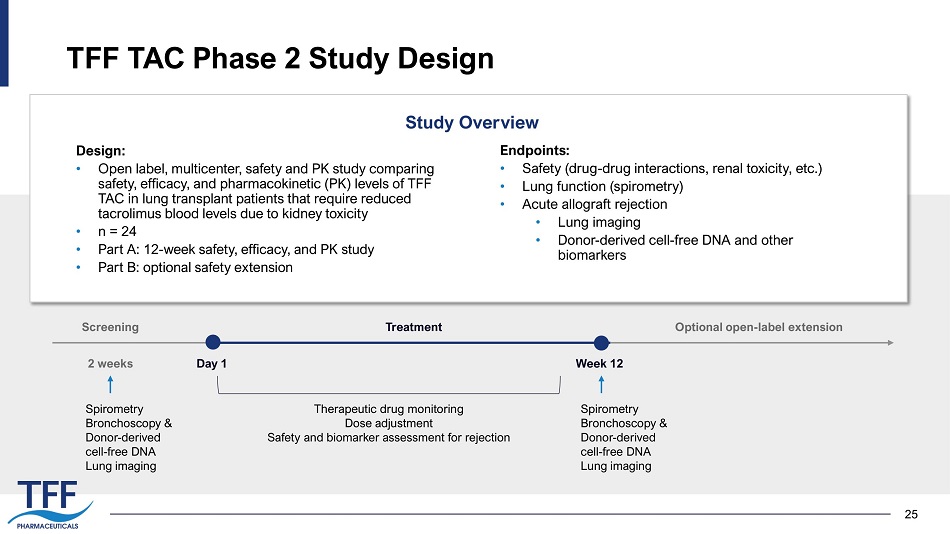
25 TFF TAC Phase 2 Study Design Design: • Open label, multicenter, safety and PK study comparing safety, efficacy, and pharmacokinetic (PK) levels of TFF TAC in lung transplant patients that require reduced tacrolimus blood levels due to kidney toxicity • n = 24 • Part A: 12 - week safety, efficacy, and PK study • Part B: optional safety extension Endpoints: • Safety (drug - drug interactions, renal toxicity, etc.) • Lung function (spirometry) • Acute allograft rejection • Lung imaging • Donor - derived cell - free DNA and other biomarkers Study Overview Spirometry Bronchoscopy & Donor - derived cell - free DNA Lung imaging Screening Day 1 2 weeks Week 12 Treatment Optional open - label extension Spirometry Bronchoscopy & Donor - derived cell - free DNA Lung imaging Therapeutic drug monitoring Dose adjustment Safety and biomarker assessment for rejection
26 26 External Pipeline Leveraging TFF’s Platform for Business Development Opportunities
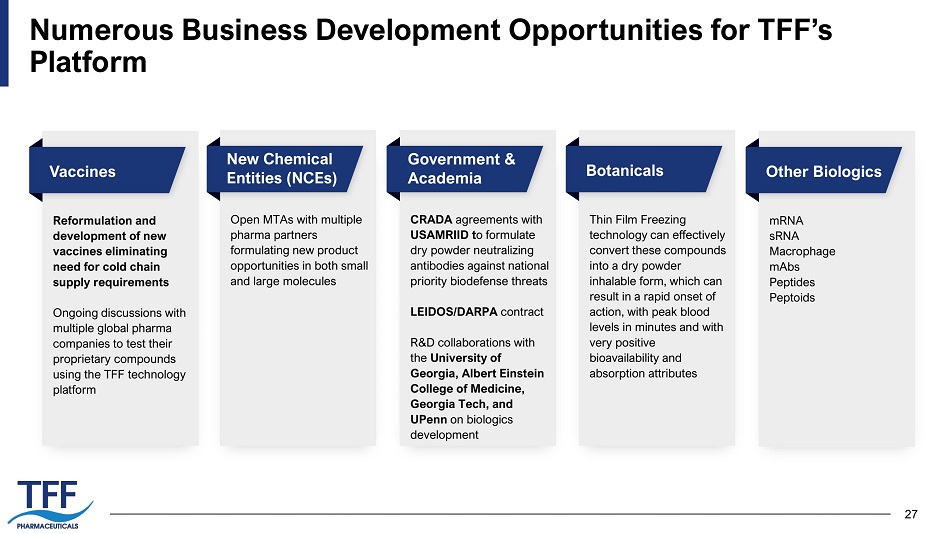
27 Numerous Business Development Opportunities for TFF’s Platform Vaccines Reformulation and development of new vaccines eliminating need for cold chain supply requirements Ongoing discussions with multiple global pharma companies to test their proprietary compounds using the TFF technology platform New Chemical Entities (NCEs) Open MTAs with multiple pharma partners formulating new product opportunities in both small and large molecules Government & Academia CRADA agreements with USAMRIID t o formulate dry powder neutralizing antibodies against national priority biodefense threats LEIDOS/DARPA contract R&D collaborations with the University of Georgia, Albert Einstein College of Medicine, Georgia Tech, and UPenn on biologics development Botanicals Thin Film Freezing technology can effectively convert these compounds into a dry powder inhalable form, which can result in a rapid onset of action, with peak blood levels in minutes and with very positive bioavailability and absorption attributes Other Biologics mRNA sRNA Macrophage mAbs Peptides Peptoids
28 28 Disclosed Collaboration Partners Pharmaceutical and Biotechnology Partners Academic and Government Partners

29 29 TFF Leadership Chris Cano Chief Operating Officer & Vice President, Business Development • 20 years of corporate development experience • Previously served in senior business development roles at Aqua Pharmaceuticals, Duchesnay USA, Inc. and Noven Pharmaceuticals Harlan F. Weisman, M.D. Interim Chief Executive Officer • 30 years experience as a senior healthcare executive • Former CEO of Flame Biosciences and Coronado Biosciences • Former Group Company Chairman and President of J&J Pharmaceutical R&D Kirk Coleman Chief Financial Officer • Over 20 years of financial and accounting experience • Previously served as an executive officer of Steelhead Capital Management, LLC and Bios Partners, LP Zamaneh Mikhak , M.D. Chief Medical Officer • Physician - scientist, board certified in Allergy/Immunology, with extensive clinical, drug development, and basic and translational research experience • Previously served in senior clinical research and development roles at Cogent Biosciences, Boston Pharmaceuticals, and Kiniksa Pharmaceuticals

30 30 TFF Board and Advisors Aaron Fletcher, Ph.D. Chairman of the Board Robert S. Mills Director Stephen Rocamboli Director Board of Directors David N. Cornfield, M.D. Professor of Pulmonary Medicine, Stanford University Prof. David Denning, FRCP, FRCPath, DCH, FMedSci Professor of Infectious Diseases, University of Manchester Jay Peters, M.D. Chief of Pulmonary and Critical Care Medicine, University of Texas Health Science Center at San Antonio Ted M. Ross, Ph.D. Professor, Center for Vaccines and Immunology, Department of Infectious Diseases, University of Georgia Mike Saag, M.D. Professor of Medicine, University of Alabama at Birmingham Drew Weissman, M.D., Ph.D. Roberts Family Professor, Vaccine Research at the Perelman School of Medicine, University of Pennsylvania Scientific Advisory Board Brandi Roberts Director Harlan F. Weisman, M.D. Interim Chief Executive Officer and Vice Chairman of the Board of Directors
31 31 Key Takeaways Revolutionary platform enabling inhalable drug delivery across the entire spectrum of pharmaceutical agents (small & large molecules, biologics, vaccines, mRNA), targeting multiple $Billion - plus markets x Two proprietary, high commercial and lower risk clinical programs with significant near - term data readouts; both with 505(b)(2) regulatory pathway x Have established POC for multiple small and large molecules x Significant number of Material Transfer Agreements in place x TFF process, drug opportunities, and new IP development x Proven management team with multiple successful exits Business development opportunities Strong patent portfolio Proven management team High commercial, low risk opportunity

32 THANK YOU































