term adjusted by the Patent Office. As a result, if valid and enforceable, it is expected to expire in 2034, exclusive of any patent term extension.
We have also filed a set of provisional patent applications that cover the use of VIB4920, at certain doses, to treat autoimmune diseases or disorders, to reduce autoantibodies, or to reduce inflammation. The expected year of expiration for this patent family, where issued, valid and enforceable and should any patents issue therefrom, is 2039, without regard to any extensions or adjustments of term that may be available under national law.
Further, we have licensed from MedImmune, LLC two international patent applications, and all corresponding family members thereof that cover (i) recombinant polypeptide scaffolds and (ii) methods of purifying an albumin-fusion protein. VIB4920 is comprised of a specific recombinant polypeptide scaffold fused to an albumin protein. The expected year of expiration for the first patent family, covering recombinant polypeptide scaffolds, where issued, valid and enforceable, is 2028, without regard to any extensions or adjustments of term that may be available under national law. The expected year of expiration for the second patent family, covering methods of purifying an albumin-fusion protein, where issued, valid and enforceable, is 2036, without regard to any extensions or restorations of term that may be available under national law.
VIB7734 Patent Coverage
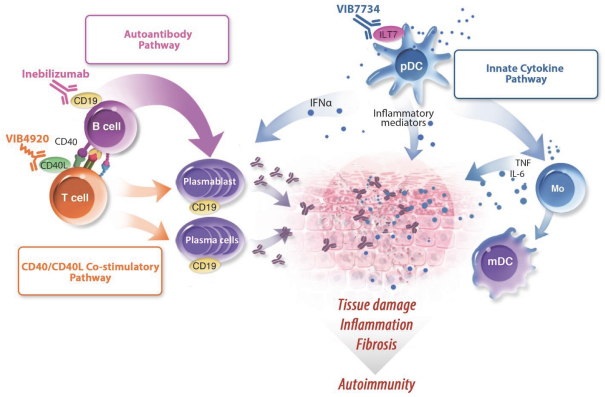
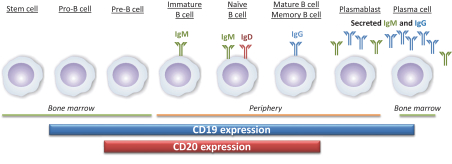
We have exclusively licensed from SBI Biotech Co. a patent family comprising one hundred and thirty four issued patents including one or more issued patents in the U.S., Australia, European, Japan, Korea, New Zealand, Russia, China, Israel, India, Mexico, Singapore, Ukraine, South Africa, Albania, Austria, Bosnia and Herzegovina, Belgium, Bulgaria, Switzerland, Cyprus, the Czech Republic, Germany, Denmark, Estonia, Spain, Finland, France, United Kingdom, Greece, Croatia, Hungary, Ireland, Iceland, Italy, Lichtenstein , Lithuania, Luxembourg, Latvia, Monaco, Montenegro, Macedonia, the Netherlands, Poland, Portugal, Romania, Serbia, Sweden, Slovenia, Slovakia, and Turkey. Additionally, the patent family has eight pending applications in Australia, Brazil, Canada, China, Hong Kong, Europe, Singapore, and the U.S. The expected year of expiration for this patent family, where issued, valid and enforceable, is 2026, without regard to any extensions or adjustments of term that may be available under national law. This family relates to, for example, monoclonal antibodies, which bind to human ILT7, binds to none of human ILT1, ILT2 and human ILT3, and suppresses interferon-a (IFNa) production from ILT7-expressing cells, or a fragment comprising its antigen binding region.
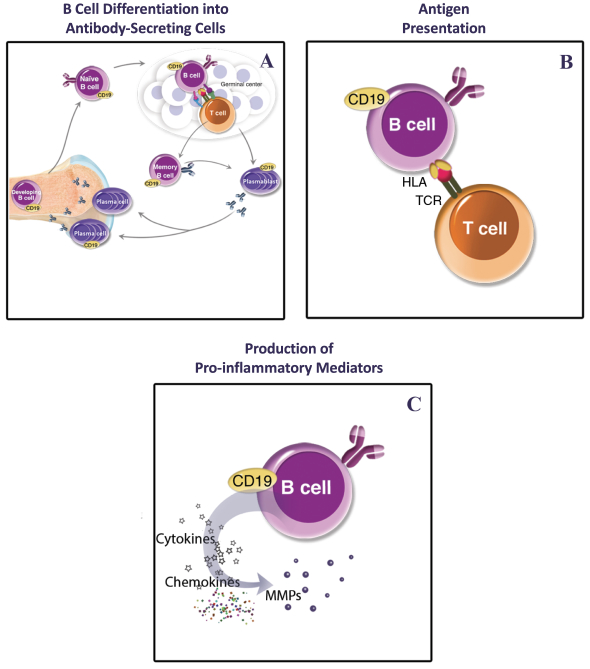
We also own a patent family covering the VIB7734composition-of-matter and its pharmaceutical composition. This family has 20 pending applications in Argentina, the Gulf Cooperation Council, Taiwan, the U.S., Australia, Brazil, Canada, China, Europe, Indonesia, Israel, India, Japan, Korea, Mexico, New Zealand, Russia, Singapore, Ukraine, and South Africa. This family covers, for example, isolated ILT7 binding proteins that can bind to the same ILT7 epitope as an antibody comprising the heavy chain variable region and the light chain variable region of VIB7734. Additionally, the family covers methods for decreasingIFN-alpha release from a plasmacytoid dendritic cell using VIB7734, as well as methods for treating or preventing an autoimmune disease in a human patient using VIB7734. The expected year of expiration for this patent family, where issued, valid and enforceable, and should any patents issue therefrom, is 2037, without regard to any extensions or adjustments of term that may be available under national law.
Trade secrets
In addition to patents, we rely upon unpatented trade secrets andknow-how and continuing technological innovation to develop and maintain our competitive position. We seek to protect our proprietary information, in part, using confidentiality agreements with our commercial partners,
135