The third patent family includes 20 pending patent applications with claims directed to compositions of matter and methods of use in the United States, Europe, Australia, China, India, Japan, Brazil, Egypt, Indonesia, Israel, Republic of Korea, Malaysia, Mexico, New Zealand, Vietnam, African Regional IPO, Philippines, Singapore, South Africa and Eurasia. Additional filings in Hong Kong and Canada will be made in due course. Patents issued from this family are expected to expire February 2, 2037, not including any patent term adjustments that may extend the patent term in certain jurisdictions.
The fourth patent family includes 19 pending patent applications with claims directed to compositions of matter and methods of use in the United States, Europe, Australia, India, Japan, Brazil, Egypt, Indonesia, Israel, Republic of Korea, Malaysia, Mexico, New Zealand, Vietnam, African Regional IPO, Philippines, Singapore, South Africa, and Eurasia. Additional filings in China, Hong Kong, and Canada will be made in due course. Patents issued from this family are expected to expire August 3, 2037, not including any patent term adjustments that may extend the patent term in certain jurisdictions.
The other two families include two international applications relating to different gene regulations platform technologies with claims directed to compositions of matter and methods of use. We expect to convert each of these international applications to U.S. and international patent filings in due course. Patents issued from these two patent families are expected to expire in 2037 and 2038, not including any patent term adjustments that may extend the patent term in certain jurisdictions.
We also own one patent relating to a vector technology developed by Vector Neurosciences Inc, acquired on October 5, 2018, with claims directed to compositions of matter. This patent is expected to expire October 21, 2025, not including any patent term adjustments that may extend the patent term in certain jurisdictions.
Licensed Intellectual Property
Certain of our issued patents and pending patent applications are exclusively licensed to us from UCL Business, Plc (“UCLB”), Brandeis University (“Brandeis”) and the National Institute of Dental and Craniofacial Research (“NIDCR”).
UCLB
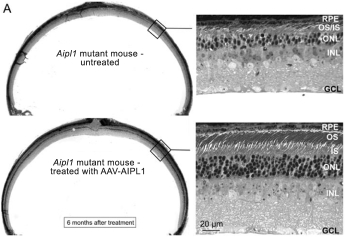
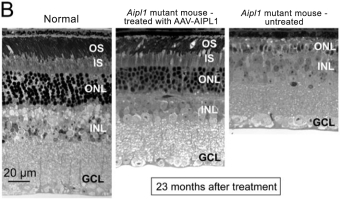
The UCLB portfolio includes three licensed patent families relating to ourRPE65,CNGA3, andRPGR gene therapy programs and one optioned patent family relating to our dry AMD gene therapy program with a combined 49 pending patent applications.
The first patent family, with claims directed to compositions of matter and methods of use relating to ourRPE65 program, and theAAV-RPE65 product candidate includes 18 pending patent applications in the United States, Europe, Australia, Canada, China, Hong Kong, India, Japan, Brazil, Egypt, Israel, Malaysia, Mexico, New Zealand, Nigeria, Philippines, Singapore, and Thailand. Patents issued from this family are expected to expire February 8, 2036, not including any patent term extensions or adjustments that may extend the patent term in certain jurisdictions.
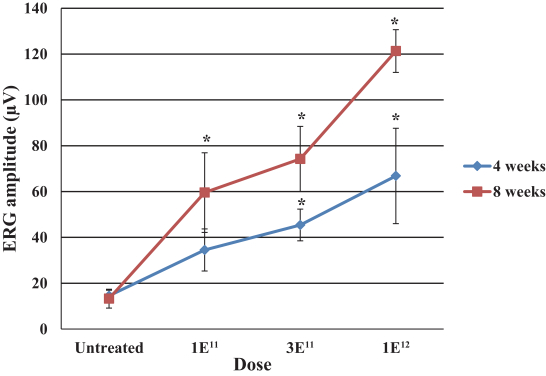
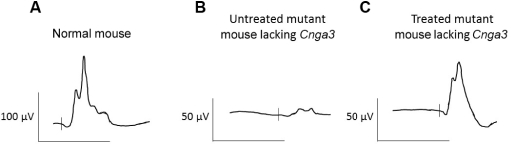
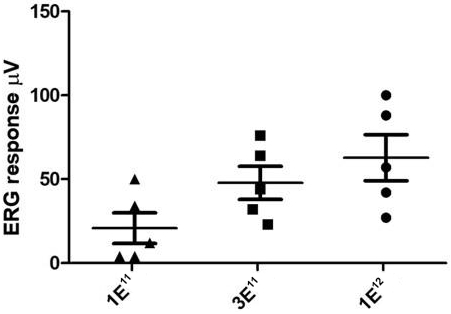
The second patent family, with claims directed to compositions of matter and methods of use relating to our achromatopsia program and theAAV-CNGA3 product candidate, includes one pending patent application, which we expect to convert to an international application and subsequent U.S. and international patent filings in due course. Patents issued from this family are expected to expire in January 2039, not including any patent term extensions or adjustments that may extend the patent term in certain jurisdictions.
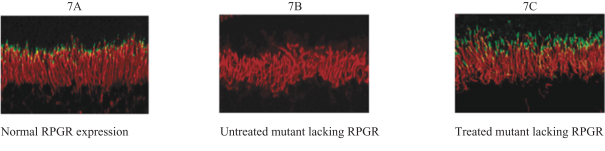
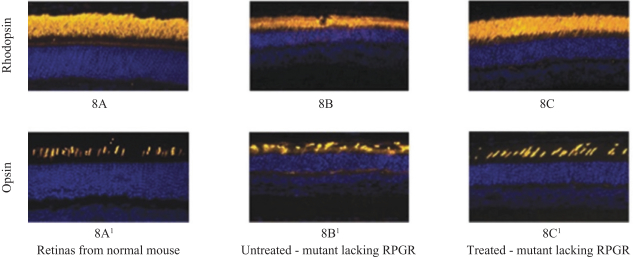
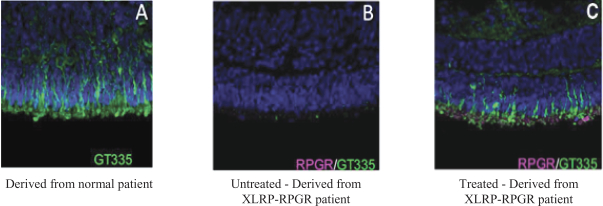
The third patent family, with claims directed to compositions of matter and methods of use relating to our retinitispigmentosa program and theAAV-RPGR product candidate, includes one allowed patent application
42