The second patent family includes 22 pending patent applications with claims directed to compositions of matter and methods of use in the United States, Europe, Australia, Canada, China, India, Japan, Brazil, Egypt, Hong Kong, Indonesia, Israel, Republic of Korea, Malaysia, Mexico, New Zealand, Vietnam, African Regional IPO, Philippines, Singapore, South Africa and Eurasia. Patents issued from this family are expected to expire February 2, 2037, not including any patent term adjustments that may extend the patent term in certain jurisdictions.
The third patent family includes 22 pending patent applications with claims directed to compositions of matter and methods of use in the United States, Europe, Australia, Canada, China, India, Japan, Brazil, Egypt, Hong Kong, Indonesia, Israel, Republic of Korea, Malaysia, Mexico, New Zealand, Vietnam, African Regional IPO, Philippines, Singapore, South Africa and Eurasia. Patents issued from this family are expected to expire February 2, 2037, not including any patent term adjustments that may extend the patent term in certain jurisdictions.
The fourth patent family includes 22 pending patent applications with claims directed to compositions of matter and methods of use in the United States, Europe, Australia, Canada, China, Hong Kong, India, Japan, Brazil, Egypt, Indonesia, Israel, Republic of Korea, Malaysia, Mexico, New Zealand, Vietnam, African Regional IPO, Philippines, Singapore, South Africa, and Eurasia. Patents issued from this family are expected to expire August 3, 2037, not including any patent term adjustments that may extend the patent term in certain jurisdictions.
The fifth patent family includes 22 pending patent applications with claims directed to compositions of matter and methods of use in the United States, Europe, Australia, Canada, China, Hong Kong, India, Japan, Brazil, Egypt, Indonesia, Israel, Republic of Korea, Malaysia, Mexico, New Zealand, Vietnam, African Regional IPO, Philippines, Singapore, South Africa, and Eurasia. Patents issued from this family are expected to expire on March 2, 2038, not including any patent term adjustments that may extend the patent term in certain jurisdictions.
The sixth patent family includes 22 pending patent applications with claims directed to compositions of matter and methods of use in the United States, Europe, Australia, Canada, China, Hong Kong, India, Japan, Brazil, Egypt, Indonesia, Israel, Republic of Korea, Malaysia, Mexico, New Zealand, Vietnam, African Regional IPO, Philippines, Singapore, South Africa, and Eurasia. Patents issued from this family are expected to expire on February 21, 2038, not including any patent term adjustments that may extend the patent term in certain jurisdictions.
Licensed Intellectual Property
Certain of our issued patents and pending patent applications are exclusively licensed to us from UCL Business, Plc (“UCLB”), Brandeis University (“Brandeis”) and the National Institute of Dental and Craniofacial Research (“NIDCR”).
UCLB
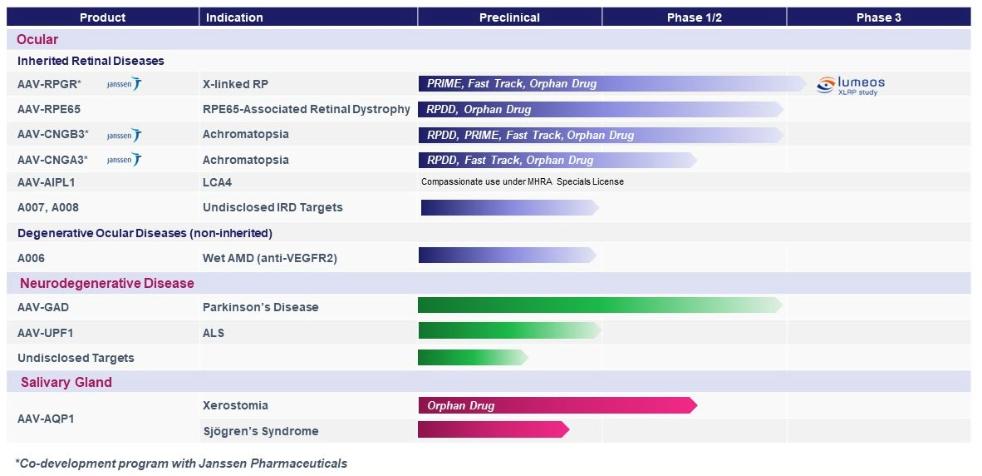
The UCLB portfolio includes three licensed patent families relating to our RPE65, CNGA3, and RPGR gene therapy programs and one optioned patent family relating to our dry AMD gene therapy program with a combined 80 United States and foreign issued patents and 68 pending patent applications.
The first patent family, with claims directed to compositions of matter and methods of use relating to our RPE65 program, and the AAV-RPE65 product candidate includes 39 issued patents in the Unites States, Singapore, Albania, Austria, Belgium, Bulgaria, Croatia, Cyprus, Czechia, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Monaco, the Netherlands, North Macedonia, Norway, Poland, Portugal, Romania, San Moreno, Serbia, Slovenia, Slovakia, Spain, Sweden, Switzerland, Turkey, and the United Kingdom and 17 pending patent applications in the United States, Europe, Australia, Canada, China, Hong Kong, India, Japan, Brazil, Egypt, Israel, Malaysia, Mexico, New Zealand, Nigeria, Philippines, and Thailand. Patents issued from this family are expected to expire February 8, 2036, not including any patent term extensions or adjustments that may extend the patent term in certain jurisdictions.