Exhibit 99.1

Pioneering a new class of RNA medicines to increase targeted gene expression Corporate Overview January 2025

Forward - Looking Statements This presentation contains forward - looking statements that are based on the beliefs and assumptions and on information currently available to CAMP4’s management. All statements other than statements of historical fact contained in this presentation are forward - looking statements. Forward - looking statements include information concerning the initiation, timing, progress and results of preclinical and clinical trials of CAMP4’s pro duc t candidates, the timing or likelihood of regulatory filings and approvals for any of its product candidates, and estimates regarding CAMP4’s expenses, future revenues , a nd future capital requirements. In some cases, you can identify forward - looking statements because they contain words such as “may,” “might,” “will,” “would,” “sha ll,” “should,” “expects,” “plans,” “anticipates,” “could,” “intends,” “target,” “projects,” “contemplates,” “believes,” “estimates,” “looks,” “seeks,” “predicts ,” “potential,” “ongoing,” or “continue” or the negative of these words or other similar terms or expressions that concern our expectations, strategy, plans or intentions, a lth ough not all forward - looking statements are accompanied by such words. Forward - looking statements involve known and unknown risks, uncertainties and other factors that may cause CAMP4’s actual results, performance or achievements to be materially different from any future results, performance or achievements expresse d o r implied by the forward - looking statements. This information was factually accurate on the date it was published. CAMP4 assumes no duty to update the information to reflect subsequent developments, except as required by law. The safety and efficacy of CAMP4’s product candidates and/or uses under investigation have not been established. There is no gua rantee that any of our product candidates will receive regulatory authority approval or become commercially available in any country for the uses being inve sti gated or that any such product candidate will achieve a particular revenue level. In particular, CAMP4’s expectations could be affected by, among other thin gs, uncertainties involved in the development of new therapeutic products; unexpected clinical trial results or unexpected new clinical data; unexpected regula tor y actions or delays or government regulation generally; CAMP4’s ability to obtain or maintain patent or other proprietary intellectual property protection; com pet ition in general; CAMP4’s ability to establish and maintain collaborations, strategic relationships and supply arrangements, or to realize the intended benefits f rom such relationships or arrangements; whether CAMP4’s cash resources will be sufficient to fund its foreseeable and unforeseeable operating expenses and capital ex pen diture requirements; CAMP4’s ability to raise additional funding on favorable terms, or at all; the rate and degree of market acceptance and clinical util ity of CAMP4’s product candidates; the ability and willingness of our third - party collaborators to continue research, development and manufacturing activities relating to our product candidates; the accuracy of our data analyses or estimates for the potential and market for our products; and government, industry, and general public pricing and other political pressures. The actual results may vary from the anticipated results and the variations may be material. Other factors that may cause the Company’s ac tual results to differ from current expectations are discussed in the Company’s filings with the SEC, including the sections titled “Risk factors,” “Management’s di scussion and analysis of financial c ondition and results of operations” and “Special note r egarding forward - looking s tatements ” in the Company’s most recent Quarterly Report on Form 10 - Q filed with the SEC on November 21, 2024. You are cautioned not to place undue reliance on these forward - looking statements, which speak on ly as of the date this presentation is given. Except as required by law, CAMP4 undertakes no obligation to publicly update any forward - looking statemen ts, whether as a result of new information, future events or otherwise. 2

CAMP4 is the leader in gene regulatory RNA ( regRNA ) discovery and regRNA - targeting antisense oligonucleotide (ASO) therapies to upregulate gene expression to restore healthy protein levels Pioneering a new class of RNA medicines to increase targeted gene expression 3 We built our Proprietary RAP Platform for the discovery of novel regRNAs that regulate the expression of every protein - coding gene Our current focus is on metabolic and CNS genetic diseases where modest increases in protein expression can be clinically meaningful Clinical data expected from MAD portion of ongoing Phase 1 study of CMP - CPS - 001 for Urea Cycle Disorders in 2H ’25 and advancement of SYNGAP1 program into GLP tox studies IPO: OCT 2024 NASDAQ: CAMP CASH RUNWAY INTO Q2 ‘26 HEADQUARTERS: CAMBRIDGE, MA There are prevalent diseases where gene upregulation is likely to have a meaningful clinical benefit

World - class management team, experienced board and advisors Josh Mandel - Brehm President & CEO David Bumcrot , PhD Chief Scientific Officer Michelle Gates Chief People Officer Caleb Moore Chief Business Operations Officer Satya Kuchimanchi , PhD SVP, Technical Operations Alla Sigova, PhD VP, Head of Platform Daniel Tardiff , PhD VP, Head of Discovery 4 Yuri Maricich , MD Chief Medical Officer Kelly Gold Chief Financial Officer Board of Directors James Boylan Ingo Chakravarty Michael Higgins Steven Holtzman Josh Mandel - Brehm Amir Nashat, ScD Paula Ragan, PhD Andy Schwab Ravi Thadhani, MD Rick Young, PhD Len Zon*, MD *Board observer, co - founder
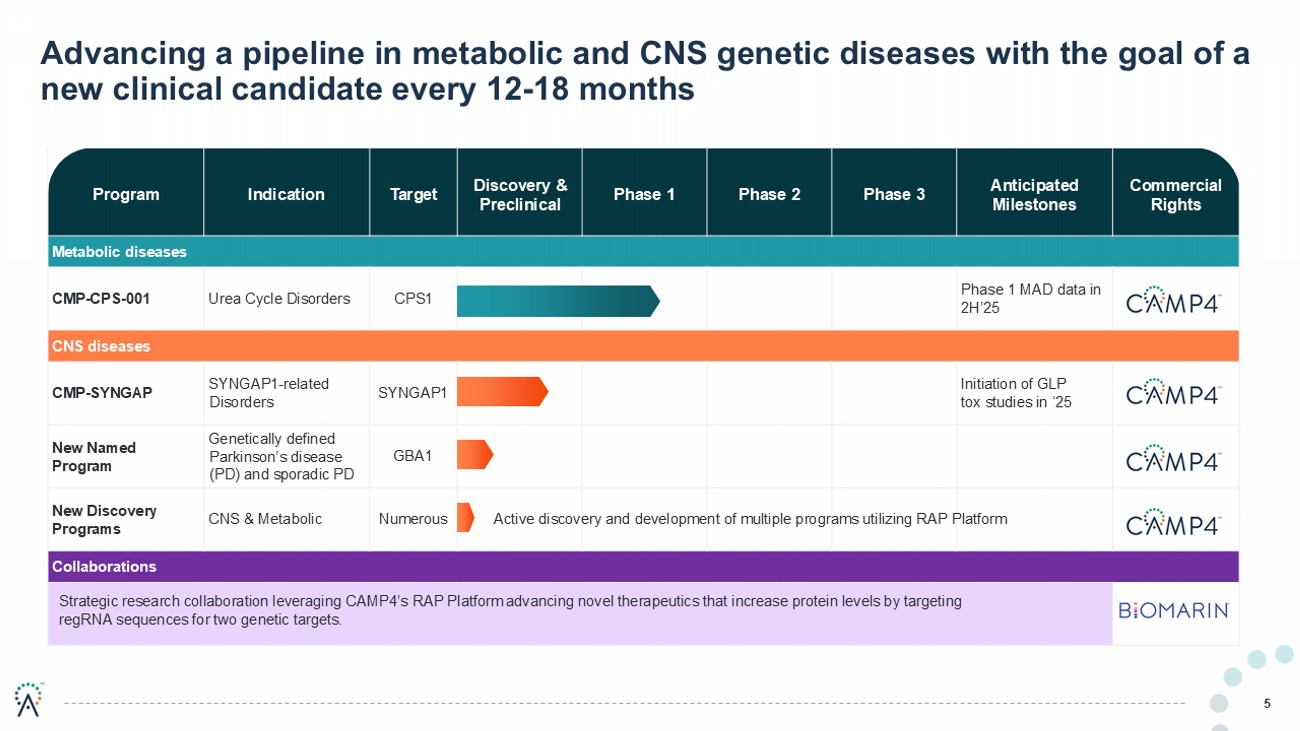
Commercial Rights Anticipated Milestones Phase 3 Phase 2 Phase 1 Discovery & Preclinical Target Indication Program Metabolic diseases Phase 1 MAD data in 2H’25 CPS1 Urea Cycle Disorders CMP - CPS - 001 CNS diseases Initiation of GLP tox studies in ‘25 SYNGAP1 SYNGAP1 - related Disorders CMP - SYNGAP GBA1 Genetically defined Parkinson’s disease (PD) and sporadic PD New Named Program Numerous CNS & Metabolic New Discovery Programs Collaborations Advancing a pipeline in metabolic and CNS genetic diseases with the goal of a new clinical candidate every 12 - 18 months 5 Strategic research collaboration leveraging CAMP4’s RAP Platform advancing novel therapeutics that increase protein levels by targeting regRNA sequences for two genetic targets. Active discovery and development of multiple programs utilizing RAP Platform

6 Agenda CAMP4 overview RAP Platform: regRNAs are master controllers of gene regulation Lead metabolic program: Urea Cycle Disorders Lead CNS program: SYNGAP1 - related Disorders

regRNAs play a central role in the regulation of every gene’s expression 7 mRNA Activators and repressors bind to regRNAs to control the transcriptional state Source: Sharp, Chakraborty, Henninger & Young, RNA 2022; Henninger, Oksuz , Shrinivas et al., Cell 2021; Sartorelli & Lauberth , NatStructMolBio 2020; Oksuz , et al (Young lab), Molecular Cell 2023 regRNAs originate from enhancers and promoters to control the expression of protein - coding genes Active Enhancer Nucleus Transcription Factors and Regulators Target Gene Promoter Activators Repressors regRNA Active Enhancer Increased mRNA expression Addresses root cause of haploinsufficient or partial loss - of - function diseases by returning targeted protein levels to within a healthy range ASOs disrupt the interactions between repressors and regRNAs enabling increases in gene expression regRNA (Modulated) Active Enhancer ASO binds regRNA 1 2 3
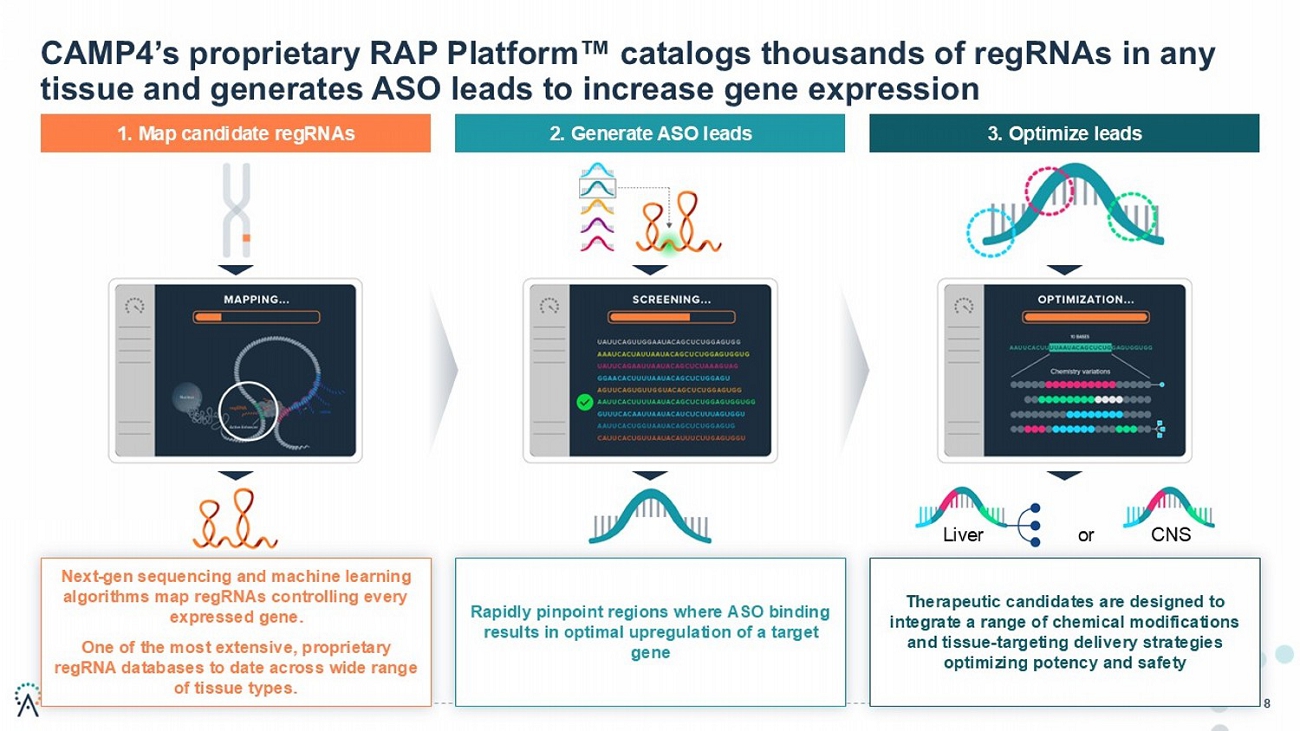
8 1. Map candidate regRNAs 2. Generate ASO leads 3. Optimize leads CAMP4’s proprietary RAP Platform catalogs thousands of regRNAs in any tissue and generates ASO leads to increase gene expression Next - gen sequencing and machine learning algorithms map regRNAs controlling every expressed gene. One of the most extensive, proprietary regRNA databases to date across wide range of tissue types. Liver or CNS Rapidly pinpoint regions where ASO binding results in optimal upregulation of a target gene Therapeutic candidates are designed to integrate range of chemical modifications and tissue - targeting delivery strategies optimizing potency and safety
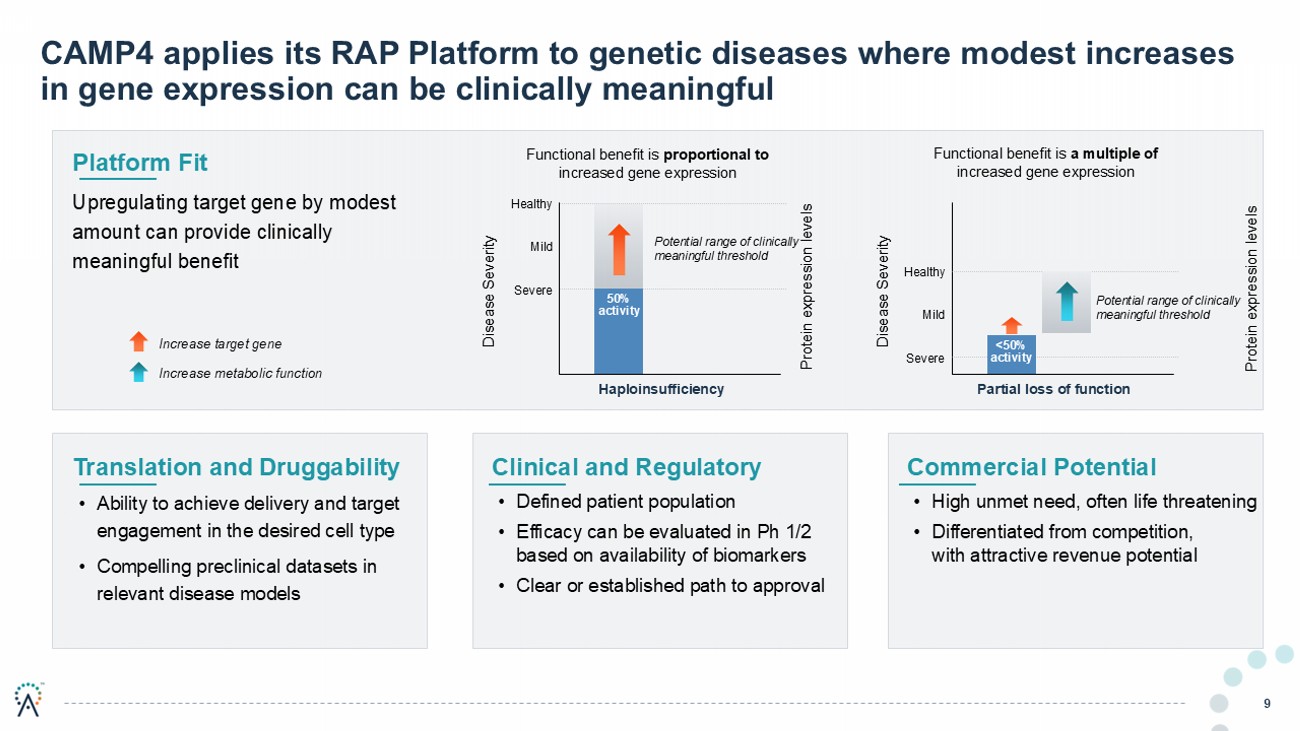
CAMP4 applies its RAP Platform to genetic diseases where modest increases in gene expression can be clinically meaningful 9 Clinical and Regulatory • Defined patient population • Efficacy can be evaluated in Ph 1/2 based on availability of biomarkers • Clear or established path to approval Platform Fit Upregulating target gene by modest amount can provide clinically meaningful benefit Translation and D ruggability • Ability to achieve delivery and target engagement in the desired cell type • Compelling preclinical datasets in relevant disease models Increase target gene Increase metabolic function Commercial Potential • High unmet need, often life threatening • Differentiated from competition, with attractive revenue potential Disease Severity Disease Severity Functional benefit is proportional to increased gene expression Functional benefit is a multiple of increased gene expression Potential range of clinically meaningful threshold Severe Healthy Mild Haploinsufficiency 50% activity Potential range of clinically meaningful threshold Severe Healthy Mild Partial loss of function <50% activity Protein expression levels Protein expression levels
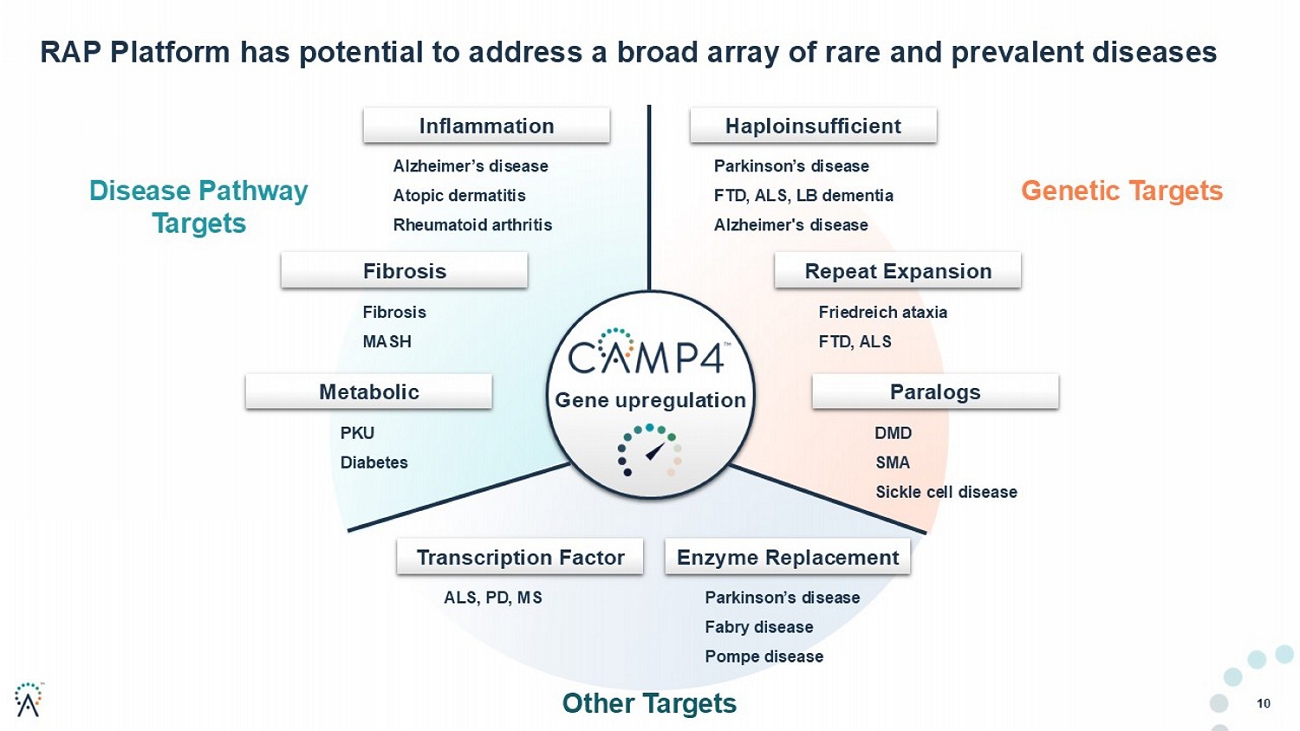
10 RAP Platform has potential to address a broad array of prevalent diseases Gene upregulation Parkinson’s disease FTD, ALS, LB dementia Alzheimer's disease Fibrosis MASH Friedreich ataxia FTD, ALS DMD SMA Sickle cell disease Fibrosis Haploinsufficient Paralogs Repeat Expansion Metabolic Enzyme Replacement Parkinson’s disease Fabry disease Pompe disease Inflammation Alzheimer’s disease Atopic dermatitis Rheumatoid arthritis PKU Diabetes Transcription Factor ALS, PD, MS Disease Pathway Targets Genetic Targets Other Targets

11 Agenda CAMP4 overview RAP Platform: regRNAs are master controllers of gene regulation Lead metabolic program: Urea Cycle Disorders Lead CNS program: SYNGAP1 - related Disorders
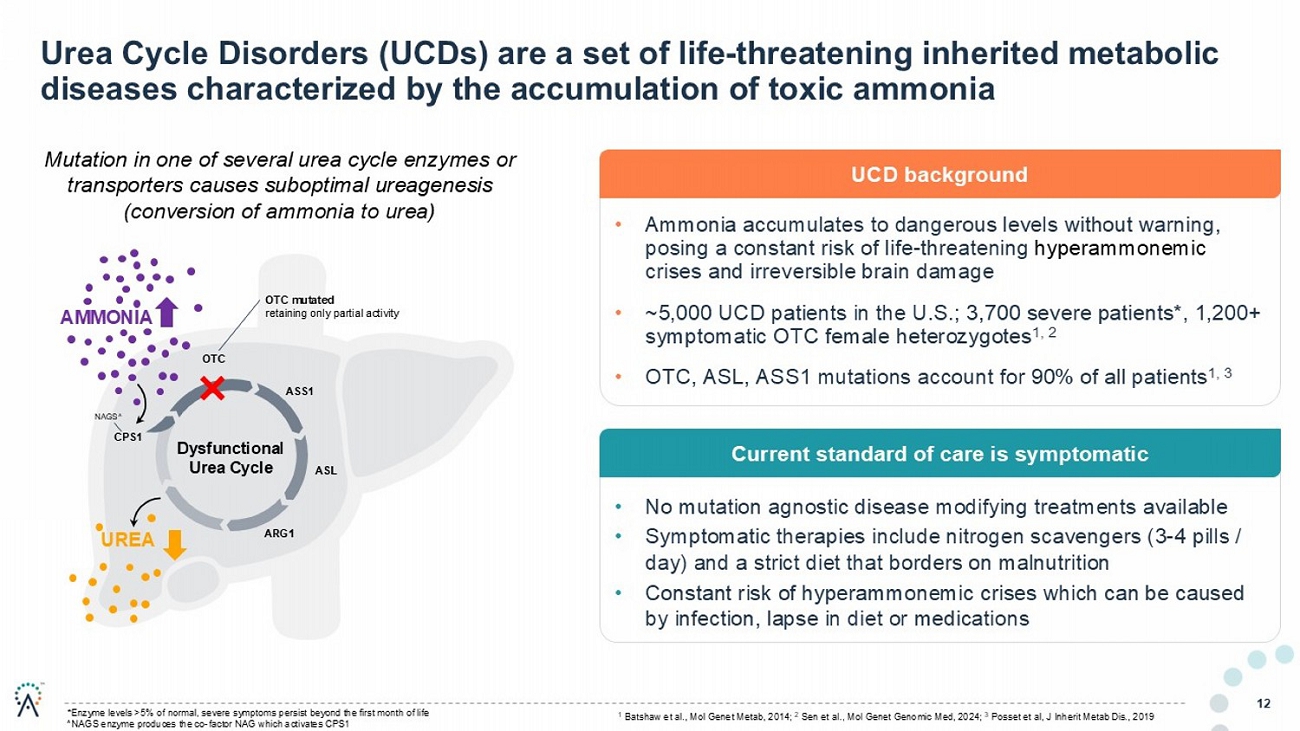
Urea Cycle Disorders (UCDs) are a set of life - threatening inherited metabolic diseases characterized by the accumulation of toxic ammonia 12 OTC ASS1 ASL ARG1 CPS1 Dysfunctional Urea Cycle NAGS^ OTC mutated retaining only partial activity UREA AMMONIA Mutation in one of several urea cycle enzymes or transporters causes suboptimal ureagenesis (conversion of ammonia to urea) • Ammonia accumulates to dangerous levels without warning, posing a constant risk of life - threatening hyperammonemic crises and irreversible brain damage • ~5,000 UCD patients in the U.S.; 3,700 severe patients*, 1,200+ symptomatic OTC female heterozygotes 1, 2 • OTC, ASL, ASS1 mutations account for 90% of all patients 1, 3 UCD background • No mutation agnostic disease modifying treatments available • Symptomatic therapies include nitrogen scavengers (3 - 4 pills / day) and a strict diet that borders on malnutrition • Constant risk of hyperammonemic crises which can be caused by infection, lapse in diet or medications Current standard of care is symptomatic *Enzyme levels >5% of normal, severe symptoms persist beyond the first month of life ^NAGS enzyme produces the co - factor NAG which activates CPS1 1 Batshaw et al., Mol Genet Metab , 2014 2 Sen et al., Mol Genet Genomic Med, 2024 3 Posset et al, J Inherit Metab Dis., 2019

CMP - CPS - 001 i s a GalNAc - conjugated ASO that binds to a CPS1 - specific regRNA to increase CPS1 expression and upregulate the expression of multiple urea cycle enzymes to amplify the conversion of ammonia to urea, potentially addressing more than 90% of patients with late onset UCDs. CAMP4 is targeting increased expression of CPS1, resulting in amplified ureagenesis and improved conversion of ammonia to urea 13 CPS1 is the gatekeeper of the urea cycle OTC ASS1 ASL ARG1 CPS1 UREA Functional Urea Cycle AMMONIA NAGS^ CMP - CPS - 001
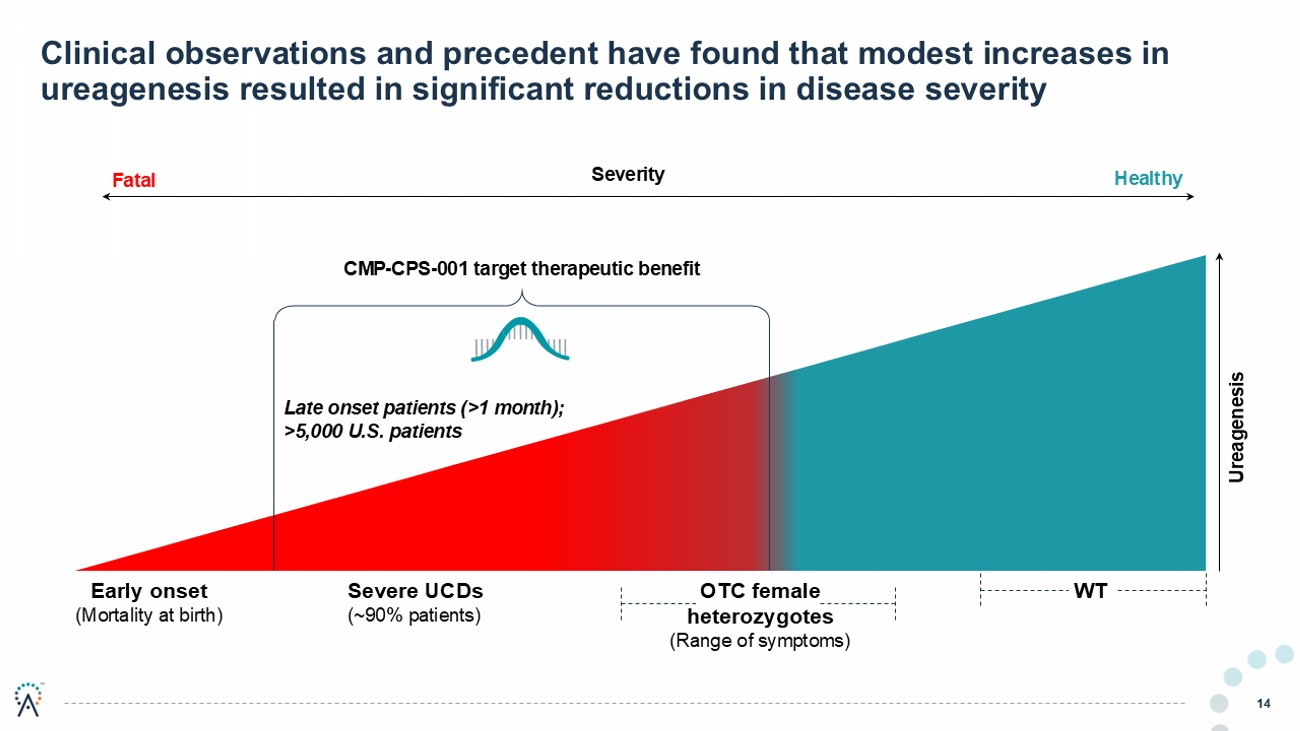
14 Clinical observations and precedent have found that modest increases in ureagenesis resulted in significant reductions in disease severity Early onset (Mortality at birth) Severe UCDs (~90% patients) OTC female heterozygotes (Range of symptoms) WT Late onset patients (>1 month); >5,000 U.S. patients CMP - CPS - 001 target therapeutic benefit Ureagenesis Severity Fatal Healthy

15 CMP - CPS - 001 has the potential to be the 1 st disease modifying pan - UCD therapy Increase ureagenesis via GalNAc - conjugated ASO *CMP - CPS - 001 has been granted a Rare Pediatric Designation and Orphan Drug Designation 1x monthly (titratable), subcutaneous • ~90% of severe UCD patients (pediatric and adult) • Symptomatic female OTC heterozygotes x Prevent hyperammonemic crises x Reduction, removal of scavengers x Protein - restricted diet liberalization AAV Gene Tx LUNAR - OTC (Ph2) • OTC - only • > 12 yrs old Nitrogen - binding agent, “scavenger” 1 - 3x daily, oral liquid Broadly applicable □ Hyperammonemic crises risk □ Significant pill burden □ Strict protein - restricted diet remains CMP - CPS - 001* Anticipated Profile Standard - of - Care (symptomatic) Pipeline DTX301 (Ph3) 1x infusion mRNA Bi - weekly infusions Description Population

• Biomarker: Urea cycle activity (ureagenesis) can be monitored in healthy volunteers and patients using the ureagenesis rate test (URT) • Phase 1 study: CAMP4 is utilizing URT in SAD / MAD Phase 1 CMP - CPS - 001 clinical study • Proof of concept: Multiple companies have utilized the URT in healthy volunteers and in patients • Human hepatocyte data: Dose - dependent increase in CPS1 expression in normal and diseased OTC - d human cells • Otc - deficient mice data: 20 - 30% ureagenesis compared to baseline, leading to ~50% ammonia (wild - type levels); ~1 month duration of action • Humanized mouse data: 20 - 30% ureagenesis compared to baseline, leading to ~70% ammonia; CPS1 + downstream enzymes • Non - human primate data: 40% ureagenesis CMP - CPS - 001 has the potential to be the first disease - modifying therapy for the treatment of the most prevalent UCDs by increasing ureagenesis 16 CAMP4 approach directly reduces toxic ammonia and increases ureagenesis Ability to measure increases in ureagenesis in healthy volunteers can translate to improved ammonia clearance in UCD patients Compelling preclinical proof of concept Phase 1 clinical design
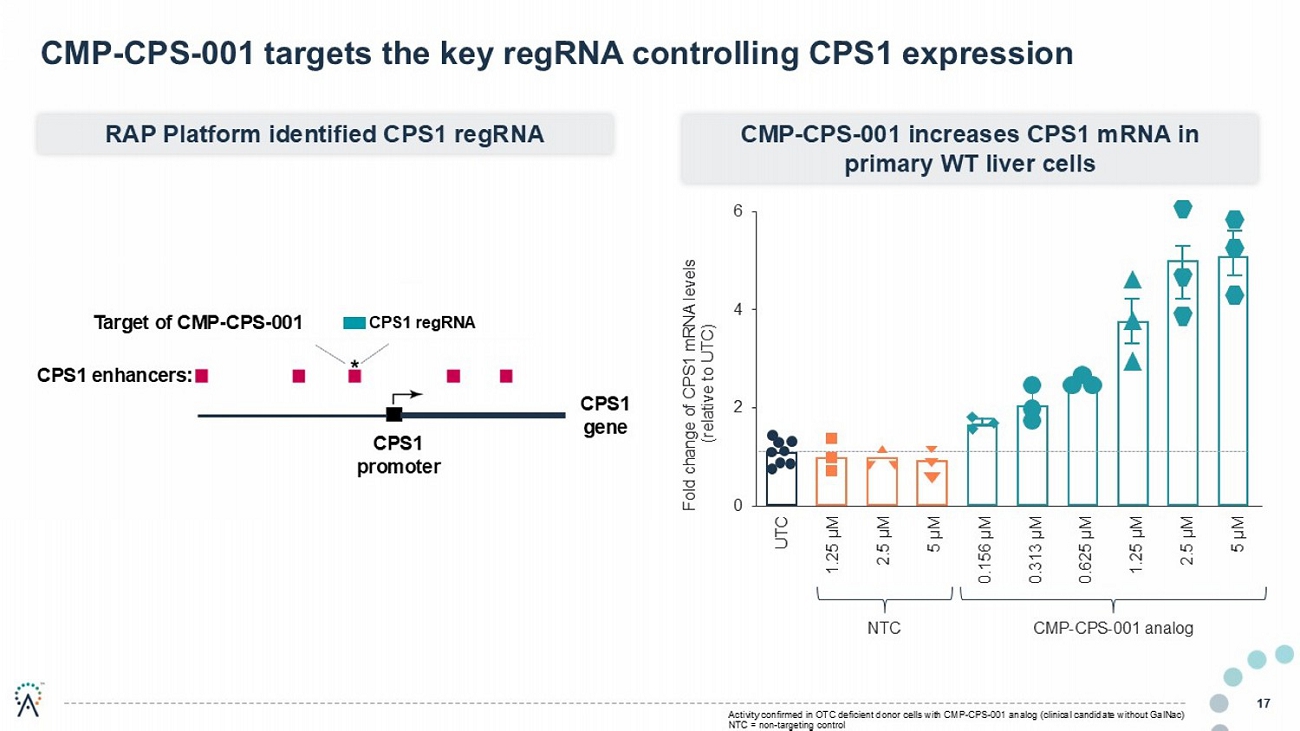
0 2 4 6 UTC 1.25 μ M 2.5 μ M 5 μ M 0.156 μ M 0.313 μ M 0.625 μ M 1.25 μ M 2.5 μ M 5 μ M Fold change of CPS1 mRNA levels (relative to UTC) 17 CMP - CPS - 001 targets the key regRNA controlling CPS1 expression RAP Platform identified CPS1 regRNA CMP - CPS - 001 increases CPS1 mRNA in primary WT liver cells NTC CMP - CPS - 001 analog CPS1 promoter CPS1 enhancers: Target of CMP - CPS - 001 CPS1 gene Activity confirmed in OTC deficient donor cells with CMP - CPS - 001 analog (clinical candidate without GalNac ) NTC = non - targeting control CPS1 regRNA

ASO targeting mouse Cps1 regRNA in Otc - deficient mice reduces ammonia and supports once - monthly dosing 18 Onset Maintenance Return (1 - 2 wks ) (~4 wks ) (~1 - 2 wks ) • The Otc spf - ash mouse model carries a patient mutation in Otc that reduces mRNA levels • Otc activity is 5% - 10% of wild - type 1 • Model displays elevated ammonia relative to wild - type mice following an acute ammonia challenge • ASO was shown to cause significant ~50% reduction in toxic ammonia (approx. WT levels) • Correlated with ~20% increase in urea production (data no shown) • Maximal effect achieved in 2 - 3 weeks • Response persisted for ~1 month 1 Hodges and Rosenberg, PNAS, 1989
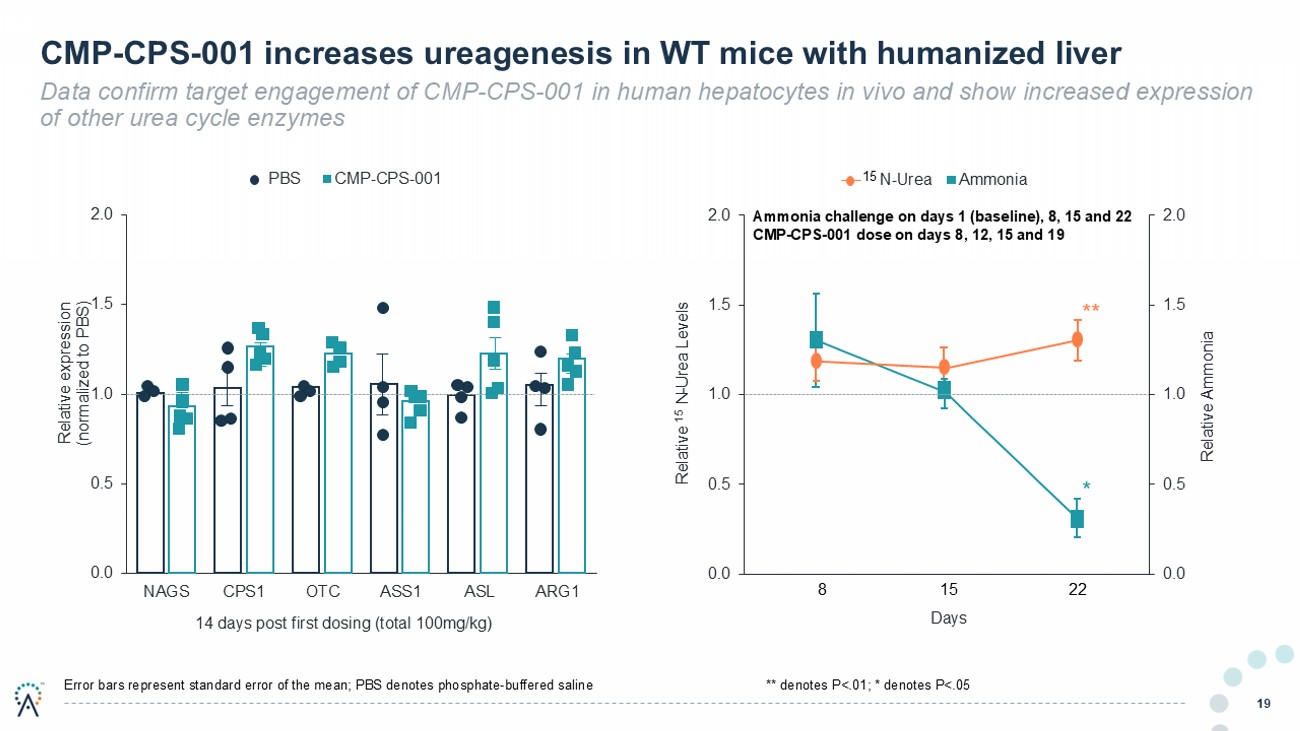
CMP - CPS - 001 increases ureagenesis in WT mice with humanized liver Data confirm target engagement of CMP - CPS - 001 in human hepatocytes in vivo and show increased expression of other urea cycle enzymes 19 0.0 0.5 1.0 1.5 2.0 NAGS CPS1 OTC ASS1 ASL ARG1 Relative expression (normalized to PBS) 14 days post first dosing (total 100mg/kg) PBS CMP-CPS-001 Error bars represent standard error of the mean; PBS denotes phosphate - buffered saline 0.0 0.5 1.0 1.5 2.0 0.0 0.5 1.0 1.5 2.0 8 15 22 Relative Ammonia Relative 15 N - Urea Levels Days N-Urea Ammonia 15 8 22 15 ** * ** denotes P<.01; * denotes P<.05 Ammonia challenge on days 1 (baseline), 8, 15 and 22 CMP - CPS - 001 dose on days 8, 12, 15 and 19
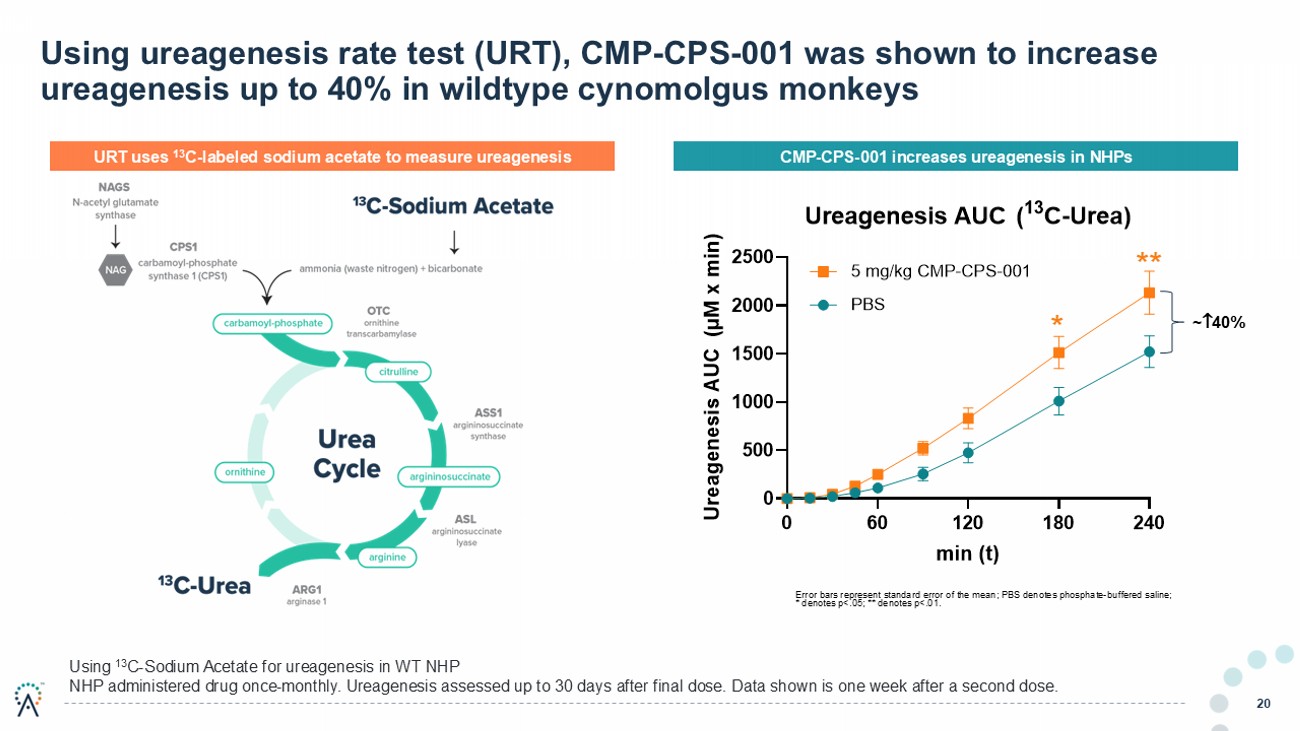
20 Using 13 C - Sodium Acetate for ureagenesis in WT NHP NHP administered drug once - monthly. Ureagenesis assessed up to 30 days after final dose. Data shown is one week after a second d ose. Error bars represent standard error of the mean; PBS denotes phosphate - buffered saline; * denotes p<.05; ** denotes p<.01. ~ 40% Using ureagenesis rate test (URT), CMP - CPS - 001 was shown to increase ureagenesis up to 40% in wildtype cynomolgus monkeys 0 60 120 180 240 0 500 1000 1500 2000 2500 min (t) U r e a g e n e s i s A U C ( μ M x m i n ) Ureagenesis AUC( 13 C-Urea) 5 mg/kg CMP-CPS-001 PBS URT uses 13 C - labeled sodium acetate to measure ureagenesis CMP - CPS - 001 increases ureagenesis in NHPs
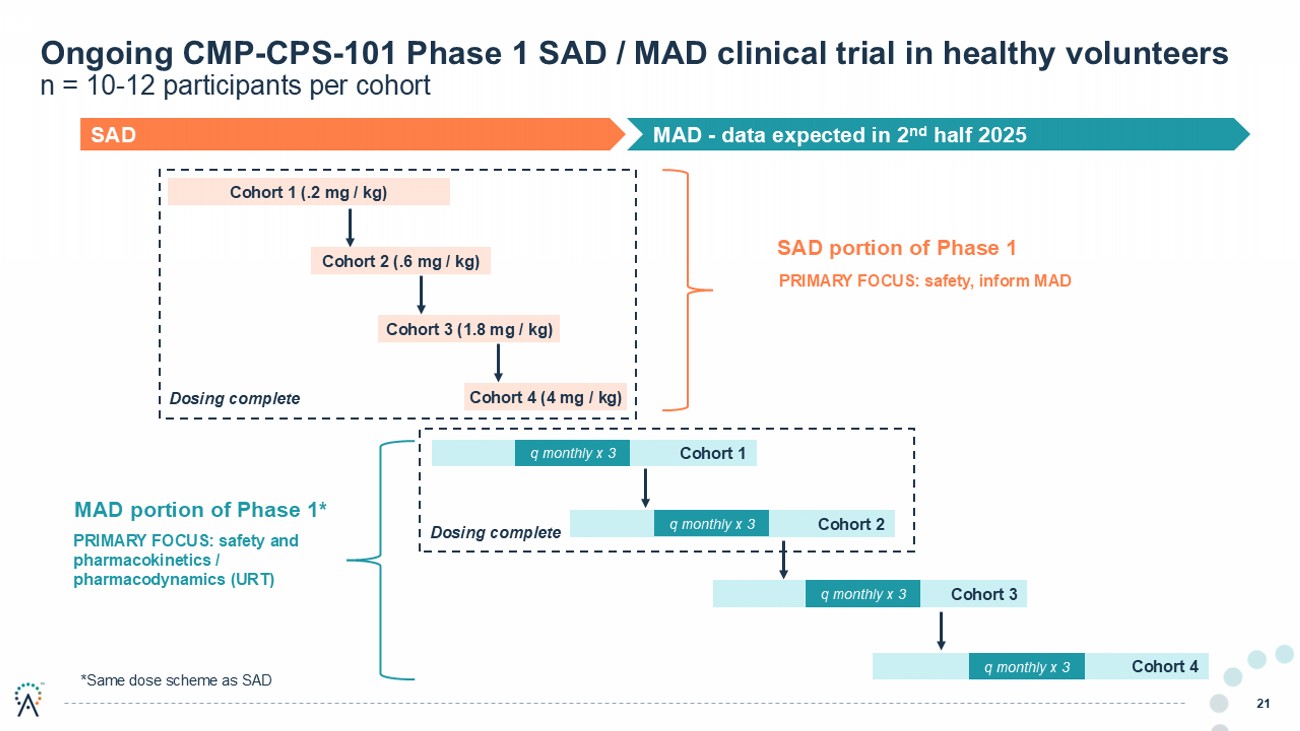
21 Ongoing CMP - CPS - 101 Phase 1 SAD / MAD clinical trial in healthy volunteers n = 10 - 12 participants per cohort Cohort 1 Cohort 1 (.2 mg / kg) Cohort 2 (.6 mg / kg) Cohort 3 (1.8 mg / kg) Cohort 4 (4 mg / kg) Cohort 2 q monthly x 3 Cohort 3 Cohort 4 SAD portion of Phase 1 MAD portion of Phase 1* SAD MAD - data expected in 2 nd half 2025 PRIMARY FOCUS: safety, inform MAD PRIMARY FOCUS: safety and pharmacokinetics / pharmacodynamics (URT) Dosing complete q monthly x 3 q monthly x 3 q monthly x 3 Dosing complete *Same dose scheme as SAD

• SAD portion of study completed. Conducted planned interim analysis of safety data for 48 normal healthy volunteer participants. • 4 Cohorts of 12 participants each, randomized 3:1 investigational product to placebo; 32 individuals received CMP - CPS - 001. • CMP - CPS - 001 has been well tolerated, with no indication of a maximum tolerated dose at the tested dose levels. No safety trends of concern have been observed, including no treatment - emergent serious adverse events. • Safety results were favorable and consistent with profiles of approved liver - targeted ASOs and with expectations based on previously reviewed safety data from SAD Cohorts 1 & 2, which showed similar findings of all TEAEs being Grade 1 (mild) or Grade 2 (moderate) with no concerning safety trends. • All treatment emergent adverse events (TEAEs) were Grade 1 (mild) or Grade 2 (moderate). The most common TEAEs by participant and number were headache (six) and nausea (four). » The Safety Review Committee has approved dose escalation and initiation of MAD Cohort 3. 22 Phase 1 SAD safety summary (all cohorts 1 – 4)
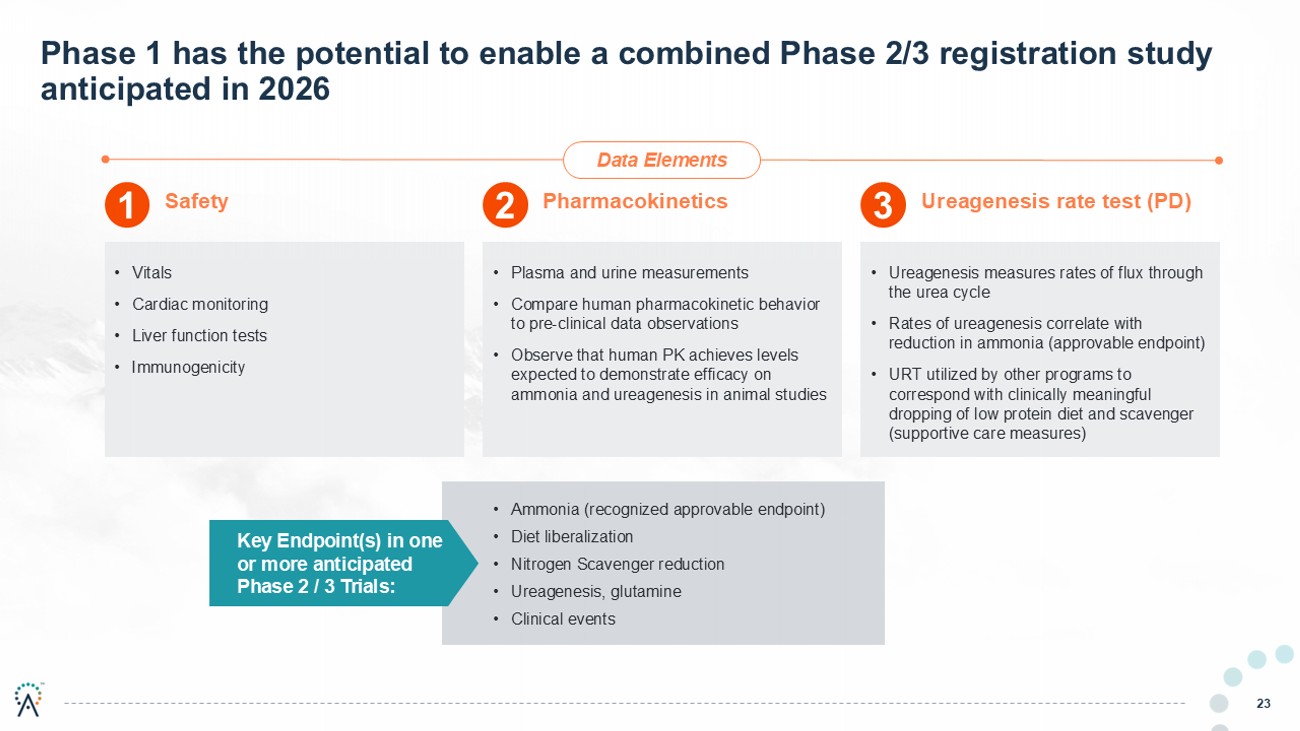
23 Phase 1 has the potential to enable a combined Phase 2/3 registration study anticipated in 2026 Safety Pharmacokinetics Ureagenesis rate test (PD) • Vitals • Cardiac monitoring • Liver function tests • Immunogenicity • Plasma and urine measurements • Compare human pharmacokinetic behavior to pre - clinical data observations • Observe that human PK achieves levels expected to demonstrate efficacy on ammonia and ureagenesis in animal studies • Ureagenesis measures rates of flux through the urea cycle • Rates of ureagenesis correlate with reduction in ammonia (approvable endpoint) • URT utilized by other programs to correspond with clinically meaningful dropping of low protein diet and scavenger (supportive care measures) Key Endpoint(s) in one or more anticipated Phase 2 / 3 Trials: • Ammonia (recognized approvable endpoint) • Diet liberalization • Nitrogen Scavenger reduction • Ureagenesis, glutamine • Clinical events 1 2 3 Data Elements

24 Agenda CAMP4 overview RAP Platform: regRNAs are master controllers of gene regulation Lead metabolic program: Urea Cycle Disorders Lead CNS program: SYNGAP1 - related Disorders

SYNGAP1 - related disorders, a severe genetic neurodevelopmental condition 25 Mutations in SYNGAP1 lead to decreased SYNGAP protein, causing increased synaptic firing • Highly burdensome symptom array: • Intellectual disability, severe behavioral problems, ASD • Generalized epilepsy • Sleep problems • Impaired motor skills, gait abnormality • Impaired communication, speech problems • ~10,000+ SYNGAP1 patients in the US 1 - 3 SYNGAP1 background • No disease modifying treatments available • Non - specific antiepileptics, sleep meds • Constant patient care needed, caregiver worry about behavioral problems, agitation Current standard of care is symptomatic 1 López - Rivera et al., Brain, 2020 2 Bahk et al., Int J Environ Res Public Health, 2019 3 Weldon et al., J Neurodev Disord , 2018 SYNGAP1 AMPAR at synapse Neuronal Excitability 1 Illustrative depiction of Electroencephalogram Presynapse Postsynapse SYNGAP1 AMPAR Glutamate EEG 1

26 SYNGAP1 AMPAR at synapse Neuronal Excitability CAMP4 aims to increase SYNGAP1 protein levels to restore SYNGAP1 at the synapse and improve disease symptoms The CMP - SYNGAP program has identified lead ASOs that bind to a SYNGAP1 - specific regRNA to increase SYNGAP1 expression. Intrathecal administration of the clinical candidate will aim to restore SYNGAP1 towards wild - type levels, normalize synaptic function, and improve symptoms of patients with mutations in SYNGAP1 causing haploinsufficiency. Restoring SYNGAP1 levels to treat disease 1 Illustrative depiction of Electroencephalogram Presynapse Postsynapse SYNGAP1 AMPAR Glutamate EEG 1 CMP - SYNGAP

CAMP4 positioned as the only transcriptional - level ASO approach 27 Discovery Preclinical Clinical Trials AAV Delivered tRNA Read - through premature stop codon, limited data ASO targeting pre - mRNA (splicing) to prevent NMD AAV Delivered dCas Target regulatory elements, limited info No industry sponsored trials ASO targeting pre - mRNA (splicing) to prevent NMD ASO targeting miRNA, 5’ UTR, or NAT limited info ASO AAV - based Transcriptional - level Post - transcriptional NMD = nonsense mediated decay; NAT = Natural Antisense Transcript ASO miRNA, splicing AAV CRISPRa, Gene Tx. ENDD = Center for Epilepsy and Neurodevelopmental Disorders (Initiative with UPenn, CHOP)
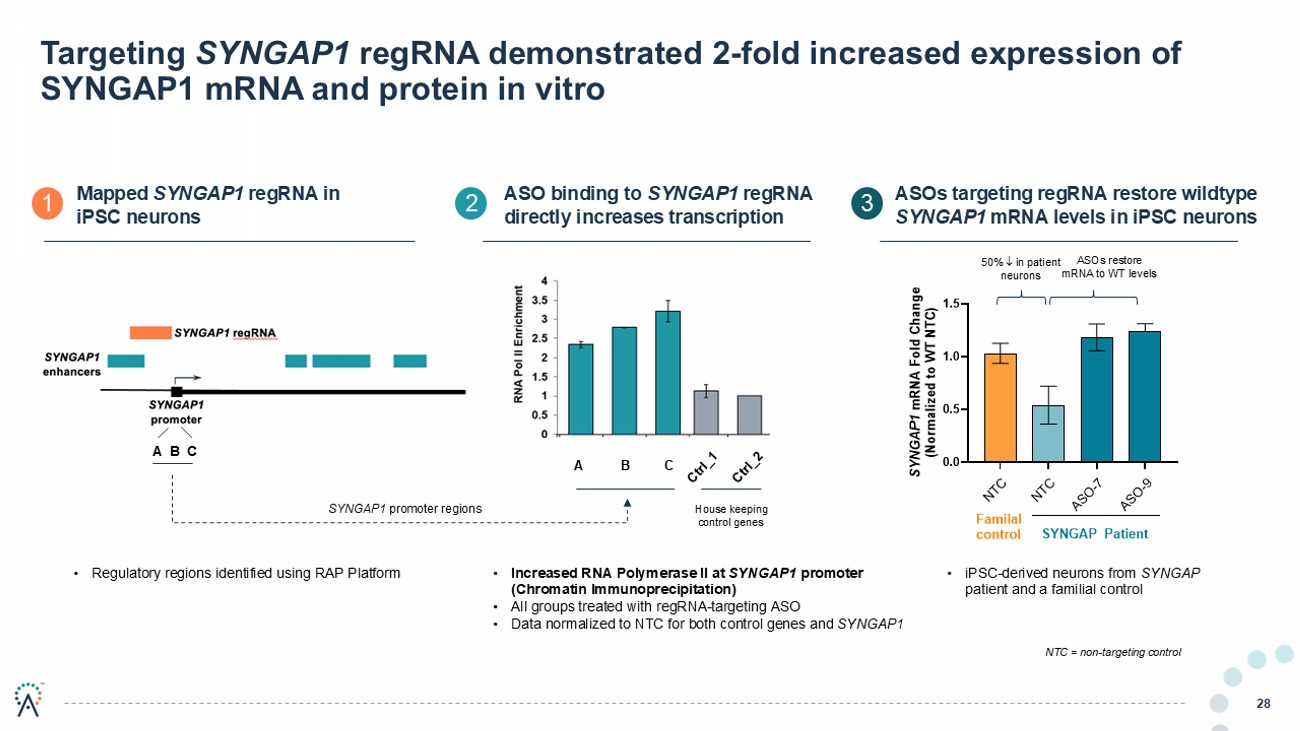
Targeting SYNGAP1 regRNA demonstrated 2 - fold increased expression of SYNGAP1 mRNA and protein in vitro 28 ASO binding to SYNGAP1 regRNA d irectly i ncreases transcription ASOs targeting regRNA restore wildtype SYNGAP1 mRNA levels in iPSC neurons A B C Mapped SYNGAP1 regRNA in iPSC neurons 1 2 3 50% in patient neurons ASOs restore mRNA to WT levels NTC = non - targeting control • iPSC - derived neurons from SYNGAP patient and a familial control House keeping control genes • Increased RNA Polymerase II at SYNGAP1 promoter (Chromatin Immunoprecipitation) • All groups treated with regRNA - targeting ASO • Data normalized to NTC for both control genes and SYNGAP1 A B C SYNGAP1 promoter regions • Regulatory regions identified using RAP Platform
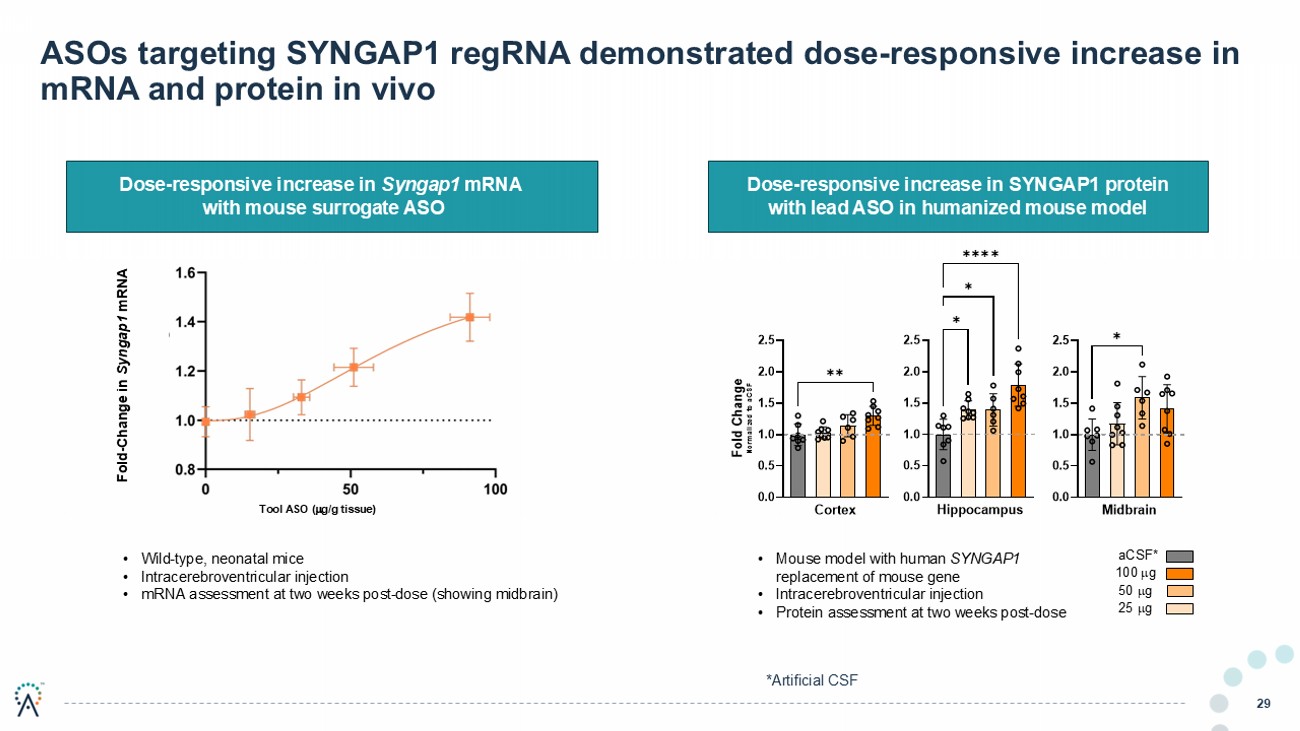
ASOs targeting SYNGAP1 regRNA demonstrated dose - responsive increase in mRNA and protein in vivo 29 Dose - responsive increase in SYNGAP1 protein with l ead ASO in humanized mouse model Dose - responsive increase in Syngap1 mRNA with mouse surrogate ASO 2024-063 P21 ICV 2-week post dose CO-19429 Dose Response in hSYNGAP mice 100 g 50 g 25 g aCSF * • Mouse model with human SYNGAP1 replacement of mouse gene • Intracerebroventricular injection • Protein assessment at two weeks post - dose Fold - Change in Syngap1 mRNA Tool ASO ( g/g tissue) • Wild - type, neonatal mice • Intracerebroventricular injection • mRNA assessment at two weeks post - dose (showing midbrain) *Artificial CSF
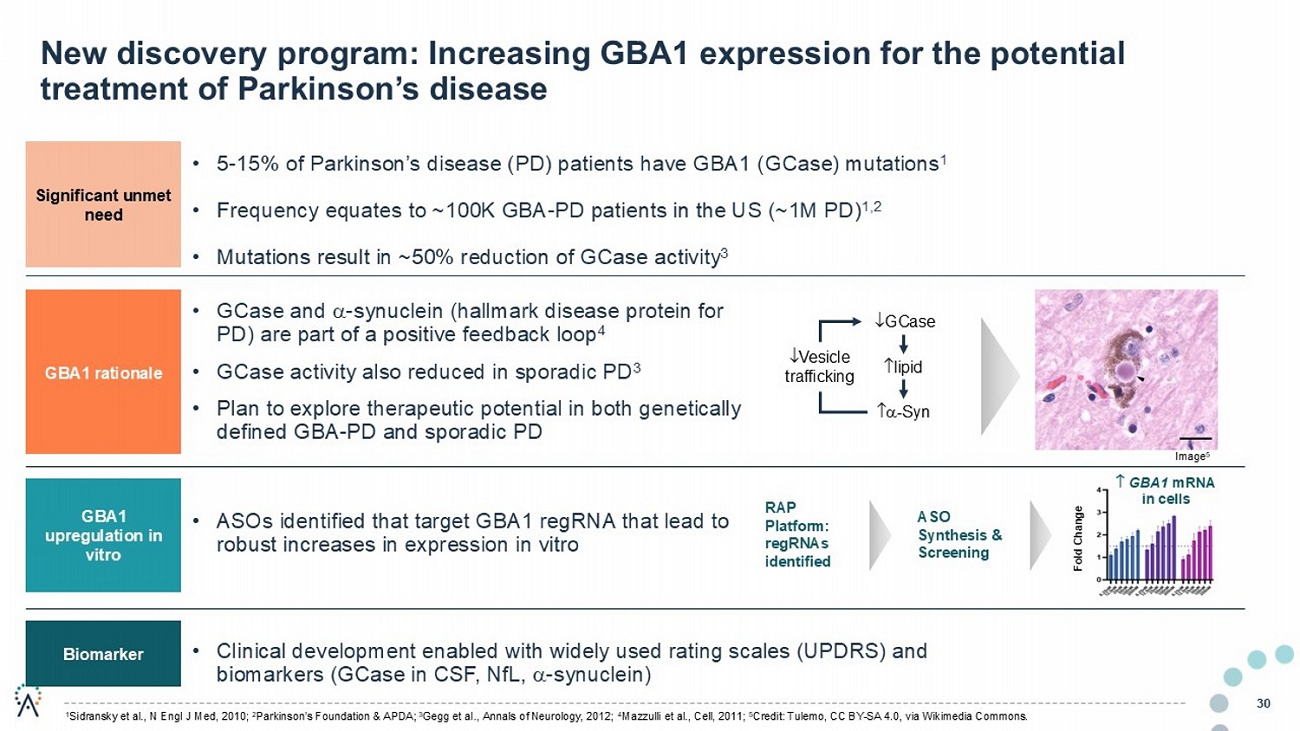
• GCase and - synuclein (hallmark disease protein for PD) are part of a positive feedback loop 4 • GCase activity also reduced in sporadic PD 3 • Plan to explore therapeutic potential in both genetically defined GBA - PD and sporadic PD 30 New discovery program: Increasing GBA1 expression for the potential treatment of Parkinson’s disease Fold Change RAP Platform: regRNAs identified GCase - Syn lipid Vesicle trafficking • 5 - 15% of Parkinson’s disease (PD) patients have GBA1 ( GCase ) mutations 1 • Frequency equates to ~100K GBA - PD patients in the US (~1M PD) 1,2 • Mutations result in ~50% reduction of GCase activity 3 • ASOs identified that target GBA1 regRNA that lead to robust increases in expression in vitro • Clinical development enabled with widely used rating scales (UPDRS) and biomarkers ( GCase in CSF, NfL , - synuclein) ASO Synthesis & Screening Significant unmet need GBA1 rationale GBA1 upregulation in vitro Biomarker 1 Sidransky et al., N Engl J Med, 2010 2 Parkinson’s Foundation & APDA 3 Gegg et al., Annals of Neurology, 2012 4 Mazzulli et al., Cell, 2011 5 Credit: Tulemo , CC BY - SA 4.0, via Wikimedia Commons. GBA1 mRNA in cells Image 5

CAMP4 is the leader in gene regulatory RNA ( regRNA ) discovery and regRNA - targeting antisense oligonucleotide (ASO) therapies to upregulate gene expression to restore healthy protein levels Pioneering a new class of RNA medicines to increase targeted gene expression 31 We built our Proprietary RAP Platform for the discovery of novel regRNAs that regulate the expression of every protein - coding gene Our current focus is on metabolic and CNS genetic diseases where modest increases in protein expression can be clinically meaningful Clinical data expected from MAD portion of ongoing Phase 1 study of CMP - CPS - 001 for Urea Cycle Disorders in 2H ’25 and advancement of SYNGAP1 program into GLP tox studies IPO: OCT 2024 NASDAQ: CAMP CASH RUNWAY INTO Q2 ‘26 HEADQUARTERS: CAMBRIDGE, MA There are prevalent diseases where gene upregulation is likely to have a meaningful clinical benefit

Thank you































