Exhibit 99.2
DO NOT DISTRIBUTE WITHOUT PERMISSION | © 2022 Compass Therapeutics Presentation May 4, 2022

DISCLAIMER This presentation has been prepared by Compass Therapeutics, Inc. ("we," "us," "our," or the “Company”). Statements contained herein are made as of the date of this presentation unless stated otherwise, and this presentation shall not under any circumstances create an implication that the information contained herein is correct as of any time after such date or that information will be updated or revised to reflect information that subsequently becomes available or changes occurring after the date hereof. This presentation includes forward - looking statements regarding our drug candidates, the timing of the start and conclusion of ongoing or planned clinical trials, including the potential impact of the ongoing COVID - 19 pandemic on our business, the timing and outcome of regulatory decisions, future availability of clinical trial data, our collaborations for our product candidates and the maintenance of those collaborations, business and results from operations, and other matters. Actual results could differ materially from those contained in any forward - looking statements as a result of various factors, including without limitation: that our drug candidates do not advance in development or result in approved products on a timely or cost effective basis or at all; the cost, timing and results of clinical trials; our ability to manage and mitigate the impact of the ongoing COVID - 19 pandemic; that many drug candidates that have completed early - stage trials do not become approved drugs on a timely or cost effective basis or at all; the ability to enroll patients in clinical trials; possible safety and efficacy concerns; regulatory developments; our ability to protect our intellectual property rights, and unexpected costs, charges or expenses that reduce cash runway. Our pipeline programs are in various stages of pre - clinical and clinical development, and the process by which such pre - clinical or clinical therapeutic candidates could potentially lead to an approved therapeutic is long and subject to significant risks and uncertainties. These and other risks and uncertainties that we face are described in our most recent Annual Report on Form 10 - K, and in other filings that we make with the Securities and Exchange Commission from time to time. We undertake no obligation to update forward - looking statements as a result of new information or otherwise. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. This presentation concerns drugs that are under clinical investigation, and which have not yet been approved for marketing by the U . S . Food and Drug Administration (FDA) . It is currently limited by Federal law to investigational use, and no representation is made as to its safety or effectiveness for the purposes for which it is being investigated . 2 DO NOT DISTRIBUTE WITHOUT PERMISSION | © 2022

Advanced Biliary Track Cancers (BTC) Richard M. Goldberg MD Professor and Director Emeritus The West Virginia University Cancer Institute
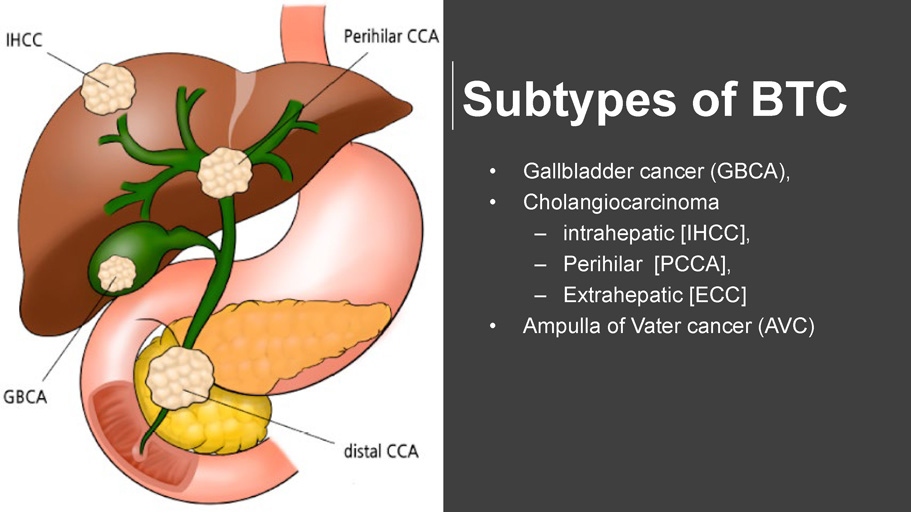
Subtypes of BTC • Gallbladder cancer (GBCA), • Cholangiocarcinoma – intrahepatic [IHCC], – Perihilar [PCCA], – Extrahepatic [ECC] • Ampulla of Vater cancer (AVC)
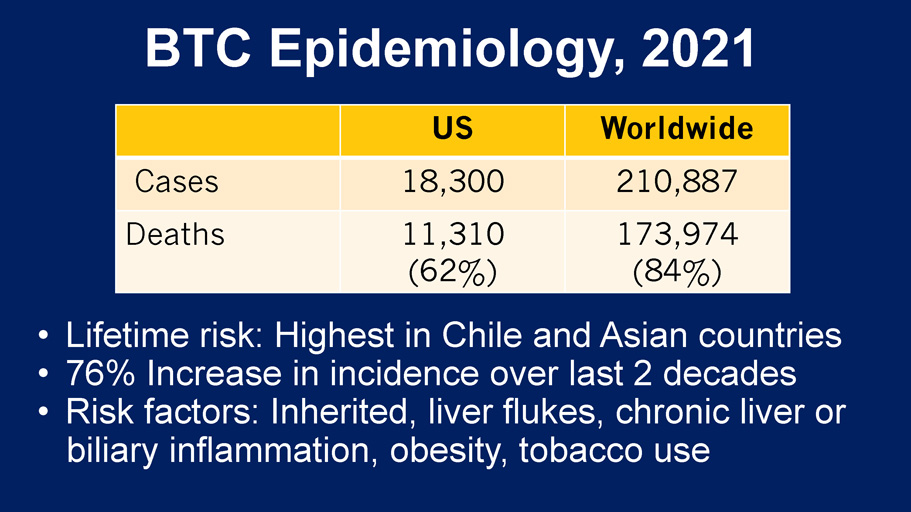
BTC Epidemiology, 2021 US Worldwide Cases 18,300 210,887 Deaths 11,310 (62%) 173,974 (84%) • Lifetime risk: Highest in Chile and Asian countries • 76% Increase in incidence over last 2 decades • Risk factors: Inherited, liver flukes, chronic liver or biliary inflammation, obesity, tobacco use

Presentation • Jaundice, yellow eyes, itching, dark urine, light colored stool • Loss of appetite and weight loss • Abdominal pain • Night sweats • Found incidentally at the time of gall bladder surgery


Original Article Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer Juan Valle, M.D., Harpreet Wasan, M.D., Daniel H. Palmer, M.D., Ph.D., David Cunningham, M.D., Alan Anthoney, M.D., Anthony Maraveyas, M.D., Ph.D., Srinivasan Madhusudan, M.D., Ph.D., Tim Iveson, M.D., Sharon Hughes, B.Sc., Stephen P. Pereira, M.D., Ph.D., Michael Roughton, M.Sc., John Bridgewater, M.D., Ph.D., for the ABC - 02 Trial Investigators N Engl J Med Volume 362(14):1273 - 1281 April 8, 2010

Patient Enrollment, Randomization, and Treatment Valle J et al. N Engl J Med 2010;362:1273 - 1281 • The ABC - 02 Study • Published 2010 • Determined the current standard of care for first line treatment of advanced CCA

ABC - 02 Outcomes Valle J et al. N Engl J Med 2010;362:1273 - 1281 Median Overall Survival Gem + Cis: Gem: 11.7 mos 8.1 mos Median Progression Free Survival Gem + Cis: Gem: 8.0 mos 5.0 mos

A Phase 3 randomized, double - blind, placebo - controlled study of durvalumab in combination with gemcitabine plus cisplatin in patients with advanced biliary tract cancer: TOPAZ - 1

TOPAZ - 1 study design
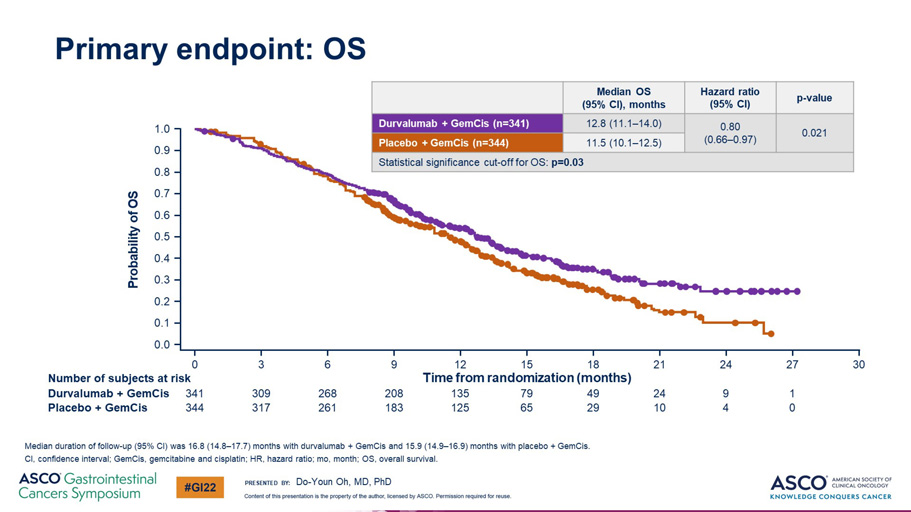
Primary endpoint: OS

Secondary endpoint: PFS
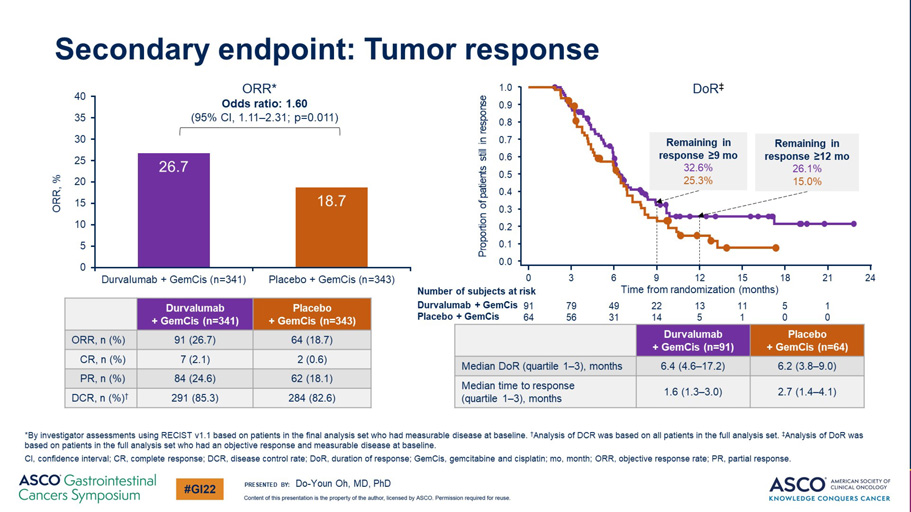
Secondary endpoint: Tumor response

Survival after 1 st Line Therapy • In ABC - 02 and Topaz - 1 median survival post progression was about 3 months

Unmet Need for Second Line Therapies for Cholangiocarcinoma ڻ Historical data of outcomes in 2L chemo - based therapies after gemcitabine/plat - based combo therapy failure result in dismal outcomes with limited progression free survival: Author Treatment Phase No. of patients PFS (mo) OS (mo) ORR (%) He et al. FOLFOX - 4 II 37 3.1 6.9 21.6 Paule et al. Gem/oxa + cetuximab II 9 4.0 7.0 11.0 Sasaki et al. Irinotecan II 13 1.8 6.7 7.7 Suzuki et al. S - 1 II 40 2.5 6.8 7.5 Fornaro et al. Gem combination Retrospective 174 3.0 6.6 3.4 Source: Ahn and Bekaii - Saab 2017* *OS (mo) reported from He et al. , and ORR (%) reported from Paule et al. and Fornaro et al. are corrected. 15
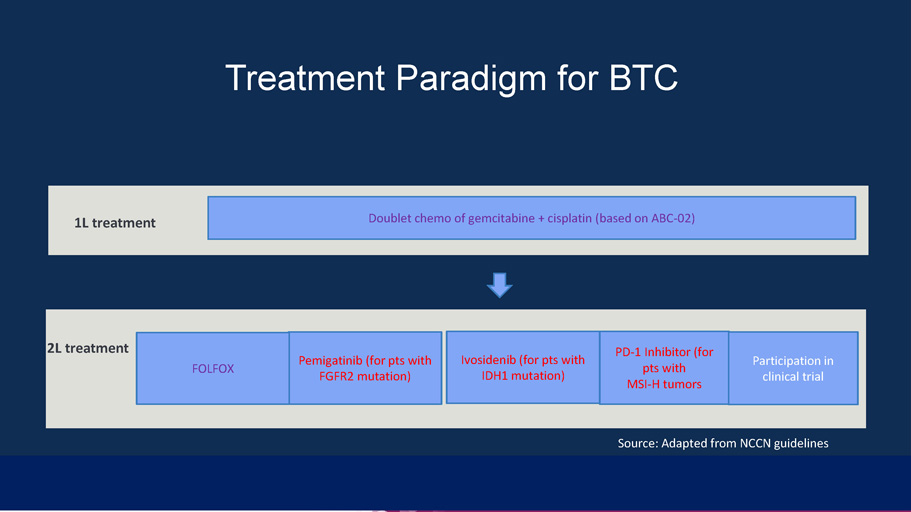
1L treatment Source: Adapted from NCCN guidelines Doublet chemo of gemcitabine + cisplatin (based on ABC - 02) Treatment Paradigm for BTC 1L treatment 2L treatment FOLFOX Pemigatinib (for pts with FGFR2 mutation) Ivosidenib (for pts with IDH1 mutation) PD - 1 Inhibitor (for pts with MSI - H tumors Participation in clinical trial

Targeted Therapy in BTC • IDH - 1 • Microsatellite Instability (MSI - H) • NTRK fusion • FGFR fusion 9.3% 4.3% 0.75% <0.50% Eligible for current targeted therapies ~14% Presented by:

Second - line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC - 06): a phase 3, open - label, randomised, controlled trial Angela Lamarca, PhD, Prof Daniel H Palmer, PhD, Harpreet Singh Wasan, MD, Paul J Ross, PhD, Yuk Ting Ma, PhD, Arvind Arora, MD, Stephen Falk, MD, Roopinder Gillmore, PhD, Prof Jonathan Wadsley, MA, Kinnari Patel, PhD, Alan Anthoney, MD, Prof Anthony Maraveyas, PhD, Prof Tim Iveson, MD, Justin S Waters, PhD, Claire Hobbs, MSc, Safia Barber, BSc, W David Ryder, Grad.IS, Prof John Ramage, MD, Prof Linda M Davies, MSc, Prof John A Bridgewater, PhD, Prof Juan W Valle, MD The Lancet Oncology Volume 22 Issue 5 Pages 690 - 701 (May 2021) DOI: 10.1016/S1470 - 2045(21)00027 - 9 Copyright © 2021 ished by Elsevier Ltd. This is an Open Access article under the CC B 4. i se. Terms and Conditions
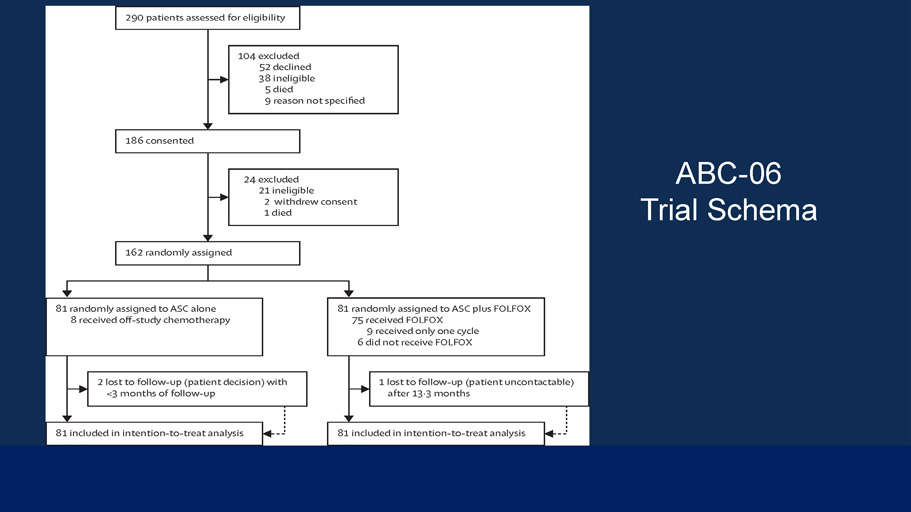
Figure 1 The Lancet Oncology 2021 2690 - 7 1DOI: (10.1016/S1470 - 2045(21)00027 - 9) Copyright © 2021 ished by Elsevier Ltd. This is an Open Access article under the CC B 4. i se. Terms and Conditions ABC - 06 Trial Schema
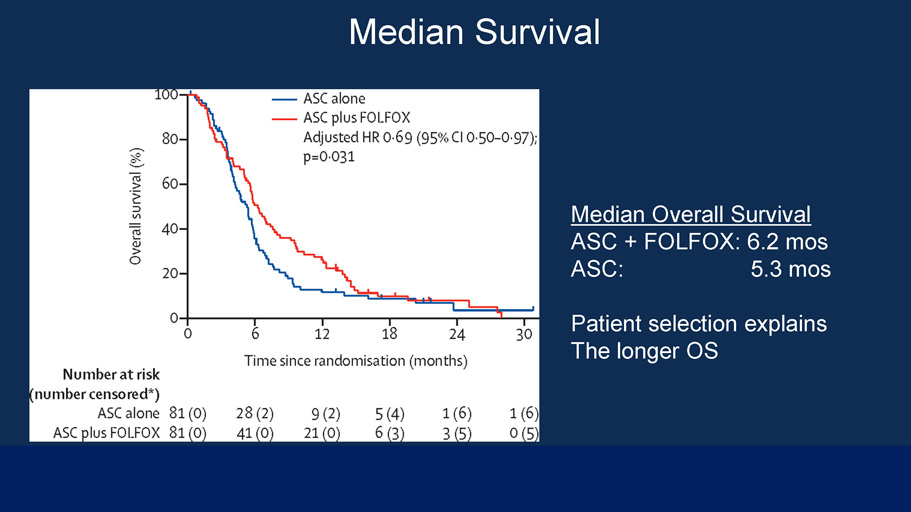
Median Survival The Lancet Oncology 2021 2690 - 7 1DOI: (10.1016/S1470 - 2045(21)00027 - 9) Copyright © 2021 ished by Elsevier Ltd. This is an Open Access article under the CC B 4. i se. Terms and Conditions Median Overall Survival ASC + FOLFOX: 6.2 mos ASC: 5.3 mos Patient selection explains The longer OS

Chemotherapy in BTC • NCCN Guidelines – First Line: Gem/Cis doublet • 26.1% ORR • 3.6 month increase in median OS vs. Gem alone (HR=0.64) • Valle, et al. (2010) – Second - line: FOLFOX • 5% ORR • 0.9 month increase in median OS vs. supportive care (HR=0.69) • Lamarca, et al. (2021) • Taxanes – Neither paclitaxel nor docetaxel are recommended by NCCN – Paclitaxel : No responses in a 15 patient first - line study [Jones, et al . ( 1996 )] – Docetaxel: No responses in a 17 patient first - and second - line study [Pazdur, et al. (1999)] – Nab - Paclitaxel is under investigation, but preferred first line regimen is Gem/Cis per NCCN Guidelines 21 DO NOT DISTRIBUTE WITHOUT PERMISSION | © 2022

There clearly are unmet needs in managing BTC Presented by:

CTX - 009 Update: Executive Summary ڻ Phase 1: 8 PRs in patients with advanced cancers both as a monotherapy and in combination with chemotherapy with an acceptable safety profile ڻ Phase 2 (Stage 1): CTX - 009 in combination with paclitaxel in patients with BTC is ongoing ڻ Interim update (data as of April 14, 2022) ڻ 24 patients with BTC have been enrolled and dosed ڻ As of 4/14; 10 PRs for a 42% ORR (10/24) ڻ Responses observed across all 4 BTC subtypes ڻ Median time on study is 6 months ڻ Adverse event profile similar to Phase 1 studies ڻ Phase 2 (Stage 2): Plan to initiate Stage 2 in the US in early Q3 DO NOT DISTRIBUTE WITHOUT PERMISSION | 2022 3
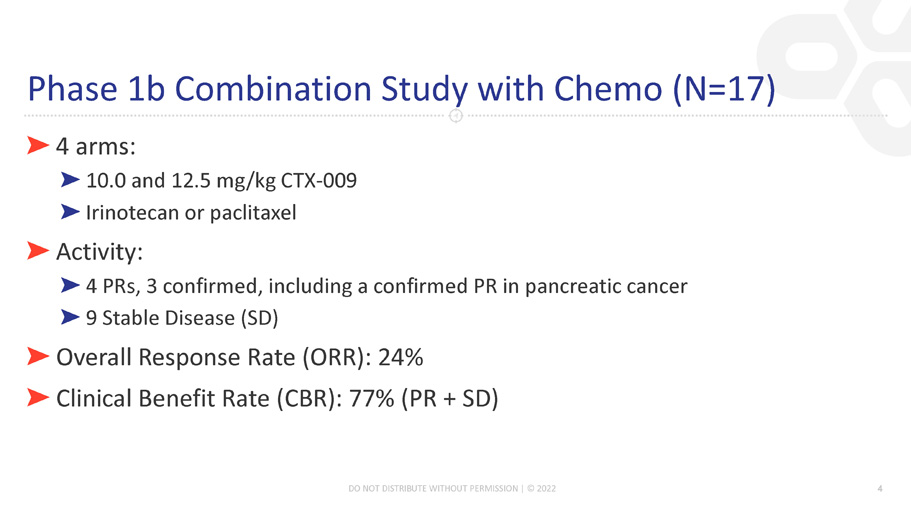
Phase 1b Combination Study with Chemo (N=17) ڻ 4 arms: ڻ 10.0 and 12.5 mg/kg CTX - 009 ڻ Irinotecan or paclitaxel ڻ Activity: ڻ 4 PRs, 3 confirmed, including a confirmed PR in pancreatic cancer ڻ 9 Stable Disease (SD) ڻ Overall Response Rate (ORR): 24% ڻ Clinical Benefit Rate (CBR): 77% (PR + SD) 4 DO NOT DISTRIBUTE WITHOUT PERMISSION | © 2022

36.8% 22.7% 16.3% 7.4% 0.0% - 0.7% - 10.4% - 12.7% - 17.0% - 18.3% - 19.7% - 20.6% - 28.0% - 31.4% - 34.9% - 41.4% - 61.6% - 30.0% - 40.0% - 50.0% - 60.0% - 70.0% - 80.0% - 10.0% - 20.0% 10.0% 0.0% 30.0% 20.0% 50.0% 40.0% 70.0% 60.0% 80.0% Tumor Growth (%) Patient Phase 1b Data (17 Patients) CTX - 009 + paclitaxel or CTX - 009 + irinotecan Phase 1b Combination Stud y Waterfall Plot 5 Waterfall of CTX - 009 (ABL001) Combination study as of November 30, 2021 (All Phase 1b Patients, 10mg/kg or 12.5 mg/kg) Gastric CRC Cholangio Pancreatic Other DO NOT DISTRIBUTE WITHOUT PERMISSION | © 2022
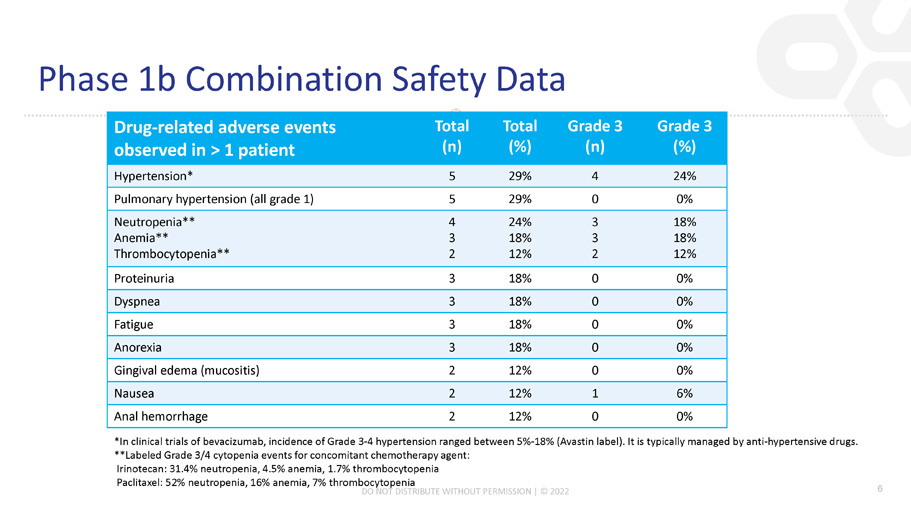
Drug - related adverse events observed in > 1 patient Total (n) Total (%) Grade 3 (n) Grade 3 (%) Hypertension* 5 29% 4 24% Pulmonary hypertension (all grade 1) 5 29% 0 0% Neutropenia** Anemia** Thrombocytopenia** 4 3 2 24% 18% 12% 3 3 2 18% 18% 12% Proteinuria 3 18% 0 0% Dyspnea 3 18% 0 0% Fatigue 3 18% 0 0% Anorexia 3 18% 0 0% Gingival edema (mucositis) 2 12% 0 0% Nausea 2 12% 1 6% Anal hemorrhage 2 12% 0 0% Phase 1b Combination Safety Data 6 *In clinical trials of bevacizumab, incidence of Grade 3 - 4 hypertension ranged between 5% - 18% (Avastin label). It is typically managed by anti - hypertensive drugs. **Labeled Grade 3/4 cytopenia events for concomitant chemotherapy agent: Irinotecan: 31.4% neutropenia, 4.5% anemia, 1.7% thrombocytopenia Paclitaxel: 52% neutropenia, 16% anemia, 7% thrombocytopenia DO NOT DISTRIBUTE WITHOUT PERMISSION | © 2022

Phase 2 Combination Study: CTX - 009 Plus Paclitaxel Phase 2 Study Design: ڻ Patients with biliary tract cancers after one or two prior therapies ڻ CTX - 009 at 10 mg/kg biweekly plus paclitaxel 80 mg/m 2 weekly 3 of 4 weeks ڻ Simon 2 Stage adaptive design: ڻ Stage 1: 21 patients ORR ڻ Stage 2: if 3 or more PRs Stage 2: 45 additional patients DO NOT DISTRIBUTE WITHOUT PERMISSION | © 2022 7
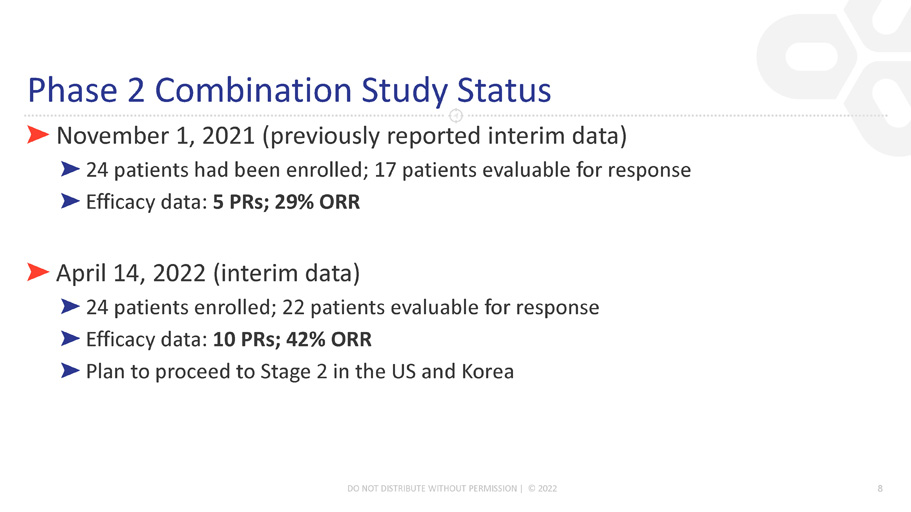
Phase 2 Combination Study Status ڻ November 1, 2021 (previously reported interim data) ڻ 24 patients had been enrolled; 17 patients evaluable for response ڻ Efficacy data: 5 PRs; 29% ORR ڻ April 14, 2022 (interim data) ڻ 24 patients enrolled; 22 patients evaluable for response ڻ Efficacy data: 10 PRs; 42% ORR ڻ Plan to proceed to Stage 2 in the US and Korea DO NOT DISTRIBUTE WITHOUT PERMISSION | © 2022 8
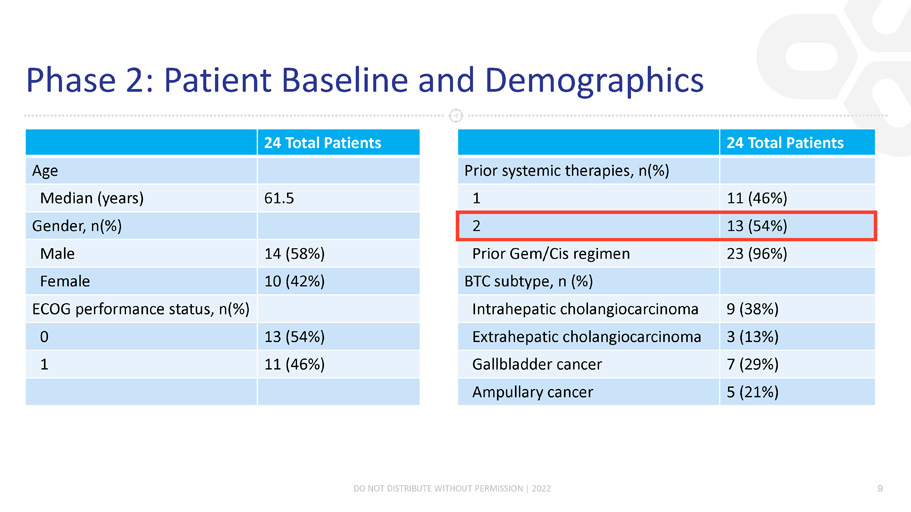
Phase 2: Patient Baseline and Demographics 24 Total Patients Age Median (years) 61.5 Gender, n(%) Male 14 (58%) Female 10 (42%) ECOG performance status, n(%) 0 13 (54%) 1 11 (46%) 24 Total Patients Prior systemic therapies, n(%) 1 11 (46%) 2 13 (54%) Prior Gem/Cis regimen 23 (96%) BTC subtype, n (%) Intrahepatic cholangiocarcinoma 9 (38%) Extrahepatic cholangiocarcinoma 3 (13%) Gallbladder cancer 7 (29%) Ampullary cancer 5 (21%) DO NOT DISTRIBUTE WITHOUT PERMISSION | 2022 9
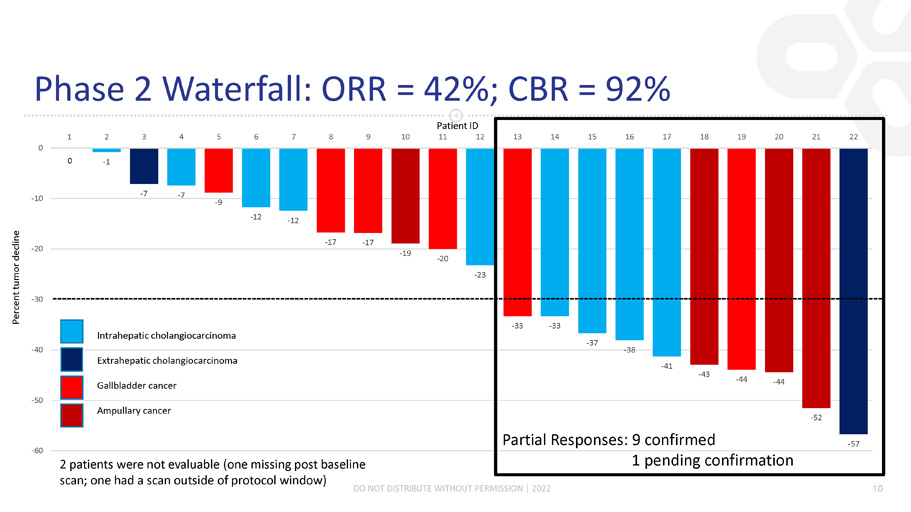
Phase 2 Waterfall: ORR = 42%; CBR = 92% DO NOT DISTRIBUTE WITHOUT PERMISSION | 2022 10 - 1 - 7 - 7 - 9 - 12 - 12 - 17 - 17 - 19 - 20 - 23 - 33 - 33 - 37 - 38 - 41 - 43 - 44 - 44 - 52 - 57 - 60 - 50 - 40 - 30 - 20 - 10 0 1 2 3 4 5 6 7 8 9 10 13 14 15 16 17 18 19 20 21 22 Intrahepatic cholangiocarcinoma Extrahepatic cholangiocarcinoma Gallbladder cancer Ampullary cancer 0 Partial Responses: 9 confirmed 1 pending confirmation 2 patients were not evaluable (one missing post baseline scan; one had a scan outside of protocol window) Patient ID 11 12 Percent tumor decline

Swimmer Plot: Median Time on Study ~ 6 Months DO NOT DISTRIBUTE WITHOUT PERMISSION | 2022 11 Days on Treatment

Safety Data: Treatment - Related ≥ Grade 3 Adverse Events Event 24 total Patients N (%) Neutropenia 12 (50.0%) Hypertension 4 (16.7%) Anemia 3 (12.5%) Thrombocytopenia 2 (8.3%) Additional events observed in 1 patient: Intestinal perforation, Asthenia, Catheter site hemorrhage, Fatigue, Cholangitis, Abdominal infection, Bacterial gastritis, Pneumonia (fatal), Post - procedure hemorrhage, Decreased appetite, Cerebral hemorrhage, Proteinuria, Embolism Event Avastin (label) Paclitaxel (label) Neutropenia 52% Hypertension 5 - 18% Anemia 16% Thrombocytopenia 7% Additional events: Additional events: GI perforation, Hypersensitivity wound healing reactions, complications, infections, bleeding, Proteinuria, neuropathy hemorrhage DO NOT DISTRIBUTE WITHOUT PERMISSION | 2022 12 Phase 2 BTC study of CTX - 009 plus paclitaxel Avastin and paclitaxel label information
CTX - 009 Next Steps ڻ Initiate Stage 2 of the Phase 2 BTC study in the US in early Q3 ڻ Initiate Phase 2/3 study in patients with colorectal cancer in the third line setting in the US in Q4 2022 ڻ Initiate Phase 2 study in patients with advanced ovarian cancer in the US in Q1 2023 ڻ Continue to evaluate additional indications for CTX - 009 both as a monotherapy and in combination with chemotherapy DO NOT DISTRIBUTE WITHOUT PERMISSION | 2022 13
CTX - 009 Interim Phase 2 Stu dy Summary ڻ 24 patients with BTC have been enrolled and dosed ڻ 10 partial responses (PRs) for a 42% ORR in patients treated in the second - and third - line settings (54% of patient were treated in the 3 rd line setting) ڻ Other regimens in BTC: ڻ FOLFOX (NCCN guidelines): 5% ORR in the second - line setting ڻ TOPAZ - 1 (Phase 3 development): 26.7% ORR for Gem/Cis/Durvalumab (anti - PD - L1) in the first - line setting ڻ Median time on study approximately 6 months, with 7 patients ongoing ڻ Adverse event profile similar to Phase 1 DO NOT DISTRIBUTE WITHOUT PERMISSION | 2022 14



































