Exhibit 99.2

UPNEEQ™ US Commercial Update August 2020

1 © 2020 Osmotica Pharmaceuticals plc. Safe Harbor This presentation contains forward - looking statements. You should not rely upon forward - looking statements as predictions of future events. All statements other than statements of historical facts contained in this presentation, including information concerning the ti ming of clinical and commercial development and launch plans with respect to our products and product candidates, are forward - looking statements. For ward - looking statements are subject to known and unknown risks, uncertainties and other factors, including that failures of or delays in clinical trials cou ld jeopardize or delay our ability to obtain regulatory approval and commence product sales for new products, as well as the other factors that are described in th e “ Risk Factors” section in our filings with the Securities and Exchange Commission. These risks, uncertainties and other factors may cause our actual result s, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward - looking statement s. Given these uncertainties, you should not place undue reliance on any forward - looking statements in this presentation. The forward - looking statements included in this presentation are made only as of the date hereof. We cannot guarantee that the f uture results, levels of activity, performance or events and circumstances reflected in the forward - looking statements will be achieved or occur. Moreover, neither we nor our advisors nor any other person assumes responsibility for the accuracy and completeness of the forward - looking statements. Neither we nor our advi sors undertake any obligation to update any forward - looking statements for any reason after the date of this presentation to conform these statements to actua l results or to changes in our expectations, except as may be required by law. You should read this presentation with the understanding that our actual futu re results, levels of activity, performance and events and circumstances may be materially different from what we expect .
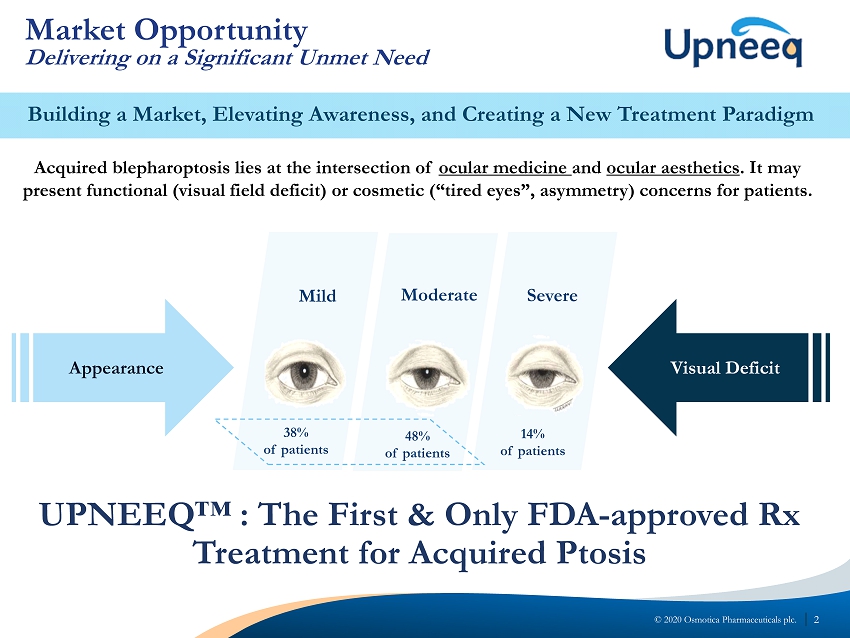
2 © 2020 Osmotica Pharmaceuticals plc. Building a Market, Elevating Awareness, and Creating a New Treatment Paradigm Market Opportunity Delivering on a Significant Unmet Need Appearance Visual Deficit Mild Moderate Severe 14% of patients 48% of patients 3 8% of patients Acquired blepharoptosis lies at the intersection of ocular medicine and ocular aesthetics . It may present functional (visual field deficit) or cosmetic (“tired eyes”, asymmetry) concerns for patients. UPNEEQ™ : The First & Only FDA - approved Rx Treatment for Acquired Ptosis

3 © 2020 Osmotica Pharmaceuticals plc. Welcome to RVL Pharmaceuticals Built For Purpose, Dedicated to Eye Care and UPNEEQ • RVL Pharmaceuticals is a new operating subsidiary that is solely dedicated to UPNEEQ’s successful launch into US eye care specialties o Increased commercial flexibility that may facilitate unique partnerships with our eye care customers • UPNEEQ & RVL Pharmaceuticals were born out of the eye care community and a desire/dedication to enhance patient care • UPNEEQ, the first and only FDA - approval prescription treatment for droopy eyelid (“acquired ptosis”), represents a significant advance for practitioners and their patients • Strong and experienced sales organization, focused exclusively on building awareness and launching Upneeq • RVL Pharmacy, another new operating unit, is built for purpose and set up to be the exclusive distributor of UPNEEQ o Consistent and seamless experience from Rx to fulfillment for provider and patient o Direct line of sight into key factors such as duration of use, abandonment, and prescriber behavior
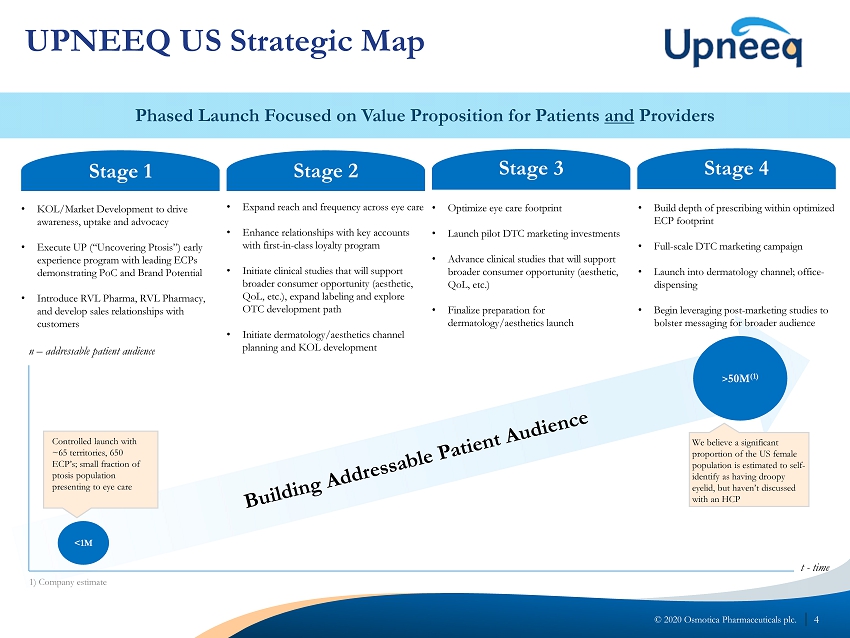
4 © 2020 Osmotica Pharmaceuticals plc. Phased Launch Focused on Value Proposition for Patients and Providers UPNEEQ US Strategic Map Stage 1 Stage 2 Stage 3 Stage 4 • KOL/Market Development to drive awareness, uptake and advocacy • Execute UP (“Uncovering Ptosis”) early experience program with leading ECPs demonstrating PoC and Brand Potential • Introduce RVL Pharma, RVL Pharmacy, and develop sales relationships with customers • Expand reach and frequency across eye care • Enhance relationships with key accounts with first - in - class loyalty program • Initiate clinical studies that will support broader consumer opportunity (aesthetic, QoL , etc.), expand labeling and explore OTC development path • Initiate dermatology/aesthetics channel planning and KOL development • Optimize eye care footprint • Launch pilot DTC marketing investments • Advance clinical studies that will support broader consumer opportunity (aesthetic, QoL , etc.) • Finalize preparation for dermatology/aesthetics launch • Build depth of prescribing within optimized ECP footprint • Full - scale DTC marketing campaign • Launch into dermatology channel; office - dispensing • Begin leveraging post - marketing studies to bolster messaging for broader audience n – addressable patient audience t - time <1M > 50M (1) Controlled launch with ~65 territories, 650 ECP’s ; small fraction of ptosis population presenting to eye care We believe a significant proportion of the US female population is estimated to self - identify as having droopy eyelid, but haven’t discussed with an HCP 1) Company estimate

5 © 2020 Osmotica Pharmaceuticals plc. Stage 1 Launch Early Experience Program Jumpstart early adoption Valuable data & insights Educate providers and patients Generate awareness of droopy eyelid and UPNEEQ Uncovering Ptosis (“UP”) Experience Program

6 © 2020 Osmotica Pharmaceuticals plc. Robust Value Proposition, Accessible to All with No Insurance Hassles Access Strategy Overview RVL Pharmaceuticals has created unrestricted access to UPNEEQ for all patients and providers through a fully transparent pricing model that removes insurance companies from the prescribing process. Pioneering Seamless Product Access Aligned w/ Existing Prescribing Habits: x Prescribers may make independent prescribing decisions without payer interference x Traditional obstacles such as PA’s, step - edits, and pre - requisites are eliminated x Consistent, predictable, and uniform pricing and customer service to every patient x Convenient direct - to - consumer delivery through owned and integrated RVL Pharmacy x Cost - adding intermediaries (wholesalers, insurance companies, retail pharmacies) removed from Rx process x Absence of CMS contract(s) allows for enhanced marketing flexibility relating to practice offerings/partnerships

7 © 2020 Osmotica Pharmaceuticals plc. Introductory Pricing that Highlights 90ct Value Proposition UPNEEQ Rx Pricing Days Supply Rx 90ct 30ct Effective Monthly Price $75.00 $105.00 Prescription Price $225.00 $105.00 Optimal Value – Maximize value of occasional user, while optimizing price for daily user – 90ct is most cost effective and convenient option for patients and providers – Additional patient discounting flexibility for refill /auto - refill – Preserve optionality for physician - dispense, loyalty programs/quantity discounts, Private Equity - backed practices and other cons olidated buying consortiums – All prices inclusive of shipping and handling costs; transparent pricing for all patients

8 © 2020 Osmotica Pharmaceuticals plc. A Real - World Perspective & Early Experiences (1) Expert Insights: Dr. Derek Cunningham Dr. Cunningham is Director of Optometry & Research at Dell Laser Consultants in Austin, Texas Before UPNEEQ Right Eye Dosed with UPNEEQ After UPNEEQ 1) Individual results with UPNEEQ may vary








