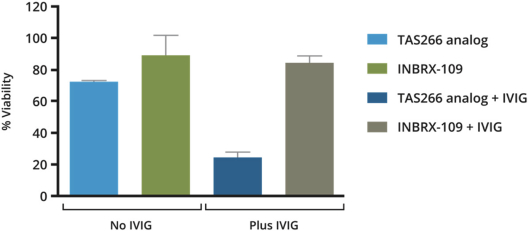
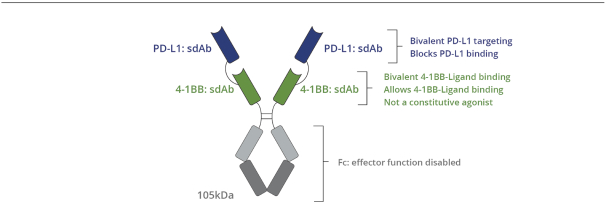
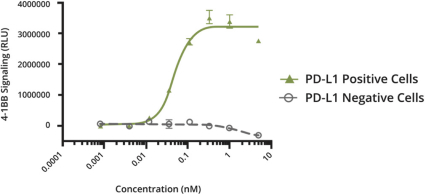
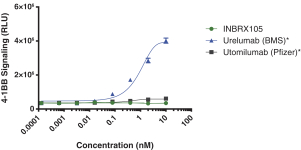
Intellectual Property
We strive to protect the proprietary technology and information commercially or strategically important to our business. We seek to obtain and maintain, patent rights intended to cover the technologies incorporated into, or used to produce, our therapeutic candidates, the compositions of matter of our therapeutic candidates and their methods of use and manufacture, as well as other inventions that are important to our business. We also seek to obtain strategic or commercially valuable patent rights in the United States and other jurisdictions.
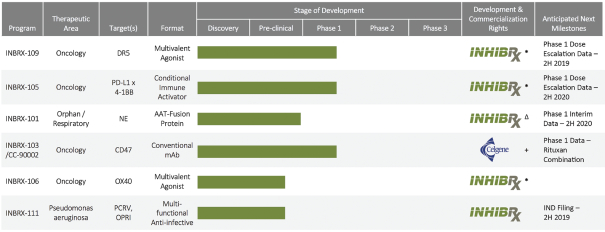
To cover our proprietary technologies and our current pipeline of proprietary products and related methods, such as methods of use, we have filed patent applications representing 28 patent families. As of April 30, 2019, our patent estate, which is solely owned, included seven issued United States patents, 15 United States pendingnon-provisional patent applications, 16 United States pending provisional patent applications, two pending Patent Cooperation Treaty, or PCT, applications, 15 issued foreign patents and 175 foreign patent applications currently pending in various foreign jurisdictions.
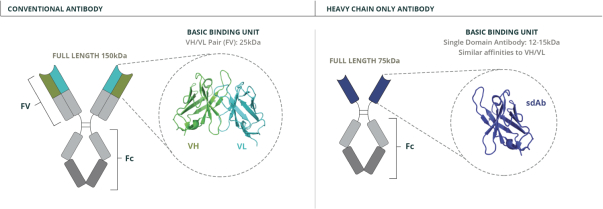
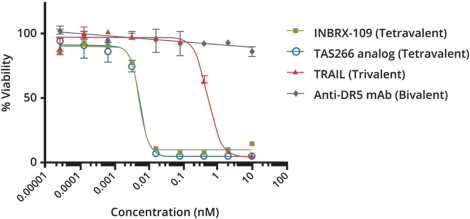
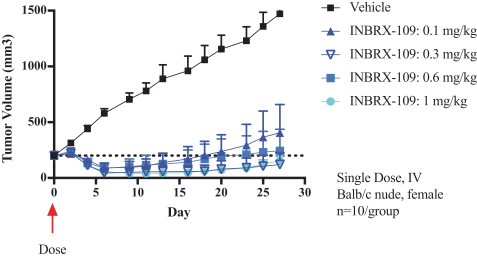
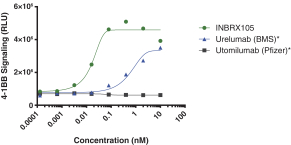
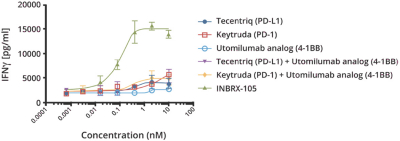
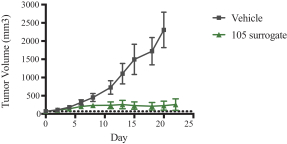
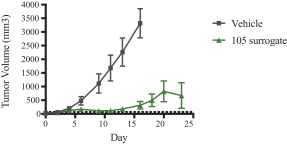
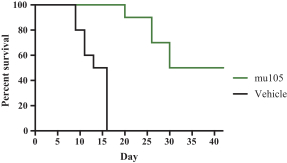
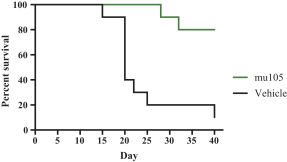
Specifically, we own more than seven patent families with claims directed to various single domain antibodies and/or multivalent therapeutic antibodies including, for example, ourINBRX-105,INBRX-106,INBRX-109, andINBRX-111 therapeutic candidates, and related methods of using the same to treat diseases, e.g., cancer, inflammatory disease, or infectious disease. Patent applications in these families are pending in multiple jurisdictions, including, for example, the United States, Australia, European Patent Organization, Canada, China, Japan, Korea, and Russia; as well as PCT applications and several U.S. provisional applications. Patents in these patent families, if granted, are expected to expire between 2036 and 2039, depending upon their respective filing dates and absent any patent term adjustments or extensions.
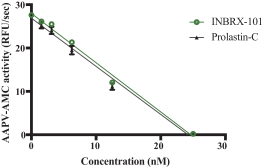
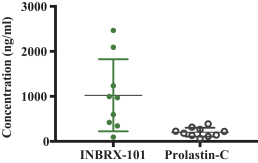
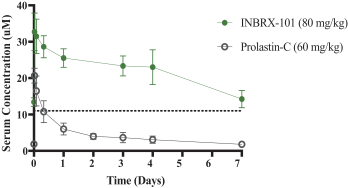
We also own two patent families directed to protease inhibitor fusion proteins including, for example, ourINBRX-101 therapeutic candidate. Two United States and four foreign patents (Australia, Mexico, New Zealand, and Russia) were granted in these families. These patents are expected to expire in 2032, absent any patent term extension. Additional patents in this patent family, if granted, are expected to expire in 2032 or 2035, depending upon their respective filing dates and absent any patent term adjustments or extensions.
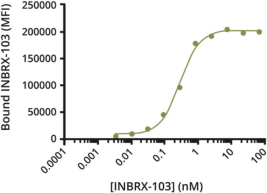
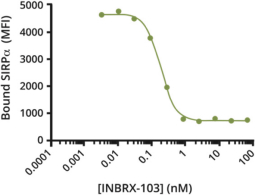
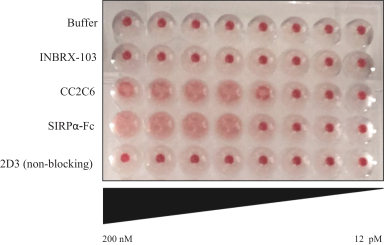
Additionally, we own two patent families relating toINBRX-103 that are licensed to Celgene. Two United States and ten foreign patents (Australia, Colombia, China, Japan, Mexico, New Zealand, Philippines, Singapore, South Africa and Ukraine) have been granted in these families and include claims directed to anti-CD47 monoclonal antibodies and/or methods of using the same to treat cancer. These patents are expected to expire in 2033, absent any patent term extension. Patent applications in these families are pending in multiple jurisdictions, including, for example, the United States, Australia, European Patent Organization, Canada, China, Japan, Korea, and the Eurasian Patent Organization. Additional patents in these patent families, if granted, are expected to expire in 2033, depending upon their respective filing dates and absent any patent term adjustments or extensions.
We continually assess and refine our intellectual property strategy as we develop new technologies and therapeutic candidates. As our business evolves, we may, among other activities, file additional patent applications in pursuit of our intellectual property strategy, to adapt to competition or to seize potential opportunities.
The term of individual patents depends upon the laws of the countries in which they are obtained. In most countries in which we file, the patent term is 20 years from the earliest date of filing of anon-provisional patent application. However, the term of United States patents may be extended for delays incurred due to compliance with the FDA requirements or by delays encountered during prosecution that are caused by the United States Patent and Trademark Office, or USPTO. For example, the Drug Price Competition and Patent Term Restoration Act of 1984, or the Hatch-Waxman Act, permits a patent term extension forFDA-approved drugs of up to five years beyond the expiration of the patent. The length of the patent term extension is related to the length of time
119