
Outcomes Focused Innovation Driven November 2022 2 This presentation contains forward-looking statements. In some cases, you can identify forward-looking statements by the words “will,” “expect,” “intend,” “plan,” “objective,” “believe,” “estimate,” “potential,” “continue” and “ongoing,” or the negative of these terms, or other comparable terminology intended to identify statements about the future. These statements are based on management’s current beliefs and expectations. These statements include but are not limited to statements regarding Inhibrx, Inc.’s (the “Company”) business strategy, the Company’s plans to develop and commercialize its product candidates, the safety and efficacy of the Company’s product candidates, the Company’s plans and expected timing with respect to clinical trials and regulatory filings and approvals, manufacturing matters, strength of intellectual property protection, and the size and growth potential of the markets for the Company’s product candidates, and any implication that pre-clinical data or preliminary or topline results will be representative of the results of later trials. This presentation also contains certain projections and estimates regarding the Company’s future financial performance, namely potential future revenue for certain of the Company’s product candidates. This information also constitutes forward-looking information and is for illustrative purposes only and should not be relied upon as necessarily being indicative of any future results. The assumptions and estimates underlying this estimated financial information are inherently uncertain and subject to a wide variety of significant business, economic competitive and other risks and uncertainties that could cause actual results to differ materially from those contained in the prospective financial information. These potential financial information and other forward-looking statements involve substantial known and unknown risks, uncertainties and other factors that may cause the Company’s actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. Additional information regarding the Company’s risks and uncertainties are described from time to time in the “Risk Factors” section of our Securities and Exchange Commission filings, including those described in our Annual Report on Form 10-K as well as our Quarterly Reports on Form 10-Q, and supplemented from time to time by our Current Reports on Form 8-K. The Company may not actually achieve the plans, intentions or expectations disclosed in its forward-looking statements, and you should not place undue reliance on the Company’s forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements the Company makes. The forward-looking statements in this presentation represent the Company’s views as of the date of this presentation. The Company anticipates that subsequent events and developments will cause its views to change. However, while the Company may elect to update these forward-looking statements at some point in the future, the Company has no current intention of doing so except to the extent required by applicable law. You should, therefore, not rely on these forward- looking statements as representing the Company’s views as of any date subsequent to the date of this presentation. The investigational product candidates discussed in this presentation have not been approved or licensed by the U.S. Food and Drug Administration or by any other regulatory authority, and they are not commercially available in any market. This presentation also contains estimates and other statistical data made by independent parties and by the Company relating to market size and growth and other data about its industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of the Company’s future performance and the future performance of the markets in which it operates are necessarily subject to a high degree of uncertainty and risk. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy securities. Presentation disclaimer 3 + Potential path to approval in chondrosarcoma by 2025 with potential profitability in 2026; potential for $600M in U.S. sales within 3 years of launch + Initial combination cohort data starting in mid-2023: mesothelioma, pancreatic adenocarcinoma, colorectal cancer and Ewing sarcoma + Potential registration-enabling trial with accelerated pathway to initiate in Q1 2023 + Favorable safety and tolerability profile w/potential to achieve normal AAT levels with monthly dosing + Potential to reach >$1.3B in U.S. sales w/in 3 years of launch, $3B peak sales potential in the U.S. INBRX-101- AATD Our Recombinant Alpha-1-Anti-trypsin Fc-Fusion Protein (AAT-Fc) THE CANDIDATES REGISTRATION- ENABLINGPHASE 1PRECLINICAL INBRX-109 Our Tetravalent DR5 Agonist Pivotal Trial Enrolling + Durable single agent activity (w/prior CPI exposure) and in combination with anti-PD-1 in CPI refractory patients + Potential to combine INBRX-106 with fast-growing IO market + Next data readouts in anti-PD-1 combination expansion cohorts in mid-2023 INBRX-106 Our Hexavalent OX40 Agonist INBRX-105 Our Tetravalent PD-L1 targeted 4-1BB Agonist + Potential to be first 4-1BB agonist with a robust therapeutic window, with potential across PD-L1 expressing tumors + Single agent responses in CPI refractory patients including a CR + Data from expansion cohorts in combination with anti-PD-1 in mid-2023 Pivotal Trial to start mid 2023 + De-risked opportunity with promising clinical data with pdAAT + Potential to reach >$600M in U.S. sales within 5 years of launch with upside potential + Potential registration-enabling trial to initiate in mid-2023 INBRX-101- GvHD Our Recombinant Alpha-1-Anti-trypsin Fc-Fusion Protein (AAT-Fc) Trial to start mid 2023 4 Anchored by innovation and protein-engineering expertise Inhibrx’s modular sdAb platform + Ability to precision engineer to specific target biology + Smaller than conventional antibodies + Antibody-like PK profile + Readily manufactured at high yields using standard processes Experienced leadership team with proven innovation and execution Expertise All platforms and programs developed in-house with strong patent protection Technology Strategy To pursue clinically validated pathways with a high unmet medical need where prior attempts have been suboptimal or ineffective
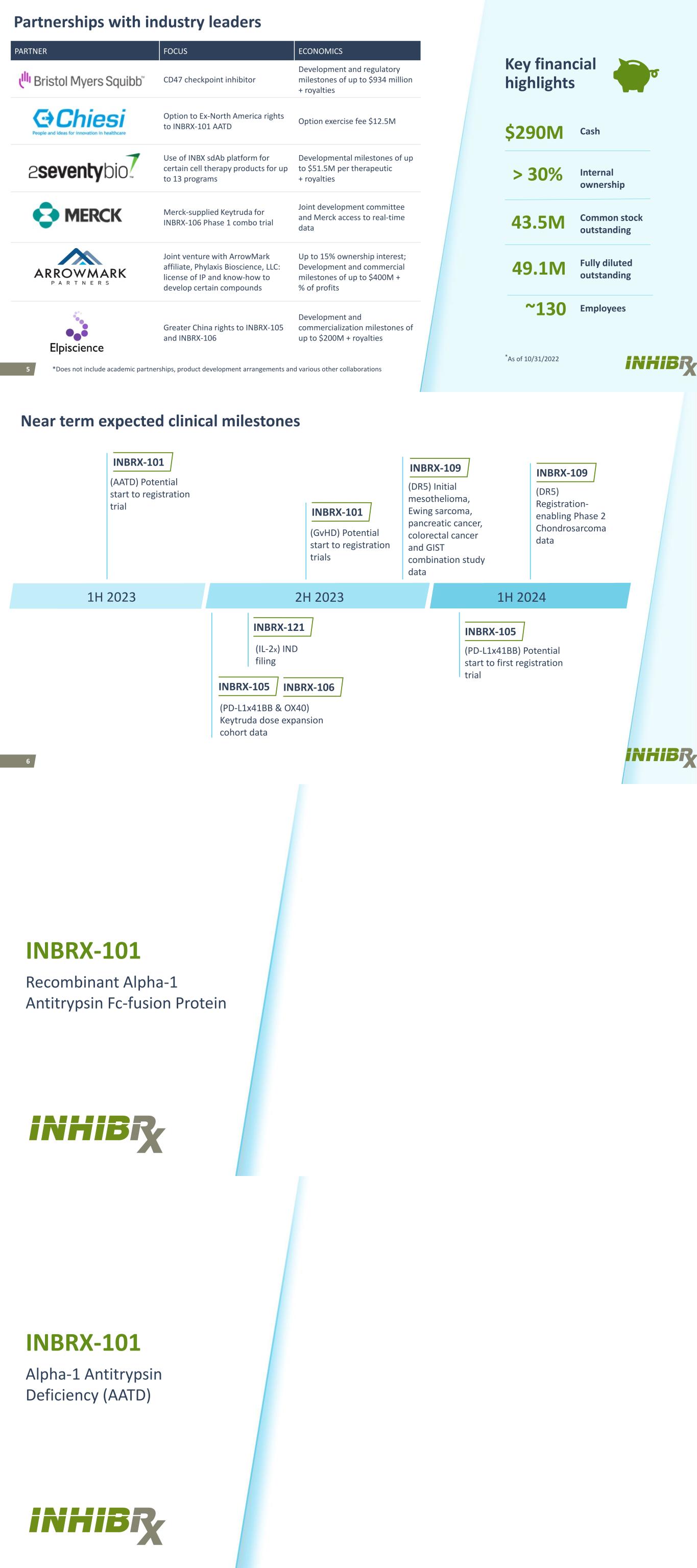
5 PARTNER FOCUS ECONOMICS CD47 checkpoint inhibitor Development and regulatory milestones of up to $934 million + royalties Option to Ex-North America rights to INBRX-101 AATD Option exercise fee $12.5M Use of INBX sdAb platform for certain cell therapy products for up to 13 programs Developmental milestones of up to $51.5M per therapeutic + royalties Merck-supplied Keytruda for INBRX-106 Phase 1 combo trial Joint development committee and Merck access to real-time data Joint venture with ArrowMark affiliate, Phylaxis Bioscience, LLC: license of IP and know-how to develop certain compounds Up to 15% ownership interest; Development and commercial milestones of up to $400M + % of profits Greater China rights to INBRX-105 and INBRX-106 Development and commercialization milestones of up to $200M + royalties Key financial highlights 43.5M $290M 49.1M Cash Common stock outstanding Fully diluted outstanding *As of 10/31/2022 > 30% Internal ownership ~130 Employees Partnerships with industry leaders *Does not include academic partnerships, product development arrangements and various other collaborations 6 Near term expected clinical milestones 1H 2023 2H 2023 (DR5) Initial mesothelioma, Ewing sarcoma, pancreatic cancer, colorectal cancer and GIST combination study data (AATD) Potential start to registration trial INBRX-101 INBRX-106 INBRX-101 (GvHD) Potential start to registration trials 1H 2024 INBRX-109 INBRX-105 (PD-L1x41BB & OX40) Keytruda dose expansion cohort data INBRX-109 INBRX-121 (IL-2x) IND filing (DR5) Registration- enabling Phase 2 Chondrosarcoma data INBRX-105 (PD-L1x41BB) Potential start to first registration trial INBRX-101 Recombinant Alpha-1 Antitrypsin Fc-fusion Protein INBRX-101 Alpha-1 Antitrypsin Deficiency (AATD)
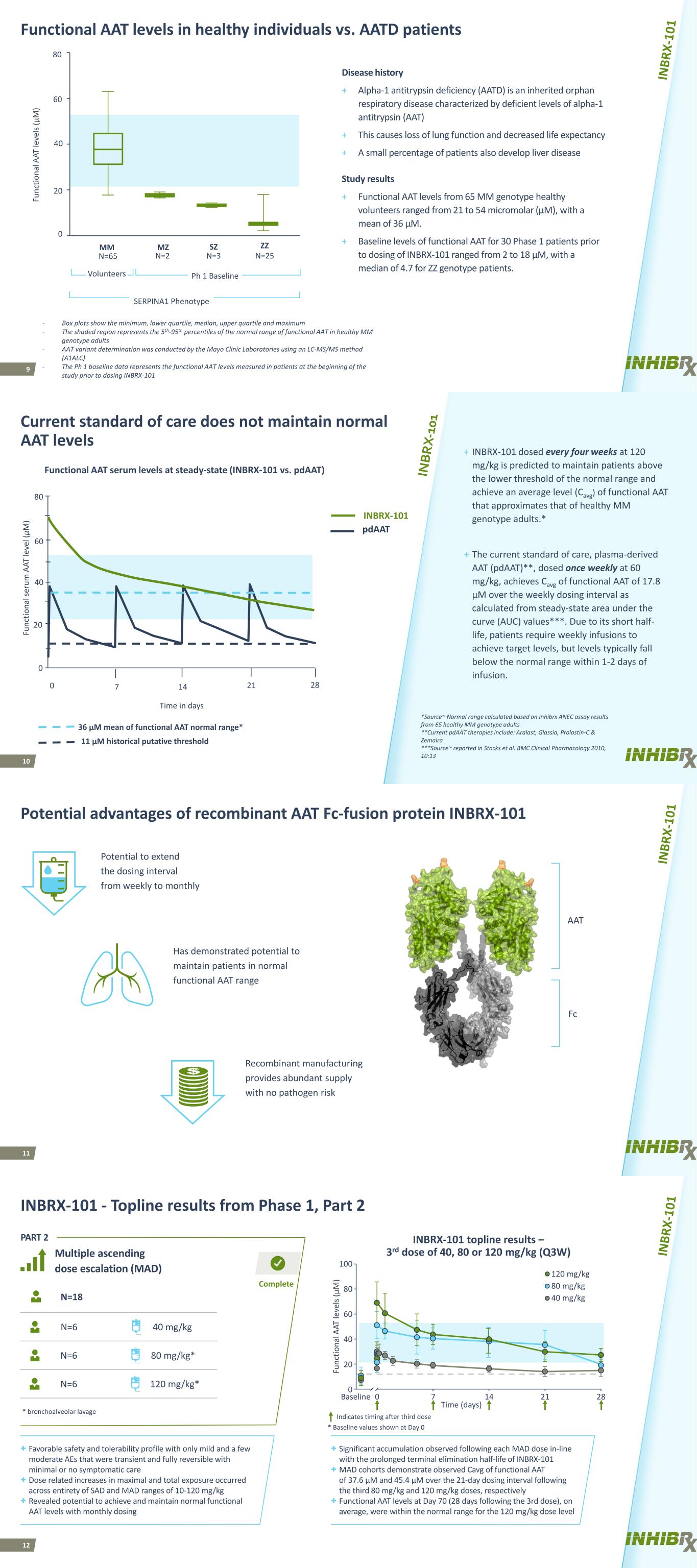
9 Functional AAT levels in healthy individuals vs. AATD patients Disease history + Alpha-1 antitrypsin deficiency (AATD) is an inherited orphan respiratory disease characterized by deficient levels of alpha-1 antitrypsin (AAT) + This causes loss of lung function and decreased life expectancy + A small percentage of patients also develop liver disease MZ SZ ZZ SERPINA1 Phenotype MM N=65 N=2 N=3 N=25 Volunteers Ph 1 Baseline Fu nc tio na l A AT le ve ls (μ M ) 80 60 0 40 20 - Box plots show the minimum, lower quartile, median, upper quartile and maximum - The shaded region represents the 5th-95th percentiles of the normal range of functional AAT in healthy MM genotype adults - AAT variant determination was conducted by the Mayo Clinic Laboratories using an LC-MS/MS method (A1ALC) - The Ph 1 baseline data represents the functional AAT levels measured in patients at the beginning of the study prior to dosing INBRX-101 Study results + Functional AAT levels from 65 MM genotype healthy volunteers ranged from 21 to 54 micromolar (µM), with a mean of 36 µM. + Baseline levels of functional AAT for 30 Phase 1 patients prior to dosing of INBRX-101 ranged from 2 to 18 µM, with a median of 4.7 for ZZ genotype patients. 10 Current standard of care does not maintain normal AAT levels + INBRX-101 dosed every four weeks at 120 mg/kg is predicted to maintain patients above the lower threshold of the normal range and achieve an average level (Cavg) of functional AAT that approximates that of healthy MM genotype adults.* + The current standard of care, plasma-derived AAT (pdAAT)**, dosed once weekly at 60 mg/kg, achieves Cavg of functional AAT of 17.8 µM over the weekly dosing interval as calculated from steady-state area under the curve (AUC) values***. Due to its short half- life, patients require weekly infusions to achieve target levels, but levels typically fall below the normal range within 1-2 days of infusion. *Source~ Normal range calculated based on Inhibrx ANEC assay results from 65 healthy MM genotype adults **Current pdAAT therapies include: Aralast, Glassia, Prolastin-C & Zemaira ***Source~ reported in Stocks et al. BMC Clinical Pharmacology 2010, 10:13 Functional AAT serum levels at steady-state (INBRX-101 vs. pdAAT) 80 Fu nc tio na l s er um A AT le ve l ( μM ) 40 20 0 0 28 Time in days 36 μM mean of functional AAT normal range* 11 μM historical putative threshold 21147 60 INBRX-101 pdAAT 11 Potential advantages of recombinant AAT Fc-fusion protein INBRX-101 AAT Fc Potential to extend the dosing interval from weekly to monthly Has demonstrated potential to maintain patients in normal functional AAT range Recombinant manufacturing provides abundant supply with no pathogen risk 12 Complete INBRX-101 - Topline results from Phase 1, Part 2 N=18 * bronchoalveolar lavage N=6 40 mg/kg N=6 80 mg/kg* N=6 120 mg/kg* INBRX-101 topline results – 3rd dose of 40, 80 or 120 mg/kg (Q3W) PART 2 Multiple ascending dose escalation (MAD) + Favorable safety and tolerability profile with only mild and a few moderate AEs that were transient and fully reversible with minimal or no symptomatic care + Dose related increases in maximal and total exposure occurred across entirety of SAD and MAD ranges of 10-120 mg/kg + Revealed potential to achieve and maintain normal functional AAT levels with monthly dosing + Significant accumulation observed following each MAD dose in-line with the prolonged terminal elimination half-life of INBRX-101 + MAD cohorts demonstrate observed Cavg of functional AAT of 37.6 µM and 45.4 µM over the 21-day dosing interval following the third 80 mg/kg and 120 mg/kg doses, respectively + Functional AAT levels at Day 70 (28 days following the 3rd dose), on average, were within the normal range for the 120 mg/kg dose level Time (days) Fu nc tio na l A AT le ve ls (μ M ) 0 7 14 28 0 40 100 120 mg/kg 80 mg/kg 40 mg/kg 60 * Baseline values shown at Day 0 21 Indicates timing after third dose 80 20 Baseline

13 INBRX-101 is present in the lung in every patient sampled following IV dosing Bronchoalveolar lavage fluid (BALF) sample collection and analysis + BALF samples were collected from 3 lobes of the lung for each patient in the 80 (N = 5)1 and 120 (N = 6) mg/kg MAD cohorts prior to dosing and two weeks after completion of multiple dosing + INBRX-101 concentrations were measured using a proprietary validated mass spectrometry assay specific to INBRX-101 - Each point represents the average INBRX-101 concentration measured across three lobes in an individual subject - Horizontal lines are the median values for each dose level - Data is preliminary and has not been fully verified BALF assessment results + At baseline, BALF samples from subjects that rolled over from the Part 1 SAD2 had measurable INBRX-101 while drug was undetectable in INBRX- 101 naïve patients (data not shown) + Post-dose, INBRX-101 was present in each lung lobe of every patient for which a bronchoscopy was performed + The Phase 1 study data provide emerging evidence of a dose-dependent increase in INBRX-101 lung exposure 1 One 80 mg/kg patient did not have a post-dose sample collected 2 In rollover patients, baseline collection was at least 84 days after the SAD 100 120 mg/kg80 mg/kg Day 56 IN BR X- 10 1 (μ g/ m L) 0 50 200 150 naive rollover naive rollover 14 INBRX-101 has the potential to achieve ~$3B in annual U.S. revenue with expected rapid uptake in patients with severe AATD Notes: *Pricing assumption is for modelling purposes only Source: KOL Qualitative Interviews (n=~25); EvaluatePharma, Datamonitor, IQVIA, Fortune Business Insights, Analog analysis of other recombinant products $0B $3B $0.3B 1721 3 $0.7B $1.3B $3.0B U. S. p ro je ct ed s al es (U SD ) Years post launch + “Severe (ZZ/SZ)” AATD patients (same as pdAATs today) + 7% CAGR throughout forecast period (conservative estimate given CAGR of ~17% from 2016 to 2020) + ~75% peak market share + 3-year time to peak share + Price parity with current pdAATs* & 2% annual price growth + Little to no generic erosion due to high barriers to entry Key assumptions 3 years to peak market share INBRX-101 top line projected U.S. sales & key assumptions 15 INBRX-101 has the potential to shift the treatment paradigm, expanding augmentation therapy to a broad group of AATD patients in the U.S. Sources: KOL Interviews, 1. Sandhaus Chronic Obstr Pulm Dis 2016; 2. Barjaktarevic and Miravitlles BMC Pulm Med 2021 TODAY FUTURE PI*ZZ or PI*SZ PI*ZZ or PI*SZ U.S. prevalence ~100K ~100K (same as today) Treatment rate ~8-10% ~40% (driven by increased diagnosis rates) Total treated U.S. patients ~8K ~40K Market revenue potential ~$1 Billion ~$4 Billion + pdAATs only utilized for severe AATD patients and market is still worth ~$1B today despite only ~8-10% treatment rate + PI*ZZ & PI*SZ AATD market is growing at ~17% annually and projected to grow to $4B due to increased diagnosis + Upside market potential from earlier intervention of augmentation therapy, which can help to prevent lung decline + Commercial viability and expansion of augmentation therapy use requires abundant supply only available via INBRX-101 Key Takeaways for AATD Market: “The results of the RAPID trial stress the importance of early intervention. Patients who started augmentation late were unable to regain lung tissue lost during placebo treatment and did not ‘catch up’ to patients who started augmentation early.” – U.S. KOL INBRX-101 Graft versus Host Disease (GvHD)
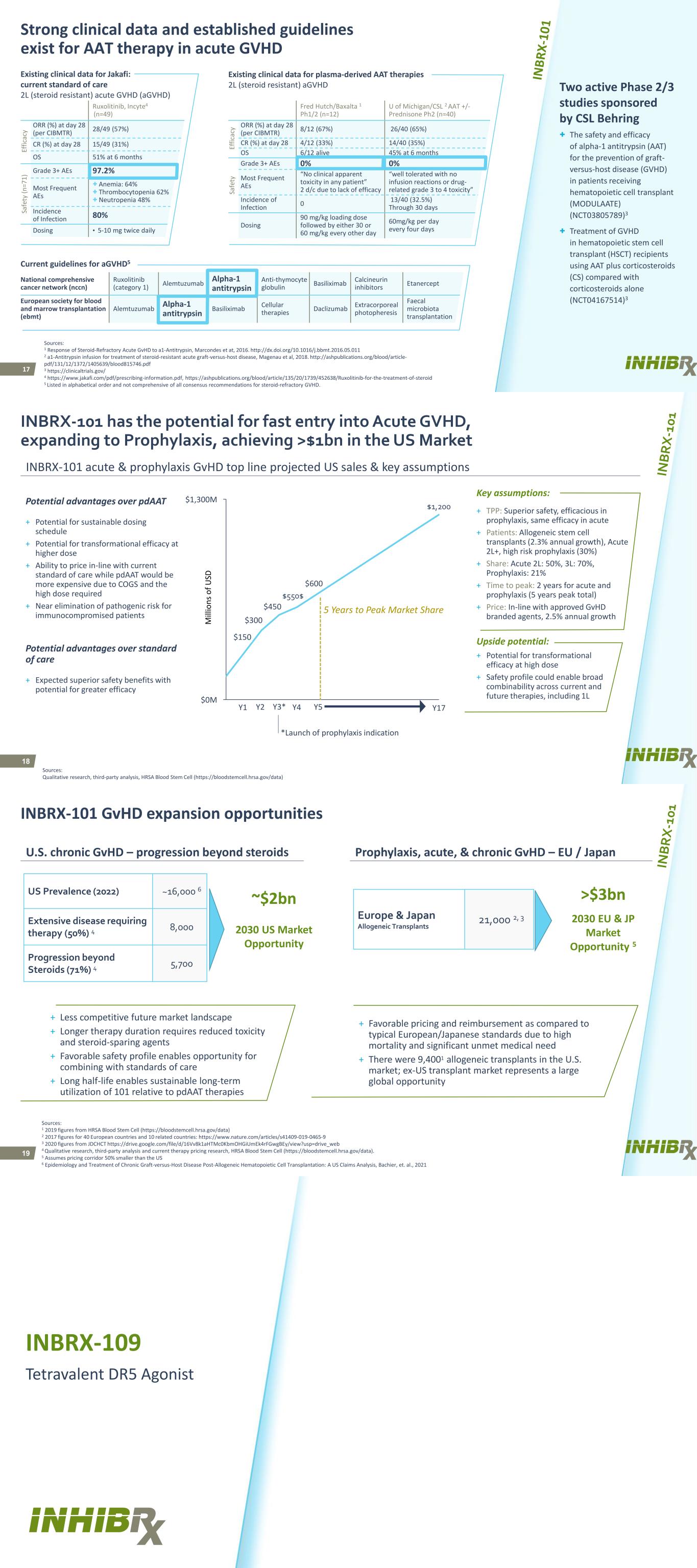
17 Fred Hutch/Baxalta 1 Ph1/2 (n=12) U of Michigan/CSL 2 AAT +/- Prednisone Ph2 (n=40) Ef fic ac y ORR (%) at day 28 (per CIBMTR) 8/12 (67%) 26/40 (65%) CR (%) at day 28 4/12 (33%) 14/40 (35%) OS 6/12 alive 45% at 6 months Sa fe ty Grade 3+ AEs 0% 0% Most Frequent AEs “No clinical apparent toxicity in any patient” 2 d/c due to lack of efficacy “well tolerated with no infusion reactions or drug- related grade 3 to 4 toxicity” Incidence of Infection 0 13/40 (32.5%) Through 30 days Dosing 90 mg/kg loading dose followed by either 30 or 60 mg/kg every other day 60mg/kg per day every four days Ruxolitinib, Incyte4 (n=49) Ef fic ac y ORR (%) at day 28 (per CIBMTR) 28/49 (57%) CR (%) at day 28 15/49 (31%) OS 51% at 6 months Sa fe ty (n =7 1) Grade 3+ AEs 97.2% Most Frequent AEs + Anemia: 64% + Thrombocytopenia 62% + Neutropenia 48% Incidence of Infection 80% Dosing • 5-10 mg twice daily National comprehensive cancer network (nccn) Ruxolitinib (category 1) Alemtuzumab Alpha-1 antitrypsin Anti-thymocyte globulin Basiliximab Calcineurin inhibitors Etanercept European society for blood and marrow transplantation (ebmt) Alemtuzumab Alpha-1 antitrypsin Basiliximab Cellular therapies Daclizumab Extracorporeal photopheresis Faecal microbiota transplantation Strong clinical data and established guidelines exist for AAT therapy in acute GVHD + The safety and efficacy of alpha-1 antitrypsin (AAT) for the prevention of graft- versus-host disease (GVHD) in patients receiving hematopoietic cell transplant (MODULAATE) (NCT03805789)3 + Treatment of GVHD in hematopoietic stem cell transplant (HSCT) recipients using AAT plus corticosteroids (CS) compared with corticosteroids alone (NCT04167514)3 Two active Phase 2/3 studies sponsored by CSL Behring Current guidelines for aGVHD5 Existing clinical data for plasma-derived AAT therapies 2L (steroid resistant) aGVHD Existing clinical data for Jakafi: current standard of care 2L (steroid resistant) acute GVHD (aGVHD) Sources: 1 Response of Steroid-Refractory Acute GvHD to a1-Antitrypsin, Marcondes et at, 2016. http://dx.doi.org/10.1016/j.bbmt.2016.05.011 2 a1-Antitrypsin infusion for treatment of steroid-resistant acute graft-versus-host disease, Magenau et al, 2018. http://ashpublications.org/blood/article- pdf/131/12/1372/1405639/blood815746.pdf 3 https://clinicaltrials.gov/ 4 https://www.jakafi.com/pdf/prescribing-information.pdf, https://ashpublications.org/blood/article/135/20/1739/452638/Ruxolitinib-for-the-treatment-of-steroid 5 Listed in alphabetical order and not comprehensive of all consensus recommendations for steroid-refractory GVHD. 18 INBRX-101 has the potential for fast entry into Acute GVHD, expanding to Prophylaxis, achieving >$1bn in the US Market Sources: Qualitative research, third-party analysis, HRSA Blood Stem Cell (https://bloodstemcell.hrsa.gov/data) + TPP: Superior safety, efficacious in prophylaxis, same efficacy in acute + Patients: Allogeneic stem cell transplants (2.3% annual growth), Acute 2L+, high risk prophylaxis (30%) + Share: Acute 2L: 50%, 3L: 70%, Prophylaxis: 21% + Time to peak: 2 years for acute and prophylaxis (5 years peak total) + Price: In-line with approved GvHD branded agents, 2.5% annual growth Key assumptions: Upside potential: + Potential for transformational efficacy at high dose + Safety profile could enable broad combinability across current and future therapies, including 1L Potential advantages over pdAAT + Potential for sustainable dosing schedule + Potential for transformational efficacy at higher dose + Ability to price in-line with current standard of care while pdAAT would be more expensive due to COGS and the high dose required + Near elimination of pathogenic risk for immunocompromised patients Potential advantages over standard of care + Expected superior safety benefits with potential for greater efficacy INBRX-101 acute & prophylaxis GvHD top line projected US sales & key assumptions $550$ $1,200 $0M $1,300M $150 $450 Y1 $300 Y4Y3*Y2 $600 Y5 Y17 5 Years to Peak Market Share *Launch of prophylaxis indication M ill io ns o f U SD 19 INBRX-101 GvHD expansion opportunities Sources: 1 2019 figures from HRSA Blood Stem Cell (https://bloodstemcell.hrsa.gov/data) 2 2017 figures for 40 European countries and 10 related countries: https://www.nature.com/articles/s41409-019-0465-9 3 2020 figures from JDCHCT https://drive.google.com/file/d/16Vv8k1aHTMc0KbmOHGiUmEk4rFGwgBEy/view?usp=drive_web 4 Qualitative research, third-party analysis and current therapy pricing research, HRSA Blood Stem Cell (https://bloodstemcell.hrsa.gov/data). 5 Assumes pricing corridor 50% smaller than the US 6 Epidemiology and Treatment of Chronic Graft-versus-Host Disease Post-Allogeneic Hematopoietic Cell Transplantation: A US Claims Analysis, Bachier, et. al., 2021 U.S. chronic GvHD – progression beyond steroids US Prevalence (2022) ~16,000 6 Extensive disease requiring therapy (50%) 4 8,000 Progression beyond Steroids (71%) 4 5,700 ~$2bn 2030 US Market Opportunity Europe & Japan Allogeneic Transplants 21,000 2, 3 Prophylaxis, acute, & chronic GvHD – EU / Japan >$3bn 2030 EU & JP Market Opportunity 5 + Less competitive future market landscape + Longer therapy duration requires reduced toxicity and steroid-sparing agents + Favorable safety profile enables opportunity for combining with standards of care + Long half-life enables sustainable long-term utilization of 101 relative to pdAAT therapies + Favorable pricing and reimbursement as compared to typical European/Japanese standards due to high mortality and significant unmet medical need + There were 9,4001 allogeneic transplants in the U.S. market; ex-US transplant market represents a large global opportunity INBRX-109 Tetravalent DR5 Agonist
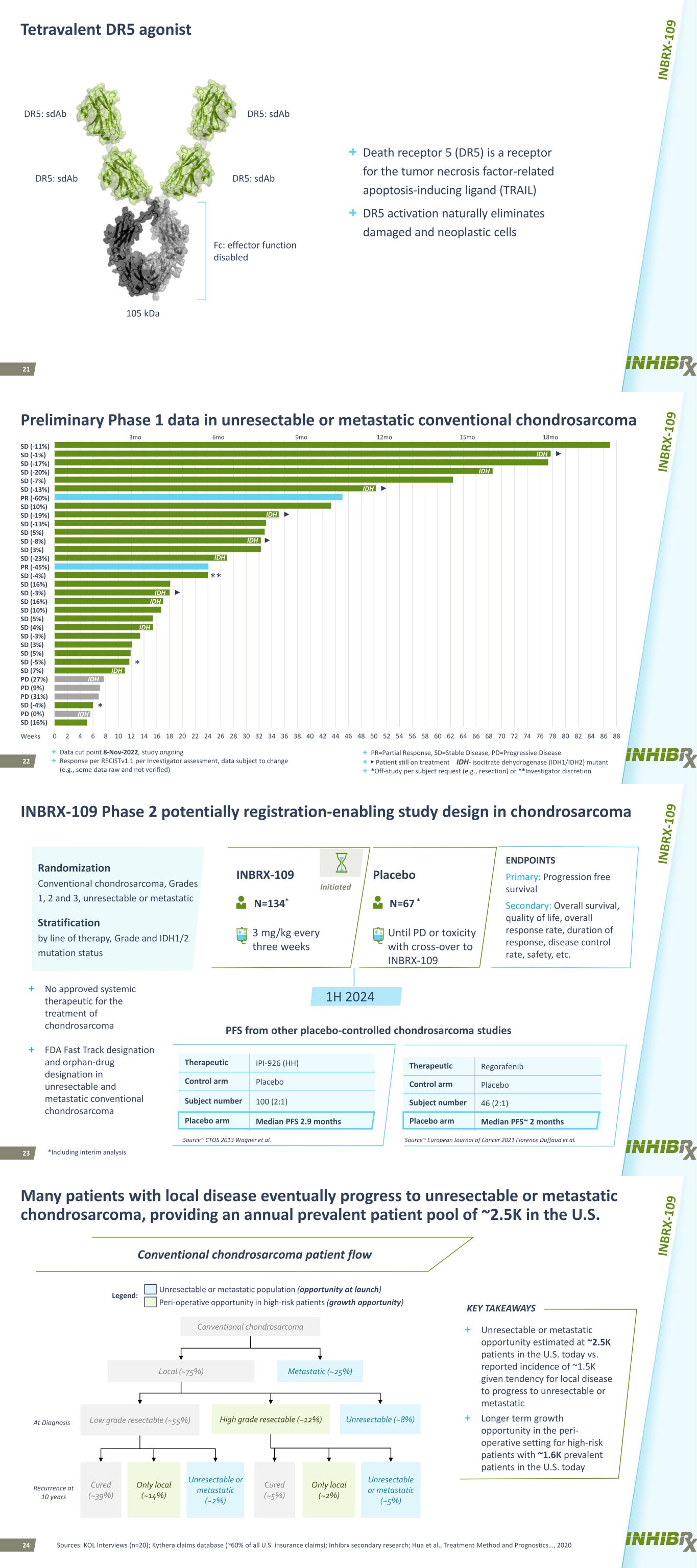
21 Tetravalent DR5 agonist + Death receptor 5 (DR5) is a receptor for the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) + DR5 activation naturally eliminates damaged and neoplastic cells DR5: sdAb DR5: sdAbDR5: sdAb DR5: sdAb 105 kDa Fc: effector function disabled 22 Preliminary Phase 1 data in unresectable or metastatic conventional chondrosarcoma + Data cut point 8-Nov-2022, study ongoing + Response per RECISTv1.1 per Investigator assessment, data subject to change (e.g., some data raw and not verified) + PR=Partial Response, SD=Stable Disease, PD=Progressive Disease + ► Patient still on treatment IDH- isocitrate dehydrogenase (IDH1/IDH2) mutant + *Off-study per subject request (e.g., resection) or **Investigator discretion * * ► ** ► ► ► ► 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 42 44 46 48 50 52 54 56 58 60 62 64 66 68 70 72 74 76 78 80 82 84 86 88 IDH IDH Weeks SD (-11%) SD (-1%) SD (-17%) SD (-20%) SD (-7%) SD (-13%) PR (-60%) SD (10%) SD (-19%) SD (-13%) SD (5%) SD (-8%) SD (3%) SD (-23%) PR (-45%) SD (-4%) SD (16%) SD (-3%) SD (16%) SD (10%) SD (5%) SD (4%) SD (-3%) SD (3%) SD (5%) SD (-5%) SD (7%) PD (27%) PD (9%) PD (31%) SD (-4%) PD (0%) SD (16%) 3mo 6mo 18mo15mo12mo9mo IDH IDH IDH IDH IDH IDH IDH IDH IDH IDH 23 ENDPOINTS Primary: Progression free survival Secondary: Overall survival, quality of life, overall response rate, duration of response, disease control rate, safety, etc. INBRX-109 Phase 2 potentially registration-enabling study design in chondrosarcoma Randomization Conventional chondrosarcoma, Grades 1, 2 and 3, unresectable or metastatic Stratification by line of therapy, Grade and IDH1/2 mutation status INBRX-109 Placebo Until PD or toxicity with cross-over to INBRX-109 *Including interim analysis N=134* N=67 * 3 mg/kg every three weeks 1H 2024 Initiated PFS from other placebo-controlled chondrosarcoma studies Therapeutic IPI-926 (HH) Control arm Placebo Subject number 100 (2:1) Placebo arm Median PFS 2.9 months Source~ European Journal of Cancer 2021 Florence Duffaud et al. Therapeutic Regorafenib Control arm Placebo Subject number 46 (2:1) Placebo arm Median PFS~ 2 months Source~ CTOS 2013 Wagner et al. + No approved systemic therapeutic for the treatment of chondrosarcoma + FDA Fast Track designation and orphan-drug designation in unresectable and metastatic conventional chondrosarcoma 24 Many patients with local disease eventually progress to unresectable or metastatic chondrosarcoma, providing an annual prevalent patient pool of ~2.5K in the U.S. At Diagnosis Recurrence at 10 years Conventional chondrosarcoma Local (~75%) Low grade resectable (~55%) Metastatic (~25%) High grade resectable (~12%) Only local (~14%) Unresectable or metastatic (~2%) Only local (~2%) Unresectable or metastatic (~5%) Cured (~39%) Cured (~5%) Unresectable (~8%) Conventional chondrosarcoma patient flow KEY TAKEAWAYS + Unresectable or metastatic opportunity estimated at ~2.5K patients in the U.S. today vs. reported incidence of ~1.5K given tendency for local disease to progress to unresectable or metastatic + Longer term growth opportunity in the peri- operative setting for high-risk patients with ~1.6K prevalent patients in the U.S. today Legend: Unresectable or metastatic population (opportunity at launch) Peri-operative opportunity in high-risk patients (growth opportunity) Sources: KOL Interviews (n=20); Kythera claims database (~60% of all U.S. insurance claims); Inhibrx secondary research; Hua et al., Treatment Method and Prognostics…, 2020

25 Based on current trends and lack of approved options, INBRX-109 has the potential to achieve ~$1B in annual revenue with rapid uptake post launch Notes: *Pricing assumption is for modelling purposes only based on analogous market research for oncology products in rare indications; Sources: KOL qualitative interviews (n=~20); Inhibrx secondary research; Hua et al., Treatment Method and Prognostics…, 2020 $0B $1B $1.0B $0.3B 3 $0.1B 21 $0.6B 17 U. S. p ro je ct ed s al es (U SD ) Years post launch + ~375K* annual price per patient + ~85% peak share + ~30% 10-year recurrence rate for local, low- grade patients + ~65% 10-year recurrence rates for local, high- grade patients Key assumptions 3 years to peak market share Incremental growth opportunity + Peri-operative setting provides an incremental ~$500M annual opportunity in the U.S. alone INBRX-109 top line projected U.S. sales in the unresectable/metastatic setting & key assumptions 26 INBRX-109 on the horizon POTENTIAL FUTURE OPPORTUNITIES Solid tumors + IAP antagonists Hematologic tumors + Bcl-2 inhibitors NSCLC + various combo agents Additional sarcoma indications Combination studies Pancreatic adenocarcinoma 2nd line with mFOLFIRIN=20 Ewing sarcoma with Irinotecan + Temozolomide PART 3 Colorectal Cancer with FOLFIRI Data releases: mid-2023 N=20 N=20 N=20 SDH-deficient GIST with Temozolomide Ongoing INBRX-105 PD-L1 x 4-1BB Multispecific 28 PD-L1 x 4-1BB multispecific + Designed to provide spatially-restricted 4-1BB agonism at sites of PD-L1 expression + Highly expressed on tumor infiltrating immune cells, 4-1BB signaling promotes prolonged T-cell survival memory formation + Constitutive 4-1BB agonism has achieved anti-tumor responses, but was limited by immune-related toxicities PD-L1: sdAb 4-1BB: sdAb Fc Effector Disabled PD-L1: sdAb 4-1BB: sdAb 105 kDa
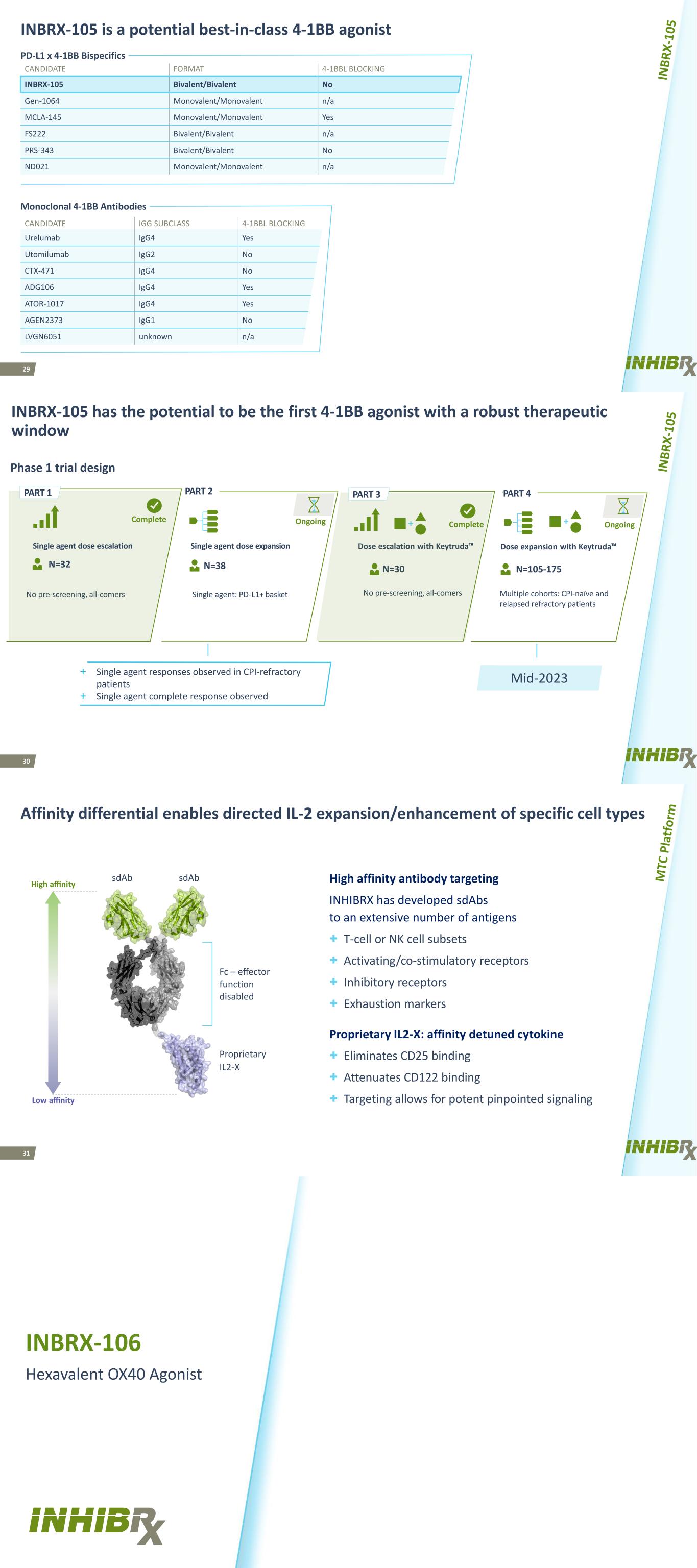
29 INBRX-105 is a potential best-in-class 4-1BB agonist CANDIDATE FORMAT 4-1BBL BLOCKING INBRX-105 Bivalent/Bivalent No Gen-1064 Monovalent/Monovalent n/a MCLA-145 Monovalent/Monovalent Yes FS222 Bivalent/Bivalent n/a PRS-343 Bivalent/Bivalent No ND021 Monovalent/Monovalent n/a CANDIDATE IGG SUBCLASS 4-1BBL BLOCKING STATUS Urelumab IgG4 Yes Discontinued Utomilumab IgG2 No Discontinued CTX-471 IgG4 No Phase I ADG106 IgG4 Yes Phase I ATOR-1017 IgG4 Yes Phase I AGEN2373 IgG1 No Phase I LVGN6051 unknown n/a Phase I PD-L1 x 4-1BB Bispecifics Monoclonal 4-1BB Antibodies 30 No pre-screening, all-comers N=30 No pre-screening, all-comers OngoingOngoingComplete INBRX-105 has the potential to be the first 4-1BB agonist with a robust therapeutic window Single agent: PD-L1+ basket Single agent dose escalation Single agent dose expansion N=105-175N=38N=32 Mid-2023 PART 2 PART 4 Dose expansion with Keytruda™Dose escalation with Keytruda™ Complete Multiple cohorts: CPI-naïve and relapsed refractory patients PART 3PART 1 Phase 1 trial design + Single agent responses observed in CPI-refractory patients + Single agent complete response observed 31 Affinity differential enables directed IL-2 expansion/enhancement of specific cell types High affinity antibody targeting INHIBRX has developed sdAbs to an extensive number of antigens + T-cell or NK cell subsets + Activating/co-stimulatory receptors + Inhibitory receptors + Exhaustion markers Proprietary IL2-X: affinity detuned cytokine + Eliminates CD25 binding + Attenuates CD122 binding + Targeting allows for potent pinpointed signaling High affinity sdAb sdAb Low affinity Fc – effector function disabled Proprietary IL2-X INBRX-106 Hexavalent OX40 Agonist
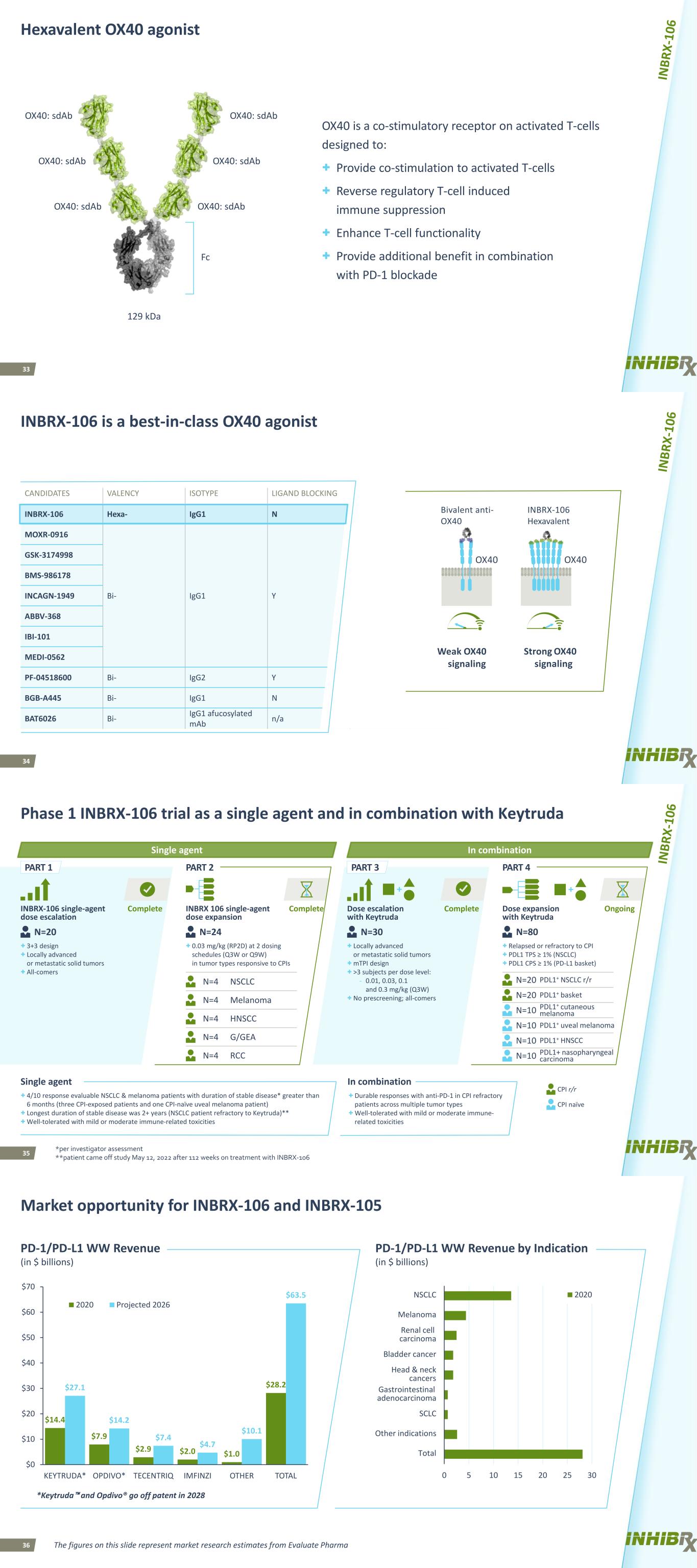
33 Hexavalent OX40 agonist OX40 is a co-stimulatory receptor on activated T-cells designed to: + Provide co-stimulation to activated T-cells + Reverse regulatory T-cell induced immune suppression + Enhance T-cell functionality + Provide additional benefit in combination with PD-1 blockade OX40: sdAb OX40: sdAb OX40: sdAbOX40: sdAb OX40: sdAb OX40: sdAb Fc 129 kDa 34 INBRX-106 is a best-in-class OX40 agonist CANDIDATES VALENCY ISOTYPE LIGAND BLOCKING STATUS INBRX-106 Hexa- IgG1 N Ph 1 (2019) MOXR-0916 Bi- IgG1 Y Discontinued GSK-3174998 Discontinued BMS-986178 Ph 1 (2016) INCAGN-1949 Ph 1 (2016) ABBV-368 Ph 1 (HNSCC, 2020) IBI-101 Ph 1 (2018) MEDI-0562 Discontinued PF-04518600 Bi- IgG2 Y Discontinued BGB-A445 Bi- IgG1 N Ph 1 (2020) BAT6026 Bi- IgG1 afucosylated mAb n/a INBRX-106 Hexavalent Strong OX40 signaling OX40 Bivalent anti- OX40 Weak OX40 signaling OX40 35 OngoingComplete PART 1 Complete Complete PART 2 PART 4PART 3 Phase 1 INBRX-106 trial as a single agent and in combination with Keytruda INBRX-106 single-agent dose escalation + 3+3 design + Locally advanced or metastatic solid tumors + All-comers Dose expansion with Keytruda Dose escalation with Keytruda INBRX 106 single-agent dose expansion N=4 + Locally advanced or metastatic solid tumors + mTPI design + >3 subjects per dose level: - 0.01, 0.03, 0.1 and 0.3 mg/kg (Q3W) + No prescreening; all-comers N=4 + 0.03 mg/kg (RP2D) at 2 dosing schedules (Q3W or Q9W) in tumor types responsive to CPIs N=4 + Relapsed or refractory to CPI + PDL1 TPS ≥ 1% (NSCLC) + PDL1 CPS ≥ 1% (PD-L1 basket) N=4 N=4 N=80N=20 N=24 NSCLC Melanoma HNSCC G/GEA RCC N=20 N=20 N=10 N=10 PDL1+ NSCLC r/r PDL1+ basket PDL1+ cutaneous melanoma PDL1+ uveal melanoma N=30 N=10 PDL1+ HNSCC N=10 PDL1+ nasopharyngeal carcinoma + Durable responses with anti-PD-1 in CPI refractory patients across multiple tumor types + Well-tolerated with mild or moderate immune- related toxicities + 4/10 response evaluable NSCLC & melanoma patients with duration of stable disease* greater than 6 months (three CPI-exposed patients and one CPI-naïve uveal melanoma patient) + Longest duration of stable disease was 2+ years (NSCLC patient refractory to Keytruda)** + Well-tolerated with mild or moderate immune-related toxicities Single agent In combination *per investigator assessment **patient came off study May 12, 2022 after 112 weeks on treatment with INBRX-106 CPI naïve CPI r/r In combinationSingle agent 36 Market opportunity for INBRX-106 and INBRX-105 The figures on this slide represent market research estimates from Evaluate Pharma *Keytruda™ and Opdivo® go off patent in 2028 $14.4 $7.9 $2.9 $2.0 $1.0 $28.2$27.1 $14.2 $7.4 $4.7 $10.1 $63.5 $0 $10 $20 $30 $40 $50 $60 $70 KEYTRUDA* OPDIVO* TECENTRIQ IMFINZI OTHER TOTAL 2020 Projected 2026 0 5 10 15 20 25 30 Total Other indications SCLC Gastrointestinal adenocarcinoma Head & neck cancers Bladder cancer Renal cell carcinoma Melanoma NSCLC 2020 PD-1/PD-L1 WW Revenue (in $ billions) PD-1/PD-L1 WW Revenue by Indication (in $ billions)
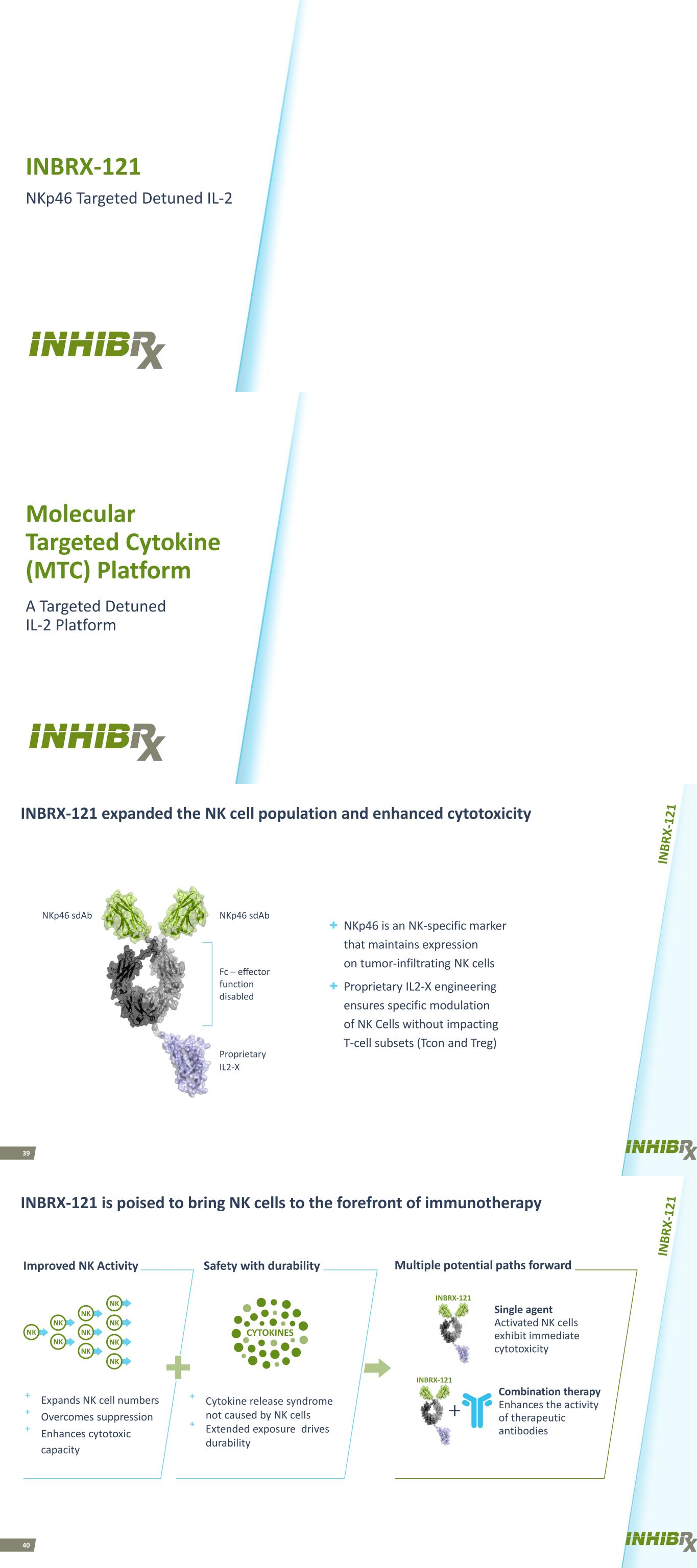
INBRX-121 NKp46 Targeted Detuned IL-2 Molecular Targeted Cytokine (MTC) Platform A Targeted Detuned IL-2 Platform 39 INBRX-121 expanded the NK cell population and enhanced cytotoxicity + NKp46 is an NK-specific marker that maintains expression on tumor-infiltrating NK cells + Proprietary IL2-X engineering ensures specific modulation of NK Cells without impacting T-cell subsets (Tcon and Treg) NKp46 sdAb NKp46 sdAb Fc – effector function disabled Proprietary IL2-X 40 INBRX-121 INBRX-121 is poised to bring NK cells to the forefront of immunotherapy NK NK NK NK NK NK NK NK NK NK Improved NK Activity Safety with durability Single agent Activated NK cells exhibit immediate cytotoxicity Multiple potential paths forward Combination therapy Enhances the activity of therapeutic antibodies ⁺ Expands NK cell numbers ⁺ Overcomes suppression ⁺ Enhances cytotoxic capacity ⁺ Cytokine release syndrome not caused by NK cells ⁺ Extended exposure drives durability CYTOKINES INBRX-121
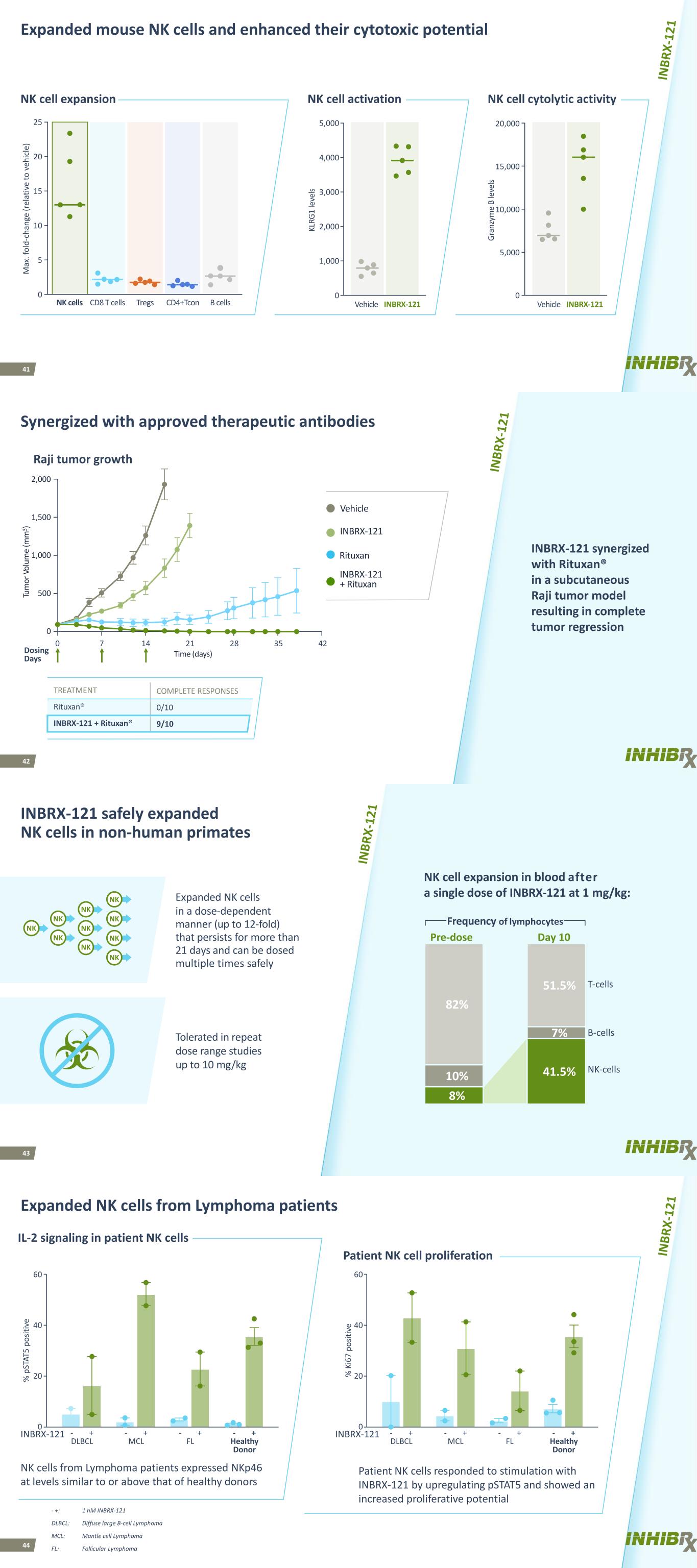
41 Expanded mouse NK cells and enhanced their cytotoxic potential 0 2,000 3,000 4,000 5,000 KL RG 1 le ve ls 5,000 10,000 15,000 20,000 Gr an zy m e B le ve ls M ax . f ol d- ch an ge (r el at iv e to v eh ic le ) 0 5 10 20 25 15 NK cells CD8 T cells Tregs CD4+Tcon B cells NK cell cytolytic activityNK cell activationNK cell expansion Vehicle INBRX-121 1,000 0 Vehicle INBRX-121 42 Synergized with approved therapeutic antibodies INBRX-121 synergized with Rituxan® in a subcutaneous Raji tumor model resulting in complete tumor regression Dosing Days TREATMENT COMPLETE RESPONSES Rituxan® 0/10 INBRX-121 + Rituxan® 9/10 Raji tumor growth 0 42 Time (days) 0 Tu m or Vo lu m e (m m 3 ) 500 1,000 1,500 2,000 7 14 21 28 35 INBRX-121 + Rituxan INBRX-121 Rituxan Vehicle 43 INBRX-121 safely expanded NK cells in non-human primates 8% 82% 10% 41.5% 51.5% 7% Expanded NK cells in a dose-dependent manner (up to 12-fold) that persists for more than 21 days and can be dosed multiple times safely Tolerated in repeat dose range studies up to 10 mg/kg NK cell expansion in blood after a single dose of INBRX-121 at 1 mg/kg: T-cells B-cells NK-cells Frequency of lymphocytes Pre-dose Day 10 NK NK NK NK NK NK NK NK NK NK 44 Expanded NK cells from Lymphoma patients NK cells from Lymphoma patients expressed NKp46 at levels similar to or above that of healthy donors Patient NK cells responded to stimulation with INBRX-121 by upregulating pSTAT5 and showed an increased proliferative potential - + Healthy Donor 0 20 40 % p ST AT 5 po sit iv e - + FL - + MCL % K i6 7 po sit iv e 60 IL-2 signaling in patient NK cells Patient NK cell proliferation - + DLBCL 0 20 40 60 - + Healthy Donor - + FL - + MCL - + DLBCL - +: 1 nM INBRX-121 DLBCL: Diffuse large B-cell Lymphoma MCL: Mantle cell Lymphoma FL: Follicular Lymphoma INBRX-121 INBRX-121

45 Expanded the number of NK cells while enhancing their individual cytotoxic capacities INBRX-121 increased NK cell-mediated killing of Raji cells in the presence of a Rituximab sequence analog (Anti-hCD20- hIgG1). Raji cell killing after INBRX-121 pre-incubation Effector: target ratio 0 % Ta rge t c ell de ath 40 80 100 120 2.5:1 5:1 10:1 20:1 40:1 Anti-hCD20-hlgG1 + INBRX-121 Anti-hCD20-hlgG1 only 60 20 11025 N. Torrey Pines Rd Ste 200 La Jolla, CA 92037 www.inhibrx.com