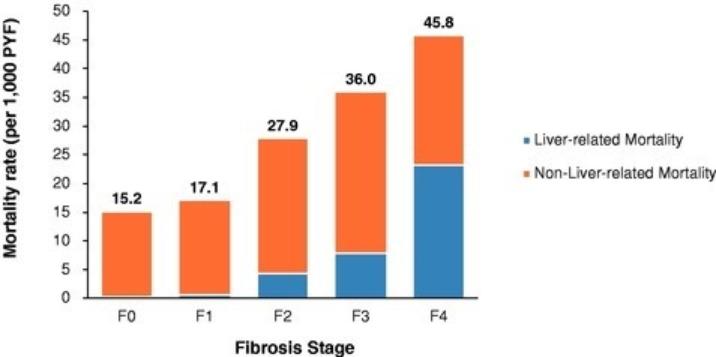
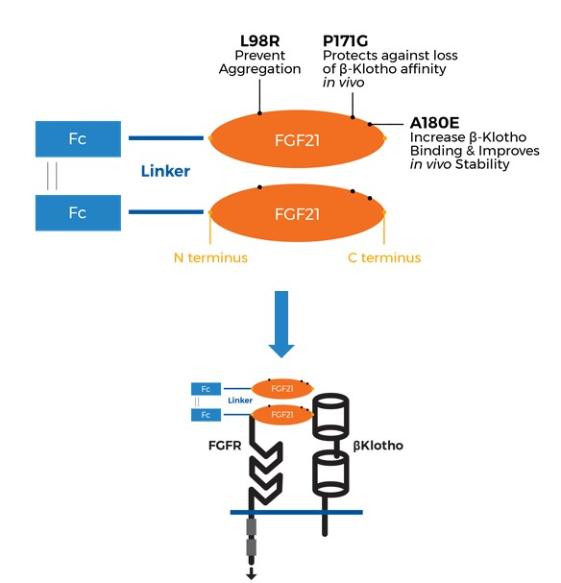
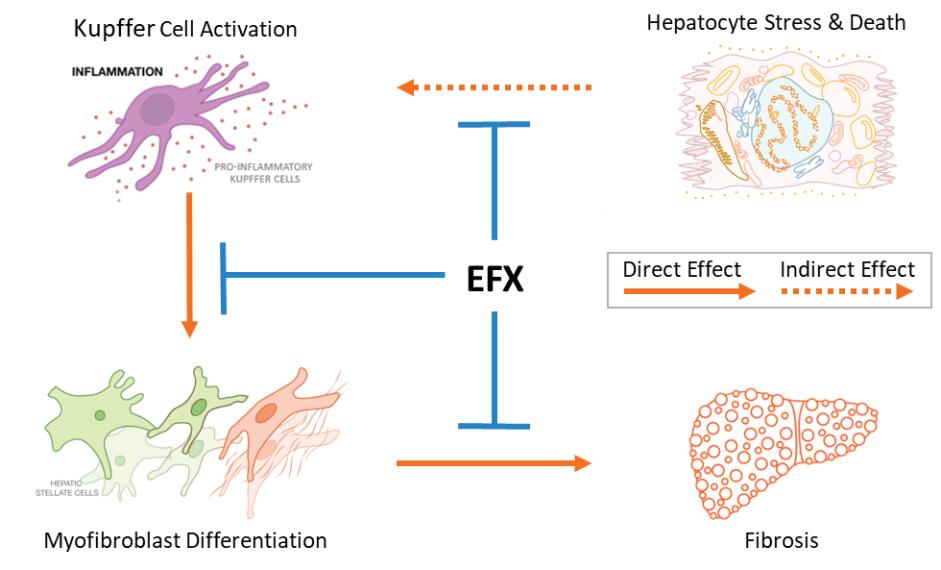
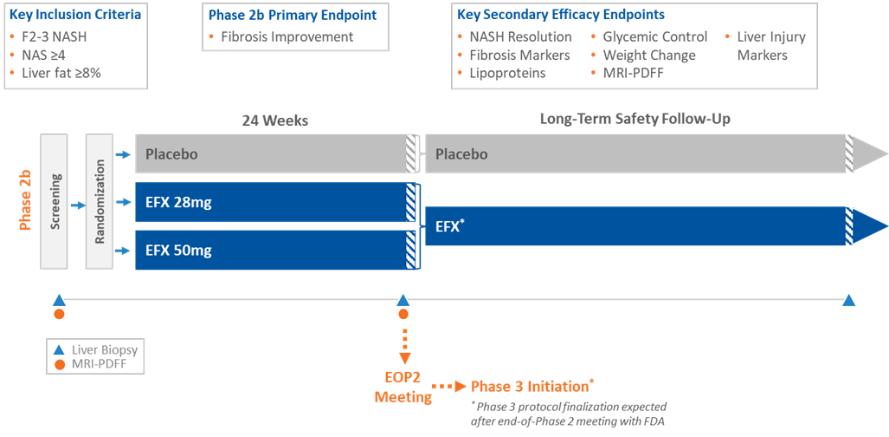
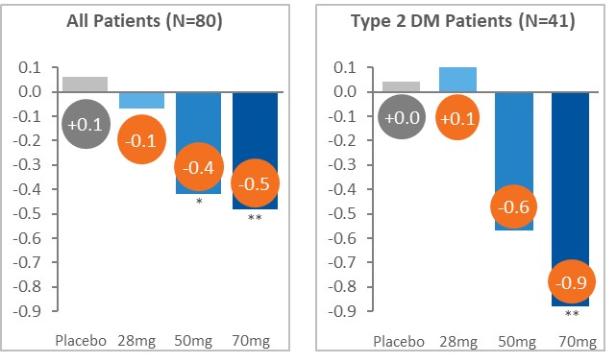
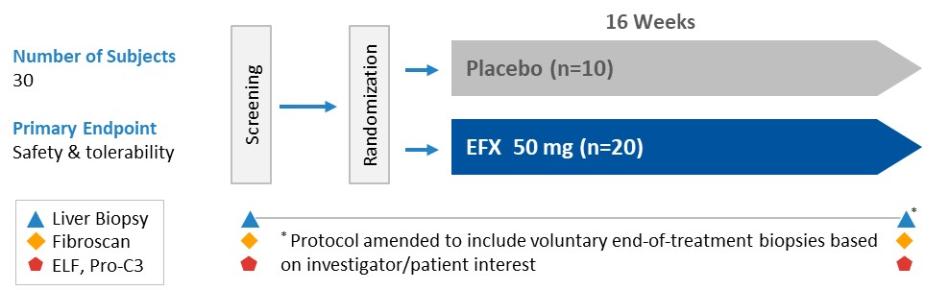
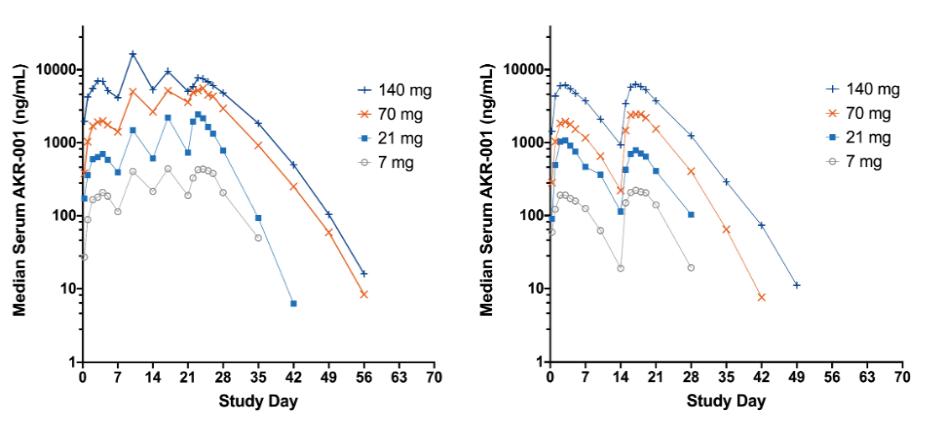
Manufacturing and supply
We manage several external commercial manufacturing organizations, or CMOs, to develop and manufacture EFX.
EFX drug substance, or DS, is manufactured by fermentation of a recombinant strain of the bacterium E. coli. Product accumulates as insoluble particles (inclusion bodies) within the cells and is recovered by cell disruption, followed by solubilization of the inclusion bodies, protein refolding and several chromatographic separation steps to yield product with target quality attributes. We have an agreement with Boehringer Ingelheim Biopharmaceuticals GmbH, or Boehringer Ingelheim, to manufacture DS for clinical development and plan to enter into a future agreement for commercial supply at the appropriate time. Whereas our recently concluded Phase 2a BALANCED study was supplied by DS acquired by Amgen, our ongoing Phase 2b HARMONY study is being supplied by DS manufactured by Boehringer Ingelheim. Yield of the Boehringer Ingelheim DS manufactured to Good Manufacturing Practice (GMP) was shown to be comparable to the Amgen DS. Analysis of the Boehringer Ingelheim GMP DS confirmed it met the same release specification as previously used for Amgen GMP DS.
We have an agreement with Vetter Pharma International GmbH, or Vetter, to manufacture EFX drug product, or DP, for clinical development and plan to enter into a future agreement for commercial supply at the appropriate time. The GMP DP being used for our ongoing HARMONY study is similar as was used for the BALANCED study, which is stored as a frozen liquid until immediately before administration to trial subjects. Analysis of the Vetter GMP DP confirmed that it met the same release specification as previously used for the DP manufactured from Amgen GMP DS.
We plan to use a newly developed freeze-dried, lyophilized DP for Phase 3 clinical trials and commercial launch, if EFX is approved. This new DP formulation is currently in the process of scale-up at Vetter. The development of a medical device is envisaged for convenient subcutaneous administration of the new formulation.
Sales and marketing
Successful marketing of a new drug for the treatment of NASH will require a targeted commercial infrastructure. We expect to begin making plans for commercialization following in parallel with our ongoing HARMONY study. We have contracted with a third-party manufacturer, Boehringer Ingelheim, to support clinical development and the potential commercialization of EFX with commercial-scale manufacturing. We intend to develop the commercial infrastructure required for bringing EFX to patients in the United States, if approved, in parallel with an anticipated Phase 3 clinical trial. We also plan to evaluate options for delivering EFX, if approved, to patients in other key markets, such as Europe, Japan and China, which may include strategic collaborations.
Competition
The biotechnology industry is intensely competitive and subject to rapid and significant technological change. Our competitors include multinational pharmaceutical companies, specialized biotechnology companies and universities and other research institutions. We understand that a number of pharmaceutical companies, including AbbVie, Inc., AstraZeneca PLC/MedImmune LLC, Boehringer Ingelheim AG, Bristol-Myers Squibb Company, Inc., Eisai, Inc., Eli Lilly and Company, Johnson & Johnson, Merck & Co., Inc., Novartis Pharmaceuticals Corporation, Novo Nordisk A/S, Pfizer Inc., Roche Holding AG, Sanofi and Takeda Pharmaceutical Company Limited, as well as large and small biotechnology companies such as Albireo Pharma, Inc., Alnylam Pharmaceuticals, Inc., Altimmune, Inc., Boston Pharmaceuticals, Inc., Cirius Therapeutics, Inc., CymaBay Therapeutics, Inc., 89bio, Enanta Pharmaceuticals, Inc., Galectin Therapeutics Inc., Galmed Pharmaceuticals Ltd., Gilead Sciences, Inc., Hanmi Pharmaceutical Company, Ltd., Intercept Pharmaceuticals, Inc., Inventiva Pharma SA, Madrigal Pharmaceuticals, Inc., MediciNova, Inc., Metacrine, Inc., NGM Biopharmaceuticals, Inc., North Sea Pharmaceuticals, Poxel SA, Sagimet Biosciences, Inc., Terns Pharmaceuticals, Inc. and Viking Therapeutics, Inc. are pursuing the development or marketing of pharmaceuticals that target NASH. It is also probable that the number of companies seeking to develop products and therapies for the treatment of serious metabolic diseases, such as NASH, will increase. Many of our competitors have substantially greater financial, technical, human and other resources than we do and may be better equipped to develop, manufacture and market technologically superior products. In addition, many of these competitors have significantly greater