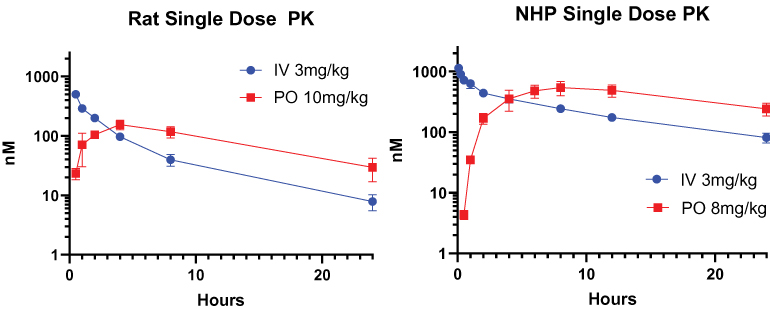
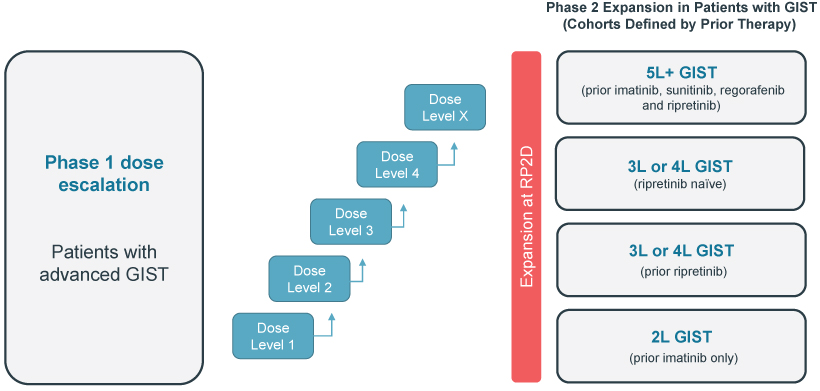
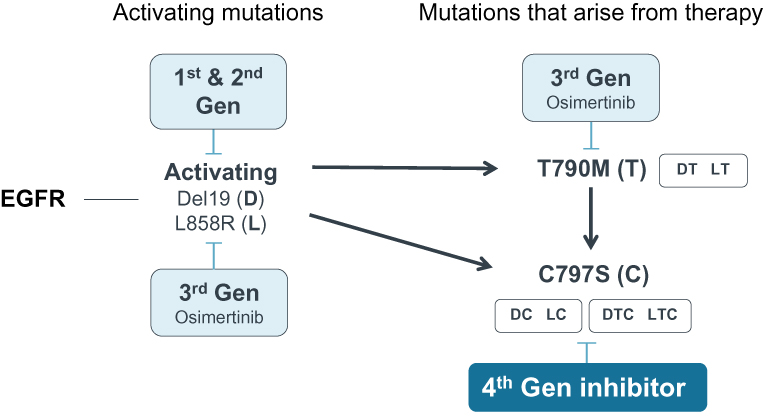
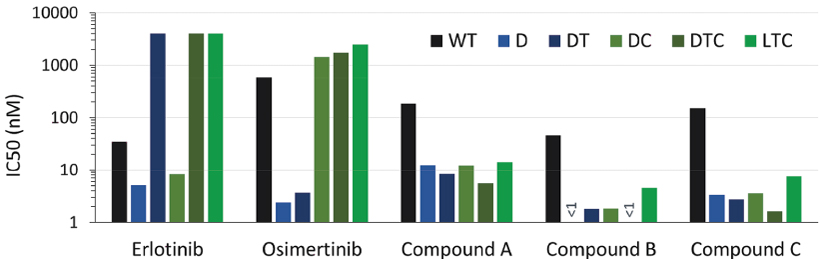
preclinical studies and clinical trials will begin or be completed on time, if at all. Human clinical trials require significant additional financial and management resources and reliance on third-party investigators and collaborators, such as medical institutions, CROs, clinical trial sites, contract manufacturing organizations, or CMOs, and potentially strategic collaborators. We may be unable to identify and contract with sufficient third-party investigators and collaborators on a timely basis or at all, or on terms that are acceptable to us. See “—Risks Related to Our Reliance on Third Parties—We rely on third parties to conduct our preclinical studies, and plan to rely on third parties to conduct clinical trials, and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of such trials, research and studies.”
We may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial, managerial and research and development resources, we must prioritize our research programs and will need to focus product candidates on the potential treatment of certain indications. As a result, we may forego or delay pursuit of opportunities with other product candidates or for other indications that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on current and future research and development programs and product candidates for specific indications may not yield any commercially viable products. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may also relinquish valuable rights to that product candidate through collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate.
We face substantial competition which may result in others discovering, developing or commercializing products before or more successfully than we do.
The pharmaceutical and biotechnology industries, particularly the field of oncology, are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary and novel products and product candidates. We have competitors both in the United States and internationally, including major multinational pharmaceutical companies, established biotechnology companies, specialty pharmaceutical companies, emerging and start-up companies, universities and other research institutions. For example, several biopharmaceutical companies, including Black Diamond Therapeutics, Inc., Blueprint Medicines Corporation, or Blueprint, Deciphera Pharmaceuticals, Inc., or Deciphera, Kinnate Biopharma Inc., Mirati Therapeutics, Inc., Nuvalent, Inc., Relay Therapeutics, Inc., Revolution Medicines, Inc., Scorpion Therapeutics, Turning Point Therapeutics, Inc. and Tyra Biosciences, Inc., are also developing precision oncology medicines.
Our competitors have developed, are developing or may develop products, product candidates and processes competitive with our product candidate. Any product candidates that we successfully develop and commercialize will compete with existing therapies and new therapies that may become available in the future. There are several approved therapies for the treatment of conditions for which we are attempting or may attempt to develop product candidates. In addition, we believe that a significant number of product candidates are currently under development, and may become commercially available in the future. We also compete to recruit and retain qualified scientific and management personnel, which could negatively affect our level of expertise and our ability to execute our business plan. As a result, our potential commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects or are more convenient than any products that we may develop.
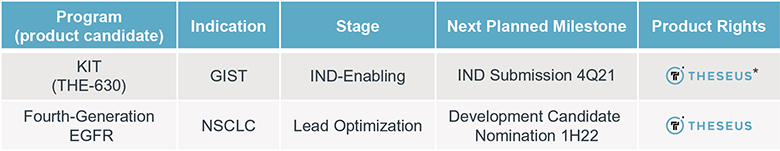
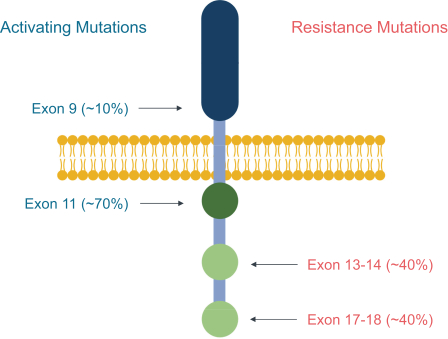
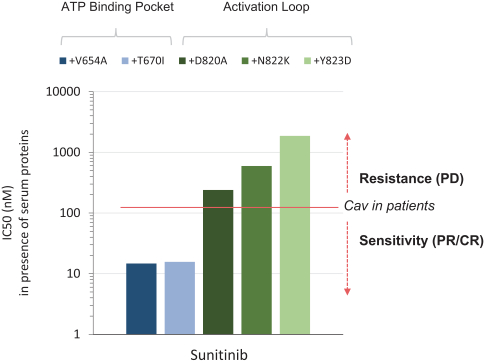
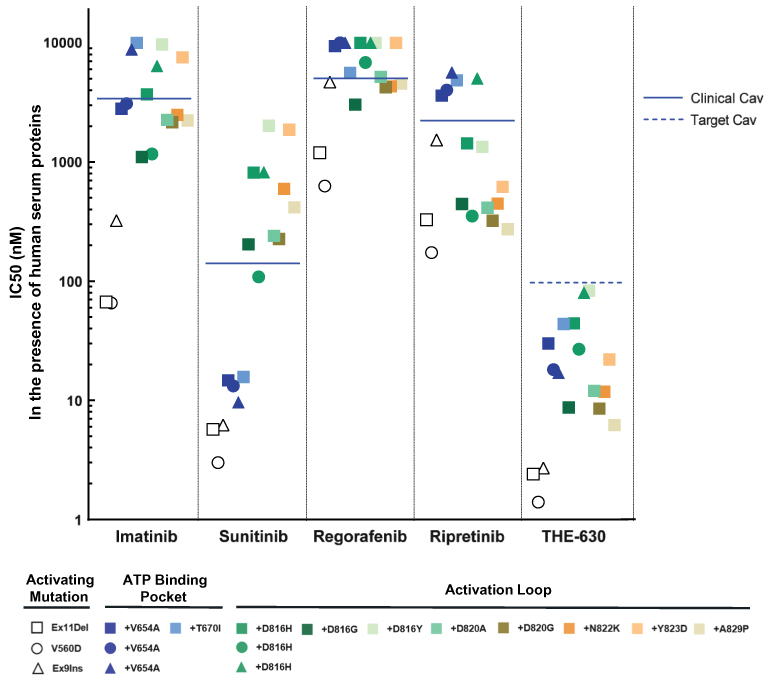
For example, with respect to our lead product candidate, THE-630, there are several large pharmaceutical companies and biotechnology companies marketing drugs for the treatment of GIST, including Blueprint, Novartis AG, Pfizer, Inc., Deciphera and Bayer AG. We are also aware of pharmaceutical and biotechnology companies developing drugs for the treatment of GIST, including AB Sciences S.A., Arog Pharmaceuticals, Inc., Chia Tai Tianqing Pharmaceutical Group CO., LTD, or CTTP, Cogent Biosciences, Inc., or Cogent, Daiichi Sankyo Company, Limited, or Daiichi, Deciphera, Exelixis, Inc., Immunicum AB, Jiangsu HengRui, Inc., Ningbo Tai Kang Medical Technology Co. Ltd., Taiho Pharmaceutical Co. Ltd, Xencor, Inc. and Merck KGaA. In particular, Deciphera is conducting its ongoing INTRIGUE phase 3 clinical study evaluating ripretinib against sunitinib, the topline results of which are expected to be announced in the fourth quarter of 2021, and Cogent has disclosed its planned
20