Exhibit 99.2

HALF - YEAR REPORT 2021 in \ MOLECULAR partners C u s t o m - b u i l t biolog y fo r p a t i en t s

2 A t a G l a n c e : K e y M i l e s t o n e s , G r o u p P r o f i l e & C o nt e nt s • A d v a n c e m e n t o f b a l a n c e d p o r t f o l i o o f d i ff e r e n t i a t e d D A R P i n ® p r o d u c t c a n d i d a t e s offering patients a new dimension of protein therapeutics for the treatment of serious diseases • K e y a d v a n c e m e n t s m a d e i n t h e a r e a s o f o n c o l o g y a n d v i r o l o g y H1 2021 Research & Development Milestones • Initiated two global Phase 2 and 3 trials of ensovibep (MP0420), to explore safety and efficacy in ambulatory patients with COVID - 19 (EMPATHY) in collaboration with Novartis, and hospitalized patients (ACTIV - 3) sponsored by the National Institutes of Health (NIH) • Received FDA Fast Track designation for ensovibep for the treatment of COVID - 19 in both hospitalized and ambulatory settings • Initiated and fully enrolled Phase 2a single arm study of ensovibep in the Netherlands in patients with mildly symptomatic COVID - 19, with data expected to be presented in a scientific conference in H2 2021 • R e p o r t e d t h a t i n v i t r o s t u d i e s i n d i c a t e t h a t e n s o v i b e p m a i n t a i n s p o t e n c y a g a i n s t a ll known SARS - CoV - 2 variants of concern, including Delta and Lambda • Presented data further supporting the MP0317, T - cell engager, and Peptide - MHC oncology programs at AACR • In August, announced receipt of global rights of abicipar pegol for the treatment of neovascular AMD (nAMD) and Diabetic Macular Edema, following termination of the license and collaboration agreement by AbbVie Inc. H 1 2021 L e a d e r s h i p & G o v e r n a n c e M i l e s t o n e s • Elected Agnete Fredriksen and Dominik Höchli to the Board of Directors at the A nn u a l G e n e r a l M ee t i n g o f A p r i l 21 , 2021 H 1 2021 F i n a n c i a l M i l e s t o n e s • Successfully completed initial public offering of American Depositary Shares (“ADSs”) on the Nasdaq, raising $63.8 million (CHF 58.8 million) in gross proceeds to support ongoing operations into H2 2023 • Ongoing strong financial position with CHF 174.3 million in cash and short - term deposits as of June 30, 2021 • Net cash outflow from operating activities of CHF 52.5 million in H1 2021 • FY 2021 expense guidance maintained at CHF 65 - 75 million

3 G r o u p P r o f i l e Molecular Partners is a clinical - stage biotech company developing DARPin ® therapeutics, a new class of custom - built protein drugs designed to address challenges current modalities cannot . The Company has formed partnerships with leading pharmaceutical companies to advance DARPin ® therapeutics in the areas of ophthalmology, oncology and infectious disease, and has compounds in various stages of clinical and preclinical development across multiple therapeutic areas . S h a r e I n f o r m a t i o n • Listed on SIX Swiss Exchange (ticker symbol: MOLN; ISIN CH0256379097) since Nov. 2014 • L i s t e d o n N a s d a q ( t i c k e r s y m b o l : M O L N ) s i n c e J u n e 2021 • 32,269,285 shares outstanding as of June 30, 2021 • CHF 608 million market capitalization as of June 30, 2021 • Free float of 84% as per SIX Swiss Exchange definition Contents A t a G l a n c e : K e y M i l e s t o n e s a n d C o n t e n t s ................................................................................ 2 H1 2021 R&D, Partnership & Team Milestones H1 2021 Financial Milestones C o m p a n y P r o f i l e & S h a r e I n f o r m a t i o n Contents S h a r e h o l d e r L e tt e r ....................................................................................................................... 5 F i n a n c i a l S u mm a r y ........................................................................................................................ 11 R e s u l t s a n d O v e r v i e w F i n a n c i a l H i g h l i g h t s Outlook 2021 and Financial Calendar D e v e l o p me n t o f E m p l o y ee B a s e F i n a n c i a l R e p o r t s .......................................................................................................................... 17 IFRS Condensed Consolidated Interim Financial Statements Auditor's Report on Review of Condensed Consolidated Interim Financial Statements

HALF - YEAR REPORT 2021 in \ MOLECULAR partners C u s o tm - bu i l b t iolog y fo r pa t ien t s

5 T o O u r S h a re h o l d er s We are committed to leveraging our leadership in DARPin ® therapeutics to deliver a unique class of custom - built protein therapeutics that go beyond the limits of current treatments for cancer, in virology as well as for other serious diseases . The COVID - 19 pandemic continued to dominate also the first half of 2021 . Nonetheless, in the course of the first semester, we were able to strongly expand our global clinical presence and collaboration with Novartis as the evolving COVID - 19 pandemic continued to underscore the need for effective antiviral therapeutics . As a now dual - listed company in Switzerland and the United States, our expanded investor base enables us to accelerate our mission to deliver a new class of medicines to people living with cancer and infectious diseases . To have entered two ongoing, late - stage trials of ensovibep further illustrates our rapid design and development capabilities, and in addition to the COVID - 19 program we are focused on expanding into new antiviral applications of the DARPin ® platform while moving towards a new phase for our immuno - oncology programs . H 1 2021 Milestones and Corporate Highlights In the following, we summarize the advancements and status of our candidates as well as the initiatives for the individual therapeutic areas virology, oncology and ophthalmology . Further, we provide an overview on the highlights in terms of Leadership & Governance of our organization . Antiviral program : Rapid development of trispecific antiviral DARPin ® c a n d i d a t e e n s o v i b e p i n m u l t i p l e i nt e r n a t i o n a l c l i n i c a l t r i a l s Molecular Partners advanced in 2021 with strong momentum for our lead antiviral candidate, ensovibep, which has progressed in the second quarter of 2021 into two ongoing, late - stage global clinical studies, EMPATHY and ACTIV - 3 , in collaboration with Novartis and the NIH, respectively . Thus far, ensovibep has provided positive Phase 1 data and continued to maintain potency in laboratory studies against all known COVID - 19 variants of concern . In March of 2021 , jointly with our collaboration partner Novartis we announced that the National Institutes of Health (NIH) selected ensovibep for inclusion in a global phase 3 randomized, controlled clinical trial as part of NIH’s Accelerating COVID - 19 Therapeutic Interventions and Vaccines (ACTIV) program . The international master protocol ACTIV - 3 is designed to evaluate the safety and efficacy of various therapies for the treatment of adults hospitalized with a COVID - 19 diagnosis . A sub - study of ACTIV - 3 evaluating ensovibep began enrolling hospitalized patients in June 2021 and is currently enrolling patients across seven countries . Topline data from this study are expected in 2022 . In May of 2021 , together with Novartis we announced the start of the clinical trial EMPATHY, a global phase 2 - 3 study, to explore the use of ensovibep for the treatment of COVID - 19 in patients who are in the early stages of infection, to prevent worsening symptoms and hospitalization . Our collaboration partner Novartis is conducting the clinical trial for ensovibep, with Molecular Partners

6 as sponsor . EMPATHY is currently enrolling patients across five countries . Topline interim data for the first 400 patients are expected towards the end of second half of 2021 with complete data expected in 2022 . . In April of 2021 , the first patient was dosed in a Phase 2 a clinical trial of ensovibep in a single arm, open label study in the Netherlands . This study enrolled a total of 12 patients with mild symptomatic COVID - 19 in two dose cohorts, and is designed to evaluate the dynamics of viral clearance, pharmacokinetics and tolerability of ensovibep . Data for this study is expected to be presented in a scientific conference in H 2 2021 . Initial results show a steady decline of viral loads in treated patients, validating the follow - up methods implemented in the Company’s ongoing registrational trials, EMPATHY and ACTIV - 3 . In March 2021 , our Company reported positive initial Phase 1 results in healthy volunteers . Ensovibep, administered intravenously (I . V . ), was seen to be safe and well tolerated, with no serious or severe adverse events reported . These preliminary results also confirmed extended exposure to ensovibep in serum, with a half - life of 2 - 3 weeks, as was expected from preclinical experiments . These data confirmed the systemic administration of ensovibep to be safe and well tolerated and supported the initiation of later stage trials . Following I . V . administration, ensovibep was also evaluated for safety and half - life when administered in bolus, and is presently being evaluated in subcutaneous (S . C . ) administration in healthy subjects . DARPin ® molecules offer a differentiated approach to treating COVID - 19 through a ‘cocktail in a molecule’ mechanism ; a single molecule that can engage three domains of the SARS - CoV - 2 virus simultaneously to inhibit viral entry into cells . This allows for a potentially broader efficacy and reduces the likelihood for the development of viral drug resistance which can result from selection pressure on any single molecular target . In addition, DARPin ® candidates are produced through rapid, high - yield microbial fermentation for potential speed and logistical advantages over mammalian cell production employed for antibodies . Based on the success seen to - date of ensovibep’s unique approach to neutralizing the virus, Molecular Partners is also evaluating the next generation of opportunities to develop antivirals against other infectious diseases with global unmet need . Immuno - oncology programs : Clinical work for AMG 506 / MP 0310 (FAP x 4 - 1 BB) ongoing ; new supportive data published across acute myeloid leukemia and MP 0317 (FAP x CD 40 ) programs as well as novel technology platforms Following the positive initial results of MP 0310 , clinical work advanced into weekly administration of MP 0310 in the Phase 1 study, to identify a dosing regimen to obtain sustained 4 - 1 bb activation . our Company expects to obtain data from this trial within 2021 , allowing for our partner, Amgen, to evaluate potential future development of MP 0310 in combination with Amgen’s oncology assets, including BiTE ® molecules . In April of 2021 , our team presented four posters highlighting research across its immuno - oncology programs at the American Association for Cancer Research (AACR) virtual Annual Meeting . Molecular Partners’ novel multi - specific DARPin ® candidates are designed to activate the immune system to fight cancer while reducing damage to healthy cells . These candidates use multiple novel DARPin ® technologies potentially applicable against a wide range of tumor types . The preclinical data shared include first results from the Company’s acute myeloid leukemia (AML) CD 3 T - cell engager program, initial results from the CD 3 prodrug program, and new data from the MP 0317 (FAP x CD 40 ) and peptide - MHC programs .

7 With respect to MP 0317 , a multi - specific DARPin ® product candidate targeting both FAP and CD 40 to enable tumor - localized immune activation, new preclinical data showed activation of B - cells and myeloid cells in ex vivo human tumor samples . This demonstrated that physiological presence of FAP, expressed in the connective tissue of a broad range of solid tumors, is mandatory and sufficient for MP 0317 to induce immune activation . Furthermore, the data presented at AACR shows that MP 0317 led to a range of pro - inflammatory activities, including macrophage repolarization and reversion of T - cell suppression only in the presence of FAP . In both assays the killing effect was comparable to that achieved by an anti - CD 40 antibody . The Company believes these data support MP 0317 ’s potential to deliver tumor - localized CD 40 - mediated immune cell activation while avoiding systemic toxicity seen with other agents . MP 0317 is anticipated to begin clinical trials in the second half of 2021 . In preclinical studies, the Company’s AML research candidates demonstrated substantial activity against different populations of AML cells in vitro and ex vivo, without significant damage to healthy cells . The candidate is further designed to bind with increased avidity as the number of relevant antigens presented increases, further strengthening its preference for tumor cells . The candidate is a single molecule designed to target three different cancer antigens simultaneously (CD 70 , CD 33 , and CD 123 ) . This multi - specific DARPin ® T - cell engager candidate is designed to deliver a highly potent and specific anti - tumor response to AML cells, with a reduced effect on healthy normal cells, and with the potential to counteract target escape mechanisms expected due to tumor heterogeneity . The AML DARPin candidate demonstrated potent induction of T - cell mediated cytotoxicity against AML cell lines and primary AML calls . In an ex vivo assay using fresh blood from healthy donors, the candidate induced profoundly less inflammatory cytokine production and reduction in platelet counts than T - cell engager candidates in development by other parties . Molecular Partners believes this candidate shows a unique avidity - driven ability to kill a broader population of AML cells while decreasing the risk of toxicity . Molecular Partners’ T - cell engager programs also include a novel prodrug DARPin ® technology for tumor - localized release of immune stimulation, through incorporation of a protease - cleavable blocker DARPin ® molecule . As CD 3 - binding T - cell engagers are highly potent and can lead to systemic toxicities, Molecular Partners has developed a DARPin ® domain designed to mask the CD 3 engager from interacting with T - cells, systemically or outside of the tumor, thus reducing toxicity by limiting immune activation to the tumor microenvironment . Molecular Partners’ prodrug research candidate, CD 3 - PDD, has demonstrated in vitro and in vivo proof - of - concept, being shown to be unable to bind and recruit T - cells in its non - cleaved state in circulation while delivering an anti - tumor effect . Finally, new data were presented supporting Molecular Partners’ peptide - MHC targeting program, which focuses on developing the capability to target cell surface protein complexes indicating disease through display of intracellular peptides . At AACR, the Company presented preclinical results demonstrating rapid and reliable generation of DARPin ® proteins against a peptide - MHC complex (pMHC) . These DARPin ® proteins were then formatted into bispecific T - cell engagers, and engineered to enable potent and specific activation of T cells . Further, the results showed that a pMHC - targeting DARPin ® candidate was able to achieve systemic half - life extension . Ophthalmology In August, the Company was updated by our collaboration partner, AbbVie Inc . of its termination of the license and collaboration agreement for the investigational drug abicipar pegol for the treatment of nAMD and DME . As such, Molecular Partners will regain the development and commercial rights of abicipar on a worldwide basis .

8 F i n a n c i a l h i g h l i g ht s i n H 1 2021 Molecular Partners remains solidly funded to capture upcoming value inflection points . In June 2021 , our Company successfully completed an initial public offering of American Depositary Shares on the Nasdaq exchange, raising $ 63 . 8 million (CHF 58 . 8 million) in gross proceeds . With the U . S . listing, we broadened our access to capital from the global investment community to support our programs and growing pipeline . In the first six months of 2021 , Molecular Partners recognized total revenues and other income of CHF 4 . 4 million (H 1 2020 : CHF 7 . 5 million) and incurred total operating expenses of CHF 39 . 2 million (H 1 2020 : CHF 30 . 6 million) . This led to an operating loss of CHF 34 . 8 million for the first six months in 2021 (H 1 2020 : Operating loss of CHF 23 . 1 million) and a net loss of CHF 33 . 6 million for H 1 2021 (H 1 2020 : Net loss of CHF 24 . 7 million) . The net cash outflow from operating activities during the first six months in 2021 was CHF 52 . 5 million ( 2020 : net cash outflow of CHF 27 . 9 million) . Including short - term time deposits, the cash and cash equivalents position increased by CHF 0 . 6 million vs . year - end 2020 to CHF 174 . 3 million as of June 30 , 2021 (December 31 , 2020 : CHF 173 . 7 million) . Total shareholders’ equity stood at CHF 134 . 6 million as of June 30 , 2021 , an increase of CHF 27 . 4 million (December 31 , 2020 : C H F 107 . 2 m i ll i o n ) . As of June 30 , 2021 , the Company employed 158 FTEs (full time equivalents), up 15 year - on - year . About 80 % of the employees are employed in R&D - related functions . H 1 2021 L e a d e r s h i p & G o v e r n a n c e h i g h l i g ht s At the Annual General Meeting of our Company on April 21 , 2021 , the shareholders of Molecular Partners elected Agnete Fredriksen and Dominik Höchli to the Board of Directors . A g n e t e F re d r i k s e n Agnete Fredriksen, Ph . D . , is a co - founder, president and chief innovation and strategy officer of Vaccibody AS of Vaccibody AS, a clinical - stage biopharmaceutical company dedicated to the discovery and development of novel immunotherapies for cancer and infectious diseases . With prior roles at Affitech AS and Medinnova AS, Agnete’s focus is on developing vaccines from idea to clinical development . She is the author of numerous scientific papers in the field of immunology, immunotherapy and vaccines, and has been awarded several patents in the field of immunotherapy . She holds an MSc and a Ph . D . from the Institute of Immunology, Rikshospitalet Medical Center in Oslo, Norway . D o m i n i k H ö c h l i Dominik Höchli has 20 years of experience in as a marketing and medical affairs executive . Since spring 2021 he is the CEO of Catapult Therapeutics, a clinical stage biotech company in the Netherlands . Previously he worked at AbbVie as Vice President, Head of Global Medical Affairs and member of the R&D and the Commercial leadership team . He led global product launches for major blockbuster products, including HUMIRA, Maviret, Venetoclax and Skyrizi, and his leadership experience ranges from smaller country organizations to large global functions . He began his corporate career at McKinsey & Co . Dominik is a Swiss national and obtained his medical degree (M . D . ) from the University of Bern .

B u s i n e ss o ut l oo k a n d p r i o r i t i e s f o r H 2 2021 a n d b e y o n d In the second half of 2021 , Molecular Partners remains focused on the rapid clinical development of ensovibep across two major global studies as well as a further bridging study to evaluate the option of subcutaneous/intramuscular administration . Topline interim data for the first 400 patients are expected towards the end of second half of 2021 with complete data expected in 2022 . We are committed to advancing other antiviral programs and is currently evaluating several potential targets with global unmet need . The Company expects to announce additional antiviral programs in the second half of 2021 . In immuno - oncology, Molecular Partners expects to provide results from the MP 0310 trial to Amgen, to inform their decision regarding future development of the program . Further, the Company intends to begin clinical studies of MP 0317 in the second half of 2021 and expects to present additional research data from its trispecific CD 3 T - cell engager for the treatment of AML . Molecular Partners is now working with AbbVie for the receipt of data and materials related to the abicipar program . We have formed a special committee to evaluate the program and determine appropriate next steps regarding abicipar . Our purpose: Deliver an entirely new class of drugs to transform care for cancer, in virology and other serious diseases At Molecular Partners, we have a strong connection to our core purpose of transforming treatment for patients with cancer and other serious diseases through delivering on the promise of DARPin ® therapeutics . As a team, we are energized about the opportunities ahead and our progress in creating and growing the capabilities of DARPin ® candidates . Our discovery and development capabilities continue to grow, as do the depth and breadth of our partnerships . We continue to demonstrate our capacity to respond to medical need and push our DARPin ® expertise into new platforms to expand the potential of this unique class of drugs . T h a n k y o u f o r y o u r c o nt i nu e d s u pp o r t o f o u r w o r k Our continued progress and value creation wouldn't be possible without the full support and tireless work of our employees, strategic partners, investors, researchers and patients . We thank all these groups for their support and look forward to sharing additional news throughout the second half of 2021 . Sincerely, B i ll B u r n s C h a i r m a n o f t h e B o a r d 9 P a t r i c k A m s t u t z C h i e f E x e c u t i v e O ff i c e r

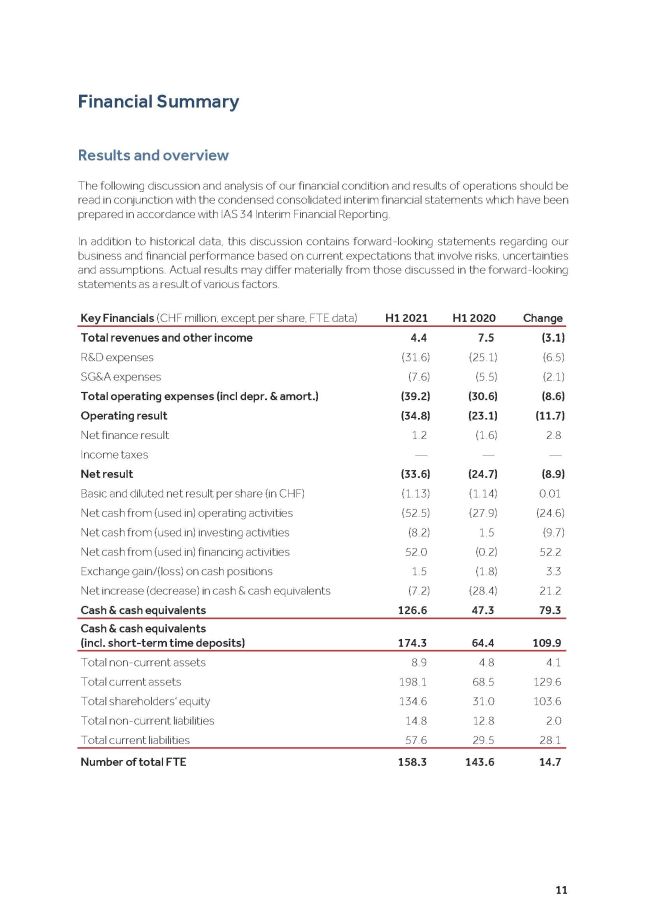
11 F i n a n c i a l S u mm a r y R e s u l t s a n d ov e r v i e w The following discussion and analysis of our financial condition and results of operations should be read in conjunction with the condensed consolidated interim financial statements which have been p r e p a r e d i n a cc o r d a n c e w i t h I A S 34 I n t e r i m F i n a n c i a l R e p o r t i n g . In addition to historical data, this discussion contains forward - looking statements regarding our business and financial performance based on current expectations that involve risks, uncertainties and assumptions . Actual results may differ materially from those discussed in the forward - looking statements as a result of various factors . Key Financials (CHF million, except per share, FTE data) H1 2021 H1 2020 Change Total revenues and other income 4.4 7.5 (3.1) R&D expenses (31.6) (25.1) (6.5) SG&A expenses (7.6) (5.5) (2.1) Total operating expenses (incl depr. & amort.) (39.2) (30.6) (8.6) Operating result (34.8) (23.1) (11.7) Net finance result 1.2 (1.6) 2.8 Income taxes — — — Net result (33.6) (24.7) (8.9) Basic and diluted net result per share (in CHF) (1.13) (1.14) 0.01 Net cash from (used in) operating activities (52.5) (27.9) (24.6) Net cash from (used in) investing activities (8.2) 1.5 (9.7) Net cash from (used in) financing activities 52.0 (0.2) 52.2 Exchange gain/(loss) on cash positions 1.5 (1.8) 3.3 Net increase (decrease) in cash & cash equivalents (7.2) (28.4) 21.2 Cash & cash equivalents 126.6 47.3 79.3 Cash & cash equivalents (incl. short - term time deposits) 174.3 64.4 109.9 Total non - current assets 8.9 4.8 4.1 Total current assets 198.1 68.5 129.6 Total shareholders’ equity 134.6 31.0 103.6 Total non - current liabilities 14.8 12.8 2.0 Total current liabilities 57.6 29.5 28.1 Number of total FTE 158.3 143.6 14.7

12 F i n a n c i a l h i g h l i g ht s Over the course of 2021 , the Group continued and is continuing to increase its investments in its clinical and preclinical programs as well as in research and development in order to progress its proprietary oncology and virology DARPin ® candidates towards value - creating milestones . The strong balance sheet, which was further reinforced with the CHF 52 . 5 million net proceeds from our Nasdaq listing in June 2021 , continues to provide the Group with financial flexibility and a forecasted cash runway into 2023 beyond the envisaged key value inflection points expected to be captured until then . Molecular Partners’ broad pipeline across multiple indications, its collaborations with blue - chip pharma companies Novartis, AbbVie and Amgen, and its strong financial position combine to provide the Group a uniquely robust position within the biotech sector . The Group continues to invest its financial and human resources into the evolution of its proprietary DARPin ® technology, the progression of innovative programs as well as the advancement of its pipeline of proprietary drug candidates in clinical development targeting high - value indications . Revenues In H 1 2021 , the Group recognized total revenues and other income of CHF 4 . 4 million, a decrease of 41 % compared to the previous year ( 2020 : CHF 7 . 5 million) . The revenue and other income in the first six months of 2021 was largely attributable to the Group's partnership with Amgen (CHF 4 . 0 m i ll i o n ) . As of June 30 , 2021 the Group has CHF 15 . 0 million of contract liabilities under the Amgen collaboration agreement . This contract liability is expected to be recognized as revenues in the 2021 and 2022 periods as the Group performs its collaboration activities . O p e r a t i n g e x p e n s e s ( i n c l . d e p r e c i a t i o n a n d a m o r t i z a t i o n ) The Group’s operating expenses consist primarily of costs associated with research, preclinical and clinical testing, personnel - related costs and, to a lesser extent, royalty and license fees, facility expenses, professional fees for legal, tax, audit and strategic purposes, administrative expenses and depreciation of property, plant and equipment . Overall, total operating expenses increased by CHF 8 . 6 million (+ 28 % ) to CHF 39 . 2 million in H 1 2021 (compared to CHF 30 . 6 million in H 1 2020 ) . The two major expense categories were personnel expenses of CHF 17 . 7 million ( 45 % of total operating expenses) and research and development projects related costs totaling CHF 17 . 3 million ( 44 % of total operating expenses) . Total R&D expenses in H 1 2021 increased by CHF 6 . 5 million (+ 26 % ) to CHF 31 . 6 million (H 1 2020 : CHF 25 . 1 million), mainly due to the growing proprietary pipeline of the Group . The Group charges all R&D expenses, including internal patent filing and patent maintenance costs, to the income statement when incurred . Total SG&A expenses in H 1 2021 went up by CHF 2 . 1 million ( 38 % ) to CHF 7 . 6 million (H 1 2020 : CHF 5 . 5 million), mainly due to personnel related costs as well and the increase in director and o ff i c e r s i n s u r a n c e f o ll o w i n g t h e N a s d a q l i s t i n g i n J u n e 2021 .

13 In 2021 , operating expenses are expected to increase, particularly related to the ongoing clinical and preclinical studies and the development of the Group's proprietary product candidates . The Group continues to expand its proprietary product pipeline and further invests in the DARPin ® technology . Moreover, hiring additional personnel (mainly in R&D) will generate additional costs . As of June 30 , 2021 , the Group had 158 full - time employees (FTEs) on its payroll, including 127 FTEs (ca . 80 % ) in R&D and 31 FTEs (ca . 20 % ) in SG&A . O p e r a t i n g p r o f i t ( l o ss ) In the first six months of 2021 , the Group generated an operating loss of CHF 34 . 8 million (compared to an operating loss of CHF 23 . 1 million in the same period in 2020 ) . The higher operating loss versus the previous year mainly reflects both the lower recognized revenues as well as further intensified R&D activities for the benefit of long - term value creation . F i n a n c i a l i n c o m e a n d e x p e n s e s In the first six months of 2021 , Molecular Partners recorded a net financial gain of CHF 1 . 2 million, compared to a net financial loss of CHF 1 . 6 million in the same period in 2020 . In the first six months of 2021 the financial income amounted to CHF 1 . 5 million, largely driven by income generated from a foreign exchange gain on the Group's cash balances . The financial expense in the first six months of 2021 of CHF 0 . 3 million arose mainly from interest expense on cash positions and short - term time deposits . The Group is not hedging for translation risks as it pursues a stringent natural hedging policy by optimizing the matching of cash in/out flows in the respective currencies . I n c o m e a n d d e f e rr e d t a x e s Molecular Partners AG did not have to pay or accrue any income taxes in the reporting periods . Future net income in Switzerland will be subject to federal, cantonal and communal income taxes . The company’s applicable income tax rate in Switzerland is 21 % . Molecular Partners Inc . , which is incorporated in the United States in the state of Delaware, is subject to statutory U . S . federal corporate income taxes and state income taxes . N e t l o ss In H1 2021, the Group recorded a net loss of CHF 33.6 million (H1 2020: CHF 24.7 million net loss).

14 Balance sheet and capital resources As of June 30 , 2021 , the Group’s position on cash and cash equivalents plus short - term time deposits increased by CHF 0 . 6 million compared to year - end 2020 to CHF 174 . 3 million (or 84 % of the total assets) . Compared to year - end 2020 , the total shareholders’ equity position increased by CHF 27 . 4 million to CHF 134 . 6 million as of June 30 , 2021 (December 31 , 2020 : CHF 107 . 2 million) . The Group’s balance sheet continued to be debt - free in 2021 . Liabilities in the balance sheet are primarily comprised of contract liabilities, trade payables and accrued expenses from our operations as well as pension liabilities as per IAS 19 . Total liabilities as of June 30 , 2021 amount to CHF 72 . 3 million (December 31 , 2020 : CHF 80 . 3 million) . The contract liabilities are the most significant liability item with a total of CHF 37 . 7 million at June 30 , 2021 (December 31 , 2020 : CHF 45 . 9 million) . The contract liabilities are expected to be recognized as revenue or as an offset of costs as the Group satisfies the related performance obligations . C a s h f l o w s t a t e m e nt In the first six months of 2021 , Molecular Partners presented a net cash outflow from operations of CHF 52 . 5 million, compared to the net cash outflow from operations of CHF 27 . 9 million in the same period in 2020 . Cash outflow from investing activities during the first six months of 2021 was CHF 8 . 2 million, compared to a CHF 1 . 5 million cash inflow in the same period of 2020 . The cash flows from investing activities are largely driven by the shift of cash into short - term time deposits and vice versa . A CHF 0 . 5 million outflow was recorded for capital expenditures in equipment and intangible assets . Net cash inflow from financing activities in the first six months of 2021 was CHF 52 . 0 million, largely driven cash generated form the Nasdaq listing . Overall, the cash flow activities resulted in a net decrease of the Group’s total cash and cash equivalents balance of CHF 7 . 2 million from CHF 133 . 7 million at the end of 2020 to CHF 126 . 5 million as per June 30 , 2021 . F i n a n c i a l r i s k m a n a g e m e nt The Group is developing several products and is currently not generating a constant revenue stream, which results in a negative cash flow from operating activities . At present, the lack of positive operating cash flow may expose the Group to financing risks in the medium term . Risk management is carried out centrally under policies approved by the Board of Directors . Furthermore, management manages financial risks such as foreign exchange risk and liquidity . Molecular Partners conducts its activities primarily in Switzerland, EU and U . S . As a result, the Group is exposed to a variety of financial risks, such as foreign exchange rate risk, credit risk, liquidity risk, cash flow and interest rate risk . The Group’s overall financial risk management program focuses on the unpredictability of financial markets and seeks to minimize potential adverse effects on the financial performance of the Group . The Group is not exposed to market price development as it has no saleable products .

15 The following is a summary of how we manage and mitigate the key financial risks : • Foreign exchange risk : In order to reduce its foreign exchange exposure, Molecular Partners may enter into currency contracts (forwards and options) with selected high - quality financial institutions to hedge against foreign currency exchange rate risks . The Group’s primary exposure to financial risk is due to fluctuation of exchange rates between CHF, EUR, GBP and USD . The Group’s hedging policy is ( 1 ) to maximize natural hedging by matching expected future cash flows in the different currencies and ( 2 ) if markets conditions allow, to consider hedging certain of the remaining expected net currency exposure as the need arises . However, due to market volatilities, the impact of negative interest rates in Switzerland and uncertainties in the cash flows, a 100 % hedging of the currency exposure is impossible or not appropriate . Molecular Partners does not engage in speculative transactions . • Interest rate risk : Molecular Partners earns interest income and pays negative interest on cash and cash equivalents and its profit and loss may be influenced by changes in market interest rates . The Group is investing part of its cash in short - term time deposits in line with its treasury guidelines . • Credit risk : The maximum credit risk on financial instruments corresponds to the carrying amounts of the Group’s cash and cash equivalents and receivables . The Group has not entered into any guarantees or similar obligations that would increase the risk over and above the carrying amounts . All cash and cash equivalents are held with three major Swiss banks with ratings between A and AAA as per Standard & Poor’s . The Group enters into partnerships with partners which have the appropriate credit history and a commitment to ethical business practices . Other receivables with credit risk mainly include interest receivables . • Liquidity risk : Based on the Group’s Business Plan 2021 - 2025 , management estimates that the Group is financed into 2023 . F i n a n c i a l O ut l oo k 2021 For the full year 2021 , at constant exchange rates, the company continues to expect total expenses of CHF 65 - 75 million, of which around CHF 7 million will be non - cash effective costs . In terms of cash outflow the company expects a gross cash utilization of CHF 85 - 95 million for FY 2021 , which includes a total of CHF 20 million payable to Novartis for the manufacturing of commercial supply (of which CHF 10 . 5 million occurred during H 1 2021 ) . This cash flow guidance does not include any potential receipts from R&D partnerships . With CHF 174 . 3 million cash at hand and no debt as per June 30 , 2021 the company expects to be funded into H 2 2023 , excluding any potential receipts from R&D partners . F i n a n c i a l C a l e n d a r 2021 The following table summarizes the scheduled financial calendar for the financial year 2021 . Date: Event: O c t o b e r 28 , 2021 I n t e r i m M a n a g eme n t S t a t eme n t Q 3 2021 D e c em b e r 15 , 2021 V i r t u a l R & D D ay


17 Condensed consolidated interim financial statements (unaudited) Condensed consolidated interim statement of financial position as of June 30, 2021 December 31, 2020 in CHF thousands Note Assets Property, plant and equipment 8,621 9,387 Intangible assets 297 347 Total non - current assets 8,918 9,734 Short - term time deposits 47,699 40,000 Prepaid expenses and accrued income 5.5 15,892 1,254 Trade and other receivables 7,912 2,837 Cash and cash equivalents 126,566 133,721 Total current assets 198,069 177,812 Total assets 206,987 187,546 Shareholders' equity and liabilities Share capital 5.6 3,227 2,915 Additional paid - in capital 5.6 353,621 299,479 Cumulative losses (222,210) (195,174) Total shareholders' equity 134,638 107,220 Contract liability 5.4 1,544 2,939 Lease liability 5,445 6,039 Employee benefits 5.11 7,741 13,678 Total non - current liabilities 14,730 22,656 Trade and other payables 10,514 5,825 Accrued expenses 9,754 7,718 Contract liability 5.4 36,167 42,948 Lease liability 1,184 1,179 Total current liabilities 57,619 57,670 Total liabilities 72,349 80,326 Total shareholders' equity and liabilities 206,987 187,546 See accompanying notes, which form an integral part of these unaudited condensed consolidated interim financial statements.

18 Condensed consolidated interim statement of comprehensive loss for the 6 months ended June 30, 2021 2020 in CHF thousands Note Revenues and other income Revenues from research and development collaborations 5.1 4,028 7,516 Other income 5.2 389 — Total revenues and other income 4,417 7,516 Operating expenses Research and development expenses (31,581) (25,141) Selling, general and administrative expenses (7,629) (5,467) Total operating expenses 5.3 (39,210) (30,608) Operating result (34,793) (23,092) Financial income 5.9 1,545 333 Financial expenses 5.9 (319) (1,976) Net finance result 1,226 (1,643) Result before income taxes (33,567) (24,735) Income taxes 5.10 — (13) Net result, attributable to shareholders (33,567) (24,748) Other comprehensive result Items that will not be reclassified to profit or loss Remeasurement of net pension liabilities, net of tax 5.11 6,527 (60) Items that are or may be reclassified subsequently to profit or loss Exchange differences on translating foreign operations 3 (15) Other comprehensive result, net of tax 6,530 (75) Total comprehensive result, attributable to shareholders (27,037) (24,823) Basic and diluted net result per share 5.12 (1.13) (1.14) See accompanying notes, which form an integral part of these unaudited condensed consolidated interim financial statements.
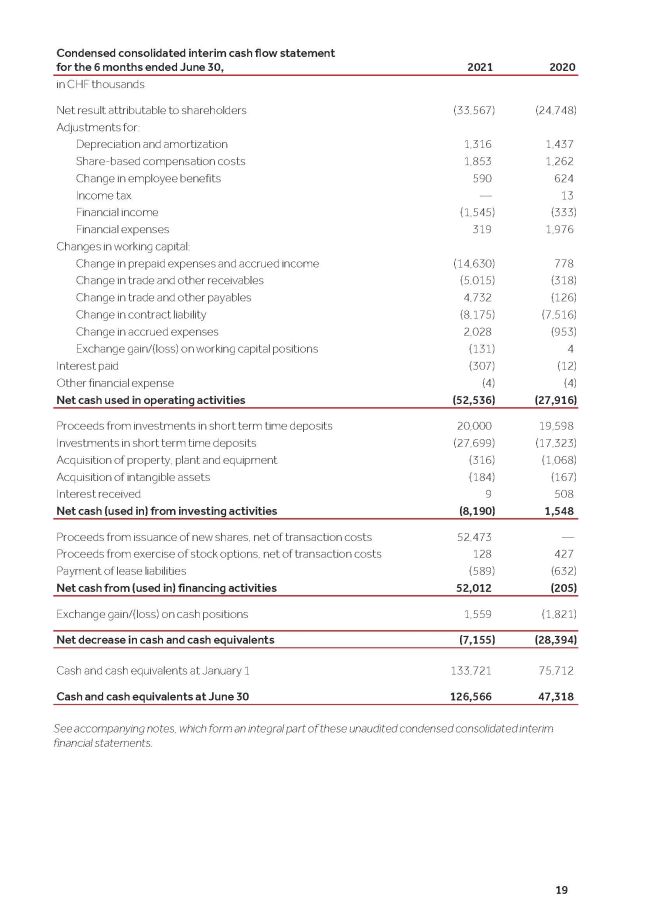
19 Condensed consolidated interim cash flow statement for the 6 months ended June 30, 2021 2020 in CHF thousands Net result attributable to shareholders (33,567) (24,748) Adjustments for: Depreciation and amortization 1,316 1,437 Share - based compensation costs 1,853 1,262 Change in employee benefits 590 624 Income tax — 13 Financial income (1,545) (333) Financial expenses 319 1,976 Changes in working capital: Change in prepaid expenses and accrued income (14,630) 778 Change in trade and other receivables (5,015) (318) Change in trade and other payables 4,732 (126) Change in contract liability (8,175) (7,516) Change in accrued expenses 2,028 (953) Exchange gain/(loss) on working capital positions (131) 4 Interest paid (307) (12) Other financial expense (4) (4) Net cash used in operating activities (52,536) (27,916) Proceeds from investments in short term time deposits 20,000 19,598 Investments in short term time deposits (27,699) (17,323) Acquisition of property, plant and equipment (316) (1,068) Acquisition of intangible assets (184) (167) Interest received 9 508 Net cash (used in) from investing activities (8,190) 1,548 Proceeds from issuance of new shares, net of transaction costs 52,473 — Proceeds from exercise of stock options, net of transaction costs 128 427 Payment of lease liabilities (589) (632) Net cash from (used in) financing activities 52,012 (205) Exchange gain/(loss) on cash positions 1,559 (1,821) Net decrease in cash and cash equivalents (7,155) (28,394) Cash and cash equivalents at January 1 133,721 75,712 Cash and cash equivalents at June 30 126,566 47,318 See accompanying notes, which form an integral part of these unaudited condensed consolidated interim financial statements.

20 Condensed consolidated interim statement of changes in equity in CHF thousands Share capital Additional paid - in capital Cumulative losses Total shareholders' equity At January 1, 2020 2,160 182,849 (130,870) 54,139 Net result — — (24,748) (24,748) Remeasurement of net pension liabilities — — (60) (60) Exchange differences on translating foreign operations — — (15) (15) Total comprehensive income — — (24,823) (24,823) Share - based compensation costs (1) — 1,262 — 1,262 Exercise of stock options, net of transaction costs 21 406 — 427 At June 30, 2020 2,181 184,517 (155,693) 31,005 At January 1, 2021 2,915 299,479 (195,174) 107,220 Net result — — (33,567) (33,567) Remeasurement of net pension liabilities — — 6,527 6,527 Exchange differences on translating foreign operations — — 3 3 Total comprehensive income — — (27,037) (27,037) Share - based compensation costs (1) — 1,853 — 1,853 Issuance of new shares, net of transaction costs (2) 300 52,173 — 52,473 Exercise of stock options, net of transaction costs 12 116 — 128 At June 30, 2021 3,227 353,621 (222,210) 134,638 (1) See n o te 5.8 (2) See n o te 5.5 See accompanying notes, which form an integral part of these unaudited condensed consolidated interim financial statements.

21 Explanatory notes to the condensed consolidated interim financial statements 1. G e n er a l I n f o r m a t i o n Molecular Partners AG ("Company'") and its subsidiary Molecular Partners Inc. (collectively "Molecular Partners", "Group") is a clinical stage biopharmaceutical company applying its pioneering DARPin ® product candidates to treat serious diseases, with a current focus on infectious disease, oncology and ophthalmology. The Company was founded on November 22, 2004, and is domiciled at Wagistrasse 14, 8952 Schlieren, Canton of Zurich, Switzerland. It is subject to the provisions of the articles of association and to article 620 et seq. of the Swiss Code of Obligations, which describe the legal requirements for limited companies (“Aktiengesellschaften”). Molecular Partners Inc. is a wholly owned subsidiary of Molecular Partners AG. Molecular Partners Inc. was incorporated in the United States in the State of Delaware on October 8, 2018. Molecular Partners Inc. is based in Cambridge, Massachusetts. The unaudited condensed consolidated interim financial statements for the six months ended June 30, 2021 were approved for issuance by the Board of Directors on August 25, 2021. On June 15, 2021 the Company announced the pricing of its initial public offering in the United States of 3,000,000 American Depositary Shares (“ADSs”) at a public offering price of $21.25 per ADS, for total gross proceeds of approximately $63.8 million. Each ADS represents one Molecular Partners ordinary share. Trading in the Company's ADSs on the Nasdaq Global Select Market takes place under the ticker symbol “MOLN' and started on June 16, 2021. The Company’s shares are listed on the SIX Swiss Exchange (Ticker: MOLN) since November 5, 2014. 2. B a s i s o f P re p a r a t i o n These unaudited condensed consolidated interim financial statements have been prepared in accordance with IAS 34 Interim Financial Reporting and should be read in conjunction with the Group's last annual consolidated financial statements as at and for the year ended December 31, 2020. They do not include all the information required for a complete set of consolidated financial statements prepared in accordance with IFRS as issued by the IASB. However, selected explanatory notes are included to explain events and transactions that are significant to gain an understanding of the changes in the Group's financial position and performance since the last annual consolidated financial statements as at and for the year ended December 31, 2020. The accounting policies set forth in the notes to those annual consolidated financial statements have been consistently applied to all periods presented, except as per below. The condensed consolidated interim financial statements are presented in thousands of Swiss Francs (TCHF), unless stated otherwise. In the course of the six months ended June 30, 2021 there were no significant events, transactions or changes in estimates that had a material impact on the condensed consolidated interim financial statements. The Group is monitoring the situation surrounding the COVID - 19 pandemic and its potential impact on patients, the team, the partners and the business. During the six months period ended June 30, 2021 as well as of the reporting date there were no major disruptions to the operations. The Group continues to comply with all local and federal instructions as it relates to the safety of our employees, patients, and citizens. The business is not subject to any seasonality. Revenues largely depend on the underlying alliance contracts and the achievement of agreed milestones, while expenses are largely affected by the phase of the respective projects, particularly with regard to external research and development expenditures.

22 Due to rounding, the numbers presented in the financial statements might not precisely equal the accompanying notes. 3. New or Revised IFRS Standards and Interpretations A number of new or amended standards became applicable for annual periods beginning on or after January 1, 2021. These standards did not have any significant impact on the Group’s accounting policies and did not require any retrospective adjustments. 4. Critical Accounting estimates and judgment The condensed consolidated interim financial statements have been prepared under the historical cost convention. In preparing these condensed consolidated interim financial statements management made judgments, estimates and assumptions that affect the application of accounting policies and the reported amounts of assets and liabilities, income and expenses. Actual results may differ from these estimates. The area involving a higher degree of judgment or complexity, or an area where assumptions and estimates are significant to the consolidated financial statements is revenue recognition. 5. O th er e x p l a n a t o r y n o t e s 1. Revenue The Group assesses and estimates the progress of its projects with alliance partners at each reporting date. The Group applies the cost based method which recognizes revenue based on the ratio of the associated costs incurred to date and the total forecasted cost to satisfy the performance obligation. In the first quarter of 2021 the Group increased its estimate of the total future costs required to satisfy the performance obligation under the Amgen collaboration. This change in estimate affects the allocation of revenue over time and has no impact on the total amount recognized or to be recognized into revenue under the agreement with Amgen. This increase in the total estimated future costs resulted in a lower amount of recognized revenue for the six months period ended June 30, 2021, as compared to the comparable prior year period. The increase in total estimated future costs is primarily related to continued development of various dosing schedules under phase 1a of the collaboration. In October 2020, the Group entered into a contract with Novartis Pharma AG ("Novartis"), granting Novartis the exclusive option to in - license global rights in relation to drug candidates MP0420 and MP0423. Under the terms of the agreement, in 2020 the Group has received an upfront, non - refundable fee of CHF 20 million for the tech transfer and manufacturing of MP0420. The Group has equally committed to utilize up to the maximum amount of this upfront fee for the manufacturing of the commercial supply for MP0420. Any such amount which is paid for manufacturing performed by the Novartis Group is considered to be a consideration payable to a customer. Given the significant inter - dependencies between the upfront fee and the manufacturing activities, the manufacturing costs paid to the Novartis Group are to be offset against the upfront non - refundable fee from the contract . During the six months ended June 30, 2021, costs paid to the Novartis Group for the manufacturing of the drug product to establish the commercial supply of MP0420 in the amount of TCHF 4,147 have b ee n o ff s e t a g a i n s t t h e u p f r o n t n o n - r e f u n d a b l e f ee ( s ee n o t e 5 . 4 ) .
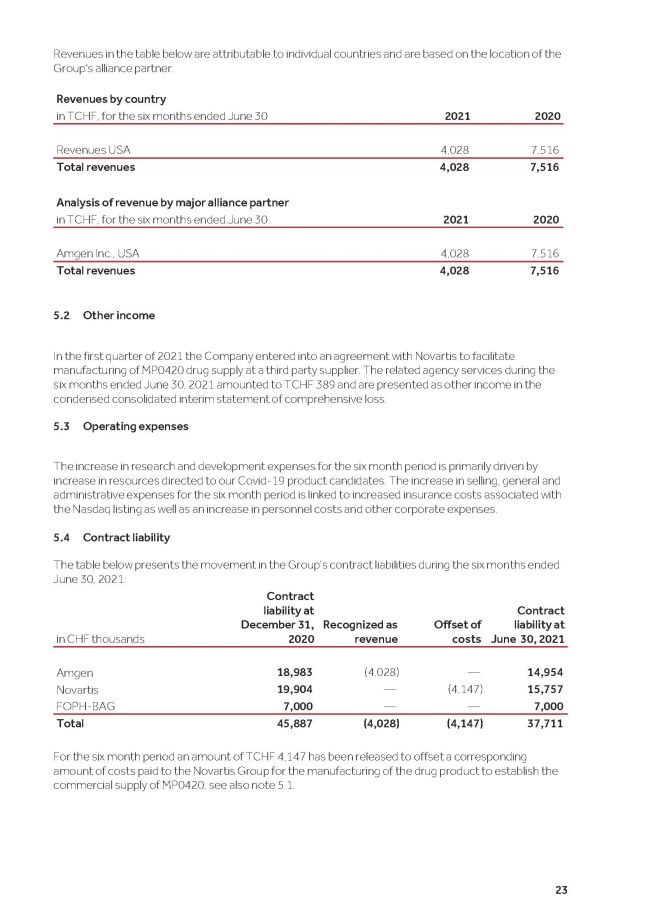
23 Revenues in the table below are attributable to individual countries and are based on the location of the Group’s alliance partner. Revenues by country in TCHF, for the six months ended June 30 2021 2020 Revenues USA 4,028 7,516 Total revenues 4,028 7,516 Analysis of revenue by major alliance partner in TCHF, for the six months ended June 30 2021 2020 Amgen Inc., USA 4,028 7,516 Total revenues 4,028 7,516 5.2 Other income In the first quarter of 2021 the Company entered into an agreement with Novartis to facilitate manufacturing of MP0420 drug supply at a third party supplier. The related agency services during the six months ended June 30, 2021 amounted to TCHF 389 and are presented as other income in the condensed consolidated interim statement of comprehensive loss. 3. O p er a t i n g e x p e n s e s The increase in research and development expenses for the six month period is primarily driven by increase in resources directed to our Covid - 19 product candidates. The increase in selling, general and administrative expenses for the six month period is linked to increased insurance costs associated with the Nasdaq listing as well as an increase in personnel costs and other corporate expenses. 4. C o nt r a c t l i a b i l i t y The table below presents the movement in the Group's contract liabilities during the six months ended June 30, 2021: Contract in CHF thousands liability at December 31, 2020 Recognized as revenue Offset of costs Contract liability at June 30, 2021 Amgen 18,983 (4,028) — 14,954 Novartis 19,904 — (4,147) 15,757 FOPH - BAG 7,000 — — 7,000 Total 45,887 (4,028) (4,147) 37,711 For the six month period an amount of TCHF 4,147 has been released to offset a corresponding amount of costs paid to the Novartis Group for the manufacturing of the drug product to establish the commercial supply of MP0420, see also note 5.1.

24 in CHF thousands Current Non - current Contract liability Amgen 13,410 1,544 14,954 Novartis 15,757 — 15,757 FOPH - BAG 7,000 — 7,000 Balance at June 30, 2021 36,167 1,544 37,711 5. P re p a i d e x p e n s e s a n d a cc r u e d i n c o m e During the six month period ended June 30, 2021 the Group recorded a prepayment of CHF 8.7 million related to the reservation of resins arising from the agreement with Novartis for the manufacturing of commercial supply for MP0420. Accordingly, the prepayment will be released to expense as the resins are received by Novartis and an offsetting reduction to the contractual liability will be recorded. The Group also recorded a prepayment of CHF 4.8 million for Directors & Officers insurance following the Group's listing on the Nasdaq. 6. I ss u a n c e s o f e q u i t y s e c u r i t i e s On June 15, 2021 the Company announced its initial public offering on the Nasdaq of an aggregate of 3,000,000 new ordinary shares, comprising 3,000,000 American Depositary Shares (“ADSs”) at a price of $21.25 per ADS. The total gross proceeds in the offering amounted to $63.8 million (CHF 58.8 million). After deducting transaction costs of CHF 6.3 million the Group reports net proceeds of CHF 52.2 million under additional paid in capital and CHF 0.3 million as increase on share capital on the consolidated statement of financial position as per June 30, 2021. As of June 30, 2021 as a result of the issuance of new shares to support the initial public offering on the Nasdaq together with the exercise of employee stock options and the vesting of Performance Share Units ("PSUs" ) and Restricted Share Units ("RSUs") under the 2018 Plan (please see also note 5.8), the outstanding issued share capital of the Company amounted to CHF 3,226,929 divided into 32,269,285 fully paid registered shares. 7. Dividends The Group has paid no dividends since its inception and does not anticipate paying dividends in the foreseeable future. 8. S h a re - b a s e d c o m p e n s a t i o n As of June 30, 2021, 341,816 options were outstanding (December 31, 2020: 382,059 options) under all active option plans. As of June 30, 2021 and December 31, 2020 all options had vested . As of June 30, 2021 a total of 563,485 PSUs and 95,635 RSUs were outstanding, of which none were vested (as of December 31, 2020 a total of 445,198 PSUs and 87,906 RSUs were outstanding, of which also none were vested). The movements in the number of share - based awards (options, RSUs and PSUs) outstanding during the six month period ended June 30, 2021 is as follows:

Share options / PSU/ RSU movements Total number s Weighte d average exercise price (CHF) Options (numbers ) Weighte d average exercise price (CHF) PSU / RSU (numbers) Weighte d average exercise price (CHF) Balance outstanding at January 1, 2021 915,163 2.74 382,059 6.42 533,104 0.10 Granted 222,903 0.10 — — 222,903 0.10 (Performance adjustment ) 1 (1,022) 0.10 — — (1,022) 0.10 (Forfeited) 2 (13,815) 0.10 — — (13,815) 0.10 (Expired) — — — — — — (Exercised options), vested PSU / RSU (122,293) 1.05 (40,243) 3.00 (82,050) 0.10 Balance outstanding at June 30, 2021 1,000,936 2.40 341,816 6.82 659,120 0.10 The share - based compensation costs recognized during the six months ended June 30, 2021 amounted to TCHF 1,853 (TCHF 1,262 for the six months ended June 30, 2020). 5.9 Financial income and expense Financial income in CHF thousands, for the six months ended June 30 2021 2020 Interest income on financial assets held at amortized cost 17 333 Net foreign exchange gain 1,528 — Total 1,545 333 Financial expense in CHF thousands, for the six months ended June 30 2021 2020 Net foreign exchange loss — (1,922) Negative interest on financial assets held at amortized costs (288) — Interest expense on leases (27) (10) Other financial expenses (4) (44) Total (319) (1,976) Exchange results primarily represent unrealized foreign exchange results on the cash and short - term time deposit balances held in USD, GBP and in EUR, respectively. 5 . 10 I n c o m e t a x e s The Company had tax loss carry - forwards in Switzerland of TCHF 157,900 as of December 31, 2020. No deferred tax assets have been recognized for these tax losses carry forwards, because it is not probable that such loss carry forwards can be utilized in the foreseeable future. In addition, no deferred tax assets were recognized on other deductible temporary differences (e.g. pension liabilities under IAS 19) for the same reasons. 1 Performance adjustments indicate forfeitures due to non - market performance conditions not achieved 2 F o r f e i t e d d u e to s e r v i c e c o n d i t i o n s n o t f u l f i ll e d 25
11. O th er c o m p re h e n s i v e re s u l t In order to recognize remeasurements of the net defined benefit obligation in the period in which they arise, the Group adopted as its accounting policy to have independent actuaries to update the calculation of the defined benefit obligation and plan assets as at each interim reporting date. The significant components of the remeasurement in the six months period ended June 30, 2021 relate to changes in demographic assumptions by adopting the new BVG / LPP 2020 tables for the first time, the increase in the discount rate, an adjustment for members who left the plan plus an improved performance of the plan's assets. 12. E a r n i n g s p er s h a re Basic net result per share is calculated by dividing the net result attributable to shareholders by the weighted average number of shares issued and outstanding during the interim periods presented, excluding any shares held as own shares. Diluted net result per share additionally takes into account the potential conversion of all dilutive potential ordinary shares. For the interim period ended June 30, 2021 and 2020 there are no dilutive effects. f o r t h e s i x m o n t h s e n d e d J u n e 30 2021 2020 Weighted average number of shares used in computing basic and diluted loss per share 29 , 705 , 254 21 , 708 , 541 13. R e l a t e d p a r t i e s The Group did not enter into any related party transactions in the interim period presented. 14. E v e nt s a f t er th e b a l a n c e s h ee t d a t e In August 2021, the Group was informed by its collaboration partner, AbbVie Inc. of the termination of the license and collaboration agreement for the investigational drug abicipar pegol for the treatment of nAMD and DME. As such, Molecular Partners will regain the development and commercial rights of abicipar on a worldwide basis. No other events occurred between the balance sheet date and the date on which these condensed consolidated interim financial statements were approved by the Board of Directors that would require adjustment to these condensed consolidated interim financial statements or disclosure under this section. 26


© 2021 KPMG AG, a Swiss corporation, is a subsidiary of KPMG Holding AG, which is a member of the KPMG global organization of independent member firms affiliated with KPMG International Lim - ited, a private English company limited by guarantee . All rights reserved . KPMG AG Badenerstrasse 172 PO Box CH - 8036 Zurich +41 58 249 31 31 kpmg.ch Report of the Independent Auditor on the Condensed Consolidated Interim Financial Statements to the Board of Directors of Molecular Partners AG, Schlieren Introduction We have been engaged to review the accompanying condensed consolidated interim statement of financial position of Molecular Partners AG as of June 30 , 2021 , and the related condensed consolidated interim statement of comprehensive loss, cash flow statement and statement of changes in equity for the six - month period then ended and selected explanatory notes (the condensed consolidated interim financial information) on pages 17 - 26 . The Board of Directors is responsible for the preparation and presentation of these condensed consolidated interim financial statements in accordance with IAS 34 , Interim Financial Reporting . Our responsibility is to express a conclusion on these condensed consolidated interim financial statements based on our review . Scope of Review We conducted our review in accordance with the International Standard on Review Engagements 2410 , Review of Interim Financial Information Performed by the Independent Auditor of the Entity . A review of condensed interim consolidated financial statements consists of making inquiries, primarily of persons responsible for financial and accounting matters, and applying analytical and other review procedures . A review is substantially less in scope than an audit conducted in accordance with International Standards on Auditing and consequently does not enable us to obtain assurance that we would become aware of all significant matters that might be identified in an audit . Accordingly, we do not express an audit opinion . Conclusion Based on our review, nothing has come to our attention that causes us to believe that the accompanying condensed consolidated interim financial statements as of June 30 , 2021 and for the six - month period then ended are not prepared, in all material respects, in accordance with IAS 34 , Interim Financial Reporting . KPMG AG Michael Blume Licensed Audit Expert Auditor in Charge Gregory Puccetti Zurich, 25 August 2021

CAUTIONARY NOTE REGARDING FORWARD - LOO KING STATEMENTS : Any statements contained in this press release that do not describe historical facts mayconstitute forward - looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995 , as amended , including , without limit at ion , implied and express statements regarding the c linic al development of Molecular Par t ners ' current or future product candidates , including timing for the potential submission of emergency use authorization for ensovibep , expectations regarding timing for reporting data from ongoing clinical trials or the initiation of future clinical tri als , the potential therapeutic andclinical benefits of Molecular Partners' product candidates , the selection and development of future antiviral or other program s , and Molecular Par t ners ' e x pected expenses and cash utilization for 2021 and that its current cash resources will be sufficient to fund its operations and capital expenditure requirements into H22023. These statements may be identified by words such as " ant icipate ", " believe ", " could" , " expect " , " intend" , " m ay ", "plan ", " potential" , " will ", "would " and similar expression s , although not all forward - looking statements maycontain these identifying words , and are based on Molecular Partners AG ' s current beliefs and expectat ions . These statements involve risks and uncertainties that could cause actual results to differ materially from those reflected in such st atement s . Some of the key factors that could cause actual results to differ from our expectations include our plans to develop and potentially commercialize our product candidates ; our reliance on third party partners andcollaborators over which we may not always have full control ; our ongoing and planned clinical trials andpreclinical studies for our product candidates , including the timing of such trials and studies ; the risk that the results of preclinical studies and clinical trials maynot be predictive of future results in connection with future clinical t rials ; the timing of andour ability to obtain andmaintain regulatory approvals for our product candidates ; the extent of clinical trials potentially required for our product candidates; the clinical utility andability to achieve market acceptance of our product candidates ; the potential impact of the COVID19 pandemic on our operations or clinical t rials ; our plans and development of any new indications for our product candidates; our commercialization , marketing and manufacturing capabilities and st rategy ; our intellectual property posit ion ; our ability to identify andin - license additional product candidates ; the adequacy of our cash resources and our anticipated cash ut ili zat ion ; and other risks and uncertainties that aredescribed in the Risk Factors section of Molecular Partners' Registration Statement on Form F - 1 filed with Securities and Exchange Commission (SEC) on June 14 , 2021 and other filings Molecular Partners makes with the SEC . These documents are available on the Investors page of Molecular Par t ners ' website at ht tp : //www . molecularpar t ners . com . Any forward - looking statements speak only as of the date of this press release and are based on information available to Molecular Partners as of the date of this release , and Molecular Partners assumes no obligation to , anddoes not intend to , update any forward - looking st atem ent s , whether as a result of newinformat ion , future events or ot herwise . Molecular Partners AG Wagistrasse 14 8952 Zurich - Schlieren Switzerland Phone : +41 44 755 77 00 Fa x : +414 4 75 5 7 7 07 m olecularpa r t ner s . com MOLECULAR partners Custom - built biology for patients