
PRECISION GENETIC MEDICINES �THROUGH BASE EDITING NASDAQ: BEAM January 2025

Cautionary note regarding forward-looking statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements include statements regarding: the initiation, timing, progress and results of preclinical studies and research and development programs, including the initiation, design and progress of clinical trials, including trials for BEAM-101, BEAM-103, BEAM-301 and BEAM-302; the advancement of our pipeline and additional liver programs in multiple preclinical studies; our current expectations and anticipated results of operations, including our expected use of capital; our estimated cash, cash equivalents and marketable securities as of December 31, 2024 and our expectations related thereto; the sufficiency of our capital resources to fund operating expenses and capital expenditure requirements and the period in which such resources are expected to be available; and the therapeutic applications and potential of our technology, including our potential to develop life-long, curative, precision genetic medicines for patients through base editing, including potential safety advantages, all of which are subject to known and unknown important risks, uncertainties and other factors that may cause our actual results, performance or achievements, market trends, or industry results to differ materially from those expressed or implied by such forward-looking statements. Therefore, any statements contained herein that are not statements of historical fact may be forward-looking statements and should be evaluated as such. Without limiting the foregoing, the words "anticipate," "expect," "suggest," "plan," "vision," “strategy,” “possibility,” “promise,” "believe," "intend," "project," "forecast," "estimates," "targets," "projections," "potential," "should," "could," "would," "may," "might," "will," and the negative thereof and similar words and expressions are intended to identify forward-looking statements. Each forward-looking statement is subject to important risks and uncertainties that could cause actual results to differ materially from those expressed or implied in such statement, including, without limitation, risks and uncertainties related to: our ability to develop, obtain regulatory approval for, and commercialize our product candidates, which may take longer or cost more than planned; our ability to raise additional funding, which may not be available; our ability to obtain, maintain and enforce patent and other intellectual property protection for our product candidates; that preclinical testing of our product candidates and preliminary or interim data from preclinical studies and clinical trials may not be predictive of the results or success of ongoing or later clinical trials; that initiation and enrollment of our clinical trials may take longer than expected; that our product candidates or the delivery modalities we rely on to administer them may cause serious adverse events; the uncertainty that our product candidates will receive regulatory approval necessary to initiate or continue human clinical trials, that our product candidates may experience manufacturing or supply interruptions or failures; risks related to competitive products; whether our actual audited results will be consistent with our estimated cash, cash equivalents and marketable securities as of December 31, 2024; and the other risks and uncertainties identified under the headings "Risk Factors Summary" and "Risk Factors" and elsewhere in our annual report on Form 10-K for the year ended December 31, 2023, and in any subsequent filings with the Securities and Exchange Commission (the "SEC") which are available on the SEC's website at www.sec.gov. Additional information will be made available by our annual and quarterly reports and other filings that we make from time to time with the SEC. These forward-looking statements speak only as of the date of this presentation. Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them. We undertake no obligation to update any forward-looking statement, whether as a result of new information, future developments or otherwise, except as may be required by applicable law. .

OUR VISION IS TO PROVIDE LIFE-LONG CURES �for patients suffering from serious diseases POTENTIAL FOR �one-time, curative therapies GENE EDITING FOR �rare and common diseases PLATFORM FOR �rapidly programmable precision medicines
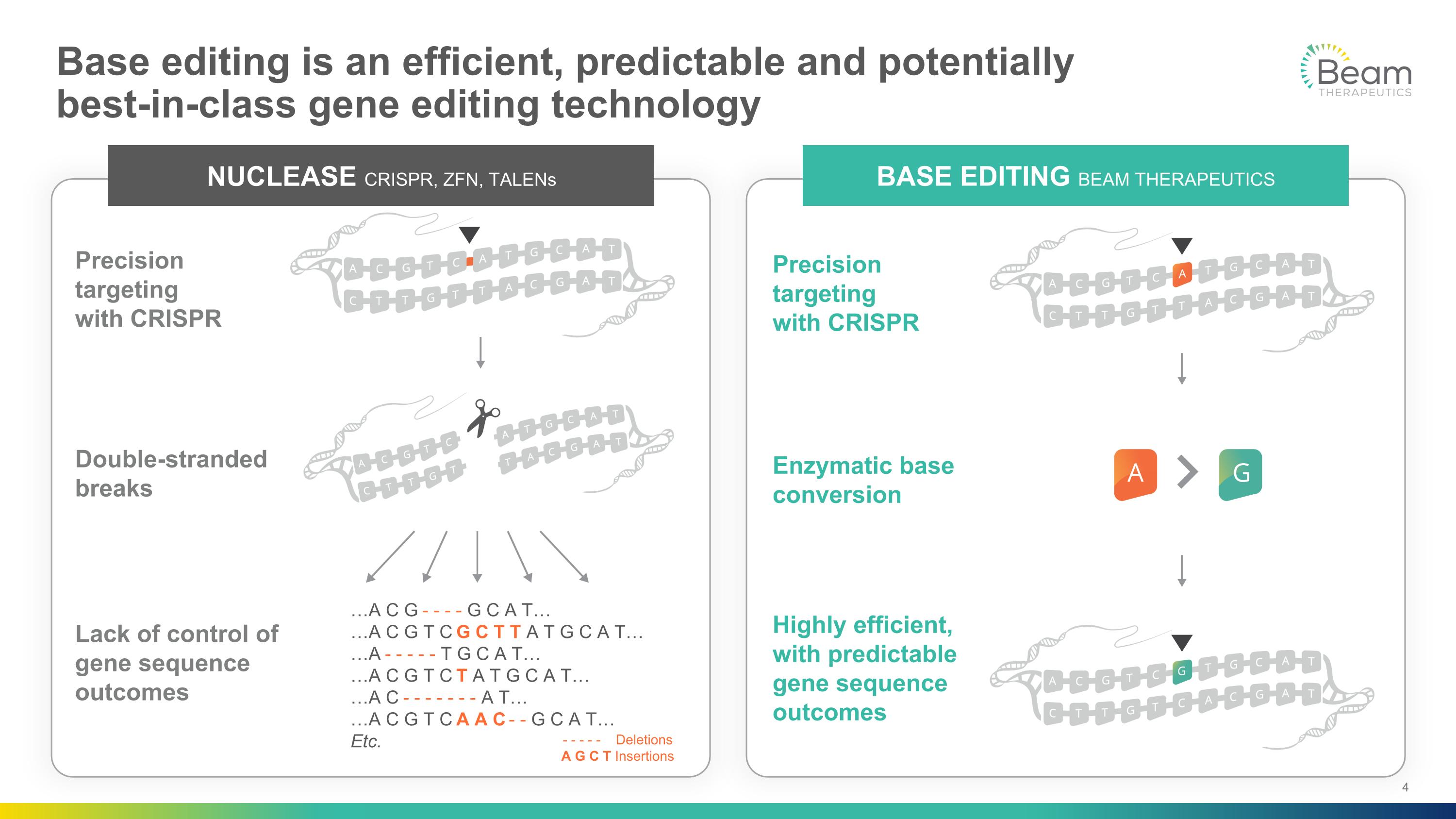
Base editing is an efficient, predictable and potentially�best-in-class gene editing technology NUCLEASE CRISPR, ZFN, TALENs Double-stranded breaks Lack of control of gene sequence outcomes Precision targeting�with CRISPR …A C G - - - - G C A T… …A C G T C G C T T A T G C A T… …A - - - - - T G C A T… …A C G T C T A T G C A T… …A C - - - - - - - A T… …A C G T C A A C - - G C A T… Etc. - - - - - Deletions A G C T Insertions Enzymatic base conversion Highly efficient, with predictable gene sequence outcomes Precision targeting�with CRISPR BASE EDITING BEAM THERAPEUTICS
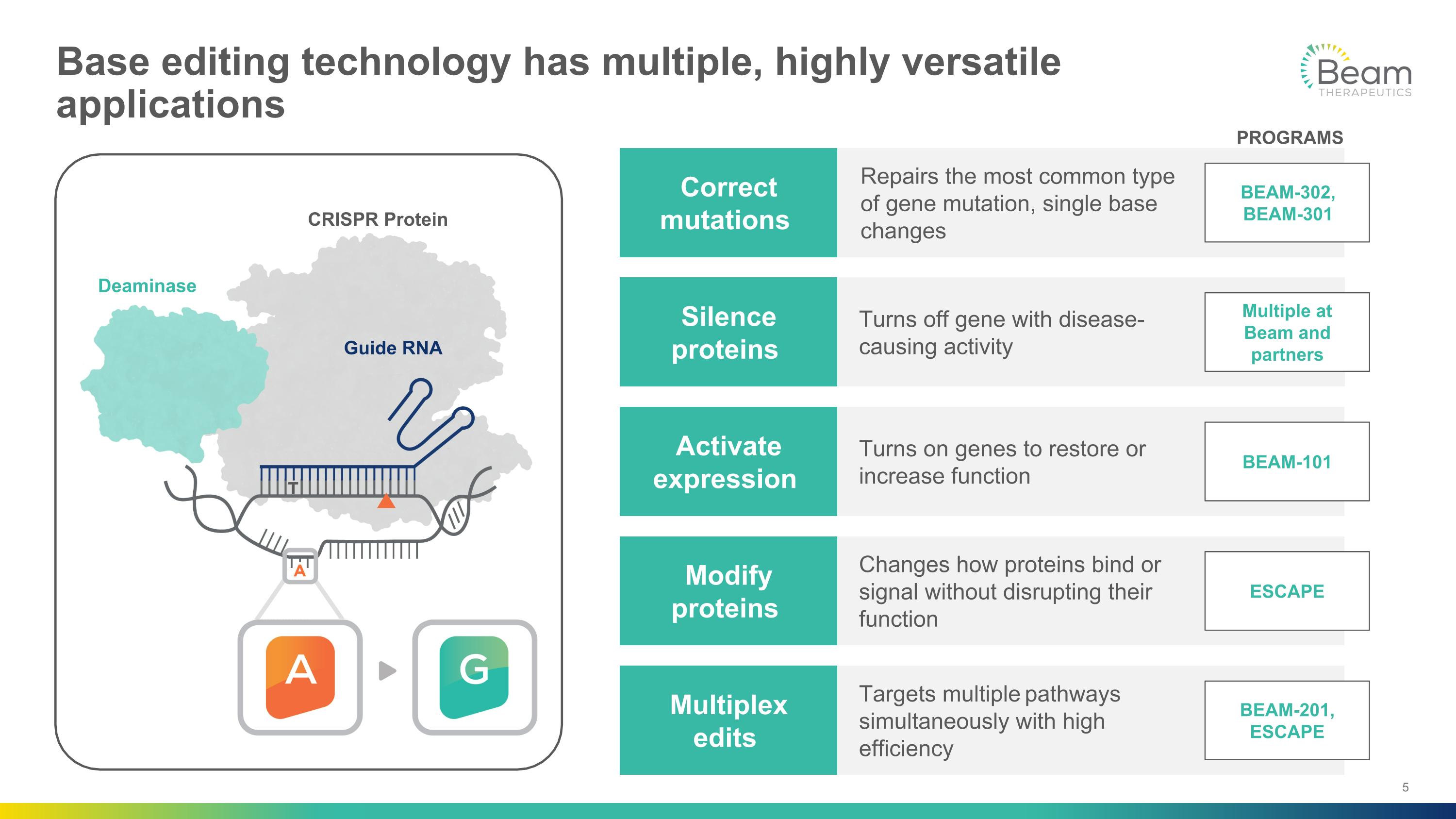
Base editing technology has multiple, highly versatile applications CRISPR Protein Deaminase Guide RNA Activate expression Silence proteins Correct mutations Multiplex edits Modify proteins Turns on genes to restore or increase function Turns off gene with disease-causing activity Repairs the most common type �of gene mutation, single base changes Targets multiple pathways simultaneously with high efficiency Changes how proteins bind or signal without disrupting their function BEAM-101 Multiple at Beam and partners BEAM-302, BEAM-301 BEAM-201, ESCAPE ESCAPE PROGRAMS
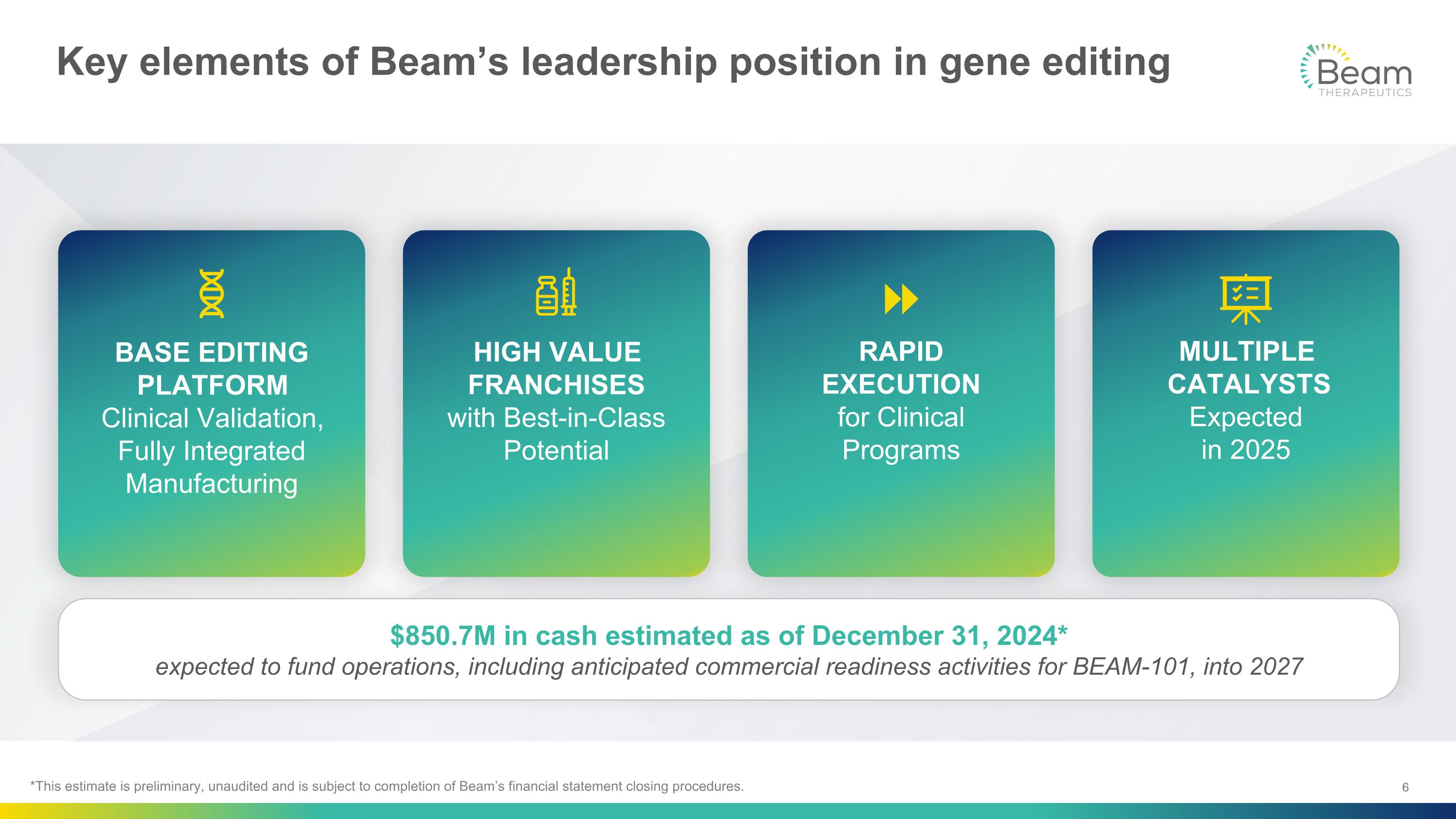
Key elements of Beam’s leadership position in gene editing BASE EDITING PLATFORM�Clinical Validation, Fully Integrated Manufacturing MULTIPLE CATALYSTS Expected �in 2025 RAPID EXECUTION�for Clinical Programs HIGH VALUE FRANCHISES�with Best-in-Class Potential $850.7M in cash estimated as of December 31, 2024* expected to fund operations, including anticipated commercial readiness activities for BEAM-101, into 2027 *This estimate is preliminary, unaudited and is subject to completion of Beam’s financial statement closing procedures.

A comprehensive, fully integrated base editing platform BASE EDITING PLATFORM�Clinical Validation, Fully Integrated Manufacturing FULLY INTEGRATED CAPABILITIES GENE EDITING AND DELIVERY TECHNOLOGIES Base editing ex vivo cell therapy in vivo LNP INTERNAL �GMP MANUFACTURING FACILITY Research Triangle, NC GMP manufacturing �at NC facility >100 GMP batches/ isolations Global regulatory filings 7 IND/CTA approvals 5 countries Global clinical operations 30+ clinical sites >20 patients treated Clinical proof of concept Clinical proof of concept
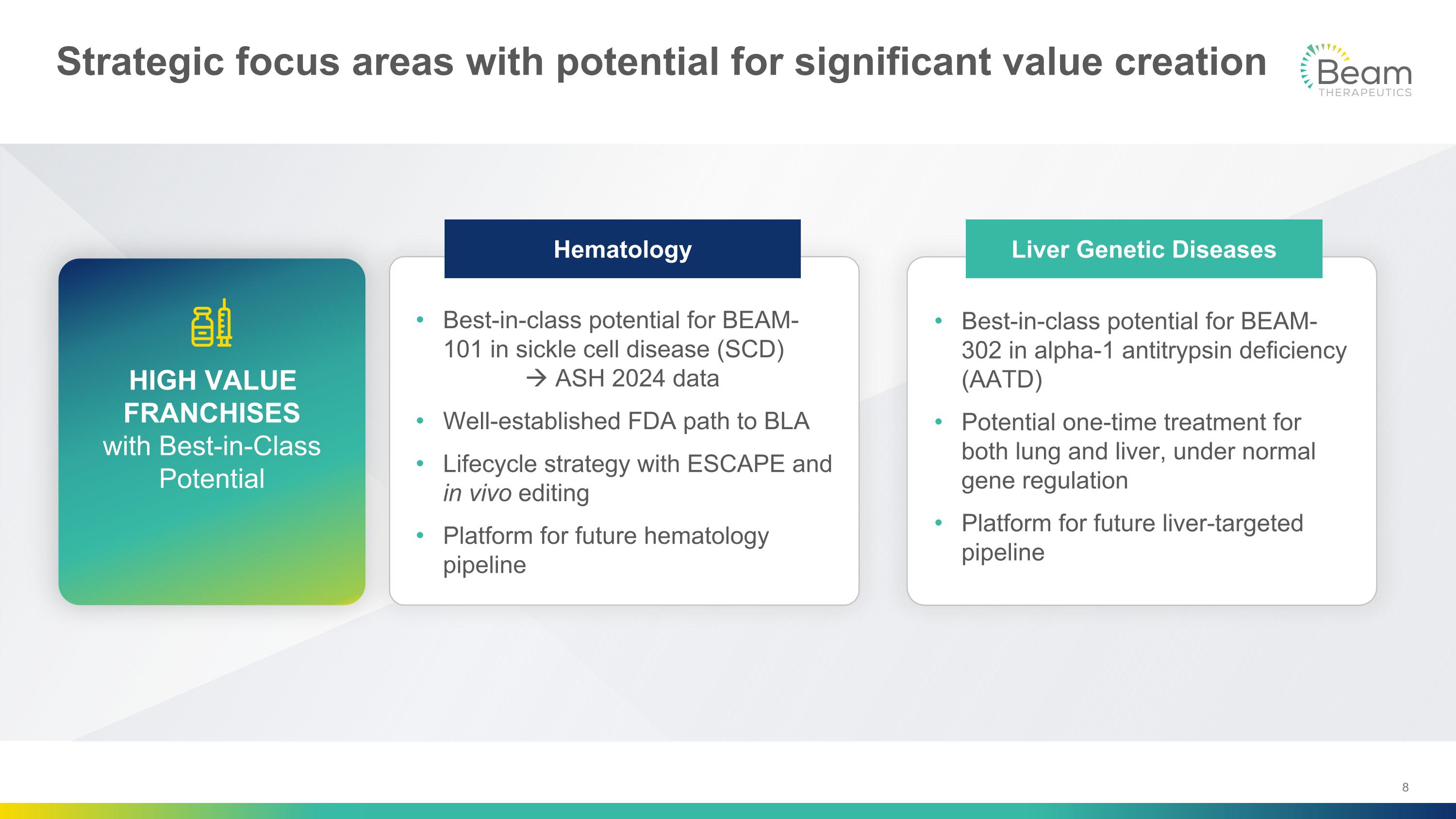
Strategic focus areas with potential for significant value creation Best-in-class potential for BEAM-101 in sickle cell disease (SCD)� ASH 2024 data Well-established FDA path to BLA Lifecycle strategy with ESCAPE and in vivo editing Platform for future hematology pipeline Best-in-class potential for BEAM-302 in alpha-1 antitrypsin deficiency (AATD) Potential one-time treatment for both lung and liver, under normal gene regulation Platform for future liver-targeted pipeline Hematology Liver Genetic Diseases HIGH VALUE FRANCHISES�with Best-in-Class Potential
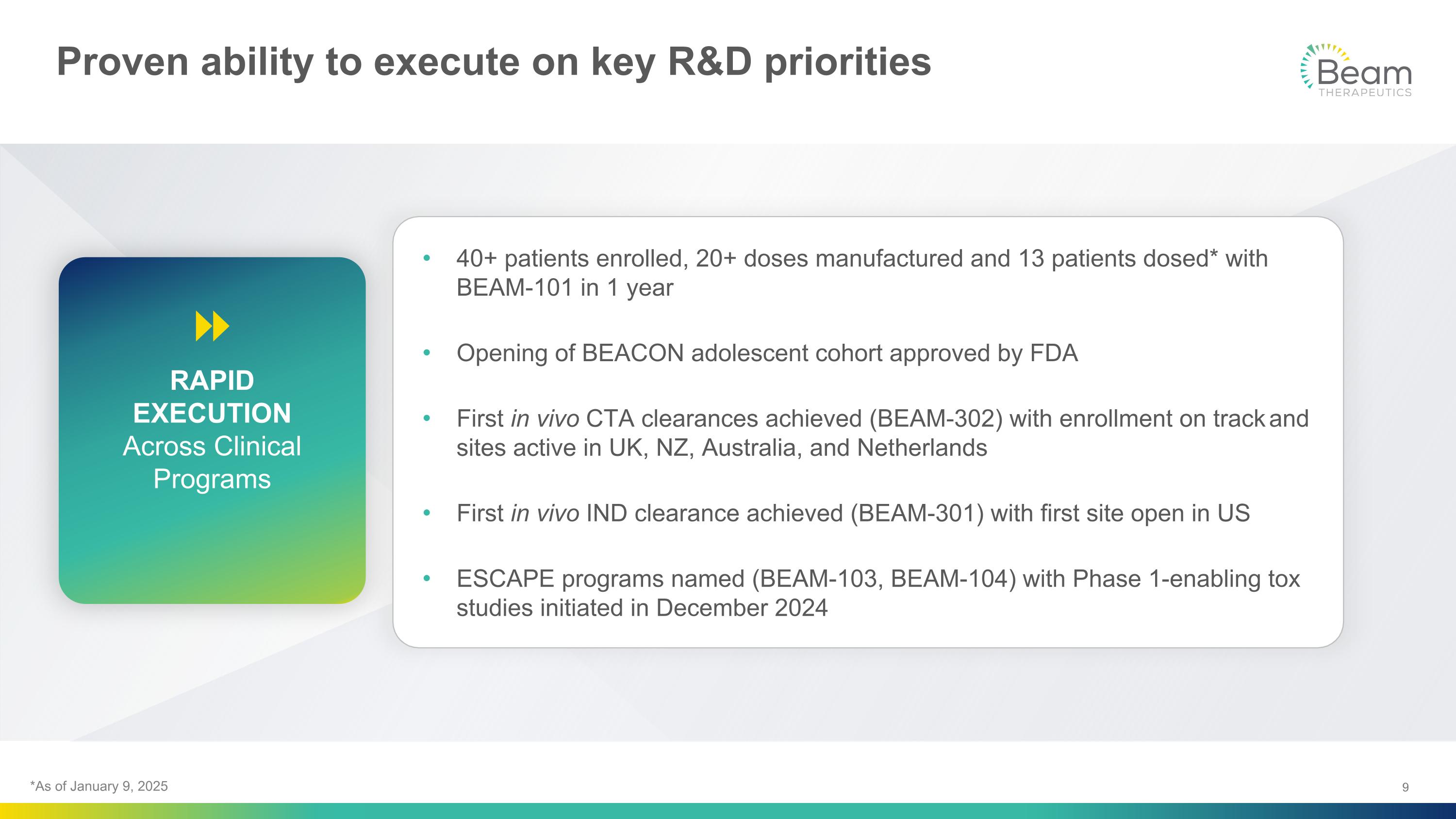
Proven ability to execute on key R&D priorities 40+ patients enrolled, 20+ doses manufactured and 13 patients dosed* with BEAM-101 in 1 year Opening of BEACON adolescent cohort approved by FDA First in vivo CTA clearances achieved (BEAM-302) with enrollment on track and sites active in UK, NZ, Australia, and Netherlands First in vivo IND clearance achieved (BEAM-301) with first site open in US ESCAPE programs named (BEAM-103, BEAM-104) with Phase 1-enabling tox studies initiated in December 2024 RAPID EXECUTION Across Clinical Programs *As of January 9, 2025
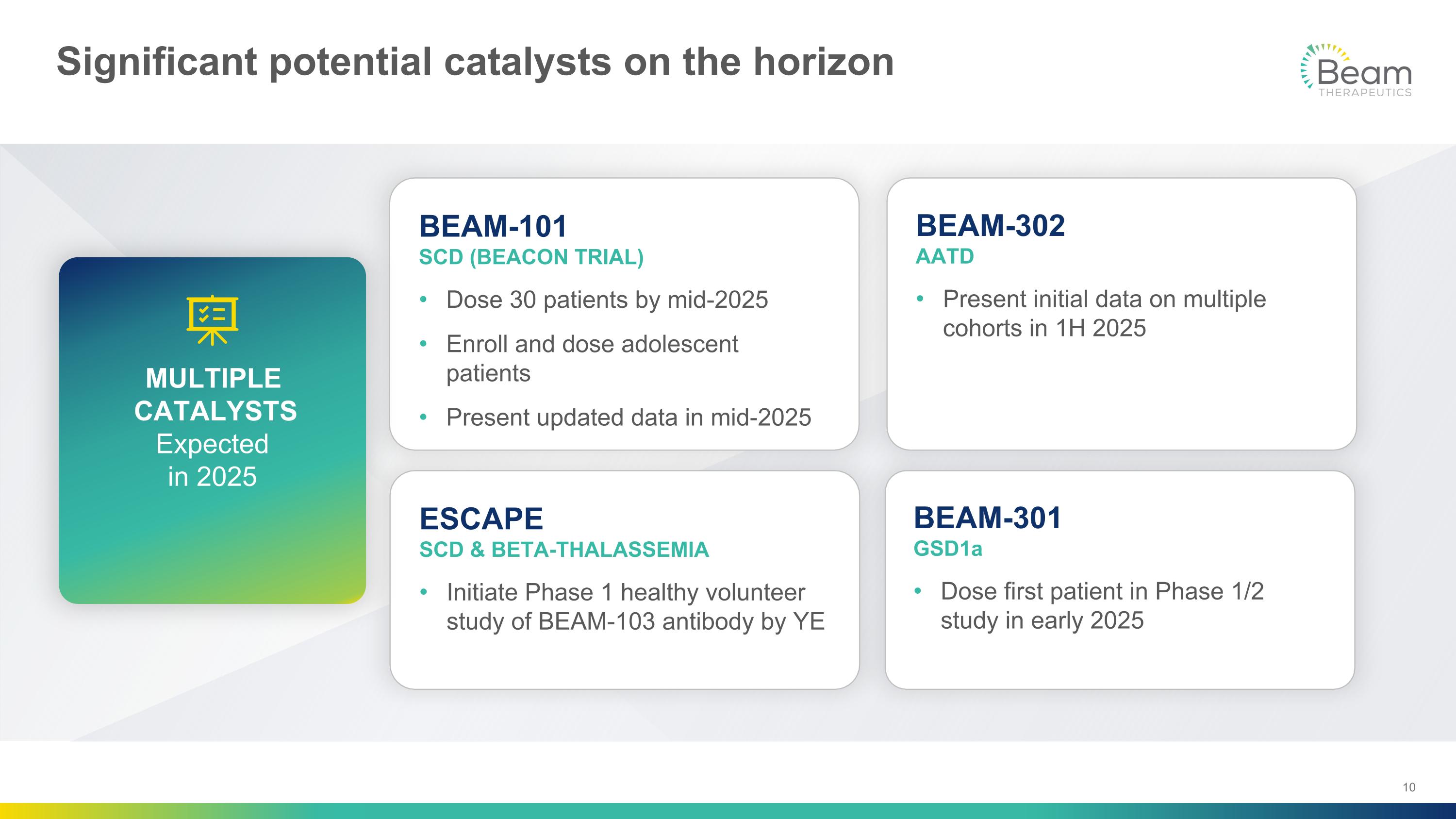
Significant potential catalysts on the horizon BEAM-101 SCD (BEACON TRIAL) Dose 30 patients by mid-2025 Enroll and dose adolescent patients Present updated data in mid-2025 BEAM-302�AATD Present initial data on multiple cohorts in 1H 2025 ESCAPE�SCD & BETA-THALASSEMIA Initiate Phase 1 healthy volunteer study of BEAM-103 antibody by YE BEAM-301�GSD1a Dose first patient in Phase 1/2 study in early 2025 MULTIPLE CATALYSTS Expected �in 2025
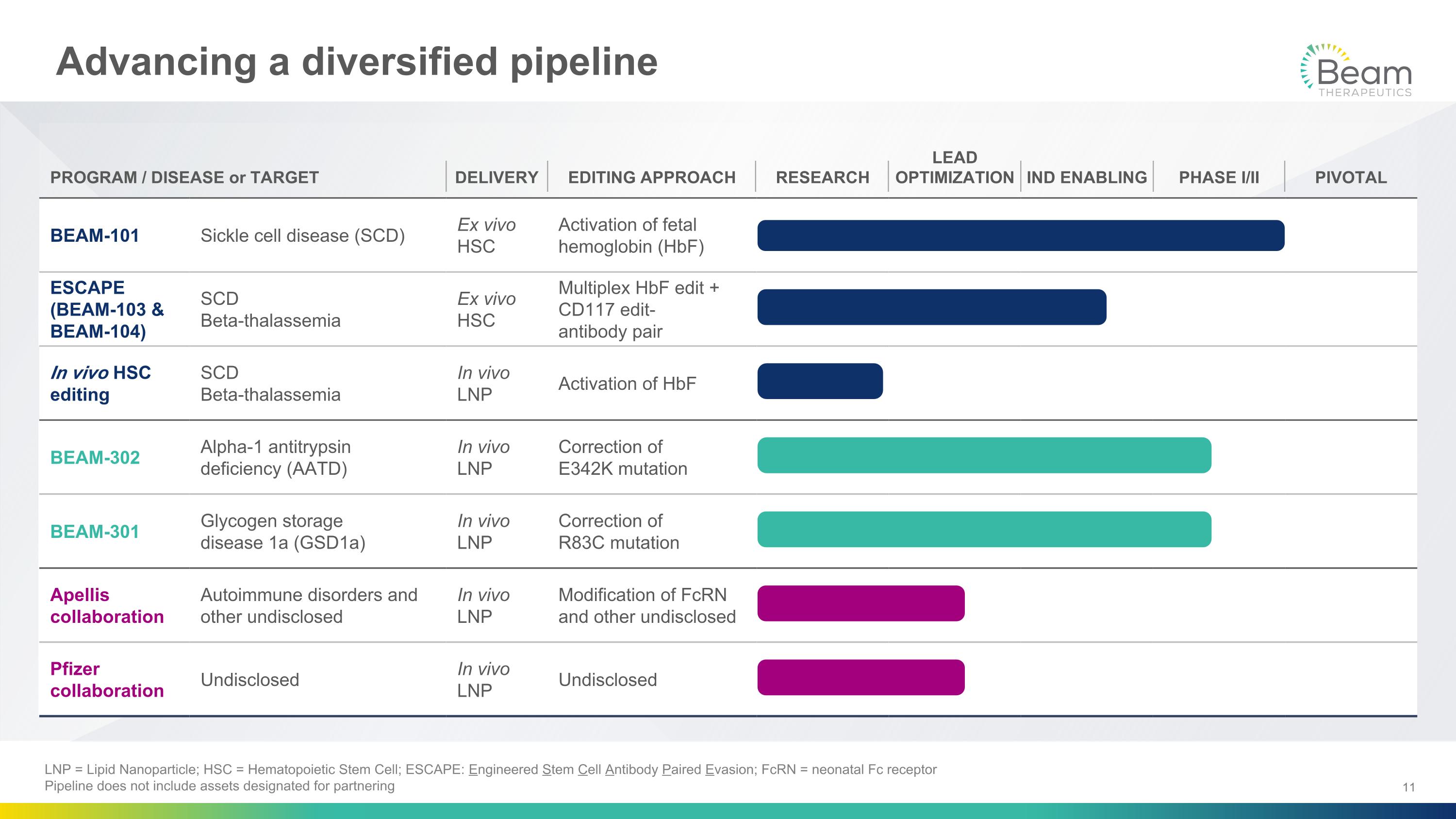
Advancing a diversified pipeline PROGRAM / DISEASE or TARGET DELIVERY EDITING APPROACH RESEARCH LEAD OPTIMIZATION IND ENABLING PHASE I/II PIVOTAL BEAM-101 Sickle cell disease (SCD) Ex vivo HSC Activation of fetal hemoglobin (HbF) ESCAPE (BEAM-103 & BEAM-104) SCD Beta-thalassemia Ex vivo HSC Multiplex HbF edit +�CD117 edit- antibody pair In vivo HSC editing SCD Beta-thalassemia In vivo LNP Activation of HbF BEAM-302 Alpha-1 antitrypsin deficiency (AATD) In vivo LNP Correction of �E342K mutation BEAM-301 Glycogen storage �disease 1a (GSD1a) In vivo LNP Correction of �R83C mutation Apellis collaboration Autoimmune disorders and other undisclosed In vivo LNP Modification of FcRN and other undisclosed Pfizer collaboration Undisclosed In vivo LNP Undisclosed LNP = Lipid Nanoparticle; HSC = Hematopoietic Stem Cell; ESCAPE: Engineered Stem Cell Antibody Paired Evasion; FcRN = neonatal Fc receptor Pipeline does not include assets designated for partnering

What if we could develop better one-time therapies for patients with SCD? SICKLE CELL DISEASE
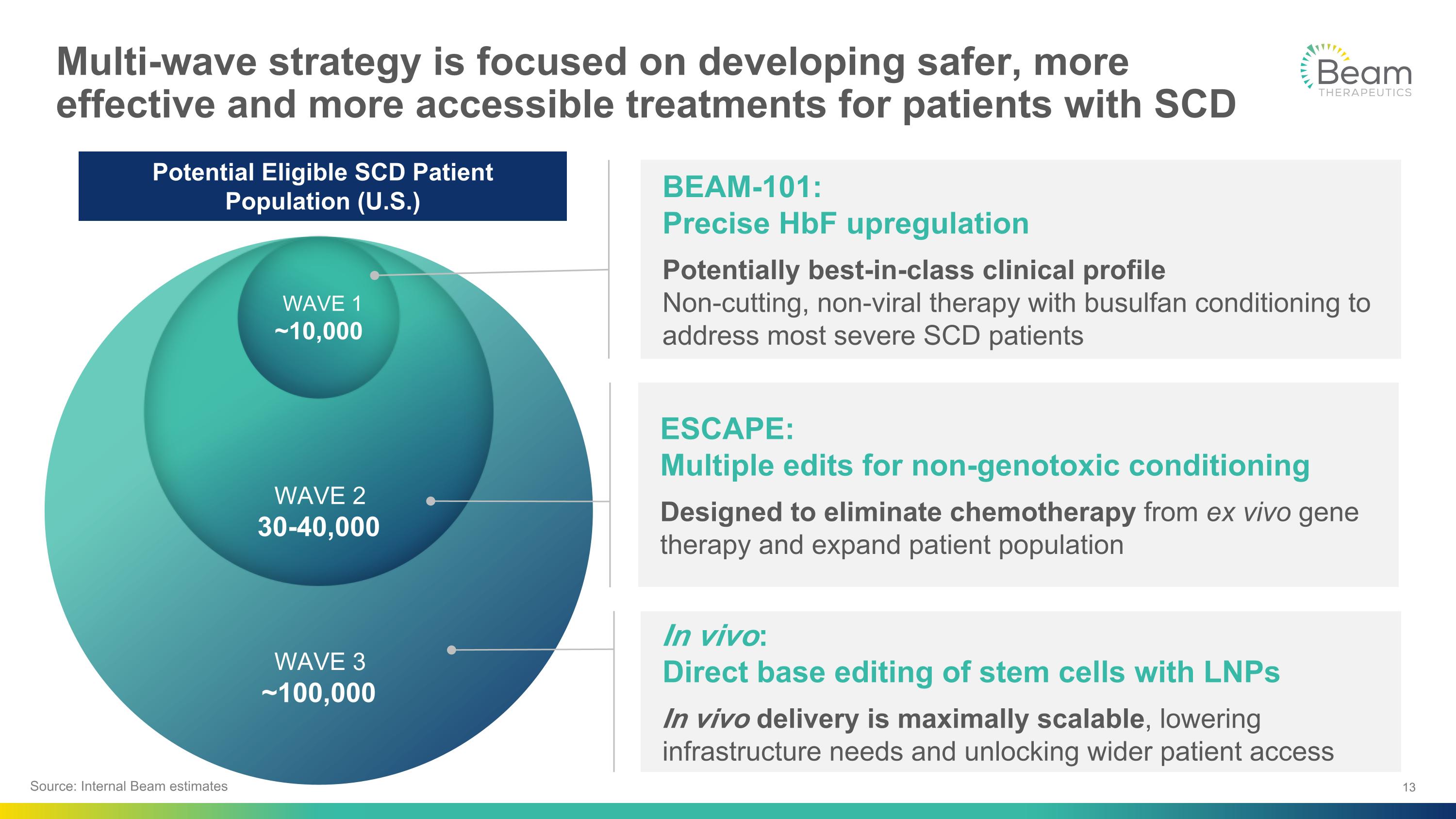
WAVE 3 ~100,000 WAVE 2 30-40,000 WAVE 1 ~10,000 Multi-wave strategy is focused on developing safer, more effective and more accessible treatments for patients with SCD ESCAPE:�Multiple edits for non-genotoxic conditioning Designed to eliminate chemotherapy from ex vivo gene therapy and expand patient population BEAM-101:�Precise HbF upregulation Potentially best-in-class clinical profile�Non-cutting, non-viral therapy with busulfan conditioning to address most severe SCD patients In vivo:�Direct base editing of stem cells with LNPs In vivo delivery is maximally scalable, lowering infrastructure needs and unlocking wider patient access Source: Internal Beam estimates Potential Eligible SCD Patient Population (U.S.)
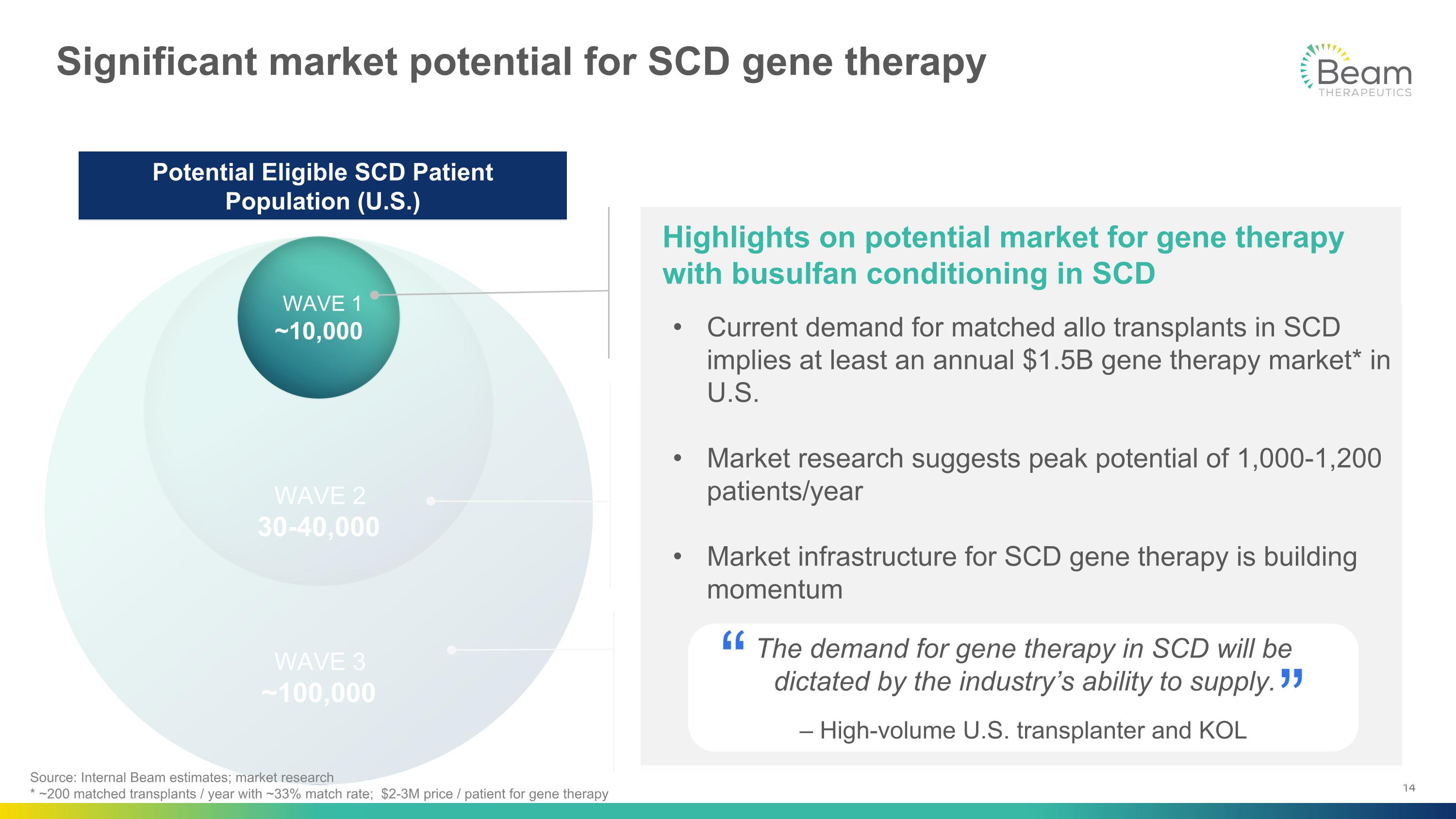
WAVE 3 ~100,000 WAVE 2 30-40,000 Significant market potential for SCD gene therapy ESCAPE: Multiple edits for non-genotoxic conditioning Designed to eliminate chemotherapy from ex vivo gene therapy and expand patient population with: Broader range of disease severity Broader age range Increased willingness-to-treat In vivo: Base editing with hemopoietic stem cell (HSC)-targeted LNPs In vivo delivery would overcome need for transplantation, lower infrastructure requirements and unlock wider patient access and geographies Potential Eligible SCD Patient Population (U.S.) WAVE 1 ~10,000 Highlights on potential market for gene therapy with busulfan conditioning in SCD Source: Internal Beam estimates; market research * ~200 matched transplants / year with ~33% match rate; $2-3M price / patient for gene therapy Current demand for matched allo transplants in SCD implies at least an annual $1.5B gene therapy market* in U.S. Market research suggests peak potential of 1,000-1,200 patients/year Market infrastructure for SCD gene therapy is building momentum The demand for gene therapy in SCD will be �dictated by the industry’s ability to supply. – High-volume U.S. transplanter and KOL “ “
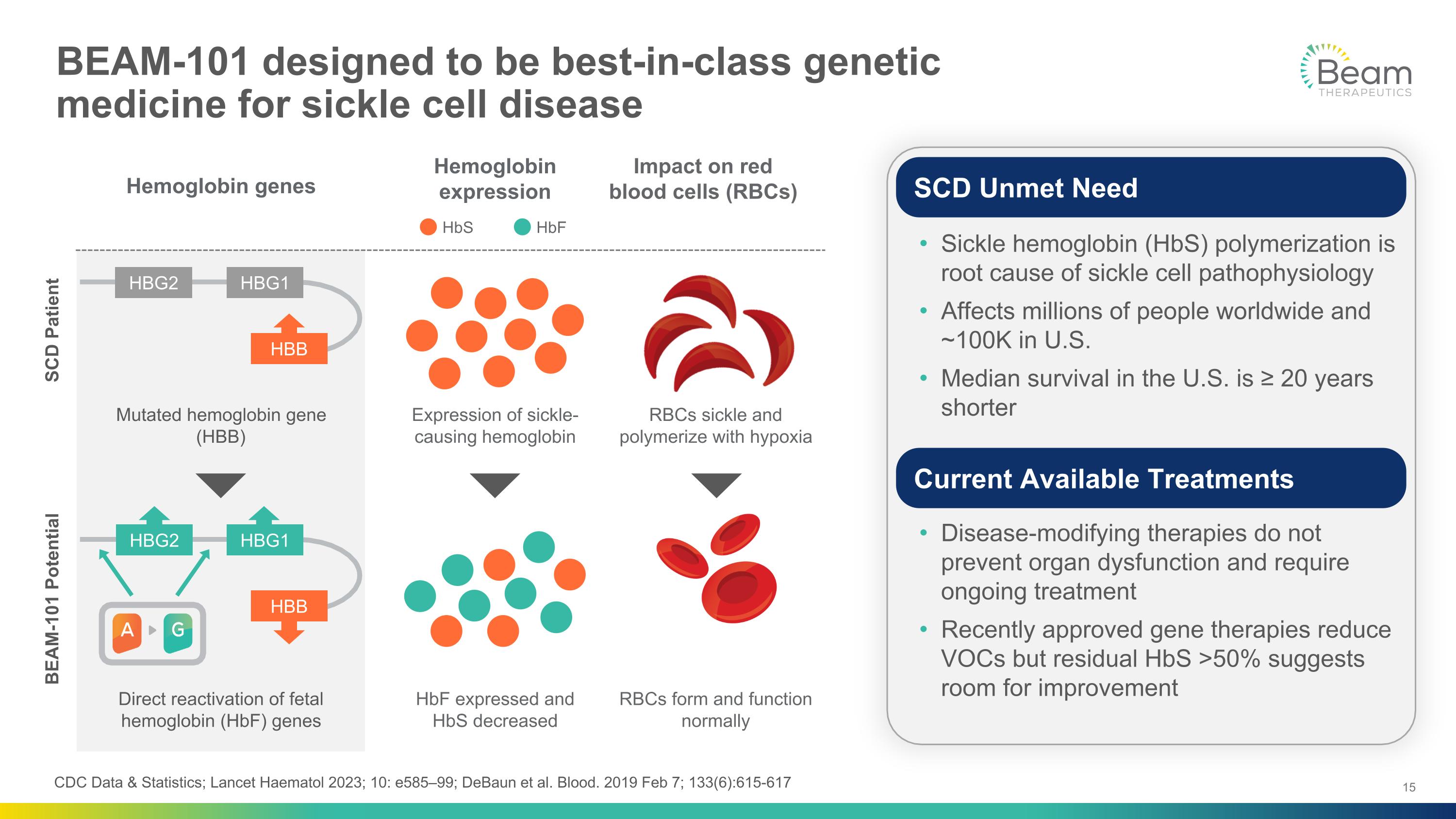
BEAM-101 designed to be best-in-class genetic�medicine for sickle cell disease BEAM-101 Potential SCD Patient HBB HBG1 HBG2 HBB HBG1 HBG2 Mutated hemoglobin gene (HBB) Direct reactivation of fetal hemoglobin (HbF) genes Hemoglobin genes Hemoglobin expression Expression of sickle-causing hemoglobin HbF expressed and �HbS decreased HbS HbF RBCs form and function normally RBCs sickle and polymerize with hypoxia Impact on red blood cells (RBCs) CDC Data & Statistics; Lancet Haematol 2023; 10: e585–99; DeBaun et al. Blood. 2019 Feb 7; 133(6):615-617 Sickle hemoglobin (HbS) polymerization is root cause of sickle cell pathophysiology Affects millions of people worldwide and ~100K in U.S. Median survival in the U.S. is ≥ 20 years shorter Disease-modifying therapies do not prevent organ dysfunction and require ongoing treatment Recently approved gene therapies reduce VOCs but residual HbS >50% suggests room for improvement SCD Unmet Need Current Available Treatments
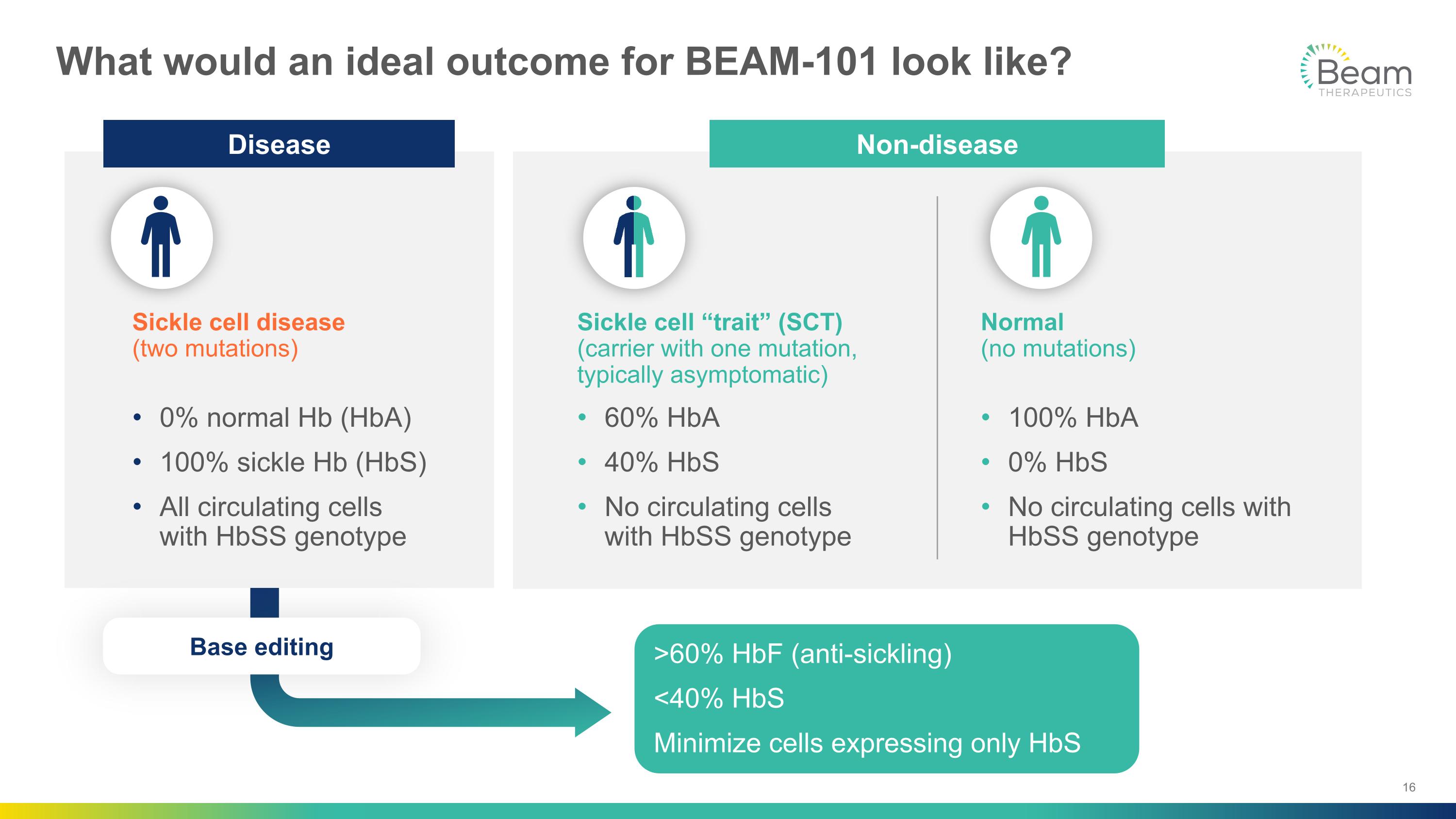
What would an ideal outcome for BEAM-101 look like? Sickle cell “trait” (SCT)�(carrier with one mutation, typically asymptomatic) 60% HbA 40% HbS No circulating cells �with HbSS genotype Normal �(no mutations) 100% HbA 0% HbS No circulating cells with HbSS genotype Sickle cell disease�(two mutations) 0% normal Hb (HbA) 100% sickle Hb (HbS) All circulating cells �with HbSS genotype >60% HbF (anti-sickling) <40% HbS Minimize cells expressing only HbS Non-disease Disease Base editing
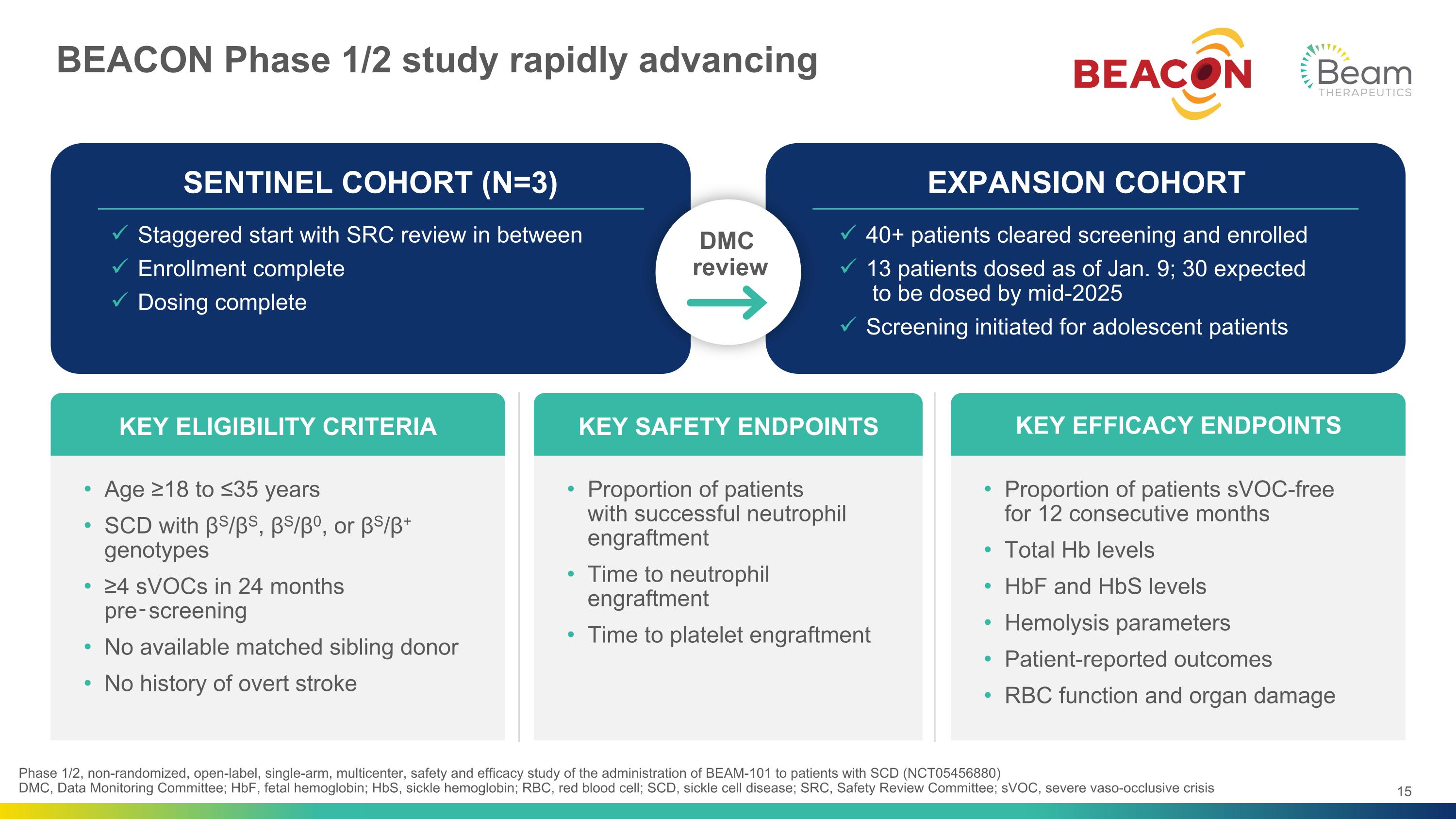
BEACON Phase 1/2 study rapidly advancing Phase 1/2, non-randomized, open-label, single-arm, multicenter, safety and efficacy study of the administration of BEAM-101 to patients with SCD (NCT05456880)�DMC, Data Monitoring Committee; HbF, fetal hemoglobin; HbS, sickle hemoglobin; RBC, red blood cell; SCD, sickle cell disease; SRC, Safety Review Committee; sVOC, severe vaso-occlusive crisis Age ≥18 to ≤35 years SCD with βS/βS, βS/β0, or βS/β+ genotypes ≥4 sVOCs in 24 months pre‑screening No available matched sibling donor No history of overt stroke KEY ELIGIBILITY CRITERIA Proportion of patients�with successful neutrophil engraftment Time to neutrophil engraftment Time to platelet engraftment KEY SAFETY ENDPOINTS Proportion of patients sVOC-free �for 12 consecutive months Total Hb levels HbF and HbS levels Hemolysis parameters Patient-reported outcomes RBC function and organ damage KEY EFFICACY ENDPOINTS SENTINEL COHORT (N=3) Staggered start with SRC review in between Enrollment complete Dosing complete EXPANSION COHORT 40+ patients cleared screening and enrolled 13 patients dosed as of Jan. 9; 30 expected� to be dosed by mid-2025 Screening initiated for adolescent patients 15 DMC review
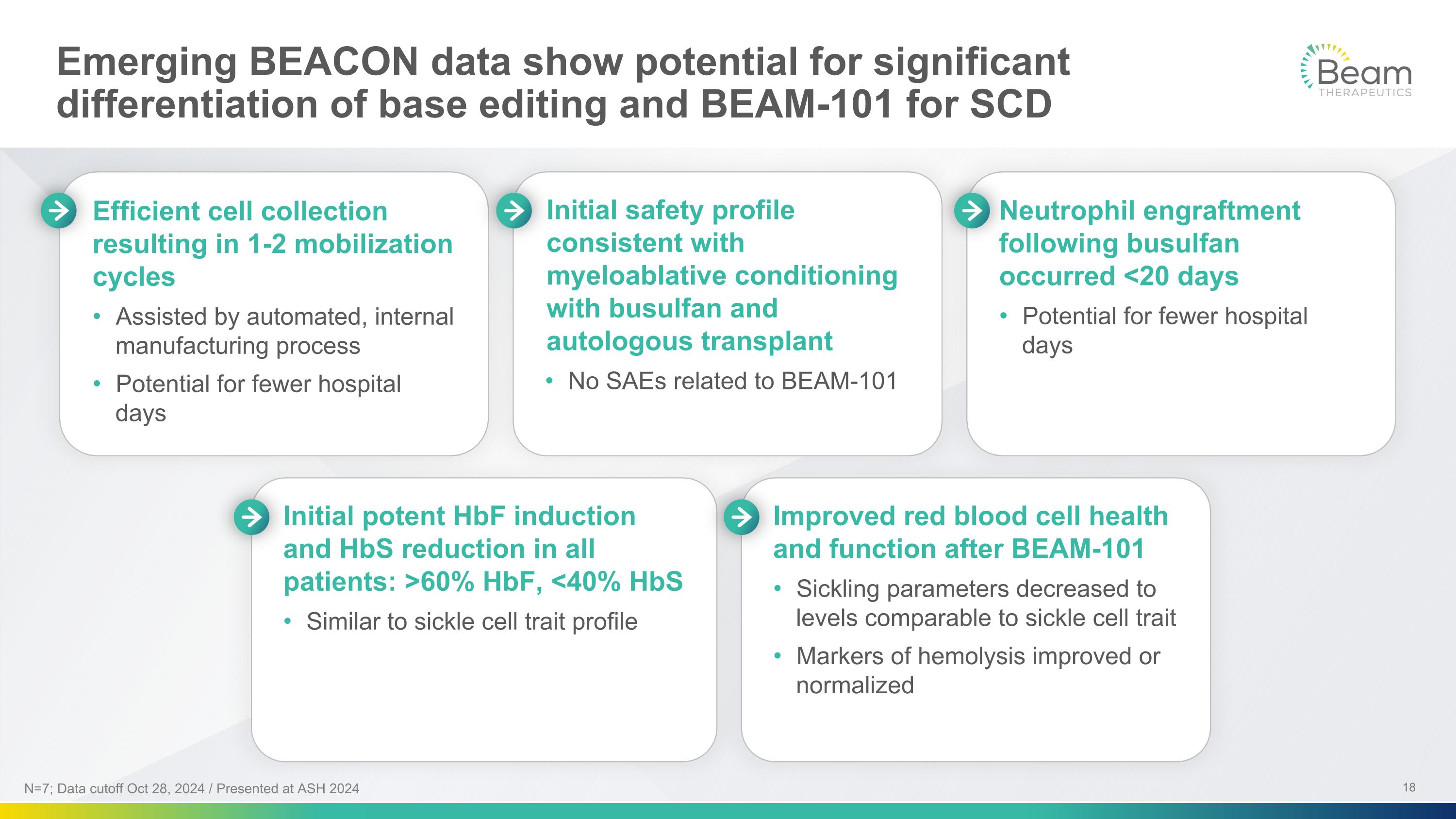
Emerging BEACON data show potential for significant differentiation of base editing and BEAM-101 for SCD Neutrophil engraftment following busulfan occurred <20 days Potential for fewer hospital days Improved red blood cell health and function after BEAM-101 Sickling parameters decreased to levels comparable to sickle cell trait Markers of hemolysis improved or normalized Initial potent HbF induction and HbS reduction in all patients: >60% HbF, <40% HbS Similar to sickle cell trait profile Efficient cell collection resulting in 1-2 mobilization cycles Assisted by automated, internal manufacturing process Potential for fewer hospital days Initial safety profile consistent with myeloablative conditioning with busulfan and autologous transplant No SAEs related to BEAM-101 N=7; Data cutoff Oct 28, 2024 / Presented at ASH 2024
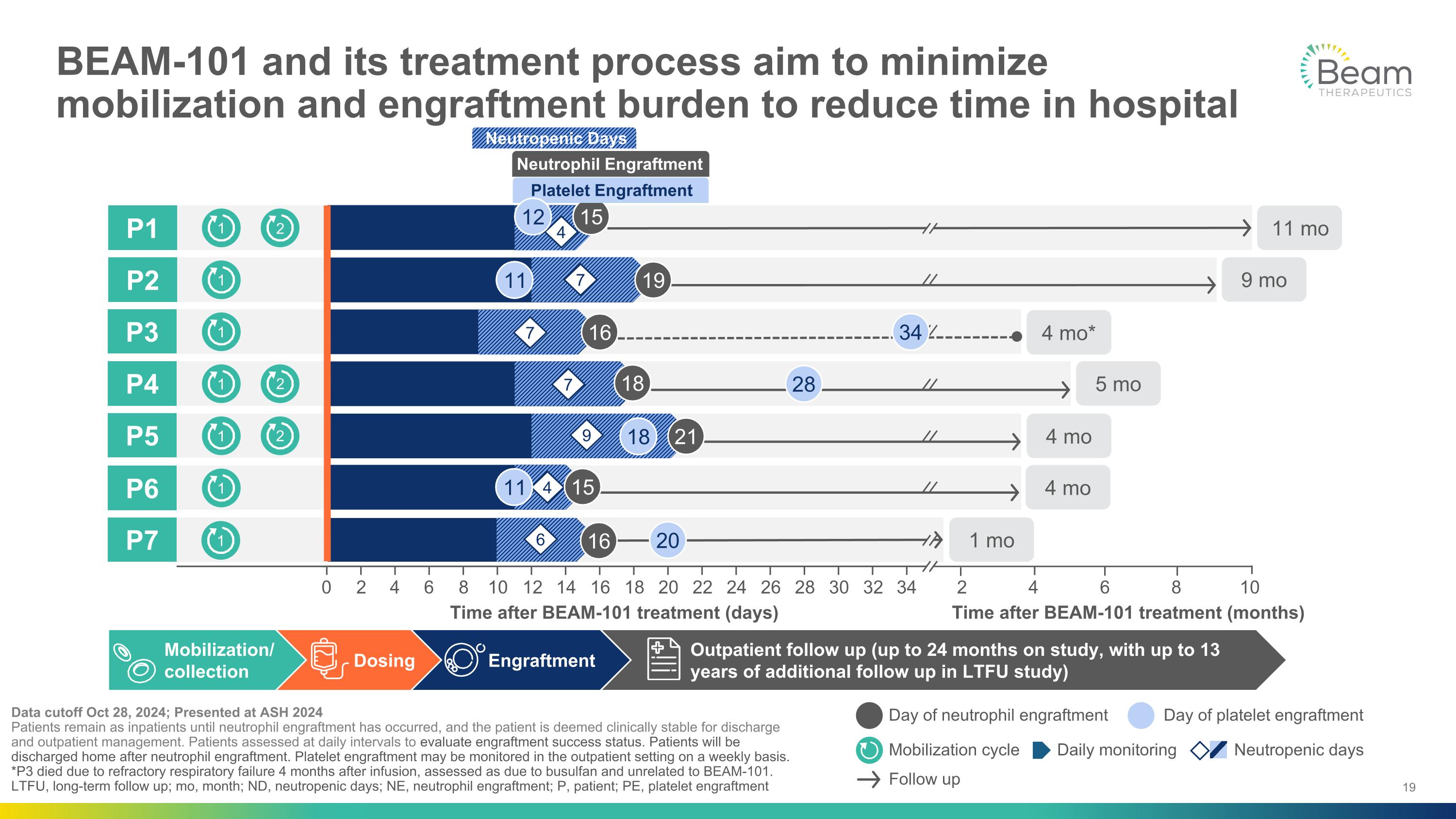
Neutropenic Days Outpatient follow up (up to 24 months on study, with up to 13 years of additional follow up in LTFU study) Engraftment Dosing BEAM-101 and its treatment process aim to minimize mobilization and engraftment burden to reduce time in hospital Data cutoff Oct 28, 2024; Presented at ASH 2024 Patients remain as inpatients until neutrophil engraftment has occurred, and the patient is deemed clinically stable for discharge and outpatient management. Patients assessed at daily intervals to evaluate engraftment success status. Patients will be discharged home after neutrophil engraftment. Platelet engraftment may be monitored in the outpatient setting on a weekly basis. *P3 died due to refractory respiratory failure 4 months after infusion, assessed as due to busulfan and unrelated to BEAM-101. LTFU, long-term follow up; mo, month; ND, neutropenic days; NE, neutrophil engraftment; P, patient; PE, platelet engraftment P1 0 34 2 10 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 1 1 1 1 1 1 2 2 2 1 2 6 4 8 P2 P3 P4 P5 P6 P7 Mobilization/�collection Follow up Daily monitoring Neutropenic days Mobilization cycle Day of neutrophil engraftment Day of platelet engraftment Time after BEAM-101 treatment (days) Time after BEAM-101 treatment (months) 7 4 7 7 9 4 6 Neutrophil Engraftment 15 19 16 18 21 15 16 11 mo 9 mo 4 mo* 5 mo 4 mo 4 mo 1 mo 34 28 20 12 Platelet Engraftment 11 18 11
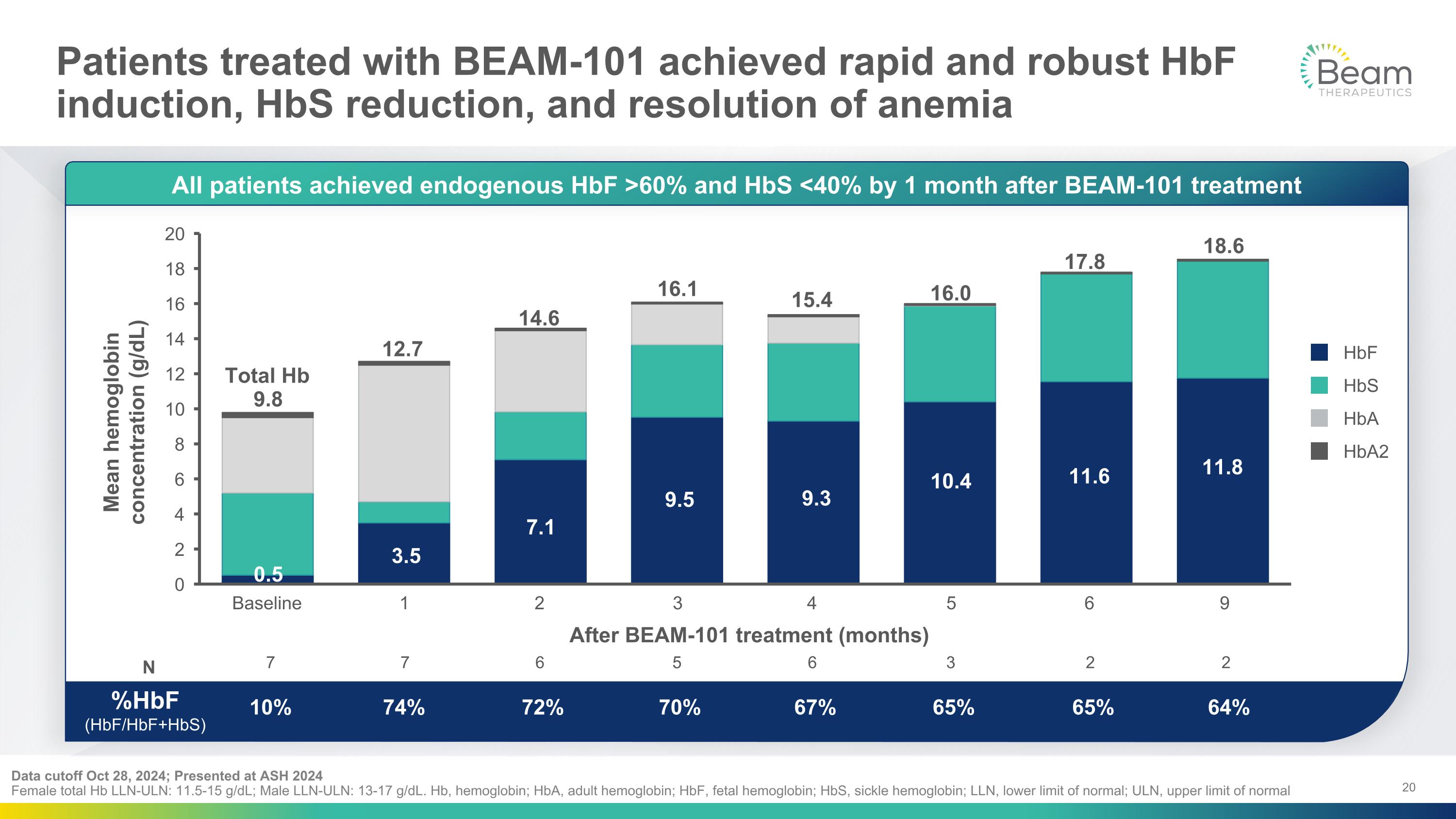
Patients treated with BEAM-101 achieved rapid and robust HbF induction, HbS reduction, and resolution of anemia Data cutoff Oct 28, 2024; Presented at ASH 2024�Female total Hb LLN-ULN: 11.5-15 g/dL; Male LLN-ULN: 13-17 g/dL. Hb, hemoglobin; HbA, adult hemoglobin; HbF, fetal hemoglobin; HbS, sickle hemoglobin; LLN, lower limit of normal; ULN, upper limit of normal N %HbF�(HbF/HbF+HbS) After BEAM-101 treatment (months) 7 10% 7 74% 6 72% 5 70% 6 67% 3 65% 2 65% 2 64% All patients achieved endogenous HbF >60% and HbS <40% by 1 month after BEAM-101 treatment HbF HbS HbA HbA2 1 2 3 4 5 6 9 Baseline 0.5 3.5 7.1 9.5 9.3 10.4 11.6 11.8 Total Hb
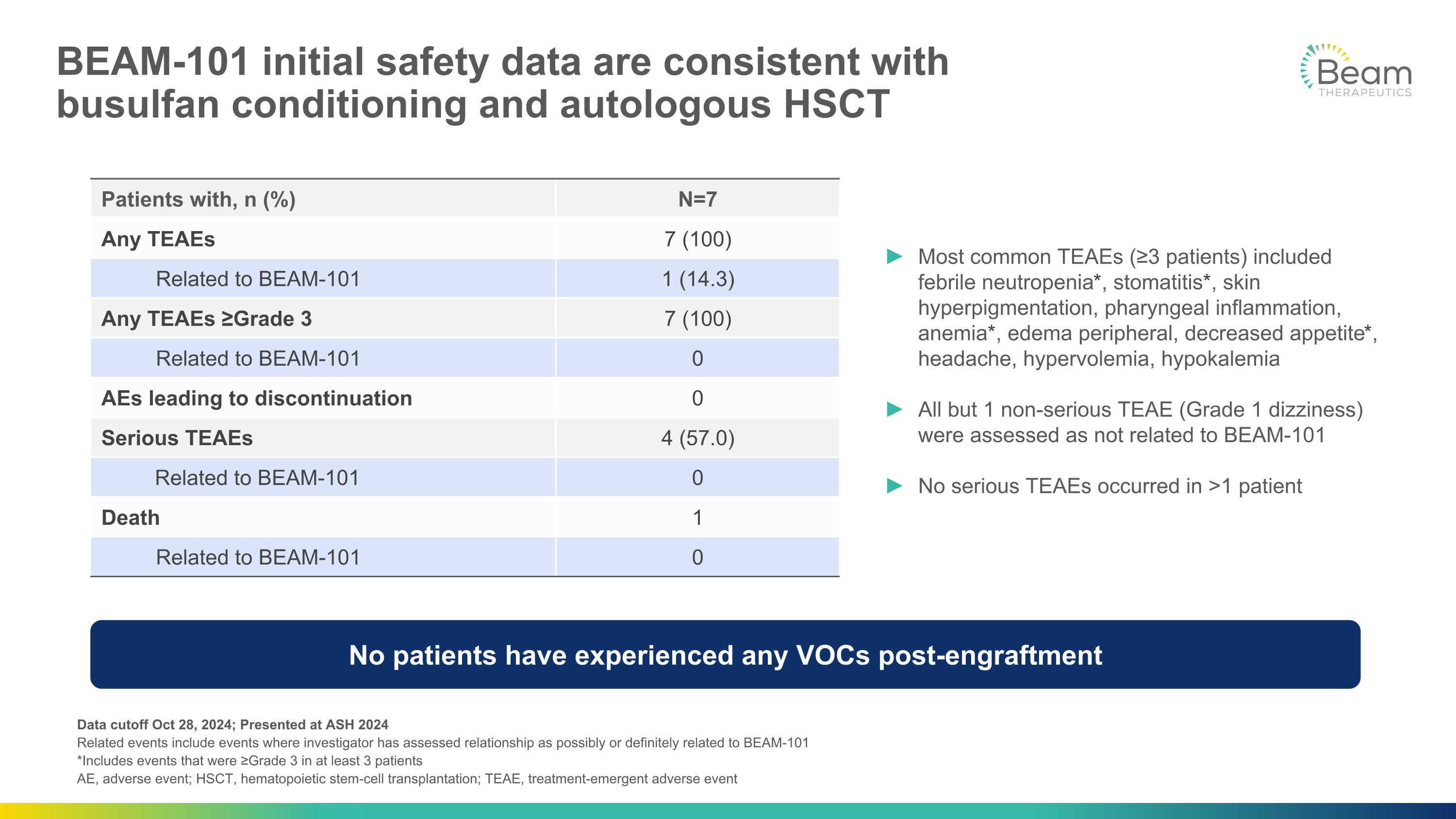
BEAM-101 initial safety data are consistent with �busulfan conditioning and autologous HSCT Data cutoff Oct 28, 2024; Presented at ASH 2024�Related events include events where investigator has assessed relationship as possibly or definitely related to BEAM-101 *Includes events that were ≥Grade 3 in at least 3 patients AE, adverse event; HSCT, hematopoietic stem-cell transplantation; TEAE, treatment-emergent adverse event No patients have experienced any VOCs post-engraftment Patients with, n (%) N=7 Any TEAEs 7 (100) Related to BEAM-101 1 (14.3) Any TEAEs ≥Grade 3 7 (100) Related to BEAM-101 0 AEs leading to discontinuation 0 Serious TEAEs 4 (57.0) Related to BEAM-101 0 Death 1 Related to BEAM-101 0 Most common TEAEs (≥3 patients) included febrile neutropenia*, stomatitis*, skin hyperpigmentation, pharyngeal inflammation, anemia*, edema peripheral, decreased appetite*, headache, hypervolemia, hypokalemia All but 1 non-serious TEAE (Grade 1 dizziness) were assessed as not related to BEAM-101 No serious TEAEs occurred in >1 patient
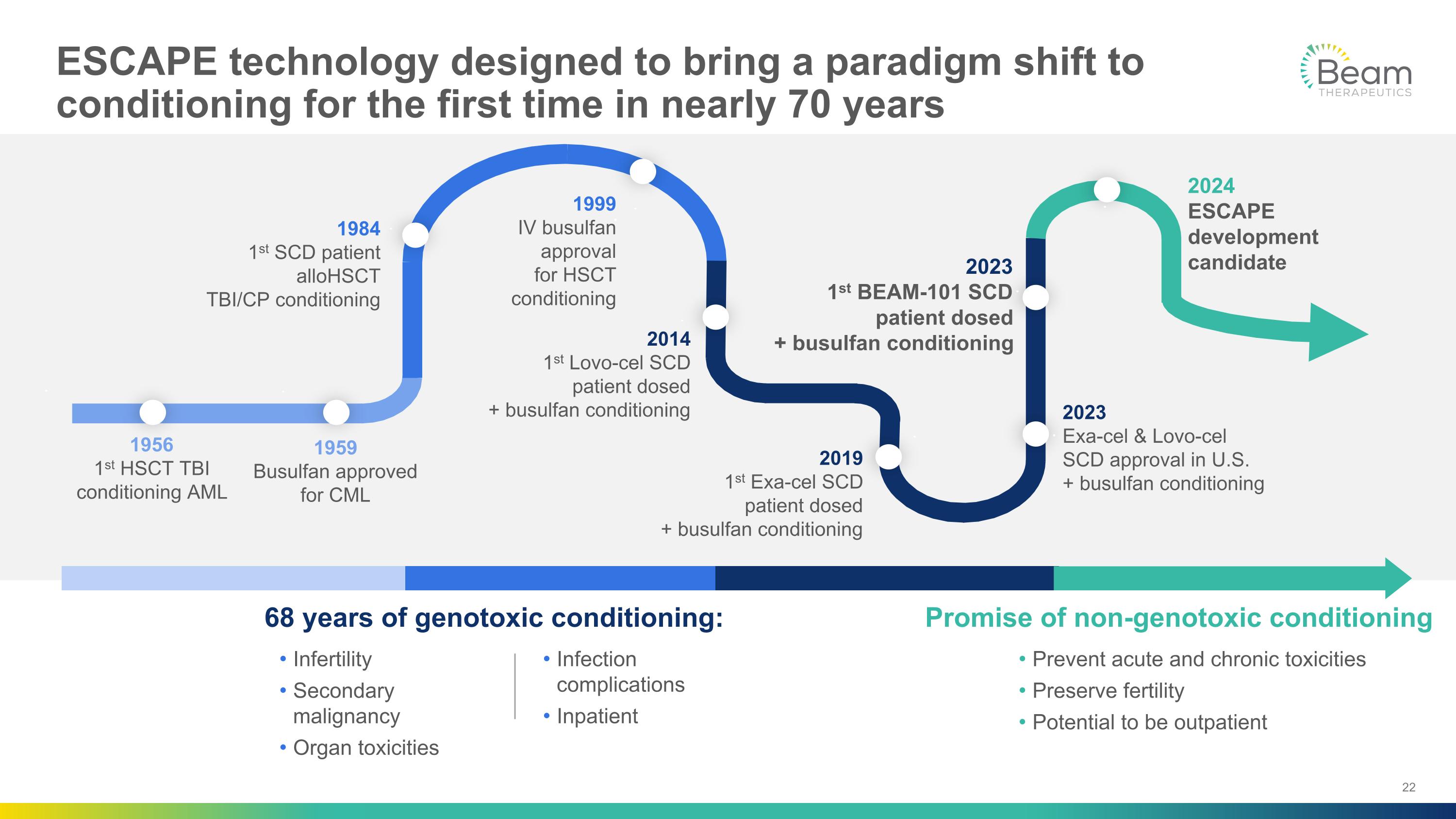
ESCAPE technology designed to bring a paradigm shift to conditioning for the first time in nearly 70 years 1956 1st HSCT TBI conditioning AML 1959 Busulfan approved for CML 1984 1st SCD patient alloHSCT TBI/CP conditioning 1999 IV busulfan approval for HSCT conditioning 2014 1st Lovo-cel SCD patient dosed + busulfan conditioning 2019 1st Exa-cel SCD patient dosed + busulfan conditioning 2023 Exa-cel & Lovo-cel SCD approval in U.S. + busulfan conditioning 2024 ESCAPE development candidate 68 years of genotoxic conditioning: Promise of non-genotoxic conditioning Prevent acute and chronic toxicities Preserve fertility Potential to be outpatient Infertility Secondary malignancy Organ toxicities Infection complications Inpatient 2023 1st BEAM-101 SCD patient dosed + busulfan conditioning
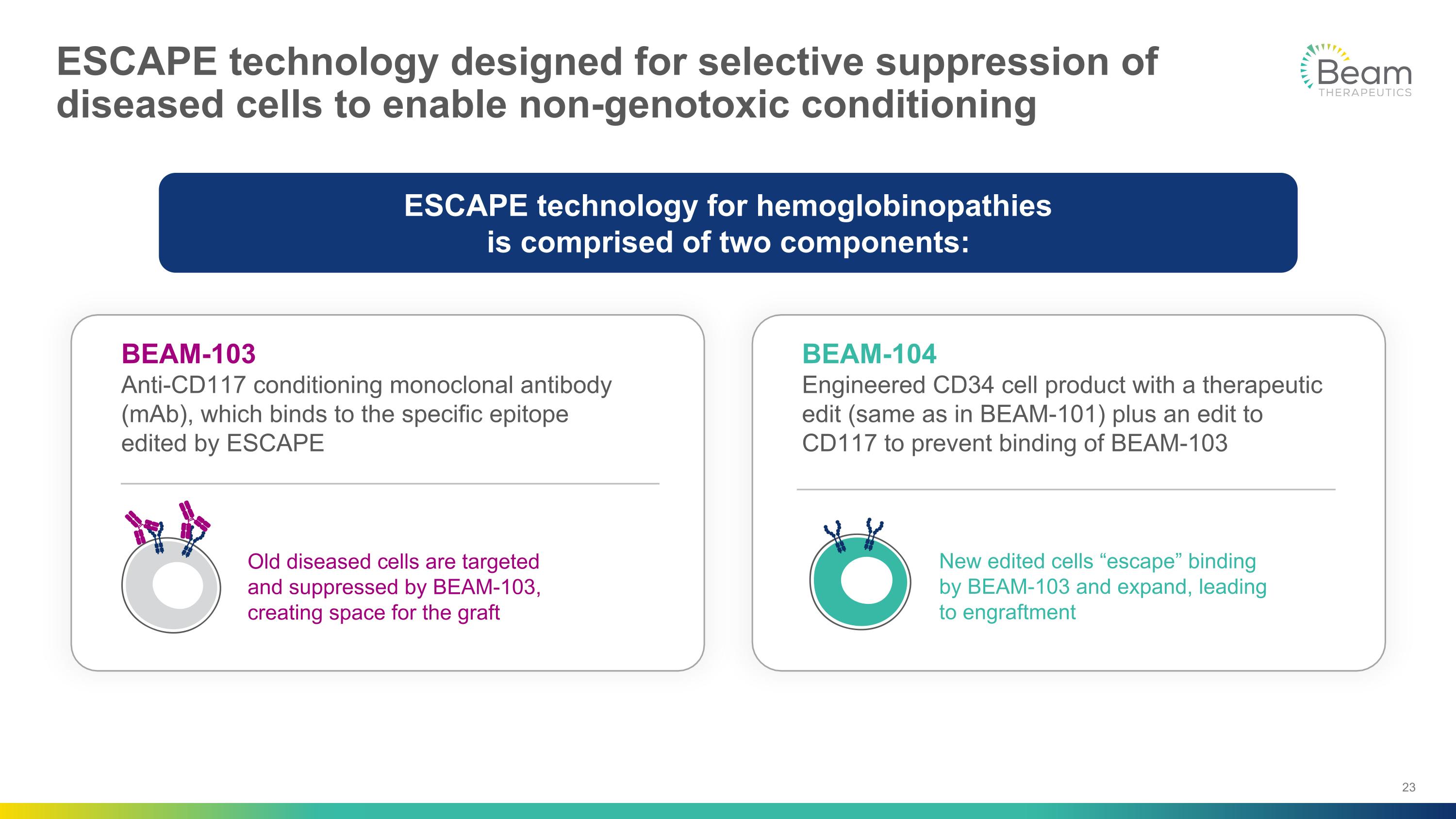
ESCAPE technology for hemoglobinopathies �is comprised of two components: ESCAPE technology designed for selective suppression of diseased cells to enable non-genotoxic conditioning Old diseased cells are targeted and suppressed by BEAM-103, creating space for the graft New edited cells “escape” binding by BEAM-103 and expand, leading to engraftment BEAM-103 Anti-CD117 conditioning monoclonal antibody (mAb), which binds to the specific epitope edited by ESCAPE BEAM-104 Engineered CD34 cell product with a therapeutic edit (same as in BEAM-101) plus an edit to CD117 to prevent binding of BEAM-103
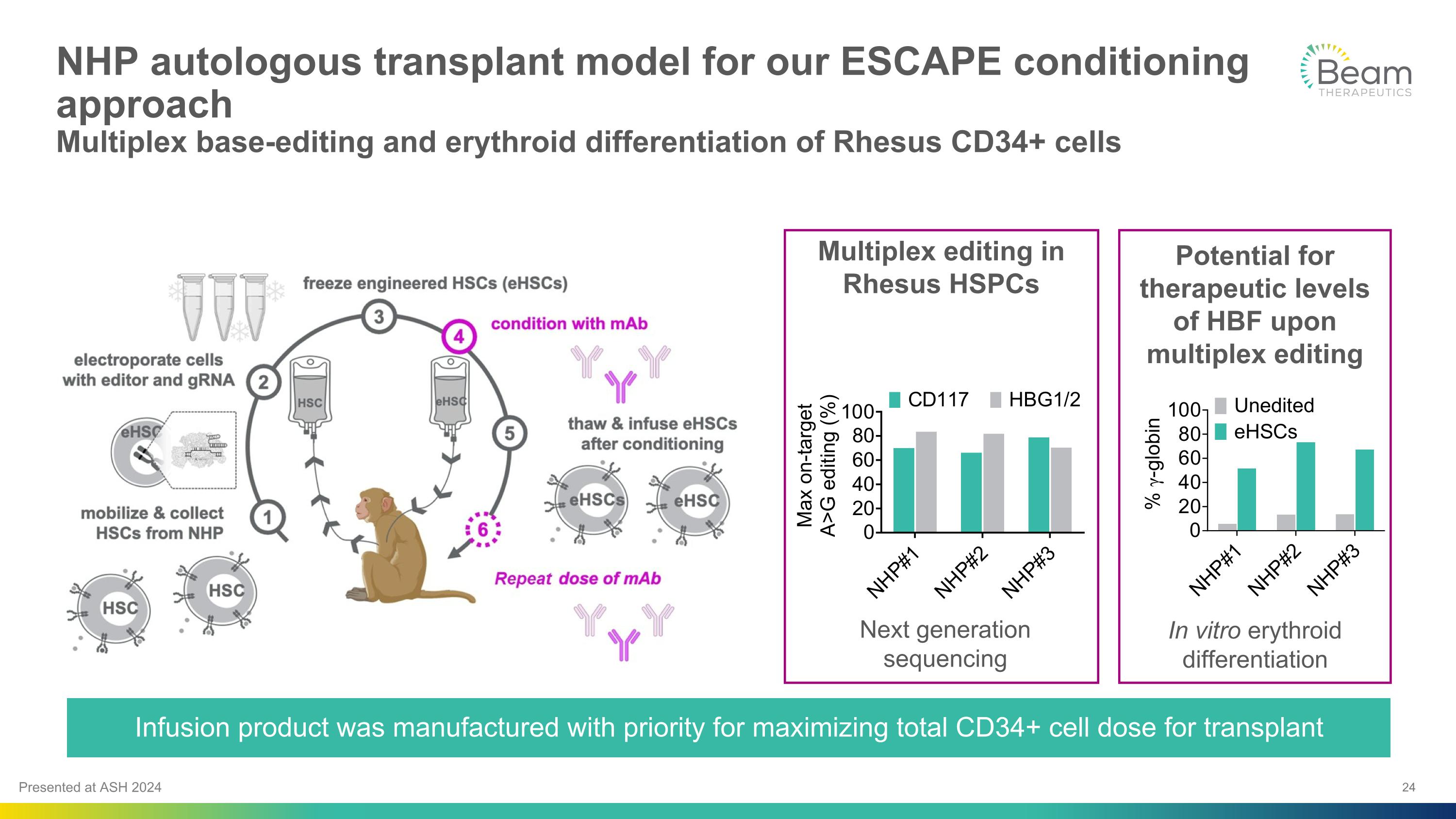
NHP autologous transplant model for our ESCAPE conditioning approach�Multiplex base-editing and erythroid differentiation of Rhesus CD34+ cells Infusion product was manufactured with priority for maximizing total CD34+ cell dose for transplant Next generation sequencing Multiplex editing in Rhesus HSPCs Potential for therapeutic levels of HBF upon multiplex editing In vitro erythroid differentiation Presented at ASH 2024
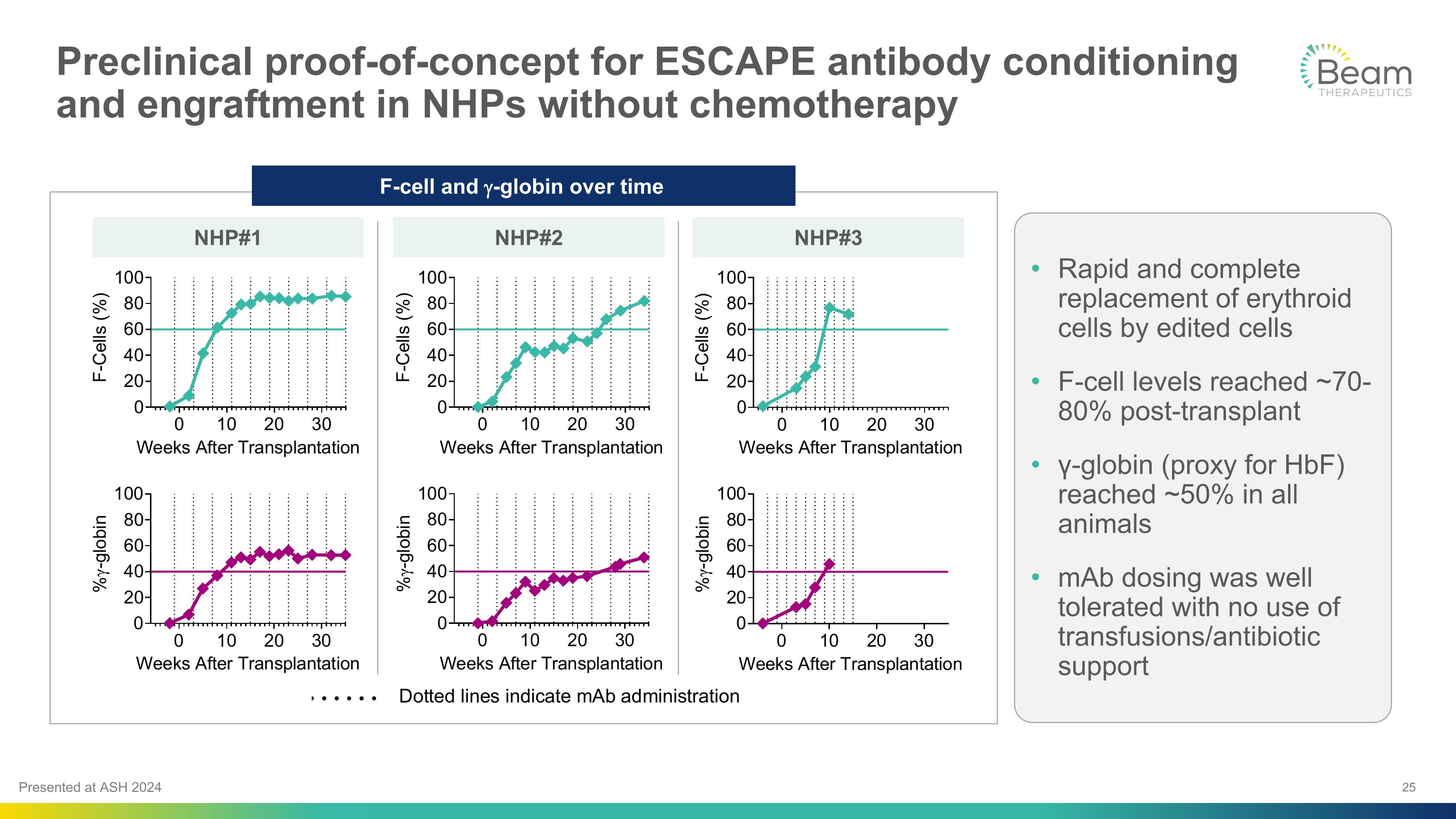
Preclinical proof-of-concept for ESCAPE antibody conditioning and engraftment in NHPs without chemotherapy Rapid and complete replacement of erythroid cells by edited cells F-cell levels reached ~70-80% post-transplant γ-globin (proxy for HbF) reached ~50% in all animals mAb dosing was well tolerated with no use of transfusions/antibiotic support F-cell and g-globin over time NHP#1 NHP#2 NHP#3 Presented at ASH 2024
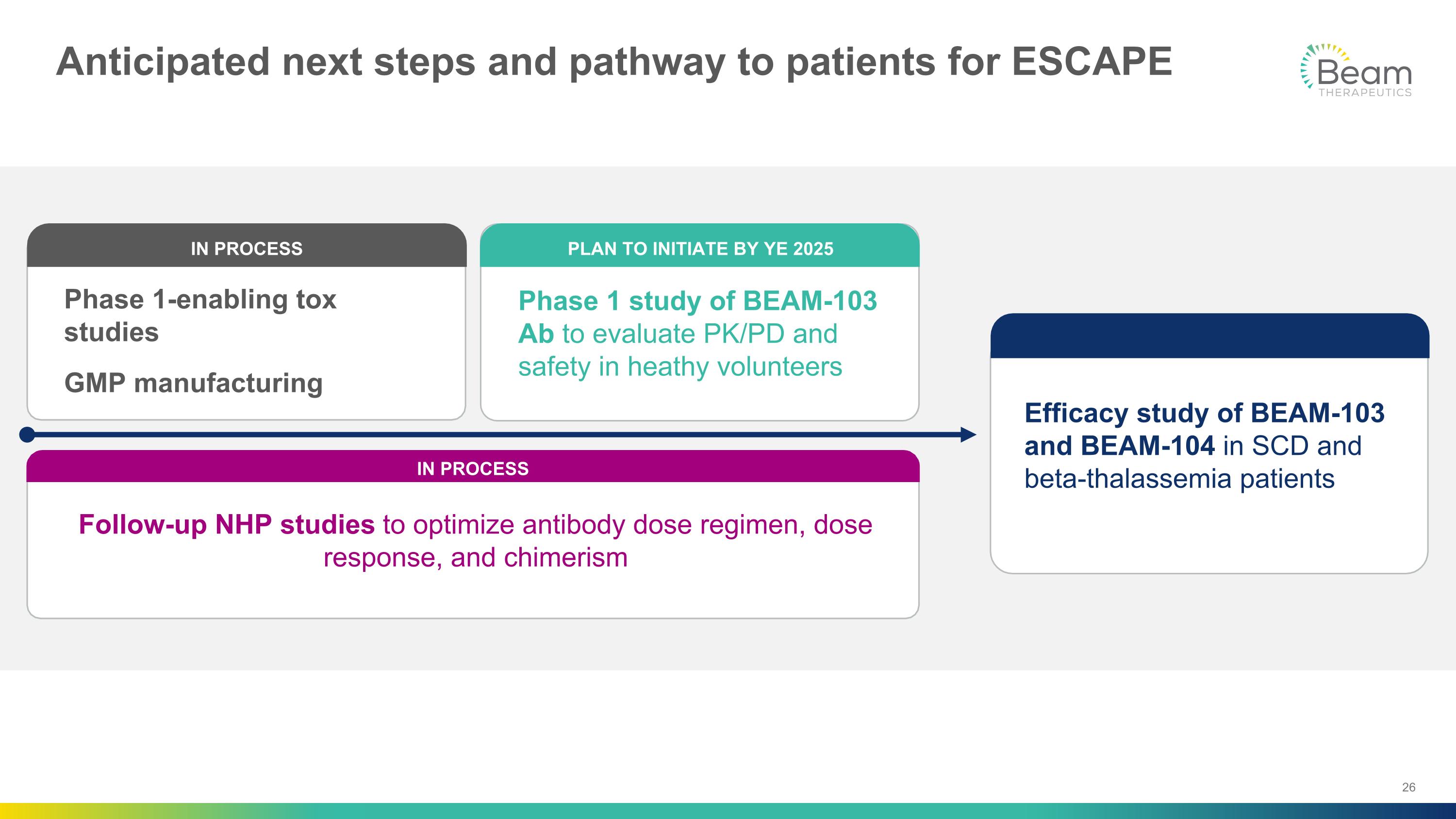
Anticipated next steps and pathway to patients for ESCAPE Phase 1 study of BEAM-103 Ab to evaluate PK/PD and safety in heathy volunteers PLAN TO INITIATE BY YE 2025 Follow-up NHP studies to optimize antibody dose regimen, dose response, and chimerism IN PROCESS Efficacy study of BEAM-103 and BEAM-104 in SCD and beta-thalassemia patients Phase 1-enabling tox studies GMP manufacturing IN PROCESS

BEAM-101 progress derisks BEAM-104 Potential to accelerate clinical trial, BLA filing, launch and commercial ramp Synergy between BEAM-101 and ESCAPE technology (BEAM-104 and BEAM-103) support efficient development in SCD BEAM-101 BEAM-104 Regulatory pathway Clinical sites + CD117 guide RNA Commercial �infrastructure BEAM-103 CD117 antibody Base editor mRNA HBG1/2 guide RNA CD34 editing and manufacturing process
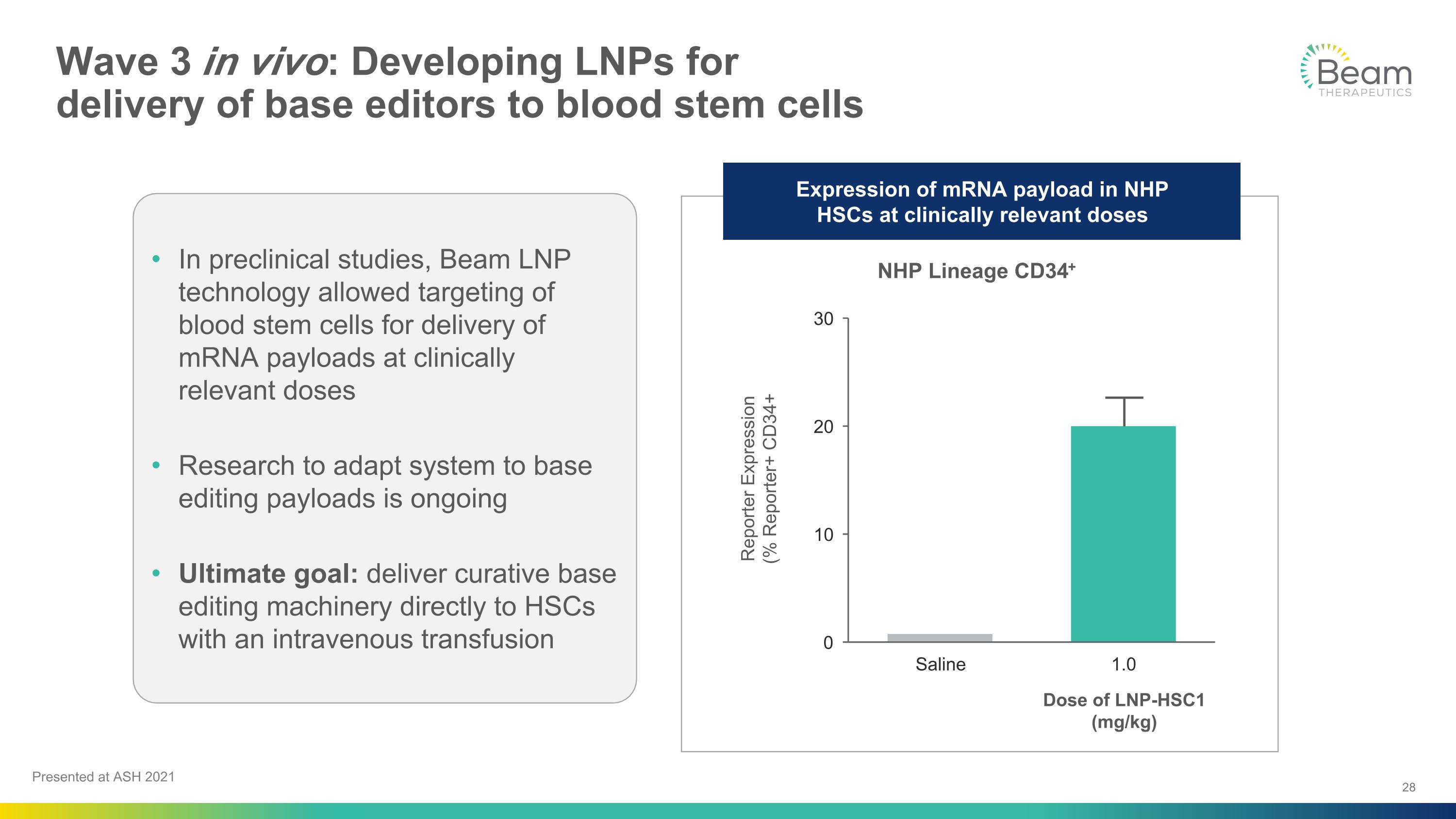
Wave 3 in vivo: Developing LNPs for �delivery of base editors to blood stem cells Presented at ASH 2021 In preclinical studies, Beam LNP technology allowed targeting of blood stem cells for delivery of mRNA payloads at clinically relevant doses Research to adapt system to base editing payloads is ongoing Ultimate goal: deliver curative base editing machinery directly to HSCs with an intravenous transfusion NHP Lineage CD34+ Dose of LNP-HSC1�(mg/kg) Expression of mRNA payload in NHP �HSCs at clinically relevant doses

What if we could use base editing to correct disease-causing mutations in vivo? GENETIC DISEASES
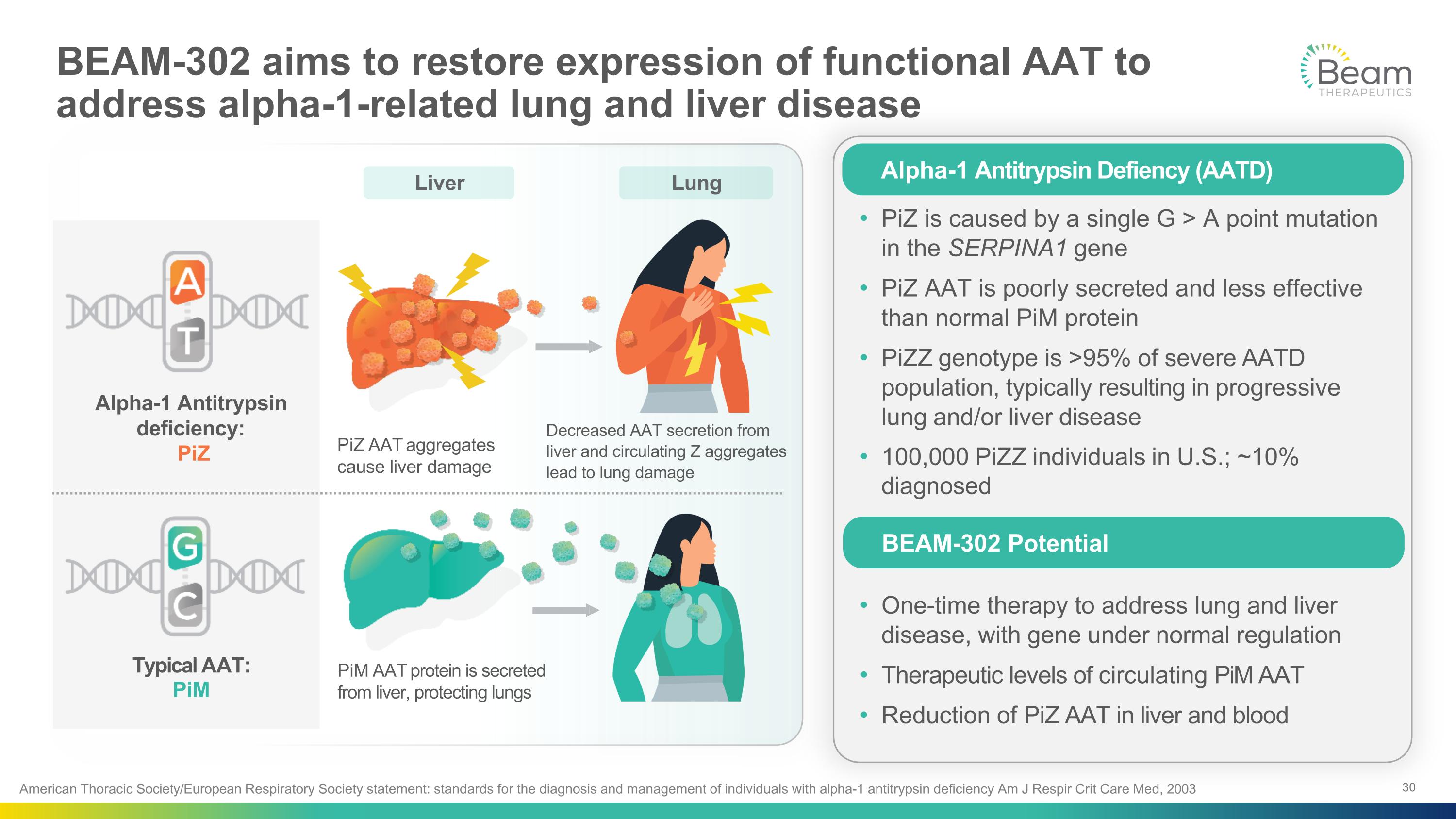
PiZ is caused by a single G > A point mutation in the SERPINA1 gene PiZ AAT is poorly secreted and less effective than normal PiM protein PiZZ genotype is >95% of severe AATD population, typically resulting in progressive lung and/or liver disease 100,000 PiZZ individuals in U.S.; ~10% diagnosed One-time therapy to address lung and liver disease, with gene under normal regulation Therapeutic levels of circulating PiM AAT Reduction of PiZ AAT in liver and blood Alpha-1 Antitrypsin Defiency (AATD) BEAM-302 Potential American Thoracic Society/European Respiratory Society statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency Am J Respir Crit Care Med, 2003 Liver Lung Typical AAT: PiM Alpha-1 Antitrypsin deficiency: PiZ PiZ AAT aggregates cause liver damage Decreased AAT secretion from liver and circulating Z aggregates lead to lung damage PiM AAT protein is secreted from liver, protecting lungs BEAM-302 aims to restore expression of functional AAT to �address alpha-1-related lung and liver disease
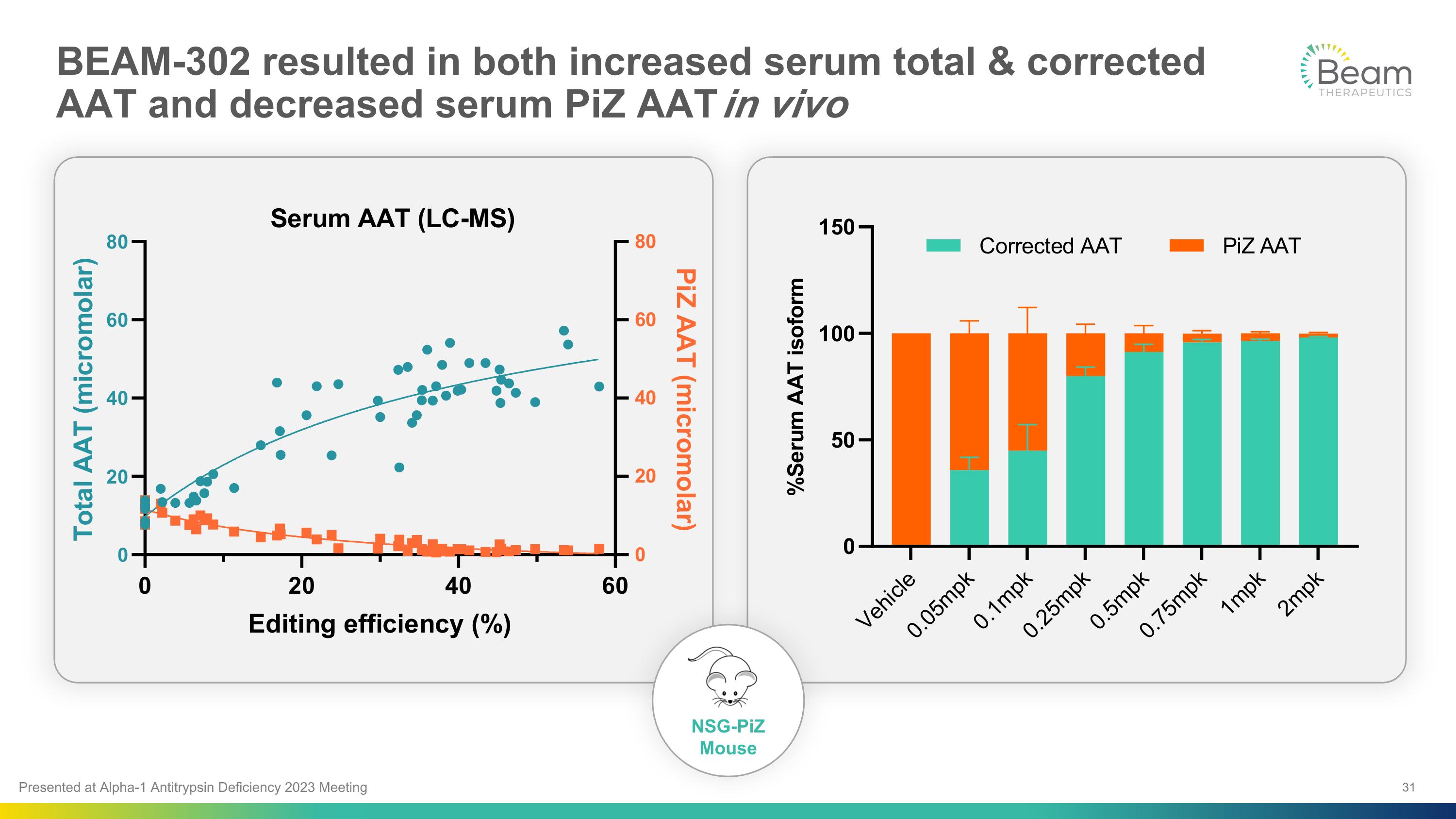
BEAM-302 resulted in both increased serum total & corrected AAT and decreased serum PiZ AAT in vivo NSG-PiZ �Mouse Presented at Alpha-1 Antitrypsin Deficiency 2023 Meeting
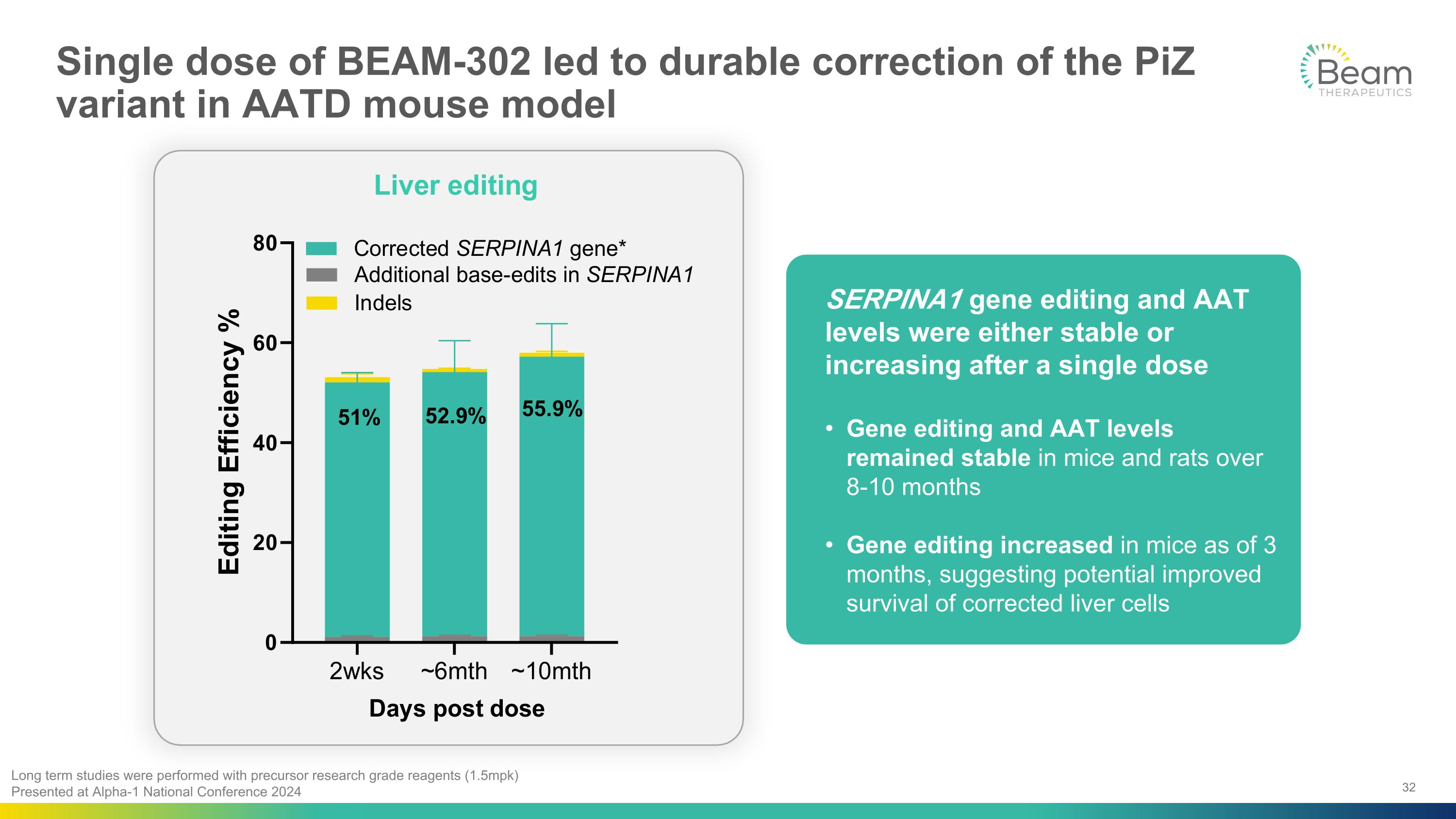
Single dose of BEAM-302 led to durable correction of the PiZ variant in AATD mouse model Long term studies were performed with precursor research grade reagents (1.5mpk) Presented at Alpha-1 National Conference 2024 SERPINA1 gene editing and AAT levels were either stable or increasing after a single dose Gene editing and AAT levels remained stable in mice and rats over 8-10 months Gene editing increased in mice as of 3 months, suggesting potential improved survival of corrected liver cells
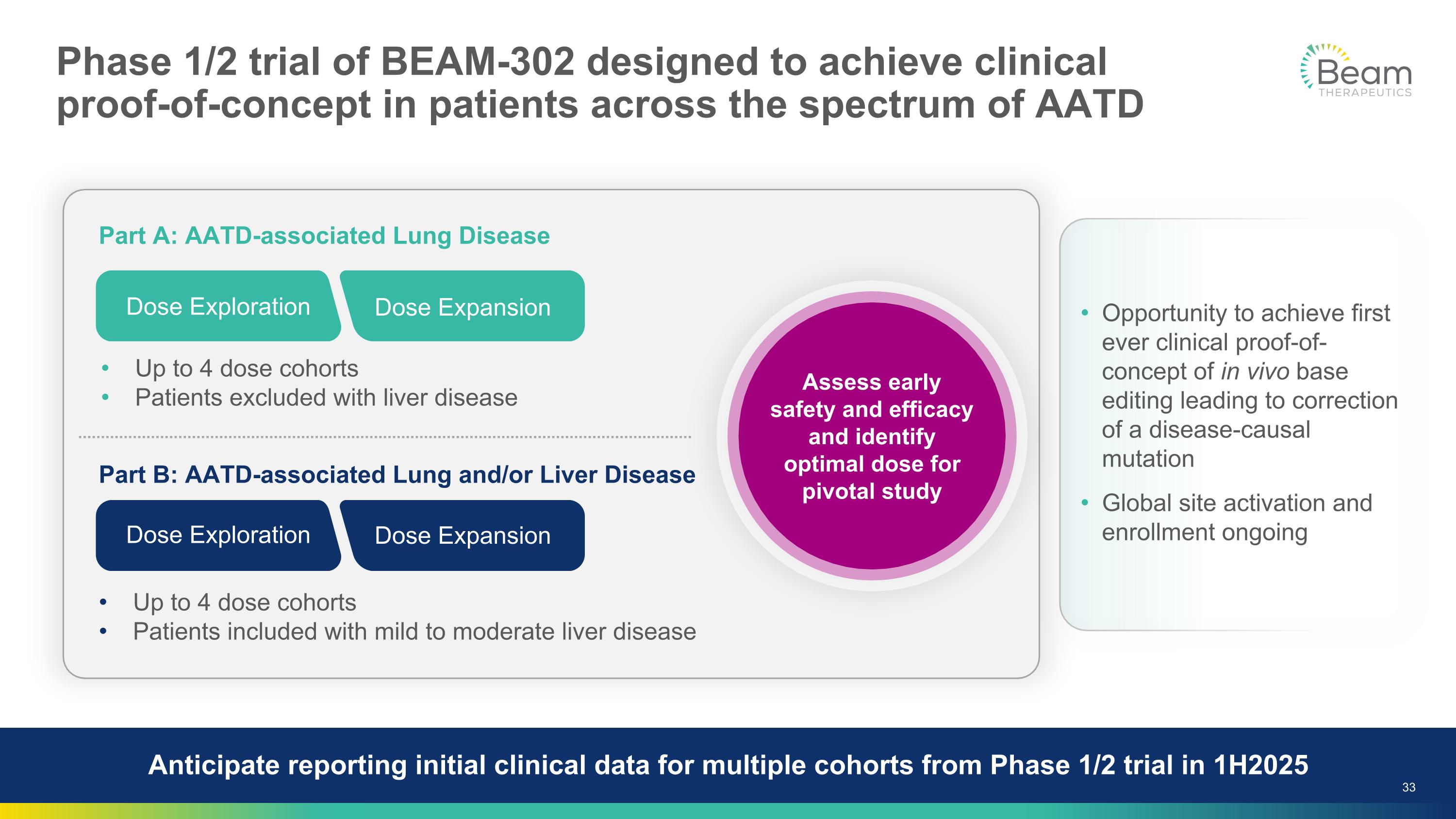
Phase 1/2 trial of BEAM-302 designed to achieve clinical �proof-of-concept in patients across the spectrum of AATD Opportunity to achieve first ever clinical proof-of-concept of in vivo base editing leading to correction of a disease-causal mutation Global site activation and enrollment ongoing Part A: AATD-associated Lung Disease Part B: AATD-associated Lung and/or Liver Disease Assess early safety and efficacy and identify optimal dose for pivotal study Up to 4 dose cohorts Patients included with mild to moderate liver disease Up to 4 dose cohorts Patients excluded with liver disease Dose Exploration Dose Expansion Dose Exploration Dose Expansion Anticipate reporting initial clinical data for multiple cohorts from Phase 1/2 trial in 1H2025
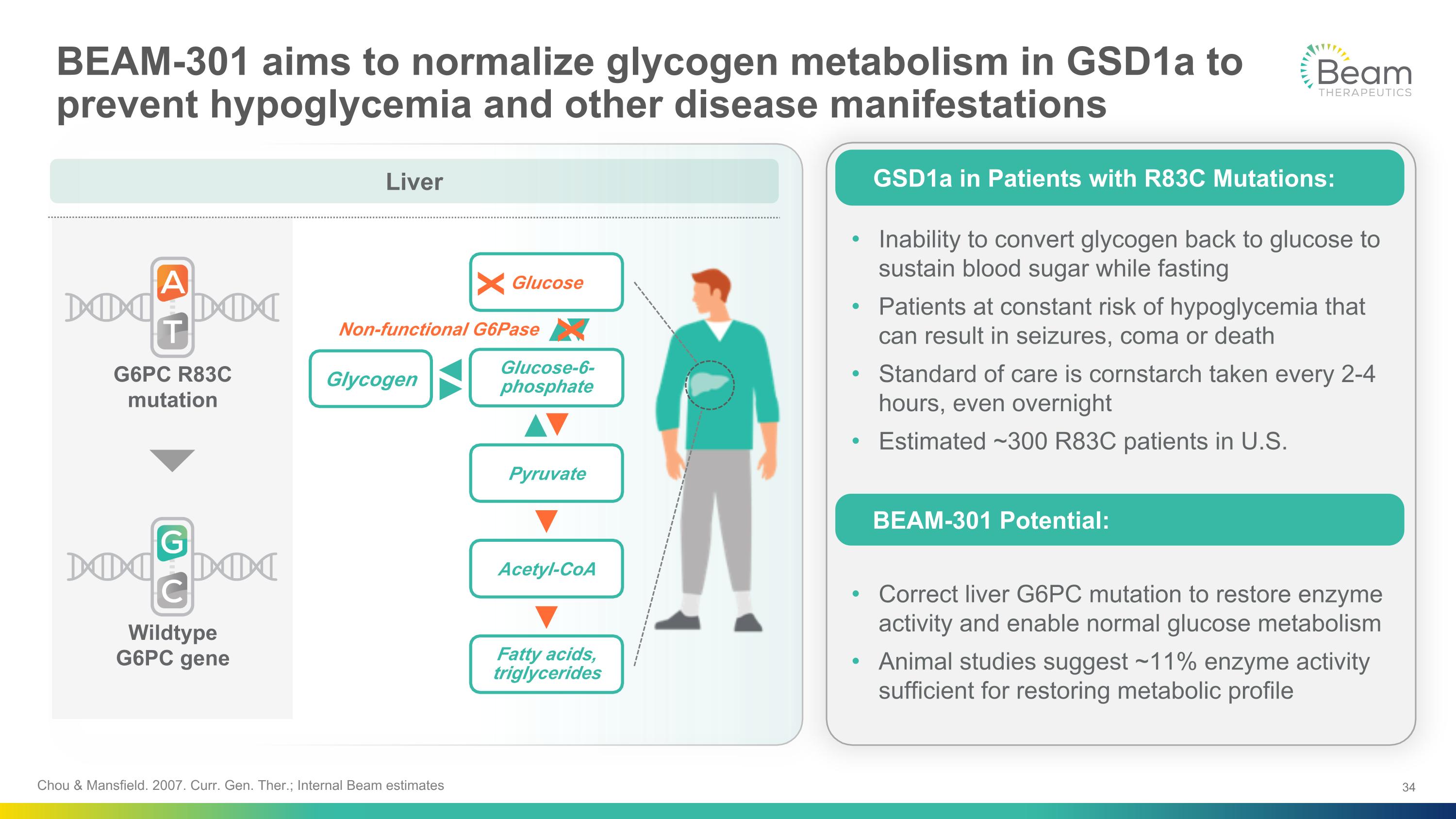
Chou & Mansfield. 2007. Curr. Gen. Ther.; Internal Beam estimates BEAM-301 aims to normalize glycogen metabolism in GSD1a to prevent hypoglycemia and other disease manifestations Liver Wildtype �G6PC gene G6PC R83C mutation Non-functional G6Pase Glycogen Glucose Glucose-6- phosphate Pyruvate Acetyl-CoA Fatty acids, triglycerides X X Inability to convert glycogen back to glucose to sustain blood sugar while fasting Patients at constant risk of hypoglycemia that can result in seizures, coma or death Standard of care is cornstarch taken every 2-4 hours, even overnight Estimated ~300 R83C patients in U.S. Correct liver G6PC mutation to restore enzyme activity and enable normal glucose metabolism Animal studies suggest ~11% enzyme activity sufficient for restoring metabolic profile GSD1a in Patients with R83C Mutations: BEAM-301 Potential:
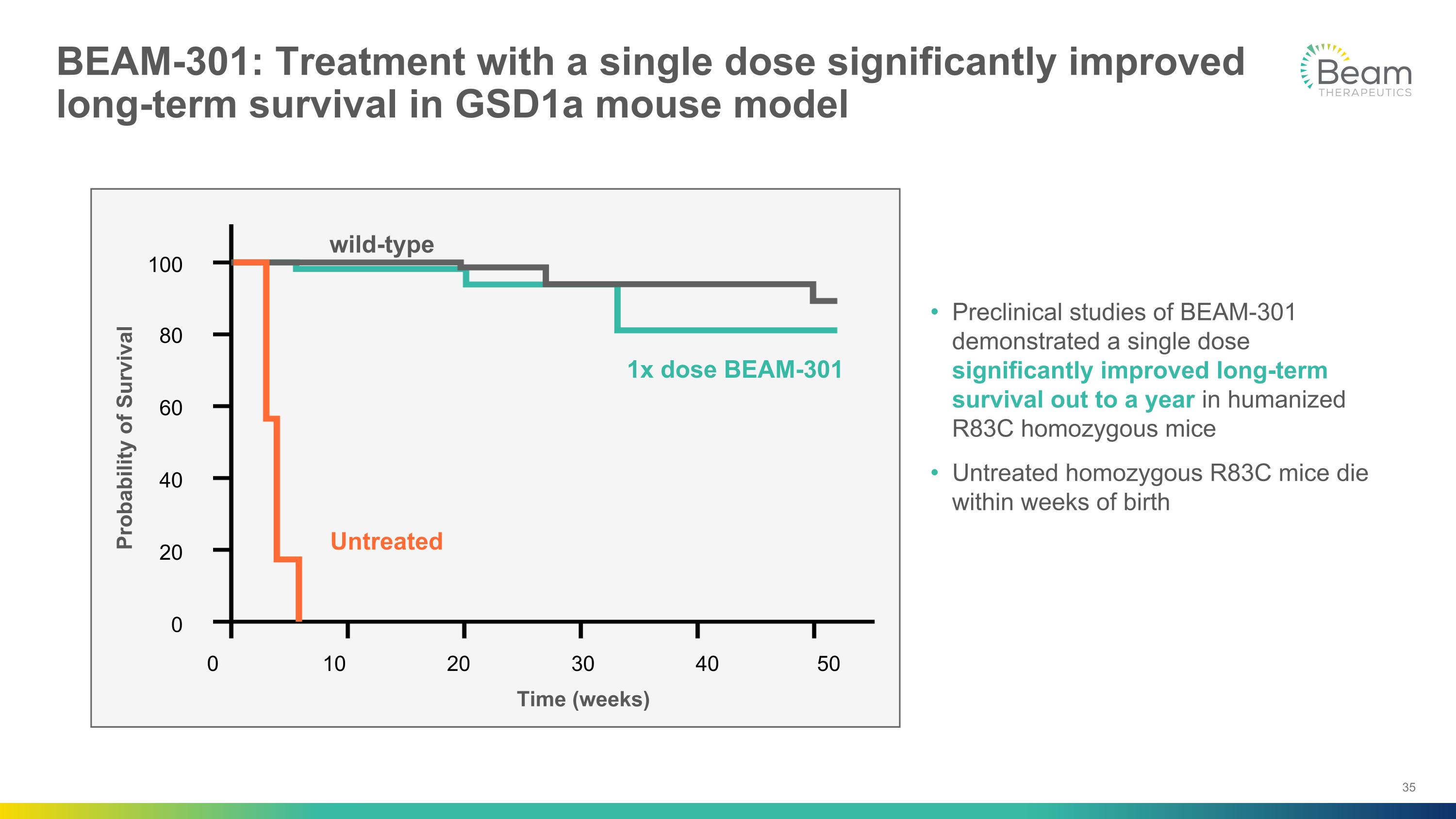
BEAM-301: Treatment with a single dose significantly improved long-term survival in GSD1a mouse model Preclinical studies of BEAM-301 demonstrated a single dose significantly improved long-term survival out to a year in humanized R83C homozygous mice Untreated homozygous R83C mice die within weeks of birth 0 Probability of Survival 20 40 60 80 100 10 20 30 40 50 1x dose BEAM-301 wild-type Untreated Time (weeks) 0
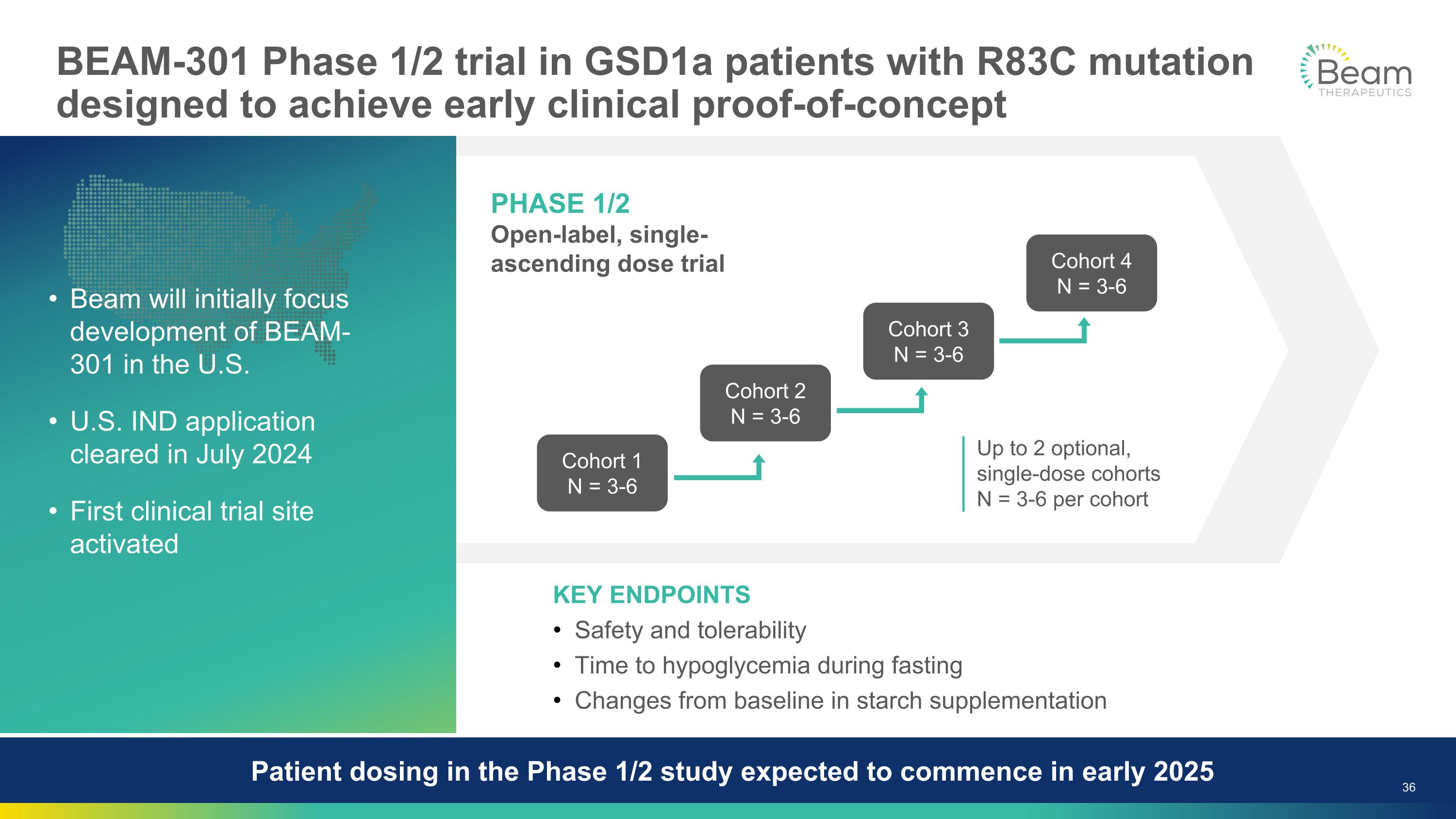
BEAM-301 Phase 1/2 trial in GSD1a patients with R83C mutation designed to achieve early clinical proof-of-concept KEY ENDPOINTS Safety and tolerability Time to hypoglycemia during fasting Changes from baseline in starch supplementation Beam will initially focus development of BEAM-301 in the U.S. U.S. IND application cleared in July 2024 First clinical trial site activated Patient dosing in the Phase 1/2 study expected to commence in early 2025 PHASE 1/2 Open-label, single-ascending dose trial Cohort 1 N = 3-6 Cohort 2 N = 3-6 Cohort 3 N = 3-6 Cohort 4 N = 3-6 Up to 2 optional, single-dose cohorts N = 3-6 per cohort
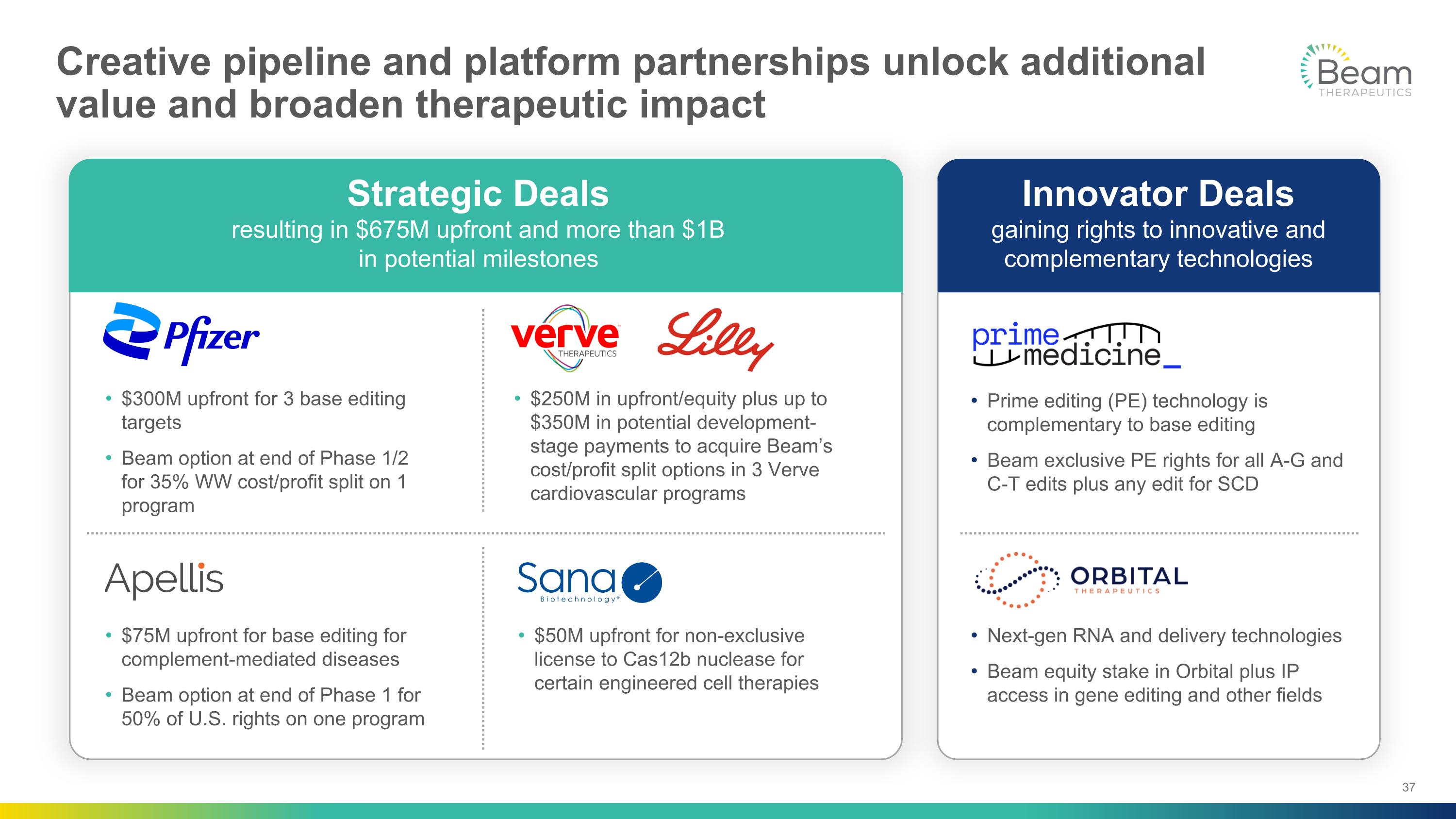
Creative pipeline and platform partnerships unlock additional value and broaden therapeutic impact Strategic Deals resulting in $675M upfront and more than $1B in potential milestones Innovator Deals gaining rights to innovative and complementary technologies $300M upfront for 3 base editing targets Beam option at end of Phase 1/2 for 35% WW cost/profit split on 1 program $75M upfront for base editing for complement-mediated diseases Beam option at end of Phase 1 for 50% of U.S. rights on one program $50M upfront for non-exclusive license to Cas12b nuclease for certain engineered cell therapies $250M in upfront/equity plus up to $350M in potential development-stage payments to acquire Beam’s cost/profit split options in 3 Verve cardiovascular programs Prime editing (PE) technology is complementary to base editing Beam exclusive PE rights for all A-G and C-T edits plus any edit for SCD Next-gen RNA and delivery technologies Beam equity stake in Orbital plus IP access in gene editing and other fields
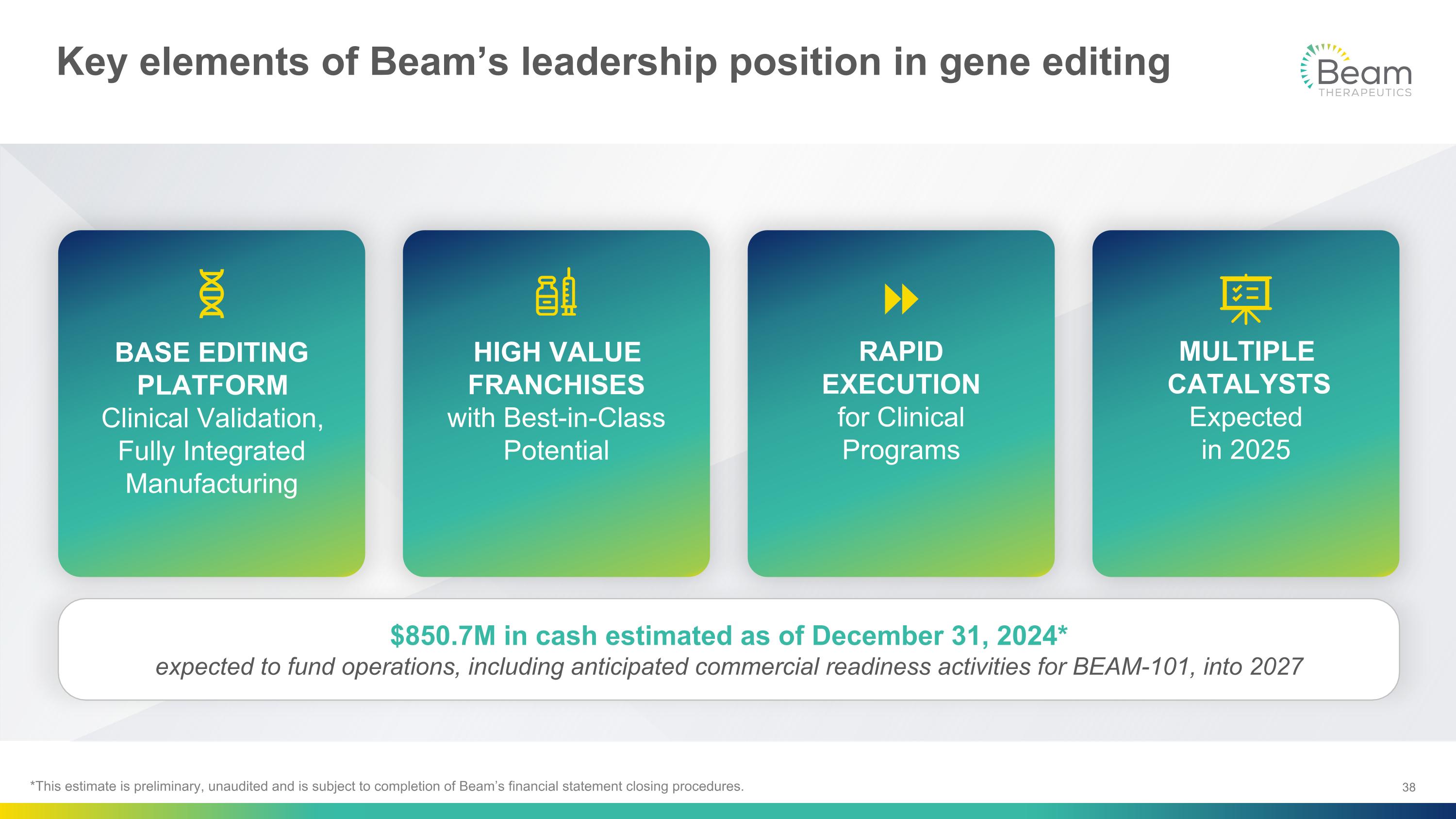
Key elements of Beam’s leadership position in gene editing BASE EDITING PLATFORM�Clinical Validation, Fully Integrated Manufacturing MULTIPLE CATALYSTS Expected �in 2025 RAPID EXECUTION�for Clinical Programs HIGH VALUE FRANCHISES�with Best-in-Class Potential $850.7M in cash estimated as of December 31, 2024* expected to fund operations, including anticipated commercial readiness activities for BEAM-101, into 2027 *This estimate is preliminary, unaudited and is subject to completion of Beam’s financial statement closing procedures.

Kyle SICKLE CELL DISEASE Dan and Kathi ALPHA-1 ANTITRYPSIN DEFICIENCY Alyssa and Gayle GLYCOGEN STORAGE DISEASE 1A Patients are at the heart of our vision






































