
Harnessing Novel Immunobiology February 2019 Exhibit 99.1

Safe Harbor Statement This presentation contains forward-looking statements about Equillium, Inc. (the “Company”). In some cases, you can identify forward-looking statements by the words “will,” “expect,” “intend,” “plan,” “objective,” “believe,” “estimate,” “potential,” “continue” and “ongoing,” or the negative of these terms, or other comparable terminology intended to identify statements about the future. These statements are based on Company management’s current beliefs and expectations. These statements include but are not limited to statements regarding the Company’s business strategy, the Company’s plans to develop and commercialize its product candidates, the safety and efficacy of the Company’s product candidates, the Company’s plans and expected timing with respect to regulatory filings and approvals, and size and growth potential of the markets for the Company’s product candidates. These statements involve substantial known and unknown risks, uncertainties and other factors that may cause the Company’s actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. The Company may not actually achieve the plans, intentions or expectations disclosed in its forward-looking statements, and you should not place undue reliance on the Company’s forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed or implied in the forward-looking statements the Company makes due to the risks and uncertainties inherent in the Company’s business, including without limitation, risk described in the Company’s filings with the Securities and Exchange Commission (“SEC”). You are cautioned not to place undue reliance on these forward-looking statements, which represent the Company’s views as of the date of this presentation. The Company’s anticipates that subsequent events and developments will cause the its views to change. However, while the Company may elect to update these forward-looking statements at some point in the future, the Company has no current intention of doing so except to the extent required by applicable law. These and other risks and uncertainties are described more fully under the caption “Risk Factors” and elsewhere in the Company’s filings and reports, which may be accessed for free by visiting EDGAR on the SEC web site at http://www.sec.gov. and on the Company’s website under the heading “Investors.” All forward-looking statements are qualified in their entirety by this cautionary statement. This caution is made under the “safe harbor” provisions of Section 21E of the Private Securities Litigation Reform Act of 1995.

Executive Summary Validated therapeutic: itolizumab has shown clinical efficacy in multiple indications – studied by Biocon in >330 patients – and is approved for the treatment of psoriasis in India Pipeline-in-a-product: EQ001 has broad potential disease modifying therapeutic utility; launching multiple clinical studies during 2019 First-in-class: Equillium is developing EQ001 (itolizumab) the first antibody targeting the novel immune checkpoint pathway CD6 – for the treatment of severe immuno-inflammatory disorders High-value partnership: Equillium acquired exclusive rights to EQ001 for the U.S. & Canada from Biocon – partnership provides clinical & commercial product, commercial scale production at FDA regulated facility Accomplished team: experienced in drug discovery, development and commercialization EQ financing: IPO completed in Q4 2018 – Jefferies, SVB Leerink, Stifel

Recent Accomplishments and Planned Milestones On track to begin Phase 1b study in Asthma in Q2 2019 On track to begin Phase 1b/2 study in aGVHD in March 2019 Acute Graft Versus Host Disease (aGVHD): awarded Fast Track designation and Orphan Drug designations for both prevention and treatment Nominated Lupus Nephritis as additional target indication with plans to initiate Phase 1b study in 2H 2019

Accomplished Management Team Dan Bradbury Chairman & Chief Executive Officer Bruce Steel, CFA President & Chief Business Officer Christine Zedelmayer Vice-President of Operations Steve Connelly, PhD Chief Scientific Officer Jason Keyes Chief Financial Officer David Essayan, MD Head of Regulatory Affairs Krishna Polu, MD Chief Medical Officer Joel Rothman Vice-President of Development Randy Adams Vice-President of Commercial
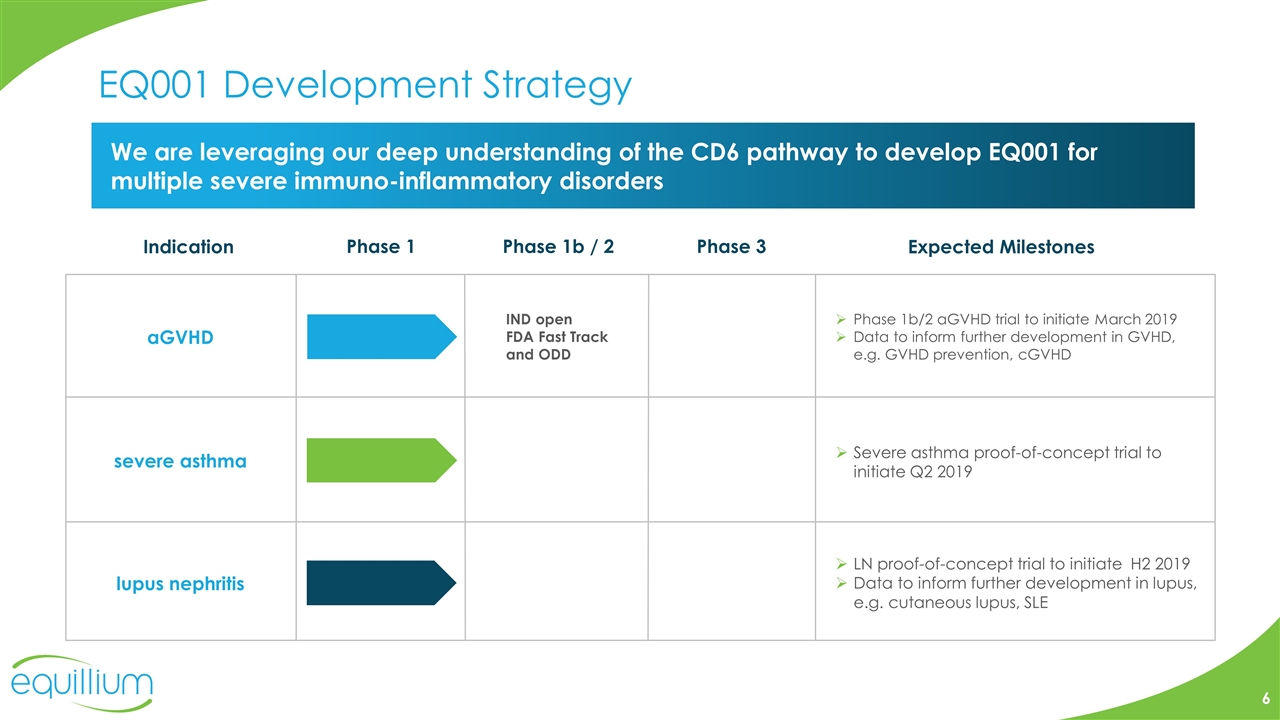
EQ001 Development Strategy We are leveraging our deep understanding of the CD6 pathway to develop EQ001 for multiple severe immuno-inflammatory disorders aGVHD severe asthma Indication Phase 1 Phase 1b / 2 Phase 3 Expected Milestones IND open FDA Fast Track and ODD lupus nephritis Severe asthma proof-of-concept trial to initiate Q2 2019 Phase 1b/2 aGVHD trial to initiate March 2019 Data to inform further development in GVHD, e.g. GVHD prevention, cGVHD LN proof-of-concept trial to initiate H2 2019 Data to inform further development in lupus, e.g. cutaneous lupus, SLE

CD6 Plays a Central Role in Immuno-inflammation
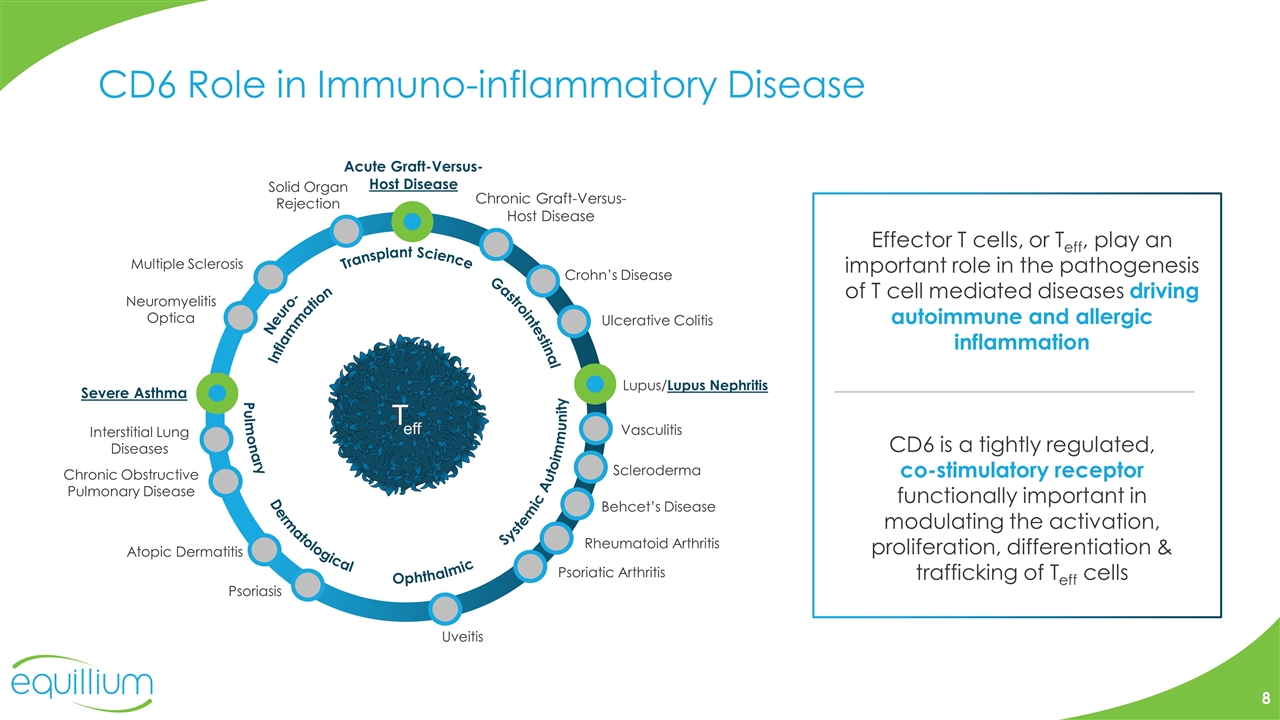
CD6 Role in Immuno-inflammatory Disease Transplant Science Neuro- Inflammation Systemic Autoimmunity Gastrointestinal Ophthalmic Pulmonary Acute Graft-Versus- Host Disease Chronic Graft-Versus-Host Disease Severe Asthma Interstitial Lung Diseases Lupus/Lupus Nephritis Scleroderma Behcet’s Disease Rheumatoid Arthritis Psoriatic Arthritis Psoriasis Uveitis Ulcerative Colitis Crohn’s Disease Multiple Sclerosis Neuromyelitis Optica Dermatological Solid Organ Rejection Chronic Obstructive Pulmonary Disease Atopic Dermatitis Vasculitis Effector T cells, or Teff, play an important role in the pathogenesis of T cell mediated diseases driving autoimmune and allergic inflammation CD6 is a tightly regulated, co-stimulatory receptor functionally important in modulating the activation, proliferation, differentiation & trafficking of Teff cells
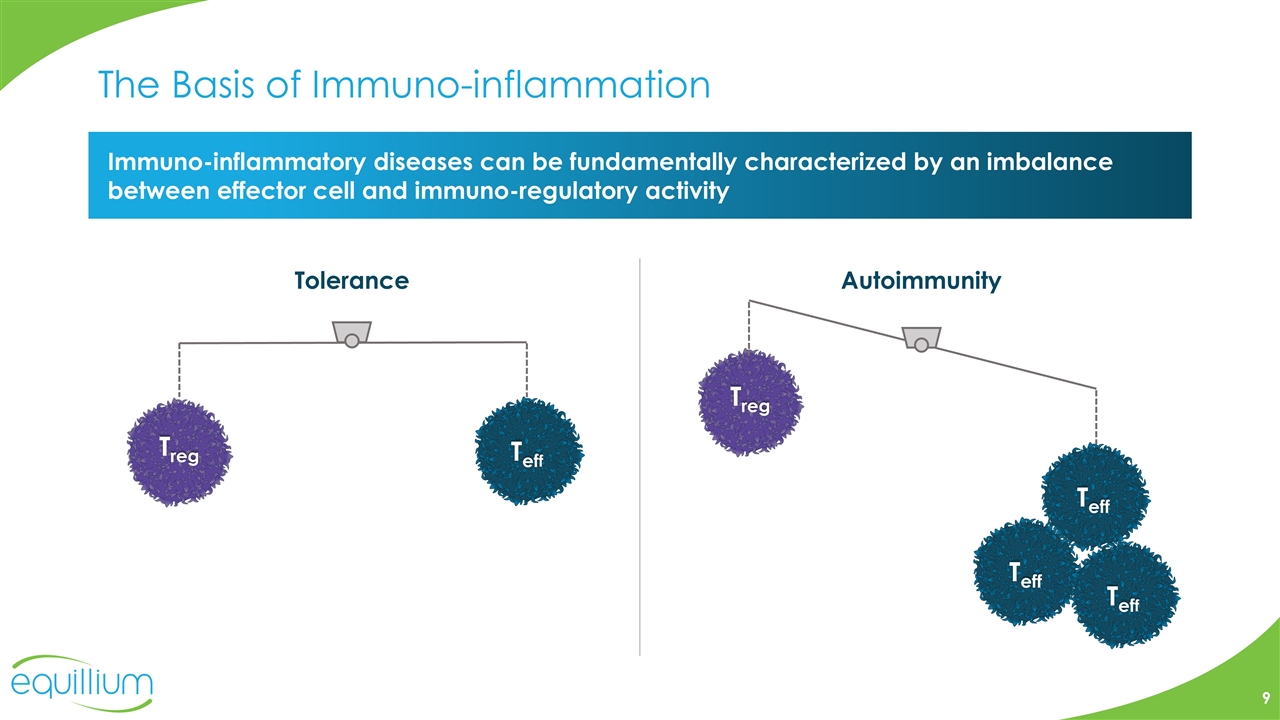
The Basis of Immuno-inflammation Immuno-inflammatory diseases can be fundamentally characterized by an imbalance between effector cell and immuno-regulatory activity Treg Teff Autoimmunity Tolerance Teff Teff Treg Teff
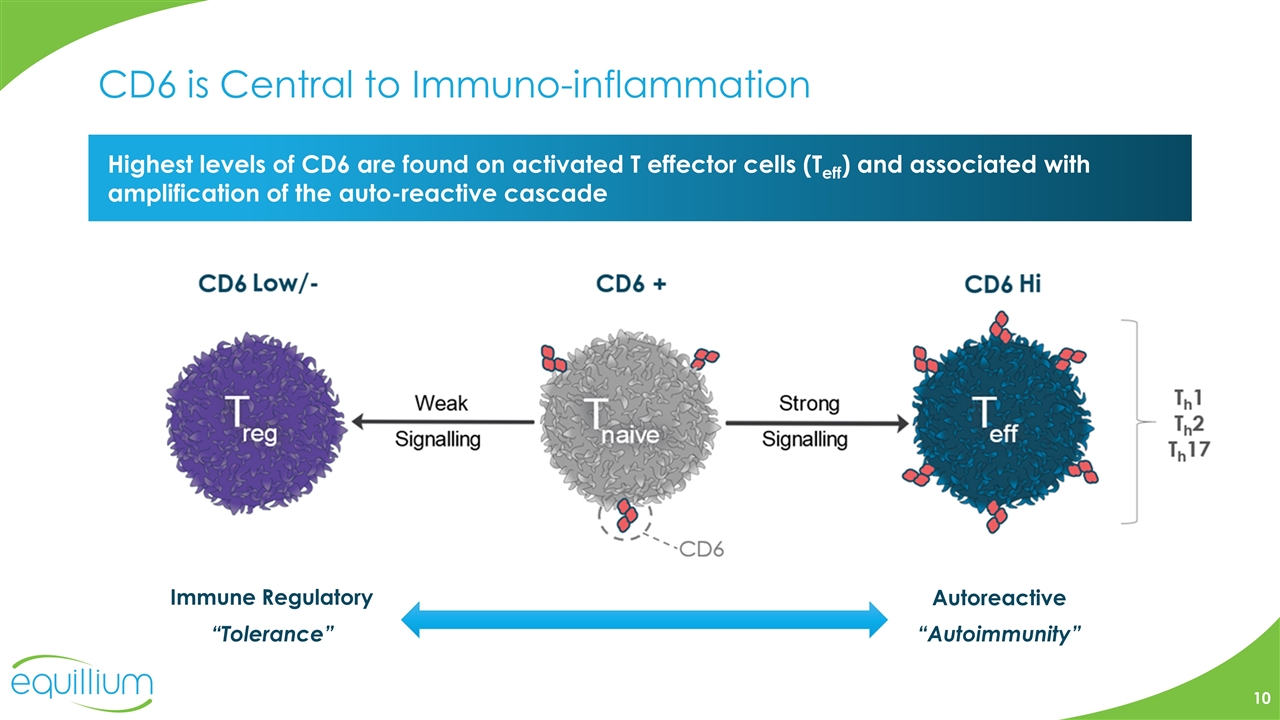
CD6 is Central to Immuno-inflammation Highest levels of CD6 are found on activated T effector cells (Teff) and associated with amplification of the auto-reactive cascade Immune Regulatory “Tolerance” Autoreactive “Autoimmunity”
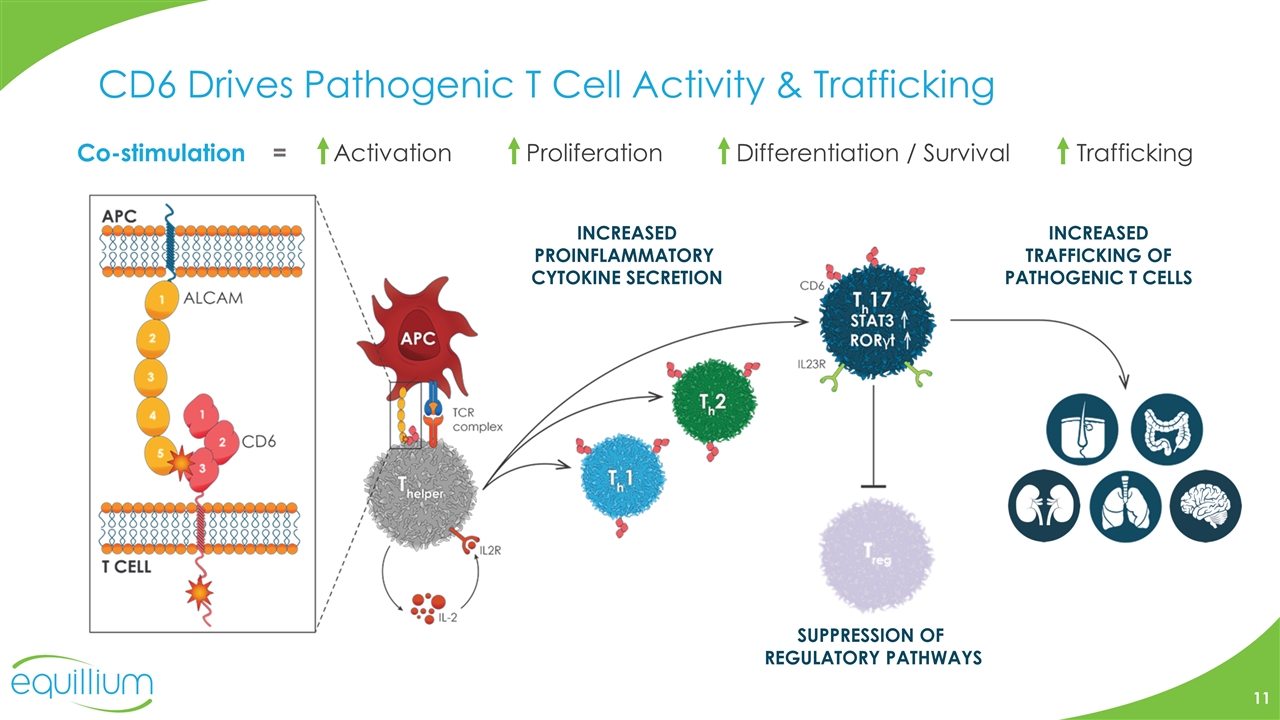
CD6 Drives Pathogenic T Cell Activity & Trafficking Co-stimulation = Activation Proliferation Differentiation / Survival Trafficking INCREASED PROINFLAMMATORY CYTOKINE SECRETION SUPPRESSION OF REGULATORY PATHWAYS INCREASED TRAFFICKING OF PATHOGENIC T CELLS
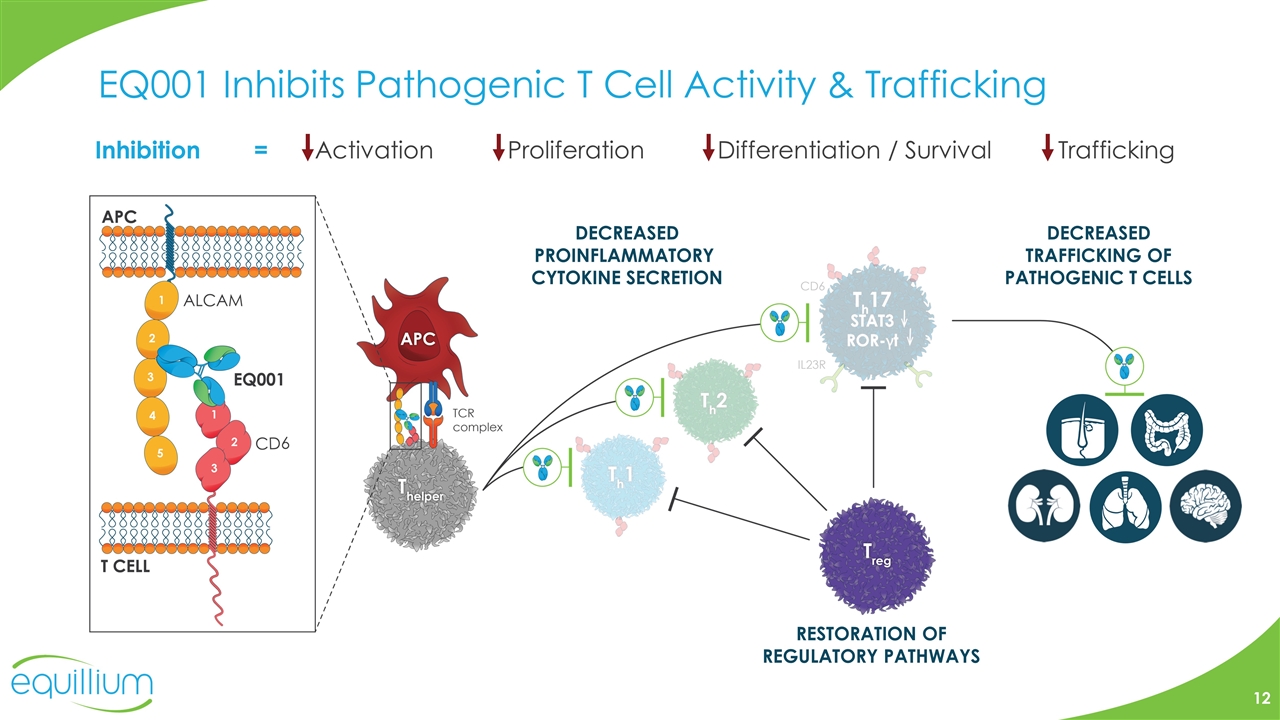
EQ001 Inhibits Pathogenic T Cell Activity & Trafficking Inhibition = Activation Proliferation Differentiation / Survival Trafficking RESTORATION OF REGULATORY PATHWAYS DECREASED PROINFLAMMATORY CYTOKINE SECRETION DECREASED TRAFFICKING OF PATHOGENIC T CELLS
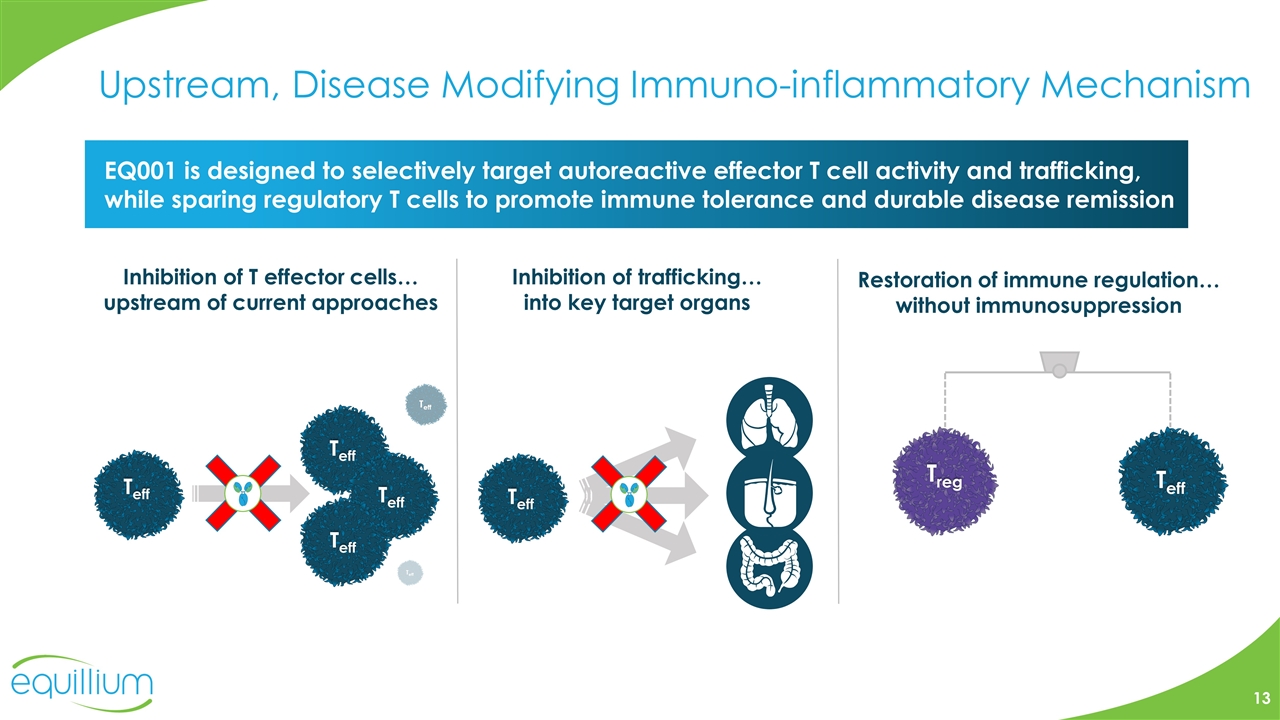
Upstream, Disease Modifying Immuno-inflammatory Mechanism Restoration of immune regulation… without immunosuppression Inhibition of trafficking… into key target organs Inhibition of T effector cells… upstream of current approaches EQ001 is designed to selectively target autoreactive effector T cell activity and trafficking, while sparing regulatory T cells to promote immune tolerance and durable disease remission Treg Teff Teff Teff Teff Teff Teff

Clinical Strategy
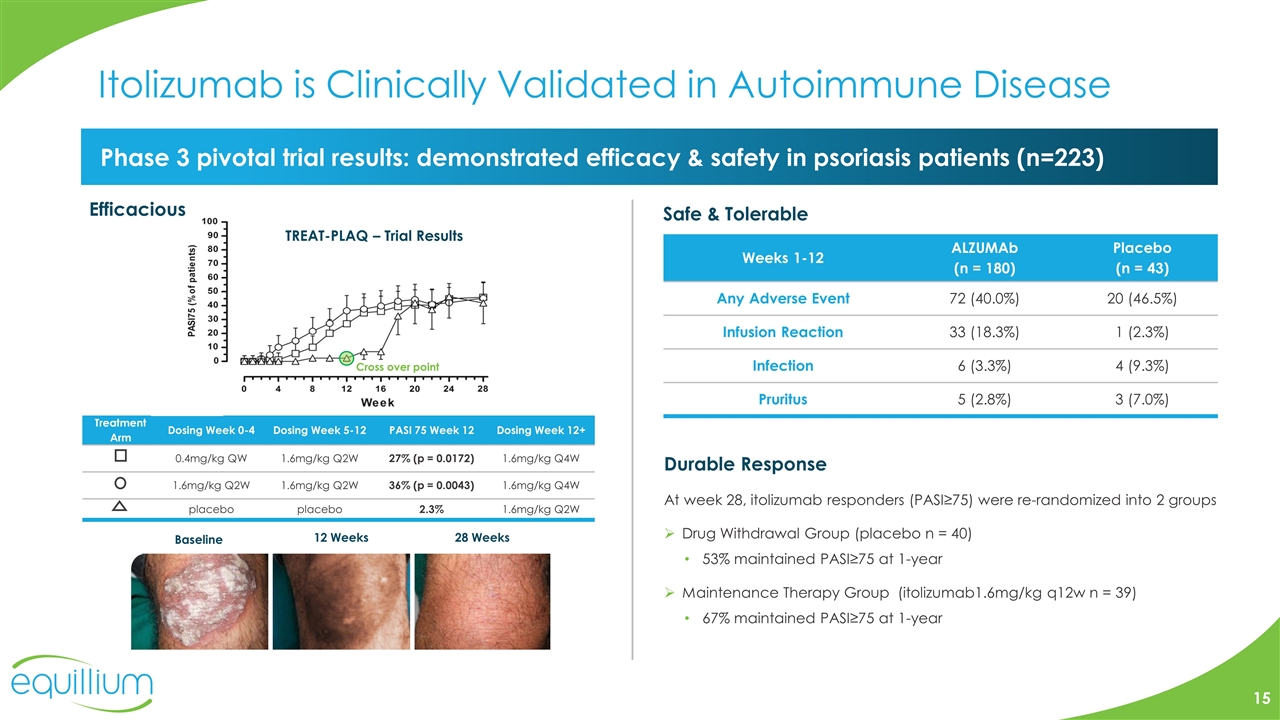
Itolizumab is Clinically Validated in Autoimmune Disease Phase 3 pivotal trial results: demonstrated efficacy & safety in psoriasis patients (n=223) Baseline 12 Weeks 28 Weeks Efficacious Treatment Arm Dosing Week 0-4 Dosing Week 5-12 PASI 75 Week 12 Dosing Week 12+ 0.4mg/kg QW 1.6mg/kg Q2W 27% (p = 0.0172) 1.6mg/kg Q4W 1.6mg/kg Q2W 1.6mg/kg Q2W 36% (p = 0.0043) 1.6mg/kg Q4W placebo placebo 2.3% 1.6mg/kg Q2W Durable Response At week 28, itolizumab responders (PASI≥75) were re-randomized into 2 groups Drug Withdrawal Group (placebo n = 40) 53% maintained PASI≥75 at 1-year Maintenance Therapy Group (itolizumab1.6mg/kg q12w n = 39) 67% maintained PASI≥75 at 1-year Safe & Tolerable Weeks 1-12 ALZUMAb (n = 180) Placebo (n = 43) Any Adverse Event 72 (40.0%) 20 (46.5%) Infusion Reaction 33 (18.3%) 1 (2.3%) Infection 6 (3.3%) 4 (9.3%) Pruritus 5 (2.8%) 3 (7.0%) Cross over point TREAT-PLAQ – Trial Results
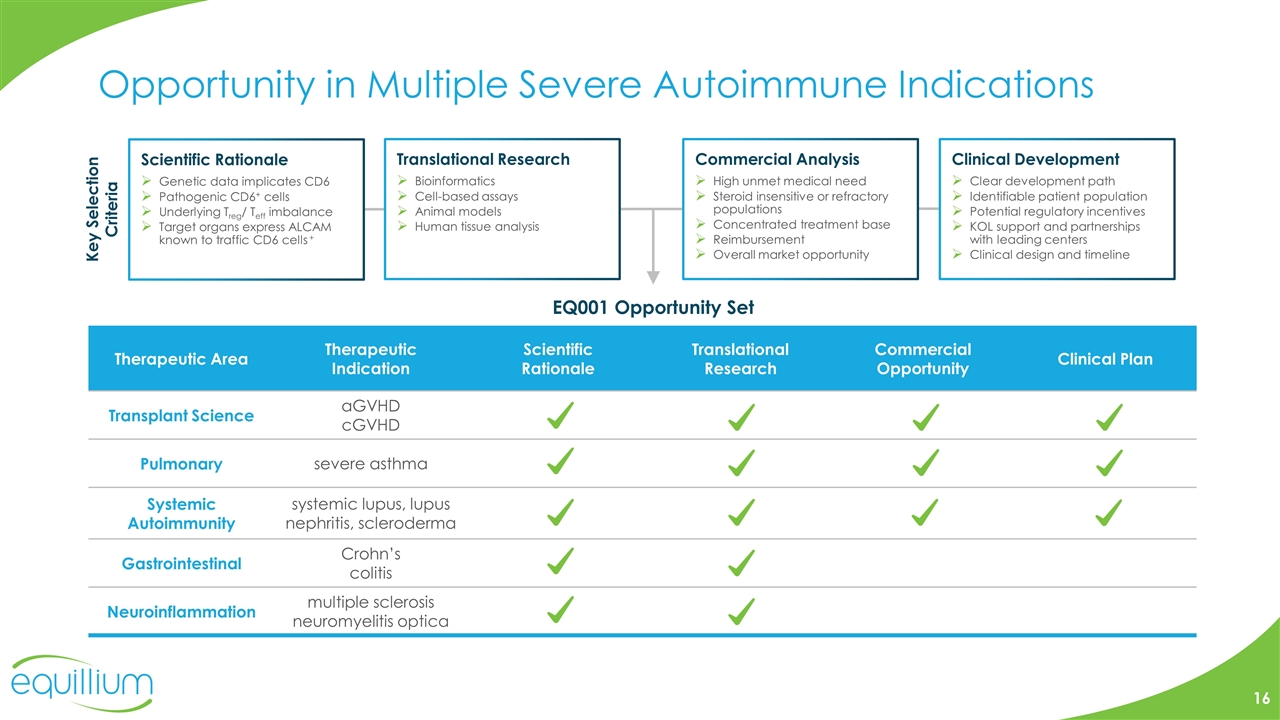
Opportunity in Multiple Severe Autoimmune Indications Therapeutic Area Therapeutic Indication Scientific Rationale Translational Research Commercial Opportunity Clinical Plan Transplant Science aGVHD cGVHD Pulmonary severe asthma Systemic Autoimmunity systemic lupus, lupus nephritis, scleroderma Gastrointestinal Crohn’s colitis Neuroinflammation multiple sclerosis neuromyelitis optica Scientific Rationale Genetic data implicates CD6 Pathogenic CD6+ cells Underlying Treg/ Teff imbalance Target organs express ALCAM known to traffic CD6 cells + Translational Research Bioinformatics Cell-based assays Animal models Human tissue analysis Clinical Development Clear development path Identifiable patient population Potential regulatory incentives KOL support and partnerships with leading centers Clinical design and timeline Commercial Analysis High unmet medical need Steroid insensitive or refractory populations Concentrated treatment base Reimbursement Overall market opportunity Key Selection Criteria EQ001 Opportunity Set
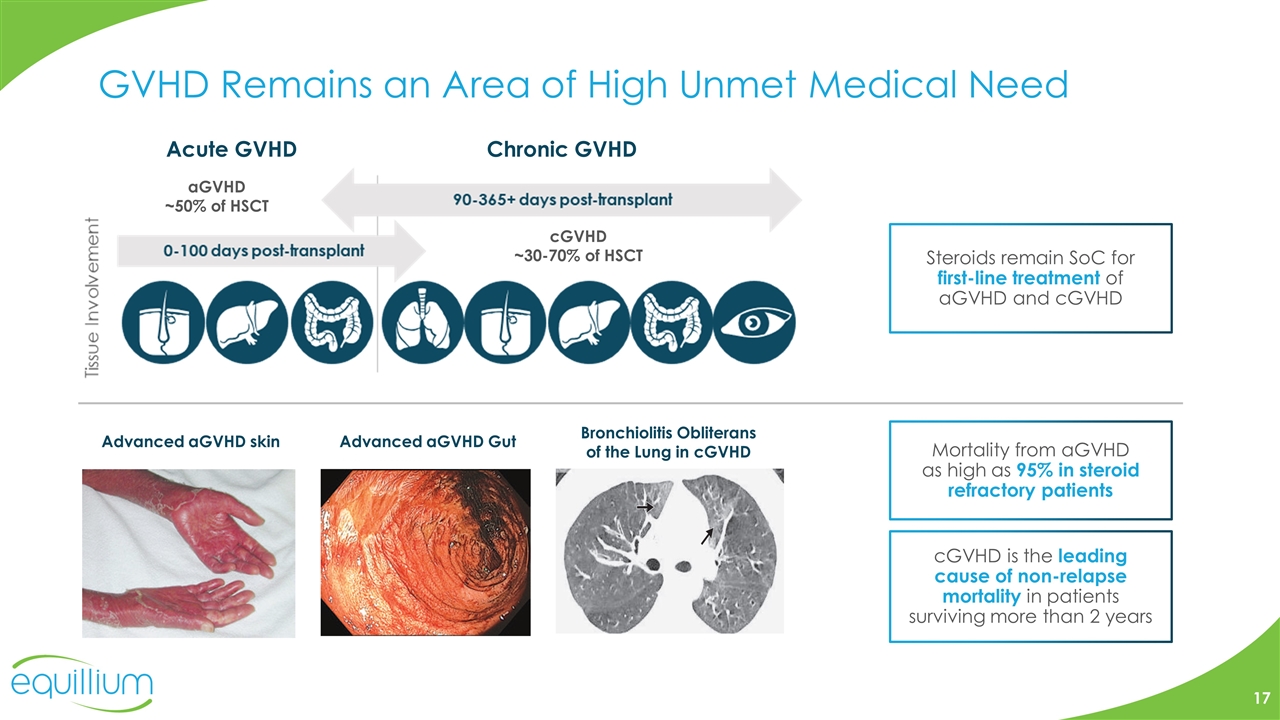
GVHD Remains an Area of High Unmet Medical Need Advanced aGVHD skin Advanced aGVHD Gut Bronchiolitis Obliterans of the Lung in cGVHD aGVHD ~50% of HSCT cGVHD ~30-70% of HSCT Mortality from aGVHD as high as 95% in steroid refractory patients Steroids remain SoC for first-line treatment of aGVHD and cGVHD cGVHD is the leading cause of non-relapse mortality in patients surviving more than 2 years Acute GVHD Chronic GVHD
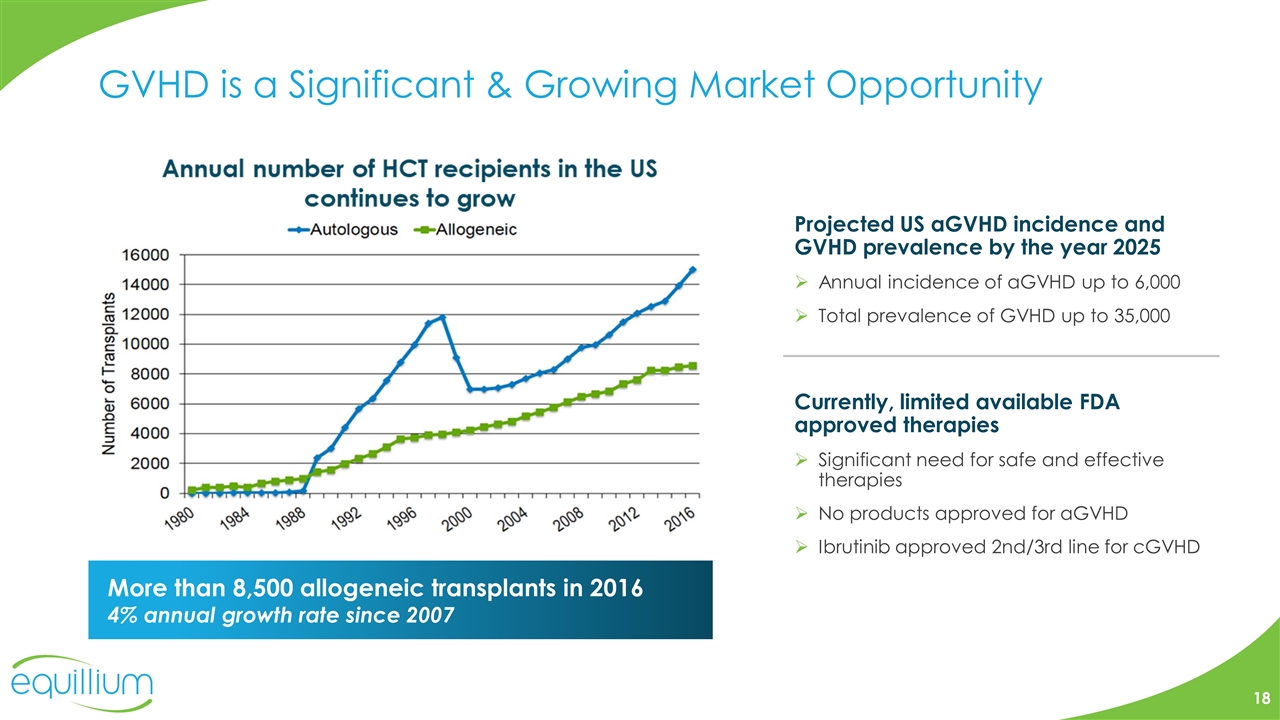
GVHD is a Significant & Growing Market Opportunity Projected US aGVHD incidence and GVHD prevalence by the year 2025 Annual incidence of aGVHD up to 6,000 Total prevalence of GVHD up to 35,000 Currently, limited available FDA approved therapies Significant need for safe and effective therapies No products approved for aGVHD Ibrutinib approved 2nd/3rd line for cGVHD More than 8,500 allogeneic transplants in 2016 4% annual growth rate since 2007
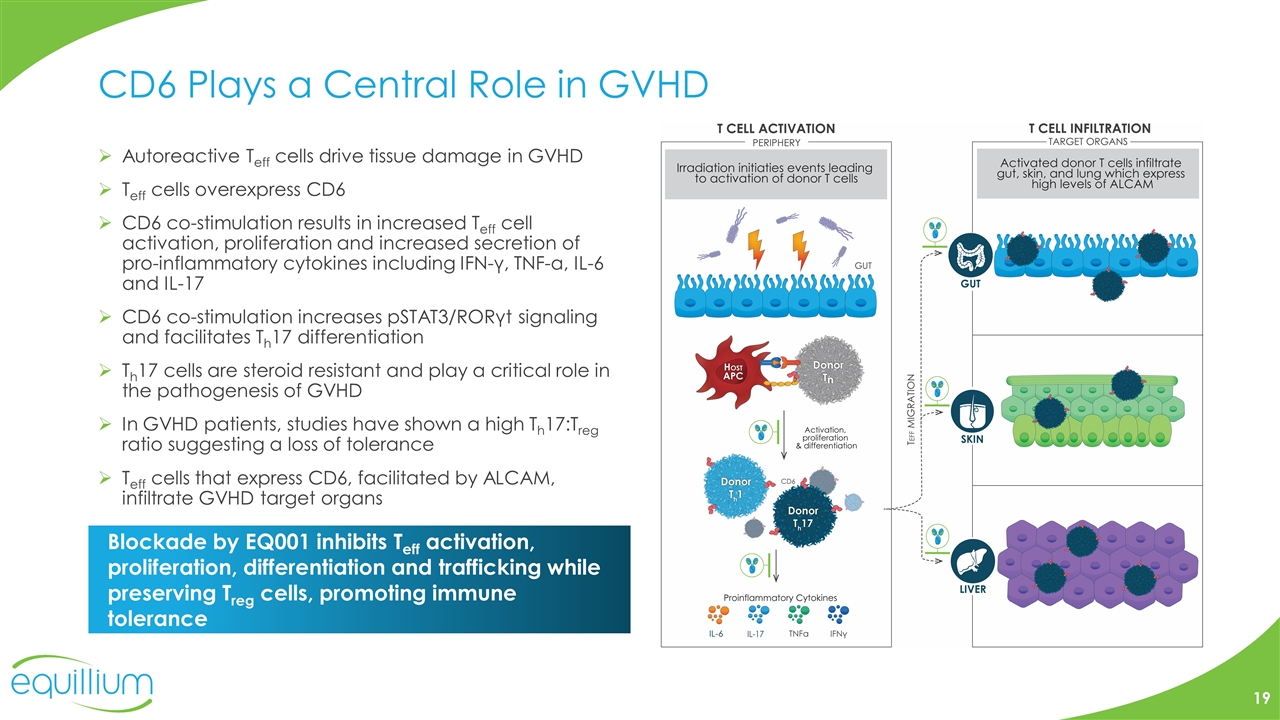
CD6 Plays a Central Role in GVHD Autoreactive Teff cells drive tissue damage in GVHD Teff cells overexpress CD6 CD6 co-stimulation results in increased Teff cell activation, proliferation and increased secretion of pro-inflammatory cytokines including IFN-γ, TNF-α, IL-6 and IL-17 CD6 co-stimulation increases pSTAT3/RORγt signaling and facilitates Th17 differentiation Th17 cells are steroid resistant and play a critical role in the pathogenesis of GVHD In GVHD patients, studies have shown a high Th17:Treg ratio suggesting a loss of tolerance Teff cells that express CD6, facilitated by ALCAM, infiltrate GVHD target organs Blockade by EQ001 inhibits Teff activation, proliferation, differentiation and trafficking while preserving Treg cells, promoting immune tolerance
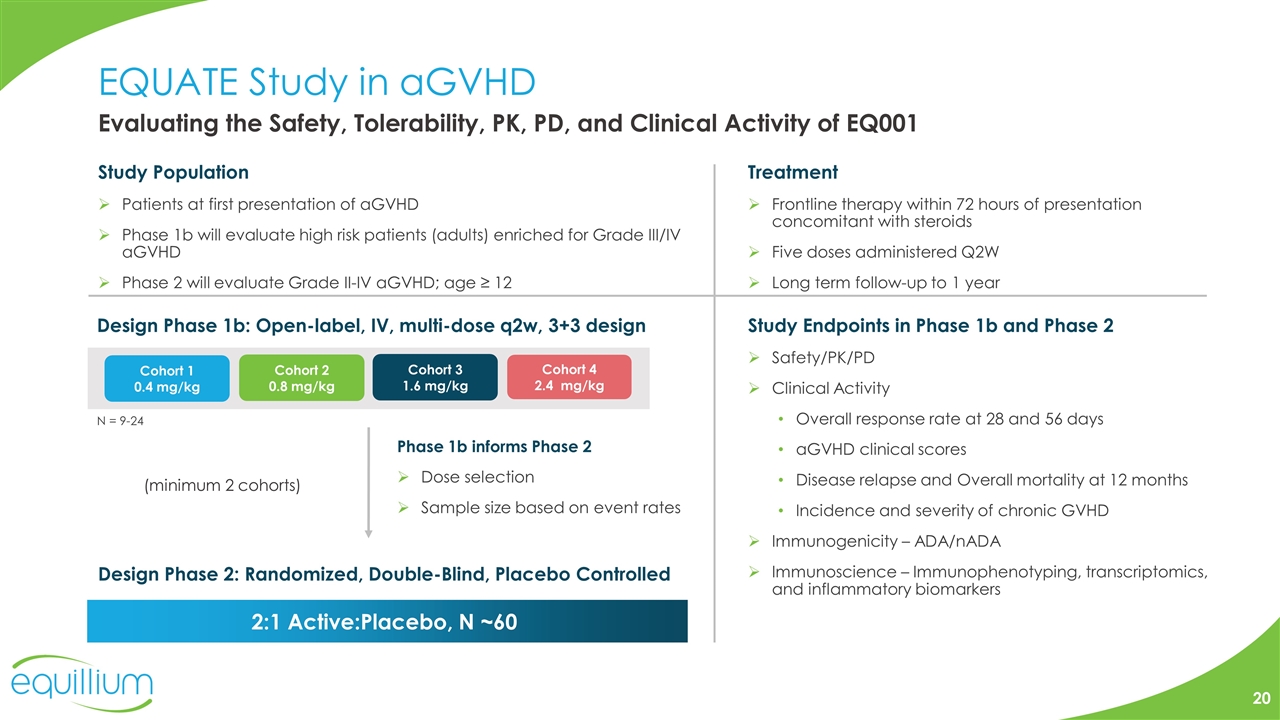
EQUATE Study in aGVHD 2:1 Active:Placebo, N ~60 Treatment Frontline therapy within 72 hours of presentation concomitant with steroids Five doses administered Q2W Long term follow-up to 1 year Study Population Patients at first presentation of aGVHD Phase 1b will evaluate high risk patients (adults) enriched for Grade III/IV aGVHD Phase 2 will evaluate Grade II-IV aGVHD; age ≥ 12 Design Phase 1b: Open-label, IV, multi-dose q2w, 3+3 design (minimum 2 cohorts) Phase 1b informs Phase 2 Dose selection Sample size based on event rates Design Phase 2: Randomized, Double-Blind, Placebo Controlled Study Endpoints in Phase 1b and Phase 2 Safety/PK/PD Clinical Activity Overall response rate at 28 and 56 days aGVHD clinical scores Disease relapse and Overall mortality at 12 months Incidence and severity of chronic GVHD Immunogenicity – ADA/nADA Immunoscience – Immunophenotyping, transcriptomics, and inflammatory biomarkers Evaluating the Safety, Tolerability, PK, PD, and Clinical Activity of EQ001 N = 9-24 Cohort 1 0.4 mg/kg Cohort 2 0.8 mg/kg Cohort 3 1.6 mg/kg Cohort 4 2.4 mg/kg
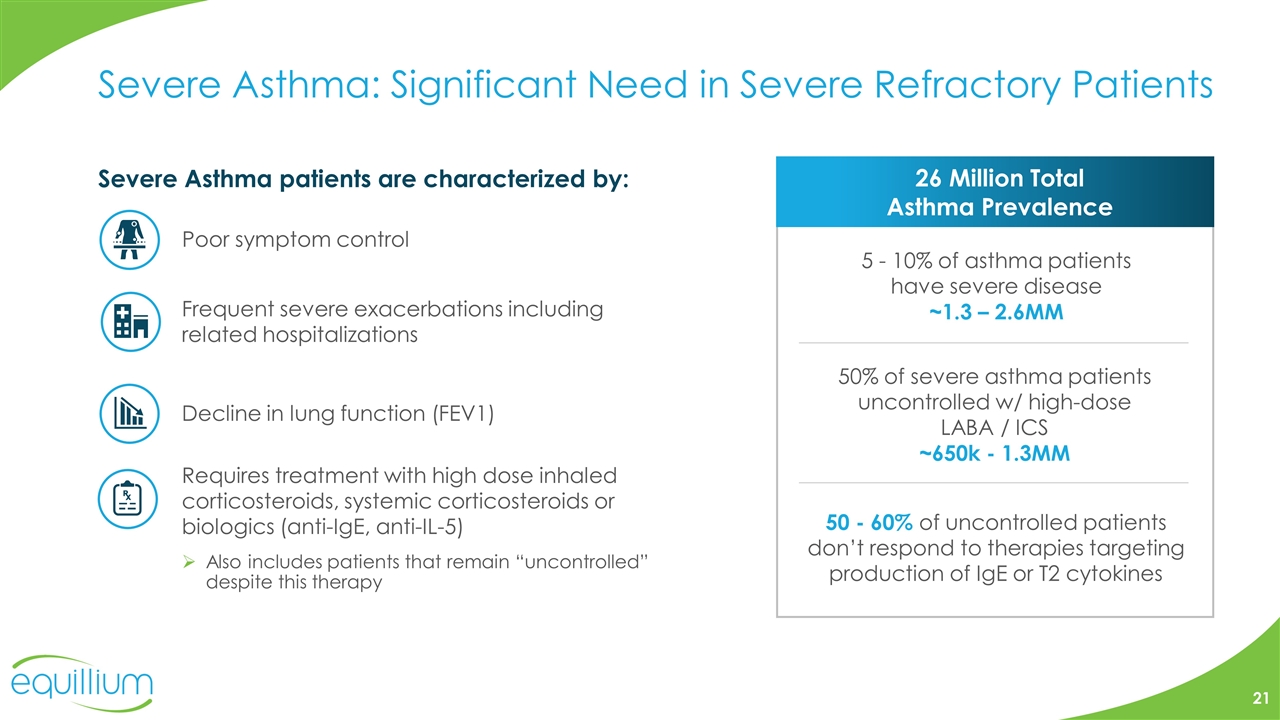
Severe Asthma: Significant Need in Severe Refractory Patients Severe Asthma patients are characterized by: Requires treatment with high dose inhaled corticosteroids, systemic corticosteroids or biologics (anti-IgE, anti-IL-5) Also includes patients that remain “uncontrolled” despite this therapy Poor symptom control Frequent severe exacerbations including related hospitalizations Decline in lung function (FEV1) 26 Million Total Asthma Prevalence 5 - 10% of asthma patients have severe disease ~1.3 – 2.6MM 50% of severe asthma patients uncontrolled w/ high-dose LABA / ICS ~650k - 1.3MM 50 - 60% of uncontrolled patients don’t respond to therapies targeting production of IgE or T2 cytokines
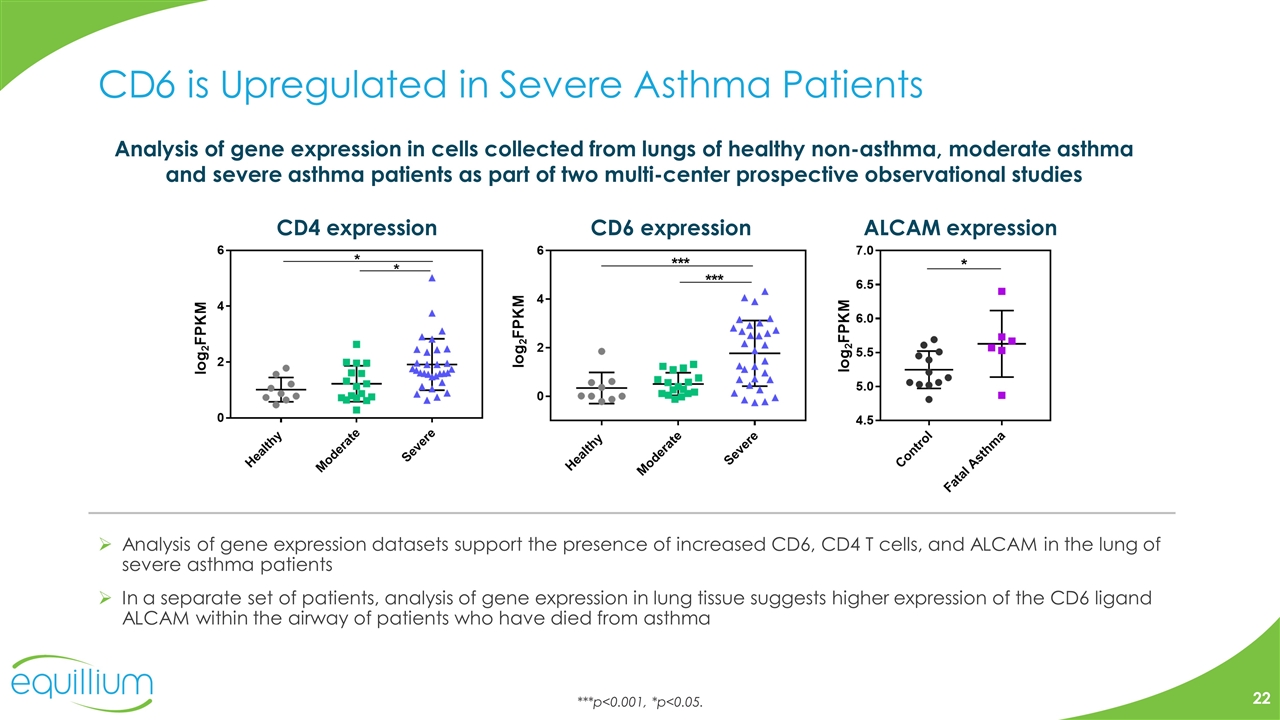
CD6 is Upregulated in Severe Asthma Patients Analysis of gene expression datasets support the presence of increased CD6, CD4 T cells, and ALCAM in the lung of severe asthma patients In a separate set of patients, analysis of gene expression in lung tissue suggests higher expression of the CD6 ligand ALCAM within the airway of patients who have died from asthma Analysis of gene expression in cells collected from lungs of healthy non-asthma, moderate asthma and severe asthma patients as part of two multi-center prospective observational studies ***p<0.001, *p<0.05. CD4 expression CD6 expression ALCAM expression
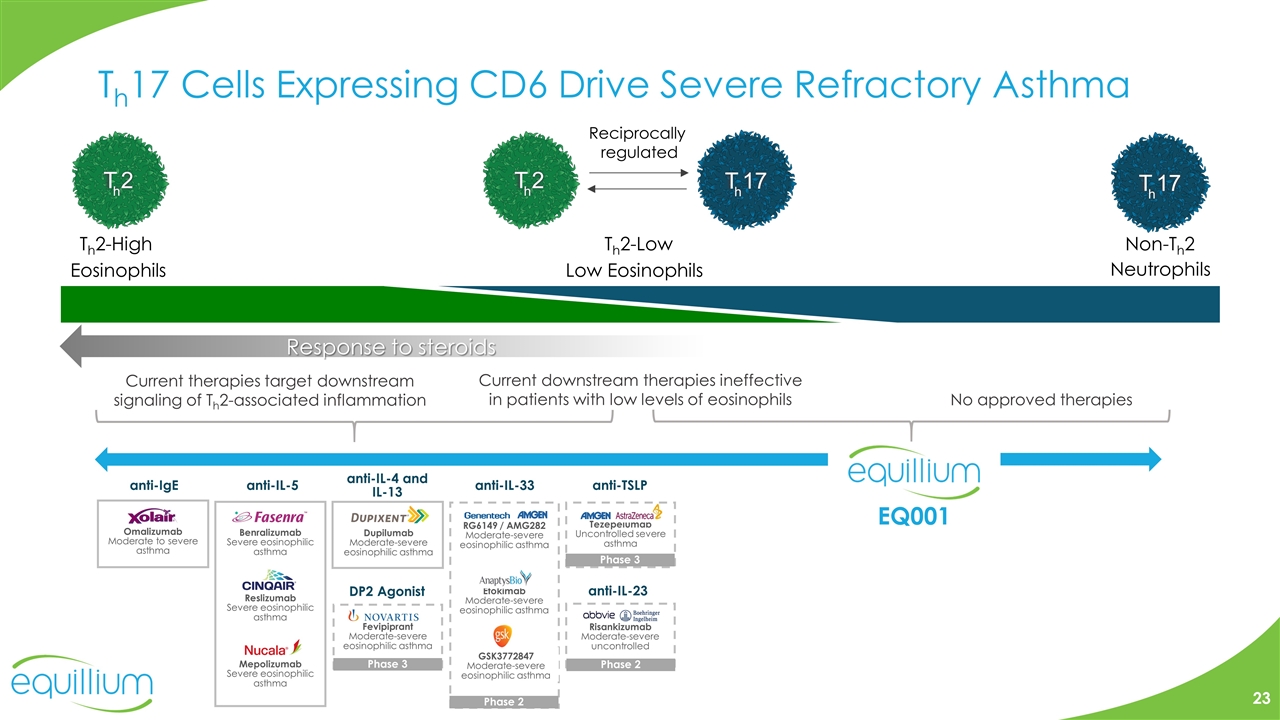
Th17 Cells Expressing CD6 Drive Severe Refractory Asthma Current therapies target downstream signaling of Th2-associated inflammation Current downstream therapies ineffective in patients with low levels of eosinophils No approved therapies EQ001 anti-TSLP anti-IgE anti-IL-5 DP2 Agonist Mepolizumab Severe eosinophilic asthma Reslizumab Severe eosinophilic asthma anti-IL-4 and IL-13 anti-IL-33 Omalizumab Moderate to severe asthma Benralizumab Severe eosinophilic asthma Dupilumab Moderate-severe eosinophilic asthma Fevipiprant Moderate-severe eosinophilic asthma Phase 3 RG6149 / AMG282 Moderate-severe eosinophilic asthma Etokimab Moderate-severe eosinophilic asthma GSK3772847 Moderate-severe eosinophilic asthma Phase 2 Tezepelumab Uncontrolled severe asthma Phase 3 anti-IL-23 Risankizumab Moderate-severe uncontrolled Phase 2 Th2-High Eosinophils Non-Th2 Neutrophils Th2-Low Low Eosinophils Reciprocally regulated Response to steroids
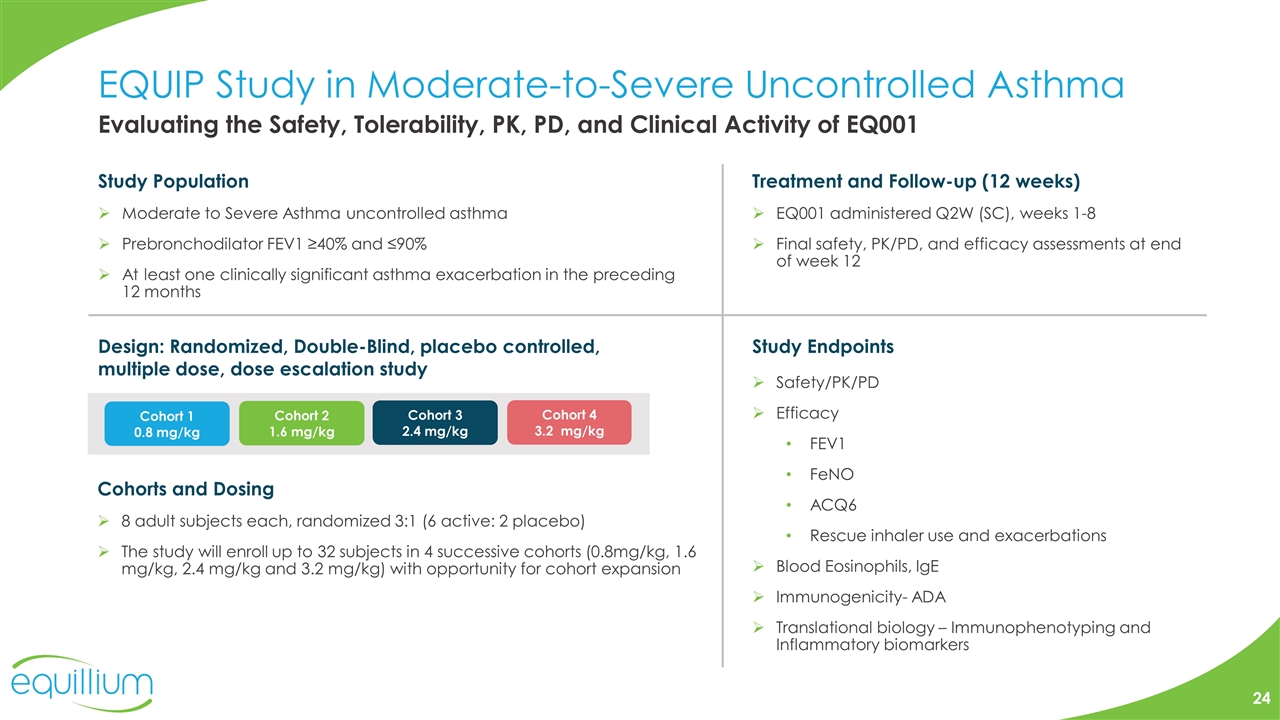
EQUIP Study in Moderate-to-Severe Uncontrolled Asthma Design: Randomized, Double-Blind, placebo controlled, multiple dose, dose escalation study Treatment and Follow-up (12 weeks) EQ001 administered Q2W (SC), weeks 1-8 Final safety, PK/PD, and efficacy assessments at end of week 12 Study Population Moderate to Severe Asthma uncontrolled asthma Prebronchodilator FEV1 ≥40% and ≤90% At least one clinically significant asthma exacerbation in the preceding 12 months Cohorts and Dosing 8 adult subjects each, randomized 3:1 (6 active: 2 placebo) The study will enroll up to 32 subjects in 4 successive cohorts (0.8mg/kg, 1.6 mg/kg, 2.4 mg/kg and 3.2 mg/kg) with opportunity for cohort expansion Study Endpoints Safety/PK/PD Efficacy FEV1 FeNO ACQ6 Rescue inhaler use and exacerbations Blood Eosinophils, IgE Immunogenicity- ADA Translational biology – Immunophenotyping and Inflammatory biomarkers Evaluating the Safety, Tolerability, PK, PD, and Clinical Activity of EQ001 Cohort 1 0.8 mg/kg Cohort 2 1.6 mg/kg Cohort 3 2.4 mg/kg Cohort 4 3.2 mg/kg
Significant Unmet Need in Lupus Nephritis Despite SoC, up to 40% of patients with severe proliferative disease will progress to end-stage renal disease (ESRD) Systemic lupus erythematosus (SLE) is a heterogeneous, multisystem, auto-immune disease characterized by the presence of multiple autoantibodies and deposition of immune complexes in various tissues Lupus Nephritis (LN) is the most frequent and serious manifestation, affecting 30-60% of SLE patients As many as 50% - 75% of LN patients are refractory to standard of care treatment; for those that respond the majority will relapse within five years There are >100,000 Lupus Nephritis Patients in the U.S.
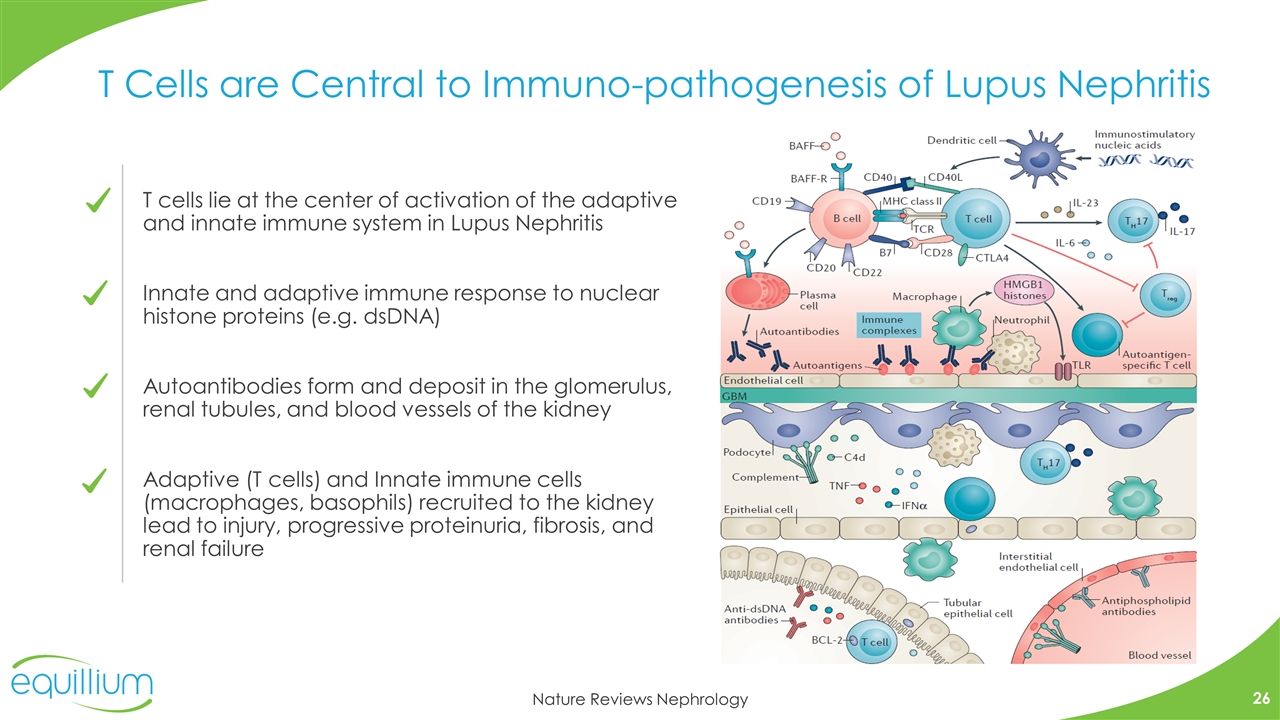
Nature Reviews Nephrology T Cells are Central to Immuno-pathogenesis of Lupus Nephritis T cells lie at the center of activation of the adaptive and innate immune system in Lupus Nephritis Innate and adaptive immune response to nuclear histone proteins (e.g. dsDNA) Autoantibodies form and deposit in the glomerulus, renal tubules, and blood vessels of the kidney Adaptive (T cells) and Innate immune cells (macrophages, basophils) recruited to the kidney lead to injury, progressive proteinuria, fibrosis, and renal failure
Supportive preclinical data from mouse models of lupus and glomerulonephritis (GN) Treatment with anti-CD6 mAb shows improvement in disease activity in Lupus model Treatment with anti-CD6 mAbs shows improvement in renal function in GN model Analysis of LN kidney biopsies show upregulation of CD6 in infiltrating T cells and an increase in ALCAM expression in APCs and broadly in resident renal cells Elevations in urinary ALCAM identifies patients with active lupus nephritis Additional translational studies ongoing to expand understanding of anti-CD6 therapy in LN and to profile patient blood and urine biomarkers for soluble CD6 and ALCAM Translational Data Supports Targeting CD6 in Lupus Nephritis
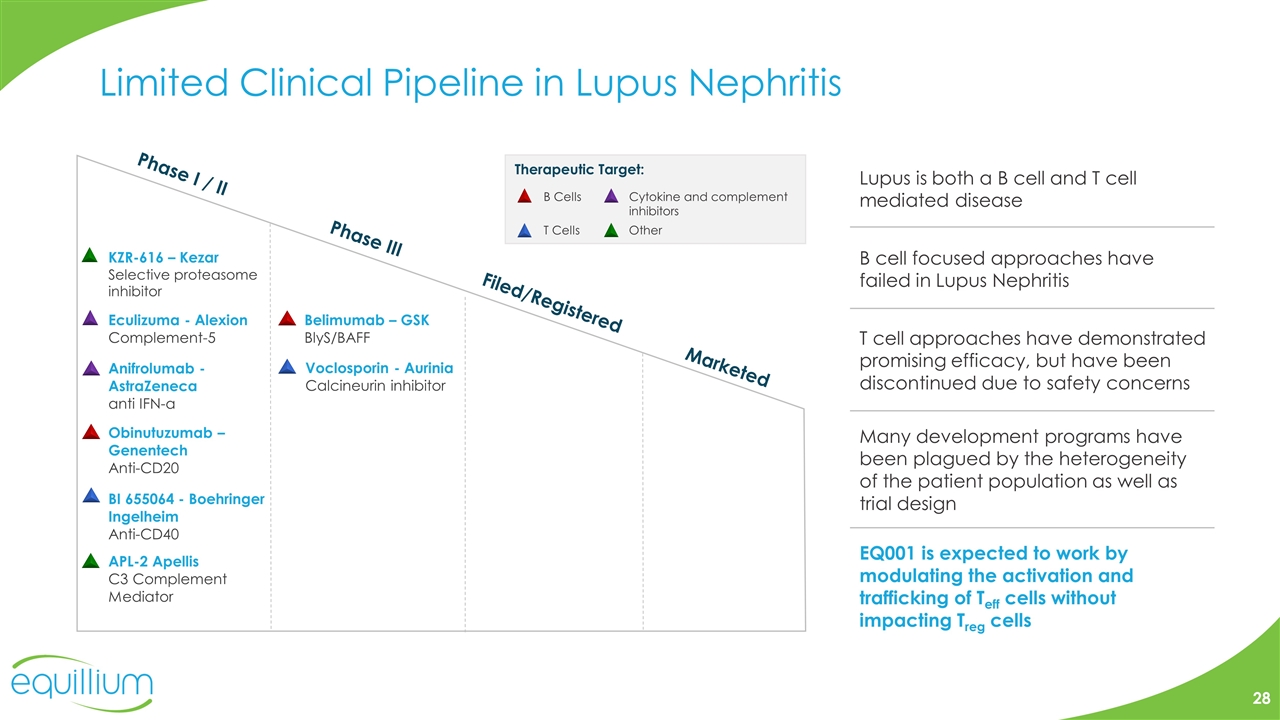
Limited Clinical Pipeline in Lupus Nephritis T cell approaches have demonstrated promising efficacy, but have been discontinued due to safety concerns B cell focused approaches have failed in Lupus Nephritis Lupus is both a B cell and T cell mediated disease Many development programs have been plagued by the heterogeneity of the patient population as well as trial design EQ001 is expected to work by modulating the activation and trafficking of Teff cells without impacting Treg cells 28 Phase I / II Phase III Filed/Registered Marketed Belimumab – GSK BlyS/BAFF Eculizuma - Alexion Complement-5 KZR-616 – Kezar Selective proteasome inhibitor Anifrolumab - AstraZeneca anti IFN-α Obinutuzumab – Genentech Anti-CD20 BI 655064 - Boehringer Ingelheim Anti-CD40 Voclosporin - Aurinia Calcineurin inhibitor APL-2 Apellis C3 Complement Mediator Other B Cells T Cells Cytokine and complement inhibitors Therapeutic Target:

Urinary Biomarkers May Guide Development Plan Leveraging urinary biomarkers can optimize drug development in Lupus Nephritis Address heterogeneity – determine patient response by urinary levels of CD6-ALCAM Optimize Therapeutic Index – urinary biomarkers to optimize dose selection and maximize therapeutic index Efficient Trial Design – Use urinary biomarker assessments to guide efficacy analysis plan and open up optionality for regulatory paths forward A number of therapies have been developed in LN and SLE that have failed. Reasons for failure have included: Trial Design Safety/ Therapeutic Index Heterogeneity of patient population – Differences in Disease Mechanisms by Patient 1 2 3 Biomarkers may help identify the right patient and maximize the benefit-risk for a given therapy and increase probability of successful drug development
Lupus Nephritis Next Steps Expand assessment of CD6-ALCAM pathway urinary biomarkers as part of companion diagnostic strategy We plan to initiate a Phase 1b proof-of-concept study in the second half of 2019 Trial will enroll adults with active proliferative Lupus Nephritis (Class III or IV +/- Class V) Study objectives will include an assessment of PK/PD, dose finding, safety, efficacy, disease biomarkers
Corporate
Partnership with a Leading Global Biopharma Company Leading global biologics manufacturer with FDA regulated cGMP facilities World’s fourth largest insulin producer, and leading bio-similar developer: insulin glargine (Lantus®), adalimumab (Humira®), bevacizumab (Avastin®), etanercept (Enbrel®) Trastuzumab (Herceptin®) & pegfilgrastim (Neulasta®) FDA approved for U.S. market India’s largest fully-integrated biopharma company ~$5 billion market capitalization

: Partnership for U.S. & Canada Equillium exclusive licensee to itolizumab for U.S. & Canada Biocon is a significant minority shareholder in Equillium Biocon partnership provides clinical and commercial supply of our drug product: Biocon has completed CMC & manufactures drug product at commercial scale in FDA regulated facility Drug product for 3 concurrent orphan indications until first U.S. approval at no cost, all other clinical supply at cost Exclusive supplier for commercial product Milestones & royalties: Biocon receives milestones and royalty on Equillium sales Equillium receives royalty on Biocon sales* * Royalty to Equillium on any approved indications leveraging Equillium clinical data
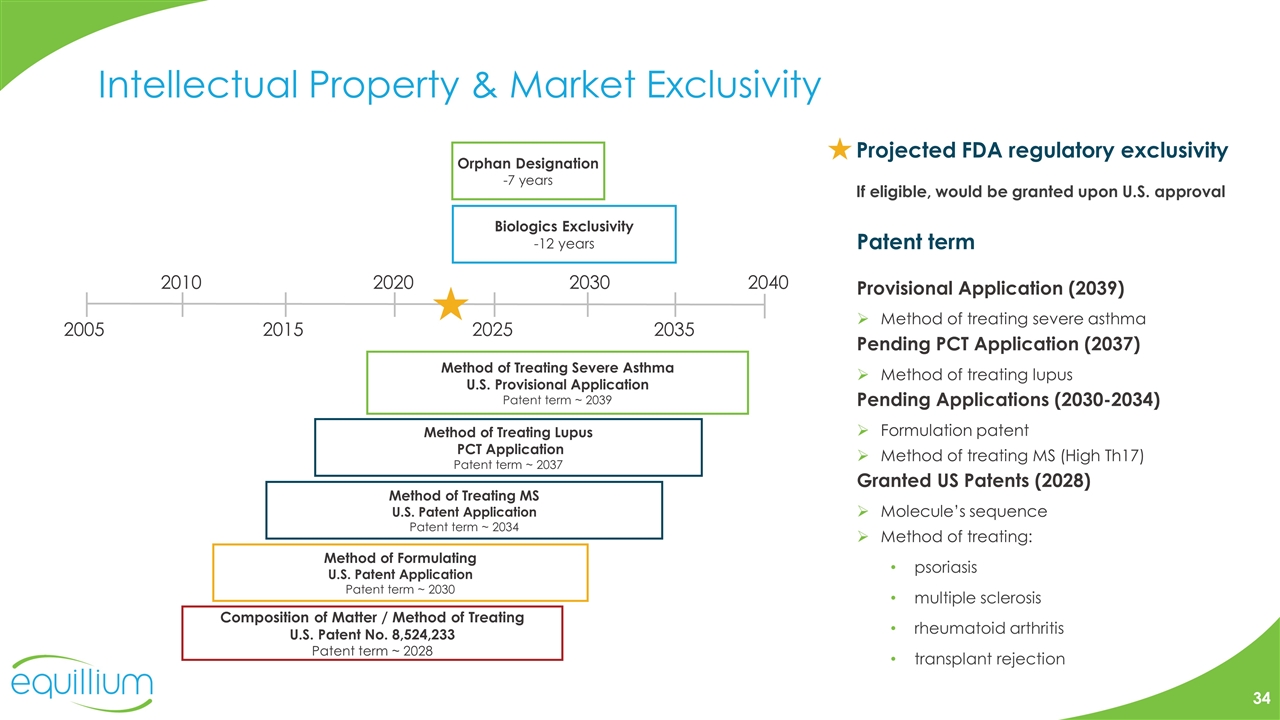
Intellectual Property & Market Exclusivity Patent term Provisional Application (2039) Method of treating severe asthma Pending PCT Application (2037) Method of treating lupus Pending Applications (2030-2034) Formulation patent Method of treating MS (High Th17) Granted US Patents (2028) Molecule’s sequence Method of treating: psoriasis multiple sclerosis rheumatoid arthritis transplant rejection Projected FDA regulatory exclusivity If eligible, would be granted upon U.S. approval Composition of Matter / Method of Treating U.S. Patent No. 8,524,233 Patent term ~ 2028 Orphan Designation -7 years Biologics Exclusivity -12 years Method of Treating Lupus PCT Application Patent term ~ 2037 2005 2015 2025 2035 Method of Treating MS U.S. Patent Application Patent term ~ 2034 Method of Formulating U.S. Patent Application Patent term ~ 2030 2010 2020 2030 2040 Method of Treating Severe Asthma U.S. Provisional Application Patent term ~ 2039

Executive Summary Validated therapeutic: itolizumab has shown clinical efficacy in multiple indications – studied by Biocon in >330 patients – and is approved for the treatment of psoriasis in India Pipeline-in-a-product: EQ001 has broad potential disease modifying therapeutic utility; launching multiple clinical studies during 2019 First-in-class: Equillium is developing EQ001 (itolizumab) the first antibody targeting the novel immune checkpoint pathway CD6 – for the treatment of severe immuno-inflammatory disorders High-value partnership: Equillium acquired exclusive rights to EQ001 for the U.S. & Canada from Biocon – partnership provides clinical & commercial product, commercial scale production at FDA regulated facility Accomplished team: experienced in drug discovery, development and commercialization EQ financing: IPO completed in Q4 2018 – Jefferies, SVB Leerink, Stifel
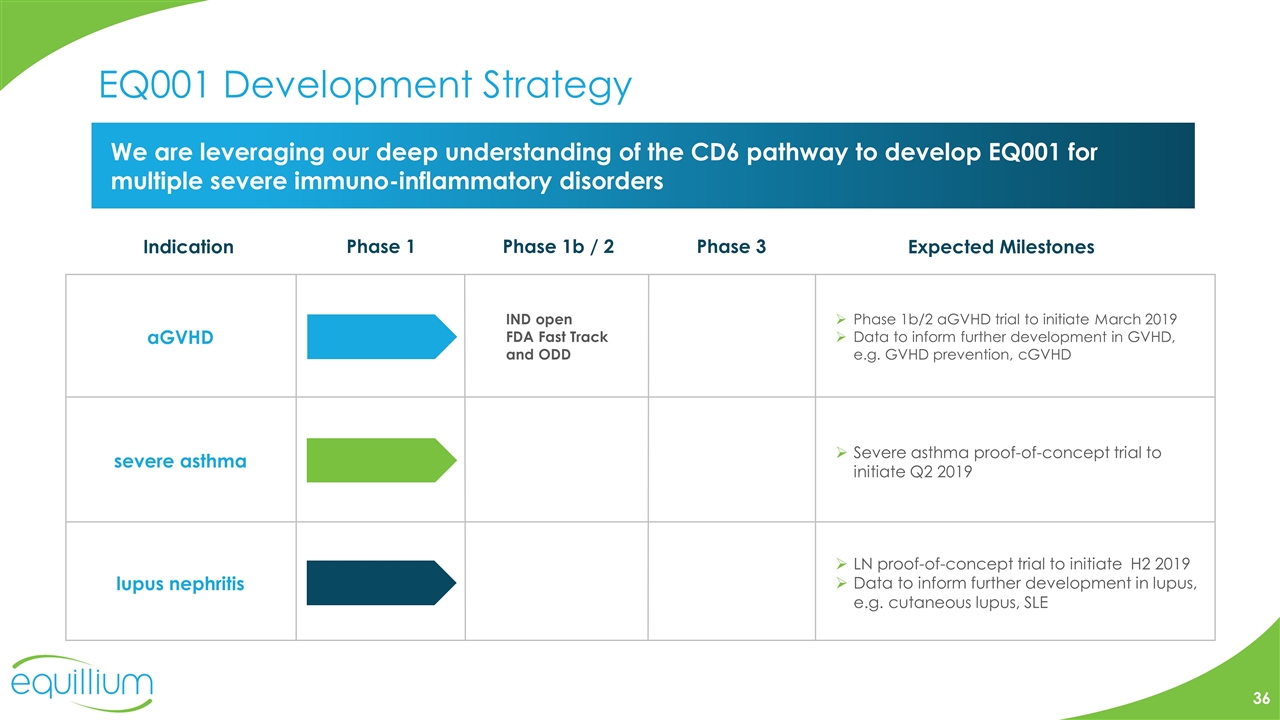
EQ001 Development Strategy We are leveraging our deep understanding of the CD6 pathway to develop EQ001 for multiple severe immuno-inflammatory disorders aGVHD severe asthma Indication Phase 1 Phase 1b / 2 Phase 3 Expected Milestones IND open FDA Fast Track and ODD lupus nephritis Severe asthma proof-of-concept trial to initiate Q2 2019 Phase 1b/2 aGVHD trial to initiate March 2019 Data to inform further development in GVHD, e.g. GVHD prevention, cGVHD LN proof-of-concept trial to initiate H2 2019 Data to inform further development in lupus, e.g. cutaneous lupus, SLE

Equillium, Inc. 2223 Avenida de la Playa / Suite 108 La Jolla, CA 92037 www.equilliumbio.com




































