Exhibit 99.1

Company Logo harnessing novel immunobiology October 2019 www. Equilliumbio.com

Safe Harbor Statement This presentation contains forward-looking statements about Equillium, Inc. (the “Company”). In some cases, you can identify forward-looking statements by the words “will,” “expect,” “intend,” “plan,” “objective,” “believe,” “estimate,” “potential,” “continue” and “ongoing,” or the negative of these terms, or other comparable terminology intended to identify statements about the future. These statements are based on Company management’s current beliefs and expectations. These statements include but are not limited to statements regarding the Company’s business strategy, the Company’s plans to develop and commercialize its product candidates, the safety and efficacy of the Company’s product candidates, the Company’s plans and expected timing with respect to regulatory filings and approvals, size and growth potential of the markets for the Company’s product candidates and cash runway. These statements involve substantial known and unknown risks, uncertainties and other factors that may cause the Company’s actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements.The Company may not actually achieve the plans, intentions or expectations disclosed in its forward-looking statements, and you should not place undue reliance on the Company’s forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed or implied in the forward-looking statements the Company makes due to the risks and uncertainties inherent in the Company’s business, including without limitation, risk described in the Company’s filings with the Securities and Exchange Commission (“SEC”). You are cautioned not to place undue reliance on these forward-looking statements, which represent the Company’s views as of the date of this presentation. The Company’s anticipates that subsequent events and developments will cause the its views to change. However, while the Company may elect to update these forward-looking statements at some point in the future, the Company has no current intention of doing so except to the extent required by applicable law. These and other risks and uncertainties are described more fully under the caption “Risk Factors” and elsewhere in the Company’s filings and reports, which may be accessed for free by visiting EDGAR on the SEC web site at http://www.sec.gov. and on the Company’s website under the heading “Investors.” All forward-looking statements are qualified in their entirety by this cautionary statement. This caution made under the “safe harbor” provisions of Section 21E of the Private Securities Litigation Reform Act of 1995.Company Logo
Delivering powerful new treatment approaches Focused on immuno-inflammatory diseases with high unmet need Driven by an accomplished team rapidly delivering key milestones Our lead asset, itolizumab, is unique, modulating both the activity and trafficking of Teff cells Initial clinical programs are targeting diseases with limited or no treatment options Three clinical studies initiated 2019

Accomplished Management Team Dan Bradbury Chairman & Chief Executive Officer Bruce Steel, CFA President & Chief Business Officer Steve Connelly, PhD Chief Scientific Officer Krishna Polu, MD Chief Medical Officer Jason Keyes Chief Financial Officer Christine Zedelmayer Vice-President of Operations amylin gsk glaxosmithkline biomed ventures anaphore Rincon pharmaceuticals ambit biosciences biomed ventures atry pharma the scripps research institute raptor cytomx therapeutics affymax amgen orexigen amylin amgen baxter amylin amgen ligand Company logo
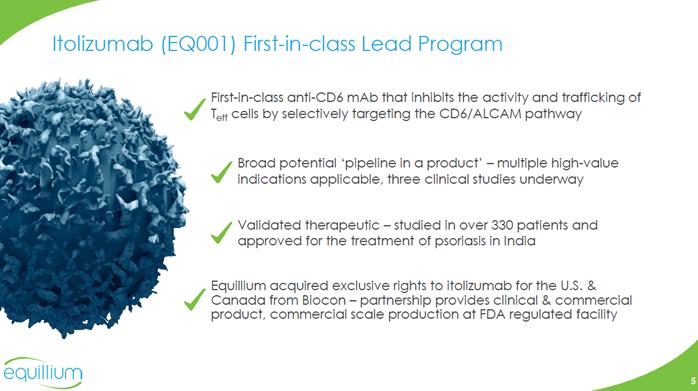
Itolizumab (EQ001) First-in-class Lead Program First-in-class anti-CD6 mAb that inhibits the activity and trafficking of Teff cells by selectively targeting the CD6/ALCAM pathway Broad potential ‘pipeline in a product’ –multiple high-value indications applicable, three clinical studies underway Validated therapeutic –studied in over 330 patients and approved for the treatment of psoriasis in India Equillium acquired exclusive rights to itolizumab for the U.S. & Canada from Biocon–partnership provides clinical & commercial product, commercial scale production at FDA regulated facility Company Logo
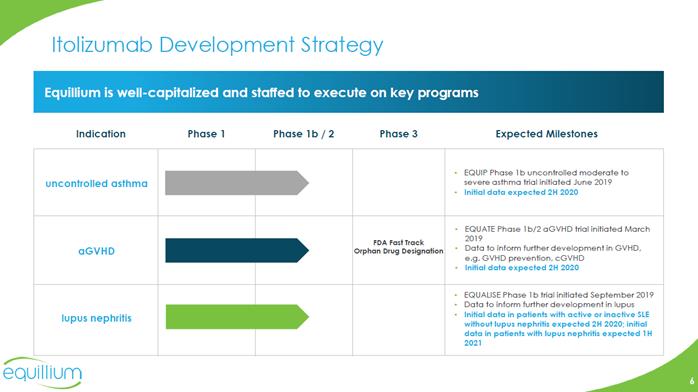
Itolizumab Development Strategy Equilliumis well-capitalized and staffed to execute on key programs uncontrolled asthma aGVHD Indication Phase 1 Phase 1b / 2 Phase 3 Expected Milestones FDA Fast Track Orphan Drug Designation Lupus nephritis EQUATE Phase 1b/2 aGVHD trial initiated March 2019 Data to inform further development in GVHD, e.g. GVHD prevention, cGVHD Initial data expected 2H 2020 EQUIP Phase 1b uncontrolled moderate to severe asthma trial initiated June 2019 Initial data expected 2H 2020 EQUALISE Phase 1b trial initiated September 2019 Data to inform further development in lupus Initial data in patients with active or inactive SLE without lupus nephritis expected 2H 2020; initial data in patients with lupus nephritis expected 1H 2021 Company logo

The cd6/alcam pathway
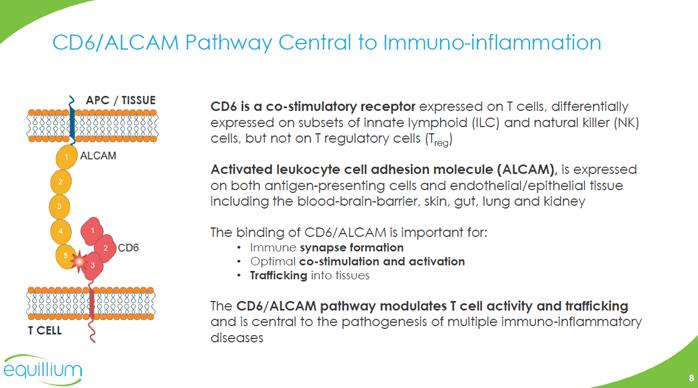
CD6/ALCAM Pathway Central to Immuno-inflammation T CELL APC / TISSUE ALCAM CD6 CD6 is a co-stimulatory receptor expressed on T cells, differentially expressed on subsets of innate lymphoid (ILC) and natural killer (NK) cells, but not on T regulatory cells (Treg) Activated leukocyte cell adhesion molecule (ALCAM), is expressed on both antigen-presenting cells and endothelial/epithelial tissue including the blood-brain-barrier, skin, gut, lung and kidney The binding of CD6/ALCAM is important for: Immune synapse formation Optimal co-stimulation and activation Trafficking into tissues The CD6/ALCAM pathway modulates T cell activity and trafficking and is central to the pathogenesis of multiple immuno-inflammatory diseases Company Logo
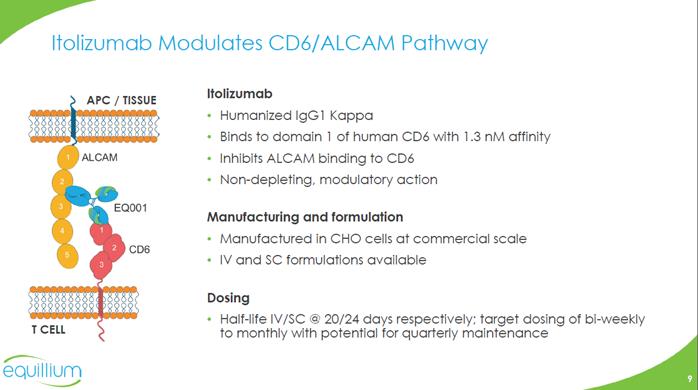
ALCAM EQ001 CD6 Itolizumab Humanized IgG1 Kappa Binds to domain 1 of human CD6 with 1.3 nM affinity Inhibits ALCAM binding to CD6 Non-depleting, modulatory action Manufacturing and formulation Manufactured in CHO cells at commercial scale IV and SC formulations available Dosing Half-life IV/SC @ 20/24 days respectively; target dosing of bi-weekly to monthly with potential for quarterly maintenance T CELL APC / TISSUE Itolizumab Modulates CD6/ALCAM Pathway Company Logo
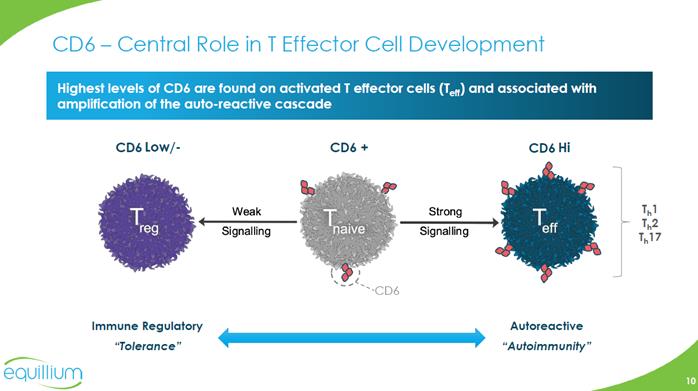
CD6 –Central Role in T Effector Cell Development Highest levels of CD6 are found on activated T effector cells (Teff) and associated with amplification of the auto-reactive cascade CD6 Low/- CD6+ CD6Hi Treg Weak Signalling Tnaive Strong Signalling Teff Th1 Th2 Th17 CD6 Immune Regulatory “Tolerance” Autoreactive “Autoimmunity” Chart Company Logo
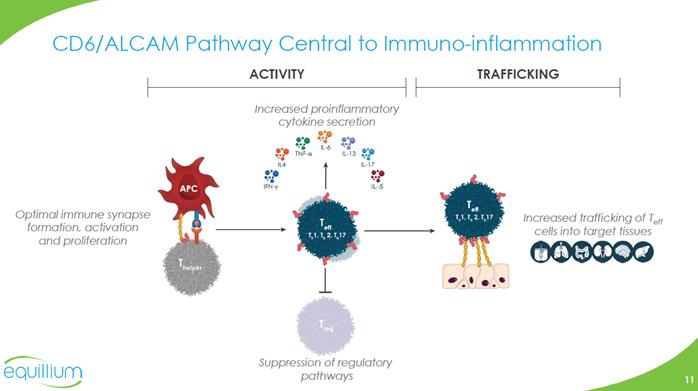
CD6/ALCAM Pathway Central to Immuno-inflammation ACTIVITY TRAFFICKING Increased proinflammatory cytokine secretion Optimal immune synapse formation, activation and proliferation Increased trafficking of Teff cells into target tissues Suppression of regulatory pathways Company Logo Chart
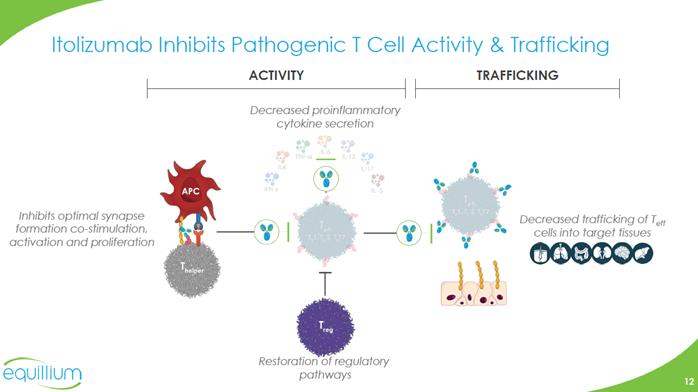
Itolizumab Inhibits Pathogenic T Cell Activity & Trafficking ACTIVITY TRAFFICKING Decreased proinflammatory cytokine secretion Inhibits optimal synapse formation co-stimulation, activation and proliferation Decreased trafficking of Teff cells into target tissues Restoration of regulatory pathways Chart Company Logo
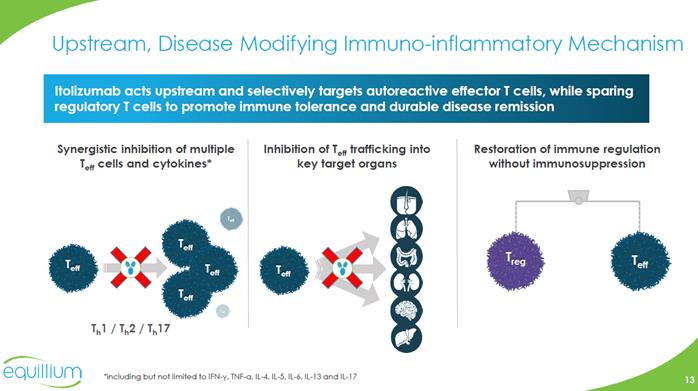
Upstream, Disease Modifying Immuno-inflammatory Mechanism Itolizumab acts upstream and selectively targets autoreactive effector T cells, while sparing regulatory T cells to promote immune tolerance and durable disease remission Synergistic inhibition of multiple Teff cells and cytokines* Inhibition of Teff trafficking into key target organs Restoration of immune regulation without immunosuppression T eff T eff T eff T eff T eff Th1 / Th2 / Th17 Teff Treg T eff *including but not limited to IFN-γ, TNF-α, IL-4, IL-5, IL-6, IL-13 and IL-17 Chart Company Logo

Clinical Strategy

Uncontrolled moderate to severe asthma A heterogeneous disease characterized by different Teff cell subtypes and other innate immune cells driving both allergic and autoimmune mechanisms, leading to chronic airway inflammation 2.6mm Severe asthma patients in the U.S. 1.3mm Patients uncontrolled by standard-of-care treatments (long-acting beta-agonists, inhaled corticosteroids, oral corticosteroids) ~50% Of uncontrolled patients do not respond to existing biologic treatments 0 Approved products covering the full spectrum of disease (Th2 High to Non-Th2 asthma) 2 Products in development that target Non-Th2 asthma 1 Product in development that modulates both the activity and trafficking of Teff cells – itolizumab All numbers are approximate and based on published reports

CD6-ALCAM Implicated Broadly in the Pathogenesis of Asthma Type 2 Inflammation Non-Type 2 / Th17 Inflammation Allergens Irritants, pollutants, microbes, and viruses TSLP IL-25 IL-33 IL-13 IL-4, IL-5, IL-13 IL-6 TGF-ΒV IL-23 CXCL8 GM-CSF IL-4 IL-5 IL-6, IL-17, IL-8 IFN-Y TNF IL-3, IL-4, IL-5, IL-9 B cell IGE Mast cell Eosinophil ALCAM Neutrophil Th1 cell Th17 cell ILC2 Th2cell Teff CD6 CD6 CD6 Asthma Figure Legend:CD6 is expressed on Th1, Th2, Th17 and ILC2 effector cells that secrete multiple proinflammatory cytokines implicated in asthma pathogenesis. ALCAM is expressed on antigen presentation cells that activate effector cells. ALCAM is also expressed on endothelial and smooth muscle cells where it plays a role in trafficking of CD6+ effector cells. Adapted from Israel E, et al., N Engl J Med. 2017;377(10):965-976.Chart Company Logo
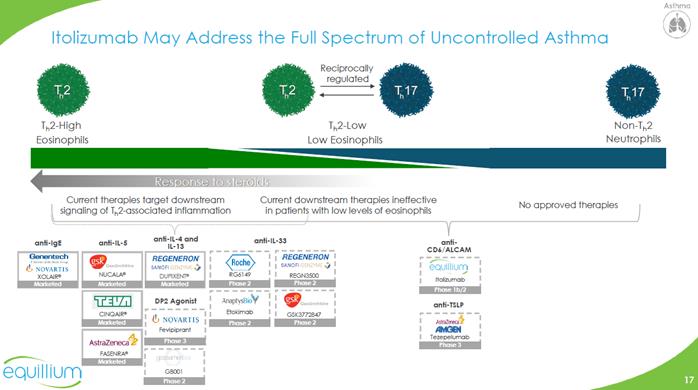
Itolizumab May Address the Full Spectrum of Uncontrolled Asthma Th2-High Eosinophils Th2-Low Low Eosinophils Non-Th2 Neutrophils Reciprocally regulated Th2 Th2 Th17 Th17 Response to steroids Current therapies target downstream signaling of Th2-associated inflammation Current downstream therapies ineffective in patients with low levels of eosinophils No approved therapies Anti-ige anti-IL-5 anti-IL-4 and IL-13 DP2 Agonist anti-CD6/ALCAM anti-TSLP CHART Company Logo

Itolizumab Targets both Th2 and Non-Th2 Asthma Pathogenesis Modulating CD6-ALCAM pathway attenuates activity and trafficking of Th1 (IFN-y), Th2 (IL-4,5,13), Th17 (IL-17) cells and cytokines in multiple models of autoimmune and allergic inflammation Transcriptional and histological analyses support the presence of increased CD6, CD4 T cells, and ALCAM in the lungs of severe asthma patients Asthma is a heterogenous disease with CD6+ Th1 (IFN-y), Th2 (IL-4,5,13), Th17 (IL-17) and ILC cells and cytokines involved in the pathogenesis Th17 and ILC cells are steroid resistant ALCAM expressed on lung tissues facilitates infiltration of CD6+ Teff cells High Th17:Treg ratio associated with asthma exacerbations Uncontrolled moderate to severe asthma proof-of-concept trial to initiate Q2 2019 Data to inform further development in Th2 and non-Th2 asthma Translational Validation Clinical Development Scientific Rationale Kim et al., 2028. “Activated leukocyte cell adhesion molecule stimulates the t-cell response in allergic asthma.” American Journal of Respiratory and Critical Care Medicine. 197(8); Li et al, 2017. “CD6 as a potential target for treating multiple sclerosis.” PNAS 114(10): 2687-2692; Bughani et al., 2018. “T cell activation and differentiation is modulated by a CD6 domain 1 antibody itolizumab.” PLoS One 12(7): e0180088 Company Logo
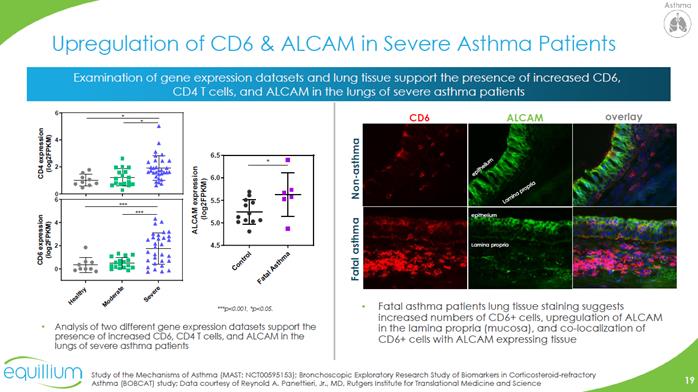
Upregulation of CD6 & ALCAM in Severe Asthma Patients Examination of gene expression datasets and lung tissue support the presence of increased CD6, CD4 T cells, and ALCAM in the lungs of severe asthma patients Analysis of two different gene expression datasets support the presence of increased CD6, CD4 T cells, and ALCAM in the lungs of severe asthma patients Fatal asthma patients lung tissue staining suggests increased numbers of CD6+ cells, upregulation of ALCAM in the lamina propria (mucosa), and co-localization of CD6+ cells with ALCAM expressing tissue Study of the Mechanisms of Asthma (MAST; NCT00595153); Bronchoscopic Exploratory Research Study of Biomarkers in Corticosteroid-refractory Asthma (BOBCAT) study; Data courtesy of Reynold A. Panettieri, Jr., MD, Rutgers Institute for Translational Medicine and Science CHART Company Logo
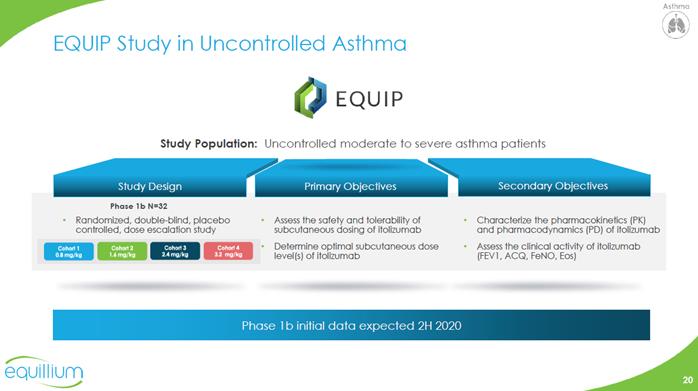
EQUIP Study in Uncontrolled Asthma EQUIP Study Population: Uncontrolled moderate to severe asthma patients Study Design Primary Objectives Secondary Objectives Phase 1b N=32 Randomized, double-blind, placebo controlled, dose escalation study Assess the safety and tolerability of subcutaneous dosing of itolizumab Determine optimal subcutaneous dose level(s) of itolizumab Characterize the pharmacokinetics (PK) and pharmacodynamics (PD) of itolizumab Assess the clinical activity of itolizumab (FEV1, ACQ, FeNO, Eos) Phase 1b initial dataexpected 2H 2020 Chart Company Logo

Acute graft-versus-host disease (aGVHD) A multisystem complication of allogeneic hematopoietic stem cell transplants, or allo-HSCT, caused by Teff cells, recognizing and attacking the recipient’s body 8,500 Allo-HSCT’s performed in 2016 4% procedural growth year-over-year 30-70% Allo-HSCT patients will develop aGVHD 53% Survival rate for steroid responders 95% Mortality rate for steroid non-responders 0 Number of approved treatments for first-line aGVHD 1 Product in development thar modulates both the activity and trafficking of Teff cells – itolizumab All numbers are approximate and based on published reports
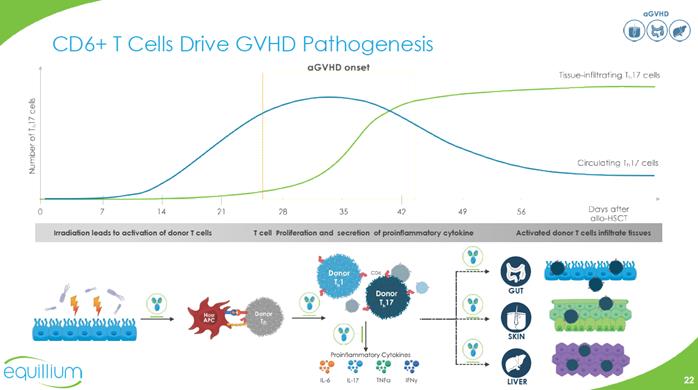
aGVHD CD 6+ T Cells Drive GVHD Bar Chart Irradiation leads to activation of donor T cells T cell Proliferation and secretion of proinflammatory cytokine Activated donor T cells infiltrate tissues (Graphic) GUT SKIN LIVER Company Logo
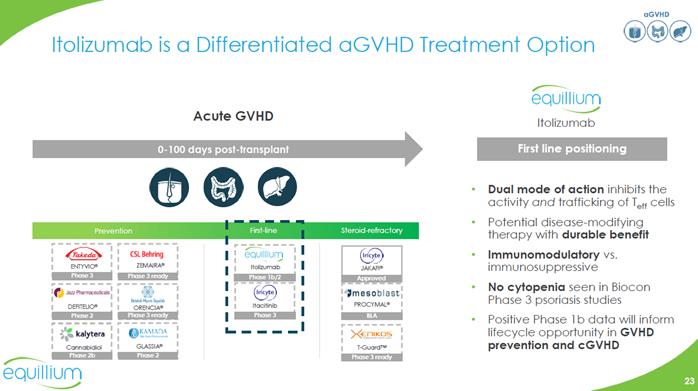
aGVHD Itolizumab is a Differentiated aGVHD Treatment Option Acute GVHD Company Logo Itolizumab 0-100 days post-transplant First line positioning Dual mode of action inhibits the activity and trafficking of Teff cells Potential disease-modifying therapy with durable benefit Immunomodulatory vs. immunosuppressive no cytopenia seen in Biocon Phase 3 psoriasis studies Positive Phase 1b data will inform lifecycle opportunity in GVHD prevention and cGVHD Prevention First-line Steroid-refractory Graphic Company Logo
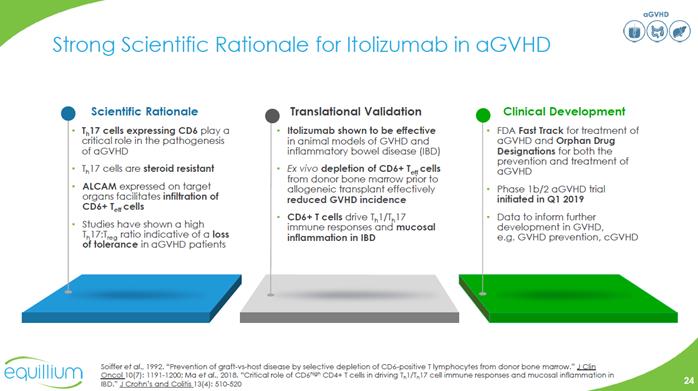
aGVHD Strong Scientific Rationale for Itolizumab in aGVHD Scientific Rationale th 17 cells expressing CD6 play a critical role in the pathogenesis of aGVHD th17 cells are steroid resistant ALCAM expressed on target organs facilitates infiltration of CD6+ Teff cells Studies have shown a high th17:Treg ratio indicative of a loss of tolerance in aGVHD patients Translational Validation Itolizumab shown to be effective in animal models of GVHD and inflammatory bowel disease (IBD) Ex vivo depletion of CD6+ Teff cells from donor bone marrow prior to allogeneic transplant effectively reduced GVHD incidence CD6+T cells drive th 1/th 17 immune responses and mucosal inflammation in IBD Clinical Development FDA Fast Track for treatment of aGVHD and Orphan Drug Designations for both the prevention and treatment of aGVHD Phase 1b/2 aGVHD trial initiated in Q1 2019 Data to inform further development in GVHD, e.g. GVHD prevention, cGVHD Soiffer et all., 1992 ‘‘Prevention of graft-vs-host disease by selective depletion of CD6-positive T lymphocytes from donor bone marrow.” J Clin Oncol 10(7): 1191-1200; Ma et al., 2018. ‘‘Critical role of CD6high CD4+ T cells in driving Th1/Th17 cells immune responses and mucosal inflammation in IBD.” J Crohn’s and Cotitis 13 (4): 510-520 Company Logo

aGVHD EQUATE Study for First-line Treatment in aGVHD EQUATE Study Population: First-line treatment of newly diagnosed aGVHD patients Study Design Phase 1b N~24 (high-risk Macmillan criteria Open-label,3x3, dose escalation Primary Objectives Assess the safety and tolerability of intravenous (IV) dosing of itolizumab Determine optimal IV dose level (S) of itolizumab Secondary Objectives Characterize the pharmacokinetics (PK) and pharmacodynamics (PD) of itolizumab Assess the clinical activity of itolizumab GVHD ORR,NRM, CGVHD, durability Phase 2 N~ 60 (Grade II – IV) Randomized, double-blind, placebo controlled Assess the clinical activity of itolizumab (GVHD ORR,NRM,CGVHD, durability Further characterize the safety, tolerability and PD of intravenous (IV) dosing of itolizumab Phase 1b initial date expected 2h 2020 Phase 1b informs Phase 2 study as well as lifecycle strategy that may include GVHD prevention and cGVHD Company Logo

Lupus nephritis a heterogeneous disease that is the most frequent and serious manifestation of system lupus erythematosus (SLE) 100,000 Lupus nephritis patients in the U.S. 50%-75% Of patients do not respond to frontline treatments 40% Of severe, proliferative patients will progress to end-stage renal disease 0 Approved treatments 9 Products in development 1 Product in development that modulates both the activity and trafficking of Teff cells -itolizumab All numbers are approximate and based on published reports
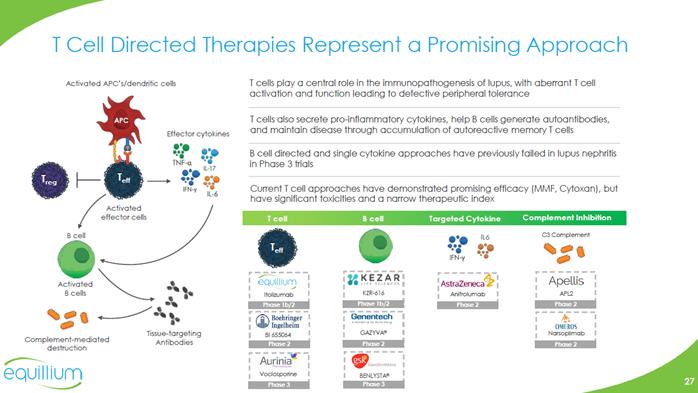
T Cell Directed Therapies Represent a Promising Approach Activated APC’s/dendritic cells T cells play a central role in the immunopathogenesis of lupus, with aberrant T cell activation and function leading to defective peripheral tolerance Effector cytokines T cells also secrete pro-inflammatory cytokines, help B cells generate autoantibodies, and maintain disease through accumulation of autoreactive memory T cells B cell directed and single cytokine approaches have previously failed in lupus nephritis in Phase 3 trials Current T cell approaches have demonstrated promising efficacy (MMF, Cytoxan), but have significant toxicities and a narrow therapeutic index Activated effector cells Tcell Bcell Targeted Cytokine Complement Inhibition (Bar Chart) Activated B cells B cell Tissue-targeting Antibodies Complement-mediated destruction Company Logo
Itolizumab Targets Lupus Nephritis Pathogenesis Scientific Rationale Teffcells play a central role in the pathogenesis of lupus Multiple Teffcells/cytokines, such Th1/IFN-γ, Th2/IL-4 and Th17/IL-17 have been implicated Elevated Th17cells are accompanied by a decrease of Tregcells, promoting loss of tolerance Translational Validation Supportive preclinical models in lupus and lupus nephritis Kidney biopsy analysis reveal upregulation of CD6 and ALCAM expression on infiltrating T cells, innate immune cells, and resident renal cells Elevation in urinary ALCAM identifies patients with active lupus nephritis Clinical Development Lupus nephritis proof-of-concept trial to initiate H2 2019 Urinary biomarker strategy to inform patient selection strategy Data to inform further lifecycle strategy in lupus(e.g. SLE and cutaneous lupus) (Company Logo) Stanley et al.,2016.Comprehensive Aptamer-Based Screening of 1129 Proteins Reveals Novel Urinary Biomarkers of Lupus Nephritis. ACR/ARHP Annual Meeting. Company logo
Treating Lupus Nephritis Today – Similar Treatment for All Patients (Graphic) Standard diagnostics unable to inform biological differences between patients All patients treated uniformly with limited choices Patients may be exposed to ineffective therapies Payers pay for treatments that are ineffective in some patients and also pay for the associated complications Company Logo

Treating Lupus Nephritis Tomorrow –Individualized Therapy (Graphic) Biomarker driven diagnostics inform differences between patients and guide treatment Therapy benefit -to-risk improved for patients who may respond Use of ineffective treatments minimized –lower overall cost to health care system Company Logo
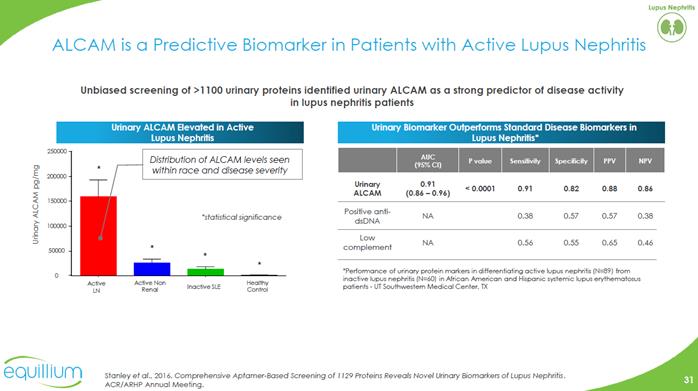
ALCAM is a Predictive Biomarker in Patients with Active Lupus Nephritis Unbiased screening of >1100 urinary proteins identified urinary ALCAM as a strong predictor of disease activity in lupus nephritis patients Urinary Biomarker Outperforms Standard Disease Biomarkers in Lupus Nephritis* Urinary ALCAM Elevated in Active Lupus Nephritis Distribution of ALCAM levels seen within race and disease severity* Performance of urinary protein markers in differentiating active lupus nephritis (N=89) from inactive lupus nephritis (N=60) in African American and Hispanic systemic lupus erythematosus patients -UT Southwestern Medical Center, TX Urinary ALCAM0.91 (0.86 –0.96) < 0.0001 0.91 0.82 0.88 0.86 Positive anti-dsDNA NA 0.38 0.57 0.57 0.38 Low complement NA 0.56 0.55 0.65 0.46Stanley et al.,2016.Comprehensive Aptamer-Based Screening of 1129 Proteins Reveals Novel Urinary Biomarkers of Lupus Nephritis. ACR/ARHP Annual Meeting. Bar Chart Company Logo
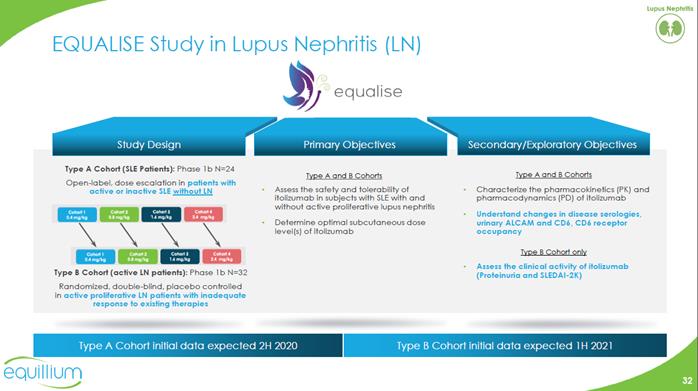
EQUALISE Study in Lupus Nephritis (LN) Study Design Primary Objectives Secondary/Exploratory Objectives Type A Cohort (SLE Patients): Phase 1b N=24 Open-label, dose escalation in patients with active or inactive SLE without LN Type B Cohort (active LN patients): Phase 1b N=32 Randomized, double-blind, placebo controlled in active proliferative LN patients with inadequate response to existing therapies Type A and B Cohorts Assess the safety and tolerability of itolizumabin subjects with SLE with and without active proliferative lupus nephritis Determine optimal subcutaneous dose level(s) of itolizumab Type A and B Cohorts Characterize the pharmacokinetics (PK) and pharmacodynamics (PD) of itolizumab Understand changes in disease serologies, urinary ALCAM and CD6, CD6 receptor occupancy Type B Cohort only Assess the clinical activity of itolizumab (Proteinuria and SLEDAI-2K) Type A Cohort initial data expected 2H2020 Type B Cohort initial data expected 1H 2021 Company Logo

Corporate

Corporate Biocon Exclusive rights for the U.S. & Canada and rights to negotiate itolizumab licensing in select ex-North America markets Robust IP portfolio and 12-year Biologics Exclusivity Biocon partnership provides risk-mitigated product supply on attractive terms CMC completed and itolizumab currently manufactured at commercial scale in FDA-regulated facility Drug product supplied at no cost for 3 concurrent orphan indicationsuntil first U.S. approval; all other clinical supply at cost Robust IP portfolio and 12-year Biologics Exclusivity Strengthened balance sheet and lean operating model expected to fund current development programs into 2H 2021 Pursuing opportunities for strategic pipeline expansion Biologics Exclusivity is subject to significant uncertainty Company Logo

Summary First in class: Itolizumab is the first antibody targeting the novel CD6/ALCAM pathway for the treatment of severe immune-inflammatory disorders Pipeline in a product: Itolizumab has broad potential disease modifying therapeutic utility Focused Development: Strong scientific rationale and translational validation supporting initial indications in areas of unmet need launching multiple clinical studies during 2019 High value partnership: Biocon partnership provides clinical & commercial product, commercial scale production at FDA regulated facility Accomplished team:Experienced in drug discovery, development and commercialization EQ resources Equillium is capitalized and staffed to deliver on key programs into 2H 2021 Company Logo

Equillium, Inc.2223 Avenida de la Playa / Suite 105La Jolla, CA 92037www.equilliumbio.comir@equilliumbio.com



































