INTEGRIS-PSC Next Steps
Pliant has recently completed enrollment of the high-dose 320 mg dose cohort of the INTEGRIS-PSC Phase 2a trial. Interim 12-week data from the 320 mg dose is expected in the first quarter of 2024, with 24-week data from this dose expected in mid-2024.
We would like to thank our INTEGRIS-PSC investigators and their study teams for their dedication in support of the successful execution of this trial. Special thanks to the INTEGRIS-PSC clinical trial participants, their families and support networks for helping us advance this promising program.
INTEGRIS-PSC Multinational Phase 2 Trial of Bexotegrast (NCT04480840)
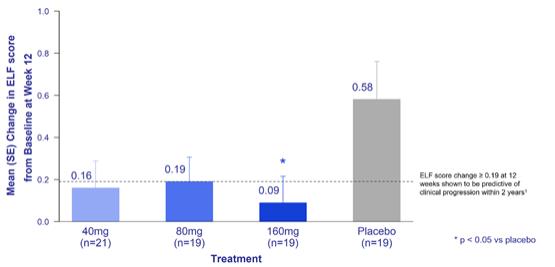
INTEGRIS-PSC is a Phase 2a, randomized, dose-ranging, double-blind, placebo-controlled trial evaluating the safety, tolerability, and pharmacokinetics of bexotegrast administered over 12 weeks in patients with IPF. Patients were enrolled in doses of 40 mg, 80 mg, 160 mg or 320 mg, with a 3:1 randomization ratio (active:placebo) and stratification based on use of ursodeoxycholic acid (UDCA). The primary endpoint is the evaluation of bexotegrast safety and tolerability and the secondary endpoint is the assessment of pharmacokinetics across a dose range. Exploratory endpoints will measure changes in liver fibrosis markers, ELF and PRO-C3, liver biochemistry and liver imaging.
Background on Primary Sclerosing Cholangitis
PSC is a rare, progressive liver disease of unknown origin, which frequently occurs in the setting of inflammatory bowel disease. PSC affects more than 30,000 patients in the United States and over 100,000 patients worldwide. The disease can occur in all ages, gender, and race. PSC is characterized by inflammation and fibrosis, with progressive liver and biliary damage leading to cirrhosis and liver failure. Currently there are no FDA or EMA-approved therapies for patients with PSC. Therefore, there is a high unmet need for new therapeutic options to address the symptoms and modify the disease progression of this grievous illness.
Conference Call and Webcast
The Company will host a conference call and webcast with a slide presentation today, Tuesday September 26 at 8:00 a.m. ET to discuss this update. Interested parties may access the live webcast on Pliant’s website at Pliant Therapeutics INTEGRIS-PSC Webcast or may participate via telephone by registering in advance at the following link: Pliant Therapeutics INTEGRIS-PSC Conference Call. Upon registration, all telephone participants will receive the dial-in number along and a unique passcode to access the call. An archived replay of the webcast will be available on Pliant’s website for 60 days following completion of the event.
About Pliant Therapeutics, Inc.
Pliant Therapeutics is a clinical stage biopharmaceutical company focused on discovering and developing novel therapies for the treatment of fibrosis. Pliant’s lead product candidate, bexotegrast (PLN-74809), is an oral, small molecule, dual selective inhibitor of αvß6 and αvß1 integrins that is in development in the lead indications for the treatment of idiopathic pulmonary fibrosis, or IPF, and primary sclerosing