OTL-201 MPS-IIIA Data IR webcast December 12, 2022 Exhibit 99.1

| Forward-looking Statements Certain information set forth in this presentation and in statements made orally during this presentation contain “forward-looking statements”. Except for statements of historical fact, information contained herein constitutes forward-looking statements and may include, but is not limited to, expectations of Orchard Therapeutics plc (the “Company” or “Orchard) regarding: (i) the safety and efficacy of Libmeldy and its product candidates; (ii) the Company’s ability to establish the infrastructure necessary to enable the treatment of eligible MLD patients and the adequacy of the Company’s supply chain and ability to commercialize Libmeldy; (iii) the expected development of the Company’s business and product candidates; (iv) the timing of regulatory submissions for approval of its product candidates; (v) the timing of interactions with regulators and regulatory submissions related to ongoing and new clinical trials for its product candidates; (vi) the timing of announcement of preclinical data for its product candidates and the likelihood that such data will be positive and support further development and regulatory approval of these product candidates; (vii) the timing and likelihood of approval of such product candidates by the applicable regulatory authorities; (viii) the adequacy of the Company’s supply chain, manufacturing capacity and plans for future investment and commercialization; (ix) execution of the Company’s vision and growth strategy, including with respect to global growth; (x) the size and value of potential markets for and commercialization of Libmeldy and the Company’s product candidates; and (xi) expected financial performance and financial condition, including its cash runway. The words “may,” “should,” “expects,” “intends,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Forward-looking statements are provided to allow investors the opportunity to understand management’s beliefs and opinions in respect of the future so that they may use such beliefs and opinions as one factor in evaluating an investment. These statements are neither promises nor guarantees of future performance. Such forward-looking statements necessarily involve known and unknown risks and uncertainties, many of which are beyond the Company’s control, which could cause actual results to differ materially from those contemplated in these forward-looking statements. In particular, these risks and uncertainties include, without limitation, the risk that Libmeldy will not be successfully commercialized, including the risk that the Company may not secure adequate pricing or reimbursement to support continued development of Libmeldy or its product candidates, if approved; the risk that any one or more of the Company’s product candidates, including OTL-200, will not be approved, successfully developed or commercialized; the risk that prior results, such as signals of safety, activity or durability of effect, observed from preclinical studies or clinical trials of Orchard’s product candidates will not be repeated or continue in ongoing or future studies or trials involving its product candidates; the risk that the market opportunity for Libmeldy or its product candidates may be lower than estimated; the risks from high inflation, macroeconomic conditions and geopolitical instability; and, the severity of the ongoing and evolving impact of the COVID-19 pandemic on Orchard’s business, including on preclinical and clinical development, its supply chain and commercial programs. You are cautioned not to place undue reliance on forward-looking statements. For additional disclosure regarding these and other risks faced by the Company, see the disclosure contained in the Company’s most recent annual or quarterly filed with the U.S. Securities and Exchange Commission (the “SEC”), as well as subsequent filings and reports filed with the SEC. These forward-looking statements speak only as of the date of this presentation. The Company undertakes no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required by law.

| ASH IR Event Agenda AGENDA TOPIC SPEAKER Orchard’s HSC Gene Therapy and MPS-IIIA Disease Overview Bobby Gaspar, M.D. Ph.D. Orchard CEO OTL-201 ASH Update: Biochemical Data Prof. Rob Wynn Royal Manchester Children's Hospital, Manchester University NHS Foundation Trust OTL-201 ASH Update: Clinical Data Dr. Simon Jones Manchester Centre for Genomic Medicine Q&A Leslie Meltzer, Ph.D. Orchard CMO
| HSC= hematopoietic stem cell Orchard’s Vision for Severe Genetic Diseases TIME VALUE Execute and deliver on rare disease portfolio Continue to build out capabilities in HSC gene therapy across regulatory, manufacturing, commercialization and access Expand on HSC gene therapy approach for larger indications and enabling technologies Seek partnership opportunities in areas where there is a compelling clinical and scientific rationale Mid-long term Near-mid term
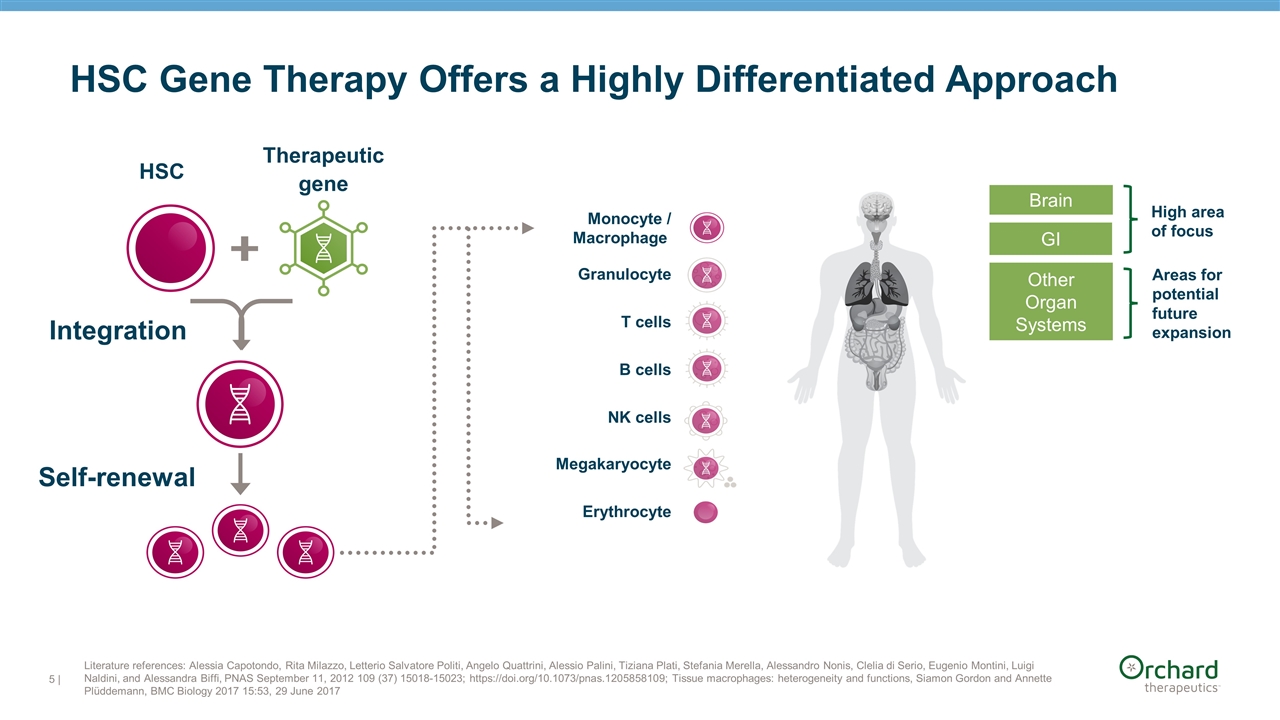
Other Organ Systems Areas for potential future expansion Monocyte / Macrophage T cells B cells NK cells Megakaryocyte Erythrocyte Granulocyte Brain High area of focus GI Integration Self-renewal | Literature references: Alessia Capotondo, Rita Milazzo, Letterio Salvatore Politi, Angelo Quattrini, Alessio Palini, Tiziana Plati, Stefania Merella, Alessandro Nonis, Clelia di Serio, Eugenio Montini, Luigi Naldini, and Alessandra Biffi, PNAS September 11, 2012 109 (37) 15018-15023; https://doi.org/10.1073/pnas.1205858109; Tissue macrophages: heterogeneity and functions, Siamon Gordon and Annette Plüddemann, BMC Biology 2017 15:53, 29 June 2017 HSC Gene Therapy Offers a Highly Differentiated Approach HSC Therapeutic gene
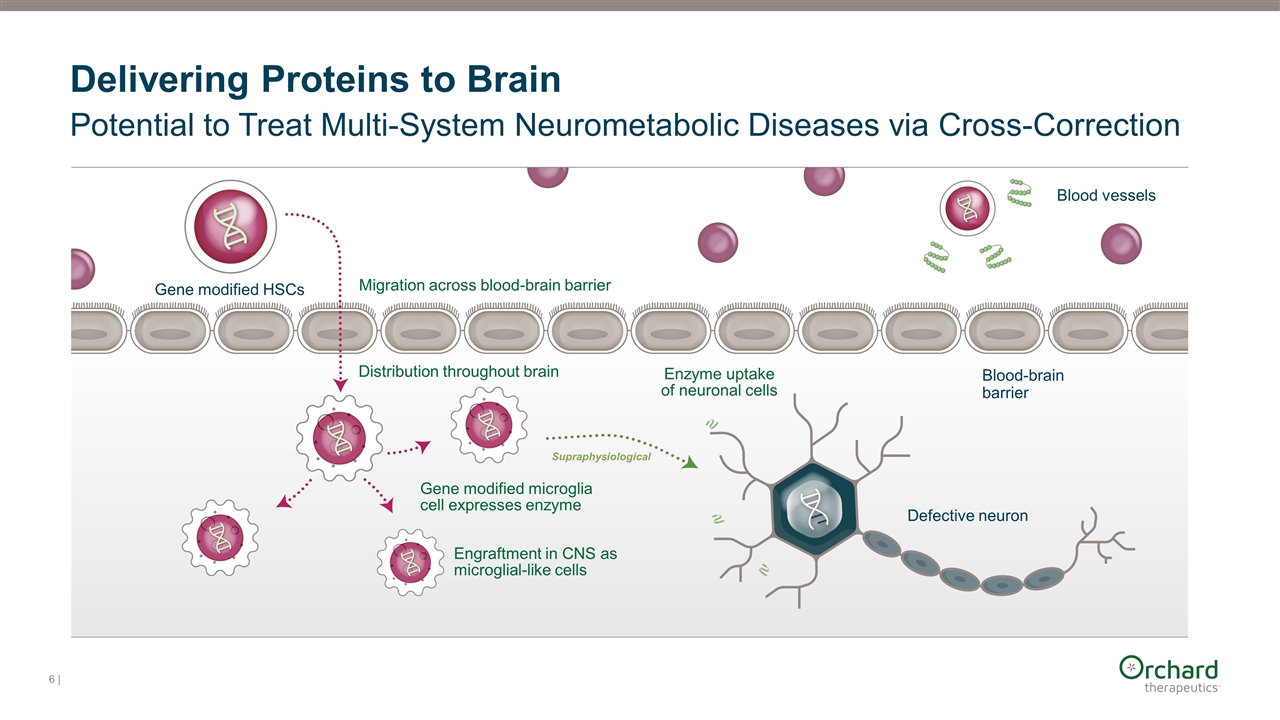
Delivering Proteins to Brain Potential to Treat Multi-System Neurometabolic Diseases via Cross-Correction Gene modified HSCs Blood vessels Blood-brain barrier Defective neuron Migration across blood-brain barrier Engraftment in CNS as microglial-like cells Distribution throughout brain Gene modified microglia cell expresses enzyme Enzyme uptake of neuronal cells Supraphysiological |
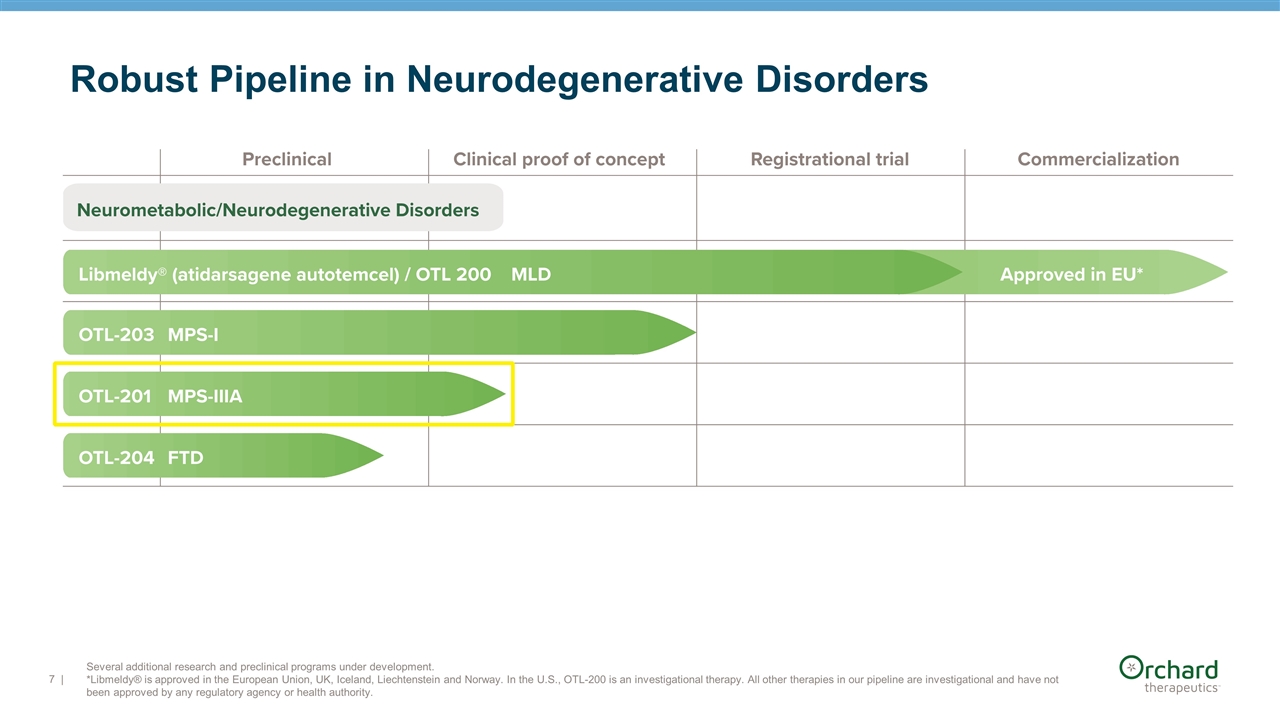
| Several additional research and preclinical programs under development. *Libmeldy® is approved in the European Union, UK, Iceland, Liechtenstein and Norway. In the U.S., OTL-200 is an investigational therapy. All other therapies in our pipeline are investigational and have not been approved by any regulatory agency or health authority. Robust Pipeline in Neurodegenerative Disorders
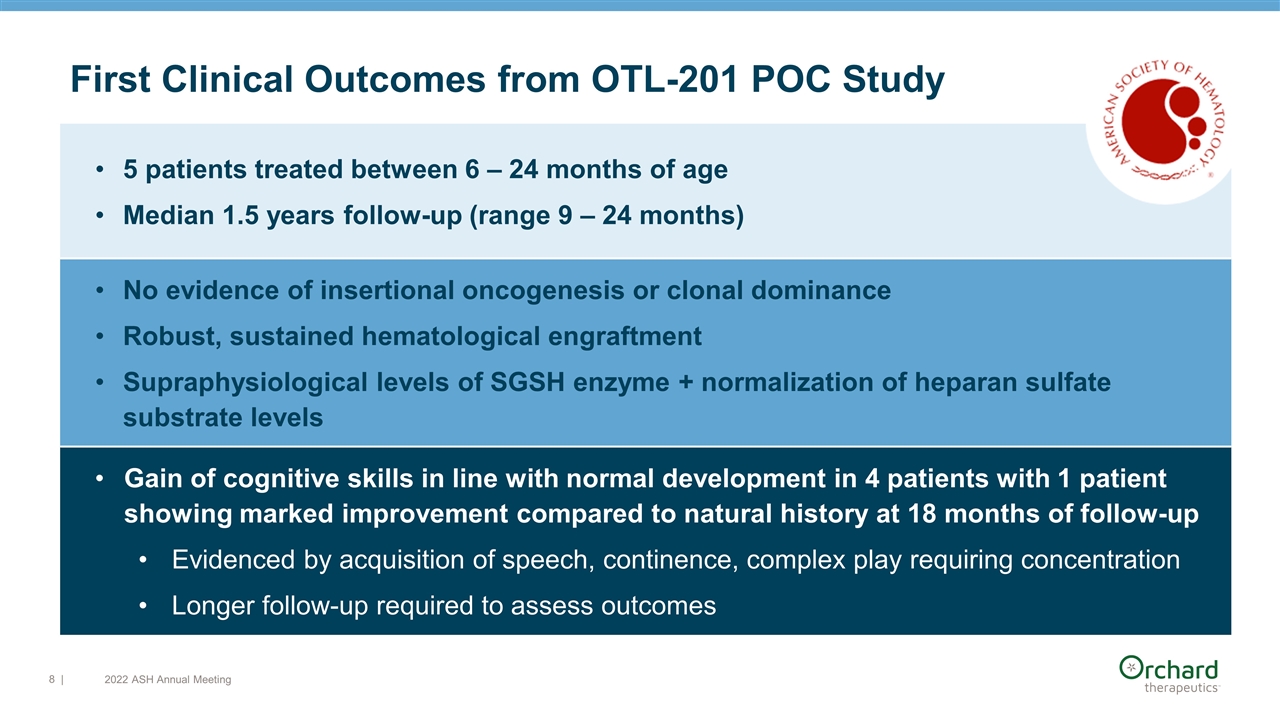
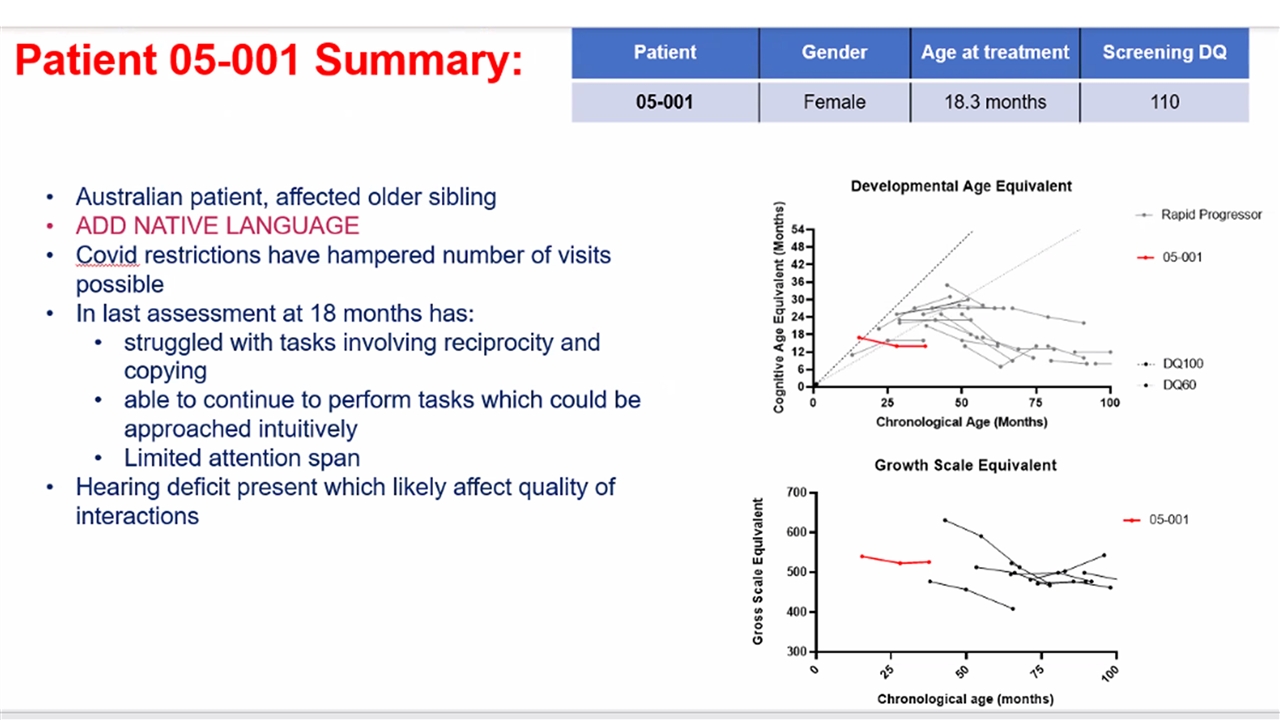
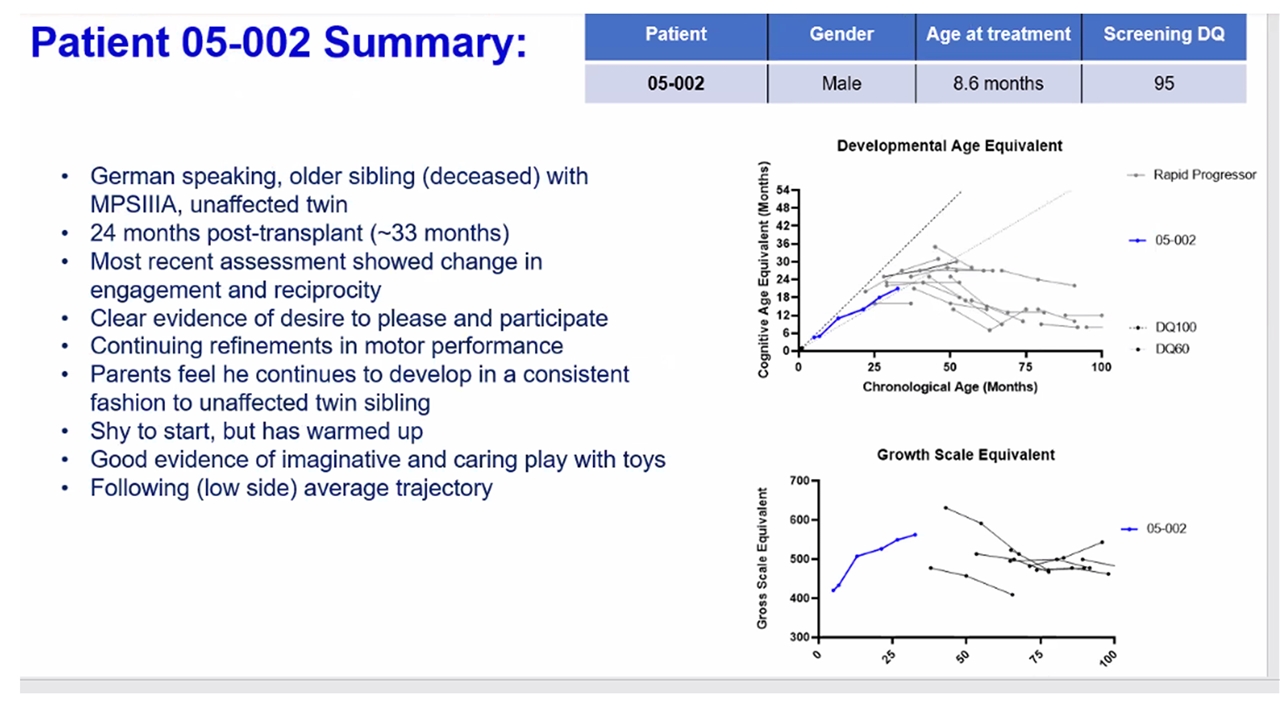
5 patients treated between 6 – 24 months of age Median 1.5 years follow-up (range 9 – 24 months) No evidence of insertional oncogenesis or clonal dominance Robust, sustained hematological engraftment Supraphysiological levels of SGSH enzyme + normalization of heparan sulfate substrate levels Gain of cognitive skills in line with normal development in 4 patients with 1 patient showing marked improvement compared to natural history at 18 months of follow-up Evidenced by acquisition of speech, continence, complex play requiring concentration Longer follow-up required to assess outcomes | First Clinical Outcomes from OTL-201 POC Study 2022 ASH Annual Meeting
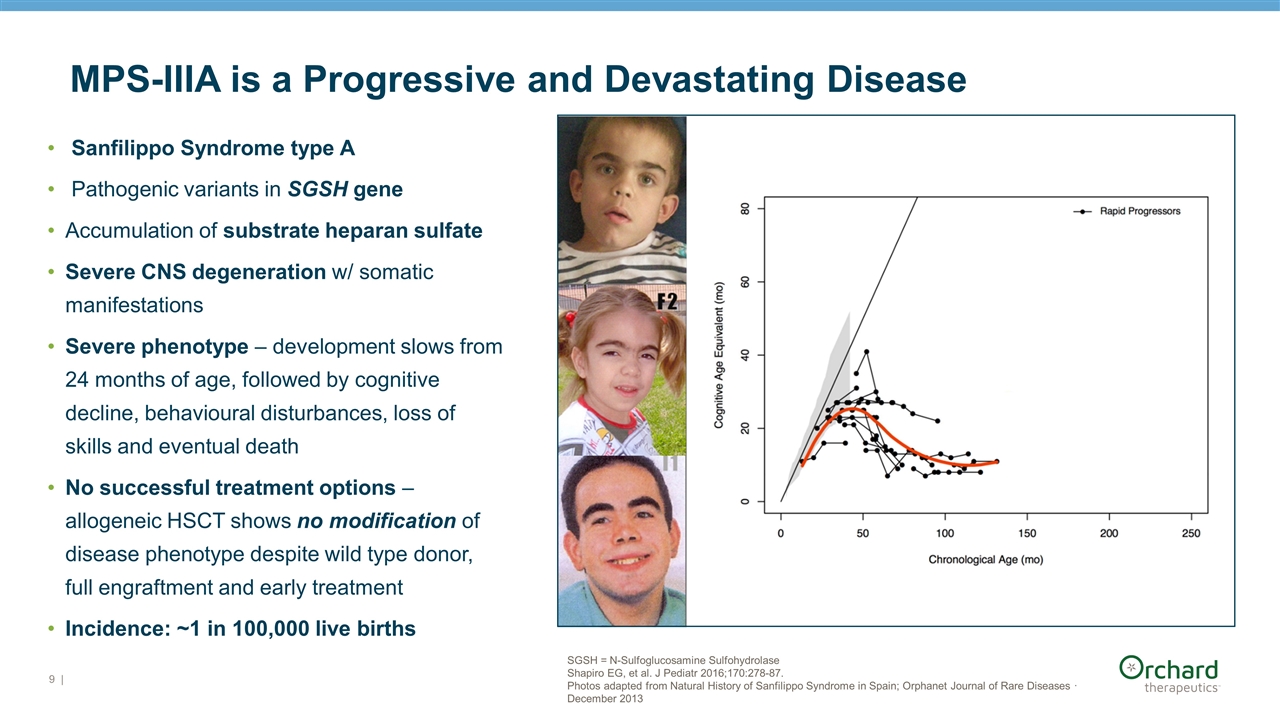
| SGSH = N-Sulfoglucosamine Sulfohydrolase Shapiro EG, et al. J Pediatr 2016;170:278-87. Photos adapted from Natural History of Sanfilippo Syndrome in Spain; Orphanet Journal of Rare Diseases · December 2013 MPS-IIIA is a Progressive and Devastating Disease Sanfilippo Syndrome type A Pathogenic variants in SGSH gene Accumulation of substrate heparan sulfate Severe CNS degeneration w/ somatic manifestations Severe phenotype – development slows from 24 months of age, followed by cognitive decline, behavioural disturbances, loss of skills and eventual death No successful treatment options – allogeneic HSCT shows no modification of disease phenotype despite wild type donor, full engraftment and early treatment Incidence: ~1 in 100,000 live births
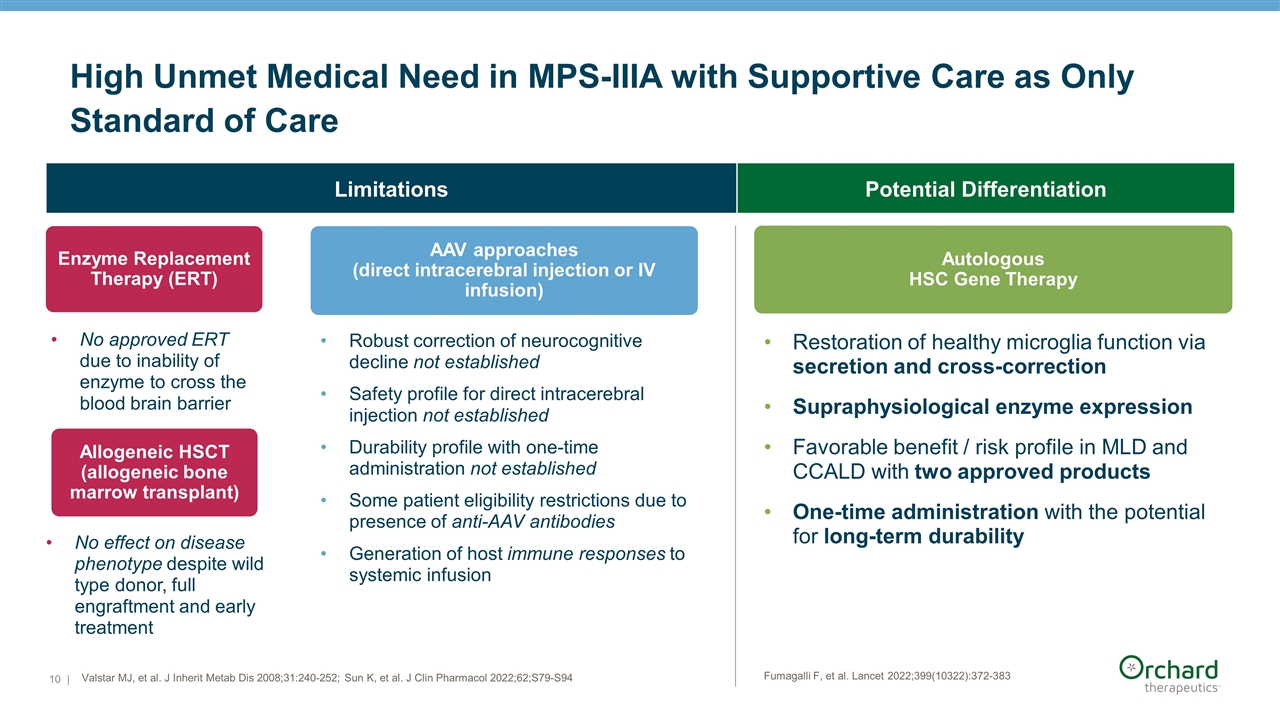
| Valstar MJ, et al. J Inherit Metab Dis 2008;31:240-252; Sun K, et al. J Clin Pharmacol 2022;62;S79-S94 High Unmet Medical Need in MPS-IIIA with Supportive Care as Only Standard of Care Autologous HSC Gene Therapy Restoration of healthy microglia function via secretion and cross-correction Supraphysiological enzyme expression Favorable benefit / risk profile in MLD and CCALD with two approved products One-time administration with the potential for long-term durability No approved ERT due to inability of enzyme to cross the blood brain barrier Enzyme Replacement Therapy (ERT) AAV approaches (direct intracerebral injection or IV infusion) Allogeneic HSCT (allogeneic bone marrow transplant) No effect on disease phenotype despite wild type donor, full engraftment and early treatment Robust correction of neurocognitive decline not established Safety profile for direct intracerebral injection not established Durability profile with one-time administration not established Some patient eligibility restrictions due to presence of anti-AAV antibodies Generation of host immune responses to systemic infusion Limitations Potential Differentiation Fumagalli F, et al. Lancet 2022;399(10322):372-383

OTL-201 Study Background and Biochemical Data Professor Rob Wynn
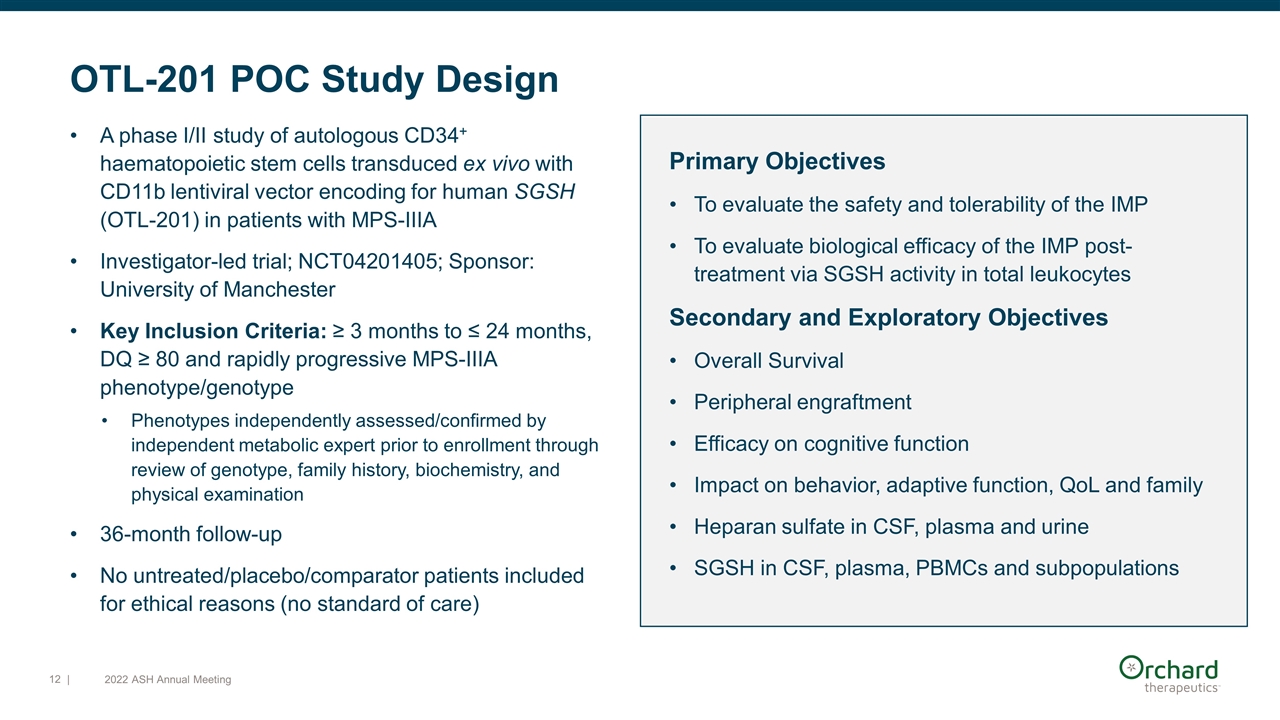
Primary Objectives To evaluate the safety and tolerability of the IMP To evaluate biological efficacy of the IMP post-treatment via SGSH activity in total leukocytes Secondary and Exploratory Objectives Overall Survival Peripheral engraftment Efficacy on cognitive function Impact on behavior, adaptive function, QoL and family Heparan sulfate in CSF, plasma and urine SGSH in CSF, plasma, PBMCs and subpopulations OTL-201 POC Study Design A phase I/II study of autologous CD34+ haematopoietic stem cells transduced ex vivo with CD11b lentiviral vector encoding for human SGSH (OTL-201) in patients with MPS-IIIA Investigator-led trial; NCT04201405; Sponsor: University of Manchester Key Inclusion Criteria: ≥ 3 months to ≤ 24 months, DQ ≥ 80 and rapidly progressive MPS-IIIA phenotype/genotype Phenotypes independently assessed/confirmed by independent metabolic expert prior to enrollment through review of genotype, family history, biochemistry, and physical examination 36-month follow-up No untreated/placebo/comparator patients included for ethical reasons (no standard of care) | 2022 ASH Annual Meeting
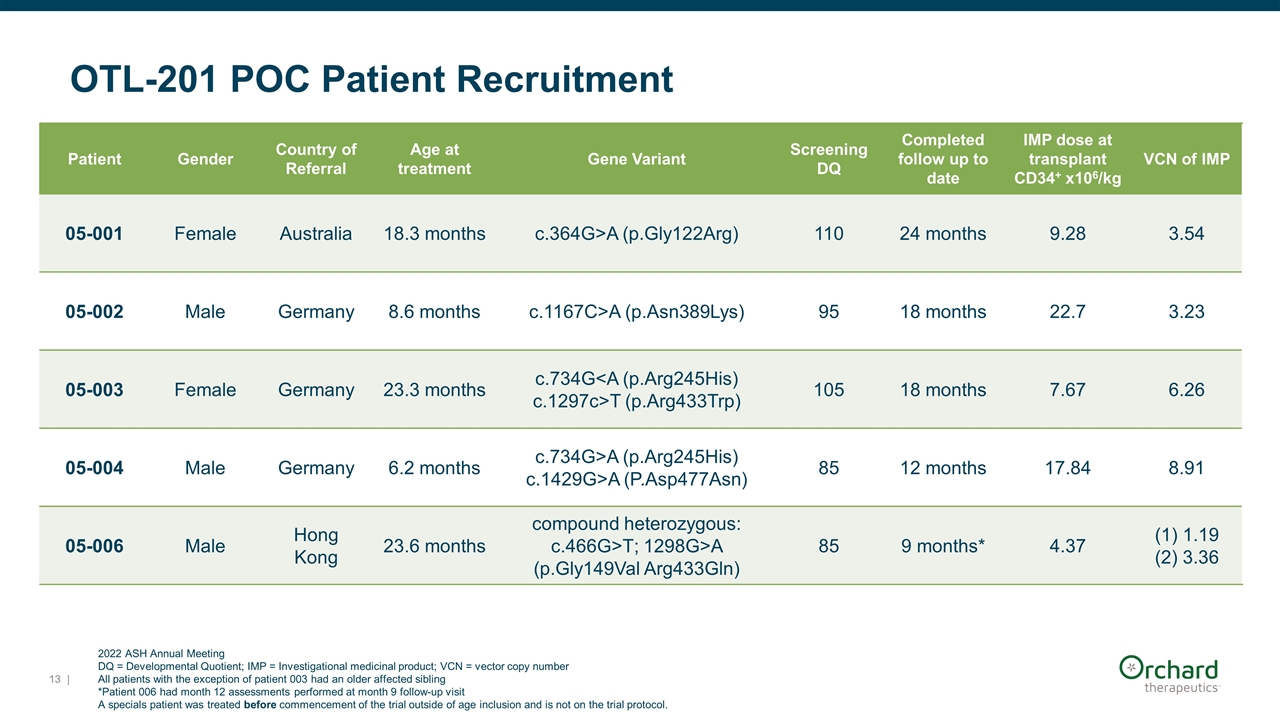
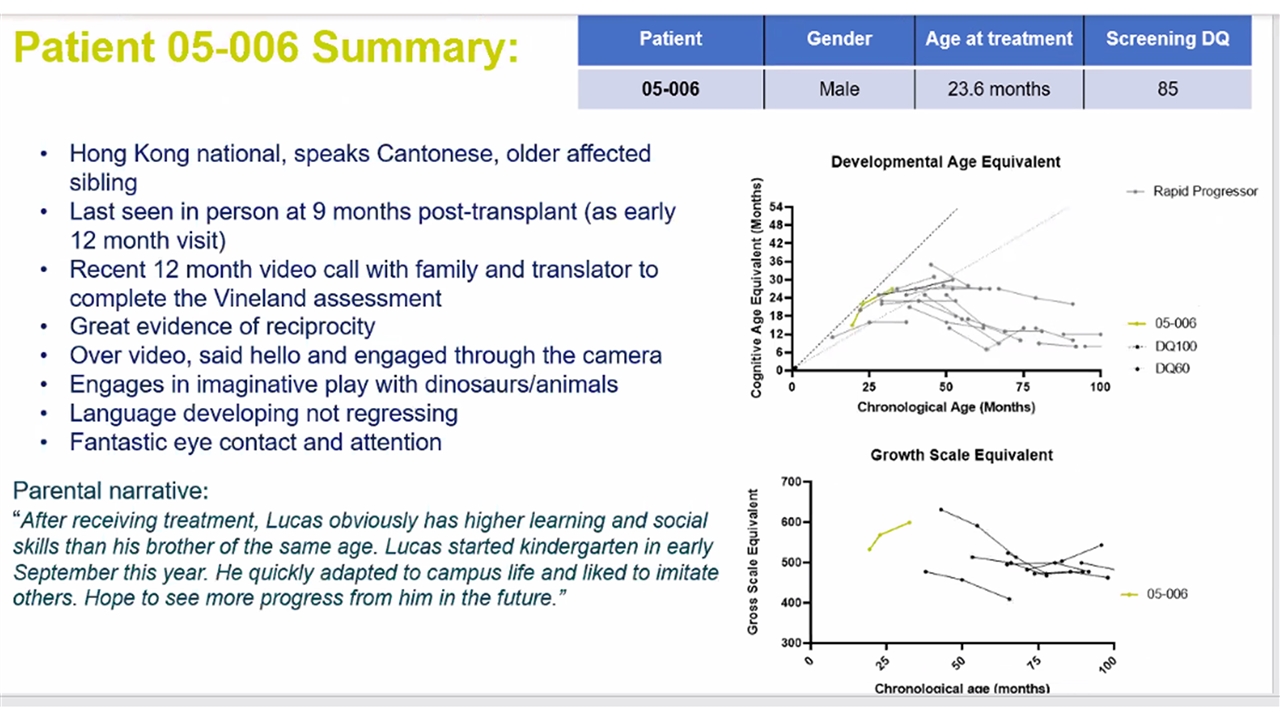
OTL-201 POC Patient Recruitment Patient Gender Country of Referral Age at treatment Gene Variant Screening DQ Completed follow up to date IMP dose at transplant CD34+ x106/kg VCN of IMP 05-001 Female Australia 18.3 months c.364G>A (p.Gly122Arg) 110 24 months 9.28 3.54 05-002 Male Germany 8.6 months c.1167C>A (p.Asn389Lys) 95 18 months 22.7 3.23 05-003 Female Germany 23.3 months c.734G<A (p.Arg245His) c.1297c>T (p.Arg433Trp) 105 18 months 7.67 6.26 05-004 Male Germany 6.2 months c.734G>A (p.Arg245His) c.1429G>A (P.Asp477Asn) 85 12 months 17.84 8.91 05-006 Male Hong Kong 23.6 months compound heterozygous: c.466G>T; 1298G>A (p.Gly149Val Arg433Gln) 85 9 months* 4.37 (1) 1.19 (2) 3.36 2022 ASH Annual Meeting DQ = Developmental Quotient; IMP = Investigational medicinal product; VCN = vector copy number All patients with the exception of patient 003 had an older affected sibling *Patient 006 had month 12 assessments performed at month 9 follow-up visit A specials patient was treated before commencement of the trial outside of age inclusion and is not on the trial protocol. |
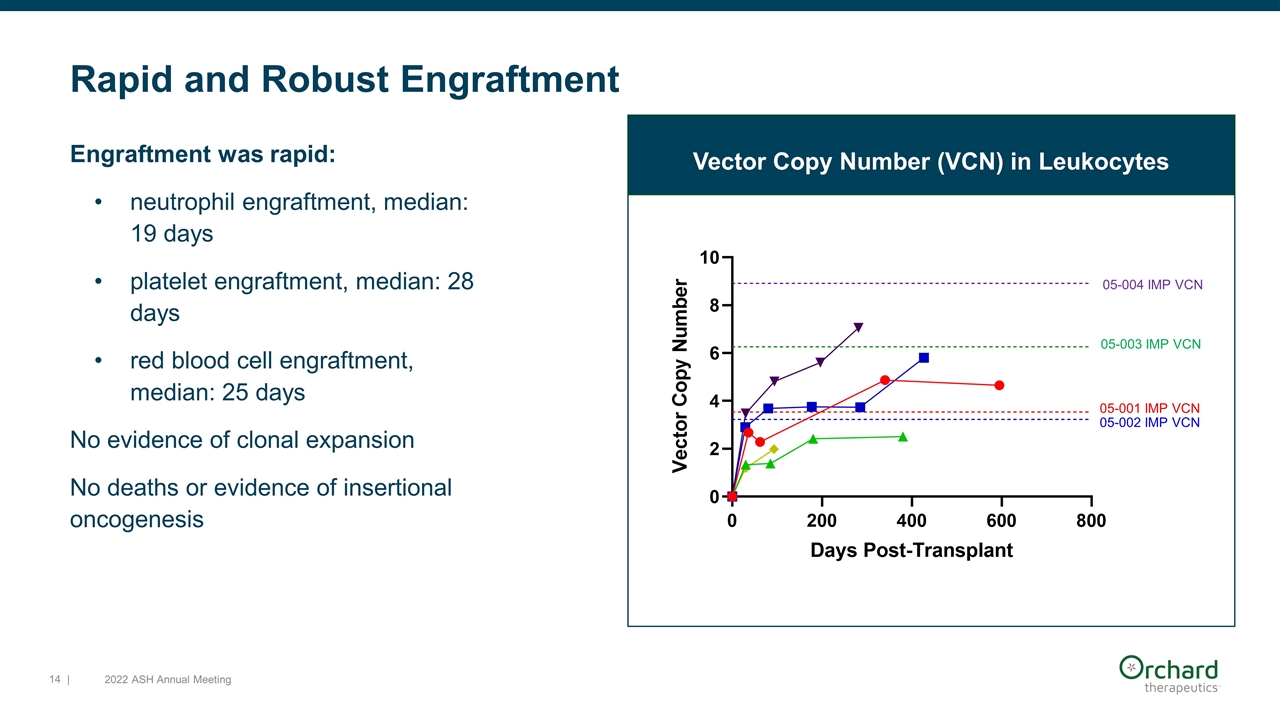
Rapid and Robust Engraftment 05-001 IMP VCN 05-002 IMP VCN 05-004 IMP VCN 05-003 IMP VCN Engraftment was rapid: neutrophil engraftment, median: 19 days platelet engraftment, median: 28 days red blood cell engraftment, median: 25 days No evidence of clonal expansion No deaths or evidence of insertional oncogenesis Vector Copy Number (VCN) in Leukocytes | 2022 ASH Annual Meeting
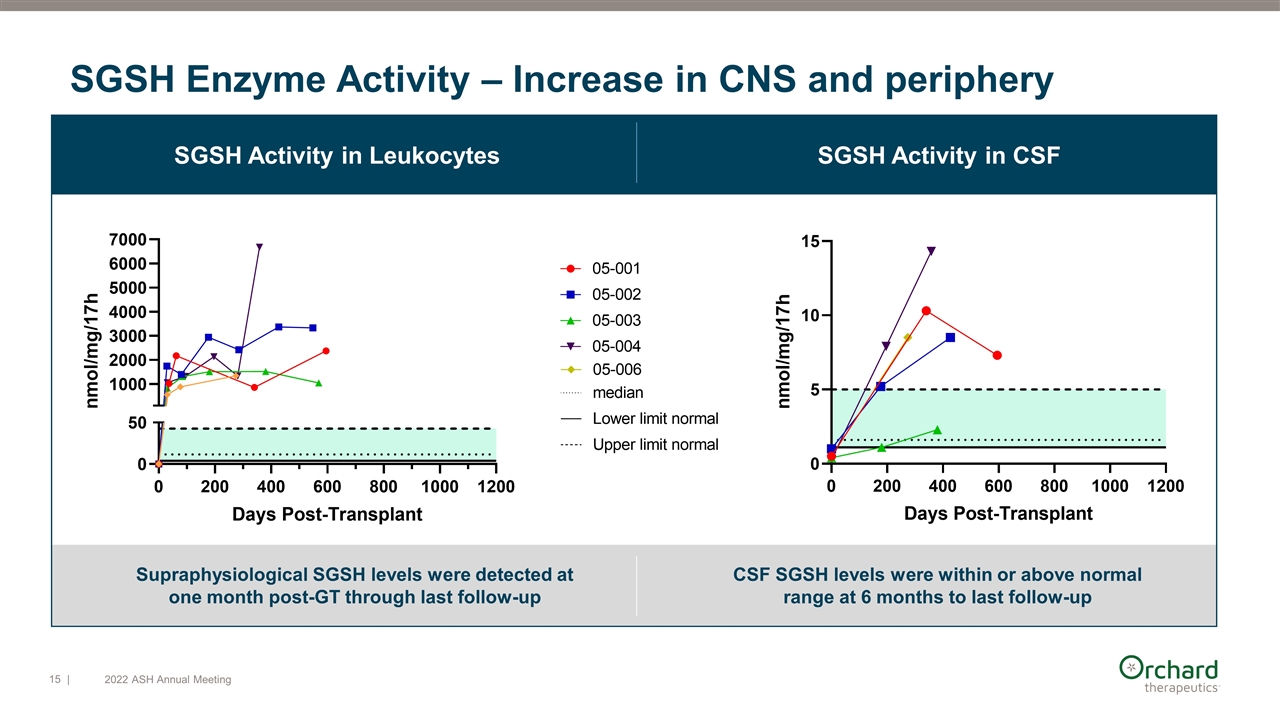
Supraphysiological SGSH levels were detected at one month post-GT through last follow-up SGSH Activity in Leukocytes SGSH Enzyme Activity – Increase in CNS and periphery SGSH Activity in CSF CSF SGSH levels were within or above normal range at 6 months to last follow-up | 2022 ASH Annual Meeting
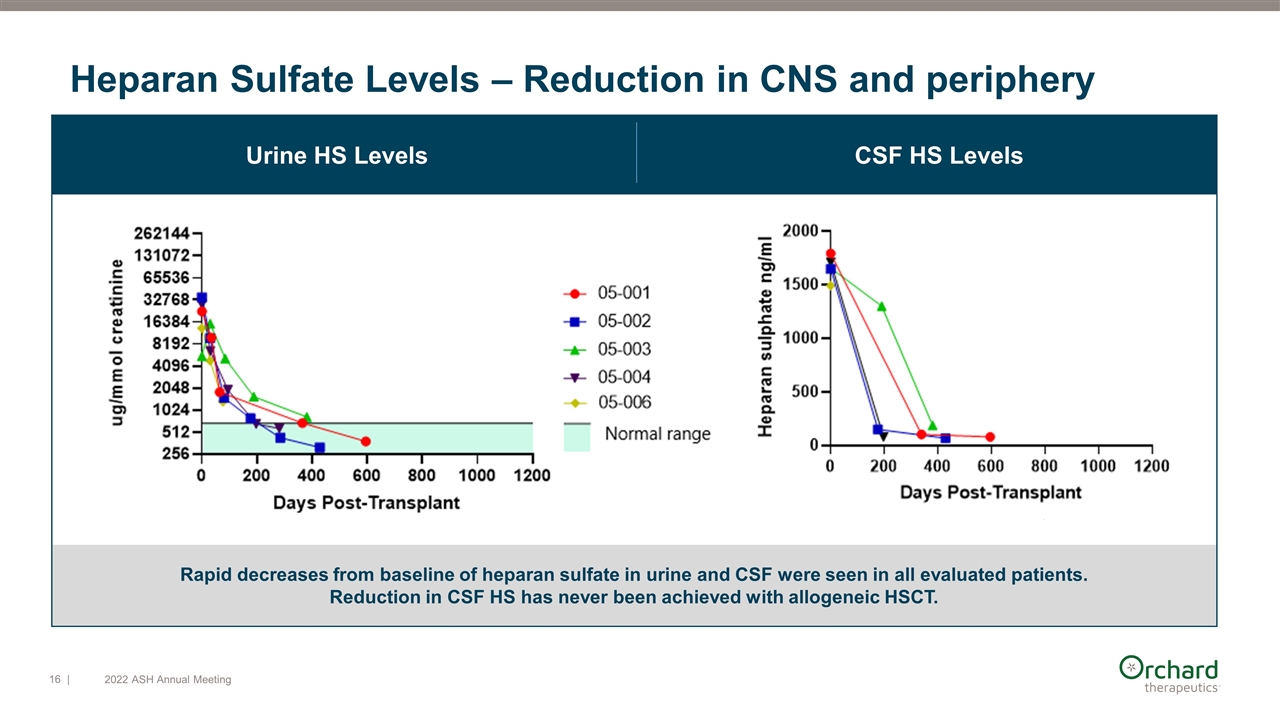
Rapid decreases from baseline of heparan sulfate in urine and CSF were seen in all evaluated patients. Reduction in CSF HS has never been achieved with allogeneic HSCT. Urine HS Levels CSF HS Levels Heparan Sulfate Levels – Reduction in CNS and periphery | 2022 ASH Annual Meeting

OTL-201 Clinical Outcomes Dr. Simon Jones
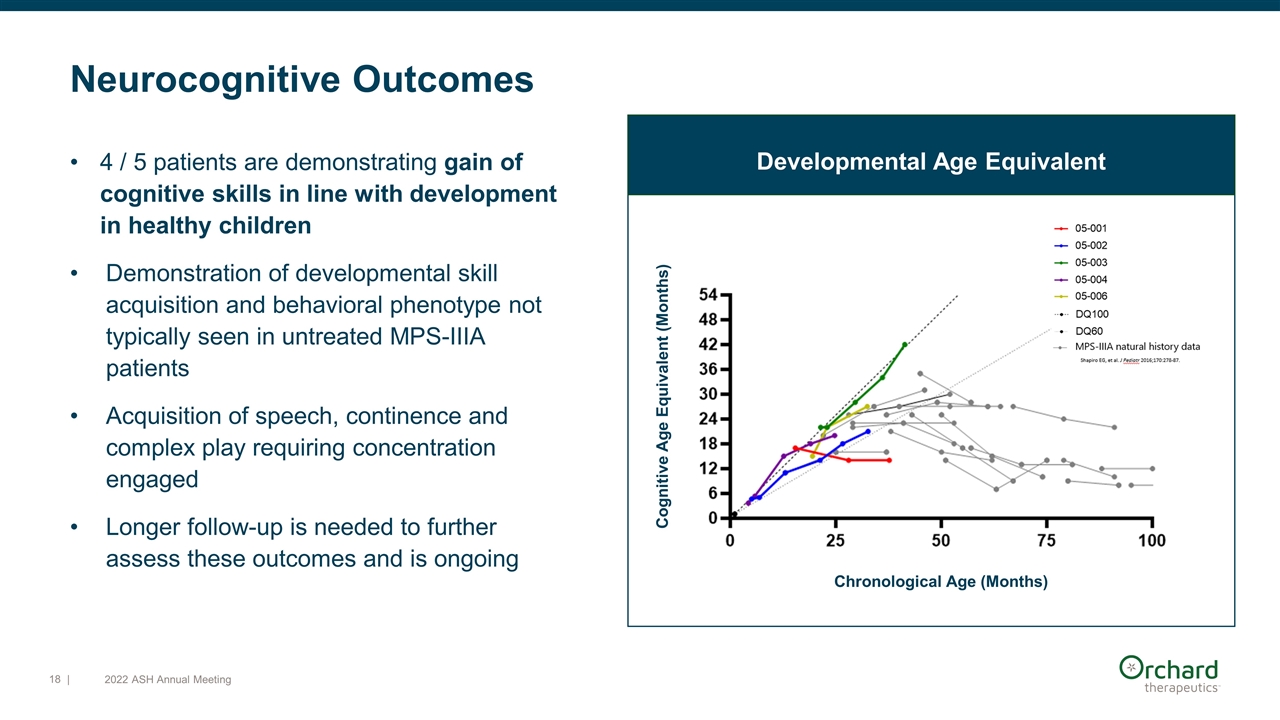
Neurocognitive Outcomes 4 / 5 patients are demonstrating gain of cognitive skills in line with development in healthy children Demonstration of developmental skill acquisition and behavioral phenotype not typically seen in untreated MPS-IIIA patients Acquisition of speech, continence and complex play requiring concentration engaged Longer follow-up is needed to further assess these outcomes and is ongoing Developmental Age Equivalent Chronological Age (Months) Cognitive Age Equivalent (Months) | 2022 ASH Annual Meeting
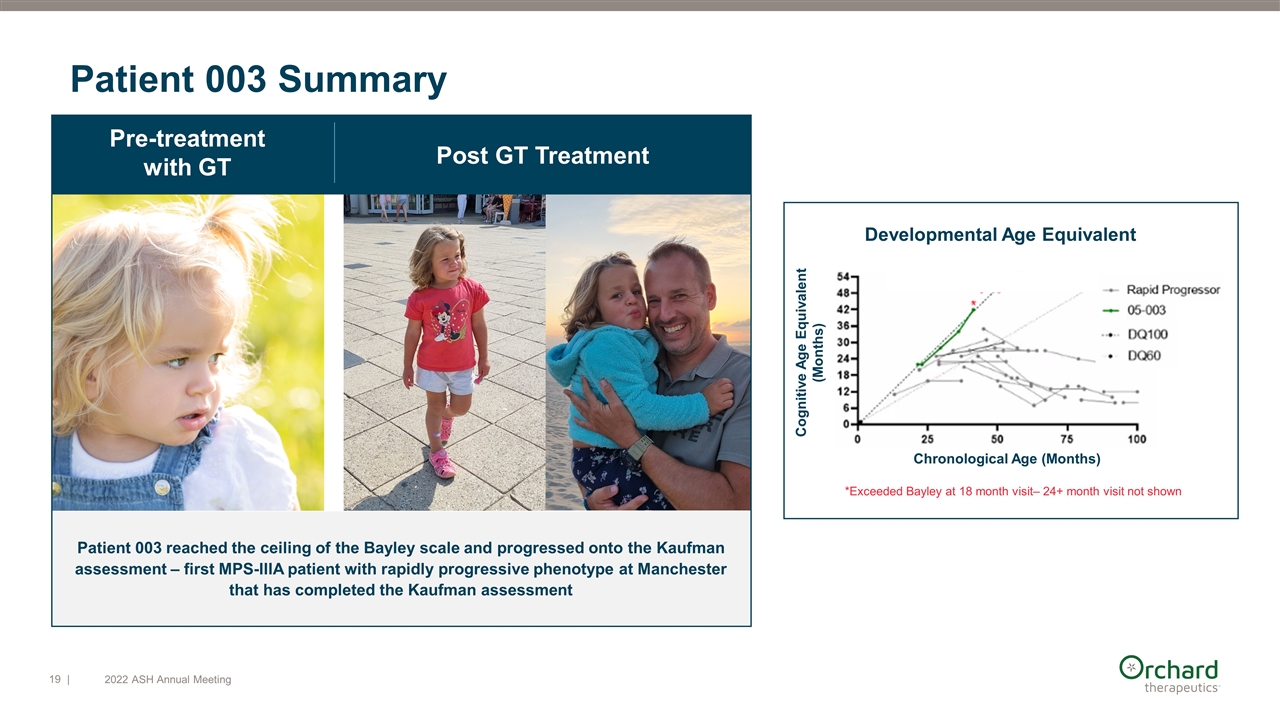
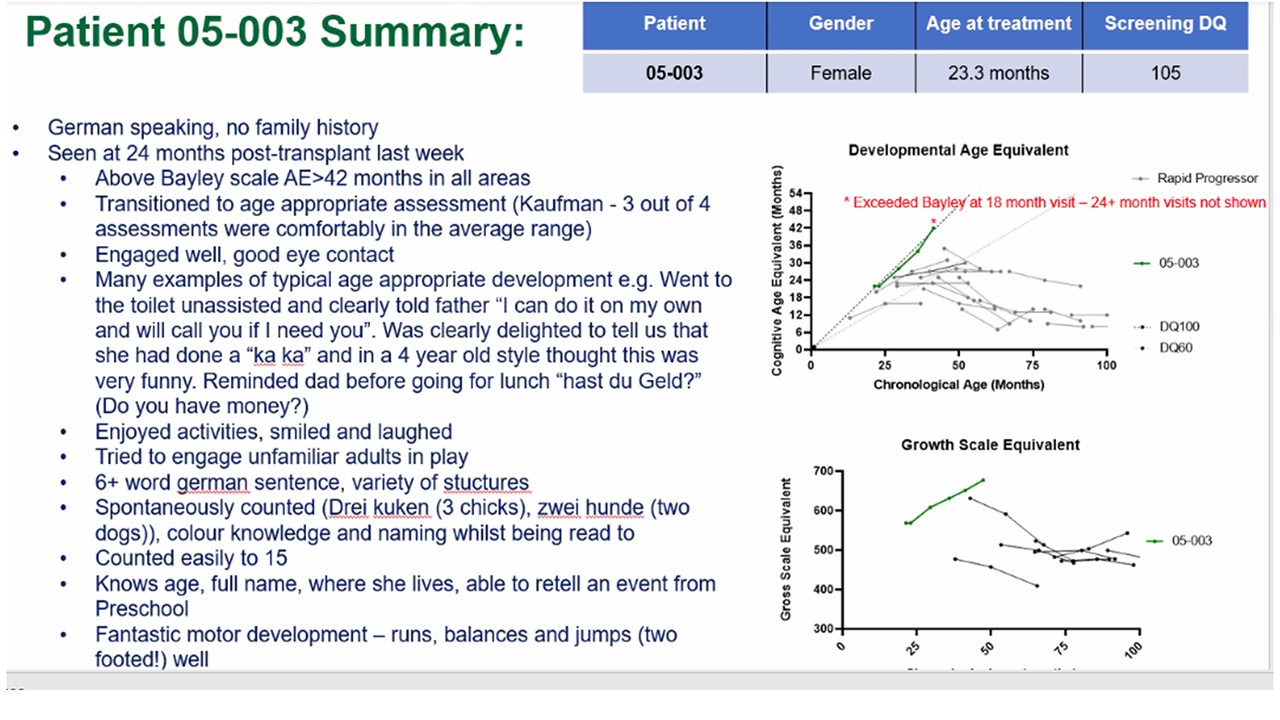
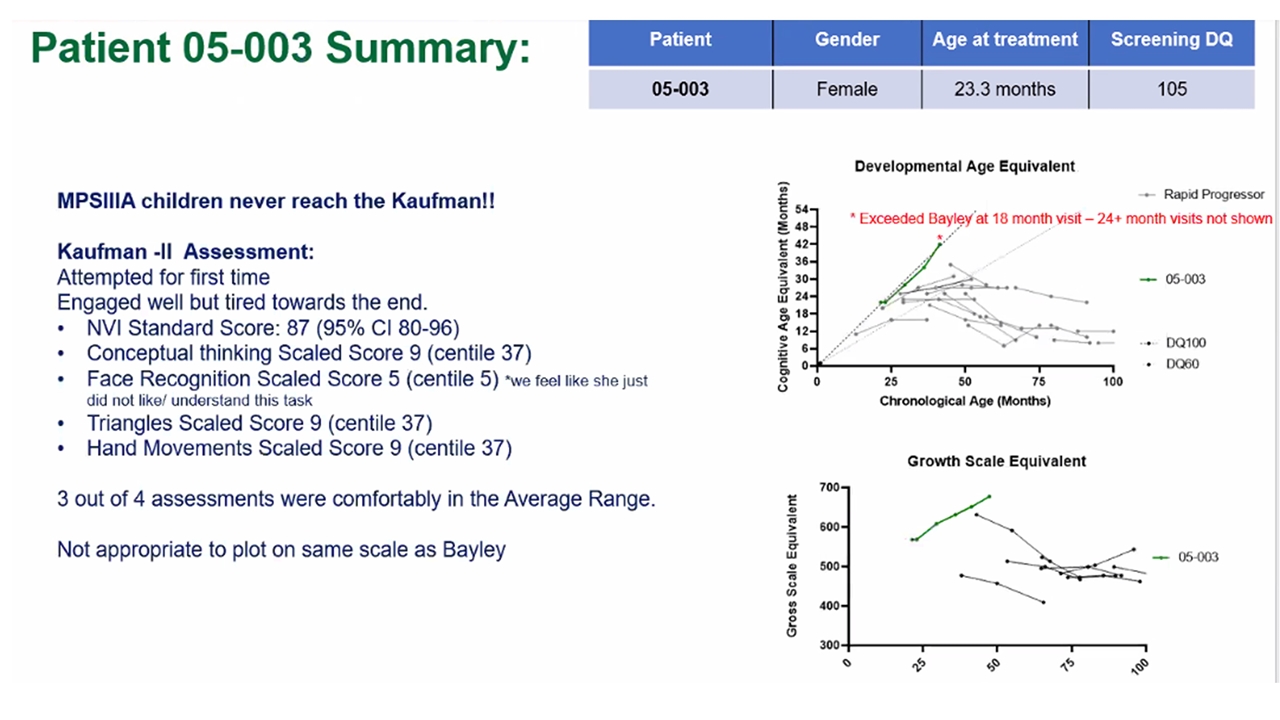
Patient 003 reached the ceiling of the Bayley scale and progressed onto the Kaufman assessment – first MPS-IIIA patient with rapidly progressive phenotype at Manchester that has completed the Kaufman assessment Pre-treatment with GT Post GT Treatment Patient 003 Summary Developmental Age Equivalent Chronological Age (Months) Cognitive Age Equivalent (Months) *Exceeded Bayley at 18 month visit– 24+ month visit not shown | 2022 ASH Annual Meeting

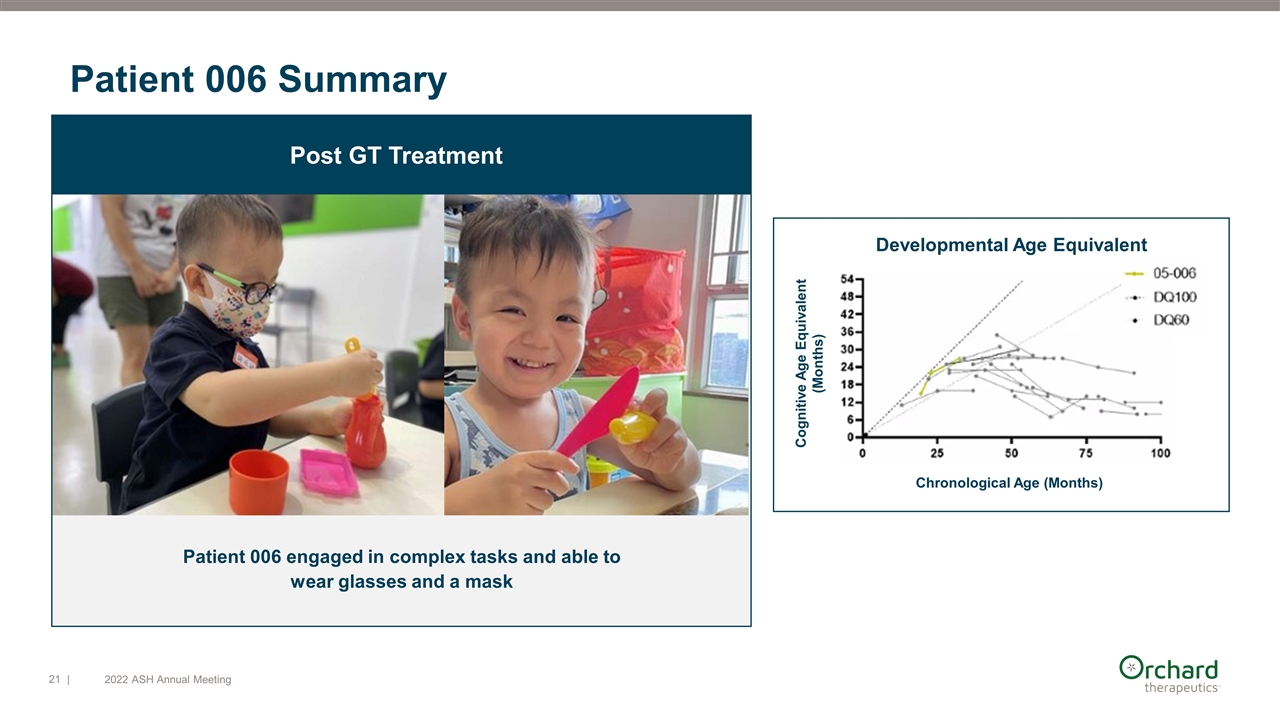
Patient 006 engaged in complex tasks and able to wear glasses and a mask Post GT Treatment Patient 006 Summary Developmental Age Equivalent Chronological Age (Months) Cognitive Age Equivalent (Months) | 2022 ASH Annual Meeting
Treatment was generally well-tolerated Robust, prompt, sustained, multi-lineage engraftment of genetically modified cells Supra-physiological levels of SGSH enzyme in leukocytes, plasma and CSF Rapid and significant reduction of substrate observed in all compartments Neurocognitive trial data is early but suggests a modification of the neurological phenotype in patients OTL-201 POC Conclusions | 2022 ASH Annual Meeting

Report additional biochemical and clinical outcomes Patients will be followed for a minimum of 3 years Significant medical need given there are no effective therapies Program presents multiple development and commercial synergies with Orchard’s other neurometabolic programs OTL-201 Program Next Steps |

Q&A Session

Back-up Appendix

| ASH IR Event Agenda AGENDA TOPIC SPEAKER Orchard’s HSC Gene Therapy and MPS-IIIA Disease Overview Bobby Gaspar OTL-201 Data Update Prof Rob Wynn Q&A Leslie Meltzer
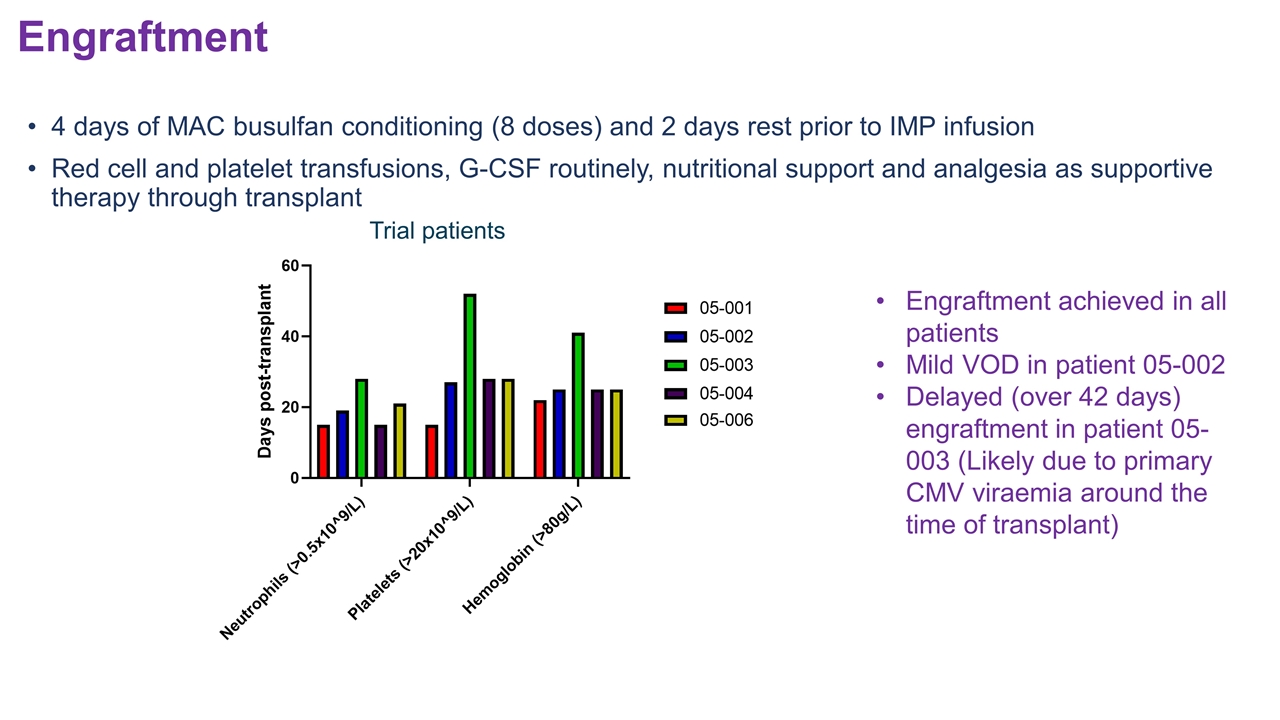
4 days of MAC busulfan conditioning (8 doses) and 2 days rest prior to IMP infusion Red cell and platelet transfusions, G-CSF routinely, nutritional support and analgesia as supportive therapy through transplant Engraftment achieved in all patients Mild VOD in patient 05-002 Delayed (over 42 days) engraftment in patient 05-003 (Likely due to primary CMV viraemia around the time of transplant) Engraftment Trial patients
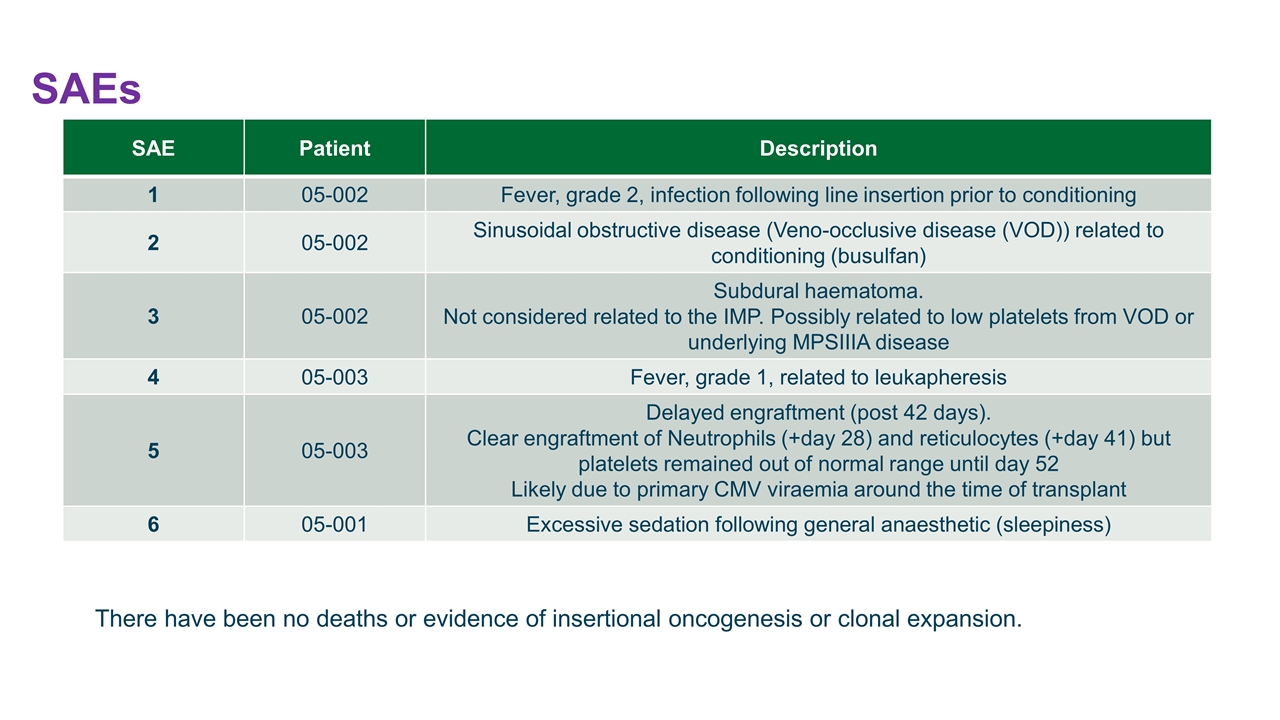
SAEs SAE Patient Description 1 05-002 Fever, grade 2, infection following line insertion prior to conditioning 2 05-002 Sinusoidal obstructive disease (Veno-occlusive disease (VOD)) related to conditioning (busulfan) 3 05-002 Subdural haematoma. Not considered related to the IMP. Possibly related to low platelets from VOD or underlying MPSIIIA disease 4 05-003 Fever, grade 1, related to leukapheresis 5 05-003 Delayed engraftment (post 42 days). Clear engraftment of Neutrophils (+day 28) and reticulocytes (+day 41) but platelets remained out of normal range until day 52 Likely due to primary CMV viraemia around the time of transplant 6 05-001 Excessive sedation following general anaesthetic (sleepiness) There have been no deaths or evidence of insertional oncogenesis or clonal expansion.
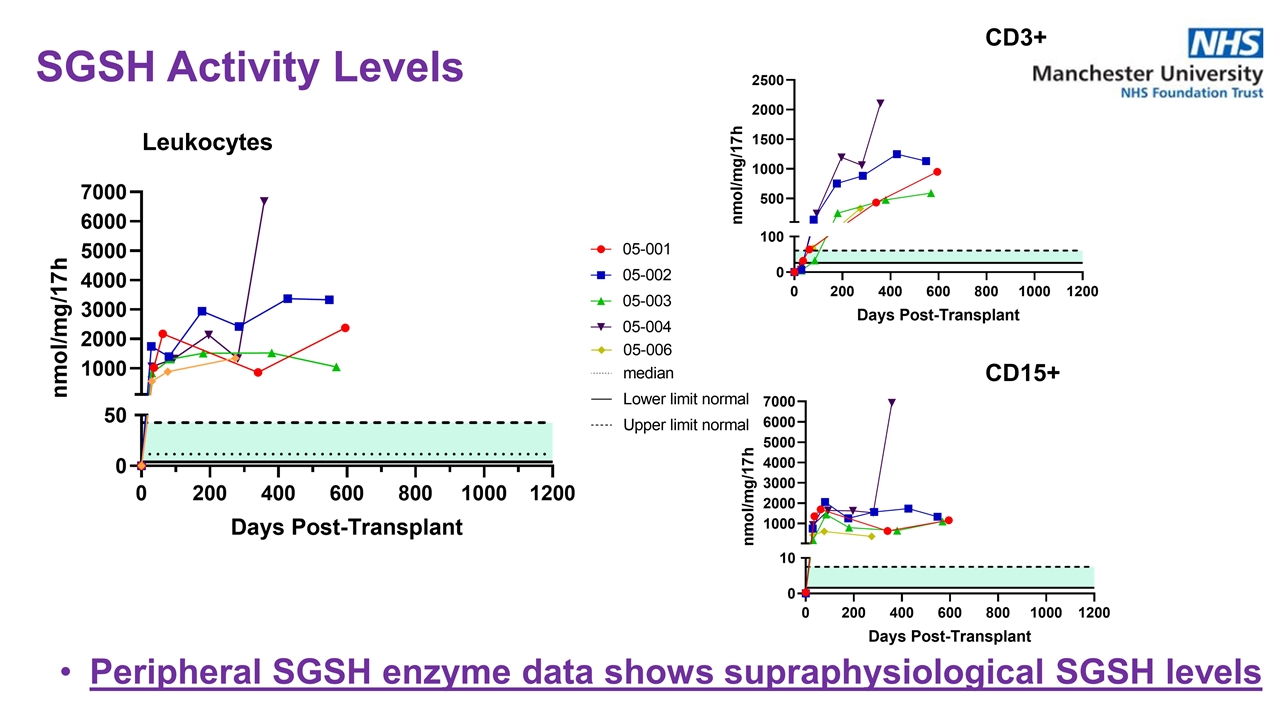
Peripheral SGSH enzyme data shows supraphysiological SGSH levels Leukocytes CD3+ CD15+ SGSH Activity Levels
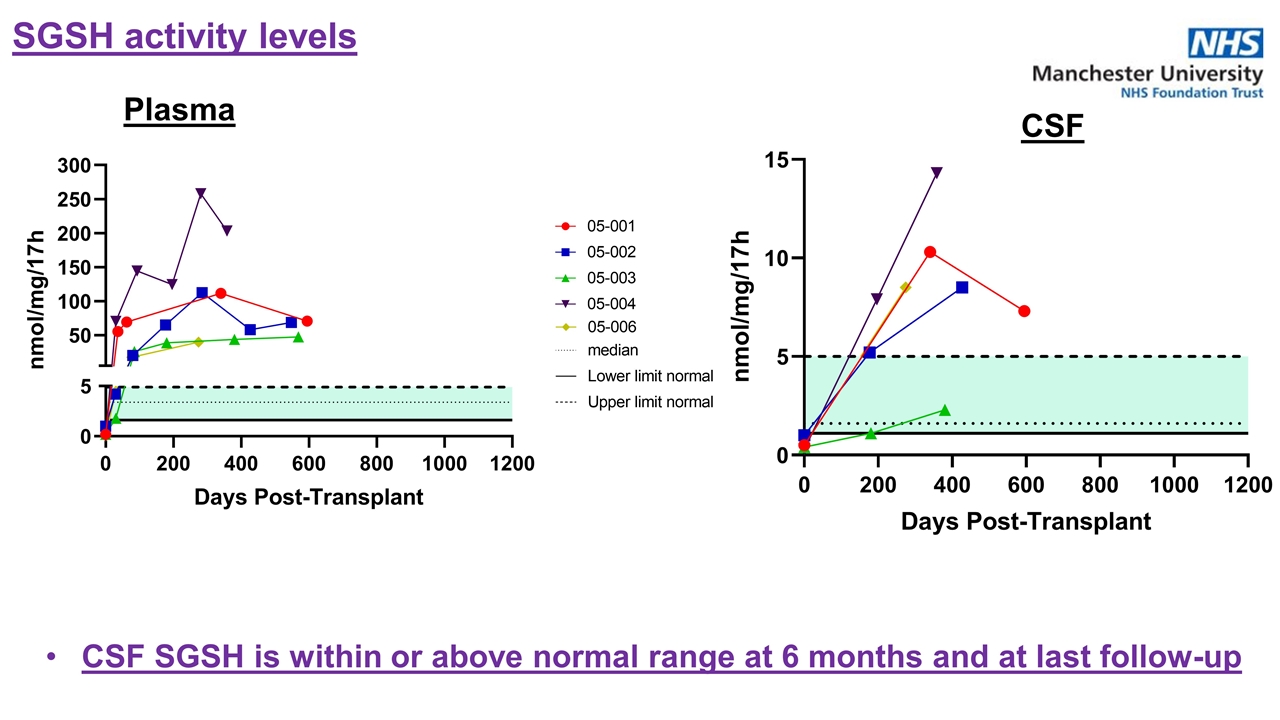
CSF SGSH is within or above normal range at 6 months and at last follow-up Plasma CSF SGSH activity levels
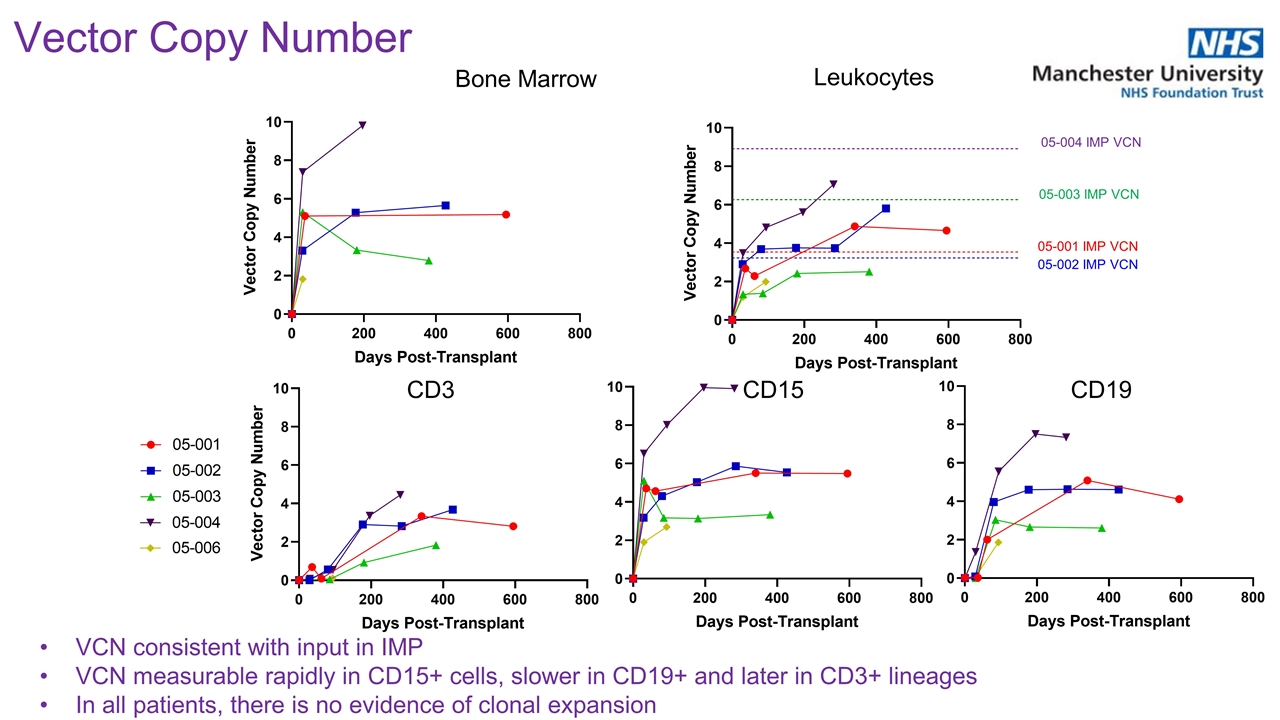
Vector Copy Number VCN consistent with input in IMP VCN measurable rapidly in CD15+ cells, slower in CD19+ and later in CD3+ lineages In all patients, there is no evidence of clonal expansion Bone Marrow Leukocytes CD3 CD15 CD19 05-001 IMP VCN 05-002 IMP VCN 05-004 IMP VCN 05-003 IMP VCN
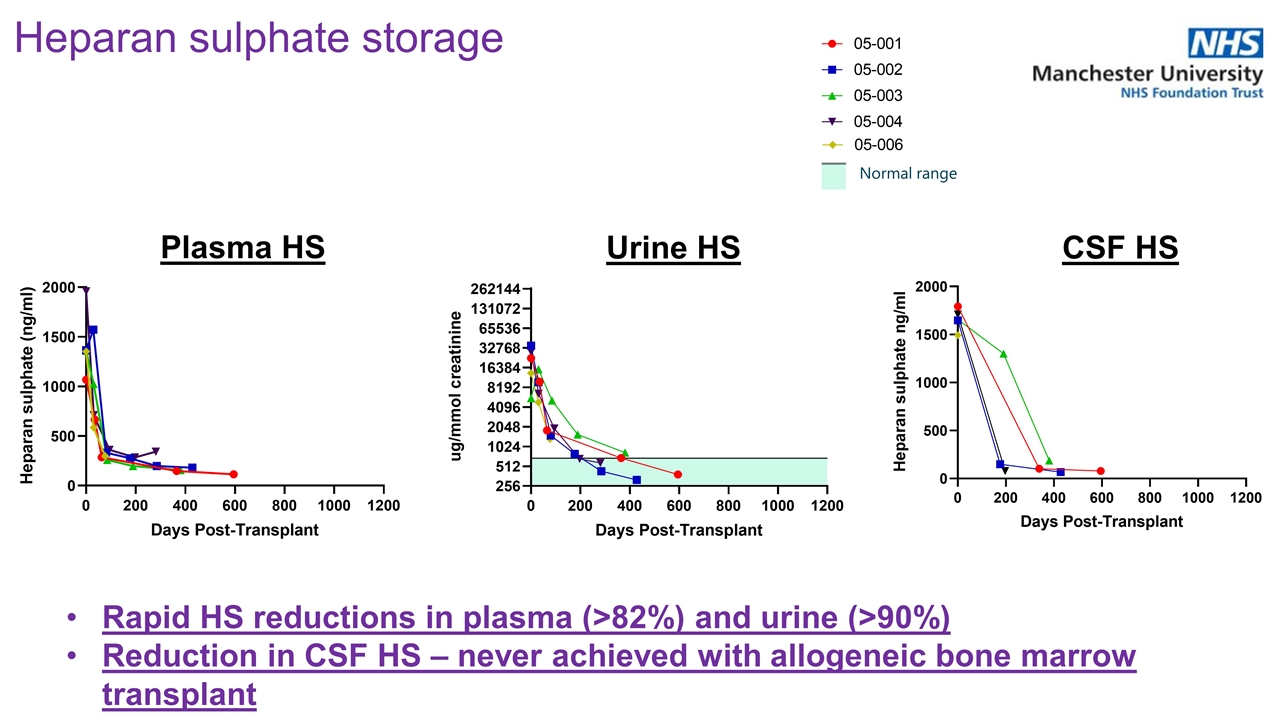
Rapid HS reductions in plasma (>82%) and urine (>90%) Reduction in CSF HS – never achieved with allogeneic bone marrow transplant Heparan sulphate storage Plasma HS Urine HS CSF HS Normal range

Neurocognitive outcomes, SUMMARY Change in cognitive function (age equivalent scores) against natural history of MPSIIIA Change in patient behaviour, patient QoL and daily living – data not formally presented, important to patients and to regulators Specials patient had suggested an attenuated phenotype compared to natural history, no loss of skills Early follow up in trial patients 4 out of 5 patients actively gaining skills in the normal range Developmental progression not seen in MPSIIIA, acquisition of speech, continence and complex play requiring concentration, engaged, wearing glasses and a mask Longer follow up needed to assess outcomes, and ongoing Patient 006
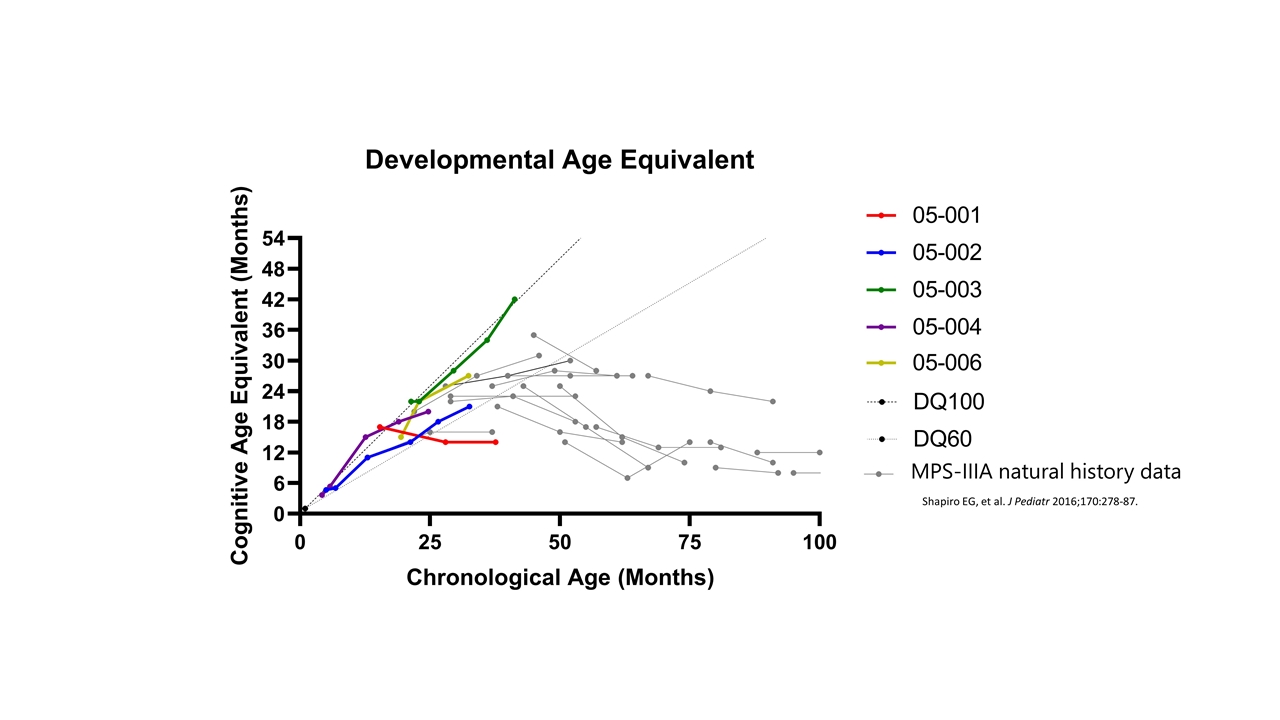
MPS-IIIA natural history data Shapiro EG, et al. J Pediatr 2016;170:278-87.