
ESMO Breast Cancer 2024�Palazestrant-Ribociclib Combination Interim Results May 15, 2024

Forward-Looking Statements This presentation contains forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Statements in this presentation that are not statements of historical fact are forward-looking statements. Words such as “anticipate,” “believe,” “estimate,” “expect,” “goal,” “intend,” “may,” “plan,” “potential,” “project,” “will,” and similar expressions are intended to identify forward-looking statements, though not all forward-looking statements necessarily contain these identifying words. Such forward-looking statements include, without limitation, statements regarding our research and clinical development plans, the scope, progress, results and costs of developing our product candidate or any other future product candidates, strategy, regulatory matters, including the timing and likelihood of success of obtaining drug approvals, the timelines for potential clinical study results and clinical trials of palazestrant (OP-1250) as a monotherapy and in combination trials, including OPERA-01, the Company’s pivotal Phase 3 monotherapy clinical trial, and OPERA-02, the Company's potential pivotal Phase 3 clinical trial of palazestrant in combination with ribociclib, the potential beneficial characteristics, profile, safety, tolerability, efficacy and therapeutic effects of palazestrant as a monotherapy and in combination trials, the potential beneficial characteristics and tolerability of a tablet formulation, the potential of palazestrant to become a best-in-class treatment option for ER+/HER2- metastatic breast cancer and a backbone therapy for women living with breast cancer, the combinability of palazestrant with other drugs, the timelines for potential preclinical studies and clinical trials for our KAT6 inhibitor, market size and opportunity, our ability to complete certain milestones, our financial condition, cash position and runway and sufficiency of our financial resources, and the sufficiency of our management team. These forward-looking statements are based on the beliefs of the Company’s management as well as assumptions made by and information currently available to the Company. Such statements reflect the current views of the Company with respect to future events and are subject to known and unknown risks, including business, regulatory, economic and competitive risks, uncertainties, contingencies and assumptions about the Company, including, without limitation, risks inherent in developing products and technologies, future results from the Company’s ongoing and planned clinical trials, the Company’s ability to obtain adequate financing to fund its planned clinical trials and other expenses, trends in the industry, the legal and regulatory framework for the industry and future expenditures, and other risks and uncertainties affecting the Company, including those described under the caption "Risk Factors" and elsewhere in the Company‘s Quarterly Reports on Form 10-Q, Annual Report on Form 10-K, and other filings and reports the Company files with the Securities and Exchange Commission from time to time. In light of these risks and uncertainties, the events or circumstances referred to in the forward-looking statements may not occur. The actual results may vary from the anticipated results and the variations may be material. These forward-looking statements should not be taken as forecasts or promises nor should they be taken as implying any indication, assurance or guarantee that the assumptions on which such forward-looking statements have been made are correct or exhaustive or, in the case of the assumptions, fully stated in this presentation. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date this presentation is given. This presentation discusses product candidates that are under clinical study and which have not yet been approved for marketing by the U.S. Food and Drug Administration. No representation is made as to the safety or effectiveness of these product candidates for the use for which such product candidates are being studied. This presentation incorporates publicly-available third-party data that we have not independently verified. There are risks inherent in conducting cross-trial comparisons and the results should be interpreted with caution. The presentation of such third-party data does not represent a head-to-head comparison of how palazestrant, in monotherapy or in combination, performed against any other third-party drug candidate or study. Rather, such third-party data has been pulled by us from publicly-available sources for supplemental informational purposes, only. We caution you that any comparisons against third-party data set forth herein should not be viewed as a side-by-side comparison, and you should not rely on the completeness or accuracy of our presentation of the results of any third-party drug candidate in these slides, due to differences in study design, how other companies quantify or qualify eligibility criteria, and how results are recorded, among other distinguishing factors and uncertainties. Because we may be unaware of or may not adequately present various distinguishing factors and uncertainties, the comparisons set forth herein may not properly present such third-party data, which may differ materially from the data as presented here. Investors are encouraged to independently review third party data and should not rely on our presentation of such data (including any such data placed in comparison with the performance of palazestrant) as a single measure to evaluate our business. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of such products.
Interim Phase 1b/2 data across 50 patients (on treatment for >4wks); 60-patient study now fully enrolled Strong investigator enthusiasm resulted in rapid study enrollment Largest data set for any CERAN/SERD in combination with ribociclib to be presented Compelling combinability Well tolerated with no DLTs, no new safety signals or increased toxicity No clinically meaningful impact on drug exposure of either therapy Promising preliminary efficacy shows best-in-class potential 85% clinical benefit rate (CBR) across all CBR-eligible patients 83% in ESR1-mutant patients, 86% in ESR1-wild-type patients, and 83% in CDK4/6i-pretreated patients Efficacy data continues to mature Palazestrant has shown best-in-class potential and significant differentiation based both on monotherapy efficacy and an ability to effectively combine with CDK4/6 inhibitors Results support continued clinical development of palazestrant in combination with ribociclib in a 1st-line pivotal Phase 3 trial Unmatched Combinability and Promising Efficacy for Palazestrant-Ribociclib Combo

Phase 1b/2 Palazestrant-Ribociclib Combination Clinical Study
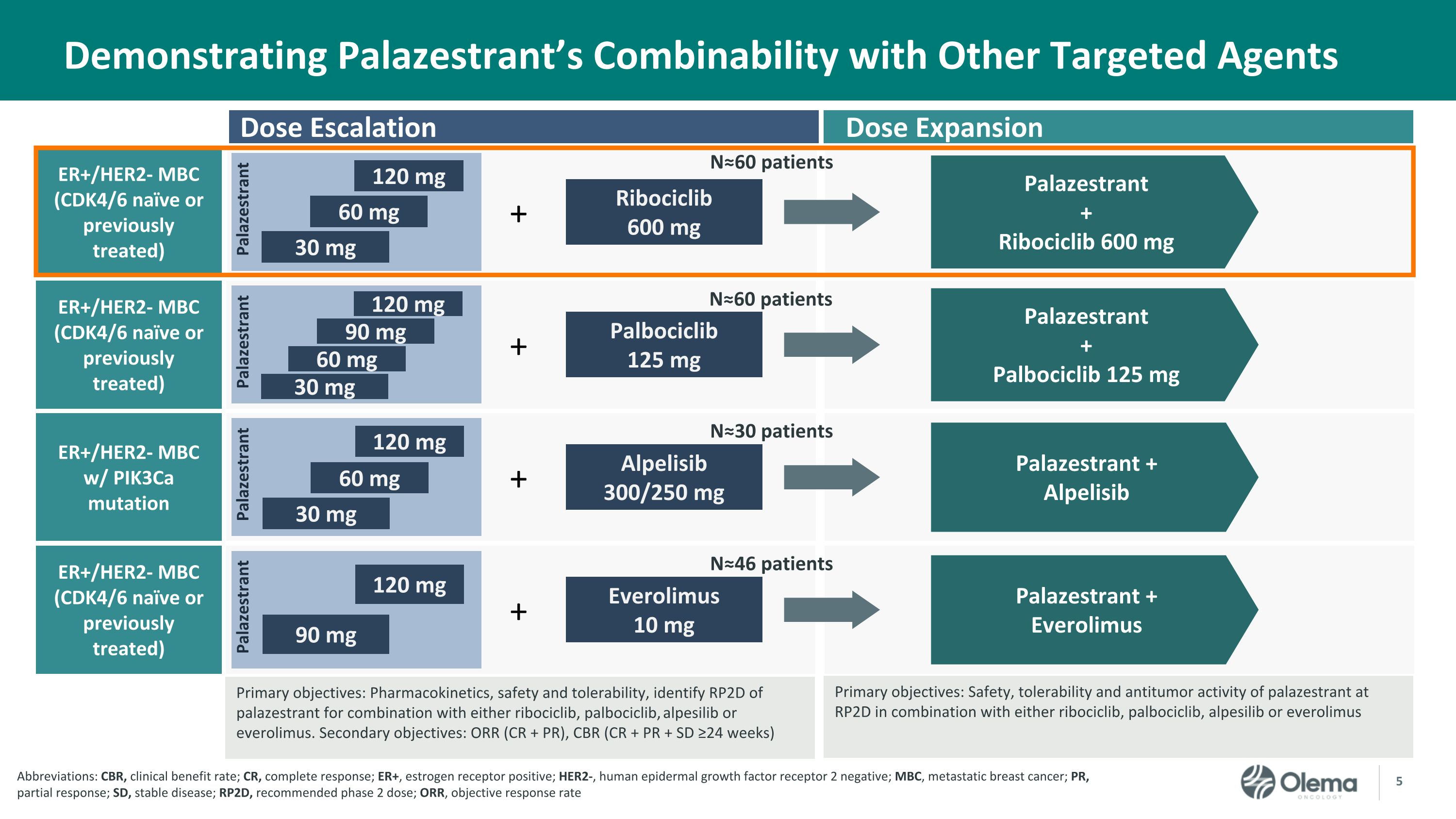
Demonstrating Palazestrant’s Combinability with Other Targeted Agents + + ER+/HER2- MBC (CDK4/6 naïve or previously treated) ER+/HER2- MBC w/ PIK3Ca mutation Palazestrant + Ribociclib 600 mg Palazestrant + Alpelisib Ribociclib 600 mg Alpelisib�300/250 mg Dose Expansion Dose Escalation Primary objectives: Safety, tolerability and antitumor activity of palazestrant at RP2D in combination with either ribociclib, palbociclib, alpesilib or everolimus Primary objectives: Pharmacokinetics, safety and tolerability, identify RP2D of palazestrant for combination with either ribociclib, palbociclib, alpesilib or everolimus. Secondary objectives: ORR (CR + PR), CBR (CR + PR + SD ≥24 weeks) N≈60 patients N≈30 patients Palazestrant Palazestrant 30 mg 60 mg 120 mg ER+/HER2- MBC (CDK4/6 naïve or previously treated) Palazestrant 90 mg 120 mg + Everolimus�10 mg N≈46 patients Palazestrant + Everolimus 30 mg 60 mg 120 mg Abbreviations: CBR, clinical benefit rate; CR, complete response; ER+, estrogen receptor positive; HER2-, human epidermal growth factor receptor 2 negative; MBC, metastatic breast cancer; PR, partial response; SD, stable disease; RP2D, recommended phase 2 dose; ORR, objective response rate + ER+/HER2- MBC (CDK4/6 naïve or previously treated) Palazestrant + Palbociclib 125 mg Palbociclib 125 mg N≈60 patients Palazestrant 30 mg 60 mg 120 mg 90 mg
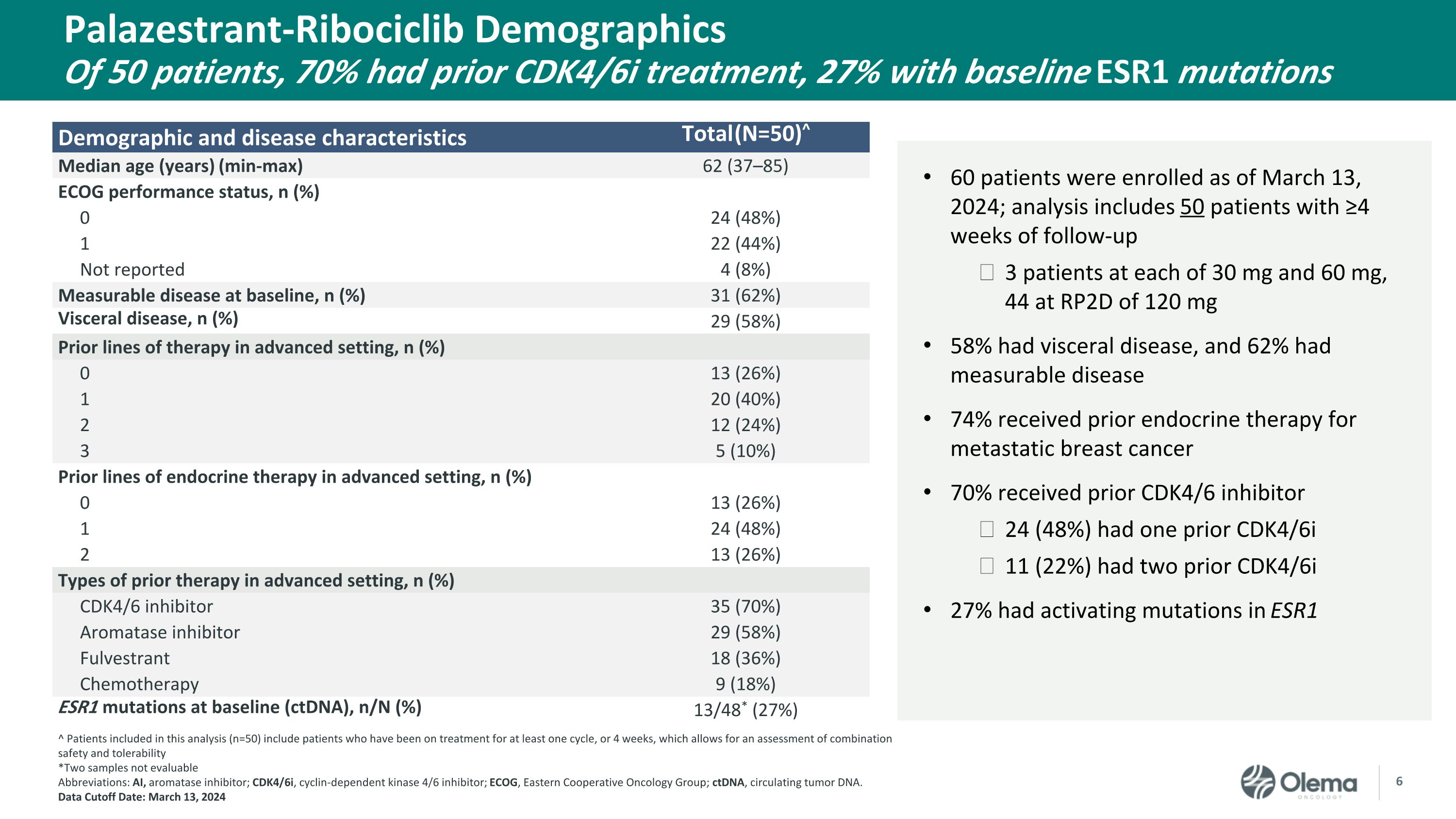
Demographic and disease characteristics Total (N=50)^ Median age (years) (min-max) 62 (37–85) ECOG performance status, n (%) 0 24 (48%) 1 22 (44%) Not reported 4 (8%) Measurable disease at baseline, n (%) 31 (62%) Visceral disease, n (%) 29 (58%) Prior lines of therapy in advanced setting, n (%) 0 13 (26%) 1 20 (40%) 2 12 (24%) 3 5 (10%) Prior lines of endocrine therapy in advanced setting, n (%) 0 13 (26%) 1 24 (48%) 2 13 (26%) Types of prior therapy in advanced setting, n (%) CDK4/6 inhibitor 35 (70%) Aromatase inhibitor 29 (58%) Fulvestrant 18 (36%) Chemotherapy 9 (18%) ESR1 mutations at baseline (ctDNA), n/N (%) 13/48* (27%) Palazestrant-Ribociclib Demographics�Of 50 patients, 70% had prior CDK4/6i treatment, 27% with baseline ESR1 mutations 60 patients were enrolled as of March 13, 2024; analysis includes 50 patients with ≥4 weeks of follow-up 3 patients at each of 30 mg and 60 mg, 44 at RP2D of 120 mg 58% had visceral disease, and 62% had measurable disease 74% received prior endocrine therapy for metastatic breast cancer 70% received prior CDK4/6 inhibitor 24 (48%) had one prior CDK4/6i 11 (22%) had two prior CDK4/6i 27% had activating mutations in ESR1 ^ Patients included in this analysis (n=50) include patients who have been on treatment for at least one cycle, or 4 weeks, which allows for an assessment of combination safety and tolerability *Two samples not evaluable Abbreviations: AI, aromatase inhibitor; CDK4/6i, cyclin-dependent kinase 4/6 inhibitor; ECOG, Eastern Cooperative Oncology Group; ctDNA, circulating tumor DNA.�Data Cutoff Date: March 13, 2024
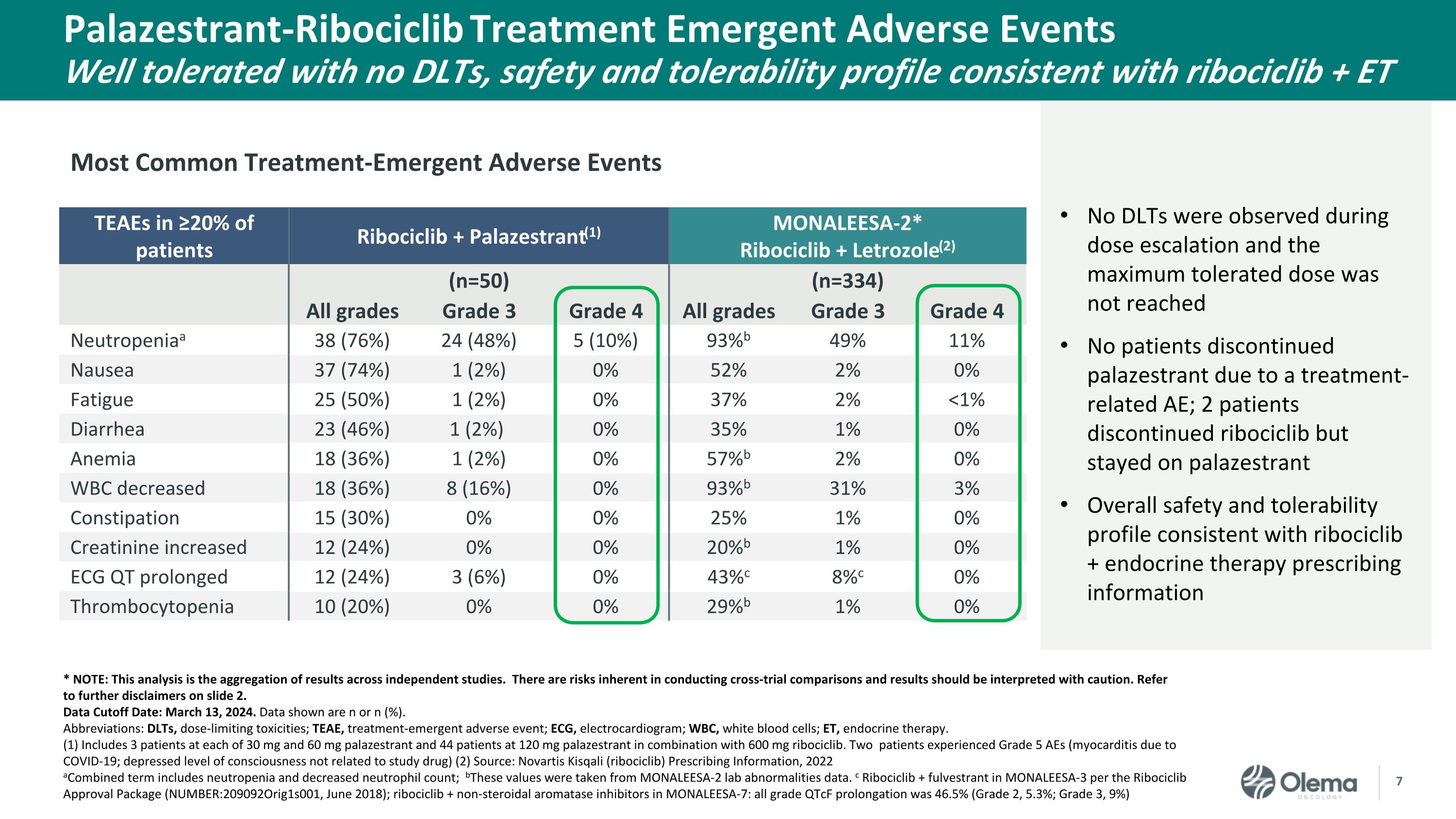
Palazestrant-Ribociclib Treatment Emergent Adverse Events �Well tolerated with no DLTs, safety and tolerability profile consistent with ribociclib + ET TEAEs in ≥20% of patients Ribociclib + Palazestrant(1) MONALEESA-2*�Ribociclib + Letrozole(2) (n=50) (n=334) All grades Grade 3 Grade 4 All grades Grade 3 Grade 4 Neutropeniaa 38 (76%) 24 (48%) 5 (10%) 93%b 49% 11% Nausea 37 (74%) 1 (2%) 0% 52% 2% 0% Fatigue 25 (50%) 1 (2%) 0% 37% 2% <1% Diarrhea 23 (46%) 1 (2%) 0% 35% 1% 0% Anemia 18 (36%) 1 (2%) 0% 57%b 2% 0% WBC decreased 18 (36%) 8 (16%) 0% 93%b 31% 3% Constipation 15 (30%) 0% 0% 25% 1% 0% Creatinine increased 12 (24%) 0% 0% 20%b 1% 0% ECG QT prolonged 12 (24%) 3 (6%) 0% 43%c 8%c 0% Thrombocytopenia 10 (20%) 0% 0% 29%b 1% 0% * NOTE: This analysis is the aggregation of results across independent studies. There are risks inherent in conducting cross-trial comparisons and results should be interpreted with caution. Refer to further disclaimers on slide 2. Data Cutoff Date: March 13, 2024. Data shown are n or n (%). Abbreviations: DLTs, dose-limiting toxicities; TEAE, treatment-emergent adverse event; ECG, electrocardiogram; WBC, white blood cells; ET, endocrine therapy. (1) Includes 3 patients at each of 30 mg and 60 mg palazestrant and 44 patients at 120 mg palazestrant in combination with 600 mg ribociclib. Two patients experienced Grade 5 AEs (myocarditis due to COVID-19; depressed level of consciousness not related to study drug) (2) Source: Novartis Kisqali (ribociclib) Prescribing Information, 2022 aCombined term includes neutropenia and decreased neutrophil count; bThese values were taken from MONALEESA-2 lab abnormalities data. c Ribociclib + fulvestrant in MONALEESA-3 per the Ribociclib Approval Package (NUMBER:209092Orig1s001, June 2018); ribociclib + non-steroidal aromatase inhibitors in MONALEESA-7: all grade QTcF prolongation was 46.5% (Grade 2, 5.3%; Grade 3, 9%) No DLTs were observed during dose escalation and the maximum tolerated dose was not reached No patients discontinued palazestrant due to a treatment-related AE; 2 patients discontinued ribociclib but stayed on palazestrant Overall safety and tolerability profile consistent with ribociclib + endocrine therapy prescribing information Most Common Treatment-Emergent Adverse Events
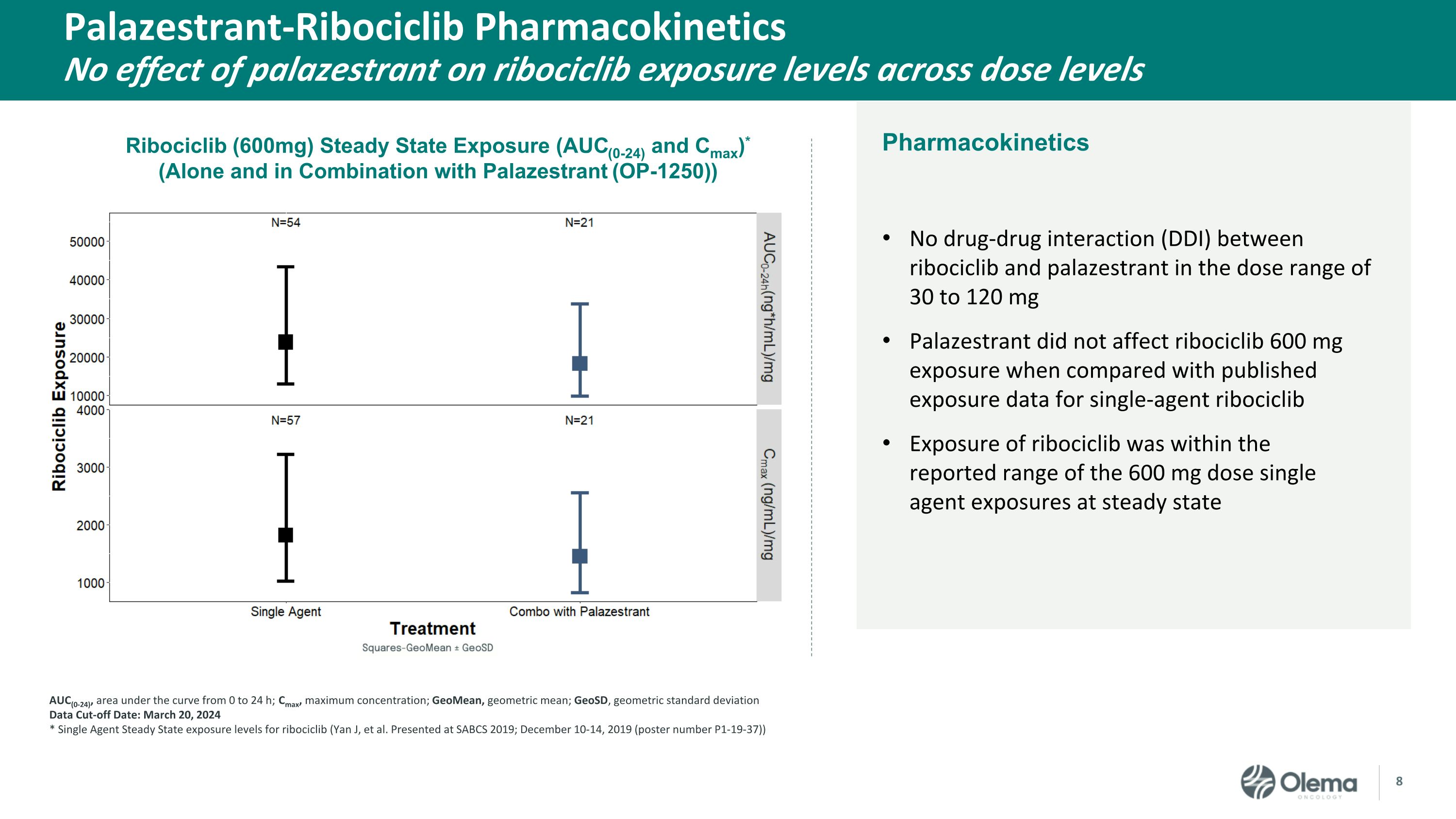
Pharmacokinetics Palazestrant-Ribociclib Pharmacokinetics�No effect of palazestrant on ribociclib exposure levels across dose levels No drug-drug interaction (DDI) between ribociclib and palazestrant in the dose range of 30 to 120 mg Palazestrant did not affect ribociclib 600 mg exposure when compared with published exposure data for single-agent ribociclib Exposure of ribociclib was within the reported range of the 600 mg dose single agent exposures at steady state AUC(0-24), area under the curve from 0 to 24 h; Cmax, maximum concentration; GeoMean, geometric mean; GeoSD, geometric standard deviation Data Cut-off Date: March 20, 2024 * Single Agent Steady State exposure levels for ribociclib (Yan J, et al. Presented at SABCS 2019; December 10-14, 2019 (poster number P1-19-37)) Ribociclib (600mg) Steady State Exposure (AUC(0-24) and Cmax)* �(Alone and in Combination with Palazestrant (OP-1250))
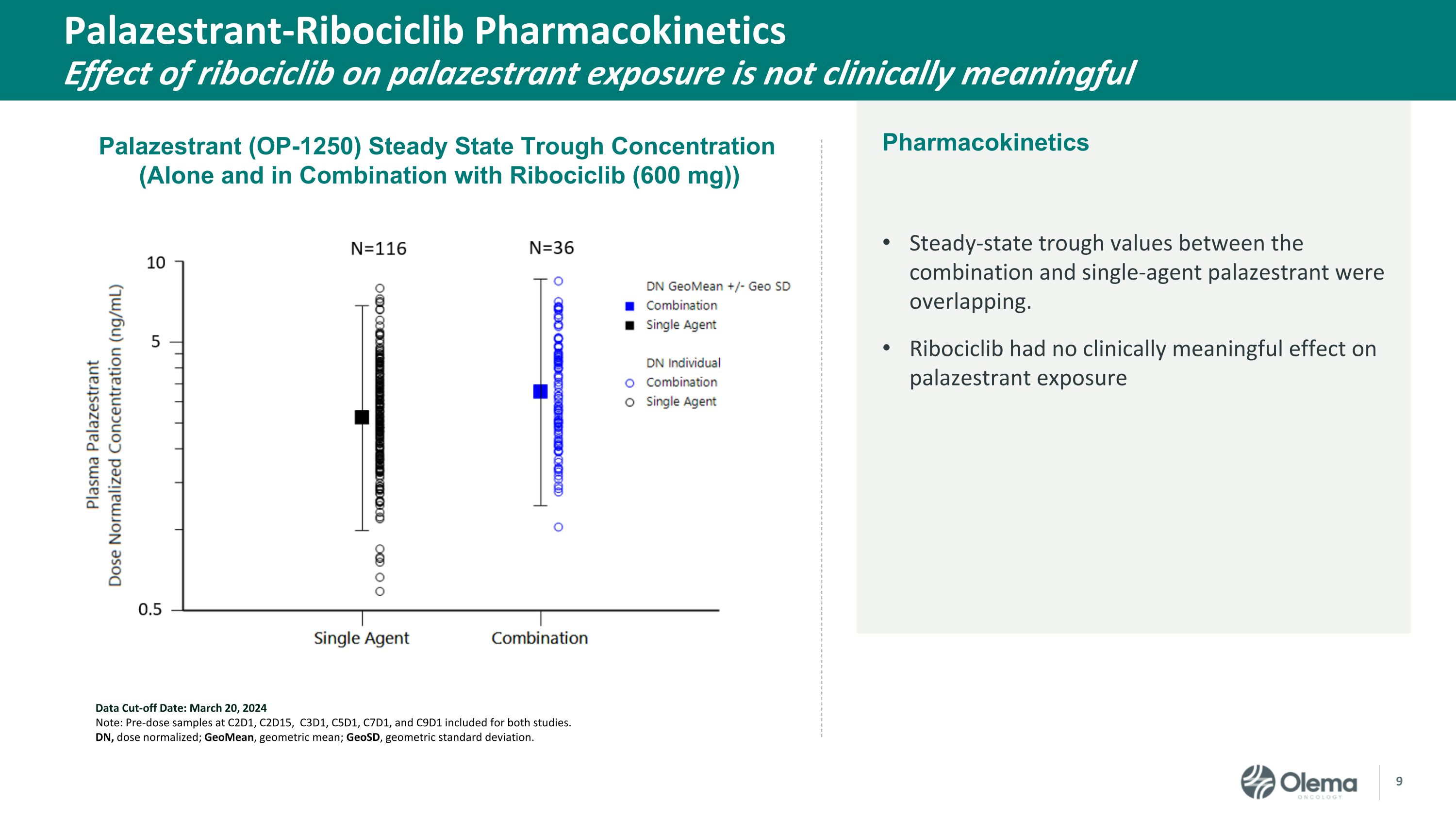
Palazestrant-Ribociclib Pharmacokinetics�Effect of ribociclib on palazestrant exposure is not clinically meaningful Steady-state trough values between the combination and single-agent palazestrant were overlapping. Ribociclib had no clinically meaningful effect on palazestrant exposure Data Cut-off Date: March 20, 2024 Note: Pre-dose samples at C2D1, C2D15, C3D1, C5D1, C7D1, and C9D1 included for both studies. DN, dose normalized; GeoMean, geometric mean; GeoSD, geometric standard deviation. Palazestrant (OP-1250) Steady State Trough Concentration �(Alone and in Combination with Ribociclib (600 mg)) Pharmacokinetics
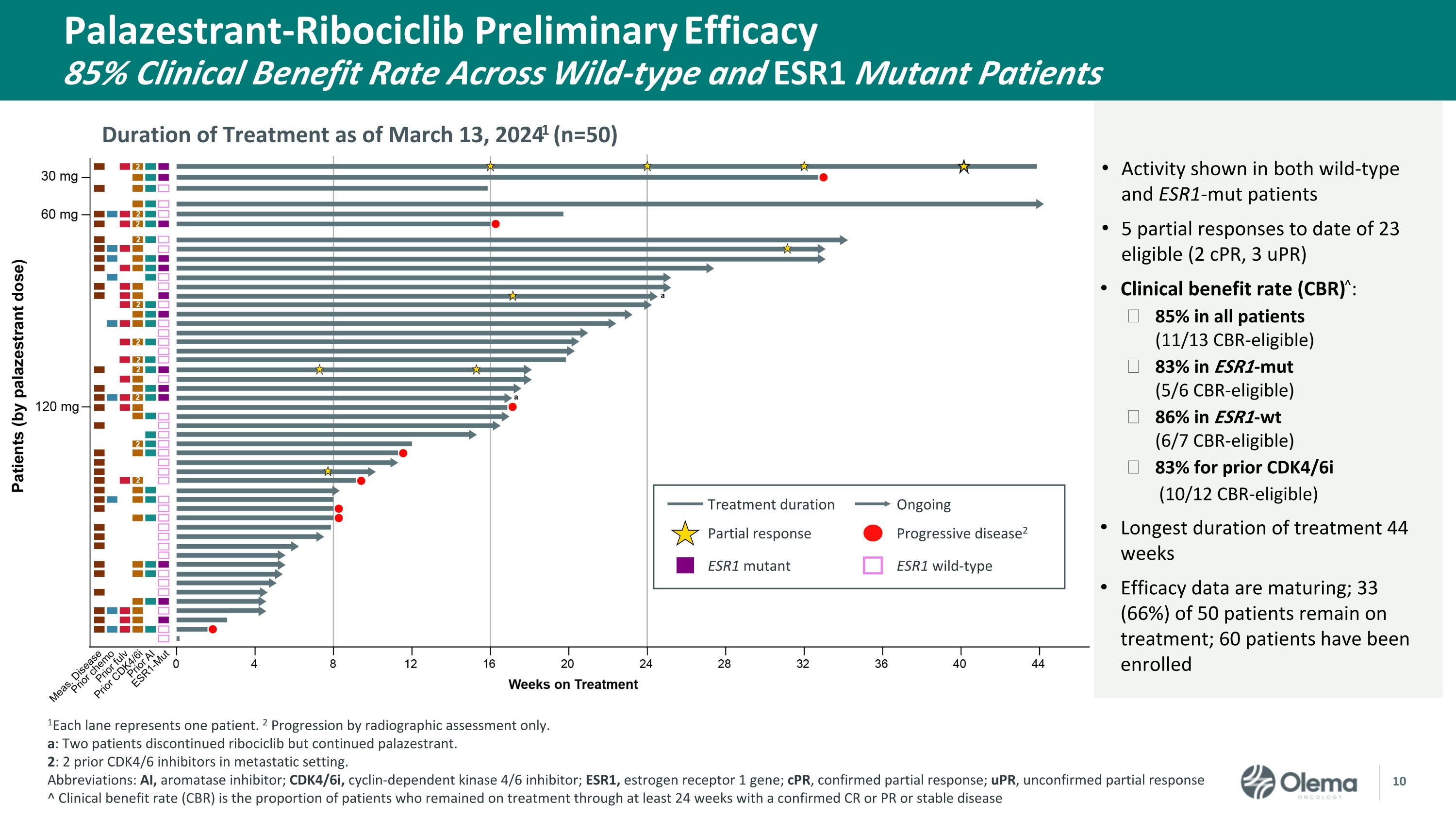
Palazestrant-Ribociclib Preliminary Efficacy�85% Clinical Benefit Rate Across Wild-type and ESR1 Mutant Patients Duration of Treatment as of March 13, 20241 (n=50) 1Each lane represents one patient. 2 Progression by radiographic assessment only. a: Two patients discontinued ribociclib but continued palazestrant. 2: 2 prior CDK4/6 inhibitors in metastatic setting.�Abbreviations: AI, aromatase inhibitor; CDK4/6i, cyclin-dependent kinase 4/6 inhibitor; ESR1, estrogen receptor 1 gene; cPR, confirmed partial response; uPR, unconfirmed partial response ^ Clinical benefit rate (CBR) is the proportion of patients who remained on treatment through at least 24 weeks with a confirmed CR or PR or stable disease Activity shown in both wild-type and ESR1-mut patients 5 partial responses to date of 23 eligible (2 cPR, 3 uPR) Clinical benefit rate (CBR)^: 85% in all patients �(11/13 CBR-eligible) 83% in ESR1-mut �(5/6 CBR-eligible) 86% in ESR1-wt �(6/7 CBR-eligible) 83% for prior CDK4/6i (10/12 CBR-eligible) Longest duration of treatment 44 weeks Efficacy data are maturing; 33 (66%) of 50 patients remain on treatment; 60 patients have been enrolled Treatment duration Partial response Mutation detected Treatment duration Ongoing Partial response Progressive disease2 ESR1 mutant ESR1 wild-type
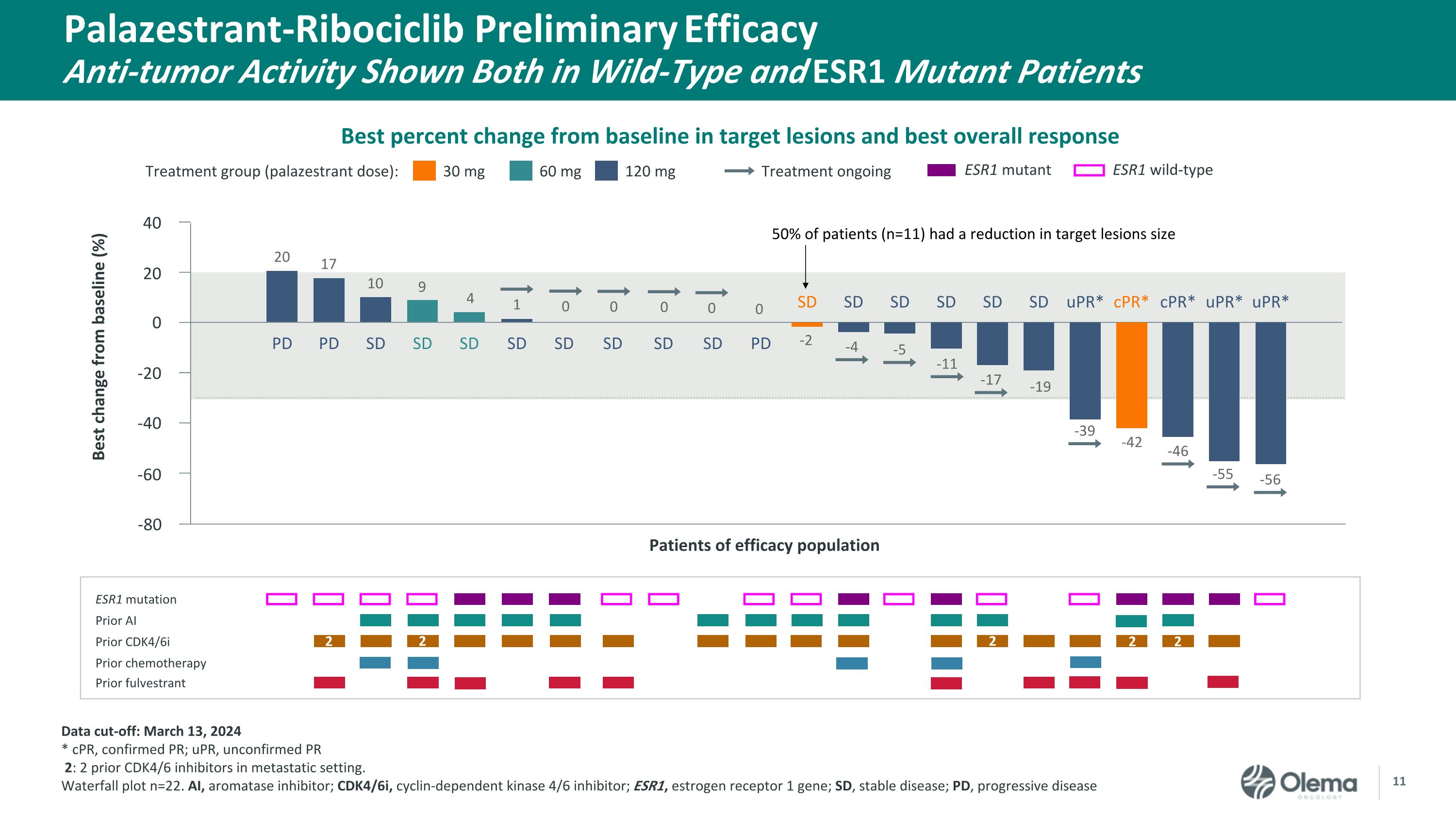
Palazestrant-Ribociclib Preliminary Efficacy�Anti-tumor Activity Shown Both in Wild-Type and ESR1 Mutant Patients 30 mg Treatment group (palazestrant dose): 60 mg 120 mg Treatment ongoing ESR1 mutation Prior AI Prior CDK4/6i Prior chemotherapy Prior fulvestrant Patients of efficacy population 40 20 0 -20 -40 -60 Best change from baseline (%) ESR1 mutant ESR1 wild-type PD cPR* SD SD uPR* PD SD SD SD SD SD uPR* cPR* uPR* PD SD SD SD SD SD 1 SD 0 SD 0 4 9 10 17 20 0 0 0 -2 -4 -5 -11 -17 -19 -39 -42 -46 -55 -56 -80 2 2 2 2 2 Data cut-off: March 13, 2024 * cPR, confirmed PR; uPR, unconfirmed PR 2: 2 prior CDK4/6 inhibitors in metastatic setting. Waterfall plot n=22. AI, aromatase inhibitor; CDK4/6i, cyclin-dependent kinase 4/6 inhibitor; ESR1, estrogen receptor 1 gene; SD, stable disease; PD, progressive disease Best percent change from baseline in target lesions and best overall response 50% of patients (n=11) had a reduction in target lesions size
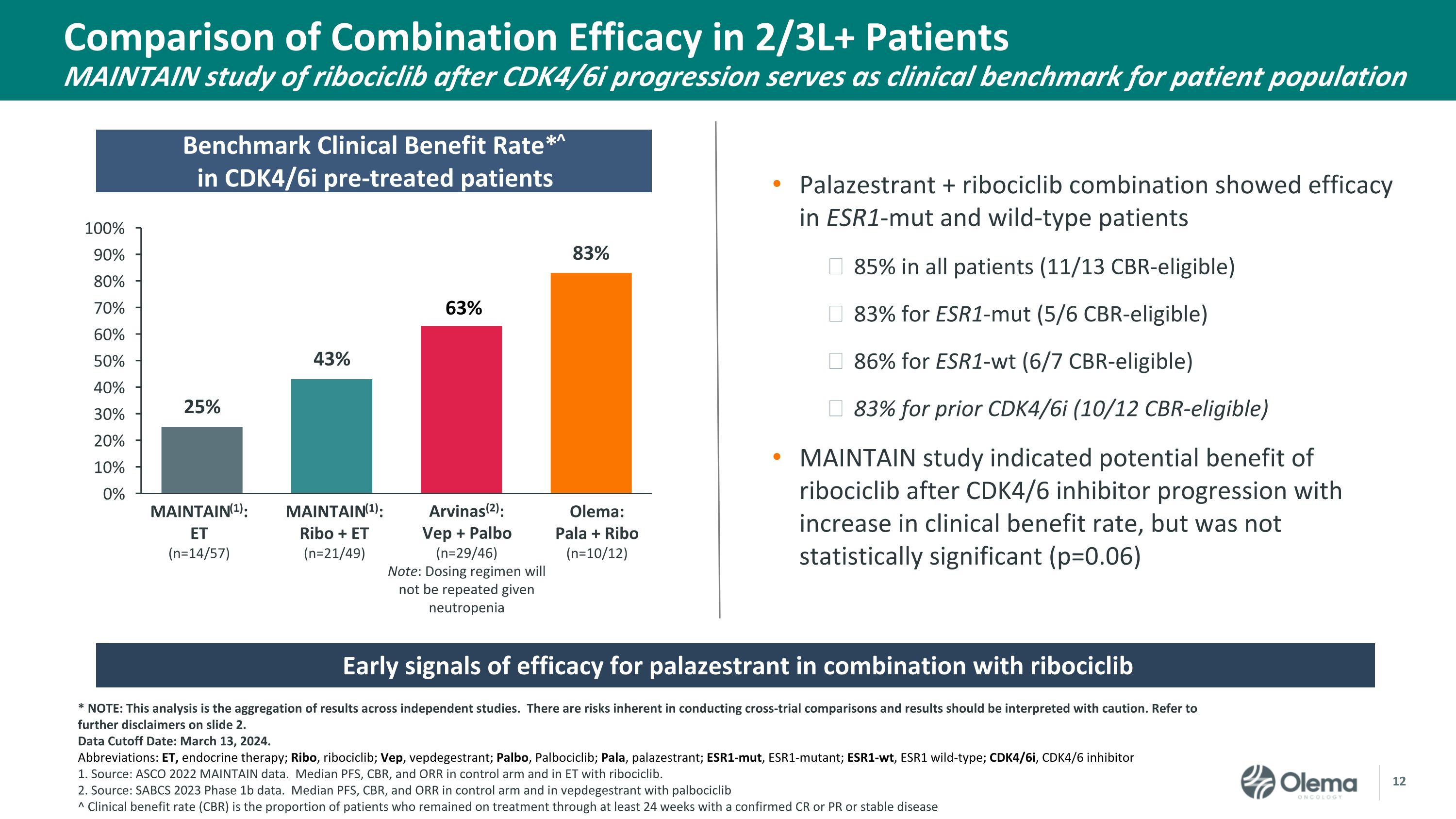
Comparison of Combination Efficacy in 2/3L+ Patients�MAINTAIN study of ribociclib after CDK4/6i progression serves as clinical benchmark for patient population Benchmark Clinical Benefit Rate*^�in CDK4/6i pre-treated patients Early signals of efficacy for palazestrant in combination with ribociclib Palazestrant + ribociclib combination showed efficacy in ESR1-mut and wild-type patients 85% in all patients (11/13 CBR-eligible) 83% for ESR1-mut (5/6 CBR-eligible) 86% for ESR1-wt (6/7 CBR-eligible) 83% for prior CDK4/6i (10/12 CBR-eligible) MAINTAIN study indicated potential benefit of ribociclib after CDK4/6 inhibitor progression with increase in clinical benefit rate, but was not statistically significant (p=0.06) * NOTE: This analysis is the aggregation of results across independent studies. There are risks inherent in conducting cross-trial comparisons and results should be interpreted with caution. Refer to further disclaimers on slide 2. Data Cutoff Date: March 13, 2024. Abbreviations: ET, endocrine therapy; Ribo, ribociclib; Vep, vepdegestrant; Palbo, Palbociclib; Pala, palazestrant; ESR1-mut, ESR1-mutant; ESR1-wt, ESR1 wild-type; CDK4/6i, CDK4/6 inhibitor 1. Source: ASCO 2022 MAINTAIN data. Median PFS, CBR, and ORR in control arm and in ET with ribociclib. 2. Source: SABCS 2023 Phase 1b data. Median PFS, CBR, and ORR in control arm and in vepdegestrant with palbociclib ^ Clinical benefit rate (CBR) is the proportion of patients who remained on treatment through at least 24 weeks with a confirmed CR or PR or stable disease MAINTAIN(1):�Ribo + ET�(n=21/49) Olema: Pala + Ribo�(n=10/12) MAINTAIN(1): �ET�(n=14/57) Arvinas(2): Vep + Palbo�(n=29/46)�Note: Dosing regimen will not be repeated given neutropenia 63%
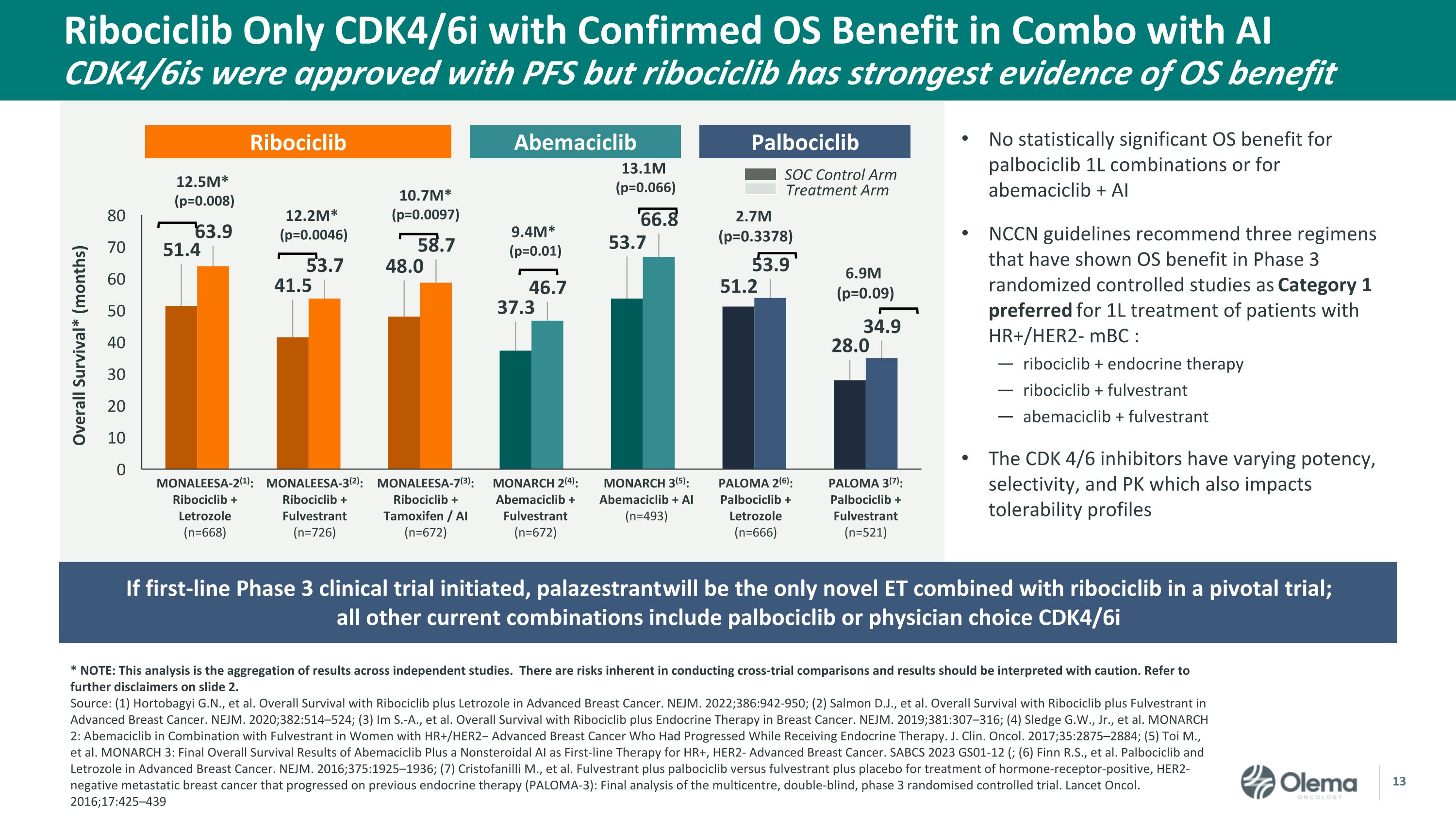
Ribociclib Only CDK4/6i with Confirmed OS Benefit in Combo with AI�CDK4/6is were approved with PFS but ribociclib has strongest evidence of OS benefit * NOTE: This analysis is the aggregation of results across independent studies. There are risks inherent in conducting cross-trial comparisons and results should be interpreted with caution. Refer to further disclaimers on slide 2. Source: (1) Hortobagyi G.N., et al. Overall Survival with Ribociclib plus Letrozole in Advanced Breast Cancer. NEJM. 2022;386:942-950; (2) Salmon D.J., et al. Overall Survival with Ribociclib plus Fulvestrant in Advanced Breast Cancer. NEJM. 2020;382:514–524; (3) Im S.-A., et al. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. NEJM. 2019;381:307–316; (4) Sledge G.W., Jr., et al. MONARCH 2: Abemaciclib in Combination with Fulvestrant in Women with HR+/HER2− Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J. Clin. Oncol. 2017;35:2875–2884; (5) Toi M., et al. MONARCH 3: Final Overall Survival Results of Abemaciclib Plus a Nonsteroidal AI as First-line Therapy for HR+, HER2- Advanced Breast Cancer. SABCS 2023 GS01-12 (; (6) Finn R.S., et al. Palbociclib and Letrozole in Advanced Breast Cancer. NEJM. 2016;375:1925–1936; (7) Cristofanilli M., et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439 No statistically significant OS benefit for palbociclib 1L combinations or for abemaciclib + AI NCCN guidelines recommend three regimens that have shown OS benefit in Phase 3 randomized controlled studies as Category 1 preferred for 1L treatment of patients with HR+/HER2- mBC : ribociclib + endocrine therapy ribociclib + fulvestrant abemaciclib + fulvestrant The CDK 4/6 inhibitors have varying potency, selectivity, and PK which also impacts tolerability profiles Ribociclib MONARCH 2(4): �Abemaciclib + Fulvestrant�(n=672) MONARCH 3(5): �Abemaciclib + AI�(n=493) MONALEESA-2(1): �Ribociclib + Letrozole�(n=668) MONALEESA-3(2): �Ribociclib + Fulvestrant�(n=726) PALOMA 2(6): �Palbociclib + Letrozole�(n=666) PALOMA 3(7): �Palbociclib + Fulvestrant�(n=521) MONALEESA-7(3): �Ribociclib + Tamoxifen / AI�(n=672) 9.4M* �(p=0.01) SOC Control Arm Treatment Arm 12.5M* �(p=0.008) 12.2M* �(p=0.0046) 10.7M* �(p=0.0097) 13.1M �(p=0.066) 2.7M �(p=0.3378) 6.9M �(p=0.09) Abemaciclib Palbociclib If first-line Phase 3 clinical trial initiated, palazestrant will be the only novel ET combined with ribociclib in a pivotal trial; �all other current combinations include palbociclib or physician choice CDK4/6i
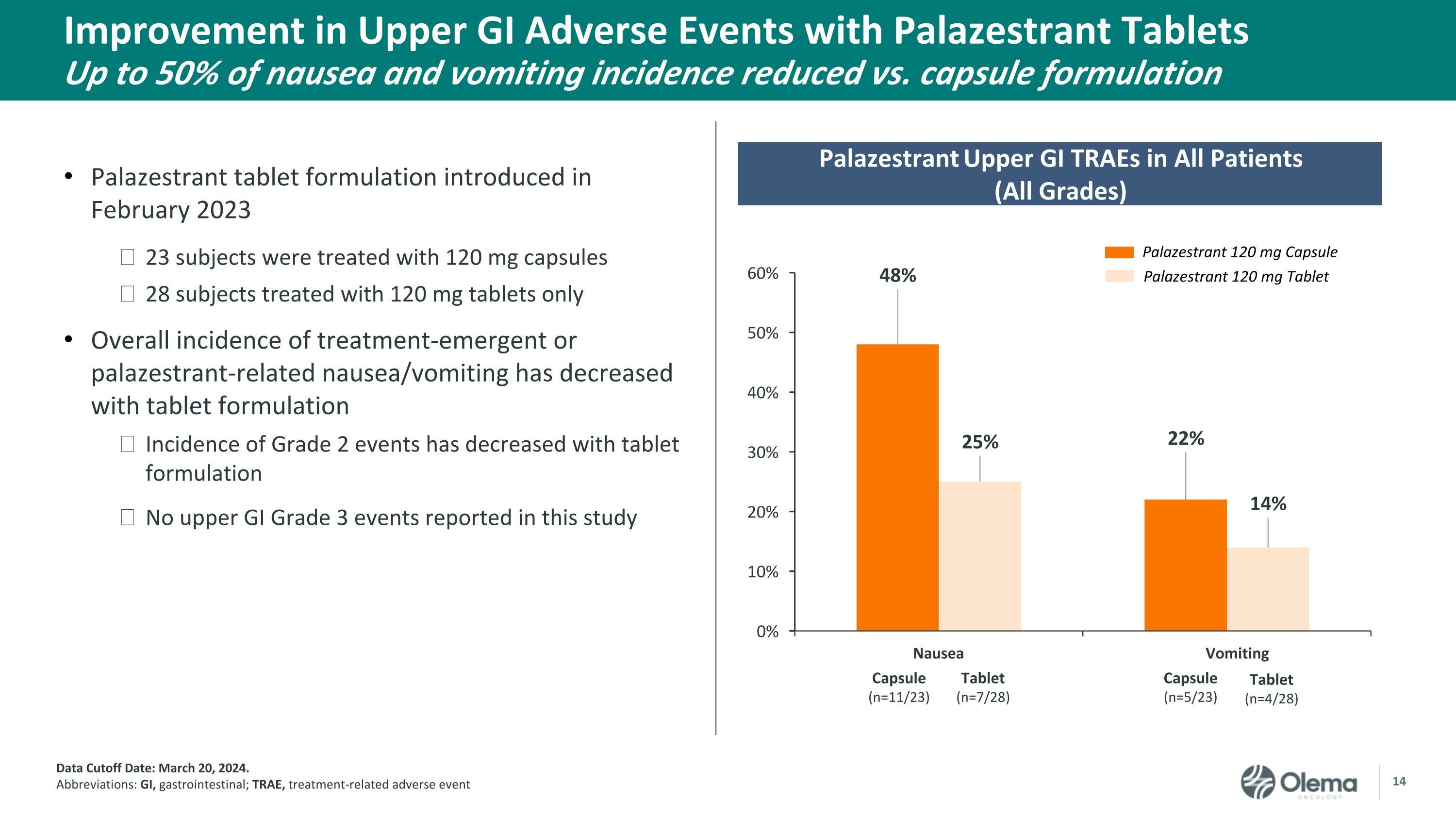
Improvement in Upper GI Adverse Events with Palazestrant Tablets�Up to 50% of nausea and vomiting incidence reduced vs. capsule formulation Data Cutoff Date: March 20, 2024. Abbreviations: GI, gastrointestinal; TRAE, treatment-related adverse event Palazestrant 120 mg Capsule Palazestrant 120 mg Tablet Palazestrant Upper GI TRAEs in All Patients�(All Grades) Nausea Vomiting Capsule �(n=11/23) Tablet �(n=7/28) Capsule �(n=5/23) Tablet �(n=4/28) Palazestrant tablet formulation introduced in February 2023 23 subjects were treated with 120 mg capsules 28 subjects treated with 120 mg tablets only Overall incidence of treatment-emergent or palazestrant-related nausea/vomiting has decreased with tablet formulation Incidence of Grade 2 events has decreased with tablet formulation No upper GI Grade 3 events reported in this study

Advancing Value – �Palazestrant and Pipeline
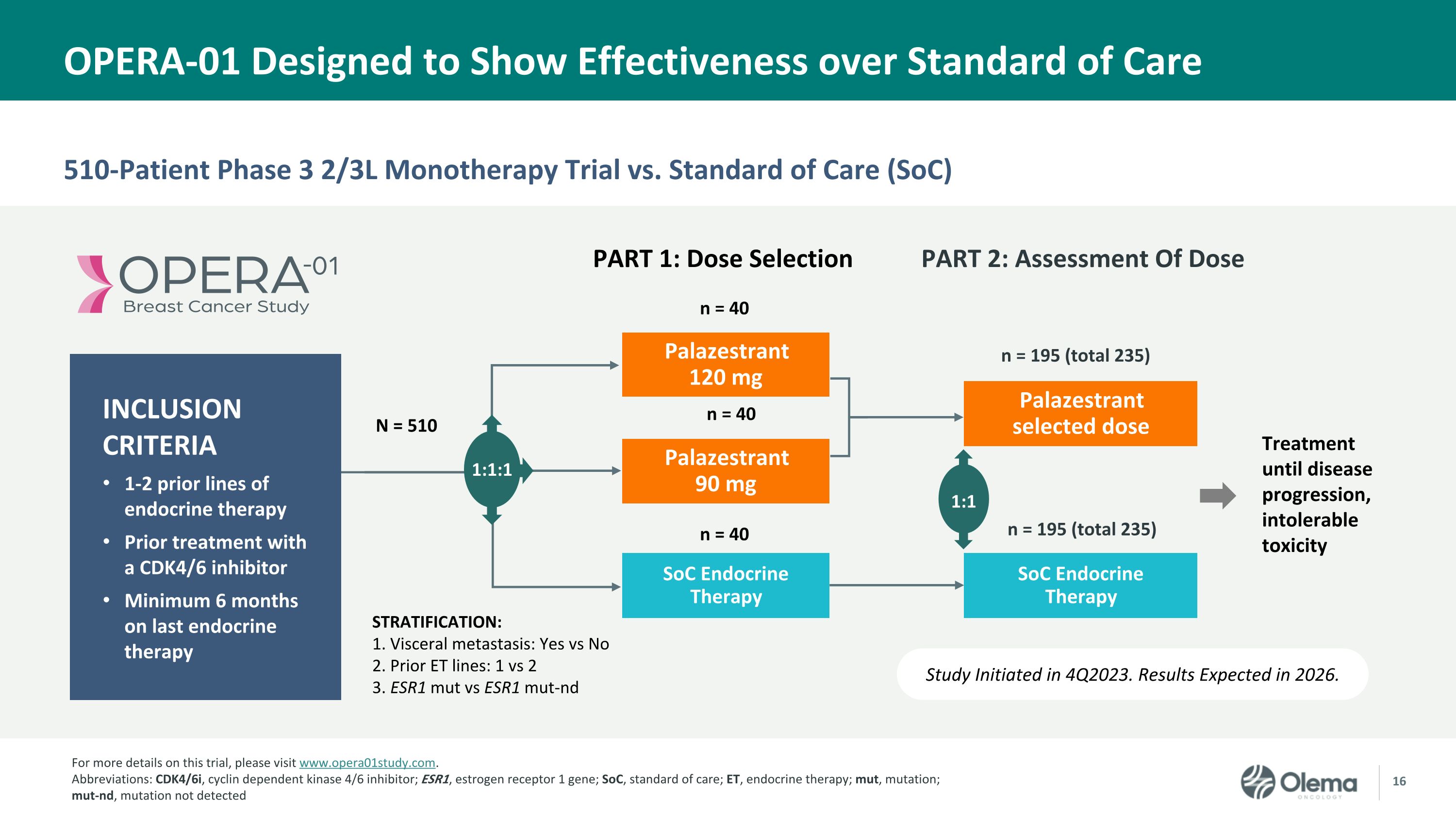
510-Patient Phase 3 2/3L Monotherapy Trial vs. Standard of Care (SoC) OPERA-01 Designed to Show Effectiveness over Standard of Care Study Initiated in 4Q2023. Results Expected in 2026. Palazestrant 120 mg SoC Endocrine Therapy STRATIFICATION: 1. Visceral metastasis: Yes vs No 2. Prior ET lines: 1 vs 2 3. ESR1 mut vs ESR1 mut-nd Palazestrant 90 mg N = 510 Palazestrant selected dose PART 1: Dose Selection 1:1 1:1:1 n = 40 n = 40 Treatment until disease progression, intolerable toxicity SoC Endocrine �Therapy n = 40 n = 195 (total 235) n = 195 (total 235) INCLUSION CRITERIA 1-2 prior lines of endocrine therapy Prior treatment with �a CDK4/6 inhibitor Minimum 6 months �on last endocrine therapy PART 2: Assessment Of Dose For more details on this trial, please visit www.opera01study.com. Abbreviations: CDK4/6i, cyclin dependent kinase 4/6 inhibitor; ESR1, estrogen receptor 1 gene; SoC, standard of care; ET, endocrine therapy; mut, mutation; mut-nd, mutation not detected
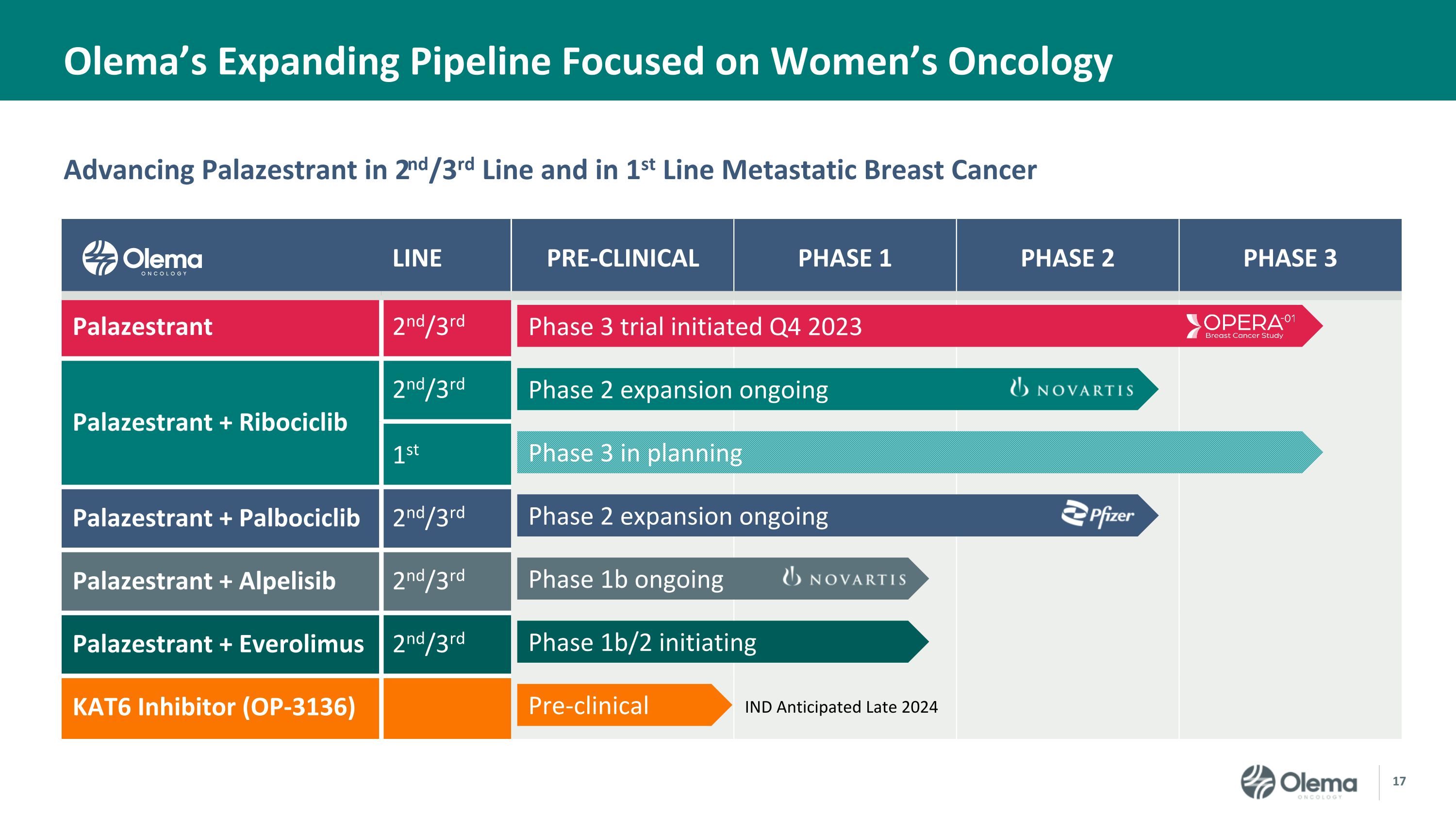
Advancing Palazestrant in 2nd/3rd Line and in 1st Line Metastatic Breast Cancer Olema’s Expanding Pipeline Focused on Women’s Oncology LINE PRE-CLINICAL PHASE 1 PHASE 2 PHASE 3 Palazestrant 2nd/3rd Palazestrant + Ribociclib 2nd/3rd 1st Palazestrant + Palbociclib 2nd/3rd Palazestrant + Alpelisib 2nd/3rd Palazestrant + Everolimus 2nd/3rd KAT6 Inhibitor (OP-3136) IND Anticipated Late 2024 Phase 3 trial initiated Q4 2023 Phase 2 expansion ongoing Phase 3 in planning Phase 2 expansion ongoing Phase 1b ongoing Phase 1b/2 initiating Pre-clinical
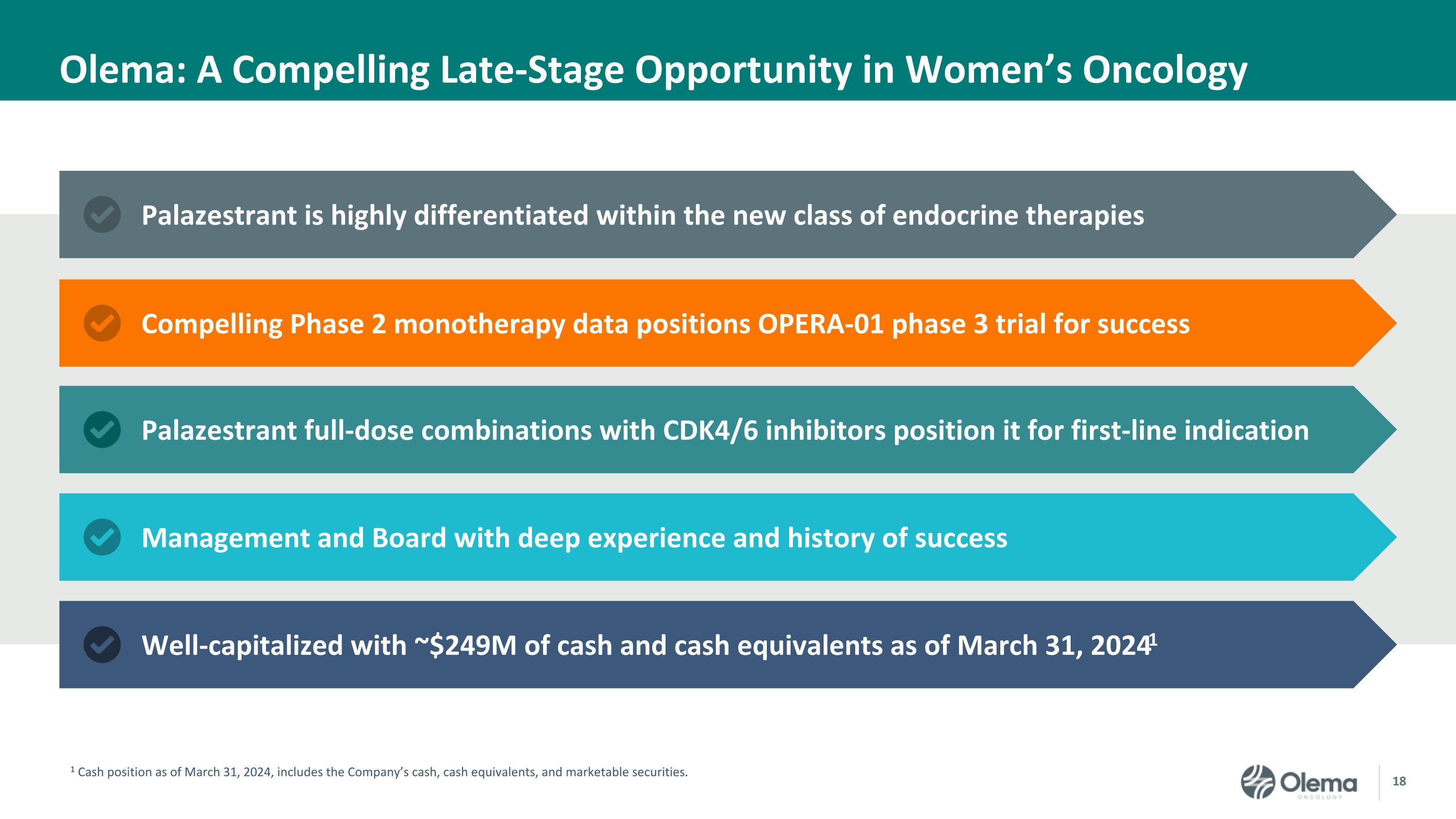
Olema: A Compelling Late-Stage Opportunity in Women’s Oncology Well-capitalized with ~$249M of cash and cash equivalents as of March 31, 20241 Palazestrant full-dose combinations with CDK4/6 inhibitors position it for first-line indication Palazestrant is highly differentiated within the new class of endocrine therapies 1 Cash position as of March 31, 2024, includes the Company’s cash, cash equivalents, and marketable securities. Compelling Phase 2 monotherapy data positions OPERA-01 phase 3 trial for success Management and Board with deep experience and history of success



















