
Palazestrant in Combination with Ribociclib Clinical Update SABCS 2024 December 10, 2024 EXHIBIT 99.3

Legal disclaimer This presentation contains forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Statements in this presentation that are not statements of historical fact are forward-looking statements. Words such as “anticipate,” “believe,” “estimate,” “expect,” “goal,” “intend,” “may,” “plan,” “potential,” “project,” “will,” and similar expressions are intended to identify forward-looking statements, though not all forward-looking statements necessarily contain these identifying words. Such forward-looking statements include, without limitation, statements regarding our research and clinical development plans, the scope, progress, results and costs of developing our product candidates or any other future product candidates, strategy, market size and opportunity, clinical trial designs, regulatory matters, including the timing and likelihood of success of obtaining drug approvals, the timelines for the potential initiation of clinical trials and the results of any such clinical trials of palazestrant (OP-1250) as a monotherapy and in combination trials, including OPERA-01, the Company’s pivotal Phase 3 monotherapy clinical trial, and OPERA-02, the Company's potential pivotal Phase 3 clinical trial of palazestrant in combination with ribociclib, the timelines for potential commercial launch and related preparatory work, the sufficiency and potential beneficial characteristics, profile, safety, tolerability, efficacy and therapeutic effects of palazestrant as a monotherapy and in combination trials, the progression-free survival rate under palazestrant in combination trials, the potential of palazestrant to become a therapeutic leader and a best-in-class treatment option for ER+/HER2- metastatic breast cancer and a backbone therapy for women living with breast cancer and beyond, the combinability of palazestrant with other drugs, the timelines for initiation of potential clinical trials for and the results of any such clinical trials in connection with our KAT6 inhibitor program, including OP-3136, the potential value and impact of a KAT6 inhibitor program, the best-in-class potential for OP-3136, the potential beneficial characteristics, profile, safety, efficacy, tolerability, and therapeutic effects of OP-3136, our ability to complete certain milestones, our financial condition, our opportunity in breast cancer and beyond, our ability to impact treatment for endocrine-driven cancers, cash position and runway and sufficiency of our financial resources, and the sufficiency and expertise of our management team. These forward-looking statements are based on the beliefs of the Company’s management as well as assumptions made by and information currently available to the Company. Such statements reflect the current views of the Company with respect to future events and are subject to known and unknown risks, including business, regulatory, economic and competitive risks, uncertainties, contingencies and assumptions about the Company, including, without limitation, risks inherent in developing products and technologies, future results from the Company’s ongoing and planned clinical trials, the Company’s ability to obtain adequate financing to fund its planned clinical trials and other expenses, trends in the industry, the legal and regulatory framework for the industry and future expenditures, and other risks and uncertainties affecting the Company, including those described under the caption "Risk Factors" and elsewhere in the Company‘s Quarterly Reports on Form 10-Q, Annual Report on Form 10-K, and other filings and reports the Company files with the Securities and Exchange Commission from time to time. In light of these risks and uncertainties, the events or circumstances referred to in the forward-looking statements may not occur. The actual results may vary from the anticipated results and the variations may be material. These forward-looking statements should not be taken as forecasts or promises nor should they be taken as implying any indication, assurance or guarantee that the assumptions on which such forward-looking statements have been made are correct or exhaustive or, in the case of the assumptions, fully stated in this presentation. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date this presentation is given. This presentation discusses product candidates that are under clinical study and which have not yet been approved for marketing by the U.S. Food and Drug Administration. No representation is made as to the safety or effectiveness of these product candidates for the use for which such product candidates are being studied. This presentation incorporates publicly-available third-party data that we have not independently verified. There are risks inherent in conducting cross-trial comparisons and the results should be interpreted with caution. The presentation of such third-party data does not represent a head-to-head comparison of how palazestrant, in monotherapy or in combination, or OP-3136 performed against any other third-party drug candidate or study. Rather, such third-party data has been pulled by us from publicly-available sources for supplemental informational purposes, only. We caution you that any comparisons against third-party data set forth herein should not be viewed as a side-by-side comparison, and you should not rely on the completeness or accuracy of our presentation of the results of any third-party drug candidate in these slides, due to differences in study design, how other companies quantify or qualify eligibility criteria, and how results are recorded, among other distinguishing factors and uncertainties. Because we may be unaware of or may not adequately present various distinguishing factors and uncertainties, the comparisons set forth herein may not properly present such third-party data, which may differ materially from the data as presented here. Investors are encouraged to independently review third party data and should not rely on our presentation of such data (including any such data placed in comparison with the performance of palazestrant) as a single measure to evaluate our business. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of such products.

— We are on a mission to elevate patient care in breast cancer �and beyond Expertise in endocrine-driven cancers with mechanistically superior scientific approach that fully inactivates estrogen receptors OP-1250, a promising potential backbone therapy for ER+/HER2- breast cancer in late-stage clinical development, forms basis of breast cancer program Exciting new and potent KAT6 inhibitor with potential to significantly impact breast cancer treatment; IND cleared by FDA and initiation of Phase 1 clinical trial anticipated early 2025 Management and Board with deep expertise developing and commercializing oncology medicines Molecular Advantage Lead Asset – Palazestrant OP-3136 Expands Pipeline Proven Leadership
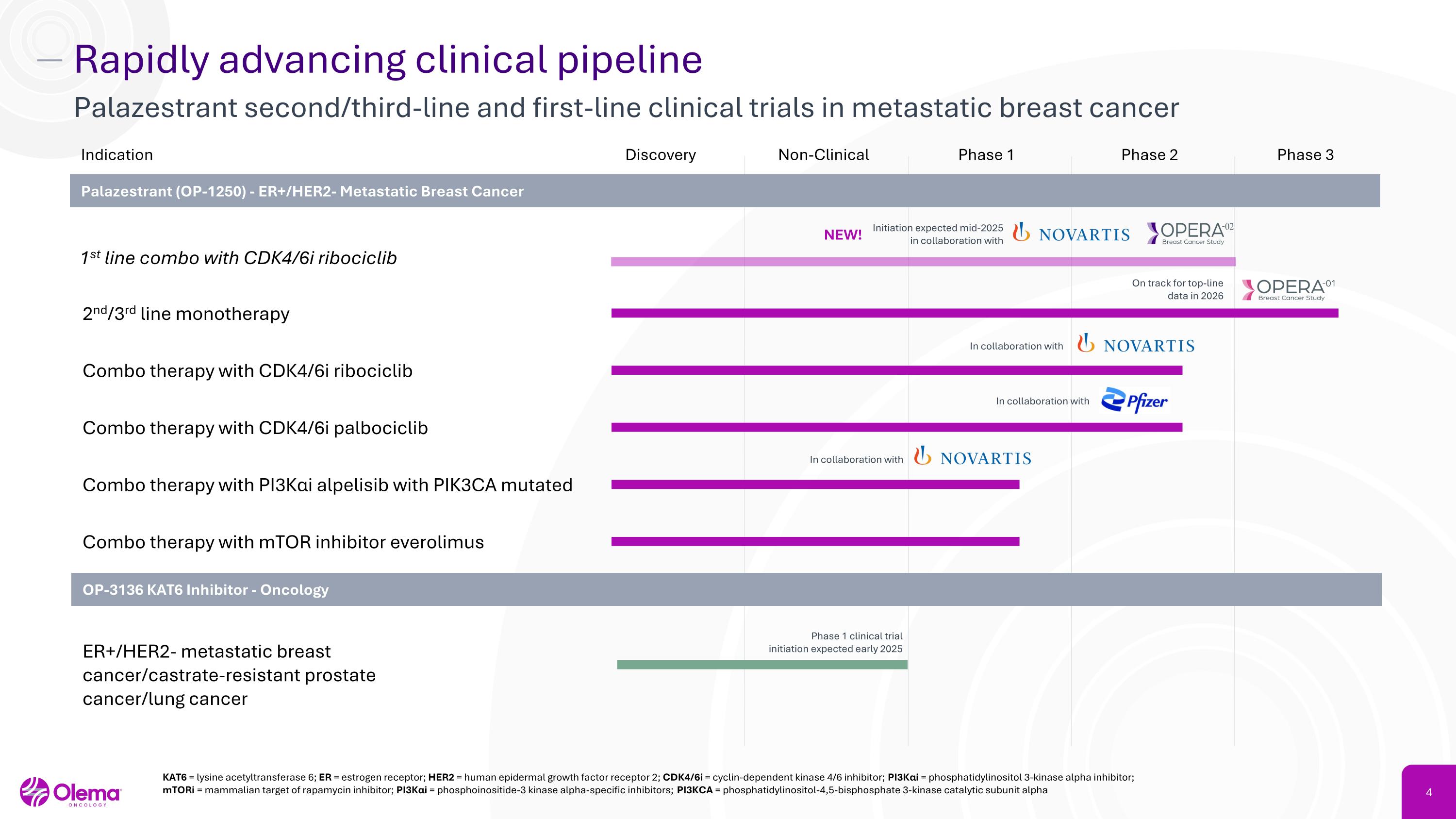
Rapidly advancing clinical pipeline Palazestrant second/third-line and first-line clinical trials in metastatic breast cancer Discovery Non-Clinical Phase 1 Phase 2 Phase 3 2nd/3rd line monotherapy Combo therapy with PI3Kαi alpelisib with PIK3CA mutated Combo therapy with mTOR inhibitor everolimus Indication Palazestrant (OP-1250) - ER+/HER2- Metastatic Breast Cancer Combo therapy with CDK4/6i ribociclib Combo therapy with CDK4/6i palbociclib OP-3136 KAT6 Inhibitor - Oncology ER+/HER2- metastatic breast cancer/castrate-resistant prostate cancer/lung cancer KAT6 = lysine acetyltransferase 6; ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; CDK4/6i = cyclin-dependent kinase 4/6 inhibitor; PI3Kαi = phosphatidylinositol 3-kinase alpha inhibitor; mTORi = mammalian target of rapamycin inhibitor; PI3Kαi = phosphoinositide-3 kinase alpha-specific inhibitors; PI3KCA = phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha 1st line combo with CDK4/6i ribociclib Initiation expected mid-2025�in collaboration with In collaboration with In collaboration with In collaboration with Phase 1 clinical trial initiation expected early 2025 NEW! On track for top-line data in 2026
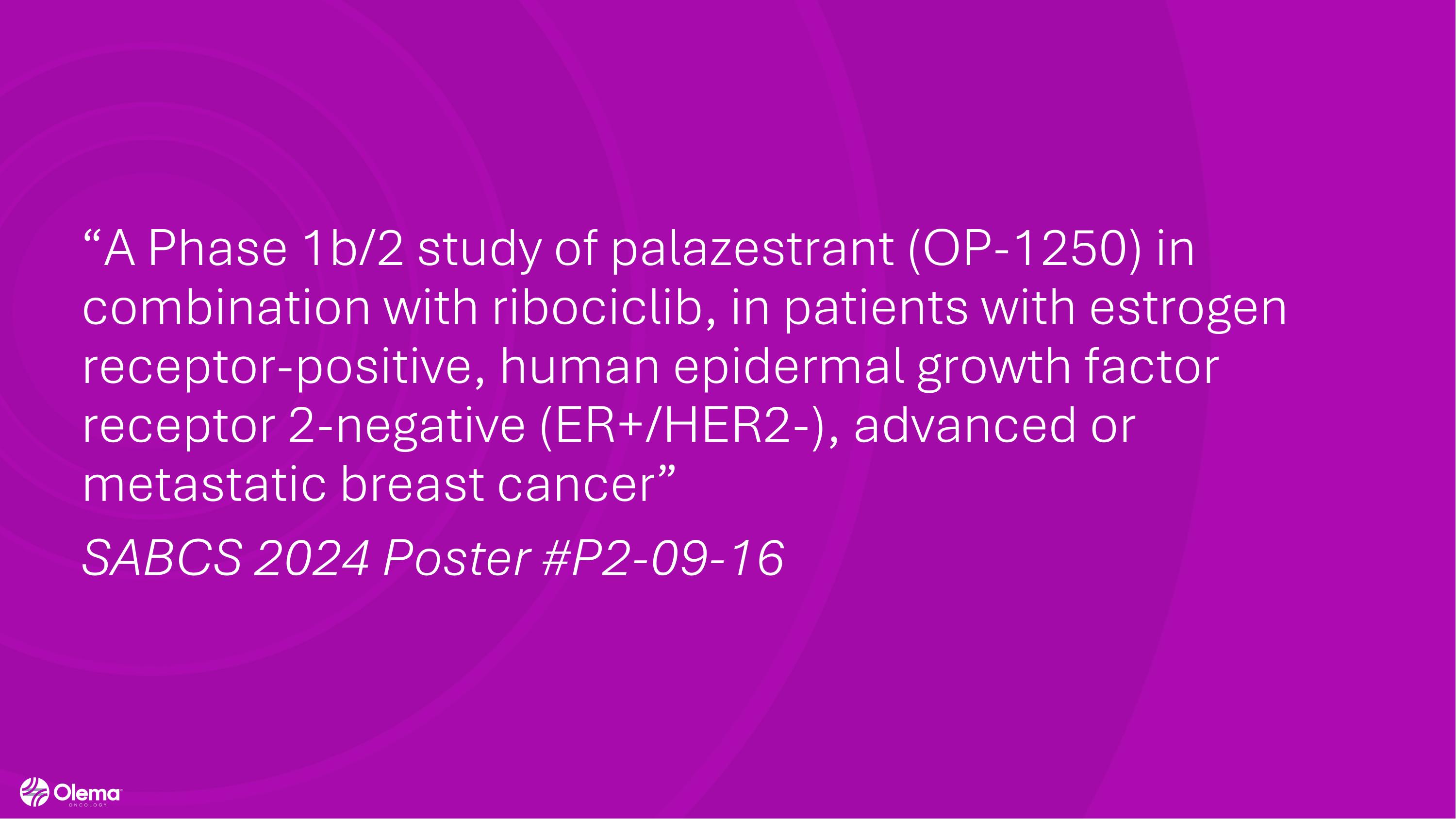
“A Phase 1b/2 study of palazestrant (OP-1250) in combination with ribociclib, in patients with estrogen receptor-positive, human epidermal growth factor receptor 2-negative (ER+/HER2-), advanced or metastatic breast cancer” SABCS 2024 Poster #P2-09-16
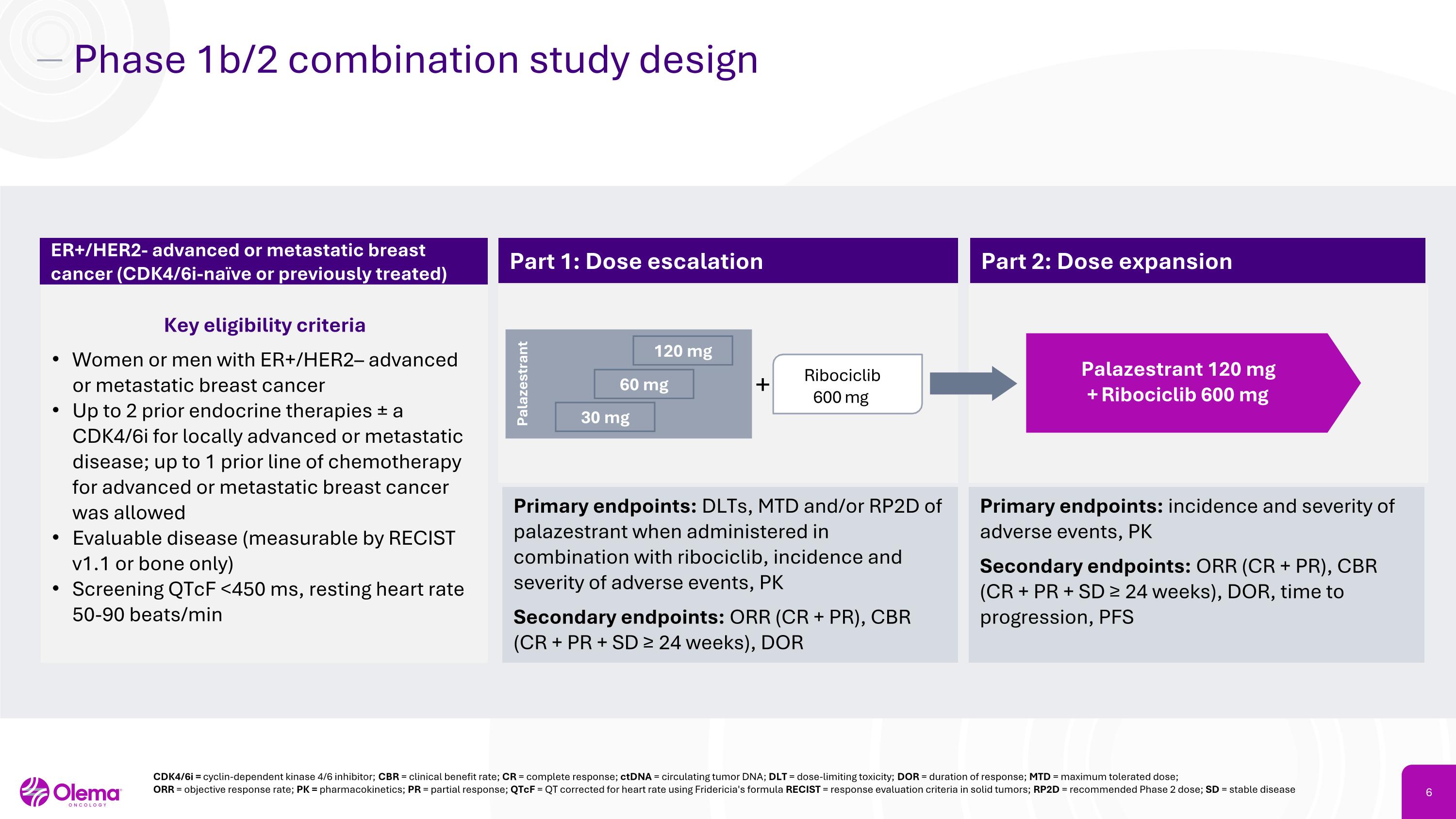
Phase 1b/2 combination study design Key eligibility criteria Women or men with ER+/HER2– advanced or metastatic breast cancer Up to 2 prior endocrine therapies ± a CDK4/6i for locally advanced or metastatic disease; up to 1 prior line of chemotherapy for advanced or metastatic breast cancer was allowed Evaluable disease (measurable by RECIST v1.1 or bone only) Screening QTcF <450 ms, resting heart rate 50-90 beats/min ER+/HER2- advanced or metastatic breast cancer (CDK4/6i-naïve or previously treated) Part 1: Dose escalation Part 2: Dose expansion Primary endpoints: DLTs, MTD and/or RP2D of palazestrant when administered in combination with ribociclib, incidence and severity of adverse events, PK Secondary endpoints: ORR (CR + PR), CBR (CR + PR + SD ≥ 24 weeks), DOR Primary endpoints: incidence and severity of adverse events, PK Secondary endpoints: ORR (CR + PR), CBR (CR + PR + SD ≥ 24 weeks), DOR, time to progression, PFS + Palazestrant 120 mg�+ Ribociclib 600 mg Ribociclib 600 mg Palazestrant 120 mg 60 mg 30 mg CDK4/6i = cyclin-dependent kinase 4/6 inhibitor; CBR = clinical benefit rate; CR = complete response; ctDNA = circulating tumor DNA; DLT = dose-limiting toxicity; DOR = duration of response; MTD = maximum tolerated dose;�ORR = objective response rate; PK = pharmacokinetics; PR = partial response; QTcF = QT corrected for heart rate using Fridericia's formula RECIST = response evaluation criteria in solid tumors; RP2D = recommended Phase 2 dose; SD = stable disease
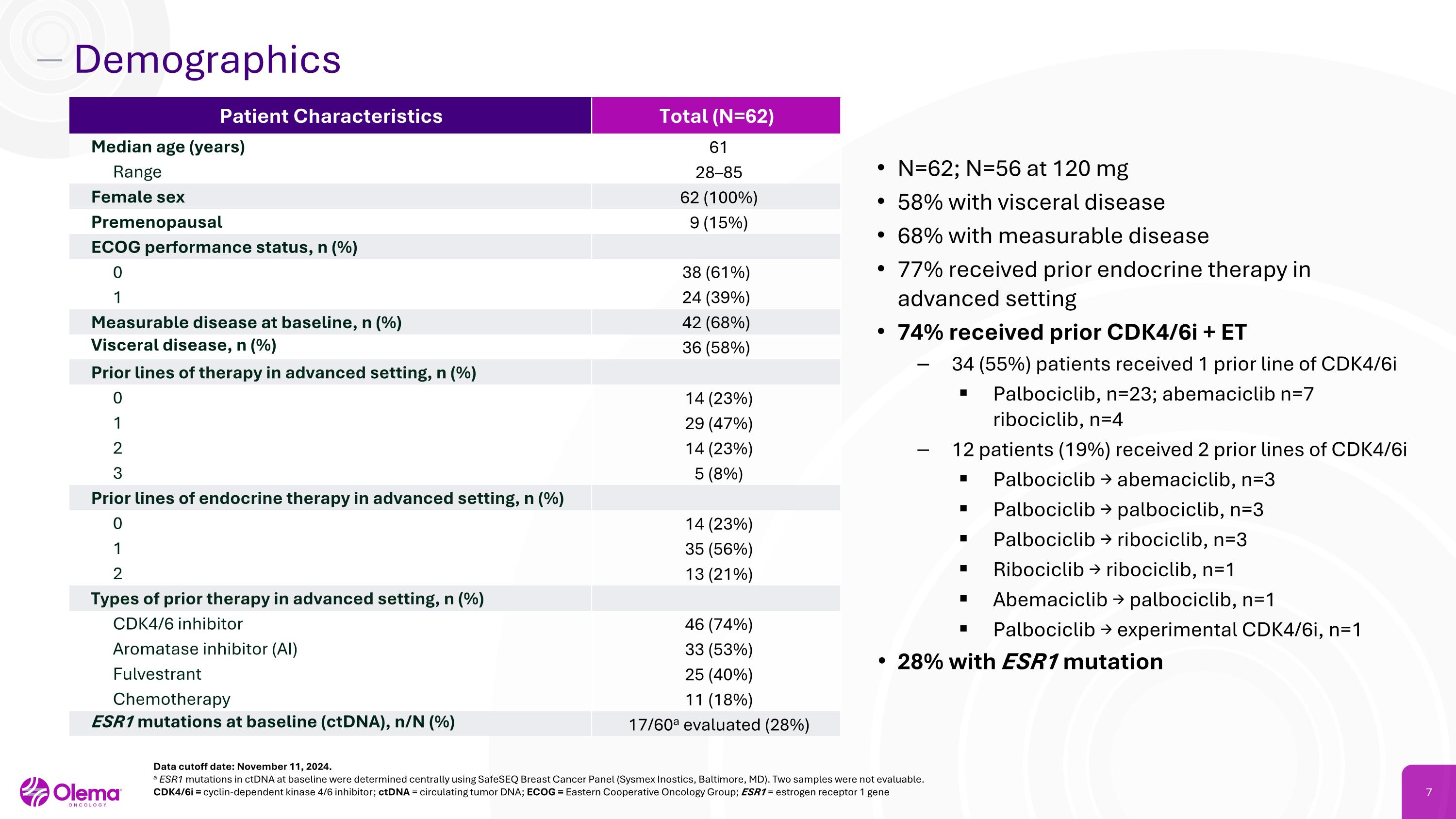
Demographics N=62; N=56 at 120 mg 58% with visceral disease 68% with measurable disease 77% received prior endocrine therapy in advanced setting 74% received prior CDK4/6i + ET 34 (55%) patients received 1 prior line of CDK4/6i Palbociclib, n=23; abemaciclib n=7 ribociclib, n=4 12 patients (19%) received 2 prior lines of CDK4/6i Palbociclib → abemaciclib, n=3 Palbociclib → palbociclib, n=3 Palbociclib → ribociclib, n=3 Ribociclib → ribociclib, n=1 Abemaciclib → palbociclib, n=1 Palbociclib → experimental CDK4/6i, n=1 28% with ESR1 mutation Data cutoff date: November 11, 2024. a ESR1 mutations in ctDNA at baseline were determined centrally using SafeSEQ Breast Cancer Panel (Sysmex Inostics, Baltimore, MD). Two samples were not evaluable. CDK4/6i = cyclin-dependent kinase 4/6 inhibitor; ctDNA = circulating tumor DNA; ECOG = Eastern Cooperative Oncology Group; ESR1 = estrogen receptor 1 gene Patient Characteristics Total (N=62) Median age (years) 61 Range 28–85 Female sex 62 (100%) Premenopausal 9 (15%) ECOG performance status, n (%) 0 38 (61%) 1 24 (39%) Measurable disease at baseline, n (%) 42 (68%) Visceral disease, n (%) 36 (58%) Prior lines of therapy in advanced setting, n (%) 0 14 (23%) 1 29 (47%) 2 14 (23%) 3 5 (8%) Prior lines of endocrine therapy in advanced setting, n (%) 0 14 (23%) 1 35 (56%) 2 13 (21%) Types of prior therapy in advanced setting, n (%) CDK4/6 inhibitor 46 (74%) Aromatase inhibitor (AI) 33 (53%) Fulvestrant 25 (40%) Chemotherapy 11 (18%) ESR1 mutations at baseline (ctDNA), n/N (%) 17/60a evaluated (28%)
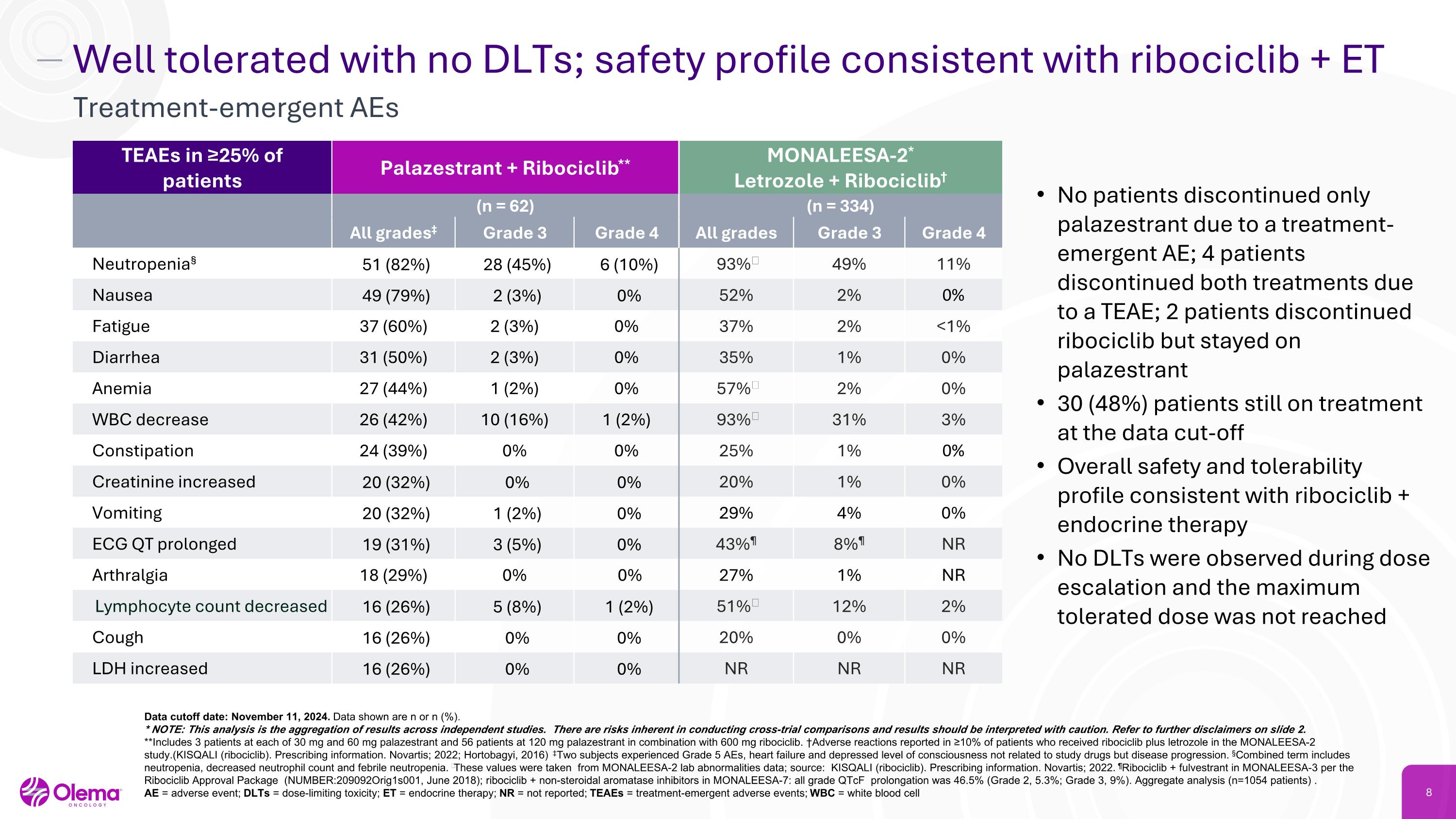
Well tolerated with no DLTs; safety profile consistent with ribociclib + ET Treatment-emergent AEs TEAEs in ≥25% of patients Palazestrant + Ribociclib** MONALEESA-2*�Letrozole + Ribociclib† (n = 62) (n = 334) All grades‡ Grade 3 Grade 4 All grades Grade 3 Grade 4 Neutropenia§ 51 (82%) 28 (45%) 6 (10%) 93%‖ 49% 11% Nausea 49 (79%) 2 (3%) 0% 52% 2% 0% Fatigue 37 (60%) 2 (3%) 0% 37% 2% <1% Diarrhea 31 (50%) 2 (3%) 0% 35% 1% 0% Anemia 27 (44%) 1 (2%) 0% 57%‖ 2% 0% WBC decrease 26 (42%) 10 (16%) 1 (2%) 93%‖ 31% 3% Constipation 24 (39%) 0% 0% 25% 1% 0% Creatinine increased 20 (32%) 0% 0% 20% 1% 0% Vomiting 20 (32%) 1 (2%) 0% 29% 4% 0% ECG QT prolonged 19 (31%) 3 (5%) 0% 43%¶ 8%¶ NR Arthralgia 18 (29%) 0% 0% 27% 1% NR Lymphocyte count decreased 16 (26%) 5 (8%) 1 (2%) 51%‖ 12% 2% Cough 16 (26%) 0% 0% 20% 0% 0% LDH increased 16 (26%) 0% 0% NR NR NR Data cutoff date: November 11, 2024. Data shown are n or n (%). * NOTE: This analysis is the aggregation of results across independent studies. There are risks inherent in conducting cross-trial comparisons and results should be interpreted with caution. Refer to further disclaimers on slide 2. **Includes 3 patients at each of 30 mg and 60 mg palazestrant and 56 patients at 120 mg palazestrant in combination with 600 mg ribociclib. †Adverse reactions reported in ≥10% of patients who received ribociclib plus letrozole in the MONALEESA-2 study.(KISQALI (ribociclib). Prescribing information. Novartis; 2022; Hortobagyi, 2016) ‡Two subjects experienced Grade 5 AEs, heart failure and depressed level of consciousness not related to study drugs but disease progression. §Combined term includes neutropenia, decreased neutrophil count and febrile neutropenia. ‖These values were taken from MONALEESA-2 lab abnormalities data; source: KISQALI (ribociclib). Prescribing information. Novartis; 2022. ¶Ribociclib + fulvestrant in MONALEESA-3 per the Ribociclib Approval Package (NUMBER:209092Orig1s001, June 2018); ribociclib + non-steroidal aromatase inhibitors in MONALEESA-7: all grade QTcF prolongation was 46.5% (Grade 2, 5.3%; Grade 3, 9%). Aggregate analysis (n=1054 patients) . �AE = adverse event; DLTs = dose-limiting toxicity; ET = endocrine therapy; NR = not reported; TEAEs = treatment-emergent adverse events; WBC = white blood cell No patients discontinued only palazestrant due to a treatment-emergent AE; 4 patients discontinued both treatments due to a TEAE; 2 patients discontinued ribociclib but stayed on palazestrant 30 (48%) patients still on treatment at the data cut-off Overall safety and tolerability profile consistent with ribociclib + endocrine therapy No DLTs were observed during dose escalation and the maximum tolerated dose was not reached
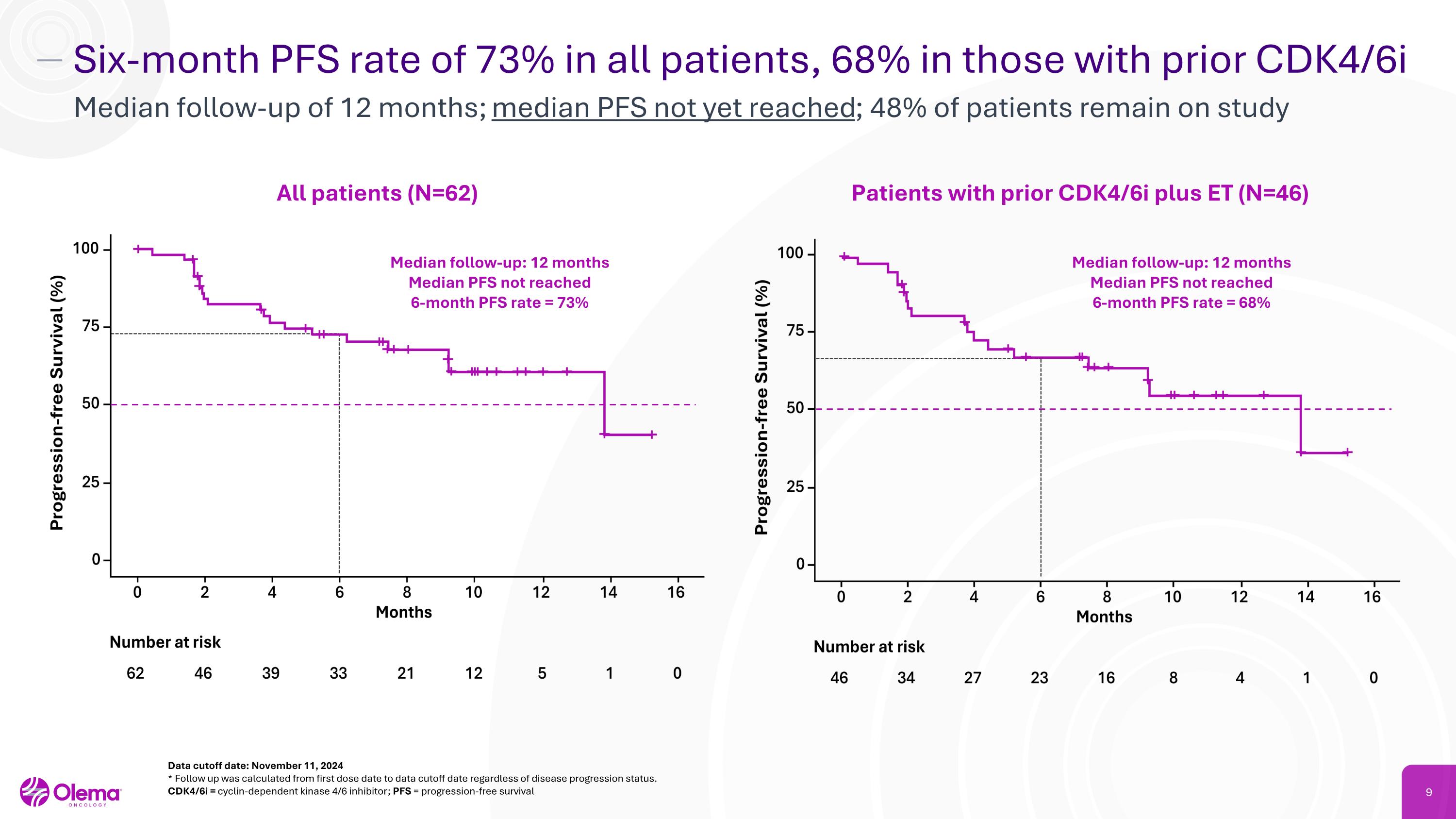
Six-month PFS rate of 73% in all patients, 68% in those with prior CDK4/6i Median follow-up of 12 months; median PFS not yet reached; 48% of patients remain on study Data cutoff date: November 11, 2024 * Follow up was calculated from first dose date to data cutoff date regardless of disease progression status.�CDK4/6i = cyclin-dependent kinase 4/6 inhibitor; PFS = progression-free survival Patients with prior CDK4/6i plus ET (N=46) All patients (N=62) Median follow-up: 12 months Median PFS not reached 6-month PFS rate = 73% Median follow-up: 12 months Median PFS not reached 6-month PFS rate = 68%
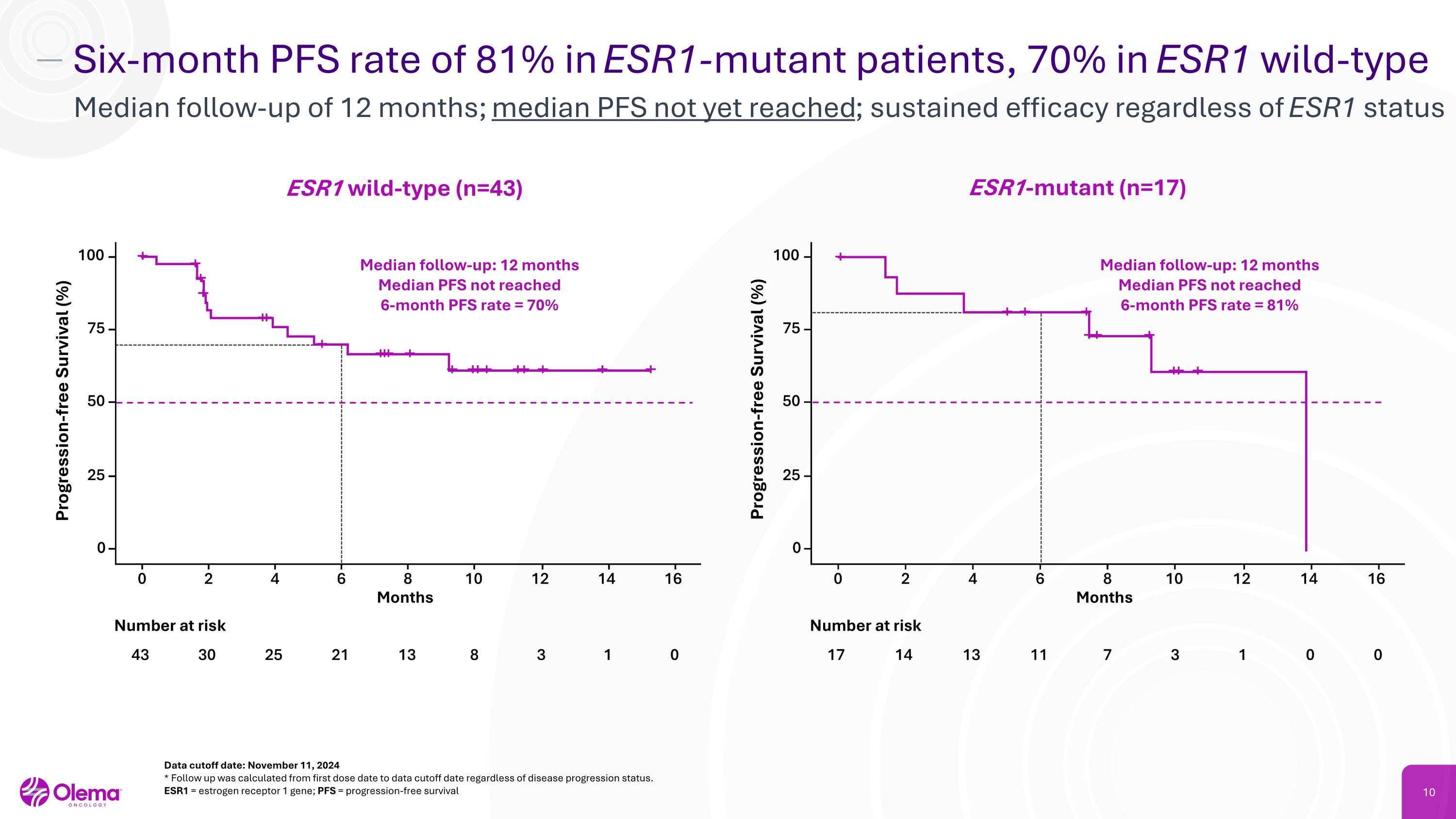
Six-month PFS rate of 81% in ESR1-mutant patients, 70% in ESR1 wild-type Median follow-up of 12 months; median PFS not yet reached; sustained efficacy regardless of ESR1 status Data cutoff date: November 11, 2024 * Follow up was calculated from first dose date to data cutoff date regardless of disease progression status. ESR1 = estrogen receptor 1 gene; PFS = progression-free survival ESR1-mutant (n=17) ESR1 wild-type (n=43) Median follow-up: 12 months Median PFS not reached 6-month PFS rate = 70% Median follow-up: 12 months Median PFS not reached 6-month PFS rate = 81%
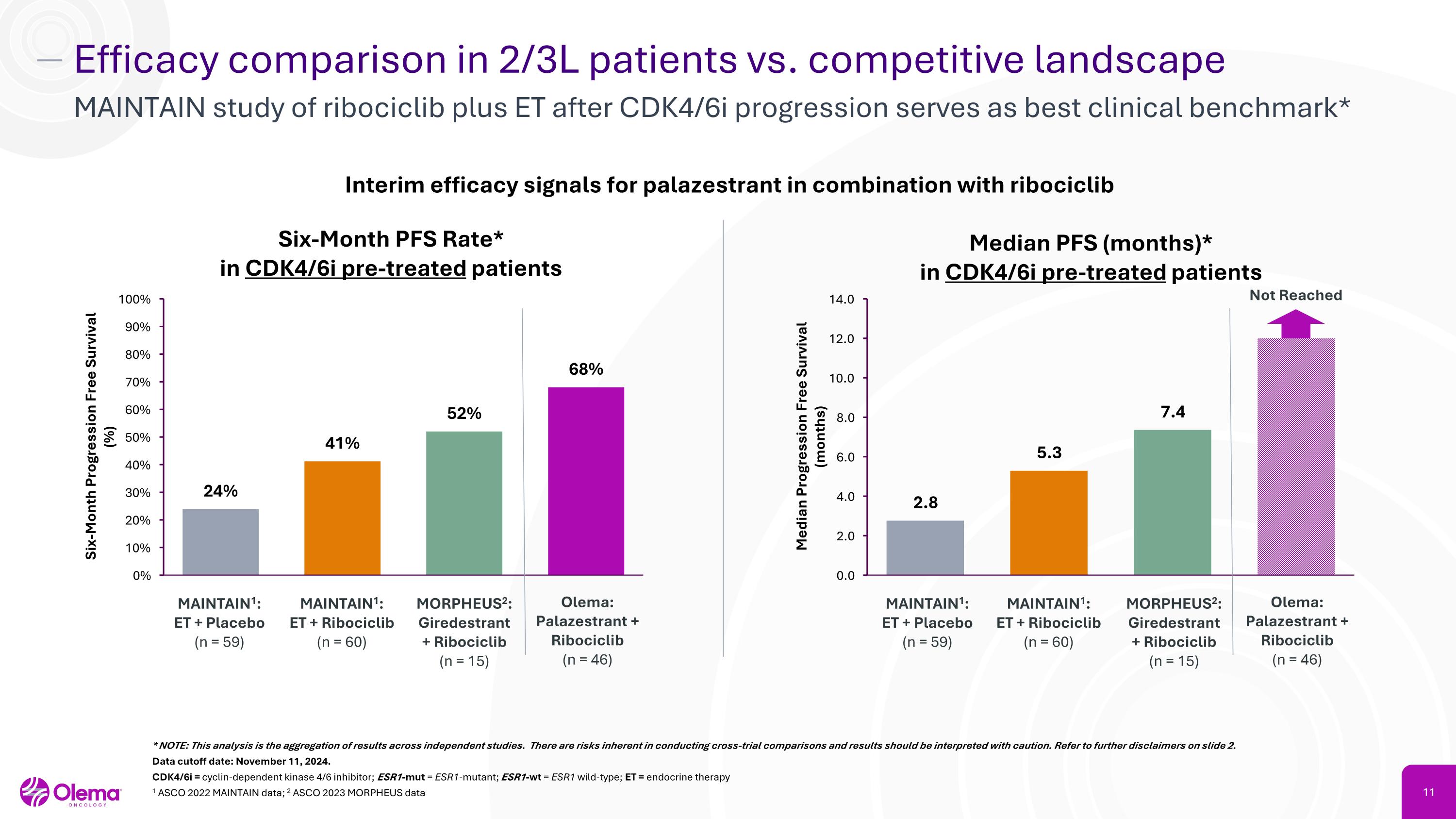
Efficacy comparison in 2/3L patients vs. competitive landscape MAINTAIN study of ribociclib plus ET after CDK4/6i progression serves as best clinical benchmark* Six-Month PFS Rate* in CDK4/6i pre-treated patients MAINTAIN1:�ET + Placebo�(n = 59) Olema: Palazestrant + Ribociclib�(n = 46) Interim efficacy signals for palazestrant in combination with ribociclib MORPHEUS2:�Giredestrant�+ Ribociclib�(n = 15) * NOTE: This analysis is the aggregation of results across independent studies. There are risks inherent in conducting cross-trial comparisons and results should be interpreted with caution. Refer to further disclaimers on slide 2. Data cutoff date: November 11, 2024. CDK4/6i = cyclin-dependent kinase 4/6 inhibitor; ESR1-mut = ESR1-mutant; ESR1-wt = ESR1 wild-type; ET = endocrine therapy 1 ASCO 2022 MAINTAIN data; 2 ASCO 2023 MORPHEUS data MAINTAIN1:�ET + Ribociclib �(n = 60) Median PFS (months)* in CDK4/6i pre-treated patients MAINTAIN1:�ET + Placebo�(n = 59) Olema: Palazestrant + Ribociclib�(n = 46) MORPHEUS2:�Giredestrant�+ Ribociclib�(n = 15) MAINTAIN1:�ET + Ribociclib �(n = 60) Not Reached
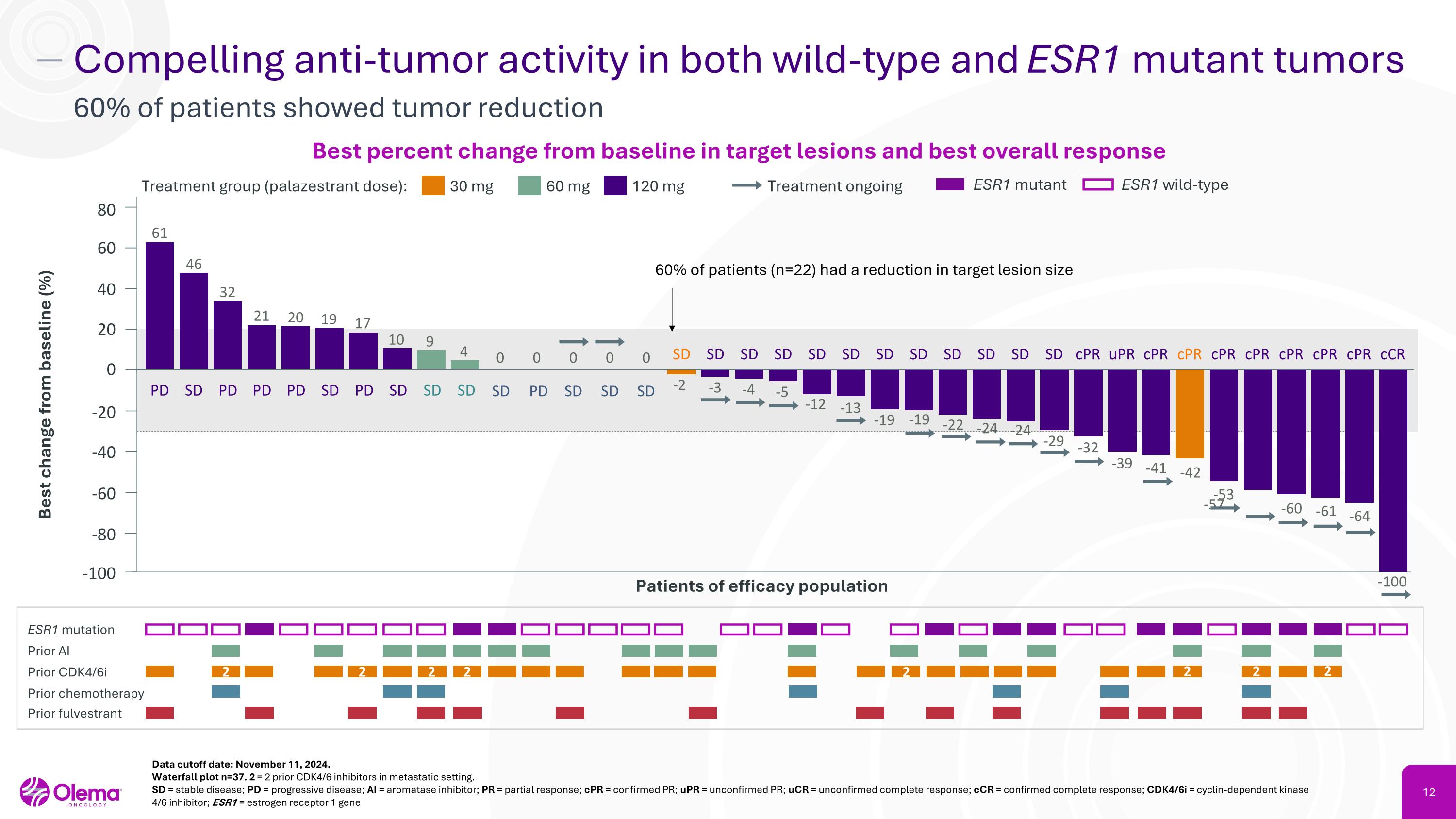
Compelling anti-tumor activity in both wild-type and ESR1 mutant tumors 60% of patients showed tumor reduction 30 mg Treatment group (palazestrant dose): 60 mg 120 mg Treatment ongoing ESR1 mutation Prior AI Prior CDK4/6i Prior chemotherapy Prior fulvestrant Patients of efficacy population 40 20 0 -20 -40 -60 Best change from baseline (%) ESR1 mutant ESR1 wild-type cPR cPR cPR uPR cPR cPR cPR cPR SD 0 PD 0 SD 0 SD 0 SD 0 SD -3 SD -12 SD -13 -19 -24 -32 -39 -53 -57 -100 Data cutoff date: November 11, 2024. Waterfall plot n=37. 2 = 2 prior CDK4/6 inhibitors in metastatic setting. SD = stable disease; PD = progressive disease; AI = aromatase inhibitor; PR = partial response; cPR = confirmed PR; uPR = unconfirmed PR; uCR = unconfirmed complete response; cCR = confirmed complete response; CDK4/6i = cyclin-dependent kinase 4/6 inhibitor; ESR1 = estrogen receptor 1 gene Best percent change from baseline in target lesions and best overall response 60% of patients (n=22) had a reduction in target lesion size -19 -22 -61 -60 -4 SD -5 SD SD SD 2 SD 19 SD 4 PD 17 SD 10 SD 9 PD 20 PD 21 PD 32 80 cPR cCR -100 -64 SD 46 PD 61 60 -80 SD -2 2 2 2 -24 -29 -41 -42 SD SD SD SD 2 2 2 2
Safety and Tolerability Palazestrant (120 mg) in combination with full dose ribociclib was well tolerated Safety was consistent with combinations of ribociclib (600 mg) with endocrine therapy Pharmacokinetics No clinically meaningful drug-drug interaction Efficacy Median follow-up of 12 months with mPFS not yet reached 6-month progression-free survival rate was: 73% across all patients 68% in patients with prior CDK4/6i exposure 70% in patients with ESR1-wt disease 81% in those with ESR1-mut disease 11 responses to date (2 confirmed CRs*, 8 confirmed PRs, and 1 unconfirmed PR) 27% (10/37) ORR among response-evaluable patients with measurable disease Clinical benefit rate (CBR)^: 76% in all patients (37/49 CBR-eligible) 81% in ESR1-mut (13/16 CBR-eligible) 74% in ESR1-wt (23/31 CBR-eligible) CBR in patients who received prior CDK4/6i: 71% in all patients (25/35 CBR-eligible) 81% in ESR1-mut (13/16 CBR-eligible) 65% in ESR1-wt (11/17 CBR-eligible) Longest duration of treatment 79 weeks and ongoing Efficacy data are maturing; 30 (48%) patients remain on treatment Highly encouraging preliminary efficacy from the combined agents, including in patients with prior CDK4/6i treatment *1 cCR in patient with non-measurable but evaluable disease. ^ CBR is the proportion of patients who remained on treatment through at least 24 weeks with a confirmed complete response or partial response, or stable disease. Abbreviations: CDK4/6i = cyclin-dependent kinase 4/6 inhibitor; ESR1 = estrogen receptor 1 gene; PR = partial response; CR = complete response.
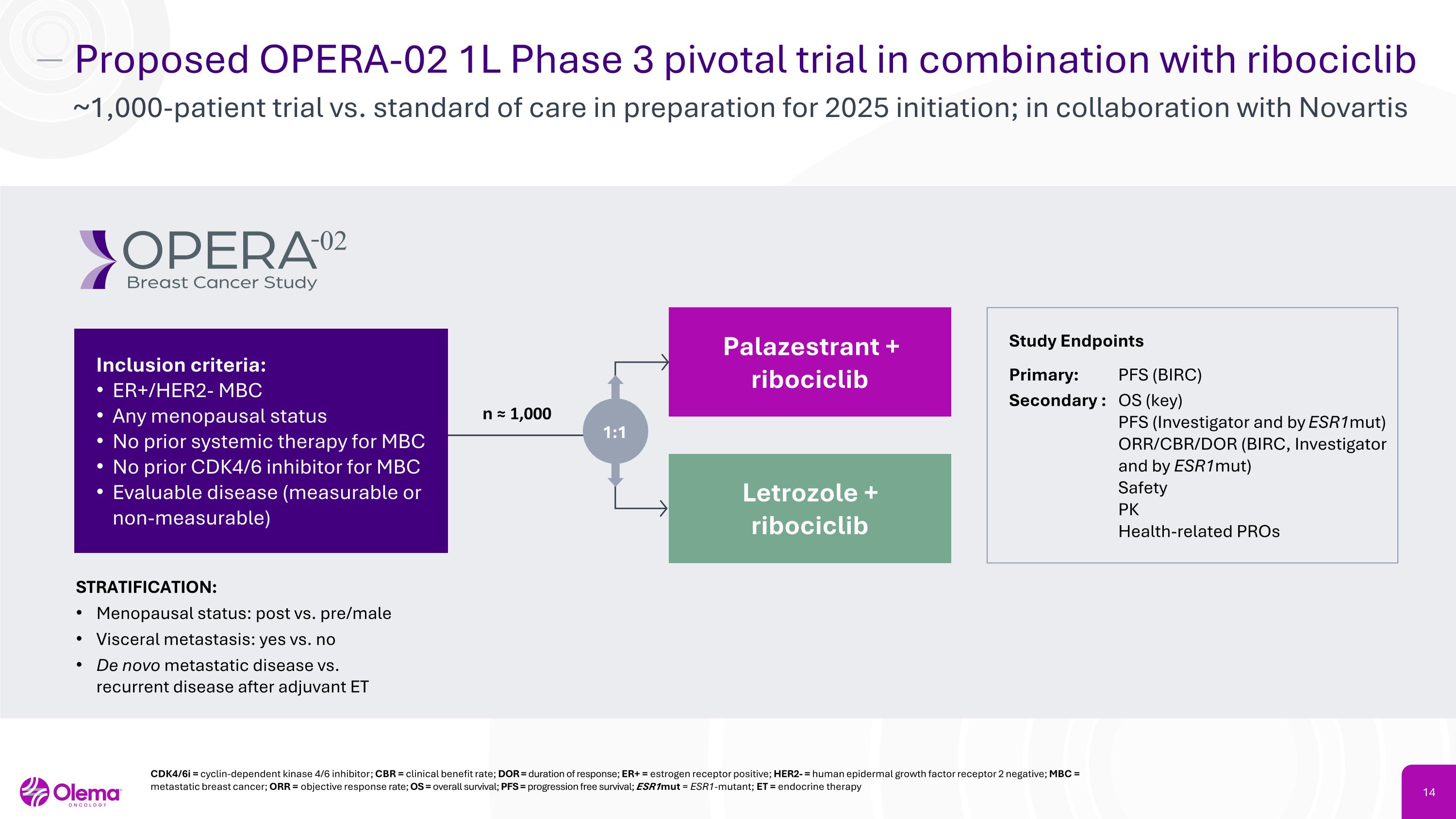
Proposed OPERA-02 1L Phase 3 pivotal trial in combination with ribociclib ~1,000-patient trial vs. standard of care in preparation for 2025 initiation; in collaboration with Novartis CDK4/6i = cyclin-dependent kinase 4/6 inhibitor; CBR = clinical benefit rate; DOR = duration of response; ER+ = estrogen receptor positive; HER2- = human epidermal growth factor receptor 2 negative; MBC = metastatic breast cancer; ORR = objective response rate; OS = overall survival; PFS = progression free survival; ESR1mut = ESR1-mutant; ET = endocrine therapy Study Endpoints Primary: PFS (BIRC) Secondary : OS (key) PFS (Investigator and by ESR1mut) ORR/CBR/DOR (BIRC, Investigator and by ESR1mut) Safety PK Health-related PROs STRATIFICATION: Menopausal status: post vs. pre/male Visceral metastasis: yes vs. no De novo metastatic disease vs. recurrent disease after adjuvant ET n ≈ 1,000 Palazestrant +�ribociclib Letrozole +�ribociclib Inclusion criteria: ER+/HER2- MBC Any menopausal status No prior systemic therapy for MBC No prior CDK4/6 inhibitor for MBC Evaluable disease (measurable or non-measurable) 1:1
Preparing to initiate OPERA-02 in 2025 New clinical trial collaboration and supply agreement with Novartis combined with $250M private placement enables execution of Olema operating plan Novartis agreement enables pivotal Phase 3 OPERA-02 clinical trial of palazestrant in combination with ribociclib in frontline ER+/HER2- advanced or metastatic breast cancer Ribociclib drug supply expected to be sufficient to conduct the planned OPERA-02 trial; valued at ~$275M Olema responsible for the day-to-day operational activities for OPERA-02 Olema retains global commercial rights to palazestrant All clinical data from OPERA-02 will be jointly owned; each party retains rights to its background IP $250M equity private placement strengthens Olema’s balance sheet Participation by new and existing high-quality institutional and accredited investors Pro forma cash and cash equivalents expected to fund research and development activities including the execution of OPERA-01, OPERA-02, OP-3136 Phase 1/2, and for working capital and general corporate purposes
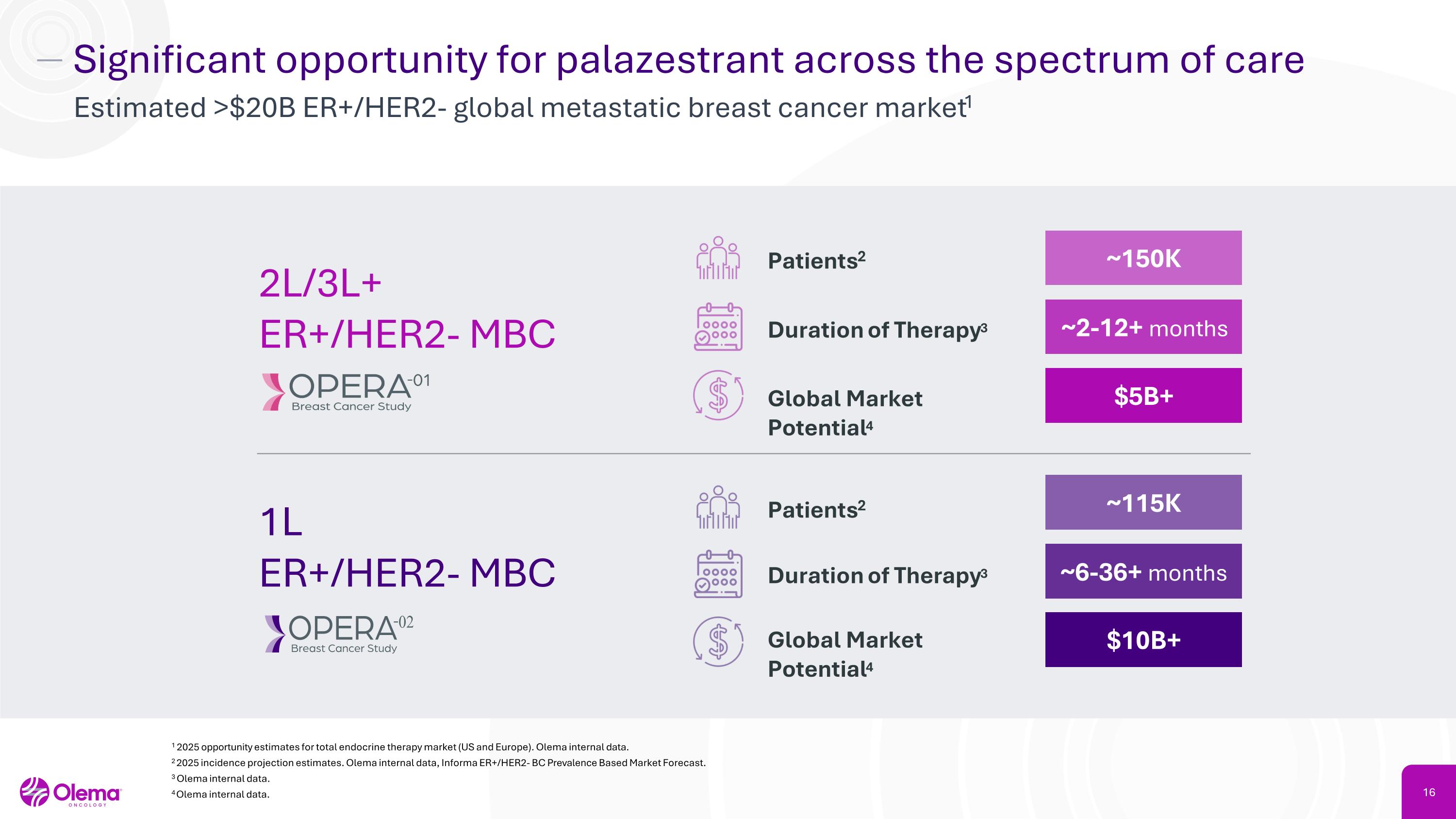
Significant opportunity for palazestrant across the spectrum of care Estimated >$20B ER+/HER2- global metastatic breast cancer market1 1 2025 opportunity estimates for total endocrine therapy market (US and Europe). Olema internal data. 2 2025 incidence projection estimates. Olema internal data, Informa ER+/HER2- BC Prevalence Based Market Forecast. 3 Olema internal data. 4Olema internal data. 2L/3L+�ER+/HER2- MBC 1L �ER+/HER2- MBC Patients2 ~150K Duration of Therapy3 ~2-12+ months Global Market Potential4 $5B+ Patients2 ~115K Duration of Therapy3 ~6-36+ months Global Market Potential4 $10B+

Olema: a compelling late-stage opportunity in breast cancer and beyond Changing the treatment paradigm for endocrine-driven cancers 1 Pro forma position includes the Company's cash, cash equivalents, and marketable securities as of September 30, 2024, plus net proceeds of approximately $237.5 million from equity private placement which closed on December 4, 2024. Palazestrant anchors Olema’s breast cancer program as a potential backbone therapy for metastatic breast cancer Highly differentiated as first oral CERAN/SERD endocrine agent Ongoing 2/3L OPERA-01 Phase 3 trial on track for top-line data in 2026 Planned 1L OPERA-02 Phase 3 trial in combination with ribociclib enabled; initiation expected in 2025 Go-to-market strategy for potential U.S. launch in 2027 OP-3136 expands pipeline with novel and validated KAT6 target IND cleared by FDA; first patient expected to enroll in Phase 1 clinical trial by early 2025 Well-capitalized with ~$452M of pro forma cash and cash equivalents as of September 30, 20241

— Advancing medicines for breast cancer and beyond

















