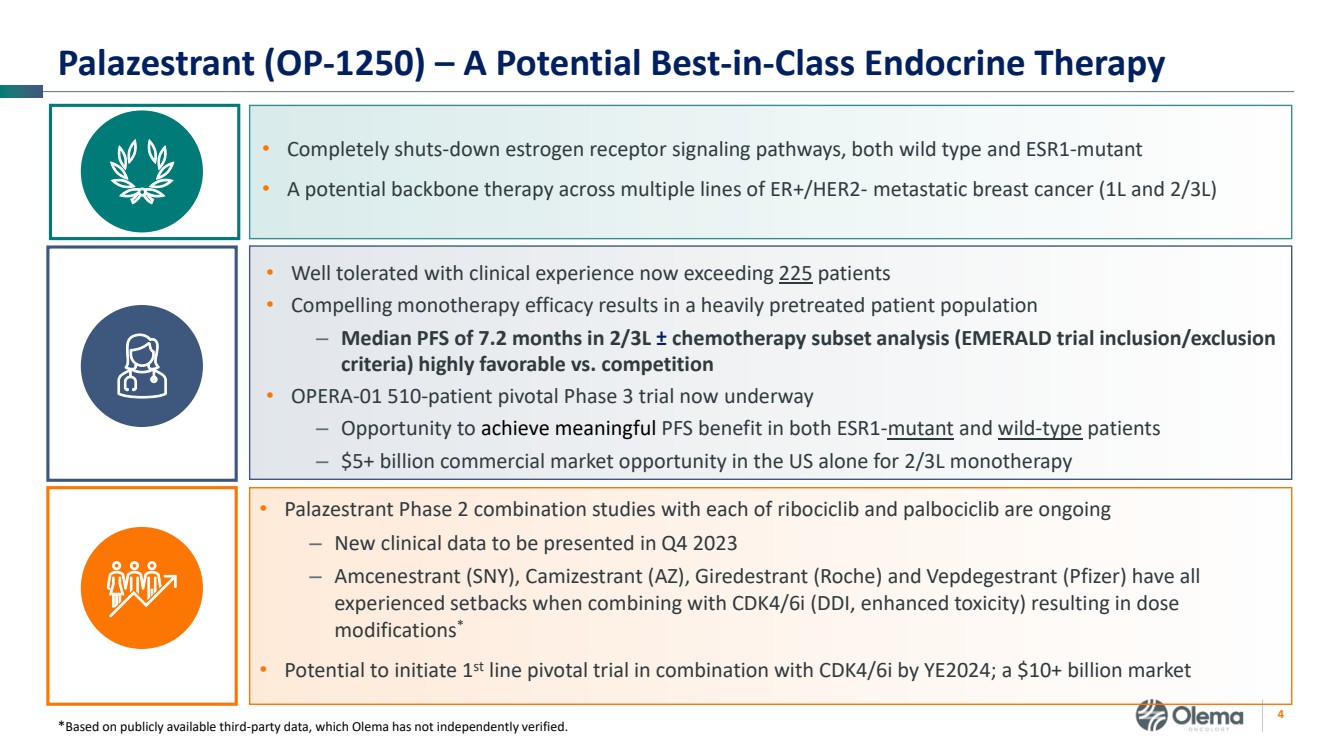
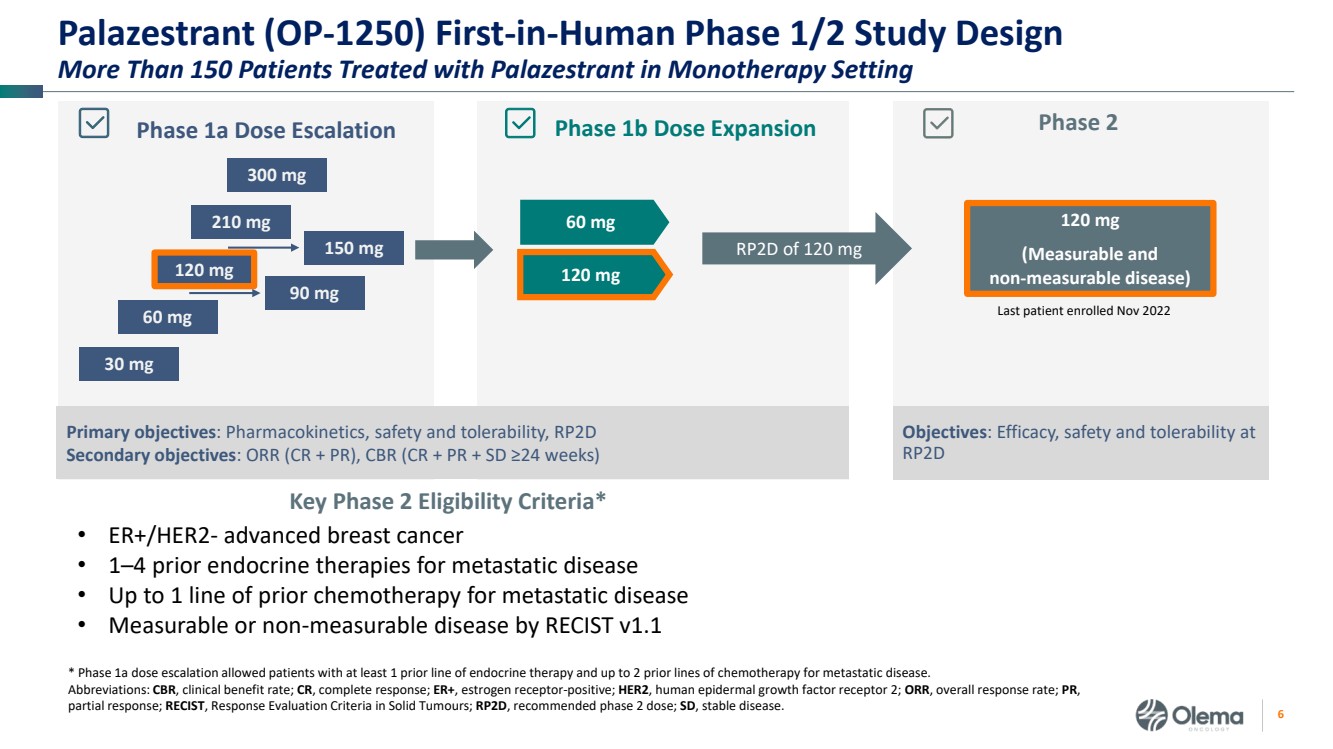
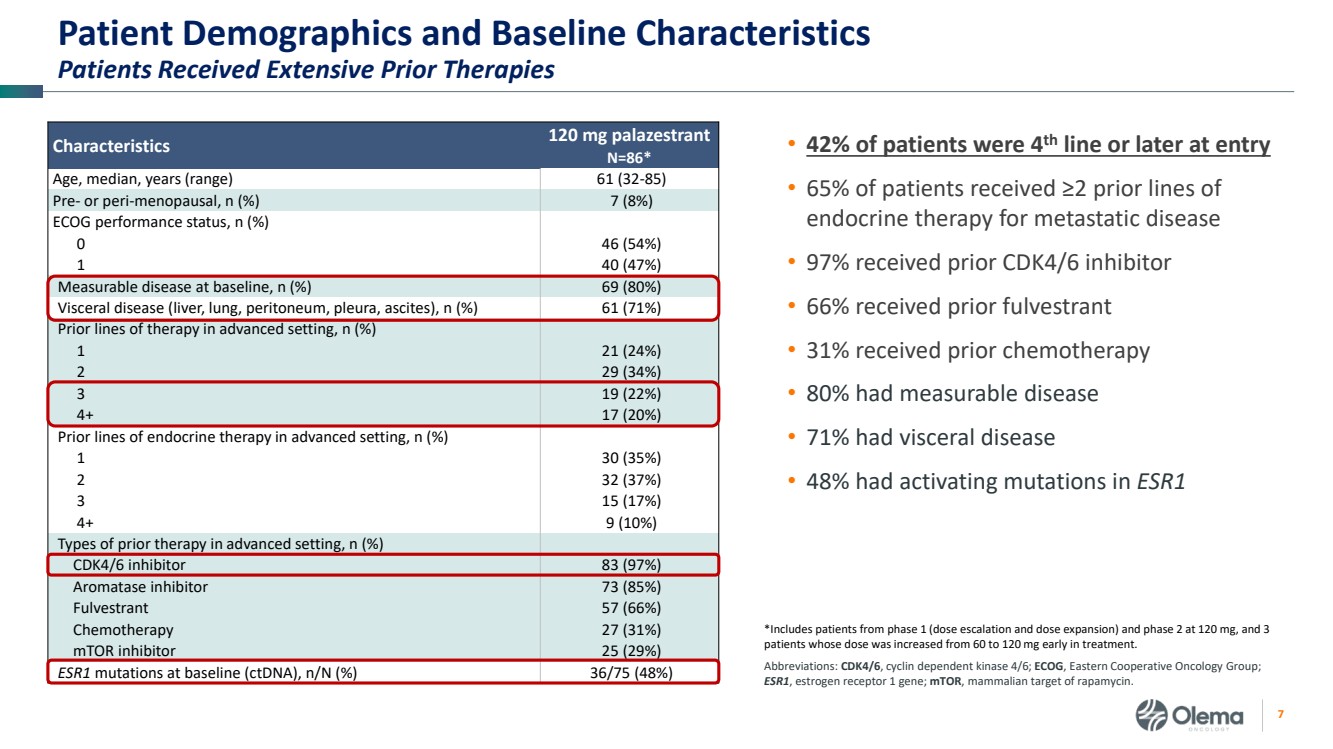
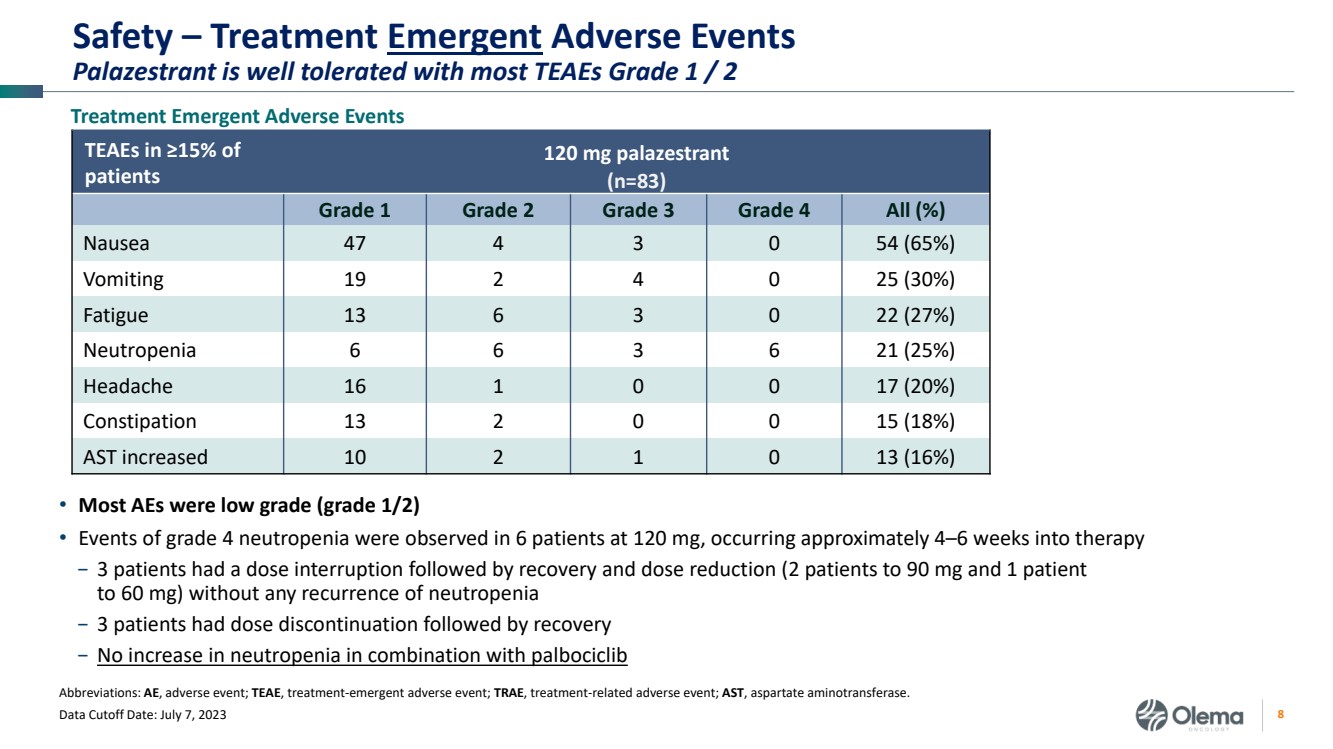
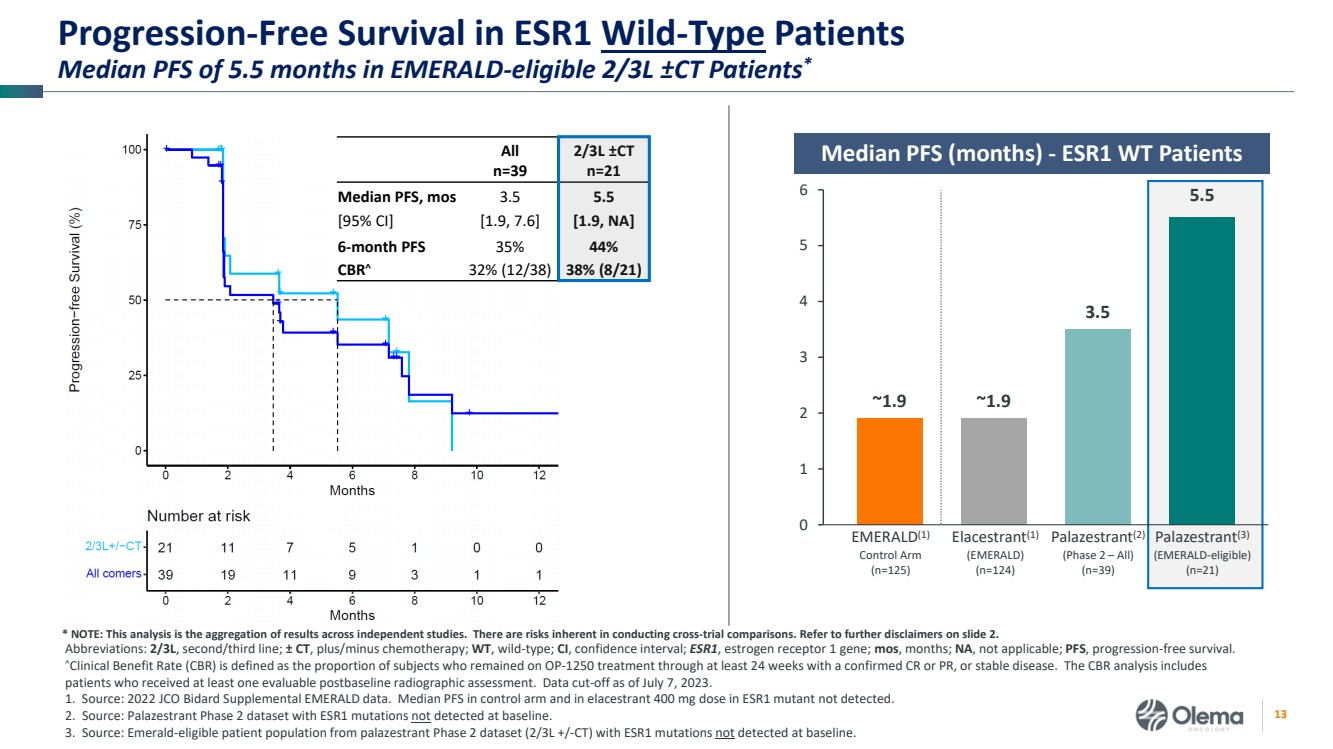
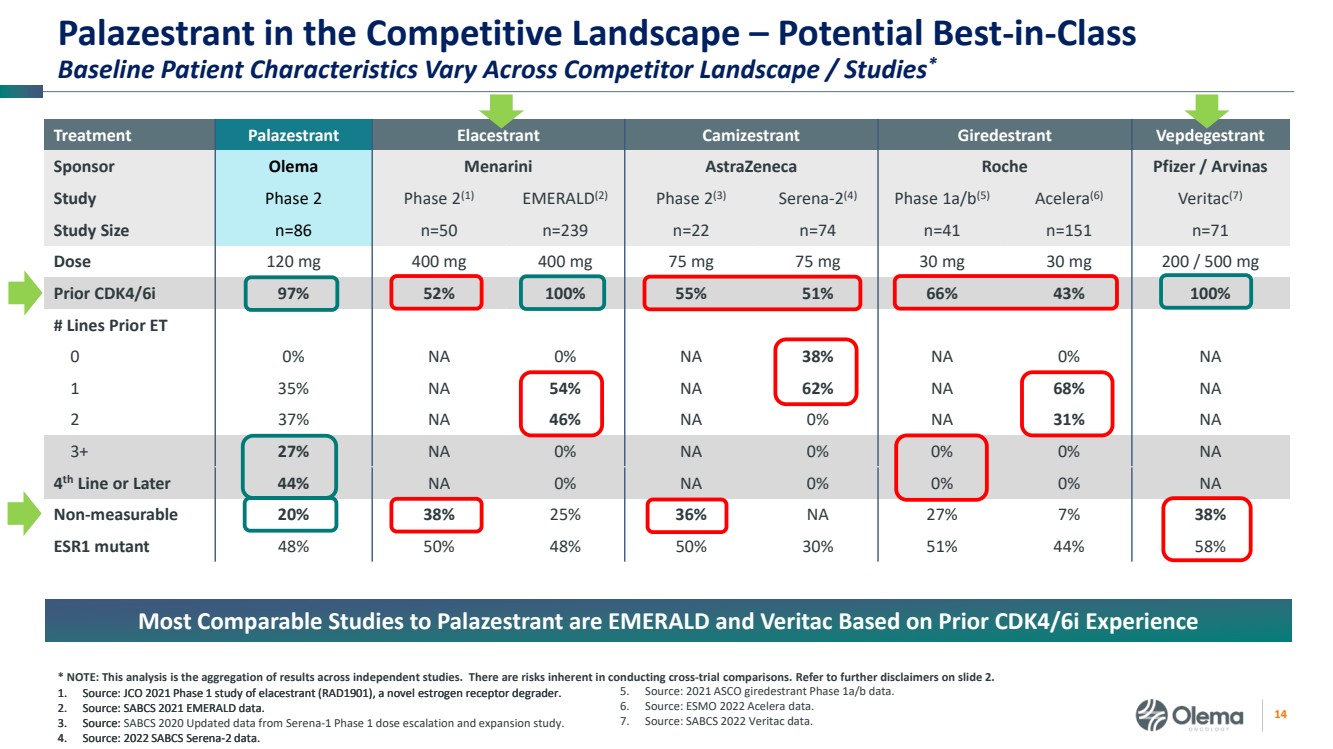
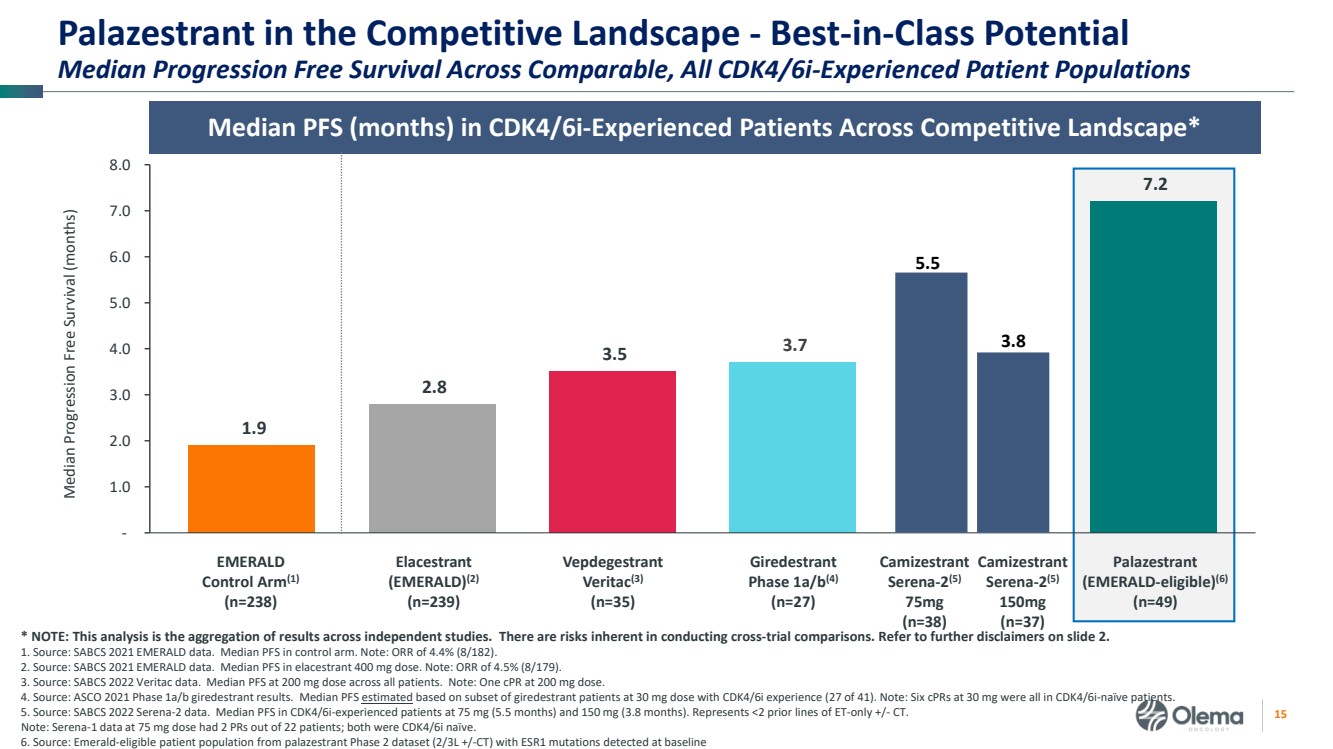
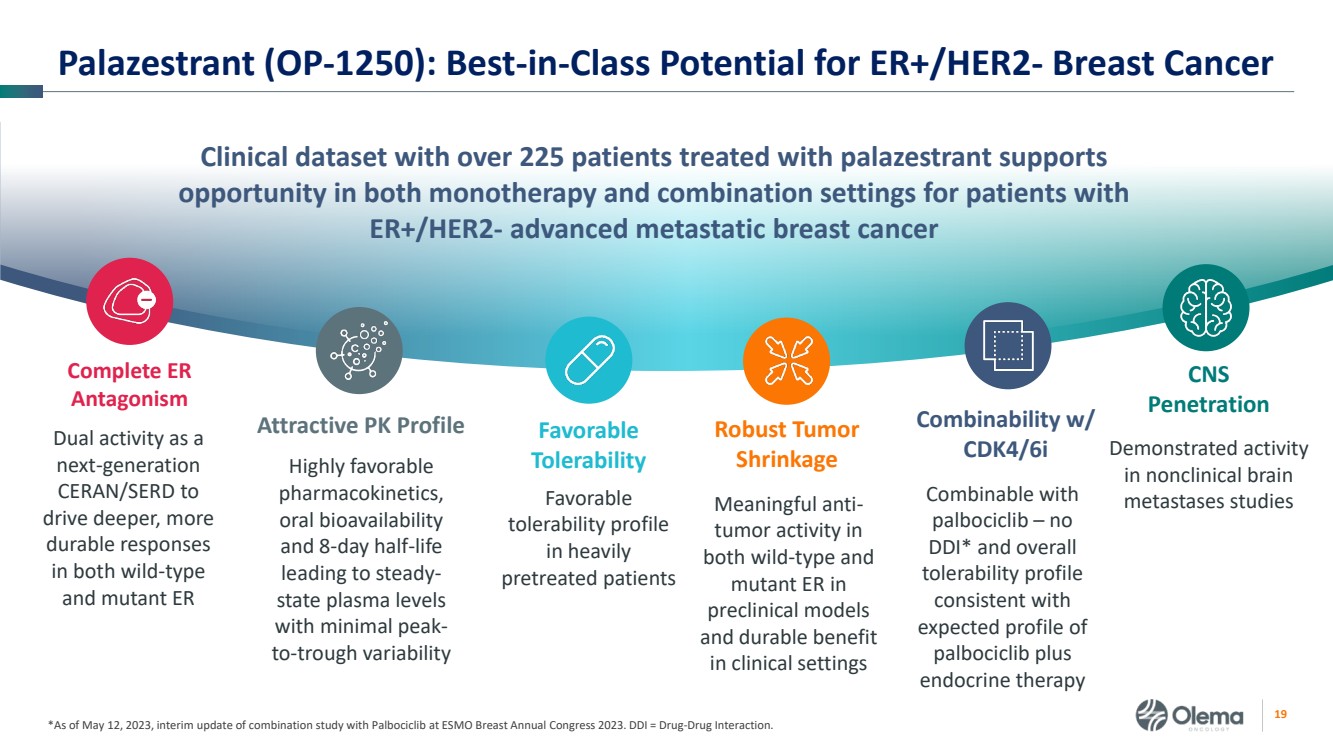
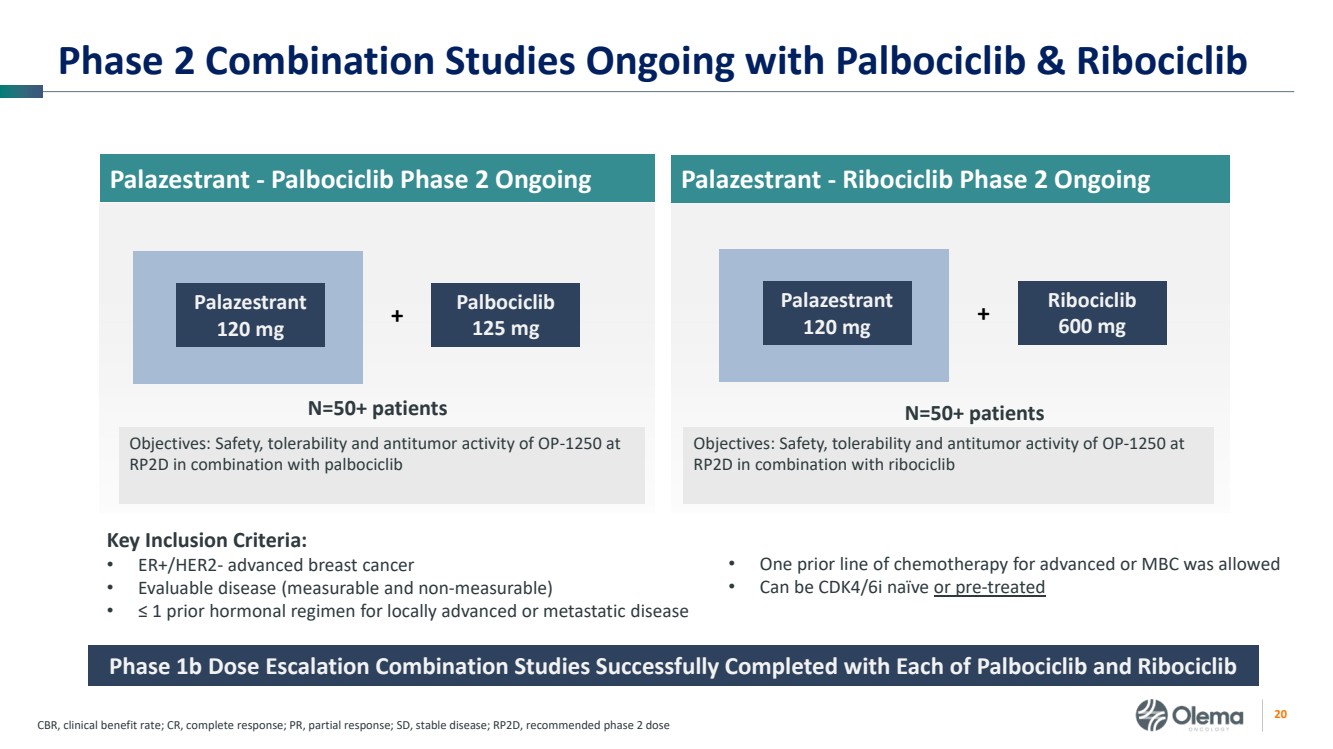
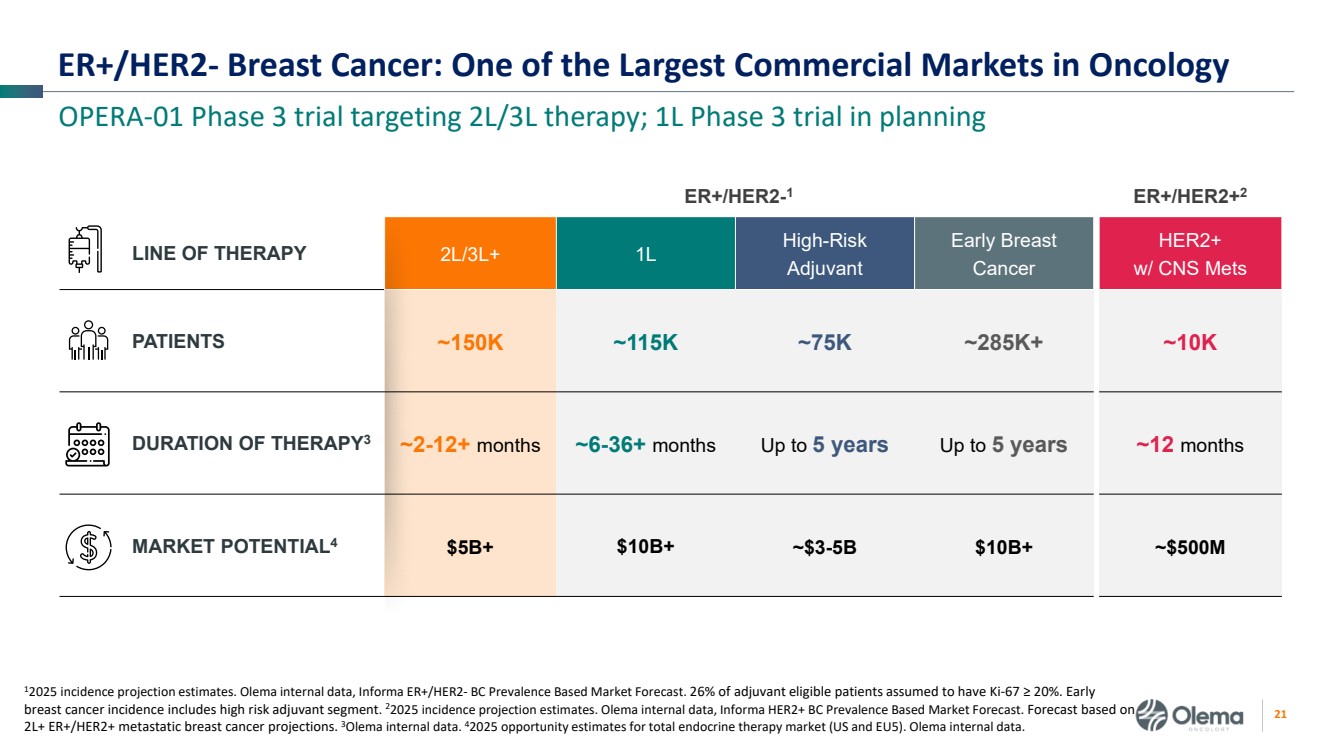
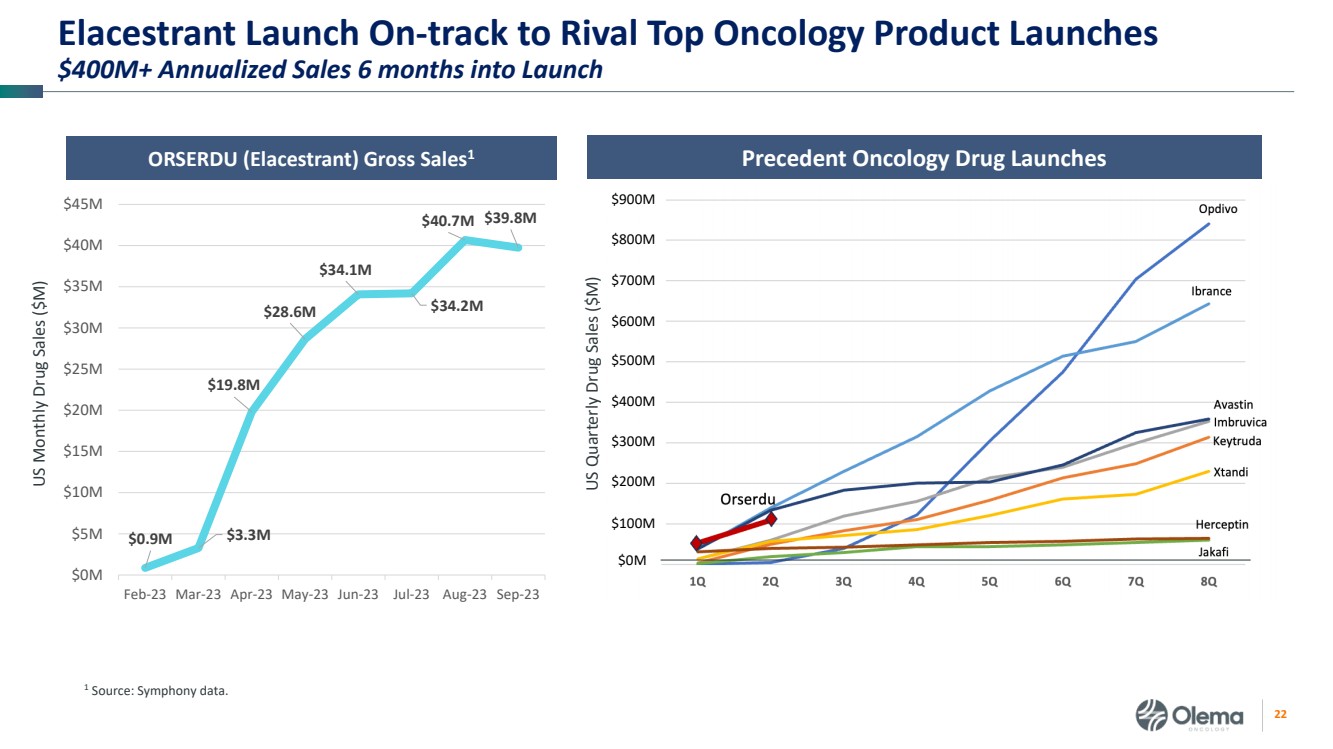
| 2 Forward-Looking Statements and Other Disclaimers This presentation contains forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Statements in this presentation that are not statements of historical fact are forward-looking statements. Such forward-looking statements include, without limitation, statements regarding our research and clinical development plans, the scope, progress, results and costs of developing our product candidate or any other future product candidates, expected manufacturing capabilities, strategy, regulatory matters, including the timing and likelihood of success of obtaining drug approvals, the timelines for potential clinical study results and initiation of clinical trials of palazestrant (OP-1250) as a monotherapy and in combination trials, including OPERA-01, the Company’s pivotal Phase 3 monotherapy clinical trial, the potential beneficial characteristics, safety, tolerability, efficacy and therapeutic effects of palazestrant, the development of palazestrant, the potential of palazestrant to become a best-in-class treatment option for ER+/HER2- metastatic breast cancer, improve the standard-of-care treatment, or become a transformative therapy for women living with breast cancer, the combinability of palazestrant with other drugs, market size and opportunity, our ability to penetrate the market,, our ability to complete certain milestones, and our financial condition, cash position, cash runway, and sufficiency of our financial resources. Words such as “believe,” “anticipate,” “plan,” “expect,” “intend,” “will,” “may,” “goal,” “project,” “estimate,” “potential” and similar expressions are intended to identify forward-looking statements, though not all forward-looking statements necessarily contain these identifying words. These forward-looking statements are based on the beliefs of the Company’s management as well as assumptions made by and information currently available to the Company. Such statements reflect the current views of the Company with respect to future events and are subject to known and unknown risks, including business, regulatory, economic and competitive risks, uncertainties, contingencies and assumptions about the Company, including, without limitation, risks inherent in developing products and technologies, future results from the Company’s ongoing and planned clinical trials, the Company’s ability to obtain adequate financing to fund its planned clinical trials and other expenses, trends in the industry, the legal and regulatory framework for the industry and future expenditures, and other risks and uncertainties affecting the Company, including those described under the caption "Risk Factors" and elsewhere in the Company‘s Quarterly Report on Form 10-Q, Annual Report on Form 10-K, and future filings and reports of the Company with the Securities and Exchange Commission. In light of these risks and uncertainties, the events or circumstances referred to in the forward-looking statements may not occur. The actual results may vary or differ from the anticipated results and the variations or differences may be material. These forward-looking statements should not be taken as forecasts or promises nor should they be taken as implying any indication, assurance or guarantee that the assumptions on which such forward-looking statements have been made are correct or exhaustive or, in the case of the assumptions, fully stated in this presentation. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date this presentation is given. This presentation discusses product candidates that are under clinical study and which have not yet been approved for marketing by the U.S. Food and Drug Administration. No representation is made as to the safety or effectiveness of these product candidates for the use for which such product candidates are being studied. This presentation incorporates publicly-available third-party data that we have not independently verified. There are risks inherent in conducting cross-trial comparisons. The presentation of such third-party data does not represent a head-to-head comparison of how palazestrant performed in a second- or third-line setting relative to elacestrant in the EMERALD study, or any other third-party drug candidate or study. Rather, such third-party data has been pulled by us from publicly-available sources for supplemental informational purposes, only. We caution you that any comparisons against third-party data set forth herein should not be viewed as a side-by-side comparison, and you should not rely on the completeness or accuracy of our presentation of the results of any third-party drug candidate in these slides, due to differences in study design, how other companies quantify or qualify eligibility criteria, and how results are recorded, among other distinguishing factors and uncertainties. Because we may be unaware of or may not adequately present various distinguishing factors and uncertainties, the comparisons set forth herein may not properly present such third-party data, which may differ materially from the data as presented here. Investors are encouraged to independently review third party data and should not rely on our presentation of such data (including any such data placed in comparison with the performance of palazestrant) as a single measure to evaluate our business. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of such products. |