
Leading a Novel Approach to Targeting the Microbiome NASDAQ: KLDO June 2021 Exhibit 99.1

Forward-Looking Statements This presentation also contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, without limitation, statements regarding our strategy, business plans and focus, including the therapeutic potential of our Microbiome Metabolic Therapy (MMT) candidates, the timing of initiation, completion and reporting of results of our clinical studies and our strategy, business plans and focus. The words “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Any forward-looking statements in this presentation are based on management’s current expectations and beliefs and are subject to a number of risks, uncertainties and important factors that may cause actual events or results to differ materially from those expressed or implied by any forward-looking statements contained in this presentation, including, without limitation, those related to planned clinical and preclinical studies and areas of interest, the preclinical and clinical development and safety profile of our MMT candidates and timelines associated with the programs for such MMT candidates, whether and when, if at all, our MMT candidates will receive approval from the U.S. Food and Drug Administration or other applicable regulatory agencies, if any, competition from other biotechnology companies, and other risks identified in our SEC filings, including the most recently filed 10-K. We caution you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. We disclaim any obligation to update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements.

Kaleido Biosciences in Brief Unique approach using synthetic glycans as modulators of the microbiome Act by targeting the resident microbiome as opposed to adding/subtracting micro-organisms Ability to induce large compound-specific microbial shifts to more favorable composition and metabolic output for therapeutic benefit Library of 1500+ diverse synthetic glycans designated Microbiome Metabolic Therapies (MMTsTM) Opportunities across broad range of therapeutic areas Small molecule modality is substantially de-risking microbiome therapeutics Increasing understanding of SAR allows targeted compound optimization and library expansion MMTs are related to a class of compounds “generally recognized as safe” (GRAS), allowing rapid progression from early discovery to clinical testing MMTs are NCE’s offering IP protection and development paths as small molecule drugs Ease and scalability of manufacture de-risking CMC Have rapidly generated multiple clinical and preclinical portfolio assets Clinical programs include mild-to-moderate COVID-19, Ulcerative Colitis, and Urea Cycle Disorders Additional preclinical programs include Immuno-Oncology and Cardiometabolic disease Strong potential for future expansion

The Kaleido Leadership Team Daniel Menichella President and Chief Executive Officer Former CEO, CureVac AG Johan van Hylckama Vlieg, Ph.D.Chief Scientific Officer Former VP for Microbiome & Human Health Innovation, Chr. Hansen William Duke Chief Financial Officer Former CFO, Pulmatrix and Valeritas Clare FisherChief Business Officer Former Group VP, Global Head of Transactions & Business Development, Shire Jerald Korn, J.D. General Counsel & Corporate Secretary Former Senior Vice President, Chief Legal and Administrative Officer, TESARO Mark Wingertzahn Senior Vice President & Head of Development Former Vice President R&D, Head of Clinical Development, GlaxoSmithKline Kimberly Hocknell Vice President, Technical Operations Former Director, GMP Facilities and Systems at LFB COMPANY BUILDING THERAPEUTIC AREA BREADTH & TECHNOLOGY DEPTH CLINICAL DEVELOPMENT & REGULATORY MANUFACTURING
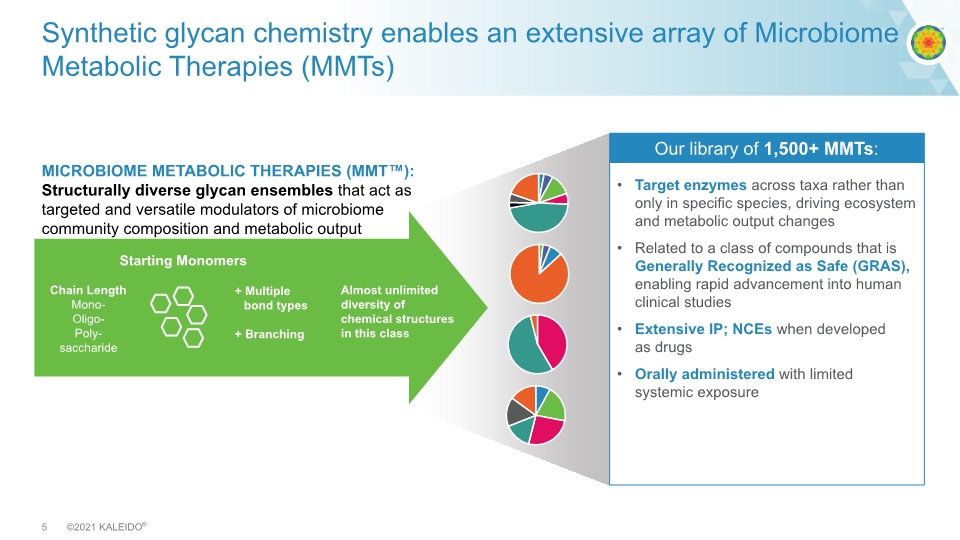
Synthetic glycan chemistry enables an extensive array of Microbiome Metabolic Therapies (MMTs) Target enzymes across taxa rather than only in specific species, driving ecosystem and metabolic output changes Related to a class of compounds that is Generally Recognized as Safe (GRAS), enabling rapid advancement into human clinical studies Extensive IP; NCEs when developed as drugs Orally administered with limited systemic exposure MICROBIOME METABOLIC THERAPIES (MMT™): Structurally diverse glycan ensembles that act as targeted and versatile modulators of microbiome community composition and metabolic output Chain Length Mono- Oligo- Poly- saccharide Our library of 1,500+ MMTs: + Multiple bond types + Branching Starting Monomers Almost unlimited diversity of chemical structures in this class
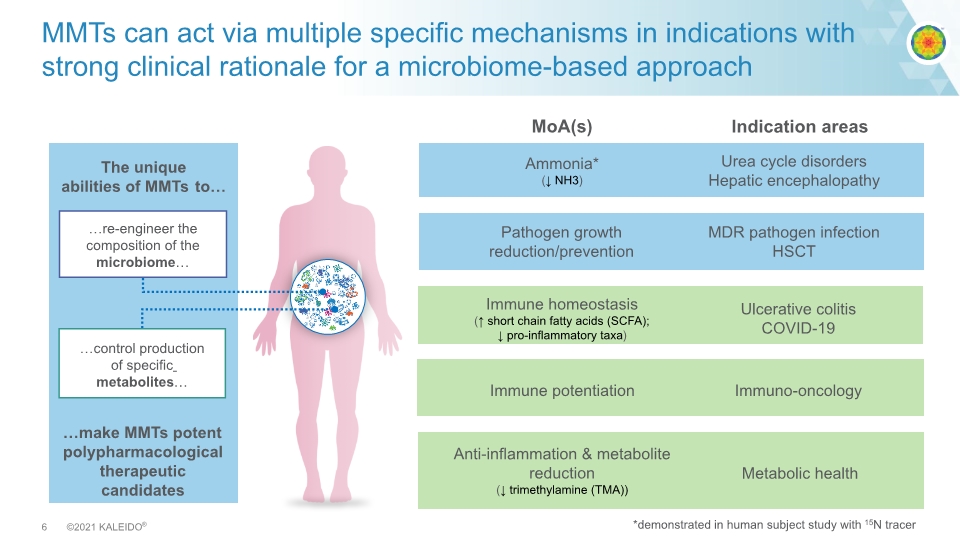
MMTs can act via multiple specific mechanisms in indications with strong clinical rationale for a microbiome-based approach The unique abilities of MMTs to… …make MMTs potent polypharmacological therapeutic candidates Urea cycle disorders Hepatic encephalopathy MDR pathogen infection HSCT Immuno-oncology Ulcerative colitis COVID-19 Metabolic health Ammonia* (↓ NH3) Pathogen growth reduction/prevention Immune potentiation Immune homeostasis (↑ short chain fatty acids (SCFA); ↓ pro-inflammatory taxa) Anti-inflammation & metabolite reduction (↓ trimethylamine (TMA)) Indication areas MoA(s) *demonstrated in human subject study with 15N tracer re-engineer the composition of the microbiome control production of specific metabolites

COVID-19 (KB109)
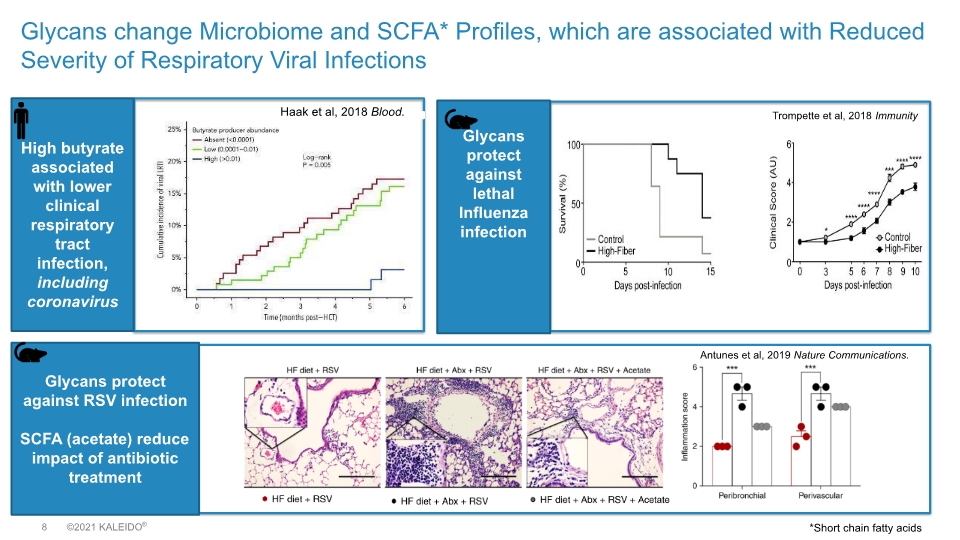
Glycans change Microbiome and SCFA* Profiles, which are associated with Reduced Severity of Respiratory Viral Infections High butyrate associated with lower clinical respiratory tract infection, including coronavirus Haak et al, 2018 Blood. Trompette et al, 2018 Immunity Glycans protect against lethal Influenza infection Glycans protect against RSV infection SCFA (acetate) reduce impact of antibiotic treatment Antunes et al, 2019 Nature Communications. *Short chain fatty acids
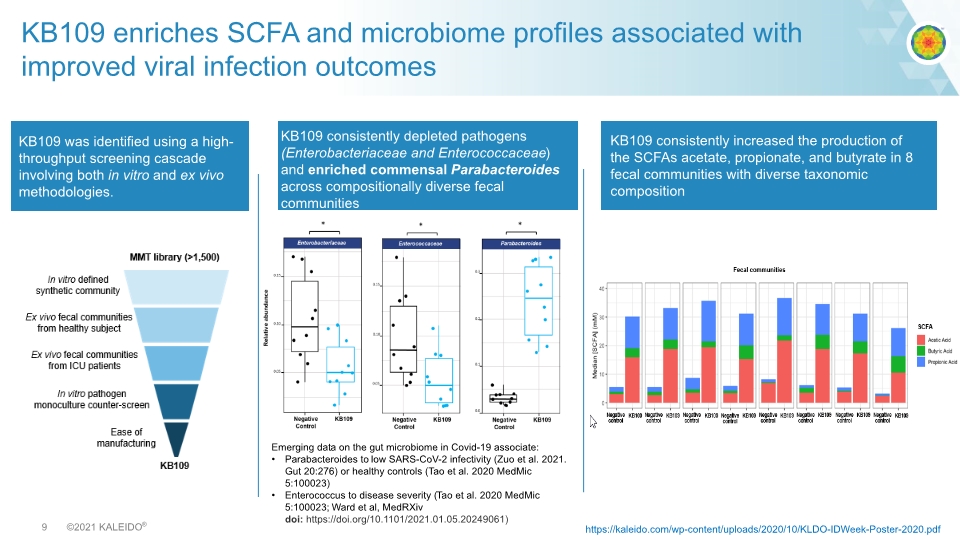
KB109 enriches SCFA and microbiome profiles associated with improved viral infection outcomes KB109 consistently depleted pathogens (Enterobacteriaceae and Enterococcaceae) and enriched commensal Parabacteroides across compositionally diverse fecal communities KB109 consistently increased the production of the SCFAs acetate, propionate, and butyrate in 8 fecal communities with diverse taxonomic composition Emerging data on the gut microbiome in Covid-19 associate: Parabacteroides to low SARS-CoV-2 infectivity (Zuo et al. 2021. Gut 20:276) or healthy controls (Tao et al. 2020 MedMic 5:100023) Enterococcus to disease severity (Tao et al. 2020 MedMic 5:100023; Ward et al, MedRXiv doi: https://doi.org/10.1101/2021.01.05.20249061) KB109 was identified using a high-throughput screening cascade involving both in vitro and ex vivo methodologies. https://kaleido.com/wp-content/uploads/2020/10/KLDO-IDWeek-Poster-2020.pdf

A Randomized, Open Label, Prospective, Parallel Group Study to Assess the Natural History of COVID-19 and Effects of KB109 in Addition to Supportive Self Care (SSC) Compared to SSC Alone on Measures of Health in Non-hospitalized Patients with Mild-to-Moderate COVID-19
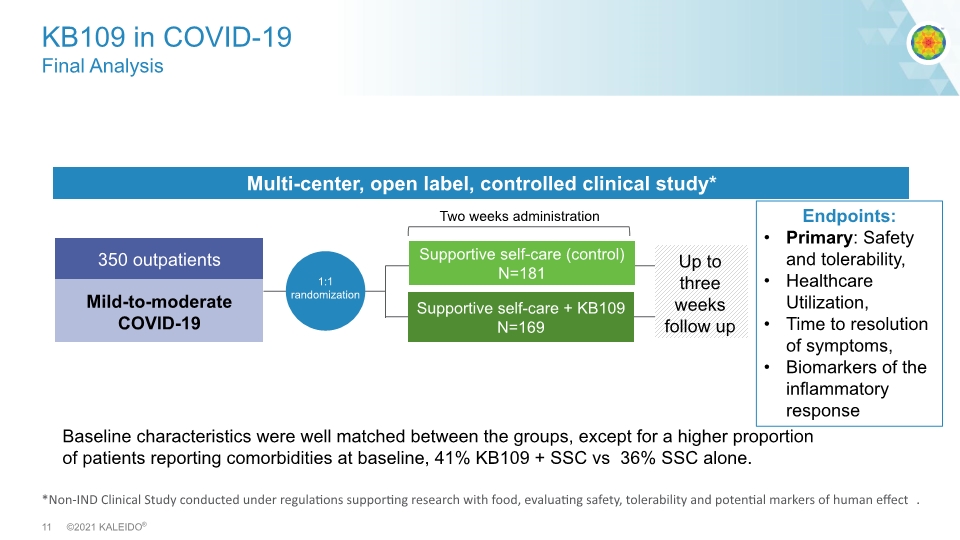
*Non-IND Clinical Study conducted under regulations supporting research with food, evaluating safety, tolerability and potential markers of human effect. KB109 in COVID-19 Final Analysis High propionate High acetate Multi-center, open label, controlled clinical study* Mild-to-moderate COVID-19 Two weeks administration Endpoints: Primary: Safety and tolerability, Healthcare Utilization, Time to resolution of symptoms, Biomarkers of the inflammatory response 350 outpatients Supportive self-care (control) N=181 Supportive self-care + KB109 N=169 Up to three weeks follow up Baseline characteristics were well matched between the groups, except for a higher proportion of patients reporting comorbidities at baseline, 41% KB109 + SSC vs 36% SSC alone.

K031 Study Objectives Primary Objective: Evaluate the safety of KB109 in addition to Supportive Self Care (SSC + KB109) compared to SSC alone in outpatients with mild-to-moderate COVID‑19. Secondary Objective: Evaluate selected measures of health in outpatients with mild-to-moderate COVID-19. Exploratory Objectives: Evaluate measures of health in outpatients with mild-to-moderate COVID-19 during the follow-up period Evaluate the changes in laboratory measures, specific biomarkers, serology and viral load in outpatients with mild-to-moderate COVID-19 (to be available in Q2/Q3 2021)
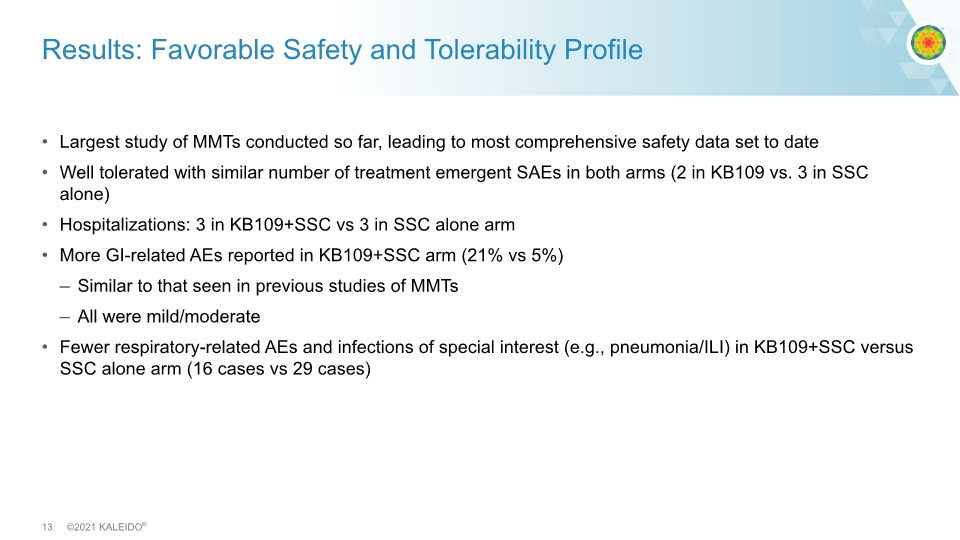
Results: Favorable Safety and Tolerability Profile Largest study of MMTs conducted so far, leading to most comprehensive safety data set to date Well tolerated with similar number of treatment emergent SAEs in both arms (2 in KB109 vs. 3 in SSC alone) Hospitalizations: 3 in KB109+SSC vs 3 in SSC alone arm More GI-related AEs reported in KB109+SSC arm (21% vs 5%) Similar to that seen in previous studies of MMTs All were mild/moderate Fewer respiratory-related AEs and infections of special interest (e.g., pneumonia/ILI) in KB109+SSC versus SSC alone arm (16 cases vs 29 cases)

COVID-19 related Hospitalization, Emergency room, or Urgent Care visits (Safety Analysis Set) KB109 – Healthcare Utilization Data Comorbidity Patient Subgroup Overall Study Population Statistics SSC + KB109 (N=169) SSC alone (N=181) Overall (N=350) % change (SSC + KB109 vs SSC alone) N (%) 7 (4.1%) 15 (8.3%) 22 (6.3%) -50.6% Statistics SSC + KB109 (N=69) SSC alone (N=66) Overall (N=135) % change (SSC + KB109 vs SSC alone) N (%) 4 (5.8%) 10 (15.2%) 14 (10.4%) -61.8%

Natural History of Mild to Moderate COVID-19: Time to Resolution of Symptoms
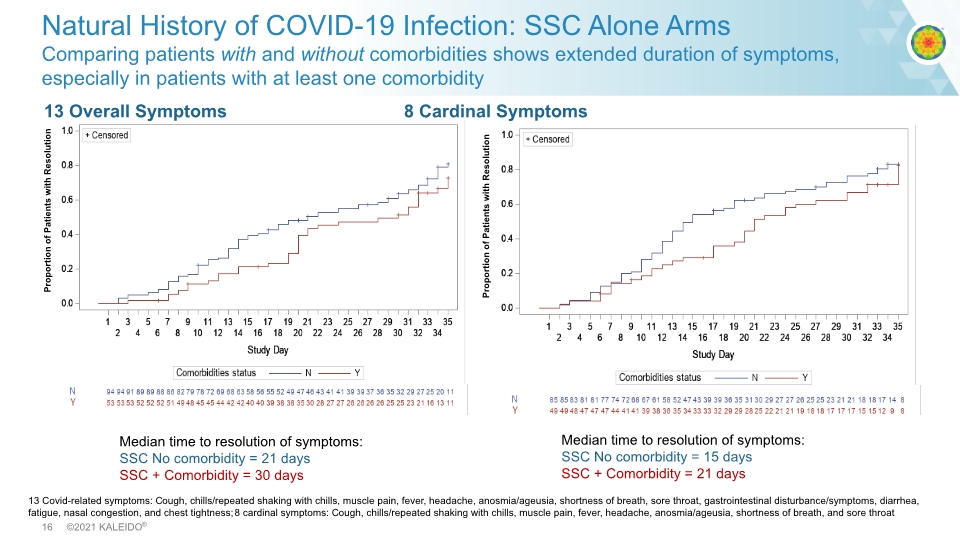
Proportion of Patients with Resolution Natural History of COVID-19 Infection: SSC Alone Arms Comparing patients with and without comorbidities shows extended duration of symptoms, especially in patients with at least one comorbidity 13 Overall Symptoms 8 Cardinal Symptoms Proportion of Patients with Resolution Median time to resolution of symptoms: SSC No comorbidity = 21 days SSC + Comorbidity = 30 days Median time to resolution of symptoms: SSC No comorbidity = 15 days SSC + Comorbidity = 21 days 13 Covid-related symptoms: Cough, chills/repeated shaking with chills, muscle pain, fever, headache, anosmia/ageusia, shortness of breath, sore throat, gastrointestinal disturbance/symptoms, diarrhea, fatigue, nasal congestion, and chest tightness; 8 cardinal symptoms: Cough, chills/repeated shaking with chills, muscle pain, fever, headache, anosmia/ageusia, shortness of breath, and sore throat

Time to Resolution of COVID-19 Related Symptoms
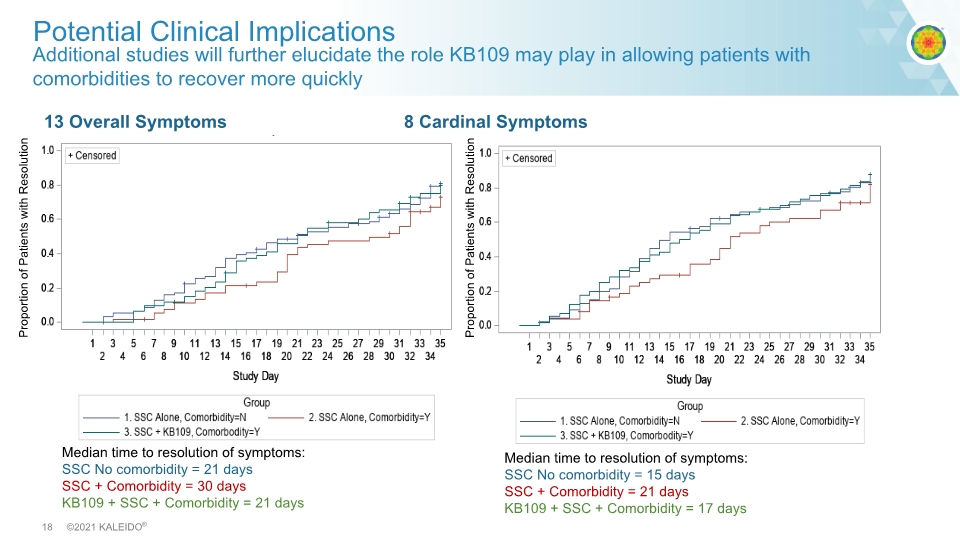
Additional studies will further elucidate the role KB109 may play in allowing patients with comorbidities to recover more quickly Potential Clinical Implications Median time to resolution of symptoms: SSC No comorbidity = 15 days SSC + Comorbidity = 21 days KB109 + SSC + Comorbidity = 17 days Median time to resolution of symptoms: SSC No comorbidity = 21 days SSC + Comorbidity = 30 days KB109 + SSC + Comorbidity = 21 days 13 Overall Symptoms 8 Cardinal Symptoms Proportion of Patients with Resolution Proportion of Patients with Resolution
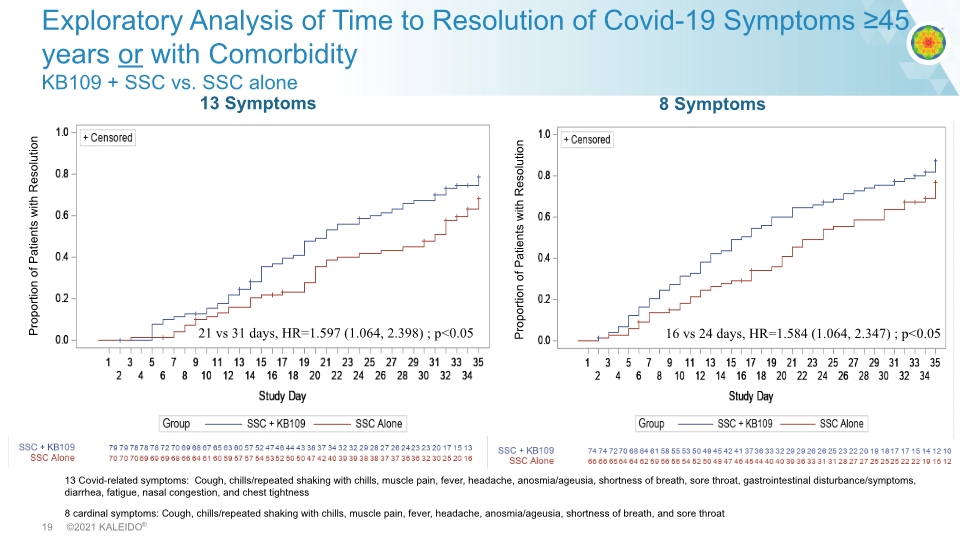
13 Symptoms Exploratory Analysis of Time to Resolution of Covid-19 Symptoms ≥45 years or with Comorbidity KB109 + SSC vs. SSC alone 13 Covid-related symptoms: Cough, chills/repeated shaking with chills, muscle pain, fever, headache, anosmia/ageusia, shortness of breath, sore throat, gastrointestinal disturbance/symptoms, diarrhea, fatigue, nasal congestion, and chest tightness 8 cardinal symptoms: Cough, chills/repeated shaking with chills, muscle pain, fever, headache, anosmia/ageusia, shortness of breath, and sore throat 8 Symptoms 21 vs 31 days, HR=1.597 (1.064, 2.398) ; p<0.05 16 vs 24 days, HR=1.584 (1.064, 2.347) ; p<0.05 Proportion of Patients with Resolution Proportion of Patients with Resolution

Inflammatory Bowel Disease (KB295)
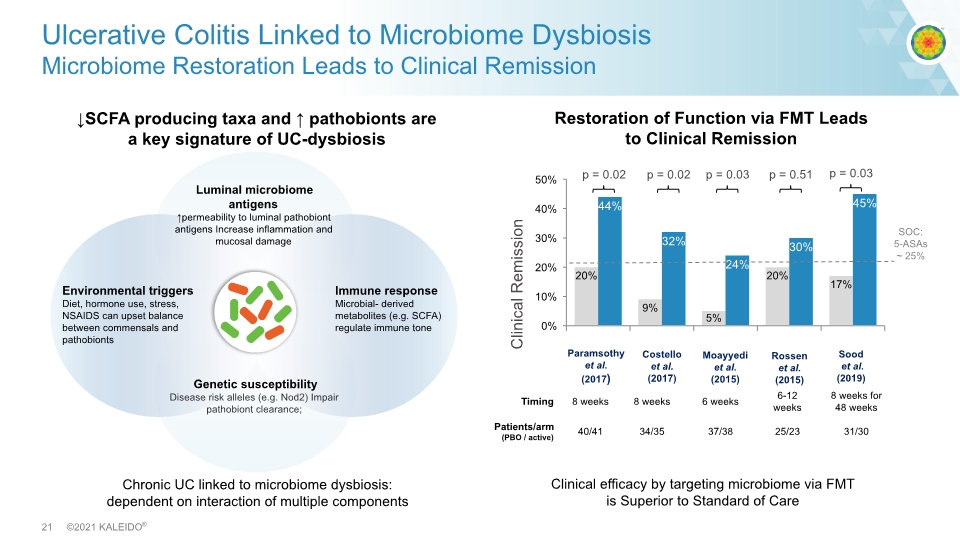
↓SCFA producing taxa and ↑ pathobionts are a key signature of UC-dysbiosis Ulcerative Colitis Linked to Microbiome Dysbiosis Microbiome Restoration Leads to Clinical Remission Clinical efficacy by targeting microbiome via FMT is Superior to Standard of Care p = 0.02 p = 0.02 p = 0.03 p = 0.51 Clinical Remission Paramsothy et al. (2017) Costello et al. (2017) Moayyedi et al. (2015) Rossen et al. (2015) SOC: 5-ASAs ~ 25% Chronic UC linked to microbiome dysbiosis: dependent on interaction of multiple components Sood et al. (2019) p = 0.03 Restoration of Function via FMT Leads to Clinical Remission Luminal microbiome antigens ↑permeability to luminal pathobiont antigens Increase inflammation and mucosal damage Genetic susceptibility Disease risk alleles (e.g. Nod2) Impair pathobiont clearance; Environmental triggers Diet, hormone use, stress, NSAIDS can upset balance between commensals and pathobionts Immune response Microbial- derived metabolites (e.g. SCFA) regulate immune tone Timing 8 weeks 8 weeks 6 weeks 6-12 weeks 8 weeks for 48 weeks Patients/arm (PBO / active) 40/41 34/35 37/38 25/23 31/30
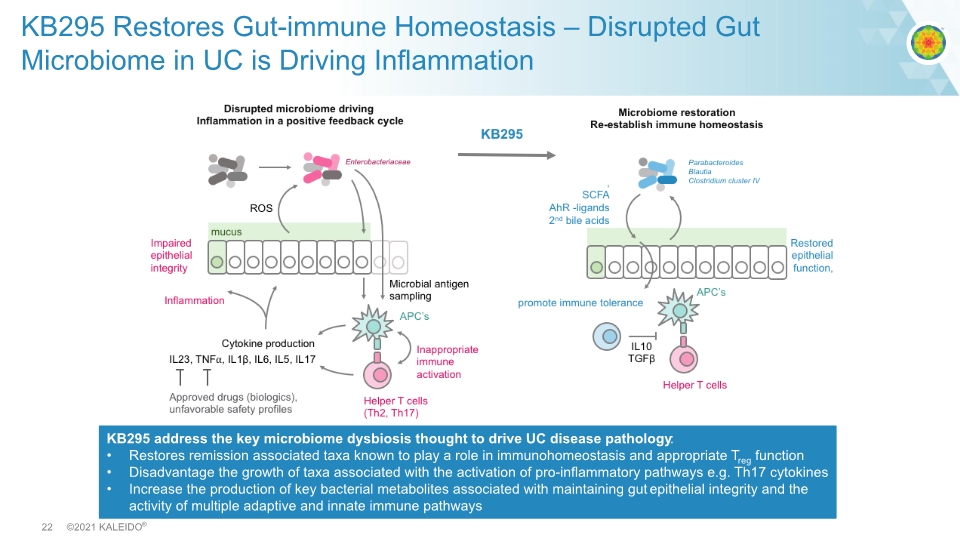
KB295 Restores Gut-immune Homeostasis – Disrupted Gut Microbiome in UC is Driving Inflammation KB295 address the key microbiome dysbiosis thought to drive UC disease pathology: Restores remission associated taxa known to play a role in immunohomeostasis and appropriate Treg function Disadvantage the growth of taxa associated with the activation of pro-inflammatory pathways e.g. Th17 cytokines Increase the production of key bacterial metabolites associated with maintaining gut epithelial integrity and the activity of multiple adaptive and innate immune pathways
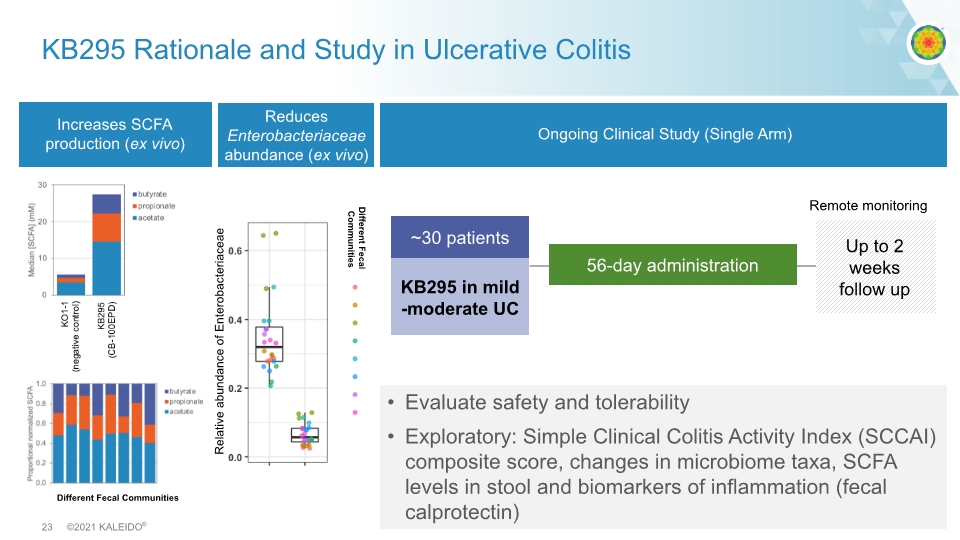
KB295 Rationale and Study in Ulcerative Colitis Increases SCFA production (ex vivo) Ongoing Clinical Study (Single Arm) Evaluate safety and tolerability Exploratory: Simple Clinical Colitis Activity Index (SCCAI) composite score, changes in microbiome taxa, SCFA levels in stool and biomarkers of inflammation (fecal calprotectin) Different Fecal Communities High propionate KB295 in mild-moderate UC ~30 patients 56-day administration Up to 2 weeks follow up Remote monitoring Reduces Enterobacteriaceae abundance (ex vivo) Relative abundance of Enterobacteriaceae
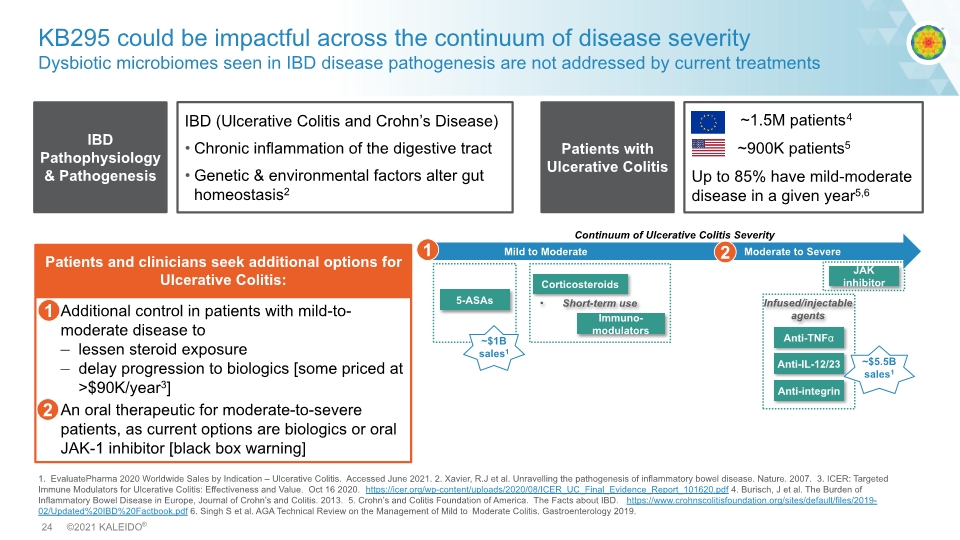
KB295 could be impactful across the continuum of disease severity Dysbiotic microbiomes seen in IBD disease pathogenesis are not addressed by current treatments 1. EvaluatePharma 2020 Worldwide Sales by Indication – Ulcerative Colitis. Accessed June 2021. 2. Xavier, R.J et al. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007. 3. ICER: Targeted Immune Modulators for Ulcerative Colitis: Effectiveness and Value. Oct 16 2020. https://icer.org/wp-content/uploads/2020/08/ICER_UC_Final_Evidence_Report_101620.pdf 4. Burisch, J et al. The Burden of Inflammatory Bowel Disease in Europe, Journal of Crohn’s and Colitis. 2013. 5. Crohn’s and Colitis Foundation of America. The Facts about IBD. https://www.crohnscolitisfoundation.org/sites/default/files/2019-02/Updated%20IBD%20Factbook.pdf 6. Singh S et al. AGA Technical Review on the Management of Mild to Moderate Colitis. Gastroenterology 2019. IBD (Ulcerative Colitis and Crohn’s Disease) Chronic inflammation of the digestive tract Genetic & environmental factors alter gut homeostasis2 IBD Pathophysiology & Pathogenesis ~1.5M patients4 ~900K patients5 Up to 85% have mild-moderate disease in a given year5,6 Patients with Ulcerative Colitis 1 2 Additional control in patients with mild-to-moderate disease to lessen steroid exposure delay progression to biologics [some priced at >$90K/year3] An oral therapeutic for moderate-to-severe patients, as current options are biologics or oral JAK-1 inhibitor [black box warning] Patients and clinicians seek additional options for Ulcerative Colitis: 1 2

Pre-Clinical Programs Intellectual Property Financials

Advancing Our MMTs for Lead Identification Poised to Deliver Multiple Clinical-Ready Programs in 2021 Jeffrey Gordon, MD Glycan chemistry & utilization Gnotobiotic preclinical models combined with computational methods to further identify molecular pathways of how MMTs can modify functional configuration of the gut microbiome and its metabolic outputs Preclinical Program Collaborator Clinical-Ready Lead MMT Expected in 2021 Prevention of infection by multi-drug resistant pathogens in highrisk patients, initially in HSCT Immuno-oncology Cardiometabolic & Liver Diseases Immune-mediated Diseases Prevention of Childhood-Onset Atopic & Immune Conditions
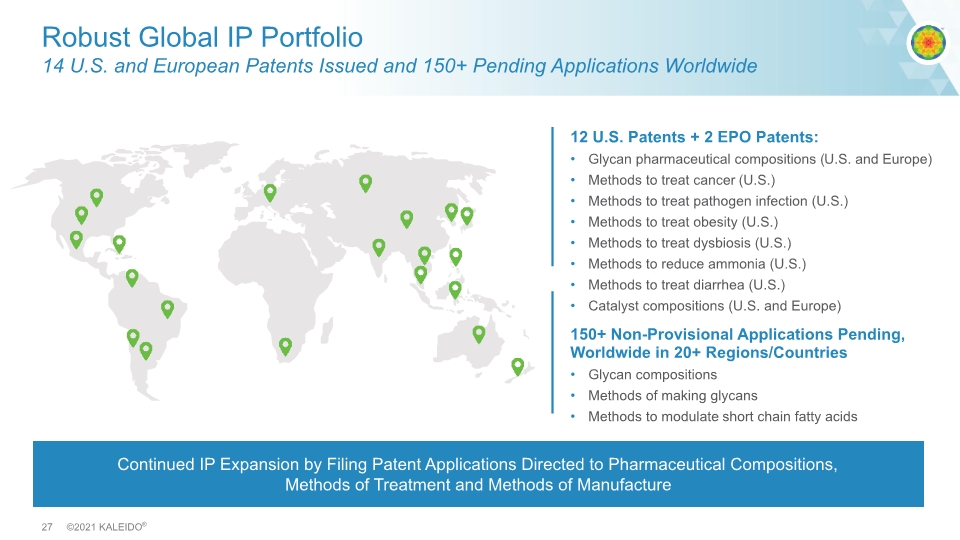
Robust Global IP Portfolio 14 U.S. and European Patents Issued and 150+ Pending Applications Worldwide 12 U.S. Patents + 2 EPO Patents: Glycan pharmaceutical compositions (U.S. and Europe) Methods to treat cancer (U.S.) Methods to treat pathogen infection (U.S.) Methods to treat obesity (U.S.) Methods to treat dysbiosis (U.S.) Methods to reduce ammonia (U.S.) Methods to treat diarrhea (U.S.) Catalyst compositions (U.S. and Europe) 150+ Non-Provisional Applications Pending, Worldwide in 20+ Regions/Countries Glycan compositions Methods of making glycans Methods to modulate short chain fatty acids Continued IP Expansion by Filing Patent Applications Directed to Pharmaceutical Compositions, Methods of Treatment and Methods of Manufacture
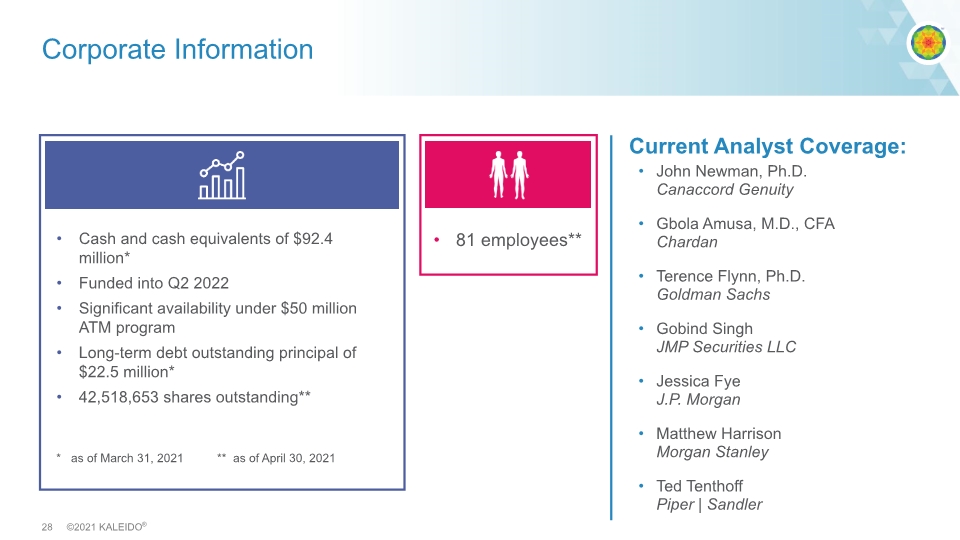
Corporate Information John Newman, Ph.D. Canaccord Genuity Gbola Amusa, M.D., CFA Chardan Terence Flynn, Ph.D. Goldman Sachs Gobind Singh JMP Securities LLC Jessica Fye J.P. Morgan Matthew Harrison Morgan Stanley Ted Tenthoff Piper | Sandler Current Analyst Coverage: Cash and cash equivalents of $92.4 million* Funded into Q2 2022 Significant availability under $50 million ATM program Long-term debt outstanding principal of $22.5 million* 42,518,653 shares outstanding** * as of March 31, 2021 ** as of April 30, 2021 81 employees**
Kaleido in Brief Highly differentiated approach Unique within the microbiome space Highly unique as a therapeutic modality overall Broad set of portfolio assets Platform makes use of compounds generally recognized as safe (GRAS) Ability to execute on near-term milestones Promising safety profile benefits Significantly improves processes for efficiency of clinical and regulatory path Clinical and preclinical programs in diverse areas Potential for future expansion from MMT approach Validation from clinical data in HE program Well-funded through two clinical data readouts in the next year

Appendix: COVID-19 Final Topline Analysis (KB109)
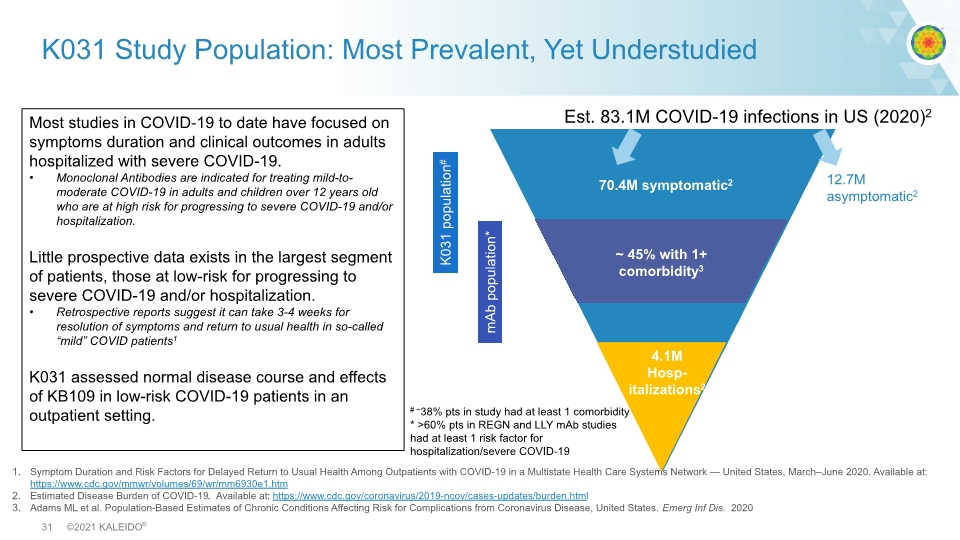
Symptom Duration and Risk Factors for Delayed Return to Usual Health Among Outpatients with COVID-19 in a Multistate Health Care Systems Network — United States, March–June 2020. Available at: https://www.cdc.gov/mmwr/volumes/69/wr/mm6930e1.htm Estimated Disease Burden of COVID-19. Available at: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/burden.html Adams ML et al. Population-Based Estimates of Chronic Conditions Affecting Risk for Complications from Coronavirus Disease, United States. Emerg Inf Dis. 2020 K031 Study Population: Most Prevalent, Yet Understudied Most studies in COVID-19 to date have focused on symptoms duration and clinical outcomes in adults hospitalized with severe COVID-19. Monoclonal Antibodies are indicated for treating mild-to-moderate COVID-19 in adults and children over 12 years old who are at high risk for progressing to severe COVID-19 and/or hospitalization. Little prospective data exists in the largest segment of patients, those at low-risk for progressing to severe COVID-19 and/or hospitalization. Retrospective reports suggest it can take 3-4 weeks for resolution of symptoms and return to usual health in so-called “mild” COVID patients1 K031 assessed normal disease course and effects of KB109 in low-risk COVID-19 patients in an outpatient setting. 70.4M symptomatic2 # ~38% pts in study had at least 1 comorbidity * >60% pts in REGN and LLY mAb studies had at least 1 risk factor for hospitalization/severe COVID-19 12.7M asymptomatic2 4.1M Hosp- italizations2 K031 population# mAb population* ~ 45% with 1+ comorbidity3
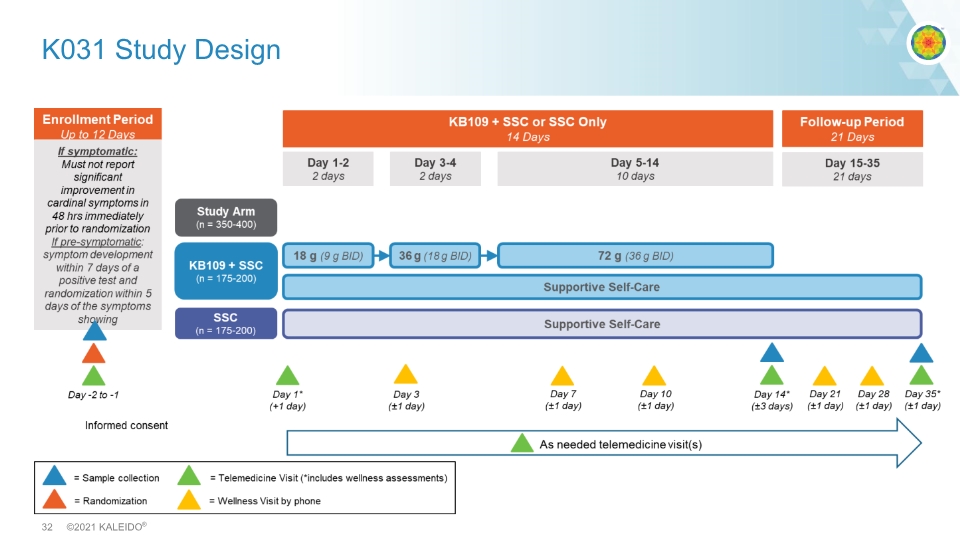
K031 Study Design
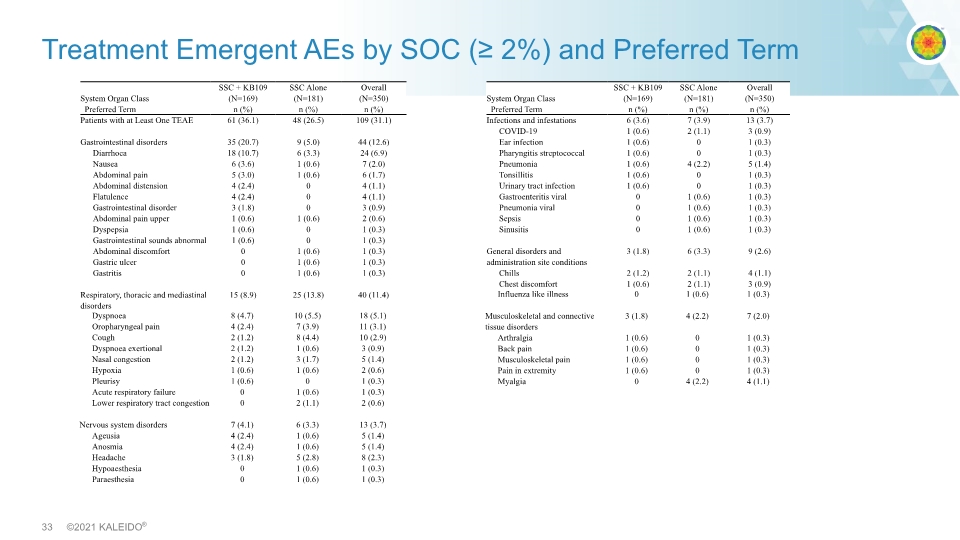
Treatment Emergent AEs by SOC (≥ 2%) and Preferred Term System Organ Class Preferred Term SSC + KB109 (N=169) n (%) SSC Alone (N=181) n (%) Overall (N=350) n (%) Patients with at Least One TEAE 61 (36.1) 48 (26.5) 109 (31.1) Gastrointestinal disorders 35 (20.7) 9 (5.0) 44 (12.6) Diarrhoea 18 (10.7) 6 (3.3) 24 (6.9) Nausea 6 (3.6) 1 (0.6) 7 (2.0) Abdominal pain 5 (3.0) 1 (0.6) 6 (1.7) Abdominal distension 4 (2.4) 0 4 (1.1) Flatulence 4 (2.4) 0 4 (1.1) Gastrointestinal disorder 3 (1.8) 0 3 (0.9) Abdominal pain upper 1 (0.6) 1 (0.6) 2 (0.6) Dyspepsia 1 (0.6) 0 1 (0.3) Gastrointestinal sounds abnormal 1 (0.6) 0 1 (0.3) Abdominal discomfort 0 1 (0.6) 1 (0.3) Gastric ulcer 0 1 (0.6) 1 (0.3) Gastritis 0 1 (0.6) 1 (0.3) Respiratory, thoracic and mediastinal disorders 15 (8.9) 25 (13.8) 40 (11.4) Dyspnoea 8 (4.7) 10 (5.5) 18 (5.1) Oropharyngeal pain 4 (2.4) 7 (3.9) 11 (3.1) Cough 2 (1.2) 8 (4.4) 10 (2.9) Dyspnoea exertional 2 (1.2) 1 (0.6) 3 (0.9) Nasal congestion 2 (1.2) 3 (1.7)5 (1.4) Hypoxia 1 (0.6) 1 (0.6) 2 (0.6) Pleurisy 1 (0.6) 0 1 (0.3) Acute respiratory failure 0 1 (0.6) 1 (0.3) Lower respiratory tract congestion 0 2 (1.1) 2 (0.6) Nervous system disorders 7 (4.1) 6 (3.3) 13 (3.7) Ageusia 4 (2.4) 1 (0.6) 5 (1.4) Anosmia 4 (2.4) 1 (0.6) 5 (1.4) Headache 3 (1.8) 5 (2.8) 8 (2.3) Hypoaesthesia 0 1 (0.6) 1 (0.3) Paraesthesia 01 (0.6) 1 (0.3) System Organ Class Preferred Term SSC + KB109 (N=169) n (%) SSC Alone (N=181) n (%) Overall (N=350) n (%) Infections and infestations 6 (3.6) 7 (3.9) 13 (3.7) COVID-19 1 (0.6) 2 (1.1)3 (0.9) Ear infection 1 (0.6) 01 (0.3) Pharyngitis streptococcal 1 (0.6) 01 (0.3) Pneumonia 1 (0.6) 4 (2.2) 5 (1.4) Tonsillitis 1 (0.6) 0 1 (0.3) Urinary tract infection 1 (0.6) 0 1 (0.3) Gastroenteritis viral 0 1 (0.6) 1 (0.3) Pneumonia viral 0 1 (0.6) 1 (0.3) Sepsis 0 1 (0.6) 1 (0.3) Sinusitis 0 1 (0.6) 1 (0.3) General disorders and administration site conditions 3 (1.8) 6 (3.3) 9 (2.6) Chills 2 (1.2) 2 (1.1) 4 (1.1) Chest discomfort 1 (0.6) 2 (1.1) 3 (0.9) Fatigue 1 (0.6) 2 (1.1) 3 (0.9) Influenza like illness 0 1 (0.6) 1 (0.3) Musculoskeletal and connective tissue disorders 3 (1.8) 4 (2.2) 7 (2.0) Arthralgia 1 (0.6) 0 1 (0.3) Back pain 1 (0.6) 0 1 (0.3) Musculoskeletal pain 1 (0.6) 0 1 (0.3) Pain in extremity 1 (0.6) 0 1 (0.3) Myalgia 0 4 (2.2) 4 (1.1)
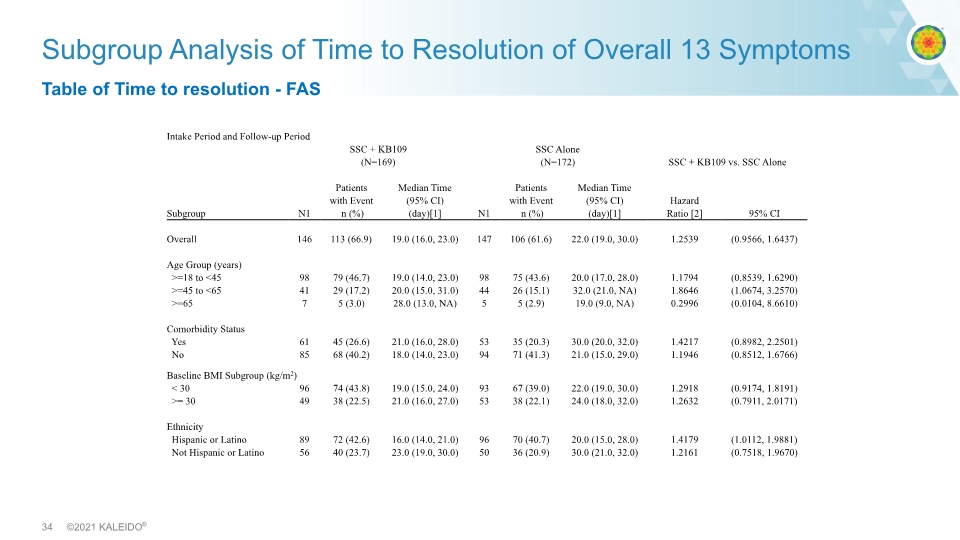
Subgroup Analysis of Time to Resolution of Overall 13 Symptoms Table of Time to resolution - FAS Intake Period and Follow-up Period SSC + KB109 (N=169) SSC Alone (N=172) SSC + KB109 vs. SSC Alone Subgroup N1 Patients with Event n (%) Median Time (95% CI) (day)[1] N1 Patients with Event n (%) Median Time (95% CI) (day)[1] Hazard Ratio [2] 95% CI Overall 146 113 (66.9) 19.0 (16.0, 23.0) 147 106 (61.6) 22.0 (19.0, 30.0) 1.2539 (0.9566, 1.6437) Age Group (years) >=18 to <45 98 79 (46.7) 19.0 (14.0, 23.0) 98 75 (43.6) 20.0 (17.0, 28.0) 1.1794 (0.8539, 1.6290) >=45 to <65 41 29 (17.2) 20.0 (15.0, 31.0) 44 26 (15.1) 32.0 (21.0, NA) 1.8646 (1.0674, 3.2570) >=65 7 5 (3.0) 28.0 (13.0, NA) 5 5 (2.9) 19.0 (9.0, NA) 0.2996 (0.0104, 8.6610) Comorbidity Status Yes 61 45 (26.6) 21.0 (16.0, 28.0) 53 35 (20.3) 30.0 (20.0, 32.0) 1.4217 (0.8982, 2.2501) No 85 68 (40.2) 18.0 (14.0, 23.0) 94 71 (41.3) 21.0 (15.0, 29.0) 1.1946 (0.8512, 1.6766) Baseline BMI Subgroup (kg/m2) < 30 96 74 (43.8) 19.0 (15.0, 24.0) 93 67 (39.0) 22.0 (19.0, 30.0) 1.2918 (0.9174, 1.8191) >= 30 49 38 (22.5) 21.0 (16.0, 27.0) 53 38 (22.1) 24.0 (18.0, 32.0) 1.2632 (0.7911, 2.0171) Ethnicity Hispanic or Latino 89 72 (42.6) 16.0 (14.0, 21.0) 96 70 (40.7) 20.0 (15.0, 28.0) 1.4179 (1.0112, 1.9881) Not Hispanic or Latino 56 40 (23.7) 23.0 (19.0, 30.0) 50 36 (20.9) 30.0 (21.0, 32.0) 1.2161 (0.7518, 1.9670)
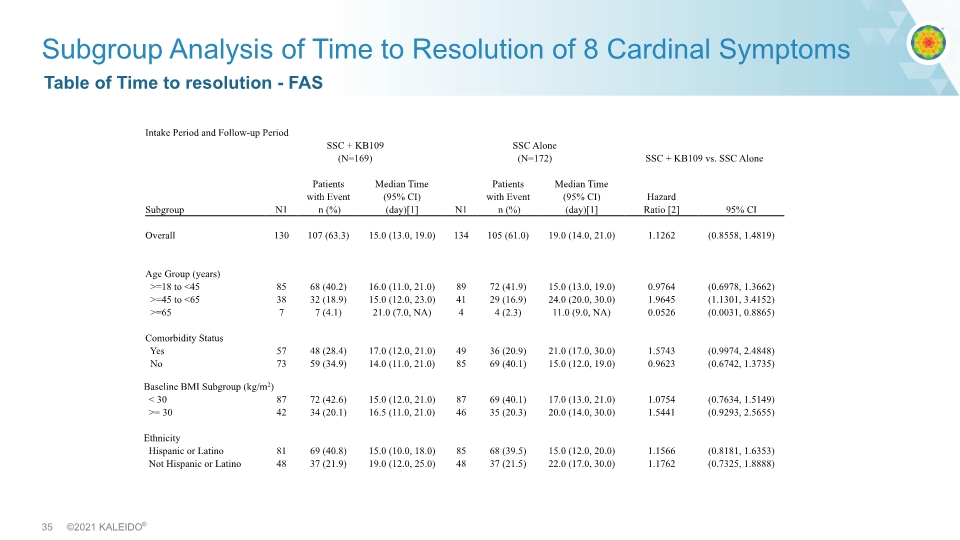
Table of Time to resolution - FAS Subgroup Analysis of Time to Resolution of 8 Cardinal Symptoms Intake Period and Follow-up Period SSC + KB109 (N=169) SSC Alone (N=172) SSC + KB109 vs. SSC Alone Subgroup N1 Patients with Event n (%) Median Time (95% CI) (day)[1] N1 Patients with Event n (%) Median Time (95% CI) (day)[1] Hazard Ratio [2] 95% CI Overall 130 107 (63.3) 15.0 (13.0, 19.0) 134 105 (61.0) 19.0 (14.0, 21.0) 1.1262 (0.8558, 1.4819) Age Group (years) >=18 to <45 85 68 (40.2) 16.0 (11.0, 21.0) 89 72 (41.9) 15.0 (13.0, 19.0) 0.9764 (0.6978, 1.3662) >=45 to <65 38 32 (18.9) 15.0 (12.0, 23.0) 41 29 (16.9) 24.0 (20.0, 30.0) 1.9645 (1.1301, 3.4152) >=65 7 7 (4.1) 21.0 (7.0, NA) 4 4 (2.3) 11.0 (9.0, NA) 0.0526 (0.0031, 0.8865) Comorbidity Status Yes 57 48 (28.4) 17.0 (12.0, 21.0) 49 36 (20.9) 21.0 (17.0, 30.0) 1.5743 (0.9974, 2.4848) No 73 59 (34.9) 14.0 (11.0, 21.0) 85 69 (40.1) 15.0 (12.0, 19.0) 0.9623 (0.6742, 1.3735) Baseline BMI Subgroup (kg/m2) < 30 87 72 (42.6) 15.0 (12.0, 21.0) 87 69 (40.1) 17.0 (13.0, 21.0) 1.0754 (0.7634, 1.5149) >= 30 42 34 (20.1) 16.5 (11.0, 21.0) 46 35 (20.3) 20.0 (14.0, 30.0) 1.5441 (0.9293, 2.5655) Ethnicity Hispanic or Latino 81 69 (40.8) 15.0 (10.0, 18.0) 85 68 (39.5) 15.0 (12.0, 20.0) 1.1566 (0.8181, 1.6353) Not Hispanic or Latino 48 37 (21.9) 19.0 (12.0, 25.0) 48 37 (21.5) 22.0 (17.0, 30.0) 1.1762 (0.7325, 1.8888)
Recent Antibody Data Provides Useful Context for K031 Data from broad population in the mAb studies did not show strong results for symptoms or outcomes, leading to conditions of FDA EUA requiring a population of patients with higher risk; comorbidities and advanced age: High risk is defined as patients who meet at least one of the following criteria: Have a body mass index (BMI) ≥35, chronic kidney disease, diabetes, immunosuppressive disease Currently receiving immunosuppressive treatment ≥65 years of age ≥55 years of age AND have CV disease, OR hypertension, OR COPD/chronic respiratory disease FDA Letter Issued February 25, 2021 [Lilly, Regeneron] will establish a process for monitoring genomic database(s) for the emergence of global viral variants of SARS-CoV-2. FDA may require [Lilly, Regeneron] to assess the activity of the authorized [mAb] against any global SARS-CoV-2 variant(s) of interest (e.g., variants that are prevalent or becoming prevalent that harbor substitutions in the target protein or in protein(s) that interact with the target protein). Age >45 was added as a risk factor to the analyses for K031, a precedent established in studies of mAbs to narrow population based on comorbidity and age
Ulcerative Colitis Prevalence and Unmet Need

Leading a Novel Approach to Targeting the Microbiome NASDAQ: KLDO June 2021





































