
Company Overview AUGUST 2019 | ⓒ 2019 KALEIDO™

Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, without limitation, statements regarding the anticipated use of our existing cash resources, including proceeds from our initial public offering, the duration for which our existing capital resources will fund our operations, statements regarding the therapeutic potential of our Microbiome Metabolic Therapy (MMT) candidates and our strategy, business plans and focus. The words “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Any forward-looking statements in this presentation are based on management’s current expectations and beliefs and are subject to a number of risks, uncertainties and important factors that may cause actual events or results to differ materially from those expressed or implied by any forward-looking statements contained in this presentation, including, without limitation, those related to the planned Phase 2 clinical trial for KB195 for the treatment of UCD, including timeline for initiation, completion and reporting of results, the preclinical and clinical development and safety profile of our MMT candidates and timelines associated with the programs for such MMT candidates, whether and when, if at all, our MMT candidates will receive approval from the U.S. Food and Drug Administration or other applicable regulatory agencies, if any, competition from other biotechnology companies, and other risks identified in our SEC filings, including our final prospectus for our initial public offering, and subsequent filings with the SEC. We caution you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. We disclaim any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements. Any forward-looking statements contained in this presentation represent our views only as of the date hereof and should not be relied upon as representing its views as of any subsequent date. We explicitly disclaim any obligation to update any forward-looking statements. 2 | ⓒ 2019 KALEIDO™

The 1st Company to Use a Chemistry-Driven Approach to Systematically DRIVE FUNCTIONAL OUTPUTS OF THE MICROBIOME ORGAN Leveraging Human-Centric Discovery and Development to Rapidly Translate the Promise of the Microbiome Into Solutions for Patients 3 | ⓒ 2019 KALEIDO™
The Kaleido Opportunity Broad Pipeline Human-Centric Proprietary Faster, More Cost with Large World-Class Discovery & Product Platform Efficient Model Market Leadership Team Development Opportunity Significant Computational Biology and Data Science Capabilities Enable Kaleido to Learn MORE, and Learn FASTER 4 | ⓒ 2019 KALEIDO™
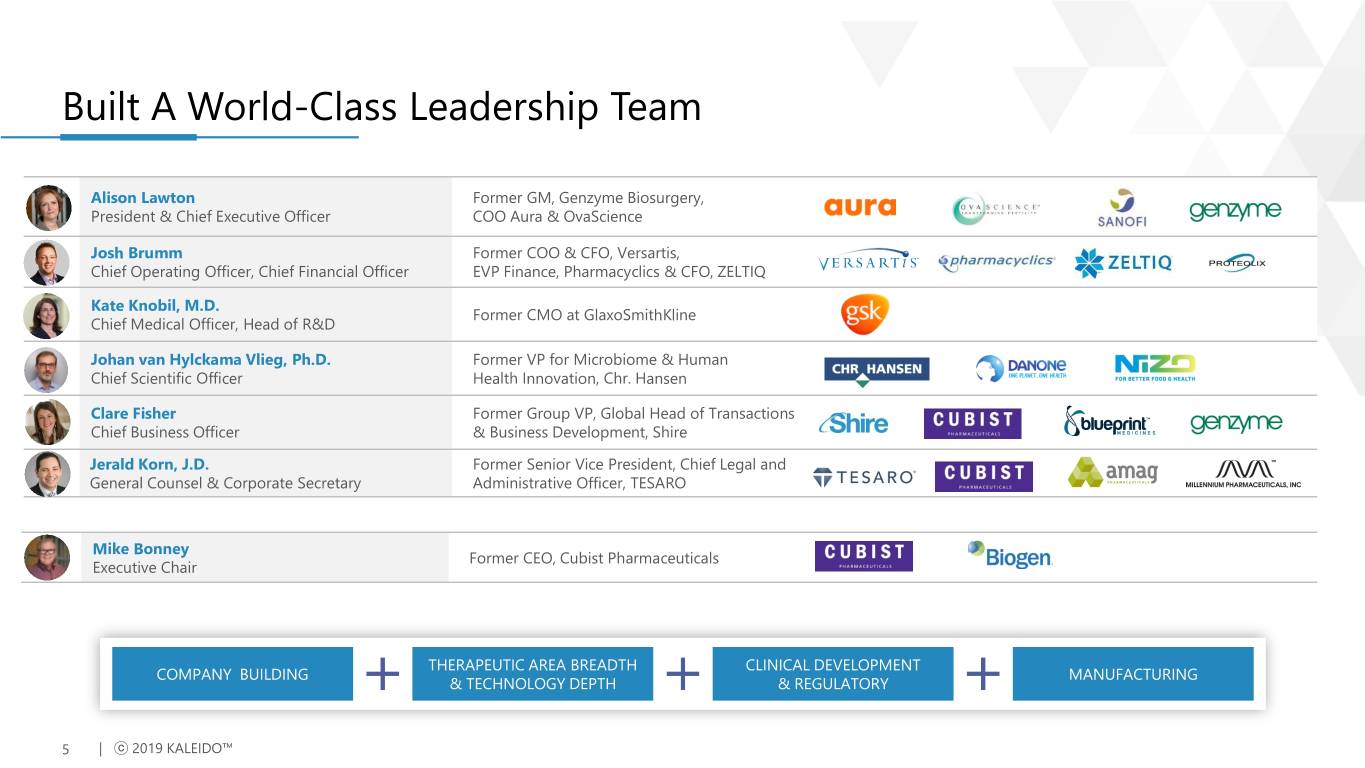
Built A World-Class Leadership Team Alison Lawton Former GM, Genzyme Biosurgery, President & Chief Executive Officer COO Aura & OvaScience Josh Brumm Former COO & CFO, Versartis, Chief Operating Officer, Chief Financial Officer EVP Finance, Pharmacyclics & CFO, ZELTIQ Kate Knobil, M.D. Former CMO at GlaxoSmithKline Chief Medical Officer, Head of R&D Johan van Hylckama Vlieg, Ph.D. Former VP for Microbiome & Human Chief Scientific Officer Health Innovation, Chr. Hansen Clare Fisher Former Group VP, Global Head of Transactions Chief Business Officer & Business Development, Shire Jerald Korn, J.D. Former Senior Vice President, Chief Legal and General Counsel & Corporate Secretary Administrative Officer, TESARO Mike Bonney Former CEO, Cubist Pharmaceuticals Executive Chair THERAPEUTIC AREA BREADTH CLINICAL DEVELOPMENT COMPANY BUILDING MANUFACTURING & TECHNOLOGY DEPTH & REGULATORY 5 | ⓒ 2019 KALEIDO™

Significant Accomplishments Since Founding IND Cleared, Phase 2 clinical trial initiated 12 non-IND human clinical studies conducted with our Microbiome Metabolic Therapy (MMT™) candidates (7 studies completed and 5 in progress) 1,000+ MMT candidates in our library today 5 current pipeline programs in multiple disease areas 100+ non-provisional applications world-wide in 20+ regions/countries, 8 US patents, 2 EPO patents $75M IPO in February 2019 Founded in 2015 to Create a New Category of Drug and Health Products 6 | ⓒ 2019 KALEIDO™
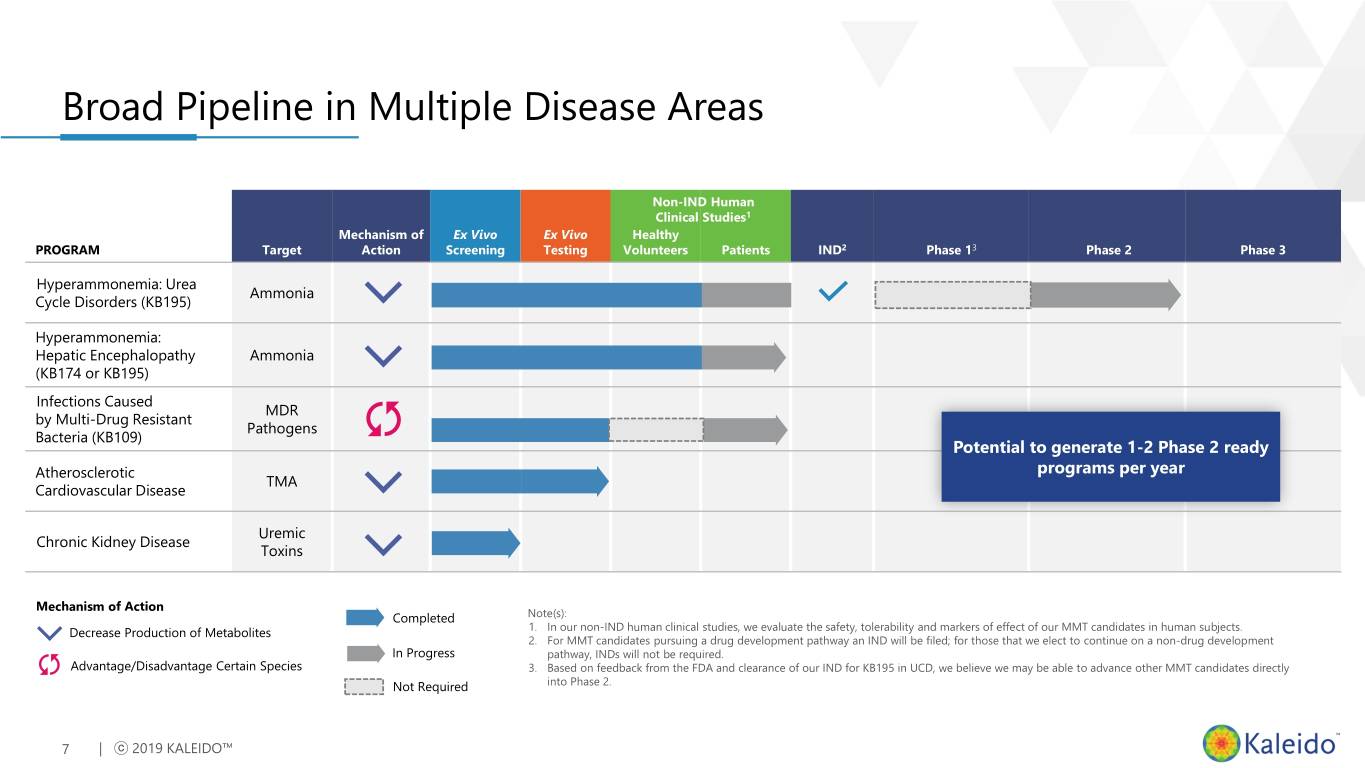
Broad Pipeline in Multiple Disease Areas Non-IND Human Clinical Studies1 Mechanism of Ex Vivo Ex Vivo Healthy PROGRAM Target Action Screening Testing Volunteers Patients IND2 Phase 13 Phase 2 Phase 3 Hyperammonemia: Urea Ammonia Cycle Disorders (KB195) Hyperammonemia: Hepatic Encephalopathy Ammonia (KB174 or KB195) Infections Caused MDR by Multi-Drug Resistant Pathogens Bacteria (KB109) Potential to generate 1-2 Phase 2 ready Atherosclerotic programs per year TMA Cardiovascular Disease Uremic Chronic Kidney Disease Toxins Mechanism of Action Completed Note(s): Decrease Production of Metabolites 1. In our non-IND human clinical studies, we evaluate the safety, tolerability and markers of effect of our MMT candidates in human subjects. 2. For MMT candidates pursuing a drug development pathway an IND will be filed; for those that we elect to continue on a non-drug development In Progress pathway, INDs will not be required. Advantage/Disadvantage Certain Species 3. Based on feedback from the FDA and clearance of our IND for KB195 in UCD, we believe we may be able to advance other MMT candidates directly Not Required into Phase 2. 7 | ⓒ 2019 KALEIDO™
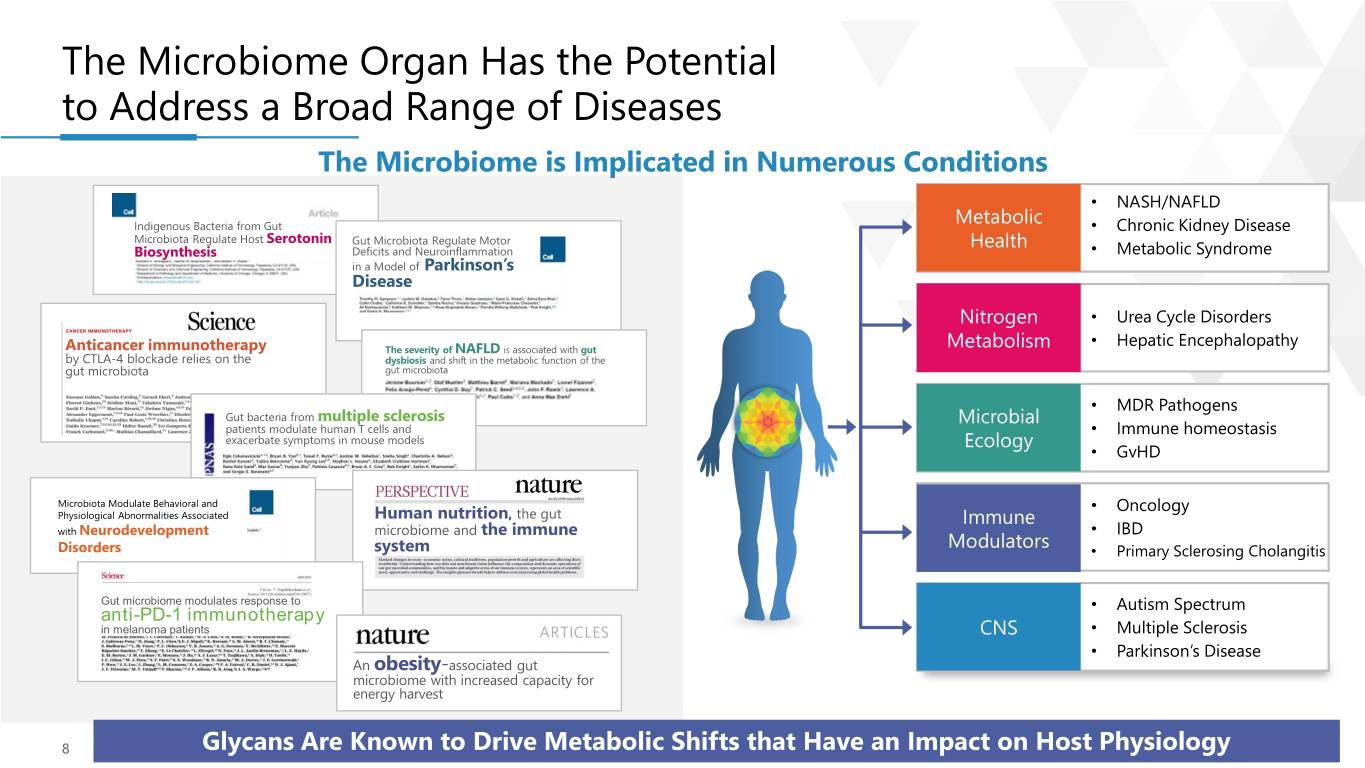
The Microbiome Organ Has the Potential to Address a Broad Range of Diseases The Microbiome is Implicated in Numerous Conditions • NASH/NAFLD Metabolic Indigenous Bacteria from Gut • Chronic Kidney Disease Microbiota Regulate Host Serotonin Gut Microbiota Regulate Motor Health Biosynthesis Deficits and Neuroinflammation • Metabolic Syndrome in a Model of Parkinson’s Disease Nitrogen • Urea Cycle Disorders • Hepatic Encephalopathy Anticancer immunotherapy The severity of NAFLD is associated with gut Metabolism by CTLA-4 blockade relies on the dysbiosis and shift in the metabolic function of the gut microbiota gut microbiota • MDR Pathogens Gut bacteria from multiple sclerosis Microbial patients modulate human T cells and • Immune homeostasis exacerbate symptoms in mouse models Ecology • GvHD Microbiota Modulate Behavioral and • Oncology Physiological Abnormalities Associated Human nutrition, the gut Immune with Neurodevelopment microbiome and the immune • IBD Disorders system Modulators • Primary Sclerosing Cholangitis Gut microbiome modulates response to • Autism Spectrum anti-PD-1 immunotherapy in melanoma patients CNS • Multiple Sclerosis • Parkinson’s Disease An obesity-associated gut microbiome with increased capacity for energy harvest 8 | ⓒ 2019 KALEIDO™Glycans Are Known to Drive Metabolic Shifts that Have an Impact on Host Physiology

We’re Leveraging 10 Years of Scientific Insights and Knowledge Growing Body of Research Powerful Enabling Tools Are Available $115M Human Microbiome Project launched More microbes than 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 human cells NUMBER Microbiome OF PAPERS Koch’s postulates structure revealed Discovery • Computational Capabilities • Shallow Shotgun Sequencing of microbes • Automation & Miniaturization • 16S RNA Sequencing 1890 1980s 1990s 2000s 2010s • Rapid, human-relevant screening • Metabolomics Now Is the Time to Develop Targeted Therapies for the Microbiome Organ 9 | ⓒ 2019 KALEIDO™
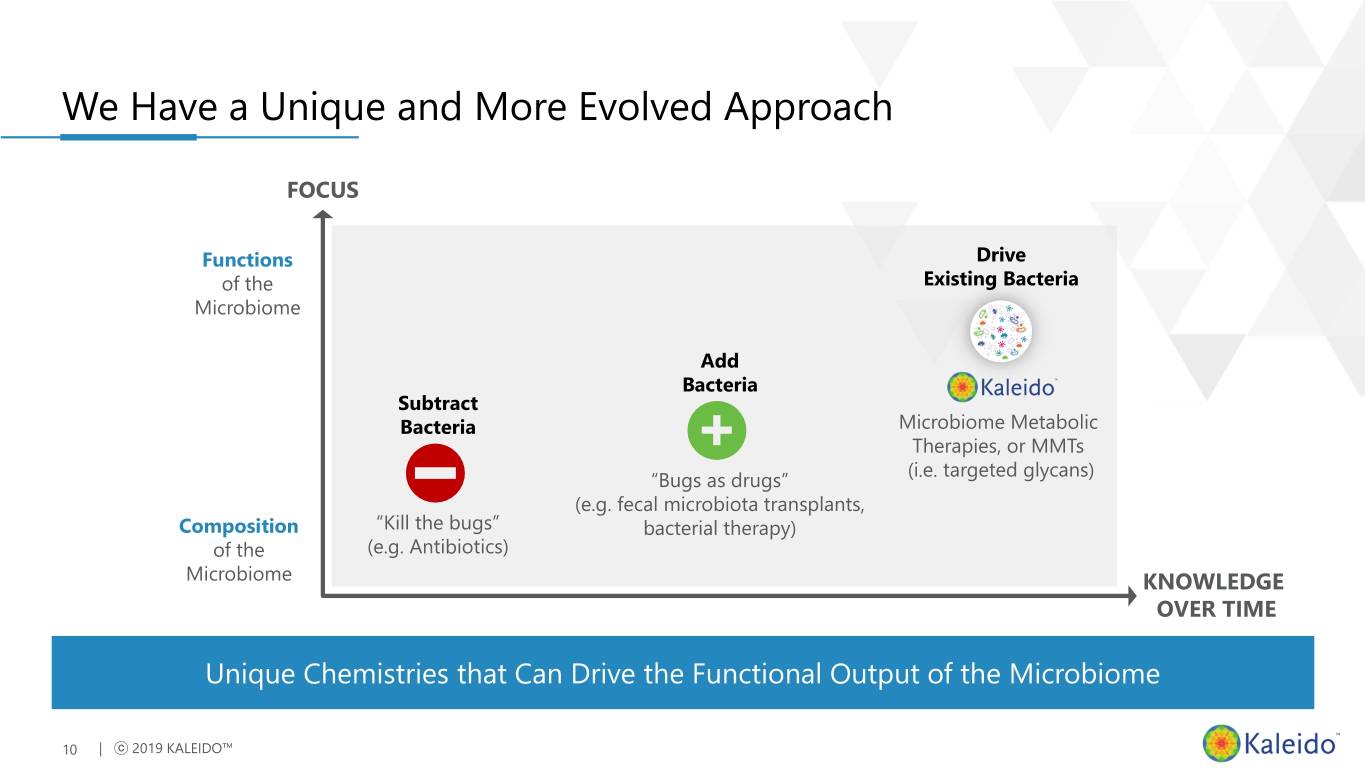
We Have a Unique and More Evolved Approach FOCUS Functions Drive of the Existing Bacteria Microbiome Add Bacteria Subtract Bacteria Microbiome Metabolic Therapies, or MMTs (i.e. targeted glycans) “Bugs as drugs” (e.g. fecal microbiota transplants, Composition “Kill the bugs” bacterial therapy) of the (e.g. Antibiotics) Microbiome KNOWLEDGE OVER TIME Unique Chemistries that Can Drive the Functional Output of the Microbiome 10 | ⓒ 2019 KALEIDO™
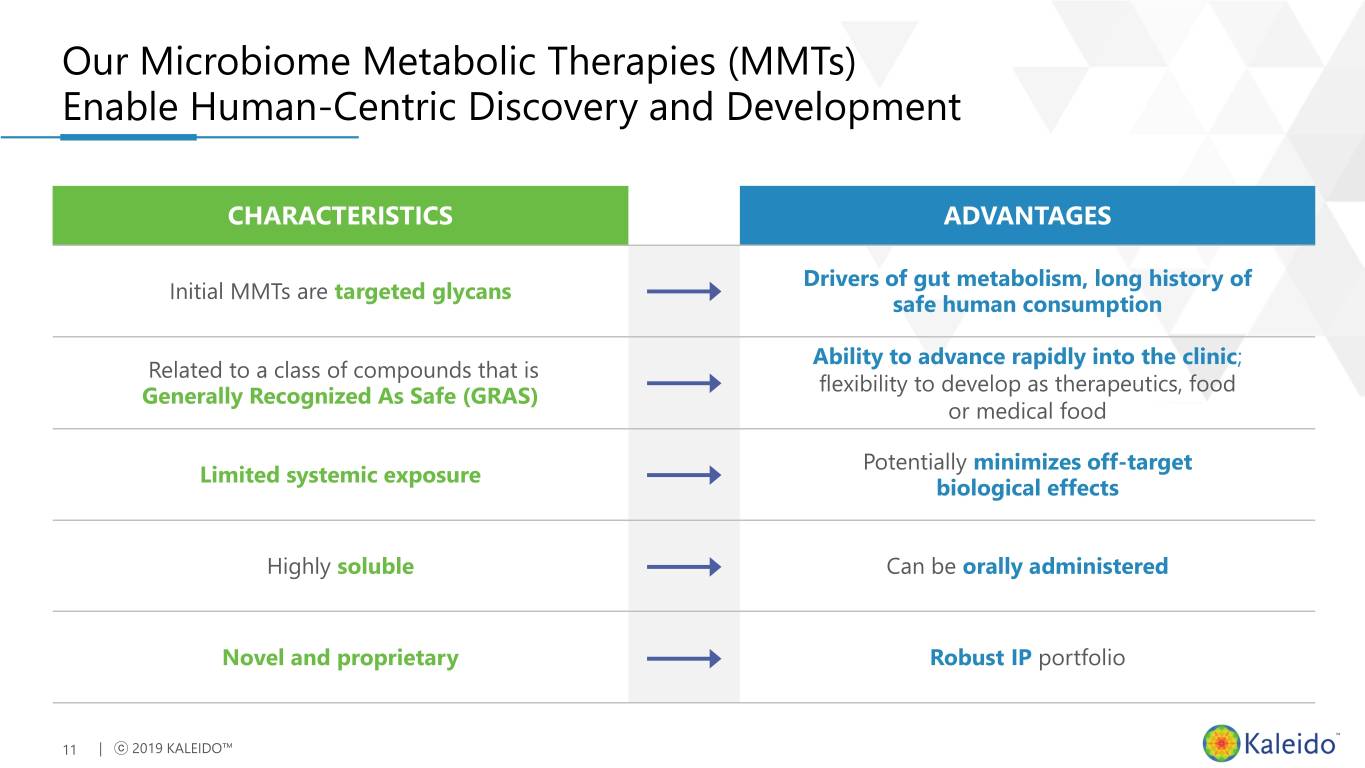
Our Microbiome Metabolic Therapies (MMTs) Enable Human-Centric Discovery and Development CHARACTERISTICS ADVANTAGES Drivers of gut metabolism, long history of Initial MMTs are targeted glycans safe human consumption Ability to advance rapidly into the clinic; Related to a class of compounds that is flexibility to develop as therapeutics, food Generally Recognized As Safe (GRAS) or medical food Potentially minimizes off-target Limited systemic exposure biological effects Highly soluble Can be orally administered Novel and proprietary Robust IP portfolio 11 | ⓒ 2019 KALEIDO™
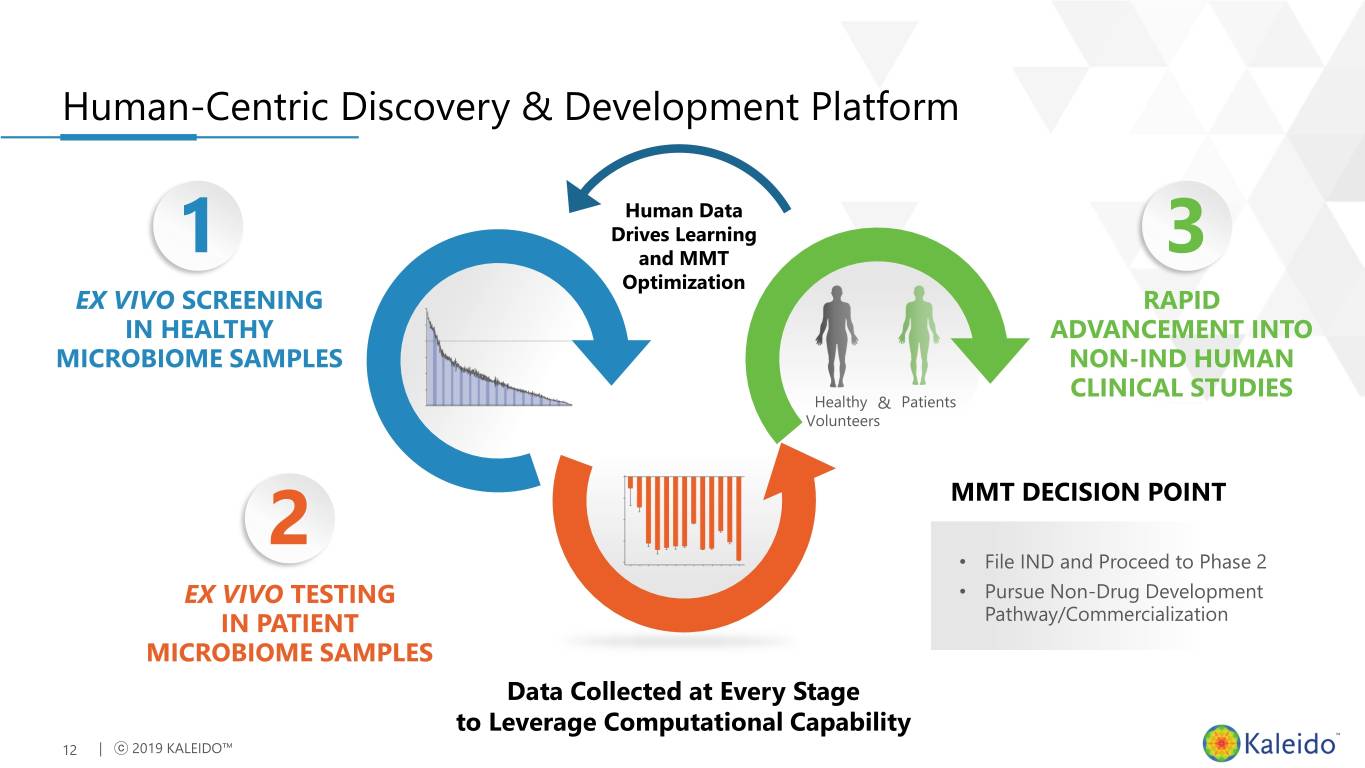
Human-Centric Discovery & Development Platform Human Data Drives Learning 1 and MMT 3 Optimization EX VIVO SCREENING RAPID IN HEALTHY ADVANCEMENT INTO MICROBIOME SAMPLES NON-IND HUMAN CLINICAL STUDIES Healthy & Patients Volunteers 2 MMT DECISION POINT • File IND and Proceed to Phase 2 EX VIVO TESTING • Pursue Non-Drug Development IN PATIENT Pathway/Commercialization MICROBIOME SAMPLES Data Collected at Every Stage to Leverage Computational Capability 12 | ⓒ 2019 KALEIDO™
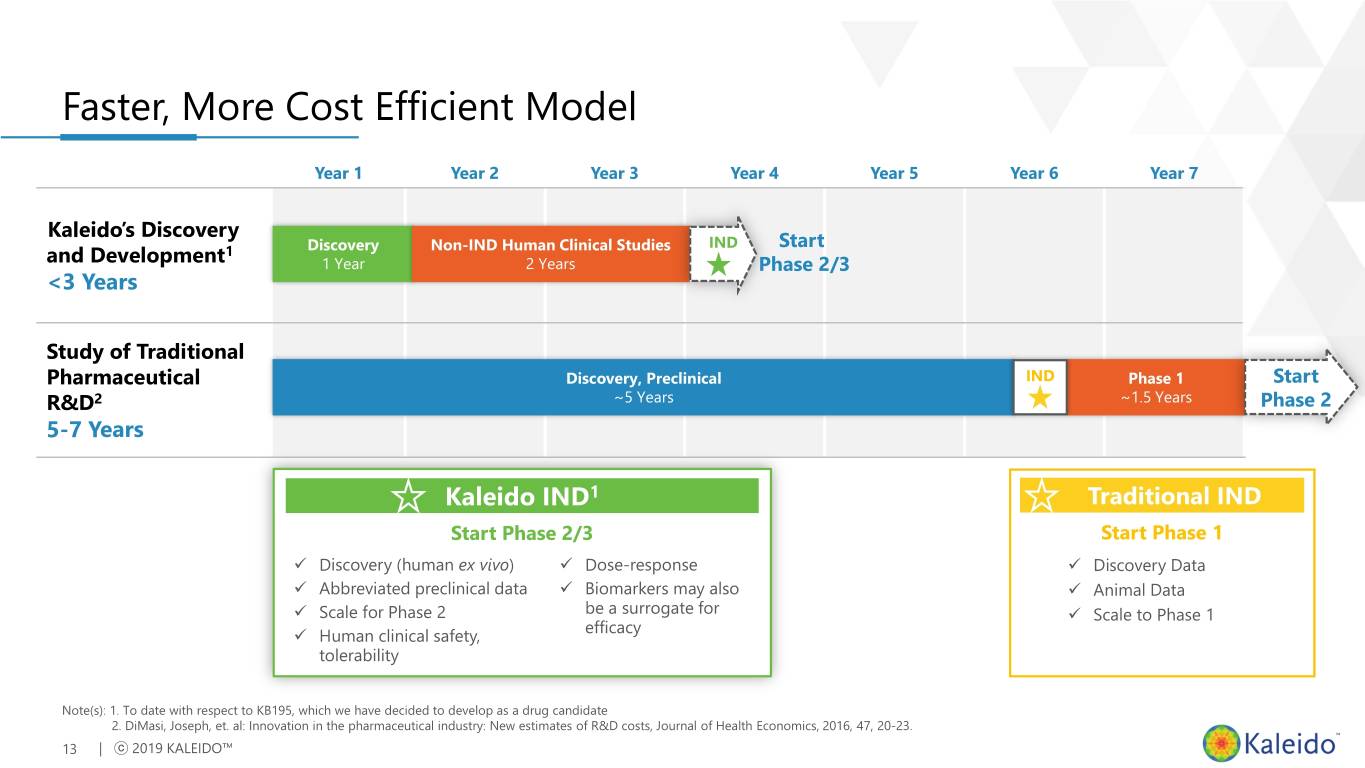
Faster, More Cost Efficient Model Year 1 Year 2 Year 3 Year 4 Year 5 Year 6 Year 7 Kaleido’s Discovery IND Start 1 Discovery Non-IND Human Clinical Studies and Development 1 Year 2 Years Phase 2/3 <3 Years Study of Traditional Pharmaceutical Discovery, Preclinical IND Phase 1 Start R&D2 ~5 Years ~1.5 Years Phase 2 5-7 Years Kaleido IND1 Traditional IND Start Phase 2/3 Start Phase 1 ✓ Discovery (human ex vivo) ✓ Dose-response ✓ Discovery Data ✓ Abbreviated preclinical data ✓ Biomarkers may also ✓ Animal Data ✓ Scale for Phase 2 be a surrogate for ✓ Scale to Phase 1 efficacy ✓ Human clinical safety, tolerability Note(s): 1. To date with respect to KB195, which we have decided to develop as a drug candidate 2. DiMasi, Joseph, et. al: Innovation in the pharmaceutical industry: New estimates of R&D costs, Journal of Health Economics, 2016, 47, 20-23. 13 | ⓒ 2019 KALEIDO™

MMTs Work Through One or More Mechanisms of Action DECREASE ADVANTAGE or DISADVANTAGE Production of Metabolites Certain Species in the Microbiome Community Ammonia MDR Pathogens Hyperammonemia: Urea Cycle Disorders Liver Transplant Patients Hepatic Encephalopathy Hematopoietic Stem Cell Transplantation Patients TMA Atherosclerotic Cardiovascular Disease Uremic Toxins INCREASE Chronic Kidney Disease Production of Metabolites Short Chain Fatty Acids 14 | ⓒ 2019 KALEIDO™
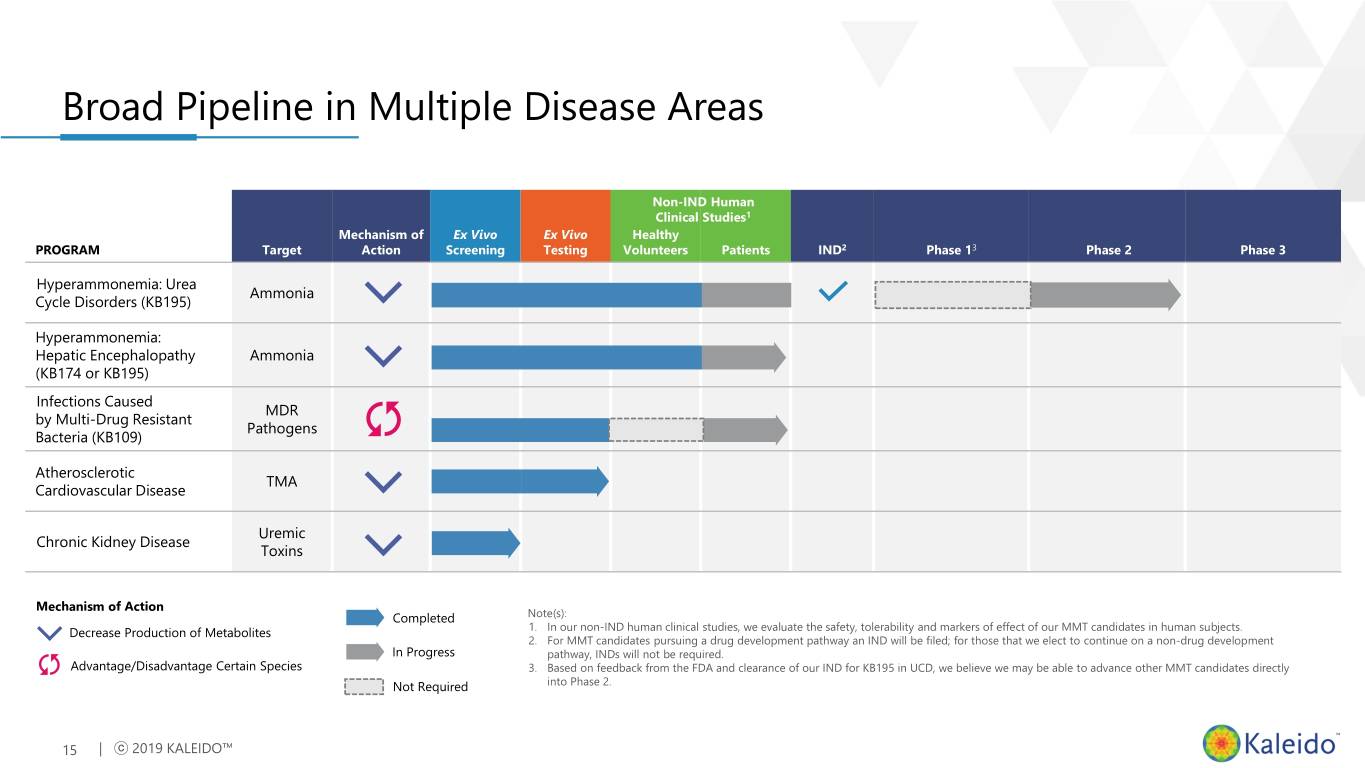
Broad Pipeline in Multiple Disease Areas Non-IND Human Clinical Studies1 Mechanism of Ex Vivo Ex Vivo Healthy PROGRAM Target Action Screening Testing Volunteers Patients IND2 Phase 13 Phase 2 Phase 3 Hyperammonemia: Urea Ammonia Cycle Disorders (KB195) Hyperammonemia: Hepatic Encephalopathy Ammonia (KB174 or KB195) Infections Caused MDR by Multi-Drug Resistant Pathogens Bacteria (KB109) Atherosclerotic TMA Cardiovascular Disease Uremic Chronic Kidney Disease Toxins Mechanism of Action Completed Note(s): Decrease Production of Metabolites 1. In our non-IND human clinical studies, we evaluate the safety, tolerability and markers of effect of our MMT candidates in human subjects. 2. For MMT candidates pursuing a drug development pathway an IND will be filed; for those that we elect to continue on a non-drug development In Progress pathway, INDs will not be required. Advantage/Disadvantage Certain Species 3. Based on feedback from the FDA and clearance of our IND for KB195 in UCD, we believe we may be able to advance other MMT candidates directly Not Required into Phase 2. 15 | ⓒ 2019 KALEIDO™
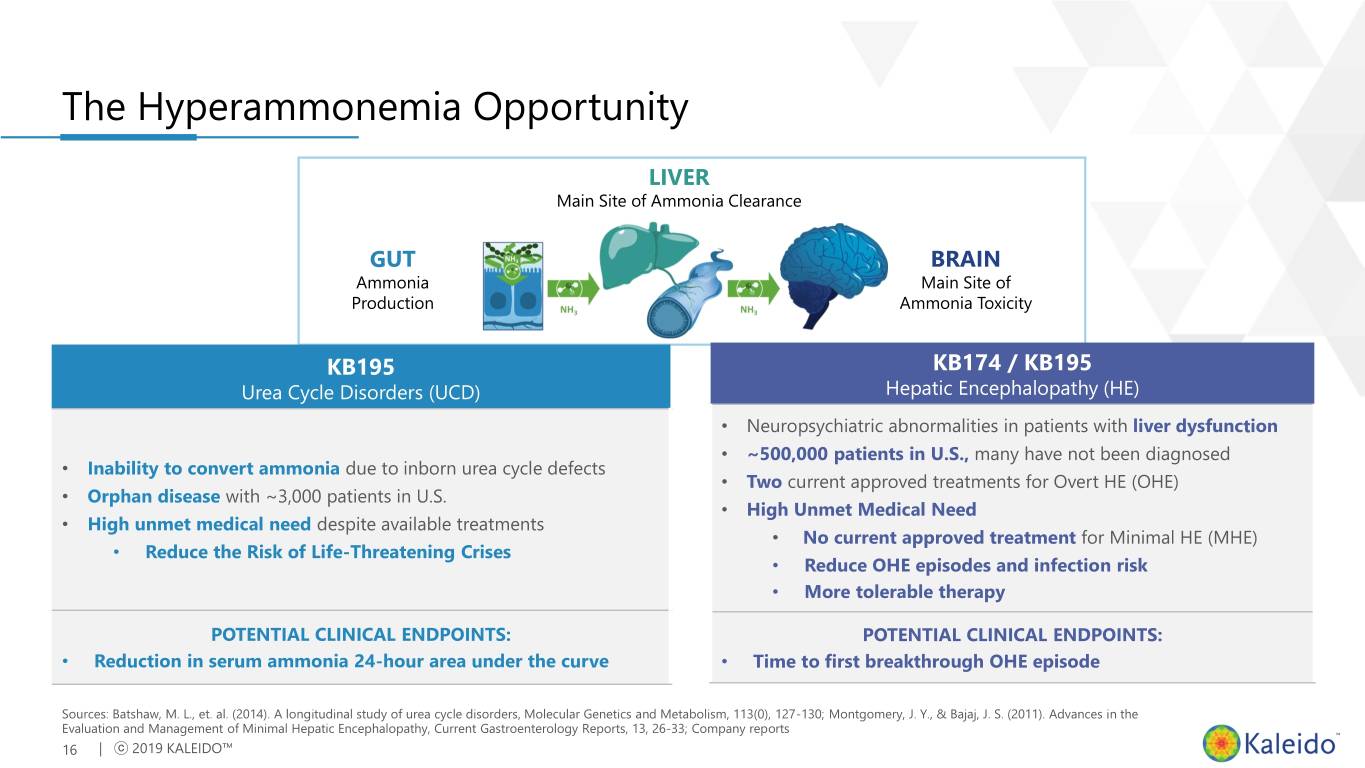
The Hyperammonemia Opportunity LIVER Main Site of Ammonia Clearance GUT BRAIN Ammonia Main Site of Production Ammonia Toxicity KB195 KB174 / KB195 Urea Cycle Disorders (UCD) Hepatic Encephalopathy (HE) • Neuropsychiatric abnormalities in patients with liver dysfunction • ~500,000 patients in U.S., many have not been diagnosed • Inability to convert ammonia due to inborn urea cycle defects • Two current approved treatments for Overt HE (OHE) • Orphan disease with ~3,000 patients in U.S. • High Unmet Medical Need • High unmet medical need despite available treatments • No current approved treatment for Minimal HE (MHE) • Reduce the Risk of Life-Threatening Crises • Reduce OHE episodes and infection risk • More tolerable therapy POTENTIAL CLINICAL ENDPOINTS: POTENTIAL CLINICAL ENDPOINTS: • Reduction in serum ammonia 24-hour area under the curve • Time to first breakthrough OHE episode Sources: Batshaw, M. L., et. al. (2014). A longitudinal study of urea cycle disorders, Molecular Genetics and Metabolism, 113(0), 127-130; Montgomery, J. Y., & Bajaj, J. S. (2011). Advances in the Evaluation and Management of Minimal Hepatic Encephalopathy, Current Gastroenterology Reports, 13, 26-33; Company reports 16 | ⓒ 2019 KALEIDO™
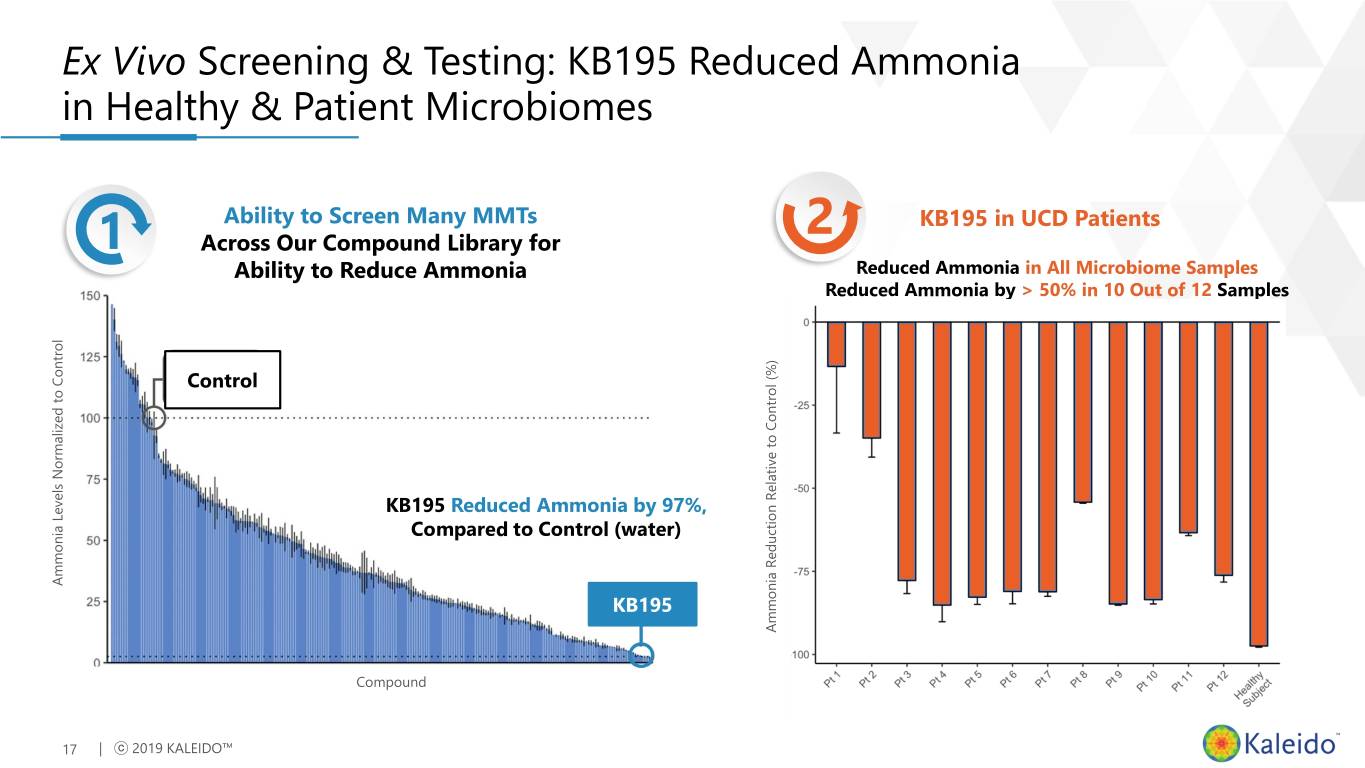
Ex Vivo Screening & Testing: KB195 Reduced Ammonia in Healthy & Patient Microbiomes Ability to Screen Many MMTs 2 KB195 in UCD Patients 1 Across Our Compound Library for Ability to Reduce Ammonia Reduced Ammonia in All Microbiome Samples Reduced Ammonia by > 50% in 10 Out of 12 Samples Control KB195 Reduced Ammonia by 97%, Compared to Control (water) Ammonia Ammonia Levels Normalized to Control KB195 Ammonia Ammonia Reduction Relative to Control (%) Compound 17 | ⓒ 2019 KALEIDO™
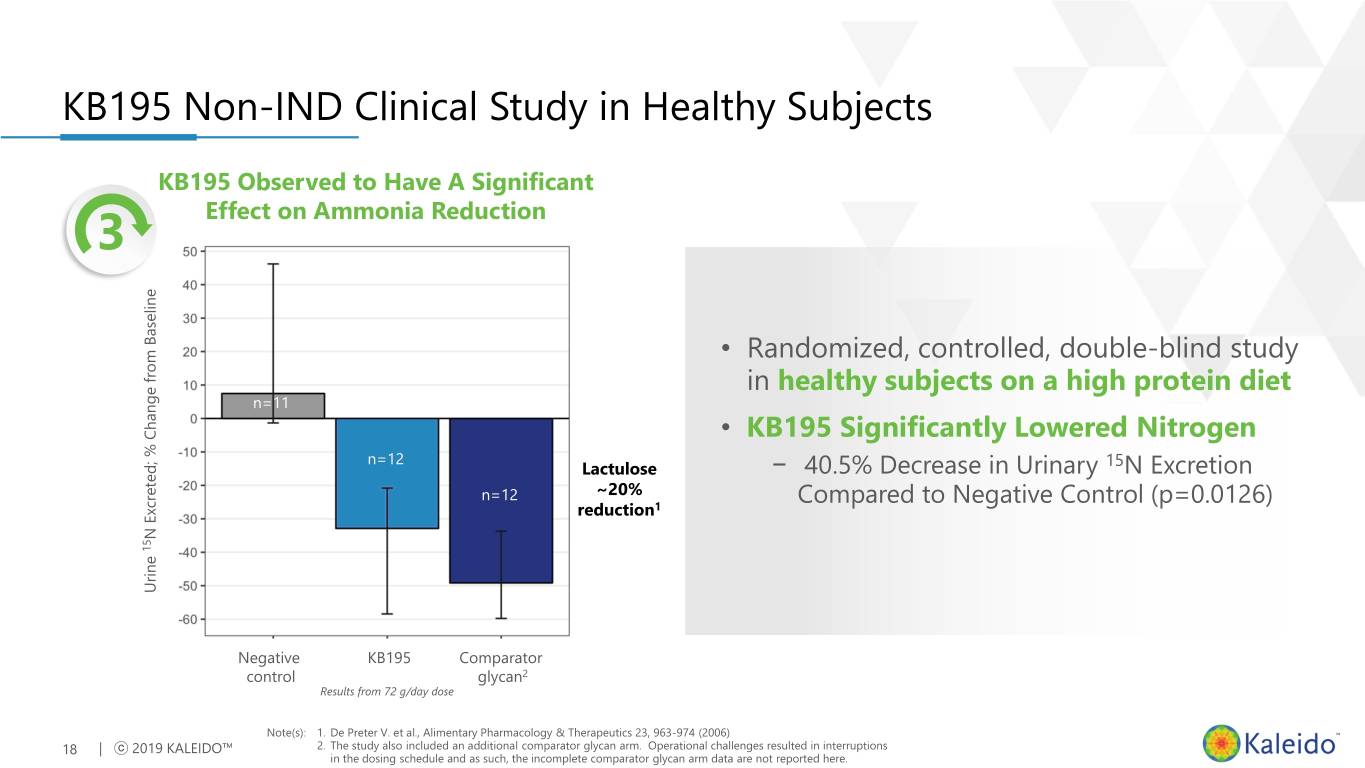
KB195 Non-IND Clinical Study in Healthy Subjects KB195 Observed to Have A Significant 3 Effect on Ammonia Reduction • Randomized, controlled, double-blind study in healthy subjects on a high protein diet n=11 • KB195 Significantly Lowered Nitrogen n=12 15 Lactulose − 40.5% Decrease in Urinary N Excretion n=12 ~20% Compared to Negative Control (p=0.0126) reduction1 N Excreted; % Change from Baseline Change % Excreted; N 15 Urine Urine Negative KB195 Comparator control glycan2 Results from 72 g/day dose Note(s): 1. De Preter V. et al., Alimentary Pharmacology & Therapeutics 23, 963-974 (2006) 18 | ⓒ 2019 KALEIDO™ 2. The study also included an additional comparator glycan arm. Operational challenges resulted in interruptions in the dosing schedule and as such, the incomplete comparator glycan arm data are not reported here.
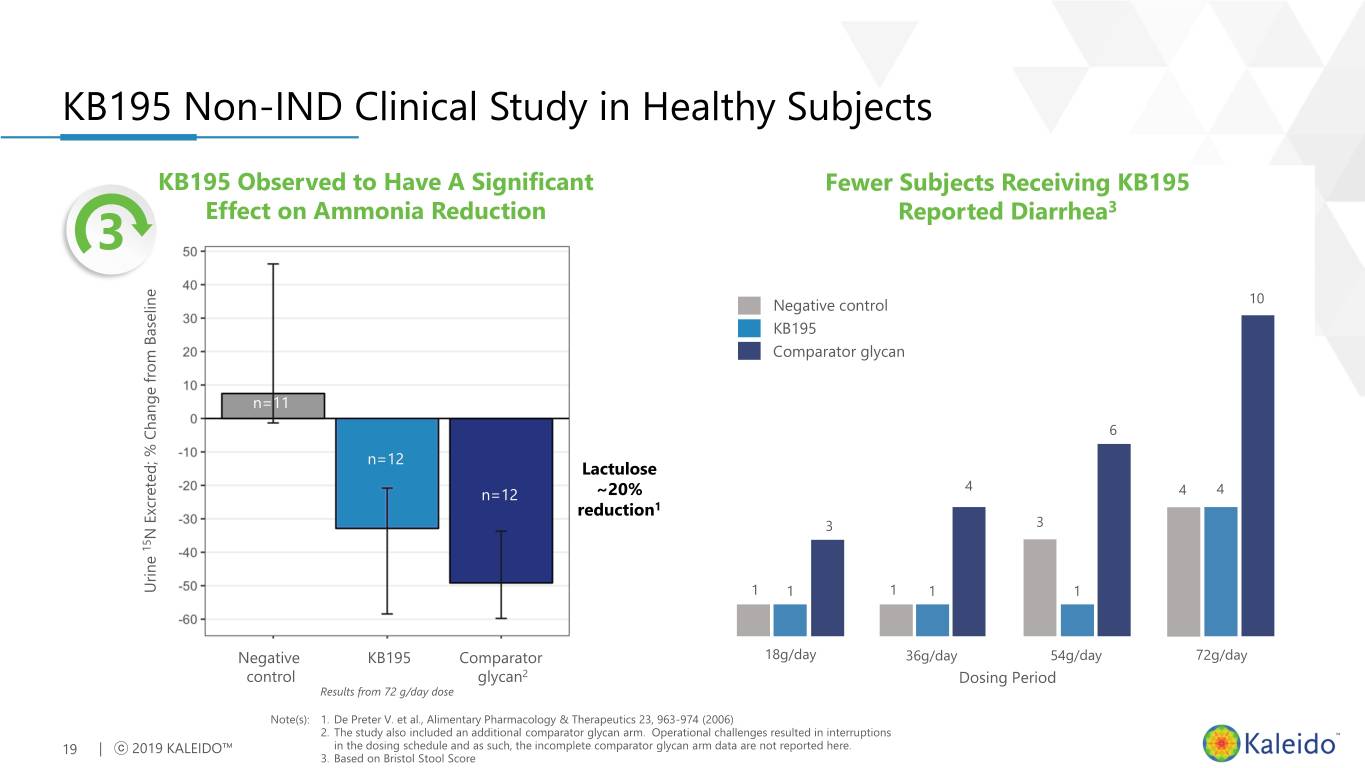
KB195 Non-IND Clinical Study in Healthy Subjects KB195 Observed to Have A Significant Fewer Subjects Receiving KB195 3 3 Effect on Ammonia Reduction Reported Diarrhea Negative control 10 KB195 Comparator glycan n=11 6 n=12 Lactulose 4 n=12 ~20% 4 4 reduction1 3 3 N Excreted; % Change from Baseline Change % Excreted; N 15 Urine Urine 1 1 1 1 1 Negative KB195 Comparator 18g/day 36g/day 54g/day 72g/day control glycan2 Dosing Period Results from 72 g/day dose Note(s): 1. De Preter V. et al., Alimentary Pharmacology & Therapeutics 23, 963-974 (2006) 2. The study also included an additional comparator glycan arm. Operational challenges resulted in interruptions 19 | ⓒ 2019 KALEIDO™ in the dosing schedule and as such, the incomplete comparator glycan arm data are not reported here. 3. Based on Bristol Stool Score
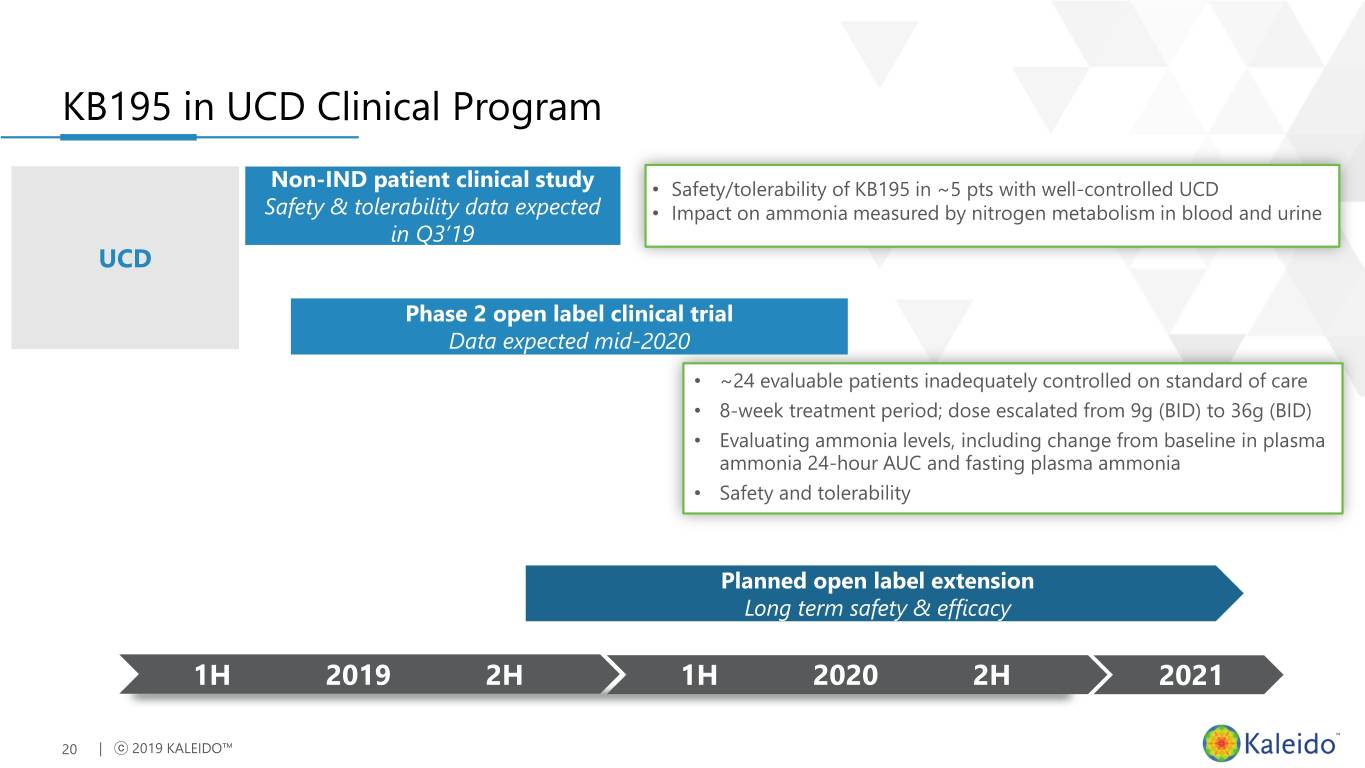
KB195 in UCD Clinical Program Non-IND patient clinical study • Safety/tolerability of KB195 in ~5 pts with well-controlled UCD Safety & tolerability data expected • Impact on ammonia measured by nitrogen metabolism in blood and urine in Q3’19 UCD Phase 2 open label clinical trial Data expected mid-2020 • ~24 evaluable patients inadequately controlled on standard of care • 8-week treatment period; dose escalated from 9g (BID) to 36g (BID) • Evaluating ammonia levels, including change from baseline in plasma ammonia 24-hour AUC and fasting plasma ammonia • Safety and tolerability Planned open label extension Long term safety & efficacy 1H 2019 2H 1H 2020 2H 2021 20 | ⓒ 2019 KALEIDO™

HE Program Advanced into Non-IND Clinical Studies 2 KB195 in Hepatically 3 Initiated two non-IND clinical studies of KB174 Impaired Patients with data expected in Q4’19 Reduced Ammonia in 18 of 19 Microbiome Samples Outperformed Lactulose in Reducing Ammonia KB174 chosen as lead based on ability to reduce Levels in 14 of 19 Samples ammonia and potential impact on infection rates Non-IND study in patients with cirrhosis • Evaluate KB174 compared to a negative control, in adult patients with well-compensated cirrhosis, including: • Nitrogen metabolism • Safety and tolerability Non-IND study in healthy volunteers to explore dosing • Assess various dosing levels of KB174 in healthy subjects evaluating: Ammonia Ammonia Reduction Relative to Control (%) • Nitrogen metabolism • Safety and tolerability 21 | ⓒ 2019 KALEIDO™
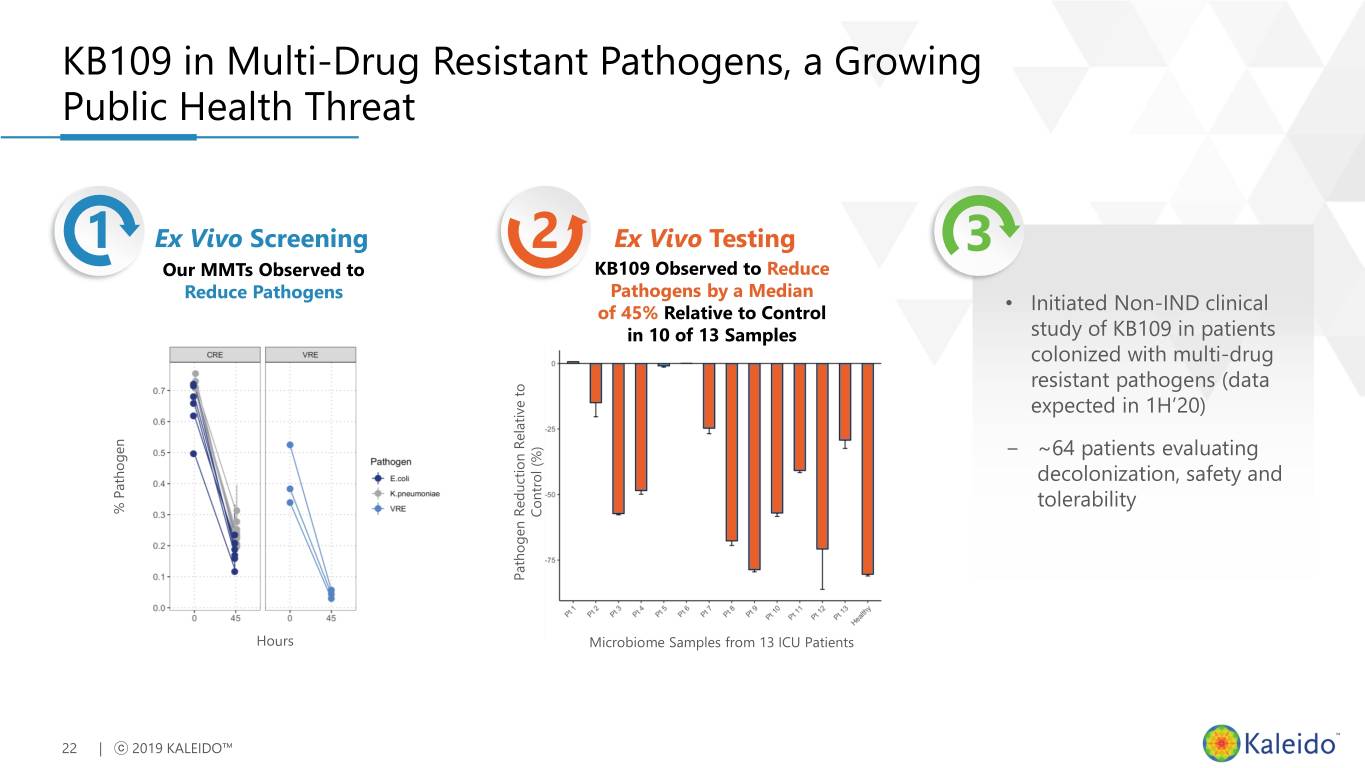
KB109 in Multi-Drug Resistant Pathogens, a Growing Public Health Threat 1 Ex Vivo Screening 2 Ex Vivo Testing 3 Our MMTs Observed to KB109 Observed to Reduce Reduce Pathogens Pathogens by a Median of 45% Relative to Control • Initiated Non-IND clinical in 10 of 13 Samples study of KB109 in patients colonized with multi-drug resistant pathogens (data expected in 1H’20) − ~64 patients evaluating decolonization, safety and tolerability % % Pathogen Control (%) Pathogen Pathogen Reduction Relative to Hours Microbiome Samples from 13 ICU Patients 22 | ⓒ 2019 KALEIDO™
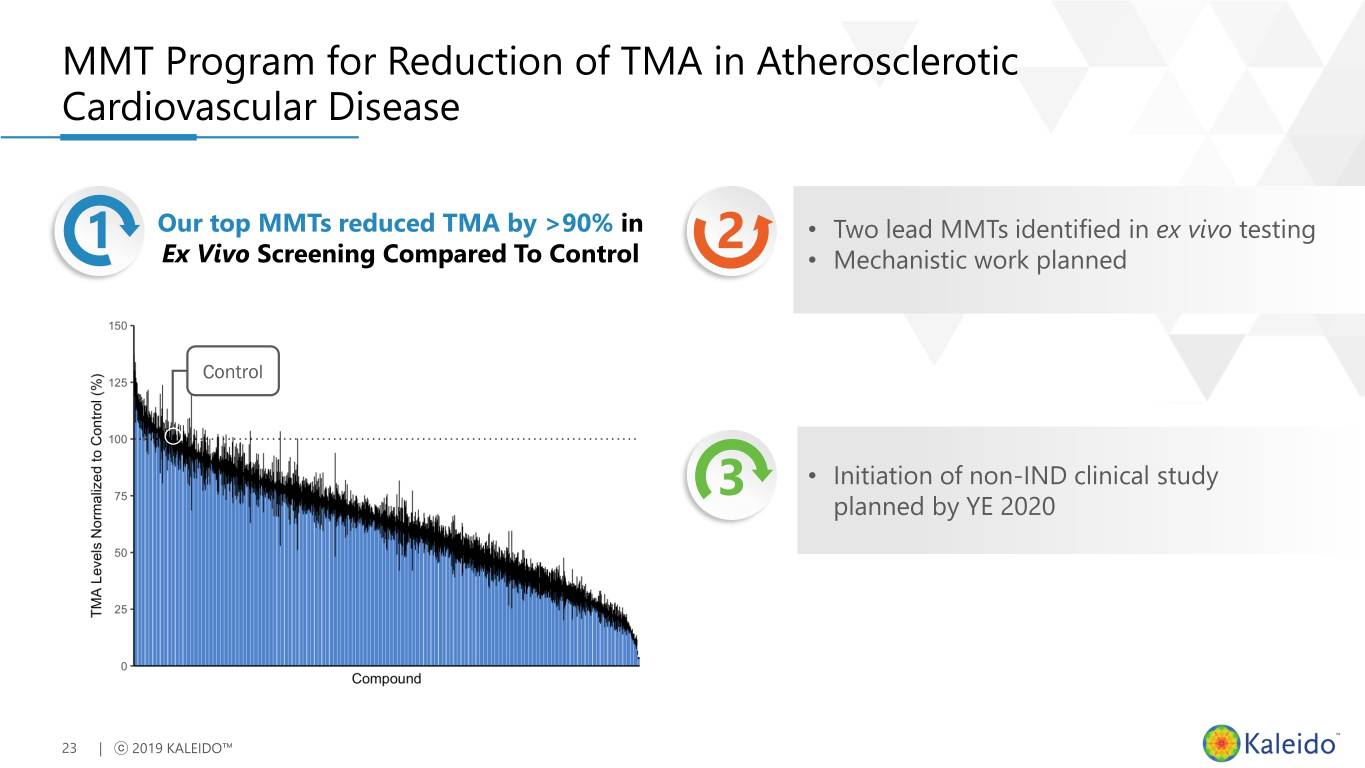
MMT Program for Reduction of TMA in Atherosclerotic Cardiovascular Disease 1 Our top MMTs reduced TMA by >90% in 2 • Two lead MMTs identified in ex vivo testing Ex Vivo Screening Compared To Control • Mechanistic work planned Control 3 • Initiation of non-IND clinical study planned by YE 2020 23 | ⓒ 2019 KALEIDO™
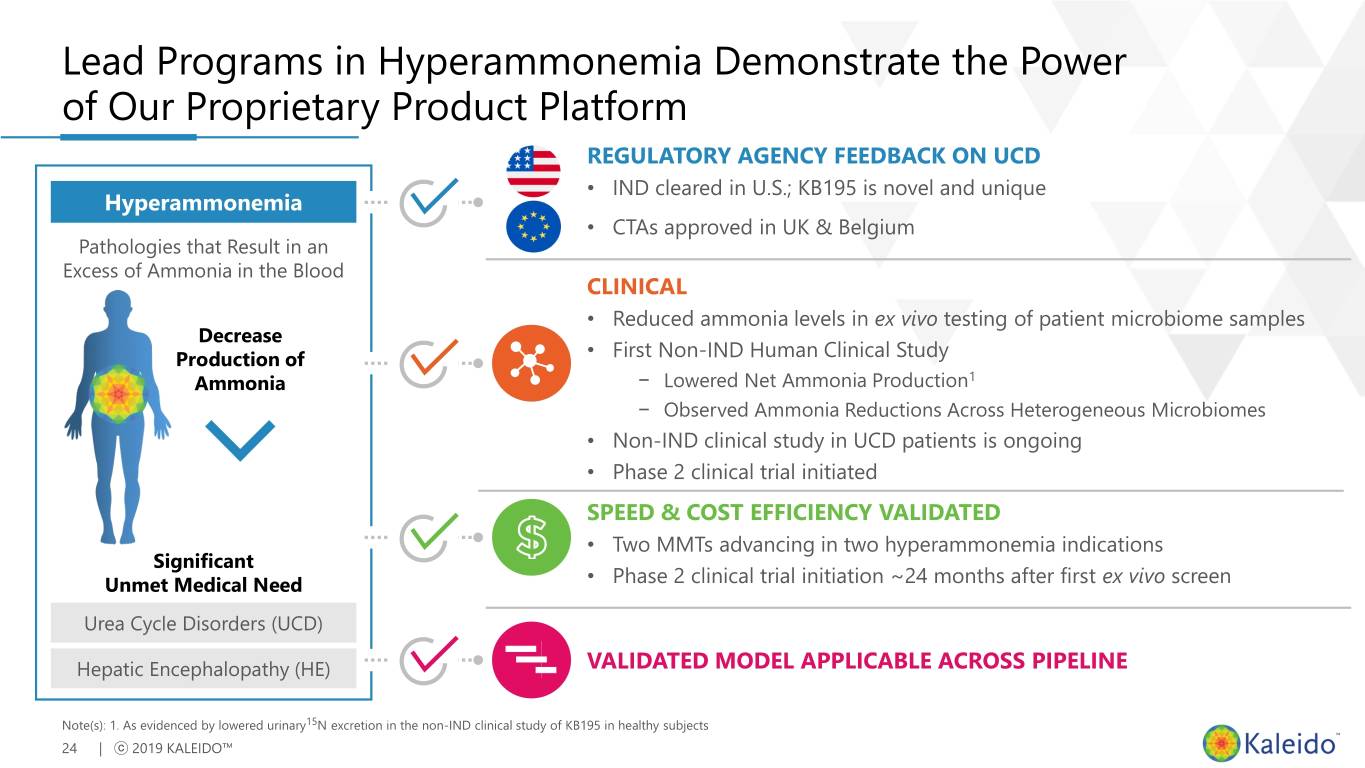
Lead Programs in Hyperammonemia Demonstrate the Power of Our Proprietary Product Platform REGULATORY AGENCY FEEDBACK ON UCD • IND cleared in U.S.; KB195 is novel and unique Hyperammonemia • CTAs approved in UK & Belgium Pathologies that Result in an Excess of Ammonia in the Blood CLINICAL • Reduced ammonia levels in ex vivo testing of patient microbiome samples Decrease Production of • First Non-IND Human Clinical Study Ammonia − Lowered Net Ammonia Production1 − Observed Ammonia Reductions Across Heterogeneous Microbiomes • Non-IND clinical study in UCD patients is ongoing • Phase 2 clinical trial initiated SPEED & COST EFFICIENCY VALIDATED • Two MMTs advancing in two hyperammonemia indications Significant Unmet Medical Need • Phase 2 clinical trial initiation ~24 months after first ex vivo screen Urea Cycle Disorders (UCD) Hepatic Encephalopathy (HE) VALIDATED MODEL APPLICABLE ACROSS PIPELINE Note(s): 1. As evidenced by lowered urinary15N excretion in the non-IND clinical study of KB195 in healthy subjects 24 | ⓒ 2019 KALEIDO™

Manufacturing is a Core Strategic Advantage Proprietary Methods Standard Small Molecule Unit Operations Scalable and Transferable Internal Manufacturing 3rd Party Manufacturers Production for ex vivo, toxicology, Large scale production for clinical trials and future and human clinical studies commercial supply (Thermo Fisher Scientific) Efficient and scalable process Internal capability to manufacture 12 MMTs per year; potential to double by early 2020 Completed tech transfer to Thermo Fisher in ~6 months, scaled to 1,000 kg with capability to increase by >40X 4 metric tons of KB195 manufactured for Phase 2 trial and toxicology 25 | ⓒ 2019 KALEIDO™

Robust Global IP Portfolio 8 U.S. Patents + 2 EPO Patents: • Glycan pharmaceutical compositions (Europe) • Methods to reduce ammonia (U.S.) • Methods to treat diarrhea (U.S.) • Methods to treat dysbiosis (U.S.) • Catalyst compositions (U.S. and Europe) 100+ Non-Provisional Applications Pending, Worldwide in 20+ Regions/Countries • Glycan compositions • Methods to treat pathogen colonization • Methods to treat immune imbalances (e.g. cancer) • Methods to modulate short chain fatty acids • Methods of making glycans Continued IP Expansion by Filing Patent Applications Directed to Pharmaceutical Compositions, Methods of Treatment and Methods of Manufacture 26 | ⓒ 2019 KALEIDO™
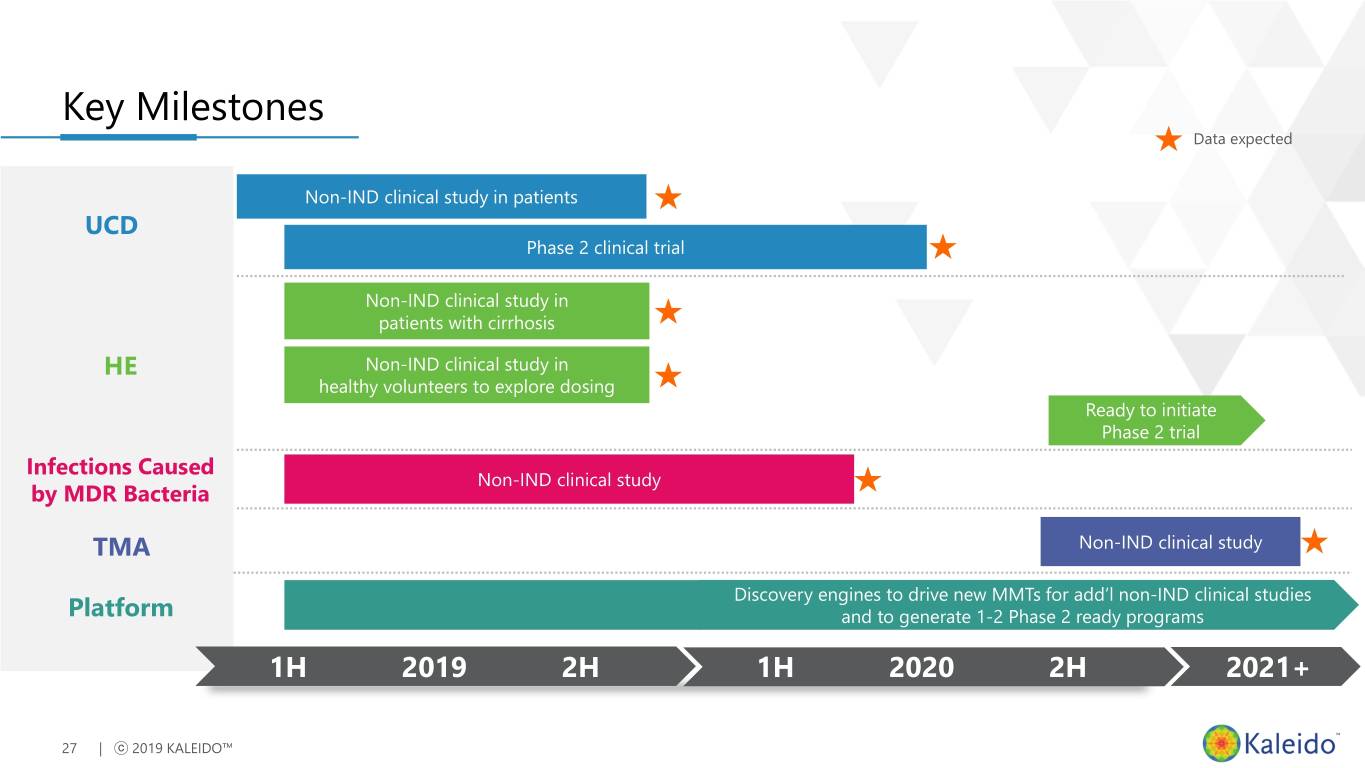
Key Milestones Data expected Non-IND clinical study in patients UCD Phase 2 clinical trial Non-IND clinical study in patients with cirrhosis HE Non-IND clinical study in healthy volunteers to explore dosing Ready to initiate Phase 2 trial Infections Caused Non-IND clinical study by MDR Bacteria TMA Non-IND clinical study Discovery engines to drive new MMTs for add’l non-IND clinical studies Platform and to generate 1-2 Phase 2 ready programs 1H 2019 2H 1H 2020 2H 2021+ 27 | ⓒ 2019 KALEIDO™
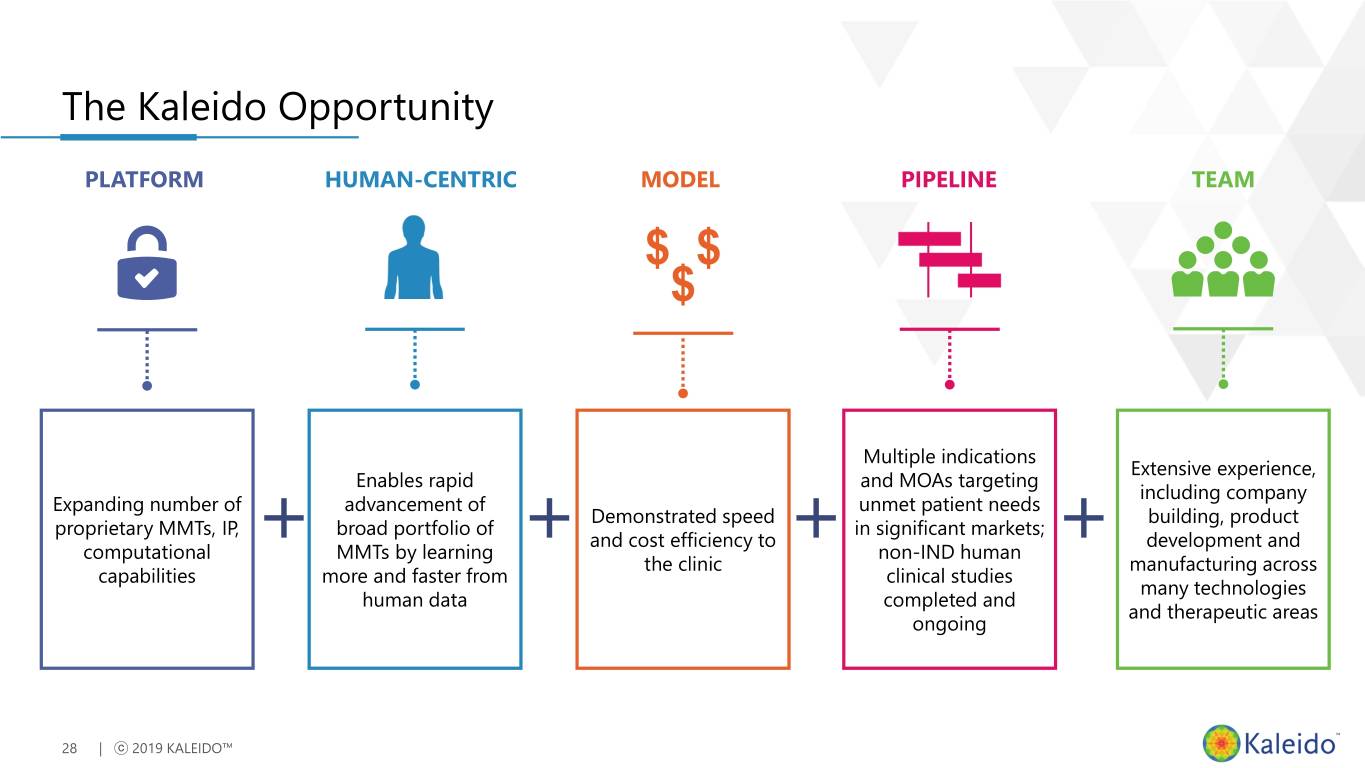
The Kaleido Opportunity PLATFORM HUMAN-CENTRIC MODEL PIPELINE TEAM Multiple indications Extensive experience, Enables rapid and MOAs targeting including company Expanding number of advancement of unmet patient needs Demonstrated speed building, product proprietary MMTs, IP, broad portfolio of in significant markets; and cost efficiency to development and computational MMTs by learning non-IND human the clinic manufacturing across capabilities more and faster from clinical studies many technologies human data completed and and therapeutic areas ongoing 28 | ⓒ 2019 KALEIDO™

29 | ⓒ 2019 KALEIDO™

Financial Overview Cash and Cash Equivalents $100.0M (as of 6/30/19) Operating Expenses (1) $22.6M (for quarter ended 6/30/19) Debt $15.0M (as of 6/30/19) Note(s): 1. Excludes stock-based compensation 30 | ⓒ 2019 KALEIDO™





























