As filed with the Securities and Exchange Commission on July 18, 2019
Registration No. 333- 232319
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_________________________________________________________
AMENDMENT NO.2 TO
FORM F-10
REGISTRATION
STATEMENT UNDER THE SECURITIES ACT OF 1933
__________________________________________________________
The Flowr Corporation
(Exact name of Registrant as specified in its charter)
Ontario, Canada
(Province or other jurisdiction of incorporation or organization)
2833
(Primary Standard Industrial Classification Code Number, if applicable)
N/A
(I.R.S. Employer Identification No., if applicable)
461 King Street W, Floor 2
Toronto, Ontario M5V 1K4
Canada
(905) 940-3993
(Address and telephone number of Registrant’s principal executive offices)
DL Services Inc.
Columbia Center
701 Fifth Avenue, Suite 6100
Seattle Washington 98104-7043
(206) 903-8800
(Name, address (including zip code) and telephone number (including area code) of agent for service in the United States)
| Copies to: | |
|
Alexander Dann
The Flowr Corporation
461 King Street W, Floor 2
Toronto, Ontario
Canada, M5V 1K4
Tel: (905) 940-3993
| |
Approximate date of commencement of proposed sale to the public:
As soon as practicable after this Registration Statement becomes effective.
Province of Ontario, Canada
(Principal jurisdiction regulating this offering)
It is proposed that this filing shall become effective (check appropriate box below):
| | | | | | |
A. | | [X] | | upon filing with the Commission pursuant to Rule 467(a) (if in connection with an offering being made contemporaneously in the United States and Canada). |
B. | | [ ] | | at some future date (check the appropriate box below): |
| | 1. | | [ ] | | pursuant to Rule 467(b) on ( ) at ( ) (designate a time not sooner than 7 calendar days after filing). |
| | 2. | | [ ] | | pursuant to Rule 467(b) on ( ) at ( ) (designate a time 7 calendar days or sooner after filing) because the securities regulatory authority in the review jurisdiction has issued a receipt or notification of clearance on ( ). |
| | 3. | | [ ] | | pursuant to Rule 467(b) as soon as practicable after notification of the Commission by the Registrant or the Canadian securities regulatory authority of the review jurisdiction that a receipt or notification of clearance has been issued with respect hereto. |
| | 4. | | [ ] | | after the filing of the next amendment to this Form (if preliminary material is being filed). |
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to the home jurisdiction’s shelf prospectus offering procedures, check the following box. [ ]
CALCULATION OF REGISTRATION FEE
Title of Each Class of Securities
to be Registered | Proposed Maximum
Aggregate Offering
Price(1) | Amount of
Registration Fee(1) |
| | | |
Common Shares | $110,136,377.57 | $13,348.53 |
(1) Calculated pursuant to Rule 457(o) under the Securities Act of 1933, as amended (the “Act”). There are being registered under this Registration Statement such number of common shares of the Registrant as shall have an aggregate offering price not to exceed US$110,136,377.57. Based on a proposed maximum aggregate offering price of CAD$125,000,000, and over-allotment amount of CAD$18,750,000. US dollar amounts are calculated based on the Bank of Canada daily average rate of US$1.00=CAD$1.3052 on July 16, 2019. The amount of registration fee includes $11,354.92 previously paid to the Commission.
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registration Statement shall become effective as provided in Rule 467 under the Act or on such date as the Commission, acting pursuant to Section 8(a) of the Act, may determine.
PART I
INFORMATION REQUIRED TO BE DELIVERED TO OFFEREES OR PURCHASERS
Information contained herein is subject to completion or amendment. A registration statement relating to these securities has been filed with the United States Securities and Exchange Commission. These securities may not be sold nor may offers to buy be accepted prior to the time the registration statement becomes effective. This prospectus shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of these securities in any state in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state.
SUBJECT TO COMPLETION, DATED JULY 18, 2019
I - 1
SHORT FORM BASE PREP PROSPECTUS

THE FLOWR CORPORATION
$125,000,000
[•] Common Shares
Price: $[•] per Offered Share |
This short form base PREP prospectus (the "Prospectus") of The Flowr Corporation ("Flowr", the "Corporation", "we", "us", "our" and similar terms) qualifies the distribution (the "Offering") of [•] common shares of the Corporation (the "Offered Shares") at a price of $[•] per Offered Share (the "Offering Price").
The Offered Shares will be issued pursuant to an underwriting agreement (the "Underwriting Agreement") dated [•], 2019, entered into among the Corporation and Barclays Capital Canada Inc., BMO Nesbitt Burns Inc. and Credit Suisse Securities (Canada), Inc. as representatives for the Underwriters (as defined below) (together, the "Representatives"), and AltaCorp Capital Inc., Clarus Securities Inc. and Sprott Capital Partners LP ( the "Canadian Underwriters" and Berenberg Capital Markets LLC, Oppenheimer & Co. Inc., Roth Capital Partners, LLC and MKM Partners, LLC (the "U.S. Underwriters, and collectively with the Representatives and the Canadian Underwriters, the "Underwriters"). The Representatives are acting as joint book-runners in connection with the Offering. See "Plan of Distribution".
Flowr operates in the cannabis industry and is focused on producing high-yield, premium cannabis for both medical and recreational markets throughout Canada, through its growing operations located in the Okanagan Valley of British Columbia, Canada.
Joint Book-Running Managers
| Barclays | BMO Capital Markets | Credit Suisse |
(continued on next page)
(continued from cover)
On June 24, 2019 the Corporation entered into a share purchase agreement which was amended on July 10, 2019 to extend the outside date to July 31, 2019 (the "Holigen Purchase Agreement") pursuant to which the Corporation has agreed to acquire (the "Acquisition") the remaining 80.2% of the outstanding equity interests in the capital of Holigen Holdings Limited ("Holigen") not already owned by the Corporation (the "Purchased Shares"). The consideration for the Purchased Shares (the "Purchase Price") is expected to be comprised of (i) share consideration; (ii) cash consideration; and (iii) the purchase of certain loans. See "Details of the Acquisition".
The share consideration for the Acquisition is expected to be satisfied by the issuance of an aggregate of 32,632,545 Series 1 Preferred Shares (as defined below) (the "Consideration Shares") at a deemed price per share of $7.15, being the volume weighted average trading price of the common shares of the Corporation (the "Common Shares") as traded on the facilities of the TSX Venture Exchange (the "TSX.V") for the five (5) trading days immediately preceding the date of the Holigen Purchase Agreement, as may be adjusted if required by the TSX.V (the "Issue Price"). Completion of the Acquisition is subject to certain closing conditions. A portion of the net proceeds of the Offering will be used to satisfy part of the Purchase Price. See "Details of the Acquisition" and "Use of Proceeds".
| | | Price to the Public | | | Underwriters’ Fee(1) | | | Net Proceeds to the
Corporation(2) | |
| Per Offered Share | $ | [•] | | $ | [•] | | $ | [•] | |
| Total(3) | $ | 125,000,000 | | $ | 7,500,000 | | $ | 117,500,000 | |
Notes:
(1) (1) In consideration for the services rendered by the Underwriters in connection with the Offering, the Underwriters will be paid a cash commission (the "Underwriters’ Fee") equal to 6% of the Offering Price for each Offered Share purchased on the Closing Date (as defined herein). See "Plan of Distribution" for additional information regarding the Underwriters’ compensation.
(2) (2) After deducting the Underwriters’ Fee, but before deducting expenses of the Offering, including expenses to prepare and file this Prospectus, which are estimated to be $2,100,000, and which will be paid from the net proceeds of the Offering.
(3) (3) The Corporation has granted the Underwriters an option (the "Underwriters’ Option"), exercisable in whole or in part, at any time and from time to time, in the sole discretion of the Underwriters, up to 30 days after and including the Closing Date (as defined below) to purchase up to an additional [•] Common Shares (the "Additional Offered Shares") at a price of $[•] per Additional Offered Share, to cover any over-allocation or for market stabilization purposes. If the Underwriters’ Option is exercised in full, the total price to the public, Underwriters’ Fee and net proceeds to the Corporation (before payment of the expenses of the Offering) will be $143,750,000, $8,625,000 and $135,125,000, respectively. This Prospectus qualifies the grant of the Underwriters’ Option and the issuance of the Additional Offered Shares on the exercise of the Underwriters’ Option. A purchaser who acquires Additional Offered Shares forming part of the Underwriters’ over-allocation position acquires those Additional Offered Shares under this Prospectus, regardless of whether the over-allocation position is ultimately filled through the exercise of the over-allocation option or secondary market purchases. Unless the context otherwise requires, references to "Offered Shares" in this Prospectus include the Additional Offered Shares. See "Plan of Distribution".
The following table sets out the number of Additional Offered Shares that may be issued by the Corporation to the Underwriters pursuant to the Underwriters’ Option:
Underwriters’ Position | Number of Securities
Available | Exercise Period | Exercise Price |
Underwriters’ Option | [•] Additional Offered Shares | Up to 30 days after the Closing Date | $[•] per Additional Offered Share |
The Common Shares are listed and posted for trading on the TSX.V under the symbol "FLWR". On June 21, 2019, the last trading day prior to the announcement of the Offering, the closing price of the Common Shares on the TSX.V was $7.26. On July 17, 2019, the last trading day prior to the date of this Prospectus the closing price of the Common Shares on the TSX.V was $4.76. The TSX.V has conditionally approved the listing of the Offered Shares and the Additional Offered Shares. Listing is subject to the Corporation fulfilling all of the listing requirements of the TSX.V on or before October 2, 2019. Concurrently with the pricing of the Offering, the Corporation intends to commence trading on the NASDAQ Capital Market (the "NASDAQ"). The Corporation has been approved for listing on the NASDAQ, subject to the effectiveness of the Corporation’s registration statement on Form 40-F filed with the SEC (as defined below) on February 5, 2019 as amended on May 28, 2019 and further amended on July 12, 2019. The Corporation intends to commence trading on NASDAQ one day after the pricing of the Offering.
The Offering is being made in each of the provinces of Canada except Quebec and the United States on an underwritten basis for 100% of the Offered Shares offered hereunder. The Underwriters, as principals, conditionally offer the Offered Shares, subject to prior sale, if, as and when issued by the Corporation and accepted by the Underwriters in accordance with the conditions contained in the Underwriting Agreement referred to under "Plan of Distribution", subject to the approval of certain Canadian legal matters on behalf of the Corporation by Fasken Martineau DuMoulin LLP and on behalf of the Underwriters by Borden Ladner Gervais LLP. The U.S. Underwriters are not registered as investment dealers in any Canadian jurisdiction and, accordingly, will only sell or solicit offers to sell the Offered Shares directly or indirectly, into the United States and will not, directly or indirectly, sell or solicit offers to sell the Offered Shares in Canada.
Subscriptions for the Offered Shares will be received subject to rejection or allotment, in whole or in part, and the Underwriters reserve the right to close the subscription books at any time without notice. Closing of the Offering is expected to take place on or about [•], 2019 or such other date as may be agreed upon by the Corporation and the Representatives, on behalf of the Underwriters, but in any event, not later than 42 days after the date of the receipt for the (final) short form base PREP prospectus (the "Closing Date"). In connection with the Offering, and subject to applicable laws, the Underwriters may over-allot or effect transactions that are intended to stabilize or maintain the market price of the Common Shares at levels other than that which might otherwise prevail in the open market. Such transactions, if commenced, may be discontinued at any time.
ii
After the Underwriters have made reasonable efforts to sell the Offered Shares at the Offering Price, the Underwriters may decrease the Offering Price for the Offered Shares and may further change the Offering Price from time to time to amounts no greater than the Offering Price. In the event the Offering Price is reduced, the compensation received by the Underwriters will be decreased by the amount that the aggregate price paid by the purchasers for the Offered Shares is less than the gross proceeds paid by the Underwriters for the Offered Shares. Any such reduction will not affect the proceeds received by the Corporation. See "Plan of Distribution".
Investing in the Offered Shares is speculative and involves a high degree of risk and should only be made by persons who can afford the total loss of their investment. A prospective purchaser should therefore review this Prospectus and the documents incorporated by reference herein in their entirety and carefully consider the risk factors described under the section "Risk Factors" in this Prospectus, prior to investing in the Offered Shares. See "Caution Regarding Forward-Looking Statements" and "Risk Factors".
Prospective purchasers are advised to consult their own tax advisors regarding the application of Canadian federal income tax laws to their particular circumstances, as well as any other provincial, foreign and other tax consequences of acquiring, holding or disposing of the Offered Shares.
It is anticipated that the Offered Shares will be delivered under the book-based system through CDS Clearing and Depository Services Inc. ("CDS") or its nominee and deposited in electronic form, or will otherwise be delivered to the Underwriters, registered as directed by the Underwriters, on the Closing Date. Except in limited circumstances, a purchaser of Offered Shares will receive only a customer confirmation from the registered dealer from or through which the Offered Shares are purchased and who is a CDS depository service participant. CDS will record the CDS participants who hold the Offered Shares on behalf of owners who have purchased the Offered Shares in accordance with the book-based system. No definitive certificates will be issued unless specifically requested or required. See "Plan of Distribution".
The Offering is made by a Canadian issuer that is permitted, under a multijurisdictional disclosure system adopted by the United States and Canada, to prepare this Prospectus in accordance with Canadian disclosure requirements. Prospective investors should be aware that such requirements are different from those applicable to issuers in the United States. Certain financial statements incorporated by reference in the Prospectus have been prepared in accordance with International Financial Reporting Standards ("IFRS") as issued by the International Accounting Standards Board ("IASB"), and may not be comparable to financial statements of United States companies.
Prospective investors should be aware that the acquisition of the Offered Shares may have tax consequences both in the United States and in Canada. Such tax consequences for investors, including investors who are resident in or citizens of the United States, may not be described fully herein.
The enforcement by investors of civil liabilities under United States federal securities laws may be affected adversely by the fact that the Corporation is amalgamated under the laws of the Province of Ontario, Canada, that most of its officers and Directors are residents of Canada, that some of the experts named in this Prospectus are residents of Canada, and that all or a substantial portion of the assets of the Corporation and said persons are located outside the United States. See "Enforceability of Civil Liabilities".
THE OFFERED SHARES HAVE NOT BEEN APPROVED OR DISAPPROVED BY THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION (THE "SEC") NOR ANY STATE SECURITIES COMMISSION AND NEITHER THE SEC NOR ANY STATE SECURITIES COMMISSIONS HAVE PASSED UPON THE ACCURACY OR ADEQUACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The Corporation does not have, and until federally legal does not intend to engage in, any direct, indirect or ancillary involvement in the United States cannabis industry (as described in CSA Staff Notice 51-352 (Revised) Issuers with U.S. Marijuana-Related Activities).
The registered and head office of the Corporation is located at 461 King Street West, Floor 2, Toronto, Ontario M5V 1K4.
iii
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
Explanatory Notes
This Prospectus describes the specific terms of the Offering and also incorporates by reference certain documents herein. In this Prospectus, the Corporation and its subsidiaries, The Flowr Canada Holdings ULC and The Flowr Group (Okanagan) Inc. are collectively referred to as the "Corporation" or "Flowr", unless the context otherwise requires. Readers of this Prospectus should rely only on information contained in this Prospectus and contained in the documents incorporated by reference in this Prospectus. The Corporation and the Underwriters have not authorized anyone to provide the reader with different information and take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give readers of this Prospectus.
The Corporation and the Underwriters are not offering to sell the Offered Shares in any jurisdictions where the offer or sale of the Offered Shares is not permitted. The information contained in this Prospectus (including the documents incorporated by reference herein) is accurate only as of the date of this Prospectus (or the date of the document incorporated by reference herein, as applicable), regardless of the time of delivery of this Prospectus or any sale of the Offered Shares. The business, financial condition, results of operations and prospects of the Corporation may have changed since those dates. The Corporation does not undertake to update the information contained or incorporated by reference herein, except as required by applicable Canadian securities laws.
This Prospectus must not be used by anyone for any purpose other than in connection with the Offering. Information contained on, or otherwise accessed through, the Corporation’s website is not and shall not be deemed to be a part of this Prospectus and such information is not incorporated by reference, despite any references to such information in this Prospectus or the documents incorporated by reference herein, and prospective purchasers should not rely on such information when deciding whether or not to invest in the Offered Shares. The information on the Underwriters’ website and any information contained in any other website maintained by the Underwriters or their affiliates is not part of this Prospectus, has not been approved and/or endorsed by the Corporation or the Underwriters and should not be relied upon by prospective purchasers.
Capitalized terms not otherwise defined in the body of this Prospectus shall have the meanings ascribed to such terms in the Glossary of Terms attached as Appendix "A" to this Prospectus.
The documents incorporated or deemed to be incorporated by reference herein contain meaningful and material information relating to the Corporation and prospective purchasers should review all information contained in this Prospectus and the documents incorporated or deemed to be incorporated by reference herein.
Trademarks
This Prospectus and the documents incorporated by reference herein include references to trademarks which are protected under applicable intellectual property laws and are the property of the Corporation, Holigen, or their respective affiliates or licensors. Solely for convenience, the trademarks of the Corporation, Holigen, their respective affiliates and/or their respective licensors referred to in this Prospectus, or documents incorporated by reference herein, may appear with or without the ® or ™ symbol, but such references or the absence thereof are not intended to indicate, in any way, that the Corporation, Holigen, or their respective affiliates or licensors will not assert, to the fullest extent under applicable law, their respective rights to these trademarks. Any other trademarks used in this Prospectus, or the accompanying Prospectus or documents incorporated by reference herein, are the property of their respective owners.
Market Data
This Prospectus and the documents incorporated by reference herein, contain certain statistical data, market research and industry forecasts that were obtained, unless otherwise indicated, from independent industry and government publications and reports or based on estimates derived from such publications and reports and management’s knowledge of, and experience in, the markets in which the Corporation operates. Industry and government publications and reports generally indicate that they have obtained their information from sources believed to be reliable, but do not guarantee the accuracy and completeness of their information. While the Corporation believes this data to be reliable, market and industry data is subject to variation and cannot be verified due to limits on the availability and reliability of raw data, the voluntary nature of the data gathering process and other limitations and uncertainties inherent in any statistical survey. The Corporation has not independently verified the accuracy or completeness of such information contained in this Prospectus or incorporated by reference herein. In addition, projections, assumptions and estimates of the Corporation’s future performance and the future performance of the industry in which the Corporation operates are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described under the heading "Risk Factors" in this Prospectus.
Information Pertaining to Holigen
The information contained in this Prospectus with respect to Holigen and its business has been provided by management of Holigen. Management and the Directors of the Corporation have relied upon Holigen for the accuracy of the information provided by Holigen. See "Risk Factors - The Corporation Does Not Currently Control Holigen and No Assurance of Holigen’s Future Performance Can be Given".
CAUTIONARY NOTE REGARDING FORWARD-LOOKING INFORMATION
Certain statements contained in this Prospectus or in documents incorporated by reference herein, constitute "forward-looking information" and "forward looking statements" within the meaning of applicable Canadian and United States securities laws ("forward-looking statements"), which are based upon the Corporation’s current internal expectations, estimates, projections, assumptions and beliefs. Statements concerning the Corporation’s objectives, goals, strategies, intentions, plans, beliefs, expectations and estimates, and the business, operations, future financial performance and condition of the Corporation and/or Holigen are forward-looking statements. The words "believe", "expect", "anticipate", "estimate", "intend", "may", "will", "would" and similar expressions, including the negative and grammatical variations of such expressions, are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These statements are not guarantees of future performance and involve known and unknown risks, uncertainties and other factors that may cause actual results or events to differ materially from those anticipated in the forward-looking statements. In addition, this Prospectus and/or the documents incorporated by reference herein, may contain forward-looking statements attributed to third-party industry sources.
By their nature, forward-looking statements involve numerous assumptions, known and unknown risks and uncertainties, both general and specific, that contribute to the possibility that the predictions, forecasts and projections that constitute forward-looking statements may not occur. Such forward-looking statements in this Prospectus or in documents incorporated by reference herein, speak only as of the date of such document. Forward-looking statements in this Prospectus or in documents incorporated by reference herein include, but are not limited to, statements with respect to:
• the completion and timing of the Offering;
• the use of net proceeds of the Offering;
• the Acquisition, including but not limited to:
• the completion and timing thereof;
• synergies and/or benefits resulting from the Acquisition; and
• the impact of the Acquisition on Flowr’s financial performance (including with respect to its growing capacity, revenues and margins);
• the performance of the Corporation’s and Holigen’s business and operations;
• the ability of the Corporation to integrate the business of Holigen with that of the Corporation;
• the retention of key personnel of the Corporation and Holigen;
• the Corporation’s capital expenditure programs;
• the future development of the Corporation, its growth strategy and the timing thereof;
• the construction, production capacity, expected completion, as well as expected receipt and timing of regulatory and GMP licensing, as applicable, of the K1 Facility, K2 Facility, Flowr Forest and R&D facility in Kelowna and Holigen’s facilities;
• the growth strategy of the Corporation;
• the estimated future contractual obligations of the Corporation;
• the Corporation’s future liquidity and financial capacity including its ability to satisfy financial obligations in future periods;
• the supply and demand for cannabis products and services similar to the Corporation’s and Holigen’s products and services;
• the ability to establish and market the Corporation’s brands within its targeted markets;
• cost and/or pricing of the Corporation’s and Holigen’s products;
• expectations regarding the Corporation’s ability to raise capital;
• the Corporation’s treatment under government regulatory and taxation regimes; and
• the Corporation’s and Holigen’s net sales of all or any one of their respective products.
With respect to the forward-looking statements contained in this Prospectus or in documents incorporated by reference herein, the Corporation has made assumptions regarding, among other things:
• the ability to develop and market future products;
• timing to launch new products;
• cost to develop and/or manufacture products;
• operating cost estimates and yield trends for the Corporation and/or Holigen;
• inventory levels;
• pricing for the Corporation’s and/or Holigen’s products;
• future market demand/trends;
• gross profitability for products;
• the ability of the Corporation to comply with its contractual obligations;
• the ability of the Corporation to complete the Acquisition and this Offering on the terms described in the Prospectus and the Underwriting Agreement;
• the successful sale of products to third parties on terms favorable to the Corporation;
• the ability of the Corporation to maintain key strategic alliances, and licensing and partnering arrangements, including in relation to the business of Holigen, now and in the future;
• the ability of the Corporation and/or Holigen to complete construction of their facilities and obtain licensing on time or at all;
• any delay in the construction and/or licensing of the facilities of the Corporation or Holigen not being material;
• the ability of the Corporation to maintain its and Holigen’s distribution networks and distribute its products effectively despite significant geographical expansion;
• the cannabis regulatory environment in which the Corporation and Holigen operate, including the areas of taxation, environmental protection, consumer safety and health regulation;
• the timely receipt of any required regulatory approvals;
• the general economic, financial, market and political conditions impacting the industry in which the Corporation and Holigen operate;
• the tax treatment of the Corporation, Holigen, and their subsidiaries and the materiality of legal proceedings;
• the ability of the Corporation to achieve or increase profitability, fund its operations with existing capital, and/or raise additional capital to fund future acquisitions and/or development, including construction and licensing of the facilities of the Corporation and Holigen;
• the ability of the Corporation to acquire any necessary technology, products or businesses and effectively integrate such acquisitions, including the Corporation’s ability to complete the Acquisition and integrate Holigen;
• reliance on third party suppliers to supply the Corporation and Holigen with products required for their respective businesses on favourable terms;
• the ability of the Corporation to generate sufficient cash flow from operations;
• the availability of raw materials and finished products necessary for the Corporation’s and Holigen’s products;
• the impact of increasing competition;
• the ability of the Corporation and Holigen to obtain and retain qualified staff, equipment and services in a timely and efficient manner;
• the ability of the Corporation and Holigen to maintain and enforce the protection afforded by trade secrets or other intellectual property rights;
• the ability of the Corporation and Holigen to conduct operations in a safe, efficient and effective manner;
• the results of continuing and future safety and efficacy studies by industry and government agencies related to the Corporation’s and Holigen’s products; and
• the ability of the Corporation to successfully market its products and services and the products and services of Holigen upon completion of the Acquisition.
Forward-looking statements contained in this Prospectus or in documents incorporated by reference herein, are based on the key assumptions described herein or therein. The reader is cautioned that such assumptions, although considered reasonable by the Corporation, may prove to be incorrect. Actual results achieved during the forecast period may vary from the information provided in this Prospectus or in documents incorporated by reference herein, as a result of numerous known and unknown risks and uncertainties. The Corporation cannot guarantee future results.
Some of the risks and other factors which could cause actual results to differ materially from those expressed in the forward-looking statements contained in this Prospectus or in documents incorporated by reference herein, include, but are not limited to, the risk factors included under the heading "Risk Factors" in this Prospectus.
Forward-looking statements contained in this Prospectus or in documents incorporated by reference herein, are based on management’s current plans, expectations, estimates, projections, beliefs and opinions and the assumptions relating to those plans, expectations, estimates, projections, beliefs and opinions may change. Management of the Corporation has included the above summary of assumptions and risks related to forward-looking statements included in this Prospectus or in documents incorporated by reference herein, for the purpose of assisting the reader in understanding management’s current views regarding those future outcomes. Readers are cautioned that this information may not be appropriate for other purposes. Readers are cautioned that the lists of assumptions and risk factors contained herein are not exhaustive. Neither the Corporation nor any other person assumes responsibility for the accuracy or completeness of the forward-looking statements contained herein.
Such forward-looking statements are made as of the date of this Prospectus or any documents incorporated by reference herein, as of the dates of such documents, and the Corporation disclaims any intention or obligation to update publicly any such forward-looking statements, whether as a result of new information, future events or results or otherwise, other than as required by applicable Canadian securities laws.
All of the forward-looking statements made in this Prospectus or in documents incorporated by reference herein are expressly qualified by these cautionary statements and other cautionary statements or factors contained or incorporated by reference herein, and there can be no assurances that the actual results or developments will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on, the Corporation. See "Risk Factors".
Actual results, performance or achievements could differ materially from those expressed in, or implied by, any forward-looking statement in this Prospectus, and, accordingly, investors should not place undue reliance on any such forward-looking statement. New factors emerge from time to time and the importance of current factors may change from time to time and it is not possible for the Corporation’s management to predict all of such factors, or changes in such factors, or to assess in advance the impact of each such factors on the business of the Corporation or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statement contained in this Prospectus.
CAUTIONARY NOTE REGARDING FUTURE-ORIENTED FINANCIAL INFORMATION
To the extent any forward-looking statements in this Prospectus or in documents incorporated by reference herein, constitute "future-oriented financial information" or "financial outlooks" within the meaning of applicable Canadian securities laws, such information is being provided to demonstrate the potential benefits of the Acquisition and the Offering described herein and the reader is cautioned that this information may not be appropriate for any other purpose and the reader should not place undue reliance on such future-oriented financial information and financial outlooks. Future-oriented financial information and financial outlooks, as with forward-looking statements generally, are, without limitation, based on the reasonable assumptions of management of the Corporation and subject to the risks set out above under the heading "Cautionary Note Regarding Forward-Looking Information".
ENFORCEABILITY OF JUDGEMENT AGAINST FOREIGN PERSONS FOR CANADIAN INVESTORS
Steve Klein, Chief Strategy Officer and Director, David Miller, Director, Don Duet, Director and J. André De Barros Teixeira, Director, each reside outside of Canada and have each appointed the Corporation, 461 King Street West, Floor 2, Toronto, Ontario M5V 1K4, as their agent for service of process in Canada. Purchasers are advised that it may not be possible for investors to enforce judgments obtained in Canada against any person that resides outside of Canada, even if the person has appointed an agent for service of process.
ENFORCEABILITY OF CIVIL LIABILITIES FOR UNITED STATES INVESTORS
Flowr is a corporation governed by the Business Corporations Act (Ontario). A number of the Corporation’s Directors and officers, and some of the experts named in this Prospectus, are residents of Canada or otherwise reside outside of the United States, and a substantial portion of their assets, and a substantial portion of the Corporation’s assets, are located outside the United States. The Corporation has appointed an agent for service of process in the United States, but it may be difficult for holders of securities who reside in the United States to effect service within the United States upon those Directors, officers and experts who are not residents of the United States. It may also be difficult for holders of securities who reside in the United States to realize in the United States upon judgments of courts of the United States predicated upon the Corporation’s civil liability and the civil liability of the Corporation’s Directors and officers and experts under the United States federal securities laws.
The Corporation filed with the SEC, concurrently with the Corporation’s registration statement on Form F-10 of which this Prospectus forms a part, an appointment of agent for service of process on Form F-X. Under the Form F-X, the Corporation appointed DL Services Inc. as its agent for service of process in the United States in connection with any investigation or administrative proceeding conducted by the SEC and any civil suit or action brought against or involving the Corporation in a United States court arising out of or related to or concerning the Offering.
CURRENCY PRESENTATION AND EXCHANGE RATE INFORMATION
All references to "$" in this Prospectus refer to Canadian dollars, all references to "US$" are to United States dollars, and all references to "€" or "EUR" are to European Union Euro, unless otherwise indicated. The following table reflects the high and low rates of exchange for one United States dollar, expressed in Canadian dollars, during the periods noted, the rates of exchange at the end of such periods, and the average rates of exchange during such periods, based on the Bank of Canada daily exchange rate.
| Three months ended
March 31, | Year ended December 31, |
| 2019 | 2018 | 2018 | 2017 |
High for the period……………………................. | 1.3600 | 1.3088 | 1.3642 | 1.3743 |
Low for the period…………………….................. | 1.3095 | 1.2288 | 1.2288 | 1.2128 |
Rate at the end of the period………….................. | 1.3363 | 1.2894 | 1.3642 | 1.2545 |
Average rate for the period…………..................... | 1.3295 | 1.2647 | 1.2957 | 1.2986 |
On July 17, 2019, the Bank of Canada rate of exchange for converting United States dollars into Canadian dollars was US$1.00 = $1.3053.
The following table reflects the high and low rates of exchange for one European Union Euro, expressed in Canadian dollars, during the periods noted, the rates of exchange at the end of such periods, and the average rates of exchange during such periods, based on the Bank of Canada daily exchange rate.
| Three months ended March 31, | Year ended December 31, |
| 2019 | 2018 | 2018 | 2017 |
High for the period……………………................. | 1.5441 | 1.6124 | 1.6124 | 1.5330 |
Low for the period…………………….................. | 1.4938 | 1.4853 | 1.4791 | 1.3832 |
Rate at the end of the period………….................. | 1.5002 | 1.5867 | 1.5613 | 1.5052 |
Average rate for the period…………..................... | 1.5098 | 1.5544 | 1.5302 | 1.4650 |
On July 17, 2019, the Bank of Canada rate of exchange for converting European Union Euros into Canadian dollars was €1.00 = $1.4649.
PRESENTATION OF FINANCIAL INFORMATION
The financial statements of the Corporation incorporated by reference in the Prospectus are reported in Canadian dollars and have been prepared in accordance with IFRS as issued by the IASB.
The financial statements of Holigen included in this Prospectus are reported in EUR and have been prepared in accordance with IFRS as issued by the IASB.
NON-IFRS MEASURES
This Prospectus contains references to certain measures that are not recognized under IFRS. These non-IFRS measures are set out in Management’s Discussion & Analysis for the three and twelve months ended December 31, 2018 and 2017 (the "YE 2018 MD&A") and Management’s Discussion & Analysis for the three months ended March 31, 2019 (the "Q1 2019 MD&A") and are used by the Corporation as indicators of financial performance and may differ from similar computations as reported by other similar entities and, accordingly, may not be comparable. The Corporation believes these measures provide useful supplemental information that may assist investors in assessing an investment in the Offered Shares.
The non-IFRS measures used in relation to the Corporation in this Prospectus are Adjusted EBITDA and Price per Gram, which have the following meanings:
"Adjusted EBITDA" is defined as net loss, plus (minus) income taxes (recovery), plus (minus) interest income (expense), net, plus depreciation and amortization, plus share-based compensation, plus (minus) non-cash fair value adjustments on biological assets and inventory sold, plus listing expense costs and plus (minus) loss (gain) on investments.
"Price per Gram" is defined as the retail price of a gram of dried cannabis flower.
Investors are cautioned that this non-IFRS measure should not be considered an alternative to net earnings for the period (as determined in accordance with IFRS) as an indicator of the Corporation’s performance, or an alternative to cash flows from operating, financing and investing activities as a measure of liquidity. The Corporation’s method of calculating such non-IFRS measures may differ from the methods used by other issuers and, accordingly, non-IFRS measures used in relation to the Corporation may not be comparable to similar measures used by other issuers. Adjusted EBITDA has limitations as an analytical tool when compared to the use of net loss, as it excludes items which others may believe are normal for the operation of our business.
Please refer to the YE 2018 MD&A and the Q1 2019 MD&A for additional details regarding the non-IFRS measures used by the Corporation.
SUMMARY
The following is a summary of the principal features of the Offering and the Acquisition and should be read together with the more detailed information and financial data and statements contained elsewhere in this Prospectus.
Capitalized terms not otherwise defined in this Summary shall have the meanings ascribed to such terms in the Glossary of Terms attached as Appendix "A" to this Prospectus.
THE FLOWR CORPORATION
General Description of the Corporation
The Corporation was incorporated under the Business Corporations Act (Alberta) on June 1, 2016. On September 21, 2018, the Corporation completed a qualifying transaction under the TSX.V rules pursuant to a business combination agreement between Flowr Privateco, Needle and Needle Subco, which resulted in a reverse takeover of Needle by the shareholders of Flowr Privateco (the "Qualifying Transaction"). In connection with the completion of the Qualifying Transaction, on September 25, 2018, the Corporation continued from being governed under the Business Corporations Act (Alberta) to being governed under the Business Corporations Act (Ontario). On January 1, 2019, the Corporation and The Flowr Group Inc. amalgamated with the name of the amalgamated company being "The Flowr Corporation". The registered and head office of Flowr is located at 461 King Street West, Floor 2, Toronto, Ontario M5V 1K4.
The Corporation’s shares are listed on the TSX.V under the symbol "FLWR" and are approved for listing on the NASDAQ under the symbol "FLWR". The listing date will be confirmed prior to closing of the Offering. The Corporation intends to commence trading on NASDAQ one day after the pricing of the Offering.
The Corporation owns 100% of the issued and outstanding common shares of Flowr ULC and two U.S. shareholders own 100% of the issued and outstanding class A preferred shares in the capital of Flowr ULC (the "Flowr ULC Class A Shares"). See "Description of Capital Structure - Flowr ULC Shares" and "Material Contracts - Share Exchange Agreement" in the AIF.
The Corporation’s interest in Flowr Okanagan is held through Flowr ULC, which owns 100% of the issued and outstanding common shares of Flowr Okanagan.
Recent Developments
The Corporation is currently in negotiations to obtain committed financing from a syndicate of lenders led by ATB Financial ("ATB") in its capacity as lead arranger and administrative agent for up to approximately $50,000,000 of committed senior secured credit facilities (the "ATB Credit Facilities"). Pursuant to the ATB Credit Facilities, the Corporation will be permitted to use the recapitalization term facility and the revolving operating credit facility for general working capital purposes and the development facility for the development of the K1 Facility, the K2 Facility and the Flowr Forest. The ATB Credit Facilities will mature in three (3) years. Under the terms of the ATB Credit Facilities, the Corporation will be subject to certain financial, positive and negative covenants. In addition, the ATB Credit Facilities will provide for an accordion of up to $70,000,000. The ATB Credit Facilities are expected to be guaranteed by the Corporation's current and future direct and indirect material subsidiaries, except Holigen (together with the Corporation, the "Loan Parties") and expected to be secured by a general security agreement granting an interest over all the current and after acquired personal property and floating charge on all lands of the Loan Parties, as well as a collateral mortgage or debenture over the Corporation's freehold and leasehold interests in certain Kelowna facilities, non-disturbance agreement among ATB, the Corporation and the landlord of the Kelowna facilities, the Corporation's pledge in respect of its interests in the capital of its subsidiaries (other than Holigen) and certain other assets. The applicable margins for the ATB Credit Facilities will be based on certain performance-pricing grids, with availability in bank acceptance, letters of credit and prime loans. The ATB Credit Facilities are subject to negotiation and documentation and we cannot assure you that such facilities will be available to the Corporation on these or different terms. See "Risk Factors- The Corporation Expects to Enter Into the ATB Credit Facilities, but Such Facilities May Not Get Finalized or Be Available. In that Event, the Corporation Would Have to Find an Alternative Form of Financing. "
THE BUSINESS
Flowr is a rapidly growing cannabis company with the mission to provide consumers and medical patients unparalleled cannabis experiences through the production, marketing, distribution and sale of premium quality cannabis products.
Who is Flowr?
Flowr’s current operations are located in Canada. Flowr focuses on producing premium cannabis products for medical patients and recreational consumers throughout Canada, utilizing its purpose-built indoor and, upon licensing, outdoor cultivation and processing facilities located in the city of Kelowna in the Okanagan Valley Region of British Columbia, Canada (the "Kelowna Campus"). Flowr is currently focused on producing, marketing and distributing premium dried cannabis flower products (i.e. packaged dried cannabis flower and pre-rolls) and intends to strategically offer new product formats such as cannabis oils, vape technology, edibles, beverages and topical products, as demand patterns evolve and regulations permit the sale of such products. We believe demand for new product formats will consistently grow and that many of these products are best when infused with ingredients derived from greenhouse-grown cannabis. On February 12, 2019, Flowr purchased a parcel of land adjacent to its current operations in Kelowna, which it plans to use for greenhouse cultivation of cannabis for extraction and use in new product form factors upon such form factors being regulated for sale (the "Flowr Forest"). The Flowr Forest is intended to cultivate cannabis for extraction and use in new classes of cannabis upon regulation of the new classes of cannabis for commercial production and sale. Flowr believes that the demand for premium cannabis products in Canada will continue to grow, and we intend to continue to focus on providing customers with access to clean, consistent, terpene-rich products produced at the Kelowna Campus.
Where is Flowr Going?
On December 20, 2018, the Corporation announced it entered into a share purchase and subscription agreement to acquire a 19.8% equity interest in Holigen (the “SPSA”). The SPSA was amended and restated on May 8, 2019 (the “Amended and Restated SPSA”). On June 24, 2019, Flowr announced the entering into of the Holigen Purchase Agreement to acquire the remaining 80.2% of the issued and outstanding equity interests in Holigen that the Corporation does not already own. Holigen is a medical cannabis company with a facility in Australia and planned facilities in Portugal that are expected to provide finished medical cannabis products, pharmaceutical ingredients and plants and seeds to medical cannabis markets globally, where regulations permit. Holigen’s Australian facility is Good Manufacturing Process (“GMP”) licensed for certain activities, with full GMP licensing expected in 2020 upon finalization of construction. Holigen’s facilities in Portugal are expected to obtain GMP certification upon completion of construction in 2020. Holigen is in the final stages of obtaining a cultivation license for its outdoor and greenhouse facilities located on 65 hectares (approximately 7 million square feet) in Portugal and which is expected to have potential production capacity of approximately 500,000 kilograms of dried cannabis flower annually. The acquisition of Holigen will provide Flowr with a global platform to effectively serve growing demand for medical cannabis in European and Australian markets, where medical cannabis is regulated. See Details of the Acquisition.
Upon completion of the acquisition of Holigen and through the combined company platform, Flowr will operate a large scale, vertically integrated, licensed cannabis company with expertise in GMP-certified cultivation and manufacturing and will have a robust distribution platform for global markets.
Flowr’s focus in Europe and Australia will be the medical cannabis market. In Canada, Flowr intends to continue to expand its product offerings in recreational and medical markets with high-quality cannabis produced cost-effectively and at scale.
Industry-Leading Cultivation Expertise
We believe the foundation of our strategy is our team’s extensive indoor cannabis cultivation and production expertise. We believe that growing high quality cannabis economically at scale is challenging. It requires expertise in multiple areas, including plant genetic development, facility design and operations, cultivation operations and post-cultivation processes. This is particularly true in markets with stringent regulatory controls, such as those prescribed by Health Canada in Canada, which sets exacting standards for product cleanliness and quality. Our cultivation and operating team members are highly experienced in cultivating high quality cannabis at scale and have amassed significant experience in the design, construction and operation of numerous cannabis production facilities in Canada, which were built to comply with a variety of stringent regulatory predecessors to the Cannabis Act, including the MMAR, MMPR and the ACMPR. Our team includes experienced engineering and construction experts and a host of cannabis-focused research and development professionals.
We believe that our Canadian operating team’s expertise is highly transferable to other regulated cannabis markets and will provide a competitive advantage for successful entry into new markets. Our operating team’s experience to date has been focused on the design, construction and operation of indoor cannabis cultivation facilities and Flowr believes that this expertise can be transferred to greenhouse and outdoor cultivation facilities at both the Flowr Forest and Holigen’s operations in Portugal. However, there can be no guarantee that we will be able to translate the experience of our operating team with indoor growing facilities into successfully designing, constructing and operating outdoor and greenhouse cultivation facilities. See “Risk Factors - The Corporation May Not Be Able to Successfully Design, Construct or Operate Outdoor or Greenhouse Cultivation Facilities”.
Purpose-Built Facilities with Exacting Control of Cultivation Environments
Producing premium cannabis products requires an exacting level of control over every aspect of the cultivation, harvest, curing and post-harvest manufacturing processes. Upon completion of the Acquisition, Flowr is expected to have a global portfolio of purpose-built cultivation, production, processing and R&D assets, many of them being built or designed to GMP specifications and awaiting GMP licensing. We believe that this will position Flowr to produce high quality cannabis optimally suited for the markets and consumers it intends to serve. The following table describes our current and planned facilities:
| Facility | Location | Type | Expected Operational
Date(1) (2) | Estimated Initial
Capacity(2)(3) | Estimated Maximum
Capacity(2)(3) | Cost to Complete
(millions)(2)(4) |
| K1 Facility(5) | British Columbia, Canada | Indoor | Q3 2019 | 10,000kg | 10,000kg | $7.2 |
K2 Facility(6) | British Columbia, Canada | Indoor | H2 2021/2022 | 785kg(8) | 40,000kg(7) | $140.2 |
Hawthorne R&D Facility(5) | British Columbia, Canada | R&D | H2 2019 | N/A | N/A | $3.8 |
Flowr Forest(9) | British Columbia, Canada | Outdoor / greenhouse | H2 2019 (first phase) | 5,000kg(10) | 10,000kg | $2.4 |
| Aljustrel(11)(12) | Aljustrel, Portugal | Outdoor | H2 2019 | 1,500kg(14) | 500,000kg(13) | $67.0 |
Sintra(11)(12) | Sintra,
Portugal | Indoor + R&D | H2 2019 | 150kg(15) | 1,800kg | $10.5 |
| Sydney -
Phase 1(11)(16) | Sydney, Australia | Indoor | H1 2020 | 500kg(17) | 1,000kg | $10.5 |
Sydney -
Phase 2(11)(16) | Sydney, Australia | Indoor + R&D | To be determined | To be determined | To be determined (18) | To be determined |
(1) The Expected Operational Date reflects the anticipated time to reach the initial phase of commercialization for each facility, which, except for the K1 Facility, will be before construction is fully completed and prior to reaching estimated maximum capacity, as we construct in stages. Other than the K1 Facility (which is expected to be fully complete/built-out and operational upon receipt of an amendment to the Corporation's Health Canada licence for new phases by Q4 2019), Flowr expects that the construction and development of the facilities described in the table above will be completed over the course of the next two to three years. Such construction is expected to be completed in stages, with completed portions of the applicable facility becoming operational while other portions are under construction.
(2) Please see "Risk Factors" and "Forward-Looking Information".
(3) Subject to licensing, zoning and permitting. Estimated Initial Capacity reflects the facility's potential annual production capacity, as at the initial phase of commercialization and in many instances, prior to full completion. Estimated Maximum Capacity reflects the facility's potential production capacity, when fully complete/built-out and operational, based on targeted yields and maximum growing capacity, where applicable. Both Estimated Initial Capacity and Estimated Maximum Capacity include dried cannabis flower and dried cannabis flower equivalents. Except as otherwise described in the notes below, Flowr expects to be able to reach the capacities listed in the table above with (i) its available cash on hand, (ii) the net proceeds of the Offering and (iii) the cash expected to be generated from Flowr's and Holigen's operations, as such cash from operations becomes available. The anticipated timelines to scale each facility to its maximum production capacity (described below) are based on the time required to reach full build-out and operation and are not subject to additional funding (except as described in the notes below).
(4) The Cost to Complete represents the forecasted remaining costs associated with the construction and development of each of the facilities to reach full completion/operation. The total aggregate amount of funding currently forecasted to be required to complete the construction of the facilities in the table above is approximately $241,600,000.
(5) K1 Facility is fully funded. The first floor of the Hawthorne R&D facility is fully funded.
(6) Expected to be funded with the proceeds from the Offering and the ATB Credit Facilities if obtained.
(7) This estimate is based on the assumption that the ATB Credit Facilities will be available to Flowr. In the event that the ATB Credit Facilities are not available, Flowr would likely construct a facility that is approximately half the size of the planned K2 Facility, which would result in half of the expected capacity of the K2 Facility from the capacity disclosed in the table above (such amount being approximately 20,000 kg). See "Use of Proceeds - Business Objectives and Milestones".
(8) Assuming achievement of the estimated initial capacity on the anticipated timeline, the annual production is expected to increase to 29,400 kilograms in the year 2022 and is expected to reach estimated maximum capacity of 40,000 kilograms in 2023.
(9) The remaining costs associated with the construction and development of the Flowr Forest are expected to be funded with the ATB Credit Facilities if obtained.
(10) Assuming achievement of the estimated initial capacity on the anticipated timeline, the annual production is expected to increase to 10,000 kilograms, reaching estimated maximum capacity, in the year 2020.
(11) Subject to the closing of the Acquisition. See "Risk Factors".
(12) Expected to be funded with the proceeds of the Offering and existing or proposed debt facilities available to Holigen.
(13) This estimate is based on the following material factors and assumptions: (i) the Aljustrel site, when fully planted and operational, is expected to have approximately 5,060,000 square feet of grow space; (ii) the anticipated yield in the outer years is expected to reach 100 grams per square foot per year; and (iii) with approximately 5,060,000 square feet of canopy at a targeted yield of 100 grams per square foot per year, the anticipated output of cannabis at the Aljustrel site is expected to be approximately 500,000 kilograms (5,060,000 multiplied by 100 grams/sq.ft./year divided by 1,000).
(14) Assuming achievement of the estimated initial capacity on the anticipated timeline, the annual production is expected to increase to 30,000 kilograms in the year 2020 and can reach the estimated maximum capacity of 500,000 kilograms in 2021, as the Aljustrel site is a low cost outdoor growing operation. Flowr will assess whether it will produce the full estimated maximum capacity in 2021 based on demand for its products.
(15) Assuming achievement of the estimated initial capacity on the anticipated timeline, the annual production is expected to increase to 1,800 kilograms, reaching estimated maximum capacity, in the year 2020.
(16) Expected to be funded with the proceeds of the Offering.
(17) Assuming achievement of the estimated initial capacity on the anticipated timeline, the annual production is expected to increase to 1,000 kilograms, reaching estimated maximum capacity, in the year 2021.
(18) Flowr has the option to construct Sydney - Phase 2 facility based on Phase 1 specifications.
R&D Engine to Drive Leadership, Enhanced by Hawthorne Partnership
Building industry-leading R&D capabilities provides us with a competitive advantage by providing proprietary insights and an intimate understanding of the full genetic potential of the cannabis plant. We believe that these capabilities will allow us to maximize cultivation yields and support the economic production of high quality derivative products and the development of new product formats to bring to market.
We believe that our cultivation and production capabilities are industry leading. The caliber of Flowr’s R&D expertise was validated in January 2018 through Flowr’s strategic R&D alliance with Hawthorne Canada Limited ("Hawthorne"). Hawthorne is a subsidiary of The Scotts Miracle-Gro Company, a NYSE-listed supplier of lawn, garden and hydroponics products.
Hawthorne conducted a review of Canadian federal cannabis licence holders in search of a partner with cultivation expertise and the ability to conduct industry-leading R&D work. Hawthorne’s search was driven by a strategic imperative to engage a federal cannabis licence holder capable of producing industry-leading yields and quality, in order to create successful case studies highlighting the effectiveness of Hawthorne’s cultivation products. Flowr and Hawthorne ultimately entered into a strategic R&D alliance, and Flowr has commenced construction of an R&D facility in collaboration with Hawthorne, as further described below. We believe that the Hawthorne-related work at this R&D facility will support the development and refinement of Hawthorne’s existing products and new products, and provide Flowr with key insights into optimal growing conditions for its products.
Focus On Quality Across Geographies and Product Formats
We cultivate, produce and market premium cannabis products targeting discerning recreational consumers in Canada under the Flowr® brand. Medical patients in Canada are targeted under the FlowrRx® brand. Following the completion of the acquisition of Holigen, Flowr intends to engage in the production and distribution of medical cannabis products in Europe and Australia under the Holigen brand.
While certain industry participants may purchase cannabis products wholesale from third parties for distribution, we believe this practice can negatively impact product quality and consistency as well as experience. As part of our premium brand strategy, we intend to maintain product quality and consistency by only using cannabis cultivated by Flowr or Holigen in Flowr-branded products. We believe that both medical patients and recreational consumers in Canada prefer premium quality cannabis products and are willing to pay a premium for such products. We believe that premium product quality, as defined by a rich sensory experience, a consistent psychoactive effect, and robust terpene profiles, is essential to our target consumers’ purchasing decisions and will continue to command a sustainable price premium in the market.
In developing premium dried cannabis flower products, we strive to deliver against the three pillars of quality that govern our processes, from seed to sale:
1. Consistency
Delivering a consistent experience for every usage occasion is essential for recreational and medical consumers to develop a trusted relationship with our brand. Doing so requires an exacting level of control over every aspect of the cultivation, harvest, curing and extraction processes. Minute variations in the environmental conditions during cultivation and harvest can drive wide variations in the chemical composition of cannabis produced with identical starting genetics. Flowr’s meticulous analysis and control of every variable in the production process provides a high level of consistency in our products and in our customers’ experiences.
2. Full Spectrum
We cultivate, harvest and cure - and, where relevant, process into cannabis concentrates, other cannabis extracts and edible cannabis - with the goal of delivering the broadest possible spectrum expression of the terpene and cannabinoid profile that results from plant’s genotype and growing conditions. Across product formats, we endeavor to produce products that are "true to the plant". The result is cannabis flower with satisfying and redolent aromas and flavors, which also have an essential role in producing a richly textured psychoactive experience. For concentrates such as live resin packaged in cartridges or vape pens, once regulated for sale, Flowr expects to also focus on extraction and manufacturing processes that preserve the full spectrum of the plant’s terpene and cannabinoid profile.
3. Clean
Due to the role played by terpenes in delivering heightened sensory experiences, we believe that preserving the terpene profile of dried cannabis flower products and, where relevant, certain derivative products, is a critical differentiating factor in producing premium products. Terpenes are components of the plant which have been shown to contribute tastes and aromas.
To date, approximately half of the Corporation’s cannabis flower products produced at the K1 Facility, which is 40% operational, have been produced without the use of irradiation. Irradiation is a "kill step" used to eliminate microbial contamination that may develop in the cultivation or curing process. Flowr is focused on reducing and ultimately eliminating the need for irradiation due to its potential negative effect on terpene profiles.
Flowr believes that operating meticulously clean, rigorously-designed indoor facilities that limit the need for irradiation is a critical factor in producing a premium dried cannabis flower product. We do not believe that other federal cannabis licence holders share the same focus to eliminate irradiation and that our achievements in reducing irradiation to date, and our targeted goal of fully eliminating the step, enhances our product quality and is a significant competitive advantage that supports our premium brand strategy.
We believe that by consistently delivering premium dried cannabis flower products, we will develop a relationship of trust with our consumers and establish a premium brand position. This relationship and associated brand equity will then be leveraged, once regulations permit, and applied to premium new form factor derivative products, including vapes, infused beverages, capsules and edibles.
Based on data received from the OCS and Nova Scotia Liquor Corporation, Flowr’s products in these provinces’ recreational markets have achieved retail price points ranging from a 25% premium to prevailing average pricing to as high as a 55% premium with an average premium of 40% between October 17, 2018 to June 16, 2019. We expect to sustain significant premiums over the medium to long term.
Our industry-leading price points are reflected in recent Ontario and Nova Scotia recreational sales data from October 17, 2018 to June 16, 2019, as illustrated below:

Since comparable recreational sales data is not currently available from the other provinces in which Flowr’s products are sold (British Columbia, Manitoba and Saskatchewan), we cannot provide similar retail price point comparisons for these provinces at this time.
Product-First Marketing Strategy Leverages Our Enthusiast Consumers
Flowr has to date executed a "product first" marketing strategy for its recreational consumers in Canada which leverages the quality of Flowr's dried cannabis flower products to build brand awareness organically through budtenders (i.e. product information, education and recommendation specialists present at retail locations), influencers, and word of mouth. To support our marketing strategy, we have hired a marketing specialist that has extensive consumer packaged goods experience with companies such as British American Tobacco and Japan Tobacco International.
Flowr’s focus is on, and its target market is, what we define as the “Cannabis Connoisseur” segment. We believe these consumers are more knowledgeable and consider themselves to be a trusted source of information for friends and family on cannabis. According to the Colorado Department of Revenue, frequent users (who consume cannabis more than 20 days per month) comprise approximately 27% of cannabis consumers but consumed over 80% of the volume of cannabis products sold in 2017 in the state of Colorado, which is considered a mature recreational market.
Our target consumer represents a potential marketing asset as an ambassador for the Flowr brand and for the premiere quality level of our products. Because the Canadian regulatory environment currently restricts the ability to use conventional marketing and promotional tactics, we believe this approach will serve us well for the near to medium term. As the market evolves, we expect the least-frequent consumers to become more knowledgeable and thus more likely to follow the lead of the “Cannabis Connoisseur” in terms of product purchases. We believe that by positioning Flowr® as a trusted premium brand, we will see some increase in market share in the long-term as the knowledge of the less-frequent consumers increases. In parallel and as the market matures further, we expect to devote additional resources to in-store merchandising, event-based education, and consultative selling within retailers, using both dedicated in-house personnel and third party brokerage and service providers. In October 2018, we also entered into a strategic partnership with Ace Hill Beer Company, a craft beer brand in Canada, to create Ace Valley, a new brand of premium cannabis products for the Canadian recreational market. The collaboration gives us information about the recreational consumer’s preferences and has allowed us to capitalize on the Ace Hill team’s expertise in marketing and brand building in a highly regulated market.
World Class Facilities Expected to Drive Industry-Leading Yields and Profit Margins
Flowr believes that the profitability of its cultivation operations is driven by yield per square foot. We believe that our yields per square foot can be increased significantly from our already elevated levels - and ultimately, we believe we can continue to achieve yields per square foot that we believe exceed industry norms.
We believe that Flowr has been able to achieve higher pricing due to the premium nature of its product offerings. Combined with Flowr’s construction, design and cultivation expertise, focus on cost optimization through standardization of production capabilities and, upon consummation of the Acquisition, lower cost international production footprint, Flowr will be positioned to maximize its profitability.
Global Footprint to Capture Worldwide Opportunities
Following the Acquisition, Flowr will have a global footprint which will allow it to leverage its cultivation expertise to supply premium products, brands and intellectual property developed in Canada, across medical and recreational markets worldwide as regulations permit. We intend to tailor our market penetration strategies to each of our markets, production assets and target customer bases.
Upon closing of the Acquisition, we will operate a large scale, vertically integrated, licensed cannabis company with expertise in GMP-certified manufacturing. We will also have a distribution platform for global markets in Europe, Australia and surrounding countries, where sales of cannabis are permitted. Driven by our cultivation expertise, we will focus on targeting the medical cannabis markets across those geographies with high-quality cannabis produced cost-effectively for extraction into active pharmaceutical ingredients (“API”).
Holigen is currently developing production, extraction and manufacturing facilities in Portugal and Australia. Holigen’s Aljustrel project in Portugal has been designated a Project of National Interest (“PIN”) by the Portuguese government. This designation provides Holigen with the ability to quickly move this project through regulatory licensing processes and potential access to funding. In Aljustrel, Portugal, Holigen is expected to build and operate a cannabis cultivation site with a combined potential production, manufacturing and R&D area of more than 7,000,000 square feet and potential annual dried cannabis flower capacity of approximately 500,000 kilograms. The Aljustrel site’s processing and manufacturing facilities will be built to GMP specifications and the cultivation area will be strategically located in proximity to a nearby dam providing reliable access to water and power. It is expected that Holigen will cultivate the majority of its cannabis product at its Aljustrel site, and distribute medical products and APIs to markets in Europe, Australia and surrounding countries, where cannabis sales are permitted, under the Holigen brand. Holigen will have an indoor cultivation and manufacturing facility in Sintra, Portugal. Upon completion of construction, this facility will include smaller indoor growing areas for high quality indoor grown cannabis, and manufacturing and packaging areas to be used for extraction and packaging of cannabis grown in Portugal.
In Australia, Holigen is licensed to cultivate and manufacture medical cannabis products, subject to additional permitting which is in process. It was also granted GMP approval by the Therapeutic Goods Administration for labelling, secondary packaging and release for supply in November 2018. Its full manufacturing facility is expected to be fully constructed in the second half of 2019 and receive GMP approval in 2020. The manufacturing space is located in Sydney and is designed with excess manufacturing capacity to process not only cannabis cultivated in Australia, but also product imported from Holigen’s Portugal operations As part of its distribution strategy, Holigen will partner with Anspec Pty Limited. (“Anspec”) in Australia, a privately-owned specialty pharmaceutical and medical distributor and exporter in Australia and abroad. Upon successful integration with Holigen, we expect that this relationship will provide Flowr with access to export pathways to 37 countries globally. To the extent that the on-site processing and manufacturing facility is not constructed, the Corporation expects to export and sell dried or fresh cannabis in countries where importation and sales of such products are permitted, such as Germany. Peter Comerford, an executive officer of Holigen and a proposed executive officer of Flowr post acquisition of Holigen, is the Chief Executive Officer of Anspec and provides a continuity of this distribution relationship to the combined company. Mr. Comerford receives compensation from Anspec for his services as Chief Executive Officer of Anspec, and is entitled to certain payments on the sale of Anspec to a third party purchaser.
Holigen’s operations represent strategic production assets and geographically optimal gateways to distribute into current and future markets in Europe, Australia and surrounding countries, where sales of cannabis are permitted, particularly given the preference displayed in these markets for locally produced cannabis. Upon closing of the Acquisition, we believe the combined company’s proprietary cannabis cultivation know-how, regulatory familiarity, complex pharmaceutical and GMP construction expertise, and pharmaceutical distribution expertise, will give it the scalability and capability needed to service the global cannabis markets.
In addition to providing access to Australia and Europe, we expect that the assets in these geographies will serve as a platform for further global expansion. As a trusted trade partner of many Asian nations, we believe that Australia is well positioned to be a leading exporter of cannabis products to the Asia-Pacific region, and that the combined company will be able to capitalize on such opportunities to enter new markets in Asia if and when regulatory regimes in this region regulate cannabis products.
FLOWR’S STRENGTHS
Flowr’s strategy is focused on leveraging its following core strengths to exploit its competitive advantage:
Cultivation expertise affirmed through partnership with a hydroponic products industry leader.
Facilities designed to maximize yield and profitability.
Highest quality products and premium brands further drive profitability.
Expertise and infrastructure to capture global opportunities.
Highly seasoned management team with aligned interests.
Cultivation Expertise Affirmed Through Partnership with a Hydroponic Products Industry Leader
Our cultivation team members have extensive experience with the production of high quality indoor cannabis. They have designed, built and operated over 15 cannabis production facilities, in aggregate, across multiple teams and under a variety of stringent regulatory structures, including the MMAR, the MMPR and the ACMPR. We believe the team’s robust cultivation-related proprietary know-how and trade secrets create a long-term strategic advantage relative to competitors - particularly as awareness grows about the high degree of difficulty involved in the consistent production of high quality cannabis.
Our cultivation infrastructure has been specifically designed to execute on our strategy to produce the highest quality cannabis flower, at the highest levels of profitability, for each of our products. For our dried cannabis flower products we use only our highest quality, premium cannabis flower. We believe that the use of premium, indoor-grown flower as an input is critical to dried cannabis flower products and important to our “Cannabis Connoisseur” target market. Flowr’s branding as a premium cannabis supplier is built on the superior quality of our indoor-grown dried cannabis flower. For products such as vapes, capsules and beverage infusions, we recognize that the quality tier of the underlying flower is less strict relative to dried cannabis flower products. We will therefore apply our cultivation expertise to produce high quality flower at lower costs from our future outdoor and greenhouse facilities to produce branded products that are expected to be market-leading.
The caliber of Flowr’s cultivation expertise was validated by Flowr’s 2018 selection as a R&D partner of Hawthorne. Hawthorne is a subsidiary of The Scotts Miracle-Gro Company, a leading supplier and distributor of lawn, garden and hydroponics products. Hawthorne conducted a review of Canadian federal cannabis licence holders in search of a partner with cultivation expertise and the ability to conduct first rate R&D work. Hawthorne’s search was driven by a strategic imperative to engage a federal cannabis licence holder capable of producing industry-leading yields and quality in order to create successful case studies highlighting the effectiveness of Hawthorne’s cultivation products. Hawthorne ultimately entered into a R&D alliance with Flowr to construct an R&D facility.
Upon completion of the Acquisition, we believe that our industry-leading R&D capabilities across the combined company’s global footprint will provide a sustainable competitive advantage. Insights drawn from our understanding of the full genetic potential of the cannabis plant have supported and will continue to support optimized cultivation yields, highly economic and efficient production of quality derivative products and the development of new product formats to bring to evolving markets.
Facilities Designed to Maximize Yield and Profitability
Similar to a real estate asset, we believe that profitability is influenced by efficiency per square footage of operations. Given Flowr’s proprietary cultivation knowledge and system utilized for growing, we expect our yields to increase with time through the optimization of growing conditions for each strain. We expect that the resulting operating leverage coupled with the premium pricing will further enhance our profitability.
We believe we can continue to achieve yields per square foot which we believe exceed industry norms through further “dialing in” the process by which the production team incrementally enhances its understanding of the nuances of the room and the environmental controls to maximize yields for each cultivar. In addition, through our R&D partnership with Hawthorne, we will have access to an array of systems, products and capabilities that may be developed within the partnership that we expect could help lower operating costs and/or increase yields resulting in incremental margin expansion. In the aggregate, we expect the compounding effect of implementing these incremental, steady improvements will result in higher yields that will drive stronger margins.
We believe we can leverage our expertise in maximizing cultivation yields at the K1 Facility to our other global indoor and outdoor facilities. We will design and build Flowr’s larger Kelowna 2 facility (the “K2 Facility”) as well as Holigen’s Sintra and Sydney indoor facilities to the same exacting standards and with the same focus on operating efficiency and cost containment as our K1 Facility. We believe this will provide us with powerful operating efficiencies and yield advantages, driving profitability across all of our global assets, regardless of cultivation format.
Highest Quality Products and Premium Brands Further Drive Profitability
We are building the Flowr® brand on the foundation of premium dried cannabis flower. Our recreational products target the “Cannabis Connoisseur”. We believe that by consistently delivering high quality cannabis flower products, we will develop a relationship of trust with our consumers and establish a premium brand position which can be directly extended to premium derivative products such as vapes, infused beverages, capsules and edibles as they become regulated for commercial production and sale.
Our strategy for growing and supplying premium cannabis products starts with the selection of cannabis genetics. We screen for and select cannabis cultivars based on what we consider to be the optimal genetic profile, factoring in growth characteristics, cannabinoid levels, terpene profiles, and anticipated distinctiveness of the end product to consumers. Based on this screening process, we cultivate multiple genetic cultivars in order to curate a targeted portfolio across consumers, usage occasions and product formats.
Based on data received from the OCS and Nova Scotia Liquor Corporation, Flowr’s products in these provinces’ recreational markets have achieved retail price points ranging from a 25% premium to prevailing average pricing to as high as a 55% premium with an average premium of 40% between October 17, 2018 to June 16, 2019. We expect to sustain significant premiums over the medium to long term. Flowr’s medical FlowrRx® dried cannabis flower products are currently the highest priced dried cannabis flower on the Medical Cannabis by Shopper's Drug Mart Inc. website
We believe that our premium price position in Canada will be sustainable over the medium to long term based on our consistent product quality and our premium brand. We further believe that this expectation aligns with the dynamics we observe in California, where producers of high quality cannabis have consistently achieved sustained premium prices, even as lower-quality cannabis flooded the market. Although the supply of low-priced cannabis has driven down the average price per gram, consumers in California still show a willingness to pay materially higher prices for higher quality cannabis. Based on 2018 California sales data provided by BDS Analytics, we estimate that approximately 40% of the total volume and 56% of the aggregate dollar value of dried cannabis flower is transacted at price points in excess of US$10. We believe that this shows a sustainable willingness of consumers to pay premium prices for higher quality cannabis.
Expertise and Infrastructure to Capture Global Opportunities
We believe our industry-leading cultivation expertise and premium brands can be leveraged across the combined company’s global portfolio of cultivation and marketing assets to execute market penetration strategies specifically tailored to each of the markets and customers we intend to target.
In Europe and Australia, upon closing of the Acquisition, Holigen will leverage Flowr’s cultivation expertise to design and build industry-leading outdoor facilities principally focused on producing quality cannabis produced cost-effectively for extraction into APIs in the medical markets. We believe the combined company’s expertise on the cannabis plant and on extraction techniques will be instrumental in securing a highly advantaged position in the European market, which is primarily oil-based. We believe that this expertise, combined with Holigen’s experience in pharmaceutical opiate extraction and pharmaceutical grade construction design, will enable the combined company to produce cannabis products and formulations that optimally address patients’ medical needs.
In Aljustrel, Portugal, Holigen is expected to construct and operate a cannabis cultivation site that is expected to have a combined potential production, manufacturing and R&D area of more than 7,000,000 square feet and potential annual dried cannabis flower capacity of approximately 500,000 kilograms.
In Australia, the combined company will leverage the Holigen brand to offer medical cannabis products. Cannabis for this market will be sourced from Holigen’s low cost operations in Portugal as well as the indoor cultivation facility in Sydney which will produce high quality dried cannabis flower.
Holigen’s Sintra, Portugal and Australia facilities also expect to have sophisticated R&D and laboratory capabilities, focused principally on the development of market-leading extraction capabilities to bring APIs and oil-based medical products to market in their respective geographies.
Upon completion of the Acquisition, the combined company expects that Holigen’s assets in Europe and Australia will serve as a platform for further global expansion. As a trusted trade partner of many Asian nations, we believe that Australia is well positioned to be a lead exporter of cannabis products to the Asia-Pacific region, and that we will be able to capitalize on such opportunities to enter new markets in Asia if and when regulatory regimes in these regions regulate cannabis.
Highly Seasoned Management Team with Aligned Interests
Flowr’s Board of Directors and executive management team is made up of former government officials, pharmaceutical and consumer goods executives, and executives with significant capital markets and M&A experience. In particular, Flowr’s Board members have consumer goods experience with companies such as The Coca-Cola Company, Interbrew, Campbells, McCain Foods and Frito-Lay. Flowr’s Chairman and its CEO, among others on the team, were early investors in both private and publicly traded cannabis firms in multiple legal markets. Through these experiences, they developed an understanding of the cannabis markets and the critical strategic factors essential for success in such markets.
We believe the strength and track record of the executive leadership team, combined with the operational excellence of the design, construction and cultivation team, will position Flowr for global expansion, access to capital and the successful execution of its strategic priorities.
With the integration of Holigen, Flowr will also gain the talents of a highly skilled management team. The Holigen team’s equity position in Flowr provides it with shareholder and company alignment. The Holigen team has extensive experience in the pharmaceutical, distribution and construction industries. Through senior engineering roles in the opiate industry, the team has gained valuable cultivation and extraction experience. The Holigen team includes senior GMP specialists, construction executives and regulatory pharmacists experienced in dealing with European regulatory environments and pharmacopeia. Flowr believes this expertise will provide it with significant operational advantages as cannabis markets develop globally.
Insiders, led by Steve Klein and Vinay Tolia, have invested approximately $32 million in the Corporation, and collectively own approximately 60% of the Corporation on a fully diluted basis (excluding equity incentives such as options, warrants and/or restricted share units) prior to completion of the Offering and the Acquisition.
THE INDUSTRY
The cannabis market in which Flowr operates is an emerging and rapidly expanding industry, with new developments occurring frequently. In many countries, cannabis has seen a paradigm shift in its regulation and perception over the last few years, resulting in the accelerated growth of the global legal addressable market. The United Nations estimates the global cannabis market (regulated and illicit) today to be worth approximately US$150 billion annually. Further regulation of medical and recreational markets is expected to lead to the absorption of illicit markets and dramatically expand the total addressable market for current legal market participants.
Canadian Market
On October 17, 2018, Canada became the first major industrialized nation to regulate the sale of cannabis for recreational use. Deloitte estimated the total cannabis market in Canada, including the regulated and illicit markets, as well as legal recreational products, is expected to generate up to $7.17 billion in total sales in 2019. Regulated sales are expected to contribute more than half of this total up to $4.34 billion in the first year. Current legal product formats in Canada include dried cannabis flower and cannabis oils; however, additional form factors, including vapes, edibles, tinctures, beverages, and topicals, are expected to be legalized in late 2019. Deloitte estimates the Canadian market for cannabis edibles and infused products to be worth approximately $2.7 billion, with approximately $1.6 billion attributable to edibles. This estimate is additive to the $7.2 billion 2019 total Canadian market estimate provided by Deloitte in an earlier report. We believe the addition of these new consumer-friendly formats will significantly expand the size of Flowr’s addressable Canadian market, as convenient, discreet, and precisely dosage-metered products such as vape pens, capsules, beverages and edibles, bring in new consumers less familiar with cannabis and less accustomed to smoking or vaping dried cannabis flower.
The Canadian medical cannabis market is experiencing rapid growth, with Health Canada reporting registered users rising more than tenfold between 2014 and 2018. Deloitte estimated that this segment will generate up to $1.79 billion in sales in 2019.
European Market
An estimated 23 million individuals in the European Union and Norway consumed cannabis at least once in 2018. With a market of approximately 743 million people and an estimated total healthcare spend of €2.3 trillion, it is estimated that Europe will be one of the largest medical cannabis markets in the world. Insurance companies in Germany, Denmark, and Italy are now covering medical cannabis prescriptions.
Further examination of various countries in the European region present different regulatory pathways that may be adopted domestically. For example, in the United Kingdom, as of November 2018, medical cannabis products became legal and the distribution model is via registered specialist doctors prescribing cannabis to patients. In Portugal, the country introduced medical cannabis reform in June 2018 that allowed physicians to prescribe medical cannabis. Similar to the United Kingdom, Germany, legalized medical cannabis in March 2017. Germany represented 68% of the total sales in kilograms of flower, representing 76% of the total revenue from cannabis sold, as retail value in Germany is significantly higher than in the Netherlands.
Ultimately, each country will have slightly differing laws for its domestic cannabis market as well as the import of cannabis into the country. Today, regulations in the United Kingdom and Germany allow for the bulk import of medical cannabis to meet the future demand at pharmacies, subject to obtaining the proper import permits adhering to each respective country’s medical cannabis regulatory laws.
Australian Market
Legislation for legalised cannabis occurred for medical use and came into effect in October 2016. The cultivation, production and manufacturing of cannabis and cannabis products for medical and scientific use is licenced by the newly established Office of Drug Control (“ODC”). The manufacturing, registration and supply of medicinal cannabis products are regulated by the Therapeutic Goods Administration (“TGA”). Medicinal cannabis products would be classified under prescription medicines, and accordingly would be subject to the Therapeutic Goods Act. Since June 2018, approximately 7,000 additional medical patients have been approved in Australia. The Australian government has also indicated that they plan to become the fourth country in the world to federally legalize medical cannabis exports to capitalize on the cannabis opportunity, with the first export completed in August 2018. The export of medicinal cannabis products was legalised in February 2018 through the Narcotic Drugs Amendment (Cannabis) Regulations 2018. The current regulations allow the following products to be exported:
• Medicinal cannabis products manufactured in Australia under a GMP Licence
• Medicinal cannabis products listed as export-only or registered in the ARTG
• Extracts of cannabis (or cannabis resin) manufactured under a Narcotic Drugs Act 1967 Licence that are not in the final dosage form
Management believes that Australia in particular, will be the key reference market for pharmaceutical quality in the region and products manufactured to the country’s highest medical standards (e.g., GMP, TGO-93 and TGO-77) will be perceived by other countries within the region to be reliable APIs.
Flowr expects the Australian market to be driven by the legal Australian medical market, which through its established trade relationships is well positioned to serve as a trade gateway to the rest of the Asia-Pacific region if and when regulatory regimes in this region legalize cannabis products.
STRATEGIES FOR GROWTH
Our advantages in cultivation, yields, premium products and management provide Flowr with opportunities to grow our business and realize our vision of being a global leader in the cannabis industry. We are focusing on driving growth in the business by executing three critical initiatives:
Expanding cultivation and production capacity at the Kelowna Campus in Canada.
Developing cultivation and production capacity at European and Australian campuses upon completion of the Acquisition.
Launching new products which leverage our core strengths.
Expand Cultivation and Production Capacity at the Kelowna Campus in Canada
We believe that demand for high quality cannabis products will continue to exceed supply in the medium to long term. To address this demand, we continue to invest in and build out our cultivation and production infrastructure.
Currently, 40% of the K1 Facility is fully licensed and operational. By the end of the third quarter of 2019, we expect construction of the K1 Facility to be completed. When fully licensed and operational, the K1 Facility will have 20 individually-controlled grow rooms, with a targeted production capacity of 10,000 kilograms of dried cannabis flower on an annualized basis.
Subject to available funding from this Offering and the ATB Credit Facilities, we intend to break ground on the adjacent K2 Facility prior to the first half of 2020 and, subject to licensing, expect to complete the K2 Facility in the second half of 2021/calendar year 2022 though certain grow rooms will come online and be operational at partial capacity prior thereto. When fully licensed and operational, the K2 Facility will comprise approximately 80 individually controlled grow rooms with a targeted production capacity of 40,000 kilograms of dried cannabis flower on an annualized basis. Collectively, the state-of-the-art K1 Facility and K2 Facility are expected to have an annual production capacity of 50,000 kilograms of premium dried cannabis flower.
In addition, when fully constructed the Flowr Forest will consist of approximately 14 acres of land for outdoor/greenhouse grow cannabis cultivation, primarily to service the Canadian market for premium derivative products such as vapes, infused beverages, capsules and edibles, once these product formats are regulated for sale. We expect, subject to obtaining necessary licences and permits, that the Flowr Forest will have a targeted annual production capacity of 10,000 kilograms of dried cannabis flower.
Develop Cultivation and Production Capacity at European and Australian Campuses Upon Completion of the Acquisition
In Aljustrel, Portugal, Holigen is expected to build and operate a cannabis cultivation site, which, is expected to have a combined potential production, manufacturing and R&D area of more than 7,000,000 square feet and licensed annual dried cannabis flower capacity of approximately 500,000 kilograms, including processing and manufacturing facilities expected to be GMP-certified in 2020. It is expected that the majority of cannabis product that the combined company will distribute to the European and Australian markets will be cultivated at this site. Holigen expects to produce cannabis at the Aljustrel site at some of the lowest production costs in the world. These European operations will also include an indoor cultivation and manufacturing facility in Sintra, Portugal. When constructed, this facility will include smaller indoor growing areas for high quality indoor grown cannabis, and manufacturing and packaging areas to be used for extraction and packaging of cannabis grown in Portugal. The Australian facility is licensed to cultivate and manufacture medical cannabis products, and was granted GMP approval by the Therapeutic Goods Administration for labelling, secondary packaging and release for supply in November 2018. Final GMP approval for manufacturing at the Australian facility is expected in 2020.
Launch New Products which Leverage our Core Strengths
We believe the brand position of our premium dried cannabis flower products produced in Canada will provide a “brand halo” which will allow us to successfully extend into new product markets based on derivative formats which are pending regulation for sale. We believe that the addition of these new consumer-friendly formats, when regulated for sale, will expand the size of our addressable market, because convenient, discreet and precisely dosage-metered products may bring in new consumers less familiar with cannabis and less accustomed to smoking or vaping dried cannabis flower.
The new classes of cannabis become saleable on October 17, 2019, subject to federal cannabis licence holders providing 60 days’ notice that a new product will be sold, as required by the Cannabis Regulations, which should result in cannabis products from within the new classes of cannabis being available for sale in both medical and recreational markets at the end of December, 2019 at the earliest.
Accordingly, in preparation for the regulation of these additional form factors following the legislative amendments expected later this year, we are investing in the buildout of a state-of-the-art, purpose-built extraction, formulation and packaging facility that will enable us to launch these new derivative products. We have leased approximately 12,000 square feet in an offsite Kelowna facility outfitted with a full test kitchen, permitting the rapid development of edible cannabis products as well as our understanding of extracts and fractionation techniques.
We are in the process of developing a Flowr branded vape product that we expect to market to recreational consumers, when regulations permit. We plan to extract all oils and resins in-house to ensure that we are able to create differentiated products that are consistent with our positioning in the dried cannabis flower market. We expect our extraction and proprietary curing processes to generate an output that is nearly identical to the chemical profile observed in cannabis from a sensory perspective. Our initial vape product is designed with quality hardware to deliver a flavour profile that is consistent throughout the use of an individual cartridge and is expected to have low failure rates. We anticipate being able to bring a vape product to market in line with the recently announced Government of Canada timeline for new classes of cannabis to be regulated for commercial production and sale.
As observed in legal U.S. markets, we believe that the new product formats will ultimately command a meaningful portion of the cannabis market in Canada, without significantly cannibalizing demand for high quality dried cannabis flower. Deloitte estimates that the annual Canadian market for cannabis products from the new classes of cannabis is worth approximately $2.7 billion, comprised of edibles ($1.6 billion), cannabis-infused beverages ($529 million), topicals ($174 million), concentrates ($140 million), tinctures ($116 million), and capsules ($114 million); with almost one in four Canadian likely to participate in the consumption of these products. The upcoming regulations are expected to create significant growth opportunities for cannabis producers in Canada.
In addition, upon completion of the Acquisition, we expect to launch a portfolio of medical and wellness cannabis derivative products designed for the European and Australian markets under the Holigen brand.
SELECTED HISTORICAL AND PRO FORMA CONSOLIDATED FINANCIAL INFORMATION
The following tables set forth Flowr’s selected consolidated financial information (i) for the year ended December 31, 2018, (ii) for the year ended December 31, 2018 pro forma after giving effect to the Acquisition, (iii) for the three months ended March 31, 2019; and (iv) for the three months ended March 31, 2019 pro forma after giving effect to the Acquisition.
The selected unaudited pro forma consolidated financial information set forth below and included elsewhere in this Prospectus is for illustrative purposes only and not necessarily indicative of results of operations that would have occurred as at or for the year ended December 31, 2018 or for the three months ended March 31, 2019 had the Acquisition been completed on January 1, 2018, or of the results of operations expected in 2019 and future years. The following pro forma financial information has been prepared by management of the Corporation on the basis of the information contained in this Prospectus or in documents incorporated by reference herein. This pro forma financial information is not a forecast or a projection of future results. The actual results of the combined operations for any period, whether before or after the completion of the Acquisition, will likely vary from the amounts set forth in the following pro forma financial information and such variation may be material. See “Non-IFRS Measures” and “Risk Factors - Risk Factors Related to the Acquisition”.

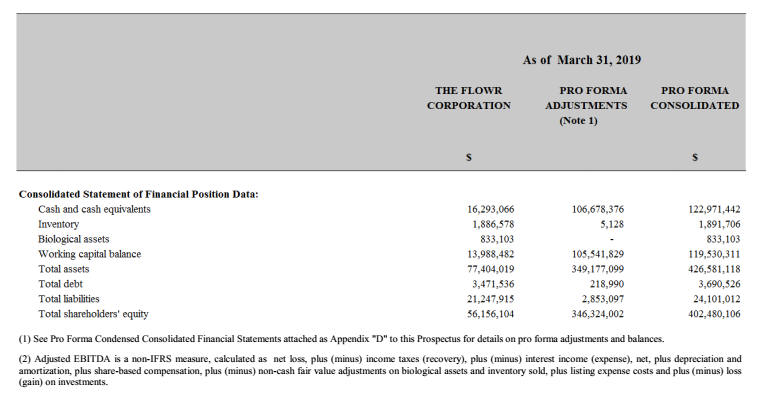
PRELIMINARY FINANCIAL RESULTS FOR THE THREE MONTHS ENDED JUNE 30, 2019
The Company has recently completed its second quarter ended June 30, 2019. While complete financial information and operating data for the three months ended June 30, 2019 are not yet available, the Corporation is in a position where it can estimate its top line revenue, working capital balance (excluding cash) and cash position for the quarter with reasonable accuracy, and the Corporation believes that such information will be helpful to an investor in making an informed investment decision. In addition to the preliminary estimates below, the Corporation expects to report a net loss for the quarter ended June 30, 2019.
Set forth below are preliminary estimates of the revenue, working capital balance (excluding cash) and cash position that we expect to report for our three months ended June 30, 2019. Our actual results may differ materially from these estimates due to the completion of our financial closing procedures (which will not occur until after the Closing Date), final adjustments and other developments that may arise between now and the time the financial results for three months ended June 30, 2019 are finalized.
The following are our preliminary estimates for the three months ended June 30, 2019:
- Revenues are expected to be between $2.1 million and $2.3 million.
- Working capital balance (excluding cash) is expected to be between ($2.0) million and ($2.2) million.
- Cash position is expected to be between $9.4 million and $9.6 million.
The foregoing estimates, which should not be viewed as a substitute for full interim financial statements prepared in accordance with IFRS, represent the most current information available to management and do not present all necessary information for an understanding of our financial condition as of and the results of operations for the three months ended June 30, 2019. The preliminary estimates included in this Prospectus have not been reviewed or audited by our independent registered public accounting firm. Accordingly, our independent auditors do not express an opinion or any other form of assurance with respect to this preliminary data.
We have provided ranges for the preliminary results described above primarily because our financial closing procedures for the three months ended June 30, 2019 are not yet complete. As a result, there is a possibility that our final results will vary from these preliminary estimates. We currently expect that our final revenue, working capital balance (excluding cash) and cash position will be within the ranges described above, but it is possible, however, that our final results will not be within the ranges we currently estimate. See "Cautionary Note Regarding Future-Oriented Financial Information," "Caution Regarding Forward-Looking Statements" and "Risk Factors".
THE OFFERING
Issuer: | The Flowr Corporation |
| | |
Offering Price: | $[•] per Offered Share |
| | |
Number of Offered Shares: | [•] |
| | |
Amount: | $125,000,000 |
| | |
Closing Date: | [•], 2019 or such other date (which date shall not be later than 42 days after the date of receipt for the (final) short form base PREP Prospectus) as may be agreed upon by the Corporation and the Representatives, on behalf of the Underwriters. |
| | |
Issue: | Common Shares. See “Description of Common Shares”. |
| | |
Underwriters’ Option: | The Corporation has granted the Underwriters the Underwriters’ Option, exercisable in whole or in part, at any time and from time to time, in the sole discretion of the Representatives, up to 30 days after and including the Closing Date, to cover sales by the Underwriters of a greater number of Common Shares than the number of Offered Shares set out above, if any. This Prospectus qualifies the grant of the Underwriters’ Option and the issuance of the Additional Offered Shares on the exercise of the Underwriters’ Option. A purchaser who acquires Additional Offered Shares on exercise of the Underwriters’ Option acquires those Additional Offered Shares under this Prospectus, regardless of whether the over-allocation position is ultimately filled through the exercise of the Underwriters’ Option or secondary market purchases. See “Plan of Distribution”. |
| | |
Use of Proceeds: | The net proceeds to the Corporation from this Offering, after deducting the Underwriters’ Fee and expenses of the Offering, are estimated to be approximately $115,400,000, or approximately $133,025,000 if the Underwriters exercise the Underwriters’ Option in full. The Corporation intends to use the net proceeds from the Offering to fund, in part: (i) the Cash Consideration; (ii) the fees and expenses incurred in connection with the Acquisition; (iii) capital required for the construction of Holigen’s and the Corporation’s development and facilities; and (iv) working capital and general corporate purposes. In the event the Underwriters’ Option is exercised, any additional net proceeds will be allocated to (i) capital required for the construction of Holigen’s and the Corporation’s development and facilities; and (ii) general corporate purposes. See “Use of Proceeds”. The Offering is not contingent on the Acquisition. In the event that the closing of the Acquisition does not occur, the net proceeds from the Offering will initially be added to the Corporation’s working capital and will subsequently be applied to development costs of the Corporation’s facilities and for general corporate purposes. See “Use of Proceeds”, “Details of the Acquisition” and “Risk Factors”. |
| | |
Listing and Trading: | The TSX.V has conditionally approved the listing of the Offered Shares and the Additional Offered Shares. Listing is subject to the Corporation fulfilling all of the listing requirements of the TSX.V on or before October 2, 2019. The Corporation has been approved for listing on the NASDAQ, subject to the effectiveness of the Corporation’s registration statement on Form 40-F filed with the SEC on February 5, 2019 as amended May 28, 2019 and further amended on July 12, 2019. See “Plan of Distribution”. |
Risk Factors: | An investment in the Offered Shares involves significant risks that should be carefully considered by prospective investors before purchasing such securities. The risks outlined in this Prospectus and in the documents incorporated by reference herein should be carefully reviewed and considered by prospective investors in connection with an investment in the Offered Shares. Certain of the risks involved in an investment in the Offered Shares and Additional Offered Shares include, but are not limited to, the following: - The Corporation’s limited operating history and uncertainty of future revenues
- The Corporation’s and Holigen’s respective business operations, including its ability to cultivate, store and sell cannabis are dependent on obtaining certain additional licences and permits and maintaining and renewing existing licences and permits
- The Corporation’s business operations are subject to regulatory risks generally applicable to the cannabis industry
- The Corporation’s business operations are susceptible to changes in laws, regulations and guidelines
- The Corporation and other federal licenced cultivators in Canada may produce more cannabis than is needed to satisfy the Canadian medical and recreational markets resulting in regional overcapacity
- Development of the cannabis industry and continued legalization may be slower or more limited in extent than originally anticipated
- The Corporation may be subject to growth-related risks
- The Corporation has incurred losses since inception and may not be able to achieve or maintain profitability and may continue to incur significant losses in the future
- The Corporation’s recurring losses from operations could cast doubt on its ability to continue as a going concern
- The Corporation faces intense competition from other companies in the cannabis industry
- The Corporation faces competition from illicit dispensaries and the illicit market more broadly
- The Corporation may fail to retain existing customers or acquire new customers
- The development and distribution of synthetic products could change the demand, volume and profitability of the cannabis industry
- The Corporation’s business might attract negative publicity which could damage the Corporation’s reputation
- The success of the Corporation will depend to a great extent upon the expertise of the Board and key executives
- The Corporation’s and Holigen’s businesses are reliant on the K1 Facility, K2 Facility, Flowr Forest, Holigen’s facilities and other proposed facilities.
- The Corporation may not be able to translate the experience of its operating team with indoor growing facilities into successfully designing, constructing and operating outdoor and greenhouse cultivation facilities
- The Corporation may not be able to realize its cannabis production targets
- A security breach at the Corporation’s facilities could expose the Corporation to additional liability, potentially costly litigation, increased expenses and may deter potential customers
- The Corporation is exposed to the risk that any of their employees, independent contractors and consultants may engage in fraudulent or other illegal activity
- The Corporation faces risk of exposure to product liability claims, regulatory action and litigation if its products are alleged to have caused significant loss or injury
- The Corporation’s products may be subject to recalls for a variety of reasons, including product defects
- The Corporation may not be able to maintain pricing premium for premium products
- The Corporation may not be able to protect or enforce all of its intellectual property rights, including trademarks, in all key jurisdictions
|
| - There exists the possibility for certain Directors and officers of the Corporation to be in a position of conflict with their duties to the Corporation
- The development of the Corporation’s business and operating results may be hindered by applicable regulatory restrictions on sales and marketing activities
- The Corporation may not be able to maintain and retain a competitive talent pool
- The Corporation’s operations in foreign countries may be subject to a higher degree of political, social and economic risk
- The Corporation may not be able to successfully expand its operations into foreign jurisdictions
- The Corporation may expand into other geographic areas, which could increase the Corporation’s operational, regulatory and other risks
- The Corporation may be responsible for corruption and anti-bribery law violations
- Holigen is an early stage development company with no operations, a history of net losses and uncertain future revenues
- The Corporation could fail to complete the Acquisition or complete the Acquisition on different terms
- The Corporation does not currently control Holigen and no assurance of Holigen’s future performance can be given
- The Corporation could fail to successfully integrate Holigen into the business of the Corporation
- The Corporation will incur substantial transaction-related costs in connection with the Acquisition
See “Cautionary Note Regarding Forward-Looking Information” and “Risk Factors”. |
RISK FACTORS
Before making an investment decision, prospective purchasers of Offered Shares should carefully consider the information described in this Prospectus and in the documents incorporated by reference herein. There are certain risks inherent in an investment in the Offered Shares, including the factors listed below, and any other factors described in a document incorporated by reference in the Prospectus, which investors should carefully consider before investing. The risk factors pertaining to the Corporation’s business, financial condition and results of operations would also apply to Holigen and its business. Some of the following risks and the risks described in the documents incorporated by reference in the Prospectus are interrelated and, consequently, investors should treat such risk factors as a whole. The risks described in this Prospectus and in the documents incorporated by reference herein, describe certain currently known material factors, any of which could have a material adverse effect on the Corporation’s business, financial condition and results of operations. If any of the following or other risks occur, it could have a material adverse effect on the business, financial condition and results of operations of the Corporation and on the trading price of the Common Shares, which could materially decline, and investors may lose all or part of their investment. It is also believed that these factors could cause actual results to be different from expected and historical results. Other sections of this Prospectus include additional risks that could have an effect on the business and financial performance of the business following the completion of the Acquisition. Additional risks and uncertainties of which the Corporation is currently unaware or that are unknown or that it currently deems to be immaterial could also have a material adverse effect on the Corporation’s business, financial condition and results of operations. The Corporation cannot assure you that it will successfully address any or all of these risks. There is no assurance that any risk management steps taken will avoid future loss due to the occurrence of any of the risks described in this Prospectus and in the documents incorporated by reference herein, or other unforeseen risks. The market in which the Corporation currently competes is very competitive and changes rapidly. Sometimes new risks emerge and management may not be able to predict all of them, or be able to predict how they may cause actual results to be different from those contained in any forward-looking statements. You should not rely upon forward-looking statements as a prediction of future results. In addition to the risks described elsewhere in this Prospectus, including in the notes to the financial statements attached hereto, each of, and the cumulative effect of all of, the following risks for the Corporation should be considered.
Risks Related to the Business
Limited Operating History and Uncertainty of Future Revenues.
The Corporation has a limited operating history and, accordingly, potential investors will have a limited basis on which to evaluate the Corporation’s ability to achieve its business objectives. The future success of the Corporation is dependent on management’s ability to implement its strategy. Although management is optimistic about the Corporation’s prospects, there is no certainty that anticipated outcomes and sustainable revenue streams will be achieved and there is no certainty that the Corporation will be able to successfully produce commercial cannabis or establish a market for its products. The Corporation faces risks frequently encountered by early-stage companies. In particular, its future growth and prospects will depend on its ability to expand its operations and gain additional revenue streams whilst at the same time maintaining effective cost controls. Any failure to expand is likely to have a material adverse effect on the Corporation’s business, financial condition and results of operations.
Future Funding Requirements.
To date, the Corporation has had negative cash flow from operating activities. To fund its current operations and anticipated capacity expansions, additional funds will be required. A portion of these funds will be funded out of the net proceeds of the Offering. However, if the Corporation continues to have negative cash flow into the future, the Corporation may need to allocate additional financing proceeds to funding this negative cash flow in addition to its operational expenses and planned capital expansions. Flowr may require additional financing to fund its operations to the point where it is generating positive cash flows. Continued negative cash flow may restrict the Corporation’s ability to pursue its business objectives which could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
The building and operation of the Corporation’s facilities and business are capital intensive. In order to execute the anticipated growth strategy, the Corporation will require additional equity and/or debt financing to support on-going operations, to undertake capital expenditures (including to complete the construction of the K1 Facility and finance the construction of the K2 Facility, Flowr Forest, and upon completion of the Acquisition, Holigen’s facilities) to undertake additional acquisitions or other business combination transactions. There can be no assurance that additional financing will be available to the Corporation when needed or on terms, which are acceptable to the Corporation. The Corporation’s inability to raise financing to support on-going operations or to fund capital expenditures or acquisitions could limit the Corporation’s growth and may have a material adverse effect upon future profitability. The Corporation may require additional financing to fund its operations to the point where it is generating positive cash flows.
If additional funds are raised through further issuances of equity or debt securities, existing shareholders (including prospective investors) could suffer significant dilution, and any new equity securities issued could have rights, preferences and privileges superior to those of holders of the Common Shares. In addition, from time to time, the Corporation may enter into transactions to acquire assets or the shares of other corporations. These transactions may be financed wholly or partially with debt and/or equity issuances, which may temporarily increase the Corporation’s debt levels above industry standards and/or further dilute shareholders significantly. Any debt financing secured in the future could involve restrictive covenants relating to the Corporation’s capital raising activities and other financial and operational matters, which may make it more difficult for the Corporation to obtain additional capital and to pursue business opportunities, including potential acquisitions.
Reliance of the Corporation on Licensing.
The Corporation’s business operations, including its ability to cultivate, store cannabis and cannabis products, and to sell cannabis products into the medical and recreational markets in Canada, and to export cannabis and cannabis products to, and import cannabis and cannabis products from, other jurisdictions for medical or scientific purposes, are dependent on the Licence. The Licence is subject to ongoing compliance and reporting requirements. In addition, all licences must be renewed from time to time. The Licence expires on December 1, 2020. Prior to expiry of the Licence, the Corporation must submit to Health Canada an application for renewal of the Licence containing information prescribed by the Cannabis Act.
Although the Corporation believes that it and Holigen are complying in all material respects with the terms of their respective licences any such failure or maintain such licences, would have a material adverse effect on the Corporation’s and Holigen’s business, financial condition and results of operations.
Although the Corporation believes it will meet the requirements of the Cannabis Act and the Cannabis Regulations for future extensions or renewals of the Licence there can be no guarantee that Health Canada will extend or renew the Licence, and if extended or renewed, that the Licence will be extended or renewed on the same or similar terms. Should the Licence not be renewed, or renewed on different terms, it would have a material adverse effect on the Corporation’s business, financial condition and results of operations. There are also various licensing requirements which the Corporation and certain of its directors, officers and employees would need to satisfy for future extensions or renewals. There is no guarantee that the Corporation and/or such persons will satisfy the licensing requirements nor that Health Canada will extend or renew the License, or, if extended or renewed, that they will be extended or renewed on the same or similar terms. In addition, the expansion of the Corporation’s operations and facilities may require new licences to be issued. Further, Health Canada has recently announced that, effective immediately, licence applications will only be reviewed in detail once construction of a site is complete. Although this new requirement is not expected to affect the Corporation’s facilities that are already under construction, it has the potential of delaying issuance of licences required for new or expanded operations, if any. Any failure or delay in obtaining licences for new or expanded operations could have a material impact on the Corporation’s ability to increase its revenue which could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
Holigen’s business operations, including its ability to cultivate, store and sell cannabis in Australia and Portugal and to any other jurisdiction, is dependent on maintaining and renewing existing licences and permits, as applicable, from INFARMED in Portugal and the regulatory authorities in Australia and on obtaining additional required licences and permits from these authorities. Although the Corporation believes that Holigen is complying in all material respects with the terms of its existing licences and permits and that it will comply with all requirements it must meet to obtain the remaining required licences and permits, there is no assurance that Holigen will be able to maintain its licences and permits, renew or extend them on the same or similar terms, or obtain the remaining licences and permits that it requires to operate.
The Corporation and Holigen may be unable to obtain, maintain or renew the necessary licences, permits, certificates, authorizations or accreditations to operate their respective businesses or on less advantageous terms. The Corporation and Holigen may not be able to comply fully with the wide variety of laws and regulations applicable to the cannabis industry in the respective jurisdictions in which they operate. Failure to comply with or to obtain the necessary licences, permits, certificates, authorizations or accreditations could result in restrictions on the Corporation’s and/or Holigen’s ability to operate in the cannabis industry in any jurisdiction, which could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
Regulatory Risks Generally Applicable to the Cannabis Industry.
The laws, regulations and guidelines generally applicable to the cannabis industry domestically and internationally may change in ways currently unforeseen by the Corporation. The Corporation’s operations are subject to a variety of laws, regulations and guidelines relating to the manufacture, extraction, management, transportation, storage, sale, health, import, export, safety and disposal of cannabis and cannabis products under the Cannabis Act, as well as those relating to taxes, labour standards and occupational health, toxic substances, land use, water use and other matters under other legislation.
The commercial cannabis industry, and the medical and recreational cannabis markets, are new in Canada and in other jurisdictions. This industry and its associated markets may not continue to exist or grow as anticipated or Flowr may ultimately be unable to succeed in this industry and market.
The Corporation anticipates that regulations governing the industry will be subject to change as the Government of Canada, the governments of individual provinces and territories and other governments, monitor operating federal licence holders. The operations of the Corporation will be subject to a variety of laws, regulations, guidelines and policies relating to the manufacture, processing, extraction, import, export, management, packaging, labelling, promoting, sale, transportation, storage and disposal of cannabis and cannabis products, but also laws and regulations relating to drugs, controlled substances, health and safety, land use, the conduct of operations and the protection of the environment. While to the knowledge of management, the Corporation is currently in material compliance with all such laws, any changes to such laws, regulations, guidelines and policies may have a material adverse effect on its business, financial condition and results of operations, including with respect to the ability of the Corporation to leverage its cultivation knowledge for expansion of operations in jurisdictions outside of Canada, including without limitation where genetic material is required by partners and/or affiliates in foreign jurisdictions for licensing purposes. No assurance can be given that new rules and regulations will not be enacted or that existing rules and regulations will not be applied in a manner which could limit or curtail the Corporation’s ability to distribute or produce cannabis and cannabis products, or the ability of its affiliates and/or partners to complete their licensing process. Amendments to current laws and regulations governing the distribution, transportation and/or production of cannabis, or more stringent implementation thereof, could cause increases in expenses, capital expenditures or production costs or reduction in levels of production or require abandonment or delays in development, which could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
Changes in Laws, Regulations and Guidelines.
The Cannabis Act and Cannabis Regulations came into force on October 17, 2018. The Cannabis Act restricts packaging, labelling and promotional activity. The Cannabis Regulations restrict and place requirements on packaging and labelling. The restrictions on packaging, labelling and promotional activity, and the requirements on packaging and labelling, or changes in such restrictions and requirements, could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
The legislative and operational framework of the provinces and territories of Canada pertaining to the distribution of cannabis products for the recreational market varies. The same is true for legislation applicable to medical cannabis in international jurisdictions. This variation among jurisdictions has resulted in additional regulations, creating additional compliance and other costs and/or limitations on the Corporation’s ability to participate in such markets. There is no guarantee that jurisdictional legislation regulating the distribution and sale of cannabis for recreational or medical purposes, as applicable, will be enacted according to all the terms announced by such jurisdictions, or at all, or that any such legislation, if enacted, will create the growth opportunities that the Corporation currently anticipates. While the impact of any new legislative framework for the regulation of the recreational cannabis market or medical market, as applicable, is uncertain, any of the foregoing could result in a material adverse effect on the Corporation’s business, financial condition and results of operations. The asymmetrical regulatory and market environment for recreational and medical cannabis in each of the provinces and territories of Canada, or of medical cannabis in jurisdictions outside of Canada, could have a material adverse effect on the Corporation’s business, financial condition and results of operations. In addition, although the Canadian government has proposed October 17, 2019 as the timeline for regulation of new classes of cannabis for sale, to the extent such timeline is delayed or such legislative proposals do not become law, additional revenue from such cannabis products will be lost, which could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
The governments of every Canadian province and territory have implemented regulatory regimes for the distribution and sale of cannabis for recreational purposes within those jurisdictions. There can be no assurance that regulation of recreational cannabis by each of the provinces and territories will continue as planned.
Regional Overcapacity.
Since the implementation of the Cannabis Act, demand for cannabis products has increased. To date, federal licence holders in Canada have not been able to produce sufficient amounts of cannabis products to satisfy demand, resulting in an undersupplied market. However, as the Corporation and other federal licenced cultivators in Canada produce more cannabis, oversupply may become an issue. If the Corporation is unable to sell or export that oversupply into other markets where cannabis use is federally regulated, it could result in a significant decline in the market price for cannabis. If this were to occur, there is no assurance that the Corporation would be able to generate sufficient revenue from the sale of recreational cannabis to result in profitability which could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
Market Development and Continued Legalization.
Development of the cannabis industry may be slower or less than originally anticipated due to a variety of factors that are out of the Corporation’s control, including: (i) delayed approvals for regulation of both medical and recreational cannabis in international markets; (ii) supply chain issues with delivery of cannabis products to end users; (iii) delayed or cancelled approvals for cannabis products that are new classes of cannabis; or (iv) lack of successful R&D for cannabis products, including those that are new classes of cannabis. Any of these could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
Management of Growth and Capacity Constraints.
The Corporation may be subject to growth-related risks including capacity constraints and pressure on its internal systems and controls. The ability of the Corporation to manage growth effectively will require it to continue to implement and improve its operational and financial systems and to expand, train and manage its employee base. The inability of the Corporation to deal with this growth may have a material adverse effect on the Corporation’s business, financial condition and results of operations.
History of Net Losses and Achieving or Maintaining Profitability.
The Corporation has incurred losses since inception. The audited annual consolidated financial statement for the year ended December 31, 2018, the only historical audited financial statements prepared after the completion of the Qualifying Transaction, only include public company costs since the completion of the Qualifying Transaction on September 21, 2018. It is possible that the Corporation’s losses would have been higher for that fiscal period as a public company. Additionally, while the Corporation’s unaudited condensed interim consolidated financial statements for the three months ended March 31, 2019 include public company costs, annualizing such costs may not be a good proxy for determining the Corporation’s public company costs for a full fiscal year. The Corporation may not be able to achieve or maintain profitability and may continue to incur significant losses in the future. In addition, the Corporation expects to continue to increase operating expenses as it implements initiatives to continue to grow its business. If the Corporation’s revenues do not increase to offset these expected increases in costs and operating expenses, the Corporation will not be profitable.
Our Recurring Losses from Operations Could Continue to Cast Doubt on Our Ability to continue as a Going Concern. Our Ability to Continue as a Going Concern Requires that We Obtain Sufficient Funding to Finance Our Operations.
The Corporation has incurred significant operating losses since its inception. Accordingly, the Corporation’s audited financial statements incorporated by reference in this Prospectus have been prepared on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities in the ordinary course of business. These financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might result from the outcome of these uncertainties related to the Corporation’s ability to operate on a going concern basis. However, in its report on the Corporation’s financial statements for the year ended December 31, 2017, the Corporation’s independent registered public accounting firm included an explanatory paragraph stating that the ability of the Corporation’s subsidiary to obtain its licence to sell medical cannabis and the fact that the Corporation required additional funding to finance its operations raised substantial doubt about the Corporation’s ability to continue as a going concern. In addition, in its report on the Holigen’s financial statements for the year ended December 31, 2018 and period from formation on July 21, 2017 to December 31, 2017, Holigen’s independent registered public accounting noted that ability of Holigen to continue as a going concern is dependent on Holigen raising sufficient capital to maintain operations and to fund further operating expenses and losses until revenues and positive cash flows can be generated from operations. The perception that the Corporation and/or Holigen may not be able to continue as a going concern may cause others to choose not to do business with the Corporation and/or Holigen due to concerns about their ability to meet their contractual obligations.
If the Corporation is unable to obtain sufficient funding, its business, prospects, financial condition and results of operations will be materially and adversely affected and it may be unable to continue as a going concern. If the Corporation is unable to continue as a going concern, it may have to liquidate assets and may receive less than the value at which those assets are carried on its audited financial statements. Investors may lose all or a part of their investment. If the Corporation seeks additional financing to fund its business activities in the future and there remains substantial doubt about its ability to continue as a going concern, investors or other financing sources may be unwilling to provide additional funding to the Corporation on commercially reasonable terms or at all.
Competition Within the Cannabis Industry.
The market in which the Corporation operates is competitive and fast moving and may become even more competitive. The Corporation faces intense competition from other companies, some of which can be expected to have a longer operating history, more financial resources, and more cultivation, processing, distribution and marketing experience than the Corporation. Increased competition by larger and better financed competitors could have a material adverse effect on the Corporation’s business, financial condition and results of operations.Because the industry in which the Corporation operates is at an early stage, the Corporation may face additional competition from new entrants. There is a risk that larger conglomerates and companies who also recognize the potential for financial success in this industry could strategically purchase or assume control of larger operations and cultivation facilities. In doing so, these larger competitors could establish price setting and cost controls which would effectively “price out” many of the individuals and small to medium-sized entities who currently make up the bulk of the participants in the medical and recreational cannabis industry. While the trend in most laws and regulations seemingly deters this type of takeover, this industry remains nascent, so what the landscape will be in the future remains largely unknown.
The number of licences granted and the number of federal cannabis licence holders ultimately authorized by Health Canada, and other applicable regulatory bodies in the markets in which the Corporation operates, could have an impact on the operations of the Corporation. To date, approximately 180 licences have been authorized within Canada. The Corporation expects to face additional competition from new market entrants that are granted licences under the Cannabis Act or existing licence holders which are not yet active in the industry. If a significant number of new licences are granted by Health Canada, or other applicable regulatory bodies in the markets in which the Corporation operates, in the near term future, the Corporation may experience increased competition for market share within the medical market and recreational markets, which could negatively impact the Corporation’s market share and demand for products.
If the demand for cannabis products in the Canadian medical and recreational markets, and the demand in other applicable jurisdictions, increases, along with an increased number of cultivation licence holders, the Corporation expects that competition will become more intense, as current and future competitors begin to offer an increasing number of diversified products, particularly within the new classes of cannabis. To remain competitive, the Corporation will require a continued high level of investment in R&D, marketing, sales and client support. The Corporation may not have sufficient resources to maintain R&D, marketing and sales support efforts on a competitive basis which could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
The Corporation expects to be a leader in technology related to cannabis. However, as new technologies related to the cultivation, processing, manufacturing and R&D of cannabis are being explored, there is potential for third party competitors to be in possession of superior technology that would reduce the relative competitiveness of the Corporation.
The Cannabis Regulations permit cultivation licence holders to conduct both outdoor and indoor cultivation of cannabis. The implications of outdoor cultivation are not yet known, but could be significant as it may reduce start-up capital required for new entrants in the cannabis industry. It may also ultimately lower prices as capital expenditure requirements related to growing outside are typically much lower than those associated with indoor growing.
Finally, the 2018 Industrial Hemp Regulations allow sale of Biomass to holders of a processing licence under the Cannabis Regulations. The Biomass may be used for extraction, which may have an unpredictable effect on the market for products rich in CBD and low in THC. All industrial hemp cultivation, and the vast majority of industrial hemp propagation, is in open fields (as opposed to inside greenhouses), and costs are much lower than with cultivation of cannabis plants under the Cannabis Act. Currently, Biomass would be primarily used for cannabis oil, but following the 2019 Amendments, Biomass may provide competitive inputs for cannabis products within the New Classes of Cannabis that are rich in CBD.
Strategic Acquisitions and Partnerships.
The Corporation currently has and may enter into further strategic acquisitions, partnerships or similar relationships that it believes will complement or augment its existing business. The Corporation’s ability to complete such strategic acquisitions or partnerships is dependent upon, and may be limited by, the availability of suitable candidates and capital. There can be no assurance that the Corporation will be able to identify acquisition or partnership opportunities that meet its strategic objectives, or to the extent such opportunities are identified, that it will be able to negotiate terms that are acceptable to it. In addition, strategic acquisitions or partnerships could present unforeseen integration obstacles or costs, may not enhance the Corporation’s business, and may involve risks that could adversely affect the ongoing business of the Corporation, including significant amounts of management time that may be diverted from operations to pursue and complete such transactions or maintain such strategic partnerships. Future strategic acquisitions or partnerships could result in the incurrence of additional debt, costs and contingent liabilities, and there can be no assurance that future strategic acquisitions or partnerships will achieve, or that the Corporation’s existing strategic partnerships will continue to achieve, the expected benefits to the Corporation’s business or that the Corporation will be able to consummate future strategic partnerships on satisfactory terms, or at all. The Corporation may also experience negative reactions from the financial markets, which could cause a decrease in the market price of the Corporation’s securities, particularly if the market price reflects market assumptions that acquisitions or partnerships will be completed or completed on certain terms.
Further, some strategic partnerships involve master supply agreements with provincial or territorial liquor control authorities in certain Canadian jurisdictions, such as Ontario and British Columbia, whereby the provinces: (i) are not obligated to order any particular volume of cannabis or order at all; (ii) can terminate the agreement for any reason and at any time with 60 or 90 days’ notice; and (iii) require the Corporation to offer them products at the lowest price at which such products are offered to any other party. Also, none of the Corporation’s customers have minimum purchase obligations pursuant to any of the Corporation’s arrangements to supply cannabis into Saskatchewan, Nova Scotia and Manitoba. Similarly, the supply agreement with Shoppers is purchase order-based, such that Shoppers is not obligated to order any particular volume of cannabis or at all. The Corporation expects to derive a significant amount of revenue through its agreements with Ontario, British Columbia and Shoppers, as well as through its arrangements to supply cannabis to licensed retailers in Saskatchewan, Manitoba and Nova Scotia. However, the amount and/or price of cannabis that the Corporation sells through these channels may vary significantly from what it expects and could be subject to material fluctuations. If any of these partners or licensed retailers decides to purchase lower volumes of cannabis than the Corporation expects or at a lower price, alters its purchasing patterns with little or no notice to the Corporation or decides not to purchase Flowr products at all, the Corporation’s revenues would be materially and adversely affected.
Additionally, certain strategic partnerships may include restrictions on the Corporation’s ability to sell its products. For example, the supply agreement with Shoppers prohibits Flowr from selling medical cannabis directly to consumers in Canada or selling its medical cannabis products for recreational or through non-medical sales channels in Canada. As a result of such restrictions, the Corporation may not realize the anticipated benefits from these partnerships.
Any of the foregoing risks and uncertainties could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
Competition from Illicit Dispensaries and the Illicit Market.
The Corporation also faces competition from illicit dispensaries and the illicit market more broadly. Participants in these markets are unlicensed and unregulated. They may be selling cannabis and cannabis products with higher concentrations of active ingredients and using classes of cannabis that are not yet regulated for sale in Canada. Various Canadian cities have seen an influx in the number of illicit dispensaries. Any inability or unwillingness of law enforcement authorities to enforce existing laws prohibiting the unlicensed cultivation and sale of cannabis and cannabis products could result in the perpetuation of the illicit market for cannabis and/or have a material adverse effect on the perception of cannabis use. Any or all these events could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
Competition from Synthetic Products.
The pharmaceutical industry may attempt to dominate the cannabis industry, and in particular, legal cannabis, through the development and distribution of synthetic products which emulate the effects of cannabis. If they are successful, the widespread popularity of such synthetic products could change the demand, volume and profitability of the cannabis industry. This could adversely affect the ability of the Corporation to secure long-term profitability and success through the sustainable and profitable operation of the anticipated businesses and investment targets, and could have a material adverse effect on the Corporation’s business, financial condition or results of operations.
Reputational Risk and Negative Public Opinion.
Damage to the Corporation’s reputation can result from the actual or perceived occurrence of any number of events, including any negative publicity, whether true or not. As a producer and distributor of cannabis, which was previously a controlled substance in Canada, and still may be associated with various other controlled substances, violence and criminal activities, there is a risk that the Corporation’s business might attract negative publicity. There is also a risk that the actions of other federal cannabis licence holders, permitted retailers or of other companies and service providers in the cannabis industry may negatively affect the reputation of the industry as a whole and thereby negatively impact the Corporation’s reputation. The increased usage of social media and other web-based tools used to generate, publish and discuss user-generated content and to connect with other users has made it increasingly easier for individuals and groups to communicate and share negative opinions and views in regards to the Corporation’s activities and the medical cannabis industry in general, whether true or not. The Corporation does not ultimately have direct control over how the Corporation or the cannabis industry is perceived by others. Reputational issues may result in decreased investor confidence, increased challenges in developing and maintaining community relations and present an impediment to the Corporation’s overall ability to advance its business strategy and realize on its growth prospects, which could have a material adverse effect on the Corporation’s business, financial condition or results of operations.
If Medical-Use Consumers Elect to Produce Cannabis for their Own Medical Purposes Under the Cannabis Regulations it Could Reduce the Addressable Market for the Corporation’s Products.
Under the Cannabis Regulations, three options are available for an individual to obtain cannabis for medical purposes: (i) registering with a holder of a licence to sell for medical purposes and purchasing products from that entity; (ii) register with Health Canada to produce a limited amount of cannabis for their own medical purposes; or (iii) designate someone else to produce cannabis for them. It is possible that (ii) and (iii) could significantly reduce the addressable market for the Corporation’s products and could materially and adversely affect the business, financial condition and results of operations of the Corporation.
Reliance on the Expertise of the Board of Directors and Key Executives.
The successful ongoing operation of the Corporation requires substantial expertise. The Board and management will have authority to make decisions and to exercise investment acquisition discretion on behalf of the Corporation. The success of the Corporation will depend to a great extent upon the expertise of the Board and management. The loss of the services of any member of the Board or one or more members of management, including as a result of the failure of such persons to be granted security clearances under the Cannabis Regulations, could have a material adverse effect on the Corporation’s business, financial condition or results of operations. In particular, given the significant operation, design and construction expertise of Flowr’s management, the loss of such expertise by way of resignation, termination and/or death could have a material adverse effect on the Corporation’s business, financial condition or results of operations.
Reliance on the Corporation’s Facilities, Holigen’s Facilities and Other Proposed Facilities.
The Corporation’s activities and resources are focused on the construction and development of the K1 Facility, K2 Facility, Flowr Forest, Holigen’s facilities and other proposed facilities. Adverse changes or developments affecting the K1 Facility, K2 Facility, Flowr Forest, Holigen’s facilities or other proposed facilities, including but not limited to a natural disaster, calamity, act of war or terrorism or other major disturbance or breach of security, could have a material and adverse effect on the Corporation’s business, financial condition or results of operations. Any breach of the security measures and other facility requirements, including any failure to comply with recommendations or requirements arising from inspections by Health Canada, or applicable regulatory authorities in Portugal and/or Australia, could also have an adverse impact on the Corporation’s or Holigen’s ability to continue operating under, or each entity’s prospects of renewing, the applicable licences and permits necessary to cultivate, manufacture, extract, grow, process and/or produce cannabis at the K1 Facility, K2 Facility, Flowr Forest, Holigen’s facilities or other proposed facilities, as applicable.
All facilities continue to operate with routine maintenance however, the buildings do have components that require replacement or repair. The Corporation will bear the costs of maintenance and upkeep at the K1 Facility, K2 Facility, Flowr Forest, Holigen’s facilities and other proposed facilities. If the Corporation is not able to keep up with such maintenance requirements it could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
Certain contemplated capital expenditures of the Corporation, including expansion and/or renovation of the K1 Facility, Holigen’s facilities and other proposed facilities, may require approval of Health Canada, or the applicable regulatory authorities in other applicable jurisdictions where the facilities are located. There is no guarantee that Health Canada, or the applicable regulatory authorities in other applicable jurisdictions where the facilities are located, will approve any contemplated capital expenditure on a timely basis or at all, which could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
In addition, there is no guarantee that, even if approved by Health Canada, or the applicable regulatory and zoning authorities in other applicable jurisdictions where the facilities are located, the Corporation will have the funds, as well as management and other resources, necessary to complete any capital expenditures or that any capital projects will be completed on time, without defect in design or construction, or in accordance with budgetary projections.
If the Corporation cannot complete construction of the K1 Facility, the K2 Facility, Flowr Forest, Holigen’s facilities or other proposed facilities, or is delayed in completing construction of these facilities, such events could have a material adverse effect on the Corporation’s business, financial condition and results of operations, including reputational damage and loss of sales. Further, there is no guarantee that the Corporation can achieve the anticipated buildout and ramp up of the K1 Facility, the K2 Facility, Flowr Forest, Holigen’s facilities or other proposed facilities’, expected capacity in the timeframe as forecasted, or at all. Even when fully built, the growing capacity of the K1 Facility, the K2 Facility, Flowr Forest, Holigen’s facilities and other proposed facilities may be less than originally anticipated. The Corporation may not be able to achieve its expected capacity due to delays in construction, project cost overruns, the inability to raise capital to fund development efforts, unanticipated crop failures and other circumstances beyond its control which could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
Further, as the Corporation completes construction, it is processing and packaging in a temporary area, which may contribute to operational delays and processing bottlenecks. Such operational delays and processing issues may lead to the Corporation being unable to sell all of the product it produces, which may have a material adverse effect on its financial condition.
Although the Corporation is designing the K1 Facility to meet GMP standards, such standards have not yet been met. An interim audit of the K1 Facility for compliance with GMP standards revealed significant deficiencies in the areas of the K1 Facility structure, staffing and documentation processes. Should such deficiencies not be rectified in a manner satisfactory to the inspector, the K1 Facility will not pass the inspection required to achieve GMP certification, which could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
The Corporation May Not Be Able to Successfully Design, Construct or Operate Outdoor or Greenhouse Cultivation Facilities.
The Corporation’s operating team’s experience to date has been in designing, constructing and operating indoor cannabis cultivation facilities. The Corporation plans to expand its operations to include outdoor and greenhouse cultivation facilities, including Flowr Forest and Holigen’s Aljustrel site. Outdoor and greenhouse growing formats pose their own challenges and risks that will be new to the Corporation and its operating team. The Corporation may not be able to translate the experience of its operating team with indoor growing facilities into successfully designing, constructing and operating outdoor and greenhouse cultivation facilities. The lack of experience in this field may lead to additional costs of construction or production, lower than expected yields, delays and diversion of management’s attention.
The Corporation May Not Be Able to Realize its Cannabis Production or Capacity Targets.
The Corporation’s ability to produce and process cannabis, at its current pace, its expected pace or at all, and the expected capacity of its facilities are affected by a number of factors, including available space, plant design errors, non-performance by third party contractors, increases in materials or labour costs, construction performance falling below expected levels of output or efficiency, environmental pollution, contractor or operator errors, breakdowns, processing bottlenecks, aging or failure of equipment or processes, labour disputes, as well as factors specifically related to indoor agricultural practices, such as reliance on provision of energy and utilities to the facility, and potential impacts of major incidents or catastrophic events on the facility, such as fires, explosions, earthquakes or storms. Should one or more of these factors materialize, the Corporation may not be able to produce cannabis at its current or expected pace, or at all, which could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
Breaches of Security at the Corporation’s or Holigen’s Facilities or in Respect of Electronic Documents and Data Storage and Risks Related to Breaches of Applicable Privacy Laws.
Given the nature of the Corporation’s product and its lack of legal availability outside of channels approved by the Government of Canada and the individual Canadian provinces and territories, and notwithstanding meeting or exceeding Health Canada’s security requirements, there remains a risk of theft. Given that the Corporation is permitted to store significant quantities of product as a federal cannabis licence holder, a security breach at the Corporation’s facilities could expose the Corporation to additional liability and to potentially costly litigation, increased expenses relating to the resolution and future prevention of these breaches and may deter potential customers from choosing the Corporation’s products.
As a federal cannabis licence holder, the Corporation is expected to collect and store personal information and is responsible for protecting that information from privacy breaches. In addition, when the Corporation sells its products through third parties, such as Shoppers, such third parties are also required to protect such information. A privacy breach may occur through procedural or process failure, information technology malfunction, or deliberate unauthorized intrusions. Theft of data for competitive purposes, particularly patient lists and preferences, is an ongoing risk whether perpetrated via employee collusion or negligence or through deliberate cyber-attack. Any such theft or privacy breach involving the Corporation and/or its partners would have a material adverse effect on the Corporation’s business, financial condition and results of operations. Moreover, there are a number of federal, provincial and territorial laws protecting the confidentiality of certain patient health information, including patient records, and restricting the use and disclosure of that protected information. In particular, the privacy rules under the Personal Information Protection and Electronic Documents Act (“PIPEDA”), protect medical records and other personal health information by limiting their use and disclosure of health information to the minimum level reasonably necessary to accomplish the intended purpose. If the Corporation or any of its partners was found to be in violation of the privacy or security rules under PIPEDA or other laws protecting the confidentiality of patient health information, such person could be subject to sanctions and civil or criminal penalties, which could increase the Corporation’s liabilities, harm its reputation and have a material adverse effect on the Corporation’s business, financial condition and results of operations.
Fraudulent or Illegal Activity by Employees, Contractors and Consultants.
The Corporation is exposed to the risk that any of their employees, independent contractors and consultants may engage in fraudulent or other illegal activity or any activity not in compliance with the Licence, the Cannabis Act or the Cannabis Regulations. Misconduct by these parties could include intentional, reckless and/or negligent conduct or disclosure of unauthorized activities that violate (i) government regulations, (ii) manufacturing standards, (iii) federal, state and provincial healthcare fraud and abuse laws and regulations, (iv) laws that require the true, complete and accurate reporting of financial information or data; or (v) the Licence. It may not always be possible for the Corporation to identify and deter misconduct by its employees and other third parties, and the precautions taken by the Corporation to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting the Corporation from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against the Corporation, and it is not successful in defending itself or asserting its rights, those actions could have a significant impact on the business of the Corporation, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of the operations of the Corporation, any of which could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
Inability to Sustain and Effectively Manage Growth and Development.
The Corporation is an early-stage company attempting to grow its business rapidly. Its ability to grow will depend on a number of factors, many of which are beyond its control, including the availability of sufficient capital on acceptable terms, potential changes in laws and regulations respecting the cultivation, production, sales and distribution of cannabis products, competition from other federal cannabis licence holders, ability to recruit and retain experienced personnel, ability to manage complex international operations and other factors outlined herein. In addition, the Corporation is subject to a variety of business risks generally associated with developing companies. As its operations grow in size, scope and complexity, and as it identifies and pursues new opportunities, it may have difficulty in implementing or maintaining required new or improved controls and such difficulty may, in the future, result in material weaknesses in the Corporation’s internal control over financial reporting or material misstatements in its future consolidated financial statements.
In addition, as the Corporation grows its business, it will need to effectively execute on business opportunities, continue to build on and deploy our assets and access new capital. The ability to execute these initiatives successfully, as well as to complete acquisitions and otherwise capitalize on other growth opportunities, may redirect the Corporation’s limited resources and require expansion of its infrastructure. As a result, the Corporation may be required to commit financial, operational and technical resources in advance of any increase in our revenues or sales volumes, and there is no assurance that revenue or sales volumes will actually increase. The Corporation may not respond adequately or quickly enough to the changing demands that expansion will impose on its management, employees and existing infrastructure, and any changes to its operating structure may result in unanticipated costs or inefficiencies. As the Corporation grows, changes may have a negative impact on its operations, and cost increases resulting from its inability to effectively manage its growth could adversely impact is profitability.
Any failure to effectively manage the Corporation’s growth could result in difficulty or delays in servicing its customers, declines in quality or consumer satisfaction, increases in costs, difficulties in introducing new products or other operational difficulties, and any of these difficulties could adversely impact the Corporation’s business and results of operations. There can be no assurance that the Corporation will be able to effectively manage its expanding operations, achieve profitability, attract and retain sufficient personnel or successfully make or integrate strategic investments or acquisitions.
Product Liability Due to the Nature of the Products.
As a distributor of products designed to be ingested by humans, the Corporation faces an inherent risk of exposure to product liability claims, regulatory action and litigation if its products are alleged to have caused significant loss or injury. In addition, the sale of the Corporation’s products involve the risk of injury to consumers due to tampering by unauthorized third parties or product contamination. Previously unknown adverse reactions resulting from human consumption of the Corporation’s products alone or in combination with other medications or substances could occur. The Corporation may be subject to various product liability claims, including, among others, that the Corporation’s products caused injury or illness, include inadequate instructions for use or include inadequate warnings concerning possible side effects or interactions with other substances. A product liability claim or regulatory action against the Corporation could result in increased costs, could adversely affect the Corporation’s reputation with Shoppers’ clients and consumers generally, and could have a material adverse effect on the business, financial condition and results of operations of the Corporation. There can be no assurances that the Corporation will be able to obtain or maintain product liability insurance on acceptable terms or with adequate coverage against potential liabilities. Such insurance is expensive and may not be available in the future on acceptable terms, or at all. The inability to obtain sufficient insurance coverage on reasonable terms or to otherwise protect against potential product liability claims could prevent or inhibit the commercialization of the Corporation’s products and could have a material adverse effect on the business, financial condition and results of operations of the Corporation.
Product Recalls Including Contamination and Unintended Harmful Side Effects.
Manufacturers and distributors of products are sometimes subject to the recall or return of their products for a variety of reasons, including product defects, such as contamination, unintended harmful side effects or interactions with other substances, packaging safety and inadequate or inaccurate labelling disclosure. If the Corporation’s products are recalled due to an alleged product defect or for any other reason, the Corporation could be required to incur the unexpected expense of the recall and any legal proceedings that might arise in connection with the recall. The Corporation may lose a significant amount of sales and may not be able to replace those sales at an acceptable margin or at all. In addition, a product recall may require significant management attention. Although the Corporation has procedures in place for testing its products, there can be no assurance that any quality, potency or contamination problems will be detected in time to avoid unforeseen product recalls, regulatory action or lawsuits. A recall for any of the foregoing reasons could lead to decreased demand for the Corporation’s products and could have a material adverse effect on the business, financial condition and results of operations of the Corporation. Additionally, product recalls may lead to increased scrutiny of the Corporation’s operations by Health Canada or other regulatory agencies, requiring further management attention and potential legal fees and other expenses.
Inability to Maintain Pricing Premium for Premium Products and a Brand that Attracts or Retains Customers.
The anticipated pricing spread between premium and non-premium products may not be as significant as originally forecasted. The Corporation’s strategy is reliant on the development of a premium and super premium segment of the recreational cannabis market and the ability to charge more for such product. A premium segment may not develop in the market and even if it does, premium pricing may be volatile. The Corporation’s business model is susceptible to erosion of profitability should the actual pricing premium for high grade products be less than expected.
Additionally, even if a premium market for cannabis does materialize, the Corporation may not be successful in creating and maintaining consumer perceptions of the value of its premium products. The promotion of cannabis is strictly regulated in Canada. Restrictions on packaging, labelling and promotional activity are summarized below in “Regulatory Framework - Canada - Packaging and Labelling and Regulatory Framework - Canada - Promotional Activity”. Such restrictions and other restrictions on advertising imposed by Canadian federal or provincial laws or regulations, or similar regulations imposed in other jurisdictions, may prevent the Corporation from creating and maintaining consumer perceptions in the value of its premium products and establishing itself as a premium producer. If the Corporation cannot successfully enter into or compete in the premium market, it may face significant challenges in gaining or maintaining a market share in Canada or in other cannabis markets in which it intends to operate, or it may be forced to sell its products at a lower price, which may materially adversely affect its revenues.
The Corporation’s success depends, in part, on its ability to attract and retain customers who in turn sell to ultimate consumers of cannabis products. To do this, the Corporation is dependent upon, among other things, continually producing desirable and effective cannabis products and the continued growth in the aggregate number of recreational cannabis consumers. Campaigns designed to enhance the Corporation’s brand and attract consumers, subject to restrictions imposed by law, can be expensive and may not result in increased sales. If the Corporation is unable to attract new consumers, it may not be able to increase its sales.
Regulation of the Recreational Cannabis Market in Canada May Have an Adverse Impact on the Corporation’s Ability to Develop and Grow a Medical Cannabis Business in Canada.
A regulated recreational market for cannabis products was implemented on October 17, 2018, and the full effect of that on the Canadian medical cannabis market remains unknown. If medical-use consumers decide to purchase cannabis products available in the recreational market instead of continuing to purchase them in the medical market, the Corporation’s ability to grow a medical cannabis business in Canada may be negatively affected.
Operational Risks Including Labour Disputes, Accidents, Fires, etc.
The Corporation may be affected by a number of operational risks and may not be adequately insured for certain risks, including: labour disputes; catastrophic accidents; fires; blockades or other acts of social activism; equipment defects, malfunction and failures; changes in the regulatory environment; impact of non-compliance with laws and regulations; natural phenomena, such as inclement weather conditions, floods, earthquakes, ground movements, accidents and explosions that can cause personal injury, loss of life, suspension of operations, damage to facilities, business interruption and damage to or destruction of property, equipment and the environment. There is no assurance that the foregoing risks and hazards will not result in damage to, or destruction of, the Corporation’s properties, facilities, grow facilities and extraction facilities, personal injury or death, environmental damage, or have an adverse impact on the Corporation’s operations, costs, monetary losses, potential legal liability and adverse governmental action, any of which could have a material adverse effect on the business, financial condition or results of operations of the Corporation. This lack of insurance coverage could have a material adverse effect on the Corporation’s business, financial condition or results of operations.
The Corporation will continuously monitor its operations for quality control and safety. However, there are no assurances that the Corporation’s safety procedures will always prevent such damages and the Corporation may be affected by liability or sustain loss in respect of certain risks and hazards. Although the Corporation will maintain insurance coverage that it believes to be adequate and customary in the industry, there can be no assurance that such insurance will be adequate to cover its liabilities. In addition, there can be no assurance that the Corporation will be able to maintain adequate insurance in the future at rates it considers reasonable and commercially justifiable. The Corporation may elect not to insure against certain risks due to cost or ease of procuring such insurance. The occurrence of a significant uninsured claim, a claim in excess of the insurance coverage limits then maintained by the Corporation, or a claim at a time when it is not able to obtain liability insurance, could have a material adverse effect on the Corporation’s business, financial condition or results of operations.
In addition, many insurance providers are not insuring companies that operate in the cannabis industry or require significant premiums in order to provide coverage. The Corporation may not be able to obtain coverage, including directors and officers liability coverage, at reasonable rates or at all as a result of such circumstances. In addition, the Corporation may choose to access lower limits of insurance. As a result, the Corporation may not have adequate insurance for covering its risks, which could have a material adverse effect on the Corporation’s business, financial condition or results of operations.
Proprietary Protection in Respect of the Corporation’s Ideas and Technology.
The success of the Corporation’s business depends in part on its ability to protect its ideas and technology. The Corporation has filed applications for a number of patents and registered trademarks in Canada and elsewhere. It is possible, however, that the Corporation will not be able to register, maintain registration for or enforce all of its intellectual property, including patents and trademarks, in all key jurisdictions.
Even where the Corporation has taken steps to protect its technology with patents, plant breeders’ rights, copyrights or by other means, the Corporation is not assured that competitors will not develop similar technology, business methods or that the Corporation will be able to exercise its legal rights to protect and maintain intellectual property monopolizing its technology.
Even where the Corporation has taken steps to protect its branding with trademarks, industrial designs, copyrights or by other means, the Corporation is not assured that competitors will not develop similar branding or that the Corporation will be able to exercise its legal rights to protect and maintain intellectual property monopolizing its branding.
Other countries may not protect intellectual property rights to the same standards as does Canada. Actions taken to protect or preserve intellectual property rights may require significant financial and other resources which may have a material adverse effect on the business, financial condition and results of operations of the Corporation.
The Corporation’s intellectual property also consists of certain trade secrets and know-how, including in the design and construction of its facilities. Such trade secrets and know-how may not be protected by applicable laws, and although the Corporation enters into confidentiality and non-use agreements to protect such intellectual property, there is no assurance that such intellectual property may not be used by a third party or employee to the Corporation’s detriment.
Conflicts of Interest in Respect of Directors and Officers of the Corporation.
All decisions to be made by the Directors and officers involving the Corporation are required to be made in accordance with their duties and obligations to act honestly and in good faith with a view to the best interests of the Corporation. In addition, such Directors and officers are required to declare their interests in, and to refrain from voting on, any matter in which they may have a material conflict of interest. However, certain of the Directors and officers of the Corporation are also directors and officers of other companies or are engaged and will continue to be engaged in activities that may put them in conflict with the business strategy of the Corporation. In addition, Peter Comerford, an executive officer of Holigen and a proposed executive officer of Flowr post acquisition of Holigen, is the Chief Executive Officer of Anspec, a distribution partner of Holigen, and receives compensation from Anspec for his services and is entitled to certain payments on the sale of Anspec to a third party purchaser. Consequently, there exists the possibility for such Directors and officers to be in a position of conflict with their duties to the Corporation and their decisions may not be in the best interests of the Corporation and its shareholders.
Constraints on Marketing Products Due to Statutory Prohibitions.
The Corporation intends to develop brand/product differentiation strategies to retain and/or grow brand market share. The potential for success of these strategies is uncertain, dependent on various factors, and subject to various challenges.
The development of the Corporation’s business and operating results may be hindered by applicable regulatory restrictions on promotional activity. Under the Cannabis Act, marketing and advertising for cannabis products is significantly limited as detailed below in “Regulatory Framework - Canada - Promotional Activity”. Accordingly, the regulatory environment in Canada limits the Corporation’s ability to compete for market share in a manner similar to other industries.
If the Corporation is unable to effectively market its cannabis products and compete for market share, or if the costs of compliance cannot be absorbed through increased selling prices for its cannabis products, it could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
Consumer Preferences May Change, and the Corporation May be Unsuccessful in Acquiring or Retaining Consumers and Keeping Pace with Changing Market Developments.
As a result of changing consumer preferences, many consumer products attain financial success for a limited period of time. Even if the Corporation’s products find success at retail, there can be no assurance that such products will continue to be profitable. The Corporation’s success will be significantly dependent upon its ability to develop new and improved product lines and adapt to consumer preferences. Even if the Corporation is successful in introducing new products or developing its current products, a failure to gain consumer acceptance or to update products could cause a decline in our products’ popularity and impair our brand. In addition, the Corporation may be required to invest significant capital in the creation of new product lines, strains, brands, marketing campaigns, packaging and other product features - none of which are guaranteed to be successful. Failure to introduce new features and product lines and to achieve and sustain market acceptance could result in the Corporation being unable to satisfy consumer preferences and generate revenue.
The Corporation’s success depends on its ability to attract and retain consumers. There are many factors which could impact its ability to attract and retain consumers, including its ability to continually produce desirable and effective products, the successful implementation of its consumer acquisition plan and the continued growth in the aggregate number of potential consumers. The Corporation’s failure to acquire and retain consumers could have a material adverse effect on the Corporation.
The legal recreational cannabis industry is in its early stages of development and it is likely that the Corporation, and its competitors, will seek to introduce new products in the future. In attempting to keep pace with any new market developments, the Corporation may need to spend significant amounts of capital in order to successfully develop and generate revenues from new products it introduces. Additionally, the Corporation may be required to obtain additional regulatory approvals from Health Canada and any other applicable regulatory authorities, which may take significant amounts of time and cost. The Corporation may not be successful in developing effective and safe new products, anticipating shifts in social trends and consumer demands, bringing such products to market in time to be effectively commercialized, or obtaining any required regulatory approvals, which, together with any capital expenditures made in the course of such product development and regulatory approval processes, may have a material adverse effect on the Corporation’s business and results of operations.
In addition, the patterns of cannabis consumption in Canada and elsewhere in the world may shift over time due to a variety of factors, including changes in demographics, social trends, public health polices and other leisure or consumption behaviors. If consumer preferences for the Corporation’s products or cannabis products in general do not develop, or if once developed, they were to move away from our products or cannabis products in general, or if the Corporation is unable to anticipate and respond effectively to shifts in consumer behaviors, it may be adversely affected.
Risks Inherent in an Agricultural Business.
Cannabis is an agricultural product. There are risks inherent in an agricultural business, such as insects, plant diseases, crop failure and similar agricultural risks. Although the products are usually grown indoors or in greenhouses under climate-controlled conditions, with conditions monitored, there can be no assurance that natural elements will not have a material adverse effect on the production of the Corporation’s products and, consequentially, on the Corporation’s business, financial condition and results of operations. In addition, the Corporation and Holigen intend to grow cannabis outdoor, in environments that are not controlled. As a result, the risk of crop failures and the loss of its crops is significantly more profound.
Although the Corporation aims to produce high quality products without the use of irradiation, the Corporation has used, and may in the future be required to use, irradiation on its products. Such irradiation is likely to impact the quality of the products, which could have a material adverse effect on the Corporation’s sales, reputation as a high-quality cannabis producer and/or selling price, and, consequentially, on the Corporation’s business, financial condition and results of operations.
Valuation of Biological Assets.
Pursuant to IFRS, the Corporation measures the value of its biological assets consisting of cannabis plants using the income approach at fair value less costs to sell up to the point of harvest. As market prices are generally not available for biological assets while they are growing, the Corporation is required to make assumptions and estimates relating to, among other things, future agricultural commodity yields, prices and production costs. The assumptions and estimates used to determine the fair value of biological assets, and any changes to such prior estimates, directly affect the Corporation’s reported results of operations. If actual yields, prices, costs, market conditions or other results differ from the Corporation’s estimates and assumptions, there could be material adjustments to the Corporation’s results of operations. In addition, the use of these future estimated metrics differs from generally accepted accounting principles in the United States. As a result, the Corporation’s financial statements and reported earnings are not directly comparable to those of similar companies in the United States.
Environmental Regulation and Risks.
The Corporation’s operations are subject to environmental regulation in the various jurisdictions in which it operates. These regulations mandate, among other things, the maintenance of air and water quality standards and land reclamation. They also set forth limitations on the generation, transportation, storage and disposal of solid and hazardous waste. Environmental legislation is evolving in a manner which will require stricter standards and enforcement, increased fines and penalties for non-compliance, more stringent environmental assessments of proposed projects and a heightened degree of responsibility for companies and their officers, directors and employees. There is no assurance that future changes in environmental regulation, if any, will not adversely affect the Corporation’s operations.
Government approvals and permits are currently, and may in the future be, required in connection with the Corporation’s operations. To the extent such approvals are required and not obtained, the Corporation may be curtailed or prohibited from its production and/or distribution of cannabis or from proceeding with the development of its operations as currently proposed.
Failure to comply with applicable laws, regulations and permitting requirements may result in enforcement actions thereunder, including orders issued by regulatory or judicial authorities causing operations to cease or be curtailed, and may include corrective measures requiring capital expenditures, installation of additional equipment, or remedial actions. The Corporation may be required to compensate those suffering loss or damage by reason of its operations and may have civil or criminal fines or penalties imposed for violations of applicable laws or regulations.
Trade of Cannabis For Non-Medical Purposes Within Canada May be Restricted by the Canadian Free Trade Agreement.
We have entered into supply agreements or arrangements with the provinces of Ontario, British Columbia, Nova Scotia, Saskatchewan and Manitoba for the supply of recreational cannabis and cannabis derivative products. The Canadian Free Trade Agreement, which generally reduces or eliminates the barriers to the free movement of persons, goods, services, and investments within Canada, specifically excludes cannabis for non-medical purposes from its scope and instead leaves the intra-Canadian movement of non-medical cannabis to future negotiations among the provinces and territories. There is a risk that the outcome of the negotiations will result in the interprovincial and interterritorial trade of cannabis for non-medical purposes in Canada being entirely restricted or subject to conditions that will negatively impact the Corporation’s ability to sell cannabis in provinces and territories in which it does not have cultivation and production facilities, including those in which the Corporation has already executed agreements or been approved to supply cannabis to retailers.
Credit Risk
Credit risk is the risk that the counterparty to a financial instrument fails to meet its contractual obligations, resulting in a financial loss to the Corporation. We have credit risk exposure based on the balance of our cash, accounts receivable, subscriptions receivable, and taxes recoverable. There are no assurances that the Corporation’s counterparties or customers will meet their contractual obligations to it.
Results of Future Clinical Research.
Research in Canada, the United States and internationally regarding the medical benefits, viability, safety, efficacy, dosing and social acceptance of cannabis or isolated phytocannabinoids (such as CBD and THC) remains in early stages. There have been relatively few clinical trials on the benefits of cannabis or isolated phytocannabinoids (such as CBD and THC). Although the Corporation believes that the articles, reports and studies support its beliefs regarding the effects of cannabis, viability, safety, efficacy, dosing and social acceptance of cannabis, future research and clinical trials may prove such statements to be incorrect, or could raise concerns regarding, and perceptions relating to, cannabis. Further, the Corporation believes the cannabis industry is highly dependent upon consumer perception regarding the safety, efficacy and quality of the cannabis produced. Consumer perception can be significantly influenced by scientific research or findings, regulatory investigations, litigation, media attention and other publicity regarding the consumption of cannabis products. There can be no assurance that future scientific research or findings, regulatory investigations, litigation, media attention or other publicity will be favorable to the cannabis market or any particular product, or consistent with earlier publicity.
Future research studies and clinical trials may reach negative conclusions regarding the medical benefits, viability, safety, efficacy, dosing, social acceptance or other facts and perceptions related to cannabis, which could have a material adverse effect on the demand for the Corporation’s products with the potential to lead to a material adverse effect on the Corporation’s business, financial condition and results of operations. There is no assurance that such adverse publicity reports or other media attention will not arise.
Transportation Disruptions Related to an Agricultural Product.
As a business revolving mainly around the growth of an agricultural product, the ability to obtain speedy, cost- effective and efficient transport services will be essential to the prolonged operations of the Corporation’s business. Should such transportation become unavailable for prolonged periods of time, it could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
Due to the nature of the Corporation’s products, security of the product during transportation to and from its facilities is of the utmost concern. A breach of security during transport or delivery could have a material adverse effect on the Corporation’s business, financial condition and results of operations. Any breach of the security measures during transport or delivery, including any failure to comply with recommendations or requirements of Health Canada, could also have an impact on the Corporation’s ability to continue operating under its licences or the prospect of renewing its licences.
Vulnerability to Rising Energy Costs.
The Corporation’s cannabis growing operations consume considerable energy, which makes the Corporation vulnerable to rising energy costs and/or the availability of stable energy sources. Accordingly, rising or volatile energy costs or the inability to access stable energy sources may have a material adverse effect on the Corporation’s business, financial condition and results of operations.
Reliance on Key Inputs.
The Corporation’s business is dependent on a number of key inputs and their related costs including raw materials and supplies related to its growing operations, as well as electricity, water and other local utilities. Any significant interruption or negative change in the availability or economics of the supply chain for key inputs could materially impact the business, financial condition and operating results of the Corporation. Some of these inputs may only be available from a single supplier or a limited group of suppliers, including access to the electricity grid. If a sole source supplier was to go out of business, the Corporation might be unable to find a replacement for such source in a timely manner or at all. If a sole source supplier were to be acquired by a competitor, that competitor may elect not to sell to the Corporation in the future. Any inability to secure required supplies and services or to do so on appropriate terms could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
Dependence on Skilled Labour and Suppliers.
The ability of the Corporation to compete and grow will be dependent on it having access, at a reasonable cost and in a timely manner, to skilled labour, equipment, parts and components. No assurances can be given that the Corporation will be successful in maintaining its required supply of skilled labour, equipment, parts and components. Qualified individuals are in high demand, and the Corporation may incur significant costs to attract and retain them. It is also possible that the final costs of the major equipment and materials, including packaging materials, contemplated by the Corporation’s capital expenditure program may be significantly greater than anticipated by the Corporation’s management, and may be greater than funds available to the Corporation, in which circumstance the Corporation may curtail, or extend the timeframes for completing, its capital expenditure plans. This could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
Security Clearance of Individuals Occupying a “Key Position” in the Corporation.
Certain people associated with cannabis licensees, including individuals occupying a “key position” such as directors, officers, large shareholders and other individuals identified by the Minister, must hold a valid security clearance issued by the Minister. Certain of Flowr’s Directors and officers have applied to the Minister for their security clearance and are currently waiting for a response. Under the Cannabis Regulations, the Minister may refuse to grant security clearance under certain circumstances, or may revoke such security clearance should certain circumstances arise. Federal cannabis licence holders were experiencing delays in Health Canada security clearances and any similar delays under the Cannabis Regulations could have an adverse effect on the Corporation’s business and results of operations. In addition, certain individuals at Holigen are, and, upon the Acquisition, certain individuals at the Corporation will be required to obtain security clearances from the applicable regulatory authorities in Portugal and Australia. There is no assurance that any of the individuals occupying a “key position”, or other individuals who are presently required, or may in the future be required, to obtain, maintain or renew a security clearance in Canada, Portugal or Australia will be able to do so. A failure by an individual to obtain, maintain or renew his or her security clearance in Canada, Portugal or Australia would result in a material adverse effect on the Corporation’s or Holigen’s, as applicable, business, financial condition and results of operations. In addition, if such an individual with security clearance leaves and Flowr or Holigen is unable to find a suitable replacement that has a security clearance in a timely manner, or at all, there could occur a material adverse effect on the Corporation’s business, financial condition and results of operations.
Third Parties with Whom the Corporation Does Business May Perceive Themselves as Being Exposed to Reputational Risk as a Result of Their Relationship with the Corporation and May, as a Result, Refuse to Do Business with the Corporation.
The parties with whom the Corporation does business, or would like to do business with, may perceive that they are exposed to reputational risk as a result of the Corporation’s business activities relating to cannabis, which could hinder the Corporation’s ability to establish or maintain business relationships. These perceptions relating to the cannabis industry may interfere with the Corporation’s relationship with service providers in Canada and other countries, particularly in the financial services industry.
The Ability to Continuously Maintain and Retain a Competitive Talent Pool.
As the Corporation grows, it will need to hire additional human resources to continue to develop its businesses. However, experienced talent in the areas of cannabis R&D, growing cannabis and extraction, as well as senior management, are difficult to source, and there can be no assurance that the appropriate individuals will be available or affordable. Without adequate personnel and expertise, the growth of the business of the Corporation may suffer. There can be no assurance that the Corporation will be able to identify, attract, hire and retain qualified personnel and expertise in the future, and any failure to do so could have a material adverse effect on the Corporation’s business, financial condition or results of operations.
Management of Growth in Respect of Staff Resources.
As the Corporation grows, the Corporation will also be required to hire, train, supervise and manage new employees. The Corporation may experience a period of significant growth in the number of personnel that will place a strain upon its management systems and resources. Its future will depend in part on the ability of its officers and other key employees to implement and improve financial and management controls, reporting systems and procedures on a timely basis and to expand, train, motivate and manage the workforce. The Corporation’s current and planned personnel, systems, procedures and controls may be inadequate to support its future operations. Failure to effectively manage any future growth could have a material adverse effect on the Corporation’s business, financial condition or results of operations.
Internal Controls Over Financial Reporting.
Effective internal controls are necessary for the Corporation to provide reliable financial reports and to help prevent fraud. Although the Corporation will undertake a number of procedures and will implement a number of safeguards, in each case, in order to help ensure the reliability of its financial reports, including those imposed on the Corporation under Canadian and United States securities law, the Corporation cannot be certain that such measures will ensure that the Corporation will maintain adequate control over financial processes and reporting. Failure to implement required new or improved controls, or difficulties encountered in their implementation, could harm the Corporation’s results of operations or cause it to fail to meet its reporting obligations. If the Corporation or its auditors discover a material weakness, the disclosure of that fact, even if quickly remedied, could reduce the market’s confidence in the Corporation’s consolidated financial statements and materially adversely affect the trading price of the Common Shares. In addition, as a TSX.V issuer, the Corporation is not required to establish and maintain disclosure controls and protocols and internal controls over financial reporting which may result in additional risks to the quality, reliability, transparency and timeliness of interim and annual filings and other reports provided by the Corporation under applicable securities legislation.
Difficulty to Forecast Sales and Other Business Metrics.
The Corporation must rely largely on its own market research to forecast sales as detailed forecasts are not generally obtainable from other sources at this early stage of the cannabis industry. If the Corporation underestimates the demand for its products, it may not be able to produce products to meet its stringent requirements, and this could result in delays in the shipment of products and failure to satisfy demand, as well as damage to reputation and partner relationships. If the Corporation overestimates the demand for its products, it could face inventory levels in excess of demand, which could result in inventory write-downs or write-offs and the sale of excess inventory at discounted prices, which would harm the Corporation’s gross margins and brand management efforts.
Due to the new nature of the recreational market, it is difficult for the Corporation to forecast demand. In particular, it is difficult to forecast the rate of the illicit cannabis market crossing over to the legal recreational market. If the recreational market does not develop as the Corporation expects, it could have a material adverse effect on its business, results of operations and financial condition.
In addition to inherent risks and difficulties forecasting sales, anticipated costs and yields are also challenging to predict with certainty as the cannabis industry is in its relative infancy and rapidly evolving. If Flowr makes capital investments based on flawed sales, costs and yields forecasts, the Corporation may not achieve its expected, or any, return on invested capital. Failure to realize forecasted sales, costs and yields could have a material adverse effect on the Corporation’s business, results of operations and financial condition.
The Corporation May Become a Party to Litigation.
The Corporation may become party to litigation from time to time in the ordinary course of business which could adversely affect its business. Should any litigation in which the Corporation becomes involved be determined against the Corporation such a decision could adversely affect the Corporation’s ability to continue operating and the market price for the Common Shares and could use significant resources and demand significant time and attention by management. Even if the Corporation is involved in litigation and wins, litigation can redirect significant resources.
The Corporation May Become Involved in Regulatory or Agency Proceedings, Investigations and Audits.
Health Canada inspectors routinely assess the Corporation for compliance with applicable regulatory requirements. The Corporation’s facilities will be inspected by Health Canada and Holigen’s facility will also be inspected for compliance by applicable regulators and will be subject to certain ongoing inspections and audits. Furthermore, the import of the Corporation’s products into other jurisdictions, such as Europe and Australia, is subject to the regulatory requirements of the respective jurisdiction. Any failure by the Corporation to comply with the applicable regulatory requirements could require extensive changes to the Corporation’s operations; result in regulatory or agency proceedings or investigations, increased compliance costs, damage awards, civil or criminal fines or penalties or restrictions on the Corporation’s operations; harm the Corporation’s reputation or give rise to material liabilities or a revocation of the Corporation’s licences and other permits. There can be no assurance that any pending or future regulatory or agency proceedings, investigations or audits will not result in substantial costs, a diversion of management’s attention and resources or other adverse consequences to the Corporation and its business.
There Can Be No Assurance That the Corporation May Not Be Treated as a “Passive Foreign Investment Company” (“PFIC”) Under the U.S. Internal Revenue Code. If the Corporation Were Treated as a PFIC, Such Treatment Could Result In Adverse Tax Consequences For Investors in the United States.
There can be no assurance that the Corporation may not be treated as a PFIC under the U.S. Internal Revenue Code. If the Corporation were treated as a PFIC, such treatment could result in adverse tax consequences for investors in the United States. Certain adverse tax consequences could apply to a U.S. holder if the Corporation were treated as a PFIC for any taxable year during which the U.S. holder owns Common Shares. The Corporation believes that it is not currently a PFIC and is not likely to become a PFIC in the foreseeable future. The determination of whether it is a PFIC, however, is made annually as of the close of each taxable year. Because the determination whether a foreign corporation is a PFIC is primarily factual and there is little administrative or judicial authority on which to rely to make a determination, the IRS might not agree that the Corporation is not a PFIC. Moreover, no assurance can be given that the Corporation would not become a PFIC for any future taxable year if there were to be changes in the Corporation’s assets, income or operations. See "Certain United States Federal Income Tax Considerations".
The Corporation is Subject to Certain Restrictions of the TSX.V Which May Constrain Its Ability to Expand Its Business Internationally.
The TSX.V required, as a condition to listing, that the Corporation deliver an undertaking (the "Undertaking") confirming that, while listed on the TSX.V, the Corporation will only conduct the business of cannabis related business as described in the Filing Statement and/or the business of the production, sale and distribution of cannabis for medical and recreational purposes in Canada as permitted under the Corporation’s licences with Health Canada. The Undertaking could have an adverse effect on the Corporation’s ability to export cannabis from Canada and on its ability to expand its business into other areas while it is still listed on the TSX.V and subject to the Undertaking. The Undertaking may prevent the Corporation from expanding into new areas of business when its competitors have no such restrictions. All such restrictions could materially and adversely affect the Corporation’s growth, business, financial condition and results of operations.
Forward-Looking Statements, Future-Oriented Financial Information, and Financial Outlooks May Prove Inaccurate.
Investors are cautioned not to place undue reliance on forward-looking statements, future-oriented financial information and financial outlooks, which include expected yields, capacity and costs of production of the Corporation. By their nature, forward-looking statements, future-oriented financial information and financial outlooks involve numerous assumptions, known and unknown risks and uncertainties, of both a general and specific nature, that could cause actual results to differ materially from those suggested by the forward-looking statements or contribute to the possibility that predictions, forecasts or projections will prove to be materially inaccurate. Future-oriented financial information and financial outlooks presented in this Prospectus are based upon many assumptions but also on the completion of the Offering and the Acquisition. If these transactions are not completed or not completed on the terms or timelines contemplated or if the underlying assumptions prove to be incorrect, this will impact the future-oriented financial information and financial outlooks provided herein and such impact may be material. Forward-looking statements also include projections about the size of the cannabis market in various jurisdictions and while these are derived from sources Management believes to be reliable, such data involve assumptions, risks and uncertainties and are subject to change. Additional information on the risks, assumptions and uncertainties are found in this Prospectus under the headings "Cautionary Note Regarding Forward-Looking Information" and "Cautionary Note Regarding Future-Oriented Financial Information".
The Corporation Expects to Enter Into the ATB Credit Facilities, but Such Facilities May Not Get Finalized or Be Available. In that Event, the Corporation Would Have to Find an Alternative Form of Financing.
The Corporation is in negotiations to obtain a loan commitment from ATB to provide the ATB Credit Facilities which, together with the net proceeds of the Offering, are expected to provide sufficient funds for its general working capital requirements over the next 12 months and to finance the construction of the K2 Facility and Flowr Forest. Such financing arrangements are subject to finalization of terms and documentation, and will contain specific terms and conditions for funding and closing to take place. If the ATB Credit Facilities are not finalized or are otherwise not available to the Corporation, the Corporation (i) would likely be required to redesign the K2 Facility and construct a facility that is approximately half the size of the planned K2 Facility, which would result in half of the expected capacity of the K2 Facility from the capacity disclosed in this Prospectus or (ii) may have to seek alternative forms of financing. If the Corporation is required to construct a smaller facility, this could result in a delay to completion and operation of the K2 Facility and reduced capacity and revenues for the Corporation. In addition, there can be no guarantee that the Corporation would be able to find an alternative form of financing on the same terms as the ATB Credit Facilities or at all. Failure to find an alternative form of financing could have a material adverse impact on the Corporation's ability to fund its working capital requirements or complete construction of the K2 Facility, which could, in turn, have a material adverse effect on the Corporation's business, financial condition and results of operations. Furthermore, if the Corporation cannot complete the Offering or find an alternative form of financing on the same terms as the ATB Credit Facilities or at all, the Corporation would likely be required to scale back certain operational and capital expenditures, including its proposed NASDAQ listing and various non-essential features of the Hawthorne R&D Facility and the K1 Facility.
The Corporation Expects to Incur Indebtedness for its General Working Capital Requirements and to Finance the Construction of the K2 Facility and Flowr Forest, which Total Indebtedness Imposes Operating And Financial Restrictions on the Corporation that, Together with the Resulting Debt Service Obligations from Such Total Indebtedness, May Limit the Corporation’s Ability to Execute its Business Plans.
The Corporation expects to enter into the ATB Credit Facilities and use them for its general working capital requirements and to finance the construction of the K2 Facility and Flowr Forest. However, if the terms of the ATB Credit Facilities differ from those currently contemplated by the Corporation, it could have a material adverse impact on the Corporation’s ability to fund its working capital requirements or complete construction of the K2 Facility and Flowr Forest, which could, in turn, have a material adverse effect on the Corporation’s business, financial condition and results of operations. In addition, the Corporation’s indebtedness under the ATB Credit Facilities may limit the Corporation’s ability to execute its business plans, which could have important consequences to holders of the Common Shares including: reducing the availability of cash flow to fund working capital, capital expenditures, development activity and other general corporate purposes, increasing the Corporation’s vulnerability to adverse general economic or industry conditions, limiting the Corporation’s ability to obtain additional financing in the future for working capital, and resulting in increased sensitivity of overall cash flow and profitability to changes in interest rates.
If the Lenders Under the ATB Credit Facilities Fail to Extend Credit Under Such Facilities, the Corporation’s Liquidity and Results of Operations May Be Adversely Affected.
The Corporation will have access to capital through the Revolving Facility, which is part of the ATB Credit Facilities. Each lender which becomes part of the syndicate for the Revolving Facility will be responsible, on a several, but not joint, basis, for providing a portion of the loans to be made under that facility. If any participant or group of participants with a significant portion of the commitments in the Revolving Facility fails to satisfy its or their respective obligations to extend credit under such facility, and the Corporation is unable to find a replacement for such participant or participants on a timely basis (or at all), the Corporation’s liquidity may be adversely affected. If the Corporation’s liquidity under the Revolving Facility is materially adversely impacted, it could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
Risks Relating to International Activities
The Corporation’s Operations in Foreign Countries May be Subject to a Higher Degree Of Political, Social, Regulatory and Economic Risk Than Jurisdictions in Which It Currently Operates.
A number of risks are inherent in international operations, including risks associated with: (i) foreign currency fluctuations and devaluations; (ii) political, social, security and economic instability in foreign countries; (iii) changes in and compliance with local laws and regulations or uncertainty regarding the interpretation and/or application of applicable laws, including export and import control laws, sanctions regulations, tax laws, labour laws, employee benefits, currency restrictions and other requirements; (iv) differences in tax regimes and potentially adverse tax consequences of operating in foreign countries or unfavourable or arbitrary tax enforcement; (v) customizing products for foreign countries; (vi) legal uncertainties regarding liability, export and import restrictions, tariffs and other trade barriers; (vii) changes in governmental regulations regarding currency or price controls, profit repatriation, labour, or health and safety matters; (viii) hiring qualified foreign employees; and (viii) difficulty in accounts receivable collection and longer collection periods. Accordingly, as the Corporation begins to operate internationally, its exposure to risks involved with operating in foreign countries will increase, which could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
Expansion into Foreign Jurisdictions.
Pursuant to the Cannabis Act, cannabis may only be exported from Canada for medical or scientific purposes and a licence is required to do so. No assurance can be given that Health Canada will grant an export licence to the Corporation, or, if granted, that such licence will be renewed or extended on the same or similar terms, or at all. In addition, the Corporation’s expansion into jurisdictions outside of Canada is also subject to risks. In jurisdictions outside of Canada, there can be no assurance that any market for the Corporation’s products will develop. The Corporation may face new or unexpected risks or significantly increase its exposure to one or more existing risk factors, including economic instability, changes in laws and regulations, and the effects of competition. These factors may limit the Corporation’s ability to successfully expand its operations into such jurisdictions and could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
Fluctuations in Foreign Currency Exchange Rates Could Harm the Corporation’s Results of Operations.
The Corporation may be exposed to fluctuations of the Canadian dollar against certain other currencies because it publishes its financial statements in Canadian dollars, while a portion of its assets, liabilities, revenues and costs are, or will be, denominated in other currencies, including the Australian dollar and euros. Exchange rates for currencies of the countries in which the Corporation intends to operate in the future, which currently include Australia and Portugal, may fluctuate in relation to the Canadian dollar, and such fluctuations may have a material adverse effect on the Corporation’s earnings or assets when translating foreign currency into Canadian dollars.
The Corporation Relies on International Advisors and Consultants.
The legal and regulatory requirements in the foreign countries in which the Corporation operates with respect to the cultivation and sale of cannabis, banking system and controls, as well as local business culture and practices are different from those in Canada. The officers and Directors of the Corporation must rely, to a great extent, on the Corporation’s local management team and employees as well as legal counsel and local consultants retained by the Corporation in order to keep abreast of material legal, regulatory and governmental developments as they pertain to and affect the Corporation’s business operations, and to assist the Corporation with its governmental relations. The Corporation also relies on the advice of local experts and professionals in connection with current and new regulations that develop in respect of the cultivation and sale of cannabis as well as in respect of banking, financing, labour, litigation and tax matters in these jurisdictions. Any developments or changes in such legal, regulatory or governmental requirements or in local business practices are beyond the control of the Corporation. The impact of any such changes could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
The Corporation May Expand into Other Geographic Areas, Which Could Increase the Corporation’s Operational, Regulatory and Other Risks.
In addition to the jurisdictions described elsewhere in this Prospectus, the Corporation may in the future expand into other geographic areas, which could increase the Corporation’s operational, regulatory, compliance, reputational and foreign exchange rate risks. The failure of the Corporation’s operating infrastructure to support such expansion could result in operational failures and regulatory fines or sanctions. In addition, to the extent the Corporation attempts to enter into the cannabis market in a developing country, these risks may be exacerbated and there may be additional risks with such emerging economies, such as hyperinflation, price controls, property seizures, war, terrorism, expropriation, nationalism, social and labour unrest, organized crime, corruption and fraud and restrictions on the acquisition or lease of properties. Future international expansion could require the Corporation to incur a number of up-front expenses, including those associated with obtaining regulatory approvals, as well as additional ongoing expenses, including those associated with infrastructure, staff and regulatory compliance. The Corporation may not be able to successfully identify suitable acquisition and expansion opportunities or integrate such operations successfully with the Corporation’s existing operations.
The Corporation’s Business May be Subject to a Variety of U.S. and Foreign Laws, Many of Which are Unsettled and Still Developing and Which Could Subject the Corporation to Claims or Otherwise Harm its Business.
In the United States, despite cannabis having been legalized at the state level for medical use in many states and for recreational in a number of states, cannabis and cannabis products, other than hemp-derived CBD under certain circumstances, continue to be categorized as a Schedule I controlled substance under the Controlled Substances Act (the "CSA"), and subject to the Controlled Substances Import and Export Act, as amended (the "CSIEA"). The Corporation believes that it is not subject to the CSA or CSIEA, because it has no active business operations in the United States and does not distribute any products in the United States. Nonetheless, it is, or may become, subject to various other U.S. federal laws and regulations, including in connection with this Offering and the listing of its Common Shares on NASDAQ, and violations of any U.S. federal laws or regulations, including the CSA and CSIEA, whether intentionally or inadvertently, could result in significant fines, penalties, administrative sanctions, convictions or settlements, arising from civil proceedings initiated by either the U.S. federal government or private citizens or criminal charges, including disgorgement of profits, cessation of business activities or divestitures. Further, the status of cannabis as a Schedule I controlled substance may cause the Corporation and its business to be negatively perceived by prospective U.S. investors or other parties, who may incorrectly believe that the CSA or CSIEA applies to the Corporation, or who may have reputational or other concerns about dealings with a cannabis grower even if it is not conducting business in, or distributing any products in, the United States.
The Corporation also is, or expects to become, subject to a variety of laws and regulations in Canada, the United States, the European Union, Australia, Portugal and elsewhere that prohibit money laundering, including the Proceeds of Crime and Terrorist Financing Act (Canada), the Money Laundering Control Act (United States), as amended, Directive (EU) 2015/849 and the rules and regulations thereunder and any related or similar rules, regulations or guidelines issued, administered or enforced by governmental authorities in Canada, the United States, the European Union, Portugal or Australia or any other jurisdiction in which we have or are developing business operations or to which we export. Although the Corporation believes that none of its activities implicate any applicable money laundering statutes, in the event that any of its business activities, any dividends or distributions therefrom, or any profits or revenue accruing thereby are found to be proceeds of crime under one or more of the statutes described above or any other applicable legislation, any persons, including investors, found to be aiding and abetting the Corporation in such violations could be subject to criminal or civil liability. Any violations of these laws, or allegations of such violations, could disrupt the Corporation’s operations, significantly distract management and involve significant costs and expenses, including legal fees. The Corporation could also suffer severe penalties, including criminal and civil penalties, disgorgement and other remedial measures.
The Corporation May Be Responsible for Corruption And Anti-Bribery Law Violations.
The Corporation’s business is subject to Canadian laws which generally prohibit companies and employees from engaging in bribery or other prohibited payments to foreign officials for the purpose of obtaining or retaining business. In addition, the Corporation is subject to the anti-bribery laws of any other countries in which it conducts business now or in the future. The Corporation’s employees or other agents may, without its knowledge and despite its efforts, engage in prohibited conduct under the Corporation’s policies and procedures and anti-bribery laws for which the Corporation may be held responsible. The Corporation’s policies mandate compliance with these anti-corruption and anti-bribery laws. However, there can be no assurance that the Corporation’s internal control policies and procedures will always protect it from recklessness, fraudulent behaviour, dishonesty or other inappropriate acts committed by its affiliates, employees, contractors or agents. If the Corporation’s employees or other agents are found to have engaged in such practices, the Corporation could suffer severe penalties and other consequences that may have a material adverse effect on its business, financial condition and results of operations.
Risk Factors Specific to Australia
Recent Changes to Legislation.
There are a significant number of laws, regulations and guidelines that are applicable to the cannabis industry in Australia. The application of such laws, regulations and guidelines to cannabis specifically has yet to be fully tested, and may be subject to amendment in the future as the prevalence of cannabis related industries in Australia grows. In particular, amendments to the Narcotics Act in relation to cannabis only came into full effect in Australia in October 2016. The ODC has published regulations and a series of guidelines which explain how the reforms operate and the application processes for licences and permits. Although this guidance is quite prescriptive, as with any new legislative regime, there remains some uncertainty as to the interpretation of the new laws and regulations and the review methodology that the ODC will adopt. In particular, specific considerations that the ODC will consider when reviewing licence and permit applications and precise weight given to each consideration are not yet widely understood.
Fit and Proper Persons.
Under the medicinal cannabis regulatory regime in Australia, in order to obtain the necessary licences, the ODC must first establish the integrity of the person applying for a licence, or who has the ability to substantially influence the conduct of activities under a licence. This is known as the 'fit and proper person’ test. In respect of an applicant who is a company, this test is applied to the directors of the company and any shareholder (or ultimate holder) who has the ability to influence the conduct of the company. Where there is a change in the board or shareholding of a licensee and that change results in a person having the ability to substantially influence the conduct of the licensee and that person does not pass the fit and proper person test, then any licences granted will be revoked.
Product Approval Risks.
There is a risk that the products produced and supplied are not approved for supply. This risk is particularly relevant in Australia as the medicinal cannabis industry is highly regulated. Medicinal cannabis products are regulated as medicines in Australia. Generally, medicines imported, supplied in, and exported from Australia must be entered in the ARTG. However, for unapproved therapeutic goods the mechanisms for access include the Special Access Scheme and Authorised Prescriber Scheme. There can be no guarantee that any or all of Holigen’s medicinal cannabis products will be approved for supply to patients through either scheme. Additionally, there is no guarantee that medical practitioners will be authorised under either scheme, or that they will elect to prescribe Holigen’s products.
Risk Factors Related to the Acquisition
Holigen is an Early Stage Development Company with No Operations, a History of Net Losses and Uncertain Future Revenues and Will Require Substantial Future Funding Requirements to Build Out its Facilities and Operate its Business.
Holigen is an early stage development company that has not previously cultivated, manufactured, produced or sold cannabis or cannabis products and, accordingly, there is no basis on which to evaluate its future prospects, revenues or the Corporation’s ability to meet its objectives with respect to the Holigen business following the closing of the Acquisition. There is no certainty that anticipated outcomes and sustainable revenue streams will be achieved or that Holigen will successfully produce commercial cannabis or establish a market for its products. Additionally, Holigen has incurred losses since inception and may not be able to achieve or maintain profitability and may continue to incur significant losses in the future. In addition, the Corporation expects that Holigen will continue to increase its operating expenses as it grows its business. If Holigen’s future revenues, if any, do not offset these expected increases in costs and operating expenses, Holigen will not be profitable.
To date Holigen has also had negative cash flows from operating activities. To fund its operations, construction of its current facilities and any anticipated future capacity expansions, significant additional funds will be required. Following the closing of the Acquisition, the Corporation will require significant additional equity and/or debt financing for these purposes. There can be no assurance that additional financing will be available to the Corporation when needed or on terms which are acceptable. The Corporation’s inability to obtain financing to support Holigen’s on-going operations or to fund capital expenditures could have a material adverse effect upon Holigen’s prospects and future revenues and profitability. The Corporation may require additional financing to fund Holigen’s operations to the point where Holigen is generating positive cash flows.
As a cannabis producer, Holigen will also be subject to similar operational, regulatory, licensing, competitive and reputational risks faced by the Corporation described in the section "Risk Factors-Risks Relating to the Business".
The Corporation Could Fail to Complete the Acquisition or Complete the Acquisition On Different Terms.
The completion of the Acquisition is subject to the satisfaction of certain conditions and may not occur. These conditions include the TSX.V’s conditional approval of the listing of the Consideration Shares, the closing of this Offering, and no legal proceedings being pending or threatened that would restrict or prohibit the Acquisition or the ability of the Corporation to conduct its business or the business of Holigen after the closing of the Acquisition. If the closing of the Acquisition has not occurred on or before July 31, 2019 (unless such date is extended by the parties), the Holigen Purchase Agreement will automatically terminate. If these conditions are not met or the Acquisition is not completed by July 31, 2019, either the Vendors or the Corporation may choose not to proceed with the Acquisition. In that case, while holders of Common Shares will have had their holdings diluted by the Offering, the Corporation would not realize any anticipated benefits from the Acquisition, which could have a material adverse effect on the Corporation’s business, financial condition, and results of operations, and the market price of the Common Shares.
Even if the Acquisition is completed, there is no assurance that it will be completed on the same or similar terms to those described in this Prospectus. If the Acquisition does not take place as contemplated, the proceeds of the Offering will not be refunded and the Corporation will use such proceeds to fund future acquisitions and for working capital and/or for general trust purposes in accordance with its investment guidelines and operating policies. If the Acquisition is not completed, or does not take place as contemplated, the Corporation may not realize the benefits described in this Prospectus and could suffer adverse consequences, including loss of investor confidence. The price of the Common Shares may decline to the extent that the relevant current market price reflects a market assumption that the Acquisition will be completed and certain costs related to the Acquisition such as legal, accounting and consulting fees must be paid even if the Acquisition is not completed. The Corporation may also experience negative reactions from its customers and employees and the Corporation may be unable to identify other investments offering financial returns comparable to those of the Acquisition. Failure to complete the Acquisition or a change in the terms of the Acquisition could each have a material adverse effect on the Corporation’s business, financial condition and results of operations and the market price of the Common Shares.
The Corporation May Have Difficulties Maintaining or Growing Holigen’s Business.
Holigen’s business is expected to involve the selling of products in markets in which the Corporation has limited experience. The Corporation may experience unanticipated challenges or difficulties maintaining Holigen’s business at its current level or growing Holigen’s business. Factors that may impair the Corporation’s ability to maintain or grow Holigen’s business, its customers and personnel may include, but are not limited to:
- receipt of licences necessary to cultivate, manufacture, extract, grow, process, produce, distribute, import and export cannabis in Europe and Australia;
the inability to complete the purchase and sale transactions on real property in Portugal and Australia;
the Corporation having sufficient funds to finance the required capital expenditures needed to construct Holigen’s production facilities;
loss of contractual relationships including as a result of the exercise of change of control provisions in connection with the Acquisition or otherwise;
loss of key members of the Holigen management team;
risk relating to infringement of third party intellectual property rights by Holigen’s business;
non-compatible business cultures;
difficulties in gaining other necessary approvals in international markets to maintain and/or expand Holigen’s business;
additional demands on resources, systems, procedures and controls; and
dealing with unfamiliar laws, customs and practices in foreign jurisdictions.
In addition, the Corporation may not have identified all risks or have fully assessed risks identified with the Acquisition. There is also a risk that the expected benefits of the Acquisition may not be achieved in the expected timeframe or to the extent expected. The individual or combined effect of these risks could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
Liability For Past Acts or Omissions.
The Corporation is purchasing the shares of Holigen and, as a result, the Corporation may become liable for acts and omissions of Holigen or its subsidiaries that occurred prior to the Acquisition Closing Date, including but not limited to matters related to taxes and governmental proceedings. Although the Vendors have provided representations and warranties in the Holigen Purchase Agreement and have agreed to indemnify the Corporation in respect of certain pre-closing acts or omissions, such contractual provisions may be of limited benefit because of time limits, enforceability, caps on claim amounts, assets of the Vendors, and other factors. In addition, even if the Corporation is indemnified by the Vendors, it may have to expend substantial resources (including the time of management) to defend any claim. Any such liability could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
Prospective investors are cautioned that: (i) neither Holigen nor the Vendors have signed the certificate page to this Prospectus, certifying that the disclosure in this Prospectus represents full, true and plain disclosure of all material facts relating to Holigen or its business and does not contain a misrepresentation relating to Holigen or its business; and (ii) the Vendors will have no liability to investors participating in the Offering in the event that the disclosure contained in this Prospectus relating to Holigen or its business contains a misrepresentation.
The Corporation Is Expanding and Diversifying its Business in Connection with the Acquisition, Which Expansion and Diversification May Not Be Successful.
The Corporation may encounter financial and operational difficulties in integrating Holigen’s business with the business of the Corporation or in managing Holigen successfully. The Corporation cannot be certain of the degree and scope of operational and integration problems that may arise. To date, the Corporation has only operated in Canada and it has no experience in the Portuguese or Australian markets. This could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
The Corporation Will Acquire Liabilities, Some of Which May Be Contingent or Latent, Through the Acquisition that Could Adversely Affect the Corporation.
The Corporation will acquire liabilities in connection with the Acquisition, some of which may be contingent or latent. Although the Corporation’s management has estimated the risks associated with these liabilities and the likelihood that contingent or latent liabilities will materialize, their estimates could differ materially from the liabilities actually incurred.
In addition, as the acquirer of Holigen, the Corporation may acquire contingent liabilities in addition to liabilities assumed pursuant to the Holigen Purchase Agreement, such as statutory liabilities imposed on the acquirer of a business pursuant to applicable laws, such as creditor protection legislation, legislation relating to the protection of personal information, and anti-bribery legislation. Any of the contingent liabilities referred to above may be material and could materially adversely affect the Corporation’s business, financial condition and results of operations.
If Goodwill or Other Intangible Assets that the Corporation Records in Connection with the Acquisition become Impaired, it may have to Take Significant Charges Against Earnings.
In connection with the accounting for the Acquisition, it is expected that the Corporation will record goodwill and other intangible assets. Under IFRS, the Corporation must assess, at least annually and potentially more frequently, whether the value of goodwill and other indefinite-lived intangible assets has been impaired. Amortizing intangible assets will be assessed for impairment in the event of an impairment indicator. Any reduction or impairment of the value of goodwill or other intangible assets will result in a charge against earnings, which could materially adversely affect the Corporation’s results of operations and shareholders’ equity in future periods.
Following the Acquisition, the Corporation’s Actual Financial Position and Results of Operations May Differ Materially From the Unaudited Pro Forma Consolidated Financial Information Included in this Prospectus.
The unaudited pro forma consolidated financial information contained in this Prospectus is presented for illustrative purposes only and may not be an indication of what the Corporation’s financial position or results of operations would have been had the Acquisition been completed on the dates indicated. The unaudited pro forma consolidated financial information has been derived from the audited and unaudited financial statements of the Corporation and Holigen since the beginning of the fiscal year in respect of which the most recent annual financial statements of the Corporation and Holigen have been filed. The assets and liabilities of the Corporation have been measured at fair value based on various preliminary estimates using assumptions that management believes are reasonable based on information currently available. The process for estimating the fair value of Holigen and assumed liabilities requires the use of judgment in determining the appropriate assumptions and estimates. These estimates and assumptions may be revised as additional information becomes available and as additional analyses are performed, but there is no guarantee these estimates and assumptions can be correct. Management may also omit certain periods from pro forma information to accord with financial reporting periods. While management believes such omissions do not have a material effect on the presentation of the pro forma financial information, there is no guarantee such belief may be correct.
Differences between preliminary estimates in the unaudited pro forma consolidated financial information and the final acquisition accounting will occur and could have a material impact on the pro forma consolidated financial information and the Corporation’s financial position and future results of operations. In addition, the assumptions used in preparing the pro forma consolidated financial information may not prove to be accurate, and other factors may affect the Corporation’s financial condition or results of operations following the Acquisition. Any potential decline in the Corporation’s financial condition or results of operations may cause significant variations in the trading price of the Common Shares following the Acquisition.
The Corporation’s Consolidated Financial Statements May Be Impacted in Future Periods Based on the Accuracy of the Corporation’s Valuations of Holigen.
Accounting for business combinations and other agreements may involve complex and subjective valuations of the assets and liabilities recorded as a result of the business combination or other agreement, and in some instances contingent consideration, which is recorded in the Corporation’s consolidated financial statements pursuant to the standards applicable for business combinations in accordance with generally accepted accounting principles. Differences between the inputs and assumptions used in the valuations and actual results could have a material effect on the Corporation’s consolidated financial statements in future periods.
The Corporation Does Not Currently Control Holigen and No Assurance of Holigen’s Future Performance Can Be Given.
Although the Corporation is currently a shareholder of Holigen, the Corporation will not control Holigen until after the completion of the Acquisition. Although the Holigen Purchase Agreement contains covenants on the part of the Vendors regarding the operation of Holigen’s business prior to the Acquisition Closing Date, the Corporation will not assume control of Holigen until completion of the Acquisition and there may be a material adverse effect on Holigen’s business, financial condition and results of operations based on events that are outside of the Corporation’s control during the intervening period. Prior to completion of the Acquisition, the Corporation cannot assure investors that Holigen will operate its business in the same way its business would be operated under the control of the Corporation. Furthermore, Holigen’s historical and current performance may not be indicative of success in future periods.
Because the Corporation does not yet control Holigen, the information contained in this Prospectus relating to Holigen has been derived from information provided to the Corporation by Holigen and the Vendors. Moreover, as Holigen is a privately held company, its internal controls and reporting procedures may not be comparable to those of a public company. The Corporation has relied on its diligence and information supplied to it by Holigen and the Vendors in the preparation of this Prospectus.
The Holigen Purchase Agreement Obligates the Corporation to Cooperate and Use Commercially Reasonable Efforts to Complete the Offering for the Acquisition.
Under the terms of the Holigen Purchase Agreement, the Corporation has agreed to use commercially reasonable efforts to complete the Offering. If the Corporation is unable to obtain this financing, it could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
If the Corporation Is Deemed To Be in Violation of Applicable Sanctions Laws and Regulations, its Reputation, Business, Results of Operations and Financial Condition Could Be Adversely Affected.
Various governments and supranational organizations, including Canada, maintain economic sanctions targeting various countries, persons and entities. The various sanctions regimes vary. In some cases, the sanctions may amount to a near-absolute prohibition on trade and investment with or involving the sanctions target for persons required to comply with the sanctions laws. In other cases, only specific goods, services or other dealings may be prohibited. Holigen operates in various jurisdictions in which the Corporation does not currently operate or have sales. Holigen may sell its products in additional jurisdictions, as it receives export licences, and neither the Corporation nor Holigen will have any experience doing business in such jurisdictions. After the closing of the Acquisition, the Corporation may have to cease Holigen’s operations or the sale of any of Holigen’s products if and to the extent that such operations or the export of such products would be in violation of the sanctions laws and regulations of Canada, the United States, Australia or the European Union with which the Corporation and Holigen are required to comply. If Holigen or the Corporation is deemed to be in violation of applicable sanctions laws and regulations, whether due to ongoing business or business conducted in the past, or to have engaged in any conduct that is sanctionable, the Corporation could be subject to sanctions or other governmental actions that could lead to civil or criminal penalties, including fines. In addition, such violations or engaging in such conduct could damage the Corporation’s reputation. All of the foregoing could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
The Acquisition May Be Consummated On Terms Different From Those Described in this Prospectus, and As A Result, The Benefits Of the Acquisition May Not Be Fully Realized.
The Corporation may, in its sole discretion, waive certain closing conditions in its favour in the Holigen Purchase Agreement or agree to amend the Holigen Purchase Agreement and consummate the Acquisition on terms that may be different from those described in this Prospectus. As a result, the expected benefits of the Acquisition may not be fully realized.
The Corporation Could Fail to Successfully Integrate Holigen Into The Business Of The Corporation.
If the Acquisition is completed, the success of the Acquisition will depend, in part, on the ability of the Corporation to realize the anticipated benefits and synergies, including cultivation, production and extraction synergies, from integrating Holigen’s business into the businesses of the Corporation. If the Corporation is not able to achieve these objectives in whole or in part, or is not able to achieve these objectives on a timely basis, the anticipated benefits of the Acquisition may not be realized fully or at all. In addition, the actual integration of Holigen may result in additional and unforeseen expenses, which could reduce the anticipated benefits and synergies of the Acquisition, and may also result in additional and unforeseen matters that require the attention of management, which could divert management’s focus and the Corporation’s resources from other strategic opportunities and operational matters. Failure to successfully integrate Holigen’s business for any of these reasons could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
The Corporation May Be Unable to Complete the Acquisition or, In Order To Do So, the Corporation and Holigen May Be Required To Comply With Material Restrictions or Satisfy Material Conditions.
The completion of the Acquisition is subject to conditions, including the absence of any orders or notices preventing completion of the Acquisition. The Corporation can provide no assurance that any required regulatory clearances will be obtained in order to complete the Acquisition. There can be no assurance as to the cost, scope or impact of the actions that may be required to obtain any required regulatory approval.
The Corporation Will Incur Substantial Transaction-Related Costs in Connection With the Acquisition Such That the Corporation May Not Be Able to Achieve the Benefits Expected to Be Realized as a Result Of the Acquisition.
The Corporation expects to incur a number of non-recurring transaction-related costs associated with assessing and completing the Acquisition, integrating Holigen and its business with the business of the Corporation and achieving desired synergies. Non-recurring transaction costs include, but are not limited to, fees paid to legal, financial and accounting advisors, filing fees and printing costs. Additional unanticipated costs may be incurred in the integration of the operations and businesses of the Corporation with Holigen. Such costs may include increases in other expenses unrelated to the Acquisition, which may offset the benefits of the Acquisition.
The estimates of benefits associated with the Acquisition reflect estimates and assumptions made by the Corporation, and it is possible that these estimates and assumptions may not ultimately reflect actual results. In addition, these estimated benefits may not actually be achieved in the timeframe anticipated or at all. If the Corporation fails to realize anticipated benefits, synergies or revenue enhancements, it could have a material adverse effect on the Corporation’s business, financial condition and results of operations. There can be no assurance that the realization of efficiencies related to the integration of Holigen into the Corporation’s business, will offset the incremental transaction-related costs over time. Thus, any net benefit may not be achieved in the near term, the long term or at all.
There Can Be No Assurance that the Acquisition Will Be Completed.
The completion of the Acquisition is subject to a number of conditions precedent as set out in "Details of the Acquisition - The Holigen Purchase Agreement - Conditions of Closing". There is no certainty that the Acquisition will be completed. The completion of the Acquisition is not a condition precedent to the closing of the Offering. Therefore, investors will become shareholders of the Corporation regardless of whether or not the Acquisition is completed.
Risk Factors Related to the Offering and the Common Shares
Ownership of the Common Shares may be Considered Unlawful in Some Jurisdictions and Holders of the Common Shares may Consequently be Subject to Liability in Such Jurisdictions.
Cannabis-related financial transactions, including investment in the securities of cannabis companies and receipt of any associated benefits, such as dividends, are currently subject to anti-money laundering and a variety of other laws that vary by jurisdiction, many of which are unsettled and still developing. While the interpretation of these laws are unclear, in some jurisdictions, such as the United Kingdom, financial benefit directly or indirectly arising from conduct that would be considered unlawful in such jurisdiction may be viewed to be within the purview of these laws, and persons receiving any such benefit, including investors in an applicable jurisdiction, may be subject to liability under such laws. Each prospective investor should therefore contact his, her or its own legal advisor regarding the ownership of the Common Shares and any related potential liability.
Enforcement of Judgments Against Foreign Persons May Not Be Possible.
Investors should be aware that some of the Directors and officers of the Corporation, are located outside of Canada and the United States and, as a result, it may not be possible for purchasers of Offered Shares to effect service of process within Canada or the United States upon these persons. All or a substantial portion of the assets of these persons are likely to be located outside of Canada and the United States and, as a result, it may not be possible to satisfy a judgment against such persons in Canada or the United States or to enforce a judgment obtained in Canadian or U.S. courts against such persons outside of Canada or the United Sates. See "Enforceability of Civil Liabilities".
Use of Proceeds from the Offering.
The Corporation intends to use the proceeds from the Offering as described under "Use of Proceeds". If the Acquisition is not consummated, the Corporation’s management will have broad discretion in the application of the net proceeds of the Offering as well of the timing of their expenditures. Accordingly, a purchaser of Offered Shares will have to rely upon the judgment of the Corporation’s management with respect to the use of the proceeds, with only limited information concerning management’s specific intentions. The Corporation’s management may spend a portion or all of the net proceeds from the Offering in ways that the Corporation’s shareholders may not desire, that may not yield a favourable return and that may not increase the value of the Common Shares. The failure by the Corporation’s management to apply such funds effectively could harm the Corporation’s business. Pending their use, the Corporation may invest the net proceeds from the Offering in a manner that does not produce income or that loses value. The results and effectiveness of the application of the net proceeds are uncertain. If the proceeds of the Offering are not applied effectively, it may have a material adverse effect on the Corporation’s business, financial condition and results of operations.
The Corporation Is Subject, or Will Be Subject, to Risks Related to Additional Regulatory Burden and Controls Over Financial Reporting.
The Corporation is subject to the continuous and timely disclosure requirements of Canadian securities laws and the rules, regulations and policies of the TSX.V and concurrently with the pricing of this Offering, intends to be subject to the continuous and timely disclosure requirements of and United States securities laws and the rules, regulations and policies of the NASDAQ. These rules, regulations and policies relate to, among other things, corporate governance, continuous disclosure and financial reporting standards. The Corporation has made, and will continue to make, changes in these and other areas. However, there is no assurance that these and other measures that it may take will be sufficient to allow the Corporation to satisfy its obligations as a public company on a timely basis. In addition, compliance with reporting and other requirements applicable to public companies create additional costs for the Corporation and require the time and attention of management of the Corporation. The Corporation cannot predict the amount of the additional costs that the Corporation may incur, the timing of such costs or the impact that management’s attention to these matters will have on the Corporation’s business.
Future Sales of the Common Shares by Existing Shareholders.
Sales of a substantial number of Common Shares in the public market could occur at any time following, or in connection with, the completion of the Offering. These sales, or the market perception that the holders of a large number of Common Shares intend to sell Common Shares, could reduce the market price of the Common Shares. Although the Common Shares of our Directors, officers and certain other shareholders of the Corporation will be subject to a 90-day lock-up imposed by the Underwriters pursuant to the Underwriting Agreement, the Underwriters may waive the provisions of the Underwriting Agreement and allow these shareholders to sell their Common Shares at any time. Similarly, although the Consideration Shares issued to the Vendors pursuant to the Holigen Purchase Agreement will be subject to a hold period ranging from one (1) to three (3) years imposed by the Corporation pursuant to the Vendor Lock-Up Agreements entered in connection with the Acquisition, the Corporation may waive the provisions of the Vendor Lock-Up Agreement and allow the Vendors to sell their Consideration Shares, or any Common Shares or other securities into which any of such Consideration Shares may from time to time convert, at any time. There are no pre-established conditions for the grant of such a waiver by the Underwriters. In addition, pursuant to the Vendor Lock-Up Agreement, such waiver by the Corporation may not be unreasonably withheld, delayed or conditioned. Any decision by the Underwriters or the Corporation to waive those conditions would depend on a number of factors, which may include market conditions, the performance of the Common Shares in the market and the Corporation’s financial condition at that time. If the restrictions in such lock-up agreements are waived, additional Common Shares will be available for sale into the public market, subject to applicable securities laws and stock exchange requirements, which could reduce the market price for the Common Shares. A decline in the market prices of the Common Shares could impair the Corporation’s ability to raise additional capital through the sale of securities should it desire to do so.
Publication of Inaccurate or Unfavourable Research and Reports.
The trading market for the Common Shares relies in part on the research and reports that securities analysts and other third parties choose to publish about the Corporation. The Corporation will not control these analysts or other third parties. The price of the Common Shares could decline if one or more securities analysts downgrade the Common Shares or if one or more securities analysts or other third parties publish inaccurate or unfavourable research about the Corporation or cease publishing reports about the Corporation. If one or more analysts cease coverage of the Corporation or fail to regularly publish reports on the Corporation, the Corporation could lose visibility in the financial markets, which in turn could cause the Corporation’s share price or trading volume to decline.
Price Volatility of the Common Shares.
The market price of the Common Shares may be subject to wide price fluctuations in response to many factors, including variations in the operating results of the Corporation and its subsidiaries, divergence in financial results from analysts’ expectations, changes in earnings estimates by stock market analysts, changes in the business prospects for the Corporation and its subsidiaries or others in the cannabis industry, general economic conditions, legislative changes, community support for the cannabis industry and other events and factors outside of the Corporation’s control, including
actual or anticipated fluctuations in the Corporation’s quarterly results of operations;
recommendations by securities research analysts;
changes in the economic performance or market valuations of companies in the industry in which the Corporation operates;
addition or departure of the Corporation’s executive officers and other key personnel;
release or expiration of transfer restrictions on outstanding Common Shares;
sales or perceived sales of additional Common Shares;
operating and financial performance that vary from the expectations of management, securities analysts and investors;
regulatory changes affecting the Corporation’s industry generally and its business and operations both domestically and abroad;
announcements of developments and other material events by the Corporation or its competitors;
fluctuations to the costs of vital production materials and services;
changes in global financial markets and global economies and general market conditions, such as interest rates and cannabis product price volatility;
significant acquisitions or business combinations, strategic partnerships, joint ventures or capital commitments by or involving the Corporation or its competitors;
operating and share price performance of other companies that investors deem comparable to the Corporation or from a lack of market comparable companies;
news reports relating to trends, concerns, technological or competitive developments, regulatory changes and other related issues in the Corporation’s industry or target markets;
investors’ general perception of the Corporation and the public’s reaction to the Corporation’s press releases, its other public announcements and its filings with the SEC and Canadian securities regulators; and
the market’s reaction to the Corporation’s reduced disclosure as a result of being an emerging growth company under the Jumpstart Our Business Startups (JOBS) Act.
Financial markets have recently experienced significant price and volume fluctuations that have particularly affected the market prices of equity securities of companies and that have often been unrelated to the operating performance, underlying asset values or prospects of such companies. Accordingly, the market price of the Common Shares may decline even if the Corporation’s operating results, underlying asset values or prospects have not changed. Additionally, these factors, as well as other related factors, may cause decreases in asset values that are deemed to be other than temporary, which may result in impairment losses. There can be no assurance that continuing fluctuations in price and volume will not occur. If such increased levels of volatility and market turmoil continue, the Corporation’s operations could be adversely impacted, and the trading price of the Common Shares may be materially adversely affected.
The listing of the Corporation’s Common Shares on NASDAQ in addition to the TSX.V may increase the trading price volatility on the TSX.V and also result in volatility of the trading price on NASDAQ because trading will be split between the two markets, resulting in less liquidity on both exchanges. In addition, different liquidity levels, volume of trading, currencies and market conditions on the TSX.V and NASDAQ may result in different prevailing trading prices.
Purchasers of Offered Shares May be Subject to Immediate and Substantial Dilution.
Purchasers of the Offered Shares offered pursuant to this Prospectus may pay more for the Offered Shares than the amounts paid by existing shareholders or securityholders of the Corporation for Common Shares. As a result, purchasers may incur immediate and substantial dilution. Convertible securities have been issued and may be issued in the future by the Corporation at a lower price than the current market value of the Common Shares and, consequently, purchasers who purchase Offered Shares under this Prospectus may also incur substantial dilution in the near future.
Decreases in the Value of the Corporation’s Assets Could Lead to Impairments in the Future.
Potential impairment of intangible assets may significantly impact the Corporation’s profitability. Intangible assets are tested for impairment annually or whenever events or changes in circumstances indicate the carrying amount of an asset exceeds its recoverable amount. If an impairment exists, the Corporation would be required to take an impairment charge with respect to the impaired asset. Events giving rise to impairment are difficult to predict and are an inherent risk. As a result of the significance of intangible assets, should such an impairment of intangible assets occur, it could have a material adverse effect on the Corporation’s business, financial condition and results of operations. It is anticipated that a large component of Holigen will be classified as goodwill in the Corporation’s financial statements, thus increasing the risk the Corporation could suffer impairments.
Investors May Suffer Dilution to their Voting Power and Economic Interest in the Corporation.
The Corporation may sell additional Common Shares or other securities that are convertible, exercisable or exchangeable into Common Shares in subsequent offerings or may issue additional Common Shares or other securities to finance future acquisitions. The Corporation cannot predict the size or nature of future sales or issuances of securities or the effect, if any, that such future sales and issuances will have on the market price of the Common Shares. Sales or issuances of substantial numbers of Common Shares or other securities that are convertible, exercisable or exchangeable into Common Shares, or the perception that such sales or issuances could occur, may adversely affect prevailing market prices of the Common Shares. With any additional sale or issuance of Common Shares or other securities that are convertible or exchangeable into Common Shares, investors will suffer dilution to their voting power and economic interest in the Corporation. Furthermore, to the extent holders of the Corporation’s stock options or other convertible securities convert or exercise their securities and sell the Common Shares they receive, the trading price of the Common Shares may decrease due to the additional amount of Common Shares available in the market.
Control by Majority Shareholders and the Corporation’s Governance.
A holder of a substantial amount of the Common Shares may be able to exercise a controlling influence on the Corporation, which may affect the Corporation’s governance and operations. For so long as such shareholders maintain their interest in the Corporation, such shareholders may be able to exercise a controlling influence over the business and affairs of the Corporation and the Corporation, the selection of senior management, the acquisition or disposition of the Corporation’s assets, access to capital markets, the payment of dividends and any change of control of the Corporation, such as a merger or take-over. The effect of this control may be to limit the price that investors are willing to pay for the Common Shares. In addition, a sale of the Common Shares by such shareholders, or the perception of the market that a sale may occur, may adversely affect the market price of the Common Shares.
As of the date of this Prospectus: (i) Tom Flow beneficially owns, or controls or directs, directly or indirectly, a total of 26,025,000 Common Shares, representing approximately 19.5% of the equity of the Corporation on a fully diluted basis (excluding equity incentives such as options, warrants and/or restricted share units); and (ii) Steven Klein, directly and indirectly through Core Flow Canada Holdings Inc., beneficially owns, or controls or directs, directly or indirectly, a total of 8,253,014 Common Shares and 41,598,000 Flowr ULC Class A Shares that are convertible into Common Shares, representing approximately 37% of the equity of the Corporation on a fully diluted basis (excluding equity incentives such as options, warrants and/or restricted share units). In addition, upon completion of the Acquisition, Pauric Duffy will indirectly hold 26,160,060 Common Shares and Series 1 Preferred Shares of the Corporation in the aggregate, and will have certain consent rights, as described under "Details of the Acquisition - Governance Agreement".
The Corporation Does Not Anticipate Paying Dividends in The Foreseeable Future.
The Corporation has no earnings or dividend record, and does not anticipate paying any dividends on the Common Shares in the foreseeable future. Dividends paid by the Corporation would be subject to tax and, potentially, withholdings for non-residents of Canada.
Limited Market for Securities.
The Offered Shares are expected to be listed on the TSX.V and NASDAQ, however, there can be no assurance that an active and liquid market for the Offered Shares will be maintained. An investor may find it difficult to resell securities of the Corporation.
The Corporation Is an "Emerging Growth Company" and Cannot be Certain if The Reduced Disclosure Requirements Applicable to Emerging Growth Companies Will Make It Less Attractive to Investors.
The Corporation is an "emerging growth company" as defined in the JOBS Act. The Corporation will continue to qualify as an "emerging growth company" until the earliest to occur of: (a) the last day of the fiscal year during which it had total annual gross revenues of US$1,070,000,000 or more; (b) the last day of its fiscal year following the fifth anniversary of the date of the first sale of its common equity securities pursuant to an effective registration statement under the U.S. Securities Act, such as the Form F-10 registration statement that is being filed concurrently with this Prospectus; (c) the date on which the Corporation, during the previous 3-year period, issued more than US$1,000,000,000 in non-convertible debt; or (d) the date on which the Corporation is deemed to be a "large accelerated filer".
For so long as the Corporation continues to qualify as an emerging growth company, it will be exempt from the requirement to include an auditor attestation report relating to internal control over financial reporting pursuant to Section 404(b) of the United States Sarbanes-Oxley Act of 2002 (the "Sarbanes-Oxley Act") in its annual reports filed under the U.S. Exchange Act even if it does not qualify as a "smaller reporting company," as well as certain other exemptions from various reporting requirements that are applicable to other public companies. The Corporation cannot predict if investors will find the Common Shares less attractive because it may rely on these exemptions. If some investors find the Common Shares less attractive as a result, there may be a less active trading market for the Common Shares and the trading price of the Common Shares may be more volatile.
The Corporation Expects to Incur Increased Costs as a Result of Being a Public Company in the United States, and Management Will be Required to Devote Substantial Time to United States Public Company Compliance Programs.
As a public company in the United States, the Corporation expects to incur significant additional legal, insurance, accounting and other expenses. In addition, its administrative staff will be required to perform additional tasks. For example, as result of becoming a public company in the United States, the Corporation is in the process of adopting additional internal controls and disclosure controls and procedures, has retained a United States transfer agent, adopted a United States compliant insider trading policy and other corporate governance programs and charters and bear all of the internal and external costs of preparing and distributing periodic public reports in compliance with its obligations under U.S. securities laws. The Corporation intends to invest resources to comply with evolving United States laws, regulations and standards, and this investment will result in increased general and administrative expenses. The Corporation’s management team may not successfully or efficiently manage the transition to being a United States public company subject to significant regulatory oversight and reporting obligations under U.S. securities laws. In particular, these new obligations will require substantial attention from the senior management and could divert their attention away from the day-to-day management of the Corporation’s business. If the efforts to comply with new United States laws, regulations and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to practice, regulatory authorities or third-parties may initiate legal proceedings against the Corporation and the business may be harmed. In connection with becoming a public company in the United States, the Corporation will be increasing its directors’ and officers’ insurance coverage, which will increase the Corporation’s insurance cost. In the future, it will be more expensive for the Corporation to obtain director and officer liability insurance, and the Corporation may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These factors could also make it more difficult for the Corporation to attract and retain qualified members to the Board in the future, particularly to serve on its audit committee, and qualified executive officers.
In addition, in order to comply with the requirements of being a United States public company, the Corporation may need to undertake various actions, including relating to implementing new internal controls and procedures and hiring new accounting or internal audit staff. The Sarbanes-Oxley Act requires the Corporation to maintain effective disclosure controls and procedures and internal control over financial reporting. The Corporation is continuing to develop and refine its disclosure controls and other procedures that are designed to ensure that information required to be disclosed by it in the reports that it files with or furnished to the SEC is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that information required to be disclosed in reports under the U.S. Exchange Act, is accumulated and communicated to the Corporation’s principal executive and financial officers. Any failure to develop or maintain effective controls could adversely affect the results of periodic management evaluations. In the event that the Corporation is not able to demonstrate compliance with the Sarbanes-Oxley Act, that its internal control over financial reporting is perceived as inadequate, or that the Corporation is unable to produce timely or accurate financial statements, investors may lose confidence in the Corporation’s results of operations and the trading price of the Common Shares could decline. In addition, if the Corporation is unable to continue to meet these requirements, it may not be able to remain listed on NASDAQ.
The Corporation is not currently required to comply with the SEC’s rules that implement Section 404 of the Sarbanes-Oxley Act, and is therefore not yet required to make a formal assessment of the effectiveness of its internal control over financial reporting under United States rules. The Corporation is required to comply with certain of the SEC’s rules implementing the Sarbanes-Oxley Act, which require management to certify financial and other information in the Corporation’s annual reports and provide an annual management report on the effectiveness of its internal control over financial reporting commencing with the Corporation’s second annual report filed with the SEC. This assessment will need to include the disclosure of any material weaknesses in the Corporation’s internal control over financial reporting identified by its management or its independent registered public accounting firm.
The Corporation’s independent registered public accounting firm will not be required to formally attest to the effectiveness of the Corporation’s internal control over financial reporting until the later of its second annual report or the first annual report required to be filed with the SEC following the date it is no longer an "emerging growth company" as defined in the JOBS Act. The Corporation cannot assure you that there will not be material weaknesses or significant deficiencies in its internal controls in the future.
As a Foreign Private Issuer, The Corporation is Subject to Different U.S. Securities Laws and Rules Than a Domestic U.S. Issuer, Which May Limit the Information Publicly Available to its Shareholders.
The Corporation is a "foreign private issuer", as such term is defined in Rule 405 under the U.S. Securities Act, and is not subject to the same requirements that are imposed upon U.S. domestic issuers by the SEC. Under the U.S. Exchange Act, the Corporation will be subject to reporting obligations that, in certain respects, are less detailed and less frequent than those of U.S. domestic reporting companies. As a result, the Corporation will not file the same reports that a U.S. domestic issuer would file with the SEC, although the Corporation will be required to file or furnish to the SEC the continuous disclosure documents that it is required to file in Canada under Canadian securities laws. In addition, the Corporation’s officers, Directors, and principal shareholders are exempt from the reporting and "short swing" profit recovery provisions of Section 16 of the U.S. Exchange Act. Therefore, the Corporation’s shareholders may not know on as timely a basis when the Corporation’s officers, Directors and principal shareholders purchase or sell shares, as the reporting deadlines under the corresponding Canadian insider reporting requirements are longer.
As a foreign private issuer, the Corporation will be exempt from the rules and regulations under the U.S. Exchange Act related to the furnishing and content of proxy statements. The Corporation will also be exempt from Regulation FD, which prohibits issuers from making selective disclosures of material non-public information. While the Corporation will comply with the corresponding requirements relating to proxy statements and disclosure of material non-public information under Canadian securities laws, these requirements differ from those under the U.S. Exchange Act and Regulation FD and shareholders should not expect to receive the same information at the same time as such information is provided by U.S. domestic companies. In addition, the Corporation will have 120 days after the end of each fiscal year to file its annual report with the SEC and will not be required under the U.S. Exchange Act to file quarterly reports with the SEC as promptly as U.S. domestic companies whose securities are registered under the U.S. Exchange Act.
In addition, as a foreign private issuer, the Corporation has the option to follow certain Canadian corporate governance practices, except to the extent that such laws would be contrary to U.S. securities laws, and provided that the Corporation discloses the requirements it is not following and describe the Canadian practices it follows instead. The Corporation may in the future elect to follow home country practices in Canada with regard to certain corporate governance matters. As a result, the Corporation’s shareholders may not have the same protections afforded to shareholders of U.S. domestic companies that are subject to all corporate governance requirements.
If the Corporation Fails to Meet Applicable Listing Requirements, the TSX.V or NASDAQ may Delist the Common Shares from Trading, In Which Case, the Liquidity and Market Price of the Common Shares Could Decline.
There is no assurance that the Corporation will be able to meet the continued listing standards of the TSX.V or the NASDAQ in the future. If the Corporation fails to comply with the applicable listing standards and the TSX.V or the NASDAQ delists the Common Shares, the Corporation and its shareholders could face significant material adverse consequences, including:
• a limited availability of market quotations for the Common Shares;
• reduced liquidity for the Common Shares;
• a determination that the Common Shares are "penny stock", which would require brokers trading in the Common Shares to adhere to more stringent rules and possibly result in a reduced level of trading activity in the secondary trading market for the Common Shares;
• a limited amount of news about the Corporation and analyst coverage of the Corporation; and
• a decreased ability for the Corporation to issue additional equity securities or obtain additional equity or debt financing in the future.
The Corporation May Lose Foreign Private Issuer Status in the Future, Which Could Result in Significant Additional Costs and Expenses.
The Corporation may in the future lose its foreign private issuer status if a majority of its shares are held in the United States and it fails to meet the additional requirements necessary to avoid loss of foreign private issuer status, such as if: (1) a majority of the Corporation’s Directors or executive officers are U.S. citizens or residents; (2) a majority of the Corporation’s assets are located in the United States; or (3) the Corporation’s business is administered principally in the United States. Although the Corporation has elected to comply with certain U.S. regulatory provisions, its loss of foreign private issuer status would make such provisions mandatory. The regulatory and compliance costs to the Corporation under U.S. securities laws as a U.S. domestic issuer will be significantly more than the costs incurred as a Canadian foreign private issuer. If the Corporation is not a foreign private issuer, it would not be eligible to use foreign issuer forms and would be required to file periodic and current reports and registration statements on U.S. domestic issuer forms with the SEC, which are generally more detailed and extensive than the forms available to a foreign private issuer. In addition, the Corporation may lose its ability to rely upon exemptions from certain corporate governance requirements on NASDAQ that are available to foreign private issuers.
The Regulated Nature of the Corporation’s Business May Impede or Discourage a Takeover, Which Could Reduce the Market Price of the Common Shares.
The Corporation requires and holds various government licences to operate its business, which would not necessarily continue to apply to an acquiror of its business following a change of control. These licensing requirements could impede a merger, amalgamation, takeover or other business combination involving the Corporation or discourage a potential acquirer from making a tender offer the Common Shares, which, under certain circumstances, could reduce the market price of our Common Shares.
DOCUMENTS INCORPORATED BY REFERENCE
Information has been incorporated by reference in this Prospectus from documents filed with the securities commissions or similar authorities in each of the provinces of Canada except Quebec. Copies of the documents incorporated herein by reference may be obtained on request without charge from the General Counsel and Secretary of the Corporation at 461 King Street West, Floor 2, Toronto, Ontario M5V 1K4, telephone 1-877-356-9726, and are also available electronically at www.sedar.com. The filings of the Corporation through SEDAR are not incorporated by reference in this Prospectus except as specifically set out herein.
The following documents, filed by the Corporation with the securities commissions or similar authorities in each of the provinces of Canada except Quebec, or filed with or furnished to the SEC, as applicable, are specifically incorporated by reference into, and form an integral part of, this Prospectus:
(a) the annual information form (the "AIF") of the Corporation dated April 3, 2019, for the fiscal year ended December 31, 2018;
(b) the management information circular of the Corporation dated November 26, 2018, in connection with the Corporation’s special shareholder meeting held on December 28, 2018;
(c) the management information circular of the Corporation dated May 9, 2019, in connection with the Corporation’s annual general and special shareholder meeting held on June 11, 2019;
(d) the material change report dated May 14, 2019;
(e) the audited consolidated financial statements of the Corporation for the fiscal years ended December 31, 2018 and 2017, together with the independent auditors’ report thereon and the notes thereto;
(f) the management’s discussion and analysis of the financial condition and results of operations of the Corporation for the fiscal year ended December 31, 2018;
(g) the unaudited condensed interim consolidated financial statements of the Corporation for the three months ended March 31, 2019 and 2018, together with the notes thereto;
(h) the management's discussion and analysis of the financial condition and results of operations of the Corporation for the three months ended March 31, 2019;
(i) the material change report dated June 28, 2019;
(j) the material change report dated July 16, 2019;
(k) the term sheet of the Corporation in respect of the Offering dated July 15, 2019; and
(l) the investor presentation of the Corporation titled "The Natural Science of Cannabis" dated July 15, 2019.
Any document of the type referred to in Section 11.1 of Form 44-101F1 Short Form Prospectus, if filed by the Corporation after the date of this Prospectus and prior to the termination of the Offering, shall be deemed to be incorporated by reference in this Prospectus for the purposes of the Offering.
Any statement contained in this Prospectus or in a document incorporated or deemed to be incorporated by reference herein shall be deemed to be modified or superseded, for purposes of this Prospectus, to the extent that a statement contained herein or in any other subsequently filed document that also is, or is deemed to be, incorporated by reference herein modifies, replaces or supersedes such statement. Any statement so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this Prospectus. The modifying or superseding statement need not state that it has modified or superseded a prior statement or include any other information set forth in the document or statement that it modifies or supersedes. The making of a modifying or superseding statement shall not be deemed an admission for any purposes that the modified or superseded statement, when made, constituted a misrepresentation, an untrue statement of a material fact or an omission to state a material fact that is required to be stated or that is necessary to make a statement not misleading in light of the circumstances in which it was made. This section shall also apply to the documents incorporated by reference in the United States per the below.
DOCUMENTS INCORPORATED BY REFERENCE IN THE UNITED STATES
To the extent that any document or information incorporated by reference into this Prospectus pursuant to the section above is also included in any report filed with or furnished to the SEC by the Corporation on Form 6-K or on Form 40-F (or any respective successor form) after the date of this Prospectus, it shall be deemed to be incorporated by reference as an exhibit to the registration statement of which this Prospectus forms a part. Further, the Corporation may incorporate by reference into the registration statement of which this Prospectus forms a part, any report on Form 6-K furnished to the SEC, including the exhibits thereto, if and to the extent provided in such report.
MARKETING MATERIALS
The Marketing Materials are not part of this Prospectus to the extent that the contents of the Marketing Materials have been modified or superseded by a statement contained in this Prospectus or any amendment. Any template version of "marketing materials" (as defined in National Instrument 41-101 - General Prospectus Requirements) filed after the date of this Prospectus and before the termination of the distribution under the Offering (including any amendments to, or an amended version of, the Marketing Materials) is deemed to be incorporated into this Prospectus.
WHERE UNITED STATES INVESTORS CAN FIND MORE INFORMATION
Substantially concurrent with the filing of this Prospectus, the Corporation will file with the SEC, under the United States Securities Act of 1933, as amended (the "U.S. Securities Act"), a registration statement on Form F-10 relating to the Offered Shares. This Prospectus, which constitutes a part of the registration statement, does not contain all of the information contained in the registration statement, certain items of which are contained in the exhibits to the registration statement as permitted by the rules and regulations of the SEC. You should refer to the registration statement and the exhibits to the registration statement for further information.
The Corporation is subject to the information requirements of the United States Securities Exchange Act of 1934, as amended (the "U.S. Exchange Act") and applicable Canadian securities legislation, and in accordance therewith, files reports and other information with the SEC and with the securities regulators in Canada. Under a multi-jurisdictional disclosure system adopted by the United States and Canada, documents and other information that the Corporation files with the SEC may be prepared in accordance with the disclosure requirements of Canada, which are different from those of the United States. As a foreign private issuer, the Corporation is exempt from the rules under the U.S. Exchange Act, prescribing the furnishing and content of proxy statements, and the Corporation’s officers, Directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the U.S. Exchange Act. In addition, the Corporation is not required to publish financial statements as promptly as U.S. companies.
The SEC maintains an Internet website that contains reports and other information regarding issuers, including the Corporation, located at www.sec.gov for further information about the public reference room. You may read and download some of the documents the Corporation has filed with the SEC’s EDGAR system at www.sec.gov. You may read and download any public document that the Corporation has filed with the Canadian securities regulatory authorities at www.sedar.com.
DOCUMENTS FILED AS PART OF THE REGISTRATION STATEMENT
The following documents have been or will be filed with the SEC as part of the registration statement of which this Prospectus forms a part insofar as required by the SEC’s Form F-10:
the Underwriting Agreement;
the documents referred to under the heading "Documents Incorporated by Reference";
the consent of the Corporation’s auditor, MNP LLP;
the consent of Holigen’s auditor, RSM Canada LLP;
the consent of the Corporation’s Canadian counsel, Fasken Martineau DuMoulin LLP;
the consent of the Underwriters’ Canadian counsel, Borden Ladner Gervais LLP; and
the powers of attorney from certain Directors and officers of the Corporation.
ELIGIBILITY FOR INVESTMENT
In the opinion of Fasken Martineau DuMoulin LLP, Canadian counsel to the Corporation, and Borden Ladner Gervais LLP, Canadian counsel to the Underwriters, based on the current provisions of the Income Tax Act (Canada) (the “Tax Act”) and the regulations thereunder (the “Regulations”) in force on the date hereof, provided that the Common Shares are listed on the date hereof on a “designated stock exchange” as defined in the Tax Act (which currently includes the TSX.V), the Offered Shares, if issued on the date hereof, would be qualified investments under the Tax Act on the date hereof for a trust governed by a registered retirement savings plan (“RRSP”), a registered retirement income fund (“RRIF”), a deferred profit sharing plan, a registered education savings plan (“RESP”), a tax-free savings account (“TFSA”), or a registered disability savings plan (“RDSP”) (collectively, “Registered Plans”).
The Offered Shares will not be “prohibited investments” for a trust governed by a TFSA, RESP, RDSP, RRSP or RRIF provided the holder of the TFSA or RDSP the annuitant of the RRSP or RRIF, or the subscriber of a RESP, as applicable: (i) deals at arm’s length with the Corporation for purposes of the Tax Act; and (ii) does not have a “significant interest” (within the meaning of subsection 207.01(4) of the Tax Act) in the Corporation. In addition, the Offered Shares will not be a “prohibited investment” for such a trust if such Offered Shares are “excluded property” for such trust as defined in subsection 207.01(1) of the Tax Act. If the Offered Shares are a “prohibited investment”, the holder, subscriber or annuitant, as the case may be, will be subject to penalty taxes as set out in the Tax Act. Such holders, subscribers or annuitants should consult their own tax advisors to ensure that the Offered Shares would not be a prohibited investment in their particular circumstances.
58
THE FLOWR CORPORATION
General Description of the Corporation
The Corporation was incorporated under the Business Corporations Act (Alberta) on June 1, 2016. On September 21, 2018, the Corporation completed the Qualifying Transaction with Flowr Privateco, Needle and Needle Subco. In connection with the completion of the Qualifying Transaction, on September 25, 2018, the Corporation continued from being governed under the Business Corporations Act (Alberta) to being governed under the Business Corporations Act (Ontario) and, on January 1, 2019, amalgamated with The Flowr Group Inc., with the name of the amalgamated company being "The Flowr Corporation". The registered and head office of Flowr is located at 461 King Street West, Floor 2, Toronto, Ontario M5V 1K4.
The Corporation’s shares are listed on the TSX.V under the symbol "FLWR" and are approved for listing on the NASDAQ under the symbol "FLWR". The listing date will be confirmed prior to closing of the Offering. The Corporation intends to commence trading on NASDAQ one day after the pricing of the Offering.
The Corporation owns 100% of the issued and outstanding common shares of Flowr ULC and two U.S. shareholders own 100% of the issued and outstanding class A preferred shares in the capital of Flowr ULC. See "Description of Capital Structure - Flowr ULC Shares" and "Material Contracts - Share Exchange Agreement" in the AIF.
The Corporation’s interest in Flowr Okanagan is held through Flowr ULC, which owns 100% of the issued and outstanding shares in Flowr Okanagan. The Corporation also has a subsidiary, FlowCo Advisory LP, which is dormant.
Recent Developments
The Corporation is currently in negotiations to obtain committed financing from a syndicate of lenders led by ATB in its capacity as lead arranger and administrative agent for the ATB Credit Facilities. Pursuant to the ATB Credit Facilities, the Corporation will be permitted to use the recapitalization term facility and the revolving operating credit facility for general working capital purposes and the development facility for the development of the K1 Facility, the K2 Facility and the Flowr Forest. The ATB Credit Facilities will mature in three (3) years. Under the terms of the ATB Credit Facilities, the Corporation will be subject to certain financial, positive and negative covenants. In addition, the ATB Credit Facilities will provide for an accordion of up to $70,000,000. The ATB Credit Facilities are expected to be guaranteed by the Loan Parties and expected to be secured by a general security agreement granting an interest over all the current and after acquired personal property and floating charge on all lands of the Loan Parties, as well as a collateral mortgage or debenture over the Corporation’s freehold and leasehold interests in certain Kelowna facilities, non-disturbance agreement among ATB, the Corporation and the landlord of the Kelowna facilities, the Corporation’s pledge in respect of its interests in the capital of its subsidiaries (other than Holigen) and certain other assets. The applicable margins for the ATB Credit Facilities will be based on certain performance-pricing grids, with availability in bank acceptance, letters of credit and prime loans. The ATB Credit Facilities are subject to negotiation and documentation and we cannot assure you that such facilities will be available to the Corporation on these or different terms. See “Risk Factors- The Corporation Expects to Enter Into the ATB Credit Facilities, but Such Facilities May Not Get Finalized or Be Available. In that Event, the Corporation Would Have to Find an Alternative Form of Financing ”
THE BUSINESS
Flowr is a rapidly growing cannabis company with the mission to provide consumers and medical patients unparalleled cannabis experiences through the production, marketing, distribution and sale of premium quality cannabis products.
Who is Flowr?
Flowr’s current operations are located in Canada. Flowr focuses on producing premium cannabis products for medical patients and recreational consumers throughout Canada, utilizing its purpose-built indoor and, upon licensing, outdoor cultivation and processing facilities at the Kelowna Campus. Flowr is currently focused on producing, marketing and distributing premium dried cannabis flower products (i.e. packaged dried cannabis flower and pre-rolls) and intends to strategically offer new product formats such as cannabis oils, vape technology, edibles, beverages and topical products, as demand patterns evolve and regulations permit the sale of such products. We believe demand for new product formats will consistently grow and that many of these products are best when infused with ingredients derived from greenhouse-grown cannabis. On February 12, 2019, Flowr purchased a parcel of land on which it is constructing the Flowr Forest, adjacent to its current operations in Kelowna, which it plans to use for greenhouse cultivation of cannabis for extraction and use in new product form factors upon such form factors being regulated for sale. The Flowr Forest is intended to cultivate cannabis for extraction and use in new classes of cannabis upon regulation of the new classes of cannabis for commercial production and sale. Flowr believes that the demand for premium cannabis products in Canada will continue to grow, and we intend to continue to focus on providing customers with access to clean, consistent, terpene-rich products produced at the Kelowna Campus.
Where is Flowr Going?
On December 20, 2018, the Corporation announced it entered into the SPSA to acquire a 19.8% equity interest in Holigen. The SPSA was amended and restated on May 8, 2019. On June 24, 2019, Flowr announced the entering into of the Holigen Purchase Agreement to acquire the remaining 80.2% of the issued and outstanding equity interests in Holigen that the Corporation does not already own. Holigen is a medical cannabis company with a facility in Australia and planned facilities in Portugal that are expected to provide finished medical cannabis products, pharmaceutical ingredients and plants and seeds to medical cannabis markets globally, where regulations permit. Holigen’s Australian facility is Good Manufacturing Process (“GMP”) licensed for certain activities, with full GMP licensing expected in 2020 upon finalization of construction. Holigen’s facilities in Portugal are expected to obtain GMP certification upon completion of construction in 2020. Holigen is in the final stages of obtaining a cultivation license for its outdoor and greenhouse facilities located on 65 hectares (approximately 7 million square feet) in Portugal and which is expected to have potential production capacity of approximately 500,000 kilograms of dried cannabis flower annually. The acquisition of Holigen will provide Flowr with a global platform to effectively serve growing demand for medical cannabis in European and Australian markets, where medical cannabis is regulated. See Details of the Acquisition.
Upon completion of the acquisition of Holigen and through the combined company platform, Flowr will operate a large scale, vertically integrated, licensed cannabis company with expertise in GMP-certified cultivation and manufacturing and will have a robust distribution platform for global markets.
Flowr’s focus in Europe and Australia will be the medical cannabis market. In Canada, Flowr intends to continue to expand its product offerings in recreational and medical markets with high-quality cannabis produced cost-effectively and at scale.
Industry-Leading Cultivation Expertise
We believe the foundation of our strategy is our team’s extensive indoor cannabis cultivation and production expertise. We believe that growing high quality cannabis economically at scale is challenging. It requires expertise in multiple areas, including plant genetic development, facility design and operations, cultivation operations, and post-cultivation processes. This is particularly true in markets with stringent regulatory controls, such as those prescribed by Health Canada in Canada, which sets exacting standards for product cleanliness and quality. Our cultivation and operating team members are highly experienced in cultivating high quality cannabis at scale and have amassed significant experience in the design, construction and operation of numerous cannabis production facilities in Canada, which were built to comply with a variety of stringent regulatory predecessors to the Cannabis Act, including the MMAR, MMPR and the ACMPR. Our team includes experienced engineering and construction experts and a host of cannabis-focused research and development professionals.
We believe that our Canadian operating team’s expertise is highly transferable to other regulated cannabis markets and will provide a competitive advantage for successful entry into new markets. Our operating team’s experience to date has been focused on the design, construction and operation of indoor cannabis cultivation facilities and Flowr believes that this expertise can be transferred to greenhouse and outdoor cultivation facilities at both the Flowr Forest and Holigen’s operations in Portugal. However, there can be no guarantee that we will be able to translate the experience of our operating team with indoor growing facilities into successfully designing, constructing and operating outdoor and greenhouse cultivation facilities. See "Risk Factors - The Corporation May Not Be Able to Successfully Design, Construct or Operate Outdoor or Greenhouse Cultivation Facilities".
Purpose-Built Facilities with Exacting Control of Cultivation Environments
Producing premium cannabis products requires an exacting level of control over every aspect of the cultivation, harvest, curing, and post-harvest manufacturing processes. Upon completion of the Acquisition, Flowr is expected to have a global portfolio of purpose-built cultivation, production, processing and R&D assets, many of them being built or designed to GMP specifications and awaiting GMP licensing. We believe that this will position Flowr to produce high quality cannabis optimally suited for the markets and consumers it intends to serve. The following table describes our current and planned facilities:
| Facility | Location | Type | Expected Operational
Date(1)(2) | Estimated Initial
Capacity(2)(3) | Estimated Maximum
Capacity(2)(3) | Cost to Complete
(millions)(2)(4) |
| K1 Facility(5) | British Columbia, Canada | Indoor | Q3 2019 | 10,000kg | 10,000kg | $7.2 |
K2 Facility(6) | British Columbia, Canada | Indoor | H2 2021/2022 | 785kg(8) | 40,000kg(7) | $140.2 |
Hawthorne R&D Facility(5) | British Columbia, Canada | R&D | H2 2019 | N/A | N/A | $3.8 |
Flowr Forest(9) | British Columbia, Canada | Outdoor / greenhouse | H2 2019
(first phase) | 5,000kg(10) | 10,000kg | $2.4 |
| Aljustrel(11)(12) | Aljustrel, Portugal | Outdoor | H2 2019 | 1,500kg(14) | 500,000kg(13) | $67.0 |
Sintra(11)(12) | Sintra,
Portugal | Indoor + R&D | H2 2019 | 150kg(15) | 1,800kg | $10.5 |
| Sydney - Phase 1(11)(16) | Sydney, Australia | Indoor | H1 2020 | 500kg(17) | 1,000kg | $10.5 |
Sydney - Phase 2(11)(16) | Sydney, Australia | Indoor + R&D | To be
determined | To be
determined | To be
determined (18) | To be
determined |
(1) The Expected Operational Date reflects the anticipated time to reach the initial phase of commercialization for each facility, which, except for the K1 Facility, will be before construction is fully completed and prior to reaching estimated maximum capacity, as we construct in stages. Other than the K1 Facility (which is expected to be fully complete/built-out and operational upon receipt of an amendment to the Corporation's Health Canada licence for new phases by Q4 2019), Flowr expects that the construction and development of the facilities described in the table above will be completed over the course of the next two to three years. Such construction is expected to be completed in stages, with completed portions of the applicable facility becoming operational while other portions are under construction.
(2) Please see "Risk Factors" and "Forward-Looking Information".
(3) Subject to licensing, zoning and permitting. Estimated Initial Capacity reflects the facility's potential annual production capacity, as at the initial phase of commercialization and in many instances, prior to full completion. Estimated Maximum Capacity reflects the facility's potential production capacity, when fully complete/built-out and operational, based on targeted yields and maximum growing capacity, where applicable. Both Estimated Initial Capacity and Estimated Maximum Capacity include dried cannabis flower and dried cannabis flower equivalents. Except as otherwise described in the notes below, Flowr expects to be able to reach the capacities listed in the table above with (i) its available cash on hand, (ii) the net proceeds of the Offering and (iii) the cash expected to be generated from Flowr's and Holigen's operations, as such cash from operations becomes available. The anticipated timelines to scale each facility to its maximum production capacity (described below) are based on the time required to reach full build-out and operation and are not subject to additional funding (except as described in the notes below).
(4) The Cost to Complete represents the forecasted remaining costs associated with the construction and development of each of the facilities to reach full completion/operation. The total aggregate amount of funding currently forecasted to be required to complete the construction of the facilities in the table above is approximately $241,600,000.
(5) K1 Facility is fully funded. The first floor of the Hawthorne R&D facility is fully funded.
(6) Expected to be funded with the proceeds from the Offering and the ATB Credit Facilities if obtained.
(7) This estimate is based on the assumption that the ATB Credit Facilities will be available to Flowr. In the event that the ATB Credit Facilities are not available, Flowr would likely construct a facility that is approximately half the size of the planned K2 Facility, which would result in half of the expected capacity of the K2 Facility from the capacity disclosed in the table above (such amount being approximately 20,000 kg). See "Use of Proceeds - Business Objectives and Milestones".
(8) Assuming achievement of the estimated initial capacity on the anticipated timeline, the annual production is expected to increase to 29,400 kilograms in the year 2022 and is expected to reach estimated maximum capacity of 40,000 kilograms in 2023.
(9) The remaining costs associated with the construction and development of the Flowr Forest are expected to be funded with the ATB Credit Facilities if obtained.
(10) Assuming achievement of the estimated initial capacity on the anticipated timeline, the annual production is expected to increase to 10,000 kilograms, reaching estimated maximum capacity, in the year 2020.
(11) Subject to the closing of the Acquisition. See "Risk Factors".
(12) Expected to be funded with the proceeds of the Offering and existing or proposed debt facilities available to Holigen.
(13) This estimate is based on the following material factors and assumptions: (i) the Aljustrel site, when fully planted and operational, is expected to have approximately 5,060,000 square feet of grow space; (ii) the anticipated yield in the outer years is expected to reach 100 grams per square foot per year; and (iii) with approximately 5,060,000 square feet of canopy at a targeted yield of 100 grams per square foot per year, the anticipated output of cannabis at the Aljustrel site is expected to be approximately 500,000 kilograms (5,060,000 multiplied by 100 grams/sq.ft./year divided by 1,000).
(14) Assuming achievement of the estimated initial capacity on the anticipated timeline, the annual production is expected to increase to 30,000 kilograms in the year 2020 and can reach the estimated maximum capacity of 500,000 kilograms in 2021, as the Aljustrel site is a low cost outdoor growing operation. Flowr will assess whether it will produce the full estimated maximum capacity in 2021 based on demand for its products.
(15) Assuming achievement of the estimated initial capacity on the anticipated timeline, the annual production is expected to increase to 1,800 kilograms, reaching estimated maximum capacity, in the year 2020.
(16) Expected to be funded with the proceeds of the Offering.
(17) Assuming achievement of the estimated initial capacity on the anticipated timeline, the annual production is expected to increase to 1,000 kilograms, reaching estimated maximum capacity, in the year 2021.
(18) Flowr has the option to construct Sydney - Phase 2 facility based on Phase 1 specifications.
R&D Engine to Drive Leadership, Enhanced by Hawthorne Partnership
Building industry-leading R&D capabilities provides us with a competitive advantage by providing proprietary insights and an intimate understanding of the full genetic potential of the cannabis plant. We believe that these capabilities will allow us to maximize cultivation yields and support the economic production of high quality derivative products and the development of new product formats to bring to market.
We believe that our cultivation and production capabilities are industry leading. The caliber of Flowr’s R&D expertise was validated in January 2018 through Flowr’s strategic R&D alliance with Hawthorne. Hawthorne is a subsidiary of The Scotts Miracle-Gro Company, a NYSE-listed supplier of lawn, garden and hydroponics products.
Hawthorne conducted a review of Canadian federal cannabis licence holders in search of a partner with cultivation expertise and the ability to conduct industry-leading R&D work. Hawthorne’s search was driven by a strategic imperative to engage a federal cannabis licence holder capable of producing industry-leading yields and quality, in order to create successful case studies highlighting the effectiveness of Hawthorne’s cultivation products. Flowr and Hawthorne ultimately entered into a strategic R&D alliance, and Flowr has commenced construction of an R&D facility in collaboration with Hawthorne, as further described below. We believe that the Hawthorne-related work at this R&D facility will support the development and refinement of Hawthorne’s existing products and new products, and provide Flowr with key insights into optimal growing conditions for its products.
Focus On Quality Across Geographies and Product Formats
We cultivate, produce and market premium cannabis products targeting discerning recreational consumers in Canada under the Flowr® brand. Medical patients in Canada are targeted under the FlowrRx® brand. Following the completion of the acquisition of Holigen, Flowr intends to engage in the production and distribution of medical cannabis products in Europe and Australia under the Holigen brand.

While certain industry participants may purchase cannabis products wholesale from third parties for distribution, we believe this practice can negatively impact product quality and consistency as well as experience. As part of our premium brand strategy, we intend to maintain product quality and consistency by only using cannabis cultivated by Flowr or Holigen in Flowr-branded products. We believe that both medical patients and recreational consumers in Canada prefer premium quality cannabis products and are willing to pay a premium for such products. We believe that premium product quality, as defined by a rich sensory experience, a consistent psychoactive effect, and robust terpene profiles, is essential to our target consumers’ purchasing decisions and will continue to command a sustainable price premium in the market.
In developing premium dried cannabis flower products, we strive to deliver against the three pillars of quality that govern our processes, from seed to sale:
1. Consistency
Delivering a consistent experience for every usage occasion is essential for recreational and medical consumers to develop a trusted relationship with our brand. Doing so requires an exacting level of control over every aspect of the cultivation, harvest, curing and extraction processes. Minute variations in the environmental conditions during cultivation and harvest can drive wide variations in the chemical composition of cannabis produced with identical starting genetics. Flowr’s meticulous analysis and control of every variable in the production process provides a high level of consistency in our products and in our customers’ experiences.
2. Full Spectrum
We cultivate, harvest and cure - and, where relevant, process into cannabis concentrates, other cannabis extracts and edible cannabis - with the goal of delivering the broadest possible spectrum expression of the terpene and cannabinoid profile that results from plant’s genotype and growing conditions. Across product formats, we endeavor to produce products that are "true to the plant". The result is cannabis flower with satisfying and redolent aromas and flavors, which also have an essential role in producing a richly textured psychoactive experience. For concentrates such as live resin packaged in cartridges or vape pens, once regulated for sale, Flowr expects to also focus on extraction and manufacturing processes that preserve the full spectrum of the plant’s terpene and cannabinoid profile.
3. Clean
Due to the role played by terpenes in delivering heightened sensory experiences, we believe that preserving the terpene profile of dried cannabis flower products and, where relevant, certain derivative products, is a critical differentiating factor in producing premium products. Terpenes are components of the plant which have been shown to contribute tastes and aromas.
To date, approximately half of the Corporation’s cannabis flower products produced at the K1 Facility, which is 40% operational, have been produced without the use of irradiation. Irradiation is a "kill step" used to eliminate microbial contamination that may develop in the cultivation or curing process. Flowr is focused on reducing and ultimately eliminating the need for irradiation due to its potential negative effect on terpene profiles.
Flowr believes that operating meticulously clean, rigorously-designed indoor facilities that limit the need for irradiation is a critical factor in producing a premium dried cannabis flower product. We do not believe that other federal cannabis licence holders share the same focus to eliminate irradiation and that our achievements in reducing irradiation to date, and our targeted goal of fully eliminating the step, enhances our product quality and is a significant competitive advantage that supports our premium brand strategy.
We believe that by consistently delivering premium dried cannabis flower products, we will develop a relationship of trust with our consumers and establish a premium brand position. This relationship and associated brand equity will then be leveraged, once regulations permit, and applied to premium new form factor derivative products, including vapes, infused beverages, capsules and edibles.
Based on data received from the OCS and Nova Scotia Liquor Corporation, Flowr’s products in these provinces' recreational markets have achieved retail price points ranging from a 25% premium to prevailing average pricing to as high as a 55% premium with an average premium of 40% between October 17, 2018 to June 16, 2019. We expect to sustain significant premiums over the medium to long term.

Since comparable recreational sales data is not currently available from the other provinces in which Flowr’s products are sold (British Columbia, Manitoba and Saskatchewan), we cannot provide similar retail price point comparisons for these provinces at this time.
Product-First Marketing Strategy Leverages Our Enthusiast Consumers
Flowr has to date executed a "product first" marketing strategy for its recreational consumers in Canada which leverages the quality of Flowr's dried cannabis flower products to build brand awareness organically through budtenders (i.e. product information, education and recommendation specialists present at retail locations), influencers, and word of mouth. To support our marketing strategy, we have hired a marketing specialist that has extensive consumer packaged goods experience with companies such as British American Tobacco and Japan Tobacco International.
Flowr's focus is on, and its target market is, what we define as the "Cannabis Connoisseur" segment. We believe these consumers are more knowledgeable and consider themselves to be a trusted source of information for friends and family on cannabis. According to the Colorado Department of Revenue, frequent users (who consume cannabis more than 20 days per month) comprise approximately 27% of cannabis consumers but consumed over 80% of the volume of cannabis products sold in 2017 in the state of Colorado, which is considered a mature recreational market. The least-frequent and least-knowledgeable consumers, on the other hand, have generated significant media interest, but represent a small percentage of sales. For example, in 2017, in Colorado, this segment accounted for 50% of cannabis consumers, but they accounted for a mere 4% of the volume of cannabis products sold. Because we believe that the Canadian market will exhibit similar characteristics over time, we focus on the "Cannabis Connoisseur" as both the largest and most attractive consumer segment, and one that represents a potential marketing asset as an ambassador for the Flowr brand and for the premiere quality level of our products. Because the Canadian regulatory environment currently restricts the ability to use conventional marketing and promotional tactics, we believe this approach will serve us well for the near to medium term. As the market evolves, we expect the least-frequent consumers to become more knowledgeable and thus more likely to follow the lead of the "Cannabis Connoisseur" in terms of product purchases. We believe that by positioning Flowr® as a trusted premium brand, we will see some increase in market share in the long-term as the knowledge of the less-frequent consumers increases. In parallel and as the market matures further, we expect to devote additional resources to in-store merchandising, event-based education, and consultative selling within retailers, using both dedicated in-house personnel and third party brokerage and service providers. In October 2018, we also entered into a strategic partnership with Ace Hill Beer Company, a craft beer brand in Canada, to create Ace Valley, a new brand of premium cannabis products for the Canadian recreational market. The collaboration gives us information about the recreational consumer's preferences and has allowed us to capitalize on the Ace Hill team's expertise in marketing and brand building in a highly regulated market.
World Class Facilities Expected to Drive Industry-Leading Yields and Profit Margins
Flowr believes that the profitability of its cultivation operations is driven by yield per square foot. We believe that our yields per square foot can be increased significantly from our already elevated levels - and ultimately, we believe we can continue to achieve yields per square foot that we believe exceed industry norms.
We believe that Flowr has been able to achieve higher pricing due to the premium nature of its product offerings. Combined with Flowr’s construction, design and cultivation expertise, focus on cost optimization through standardization of production capabilities and, upon consummation of the Acquisition, lower cost international production footprint, Flowr will be positioned to maximize its profitability.
Global Footprint to Capture Worldwide Opportunities
Following the Acquisition, Flowr will have a global footprint which will allow it to leverage its cultivation expertise to supply premium products, brands and intellectual property developed in Canada, across medical and recreational markets worldwide as regulations permit. We intend to tailor our market penetration strategies to each of our markets, production assets and target customer bases.
Upon closing of the Acquisition, we will operate a large scale, vertically integrated, licensed cannabis company with expertise in GMP-certified manufacturing. We will also have a distribution platform for global markets in Europe, Australia and surrounding countries, where sales of cannabis are permitted. Driven by our cultivation expertise, we will focus on targeting the medical cannabis markets across those geographies with high-quality cannabis produced cost-effectively for extraction into APIs.
Holigen is currently developing production, extraction and manufacturing facilities in Portugal and Australia. Holigen’s Aljustrel project in Portugal has been designated a Project of National Interest (“PIN”) by the Portuguese government. This designation provides Holigen with the ability to quickly move this project through regulatory licensing processes and potential access to funding. In Aljustrel, Portugal, Holigen is expected to build and operate a cannabis cultivation site with a combined potential production, manufacturing and R&D area of more than 7,000,000 square feet and potential annual dried cannabis flower capacity of approximately 500,000 kilograms. The Aljustrel site’s processing and manufacturing facilities will be built to GMP specifications and the cultivation area will be strategically located in proximity to a nearby dam providing reliable access to water and power. It is expected that Holigen will cultivate the majority of its cannabis product at its Aljustrel site, and distribute medical products and APIs to markets in Europe, Australia and surrounding countries, where cannabis sales are permitted, under the Holigen brand. Holigen will have an indoor cultivation and manufacturing facility in Sintra, Portugal. Upon completion of construction, this facility will include smaller indoor growing areas for high quality indoor grown cannabis, and manufacturing and packaging areas to be used for extraction and packaging of cannabis grown in Portugal.
In Australia, Holigen is licensed to cultivate and manufacture medical cannabis products, subject to additional permitting which is in process. It was also granted GMP approval by the Therapeutic Goods Administration for labelling, secondary packaging and release for supply in November 2018. Its full manufacturing facility is expected to be fully constructed in the second half of 2019 and receive GMP approval in 2020. The manufacturing space is located in Sydney and is designed with excess manufacturing capacity to process not only cannabis cultivated in Australia, but also product imported from Holigen’s Portugal operations. As part of its distribution strategy, Holigen will partner with Anspec in Australia, a privately-owned specialty pharmaceutical and medical distributor and exporter in Australia and abroad. Upon successful integration with Holigen, we expect that this relationship will provide Flowr with access to export pathways to 37 countries globally. To the extent that the on-site processing and manufacturing facility is not constructed, the Corporation expects to export and sell dried or fresh cannabis in countries where importation and sales of such products are permitted, such as Germany. Peter Comerford, an executive officer of Holigen and a proposed executive officer of Flowr post acquisition of Holigen, is the Chief Executive Officer of Anspec and provides a continuity of this distribution relationship to the combined company. Mr. Comerford receives compensation from Anspec for his services as Chief Executive Officer of Anspec, and is entitled to certain payments on the sale of Anspec to a third party purchaser. See "Risk Factors - Conflicts of Interest in Respect of Directors and Officers of the Corporation".
Holigen’s operations represent strategic production assets and geographically optimal gateways to distribute into current and future markets in Europe, Australia and surrounding countries, where sales of cannabis are permitted, particularly given the preference displayed in these markets for locally produced cannabis. Upon closing of the Acquisition, we believe the combined company’s proprietary cannabis cultivation know-how, regulatory familiarity, complex pharmaceutical and GMP construction expertise, and pharmaceutical distribution expertise, will give it the scalability and capability needed to service the global cannabis markets.
In addition to providing access to Australia and Europe, we expect that the assets in these geographies will serve as a platform for further global expansion. As a trusted trade partner of many Asian nations, we believe that Australia is well positioned to be a leading exporter of cannabis products to the Asia-Pacific region, and that the combined company will be able to capitalize on such opportunities to enter new markets in Asia if and when regulatory regimes in this region regulate cannabis products.
THE INDUSTRY
The cannabis market in which Flowr operates is an emerging and rapidly expanding industry, with new developments occurring frequently. In many countries, cannabis has seen a paradigm shift in its regulation and perception over the last few years, resulting in the accelerated growth of the global legal addressable market. The United Nations estimates the global cannabis market (regulated and illicit) today to be worth approximately US$150 billion annually. Further regulation of medical and recreational markets is expected to lead to the absorption of illicit markets and dramatically expand the total addressable market for current legal market participants.
Canadian Market
On October 17, 2018, Canada became the first major industrialized nation to regulate the sale of cannabis for recreational use. Deloitte estimated the total cannabis market in Canada, including the regulated and illicit markets, as well as legal recreational products, is expected to generate up to $7.17 billion in total sales in 2019. Regulated sales are expected to contribute more than half of this total up to $4.34 billion in the first year. Current legal product formats in Canada include dried cannabis flower and cannabis oils; however, additional form factors, including vapes, edibles, tinctures, beverages, and topicals, are expected to be legalized in late 2019. Deloitte estimates the Canadian market for cannabis edibles and infused products to be worth approximately $2.7 billion, with approximately $1.6 billion attributable to edibles. This estimate is additive to the $7.2 billion 2019 total Canadian market estimate provided by Deloitte in an earlier report. We believe the addition of these new consumer-friendly formats will significantly expand the size of Flowr’s addressable Canadian market, as convenient, discreet, and precisely dosage-metered products such as vape pens, capsules, beverages and edibles, bring in new consumers less familiar with cannabis and less accustomed to smoking or vaping dried cannabis flower.
The Canadian medical cannabis market is experiencing rapid growth, with Health Canada reporting registered users rising more than tenfold between 2014 and 2018. Deloitte estimated that this segment will generate up to $1.79 billion in sales in 2019.
European Market
An estimated 23 million individuals in the European Union and Norway consumed cannabis at least once in 2018. With a market of approximately 743 million people and an estimated total healthcare spend of €2.3 trillion, it is estimated that Europe will be one of the largest medical cannabis markets in the world. Insurance companies in Germany, Denmark, and Italy are now covering medical cannabis prescriptions.
Further examination of various countries in the European region present different regulatory pathways that may be adopted domestically. For example, in the United Kingdom, as of November 2018, medical cannabis products became legal and the distribution model is via registered specialist doctors prescribing cannabis to patients. In Portugal, the country introduced medical cannabis reform in June 2018 that allowed physicians to prescribe medical cannabis. Similar to the United Kingdom, Germany, legalized medical cannabis in March 2017. Germany represented 68% of the total sales in kilograms of flower, representing 76% of the total revenue from cannabis sold, as retail value in Germany is significantly higher than in the Netherlands.
Ultimately, each country will have slightly differing laws for its domestic cannabis market as well as the import of cannabis into the country. Today, regulations in the United Kingdom and Germany allow for the bulk import of medical cannabis to meet the future demand at pharmacies, subject to obtaining the proper import permits adhering to each respective country’s medical cannabis regulatory laws.
Australian Market
Legislation for legalised cannabis occurred for medical use and came into effect in October 2016. The cultivation, production and manufacturing of cannabis and cannabis products for medical and scientific use is licenced by the newly established the ODC. The manufacturing, registration and supply of medicinal cannabis products are regulated by the TGA. Medicinal cannabis products would be classified under prescription medicines, and accordingly would be subject to the Therapeutic Goods Act. Since June 2018, approximately 7,000 additional medical patients have been approved in Australia. The Australian government has also indicated that they plan to become the fourth country in the world to federally legalize medical cannabis exports to capitalize on the cannabis opportunity, with the first export completed in August 2018. The export of medicinal cannabis products was legalised in February 2018 through the Narcotic Drugs Amendment (Cannabis) Regulations 2018. The current regulations allow the following products to be exported:
• Medicinal cannabis products manufactured in Australia under a GMP Licence
• Medicinal cannabis products listed as export-only or registered in the ARTG
• Extracts of cannabis (or cannabis resin) manufactured under a Narcotic Drugs Act 1967 Licence that are not in the final dosage form
Management believes that Australia in particular, will be the key reference market for pharmaceutical quality in the region and products manufactured to the country’s highest medical standards (e.g., GMP, TGO-93 and TGO-77) will be perceived by other countries within the region to be reliable APIs.
Flowr expects the Australian market to be driven by the legal Australian medical market, which through its established trade relationships is well positioned to serve as a trade gateway to the rest of the Asia-Pacific region if and when regulatory regimes in this region legalize cannabis products.
FLOWR’S MARKETS
Our Approach to the Markets We Address
We want to leverage our industry-leading cultivation expertise and premium products to execute market penetration strategies specifically tailored to each of our markets, production assets and target customer bases. We believe this approach will allow us to establish our brand as a recognized producer of premium cannabis.
In Canada, Flowr has focused on producing indoor-grown premium dried cannabis flower product for medical and "Cannabis Connoisseur" recreational consumers primarily under the FlowrRx® and Flowr® brands respectively. When Canadian regulations permit the sale of additional form factors, we intend to launch additional premium products in new form factors such as vapes, infused beverages, capsules and edibles. In some cases, we expect that the production of new form factors will utilize concentrate cannabis extract from cannabis cultivated at our Flowr Forest outdoor and greenhouse facilities.
Through the acquisition of Holigen, Flowr intends to leverage its expertise to design and build indoor production facilities to GMP standards in Europe and Australia. These facilities will principally focus on the medical markets, with high quality cannabis produced cost-effectively for extraction into APIs for patients, and for consumer packaged goods, personal care and pharmaceutical customers and partners.
Canadian Recreational Market
We believe that demand for high quality cannabis flower in Canada will continue to outstrip supply for the foreseeable future. Because of the widely-reported challenges in production, certain federal cannabis licence holders have shifted resources toward building cultivation infrastructure requiring a lower degree of expertise to build and operate, but consequently produces lower quality cannabis. We believe the majority of planned industry capacity in Canada is expected to come from greenhouses, which could result in a supply glut of lower quality cannabis and a shortage of high quality cannabis.
Our belief that high quality cannabis will command a price premium over the medium to long term is supported by the presence of persistent pricing premiums for high quality cannabis in mature U.S. markets where recreational cannabis use is legal at the state level. In California for example, where the supply of low-priced cannabis has driven down the average price per gram, consumers still show a willingness to pay materially higher prices for high quality cannabis. Based on 2018 California sales data provided by BDS Analytics, we estimate that approximately 40% of the total volume and 56% of the aggregate dollar value of dried cannabis flower is transacted at price points in excess of US$10. We believe that this shows a sustainable willingness of consumers to pay premium prices for higher quality cannabis.
Our strategy within the Canadian recreational market focuses on developing high quality dried cannabis flower and pre-roll products offered under the Flowr® brand. While new product formats derived from extracts, such as edibles and concentrates, are surging in popularity in mature markets outside of Canada, combustible products continue to experience strong sales growth in absolute terms and remain the predominant format used by consumers for recreational use. Given that current regulations in the Canadian recreational market permit limited product formats, we believe that premium combustible products are essential to establishing a premium brand that can command premium prices.
Our target customer is what we define as the "Cannabis Connoisseur". We believe these consumers are more knowledgeable and consider themselves to be a trusted source of information for friends and family on cannabis. According to the Colorado Department of Revenue, frequent users (who consume cannabis more than 20 days per month) comprise approximately 27% of cannabis consumers but consumed over 80% of the volume of cannabis products sold in 2017 in the state of Colorado, which is considered a mature recreational market. We believe that premium product quality, as defined by a rich sensory experience, consistent psychoactive effects, and robust terpene profiles, is essential to frequent consumers’ purchasing decisions and will continue to command a sustainable price premium in the market. We believe that by consistently delivering high quality cannabis products, we will develop a relationship of trust with our consumers and establish a premium brand position, which can be directly extended to premium derivative products such as vapes, infused beverages, capsules and edibles as they become legal.
We believe that premium dried cannabis flower will remain the largest Canadian recreational market segment. Given that the chemical composition of the cannabis flower is highly dependent on precise control over every facet of the environmental conditions in which it is grown, we believe that high quality dried cannabis flower can only be produced in purpose-built indoor facilities such as our K1 Facility. We therefore produce our dried cannabis flower products exclusively through indoor growing facilities designed to GMP standards and awaiting GMP licensing, with small, self-contained flowering rooms that can isolate any potential pathogens as well as provide unique environmental conditions specifically calibrated to produce the fullest expression of the potential of each cultivar.
Based on data received from the OCS and Nova Scotia Liquor Corporation, Flowr’s products in these provinces' recreational markets have achieved retail price points ranging from a 25% premium to prevailing average pricing to as high as a 55% premium with an average premium of 40% between October 17, 2018 to June 16, 2019. We expect to sustain significant premiums over the medium to long term.
In Canada, our products are distributed through supply agreements or arrangements with liquor control authorities, distributors or directly with retailers, as applicable (see "Regulatory Framework - Canada - Provincial Regulatory Framework") in the provinces of Ontario, British Columbia, Nova Scotia, Manitoba, and Saskatchewan. We are in advanced discussions with the Province of Alberta and expect a supply agreement to be in place and product available for sale in the province by the second half of 2019. Flowr’s current provincial distribution agreements and the future expected agreement with Alberta will address approximately 70% of the Canadian population.
Canadian Medical Market
Flowr’s medical products in Canada are offered under the FlowrRx® brand. Patients medicating with cannabis expect consistency across dosages, similar to other pharmaceutical products. As such, product quality and consistency is critical to a patient’s experience and the creation of brand trust and loyalty. Our medical cannabis products are produced with the highest level of attention and care and are intended to deliver patients a consistent high-quality product and repeatable experience that they can rely on to treat their medical conditions.
Similar to the recreational market, our medical product line is focused on premium dried cannabis flower and supported through our state-of-the-art K1 Facility. As consumption methods in the medical market continue to shift towards alternative form factors and as regulations permit the sale of such products, we intend to expand our product offering to include oils, extracts and capsules, utilizing cannabis inputs from the Flowr Forest. We believe that additional medical products will benefit from our developing a medical brand position as a trusted provider of premium dried cannabis flower products produced from the K1 Facility and the K2 Facility, upon completion and acquisition of the required licences.
We believe that by using our own cultivated cannabis for extraction and processing of Flowr branded products, medical patients will be provided with a heightened level of comfort knowing the product was manufactured with the same mindset and cultivation approach that we take with our indoor grown cannabis flower.
Under the Cannabis Regulations, medical cannabis can only be sold by federal medical sales licence holders. Shoppers, through Medical Cannabis by Shoppers Drug Mart Inc., holds a processing licence and a sale for medical purposes licence, and sources cannabis products from other federal processing licence holders. Shoppers is Canada’s largest drug store retailer with approximately 1,300 locations across Canada. We made a strategic decision to distribute our FlowrRx® cannabis products through Shoppers and supply to Shoppers on a purchase order basis. We believe that Shoppers’ entry into the medical cannabis market will hasten the acceptance of medical cannabis in Canada. We believe the recent decision by Great West Life to reimburse plan members for certain medical cannabis purchases is evidence of this shift. We believe direct-to-consumer distribution will become less relevant in the future, reinforcing the value of a strong relationship with retail outlets for medical cannabis, such as Shoppers.
European, Australian and Potential Asian Medical Markets
After the closing of the Acquisition, we expect to establish a local presence in the rapidly growing European and Australian regions. We believe that Flowr’s ecosystem of cultivation techniques, design of GMP compliant facilities, and operational expertise established in the Canadian market will enable us to capture market share internationally through Holigen. The expertise of Flowr’s R&D team, particularly with regards to plant science and operations, will help Holigen to execute on its manufacturing and extraction protocols. This will be critical to success in the European and Australian markets as they are primarily oil-based markets rather than dried cannabis flower markets. We believe that this expertise, combined with the Holigen management team’s experience in pharmaceutical opiate extraction and GMP pharmaceutical grade construction design and strong global pharmaceutical distribution relationships, will enable the combined company to produce and distribute consistent, high-quality, cannabis products and APIs that are ideally tailored to the needs of the international markets Flowr gains exposure to through Holigen.
In addition to providing access to Australia and Europe, we expect that the combined company’s assets in these geographies will serve as platforms for further global expansion. As a trusted trade partner of many Asian nations, we believe that Australia is well positioned to be a lead exporter of cannabis products to the Asia-Pacific region if and when regulatory regimes in this region legalize medical cannabis.
Europe
In Europe, the combined company’s medical cannabis products are expected to be offered under the Holigen brand and will be supplied through Holigen’s facilities based in Sintra and Aljustrel, Portugal. In accordance with European regulations, the combined company intends to operate GMP-certified production facilities in Portugal. Due to favorable labor costs and an ideal climate, we expect the cannabis produced at the Aljustrel site will be among some of the lowest cost cannabis in the world without sacrificing product quality. In addition, we believe that the indoor growing, extraction and processing facility at the Sintra site will be a significant point of differentiation for the combined company as this provides ready-access for other companies to operate within a facility, which is expected to become GMP-certified in 2019, should they decide to pursue joint ventures/strategic partnerships to create innovative cannabis products. As the market evolves, the Sintra facility will allow Holigen to supply high quality cannabis to the European markets as well as conduct R&D.
Australia
Upon completion of the acquisition of Holigen, Flowr intends to leverage the Holigen brand to market and sell medical cannabis products in the Australian market and, if and when legal, Asia. Cannabis for this market will be sourced from Holigen’s low cost facility in Aljustrel as well as from a local indoor cultivation facility located in Sydney, Australia which will produce high quality dried cannabis flower. In Australia, Holigen is licensed to cultivate and manufacture medical cannabis products, and was granted GMP approval by the Therapeutic Goods Administration for labelling, secondary packaging and release for supply in November 2018. Holigen believes that it is on track to have its full manufacturing facility in Australia approved for GMP accreditation in 2020. The Sydney facility will also include manufacturing and packaging areas to be used for extraction and packaging of cannabis. Subject to available funding from this Offering, we expect construction at the Australian facility to be finalized in the second half of 2019, in preparation for a final inspection by authorities. Upon completion of construction, inspections, permitting and licensing, Holigen expects to commence manufacturing in Australia by the first half of 2020.
While the primary focus remains on Australia, if other jurisdictions in the region introduce regulatory regimes to govern the legal use of medical cannabis, the combined company of Flowr and Holigen will utilize its local presence in Australia as well as the country’s status as a trusted trading partner for many Asia-Pacific states, to export medical cannabis products to new markets.
Future Expansion of Canadian Recreational Market and Products
Other concentrate-based form factors including vapes, edibles, tinctures, beverages and topicals are expected to be regulated for sale in Canada. We intend to leverage our developing brand strength and use lower cost product inputs to increase market penetration, capture new market segments and retain stronger margins. To support new form factor products, we are expanding our cultivation infrastructure in Canada to include our lower cost Flowr Forest greenhouse and outdoor cultivation facility, which is currently under development. The Flowr Forest will produce lower-cost cannabis, grown with the same level of care afforded to our indoor production, that will be extracted and processed into concentrates to be used in the development and future sales of new products. By utilizing this lower cost production environment, we expect to capture higher margins without compromising quality.
We believe that our developing reputation as a premium cannabis brand in Canada will enable us to capture market share for these other form factors once they are regulated for commercial production and sale. We believe consumers will attribute the same premium position to these new cannabis products. We anticipate bringing extracts for vaping, beverages, cannabis oils, APIs, edibles, capsules, tinctures and beverages to market in both psychoactive and wellness-focused varieties, with the latter being a focus on certain customers making purchasing decisions based on healthier alternatives.
OUR PRODUCTS AND BRANDS
Together, Flowr and Holigen, will market their products in the recreational and medical markets in Canada and the medical markets in Europe and Australia.
Recreational Brands
Flowr® is our primary brand for the Canadian recreational cannabis market and is targeted at the "Cannabis Connoisseur" market segment. We also plan to continue entering into select strategic partnerships to bring new brands of cannabis products to a broader range of recreational consumers, as we have done with our Ace Valley® brand through a partnership with Ace Hill. We expect to enter into similar partnerships to maintain a connection between the quality of the product a recreational user consumes and the Flowr brand.
Medical Brands
In Canada, we sell our products to medical patients under the FlowrRx® brand and expect to leverage the Holigen brand to address the global medical market, with an initial focus on Australia and Europe. The Holigen brand may also be used to sell products to the branded cannabis ingredient market.

The Flowr brand has been built on a foundation of premium dried cannabis flower with our recreational products in Canada targeting the "Cannabis Connoisseur". We believe that by consistently delivering high quality cannabis flower products, we will develop a relationship of trust with our consumers and establish a premium brand position, which can be directly extended to premium derivative products such as vapes, infused beverages, capsules and edibles as they become legal.
Cannabis as an Ingredient
Upon the completion of the Acquisition, Flowr will benefit from having access to a cannabis cultivation site in Aljustrel, Portugal that is expected to have a combined potential production, manufacturing and R&D area of more than 7,000,000 square feet and potential annual dried cannabis flower capacity of approximately 500,000 kilograms. We expect to produce cannabis that can be converted and formulated into high quality APIs across indoor, greenhouses and outdoor facilities in Portugal. The Acquisition also facilitates a robust global distribution platform for APIs and finished goods across the European and Australian markets where regulations permit. Management believes that the low-cost characteristics of the Portuguese outdoor grow as well as the extraction and production capabilities in our facilities, combined with the Holigen management’s expertise in producing high quality APIs, will make the combined company a partner of choice for global pharmaceutical and consumer goods companies seeking to commercialize products utilizing cannabis as the active ingredient
In addition, Holigen’s Sintra facility is designed to house several production, manufacturing and packaging lines. We believe that, once completed, fully licensed and GMP-certified, this cannabis production infrastructure will attract pharmaceutical product companies who will want to use Holigen’s facilities for their cannabis product development and production capabilities. We believe this structure will offer pharmaceutical and consumer goods companies a path to commercialize and distribute cannabis products quickly and efficiently within Europe, while ensuring the highest standard of APIs and manufacturing processes. In return for access to Holigen’s facilities and APIs, the combined company intends to generate revenues through the sale of APIs, charging a fee for access and services and/or equity or royalty participation in products developed and sold.
Canada Clones and Genetics Products
In addition to our cannabis products for the medical and recreational markets, Flowr plans to sell a wide selection of cannabis cultivars in both clone and seed form to other licensed cannabis producers. Flowr expects that it will be able to produce more than 3.2 million high quality clones on an annualized basis once the K1 Facility is completed. The sale of these clones is not expected to impact Flowr’s cultivation operations or ability to produce flower for the Corporation’s recreational and medical markets.
Flowr believes the current demand for high quality clones that are free from pests and pathogens and grown in a controlled environment exceeds supply due to the rapid expansion of cultivation facilities commencing construction across Canada and globally, subject to applicable licensing requirements.
Notwithstanding market demand for these clones, Flowr does not expect to distribute its highest quality clones to other federal cannabis licence holders, thus preserving its cultivation advantage for premium products. Flowr also believes that its cultivation proprietary know-how is critical to realizing the full potential and scalability of its cultivars. Therefore, we believe the sale of our cultivars has the potential to generate meaningful revenues from recurring nursery sales, while preserving for Flowr the value of its most valuable proprietary genetics.
OUR CURRENT AND PROPOSED CULTIVATION AND R&D FACILITIES
| Facility | Location | Type | Expected Operational
Date(1) (2) | Estimated Initial
Capacity(2)(3) | Estimated Maximum
Capacity(2)(3) | Cost to Complete
(millions)(2)(4) |
| K1 Facility(5) | British Columbia, Canada | Indoor | Q3 2019 | 10,000kg | 10,000kg | $7.2 |
K2 Facility(6) | British Columbia, Canada | Indoor | H2 2021/2022 | 785kg(8) | 40,000kg(7) | $140.2 |
Hawthorne R&D Facility(5) | British Columbia, Canada | R&D | H2 2019 | N/A | N/A | $3.8 |
Flowr Forest(9) | British Columbia, Canada | Outdoor / greenhouse | H2 2019 (first phase) | 5,000kg(10) | 10,000kg | $2.4 |
| Aljustrel(11)(12) | Aljustrel, Portugal | Outdoor | H2 2019 | 1,500kg(14) | 500,000kg(13) | $67.0 |
Sintra(11)(12) | Sintra,
Portugal | Indoor + R&D | H2 2019 | 150kg(15) | 1,800kg | $10.5 |
| Sydney -
Phase 1(11)(16) | Sydney, Australia | Indoor | H1 2020 | 500kg(17) | 1,000kg | $10.5 |
Sydney -
Phase 2(11)(16) | Sydney, Australia | Indoor + R&D | To be determined | To be determined | To be determined (18) | To be determined |
(1) The Expected Operational Date reflects the anticipated time to reach the initial phase of commercialization for each facility, which, except for the K1 Facility, will be before construction is fully completed and prior to reaching estimated maximum capacity, as we construct in stages. Other than the K1 Facility (which is expected to be fully complete/built-out and operational upon receipt of an amendment to the Corporation's Health Canada licence for new phases by Q4 2019), Flowr expects that the construction and development of the facilities described in the table above will be completed over the course of the next two to three years. Such construction is expected to be completed in stages, with completed portions of the applicable facility becoming operational while other portions are under construction.
(2) Please see "Risk Factors" and "Forward-Looking Information".
(3) Subject to licensing, zoning and permitting. Estimated Initial Capacity reflects the facility's potential annual production capacity, as at the initial phase of commercialization and in many instances, prior to full completion. Estimated Maximum Capacity reflects the facility's potential production capacity, when fully complete/built-out and operational, based on targeted yields and maximum growing capacity, where applicable. Both Estimated Initial Capacity and Estimated Maximum Capacity include dried cannabis flower and dried cannabis flower equivalents. Except as otherwise described in the notes below, Flowr expects to be able to reach the capacities listed in the table above with (i) its available cash on hand, (ii) the net proceeds of the Offering and (iii) the cash expected to be generated from Flowr's and Holigen's operations, as such cash from operations becomes available. The anticipated timelines to scale each facility to its maximum production capacity (described below) are based on the time required to reach full build-out and operation and are not subject to additional funding (except as described in the notes below).
(4) The Cost to Complete represents the forecasted remaining costs associated with the construction and development of each of the facilities to reach full completion/operation. The total aggregate amount of funding currently forecasted to be required to complete the construction of the facilities in the table above is approximately $241,600,000.
(5) K1 Facility is fully funded. The first floor of the Hawthorne R&D facility is fully funded.
(6) Expected to be funded with the proceeds from the Offering and the ATB Credit Facilities if obtained.
(7) This estimate is based on the assumption that the ATB Credit Facilities will be available to Flowr. In the event that the ATB Credit Facilities are not available, Flowr would likely construct a facility that is approximately half the size of the planned K2 Facility, which would result in half of the expected capacity of the K2 Facility from the capacity disclosed in the table above (such amount being approximately 20,000 kg). See "Use of Proceeds - Business Objectives and Milestones".
(8) Assuming achievement of the estimated initial capacity on the anticipated timeline, the annual production is expected to increase to 29,400 kilograms in the year 2022 and is expected to reach estimated maximum capacity of 40,000 kilograms in 2023.
(9) The remaining costs associated with the construction and development of the Flowr Forest are expected to be funded with the ATB Credit Facilities if obtained.
(10) Assuming achievement of the estimated initial capacity on the anticipated timeline, the annual production is expected to increase to 10,000 kilograms, reaching estimated maximum capacity, in the year 2020.
(11) Subject to the closing of the Acquisition. See "Risk Factors".
(12) Expected to be funded with the proceeds of the Offering and existing or proposed debt facilities available to Holigen.
(13) This estimate is based on the following material factors and assumptions: (i) the Aljustrel site, when fully planted and operational, is expected to have approximately 5,060,000 square feet of grow space; (ii) the anticipated yield in the outer years is expected to reach 100 grams per square foot per year; and (iii) with approximately 5,060,000 square feet of canopy at a targeted yield of 100 grams per square foot per year, the anticipated output of cannabis at the Aljustrel site is expected to be approximately 500,000 kilograms (5,060,000 multiplied by 100 grams/sq.ft./year divided by 1,000).
(14) Assuming achievement of the estimated initial capacity on the anticipated timeline, the annual production is expected to increase to 30,000 kilograms in the year 2020 and can reach the estimated maximum capacity of 500,000 kilograms in 2021, as the Aljustrel site is a low cost outdoor growing operation. Flowr will assess whether it will produce the full estimated maximum capacity in 2021 based on demand for its products.
(15) Assuming achievement of the estimated initial capacity on the anticipated timeline, the annual production is expected to increase to 1,800 kilograms, reaching estimated maximum capacity, in the year 2020.
(16) Expected to be funded with the proceeds of the Offering.
(17) Assuming achievement of the estimated initial capacity on the anticipated timeline, the annual production is expected to increase to 1,000 kilograms, reaching estimated maximum capacity, in the year 2021.
(18) Flowr has the option to construct Sydney - Phase 2 facility based on Phase 1 specifications.
Other than the K2 Facility, the design of each of the facilities listed above has been completed or substantially completed. With respect to the K2 Facility, the Corporation has conducted design specifications, and continues to refine the design work towards completion. The next steps for completion of the facilities would then be funding (with cash on hand, the proceeds of the Offering and the expected ATB Credit Facilities), construction (in many cases in stages, with completed portions of the applicable facility becoming operational while other portions are under construction), and then final operation of the completed facility.
Canada
Our Canadian cultivation assets are located at our Kelowna Campus in Kelowna, British Columbia.
Flowr holds a licence authorizing Standard Cultivation, Standard Processing and Sale for Medical Purposes (the "Licence"). The Licence authorizes Flowr to cultivate, process and sell cannabis products under the Cannabis Act and the Cannabis Regulations. Flowr carries on its current cultivation operations at the K1 Facility.
Our cultivation team members have extensive experience with the mass production of high quality indoor cannabis and have collectively designed, built and operated over 15 cannabis production facilities under the MMAR, and also under the stringent regulatory structures of the MMPR and ACMPR. We believe the team’s robust cultivation, proprietary know-how and trade secrets create a long-term strategic advantage relative to our competitors, particularly as awareness grows about the high degree of difficulty involved in the consistent production of high quality cannabis. We also believe that we enjoy a distinct operational advantage from having a single, centralized facility for all of our Canadian cultivation, processing, and sale assets, rather than having them diffused across multiple Canadian locations.
The K1 Facility features industry-leading climate control, lighting, cultivation systems, and other proprietary trade secrets that aid the realization of operational efficiencies and maximized yields. The K1 Facility is built with small, self-contained flowering rooms, which in addition to allowing us to isolate any potential pathogens, enable us to create unique environmental conditions specifically designed to produce the fullest potential of each cultivar to maximize yields.
Furthermore, we expect our yields to increase over time through further refinement and optimization of our grow rooms and systems (so-called "dialing in"), tailoring environmental conditions to specific cultivars, new processes, procedures, and innovations. As we optimize our facilities and systems we expect over the medium term to continue to achieve yields per square foot that we believe exceed industry norms.
The K1 Facility is designed to be compliant with GMP standards, including the requirements of The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use ("ICH"). ICH Good Manufacturing Practices are the standards and procedures that pharmaceutical companies must adhere to in manufacturing products in North America. The ICH Good Manufacturing Practices exceed the good pharmacy practice standards required by Health Canada for growing and cultivating medical cannabis. The K1 Facility was assessed by a consultant to determine whether its production processes meet the ICH Good Manufacturing Practices requirements. Flowr expects that the ICH Good Manufacturing Practices certification will be received in 2020 after completion of the K1 Facility. If obtained, this certification will indicate that Flowr’s production practices meet stringent pharmaceutical manufacturing requirements that are internationally harmonized in 17 countries including the United States, Canada, Singapore, Japan, Australia and several European nations. Flowr expects that such certification, if obtained, will provide it with a competitive advantage relative to other producers that do not produce cannabis in compliance with these standards.
Flowr expects to complete construction of the K1 Facility by the end of the third quarter of 2019. When complete, Flowr expects the K1 Facility to attain targeted production of approximately 10,000 kilograms of high quality cannabis flower on an annualized basis. Subject to obtaining the required financing, Flowr also anticipates beginning construction of the K2 Facility located on the Kelowna Campus prior to the first half of 2020. We project that the K2 Facility will have targeted production of approximately 40,000 kilograms on an annualized basis after its completion and licensing. Subject to obtaining the proceeds of this Offering and the funding under the ATB Credit Facilities, we expect construction of the K2 Facility to be completed by the second half of 2021/calendar year 2022.
In the first quarter of 2019, Flowr commenced construction of the Flowr Forest. On June 25, 2019 Flowr filed a new site application with Health Canada for the Flowr Forest and on July 12, 2019 received its cultivation license for this facility. We have begun cultivation at the Flowr Forest and expect to complete a first harvest in the fourth quarter of 2019. The Flowr Forest will consist of modular capital-light greenhouses that leverage our knowledge of plant genetics and cultivation expertise while producing cannabis at a lower cost for extraction and production of cannabis products in the new classes of cannabis.
Europe
The Sintra facility in Portugal will have 21,500 square feet for cultivation, processing, GMP manufacturing and R&D operations when construction is completed and GMP certification is received. Holigen has been granted a cultivation license for its Sintra indoor facility which currently has mother plants growing and has applied for a license to manufacture and distribute cannabis at Sintra. The GMP-designed extraction and packaging areas in the Sintra facility are complete and the extraction equipment is in place and undergoing testing. The GMP manufacturing inspection for Sintra’s indoor facility is scheduled and expected to occur in August 2019.
In Europe, regulations stipulate that medical cannabis products can only be sold if they are extracted and manufactured in a GMP-compliant facility. We believe our experience designing our K1 Facility to GMP standards as well as our operation team’s experience in constructing GMP compliant facilities provide the combined company with a significant competitive advantage in Europe.
In Aljustrel, Portugal, Holigen expects to initiate the construction of a cannabis cultivation site that, upon completion, is expected to have a combined potential production, manufacturing and R&D area of more than 7,000,000 square feet and potential annual dried cannabis flower capacity of approximately 500,000 kilograms. Holigen's Aljustrel site is now security fenced and the Portuguese authorities have completed their inspection of the site. Holigen expects to receive its cultivation licence at this site in July 2019. The outdoor cultivation facility at Holigen's Aljustrel site consists of a raised bed grow system with drip feed irrigation and cleanroom modular construction units for mothering with enough modular construction of capital-light greenhouses that will leverage Flowr's deep knowledge of plant genetics and cultivation expertise while producing cannabis at a lower cost for extraction into form factors and APIs. Certain funds raised pursuant to this Offering will be used to finalize the construction at the Aljustrel site in the second half of 2019. See "Use of Proceeds".
Holigen’s Aljustrel site was recognized as a PIN by Agência para o Investimento e Comércio Externo de Portugal. To receive the PIN designation, Holigen was required to fulfill certain conditions, including, certain investment and job creation requirements. In addition, the Comissão Permanente de Apoio ao Investidor requested opinions from other government entities involved in the process, including the Mayor of Aljustrel, INFARMED and Direção-Geral de Agricultura e Desenvolvimento Rural, each of which supported awarding the PIN designation. Recognition as a PIN by the Portuguese government provides Holigen with the ability to fast-track through regulatory licensing processes and potential access to funding.
Australia
In Australia, Holigen is licensed to cultivate and manufacture medical cannabis products, and was granted GMP approval by the Therapeutic Goods Administration for labelling, secondary packaging and release for supply in November 2018. Holigen believes that it is on track to have its full manufacturing facility in Australia approved for GMP accreditation in 2020.
Subject to available funding from this Offering, Flowr expects construction at the Australian facility to be finalized in the second half of 2019, in preparation for a final inspection by authorities. Upon completion of construction, inspections, permitting and licensing, Holigen expects to commence manufacturing in Australia by the first half of 2020.
Research & Development
Flowr and Hawthorne have entered into a strategic R&D alliance, for a term of twenty years commencing on December 14, 2018. Under the terms of the agreement with Hawthorne, Flowr oversees the construction of the R&D facility on land owned by the Corporation, in consideration for a $1.5 million design and construction fee payable by Hawthorne. Once the R&D facility is operational, Flowr will also provide certain research and development services to Hawthorne, in consideration for a monthly fee of $29,000 (adjustable by the lesser of 3% or annual Canadian inflation rate over the prior year, commencing with the 11th year after the R&D facility opens), as well as certain other payments and contributions as further described below.
Construction of a two floor R&D facility has commenced, and is expected to be completed during the second half of 2019. Hawthorne will fund the first $11.5 million of costs associated with (i) the design and construction of the first floor of the R&D facility (expected to total approximately 20,000 square feet), (ii) the shell for a second floor and rooftop greenhouse (the "Additional Floors"), and (iii) certain structural and other improvements required to construct the Additional Floors, by a loan in the same amount from Hawthorne to the Corporation. The loan bears interest at a rate of 4% per annum, repayable monthly, commencing with the first month that a management fee is payable from Hawthorne to the Corporation under the terms of the agreement. The monthly fee payable from Hawthorne to the Corporation in consideration for its research and development services will include an amount equivalent to the monthly principal and interest payment under the loan due from the Corporation to Hawthorne. The Corporation will be solely responsible for any costs of design and construction of the first floor of the R&D facility in excess of $11.5 million (costs of which are expected to amount to $3.7 million) as well as the costs of the design and construction of the Additional Floors other than the shell and certain structural and other improvements (costs of which are not confirmed at this time.)
Hawthorne and the Corporation will each be responsible for the acquisition and maintenance costs of the facility equipment installed in their respective areas of the R&D facility, and will each separately own such equipment. Hawthorne has a first ranking mortgage and security interest over the Corporation’s Kelowna property located at 9580 McCarthy Road, Kelowna, BC, including, without limitation, the R&D facility, to secure the amount of the loan and the value of any of its equipment which it does not remove from the R&D facility, which amounts the Corporation would be obligated to repay to Hawthorne in the event that Hawthorne prematurely terminates the agreement following a default thereunder by the Corporation.
Once constructed, at least 80% of the floor space of the first floor of the R&D facility will be dedicated to research, development and testing of Hawthorne products (the “Hawthorne R&D”), which we believe will support the development and refinement of Hawthorne’s existing products and new products. All rights to intellectual property and know-how derived from the conduct of the Hawthorne R&D will be exclusively owned by Hawthorne. The Corporation will be allocated 20% of the floor space of the first floor of the R&D facility (the “Flowr Area”) for its own, discrete, unlimited full use, as well as have the right to use certain common testing equipment for 20% of the time for its own purposes. The research and development to be conducted by the Corporation in the Flowr Area is expected to include determining cultivar specific nutrient demand curves, maximizing terpene profiles and cannabinoid output and optimizing cultivars for different climates and growing conditions. Any intellectual property derived from the conduct of research and development in the Flowr Area will be exclusively owned by the Corporation. Upon completion, Flowr will own the R&D facility. All staffing costs related to staff dedicated to the Hawthorne R&D, as well as 80% of the ongoing costs of operating the first floor of the R&D facility, will be funded by Hawthorne. Hawthorne has the right to participate in the design, development and construction of the Additional Floors under the terms of the agreement, but the Corporation and Hawthorne have not yet determined the terms of such participation, if any. If Hawthorne elects to participate, the allocation of operating costs and use of the Additional Floors will be determined through negotiation between the Corporation and Hawthorne.
We also expect the R&D facility will allow us to conduct test work on live plants without negatively impacting ongoing operations at our cultivation facilities. The Flowr R&D team is led by Jason Broome, who has amassed a 15-year career in the pharmaceutical industry. Mr. Broome led the commercialization of major pharmaceutical brands and built, operated and sold several healthcare-related companies. The R&D team also includes Dr. Deron Caplan, a Doctor of Philosophy (PhD) with research focused on cannabis production and Chef Ryan Reed, a past winner of Chopped and Victoria’s 2011 Chef of the Year. This team is highly focused on Flowr’s R&D activities, including a focus on optimal growing conditions for certain cultivar plants and the development of a variety of cannabis form factors as regulations permit them to be marketed and sold in Flowr’s target markets.
Accordingly, in preparation for the regulation of these additional form factors following the legislative amendments expected later this year, we are investing in the buildout of a state-of-the-art, purpose-built extraction, formulation and packaging facility that will enable us to launch these new derivative products. We have leased approximately 12,000 square feet in an offsite Kelowna facility outfitted with a full test kitchen, permitting the rapid development of edible cannabis products as well as our understanding of extracts and fractionation techniques.
In addition, upon completion of the Acquisition, Flowr intends to use the Sintra and Australia facilities to develop extraction capabilities to bring APIs and oil-based medical products to market and to produce extracts specially designed for individual customers as APIs and finished products for the medical and wellness markets.
FLOWR’S STRENGTHS
Flowr’s strategy is focused on leveraging its following core strengths to exploit its competitive advantage:
Cultivation expertise affirmed through partnership with a hydroponic products industry leader.
Facilities designed to maximize yield and profitability.
Highest quality products and premium brands further drive profitability.
Expertise and infrastructure to capture global opportunities.
Highly seasoned management team with aligned interests.
Cultivation Expertise Affirmed Through Partnership with a Hydroponic Products Industry Leader
Our cultivation team members have extensive experience with the production of high quality indoor cannabis. They have designed, built and operated over 15 cannabis production facilities, in aggregate, across multiple teams and under a variety of stringent regulatory structures, including the MMAR, the MMPR and the ACMPR. We believe the team’s robust cultivation-related proprietary know-how and trade secrets create a long-term strategic advantage relative to competitors - particularly as awareness grows about the high degree of difficulty involved in the consistent production of high quality cannabis.
Our cultivation infrastructure has been specifically designed to execute on our strategy to produce the highest quality cannabis flower, at the highest levels of profitability, for each of our products. For our dried cannabis flower products we use only our highest quality, premium cannabis flower. We believe that the use of premium, indoor-grown flower as an input is critical to dried cannabis flower products and important to our "Cannabis Connoisseur" target market. Flowr’s branding as a premium cannabis supplier is built on the superior quality of our indoor-grown dried cannabis flower. For products such as vapes, capsules and beverage infusions, we recognize that the quality tier of the underlying flower is less strict relative to dried cannabis flower products. We will therefore apply our cultivation expertise to produce high quality flower at lower costs from our future outdoor and greenhouse facilities to produce branded products that are expected to be market-leading.
The caliber of Flowr’s cultivation expertise was validated by Flowr’s 2018 selection as a R&D partner of Hawthorne. Hawthorne is a subsidiary of The Scotts Miracle-Gro Company, a leading supplier and distributor of lawn, garden and hydroponics products. Hawthorne conducted a review of Canadian federal cannabis licence holders in search of a partner with cultivation expertise and the ability to conduct first rate R&D work. Hawthorne’s search was driven by a strategic imperative to engage a federal cannabis licence holder capable of producing industry-leading yields and quality in order to create successful case studies highlighting the effectiveness of Hawthorne’s cultivation products. Hawthorne ultimately entered into a R&D alliance with Flowr to construct a R&D facility.
Upon completion of the Acquisition, we believe that our industry-leading R&D capabilities across the combined company’s global footprint will provide a sustainable competitive advantage. Insights drawn from our understanding of the full genetic potential of the cannabis plant have supported and will continue to support optimized cultivation yields, highly economic and efficient production of quality derivative products and the development of new product formats to bring to evolving markets.
Facilities Designed to Maximize Yield and Profitability
Similar to a real estate asset, we believe that profitability is influenced by efficiency per square footage of operations. Given Flowr’s proprietary cultivation knowledge and system utilized for growing, we expect our yields to increase with time through the optimization of growing conditions for each strain. We expect that the resulting operating leverage coupled with the premium pricing will further enhance our profitability.
We believe we can continue to achieve yields per square foot which we believe exceed industry norms through further "dialing in" the process by which the production team incrementally enhances its understanding of the nuances of the room and the environmental controls to maximize yields for each cultivar. In addition, through our R&D partnership with Hawthorne, we will have access to an array of systems, products and capabilities that may be developed within the partnership that we expect could help lower operating costs and/or increase yields resulting in incremental margin expansion. In the aggregate, we expect the compounding effect of implementing these incremental, steady improvements will result in higher yields that will drive stronger margins.
We believe we can leverage our expertise in maximizing cultivation yields at the K1 Facility to our and Holigen’s other global indoor and outdoor facilities. We will design and build the K2 Facility, and continue to build Holigen’s Sintra and Sydney indoor facilities to the same exacting standards and with the same focus on operating efficiency and cost containment as our K1 Facility. We believe this will provide us with powerful operating efficiencies and yield advantages, driving profitability across all of our global assets, regardless of cultivation format.
Highest Quality Products and Premium Brands Further Drive Profitability
We are building the Flowr® brand on the foundation of premium dried cannabis flower. Our recreational products target the "Cannabis Connoisseur". We believe that by consistently delivering high quality cannabis flower products, we will develop a relationship of trust with our consumers and establish a premium brand position which can be directly extended to premium derivative products such as vapes, infused beverages, capsules and edibles as they become regulated for commercial production and sale.
Our strategy for growing and supplying premium cannabis products starts with the selection of cannabis genetics. We screen for and select cannabis cultivars based on what we consider to be the optimal genetic profile, factoring in growth characteristics, cannabinoid levels, terpene profiles, and anticipated distinctiveness of the end product to consumers. Based on this screening process, we cultivate multiple genetic cultivars in order to curate a targeted portfolio across consumers, usage occasions and product formats.
Based on data received from the OCS and Nova Scotia Liquor Corporation, Flowr’s products in these provinces' recreational markets have achieved retail price points ranging from a 25% premium to prevailing average pricing to as high as a 55% premium with an average premium of 40% between October 17, 2018 to June 16, 2019. We expect to sustain significant premiums over the medium to long term. Flowr’s medical FlowrRx® dried cannabis flower products are currently the highest priced dried cannabis flower on the Medical Cannabis by Shopper’s Drug Mart Inc. website.
Our industry-leading price points are reflected in recent Ontario and Nova Scotia sales data from October 17, 2018 to June 16, 2019, as illustrated below:

Since comparable recreational sales data is not currently available from the other provinces in which Flowr’s products are sold (British Columbia, Manitoba and Saskatchewan), we cannot provide similar retail price point comparisons for these provinces at this time.
We believe that our premium price position in Canada will be sustainable over the medium to long term based on our consistent product quality and our premium brand. We further believe that this expectation aligns with the dynamics we observe in California, where producers of high quality cannabis have consistently achieved sustained premium prices, even as lower-quality cannabis flooded the market. Although the supply of low-priced cannabis has driven down the average price per gram, consumers in California still show a willingness to pay materially higher prices for higher quality cannabis. Based on 2018 California sales data provided by BDS Analytics, we estimate that approximately 40% of the total volume and 56% of the aggregate dollar value of dried cannabis flower is transacted at price points in excess of US$10. We believe that this shows a sustainable willingness of consumers to pay premium prices for higher quality cannabis.
Expertise and Infrastructure to Capture Global Opportunities
We believe our industry-leading cultivation expertise and premium brands can be leveraged across the combined company’s global portfolio of cultivation and marketing assets to execute market penetration strategies specifically tailored to each of the markets and customers we intend to target.
In Europe and Australia, upon closing of the Acquisition, Holigen will leverage Flowr’s cultivation expertise to design and build industry-leading outdoor facilities principally focused on producing quality cannabis produced cost-effectively for extraction into APIs in the medical markets. We believe the combined company’s expertise on the cannabis plant and on extraction techniques will be instrumental in securing a highly advantaged position in the European market, which is primarily oil-based. We believe that this expertise, combined with Holigen’s experience in pharmaceutical opiate extraction and pharmaceutical grade construction design, will enable the combined company to produce cannabis products and formulations that optimally address patients’ medical needs.
In Aljustrel, Portugal, Holigen is expected to construct and operate a cannabis cultivation site that is expected to have a combined potential production, manufacturing and R&D area of more than 7,000,000 square feet and potential annual dried cannabis flower capacity of approximately 500,000 kilograms.
In Australia, the combined company will leverage the Holigen brand to offer medical cannabis products. Cannabis for this market will be sourced from Holigen’s low cost operations in Portugal as well as the indoor cultivation facility in Sydney which will produce high quality dried cannabis flower.
Holigen’s Sintra, Portugal and Australia facilities also expect to have sophisticated R&D and laboratory capabilities, focused principally on the development of market-leading extraction capabilities to bring APIs and oil-based medical products to market in their respective geographies.
Upon completion of the Acquisition, the combined company expects that Holigen’s assets in Europe and Australia will serve as a platform for further global expansion. As a trusted trade partner of many Asian nations, we believe that Australia is well positioned to be a lead exporter of cannabis products to the Asia-Pacific region, and that we will be able to capitalize on such opportunities to enter new markets in Asia if and when regulatory regimes in these regions regulate cannabis.
Highly Seasoned Management Team with Aligned Interests
Flowr’s Board of Directors and executive management team is made up of former government officials, pharmaceutical and consumer goods executives, and executives with significant capital markets and M&A experience. In particular, Flowr’s Board members have consumer goods experience with companies such as The Coca-Cola Company, Interbrew, Campbells, McCain Foods and Frito-Lay. Flowr’s Chairman and its CEO, among others on the team, were early investors in both private and publicly traded cannabis firms in multiple legal markets. Through these experiences, they developed an understanding of the cannabis markets and the critical strategic factors essential for success in such markets.
We believe the strength and track record of the executive leadership team, combined with the operational excellence of the design, construction and cultivation team, will position Flowr for global expansion, access to capital and the successful execution of its strategic priorities.
With the integration of Holigen, Flowr will also gain the talents of a highly skilled management team. The Holigen team has extensive experience in the pharmaceutical, distribution and construction industries. Through senior engineering roles in the opiate industry, the team has gained valuable cultivation and extraction experience. The Holigen team includes senior GMP specialists, construction executives and regulatory pharmacists experienced in dealing with European regulatory environments and pharmacopeia. Flowr believes this expertise will provide it with significant operational advantages as cannabis markets develop globally. The Holigen team’s equity position in Flowr provides it with shareholder and company alignment.
Insiders, led by Steve Klein and Vinay Tolia, have invested approximately $32 million in the Corporation, and collectively own approximately 60% of the Corporation on a fully diluted basis (excluding equity incentives such as options, warrants and/or restricted share units) prior to completion of the Offering and the Acquisition.
STRATEGIES FOR GROWTH
Our advantages in cultivation, yields, premium products and management provide Flowr with opportunities to grow our business and realize our vision of being a global leader in the cannabis industry. We are focusing on driving growth in the business by executing three critical initiatives:
Expanding cultivation and production capacity at the Kelowna Campus in Canada.
Developing cultivation and production capacity at European and Australian campuses upon completion of the Acquisition.
Launching new products which leverage our core strengths.
Expand Cultivation and Production Capacity at the Kelowna Campus in Canada
We believe that demand for high quality cannabis products will continue to exceed supply in the medium to long term. To address this demand, we continue to invest in and build out our cultivation and production infrastructure.
Currently, 40% of the K1 Facility is fully licensed and operational. By the end of the third quarter of 2019, we expect construction of the K1 Facility to be completed. When fully licensed and operational, the K1 Facility will have 20 individually-controlled grow rooms, with a targeted production capacity of 10,000 kilograms of dried cannabis flower on an annualized basis.
Subject to available funding from this Offering and the ATB Credit Facilities, we intend to break ground on the adjacent K2 Facility prior to the first half of 2020 and, subject to licensing, expect to complete the K2 Facility in the second half of 2021/calendar year 2022 though certain grow rooms will come online and be operational at partial capacity prior thereto. When fully licensed and operational, the K2 Facility will comprise approximately 80 individually controlled grow rooms with a targeted production capacity of 40,000 kilograms of dried cannabis flower on an annualized basis. Collectively, the state-of-the-art K1 Facility and K2 Facility are expected to have an annual production capacity of 50,000 kilograms of premium dried cannabis flower.
In addition, when fully constructed, the Flowr Forest will consist of approximately 14 acres of land for outdoor/greenhouse grow cannabis cultivation, primarily to service the Canadian market for premium derivative products such as vapes, infused beverages, capsules and edibles, once these product formats are regulated for sale. We expect, subject to obtaining necessary licences and permits, that the Flowr Forest will have a targeted annual production capacity of 10,000 kilograms of dried cannabis flower.
Develop Cultivation and Production Capacity at European and Australian Campuses Upon Completion of the Acquisition
In Aljustrel, Portugal, Holigen is expected to build and operate a cannabis cultivation site, which is expected to have a combined potential production, manufacturing and R&D area of more than 7,000,000 square feet and licensed annual dried cannabis flower capacity of approximately 500,000 kilograms, including processing and manufacturing facilities expected to be GMP-certified in 2020. It is expected that the majority of cannabis product that the combined company will distribute to the European and Australian markets will be cultivated at this site. Holigen expects to produce cannabis at the Aljustrel site at some of the lowest production costs in the world. These European operations will also include an indoor cultivation and manufacturing facility in Sintra, Portugal. When constructed, this facility will include smaller indoor growing areas for high quality indoor grown cannabis, and manufacturing and packaging areas to be used for extraction and packaging of cannabis grown in Portugal. The Australian facility is licensed to cultivate and manufacture medical cannabis products, and was granted GMP approval by the Therapeutic Goods Administration for labelling, secondary packaging and release for supply in November 2018. Final GMP approval for manufacturing at the Australian facility is expected in 2020.
Launch New Products which Leverage our Core Strengths
We believe the brand position of our premium dried cannabis flower products produced in Canada will provide a "brand halo" which will allow us to successfully extend into new product markets based on derivative formats which are pending regulation for sale. We believe that the addition of these new consumer-friendly formats, when regulated for sale, will expand the size of our addressable market, because convenient, discreet and precisely dosage-metered products may bring in new consumers less familiar with cannabis and less accustomed to smoking or vaping dried cannabis flower.
The new classes of cannabis become saleable on October 17, 2019, subject to federal cannabis licence holders providing 60 days’ notice that a new product will be sold, as required by the Cannabis Regulations, which should result in cannabis products from within the new classes of cannabis being available for sale in both medical and recreational markets at the end of December, 2019 at the earliest.
Accordingly, in preparation for the regulation of these additional form factors following the legislative amendments expected later this year, we are investing in the buildout of a state-of-the-art, purpose-built extraction, formulation and packaging facility that will enable us to launch these new derivative products. We have leased approximately 12,000 square feet in an offsite Kelowna facility outfitted with a full test kitchen, permitting the rapid development of edible cannabis products as well as our understanding of extracts and fractionation techniques.
We are in the process of developing a Flowr branded vape product that we expect to market to recreational consumers, when regulations permit. We plan to extract all oils and resins in-house to ensure that we are able to create differentiated products that are consistent with our positioning in the dried cannabis flower market. We expect our extraction and proprietary curing processes to generate an output that is nearly identical to the chemical profile observed in cannabis from a sensory perspective. Our initial vape product is designed with quality hardware to deliver a flavour profile that is consistent throughout the use of an individual cartridge and is expected to have low failure rates. We anticipate being able to bring a vape product to market in line with the recently announced Government of Canada timeline for new classes of cannabis to be regulated for commercial production and sale.
As observed in legal U.S. markets, we believe that the new product formats will ultimately command a meaningful portion of the cannabis market in Canada, without significantly cannibalizing demand for high quality dried cannabis flower. Deloitte estimates that the annual Canadian market for cannabis products from the new classes of cannabis is worth approximately $2.7 billion, comprised of edibles ($1.6 billion), cannabis-infused beverages ($529 million), topicals ($174 million), concentrates ($140 million), tinctures ($116 million), and capsules ($114 million); with almost one in four Canadian likely to participate in the consumption of these products. The upcoming regulations are expected to create significant growth opportunities for cannabis producers in Canada.
In addition, upon completion of the Acquisition, we expect to launch a portfolio of medical and wellness cannabis derivative products designed for the European and Australian markets under the Holigen brand.
THE ACQUISITION OF HOLIGEN
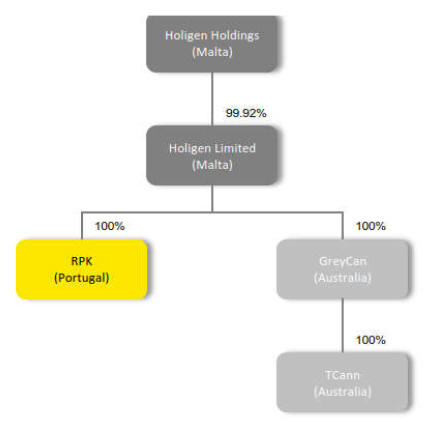
Overview of the Acquisition
On December 19, 2018, Flowr Okanagan entered into a licence agreement with certain subsidiaries of Holigen and loaned $6,000,000 to Pleiades Trading Ltd. and DFT Trading Limited (together, the "Vendors"). On June 3, 2019, the Corporation completed an initial purchase from DFT Trading Limited of 180 Ordinary "A" shares of Holigen for €1.00 per share and on June 18, 2019, the corporation completed a subscription for an additional 72 Ordinary "A" shares of Holigen in satisfaction of the debt of $6,000,000 pursuant to the Amended and Restated SPSA. After giving effect to the initial purchase and subscription, the Corporation owns 19.8% of the outstanding shares of Holigen on a fully diluted basis. Pursuant to the Amended and Restated SPSA, the Corporation is permitted to nominate one director to the board of directors of each of Holigen and Holigen Subco, which nominee director is, initially, Vinay Tolia. The Corporation has agreed not to, for a period of three years from the closing date of the initial purchase and subscription, sell, transfer or otherwise dispose of the purchase and subscription shares without the prior written consent of the board of directors of Holigen (excluding the vote of Vinay Tolia or such other director nominee of the Corporation from time to time). In the event Holigen desires to approve a sale of shares to a third party, then the Corporation will have, in its sole discretion, the option to transfer the purchase and subscription Shares to such third party. The Amended and Restated SPSA also governs the conduct of Holigen and the shareholders of Holigen on a go-forward basis and, as such, contains certain pre-emptive, tag-along, and drag-along rights in respect of Holigen.
Pursuant to the Holigen Purchase Agreement, the Corporation has agreed to acquire the Purchased Shares. The Purchase Price is expected to be comprised of (i) the Share Consideration; (ii) the Cash Consideration; and (iii) the Loan Consideration. See "Details of the Acquisition".
The goodwill associated with the Acquisition includes the expected synergies to be realized between Holigen and the Corporation including increased production capacity, international presence and reach, and target revenue and profitability growth. Management reasonably believes that Holigen's potential large scale grow operations in the foreseeable future (including its Aljustrel site which is expected to have approximately 5,060,000 square feet of outdoor growing area and potential annual dried cannabis flower capacity of approximately 500,000 kilograms) combined with low cost operations, and access to Holigen's distribution partners in Europe and Australia, will generate significant cash flows. The Corporation has estimated the recoverable amounts of goodwill utilizing net present value calculations based on assumptions that included internal net cash projections of the Holigen assets and an average cost of capital that ranged from 15% to 20%. The Corporation currently does not anticipate any factors that would cause the recoverable amount of goodwill estimated by the Corporation to be less than the goodwill amount recognized. Furthermore, the Corporation reasonably believes that its expertise in designing and constructing cultivation facilities will de-risk Holigen, including the risk of an impairment to the associated goodwill, as Flowr's know-how is expected to be transferred to the Holigen business units.
General Description of the Holigen Business
Holigen was formed in 2018 to bring together RPK and TCann to create an international cannabis company with access to medical markets in Europe, Australia and surrounding countries, where sales of cannabis are permitted. The current business of Holigen is conducted through its operating subsidiaries RPK and TCann. TCann is an Australian company that holds certain Australian cannabis cultivation and manufacturing licences and a lease for a previous GMP pharmaceutical facility in Sydney. RPK, headquartered in Lisbon, Portugal, has a cultivation, extraction, import and export licence for cannabis as issued INFARMED for its facility in Sintra, Portugal. RPK also leases certain land in Aljustrel, where it proposes to operate both an indoor and outdoor grow facility.
In connection with the SPSA, Flowr agreed to provide Holigen with access to its startup plant genetics and intellectual property under the License Agreement for use at Holigen’s operations in Europe and Australia. The License Agreement also provides Holigen with access to design and construction know-how and standard operating procedures to enable Holigen to begin construction of its facilities in Portugal and Australia to GMP standards. Holigen has finalized the first stage of facility designs in Australia and Portugal and is in the process of commencing the construction/fit-out of same.
It is expected that all of Holigen's planting milestones will be completed within three years of the closing of the Acquisition. Holigen expects to begin planting in the second half of 2019, primarily using clones supplied by the Corporation and the use of the Resource Commitment (as defined below) provided by the Corporation pursuant to the Conversion Agreement (as defined below). See "Details of the Acquisition - Conversion Agreement - Milestones & Resource Commitments" for additional information regarding Holigen's planting milestones.
Facilities
Sintra, Portugal
Holigen will start its cultivation, processing and GMP manufacturing operations from the Sintra facility in Lisbon. The interior of this facility is currently under construction which is being completed in stages. We expect Holigen to be inspected by INFARMED by August 2019 and, assuming no major non-conformities in the inspection, to receive its license for manufacturing of cannabis in the third quarter of 2019. We anticipate Holigen to complete construction of this facility, and supporting infrastructure, in the second half of 2019. Upon completion of construction, this facility will total approximate 21,500 square feet, of which approximately 9,300 square feet will be dedicated to cultivation in addition to the clone and mother rooms. The balance of the space will be divided between distribution and manufacturing. The bulk of the cannabis plant product for manufacturing is expected to come from Holigen’s outdoor cultivation operations in Aljustrel (south of Lisbon).
The Sintra facility was designed in a manner that is intended to comply with GMP standards for the production of narcotics and other pharmaceuticals, mandated by INFARMED and other European Health Authorities. The quality of the Sintra facility and its ability to meet these standards will play an important role in the ability of Holigen to accommodate expanding markets. The expert and hands-on input of the Flowr design and cultivation teams is expected to accelerate Holigen’s ramp-up and delivery of the economies that can be achieved through outdoor grow. See "Our Current and Proposed Cultivation and R&D Facilities - Europe"
Aljustrel, Portugal
Holigen has completed the construction of the security fencing and installation of the security system of its outdoor cultivation site, located in Aljustrel. INFARMED has completed its outdoor inspection of the site and Holigen expects to receive its cultivation licence in July 2019. This site has combined potential production, manufacturing and R&D area of more than 7,000,000, of which, Holigen holds a lease, expiring on August 20, 2025, on the land on which the Aljustrel site is located.
In addition to the above-mentioned site, Holigen is targeting the end of the second half of 2019 to commence construction of a manufacturing and greenhouse cultivation facility in Aljustrel. This facility is expected to add approximately 250,000 square feet of cultivation area (not including the clone and mother rooms) and approximately 86,000 square feet of processing and GMP manufacturing. The inspection by INFARMED to grant the licence for cultivation and manufacturing is targeted to occur in mid-2020. See "Our Current and Proposed Cultivation and R&D Facilities - Europe"
Australia
Holigen has been licensed by the Office of Drug Control (the "ODC") to cultivate and manufacture medical cannabis products, and was granted GMP accreditation by the Therapeutic Goods Administration for labelling, secondary packaging and release for supply in November 2018.
Subject to available funding from this Offering, phase 1 construction at the Australian facility is expected to be finalized in the first quarter of 2020. We expect Holigen to have its full manufacturing facility in Australia inspected to GMP before the first quarter of 2020. Upon completion of construction, inspections, permitting and licensing, we expect Holigen to start manufacturing in Australia by the first half of 2020. See "Our Current and Proposed Cultivation and R&D Facilities - Australia".
Licensing
Sintra, Portugal
Below is a list of the current licences and permits that Holigen holds in Sintra, Portugal through its subsidiary RPK:
- Permits for importing seeds issued by INFARMED on November 28, 2018; and
- Cultivation, Extraction, Import and Export licence for medical cannabis issued by INFARMED on February 1, 2019.
Below is a list of the current activities that Holigen is undertaking in Sintra, Portugal, through RPK, in order to commence sales of medical cannabis products through this site:
- Inspection by INFARMED for licensing for manufacturing and wholesale of cannabis for medical use, which is expected after construction of the Sintra facility to allow GMP operations. Holigen expects this to be completed in the third quarter of 2019; and
- Licence for manufacturing of cannabis and derived products for medical use. To be issued by INFARMED upon completed inspection without major non-conformities. Holigen expects this to be completed in the third quarter of 2019.
Through Holigen, the Corporation will have a Cultivation, Extraction, Import and Export licence for medical cannabis issued by INFARMED and will therefore have the necessary licences to export cannabis products to countries where importation and sales of such products are legally permitted. Please see the "Regulatory Framework" section of this Prospectus for further information regarding the regulatory framework the Corporation operates in which permits the export of cannabis products from Portugal.
Aljustrel, Portugal
Below is a list of the current activities that Holigen is undertaking in Aljustrel, Portugal, through RPK, in order to commence sales of medical cannabis products in Portugal through this site:
- Licence for cultivation and extraction of medical cannabis. Holigen expects these licences to be issued by INFARMED in 2020.
Australia
Below is a list of the current licences and permits that Holigen holds in Australia through its subsidiary TCann:
- Medical cannabis licence for cultivation of cannabis plants and production of cannabis or cannabis resin issued by the ODC on March 29, 2018, expiring April 2, 2020;
- Cannabis research licence issued by the ODC on March 29, 2018, expiring April 2, 2020;
- Manufacture licence for specified activities relating to the manufacture and supply of extracts and tinctures of cannabis and cannabis resin issued by the ODC on November 23, 2018, expiring November 23, 2019; and
- GMP licence to manufacture therapeutic goods authorizing the labelling, secondary packaging and release for supply of cannabis products for therapeutic use issued by the TGA on November 13, 2018, subject to on-going audit by the TGA.
The Corporation and Holigen are in frequent contact with the applicable regulatory authorities in respect of the above licences and permits and continuously prepare with their regulatory teams to ensure that all licences and permits are renewed prior to expiry. The Corporation and Holigen expect that the above licences and permits will be renewed by the applicable regulatory authorities prior to the expiry dates noted above. See "Risk Factors - Reliance of the Corporation on Licensing."
Below is a list of the current activities that Holigen is undertaking in Australia, through TCann, in order to commence sales of medical cannabis products in Australia:
- Medical cannabis permit, expected to be issued by the ODC in the third quarter of 2019;
- Cannabis research permit, expected to be issued by the ODC in the third quarter of 2019;
- Manufacture permit for cannabis, expected to be issued by the ODC in the first quarter of 2020;
- New South Wales S8 and Wholesale License, expected to be issued by the State of New South Wales in the first quarter of 2020; and
- GMP license to manufacture therapeutic goods, expected to be issued by the TGA in first quarter of 2020.
Production and Development
Holigen believes that its facilities for both indoor and outdoor cultivation will provide a significant competitive advantage over other cannabis producers, on the basis that they were designed by a team of experienced pharmaceutical engineers with the support of Flowr, combining decades of experience in (i) designing and building pharmaceutical facilities compliant with GMP standards, (ii) developing, validating and registering of pharmaceutical products, (iii) designing and building cannabis cultivation facilities, and (iv) cultivating cannabis plants. This combination of construction, design, cultivation and pharmaceutical products development and validation and registration experience should enable Holigen to have significant control over the cultivation environment, complying with the most stringent pharmaceutical regulations applicable to the global cannabis industry. Holigen’s facilities will include industry-leading security systems, climate control, lighting, cultivation systems, and other proprietary cultivation equipment to realize operational efficiencies.
Acquisition Rationale
The Corporation believes the Acquisition is highly complementary to its existing business and is expected to result in the following significant strategic and financial benefits to Flowr:
Platform for International Expansion and Distribution
Flowr expects that Holigen’s strategic location in Portugal will provide it with the capability to supply customers across the European Union, where sales of cannabis are permitted. The Corporation believes that Holigen’s operations within the European Union will facilitate easier, and lower cost supply to countries across the region that have frameworks for legal sales of cannabis. Similarly, Flowr expects Holigen’s operations in Australia to provide local production capabilities that can better serve Australia and surrounding countries, where sales of cannabis are permitted.
Access to Low Cost Production at Significant Scale
Holigen's Aljustrel outdoor cultivation site in Portugal is expected to provide Flowr with approximately 65 hectares of potential outdoor growing capacity, which Flowr expects to be fully licensed by the end of the third quarter of 2019. At its fully built-out licensed capacity, this location is expected to have approximately 5,060,000 square feet of grow space and potential annual dried cannabis flower capacity of approximately 500,000 kilograms. Flowr expects that outdoor production at Aljustrel can be cultivated at some of the lowest production costs in the world. Outdoor cultivation will provide Flowr the ability to produce high volume, low cost cannabis that can serve as feedstock for the production of cannabis oil for export globally.
Holigen provides Flowr with an operational foundation in two strategic locations in Portugal and Australia that grants the combined company, upon completion of the Acquisition, the ability to target the European and Australian medical cannabis markets. Once complete, assuming full funding for its facilities, Flowr expects these operations to have potential capacity to grow approximately 500,000 kilograms of dried cannabis flower annually.
The combined potential production, manufacturing and R&D area at Holigen's Aljustrel, Portugal site is more than 7,000,000 square feet, of which 72%, or approximately 5,060,000 square feet, is expected to be used as canopy or net grow space. The Corporation believes that the anticipated yield in the outer years will reach 100 grams per square foot per year. With over 5 million square feet of canopy at a targeted yield of 100 grams per square foot per year, the anticipated output of cannabis from Holigen's Aljustrel, Portugal site is expected to be approximately 500,000 kilograms (5,000,000 multiplied by 100 grams/sq.ft./year divided by 1,000).
Flowr expects that the combination of its experience designing and producing highly controlled, consistent indoor growing environments, with large outdoor capacity will enable it to produce significant volumes of premium dried cannabis flower, as well as a low cost, high volume feedstock to be extracted and utilized in products targeting the global medical market. The strategic location and the anticipated demand in both locations are expected to provide Flowr with significant international growth for multiple years.
Experienced Management Team
Holigen is led by seasoned industry executives Pauric Duffy (co-founder and sole initial private investor in Holigen) and Peter Comerford and a management team with over 40 years of combined experience across multiple industries that are required for a cannabis operation, including construction, life science, distribution and medicine.
Management incentives are aligned with the success of the combined Corporation due to the contemplated earn-out structure of the transaction, large equity component of the purchase consideration and lock-up provisions in place.
Favourable and Stable Climate for Growing
Flowr believes that Holigen’s large scale outdoor production in Portugal could produce one of the lowest cost cannabis cultivation opportunities in the world due to Portugal’s favorable climate and labour conditions in combination with Flowr’s cultivation expertise. Benefitting from Portugal’s climate, cost-effective land and labour, and the high crop yields it expects to generate, Flowr believes Holigen could position itself to be among the lowest cost cannabis producers in the world. Portugal and Australia also provide stable political and regulatory environments.
DETAILS OF THE ACQUISITION
The following is a summary of the material terms of the Holigen Purchase Agreement and associated agreements and share terms. This summary does not purport to be complete and is subject to, and is qualified in its entirety by reference to, the provisions of such agreements or terms. A copy of the Holigen Purchase Agreement, which the Corporation considers to be a "material contract" (as such term is defined pursuant to National Instrument 51-102 - Continuous Disclosure Obligations) has been filed on SEDAR at www.sedar.com. The other material contracts and the terms of the Series 1 Preferred Shares will be filed on SEDAR on or after the Acquisition Closing Date pursuant to applicable law and reference should be made to such documents.
The Holigen Purchase Agreement
On June 24, 2019 the Corporation entered into the Holigen Purchase Agreement with the Vendors, and Pauric Duffy, Peter Comerford, Pleiades Holdings Ltd. and DFT Holdings Limited. (as guarantors) (together, the "Guarantors") pursuant to which the Corporation has agreed to acquire the Purchased Shares. On July 10, 2019, the parties agreed to extend the outside date to July 31, 2019, or such other date as agreed to by the parties. The Purchase Price is expected to be comprised of (i) an amount equal to the number of Consideration Shares multiplied by the Issue Price (as defined below) (the "Share Consideration"); (ii) an amount equal to the Canadian dollar equivalent of €4,269,927.31 (the "Cash Consideration") based on the Bank of Canada exchange rate on the business day prior to the closing date of the Acquisition (the "Acquisition Closing Date"); and (iii) an amount equal to the Canadian dollar equivalent of any amounts loaned by Pauric Duffy (or certain parties related to Pauric Duffy) to Holigen or a subsidiary of Holigen (the "Target Corporations") between the dates of January 1, 2019 and the Acquisition Closing Date (the "PD Loans"), up to a maximum amount of $2,000,000 (the "Loan Consideration").
82
The Share Consideration is expected to be satisfied by the issuance of Consideration Shares to the Vendors at a deemed share price of $7.15, being the volume weighted average price of the Common Shares as traded on the facilities of the TSX.V for the five (5) trading days immediately preceding the date of the Holigen Purchase Agreement, as may be adjusted if required by the TSX.V.
At closing, 3,263,255 Consideration Shares will, in accordance with their terms, automatically convert into Common Shares. The remaining 29,369,290 Consideration Shares will be delivered to a third party escrow agent (the "Escrow Agent") and held in escrow and released therefrom in accordance with an escrow agreement dated the Acquisition Closing Date (the "Escrow Agreement"). The escrow release will be triggered upon the satisfaction of certain milestones. See "Terms of Consideration Shares - Conversion into Common Shares".
The Cash Consideration and Loan Consideration are expected to be satisfied by a wire payment on the Acquisition Closing Date as directed by the Vendors.
In addition, within ten (10) business days of the Acquisition Closing Date, Flowr has agreed to pay the aggregate amount of €1,378,106.53 to certain creditors of the Target Corporations.
The Purchase Price is subject to adjustment based on the working capital of Holigen on the Acquisition Closing Date.
Representations, Warranties and Covenants
Pursuant to the Holigen Purchase Agreement, the Vendors have given to the Corporation customary representations and warranties on the Holigen business and other matters, subject to customary exceptions and limitations.
The Vendors have also agreed that, until the Acquisition Closing Date, they will operate the business of Holigen in the ordinary course (including maintaining all licences required to operate the business) and, subject to certain exceptions, also refrain from taking certain actions including incurring additional indebtedness, issuing any securities and materially increasing compensation entitlements for directors, officers and employees of Holigen and its subsidiaries, among other covenants as set out in Article 6 of the Holigen Purchase Agreement.
Pursuant to the Holigen Purchase Agreement, the Corporation has agreed to appoint Mr. Duffy to the Board on the Acquisition Closing Date, subject to TSX.V approval.
The Vendors and the Guarantors have agreed to indemnify the Corporation and its shareholders, Directors, officers, employees, agents and representatives harmless from damages resulting from certain specified matters in connection with the Acquisition. The aggregate liability of the Vendors and the Guarantors for a breach of a representation or warranty is limited to 30% of the Purchase Price, except in the case of liability arising from a breach of certain fundamental representations of the Vendors, in which case aggregate liability is limited to the Purchase Price. No claims for damages may be made unless the aggregate amount of damages for which the claiming party is entitled to be indemnified exceeds 1% of the Purchase Price (the "Deductible") in which event the accumulated aggregate amount of all damages in excess of the Deductible may be recovered by the claiming Party, subject to the caps set forth in the previous sentence. See "Risk Factors".
Conditions of Closing
The closing of the Acquisition is subject to, among other things, the TSX.V’s conditional approval of the listing of the Common Shares that may be issued upon conversion of the Consideration Shares, the closing of this Offering, and no legal proceedings being pending or threatened that would restrict or prohibit the Acquisition or the ability of the Corporation to conduct its business or the business of Holigen after the closing of the Acquisition. If the closing of the Acquisition has not occurred on or before July 31, 2019 (unless such date is extended by the parties), Flowr or the Vendors may terminate the Holigen Purchase Agreement.
On the Acquisition Closing Date, the Corporation will enter into the Governance Agreement and the Conversion Agreement, each as described below.
Terms of Consideration Shares
In connection with the Acquisition, the Corporation will designate the first series of preferred shares as "Series 1 Voting Convertible Redeemable Preferred Shares" (the "Series 1 Preferred Shares"). Such shares will constitute the "Consideration Shares".
Conversion into Common Shares
Subject to the terms of the Conversion Agreement (as defined below):
(i) immediately after the issuance of the Series 1 Preferred Shares on the Acquisition Closing Date, ten percent (10%) of the aggregate number of Series 1 Preferred Shares issued on such date shall automatically convert into an aggregate of 3,263,255 Common Shares;
(ii) forty percent (40%) of the aggregate number of the Series 1 Preferred Shares issued on the Acquisition Closing Date, as adjusted, shall automatically convert six (6) months from the Acquisition Closing Date into an aggregate number of Common Shares equal to the Conversion Rate (as defined below) multiplied by the number of Series 1 Preferred Shares so converted;
(iii) if the "Regulatory Milestone" (as defined below) has been satisfactorily achieved prior to the third anniversary of the Acquisition Closing Date (the "Three-Year Conversion Expiry Date"), fifteen percent (15%) of the aggregate number of the Series 1 Preferred Shares issued on the Acquisition Closing Date, as adjusted, shall automatically convert on the later of (A) twelve (12) months from the Acquisition Closing Date and (B) the date specified in the applicable conversion notice in respect of the achievement of the Milestone, into an aggregate number of Common Shares equal to the Conversion Rate multiplied by the number of Series 1 Preferred Shares so converted;
(iv) if the Australia Planting Milestone (as defined below) has been satisfactorily achieved prior to the second anniversary of the Acquisition Closing Date (the "Two-Year Conversion Expiry Date", and together with the Three-Year Conversion Expiry Date, the "Conversion Expiry Date"), seventeen and one-half percent (17.5%) of the aggregate number of the Series 1 Preferred Shares issued on the Acquisition Closing Date, as adjusted, automatically convert on the date specified in the applicable conversion notice in respect of the achievement of the Australia Planting Milestone, into an aggregate number of Common Shares equal to the Conversion Rate multiplied by the number of Series 1 Preferred Shares so converted;
(v) if the Portugal Planting Milestone (Aljustrel) (as defined below) has been satisfactorily achieved prior to Three-Year Conversion Expiry Date, ten percent (10%) of the aggregate number of the Series 1 Preferred Shares issued on the Acquisition Closing Date, as adjusted, shall automatically convert on the date specified in the applicable conversion notice in respect of the achievement of the Portugal Planting Milestone (Aljustrel), into an aggregate number of Common Shares equal to the Conversion Rate multiplied by the number of Series 1 Preferred Shares so converted; and
(vi) if the Portugal Planting Milestone (Sintra) (as defined below) has been satisfactorily achieved prior to the Two-Year Conversion Expiry Date, seven and one-half percent (7.5%) of the aggregate number of the Series 1 Preferred Shares issued on the Acquisition Closing Date, as adjusted, shall automatically convert on the date specified in the applicable conversion notice in respect of the achievement of the Portugal Planting Milestone (Sintra), into an aggregate number of Common Shares equal to the Conversion Rate multiplied by the number of Series 1 Preferred Shares so converted.
Notwithstanding the above conversion dates and subject to the Conversion Agreement, if the Corporation does not make the Resource Commitment (as defined below) to Holigen on the dates set forth in the Conversion Agreement, the satisfaction of the milestones ("Milestones") set out above in respect of planting in Australia, Sintra and Aljustrel and in the Conversion Agreement will not be a condition of conversion of the Consideration Shares associated with such planting Milestones.
The conversion rate (the "Conversion Rate") at the Acquisition Closing Date will be 1:1. Such rate is subject to customary adjustments in the event of a subdivision or consolidation of the Common Shares and certain other corporate events.
Notwithstanding the above conversion dates and subject to the Conversion Agreement, if Flowr completes certain fundamental transactions and at the time of the completion of such transaction, any Series 1 Preferred Shares are issued and outstanding, all Milestones shall be deemed satisfied and the achievement of any Milestone shall not be a condition of conversion of the issued and outstanding Series 1 Preferred Shares. Such transactions are: (A) a merger, amalgamation, arrangement, reorganization, or other business combination or similar transaction involving Flowr in which (i) the consent or approval of the Board and the holders of Common Shares is required to complete the transaction, (ii) less than 10% of the total consideration payable pursuant to the transaction is cash consideration, and (iii) the holders of Common Shares on a partially-diluted basis immediately before the completion of the transaction would hold less than 50% of the common shares or other equity securities of Flowr’s successor or of the continuing or surviving entity immediately following the completion of such transaction, assuming the conversion of all of the Series 1 Preferred Shares and Flowr ULC Class A Shares immediately prior to the completion of the transaction; or (B) (i) a share sale transaction that would result in a direct or indirect change of control of Flowr; or (ii) the sale, lease, exchange or transfer of all or substantially all of the assets of the Flowr (in the case of (i) and (ii), to a person that is not an affiliate of Flowr, but for avoidance of doubt does not include an internal reorganization the result of which would have Flowr continue to have ultimate control of such entities). Conversion in the foregoing circumstances would not occur if any redemption notice has been issued in respect of the Series 1 Preferred Shares as permitted pursuant to the Conversion Agreement or if the Corporation has given notice to the holders of the Series 1 Preferred Shares that there are bona fide claims or damages under the Holigen Purchase Agreement relating to or involving fraud, gross negligence, bad faith or wilful misconduct or an intentional misrepresentation, or breach of a fundamental representation of the Vendor.
Voting Rights
Until the Conversion Expiry Date, the holders of Series 1 Preferred Shares are entitled to vote with the holders of outstanding Common Shares, voting together as a single class, with respect to any and all matters presented to the shareholders of the Corporation for their action or consideration, except, in each case, as provided by law. In any such vote, each Series 1 Preferred Share shall be entitled to a number of votes equal to the number of Common Shares into which such Series 1 Preferred Share is convertible as of the record date, or, failing a record date, the date of any such vote (and assuming all such Series 1 Preferred Shares are convertible into Common shares on the applicable date).
Dividends
If the Corporation declares or pays a dividend or distribution on the Common Shares, the Corporation shall simultaneously declare and pay, in preference to any Common Shares or other specifically-designated junior shares (together, "Junior Shares"), a dividend on the Series 1 Preferred Shares on a pro rata basis with the Common Shares based on the number of Common Shares into which an outstanding Series 1 Preferred Share is convertible as of the record date of the applicable dividend or if no record date is fixed, the date as of which the registered holders of Common Shares entitled to such dividends are to be determined (and for such purpose it shall be assumed that all outstanding Series 1 Preferred Shares are fully convertible on the applicable date unless otherwise provided in the Conversion Agreement).
Liquidation, Dissolution and Winding-Up
In the event of the liquidation, dissolution or winding up of the Corporation (whether voluntary or involuntary) or any other distribution of assets of the Corporation among its shareholders for the purposes of winding up its affairs (collectively, a "Liquidation"), the holders of Series 1 Preferred Shares are entitled to be paid out of the assets of the Corporation available for distribution to its shareholders, in priority to holders of Junior Shares, $0.00001 per share held by such Series 1 Preferred Share holder (subject to adjustment) (the "Liquidation Value"). In addition to payment of such preferential amount, the holders of Series 1 Preferred Shares will be entitled to participate with the holders of Junior Shares, pro rata as a single class based on the aggregate number of outstanding Junior Shares on an as-converted basis immediately before the Liquidation, in the distribution of all the remaining assets and funds of the Corporation available for distribution to its shareholders (and for such purpose it shall be assumed that all outstanding Series 1 Preferred Shares are fully convertible on the applicable date unless otherwise provided in the Conversion Agreement).
Redemption
Following the date that is three years from the Acquisition Closing Date, or at any other time permitted pursuant to the Conversion Agreement, the Corporation will redeem all of the then-outstanding Series 1 Preferred Shares for a price per share equal to the Liquidation Value, plus any declared and unpaid dividends, minus any tax required to be deducted or withheld by the Corporation.
Conversion Agreement
In connection with the issue of the Consideration Shares pursuant to the Acquisition, on the Acquisition Closing Date, the Corporation and the Vendors will enter into the share conversion agreement (the "Conversion Agreement") to set out the process for, and conditions to, conversion of the Consideration Shares into Common Shares.
Milestones & Resource Commitments
The following Milestones are set forth in the Conversion Agreement:
The "Regulatory Milestone" shall be considered achieved upon the earlier of (A) the lodging by the Corporation (or one of its affiliates) of a certain regulatory product application, or (B) the lodging by the Corporation (or one of its affiliates) of a different application as agreed by the Corporation and the holders of Series 1 Preferred Shares. In connection with achieving the Regulatory Milestone, if requested by such shareholders, the Corporation has agreed to provide: certain stability data relating to genetic lines put forward for testing in certain laboratories ("Stability Data"), information about the Corporation required or requested by applicable governmental entities ("Information"), genetic materials (e.g. seeds and cuttings) ("Genetics"), and any further information or genetics that in the future may be required by an applicable governmental entity. Notwithstanding the Conversion Expiry Date, (i) the holders of Series 1 Preferred Shares shall have no more than twelve months after receiving the Stability Data, Information and Genetics to submit such application in order for the Regulatory Milestone to be considered satisfied prior to the expiry of the Conversion Expiry Date; and (ii) if the Corporation does not provide the Stability Data within six months after signing the Holigen Purchase Agreement (subject to a six month extension due to a delay by a third party lab) then the Regulatory Milestone shall be deemed satisfied on the date that is six months after the Acquisition Closing Date (as may have been extended) and the applicable Series 1 Preferred Shares to be converted on meeting the Regulatory Milestone shall be subject to conversion into Common Shares.
In the event of any such request(s), the Corporation covenants to appoint the shareholders to complete and submit the applicable application on behalf of the Corporation or one of its affiliates. The shareholders shall advise the Corporation, within two months after the Closing Date, of all required Information and Genetics, subject to changes that may in the future be required as a result of a request made by an applicable Governmental Entity (the "Further Assistance"). In the event that Further Assistance is required as a result of a request made by an applicable Governmental Entity in respect of either (A) or (B), the Parties agree to act reasonably to request and provide such Further Assistance promptly.
Please refer to the Use of Proceeds - Principal Purposes Assuming Completion of the Acquisition of the Prospectus.
The "Australia Planting Milestone" shall be considered achieved upon the planting by the Corporation (or one of its affiliates) in the country of Australia, in compliance with applicable laws, of cannabis plants in the aggregate of over 5,000 square feet of space, within two years of the Acquisition Closing Date.
Please refer to the Use of Proceeds - Principal Purposes Assuming Completion of the Acquisition of the Prospectus.
The "Portugal Planting Milestone (Aljustrel)" shall be considered achieved upon the planting by the Corporation (or one of its affiliates) in the town of Aljustrel, in the country of Portugal, in compliance with applicable laws, of cannabis plants in the aggregate of at least 10,000 square metres of space, within three years of the Acquisition Closing Date.
Please refer to the Use of Proceeds - Principal Purposes Assuming Completion of the Acquisition of the Prospectus.
The "Portugal Planting Milestone (Sintra)" shall be considered achieved upon the planting by the Corporation (or one of its affiliates) in the town of Sintra, in the country of Portugal, in compliance with applicable Laws, of cannabis plants in six rooms of the building located at Avenida Santa Isabel, no. 7, EN 249-4, Sintra, Rio de Mouro, 2635 047, Rio de Mouro, within two years of the date hereof. In respect of the Portugal Planting Milestone (Sintra), the conversion expiry date is within two years of the Acquisition Closing Date.
Please refer to the Use of Proceeds - Principal Purposes Assuming Completion of the Acquisition of the Prospectus.
- Pursuant to the Conversion Agreement, the Corporation has agreed to expend an aggregate of AUD$11,000,000 and €17,000,000 by January 2020 (the "Resource Commitment") consistent with the timing and budget set out in the Conversion Agreement, in order to achieve the Australia Planting Milestone, the Portugal Planting Milestone (Sintra) and the Portugal Planting Milestone (Aljustrel).
Restriction on Transfer
In connection with the issuance of the Series 1 Preferred Shares, the Vendors have agreed not to directly or indirectly, without the prior written consent of the Corporation, or waiver by the Corporation (not to be unreasonably withheld, delayed or conditioned) offer, sell, contract to sell, grant or sell any option to purchase, hypothecate, encumber, pledge, transfer, assign, purchase any option or contract to sell, lend, swap or enter into any other agreement to transfer the economic or voting consequences of, any right, title or interest in, or otherwise dispose of or deal with the Series 1 Preferred Shares or Common Shares (the "Subject Shares"), whether through the facilities of a stock exchange, by private placement or otherwise, except to certain permitted transferees (including the other Vendor, certain family members of a Vendor, or a corporation controlled by any of the foregoing) (each, a "Permitted Transferee").
Governance Agreement
In connection with the Acquisition, Mr. Duffy and his affiliates will indirectly receive, among other consideration, Consideration Shares representing over 10% of the outstanding Common Shares on a partially-diluted basis (except for dilution to account for the conversion of Consideration Shares into Common Shares) immediately after the Acquisition (and prior to giving effect to the Offering). As a result, the Vendors have agreed with the Corporation that, concurrently with the completion of the Acquisition, DFT Trading Limited and Mr. Duffy will enter into a governance agreement (the "Governance Agreement") with the Corporation. Mr. Comerford will not be party to the Governance Agreement or any governance agreement.
Transfer Restrictions
Until the first business day on which Mr. Duffy and his affiliates collectively beneficially own Series 1 Preferred Shares, Common Shares, and other equity rights convertible into shares or equity interests of the Corporation (collectively, "Shares") representing less than 10% of the then issued and outstanding Common Shares on a partially-diluted basis, Mr. Duffy will not transfer in any calendar month an aggregate number of Common Shares representing more than 2% of the then issued and outstanding Common Shares on a partially-diluted basis, other than with the Corporation’s prior written consent (not to be unreasonably withheld, delayed or conditioned). Subject to the foregoing, if Mr. Duffy or an affiliate desires to transfer in any calendar month an aggregate number of Common Shares representing more than 2% of the then issued and outstanding Common Shares on a partially-diluted basis, Mr. Duffy will in good faith engage the Corporation to seek to implement a marketed transfer of such Common Shares, provided that in any event Mr. Duffy will not be prohibited from transferring such Common Shares through a non-publicized pre-arranged off-exchange block trade using a securities dealer that is acceptable to Mr. Duffy and the Corporation (each acting reasonably).
Voting
Subject to compliance with applicable laws, in the event that the holders of Series 1 Preferred Shares are entitled to vote as a separate class on a shareholder proposal, Mr. Duffy has agreed to vote any Series 1 Preferred Shares (and use commercially reasonable efforts to cause all of the Series 1 Preferred Shares owned or beneficially owned by Mr. Duffy or any of his affiliates or over which Mr. Duffy or any of his affiliates has voting control or the power to direct voting control), to be voted in accordance with a recommendation of the Board.
Standstill
Until the first business day on which Mr. Duffy and his affiliates collectively beneficially own Shares representing less than 5% of the then issued and outstanding Common Shares (on a partially-diluted basis), Mr. Duffy and his affiliates will not, directly or indirectly, without the prior written consent or waiver by the Corporation:
(i) acquire, or agree to acquire, or make any proposal to acquire, directly or indirectly, by means of purchase, merger, consolidation, take-over bid, exchange offer, tender offer, business combination, arrangement, amalgamation or in any other manner, whether in one transaction or a series of transactions, any securities or assets of the Corporation or any of its subsidiaries, other than conversion of Series 1 Preferred Shares into Common Shares through the process set out in the Conversion Agreement; or
(ii) undertake certain additional actions that may affect control of or which are hostile to the Corporation (including, among others as set out in the Governance Agreement, offering to enter into any extraordinary transaction involving the Corporation or acquire a material portion of the assets of the Corporation; initiating any shareholder proposals or soliciting proxies, commencing any take-over bid or similar transaction; acting alone or in concert with others to control the Corporation or any of its subsidiaries, assisting with the foregoing or announcing any intention with respect to the foregoing).
Consent Right
Until the earlier of the first business day on which (i) Mr. Duffy beneficially owns Shares representing less than 13% of the then issued and outstanding Common Shares on a partially-diluted basis, and (ii) the conversion or redemption of all of the Series 1 Preferred Shares held by Mr. Duffy and his affiliates, the completion of certain transactions (as set out in the Governance Agreement) by the Corporation will require the prior written consent of Mr. Duffy (not to be unreasonably withheld). This consent right, however, will not restrict the Board from exercising its fiduciary duties to the Corporation.
Board Observer Right
Until the earlier of (i) the first business day on which Mr. Duffy beneficially owns Shares representing less than 10% of the then issued and outstanding Common Shares on a partially-diluted basis, and (ii) the first business day on which Mr. Duffy ceases to be a Director of the Corporation, Mr. Duffy has the right to appoint one non-voting observer (who shall, initially, be Peter Comerford) to attend and observe meetings of the Board, subject to such observer first providing the Corporation a confidentiality agreement in form and content satisfactory to the Corporation, acting reasonably.
Financings
The Corporation has provided a covenant to use commercially reasonable efforts to allocate proceeds of this Offering towards the completion of the Corporation’s projects as set out in an agreed upon budget, including in support of the business and projects of the Target Corporations.
Other Agreements
In connection with the closing of the Acquisition, each Vendor will enter into an Escrow Agreement and a lock-up agreement (each, a "Vendor Lock-Up Agreement") with the Corporation or an affiliate.
Pursuant the Escrow Agreements, the applicable Consideration Shares will be held in escrow with the Escrow Agent until converted into Common Shares pursuant to their terms and the Conversion Agreement, in which case such Common Shares will be delivered to the Vendor (or redeemed pursuant to their terms and the Conversion Agreement, in which case such Series 1 Preferred Shares shall be delivered to the Corporation).
Pursuant to the Vendor Lock-Up Agreements, the Vendors will agree not to undertake a Transfer except to a Permitted Transferee (and not announce any intention to undertake a Transfer) commencing on the Acquisition Closing Date and (i) with respect to 34% of the Subject Shares, continuing through the close of trading the date that is one year after the Acquisition Closing Date; (ii) with respect to 33% of the Subject Shares, continuing through the close of trading on the date that is two years after the Acquisition Closing Date; and (iii) with respect to the remaining 33% of the Subject Shares, continuing through the close of trading on the date that is three years after the Acquisition Closing Date.
In addition, on the Acquisition Closing Date, the Corporation or an affiliate will enter into employment and/or consulting agreements with Mr. Pauric Duffy as Managing Director, Portugal and Mr. Peter Comerford as Managing Director, Australia, generally in accordance with the Corporation’s form of employment and/or consulting agreement.
Parties to the Acquisition
Andre Teixeira is a Director of the Corporation. Since December 2018, Mr. Teixeira has provided Holigen with certain consulting services. As at March 31, 2019, Mr. Teixeira has billed €66,664 for such services. See "Risk Factors - Conflicts of Interest in Respect of Directors and Officers of the Corporation".
SELECTED HISTORICAL AND PRO FORMA CONSOLIDATED FINANCIAL INFORMATION
The following tables set forth Flowr’s selected consolidated financial information (i) for the year ended December 31, 2018, (ii) for the year ended December 31, 2018 pro forma after giving effect to the Acquisition, (iii) for the three months ended March 31, 2019; and (iv) for the three months ended March 31, 2019 pro forma after giving effect to the Acquisition.
The selected unaudited pro forma consolidated financial information set forth below and included elsewhere in this Prospectus is for illustrative purposes only and not necessarily indicative of results of operations that would have occurred as at or for the year ended December 31, 2018 or for the three months ended March 31, 2019 had the Acquisition been effective since January 1, 2018, or of the results of operations expected in 2019 and future years. The following pro forma financial information has been prepared by management of the Corporation on the basis of the information contained in this Prospectus or in documents incorporated by reference herein. This pro forma financial information is not a forecast or a projection of future results. The actual results of the combined operations for any period, whether before or after the closing of the Acquisition, will likely vary from the amounts set forth in the following pro forma financial information and such variation may be material. See "Non-IFRS Measures" and "Risk Factors - Risk Factors Related to the Acquisition".


(1) See Pro Forma Condensed Consolidated Financial Statements attached as Appendix "D" to this Prospectus for details on pro forma adjustments and balances.
(2) Adjusted EBITDA is a non-IFRS measure, calculated as net loss, plus (minus) income taxes (recovery), plus (minus) interest income (expense), net, plus depreciation and amortization, plus share-based compensation, plus (minus) non-cash fair value adjustments on biological assets and inventory sold, plus listing expense costs and plus (minus) loss (gain) on investments.
For the purposes of the pro forma financial statements, the Corporation has not disaggregated the intangible assets of Holigen into smaller asset classes since the Corporation has not, at this time, undertaken the identification of the sub-classes of the ascribed intangible amounts. In accordance with IFRS, the Corporation has up to one year following the closing of the Acquisition to complete the purchase price allocation. Accordingly, during the one year period following the completion of the Acquisition, the Corporation will, to the extent required by IFRS as applied to the Corporation's circumstances, disaggregate intangible assets into smaller asset classes.
PRELIMINARY FINANCIAL RESULTS FOR THE THREE MONTHS ENDED JUNE 30, 2019
The Company has recently completed its second quarter ended June 30, 2019. While complete financial information and operating data for the three months ended June 30, 2019 are not yet available, the Corporation is in a position where it can estimate its top line revenue, working capital balance (excluding cash) and cash position for the quarter with reasonable accuracy, and the Corporation believes that such information will be helpful to an investor in making an informed investment decision. In addition to the preliminary estimates below, the Corporation expects to report a net loss for the quarter ended June 30, 2019.
Set forth below are preliminary estimates of the revenue, working capital balance (excluding cash) and cash position that we expect to report for our three months ended June 30, 2019. Our actual results may differ materially from these estimates due to the completion of our financial closing procedures (which will not occur until after the Closing Date), final adjustments and other developments that may arise between now and the time the financial results for three months ended June 30, 2019 are finalized.
The following are our preliminary estimates for the three months ended June 30, 2019:
- Revenues are expected to be between $2.1 million and $2.3 million.
- Working capital balance (excluding cash) is expected to be between ($2.0) million and ($2.2) million.
- Cash position is expected to be between $9.4 million and $9.6 million
The foregoing estimates, which should not be viewed as a substitute for full interim financial statements prepared in accordance with IFRS, represent the most current information available to management and do not present all necessary information for an understanding of our financial condition as of and the results of operations for the three months ended June 30, 2019. The preliminary estimates included in this Prospectus have not been reviewed or audited by our independent registered public accounting firm. Accordingly, our independent auditors do not express an opinion or any other form of assurance with respect to this preliminary data.
We have provided ranges for the preliminary results described above primarily because our financial closing procedures for the three months ended June 30, 2019 are not yet complete. As a result, there is a possibility that our final results will vary from these preliminary estimates. We currently expect that our final revenue, working capital balance (excluding cash) and cash position will be within the ranges described above, but it is possible, however, that our final results will not be within the ranges we currently estimate. See “Cautionary Note Regarding Future-Oriented Financial Information,” “Caution Regarding Forward-Looking Statements” and “Risk Factors”.
REGULATORY FRAMEWORK
The following summary addresses the primary laws and regulations of Canada, Australia and Portugal that are associated with the cultivation, processing and distribution of cannabis, and the processing and distribution of cannabis products. It does not address the laws and regulations of any other jurisdiction. The Corporation believes that, as of the date of this Prospectus, the Corporation and Holigen are in material compliance with all laws and regulations summarized below and the terms of all permits and licenses (which are in full force and effect) necessary for the conduct of their respective businesses.
Canada
Background
On October 17, 2018, the Cannabis Act and the Cannabis Regulations came into force, removing cannabis from schedule II of the CDSA and regulating sale of cannabis in recreational markets in Canada. Prior to the Cannabis Act and the Cannabis Regulations coming into force, sale of cannabis was regulated for medical purposes only under the ACMPR, the NCR and the CDSA. The Cannabis Act and the Cannabis Regulations replaced the CDSA, the ACMPR and the NCR as the governing laws and regulations in respect of the cultivation, processing, sale and distribution of medical cannabis and further created the regulatory regime and licensing framework for the production, testing, packaging, labelling, sending, delivery, transportation, sale, possession and disposal of cannabis for non-medical (i.e. recreational) use. The Cannabis Act and the Cannabis Regulations maintain federally regulated medical access, and importation and exportation of cannabis for medical or scientific purposes. Each province and territory has implemented programs for the sale of recreational cannabis products, as described below. On October 17, 2018, the Industrial Hemp Regulations, SOR/98-156 of the CDSA were replaced by the Industrial Hemp Regulations, SOR/2018-145 under the Cannabis Act (the "2018 Industrial Hemp Regulations"). Given that the Cannabis Act, the Cannabis Regulations and the 2018 Industrial Hemp Regulations are very recently in force, the impact of such regulatory changes on the Corporation’s business is unknown. See "Risk Factors - Changes in Laws, Regulations and Guidelines".
The purpose of the Cannabis Act is to protect public health and public safety and, in particular, to (a) protect the health of young persons by restricting their access to cannabis; (b) protect young persons and others from inducements to use cannabis; (c) provide for the licit production of cannabis to reduce illicit activities in relation to cannabis; (d) deter illicit activities in relation to cannabis through appropriate sanctions and enforcement measures; (e) reduce the burden on the criminal justice system in relation to cannabis; (f) provide access to a quality-controlled supply of cannabis; and (g) enhance public awareness of the health risks associated with cannabis use.
In furtherance of the purposes of the Cannabis Act in the previous paragraph, the Cannabis Act and the Cannabis Regulations provide a licensing and permitting scheme for the production, importation, exportation, testing, packaging, labelling, sending, delivery, transportation, sale, possession and disposal of cannabis. The Cannabis Act and the Cannabis Regulations regulate both the medical and recreational markets. Import and export permits are only issued in respect of cannabis for medical or scientific purposes or in respect of industrial hemp. The Cannabis Act empowers the Minister, now the Minister of Border Security and Organized Crime Reduction, to establish and maintain a national cannabis tracking system. The Cannabis Tracking and Licensing System (the "CTLS") was established for this purpose and is described under Regulatory Framework - Canada - Cannabis Tracking and Licensing System.
The Cannabis Regulations, among other things, set out requirements relating to the following matters: (1) Licences; (2) Security Clearances; (3) Cannabis Products; (4) Packaging and Labelling; and (5) Cannabis for Medical Purposes. Provincial legislation regulates sale of cannabis products in the recreational market. The Cannabis Act provides for restrictions on promotional activity of cannabis, cannabis accessories and services related to cannabis. The 2018 Industrial Hemp Regulations provide for licensing allowing cultivation, propagation, processing (as defined in the 2018 Industrial Hemp Regulations, and not as defined in the Cannabis Regulations) and sale of industrial hemp. The 2019 Amendments will regulate the new classes of cannabis for sale to consumers as cannabis products in both the recreational and medical markets. Each of these points is briefly summarized below.
Licences
The Cannabis Regulations establish six classes of licences under the Cannabis Act: cultivation; processing; analytical testing; sale to individual clients for medical purposes; research; and cannabis drug production. Subclasses for cultivation (standard cultivation, micro-cultivation and nursery) and processing (standard processing and micro-processing) are also provided for in the Cannabis Regulations. The various licences and subclasses carry differing rules and requirements.
Security Clearances
The Cannabis Regulations require that certain people associated with cannabis licensees must hold a valid security clearance issued by the Minister. Security clearances must be held by directors, officers, individuals who exercise, or are in a position to exercise, direct control over a corporate licensee, (iii) directors and officers of any corporation that exercises, or is in a position to exercise, direct control over a corporate licensee and key operational personnel (master grower, quality assurance person, head of security and a responsible person who serves as the licence holder’s contact at Health Canada), and any individuals identified by the Minister. The Minister may refuse to grant security clearances to individuals with associations to organized crime or with past convictions for, or an association with, drug trafficking, corruption or violent offences. The scope of bases upon which the Minister may decline a security clearance has expanded under the Cannabis Regulations compared with the ACMPR, but the overall approach is largely the same as under the ACMPR, except that the security clearance requirements apply to parent companies as well as corporate licensees. Individuals who have histories of non-violent, lower-risk criminal activity (for example, simple possession of cannabis, or small-scale cultivation of cannabis plants) are not precluded from participating in the legal cannabis industry. See "Risk Factors - Dependence on Skilled Labour and Suppliers".
Cannabis Tracking System
Under the Cannabis Act, the Minister is authorized to establish and maintain a national cannabis tracking system, which is now operational and is called the CTLS. As the title implies, the CTLS is used in both tracking and licensing. The CTLS provides a single entry point online secure platform for filing applications for security clearances and licences under the Cannabis Regulations. The CTLS also tracks cannabis through the supply chain from federal cannabis licence holders to individual medical clients, or from federal cannabis licence holders to recreational market channels (which vary by province). Federal cannabis licence holders are required to enter data into the CTLS and submit monthly reports to the Minister, among other things. The tracking function of the CTLS mitigates diversion of cannabis into, and out of, the regulated medical and recreational markets. The Cannabis Act provides the Minister with authority to order that any particular persons report specific information about their authorized activities with cannabis, in a form and manner specified by the Minister.
Cannabis Products
Currently saleable classes of cannabis are described in this heading. To avoid confusion, imminent expansion of saleable classes of cannabis through a proposed amendment of the Cannabis Act is described separately below. The Cannabis Regulations set standards for products being safe to use, in terms of being free of chemical and biological contaminants. The Cannabis Regulations also limit THC concentrations in cannabis oil to mitigate the chances of unintentional over-consumption, as cannabis oil has a much greater delay between dosing and perceived effects than is the case with vaporizing or smoking dried cannabis, or vaporizing cannabis derivatives or other extracts.
Saleable classes of cannabis are regulated by the Cannabis Act, and with the exception of plants and seeds (prohibited in Manitoba and Québec other than through medical access), are uniform across Canada. The Cannabis Act allows sale of dried cannabis, cannabis oil, fresh cannabis, cannabis plants, and cannabis seeds. Dried cannabis is typically sold as intact flower, milled flower or decarboxylated flower, and can be sold in pre-rolls, pre-loaded vaporization pods or other dosage forms. Cannabis oil can be sold in dropper bottles, spray bottles or other containers without dosage form, or in gel caps, softgels or other dosage forms.
Packaging and Labelling
The Cannabis Act sets out prohibitions on labelling, such as packaging or labelling (a) in a way that could be appealing to young people, (b) that includes an endorsement, (c) that depicts a person, character or animal, whether real or fictional, (d) presents in way that evokes a positive or negative emotion about or image of, a way of life such as one that includes glamour, recreation, excitement, vitality, risk or daring, or (e) that includes any information that is false, misleading or deceptive or that is likely to create an erroneous impression about its characteristics, value, quantity, composition, strength, concentration, potency, purity, quality, merit, safety, health effects or health risks.
The Cannabis Regulations also set out positive requirements and prohibitions on the packaging and labelling of cannabis products to promote informed consumer choice, allow for the safe handling and transportation of cannabis, ensure child-proofing on containers, reducing the appeal of cannabis to youth and promoting safe consumption. The size and colour of logos, names and other branding are restricted. In addition to being child-resistant, packaging must be tamper-evident. Cannabis package labels must include specific information, such as: (i) product source information, including the class of cannabis and the name, phone number and email of the processor; (ii) a mandatory health warning, rotating between Heath Canada’s list of standard health warnings; (iii) the Health Canada standardized cannabis symbol; and (iv) information specifying THC and Cannabidiol ("CBD") content.
Cannabis for Medical Purposes
With the Cannabis Act and the Cannabis Regulations coming into force on October 17, 2018, the medical cannabis regime migrated from the CDSA and the ACMPR to the Cannabis Act and the Cannabis Regulations. The medical cannabis regulatory framework under the Cannabis Act and the Cannabis Regulations remains substantively the same as it existed under the CDSA and the ACMPR, with adjustments to create consistency with rules for recreational use, improve patient access, and reduce the risk of abuse within the medical access system. Under Part 14 of the Cannabis Regulations, three options are available for obtaining cannabis for medical purposes: (i) registering with a holder of a licence to sell for medical purposes; (ii) register with Health Canada to produce a limited amount of cannabis for their own medical purposes; or (iii) designate someone else to produce cannabis for them. With respect to (ii) and (iii), starting materials, such as cannabis plants or seeds, must be obtained from medical sales licence holders. It is possible that (ii) and (iii) could significantly reduce the addressable market for the Corporation’s products. However, management of the Corporation believes that many patients may be deterred from opting to proceed with options (ii) or (iii) since such steps require applying for and obtaining registration from Health Canada to grow cannabis, as well as the up-front costs of obtaining equipment and materials to produce such cannabis. See "Risk Factors - If Medical-Use Consumers Elect to Produce Cannabis for their Own Medical Purposes Under the Cannabis Regulations it Could Reduce the Addressable Market for the Corporation’s Products"
Provincial Regulatory Framework
The Cannabis Act allows the possession, sale, and distribution of cannabis by persons authorized under provincial legislation. Under the Cannabis Regulations, such provincially authorized persons may only source cannabis products for retail sale from federally licensed cannabis producers.
All Canadian provinces and territories have legislation in effect regulating the distribution and sale of cannabis for recreational purposes, allowing all Canadians over the age of 19 (18 in Alberta and Québec) to purchase cannabis products without medical access. The only provinces with restrictions on classes of cannabis that may be sold in the recreational markets are Québec and Manitoba, where plants and seeds are not sold because personal cultivation for recreational purposes is prohibited in those two provinces. Regardless of the framework, all cannabis products for the recreational cannabis market are ultimately supplied by federally licensed cultivators (plants and seeds only) and processors (all saleable classes of cannabis - currently dried cannabis, cannabis oil, plants and seeds; fresh cannabis is also saleable but the Corporation is unaware of any entity selling fresh cannabis as a cannabis product).
At present, the Corporation has entered into supply agreements or arrangements with provincial liquor authorities of, or
as applicable with retailers operating in, the provinces of Ontario, Manitoba, Saskatchewan, Nova Scotia and British Columbia. In most provinces and territories, a liquor or cannabis authority operated by the province serves as a wholesaler, with retailers purchasing cannabis products from the liquor or cannabis authority or from provincially licensed distributors. The wholesalers, in turn, acquire the cannabis products from federally licensed cultivators and processors. Storefront and online sales of recreational cannabis products are regulated as part of the private sector or as public entities as in the following chart:
Activity | Privately Operated | Publicly Operated |
Storefront recreational sale | British Columbia
Alberta
Saskatchewan
Manitoba
Ontario
Newfoundland
Nunavut
Northwest Territories
Yukon | British Columbia
Québec
New Brunswick
Nova Scotia
Prince Edward Island
Yukon
Northwest Territories |
Online recreational sale | Manitoba
Saskatchewan | British Columbia
Alberta
Ontario
Québec
New Brunswick
Nova Scotia
Prince Edward Island
Newfoundland
Yukon
Northwest Territories
Nunavut |
Originally, only twenty five retail licences were issued in all of Ontario, with only five in the City of Toronto, pending alleviation of shortages of cannabis products. The Ontario Government recently announced that it plans to issue retail licences for up to fifty additional cannabis retail locations beginning in October, 2019.
Promotional Activity
The Cannabis Act generally prohibits promotions of cannabis, cannabis accessories, and services related to cannabis, subject to exceptions. As a balance point, brand preference or informational promotion is permitted provided that it is communicated in a fashion that excludes young people. Within permitted channels for promotional activity, content restrictions prohibit any promotional activity that (a) communicates price or distribution, (b) could be appealing to young persons, (c) includes a testimonial or endorsement, (d) depicts a person, character or animal, whether real or fictional or (e) presents in way that evokes a positive or negative emotion about or image of, a way of life such as one that includes glamour, recreation, excitement, vitality, risk or daring. It is also prohibited to promote cannabis or a cannabis accessory in a manner that is false, misleading or deceptive or that is likely to create an erroneous impression about its characteristics, value, quantity, composition, strength, concentration, potency, purity, quality, merit, safety, health effects or health risks. Display of a brand element in sponsorship of a person, event, entity, activity or facility, and naming of a sports or cultural facility with a cannabis brand element, are also prohibited. The Cannabis Act also prohibits offering cannabis or a cannabis accessory without consideration or as consideration for other purchases or transactions. Similarly, it is prohibited to offer benefits conditional on purchase of cannabis or a cannabis accessory.
Industrial Hemp Regulations
The Cannabis Act regulates all commercial and personal cultivation of cannabis plants. The 2018 Industrial Hemp Regulations define "industrial hemp" as a subset of cannabis: a cannabis plant - or any part of that plant - in which the concentration of THC is 0.3% w/w or less in the flowering heads and leaves. Licences to cultivate and propagate and process seed or grain of industrial hemp are issued under the 2018 Industrial Hemp Regulations. Cultivation is for commercial production while propagation is for breeding new varieties of industrial hemp. Processing under a 2018 Industrial Hemp Regulations licence is restricted to processing grain. Flowering tops, leaves and branches of industrial hemp ("Biomass") may not be processed under an IHR licence other than harvesting and drying for storage. Biomass may be sold to a holder of a processing licence under the Cannabis Regulations for extraction or other processing into cannabis products.
Industrial hemp generally has lower levels of phytocannabinoids than other varieties of cannabis plants, but Biomass may be a viable source of CBD. When proposed amendments to the Cannabis Act come into force, CBD extracted from Biomass (or less likely, the Biomass itself without extraction), may be used by the holder of a processing licence under the Cannabis Regulations to manufacture cannabis products including CBD, other phytocannabinoids, and any terpenoids or other compounds found in the Biomass. The Corporation believes that the New Classes of Cannabis will provide a strong market for cannabis products including CBD, other phytocannabinoids, and any terpenoids or other compounds found in the Biomass.
New Classes of Cannabis - 2019 Amendments
Draft amendments to the Cannabis Act and the Cannabis Regulations were distributed to industry on December 20, 2018 and published in the Canada Gazette on December 22, 2018 (the "2019 Amendments"). The 2019 Amendments were re-released to industry in final form on June 14, 2019 and published in the Canada Gazette on June 26, 2019. Generally, the 2019 Amendments expand the saleable classes of ready-to-use cannabis to include cannabis extracts, cannabis topicals and edible cannabis. Dried cannabis, fresh cannabis, cannabis plants and seeds will also remain saleable. Other amendments are also included in the 2019 Amendments.
The Federal Government has indicated that the 2019 Amendments will come into effect no later than October 17, 2019. The 2019 Amendments include many details about the new classes of cannabis. A few significant points relevant to cannabis extracts and edible cannabis are summarized herein.
The 2019 Amendments will eliminate the class of cannabis currently referred to as "cannabis oil", which will be subsumed within the cannabis extracts or edible cannabis class, depending on the details of how the particular cannabis product is packaged, the THC content and the presence of other ingredients. Most cannabis oil currently on the market will be sold as cannabis extracts after the 2019 Amendments take effect. Certain packaging standards for cannabis extracts are to be made more strict, and any current packaging that is non-compliant must all be sold within twelve months of the 2019 Amendments taking effect. Following these amendments, cannabis extracts that are suitable for vaporizing will be saleable, including in the popular cartridges that fit 510 threaded batteries, as are commonly known in both the Canadian illicit market and in the various regulated U.S. state markets. This product in particular is expected to increase the appeal of the regulated market relative to the illicit market.
The 2019 Amendments include a proposed limit of 10 mg THC per package of any edible product in the edible cannabis category. Edible cannabis products will not be able to be processed at sites co-located with standard food manufacturing to avoid the risk of cross over, which could be particularly problematic with food destined for export. The 2019 Amendments regulate edible cannabis to include beverages, provided that no more than 30 mg of caffeine (which must be naturally present in the food ingredients and not added) or 0.5% alcohol (the same cutoff for dealcoholized beverage alcohol). Products that require refrigeration prior to being opened are excluded.
In order to sell new cannabis products, federal cannabis licence holders will need to apply for an amendment to their licence and attest that all the regulatory requirements specific to the new cannabis products have been met before being authorized to sell them. Federal licence holders will need to provide Health Canada with written notice at least 60 days before making a new cannabis product available for sale. The Federal Government has indicated that Health Canada will begin approving amendments to licences to authorize the production and sale of the new cannabis products and reviewing notifications from federal licence holders of intent to sell new products once the 2019 Amendments come into force on October 17, 2019.
Australia
The summary below does not purport to be a complete and comprehensive overview of the entire regulatory regime in Australia. Rather the summary below discusses generally certain aspects of the regulatory regime in Australia and focuses on the regulatory regime in New South Wales as that is the current location of Holigen’s assets. There are various state laws and territorial laws which may vary from those laws applicable in New South Wales.
Australia’s federal, state and territory regulatory framework is relatively new, as Australia transitions from a prohibition-based framework to a framework in which certain medical cannabis activities are permitted, but strictly regulated. Under the Criminal Code Act 1995 (Cth), it is an offence to (amongst other things) cultivate or sell a "controlled plant", manufacture, traffic (including sell) or possess a "controlled drug" or import a "border controlled drug/plant". Under the Criminal Code Regulations 2002, any plant of the genus of cannabis is defined as both a "controlled plant" and a "border controlled plant", whilst any form of cannabis is defined as both a "controlled drug" and a "border controlled drug". However, the relevant provisions that make these activities an offence do not apply where a person engages in activities that are justified or excused under a law of the Commonwealth of Australia or the Australian state or territory where the activities take place. Assuming the relevant entity complies with the relevant licensing regimes set out below, its activities will not constitute an offence under the Criminal Code Act 1995. This does not necessarily assist at a state level, where further prohibitions regarding controlled plants and drugs of addiction exist.
The cultivation, production, manufacturing, distribution and use of cannabis and cannabis products in Australia is governed by the Narcotic Drugs Act 1967 (Cth) (the "Narcotics Act") and the Therapeutic Goods Act 1989 (Cth) (the "TG Act") (and their respective subordinate legislation) as well as drugs and poisons legislation in the various States and Territories. Further, Australia’s federal laws must conform to the country’s treaty obligations, including the Single Convention on Narcotic Drugs 1961 which restricts the possession, use, trade, distribution, import, export, manufacture and production of narcotic drugs (including cannabis) to medical and scientific purposes.
The Australian Government Department of Health regulates medical cannabis products through the ODC, which oversees controlled substances, including cannabis. The ODC is responsible for granting licences and permits for the cultivation and production, manufacture and importation of cannabis and cannabis products for medical and scientific purposes. In addition, the Therapeutic Goods Administration (the "TGA") also regulates the manufacture, registration and supply of medical cannabis. Under the Narcotics Act, cultivation and production, manufacturing and import licences are subject to screening by the ODC, and applicants for, and holders of, licences must satisfy certain "fit and proper person" requirements which take into account: (i) any convictions or civil penalties under the laws of the Commonwealth, states or territories; (ii) the associations of the directors and officers of the licensee; (iii) previous business experience of the directors and officers of the licensee; (iv) whether the directors and officers of the licensee are of good repute, having regard to their character, honesty and integrity; (v) the financial capacity and circumstances of the licensee; and (vi) and the ability of the licensee to meet the conditions of the licence.
State and territory governments also regulate the prescribing, storage and supply of medical cannabis and medical cannabis products.
Cultivation and Production
Cultivation, including production, requires a cultivation licence issued by the ODC. The Narcotics Act sets out two types of cultivation and production cannabis licences that can be issued by the ODC, a medical cannabis licence and a cannabis research licence. Broadly, a cannabis research licence may authorise the cultivation or production of cannabis or cannabis resin for the purposes of activities relating to research and related scientific purposes. A medical cannabis licence may authorise the cultivation or production of cannabis and cannabis resin for medical purposes. Cultivation includes all steps up to, but not including, harvest. Production consists of harvest and placing in a container for the purpose of manufacture or research. Before starting cultivation and/or production, licensees must also obtain a cultivation permit and/or production permit from the ODC. Cultivation permits authorize specific activities relating to the cultivation of cannabis, and include restrictions on the types and strains of plants that may be cultivated, the maximum number of plants that may be cultivated, and limitations on the concentrations of THC and CBD that cannabis products may contain. Production permits authorise specific activities relating to the production of cannabis products, and include restrictions on the maximum quantity of dried cannabis or cannabis resin to be produced, the maximum quantity of dried cannabis and cannabis resin the licensee may hold, and the time period during which cultivation activities may occur. Cultivation licences and cultivation permits are only granted for a specified period of time, as outlined in each licence and permit granted. As such, a new licence and permit will need to be obtained, according to their respective expiry date.
Manufacturing
Similarly, manufacturing of cannabis products also requires a manufacturing licence and permits issued by the ODC. Manufacturing licences allow the licensee to manufacture cannabis products and to engage in certain related activities such as packaging, transport, storage, supply and destruction. A manufacturing permit must also be obtained before starting to manufacture cannabis products. Manufacturing permits authorize certain activities and prescribe the maximum quantity of cannabis products that may be manufactured, the maximum quantity of cannabis products the licensee may hold, and the period during which manufacturing may occur. Manufacturing licences and permits are granted for a specified period of time as outlined in each licence and permit granted. As such, a new licence and permit will need to be obtained, in accordance with each respective expiry date.
Manufacturers of cannabis products must also comply with the Good Manufacturing Practice for Medicinal Products in order to satisfy the requirements of the TGA. Manufacturing must occur at facilities inspected and approved by the TGA. Further, the TGA has also issued a Therapeutic Goods Order which imposes certain standards relating to decontamination, identification, chemical testing, adulteration and quality testing for medical cannabis and cannabis products in Australia.
Import
The Customs Act 1901 (Cth) confers on the Governor-General of Australia the power to prohibit (absolutely or under certain circumstances / conditions) the importation of goods into Australia. Broadly, under regulation 5 of the Customs (Prohibited Imports) Regulations 1956, the importation of cannabinoids, cannabis and cannabis resin is prohibited unless the person importing the drug is the holder of a licence to import drugs granted by the Secretary of the Commonwealth Department of Health (or an authorised person) and permission to import the drug granted by the Secretary (or authorised person). An importation licence will not be granted unless the applicant furnishes all the information reasonably required in relation to the application; is a fit and proper person to be granted the licence (including any agents and employees); and the premises on which the drugs will be stored is secure for that purpose. Importers may apply for an importation licence and importation permission if it is established that the drug will be used for a Special Access Scheme ("SAS"), Authorised Prescriber Scheme ("APS") or clinical trial under the TG Act (as discussed below). In the event that an importation licence and an importation permission are granted, the licence holder must keep the drugs in safe custody at all times (including transport); only dispose of the drugs solely for medical or scientific purposes; keep records in compliance with the regulations; furnish information, records or drugs when requested for inspection; and ensure importation of drugs is in accordance with the terms of an importation permission. Separately, an exportation licence and/or permit from the country of origin may be required depending on the laws of that country.
Prospective import licensees must submit to an ODC inspection of their storage and security measures. Import licensees must obtain an import permit prior to each shipment, which discloses the details of the substance to be imported. Once cannabis products are imported, the licensee must comply with a number of record-keeping obligations, including maintaining records of the quantity of cannabis held and supplied. To import nursery stock, an additional permit is also required from the Department of Agriculture and Water Resources. Any plant material, including cannabis, imported into Australia is subject to biosecurity screening before entering the country as well as quarantine requirements before being released for cultivation.
Prescription, Supply and Storage
The TG Act provides for the establishment and maintenance of a national system of controls for the quality, safety, efficacy and timely availability of therapeutic goods that are imported into, manufactured in, supplied in, used in, or exported from, Australia. Under the TG Act, the ARTG is administered by the TGA and compiles information in relation to, and for the evaluation of, therapeutic goods for humans. Generally, medicines (including medical cannabis) imported into, manufactured in, supplied in, and exported from Australia must be entered into the ARTG. However, for unapproved therapeutic goods, the TG Act and guidance from the Department of Health provide a series of mechanisms to enable these activities. For medical cannabis, the pathways include the SAS; APS; or access as part of a clinical trial. Both SAS and APS permit medical practitioners to prescribe cannabis and cannabis products to patients; however, under the SAS, medical practitioners must seek approval from the TGA prior to writing each prescription on an individual patient basis, whereas the APS allows approved medical practitioners to prescribe cannabis to specific classes of patients with defined illnesses or which meet certain criteria. Medical practitioners that wish to prescribe cannabis under the APS, must first be approved by a Human Research Ethics Committee. State and Territory laws will also limit prescribing of medical cannabis, and approval must be obtained from the applicable state or territory Health Departments.
The supply of medical cannabis products by retail, other than by a pharmacist on prescription, is prohibited in Australia. Cannabis may only be supplied to certain categories of person expressly named in the applicable statutes, which are typically limited to medical practitioners and pharmacists. Each state and territory has specific requirements for companies that wish to supply medical cannabis products. Medical cannabis suppliers must first obtain a licence from the applicable state or territory Department of Health. A supply licence is generally only required in the state or territory in which the supplier will store products. This is not expressly stated in the relevant statutes, but is accepted practice in the market and by the relevant Departments.
New South Wales
In addition to the above, each state and territory has individual requirements relating to cannabis. In relation to NSW, where TCann is operating, the following licences and approvals are required:
(1) a wholesale supply licence under the Poisons and Therapeutic Goods Regulation 2008 (NSW);
(2) a manufacture licence under the Poisons and Therapeutic Goods Regulations 2008 (NSW); and
(3) medical practitioners who are "authorised prescribers" must also apply for approval under the Poisons and Therapeutic Goods Act 1966 (NSW).
Poisons and Therapeutic Goods Act 1966 (NSW) (the "PTG Act")
The Poisons and Therapeutic Goods Regulations 2008 (the "PTG Regs") sets out the licensing regimes for the possession, manufacture and supply of medical cannabis products.
(1) Wholesale Supply
In order to supply medical cannabis products in NSW, a wholesale supply licence under Part 8 of the PTG Regs is required. The licence application process involves producing required documentation (including documents confirming the proposed licensee is a ’fit and proper person’) and gives the NSW Director-General of Health broad powers to refuse applications or, if granted, impose conditions in respect of the licence. Under the PTG Regs, the holder of the wholesale licence can only supply medical cannabis products to another person if:
(i) the person to whom the product is supplied is a medical practitioner and the product is supplied for the treatment of patients of the medical practitioner (including patients in a clinical trial);
(ii) the person to whom the product is supplied is a pharmacist and the product is supplied for the purposes of the pharmacist supplying the product on the prescription of a medical practitioner; or
(iii) the product is supplied for the purposes of a clinical trial and the person responsible for the conduct of the trial holds a licence or authority under Part 8, or under the Drug Misuse and Trafficking Act 1985, authorising the use of the product in the trial.
The NSW wholesale supply licence is a prerequisite for an application for an Importation Licence / Permission under the Customs Act (as described above).
(2) Manufacture
In addition to the licences required under the ND Act and the TG Act, a person seeking to manufacture medical cannabis products in NSW is also required to obtain a licence to manufacture in NSW under Part 8 of the PTG Regs.
The PTG Regs also set out requirements for handling, including storage, maintenance of registers, packaging, labelling, and procedures for orders from authorised practitioners.
(3) Access
In addition to the requirements under the APS, a medical practitioner must also apply for approval from the Secretary under the PTG Act to prescribe cannabis products. A similar obligation exists under the relevant drugs and poisons legislation in the other States and Territories.
Drugs Misuse and Trafficking Act 1985 (NSW) (the "DMT Act")
Division 2 of Part 2 of the DMT sets out a variety of indictable offences relating to the possession, cultivation and supply of prohibited plants and drugs. The definition of "prohibited plant" includes any growing plant of the genus cannabis. Additionally, "prohibited drug" includes any growing plant of the genus cannabis, cannabis oil and cannabis resin.
Section 8 of the DMT Act notes that nothing within the DMT Act affects any provision made by or under the PTG Act or renders unlawful anything done in accordance with any such provision. As a result, any activities by a licence holder under the PTG Act (or PTG Regs) that are done in accordance with the relevant licence will not constitute an offence under the DMT Act.
Portugal
Decree-Law no. 15/93, of January 1993 ("DL 15/93") provides the legal framework in Portugal for the cultivation, production, manufacture, distribution, wholesale trade, import, export, transit, transportation, possession by any title and use of narcotic drugs and psychotropic substances, including certain cannabis substances. DL 15/93, is regulated by Regulatory-Decree 61/94 ("RD 61/94"), which among other things, sets out regulations relating to: (i) cultivation and production; (ii) wholesale trade; (iii) delivery conditions; (iv) record keeping; (v) packaging and labelling; (vi) licensing requirements; and (vi) reporting obligations.
In July 2018, Law 33/2018, of 18 July ("Law 33/2018") was published, and subsequently came into effect, providing for the use of drugs, preparations and substances based on the cannabis plant, for medical use. New regulations were published on January 15, 2019 (Decree-Law 8/2019) and came into effect in February 2019, which provide that the sale authorization for placing the Cannabis-based drugs, preparations and substances for medical use in the market can be issued for a period of five years and be renewed. In addition, these new regulations will: (i) require that licensees annually update the information provided in their initial licence application; (ii) provide that licensees must follow a system of good practices in the activities of cultivation, manufacture and distribution of Cannabis-based drugs, preparations and substances for medical use, which must follow the European and National guidelines; and (iii) impose regulations on the prescription of cannabis-based drugs by doctors, which must follow the lists and regulations of the National Institute of Pharmacy and Medicine ("INFARMED") for the conditions cannabis-based drugs may be prescribed to treat. Under DL 15/93 and RD 61/94, applicants for licences are subject to screening by INFARMED to verify that their owners and legal representatives offer sufficient guarantees of moral and professional integrity. Each owner and legal representative of an applicant must submit a criminal registry certificate and may also be subject to searches by INFARMED in the records of the Gabinete do Combate à Droga do Ministério da Justiça.
According to the current regulatory framework, entities that undertake the activities of cultivation, import, export, manufacture or wholesale of cannabis-based drugs, preparations and substances for medical use, are obliged to adopt security measures and provide INFARMED with proof of the implementation of such measures.
The specific security measures that should to be adopted in this matter will still be defined by a Ministerial Order, which, to date, has not been approved. In the meantime, this legislation refers to the technical requirements provided for in former regulation applicable to the private security actives in general.
Therefore, and until the approval of the relevant Ministerial Order on this matter, the security system on the activities mentioned above shall comply, at least, with the following requirements: i) it must be equipped with a video-surveillance system composed by video cameras for the capturing and recording of images covering the access areas to the facilities, ii) it must be equipped with a system of intrusion detection, and iii) it must be connected to a central control unit.
In addition, for the activities of cultivation, manufacture and wholesale of cannabis-based drugs, preparations and substances for medical use, a security director is also required. The security director has to meet a number of requirements, such as: (i) be a Portuguese citizen, or be a citizen of a Member State of the European Union, a State party to the EEA Agreement or, under conditions of reciprocity, a State of Portuguese official language; (ii) hold a 12th grade diploma or equivalent (secondary education); (iii) do not to exercise, or never have exercised, in any capacity, a position or function of supervision over exercise of private security activity in the preceding three years and (iv) hold the specific professional title of "security director", issued by the Portuguese public security policy (PSP - Polícia de Segurança Pública).
INFARMED is a public institute and an instrumentality entity of the Portuguese Republic invested in the powers to issue license, regulate and supervise activities relating to the cultivation, production, extraction and manufacture, wholesale trade, distribution to pharmacies, import and export, transit, purchase, sale and delivery of drugs, preparations and substances based on the cannabis plant, for medical purposes, as provided for in the applicable laws. INFARMED is also responsible for Portugal’s cannabis licensing regime.
CONSOLIDATED CAPITALIZATION
There have been no material changes in the share and loan capital of the Corporation since the date of the interim financial statements except as set forth under the sections entitled “Prior Sales”, “Details of the Acquisition” and “Recent Events”. After giving effect to the Offering, assuming an estimated [17,217,631] Common Shares at a price of [$7.26] will be issued in connection with the Offering (excluding the Underwriters’ Option) and assuming the Acquisition has been consummated on such date. there will be a total of [138,761,071] Common Shares issued and outstanding.
The table below sets forth the Corporation’s total capitalization as of March 31, 2019: (i) on an actual basis; (ii) on an as adjusted basis to give effect to the Offering; and (iii) on a pro forma as adjusted basis, to reflect the Acquisition and to give effect to the Offering. The following table should be reviewed in conjunction with the Pro Forma Condensed Consolidated Financial Statements attached as Appendix “D” to this Prospectus, as well as the unaudited condensed interim consolidated financial statements of the Corporation for the three months ended March 31, 2019 and 2018, together with the notes thereto.

(1) Pursuant to the closing of a private placement on May 10, 2019, 2,165,547 shares were issued for gross proceeds of $13,534,668
(2) A holder of Flowr ULC Class A Shares elected to convert at no cost, 150,000 Flowr ULC Class A Shares into Common Shares
(3) An estimated [17,217,631] Common Shares at a price of [$7.26] will be issued in connection with the Offering, with estimated net proceeds of $115,400,000
(4) 1,652,500 stock options granted to employees at an exercise price of $5.93 per Common Share
(5) 75,000 stock options granted to an employee and directors at an exercise price of $5.70 per Common Share
(6) 372,250 restricted share units granted to employees between April 24, 2019 - June 3, 2019
(7) 32,632,545 Common Shares to be issued in connection with the Acquisition
(8) Absorption of the Holigen accounts in the consolidated pro forma
PLAN OF DISTRIBUTION
The Corporation and the Underwriters have entered into the Underwriting Agreement with respect to the Offered Shares. Subject to certain conditions, the Underwriters have severally and not jointly agreed to purchase, as principals, and the Corporation has agreed to sell, on the Closing Date, the Offered Shares at the Offering Price.
The obligations of the Underwriters under the Underwriting Agreement are several and not joint and may be terminated at their discretion upon the occurrence of certain stated events, including an event such as: (a) there has been, in the judgement of the Representatives since the time of the execution of the Underwriting Agreement, any material adverse change in the financial condition, earnings, business affairs or prospects of the Corporation and its subsidiaries; (b) any inquiry, action, suit, investigation or other proceeding is instituted or announced or any order is made by a Governmental Authority in relation to the Corporation or its subsidiaries or any change in law, or the interpretation or administration thereof; (c) there is a general moratorium on banking activities in Canada or the United States declared by relevant authorities; (d) a material disruption in commercial banking or securities settlement or clearance services; (e) trading generally has been suspended or materially limited on, or by, as the case may be, any of the TSX.V or the NASDAQ, or minimum or maximum prices for trading have been fixed, or maximum ranges for prices have been required, by TSX.V or the NASDAQ, or by order of a Canadian Securities Regulator, the SEC, FINRA or any other Governmental Authority; (f) trading of any securities of the Corporation shall have been suspended on the TSX.V or the NASDAQ; (g) any event, action, state, condition or major financial occurrence of national or international consequence or any outbreak or escalation of national or international hostilities or any crisis or calamity or any governmental action, law, regulation, inquiry or other similar occurrence which, in the opinion of any of the Underwriters, materially adversely affects or may materially adversely affect the financial markets in Canada or in the United States or the business, operations or affairs of the Corporation and the Subsidiaries taken as a whole, or the market price or value of the Offered Shares or any of them; (h) any inquiry, investigation or other proceeding, or order, ruling or other pronouncement is issued or announced under or pursuant to any relevant statute or by any stock exchange or other regulatory authority, which, in the reasonable opinion of any of the Underwriters, operates to prevent, suspend, restrict, inhibit or otherwise adversely affect the trading in, or which materially adversely impacts the distribution of the Offered Shares (i) a material change or change in any material fact which in the opinion of the Representatives has or could be reasonably expected to have a significant adverse effect on the market price or value of the Common Shares; (j) breach of certain conditions by the Corporations in the Underwriting Agreement; or (k) the state of financial markets in Canada or the United States is such that, in the reasonable opinion of the Representatives on behalf of the Underwriters, the Offered Shares cannot be marketed profitably.
In consideration of the agreement of the Underwriters to purchase the Offered Shares and for the services rendered by the Underwriters in connection with the Offering, the Underwriters will be paid an aggregate cash commission representing 6% of the Offering Price per Offered Share (including gross proceeds realized on the sale of Offered Shares pursuant to the exercise of the Underwriters’ Option). Pursuant to the Underwriting Agreement, the Corporation has also agreed to reimburse the Underwriters for fees and expenses of counsel to the Underwriters, up to a maximum of $900,000, and for other expenses of the Underwriters not to exceed $[•].
The Corporation has granted the Underwriters the Underwriters’ Option, exercisable in whole or in part, at any time and from time to time, in the sole discretion of the Representatives, no later than 30 days following the Closing Date, to purchase up to [•] Additional Offered Shares at the Offering Price of $[•] per Additional Offered Share. This Prospectus qualifies the grant of the Underwriters’ Option and the issuance of the Additional Offered Shares on the exercise of the Underwriters’ Option. A purchaser who acquires Additional Offered Shares forming part of the Underwriters’ over-allocation position acquires those Additional Offered Shares under this Prospectus, regardless of whether the over-allocation position is ultimately filled through the exercise of the over-allocation option or secondary market purchases.
The Underwriters are entitled under the Underwriting Agreement to customary indemnification by the Corporation against certain liabilities (including liabilities under the U.S. Securities Act), and expenses.
Pursuant to the Underwriting Agreement, the Corporation hereby agrees that, without the prior written consent of the Representatives on behalf of the Underwriters, it will not, directly or indirectly, during the period ending 90 days after the Closing of the Offering (the “Restricted Period”), (1) create, allot, authorize, offer, issue, secure, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise lend, transfer or dispose of, directly or indirectly, any Common Shares, rights to purchase such Common Shares or any securities convertible into or exercisable or exchangeable for such Common Shares or (2) enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of the Common Shares, whether any such transaction described in clause (1) or (2) above is to be settled by delivery of Common Shares or such other securities, in cash or otherwise or (3) file or confidentially submit any registration statement with respect to any Common Shares or any securities convertible into or exercisable or exchangeable for Common Shares; or (4) publicly announce the intention to do any of the foregoing, whether any such transaction described in clause (1) or (2) above is to be settled by delivery of such Common Shares or such other securities or interests, in cash or otherwise, or agree to do any of the foregoing, save and except (a) the Securities to be sold hereunder; (b) for purposes of director, officer, employee or consultant incentive plans, including the grant or issuance of incentive securities and the conversion or exercise of incentive securities into Common Shares (including, without limitation, the filing of a S-8 registration statement with respect thereto), (c) to satisfy existing instruments including with respect to Flowr ULC Convertible Shares issued at the date hereof, or (d) in connection with any acquisition, merger, business combination, tender offer, take-over bid, arrangement, asset purchase, joint venture or similar transaction, including the acquisition of Holigen, provided that in the event that any such transaction is completed.
Additionally, senior officers and directors of the Corporation have executed agreements in favour of the Underwriters agreeing not to, for a period ending on the date that is 90 days following the Closing Date, directly or indirectly: (i) offer, sell, contract to sell, transfer, assign, secure, pledge, lend or otherwise dispose of or deal with, whether through the facilities of a stock exchange, by private placement or otherwise, any Common Shares or other securities of the Corporation owned, directly or indirectly, by such officers or directors or their associates; or (ii) make any short sale, engage in any hedging transaction, or enter into any swap or other arrangement or agreement that transfers any of the economic consequences of ownership of any Common Shares or securities of the Corporation; or (iii) otherwise publicly announce any intention to do any of the restricted activities, whether through the facilities of a stock exchange, by private placement or otherwise, unless (a) the prior written consent of the Underwriters has been obtained, (b) there is a take-over bid or similar transaction, or plan of arrangement or other business combination, involving a change of control of the Corporation generally made to all shareholders of the Corporation to which such persons will tender their Common Shares, (c) the transfer of any or all of the locked up securities to wholly-owned affiliated entities of the undersigned (so long as any such affiliated entity remains an affiliate of the undersigned) or any company, trust or other entity wholly-owned or maintained for the benefit of the undersigned, provided, however, that it shall be a condition precedent to any transaction contemplated in clause (ii) above that (x) the transfer will not trigger the filing of an insider report or any disclosure document or otherwise trigger any “late” filing following expiration of the Restricted Period and (y) the transferee executes and delivers to the Representatives on behalf of the Underwriters an agreement stating that the transferee is receiving the locked up securities subject to the provisions of this agreement and that such transferee shall be subject to the provisions of the lock up agreement as if it were a signatory.
Pursuant to policy statements of certain Canadian provincial securities regulators, the Underwriters may not, throughout the period of distribution under this Prospectus, bid for or purchase Common Shares for their own account or for accounts over which they exercise control or direction. The foregoing restriction is subject to exceptions, provided the bid or purchase is not engaged in for the purposes of creating actual or apparent trading in, or raising the price of, the Common Shares. These exceptions include bid or purchases permitted by the by-laws and rules of applicable regulatory authorities and stock exchanges, including the Universal Market Integrity Rules for Canadian Marketplaces administered by the Investment Industry Regulatory Organization of Canada, relating to passive market making activities, and a bid or purchase made for and on behalf of a customer where the order was not solicited during the period of distribution. Subject to applicable laws, the Underwriters may, pursuant to the first mentioned exception, in connection with the Offering, over-allot or effect transactions intended to maintain the market price of the Common Shares at levels other than those which might otherwise prevail in the open market. Such transactions, if commenced, may be discontinued at any time.
If the Underwriters create a short position in the Common Shares in connection with the Offering, i.e., if they sell more Offered Shares than are listed on the cover of this Prospectus, the Underwriters may reduce that short position by purchasing Common Shares in the open market. The Underwriters may also elect to reduce any short position by exercising the Underwriters’ Option to purchase Additional Offered Shares described above. Purchases of Common Shares to stabilize the price or to reduce a short position may cause the price of the Common Shares to be higher than it might otherwise be in the absence of such purchases. No representation is made as to the magnitude or effect of any such stabilization or other activities. The Underwriters are not required to engage in these activities.
After the Underwriters have made reasonable efforts to sell the Offered Shares at the Offering Price, the Underwriters may decrease the Offering Price for the Offered Shares and may further change the Offering Price from time to time to amounts no greater than the Offering Price. In the event the Offering Price of the Offered Shares is reduced, the compensation received by the Underwriters will be decreased by the amount that the aggregate price paid by the purchasers for the Offered Shares is less than the gross proceeds paid by the Underwriters for the Offered Shares. Any such reduction will not affect the proceeds received by the Corporation.
The TSX.V has conditionally approved the listing of the Offered Shares and the Additional Offered Shares. Listing is subject to the Corporation fulfilling all of the listing requirements of the TSX.V on or before October 2, 2019. The Corporation has been approved for listing on the NASDAQ, subject to the effectiveness of the Corporation's registration statement on Form 40-F filed with the SEC on February 5, 2019 as amended on May 28, 2019 and further amended on July 12, 2019.
The Offering is being made in each of the provinces of Canada except Quebec on an underwritten basis for 100% of the Offered Shares offered hereunder. The Underwriters conditionally offer the Offered Shares, subject to prior sale, if, as and when issued by the Corporation and accepted by the Underwriters in accordance with the conditions contained in the Underwriting Agreement. The Offering is also being made in the United States pursuant to the multijurisdictional disclosure system implemented by the securities regulatory authorities in the United States and Canada through the Underwriters directly or through their respective broker-dealer affiliates registered in the United States. The U.S. Underwriters are not registered as investment dealers in any Canadian jurisdiction and, accordingly, will only sell or solicit offers to sell the Offered Shares, directly or indirectly, into the United States and will not, directly or indirectly, sell or solicit offers to sell the Offered Shares in Canada.
In the ordinary course of their various business activities, the Underwriters and their respective affiliates, officers, directors and employees may purchase, sell or hold a broad array of investments and actively trade securities, derivatives, loans, commodities, currencies, credit default swaps and other financial instruments for their own account and for the accounts of their customers, and such investment and trading activities may involve or relate to assets, securities and/or instruments of the Corporation (directly, as collateral securing other obligations or otherwise) and/or persons and entities with relationships with the Corporation. The Underwriters and their respective affiliates may also communicate independent investment recommendations, market color or trading ideas and/or publish or express independent research views in respect of such assets, securities or instruments and may at any time hold, or recommend to clients that they should acquire, long and/or short positions in such assets, securities and instruments.
The foregoing is a summary only and does not purport to be a complete description of all of the terms, provisions, covenants, and agreements contained in the Underwriting Agreement, and is subject to and qualified in its entirety by reference to the full text of the Underwriting Agreement.
Selling Restrictions
Other than in the United States and the provinces of Canada, except the province of Québec, no action has been taken by us that would permit a public offering of the Common Shares in any jurisdiction where action for that purpose is required. The Common Shares may not be offered or sold, directly or indirectly, nor may this Prospectus or any other offering material or advertisements in connection with this Offering be distributed or published in any jurisdiction, except under circumstances that will result in compliance with applicable rules and regulations of that jurisdiction. Persons into whose possession this Prospectus comes are advised to inform themselves about and to observe any restrictions relating to this Offering and the distribution of this Prospectus. This Prospectus does not constitute an offer to sell or a solicitation of an offer to buy any Common Shares in any jurisdiction in which such an offer or solicitation is unlawful.
Bermuda
The Common Shares may not be marketed, offered or sold directly or indirectly to the public in Bermuda and neither this Prospectus nor any other offering material or advertisements in connection with this Offering or information contained herein relating to the Offering may be supplied to the public in Bermuda or used in connection with or constitute any offer for the subscription or sale of the Common Shares to the public in Bermuda.
Cayman Islands
This Prospectus does not constitute an offer or invitation to the public in the Cayman Islands to subscribe for the Common Shares.
USE OF PROCEEDS
Proceeds
The net proceeds to the Corporation from this Offering, after deducting the Underwriters’ Fee and expenses of the Offering, are estimated to be approximately $115,400,000, or approximately $133,025,000 if the Underwriters exercise the Underwriters’ Option in full. See “Plan of Distribution”.
Principal Purposes Assuming Completion of the Acquisition
The Corporation intends to use the net proceeds from the Offering to fund, in part: (i) the Cash Consideration; (ii) the fees and expenses incurred in connection with the Acquisition; (iii) capital required for the construction and development of Holigen’s and the Corporation’s facilities; and (iv) working capital and general corporate purposes.
The following table illustrates the estimated uses of the proceeds of the Offering.
Use of Proceeds | Amount
(CAD millions)(1) |
Full satisfaction of the Cash Consideration(2) | $8.2 |
Full satisfaction of fees and expenses (including underwriting fees) incurred in connection with the Offering & Acquisition | $12.0 |
| Capital required for the construction and development of the K2 Facility | $54.0 |
Capital required for the construction and development of Holigen's facilities in Sintra, Portgual | $11.0 |
| Capital required for the construction and development of Holigen's facilities in Aljustrel, Portgual | $20.5 |
| Capital required for the construction and development of Holigen's facilities in Australia | $10.5 |
Corporate, general & administration and working capital | $8.8 |
Gross proceeds to the Corporation(3) | $125.0 |
(1) Without giving effect to the exercise of the Underwriters' Option.
(2) The Cash Consideration portion of the Purchase Price will be financed through the net proceeds of the Offering. In addition, a portion of the Purchase Price will be satisfied through the issuance of the Consideration Shares. The partial satisfaction of the Purchase Price attributable to the proceeds of the Offering is approximately $8.2 million, representing the entire portion of the Cash Consideration in the Acquisition. For a discussion of the Acquisition, see "The Acquisition of Holigen".
(3) The proceeds before deducting the underwriting fees and estimated expenses that are payable by the Corporation in relation to the Offering and Acquisition.
In the event the Underwriters’ Option is exercised, any additional net proceeds will be allocated to: (i) working capital required for the construction of Holigen’s and the Corporation’s development and facilities; and (ii) general corporate purposes.
The Corporation has negative cash flow from operating activities as currently disclosed in the audited consolidated financial statements of the Corporation for the fiscal years ended December 31, 2018 and 2017. As at May 31, 2019, the Corporation had a negative working capital balance of approximately $3.6 million and its total funds available were approximately $17.6 million. If the Corporation continues to have negative cash flows, the Corporation may need to allocate additional financing proceeds to fund the negative cash flow in addition to its operational expenses and planned capital expansions. The Corporation may require additional financing to fund its operations to the point where it is generating positive cash flows. See "Risk Factors - Future Funding Requirements."
Principal Purposes if the Acquisition is Not Completed
In the event that the closing of the Acquisition does not occur, the net proceeds from the Offering (including any proceeds from the exercise of the Underwriters’ Option before deducting expenses of the Offering of $2,100,000) will be used to fund the Corporation’s working capital, including construction and development of the Corporation’s facilities and for general corporate purposes. Other than the Acquisition, the Corporation does not currently have an intention to use the net proceeds of the Offering for any specific acquisition. See “Cautionary Note Regarding Forward-Looking Information”, “Plan of Distribution” and “Risk Factors”. From time to time, the Corporation evaluates various potential acquisition opportunities, some of which could, if consummated, have a material impact on the Corporation. There can be no assurance that the Corporation will be able to identify acquisition opportunities that meet its strategic objectives, or to the extent such opportunities are identified, that it will be able to negotiate acquisition terms that are acceptable to it or complete any transaction. No commitments have been made with respect to any transactions. However, the Corporation cannot preclude the possibility that agreement on one or more acquisition transactions will be reached in the weeks or months following the Closing Date. As noted above, if the closing of the Acquisition does not occur, all or a portion of the net proceeds of the Offering received by the Corporation may be allocated to effect acquisitions.
The Corporation intends to use the funds available to it as stated in this Prospectus; however, there may be circumstances where, for sound business reasons, a reallocation of funds may be deemed prudent or necessary. See “Risk Factors - Risks Relating to the Offering”.
Business Objectives and Milestones
The Corporation expects that the proceeds of the Offering and the Corporation's existing resources will enable it to continue operations for the next 12 months.
The next milestones for the Corporation are (i) completion of the remaining construction of the K1 Facility and Flowr Forest and (ii) construction of the K2 Facility and Holigen facilities. The Corporation expects to fully fund these milestones with its available cash on hand, the proceeds of the Offering, the expected proceeds of the ATB Credit Facilities and the cash generated from Flowr's and Holigen's operations. See "Risk Factors - The Corporation Expects to Enter Into the ATB Credit Facilities, but Such Facilities May Not Get Finalized or Be Available. In that Event, the Corporation Would Have to Find an Alternative Form of Financing." The table under "Our Current and Proposed Cultivation and R&D Facilities" describes the stages of development and proposed time period for the build out of the Corporation's and Holigen's facilities.
The Corporation is in negotiations to obtain a loan commitment from ATB to provide the ATB Credit Facilities which, if obtained, together with the net proceeds of the Offering, are expected to provide sufficient funds for its general working capital requirements over the next 12 months and to finance the construction of the K2 Facility and Flowr Forest. Although the Corporation expects to enter into the ATB Credit Facilities, such facilities are subject to finalization of terms and documentation and will contain specific terms and conditions for funding and closing to take place. The Corporation expects that the closing of the ATB Credit Facilities will be completed concurrent with closing of the Offering and has no reason to believe that the ATB Credit Facilities will not be available. However, in the event that the ATB Credit Facilities are not finalized and funds are not made available, the Corporation would likely be required to redesign the K2 Facility and construct a facility that is approximately half the size of the planned K2 Facility, which would result in half of the expected capacity of the K2 Facility from the capacity disclosed in this Prospectus, or may seek alternative forms of financing. If the Corporation is required to construct a smaller K2 Facility, it would be constructed in multiple stages, similar to the construction of the K1 Facility. As construction proceeds, the Corporation would be able to generate revenue from completed portions of the K2 Facility. Thereafter, if and as funds become available to the Corporation, including funds from external creditors and internally generated cash flows, the Corporation would assess the possibility of expanding the K2 Facility to the proposed annual capacity of 40,000 kilograms of dried cannabis flower. If a smaller K2 Facility is constructed, it could result in a delay to completion and operation of the K2 Facility and reduced capacity and revenues for the Corporation. There is also no guarantee that the Corporation would be able to find an alternative form of financing on the same terms as the ATB Credit Facilities or at all. Furthermore, failure to find an alternative form of financing could have a material adverse impact on the Corporation's ability to fund its working capital requirements or complete construction of the K2 Facility, which could, in turn, have a material adverse effect on the Corporation's business, financial condition and results of operations. If the Corporation cannot complete the Offering or find an alternative form of financing on the same terms as the ATB Credit Facilities or at all, the Corporation would likely be required to scale back certain operational and capital expenditures, including its proposed NASDAQ listing and various non-essential features of the Hawthorne R&D Facility and the K1 Facility. See "Risk Factors - The Corporation Expects to Enter Into the ATB Credit Facilities, but Such Facilities May Not Get Finalized or Be Available. In that Event, the Corporation Would Have to Find an Alternative Form of Financing."
Holigen has entered into a debt facility with Credito Agricola Bank, which provides for an aggregate loan of €5.22 million, and is in discussions with various financial institutions in Portugal with respect to additional sources of financing. In addition, recognition of Holigen's Aljustrel site as a PIN by the Portuguese government provides Holigen with the ability to fast-track through regulatory licensing processes and access to potential funding. As at the date of this Prospectus, the entire Aljustrel site has been security fenced and, with part of the proceeds of the Offering allocated thereto, the Corporation expects to begin planting of 1,000,000 square feet in the Aljustrel site. In addition, the Corporation expects to be able to plant the additional square footage of the Aljustrel site using clone plants sourced from its current operations. The balance of the capital costs for the Aljustrel site relate to the costs to build an on-site processing and manufacturing facility, which the Corporation could elect to delay, if funds are not available for such purpose, until additional cash from operations becomes available.
The growing areas of both the Aljustrel site and Flowr Forest are low capital cost outdoor growing facilities, unlike the K2 Facility which is a purpose built indoor growing facility. In the event that secured funding is not available, construction of the growing area of these facilities is expected to be funded with cash flow generated from the Company's operations and, in the case of Aljustrel, using part of the proceeds of the Offering. Such construction is expected to be completed in stages, with subsequent phases of the facilities being completed in later years as more cash from operations becomes available.
DESCRIPTION OF SHARES
The authorized capital of the Corporation consists of an unlimited number of Common Shares and an unlimited number of preferred shares issuable in series without nominal or par value (the “Preferred Shares”). As at July 17, 2019, 89,255,067 Common Shares were issued and outstanding. As at the date hereof, there are no preferred shares issued and outstanding.
Common Shares
The holders of Common Shares are entitled to receive notice of, and attend, all meetings of shareholders convened by the Corporation. At any general meeting, on a show of hands every shareholder who is present in person or by proxy and entitled to vote has one vote, and on a poll, every shareholder who is entitled to vote has one vote for each Common Share held and may exercise such vote either in person or by proxy, except for meetings at which only holders of another class or series of shares of the Corporation are entitled to vote separately as a class or series.
Holders of Common Shares have the right to receive dividends out of monies of the Corporation properly applicable to the payment of dividends if and when declared by the Board provided that the Board shall declare dividends on the Preferred Shares, or any other class of shares without being obliged to declare any dividends on the Common Shares of the Corporation. In the event of a liquidation, dissolution or winding up of the Corporation, whether voluntary or involuntary, or any other distribution of assets of the Corporation among its shareholders for the purpose of winding up its affairs, subject to the prior rights of the holders of the Preferred Shares and to any other shares ranking equal to the Common Shares, the holders of the Common Shares shall be entitled to receive the remaining property and assets of the Corporation.
Preferred Shares
The Board may fix from time to time before each issuance of a series of Preferred Shares, the number of Preferred Shares comprising each series and the designation, rights, privileges, restrictions and conditions attaching to each series of Preferred Shares including, any voting rights, the rate or amount of dividends or the method of calculating dividends, the dates of payment thereof, the terms and conditions of redemption, purchase and conversion, if any, and any sinking fund or other provisions.
The Preferred Shares of each series is entitled, with respect to the payment of dividends and the distribution of assets or return of capital in the event of liquidation, dissolution or winding-up of the Corporation, or any other return of capital or distribution, to a preference over the Common Shares and over any other shares of the Corporation ranking by their terms junior to the Preferred Shares of that series. As at the date hereof, there were and are no preferred shares issued and outstanding. The Corporation is expected to issue Consideration Shares, which are Preferred Shares, to the Vendors at a deemed share price of $7.15, being the volume weighted average price of the Common Shares as traded on the facilities of the TSX.V for the five (5) trading days immediately preceding the date of the Holigen Purchase Agreement, as may be adjusted if required by the TSX.V. in order to satisfy the Share Consideration. Please see “The Acquisition”.
Flowr ULC Shares
In addition, there are currently 43,950,000 Flowr ULC Class A Shares issued by Flowr ULC, exchangeable at no additional consideration for Common Shares on a one for one basis at any time at the option of the holders thereof pursuant to the share exchange agreement dated August 2018 among the Corporation, Flowr ULC and certain shareholders of the Corporation. See “Description of Capital Structure - Flowr ULC Shares” and “Material Contracts - Share Exchange Agreement” in the AIF.
DIVIDEND POLICY
There are no restrictions in the Corporation’s articles preventing the Corporation from paying dividends. All of the Common Shares are entitled to an equal share in any dividends declared and paid. The non-voting preferred shares issuable in series are entitled to any dividends declared and paid, and are entitled to preference over the Common Shares. The Board will determine if, and when, dividends will be declared and paid in the future from funds properly applicable to the payment of dividends based on the Corporation’s financial position at the relevant time. In addition, under the terms of the ATB Credit Facilities, the Corporation will be subject to certain negative covenants restricting its ability to pay dividends.
PRIOR SALES
The following tables set forth details regarding issuances of Common Shares during the 12-month period before the date of this Prospectus.
Date | Type of Security Issued | Issuance Price per Security | Number of Securities Issued |
September 21, 2018 | Common Shares(1) | $2.60(2) | 86,282,864 |
November 10, 2018 | Common Shares(3) | $0.05(3) | 162,500 |
November 10, 2018 | Common Shares(4) | $1.30(4) | 18,461 |
December 7, 2018 | Common Shares(5) | $0.05(5) | 225,000 |
October 2018 - January 2019(6) | Common Shares(6) | $1.30(6) | 16,587 |
| February 25, 2019 | Common Shares(7) | $2.60(7) | 13,252 |
| May 10, 2019(8) | Common Shares | $6.25 | 2,165,547 |
| June 28, 2019 | Common Shares(9) | $2.60(9) | 44,172 |
(1) Issued to former shareholders of Flowr Privateco pursuant to the Business Combination Agreement between the Corporation, Flowr Privateco and Needle Subco Inc. (the “Business Combination Agreement”). Pursuant to the private placement of subscription receipts (the “Subscription Receipts”) completed prior to the closing of the Qualifying Transaction, Flowr Privateco issued 13,807,754 Subscription Receipts, which were exchanged for one common share of Flowr Privateco, which common shares were then exchanged for Common Shares of the Corporation on a one-for-one basis pursuant to the Qualifying Transaction. This figure includes the Common Shares issued to holders of such Subscription Receipts and 36,923 stock options exercised by former officers of the Corporation.
(2) Deemed price on a post-consolidation basis of one (1) post-consolidation Common Share for every thirteen (13) pre-consolidation Common Shares (the “Consolidation”), completed prior to the closing of the Qualifying Transaction.
(3) Issued upon exercise of 162,500 stock options by former employees of the Corporation. These stock options were granted by Flowr Privateco on October 30, 2017 with each option having an exercise price of $0.05.
(4) Issued upon exercise of 18,461 stock options by a former Director of the Corporation. These stock options were granted in connection with the Corporation’s initial public offering completed on September 15, 2017 with each option having an exercise price of $0.10 for each share (or an exercise price of $1.30 on a post-Consolidation basis).
(5) Issued upon exercise of 225,500 stock options by a former officer/employee of the Corporation. These stock options were granted by Flowr Privateco on October 30, 2017 with each option having an exercise price of $0.05.
(6) Issued upon exercise of 16,587 warrants held by agents of the Corporation between October 3, 2018 and January 23, 2019. These warrants were issued in connection with the Corporation's initial public offering completed on September 15, 2017 with each warrant having an exercise price of $0.10 for each share (or an exercise price of $1.30 on a post-Consolidation basis).
(7) Issued upon exercise of 13,252 warrants held by agents of the Corporation. These warrants were issued in connection with the financing completed concurrently with the Qualifying Transaction with each warrant having an exercise price of $2.60 for each share.
(8) Issued in connection with a non-brokered private placement completed on May 10, 2019.
(9) Issued upon exercise of 44,172 warrants held by agents of the Corporation. These warrants were issued in connection with the financing completed concurrently with the Qualifying Transaction with each warrant having an exercise price of $2.60 for each share.
The following table set forth details regarding issuances of stock options, warrants and restricted share units by the Corporation during the 12-month period before the date of this Prospectus.
Date | Type of Security Issued | Issuance Price per Security | Number of Securities Issued |
September - October 2018 | Stock Options(1) | $0.05 | 4,000,000 |
September 21, 2018 | Stock Options(2) | $2.60 | 8,035,530 |
September 21, 2018 | Stock Options(3) | $1.30 | 18,461 |
September 21, 2018 | Warrants(4) | $2.60 | 441,720 |
September 21, 2018 | Warrants(5) | $1.30 | 23,077 |
October 2, 2018 | Stock Options(6) | $5.24 | 325,000 |
October 23, 2018 | Stock Options(7) | $3.43 | 20,000 |
November 6, 2018 | Stock Options(8) | $3.60 | 20,000 |
April 24, 2019 | Stock Options(9) | $5.93 | 1,652,500 |
April 24, 2019 | Restricted Share Units(10) | N/A | 197,250 |
May 2, 2019 | Stock Options(11) | $5.70 | 75,000 |
May 9, 2019 | Restricted Share Units(12) | N/A | 80,000 |
June 3, 2019 | Restricted Share Units(13) | N/A | 95,000 |
| June 10, 2019 | Restricted Share Units(14) | N/A | 85,000 |
(1) Pursuant to the Business Combination Agreement and the Qualifying Transaction, these stock options were re-issued on September 10, 2018 and October 1, 2018 to employees and former employees in connection with the exchange of the stock options of Flowr Privateco that were originally issued on October 30, 2017 at a price of $0.05 per share. These options expire on October 30, 2022. See “The Flowr Corporation - Company Overview”.
(2) Pursuant to the Business Combination Agreement and the Qualifying Transaction, these stock options were issued to employees, directors and an agent in connection with the exchange of the stock options of Flowr Privateco held by such employees, directors and an agent that were originally issued between June 1, 2018 and September 14, 2018 at a price of $2.60 per share. These options expire between May 31, 2023 and September 14, 2023.
(3) These stock options were issued to a former Director of the Corporation that were originally granted in connection with the Corporation’s initial public offering completed on September 15, 2017 with each option having an exercise price of $0.10 for each share (or an exercise price of $1.30 on a post-Consolidation basis). These options were fully exercised on November 10, 2018 in exchange for Common Shares.
(4) These warrants were issued to certain agents of the Corporation in connection with the financing completed concurrently with the Qualifying Transaction and expire on September 20, 2021.
(5) These warrants were issued to agents in connection with the Corporation’s initial public offering completed on September 15, 2017 with each warrant having an exercise price of $0.10 for each share (or an exercise price of $1.30 on a post-Consolidation basis).
(6) Stock options granted to an officer of the Corporation expiring on October 2, 2023.
(7) Stock options granted to an employee of the Corporation expiring on October 23, 2023.
(8) Stock options granted to an employee of the Corporation expiring on November 6, 2023.
(9) Stock options granted to employees of the Corporation expiring on April 24, 2024.
(10) Restricted share units granted to employees of the Corporation expiring on April 24, 2024.
(11) Stock options granted to an employee and directors of the Corporations expiring on May 2, 2024.
(12) Restricted share units granted to employees of the Corporation expiring on May 9, 2024.
(13) Restricted share units granted to employees of the Corporation expiring on June 3, 2024. 60,000 of the restricted share units granted on this date were subsequently cancelled following the termination of employment of the grantee.
(14) Restricted share units granted to employees of the Corporation expiring on June 10, 2024.
TRADING PRICE AND VOLUME
The Common Shares are currently listed on the TSX.V under the trading symbol "FLWR". On June 21, 2019, the last trading day prior to the announcement of the Offering, the closing price of the Common Shares on the TSX.V was $7.26. On July 17, 2019, the last trading day prior to the date of this Prospectus the closing price of the Common Shares on the TSX.V was $4.76.
The following table sets out the high and low trading prices, as well as the trading volume for the Common Shares on the TSX.V (as reported by the TSX.V) for the periods indicated.
Month | High
($) | Low
($) | Volume |
2019 | | | |
| July 1 - 17 | 6.38 | 4.65 | 1,782,899 |
June | 7.50 | 5.56 | 2,907,879 |
May…………………………... | 7.75 | 6.37 | 2,878,729 |
April………………………….. | 7.00 | 5.85 | 3,965,770 |
March……………………….... | 8.42 | 5.33 | 11,221,170 |
February……………………… | 5.50 | 3.90 | 3,475,066 |
January……………………….. | 4.70 | 3.58 | 1,648,595 |
2018 | | | |
December…………………….. | 4.39 | 2.74 | 1,799,895 |
November…………………….. | 3.94 | 2.96 | 1,099,260 |
October……………………….. | 7.19 | 3.11 | 3,350,790 |
September…………………….. | 8.00 | 4.00 | 2,438,254 |
RELATIONSHIP BETWEEN THE UNDERWRITERS AND THE CORPORATION
The Underwriters and their respective affiliates are full service financial institutions engaged in various activities, which may include sales and trading, commercial and investment banking, advisory, investment management, investment research, principal investment, hedging, market making, brokerage and other financial and non-financial activities and services. Certain of the Underwriters and their respective affiliates have provided, and may in the future provide, a variety of these services to the Corporation and to persons and entities with relationships with the Corporation, for which they received or will receive customary fees and expenses.
The decision to issue the Offered Shares and the determination of the terms of the distribution of the Offered Shares were made through negotiation between the Corporation and the Representatives, on behalf of the Underwriters. The Canadian financial institutions of which Barclays Capital Canada Inc., BMO Capital Markets or Credit Suisse Securities (Canada), Inc. are an affiliate do not have any involvement in such decision or determination. As a consequence of the Offering, each of the Underwriters will receive its proportionate share of the Underwriters’ Fee.
CERTAIN CANADIAN FEDERAL INCOME TAX CONSIDERATIONS
In the opinion of Fasken Martineau DuMoulin LLP, counsel to the Corporation, and Borden Ladner Gervais LLP, counsel to the Underwriters, the following summary describes the principal Canadian federal income tax considerations that generally apply to a purchaser who acquires Offered Shares as beneficial owner pursuant to the Offering and who, at all relevant times, for purposes of the Tax Act: (i) deals at arm’s length with the Corporation and the Underwriters; (ii) is not affiliated with the Corporation or the Underwriters; and (iii) holds the Offered Shares as capital property (a “Holder”). Generally, the Offered Shares will be capital property to a Holder provided the Holder does not acquire or hold them in the course of carrying on a business or as part of an adventure or concern in the nature of trade.
This summary is based on the current provisions of the Tax Act and the Regulations, and counsel’s understanding of the current administrative policies and assessing practices of the Canada Revenue Agency made publicly available prior to the date hereof. This summary takes into account all specific proposals to amend the Tax Act and the Regulations publicly announced by or on behalf of the Minister of Finance (Canada) prior to the date hereof (the "Proposed Amendments") and assumes that all Proposed Amendments will be enacted in the form proposed. However, no assurances can be given that the Proposed Amendments will be enacted as proposed, or at all. This summary does not otherwise take into account or anticipate any changes in law or administrative policy or assessing practice whether by legislative, administrative or judicial action nor does it take into account tax legislation or considerations of any province, territory or foreign jurisdiction, which may differ from those discussed herein.
In general, amounts relevant to the computation of income under the Tax Act are reported in Canadian dollars. Accordingly, adjusted cost base, dividends and proceeds of disposition in respect of the Offered Shares must be converted into Canadian dollars generally based on the relevant spot rate quoted by the Bank of Canada on the date each such amount arises.
This summary is of a general nature only and is not, and is not intended to be, legal or tax advice to any particular holder. This summary is not exhaustive of all Canadian federal income tax considerations. Accordingly, prospective purchasers of Offered Shares should consult their own tax advisors having regard to their own particular circumstances.
Holders Resident in Canada
This portion of the summary only applies to a Holder who, at all relevant times, for purposes of the Tax Act, is, or is deemed to be, resident in Canada (a “Resident Holder”). Certain Resident Holders who may not otherwise be considered to hold their Offered Shares as capital property may be entitled to make the irrevocable election permitted by subsection 39(4) of the Tax Act to have their Offered Shares (and all other “Canadian securities”, as defined in the Tax Act) owned by such Resident Holder in the taxation year in which the election is made and in all subsequent taxation years deemed to be capital property. Resident Holders whose Offered Shares might not otherwise be considered to be capital property should consult their own tax advisors. This portion of the summary does not apply to: (i) a purchaser that is a “specified financial institution”; (ii) a purchaser an interest in which would be a “tax shelter investment”; (iii) a purchaser that is, for purposes of certain rules (referred to as the mark-to-market rules) applicable to securities held by financial institutions, a “financial institution”; (iv) a purchaser that reports its “Canadian tax results” in a currency other than Canadian currency; or (v) a purchaser that has entered into or will enter into with respect to the purchaser’s Offered Shares, a “derivative forward agreement”, as defined in the Tax Act. Such purchasers should consult their own tax advisors.
Dispositions of Offered Shares
On the disposition or deemed disposition of an Offered Share (other than to the Corporation, unless purchased by the Corporation in the open market in the manner in which shares are normally purchased by any member of the public in the open market, in which case other considerations may arise), a Resident Holder will generally realize a capital gain (or a capital loss) equal to the amount by which the proceeds of disposition exceed (or are exceeded by) the aggregate of the Resident Holder’s adjusted cost base of the Offered Share and any reasonable costs of disposition. See "Tax Treatment of Capital Gains and Losses" below. The cost to a Resident Holder of an Offered Share will generally be the amount paid to acquire the Offered Share. The adjusted cost base to a Resident Holder of Offered Shares acquired pursuant to this Offering will be determined by averaging the cost of such Offered Shares with the adjusted cost base of all other Common Shares (if any) held by the Resident Holder immediately before the time of acquisition.
Dividends on Offered Shares
A Resident Holder will be required to include in computing its income for a taxation year any dividends received (or deemed to be received) on the Offered Shares. In the case of a Resident Holder that is an individual (other than certain trusts), such dividends will be subject to the gross-up and dividend tax credit rules that apply to taxable dividends received from taxable Canadian corporations, including the enhanced gross-up and dividend tax credit applicable to any dividends designated by the Corporation as "eligible dividends" in accordance with the provisions of the Tax Act. There may be limitations on the Corporation’s ability to designate its dividends on the Offered Shares as "eligible dividends".
In the case of a Resident Holder that is a corporation, such dividends received or deemed to be received on Offered Shares held by the Resident Holder generally will be deductible in computing its taxable income. In certain circumstances, subsection 55(2) of the Tax Act will treat a taxable dividend received by a Resident Holder that is a corporation as proceeds of disposition or a capital gain.
A Resident Holder that is a "private corporation", for the purposes of Part IV of the Tax Act, or any other corporation resident in Canada controlled, whether because of a beneficial interest in one or more trusts or otherwise, by or for the benefit of an individual (other than a trust) or a related group of individuals (other than trusts), will generally be liable to pay a refundable tax of 38⅓% under Part IV of the Tax Act on dividends received (or deemed to be received) on the Offered Shares to the extent such dividends are deductible in computing the Resident Holder’s taxable income for the taxation year.
A dividend received by a Resident Holder who is an individual (other than certain trusts) on the Offered Shares may give rise to alternative minimum tax under the Tax Act, depending on the individual’s circumstances.
Tax Treatment of Capital Gains and Losses
Generally, a Resident Holder is required to include in computing its income for a taxation year one-half of the amount of any capital gain (a "taxable capital gain") realized in the year. Subject to and in accordance with the provisions of the Tax Act, a Resident Holder is required to deduct one-half of the amount of any capital loss (an "allowable capital loss") realized in a taxation year from taxable capital gains realized by the Resident Holder in the year. Allowable capital losses in excess of taxable capital gains realized in a taxation year may be carried back and deducted in any of the three preceding taxation years or carried forward and deducted in any subsequent taxation year against net taxable capital gains realized in such years subject to the detailed rules in the Tax Act.
The amount of any capital loss realized by a Resident Holder that is a corporation on the disposition of an Offered Share may be reduced by the amount of any dividends received (or deemed to be received) by the Resident Holder on such Offered Share or a share for which the Offered Share is substituted or exchanged to the extent and under the circumstances described by the Tax Act. Similar rules may apply where an Offered Share is owned by a partnership or trust of which a corporation, trust or partnership is a member or beneficiary. Such Resident Holders should consult their own advisors.
Capital gains realized by a Resident Holder who is an individual (other than certain trusts) may give rise to a liability for alternative minimum tax under the Tax Act.
If the Resident Holder is throughout the relevant taxation year a "Canadian-controlled private corporation" (as defined in the Tax Act), the Resident Holder may also be liable to pay a refundable tax on its "aggregate investment income", which is defined in the Tax Act to include taxable capital gains.
Holders Not Resident in Canada
This portion of the summary only applies to a Holder who, at all relevant times, for purposes of the Tax Act, is not, and is not deemed to be, resident in Canada and does not use or hold and is not deemed to use or hold the Offered Shares in a business carried on in Canada (a "Non-Resident Holder"). Special rules, which are not discussed in this summary, may apply to a non-Canadian Holder that is an insurer that carries on an insurance business in Canada and elsewhere.
Dividends on Offered Shares
Dividends paid or credited on the Offered Shares or deemed to be paid or credited on the Offered Shares to a Non-Resident Holder will be subject to Canadian withholding tax at the rate of 25%, subject to any reduction in the rate of withholding to which the Non-Resident Holder is entitled under any applicable income tax convention. For example, under the Canada-U.S. Income Tax Convention (1980) (the "Convention"), as amended, where dividends on the Offered Shares are considered to be paid to or derived by a Non-Resident Holder that is the beneficial owner of the dividends and is a United States resident for the purposes of, and is entitled to full benefits in accordance with, the provisions of the Convention, the applicable rate of Canadian withholding tax is generally reduced to 15%.
Dispositions of Offered Shares
A Non-Resident Holder will not be subject to tax under the Tax Act on any capital gain realized on a disposition or deemed disposition of Offered Shares (other than to the Corporation, unless purchased by the Corporation in the open market in the manner in which shares are normally purchased by any member of the public in the open market, in which case other considerations may arise), unless the Offered Shares are "taxable Canadian property" of the Non-Resident Holder for purposes of the Tax Act and the Non-Resident Holder is not entitled to relief under an applicable income tax convention between Canada and the country in which the Non-Resident Holder is resident.
Generally, the Offered Shares will not constitute "taxable Canadian property" of a Non-Resident Holder at a particular time provided that the Offered Shares are listed at that time on a "designated stock exchange" for purposes of the Tax Act (which currently includes the TSX.V), unless at any particular time during the 60-month period that ends at that time: both: (i) (A) the Non-Resident Holder, (B) persons with whom the Non-Resident Holder does not deal with at arm’s length, (C) partnerships in which the Non-Resident Holder or a person described in (B) holds an interest directly or indirectly through one or more partnerships or (D) any combination of (A) to (C) owned 25% or more of the issued shares of any class or series of the capital stock of the Corporation; and (ii) more than 50% of the fair market value of the Offered Shares was derived directly or indirectly from one or any combination of: (a) real or immovable properties situated in Canada, (b) "Canadian resource properties" (as defined in the Tax Act), (c) "timber resource properties" (as defined in the Tax Act), and (d) options in respect of, or interests in, or for civil law rights in, property in any of the foregoing whether or not the property exists. Notwithstanding the foregoing, in certain circumstances set out in the Tax Act, Offered Shares may be deemed to be taxable Canadian property. Non-Resident Holders whose Offered Shares may constitute taxable Canadian property should consult their own tax advisors.
CERTAIN UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS
The following is a general summary of certain material U.S. federal income tax considerations applicable to a U.S. Holder (as defined below) arising from and relating to the acquisition, ownership, and disposition of Offered Shares acquired pursuant to this Prospectus.
This summary is for general information purposes only and does not purport to be a complete analysis or listing of all potential U.S. federal income tax considerations that may apply to a U.S. Holder arising from and relating to the acquisition, ownership, and disposition of Offered Shares. In addition, this summary does not take into account the individual facts and circumstances of any particular U.S. Holder that may affect the U.S. federal income tax consequences to such U.S. Holder, including, without limitation, specific tax consequences to a U.S. Holder under an applicable income tax treaty. Accordingly, this summary is not intended to be, and should not be construed as, legal or U.S. federal income tax advice with respect to any U.S. Holder. This summary does not address the U.S. federal net investment income, U.S. alternative minimum, U.S. federal estate and gift, U.S. state and local, and non-U.S. tax consequences to U.S. Holders of the acquisition, ownership, and disposition of Offered Shares. In addition, except as specifically set forth below, this summary does not discuss applicable tax reporting requirements. Each prospective U.S. Holder should consult its own tax advisor regarding the U.S. federal, U.S. federal net investment income, U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and non-U.S. tax consequences relating to the acquisition, ownership, and disposition of Offered Shares.
No legal opinion from U.S. legal counsel or ruling from the Internal Revenue Service (the "IRS") has been requested, or will be obtained, regarding the U.S. federal income tax consequences of the acquisition, ownership, and disposition of Offered Shares. This summary is not binding on the IRS, and the IRS is not precluded from taking a position that is different from, and contrary to, the positions taken in this summary. In addition, because the authorities on which this summary are based are subject to various interpretations, the IRS and the U.S. courts could disagree with one or more of the conclusions described in this summary.
Scope of this Summary
Authorities
This summary is based on the Internal Revenue Code of 1986, as amended (the "Code"), Treasury Regulations (whether final, temporary, or proposed), published rulings of the IRS, published administrative positions of the IRS, the Convention Between Canada and the United States of America with Respect to Taxes on Income and on Capital, signed September 26, 1980, as amended (the "Canada-U.S. Tax Convention"), and U.S. court decisions that are applicable, and, in each case, as in effect and available, as of the date of this document. Any of the authorities on which this summary is based could be changed in a material and adverse manner at any time, and any such change could be applied retroactively. This summary does not discuss the potential effects, whether adverse or beneficial, of any proposed legislation.
U.S. Holders
For purposes of this summary, the term "U.S. Holder" means a beneficial owner of Offered Shares acquired pursuant to this Offering that is for U.S. federal income tax purposes:
- an individual who is a citizen or resident of the United States;
- a corporation (or other entity treated as a corporation for U.S. federal income tax purposes) organized under the laws of the United States, any state thereof or the District of Columbia;
- an estate whose income is subject to U.S. federal income taxation regardless of its source; or
- a trust that (1) is subject to the primary supervision of a court within the United States and the control of one or more U.S. persons for all substantial decisions or (2) has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person.
U.S. Holders Subject to Special U.S. Federal Income Tax Rules Not Addressed
This summary does not address the U.S. federal income tax considerations applicable to U.S. Holders that are subject to special provisions under the Code, including, but not limited to, U.S. Holders that: (a) are tax-exempt organizations, qualified retirement plans, individual retirement accounts, or other tax-deferred accounts; (b) are financial institutions, underwriters, insurance companies, real estate investment trusts, or regulated investment companies; (c) are broker-dealers, dealers, or traders in securities or currencies that elect to apply a mark-to-market accounting method; (d) have a "functional currency" other than the U.S. dollar; (e) own Offered Shares as part of a straddle, hedging transaction, conversion transaction, constructive sale, or other integrated transaction; (f) acquire Offered Shares in connection with the exercise of employee stock options or otherwise as compensation for services; (g) hold Offered Shares other than as a capital asset within the meaning of Section 1221 of the Code (generally, property held for investment purposes); (h) are required to accelerate the recognition of any item of gross income with respect to Offered Shares as a result of such income being recognized on an applicable financial statement; or (i) own, have owned or will own (directly, indirectly, or by attribution) 10% or more of the total combined voting power or value of the outstanding shares of the Corporation. This summary also does not address the U.S. federal income tax considerations applicable to U.S. Holders who are: (a) U.S. expatriates or former long-term residents of the U.S.; (b) persons that have been, are, or will be a resident or deemed to be a resident in Canada for purposes of the Tax Act; (c) persons that use or hold, will use or hold, or that are or will be deemed to use or hold Offered Shares in connection with carrying on a business in Canada; (d) persons whose Offered Shares constitute "taxable Canadian property" under the Tax Act; or (e) persons that have a permanent establishment in Canada for the purposes of the Canada-U.S. Tax Convention. This discussion does not address the alternative minimum tax, nor does it address U.S. federal estate and gift tax or state, local or non-U.S. tax consequences. U.S. Holders should consult their own tax advisors regarding the particular U.S. federal income tax consequences to them of holding and disposing of the Offered Shares, any tax consequences that may arise under any other U.S. federal tax laws, the laws of any relevant non-U.S., state, local, or other taxing jurisdiction or under any applicable tax treaty, and the possible effects of changes in the U.S. federal or other tax laws.
If an entity or arrangement that is classified as a partnership (or other "pass-through" entity) for U.S. federal income tax purposes holds Offered Shares, the U.S. federal income tax consequences to such entity and the partners (or other owners) of such entity generally will depend on the activities of the entity and the status of such partners (or owners). This summary does not address the tax consequences to any such partner (or owner). Partners (or other owners) of entities or arrangements that are classified as partnerships or as "pass-through" entities for U.S. federal income tax purposes should consult their own tax advisors regarding the U.S. federal income tax consequences arising from and relating to the acquisition, ownership, and disposition of Offered Shares.
Ownership and Disposition of Offered Shares
The following discussion is subject in its entirety to the rules described below under the heading "Passive Foreign Investment Company Rules".
Taxation of Distributions
A U.S. Holder that receives a distribution, including a constructive distribution, with respect to an Offered Share will be required to include the amount of such distribution in gross income as a dividend (without reduction for any foreign income tax withheld from such distribution) to the extent of the current or accumulated "earnings and profits" of the Corporation, as computed for U.S. federal income tax purposes. To the extent that a distribution exceeds the current and accumulated "earnings and profits" of the Corporation, such distribution will be treated first as a tax-free return of capital to the extent of a U.S. Holder’s tax basis in the Offered Shares and thereafter as gain from the sale or exchange of such Offered Shares (see "Sale or Other Taxable Disposition of Offered Shares" below). However, the Corporation may not maintain the calculations of its earnings and profits in accordance with U.S. federal income tax principles, and each U.S. Holder may have to assume that any distribution by the Corporation with respect to the Offered Shares will constitute ordinary dividend income. Dividends received on Offered Shares by corporate U.S. Holders generally will not be eligible for the "dividends received deduction". Subject to applicable limitations and provided the Corporation is eligible for the benefits of the Canada-U.S. Tax Convention or the Offered Shares are readily tradable on a United States securities market, dividends paid by the Corporation to non-corporate U.S. Holders, including individuals, generally will be eligible for the preferential tax rates applicable to long-term capital gains for dividends, provided certain holding period and other conditions are satisfied, including that the Corporation not be classified as a PFIC (as defined below) in the tax year of distribution or in the preceding tax year. The dividend rules are complex, and each U.S. Holder should consult its own tax advisor regarding the application of such rules.
Sale or Other Taxable Disposition of Offered Shares
A U.S. Holder will generally recognize gain or loss on the sale or other taxable disposition of Offered Shares in an amount equal to the difference, if any, between (a) the amount of cash plus the fair market value of any property received and (b) such U.S. Holder’s tax basis in such Offered Shares sold or otherwise disposed of. A U.S. Holder’s basis in the Offered Shares generally will be the amount that such U.S. Holder paid for the Offered Shares. Any such gain or loss generally will be capital gain or loss, which will be long-term capital gain or loss if, at the time of the sale or other disposition, such Offered Shares are held for more than one year.
Preferential tax rates apply to long-term capital gains of a U.S. Holder that is an individual, estate, or trust. There are currently no preferential tax rates for long-term capital gains of a U.S. Holder that is a corporation. Deductions for capital losses are subject to significant limitations under the Code.
Passive Foreign Investment Company Rules
If the Corporation were to constitute a "passive foreign investment company" ("PFIC") for any year during a U.S. Holder’s holding period, then certain potentially adverse rules would affect the U.S. federal income tax consequences to a U.S. Holder resulting from the acquisition, ownership and disposition of Offered Shares. Based on current business plans and financial expectations, the Corporation expects that it should not be a PFIC for its current tax year and expects that it should not be a PFIC for the foreseeable future. No opinion of legal counsel or ruling from the IRS concerning the status of the Corporation as a PFIC has been obtained or is currently planned to be requested. However, PFIC classification is fundamentally factual in nature, generally cannot be determined until the close of the tax year in question, and is determined annually. Additionally, the analysis depends, in part, on the application of complex U.S. federal income tax rules, which are subject to differing interpretations. Consequently, there can be no assurance that the Corporation has never been and will not become a PFIC for any tax year during which U.S. Holders hold Offered Shares.
In any year in which the Corporation is classified as a PFIC, a U.S. Holder will be required to file an annual report with the IRS containing such information as Treasury Regulations and/or other IRS guidance may require. In addition to penalties, a failure to satisfy such reporting requirements may result in an extension of the time period during which the IRS can assess a tax. U.S. Holders should consult their own tax advisors regarding the requirements of filing such information returns under these rules, including the requirement to file an IRS Form 8621 annually.
The Corporation generally will be a PFIC if, after the application of certain "look-through" rules with respect to subsidiaries in which the Corporation holds at least 25% of the value of such subsidiary, for a tax year, (a) 75% or more of the gross income of the Corporation for such tax year is passive income (the "income test") or (b) 50% or more of the value of the Corporation’s assets either produce passive income or are held for the production of passive income (the "asset test"), based on the quarterly average of the fair market value of such assets. "Gross income" generally includes all sales revenues less the cost of goods sold, plus income from investments and from incidental or outside operations or sources, and "passive income" generally includes, for example, dividends, interest, certain rents and royalties, certain gains from the sale of stock and securities, and certain gains from commodities transactions.
If the Corporation were a PFIC in any tax year during which a U.S. Holder held Offered Shares, such holder generally would be subject to special rules with respect to "excess distributions" made by the Corporation on the Offered Shares and with respect to gain from the disposition of Offered Shares. An "excess distribution" generally is defined as the excess of distributions with respect to the Offered Shares received by a U.S Holder in any tax year over 125% of the average annual distributions such U.S. Holder has received from the Corporation during the shorter of the three preceding tax years, or such U.S. Holder’s holding period for the Offered Shares. Generally, a U.S. Holder would be required to allocate any excess distribution or gain from the disposition of the Offered Shares ratably over its holding period for the Offered Shares. Such amounts allocated to the year of the disposition or excess distribution would be taxed as ordinary income, and amounts allocated to prior tax years would be taxed as ordinary income at the highest tax rate in effect for each such year and an interest charge at a rate applicable to underpayments of tax would apply.
If the Corporation were a PFIC for the taxable year in which a dividend is paid or in the preceding taxable year, the dividend would not be eligible for the preferential tax rates applicable to qualified dividends. Special rules also apply to foreign tax credits that a U.S. Holder may claim on a distribution from a PFIC.
If the Corporation is a PFIC for any taxable year during which a U.S. Holder holds the Offered Shares, the Corporation will continue to be treated as a PFIC with respect to such U.S. Holder for any subsequent taxable year in which such U.S. Holder continues to hold the Offered Shares, regardless of whether the Corporation ceases to be a PFIC in one or more subsequent taxable years. A U.S. Holder may terminate this deemed PFIC status by electing to recognize gain (which will be taxed under the special rules discussed above) as if such Offered Shares were sold on the last day of the last taxable year for which the Corporation was a PFIC.
While there are U.S. federal income tax elections that can sometimes be made to mitigate these adverse tax consequences (including the "QEF Election" under Section 1295 of the Code and the "Mark-to-Market Election" under Section 1296 of the Code), such elections are available in limited circumstances and must be made in a timely manner.
U.S. Holders should be aware that, for each tax year, if any, that the Corporation is a PFIC, the Corporation can provide no assurances that it will satisfy the record keeping requirements or make available to U.S. Holders the information such U.S. Holders require to make a QEF Election with respect to the Corporation or any subsidiary that is also classified as a PFIC.
Certain additional adverse rules may apply with respect to a U.S. Holder if the Corporation is a PFIC, regardless of whether the U.S. Holder makes a QEF Election. These rules include special rules that apply to the amount of foreign tax credit that a U.S. Holder may claim on a distribution from a PFIC. Subject to these special rules, foreign taxes paid with respect to any distribution in respect of stock in a PFIC are generally available for the foreign tax credit. U.S. Holders should consult their own tax advisors regarding the potential application of the PFIC rules to the ownership and disposition of Offered Shares, and the availability of certain U.S. tax elections under the PFIC rules.
Additional Considerations
Receipt of Foreign Currency
The amount of any distribution paid to a U.S. Holder in foreign currency, or on the sale, exchange or other taxable disposition of Offered Shares, generally will be equal to the U.S. dollar value of such foreign currency based on the exchange rate applicable on the date of receipt (regardless of whether such foreign currency is converted into U.S. dollars at that time). A U.S. Holder will have a basis in the foreign currency equal to its U.S. dollar value on the date of receipt. Any U.S. Holder who converts or otherwise disposes of the foreign currency after the date of receipt may have a foreign currency exchange gain or loss that would be treated as ordinary income or loss, and generally will be U.S. source income or loss for foreign tax credit purposes. Different rules apply to U.S. Holders who use the accrual method of tax accounting. Each U.S. Holder should consult its own U.S. tax advisor regarding the U.S. federal income tax consequences of receiving, owning, and disposing of foreign currency.
Foreign Tax Credit
Subject to the PFIC rules discussed above, a U.S. Holder that pays (whether directly or through withholding) Canadian income tax with respect to dividends paid on the Offered Shares generally will be entitled, at the election of such U.S. Holder, to receive either a deduction or a credit for such Canadian income tax. Generally, a credit will reduce a U.S. Holder’s U.S. federal income tax liability on a dollar-for-dollar basis, whereas a deduction will reduce a U.S. Holder’s income that is subject to U.S. federal income tax. This election is made on a year-by-year basis and applies to all foreign taxes paid (whether directly or through withholding) by a U.S. Holder during a year.
The foreign tax credit rules are complex, and involve the application of rules that depend on a U.S. Holders’ particular circumstances. Accordingly, each U.S. Holder should consult its own U.S. tax advisors regarding the foreign tax credit rules.
Backup Withholding and Information Reporting
Under U.S. federal income tax law and Treasury Regulations, certain categories of U.S. Holders must file information returns with respect to their investment in, or involvement in, a foreign corporation. For example, U.S. return disclosure obligations (and related penalties) are imposed on individuals who are U.S. Holders that hold certain specified foreign financial assets in excess of certain threshold amounts. The definition of specified foreign financial assets includes not only financial accounts maintained in foreign financial institutions, but also, unless held in accounts maintained by a financial institution, any stock or security issued by a non-U.S. person, any financial instrument or contract held for investment that has an issuer or counterparty other than a U.S. person and any interest in a foreign entity. U. S. Holders may be subject to these reporting requirements unless their Offered Shares are held in an account at certain financial institutions. Penalties for failure to file certain of these information returns are substantial. U.S. Holders should consult their own tax advisors regarding the requirements of filing information returns, including the requirement to file an IRS Form 8938.
Payments made within the U.S. or by a U.S. payor or U.S. middleman, of dividends on, and proceeds arising from the sale or other taxable disposition of, Offered Shares will generally be subject to information reporting and backup withholding tax, at the current rate of 24%, if a U.S. Holder (a) fails to furnish such U.S. Holder’s correct U.S. taxpayer identification number (generally on Form W-9), (b) furnishes an incorrect U.S. taxpayer identification number, (c) is notified by the IRS that such U.S. Holder has previously failed to properly report items subject to backup withholding tax, or (d) fails to certify, under penalty of perjury, that such U.S. Holder has furnished its correct U.S. taxpayer identification number and that the IRS has not notified such U.S. Holder that it is subject to backup withholding tax. However, certain exempt persons generally are excluded from these information reporting and backup withholding rules. Backup withholding is not an additional tax. Any amounts withheld under the U.S. backup withholding tax rules will be allowed as a credit against a U.S. Holder’s U.S. federal income tax liability, if any, or will be refunded, if such U.S. Holder furnishes required information to the IRS in a timely manner.
The discussion of reporting requirements set forth above is not intended to constitute a complete description of all reporting requirements that may apply to a U.S. Holder. A failure to satisfy certain reporting requirements may result in an extension of the time period during which the IRS can assess a tax, and under certain circumstances, such an extension may apply to assessments of amounts unrelated to any unsatisfied reporting requirement. Each U.S. Holder should consult its own tax advisor regarding the information reporting and backup withholding rules.
THE ABOVE SUMMARY IS NOT INTENDED TO CONSTITUTE A COMPLETE ANALYSIS OF ALL TAX CONSIDERATIONS APPLICABLE TO U.S. HOLDERS WITH RESPECT TO THE ACQUISITION, OWNERSHIP, AND DISPOSITION OF OFFERED SHARES. U.S. HOLDERS SHOULD CONSULT THEIR OWN TAX ADVISORS AS TO THE TAX CONSIDERATIONS APPLICABLE TO THEM IN THEIR OWN PARTICULAR CIRCUMSTANCES.
LEGAL MATTERS
Certain Canadian legal matters relating to the Offering will be passed upon on behalf of the Corporation by Fasken Martineau DuMoulin LLP, and on behalf of the Underwriters by Borden Ladner Gervais LLP. As of the date hereof, Fasken Martineau DuMoulin LLP, and its partners and associates, and Borden Ladner Gervais LLP, and its partners and associates, beneficially own, directly or indirectly, in their respective groups, less than 1% of any class of outstanding securities of the Corporation. The Underwriters are represented by Shearman & Sterling LLP with respect to certain U.S. legal matters.
AUDITORS, TRANSFER AGENT AND REGISTRAR
MNP LLP is the auditor of the Corporation and has confirmed that they are independent within the meaning of the relevant rules and related interpretations prescribed by the relevant professional bodies in Canada and any applicable legislation or regulations.
The transfer agent and registrar for the Common Shares is Computershare Investor Services Inc. at its principal offices in Toronto, Ontario.
RSM Canada LLP is the auditor of Holigen and has confirmed that they are independent within the meaning of the relevant rules and related interpretations prescribed by the relevant professional bodies in Canada and any applicable legislation or regulations.
INTEREST OF EXPERTS
The following are persons or companies whose profession or business gives authority to a statement made in this Prospectus as having prepared or certified a part of that document or report described in this Prospectus:
- Fasken Martineau DuMoulin LLP is the Corporation’s counsel with respect to Canadian legal matters herein;
- Borden Ladner Gervais LLP is the Underwriters’ counsel with respect to Canadian legal matters herein;
- MNP LLP is the external auditor of the Corporation and reported on the Corporation’s audited financial statements for the years ended on December 31, 2018 and 2017;
- RSM Canada LLP is the external auditor of Holigen and reported on Holigen’s audited financial statements for the year ended on December 31, 2018 and for the period ended December 31, 2017.
To the knowledge of management, as of the date hereof, the aforementioned firms held either less than one percent or no securities of the Corporation or of any associate or affiliate of the Corporation.
PROMOTERS
As of the date of this Prospectus: (i) Tom Flow beneficially owns, or controls or directs, directly or indirectly, a total of 26,025,000 Common Shares, representing approximately 19.5% of the equity of the Corporation on a fully diluted basis (excluding equity incentives such as options, warrants and/or restricted share units); and (ii) Steven Klein, directly and indirectly through Core Flow Canada Holdings Inc., beneficially owns, or controls or directs, directly or indirectly, a total of 8,253,014 Common Shares and 41,598,000 Flowr ULC Class A Shares that are convertible into Common Shares, representing approximately 37% of the equity of the Corporation on a fully diluted basis (excluding equity incentives such as options, warrants and/or restricted share units). Other than as disclosed in this section or elsewhere in this Prospectus or the documents incorporated by reference herein, no person who was a promoter of the Corporation:
- received anything of value directly or indirectly from the Corporation or a subsidiary within the last two years;
- sold or otherwise transferred any asset to the Corporation or a subsidiary within the last two years;
has been a director, chief executive officer or chief financial officer of any company that during the past 10 years was the subject of a cease trade order or similar order or an order that denied the Corporation access to any exemptions under securities legislation for a period of more than 30 consecutive days or became bankrupt, made a proposal under any legislation relating to bankruptcy or insolvency or been subject to or instituted any proceedings, arrangement or compromise with creditors or had a receiver or receiver manager or trustee appointed to hold its assets;
115
- has been subject to any penalties or sanctions imposed by a court relating to Canadian securities legislation or by a Canadian securities regulatory authority or has entered into a settlement agreement with a Canadian securities regulatory authority within the last two years;
- has been subject to any other penalties or sanctions imposed by a court or regulatory body that would be likely to be considered important to a reasonable investor making an investment decision within the last two years; or
- has within the past 10 years become bankrupt, made a proposal under any legislation relating to bankruptcy or insolvency or been subject to or instituted any proceedings, arrangement or compromise with creditors or had a receiver or receiver manager or trustee appointed to hold its assets.
APPENDIX "A" - GLOSSARY OF TERMS
In addition to terms defined elsewhere in this Prospectus, the following are defined terms used in this Prospectus:
2018 Industrial Hemp Regulations | has the meaning ascribed to that term under the heading "Regulatory Framework - Canada - Background" in this Prospectus. |
2019 Amendments | has the meaning ascribed to that term under the heading "Regulatory Framework - Canada - Classes of Cannabis" in this Prospectus. |
ACMPR | means the Access to Cannabis for Medical Purposes Regulations, SOR/2016-230. |
Acquisition | has the meaning ascribed to that term on the cover page of this Prospectus. |
Acquisition Closing Date | has the meaning ascribed to that term on the cover page of this Prospectus. |
Additional Floors | has the meaning ascribed to that term under the heading "Our Current and Proposed Cultivation and R&D Facilities - Research & Development" in this Prospectus. |
Additional Offered Shares | has the meaning ascribed to that term on the cover page of this Prospectus. |
AIF | has the meaning ascribed to that term under the heading "Documents Incorporated by Reference" in this Prospectus. |
allowable capital loss | has the meaning ascribed to that term under the heading "Certain Canadian Federal Income Tax Considerations - Holders Resident in Canada - Tax Treatment of Capital Gains and Losses" in this Prospectus. |
Amended and Restated SPSA | has the meaning ascribed to that term under the heading "The Business - Where is Flowr Going?" in this Prospectus. |
Anspec | has the meaning ascribed to that term under the heading "Summary - The Business - Global Footprint to Capture Worldwide Opportunities in this Prospectus. |
API | means active pharmaceutical ingredient. |
APS | has the meaning ascribed to that term under the heading "Regulatory Framework - Australia - Import" in this Prospectus. |
ARTG | means the Australian Register of Therapeutic Goods |
asset test | has the meaning ascribed to that term under the heading "Certain United States Federal Income Tax Considerations - Passive Foreign Investment Company Rules" in this Prospectus. |
ATB Credit Facilities | has the meaning ascribed to that term under the heading "The Flowr Corporation - Recent Developments" " in this Prospectus. |
Australia Planting Milestone | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement - Milestones & Resource Commitments" in this Prospectus. |
BDS Analytics | means BDS Analytics, Inc. |
Biomass | has the meaning ascribed to that term under the heading "Regulatory Framework - Canada - Industrial Hemp Regulations" in this Prospectus. |
Board | means the board of directors of the Corporation. |
Business Combination Agreement | has the meaning ascribed to that term under the heading "Prior Sales" in this Prospectus. |
Canada-U.S. Tax Convention | has the meaning ascribed to that term under the heading "Certain United States Federal Income Tax Considerations - Scope of this Summary - Authorities" in this Prospectus. |
Canadian securities | has the meaning ascribed to that term under the heading "Certain Canadian Federal Income Tax Considerations - Holders Resident in Canada" in this Prospectus. |
Cannabis Act | means the Cannabis Act, SC 2018, c 16 (Canada). |
Cannabis Regulations | means the Cannabis Regulations, SOR/2018-144 of the Cannabis Act. |
Cannatech | has the meaning ascribed to that term under the heading "The Flowr Corporation - Company History" in this Prospectus. |
Cash Consideration | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement" in this Prospectus. |
CBD | means cannabidiol. |
CDS | has the meaning ascribed to that term on the cover page of this Prospectus. |
CDSA | means the Controlled Drugs and Substances Act , SC 1996, c 19 (Canada). |
Closing Date | has the meaning ascribed to that term on the cover page of this Prospectus. |
Code | has the meaning ascribed to that term under the heading "Certain United States Federal Income Tax Considerations - Scope of this Summary - Authorities" in this Prospectus. |
Common Shares | has the meaning ascribed to that term on the cover page of this Prospectus. |
Consideration Shares | has the meaning ascribed to that term on the cover page of this Prospectus. |
Consolidation | means the consolidation of the Needle Shares on the basis of one (1) post-consolidation Needle Share for every thirteen (13) pre-consolidation Needle Shares completed on September 20, 2018. |
Convention | has the meaning ascribed to that term under the heading "Certain Canadian Federal Income Tax Considerations - Holders Not Resident in Canada - Dividends on Offered Shares" in this Prospectus. |
Conversion Expiry Date | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement - Terms of Consideration Shares - Conversion into Common Shares" in this Prospectus. |
Conversion Agreement | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement - Conversion Agreement" in this Prospectus. |
Conversion Rate | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement - Terms of Consideration Shares - Conversion into Common Shares" in this Prospectus. |
Corporation or Flowr | means The Flowr Corporation, and unless the context otherwise requires, includes each of the subsidiaries of The Flowr Corporation. |
CSA | has the meaning ascribed to that term under the heading "Risk Factors" in this Prospectus. |
CSIEA | has the meaning ascribed to that term under the heading "Risk Factors" in this Prospectus. |
CTLS | has the meaning ascribed to that term under the heading "Regulatory Framework - Canada - Background" in this Prospectus. |
Deductible | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement - Representations, Warranties and Covenants" in this Prospectus. |
dialing in | has the meaning ascribed to that term under the heading "Our Current and Proposed Cultivation and R&D Facilities - Canada" in this Prospectus. |
Director | means a member of the Board. |
DL 15/93 | has the meaning ascribed to that term under the heading "Regulatory Framework - Portugal" in this Prospectus. |
DMT Act | means Drugs Misuse and Trafficking Act 1985 (NSW) |
Holder | has the meaning ascribed to that term under the heading "Certain Canadian Federal Income Tax Considerations" in this Prospectus. |
EBITDA | has the meaning ascribed to that term under the heading "Non-IFRS Measures" in this Prospectus. |
Escrow Agent | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement" in this Prospectus. |
Escrow Agreement | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement" in this Prospectus. |
Filing Statement | means the filing statement of Needle in respect of the Qualifying Transaction dated September 19, 2018. |
Flowr Area | has the meaning ascribed to that term under the heading "Our Current and Proposed Cultivation and R&D Facilities - Research & Development" in this Prospectus. |
Flowr Common Shares | means the common shares in the capital of Flowr Privateco. |
Flowr Forest | has the meaning ascribed to that term under the heading "Summary - The Business - Who is Flowr" in this Prospectus. |
Flowr Okanagan | has the meaning ascribed to that term under the heading "The Flowr Corporation - Company History" in this Prospectus. |
Flowr Privateco | means The Flowr Corporation, as it existed prior to the Qualifying Transaction. |
Flowr ULC | has the meaning ascribed to that term under the heading "The Flowr Corporation - Company History" in this Prospectus. |
Flowr ULC Class A Shares | means the class A preferred shares in the capital of Flowr ULC. |
Forward-looking statements | has the meaning ascribed to that term under the heading "Cautionary Note Regarding Forward-Looking Information" in this Prospectus. |
Genetics | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement - Milestones & Resource Commitments" in this Prospectus. |
GMP | means the ICH good manufacturing practices standards. |
Governance Agreement | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement - Governance Agreement" in this Prospectus. |
Guarantors | has the meaning ascribed to that term under the heading "The Acquisition of Holigen- Overview of the Acquisition" in this Prospectus. |
Hawthorne | has the meaning ascribed to that term under the heading "Summary - The Business - R&D Engine to Drive Leadership, Enhanced by Hawthorne Partnership" in this Prospectus. |
Hawthorne R&D | has the meaning ascribed to that term under the heading "Our Current and Proposed Cultivation and R&D Facilities - Research & Development" in this Prospectus. |
Holigen | has the meaning ascribed to that term on the cover page of this Prospectus. |
Holigen Australia | has the meaning ascribed to that term under the heading "The Business - Where is Flowr Going" in this Prospectus. |
Holigen Portugal | has the meaning ascribed to that term under the heading "The Business - Where is Flowr Going" in this Prospectus. |
Holigen Purchase Agreement | has the meaning ascribed to that term on the cover page of this Prospectus. |
IASB | has the meaning ascribed to that term on the cover page of this Prospectus. |
ICH | means the International Council for Harmonisation. |
IFRS | has the meaning ascribed to that term on the cover page of this Prospectus. |
Immediate Conversion Date | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement - Terms of Consideration Shares - Conversion into Common Shares" in this Prospectus. |
income test | has the meaning ascribed to that term under the heading "Certain United States Federal Income Tax Considerations - Passive Foreign Investment Company Rules" in this Prospectus. |
INFARMED | has the meaning ascribed to that term under the heading "Regulatory Framework - Portugal" in this Prospectus. |
Information | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement - Milestones & Resource Commitments" in this Prospectus. |
IRS | has the meaning ascribed to that term under the heading "Certain United States Federal Income Tax Considerations" in this Prospectus. |
Issue Price | has the meaning ascribed to that term on the cover page of this Prospectus. |
Junior Shares | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement - Terms of Consideration Shares - Dividends" in this Prospectus. |
K1 Facility | has the meaning ascribed to that term under the heading "Summary - The Business - Focus On Quality Across Geographies and Product Formats?" in this Prospectus. |
K2 Facility | has the meaning ascribed to that term under the heading "Summary - Flowr’s Strengths - Facilities Designed to Maximize Yield and Profitability" in this Prospectus. |
Kelowna Campus | has the meaning ascribed to that term under the heading "Summary - The Business - Who is Flowr?" in this Prospectus. |
Law 33/2018 | has the meaning ascribed to that term under the heading "Regulatory Framework - Portugal" in this Prospectus. |
Licence | has the meaning ascribed to that term under the heading "Our Current and Proposed Cultivation and R&D Facilities - Canada" in this Prospectus. |
Liquidation | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement - Terms of Consideration Shares - Liquidation, Dissolution and Winding-Up" in this Prospectus. |
Liquidation Value | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement - Terms of Consideration Shares - Liquidation, Dissolution and Winding-Up" in this Prospectus. |
Loan Consideration | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement" in this Prospectus |
Management | means the management team of the Corporation. |
MD&A | has the meaning ascribed to that term under the heading "Non-IFRS Measures". |
Milestones | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement - Terms of Consideration Shares - Conversion into Common Shares" in this Prospectus. |
Minister | means the Minister of Border Security and Organized Crime Reduction. |
MMAR | means the Marihuana Medical Access Regulations, SOR/2001-227 of the CDSA. |
MMPR | means the Marihuana for Medical Purposes Regulations, SOR/2013-119 of the CDSA. |
Narcotics Act | has the meaning ascribed to that term under the heading "Regulatory Framework - Australia" in this Prospectus. |
NASDAQ | has the meaning ascribed to that term on the cover page of this Prospectus. |
NCR | means the Narcotic Control Regulations, CRC, c 1041 of the CDSA. |
Needle | means The Needle Capital Corp. |
Needle Subco | means 2652253 Ontario Inc., a subsidiary of Needle. |
Non-Resident Holder | has the meaning ascribed to that term under the heading "Certain Canadian Federal Income Tax Considerations - Holders Not Resident in Canada" in this Prospectus. |
OCS | means the Ontario Cannabis Store, a subsidiary of the Liquor Control Board of Ontario. |
ODC | has the meaning ascribed to that term under the heading "The Acquisition of Holigen - Facilities - Australia" in this Prospectus. |
Offered Shares | has the meaning ascribed to that term on the cover page of this Prospectus. |
Offering | has the meaning ascribed to that term on the cover page of this Prospectus. |
Offering Price | has the meaning ascribed to that term on the cover page of this Prospectus. |
PD Loans | has the meaning ascribed to that term under the heading "The Acquisition of Holigen - Overview of the Acquisition" in this Prospectus. |
Permitted Transferee | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement - Restriction on Transfer" in this Prospectus. |
PFIC | has the meaning ascribed to that term under the heading "Certain United States Federal Income Tax Considerations - Passive Foreign Investment Company Rules" in this Prospectus. |
PIN | has the meaning ascribed to that term under the heading "Our Current and Proposed Cultivation and R&D Facilities - Europe" |
PIPEDA | has the meaning ascribed to that term under the heading "Risk Factors" in this Prospectus. |
Portugal Planting Milestone (Aljustrel) | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement - Milestones & Resource Commitments" in this Prospectus. |
Portugal Planting Milestone (Sintra) | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement - Milestones & Resource Commitments" in this Prospectus. |
Preferred Shares | has the meaning ascribed to that term under the heading "Description of Shares" in this Prospectus. |
Proposed Amendments | has the meaning ascribed to that term under the heading "Certain Canadian Federal Income Tax Considerations" in this Prospectus. |
Prospectus | has the meaning ascribed to that term on the cover page of this Prospectus. |
PTG Act | means the Poisons and Therapeutic Goods Act 1966 (NSW). |
PTG Regs | has the meaning ascribed to that term under the heading "Regulatory Framework - Australia - New South Wales" in this Prospectus. |
Purchase Price | has the meaning ascribed to that term on the cover page of this Prospectus. |
Purchased Shares | has the meaning ascribed to that term under the heading "The Acquisition of Holigen - Overview of the Acquisition" in this Prospectus. |
Qualifying Transaction | means the qualifying transaction completed under the TSX.V rules pursuant to the Business Combination Agreement which resulted in a reverse takeover of Needle by the shareholders of Flowr Privateco. |
R&D | means research and development. |
RD 61/94 | has the meaning ascribed to that term under the heading "Regulatory Framework - Portugal" in this Prospectus. |
RDSP | has the meaning ascribed to that term under the heading "Eligibility for Investment" in this Prospectus. |
Registered Plans | has the meaning ascribed to that term under the heading "Eligibility for Investment" in this Prospectus. |
Regulations | has the meaning ascribed to that term under the heading "Eligibility for Investment" in this Prospectus. |
Regulatory Milestone | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement - Milestones & Resource Commitments" in this Prospectus. |
Representatives | has the meaning ascribed to that term on the cover page of this Prospectus. |
Resident Holder | has the meaning ascribed to that term under the heading "Certain Canadian Federal Income Tax Considerations - Holders Resident in Canada" in this Prospectus. |
Resource Commitment | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement - Milestones & Resource Commitments" in this Prospectus. |
RESP | has the meaning ascribed to that term under the heading "Eligibility for Investment" in this Prospectus. |
RPK | means RPK Biopharma, Unipessoal Lda. |
RRIF | has the meaning ascribed to that term under the heading "Eligibility for Investment" in this Prospectus. |
RRSP | has the meaning ascribed to that term under the heading "Eligibility for Investment" in this Prospectus. |
Sarbanes-Oxley Act | has the meaning ascribed to that term under the heading "Risk Factors" in this Prospectus. |
Revolving Facility | means the $500,000 revolving operating credit facility under the ATB Credit Facilities. |
SAS | has the meaning ascribed to that term under the heading "Regulatory Framework - Australia - Import" in this Prospectus. |
SEC | has the meaning ascribed to that term on the cover page of this Prospectus. |
SEDAR | means the System for Electronic Document Analysis and Retrieval. |
Series 1 Preferred Shares | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement - Terms of Consideration Shares" in this Prospectus. |
Share Consideration | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement" in this Prospectus. |
Shares | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement - Transfer Restrictions" in this Prospectus. |
Shoppers | means Shoppers Drug Mart Inc. |
SPSA | has the meaning ascribed to that term under the heading "The Business - Where is Flowr Going?" in this Prospectus. |
Stability Data | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement - Milestones & Resource Commitments" in this Prospectus. |
Subject Shares | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement - Restriction on Transfer" in this Prospectus. |
Subscription Receipt | has the meaning ascribed to that term under the heading "Prior Sales" in this Prospectus. |
Target Corporations | has the meaning ascribed to that term under the heading "The Acquisition of Holigen - Overview of the Acquisition" in this Prospectus. |
Tax Act | has the meaning ascribed to that term under the heading "Eligibility for Investment" in this Prospectus. |
taxable capital gain | has the meaning ascribed to that term under the heading "Certain Canadian Federal Income Tax Considerations - Holders Resident in Canada - Tax Treatment of Capital Gains and Losses" in this Prospectus. |
TCann | means TCann Pty Ltd. |
TFSA | has the meaning ascribed to that term under the heading "Eligibility for Investment" in this Prospectus. |
TG Act | has the meaning ascribed to that term under the heading "Regulatory Framework - Australia" in this Prospectus. |
TGA | has the meaning ascribed to that term under the heading "Regulatory Framework - Australia" in this Prospectus. |
TGO-77 | means Therapeutic Goods Order No. 77 - Microbiological Standards for Medicines. |
TGO-93 | means Therapeutic Goods Order No. 93 - Standard for Medicinal Cannabis. |
THC | means tetrahydrocannabinol. |
Three-Year Conversion Expiry Date | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement - Terms of Consideration Shares - Conversion into Common Shares" in this Prospectus. |
Transfer | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement - Restriction on Transfer" in this Prospectus. |
TSX.V | has the meaning ascribed to that term on the cover page of this Prospectus. |
Two-Year Conversion Expiry Date | has the meaning ascribed to that term under the heading "Details of the Acquisition - The Holigen Purchase Agreement - Terms of Consideration Shares - Conversion into Common Shares" in this Prospectus. |
U.S. Exchange Act | has the meaning ascribed to that term under the heading "Where United States Investors Can Find More Information" in this Prospectus. |
U.S. Securities Act | has the meaning ascribed to that term under the heading "Where United States Investors Can Find More Information" in this Prospectus. |
Undertaking | has the meaning ascribed to that term under the heading "Risk Factors" in this Prospectus. |
Underwriters | has the meaning ascribed to that term on the cover page of this Prospectus. |
Underwriters’ Fee | has the meaning ascribed to that term on the cover page of this Prospectus. |
Underwriters’ Option | has the meaning ascribed to that term on the cover page of this Prospectus. |
Underwriting Agreement | has the meaning ascribed to that term on the cover page of this Prospectus. |
Vendor Lock-Up Agreement | has the meaning ascribed to that term under the heading "Details of the Acquisition - Other Agreements" in this Prospectus. |
Vendors | has the meaning ascribed to that term under the heading "The Acquisition of Holigen - Overview of the Acquisition" in this Prospectus. |
APPENDIX "B" - AUDITED COMBINED AND CONSOLIDATED FINANCIAL STATEMENTS OF HOLIGEN FOR THE PERIOD ENDED DECEMBER 31, 2017 AND THE YEAR ENDED DECEMBER 31, 2018
B-1

HOLIGEN HOLDINGS LIMITED
COMBINED AND CONSOLIDATED FINANCIAL STATEMENTS
For the year ended December 31, 2018 and period from
formation on July 21, 2017 to December 31, 2017
(Expressed in Euros)
B-2
Holigen Holdings Limited
Combined and Consolidated Financial Statements
December 31, 2018
B-3

INDEPENDENT AUDITOR’S REPORT
To the Shareholders of Holigen Holdings Limited
Opinion
We have audited the combined and consolidated financial statements of Holigen Holdings Limited, (the Group), which comprise the combined and consolidated statements of financial position as at December 31, 2018 and December 31, 2017 and the combined and consolidated statements of comprehensive loss, changes in shareholders’ (deficit) equity and cash flows for the year ended December 31, 2018 and the period from formation on July 21, 2017 to December 31, 2017, and notes to the combined and consolidated financial statements, including a summary of significant accounting policies.
In our opinion, the accompanying combined and consolidated financial statements present fairly, in all material respects, the combined and consolidated financial position of the Group as at December 31, 2018 and December 31, 2017, and its combined and consolidated financial performance and its combined and consolidated cash flows for the year ended December 31, 2018 and the period from formation on July 21, 2017 to December 31, 2017 in accordance with International Financial Reporting Standards, as issued by the International Accounting Standards Board.
Basis for Opinion
We conducted our audit in accordance with Canadian generally accepted auditing standards. Our responsibilities under those standards are further described in theAuditor’s Responsibilities for the Audit of the Consolidated Financial Statements section of our report. We are independent of the Group in accordance with the ethical requirements that are relevant to our audit of the consolidated financial statements in Canada, and we have fulfilled our other ethical responsibilities in accordance with these requirements. We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our opinion.
Material Uncertainty Related to Going Concern
We draw attention to Note 2 in the combined and consolidated financial statements, which indicates that the Group incurred a comprehensive loss of €1,313,705 during the year ended December 31, 2018 and, as of that date, the Group’s current liabilities exceeded its current assets by €3,836,311. As stated in Note 2, these events or conditions indicate that a material uncertainty exists that may cast significant doubt on the Group’s ability to continue as a going concern. Our opinion is not modified in respect of this matter.
Responsibilities of Management for the Combined and Consolidated Financial Statements
Management is responsible for the preparation and fair presentation of the combined and consolidated financial statements in accordance with International Financial Reporting Standards, as issued by the International Accounting Standards Board, and for such internal control as management determines is necessary to enable the preparation of combined and consolidated financial statements that are free from material misstatement, whether due to fraud or error.
In preparing the combined and consolidated financial statements, management is responsible for assessing the Group’s ability to continue as a going concern, disclosing, as applicable, matters related to going concern and using the going concern basis of accounting unless management either intends to liquidate the Group or to cease operations, or has no realistic alternative but to do so.

Auditor’s Responsibilities for the Audit of the Combined and Consolidated Financial Statements
Our objectives are to obtain reasonable assurance about whether the combined and consolidated financial statements as a whole are free from material misstatement, whether due to fraud or error, and to issue an auditor’s report that includes our opinion. Reasonable assurance is a high level of assurance, but is not a guarantee that an audit conducted in accordance with Canadian generally accepted auditing standards will always detect a material misstatement when it exists. Misstatements can arise from fraud or error and are considered material if, individually or in the aggregate, they could reasonably be expected to influence the economic decisions of users taken on the basis of these consolidated financial statements.
As part of an audit in accordance with Canadian generally accepted auditing standards, we exercise professional judgment and maintain professional skepticism throughout the audit. We also:
Identify and assess the risks of material misstatement of the combined and consolidated financial statements, whether due to fraud or error, design and perform audit procedures responsive to those risks, and obtain audit evidence that is sufficient and appropriate to provide a basis for our opinion. The risk of not detecting a material misstatement resulting from fraud is higher than for one resulting from error, as fraud may involve collusion, forgery, intentional omissions, misrepresentations, or the override of internal control.
Obtain an understanding of internal control relevant to the audit in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Group’s internal control.
Evaluate the appropriateness of accounting policies used and the reasonableness of accounting estimates and related disclosures made by management.
Conclude on the appropriateness of management’s use of the going concern basis of accounting and, based on the audit evidence obtained, whether a material uncertainty exists related to events or conditions that may cast significant doubt on the Group’s ability to continue as a going concern. If we conclude that a material uncertainty exists, we are required to draw attention in our auditor’s report to the related disclosures in the consolidated financial statements or, if such disclosures are inadequate, to modify our opinion. Our conclusions are based on the audit evidence obtained up to the date of our auditor’s report. However, future events or conditions may cause the Group to cease to continue as a going concern.
Evaluate the overall presentation, structure and content of the combined and consolidated financial statements, including the disclosures, and whether the combined and consolidated financial statements represent the underlying transactions and events in a manner that achieves fair presentation.
Obtain sufficient appropriate audit evidence regarding the financial information of the entities or business activities within the Group to express an opinion on the combined and consolidated financial statements. We are responsible for the direction, supervision and performance of the group audit. We remain solely responsible for our audit opinion.
We communicate with those charged with governance regarding, among other matters, the planned scope and timing of the audit and significant audit findings, including any significant deficiencies in internal control that we identify during our audit.

Chartered Professional Accountants
Licensed Public Accountants
June 7, 2019
Toronto, Ontario
B-5
| Holigen Holdings Limited |
| Combined and Consolidated Statements of Financial Position |
| As at December 31, 2018 and December 31, 2017 |
| (Expressed in euros) |
| | | 2018 | | | 2017 | |
| | | € | | | € | |
| ASSETS | | | | | | |
| Current | | | | | | |
| Cash | | 1,654,176 | | | 82,549 | |
| Sales tax recoverable | | 40,031 | | | 7,045 | |
| Prepaid expense | | 57,837 | | | - | |
| Inventory | | 12,904 | | | 26,828 | |
| | | | | | | |
| | | 1,764,948 | | | 116,422 | |
| | | | | | | |
| Non-current | | | | | | |
| Security deposit | | 23,994 | | | 25,378 | |
| Property, plant and equipment(Note 6) | | 2,234,342 | | | - | |
| | | | | | | |
| TOTAL ASSETS | | 4,023,284 | | | 141,800 | |
| | | | | | | |
| LIABILITIES | | | | | | |
| Current | | | | | | |
| Accounts payable and accrued liabilities(Note 7) | | 1,215,082 | | | 239,291 | |
| Loans payable to related parties(Note 8) | | 426,533 | | | 105,328 | |
| Derivative liability(Note 9) | | 1,032,838 | | | | |
| Convertible loan(Note 9) | | 2,926,806 | | | - | |
| | | | | | | |
| | | 5,601,259 | | | 344,619 | |
| | | | | | | |
| SHAREHOLDERS’ (DEFICIT) EQUITY | | | | | | |
| Share capital(Note 10) | | 1,200 | | | 62,652 | |
| Contributed surplus | | 24,333 | | | 24,333 | |
| Deficit | | (1,636,878 | ) | | (297,917 | ) |
| Accumulated other comprehensive income | | 34,486 | | | 8,113 | |
| | | | | | | |
| Equity attributable to owners of the parent | | (1,576,859 | ) | | (202,819 | ) |
| Non-controlling interest(Note 10) | | (1,116 | ) | | - | |
| | | | | | | |
| | | (1,577,975 | ) | | (202,819 | ) |
| | | | | | | |
| TOTAL LIABILITIES AND SHAREHOLDERS’ EQUITY | | 4,023,284 | | | 141,800 | |
See Note 2 – Basis of preparation, going concern
The accompanying notes are an integral part of these combined and consolidated financial statements.
Approved by the Board of Directors on June 7, 2019 and signed on its behalf by:
________________________________________
Pauric Duffy, Director
B-6
| Holigen Holdings Limited |
| Combined and Consolidated Statements of Changes in Shareholders’ (Deficit) Equity |
| For the year ended December 31, 2018 and period from formation on July 21, 2017 to December 31, 2017 |
| (Expressed in euros) |
| | | | | | | | | | | | Accumulated | | | | | | | |
| | | | | | | | | | | | Other | | | Non- | | | | |
| | | Share | | | Contributed | | | | | | Comprehensive | | | controlling | | | | |
| | | capital | | | Surplus | | | Deficit | | | Income | | | interest | | | Total | |
| | | € | | | | | | € | | | € | | | € | | | € | |
| | | (Note 10) | | | | | | | | | | | | (Note 10) | | | | |
| | | | | | | | | | | | | | | | | | | |
| Balances, July 21, 2017 | | - | | | - | | | - | | | - | | | - | | | - | |
| Issuance of Ordinary shares of: | | | | | | | | | | | | | | | | | | |
| RPK | | 1,000 | | | - | | | - | | | - | | | - | | | 1,000 | |
| GreyCan | | 61,652 | | | - | | | - | | | - | | | - | | | 61,652 | |
| Debt forgiveness by shareholder | | - | | | 24,333 | | | - | | | - | | | - | | | 24,333 | |
| Net loss for the period | | - | | | - | | | (297,917 | ) | | - | | | - | | | (297,917 | ) |
| Unrealized gain on translation of foreign operation | | - | | | - | | | - | | | 8,113 | | | - | | | 8,113 | |
| | | | | | | | | | | | | | | | | | | |
| Balances, December 31, 2017 | | 62,652 | | | 24,333 | | | (297,917 | ) | | 8,113 | | | - | | | (202,819 | ) |
| | | | | | | | | | | | | | | | | | | |
| Issuance of ordinary shares of: | | | | | | | | | | | | | | | | | | |
| RPK | | 124,000 | | | - | | | - | | | - | | | - | | | 124,000 | |
| Holigen Limited(Note 10) | | - | | | - | | | - | | | - | | | 1 | | | 1 | |
| Reorganization(Notes 2 & 10) | | (186,652 | ) | | - | | | - | | | - | | | - | | | (186,652 | ) |
| | | | | | | | | | | | | | | | | | | |
| Balances, November 7, 2018 | | - | | | 24,333 | | | (297,917 | ) | | 8,113 | | | 1 | | | (265,470 | ) |
| Issuance of ordinary shares of: | | | | | | | | | | | | | | | | | | |
| Holigen Holdings Limited | | 1,200 | | | - | | | - | | | - | | | - | | | 1,200 | |
| Net loss for the year | | - | | | - | | | (1,338,961 | ) | | - | | | (1,117 | ) | | (1,340,078 | ) |
| Unrealized gain on translation of foreign operation | | - | | | - | | | - | | | 26,373 | | | - | | | 26,373 | |
| | | | | | | | | | | | | | | | | | | |
| Balances, December 31, 2018 | | 1,200 | | | 24,333 | | | (1,636,878 | ) | | 34,486 | | | (1,116 | ) | | (1,577,975 | ) |
The accompanying notes are an integral part of these combined and consolidated financial statements.
B-7
| Holigen Holdings Limited |
| Combined and Consolidated Statements of Comprehensive Loss |
| For the year ended December 31, 2018 and period from formation on July 21, 2017 to December 31, 2017 |
| (Expressed in euros) |
| | | 2018 | | | 2017 | |
| | | € | | | € | |
| | | | | | | |
| Revenue(Note 11) | | 32,689 | | | - | |
| | | | | | | |
| Expenses | | | | | | |
| Cost of goods sold | | 10,894 | | | - | |
| Accretion on convertible loan | | 94,272 | | | | |
| Consulting(Note 14(i)) | | 532,905 | | | 15,045 | |
| Licenses and development costs(Note 14(ii)) | | 394,005 | | | 257,988 | |
| Rent and occupancy(Note 14(iii)) | | 169,441 | | | 19,135 | |
| Office and general | | 87,938 | | | 4,085 | |
| Professional fees | | 142,165 | | | 1,664 | |
| Depreciation | | 13,831 | | | - | |
| Gain on derivative liability | | 9,864 | | | | |
| Foreign exchange gain | | (82,548 | ) | | - | |
| | | | | | | |
| | | 1,372,767 | | | 297,917 | |
| | | | | | | |
| Net loss | | (1,340,078 | ) | | (297,917 | ) |
| Item that will be reclassified to profit or loss: | | | | | | |
| Unrealized gain on retranslation of subsidiary | | 26,373 | | | 8,113 | |
| | | | | | | |
| Comprehensive loss | | (1,313,705 | ) | | (289,804 | ) |
| | | | | | | |
| Net loss attributable to: | | | | | | |
| Equity holders of Holigen Holdings Limited | | (1,338,961 | ) | | (297,917 | ) |
| Non-controlling interest | | (1,117 | ) | | - | |
| | | | | | | |
| Net loss | | (1,340,078 | ) | | (297,917 | ) |
| | | | | | | |
| Comprehensive loss attributable to: | | | | | | |
| Equity holders of Holigen Holdings Limited | | (1,312,610 | ) | | (289,804 | ) |
| Non-controlling interest | | (1,095 | ) | | - | |
| | | | | | | |
| Comprehensive loss | | (1,313,705 | ) | | (289,804 | ) |
The accompanying notes are an integral part of these combined and consolidated financial statements.
B-8
| Holigen Holdings Limited |
| Combined and Consolidated Statements of Cash Flows |
| For the year ended December 31, 2018 and period from formation on July 21, 2017 to December 31, 2017 |
| (Expressed in euros) |
| | | 2018 | | | 2017 | |
| | | € | | | € | |
| | | | | | | |
| Operating activities | | | | | | |
| Net loss | | (1,340,078 | ) | | (297,917 | ) |
| Adjustments for non-cash items: | | | | | | |
| Depreciation | | 13,831 | | | - | |
| Accretion on convertible loan | | 94,272 | | | - | |
| Unrealized foreign exchange gain (loss) | | (73,196 | ) | | 1,975 | |
| | | | | | | |
| Operating cash flows before non-cash working capital changes | | (1,305,171 | ) | | (295,942 | ) |
| | | | | | | |
| Changes in non-cash working capital: | | | | | | |
| Prepaid expense | | (57,837 | ) | | - | |
| Sales tax recoverable | | (32,986 | ) | | (7,045 | ) |
| Inventory | | 13,923 | | | - | |
| Accounts payable and accrued liabilities | | 1,032,648 | | | 239,291 | |
| | | | | | | |
| Cash used in operating activities | | (349,423 | ) | | (63,696 | ) |
| | | | | | | |
| Financing activities | | | | | | |
| Convertible loan issued(Note 9) | | 3,960,830 | | | - | |
| Proceeds from loans payable to related parties, net | | 201,697 | | | 106,078 | |
| Proceeds from issuance of ordinary shares (Note 10) | | 1,200 | | | 61,902 | |
| | | | | | | |
| Cash provided by financing activities | | 4,163,727 | | | 167,980 | |
| | | | | | | |
| Investing activities | | | | | | |
| Purchase of property, plant and equipment | | (2,248,173 | ) | | - | |
| Payment of security deposit | | - | | | (25,378 | ) |
| | | | | | | |
| Cash used in investing activities | | (2,248,173 | ) | | (25,378 | ) |
| | | | | | | |
| Change in cash during the year | | 1,566,131 | | | 78,906 | |
| Impact of foreign exchange on cash held | | 5,496 | | | 3,643 | |
| Cash, beginning of period | | 82,549 | | | - | |
| | | | | | | |
| Cash, end of period | | 1,654,176 | | | 82,549 | |
| | | | | | | |
| Non-cash transaction | | | | | | |
| | | | | | | |
| Acquisitions, in exchange for loans payable to related party(Notes 2 & 10) | | 186,652 | | | - | |
The accompanying notes are an integral part of these combined and consolidated financial statements
B-9
| Holigen Holdings Limited |
| Notes to the Combined and Consolidated Financial Statements |
| For the year ended December 31, 2018 and period from formation on July 21, 2017 to December 31, 2017 |
| (Expressed in euros) |
1. NATURE OF BUSINESS
Holigen Holdings Limited (theCompany) was incorporated under the Companies Act (Malta) on June 28, 2018 and is domiciled and registered in Malta as a limited liability company. The Company was named RPK Holdings Limited prior to August 7, 2018. The Company’s registered office is ‘Lara Buildings’, Level 1, Guzeppi Calleja Street, Iklin 1KL262, Malta.
The Company had no immediate parent prior to January 28, 2019 (see Note 17(i) – Subsequent events) and has no ultimate parent.
The combined and consolidated statements of the Company (the ‘Group’) as at December 31, 2018 are comprised of Holigen Holdings Limited and its 99.92% owned subsidiary Holigen Limited along with its wholly-owned subsidiaries RPK Biopharma Unipessoal Lda. (‘RPK’), and GreyCan Pty Ltd. (‘GreyCan’) and GreyCan’s wholly owned subsidiary TCann Pty Ltd. (‘TCann’).
The Company is pursuing its strategic objectives to commence production and research related to medical marijuana. Through an Australian subsidiary, the Company has been granted licenses for Cultivation, R&D and Manufacturing from the Office of Drug Control and Drug Control Section Australia (Therapeutic Goods Administration) and Good Manufacturing Process License from the TGA, which represents a significant milestone in the Company’s strategic objectives. In addition, the Group is progressing through the required inspections to commence operations in Portugal.
2. BASIS OF PREPARATION AND GOING CONCERN
Basis of consolidation and combination and reorganization
These combined and consolidated financial statements are prepared under the name of Holigen Holdings Limited based on the corporate structure as at December 31, 2018. The Company has elected to restate the comparative period information to reflect the effects of the reorganization that took place in 2018, as though the post-reorganization structure had always been in place. This continuity of interests method has resulted in the Company assuming the deficit and other comprehensive income balances as at July 21, 2017. However, since Holigen Holdings Limited was not incorporated until June 28, 2018, the financial statements for the period from formation of the Group on July 21, 2017 to December 31, 2017 and up until June 28, 2018 are prepared on a combined basis, as a combination of the financial position and performance of RPK, GreyCan and TCann since the dates of their respective incorporations. Equity presented on the combined statement of financial position as at December 31, 2017 is described in Note 10 – Share capital. All entities included were under common control throughout the period from July 21, 2017 to June 28, 2018.
Statement of compliance
These combined and consolidated financial statements have been prepared in accordance with International Financial Reporting Standards (IFRS) as issued by the International Accounting Standards Board (IASB). These combined and consolidated financial statements are the Company’s first general-purpose financial statements and therefore the Company has also applied IFRS 1First-time Adoption of International Financial Reporting Standards.
Basis of presentation
These combined and consolidated financial statements have been prepared on a historical cost basis, except as detailed in the accounting policies disclosed in Note 4. All accounting policies and methods of computation followed in the preparation of these combined and consolidated financial statements are consistent with those of the previous financial period.
B-10
| Holigen Holdings Limited |
| Notes to the Combined and Consolidated Financial Statements |
| For the year ended December 31, 2018 and period from formation on July 21, 2017 to December 31, 2017 |
| (Expressed in euros) |
The combined and consolidated financial statements include the accounts of the Company and the following subsidiaries (collectively, the ‘Group’):
| Company name | Domicile | Incorporation date | Ownership |
| | | | |
| Holigen Limited2(‘Holigen’) | Malta | July 2, 2018 | 99.92% directly owned1 |
| RPK Biopharma, Unipessoal Lda. (‘RPK’) | Portugal | October 24, 2017 | 100% owned by Holigen |
| GreyCan Pty Ltd. (‘GreyCan’) | Australia | July 21, 2017 | 100% owned by Holigen |
| TCann Pty Ltd. (‘TCann’) | Australia | July 21, 2017 | 100% owned by GreyCan |
Note 1 – See Note 10 – Non-controlling interest
Note 2 – Formerly RPK Trading Limited, prior to August 7, 2018 |
During the year ended December 31, 2018, the Company effected a corporate reorganization of entities under common control and management, through the following steps:
| (i) | on July 6, 2018, all issued and outstanding shares of GreyCan were transferred to Holigen, in exchange for a loan payable of €61,652; and |
| | |
| (ii) | on November 7, 2018, all issued and outstanding shares of RPK were transferred to Holigen, in exchange for a loan payable of €125,000 (see Note 8 – Loans payable to related parties). |
The Company used the continuity of interests accounting with respect to the reorganization transactions which are fully restrospective rather than applying the acquisition method of IFRS 3Business Combinations. Subsidiaries are under the control of a parent, and therefore consolidated, when an investor is exposed, or has rights, to variable returns from the investee and has the ability to affect those returns through its power over the investee. Uniform accounting policies have been applied by the Group throughout, and all intra-Group balances and items of revenue and expense have been eliminated from both periods presented.
Going concern
These combined and consolidated financial statements have been prepared under the going concern assumption, which contemplates the realization of assets and settlement of liabilities in the normal course of business. During the year ended December 31, 2018, the Company incurred a comprehensive loss of €1,313,705 (2017 - €289,804) and had a net outflow of cash from operations of €349,423 (2017 - €63,696). As at December 31, 2018, the Company had a working capital deficit of €3,836,311 (2017 – €228,197) and an accumulated deficit of €1,636,878 (2017 - €297,917).
The ability of the Company to continue as a going concern is dependent on management raising sufficient capital to maintain operations and to fund further operating expenses and losses until revenues and positive cash flows can be generated from operations. These are significant uncertainties that may cast significant doubt on the ability of the Company to continue as a going concern.
Management believes that the Company has access to sufficient funding on reasonable commercial terms necessary to achieve its strategic objectives. Therefore, the combined and consolidated financial statements do not give effect to adjustments that would be necessary to the carrying amounts and classifications of assets and liabilities should the going concern assumption not be appropriate.
Presentation and functional currencies
The combined and consolidated financial statements are presented in euros (or €s) as the presentation currency of the Group.
The functional currency of the Company, Holigen and RPK is the euro. The functional currency of GreyCan and TCann is the Australian dollar (AUD). Revenue and expenses of GreyCan and TCann are translated into euros using the average Australian exchange rate with the euro for the respective reporting period. Assets and liabilities are translated at the spot rate as at the statement of financial position date. Exchange differences arising from translation are recognized in other comprehensive income.
Monetary items denominated in currencies other than the functional currency of the entity are initially measured using the exchange rate on the date of the transaction. Gains and losses arising from retranslation at subsequent reporting dates are recognized in net income or loss.
B-11
| Holigen Holdings Limited |
| Notes to the Combined and Consolidated Financial Statements |
| For the year ended December 31, 2018 and period from formation on July 21, 2017 to December 31, 2017 |
| (Expressed in euros) |
3. ESTIMATES AND JUDGEMENTS
The preparation of financial statements requires management to make judgements, estimates and underlying assumptions that could affect the reported amounts in the financial statements. Management bases its judgements and estimates on historical experience and other factors, including forward looking information, that it believes to be reasonable under the circumstances, but which are inherently uncertain and unpredictable. The results of these estimates form the basis of the carrying amounts of assets and liabilities.
Imprecision in exercising judgement and making estimates and assumptions could require adjustment to the carrying amounts of the affected asset or liability, and those adjustments could be material. Any adjustment will be recognized in the period in which it is identified and in any future periods affected.
The most significant judgements include those related to determining the functional currency of the Company, assessing whether the criteria are met for recognition of deferred tax assets, consisting of tax losses available and research and development tax credits, the ability of the Company to continue as a going concern, the determination of when property, plant and equipment are available for use as well as their useful lives, valuation and recoverability of deferred taxes, and its impairment of its financial and non-financial assets. The Company is subject to a number of risks and uncertainties associated with the going concern assumption and exercises judgment to assess the uncertainties relating to the determination of the Company’s ability to continue as a going concern.
The most significant estimates and assumptions include those related to the inputs used in accounting for the valuation of the foreign exchange derivative liability, including volatility, and the fair value of financial instruments. Management has determined that judgments, estimates and assumptions reflected in these consolidated financial statements are reasonable.
4. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Property, plant and equipment
Property, plant and equipment are recorded at cost less accumulated depreciation. Depreciation commences once the installation is complete and the asset is available for use.
Land is not depreciated. Depreciation is calculated on the Company’s other asset classes on a straight-line basis using the following rates and periods:
| Building | 20 years |
| Leasehold improvements | Over the lease term |
| Fixtures, fitting and equipment | 3 – 10 years |
The useful economic lives and residual amounts are reviewed annually and depreciation rates are adjusted accordingly on a prospective basis. Gains and losses on disposal of property, plant and equipment are determined by comparing the proceeds with the carrying amount for the asset and are recognized in net income or loss.
Inventory
Inventory consists of purchased goods and is valued at the lower of cost and net realizable value, with cost determined based on an average cost basis. Net realizable value is determined as the estimated selling price in the ordinary course of business less estimated costs necessary to make the sale.
Impairment of long-lived assets
Long-lived assets are reviewed at each reporting date to determine whether there is any indication of impairment. The Company tests for impairment whenever indications of impairment exist by calculating an estimate of the asset’s recoverable amount. The recoverable amount is the higher of an asset’s fair value less costs to sell or its value in use. Fair value is determined as the amount that would be obtained from the sale of the asset in an arm’s length transaction between knowledgeable and willing parties. In assessing the value in use, the estimated future cash flows are discounted to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money and the risks specific to the asset.
B-12
| Holigen Holdings Limited |
| Notes to the Combined and Consolidated Financial Statements |
| For the year ended December 31, 2018 and period from formation on July 21, 2017 to December 31, 2017 |
| (Expressed in euros) |
If the recoverable amount of an asset is estimated to be less than its carrying amount, the carrying amount of the asset is reduced to its recoverable amount and the impairment loss is recognized in the consolidated statement of loss and comprehensive loss for the period.
Where an impairment loss subsequently reverses, the carrying amount of the asset is increased to the revised estimate of its recoverable amount up to the point that the amount does not exceed the carrying amount that would have been determined had no impairment loss been recognized for the asset in prior years. A reversal of an impairment loss is recognized immediately in income for the period. The Company has assessed the assets of its operating entities and has determined that there is no indication of impairment of its long-lived assets.
Hybrid financial instruments
Hybrid financial instruments issued by the Company consist of a convertible loan. As the loan is denominated in currency other than the Company’s functional currency, the conversion feature is considered to be a derivative liability, and is initially measured at fair value using the Black-Scholes option pricing model. Any residual amount of the loan is allocated to the host liability instrument. Subsequent to initial recognition, the derivative liability component is remeasured at fair value at each financial reporting date. The host liability is measured at amortized cost. Upon conversion, the liability component and conversion feature are reclassified to share capital.
Related party transactions
Parties are considered to be related if one party has the ability, directly or indirectly, to control the other party or exercise significant influence over the other party in making financial and operating decisions. Parties are also considered to be related if they are subject to common control or common significant influence. Related parties may be individuals or corporate entities. A transaction is considered to be a related party transaction when there is a transfer of resources or obligations between related parties. Related party transactions are measured at the amounts agreed upon by the parties.
Income taxes
Income tax on the consolidated statement of loss and comprehensive loss for the periods presented comprises current and deferred tax. Income tax is recognized in the Consolidated Statement of Loss and Comprehensive Loss except to the extent that it relates to items recognized directly in equity, in which case it is recognized in equity.
Current tax expense is the expected tax payable on the taxable income for the year, using tax rates enacted or substantively enacted at the end of the reporting period, adjusted for amendments to tax payable with regards to previous years.
Deferred tax is provided using the asset and liability method, providing for temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for taxation purposes. The following temporary differences are not provided for: goodwill not deductible for tax purposes; the initial recognition of assets or liabilities that affect neither accounting or taxable income; nor differences relating to investments to the extent that they will probably not reverse in the foreseeable future. The amount of deferred tax provided is based on the expected manner of realization or settlement of the carrying amount of assets and liabilities, using tax rates enacted or substantively enacted at the end of each financial reporting period.
A deferred tax asset is recognized only to the extent that it is probable that future taxable income will be available against which the asset can be utilized. To the extent that the Company does not consider it probable that a deferred tax asset will be recovered, it does not recognize a deferred tax asset.
B-13
| Holigen Holdings Limited |
| Notes to the Combined and Consolidated Financial Statements |
| For the year ended December 31, 2018 and period from formation on July 21, 2017 to December 31, 2017 |
| (Expressed in euros) |
Financial Instruments
Under IFRS 9, in the case of a financial asset not at fair value through profit and loss (“FVTPL”), financial assets are initially measured at fair value plus transaction costs. Financial assets are subsequently measured at:
| | i) | FVTPL; |
| | ii) | amortized cost; |
| | iii) | equity investments designated at FVOCI; or |
| | iv) | debt measured at fair value through other comprehensive income (“FVOCI”) |
The classification is based on whether the contractual cash flow characteristics represent “solely payment of principal and interest” (the “SPPI test”) as well as the business model under which the financial assets are managed. Financial assets are required to be reclassified only when the business model under which they are managed has changed. All reclassifications are to be applied prospectively from the reclassification date.
Debt investments are recorded at amortized cost for financial assets that are held within a business model with the objective to hold the financial assets in order to collect contractual cash flows that meet the SPPI test.
All financial assets and liabilities held by the Company are initially measured at fair value and, other than the derivative liability, are subsequently measured at amortized cost, using the effective interest method. The effective interest method is a method of calculating the amortized cost of an instrument and of allocating interest income over the relevant period. The effective interest rate is the rate that discounts future cash receipts (including all transaction costs and other premiums or discounts) through the expected life of the debt instrument to the net carrying amount on initial recognition.
Revenue recognition
Revenue is recognized when control of the product transfers to the customer, and the collectability is reasonably assured. Control is transferred at the point that the customer takes physical possession of the goods and assumes the risks and rewards of ownership, and is measured at the consideration received or receivable after taking into account any trade discounts, volume rebates allowed and delayed or advanced payment terms if the financing effect is significant. The point at which control transfers is also when the performance obligations have been fulfilled under the terms of the related sales contract.
Research and development
Development costs are capitalized only if they are reliably measurable, the development is technically and commercially feasible, future economic benefits are probable and the Company has the intent and resources to exploit the resulting asset. Development costs that do not meet the criteria for capitalization and all research costs are expensed as incurred.
Leases
Leases are classified as finance leases when they transfer substantially all the risks and rewards of ownership to the Company. All other leases are accounted for as operating leases. Payments in connection with operating leases are expensed on a straight-line basis over the term of the lease. Associated costs, such as realty taxes and operating costs, are expensed as incurred. The Company has no finance leases during the current year or prior period.
B-14
| Holigen Holdings Limited |
| Notes to the Combined and Consolidated Financial Statements |
| For the year ended December 31, 2018 and period from formation on July 21, 2017 to December 31, 2017 |
| (Expressed in euros) |
5. STANDARDS AND AMENDMENTS ISSUED BUT NOT YET EFFECTIVE
The following new standard and amendment have been issued with early adoption permitted, unless otherwise noted.
IFRS 16Leases
IFRS 16 sets out principles for the recognition, measurement, presentation and disclosure of leases. The standard replaces IAS 17Leases, IFRIC 4Determining whether an Arrangement Contains a Lease, SIC 15Operating Leases – Incentives and SIC 27Evaluating the Substance of Transactions Involving the Legal Form of a Lease. IFRS 16 requires a lessee to recognize a liability and a right-of-use asset. Subsequent to initial recognition, the liability is accounted for at amortized cost using the effective interest method and the right-of-use asset is depreciated over the short of the asset’s useful economic life and the lease term. For any leases that are shorter than 12 months in duration or where the underlying asset is of low value an election can be made to expense lease payments as incurred.
The Company intends adopting IFRS 16 when it becomes effective for the year ended December 31, 2019. The date of initial application of the standard will be January 1, 2019. A preliminary assessment indicates that the Company’s operating lease for premises will meet the definition of a lease under IFRS 16 and hence the Company will recognize a right-of-use asset and a corresponding liability in respect of this lease as at January 1, 2019. Management is in the process of quantifying the financial effect of applying the new standard.
IFRIC 23 Uncertainty over Income Tax Treatments
IFRIC 23 was issued to provide guidance on how to reflect uncertainty in the recognition and measurement of income taxes. An uncertain tax treatment is a tax treatment for which there is uncertainty over whether the relevant tax authority will accept the filing. The IFRIC is effective for annual periods beginning on or after January 1, 2019 and will be adopted on the effective date. Adoption is not expected to have any impact on the Company’s financial statement.
6. PROPERTY, PLANT AND EQUIPMENT
| | | | | | | | | | | | Fixtures, | | | | |
| | | | | | | | | Leasehold | | | fittings & | | | | |
| | | Land | | | Building | | | improvements | | | equipment | | | Total | |
| | | € | | | € | | | € | | | € | | | € | |
| Cost | | | | | | | | | | | | | | | |
| Balances, July 21 & December 31, 2017 | | - | | | - | | | - | | | - | | | - | |
| Additions | | 306,365 | | | 1,752,651 | | | 128,942 | | | 60,215 | | | 2,248,173 | |
| Balances, December 31, 2018 | | 306,365 | | | 1,752,651 | | | 128,942 | | | 60,215 | | | 2,248,173 | |
| | | | | | | | | | | | | | | | |
| Depreciation | | | | | | | | | | | | | | | |
| Balances, July 21 & December 31, 2017 | | - | | | - | | | - | | | - | | | - | |
| Expense | | - | | | 12,621 | | | - | | | 1,210 | | | 13,831 | |
| Balances, December 31, 2018 | | - | | | 12,621 | | | - | | | 1,210 | | | 13,831 | |
| | | | | | | | | | | | | | | | |
| Net book values | | | | | | | | | | | | | | | |
| December 31, 2018 | | 306,365 | | | 1,740,030 | | | 128,942 | | | 59,005 | | | 2,234,342 | |
| December 31, 2017 | | - | | | - | | | - | | | - | | | - | |
The cost of leasehold improvements recognized during the year ended December 31, 2018 relates to improvements made to newly leased space. The Company expects to commence occupancy of the new space during the first half of fiscal 2019. Since the leasehold improvements were not yet available for use, the cost of these assets has been capitalized but not yet amortized.
B-15
| Holigen Holdings Limited |
| Notes to the Combined and Consolidated Financial Statements |
| For the year ended December 31, 2018 and period from formation on July 21, 2017 to December 31, 2017 |
| (Expressed in euros) |
7. ACCOUNTS PAYABLE AND ACCRUED LIABILITIES
| | | 2018 | | | 2017 | |
| | | € | | | € | |
| Trade payables | | 444,984 | | | 76 | |
| Accrued liabilities | | 766,710 | | | 239,215 | |
| Withholding tax payable | | 3,388 | | | - | |
| | | | | | | |
| | | 1,215,082 | | | 239,291 | |
8. LOANS PAYABLE TO RELATED PARTIES
| | | 2018 | | | 2017 | |
| | | € | | | € | |
| Payable to a trust controlled by the spouse of the Company’s controlling shareholder | | 236,873 | | | 85,328 | |
| Payable to the Company’s controlling shareholder | | 189,660 | | | 20,000 | |
| | | | | | | |
| | | 426,533 | | | 105,328 | |
The loans payable to related parties are non-interest bearing and unsecured and have no fixed terms of repayment. The shareholder loan is due on demand and has no stated rate of interest.
9. CONVERTIBLE LOAN
On November 19, 2018, the Company entered into a binding term sheet with The Flowr Corporation (‘Flowr’), pursuant to which Flowr agreed to loan CAD $6,000,000 to the Company. The loan is interest free and includes an option that would allow Flowr to convert the loan into Class A shares of the Company at a ratio of 8 shares for each CAD $1 million if the Company and Flowr did not enter into a subscription and purchase agreement by January 19, 2019.
The Company and Flowr entered into a subscription and purchase agreement on December 19, 2018 (the ‘Subscription and Purchase Agreement’), pursuant to which Flowr agreed to purchase 15% of the ordinary class A shares of the Company from the controlling shareholder (the Share Purchase). The Company and Flowr agreed to exchange, immediately following the Share Purchase, an additional 4.8% of the ordinary class A shares of the Company in settlement of the closing date euro equivalent of the CAD $6,000,000 loan (the Settlement). These transactions will close when all conditions and criteria have been met and will result in Flowr becoming the holder of 19.8% of the ordinary A shares of the Company.
The Company’s functional currency is the euro. As the convertible loan is in a currency different than the functional currency, the loan is accounted for as a financial liability and the conversion privilege is considered to be a derivative liability as it fails the fixed for fixed criteria. The derivative liability is initially measured at fair value using the Black-Scholes option pricing model and is revalued at each financial reporting date with any gains and losses recognized in net income or loss for the period. The assumptions used in the initial valuation included the following: volatility of 65%, expected life of 1 year, risk free interest rate of 2.10%, and annual dividend yield of nil.
The derivative liability component was initially fair valued at €1,042,702 resulting in a valuation of the host liability of €2,940,832. At December 31, 2018 the fair value of the derivative liability was determined to be €1,032,838. The change in value is included in the statement of loss for the year ended December 31, 2018. The host liability was amortized at the effective interest rate, and the accretion expense is included in the statement of loss for the year ended December 31, 2018.
B-16
| Holigen Holdings Limited |
| Notes to the Combined and Consolidated Financial Statements |
| For the year ended December 31, 2018 and period from formation on July 21, 2017 to December 31, 2017 |
| (Expressed in euros) |
10. SHARE CAPITAL
The Company’s statement of financial position as at December 31, 2017 and 2018 included the combination of the following share capital amounts:
| | | RPK | | | GreyCan | | | Total | |
| | | Ordinary | | | Share | | | Ordinary | | | Share | | | Share | |
| | | Shares | | | capital | | | Shares | | | capital | | | capital | |
| | | Number | | | € | | | Number | | | € | | | € | |
| Balances, July 21, 2017 | | - | | | - | | | - | | | - | | | - | |
| Issuance of Ordinary shares | | 1,000 | | | 1,000 | | | 100 | | | 61,652 | | | 62,652 | |
| Balances, December 31, 2017 | | 1,000 | | | 1,000 | | | 100 | | | 61,652 | | | 62,652 | |
| Issuance of ordinary shares | | 124,000 | | | 124,000 | | | - | | | - | | | 124,000 | |
| Reorganization(Note 2) | | (125,000 | ) | | (125,000 | ) | | (100 | ) | | (61,652 | ) | | (186,652 | ) |
| | | | | | | | | | | | | | | | |
| Balances, November 7, 2018 | | - | | | - | | | - | | | - | | | - | |
GreyCan and RPK are each authorized to issue an unlimited number of ordinary shares, with par value of €1 each. The reorganization described in Note 2 – Basis of preparation involving GreyCan and RPK had the effect of these companies becoming subsidiaries of Holigen Holdings Limited and therefore their share capital balances were eliminated on consolidation with effect from July 6 and November 7, 2018, respectively.
Holigen Holdings Limited is authorized to issue an unlimited number of Ordinary A Shares and Ordinary B Nominee Shares, the shares of each class having a par value of €1 each. The Ordinary B Nominee Shares are non-voting, have no dividend entitlement and do not share in the assets of the Company on a winding-up event.
On incorporation, the Company issued 1,199 Ordinary A Shares and 1 Ordinary B Share for fully paid-up cash consideration of €1,199 and €1, respectively, which were outstanding as of December 31, 2018.
11. REVENUE
The revenue recognized during the year ended December 31, 2018 was € 32,689 (2017 - €nil) for the sale of bottled product to an authorized Australian distribution agent. The sale was made to a company related by virtue of common management, and the receivable was netted against amounts owing to the related party for rent.
12. INCOME TAXES
Holigen Holdings Limited’s jurisdiction for income tax purposes is Malta, which has a statutory income tax rate of 35.00% (2017 - 35.00%) . The theoretical tax recovery, derived by applying the Company’s statutory tax rate to the loss before tax for the year, can be reconciled to the recovery recognized in the statement of comprehensive income as follows:
| | | 2018 | | | 2017 | |
| Loss before tax | | (1,340,078 | ) | | (297,917 | ) |
| Maltese statutory income tax rate | | 35.00% | | | 35.00% | |
| Income tax expense based on the statutory income tax rate | | (469,027 | ) | | (104,271 | ) |
| Difference in tax rates in foreign jurisdictions | | 45,416 | | | 1,612 | |
| Expenses claimed for Australian SRED | | 249,517 | | | - | |
| Future tax benefit of losses carried forward not recognized | | 174,094 | | | 102,659 | |
| Income tax recovery | | - | | | - | |
B-17
| Holigen Holdings Limited |
| Notes to the Combined and Consolidated Financial Statements |
| For the year ended December 31, 2018 and period from formation on July 21, 2017 to December 31, 2017 |
| (Expressed in euros) |
The Company has filed a claim in Australia under the scientific research and experimental (‘SRED’) program that offers refundable credits at a rate of 38.5% of qualifying expenditures. The potential benefit of the refundable tax credit as at December 31, 2018 is estimated to be €249,517.
Loss Carry Forwards
As at December 31, 2018, the Company has non-capital tax loss carry forwards of €1,020,000 expiring as follows:
| 2030 | | 193,000 | |
| 2031 | | 827,000 | |
| | | 1,020,000 | |
13. COMMITMENT AND CONTINGENCIES
| (i) | The Licenses held by the Company require payment of annual renewal fees for each license which are expected to be approximately €20,000 (AUD$30,000). |
| | |
| (ii) | The Company is committed under an operating lease for premises at 9-15 Chilvers Road, Thornleigh, New South Wales, 2120, Australia. The lease is for a five-year term, that commenced November 1, 2017, with annual rent in the first year of €109,703, escalating by 3% with effect from April 1, 2020 and each anniversary thereafter. The Company paid a security deposit of €23,994 with respect to the lease commitment. The lease contains an option to extend for an additional two years and 11 months. |
| | |
| (iii) | The Company is also committed to an operating lease for approximately 65 hectares of land in the municipality of Aljustrel, Portugal. The lease is for seven years ending in October 2025 with annual rent of €123,500 for the first five years, then increasing by the change in the consumer price index for the sixth and seventh years. |
| | |
| (iv) | The Company has entered into a purchase and sale agreement for the acquisition of an industrial site covering 6.43 hectares in the municipality of Aljustrel, Portugal for €300,000, which is expected to close in June 2019, provided certain conditions are satisfied. |
| | |
| (v) | The Company entered into an agreement with an entity to perform various services regarding an application process for an incentive program available in Portugal. The agreement contains fixed fees and variable fees. The variable fees are contingent upon (i) the Company successfully obtaining an approved investment via the incentive program application (1% of the approved investment amount), and (ii) a financing advice fee amounting to 0.5% of any financing obtained by the Company resulting from this agreement. The Company has not pursued any programs under this agreement to date. |
Future minimum annual lease payments (excluding the requirement to pay realty taxes and operating costs) for the leases described in (iii) and (iv) are disclosed in Note 15. Lease payments expensed during the year are included in rent and occupancy in the statement of comprehensive income and totalled €169,441 (2017 - €19,135).
In the ordinary course of business activities, the Company may be contingently liable for litigation and claims with customers, suppliers, former employees or other parties. There were no litigation and claims during the year ended December 31, 2018.
B-18
| Holigen Holdings Limited |
| Notes to the Combined and Consolidated Financial Statements |
| For the year ended December 31, 2018 and period from formation on July 21, 2017 to December 31, 2017 |
| (Expressed in euros) |
14. RELATED PARTY TRANSACTIONS
Transactions with related parties are in the normal course of operations and included:
| (i) | Key management is defined as the Board of Directors, the CEO and the CFO of the Company and the CEO of TCann. During the year ended December 31, 2018, short-term benefits in the amount of €471,494 (2017 - €nil) were paid or accrued to key management personnel and are included in consulting expense. |
| | |
| (ii) | Included in licensing and development costs is €218,032 (2017 - €228,182) paid or accrued to a company controlled by a close relative of the CEO of the Company, and €113,490 (2017 - €nil) paid to a company controlled by the spouse of the CEO of the Company, and €21,784 (2017 - €nil) paid to a company under common management. |
| | |
| (iii) | Included in rent and occupancy costs is rent of €111,044 (2017 - €19,135) paid to a Company under common management. |
| | |
| (iv) | During 2018 the Company acquired property, plant and equipment from a company under common management in the amount of €4,864 (2017 - €nil) and from a company controlled by the spouse of the CEO of the Company in the amount of €95,220 (2017 - €nil). |
| | |
| (v) | At December 31, 2018 the following amounts were included in accounts payable and accrued liabilities: |
| • | Payable to a company controlled a close relative of the CEO of the Company: €423,894 (2017 - €224,170) for license and development costs; |
| • | Payable to a company controlled by the spouse of the CEO : €208,710 (2017 - €nil) for license and development costs and acquisition of property, plant and equipment; |
| • | Payable to a company controlled by the CFO of the Company €395,080 (2017 - €nil) for accounting and management services, which are included in consulting fees. |
| (vi) | During 2018, the Company signed a loan facility agreement with a company controlled by a close family member of the controlling shareholder. Interest on any loans would be at the benchmark interest rate prescribed by or under Section 109N of the Income Tax Assessment Act (Australia), and each loan must be repaid within 7 years, unless it is converted to a secured loan. As at December 31, 2018 no balance is outstanding under the facility. |
| | |
| (vii) | During 2018, the Company recorded revenue in the amount of €32,689 from a Company related by virtue of common management– see Note 11. |
15. FINANCIAL INSTRUMENTS
Fair value
Where the Company measures financial instruments for disclosure or measurement purposes, it classifies those fair values according with the following hierarchy, which is based on the inputs used to value the instrument:
Level 1 – inputs to the valuation methodology are unadjusted quoted prices for identical assets or liabilities in active markets.
Level 2 – inputs to the valuation methodology include quoted prices for similar assets or liabilities in active markets, and inputs that are observable for the asset or liability, either directly or indirectly, for substantially the full term of the financial instrument.
Level 3 – inputs to the valuation methodology are unobservable and significant to the fair value measurement.
The carrying amounts of cash, accounts payable and accrued liabilities approximate their fair values due to the short-term nature of these instruments. Loans payable to related parties have no fixed terms of repayment and are non-interest bearing. The carrying amount of these instruments are considered to represent fair value due to their short term nature. The convertible loan is valued at amortized cost and the derivative liability has been valued at its estimated fair value. The derivative liability is a level 2 valuation.
B-19
| Holigen Holdings Limited |
| Notes to the Combined and Consolidated Financial Statements |
| For the year ended December 31, 2018 and period from formation on July 21, 2017 to December 31, 2017 |
| (Expressed in euros) |
Financial risks
Financial instruments give rise to a variety of risks: market risks (including foreign exchange risk, interest rate risk and other price risks), credit risk and liquidity risk. It is management’s opinion that the Company is not exposed to significant other price risks arising from its financial instruments.
The Company manages its exposure to risks by operating in a manner that minimizes that exposure to the extent practicable. The Company does not make use of derivative instruments in managing these risk exposures. There have been no significant changes in the management of risk since the prior year and in the underlying risks, except as described in the following.
Credit risk
The Company’s financial instrument assets include cash. No allowance for credit losses has been recorded as at December 31, 2018 or 2017. The Group’s cash is held at significant banking institutions in Australia and Portugal. While the balance at any one time may exceed the government-insured limits, management monitors changes in the financial strength of these banks and considers the credit risk to be minimal.
Foreign currency risk
The functional currency of GreyCan and TCann is the Australian dollar (AUD). Fluctuations in the value of the Australian dollar compared to the euro, which is the Group’s functional currency, can cause fluctuation in the measurement of the net assets and comprehensive loss of these foreign subsidiaries on consolidation. The Company’s exposure to this risk is comparable to the prior period, except for the increase in AUD denominated net assets during the current year. The effect on the euro value of net assets of the Group of a reasonably possible 12-month fluctuation of the Australian dollar exchange rate compared to the euro is as follows:
| | | € | | | € | |
| Exchange rate at year end | | 0.615187/AUD | | | 0.650664/AUD | |
| Australian net assets of the subsidiaries, denominated in euros | | (797,404 | ) | | (221,144 | ) |
| Effect of a 5% increase or decrease in the AUD/€ exchange rate | | 38,000 | | | 11,000 | |
During the year the Company became exposed to fluctuations in the value of the Canadian dollar compared to the euro due to the convertible loan which is denominated in Canadian dollars. The loan was outstanding from November 19, 2018. A fluctuation of 6% in the Canadian dollar to euro exchange rate would cause an increase or decrease in net income or loss of €230,577.
Liquidity risk
Liquidity risk is the risk that the Company experiences difficulty in settling its contractual and other obligations as they come due, or that the Company will only be able to do so at adverse finance rates. The Company is exposed to liquidity risk as a result of accounts payable and accrued liabilities, convertible loan and derivative liability, its purchase commitment and lease commitments. Management continuously monitors its funding needs. The exposure to liquidity risk is unchanged from the prior period except for the quantum of the risk given the additional debt incurred and new commitments entered into by the Company during the current year.
B-20
| Holigen Holdings Limited |
| Notes to the Combined and Consolidated Financial Statements |
| For the year ended December 31, 2018 and period from formation on July 21, 2017 to December 31, 2017 |
| (Expressed in euros) |
The Company’s undiscounted contractual payments of financial liabilities and settlement of other commitments is as follows:
| | | Total | | | 2019 | | | 2020 | | | 2021 | | | 2022 | | | 2023 | | | Beyond | |
| | | € | | | € | | | € | | | € | | | € | | | € | | | € | |
| Financial liabilities recognized on the statement of financial position: | | | | | | | | | | | | | | | | |
| Accounts payable and accrued liabilities | | 1,215,082 | | | 1,215,082 | | | | | | | | | | | | | | | | |
| Loans payable to related parties | | 426,533 | | | 426,533 | | | | | | | | | | | | | | | | |
| Convertible loan | | 3,959,644 | | | 3,959,644 | | | | | | | | | | | | | | | | |
| Other obligations: | | | | | | | | | | | | | | | | | | | | | |
| Purchase commitment | | 300,000 | | | 300,000 | | | | | | | | | | | | | | | | |
| Lease commitments | | 1,280,353 | | | 233,203 | | | 235,672 | | | 239,037 | | | 222,524 | | | 123,500 | | | 226,417 | |
| | | | | | | | | | | | | | | | | | | | | | |
| Total | | 7,181,612 | | | 6,134,462 | | | 235,672 | | | 239,037 | | | 222,524 | | | 123,500 | | | 226,417 | |
The loans payable to related parties are included in the within one-year column to be consistent with the statement of financial position presentation because they are either due on demand or have no fixed terms of repayment. The nature of these payables, being with common control individuals and entities, means their settlement is within the control of the respective creditor. See Note 2 –Basis of preparation and going concern. The lease commitments obligation excludes the effect of any rent escalation in years 6 and 7.
16.CAPITAL MANAGEMENT
The Company defines capital as the aggregate of its shareholders’ (deficit) equity and convertible loan and derivative liability. The Company’s objectives in managing capital, which have not changed since the prior period, are to ensure that the Company’s ability to operate as a going concern is protected and that it has sufficient liquidity to allow progress to be made towards achieving the Company’s strategic objectives until such time as positive revenues and cash flows from operations are achieved. Future financing needs are anticipated and managed on a continuous basis. There have been no changes in the objectives, policies and processes of managing capital since the prior period. See Note 2 – Basis of presentation and going concern.
17.SUBSEQUENT EVENTS
| (i) | On 28 January 2019, all of the issued and outstanding shares of Holigen Holdings Limited were transferred to DFT Trading Limited (a company incorporated and domiciled in Malta). DFT Trading Limited became the Company’s immediate parent with effect from that date. |
| | |
| (ii) | The Company purchased vehicles for a total of €200,000, financed at a rate of 2.4% per annum and repayable over a term of 60 months. |
| | |
| (iii) | On 4 February 2019, the Company entered into a credit facility for up to €5,220,000, bearing interest at 10% and secured by a first mortgage over the Company’s land and building. |
| | |
| (iv) | On May 22, 2019 the Company signed a Convertible Credit Agreement with Flowr whereby Flowr agreed to provide a Credit Facility in the amount of CAD $4,500,000 to the Company. The loan is non-interest bearing and will terminate upon the earliest of: (a) August 31, 2019; (b) the repayment in full of the Credit Facility; (c) the exercise of the conversion option; and (d) the termination of the Credit Facility. The loan is convertible at the option of Flowr into Class A shares of the Company such that Flowr would receive the number of shares in the Company that would provide Flowr with 3.0% of the issued and outstanding shares in the Company. |
B-21
APPENDIX “C” - UNAUDITED CONDENSED INTERIM COMBINED AND CONSOLIDATED FINANCIAL STATEMENTS OF HOLIGEN FOR THE THREE MONTHS ENDED MARCH 31, 2019 AND 2018
C-1

HOLIGEN HOLDINGS LIMITED
CONDENSED INTERIM COMBINED AND CONSOLIDATED FINANCIAL STATEMENTS
For the Three Months Ended March 31, 2019 and 2018
Unaudited
(Expressed in Euros)
C-2
Holigen Holdings Limited
Condensed Interim Combined and Consolidated Financial Statements
For the Three Months Ended March 31, 2019 and 2018
(Unaudited)
| | Page |
| | |
| Condensed Interim Combined and Consolidated Statements of Financial Position | C-4 |
| Condensed Interim Combined and Consolidated Statements of Changes in Shareholders’ (Deficit) Equity | C-5 |
| Condensed Interim Combined and Consolidated Statements of Comprehensive Loss | C-6 |
| Condensed Interim Combined and Consolidated Statements of Cash Flows | C-7 |
| Notes to the Condensed Interim Combined and Consolidated Financial Statements | C-8 - C-16 |
C-3
| Holigen Holdings Limited |
| Condensed Interim Combined and Consolidated Statements of Financial Position |
| As at March 31, 2019 and December 31, 2018 |
| (Unaudited - expressed in Euros) |
| | | March 31, | | | December | |
| | | 2019 | | | 31, | |
| | | | | | 2018 | |
| | | € | | | € | |
| ASSETS | | | | | | |
| Current | | | | | | |
| Cash | | 1,283,768 | | | 1,654,176 | |
| Sales tax recoverable | | 88,195 | | | 40,031 | |
| Prepaid expense | | 57,837 | | | 57,837 | |
| Inventory | | 3,420 | | | 12,904 | |
| | | 1,433,220 | | | 1,764,948 | |
| Non-current | | | | | | |
| Security deposit | | 24,608 | | | 23,994 | |
| Property, plant and equipment(Notes 4 and5) | | 4,125,303 | | | 2,234,342 | |
| | | | | | | |
| TOTAL ASSETS | | 5,583,131 | | | 4,023,284 | |
| | | | | | | |
| LIABILITIES | | | | | | |
| Current | | | | | | |
| Accounts payable and accrued liabilities | | 1,444,515 | | | 1,215,082 | |
| Current portion of lease liability (Note 4) | | 228,808 | | | - | |
| Current portion of loans payable(Note 7) | | 3,342,089 | | | 2,926,806 | |
| Loans payable to related parties(Note 6) | | 432,590 | | | 426,533 | |
| Derivative liability(Note 7) | | 942,531 | | | 1,032,838 | |
| | | 6,390,533 | | | 5,601,259 | |
| Non-current | | | | | | |
| Loans payable (Note 7) | | 1,055,418 | | | - | |
| Lease liability (Note 4) | | 880,988 | | | - | |
| | | | | | | |
| TOTAL LIABILITIES | | 8,326,939 | | | 5,601,259 | |
| | | | | | | |
| SHAREHOLDERS’ DEFICIT | | | | | | |
| Share capital(Note 8) | | 1,200 | | | 1,200 | |
| Contributed surplus | | 24,333 | | | 24,333 | |
| Deficit | | (2,781,522 | ) | | (1,636,878 | ) |
| Accumulated other comprehensive income | | 14,252 | | | 34,486 | |
| Equity attributable to owners of the parent | | (2,741,737 | ) | | (1,576,859 | ) |
| Non-controlling interest | | (2,071 | ) | | (1,116 | ) |
| | | (2,743,808 | ) | | (1,577,975 | ) |
| | | | | | | |
| TOTAL LIABILITIES ANDSHAREHOLDERS’ DEFICIT | | 5,583,131 | | | 4,023,284 | |
See Note 2 – Basis of preparation, Going concern
Approved by the Board of Directors on June 14, 2019 and signed on its behalf by:
| “Pauric Duffy” | |
| Pauric Duffy, Director | |
See accompanying notes to condensed interim combined and consolidated financial statements.
C-4
| Holigen Holdings Limited |
| Condensed Interim Combined and Consolidated Statements of Changes in Shareholders’(Deficit) Equity |
| For the Three Months Ended March 31, 2019 and 2018 |
| (Unaudited - expressed in Euros) |
| | | | | | | | | | | | Accumulated | | | | | | | |
| | | | | | | | | | | | other | | | Non- | | | | |
| | | Share | | | Contributed | | | | | | comprehensive | | | controlling | | | | |
| | | capital | | | surplus | | | Deficit | | | income | | | interest | | | Total | |
| | | € | | | € | | | € | | | € | | | € | | | € | |
| | | (Note 8 | ) | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | |
| Balances, December 31, 2017 | | 62,652 | | | 24,333 | | | (297,917 | ) | | 8,113 | | | - | | | (202,819 | ) |
| Issuance of Ordinary shares of: | | | | | | | | | | | | | | | | | | |
| RPK | | 124,000 | | | - | | | - | | | - | | | - | | | 124,000 | |
| Net loss for the period | | - | | | - | | | (190,873 | ) | | - | | | (159 | ) | | (191,032 | ) |
| Unrealized loss on translation of foreign operation | | - | | | - | | | - | | | 9,114 | | | - | | | 9,114 | |
| | | | | | | | | | | | | | | | | | | |
| Balances, March 31, 2018 | | 186,652 | | | 24,333 | | | (488,790 | ) | | 17,227 | | | (159 | ) | | (260,737 | ) |
| | | | | | | | | | | | | | | | | | | |
| Issuance of ordinary shares of: | | | | | | | | | | | | | | | | | | |
| Holigen Limited(Note 8) | | - | | | - | | | - | | | - | | | 1 | | | 1 | |
| Reorganization(Notes 2 & 8) | | (186,652 | ) | | - | | | - | | | - | | | - | | | (186,652 | ) |
| Issuance of ordinary shares of: | | | | | | | | | | | | | | | | | | |
| Holigen Holdings Limited | | 1,200 | | | - | | | - | | | - | | | - | | | 1,200 | |
| Net loss for the period | | - | | | - | | | (1,148,088 | ) | | - | | | (958 | ) | | (1,149,046 | ) |
| Unrealized gain on translation of foreign operation | | - | | | - | | | - | | | 17,259 | | | - | | | 17,259 | |
| Balances, December 31, 2018 | | 1,200 | | | 24,333 | | | (1,636,878 | ) | | 34,486 | | | (1,116 | ) | | (1,577,975 | ) |
| Net loss for the period | | - | | | - | | | (1,144,644 | ) | | - | | | (955 | ) | | (1,145,599 | ) |
| Unrealized loss on translation of foreign operation | | - | | | - | | | - | | | (20,234 | ) | | - | | | (20,234 | ) |
| | | | | | | | | | | | | | | | | | | |
| Balances, March 31, 2019 | | 1,200 | | | 24,333 | | | (2,781,522 | ) | | 14,252 | | | (2,071 | ) | | (2,743,808 | ) |
See accompanying notes to condensed interim combined and consolidated financial statements.
C-5
| Holigen Holdings Limited |
| Condensed Interim Combined and Consolidated Statements of Comprehensive Loss |
| For the Three Months Ended March 31, 2019 and 2018 |
| (Unaudited - expressed in Euros) |
| | | Three Months Ended March 31 | |
| | | 2019 | | | 2018 | |
| | | € | | | € | |
| Revenue | | | | | | |
| Sales(Note 9) | | 29,342 | | | - | |
| | | | | | | |
| Expenses | | | | | | |
| Cost of goods sold | | 9,759 | | | - | |
| Accretion on convertible loan | | 258,529 | | | - | |
| Consulting(Note 11(i)) | | 275,348 | | | 21,467 | |
| Depreciation(Note 5) | | 96,620 | | | - | |
| Licenses and development costs(Note 11(ii)) | | 2,880 | | | 114,143 | |
| Office and general | | 182,149 | | | 21,147 | |
| Professional fees | | 171,200 | | | 872 | |
| Rent and occupancy(Note 11(iii)) | | 10,326 | | | 32,660 | |
| Staff cost | | 142,736 | | | 743 | |
| Fair value change in derivative liability(Note7(i)) | | (90,307 | ) | | - | |
| Interest on long-term debt | | 9,325 | | | - | |
| Foreign exchange loss | | 106,376 | | | - | |
| | | | | | | |
| | | 1,174,941 | | | 191,032 | |
| Net loss | | (1,145,599 | ) | | (191,032 | ) |
| Item that will be reclassified to profit or loss: | | | | | | |
| Unrealized (loss) gain on retranslation of subsidiary | | (20,234 | ) | | 9,114 | |
| | | | | | | |
| Comprehensive loss | | (1,165,833 | ) | | (181,918 | ) |
| | | | | | | |
| Net loss attributable to: | | | | | | |
| Equity-holders of Holigen Holdings Limited | | (1,144,644 | ) | | (190,873 | ) |
| Non-controlling interest | | (955 | ) | | (159 | ) |
| | | | | | | |
| Net loss | | (1,145,599 | ) | | (191,032 | ) |
| | | | | | | |
| Comprehensive loss attributable to: | | | | | | |
| Equity-holders of Holigen Holdings Limited | | (1,164,861 | ) | | (181,766 | ) |
| Non-controlling interest | | (972 | ) | | (152 | ) |
| | | | | | | |
| Comprehensive loss | | (1,165,833 | ) | | (181,918 | ) |
See accompanying notes to condensed interim combined and consolidated financial statements.
C-6
Holigen Holdings Limited
Condensed Interim Combined and Consolidated Statements of Cash Flows
For the Three Months Ended March 31, 2019 and 2018
(Unaudited - expressed in Euros)
| | | Three Months Ended March 31 | |
| | | 2019 | | | 2018 | |
| | | € | | | € | |
| Operating activities | | | | | | |
| Net loss | | (1,145,599 | ) | | (191,032 | ) |
| Adjustments for non-cash items: | | | | | | |
| Depreciation | | 96,620 | | | - | |
| Accretion on convertible loan | | 258,529 | | | - | |
| Gain on fair value change in derivative liability | | (90,307 | ) | | - | |
| Unrealized foreign exchange loss | | 120,436 | | | 11,297 | |
| | | | | | | |
| Operating cash flows before working capital changes | | (760,321 | ) | | (179,735 | ) |
| Changes in non-cash working capital: | | | | | | |
| Sales tax recoverable | | (48,164 | ) | | 2,656 | |
| Inventory | | 9,484 | | | - | |
| Accounts payable and accrued liabilities | | 229,432 | | | 122,672 | |
| | | | | | | |
| Cash used in operating activities | | (569,569 | ) | | (54,407 | ) |
| | | | | | | |
| Financing activities | | | | | | |
| Proceeds from loan payable | | 1,093,406 | | | - | |
| Payments on principal portion of lease liability | | (49,462 | ) | | | |
| Loans from related parties | | - | | | 7,903 | |
| Repayments of loan payable | | (6,350 | ) | | - | |
| | | | | | | |
| Cash provided by financing activities | | 1,037,594 | | | 7,903 | |
| | | | | | | |
| Investing activities | | | | | | |
| Purchase of property, plant and equipment | | (828,322 | ) | | - | |
| | | | | | | |
| Cash used in investing activities | | (828,322 | ) | | - | |
| | | | | | | |
| Impact of foreign exchange on cash held | | (10,110 | ) | | - | |
| | | | | | | |
| Change in cash during the period | | (370,408 | ) | | (46,504 | ) |
| Cash, beginning of period | | 1,654,176 | | | 82,549 | |
| | | | | | | |
| Cash, end of period | | 1,283,768 | | | 36,045 | |
See accompanying notes to condensed interim combined and consolidated financial statements.
C-7
| Holigen Holdings Limited |
| Notes to the Condensed Interim Combined and Consolidated Financial Statements |
| For the Three Months Ended March 31, 2019 and 2018 |
| (Unaudited - expressed in Euros) |
1. NATURE OF BUSINESS
Holigen Holdings Limited (the ‘Company’) was incorporated under the Companies Act (Malta) on June 28, 2018 and is domiciled and registered in Malta as a limited liability company. The Company was named RPK Holdings Limited prior to August 7, 2018. The Company’s registered office is ‘Lara Buildings’, Level 1, Guzeppi Calleja Street, Iklin 1KL262, Malta.
On January 28, 2019, all of the issued and outstanding shares of Holigen Holdings Limited were transferred to DFT Trading Limited (a company registered and domiciled in Malta), which became the Company’s immediate parent.
The condensed interim combined and consolidated statements of the Company (the ‘Group’) as at March 31, 2019 are comprised of Holigen Holdings Limited and its 99.92% owned subsidiary Holigen Limited along with its wholly-owned subsidiaries RPK Biopharma Unipessoal Lda. (‘RPK’), and GreyCan Pty Ltd. (‘GreyCan’) and GreyCan’s wholly owned subsidiary TCann Pty Ltd. (‘TCann’).
The Company is pursuing its strategic objectives to commence production and research related to medicinal marijuana. Through an Australian subsidiary, the Company has been granted licenses for Cultivation, R&D and Manufacturing from the Office of Drug Control and Drug Control Section Australia (Therapeutic Goods Administration) and a Good Manufacturing Process License from the TGA. On February 1, 2019, the Company’s Portuguese subsidiary was granted permission to operate a medicinal cannabis business. These awards represent significant milestones in the Company’s strategic objectives.
2. BASIS OF PREPARATION AND GOING CONCERN
Basis of consolidation and combination and reorganization
These combined and consolidated financial statements are prepared under the name of Holigen Holdings Limited based on the corporate structure as at March 31, 2019. The Company has elected to restate the comparative period information to reflect the effects of the reorganization that took place in 2018, as though the post-reorganization structure had always been in place. This continuity of interests method has resulted in the Company assuming the deficit and other comprehensive income balances as at July 21, 2017. However, since Holigen Holdings Limited was not incorporated until June 28, 2018, the financial statements for the period from formation of the Group on July 21, 2017 to December 31, 2017 and up until June 28, 2018 are prepared on a combined basis, as a combination of the financial position and performance of RPK, GreyCan and TCann since the dates of their respective incorporations. All entities included were under common control throughout the period from July 21, 2017 to June 28, 2018.
Statement of compliance
These condensed interim combined and consolidated financial statements have been prepared in accordance with International Accounting Standard (IAS) 34Interim Financial Reporting,are unaudited (except for the information noted) and should be read in conjunction with the audited financial statements for December 31, 2018.
Basis of presentation
These condensed interim combined and consolidated financial statements have been prepared on a historical cost basis. All accounting policies and methods of computation followed in the preparation of these condensed interim combined and consolidated financial statements are consistent with those of the year ended December 31, 2018, except as detailed in Note 4 of those statements.
C-8
| Holigen Holdings Limited |
| Notes to the Condensed Interim Combined and Consolidated Financial Statements |
| For the Three Months Ended March 31, 2019 and 2018 |
| (Unaudited - expressed in Euros) |
The condensed interim combined and consolidated financial statements include the accounts of the Company and the following subsidiaries (collectively, the ‘Group’):
| Company name | Domicile | Incorporation date | Ownership |
| | | | |
| Holigen Limited1(‘Holigen’) | Malta | July 2, 2018 | 99.92% directly owned |
| RPK Biopharma, Unipessoal Lda. (‘RPK’) | Portugal | October 24, 2017 | 100% owned by Holigen |
| GreyCan Pty Ltd. (‘GreyCan’) | Australia | July 21, 2017 | 100% owned by Holigen |
| TCann Pty Ltd. (‘TCann’) | Australia | July 21, 2017 | 100% owned by GreyCan |
Note 1– Formerly RPK Trading Limited, prior to August 7, 2018
During the year ended December 31, 2018, the Company effected a corporate reorganization of entities under common control and management, through the following steps:
| (i) | on July 6, 2018, all issued and outstanding shares of GreyCan were transferred to Holigen, in exchange for a loan payable of €61,652; and |
| | |
| (ii) | on November 7, 2018, all issued and outstanding shares of RPK were transferred to Holigen, in exchange for a loan payable of €125,000 (see Note 6 – Loans payable to related parties). |
The Company used the continuity of interests accounting with respect to the reorganization transactions which are fully restrospective rather than applying the acquisition method of IFRS 3Business Combinations.
Subsidiaries are under the control of a parent, and therefore consolidated, when an investor is exposed, or has rights, to variable returns from the investee and has the ability to affect those returns through its power over the investee. Uniform accounting policies have been applied by the Group throughout, and all intra-Group balances and items of revenue and expense have been eliminated from both periods presented.
Going concern
These condensed interim combined and consolidated financial statements have been prepared under the going concern assumption, which contemplates the realization of assets and settlement of liabilities in the normal course of business. During the period ended March 31, 2019, the Company incurred a comprehensive loss of €1,165,833 (2018 - €181,918) and had a net outflow of cash from operations of €563,259 (2018 - €54,407). As at March 31, 2019, the Company had a working capital deficit of €4,957,313 (December 31, 2018 – €3,836,311) and an accumulated deficit of €2,781,522 (December 31, 2018 - €1,636,878).
The ability of the Company to continue as a going concern is dependent on management raising sufficient capital to maintain operations and to fund further operating expenses and losses until revenues and positive cash flows can be generated from operations. These are significant uncertainties that may cast significant doubt on the ability of the Company to continue as a going concern.
Management believes that the Company has access to sufficient funding on reasonable commercial terms necessary to achieve its strategic objectives. Therefore, the condensed interim combined and consolidated financial statements do not give effect to adjustments that would be necessary to the carrying amounts and classifications of assets and liabilities should the going concern assumption not be appropriate.
Presentation and functional currencies
The condensed interim combined and consolidated financial statements are presented in euros (or €s) as the presentation currency of the Group.
The functional currency of the Company, Holigen and RPK is the euro. The functional currency of GreyCan and TCann is the Australian dollar (AUD). Revenue and expenses of GreyCan and TCann are translated into euros using the average Australian exchange rate with the euro for the respective reporting period. Assets and liabilities are translated at the spot rate as at the statement of financial position date. Exchange differences arising from translation are recognized in other comprehensive income.
Monetary items denominated in currencies other than the functional currency of the entity are initially measured using the exchange rate on the date of the transaction. Gains and losses arising from retranslation at subsequent reporting dates are recognized in net income or loss.
C-9
| Holigen Holdings Limited |
| Notes to the Condensed Interim Combined and Consolidated Financial Statements |
| For the Three Months Ended March 31, 2019 and 2018 |
| (Unaudited - expressed in Euros) |
3. ESTIMATES AND JUDGEMENTS
The preparation of interim financial statements requires management to make judgements, estimates and underlying assumptions that could affect the reported amounts in the financial statements. Management bases its judgements and estimates on historical experience and other factors, including forward looking information, that it believes to be reasonable under the circumstances, but which are inherently uncertain and unpredictable. The results of these estimates form the basis of the carrying amounts of assets and liabilities.
Imprecision in exercising judgement and making estimates and assumptions could require adjustment to the carrying amounts of the affected asset or liability, and those adjustments could be material. Any adjustment will be recognized in the period in which it is identified and in any future periods affected.
The most significant judgements include those related to determining the functional currency of the Company, assessing whether the criteria are met for recognition of deferred tax assets, consisting of tax losses available and research and development tax credits, the ability of the Company to continue as a going concern, the determination of when property, plant and equipment are available for use as well as their useful lives, valuation and recoverability of deferred taxes, and impairment of financial and non-financial assets. The Company is subject to a number of risks and uncertainties associated with the going concern assumption and exercises judgment to assess the uncertainties relating to the determination of the Company’s ability to continue as a going concern.
The most significant estimates and assumptions include those related to the inputs used in accounting for the valuation of the foreign exchange derivative liability, including volatility, and the fair value of financial instruments. Management has determined that judgments, estimates and assumptions reflected in these condensed interim combined and consolidated financial statements are reasonable.
4. NEW ACCOUNTING STANDARDS ADOPTED
IFRS 16 -Leases
IFRS 16,Leases eliminates the classification of leases as either operating or finance leases and introduces a single lessee model which requires the lessee to recognize assets and liabilities for all leases with a term of longer than 12 months, with the exception of low-value assets. The Company adopted IFRS 16 effective January 1, 2019, using the modified retrospective approach. Accordingly, the information presented for 2018 has not been restated and is accounted for under IAS 17,Leases.
New leases accounting policy effective January 1, 2019
At the inception of a contract, to determine if it contains a lease, the Company assesses whether it conveys the right to control and obtain substantially all of the economic benefits of an identified asset, for a period of time, in exchange for consideration. Where a contract contains a lease, the Company recognizes a right-of-use asset and a lease liability at the commencement date of the lease.
The right-of-use asset is measured at cost less any accumulated depreciation and impairment losses and may be adjusted for any remeasurement of the lease liability. Cost is the amount of the initial lease liability plus any initial direct costs incurred and any lease payments made at or before commencement date less any incentives received.
The right-of-use assets are included in the cost of property, plant and equipment on the balance sheet. They are depreciated, in accordance with the Company’s existing accounting policy, over the shorter of the term of the lease or the life of the asset.
The lease liability is initially measured at the present value of future lease payments discounted at the interest rate implicit in the contract. If the implicit rate cannot be determined, the incremental borrowing rate over a similar term and with similar security for the funds necessary to obtain an asset of similar value in a similar economic environment is used. The lease payments include fixed payments less any incentives receivable, variable lease payments that depend on an index or rate and amounts expected to be paid under residual value guarantees. Where the lease contains an extension or purchase option, the costs associated with the option are included if it is reasonably expected to be exercised by the Company.
C-10
| Holigen Holdings Limited |
| Notes to the Condensed Interim Combined and Consolidated Financial Statements |
| For the Three Months Ended March 31, 2019 and 2018 |
| (Unaudited - expressed in Euros) |
Thereafter, the amount of the lease liability is increased to reflect the accretion of interest and reduced for the lease payments made. In addition, the carrying amount of the lease liability is remeasured to reflect any modifications to the contract terms.
The Company has elected not to recognize right-of-use assets and lease liabilities for contracts that have a lease term of 12 months or less or are for the use of low value assets. The Company has also elected not to reassess whether a contract is, or contains a lease at the date of initial application. Instead for contracts entered into before the transition date the Company relied on its assessment made applying IAS 17 and IFRIC 4 Determining whether an Arrangement contains a Lease. These contracts are recognized as an expense in the condensed interim combined and consolidated statements of operations in the period the cost is incurred.
Impact of adopting IFRS 16
Upon transition to IFRS 16, the Company recognized right-of-use assets and lease liabilities totalling €1,132,748. The lease liabilities have remaining terms of between 4 and 7 years and are discounted using its incremental borrowing rate at January 1, 2019. The weighted-average rate applied was 3.0% . The following table outlines the difference between operating lease commitments immediately preceding the date of initial application and lease liabilities recognised on the condensed interim combined and consolidated statements of financial position at application:
| | | € | |
| | | | |
| Operating lease commitments at December 31, 2018 | | 1,280,353 | |
| Effect of discounting at January 1, 2019 | | 107,080 | |
| Lease liabilities arising on initial application of IFRS 16 at January 1, 2019 | | 1,173,273 | |
The Company applied the following practical expedients in the adoption of IFRS 16:
- Applied the exception not to recognize right-of-use assets for leases with a term of 12 months or less remaining at January 1, 2019;
- For contracts previously determined to contain a finance lease under IAS 17, used the carrying amount of the right-of-use asset and lease liability determined under IAS 17;
- Excluded initial direct costs from measuring right-of-use assets at the date of initial adoption;
- For certain classes of assets, the Company has elected to account for both the lease and non-lease components as a single lease component.
During the first quarter of 2019, the Company recognized depreciation of €54,735 on new right-of-use assets recognized under IFRS 16 in the condensed interim combined and consolidated statements of operations and made payments on these leases of €58,217.
5. PROPERTY, PLANT AND EQUIPMENT
Total depreciation for the three months ended March 31, 2019 was €96,620 (2018 – nil).
At March 31, 2019, leasehold improvements with a carrying value of €130,883 was not yet available for use. As such, the asset has not yet commenced amortization.
Included in additions for the three months ended March31, 2019 was a right-of-use asset in the amount of €1,173,273 related to the adoption of IFRS 16 – see note 4.
C-11
| Holigen Holdings Limited |
| Notes to the Condensed Interim Combined and Consolidated Financial Statements |
| For the Three Months Ended March 31, 2019 and 2018 |
| (Unaudited - expressed in Euros) |
6. LOANS PAYABLE TO RELATED PARTIES
| | | March 31 | | | December 31 | |
| | | 2019 | | | 2018 | |
| | | € | | | € | |
| Payable to a trust controlled by the spouse of the Company’s controlling shareholder | | 242,930 | | | 236,873 | |
| Payable to the Company’s controlling shareholder | | 189,660 | | | 189,660 | |
| | | | | | | |
| | | 432,590 | | | 426,533 | |
The loans payable to related parties are non-interest bearing and unsecured and have no fixed terms of repayment. The shareholder loan is due on demand and has no stated rate of interest.
7. LOANS PAYABLE
Loans payable include the following. See also Note 12 – Financial instruments, Financial risks, Liquidity risk for the future principal repayments.
| | | March 31 | | | December 31 | |
| | | 2019 | | | 2018 | |
| | | € | | | € | |
| Convertible loan - see (i) | | 3,310,452 | | | 2,926,806 | |
| Term loan - see (ii) | | 941,000 | | | - | |
| Car loan – see (iii) | | 193,650 | | | - | |
| Less unamortized financing cost of term loan | | (47,595 | ) | | - | |
| | | 4,397,507 | | | 2,926,806 | |
| | | | | | | |
| Less current portion | | (3,342,089 | ) | | (2,926,806 | ) |
| | | | | | | |
| Non-current | | 1,055,418 | | | - | |
(i) Convertible loan - On November 19, 2018, the Company entered into a binding term sheet with The Flowr Corporation (Flowr), pursuant to which Flowr agreed to loan CAD $6,000,000 to the Company. The loan is interest free and includes an option that would allow Flowr to convert the loan into Class A shares of the Company at a ratio of 8 shares for each CAD $1 million if the Company and Flowr did not enter into a subscription and purchase agreement by January 19, 2019.
The Company and Flowr entered into a subscription and purchase agreement on December 19, 2018 (the ‘Subscription and Purchase Agreement’), pursuant to which Flowr agreed to purchase 15% of the ordinary class A shares of the Company from the controlling shareholder (the Share Purchase). The Company and Flowr agreed to exchange, immediately following the Share Purchase, an additional 4.8% of the ordinary class A shares of the Company in settlement of the closing date euro equivalent of the CAD $6,000,000 loan (the Settlement). These transactions will close when all conditions and criteria have been met and will result in Flowr becoming the holder of 19.8% of the ordinary A shares of the Company.
The Company’s functional currency is the euro. As the convertible loan is in a currency different than the functional currency, the loan is accounted for as a financial liability and the conversion privilege is considered to be a derivative liability as it fails the fixed for fixed criteria. The derivative liability is initially measured at fair value using the Black-Scholes option pricing model and is revalued at fair value at each financial reporting date with gains and losses recognized in net income or loss. At initial recognition, fair value measurement was based on the following assumptions: volatility of 65%, expected life of 1 year, risk free interest rate of 2.10%, and annual dividend yield of nil. The derivative liability component was initially fair valued at €1,042,702 resulting in a valuation of the host liability of €2,940,832
At March 31, 2019, the fair value of the derivative liability was determined to be €942,531 (December 31, 2018 – €1,032,838) with the change in fair value included in the statement of comprehensive loss.
C-12
| Holigen Holdings Limited |
| Notes to the Condensed Interim Combined and Consolidated Financial Statements |
| For the Three Months Ended March 31, 2019 and 2018 |
| (Unaudited - expressed in Euros) |
(ii) Under the term loan with Credito Agricola, proceeds are available up to €5,220,000, maturing February 2026, bearing interest at a variable rate of the 12-month EURIBOR rate plus 3% per annum with a floor of 3% per annum, interest only for the first 12 months and secured by land and building with a carrying amount of €2,337,847 at March 31, 2019. The balance drawn down at March 31, 2019 is repayable in quarterly payments of: €27,068 in the second year; €29,480 for the third and fourth years; €49,741 in the fifth, sixth and seventh years. See also Note 14(i) – Subsequent events.
(iii) The Company acquired vehicles financed by a loan maturing January 2024, bearing interest at a variable rate of the 12-month EURIBOR rate plus 2% per annum with a floor of 2% per annum, repayable in monthly principal repayments of €3,203 plus interest, secured by vehicles with a carrying amount of €244,230 at March 31, 2019
8. SHARE CAPITAL
The Company’s statement of financial position as at December 31, 2017 and 2018 included the combination of the following share capital amounts :
| | | RPK | | | GreyCan | | | Total | |
| | | Ordinary | | | Share | | | Ordinary | | | Share | | | Share | |
| | | Shares | | | capital | | | Shares | | | capital | | | capital | |
| | | Number | | | € | | | Number | | | € | | | € | |
| Balances, December 31, 2017 | | 1,000 | | | 1,000 | | | 100 | | | 61,652 | | | 62,652 | |
| Issuance of ordinary shares | | 124,000 | | | 124,000 | | | - | | | - | | | 124,000 | |
| Reorganization(Note 2) | | (125,000 | ) | | (125,000 | ) | | (100 | ) | | (61,652 | ) | | (186,652 | ) |
| | | | | | | | | | | | | | | | |
| Balances, November 7, 2018 | | - | | | - | | | - | | | - | | | - | |
GreyCan and RPK are each authorized to issue an unlimited number of ordinary shares, with par value of €1 each. The reorganization described in Note 2 – Basis of preparation involving GreyCan and RPK had the effect of these companies becoming subsidiaries of Holigen Holdings Limited and therefore their share capital balances were eliminated on consolidation with effect from July 6 and November 7, 2018, respectively.
Holigen Holdings Limited is authorized to issue an unlimited number of Ordinary A Shares and Ordinary B Nominee Shares, the shares of each class having a par value of €1 each. The Ordinary B Nominee Shares are non-voting, have no dividend entitlement and do not share in the assets of the Company on a winding-up event.
On incorporation, the Company issued 1,199 Ordinary A Shares and 1 Ordinary B Share for fully paid-up cash consideration of €1,199 and €1, respectively, which were outstanding as of March 31, 2019 and December 31, 2018.
9. REVENUE
The revenue recognized during the period ended March 31, 2019 (2018 - €nil) is for the sale of bottled product to an authorized Australian distribution agent. The sale was made to a company related by virtue of common management, and the receivable was netted against amounts owing to the related party for rent.
10. COMMITMENTS AND CONTINGENCIES
| (i) | In connection with the Subscription and Purchase Agreement described in Note 7 – Loan payable, The Flowr Group (Okanagan) Inc. (Flowr Okanagan), a subsidiary of Flowr, entered into an exclusive intellectual property licensing agreement, pursuant to which Flowr Okanagan agreed to provide certain intellectual property know-how to the Company. |
| | |
| (ii) | The Australian Licenses held by the Company, as described in Note 1 – Nature of business, require payment of annual renewal fees for each license which are expected to be approximately €20,000 (AUD$30,000). |
C-13
| Holigen Holdings Limited |
| Notes to the Condensed Interim Combined and Consolidated Financial Statements |
| For the Three Months Ended March 31, 2019 and 2018 |
| (Unaudited - expressed in Euros) |
| (iii) | The Company is committed under a lease for premises at 9-15 Chilvers Road, Thornleigh, New South Wales, 2120, Australia. The lease is for a five-year term, that commenced November 1, 2017, with annual rent in the first year of €109,703, escalating by 3% with effect from April 1, 2020 and each anniversary thereafter. The Company paid a security deposit of €23,994 with respect to the lease commitment. The lease contains an option to extend for an additional two years and 11 months. |
| | |
| (iv) | The Company is also committed to a lease for approximately 65 hectares of land in the municipality of Aljustrel, Portugal. The lease is for seven years ending in October 2025 with annual rent of €123,500 for the first five years, then increasing by the change in the consumer price index for the sixth and seventh years. |
| | |
| (v) | The Company has entered into a purchase and sale agreement for the acquisition of an industrial site covering 6.43 hectares in the municipality of Aljustrel, Portugal for €300,000, which is expected to close in June 2019, provided certain conditions are satisfied. |
| | |
| (vi) | The Company entered into an agreement with an entity to perform various services regarding an application process for an incentive program available in Portugal. The agreement contains fixed fees and variable fees. The variable fees are contingent upon (i) the Company successfully obtaining an approved investment via the incentive program application (1% of the approved investment amount), and (ii) a financing advice fee amounting to 0.5% of any financing obtained by the Company resulting from this agreement. The Company has not pursued any programs under this agreement to date |
In the ordinary course of business activities, the Company may be contingently liable for litigation and claims with customers, suppliers, former employees or other parties. There were no litigation and claims during the period ended March 31, 2019.
11. RELATED PARTY TRANSACTIONS
Transactions with related parties are in the normal course of operations and included:
| (i) | Key management is defined as the Board of Directors, the CEO and the CFO of the Company and the CEO of TCann. During the three months ended March 31, 2019, short-term benefits in the amount of €244,415 (2018 - €nil) were paid or accrued to key management personnel and are included in consulting expense. |
| | |
| (ii) | Included in licensing and development costs is €nil (2018 - €109,016) paid or accrued to a company controlled by a close relative of the CEO of the Company, and €nil (2018 - €4,941) paid to a company controlled by the spouse of the CEO of the Company. |
| | |
| (iii) | During the three months ended March 31, 2019, lease payments of €35,417 were paid to a Company under common management and were recorded as a reduction in the lease liability under IFRS 16. During the three months ended March 31, 2018 prior to the adoption of IFRS 16 (see Note 4), €30,275 paid to a Company under common management was recorded in rent and occupancy expense. |
| | |
| (iv) | At March 31, 2019 the following amounts were included in accounts payable and accrued liabilities: |
| | • | Payable to a company controlled a close relative of the CEO of the Company: €434,733 (2018 - €423,894) for license and development costs; |
| | • | Payable to a company controlled by the spouse of the CEO: €50,664 (2018 - €208,710) for license and development costs and acquisition of property, plant and equipment; |
| | • | Payable to a company controlled by the CFO of the Company: €474,580 (2018 - €395,080) for accounting and management services, which are included in consulting fees; |
| | • | Payable to a company controlled by the CEO of the Company: €96,974 (2018 - €nil) for management services, which are included in consulting fees. |
| (vi) | During 2018, the Company signed a loan facility agreement with a company controlled by a close family member of the controlling shareholder. Interest on any loans would be at the benchmark interest rate prescribed by or under Section 109N of the Income Tax Assessment Act (Australia), and each loan must be repaid within 7 years, unless it is converted to a secured loan. As at December 31, 2018 and March 31, 2019 no balance is outstanding under the facility. |
C-14
| Holigen Holdings Limited |
| Notes to the Condensed Interim Combined and Consolidated Financial Statements |
| For the Three Months Ended March 31, 2019 and 2018 |
| (Unaudited - expressed in Euros) |
| (vii) | During the three months ended March 31, 2019, the Company recorded revenue in the amount of €29,342 (2018 – €nil) from a Company related by virtue of common management– see Note 9. |
12. FINANCIAL INSTRUMENTS
Fair value
Where the Company measures financial instruments for disclosure or measurement purposes, it classifies those fair values according with the following hierarchy, which is based on the inputs used to value the instrument:
Level 1 – inputs to the valuation methodology are unadjusted quoted prices for identical assets or liabilities in active markets.
Level 2 – inputs to the valuation methodology include quoted prices for similar assets or liabilities in active markets, and inputs that are observable for the asset or liability, either directly or indirectly, for substantially the full term of the financial instrument.
Level 3 – inputs to the valuation methodology are unobservable and significant to the fair value measurement.
The carrying amounts of cash and accounts payable and accrued liabilities approximate their fair values due to the short-term nature of these instruments. Loans payable to related parties either have no fixed terms of repayment or are due on demand and the carrying amount of these instruments is therefore considered to represent fair value. The convertible loan is valued at amortized cost and the derivative liability has been valued at its estimated fair value. The derivative liability is a level 2 valuation. The other loans payable are representative of their fair values as the interest rates are representative of current market rates for similar loans.
Financial risks
Financial instruments give rise to a variety of risks: market risks (including foreign exchange risk, interest rate risk and other price risks), credit risk and liquidity risk. It is management’s opinion that the Company is not exposed to significant other price risks arising from its financial instruments.
The Company manages its exposure to risks by operating in a manner that minimizes that exposure to the extent practicable. The Company does not make use of derivative instruments in managing these risk exposures. There have been no significant changes in the management of risk since the prior year and in the underlying risks, except as described in the following.
Credit risk
The Company’s financial instrument assets include cash. No allowance for credit losses has been recorded as at March 31, 2019 or at December 31, 2018. The Group’s cash is held at significant banking institutions in Australia and Portugal. While the balance at any one time may exceed the government-insured limits, management monitors changes in the financial strength of these banks and considers the credit risk to be minimal.
Foreign currency risk
The Company is exposed to foreign exchange risk in Australian dollars. Foreign exchange risk is the risk that the exchange rate that was in effect on the date that an obligation in a foreign currency was made to the Company by a customer or lender, or that an obligation in a foreign currency was made by the Company to a supplier or a partner, is different at the time of settlement than it was at the time that the obligation was determined. The Company reduces its exposure to foreign exchange risk by carefully monitoring exchange rates on obligations that are made to the Company. The Company did not have any hedges that the financial statements were issued. The Company does not utilise financial instruments to manage its foreign exchange risk.
Liquidity risk
Liquidity risk is the risk that the Company experiences difficulty in settling its contractual and other obligations as they come due, or that the Company will only be able to do so at adverse finance rates. The
C-15
| Holigen Holdings Limited |
| Notes to the Condensed Interim Combined and Consolidated Financial Statements |
| For the Three Months Ended March 31, 2019 and 2018 |
| (Unaudited - expressed in Euros) |
Company is exposed to liquidity risk as a result of accounts payable and accrued liabilities, convertible loan and derivative liability, its purchase commitment and lease commitments. Management continuously monitors its funding needs. The exposure to liquidity risk is unchanged from the prior period except for the quantum of the risk given the additional debt incurred and new commitments entered into by the Company during the current year.
13. CAPITAL MANAGEMENT
The Company defines capital as the aggregate of its shareholders’ (deficit) equity and convertible loan and derivative liability. The Company’s objectives in managing capital, which have not changed since the prior period, are to ensure that the Company’s ability to operate as a going concern is protected and that it has sufficient liquidity to allow progress to be made towards achieving the Company’s strategic objectives until such time as positive revenues and cash flows from operations are achieved. Future financing needs are anticipated and managed on a continuous basis. There have been no changes in the objectives, policies and processes of managing capital since the prior period. See Note 2 – Basis of presentation and going concern.
14. SUBSEQUENT EVENTS
| (i) | On April 30, 2019, an amount of €398,100 was drawn down under the Company’s term loan (as described in Note 7 – Loans payable). On May 28, 2019, an additional €202,000 was drawn down under this term loan. |
| | |
| (ii) | On May 22, 2019 the Company signed a Convertible Credit Agreement with Flowr whereby Flowr agreed to provide a Credit Facility in the amount of CAD $4,500,000 to the Company. The loan is non-interest bearing and will terminate upon the earliest of: (a) August 31, 2019; (b) the repayment in full of the Credit Facility; (c) the exercise of the conversion option; and (d) the termination of the Credit Facility. The loan is convertible at the option of Flowr into Class A shares of the Company such that Flowr would receive the number of shares in the Company that would provide Flowr with 3.0% of the issued and outstanding shares in the Company. |
C-16
APPENDIX “D” - PRO FORMA FINANCIAL INFORMATION
D-1

Pro Forma Condensed Consolidated Financial Statements
(unaudited)
For the periods ended
March 31, 2019 and December 31, 2018
(in Canadian dollars)
D-2
| PRO FORMA CONSOLIDATED STATEMENT OF FINANCIAL POSITION |
| As at March 31, 2019 |
| Unaudited |
| | | | | | HOLIGEN | | | | | | | | |
| | | THE FLOWR | | | HOLDINGS | | | | PRO FORMA | | | PRO FORMA | |
| | | CORPORATION | | | LIMITED | | | | ADJUSTMENTS | | | CONSOLIDATED | |
| | | | | | (Note 1(d)) | | | | | | | | |
| | | $ | | | $ | | Note | | $ | | | $ | |
| | | | | | | | | | | | | | |
| Assets | | | | | | | | | | | | | |
| Current | | | | | | | | | | | | | |
| Cash and cash equivalents | | 16,293,066 | | | 1,924,823 | | 2(a) | | 115,400,000 | | | 122,971,442 | |
| | | | | | | | 2(b) | | (2,400,000 | ) | | | |
| | | | | | | | 2(c) | | (8,246,447 | ) | | | |
| Amounts receivable | | 3,163,838 | | | 132,236 | | | | - | | | 3,296,074 | |
| Prepaids and other current assets | | 905,440 | | | 86,718 | | | | - | | | 992,158 | |
| Inventory | | 1,886,578 | | | 5,128 | | | | - | | | 1,891,706 | |
| Biological assets | | 833,103 | | | - | | | | - | | | 833,103 | |
| Total Current Assets | | 23,082,025 | | | 2,148,905 | | | | 104,753,553 | | | 129,984,483 | |
| Non-current assets | | | | | | | | | | | | | |
| Investments at fair value | | 739,909 | | | - | | | | - | | | 739,909 | |
| Property, plant & equipment | | 44,346,851 | | | 6,185,290 | | | | - | | | 50,532,141 | |
| Convertible loan receivable | | 6,000,000 | | | - | | 2(c) | | (6,000,000 | ) | | - | |
| Other long-term assets | | 234,087 | | | 36,896 | | | | - | | | 270,983 | |
| Intangible assets | | 3,001,147 | | | | | 2(c) | | 42,482,006 | | | 45,483,153 | |
| Unallocated acquisition - goodwill | | - | | | - | | 2(c) | | 199,570,449 | | | 199,570,449 | |
| | | 54,321,994 | | | 6,222,186 | | | | 236,052,455 | | | 296,596,635 | |
| Total Assets | | 77,404,019 | | | 8,371,091 | | | | 340,806,008 | | | 426,581,118 | |
| | | | | | | | | | | | | | |
| Liabilities | | | | | | | | | | | | | |
| Current | | | | | | | | | | | | | |
| Accounts payable and accrued liabilities | | 6,805,011 | | | 2,165,839 | | | | (1,195,709 | ) | | 7,775,141 | |
| Due to related parties | | 51,212 | | | 648,606 | | | | (648,606 | ) | | 51,212 | |
| Deferred revenue | | 1,114,881 | | | - | | | | - | | | 1,114,881 | |
| Short-term lease obligations | | 787,020 | | | 343,064 | | | | - | | | 1,130,084 | |
| Derivative liability | | - | | | 1,413,188 | | 2(c) | | (1,413,188 | ) | | - | |
| Loans payable - current | | 335,419 | | | 5,010,975 | | 2(c) | | (4,963,540 | ) | | 382,854 | |
| Total Current Liabilities | | 9,093,543 | | | 9,581,672 | | | | (8,221,043 | ) | | 10,454,172 | |
| Non-current liabilities | | | | | | | | | | | | | |
| Loans payable - long-term | | 3,136,117 | | | 1,582,445 | | | | (1,410,890 | ) | | 3,307,672 | |
| Lease obligations | | 9,018,255 | | | 1,320,913 | | | | - | | | 10,339,168 | |
| | | 12,154,372 | | | 2,903,358 | | | | (1,410,890 | ) | | 13,646,840 | |
| Total Liabilities | | 21,247,915 | | | 12,485,030 | | | | (9,631,933 | ) | | 24,101,012 | |
| | | | | | | | | | | | | | |
| Shareholders' Equity | | | | | | | | | | | | | |
| Share capital | | 70,737,936 | | | 1,799 | | 2(c) | | (1,799 | ) | | 419,461,938 | |
| | | | | | | | 2(c) | | 233,324,002 | | | | |
| | | | | | | | 2(a) | | 115,400,000 | | | | |
| Contributed surplus | | (14,062,322 | ) | | 36,484 | | 2(c) | | (36,484 | ) | | (14,062,322 | ) |
| Deficit | | (21,576,844 | ) | | (4,170,486 | ) | 2(c) | | 4,170,486 | | | (23,976,844 | ) |
| | | | | | | | 2(b) | | (2,400,000 | ) | | | |
| Other comprehensive income | | (148,089 | ) | | 21,369 | | 2(c) | | (21,369 | ) | | (148,089 | ) |
| Equity attributable to commonshareholders of the Company | | 34,950,681 | | | (4,110,834 | ) | | | 350,434,836 | | | 381,274,683 | |
| Non-controlling interests | | 21,205,423 | | | (3,105 | ) | | | 3,105 | | | 21,205,423 | |
| Total Equity | | 56,156,104 | | | (4,113,939 | ) | | | 350,437,941 | | | 402,480,106 | |
| Total Liabilities and Equity | | 77,404,019 | | | 8,371,091 | | | | 340,806,008 | | | 426,581,118 | |
See accompanying notes to the pro forma condensed consolidated financial statements.
D-3
| PRO FORMA CONSOLIDATED STATEMENT OF LOSS AND COMPREHENSIVE LOSS |
| For the three months ended March 31, 2019 |
| Unaudited |
| | | | | | HO LIGEN | | | | | | | | |
| | | THE FLOWR | | | HOLDINGS | | | | PRO FORMA | | | PRO FORMA | |
| | | CORPORATION | | | LIMITED | | | | ADJUSTMENTS | | | CONSOLIDATED | |
| | | | | | (Note 1(d)) | | | | | | | | |
| | | $ | | | $ | | Note | | $ | | | $ | |
| | | | | | | | | | | | | | |
| Revenue | | 1,625,694 | | | 44,285 | | | | - | | | 1,669,979 | |
| Cost of sales | | 1,512,114 | | | 14,729 | | | | - | | | 1,526,843 | |
| Gross profit (loss) before fairvalue adjustments | | 113,580 | | | 29,556 | | | | - | | | 143,136 | |
| Fair value adjustments on inventory sold | | (42,237 | ) | | - | | | | - | | | (42,237 | ) |
| Unrealized (gains) losses on fair value of biological assets | | (206,180 | ) | | - | | | | - | | | (206,180 | ) |
| | | 361,997 | | | 29,556 | | | | - | | | 391,553 | |
| Selling and marketing | | 25,843 | | | - | | | | | | | 25,843 | |
| General and administrative | | 3,675,339 | | | 1,179,897 | | | | | | | 4,855,236 | |
| Research and development | | 59 | | | 4,347 | | | | | | | 4,406 | |
| Share-based compensation | | 2,103,109 | | | - | | | | | | | 2,103,109 | |
| Design and construction income | | (249,875 | ) | | - | | | | | | | (249,875 | ) |
| Depreciation and amortization | | 260,901 | | | 145,827 | | | | | | | 406,728 | |
| Finance costs | | 98,634 | | | - | | | | | | | 98,634 | |
| Other expense (income) | | 297,656 | | | 428,521 | | 2 (d) | | (253,895 | ) | | 472,282 | |
| Loss before income taxes | | (5,849,669 | ) | | (1,729,036 | ) | | | 253,895 | | | (7,324,810 | ) |
| Income taxes | | - | | | - | | | | | | | - | |
| Net loss | | (5,849,669 | ) | | (1,729,036 | ) | | | 253,895 | | | (7,324,810 | ) |
| | | | | | | | | | | | | | |
| Item that will be reclassified to profit or loss: | | | | | | | | | | | | | |
| Unrealized loss on retranslation of subsidiary | | - | | | (30,539 | ) | | | | | | (30,539 | ) |
| Comprehensive loss | | (5,849,669 | ) | | (1,759,575 | ) | | | 253,895 | | | (7,355,349 | ) |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| Net loss attributable to: | | | | | | | | | | | | | |
| Common shareholders of the Company | | (5,044,280 | ) | | (1,727,594 | ) | | | 253,895 | | | (6,517,979 | ) |
| Non-controlling interests | | (805,389 | ) | | (1,441 | ) | | | - | | | (806,830 | ) |
| Net loss | | (5,849,669 | ) | | (1,729,035 | ) | | | 253,895 | | | (7,324,809 | ) |
| | | | | | | | | | | | | | |
| Comprehensive loss attributable to: | | | | | | | | | | | | | |
| Common shareholders of the Company | | (5,044,280 | ) | | (1,758,107 | ) | | | 253,895 | | | (6,548,492 | ) |
| Non-controlling interests | | (805,389 | ) | | (1,467 | ) | | | - | | | (806,856 | ) |
| Comprehensive loss | | (5,849,669 | ) | | (1,759,574 | ) | | | 253,895 | | | (7,355,348 | ) |
See accompanying notes to the pro forma condensed consolidated financial statements.
D-4
| PRO FORMA CONSOLIDATED STATEMENT OF LOSS AND COMPREHENSIVE LOSS |
| For the year ended December 31, 2018 |
| Unaudited |
| | | | | | HO LIGEN | | | | | | | | |
| | | THE FLOWR | | | HOLDINGS | | | | PRO FORMA | | | PRO FORMA | |
| | | CORPORATION | | | LIMITED | | | | ADJUSTMENTS | | | CONSOLIDATED | |
| | | | | | (Note 1(c)) | | | | | | | | |
| | | $ | | | $ | | Note | | $ | | | $ | |
| | | | | | | | | | | | | | |
| Revenue | | 2,870,408 | | | 50,032 | | | | - | | | 2,920,440 | |
| Cost of sales | | 3,260,799 | | | 16,674 | | | | - | | | 3,277,473 | |
| Gross profit (loss) before fairvalue adjustments | | (390,391 | ) | | 33,358 | | | | - | | | (357,033 | ) |
| Fair value adjustments on inventory sold | | (499,793 | ) | | - | | | | - | | | (499,793 | ) |
| Unrealized (gains) losses on fair value of biological assets | | 420,410 | | | - | | | | - | | | 420,410 | |
| | | (311,008 | ) | | 33,358 | | | | - | | | (277,650 | ) |
| Selling and marketing | | 1,266,320 | | | - | | | | | | | 1,266,320 | |
| General and administrative | | 7,265,082 | | | 1,427,150 | | | | | | | 8,692,232 | |
| Research and development | | 321,824 | | | 603,040 | | | | | | | 924,864 | |
| Share-based compensation | | 7,208,273 | | | - | | | | | | | 7,208,273 | |
| Listing expense | | 1,802,830 | | | - | | | | | | | 1,802,830 | |
| Design and construction income | | (172,135 | ) | | - | | | | | | | (172,135 | ) |
| Depreciation and amortization | | 174,446 | | | 21,169 | | | | | | | 195,615 | |
| Other expense (income) | | (270,460 | ) | | 33,041 | | 2 (d) | | (159,384 | ) | | 2,003,197 | |
| | | | | | | | 2 (b) | | 2,400,000 | | | | |
| Loss before income taxes | | (17,907,188 | ) | | (2,051,042 | ) | | | (2,240,616 | ) | | (22,198,846 | ) |
| Income taxes | | - | | | - | | | | | | | - | |
| Net loss | | (17,907,188 | ) | | (2,051,042 | ) | | | (2,240,616 | ) | | (22,198,846 | ) |
| | | | | | | | | | | | | | |
| Item that will be reclassified to profit or loss: | | | | | | | | | | | | | |
| Unrealized gain on retranslation of subsidiary | | - | | | 40,365 | | | | - | | | 40,365 | |
| Comprehensive loss | | (17,907,188 | ) | | (2,010,677 | ) | | | (2,240,616 | ) | | (22,158,481 | ) |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| Net loss attributable to: | | | | | | | | | | | | | |
| Common shareholders of the Company | | (15,401,298 | ) | | (2,049,333 | ) | | | (2,240,616 | ) | | (19,691,247 | ) |
| Non-controlling interests | | (2,505,890 | ) | | (1,710 | ) | | | - | | | (2,507,600 | ) |
| Net loss | | (17,907,188 | ) | | (2,051,043 | ) | | | (2,240,616 | ) | | (22,198,847 | ) |
| | | | | | | | | | | | | | |
| Comprehensive loss attributable to: | | | | | | | | | | | | | |
| Common shareholders of the Company | | (15,401,298 | ) | | (2,009,002 | ) | | | (2,240,616 | ) | | (19,650,916 | ) |
| Non-controlling interests | | (2,505,890 | ) | | (1,675 | ) | | | - | | | (2,507,565 | ) |
| Comprehensive loss | | (17,907,188 | ) | | (2,010,677 | ) | | | (2,240,616 | ) | | (22,158,481 | ) |
See accompanying notes to the pro forma condensed consolidated financial statements.
D-5
1. Basis of presentation:
The Flowr Corporation (the "Company" or "Flowr"), is a publicly traded, cannabis cultivation company that was incorporated under the Business Corporations Act (Alberta) (“ABCA”) on June 1, 2016. On September 25, 2018, the Company continued from the ABCA to the Business Corporations Act (Ontario) as part of the completion of its qualifying transaction. The Head Office of the Company is located at 461 King Street West, Suite 200, Toronto, Ontario, M5V 1K4. The principal activity of the Company is the production and sale of premium cannabis in Canada, with a focus on the natural science of cannabis. In December 2017, through its subsidiary The Flowr Group (Okanagan) Inc. (“Flowr Okanagan”), formerly Cannatech Plant Systems Inc., the Company received its license from Health Canada to operate as a licensed producer, under the provisions of Access to Cannabis for Medical Purposes Regulations. On August 10, 2018, Flowr Okanagan received its sales license from Health Canada, allowing the Company to sell to the Canadian medical market and to the adult-use recreational (“recreational”) market post legalization on October 17, 2018. The Company’s common shares are listed on the TSX Venture Exchange (the “Exchange”), under the trading symbol “FLWR”. These unaudited pro forma condensed consolidated financial statements ("pro forma financial statements") have been prepared for illustrative purposes in compliance with National Instrument 51-102 by the Company for inclusion in the short form prospectus (the "Prospectus"), dated June 24, 2019, relating to the offering of [17,217,631] common shares of the Company (the “Offered Shares”) at a price of $[7.26] per Offered Share (the “Offering Price”). On June 24, 2019 the Company entered into a share purchase agreement, which was amended on July 10, 2019 to extend the outside date to July 31, 2019 (the “Holigen Purchase Agreement”) pursuant to which the Company has agreed to acquire (the “Acquisition”) all of the outstanding shares in the capital of Holigen Holdings Limited and its subsidiaries, RPK Biopharma Unipessoal Lda., GreyCan Pty Ltd. and TCann Pty Ltd. (collectively referred to as "Holigen") not already owned by the Company (the “Purchased Shares”). The pro forma financial statements give effect to the Acquisition, which is expected to close in the third quarter of 2019.
The accounting policies used in the preparation of the unaudited pro forma condensed consolidated statement of financial position as at March 31, 2019, and the unaudited pro forma condensed consolidated statements of loss and comprehensive loss for the three-month period ended March 31, 2019 and for the year ended December 31, 2018, include all the adjustments necessary for the fair presentation of the transactions in note 2 in accordance with the recognition and measurement principles of International Financial Reporting Standards ("IFRS") as issued by the International Accounting Standards Board ("IASB") and incorporate the significant accounting policies expected to be used to prepare the Company's consolidated financial statements.
The accounting policies used in the preparation of these pro forma financial statements are consistent with those described in the audited consolidated financial statements for the year ended December 31, 2018 of the Company.
Management of the Company has conducted a review of Holigen's accounting policies and has identified no material differences in the significant accounting policies adopted by Holigen. In addition, management has reclassified certain items related to the financial statements of Holigen within the unaudited pro forma condensed consolidated statement of financial position and unaudited pro forma condensed consolidated statements of earnings and comprehensive income in order to conform with the presentation adopted by the Company.
D-6
These financial statements have been prepared from:
| (a) | the audited statement of consolidated comprehensive loss for the year ended December 31, 2018 of the Company; |
| | |
| (b) | the unaudited condensed interim consolidated statement of financial position as at March 31, 2019 and the unaudited condensed interim consolidated statement of comprehensive loss for the three-month period ended March 31, 2019 of the Company; |
| | |
| (c) | the audited combined and consolidated statement of comprehensive loss for the year ended December 31, 2018 of Holigen (translated into Canadian dollars using the average exchange rate for the year ended December 31, 2018 of $1.5305) prepared in accordance with IFRS as follows: |
HOLIGEN HOLDINGS LIMITED
STATEMENT OF LOSS AND COMPREHENSIVE LOSS
TRANSLATED TO $CAD
For the year ended December 31, 2018
| | | | | | Exchange | | | Canadian | |
| | | Euros | | | rate | | | dollars | |
| | | € | | | | | | $ | |
| | | | | | | | | | |
| | | | | | | | | | |
| Revenue | | 32,689 | | | 1.5305 | | | 50,032 | |
| Cost of sales | | 10,894 | | | 1.5305 | | | 16,674 | |
| Gross profit (loss) | | 21,795 | | | | | | 33,358 | |
| General and administrative | | 932,449 | | | 1.5305 | | | 1,427,150 | |
| Depreciation and amortization | | 13,831 | | | 1.5305 | | | 21,169 | |
| Research and development | | 394,005 | | | 1.5305 | | | 603,040 | |
| Other expense (income) | | 21,588 | | | 1.5305 | | | 33,041 | |
| Loss before income taxes | | (1,340,078 | ) | | | | | (2,051,042 | ) |
| Income taxes | | - | | | 1.5305 | | | - | |
| Net loss | | (1,340,078 | ) | | | | | (2,051,042 | ) |
| | | | | | | | | | |
| Item that will be reclassified to profit or loss: | | | | | | | | | |
| Unrealized gain on retranslation of subsidiary | | 26,373 | | | 1.5305 | | | 40,365 | |
| Comprehensive loss | | (1,313,705 | ) | | | | | (2,010,677 | ) |
| | | | | | | | | | |
| Net loss attributable to: | | | | | | | | | |
| Common shareholders of the Company | | (1,338,961 | ) | | 1.5305 | | | (2,049,333 | ) |
| Non-controlling interests | | (1,117 | ) | | 1.5305 | | | (1,710 | ) |
| Net loss | | (1,340,078 | ) | | | | | (2,051,043 | ) |
| | | | | | | | | | |
| Comprehensive loss attributable to: | | | | | | | | | |
| Common shareholders of the Company | | (1,312,610 | ) | | 1.5305 | | | (2,009,002 | ) |
| Non-controlling interests | | (1,095 | ) | | 1.5305 | | | (1,675 | ) |
| Comprehensive loss | | (1,313,705 | ) | | | | | (2,010,677 | ) |
D-7
| (d) | the unaudited combined and consolidated statement of financial position as at March 31, 2019 (translated into Canadian dollars using the exchange rate as at March 31, 2019 of $1.4994) and the unaudited combined and consolidated statement of operations for the three-month period ended March 31, 2019 (translated into Canadian dollars using the average exchange rate for the three month period ended March 31, 2019 of $1.5093) of Holigen prepared in accordance with IFRS as follows: |
HOLIGEN HOLDINGS LIMITED
STATEMENT OF LOSS AND COMPREHENSIVE LOSS
TRANSLATED TO $CAD
For the three months ended March 31, 2019
| | | | | | Exchange | | | Canadian | |
| | | Euros | | | rate | | | dollars | |
| | | € | | | | | | $ | |
| | | | | | | | | | |
| | | | | | | | | | |
| Revenue | | 29,342 | | | 1.5093 | | | 44,285 | |
| Cost of sales | | 9,759 | | | 1.5093 | | | 14,729 | |
| Gross profit (loss) | | 19,583 | | | | | | 29,556 | |
| General and administrative | | 781,759 | | | 1.5093 | | | 1,179,897 | |
| Depreciation and amortization | | 96,620 | | | 1.5093 | | | 145,827 | |
| Research and development | | 2,880 | | | 1.5093 | | | 4,347 | |
| Other expense (income) | | 283,923 | | | 1.5093 | | | 428,521 | |
| Loss before income taxes | | (1,145,599 | ) | | | | | (1,729,036 | ) |
| Income taxes | | - | | | 1.5093 | | | - | |
| Net loss | | (1,145,599 | ) | | | | | (1,729,036 | ) |
| | | | | | | | | | |
| Item that will be reclassified to profit or loss: | | | | | | | | | |
| Unrealized loss on retranslation of subsidiary | | (20,234 | ) | | 1.5093 | | | (30,539 | ) |
| Comprehensive loss | | (1,165,833 | ) | | | | | (1,759,575 | ) |
| | | | | | | | | | |
| | | | | | | | | | |
| Net loss attributable to: | | | | | | | | | |
| Common shareholders of the Company | | (1,144,644 | ) | | 1.5093 | | | (1,727,594 | ) |
| Non-controlling interests | | (955 | ) | | 1.5093 | | | (1,441 | ) |
| Net loss | | (1,145,599 | ) | | | | | (1,729,035 | ) |
| | | | | | | | | | |
| Comprehensive loss attributable to: | | | | | | | | | |
| Common shareholders of the Company | | (1,164,861 | ) | | 1.5093 | | | (1,758,107 | ) |
| Non-controlling interests | | (972 | ) | | 1.5093 | | | (1,467 | ) |
| Comprehensive loss | | (1,165,833 | ) | | | | | (1,759,574 | ) |
D-8
HOLIGEN HOLDINGS LIMITED
STATEMENT OF FINANCIAL POSITION
TRANSLATED TO $CAD
As at March 31, 2019
| | | | | | Exchange | | | Canadian | |
| | | Euros | | | rate | | | dollars | |
| | | € | | | | | | $ | |
| | | | | | | | | | |
| Assets | | | | | | | | | |
| Current | | | | | | | | | |
| Cash and cash equivalents | | 1,283,768 | | | 1.4994 | | | 1,924,823 | |
| Prepaids and other current assets | | 57,837 | | | 1.4994 | | | 86,718 | |
| Amounts receivable | | 88,195 | | | 1.4994 | | | 132,236 | |
| Inventory | | 3,420 | | | 1.4994 | | | 5,128 | |
| Other receivable | | - | | | 1.4994 | | | - | |
| Total Current Assets | | 1,433,220 | | | | | | 2,148,905 | |
| Non-current assets | | | | | | | | | |
| Property, plant & equipment | | 4,125,303 | | | 1.4994 | | | 6,185,290 | |
| Other long-term assets | | 24,608 | | | 1.4994 | | | 36,896 | |
| | | 4,149,911 | | | | | | 6,222,186 | |
| Total Assets | | 5,583,131 | | | | | | 8,371,091 | |
| | | | | | | | | | |
| Liabilities | | | | | | | | | |
| Current | | | | | | | | | |
| Accounts payable and accrued liabilities | | 1,444,515 | | | 1.4994 | | | 2,165,839 | |
| Due to related parties - loan payable | | 432,590 | | | 1.4994 | | | 648,606 | |
| Lease liability | | 228,808 | | | 1.4994 | | | 343,064 | |
| Loan payable | | 3,342,089 | | | 1.4994 | | | 5,010,975 | |
| Derivative Liability | | 942,531 | | | 1.4994 | | | 1,413,188 | |
| Total Current Liabilities | | 6,390,533 | | | | | | 9,581,672 | |
| Non-current liabilities | | | | | | | | | |
| Lease liability | | 880,988 | | | 1.4994 | | | 1,320,913 | |
| Loan payable | | 1,055,418 | | | 1.4994 | | | 1,582,445 | |
| Total Non-current Liabilities | | 1,936,406 | | | | | | 2,903,358 | |
| Total Liabilities | | 8,326,939 | | | | | | 12,485,030 | |
| | | | | | | | | | |
| Shareholders' Equity | | | | | | | | | |
| Share capital | | 1,200 | | | 1.4994 | | | 1,799 | |
| Contributed suprlus | | 24,333 | | | 1.4994 | | | 36,484 | |
| Deficit | | (2,781,522 | ) | | 1.4994 | | | (4,170,486 | ) |
| Other comprehensive income | | 14,252 | | | 1.4994 | | | 21,369 | |
| Equity attributable to commonshareholders of the Company | | (2,741,737 | ) | | | | | (4,110,834 | ) |
| | | | | | | | | | |
| Non-controlling interests | | (2,071 | ) | | 1.4994 | | | (3,105 | ) |
| Total Equity | | (2,743,808 | ) | | | | | (4,113,939 | ) |
| Total Liabilities and Equity | | 5,583,131 | | | | | | 8,371,091 | |
D-9
The unaudited pro forma condensed consolidated statement of financial position gives effect to the transactions in note 2 as if they had occurred on March 31, 2019. The unaudited pro forma condensed consolidated statements of comprehensive loss give the effect to the transactions in note 2 as if they had occurred on January 1, 2018.
The pro forma financial statements may not be indicative of the financial position that would have prevailed and operating results that would have been obtained if the transactions in note 2 had taken place on the dates indicated.
2. Pro forma assumptions and financial statement adjustments:
The pro forma adjustments to the unaudited pro forma consolidated statement of financial position and unaudited pro forma consolidated statements of earnings and comprehensive income have been prepared to account for the issuance of common shares and the acquisition of Holigen as described below.
(a) Issuance of common shares:
The pro forma consolidated financial statements assume that on closing (the "Closing"), the Company will raise gross proceeds of $125,000,000 (excluding any over-allotment option) (the "Offering") through the issuance of [17,217,631] common shares at $[7.26] per common share. Costs relating to the Offering, including underwriters' fees, are estimated to be $9,600,000 and are recorded as a reduction of proceeds from the Offering.
(b) Acquisition of Holigen:
The pro forma financial statements have been adjusted to reflect the Company's acquisition of 100% of the common shares of Holigen pursuant to the terms and conditions set forth in the share purchase agreement dated June 24, 2019, as amended July 10, 2019. The fair value of the consideration is comprised of cash paid on closing of $8,246,447 (Euro €5,500,000), of which $1,844,315 (Euro €1,230,073) will be used to settle certain accounts payable and amounts due to related parties of Holigen and additional consideration payable in common shares of the Company (the "Additional Consideration"). Costs of $2,400,000 to complete the acquisition of Holigen have been expensed immediately in the unaudited pro forma condensed interim consolidated statement of earnings and comprehensive income for the year ended December 31, 2018.
D-10
(c) Assets acquired and liabilities assumed in the Holigen acquisition:
The following is a preliminary fair value estimate of the assets acquired and the liabilities assumed by the Company in connection with the acquisition of Holigen. The acquisition has been accounted for using the acquisition method of accounting in accordance with IFRS 3 - Business Combinations. The Company is in the process of conducting a detailed valuation of all assets acquired and liabilities assumed as of the acquisition date. As a result, the fair value of the assets acquired and liabilities assumed may differ materially from those presented below:
PURCHASE PRICE ALLOCATION
As at March 31, 2019
| | | | | | | | | Book Value | | | | | | Fair Value | |
| | | | | | Exchange | | | Canadian | | | | | | Canadian | |
| | | Euros | | | rate | | | dollars | | | Adjustments | | | dollars | |
| | | € | | | | | | $ | | | $ | | | $ | |
| | | | | | | | | | | | | | | | |
| Consideration given | | | | | | | | | | | | | | | |
| Cash - Euro [1] | | 5,500,000 | | | 1.4994 | | | 8,246,447 | | | - | | | 8,246,447 | |
| Cash - Canadian Dollar | | | | | | | | 6,000,000 | | | | | | 6,000,000 | |
| Common shares - 32,632,545 @ 7.15 | | | | | | | | | | | 233,324,002 | | | 233,324,002 | |
| | | | | | | | | | | | | | | 247,570,449 | |
| | | | | | | | | | | | | | | | |
| Identified net assets acquired | | | | | | | | | | | | | | | |
| Cash and cash equivalents | | 1,283,768 | | | 1.4994 | | | 1,924,823 | | | - | | | 1,924,823 | |
| Amounts receivable | | 88,195 | | | 1.4994 | | | 132,236 | | | - | | | 132,236 | |
| Prepaids and other current assets | | 57,837 | | | 1.4994 | | | 86,718 | | | - | | | 86,718 | |
| Inventory | | 3,420 | | | 1.4994 | | | 5,128 | | | - | | | 5,128 | |
| Property, plant & equipment | | 4,125,303 | | | 1.4994 | | | 6,185,290 | | | - | | | 6,185,290 | |
| Security deposit | | 24,608 | | | 1.4994 | | | 36,896 | | | - | | | 36,896 | |
| Accounts payable and accrued liabilities | | (1,444,515 | ) | | 1.4994 | | | (2,165,839 | ) | | 1,195,709 | | | (970,130 | ) |
| Lease liability - current | | (228,808 | ) | | 1.4994 | | | (343,064 | ) | | - | | | (343,064 | ) |
| Loans payable - current | | (3,342,089 | ) | | 1.4994 | | | (5,010,975 | ) | | 4,963,540 | | | (47,435 | ) |
| Derivative Liability | | (942,531 | ) | | 1.4994 | | | (1,413,188 | ) | | 1,413,188 | | | - | |
| Due to related parties - loan payable | | (432,590 | ) | | 1.4994 | | | (648,606 | ) | | 648,606 | | | - | |
| Lease liability - long-term | | (880,988 | ) | | 1.4994 | | | (1,320,913 | ) | | - | | | (1,320,913 | ) |
| Loan payables - long-term | | (1,055,418 | ) | | 1.4994 | | | (1,582,445 | ) | | 1,410,890 | | | (171,555 | ) |
| Intangible assets | | - | | | 1.4994 | | | - | | | 42,482,006 | | | 42,482,006 | |
| Total identified net assets | | (2,743,808 | ) | | | | | (4,113,939 | ) | | 52,113,939 | | | 48,000,000 | |
| Unallocated proceeds - goodwill | | | | | | | | | | | | | | 199,570,449 | |
| | | | | | | | | | | | | | | 247,570,449 | |
[1] A part of the consideration will be allocated to repay amounts due to related parties and certain accounts payable balances.
| | | | | | | | | Canadian | |
| | | Euros | | | Exchange | | | dollars | |
| | | € | | | rate | | | $ | |
| Amount to shareholders | | 4,269,927 | | | 1.4994 | | | 6,402,132 | |
| To settle related party debt and accounts payable | | 1,230,073 | | | 1.4994 | | | 1,844,315 | |
| Total consideration | | 5,500,000 | | | | | | 8,246,447 | |
D-11
(d) Elimination of convertible loan:
The Company advanced a $6,000,000 convertible loan to Holigen during the year ended December 31, 2018. As a result of consolidation, the convertible loan and associated impact on the statement of loss and comprehensive loss is eliminated. The elimination of the convertible loan asset of the Company and convertible loan liability of Holigen on the statement of financial position has been reflected through the pro forma adjustment in note 2(b). The elimination of the associated impact on the statement of loss and comprehensive loss is as follows:
| | | | | | Exchange | | | Canadian | |
| | | Euros | | | rate | | | dollars | |
| | | € | | | | | | $ | |
| | | | | | | | | | |
| | | | | | | | | | |
| March 31, 2019: | | | | | | | | | |
| Fair value change in derivative liability | | (90,307 | ) | | 1.5093 | | | (136,299 | ) |
| Accretion on convertible loan | | 258,529 | | | 1.5093 | | | 390,194 | |
| | | 168,222 | | | | | | 253,895 | |
| | | | | | | | | | |
| December 31, 2018 | | | | | | | | | |
| Fair value change in derivative liability | | 9,864 | | | 1.5305 | | | 15,097 | |
| Accretion on convertible loan | | 94,272 | | | 1.5305 | | | 144,287 | |
| | | 104,136 | | | | | | 159,384 | |
3. Pro forma basic and diluted loss and comprehensive loss per share:
| | | March 31, | | | December 31, | |
| | | 2019 | | | 2018 | |
| | | | | | | |
| | | | | | | |
| Basic and diluted weighted average number of common shares outstanding | | 86,799,052 | | | 71,335,830 | |
| | | | | | | |
| After giving the effect to the transactions in note 2 as if they had occurred on January 1, 2018: | | | | | | |
| Issuance of common shares (note 2(a)) | | [17,217,631] | | | [17,217,631] | |
| Acquisition of Holigen (note 2(b)) | | 32,632,545 | | | 32,632,545 | |
| Adjusted Basic and diluted weighted average number of common shares outstanding | | 136,649,228 | | | 121,186,006 | |
| | | | | | | |
| Pro forma net loss attributable to common shareholders of the Company | | (6,517,979 | ) | | (19,691,247 | ) |
| Pro forma net loss basic and diluted loss per share | | (0.05 | ) | | (0.16 | ) |
| | | | | | | |
| Pro forma net comprehensive loss attributable to common shareholders of the Company | | (6,548,492 | ) | | (19,650,916 | ) |
| Pro forma net loss basic and diluted comprehensive loss per share | | (0.05 | ) | | (0.16 | ) |
D-12

PART II
INFORMATION NOT REQUIRED TO BE DELIVERED
TO OFFEREES OR PURCHASERS
Indemnification of Directors and Officers
Under the Business Corporations Act (Ontario), the Registrant may indemnify a director or officer of the Registrant, a former director or officer of the Registrant or another individual who acts or acted at the Registrant’s request as a director or officer, or an individual acting in a similar capacity, of another entity (each of the foregoing, an "Individual"), against all costs, charges and expenses, including an amount paid to settle an action or satisfy a judgment, reasonably incurred by the Individual in respect of any civil, criminal, administrative, investigative or other proceeding in which the Individual is involved because of that association with the Registrant or other entity, on the condition that:
(i) the Individual acted honestly and in good faith with a view to the best interests of the Registrant or, as the case may be, to the best interests of the other entity for which the Individual acted as a director or officer or in a similar capacity at the Registrant’s request; and
(ii) if the matter is a criminal or administrative action or proceeding that is enforced by a monetary penalty, the Registrant shall not indemnify the Individual unless the Individual had reasonable grounds for believing that his or her conduct was lawful.
The Registrant may advance money to a director, officer, or other Individual in relation to the foregoing matters, but the Individual shall repay the money if the Individual does not fulfill the conditions set out in (i) and (ii) above.
Further, the Registrant may, with the approval of a court, indemnify an Individual in respect of an action by or on behalf of the Registrant or other entity, or advance moneys as set out above, to obtain a judgment in its favor, to which the Individual is made a party because of the Individual’s association with the Registrant or other entity as a director or officer, a former director or officer, an Individual who acts or acted at the Registrant’s request as a director or officer, or an Individual acting in a similar capacity, against all costs, charges and expenses reasonably incurred by the Individual in connection with such action, if the Individual fulfills the conditions in (i) and (ii) above. Such Individuals are entitled to indemnification from the Registrant in respect of all costs, charges and expenses reasonably incurred by the Individual in connection with the defense of any civil, criminal, administrative, investigative or other proceeding to which the Individual is subject because of the Individual’s association with the Registrant or other entity as described above, provided the Individual seeking an indemnity: (A) was not judged by a court or other competent authority to have committed any fault or omitted to do anything that the Individual ought to have done; and (B) fulfills the conditions in (i) and (ii) above.
The by-laws of the Registrant provide that, subject to the Business Corporations Act (Ontario), the Registrant shall indemnify a director or officer of the Registrant, a former director or officer of the Registrant or another Individual who acts or acted at the Registrant’s request as a director or officer of a body corporate of which the Registrant is or was a shareholder or creditor, and the heirs and legal representatives of such person, against all costs, charges and expenses, including an amount paid to settle an action or satisfy a judgment, reasonably incurred by the Individual in respect of any civil, criminal or administrative, or other proceeding in which the Individual is made a party by reason of being or having been a director or officer of the Registrant or body corporate, if: (i) the Individual acted honestly and in good faith with a view to the best interests of the Registrant and (ii) in the case of a criminal or administrative action or proceeding that is enforced by a monetary penalty, the person had reasonable grounds for believing that the Individual’s conduct was lawful.
Directors and officers of the Registrant have entered into indemnity agreements with the Registrant whereby the Registrant has agreed to indemnify and save harmless the directors and officers of the Registrant (the "Indemnified Party") in respect of (i) any civil, criminal, administrative, investigative, or other proceeding to which the director or officer of the Registrant was involved by reason of being or having been a director or officer of the Registrant; and (ii) all expenses including all costs, charges, damages, awards, settlements, liabilities, interest, judgments, fines, penalties, statutory obligations, professional fees and retainers and other expenses of whatever nature or kind, provided that any such costs, charges, professional fees and other expenses are reasonable.
The form of indemnity agreement provides that the rights therein will, subject to applicable law, apply without reduction to an Indemnified Party provided that, among other things: (a) the Indemnified Party acted honestly and in good faith with a view to the best interests of the Registrant; (b) in the case of a criminal or administrative action or proceeding that is enforced by a monetary penalty, the Indemnified Party had reasonable grounds for believing that his or her conduct was lawful; and (c) in the case of claims by the Registrant or any of subsidiary of the Registrant, for the forfeiture or recovery by the Registrant or any of subsidiary of the Registrant, of bonuses or other compensation received by the Indemnified Party from the Registrant or any of subsidiary of the Registrant, as long as the Indemnified Party did not violate applicable laws related to forfeiture and recovery by the Registrant of bonuses and other compensation and there are no grounds upon which the Registrant is entitled to effect forfeiture or recovery of bonuses or other compensation received by the Indemnified Party.
The Registrant maintains directors’ and officers’ liability insurance which insures directors and officers for losses as a result of claims against the directors and officers of the Registrant in their capacity as directors and officers, subject to certain limitations and exclusions, and also reimburses the Registrant for payments made pursuant to the indemnity provisions under the by-laws of the Registrant and the Business Corporations Act (Ontario).
* * *
Insofar as indemnification for liabilities arising under the Securities Act of 1933, as amended, may be permitted to directors, officers or persons controlling the Registrant pursuant to the foregoing provisions, the Registrant has been informed that in the opinion of the U.S. Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933, as amended, and is therefore unenforceable.
The exhibits listed in the exhibit index, appearing elsewhere in this Registration Statement, have been filed as part of this Registration Statement.
PART III
UNDERTAKING AND CONSENT TO SERVICE OF PROCESS
Item 1. Undertaking
The Registrant undertakes to make available, in person or by telephone, representatives to respond to inquiries made by the Commission staff, and to furnish promptly, when requested to do so by the Commission staff, information relating to the securities registered pursuant to Form F-10 or to transactions in said securities.
Item 2. Consent to Service of Process
A written Appointment of Agent for Service of Process and Undertaking on Form F-X for the Registrant and its agent for service of process was filed concurrently with the initial filing of this Registration Statement on F-10..
Any change to the name or address of the agent for service of process of the Registrant shall be communicated promptly to the Commission by amendment to Form F-X referencing the file number of this Registration Statement on Form F-10.
EXHIBIT INDEX
| | |
Exhibit
Number | | Description |
3.1* | | Form of Underwriting Agreement |
| | | |
4.1 | | Annual Information Form of the Registrant dated April 3, 2019 for the fiscal year ended December 31, 2018 (incorporated by reference from Exhibit No. 99.65 to the Registrant’s Form 40-F/A, filed with the Commission on May 28, 2019) |
| | | |
4.2 | | Management Information Circular dated November 26, 2018 in connection with the December 28, 2018 Special Meeting of Shareholders (incorporated by reference from Exhibit No. 99.42 to the Registrant’s Form 40-F, filed with the Commission on February 5, 2019) |
| | | |
4.3 | | Audited Consolidated Financial Statements of the Registrant for the fiscal year ended December 31, 2018 and 2017, together with the independent auditors’ report thereon and the notes thereto (incorporated by reference from Exhibit No. 99.69 to the Registrant’s Form 40-F/A, filed with the Commission on May 28, 2019) |
| | |
4.4 | | Management’s Discussion and Analysis of the Financial Condition and Results of Operations of the Registrant for the fiscal year ended December 31, 2018 (incorporated by reference from Exhibit No. 99.66 to the Registrant’s Form 40-F/A, filed with the Commission on May 28, 2019) |
| | |
4.5 | | Unaudited Condensed Interim Consolidated Financial Statements of the Registrant for the three months ended March 31, 2019 and 2018, together with the notes thereto (incorporated by reference from Exhibit No. 99.81 to the Registrant’s Form 40-F/A, filed with the Commission on May 28, 2019) |
| | |
4.6 | | Management’s Discussion and Analysis of the Financial Condition and Results of Operations of the Registrant for the three months ended March 31, 2019 (incorporated by reference from Exhibit No. 99.78 to the Registrant’s Form 40-F/A, filed with the Commission on May 28, 2019) |
| | |
4.7 | | Material Change Report dated June 28, 2019 (incorporated by reference from Exhibit No. 99.92 to the Registrant’s Form 40-F/A, filed with the Commission on July 12, 2019) |
| | | |
| 4.8 | | Investor Presentation dated July 15, 2019 |
| | | |
| 4.9 | | Term Sheet dated July 15, 2019 |
| | | |
4.10 | | Material Change Report dated July 16, 2019 |
| | | |
5.1 | | Consent of MNP LLP and Sandra Alison Solecki |
| | |
5.2 | | Consent of RSM Canada LLP |
| | |
5.3** | | Consent of Fasken Martineau DuMoulin LLP |
| | |
5.4** | | Consent of Borden Ladner Gervais LLP |
| | |
6.1 | | Powers of Attorney (contained in the signature page of the Form F-10 Registration Statement filed with the Commission on June 25, 2019). |
* To be filed pursuant to Form 6-K.
**Previously filed.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form F-10 and has duly caused this Amendment No. 2 to the Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Toronto, Country of Canada on July 18, 2019.
| THE FLOWR CORPORATION |
| | | |
| By: | /s/ Alexander Dann |
| | Name: Alexander Dann |
| | Title: Chief Financial Officer |
Pursuant to the requirements of the Securities Act of 1933, this Amendment No. 2 to the Registration Statement has been signed below by the following persons in the capacities indicated and on the dates indicated.
| Signature | | Capacity | | Date |
| | | | | |
| * | | | | |
| Vinay Tolia | | Executive Director, Chief Executive Officer | | July 18, 2019 |
| | | | | |
| /s/ Alexander Dann | | | | |
| Alexander Dann | | Chief Financial Officer | | July 18, 2019 |
| | | | | |
| * | | | | |
| Steve Klein | | Chairman of the Board | | July 18, 2019 |
| * | | | | |
| Dr. Lyle Oberg | | Executive Director, Chief Policy & Medical Officer | | July 18, 2019 |
| | | | | |
| * | | | | |
| David Miller | | Director | | July 18, 2019 |
| | | | | |
| * | | | | |
| Karen Basian | | Lead Independent Director | | July 18, 2019 |
| | | | | |
| * | | | | |
| Don Duet | | Independent Director | | July 18, 2019 |
| | | | | |
| * | | | | |
| Dr. J. André de Barros Teixeira | | Independent Director | | July 18, 2019 |
| | | | | |
| * | | | | |
| Maurice Levesque | | Independent Director | | July 18, 2019 |
*By: | /s/ Alexander Dann |
| Name: Alexander Dann |
| Title: Attorney-in-fact |
AUTHORIZED REPRESENTATIVE
Pursuant to the requirements of Section 6(a) of the Securities Act of 1933, as amended, the undersigned has signed this Amendment No. 2 to the Registration Statement, in the capacity of the duly authorized representative of the Registrant in the United States, on July 18, 2019.
| | | /s/ Steve Klein |
| | | Name: Steve Klein |
| | | Title: Chairman of the Board |














