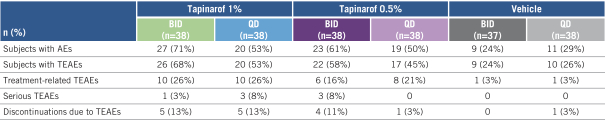
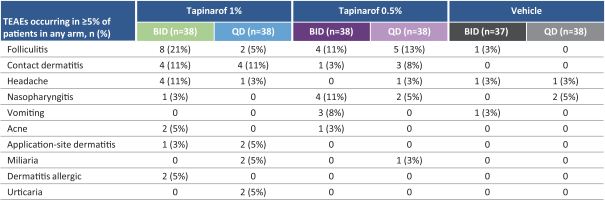
company that receives NDA approval will be eligible for the PTE which can be applied to one patent that is listed in the USFDA’s Orange Book. Accordingly, should we receive NDA approval for cerdulatinib before Portola, cerdulatinib composition of matter may be eligible for up to five years of PTE, which would bring expiration to 2034. If we receive a PWR from the FDA and complete pediatric studies according to the terms of the PWR, we will be awarded asix-month Pediatric Exclusivity which is added to all exclusivities listed in the Orange Book.
Similar provisions are available in Europe, Japan and certain other jurisdictions to extend the exclusivity of a patent that covers an approved drug. In Europe, we believe tapinarof, lotamilast andDMVT-503 will be eligible for 10 years of regulatory exclusivity from European Marketing Application, or EMA, approval. In Japan, we believe tapinarof, lotamilast andDMVT-503 will be eligible for eight years of regulatory exclusivity from a Japanese new drug application, orJ-NDA, approval.
We believe cerdulatinib will also receive such regulatory exclusivity in Europe and Japan should we receive approval before Portola. Additionally, the cerdulatinib composition of matter patent has issued in Europe and Japan and has a natural expiration date of 2029. We believe this composition of matter patent will be eligible for up to a five-year extension in both Europe and Japan.
Trademarks and Trade Secrets
As of March 31, 2019, our trademark portfolio contained more than 35 trademark registrations and applications for DERMAVANT, including four U.S. trademark registrations and three pending U.S. trademark applications.
Furthermore, we rely upon tradesecrets, know-how, continuing technological innovation and potentialin-licensing opportunities to develop and maintain our competitive position. We seek to protect our proprietary information, in part, using confidentiality and invention assignment agreements with our commercial partners, collaborators, employees and consultants. These agreements are designed to protect our proprietary information and, in the case of the invention assignment agreements, to grant us ownership of technologies that are developed through a relationship with an employee or a third party. These agreements may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our commercial partners, collaborators, employees and consultants use intellectual property owned by others in their work for us, disputes may arise as to the rights in related orresulting know-how and inventions.
Government Regulation
Government authorities in the United States at the federal, state and local level and in other countries regulate, among other things, the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, post-approval monitoring and reporting, marketing and export and import of drugs. Generally, before a new drug can be marketed, considerable data demonstrating its quality, safety and efficacy must be obtained, organized into a format specific for each regulatory authority, submitted for review and approved by the regulatory authority.
FDA Drug Approval Process
In the United States, pharmaceutical products are subject to extensive regulation by the FDA. The Federal Food, Drug, and Cosmetic Act, and other federal and state statutes and regulations, govern, among other things, the research, development, testing, quality control, manufacture, storage, recordkeeping, safety, effectiveness, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling, and import and export of pharmaceutical products. Failure to comply with applicable U.S. requirements may subject a company to a variety of administrative or judicial sanctions, such as FDA refusal to approve pending NDAs, warning or untitled letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties, and criminal prosecution.
We cannot market a drug product candidate in the United States until the drug has received FDA approval. The steps required before a drug may be marketed in the United States generally include the following:
| | ∎ | | completion of extensive nonclinical laboratory tests, animal studies, and formulation studies in accordance with the FDA’s GLP regulations; |
131