
Thinking differently about cognition CORPORATE Presentation June 2022 Exhibit 99.2

Safe harbor statement This presentation is for informational purposes only and is not an offer to sell nor a solicitation of an offer to buy any securities of Cyclerion Therapeutics, Inc. (the “Company”). This presentation includes or may include certain information obtained from trade and statistical services or sources, third party publications and other sources. The Company has not independently verified such information and there can be no assurance as to its accuracy. Certain matters discussed in this presentation are “forward-looking statements”. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “positive,” “should” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. In particular, the Company’s statements regarding trends and potential future results are examples of such forward-looking statements. The forward-looking statements include risks and uncertainties, including, but not limited to, the success, timing and cost of our ongoing or future clinical trials and anticipated clinical trials for our current product candidates, including statements regarding the timing of initiation and completion of the trials, futility analyses and receipt of interim results, which are not necessarily indicative of or supported by the final results of our ongoing or subsequent clinical trials; any results of clinical studies, including in particular single-arm open-label studies involving a number of patients that is not statistically significant such as described in this presentation, not necessarily being indicative of or supported by the final results of our ongoing or subsequent clinical trials; our ability to fund additional clinical trials to continue the advancement of our product candidates; the timing of and our ability to obtain and maintain U.S. Food and Drug Administration (“FDA”) or other regulatory authority approval of, or other action with respect to, our product candidates; the potential for the CY6463 clinical trial to provide a basis for approval for treatment of MELAS; the Company’s ability to successfully defend its intellectual property or obtain necessary licenses at a cost acceptable to the Company, if at all; the successful implementation of the Company’s research and development programs and collaborations; the success of the Company’s license agreements; the acceptance by the market of the Company’s product candidates, if approved; and other factors, including general economic conditions and regulatory developments, not within the Company’s control. The factors discussed herein could cause actual results and developments to be materially different from those expressed in or implied by such statements. The forward-looking statements are made only as of the date of this presentation and the Company undertakes no obligation to publicly update such forward-looking statements to reflect subsequent events or circumstance. Other important factors that could cause actual results to differ from those reflected in any forward-looking statements herein are described in the Company’s most recent Form 10-K as well as the Company’s subsequent filings with the Securities and Exchange Commission (the “SEC”). All of the Company’s development plans may be subject to adjustment depending on funding, recruitment rate, regulatory review, preclinical and clinical results, and other factors any of which could result in changes to the Company’s development plans and programs or delay the initiation, enrollment, completion, or reporting of clinical trials. In addition to the risks described above and in the Company’s filings with the SEC, other unknown or unpredictable factors could affect the Company’s results. No froward-looking statements can be guaranteed, and actual results may materially differ from such statements. The information in this presentation is provided only as of June 10, 2022, and the Company undertakes no obligation to update any forward-looking statements contained in this presentation on account of new information, future events, or otherwise, except as required by law.
Developing therapies to restore cognitive function Experienced team of scientific leaders, drug hunters, company builders, and CNS experts Developed and launched first-in-class therapies Raised billions in capital Built leading strategic partnerships Neuroinnovation engine enables faster, more precise drug development Advancing CY6463, potential breakthrough, first-in-class CNS therapy Improvement trends in MELAS patients across biomarkers of mitochondrial function, inflammation, cerebral blood flow and functional connectivity CIAS study results expected in Q3’22; ADv study actively enrolling Evaluating collaborative development opportunities Developing disease and mechanism specific translational biomarkers Continuously refining signal-to-noise Identifying most promising patient populations early

Neuroinnovation Engine
Efficiently developing drugs that matter Identify most promising patient populations early, increase signal-to-noise ratio Leverage deep understanding of brain biology, pathobiology of cognitive dysfunction and small molecule mechanisms Partner with academic and industry leaders to access leading-edge data and technologies Apply advanced analytics to robust multimodal nonclinical and clinical data sets to extract actionable insights
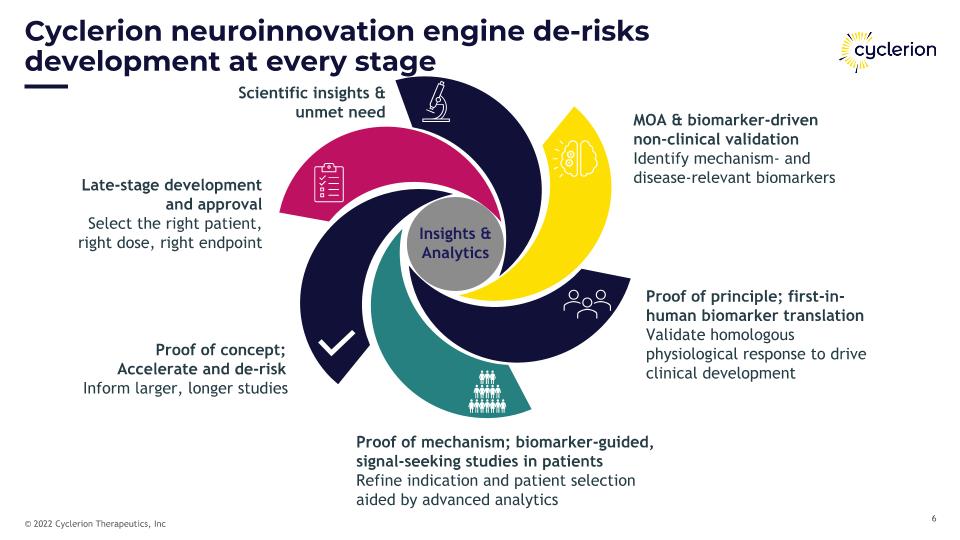
MOA & biomarker-driven non-clinical validation Identify mechanism- and disease-relevant biomarkers Proof of principle; first-in-human biomarker translation Validate homologous physiological response to drive clinical development Proof of mechanism; biomarker-guided, signal-seeking studies in patients Refine indication and patient selection aided by advanced analytics Proof of concept; Accelerate and de-risk Inform larger, longer studies Late-stage development and approval Select the right patient, right dose, right endpoint Scientific insights & unmet need Cyclerion neuroinnovation engine de-risks development at every stage Insights & Analytics

Deploying our NeuroInnovation ENGINE
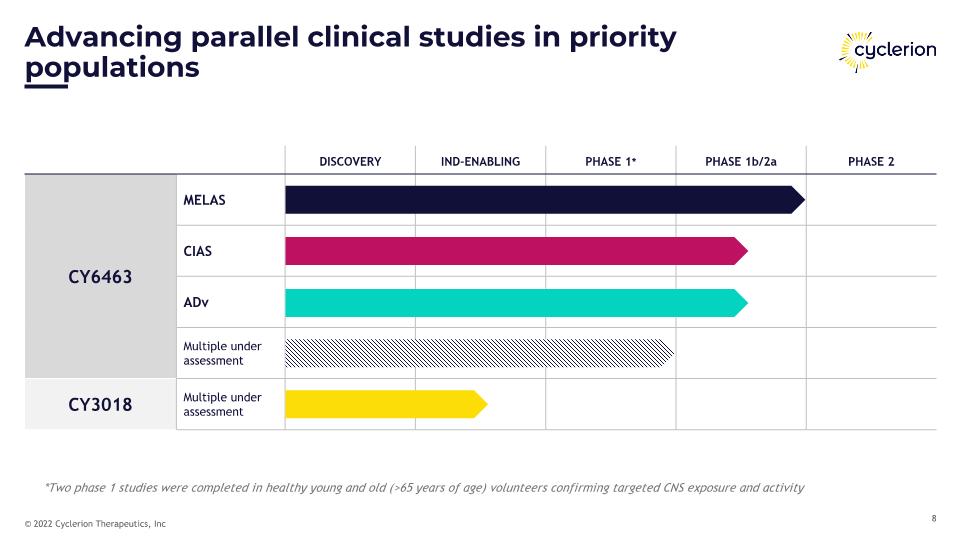
DISCOVERY IND-ENABLING PHASE 1* PHASE 1b/2a PHASE 2 CY6463 MELAS CIAS ADv Multiple under assessment CY3018 Multiple under assessment Advancing parallel clinical studies in priority populations *Two phase 1 studies were completed in healthy young and old (>65 years of age) volunteers confirming targeted CNS exposure and activity
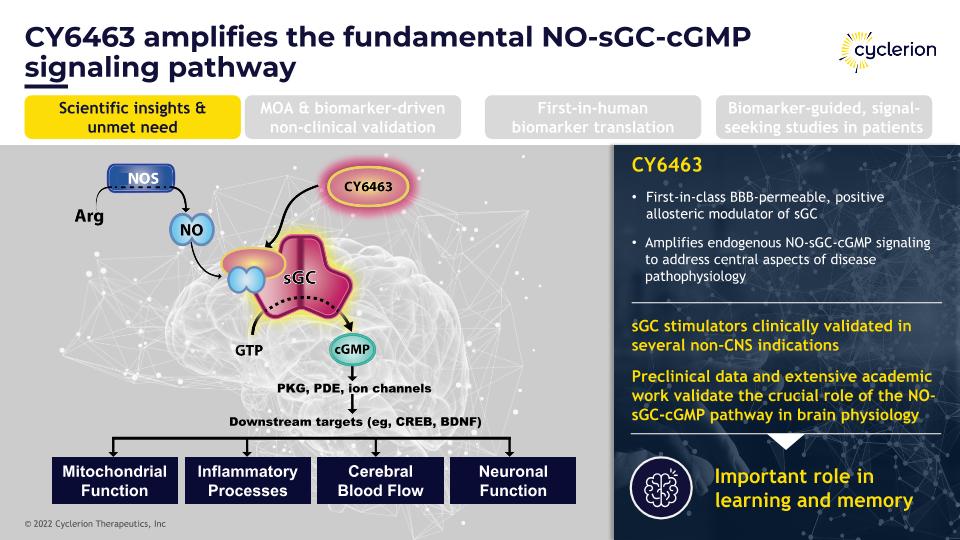
CY6463 amplifies the fundamental NO-sGC-cGMP signaling pathway CY6463 First-in-class BBB-permeable, positive allosteric modulator of sGC Amplifies endogenous NO-sGC-cGMP signaling to address central aspects of disease pathophysiology sGC stimulators clinically validated in several non-CNS indications Preclinical data and extensive academic work validate the crucial role of the NO-sGC-cGMP pathway in brain physiology Important role in learning and memory Scientific insights & unmet need MOA & biomarker-driven non-clinical validation First-in-human biomarker translation Biomarker-guided, signal-seeking studies in patients Mitochondrial Function Inflammatory Processes Cerebral �Blood Flow Neuronal Function PKG, PDE, ion channels Downstream targets (eg, CREB, BDNF)
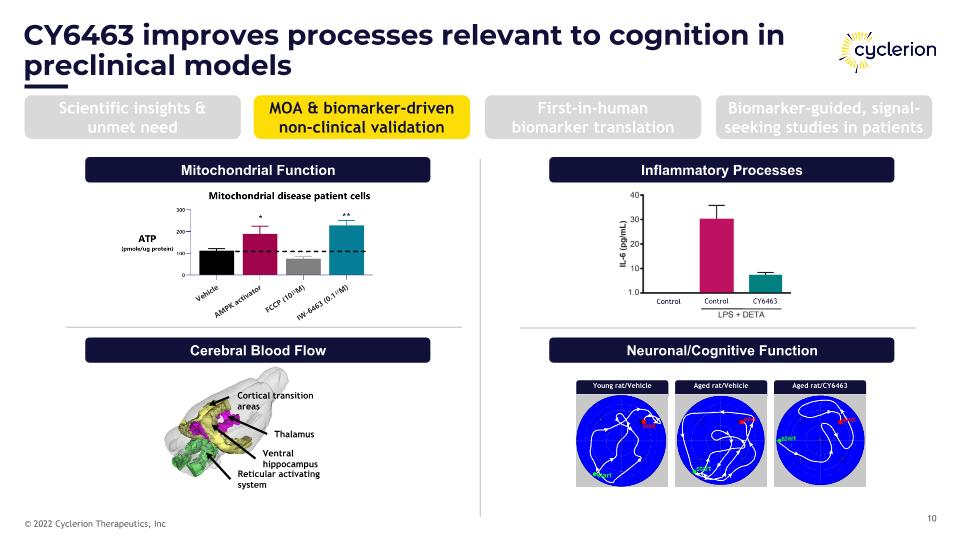
CY6463 improves processes relevant to cognition in preclinical models Mitochondrial Function Cerebral Blood Flow Inflammatory Processes Neuronal/Cognitive Function Reticular activating system Thalamus Cortical transition areas Ventral hippocampus Young rat/Vehicle Aged rat/Vehicle Aged rat/CY6463 Scientific insights & unmet need MOA & biomarker-driven non-clinical validation First-in-human biomarker translation Biomarker-guided, signal-seeking studies in patients
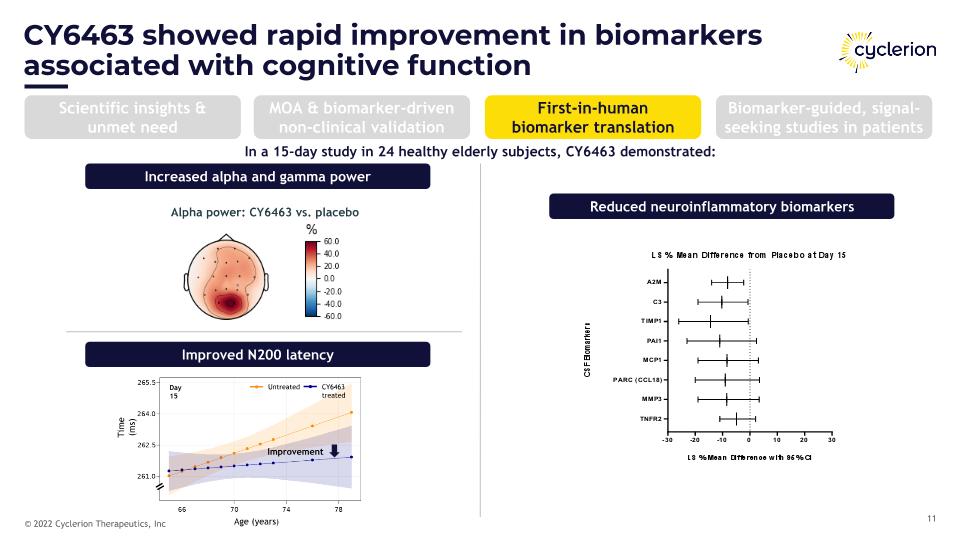
CY6463 showed rapid improvement in biomarkers �associated with cognitive function Increased alpha and gamma power Improved N200 latency Alpha power: CY6463 vs. placebo Day 15 Improvement Age (years) Untreated CY6463 treated Time (ms) Reduced neuroinflammatory biomarkers In a 15-day study in 24 healthy elderly subjects, CY6463 demonstrated: Scientific insights & unmet need MOA & biomarker-driven non-clinical validation First-in-human biomarker translation Biomarker-guided, signal-seeking studies in patients
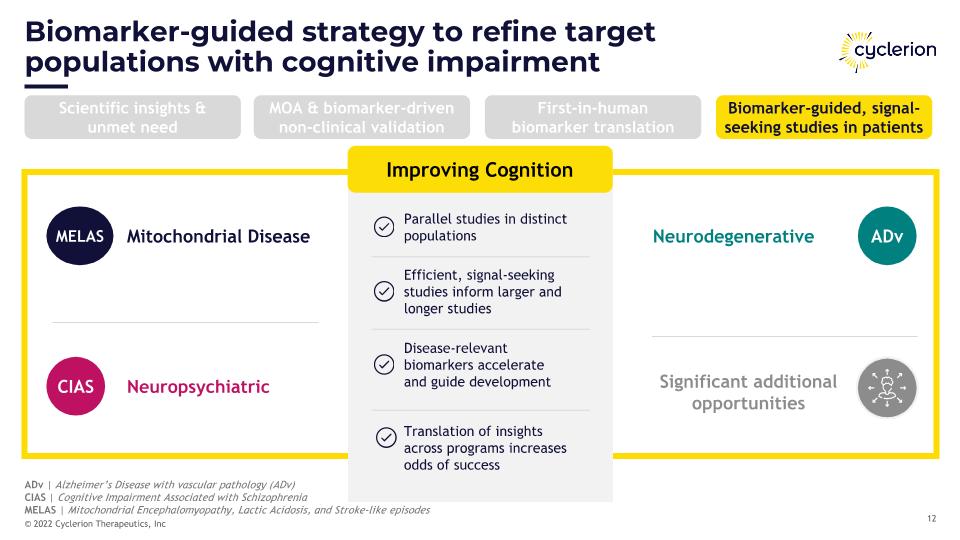
Biomarker-guided strategy to refine target populations with cognitive impairment ADv | Alzheimer’s Disease with vascular pathology (ADv) CIAS | Cognitive Impairment Associated with Schizophrenia MELAS | Mitochondrial Encephalomyopathy, Lactic Acidosis, and Stroke-like episodes ADv MELAS Mitochondrial Disease Parallel studies in distinct populations Efficient, signal-seeking studies inform larger and longer studies Disease-relevant �biomarkers accelerate �and guide development Improving Cognition Neuropsychiatric CIAS Neurodegenerative Significant additional opportunities Translation of insights across programs increases odds of success Scientific insights & unmet need MOA & biomarker-driven non-clinical validation First-in-human biomarker translation Biomarker-guided, signal-seeking studies in patients

MELAS Study Results
MELAS clinical data demonstrate that CY6463 has potential as breakthrough CNS therapeutic MELAS Devastating genetically and phenotypically defined mitochondrial disease (MD) with no approved therapies CY6463 Positive allosteric modulator of sGC which amplifies endogenous NO signaling Study design Open label, 29-day study of once-daily, oral, CY6463 (n=8) Improvement observed across important biomarkers associated with MELAS after CY6463 treatment Well tolerated with no serious adverse events or treatment discontinuation Oral once-daily administration provided expected CNS exposure Improvements observed across multiple domains of disease activity: Biomarkers associated with mitochondrial function, including lactate and GDF-15 Broad panel of inflammatory biomarkers with the potential to translate to CNS diseases with mitochondrial dysfunction Cerebral blood flow (CBF) across all brain regions Functional connectivity between brain regions and activation of occipital brain regions in response to the visual stimulus as measured by fMRI BOLD Supported by correlations across several endpoints and more pronounced in patients with heavier baseline disease burden Return toward baseline levels observed across several biomarkers after dosing discontinuation
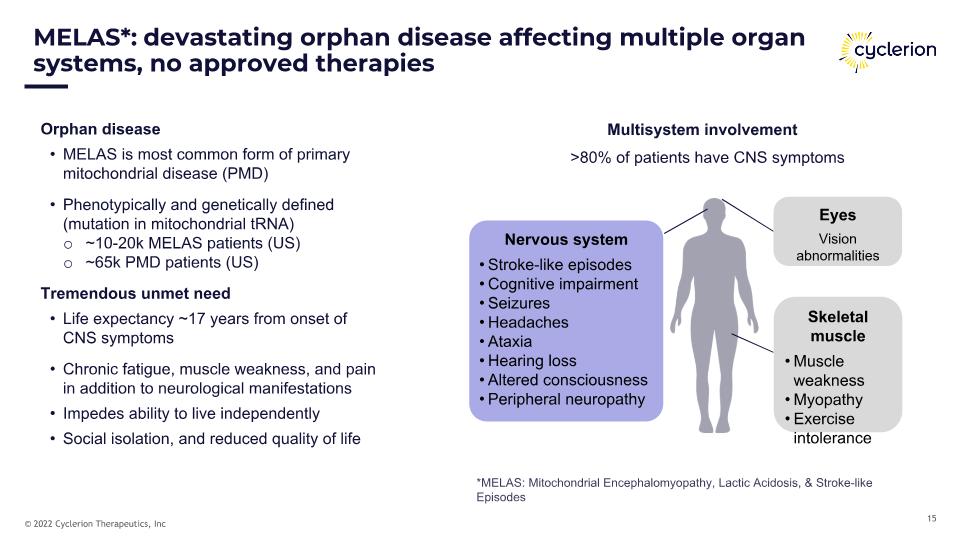
MELAS*: devastating orphan disease affecting multiple organ systems, no approved therapies Multisystem involvement Skeletal muscle Muscle weakness Myopathy Exercise intolerance Eyes Vision abnormalities Orphan disease MELAS is most common form of primary mitochondrial disease (PMD) Phenotypically and genetically defined (mutation in mitochondrial tRNA) ~10-20k MELAS patients (US) ~65k PMD patients (US) Tremendous unmet need Life expectancy ~17 years from onset of CNS symptoms Chronic fatigue, muscle weakness, and pain in addition to neurological manifestations Impedes ability to live independently Social isolation, and reduced quality of life >80% of patients have CNS symptoms Nervous system Stroke-like episodes Cognitive impairment Seizures Headaches Ataxia Hearing loss Altered consciousness Peripheral neuropathy *MELAS: Mitochondrial Encephalomyopathy, Lactic Acidosis, & Stroke-like Episodes
Strong therapeutic rationale for stimulating NO-sGC-cGMP pathway to treat mitochondrial disease CY6463 is a positive allosteric modulator of sGC and amplifies endogenous NO signaling Literature links NO-sGC-cGMP pathway to mitochondrial biogenesis and function NO deficiency in mitochondrial disease has been linked to impaired blood flow, inflammation, angiopathy, and endothelial dysfunction Use of NO precursors recommended by Mitochondrial Medicine Society CYCN preclinical data demonstrate CY6463 affects multiple aspects of mitochondrial disease pathophysiology
Open-label, 29-day study of CY6463 in MELAS patients to assess safety, PK, PD and impact on important domains of mitochondrial disease CNS/PD Safety Safety and tolerability profile with 15-mg QD dosing Safety on top of NO precursors and other stable medications PK Study population (n=8) Genetically confirmed with history of CNS symptoms such as stroke, seizure, headache Stable medications including NO precursors (e.g., arginine and citrulline) permitted (6 of 8) Plasma and, when available, cerebrospinal fluid (CSF) concentrations of CY6463 Measures of key domains of MELAS Mitochondrial function Inflammatory processes Cerebral blood flow Neuronal/cognitive function Patient-reported outcomes (PROs)
Strong safety/tolerability and once-daily profile extended to participants with MELAS CY6463 well tolerated with and without NO precursors (L-arginine and L-citrulline) Mostly mild adverse events (AEs), no severe AEs No SAEs, no discontinuations due to AEs Most common AE was headache, all but 1 mild No signals on clinical labs, vital signs, ECGs, or suicidal rating scale Once-daily dosing with consistent pharmacokinetics Pharmacokinetics (AUCtau, Cmax, and Ctrough) in MELAS participants consistent with PK studies in healthy volunteers Confirmed CNS exposure with CSF:plasma ratio consistent with that observed in healthy volunteers
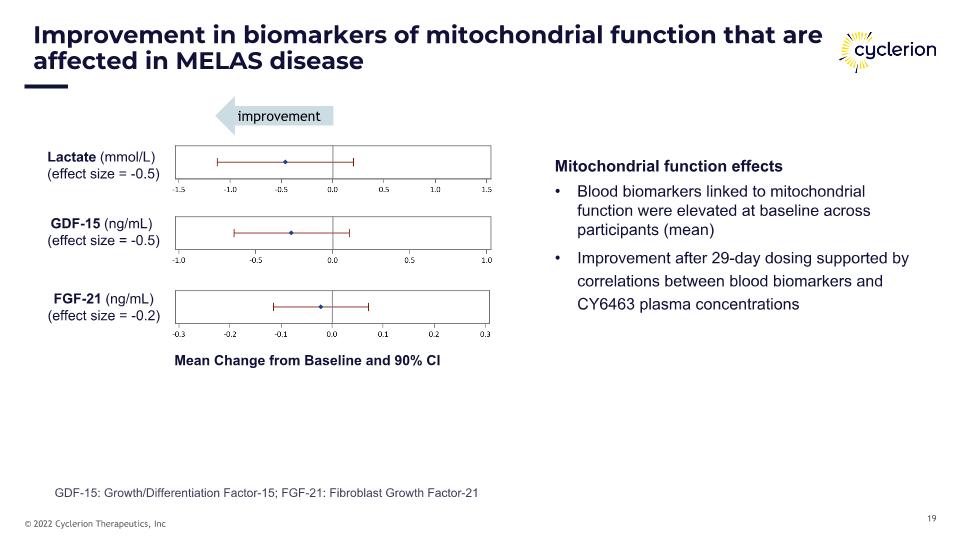
Lactate (mmol/L) (effect size = -0.5) GDF-15 (ng/mL) (effect size = -0.5) FGF-21 (ng/mL) (effect size = -0.2) Improvement in biomarkers of mitochondrial function that are affected in MELAS disease Mitochondrial function effects Blood biomarkers linked to mitochondrial function were elevated at baseline across participants (mean) Improvement after 29-day dosing supported by correlations between blood biomarkers and CY6463 plasma concentrations Mean Change from Baseline and 90% CI improvement GDF-15: Growth/Differentiation Factor-15; FGF-21: Fibroblast Growth Factor-21
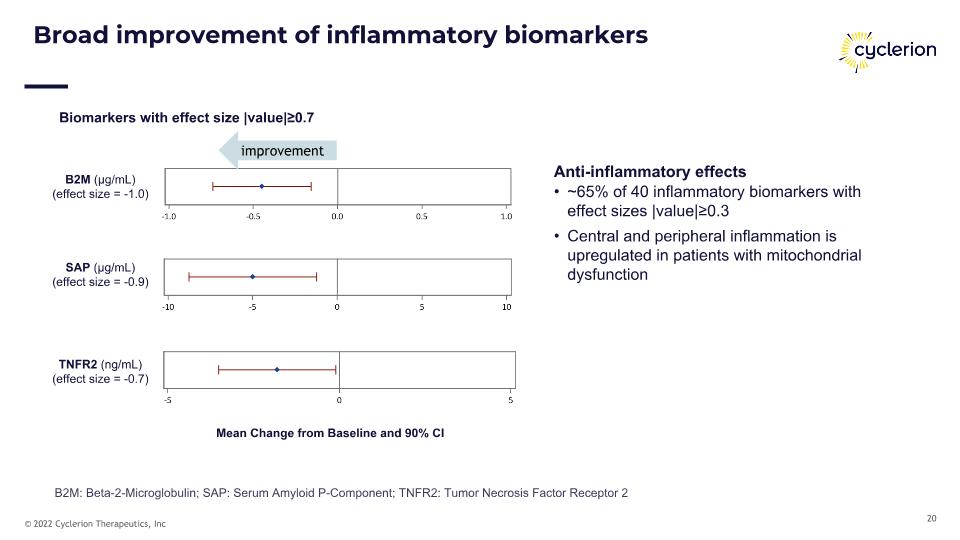
B2M (μg/mL) (effect size = -1.0) SAP (μg/mL) (effect size = -0.9) Broad improvement of inflammatory biomarkers Anti-inflammatory effects ~65% of 40 inflammatory biomarkers with effect sizes |value|≥0.3 Central and peripheral inflammation is upregulated in patients with mitochondrial dysfunction TNFR2 (ng/mL) (effect size = -0.7) Mean Change from Baseline and 90% CI Biomarkers with effect size |value|≥0.7 improvement B2M: Beta-2-Microglobulin; SAP: Serum Amyloid P-Component; TNFR2: Tumor Necrosis Factor Receptor 2
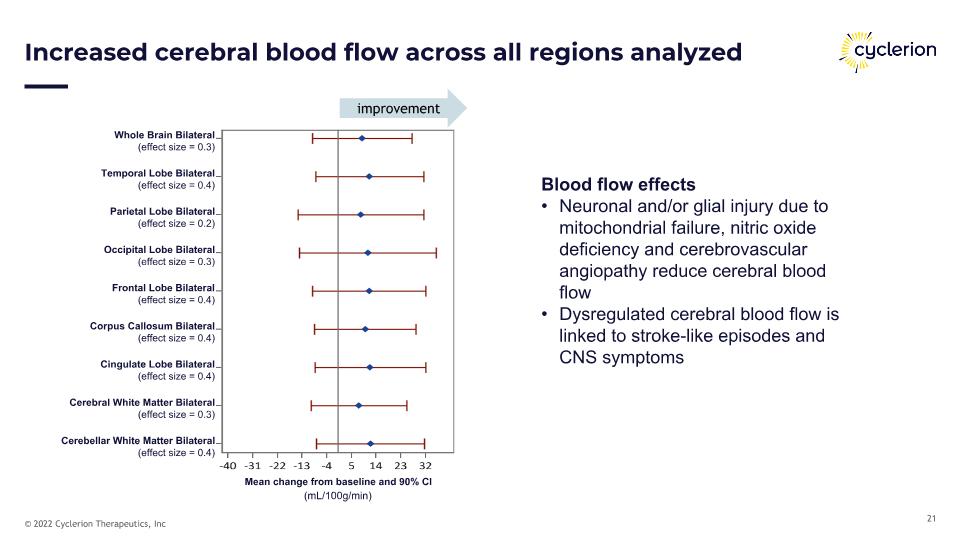
Increased cerebral blood flow across all regions analyzed Blood flow effects Neuronal and/or glial injury due to mitochondrial failure, nitric oxide deficiency and cerebrovascular angiopathy reduce cerebral blood flow Dysregulated cerebral blood flow is linked to stroke-like episodes and CNS symptoms (mL/100g/min) Whole Brain Bilateral (effect size = 0.3) Temporal Lobe Bilateral (effect size = 0.4) Parietal Lobe Bilateral (effect size = 0.2) Occipital Lobe Bilateral (effect size = 0.3) Frontal Lobe Bilateral (effect size = 0.4) Corpus Callosum Bilateral (effect size = 0.4) Cingulate Lobe Bilateral (effect size = 0.4) Cerebral White Matter Bilateral (effect size = 0.3) Cerebellar White Matter Bilateral (effect size = 0.4) Mean change from baseline and 90% CI improvement
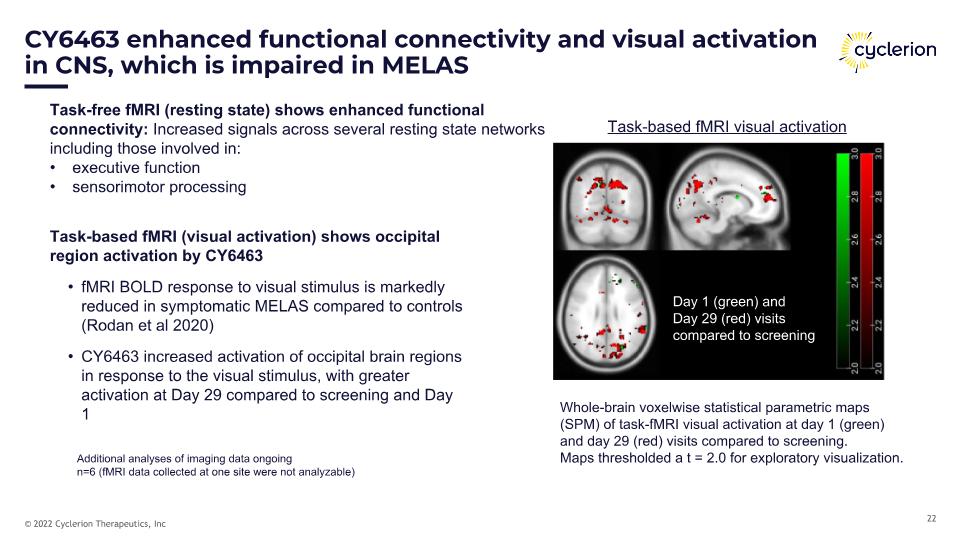
CY6463 enhanced functional connectivity and visual activation in CNS, which is impaired in MELAS Task-free fMRI (resting state) shows enhanced functional connectivity: Increased signals across several resting state networks including those involved in: executive function sensorimotor processing Task-based fMRI (visual activation) shows occipital region activation by CY6463 fMRI BOLD response to visual stimulus is markedly reduced in symptomatic MELAS compared to controls (Rodan et al 2020) CY6463 increased activation of occipital brain regions in response to the visual stimulus, with greater activation at Day 29 compared to screening and Day 1 Additional analyses of imaging data ongoing n=6 (fMRI data collected at one site were not analyzable) Whole-brain voxelwise statistical parametric maps (SPM) of task-fMRI visual activation at day 1 (green) and day 29 (red) visits compared to screening. Maps thresholded a t = 2.0 for exploratory visualization. Day 1 (green) and �Day 29 (red) visits compared to screening Task-based fMRI visual activation
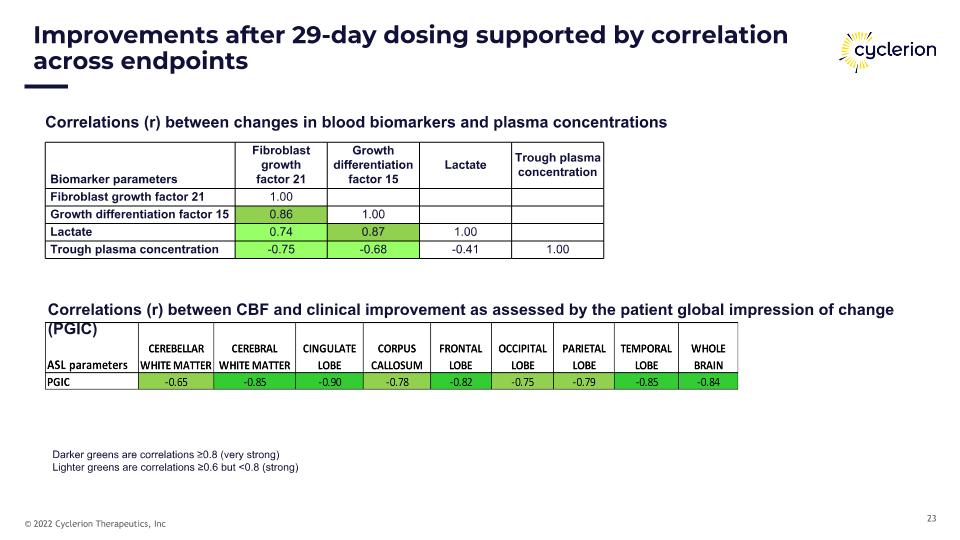
Improvements after 29-day dosing supported by correlation across endpoints Correlations (r) between CBF and clinical improvement as assessed by the patient global impression of change (PGIC) Correlations (r) between changes in blood biomarkers and plasma concentrations Biomarker parameters Fibroblast �growth �factor 21 Growth differentiation factor 15 Lactate Trough plasma concentration Fibroblast growth factor 21 1.00 Growth differentiation factor 15 0.86 1.00 Lactate 0.74 0.87 1.00 Trough plasma concentration -0.75 -0.68 -0.41 1.00 Darker greens are correlations ≥0.8 (very strong) Lighter greens are correlations ≥0.6 but <0.8 (strong)

Ongoing clinical trials

CIAS study ongoing; data expected Q3 2022 Objectives Exploratory, signal-seeking study to evaluate safety, tolerability, and pharmacodynamic effects (qEEG, ERP, digital cognitive performance battery) Study design In-clinic, randomized, placebo-controlled, double-blind, multiple-ascending-dose design 14-day treatment with Once-daily CY6463 or placebo 48 participants across 4 sequential cohorts Patient targeting Psychiatrically stable adults with schizophrenia, no more than moderate symptoms On stable, single antipsychotic regimen Collaborations Study conducted at experienced, partner sites: Hassman Research Institute and Collaborative Neuroscience Exploratory, AI-driven, integrated analysis of data with Ariana Pharma Scientific insights & unmet need MOA & biomarker-driven non-clinical validation First-in-human biomarker translation Biomarker-guided, signal-seeking studies in patients
ADv study ongoing Exploratory, signal-seeking study to evaluate safety, tolerability, and pharmacodynamic effects (EEG, MRI, neuroinflammatory biomarkers, cognition) Once-daily CY6463 vs. placebo 12 weeks 30 participants Confirmed AD pathology (PET, CSF) 2+ cardiovascular risk factors Mild-moderate subcortical small-vessel disease on MRI Mini mental state exam score (20-26) Objectives Study design Patient targeting Partially funded by the Alzheimer's Association’s Part the Cloud-Gates Partnership Collaborating with Dr. Andrew Budson at Boston University on a study to examine the relationship between ERP/EEG and cognitive measures in dementias Collaborations Scientific insights & unmet need MOA & biomarker-driven non-clinical validation First-in-human biomarker translation Biomarker-guided, signal-seeking studies in patients

Next-generation sGC stimulator program
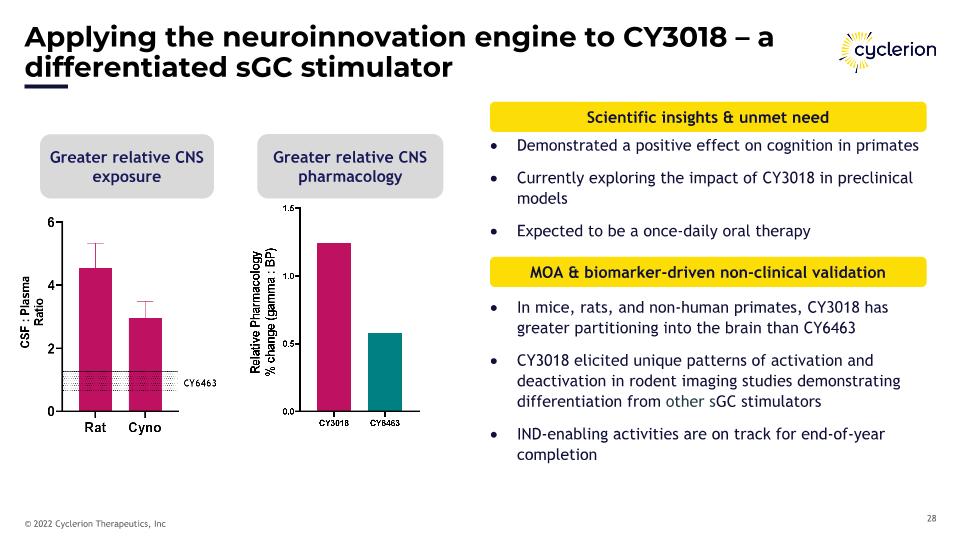
Applying the neuroinnovation engine to CY3018 – a differentiated sGC stimulator In mice, rats, and non-human primates, CY3018 has greater partitioning into the brain than CY6463 CY3018 elicited unique patterns of activation and deactivation in rodent imaging studies demonstrating differentiation from other sGC stimulators IND-enabling activities are on track for end-of-year completion Greater relative CNS exposure Greater relative CNS pharmacology Scientific insights & unmet need MOA & biomarker-driven non-clinical validation Demonstrated a positive effect on cognition in primates Currently exploring the impact of CY3018 in preclinical models Expected to be a once-daily oral therapy

Track record of success

Leadership Team with Track Record of Success Peter Hecht, PhD Chief Executive Officer Andy Busch, PhD Chief Scientific Officer Chris Winrow, PhD Vice President, Translational Medicine & Development Program Lead Jennifer Chickering, PhD Vice President, Clinical Strategy Anjeza Gjino, MBA Chief Financial Officer Cheryl Gault Chief Operating Officer Todd Milne, PhD Sr. Vice President, Corporate Development Bill Kissel, PhD Vice President, Pharmaceutical Development Bruce Kinon, MD Vice President, Clinical Development Kevin Durfee Vice President, Information Technology & Facilities

George Conrades Errol De Souza, PhD Marsha Fanucci, Chair Peter Hecht, PhD, CEO Ole Isacson, MD, PhD Stephanie Lovell Terrance McGuire Michael Mendelsohn, MD Experienced Board of Directors and Scientific Advisors Board of Directors Scientific Advisors Claudio Babiloni, PhD University of Rome Andrew E. Budson, MD VA Boston Healthcare System Boston University Harvard Medical School Mark Currie, PhD Ironwood Pharmaceuticals Science Exchange Marni J. Falk, MD The Children’s Hospital of Philadelphia University of Pennsylvania Michael Heneka, MD, PhD Luxembourg Centre for Systems Biology University of Massachusetts Robert C. Malenka, MD, PhD Nancy Pritzker Laboratory Stanford University David H. Salat, PhD MGH / Harvard Medical School VA Boston Healthcare System Daniela Salvemini, PhD St. Louis University Harald H.H.W. Schmidt, MD, PhD, PharmD Maastricht University Eric Smith, MD University of Calgary M Brandon Westover, MD, PhD MGH / Harvard Medical School Beacon Biosignals Chris Wright, MD, PhD AavantiBio
Developing therapies to restore cognitive function Experienced team of scientific leaders, drug hunters, company builders, and CNS experts Developed and launched first-in-class therapies Raised billions in capital Built leading strategic partnerships Neuroinnovation engine enables faster, more precise drug development Advancing CY6463, potential breakthrough, first-in-class CNS therapy Improvement trends in MELAS patients across biomarkers of mitochondrial function, inflammation, cerebral blood flow and functional connectivity CIAS study results expected in Q3’22; ADv study actively enrolling Evaluating collaborative development opportunities Developing disease and mechanism specific translational biomarkers Continuously refining signal-to-noise Identifying most promising patient populations early

Appendices Preclinical, Phase 1 and translational pharmacology studies, references
Preclinical Data
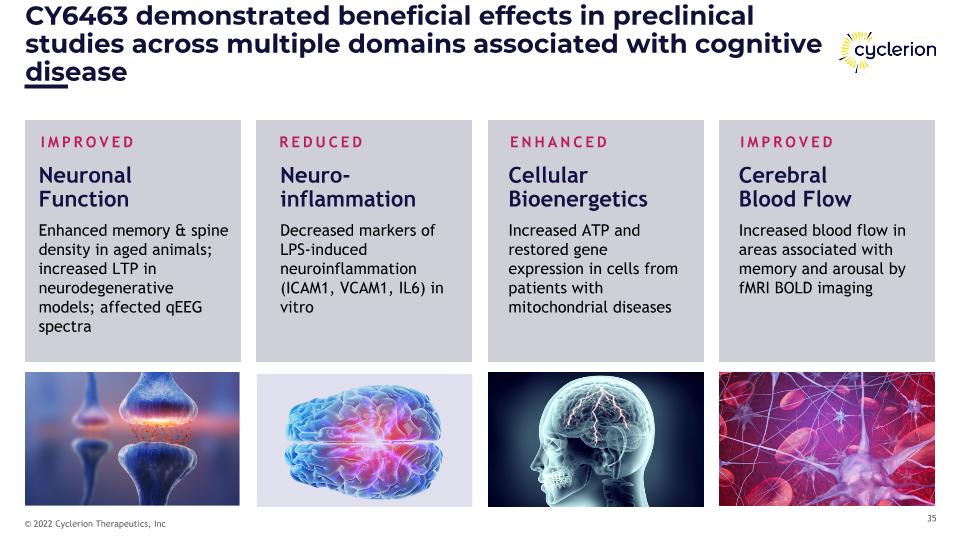
CY6463 demonstrated beneficial effects in preclinical studies across multiple domains associated with cognitive disease Cerebral �Blood Flow Increased blood flow in areas associated with memory and arousal by fMRI BOLD imaging IMPROVED Cellular�Bioenergetics Increased ATP and restored gene expression in cells from patients with mitochondrial diseases ENHANCED Neuronal �Function Enhanced memory & spine density in aged animals; increased LTP in neurodegenerative models; affected qEEG spectra IMPROVED Neuro- �inflammation Decreased markers of LPS-induced neuroinflammation (ICAM1, VCAM1, IL6) in vitro REDUCED
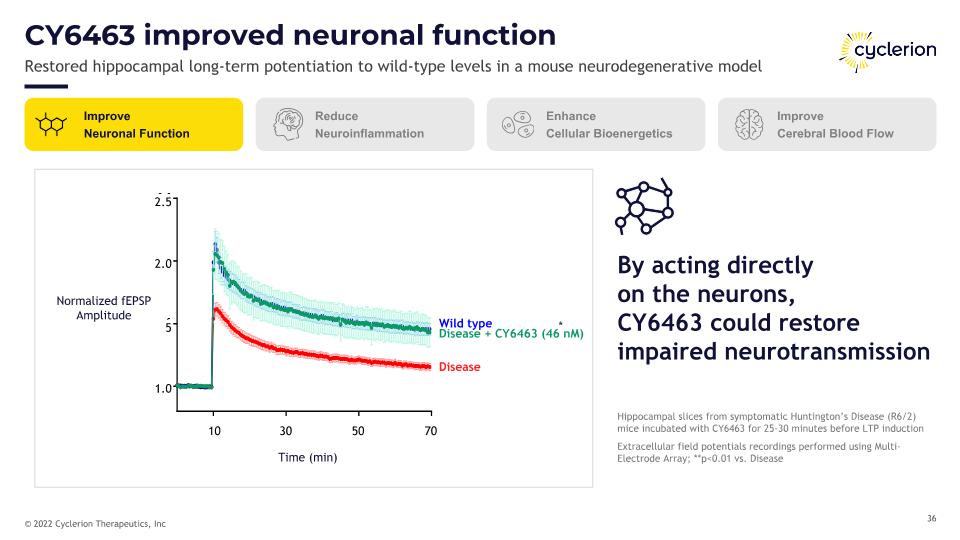
CY6463 improved neuronal function Restored hippocampal long-term potentiation to wild-type levels in a mouse neurodegenerative model Wild type Disease Disease + CY6463 (46 nM) * Normalized fEPSP Amplitude Time (min) 10 30 50 70 1.0 5 2.0 2.5 Improve Neuronal Function Reduce Neuroinflammation Enhance Cellular Bioenergetics Improve Cerebral Blood Flow Hippocampal slices from symptomatic Huntington’s Disease (R6/2) mice incubated with CY6463 for 25-30 minutes before LTP induction Extracellular field potentials recordings performed using Multi-Electrode Array; **p<0.01 vs. Disease By acting directly�on the neurons,�CY6463 could restore impaired neurotransmission
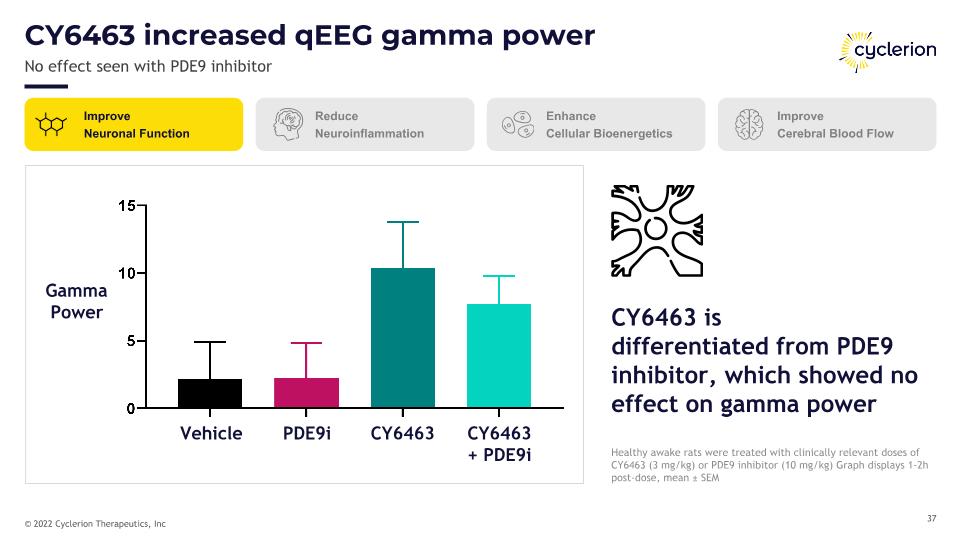
CY6463 increased qEEG gamma power No effect seen with PDE9 inhibitor Healthy awake rats were treated with clinically relevant doses of CY6463 (3 mg/kg) or PDE9 inhibitor (10 mg/kg) Graph displays 1-2h post-dose, mean ± SEM Improve Neuronal Function Reduce Neuroinflammation Enhance Cellular Bioenergetics Improve Cerebral Blood Flow CY6463 is�differentiated from PDE9 inhibitor, which showed no effect on gamma power Gamma Power Vehicle PDE9i CY6463 CY6463 + PDE9i
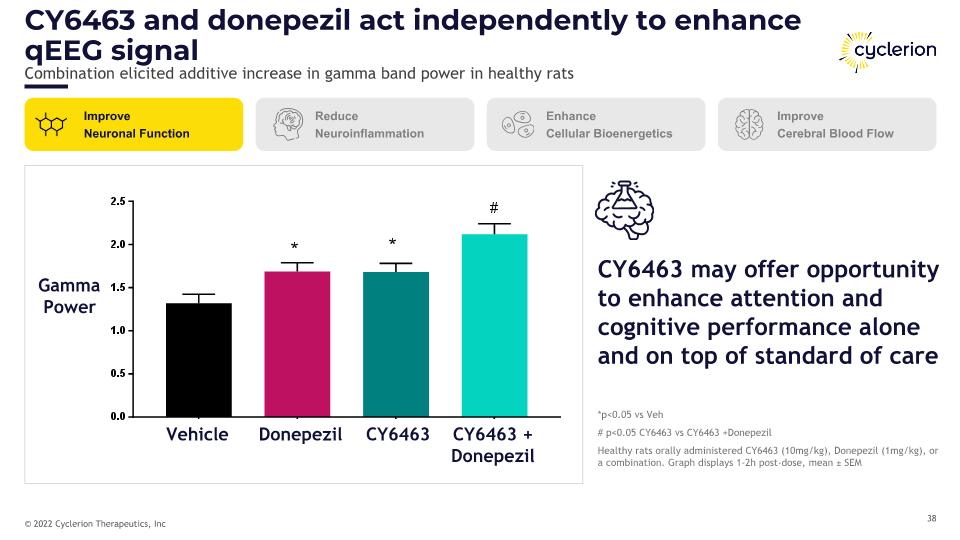
CY6463 and donepezil act independently to enhance qEEG signal Combination elicited additive increase in gamma band power in healthy rats Improve Neuronal Function Reduce Neuroinflammation Enhance Cellular Bioenergetics Improve Cerebral Blood Flow *p<0.05 vs Veh # p<0.05 CY6463 vs CY6463 +Donepezil Healthy rats orally administered CY6463 (10mg/kg), Donepezil (1mg/kg), or a combination. Graph displays 1-2h post-dose, mean ± SEM CY6463 may offer opportunity to enhance attention and cognitive performance alone and on top of standard of care Gamma Power Vehicle Donepezil CY6463 CY6463 + Donepezil
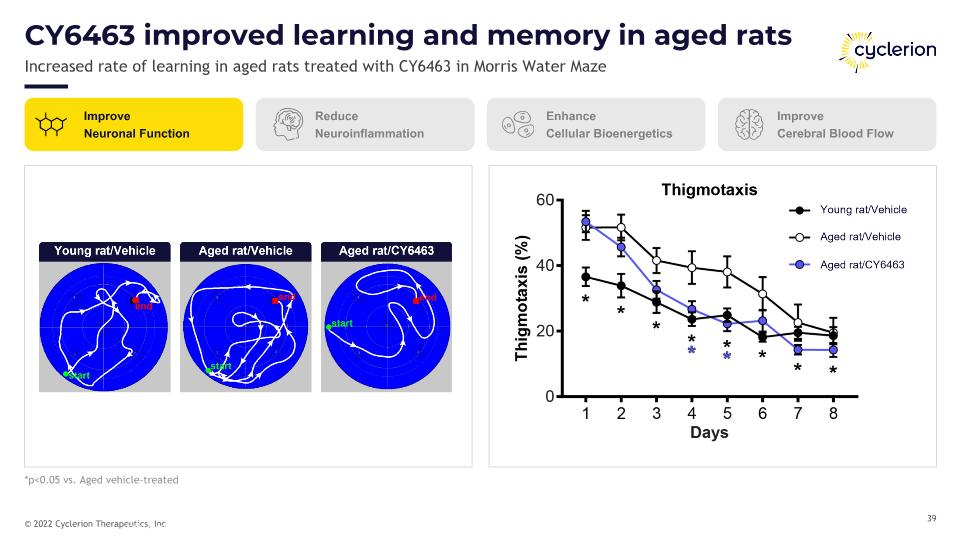
CY6463 improved learning and memory in aged rats Increased rate of learning in aged rats treated with CY6463 in Morris Water Maze Healthy aged male rats were administered CY6463 (10 mg/kg, p.o.) daily during Morris Water Maze training *p<0.05 vs. Aged vehicle-treated Improve Neuronal Function Reduce Neuroinflammation Enhance Cellular Bioenergetics Improve Cerebral Blood Flow
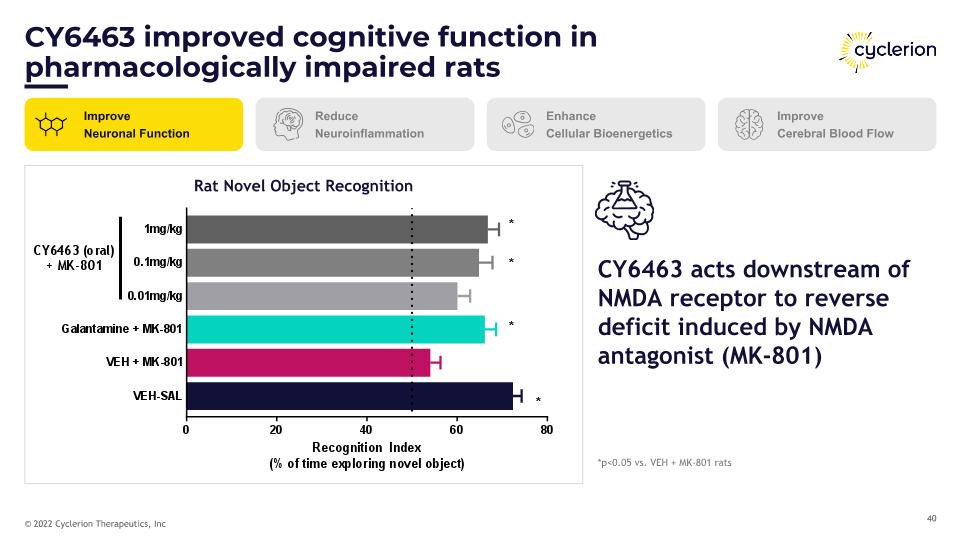
CY6463 improved cognitive function in pharmacologically impaired rats Improve Neuronal Function Reduce Neuroinflammation Enhance Cellular Bioenergetics Improve Cerebral Blood Flow Rat Novel Object Recognition *p<0.05 vs. VEH + MK-801 rats CY6463 acts downstream of NMDA receptor to reverse deficit induced by NMDA antagonist (MK-801)
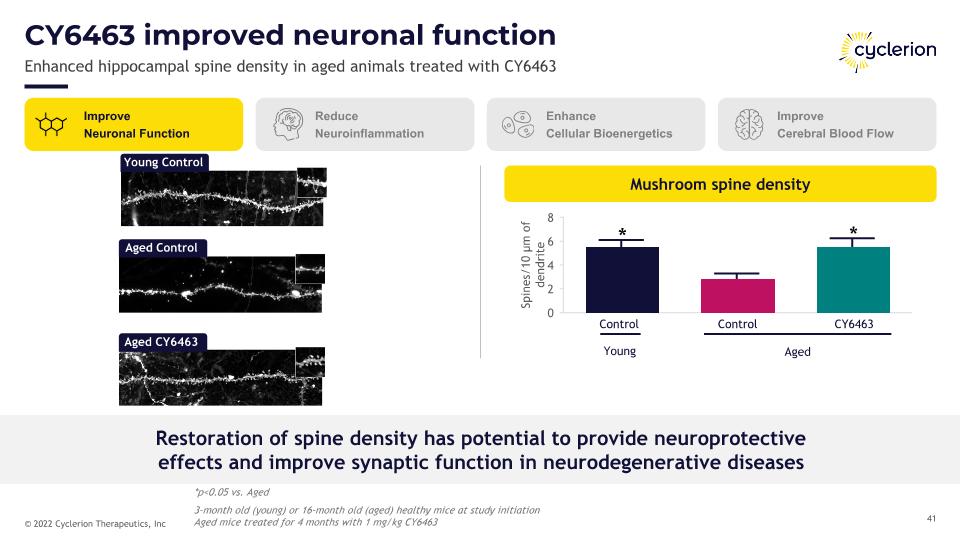
Mushroom spine density Restoration of spine density has potential to provide neuroprotective�effects and improve synaptic function in neurodegenerative diseases CY6463 improved neuronal function Enhanced hippocampal spine density in aged animals treated with CY6463 Young Control Aged Control Aged CY6463 Control Young CY6463 Control Aged * * Improve Neuronal Function Reduce Neuroinflammation Enhance Cellular Bioenergetics Improve Cerebral Blood Flow *p<0.05 vs. Aged 3-month old (young) or 16-month old (aged) healthy mice at study initiation�Aged mice treated for 4 months with 1 mg/kg CY6463
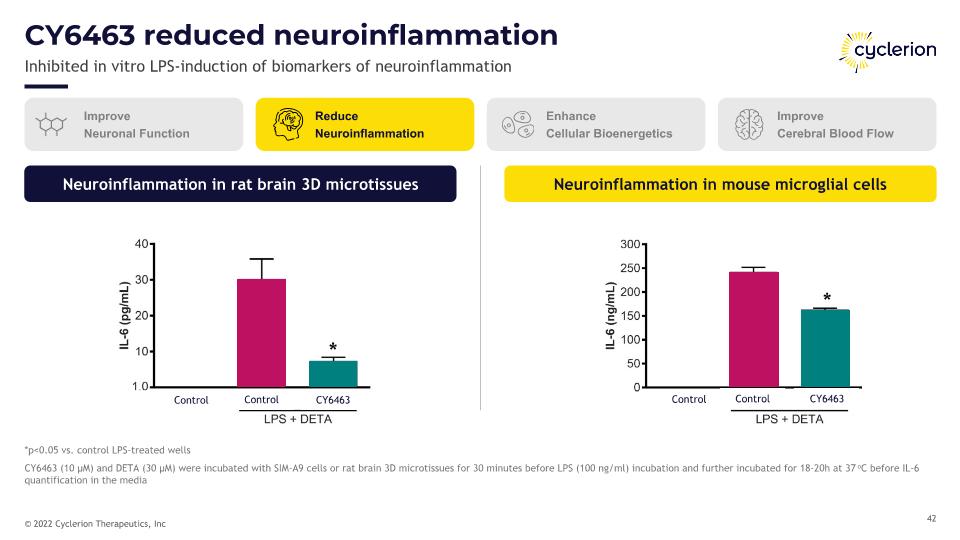
CY6463 reduced neuroinflammation Inhibited in vitro LPS-induction of biomarkers of neuroinflammation *p<0.05 vs. control LPS-treated wells CY6463 (10 µM) and DETA (30 µM) were incubated with SIM-A9 cells or rat brain 3D microtissues for 30 minutes before LPS (100 ng/ml) incubation and further incubated for 18-20h at 37oC before IL-6 quantification in the media Neuroinflammation in mouse microglial cells Neuroinflammation in rat brain 3D microtissues * Improve Neuronal Function Reduce Neuroinflammation Enhance Cellular Bioenergetics Improve Cerebral Blood Flow CY6463 Control Control CY6463 Control Control * *
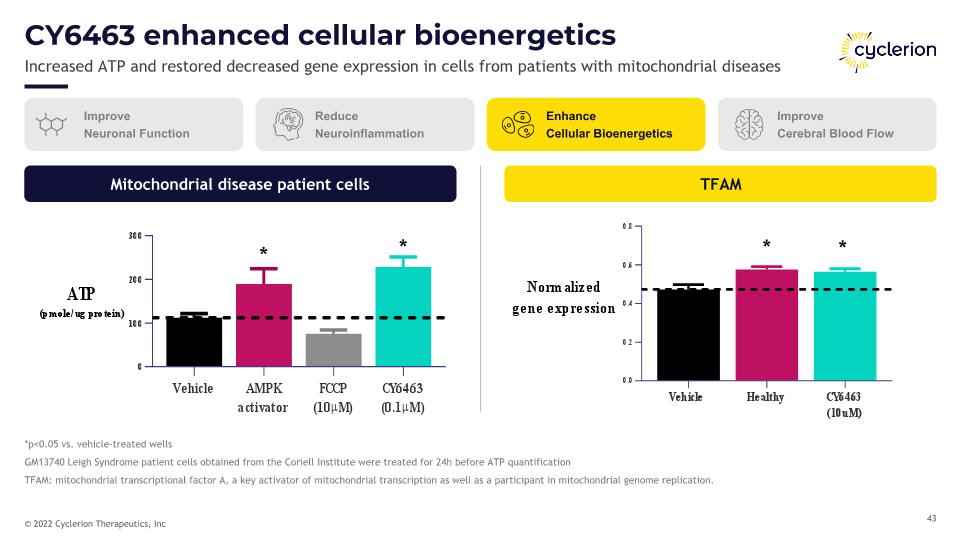
CY6463 enhanced cellular bioenergetics Increased ATP and restored decreased gene expression in cells from patients with mitochondrial diseases *p<0.05 vs. vehicle-treated wells GM13740 Leigh Syndrome patient cells obtained from the Coriell Institute were treated for 24h before ATP quantification TFAM: mitochondrial transcriptional factor A, a key activator of mitochondrial transcription as well as a participant in mitochondrial genome replication. Improve Neuronal Function Reduce Neuroinflammation Enhance Cellular Bioenergetics Improve Cerebral Blood Flow TFAM Mitochondrial disease patient cells * * * *
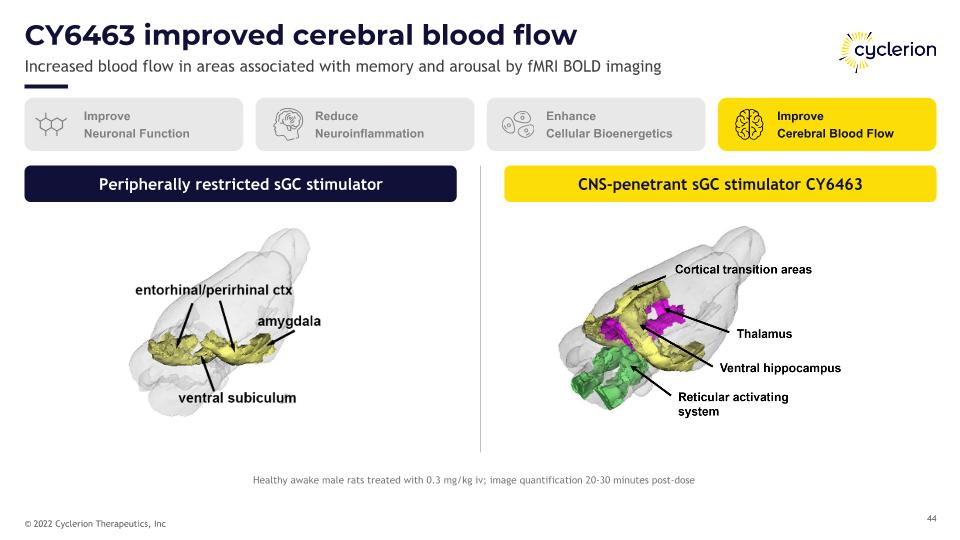
CY6463 improved cerebral blood flow Increased blood flow in areas associated with memory and arousal by fMRI BOLD imaging Peripherally restricted sGC stimulator CNS-penetrant sGC stimulator CY6463 Improve Neuronal Function Reduce Neuroinflammation Enhance Cellular Bioenergetics Improve Cerebral Blood Flow Healthy awake male rats treated with 0.3 mg/kg iv; image quantification 20-30 minutes post-dose

Phase 1 study results
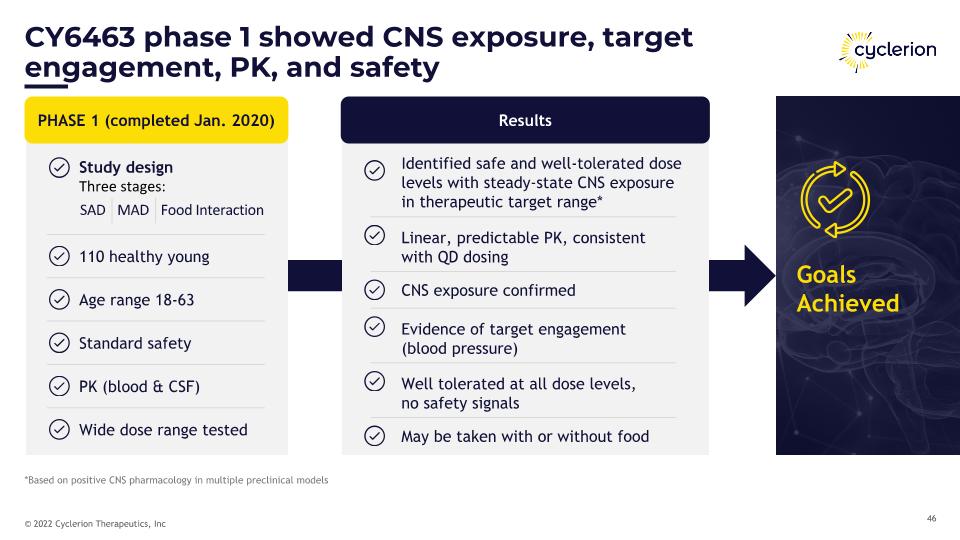
Results CY6463 phase 1 showed CNS exposure, target engagement, PK, and safety PHASE 1 (completed Jan. 2020) 110 healthy young Age range 18-63 Standard safety Identified safe and well-tolerated dose levels with steady-state CNS exposure in therapeutic target range* Linear, predictable PK, consistent�with QD dosing CNS exposure confirmed Evidence of target engagement�(blood pressure) Goals�Achieved Study design�Three stages: SAD MAD Food Interaction PK (blood & CSF) Wide dose range tested Well tolerated at all dose levels,�no safety signals May be taken with or without food *Based on positive CNS pharmacology in multiple preclinical models

Translational Pharmacology Study Results
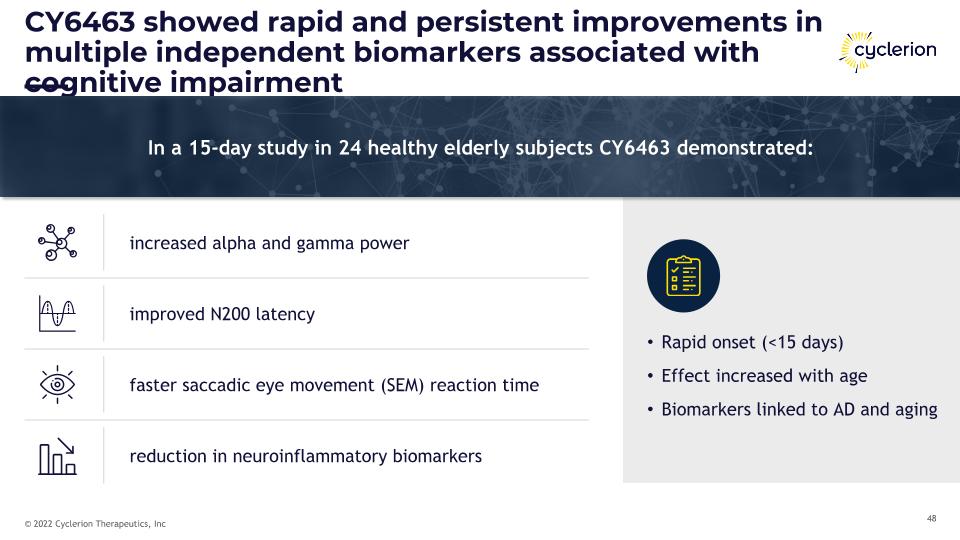
CY6463 showed rapid and persistent improvements in multiple independent biomarkers associated with cognitive impairment In a 15-day study in 24 healthy elderly subjects CY6463 demonstrated: increased alpha and gamma power improved N200 latency faster saccadic eye movement (SEM) reaction time reduction in neuroinflammatory biomarkers Rapid onset (<15 days) Effect increased with age Biomarkers linked to AD and aging
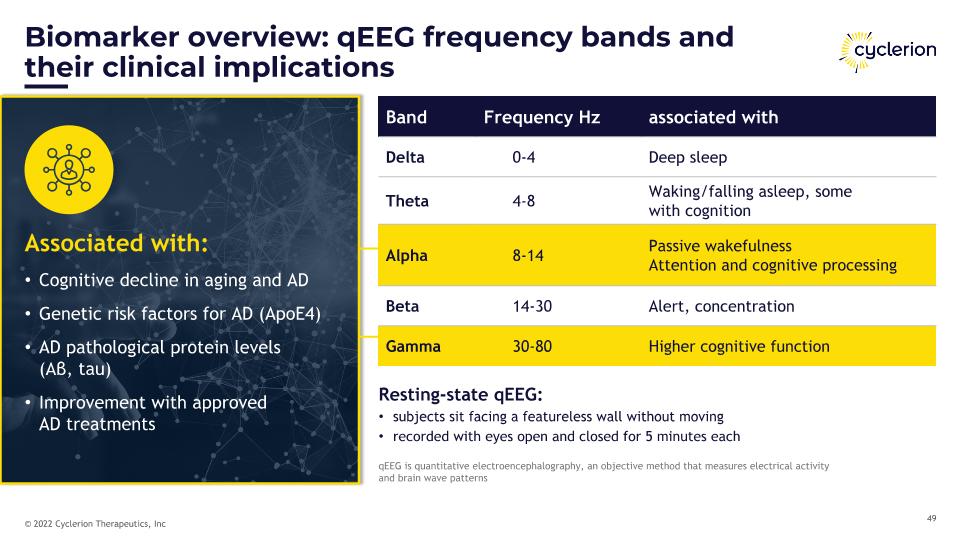
Biomarker overview: qEEG frequency bands and their clinical implications qEEG is quantitative electroencephalography, an objective method that measures electrical activity �and brain wave patterns Resting-state qEEG: subjects sit facing a featureless wall without moving recorded with eyes open and closed for 5 minutes each Band Frequency Hz associated with Delta 0-4 Deep sleep Theta 4-8 Waking/falling asleep, some �with cognition Alpha 8-14 Passive wakefulness Attention and cognitive processing Beta 14-30 Alert, concentration Gamma 30-80 Higher cognitive function Associated with: Cognitive decline in aging and AD Genetic risk factors for AD (ApoE4) AD pathological protein levels �(Aβ, tau) Improvement with approved �AD treatments
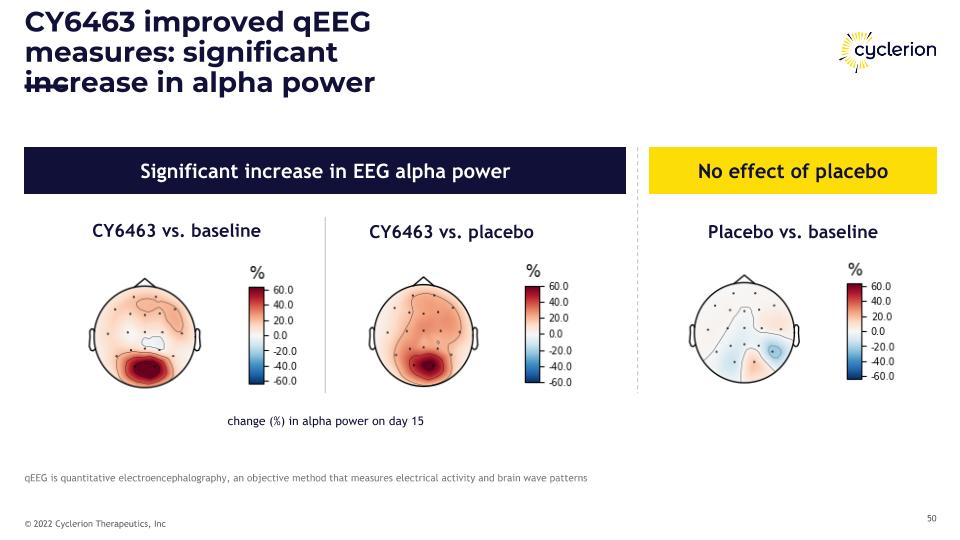
CY6463 improved qEEG measures: significant increase in alpha power qEEG is quantitative electroencephalography, an objective method that measures electrical activity and brain wave patterns CY6463 vs. baseline Significant increase in EEG alpha power No effect of placebo Placebo vs. baseline CY6463 vs. placebo change (%) in alpha power on day 15
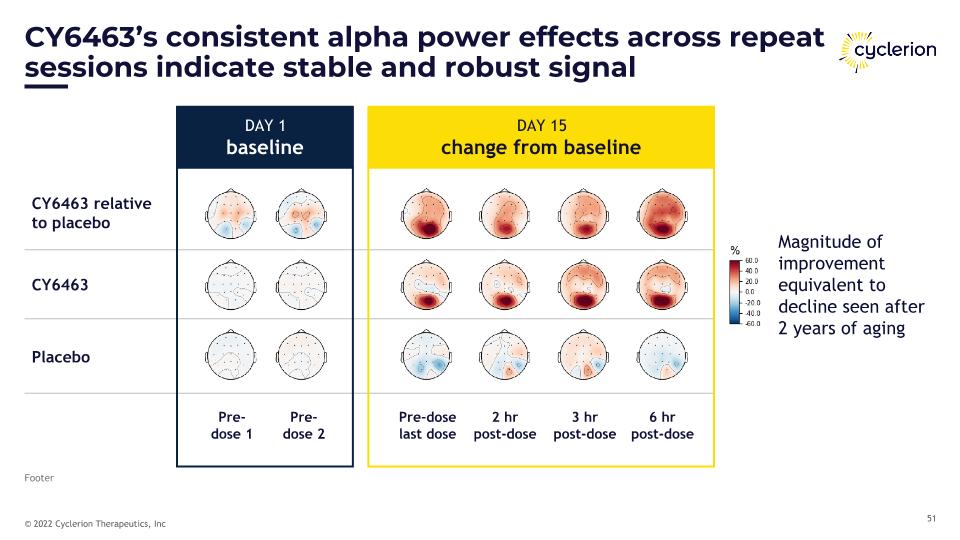
CY6463’s consistent alpha power effects across repeat sessions indicate stable and robust signal Footer DAY 1 baseline DAY 15 change from baseline CY6463 relative �to placebo CY6463 Placebo Pre-�dose 1 Pre-�dose 2 Pre-dose�last dose 2 hr �post-dose 3 hr �post-dose 6 hr �post-dose Magnitude of improvement equivalent to decline seen after 2 years of aging
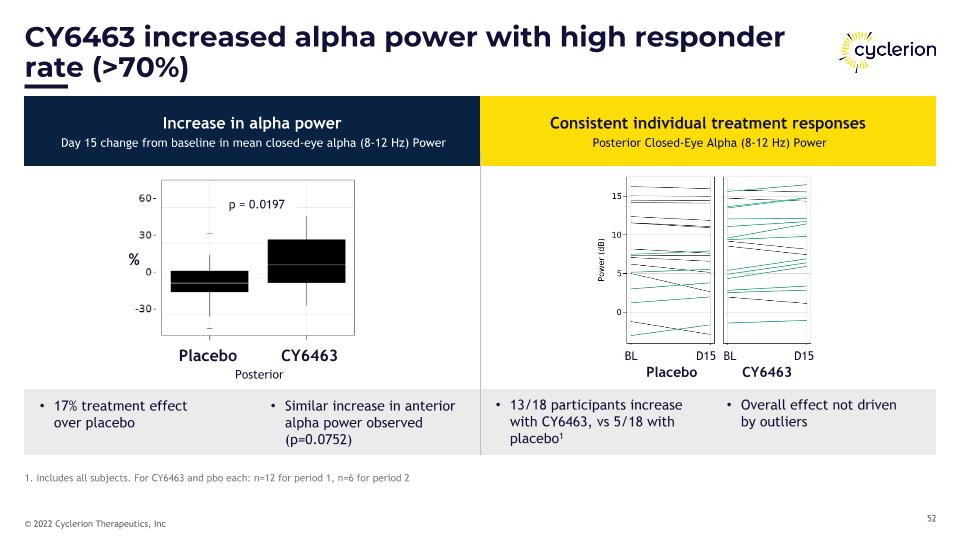
CY6463 increased alpha power with high responder rate (>70%) 1. Includes all subjects. For CY6463 and pbo each: n=12 for period 1, n=6 for period 2 Increase in alpha power Day 15 change from baseline in mean closed-eye alpha (8-12 Hz) Power Consistent individual treatment responses Posterior Closed-Eye Alpha (8-12 Hz) Power Placebo CY6463 Placebo CY6463 Posterior p = 0.0197 % 17% treatment effect �over placebo Similar increase in anterior alpha power observed (p=0.0752) 13/18 participants increase with CY6463, vs 5/18 with placebo1 Overall effect not driven �by outliers Placebo CY6463 BL BL D15 D15
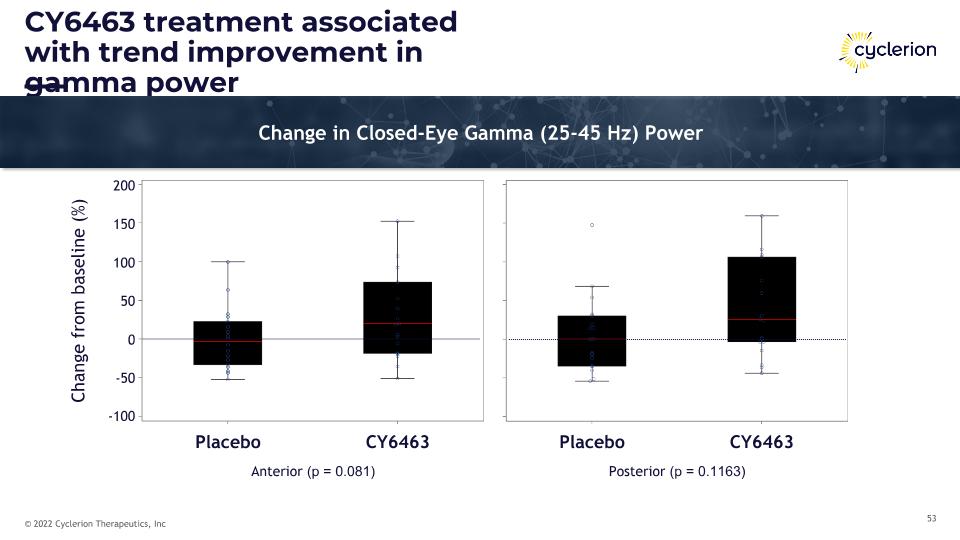
CY6463 treatment associated with trend improvement in gamma power Change in Closed-Eye Gamma (25-45 Hz) Power Placebo CY6463 Placebo CY6463 200 150 100 50 0 -50 -100 Anterior (p = 0.081) Posterior (p = 0.1163) Change from baseline (%)
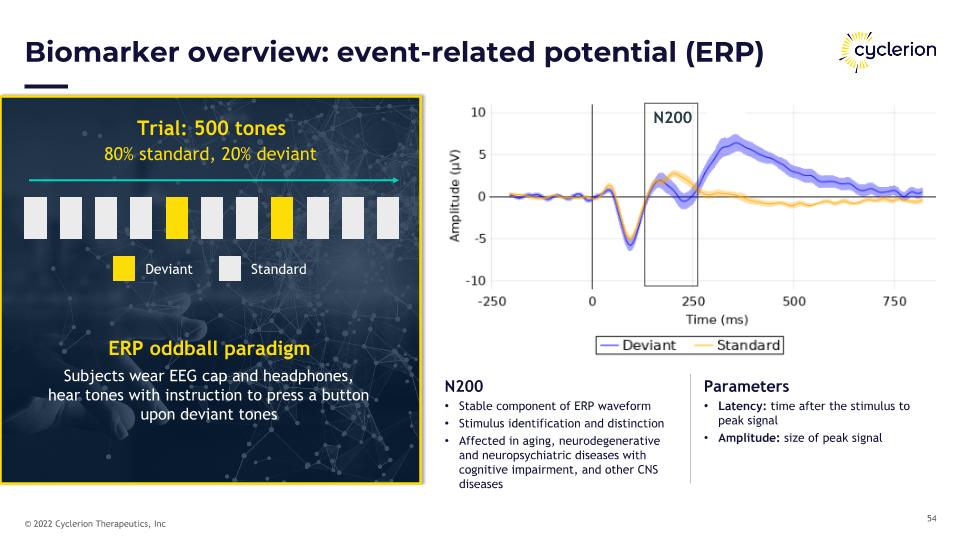
Biomarker overview: event-related potential (ERP) Trial: 500 tones 80% standard, 20% deviant Deviant Standard ERP oddball paradigm Subjects wear EEG cap and headphones, �hear tones with instruction to press a button �upon deviant tones P300 N200 N200 Stable component of ERP waveform Stimulus identification and distinction Affected in aging, neurodegenerative and neuropsychiatric diseases with cognitive impairment, and other CNS diseases Parameters Latency: time after the stimulus to peak signal Amplitude: size of peak signal
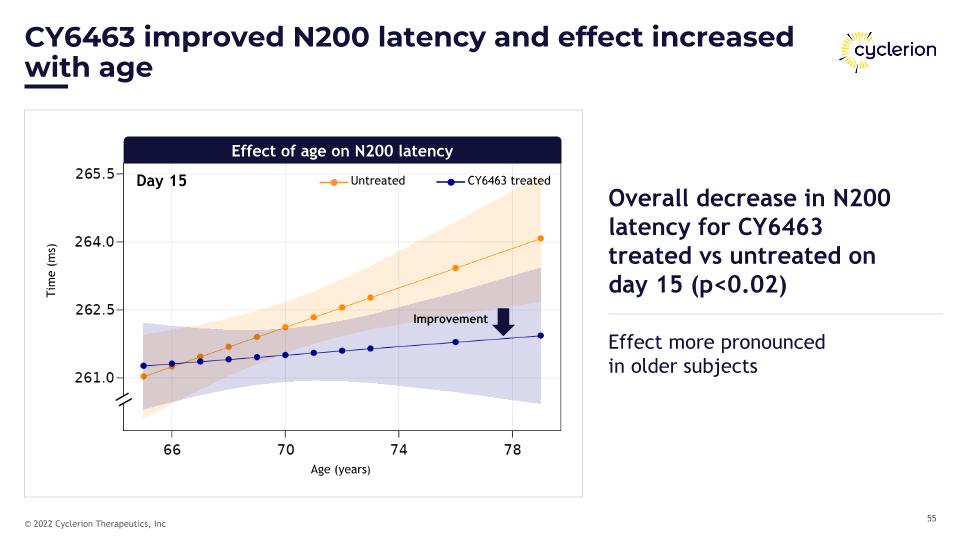
CY6463 improved N200 latency and effect increased with age Day 15 Improvement Age (years) Untreated CY6463 treated Time (ms) Effect of age on N200 latency Overall decrease in N200 latency for CY6463 treated vs untreated on day 15 (p<0.02) Effect more pronounced�in older subjects
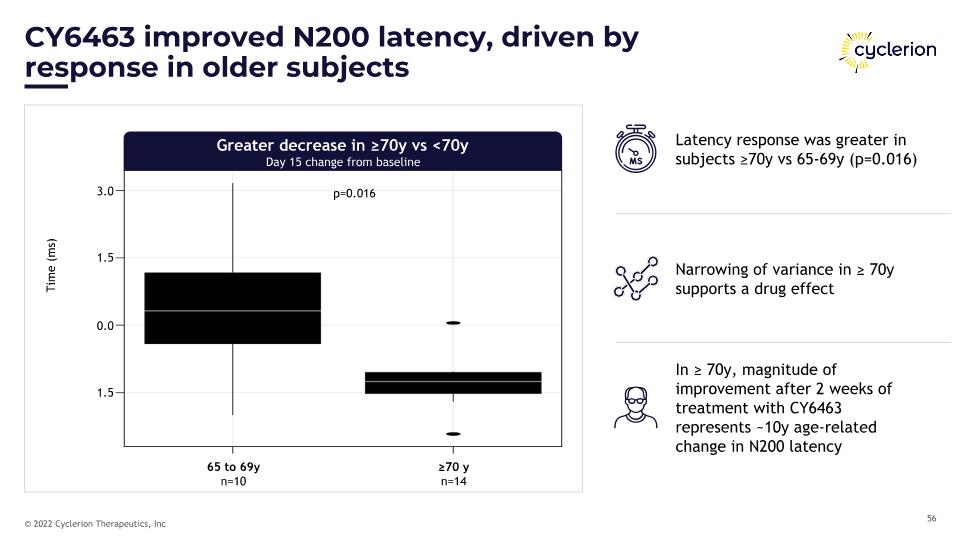
65 to 69y�n=10 ≥70 y�n=14 p=0.016 CY6463 improved N200 latency, driven by�response in older subjects Greater decrease in ≥70y vs <70y�Day 15 change from baseline Latency response was greater in subjects ≥70y vs 65-69y (p=0.016) Narrowing of variance in ≥ 70y supports a drug effect In ≥ 70y, magnitude of improvement after 2 weeks of treatment with CY6463 represents ~10y age-related change in N200 latency Time (ms) 3.0 1.5 0.0 1.5
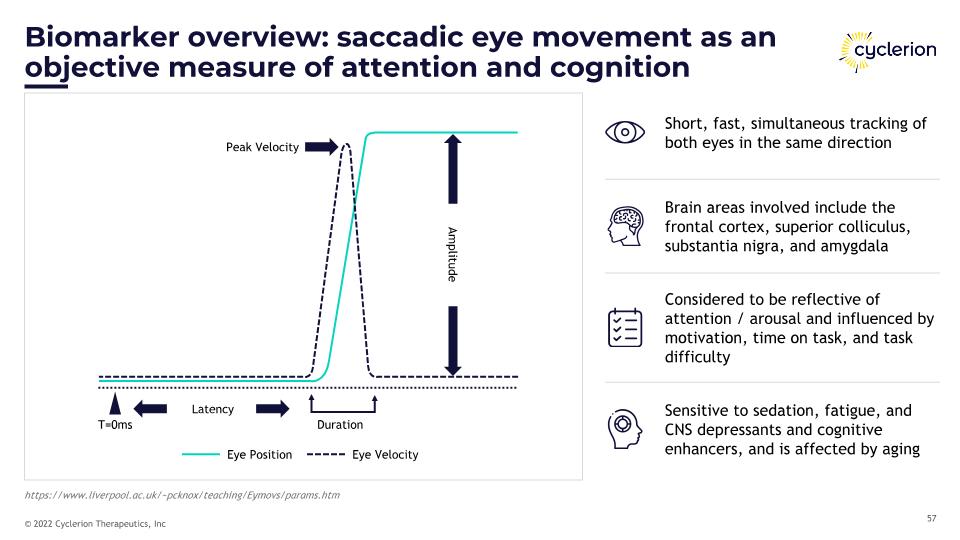
Biomarker overview: saccadic eye movement as an objective measure of attention and cognition Short, fast, simultaneous tracking of both eyes in the same direction Brain areas involved include the frontal cortex, superior colliculus, substantia nigra, and amygdala Considered to be reflective of attention / arousal and influenced by motivation, time on task, and task difficulty Sensitive to sedation, fatigue, and CNS depressants and cognitive enhancers, and is affected by aging Peak Velocity Amplitude Latency Duration T=0ms Eye Position Eye Velocity https://www.liverpool.ac.uk/~pcknox/teaching/Eymovs/params.htm
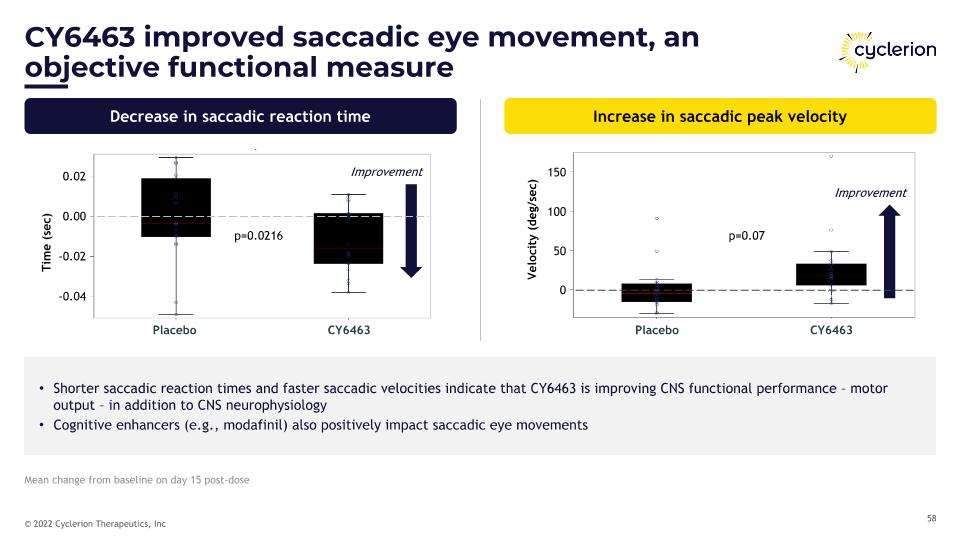
CY6463 improved saccadic eye movement, an objective functional measure Decrease in saccadic reaction time Increase in saccadic peak velocity Shorter saccadic reaction times and faster saccadic velocities indicate that CY6463 is improving CNS functional performance – motor output – in addition to CNS neurophysiology Cognitive enhancers (e.g., modafinil) also positively impact saccadic eye movements Time (sec) p=0.0216 Placebo CY6463 Placebo CY6463 p=0.0216 p=0.07 Improvement Velocity (deg/sec) Improvement Mean change from baseline on day 15 post-dose 0.02 0.00 -0.02 -0.04 150 100 50 0
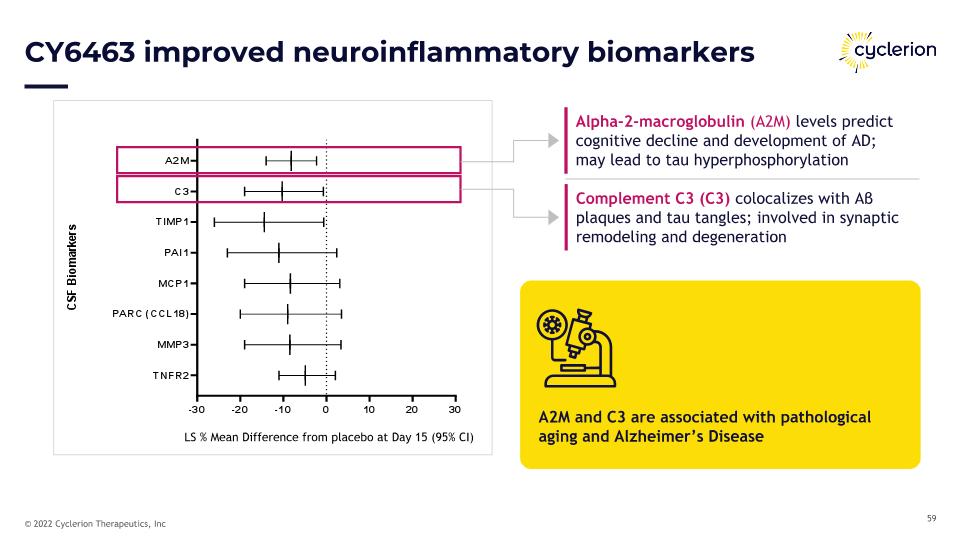
CY6463 improved neuroinflammatory biomarkers A2M and C3 are associated with pathological aging and Alzheimer’s Disease Alpha-2-macroglobulin (A2M) levels predict cognitive decline and development of AD; may lead to tau hyperphosphorylation Complement C3 (C3) colocalizes with Aβ plaques and tau tangles; involved in synaptic remodeling and degeneration LS % Mean Difference from placebo at Day 15 (95% CI)

Relevant reference publications

Relevant reference publications (1 of 2) NO-sGC-cGMP signaling in the CNS Garthwaite, John. “Nitric oxide as a multimodal brain transmitter.” Brain and neuroscience advances vol. 2 2398212818810683.�4 Dec. 2018 Kleppisch T, Feil R. cGMP signaling in the mammalian brain: role in synaptic plasticity and behaviour. Handb Exp Pharmacol. 2009;(191):549-79 Ben Aissa M, Lee SH, Bennett BM, Thatcher GR. Targeting NO/cGMP Signaling in the CNS for Neurodegeneration and Alzheimer's Disease. Curr Med Chem. 2016;23(24):2770-2788 Hollas MA, Ben Aissa M, Lee SH, Gordon-Blake JM, Thatcher GRJ. Pharmacological manipulation of cGMP and NO/cGMP in CNS drug discovery. Nitric Oxide. 2019 Jan 1;82:59-74 qEEG spectral frequency analysis Ishii et al. Healthy and Pathological Brain Aging: From the Perspective of Oscillations, Functional Connectivity, and Signal Complexity. Neuropsychobiology, 2018 Babiloni, et al. Resting-state posterior alpha rhythms are abnormal in subjective memory complaint seniors with preclinical Alzheimer's neuropathology and high education level: the INSIGHT-preAD study. Neurobiol Aging. 2020;90:43-59

Relevant reference publications (2 of 2) Event-related potential (ERP): MMN, N200 and P300 Bennys K, Portet F, Touchon J. Diagnostic value of event-related evoked potentials N200 and P300 subcomponents in early diagnosis of Alzheimer’s disease and mild cognitive impairment. J Clin Neurophysiol 2007;24:405–12 Fruehwirt et al. Associations of event-related brain potentials and Alzheimer’s disease severity: A longitudinal study. Progress in Neuropsychopharmacology and Biological Psychiatry 92 (2019) 31-38 Saccadic eye movement (SEM) Wilcockson et al. Abnormalities of saccadic eye movements in dementia due to Alzheimer’s disease and mild cognitive impairment. Aging 2019, Vol.11, No.15 James A. Sharpe & David H. Zackon (1987) Senescent Saccades: Effects of Aging on Their Accuracy, Latency and Velocity, Acta Oto-Laryngologica, 104:5-6, 422-428 ADv Cortes-Canteli M, Iadecola C. Alzheimer’s Disease and Vascular Aging: JACC Focus Seminar. J Am Coll Cardiol. 2020;75(8):942-951 MELAS El-Hattab AW, Adesina AM, Jones J, Scaglia F. MELAS syndrome: Clinical manifestations, pathogenesis, and treatment options. Mol Genet Metab. 2015;116(1-2):4-12 CIAS Keefe RS, Harvey PD. Cognitive impairment in schizophrenia. Handb Exp Pharmacol. 2012;(213):11-37































































