Exhibit 99.1

Delivering impact in CNS diseases Investor webinar July 9, 2020

Safe Harbor Statement This presentation contains forward - looking statements. Any statements contained in this presentation that are not historical fac ts may be deemed to be forward looking statements. Words such as “anticipate,” “believe,” “potential,” “expect,” “may,” “will,” “should,” “could,” “plan,” “estimate,” “target,” “project,” “contemplate,” “intend,” “future,” “will,” “predict,” “continue,” an d the negative of these terms and similar expressions are intended to identify these forward - looking statements. These forward - looking statements are based on Cyclerion’s current expectations, projections and trends, are only predictions and involve known and unknown risks and uncertainties that could cause actual results to differ materially from those expressed o r implied in such statements. Investors are cautioned not to place undue reliance on these forward - looking statements, which include but are not limited to statements about possible or assumed future results of operations; preclinical, clinical and n on - clinical studies, the interpretation of data therefrom and the ability to replicate findings from such studies; business stra teg ies, research and development plans, collaborations, partnerships, out - licensing (including without limitation with respect to praliciguat), regulatory activities and any timing thereof; competitive position, potential growth or commercial opportunitie s; the clinical potential, application, commercialization or potential markets of or for any proposed products; the anticipated timi ng of release of data from any clinical trials; and the size and design of those clinical trials. Applicable risks and uncertainties include those listed under the heading “Risk Factors” and elsewhere in our most recent For m 1 0 - K filed with the SEC on March 12, 2020, and in our subsequent SEC filings, including our Quarterly Report on Form 10 - Q filed wit h the SEC on May 4, 2020. These forward - looking statements speak only as of the date of this presentation, and we undertake no obligation and do not intend to update these forward - looking statements. 2

Two priority disease areas creating multiple potential ways to win in sickle cell and CNS • genetically and phenotypically defined populations with high unmet need • harness power, signaling precision of sGC • biomarker - guided fast - to - POC trials underway • supported by discovery platform • attractive to investors and partners Innovative sGC platform for the NO - sGC - cGMP pathway • multi - dimensional pharmacology well - suited to disease biology • molecules tailored to the tissues relevant to the disease • wholly owned IP • validated class Creating value from pioneering approaches to SCD and CNS Sickle Cell CNS Building a company: our sGC science, pipeline and our team 3
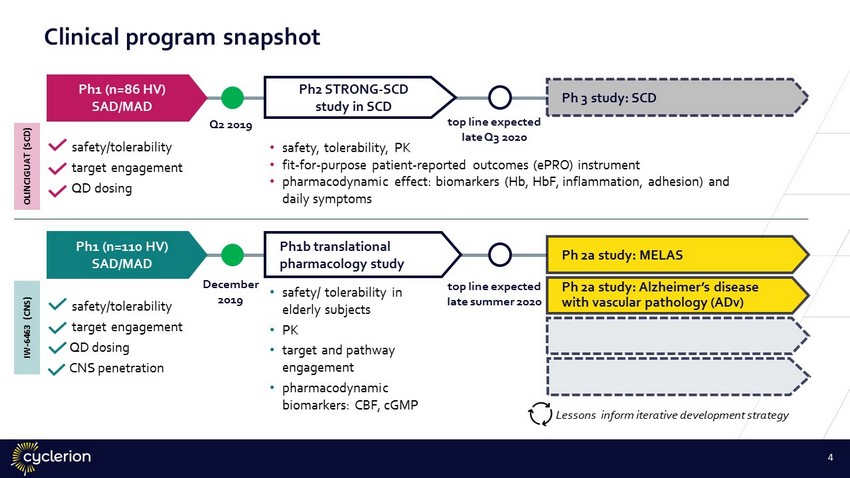
Clinical program snapshot 4 Ph1 (n=110 HV) SAD/MAD Ph 2a study: Alzheimer’s disease with vascular pathology (ADv) • safety/ tolerability in elderly subjects • PK • target and pathway engagement • pharmacodynamic biomarkers: CBF, cGMP December 2019 t op line expected late summer 2020 Ph1b translational pharmacology study Ph 2a study: MELAS safety/tolerability target engagement QD dosing CNS penetration Lessons inform iterative development strategy Ph2 STRONG - SCD study in SCD • safety, tolerability, PK • fit - for - purpose patient - reported outcomes (ePRO) instrument • pharmacodynamic effect: biomarkers (Hb, HbF, inflammation, adhesion) and daily symptoms Ph1 (n=86 HV) SAD/MAD safety/tolerability target engagement QD dosing t op line expected late Q3 2020 Ph 3 study: SCD Q2 2019 OLINCIGUAT (SCD) IW - 6463 (CNS)

Sickle Cell • potential for broad clinical utility in SCD • multi - dimensional mechanism that offers both upstream and downstream pharmacology • 70 patients enrolled; dosing completed • TL expected late Q3 2020 • Ph3 long - lead items underway: CMC, protocols, global ad board, regulatory plans • plan to develop and commercialize ourselves, but partnerships will be considered 5 Olinciguat: potential to raise the standard of care for sickle cell disease patients

• newly approved therapies each target a single clinical domain… • …olinciguat has the potential to improve four • daily symptoms and end - organ protection remain unaddressed, decreasing QoL and increasing mortality • further improvement in anemia and/or VOC possible with olinciguat alone or as add - on therapy serving broad SCD patient population 6 Potential for broad clinical utility improve daily symptoms olinciguat reduce anemia reduce painful crises (VOC) preserve organ function
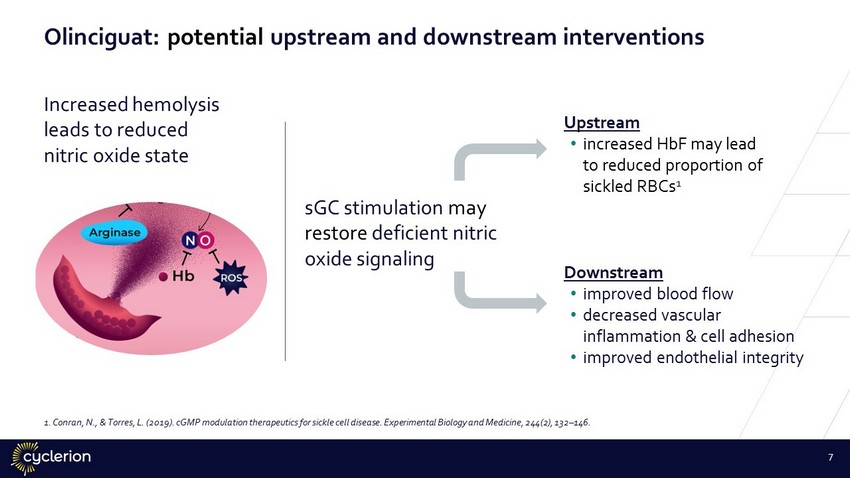
OLINCIGUAT sGC stimulation may restore deficient nitric oxide signaling Increased hemolysis leads to reduced nitric oxide state Upstream • increased HbF may lead to reduced proportion of sickled RBCs 1 Downstream • improved blood flow • decreased vascular inflammation & cell adhesion • improved endothelial integrity 1. Conran, N., & Torres, L. (2019). cGMP modulation therapeutics for sickle cell disease. Experimental Biology and Medicine, 244 (2), 132 – 146. 7 Olinciguat: potential upstream and downstream interventions
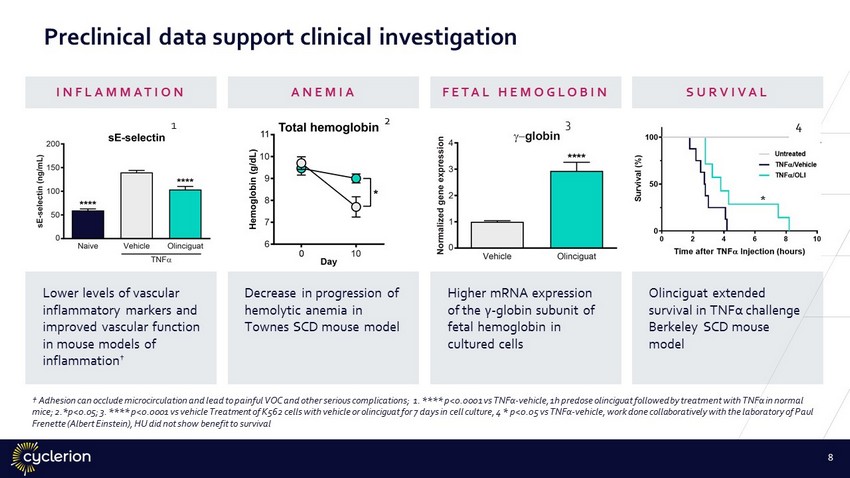
1 2 3 4 * Decrease in progression of hemolytic anemia in Townes SCD mouse model Higher mRNA expression of the γ - globin subunit of fetal hemoglobin in cultured cells Olinciguat extended survival in TNF α challenge Berkeley SCD mouse model † Adhesion can occlude microcirculation and lead to painful VOC and other serious complications; 1. **** p<0.0001 vs TNF α - vehicle, 1h predose olinciguat followed by treatment with TNFα in normal mice; 2.*p<0.05; 3. **** p<0.0001 vs vehicle Treatment of K562 cells with vehicle or olinciguat for 7 days in cell culture, 4 * p<0.05 vs TNF α - vehicle, work done collaboratively with the laboratory of Paul Frenette (Albert Einstein), HU did not show benefit to survival 8 Preclinical data support clinical investigation Lower levels of vascular inflammatory markers and improved vascular function in mouse models of inflammation † INFLAMMATION ANEMIA FETAL HEMOGLOBIN SURVIVAL

1h predose olinciguat followed by treatment with TNF α in normal mice Lower expression of cellular adhesion molecules associated with olinciguat treatment in preclinical model † † in models of vascular inflammation, * p<0.05, ** p<0.001; **** p<0.0001 vs TNF α - vehicle 9
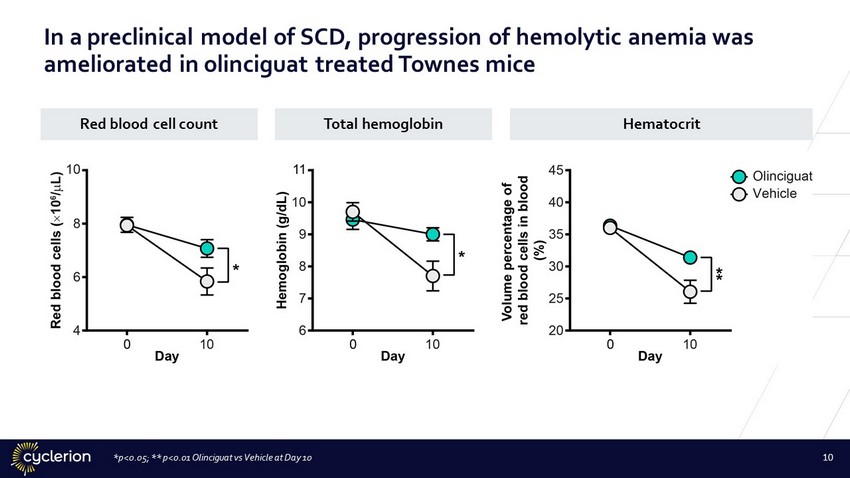
*p<0.05; ** p<0.01 Olinciguat vs Vehicle at Day 10 10 In a preclinical model of SCD, progression of hemolytic anemia was ameliorated in olinciguat treated Townes mice Red blood cell count Total hemoglobin Hematocrit
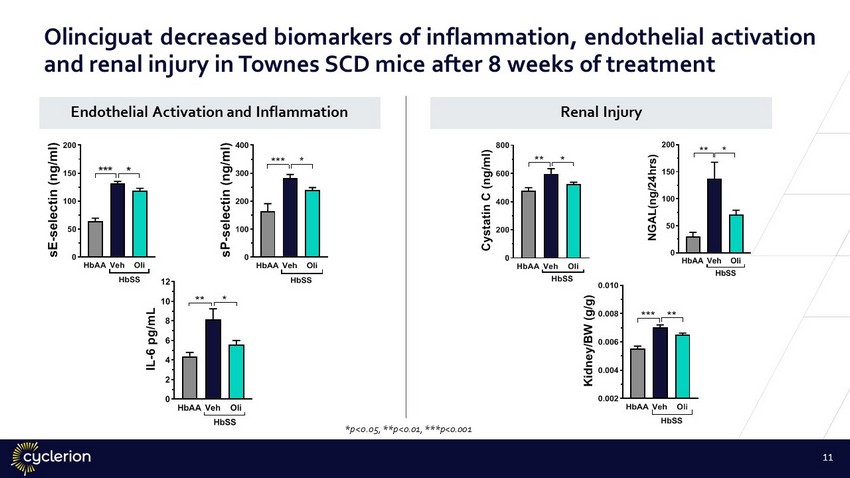
HbAAVeh Oli 0 100 200 300 400 s P - s e l e c t i n ( n g / m l ) HbSS *** * HbAAVeh Oli 0 50 100 150 200 s E - s e l e c t i n ( n g / m l ) HbSS *** * HbAAVeh Oli 0 2 4 6 8 10 12 I L - 6 p g / m L ** * HbSS HbAAVeh Oli 0 200 400 600 800 C y s t a t i n C ( n g / m l ) ** * HbSS HbAAVeh Oli 0.002 0.004 0.006 0.008 0.010 K i d n e y / B W ( g / g ) HbSS *** ** Endothelial Activation and Inflammation HbAAVeh Oli 0 50 100 150 200 N G A L ( n g / 2 4 h r s ) ** * HbSS Renal Injury *p<0.05, **p<0.01, ***p<0.001 11 Olinciguat decreased biomarkers of inflammation, endothelial activation and renal injury in Townes SCD mice after 8 weeks of treatment
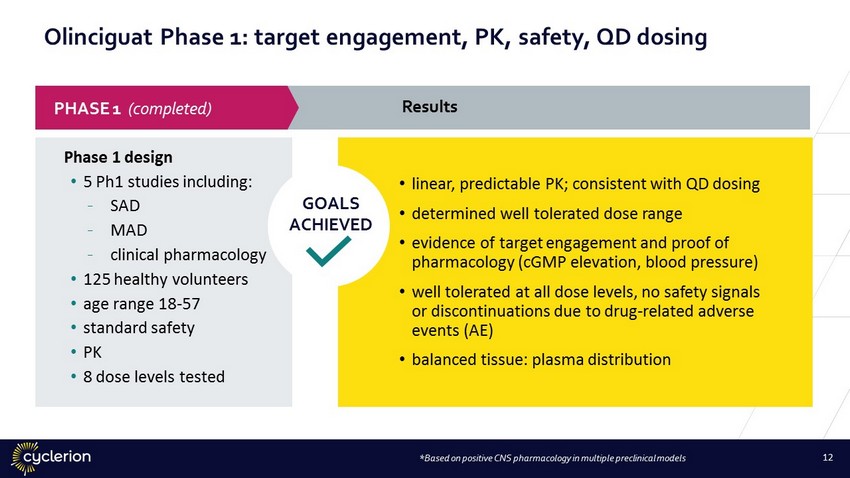
Olinciguat Phase 1: target engagement, PK, safety, QD dosing Phase 1 design • 5 Ph1 studies including: - SAD - MAD - clinical pharmacology • 125 healthy volunteers • age range 18 - 57 • standard safety • PK • 8 dose levels tested • linear, predictable PK; consistent with QD dosing • determined well tolerated dose range • evidence of target engagement and proof of pharmacology (cGMP elevation, blood pressure) • well tolerated at all dose levels, no safety signals or discontinuations due to drug - related adverse events (AE) • balanced tissue: plasma distribution *Based on positive CNS pharmacology in multiple preclinical models Results PHASE 1 (completed) GOALS ACHIEVED 12

Structure • 70 patients enrolled in all SCD genotypes, aged 16 – 70 • placebo controlled, double blind • 4 dose levels • 12 - week treatment Objectives • assess safety and tolerability • confirm PK profile in SCD patients • development of the CYCN fit - for - purpose patient - reported outcomes (ePRO) instrument • signs of pharmacodynamic effect: biomarkers ( Hb, HbF , inflammation, adheion) and daily symptom effects Insights for Phase 3 design • endpoint selection based on results (biomarkers and ePRO) • determine study sample size and dose(s) Topline results expected late Q3 2020 Olinciguat phase 2 trial designed to support rapid advancement 13
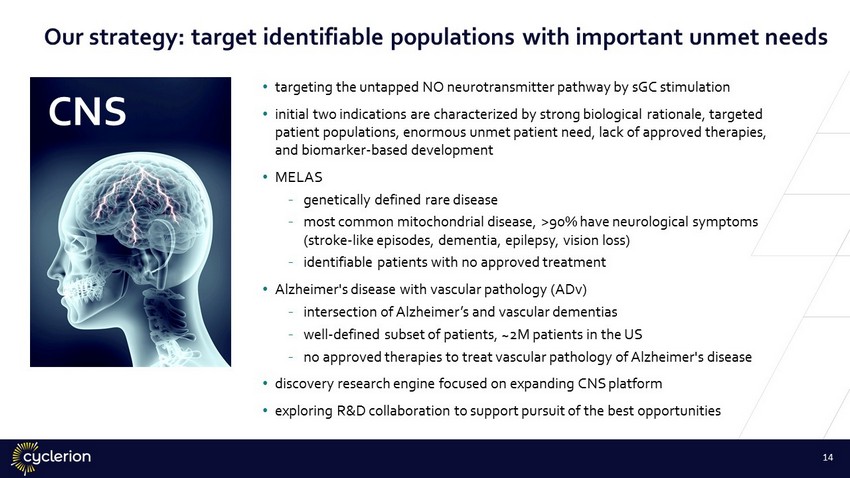
• targeting the untapped NO neurotransmitter pathway by sGC stimulation • initial two indications are characterized by strong biological rationale, targeted patient populations, enormous unmet patient need, lack of approved therapies, and biomarker - based development • MELAS - genetically defined rare disease - most common mitochondrial disease, >90% have neurological symptoms (stroke - like episodes, dementia, epilepsy, vision loss) - identifiable patients with no approved treatment • Alzheimer's disease with vascular pathology (ADv) - intersection of Alzheimer’s and vascular dementias - well - defined subset of patients, ~2M patients in the US - no approved therapies to treat vascular pathology of Alzheimer's disease • discovery research engine focused on expanding CNS platform • exploring R&D collaboration to support pursuit of the best opportunities CNS 14 Our strategy: target identifiable populations with important unmet needs

clear biological rationale geno/ phenotypically defined populations • pursue multiple indications in parallel • leverage biomarkers to drive development • implement nimble trials with leading edge investigators and imaging analytics • investigate a strategic R&D partnership to explore full potential of sGC in the CNS Raising the odds of success: important patient need IW - 6463 Our approach: intersection of patients and biology 15

Nitric oxide • IW - 6463 GABAergic • Valium® (1963) • Ambien® (1992) Dopaminergic • Levodopa (1970) • Risperdal® (1993) Cholinergic • Scopolamine (1979) • Aricept® (1996) Adrenergic/Serotonergic • Amitriptyline (1961) • Prozac® (1987) • Paxil® (1992) Successfully drugged neurotransmitter systems Glutamatergic • Ketamine (1970) • Namenda® (2003) 16 sGC stimulators: potential to be next druggable neurotransmitter system
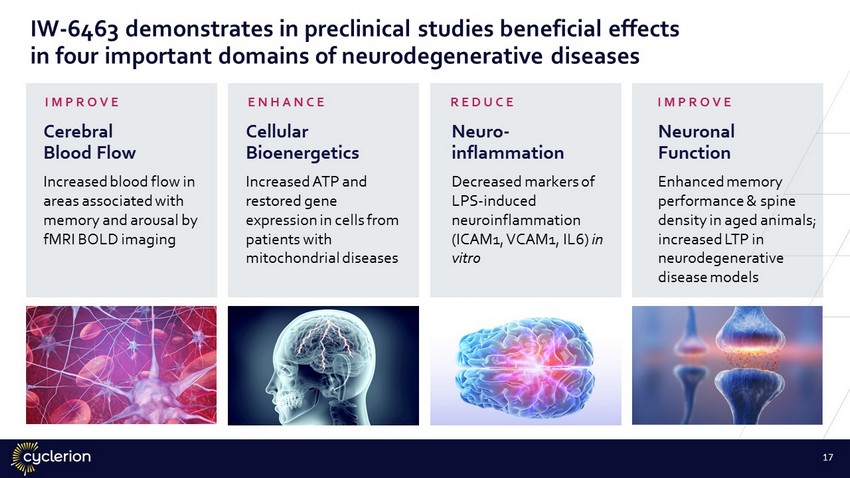
IW - 6463 demonstrates in preclinical studies beneficial effects in four important domains of neurodegenerative diseases Cellular Bioenergetics Increased ATP and restored gene expression in cells from patients with mitochondrial diseases Cerebral Blood Flow Increased blood flow in areas associated with memory and arousal by fMRI BOLD imaging Neuro - inflammation Decreased markers of LPS - induced neuroinflammation (ICAM1, VCAM1, IL6) in vitro Neuronal Function Enhanced memory performance & spine density in aged animals; increased LTP in neurodegenerative disease models ENHANCE IMPROVE REDUCE IMPROVE 17

IW - 6463 preclinical results support potential broad use in CNS treatment Cellular Bioenergetics Cerebral Blood Flow Neuro - inflammation Neuronal Function ENHANCE IMPROVE REDUCE IMPROVE Inflammatory cytokine in rat brain 3D microtissues V e h i c l e A M P K a c t i v a t o r F C C P ( 1 0M ) I W - 6 4 6 3 ( 0 . 1 M ) 0 100 200 300 ATP (pmole/ug protein) * ** Mitochondrial disease patient cells Maze performance in aged rats 18
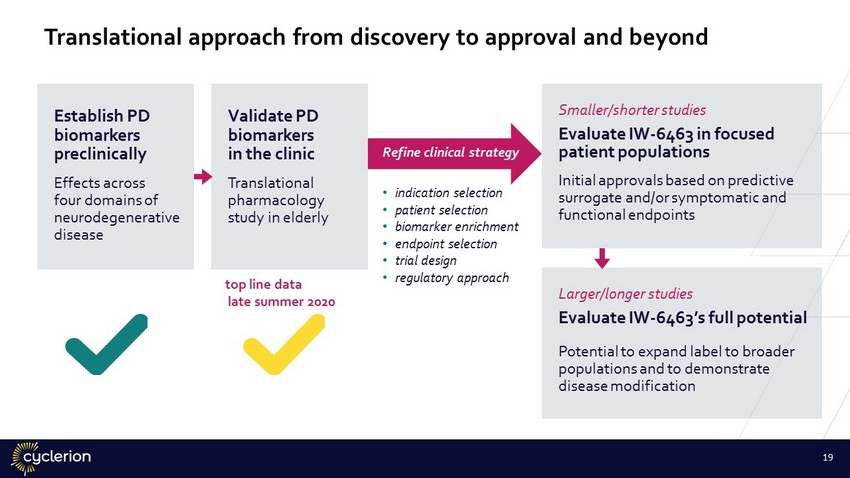
Translational approach from discovery to approval and beyond • indication selection • patient selection • biomarker enrichment • endpoint selection • trial design • regulatory approach top line data late summer 2020 Establish PD biomarkers preclinically Effects across four domains of neurodegenerative disease Validate PD biomarkers in the clinic Translational pharmacology study in elderly Smaller/shorter studies Evaluate IW - 6463 in focused patient populations Initial approvals based on predictive surrogate and/or symptomatic and functional endpoints Larger/longer studies Evaluate IW - 6463’s full potential Potential to expand label to broader populations and to demonstrate disease modification Refine clinical strategy 19
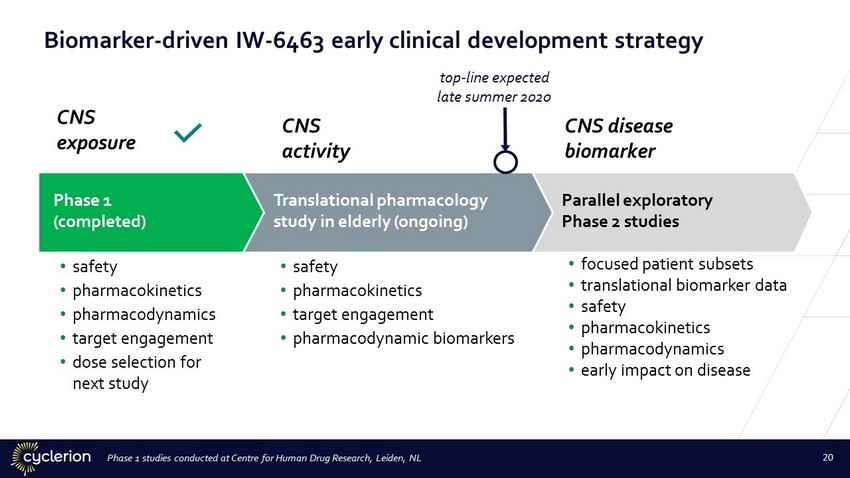
Translational pharmacology study in elderly (ongoing) • safety • pharmacokinetics • target engagement • pharmacodynamic biomarkers Phase 1 (completed) • safety • pharmacokinetics • pharmacodynamics • target engagement • dose selection for next study Parallel exploratory Phase 2 studies • focused patient subsets • translational biomarker data • safety • pharmacokinetics • pharmacodynamics • early impact on disease Biomarker - driven IW - 6463 early clinical development strategy CNS exposure CNS activity CNS disease biomarker t op - line expected late summer 2020 Phase 1 studies conducted at Centre for Human Drug Research, Leiden, NL 20

IW - 6463 Phase 1: CNS exposure, target engagement, PK, and safety Study design • three stages: - SAD - MAD - food interaction • 110 healthy volunteers • age range 18 - 63 • standard safety • PK (blood & CSF) • wide dose range tested • identified safe and well - tolerated dose levels with steady - state CNS exposure in therapeutic target range* • linear, predictable PK; consistent with QD dosing • CNS exposure confirmed • evidence of target engagement (blood pressure) • well tolerated at all dose levels, no safety signals • may be taken with or without food *Based on positive CNS pharmacology in multiple preclinical models Results PHASE 1 (completed) GOALS ACHIEVED 21

Cellular Bioenergetics • brain metabolism via magnetic resonance spectroscopy (MRS) Cerebral Blood Flow • MRI arterial spin labeling (ASL ) Neuro - inflammation • cytokines, adhesion molecules Neuronal Function • qEEG • measures of cognition and behavior ( NeuroCart ®) ENHANCE IMPROVE REDUCE IMPROVE Assessing safety, PK and target engagement in CNS (cGMP) Top line data expected late summer 2020 24 elderly subjects IW - 6463 QD placebo 15 days 15 days IW - 6463 QD placebo washout 22 Translational study design: pharmacodynamic biomarkers and safety
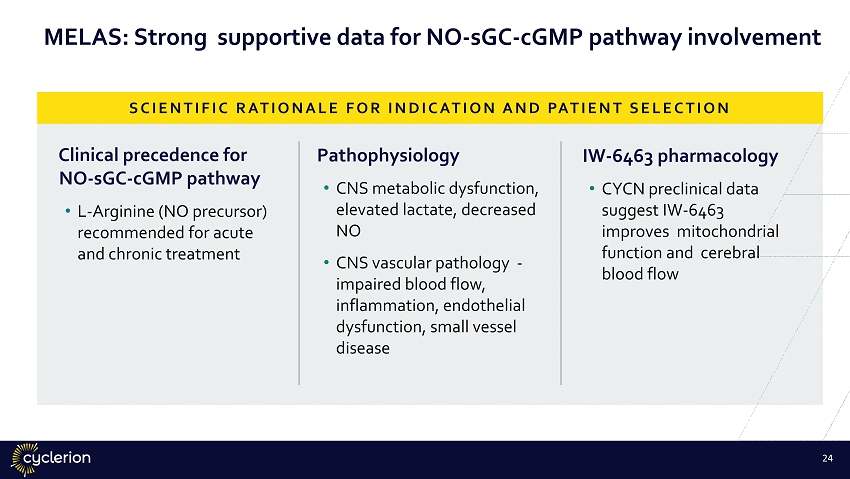
MELAS: Strong supportive data for NO - sGC - cGMP pathway involvement Clinical precedence for NO - sGC - cGMP pathway • L - Arginine (NO precursor) recommended for acute and chronic treatment Pathophysiology • CNS metabolic dysfunction, elevated lactate, decreased NO • CNS vascular pathology - impaired blood flow, inflammation, endothelial dysfunction, small vessel disease IW - 6463 pharmacology • CYCN preclinical data suggest IW - 6463 improves mitochondrial function and cerebral blood flow SCIENTIFIC RATIONALE FOR INDICATION AND PATIENT SELECTION 23
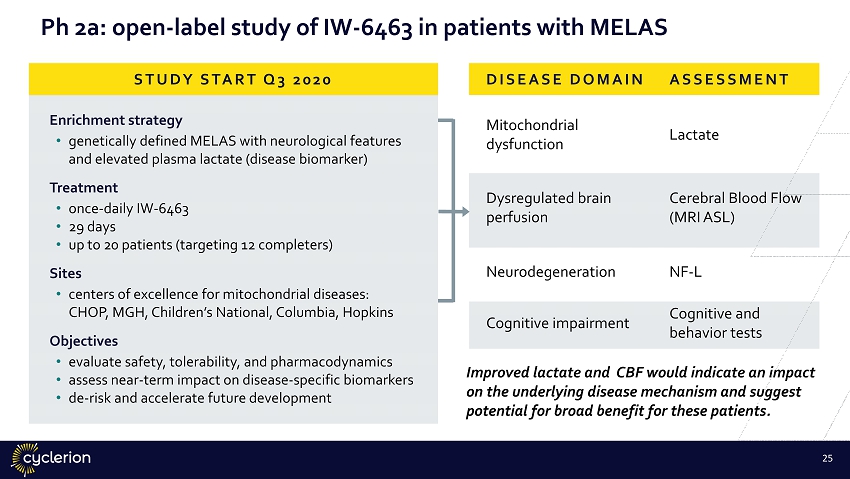
Ph 2a: open - label study of IW - 6463 in patients with MELAS Enrichment strategy • genetically defined MELAS with neurological features and elevated plasma lactate (disease biomarker) Treatment • once - daily IW - 6463 • 29 days • up to 20 patients (targeting 12 completers) Sites • centers of excellence for mitochondrial diseases: CHOP, MGH, Children’s National, Columbia, Hopkins Objectives • evaluate safety, tolerability, and pharmacodynamics • assess near - term impact on disease - specific biomarkers • de - risk and accelerate future development DISEASE DOMAIN ASSESSMENT Mitochondrial dysfunction Lactate Dysregulated brain perfusion Cerebral Blood Flow (MRI ASL) Neurodegeneration NF - L Cognitive impairment Cognitive and behavior tests STUDY START Q3 2020 Improved lactate and CBF would indicate an impact on the underlying disease mechanism and suggest potential for broad benefit for these patients. 24
Alzheimer’s Vascular mixed dementia Pathophysiology NO dysregulation, endothelial cell loss, impaired blood flow, vascular leakage, inflammation, neuronal dysfunction, and neuronal loss are major contributing factors to rapid disease progression Standard of care No approved therapies to treat vascular dementia. AD therapies offer limited benefits; not disease modifying Pharmacology Our preclinical data suggests IW - 6463 has potential to improve cerebral blood flow, endothelial health, neuroinflammation, and cellular energetics as well as prevent neurodegeneration Alzheimer’s Association,, Rizzi et al., NCI Analysis Target population ADv : an identifiable subset of mixed dementia patients with: • AD pathology AND • sub - cortical vascular disease AND • CV risk factors ADv AD with vascular pathology (ADv) – focused mixed dementia subset Defined population well suited for treatment with IW - 6463 DISEASE RATIONALE FOR PATIENT SELECTION 25

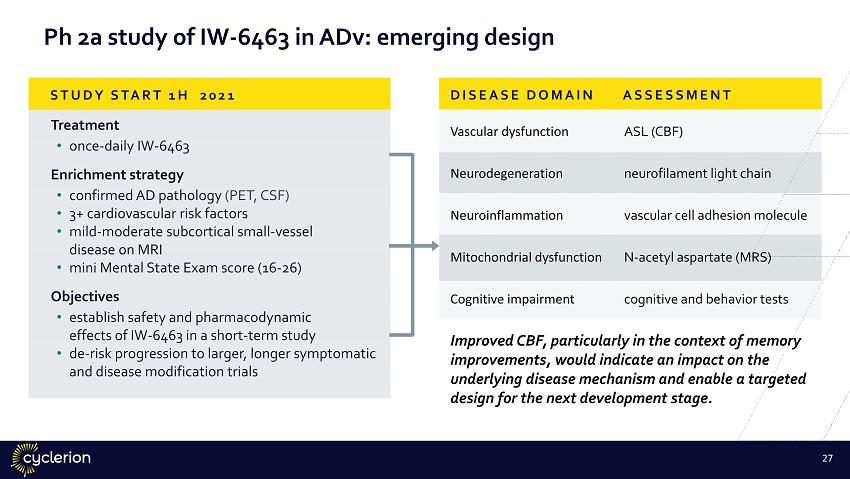
Treatment • once - daily IW - 6463 Enrichment strategy • confirmed AD pathology (PET, CSF) • 3+ cardiovascular risk factors • mild - moderate subcortical small - vessel disease on MRI • mini Mental State Exam score (16 - 26) Objectives • establish safety and pharmacodynamic effects of IW - 6463 in a short - term study • de - risk progression to larger, longer symptomatic and disease modification trials Vascular dysfunction ASL (CBF) Neurodegeneration neurofilament light chain Neuroinflammation vascular cell adhesion molecule Mitochondrial dysfunction N - acetyl aspartate (MRS) Cognitive impairment cognitive and behavior tests STUDY START 1H 2021 DISEASE DOMAIN ASSESSMENT Ph 2a study of IW - 6463 in ADv: emerging design Improved CBF, particularly in the context of memory improvements, would indicate an impact on the underlying disease mechanism and enable a targeted design for the next development stage. 26
• sGC stimulators: powerful intervention in a genetically and clinically validated pathway • a wholly owned pipeline of differentiated molecules • exploring partnerships across programs; praliciguat out - licensing scope expanded • experienced leadership team with a distinctive track record of innovative drug discovery and development • starting Q3 2020 with ~$61M cash*; supports our priorities into second half of 2021 • limited disruption from Covid - 19 Sickle Cell CNS * Preliminary, unaudited unrestricted cash, cash equivalents and restricted cash balance as of June 3o, 2020 27 Building our company: the science, the pipeline and the team Building a company: our sGC science, pipeline and our team
• multiple successful drugs target the NO - sGC - cGMP pathway for the treatment of CV diseases NO donors, PDE5 inhibitors, sGC stimulators • NO - sGC - cGMP pathway plays central role in CNS diseases Network analysis delivers z - scores for CNS diseases similar to validated CV diseases • sGC: optimal target for pathway intervention Broadly expressed in CNS, amplifies endogenous signaling, increases cGMP levels at the source with no attenuation of response Therapeutic effects PDE sGC stimulators: ideal intervention in a genetically and clinically validated pathway 28

SMOOTH MUSCLE & VASCULAR FUNCTION METABOLISM CELLULAR BIOENERGETICS NEURONAL FUNCTION INFLAMMATION 29 Broad impact in the NO - sGC - cGMP pathway
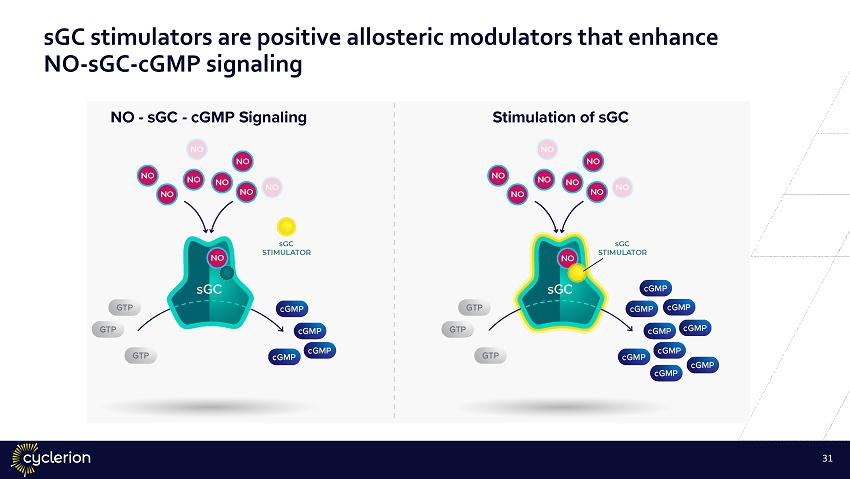
30 sGC stimulators are positive allosteric modulators that enhance NO - sGC - cGMP signaling
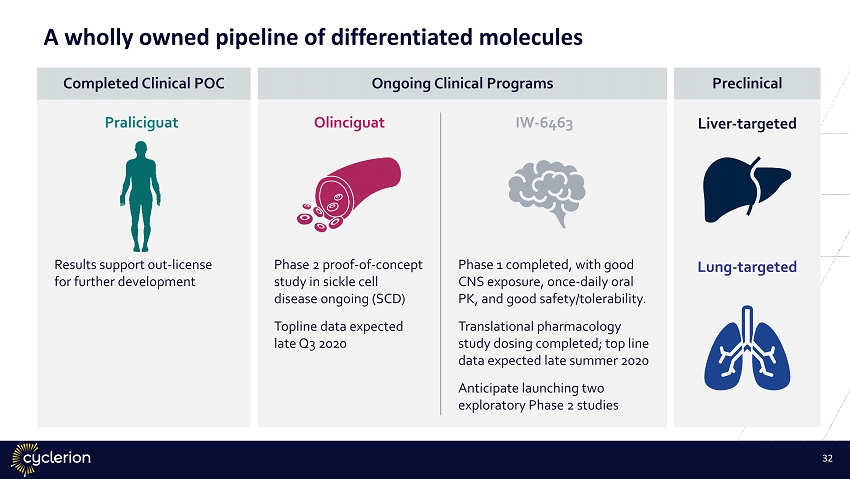
Liver - targeted Lung - targeted Preclinical Olinciguat Phase 2 proof - of - concept study in sickle cell disease ongoing (SCD) Topline data expected late Q3 2020 Praliciguat Results support out - license for further development IW - 6463 Phase 1 completed, with good CNS exposure, once - daily oral PK, and good safety/tolerability. Translational pharmacology study dosing completed; top line data expected late summer 2020 Anticipate launching two exploratory Phase 2 studies Ongoing Clinical Programs Completed Clinical POC 31 A wholly owned pipeline of differentiated molecules
• promising DN results: - UACR reductions on top of standard of care - reductions in blood pressure, HbA1c, total and LDL cholesterol - favorable safety profile, consistent with previous studies - attractive dosing and PK relative to others in class • VICTORIA results further validate cardiometabolic potential of the class and suggest potential for praliciguat as a cardio metabolic therapeutic Out - licensing discussions ongoing Data support further development • continuing discussions to out - license global rights to praliciguat • expanded the scope of its out - licensing discussions beyond cardiometabolic disorders to include additional indications where sGC stimulators have shown efficacy • no assurances on the prospects or timing of any partnership or licensing transactions -- generally or specifically on praliciguat 32 Praliciguat out - licensing discussions ongoing with expanded scope
• distinctive track record of innovative drug discovery/development (e.g. -- CELEBREX ® , KALYDECO ® , LINZESS ® , LUNESTA ® , OPDIVO ® , ORKAMBI ® , YERVOY ® ) • successful sGC/cGMP drug hunters; deep knowledge of nitric oxide (NO) - cGMP pathway • broad experience in creating strong organizations and commercializing products Experienced team and successful leadership 33

Delivering impact in CNS diseases Investor webinar July 9, 2020



































