Exhibit 99.3

Delivering impact in CNS diseases INVESTOR CALL - OCTOBER 14, 2020

Safe Harbor Statement This presentation contains forward - looking statements. Any statements contained in this presentation that are not historical fac ts may be deemed to be forward looking statements. Words such as “anticipate,” “believe,” “potential,” “expect,” “may,” “will,” “should ,” “could,” “plan,” “estimate,” “target,” “project,” “contemplate,” “intend,” “future,” “will,” “predict,” “continue,” and the negative of these ter ms and similar expressions are intended to identify these forward - looking statements. These forward - looking statements are based on Cyclerion’s current expectations, projections and trends, are only predictions and involve known and unknown risks and uncertainties that could cause actual results to differ materially from those expressed or implie d i n such statements. Investors are cautioned not to place undue reliance on these forward - looking statements, which include but are not l imited to statements about possible or assumed future results of operations; preclinical, clinical and non - clinical studies, the interpret ation of data therefrom and the ability to replicate findings from such studies; including statements about the results and conduct of our Pha se 1 translational pharmacology clinical trial of IW - 6463; our interpretation of the data from the clinical trial; the potential of f urther evaluation of IW - 6463; the clinical potential of IW - 6463; our future business focus; the anticipated timing of our planned clinical trials; bu siness strategies, research and development plans, collaborations, partnerships, out - licensing, regulatory activities and any timing thereof; compe titive position, potential growth or commercial opportunities; the clinical potential, application, commercialization or potential markets of or for any proposed products; the anticipated timing of release of data from any clinical trials; and the size and design of those clinical trial s. Applicable risks and uncertainties include those listed under the heading “Risk Factors” and elsewhere in our 2019 Form 10 - K filed on March 12, 2020, and in Cyclerion’s subsequent SEC filings, including the Form 10 - Q filed on May 4, 2020 and the Form 10 - Q filed on Aug ust 3, 2020 . These forward - looking statements speak only as of the date of this presentation, and we undertake no obligation and do not int end to update these forward - looking statements, except as required by law. 2

INTRODUCTION Peter Hecht, Chief Executive Officer 3

Results in healthy elderly subjects x favorable safety and tolerability x crossed the blood brain barrier (BBB) x pathway target engagement confirmed x delivered rapid, robust, and selective neurophysiological changes 4

Independent expert joining us today Andrew E. Budson , MD Chief of Cognitive & Behavioral Neurology, Associate Chief of Staff for Education, and Director of the Center for Translational Cognitive Neuroscience, Veterans Affairs (VA) Boston Healthcare System AFFILIATIONS: Associate Director for Research, Boston University Alzheimer’s Disease Center; Professor of Neurology, Boston University School of Medicine; Lecturer in Neurology, Harvard Medical School; and Medical Director, Boston Center for Memory (Newton, MA) 5

Agenda Translational pharmacology results 2 Introduction and overview 1 Q&A 4 Strategic focus 3 6
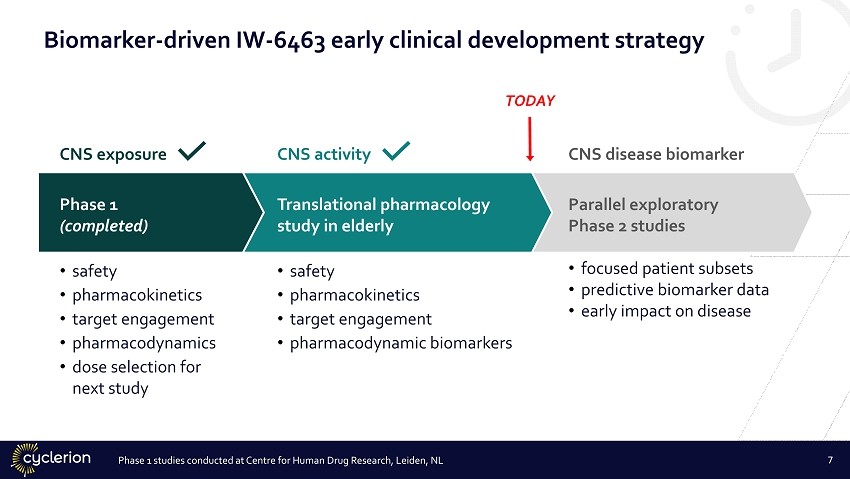
Translational pharmacology study in elderly • safety • pharmacokinetics • target engagement • pharmacodynamic biomarkers Phase 1 (completed) • safety • pharmacokinetics • target engagement • pharmacodynamics • dose selection for next study Parallel exploratory Phase 2 studies • focused patient subsets • predictive biomarker data • early impact on disease CNS exposure CNS activity CNS disease biomarker TODAY Phase 1 studies conducted at Centre for Human Drug Research, Leiden, NL Biomarker - driven IW - 6463 early clinical development strategy 7
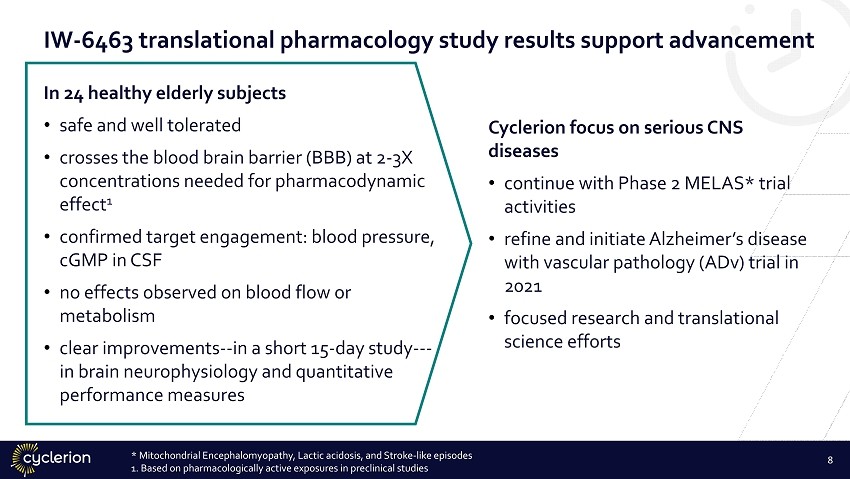
IW - 6463 translational pharmacology study results support advancement In 24 healthy elderly subjects • safe and well tolerated • crosses the blood brain barrier (BBB) at 2 - 3X concentrations needed for pharmacodynamic effect 1 • confirmed target engagement: blood pressure, cGMP in CSF • no effects observed on blood flow or metabolism • clear improvements -- in a short 15 - day study --- in brain neurophysiology and quantitative performance measures Cyclerion focus on serious CNS diseases • continue with Phase 2 MELAS* trial activities • refine and initiate Alzheimer’s disease with vascular pathology (ADv) trial in 2021 • focused research and translational science efforts * Mitochondrial Encephalomyopathy, Lactic acidosis, and Stroke - like episodes 1. Based on pharmacologically active exposures in preclinical studies 8

C6463 - 102 s tudy design and objectives 24 healthy elderly subjects completed period 1 IW - 6463 QD Placebo 15 days 15 days IW - 6463 QD Placebo washout 12 subjects completed both crossover periods* *Period 2 participation was reduced due to Covid - 19 related restrictions in the Netherlands OBJECTIVES TO ASSESS: Cerebral Blood Flow • MRI arterial spin labeling (ASL) Neuro - inflammation • cytokines, adhesion molecules x Activity in the CNS as measured in one or more of the following: Cellular Bioenergetics • brain metabolism via magnetic resonance spectroscopy (MRS) Neuronal Function • qEEG , ERP • cognition and behavior measures x Safety x PK x Target engagement (cGMP) 9

Activity in CNS: neuronal function Cellular Bioenergetics Cerebral Blood Flow Neuro Inflammation Neuronal Function Data analysis ongoing No significant effects observed in this healthy elderly study. MELAS study to assess effects of IW - 6463 on dysregulated metabolism in mitochondrial disease Encouraging impact on measures associated with aging/cognitive decline • significant increase in alpha power and increase in gamma • improvements in N200 ERP latencies (greater effects at older age) • Significantly shorter saccadic reaction times, trend increase in saccadic velocity No significant effects observed in this healthy elderly study 10
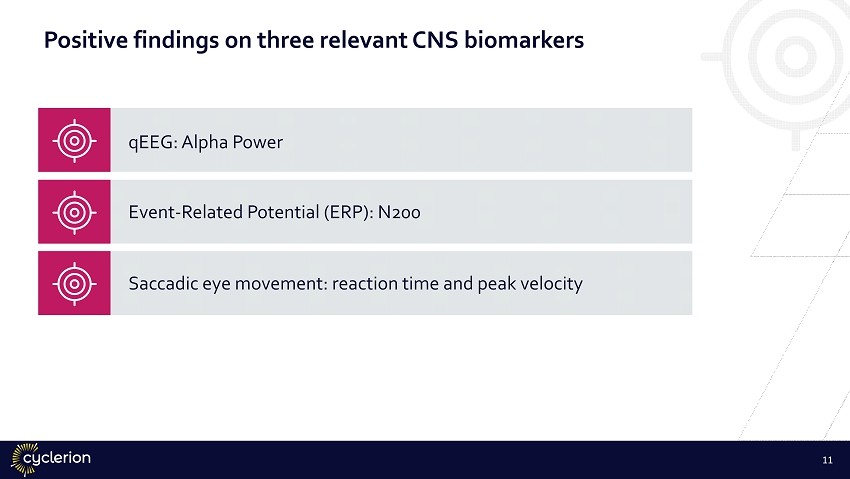
Positive findings on three relevant CNS biomarkers qEEG : Alpha Power Event - Related Potential (ERP): N200 Saccadic eye movement: reaction time and peak velocity 11
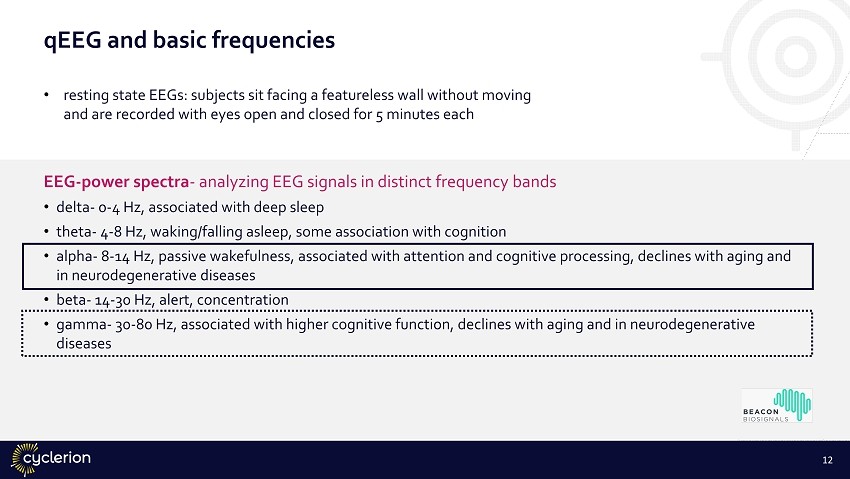
qEEG and basic frequencies EEG - power spectra - analyzing EEG signals in distinct frequency bands • delta - 0 - 4 Hz, associated with deep sleep • theta - 4 - 8 Hz, waking/falling asleep, some association with cognition • alpha - 8 - 14 Hz, passive wakefulness, associated with attention and cognitive processing, declines with aging and in neurodegenerative diseases • beta - 14 - 30 Hz, alert, concentration • gamma - 30 - 80 Hz, associated with higher cognitive function, declines with aging and in neurodegenerative diseases • resting state EEGs: subjects sit facing a featureless wall without moving and are recorded with eyes open and closed for 5 minutes each 12
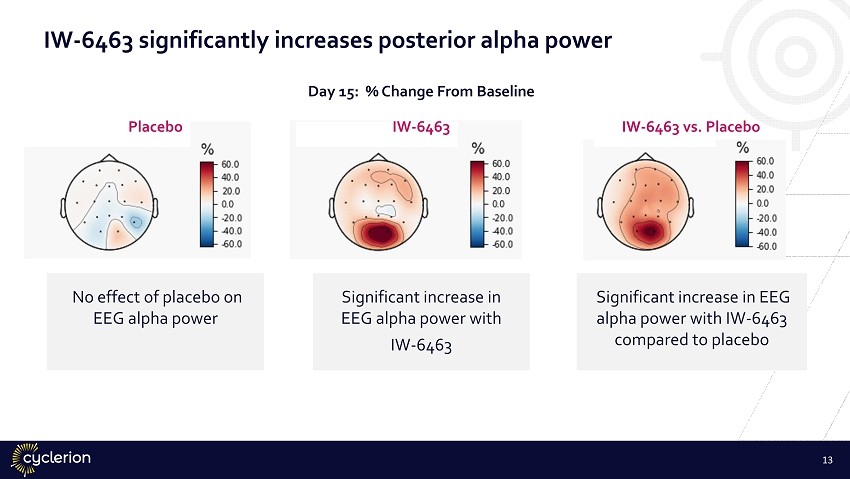
IW - 6463 significantly increases posterior alpha power Placebo IW - 6463 IW - 6463 vs. Placebo No effect of placebo on EEG alpha power Significant increase in EEG alpha power with IW - 6463 Significant increase in EEG alpha power with IW - 6463 compared to placebo 13 Day 15: % Change From Baseline
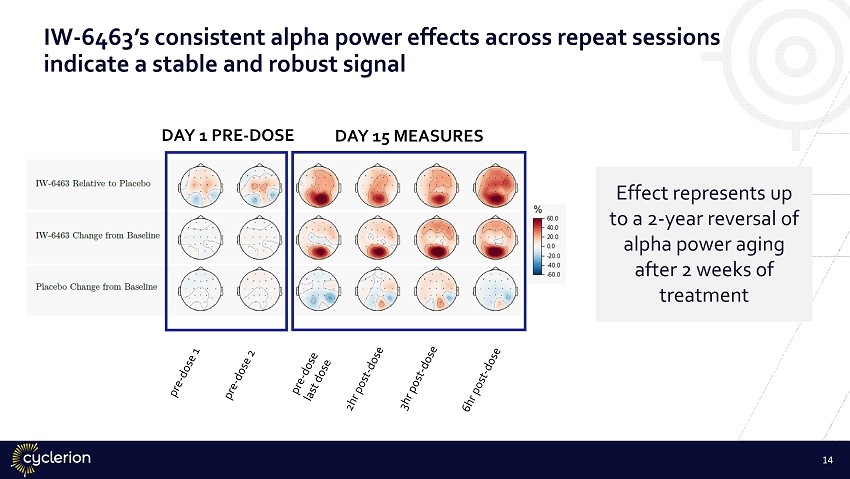
IW - 6463’s consistent alpha power effects across repeat sessions indicate a stable and robust signal DAY 1 PRE - DOSE DAY 15 MEASURES Effect represents up to a 2 - year reversal of alpha power aging after 2 weeks of treatment 14

IW - 6463 increases alpha power with consistent treatment responses 60 30 0 - 30 Placebo IW - 6463 Anterior (p = 0.0752) Change from Baseline (%) 15 Statistically significant alpha power increases Change in Closed - Eye Alpha (8 - 12 Hz) Power Persistent, consistent treatment responses Posterior Closed - Eye Alpha (8 - 12 Hz) Power • substantial 17% treatment effect • similar trends in anterior • 13/18 participants exhibit increasing alpha power with IW - 6463, vs 5/18 with placebo 1 • not driven by outliers 1. Includes all subjects. For IW - 6463 and pbo each: n=12 for period 1, n=6 for period 2
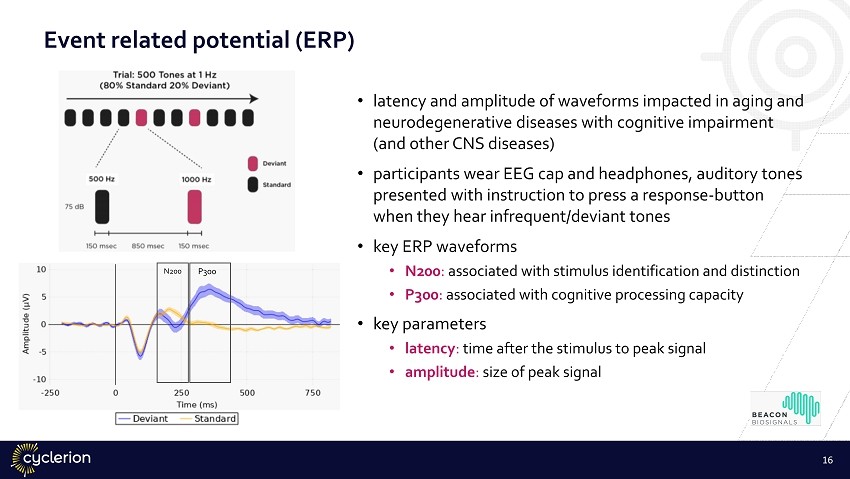
Event related potential (ERP) • latency and amplitude of waveforms impacted in aging and neurodegenerative diseases with cognitive impairment (and other CNS diseases) • participants wear EEG cap and headphones, auditory tones presented with instruction to press a response - button when they hear infrequent/deviant tones • key ERP waveforms • N200 : associated with stimulus identification and distinction • P300 : associated with cognitive processing capacity • key parameters • latency : time after the stimulus to peak signal • amplitude : size of peak signal 16 N200 P300

IW - 6463 improves N200 latency with a greater age - associated effect p=.016 p=.02 • significant latency reduction of N200 response to IW - 6463 in participants treated for 15 days compared to those untreated. • N200 response to IW - 6463 in participants older and younger than 70. • latency response is significantly stronger with older age. • narrowing of the variance in 70+ supports a drug effect. 17
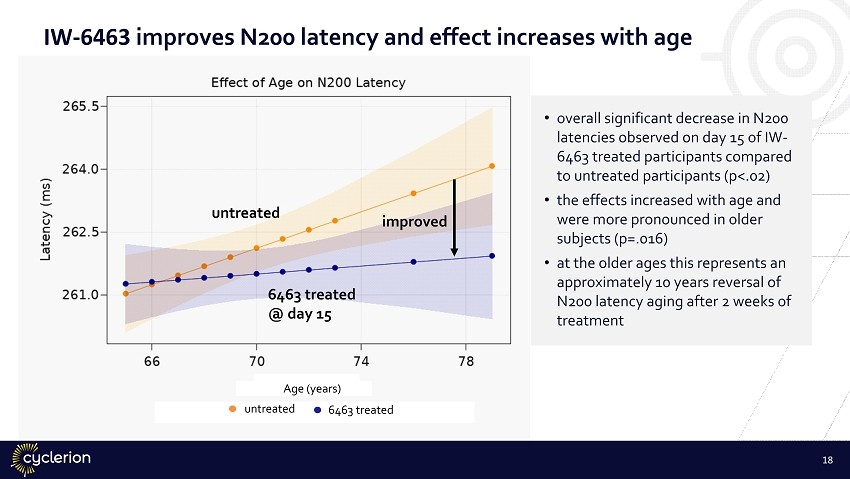
IW - 6463 improves N200 latency and effect increases with age untreated 6463 treated @ day 15 untreated 6463 treated • overall significant decrease in N200 latencies observed on day 15 of IW - 6463 treated participants compared to untreated participants (p<.02) • the effects increased with age and were more pronounced in older subjects (p=.016) • at the older ages this represents an approximately 10 years reversal of N200 latency aging after 2 weeks of treatment Age (years) improved 18
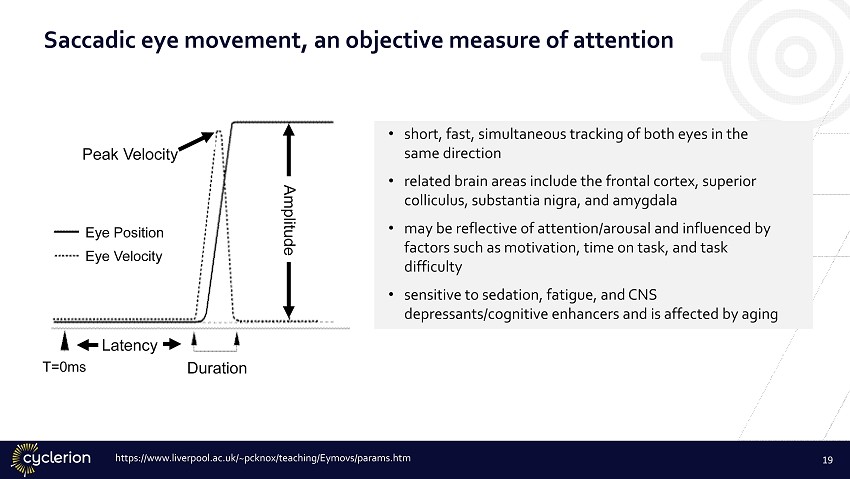
Saccadic eye movement, an objective measure of attention • short, fast, simultaneous tracking of both eyes in the same direction • related brain areas include the frontal cortex, superior colliculus, substantia nigra, and amygdala • may be reflective of attention/arousal and influenced by factors such as motivation, time on task, and task difficulty • sensitive to sedation, fatigue, and CNS depressants/cognitive enhancers and is affected by aging https://www.liverpool.ac.uk/~pcknox/teaching/Eymovs/params.htm Peak Velocity Amplitude Eye Position Eye Velocity Latency Duration T=0ms 19
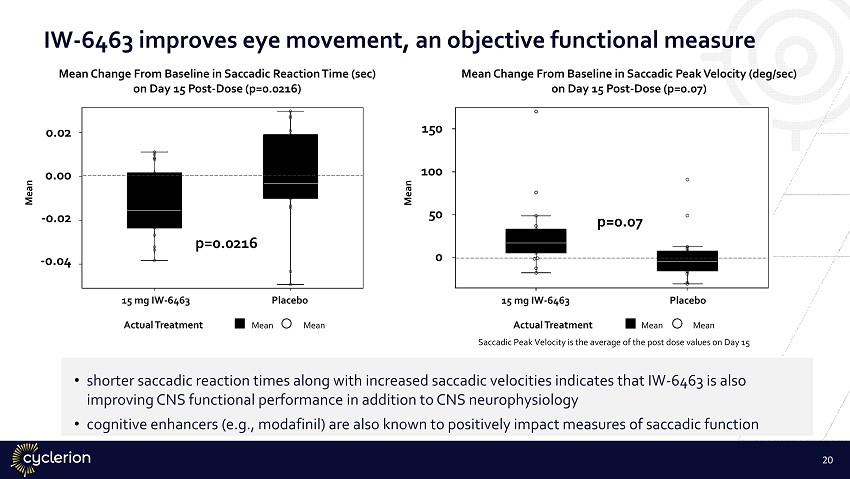
IW - 6463 improves eye movement, an objective functional measure Actual Treatment Mean Mean 0.02 0.00 - 0.02 - 0.04 15 mg IW - 6463 Placebo Mean Mean Change From Baseline in Saccadic Reaction Time (sec) on Day 15 Post - Dose (p=0.0216) Actual Treatment Mean Mean Saccadic Peak Velocity is the average of the post dose values on Day 15 150 100 50 0 15 mg IW - 6463 Placebo Mean Mean Change From Baseline in Saccadic Peak Velocity (deg/sec) on Day 15 Post - Dose (p=0.07) • shorter saccadic reaction times along with increased saccadic velocities indicates that IW - 6463 is also improving CNS functional performance in addition to CNS neurophysiology • cognitive enhancers (e.g., modafinil) are also known to positively impact measures of saccadic function 20 p=0.0216 p=0.07
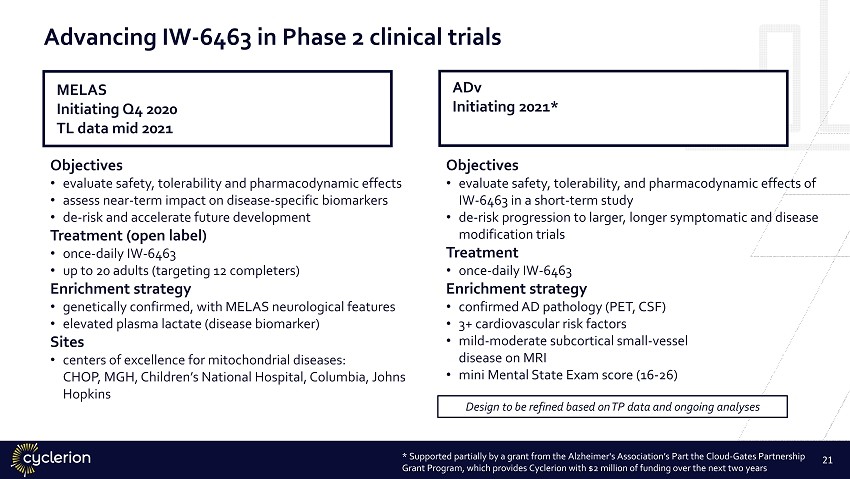
Advancing IW - 6463 in Phase 2 clinical trials 21 Objectives • evaluate safety, tolerability, and pharmacodynamic effects of IW - 6463 in a short - term study • de - risk progression to larger, longer symptomatic and disease modification trials Treatment • once - daily IW - 6463 Enrichment strategy • confirmed AD pathology (PET, CSF) • 3+ cardiovascular risk factors • mild - moderate subcortical small - vessel disease on MRI • mini Mental State Exam score (16 - 26) Objectives • evaluate safety, tolerability and pharmacodynamic effects • assess near - term impact on disease - specific biomarkers • de - risk and accelerate future development Treatment (open label) • once - daily IW - 6463 • up to 20 adults (targeting 12 completers) Enrichment strategy • genetically confirmed, with MELAS neurological features • elevated plasma lactate (disease biomarker) Sites • centers of excellence for mitochondrial diseases: CHOP, MGH, Children’s National Hospital, Columbia, Johns Hopkins MELAS Initiating Q4 2020 TL data mid 2021 ADv Initiating 2021* Design to be refined based on TP data and ongoing analyses * Supported partially by a grant from the Alzheimer's Association’s Part the Cloud - Gates Partnership Grant Program, which provides Cyclerion with $2 million of funding over the next two years
Immediate Execution • deepen understanding of biology and powerful pharmacology • MELAS study • ADv study • strategic partnership Portfolio Growth Our commitment to CNS 22 Portfolio Advancement Focused CNS company foundation • capital allocation to CNS development and pipeline building • pursuing a risk - reduced development approach • organization size and capabilities for CNS distinctiveness Mid - term Long - term Current Now

23 Thank you for joining x significant improvements observed in neurophysiological and objective performance measures x moving forward in MELAS and ADv , informed by these data x Phase 2 data in 2021 x CNS focus as a company

Delivering impact in CNS diseases INVESTOR CALL - OCTOBER 14, 2020























