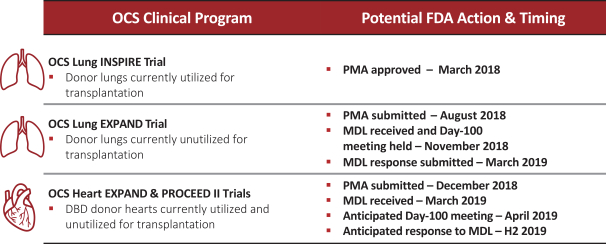
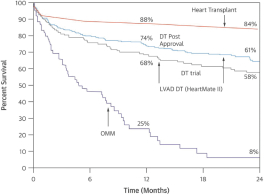
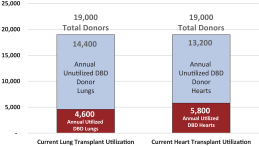
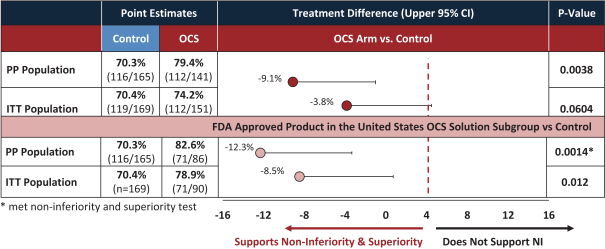
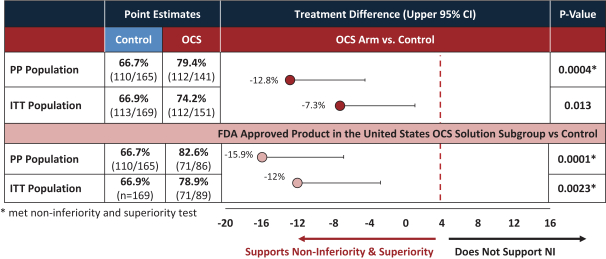
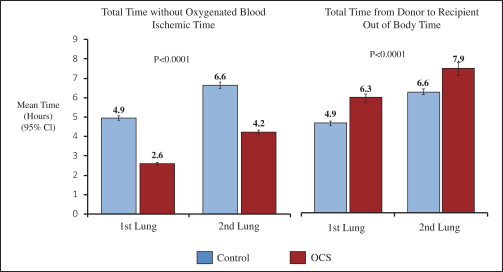
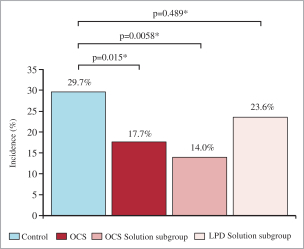
intended for transplantation. This is a two-armed, multi-center, randomized, controlled pivotal trial with participants assigned to the OCS treatment arm or the control arm, which uses cold storage. Target enrollment for completion of the study is a total of 300 patients. The trial is currently enrolling patients with 199 out of 300 patients enrolled at leading academic U.S. liver transplant centers as of March 30, 2019.
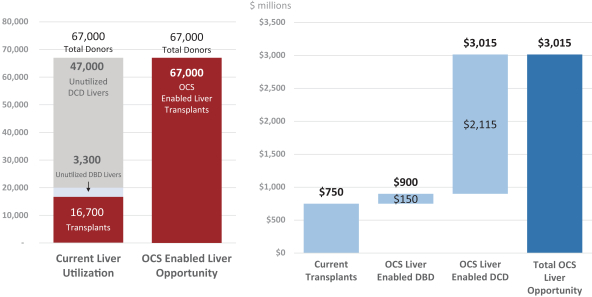
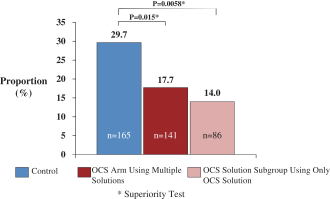
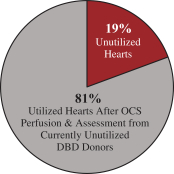
Additionally, our OCS Liver European REVIVE Trial, which was a single arm, prospective trial of 25 transplanted liver recipients, evaluated the safety and performance of the OCS Liver. The primary performance endpoint was the number of donor livers preserved by the OCS Liver in a near-physiologic state. The primary safety endpoint was the number of events directly related to the use of the OCS Liver that led to the donor liver being deemed not clinically acceptable and, consequently, not transplanted. Results from the OCS Liver European REVIVE Trial demonstrated that the OCS Liver resulted in 100% utilization of DBD and DCD donor livers.
Intellectual Property
Patents and Trade Secrets
We rely on a combination of patent, trademark, copyright, trade secret and other intellectual property laws, nondisclosure and assignment of inventions agreements and other measures to protect our intellectual property. Our patent portfolio includes patents and patent applications that we own or license from third parties.
As of March 30, 2019, our owned and licensed patent portfolio consisted of approximately 188 issued patents and pending patent applications worldwide, including in Australia, Europe, Canada, China, Israel, New Zealand and Japan. Our licensed portfolio includes one issued unexpired United States patent licensed from the VA. Several other licensed U.S. and international patents expired in 2018. The issued unexpired licensed VA patent includes claims directed to portable perfusion apparatus for preserving a harvested donor heart in a viable state. Our owned portfolio includes patents and applications related to one or more of the OCS Lung, OCS Heart, OCS Liver and solutions. In the United States, our owned portfolio includes about 22 issued patents and 9 pending applications. Worldwide, our owned portfolio includes about 99 issued patents and 58 pending applications. Issued patents in our portfolio are expected to expire between 2019 and 2036, excluding any potential additional patent term for patent term adjustments or patent term extensions, if applicable. If granted, the pending U.S. and foreign patent applications in our portfolio are expected to expire between 2023 and 2036, excluding any potential additional patent term for patent term adjustments or patent term extensions, if applicable.
As of March 30, 2019, our patent portfolio relating to the OCS Lung includes a family comprised of patents and patent applications with claims that are generally directed to certain methods and systems for preserving a lungex vivo using both perfusion and ventilation. Such patents are issued in the United States, Australia, Canada, China, Israel, Japan, Hong Kong and New Zealand and patent applications are pending in the United States, Australia, Canada, China, Europe, Hong Kong, Israel, Japan and New Zealand. These patents, and any patents issued from pending patent applications, are expected to expire in 2029, excluding any potential additional patent term for patent term adjustments or patent term extensions, if applicable.
As of March 30, 2019, our patent portfolio relating to the OCS Heart includes a family comprised of patents and patent applications with claims that are generally directed to certain methods and systems for preserving a heartex vivo. Such patents are issued in the United States, Australia, Belgium, Canada, China, Germany, Denmark, Europe, Spain, France, United Kingdom, Hong Kong, Ireland, Israel, Italy, Japan, The Netherlands, New Zealand and Sweden and patent applications are pending in the United States, Australia, Canada, Israel, Japan and New Zealand. These patents, and any patents issued from pending patent applications, are expected to expire in 2025, excluding any potential additional patent term for patent term adjustments or patent term extensions, if applicable.
122