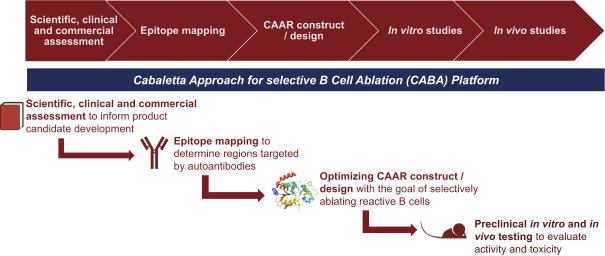
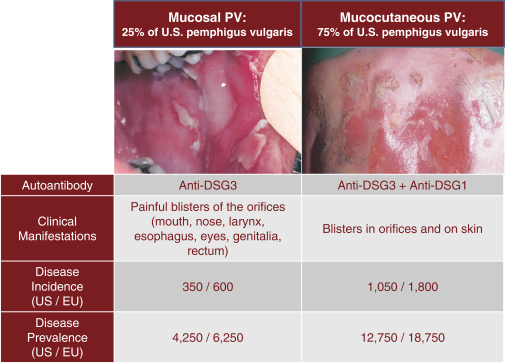
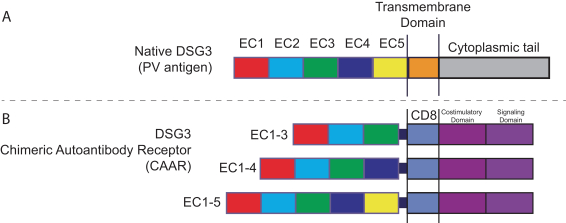
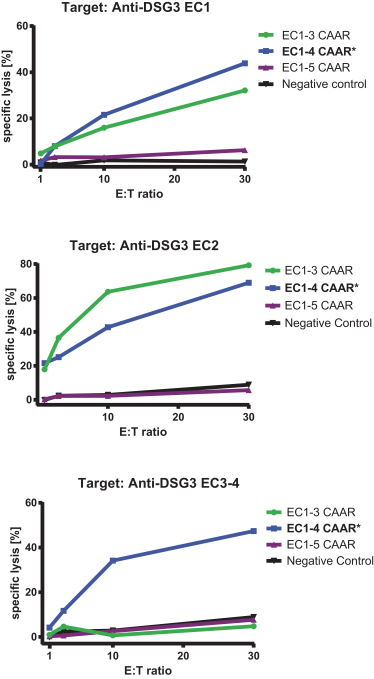
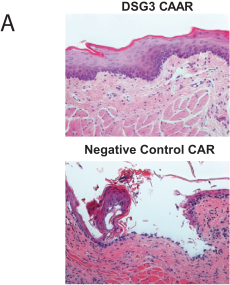
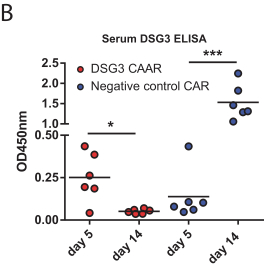
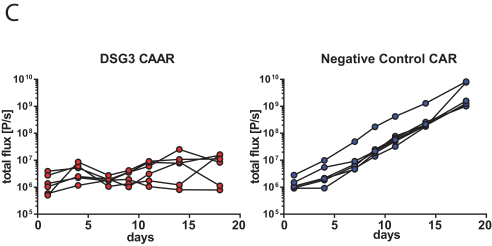
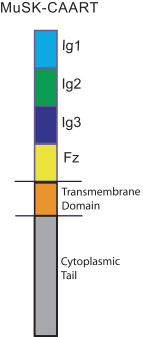
With regard to our FVIII-CAAR T cell product candidate, we have one pending U.S. patent application and counterpart patent applications pending in Australia, Canada, China, Europe, Japan, Korea, Mexico, New Zealand, and Russia, which if issued, would be expected to expire in 2037. This patent family isco-owned by Penn and CHOP and is exclusively licensed to us in the field of the license.
The term of individual patents depends upon the legal term of the patents in the countries in which they are obtained. In most countries in which we file, the patent term is 20 years from the earliest date of filing anon-provisional patent application.
In the United States, the term of a patent covering anFDA-approved drug may be eligible for a patent term extension under the Hatch-Waxman Act as compensation for the loss of patent term during the FDA regulatory review process. The period of extension may be up to five years beyond the expiration of the patent, but cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval. Only one patent among those eligible for an extension may be extended, and a given patent may only be extended once. Similar provisions are available in Europe and in certain other jurisdictions to extend the term of a patent that covers an approved drug. It is possible that issued U.S. patents covering each of our product candidates may be entitled to patent term extensions. If our product candidates receive FDA approval, we intend to apply for patent term extensions, if available, to extend the term of patents that cover the approved product candidates. We also intend to seek patent term extensions in any jurisdictions where they are available, however, there is no guarantee that the applicable authorities, including the FDA, will agree with our assessment of whether such extensions should be granted, and even if granted, the length of such extensions.
In addition to patent protection, we also rely onknow-how and trade secret protection for our proprietary information that is not amenable to, or that we do not consider appropriate for, patent protection, to develop and maintain our proprietary position. However, trade secrets can be difficult to protect. Although we take steps to protect our proprietary information, including restricting access to our premises and our confidential information, as well as entering into agreements with our employees, consultants, advisors and potential collaborators, third parties may independently develop the same or similar proprietary information or may otherwise gain access to our proprietary information. As a result, we may be unable to meaningfully protect ourknow-how, trade secrets, and other proprietary information.
In addition, we plan to rely on regulatory protection based on orphan drug exclusivities, data exclusivities, and market exclusivities. See “—Government Regulation” for additional information.
Our Material Agreements
Amended and Restated License Agreement with Penn
In July 2019, we entered into an amended and restated license agreement, or the License Agreement, with Penn and the Children’s Hospital of Philadelphia, or CHOP, and together with Penn, the Institutions, pursuant to which we obtained (a) anon-exclusive,non-sublicensable, worldwide research license to make, have made and use products in two subfields of use, (b) effective as of October 2018, an exclusive, worldwide, royalty-bearing license, with the right to sublicense, under certain patent rights of the Institution to make, use, sell, offer for sale and import products in the same two subfields of use, and (c) effective as of October 2018, anon-exclusive, worldwide, royalty-bearing license, with limited rights to sublicense, under certain of Penn’sknow-how, whichknow-how satisfies certain criteria and is listed on a mutuallyagreed-to schedule, to make, have made, use, sell, offer for sale, import and have imported products in the same two subfields of use. As of July 2019, no suchknow-how has been scheduled. Our rights are subject to the rights of the U.S. government and certain rights retained by the Institutions.
Unless earlier terminated, the License Agreement will expire with respect to a product upon the later of (a) the expiration of the last to expire patent or patent application covering such product or (b) 10 years after the
134