
CABA-201 Initial Clinical Data from the RESET-Myositis™ & RESET-SLE™ Phase 1/2 Trials JUNE 2024 Exhibit 99.1

Disclaimer The following presentation, including any printed or electronic copy of these slides, the talks given by the presenters, the information communicated during any delivery of the presentation and any question and answer session and any document or material distributed at or in connection with the presentation (collectively, the “Presentation”) has been prepared by Cabaletta Bio, Inc. (“we,” “us,” “our,” “Cabaletta” or the “Company”) and is made for informational purposes only. This Presentation does not purport to be a prospectus, to be complete or to contain all of the information you may desire. Statements contained herein are made as of the date of this Presentation unless stated otherwise, and this Presentation shall not under any circumstances create an implication that the information contained herein is correct as of any time after such date or that information will be updated or revised to reflect information that subsequently becomes available or changes occurring after the date hereof. This Presentation may contain “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 relating to our business, operations, and financial conditions, and include, but are not limited to, express or implied statements regarding our current beliefs, expectations and assumptions regarding: our business, future plans and strategies for our CAAR T and CARTA technologies; our ability to grow our autoimmune-focused pipeline; the ability to capitalize on and potential benefits resulting from our research and translational insights; including those related to any similarly-designed constructs or dosing regimens; the anticipated market opportunities for CABA-201 in patients with autoimmune diseases; the Company’s business plans and objectives; our expectations around the potential success and therapeutic benefits of CABA-201, including our belief that CABA-201 may enable an “immune system reset”; Cabaletta's belief of the potential for CAR T to enable a paradigm shift in autoimmunity, including its potential achieve durable remissions without chronic therapy;; our plans for Phase 1/2 clinical trials of CABA-201 in patients with systemic lupus erythematosus (SLE), myositis, SSc, and generalized myasthenia gravis (gMG), and for advancement of a RESET-PV sub-study within the ongoing DesCAARTes trial in PV, including the timing thereof, including our anticipated progress, timing of enrollment, expectations for the efficiency of the clinical trial designs, updates related to status, safety data, or otherwise and the expected timing of the related data read-outs, and ability to leverage our experience in autoimmune cell therapy; our initial clinical data read-out in the first half of 2024 at the EULAR 2024 symposium for patients with myositis and SLE treated with CABA-201; our planned initial clinical data read-out in the second half of 2024 for patients with SSc and gMG treated with CABA-201 and additional data; our ability to enroll the requisite number of patients, dose each dosing cohort in the intended manner, and advance the trial as planned in our Phase 1/2 clinical trials of CABA-201; the timing any planned regulatory filings for our development programs, including IND applications; the progress and results of our DesCAARTes™ Phase 1 trial, including the significance and impact around reported safety and clinical and translational data of cohorts from our DesCAARTes™ and MusCAARTes™ Phase 1 trials; Cabaletta's potential to eliminate the need for apheresis by using a simpler collection process to obtain the starting material for the CABA-201 manufacturing process the therapeutic potential and clinical benefits of our product candidates; the expectation that Cabaletta may improve outcomes for patients suffering from SLE, SSc, myositis, gMG, mucosal pemphigus vulgaris, MuSK myasthenia gravis, or other autoimmune diseases; the ability of our clinical strategy to reduce risk, maximize reach and accelerate timelines of our Phase 1/2 clinical trials of CABA-201; our ability to successfully complete our preclinical and clinical studies for our product candidates, including our ability to enroll the requisite number of patients, dose each dosing cohort in the intended manner, and progress the trial; our ability to obtain and maintain regulatory approval of our product candidates, including our expectations regarding the intended incentives conferred by and ability to retain Orphan Drug Designation and Fast Track Designations for our product candidates, as applicable; our ability to accelerate our pipeline and to develop meaningful therapies for patients, including in collaboration with academic and industry partners and the ability to optimize such collaborations on our development programs; our ability to contract with third-party suppliers and manufacturers and retain such manufacturers, whether due to legislative action or otherwise; to implement an enhanced manufacturing process and further develop our internal manufacturing strategy, capabilities and facilities; our potential commercial opportunities, including value and addressable market, for our product candidates; and our expectations regarding our use of capital and other financial results, including our ability to fund operations into the first half of 2026. Words such as, but not limited to, “look forward to,” “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” “would,” “should” and “could,” and similar expressions or words, identify forward-looking statements. Various risks, uncertainties and assumptions could cause actual results to differ materially from those anticipated or implied in our forward-looking statements. Such risks and uncertainties include, but are not limited to, risks related to the success, cost, and timing of our product candidate development activities and preclinical studies and clinical trials, risks related to our ability to demonstrate sufficient evidence of safety, efficacy and tolerability in our preclinical studies and clinical trials of CABA-201, DSG3-CAART and MuSK-CAART, the risk that the results observed with the similarly-designed construct, including, but not limited to, due to dosing regimen, are not indicative of the results we seek to achieve with CABA-201, our plans to evaluate additional cohorts in the DesCAARTes™ trial, including a cohort implementing a pre-treatment regimen, the risk that signs of biologic activity or persistence may not inform long-term results, the risk that persistence observed with effective CD19-CAR T oncology studies in combination with lymphodepletion is not indicative of, or applicable to, clinical responses in patients with mPV, risks related to clinical trial site activation or enrollment rates that are lower than expected, our ability to protect and maintain our intellectual property position, risks related to our relationships with third parties, uncertainties related to regulatory agencies’ evaluation of regulatory filings and other information related to our product candidates, our ability to retain and recognize the intended incentives conferred by any Orphan Drug Designations and Fast Track Designations, risks related to regulatory filings and potential clearance, the risk that any one or more of our product candidates will not be successfully developed and commercialized, the risk that the results of preclinical studies or clinical studies will not be predictive of future results in connection with future studies, and risks related to volatile market and economic conditions and public health crises. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. Accordingly, you are cautioned not to place undue reliance on these forward-looking statements. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause our actual results to differ materially from those contained in the forward-looking statements, see the section entitled "Risk Factors" in our most recent annual report on Form 10-K, as well as discussions of potential risks, uncertainties, and other important factors in our other and subsequent filings with the Securities and Exchange Commission. Certain information contained in this Presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this Presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. The Company is the owner of various trademarks, trade names and service marks. Certain other trademarks, trade names and service marks appearing in this Presentation are the property of third parties. Solely for convenience, the trademarks and trade names in this Presentation are referred to without the ® and TM symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto.
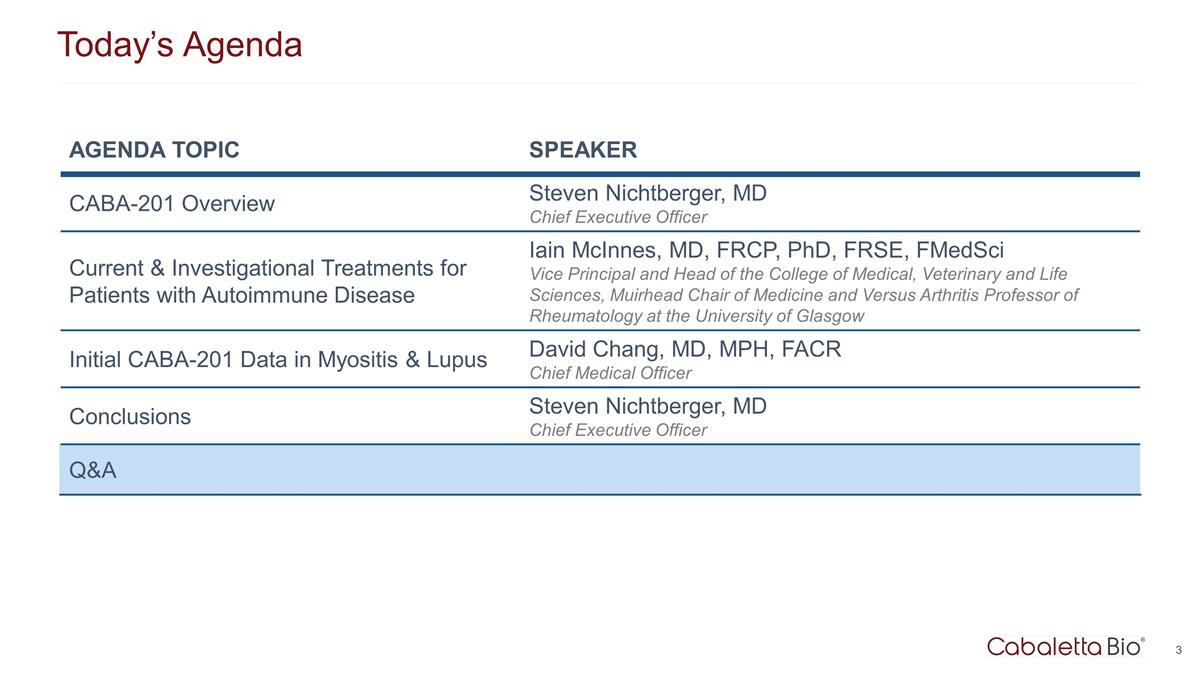
Today’s Agenda AGENDA TOPIC SPEAKER CABA-201 Overview Steven Nichtberger, MD Chief Executive Officer Current & Investigational Treatments for Patients with Autoimmune Disease Iain McInnes, MD, FRCP, PhD, FRSE, FMedSci Vice Principal and Head of the College of Medical, Veterinary and Life Sciences, Muirhead Chair of Medicine and Versus Arthritis Professor of Rheumatology at the University of Glasgow Initial CABA-201 Data in Myositis & Lupus David Chang, MD, MPH, FACR Chief Medical Officer Conclusions Steven Nichtberger, MD Chief Executive Officer Q&A
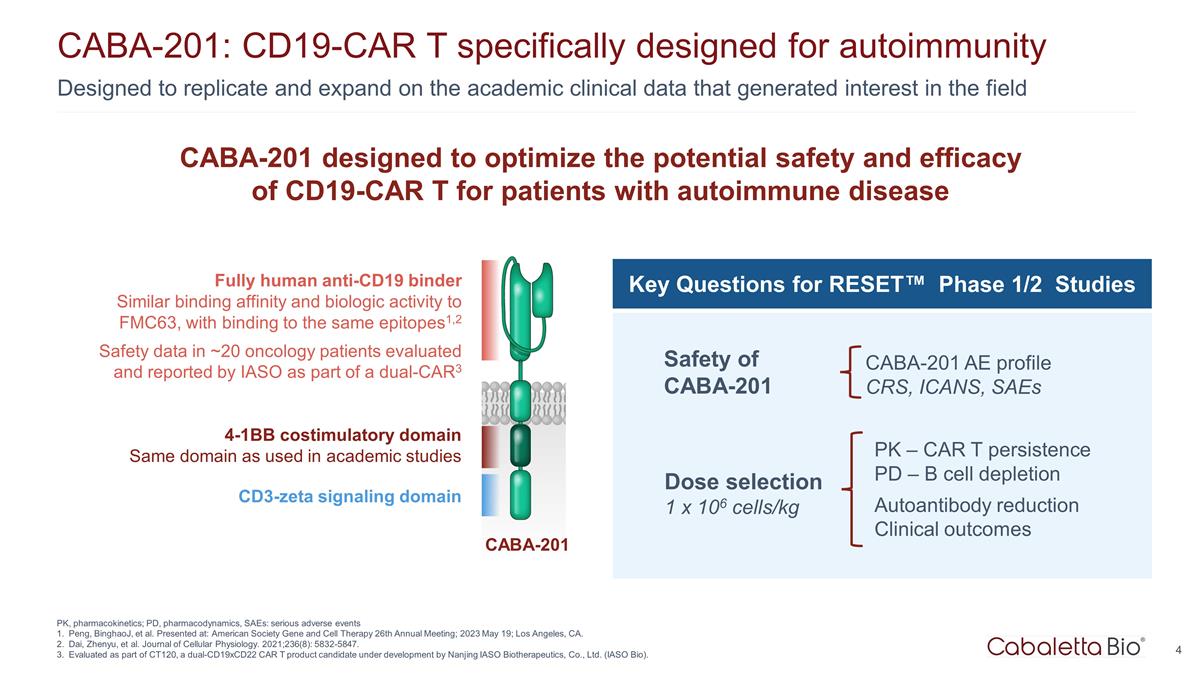
4 Designed to replicate and expand on the academic clinical data that generated interest in the field CABA-201: CD19-CAR T specifically designed for autoimmunity PK, pharmacokinetics; PD, pharmacodynamics, SAEs: serious adverse events Peng, BinghaoJ, et al. Presented at: American Society Gene and Cell Therapy 26th Annual Meeting; 2023 May 19; Los Angeles, CA. Dai, Zhenyu, et al. Journal of Cellular Physiology. 2021;236(8): 5832-5847. Evaluated as part of CT120, a dual-CD19xCD22 CAR T product candidate under development by Nanjing IASO Biotherapeutics, Co., Ltd. (IASO Bio). Fully human anti-CD19 binder Similar binding affinity and biologic activity to FMC63, with binding to the same epitopes1,2 Safety data in ~20 oncology patients evaluated and reported by IASO as part of a dual-CAR3 4-1BB costimulatory domain Same domain as used in academic studies CD3-zeta signaling domain CABA-201 CABA-201 designed to optimize the potential safety and efficacy of CD19-CAR T for patients with autoimmune disease Key Questions for RESET™ Phase 1/2 Studies Safety of CABA-201 CABA-201 AE profile CRS, ICANS, SAEs Dose selection 1 x 106 cells/kg PK – CAR T persistence PD – B cell depletion Autoantibody reduction Clinical outcomes
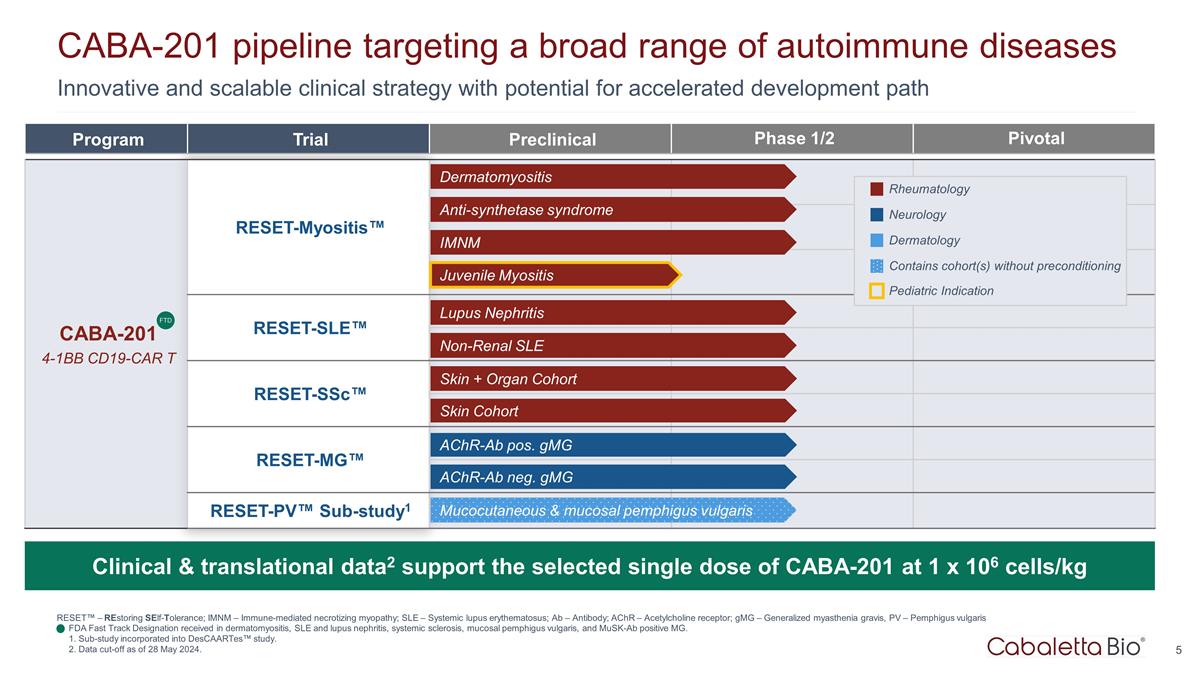
Innovative and scalable clinical strategy with potential for accelerated development path CABA-201 pipeline targeting a broad range of autoimmune diseases RESET™ – REstoring SElf-Tolerance; IMNM – Immune-mediated necrotizing myopathy; SLE – Systemic lupus erythematosus; Ab – Antibody; AChR – Acetylcholine receptor; gMG – Generalized myasthenia gravis, PV – Pemphigus vulgaris FDA Fast Track Designation received in dermatomyositis, SLE and lupus nephritis, systemic sclerosis, mucosal pemphigus vulgaris, and MuSK-Ab positive MG. 1. Sub-study incorporated into DesCAARTes™ study. 2. Data cut-off as of 28 May 2024. Program Trial Preclinical Phase 1/2 Pivotal CABA-201 4-1BB CD19-CAR T RESET-Myositis™ CARTA Chimeric Antigen Receptor T cells for Autoimmunity RESET-SLE™ RESET-SSc™ RESET-MG™ RESET-PV™ Sub-study1 Dermatomyositis Anti-synthetase syndrome IMNM Lupus Nephritis Non-Renal SLE Skin + Organ Cohort AChR-Ab neg. gMG AChR-Ab pos. gMG Skin Cohort FTD Mucocutaneous & mucosal pemphigus vulgaris Juvenile Myositis Clinical & translational data2 support the selected single dose of CABA-201 at 1 x 106 cells/kg Rheumatology Neurology Dermatology Contains cohort(s) without preconditioning Pediatric Indication
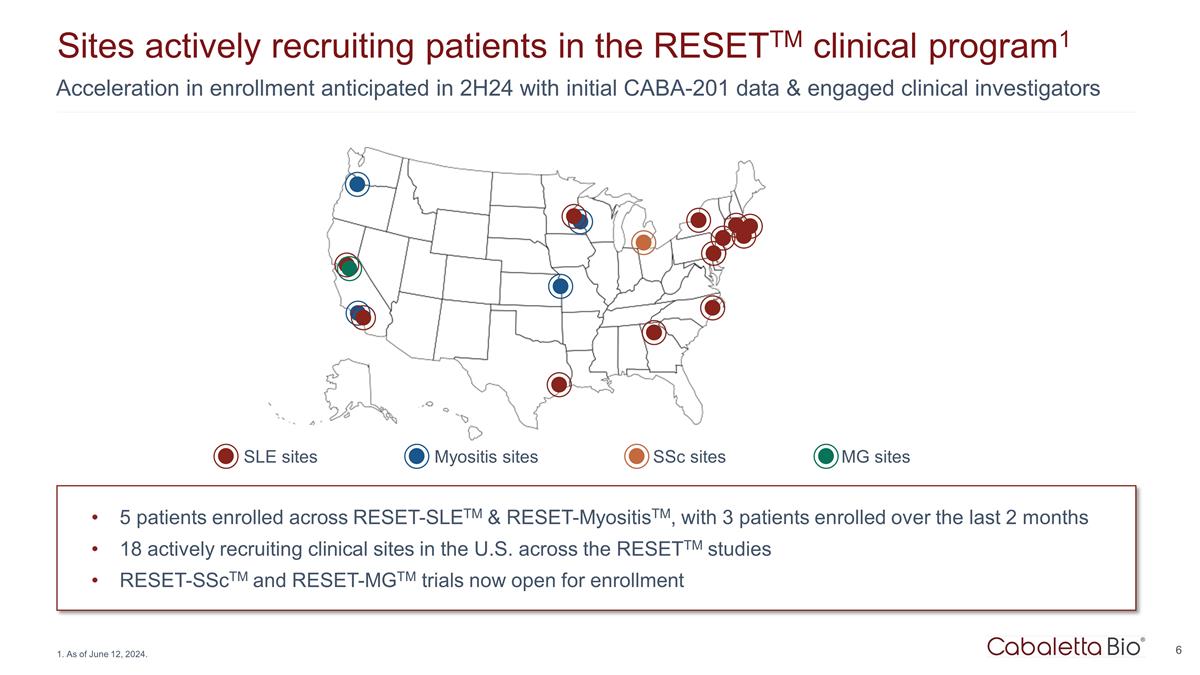
Acceleration in enrollment anticipated in 2H24 with initial CABA-201 data & engaged clinical investigators Sites actively recruiting patients in the RESETTM clinical program1 5 patients enrolled across RESET-SLETM & RESET-MyositisTM, with 3 patients enrolled over the last 2 months 18 actively recruiting clinical sites in the U.S. across the RESETTM studies RESET-SScTM and RESET-MGTM trials now open for enrollment 1. As of June 12, 2024. SLE sites Myositis sites SSc sites MG sites

Current & Investigational Treatments for Patients with Autoimmune Diseases

Broad immunosuppression and chronic administration often required to achieve partial, transient responses Current therapies for autoimmunity do not achieve drug-free remission (Hematopoietic stem cell transplant has been shown to be curative in systemic sclerosis but has increased mortality in the first year6) There is a need for durable, effective and safe therapies that reestablish immune tolerance to eliminate the need for long-term therapy5,6 Khoo T, et al. Nat Rev Rheumatol. 2023;19(11):695-712. Octapharma. Accessed June 10, 2024. Hoover PJ, Costenbader KH. Kidney Int. 2016;90(3):487-92. High Unmet Clinical Need in SLE & Myositis Potential for life-threatening complications ~40% of patients with SLE develop LN3,4 ~25% risk of death or ESRD within 10y Incomplete responses despite chronic therapy High mortality due to lung & cardiac involvement1 Only FDA & EMA-approved therapy is IVIg in DM2 Many patients remain refractory to standard of care therapies – particularly high unmet need in IMNM1 Lupus Current Therapies in Autoimmunity Broad immunosuppression Modest & inconsistent clinical responses Chronic therapy requirements Hahn BH, et al. Arthritis Care Res (Hoboken). 2012; 64(6): 797–808. Rosenblum MD, et al. Sci Transl Med. 2012;4(125):125sr1. Swart J, et al. Nat Rev Rheumatol. 2017;13:244-256. Myositis
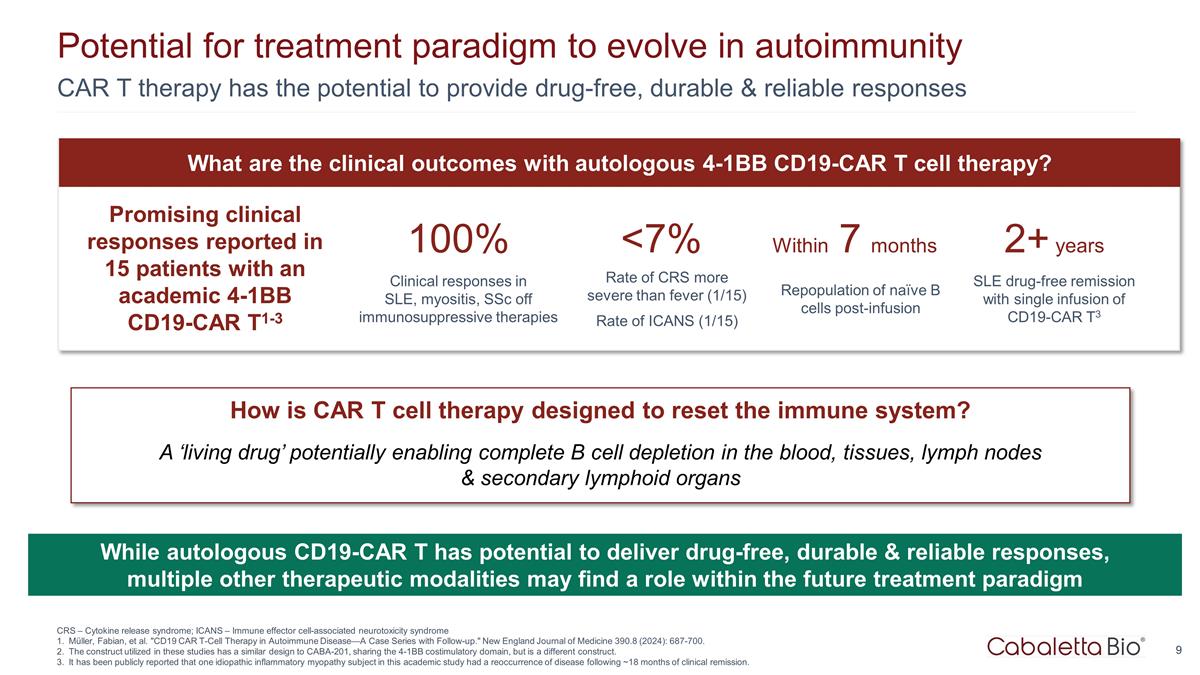
Potential for treatment paradigm to evolve in autoimmunity CAR T therapy has the potential to provide drug-free, durable & reliable responses Promising clinical responses reported in 15 patients with an academic 4-1BB CD19-CAR T1-3 100% Clinical responses in SLE, myositis, SSc off immunosuppressive therapies 2+ years SLE drug-free remission with single infusion of CD19-CAR T3 Within 7 months Repopulation of naïve B cells post-infusion <7% Rate of CRS more severe than fever (1/15) Rate of ICANS (1/15) What are the clinical outcomes with autologous 4-1BB CD19-CAR T cell therapy? CRS – Cytokine release syndrome; ICANS – Immune effector cell-associated neurotoxicity syndrome Müller, Fabian, et al. "CD19 CAR T-Cell Therapy in Autoimmune Disease—A Case Series with Follow-up." New England Journal of Medicine 390.8 (2024): 687-700. The construct utilized in these studies has a similar design to CABA-201, sharing the 4-1BB costimulatory domain, but is a different construct. It has been publicly reported that one idiopathic inflammatory myopathy subject in this academic study had a reoccurrence of disease following ~18 months of clinical remission. How is CAR T cell therapy designed to reset the immune system? A ‘living drug’ potentially enabling complete B cell depletion in the blood, tissues, lymph nodes & secondary lymphoid organs While autologous CD19-CAR T has potential to deliver drug-free, durable & reliable responses, multiple other therapeutic modalities may find a role within the future treatment paradigm

Initial CABA-201 Data in Myositis & Lupus
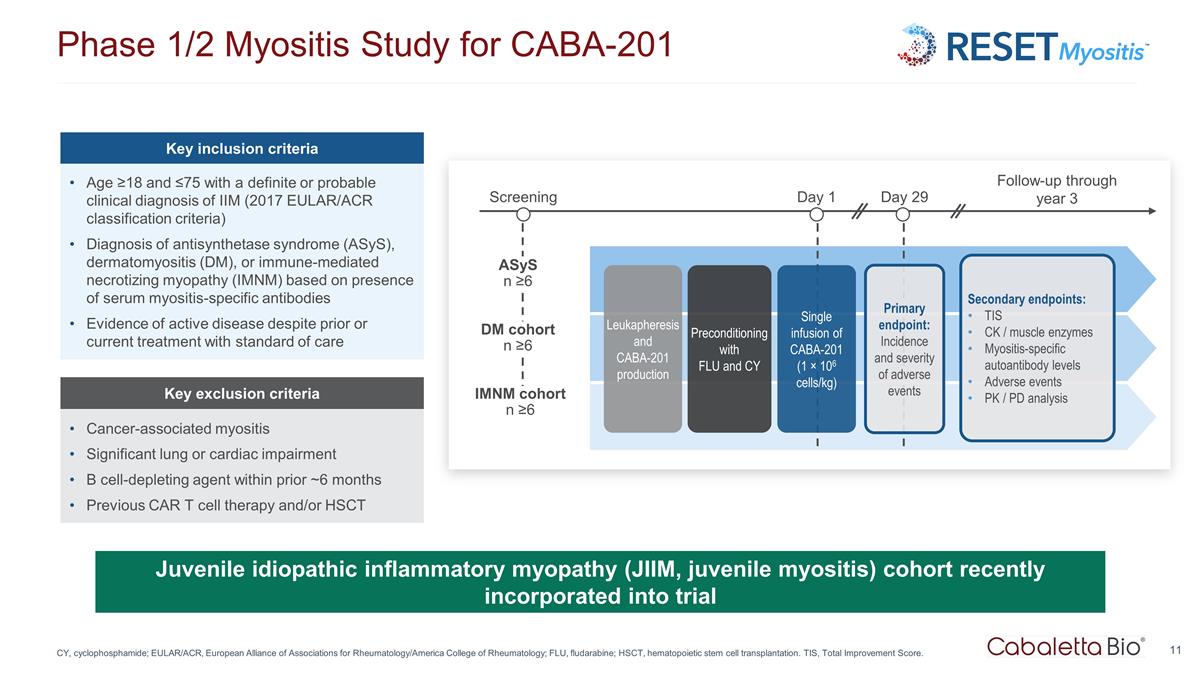
Phase 1/2 Myositis Study for CABA-201 CY, cyclophosphamide; EULAR/ACR, European Alliance of Associations for Rheumatology/America College of Rheumatology; FLU, fludarabine; HSCT, hematopoietic stem cell transplantation. TIS, Total Improvement Score. Juvenile idiopathic inflammatory myopathy (JIIM, juvenile myositis) cohort recently incorporated into trial Key inclusion criteria Age ≥18 and ≤75 with a definite or probable clinical diagnosis of IIM (2017 EULAR/ACR classification criteria) Diagnosis of antisynthetase syndrome (ASyS), dermatomyositis (DM), or immune-mediated necrotizing myopathy (IMNM) based on presence of serum myositis-specific antibodies Evidence of active disease despite prior or current treatment with standard of care Key exclusion criteria Cancer-associated myositis Significant lung or cardiac impairment B cell-depleting agent within prior ~6 months Previous CAR T cell therapy and/or HSCT Screening ASyS n ≥6 IMNM cohort n ≥6 DM cohort n ≥6 Day 1 Day 29 Follow-up through year 3 Leukapheresis and CABA-201 production Preconditioning with FLU and CY Single infusion of CABA-201 (1 × 106 cells/kg) Primary endpoint: Incidence and severity of adverse events Secondary endpoints: TIS CK / muscle enzymes Myositis-specific autoantibody levels Adverse events PK / PD analysis
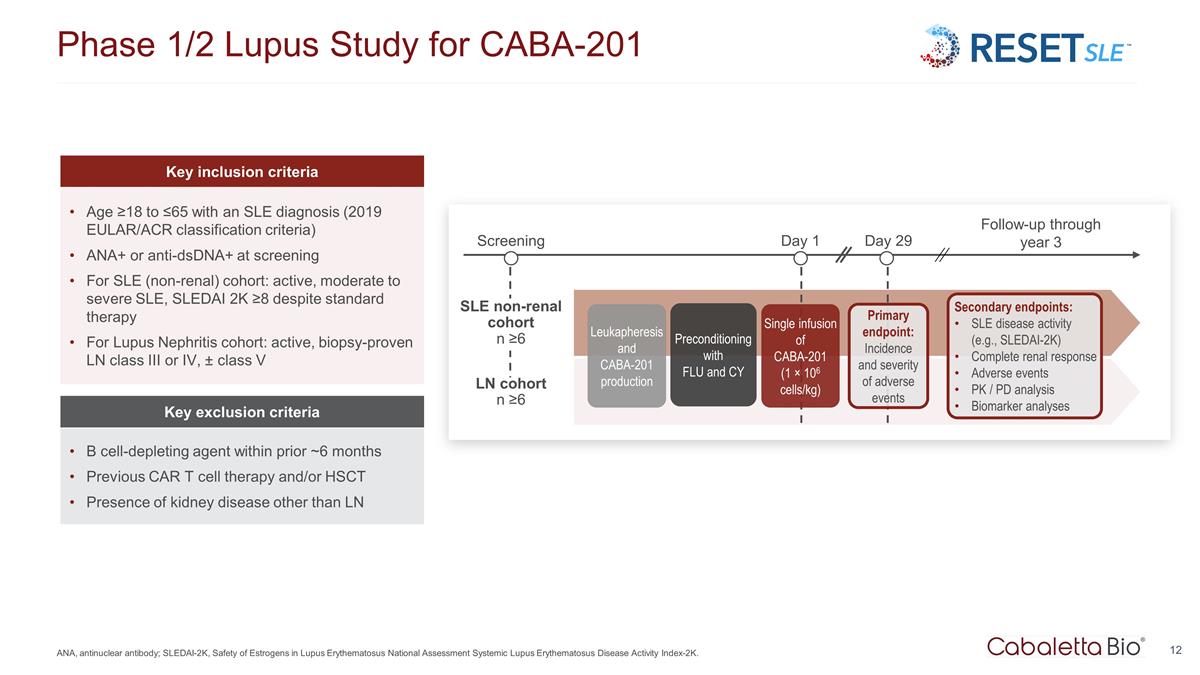
Phase 1/2 Lupus Study for CABA-201 ANA, antinuclear antibody; SLEDAI-2K, Safety of Estrogens in Lupus Erythematosus National Assessment Systemic Lupus Erythematosus Disease Activity Index-2K. Key inclusion criteria Age ≥18 to ≤65 with an SLE diagnosis (2019 EULAR/ACR classification criteria) ANA+ or anti-dsDNA+ at screening For SLE (non-renal) cohort: active, moderate to severe SLE, SLEDAI 2K ≥8 despite standard therapy For Lupus Nephritis cohort: active, biopsy-proven LN class III or IV, ± class V Key exclusion criteria B cell-depleting agent within prior ~6 months Previous CAR T cell therapy and/or HSCT Presence of kidney disease other than LN Screening LN cohort n ≥6 Day 1 Day 29 Follow-up through year 3 Leukapheresis and CABA-201 production Preconditioning with FLU and CY Single infusion of CABA-201 (1 × 106 cells/kg) Primary endpoint: Incidence and severity of adverse events SLE non-renal cohort n ≥6 Secondary endpoints: SLE disease activity (e.g., SLEDAI-2K) Complete renal response Adverse events PK / PD analysis Biomarker analyses
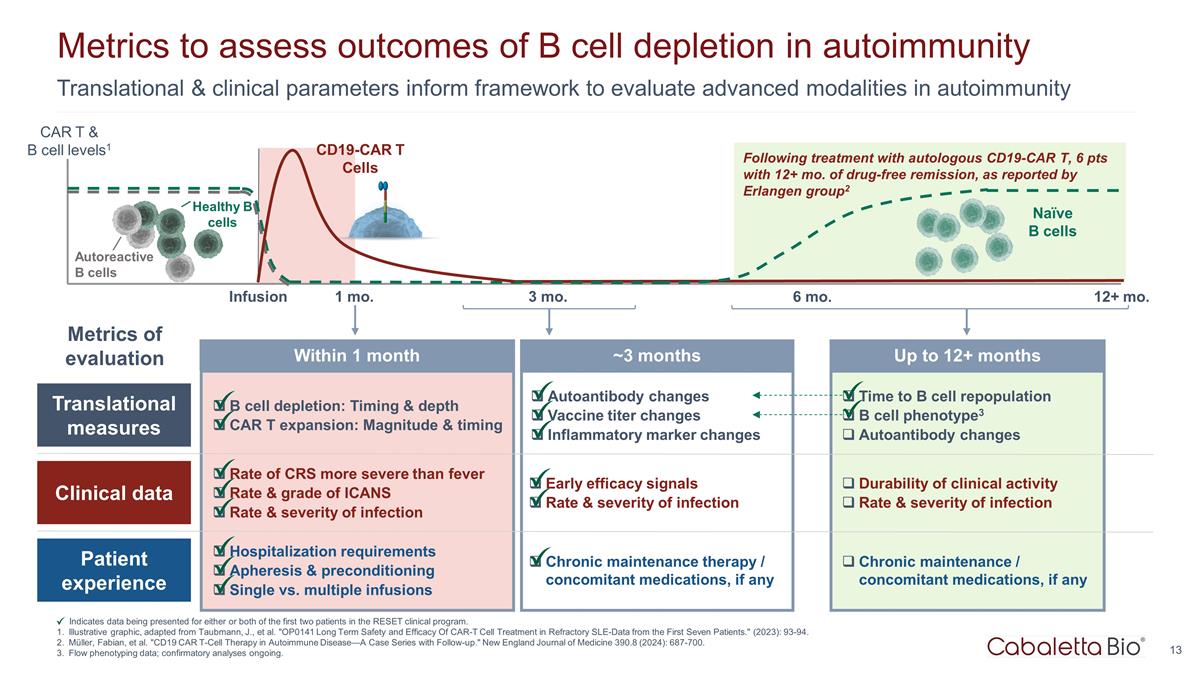
Time to B cell repopulation B cell phenotype3 Autoantibody changes Durability of clinical activity Rate & severity of infection Chronic maintenance / concomitant medications, if any Up to 12+ months Translational & clinical parameters inform framework to evaluate advanced modalities in autoimmunity Metrics to assess outcomes of B cell depletion in autoimmunity Indicates data being presented for either or both of the first two patients in the RESET clinical program. Illustrative graphic, adapted from Taubmann, J., et al. "OP0141 Long Term Safety and Efficacy Of CAR-T Cell Treatment in Refractory SLE-Data from the First Seven Patients." (2023): 93-94. Müller, Fabian, et al. "CD19 CAR T-Cell Therapy in Autoimmune Disease—A Case Series with Follow-up." New England Journal of Medicine 390.8 (2024): 687-700. Flow phenotyping data; confirmatory analyses ongoing. Following treatment with autologous CD19-CAR T, 6 pts with 12+ mo. of drug-free remission, as reported by Erlangen group2 Infusion 1 mo. 3 mo. 12+ mo. 6 mo. CD19-CAR T Cells Naïve B cells Healthy B cells Autoreactive B cells CAR T & B cell levels1 Translational measures Clinical data Patient experience B cell depletion: Timing & depth CAR T expansion: Magnitude & timing Rate of CRS more severe than fever Rate & grade of ICANS Rate & severity of infection Hospitalization requirements Apheresis & preconditioning Single vs. multiple infusions Autoantibody changes Vaccine titer changes Inflammatory marker changes Early efficacy signals Rate & severity of infection Chronic maintenance therapy / concomitant medications, if any Within 1 month ~3 months Metrics of evaluation
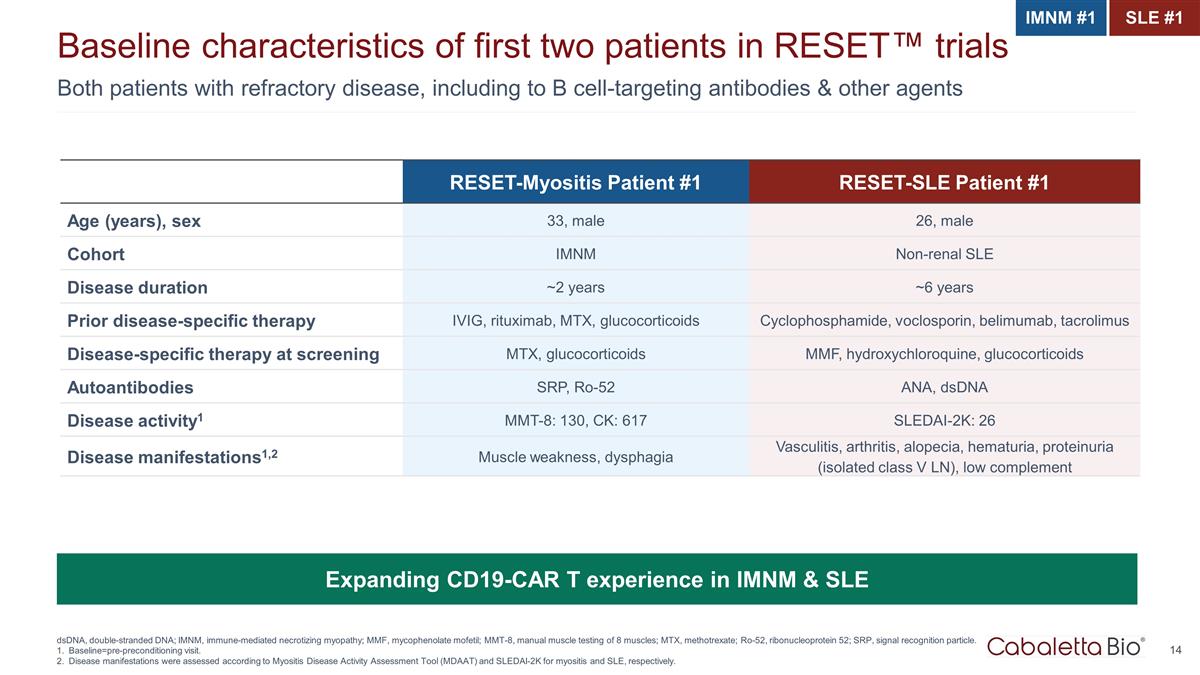
Both patients with refractory disease, including to B cell-targeting antibodies & other agents Baseline characteristics of first two patients in RESET™ trials dsDNA, double-stranded DNA; IMNM, immune-mediated necrotizing myopathy; MMF, mycophenolate mofetil; MMT-8, manual muscle testing of 8 muscles; MTX, methotrexate; Ro-52, ribonucleoprotein 52; SRP, signal recognition particle. Baseline=pre-preconditioning visit. Disease manifestations were assessed according to Myositis Disease Activity Assessment Tool (MDAAT) and SLEDAI-2K for myositis and SLE, respectively. RESET-Myositis Patient #1 RESET-SLE Patient #1 Age (years), sex 33, male 26, male Cohort IMNM Non-renal SLE Disease duration ~2 years ~6 years Prior disease-specific therapy IVIG, rituximab, MTX, glucocorticoids Cyclophosphamide, voclosporin, belimumab, tacrolimus Disease-specific therapy at screening MTX, glucocorticoids MMF, hydroxychloroquine, glucocorticoids Autoantibodies SRP, Ro-52 ANA, dsDNA Disease activity1 MMT-8: 130, CK: 617 SLEDAI-2K: 26 Disease manifestations1,2 Muscle weakness, dysphagia Vasculitis, arthritis, alopecia, hematuria, proteinuria (isolated class V LN), low complement Expanding CD19-CAR T experience in IMNM & SLE IMNM #1 SLE #1
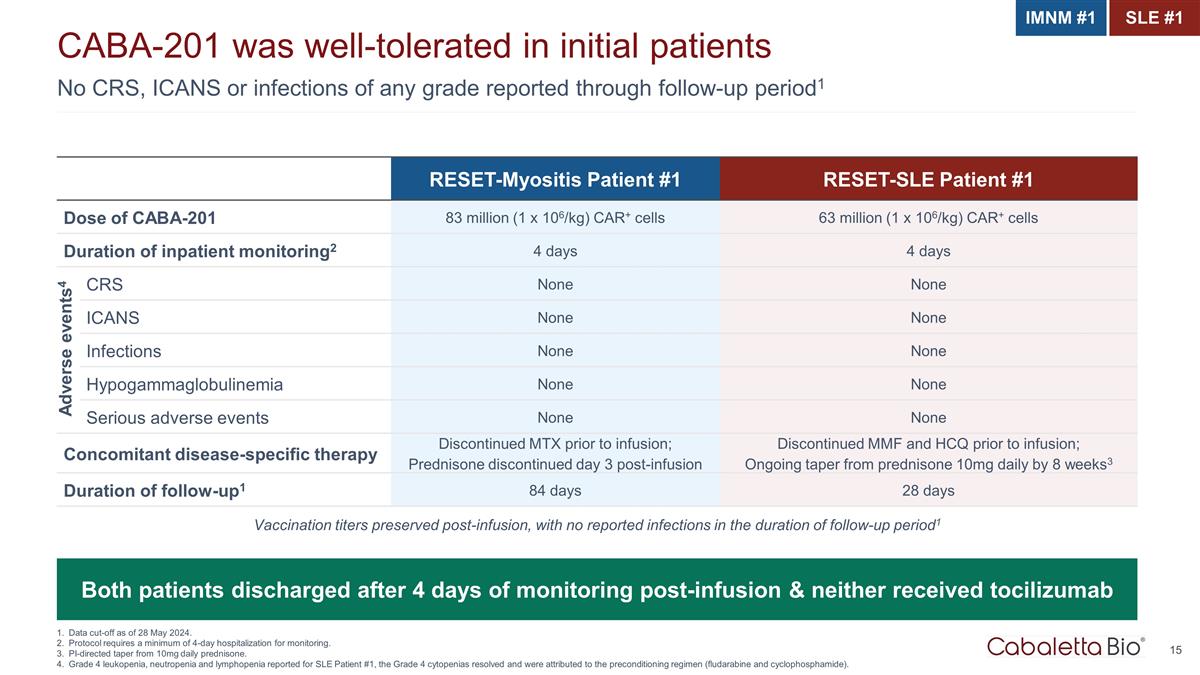
No CRS, ICANS or infections of any grade reported through follow-up period1 CABA-201 was well-tolerated in initial patients Data cut-off as of 28 May 2024. Protocol requires a minimum of 4-day hospitalization for monitoring. PI-directed taper from 10mg daily prednisone. Grade 4 leukopenia, neutropenia and lymphopenia reported for SLE Patient #1, the Grade 4 cytopenias resolved and were attributed to the preconditioning regimen (fludarabine and cyclophosphamide). RESET-Myositis Patient #1 RESET-SLE Patient #1 Dose of CABA-201 83 million (1 x 106/kg) CAR+ cells 63 million (1 x 106/kg) CAR+ cells Duration of inpatient monitoring2 4 days 4 days CRS None None ICANS None None Infections None None Hypogammaglobulinemia None None Serious adverse events None None Concomitant disease-specific therapy Discontinued MTX prior to infusion; Prednisone discontinued day 3 post-infusion Discontinued MMF and HCQ prior to infusion; Ongoing taper from prednisone 10mg daily by 8 weeks3 Duration of follow-up1 84 days 28 days Both patients discharged after 4 days of monitoring post-infusion & neither received tocilizumab Vaccination titers preserved post-infusion, with no reported infections in the duration of follow-up period1 IMNM #1 SLE #1 Adverse events4
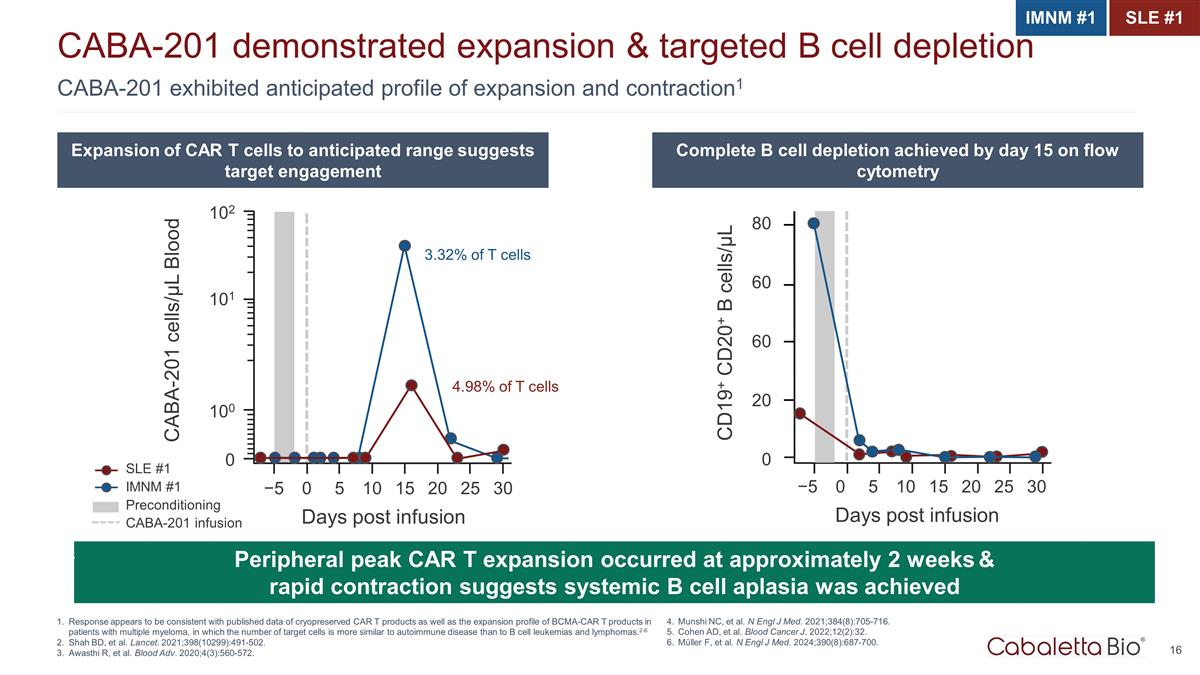
CABA-201 exhibited anticipated profile of expansion and contraction1 CABA-201 demonstrated expansion & targeted B cell depletion Response appears to be consistent with published data of cryopreserved CAR T products as well as the expansion profile of BCMA-CAR T products in patients with multiple myeloma, in which the number of target cells is more similar to autoimmune disease than to B cell leukemias and lymphomas.2-6 Shah BD, et al. Lancet. 2021;398(10299):491-502. Awasthi R, et al. Blood Adv. 2020;4(3):560-572. IMNM #1 SLE #1 Expansion of CAR T cells to anticipated range suggests target engagement Complete B cell depletion achieved by day 15 on flow cytometry Peripheral peak CAR T expansion occurred at approximately 2 weeks & rapid contraction suggests systemic B cell aplasia was achieved 4.98% of T cells 3.32% of T cells CABA-201 cells/µL Blood 102 101 100 0 −5 0 5 10 15 20 25 30 Days post infusion SLE #1 IMNM #1 Preconditioning CABA-201 infusion CD19+ CD20+ B cells/µL 60 80 60 20 0 −5 0 5 10 15 20 25 30 Days post infusion Munshi NC, et al. N Engl J Med. 2021;384(8):705-716. Cohen AD, et al. Blood Cancer J. 2022;12(2):32. Müller F, et al. N Engl J Med. 2024;390(8):687-700.
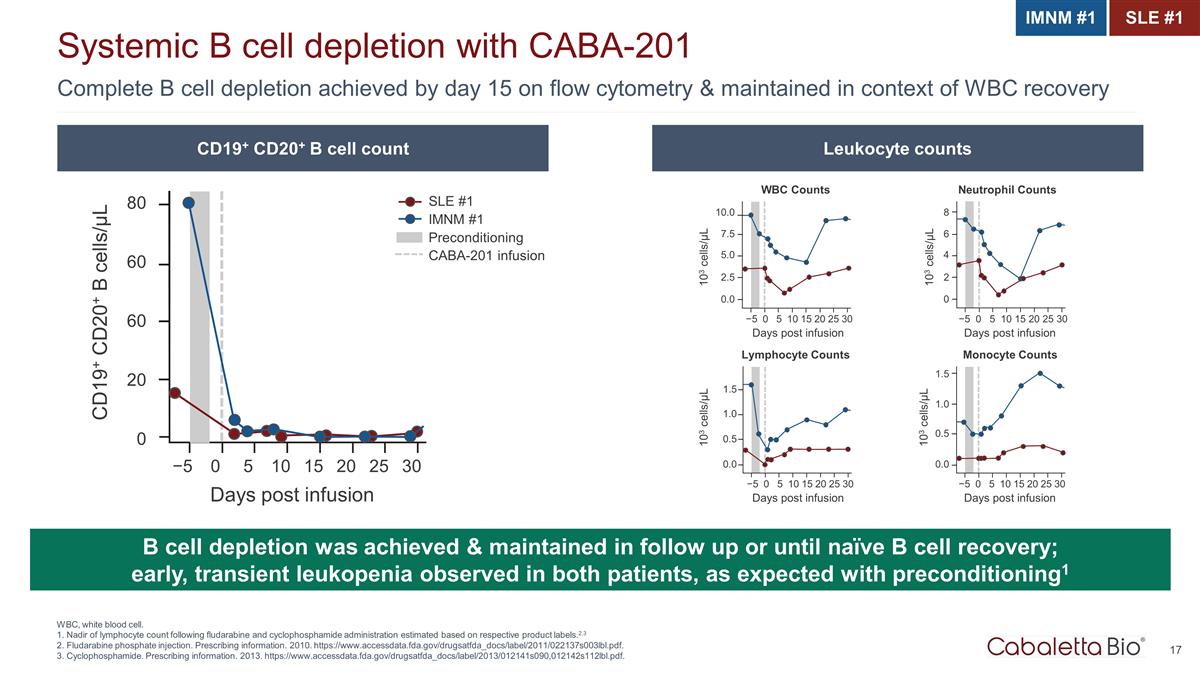
Complete B cell depletion achieved by day 15 on flow cytometry & maintained in context of WBC recovery Systemic B cell depletion with CABA-201 WBC, white blood cell. 1. Nadir of lymphocyte count following fludarabine and cyclophosphamide administration estimated based on respective product labels.2,3 2. Fludarabine phosphate injection. Prescribing information. 2010. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/022137s003lbl.pdf. 3. Cyclophosphamide. Prescribing information. 2013. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/012141s090,012142s112lbl.pdf. B cell depletion was achieved & maintained in follow up or until naïve B cell recovery; early, transient leukopenia observed in both patients, as expected with preconditioning1 CD19+ CD20+ B cells/µL SLE #1 IMNM #1 Preconditioning CABA-201 infusion 0.0 −5 0 5 10 15 20 25 30 Days post infusion 2.5 5.0 7.5 10.0 103 cells/µL WBC Counts 0 −5 0 5 10 15 20 25 30 Days post infusion 2 4 6 8 103 cells/µL Neutrophil Counts 0.0 −5 0 5 10 15 20 25 30 Days post infusion 0.5 1.0 1.5 103 cells/µL Lymphocyte Counts 0.0 −5 0 5 10 15 20 25 30 Days post infusion 0.5 1.0 1.5 103 cells/µL Monocyte Counts 60 80 60 20 0 −5 0 5 10 15 20 25 30 Days post infusion CD19+ CD20+ B cell count Leukocyte counts IMNM #1 SLE #1
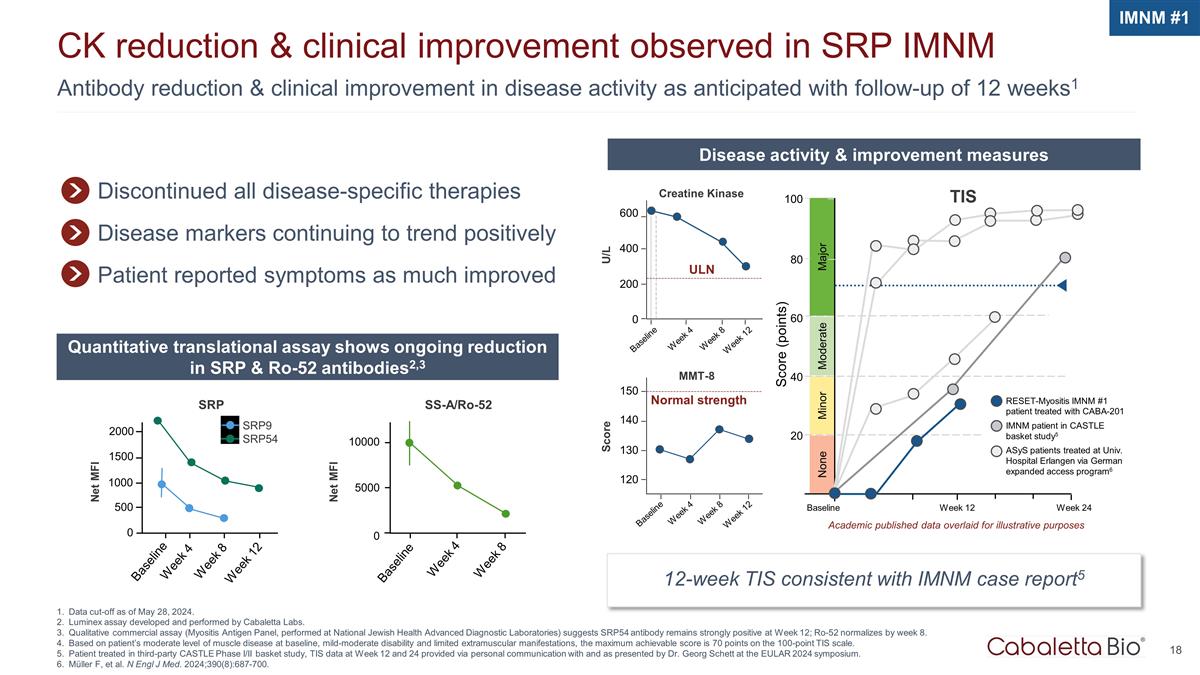
Antibody reduction & clinical improvement in disease activity as anticipated with follow-up of 12 weeks1 CK reduction & clinical improvement observed in SRP IMNM Data cut-off as of May 28, 2024. Luminex assay developed and performed by Cabaletta Labs. Qualitative commercial assay (Myositis Antigen Panel, performed at National Jewish Health Advanced Diagnostic Laboratories) suggests SRP54 antibody remains strongly positive at Week 12; Ro-52 normalizes by week 8. Based on patient’s moderate level of muscle disease at baseline, mild-moderate disability and limited extramuscular manifestations, the maximum achievable score is 70 points on the 100-point TIS scale. Patient treated in third-party CASTLE Phase I/II basket study, TIS data at Week 12 and 24 provided via personal communication with and as presented by Dr. Georg Schett at the EULAR 2024 symposium. Müller F, et al. N Engl J Med. 2024;390(8):687-700. IMNM #1 Discontinued all disease-specific therapies Disease markers continuing to trend positively Patient reported symptoms as much improved Disease activity & improvement measures Quantitative translational assay shows ongoing reduction in SRP & Ro-52 antibodies2,3 12-week TIS consistent with IMNM case report5 Baseline Week 4 ULN U/L 600 400 200 0 Week 8 Week 12 Creatine Kinase Normal strength MMT-8 150 140 130 120 Baseline Week 4 Week 8 Week 12 Score Major Score (points) 100 80 Minor Moderate 60 40 20 Week 24 Baseline Week 12 None TIS RESET-Myositis IMNM #1 patient treated with CABA-201 IMNM patient in CASTLE basket study5 ASyS patients treated at Univ. Hospital Erlangen via German expanded access program6 Academic published data overlaid for illustrative purposes SRP9 SRP54 SS-A/Ro-52 Week 8 Week 4 Baseline 10000 5000 0 Week 8 Week 4 Baseline 1500 500 2000 Week 12 1000 0 Net MFI Net MFI SRP

0 0 Month 3 Month 6 SLEDAI-2K Score 25 20 30 10 5 15 Academic published data for illustrative purposes 19 Trend toward improvement in disease manifestations with follow up of 4 weeks2 Early efficacy signals in first patient in non-renal SLE cohort1 Patient in non-renal SLE cohort due to isolated Class V LN. Data cut-off as of 28 May 2024. Baseline and Day 29 SLEDAI-2K score are reflective of disease activity at study visit day. SLE #1 Vasculitis, arthritis and hematuria resolved within 4 weeks despite discontinuation of all therapies at infusion other than ongoing taper from prednisone 10mg per day Alopecia Low complement Increased DNA binding4 Proteinuria5 Hematuria Arthritis Vasculitis 26 10 20 SLEDAI-2K3 10 0 Baseline Week 4 30 SLE patient #1 Academic SLE data6 4. Anti-dsDNA antibody titer decreased from 1:40 to 1:10 from Baseline to Week 4. 5. Urine Protein Creatinine Ratio decreased from 1.08 to 0.80 from Baseline to Week 4. 6. SLE patients treated at Univ. Hospital Erlangen via German expanded access program; Müller F, et al. N Engl J Med. 2024;390(8):687-700.
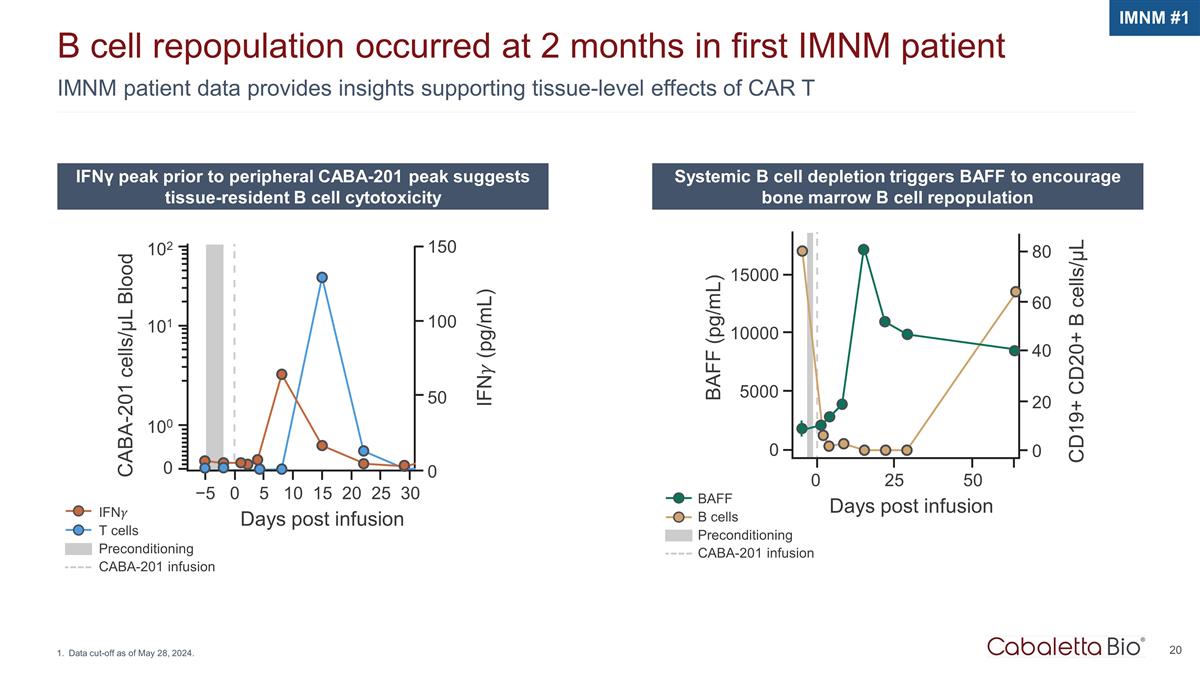
BAFF B cells Preconditioning CABA-201 infusion IMNM patient data provides insights supporting tissue-level effects of CAR T B cell repopulation occurred at 2 months in first IMNM patient Data cut-off as of May 28, 2024. IFNγ peak prior to peripheral CABA-201 peak suggests tissue-resident B cell cytotoxicity Systemic B cell depletion triggers BAFF to encourage bone marrow B cell repopulation IFN�� T cells Preconditioning CABA-201 infusion CABA-201 cells/µL Blood 102 101 100 0 −5 0 5 10 15 20 25 30 Days post infusion IFN�� (pg/mL) 150 100 50 0 IMNM #1 15000 10000 5000 0 0 25 50 Days post infusion CD19+ CD20+ B cells/µL BAFF (pg/mL) 80 60 40 20 0
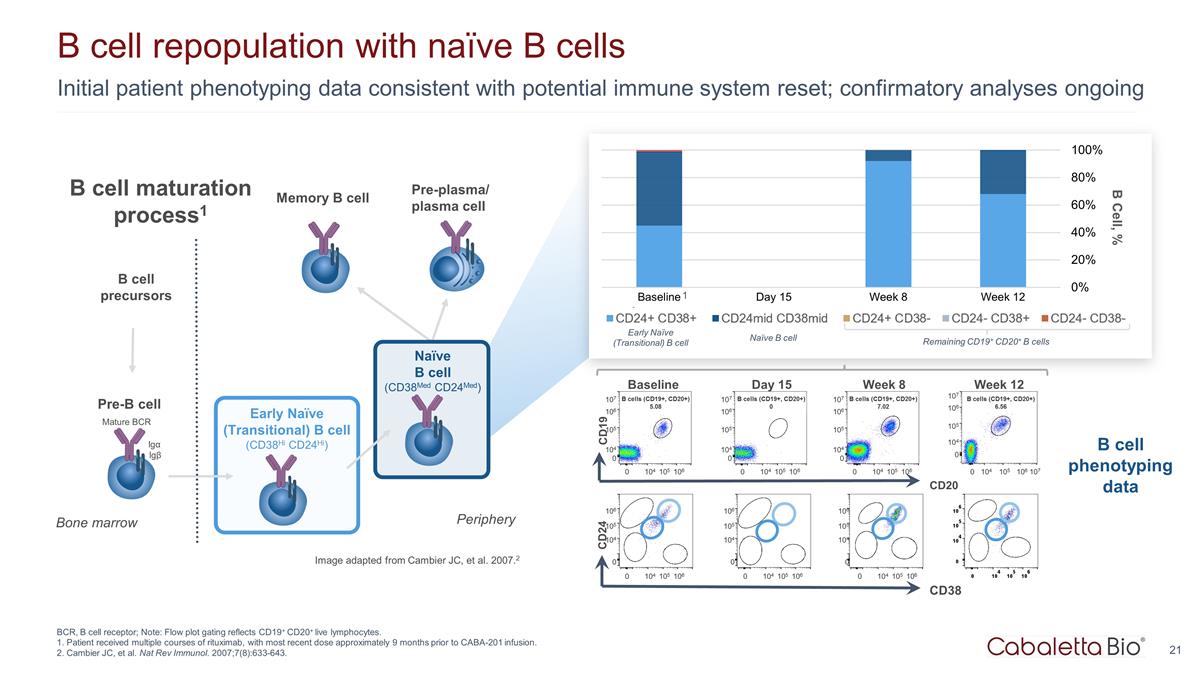
Initial patient phenotyping data consistent with potential immune system reset; confirmatory analyses ongoing B cell repopulation with naïve B cells BCR, B cell receptor; Note: Flow plot gating reflects CD19+ CD20+ live lymphocytes. 1. Patient received multiple courses of rituximab, with most recent dose approximately 9 months prior to CABA-201 infusion. 2. Cambier JC, et al. Nat Rev Immunol. 2007;7(8):633-643. Pre-B cell Early Naïve (Transitional) B cell (CD38Hi CD24Hi) Naïve B cell (CD38Med CD24Med) Mature BCR Igα Igβ Bone marrow Periphery B cell precursors Image adapted from Cambier JC, et al. 2007.2 Pre-plasma/ plasma cell B Cell, % CD20 Baseline CD19 107 106 105 104 0 0 104 106 105 B cells (CD19+, CD20+) 5.08 Week 12 107 106 105 104 0 0 104 106 105 B cells (CD19+, CD20+) 6.56 107 Day 15 107 106 105 104 0 0 104 106 105 B cells (CD19+, CD20+) 0 Week 8 107 106 105 104 0 0 104 106 105 B cells (CD19+, CD20+) 7.02 CD38 CD24 106 105 104 0 0 104 106 105 106 105 104 0 0 104 106 105 106 105 104 0 0 104 106 105 B cell phenotyping data 1 Early Naïve (Transitional) B cell Naïve B cell Remaining CD19+ CD20+ B cells B cell maturation process1 Memory B cell

Conclusions
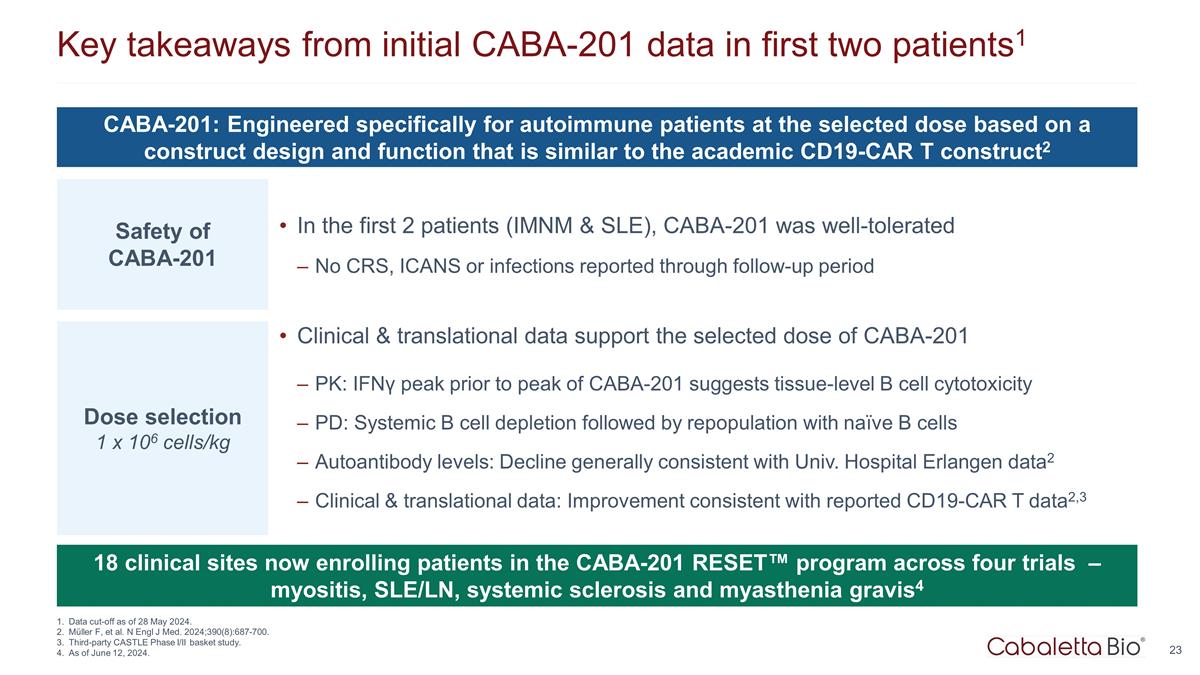
Key takeaways from initial CABA-201 data in first two patients1 Data cut-off as of 28 May 2024. Müller F, et al. N Engl J Med. 2024;390(8):687-700. Third-party CASTLE Phase I/II basket study. As of June 12, 2024. 18 clinical sites now enrolling patients in the CABA-201 RESET™ program across four trials – myositis, SLE/LN, systemic sclerosis and myasthenia gravis4 CABA-201: Engineered specifically for autoimmune patients at the selected dose based on a construct design and function that is similar to the academic CD19-CAR T construct2 Safety of CABA-201 Dose selection 1 x 106 cells/kg Clinical & translational data support the selected dose of CABA-201 PK: IFNγ peak prior to peak of CABA-201 suggests tissue-level B cell cytotoxicity PD: Systemic B cell depletion followed by repopulation with naïve B cells Autoantibody levels: Decline generally consistent with Univ. Hospital Erlangen data2 Clinical & translational data: Improvement consistent with reported CD19-CAR T data2,3 In the first 2 patients (IMNM & SLE), CABA-201 was well-tolerated No CRS, ICANS or infections reported through follow-up period
24 Realizing the vision to transform autoimmune disease treatment 1H24: No reported CRS or ICANS in first myositis or SLE patients2 Clinical update on each patient at EULAR symposium2 Evaluating CABA-201 without preconditioning in PV study Advancing program to potentially eliminate apheresis Securing scalable commercial manufacturing Cash runway into 1H26 Engineered CABA-201 specifically for use in autoimmune patients Leveraging data from an academic 4-1BB CD19-CAR T construct with favorable safety data & durable, drug free remissions1 Designed & implemented novel Phase 1/2 clinical program to accelerate path to approval No requirement for dose escalation Independent, parallel 6-patient cohorts Broad portfolio of trials in autoimmunity 2H24: Additional data from myositis & SLE trials Initial clinical data in SSc & gMG trials Initiated CABA-201 dosing in two company-sponsored studies SLE – Systemic lupus erythematosus; SSc – Systemic sclerosis; gMG – Generalized myasthenia gravis; PV – Pemphigus vulgaris Müller, Fabian, et al. "CD19 CAR T-Cell Therapy in Autoimmune Disease—A Case Series with Follow-up." New England Journal of Medicine 390.8 (2024): 687-700. The construct utilized in these studies has a similar design to CABA-201, sharing the 4-1BB costimulatory domain, but is a different construct. Data cut-off as of 28 May 2024.

Q&A
























