- SRGAQ Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
-
ETFs
- Insider
- Institutional
- Shorts
-
424B3 Filing
Surgalign (SRGAQ) 424B3Prospectus supplement
Filed: 7 Feb 19, 12:00am
Filed Pursuant to Rule 424(b)(3)
Registration No. 333-228694
MERGER PROPOSED—YOUR VOTE IS VERY IMPORTANT
To the Stockholders of RTI Surgical, Inc. and the Unitholders of PS Spine Holdco, LLC:
RTI Surgical, Inc., a Delaware corporation (“RTI”), PS Spine Holdco, LLC, a Delaware limited liability company (“PS Spine”), Bears Holding Sub, Inc., a Delaware corporation and direct wholly owned subsidiary of RTI (“Holdco”), and Bears Merger Sub, Inc., a Delaware corporation and direct wholly owned subsidiary of Holdco (“Merger Sub”), have entered into a Master Transaction Agreement, dated as of November 1, 2018 (as may be amended from time to time, the “Master Transaction Agreement”). Pursuant to the Master Transaction Agreement and subject to its terms and conditions, at the closing contemplated by the Master Transaction Agreement (the “closing”), Holdco will acquire all of the outstanding equity interests in Paradigm Spine, LLC, a Delaware limited liability company and wholly owned subsidiary of PS Spine (“Paradigm”), through a transaction in which: (i) PS Spine will contribute all of the issued and outstanding equity interests in Paradigm to Holdco (the “Contribution”), (ii) Merger Sub will be merged with and into RTI (the “Merger”), with RTI surviving as a wholly owned direct subsidiary of Holdco and (iii) Holdco will be renamed “RTI Surgical Holdings, Inc.” (collectively, the “Transaction”). The board of directors of RTI and the board of managers of PS Spine have each unanimously approved the Master Transaction Agreement and the transactions contemplated thereby, including the Merger and the Contribution.
If the Transaction is completed, as consideration for the Contribution, at closing Holdco will pay PS Spine $100.0 million in cash, subject to adjustment as described below (the “Cash Consideration Amount”), and will issue 10,729,614 fully paid andnon-assessable shares of Holdco common stock (as defined below) (the “Stock Consideration Amount”) in the aggregate to PS Spine and to the lenders under Paradigm’s existing senior secured credit agreement and their affiliates. The Stock Consideration Amount was determined by dividing $50.0 million by the volume weighted average closing price of common stock, par value $0.001 per share, of RTI (“RTI common stock”) for the five business days prior to the date of the Master Transaction Agreement (the “RTI Price”). The Cash Consideration Amount is subject to the following adjustments: (i) positive dollar-for-dollar adjustment based on the amount of Paradigm’s cash and cash equivalents at closing, (ii) negative dollar-for-dollar adjustment based on the amount of outstanding indebtedness and unpaid transaction expenses of Paradigm at closing and (iii) negative dollar-for-dollar adjustment to the extent that Paradigm’s working capital (excluding cash, cash equivalents, indebtedness and transaction expenses) at closing is less than the working capital target of $7.0 million. At the closing, due to the expected amount of Paradigm’s indebtedness and transaction expenses, all cash that would otherwise be paid at closing pursuant to the Master Transaction Agreement to PS Spine, as well as a portion of the Stock Consideration Amount, is expected to be used to satisfy Paradigm’s outstanding indebtedness and unpaid transaction expenses.
In addition to the Cash Consideration Amount and the Stock Consideration Amount payable at the closing, Holdco may be required to make further cash payments or issue additional shares of common stock, par value $0.001 per share, of Holdco (“Holdco common stock”) to PS Spine in an amount up to an additional $50.0 million of shares of Holdco common stock to be valued based upon the RTI Price and an additional $100.0 million of cash and/or Holdco common stock to be valued at the time of issuance (the “Earnout Consideration”), in each case, if certain revenue targets, which are described in the accompanying joint proxy and consent solicitation statement/prospectus, are achieved between closing and December 31, 2022. In certain circumstances, described in greater detail in the accompanying joint proxy and consent solicitation statement/prospectus, $10.0 million of the Earnout Consideration (which can take the form of cash, stock or a combination of both) will be paid to the lenders under Paradigm’s existing senior secured credit agreement and/or their affiliates.
If the Transaction is completed, (a) each share of RTI common stock issued and outstanding immediately prior to the effective time of the Merger (other than shares held by RTI as treasury shares or by Holdco or Merger Sub immediately prior to the effective time, which shall be automatically cancelled and cease to exist) will be converted automatically into one fully paid andnon-assessable share of Holdco common stock, (b) each share of Series A convertible preferred stock, par value $0.001 per share, of RTI issued and outstanding immediately prior to the effective time of the Merger (other than shares held by RTI as treasury shares or by
Holdco or Merger Sub immediately prior to the effective time which shall be automatically cancelled and cease to exist) shall be converted automatically into one fully paid and non-assessable share of Series A preferred stock, par value $0.001 per share, of Holdco and (c) each stock option and restricted stock award granted by RTI will be converted into a stock option or restricted stock award, as applicable, of Holdco with respect to an equivalent number of shares of Holdco common stock on the same terms and conditions as were applicable prior to the closing.
After the consummation of the Transaction, Holdco will own both RTI and Paradigm as wholly owned subsidiaries. Subject to certain assumptions set forth in the accompanying joint proxy and consent solicitation statement/prospectus, it is anticipated that, immediately upon completion of the Transaction, the former stockholders of RTI will own approximately 87.83% of Holdco and PS Spine will own approximately 5.31% of Holdco, and the lenders under Paradigm’s existing senior secured credit agreement and their affiliates will own approximately 6.86% of Holdco. We expect that Holdco common stock will be listed on the Nasdaq Global Market under the symbol “RTIX”.
The closing is subject to certain conditions, including (i) RTI stockholders approving a proposal to adopt the Master Transaction Agreement and approve the transactions contemplated thereby, including the Merger (the “Merger Proposal”) and to comply with applicable provisions of Nasdaq Stock Market LLC Listing Rule 5635, the potential issuance of more than twenty percent (20%) of Holdco’s issued and outstanding shares in connection with the Transaction (the “Share Issuance Proposal”) and (ii) PS Spine unitholders approving, by written consent, the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution.To obtain these approvals, RTI will hold a special meeting of RTI stockholders (the “RTI special meeting”), and PS Spine will conduct a consent solicitation in order to obtain the requisite approval from PS Spine unitholders (the “PS Spine consent solicitation”). The RTI special meeting will be held on March 7, 2019 at 10:00 a.m., local time, at 520 Lake Cook Road, Suite 315, Deerfield, Illinois 60015.
Simultaneously with the execution of the Master Transaction Agreement, certain holders of 5% or more of the voting units of PS Spine, representing approximately 67.4% of the outstanding Common Units, Class A Common Units, Class A Preferred Units, Class B Preferred Units,Class E-1 Preferred Units and Class F Preferred Units, including a majority of the Class A Preferred Units and a majority of the Class B Preferred Units, of PS Spine have each entered into support agreements with RTI and Holdco pursuant to which, among other things, they have agreed to execute and return written consents approving the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution. PS Spine expects that the written consents to be delivered by unitholders pursuant to the support agreements will represent a sufficient number of voting units of PS Spine to satisfy the approval requirement described above with respect to the approval of the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution. Additionally, certain executive officers, directors and holders of five percent (5%) or more of the voting shares of RTI stock, representing approximately 2.08% of the outstanding RTI common stock and all of the outstanding RTI preferred stock as of the date of the Master Transaction Agreement, have each entered into support agreements with PS Spine pursuant to which, among other things, they have agreed to vote all of the shares of RTI common stock and RTI preferred stock owned by them in favor of (i) the Merger Proposal, (ii) the Share Issuance Proposal and (iii) a proposal to adjourn the RTI special meeting, if necessary or appropriate, to solicit additional proxies if there are not sufficient votes to approve the Merger Proposal or the Share Issuance Proposal.
At the RTI special meeting, RTI stockholders will be asked to vote on (i) the Merger Proposal, (ii) the Share Issuance Proposal and (iii) a proposal to adjourn the RTI special meeting, if necessary or appropriate, to solicit additional proxies if there are not sufficient votes to approve the Merger Proposal or the Share Issuance Proposal.
The RTI board of directors unanimously recommends that RTI stockholders vote “FOR” each of these proposals at the RTI special meeting.
The PS Spine board of managers unanimously recommends that PS Spine unitholders provide their written consent to the proposal to approve the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution.
The accompanying document is a proxy statement for RTI, as well as a consent solicitation statement for PS Spine, and provides you with important information about the RTI special meeting, the PS Spine consent solicitation and other matters contemplated by the Master Transaction Agreement. The accompanying document is also the prospectus of Holdco to register the shares of Holdco common stock and Holdco preferred stock to be issued in the Transaction.RTI and PS Spine encourage you to read this entire joint proxy and consent solicitation statement/prospectus carefully, including the section entitled “Risk Factors”, beginning on page38.
The Transaction cannot be completed unless RTI stockholders approve the Merger Proposal and the Share Issuance Proposal. Your vote is very important, regardless of the number of shares you own. Whether or not RTI stockholders plan to attend the RTI special meeting, we ask RTI stockholders to please promptly mark, sign and date the accompanying proxy card and return it promptly in the enclosed postage-paid envelope, or authorize the individuals named on your proxy card to vote your shares by calling the toll-free telephone number or by logging on to the Internet website specified in the instructions included with your proxy card. We ask PS Spine unitholders to please promptly complete the written consent furnished with this joint proxy and consent solicitation statement/prospectus and return it promptly to PS Spine.
Sincerely,
| Sincerely,
| |
| Curtis M. Selquist | Marc R. Viscogliosi | |
| Chairman | Chairman and Chief Executive Officer | |
| RTI Surgical, Inc. | PS Spine Holdco, LLC |
Neither the Securities and Exchange Commission nor any state or provincial securities regulator has approved or disapproved of the proposed transactions described in this joint proxy and consent solicitation statement/prospectus or the securities to be issued pursuant to the Master Transaction Agreement or determined if the information contained in this joint proxy and consent solicitation statement/prospectus is accurate or adequate. Any representation to the contrary is a criminal offense.
This joint proxy and consent solicitation statement/prospectus is dated February 6, 2019, and is being mailed to RTI stockholders and PS Spine unitholders on or about February 6, 2019.
RTI SURGICAL, INC.
11621 Research Circle
Alachua, Florida 32615
NOTICE OF SPECIAL MEETING OF STOCKHOLDERS
TO BE HELD ON MARCH 7, 2019
To the Stockholdersof RTI Surgical, Inc. (“RTI”):
RTI will hold a special meeting (the “RTI special meeting”) of stockholders of RTI at 520 Lake Cook Road, Suite 315, Deerfield, Illinois 60015, on March 7, 2019, at 10:00 a.m., local time, for the following purposes:
| 1. | To consider and vote upon a proposal to adopt the Master Transaction Agreement, dated as of November 1, 2018 (as may be amended from time to time, the “Master Transaction Agreement”), by and among RTI, PS Spine Holdco, LLC, a Delaware limited liability company (“PS Spine”), Bears Holding Sub, Inc., a Delaware corporation and direct wholly owned subsidiary of RTI (“Holdco”), and Bears Merger Sub, Inc., a Delaware corporation and direct wholly owned subsidiary of Holdco (“Merger Sub”), and approve the transactions contemplated thereby, including the merger of Merger Sub with and into RTI, with RTI surviving as a wholly owned subsidiary of Holdco (the “Merger”), (the “Merger Proposal”). |
| 2. | To consider and vote upon a proposal to approve, for purposes of complying with applicable provisions of Nasdaq Stock Market LLC Listing Rule 5635 (“Nasdaq Listing Rule 5635”), the potential issuance of more than twenty percent (20%) of Holdco’s issued and outstanding common stock in connection with the transactions contemplated by the Master Transaction Agreement (the “Share Issuance Proposal”). |
| 3. | To consider and vote upon a proposal to adjourn the RTI special meeting, if necessary or appropriate, including to permit further solicitation of proxies in favor of the Merger Proposal or the Share Issuance Proposal if there are insufficient votes at the time of the RTI special meeting to approve the Merger Proposal or the Share Issuance Proposal (the “Meeting Adjournment Proposal”). |
| 4. | To transact such other business as may properly come before the RTI special meeting or any adjournments or postponements of the RTI special meeting. |
The board of directors of RTI has fixed the close of business on February 1, 2019 as the record date for the determination of the stockholders of RTI entitled to receive notice of the RTI special meeting. Only RTI stockholders of record at the close of business on the record date for the RTI special meeting are entitled to notice of the RTI special meeting and any adjournment or postponements of the RTI special meeting. Only holders of record of RTI common stock and RTI preferred stock at the close of business on the record date for the RTI special meeting are entitled to vote at the RTI special meeting and any adjournment or postponements of the RTI special meeting. A complete list of RTI stockholders entitled to vote at the RTI special meeting will be available for review at the location of the RTI special meeting during the course of the meeting and at the executive offices of RTI during ordinary business hours for a period of ten (10) days before the RTI special meeting.
The board of directors of RTI unanimously recommends that RTI stockholders vote “FOR” the Merger Proposal, “FOR” the Share Issuance Proposal and “FOR” the Meeting Adjournment Proposal.
As holders of RTI stock, your vote is very important. We cannot complete the Transaction described in this joint proxy and consent solicitation statement/prospectus unless (A) the Merger Proposal receives (i) the affirmative vote of the holders of a majority of the outstanding shares of RTI common stock and RTI preferred stock (on a fully converted basis) entitled to vote thereon and (ii) the written consent or affirmative vote of the holders of a majority of the outstanding shares of RTI preferred stock entitled to vote thereon, voting separately as a class and (B) the Share Issuance Proposal receives (i) the affirmative vote of a majority of votes cast on the proposal by RTI common stock and RTI preferred stock (on a fully converted basis) and (ii) the written consent or affirmative vote of the holders of a majority of the outstanding shares of RTI preferred stock, voting
separately as a class, in each case assuming a quorum is present. Abstentions and brokernon-votes will have the same effect as a vote “AGAINST” the Merger Proposal. Abstentions will have (i) the same effect as a vote “AGAINST” the Share Issuance Proposal for holders of RTI preferred stock, voting separately as a class and (ii) no effect on the Share Issuance Proposal for the holders of RTI common stock and RTI preferred stock (on a fully converted basis). Brokernon-votes will have (i) the same effect as a vote “AGAINST” the Share Issuance Proposal for holders of RTI preferred stock, voting separately as a class and (ii) no effect on the Share Issuance Proposal for the holders of RTI common stock and RTI preferred stock (on a fully converted basis).
It is important that your shares be represented and voted whether or not you plan to attend the RTI special meeting in person. Instructions regarding the different methods for voting your shares are provided under the section entitled “Questions and Answers about the Special Meeting of RTI Stockholders and the Consent Solicitation of PS Spine Unitholders.”
| By | Order of the Board of Directors
| |
Jonathon M. Singer Chief Financial and Administrative Officer, Corporate Secretary | ||
February 6, 2019
PS SPINE HOLDCO, LLC
505 Park Avenue, 14th Floor
New York, New York 10022
NOTICE OF SOLICITATION OF WRITTEN CONSENT
To the Unitholdersof PS Spine Holdco, LLC (“PS Spine”):
Pursuant to a Master Transaction Agreement, dated as of November 1, 2018 (as may be amended from time to time, the “Master Transaction Agreement”), by and among RTI Surgical, Inc. a Delaware corporation (“RTI”), PS Spine, Bears Holding Sub, Inc., a Delaware corporation and direct wholly owned subsidiary of RTI (“Holdco”), and Bears Merger Sub, Inc., a Delaware corporation and direct wholly owned subsidiary of Holdco (“Merger Sub”), and subject to the terms and conditions of the Master Transaction Agreement, (i) PS Spine will contribute all of the issued and outstanding equity interests of Paradigm Spine, LLC, a Delaware limited liability company and wholly owned subsidiary of PS Spine, to Holdco (the “Contribution”), (ii) Merger Sub will be merged with and into RTI, with RTI surviving as a wholly owned subsidiary of Holdco, and (iii) Holdco will be renamed “RTI Surgical Holdings, Inc.” (collectively, the “Transaction”).
As more specifically described in the enclosed joint proxy and consent solicitation statement/prospectus, the approval of the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution, by the unitholders of PS Spine requires the affirmative consent or vote of the holders of66-2/3% of the outstanding Common Units, Class A Common Units, Class A Preferred Units, Class B Preferred Units,Class E-1 Preferred Units and Class F Preferred Units of PS Spine, given in writing or by vote at a meeting, consenting or voting as a single class, as well as the affirmative consent or vote of the holders of a majority of the outstanding Class A Preferred Units and a majority of the outstanding Class B Preferred Units of PS Spine, given in writing or by vote at a meeting, each consenting or voting as a separate class.
The enclosed joint proxy and consent solicitation statement/prospectus is being delivered to you on behalf of the board of managers of PS Spine to request that unitholders of PS Spine, as of the record date of February 6, 2019, execute and return written consents to approve the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution.
The joint proxy and consent solicitation statement/prospectus describes the proposed Transaction and the actions to be taken in connection with the Transaction and provides additional information about the parties involved. We encourage you to read carefully the entire joint proxy and consent solicitation statement/prospectus, including all its annexes, the documents incorporated by reference therein and the section entitled “Risk Factors”, beginning on page 38.
The board of managers of PS Spine has carefully considered the Master Transaction Agreement, the terms thereof and the transactions contemplated thereby, including the Contribution, and has declared that the Master Transaction Agreement, the terms thereof and the transactions contemplated thereby, including the Contribution, are advisable and fair to and in the best interests of PS Spine and its unitholders. Accordingly, the board of managers of PS Spine unanimously recommends that unitholders of PS Spine approve the Master Transaction Agreement and the transactions contemplated thereby.
After your review of the joint proxy and consent solicitation statement/prospectus and assuming your approval thereof,please sign and complete the written consent furnished with the enclosed joint proxy and consent solicitation statement/prospectus and return it to PS Spine by one of the means described under “The PS Spine Solicitation of Written Consents.”Time is of the essence, and, assuming your approval thereof, you must return the written consent by February 13, 2019.
Thank you for your prompt attention to these matters.
Yours truly,
|
| Marc R. Viscogliosi |
| Chairman and Chief Executive Officer |
NO MEETING OF THE UNITHOLDERS OF PS SPINE IS BEING HELD IN CONNECTION WITH THE PROPOSED TRANSACTION. PS SPINE IS SOLICITING BY THE ENCLOSED CONSENT MATERIALS YOUR WRITTEN CONSENT TO THE MASTER TRANSACTION AGREEMENT AND THE TRANSACTIONS CONTEMPLATED THEREBY, INCLUDING THE CONTRIBUTION.
ADDITIONAL INFORMATION
The accompanying joint proxy and consent solicitation statement/prospectus incorporates by reference important business and financial information about RTI from documents that are not included in or delivered with the accompanying joint proxy and consent solicitation statement/prospectus. You can obtain the documents that are incorporated by reference into the accompanying joint proxy and consent solicitation statement/prospectus (other than certain exhibits or schedules to those documents), without charge, by requesting them in writing or by telephone from RTI at the following addresses and telephone numbers, or through the Securities and Exchange Commission website at www.sec.gov:
RTI Surgical, Inc.
11621 Research Circle
Alachua, Florida 32615
Attn: Investor Relations, Molly Poarch
Telephone:(386) 418-8888
To obtain timely delivery of the documents, you must request them no later than five business days before the date of the RTI special meeting or the PS Spine deadline for submitting written consents. Therefore, if you would like to request documents from RTI, please do so by February 28, 2019 in order to receive them before the RTI special meeting or by February 6, 2019 in order to receive them before the PS Spine deadline for submitting written consents.
The accompanying joint proxy and consent solicitation statement/prospectus includes and contains calculations based upon shares of RTI common stock outstanding and entitled to vote and holders of record as of January 15, 2019, the most recent practicable date prior to the date of filing of the joint proxy and consent solicitation statement/prospectus. RTI does not believe such totals will change materially as of the RTI record date and undertakes to file a Current Report on Form 8-K with such updated totals promptly after the RTI record date.
In addition, if you have questions about the proposed transaction or the accompanying joint proxy and consent solicitation statement/prospectus, would like additional copies of the accompanying joint proxy and consent solicitation statement/prospectus or need to obtain proxy cards or other information related to the joint proxy and consent solicitation, please contact Georgeson LLC, the proxy solicitor for RTI, toll-free at (866)391-6921 or PS Spine toll-free at (888)273-9897 or by email at InvestorRelations@paradigmspine.com. You will not be charged for any of these documents that you request.
For more information, see the section entitled “Where You Can Find More Information”, beginning on page 187 of the accompanying joint proxy and consent solicitation statement/prospectus.
Unless otherwise indicated or as the context otherwise requires, all references in this joint proxy and consent solicitation statement/prospectus to:
| • | “combined company” refer collectively to Holdco, RTI and Paradigm, following completion of the Transaction; |
| • | “Contribution” refer to the contribution by PS Spine of all of the issued and outstanding equity interests in Paradigm to Holdco; |
| • | “Holdco” refer to Bears Holding Sub, Inc., a Delaware corporation and direct wholly owned subsidiary of RTI; |
| • | “Holdco common stock” refer to shares of common stock of Holdco, par value $0.001 per share; |
| • | “Holdco preferred stock” refer to shares of Series A preferred stock of Holdco, par value $0.001 per share; |
| • | “Master Transaction Agreement” refer to the Master Transaction Agreement, dated as of November 1, 2018, as it may be amended from time to time, by and among RTI, PS Spine, Holdco and Merger Sub, a copy of which is attached asAnnex A to this joint proxy and consent solicitation statement/prospectus and is incorporated herein by reference; |
| • | “Merger” refer to the merger of Merger Sub with and into RTI such that the separate corporate existence of Merger Sub will thereupon cease; |
| • | “Merger Sub” refer to Bears Merger Sub, Inc., a Delaware corporation and direct wholly owned subsidiary of Holdco; |
| • | “Paradigm” refer to Paradigm Spine, LLC, a Delaware limited liability company and wholly owned subsidiary of PS Spine; |
| • | “PS Spine” refer to PS Spine Holdco, LLC, a Delaware limited liability company; |
| • | “PS Spine unitholders” refer to holders of PS Spine units; |
| • | “RTI” refer to RTI Surgical, Inc., a Delaware corporation; |
| • | “RTI common stock” refer to shares of common stock of RTI, par value $0.001 per share; |
| • | “RTI preferred stock” refer to shares of Series A preferred stock of RTI, par value $0.001 per share; |
| • | “RTI stockholders” refer to collectively to holders of RTI common stock and holders of RTI preferred stock; |
| • | “Transaction” refer collectively to the Merger and the Contribution; and |
| • | “we”, “our” and “us” refer to RTI and PS Spine, collectively. |
For an index of other defined terms used in this joint proxy and consent solicitation statement/prospectus, see “Index of Defined Terms”.
ABOUT THIS JOINT PROXY AND CONSENT SOLICITATION STATEMENT/PROSPECTUS
This joint proxy and consent solicitation statement/prospectus, which forms part of a registration statement on FormS-4 filed with the U.S. Securities and Exchange Commission by Holdco (FileNo. 333-228694), constitutes a prospectus of Holdco under Section 5 of the Securities Act of 1933, as amended, with respect to the Holdco shares to be issued in connection with the transactions pursuant to the Master Transaction Agreement. This joint proxy and consent solicitation statement/prospectus also constitutes a proxy statement under Section 14(a) of the Securities Exchange Act of 1934, as amended. It also constitutes a notice of meeting with respect to the RTI special meeting and a notice of solicitation of written consent with respect to the PS Spine consent solicitation.
You should rely only on the information contained in or incorporated by reference into this joint proxy and consent solicitation statement/prospectus. No one has been authorized to provide you with information that is different from that contained in, or incorporated by reference into, this joint proxy and consent solicitation statement/prospectus. This joint proxy and consent solicitation statement/prospectus is dated February 6, 2019. You should not assume that the information contained in, or incorporated by reference into, this joint proxy and consent solicitation statement/prospectus is accurate as of any date other than the date of the applicable document that contains that information. Neither our mailing of this joint proxy and consent solicitation statement/prospectus to RTI stockholders and PS Spine unitholders, nor the issuance by Holdco of its common stock and preferred stock in connection with the transactions pursuant to the Master Transaction Agreement, will create any implication to the contrary.
This joint proxy and consent solicitation statement/prospectus does not constitute an offer to sell, or a solicitation of an offer to buy, any securities, or the solicitation of a proxy or a written consent, in any jurisdiction to or from any person to whom it is unlawful to make any such offer or solicitation in such jurisdiction. Information contained in this joint proxy and consent solicitation statement/prospectus regarding RTI and Holdco has been provided by RTI and Holdco and information contained in this joint proxy and consent solicitation statement/prospectus regarding PS Spine and Paradigm has been provided by PS Spine.
| 1 | ||||
| 3 | ||||
| 3 | ||||
| 8 | ||||
| 13 | ||||
| 16 | ||||
| 16 | ||||
| 18 | ||||
| 20 | ||||
| 21 | ||||
| 21 | ||||
| 22 | ||||
| 22 | ||||
| 22 | ||||
| 23 | ||||
| 23 | ||||
Board of Directors and Management of Holdco after the Transaction | 23 | |||
| 23 | ||||
| 24 | ||||
| 25 | ||||
| 25 | ||||
| 25 | ||||
| 25 | ||||
| 26 | ||||
| 27 | ||||
| 27 | ||||
| 28 | ||||
| 28 | ||||
| 28 | ||||
Comparison of Rights of Holdco Stockholders, RTI Stockholders and the Paradigm Member | 29 | |||
Summary Selected Historical Consolidated Financial Data for RTI | 30 | |||
Summary Selected Historical Consolidated Financial Data for Paradigm | 32 | |||
Summary Selected Unaudited Pro Forma Condensed Combined Financial Information | 33 | |||
Unaudited Pro Forma Combined Per Share and Per Unit Information | 34 | |||
| 36 | ||||
i
| 38 | ||||
| 38 | ||||
| 48 | ||||
| 48 | ||||
| 61 | ||||
| 61 | ||||
| 61 | ||||
| 61 | ||||
| 61 | ||||
| 62 | ||||
| 62 | ||||
| 62 | ||||
| 63 | ||||
| 64 | ||||
| 64 | ||||
| 65 | ||||
| 66 | ||||
| 68 | ||||
| 69 | ||||
| 69 | ||||
| 69 | ||||
| 69 | ||||
| 69 | ||||
| 69 | ||||
| 70 | ||||
| 70 | ||||
| 71 | ||||
PS SPINE PROPOSAL 1—APPROVAL OF THE MASTER TRANSACTION AGREEMENT | 72 | |||
| 73 | ||||
| 73 | ||||
| 73 | ||||
Recommendation of the RTI Board and Its Reasons for the Transaction | 79 | |||
Recommendation of PS Spine Board and Its Reasons for the Transaction | 80 | |||
| 84 | ||||
| 102 | ||||
| 108 | ||||
| 108 | ||||
| 109 | ||||
| 109 | ||||
ii
| 109 | ||||
| 113 | ||||
| 113 | ||||
| 114 | ||||
Restrictions on Sales of Shares of Holdco Received in the Transaction | 114 | |||
Interests of PS Spine’s and Paradigm’s Managers and Executive Officers in the Transaction | 114 | |||
Board of Directors and Management of Holdco after the Transaction | 119 | |||
Board of Directors and Management of the Surviving Corporation after the Transaction | 119 | |||
| 119 | ||||
| 120 | ||||
| 121 | ||||
| 121 | ||||
| 121 | ||||
| 121 | ||||
| 123 | ||||
| 123 | ||||
| 123 | ||||
| 124 | ||||
| 124 | ||||
| 125 | ||||
| 126 | ||||
| 128 | ||||
| 130 | ||||
| 139 | ||||
| 141 | ||||
| 142 | ||||
| 144 | ||||
| 145 | ||||
| 147 | ||||
| 147 | ||||
| 147 | ||||
| 147 | ||||
| 149 | ||||
| 149 | ||||
| 149 | ||||
| 150 | ||||
| 150 | ||||
| 151 | ||||
iii
| 151 | ||||
| 151 | ||||
| 152 | ||||
| 152 | ||||
| 152 | ||||
| 152 | ||||
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS OF PARADIGM | 153 | |||
| 153 | ||||
Critical Accounting Policies and Significant Judgments and Estimates | 154 | |||
| 155 | ||||
| 156 | ||||
| 157 | ||||
| 159 | ||||
| 159 | ||||
| 159 | ||||
| 159 | ||||
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT OF RTI | 160 | |||
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT OF PS SPINE | 163 | |||
| 166 | ||||
| 166 | ||||
| 168 | ||||
| 168 | ||||
| 170 | ||||
COMPARISON OF RIGHTS OF HOLDCO STOCKHOLDERS, RTI STOCKHOLDERS AND THE PARADIGM MEMBER | 172 | |||
| 179 | ||||
NOTES TO UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION | 183 | |||
| 186 | ||||
| 186 | ||||
| 186 | ||||
| 186 | ||||
| 186 | ||||
| 186 | ||||
| 187 | ||||
| 187 | ||||
| 189 | ||||
| F-1 | ||||
iv
CAUTIONARY STATEMENT CONCERNINGFORWARD-LOOKING STATEMENTS
This joint proxy and consent solicitation statement/prospectus and the documents that are incorporated into this joint proxy and consent solicitation statement/prospectus by reference contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on the current expectations, estimates and projections of RTI, Holdco, PS Spine and Paradigm about their industry, the respective beliefs of the management of RTI, Holdco, PS Spine and Paradigm, and certain assumptions made by the management of RTI, Holdco, PS Spine and Paradigm. Words such as “anticipates,” “believes,” “estimates,” “expects,” “intends,” “plans,” “projects,” “seeks,” “targets,” variations of such words and similar expressions are intended to identify such forward-looking statements. In addition, except for historical information, any statements made in this joint proxy and consent solicitation statement/prospectus or any of the documents that are incorporated into this joint proxy and consent solicitation statement/prospectus by reference about: the respective financial condition of RTI, Holdco, PS Spine or Paradigm; expected financial results of the Transaction, including any projections or pro forma financial statements; the impact of the Transaction on the complexity of combined company’s operations; any benefits of scaling caused by the Transaction; the impact of the Transaction on the combined company’s growth rates; potential long-term growth for coflex® products; the impact of the Transaction on the combined company’s market share; the retention of current customers or the acquisition of additional customers; and the payment of any potential Earnout Consideration (as defined below) to PS Spine also are forward-looking statements. Many factors could affect the actual financial results of RTI, Holdco, PS Spine and Paradigm and cause them to vary materially from the expectations contained in the forward-looking statements, including those set forth in, or incorporated by reference into, this joint proxy and consent solicitation statement/prospectus. These statements are not guarantees of future performance and are subject to risks and uncertainties. In addition to the risk factors described under the section entitled “Risk Factors”, beginning on page38, these risks and uncertainties include, among other things:
| • | those identified and disclosed in Part I, Item 1.A., Risk Factors, of RTI’s Annual Report on Form10-K for the year ended December 31, 2017, as filed with the United States Securities and Exchange Commission (the “SEC”) on March 2, 2018; |
| • | the failure to obtain RTI stockholder approval of the proposed Transaction; |
| • | the failure to obtain PS Spine unitholder approval of the proposed Transaction; |
| • | the possibility that the closing conditions to the proposed Transaction may not be satisfied or may be waived, including that a governmental entity may prohibit, delay or refuse to grant a necessary regulatory approval and any conditions imposed in connection with consummation of the proposed Transaction; |
| • | delay in closing the proposed Transaction or the possibility ofnon-consummation of the proposed Transaction; |
| • | the risk that the cost savings and any other synergies from the proposed Transaction may not be fully realized or may take longer to realize than expected, including that the proposed Transaction may not be accretive within the expected timeframe or to the extent anticipated; |
| • | the occurrence of any event that could give rise to termination of the Master Transaction Agreement; |
| • | the risk that litigation in connection with the proposed Transaction may affect the timing or occurrence of the proposed Transaction or result in significant costs of defense, indemnification and liability; |
| • | risks related to the disruption of the proposed Transaction to RTI, Holdco or Paradigm and their management; |
| • | the effect of the announcement of the proposed Transaction on the ability of RTI, Holdco or Paradigm to retain and hire key personnel and maintain relationships with customers, suppliers, third-party distributors and other third parties; |
| • | RTI’s and Paradigm’s inability to pursue alternative business opportunities or make appropriate changes to their respective businesses because of requirements in the Master Transaction Agreement that they conduct their businesses in all material respects in the ordinary course of business consistent with past practice and not engage in certain activities prior to the completion of the Transaction; |
| • | delays or deferments of certain business decisions by RTI’s and Paradigm’s customers, suppliers, third-party distributors and other business partners; |
| • | the ability and timing to obtain required regulatory approvals and satisfy other closing conditions; |
1
| • | the incurrence of significant costs, expenses and fees for professional services and other transaction costs associated with the Transaction; |
| • | the reduction in the ownership percentage of RTI’s stockholders and PS Spine in the combined company after the consummation of the Transaction; |
| • | the risk that RTI’s share price may fluctuate prior to the completion of the Transaction, and the combined company’s share price may fluctuate following the completion of the Transaction; |
| • | the risk that the monetary value of the Stock Consideration Amount (as defined below) and certain portions of the Earnout Consideration may change following the time of the signing of the Master Transaction Agreement or the date of this joint proxy and consent solicitation statement/prospectus due to a change in the value of Holdco common stock; |
| • | the risk that a significant portion of the consideration to be paid to PS Spine pursuant to the Master Transaction Agreement is contingent on the occurrence of certain events in the future; |
| • | the risk that the market price of the common stock of the combined company may be affected by factors different from those affecting the market price for shares of RTI common stock; |
| • | the risks associated with the incurrence of additional indebtedness to finance the Cash Consideration Amount (as defined below) and pay other costs and expenses incurred in connection with the Transaction, which may adversely affect the business, financial condition and operating results of the combined company; |
| • | the risk that a significant portion of the consideration that would otherwise be paid to PS Spine pursuant to the Master Transaction Agreement will be used to pay amounts owed to the lenders under Paradigm’s existing senior secured credit agreement and their affiliates; and |
| • | the risk that PS Spine and Paradigm managers and executive officers may have interests in the Transaction different from the interests of PS Spine unitholders. |
The areas of risk and uncertainty described above should be considered in connection with any written or oral forward-looking statements that may be made by RTI, Holdco, PS Spine or Paradigm or anyone acting for any or all of them. Except for their ongoing obligations to disclose material information under the U.S. federal securities laws, none of RTI, Holdco, PS Spine and Paradigm undertakes any obligation to release publicly any revisions to any forward-looking statements, to report events or circumstances after the date of this joint proxy and consent solicitation statement/prospectus or to report the occurrence of unanticipated events or actual outcomes.
For additional information about factors that could cause actual results to differ materially from those described in the forward-looking statements, see the note regarding forward-looking statements in Item 7 of RTI’s Annual Report on Form10-K for the year ended December 31, 2017, as filed with the SEC on March 2, 2018, and incorporated by reference in this joint proxy and consent solicitation statement/prospectus. See the sections entitled “Additional Information” and “Where You Can Find More Information”.
RTI, Holdco, PS Spine and Paradigm also caution you that undue reliance should not be placed on any forward-looking statements, which speak only as of the date of this joint proxy and consent solicitation statement/prospectus. Actual results may differ materially from the expected results reflected in forward-looking statements. Copies of RTI’s SEC filings may be obtained by contacting RTI or the SEC or by visiting RTI’s website at www.rtix.com or the SEC’s website at www.sec.gov.
2
QUESTIONS AND ANSWERS ABOUT THE SPECIAL MEETING OF RTI STOCKHOLDERS AND THE CONSENT SOLICITATION OF PS SPINE UNITHOLDERS
The following are some questions that you, as an RTI stockholder or a PS Spine unitholder, may have regarding the special meeting of RTI stockholders (the “RTI special meeting”) or the consent solicitation of PS Spine unitholders (the “PS Spine consent solicitation”), and brief answers to those questions. For more information about the matters discussed in these questions and answers, see the sections entitled “The RTI Special Meeting” and “The PS Spine Solicitation of Written Consents”, beginning on pages 61 and 69, respectively, of this joint proxy and consent solicitation statement/prospectus. RTI and PS Spine encourage you to read carefully the remainder of this joint proxy and consent solicitation statement/prospectus because the information in this section does not provide all of the information that might be important to you with respect to the matters being considered at the RTI special meeting or the PS Spine consent solicitation. Additional important information is also contained in the Annexes to, and in the documents incorporated by reference into, this joint proxy and consent solicitation statement/prospectus. See the section entitled “Where You Can Find More Information”, beginning on page 187 of this joint proxy and consent solicitation statement/prospectus. RTI and PS Spine are collectively referred to as “we,” “us,” and “our.”
| Q: | What is the proposed transaction on which RTI stockholders are being asked to vote and to which PS Spine unitholders are being asked to consent? |
| A: | RTI, PS Spine, Holdco and Bears Merger Sub, Inc., a Delaware corporation and direct wholly owned subsidiary of Holdco (“Merger Sub”), entered into the Master Transaction Agreement that is described in this joint proxy and consent solicitation statement/prospectus, and a copy of which is attached asAnnex A. Subject to the terms and conditions set forth in the Master Transaction Agreement, at the closing contemplated by the Master Transaction Agreement (the “closing”), Holdco will acquire all of the outstanding equity interests in Paradigm, a wholly owned subsidiary of PS Spine, through a transaction in which: (i) PS Spine will contribute all of the issued and outstanding equity interests in Paradigm to Holdco (i.e., the Contribution), (ii) Merger Sub will be merged with and into RTI (i.e., the Merger), with RTI surviving the Merger as a wholly owned direct subsidiary of Holdco and (iii) Holdco will be renamed “RTI Surgical Holdings, Inc.” (collectively, the “Transaction”). The Merger and the Contribution will become effective concurrently (such time as the Merger and the Contribution become effective, the “effective time”). |
If the Transaction is completed, as consideration for the Contribution, at the closing Holdco will pay PS Spine $100.0 million in cash, subject to adjustment as described below (the “Cash Consideration Amount”), and will issue 10,729,614 fully paid andnon-assessable shares of Holdco common stock in the aggregate (the “Stock Consideration Amount”) to PS Spine and to the lenders under Paradigm’s existing senior secured credit agreement and their affiliates. The Stock Consideration Amount was determined by dividing $50.0 million by the volume weighted average closing price of RTI common stock for the five business days prior to the date of the Master Transaction Agreement, which was $4.66 per share of RTI common stock (the “RTI Price”). The Cash Consideration Amount is subject to the following adjustments: (i) positive dollar-for-dollar adjustment based on the amount of Paradigm’s cash and cash equivalents at closing, (ii) negative dollar-for-dollar adjustment based on the amount of outstanding indebtedness and unpaid transaction expenses of Paradigm at closing and (iii) negative dollar-for-dollar adjustment to the extent that Paradigm’s working capital (excluding cash, cash equivalents, indebtedness and transaction expenses) at closing is less than the working capital target of $7.0 million. At the closing, due to the expected amount of Paradigm’s indebtedness and transaction expenses, all cash that would otherwise be paid at closing pursuant to the Master Transaction Agreement to PS Spine, as well as a portion of the Stock Consideration Amount, is expected to be used to satisfy Paradigm’s outstanding indebtedness and unpaid transaction expenses.
In addition to the Cash Consideration Amount and the Stock Consideration Amount payable at the closing, Holdco may be required to make further cash payments or issue additional shares of Holdco common stock to PS Spine in an amount up to an additional $50.0 million of shares of Holdco common stock to be valued based upon
3
the RTI Price and an additional $100.0 million of cash and/or Holdco common stock to be valued at the time of issuance (the “Earnout Consideration”), in each case, if certain revenue targets, which are described in section entitled “The Master Transaction Agreement—Purchase Price—Contingent Consideration”, beginning on page 127, are achieved between closing and December 31, 2022. In certain circumstances, described in greater detail in this joint proxy and consent solicitation statement/prospectus, $10.0 million of the Earnout Consideration (which can take the form of cash, stock or a combination of both) will be paid to the lenders under Paradigm’s existing senior secured credit agreement and/or their affiliates.
If the Transaction is completed, (a) each share of RTI common stock issued and outstanding immediately prior to the effective time of the Merger (other than shares held by RTI as treasury shares or by Holdco or Merger Sub immediately prior to the effective time, which shall be automatically cancelled and cease to exist) will be converted automatically into one fully paid andnon-assessable share of Holdco common stock, (b) each share of RTI preferred stock issued and outstanding immediately prior to the effective time of the Merger (other than shares held by RTI as treasury shares or by Holdco or Merger Sub immediately prior to the effective time which shall be automatically cancelled and cease to exist) shall be converted automatically into one fully paid and non-assessable share of Holdco preferred stock and (c) each stock option and restricted stock award granted by RTI will be converted into a stock option or restricted stock award, as applicable, of Holdco with respect to an equivalent number of shares of Holdco common stock on the same terms and conditions as were applicable prior to the closing.
After the consummation of the Transaction, Holdco will own both RTI and Paradigm as wholly owned subsidiaries.
| Q: | Why am I receiving this joint proxy and consent solicitation statement/prospectus? |
| A: | If you are an RTI stockholder, you are receiving this joint proxy and consent solicitation statement/prospectus because you were a holder of record of RTI shares as of the close of business on February 1, 2019 (the “RTIrecord date”). RTI common stock and RTI preferred stock are collectively referred to as “RTI shares” or “RTI stock”. If you are a PS Spine unitholder, you are receiving this joint proxy and consent solicitation statement/prospectus because you were a holder of record of PS Spine units as of the close of business on February 6, 2019 (the “PS Spinerecord date”). |
This joint proxy and consent solicitation statement/prospectus serves as the proxy statement through which RTI will solicit proxies to obtain the necessary RTI stockholder approval of the Merger Proposal and the Share Issuance Proposal (each, as defined below) and as the consent solicitation statement for PS Spine to obtain the necessary written consent of its unitholders approving the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution. It also serves as the prospectus of Holdco to register the shares of Holdco common stock and Holdco preferred stock to be issued in the Transaction.
RTI is holding a special meeting of its stockholders in order to obtain the stockholder approval necessary to approve the Merger Proposal and the Share Issuance Proposal. RTI stockholders will also be asked to approve the adjournment of the RTI special meeting (if necessary or appropriate to solicit additional proxies if there are not sufficient votes to adopt the Merger Proposal or the Share Issuance Proposal).
PS Spine is seeking the written consent of its unitholders to approve the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution.
We will be unable to complete the Transaction unless, among other things, the RTI stockholders vote to adopt the Merger Proposal and the Share Issuance Proposal and the PS Spine unitholders provide their written consent to adopt the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution.
This joint proxy and consent solicitation statement/prospectus contains important information about the Merger, the Contribution, the Master Transaction Agreement, the RTI special meeting and the PS Spine consent solicitation. You should read this information carefully and in its entirety. The enclosed voting materials allow RTI stockholders to vote RTI shares without attending the RTI special meeting. No meeting of the unitholders of PS Spine is being held in connection with the proposed Transaction. PS Spine is soliciting the consent of PS Spine unitholders by the enclosed consent materials.
4
| Q: | What will RTI stockholders receive in connection with the Transaction? |
| A: | If the Transaction is completed, holders of RTI common stock will receive one share of Holdco common stock for each share of RTI common stock, and holders of RTI preferred stock will receive one share of Holdco preferred stock for each share of RTI preferred stock. |
| Q: | What will PS Spine receive in the Transaction? |
| A: | If the Transaction is completed, as consideration for the Contribution, Holdco will pay PS Spine $100.0 million in cash, subject to adjustment as described below, representing the Cash Consideration Amount, and will issue 10,729,614 fully paid andnon-assessable shares of Holdco common stock in the aggregate, representing the Stock Consideration Amount, to PS Spine and to the lenders under Paradigm’s existing senior secured credit agreement and their affiliates. The Stock Consideration Amount was determined by dividing $50.0 million by the RTI Price. The Cash Consideration Amount is subject to the following adjustments: (i) positive dollar-for-dollar adjustment based on the amount of Paradigm’s cash and cash equivalents at closing, (ii) negative dollar-for-dollar adjustment based on the amount of outstanding indebtedness and unpaid transaction expenses of Paradigm at closing and (iii) negative dollar-for-dollar adjustment to the extent that Paradigm’s working capital (excluding cash, cash equivalents, indebtedness and transaction expenses) at closing is less than the working capital target of $7.0 million. At the closing, due to the expected amount of Paradigm’s indebtedness and transaction expenses, all cash that would otherwise be paid at closing pursuant to the Master Transaction Agreement to PS Spine, as well as a portion of the Stock Consideration Amount, is expected to be used to satisfy Paradigm’s outstanding indebtedness and unpaid transaction expenses. |
In addition to the Cash Consideration Amount and the Stock Consideration Amount payable at the closing, Holdco may be required to make further cash payments or issue additional shares of Holdco common stock to PS Spine in an amount up to an additional $50.0 million of shares of Holdco common stock to be valued based upon the RTI Price and an additional $100.0 million of cash and/or Holdco common stock to be valued at the time of issuance, representing the Earnout Consideration, in each case, if certain revenue targets, which are described in the section entitled “The Master Transaction Agreement—Purchase Price—Contingent Consideration”, beginning on page 127, are achieved between closing and December 31, 2022. In certain circumstances, described in greater detail in this joint proxy and consent solicitation statement/prospectus, $10.0 million of the Earnout Consideration (which can take the form of cash, stock or a combination of both) will be paid to the lenders under Paradigm’s existing senior secured credit agreement and/or their affiliates.
PS Spine intends to distribute the consideration that it receives pursuant to the Master Transaction Agreement (net of transaction and other expenses and amounts due to its senior lenders and their affiliates) to its unitholders, in accordance with PS Spine’s organizational documents, as soon as reasonably practicable following its receipt thereof.
| Q: | What ownership percentage of Holdco will former RTI stockholders and PS Spine have after the Transaction is completed? |
| A: | If the Transaction occurs, 10,729,614 shares of common stock of Holdco will be issued at closing as partial consideration for the Contribution. A significant portion of these shares will be used, along with cash that would otherwise be paid at closing, to pay the amounts outstanding under Paradigm’s existing senior secured credit agreement. Assuming (i) the closing occurs on March 15, 2019, (ii) 62,245,112 shares of RTI common stock (based on the number of shares outstanding as of January 15, 2019) and 50,000 shares of RTI preferred stock are issued and outstanding immediately prior to the effective time and (iii) the amount of cash paid to the lenders under Paradigm’s existing senior secured credit agreement at closing does not exceed $95.0 million, and taking into account the $3.0 million Paradigm borrowed from the lenders under its existing senior secured credit agreement on December 6, 2018, approximately 85.30% of the issued and outstanding Holdco common stock and 100% of the issued and outstanding Holdco preferred stock immediately following the closing will be held by former RTI stockholders, approximately 6.42% of the issued and outstanding Holdco common stock immediately following the closing will be held by PS Spine and approximately 8.28% of the issued and outstanding Holdco common stock immediately following the closing will be held by the lenders under Paradigm’s existing |
5
| senior secured credit agreement and their affiliates. Using the same assumptions set forth above and assuming the conversion of the RTI preferred stock, immediately following the closing, former holders of RTI common stock and RTI preferred stock will collectively hold 87.37% of the voting power of Holdco, PS Spine will hold 5.51% of the voting power of Holdco and the lenders under Paradigm’s existing senior secured credit agreement and their affiliates will hold 7.12% of the voting power of Holdco. As described in the section entitled “The Transaction—Interests of PS Spine’s and Paradigm’s Managers and Executive Officers in the Transaction—The Settlement Agreement”, the lenders under Paradigm’s existing senior secured credit agreement and their affiliates may receive a smaller or larger portion of the Stock Consideration Amount than as assumed for purposes of this example. If the lenders under Paradigm’s existing senior secured credit agreement and their affiliates receive a larger portion of the Stock Consideration Amount than as assumed for purposes of this example, the percentage of the issued and outstanding Holdco common stock immediately following the closing that will be held by the lenders under Paradigm’s existing senior secured credit agreement and their affiliates will be greater than set forth in this example and the percentage that will be held by PS Spine will be less than set forth in this example. If the lenders under Paradigm’s existing senior secured credit agreement and their affiliates receive a smaller portion of the Stock Consideration Amount than as assumed for purposes of this example, the percentage of the issued and outstanding Holdco common stock immediately following the closing that will be held by the lenders under Paradigm’s existing senior secured credit agreement and their affiliates will be less than set forth in this example and the percentage that will be held by PS Spine will be greater than set forth in this example. In addition, as described in greater detail herein, the earnout provisions of the Master Transaction Agreement provide for the possibility that PS Spine may receive additional shares of Holdco common stock, which would cause former RTI stockholders ownership of Holdco to be further diluted. |
For more information regarding the risk associated with RTI stockholders’ and PS Spine unitholders’ reduced ownership in the combined company, see “Risk Factors—Risks Related to the Transaction and the Combined Company—RTI stockholders and PS Spine unitholders will have a reduced ownership and voting interest after the Transaction and will each exercise less influence over management”.
| Q: | Should I, as an RTI stockholder, send in my stock certificates now for the exchange? |
| A: | RTI stockholders are not required to take any action to receive shares of Holdco stock. At the effective time, each share of RTI common stock will automatically be converted into a share of Holdco common stock. Similarly, at the effective time, each share of RTI preferred stock will automatically be converted into a share of Holdco preferred stock. At the effective time, each valid certificate or certificates which immediately prior to the effective time represented any such shares of RTI common stock or RTI preferred stock ornon-certificated shares of RTI common stock or RTI preferred stock held in book entry form, shall automatically represent an equivalent number of shares of Holdco common stock or Holdco preferred stock, as applicable (without any requirement for the surrender of any such certificates ornon-certificated shares). |
| Q: | Is there an exchange agent for the Transaction? |
| A: | No. Because each valid certificate of RTI stock shall, upon the effective time, automatically represent an equivalent number of shares of Holdco common stock or Holdco preferred stock, as applicable, no exchange of RTI stock certificates for Holdco stock certificates will take place. Therefore, an exchange agent is not needed for the Transaction. |
| Q: | When do you expect the Transaction to be completed? |
| A: | We are currently targeting completion of the Transaction by the end of the first quarter of 2019, subject to the required RTI stockholder approval, the required written consent of PS Spine unitholders and the satisfaction or waiver of the other closing conditions. It is possible that factors outside the control of RTI or PS Spine could result in the Transaction being completed at a later time, or not at all. |
6
| Q: | What effects will the Transaction have on RTI and Paradigm? |
| A: | Upon completion of the Transaction, RTI will cease to be a publicly traded company. Merger Sub will merge with and into RTI, with RTI surviving as a wholly owned subsidiary of Holdco. In addition, upon completion of the Transaction, Holdco will own all of the outstanding equity interests in Paradigm. As a result of the Transaction, RTI stockholders will own shares of Holdco and will not directly own any shares of RTI, and PS Spine will own shares of Holdco and will not directly own any equity interest in Paradigm. |
Following the completion of the Transaction, the registration of the RTI common stock and RTI preferred stock and their respective reporting obligations under the Securities Exchange Act of 1934, as amended (the “Exchange Act”) will be terminated. In addition, following the completion of the Transaction, shares of RTI common stock will no longer be listed on the Nasdaq Global Select Market (“Nasdaq”) or any other stock exchange or quotation system. However, as explained below, we expect that Holdco common stock will be listed on the Nasdaq Global Market under the symbol “RTIX”.
Although current RTI stockholders will no longer be stockholders of RTI after the Transaction, current RTI stockholders will have an indirect interest in both RTI and Paradigm through their ownership of Holdco common stock or Holdco preferred stock. Likewise, although PS Spine will no longer directly own any equity interest in Paradigm, PS Spine will have an indirect interest in both RTI and Paradigm through its ownership of Holdco common stock.
| Q: | What effects will the Transaction have on Holdco? |
| A: | Upon completion of the Transaction, Holdco will become the holding company of RTI and Paradigm, and Holdco will be renamed “RTI Surgical Holdings, Inc.” We expect that Holdco common stock will be listed on the Nasdaq Global Market under the symbol “RTIX”. |
| Q: | Who will manage the combined company after the Transaction? |
| A: | The executive officers of RTI immediately prior to the closing will become the executive officers of Holdco as of the effective time. Camille I. Farhat is anticipated to remain as Chief Executive Officer of Holdco. The directors of RTI immediately prior to the closing, as well as Jeffrey C. Lightcap, who is currently a manager of PS Spine and Paradigm, will become the directors of Holdco as of the effective time. |
| Q: | What will happen to outstanding RTI equity awards in the Transaction? |
| A: | At the effective time, each RTI Stock Option that is outstanding immediately prior to the effective time, whether vested or unvested, will be converted into a stock option in respect of shares of Holdco common stock, on the same terms and conditions as were applicable under such RTI Stock Option immediately prior to the effective time (including with respect to vesting). At the effective time, each RTI Restricted Stock Award that is outstanding immediately prior to the effective time will be converted into a restricted stock award with the same terms and conditions as were applicable under such RTI Restricted Stock Award immediately prior to the effective time (including with respect to vesting). For more information, see the section entitled “The Master Transaction Agreement—Treatment of RTI Equity Awards”. |
| Q: | Will RTI stockholders or PS Spine unitholders be paid dividends prior to the Transaction? |
| A: | Pursuant to the Master Transaction Agreement, RTI is not permitted to declare, set aside or pay dividends or other distributions (whether payable in cash, stock, property or a combination thereof) in respect of the stock of RTI prior to the closing of the Transaction without the consent of PS Spine. Under RTI’s current credit agreement with JPMorgan Chase Bank, N.A. (“JPM”), as lender, it is restricted from paying dividends on its common stock without the prior written consent of the administrative agent. Pursuant to the Master Transaction Agreement, PS Spine is not permitted to declare any equity dividend in respect of the PS Spine units prior to the closing of the |
7
| Transaction without the consent of RTI. Under Paradigm’s existing senior secured credit agreement, PS Spine is restricted from paying dividends or distributions to its unitholders. Any future dividends following the closing of the Transaction will be at the discretion of the Holdco board of directors (the “Holdco board”). |
| Q: | Are there any risks in the Transaction that I should consider? |
| A: | Yes. There are risks associated with all business combinations, including the Transaction between RTI and Paradigm. These risks are discussed in more detail in the section entitled “Risk Factors”. |
| Q: | What is householding and how does it affect RTI stockholders? |
| A: | The SEC permits RTI to deliver a single copy of its proxy statements and annual reports to RTI stockholders who have the same address and last name, unless RTI has received contrary instructions from such RTI stockholders. Each RTI stockholder will continue to receive a separate proxy card. This procedure, called “householding”, will reduce the volume of duplicate information RTI stockholders receive and reduce RTI’s printing and postage costs. RTI will promptly deliver a separate copy of this joint proxy and consent solicitation statement/prospectus to any such RTI stockholder upon written or oral request. A stockholder wishing to receive a separate joint proxy and consent solicitation statement/prospectus can notify RTI at RTI Surgical, Inc., 11621 Research Circle, Alachua, Florida 32615, Attention: Investor Relations, Molly Poarch, telephone: (386)418-8888. Similarly, RTI stockholders currently receiving multiple copies of these documents can request the elimination of duplicate documents by contacting RTI as described above. |
| Q: | When and where will the RTI special meeting be held? |
| A: | The RTI special meeting will be held at 520 Lake Cook Road, Suite 315, Deerfield, Illinois 60015, on March 7, 2019, at 10:00 a.m., local time. |
| Q: | Who is entitled to vote at the RTI special meeting? |
| A: | Only holders of record of RTI common stock and RTI preferred stock at the close of business on the RTI record date are entitled to notice of, and to vote at, the RTI special meeting and at any adjournment or postponement of the RTI special meeting. |
| Q: | How can I, as an RTI stockholder, attend the RTI special meeting? |
| A: | All RTI stockholders are invited to attend the RTI special meeting. You may be asked to present a valid photo identification, such as a driver’s license or passport, before being admitted to the RTI special meeting. If you hold your RTI shares in “street name”, you also may be asked to present proof of ownership to be admitted to the RTI special meeting. A brokerage statement or letter from your broker, bank, trust company or other nominee proving ownership of the shares on the RTI record date are examples of proof of ownership. To help RTI plan for the RTI special meeting, please indicate whether you expect to attend by responding affirmatively when prompted during Internet or telephone proxy submission or by following the instructions included on your proxy card. |
| Q: | What proposals will be considered at the RTI special meeting? |
| A: | At the RTI special meeting, RTI stockholders will be asked: |
| • | to consider and vote on a proposal to adopt the Master Transaction Agreement, a copy of which is attached asAnnex A to this joint proxy and consent solicitation statement/prospectus, and to approve the transactions contemplated thereby, including the Merger (see the section entitled “RTI Proposal 1: Merger Proposal”); |
8
| • | to consider and vote upon a proposal to approve, for purposes of complying with applicable provisions of Nasdaq Listing Rule 5635, the potential issuance of more than twenty percent (20%) of Holdco’s issued and outstanding common stock in connection with the transactions contemplated by the Master Transaction Agreement (see the section entitled “RTI Proposal 2: Share Issuance Proposal”); and |
| • | to consider and vote upon a proposal to adjourn the RTI special meeting, if necessary or appropriate, including to permit further solicitation of proxies in favor of the Merger Proposal or the Share Issuance Proposal if there are insufficient votes at the time of the RTI special meeting to approve the Merger Proposal or the Share Issuance Proposal (see the section entitled “RTI Proposal 3: Meeting Adjournment Proposal”). |
RTI will not transact other business at its special meeting, except such other business as may properly be brought before the RTI special meeting or any adjournments or postponements thereof.
| Q: | How does the RTI board of directors recommend that I vote? |
| A: | The RTI board of directors (the “RTI board”), on October 30, 2018, unanimously approved and adopted the Master Transaction Agreement and the transactions contemplated thereby, including the Merger, on the terms and subject to the conditions set forth therein, determined and declared that the terms of the Master Transaction Agreement and the transactions contemplated thereby, including the Merger, are advisable and in the best interests of RTI and its stockholders, and recommended that RTI stockholders approve and adopt the Master Transaction Agreement and the transactions contemplated thereby, including the Merger. The RTI board unanimously recommends that RTI stockholders vote “FOR” each of the Merger Proposal, the Share Issuance Proposal and the Meeting Adjournment Proposal. |
| Q: | How many shares of RTI common stock and RTI preferred stock are eligible to be voted? |
| A: | As of January 15, 2019, there were 62,245,112 shares of RTI common stock outstanding and entitled to vote at the RTI special meeting (including 1,056,028 unvested restricted shares of RTI common stock), held by approximately 291 holders of record. Each holder of RTI common stock is entitled to one vote for each share of RTI common stock owned as of the RTI record date. This joint proxy and consent solicitation statement/prospectus includes and contains calculations based upon shares of RTI common stock outstanding and entitled to vote and holders of record as of January 15, 2019, the most recent practicable date prior to the date of filing of this joint proxy and consent solicitation statement/prospectus. RTI does not believe such totals will change materially as of the RTI record date and undertakes to file a Current Report on Form 8-K with such updated totals promptly after the RTI record date. |
As of the RTI record date, there were 50,000 shares of RTI preferred stock outstanding and entitled to vote at the RTI special meeting, held by one holder of record. Each holder of RTI preferred stock is entitled to 239 votes for each share of RTI preferred stock owned as of the RTI record date.
| Q: | What vote is required to approve each RTI proposal? |
| A: | Assuming a quorum is present, the Merger Proposal requires (i) the affirmative vote of the holders of a majority of the outstanding shares of RTI common stock and RTI preferred stock (on a fully converted basis) entitled to vote thereon and (ii) the written consent or affirmative vote of the holders of a majority of the outstanding shares of RTI preferred stock entitled to vote thereon, voting separately as a class. Abstentions and brokernon-votes will have the same effect as a vote “AGAINST” the Merger Proposal. |
Assuming a quorum is present, the Share Issuance Proposal requires (i) the affirmative vote of a majority of votes cast on the proposal by RTI common stock and RTI preferred stock (on a fully converted basis) and (ii) the written consent or affirmative vote of the holders of a majority of the outstanding shares of RTI preferred stock, voting separately as a class. Abstentions will have (i) the same effect as a vote “AGAINST” the Share Issuance Proposal for holders of RTI preferred stock, voting separately as a class and (ii) no effect on the Share Issuance Proposal for the holders of RTI common stock and RTI preferred stock (on a fully converted basis). Brokernon-votes will have (i) the same effect as a vote “AGAINST” the Share Issuance Proposal for holders of RTI preferred stock, voting separately as a class and (ii) no effect on the Share Issuance Proposal for the holders of RTI common stock and RTI preferred stock (on a fully converted basis).
9
Assuming a quorum is present, the Meeting Adjournment Proposal requires the affirmative vote of the holders of a majority of the shares having voting power present in person or represented by proxy at the RTI special meeting. Abstentions will have the same effect as a vote “AGAINST” the Meeting Adjournment Proposal, and brokernon-votes will not affect the outcome of the vote on the Meeting Adjournment Proposal.
| Q: | How do I, as an RTI stockholder, vote? |
| A: | If you are a holder of record of RTI shares as of the RTI record date, you may vote in person by attending the RTI special meeting. Shares held beneficially in street name may be voted by you in person at the RTI special meeting only if you obtain a legal proxy from the broker, trustee or nominee that holds your shares giving you the right to vote the shares. Even if you plan to attend the RTI special meeting, RTI recommends that you also submit your proxy or voting instructions as described below so that your vote will be counted if you later decide not to attend the special meeting. |
Whether you hold shares directly as the stockholder of record or beneficially in street name, you may direct how your shares are voted without attending the RTI special meeting. If you are an RTI stockholder of record, you may vote by submitting a proxy by one of the methods described below. If you hold shares beneficially in street name, you may vote by submitting voting instructions to your broker, trustee or nominee.
| • | By Internet- RTI stockholders of record with internet access may submit proxies by following the “Vote by Internet” instructions on their proxy cards. Most RTI stockholders who hold shares beneficially in street name may vote by accessing the web site specified on the voting instruction cards provided by their brokers, trustees or nominees. |
| • | By Telephone-RTI stockholders of record who live in the United States or Canada may submit proxies by following the “Vote by Phone” instructions on their proxy cards. Most RTI stockholders who hold shares beneficially in street name and live in the United States or Canada may vote by phone by calling the number specified on the voting instruction cards provided by their brokers, trustees or nominees. |
| • | By Mail- RTI stockholders of record may submit proxies by completing, signing and dating their proxy cards and mailing them in the accompanyingpre-addressed envelopes. RTI stockholders who hold shares beneficially in street name may vote by mail by completing, signing and dating the voting instruction cards provided by their brokers, trustees or nominees and mailing them in the accompanyingpre-addressed envelopes. |
| Q: | What will happen if I, as an RTI stockholder, abstain from voting? |
| A: | Assuming a quorum is present, abstentions will have (i) the same effect as (A) a vote “AGAINST” the Merger Proposal and the Adjournment Proposal, and (B) a vote “AGAINST” the Share Issuance Proposal for holders of RTI preferred stock, voting separately as a class and (ii) no effect on the Share Issuance Proposal for the holders of RTI common stock and RTI preferred stock (on a fully converted basis). Brokernon-votes will have no effect on the outcome of the proposals, assuming a quorum is present, except with respect (i) to the Merger Proposal and (ii) the vote of the RTI preferred stock, voting separately as a class, on the Share Issuance Proposal, in each case, whichbroker-non votes will have the same effect as a vote “AGAINST” such proposal. |
| Q: | What constitutes a quorum? |
| A: | The holders of a majority of the outstanding shares of RTI common stock and RTI preferred stock (on anas-converted basis) entitled to vote at the RTI special meeting, either present in person or represented by proxy, will constitute a quorum. Both abstentions and brokernon-votes are counted for the purpose of determining the presence of a quorum. |
| Q: | If my shares are held in “street name” by my broker, will my broker automatically vote my shares for me? |
| A: | No. If your brokerage firm, bank or other similar organization is the holder of record of your shares (i.e., your shares are held in “street name”), you may instruct the holder of record to vote your RTI shares by following the |
10
| instructions that the holder of record provides you with these materials. You must follow these instructions in order for your shares to be voted, and your broker, bank or similar organization is required to vote those shares in accordance with your instructions. |
“Brokernon-votes” are shares held by brokers or nominees which are present in person or represented by proxy, but which are not voted on a particular matter because instructions have not been received from the beneficial owner. Under the rules of the Financial Industry Regularity Authority, member brokers generally may not vote shares held by them in street name for customers unless they are permitted to do so under the rules of any national securities exchange of which they are a member. Brokernon-votes, if any, are counted for the purpose of determining the presence of a quorum. Brokernon-votes will have no effect on the outcome of the proposals, assuming a quorum is present, except with respect (i) to the Merger Proposal and (ii) the vote of the RTI preferred stock, voting separately as a class, on the Share Issuance Proposal, in each case, whichbroker-non votes will have the same effect as a vote “AGAINST” such proposal.
| Q: | What will happen if I return my proxy card without indicating how to vote? |
| A: | If a signed proxy card is returned without an indication as to how the RTI shares represented are to be voted with regard to a particular proposal, the RTI shares represented by the proxy card will be voted “FOR” each such proposal. |
| Q: | Can I change my vote after I have returned a proxy card or voting instruction card? |
| A: | Any holder of RTI common stock or RTI preferred stock has the right to revoke his or her proxy at any time prior to the voting thereof at the RTI special meeting by: (1) filing a written revocation with RTI’s Secretary prior to the voting of such proxy; (2) giving a duly executed proxy bearing a later date; or (3) attending the RTI special meeting and voting in person. Attendance by an RTI stockholder at the RTI special meeting will not itself revoke his or her proxy. If you hold your shares in the name of a bank, broker or other nominee, you should follow the instructions provided by your bank, broker or nominee in revoking your previously granted proxy. |
| Q: | What should RTI stockholders do if they receive more than one set of voting materials? |
| A: | You may receive more than one set of voting materials with respect to the proposals described in this joint proxy and consent solicitation statement/prospectus, including multiple copies of this joint proxy and consent solicitation statement/prospectus and multiple proxy cards or voting instruction cards. For example, if you hold your RTI shares in more than one brokerage account, you will receive a separate voting instruction card for each brokerage account in which you hold shares. If you are a holder of record of RTI shares and your RTI shares are registered in more than one name, you will receive more than one proxy card. In each case, please complete, sign, date and return each proxy card and voting instruction card that you receive to ensure that all of your shares are voted. |
| Q: | Are there any support agreements entered into by holders of RTI stock that I should be aware of? |
| A: | Yes. Each of Camille I. Farhat, Jonathon M. Singer and WSHP Biologics Holdings, LLC entered into support agreements on the date of the Master Transaction Agreement to vote in favor of each of the proposals. As of the close of business on January 15, 2019, Mr. Farhat owned approximately 1.78% of the outstanding shares of RTI common stock representing 1.50% of the voting power of the outstanding shares of RTI stock. As of the close of business on January 15, 2019, Mr. Singer owned approximately 0.43% of the outstanding shares of RTI common stock representing 0.36% of the voting power of the outstanding shares of RTI stock. As of the close of business on January 15, 2019, WSHP Biologics Holdings, LLC owned 100% of the outstanding shares of RTI preferred stock representing 16.15% of the voting power of the outstanding shares of RTI stock. |
| Q: | How will the votes be tabulated? |
| A: | The inspector of elections appointed for the RTI special meeting will tabulate the votes cast, in person or by proxy, at the RTI special meeting and will determine whether a quorum is present. The RTI board has appointed a |
11
| representative of Broadridge Financial Solutions, Inc. (“Broadridge”) to act as the inspector of election for the RTI special meeting. |
| Q: | Do I, as an RTI stockholder, have appraisal rights if I dissent from voting on a matter at the RTI special meeting? |
| A: | There are no statutory or contractual rights of appraisal or similar remedies available to those RTI stockholders who dissent from any matter to be acted on at the RTI special meeting. |
| Q: | What happens if I transfer my shares of RTI common stock or RTI preferred stock before the RTI special meeting? |
| A: | The RTI record date for the RTI special meeting is earlier than both the date of the RTI special meeting and the date that the Transaction is expected to be completed. If you transfer your shares of RTI common stock or RTI preferred stock after the RTI record date but before the RTI special meeting, you will retain your right to vote at the RTI special meeting. However, in order for you, as a current RTI stockholder, to receive shares of Holdco pursuant to the Transaction, you must hold your shares of RTI common stock or RTI preferred stock through the completion of the Transaction. |
| Q: | What happens if I transfer my shares of RTI common stock or RTI preferred stock after the RTI special meeting but before the completion of the Transaction? |
| A: | If you transfer your shares of RTI common stock or RTI preferred stock after the RTI special meeting, but before the completion of the Transaction, you, as a current RTI stockholder, will have transferred your right to receive shares of Holdco pursuant to the Transaction. In order for you, as a current RTI stockholder, to receive shares of Holdco pursuant to the Transaction, you must hold your shares of RTI common stock or RTI preferred stock through the completion of the Transaction. |
| Q: | Where can I find the voting results of the RTI special meeting? |
| A: | Voting results will be disclosed on a Form8-K filed with the SEC within four business days after the RTI special meeting and will be available on RTI’s website. |
| Q: | What will happen if all of the proposals to be considered at the RTI special meeting are not approved? |
| A: | As a condition to the completion of the Transaction, RTI stockholders must approve the Merger Proposal and the Share Issuance Proposal. Each such proposal is cross-conditioned upon the approval of the other proposal. |
| Q: | What do I, as an RTI stockholder, need to do now? |
| A: | Carefully read and consider the information contained in and incorporated by reference into this joint proxy and consent solicitation statement/prospectus, including its annexes. |
If you are a holder of record of RTI shares as of the close of business on the RTI record date, in order for your RTI shares to be represented at the RTI special meeting, you must:
| • | vote in person by attending the RTI special meeting; or |
| • | mark, sign and date the accompanying proxy card and return it in the enclosed postage-paid envelope so that it is received by the Corporate Secretary of RTI prior to the RTI special meeting; or |
| • | submit your proxy by following the “Vote by Internet” instructions on your proxy card; or |
| • | submit your proxy by following the “Vote by Phone” instructions on your proxy card. |
12
If you hold RTI shares through a broker or other nominee, you may instruct your broker or other nominee to vote your RTI shares by following the instructions that the broker or other nominee provides to you with these materials.
| Q: | Who can help answer my questions? |
| A: | RTI stockholders who have questions about the Master Transaction Agreement, the Merger, the Contribution or the other matters to be voted upon at the RTI special meeting or who desire additional copies of this joint proxy and consent solicitation statement/prospectus or additional proxy cards or election forms should contact RTI’s proxy solicitor: |

1290 Avenue of the Americas, 9th Floor
New York, NY 10104
All Stockholders Call Toll-Free: (866)391-6921
About the PS Spine Consent Solicitation
| Q: | What am I being asked to approve? |
| A: | You are being asked to approve the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution. |
| Q: | What is the record date and what does it mean? |
| A: | The record date to determine the unitholders of PS Spine entitled to sign and deliver written consents with respect to the approval of the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution, is the close of business on February 6, 2019. The record date was established by the board of managers of PS Spine in accordance with PS Spine’s organizational documents and Delaware law. |
| Q: | Who is entitled to give a written consent? |
| A: | Only holders of outstanding Common Units, Class A Common Units, Class A Preferred Units, Class B Preferred Units,Class E-1 Preferred Units and Class F Preferred Units of PS Spine as of the close of business on the PS Spine record date are entitled to execute and deliver written consents with respect to the approval of the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution. As of the close of business on the PS Spine record date, there were 2,075,282 Common Units, 1,140,708 Class A Common Units, 2,717,886 Class A Preferred Units, 7,895,271 Class B Preferred Units, 14,786,878Class E-1 Preferred Units and no Class F Preferred Units of PS Spine issued and outstanding and entitled to execute and deliver written consents with respect to the approval of the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution. |
| Q: | What unitholder consent is required to approve the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution? |
| A: | The Contribution cannot be completed unless the unitholders of PS Spine approve the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution. Approval requires written consent from holders of66-2/3% of the outstanding Common Units, Class A Common Units, Class A Preferred Units, Class B Preferred Units,Class E-1 Preferred Units and Class F Preferred Units of PS Spine, as of the close of business on the PS Spine record date, consenting together as a single class, in addition to written consents from |
13
| holders of a majority of the outstanding Class A Preferred Units and a majority of the outstanding Class B Preferred Units of PS Spine, as of the close of business on the PS Spine record date, each consenting as a separate class. |
Certain holders of 5% or more of the voting units of PS Spine, representing approximately 67.4% of the outstanding Common Units, Class A Common Units, Class A Preferred Units, Class B Preferred Units,Class E-1 Preferred Units and Class F Preferred Units, including a majority of the Class A Preferred Units and a majority of the Class B Preferred Units, of PS Spine as of the close of business on the PS Spine record date, have entered into support agreements with RTI and Holdco pursuant to which, among other things, they have agreed to execute and return written consents approving the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution. PS Spine expects that the written consents to be delivered by unitholders pursuant to the support agreements will represent a sufficient number of voting units of PS Spine to satisfy the approval requirement described above with respect to the approval of the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution.
| Q: | What options do I have with respect to the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution? |
| A: | With respect to the Common Units, Class A Common Units, Class A Preferred Units, Class B Preferred Units,Class E-1 Preferred Units or Class F Preferred Units of PS Spine that you hold, you may execute a written consent to approve the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution, or to disapprove the same. If you fail to execute and return your written consent, or otherwise withhold your written consent or abstain, it has the same effect as voting against the approval of the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution. |
| Q: | Are there any support agreements entered into by PS Spine unitholders that I should be aware of? |
| A: | Yes, as indicated above, certain holders of 5% or more of the voting units of PS Spine, representing approximately 67.4% of the outstanding Common Units, Class A Common Units, Class A Preferred Units, Class B Preferred Units,Class E-1 Preferred Units and Class F Preferred Units, including a majority of the Class A Preferred Units and a majority of the Class B Preferred Units, of PS Spine as of the close of business on the PS Spine record date, have entered into support agreements with RTI and Holdco pursuant to which, among other things, they have agreed to execute and return written consents approving the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution. PS Spine expects that the written consents to be delivered by unitholders pursuant to the support agreements will represent a sufficient number of voting units of PS Spine to satisfy the approval requirement described above with respect to the approval of the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution. |
| Q: | Can I dissent and require appraisal of my units? |
| A: | There are no statutory or contractual rights of appraisal, dissenters’ rights or similar remedies available to those unitholders of PS Spine who dissent from any matter to be approved though the written consent, including the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution. |
| Q: | How do I return my written consent? |
| A: | You may return your written consent to PS Spine by emailing a “.pdf” copy of your written consent to InvestorRelations@paradigmspine.com or by mailing your written consent to PS Spine at 505 Park Avenue, 14th Floor, New York, NY 10022, Attention: Secretary. |
| Q: | What is the deadline for returning PS Spine written consents? |
| A: | The board of managers of PS Spine has set February 13, 2019 as the final date for receipt of written consents. PS Spine reserves the right to extend the final date for receipt of written consents beyond February 13, 2019. Any such extension may be made without notice to you. |
14
| Q: | What if I am a unitholder as of the PS Spine record date and return my signed written consent without indicating a decision with respect to the approval of the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution? |
| A: | If you are a holder of voting units of PS Spine as of the PS Spine record date and you return a signed written consent without indicating your decision on the approval of the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution, you will have given your consent to such approval. |
| Q: | Can I change or revoke my written consent? |
| A: | Yes. If you are a unitholder on the PS Spine record date of Common Units, Class A Common Units, Class A Preferred Units, Class B Preferred Units,Class E-1 Preferred Units or Class F Preferred Units of PS Spine, you may change or revoke your consent to the approval of the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution, subject to any contractual obligation you may have, at any time prior to February 13, 2019 or, if earlier, at any time before the consents of a sufficient number of voting units of PS Spine to approve the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution, have been filed with PS Spine. If you wish to change or revoke your consent before that time, you may do so by emailing a “.pdf” copy of such change or revocation to InvestorRelations@paradigmspine.com or by mailing such change or revocation to PS Spine at 505 Park Avenue, 14th Floor, New York, NY 10022, Attention: Secretary. |
| Q: | What is the recommendation of the board of managers of PS Spine? |
| A: | The board of managers of PS Spine unanimously recommends that you execute a written consent to approve the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution. |
| Q: | What will happen if the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution, are not approved? |
| A: | As a condition to the completion of the Transaction, PS Spine unitholders must approve the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution. |
| Q: | What do I, as PS Spine unitholder, need to do now? |
| A: | Carefully read and consider the information contained in and incorporated by reference into this joint proxy and consent solicitation statement/prospectus, including its annexes. If you are a holder of voting units of PS Spine as of the PS Spine record date, you may return your written consent to PS Spine by emailing a “.pdf” copy of your written consent to InvestorRelations@paradigmspine.com or by mailing your written consent to PS Spine at 505 Park Avenue, 14th Floor, New York, NY 10022, Attention: Secretary. The board of managers of PS Spine has set February 13, 2019 as the final date for receipt of written consents. PS Spine reserves the right to extend the final date for receipt of written consents beyond February 13, 2019. Any such extension may be made without notice to you. |
| Q: | Who can help answer my questions? |
| A: | PS Spine unitholders who have questions about the Master Transaction Agreement, the Merger, the Contribution or the matters to be approved through the consent solicitation or desire additional copies of this joint proxy and consent solicitation statement/prospectus or a replacement written consent should contact PS Spine at: |
PS Spine Holdco, LLC
505 Park Avenue, 14th Floor
New York, NY 10022
Attn: Secretary
Telephone: (888)273-9897
Email: InvestorRelations@paradigmspine.com
15
The following is a summary that highlights information contained in this joint proxy and consent solicitation statement/prospectus. This summary does not contain all of the information that might be important to you. For a more complete description of the Master Transaction Agreement and the transactions contemplated by the Master Transaction Agreement, including the Merger and the Contribution and the potential issuance of shares of Holdco common stock and preferred stock pursuant to the Master Transaction Agreement, we encourage you to read carefully this entire joint proxy and consent solicitation statement/prospectus, including the attached Annexes. In addition, we encourage you to read carefully the information incorporated by reference into this joint proxy and consent solicitation statement/prospectus, which includes important business and financial information about RTI that has been filed with the SEC. You may obtain the information incorporated by reference into this joint proxy and consent solicitation statement/prospectus without charge by following the instructions in the section entitled “Where You Can Find More Information”, beginning on page 187. Each item in this summary refers to the page of this joint proxy and consent solicitation statement/prospectus on which that subject is discussed in more detail.
This summary and the balance of this joint proxy and consent solicitation statement/prospectus contain forward-looking statements about events that are not certain to occur, and you should not place undue reliance on those statements. Please carefully read “Cautionary Statement Concerning Forward-Looking Statements”, beginning on page 1.
Information about the Companies
RTI (see page 147)
RTI Surgical, Inc.
11621 Research Circle
Alachua, Florida 32615
Telephone: (386)418-8888
RTI is a global surgical implant company that designs, develops, manufactures and distributes biologic, metal and synthetic implants. RTI’s implants are used in orthopedic, spine, sports medicine, plastic surgery, trauma and other surgical procedures to repair and promote the natural healing of human bone and other human tissues and improve surgical outcomes. RTI manufactures metal and synthetic implants and processes donated human musculoskeletal and other tissue and bovine and porcine animal tissue in producing allograft and xenograft implants using its proprietary BIOCLEANSE®, TUTOPLAST® and CANCELLE® SP sterilization processes. RTI processes tissue at its facilities in Alachua, Florida and Neunkirchen, Germany and manufactures metal and synthetic implants in Marquette, Michigan and Greenville, North Carolina, respectively. RTI is accredited in the U.S. by the American Association of Tissue Banks and is a member of AdvaMed. RTI’s implants are distributed directly to hospitals and free-standing surgery centers throughout the U.S. and in more than 40 countries worldwide with the support of both its and third-party representatives as well as through larger purchasing companies.
RTI common stock is listed on Nasdaq under the symbol “RTIX”.
Additional information about RTI and its subsidiaries is included in documents incorporated by reference in this joint proxy and consent solicitation statement/prospectus. See the section entitled “Where You Can Find More Information”, beginning on page 187.
Holdco (see page 147)
Bears Holding Sub, Inc.
c/o RTI Surgical, Inc.
520 Lake Cook Road, Suite 315
Deerfield, Illinois 60015
Telephone: (386)418-8888
16
Bears Holding Sub, Inc., a wholly owned subsidiary of RTI, is a Delaware corporation that was incorporated on October 26, 2018, for the purpose of effecting the matters set forth in the Master Transaction Agreement. To date, Holdco has not conducted any activities other than those incidental to its incorporation and the matters contemplated by the Master Transaction Agreement. Pursuant to the Master Transaction Agreement, at the effective time, the certificate of incorporation of Holdco will be amended and restated, and the name of Holdco will be changed to “RTI Surgical Holdings, Inc.” After the consummation of the Transaction, Holdco will own both RTI and Paradigm as wholly owned subsidiaries. It is expected that the Holdco common stock will be listed on the Nasdaq Global Market under the symbol “RTIX”. The business of Holdco will be the combined businesses currently conducted by RTI and Paradigm.
Merger Sub (see page 147)
Bears Merger Sub, Inc.
c/o RTI Surgical, Inc.
11621 Research Circle
Alachua, Florida 32615
Telephone: (386)418-8888
Bears Merger Sub, Inc., a wholly owned subsidiary of Holdco, is a Delaware corporation that was incorporated on October 26, 2018, for the purpose of effecting the matters set forth in the Master Transaction Agreement. To date, Merger Sub has not conducted any activities other than those incidental to its formation and the matters contemplated by the Master Transaction Agreement. Pursuant to the Master Transaction Agreement, Merger Sub will be merged with and into RTI, with RTI surviving as a wholly owned subsidiary of Holdco.
PS Spine (see page 149)
PS Spine Holdco, LLC
505 Park Avenue, 14th Floor
New York, New York 10022
Telephone: (212)367-7274
PS Spine is a Delaware limited liability company that was originally formed on January 28, 2008, under the name Fourth Dimension Holding, LLC. PS Spine changed its name to Paradigm Spine Holdings, LLC on October 6, 2010, to PS 2016, LLC on April 5, 2016 and to PS Spine Holdco, LLC on August 9, 2018.
PS Spine is not engaged in any business and has no material assets, other than owning all of the outstanding equity interests in Paradigm.
Paradigm (see page 149)
Paradigm Spine, LLC
505 Park Avenue, 14th Floor
New York, New York 10022
Telephone: (212)367-7274
Paradigm is a Delaware limited liability company. Paradigm was originally formed as a Delaware corporation on September 27, 2002 under the name Spine Motion, Inc. Paradigm converted from a Delaware corporation to a Delaware limited liability company on April 5, 2005, at which time it changed its name to Spine Motion, LLC. Paradigm then changed its name to Paradigm Spine, LLC on July 14, 2005.
Paradigm is focused on the design and development of solutions for the disease management of spinal stenosis. Paradigm’s signature product is the coflex® Interlaminar Stabilization® device, which is currently used in over 40 countries worldwide. Coflex® is the only lumbar spinal device that has produced Level I evidence in two separate prospective, randomized, controlled studies against two different surgical control groups, changing the standard of care for lumbar spinal stenosis treatment.
17
The Merger and the Contribution (see page 73)
The Merger
On the terms and subject to the conditions set forth in the Master Transaction Agreement, at the effective time, and in accordance with the General Corporation Law of the State of Delaware (the “DGCL”), (i) Merger Sub will be merged with and into RTI such that the separate corporate existence of Merger Sub will thereupon cease, (ii) RTI will continue as the surviving corporation (the “Surviving Corporation”) and (iii) the Merger will have the effects set forth in the Master Transaction Agreement, the certificate of merger relating to the Merger to be executed, acknowledged and filed with the Secretary of State of the State of Delaware and the DGCL. As a result of the Merger, RTI will become a wholly owned subsidiary of Holdco.
The Contribution
On the terms and subject to the conditions set forth in the Master Transaction Agreement, at the effective time, PS Spine will contribute all of the issued and outstanding equity interests of Paradigm to Holdco, free and clear of all encumbrances. As a result of the Contribution, Paradigm will become a wholly owned subsidiary of Holdco.
Organizational Structure
The organization of Holdco, RTI, Merger Sub, Paradigm and PS Spine before and after the Contribution and the Merger is illustrated below.
Prior to the Transaction
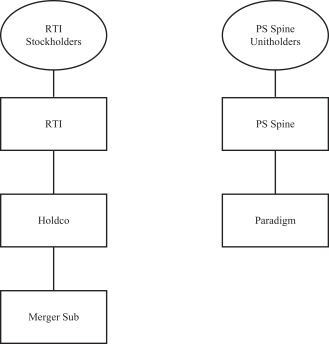
18
The Transaction
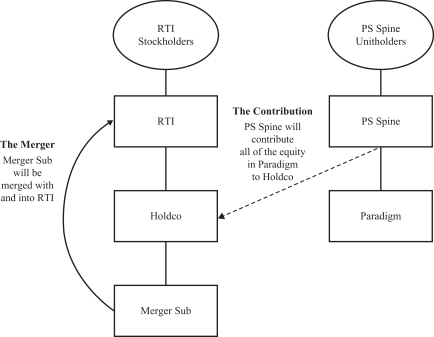
After the Transaction
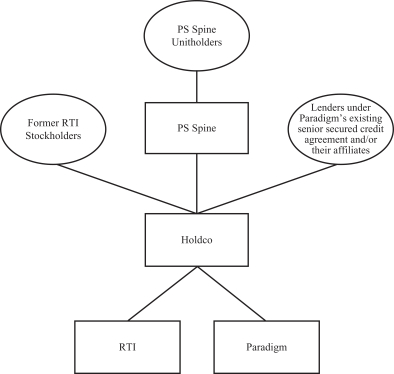
19
Consideration in the Transaction (see page 126)
The consideration for the Contribution payable by Holdco is generally payable in cash (without interest) and shares of Holdco common stock and is in addition to the contingent consideration that may become payable upon the occurrence of certain events in the future in accordance with the terms and conditions of the Master Transaction Agreement.
Cash Consideration Payable at the Closing
PS Spine will initially receive at the closing the Cash Consideration Amount as follows:
| • | $100,000,000,minus (i) the aggregate amount of outstanding indebtedness and unpaid transaction expenses of Paradigm (to the extent not covered by the issuance of Holdco common stock to any lender under Paradigm’s existing senior secured credit agreement and any applicable interim debt financing of Paradigm and/or their affiliates) at closing,minus (ii) the amount, if any, by which the working capital of Paradigm is less than the target working capital amount of $7,000,000,plus (iii) the aggregate amount of cash of Paradigm at closing. The Cash Consideration Amount is subject to a customary post-closing adjustment. |
At the closing, due to the expected amount of Paradigm’s indebtedness and transaction expenses, all cash that would otherwise be paid at closing pursuant to the Master Transaction Agreement to PS Spine, as well as a portion of the Stock Consideration Amount, is expected to be used to satisfy Paradigm’s outstanding indebtedness and unpaid transaction expenses.
Stock Consideration Payable at the Closing
PS Spine will initially receive at the closing the Stock Consideration Amount as follows:
| • | 10,729,614 shares of Holdco common stock, minus the number of shares of Holdco common stock necessary to pay the lenders under Paradigm’s existing senior secured credit agreement and any applicable interim debt financing of Paradigm and/or their affiliates, which shall be issued directly to such lenders at the closing, as further described in the sections entitled “The Master Transaction Agreement—Purchase Price” and “The Transaction—Interests of PS Spine’s and Paradigm’s Managers and Executive Officers in the Transaction—Settlement Agreement”. |
Contingent Consideration
Holdco has agreed to pay contingent consideration to PS Spine upon the occurrence of certain events in the future, on the terms and conditions set forth in the Master Transaction Agreement. The contingent consideration includes the following:
| • | if on or prior to December 31, 2020, the Giants Revenue is equal to or exceeds $65,000,000 in any LTM Period, then Holdco shall pay to PS Spine or its designee, no earlier than December 31, 2020, by wire transfer of immediately available funds, $20,000,000; |
| • | if in any LTM Period ending on or prior to December 31, 2021, the net revenue of Holdco and its subsidiaries in respect of specified legacy products of Paradigm and specified RTI Zyga products (the “Giants/Zyga Revenue”) is greater than $85,000,000, then Holdco shall issue to PS Spine the number of Initial Earnout Shares (as defined below) payable to PS Spine with respect to such LTM Period (less the total number of Initial Earnout Shares that have previously been issued to PS Spine in any prior LTM Period); and |
| • | if in any LTM Period ending on or prior to December 31, 2022, the Giants/Zyga Revenue is greater than $105,000,000, then Holdco shall, at its option, either (A) issue to PS Spine the Final Earnout |
20
Shares (as defined below), or (B) pay PS Spine, by wire transfer of immediately available funds, the Cash Election Amount (as defined below). |
If the Cash Earnout Amount is earned, PS Spine has agreed to pay $10.0 million of the Earnout Consideration (which can take the form of cash, stock or a combination of both) that would otherwise be payable to PS Spine to the lenders under Paradigm’s existing senior secured credit agreement and/or their affiliates. See the section entitled “The Transaction—Interests of PS Spine’s and Paradigm’s Managers and Executive Officers in the Transaction”, beginning on page 114.
For a complete description of the contingent consideration payable to PS Spine upon the occurrence of certain events in the future, see the section entitled The Master Transaction Agreement—Purchase Price—Contingent Consideration.”
Treatment of Equity Awards in the Transaction (see page 125)
At the effective time, each RTI Stock Option that is outstanding immediately prior to the effective time, whether vested or unvested, will be converted into a stock option in respect of shares of Holdco common stock, on the same terms and conditions as were applicable under such RTI Stock Option immediately prior to the effective time (including with respect to vesting). At the effective time, each RTI Restricted Stock Award that is outstanding immediately prior to the effective time will be converted into a restricted stock award with the same terms and conditions as were applicable under such RTI Restricted Stock Award immediately prior to the effective time (including with respect to vesting). For more information, see the section entitled “The Master Transaction Agreement—Treatment of RTI Equity Awards”.
Ownership of Holdco after the Transaction
Assuming (i) the closing occurs on March 15, 2019 (ii) 62,245,112 shares of RTI common stock (based on the number of shares outstanding as of January 15, 2019) and 50,000 shares of RTI preferred stock are issued and outstanding immediately prior to the effective time and (iii) the amount of cash paid to the lenders under Paradigm’s existing senior secured credit agreement at closing does not exceed $95.0 million, and taking into account the $3.0 million Paradigm borrowed from the lenders under its existing senior secured credit agreement on December 6, 2018, approximately 85.30% of the issued and outstanding Holdco common stock and 100% of the issued and outstanding Holdco preferred stock immediately following the closing will be held by former RTI stockholders, approximately 6.42% of the issued and outstanding Holdco common stock immediately following the closing will be held by PS Spine and approximately 8.28% of the issued and outstanding Holdco common stock immediately following the closing will be held by the lenders under Paradigm’s existing senior secured credit agreement and their affiliates. Using the same assumptions set forth above and assuming the conversion of the RTI preferred stock, immediately following the closing, former holders of RTI common stock and RTI preferred stock will collectively hold 87.37% of the voting power of Holdco, PS Spine will hold 5.51% of the voting power of Holdco and the lenders under Paradigm’s existing senior secured credit agreement and their affiliates will hold 7.12% of the voting power of Holdco. As described in the section entitled “The Transaction—Interests of PS Spine’s and Paradigm’s Managers and Executive Officers in the Transaction—The Settlement Agreement”, the lenders under Paradigm’s existing senior secured credit agreement and their affiliates may receive a smaller or larger portion of the Stock Consideration Amount than as assumed for purposes of this example. If the lenders under Paradigm’s existing senior secured credit agreement and their affiliates receive a larger portion of the Stock Consideration Amount than as assumed for purposes of this example, the percentage of the issued and outstanding Holdco common stock immediately following the closing that will be held by the lenders under Paradigm’s existing senior secured credit agreement and their affiliates will be greater than set forth in this example and the percentage that will be held by PS Spine will be less than set forth in this example. If the lenders under Paradigm’s existing senior secured credit agreement and their affiliates receive a smaller portion of the Stock Consideration Amount than as assumed for purposes of this example, the percentage of the issued and outstanding Holdco common stock immediately following the closing that will be held by the lenders under Paradigm’s existing senior secured credit agreement and their affiliates will be less than set forth in this example and the percentage that will be held by PS Spine will be greater than set forth in this example. In addition, the earnout provisions of the Master Transaction Agreement provide for the possibility that PS Spine may receive additional shares of Holdco common stock, which would cause former RTI stockholders ownership of Holdco to be further diluted.
21
Share Ownership of RTI Directors and Officers
As of January 15, 2019, RTI’s directors and executive officers beneficially owned and were entitled to vote an aggregate of 2,370,591 shares of RTI common stock, or the right to vote approximately 3.19% of the total RTI voting stock outstanding on January 15, 2019. Assuming a quorum is present, approval of the Merger Proposal requires (i) the affirmative vote of the holders of a majority of the outstanding shares of RTI common stock and RTI preferred stock (on a fully converted basis) entitled to vote thereon and (ii) the written consent or affirmative vote of the holders of a majority of the outstanding shares of RTI preferred stock entitled to vote thereon, voting separately as a class. Assuming a quorum is present, approval of the Share Issuance proposal requires (i) the affirmative vote of a majority of votes cast on the proposal by RTI common stock and RTI preferred stock (on a fully converted basis) and (ii) the written consent or affirmative vote of the holders of a majority of the outstanding shares of RTI preferred stock, voting separately as a class. Camille I. Farhat, who has entered into an RTI Support Agreement, as of January 15, 2019, beneficially owned and was entitled to vote an aggregate of 1,110,619 shares of RTI common stock, or the right to vote approximately 1.50% of the total RTI voting stock outstanding on January 15, 2019. Jonathon M. Singer, who has entered into an RTI Support Agreement, as of January 15, 2019, beneficially owned and was entitled to vote an aggregate of 264,844 shares of RTI common stock, or the right to vote approximately 0.36% of the total RTI voting stock outstanding on January 15, 2019. Pursuant to the RTI Support Agreements, Camille I. Farhat and Jonathon M. Singer will be casting their votes in favor of the Merger Proposal and the Share Issuance Proposal. For more information about the support agreements, see the section entitled “The Transaction—Support Agreements”, beginning on page 119.
Unit Ownership of PS Spine Managers and Executive Officers
As of the close of business on the PS Spine record date, the managers and executive officers of PS Spine, and their respective affiliates, collectively owned and were entitled to execute and deliver written consents with respect to 1,354,253 Common Units, 701,691 Class A Common Units, 1,880,474 Class A Preferred Units, 7,124,650 Class B Preferred Units, 11,862,157Class E-1 Preferred Units and no Class F Preferred Units of PS Spine, which represent, in the aggregate, approximately 80.1% of the outstanding voting power of the unitholders of PS Spine. Written consents from holders of66-2/3% of the outstanding Common Units, Class A Common Units, Class A Preferred Units, Class B Preferred Units,Class E-1 Preferred Units and Class F Preferred Units of PS Spine, as of the close of business on the PS Spine record date, consenting together as a single class, in addition to written consents from holders of a majority of the outstanding Class A Preferred Units and a majority of the outstanding Class B Preferred Units of PS Spine, as of the close of business on the PS Spine record date, each consenting as a separate class, are required to approve the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution. Certain holders of 5% or more of the voting units of PS Spine that are affiliated with certain managers and executive officers of PS Spine, representing approximately 67.4% of the outstanding Common Units, Class A Common Units, Class A Preferred Units, Class B Preferred Units,Class E-1 Preferred Units and Class F Preferred Units, including a majority of the Class A Preferred Units and a majority of the Class B Preferred Units, of PS Spine as of the close of business on PS Spine record date, have entered into support agreements with RTI and Holdco pursuant to which, among other things, they have agreed to execute and return written consents approving the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution. PS Spine expects that the written consents to be delivered by unitholders pursuant to the support agreements will represent a sufficient number of voting units of PS Spine to satisfy the approval requirement described above with respect to the approval of the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution. For more information about the support agreements, see the section entitled “The Transaction—Support Agreements”, beginning on page 119.
Recommendation of the RTI Board (see page 79)
The RTI board unanimously approved and adopted the Master Transaction Agreement and the transactions contemplated thereby, including the Merger, on the terms and subject to the conditions set forth therein, determined and declared that the terms of the Master Transaction Agreement and the transactions contemplated thereby, including the Merger, are advisable and in the best interests of RTI and its stockholders, and recommended that RTI stockholders approve and adopt the Master Transaction Agreement and the Merger.
22
Recommendation of the PS Spine Board (see page 80)
The board of managers of PS Spine (“PS Spine board”) has determined that the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution, are advisable and in the best interests of PS Spine and its unitholders and has unanimously approved the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution. The PS Spine board unanimously recommends that unitholders of PS Spine consent to the approval of the Master Transaction Agreement and the transactions contemplated by the Master Transaction Agreement, including the Contribution.
Opinion of RTI’s Financial Advisor (see page 84)
On October 30, 2018, Piper Jaffray & Co. (“Piper Jaffray”) rendered its oral opinion to the RTI board (which was subsequently confirmed in writing by delivery of Piper Jaffray’s written opinion, dated November 1, 2018) to the effect that, as of November 1, 2018, and based upon and subject to the various assumptions and limitations set forth therein, the Aggregate Consideration (as defined below) to be paid by Holdco in the Transaction was fair, from a financial point of view, to Holdco. “Aggregate Consideration” refers to the aggregate of the (i) Cash Consideration Amount (which Piper Jaffray assumed to be $100,000,000), (ii) Stock Consideration Amount, (iii) Initial Earnout Shares, (iv) Final Earnout Shares and (v) Cash Earnout Amount (clauses (iii) through (v), in each case, as defined in the section entitled “The Master Transaction Agreement—Purchase Price”, beginning on page 126).
Piper Jaffray’s opinion was directed to the RTI board, and only addressed the fairness, from a financial point of view, to Holdco of the Aggregate Consideration to be paid by Holdco in the Transaction and did not address any other aspect or implication of the Transaction. The summary of Piper Jaffray’s opinion in this joint proxy and consent solicitation statement/prospectus is qualified in its entirety by reference to the full text of its written opinion, which is included asAnnex B to this joint proxy and consent solicitation statement/prospectus and sets forth the assumptions made, procedures followed, matters considered and limitations on the scope of the review undertaken by Piper Jaffray in preparing its opinion. However, neither Piper Jaffray’s written opinion nor the summary of its opinion and the related analyses set forth in this joint proxy and consent solicitation statement/prospectus is intended to be, and they do not constitute, a recommendation to any RTI stockholder as to how such stockholder should act or vote with respect to the Transaction or any other matter.
SeeAnnex B and the section of this joint proxy and consent solicitation statement/prospectus entitled “The Transaction—Opinion of RTI’s Financial Advisor”, beginning on page 84.
Board of Directors and Management of Holdco after the Transaction (see page 119)
Pursuant to the Master Transaction Agreement, the directors of RTI in office immediately prior to the effective time, as well as Jeffrey C. Lightcap, currently a manager of PS Spine and Paradigm, will, from and after the effective time, be the directors of Holdco, and the officers of RTI in office immediately prior to the effective time will, from and after the effective time, be the officers of Holdco.
Listing of Holdco Common Stock (see page 114)
Pursuant to the Master Transaction Agreement, RTI will cause the shares of Holdco common stock to be issued in the Transaction to be approved for listing on the Nasdaq Global Market. It is a condition to the completion of the Transaction that the Holdco common stock be approved for listing on the Nasdaq Global Market.
Following the Transaction, RTI expects Holdco’s common stock will be listed on the Nasdaq Global Market and will trade under the name “RTI Surgical Holdings, Inc.” and symbol “RTIX”.
23
Conditions to Completion of the Transaction (see page 139)
The respective obligations of RTI, Holdco, Merger Sub and PS Spine to complete the transactions contemplated by the Master Transaction Agreement are each subject to the satisfaction (or, if permitted by applicable law, waiver) of certain customary conditions, including, among other things:
| • | the accuracy of the representations and warranties of the parties and compliance by the parties with their respective obligations under the Master Transaction Agreement, in each case subject to certain materiality qualifiers; |
| • | the written consent of PS Spine’s unitholders approving the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution; |
| • | the receipt of RTI stockholder approval of the Merger Proposal; |
| • | the expiration or termination of the applicable waiting periods (and any extensions thereof) under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended (the “HSR Act”); |
| • | the absence of any law or order in effect that prevents, makes unlawful or prohibits the consummation of the Transaction; |
| • | the absence of any material adverse effect on the business, condition (financial or otherwise) or results of operations of RTI and its subsidiaries, taken as a whole, or Paradigm and its subsidiaries, taken as a whole, in each case subject to certain exceptions, since November 1, 2018, the date of the Master Transaction Agreement; |
| • | the Cash Consideration Amount being equal to or greater than $0; |
| • | the shares of Holdco common stock issuable pursuant to the Merger and shares of Holdco common stock representing the Stock Consideration Amount being approved for listing on the Nasdaq Global Market, subject to official notice of issuance; |
| • | the registration statement on FormS-4, of which this joint proxy and consent solicitation statement/prospectus forms a part, becoming effective under the Securities Act of 1933, as amended (the “Securities Act”); |
| • | the receipt of representation letters from officers of RTI, Holdco and PS Spine regarding the Intended Tax Treatment (as defined below) of the Transaction (provided this condition will be deemed not to be satisfied if (i) Sidley Austin LLP (“Sidley Austin”) or Dorsey & Whitney LLP (“Dorsey & Whitney”), as the case may be, delivers an opinion that, as a result of a change in law occurring after November 1, 2018, it is unable to provide an opinion to the effect that the Merger and the Contribution, taken together, will qualify as a transaction described in Section 351 of the Code (and, in the case of Sidley Austin LLP, that the Merger will not qualify as a “reorganization” within the meaning of Section 368(a) of the Code) and (ii) RTI or PS Spine, as the case may be, is unable to obtain such opinion from an alternative tax counsel); |
| • | neither Marc Viscogliosi nor Francis Magee rescinding his obligations under his applicable consulting agreement with RTI, and RTI not rescinding its obligations under those consulting agreements; |
| • | none of Viscogliosi Brothers, LLC, HealthCor Paradigm Blocker Company Two, Inc. and HealthCor AIV, L.P. rescinding its obligations under its applicableNon-Competition Agreement with Holdco; and |
| • | none of the parties to the Settlement Agreement (as defined below) rescinding their obligations under the Settlement Agreement, the Settlement Agreement being in full force and effect and not having been amended in any manner adverse to Holdco, RTI or Merger Sub and the transactions contemplated by the Settlement Agreement having been performed in accordance with the terms of the Settlement Agreement. |
24
We cannot provide any assurances that these conditions will be satisfied in a timely manner or at all or that the Transaction will occur.
Regulatory Approvals (see page 109)
Under the HSR Act and the rules that have been promulgated thereunder by the U.S. Federal Trade Commission (the “FTC”), certain transactions may not be consummated unless information has been furnished to the Antitrust Division of the Department of Justice (“Antitrust Division”) and the FTC and certain waiting period requirements have been satisfied. The Transaction is subject to the requirements of the HSR Act, which prevents RTI and PS Spine from completing the Transaction until the applicable waiting period under the HSR Act is terminated or expires. Early termination of the waiting period under the HSR Act was granted by the FTC on November 26, 2018.
Neither RTI nor PS Spine can assure you that the Antitrust Division, the FTC, any state attorney general, or any other government authority will not attempt to challenge the Transaction on antitrust grounds, and, if such a challenge is made, neither RTI nor PS Spine can assure you as to its result. Neither RTI nor PS Spine is aware of any material regulatory approvals or actions that are required for completion of the Transaction that have not been obtained. It is presently contemplated that if any such additional regulatory approvals or actions are required, those approvals or actions will be sought. There can be no assurance, however, that any additional approvals or actions will be obtained.
On November 1, 2018, in connection with its entry into the Master Transaction Agreement, RTI entered into (i) a Consent Under Credit Agreement with JPM pursuant to which JPM agreed to amend the existing Credit Agreement dated as of June 5, 2018 among JPM, RTI and certain of RTI’s subsidiaries (the “Existing Revolving Credit Agreement”) in order to, among other things, (a) reduce the aggregate revolving credit commitments under the Existing Revolving Credit Agreement from $100 million to $75 million and (b) consent to the incurrence of the Second Lien Facility (as defined below) and (ii) a Commitment Letter with Ares Capital Corporation pursuant to which Ares agreed to provide a $100 million second lien term loan (the “Second Lien Facility”) in a single draw on the closing date in connection with the Transaction.
Exclusive Dealing by PS Spine (see page 133)
Until the closing or earlier termination of the Master Transaction Agreement, the Master Transaction Agreement generally prohibits PS Spine, Paradigm and its subsidiaries from taking any action to directly or indirectly initiate, solicit or engage in discussions or negotiations with, or knowingly provide any information to, any person concerning any purchase of any of the equity interests of Paradigm or any merger, sale of substantially all of the assets of Paradigm or its subsidiaries or similar transactions involving Paradigm or its subsidiaries.
RTI Board Change in Recommendation (see page 137)
Except as described in the immediately following paragraph, the RTI board and each committee thereof may not change, withhold, withdraw, qualify or modify (or publicly propose or resolve to change, withhold, withdraw, qualify or modify) in a manner adverse to PS Spine, its recommendation that the stockholders of RTI vote in favor of the Merger Proposal, Share Issuance Proposal and the Meeting Adjournment Proposal.
Notwithstanding the foregoing, at any time prior to the approval of the Master Transaction Agreement by RTI’s stockholders, the RTI board may change, withhold, withdraw, qualify or modify (or publicly propose or resolve to change, withhold, withdraw, qualify or modify) its recommendation in a manner adverse to PS Spine (a “change in recommendation”) if the RTI board determines in good faith, after consultation with outside counsel and, if the RTI board reasonably determines, after consultation with outside counsel, it to be necessary in connection with its fulfillment of its fiduciary duties under applicable law, a financial advisor of nationally recognized reputation, that the failure to take such action would be inconsistent with its fiduciary duties under applicable law as a result of any event, fact, circumstances, development or occurrence relating to or affecting
25
RTI or its subsidiaries, including the business, operations, assets or liabilities of RTI or its subsidiaries, and which was not known or reasonably foreseeable to the RTI board as of the date of the Master Transaction Agreement (an “InterveningEvent”); provided, that neither an actual or anticipated financing failure nor the actual or anticipated consequences thereof will be an Intervening Event.
No change in recommendation by the RTI board may be made until after 5:00 p.m., New York City time, on the fifth (5th) business day following PS Spine’s receipt of written notice from RTI advising that the RTI board intends to take such action and specifying the reasons therefor. After providing such notice and prior to effecting such change in recommendation, (i) RTI will, during such five business day period, negotiate in good faith with PS Spine and its representatives, to the extent PS Spine wishes to negotiate, with respect to any revisions to the terms of the transactions contemplated by the Master Transaction Agreement proposed by PS Spine, and (ii) in determining whether it may still under the terms of the Master Transaction Agreement make a change in recommendation, the RTI board will take into account any changes to the terms of the Master Transaction Agreement proposed by PS Spine and any other information provided by PS Spine in response to such notice during such five business day period.
Termination of the Agreement (see page 142)
In general, the Master Transaction Agreement may be terminated at any time prior to closing in the following ways:
| • | By the mutual written consent of RTI and PS Spine. |
| • | By either RTI or PS Spine: |
| • | if the Required Merger Proposal Vote is not obtained at the RTI special meeting, or any adjournment or postponement thereof; |
| • | if the closing has not occurred on or prior to January 31, 2019 (as it may be extended, the “outside date”), provided that if the closing has not occurred by January 31, 2019, the outside date may be extended by RTI to February 28, 2019 and provided, further, that if the closing has not occurred by February 28, 2019, the outside date may be extended by RTI to March 31, 2019 (which extensions RTI intends to exercise, as necessary), provided, further that the party seeking to terminate pursuant to this clause will not have breached in any material respect the Master Transaction Agreement in any manner that would have proximately caused the failure to consummate the transactions contemplated by the Master Transaction Agreement on or prior to the outside date; or |
| • | if any law or order is enacted, issued, promulgated or entered by a governmental entity permanently restraining, enjoining or otherwise prohibiting the consummation of the Merger or the Contribution which is final andnon-appealable; provided that the party seeking to terminate will not have breached in any material respect the Master Transaction Agreement in any manner that will have caused such law or order to be enacted, issued, promulgated or entered. |
| • | By RTI: |
| • | if the PS Spine written consent is not delivered to RTI within five business days following the effectiveness of the registration statement on FormS-4, of which this joint proxy and consent solicitation statement/prospectus forms a part; or |
| • | if any of PS Spine’s representations and warranties will not be true and correct, or PS Spine has failed to perform any covenant or agreement on the part of PS Spine, such that the conditions to closing would not be satisfied at or prior to the outside date and the breach or failure to perform is not cured or capable of being cured within thirty (30) days after written notice thereof is delivered to PS Spine (provided that RTI is not permitted to terminate pursuant to this clause if it, Holdco or Merger Sub is then in breach of any of its respective representations, warranties, covenants or agreements such that the conditions to closing would not be satisfied). |
26
| • | By PS Spine: |
| • | if any of RTI’s, Holdco’s or Merger Sub’s representations and warranties will not be true and correct, or RTI, Holdco or Merger Sub has failed to perform any covenant or agreement on the part of RTI, Holdco or Merger Sub, such that the conditions to closing would not be satisfied at or prior to the outside date and the breach or failure to perform is not cured or capable of being cured within thirty (30) days after written notice thereof is delivered to RTI (provided that PS Spine is not permitted to terminate pursuant to this clause if it is then in breach of any of its respective representations, warranties, covenants or agreements such that the conditions to closing would not be satisfied); |
| • | if the RTI board shall have made a change in recommendation that the RTI stockholders vote to approve the Merger Proposal, Share Issuance Proposal and the Meeting Adjournment Proposal, provided that PS Spine may not terminate pursuant to this clause if the Required Merger Proposal Vote has been obtained; or |
| • | if (i) all of the conditions to closing are satisfied (other than conditions that by their terms will be satisfied at closing or conditions that have not been or will not be satisfied as a result of RTI, Holdco or Merger Sub’s breach of, or failure to perform any of its covenants set forth in, the Master Transaction Agreement), (ii) PS Spine has provided notice to RTI that it is prepared, ready, willing and able to consummate the closing and (iii) Holdco, RTI and Merger Sub fail to consummate the closing within three business days after the delivery of such notice and PS Spine stood ready, willing and able to consummate the closing throughout such period. |
Termination Fees and Expenses (see page 143)
RTI is required to pay a termination fee of $4,500,000 in the event the Master Transaction Agreement is terminated (i) by PS Spine if the RTI board has made a change in recommendation or (ii) by PS Spine or RTI due to the occurrence of the outside date or if the Required Merger Proposal Vote shall not have been obtained and, at the time of such termination, PS Spine had the right to terminate the Master Transaction Agreement due to the RTI board having made a change in recommendation.
RTI is required to pay a termination fee of $9,000,000 in the event the Master Transaction Agreement is terminated (i) by PS Spine if (A) all of the conditions to closing are satisfied (other than conditions that by their terms will be satisfied at closing or conditions that have not been or will not be satisfied as a result of RTI, Holdco or Merger Sub’s breach of, or failure to perform any of its covenants set forth in, the Master Transaction Agreement, (B) PS Spine has provided notice to RTI that it is prepared, ready, willing and able to consummate the closing and (C) Holdco, RTI and Merger Sub fail to consummate the closing within three business days after the delivery of such notice and PS Spine stood ready, willing and able to consummate the closing throughout such period, or (ii) by PS Spine or RTI due to the occurrence of the outside date and, at the time of such termination, PS Spine had the right to terminate the Master Transaction Agreement pursuant to clause (i) of this paragraph.
Material U.S. Federal Income Tax Consequences (see page 109)
RTI and PS Spine intend to take the position that the Merger and the Contribution, taken together, qualify as a transaction described in Section 351 of the Internal Revenue Code of 1986, as amended from time to time, and the rules and regulations promulgated thereunder (the “Code”) (or, in the case of RTI, that the Merger qualifies as a reorganization within the meaning of Section 368(a) of the Code) (the “Intended Tax Treatment”). The obligations of PS Spine and RTI to consummate the Merger and the Contribution are conditioned on the receipt of certain representations and covenants in certificates of officers of RTI, PS Spine, and Holdco, which representations and covenants are intended to support the ability of each of PS Spine and RTI to take the position that the Merger and/or the Contribution will qualify for the Intended Tax Treatment. If any of the representations or covenants upon which the Intended Tax Treatment is based is inaccurate, the United States federal income tax consequences of the Merger and/or the Contribution could differ from the Intended Tax Treatment.
27
Assuming that the receipt of Holdco common stock in exchange for RTI shares pursuant to the Merger, taken together with the receipt of Holdco common stock in exchange for Paradigm equity interests pursuant to the Contribution, qualifies as an “exchange” described in Section 351(a) of the Code (or, in the case of RTI stockholders, that the Merger, taken alone, qualifies as a “reorganization” within the meaning of Section 368(a) of the Code), and subject to the limitations and qualifications described in “The Transaction—Material U.S. Federal Income Tax Consequences”:
| • | a U.S. holder of RTI shares receiving Holdco shares pursuant to the Merger will not recognize gain or loss with respect to such receipt of Holdco shares; and |
| • | PS Spine will recognize gain (but not loss) in connection with the Contribution, but not in excess of the amount of cash (including cash received as part of the Earnout Consideration) received in the Contribution. |
Please carefully review the information set forth in the section entitled “The Transaction—Material U.S. Federal Income Tax Consequences”, beginning on page 109, for a description of the material U.S. federal income tax consequences of the Merger and the Contribution.The tax consequences to you of the Merger and the Contribution will depend on your own situation. Please consult your own tax advisors as to the specific tax consequences to you of the Merger and the Contribution.
Accounting Treatment (see page 113)
The Transaction will be accounted for as an acquisition of a business. Holdco will account for the acquisition pursuant to the Master Transaction Agreement using the acquisition method of accounting in accordance with United States generally accepted accounting principles (“GAAP”). Holdco will measure the assets acquired and liabilities assumed at their fair values including net tangible and identifiable intangible assets acquired and liabilities assumed as of the closing of the Transaction. Any excess of the purchase price over those fair values will be recorded as goodwill. Definite lived intangible assets will be amortized over their estimated useful lives. Intangible assets with indefinite useful lives and goodwill will not be amortized but will be tested for impairment at least annually, and all assets including goodwill will be tested for impairment when certain indicators are present. If, in the future, Holdco determines that tangible or intangible assets (including goodwill) are impaired, Holdco will record an impairment charge at that time. The final purchase price and fair value assessment of assets and liabilities will be based in part on a detailed valuation which has not yet been completed.
In evaluating the proposals described herein, you should carefully read this joint proxy and consent solicitation statement/prospectus and especially consider the factors discussed in the section entitled “Risk Factors”, beginning on page 38.
Other Ancillary Agreements (see page 119)
In connection with the Transaction, RTI and Holdco have entered into the Paradigm Support Agreements with the Paradigm Support Unitholders, PS Spine has entered into the RTI Support Agreements with the RTI Support Stockholders, Holdco has entered into theNon-Competition Agreements with Viscogliosi Brothers, LLC, HealthCor Paradigm Blocker Company Two, Inc. and HealthCor AIV, L.P. and RTI has entered into consulting agreements with each of Marc. R. Viscogliosi and Francis Magee. At the closing, PS Spine will enter into aLock-Up Agreement. Additionally, at the closing, RTI, Holdco and the parties to the Settlement Agreement, apart from PS Spine and Paradigm, will enter into aLock-Up and Permitted Sale Agreement. For a description of these agreements, please see the section entitled “The Transaction.”
28
Comparison of Rights of Holdco Stockholders, RTI Stockholders and the Paradigm Member (see page 172)
For a comparison of the rights of Holdco Stockholders as compared to RTI Stockholders and the sole member of Paradigm, see the section entitled “Comparison of Rights of Holdco Stockholders, RTI Stockholders and the Paradigm Member”, beginning on page 172.
29
Summary Selected Historical Consolidated Financial Data for RTI
The summary selected historical consolidated financial data set forth below for each of the years ended December 31, 2017, 2016 and 2015, and as of December 31, 2017 and 2016, have been derived from audited consolidated financial statements of RTI which are incorporated by reference into this joint proxy and consent solicitation statement/prospectus. The summary selected historical consolidated financial data set forth below as of December 31, 2015, 2014 and 2013 and for each of the years ended December 31, 2014 and 2013, have been derived from consolidated financial statements of RTI which are not included or incorporated by reference into this joint proxy and consent solicitation statement/prospectus. The data as of September 30, 2018 and the nine months ended September 30, 2018 and 2017 have been derived from unaudited consolidated condensed financial statements of RTI in its Quarterly Report on Form10-Q for the quarterly period ended September 30, 2018, which is incorporated by reference into this joint proxy and consent solicitation statement/prospectus.
The following information should be read together with RTI’s consolidated financial statements, the notes related thereto and the related “Management’s Discussion and Analysis of Financial Condition and Results of Operations” contained in RTI’s Annual Report on Form10-K for the year ended December 31, 2017 and Quarterly Report on Form10-Q for the quarterly period ended September 30, 2018, each of which is incorporated herein by reference. See the section entitled “Where You Can Find More Information”, beginning on page 187. RTI’s historical consolidated financial information may not be indicative of the future performance of RTI or the combined company.
| Nine Months Ended September 30, | Year Ended December 31, | |||||||||||||||||||||||||||
| 2018 | 2017 | 2017 | 2016 | 2015 | 2014 | 2013 | ||||||||||||||||||||||
| (in thousands, except per share data) | ||||||||||||||||||||||||||||
Statements of Operations Data: | ||||||||||||||||||||||||||||
Revenues | $ | 209,639 | $ | 208,747 | $ | 279,563 | $ | 272,865 | $ | 282,293 | $ | 262,810 | $ | 197,979 | ||||||||||||||
Operating (loss) income | (2,443 | ) | 32,646 | 28,810 | (15,681 | ) | 24,625 | 5,625 | (28,656 | ) | ||||||||||||||||||
Net (loss) income | (5,441 | ) | 11,153 | 2,549 | (17,907 | ) | 11,610 | (417 | ) | (19,189 | ) | |||||||||||||||||
Net (loss) income per share—basic | (0.09 | ) | 0.19 | 0.04 | (0.31 | ) | 0.20 | (0.01 | ) | (0.34 | ) | |||||||||||||||||
Net (loss) income per share—diluted | (0.09 | ) | 0.19 | 0.04 | (0.31 | ) | 0.20 | (0.01 | ) | (0.34 | ) | |||||||||||||||||
Weighted average common shares outstanding (basic and diluted) | 63,518 | 59,045 | 59,684 | 58,237 | 57,611 | 56,736 | 56,259 | |||||||||||||||||||||
| 63,518 | 59,955 | 60,600 | 58,237 | 58,590 | 56,736 | 56,259 | ||||||||||||||||||||||
Balance Sheet Data: | ||||||||||||||||||||||||||||
Cash and cash equivalents | 10,022 | 22,381 | 13,849 | 12,614 | 15,703 | 18,721 | ||||||||||||||||||||||
Total working capital | 120,274 | 132,676 | 121,329 | 131,941 | 133,510 | 134,302 | ||||||||||||||||||||||
Total assets | 350,097 | 345,906 | 368,031 | 380,662 | 378,135 | 369,854 | ||||||||||||||||||||||
Long-term debt—less current portion | 49,021 | 42,076 | 77,267 | 73,384 | 69,413 | 67,706 | ||||||||||||||||||||||
Preferred stock | 66,180 | 63,923 | 60,016 | 56,323 | 52,834 | 49,537 | ||||||||||||||||||||||
Accumulated deficit | (239,515 | ) | (237,066 | ) | (243,338 | ) | (228,939 | ) | (243,854 | ) | (246,550 | ) | ||||||||||||||||
Stockholders’ equity | $ | 180,776 | $ | 181,737 | $ | 164,916 | $ | 181,356 | $ | 167,835 | $ | 168,053 | ||||||||||||||||
30
Effective January 1, 2018, the reporting of RTI’s lines of business are composed primarily of four categories: spine; sports; original equipment manufacturer (“OEM”) and international. RTI’s previous lines of business were composed of: spine; sports medicine and orthopedics; surgical specialties; cardiothoracic; international; OEM; and other. Effective January 1, 2018, the other revenues category is included in the OEM line of business. RTI believes that the changes in the reporting of RTI’s lines of business are aligned with RTI’s focused strategy of reducing complexity and a better understanding of RTI’s lines of business. Additionally, on August 3, 2017, RTI completed the sale of substantially all of the assets related to its Cardiothoracic closure business (the “CT Business”) to A&E Advanced Closure Systems, LLC (a subsidiary of A&E Medical Corporation) (“A&E”). In connection with the CT Business sale, RTI entered into a multi-year Contract Manufacturing Agreement with A&E whereby RTI continues to support the CT Business under A&E’s ownership through the manufacturing of existing products, which generates revenue for RTI’s OEM business. Discrete financial information is not available for these four lines of business. The following table presents revenues from these five categories for the year ended December 31, 2017, 2016 and 2015:
| 2017 | 2016 | 2015 | ||||||||||
Revenues: | ||||||||||||
Spine | $ | 77,514 | $ | 73,907 | $ | 57,983 | ||||||
Sports | 57,211 | 54,609 | 53,741 | |||||||||
OEM | 110,710 | 108,093 | 139,964 | |||||||||
International | 25,964 | 25,109 | 21,906 | |||||||||
Cardiothoracic | 8,164 | 11,147 | 8,699 | |||||||||
|
|
|
|
|
| |||||||
Total revenues | $ | 279,563 | $ | 272,865 | $ | 282,293 | ||||||
|
|
|
|
|
| |||||||
RTI expects the amounts previously disclosed as of and for the years ended December 31, 2017 and 2016, in its Annual Report on Form 10-K for the year ended December 31, 2017 will be restated to equal amounts in the table above when RTI’s Annual Report on From 10-K for the year ended December 31, 2018 is filed with the SEC.
31
Summary Selected Historical Consolidated Financial Data for Paradigm
The summary selected historical consolidated financial data set forth below as of and for each of the years ended December 31, 2017 and 2016 have been derived from audited consolidated financial statements of Paradigm which are included in this joint proxy and consent solicitation statement/prospectus. The summary selected historical consolidated financial data set forth below as of and for the years ended December 31, 2015, 2014 and 2013 have been derived from audited financial statements of Paradigm which are not included in or incorporated by reference into this joint proxy and consent solicitation statement/prospectus. The summary selected historical consolidated financial data set forth below as of September 30, 2018 and for the nine months ended September 30, 2018 and 2017 have been derived from unaudited consolidated condensed financial statements of Paradigm which are included in this joint proxy and consent solicitation statement/prospectus. In the opinion of Paradigm, such unaudited consolidated condensed financial statements include all adjustments, including those of a normal recurring nature, necessary for a fair presentation of its financial position and results of operations for such periods. Interim results for the nine months ended September 30, 2018 are not necessarily indicative of, and are not projections for, the results to be expected for the full year ending December 31, 2018.
The summary selected historical consolidated financial data below should be read in conjunction with the section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations of Paradigm”, beginning on page 153, as well as the consolidated financial statements of Paradigm and their accompanying notes that are included in this joint proxy and consent solicitation statement/prospectus.
| Nine Months Ended September 30, | Year Ended December 31, | |||||||||||||||||||||||||||
| 2018 | 2017 | 2017 | 2016 | 2015 | 2014 | 2013 | ||||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||||||||||
Statements of Operations Data: | ||||||||||||||||||||||||||||
Net sales | $ | 30,532 | $ | 33,925 | $ | 44,832 | $ | 55,641 | $ | 51,768 | $ | 50,010 | $ | 37,404 | ||||||||||||||
Operating (loss) income | (13,458 | ) | (7,076 | ) | (11,285 | ) | 3,579 | 1,972 | 4,384 | 1,068 | ||||||||||||||||||
Net loss | (30,600 | ) | (9,461 | ) | (42,222 | ) | (3,626 | ) | (7,558 | ) | (12,610 | ) | (6,104 | ) | ||||||||||||||
Balance Sheet Data: | ||||||||||||||||||||||||||||
Cash and cash equivalents | 1,075 | 10,747 | 49,712 | 10,475 | 9,151 | 11,810 | ||||||||||||||||||||||
Total working capital | (123,976 | ) | (102,050 | ) | (43,780 | ) | 12,134 | 17,499 | 11,181 | |||||||||||||||||||
Total assets | 16,203 | 26,734 | 66,196 | 25,355 | 25,785 | 28,453 | ||||||||||||||||||||||
Long-term debt—less current portion | — | — | — | 47,740 | 48,482 | 37,935 | ||||||||||||||||||||||
Preferred units | 102,940 | 102,940 | 103,544 | 103,544 | 103,789 | 103,839 | ||||||||||||||||||||||
Accumulated deficit | (245,029 | ) | (214,429 | ) | (172,207 | ) | (168,581 | ) | (161,023 | ) | (148,413 | ) | ||||||||||||||||
Members’ deficit | $ | (122,632 | ) | $ | (91,749 | ) | $ | (50,104 | ) | $ | (47,015 | ) | $ | (39,824 | ) | $ | (27,129 | ) | ||||||||||
32
Summary Selected Unaudited Pro Forma Condensed Combined Financial Information
The following summary selected unaudited pro forma condensed combined financial information is designed to show how the Transaction between RTI and Paradigm might have affected historical financial statements if the Transaction had been completed at an earlier time and was prepared based on the historical financial results reported by RTI and Paradigm. The following should be read in connection with the “Bears Holding Sub, Inc. and Subsidiaries Unaudited Pro Forma Condensed Combined Financial Information”, beginning on page 179, and the RTI consolidated financial statements, which are incorporated by reference into this joint proxy and consent solicitation statement/prospectus, and the Paradigm consolidated financial statements included elsewhere in this this joint proxy and consent solicitation statement/prospectus.
The summary selected unaudited pro forma condensed combined balance sheet data assumes that the Transaction took place on September 30, 2018 and combines RTI’s consolidated balance sheet as of September 30, 2018 with Paradigm’s consolidated balance sheet as of September 30, 2018. The summary selected unaudited pro forma condensed combined statements of operations data for the nine months ended September 30, 2018 and for the year ended December 31, 2017 give effect to the Transaction as if it occurred on January 1, 2017.
The summary selected unaudited pro forma condensed combined financial data is presented for illustrative purposes only and is not necessarily indicative of the financial condition or results of operations of future periods or the financial condition or results of operations that actually would have been realized had the entities been a single company during these periods. Future results may vary significantly from the results reflected because of various factors, including those discussed in the section entitled “Risk Factors”, beginning on page 38.
| Year Ended December 31, 2017 | Nine Months Ended September 30, 2018 | |||||||
| (in thousands, except per share data) | ||||||||
Statements of Operations Data: | ||||||||
Revenues | $ | 324,395 | $ | 240,171 | ||||
Operating income (loss) | 8,858 | (22,401 | ) | |||||
Net loss applicable to common shares | (26,612 | ) | (34,235 | ) | ||||
Net loss per common share—basic | (0.38 | ) | (0.46 | ) | ||||
Net loss per common share—diluted | (0.38 | ) | (0.46 | ) | ||||
| As of September 30, 2018 | ||||
| (in thousands) | ||||
Balance Sheet Data: | ||||
Cash and cash equivalents | $ | 10,022 | ||
Working capital | 116,167 | |||
Total assets | 613,799 | |||
Long-term obligations—less current portion | 143,664 | |||
Accumulated deficit | (245,265 | ) | ||
Stockholders’ equity | 219,768 | |||
33
Unaudited Pro Forma Combined Per Share and Per Unit Information
The following table sets forth certain historical per share information for RTI, certain historical per unit information for Paradigm and certain unaudited pro forma combined per share and per unit information. The unaudited pro forma book value per share and per unit information was computed as if the Transaction had been completed on December 31, 2017 and September 30, 2018. The pro forma net income (loss) from continuing operations information was computed as if the Transaction had been completed on January 1, 2017 and 2018. These amounts do not necessarily reflect future per share amounts of net income (loss) from operations and book value per share of the combined company.
The following unaudited comparative per share and per unit information is derived from the historical consolidated financial statements of each of RTI and Paradigm included in or incorporated by reference into this joint proxy and consent solicitation statement/prospectus, as well as the unaudited pro forma condensed combined financial information and accompanying notes included in this joint proxy and consent solicitation statement/prospectus.
The unaudited pro forma per share and per unit information below is presented for illustrative purposes only. Either company’s actual historical financial condition and results of operations may have been different had the companies always been combined. You should not rely on this information as being indicative of the historical financial condition and results of operations that would have actually been achieved or of the future results of Holdco after the completion of the Transaction.
The information below should be read in conjunction with the consolidated financial statements and accompanying notes of RTI and Paradigm, which are included in or incorporated by reference into this joint proxy and consent solicitation statement/prospectus. We urge you also to read the “Bears Holding Sub, Inc. and Subsidiaries Unaudited Pro Forma Condensed Combined Financial Information”, beginning on page 179.
| As of and For the Year Ended December 31, 2017 | As of and For the Year Ended September 30, 2018 | |||||||
RTI—Historical | ||||||||
Net income (loss) per common share—basic | $ | 0.04 | $ | (0.09 | ) | |||
Net income (loss) per common share—diluted | 0.04 | (0.09 | ) | |||||
Book value at end of period—per common share outstanding | 3.00 | 2.85 | ||||||
Paradigm—Historical | ||||||||
Net loss per common unit—basic | $ | (0.60 | ) | $ | (0.41 | ) | ||
Net loss per common unit—diluted | (0.60 | ) | (0.41 | ) | ||||
Book value at end of period—per common unit outstanding | (2.76 | ) | (3.04 | ) | ||||
34
| As of and For the Year Ended December 31, 2017 | As of and For the Nine Months Ended September 30, 2018 | |||||||
Unaudited Bears Holding Sub, Inc. | ||||||||
Pro Forma Combined | ||||||||
Net loss per common share—basic | $ | (0.38 | ) | $ | (0.46 | ) | ||
Net loss per common share—diluted | (0.38 | ) | (0.46 | ) | ||||
Book value at end of period—per common share outstanding | 3.29 | 2.96 | ||||||
| As of and For the Year Ended December 31, 2017 | As of and For the Nine Months Ended September 30, 2018 | |||||||
Paradigm Pro Forma Equivalent | ||||||||
Net loss per common unit—basic | $ | (3.94 | ) | $ | (2.85 | ) | ||
Net loss per common unit—diluted | (3.94 | ) | (2.85 | ) | ||||
Book value at end of period—per common unit outstanding | (18.15 | ) | (21.02 | ) | ||||
35
Per Share/Unit Market Price Data and Dividend Information
RTI common stock trades on Nasdaq under the symbol “RTIX”. The following table presents the range of high and low per share sales prices for RTI common stock as reported on Nasdaq for each of the periods set forth below. PS Spine and Paradigm are each limited liability companies. There is no established public trading market for PS Spine or Paradigm units, and neither PS Spine nor Paradigm has declared any dividends or distributions during the periods set forth in the table below. These per share sales prices do not give effect to the Transaction.
RTI Common Stock
| High | Low | Cash Dividends Declared | ||||||||||
Fiscal Year 2017 | ||||||||||||
First quarter | 4.10 | 3.01 | — | |||||||||
Second quarter | 5.95 | 3.80 | — | |||||||||
Third quarter | 6.00 | 4.25 | — | |||||||||
Fourth quarter | 5.08 | 3.85 | — | |||||||||
Fiscal Year 2018 | ||||||||||||
First quarter | 5.10 | 4.05 | — | |||||||||
Second quarter | 4.95 | 4.20 | — | |||||||||
Third quarter | 4.95 | 4.25 | — | |||||||||
Fourth quarter | 4.89 | 3.53 | — | |||||||||
Fiscal Year 2019 | ||||||||||||
First quarter (through January 17, 2019) | 4.46 | 3.51 | — | |||||||||
PS Spine Units
| High | Low | Cash Dividends and Distributions Declared | ||||||||||
Fiscal Year 2017 | ||||||||||||
First quarter | N/A | N/A | — | |||||||||
Second quarter | N/A | N/A | — | |||||||||
Third quarter | N/A | N/A | — | |||||||||
Fourth quarter | N/A | N/A | — | |||||||||
Fiscal Year 2018 | ||||||||||||
First quarter | N/A | N/A | — | |||||||||
Second quarter | N/A | N/A | — | |||||||||
Third quarter | N/A | N/A | — | |||||||||
Fourth quarter | N/A | N/A | — | |||||||||
Fiscal Year 2019 | ||||||||||||
First quarter (through January 17, 2019) | N/A | N/A | — | |||||||||
36
Paradigm Units
| High | Low | Cash Dividends and Distributions Declared | ||||||||||
Fiscal Year 2017 | ||||||||||||
First quarter | N/A | N/A | — | |||||||||
Second quarter | N/A | N/A | — | |||||||||
Third quarter | N/A | N/A | — | |||||||||
Fourth quarter | N/A | N/A | — | |||||||||
Fiscal Year 2018 | ||||||||||||
First quarter | N/A | N/A | — | |||||||||
Second quarter | N/A | N/A | — | |||||||||
Third quarter | N/A | N/A | — | |||||||||
Fourth quarter | N/A | N/A | — | |||||||||
Fiscal Year 2019 | ||||||||||||
First quarter (through January 17, 2019) | N/A | N/A | — | |||||||||
On November 1, 2018, the last trading day before the public announcement of the execution of the Master Transaction Agreement, the last reported sale price of RTI common stock on Nasdaq was $4.57 per share. On January 17, 2019, the last reported sale price of RTI common stock on Nasdaq was $4.32 per share.
Following the Transaction, RTI expects Holdco’s common stock will be listed on the Nasdaq Global Market and will trade under the name “RTI Surgical Holdings, Inc.” and symbol “RTIX”.
As of January 15, 2019, there were approximately 291 stockholders of record of RTI common stock.
As of both January 15, 2019 and the PS Spine record date, there were approximately 58 holders of record of Common Units of PS Spine, approximately 19 holders of record of Class A Common Units of PS Spine, approximately 16 holders of record of Class A Preferred Units of PS Spine, approximately 17 holders of record of Class B Preferred Units of PS Spine, approximately 97 holders of record of Class C Preferred Units of PS Spine, approximately 66 holders of record of Class D Preferred Units of PS Spine, approximately 72 holders of record ofClass E-1 Preferred Units of PS Spine, approximately 66 holders of record ofClass E-2 Preferred Units of PS Spine and no holders of record of Class F Preferred Units of PS Spine. Holders of Class C Preferred Units, Class D Preferred Units andClass E-1 Preferred Units of PS Spine generally do not have voting rights. As of both January 15, 2019 and the PS Spine record date, PS Spine was the sole holder of Paradigm units.
37
The combined company will be faced with a market environment that cannot be predicted and that involves significant risks, many of which will be beyond its control. In addition to the other information contained in this joint proxy and consent solicitation statement/prospectus, you should carefully consider the material risks described below before you, as an RTI stockholder decide how to vote your shares of stock, or you, as a PS Spine unitholder, decide if you will provide written consent. You should also read and consider the other information in this joint proxy and consent solicitation statement/prospectus and the other documents incorporated by reference into this joint proxy and consent solicitation statement/prospectus, including the matters addressed in the section entitled “Cautionary Statement Concerning Forward-Looking Statements”, beginning on page 1.See also“Where You Can Find More Information”, beginning on page 187.
Risks Relating to the Transaction and the Combined Company
The announcement and pendency of the Transaction may adversely affect the business, financial condition and results of operations of RTI, Paradigm and, consequently, if the Transaction is consummated, the combined company.
Uncertainty about the effect of the Transaction on employees, customers, suppliers, third-party distributors and other parties, may have an adverse effect on each of the respective businesses, financial conditions and results of operations of RTI and Paradigm, regardless of whether the Transaction is completed, and may have an adverse effect on the business, financial condition and results of operations of the combined company if the Transaction is completed. These risks include the following, all of which could be exacerbated by a delay in the completion of the Transaction:
| • | the impairment of RTI’s and Paradigm’s ability to attract, retain and motivate current and prospective employees, including key personnel; |
| • | the diversion of significant time and resources of RTI’s and Paradigm’s management; |
| • | difficulties maintaining relationships with RTI’s and Paradigm’s customers, suppliers, third-party distributors and other business partners; |
| • | delays or deferments of certain business decisions by RTI’s and Paradigm’s customers, suppliers, third-party distributors and other business partners; |
| • | RTI’s and Paradigm’s inability to pursue alternative business opportunities or make appropriate changes to their respective businesses because of requirements in the Master Transaction Agreement that they conduct their businesses in all material respects in the ordinary course of business consistent with past practice and not engage in certain activities prior to the completion of the Transaction; |
| • | any litigation relating to the Transaction and the costs related thereto; and |
| • | the incurrence of significant costs, expenses and fees for professional services and other transaction costs in connection with the Transaction. |
Failure to consummate the Transaction within the expected timeframe or at all could have a material adverse impact on the business, financial condition and results of operations of RTI, Paradigm and consequently, if the Transaction is consummated, the combined company.
There can be no assurance that the Transaction will occur within the expected timeframe or at all. Consummation of the Transaction is subject to specified conditions, including:
| • | the accuracy of the representations and warranties of the parties and compliance by the parties with their respective obligations under the Master Transaction Agreement, in each case subject to certain materiality qualifiers; |
| • | the written consent of PS Spine’s unitholders approving the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution; |
38
| • | the receipt of RTI stockholder approval; |
| • | the expiration or termination of the applicable waiting periods (and any extension thereof) under the HSR Act; |
| • | the absence of any law or order in effect that prevents, makes unlawful or prohibits the consummation of the Transaction; |
| • | the absence of any material adverse effect on the business, condition (financial or otherwise) or results of operations of RTI and its subsidiaries, taken as a whole, or Paradigm and its subsidiaries, taken as a whole, in each case subject to certain exceptions, since November 1, 2018, the date of the Master Transaction Agreement; |
| • | the Cash Consideration Amount being equal to or greater than $0; |
| • | the shares of Holdco common stock issuable pursuant to the Merger and shares of Holdco common stock representing the Stock Consideration Amount being approved for listing on the Nasdaq Global Market, subject to official notice of issuance; |
| • | the registration statement on FormS-4, of which this joint proxy and consent solicitation statement/prospectus forms a part, becoming effective under the Securities Act; |
| • | the receipt of representation letters from officers of RTI, Holdco and PS Spine regarding the intended tax treatment of the Transaction (provided this condition will be deemed not to be satisfied if (i) Sidley Austin or Dorsey & Whitney, as the case may be, delivers an opinion that, as a result of a change in law occurring after November 1, 2018, it is unable to provide an opinion to the effect that the Merger and the Contribution, taken together, will qualify as a transaction described in Section 351 of the Code (and, in the case of Sidley Austin, that the Merger will not qualify as a “reorganization” within the meaning of Section 368(a) of the Code) and (ii) RTI or PS Spine, as the case may be, is unable to obtain such opinion from an alternative tax counsel); |
| • | neither Marc Viscogliosi nor Francis Magee rescinding his obligations under his applicable consulting agreement with RTI, and RTI not rescinding its obligations under those consulting agreements; |
| • | none of Viscogliosi Brothers, LLC, HealthCor Paradigm Blocker Company Two, Inc. and HealthCor AIV, L.P. rescinding its obligations under its applicableNon-Competition Agreement with Holdco; and |
| • | none of the parties to the Settlement Agreement rescinding their obligations under the Settlement Agreement, the Settlement Agreement being in full force and effect and not having been amended in any manner adverse to Holdco, RTI or Merger Sub and the transactions contemplated by the Settlement Agreement having been performed in accordance with the terms of the Settlement Agreement. |
For additional information regarding the specified conditions to the closing of the Transaction, see the section entitled “The Master Transaction Agreement—Conditions to the Closing”.
We cannot provide any assurances that these conditions will be satisfied in a timely manner or at all or that the Transaction will occur.
In addition, the Master Transaction Agreement contains certain termination rights, including (i) for RTI and PS Spine if the Transaction is not consummated on or prior to January 31, 2019 (which such deadline may be extended by RTI under certain circumstances to March 31, 2019 and which extensions RTI intends to exercise, as necessary), (ii) for RTI and PS Spine if the Required Merger Proposal Vote is not obtained at the RTI special meeting, or any adjournment or postponement thereof, (iii) for RTI if the PS Spine written consent is not provided to RTI within five business days following the effectiveness of the registration statement on FormS-4, of which this joint proxy and consent solicitation statement/prospectus forms a part and (iv) for PS Spine if the RTI board changes its recommendation that the RTI stockholders vote to approve the Merger Proposal, Share Issuance Proposal and the Meeting Adjournment Proposal, provided that PS Spine may not terminate pursuant to this clause if the Required Merger Proposal Vote has been obtained. For additional information regarding the circumstances under which RTI and PS Spine are able to terminate the Master Transaction Agreement, see the section entitled “The Master Transaction Agreement—Termination”.
39
In addition, satisfying the conditions to the Transaction may take longer, and could cost more, than RTI and Paradigm expect. The occurrence of any of these events individually or in combination may adversely affect the benefits RTI and Paradigm expect to achieve from the Transaction and the trading prices of, prior to the consummation of the Transaction, RTI shares and, following the consummation of the Transaction, Holdco shares. In addition, if the Transaction does not close, the attention of RTI’s management and Paradigm’s management will have been diverted to the consummation of the Transaction, rather than their respective operations and pursuit of other opportunities, in certain circumstances RTI would be required to pay a reverse termination fee equal to $4.5 million or $9.0 million to PS Spine and RTI and Paradigm may suffer reputational or other harm due to the adverse perception of any failure to successfully complete the Transaction.
Competition authorities may take certain actions in connection with the Transaction under applicable antitrust laws, including enjoining consummation of the Transaction.
Under the HSR Act and the rules that have been promulgated thereunder by the FTC, certain transactions may not be consummated unless information has been furnished to the Antitrust Division and the FTC and certain waiting period requirements have been satisfied. The Transaction is subject to these requirements and may not be completed until the expiration of a30-day waiting period following the filing of the required Notification and Report Forms with the Antitrust Division and the FTC or until early termination is granted. On November 13, 2018, Holdco and PS Spine filed the required forms under the HSR Act with the Antitrust Division and the FTC and requested early termination, which was granted on November 26, 2018. Even though early termination has been granted under the HSR Act, at any time before or after consummation of the Transaction, notwithstanding such termination, the applicable competition authorities could take such action under applicable antitrust laws as each deems necessary or desirable in the public interest, including seeking to enjoin the consummation of the Transaction. For additional information regarding the regulatory approvals required for closing of the Transaction, see the section entitled “The Transaction—Regulatory Approvals”.
If the Merger and the Contribution, taken together, do not qualify as a transaction described in Section 351 of the Code (or, in the case of the RTI stockholders, if the Merger does not qualify as a reorganization within the meaning of Section 368(a) of the Code), RTI stockholders may recognize substantial gain as a result of the Merger or the Contribution and may be required to pay substantial incremental U.S. federal income taxes, and PS Spine may recognize substantial additional gain as a result of the Contribution, in the taxable year in which the Merger and Contribution occur.
The obligations of PS Spine and RTI to consummate the Contribution and the Merger are conditioned on the receipt of certain representations and covenants in certificates of officers of RTI, PS Spine, and Holdco, which representations and covenants are intended to support the ability of each of PS Spine and RTI to take the position that the Merger and/or the Contribution will qualify for the Intended Tax Treatment. The positions of RTI and PS Spine are not binding on the Internal Revenue Service (the “IRS”) or the courts, and the parties do not intend to request a ruling from the IRS with respect to the Merger and/or the Contribution. Accordingly, there can be no assurance that the IRS will not challenge the qualification of the Merger and the Contribution, taken together, as a transaction described in Section 351 of the Code (or, in the case of RTI, the qualification of the Merger as a reorganization within the meaning of Section 368(a) of the Code) or that a court will not sustain such a challenge. If the IRS were to be successful in any such contention, or if for any other reason the Merger and the Contribution, taken together, were not treated as a transaction described in Section 351 of the Code (and, in the case of the RTI stockholders, if the Merger did not qualify as a reorganization within the meaning of Section 368(a) of the Code), then the RTI stockholders and/or PS Spine, as the case may be, would not be entitled to defer any portion of the gain realized as a result of receiving Holdco shares in the Merger or the Contribution and may be required to pay substantial incremental U.S. federal income taxes with respect to the taxable year in which the Merger and Contribution occur. For additional information regarding the U.S. federal income tax consequences of the Merger and the Contribution, see the section entitled “The Transaction—Material U.S. Federal Income Tax Consequences”, beginning on page 109.
The U.S. federal income tax treatment of the Earnout Consideration is not entirely clear under current law.
RTI and PS Spine intend to take the position that the recognition of gain upon receipt of the Holdco common stock component of the Earnout Consideration will be deferred pursuant to Section 351(a) of the Code. The U.S. federal income tax treatment of the receipt, after the close of the taxable year in which the Contribution occurs, of Holdco common stock as contingent consideration in a transaction that otherwise qualifies as a transaction described in
40
Section 351 of the Code is not entirely clear under current law, and there is no direct authority with respect to the tax treatment of the Earnout Consideration. If the recognition of gain upon the receipt of the Holdco common stock component of the Earnout Consideration is not eligible for deferral under Section 351 of the Code, then the value of such Holdco common stock would be treated in the same manner as the cash component of the Earnout Consideration. In that case, PS Spine would recognize gain (but not loss) as a result of the receipt of such Holdco common stock. Such gain may be eligible to be reported under the installment method in accordance with the rules (including the imposition of an interest charge), described in the section entitled “The Transaction—Material U.S. Federal Income Tax Consequences—Treatment of the Earnout Consideration”, beginning on page 112. Regardless of whether Section 351(a) applies to the Holdco common stock component of the Earnout Consideration, a portion of Holdco common stock received as Earnout Consideration may be recharacterized as imputed interest under the rules described in the section entitled “The Transaction—Material U.S. Federal Income Tax Consequences—Imputed Interest”, beginning on page 113.
RTI and PS Spine may be the target of securities class action and derivative lawsuits which could result in substantial costs and may delay or prevent the Transaction from being completed.
Securities class action lawsuits and derivative lawsuits are often brought against companies that have entered into merger agreements or other business combinations. Even if the lawsuits are without merit, defending against these claims can result in substantial costs and divert management time and resources. An adverse judgment could result in monetary damages, which could have a negative impact on RTI’s or PS Spine’s liquidity and financial condition. Additionally, if a plaintiff is successful in obtaining an injunction prohibiting the closing of the Transaction, then that injunction may delay or prevent the Transaction from being consummated, which may adversely affect RTI’s and Paradigm’s respective business, financial position and results of operation. As of the date of this joint proxy and consent solicitation statement/prospectus, neither RTI nor Paradigm is aware of any securities class action lawsuits or derivative lawsuits having been filed in connection with the Transaction.
RTI stockholders and PS Spine unitholders will have a reduced ownership and voting interest after the Transaction and will each exercise less influence over management.
The Holdco shares that each RTI stockholder receives in the Transaction in exchange for shares of RTI will represent a percentage ownership of Holdco that is smaller than such RTI stockholder’s percentage ownership of RTI. Similarly, the Holdco shares that PS Spine receives in the Transaction in exchange for equity interests in Paradigm will represent a percentage ownership of Holdco that is significantly smaller than PS Spine’s percentage ownership of Paradigm. If the Transaction occurs, 10,729,614 shares of common stock of Holdco will be issued at closing as partial consideration for the Contribution. A significant portion of these shares will be used, along with cash that would otherwise be paid at closing, to pay the amounts outstanding under Paradigm’s existing senior secured credit agreement. Assuming (i) the closing occurs on March 15, 2019, (ii) 62,245,112 shares of RTI common stock (based on the number of shares outstanding as of January 15, 2019) and 50,000 shares of RTI preferred stock are issued and outstanding immediately prior to the effective time and (iii) the amount of cash paid to the lenders under Paradigm’s existing senior secured credit agreement at closing does not exceed $95.0 million, and taking into account the $3.0 million Paradigm borrowed from the lenders under its existing senior secured credit agreement on December 6, 2018, approximately 85.30% of the issued and outstanding Holdco common stock and 100% of the issued and outstanding Holdco preferred stock immediately following the closing will be held by former RTI stockholders, approximately 6.42% of the issued and outstanding Holdco common stock immediately following the closing will be held by PS Spine and approximately 8.28% of the issued and outstanding Holdco common stock immediately following the closing will be held by the lenders under Paradigm’s existing senior secured credit agreement and their affiliates. Using the same assumptions set forth above and assuming the conversion of the RTI preferred stock, immediately following the closing, former holders of RTI common stock and RTI preferred stock will collectively hold 87.37% of the voting power of Holdco, PS Spine will hold 5.51% of the voting power of Holdco and the lenders under Paradigm’s existing senior secured credit agreement and their affiliates will hold 7.12% of the voting power of Holdco. As a result of this reduced ownership percentage, former RTI stockholders will have less influence on the management and policies of Holdco than they now have with respect to RTI and PS Spine unitholders will have less influence on the management and policies of Holdco than they now have with respect to Paradigm. As described in the section entitled “The Transaction—Interests of PS Spine’s and Paradigm’s Managers and Executive Officers in the Transaction—The Settlement Agreement”, the lenders under Paradigm’s existing senior secured credit agreement and their affiliates may receive a smaller or larger portion of the Stock Consideration Amount than as assumed for purposes of this example. If the lenders under Paradigm’s
41
existing senior secured credit agreement and their affiliates receive a larger portion of the Stock Consideration Amount than as assumed for purposes of this example, the percentage of the issued and outstanding Holdco common stock immediately following the closing that will be held by the lenders under Paradigm’s existing senior secured credit agreement and their affiliates will be greater than set forth in this example and the percentage that will be held by PS Spine will be less than set forth in this example. If the lenders under Paradigm’s existing senior secured credit agreement and their affiliates receive a smaller portion of the Stock Consideration Amount than as assumed for purposes of this example, the percentage of the issued and outstanding Holdco common stock immediately following the closing that will be held by the lenders under Paradigm’s existing senior secured credit agreement and their affiliates will be less than set forth in this example and the percentage that will be held by PS Spine will be greater than set forth in this example. In addition, the earnout provisions of the Master Transaction Agreement provide for the possibility that PS Spine may receive additional shares of Holdco common stock, which would cause former RTI stockholders ownership of Holdco to be further diluted. For a description of the earnout provisions, including a description of the number of additional shares that may be issued, see the section entitled “The Master Transaction Agreement—Purchase Price—Contingent Consideration”.
The opinion of RTI’s financial advisor will not be updated to reflect changes in circumstances between signing of the Master Transaction Agreement in November 2018 and the completion of the Transaction.
RTI has not obtained an updated opinion from its financial advisor as of the date of this joint proxy and consent solicitation statement/prospectus, and RTI does not anticipate asking its financial advisor to update its opinion. Changes in the operations and prospects of RTI or Paradigm, general market and economic conditions and other factors that may be beyond the control of RTI or Paradigm, and on which RTI’s financial advisor’s opinions were based, may significantly alter the value of RTI or Paradigm or the trading prices of RTI shares by the time the Transaction is completed. The opinion does not speak as of the time the Transaction will be completed or as of any date other than the date on which such opinion was delivered. Because RTI’s financial advisor will not be updating its opinion, which was issued in connection with the signing of the Master Transaction Agreement on November 1, 2018, the opinion will not address the fairness of the Aggregate Consideration from a financial point of view at the time the Transaction is completed. For a description of the opinion that RTI received from its financial advisor, see the section entitled “The Transaction—Opinion of RTI’s Financial Advisor”.
The Transaction may be completed even though material adverse changes may result from the announcement of the Transaction, industry-wide changes or other causes.
In general, RTI may refuse to complete the Transaction if there is a change causing a material adverse effect on the business, condition (financial or otherwise) or results of operations of Paradigm and its subsidiaries, taken as a whole, prior to the closing. Similarly, in general, PS Spine may refuse to complete the Transaction if there is a change causing a material adverse effect on the business, condition (financial or otherwise) or results of operations of RTI and its subsidiaries, taken as a whole, prior to the closing. However, some types of changes do not permit either party to refuse to complete the Transaction, even if such changes would have a material adverse effect on RTI or Paradigm. If adverse changes occur but RTI and PS Spine must still complete the Transaction, the market price of the combined company’s stock may suffer. For a more complete discussion of what constitutes a material adverse effect on RTI or Paradigm under the Master Transaction Agreement, see the section entitled “The Master Transaction Agreement—Representations and Warranties”.
The combined company may fail to realize the anticipated benefits of the Transaction.
The success of the Transaction will depend on, among other things, the combined company’s ability to combine the RTI and Paradigm businesses in a manner that facilitates growth opportunities, including with respect to the acceleration of reimbursement of coflex®, and realizing anticipated cost synergies and performance improvements.
However, the combined company must successfully combine the businesses of RTI and Paradigm in a manner that permits these anticipated cost synergies and performance improvements to be realized. In addition, the combined company must achieve the anticipated synergies and improvements without adversely affecting current revenues and investments in future growth. If the combined company is not able to successfully achieve these objectives, the anticipated benefits of the Transaction may not be realized fully or at all or may take longer to realize than expected.
42
The failure to integrate successfully certain businesses and operations of RTI and Paradigm in the expected time frame may adversely affect the combined company’s future results.
Historically, RTI and Paradigm have operated as independent companies, and they will continue to do so until the completion of the Transaction. The management of the combined company may face significant challenges in integrating and consolidating certain businesses and the functions of RTI and Paradigm, integrating their technologies, organizations, procedures, policies and operations, addressing differences in the business cultures of the two companies and retaining key personnel. The integration may also be complex and time consuming, and require substantial resources and effort. The integration process and other disruptions resulting from the Transaction may also disrupt RTI’s and Paradigm’s ongoing businesses or cause inconsistencies in standards, controls, procedures and policies that adversely affect the combined company’s relationships with employees, suppliers, customers and others with whom RTI and Paradigm have business or other dealings or limit the combined company’s ability to achieve the anticipated benefits of the Transaction. In addition, difficulties in integrating the businesses or regulatory functions of RTI and Paradigm could harm the reputation of the combined company.
Combining the businesses of RTI and Paradigm may be more difficult, costly or time-consuming than expected, which may adversely affect the combined company’s results and negatively affect the value of Holdco shares following the Transaction.
RTI and PS Spine have entered into the Master Transaction Agreement because RTI and PS Spine each believes that the Transaction will be beneficial to, in the case of RTI, its company and its stockholders, and in the case of PS Spine, Paradigm and PS Spine’s unitholders, and that combining the businesses of RTI and Paradigm will produce cost synergies and performance improvements. If the combined company is not able to successfully combine the businesses of RTI and Paradigm in an efficient, effective and timely manner, the anticipated synergies and improvements of the Transaction may not be realized fully, or at all, or may take longer to realize than expected, and the value of Holdco shares may be affected adversely.
An inability to realize the full extent of the anticipated benefits of the Transaction and the other transactions contemplated by the Master Transaction Agreement, as well as any delays encountered in the integration process, could have an adverse effect upon the revenues, level of expenses and operating results of the combined company, which may adversely affect the value of Holdco shares after the completion of the Transaction.
In addition, the actual integration may result in additional and unforeseen expenses, and the anticipated benefits of the integration plan may not be realized. Actual synergies, if achieved, may be lower than what RTI and Holdco expect and may take longer to achieve than anticipated. If the combined company is not able to adequately address integration challenges, the combined company may be unable to successfully integrate RTI’s and Paradigm’s operations or to realize the anticipated benefits of the integration of the two companies.
RTI, Holdco, PS Spine and Paradigm will incur significant transaction costs in connection with the Transaction.
RTI, Holdco, PS Spine and Paradigm have incurred and expect to incur a number ofnon-recurring costs associated with the Transaction. These costs and expenses include financial advisory, debt origination fees, legal, accounting, consulting and other advisory fees and expenses, reorganization and restructuring costs, severance and employee benefit-related expenses, filing fees, printing expenses and other related charges. RTI expects that its transaction expenses will equal approximately $11.7 million, and PS Spine and Paradigm expect that their collective transaction expenses will equal approximately $4.0 million. Some of these costs are payable by RTI, Holdco, PS Spine and Paradigm regardless of whether the Transaction is completed. There is also a large number of processes, policies, procedures, operations, technologies and systems that must be integrated in connection with the Transaction. While both RTI and PS Spine have assumed that a certain level of expenses would be incurred in connection with the Transaction and the other transactions contemplated by the Master Transaction Agreement, there are many factors beyond their control that could affect the total amount or the timing of the integration and implementation expenses.
There may also be additional unanticipated significant costs in connection with the Transaction that the combined company may not recoup. These costs and expenses could reduce the benefits and additional income Holdco expects to
43
achieve from the Transaction. Although Holdco expects that these benefits will offset the transaction expenses and implementation costs over time, this expected net benefit may not be achieved in the near term or at all.
The combined company expects to have significant additional indebtedness following the Transaction and the credit ratings of the combined company or its subsidiaries may be different from current ratings and what RTI and PS Spine currently expect.
RTI expects to incur significant additional indebtedness in order to finance the Cash Consideration Amount and pay other costs and expenses incurred in connection with the Master Transaction Agreement and the transactions contemplated thereby. The receipt of financing by RTI or its affiliates, however, is not a condition to completion of the Transaction. Following completion of the Transaction, the combined company will have substantial indebtedness, which may adversely affect the business, financial condition and operating results of the combined company, including:
| • | making it more difficult for the combined company to satisfy its debt service obligations; |
| • | requiring the combined company to dedicate a substantial portion of its cash flows to debt service obligations, thereby potentially reducing the availability of cash flows to pay any future cash dividends and to fund working capital, capital expenditures, acquisitions, investments and other general operating requirements; |
| • | limiting the ability of the combined company to obtain additional financing to fund its working capital requirements, capital expenditures, acquisitions, investments, debt service obligations and other general operating requirements; |
| • | restricting the combined company from making investments in research and development, strategic acquisitions or taking advantage of favorable business opportunities; |
| • | placing the combined company at a relative competitive disadvantage compared to competitors that have less debt; |
| • | limiting flexibility to plan for, or react to, changes in the businesses and industries in which the combined company operates, which may adversely affect the combined company’s operating results and ability to meet its debt service obligations; |
| • | increasing the vulnerability of the combined company to adverse general economic and industry conditions, including changes in interest rates; and |
| • | limiting the ability of the combined company to refinance its indebtedness or increasing the cost of such indebtedness. |
If the combined company incurs additional indebtedness following the Transaction, the risks related to the substantial indebtedness of the combined company may intensify.
PS Spine and Paradigm managers and executive officers may have interests in the Transaction different from the interests of PS Spine Unitholders.
Although the PS Spine board recommends to PS Spine unitholders that they approve the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution, PS Spine unitholders should be aware that certain members of the PS Spine and Paradigm boards and executive officers of PS Spine and Paradigm have interests in the transactions contemplated by the Master Transaction Agreement that may be different from, or are in addition to, the general interests of PS Spine unitholders, as described in the section entitled “The Transaction—Interests of PS Spine’s and Paradigm’s Managers and Executive Officers in the Transaction”. These interests include, among other things: arrangements that provide for severance payments to certain of Paradigm’s executive officers in certain circumstances pursuant to the terms of their respective employment agreements with Paradigm; RTI’s expected execution of an employment agreement with William L. Pfost, Paradigm’s Executive Vice President, Sales and Marketing, and RTI’s execution of consulting agreements with Marc R. Viscogliosi, the Chairman and Chief Executive
44
Officer of PS Spine and Paradigm, and Francis Magee, Paradigm’s Chief Technology Officer; the election of Jeffrey C. Lightcap, a manager of PS Spine and Paradigm, to the Holdco board of directors upon the effective time; Paradigm’s expected execution of a consulting agreement with an entity in which certain managers and executive officers of PS Spine and Paradigm have an ownership interest; the ability of certain unitholders affiliated with certain managers of PS Spine and Holdco to receive distributions from PS Spine prior to other unitholders; a significant portion of the consideration that would otherwise be paid directly to PS Spine pursuant to the Master Transaction Agreement will be used to pay amounts owed to the lenders under Paradigm’s existing senior secured credit facility and their affiliates, and that one of PS Spine’s and Paradigm’s managers is a Managing Director at an entity related to such lenders and their affiliates; and rights to indemnification and directors’ and officers’ liability insurance that will survive the closing. PS Spine unitholders should consider whether these interests may have influenced the managers and executive officers of PS Spine and Paradigm to support or recommend the Transaction.
RTI’s share price may fluctuate prior to the completion of the Transaction, the combined company’s share price may fluctuate following the completion of the Transaction, and the monetary value of the Stock Consideration Amount and certain portions of the Earnout Consideration may change following the time of the signing of the Master Transaction Agreement or the date of this joint proxy and consent solicitation statement/prospectus due to a change in the value of Holdco common stock.
The Stock Consideration Amount and certain portions of the Earnout Consideration consist of a fixed number of shares of Holdco common stock, as set forth in the Master Transaction Agreement. Accordingly, any change in the market price of RTI common stock prior to completion of the Transaction, and any change in the market price of Holdco common stock following the completion of the Transaction, will affect the dollar value of such shares of Holdco common stock. Changes in the market price of RTI and/or Holdco common stock could result from a variety of factors, many of which are beyond RTI’s and/or the combined company’s control, including:
| • | general market and economic conditions, including market conditions in the medical device and healthcare industry; |
| • | technological innovations or new products by RTI’s or the combined company’s competitors; |
| • | actual or expected variations in results of operations; |
| • | changes in recommendations by securities analysts; |
| • | operations and stock performance of industry participants; |
| • | significant acquisitions or strategic alliances by competitors; |
| • | sales of RTI and/or combined company common stock, particularly as part of an underwritten offering; |
| • | recruitment or departure of key personnel; |
| • | loss of any strategic relationship; and |
| • | failure to achieve the perceived benefits of the Transaction as rapidly as, or to the extent, expected. |
The market price of the common stock of the combined company may be affected by factors different from those affecting the market price for shares of RTI common stock.
Upon the closing, holders of RTI common stock and PS Spine will become holders of Holdco common stock. Paradigm’s business differs from that of RTI, and the business of the combined company will differ from that of RTI. Accordingly, the results of operations for the combined company may be affected by factors different from those currently affecting the results of operations of RTI. For a discussion of the businesses of RTI and of certain factors to consider in connection with those businesses, see the see the section entitled “Information About RTI, Holdco and Merger Sub” and the documents incorporated by reference in this joint proxy and consent solicitation statement/prospectus and referred to in the section entitled “Where You Can Find More Information”. For a discussion of the
45
businesses of Paradigm and of certain factors to consider in connection with those businesses, see the sections entitled “Information About PS Spine and Paradigm” and “Paradigm’s Business”. See also the section entitled “Summary—Per Share Market Price Data and Dividend Information” for additional information on the market value of shares of RTI common stock and units of Paradigm.
There has been no public market for membership units in PS Spine and the lack of a public market makes it difficult to determine the fair market value of Paradigm.
The outstanding membership units in PS Spine are privately held and not traded on any public market. The lack of a public market may make it more difficult to determine the fair market value of Paradigm than if membership units in PS Spine were traded publicly. The consideration to be paid to PS Spine pursuant to the Master Transaction Agreement was determined based on negotiations between the parties and, even though the RTI board received a fairness opinion in connection with its analysis of the Transaction, such consideration may not be indicative of the price at which PS Spine membership units may have traded if they were traded on a public market.
A significant portion of the consideration to be paid to PS Spine pursuant to the Master Transaction Agreement will be used to pay amounts owed to the lenders under Paradigm’s existing senior secured credit agreement and their affiliates.
Hayfin Services LLP is the administrative agent, and certain of its affiliates are the lenders, under Paradigm’s existing senior secured credit agreement. PS Spine and Paradigm have entered into the Settlement Agreement with the Hayfin Entities, pursuant to which:
| • | all of the Cash Consideration Amount not used to pay transaction expenses of PS Spine or Paradigm (the “Closing Debt Cash Amount”); |
| • | 5,364,807 shares of Holdco common stock to be issued as part of the Stock Consideration Amount; |
| • | a number of shares of Holdco common stock, to be issued as part of the Stock Consideration Amount, equal to (a)(i) the amount of any loans made by the lenders under Paradigm’s existing senior secured credit agreement to Paradigm or any of its subsidiaries following the date of the Master Transaction Agreement and prior to the closing, plus accrued and unpaid interest thereon and any additional payments due to such lenders as a result thereof, minus (ii) the amount by which the Closing Debt Cash Amount, less anyout-of-pocket fees and expenses of such lenders, exceeds $95.0 million, divided by (b) $4.66; and |
| • | $10.0 million of the Earnout Consideration (which can take the form of cash, stock or a combination of both) that would otherwise be payable to PS Spine if Giants Revenue (as defined below) is equal to or exceeds $65.0 million in any LTM Period (as defined below) ending on or prior to December 31, 2020, |
will be used to pay amounts outstanding under Paradigm’s existing senior secured credit agreement and other amounts due to the lenders thereunder and their affiliates. Payments of the above amounts to the lenders under Paradigm’s existing senior secured credit agreement and their affiliates will decrease the amount of consideration paid directly to PS Spine pursuant to the Master Transaction Agreement, which in turn will decrease the amount of consideration available for PS Spine to distribute to its unitholders.
A significant portion of the consideration to be paid to PS Spine pursuant to the Master Transaction Agreement is contingent on the occurrence of certain events in the future.
The payment of the Earnout Consideration, including additional shares of Holdco common stock, is contingent upon the occurrence of certain events in the future, subject to the terms and conditions set forth in the Master Transaction Agreement. For a more complete discussion of the Earnout Consideration, see the section entitled “The Master Transaction Agreement—Purchase Price”, beginning on page 126. There can be no assurance that any of the conditions to the payment of all or a portion of the Earnout Consideration will be satisfied in a timely manner or at all, or that an effect, event, development or change will not transpire that could delay or prevent these conditions from being satisfied.
46
In addition, after the closing, the combined company will have the right to setoff certain indemnification claims against the Earnout Consideration, as further described in the section entitled “The Master Transaction Agreement—Indemnification”, beginning on page 141.
Accordingly, there can be no guarantee with respect to whether or when any of the Earnout Consideration will be paid, if at all. As a result, the exact amounts of cash and shares of Holdco common stock that PS Spine will be entitled to receive pursuant to the Master Transaction Agreement will not be determined until subsequent to the closing and could vary significantly.
Expenses that may be incurred by PS Spine will reduce the amounts otherwise available for distribution to its unitholders.
PS Spine intends to distribute the consideration that it receives pursuant to the Master Transaction Agreement (net of transaction and other expenses and amounts due to its senior lenders and their affiliates) to its unitholders in accordance with PS Spine’s organizational documents as soon as reasonably practicable following its receipt thereof. Expenses that may be incurred by PS Spine, whether in connection with the Master Transaction Agreement, in connection with the distribution of consideration PS Spine receives pursuant to the Master Transaction Agreement or otherwise, will reduce the amounts otherwise available for distribution to PS Spine unitholders, and accordingly may reduce the payments that PS Spine unitholders would otherwise receive in connection with the Transaction.
The unaudited pro forma condensed combined financial information is presented for illustrative purposes only and may not be an indication of the combined company’s financial condition or results of operations following the completion of the Transaction.
The unaudited pro forma condensed combined financial information contained elsewhere in this joint proxy and consent solicitation statement/prospectus is presented for illustrative purposes only and may not be an indication of the combined company’s financial condition or results of operations following the completion of the Transaction for several reasons. The unaudited pro forma condensed combined financial information has been derived from the historical financial statements of RTI and Paradigm and adjustments and assumptions have been made regarding the combined company after giving effect to the Transaction. The information upon which these adjustments and assumptions have been made is preliminary, and these kinds of adjustments and assumptions are difficult to make with accuracy. Moreover, the unaudited pro forma condensed combined financial information does not reflect all costs that are expected to be incurred by the combined company in connection with the Transaction. For example, the impact of any incremental costs incurred in integrating RTI and Paradigm are not reflected in the unaudited pro forma condensed combined financial information. As a result, the actual financial condition and results of operations of the combined company following the completion of the Transaction may not be consistent with, or evident from, the unaudited pro forma condensed combined financial information. The assumptions used in preparing the unaudited pro forma condensed combined financial information may not prove to be accurate, and other factors may affect the combined company’s financial condition or results of operations following the Transaction. Any decline or potential decline in the combined company’s financial condition or results of operations may cause significant variations in the market price of the combined company’s stock.
The unaudited prospective financial information included in this joint proxy and consent solicitation statement/prospectus may not prove to be accurate and is not necessarily indicative of current values or future performance.
The unaudited prospective financial information of RTI and Paradigm contained in this joint proxy and consent solicitation statement/prospectus involves risks, uncertainties and assumptions and is not a guarantee of future performance. The assumptions used in preparing the unaudited prospective financial information may not prove to be accurate, and other factors may affect the combined company’s financial condition or results of operations following the Transaction. Neither RTI nor Paradigm can provide any assurance that the results indicated in RTI’s or Paradigm’s unaudited prospective financial information will be realized or that RTI’s or Paradigm’s future financial results will not materially vary from the unaudited prospective financial information. See “Certain Unaudited Prospective Financial Information.”
47
If the issuance of Holdco common stock as Earnout Consideration is at a price less than the applicable conversion price of Holdco preferred stock (which is expected to be $4.39 per share immediately following the closing), there will be a downward adjustment to the conversion price of Holdco preferred stock, which would result in dilution to Holdco’s common stockholders.
The conversion price of Holdco preferred stock will be subject to adjustment in certain events. The issuance of Initial Earnout Shares or Final Earnout Shares at a price below the then-applicable conversion price of the Holdco preferred stock (which is expected to be $4.39 per share immediately following the closing) would result in a downward adjustment of the conversion price of the Holdco preferred stock. This potential reduction in conversion price would increase the number of shares of Holdco common stock that would be issued upon conversion of the Holdco preferred stock and would result in dilution to the other holders of Holdco common stock. Such dilution could adversely affect the trading price of Holdco common stock. See the section entitled “Description of Holdco Capital Stock—Preferred Stock”, beginning on page 168, for a discussion of the terms of a potential adjustment of the conversion price of the Holdco preferred stock.
Third parties may terminate or alter existing contracts or relationships with RTI or Paradigm.
Each of RTI and Paradigm has contracts with customers, suppliers, third-party distributors, vendors, landlords, licensors and other business partners which may require RTI or Paradigm, as applicable, to obtain consent from these other parties in connection with the Transaction. If these consents cannot be obtained, the combined company may suffer a loss of potential future revenue and may lose rights that are material to its business. In addition, third parties with which RTI or Paradigm currently have relationships may terminate or otherwise reduce the scope of their relationships with either or both parties in anticipation of the Transaction, or with the combined company following the Transaction. Any such disruptions could limit the combined company’s ability to achieve the anticipated benefits of the Transaction. The adverse effect of such disruptions could also be exacerbated by a delay in the anticipated completion of the Transaction or the termination of the Master Transaction Agreement.
RTI is, and will continue to be, subject to the risks described in Part I, Item 1A in RTI’s Annual Report on Form10-K for the year ended December 31, 2017, as filed with the SEC and incorporated by reference into this joint proxy and consent solicitation statement/prospectus. See the section entitled “Where You Can Find More Information”, beginning on page 187.
Paradigm is dependent on its key personnel, including senior management.
Paradigm is highly dependent upon its senior management. The loss of services of members of Paradigm’s senior management could have a material adverse effect on Paradigm’s business, prospects, financial condition and results of operations. The employment of certain members of Paradigm’s senior management is “at will,” and any member of Paradigm’s senior management can terminate his or her employment with Paradigm at any time. In connection with the Transaction, it is expected that at closing two members of Paradigm’s senior management, Marc R. Viscogliosi, Paradigm’s Chairman and Chief Executive Officer, and Francis Magee, Paradigm’s Chief Technology Officer, will voluntarily terminate their employment with Paradigm, and commence providing services under their respective consulting agreements with RTI, which become effective at the closing. For more information on the consulting agreements, see the sections entitled “The Transaction—Consulting Agreement Between RTI and Marc. R. Viscogliosi” and “The Transaction—Consulting Agreement Between RTI and Francis Magee”, beginning on pages 121 and 121, respectively. If any member of Paradigm’s senior management should die, become disabled or otherwise cease to participate in Paradigm’s business, its ability to manage its operations could be severely impaired. There can be no assurance that any of these individuals will remain in the employ of Paradigm, or otherwise continue to be able to carry on their current duties or otherwise provide services to Paradigm.
48
In addition, Paradigm may need to hire additional qualified management, sales, scientific, regulatory, quality assurance and control and administrative personnel as it continues to expand its business activities. It may not be able to attract and retain qualified personnel on acceptable terms given the competition for such personnel among companies that operate in its markets. The trend in the orthopedics industry of requiring sales and other personnel to enter intonon-competition agreements prior to starting employment exacerbates this problem, since personnel who have made such a commitment to their current employers are more difficult to recruit. If Paradigm fails to identify, attract, retain and motivate these highly skilled personnel, or if it loses current skilled personnel, its business, prospects, financial condition and results of operations could be adversely affected.
Paradigm has a history of net losses and may never become profitable.
Paradigm has a history of incurring losses and has earned no profits as of the date of this joint proxy and consent solicitation statement/prospectus. Because of the risks and uncertainties associated with researching, developing and commercializing medical device products, it is not possible to predict with any reasonable degree of certainty the extent of any future losses or when Paradigm will become profitable, if at all. The report of Paradigm’s independent auditors on its financial statements as of December 31, 2017 and 2016 and for each of the two years in the period ended December 31, 2017, includes an explanatory paragraph relating to Paradigm’s ability to continue as a going concern. As of December 31, 2017, Paradigm had an accumulated deficit of approximately $214.4 million, primarily because of costs relating to the development, regulatory approvals, clinical studies and commercialization of its products. Paradigm expects its operating expenses relating to sales and marketing activities and product development will continue during the foreseeable future. To achieve profitability, Paradigm would need to generate substantially more revenue than it has in prior years. Paradigm’s ability to achieve significant revenue growth will depend, in large part, on its ability to achieve widespread market acceptance and third-party reimbursement for coflex® and successfully expand its business in the United States. Paradigm may never achieve such substantial market acceptance or realize significant revenue from the sale of its products, and therefore may never reach profitability.
The markets for Paradigm’s products are highly competitive. If competitors are better able to develop and market products that are more effective, or gain greater acceptance in the marketplace than products Paradigm markets, its commercial opportunities may be reduced or eliminated.
The orthopedic device industry, and the medical device industry, in general, are constantly evolving, and scientific advances are expected to continue at a rapid pace. This results in intense competition among companies operating in Paradigm’s industry. Other, larger companies may have, or may be developing, products that compete with Paradigm products and may significantly limit the market acceptance of its products or render them obsolete. Paradigm’s technical and/or business competitors include universities and nonprofit research institutions and foundations and large and small medical device companies, such as Smith & Nephew, plc, Wright Medical Group, Inc., Integra LifeSciences Holdings Corporation, Tornier, Inc., Ascension Orthopedics, Inc., Zimmer Holdings, Inc., Depuy Synthes, Stryker Corporation, Exactech, Inc. and Medtronic, Inc., among others. Most of these competitors have significantly greater research and development capabilities than Paradigm has, as well as substantial marketing, financial and managerial resources. These organizations also compete with Paradigm for acquisitions, joint ventures and other collaborations, as well as with respect to attracting qualified personnel.
The primary competitive factors facing Paradigm include price, quality, innovative design and technical capability, breadth of product line, scale of operations, service and distribution capabilities. Paradigm’s current and future competitors may have greater resources, more widely accepted and innovative products, less invasive therapies, greater technical capabilities and stronger name recognition than Paradigm. Paradigm’s ability to compete is affected by its ability to:
| • | develop or acquire new products and innovative technologies; |
| • | obtain regulatory clearance and compliance for products; |
| • | manufacture and sell products cost-effectively; |
| • | meet all relevant quality standards for products in their particular markets; |
49
| • | respond to competitive pressures specific to each geographic and product market; |
| • | protect the proprietary technology of products and avoid infringement of the proprietary rights of others; |
| • | market products; |
| • | attract and retain skilled employees, including sales representatives; and |
| • | maintain and establish distribution relationships. |
Competitors could develop products that are more effective, achieve more favorable reimbursement status from third-party payors, cost less or are ready for commercial introduction before Paradigm’s products. If competitors are better able to develop and patent products earlier than Paradigm can, or develop more effective and/or less expensive products that render Paradigm’s products obsolete ornon-competitive, its business will be harmed and commercial opportunities will be reduced or eliminated.
Market acceptance of proposed and existing products is vital to Paradigm’s future success.
The commercial success of Paradigm’s products is dependent upon the acceptance of its products. Its products may not gain and maintain a sufficient degree of market acceptance among surgeons, other healthcare providers and/or patients, or acceptance by third-party payors, such as private health insurance companies or federal healthcare programs, such as Medicare and Medicaid. The medical indications that Paradigm’s products treat can also be treated by other healthcare items and services, including other medical devices. The medical community widely accepts many alternative treatments for the indications that Paradigm’s products target, and certain of these other treatments have a long history of use. We cannot be certain that Paradigm’s products and the procedures in which they are used will be able to replace those established treatments or that either surgeons or the medical community in general, will accept and utilize Paradigm’s products or any other medical products that Paradigm may market.
Market acceptance will depend upon numerous factors, many of which are not under Paradigm’s control, including:
| • | the safety and efficacy of Paradigm’s products, as demonstrated in clinical trials and the acceptance of this by the medical community, patients, and payors; |
| • | the timing of market introduction of products in Paradigm’s developmental pipeline as well as competitors’ products; |
| • | favorable regulatory approval of medical indications and product labeling claims; |
| • | the ease of use of Paradigm’s implants and any related instrumentation that accompanies those products; |
| • | the availability, safety, efficacy and ease of use of alternative products or treatments; |
| • | the potential and perceived advantages of Paradigm’s products over alternative treatments; |
| • | the prevalence and severity of adverse side effects of Paradigm’s products and related procedures; |
| • | Paradigm’s ability to effectively market and sell its products; |
| • | Paradigm’s ability to educate and train surgeons and other healthcare providers on the advantages of its products; |
| • | the price of Paradigm’s products relative to alternative technologies and therapies; and |
| • | the availability of third-party coverage and reimbursement of Paradigm’s products. |
In addition, Paradigm’s future success depends, in part, on its ability to develop and acquire new products and technologies. Even if Paradigm determines that a product candidate has medical benefits, the cost of commercializing that product candidate could be too high to justify development.
50
Sales of Paradigm’s products are largely dependent upon third-party coverage and reimbursement and could be harmed by healthcare cost containment initiatives.
In the United States, hospitals, ambulatory surgery centers and other healthcare providers and facilities that purchase medical devices generally rely on third-party payors, such as Medicare, Medicaid, private health insurance plans and health maintenance organizations, to cover and reimburse all or a portion of the cost of the devices, as well as any related healthcare services. Coverage and reimbursement by third-party payors may depend on a number of factors, including the payor’s determination that the use of any of Paradigm’s products is clinically useful and cost-effective, medically necessary and not experimental or investigational. Payors continue to review their coverage policies carefully for existing and new therapies and can, without notice, deny coverage for treatments that include the use of Paradigm’s products. In addition, third-party payors may limit reimbursement amounts paid for medical products and services. Since coverage and reimbursement for healthcare items and services are determined on apayor-by-payor basis, ensuring coverage and reimbursement of Paradigm’s products can be a time-consuming and costly process. This can require Paradigm to provide supporting scientific, clinical and cost-effectiveness data for the use of its products to each payor separately, significantly increasing the cost of securing coverage and reimbursement.
In addition, some healthcare providers in the United States have adopted or are considering a managed care system in which the providers contract with third-party payors to provide comprehensive health care for a fixed cost per person. Third-party payors may also attempt to control costs by authorizing fewer elective surgical procedures, including spinal surgeries, or by requiring the use of the least expensive implant available. Eligibility of a procedure for reimbursement does not guarantee that reimbursement levels will be adequate to justify use of Paradigm’s products.
Moreover, if adequate levels of reimbursement from third-party payors outside of the United States are not obtained, international sales of Paradigm’s products may decline. Outside of the United States, reimbursement systems vary significantly by country. Many foreign markets have government-managed healthcare systems that govern reimbursement for medical devices and procedures. Canada, and some European Union and Asian countries, in particular France, Japan, Taiwan and Korea, have tightened reimbursement rates. Additionally, in the European Union, reference pricing systems and/or arbitrage betweenlow-priced and high-priced European Union countries may lead to cost containment and further reduced prices. Publication of discounts by European Union third-party payors or other European Union reimbursement authorities may lead to further pressure on prices or reimbursement levels within the European Union country of publication and other European Union countries. The inability to promptly obtain coverage and profitable payment rates from both European Union government-funded and European Union private payors for Paradigm’s products could have a material adverse effect on Paradigm’s operating results, Paradigm’s ability to raise capital needed to commercialize products and Paradigm’s overall financial condition.
Third-party reimbursement coverage may not be available or adequate for any products that Paradigm owns, develops, markets or acquires. Surgeons, hospitals and other healthcare providers may not purchase Paradigm’s products if they do not believe they will receive sufficient reimbursement to cover their cost of using the products from third-party payors. Failure to consistently obtain and maintain sufficient coverage and reimbursement could materially adversely affect Paradigm’s financial performance. In addition, changes in the policies of third-party payors could materially adversely affect Paradigm’s financial performance.
Healthcare policy changes, including the 2010 Health Care Reform Laws, may have a material adverse effect on Paradigm.
In response to perceived increases in healthcare costs in recent years, there have been and continue to be proposals by the federal government, state governments, regulators and third-party payors to control these costs and, more generally, to reform the U.S. healthcare system. Certain of these proposals could limit the prices Paradigm is able to charge for its products or the amounts of reimbursement available for its products, and could limit the acceptance and availability of its products. Moreover, as discussed below, the 2010 federal legislation imposed significant new taxes on medical device makers such as Paradigm, which could have a material adverse effect on Paradigm’s financial position and results of operations.
51
In 2010, the Patient Protection and Affordable Care Act (Public Law111-148) (“PPACA”), as amended by the Health Care and Education Reconciliation Act of 2010 (Public Law111-152) (collectively, the “2010 Health Care Reform Laws”) imposed, among other things, significant new taxes on medical device makers. Although the imposition of the medical device tax has been postponed until January 1, 2020, there is no assurance that further postponements will occur. These taxes would result in a significant increase in the tax burden on the medical device industry, which could have a material adverse effect on Paradigm’s results of operations and cash flows. Other elements of the 2010 Health Care Reform Laws, such as shared savings pilots and comparative effectiveness research, may result in cost containment measures, and reforms that could meaningfully change the way healthcare is developed and delivered, and may materially impact numerous aspects of Paradigm’s business. Although it remains impossible to predict the extent of the regulation and the full impact of the 2010 Health Care Reform Laws, any changes that lower reimbursement for Paradigm’s products or reduce medical procedure volumes could adversely affect its business and results of operations.
The 2010 Health Care Reform Laws also included theso-called federal Physician Payments Sunshine Act, which requires drug and device manufacturers, among others, to report any payments or other transfers of value they make to U.S.-licensed physicians and U.S. teaching hospitals (referred to as “Covered Recipients”), including payments in the form of gifts, honoraria, consulting fees, travel, and ownership or investment interests held by a Covered Recipient in the applicable manufacturer. The penalties for failure to report range from $10,000 to $100,000 per violation. Further, pursuant to this law, the Secretary of the U.S. Department of Health and Human Services (“HHS”) created a public website, known as Open Payments, to post the payment or ownership or investment interest information in a clear and understandable manner. Certain payments Paradigm makes to Covered Recipients are subject to reporting under the Physician Payments Sunshine Act and disclosure on the Open Payments website.
Increased scrutiny, particularly at the U.S. Food and Drug Administration (“FDA”), and new regulation, may delay Paradigm’s introduction of new products and adversely affect Paradigm’s business.
The FDA has increased significantly the scrutiny applied to products that require premarket notification under Section 510(k) of the Federal Food, Drug, and Cosmetic Act (“510(k)”) submissions, and it may also focus more scrutiny on other regulation within its purview. The FDA and the U.S. Congress are influenced by high profile events, injuries and cases that generate publicity and public attention, and new legislation is often generated as a result of those events. Paradigm may be required to devote substantial time and financial resources to further develop and implement policies, systems and processes to comply with enhanced legal and regulatory requirements, which may impact its business. Paradigm anticipates that governmental authorities will continue to scrutinize the medical device industry closely, and that additional regulation may increase compliance and legal costs and expose Paradigm to litigation, each of which could have an adverse effect on Paradigm’s business. In addition, there can be no assurance that new products Paradigm introduces will not be delayed by the current level of scrutiny applied to applications at the FDA or that new laws and regulations will not be adopted that negatively impact the cost of production and marketing of Paradigm’s existing products, each of which could adversely affect Paradigm’s business.
The spine and orthopedics industries are subject to extensive and complex healthcare regulation. Any determination that Paradigm has violated federal or state laws applicable to it that regulate healthcare would have a material adverse effect on its business, prospects and financial condition.
Paradigm and its products are subject to regulation by HHS, including the Centers for Medicare & Medicaid Services (“CMS”), the HHS Office of Inspector General (“OIG”), as well as comparable state andnon-U.S. agencies responsible for coverage, reimbursement and fraud and abuse of healthcare companies and healthcare items and services. U.S. federal government healthcare laws apply when Paradigm submits a claim on behalf of a U.S. federal healthcare program beneficiary, or when a customer submits a claim for an item or service that is reimbursed (in whole or in part) by a U.S. federal healthcare program, such as Medicare or Medicaid. The principal U.S. federal law implicated is the federal False Claims Act, which imposes liability on any person, who among other things, knowingly presents, or causes to be presented, a false or fraudulent claim to the federal government for payment or approval. Although manufacturers like Paradigm do not typically submit claims to the government, the government andqui tamrelators pursue False Claims Act claims under the theory that the manufacturer’s conduct causes providers to submit false or fraudulent claims for reimbursement to the federal healthcare programs.
52
The federal Anti-Kickback Statute is a criminal law that prohibits the solicitation, payment, receipt or offering of any direct or indirect remuneration in return for, or as an inducement for, referrals of items or services that may be reimbursable (in whole or in part) under Medicare, Medicaid or other federal healthcare programs. Violation of the federal Anti-Kickback Statute can lead to the imposition of civil and criminal penalties upon all parties involved, including substantial monetary penalties and exclusion from participation in Medicare, Medicaid and other federally funded healthcare programs. A violation of the federal Anti-Kickback Statute can also form the basis for a claim of fraud under the federal False Claims Act. The scope of prohibited conduct under the federal Anti-Kickback Statute is broad and it extends to economic arrangements involving hospitals, physicians and their business partners, among others. Applicable state laws may also prohibit the payment of remuneration in return for referrals and may not be limited to services reimbursed by a federal healthcare program. Violation of these laws may not only result in the imposition of monetary penalties on physicians or othernon-physicians involved in the arrangement, and suspension or revocation of a physician’s license, but also criminal charges against Paradigm. There can be no assurance that the regulatory authorities or other parties will not assert that Paradigm is in violation of these laws, and if such an action is taken or such a determination is made, it could materially adversely affect Paradigm’s business, prospects and financial condition.
The federal physician self-referral law, known as the Stark Law, imposes restrictions on physicians’ referrals for certain designated health services reimbursable by Medicare or Medicaid to entities with which any such physician (or an immediate family member) has a financial relationship, whether through an ownership, debt or compensation arrangement, and it prohibits an entity from billing Medicare or Medicaid, or any other federally funded health benefit program, for services rendered pursuant to a prohibited referral. Violations can result in denial and recoupment of payments for services, imposition of substantial civil monetary penalties and exclusion from Medicare and Medicaid. A violation of the federal physician-self-referral law can also form the basis for a claim of fraud under the federal False Claims Act. In addition, several states have adopted physician self-referral laws, some of which are not limited to Medicare or Medicaid reimbursed services or to certain designated health services. Violation of any such laws may result in the imposition of penalties on the physicians pursuant to applicable regulations, including fines and suspension or revocation of the physician’s license. Any such penalties on a physician due to his or her relationship with Paradigm could result in adverse media reports and public perception of Paradigm, which could result in a material adverse effect on its business, prospects and financial condition.
Most states have laws and regulations to regulate the provision of healthcare services and some states prohibit companies, such as Paradigm, from practicing medicine or otherwise providing healthcare items and services without a medical license and from exercising control over the medical judgments or decisions of physicians and licensed providers. Violation of these requirements could result in civil and criminal sanctions against Paradigm. There has also been recent increased government focus, including from the OIG, on the financial interests of doctors in medical device companies (often referred to as physician-owned distributors) and conflicts of interest that may develop as a result of such financial interests. For example, OIG issued a report in May 2018 on the overlap between physician-owned hospitals and physician-owned distributors of products such as spinal devices, finding a lack of transparency in the ownership structures. Any proposed or actual legislation addressing this issue may result in a more rigorous clinical trial review process and delay Paradigm’s requests for 510(k) clearance or futurepre-market approval (“PMA”) of new products or new intended uses of existing products. This could, in turn, materially adversely affect Paradigm’s ability to bring any applicable products to market.
If Paradigm fails to comply with FDA regulations, its business could suffer.
The manufacture and marketing of spine medical devices are subject to extensive regulation by the FDA and foreign and state regulatory authorities. In the United States, orthopedic medical device companies must comply with laws and regulations promulgated by the FDA. These laws and regulations require various authorizations prior to a product being marketed in the United States. Manufacturing facilities and practices are also subject to FDA regulations. The FDA regulates the clinical development, manufacture, labeling, packaging, sale, distribution, advertising, promotion, import and export of medical devices in the United States. Paradigm’s failure to comply with regulatory requirements, including any future changes to those requirements, could have a material adverse effect on its business, prospects, financial condition and results of operations.
53
Even after clearance or approval of a product, Paradigm is subject to continuing regulation by the FDA, including the requirements of registering its facilities and listing its products with the FDA. Paradigm is subject to medical device reporting and monitoring regulations. These regulations require Paradigm to report to the FDA if any of its products may have caused or contributed to an adverse event such as death or serious injury, or have malfunctioned, and such product or a similar product that Paradigm markets would likely cause or contribute to a death or serious injury if the malfunction were to recur. Unless an exemption applies, Paradigm must report corrections and removals to the FDA where the correction or removal was initiated to reduce a risk to health posed by the device or to remedy a violation of the Federal Food, Drug and Cosmetic Act. The FDA also requires that Paradigm maintain records of corrections or removals, regardless of whether such corrections and removals are required to be reported to the FDA. In addition, the FDA closely regulates promotion and advertising, and Paradigm’s promotional and advertising activities could come under scrutiny by the FDA.
The FDA also requires that Paradigm, or its contract manufacturers and suppliers, manufacture its products in accordance with its Quality System Regulation (“QSR”). The QSR covers the methods and documentation of the design, testing, control, manufacturing, labeling, quality assurance, packaging, storage and shipping of Paradigm’s products. Paradigm’s failure to maintain compliance with the QSR requirements could result in the shut-down of, or restrictions on, its manufacturing operations and the recall or seizure of its products, which would have a material adverse effect on its business. In the event that one of Paradigm’s suppliers fails to maintain compliance with its quality requirements, it may have to qualify a new supplier and could experience manufacturing delays as a result.
The FDA has broad enforcement powers. If Paradigm violates applicable regulatory requirements, the FDA may take regulatory actions or bring enforcement actions against it, which may include any of the following:
| • | issuance of Form 483s (Inspection Observations), warning letters, fines, injunctions, consent decrees and civil penalties; |
| • | mandatory repair, replacement, recall, seizure or import bans of products, which may include refunds by Paradigm of the purchase price of its products; |
| • | operating restrictions, partial suspension or total shutdown of production; |
| • | disqualification of an investigator fornon-compliance with FDA regulations; |
| • | refusing or delaying Paradigm’s requests for 510(k) clearance or PMA of new products or new intended uses of existing products; |
| • | withdrawing 510(k) clearances, PMA approvals or humanitarian device exception approvals that have already been granted; or |
| • | criminal prosecution. |
If any of these events were to occur, they could have a material adverse effect on Paradigm’s business, prospects, financial condition and results of operations.
Failure to maintain or obtain regulatory approval in foreign jurisdictions will prevent Paradigm from marketing products abroad.
Paradigm markets products in foreign jurisdictions. In order to market its products in the European Union and many other foreign jurisdictions, it must obtain separate regulatory approvals from the FDA approvals referred to above. The approval procedure varies among foreign jurisdictions and can involve additional testing and cost, and the time and effort required to obtain approval may differ substantially from that required to obtain FDA approval. The foreign regulatory approval process may include all of the risks associated with obtaining FDA approval, in addition to other risks. Paradigm may not obtain foreign regulatory approvals on a timely basis, if at all. Importantly, approval by the FDA does not ensure approval by regulatory authorities in other foreign jurisdictions, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign jurisdictions or by the FDA.
54
Paradigm may not be able to file for regulatory approvals and may not receive necessary approvals to commercialize its products in any foreign jurisdictions. Failure to obtain regulatory approvals would have a material adverse effect on Paradigm’s business, prospects, financial condition and results of operations.
To market in the European Union, a medical device manufacturer must obtain and maintain a CE Mark for its products. A CE Mark indicates that a manufacturer’s medical devices comply with the applicable EU regulations (e.g., the EU Medical Devices Directive (93/43/EEC) and related guidance) and enables the marketing of the medical devices in the European Union. In order to obtain and to maintain a CE Mark, a medical device manufacturer must: (1) implement a quality management system; customarily manufacturers use International Standards Organization (“ISO”) 13485 to meet the relevant requirements; (2) prepare a CE Mark technical file or a design dossier; (3) prepare a Clinical Evaluation Report; and (4) obtain a CE Mark and ISO certification from a recognized European Union notified body, following an audit (by the recognized European Union notified body) of the manufacturer’s quality management system and technical file or design dossier; (Class I andnon-sterile medical devices as well as those medical devices that have no measuring function do not require notified body audits). Lastly, medical device manufacturers are required to prepare a declaration of conformity which states that their product complies with the requirements of the applicable EU regulations. Certain individual countries within Europe require further approval by their national regulatory authorities. In addition, regulatory authorities periodically inspect manufacturing facilities to make sure that quality, safety and efficacy standards required for CE marking and certification continue to be met.
Paradigm’s wholly owned German subsidiary, Paradigm Spine GmbH, manages all aspects of its international operations (research and development, sales and marketing, manufacturing and purchasing, logistics, quality assurance, and international regulatory approvals). Paradigm distributes its products worldwide and has distribution contracts with third-party distributors in 48 countries. The main task of Paradigm’s regulatory affairs department is to support distributors with the registration of its products in their countries. Failure to obtain the necessary regulatory approval needed to register products for distribution internationally would materially affect Paradigm’s ability to market and commercialize its products foreign jurisdictions.
Even if Paradigm obtains the necessary international regulatory approvals, it must continue to comply with regulatory requirements. For example, submitting a vigilance report is required for devices marketed in the European Union which carry a CE Mark. Vigilance reports refer both to incident reports and field safety corrective action reports. Incidents which need to be reported under European Union regulations and guidance are those which result in death, serious injury, or may lead to death or serious deterioration in state of health if it were to recur. A field safety corrective action report must be distributed to regulatory authorities in the European Union countries where the medical device is being marketed as well as the regulatory authorities where the manufacturer is located if a manufacturer takes an action to reduce the risk of death or serious deterioration in health, such as a recall.
Paradigm relies on third parties to supply and manufacture all of its products. If these third parties do not perform as expected or if Paradigm’s agreements with them are terminated, its business, prospects, financial condition and results of operations would be materially adversely affected.
Paradigm’s reliance on contract manufacturers and suppliers exposes it to risks, including the following:
| • | Paradigm relies on suppliers and manufacturers to provide it with the needed products or components in a timely fashion and of an acceptable quality. An uncorrected defect or supplier’s variation in a component could harm its or its third party manufacturers’ ability to manufacture, and Paradigm’s ability to sell, products, and may subject it to product liability claims. |
| • | The facilities of Paradigm’s worldwide third-party manufacturers must satisfy production and quality standards set by applicable regulatory authorities. Regulatory authorities periodically inspect manufacturing facilities to determine compliance with these standards. If Paradigm’s third-party manufacturers fail to satisfy these requirements, their respective facilities could be shut down. |
| • | These manufacturing operations could also be disrupted or delayed by fire, earthquake or other natural disaster, a work stoppage or other labor-related disruption, failure in supply or other logistical channels, |
55
electrical outages or other reasons. If there was any such disruption to any of these manufacturing facilities, Paradigm’s third-party manufacturers would potentially be unable to manufacture its products. |
| • | A third-party manufacturer or supplier could decide to terminate Paradigm’s manufacturing or supply arrangement, including due to a disagreement between Paradigm and that third-party manufacturer, if the third-party manufacturer is acquired by one of Paradigm’s competitors and determines not to further manufacture its products or if Paradigm fails to comply with its obligations under its applicable arrangements. |
| • | If any third-party manufacturer makes improvements in the manufacturing process for Paradigm’s products, Paradigm may not own, or may have to share, the intellectual property rights to the innovation. |
To the extent Paradigm is able to identify alternative suppliers, qualifying suppliers is a lengthy process. There are a limited number of manufacturers and suppliers that are ISO and FDA approved. In addition, FDA regulations may require additional testing of any raw materials or components from new suppliers prior to Paradigm’s use of these materials or components, which testing could delay or prevent the supply of raw materials and components. Moreover, a new manufacturer would have to be educated in, or develop substantially equivalent processes for, production of Paradigm’s products, which could take a significant period of time.
Each of these risks could delay the development or commercialization of Paradigm’s products or result in higher costs or deprive Paradigm of its potential product revenues. Furthermore, delays or interruptions in the manufacturing process could limit or curtail Paradigm’s ability to meet demand for products and/or make commercial sales, unless and until the manufacturing capability at the facilities are restored andre-qualified or alternative manufacturing facilities are developed or broughton-line and “scaled up.” Any such delay or interruption could have a material adverse effect on Paradigm’s business, prospects, financial condition and results of operations.
Paradigm may be subject to product liability claims and its insurance may not be sufficient to cover these claims, or it may be required to recall its products.
Paradigm manufactures and markets medical devices that are used to treat patients in surgical procedures. As a result, it faces an inherent risk of product liability claims. The medical device industry has been historically litigious. Since Paradigm’s products are implanted in the human body, manufacturing errors, design defects or packaging defects could result in injury or death to the patient. This could result in a recall of one or more of Paradigm’s products and substantial monetary damages. Any product liability claim brought against Paradigm, with or without merit, could result in a diversion of its resources, an increase in product liability insurance premiums and/or an inability to secure coverage in the future. Paradigm might also have to pay any amount awarded by a court in excess of its policy limits. In addition, any recall of products, whether initiated by Paradigm or by a regulatory agency, may result in adverse publicity for Paradigm that could have a material adverse effect on its business, prospects, financial condition and results of operations. Paradigm’s product liability insurance policies have various exclusions. Accordingly, Paradigm may be subject to a product liability claim or recall for which it has no insurance coverage. In such a case, Paradigm may have to pay the entire amount of the award or costs of the recall. Finally, product liability insurance is expensive and may not be available in the future on acceptable terms, or at all.
Each of Paradigm’s products is subject to instructions for use, which surgeons are required to follow. Proper surgical technique in the use of its products can be critical to obtaining a successful outcome for the patient. Failure by a surgeon to follow the instructions for use of one of Paradigm’s products or theoff-label use by the surgeon of one of its products could compromise the integrity of the product and cause the procedure to fail, which could result in product liability claims, adverse publicity and other risks described above.
Studies of the long-term effects of Paradigm’s products in the human body are limited.
Some of Paradigm’s products are newly developed. Consequently, it is not possible to predict the long-term effects of many of those products after their implant in the human body, or whether additional procedures would need
56
to be performed at a later date to either correct product deficiencies or replace the implanted product with a next generation product. It is not possible to accurately predict the longevity or clinical efficacy of Paradigm’s products once implanted, except through long-term monitoring andfollow-up.
Paradigm’s ability to market products may be impaired by the intellectual property rights of third parties.
Paradigm’s success depends in part on its products not infringing on the patents and proprietary rights of other parties. In the United States, patent applications filed in recent years are confidential for eighteen (18) months, while older applications are not published until the patent issues. As a result, there may be patents and patent applications of which Paradigm is unaware, and avoiding patent infringement may be difficult.
The spine industry is characterized by a large number of patents, patent applications and frequent litigation based on allegations of patent infringement. Competitors may own patents or proprietary rights, or have filed patent applications, related to products that are similar to those of Paradigm. Paradigm may not be aware of all of the patents and pending applications potentially adverse to its interests that may have been issued to others. Moreover, since there may be unpublished patent applications that could result in patents with claims relating to Paradigm’s products, it cannot be sure that its current products will not infringe any patents which might be issued or filed in the future. Based on the litigious nature of the industry and the fact that Paradigm may pose a competitive threat to some companies who own or control various patents, it is possible that one or more third parties may assert a patent infringement claim seeking damages or enjoining Paradigm from the manufacture or marketing of one or more of its products. Such a lawsuit may have already been filed against Paradigm without its knowledge. If any future claim of infringement against Paradigm is successful, it may be required to pay substantial damages, cease the infringing activity or obtain the requisite licenses or rights to use the technology, which may not be available to it on acceptable terms, if at all. Even if Paradigm were able to obtain rights to a third party’s intellectual property rights, these rights may benon-exclusive, thereby giving Paradigm’s competitors potential access to the same rights and weakening its market position. Moreover, regardless of the outcome, patent litigation could significantly disrupt Paradigm’s business, divert its management’s attention and consume its financial resources. Paradigm cannot predict if or when any third-party patent holder will file suit for patent infringement.
Paradigm may become involved in lawsuits or proceedings to protect or enforce its intellectual property rights or to defend against infringement claims, which could be expensive and time consuming.
Litigation may be necessary in the future to enforce Paradigm’s intellectual property rights, protect Paradigm’s trade secrets or determine the validity and scope of the proprietary rights of others. Interference proceedings conducted by the U.S. Patent and Trademark Office may be necessary to determine the priority of inventions with respect to patent applications. Litigation or interference proceedings could result in substantial costs and diversion of resources and management attention. In addition, in an infringement proceeding, a court may decide that a patent of Paradigm’s is not valid or is unenforceable or may refuse to stop the other party from using the technology at issue on the grounds that Paradigm’s patents do not cover the technology. An adverse determination of any litigation or defense proceedings could put one or more of Paradigm’s patents at risk of being invalidated or interpreted narrowly and could put its patent applications at risk of not issuing. In addition, Paradigm may be enjoined from marketing one or more of its products if a court finds that such products infringe the intellectual property rights of a third party.
During litigation, Paradigm may not be able to protect the confidentiality of certain of its proprietary rights because of the substantial amount of discovery required in connection with intellectual property litigation. In addition, during the course of litigation, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If investors or customers perceive these results to be negative, it could have a material adverse effect on Paradigm’s business, prospects, financial condition and results of operations.
Product introductions or modifications may be delayed or canceled if Paradigm is unable to obtain FDA approval.
The process of obtaining regulatory approvals to market a medical device, particularly from the FDA, can be costly and time-consuming. If clinical testing is required for product clearance or approval, there can be no assurance that testing would produce favorable results, that clearances or approvals would be granted on a timely basis, if at all,
57
or that approvals would not be subject to conditions that Paradigm would find unacceptable or that could inhibit the sale or marketing of one or more of its products. The regulatory process may delay the marketing of new products for lengthy periods and impose substantial additional costs or it may prevent the introduction of new products altogether. In particular, the FDA permits commercial distribution of a new medical device only after the device has met the established regulatory compliance guidelines. The FDA will clear a medical device for marketing through the 510(k) premarket notification process if it is demonstrated that the new product is substantially equivalent to other legally marketed products. If the device is not eligible for clearance through the 510(k) process, the device sponsor must file a PMA application, which is typically preceded by the submission of an Investigational Device Exemption (“IDE”) application. For devices entering the market through the IDE/PMA pathway, the FDA usually requires extensive data, including, but not limited to, technical, preclinical, clinical, manufacturing, and labeling information to demonstrate to the FDA’s satisfaction the safety and efficacy of the device for the intended use and for the intended patient population.
Both 510(k) and PMA pathways can be expensive and lengthy and entail significant user fees. The 510(k) process is typically faster and less expensive with lower risk. The FDA usually responds to a 510(k) premarket notification within 90 days of submission of the notification, but the response may be a request for additional information or data, sometimes including clinical data. As a practical matter, 510(k) clearance can take six months to one year or more. The PMA process is much more costly, demanding and uncertain than the 510(k) clearance process and may require five to eight years from the initiation of a clinical trial until the FDA issues a decision on whether to grant or deny a PMA approval for a device. It usually takes five years to submit the PMA application to the FDA, which involves one to two years to enroll the required number of subjects in the IDE clinical trial, two additional years to allow all patients to contribute sufficient post-operativefollow-up data, and one year to prepare the PMA. After the PMA application is submitted to the FDA, it generally takes from one to three years, or even longer, from the date of the submission of the PMA application until an approval is obtained, if at all. There can be no assurance that any new products that Paradigm develops, licenses or acquires will be subject to the shorter 510(k) clearance process.
Any modification to a PMA approved device that affects its safety or efficacy requires the submission and approval of a PMA Supplement, or in certain instances, a new PMA. With respect to 510(k)-cleared products, if a manufacturer determines that a modification to anFDA-cleared device could significantly affect its safety or efficacy, or would constitute a major change in its intended use, then the manufacturer must file for a new 510(k) clearance or possibly a PMA. The FDA requires every manufacturer to make the determination in the first instance regarding whether a new approval or clearance is required, but the FDA can review a manufacturer’s decision and may disagree. The FDA may also, on its own initiative, determine that a new clearance or approval is required. If the FDA requires new clearances or approvals for product modifications, Paradigm may be required to recall and to stop marketing its products as modified, which could require it to redesign its products and result in a material adverse effect on its business, prospects, financial condition and results of operations.
Medical devices may be marketed only for indications, or uses, for which they have been approved or cleared. The FDA may not approve or clear indications that are necessary or desirable for successful commercialization. The FDA may refuse requests for 510(k) clearance or PMA approval of new products, new uses of previously approved or cleared products or modifications to existing products. Moreover, any clearances or approvals that Paradigm obtains may not be sufficiently broad, as to the indication for which Paradigm would like to market, to permit successful commercialization. Paradigm’s clearances and approvals can be revoked if safety or efficacy problems develop. Any of these outcomes could materially and adversely affect Paradigm’s competitiveness in the marketplace and consequently have a material adverse effect on its business, prospects, financial condition and results of operations.
Extensive“off-label” use of its products by surgeons may adversely affect Paradigm.
Paradigm is subject to ongoing oversight by the FDA and other regulatory authorities. Medical devices may be marketed only for approved or cleared indications in accordance with their labeling, and Paradigm can only promote its products for indications that have been approved or cleared. While a medical device manufacturer may not promote an“off-label” use of a product, doctors are generally allowed, in the exercise of their professional judgment in the practice of medicine, to use a product in ways not approved by regulatory authorities. However, a pattern of widespread
58
off-label use by doctors could cause regulatory authorities to scrutinize the marketing activities of a medical device manufacturer.
Off-label marketing regulations are subject to varying and evolving interpretations, and regulatory authorities have broad enforcement power. If Paradigm does not comply with these regulations, it could become subject to a wide variety of penalties, both monetary and operational. Threat of such enforcement action, such as through Untitled and Warning Letters, can also have a negative impact on Paradigm or its business. Similarly, Paradigm also may get regulatory enforcement if its marketing is false or misleading.
Paradigm’s international sales are subject to numerous risks.
Paradigm’s international sales are subject to numerous risks. Regulatory requirements, as well as pricing, marketing and distribution structures, vary significantly from country to country in foreign jurisdictions. Additionally, international sales can be adversely affected by, among other things, disruptions caused by political instability, the imposition of government regulatory and other controls, trade restrictions, changes in tariffs, competition and difficulties coordinating foreign distribution. Moreover, Paradigm’s sources of international revenue may be adversely affected by fluctuations in overseas economic conditions, international currency exchange rates, difficulty gaining market acceptance in each country’s medical community and other constraints on its ability to maintain or increase prices. Paradigm may not be able to successfully commercialize its products in a manner sufficient to support its business plan.
Security breaches and other disruptions could compromise Paradigm’s information and expose it to liability, which would cause its business and reputation to suffer.
In the ordinary course of Paradigm’s business, it uses its networks to collect and store sensitive data, including intellectual property, its proprietary business information and that of its customers, suppliers and business partners, personally identifiable information of its customers and employees and data relating to patients who use its products. The secure processing, maintenance and transmission of this information is critical to Paradigm’s operations. Despite Paradigm’s security measures, its information technology and infrastructure may be vulnerable to attacks by hackers or breached due to employee error, malfeasance or other disruptions. Any such breach could compromise Paradigm’s networks and the information stored there could be accessed, publicly disclosed, lost or stolen. Any such access, disclosure or other loss of information could result in legal claims or proceedings, liability under laws that protect the privacy of personal information, regulatory penalties, disrupt Paradigm’s operations, damage its reputation and cause a loss of confidence in its products, all of which could adversely affect its operating margins, revenues and competitive position.
Paradigm’s operating results may fluctuate.
Paradigm’s operating results have fluctuated in the past and can be expected to fluctuate from time to time in the future. Some of the factors that may cause these fluctuations include:
| • | the collectability of accounts receivable from customers and write-offs of intangibles; |
| • | market acceptance of existing products, as well as products in development; |
| • | the timing of regulatory approvals; |
| • | changes in the rates of exchange between the U.S. dollar and currencies of foreign countries in which it does business; |
| • | its ability or the ability of its contract manufacturers to manufacture products efficiently; and |
| • | the timing of research and development expenditures. |
59
If Paradigm needs to raise funding in the future, it may be unable to raise capital when needed or on acceptable terms, or at all.
If Paradigm needs to raise funding in the future, it may be unable to raise capital when needed or on acceptable terms, or at all. If Paradigm does not receive additional capital, it may be required to delay, scale back or stop the marketing of its existing products, the development of its proposed products or acquisition of new technologies or products and/or cease its operations.
PS Spine and Paradigm are in default under their existing senior secured credit facility.
PS Spine and Paradigm are in default under their existing senior secured credit facility, due primarily to the failure to satisfy certain financial covenants. The lenders under PS Spine and Paradigm’s existing senior secured credit facility have approved PS Spine’s adoption of the Master Transaction Agreement and its participation in the transactions contemplated thereby. However, the lenders retain the right to exercise any and all remedies under the senior secured credit facility, including the right to take control of PS Spine’s and Paradigm’s boards of managers and to cause the sale of Paradigm.
Paradigm’s business transactions with Viscogliosi Brothers, LLC and Musculoskeletal Clinical Regulatory Advisors, LLC may create conflicts of interest.
Paradigm has entered into agreements with or has had certain transactions with related parties, including Viscogliosi Brothers, LLC and Musculoskeletal Clinical Regulatory Advisors, LLC (“MCRA”). The principals of Viscogliosi Brothers, LLC, and the indirect 100% owners of MCRA, are Marc R. Viscogliosi, John J. Viscogliosi and Anthony G. Viscogliosi. Anthony G. Viscogliosi is a member of both the PS Spine board and the board of managers of Paradigm (the “Paradigm board”) and Marc R. Viscogliosi is a member of the PS Spine board, Paradigm board and Chairman and Chief Executive Officer of both PS Spine and Paradigm. In addition, RTI and Marc R. Viscogliosi have entered into a consulting agreement, pursuant to which Mr. Viscogliosi has agreed, following the closing, to provide certain consulting services, to be set forth in statements of work to be agreed upon by the parties, to RTI. For a description of the consulting agreement between Marc R. Viscogliosi and RTI, see the section entitled “The Transaction—Consulting Agreement Between RTI and Marc. R. Viscogliosi”, beginning on page 121.
Paradigm has entered into a management services agreement with Viscogliosi Brothers, LLC, pursuant to which Viscogliosi Brothers, LLC provides space rental, administrative, information technology and shared office services to Paradigm and is also a party to a master consulting agreement with MCRA pursuant to which MCRA provides it with clinical and regulatory consulting services in connection with its products. In addition, the Master Transaction Agreement provides that, prior to closing, PS Spine will use its reasonable best efforts to cause Paradigm to negotiate and enter into a written master consulting agreement, including statements of work, with MCRA, in substantially the same form, and for substantially the same terms and conditions, as Paradigm’s current master consulting agreement with MCRA. Each of the above affiliations and related party transactions may create conflicts of interest.
60
This joint proxy and consent solicitation statement/prospectus is being provided to RTI stockholders as of the record date as part of a solicitation of proxies by the RTI board for use at the RTI special meeting and any adjournment or postponement thereof. This joint proxy and consent solicitation statement/prospectus provides RTI stockholders with important information they need to know to be able to vote, or instruct their bank, broker, trust or other nominee to vote, at the RTI special meeting or any adjournment or postponement thereof.
Date, Time, Place and Purpose of the RTI Special Meeting
The RTI special meeting will be held at 520 Lake Cook Road, Suite 315, Deerfield, Illinois 60015, on March 7, 2019, at 10:00 a.m., local time.
The RTI special meeting is being held for the following purposes:
| • | to consider and vote on the Merger Proposal; |
| • | to consider and vote on the Share Issuance Proposal; and |
| • | to consider and vote on the Meeting Adjournment Proposal. |
Recommendation of the RTI Board
After careful consideration, the RTI board, on October 30, 2018, unanimously approved and adopted the Master Transaction Agreement and the transactions contemplated thereby, including the Merger, on the terms and subject to the conditions set forth therein, determined and declared that the terms of the Master Transaction Agreement and the transactions contemplated thereby, including the Merger, are advisable and in the best interests of RTI and its stockholders, and recommended that RTI stockholders approve and adopt the Master Transaction Agreement and the Merger.
For a summary of the factors considered by the RTI board in reaching its decision to approve the Master Transaction Agreement as well as the RTI board’s reasons for approving, and certain risks related to, the Transaction, see “The Transaction—Recommendation of the RTI Board and Its Reasons for the Transaction”, beginning on page 79.
The RTI board unanimously recommends that RTI stockholders vote “FOR” each of the Merger Proposal, the Share Issuance Proposal and the Meeting Adjournment Proposal.
Record Date; Outstanding Shares; Shares Entitled to Vote
Only holders of record of RTI common stock and RTI preferred stock at the close of business on the RTI record date, February 1, 2019, are entitled to notice of and to vote at the RTI special meeting. As of January 15, 2019, there were 62,245,112 shares of RTI common stock outstanding and entitled to vote at the RTI special meeting (including 1,056,028 unvested restricted shares of RTI common stock), held by approximately 291 holders of record. Each holder of RTI common stock is entitled to one vote for each share of RTI common stock owned as of the RTI record date. As of the RTI record date, there were 50,000 shares of RTI preferred stock outstanding and entitled to vote at the RTI special meeting, held by one holder of record. Each holder of RTI preferred stock is entitled to 239 votes for each share of RTI preferred stock owned as of the RTI record date.
A complete list of RTI stockholders entitled to vote at the RTI special meeting will be available for review at the location of the RTI special meeting during the course of the meeting and at the executive offices of RTI during ordinary business hours for a period of ten (10) days before the RTI special meeting.
61
A quorum is necessary to transact business at the RTI special meeting. The holders of a majority of the outstanding shares of RTI common stock and RTI preferred stock (on anas-converted basis) entitled to vote at the RTI special meeting, either present in person or represented by proxy, will constitute a quorum. Both abstentions and brokernon-votes are counted for the purpose of determining the presence of a quorum.
Assuming a quorum is present, the Merger Proposal requires (i) the affirmative vote of the holders of a majority of the outstanding shares of RTI common stock and RTI preferred stock (on a fully converted basis) entitled to vote thereon and (ii) the written consent or affirmative vote of the holders of a majority of the outstanding shares of RTI preferred stock entitled to vote thereon, voting separately as a class. Abstentions and brokernon-votes will have the same effect as a vote “AGAINST” the Merger Proposal.
Assuming a quorum is present, the Share Issuance Proposal requires (i) the affirmative vote of a majority of votes cast on the proposal by RTI common stock and RTI preferred stock (on a fully converted basis) and (ii) the written consent or affirmative vote of the holders of a majority of the outstanding shares of RTI preferred stock, voting separately as a class. Abstentions will have (i) the same effect as a vote “AGAINST” the Share Issuance Proposal for holders of RTI preferred stock, voting separately as a class and (ii) no effect on the Share Issuance Proposal for the holders of RTI common stock and RTI preferred stock (on a fully converted basis). Brokernon-votes will have (i) the same effect as a vote “AGAINST” the Share Issuance Proposal for holders of RTI preferred stock, voting separately as a class and (ii) no effect on the Share Issuance Proposal for the holders of RTI common stock and RTI preferred stock (on a fully converted basis).
Assuming a quorum is present, the Meeting Adjournment Proposal requires the affirmative vote of the holders of a majority of the shares having voting power present in person or represented by proxy at the RTI special meeting. Abstentions will have the same effect as a vote “AGAINST” the Meeting Adjournment Proposal, and brokernon-votes will not affect the outcome of the vote on the Meeting Adjournment Proposal.
Voting by RTI’s Directors and Executive Officers
As of January 15, 2019, RTI’s directors and executive officers beneficially owned and were entitled to vote an aggregate of 2,370,591 shares of RTI common stock, or the right to vote approximately 3.19% of the total RTI voting stock outstanding on January 15, 2019. Camille I. Farhat, who has entered into an RTI Support Agreement (as defined in the section entitled “The Transaction—Support Agreements”, beginning on page 119), as of January 15, 2019, beneficially owned and was entitled to vote an aggregate of 1,110,619 shares of RTI common stock, or the right to vote approximately 1.50% of the total RTI voting stock outstanding on January 15, 2019. Jonathon M. Singer, who has entered into an RTI Support Agreement, as of January 15, 2019, beneficially owned and was entitled to vote an aggregate of 264,844 shares of RTI common stock, or the right to vote approximately 0.36% of the total RTI voting stock outstanding on January 15, 2019. Pursuant to the RTI Support Agreements, Camille I. Farhat and Jonathon M. Singer will be casting their votes in favor of the Merger Proposal and the Share Issuance Proposal. For more information about the support agreements, see the section entitled “The Transaction—Support Agreements”, beginning on page 119.
Voting in Person
RTI shares held in your name as the stockholder of record may be voted by you in person at the RTI special meeting. RTI shares held beneficially in street name may be voted by you in person at the RTI special meeting only if you obtain a legal proxy from the broker, trustee or nominee that holds your shares giving you the right to vote the shares. Even if you plan to attend the RTI special meeting, RTI recommends that you also submit your proxy or voting instructions as described below so that your vote will be counted if you later decide not to attend the special meeting.
62
Voting by Proxy
Whether you hold shares directly as the stockholder of record or beneficially in street name, you may direct how your shares are voted without attending the RTI special meeting. If you are a stockholder of record, you may vote by submitting a proxy by one of the methods described below. If you hold shares beneficially in street name, you may vote by submitting voting instructions to your broker, trustee or nominee. For directions on how to vote, please refer to the instructions below and those included on your proxy card or, for shares held beneficially in street name, the voting instruction card provided by your broker, trustee or nominee.
| • | By Internet-Stockholders of record with internet access may submit proxies by following the “Vote by Internet” instructions on their proxy cards until 11:59 p.m. local time on March 6, 2019. Most stockholders who hold shares beneficially in street name may vote by accessing the web site specified on the voting instruction cards provided by their brokers, trustees or nominees. Please check the voting instruction card for internet voting availability. |
| • | By Telephone-Stockholders of record who live in the United States or Canada may submit proxies by following the “Vote by Phone” instructions on their proxy cards until 11:59 p.m. local time on March 6, 2019. Most stockholders who hold shares beneficially in street name and live in the United States or Canada may vote by phone by calling the number specified on the voting instruction cards provided by their brokers, trustees or nominees. Please check the voting instruction card for telephone voting availability. |
| • | By Mail-Stockholders of record may submit proxies by completing, signing and dating their proxy cards and mailing them in the accompanyingpre-addressed envelopes. Proxy cards submitted by mail must be received by the time of the special meeting in order for your shares to be voted. Stockholders who hold shares beneficially in street name may vote by mail by completing, signing and dating the voting instruction cards provided by their brokers, trustees or nominees and mailing them in the accompanyingpre-addressed envelopes. |
If a proxy card is signed and returned without an indication as to how the shares represented by the proxy are to be voted with regard to a particular proposal, the shares represented by the proxy will be voted “FOR” each such proposal.
Votes cast by proxy or in person at the RTI special meeting will be tabulated and certified by Broadridge, which will serve as the inspector of election for the RTI special meeting.
If any matters are properly presented for consideration at the RTI special meeting, the persons named in the enclosed proxy card and acting thereunder will have discretion to vote on those matters in accordance with their best judgment. We do not currently anticipate that any other matters will be raised at the RTI special meeting.
Revocation of Proxy
Any holder of RTI common stock or RTI preferred stock has the right to revoke his or her proxy at any time prior to the voting thereof at the RTI special meeting by: (1) filing a written revocation with the Secretary of RTI prior to the voting of such proxy; (2) giving a duly executed proxy bearing a later date; or (3) attending the RTI special meeting and voting in person. Attendance by a stockholder at the RTI special meeting will not itself revoke his or her proxy. If you hold your shares in the name of a bank, broker or other nominee, you should follow the instructions provided by your bank, broker or nominee in revoking your previously granted proxy.
RTI will bear the entire cost of soliciting proxies from its stockholders. In addition to the solicitation of proxies by mail, RTI will request that banks, brokers and other record holders send proxies and proxy material to the beneficial owners of RTI shares and secure their voting instructions, if necessary. RTI will reimburse the record holders for their reasonable expenses in taking those actions.
63
RTI has also made arrangements with Georgeson LLC to assist in soliciting proxies and in communicating with RTI stockholders. RTI will pay Georgeson LLC a base fee of $15,000 for these services, plus its related costs and expenses. If necessary, RTI may also use several of its employees, who will not be specially compensated, to solicit proxies from RTI stockholders, either personally or by telephone, the Internet, facsimile or letter.
If a quorum is not present, a meeting of RTI stockholders may be adjourned for such periods as the holders of a majority of the shares of RTI common stock and RTI preferred stock (on anas-converted basis) entitled to vote, present in person or represented by proxy, at the RTI special meeting stockholders shall direct. If a quorum is present at the RTI special meeting but there are not sufficient votes at the time of the RTI special meeting to approve the Merger Proposal or the Share Issuance Proposal, then RTI stockholders may be asked to vote on the Meeting Adjournment Proposal. Notice need not be given of the adjourned meeting if the date, hour and place thereof are announced at the meeting at which the adjournment is taken, unless the adjournment is for more than 30 days thereafter or the RTI board sets a new record date for such meeting, in which case a notice of the adjourned meeting will be given to each RTI stockholder of record entitled to vote at the meeting. At any subsequent reconvening of the RTI special meeting at which a quorum is present, any business may be transacted that might have been transacted at the original meeting and all proxies will be voted in the same manner as they would have been voted at the original convening of the RTI special meeting, except for any proxies that have been effectively revoked or withdrawn prior to the time the proxy is voted at the reconvened meeting.
If you need assistance in completing your proxy card or have questions regarding the RTI special meeting, please contact Georgeson LLC, the proxy solicitation agent for RTI, at (866)391-6921.
64
RTI PROPOSAL 1: MERGER PROPOSAL
RTI is asking its stockholders to adopt the Master Transaction Agreement and approve the transactions contemplated thereby, including the Merger (the “Merger Proposal”). RTI stockholders should read carefully this joint proxy and consent solicitation statement/prospectus in its entirety for more detailed information concerning the Master Transaction Agreement, which is attached asAnnex Ato this joint proxy and consent solicitation statement/prospectus.
Please see the section entitled “The Transaction”, beginning on page73of this joint proxy and consent solicitation statement/prospectus for additional information and a summary of certain terms of the Merger, and please see the section entitled “The Master Transaction Agreement”, beginning on page123of this joint proxy and consent solicitation statement/prospectus for additional information and a summary of certain terms of the Master Transaction Agreement.You are urged to readcarefully this joint proxy and consent solicitation statement/prospectus and the Master Transaction Agreement in their entirety before voting on this proposal.
Vote Required for Approval
Each of the Merger Proposal and the Share Issuance Proposal are cross-conditioned on the approval of each other.
Assuming a quorum is present, the Merger Proposal requires (i) the affirmative vote of the holders of a majority of the outstanding shares of RTI common stock and RTI preferred stock (on a fully converted basis) entitled to vote thereon and (ii) the written consent or affirmative vote of the holders of a majority of the outstanding shares of RTI preferred stock entitled to vote thereon, voting separately as a class (the “Required Merger Proposal Vote”). Abstentions and brokernon-votes will have the same effect as a vote “AGAINST” the Merger Proposal.
THE RTI BOARD UNANIMOUSLY RECOMMENDS
THAT YOU VOTE “FOR” THE MERGER PROPOSAL.
65
RTI PROPOSAL 2: SHARE ISSUANCE PROPOSAL
As described below, RTI is asking its stockholders to consider and vote upon a proposal to approve, for purposes of complying with applicable provisions of Nasdaq Listing Rule 5635, the potential issuance of more than twenty percent (20%) of Holdco’s issued and outstanding common stock in connection with the transactions contemplated by the Master Transaction Agreement (the “Share Issuance Proposal”).
Overview
Assuming the Merger Proposal is approved, as consideration for the Contribution, at closing Holdco will pay PS Spine $100.0 million in cash, subject to adjustment as described below, and will issue 10,729,614 fully paid andnon-assessable shares of Holdco common stock in the aggregate to PS Spine and to the lenders under Paradigm’s existing senior secured credit agreement and their affiliates. The Stock Consideration Amount was determined by dividing $50.0 million by the RTI Price. The Cash Consideration Amount is subject to the following adjustments: (i) positive dollar-for-dollar adjustment based on the amount of Paradigm’s cash and cash equivalents at closing, (ii) negative dollar-for-dollar adjustment based on the amount of outstanding indebtedness and unpaid transaction expenses of Paradigm at closing and (iii) negative dollar-for-dollar adjustment to the extent that Paradigm’s working capital (excluding cash, cash equivalents, indebtedness and transaction expenses) at closing is less than the working capital target of $7.0 million. At the closing, due to the expected amount of Paradigm’s indebtedness and transaction expenses all cash that would otherwise be paid at closing pursuant to the Master Transaction Agreement to PS Spine, as well as a portion of the Stock Consideration Amount, is expected to be used to satisfy Paradigm’s outstanding indebtedness and unpaid transaction expenses.
In addition to the Cash Consideration Amount and the Stock Consideration Amount payable at the closing, Holdco may be required to make further cash payments or issue additional shares of Holdco common stock to PS Spine in an amount up to an additional $50.0 million of shares of Holdco common stock to be valued based upon the RTI Price and an additional $100.0 million of cash and/or Holdco common stock to be valued at the time of issuance, representing the Earnout Consideration, in each case, if certain revenue targets, which are described in the section entitled “The Master Transaction Agreement—Purchase Price—Contingent Consideration”, beginning on page 127, are achieved between closing and December 31, 2022. In certain circumstances, described in greater detail in this joint proxy and consent solicitation statement/prospectus, $10.0 million of the Earnout Consideration (which can take the form of cash, stock or a combination of both) will be paid to the lenders under Paradigm’s existing senior secured credit agreement and/or their affiliates.
The terms of the consideration to be paid pursuant to the Master Transaction Agreement are complex and only briefly summarized above. For further information, please see the full text of the Master Transaction Agreement, which is attached asAnnex A hereto. The discussion herein is qualified in its entirety by reference to such document.
Why RTI Needs Stockholder Approval
RTI is seeking approval of its stockholders in order to comply with Nasdaq Listing Rule 5635(a).
Under Nasdaq Listing Rule 5635(a), stockholder approval is required prior to the issuance of securities in connection with the acquisition of another company if such securities are not issued in a public offering and (A) have, or will have upon issuance, voting power equal to or in excess of twenty percent (20%) of the voting power outstanding before the issuance of common stock (or securities convertible into or exercisable for common stock); or (B) the number of shares of common stock to be issued is or will be equal to or in excess of twenty percent (20%) of the number of shares of common stock outstanding before the issuance of the stock or securities. Because the number of shares of Holdco common stock that may be issued in connection with the Transaction, including the Earnout Consideration, will have, upon issuance, voting power equal to or in excess of twenty percent (20%) of the voting power of RTI outstanding before such issuance, Nasdaq Listing Rule 5635(a) requires the approval of RTI stockholders.
66
Effect of Proposal on Current RTI Stockholders
The issuance of such shares would result in significant dilution to RTI stockholders, and would afford RTI stockholders a smaller percentage interest in the voting power, liquidation value and aggregate book value of the combined company.
Vote Required for Approval
Each of the Merger Proposal and the Share Issuance Proposal are cross-conditioned on the approval of each other.
Assuming a quorum is present, the Share Issuance Proposal requires (i) the affirmative vote of a majority of votes cast on the proposal by RTI common stock and RTI preferred stock (on a fully converted basis) and (ii) the written consent or affirmative vote of the holders of a majority of the outstanding shares of RTI preferred stock, voting separately as a class (the “Required Share Issuance Proposal Vote”). Abstentions will have (i) the same effect as a vote “AGAINST” the Share Issuance Proposal for holders of RTI preferred stock, voting separately as a class and (ii) no effect on the Share Issuance Proposal for the holders of RTI common stock and RTI preferred stock (on a fully converted basis). Brokernon-votes will have (i) the same effect as a vote “AGAINST” the Share Issuance Proposal for holders of RTI preferred stock, voting separately as a class and (ii) no effect on the Share Issuance Proposal for the holders of RTI common stock and RTI preferred stock (on a fully converted basis).
THE RTI BOARD UNANIMOUSLY RECOMMENDS
THAT YOU VOTE “FOR” THE SHARE ISSUANCE PROPOSAL.
67
RTI PROPOSAL 3: MEETING ADJOURNMENT PROPOSAL
The RTI special meeting may be adjourned to another time and place to permit further solicitation of proxies, if necessary or appropriate, to obtain additional proxies if there are not sufficient votes to approve the Merger Proposal or the Share Issuance Proposal. The RTI board does not intend to propose adjournment of the RTI special meeting if there are sufficient votes to approve the RTI Merger Proposal and the Share Issuance Proposal.
RTI is asking you to authorize the holder of any proxy solicited by RTI’s board to vote in favor of any adjournment of the RTI special meeting, if necessary or appropriate, to solicit additional proxies if there are not sufficient votes to approve the Merger Proposal or the Share Issuance Proposal (the “Meeting Adjournment Proposal”).
Assuming a quorum is present, the Meeting Adjournment Proposal requires the affirmative vote of the holders of a majority of the shares having voting power present in person or represented by proxy at the RTI special meeting. Abstentions will have the same effect as a vote “AGAINST” the Meeting Adjournment Proposal, and brokernon-votes will not affect the outcome of the vote on the Meeting Adjournment Proposal.
THE RTI BOARD UNANIMOUSLY RECOMMENDS
THAT YOU VOTE “FOR” THE MEETING ADJOURNMENT PROPOSAL.
68
THE PS SPINE SOLICITATION OF WRITTEN CONSENTS
The following contains information for unitholders of PS Spine regarding the solicitation of written consents from the unitholders of PS Spine to approve the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution.
Purpose of the Consent Solicitation
The PS Spine board is providing these consent solicitation materials to unitholders of PS Spine. Unitholders of PS Spine are being asked to approve the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution, by executing and delivering the written consent furnished with this joint proxy and consent solicitation statement/prospectus.
The PS Spine board has set February 6, 2019, as the PS Spine record date for determining unitholders of PS Spine entitled to execute and deliver written consents with respect to the approval of the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution.
Unitholders of PS Spine Entitled to Consent
Only holders of outstanding Common Units, Class A Common Units, Class A Preferred Units, Class B Preferred Units,Class E-1 Preferred Units and Class F Preferred Units of PS Spine as of the close of business on the PS Spine record date are entitled to execute and deliver written consents with respect to the approval of the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution. As of the close of business on the PS Spine record date, there were 2,075,282 Common Units, 1,140,708 Class A Common Units, 2,717,886 Class A Preferred Units, 7,895,271 Class B Preferred Units, 14,786,878Class E-1 Preferred Units and no Class F Preferred Units of PS Spine issued and outstanding and entitled to execute and deliver written consents with respect to the approval of the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution.
As of the close of business on the PS Spine record date, the managers and executive officers of PS Spine, and their respective affiliates, collectively owned and were entitled to execute and deliver written consents with respect to 1,354,253 Common Units, 701,691 Class A Common Units, 1,880,474 Class A Preferred Units, 7,124,650 Class B Preferred Units, 11,862,157Class E-1 Preferred Units and no Class F Preferred Units of PS Spine, which represent, in the aggregate, approximately 80.1% of the outstanding voting power of the unitholders of PS Spine.
Recommendation of the Board of Managers of PS Spine
The PS Spine board has unanimously approved and declared advisable the Master Transaction Agreement and the transactions contemplated by the Master Transaction Agreement, including the Contribution, and has determined that the Master Transaction Agreement and the transactions contemplated by the Master Transaction Agreement, including the Contribution, are fair to, and in the best interests of, PS Spine and its unitholders.
The PS Spine board unanimously recommends that unitholders of PS Spine approve the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution.
Written consents from holders of66-2/3% of the outstanding Common Units, Class A Common Units, Class A Preferred Units, Class B Preferred Units,Class E-1 Preferred Units and Class F Preferred Units of PS Spine, as of the close of business on the PS Spine record date, consenting together as a single class, in addition to written consents from holders of a majority of the outstanding Class A Preferred Units and a majority of the outstanding Class B Preferred
69
Units of PS Spine, as of the close of business on the PS Spine record date, each consenting as a separate class, are required to approve the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution (the “PS Spine written consent”).
Certain holders of 5% or more of the voting units of PS Spine, representing approximately 67.4% of the outstanding Common Units, Class A Common Units, Class A Preferred Units, Class B Preferred Units,Class E-1 Preferred Units and Class F Preferred Units, including a majority of the Class A Preferred Units and a majority of the Class B Preferred Units, of PS Spine as of the close of business on the PS Spine record date, have entered into support agreements with RTI and Holdco pursuant to which, among other things, they have agreed to execute and return written consents approving the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution. PS Spine expects that the written consents to be delivered by unitholders pursuant to the support agreements will represent a sufficient number of voting units of PS Spine to satisfy the approval requirement described above with respect to the approval of the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution. For more information about the support agreements, see the section entitled “The Transaction—Support Agreements”, beginning on page 119.
Unitholders of PS Spine may consent to the approval of the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution, with respect to their units of PS Spine by completing, dating and signing the written consent enclosed with this joint proxy and consent solicitation statement/prospectus and returning it to PS Spine. The PS Spine board has set February 13, 2019, as the final date for receipt of written consents. PS Spine reserves the right to extend the final date for receipt of written consents beyond February 13, 2019. Any such extension may be made without notice to the unitholders of PS Spine. Once a sufficient number of consents to approve the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution, have been received, the consent solicitation will conclude.
If you hold voting units of PS Spine as of the close of business on the PS Spine record date and you wish to give your written consent, you must fill out the enclosed written consent, date and sign it, and promptly return it to PS Spine. Once you have completed, dated and signed the written consent, you may deliver it to PS Spine by emailing a “.pdf” copy of your written consent to InvestorRelations@paradigmspine.com or by mailing your written consent to PS Spine at 505 Park Avenue, 14th Floor, New York, NY 10022, Attention: Secretary.
Executing Consents; Revocation of Consents
Holders of voting units of PS Spine as of the close of business on the PS Spine record date may execute written consents to approve the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution. If a holder of voting units of PS Spine does not provide such holder’s written consent to approve the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution, it will have the same effect as a vote against the approval of the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution. If you are a record holder of voting units of PS Spine and you return a signed written consent without indicating your decision on the approval of the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution, you will have consented to such approval.
If you are a unitholder on the PS Spine record date of Common Units, Class A Common Units, Class A Preferred Units, Class B Preferred Units,Class E-1 Preferred Units or Class F Preferred Units of PS Spine, you may change or revoke your consent to the approval of the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution, subject to any contractual obligation you may have, at any time prior to February 13, 2019 or, if earlier, at any time before the consents of a sufficient number of voting units of PS Spine to approve the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution, have been filed with PS Spine. If you wish to change or revoke your consent before that time, you may do so by emailing a “.pdf” copy of such change or revocation to InvestorRelations@paradigmspine.com or by mailing such change or revocation to PS Spine at 505 Park Avenue, 14th Floor, New York, NY 10022, Attention: Secretary.
70
Solicitation of Consents; Expenses
PS Spine will bear the entire cost of preparing, printing and mailing these consent solicitation materials to unitholders of PS Spine. Officers and employees of PS Spine and Paradigm may solicit consents by telephone and personally, in addition to solicitation by mail. These persons will receive their regular salaries but no special compensation for soliciting consents.
71
PS SPINE PROPOSAL 1—APPROVAL OF THE MASTER TRANSACTION AGREEMENT
As discussed in this joint proxy and consent solicitation statement/prospectus, PS Spine is asking its unitholders to approve the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution. For a summary of and detailed information regarding this proposal, see the information about the Master Transaction Agreement throughout this joint proxy and consent solicitation statement/prospectus, including the information set forth in the section entitled “The Transaction”, beginning on page 73, and “The Master Transaction Agreement”, beginning on page 123. A copy of the Master Transaction Agreement is attached asAnnex A to this joint proxy and consent solicitation statement/prospectus and incorporated herein by reference. Unitholders of PS Spine are urged to read carefully this joint proxy and consent solicitation statement/prospectus and the Master Transaction Agreement in their entirety before executing and delivering a written consent with respect to the approval of the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution.
The Master Transaction Agreement provides, among other things, that PS Spine will contribute all of the issued and outstanding equity interests in Paradigm to Holdco in exchange for the right to receive the consideration therefor described herein. PS Spine intends to distribute such consideration (net of transaction and other expenses and amounts due to its senior lenders and their affiliates) to its unitholders, in accordance with PS Spine’s organizational documents, as soon as reasonably practicable following its receipt thereof.
Required Vote
Written consents from holders of66-2/3% of the outstanding Common Units, Class A Common Units, Class A Preferred Units, Class B Preferred Units,Class E-1 Preferred Units and Class F Preferred Units of PS Spine, as of the close of business on the PS Spine record date, consenting together as a single class, in addition to written consents from holders of a majority of the outstanding Class A Preferred Units and a majority of the outstanding Class B Preferred Units of PS Spine, as of the close of business on the PS Spine record date, each consenting as a separate class, are required to approve the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution (the “Required PS Spine Vote”).
Recommendation of the Board of Managers of PS Spine
The PS Spine board unanimously recommends that the unitholders of PS Spine consent to “APPROVE” the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution.
72
The following is a description of the material aspects of the Transaction. While we believe that the following description covers the material terms of the Transaction, the description may not contain all of the information that is important to you. We encourage you to read carefully this entire joint proxy and consent solicitation statement/prospectus, including the Master Transaction Agreement attached to this joint proxy and consent solicitation statement/prospectus asAnnex A, for a more complete understanding of the Transaction.
On, November 1, 2018, RTI, PS Spine, Holdco and Merger Sub entered into the Master Transaction Agreement, dated as of November 1, 2018. Subject to the terms and conditions set forth in the Master Transaction Agreement, at the closing, Holdco will acquire all of the outstanding equity interests in Paradigm, through a transaction in which: (i) PS Spine will contribute all of the issued and outstanding equity interests in Paradigm to Holdco (i.e., the Contribution as defined above), (ii) Merger Sub will be merged with and into RTI (i.e., the Merger as above), with RTI surviving the Merger as a wholly owned direct subsidiary of Holdco and (iii) Holdco will be renamed “RTI Surgical Holdings, Inc.” The Merger and the Contribution will become effective concurrently. A copy of the Master Transaction Agreement that is described in this joint proxy and consent solicitation statement/prospectus is attached asAnnex A.
Background of the Merger and the Contribution
The RTI board and senior management routinely review strategic alternatives with the goal of enhancing stockholder value. From time to time, these reviews have included discussions and analyses of potential acquisition transactions.
The Paradigm board (the members of which are the same as the members of the PS Spine board) and senior management routinely review strategic alternatives with the goal of enhancing Paradigm or PS Spine unitholder value. From time to time, these reviews have included discussions and analyses of potential strategic transactions.
On November 1, 2017, in connection with the amendment and restatement of Paradigm’s existing senior secured credit agreement, Paradigm entered into an agreement with its senior lenders pursuant to which Paradigm agreed to appoint a designee of the lenders’ representative, Mr. Howard Rowe, as a member of the Paradigm board, to establish a strategic transaction committee of the Paradigm board, comprising Mr. Rowe, Mr. Jeffrey C. Lightcap, a member of both the PS Spine and Paradigm boards, and Mr. Anthony G. Viscogliosi, a member of both the PS Spine and Paradigm boards of managers, and to commence a sale process that would culminate in the execution of an agreement to sell Paradigm’s business no later than November 15, 2018.
On April 16, 2018, Mr. Jonathon Singer, the Chief Financial and Administrative Officer of RTI, and other members of RTI’s senior management met with representatives from Piper Jaffray to discuss a broad cross section of strategic opportunities focused on differentiated technologies in the spinal market, including a potential acquisition of Paradigm. During the week of April 23, 2018, a representative of Piper Jaffray reached out to Mr. Anthony Viscogliosi to discuss Paradigm’s potential interest in exploring a strategic transaction with RTI and to schedule a meeting between RTI and Paradigm.
On April 23, 2018, Mr. Camille Farhat, the Chief Executive Officer of RTI, and Mr. Singer met with Mr. Marc Viscogliosi, the Chairman and Chief Executive Officer of each of PS Spine and Paradigm, at Paradigm’s offices in New York City to discuss a potential strategic business transaction between RTI and Paradigm.
During April 23, 2018 and April 24, 2018, representatives of RTI and Paradigm negotiated anon-disclosure agreement and on April 24, 2018, RTI and Paradigm executed thenon-disclosure agreement to share confidential information regarding each others’ businesses and continue discussions regarding a potential strategic business transaction.
On May 1, 2018, the RTI board had a meeting, with a representative of Holland & Knight, financing and corporate governance counsel to RTI, participating, in which certain members of RTI senior management provided a
73
strategic update on RTI. The strategic update provided a general overview of opportunities RTI was considering to accelerate growth, including a discussion of Paradigm and its coflex® product. The RTI board supported management’s strategic direction, including continuing discussions with Paradigm regarding a potential strategic business transaction.
On May 4, 2018, Mr. Farhat, Mr. Singer and other members of RTI management participated in a conference call with Mr. Marc Viscogliosi and Mr. Anthony Viscogliosi to discuss Paradigm’s business operations.
On May 8, 2018, representatives of Piper Jaffray, at the request of senior management of RTI, held a conference call with Mr. Anthony Viscogliosi, Mr. Marc Viscogliosi and Mr. Lightcap to convey RTI’s interest in an acquisition of Paradigm for a potential purchase price of up to $350 million in the aggregate, consisting of $100 million in cash at closing, $50 million in RTI common stock at closing and two contingent consideration payments of $100 million each if specific milestones were met after closing. Any RTI common stock issued as contingent consideration would be priced when earned. Mr. Anthony Viscogliosi and Mr. Lightcap told the representatives of Piper Jaffray that Paradigm would want a higher amount of both cash and stock consideration in any potential transaction.
Following discussions with senior management of RTI regarding Paradigm’s initial response to the May 8, 2018 proposal, representatives of Piper Jaffray met with Mr. Anthony Viscogliosi, Mr. Marc Viscogliosi and Mr. Lightcap on May 15, 2018 to discuss RTI’s proposal. At that meeting representatives of Piper Jaffray, at the request of senior management of RTI, delivered a revised proposal set forth in a draft,non-binding memorandum of understanding, which included a potential purchase price of up to $350 million in the aggregate, consisting of $100 million in cash at closing, $50 million in RTI common stock at closing, two contingent consideration payments of $100 million each if specific milestones were met after closing and a grant of options to purchase five million shares of RTI common stock at closing. Any RTI common stock issued as contingent consideration would be priced when earned.
Between the May 15, 2018 meeting and June 7, 2018, representatives of both RTI and Paradigm performed preliminary due diligence on each other, including a meeting in New York, New York together with a representative of Piper Jaffray at Piper Jaffray’s office on May 30, 2018 to discuss RTI’s business and the proposed strategy for the potential business combination after closing.
On June 8, 2018, Mr. Lightcap called representatives at Piper Jaffray to verbally counter the terms in RTI’s May 15, 2018 proposal, requesting a purchase price of up to $375 million in the aggregate, consisting of a $125 million cash payment at closing, $150 million of RTI common stock at closing and one contingent consideration payment of $100 million if specific milestones were met after closing. Any RTI common stock issued as contingent consideration would be priced when earned.
On June 12, 2018, the Transaction Committee of the RTI board held a conference call to receive an update on the progression of the discussions with Paradigm and to discuss Paradigm’s June 8, 2018 position on valuation. On that call the Transaction Committee of the RTI board indicated that they were supportive of RTI’s senior management continuing discussions with Paradigm.
On June 13, 2018, representatives of Piper Jaffray, at the request of senior management of RTI, sent to Mr. Anthony Viscogliosi and Mr. Lightcap a revised draft memorandum of understanding proposing a business combination with Paradigm at a purchase price of up to $350 million in the aggregate, consisting of $100 million in cash at closing, $50 million in RTI common stock at closing and two contingent consideration payments of $100 million each if specific milestones were met after closing. The revised proposal reflected that RTI shares issuable for the first contingent consideration payment would be based on RTI’s common stock price at signing of the transaction, whereas the shares issuable for the second contingent consideration payment would be based on RTI’s common stock price at the time that the contingent consideration was earned. Following delivery of the revised proposal, representatives of Piper Jaffray participated in a telephonic meeting with Mr. Anthony Viscogliosi and Mr. Lightcap to discuss the revised proposal.
Following the conference call on June 13, 2018, representatives of RTI and Paradigm engaged in further discussions and negotiations and on June 17, 2018 agreed to continue discussions on the basis of the terms set forth
74
in anon-binding memorandum of understanding, which reflected a purchase price of up to $340 million in the aggregate, consisting of a $110 million cash payment at closing, $65 million in RTI common stock at closing and three contingent consideration payments of: (i) up to $85 million of RTI common stock to vest ratably within a range if specific revenue thresholds for specific Paradigm and RTI products were achieved by December 31, 2021; (ii) $15 million in RTI common stock if a specific amount of revenue for specific Paradigm and RTI products was achieved by December 31, 2021; and (iii) $65 million in RTI common stock or cash at RTI’s election if a specific amount of revenue for specific Paradigm products was achieved by December 31, 2023. Thenon-binding memorandum of understanding also reflected that RTI shares issuable for the first two contingent consideration payments would be based on RTI’s common stock price at the signing of the transaction. The terms in thenon-binding memorandum of understanding were subject to approval by RTI’s board of the proposed transaction and upon the completion of due diligence by both RTI and Paradigm.
On June 18, 2018, members of RTI’s senior management updated the RTI board with respect to the proposed transaction with Paradigm, with a representative of Holland & Knight participating. Following that update, RTI engaged Sidley Austin to act as legal counsel to RTI in connection with the proposed transaction with Paradigm.
On June 21, 2018, Mr. Farhat and Mr. Singer met with Mr. Lightcap to discuss the proposed transaction and post-closing corporate governance matters, including the possibility of Paradigm’s equityholders having the right to designate an individual to serve on the RTI board following the closing.
On June 21, 2018, Paradigm provided RTI and its advisors with access to a virtual data room for purposes of their due diligence review. From June 21, 2018 until the execution of the Master Transaction Agreement, RTI and its advisors conducted due diligence on Paradigm, which included reviewing the materials provided in the virtual data room and other materials provided by Paradigm and participating in conference calls with Paradigm and Paradigm’s advisors.
Between June 27, 2018 and July 12, 2018, certain members of management of RTI and Paradigm and representatives of each of Piper Jaffray, Sidley Austin, PricewaterhouseCoopers LLP (tax advisor to RTI) and Dorsey & Whitney (legal counsel to PS Spine and Paradigm) participated in several conference calls about different potential transaction structures and the anticipated tax implications of such structures.
On July 12, 2018, RTI and Paradigm orally agreed to proceed with the transaction structure that was ultimately reflected in the Master Transaction Agreement.
On July 25, 2018, RTI provided Paradigm and its advisors with access to a virtual data room for purposes of their due diligence review. From July 25, 2018 until the execution of the Master Transaction Agreement, PS Spine, Paradigm and their advisors conducted due diligence on RTI, which included reviewing the materials provided in the virtual data room and other materials provided by RTI and participating in conference calls with RTI and RTI’s advisors.
On July 26, 2018, representatives of Sidley Austin circulated an initial draft of the Master Transaction Agreement to Dorsey & Whitney reflecting the transaction structure agreed to between the parties and the terms of the revisednon-binding memorandum of understanding from June 17, 2018.
From July 26, 2018 until August 4, 2018, certain members of management of RTI and Paradigm, and representatives of each of Sidley Austin and Dorsey & Whitney negotiated the terms of the Master Transaction Agreement.
On July 30, 2018, the Paradigm board met and discussed the proposed transaction, with representatives of Dorsey & Whitney participating. After the meeting, the strategic transaction committee of the Paradigm board met with representatives of Dorsey & Whitney to discuss the initial draft of the Master Transaction Agreement and Paradigm’s potential response thereto.
On August 4, 2018, Dorsey & Whitney distributed a revised draft of the Master Transaction Agreement back to Sidley Austin.
75
During the week of August 6, 2018, representatives of each of RTI, Paradigm and Piper Jaffray met in Chicago at Sidley Austin’s office to conduct additional due diligence, including Paradigm’s due diligence of RTI, and to discuss Paradigm’s updated financial performance.
On August 8, 2018, representatives of each of RTI and Piper Jaffray discussed Paradigm’s recent financial performance and concluded that a reduction in the proposed purchase price would be needed in order for RTI to continue to move forward with the proposed transaction.
On August 9, 2018, representatives of Piper Jaffray, at the request of senior management of RTI, spoke with senior management at Paradigm and indicated that RTI believed that Paradigm’s updated financial performance did not support the price RTI had offered in the revisednon-binding memorandum of understanding on June 17, 2018, and as such RTI was considering a reduction in the portion of the purchase price payable at the closing.
On August 10, 2018, the RTI board held a telephonic meeting, which included Mr. Singer, and representatives of each of Piper Jaffray, Holland & Knight and Sidley Austin. At that meeting Mr. Singer provided an update on the proposed transaction and noted that Paradigm’s current revenue projections no longer supported the price RTI had offered in the revisednon-binding memorandum of understanding on June 17, 2018. Mr. Singer also updated the RTI board on the key findings from RTI’s and its advisors’ due diligence for the transaction. Mr. Singer indicated that RTI expected to renegotiate the purchase price for the transaction and would keep the RTI board updated on the status of those negotiations.
On August 13 and 14, 2018, representatives of RTI, Paradigm and Piper Jaffray met at RTI’s office in Deerfield, Illinois to discuss potential transaction synergies and the revised forecast for the Paradigm business. Following those meetings, management of RTI and representatives of Piper Jaffray reviewed and discussed revisions to the financial model for the transaction in light of Paradigm’s financial performance.
On August 20 and 21, 2018, the RTI board met, with Mr. Singer and a representative of Holland & Knight participating as well. During the meeting the RTI board discussed RTI’s spine industry strategy and the expected contributions thereto from the potential business combination with Paradigm. The RTI board also discussed the timing of and execution risk associated with the potential transaction with Paradigm. For a portion of the meeting, representatives from Piper Jaffray participated telephonically to review the proposed financial terms of the transaction in detail and the preliminary financial analysis Piper Jaffray had performed with respect to the proposed consideration to be paid in the transaction. Representatives of Piper Jaffray also discussed RTI’s ongoing debt capacity and a leverage analysis, as well as preliminarypro-forma estimates for the combined business post-closing. Following this presentation, the RTI board met in executive session and following that meeting reviewed with Mr. Farhat and Mr. Singer the purchase price terms that the RTI board believed needed to bere-negotiated with Paradigm.
On August 23, 2018, the RTI board held a special meeting, with a representative of Holland & Knight participating, to discuss the potential business combination with Paradigm and the revised terms that had been prepared by RTI’s senior management and representatives of Piper Jaffray based on the input received from the RTI board at the August 20 and 21, 2018 meeting of the RTI board.
Following the RTI board meeting on August 23, 2018, at the direction of the RTI board Piper Jaffray sent a revisednon-binding memorandum of understanding to representatives of Paradigm, reflecting a purchase price of up to $340 million in the aggregate, consisting of a $100 million cash payment at closing, $50 million in Holdco common stock at closing and three contingent consideration payments of (i) up to $85 million of Holdco common stock, (ii) up to $40 million of Holdco common stock and (iii) $65 million in cash and/or Holdco common stock at RTI’s election, in each case if specific revenue milestones were met by specific dates after closing. The revisednon-binding memorandum of understanding generally provided that any Holdco common stock issued as contingent consideration would be priced when earned.
Between August 23, 2018 and August 31, 2018, the senior management of RTI, representatives of Piper Jaffray and representatives of Paradigm negotiated the proposed financial terms of the transaction, before ultimately agreeing to proceed with the proposed transaction based on a purchase price of up to $300 million in the aggregate, consisting of
76
a $100 million cash payment at closing, $50 million in Holdco common stock at closing and three contingent consideration payments of (i) $20 million in cash if specific Paradigm products achieve specific revenue targets by December 31, 2020, (ii) up to $85 million in Holdco common stock (with $50 million to be priced at the Parent Price and $35 million to be priced when earned) if specific Paradigm and RTI products achieve specific revenue targets by December 31, 2021 and up to $45 million in cash or Holdco common stock (to be priced when earned) at RTI’s election if specific Paradigm and RTI products achieve specific revenue targets by December 31, 2022.
The RTI board held a telephonic meeting on September 4, 2018 to review and discuss the financial terms of the revised proposed business combination. Representatives from Piper Jaffray and Holland & Knight also participated in the meeting. Following further discussion, the RTI board authorized senior management to proceed with the proposed business combination on the revised financial terms agreed between the parties on August 31, 2018, subject to the satisfactory completion of RTI’s due diligence on Paradigm.
On September 6, 2018, Mr. Farhat and Mr. Singer met with Mr. Marc Viscogliosi and Mr. Francis Magee, the Chief Technology Officer of Paradigm, to discuss integration and operational matters.
On September 7, 2018, Sidley Austin circulated a revised draft of the Master Transaction Agreement.
On September 13, 2018, the strategic transaction committee of the Paradigm board and representatives of Dorsey & Whitney had a conference call to review the status of the transaction.
Managements of each of RTI and Paradigm, and representatives of each of Sidley Austin and Dorsey & Whitney participated in a conference call on September 18, 2018 to negotiate the terms of the Master Transaction Agreement. Between September 7, 2018 and October 11, 2018 representatives of each of Sidley Austin and Dorsey & Whitney continued to negotiate the terms of the Master Transaction Agreement and also shared drafts of disclosure schedules prepared by both RTI and Paradigm.
Throughout October, 2018, Paradigm and Dorsey & Whitney kept Paradigm’s senior lenders’ representative and its counsel apprised of all material developments relating to the Master Transaction Agreement and discussed their respective comments on the Master Transaction Agreement and ancillary documents.
Dorsey & Whitney circulated an initial draft of the support agreement for WSHP Biologics Holdings, LLC, Mr. Farhat and Mr. Singer to execute in connection with the transaction on October 5, 2018.
On October 7, 2018, the strategic transaction committee of the Paradigm board and representatives of Dorsey & Whitney had a conference call to review the status of the transaction.
On October 8, 2018, RTI and Paradigm entered into an exclusivity letter agreement providing for Paradigm’s agreement to exclusively negotiate with RTI through October 15, 2018, and Dorsey & Whitney sent Sidley Austin a revised draft of the Master Transaction Agreement.
On October 9, 2018, the Transaction Committee of the RTI board had a conference call, with Mr. Singer updating the committee on the details of the transaction and the status of the negotiations.
On October 11, 2018, the RTI board held a telephonic meeting, with Mr. Singer and representatives of each of Holland & Knight, Piper Jaffray and Sidley Austin participating. During that meeting, a representative of Holland & Knight provided the RTI board with a summary of their fiduciary duties in respect of the proposed transaction with Paradigm. Mr. Farhat and Mr. Singer then provided the RTI board with a summary of the recent negotiations and status of the proposed transaction. A representative of Sidley Austin then led a detailed review of the current proposed terms of the Master Transaction Agreement, noting the remaining open items between the parties and answered various questions from the RTI board. Representatives of Piper Jaffray then provided the RTI board with updated financial analyses and discussed such analyses with the RTI board.
Between October 11, 2018 and October 29, 2018, the parties continued to negotiate the terms of the Master Transaction Agreement and the disclosure schedules.
77
Between October 11, 2018 and October 26, 2018, Mr. Lightcap and Mr. Anthony Viscogliosi, on behalf of Paradigm, and Mr. Rowe, on behalf of Paradigm’s senior lenders’ representative, negotiated and agreed in principle on the terms of repayment of the senior debt and associated obligations of Paradigm.
On October 14, 2018, the strategic transaction committee of the Paradigm board and representatives of Dorsey & Whitney had a conference call to review the status of the transaction.
On October 17, 2018 Sidley Austin circulated to Dorsey & Whitney a form of support agreement to be executed by the Paradigm Support Unitholders.
On October 18, 2018, RTI and Paradigm extended the period during which Paradigm agreed to exclusively negotiate with RTI through October 31, 2018.
On October 19, 2018, the Paradigm board held a telephonic meeting to, among other things, review the status of the transaction, with representatives of Dorsey & Whitney participating. In that meeting, a representative of Dorsey & Whitney provided the Paradigm board with a summary of their duties in respect of the proposed transaction with RTI.
Between October 27, 2018 and October 31, 2018, Dorsey & Whitney, counsel for Paradigm’s senior lenders’ representative and Sidley Austin negotiated an agreement relating to the application of transaction consideration to the satisfaction of Paradigm’s senior debt and associated obligations and the senior lenders’ release of liens relating to Paradigm as of the closing under the Master Transaction Agreement.
On October 30, 2018, the RTI board held a meeting to approve the Master Transaction Agreement and the transactions contemplated thereby, with Mr. Singer and representatives of each of Holland & Knight, Piper Jaffray and Sidley Austin participating. Representatives of Sidley Austin provided an update to their review of the terms of the Master Transaction Agreement based on the negotiations that had occurred since the October 11, 2018 meeting of the RTI board, and representatives of Piper Jaffray provided an update to their financial analyses previously provided to the RTI board and provided an oral opinion to the RTI board (which was subsequently confirmed in writing by delivery of Piper Jaffray’s written opinion dated November 1, 2018) to the effect that, as of November 1, 2018, and based upon and subject to the various assumptions and limitations set forth therein, the consideration to be paid by Holdco was fair, from a financial point of view, to Holdco. The RTI board then approved the Master Transaction Agreement and the transactions contemplated thereby.
Over the course of October 30 and 31, 2018, the parties finalized the terms of the Master Transaction Agreement, disclosure schedules and other ancillary documentation to be executed concurrently with the Master Transaction Agreement and RTI, Paradigm and their respective affiliates and advisors coordinated on their communications strategies in preparation for the execution of the Master Transaction Agreement.
On October 31, 2018, the Paradigm board held a telephonic meeting, with representatives of Dorsey & Whitney providing an update regarding the Master Transaction Agreement, the disclosure schedules and the ancillary documentation relating thereto.
Later on October 31, 2018, the board of PS Spine, via unanimous written consent, resolved that it was advisable, fair to and in the best interests of PS Spine and its unitholders for PS Spine to enter into the Master Transaction Agreement, approved the Master Transaction Agreement and the transactions contemplated thereby, resolved to submit the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution, to PS Spine’s voting unitholders for approval and recommended that the unitholders of PS Spine approve the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution.
At approximately 4:05 pm (Eastern Time) on November 1, 2018, RTI, Holdco, Merger Sub and PS Spine executed and delivered the Master Transaction Agreement and delivered the applicable support agreements. During the evening of November 1, 2018, RTI and Paradigm issued a joint press release announcing the transaction.
78
Recommendation of the RTI Board and Its Reasons for the Transaction
After careful consideration, the RTI board, on October 30, 2018, unanimously approved and adopted the Master Transaction Agreement and the transactions contemplated thereby, including the Merger, on the terms and subject to the conditions set forth therein, determined and declared that the terms of the Master Transaction Agreement and the transactions contemplated thereby, including the Merger, are advisable and in the best interests of RTI and its stockholders, and recommended that RTI stockholders approve and adopt the Master Transaction Agreement and the Merger.
The RTI board unanimously recommends that RTI stockholders vote “FOR” each of the Merger Proposal, the Share Issuance Proposal and the Meeting Adjournment Proposal.
In reaching its decision to approve and adopt the Master Transaction Agreement, the Transaction and the other transactions contemplated by the Master Transaction Agreement, including recommending that its stockholders approve: (i) the Merger Proposal; (ii) the Share Issuance Proposal and (iii) the Meeting Adjournment Proposal, the RTI board consulted with RTI management, as well as outside financial and legal advisors and considered a number of factors, including the following material factors:
| • | the alignment with the strategic priorities of RTI to invest in differentiated high growth products in the spine market while building scale, as Paradigm provides apre-market approval product and the businesses of the two entities are synergistic, including their complementary models, business lines, research capabilities and sales infrastructure; |
| • | each of RTI’s and Paradigm’s business, operations, financial condition, quarterly earnings and prospects; |
| • | the amounts and timing of cost savings, related expenses and revenue synergies expected to result from the Transaction; |
| • | the expectation that Holdco would create long-term stockholder value by leveraging the respective strengths of each business and expanding Holdco’s product portfolio and sales and marketing capabilities; |
| • | the potential for expansion in the growing spine market and the ability to leverage the sales channels of RTI and Paradigm to market a product portfolio of differentiated spine products and treatments along with Holdco’s suite of products; |
| • | the demonstrated clinical efficiency and market potential of Paradigm’s coflex® product; |
| • | the status of reimbursement decisions by Medicare and Medicaid for the coflex® product; |
| • | its understanding of the current and prospective business environment in which RTI and Paradigm operate, including international, U.S., state and local economic conditions, the competitive environment for entities in the spine treatment market and the likely effect of these factors on RTI both with and without the Transaction; |
| • | its review and discussion with RTI’s management concerning the due diligence investigation of Paradigm, including its review of Paradigm’s financial condition, results of operations, asset quality, growth potential, regulatory status and intellectual property; |
| • | the opinion of Piper Jaffray, delivered to the RTI board on November 1, 2018, to the effect that, as of such date and based on and subject to the assumptions made, procedures followed, matters considered and limitations on the scope of the review undertaken by Piper Jaffray, as described in its written opinion, the Aggregate Consideration (as defined above) to be paid by Holdco in the Transaction was fair, from a financial point of view, to Holdco, as more fully described below under ��The Transaction—Opinion of RTI’s Financial Advisor”, beginning on page 84; and |
| • | the financial and other terms of the Master Transaction Agreement, including the limitations on the aggregate consideration paid in cash and stock, the portion of the total consideration based upon future events and contingent performance measures, expected tax treatment, prohibitions on PS Spine’s ability to seek |
79
alternative acquisition proposals, the other deal protection and termination provisions, and the restrictions on the conduct of Paradigm’s business between the date of the Master Transaction Agreement and the closing, which it reviewed with its outside legal counsel. |
The RTI board also considered potential risks related to the Transaction and other transactions contemplated by the Master Transaction Agreement, including the following:
| • | RTI’s management’s attention and RTI’s resources may be diverted from the operation of RTI’s business and towards the completion of the Transaction; |
| • | RTI may not realize all of the expected benefits of the Transaction, including maintenance of existing customer and employee relationships, and minimal disruption in the integration of Paradigm’s operations and sales infrastructure with RTI; |
| • | the likelihood that the acquisition would be dilutive in 2019; |
| • | the challenges and difficulties relating to integrating the operations of Paradigm and RTI; |
| • | the nature and amount of payments and other benefits to be received by PS Spine in connection with the Transaction pursuant to the Master Transaction Agreement; |
| • | the risk that because the exchange ratio related to the stock portion of the initial consideration to be paid to PS Spine is fixed, the value of the stock portion of the initial consideration to be paid by RTI could fluctuate and increase between the original signing of the Master Transaction Agreement and the completion of the transactions contemplated by the Master Transaction Agreement; |
| • | the risk that the combination with Paradigm might not be completed in a timely manner, or at all, and the attendant adverse consequences for RTI’s and Paradigm’s respective businesses, as a result of the pending of the combination and potential operational disruptions; |
| • | the requirement that RTI pay PS Spine a termination fee under certain circumstances prompting the termination of the Master Transaction Agreement; |
| • | the substantial costs that RTI will incur in connection with the Transaction and other transactions contemplated by the Master Transaction Agreement, even if they are not consummated; and |
| • | the risks associated with the increase in RTI’s leverage upon the consummation of the Transaction. |
The above discussion of the factors considered by the RTI board is not intended to be exhaustive, but, rather, includes the material factors considered by the RTI board. In reaching its decision to approve and adopt the Master Transaction Agreement, the Transaction and the other transactions contemplated by the Master Transaction Agreement, including the issuance of shares of Holdco common stock and Holdco preferred stock in the Transaction, the RTI board did not quantify or assign any relative weights to the factors considered, and individual directors may have given different weights to different factors. The RTI board considered all these factors as a whole, including discussions with, and questioning of, RTI’s management and RTI’s outside financial and legal advisors, and overall considered the factors to be favorable to, and to support, its determination.
Recommendation of PS Spine Board and Its Reasons for the Transaction
The PS Spine board has determined that the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution, are advisable and in the best interests of PS Spine and its unitholders and has unanimously approved the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution.
The PS Spine board unanimously recommends that unitholders of PS Spine consent to the approval of the Master Transaction Agreement and the transactions contemplated by the Master Transaction Agreement, including the Contribution.
80
The PS Spine board considered many factors in making its decision to recommend that unitholders of PS Spine consent to the approval of the Master Transaction Agreement and the transactions contemplated by the Master Transaction Agreement, including the Contribution. In arriving at its decision, the PS Spine board consulted with PS Spine’s senior management and legal advisors, reviewed a significant amount of information and considered a number of factors, including, without limitation, the following:
| • | historical and current information concerning Paradigm’s business, financial performance, financial condition, operations and management, including financial projections of Paradigm under various scenarios and its short- and long-term strategic objectives and the significant risks associated therewith; |
| • | the consideration of strategic alternatives to the proposed transaction with RTI, including continuing to operate Paradigm’s business on a stand-alone basis, and the belief that the proposed transaction with RTI would provide the unitholders of PS Spine with a greater potential opportunity to realize a return on their investment than any other alternative reasonably available to PS Spine and its unitholders at that time; |
| • | the belief that the combination of Paradigm’s and RTI’s businesses would create more value for the unitholders of PS Spine in the long-term than PS Spine could create with Paradigm as an independent, stand-alone company, given the anticipated costs, timing and risks associated with Paradigm’s business at that time; |
| • | the belief that the consideration to be paid by RTI and Holdco pursuant to the Master Transaction Agreement was the highest price that RTI was willing to offer, taking into account the extensive negotiations between the parties and RTI’s indication that such consideration was its best and final offer; |
| • | the complementary nature of Paradigm’s and RTI’s businesses and the additional revenue growth opportunities presented by the combined company’s expanded and comprehensive product offering; |
| • | Paradigm’s debt obligations, cash needs and future financing opportunities; |
| • | the fact that Paradigm was in default under its existing senior secured credit agreement, and that the lenders thereunder had the right to compel the sale of Paradigm; |
| • | the expectation that the combined company could achieve annual cost savings and synergies from, among other things, reductions in corporate overhead and administrative costs in comparison to both companies on a stand-alone basis; |
| • | the status of reimbursement decisions by insurance providers for Paradigm’s products; |
| • | the Stock Consideration Amount and a portion of the Earnout Consideration to be paid in shares of Holdco common stock are set at a fixed number of shares, the value of which could fluctuate and increase or decrease; |
| • | historical and current information concerning RTI’s business, financial performance, financial condition, operations and management, and the results of a due diligence investigation of RTI conducted by PS Spine’s management and advisors; |
| • | the opportunity for the unitholders of PS Spine to participate in the potential future value of the combined company, including future potential value from RTI’s products and products pipeline; |
| • | the tax treatment of the consideration to be paid by RTI and Holdco pursuant to the Master Transaction Agreement; |
| • | the likelihood that the transaction will be completed based on, among other things: |
| • | the fact that RTI has obtained committed debt financing for the transaction, the amount of the debt financing commitment, the limited number and nature of conditions to the debt financing and the obligation of RTI to use its reasonable best efforts to obtain the debt financing; |
| • | RTI’s interest in and knowledge of Paradigm’s business; |
81
| • | the fact that WSHP Biologics Holdings, LLC, Camille I. Farhat, and Jonathon M. Singer have generally agreed, pursuant to support agreements executed with PS Spine, to vote all of the RTI capital stock owned by them, which represents approximately 18.01% of the voting power of RTI, in favor of the Merger Proposal and the Share Issuance Proposal, unless the applicable support agreement is earlier terminated (for more information, see the section entitled “The Transaction—Support Agreements”, beginning on page 119); |
| • | the fact that HealthCor Paradigm Blocker Company Two, Inc., HealthCor AIV, L.P., Trevi Health Ventures LP, Trevi AIV, LP, Viscogliosi Brothers LLC and VB Acquisition Co I LLC have generally agreed, pursuant to support agreements executed with RTI and Holdco, to deliver written consents with respect to the Master Transaction Agreement and the transactions contemplated thereby, unless the applicable support agreement is earlier terminated, and that such written consents are expected to be sufficient for the unitholders of PS Spine to approve the Master Transaction Agreement and the transactions contemplated thereby (for more information, see the section entitled “The Transaction—Support Agreements”, beginning on page 119); and |
| • | the terms and conditions of the Master Transaction Agreement, including, without limitation, the following: |
| • | the structure of the transactions contemplated by the Master Transaction Agreement and the percentage of the total shares of capital stock of the combined company that current RTI stockholders and unitholders of PS Spine are expected to own after the closing; |
| • | the absence of a financing or due diligence condition to the Transaction; |
| • | the limited number of conditions to the closing, including the fact that there were not expected to be any substantive issues in connection with the expiration or termination of the HSR Act waiting period (for more information, see the section entitled “The Master Transaction Agreement—Conditions to the Closing”, beginning on page 139); |
| • | the requirement that RTI pay either a $4.5 million termination fee or a $9.0 million termination fee if the Master Transaction Agreement is terminated in certain circumstances (for more information, see the section entitled “The Master Transaction Agreement—Termination”, beginning on page 142); |
| • | PS Spine’s ability, under certain circumstances, to seek specific performance of the obligations of RTI, Holdco and Merger Sub under the Master Transaction Agreement; |
| • | the fact that, if PS Spine is required to indemnify RTI pursuant to the terms of the Master Transaction Agreement, RTI’s sole recourse is to withhold, reduce and/or set off the amount subject to indemnification against the Earnout Consideration (for more information, see the section entitled “The Master Transaction Agreement—Indemnification”, beginning on page 141); and |
| • | the belief that the parties’ respective representations, warranties, and covenants, and the conditions to their respective obligations, are reasonable under the circumstances. |
In the course of its deliberations, the PS Spine board also considered a variety of uncertainties, risks and other potentially negative factors relevant to the transactions contemplated by the Master Transaction Agreement, including, without limitation, the following:
| • | the fact that unitholders of PS Spine will own a significantly smaller percentage in the combined company than such unitholders indirectly own in Paradigm currently; |
| • | the fact that all of the Cash Consideration Amount, a significant portion of the Stock Consideration Amount and a significant portion of the Earnout Consideration will be used to pay transaction expenses, amounts outstanding under Paradigm’s existing senior secured credit agreement and other amounts due to Paradigm’s senior lenders and their affiliates (for more information, see the section entitled “—Interests of PS Spine’s and Paradigm’s Managers and Executive Officers in the Transaction—Settlement Agreement”, beginning on page 117); |
82
| • | the fact that the Earnout Consideration represents a significant portion of the total consideration to be paid by RTI and Holdco pursuant to the Master Transaction Agreement, and will not be paid if the applicable revenue targets are not achieved (for more information, see the section entitled “The Master Transaction Agreement—Purchase Price—Contingent Consideration”, beginning on page 127); |
| • | the difficulty, complexity and costs inherent in the combination of two businesses of the size of PS Spine and RTI, and the risk that the cost savings, synergies and other benefits expected to be obtained as a result of the Transaction might not be fully or timely realized if at all; |
| • | the restrictions on the conduct of Paradigm’s business during the pendency of the Transaction, which may delay or prevent Paradigm from undertaking potential business opportunities that may arise or may negatively affect Paradigm’s ability to attract, retain and motivate key personnel; |
| • | the inability of the PS Spine board, pursuant to the terms of the Master Transaction Agreement, to withdraw, modify or amend its recommendation that the unitholders of PS Spine vote in favor of the approval of the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution; |
| • | the adverse impact that business uncertainty prior to the effective time could have on Paradigm’s ability to retain and motivate key personnel; |
| • | the time, effort and substantial costs involved in connection with entering into the Master Transaction Agreement and consummating the Transaction, and the related disruptions to the operation of Paradigm’s business, including the risk of diverting management’s attention from other strategic priorities to implement integration efforts, and the risk that the operations of Paradigm would be disrupted by employee concerns or departures or by changes to or termination of Paradigm’s relationships with its customers, suppliers, independent sales representatives and third-party distributors following the public announcement of the Master Transaction Agreement; |
| • | the fact that PS Spine has incurred and will continue to incur significant transaction costs and expenses in connection with the Transaction, regardless of whether the Transaction is consummated; |
| • | the risk that RTI’s stockholders may fail to approve the Merger Proposal and the Share Issuance Proposal; |
| • | the risk that RTI may fail to obtain financing to complete the Transaction, in which case RTI may under certain circumstances be obligated to pay a termination fee of $9.0 million pursuant to the terms of Master Transaction Agreement; |
| • | the possibility that the Transaction might be unduly delayed and the potential for such a delay to reduce or eliminate the expected benefits of the Transaction; and |
| • | the risk that the combined company’s financial forecasts are not attained at the level or within the timeframe expected if at all. |
In addition to considering the factors described above, the PS Spine board considered that some members of the PS Spine board, Paradigm board and certain executive officers of PS Spine and Paradigm have interests in the transactions contemplated by the Master Transaction Agreement that are in addition to, and that may be different from, the interests of the unitholders of PS Spine generally, as described in “The Transaction—Interests of PS Spine’s and Paradigm’s Managers and Executive Officers in the Transaction”, beginning on page 114.
The above discussion of the information and factors considered by the PS Spine board is not exhaustive, but is intended to reflect the material factors considered by the PS Spine board in its consideration of the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution. In view of the complexity, and the large number, of the factors considered, the managers of PS Spine, both individually and collectively, did not find it practicable to and did not attempt to quantify or assign any relative or specific weight to the various factors. Rather, the PS Spine board based its recommendation on the totality of the information presented to and considered by it. In addition, individual members of the PS Spine board may have given different weight to different factors.
83
Opinion of RTI’s Financial Advisor
On October 30, 2018, Piper Jaffray rendered its oral opinion to the RTI board (which was subsequently confirmed in writing by delivery of Piper Jaffray’s written opinion, dated November 1, 2018) to the effect that, as of November 1, 2018, and based upon and subject to the various assumptions and limitations set forth therein, the Aggregate Consideration (as defined above) to be paid by Holdco in the Transaction was fair, from a financial point of view, to Holdco.
The full text of the Piper Jaffray written opinion, dated November 1, 2018, which sets forth, among other things, the assumptions made, procedures followed, matters considered and limitations on the scope of the review undertaken by Piper Jaffray in rendering its opinion, is attached asAnnex B to this joint proxy and consent solicitation statement/prospectus. The Piper Jaffray opinion addresses only the fairness, from a financial point of view and as of the date of the opinion, of the Aggregate Consideration to be paid by Holdco in the Transaction. Piper Jaffray’s opinion was directed to the RTI board in connection with its consideration of the Transaction and was not intended to be, and does not constitute, a recommendation to any RTI stockholder as to how such stockholder should act or vote with respect to the Transaction or any other matter. Piper Jaffray’s opinion was approved for issuance by the Piper Jaffray opinion committee.
In connection with rendering the opinion described above and performing its related financial analyses, Piper Jaffray, among other things:
| • | reviewed and analyzed the financial terms of a draft of the Master Transaction Agreement; |
| • | reviewed and analyzed certain information, including financial forecasts, relating to the business, earnings, cash flow, assets, liabilities and prospects of each of RTI (including revenue projections relating to Zyga Technology, Inc. (“Zyga”), a subsidiary of RTI) and Paradigm that were furnished to Piper Jaffray by RTI (which included the RTI adjusted Paradigm financial projections, which are set forth in the section entitled “The Transaction—Certain Unaudited Prospective Financial Information”, beginning on page 102), which included the analyses and forecasts of certain cost savings and operating efficiencies expected by management of RTI and Paradigm to result from the Transaction (the “Synergies”); |
| • | conducted discussions with members of senior management and representatives of RTI and Paradigm concerning the immediately preceding matter described above, as well as the business of Paradigm, Zyga and their respective prospects before and after giving effect to the Transaction and the Synergies; |
| • | compared the financial performance of Paradigm with that of certainpublicly-traded companies that Piper Jaffray deemed relevant; and |
| • | reviewed the financial terms of certain business combination transactions that Piper Jaffray deemed relevant. |
In addition, Piper Jaffray conducted such other analyses, examinations and inquiries and considered such other financial, economic and market criteria as Piper Jaffray deemed necessary in arriving at its opinion.
The following is a summary of the material financial analyses performed by Piper Jaffray in connection with the preparation of its fairness opinion and reviewed with the RTI board at a meeting held on October 30, 2018.
This summary includes information presented in tabular format, which tables must be read together with the text of each analysis summary and considered as a whole in order to fully understand the financial analyses presented by Piper Jaffray. The tables alone do not constitute a complete summary of the financial analyses. The order in which these analyses are presented below, and the results of those analyses, should not be taken as any indication of the relative importance or weight given to these analyses by Piper Jaffray or the RTI board. Except as otherwise noted, the following quantitative information, to the extent that it is based on market data, is based on market data as it existed on or before October 25, 2018, and is not necessarily indicative of current market conditions.
For purposes of its financial analyses, Piper Jaffray assumed the aggregate value to be paid to PS Spine at the closing of the Transaction in respect of the Cash Consideration Amount, together with the Stock Consideration Amount
84
(collectively, the “Upfront Aggregate Consideration”) would be $150 million. Piper Jaffray also calculated the estimated net present value of the total projected Aggregate Consideration, including projected earnout payments, to be $262 million (the “NPV Aggregate Consideration”). Piper Jaffray calculated the NPV Aggregate Consideration using assumptions as to the timing of potential earnout payments to be made to PS Spine through December 31, 2022 based on the RTI adjusted Paradigm financial projections, which are set forth in the section entitled “The Transaction—Certain Unaudited Prospective Financial Information”, beginning on page 102. Such projections assumed that the maximum amount of Earnout Consideration would be paid to PS Spine, and that Holdco would elect to pay the Cash Election Amount in lieu of the Final Earnout Shares (in each case, as defined below in the section entitled “The Master Transaction Agreement—Purchase Price”, beginning on page 126). Piper Jaffray discounted the assumed future earnout payments back to September 30, 2018 using a discount rate of 11.5%, the midpoint of the range of discount rates that Piper Jaffray used as the estimated weighted average cost of capital for Paradigm (as described below under “—Discounted Cash Flows Analysis”, beginning on page 99).
Piper Jaffray also performed certain additional illustrative, theoretical future valuation sensitivity analyses of the Earnout Consideration with respect to the aggregate value of the Aggregate Consideration as a function of the value of Holdco common stock under different scenarios. Specifically, Piper Jaffray performed such illustrative, theoretical valuation analyses as of December 31, 2022, assuming (a) the maximum amount of Earnout Consideration would be paid to PS Spine, (b) the VWAP (as defined below) used to calculate a portion of the number of shares of Holdco common stock to be issued in connection with the Initial Earnout Shares (as more fully described below in the section entitled “The Master Transaction Agreement—Purchase Price”, beginning on page 126) would be $8.00 per share under the scenarios described in clauses (ii) and (iii) below, and (c) Holdco would elect to pay the Cash Election Amount (as defined below) in lieu of the Final Earnout Shares (as defined below). Based on these assumptions, Piper Jaffray calculated three illustrative, theoretical future valuations of aggregate Aggregate Consideration, consisting of (i) $300 million (the nominal maximum Earnout Consideration), based on a Holdco common stock price of $4.59 per share as of December 31, 2022 (the RTI common VWAP as of October 25, 2018) (the “Full Earnout” scenario), (ii) an aggregate Aggregate Consideration based on a Holdco common stock price of $9.18 per share as of December 31, 2022 (the “Stock Price Doubles” scenario), and (iii) an aggregate Aggregate Consideration based on a Holdco common stock price of $13.77 per share as of December 31, 2022 (the “Stock Price Triples” scenario).
In calculating the various components of Aggregate Consideration, Piper Jaffray did not assume any potential tax benefits for RTI that may result from the structure of Paradigm’s business.
In addition, for purposes of its analyses, and unless the context indicates otherwise, Piper Jaffray calculated enterprise value (“EV”) to be implied equity value, plus net debt (calculated to be outstanding debt less cash and cash equivalents) (i) for the selected public companies described below, using implied equity values based on their respective closing prices per share on October 25, 2018, and net debt amounts based on the most recent balance sheets set forth in their most recent respective public filings as of October 25, 2018, and (ii) for the selected merger and acquisition transactions described below, using, in the case of mergers and acquisition transactions involving (a) public target companies, implied equity values based on the implied announced purchase price for the equity securities of the respective target companies, and net debt amounts based on the most recent balance sheets set forth in the most recent public filings for the respective target companies as of the announcement date of the respective merger and acquisition transactions, and (b) private target companies, the announced transaction value.
Selected Public Companies Analysis
Medical Technology—Spine Business Profile
Piper Jaffray reviewed projected financial data for Paradigm prepared by management of RTI for the years ending December 31, 2018 and 2019, and compared such data to corresponding Wall Street consensus research estimates for publicly traded U.S.-based spinal implant companies in the medical technology industry that Piper Jaffray believed were comparable to Paradigm’s business profile.
85
Piper Jaffray selected the following companies:
| • | Alphatec Holdings, Inc. |
| • | Globus Medical, Inc. |
| • | NuVasive, Inc. |
| • | Orthofix Holdings, Inc. |
| • | RTI Surgical, Inc. |
| • | SeaSpine Holdings Corporation |
| • | Xtant Medical Holdings, Inc. |
For the selected business profile public company analysis, Piper Jaffray compared, among other things, implied multiples of projected 2018 and 2019 EV/revenue and EV/gross profit for Paradigm, based on the Aggregate Consideration, to the corresponding implied EV multiples for the selected public companies. Projected 2018 and 2019 revenue and gross profit for Paradigm were based on the RTI adjusted Paradigm financial projections. Projected 2018 and 2019 revenue and gross profit for the selected public companies were based on Wall Street consensus research estimates, public filings and press releases of such companies.
The analysis indicated the following multiples:
| Paradigm | Selected Publicly Traded U.S.-Based Spinal Implant Companies | |||||||||||||||||||||||||||||||
| Upfront(1) | NPV(2) | Maximum | 75th% | Mean | Median | 25th% | Minimum | |||||||||||||||||||||||||
EV to projected 2018 revenue | 3.7x | 6.4x | 7.1x | 3.6x | 2.8x | 2.0x | 1.6x | 1.4x | ||||||||||||||||||||||||
EV to projected 2019 revenue | 2.6x | 4.5x | 6.6x | 3.4x | 2.7x | 1.9x | 1.5x | 1.4x | ||||||||||||||||||||||||
EV to projected 2018 gross profit | 4.2x | 7.3x | 9.3x | 4.9x | 4.1x | 3.1x | 2.8x | 2.4x | ||||||||||||||||||||||||
EV to projected 2019 gross profit | 2.9x | 5.1x | 8.6x | 4.6x | 3.8x | 2.9x | 2.5x | 2.3x | ||||||||||||||||||||||||
| (1) | Based on the Upfront Aggregate Consideration. |
| (2) | Based on the NPV Aggregate Consideration. |
Based on this analysis, with respect to the Upfront Aggregate Consideration, Piper Jaffray noted that, with respect to Paradigm, (i) the EV/2018 revenue multiple fell between the “Maximum” and the 75th percentile range of implied EV multiples for the selected public companies, (ii) the EV/2019 revenue multiple fell between the mean and the median range of implied EV multiples for the selected public companies, (iii) the EV/2018 gross profit multiple fell between the 75th percentile and the mean range of implied EV multiples for the selected public companies, and (iv) the EV/2019 gross profit multiple fell at the median of implied EV multiples for the selected public companies.
Based on this analysis, with respect to the NPV Aggregate Consideration, Piper Jaffray noted that, with respect to Paradigm, each EV/revenue multiple and each EV/gross profit multiple fell between the “Maximum” and the 75th percentile range of implied EV multiples for the selected public companies.
86
In addition, Piper Jaffray observed that the range of implied EVs of Paradigm based on the mean and median for each valuation metric yielded the following, as compared to the Aggregate Consideration:
| Implied Enterprise Value of Paradigm (in millions) | ||||
2018 revenue (mean-median range) | $81 - $117 | |||
2019 revenue (mean-median range) | $108 - $154 | |||
2018 gross profit (mean-median range) | $110 - $145 | |||
2019 gross profit (mean-median range) | $148 - $192 | |||
Upfront Aggregate Consideration | $150 | |||
NPV Aggregate Consideration | $262 | |||
Additionally, Piper Jaffray performed an illustrative, theoretical valuation sensitivity analysis referred to above. For purposes of such sensitivity analysis, Piper Jaffray assumed (i) currently projected 2022 revenue and gross profit amounts for each of Paradigm and Zyga will be met, (ii) projected 2023 revenue and gross profit amounts for each of Paradigm and Zyga will not change through December 31, 2022, and (iii) the implied valuation multiples derived from its review of certain publicly-traded companies and certain business combination transactions described more fully in this section will not change through December 31, 2022 (such assumptions set forth in clauses (i) through (iii), the “Sensitivity Assumptions”). The resulting ranges of implied EVs of Paradigm based on the mean and median for each valuation metric, as compared to the Aggregate Consideration sensitivities, are as follows:
| Implied Enterprise Value of Paradigm (in millions) | ||||
2022 revenue (mean-median range) | $242 - $348 | |||
2023 revenue (mean-median range) | $250 - $355 | |||
2022 gross profit (mean-median range) | $334 - $442 | |||
2023 gross profit (mean-median range) | $344 - $447 | |||
Aggregate Consideration (Full Earnout) | $300 | |||
Aggregate Consideration (Stock Price Doubles) | $405 | |||
Aggregate Consideration (Stock Price Triples) | $525 | |||
Medical Technology—Low Growth Financial Profile
Piper Jaffray reviewed projected financial data for Paradigm prepared by management of RTI for the years ending December 31, 2018 and 2019, and compared such data to corresponding Wall Street consensus research estimates for publicly traded U.S.-based low growth companies in the medical technology industry that Piper Jaffray believed were comparable to Paradigm’s financial profile because of their low growth prospects, which are consistent with Paradigm’s historical financial performance. Piper Jaffray selected U.S.-listed public companies that it considered to be low growth medical technology companies that had: (i) revenue for the latest twelve month period for which financial information was publicly available (“TTM”) greater than $25 million and less than $500 million, (ii) projected 2018 and 2019 revenue growth of less than 10%, and (iii) TTM gross margin greater than 60%.
Piper Jaffray selected the following companies:
| • | Anika Therapeutics, Inc. |
| • | Endologix, Inc. |
| • | LeMaitre Vascular, Inc. |
| • | Orthofix Holdings, Inc. |
87
| • | SeaSpine Holdings Corporation |
| • | Xtant Medical Holdings, Inc. |
For the selected financial profile public company analysis, Piper Jaffray compared, among other things, implied multiples of projected 2018 and 2019 EV/revenue and EV/gross profit for Paradigm, based on the Aggregate Consideration, to the corresponding implied EV multiples for the selected public companies. Projected 2018 and 2019 revenue and gross profit for Paradigm were based on the RTI adjusted Paradigm financial projections. Projected 2018 and 2019 revenue and gross profit for the selected public companies were based on Wall Street consensus research estimates, public filings and press releases of such companies.
The analysis indicated the following multiples:
| Paradigm | Selected Publicly Traded U.S.-Based Low Growth Med Tech Companies | |||||||||||||||||||||||||||||||
| Upfront(1) | NPV(2) | Maximum | 75th% | Mean | Median | 25th% | Minimum | |||||||||||||||||||||||||
EV to projected 2018 revenue | 3.7x | 6.4x | 5.0x | 3.9x | 2.8x | 2.2x | 1.9x | 1.6x | ||||||||||||||||||||||||
EV to projected 2019 revenue | 2.6x | 4.5x | 4.6x | 3.6x | 2.6x | 2.2x | 1.8x | 1.6x | ||||||||||||||||||||||||
EV to projected 2018 gross profit | 4.2x | 7.3x | 7.0x | 5.7x | 4.0x | 3.1x | 2.9x | 2.4x | ||||||||||||||||||||||||
EV to projected 2019 gross profit | 2.9x | 5.1x | 6.4x | 5.1x | 3.7x | 3.1x | 2.7x | 2.3x | ||||||||||||||||||||||||
| (1) | Based on the Upfront Aggregate Consideration. |
| (2) | Based on the NPV Aggregate Consideration. |
Based on this analysis, with respect to the Upfront Aggregate Consideration, Piper Jaffray noted that, with respect to Paradigm, (i) each of the EV/2018 revenue and EV/2018 gross profit multiples fell between the 75th percentile and the mean range of implied EV multiples for the selected public companies, (ii) the EV/2019 revenue multiple fell at the mean of implied EV multiples for the selected public companies, and (iii) the EV/2019 gross profit multiple fell between the median and the 25th percentile range of implied EV multiples for the selected public companies.
Based on this analysis, with respect to the NPV Aggregate Consideration, Piper Jaffray noted that, with respect to Paradigm, (i) each of the EV/2018 revenue and EV/2018 gross profit multiples fell above the “Maximum” of implied EV multiples for the selected public companies, (ii) the EV/2019 revenue multiple fell between the “Maximum” and the 75th percentile range of implied EV multiples for the selected public companies, and (iii) the EV/2019 gross profit multiple fell at the 75th percentile of implied EV multiples for the selected public companies.
In addition, Piper Jaffray observed that the range of implied EVs of Paradigm based on the mean and median for each valuation metric yielded the following, as compared to the Aggregate Consideration:
| Implied Enterprise Value of Paradigm (in millions) | ||
2018 revenue (mean-median range) | $90 - $113 | |
2019 revenue (mean-median range) | $127 - $152 | |
2018 gross profit (mean-median range) | $112 - $143 | |
2019 gross profit (mean-median range) | $158 - $191 | |
Upfront Aggregate Consideration | $150 | |
NPV Aggregate Consideration | $262 |
Additionally, Piper Jaffray performed an illustrative, theoretical valuation sensitivity analysis referred to above. For purposes of such sensitivity analysis, Piper Jaffray used the Sensitivity Assumptions described above. The resulting
88
ranges of implied EVs of Paradigm based on the mean and median for each valuation metric, as compared to the Aggregate Consideration sensitivities, are as follows:
| Implied Enterprise Value of Paradigm (in millions | ||||
2022 revenue (mean-median range) | $269 - $338 | |||
2023 revenue (mean-median range) | $292 - $350 | |||
2022 gross profit (mean-median range) | $342 - $434 | |||
2023 gross profit (mean-median range) | $368 - $444 | |||
Aggregate Consideration (Full Earnout) | $300 | |||
Aggregate Consideration (Stock Price Doubles) | $405 | |||
Aggregate Consideration (Stock Price Triples) | $525 | |||
Medical Technology—High Growth Financial Profile
Piper Jaffray reviewed projected financial data for Paradigm prepared by management of RTI for the years ending December 31, 2018 and 2019, and compared such data to corresponding Wall Street consensus research estimates for publicly traded U.S.-based high growth companies in the medical technology industry that Piper Jaffray believed were comparable to Paradigm’s financial profile because of their high growth prospects, which are consistent with Paradigm’s projected future financial performance. Piper Jaffray selected U.S.-listed public companies that it considered to be high growth medical technology companies that had: (i) TTM revenue greater than $25 million and less than $500 million, (ii) projected 2018 and 2019 revenue growth greater than 15% and less than 50%, and (iii) TTM gross margin greater than 60%.
Piper Jaffray selected the following companies:
| • | AxoGen, Inc.(1)(2)(3) |
| • | Insulet Corporation |
| • | Iradimed Corporation |
| • | iRhythm Technologies, Inc. (1)(3) |
| • | Misonix, Inc. |
| • | Neuronetics, Inc. |
| • | NovoCure Ltd. (3) |
| • | OrthoPediatrics Corp. |
| • | Penumbra, Inc. |
| • | STAAR Surgical Company(1)(3)(4) |
| • | Tactile Systems Technology, Inc. |
| (1) | EV/TTM revenue was deemed not material if greater than 15.0x. |
| (2) | EV/2018 revenue was deemed not material if greater than 15.0x. |
| (3) | EV/TTM gross profit was deemed not material if greater than 20.0x. |
| (4) | EV/2018 gross profit was deemed not material if greater than 20.0x. |
For the selected financial profile public company analysis, Piper Jaffray compared, among other things, implied multiples of projected 2018 and 2019 EV/revenue and EV/gross profit for Paradigm, based on the Aggregate Consideration, to the corresponding implied EV multiples for the selected public companies. Projected 2018 and 2019 revenue and gross profit for Paradigm were based on the RTI adjusted Paradigm financial projections. Projected 2018 and 2019 revenue and gross profit for the selected public companies were based on Wall Street consensus research estimates, public filings and press releases of such companies.
89
The analysis indicated the following multiples:
| Paradigm | Selected Publicly Traded U.S.-Based High Growth Med Tech Companies | |||||||||||||||||||||||||||||||
| Upfront(1) | NPV(2) | Maximum | 75th% | Mean | Median | 25th% | Minimum | |||||||||||||||||||||||||
EV to projected 2018 revenue | 3.7x | 6.4x | 14.7x | 13.6x | 10.1x | 9.8x | 7.5x | 4.0x | ||||||||||||||||||||||||
EV to projected 2019 revenue | 2.6x | 4.5x | 12.4x | 10.3x | 8.3x | 7.7x | 6.3x | 3.4x | ||||||||||||||||||||||||
EV to projected 2018 gross profit | 4.2x | 7.3x | 19.9x | 19.1x | 14.1x | 13.9x | 10.0x | 5.8x | ||||||||||||||||||||||||
EV to projected 2019 gross profit | 2.9x | 5.1x | 16.4x | 13.9x | 11.2x | 11.6x | 9.0x | 4.9x | ||||||||||||||||||||||||
| (1) | Based on the Upfront Aggregate Consideration. |
| (2) | Based on the NPV Aggregate Consideration. |
Based on this analysis, with respect to the Upfront Aggregate Consideration, Piper Jaffray noted that, with respect to Paradigm, each of the EV/revenue and EV/gross profit multiples fell below the “Minimum” of implied EV multiples for the selected public companies.
Based on this analysis, with respect to the NPV Aggregate Consideration, Piper Jaffray noted that, with respect to Paradigm, each of the EV/revenue and EV/gross profit multiples fell between the 25th percentile range and the “Minimum” of implied EV multiples for the selected public companies.
In addition, Piper Jaffray observed that the range of implied EVs of Paradigm based on the mean and median for each valuation metric yielded the following, as compared to the Aggregate Consideration:
| Implied Enterprise Value of Paradigm (in millions) | ||||
2018 revenue (mean-median range) | $402 - $413 | |||
2019 revenue (mean-median range) | $449 - $481 | |||
2018 gross profit (mean-median range) | $497 - $503 | |||
2019 gross profit (mean-median range) | $572 - $592 | |||
Upfront Aggregate Consideration | $150 | |||
NPV Aggregate Consideration | $262 | |||
Additionally, Piper Jaffray performed an illustrative, theoretical valuation sensitivity analysis referred to above. For purposes of such sensitivity analysis, Piper Jaffray used the Sensitivity Assumptions described above. The resulting ranges of implied EVs of Paradigm based on the mean and median for each valuation metric, as compared to the Aggregate Consideration sensitivities, are as follows:
| Implied Enterprise Value of Paradigm (in millions) | ||||
2022 revenue (mean-median range) | $1,201 - $1,233 | |||
2023 revenue (mean-median range) | $1,035 - $1,110 | |||
2022 gross profit (mean-median range) | $1,514 - $1,531 | |||
2023 gross profit (mean-median range) | $1,331 - $1,379 | |||
Aggregate Consideration (Full Earnout) | $300 | |||
Aggregate Consideration (Stock Price Doubles) | $405 | |||
Aggregate Consideration (Stock Price Triples) | $525 | |||
90
Selected M&A Transaction Analysis
Medical Technology—Spine PMA Business Profile
Piper Jaffray reviewed merger and acquisition (“M&A”) transactions involving target companies in the medical technology industry that Piper Jaffray believed were comparable to Paradigm’s business profile. Piper Jaffray selected transactions involving spine focused PMA device companies in the medical technology industry as targets that had one or more PMA approved or pending spine products.
Based on these criteria, the following six transactions were selected:
Target | Acquiror | Date of Transaction Announcement | ||
| LDR Holding Corp | Zimmer Biomet Holdings, Inc. | June 7, 2016 | ||
| Kyphon, Inc. | Medtronic Inc. | July 27, 2007 | ||
| St. Francis Medical Technologies, Inc. | Kyphon, Inc. | December 4, 2006 | ||
| Link Spine Group, Inc. | Johnson & Johnson — DePuy Acromed, Inc. | May 6, 2003 | ||
| Sofamor Danek Group Inc. | Medtronic Inc. | November 2, 1998 | ||
| Spine-Tech Inc. | Sulzer Medica Ltd. | December 16, 1997 |
For the selected M&A transactions analysis, Piper Jaffray compared, among other things, implied EV/TTM revenue and EV/TTM gross profit multiples for Paradigm, based on the Aggregate Consideration, to the corresponding multiples for each selected transaction, as well as Paradigm’s implied multiples of EV/projected revenue for the 12 month period immediately following the TTM period (“FTM”) and EV/projected FTM gross profit, based on the Aggregate Consideration, to the corresponding multiples for each selected transaction. TTM revenues and TTM gross profit for (i) Paradigm were based on historical financial data for the 12 months ended September 30, 2018 and (ii) the selected target companies were based on publicly available historical data as of the respective announcement date of the applicable M&A transaction. Projected FTM revenues and FTM gross profit for Paradigm were for the 12 months beginning September 30, 2018 and were based on the RTI adjusted Paradigm financial projections, and (ii) for the selected transactions were based on selected Wall Street research estimates available as of the respective announcement date of the applicable M&A transaction.
The analysis indicated the following multiples:
| Paradigm | Selected M&A Transactions | |||||||||||||||||||||||||||||||
| Upfront(1) | NPV(2) | Maximum | 75th% | Mean | Median | 25th% | Minimum | |||||||||||||||||||||||||
EV to TTM revenue | 3.6x | 6.3x | 13.2x | 13.2x | 10.5x | 11.2x | 7.6x | 6.1x | ||||||||||||||||||||||||
EV to FTM revenue | 2.8x | 4.9x | 7.6x | 7.5x | 6.4x | 6.2x | 5.7x | 5.3x | ||||||||||||||||||||||||
EV to TTM gross profit | 4.1x | 7.2x | 17.1x | 15.7x | 12.6x | 13.1x | 8.9x | 7.4x | ||||||||||||||||||||||||
EV to FTM gross profit | 3.2x | 5.6x | 9.2x | 9.0x | 7.7x | 7.5x | 6.6x | 6.3x | ||||||||||||||||||||||||
| (1) | Based on the Upfront Aggregate Consideration. |
| (2) | Based on the NPV Aggregate Consideration. |
Based on this analysis, with respect to the Upfront Aggregate Consideration, Piper Jaffray noted that, with respect to Paradigm, each of the EV/revenue and EV/gross profit multiples fell below the “Minimum” of implied EV multiples for the selected M&A transactions.
Based on this analysis, with respect to the NPV Aggregate Consideration, Piper Jaffray noted that, with respect to Paradigm, (i) the EV/TTM revenue multiple fell between the 25th percentile range and the “Minimum” of implied EV multiples for the selected M&A transactions, and (ii) the EV/FTM revenue multiple and each of the EV/gross profit multiples fell below the “Minimum” of implied EV multiples for the selected M&A transactions.
91
In addition, Piper Jaffray observed that the range of implied EVs of Paradigm based on the mean and median for each valuation metric yielded the following, as compared to the Aggregate Consideration:
| Implied Enterprise Value of Paradigm (in millions | ||||
TTM revenue (mean-median range) | $ | 435 - $464 | ||
FTM revenue (mean-median range) | $331 - $344 | |||
TTM gross profit (mean-median range) | $456 - $475 | |||
FTM gross profit (mean-median range) | $355 - $363 | |||
Upfront Aggregate Consideration | $150 | |||
NPV Aggregate Consideration | $262 | |||
Additionally, Piper Jaffray performed an illustrative, theoretical valuation sensitivity analysis referred to above. For purposes of such sensitivity analysis, Piper Jaffray used the Sensitivity Assumptions described above. The resulting ranges of implied EVs of Paradigm based on the mean and median for each valuation metric, as compared to the Aggregate Consideration sensitivities, are as follows:
| Implied Enterprise Value of Paradigm (in millions) | ||||
TTM revenue (mean-median range) | $1,283 - $1,367 | |||
FTM revenue (mean-median range) | $825 - $859 | |||
TTM gross profit (mean-median range) | $1,364 - $1,422 | |||
FTM gross profit (mean-median range) | $897 - $916 | |||
Aggregate Consideration (Full Earnout) | $300 | |||
Aggregate Consideration (Stock Price Doubles) | $405 | |||
Aggregate Consideration (Stock Price Triples) | $525 | |||
Medical Technology—Spine Business Profile
Piper Jaffray reviewed M&A transactions involving target companies in the medical technology industry that Piper Jaffray believed were comparable to Paradigm’s business profile. Piper Jaffray selected transactions that were announced after January 1, 2010 involving spine focused companies in the medical technology industry as targets.
Based on these criteria, the following 16 transactions were selected:
Target | Acquiror | Date of Transaction Announcement | ||
| Mazor Robotics Ltd. | Medtronic Public Limited Company | September 25, 2018 | ||
| K2M Group Holdings, Inc. | Stryker Corporation | August 31, 2018 | ||
| Vexim SA | Stryker Corporation | October 24, 2017 | ||
| Medtech SA(1) | Zimmer Biomet Holdings, Inc. | July 18, 2016 | ||
| DFINE, Inc. | Merit Medical Systems, Inc. | July 6, 2016 | ||
| LDR Holding Corp | Zimmer Biomet Holdings, Inc. | June 7, 2016 | ||
| Ellipse Technologies, Inc. | NuVasive, Inc. | January 5, 2016 | ||
| X-spine Systems, Inc. | Bacterin International, Inc. | July 28, 2015 | ||
| Lanx, Inc. | Biomet, Inc. | October 7, 2013 | ||
| Pioneer Surgical Technology, Inc. | RTI Biologics Inc. | June 12, 2013 | ||
| Impulse Monitoring, Inc. | NuVasive, Inc. | September 28, 2011 | ||
| Salient Surgical Technologies, Inc. | Medtronic Inc. | July 7, 2011 | ||
| SeaSpine, Inc. | Integra LifeSciences Holdings Corporation | May 24, 2011 | ||
| Orthovita, Inc. | Stryker Corporation | May 16, 2011 | ||
| Osteotech, Inc. | Medtronic Inc. | August 17, 2010 | ||
| K2M Group Holdings, Inc. | Welsh, Carson, Anderson & Stowe XI, L.P. | July 8, 2010 |
| (1) | EV/TTM gross profit and EV/FTM gross profit multiples were not available. |
For the selected M&A transactions analysis, Piper Jaffray compared, among other things, implied EV/TTM revenue and EV/TTM gross profit multiples for Paradigm, based on the Aggregate Consideration, to the corresponding
92
multiples for each selected transaction, as well as Paradigm’s implied multiples of EV/projected FTM revenue and EV/projected FTM gross profit, based on the Aggregate Consideration, to the corresponding multiples for each selected transaction. TTM revenues and TTM gross profit for (i) Paradigm were based on historical financial data for the 12 months ended September 30, 2018 and (ii) the selected target companies were based on publicly available historical data as of the respective announcement date of the applicable M&A transaction. Projected FTM revenues and FTM gross profit for (i) Paradigm were for the 12 months beginning September 30, 2018 and were based on the RTI adjusted Paradigm financial projections, and (ii) for the selected transactions were based on selected Wall Street research estimates available as of the respective announcement date of the applicable M&A transaction.
The analysis indicated the following multiples:
| Paradigm | Selected M&A Transactions | |||||||||||||||||||||||||||||||
| Upfront(1) | NPV(2) | Maximum | 75th% | Mean | Median | 25th% | Minimum | |||||||||||||||||||||||||
EV to TTM revenue | 3.6x | 6.3x | 23.4x | 7.5x | 5.5x | 3.3x | 1.8x | 1.3x | ||||||||||||||||||||||||
EV to FTM revenue | 2.8x | 4.9x | 20.7x | 5.3x | 4.3x | 2.7x | 1.6x | 1.2x | ||||||||||||||||||||||||
EV to TTM gross profit | 4.1x | 7.2x | 39.5x | 7.7x | 7.8x | 5.2x | 2.6x | 2.0x | ||||||||||||||||||||||||
EV to FTM gross profit | 3.2x | 5.6x | 35.0x | 6.7x | 6.4x | 4.4x | 2.3x | 1.8x | ||||||||||||||||||||||||
| (1) | Based on the Upfront Aggregate Consideration. |
| (2) | Based on the NPV Aggregate Consideration. |
Based on this analysis, with respect to the Upfront Aggregate Consideration, Piper Jaffray noted that, with respect to Paradigm, (i) each of the EV/revenue multiples fell between the mean and the median range of implied EV multiples for the selected M&A transactions, and (ii) each of the EV/gross profit multiples fell between the 25th percentile and the median range of implied EV multiples for the selected M&A transactions.
Based on this analysis, with respect to the NPV Aggregate Consideration, Piper Jaffray noted that, with respect to Paradigm, (i) each of the EV/revenue multiples fell between the 75th percentile and the mean range of implied EV multiples for the selected M&A transactions, and (ii) each of the EV/gross profit multiples fell between the mean and the median range of implied EV multiples for the selected M&A transactions.
In addition, Piper Jaffray observed that the range of implied EVs of Paradigm based on the mean and median for each valuation metric yielded the following, as compared to the Aggregate Consideration:
| Implied Enterprise Value of Paradigm (in millions) | ||
TTM revenue (mean-median range) | $138 - $229 | |
FTM revenue (mean-median range) | $147 - $232 | |
TTM gross profit (mean-median range) | $188 - $282 | |
FTM gross profit (mean-median range) | $207 - $302 | |
Upfront Aggregate Consideration | $150 | |
NPV Aggregate Consideration | $262 |
93
Additionally, Piper Jaffray performed an illustrative, theoretical valuation sensitivity analysis referred to above. For purposes of such sensitivity analysis, Piper Jaffray used the Sensitivity Assumptions described above. The resulting ranges of implied EVs of Paradigm based on the mean and median for each valuation metric, as compared to the Aggregate Consideration sensitivities, are as follows:
| Implied Enterprise Value of Paradigm (in millions) | ||
TTM revenue (mean-median range) | $407 - $674 | |
FTM revenue (mean-median range) | $365 - $578 | |
TTM gross profit (mean-median range) | $562 - $843 | |
FTM gross profit (mean-median range) | $522 - $762 | |
Aggregate Consideration (Full Earnout) | $300 | |
Aggregate Consideration (Stock Price Doubles) | $405 | |
Aggregate Consideration (Stock Price Triples) | $525 |
Medical Technology—PMA
Piper Jaffray reviewed M&A transactions involving target companies in the medical technology industry that Piper Jaffray believed were comparable to Paradigm’s business profile. Piper Jaffray selected transactions that were announced after January 1, 2010 involving companies in the medical technology industry as targets that had one or more PMA approved or pending products.
Based on these criteria, the following twelve (12) transactions were selected:
Target | Acquiror | Date of Transaction Announcement | ||
| Cartiva, Inc. | Wright Medical Group N.V. | August 27, 2018 | ||
| LDR Holding Corp | Zimmer Biomet Holdings, Inc. | June 7, 2016 | ||
| On-X Life Technologies Holdings, Inc. | CryoLife, Inc. | December 22, 2015 | ||
| Thoratec Corporation | St. Jude Medical, Inc. | July 22, 2015 | ||
| Endo — AMS Urology Business | Boston Scientific Corporation | March 2, 2015 | ||
| Small Bone Innovations, Inc. | Stryker Corporation | June 30, 2014 | ||
| IDEV Technologies, Inc. | Abbott Laboratories | July 15, 2013 | ||
| Conceptus, Inc. | Bayer Healthcare LLC | April 29, 2013 | ||
| Neomend, Inc.(1) | C.R. Bard, Inc. | October 23, 2012 | ||
| AGA Medical Holdings, Inc. | St. Jude Medical, Inc. | October 18, 2010 | ||
| ATS Medical, Inc. | Medtronic Inc. | April 29, 2010 | ||
| Invatec S.p.A. | Medtronic Inc. | January 25, 2010 |
| (1) | EV/FTM revenue and EV/gross profit were not available. |
For the selected M&A transactions analysis, Piper Jaffray compared, among other things, implied EV/TTM revenue and EV/TTM gross profit multiples for Paradigm, based on the Aggregate Consideration, to the corresponding multiples for each selected transaction, as well as Paradigm’s implied multiples of EV/projected FTM revenue and EV/projected FTM gross profit, based on the Aggregate Consideration, to the corresponding multiples for each selected transaction. TTM revenues and TTM gross profit for (i) Paradigm were based on historical financial data for the 12 months ended September 30, 2018 and (ii) the selected target companies were based on publicly available historical data as of the respective announcement date of the applicable M&A transaction. Projected FTM revenues and FTM gross profit for (i) Paradigm were for the 12 months beginning September 30, 2018 and were based on the RTI adjusted Paradigm financial projections, and (ii) for the selected transactions were based on selected Wall Street research estimates available as of the respective announcement date of the applicable M&A transaction.
94
The analysis indicated the following multiples:
| Paradigm | Selected M&A Transactions | |||||||||||||||||||||||||||||||
| Upfront(1) | NPV(2) | Maximum | 75th% | Mean | Median | 25th% | Minimum | |||||||||||||||||||||||||
EV to TTM revenue | 3.6x | 6.3x | 18.6x | 8.2x | 7.2x | 6.8x | 4.2x | 3.3x | ||||||||||||||||||||||||
EV to FTM revenue | 2.8x | 4.9x | 12.4x | �� | 6.8x | 5.7x | 5.3x | 4.1x | 2.7x | |||||||||||||||||||||||
EV to TTM gross profit | 4.1x | 7.2x | 19.6x | 10.1x | 8.9x | 7.4x | 6.5x | 4.9x | ||||||||||||||||||||||||
EV to FTM gross profit | 3.2x | 5.6x | 13.1x | 8.9x | 7.1x | 6.3x | 5.3x | 4.0x | ||||||||||||||||||||||||
| (1) | Based on the Upfront Aggregate Consideration. |
| (2) | Based on the NPV Aggregate Consideration. |
Based on this analysis, with respect to the Upfront Aggregate Consideration, Piper Jaffray noted that, with respect to Paradigm, (i) each of the EV/revenue multiples fell between the 25th percentile and the “Minimum” of implied EV multiples for the selected M&A transactions, and (ii) each of the EV/gross profit multiples fell below the “Minimum” of implied EV multiples for the selected M&A transactions.
Based on this analysis, with respect to the NPV Aggregate Consideration, Piper Jaffray noted that, with respect to Paradigm, each of the EV/revenue and EV/gross profit multiples fell between the 25th percentile and the median range of implied EV multiples for the selected M&A transactions.
In addition, Piper Jaffray observed that the range of implied EVs of Paradigm based on the mean and median for each valuation metric yielded the following, as compared to the Aggregate Consideration:
| Implied Enterprise Value of Paradigm (in millions) | ||||
TTM revenue (mean-median range) | $282 - $298 | |||
FTM revenue (mean-median range) | $284 - $305 | |||
TTM gross profit (mean-median range) | $268 - $323 | |||
FTM gross profit (mean-median range) | $299 - $336 | |||
Upfront Aggregate Consideration | $150 | |||
NPV Aggregate Consideration | $262 | |||
Additionally, Piper Jaffray performed an illustrative, theoretical valuation sensitivity analysis referred to above. For purposes of such sensitivity analysis, Piper Jaffray used the Sensitivity Assumptions described above. The resulting ranges of implied EVs of Paradigm based on the mean and median for each valuation metric, as compared to the Aggregate Consideration sensitivities, are as follows:
| Implied Enterprise Value of Paradigm (in millions) | ||||
TTM revenue (mean-median range) | $833 - $879 | |||
FTM revenue (mean-median range) | $709 - $760 | |||
TTM gross profit (mean-median range) | $802 - $966 | |||
FTM gross profit (mean-median range) | $755 - $848 | |||
Aggregate Consideration (Full Earnout) | $300 | |||
Aggregate Consideration (Stock Price Doubles) | $405 | |||
Aggregate Consideration (Stock Price Triples) | $525 | |||
Medical Technology—Low Growth Financial Profile
Piper Jaffray reviewed M&A transactions involving low growth target companies in the medical technology industry that Piper Jaffray believed were comparable to Paradigm’s financial profile because of their low growth
95
prospects, which are consistent with Paradigm’s historical financial performance. Piper Jaffray selected transactions that were announced after January 1, 2010 involving low growth companies in the medical technology industry as targets that (i) had TTM revenue greater than $25 million and less than $500 million, (ii) had FTM revenue growth of less than 10%, and (iii) had TTM gross margin greater than 60%. Piper Jaffray excluded asset and product line divestitures.
Based on these criteria, the following 9 transactions were selected:
Target | Acquiror | Date of Transaction Announcement | ||
| Exactech, Inc. | TPG Capital, L.P. | October 23, 2017 | ||
| TEI Biosciences Inc., and TEI Medical Inc. | Integra LifeSciences Holdings Corporation | June 29, 2015 | ||
| Volcano Corporation | Koninklijke Philips N.V. | December 17, 2014 | ||
| AngioScore Inc. | Spectranetics Corporation | May 27, 2014 | ||
| ArthroCare Corp. | Smith & Nephew plc | February 3, 2014 | ||
| Solta Medical, Inc. | Valeant Pharmaceuticals International, Inc. | December 16, 2013 | ||
| Orthovita, Inc. | Stryker Corporation | May 16, 2011 | ||
| Somanetics Corporation | Covidien plc | June 16, 2010 | ||
| ATS Medical, Inc. | Medtronic Inc. | April 29, 2010 |
For the selected M&A transactions analysis, Piper Jaffray compared, among other things, implied EV/TTM revenue and EV/TTM gross profit multiples for Paradigm, based on the Aggregate Consideration, to the corresponding multiples for each selected transaction, as well as Paradigm’s implied multiples of EV/projected FTM revenue and EV/projected FTM gross profit, based on the Aggregate Consideration, to the corresponding multiples for each selected transaction. TTM revenues and TTM gross profit for (i) Paradigm were based on historical financial data for the 12 months ended September 30, 2018, and (ii) the selected target companies were based on publicly available historical data as of the respective announcement date of the applicable M&A transaction. Projected FTM revenues and FTM gross profit for (i) Paradigm were for the 12 months beginning September 30, 2018 and were based on the RTI adjusted Paradigm financial projections, and (ii) for the selected transactions were based on selected Wall Street research estimates available as of the respective announcement date of the applicable M&A transaction.
The analysis indicated the following multiples:
| Paradigm | Selected M&A Transactions | |||||||||||||||||||||||||||||||
| Upfront(1) | NPV(2) | Maximum | 75th% | Mean | Median | 25th% | Minimum | |||||||||||||||||||||||||
EV to TTM revenue | 3.6x | 6.3x | 5.3x | 4.7x | 3.6x | 3.3x | 2.9x | 1.7x | ||||||||||||||||||||||||
EV to FTM revenue | 2.8x | 4.9x | 5.0x | 4.3x | 3.4x | 3.1x | 2.8x | 1.6x | ||||||||||||||||||||||||
EV to TTM gross profit | 4.1x | 7.2x | 6.8x | 6.1x | 5.2x | 5.2x | 4.3x | 2.6x | ||||||||||||||||||||||||
EV to FTM gross profit | 3.2x | 5.6x | 6.2x | 5.6x | 4.7x | 4.8x | 4.1x | 2.4x | ||||||||||||||||||||||||
| (1) | Based on the Upfront Aggregate Consideration. |
| (2) | Based on the NPV Aggregate Consideration. |
Based on this analysis, with respect to the Upfront Aggregate Consideration, Piper Jaffray noted that, with respect to Paradigm, (i) the EV/TTM revenue multiple fell at the mean of implied EV multiples for the selected M&A transactions, (ii) the EV/FTM revenue multiple fell at the 25th percentile range of implied EV multiples for the selected M&A transactions, and (iii) each of the EV/gross profit multiples fell between the 25th percentile and “Minimum” of implied EV multiples for the selected M&A transactions.
Based on this analysis, with respect to the NPV Aggregate Consideration, Piper Jaffray noted that, with respect to Paradigm, (i) each of the EV/TTM revenue and the EV/TTM gross profit multiples fell above the “Maximum” of implied EV multiples for the selected M&A transactions, (ii) the EV/FTM revenue multiple fell between the
96
“Maximum” and the 75th percentile range of implied EV multiples for the selected M&A transactions, and (iii) the EV/FTM gross profit multiple fell at the 75th percentile range of implied EV multiples for the selected M&A transactions.
In addition, Piper Jaffray observed that the range of implied EVs of Paradigm based on the mean and median for each valuation metric yielded the following, as compared to the Aggregate Consideration:
| Implied Enterprise Value of Paradigm (in millions) | ||
TTM revenue (mean-median range) | $135 - $151 | |
FTM revenue (mean-median range) | $165 - $184 | |
TTM gross profit (mean-median range) | $187 - $189 | |
FTM gross profit (mean-median range) | $223 - $228 | |
Upfront Aggregate Consideration | $150 | |
NPV Aggregate Consideration | $262 |
Additionally, Piper Jaffray performed an illustrative, theoretical valuation sensitivity analysis referred to above. For purposes of such sensitivity analysis, Piper Jaffray used the Sensitivity Assumptions described above. The resulting ranges of implied EVs of Paradigm based on the mean and median for each valuation metric, as compared to the Aggregate Consideration sensitivities, are as follows:
| Implied Enterprise Value of Paradigm (in millions) | ||||
TTM revenue (mean-median range) | $398 - $444 | |||
FTM revenue (mean-median range) | $410 - $458 | |||
TTM gross profit (mean-median range) | $561 - $567 | |||
FTM gross profit (mean-median range) | $564 - $577 | |||
Aggregate Consideration (Full Earnout) | $300 | |||
Aggregate Consideration (Stock Price Doubles) | $405 | |||
Aggregate Consideration (Stock Price Triples) | $525 | |||
Medical Technology—High Growth Financial Profile
Piper Jaffray reviewed M&A transactions involving high growth target companies in the medical technology industry that Piper Jaffray believed were comparable to Paradigm’s financial profile because of their high growth prospects, which are consistent with Paradigm’s projected future financial performance. Piper Jaffray selected transactions that were announced after January 1, 2010 involving high growth companies in the medical technology industry as targets that (i) had TTM revenue greater than $25 million and less than $500 million, (ii) had FTM revenue growth of greater than 15% and less than 50%, and (iii) had TTM gross margin greater than 60%. Piper Jaffray excluded asset and product line divestitures.
Based on these criteria, the followingtwenty-six (26) transactions were selected:
Target | Acquiror | Date of Transaction Announcement | ||
| Cianna Medical, Inc. | Merit Medical Systems, Inc. | October 1, 2018 | ||
| Invuity, Inc. | Stryker Corporation | September 11, 2018 | ||
| Cogentix Medical, Inc. | Laborie Medical Technologies, Inc. | March 12, 2018 | ||
| Entellus Medical, Inc. | Stryker Corporation | December 7, 2017 | ||
| NOVADAQ Technologies Inc. | Stryker Corporation | June 19, 2017 | ||
| ZELTIQ Aesthetics, Inc. | Allergan plc | February 13, 2017 | ||
| DFINE, Inc. | Merit Medical Systems, Inc. | July 6, 2016 | ||
| LDR Holding Corp | Zimmer Biomet Holdings, Inc. | June 7, 2016 | ||
| Ellipse Technologies, Inc. | NuVasive, Inc. | January 5, 2016 | ||
| On-X Life Technologies Holdings, Inc. | CryoLife, Inc. | December 22, 2015 | ||
| TriVascular Technologies, Inc. | Endologix, Inc. | October 26, 2015 | ||
| Thoratec Corporation | St. Jude Medical, Inc. | July 22, 2015 |
97
Target | Acquiror | Date of Transaction Announcement | ||
| MAKO Surgical Corp. | Stryker Corporation | September 25, 2013 | ||
| IDEV Technologies, Inc. | Abbott Laboratories | July 15, 2013 | ||
| Conceptus, Inc. | Bayer Healthcare LLC | April 29, 2013 | ||
| Kensey Nash Corp. | Koninklijke DSM N.V. | May 3, 2012 | ||
| Synovis Life Technologies, Inc. | Baxter International Inc. | January 1, 2012 | ||
| SonoSite, Inc. | FUJIIM Holdings Corporation | December 15, 2011 | ||
| Salient Surgical Technologies, Inc. | Medtronic Inc. | July 7, 2011 | ||
| Advanced Biohealing, Inc. | Shire plc | May 18, 2011 | ||
| Biocompatibles International plc | BTG plc | November 19, 2010 | ||
| AGA Medical Holdings, Inc. | St. Jude Medical, Inc. | October 18, 2010 | ||
| K2M Group Holdings, Inc. | Welsh, Carson, Anderson & Stowe XI, L.P. | July 8, 2010 | ||
| ev3 Inc. | Covidien plc | June 1, 2010 | ||
| SenoRx, Inc. | C.R. Bard, Inc. | May 4, 2010 | ||
| Invatec S.p.A. | Medtronic Inc. | January 25, 2010 |
For the selected M&A transactions analysis, Piper Jaffray compared, among other things, implied EV/TTM revenue and EV/TTM gross profit multiples for Paradigm, based on the Aggregate Consideration, to the corresponding multiples for each selected transaction, as well as Paradigm’s implied multiples of EV/projected FTM revenue and EV/projected FTM gross profit, based on the Aggregate Consideration, to the corresponding multiples for each selected transaction. TTM revenues and TTM gross profit for (i) Paradigm were based on historical financial data for the 12 months ended September 30, 2018 and (ii) the selected target companies were based on publicly available historical data as of the respective announcement date of the applicable M&A transaction. Projected FTM revenues and FTM gross profit for (i) Paradigm were for the 12 months beginning September 30, 2018 and were based on the RTI adjusted Paradigm financial projections, and (ii) for the selected transactions were based on selected Wall Street research estimates available as of the respective announcement date of the applicable M&A transaction.
The analysis indicated the following multiples:
| Paradigm | Selected M&A Transactions | |||||||||||||||||||||||||||||||
| Upfront(1) | NPV(2) | Maximum | 75th% | Mean | Median | 25th% | Minimum | |||||||||||||||||||||||||
EV to TTM revenue | 3.6x | 6.3x | 15.2x | 7.5x | 5.8x | 5.2x | 3.8x | 2.9x | ||||||||||||||||||||||||
EV to FTM revenue | 2.8x | 4.9x | 10.6x | 5.8x | 4.6x | 4.1x | 2.9x | 2.4x | ||||||||||||||||||||||||
EV to TTM gross profit | 4.1x | 7.2x | 22.5x | 9.7x | 8.0x | 6.9x | 5.5x | 3.9x | ||||||||||||||||||||||||
EV to FTM gross profit | 3.2x | 5.6x | 14.8x | 8.1x | 6.2x | 5.7x | 4.3x | 3.2x | ||||||||||||||||||||||||
| (1) | Based on the Upfront Aggregate Consideration. |
| (2) | Based on the NPV Aggregate Consideration. |
Based on this analysis, with respect to the Upfront Aggregate Consideration, Piper Jaffray noted that, with respect to Paradigm, (i) each of the EV/revenue multiples and the EV/TTM gross profit multiple fell between the 25th percentile and the “Minimum” of implied EV multiples for the selected M&A transactions and (ii) the EV/FTM gross profit multiple fell at the “Minimum” of implied EV multiples for the selected M&A transactions.
Based on this analysis, with respect to the NPV Aggregate Consideration, Piper Jaffray noted that, with respect to Paradigm, (i) each of the EV/revenue multiples fell between the 75th percentile and the mean range of implied EV multiples for the selected M&A transactions, (ii) the EV/TTM gross profit multiple fell between the mean and the median range of implied EV multiples for the selected M&A transactions, and (iii) the EV/FTM gross profit multiple fell between the 25th percentile and the median range of implied EV multiples for the selected M&A transactions.
98
In addition, Piper Jaffray observed that the range of implied EVs of Paradigm based on the mean and median for each valuation metric yielded the following, as compared to the Aggregate Consideration:
| Implied Enterprise Value of Paradigm (in millions) | ||||
TTM revenue (mean-median range) | $215 - $241 | |||
FTM revenue (mean-median range) | $222 - $247 | |||
TTM gross profit (mean-median range) | $252 - $291 | |||
FTM gross profit (mean-median range) | $267 - $293 | |||
Upfront Aggregate Consideration | $150 | |||
NPV Aggregate Consideration | $262 | |||
Additionally, Piper Jaffray performed an illustrative, theoretical valuation sensitivity analysis referred to above. For purposes of such sensitivity analysis, Piper Jaffray used the Sensitivity Assumptions described above. The resulting ranges of implied EVs of Paradigm based on the mean and median for each valuation metric, as compared to the Aggregate Consideration sensitivities, are as follows:
| Implied Enterprise Value of Paradigm (in millions | ||||
TTM revenue (mean-median range) | $633 - $712 | |||
FTM revenue (mean-median range) | $553 - $615 | |||
TTM gross profit (mean-median range) | $755 - $870 | |||
FTM gross profit (mean-median range) | $674 - $741 | |||
Aggregate Consideration (Full Earnout) | $300 | |||
Aggregate Consideration (Stock Price Doubles) | $405 | |||
Aggregate Consideration (Stock Price Triples) | $525 | |||
Discounted Cash Flows Analysis
Using a discounted cash flows analysis, Piper Jaffray calculated an estimated range of theoretical enterprise values for Paradigm based on the net present value of (i) projected unlevered free cash flows, including projected Synergies, but excluding the tax benefit of existing net operating losses, from September 30, 2018 to December 31, 2022, discounted back to September 30, 2018, and (ii) a projected terminal value at December 31, 2022 based upon revenue exit multiples ranging from 3.0x to 4.0x, discounted back to September 30, 2018. The free cash flows for each year and terminal year revenue were calculated from operating financial projections for such periods provided to Piper Jaffray by management of RTI, including the RTI adjusted Paradigm financial projections. Piper Jaffray calculated the range of net present values for unlevered free cash flows for such periods, as well as the present value of the projected terminal value, based on a range of discount rates ranging from 10.5% to 12.5%, based on its estimation of Paradigm’s weighted average cost of capital using the capital asset pricing model, together with a size premium.
Piper Jaffray observed that the range of implied EV of Paradigm based on the discounted cash flows analysis yielded the following, as compared to the Aggregate Consideration:
| Implied Enterprise Value of Paradigm (in millions) | ||||
Discounted cash flows | $252 - $351 | |||
Upfront Aggregate Consideration | $150 | |||
NPV Aggregate Consideration | $262 | |||
99
Miscellaneous
The summary set forth above does not contain a complete description of the analyses performed by Piper Jaffray and reviewed with the RTI board, but summarizes the material analyses performed by Piper Jaffray in rendering its opinion. The preparation of a fairness opinion is not necessarily susceptible to partial analysis or summary description. Piper Jaffray believes that its analyses and the summary set forth above must be considered as a whole and that selecting portions of its analyses or of the summary, without considering the analyses as a whole or all of the factors included in its analyses, would create an incomplete view of the processes underlying the analyses set forth in the Piper Jaffray opinion. In arriving at its opinion, Piper Jaffray considered the results of all of its analyses and did not attribute any particular weight to any factor or analysis. Instead, Piper Jaffray made its determination as to fairness on the basis of its experience and financial judgment after considering the results of all of its analyses. In addition, the ranges of valuations resulting from any particular analysis described above should not be taken to be Piper Jaffray’s view of the actual value of RTI common stock or Holdco common stock.
None of the selected companies or transactions used in the analyses above for purposes of comparison is identical to any of Holdco, RTI or Paradigm or the Transaction. Accordingly, an analysis of the results of the comparisons is not mathematical; rather, it involves considerations and judgments about differences in the companies and transactions to which each of Holdco, RTI and Paradigm and the Transaction were compared and other factors that could affect the public trading value or transaction value of the companies involved.
Piper Jaffray performed its analyses for purposes of providing its opinion to the RTI board. In performing its analyses, Piper Jaffray made numerous assumptions with respect to industry performance, general business and economic conditions and other matters. Certain of the analyses performed by Piper Jaffray are based upon financial projections of future results furnished to Piper Jaffray by the management of RTI, which are not necessarily indicative of actual future results and may be significantly more or less favorable than actual future results. These financial projections are inherently subject to uncertainty because, among other things, they are based upon numerous factors or events beyond the control of the parties or their respective advisors. Piper Jaffray does not assume responsibility if future results are materially different from projected financial results.
Piper Jaffray’s opinion was one of many factors taken into consideration by the RTI board in making the determination to approve the Master Transaction Agreement. While Piper Jaffray provided advice to the RTI board during RTI’s negotiations with PS Spine and Paradigm, Piper Jaffray did not recommend any specific amount or type of Aggregate Consideration.
Piper Jaffray relied upon and assumed, without assuming liability or responsibility for independent verification, the accuracy and completeness of all information that was publicly available or was furnished, or otherwise made available, to Piper Jaffray or discussed with or reviewed by Piper Jaffray. Piper Jaffray further relied upon the assurances of the management of RTI that the financial information provided to Piper Jaffray by the management of RTI was prepared on a reasonable basis in accordance with industry practice, and that the management of RTI was not aware of any information or facts that would make any information provided to Piper Jaffray incomplete or misleading. Without limiting the generality of the foregoing, for the purpose of its opinion, Piper Jaffray assumed that with respect to financial forecasts, estimates and other forward-looking information (including the Synergies) reviewed by Piper Jaffray, that such information was reasonably prepared based on assumptions reflecting the best currently available estimates and judgments of the management of RTI as to the expected future revenue of Zyga and future results of operations and financial condition of Paradigm. Piper Jaffray expressed no opinion as to any such financial forecasts, estimates or forward-looking information (including the Synergies) or the assumptions on which they were based. Piper Jaffray relied, with consent of the RTI board, on advice of the outside counsel and the independent accountants to RTI, and on the assumptions of the management of RTI, as to all accounting, legal, tax and financial reporting matters with respect to each of Holdco, RTI, Paradigm and the Master Transaction Agreement.
In arriving at its opinion, Piper Jaffray assumed that the executed Master Transaction Agreement would be in all material respects identical to the last draft reviewed by Piper Jaffray. Piper Jaffray relied upon and assumed, without
100
independent verification, that (i) the representations and warranties of all parties to the Master Transaction Agreement and all other related documents and instruments that are referred to therein are true and correct, (ii) each party to such agreements will fully and timely perform all of the covenants and agreements required to be performed by such party, (iii) the Transaction will be consummated pursuant to the terms of the Master Transaction Agreement without amendments thereto, (iv) all conditions to the consummation of the Transaction will be satisfied without waiver by any party of any conditions or obligations thereunder, and (v) any adjustments to any of the components of the Aggregate Consideration will not result in any adjustment to the Aggregate Consideration that is material to Piper Jaffray’s analysis. Additionally, Piper Jaffray assumed that all the necessary regulatory approvals and consents required for the Transaction will be obtained in a manner that would not adversely affect any of Holdco, RTI, Paradigm or the contemplated benefits of the Transaction.
In arriving at its opinion, Piper Jaffray did not perform any appraisals or valuations of any specific assets or liabilities (fixed, contingent or other) of any of Holdco, RTI or Paradigm, and Piper Jaffray was not furnished or provided with any such appraisals or valuations, nor did Piper Jaffray evaluate the solvency of any of Holdco, RTI or Paradigm under any state or federal law relating to bankruptcy, insolvency or similar matters. The analyses performed by Piper Jaffray in connection with its opinion were going concern analyses. Piper Jaffray expressed no opinion regarding the liquidation value of any of Holdco, RTI, Paradigm or any other entity. Without limiting the generality of the foregoing, Piper Jaffray undertook no independent analysis of any pending or threatened litigation, regulatory action, possible unasserted claims or other contingent liabilities, to which any of Holdco, RTI, Paradigm or any of its affiliates is a party or may be subject, and at RTI’s direction and with its consent, Piper Jaffray’s opinion made no assumption concerning, and therefore did not consider, the possible assertion of claims, outcomes or damages arising out of any such matters. Piper Jaffray also assumed that none of Holdco, RTI nor Paradigm is party to any material pending transaction, including without limitation, any financing, recapitalization, acquisition or merger, divestiture orspin-off, other than the Transaction.
Piper Jaffray’s opinion was necessarily based upon the information available to it and facts and circumstances as they existed and were subject to evaluation on the date of its opinion. Events occurring after the date of its opinion could materially affect the assumptions used in preparing its opinion. Piper Jaffray did not express any opinion as to the price at which shares of RTI common stock or Holdco common stock may trade following announcement of the Transaction or at any future time. Piper Jaffray did not undertake to reaffirm or revise its opinion or otherwise comment upon any events occurring after the date of its opinion and does not have any obligation to update, revise or reaffirm its opinion.
Piper Jaffray’s opinion addressed solely the fairness, from a financial point of view, to Holdco of the proposed Aggregate Consideration set forth in the Master Transaction Agreement and did not address any other terms or agreement relating to the Transaction or any other terms of the Master Transaction Agreement. Piper Jaffray was not requested to opine as to, and its opinion does not address, (i) the basic business decision to proceed with or effect the Transaction, (ii) the merits of the Transaction relative to any alternative transaction or business strategy that may be available to RTI, (iii) any other terms contemplated by the Master Transaction Agreement or the fairness of the Transaction to, or any consideration received in connection therewith by, any creditor or other constituency of RTI, or (iv) the solvency or financial viability of any of Holdco, RTI or Paradigm at the date of Piper Jaffray’s opinion, upon consummation of the Transaction, or at any future time. Furthermore, Piper Jaffray expressed no opinion with respect to the amount or nature of compensation to any officer, director, or employee of any party to the Transaction, or any class of such persons, relative to the Aggregate Consideration to be paid by Holdco in the Transaction or with respect to the fairness of any such compensation.
Information about Piper Jaffray
As a part of its investment banking business, Piper Jaffray is regularly engaged in the valuation of businesses in the medical technology and other industries and their securities in connection with mergers and acquisitions, underwritings, secondary distributions of listed and unlisted securities, private placements, and valuations for corporate and other purposes. The RTI board selected Piper Jaffray to be its financial advisor and render its fairness opinion in connection with the Transaction on the basis of such experience and its familiarity with RTI and Paradigm.
101
Piper Jaffray acted as a financial advisor to RTI in connection with the Transaction and will receive a fee of $3.5 million from RTI, which is contingent upon the consummation of the Transaction, except for $500,000 of such fee which has been earned by Piper Jaffray for rendering its fairness opinion and is creditable against the total fee. In addition, at RTI’s sole discretion, RTI may pay Piper Jaffray an additional, discretionary fee of $500,000 upon consummation of the Transaction. The opinion fee was not contingent upon the consummation of the Transaction or the conclusions reached in Piper Jaffray’s opinion. RTI has also agreed to indemnify Piper Jaffray against certain liabilities and reimburse Piper Jaffray for certain expenses in connection with its services. In the past three years, Piper Jaffray has provided financial advisory and financing services to affiliates of Viscogliosi Brothers, LLC, a unitholder of PS Spine, including serving as arranger and placement agent for debt and equity financings for which it received aggregate fees of approximately $4.3 million. Piper Jaffray may continue to provide financial advisory and financing services to affiliates of such unitholder, and may receive fees for rendering such services. In addition, in the ordinary course of its business, Piper Jaffray and its affiliates may actively trade securities of RTI for its own account or the account of its customers and, accordingly, may at any time hold a long or short position in such securities. Piper Jaffray may also, in the future, provide investment banking and financial advisory services to Holdco, RTI, Paradigm, PS Spine or entities that are affiliated with Holdco, RTI, Paradigm or PS Spine, for which Piper Jaffray would expect to receive compensation.
Certain Unaudited Prospective Financial Information
Given the unpredictability of the underlying assumptions and estimates inherent in preparing financial projections, RTI does not as a matter of general practice publicly disclose detailed projections as to its anticipated financial position or results of operations, other than providing, from time to time, guidance with respect to the then-current fiscal year for certain expected financial results in its regular earnings press releases and communications and other investor materials. Similarly, Paradigm, as a private company, does not as a matter of general practice publicly disclose financial information, results of operations or projections thereof. However, in connection with the evaluation of a possible transaction:
| • | In August 2018, Paradigm’s management prepared and provided to RTI forward-looking financial information of Paradigm with respect to fiscal years 2018 through 2022, reflecting operating expense synergies based on a combination with RTI, in addition to the incorporation of incremental revenue and associated cost of goods sold for RTI’s SImmetry product, which were included because RTI’s SImmetry product was viewed by Paradigm as a key component of its analysis of the potential Earnout Consideration (the “Paradigm financial projections”), which Paradigm financial projections are summarized below. The Paradigm financial projections were provided to RTI management and the RTI board in connection with RTI’s due diligence investigation with respect to Paradigm and evaluation of a possible transaction and were used as the basis for the RTI board and RTI management to continue discussions with PS Spine with respect to a potential transaction. |
| • | Beginning in August 2018, RTI management adjusted the Paradigm financial projections in light of, among other things, a review of the Paradigm financial projections, discussions with Paradigm’s management regarding its business and future prospects, RTI’s management’s views on Paradigm’s businesses and future prospects and certain macroeconomic and industry trends developed during due diligence through extensive primary and secondary research, which is described in greater detail below, and RTI’s management’s judgment of its best available estimates regarding Paradigm’s business and future prospects (the “RTI adjusted Paradigm financial projections”), which RTI adjusted Paradigm financial projections are summarized below. As referenced above, RTI, in conjunction with a market research firm, conducted extensive market research to develop an independent view of Paradigm’s near-term and long-term projections and coflex® adoption. Primary and secondary research was conducted with surgeons, Integrated Delivery Networks, spine distributors, payors, and other stakeholders to form the basis for RTI’s view of coflex® adoption and shift in commercial reimbursement from investigative use to standard of care, as reflected in the RTI adjusted Paradigm financial projections. The RTI adjusted Paradigm financial projections were provided to the RTI board in connection with RTI’s due diligence investigation with respect |
102
to Paradigm and its financial analysis of Paradigm and by RTI to representatives of Piper Jaffray for its use in connection with its financial analysis and fairness opinion with respect to the Aggregate Consideration. |
| • | In August 2018, RTI’s management prepared and provided to the RTI board, PS Spine and potential financing sources forward-looking financial information of the combined company with respect to fiscal years 2018 through 2022 based on RTI’s internal long-range plan, which was prepared to reflect certain anticipated cost synergies and the operations of an entity combining RTI and Paradigm (the “August RTI combined financial projections”), which August RTI combined financial projections are summarized below. |
| • | In October 2018, RTI’s management prepared and provided to the RTI board, PS Spine and potential financing sources updated forward-looking financial information of the combined company with respect to fiscal years 2018 through 2022, which was prepared to reflect certain anticipated cost synergies and the operations of an entity combining RTI and Paradigm, updated to reflect RTI’s updated long range plan prepared in September 2018 in light of RTI’s initial view of its 2019 operating plan as further described below (the “October RTI combined financial projections”), which October RTI combined financial projections are summarized below. |
Other than with respect to discussions between RTI and Paradigm concerning anticipated operating expenses (including with respect to anticipated synergies and the anticipated operating infrastructure of the combined company), the Paradigm financial projections were not prepared with the cooperation or agreement of RTI and, in any event, are not consensus financial projections. Similarly, other than with respect to discussions between RTI and Paradigm concerning anticipated operating expenses (including with respect to anticipated synergies and the anticipated operating infrastructure of the combined company), the RTI adjusted Paradigm financial projections, the August RTI combined financial projections and the October RTI combined financial projections were not prepared with the cooperation or agreement of Paradigm and, in any event, are not consensus financial projections. The Paradigm financial projections, the RTI adjusted Paradigm financial projections, the August RTI combined financial projections and the October RTI combined financial projections are referred to herein collectively as the “financial projections.”
Summaries of the financial projections are included in this joint proxy and consent solicitation statement/prospectus only because certain of the financial projections were made available to RTI, the RTI board, Paradigm, PS Spine, representatives of Piper Jaffray and potential financing sources, as applicable. RTI has included below a summary of these financial projections to provide its stockholders access to certainnon-public information that was furnished to the above-listed parties and considered by Piper Jaffray in connection with its financial analysis. The inclusion of the financial projections in this joint proxy and consent solicitation statement/prospectus does not constitute an admission or representation by RTI, PS Spine or Paradigm that the financial projections or the information contained therein is material.
The financial projections are unaudited and were not prepared with a view toward public disclosure or compliance with GAAP, the guidelines established by the American Institute of Certified Public Accountants for preparation and presentation of prospective financial information or the published guidelines of the SEC regarding projections and the use ofnon-GAAP financial measures. None of RTI’s nor Paradigm’s independent registered public accounting firm or any other independent accountant has compiled, examined or performed any procedures with respect to the financial projections or expressed any opinion or any other form of assurance on the financial projections or their achievability, and each of RTI’s and Paradigm’s independent registered public accounting firms assumes no responsibility for, and disclaims any association with, the financial projections.
In the view of Paradigm’s management, the Paradigm financial projections were prepared on a reasonable basis reflecting Paradigm’s management’s best available estimates and judgments regarding Paradigm’s future financial performance (with synergies related to the Transaction). In the view of RTI’s management, the RTI adjusted Paradigm financial projections, the August RTI combined financial projections and the October RTI combined financial projections were prepared on a reasonable basis reflecting RTI’s management’s best available estimates and judgments regarding RTI’s and Paradigm’s future financial performance (with synergies related to the Transaction). The financial projections are not facts and should not be relied upon as necessarily predictive of actual future results. You are
103
cautioned not to place undue reliance upon the financial projections. Some or all of the assumptions that have been made in connection with the preparation of the financial projections may have changed since the date the financial projections were prepared. In addition, certain information below provides summaries of the key assumptions and does not purport to be a comprehensive overview of all assumptions reflected in the financial projections. The ultimate performance of Paradigm or RTI could be materially different than the applicable financial projections. None of PS Spine, Paradigm, RTI or any of their respective affiliates, advisors or other representatives assumes any responsibility for or makes any representation as to the ultimate performance of Paradigm or RTI relative to the financial projections or otherwise. Except as required by applicable law, none of PS Spine, Paradigm, RTI or any of their respective affiliates, advisors or other representatives intends to, and each of them disclaims any obligation to, update, correct or otherwise revise the financial projections if any or all of them have changed or change or otherwise are or become inaccurate, even in the event that any or all assumptions are shown to be in error (even in the short term). These considerations should be taken into account if reviewing or evaluating the financial projections, which were prepared as of an earlier date.
The financial projections do not necessarily reflect changes in general business or economic conditions since the time they were prepared, including the effect of the Transaction, changes in Paradigm’s or RTI’s businesses or prospects or any other transactions or events that have occurred or that may occur, including any failure of the Transaction to be consummated, that were not anticipated at the time the financial projections were prepared, and the financial projections are not necessarily indicative of current values or predictive of future performance, which may be significantly more favorable or less favorable than as set forth therein and should not be regarded as a representation that the financial forecasts, projected results or other estimates and assumptions therein will be achieved.
Because the financial projections reflect subjective judgment in many respects, they are susceptible to multiple interpretations and frequent revisions based on actual experience and business developments. The financial projections also cover multiple fiscal years, and such information by its nature becomes less predictive with each succeeding fiscal year. The financial projections constitute forward-looking information and are subject to a wide variety of significant risks and uncertainties that could cause the actual results to materially differ from the projected results, including, without limitation, the risks and uncertainties described in “Risk Factors” beginning on page 38, the risks and uncertainties described in Part I, Item 1.A., Risk Factors, of RTI’s Annual Report on Form10-K for the year ended December 31, 2017, as filed with the SEC on March 2, 2018, and in RTI’s other public filings with the SEC. For additional information on factors that may cause Paradigm’s or RTI’s future financial results to materially vary from the projected results summarized below, see “Cautionary Statement Concerning Forward-Looking Statements” beginning on page 1. Accordingly, there can be no assurance that the projected results summarized below will be realized or that actual results will not differ materially from the projected results summarized below, and the financial projections cannot be considered a guarantee of future operating results and should not be relied upon as such.
PS Spine has not made any representation to RTI, Holdco or Merger Sub in the Master Transaction Agreement or otherwise concerning the Paradigm financial projections, and none of RTI, Holdco or Merger Sub has made any representation to PS Spine in the Master Transaction Agreement or otherwise concerning the RTI adjusted Paradigm financial projections, the August RTI combined financial projections or the October RTI combined financial projections. The financial projections are not included in this joint proxy and consent solicitation statement/prospectus in order to induce any RTI stockholder to vote in favor of the Merger Proposal, the Share Issuance Proposal or any other proposal to be voted on at the RTI special meeting or to influence any RTI stockholder to make any investment decision. Similarly, the financial projections are not included in this joint proxy and consent solicitation statement/prospectus in order to induce any PS Spine unitholder to provide their written consent with respect to the approval of the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution, or to influence any PS Spine unitholder to make any investment decision.
The financial projections should be evaluated, if at all, in conjunction with the historical financial statements and other information regarding Paradigm and RTI, as applicable, contained in RTI’s public filings with the SEC and herein. See “Where You Can Find More Information” beginning on page 187. In addition, stockholders of RTI and unitholders of PS Spine are urged to review “Risk Factors” beginning on page 38. The financial projections do not
104
take into account any circumstances or events occurring after the date they were prepared, including any failure of the Transaction to be consummated and related matters, and should not be viewed in any manner in that context.
Paradigm Financial Projections
The following table presents a summary of the Paradigm financial projections:
Paradigm Financial Projections (Prepared by Paradigm’s Management) (Unaudited)
| Year Ended December 31, | ||||||||||||||||||||
| 2018P | 2019P | 2020P | 2021P | 2022P | ||||||||||||||||
| (dollars in thousands) | ||||||||||||||||||||
Paradigm Product Revenue | $ | 43,422 | $ | 53,383 | $ | 68,929 | $ | 83,208 | $ | 97,414 | ||||||||||
SImmetry Revenue | $ | 1,500 | $ | 7,500 | $ | 7,813 | $ | 9,766 | $ | 11,914 | ||||||||||
Total Revenue | $ | 44,922 | $ | 60,883 | $ | 76,741 | $ | 92,973 | $ | 109,328 | ||||||||||
Gross Profit | $ | 39,257 | $ | 53,519 | $ | 67,756 | $ | 81,923 | $ | 96,487 | ||||||||||
Net Income | $ | (37,110 | ) | $ | 3,423 | $ | 10,631 | $ | 12,463 | $ | 18,731 | |||||||||
EBITDA1 | $ | (15,143 | ) | $ | 3,994 | $ | 14,549 | $ | 21,002 | $ | 31,277 | |||||||||
| 1 | See“—Non-GAAP Financial Measures” for the definition of EBITDA. |
The Paradigm financial projections reflect various estimates, assumptions and methodologies of Paradigm’s management, all of which are difficult to predict and many of which are beyond Paradigm’s and RTI’s control, including, among others, assumptions with respect to industry performance, general business, economic, regulatory, litigation, market and financial conditions and matters specific to Paradigm’s and RTI’s businesses. Such estimates, assumptions and methodologies of Paradigm’s management include, but are not limited to, the following:
| • | revenue will grow based on surgeon training and surgeon conversion to use of coflex®; and |
| • | cost saving synergies related to the downsizing of Paradigm’s general and administrative department and the elimination of overlapping marketing and advertising vendors will result in decreased operating expenses. |
RTI Adjusted Paradigm Financial Projections
The following table presents a summary of the RTI adjusted Paradigm financial projections:
RTI Adjusted Paradigm Financial Projections (Prepared by RTI’s Management) (Unaudited)
| Year Ended December 31, | ||||||||||||||||||||
| 2018P | 2019P | 2020P | 2021P | 2022P | ||||||||||||||||
| (dollars in thousands) | ||||||||||||||||||||
Total Revenue | $ | 40,930 | $ | 58,054 | $ | 83,274 | $ | 100,972 | $ | 122,171 | ||||||||||
Gross Profit | $ | 35,698 | $ | 51,105 | $ | 74,079 | $ | 89,598 | $ | 108,659 | ||||||||||
Adjusted EBITDA1 | $ | (12,983 | ) | $ | 1,842 | $ | 18,076 | $ | 28,032 | $ | 41,743 | |||||||||
Free Cash Flow1 | $ | NM | 2 | $ | (3,361 | ) | $ | 8,087 | $ | 16,100 | $ | 25,569 | ||||||||
| 1 | See“—Non-GAAP Financial Measures” for the definitions of Adjusted EBITDA and Free Cash Flow. |
| 2 | As the adjustments to Free Cash Flow for 2018 only represent figures for the fourth quarter of 2018, this figure is not meaningful. |
The RTI adjusted Paradigm financial projections reflect various estimates, assumptions and methodologies of RTI’s management, all of which are difficult to predict and many of which are beyond Paradigm’s and RTI’s control,
105
including, among others, assumptions with respect to industry performance, general business, economic, regulatory, litigation, market and financial conditions and matters specific to Paradigm’s and RTI’s businesses. Such estimates, assumptions and methodologies of RTI’s management are based upon and include, but are not limited to, the assumptions set forth under “—Paradigm Financial Projections” above. Further, the Paradigm financial projections were adjusted by RTI to reflect lower than expected recent results for Paradigm due to slower than anticipated commercial payor reimbursement coverage, as well as assumptions from RTI’s market research about commercial payor reimbursement coverage in future years.
August RTI Combined Financial Projections
The following table presents a summary of the August RTI combined financial projections:
August RTI Combined Financial Projections (Prepared by RTI Management) (Unaudited)
| Year Ended December 31, | ||||||||||||||||||||
| 2018P | 2019P | 2020P | 2021P | 2022P | ||||||||||||||||
| (dollars in thousands) | ||||||||||||||||||||
Total Revenue | $ | 323,087 | $ | 363,485 | $ | 408,946 | $ | 443,286 | $ | 484,701 | ||||||||||
Gross Profit | $ | 187,940 | $ | 226,890 | $ | 265,539 | $ | 292,576 | $ | 325,570 | ||||||||||
Adjusted EBITDA1 | $ | 20,671 | $ | 64,768 | $ | 89,915 | $ | 104,218 | $ | 124,659 | ||||||||||
| 1 | See“—Non-GAAP Financial Measures” for the definition of Adjusted EBITDA. |
The August RTI combined financial projections reflect various estimates, assumptions and methodologies of RTI’s management, all of which are difficult to predict and many of which are beyond Paradigm’s and RTI’s control, including, among others, assumptions with respect to industry performance, general business, economic, regulatory, litigation, market and financial conditions and matters specific to the combined company’s businesses. Such estimates, assumptions and methodologies of RTI’s management include, but are not limited to, the following:
| • | continued execution of RTI’s strategy to reduce complexity, drive operating excellence and accelerate growth and the related impact on sales growth and margin enhancement through manufacturing cost reduction initiatives; |
| • | a reduced sales and marketing cost structure for Paradigm reflecting sales and marketing integration with RTI’s existing Spine franchise; |
| • | reduced operational and administrative cost structure of Paradigm reflecting integration of key functions including finance, human resources, legal and information technology; and |
| • | additional synergies of approximately $8 million not specifically identified but assumed to be recognized through additional sales and marketing integration and acceleration of sales for certain existing RTI spine products through the efforts of the combined sales infrastructure. |
106
October RTI Combined Financial Projections
The following table presents a summary of the October RTI combined financial projections:
October RTI Combined Financial Projections (Prepared by RTI Management) (Unaudited)
| Year Ended December 31, | ||||||||||||||||||||
| 2018P | 2019P | 2020P | 2021P | 2022P | ||||||||||||||||
| (dollars in thousands) | ||||||||||||||||||||
Total Revenue | $ | 323,254 | $ | 352,440 | $ | 403,055 | $ | 441,830 | $ | 486,125 | ||||||||||
Gross Profit | $ | 188,365 | $ | 220,496 | $ | 262,156 | $ | 289,359 | $ | 323,512 | ||||||||||
Adjusted EBITDA1 | $ | 20,137 | $ | 52,266 | $ | 78,387 | $ | 91,036 | $ | 111,589 | ||||||||||
| 1 | See“—Non-GAAP Financial Measures” for the definition of Adjusted EBITDA |
The October RTI combined financial projections reflect various estimates, assumptions and methodologies of RTI’s management, all of which are difficult to predict and many of which are beyond Paradigm’s and RTI’s control, including, among others, assumptions with respect to industry performance, general business, economic, regulatory, litigation, market and financial conditions and matters specific to the combined company’s businesses. Such estimates, assumptions and methodologies of RTI’s management include, but are not limited to, those set forth above under “—August RTI Combined Financial Projections”. The October RTI combined financial projections reflect an update to RTI’s internal long-range plan prepared by RTI’s management in September 2018 in which:
| • | the combined synergies were revised to reflect a strategic marketing focus on key markets and driving reimbursement for coflex®; |
| • | sales and marketing synergies were reduced by approximately $8 million to reflect a revised sales and marketing organization, slower recognition of certain targeted cost synergies and the impact of slower private insurance coverage decisions for coflex® and a more conservative view of the contribution from the sale of certain existing RTI spine products; |
| • | during the third quarter of 2018, RTI decided that it would not pursue the more rigorous FDA requirements applicable to biological drug products and concluded that it would stop distributing its map3® implants effective October 31, 2018; and |
| • | RTI’s forecasts were updated to reflect August and September 2018 actual financial results and RTI’s decision to discontinue the sales and marketing of its map3® product. |
Non-GAAP Financial Measures
Certain of the financial projections arenon-GAAP financial measures, including Free Cash Flow, EBITDA and Adjusted EBITDA.Non-GAAP financial measures should not be considered in isolated form or as a substitute for financial information presented in compliance with GAAP, andnon-GAAP financial measures as used by Paradigm and RTI may not be comparable to similarly titled financial measures used by other companies.
RTI defines “Adjusted EBITDA” to mean earnings before interest expense, stock-based compensation, income taxes, depreciation and amortization.
Paradigm defines “EBITDA” to mean earnings before interest expense, income taxes, depreciation and amortization.
RTI defines “Free Cash Flow” to meanafter-tax operating income, adjusted for depreciation and amortization, capital expenditures and changes in net working capital.
107
RTI management uses Adjusted EBITDA to focus on RTI’son-going operations, and believes Adjusted EBITDA is useful to investors because it enables investors to perform meaningful comparisons of past and present operating results. RTI also believes that Adjusted EBITDA provides useful information to investors because it improves the comparability of the financial results between periods and provides for greater transparency to key measures used to evaluate the performance of RTI. In addition, RTI management uses Adjusted EBITDA for evaluating its performance against competitors and as a performance metric.
Paradigm management uses EBITDA to focus on Paradigm’son-going operations, and believes EBITDA is useful to investors because it enables investors to perform meaningful comparisons of past and present operating results. Paradigm also believes that EBITDA provides useful information to investors because it provides for greater transparency to key measures used to evaluate the performance of Paradigm. In addition, Paradigm management uses EBITDA for evaluating its performance against competitors.
Free Cash Flow provides another measure by which to evaluate Paradigm’s core operating performance and trends, but is not used by Paradigm or RTI for financial and operational decision making or as a means to evaluateperiod-to-period comparisons. Free Cash Flow was calculated by Piper Jaffray at the direction of RTI management solely for purposes of the discounted cash flow analysis in connection with Piper Jaffray’s opinion, and none of RTI, Paradigm, PS Spine or Piper Jaffray assumes any responsibility for any use of such estimates, or reliance on such estimates, for any other purpose.
NONE OF RTI, PS SPINE, PARADIGM NOR ANY OF THEIR RESPECTIVE AFFILIATES INTENDS TO, AND EACH OF THEM DISCLAIMS ANY OBLIGATION TO, UPDATE, CORRECT OR OTHERWISE REVISE THE FINANCIAL PROJECTIONS TO REFLECT CIRCUMSTANCES EXISTING OR EVENTS OCCURRING AFTER THE RESPECTIVE DATES WHEN THE FINANCIAL PROJECTIONS WERE PREPARED OR TO REFLECT THE EXISTENCE OF FUTURE CIRCUMSTANCES OR THE OCCURRENCE OF FUTURE EVENTS, EVEN IN THE EVENT THAT ANY OR ALL OF THE ASSUMPTIONS UNDERLYING THE FINANCIAL PROJECTIONS ARE INACCURATE OR NO LONGER APPROPRIATE.
RTI has never paid cash dividends to holders of its common stock. It does not expect to declare or pay any dividends on its common stock in the foreseeable future. Pursuant to the Master Transaction Agreement, RTI is not permitted to declare, set aside or pay dividends or other distributions (whether payable in cash, stock, property or a combination thereof) in respect of the stock of RTI prior to the closing of the Transaction without the consent of PS Spine. Under RTI’s current credit agreement with JPM, as lender, it is restricted from paying cash dividends on its common stock without the prior written consent of the administrative agent, unless certain payment conditions with respect to available borrowing capacity under RTI’s current credit agreement and fixed charge coverage are satisfied. Pursuant to the terms of the RTI Certificate of Designation, so long as any shares of RTI preferred stock remain outstanding, RTI may not pay any dividend or make any distribution upon any junior securities (including its common stock) until all accrued and accumulated but unpaid dividends on RTI preferred stock have been paid or declared with funds irrevocably set apart for payment. In addition, pursuant to the terms of the RTI Certificate of Designation, so long as any shares of RTI preferred stock remain outstanding, RTI may not pay any dividend or make any distribution upon any junior securities (including its common stock) without the prior written consent of the holders of a majority of RTI preferred stock.
The payment of future dividends, if any, will be at the discretion of Holdco’s board of directors and will depend upon Holdco’s: future earnings, if any; capital requirements; financial condition; debt covenant terms, which will be substantially similar to those in effect as of the date of this joint proxy and consent solicitation statement/prospectus under RTI’s current credit agreement; ability to do so under applicable laws; and other relevant factors. Pursuant to the
108
terms of the Holdco Certificate of Designation, so long as any shares of Holdco preferred stock remain outstanding, Holdco may not pay any dividend or make any distribution upon any junior securities (including its common stock) until all accrued and accumulated but unpaid dividends on Holdco preferred stock have been paid or declared with funds irrevocably set apart for payment. In addition, pursuant to the terms of the Holdco Certificate of Designation, so long as any shares of Holdco preferred stock remain outstanding, Holdco may not pay any dividend or make any distribution upon any junior securities (including its common stock) without the prior written consent of the holders of a majority of Holdco preferred stock.
PS Spine’s and Paradigm’s Dividend Policy
Neither PS Spine nor Paradigm has ever paid cash dividends or distributions to their respective unitholders. Paradigm does not expect to declare or pay any dividends or distributions with respect to its equity interests in the foreseeable future. PS Spine intends to distribute the consideration that it receives pursuant to the Master Transaction Agreement (net of transaction and other expenses and amounts due to its senior lenders and their affiliates) to its unitholders, in accordance with PS Spine’s organizational documents, as soon as reasonably practicable following its receipt thereof. Pursuant to the Master Transaction Agreement, Paradigm is not permitted to declare or pay any cash dividend or distribution in respect of its equity interests prior to the closing of the Transaction without the consent of RTI. Under Paradigm’s existing senior secured credit agreement, PS Spine and Paradigm are restricted from paying dividends or distributions in respect of their equity interests.
Under the HSR Act and the rules that have been promulgated thereunder by the FTC, certain transactions may not be consummated unless information has been furnished to the Antitrust Division and the FTC and certain waiting period requirements have been satisfied. The Transaction is subject to these requirements and may not be completed until the expiration of a30-day waiting period following the filing of the required Notification and Report Forms with the Antitrust Division and the FTC or until early termination is granted. On November 13, 2018, Holdco and PS Spine filed the required forms under the HSR Act with the Antitrust Division and the FTC and requested early termination, which was granted on November 26, 2018.
At any time before or after consummation of the Transaction, notwithstanding the termination of the waiting period under the HSR Act, the applicable competition authorities could take such action under applicable antitrust laws as each deems necessary or desirable in the public interest, including seeking to enjoin the consummation of the Transaction. Private parties may also seek to take legal action under the antitrust laws under certain circumstances. Neither RTI nor PS Spine can assure you that the Antitrust Division, the FTC, any state attorney general, or any other government authority will not attempt to challenge the Transaction on antitrust grounds, and, if such a challenge is made, neither RTI nor PS Spine can assure you as to its result. Neither RTI nor PS Spine is aware of any material regulatory approvals or actions that are required for completion of the Transaction that have not been obtained. It is presently contemplated that if any such additional regulatory approvals or actions are required, those approvals or actions will be sought. There can be no assurance, however, that any additional approvals or actions will be obtained.
Material U.S. Federal Income Tax Consequences
The following is a summary of (i) the material United States federal income tax consequences of the Merger to U.S. holders (as defined below) of RTI shares and (ii) the material United States federal income tax consequences of the Contribution to PS Spine as the holder of membership interests in Paradigm. This summary does not discuss the United States federal income tax consequences of the Merger or the Contribution to persons who are not U.S. holders and does not address the tax consequences of any transaction other than the Merger and the Contribution.
109
This summary is based on current provisions of the Code, Treasury Regulations promulgated thereunder and administrative and judicial interpretations thereof. All of the foregoing is subject to change, possibly with retroactive effect, and could affect the tax consequences described below. This summary deals only with RTI shares held as capital assets within the meaning of Section 1221 of the Code. This summary does not address all aspects of United States federal income taxation which may be relevant to particular RTI stockholders or to PS Spine in light of their or its individual circumstances, such as stockholders subject to special tax rules including, without limitation, the following: persons that are subject to special expatriation rules; financial institutions; insurance companies; dealers or traders in securities or currencies or notional principal contracts;tax-exempt entities; 401(k) and othertax-free qualified plans; persons that hold their stock as part of a “hedging” or “conversion” transaction or as a position in a “straddle” or as part of a “synthetic security” or other integrated transaction for United States federal income tax purposes; stockholders subject to the alternative minimum tax; regulated investment companies; real estate investment trusts; persons that own (or are deemed to own) five percent or more of the outstanding stock of RTI; partnerships and other pass-through entities and persons who hold stock through such partnerships or other pass-through entities; persons that have a “functional currency” other than the U.S. dollar; and stockholders that acquired (or will acquire) common stock through exercise of employee stock options or otherwise as compensation, all of whom may be subject to United States federal income tax rules that differ significantly from those discussed below. Also, this discussion does not address any United States federal income tax considerations applicable to holders of options or warrants to purchase RTI, PS Spine, Paradigm or Holdco stock or units, or holders of debt instruments convertible into RTI, PS Spine, Paradigm or Holdco stock or units or any tax considerations arising under the unearned income Medicare contribution tax pursuant to the Health Care and Education Reconciliation Act of 2010 or under the Foreign Account Tax Compliance Act (Sections 1471 through 1474 of the Code, the Treasury regulations and administrative guidance thereunder or any intergovernmental agreement entered into in connection therewith).
This summary does not address the tax consequences of the Contribution to PS Spine unitholders. The tax consequences of the Contribution and any transactions related to the Contribution to PS Spine unitholders will generally depend upon the particular circumstances and status of PS Spine and each such PS Spine unitholder. PS Spine unitholders should consult their own tax advisors regarding the tax consequences of the Contribution to them in light of their particular tax circumstances and the tax status and circumstances of PS Spine.
This discussion is a summary and does not purport to be a comprehensive analysis or description of all potential U.S. federal income tax consequences of the Merger or the Contribution. No rulings will be sought by Holdco, RTI or PS Spine from the IRS with respect to the Merger or the Contribution, and there can be no assurance that the IRS or a court will not take a position contrary to the tax consequences described herein. The discussion does not address anynon-income tax considerations or any foreign, state or local tax consequences. We urge you to consult your tax advisor with respect to the particular U.S. federal, state and local or foreign tax consequences of the Merger or the Contribution to you.
For purposes of this discussion, the term “U.S. holder” means an RTI stockholder who, for United States federal income tax purposes is:
| • | an individual who is a citizen or resident of the United States; |
| • | a corporation or other entity taxable as a corporation that is created in, or organized under the law of, the United States or any state or political subdivision thereof; |
| • | a trust (1) the administration of which is subject to the primary supervision of a court within the United States and which has one or more United States persons who have the authority to control all substantial decisions of the trust or (2) that has a valid election in effect under applicable United States Treasury Regulations to be treated as a United States person for United States federal income tax purposes; or |
| • | an estate, the income of which is includible in gross income for United States federal income tax purposes regardless of its source. |
If a partnership (or other entity or arrangement treated as a partnership for United States federal income tax purposes) holds RTI shares, the tax treatment of a partner will generally depend on the status of the partner and the
110
activities of the partnership. A partnership, or a U.S. holder that is a partner in a partnership, holding RTI shares should consult its tax advisors regarding the U.S. federal income tax consequences of the Merger and the Contribution.
HOLDERS OF RTI SHARES AND HOLDERS OF PS SPINE UNITS SHOULD CONSULT WITH THEIR TAX ADVISORS REGARDING THE TAX CONSEQUENCES OF THE MERGER AND THE CONTRIBUTION TO THEM, INCLUDING THE EFFECTS OF THE MERGER AND THE CONTRIBUTION UNDER UNITED STATES FEDERAL, STATE AND LOCAL, AND FOREIGN INCOME AND OTHER TAX LAWS.
The following discussion assumes that the Merger and the Contribution will be consummated as described in the Master Transaction Agreement and this joint proxy and consent solicitation statement/prospectus. RTI and PS Spine intend to take the position that the Merger and the Contribution, taken together, qualify as a transaction described in Section 351 of the Code (or, in the case of RTI, that the Merger, considered alone, qualifies as a reorganization within the meaning of Section 368(a) of the Code) (the “Intended Tax Treatment”). The obligations of PS Spine and RTI to consummate the Contribution and the Merger are conditioned on the receipt of certain representations and covenants in certificates of officers of RTI, PS Spine, and Holdco, which representations and covenants are intended to support the ability of each of PS Spine and RTI to take the position that the Merger and/or the Contribution will qualify for the Intended Tax Treatment. If any of the representations or covenants upon which the Intended Tax Treatment is based is inaccurate, the United States federal income tax consequences of the Merger and/or the Contribution could differ from the Intended Tax Treatment. RTI and PS Spine also intend to take the position that the recognition of gain upon receipt of the Holdco common stock component of the Earnout Consideration will be deferred pursuant to Section 351(a) of the Code.
In connection with the effectiveness of the registration statement on Form S-4 of which this joint proxy and consent solicitation statement/prospectus forms a part, (a) RTI’s counsel, Sidley Austin, delivered an opinion to RTI (which opinion is filed as Exhibit 8.1 to such registration statement) to the effect that (i) the Merger and the Contribution, taken together, will qualify as a transaction described in Section 351 of the Code or (ii) the Merger will qualify as a “reorganization” within the meaning of Section 368(a) of the Code, with the material U.S. federal income tax consequences of the Merger to holders of RTI shares described below in the section entitled “United States Federal Income Tax Consequences to U.S. Holders of RTI Shares,” and (b) Paradigm’s and PS Spine’s counsel, Dorsey & Whitney, delivered an opinion to PS Spine (which opinion is filed as Exhibit 8.2 to such registration statement) to the effect that the Merger and the Contribution, taken together, will qualify as a transaction described in Section 351(a) of the Code, with the material U.S. federal income tax consequences of the Merger and the Contribution to PS Spine described below in the section entitled “United States Federal Income Tax Consequences to PS Spine.” Such opinions are conditioned on the accuracy, as of the date of the opinion and the effective date of such registration statement, of representations contained in letters and certificates received from RTI, Holdco, and PS Spine, and are based on customary factual assumptions, as well as certain covenants and undertakings of RTI, Holdco, and PS Spine.
The positions of RTI and PS Spine are not binding on the IRS or the courts, and the parties do not intend to request a ruling from the IRS with respect to the Merger and/or the Contribution. Accordingly, there can be no assurance that the IRS will not challenge either the qualification of the Merger and the Contribution, taken together, as a transaction described in Section 351 of the Code (or the qualification of the Merger, taken alone, as a reorganization within the meaning of Section 368(a) of the Code), or the deferral of gain recognition upon the receipt of the Holdco common stock component of the Earnout Consideration pursuant to Section 351(a) of the Code, or that a court will not sustain such challenge. If the IRS were to successfully make either such challenge, the tax consequences could differ from those described in this joint proxy and consent solicitation statement/prospectus.
United States Federal Income Tax Consequences to U.S. Holders of RTI Shares
Assuming that the receipt of Holdco shares in exchange for RTI shares pursuant to the Merger, taken together with the receipt of Holdco common stock in exchange for Paradigm membership interests pursuant to the Contribution, qualifies as an “exchange” described in Section 351(a) of the Code and/or, taken alone, qualifies as a “reorganization” within the meaning of Section 368(a) of the Code, a U.S. holder of RTI shares receiving Holdco shares pursuant to the
111
Merger will not recognize gain or loss with respect to such receipt of Holdco shares. The aggregate adjusted tax basis of the Holdco shares the U.S. holder of RTI shares receives will be equal to the aggregate adjusted tax basis of the RTI shares the U.S. holder surrendered pursuant to the Merger, and the holding period of the Holdco shares will include the U.S. holder’s holding period of the RTI shares surrendered therefor.
United States Federal Income Tax Consequences to PS Spine
Assuming that the receipt of Holdco common stock (including as a component of the Earnout Consideration) in exchange for Paradigm equity interests pursuant to the Contribution, taken together with the receipt of Holdco shares in exchange for RTI shares pursuant to the Merger, qualifies as an “exchange” described in Section 351(a) of the Code, the material United States federal income tax consequences to PS Spine generally will be as follows, subject to the discussion below in the sections entitled “Treatment of the Earnout Consideration” and “Imputed Interest”:
| • | gain (but not loss) will be recognized in an amount equal to the lesser of: |
| • | the excess of (i) the sum of the fair market value of the Holdco common stock and the amount of cash received by PS Spine in the Contribution (including as part of the Earnout Consideration) over (ii) PS Spine’s adjusted tax basis in Paradigm’s assets at the time of the Contribution; and |
| • | the amount of cash received by PS Spine in the Contribution (including as part of the Earnout Consideration); |
| • | the aggregate adjusted tax basis of the Holdco common stock received by PS Spine in the Contribution will generally be equal to the aggregate adjusted tax basis of Paradigm’s assets (but not in excess of the fair market value of such Holdco common stock), reduced by the Cash Consideration Amount that PS Spine receives (including any Cash Consideration Amount used to satisfy PS Spine’s or Paradigm’s outstanding indebtedness and unpaid transaction expenses) and any liabilities of Paradigm deemed to be assumed in the Contribution, and increased by the amount of gain that PS Spine recognizes; and |
| • | the holding period of the Holdco common stock received in the Contribution will include the holding period of the Paradigm assets. |
The character and amount of any gain recognized by PS Spine in connection with the Contribution will be determined by reference to the character and fair market value of the assets held by Paradigm, as if all the assets of Paradigm had been transferred to Holdco. Such gain may include a mixture of long-term capital gain, short-term capital gain and ordinary income.
PS Spine will recognize gain or loss on Holdco common stock issued in the Contribution that is transferred in satisfaction of PS Spine’s or Paradigm’s outstanding indebtedness. The amount of such gain or loss will equal the difference between the fair market value of the Holdco common stock on the closing date transferred in satisfaction of indebtedness and PS Spine’s adjusted tax basis (determined as described in the second bullet point above) in the Holdco common stock so transferred.
Treatment of the Earnout Consideration
Because all or a portion of the Earnout Consideration may be received by PS Spine after the close of the taxable year in which the Contribution occurs, the installment method may apply to the cash components of the Earnout Consideration. Under the installment method, PS Spine will defer the recognition of a portion of any gain realized in the Contribution until such times as PS Spine actually or constructively receives the Earnout Consideration. The rules governing such deferrals are complex, and their applicability to the Earnout Consideration is not free from doubt.
If a taxpayer has installment obligations arising during the year and outstanding at the close of the year exceeding $5.0 million in total, an interest charge, payable by the taxpayer, is imposed on the deferred tax liability. A taxpayer may elect out of the installment method (unless the taxpayer is otherwise ineligible for installment method reporting) by timely filing the appropriate form with its tax return for the tax year in which the Contribution occurs. If PS Spine elects out of the installment method or the installment method does not otherwise apply, the fair market value of PS
112
Spine’s right to receive the cash components of the Earnout Consideration may be treated as taxable consideration received at the time of the Contribution. There is, however, no direct authority with respect to the tax treatment of the Earnout Consideration under such circumstances.
The foregoing discussion assumes that the recognition of gain upon receipt of the Holdco common stock component of the Earnout Consideration will be deferred pursuant to Section 351(a) of the Code. The U.S. federal income tax treatment of the receipt, after the close of the taxable year in which the Contribution occurs, of Holdco common stock as contingent consideration in a transaction that otherwise qualifies as a transaction described in Section 351 of the Code is not entirely clear under current law, and there is no direct authority with respect to the tax treatment of the Earnout Consideration. If the recognition of gain upon the receipt of the Holdco common stock component of the Earnout Consideration is not eligible for deferral under Section 351 of the Code, then the value of such Holdco common stock would be treated in the same manner as the cash component of the Earnout Consideration. In that case, PS Spine would recognize gain (but not loss) as a result of the receipt of such Holdco common stock. Such gain may be eligible to be reported under the installment method in accordance with the rules (including the imposition of an interest charge), described above.
Imputed Interest
A portion of any payment of the Earnout Consideration will be treated as interest income taxable at ordinary income rates when received (regardless of whether PS Spine reports under the installment method). The portion of any payment of the Earnout Consideration that will be treated as interest income is determined by discounting the actual amount of the payment using the appropriate applicable federal rate, back to the date of the Contribution.
TAX MATTERS ARE VERY COMPLICATED, AND THE TAX CONSEQUENCES OF THE MERGER AND THE CONTRIBUTION TO HOLDERS OF RTI SHARES AND PS SPINE UNITS WILL DEPEND ON EACH SUCH HOLDER’S PARTICULAR SITUATION. HOLDERS OF RTI SHARES AND PS SPINE UNITS ARE URGED TO CONSULT WITH THEIR TAX ADVISORS REGARDING THE TAX CONSEQUENCES OF THE MERGER AND THE CONTRIBUTION TO THEM IN LIGHT OF THEIR PARTICULAR CIRCUMSTANCES, INCLUDING THE EFFECTS OF UNITED STATES FEDERAL, STATE AND LOCAL, AND FOREIGN INCOME AND OTHER TAX LAWS AND APPLICABLE REPORTING REQUIREMENTS.
The Transaction will be accounted for as an acquisition of a business. Holdco will account for the acquisition pursuant to the Master Transaction Agreement using the acquisition method of accounting in accordance with GAAP. Holdco will measure the assets acquired and liabilities assumed at their fair values including net tangible and identifiable intangible assets acquired and liabilities assumed as of the closing of the Transaction. Any excess of the purchase price over those fair values will be recorded as goodwill. Definite lived intangible assets will be amortized over their estimated useful lives. Intangible assets with indefinite useful lives and goodwill will not be amortized but will be tested for impairment at least annually, and all assets including goodwill will be tested for impairment when certain indicators are present. If, in the future, Holdco determines that tangible or intangible assets (including goodwill) are impaired, Holdco will record an impairment charge at that time. The final purchase price and fair value assessment of assets and liabilities will be based in part on a detailed valuation which has not yet been completed.
RTI stockholders are not entitled to appraisal or dissenters’ rights in connection with the Merger or any of the other transactions contemplated by the Master Transaction Agreement, and unitholders of PS Spine are not entitled to appraisal or dissenters’ rights in connection with the Contribution or any of the other transactions contemplated by the Master Transaction Agreement.
113
Listing of Holdco Common Stock
Pursuant to the Master Transaction Agreement, RTI will cause the shares of Holdco common stock to be issued in the Transaction to be approved for listing on the Nasdaq Global Market. It is a condition to the completion of the Transaction that the Holdco common stock be approved for listing on the Nasdaq Global Market.
Following the Transaction, RTI expects Holdco’s common stock will be listed on the Nasdaq Global Market and will trade under the name “RTI Surgical Holdings, Inc.” and symbol “RTIX”.
Restrictions on Sales of Shares of Holdco Received in the Transaction
Except as set forth below, shares of Holdco common stock issued pursuant to the Transaction will be freely tradable for purposes of the Securities Act and the Exchange Act unless such holders of Holdco common stock are “affiliates” of Holdco for purposes of Rule 145 under the Securities Act after the completion of the Transaction. Persons who may be deemed to be affiliates include individuals or entities that control, are controlled by, or are under common control with, Holdco and may include the executive officers, directors and significant stockholders of Holdco, such as the Designated Director (as defined in the section entitled “—Interests of PS Spine’s and Paradigm’s Managers and Executive Officers in the Transaction—Designated Director”, beginning on page 114) who will join the Holdco board of directors. This prospectus does not cover resales of Holdco common stock received by any person upon the completion of the Transaction, and no person is authorized to make any use of this prospectus in connection with any resale.
Pursuant to aLock-Up Agreement in the form attached to the Master Transaction Agreement that PS Spine is required to enter into at the closing (the “Lock-Up Agreement”), at the closing, PS Spine will agree to not sell or otherwise transfer any shares of Holdco common stock constituting part of the Stock Consideration Amount, subject to certain limited exceptions (including, among others, the transfer of such shares to PS Spine’s unitholders that agree to receive such shares subject to the provisions of theLock-Up Agreement that are applicable to PS Spine), during the period commencing from the closing and ending on the earlier of (x) the date that is one hundred eighty (180) days after the closing date and (y) the date on which Holdco consummates a liquidation, merger, share exchange or other similar transaction with an unaffiliated third party that results in all of Holdco’s stockholders having the right to exchange their equity holdings in Holdco for cash, securities or other property (for more information, see the section entitled “TheTransaction—Lock-Up Agreement”, beginning on page 121).
Pursuant to aLock-Up and Permitted Sale Agreement in the form attached to the Master Transaction Agreement that each of Holdco and the parties to the Settlement Agreement, apart from PS Spine and Paradigm, are required to enter into at the closing (the “Lock-Up and Permitted Sale Agreement”), at the closing, the parties to theLock-Up and Permitted Sale Agreement will agree to not sell or otherwise transfer any shares of Holdco common stock during the period commencing from the closing and ending on the earlier of (x) the date that is one hundred eighty (180) days after the closing date and (y) the date on which Holdco consummates a liquidation, merger, share exchange or other similar transaction with an unaffiliated third party that results in all of Holdco’s stockholders having the right to exchange their equity holdings in Holdco for cash, securities or other property (the “Lock-Up Period”); provided, however, such transfer restrictions are subject to certain limited exceptions, including the right to transfer, subject to the prior written approval of Holdco, which shall not be unreasonably withheld, conditioned or delayed, pursuant to an arrangement with any nationally recognized financial institution (for more information, see the section entitled “The Transaction—Lock-Up and Permitted Sale Agreement”, beginning on page 121).
Interests of PS Spine’s and Paradigm’s Managers and Executive Officers in the Transaction
In considering the recommendation of the PS Spine board that unitholders of PS Spine approve the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution, unitholders of PS Spine should be aware that certain of the managers of PS Spine and Paradigm and certain executive officers of PS Spine and Paradigm have interests in the transactions contemplated by the Master Transaction Agreement, including the Merger and the Contribution, that may be different from, or in addition to, the interests of the unitholders of PS Spine generally. The PS Spine board was aware of these interests and considered them, among other matters, in approving and declaring advisable the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution. These interests are described below.
Designated Director
The Master Transaction Agreement provides that, following the closing, until the later of (i) December 31, 2022, and (ii) the time at which PS Spine and its unitholders as of immediately prior to the Contribution own less than 10%
114
of Holdco’s outstanding common stock (assuming the conversion of all of Holdco’s outstanding convertible preferred stock into Holdco common stock), PS Spine will be entitled to nominate one director to the Holdco board (the “Designated Director”), which Designated Director will be Jeffrey C. Lightcap, Anthony G. Viscogliosi or any other person mutually agreed by Holdco and PS Spine. The Master Transaction Agreement also provides that, at each meeting of Holdco’s stockholders at which the election of directors is to be considered, Holdco will nominate the Designated Director designated by PS Spine for election to the Holdco board, solicit proxies from Holdco’s stockholders in favor of the election of such Designated Director and use its reasonable best efforts to cause such Designated Director to be elected to the Holdco board, including voting all unrestricted proxies in favor of the election of such Designated Director, recommending approval of such Designated Director’s election to the Holdco board and not taking any action designed to diminish the prospects of such Designated Director being elected to the Holdco board. The Designated Director will be entitled to compensation (including committee and committee chair compensation), reimbursement of expenses incurred in the Designated Director’s capacity as director and indemnification and advancement of expenses on the same basis as Holdco provides to its othernon-management directors.
Jeffrey C. Lightcap, currently a manager of PS Spine and Paradigm, will be the initial Designated Director and, accordingly, will be elected to the Holdco board upon the effective time of the Merger, to hold such office until the next annual meeting of stockholders of Holdco or until his successor has been duly elected and qualified.
Employment Agreement Between Paradigm and Steven D. Ward
Paradigm previously entered into an employment agreement with Steven D. Ward to serve as Paradigm’s Chief Financial Officer. The employment agreement provides that, if Mr. Ward’s employment is terminated as a result of the change of control of Paradigm, then Mr. Ward will be entitled to an amount of cash equal to Mr. Ward’s base salary for six months, as well as the continuation of the medical, dental and vision benefits provided to Mr. Ward and his family for the same period. The Transaction will constitute a change of control of Paradigm for purposes of Mr. Ward’s employment agreement. Accordingly, if the Transaction had been consummated on January 15, 2019, and Mr. Ward’s employment was terminated in connection therewith, Mr. Ward would have been entitled to receive payments equal to $175,000 in the aggregate, representing Mr. Ward’s base salary for six months, as well as the continuation of the medical, dental and vision benefits provided to Mr. Ward and his family for thatsix-month period.
Employment Agreement Between Paradigm and William L. Pfost
Paradigm previously entered into an employment agreement with William L. Pfost to serve as Paradigm’s Executive Vice President, Sales and Marketing. The employment agreement provides that, in the event of a change of control of Paradigm in which the acquiror of Paradigm does not offer Mr. Pfost an offer of employment on terms that are comparable to the terms of Mr. Pfost’s employment agreement with Paradigm, then Mr. Pfost will be entitled to severance that will include:
| • | a payment in an amount equal to the sum of (i) three months of Mr. Pfost’s base salary (at the rate in effect at the time of termination or, if higher, as in effect immediately prior to the change of control), plus (ii) the monthly average of Mr. Pfost’s variable compensation (bonus and commissions) for the most recently completed three calendar months prior to the date of termination multiplied by three, which payment will be paid over three months; and |
| • | the continuation of the medical, dental and vision benefits provided to Mr. Pfost and his family for the three-month period following the date of termination. |
Accordingly, if the Transaction had been consummated on January 15, 2019, and neither RTI nor Holdco offered Mr. Pfost employment on terms that are comparable to the terms of Mr. Pfost’s employment agreement with Paradigm, Mr. Pfost would have been entitled to payments of $87,500, representing three months of Mr. Pfost’s base salary, at the rate in effect at the time of termination, and $79,740, representing the monthly average of Mr. Pfost’s variable compensation (bonus and commissions) for the most recently completed three calendar months prior to the date of termination multiplied by three, in each case, payable over three months, as well as the continuation of the medical, dental and vision benefits provided to Mr. Pfost and his family for the three-month period following the date of
115
termination. At closing, as described in greater detail below, RTI is expected to enter into an employment agreement with Mr. Pfost on terms that are comparable to the terms of Mr. Pfost’s employment agreement with Paradigm. Accordingly, none of the above-described severance payments are expected to be made to Mr. Pfost in connection with the Transaction.
Employment Agreement Between RTI and William L. Pfost
At closing, RTI is expected to enter into an employment agreement with William L. Pfost, Paradigm’s Executive Vice President, Sales and Marketing, which would set forth the terms of Mr. Pfost’s service as RTI’s Vice President, Sales. The employment agreement is expected to have an initial term of one year, which would automatically renew annually. The employment agreement is also expected to provide that RTI will pay Mr. Pfost a base salary of at least $350,000 annually and that Mr. Pfost will be eligible to receive monthly bonus payments of up to $35,000, based on the attainment ofpre-established performance goals, and a car allowance of $600 per month. In addition, the employment agreement is expected to contain customary covenants regarding confidentiality,non-competition,non-solicitation, andnon-interference, as well as customary provisions regarding the termination of the employment agreement and the consequences thereof.
Consulting Agreement Between RTI and Marc. R. Viscogliosi
RTI and Marc R. Viscogliosi, Chairman and Chief Executive Officer of PS Spine and Paradigm, have entered into a consulting agreement. See the section below entitled “The Transaction—Consulting Agreement Between RTI and Marc R. Viscogliosi”, beginning on page 121, for more information.
Consulting Agreement Between RTI and Francis Magee
RTI and Francis Magee, Paradigm’s Chief Technology Officer, have entered into a consulting agreement. See the section below entitled “The Transaction—Consulting Agreement Between RTI and Francis Magee”, beginning on page 121, for more information.
Consulting Agreement Between Paradigm and Musculoskeletal Clinical Regulatory Advisors
The Master Transaction Agreement provides that, prior to closing, PS Spine will use its reasonable best efforts to cause Paradigm to negotiate and enter into a written master consulting agreement, including statements of work, with MCRA, in substantially the same form, and for substantially the same terms and conditions, as Paradigm’s current consulting agreement with MCRA. Marc R. Viscogliosi, Chairman and Chief Executive Officer of PS Spine and Paradigm, and Anthony G. Viscogliosi, a manager of PS Spine and Paradigm, each have an ownership interest in MCRA.
Class F Preferred Units
As of the date of this joint proxy and consent solicitation statement/prospectus, no Class F Preferred Units of PS Spine have been issued or are outstanding. However, pursuant to certain agreements previously entered into with PS Spine, certain unitholders of PS Spine, including certain entities affiliated with HealthCor Partners Management, L.P. and Trevi Health Capital LLC, have the right, prior to the closing, to exchange their Class D Preferred Units and/orClass E-1 Preferred Units of PS Spine for an equal number of Class F Preferred Units of PS Spine. Under PS Spine’s organizational documents, all distributions that it may make to its unitholders, including distributions in connection with the Transaction, must be made first to the holders of Class F Preferred Units, until each holder thereof has received an amount equal to that holder’s capital contributions to PS Spine. In addition, pursuant to an agreement previously entered into with PS Spine, all of the potential holders of Class F Preferred Units have agreed that if PS Spine makes a distribution to the holders of its Class F Preferred Units, including a distribution in connection with the Transaction, that distribution must be made first to the holders of Class F Preferred Units that are affiliated with HealthCor Partners Management, L.P. and Trevi Health Capital LLC, until those holders have received an amount equal to their respective capital contributions to PS Spine. Jeffrey C. Lightcap, a manager of PS Spine and Paradigm, is
116
Senior Managing Director at HealthCor Partners Management, L.P., Michael Y. Mashaal, a manager of PS Spine and Paradigm, is a Managing Director at HealthCor Partners Management, L.P. and David Robbins, a manager of PS Spine and Paradigm, is an Advisor andCo-Founder of Trevi Health Capital LLC.
Settlement Agreement
Hayfin Services LLP is the administrative agent, and certain of its affiliates are the lenders, under Paradigm’s existing senior secured credit agreement. PS Spine and Paradigm have entered into an agreement (the “Settlement Agreement”) with Hayfin Services LLP and certain of its affiliates (collectively, the “Hayfin Entities”), pursuant to which:
| • | the Closing Debt Cash Amount; |
| • | 5,364,807 shares of Holdco common stock to be issued as part of the Stock Consideration Amount; |
| • | a number of shares of Holdco common stock, to be issued as part of the Stock Consideration Amount, equal to (a)(i) the amount of any loans made by the lenders under Paradigm’s existing senior secured credit agreement to Paradigm or any of its subsidiaries following the date of the Master Transaction Agreement and prior to the closing, plus accrued and unpaid interest thereon and any additional payments due to such lenders as a result thereof, minus (ii) the amount by which the Closing Debt Cash Amount, less anyout-of-pocket fees and expenses of such lenders, exceeds $95.0 million, divided by (b) $4.66; and |
| • | $10.0 million of the Earnout Consideration (which can take the form of cash, stock or a combination of both) that would otherwise be payable to PS Spine if Giants Revenue is equal to or exceeds $65.0 million in any LTM Period ending on or prior to December 31, 2020, |
will be used to pay amounts outstanding under Paradigm’s existing senior secured credit agreement and other amounts due to the lenders thereunder and their affiliates. Howard Rowe, a manager of PS Spine and Paradigm, is a Managing Director at Hayfin Capital Management LLP, an affiliate of the Hayfin Entities.
Lock-Up and Permitted Sale Agreement
At the closing, RTI and Holdco will enter into alock-up and permitted sale agreement with the Hayfin Entities. See the section below entitled “TheTransaction—Lock-Up and Permitted Sale Agreement”, beginning on page 121, for more information. Howard Rowe, a manager of PS Spine and Paradigm, is a Managing Director at Hayfin Capital Management LLP, an affiliate of the Hayfin Entities.
Indemnification and Directors’ and Officers’ Insurance and Fiduciary Liability Insurance
Pursuant to the Master Transaction Agreement, Holdco has agreed that, for a period of at least six years following the closing, it will, or will cause its applicable subsidiary to, to the fullest extent permitted by law, indemnify the current and former employees, directors, managers and officers of Paradigm and its subsidiaries, to the same extent as provided in the organizational documents of Paradigm and its subsidiaries as of the date of the Master Transaction Agreement, with respect to their activities on behalf of Paradigm or any of its subsidiaries prior to the closing.
In addition, prior to the closing, Paradigm will purchase, at RTI’s sole cost and expense, asix-year prepaid directors’ and officers’ insurance and fiduciary liability insurance tail policy, with respect to matters existing or occurring at or prior to the closing, covering, without limitation, the transactions contemplated by the Master Transaction Agreement. Holdco has agreed that, following the closing, it will not terminate or amend that tail policy and it will cause Paradigm and its subsidiaries to use commercially reasonable efforts to recover amounts under that tail policy. The Master Transaction Agreement also provides that PS Spine will indemnify Holdco, by withholding, reducing and/or setting off against the Earnout Consideration otherwise payable pursuant to the Master Transaction Agreement, for any amount by the which the liabilities of Paradigm and its subsidiaries to indemnify or hold harmless any director, manager, officer or employee of Paradigm or any of its subsidiaries for claims that relate to periods prior to the closing exceeds the amount of all insurance proceeds received from the tail policy on account of those liabilities.
117
The current and former employees, directors, managers and officers of Paradigm and its subsidiaries will have the right to enforce the provisions of the Master Transaction Agreement relating to their indemnification and are express third-party beneficiaries of the Master Transaction Agreement for this purpose.
Agreements with the Combined Company
As of the date of this joint proxy and consent solicitation statement/prospectus, except as set forth above, no members of Paradigm’s management have entered into any agreement, arrangement or understanding with RTI, Holdco or any of their affiliates regarding employment with RTI, Holdco or any of their subsidiaries, and no members of Paradigm’s management currently expect to enter into any of these type of agreements, arrangements or understandings prior to the closing. Moreover, as of the date of this joint proxy and consent solicitation statement/prospectus, except as set forth above, no discussions have occurred between members of Paradigm’s management and representatives of RTI, Holdco or their affiliates with respect to any of these type of agreements, arrangements and understandings.
However, the Master Transaction Agreement provides that:
| • | for a period of at least one year following the closing, Holdco will, or will cause its applicable subsidiary to, provide all individuals who are employees of Paradigm or any of its subsidiaries as of the closing, while employed by Holdco, or any of its subsidiaries, with salaries, annual bonus opportunities (excluding equity and long-term incentive awards) and employee benefits that are substantially comparable in the aggregate to those provided by Paradigm and its subsidiaries immediately prior to the closing; |
| • | from and after the closing, Holdco will cause Paradigm and its subsidiaries to comply with the terms (including terms which provide for or permit amendment or termination) of all contracts, agreements, plans and commitments of Paradigm and its subsidiaries as in effect immediately prior to the closing that are applicable to any employees or current or former directors or managers of Paradigm or any of its subsidiaries; |
| • | Holdco will provide all individuals who are employees of Paradigm or any of its subsidiaries as of the closing with full credit for their prior service with Paradigm or its applicable subsidiary for all purposes (including for purposes of eligibility, vesting and benefit accrual, but excluding benefit accrual under any defined benefit pension plan), under any employee benefit plan, program or arrangement established or maintained by Holdco or any of its subsidiaries under which those individuals may be eligible to participate from or after the closing to the same extent recognized by Paradigm or its applicable subsidiary immediately prior to the closing (except to the extent that it would result in a duplication of benefits); and |
| • | Holdco will use its commercially reasonable efforts to: |
| • | cause to be waived all limitations as toat-work conditions, to the extent those limitations are waived under a comparable plan, program or arrangement of Paradigm or any of its subsidiaries; |
| • | cause to be recognized deductible,co-insurance andout-of-pocket expenses paid by employees of Paradigm or any of its subsidiaries during the plan year in which the closing occurs, to the same extent those payments are recognized under a comparable plan, program or arrangement of Paradigm or any of its subsidiaries; and |
| • | waive any waiting period or evidence of insurability requirement that would otherwise affect employees of Paradigm or any of its subsidiaries who remain in the employment of RTI, Holdco or any of their subsidiaries, and their eligible dependents, from or after the closing during the plan year in which such closing occurs. |
However, the above-described provisions of the Master Transaction Agreement are not enforceable by employees or others as third-party beneficiaries.
118
Support Agreements
Concurrently with and as a condition to the execution of the Master Transaction Agreement, the Paradigm Support Unitholders (as defined below) entered into the Paradigm Support Agreements (as defined below). See the section entitled “The Transaction—Support Agreements”, beginning on page 119, for more information. Certain of PS Spine’s and Paradigm’s managers and executive officers are affiliated with entities that are affiliated with the Paradigm Support Unitholders. Specifically, Marc R. Viscogliosi, Chairman and Chief Executive Officer of PS Spine and Paradigm, and Anthony G. Viscogliosi, a manager of PS Spine and Paradigm, are each members of Viscogliosi Brothers, LLC, which is an affiliate of VB Acquisition Co I LLC, Jeffrey C. Lightcap, a manager of PS Spine and Paradigm, is Senior Managing Director at HealthCor Partners Management, L.P., which is an affiliate of HealthCor Paradigm Blocker Company Two, Inc. and HealthCor AIV, L.P., Michael Y. Mashaal, a manager of PS Spine and Paradigm, is a Managing Director at HealthCor Partners Management, L.P., which is an affiliate of HealthCor Paradigm Blocker Company Two, Inc. and HealthCor AIV, L.P., and David Robbins, a manager of PS Spine and Paradigm, is an Advisor andCo-Founder of Trevi Health Capital LLC, which is an affiliate of Trevi Health Ventures LP and Trevi AIV, LP.
Non-Competition Agreements
Concurrently with and as a condition to the execution of the Master Transaction Agreement, Viscogliosi Brothers, LLC, HealthCor AIV, L.P. and HealthCor Paradigm Blocker Company Two, Inc., entered into theNon-Competition Agreements (as defined below). See the section entitled “TheTransaction—Non-Competition Agreements” beginning on page 120 for more information). Marc R. Viscogliosi, Chairman and Chief Executive Officer of PS Spine and Paradigm, and Anthony G. Viscogliosi, a manager of PS Spine and Paradigm, are each members of Viscogliosi Brothers, LLC, Jeffrey C. Lightcap, a manager of PS Spine and Paradigm, is Senior Managing Director at HealthCor Partners Management, L.P., which is an affiliate of HealthCor AIV, L.P. and HealthCor Paradigm Blocker Company Two, Inc., and Michael Y. Mashaal, a manager of PS Spine and Paradigm, is a Managing Director at HealthCor Partners Management, L.P.
Board of Directors and Management of Holdco after the Transaction
Pursuant to the Master Transaction Agreement, the directors of RTI in office immediately prior to the effective time, as well as Jeffrey C. Lightcap, will, from and after the effective time, be the directors of Holdco, and the officers of RTI in office immediately prior to the effective time will, from and after the effective time, be the officers of Holdco.
Board of Directors and Management of the Surviving Corporation after the Transaction
As described in the section entitled “The Master Transaction Agreement—Effects of the Transaction; Closing; Effective Time”, beginning on page 123, Merger Sub will be merged with and into RTI such that the separate corporate existence of Merger Sub will thereupon cease, and (ii) RTI will continue as the surviving corporation. Pursuant to the Master Transaction Agreement, the directors of Merger Sub in office immediately prior to the effective time will, from and after the effective time, be the directors of the Surviving Corporation, and the officers of RTI in office immediately prior to the effective time will, from and after the effective time, be the officers of the Surviving Corporation.
Concurrently with and as a condition to the execution of the Master Transaction Agreement, RTI along with Holdco entered into support agreements (the “Paradigm Support Agreements”) with the following equity holders of PS Spine: (i) HealthCor Paradigm Blocker Company Two, Inc. and HealthCor AIV, L.P.; (ii) Trevi Health Ventures LP and Trevi AIV, LP; and (iii) Viscogliosi Brothers LLC and VB Acquisition Co I LLC (collectively, the “Paradigm Support Unitholders”). Pursuant to the Paradigm Support Agreements, the Paradigm Support Unitholders have agreed to vote all of the voting units of PS Spine owned by them (the “Covered Units”), including by written consent, in favor of PS Spine’s entry into the Master Transaction Agreement and the transactions contemplated by the Master Transaction Agreement and against any action that would interfere with the consummation of the transactions
119
contemplated by the Master Transaction Agreement. The Paradigm Support Agreements also contain certain restrictions on the sale or transfer of the Covered Units. The Paradigm Support Agreements will terminate upon the earlier of: (i) the termination of the Master Transaction Agreement; (ii) the effective time; (iii) the adoption and approval of the Master Transaction Agreement and the transactions contemplated by the Master Transaction Agreement by the unitholders of PS Spine; and (iv) any amendment or modification of the Master Transaction Agreement that is material or adverse to the Paradigm Support Unitholders. The written consent of the Paradigm Support Unitholders in favor of the Transaction, following the effectiveness of the Registration Statement on FormS-4, of which this joint proxy and consent solicitation statement/prospectus forms a part, is expected to be sufficient for the unitholders of PS Spine to approve the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution.
In addition to the Paradigm Support Agreements, WSHP Biologics Holdings, LLC, Camille I. Farhat, and Jonathon M. Singer (collectively, the “RTI Support Stockholders”) entered into support agreements with PS Spine (collectively, the “RTI Support Agreements”). Pursuant to the RTI Support Agreements, the RTI Support Stockholders have agreed to vote all of the shares of RTI capital stock owned by them (the “Covered Shares”) in favor of RTI’s entry into the Master Transaction Agreement and the transactions contemplated by the Master Transaction Agreement and against any action that would interfere with the consummation of the transactions contemplated by the Master Transaction Agreement. The RTI Support Agreements also contain certain restrictions on the sale or transfer of the Covered Shares. The RTI Support Agreements will terminate upon the earlier of: (i) the termination of the Master Transaction Agreement; (ii) the occurrence of a change in recommendation; (iii) the effective time; (iv) the adoption and approval of the Master Transaction Agreement and the transactions contemplated by the Master Transaction Agreement by the RTI stockholders; and (v) any amendment or modification of the Master Transaction Agreement that is material or adverse to RTI Support Stockholders.
Concurrently with and as a condition to the execution of the Master Transaction Agreement, Holdco entered intonon-competition agreements with HealthCor AIV, L.P. and HealthCor Paradigm Blocker Company Two, Inc. and with Viscogliosi Brothers, LLC (the “Non-Competition Agreements”). Pursuant to theNon-Competition Agreements, HealthCor AIV, L.P., HealthCor Paradigm Blocker Company Two, Inc. and Viscogliosi Brothers, LLC have agreed, during a period commencing at the closing and ending December 31, 2022 and subject to certain exceptions, not to (i) engage in or assist in the operations or management of any business that designs, develops, manufactures, sells, markets and/or provides Restricted Products (as defined below), (ii) design, develop, improve, manufacture, market, sell or provide any Restricted Products anywhere within the United States of America, Canada, Mexico, South America, any country that is a contracting state of the Patent Cooperation Treaty and all other countries or areas in which Paradigm has applied or filed for patent or other intellectual property protection with respect to any of its products, (iii) sell, market, provide, attempt to sell, market or provide, or assist in the selling, marketing or provision of, any Restricted Products to certain customers, (iv) induce or attempt to induce any distributor, customer, contractor, broker, supplier, vendor or other person with whom Holdco has or had relationships relating to the business of developing, manufacturing, marketing and selling spinal fixation products to curtail or cancel any such relationship or otherwise interfere with the relationship between Holdco and any of its distributors, customers, contractors, brokers, suppliers, vendors or other person involving the business of Holdco, (v) recruit, hire, or attempt to hire or engage any employee of Holdco involved in its business and/or (vi) make or publish any statements or comments that are designed or intended to disparage or injure the reputation or goodwill of the business of Holdco or any product of Paradigm. For purposes of theNon-Competition Agreements, “Restricted Products” means any spinal fixation product manufactured using a metal or synthetic material that (i) is the subject of an FDA approved premarket approval application or 510(k) clearance, (ii) has the indication set forth in the Indications for Use approved by the FDA on theFDA-approved label for coflex®, and (iii) can be implanted using minimally invasive surgical technique. TheNon-Competition Agreements also contain anon-disclosure provision which, subject to certain exceptions, prohibits HealthCor AIV, L.P., HealthCor Paradigm Blocker Company Two, Inc. and Viscogliosi Brothers, LLC from using or disclosing Paradigm’s confidential information from and after the closing date.
120
At the closing, PS Spine will enter into aLock-Up Agreement in substantially the form attached as Exhibit D to the Master Transaction Agreement. Pursuant to theLock-Up Agreement, PS Spine will agree not to sell or otherwise transfer any shares of Holdco common stock constituting part of the Stock Consideration Amount, subject to certain limited exceptions (including, among others, the transfer of such shares to PS Spine’s unitholders that agree to receive such shares subject to the provisions of theLock-Up Agreement that are applicable to PS Spine), during the period commencing from the closing and ending on the earlier of (x) the date that is one hundred eighty (180) days after the closing date and (y) the date on which Holdco consummates a liquidation, merger, share exchange or other similar transaction with an unaffiliated third party that results in all of Holdco’s stockholders having the right to exchange their equity holdings in Holdco for cash, securities or other property.
Lock-Up and Permitted Sale Agreement
At the closing, Holdco and the parties to the Settlement Agreement, apart from PS Spine and Paradigm, will enter into aLock-Up and Permitted Sale Agreement in substantially the form attached as Exhibit E to the Master Transaction Agreement. Pursuant to theLock-Up and Permitted Sale Agreement, the parties to theLock-Up and Permitted Sale Agreement will agree to not sell or otherwise transfer any shares of Holdco common stock during the period commencing from the closing and ending on the earlier of (x) the date that is one hundred eighty (180) days after the closing date and (y) the date on which Holdco consummates a liquidation, merger, share exchange or other similar transaction with an unaffiliated third party that results in all of Holdco’s stockholders having the right to exchange their equity holdings in Holdco for cash, securities or other property; provided, however, such transfer restrictions are subject to certain limited exceptions, including the right to transfer, subject to the prior written approval of Holdco, which shall not be unreasonably withheld, conditioned or delayed, pursuant to an arrangement with any nationally recognized financial institution.
Consulting Agreement Between RTI and Marc. R. Viscogliosi
RTI and Marc R. Viscogliosi, Chairman and Chief Executive Officer of PS Spine and Paradigm, have entered into a consulting agreement, pursuant to which Mr. Viscogliosi has agreed, following the closing, to provide certain consulting services, to be set forth in statements of work to be agreed upon by the parties, to RTI. The consulting agreement will terminate on December 31, 2022, unless earlier terminated for cause, upon the consummation of a change of control of Holdco or if Holdco disposes of all or substantially all of the assets of the business of developing, manufacturing, marketing and selling coflex® spinal fixation products. Pursuant to the consulting agreement, Mr. Viscogliosi will be entitled, at RTI’s sole cost and expense, to occupy the office he occupied as an employee of Paradigm immediately prior to the closing and to receive administrative assistant services of the same type and to the same extent as such services were provided to him as an employee of Paradigm immediately prior the closing, and be permitted to select his administrative assistant.
The initial statement of work under the consulting agreement, which runs through December 31, 2019, provides that Mr. Viscogliosi will support RTI’s reimbursement and physician engagement for differentiated spinal products including, but not limited to, coflex® and SImmetry®. The initial statement of work also provides that RTI will pay Mr. Viscogliosi a base consulting fee of $25,000 per month and that Mr. Viscogliosi will be granted $1.0 million of restricted shares of Holdco common stock upon the closing, which restricted shares will vest upon the attainment of certainpre-established performance goals through December 31, 2022.
Consulting Agreement Between RTI and Francis Magee
RTI and Francis Magee, Paradigm’s Chief Technology Officer, have entered into a consulting agreement, pursuant to which Mr. Magee has agreed, following the closing, to provide certain consulting services, to be set forth in statements of work to be agreed upon by the parties, to RTI. The consulting agreement will terminate on December 31, 2022, unless earlier terminated for cause, upon the consummation of a change of control of Holdco or if Holdco
121
disposes of all or substantially all of the assets of the business of developing, manufacturing, marketing and selling coflex® spinal fixation products. Pursuant to the consulting agreement, Mr. Magee will be entitled, at RTI’s sole cost and expense, to occupy the office he occupied as an employee of Paradigm immediately prior to the closing and to receive administrative assistant services of the same type and to the same extent as such services were provided to him as an employee of Paradigm immediately prior the closing, and be permitted to select his administrative assistant.
The initial statement of work under the consulting agreement, which runs through December 31, 2019, provides that Mr. Magee will support RTI’s reimbursement and physician engagement for differentiated spinal products including, but not limited to coflex® and SImmetry®. The initial statement of work also provides that RTI will pay Mr. Magee a base consulting fee of $37,500 per month, grant Mr. Magee $1.0 million of restricted shares of Holdco common stock upon the closing, which restricted shares will vest upon the attainment of certainpre-established performance goals through December 31, 2022, and provide Mr. Magee with the same housing that he was provided prior to the closing by Paradigm, not to exceed $6,500 per month.
122
THE MASTER TRANSACTION AGREEMENT
The following describes the material terms of the Master Transaction Agreement. The description of the Master Transaction Agreement in this section and elsewhere in this joint proxy and consent solicitation statement/prospectus is qualified in its entirety by reference to the complete text of the Master Transaction Agreement, a copy of which is attached asAnnex A and is incorporated by reference into this joint proxy and consent solicitation statement/prospectus. This summary does not purport to be complete and may not contain all of the information about the Master Transaction Agreement that is important to you. We encourage you to read the Master Transaction Agreement carefully and in its entirety.
Explanatory Note Regarding the Master Transaction Agreement
The Master Transaction Agreement, a copy of which is attached asAnnex A, and this summary of its terms are included to provide you with information regarding its terms. The representations, warranties and covenants made in the Master Transaction Agreement by RTI, PS Spine, Holdco and Merger Sub were made solely to the parties to, and solely for the purposes of, the Master Transaction Agreement and as of specific dates and were qualified and subject to important limitations agreed to by RTI, PS Spine, Holdco and Merger Sub in connection with negotiating the terms of the Master Transaction Agreement. In particular, in your review of the representations and warranties contained in the Master Transaction Agreement and described in this summary, it is important to bear in mind that the representations and warranties were negotiated with the principal purposes of establishing the circumstances in which a party to the Master Transaction Agreement may have the right not to effect the Transaction if the representations and warranties of the other party prove to be untrue due to a change in circumstance or otherwise, and allocating risk between the parties to the Master Transaction Agreement, rather than establishing matters as facts. The representations and warranties may also be subject to a contractual standard of materiality different from those generally applicable to stockholders and reports and documents filed with the SEC and in some cases were qualified by the matters contained in the PS Spine disclosure letter that PS Spine provided to RTI in connection with the Master Transaction Agreement (the “PS Spine disclosure letter”) or the disclosure letter that RTI provided to PS Spine in connection with the Master Transaction Agreement (the “RTI disclosure letter”), which disclosures were not reflected in the Master Transaction Agreement. Moreover, information concerning the subject matter of the representations and warranties may have changed since the date of the Master Transaction Agreement. Investors should not rely on the representations, warranties and covenants or any description thereof as characterizations of the actual state of facts of RTI, PS Spine, Holdco, Merger Sub or any of their respective subsidiaries or affiliates.
Effects of the Transaction; Closing; Effective Time
The Contribution
On the terms and subject to the conditions set forth in the Master Transaction Agreement, at the effective time, PS Spine shall contribute all of the issued and outstanding equity interests of Paradigm to Holdco, free and clear of all encumbrances. As a result of the Contribution, Paradigm will become a wholly owned subsidiary of Holdco.
The Merger
On the terms and subject to the conditions set forth in the Master Transaction Agreement, at the effective time, and in accordance with the DGCL, (i) Merger Sub will be merged with and into RTI such that the separate corporate existence of Merger Sub will thereupon cease, (ii) RTI will continue as the Surviving Corporation and (iii) the Merger will have the effects set forth in the Master Transaction Agreement, the certificate of merger relating to the Merger to be executed, acknowledged and filed with the Secretary of State of the State of Delaware and the DGCL. As a result of the Merger, RTI will become a wholly owned subsidiary of Holdco.
Closing
Subject to the satisfaction or waiver of all of the conditions precedent set forth in the Master Transaction Agreement, the closing of the Merger and the Contribution will take place on the fifth business day following the
123
satisfaction or waiver of all of the conditions precedent set forth in the Master Transaction Agreement (other than those conditions that by their nature are to be satisfied at the closing, but subject to the satisfaction or waiver thereof at the closing), unless another date is agreed to in writing by RTI, PS Spine, Holdco and Merger Sub. The date on which the closing actually occurs is referred to as the “closing date”.
Effective Time
On the terms and subject to the conditions set forth in the Master Transaction Agreement, on the closing date, (i) RTI shall file the certificate of merger relating to the Merger to be executed, acknowledged and filed with the Secretary of State of the State of Delaware and (ii) PS Spine shall effect the Contribution. The Merger and Contribution will become effective concurrently at the time on the closing date as RTI and PS Spine will agree and specify in the certificate of merger.
Governing Documents; Directors and Officers
Certificates of Incorporation, Certificate of Designation and Bylaws
The Holdco charter and Holdco bylaws shall be amended and restated at the effective time to contain provisions substantially identical to the certificate of incorporation and bylaws of RTI immediately prior to the effective time, and the Holdco charter will be amended to change the name of Holdco to “RTI Surgical Holdings, Inc.”, effective as of the effective time. At the effective time, Holdco shall adopt a certificate of designation containing provisions substantially identical to the certificate of designation of RTI immediately prior to the effective time. For additional information on the Holdco charter, Holdco certificate of designation and Holdco bylaws, see the sections entitled “Description of Holdco Capital Stock”, beginning on page 166, and “Comparison of Rights of Holdco Stockholders, RTI Stockholders and the Paradigm Member”, beginning on page 172.
At the effective time, the certificate of incorporation and the bylaws of Merger Sub as in effect immediately prior to the effective time shall be the certificate of incorporation and bylaws of RTI until thereafter amended in accordance with applicable law, except that the name of RTI reflected therein shall be as reasonably determined by RTI prior to the closing.
Directors and Officers of Holdco and the Surviving Corporation
Pursuant to the terms of the Master Transaction Agreement, the directors of RTI in office immediately prior to the effective time will, together with Jeffrey C. Lightcap, currently a member of PS Spine’s and Paradigm’s board of managers, from and after the effective time, be directors of Holdco, and the officers of RTI in office immediately prior to the effective time will, from and after the effective time, be the officers of Holdco, in each case until their respective successors have been duly elected, designated or qualified, or until their earlier death, resignation or removal. Subject to the closing of the Transaction, Holdco expects to increase the number of directors on the Holdco board of directors by one and, immediately following the effective time, elect Jeffrey C. Lightcap to fill the vacancy established thereby, to serve until his successor has been duly elected or appointed and qualified or until his earlier death, resignation or removal.
The directors of Merger Sub in office immediately prior to the effective time will, from and after the effective time, be the directors of the Surviving Corporation, and the officers of RTI in office immediately prior to the effective time will, from and after the effective time, be the officers of the Surviving Corporation, in each case until their respective successors have been duly elected, designated or qualified, or until their earlier death, resignation or removal.
At the effective time, by virtue of the Merger and without action on the part of RTI, Holdco, Merger Sub or holders of any RTI shares, Holdco shares or the capital stock of Merger Sub, each issued and outstanding share of common stock of Merger Sub will be converted into one share of common stock of the Surviving Corporation.
124
At the effective time, and without any action on the part of RTI, Holdco, the Surviving Corporation or the holders of RTI shares, Holdco shares or the capital stock of the Surviving Corporation, each (i) share of RTI common stock issued and outstanding immediately prior to the effective time (other than RTI shares to be converted pursuant to the following sentence) shall be converted automatically into one fully paid and non-assessable share of Holdco common stock and (ii) share of RTI preferred stock issued and outstanding immediately prior to the effective time (other than RTI shares to be cancelled pursuant to the following sentence) shall be converted automatically into one fully paid and non-assessable share of Holdco preferred stock. As of the effective time, each share of RTI common stock or RTI preferred stock that is owned by RTI as a treasury share or owned by Holdco or Merger Sub immediately prior to the effective time will be cancelled and shall cease to exist.
As of the effective time, all RTI shares converted into Holdco common stock or Holdco preferred stock, as applicable, will no longer be issued and outstanding and will automatically be cancelled and will cease to exist, and each valid certificate or certificates which immediately prior to the effective time represented any such RTI sharesor non-certificated RTI shares held in book entry form shall, upon the effective time, automatically represent an equivalent number of shares of Holdco common stock or Holdco preferred stock, as applicable (without any requirement for the surrender of any suchcertificates or non-certificated shares). At the effective time, Holdco shall cause Broadridge Financial Solutions, Inc. (or such other person as may be mutually agreed upon by Holdco and PS Spine) to credit in the stock ledger and other appropriate books and records of Holdco an equivalent number of shares of Holdco common stock or Holdco preferred stock, as applicable, for any uncertificated RTI shares (other than any share of RTI common stock or RTI preferred stock that is owned by RTI as a treasury share or owned by Holdco or Merger Sub immediately prior to the effective time). For the avoidance of doubt, from and after the effective time, the former holders of RTI shares (i) which have been converted into Holdco common stock at the effective time, shall be entitled to receive any dividends and distributions which may be made with respect to such shares of Holdco common stock and (ii) which have been converted into Holdco preferred stock at the effective time, shall be entitled to receive any dividends and distributions which may be made with respect to such shares of Holdco preferred stock, as applicable.
At the effective time, each share of capital stock of Holdco issued and outstanding immediately prior to the effective time and owned by RTI shall remain outstanding. At the effective time, each share of capital stock of Holdco issued and outstanding immediately prior to the effective time and owned by a person other than RTI shall be automatically cancelled and shall cease to exist, and no consideration shall be delivered in exchange therefor. Immediately following the effective time, shares of capital stock of Holdco owned by RTI shall be surrendered to Holdco without payment therefor.
Treatment of RTI Equity Awards
At the effective time, each compensatory option to purchase shares of RTI common stock (an “RTI Stock Option”) that is outstanding immediately prior to the effective time, whether vested or unvested, shall be converted into a stock option in respect of shares of Holdco common stock, on the same terms and conditions as were applicable under such RTI Stock Option immediately prior to the effective time (including with respect to vesting), relating to the number of shares of Holdco common stock equal to the total number of shares of RTI common stock subject to such RTI Stock Option immediately prior to the effective time and with an exercise price per share of Holdco common stock equal to the exercise price per share of RTI common stock subject to such RTI Stock Option immediately prior to the effective time.
At the effective time, each compensatory restricted stock award with respect to shares of RTI common stock (an “RTI Restricted Stock Award”) that is outstanding immediately prior to the effective time shall be converted into a restricted stock award with the same terms and conditions as were applicable under such RTI Restricted Stock Award immediately prior to the effective time (including with respect to vesting), and relating to the number of shares of Holdco common stock equal to the total number of shares of RTI common stock subject to such RTI Restricted Stock Award immediately prior to the effective time. Any accrued but unpaid dividend equivalents with respect to any RTI Restricted Stock Award will be assumed and become an obligation with respect to the applicable converted RTI Restricted Stock Award.
125
Prior to the effective time, RTI shall take all actions necessary (including adopting such resolutions of the RTI board or any committee of the RTI board) to effectuate the treatment of the RTI Stock Options and RTI Restricted Stock Awards set forth above. Holdco shall also take all corporate action necessary to reserve for issuance a sufficient number of shares of Holdco common stock for delivery with respect to the settlement of converted RTI Stock Options and RTI Restricted Stock Awards assumed by it. Holdco will file with the SEC, as soon as practicable following the effective time, a post-effective amendment to the registration statement on FormS-4, of which this joint proxy and consent solicitation statement/prospectus forms a part, or a registration statementon Form S-8 (or any successor form), to the extent such form is available, relating to such shares of Holdco common stock.
The consideration for the Contribution is generally payable in cash (without interest) and shares of Holdco common stock and is in addition to the contingent consideration that may become payable upon the occurrence of certain events in the future in accordance with the terms and conditions of the Master Transaction Agreement. PS Spine will initially receive at closing the Stock Consideration Amount and the Cash Consideration Amount, as follows:
| • | 10,729,614 shares of Holdco common stock,minus the number of shares of Holdco common stock necessary to pay the lenders under Paradigm’s existing senior secured credit agreement and any applicable interim debt financing of Paradigm, which shall be issued directly to such lenders and/or their affiliates at the closing, as further described in the sections entitled “—Issuances of Holdco Common Stock at Closing” below and “The Transaction—Interests of PS Spine’s and Paradigm’s Managers and Executive Officers in the Transaction—Settlement Agreement”, beginning on page 117; and |
| • | $100,000,000,minus (i) the aggregate amount of outstanding indebtedness and unpaid transaction expenses of Paradigm (to the extent not covered by the issuance of Holdco common stock to any lender under Paradigm’s existing senior secured credit agreement and any applicable interim debt financing of Paradigm and/or their affiliates) at closing,minus (ii) the amount, if any, by which the working capital of Paradigm is less than the target working capital amount of $7,000,000,plus (iii) the aggregate amount of cash of Paradigm at closing. |
Issuances of Holdco Common Stock at Closing
At the effective time, Holdco shall:
| • | Issue to the lenders under Paradigm’s senior secured credit agreement and any applicable interim debt financing of Paradigm, in a registered transaction pursuant to a registration statement on FormS-4, of which this joint proxy and consent solicitation statement/prospectus forms a part of, the number of shares of Holdco common stock to be paid to such lenders pursuant to the payoff letters delivered by Paradigm prior to closing, which amount shall be in accordance with the Settlement Agreement (as further described in “The Transaction—Interests of PS Spine’s and Paradigm’s Managers and Executive Officers in the Transaction—Settlement Agreement”, beginning on page 117); and |
| • | Issue to PS Spine the number of shares of Holdco common stock sufficient to deliver the aggregate Stock Consideration not issued to the lenders under Paradigm’s senior secured credit agreement and any applicable interim debt financing of Paradigm. |
Post-Closing Adjustment
Following the closing, Holdco and PS Spine will determine any adjustment to the Cash Consideration Amount based on the variance of the amounts of final indebtedness, unpaid transaction expenses, cash and working capital to the amounts of indebtedness, unpaid transaction expenses, cash and working capital estimated by PS Spine prior to closing. If the Cash Consideration Amount, as calculated by Holdco within 60 days of the closing, exceeds the Cash Consideration Amount calculated by PS Spine prior to the closing, Holdco will pay the difference by wire transfer of immediately available funds to PS Spine. Conversely, if the Cash Consideration Amount calculated by PS Spine prior to the closing exceeds the Cash Consideration Amount, as calculated by Holdco within 60 days of the closing, PS Spine shall pay the difference by wire transfer of immediately available funds to Holdco.
126
Contingent Consideration
Holdco has agreed to pay contingent consideration to PS Spine upon the occurrence of certain events in the future, on the terms and conditions set forth in the Master Transaction Agreement. The contingent consideration includes the following:
| • | if on or prior to December 31, 2020, the net revenue of Holdco and its subsidiaries in respect of specified legacy products of Paradigm (the “Giants Revenue”) is equal to or exceeds $65,000,000 in any consecutive twelve month period ending upon the completion of any fiscal quarter at least twelve months after the closing date (the “LTM Period”), then Holdco shall pay to PS Spine or its designee, no earlier than December 31, 2020, by wire transfer of immediately available funds, $20,000,000 (the “Cash Earnout Amount”); |
| • | if in any LTM Period ending on or prior to December 31, 2021, the net revenue of Holdco and its subsidiaries in respect of specified legacy products of Paradigm and specified RTI Zyga products (the “Giants/Zyga Revenue”) is greater than $85,000,000, then Holdco shall issue to PS Spine the number of Initial Earnout Shares (as defined below) payable to PS Spine with respect to such LTM Period (less the total number of Initial Earnout Shares that have previously been issued to PS Spine in any prior LTM Period); and |
| • | if in any LTM Period ending on or prior to December 31, 2022, the Giants/Zyga Revenue is greater than $105,000,000, then Holdco shall, at its option, either (A) issue to PS Spine the Final Earnout Shares (as defined below), or (B) pay PS Spine, by wire transfer of immediately available funds, the Cash Election Amount (as defined below). |
The number of Initial Earnout Shares and Final Earnout Shares and the Cash Election Amount shall be determined as follows:
| • | “Initial Earnout Shares” shall be equal to the number of shares of Holdco common stock (rounded to the nearest whole share) equal to the sum of: |
| • | (i) (A) 10,729,614 shares of Holdco common stock,multiplied by (B) the quotient of (1) (I) the Giants/Zyga Revenue for the applicable LTM Period, minus (II) $85,000,000, divided by (2) $11,764,705.88, provided, that if the Giants/Zyga Revenue for the applicable LTM Period is greater than $96,764,705.88, for purposes of this calculation, the Giants/Zyga Revenue shall be deemed to equal $96,764,705.88; and |
| • | (ii) the greater of (A) zero (0) shares of Holdco common stock and (B) (1) the number of shares (rounded to the nearest whole share) of Holdco common stock with an aggregate value of $35,000,000 based on the volume weighted average closing trading price of the Holdco common stock on the Nasdaq Global Market (or any other national stock exchange or quotation system on which Holdco common stock is then listed or quoted) for the last five trading days in the applicable fiscal period in which such shares are earned as reported by the Wall Street Journal or, if not reported therein, in another authoritative source mutually selected by RTI and PS Spine (the “VWAP”), multiplied by (2) the quotient of (I) (a) the Giants/Zyga Revenue for the applicable LTM Period, minus (b) $96,764,705.88, divided by (II) $8,235,294.12, provided, that if the Giants/Zyga Revenue for the applicable LTM Period is greater than $105,000,000, for purposes of this calculation, the Giants/Zyga Revenue shall be deemed to equal $105,000,000. |
| • | “Final Earnout Shares” shall be equal to the: |
| • | (i) number of shares (rounded to the nearest whole share) of Holdco common stock with an aggregate value of $45,000,000 based on the VWAP,multiplied by (i) the quotient of (A) (1) the Giants/Zyga Revenue for the applicable LTM Period, minus (2) $105,000,000, divided by (B) $20,000,000, provided, that if the Giants/Zyga Revenue for the applicable LTM Period is greater than $125,000,000, for purposes of this calculation, the Giants/Zyga Revenue shall be deemed to equal $125,000,000. |
| • | “Cash Election Amount” shall be equal to the amount in cash (which, for the avoidance of doubt, cannot exceed $45,000,000) (rounded to the nearest whole U.S. Dollar) equal to (i) $45,000,000, multiplied by |
127
(ii) the quotient of (A) (1) the Giants/Zyga Revenue for the applicable LTM Period, minus (2) $105,000,000, divided by (B) $20,000,000, provided, that if the Giants/Zyga Revenue for the applicable LTM Period is greater than $125,000,000, for purposes of this calculation the Giants/Zyga Revenue shall be deemed to equal $125,000,000. |
In addition, if a change of control of Holdco occurs or if Holdco disposes of all or substantially all of the assets of the business of developing, manufacturing, marketing and selling coflex® spinal fixation products, any remaining contingent consideration that has not been paid or issued to PS Spine (other than any contingent consideration that cannot be earned due to the expiration of the applicable time periods set forth above) shall accelerate and be payable to PS Spine at their respective maximum potential amounts upon the occurrence of such change of control or sale of coflex® spinal fixation products.
Withholding Rights
Each of RTI and Holdco will be entitled to deduct and withhold from the consideration payable pursuant to the Master Transaction Agreement such amounts as it is required to deduct and withhold with respect to the making of such payment under any applicable tax law. All amounts deducted or withheld shall be treated for all purposes under the Master Transaction Agreement as having been paid to the person in respect of which such deduction and withholding was made.
Representations and Warranties
The Master Transaction Agreement contains representations and warranties made by PS Spine to RTI and by RTI, Holdco and Merger Sub to PS Spine. The representations and warranties and other provisions of the Master Transaction Agreement should not be read alone, but instead should be read only in conjunction with the information provided elsewhere in this joint proxy and consent solicitation statement/prospectus and in the documents incorporated by reference into this joint proxy and consent solicitation statement/prospectus. See the section entitled “Where You Can Find More Information”, beginning on page 187.
PS Spine made a number of representations and warranties to RTI in the Master Transaction Agreement, including representations and warranties relating to the following matters:
| • | organization, organizational power, qualifications to do business and good standing in each applicable jurisdiction of PS Spine and Paradigm; |
| • | authorization and board approval for PS Spine to enter into and carry out its obligations under the Master Transaction Agreement; |
| • | absence of certain breaches, violations or conflicts arising out of the execution or performance of the Master Transaction Agreement or completion of the transactions contemplated thereby by PS Spine or Paradigm; |
| • | required governmental approvals or consents; |
| • | title to units of Paradigm, capitalization and capital structure of Paradigm and its subsidiaries; |
| • | financial statements; |
| • | absence of undisclosed liabilities; |
| • | internal controls and accounts receivable; |
| • | absence of certain changes since August 31, 2018; |
| • | real property; |
| • | tax matters; |
| • | disclosure of certain material contracts and the absence of breaches of such contracts; |
128
| • | intellectual property rights and information technology matters; |
| • | absence of litigation or outstanding governmental orders; |
| • | employee benefits; |
| • | insurance; |
| • | compliance with applicable legal requirements and permits; |
| • | environmental matters; |
| • | related party transactions; |
| • | employment related matters; |
| • | material customers and suppliers; |
| • | broker’s fees; |
| • | compliance with anti-corruption laws and the absence of certain payments; |
| • | device regulatory matters; |
| • | healthcare regulatory matters; |
| • | title, condition and sufficiency of assets; |
| • | data privacy and protection matters; |
| • | product warranty matters; |
| • | information supplied for inclusion in this joint proxy and consent solicitation statement/prospectus and the registration statement on FormS-4, of which this joint proxy and consent solicitation statement/prospectus forms a part; and |
| • | the Required PS Spine Vote (as defined above). |
RTI, Holdco and Merger Sub made a number of representations and warranties to RTI in the Master Transaction Agreement, including representations and warranties relating to the following matters:
| • | organization, organizational power, qualifications to do business and good standing in each applicable jurisdiction; |
| • | authorization and board approval to enter into and carry out its obligations under the Master Transaction Agreement; |
| • | absence of certain breaches, violations or conflicts arising out of the execution or performance of the Master Transaction Agreement or completion of the transactions contemplated thereby; |
| • | required governmental approvals or consents; |
| • | capitalization and capital structure of RTI and its subsidiaries; |
| • | subsidiaries of RTI; |
| • | absence of litigation or outstanding governmental orders; |
| • | RTI’s SEC filings since January 1, 2016 and the financial disclosures included therein; |
| • | RTI’s internal disclosure controls; |
| • | absence of certain changes since June 30, 2018; |
| • | absence of undisclosed liabilities; |
| • | compliance with applicable legal requirements; |
129
| • | tax matters; |
| • | intellectual property rights; |
| • | healthcare regulatory matters; |
| • | brokers’ fees; |
| • | financing and solvency; |
| • | information supplied for inclusion in this joint proxy and consent solicitation statement/prospectus and the registration statement on FormS-4, of which this joint proxy and consent solicitation statement/prospectus forms a part; and |
| • | state takeover laws. |
Many of the representations and warranties of PS Spine and RTI, Holdco and Merger Sub are qualified by, among other things, exceptions relating to the absence of a “PS Spine material adverse effect” or an “RTI material adverse effect”, which means any event, change, occurrence, fact, development or effect that (a) would reasonably be expected to, individually or in the aggregate, prevent, materially delay or materially impair the ability of PS Spine or its subsidiaries, or RTI and its subsidiaries, as applicable, to consummate the transactions contemplated by the Master Transaction Agreement or (b) has had or would reasonably be expected to have, individually or in the aggregate, a material adverse effect on the business, condition (financial or otherwise) or results of operations of PS Spine or its subsidiaries, or RTI and its subsidiaries, as applicable, in each case taken as a whole, other than any event, change, occurrence, fact, development or effect arising out of, attributable to or resulting from:
| • | general business or economic conditions generally affecting the industries in which such party and its subsidiaries operate; |
| • | changes in political conditions or general economic, business, regulatory, political or market conditions; |
| • | epidemics, pandemics, natural disasters, calamities and other force majeure events; |
| • | changes in applicable laws or accounting regulations or principles or interpretations thereof; |
| • | changes or effects primarily caused by the announcement or pendency of the transactions contemplated by the Master Transaction Agreement; |
| • | any failure to meet projects or forecasts; |
| • | in respect of RTI, any decline in the price or trading volume of RTI’s common stock on Nasdaq; |
| • | any actions expressly required by the Master Transaction Agreement or the failure to take any specific action expressly prohibited by the Master Transaction Agreement; and |
| • | any change resulting or arising from the identity of the other party. |
Except that, with respect to the first four bullets above, such changes will be considered in determining whether a material adverse effect has occurred, or would reasonably be expected to occur, to the extent that it has, individually or in the aggregate, a disproportionate effect on such party as compared to other companies operating in the same industries.
Conduct of Business Prior to Closing
PS Spine has agreed that, prior to the closing of the Transaction, unless RTI shall have consented, as required by applicable law, with respect to any interim financing or as expressly contemplated by the Master Transaction Agreement, it will cause Paradigm and its subsidiaries to conduct their business in the ordinary course consistent in all material respects with past practice and to use their commercially reasonably efforts to preserve intact its current
130
business organization and ongoing operations and maintain relations and goodwill with suppliers, customers, landlords, employees and creditors with whom such entity has a relationship, and maintain the properties and assets of such entity in their current state of repair and condition (excluding normal wear and tear).
In addition, PS Spine has agreed, until the closing date, unless RTI shall have consented, as required by applicable law, with respect to any interim financing or as expressly contemplated by the Master Transaction Agreement, it will not and will not permit Paradigm or any of its subsidiaries to, among other things:
| • | issue, sell or deliver any of Paradigm’s or any of its subsidiaries equity securities or issue or sell any securities convertible into, or options with respect to, or warrants to purchase or rights to subscribe for, any of Paradigm’s or any of its subsidiaries’ equity securities; |
| • | recapitalize, reclassify, combine, split, subdivide or redeem, declare any stock or equity dividend, purchase or otherwise acquire or otherwise make any change in, directly or indirectly, Paradigm’s or any of its subsidiaries’ equity interests or make any other change with respect to Paradigm’s or any of its subsidiaries’ capital structure; |
| • | amend its governing documents; |
| • | make any redemption or purchase of its equity interests; |
| • | create any new subsidiary; |
| • | sell, assign or transfer any material portion of its tangible assets (other than sales of inventory in the ordinary course of business), or mortgage, encumber, pledge, or impose any encumbrance upon any of its assets, other than permitted liens; |
| • | adopt a plan of complete or partial liquidation, dissolution, merger or consolidation of any of Paradigm or any of its subsidiaries; |
| • | sell, assign, transfer or exclusively license any material patents, trademarks, trade names or copyrights; |
| • | enter into any contract, agreement or arrangement that would be a mater contract if entered into prior to the date of the Master Transaction Agreement (other than a renewal or replacement of any existing contract that is expiring by its terms, so long as the terms and conditions of such renewal or replacement contract, agreement or arrangement, in the aggregate, are not materially less favorable to Paradigm or its applicable subsidiary than the existing contract); |
| • | terminate, cause the termination of, amend or modify any material contract (subject to limited exceptions) in any material respect (other than in the ordinary course of business), or waive or release any rights or claims thereunder; |
| • | pay, discharge or satisfy any material claims or liabilities other than in the ordinary course of business, or fail to pay or otherwise satisfy (except if being contested in good faith) any material accounts payable, liabilities, or obligations when due and payable, in each case, other than liabilities or obligations due and payable under certain limited exceptions; |
| • | directly or indirectly, merge with or into, consolidate with or acquire any material asset out of the ordinary course of business; |
| • | make any capital contributions to, or investments in, or any advance or loan to, or acquire the securities of, any person other than a wholly owned subsidiary of Paradigm; |
| • | make any capital expenditures or commitments therefor other than those reflected in Paradigm’s budget as of the date of the Master Transaction Agreement or in an amount not to exceed $250,000; |
| • | in the case of Paradigm or any subsidiary of Paradigm, enter into any transaction with any of its directors, officers or employees outside the ordinary course of business; |
| • | except as required under the terms of any written agreement or Paradigm employee benefit plan as in effect on the date of the Master Transaction Agreement, (A) increase salaries, bonuses or other compensation or |
131
remuneration and benefits payable by Paradigm or any of its subsidiaries to any of its employees, officers, directors or other service providers other than increases in base compensation in the ordinary course of business; (B) increase the benefits provided to any person under any Paradigm employee benefit plan; (C) hire or engage the services of any person with annual base compensation in excess of $200,000; or (D) terminate or amend any Paradigm employee benefit plan or adopt any new arrangement for the benefit or welfare of any officer or employee, director or other service provider of Paradigm or any of its subsidiaries that would be a Paradigm employee benefit plan if it were in existence as of the date of the Master Transaction Agreement (other than in conjunction with agreements or arrangements that are entered into in the ordinary course of business with new hire employees); |
| • | settle any legal proceeding if (A) the amount payable by Paradigm or any of its subsidiaries in connection therewith would exceed $200,000 or (B) would be reasonably likely to have a material effect on the post-closing operations of the business of Paradigm or any of its subsidiaries or agree to any injunctive or other equitable relief; |
| • | cancel any material third-party indebtedness owed to Paradigm or any of its subsidiaries; |
| • | recognize any labor union or enter into, modify, or amend any collective bargaining agreement or engage in any substantive communications with any labor union regarding any party’s anticipated actions on or after the closing with respect to such union; |
| • | in the case of Paradigm or any of its subsidiaries, prepare or file any tax return inconsistent with past practice or, on any such tax return, take any position or adopt any method that is inconsistent with positions taken or methods used in preparing or filing similar tax returns in prior periods, settle or compromise any claim relating to taxes, or otherwise settle any dispute relating to taxes; |
| • | in the case of Paradigm or any of its subsidiaries, make, modify or revoke any income or other material election with respect to taxes, consent to any waiver or extension of time to assess or collect any taxes payable by Paradigm or any of its subsidiaries, file any amended tax returns or fail to file for or otherwise surrender any refund claim on behalf of Paradigm or any of its subsidiaries; or |
| • | agree, whether orally or in writing, to do any of the foregoing, or agree, whether orally or in writing, to any action or omission that would result in any of the foregoing. |
RTI has agreed that, prior to the closing of the Transaction, unless PS Spine shall have consented, as required by applicable law or as expressly contemplated by the Master Transaction Agreement, it will and will cause its subsidiaries to conduct their business in the ordinary course consistent in all material respects with past practice and in compliance with applicable law.
In addition, RTI has agreed, until the closing date, unless PS Spine shall have consented, as required by applicable law, as set forth in RTI’s SEC reports filed or furnished to the SEC after January 1, 2017 or as expressly contemplated by the Master Transaction Agreement, it will not and will not permit any of its subsidiaries to, among other things:
| • | make any amendment or modification to any of RTI’s governing documents; |
| • | issue any additional shares of capital stock, membership interests or partnership interests or other equity securities or grant any option, warrant or right to acquire any capital stock, membership interests or partnership interests or other equity securities or issue any security convertible into or exchangeable for such securities or alter in any way any of its outstanding securities or make any change in outstanding shares of capital stock, membership interests or partnership interests or other ownership interests or its capitalization, whether by reason of a reclassification, recapitalization, stock split or combination, exchange or readjustment of shares, stock dividend or otherwise, except, in each case, for (A) grants of RTI equity awards in the ordinary course of business, or (B) shares of RTI common stock issuable upon settlement or exercise, as applicable, of outstanding RTI equity awards in accordance with their terms; |
| • | declare, set aside or pay dividends or other distributions (whether payable in cash, stock, property or a combination thereof) in respect of the capital stock of RTI; |
132
| • | redeem, retire, purchase or otherwise acquire, directly or indirectly, any shares of the capital stock, membership interests or partnership interests or other ownership interests of RTI or any of its subsidiaries, other than (A) in connection with (x) required tax withholding in connection with the vesting and/or exercise of RTI equity awards and (y) forfeitures of RTI equity awards pursuant to their terms as in effect on the date of the Master Transaction Agreement or (B) purchases or acquisitions of RTI common stock to offset dilution from RTI stock options and other RTI equity awards; |
| • | adopt a plan of complete or partial liquidation or dissolution, or acquire another business, in each case, to the extent such action would, or would reasonably be expected to, prevent, materially delay or materially impair the consummation of the transactions contemplated by the Master Transaction Agreement; |
| • | adopt a plan of merger or consolidation, enter into any binding share exchange, business combination or similar transaction with another person or restructure or reorganize, in each case, to the extent (i) such action is not an action contemplated by the immediately preceding bullet, (ii) such action would, or would reasonably be expected to, prevent, materially delay or materially impair the consummation of the transactions contemplated by the Master Transaction Agreement and (iii) the RTI board has not effected a change in recommendation in connection with such action; or |
| • | agree, whether orally or in writing, to do any of the foregoing, or agree, whether orally or in writing, to any action or omission that would result in any of the foregoing. |
Exclusive Dealing
Until the closing or earlier termination of the Master Transaction Agreement, the Master Transaction Agreement generally prohibits PS Spine, Paradigm and its subsidiaries from taking any action to directly or indirectly initiate, solicit or engage in discussions or negotiations with, or knowingly provide any information to, any person concerning any purchase of any of the equity interests of Paradigm or any merger, sale of substantially all of the assets of Paradigm or its subsidiaries or similar transactions involving Paradigm or its subsidiaries.
Efforts to Consummate
Subject to the terms and conditions of the Master Transaction Agreement, RTI, Holdco, Merger Sub and PS Spine will each use their respective reasonable best efforts to take, or cause to be taken, all actions and to do, or cause to be done, all things reasonably necessary, proper or advisable to consummate and make effective as promptly as practicable the transactions contemplated by the Master Transaction Agreement and will not take any action specifically intended to prevent the closing. During the period prior to the earlier termination of the Master Transaction Agreement and the closing date, RTI shall, and shall cause its subsidiaries to, and PS Spine shall, and shall cause its subsidiaries to, use their respective commercially reasonable efforts to secure any consents, waivers and approvals of any third party (other than in respect of any regulatory approvals) required to be obtained by RTI, PS Spine or any of their subsidiaries, as applicable, to consummate the transactions contemplated by the Master Transaction Agreement.
In furtherance and not in limitation of the foregoing, in the event that any “fair price,” “moratorium,” “control share acquisition” or other anti-takeover law is or becomes applicable to any of the transactions contemplated by the Master Transaction Agreement, any Paradigm Support Agreement or any RTI Support Agreement, PS Holdco, RTI, Holdco and Merger Sub shall use their reasonable best efforts to ensure that the transactions contemplated by the Master Transaction Agreement, the Paradigm Support Agreements and the RTI Support Agreements may be consummated as promptly as reasonably practicable on the terms and subject to the conditions set forth in the Master Transaction Agreement and otherwise eliminate or minimize the effects of such law on the Master Transaction Agreement, the Paradigm Support Agreements and the RTI Support Agreements and the transactions contemplated thereby.
133
Antitrust Filings and Related Actions
RTI, PS Spine, Holdco and Merger Sub agreed to:
| • | make an appropriate filing of a notification and report form pursuant to the HSR Act within ten business days after the date of the Master Transaction Agreement, and any other applicable regulatory law with respect to the transactions contemplated by Master Transaction Agreement as promptly as practicable and advisable following the date of the Master Transaction Agreement; |
| • | respond promptly to any requests for additional information made by any governmental entity; and |
| • | upon the terms and subject to the conditions of the Master Transaction Agreement, use their reasonable best efforts to cause the expiration or termination of any applicable waiting periods under any applicable laws as soon as reasonably practicable. |
In connection with the efforts referenced above, to obtain all necessary consents, approvals, waivers and authorizations of any governmental entity required, each of RTI, PS Spine, Holdco and Merger Sub agreed to use their reasonable best efforts to lift any restraint, injunction or other legal bar to the transactions contemplated by the Master Transaction Agreement, including if necessary to obtain clearance before the outside date committing, agreeing or submitting (or offering to commit, agree or submit) to any consent decree, hold separate order, sale, divestiture, lease, license, transfer, disposal, encumbrance, other change or restructuring of, or operating restriction with respect to the businesses, properties, product lines, assets, permits, operations, rights, or interest therein of RTI or its subsidiaries, or Paradigm or its subsidiaries, and any other actions that limit the freedom of action with respect to, or the ability to retain, any business or assets of RTI or its subsidiaries or Paradigm or its subsidiaries (all of the foregoing, a “Divestiture Action”).
Notwithstanding the foregoing, neither RTI nor any of its subsidiaries shall be required to, and Paradigm and its subsidiaries shall not, without the prior written consent of RTI:
| • | commit, agree, submit (or offer to commit, agree or submit) to any Divestiture Action, if doing so would, individually or in the aggregate, reasonably be expected to be material to (1) RTI and its subsidiaries or (2) Paradigm and its subsidiaries (in each case of (1) and (2), as measured on a scale relative to Paradigm and its subsidiaries, taken as a whole); or |
| • | commit, agree, or submit (or offer to commit, agree, or submit) to any Divestiture Action not conditioned on the consummation of the Transaction. |
In addition, each of RTI, PS Spine, Holdco and Merger Sub shall use its commercially reasonable efforts to defend through litigation on the merits any claim asserted in court by any person in order to avoid entry of, or to have vacated or terminated, any order (whether temporary, preliminary or permanent) that would prevent the closing.
Registration, RTI Stockholder Approval and PS Spine Unitholder Approval
RTI, Holdco and PS Spine have agreed to cause this joint proxy and consent solicitation statement/prospectus to be mailed to RTI stockholders and PS Spine equity holders as soon as reasonably practicable after the registration statement on FormS-4, of which this joint proxy and consent solicitation statement/prospectus forms a part, is declared effective, and to otherwise use their respective reasonable best efforts to solicit approval of their respective stockholders or unitholders, as applicable, either by written consent or by holding a special meeting of the stockholders.
RTI has agreed to solicit, by holding a special meeting of the RTI stockholders, adoption of the Master Transaction Agreement. See the section entitled “RTI Proposal 1: Merger Proposal”, beginning on page 65.
Similarly, PS Spine has agreed to solicit, by written consent, the adoption and approval of the Master Transaction Agreement by the affirmative vote of its unitholders holdingtwo-thirds (2/3) of the outstanding Common Units, Class A Common Units, Class A Preferred Units, Class B Preferred Units,Class E-1 Preferred Units and Class F Preferred Units of PS Spine.
134
Holdco and RTI have agreed to use their reasonable best efforts to cause the shares of Holdco common stock to be issued in connection with the transactions contemplated by the Master Transaction Agreement to be approved for listing on the Nasdaq Global Market prior to the closing.
Financing and Financing Cooperation
Financing Cooperation
PS Spine and each of its subsidiaries will assist RTI in connection with the financing arrangements contemplated by the First Lien Consent and Second Lien Commitment Letters (the “Commitment Letters”). Specifically, PS Spine and each of its subsidiaries will, at RTI’s sole expense: (i) participate and make the appropriate senior offices of Paradigm available for meetings, including in particular, due diligence sessions and sessions with prospective rating agencies; (ii) cooperate with the marketing efforts and due diligence efforts of RTI and its financing sources, including assisting with private placement memoranda, syndication memoranda and other marketing materials; (iii) provide RTI and its financing sources with the required financial statements and other pertinent information regarding the business of Paradigm and its subsidiaries and promptly provide any additional information to supplement this information, if to the knowledge of PS Spine, such information contains any material misstatement of fact or omits to state any material fact; (iv) execute and deliver any loan documents, including, but not limited to, credit agreements, notes, guarantees and solvency certificates; (v) take certain actions as are reasonably requested by RTI to assist with collateral audits and due diligence examinations in connection with determining the collateral eligibility criteria and other related criteria contemplated by the Commitment Letters; (vi) assist with the borrowing base certificate and facilitate the grant of the security interest in the collateral; and (vii) at least five business days prior to closing, provide all documentation and other information of Paradigm and its subsidiaries required to comply with the applicable “know your customer” and anti-money laundering rules and regulations. In connection with the “know your customer” requirements, RTI will request from the financing sources to the Commitment Letters a list of the documents and other information required to comply with such rules and regulations.
Notwithstanding the foregoing, PS Spine and its subsidiaries will not be required to provide documentation or cooperate with the financing arrangements contemplated by the Commitment Letters to the extent it would prevent a closing condition under the Master Transaction Agreement to be satisfied, cause a breach under the Master Transaction Agreement, conflict with or violate PS Spine’s or its subsidiaries’ organizational documents or any applicable laws, or require PS Spine or any of its subsidiaries to provide legal opinions or reliance letters which are not required under the Master Transaction Agreement.
Upon the request of PS Spine, RTI will promptly reimburse PS Spine for all reasonable and documentedout-of-pocket costs and expenses incurred by PS Spine and its subsidiaries in connection with cooperating and assisting with the financing arrangements contemplated by the Commitment Letters. Additionally, RTI and Holdco will jointly and severally indemnify and hold harmless PS Spine, its subsidiaries and their respective representatives from and against all liabilities, losses, damages, claims and reasonable and documentedout-of-pocket costs or expenses suffered in connection with the financing arrangements contemplated by the Commitment Letters.
Financing
RTI will use reasonable efforts to consummate the financing on a timely basis in accordance with the terms and conditions of the Commitment Letters and cause the financing sources to such Commitment Letters to fund the full amount of the financing on the closing date. If any portion of the financing becomes unavailable on the terms and conditions contemplated in the Commitment Letters, then RTI will provide prompt notice to PS Spine and use reasonable best efforts to obtain alternative financing on terms that are not materially less favorable to RTI than the terms of the Commitment Letters (including flex provisions). Additionally, RTI will keep PS Spine informed regarding the financing and will provide prompt notice if RTI becomes aware of any material adverse change with respect to the availability of the financing, including, but not limited to, any material breach, cancellation or termination of the Commitment Letters.
RTI may amend, modify, supplement or waive any provision of the Commitment Letters or definitive agreements relating to the financing to add or replace lenders, modify pricing (including the exercise of any of the flex provisions) or
135
reallocate commitments. Without the prior written consent of PS Spine, RTI will not amend, modify, supplement or waive any provision of the Commitment Letters that would (i) decrease the aggregate amount of the financing below the amount to fund the Acquisition, pay existing indebtedness and fees and expenses or (ii) impose new or additional conditions that would reasonably be expected to delay the consummation of the transactions contemplated by the Master Transaction Agreement.
A violation of the agreements between RTI and PS Spine will not give rise to a termination right in favor of PS Spine to decline to consummate the closing on the terms and conditions set forth in the Master Transaction Agreement, if RTI and the other parties satisfy their obligations to consummate the closing.
Employee Matters
For at least one year following the closing, Holdco has agreed to provide employees of Paradigm and its subsidiaries as of the closing who continue employment with Holdco and its subsidiaries (“Affected Employees”) with salaries, annual bonus opportunities (excluding equity and long-term incentive awards), and employee benefits that are substantially comparable in the aggregate to those provided to the Affected Employees immediately prior to the closing. Holdco will also cause each Affected Employee to receive full credit for service accrued with Paradigm and its subsidiaries as of immediately prior to the closing for all purposes (except for benefit accrual under any defined benefit pension plan) under employee benefit plans maintained by Holdco (or any of its subsidiaries) in which the Affected Employee may be eligible to participate. Holdco has also agreed that it will use commercially reasonable efforts to (1) waive all limitations as toat-work conditions with respect to participation and coverage requirements under any Holdco welfare benefit plan to the same extent waived under an analogous Paradigm welfare benefit plan; (2) take into account eligible expenses incurred by any Affected Employee or dependent under a welfare employee benefit plan during the plan year in which the individual transitions coverage to an analogous Holdco welfare benefit plan for purposes of satisfying deductible,co-insurance, and maximumout-of-pocket requirements; and (3) waive waiting period limitations or evidence of insurability requirements that would otherwise be applicable to an Affected Employee and dependents during the plan year in which the individuals transition coverage to an analogous Holdco welfare benefit plan.
The Master Transaction Agreement requires PS Spine to cause Paradigm and its subsidiaries to take all actions necessary or appropriate, prior to the closing date, to terminate each 401(k) plan in which employees of Paradigm or its subsidiaries participate (a “401(k) Plan”), unless RTI agrees to sponsor any 401(k) Plan by providing notice to PS Spine prior to the closing date. If RTI does not agree to sponsor any 401(k) Plan, PS Spine must terminate such 401(k) Plan or Paradigm’s and its subsidiaries’ participation in such 401(k) Plan prior to the closing date.
Indemnification and Directors’ and Officers’ Insurance
Pursuant to the Master Transaction Agreement, Holdco has agreed that, for a period of at least six years following the closing, it will, or will cause its applicable subsidiary to, to the fullest extent permitted by law, indemnify the current and former employees, directors, managers and officers of Paradigm and its subsidiaries, to the same extent as provided in the organizational documents of Paradigm and its subsidiaries as of the date of the Master Transaction Agreement, with respect to their activities on behalf of Paradigm or any of its subsidiaries prior to the closing.
In addition, prior to the closing, Paradigm will purchase, at RTI’s sole cost and expense, asix-year prepaid directors’ and officers’ insurance and fiduciary liability insurance tail policy, with respect to matters existing or occurring at or prior to the closing, covering, without limitation, the transactions contemplated by the Master Transaction Agreement. Holdco has agreed that, following the closing, it will not terminate or amend that tail policy and it will cause Paradigm and its subsidiaries to use commercially reasonable efforts to recover amounts under that tail policy. The Master Transaction Agreement also provides that PS Spine will indemnify Holdco, by withholding, reducing and/or setting off against the Earnout Consideration otherwise payable pursuant to the Master Transaction Agreement, for any amount by the which the liabilities of Paradigm and its subsidiaries to indemnify or hold harmless any director, manager, officer or employee of Paradigm or any of its subsidiaries for claims that relate to periods prior to the closing exceeds the amount of all insurance proceeds received from the tail policy on account of those liabilities.
136
The current and former employees, directors, managers and officers of Paradigm and its subsidiaries will have the right to enforce the provisions of the Master Transaction Agreement relating to their indemnification and are express third-party beneficiaries of the Master Transaction Agreement for this purpose.
Change in Recommendation
Except as permitted by the immediately following paragraph, the RTI board and each committee thereof shall not change, withhold, withdraw, qualify or modify (or publicly propose or resolve to change, withhold, withdraw, qualify or modify) in a manner adverse to PS Spine, its recommendation that the stockholders of RTI vote in favor of the Merger Proposal, Share Issuance Proposal and the Adjournment Proposal.
Notwithstanding the foregoing, at any time prior to the approval of the Master Transaction Agreement by RTI’s stockholders, the RTI board may change, withhold, withdraw, qualify or modify (or publicly propose or resolve to change, withhold, withdraw, qualify or modify) its recommendation in a manner adverse to PS Spine if the RTI board determines in good faith, after consultation with outside counsel and, if the RTI board reasonably determines, after consultation with outside counsel, it to be necessary in connection with its fulfillment of its fiduciary duties under applicable law, a financial advisor of nationally recognized reputation, that the failure to take such action would be inconsistent with its fiduciary duties under applicable law as a result of any event, fact, circumstances, development or occurrence relating to or affecting RTI or its subsidiaries, including the business, operations, assets or liabilities of RTI or its subsidiaries, and which was not known or reasonably foreseeable to the RTI board as of the date of the Master Transaction Agreement; provided, that neither an actual or anticipated financing failure nor the actual or anticipated consequences thereof shall be an Intervening Event.
No change in recommendation by the RTI board may be made until after 5:00 p.m., New York City time, on the fifth (5th) business day following PS Spine’s receipt of written notice from RTI advising that the RTI board intends to take such action and specifying the reasons therefor. After providing such notice and prior to effecting such change in recommendation, (i) RTI shall, during such five business day period, negotiate in good faith with PS Spine and its representatives, to the extent PS Spine wishes to negotiate, with respect to any revisions to the terms of the transactions contemplated by the Master Transaction Agreement proposed by PS Spine, and (ii) in determining whether it may still under the terms of the Master Transaction Agreement make a change in recommendation, the RTI board shall take into account any changes to the terms of the Master Transaction Agreement proposed by PS Spine and any other information provided by PS Spine in response to such notice during such five business day period.
Tax Matters
The Master Transaction Agreement provides that PS Spine will indemnify and hold harmless RTI, its affiliates and representatives from and against any damages suffered by RTI, its affiliates or representatives or that arise from, are connected with or as a result of, directly and indirectly (i) taxes imposed on Paradigm or its subsidiaries or for which Paradigm or its subsidiaries are liable, as a result of having been Paradigm or its subsidiary during any taxable year or period that ends on or before the closing date, and the portion of any Straddle Period ending on and including the closing date, (ii) taxes imposed on Paradigm and its subsidiaries or for which Paradigm and its subsidiaries are liable, for any taxable year or period that ends on or before the closing date, and with respect to any Straddle Period, the portion of such Straddle Period ending on and including the closing date, (iii) taxes imposed on RTI or its subsidiaries as a result of it being a United States shareholder (within the meaning of Section 951(b) of the Code) of Paradigm or its subsidiaries, to the extent such amounts are attributable to Subpart F income (within the meaning of Section 952(a) of the Code) arising in a taxable year of Paradigm or its subsidiaries ending on or prior to the closing date, or, with respect to any Straddle Period, the portion of such Straddle Period ending on and including the closing date, and (iv) taxes imposed on any cancellation of indebtedness income arising as a result of the transactions contemplated by the Master Transaction Agreement. “Straddle Period” means any taxable period that includes (but does not end on) the closing date.
PS Spine has agreed to prepare or cause to be prepared and timely file or cause to be timely filed when due (i) all tax returns that are required to be filed by or with respect to Paradigm and its subsidiaries on a combined, consolidated
137
or unitary basis with PS Spine, (ii) all income tax returns of or with respect to Paradigm and its subsidiaries for any taxable year or period that ends on or before the closing date and (iii) all other tax returns that are required to be filed by or with respect to Paradigm and its subsidiaries on or prior to the closing date. RTI has agreed to timely file or cause to be timely filed when due all other tax returns that are required to be filed by or with respect to Paradigm and its subsidiaries after the closing date and will remit or cause to be remitted any taxes due in respect of such tax returns.
Subject to limited exceptions, PS Spine will be entitled to receive from Paradigm or its subsidiaries all refunds (or credits for overpayments) of taxes of Paradigm or its subsidiaries (including interest) for any taxable year that ends on or before the closing date and, with respect to any Straddle Period, the portion of such Straddle Period ending on and including the closing date (a “Pre-Closing Refund”).
RTI, its affiliates and representatives sole and exclusive remedy under the Master Transaction Agreement for any indemnifiable taxes is to either (i) withhold and set off against any contingent consideration equal to the amount of damages to which RTI, its affiliates or representatives is entitled to, (ii) permanently reduce the amount of the contingent consideration available to be issued or paid to PS Spine in an amount equal to the amount of damages to which RTI, its affiliates or representatives is entitled to or (iii) reduce the amount of anyPre-Closing Tax Refund due to PS Spine by the amount of damages to which RTI, its affiliates or representatives is entitled to.
Designated Director
Following the closing until the later of (i) December 31, 2022 and (ii) the time at which PS Spine and its unitholders own less than 10% of the outstanding shares of Holdco common stock (calculated on a fully diluted basis), PS Spine shall be entitled to nominate one director to the Holdco board, who shall be Jeffrey C. Lightcap, Anthony G. Viscogliosi or any other person mutually agreed by Holdco and PS Spine. Holdco has agreed to nominate such designated director for election to the Holdco board and use its reasonable best efforts to cause such designated director to be elected to the Holdco board.
Other Covenants
Subject to certain customary limitations, until the earlier termination of the Master Transaction Agreement and the closing date, PS Spine will provide RTI and its authorized representatives with reasonable access during normal business hours, and upon reasonable notice, to the offices, properties, personnel, and all financial books and records of Paradigm and its subsidiaries.
PS Spine has also agreed until the earlier termination of the Master Transaction Agreement and the closing date to cause Paradigm to prepare in the ordinary course of business consistent with past practice, and deliver to RTI promptly upon completion but in any event no later than fifteen days after the end of each fiscal month, consolidated financial statements for Paradigm and its subsidiaries for each fiscal month ending after September 30, 2018.
PS Spine has also agreed to, at least three business days prior to the anticipated closing, deliver to RTI customary payoff letters executed by or on behalf of the lenders of Paradigm’s indebtedness under its existing senior secured credit agreement and any applicable interim debt financing of Paradigm providing for the total amount of cash and shares of Holdco common stock required to be paid at the closing to satisfy such indebtedness and setting forth the lenders’ obligation to release all liens and other security securing such indebtedness.
PS Spine has also agreed to use its reasonable best efforts to cause Paradigm to negotiate and enter into a written master consulting agreement with Musculoskeletal Clinical Regulatory Advisors, LLC in substantially the same form and for substantially the same terms and conditions (and, in each case, in a form and substance reasonably satisfactory to RTI) as set forth in Paradigm’s existing agreement with Musculoskeletal Clinical Regulatory Advisors, LLC, provided that the consideration to be paid by Paradigm under such new agreement shall be equal to in all material respects the consideration paid for the same or similar services set forth in the existing agreement with Musculoskeletal Clinical Regulatory Advisors, LLC.
138
Following the closing until December 31, 2022, Holdco has agreed to use its reasonable best efforts to cause the leader of the sales force for coflex® and SImmetry® products to be William L. Pfost, and shall use its reasonable best efforts to cause progress meetings to be conducted and progress reports to be prepared in respect of such sales on a monthly basis.
Conditions to RTI’s, Holdco’s and Merger Sub’s Obligations to Close
The obligations of RTI, Holdco and Merger Sub to consummate the transactions contemplated by the Master Transaction Agreement are subject to the satisfaction (or, if permitted by applicable law, waiver) of the following conditions:
| • | PS Spine’s representations pertaining to the equity interests of Paradigm and PS Spine’s ownership thereof being true and correct, subject only to de minimis inaccuracies, as of the date of the Master Transaction Agreement and as of the closing date; |
| • | PS Spine’s representations pertaining to existence and good standing, authority and enforceability, subsidiaries, tax matters and broker’s fees being true and correct in all material respects as of the date of the Master Transaction Agreement and as of the closing date; |
| • | all other representations and warranties of PS Spine contained in the Master Transaction Agreement being true and correct (without giving effect to any limitation as to materiality or material adverse effect set forth therein) as of the date of the Master Transaction Agreement and as of the closing date (except to the extent expressly made as of an earlier date, in which case only as of such date), except where the failure of such representations and warranties to be so true and correct has not had a material adverse effect; |
| • | PS Spine having performed and complied in all material respects with all of its covenants and agreements required to be performed by it under the Master Transaction Agreement at or prior to the closing; |
| • | there not having been a material adverse effect since the date of the Master Transaction Agreement; |
| • | PS Spine having delivered to RTI a certificate of an officer of PS Spine stating that the conditions set forth in the five bullet points above have been satisfied; |
| • | the representations pertaining to the capitalization of RTI, Holdco and Merger Sub being true and correct, subject only to de minimis inaccuracies, as of the date of the Master Transaction Agreement and as of the closing date; |
| • | PS Spine having obtained the PS Spine written consent; |
| • | the Required Merger Proposal Vote having been obtained; |
| • | the waiting period under the HSR Act having expired or been terminated; |
| • | no governmental entity of competent jurisdiction having enacted, issued, promulgated or entered any order or law that is in effect and that prevents the performance of the Master Transaction Agreement or the consummation of the transactions contemplated thereby, declares unlawful the transactions contemplated by the Master Transaction Agreement or causes such transactions to be rescinded; |
| • | the Cash Consideration Amount being equal to or greater than zero dollars; |
| • | the shares of Holdco common stock to be issued at the closing having been authorized for listing on the Nasdaq Global Market, subject to official notice of issuance; |
| • | the registration statement on FormS-4, of which this joint proxy and consent solicitation statement/prospectus forms a part, having become effective under the Securities Act and no stop order suspending the effectiveness of the registration statement on FormS-4 having been issued by the SEC and remaining in effect and no proceedings for that purpose having been initiated or threatened by the SEC unless subsequently withdrawn; |
139
| • | RTI having received certain representations and covenants in certificates of officers of RTI, PS Spine, and Holdco, which representations and covenants are intended to support the ability of each of PS Spine and RTI to take the position that the Merger and/or the Contribution will qualify for the Intended Tax Treatment; |
| • | neither Marc Viscogliosi nor Francis Magee rescinding his obligations under his applicable consulting agreement with RTI; |
| • | none of Viscogliosi Brothers, LLC, HealthCor Paradigm Blocker Company Two, Inc. and HealthCor AIV, L.P. rescinding its obligations under its applicablenon-competition agreement; and |
| • | none of the parties to the Settlement Agreement (as defined above) rescinding their obligations under the Settlement Agreement, the Settlement Agreement being in full force and effect and not having been amended in any manner adverse to Holdco, RTI or Merger Sub and the transactions contemplated by the Settlement Agreement having been performed in accordance with the terms of the Settlement Agreement. |
The condition that the waiting period under the HSR Act expire or terminate has been satisfied, because the termination of the waiting period under the HSR Act was granted by the FTC on November 26, 2018.
Conditions to PS Spine’s Obligation to Close
The obligations of PS Spine to consummate the transactions contemplated by the Master Transaction Agreement are subject to the satisfaction (or, if permitted by applicable law, waiver) of the following conditions:
| • | the representations pertaining to the capitalization of RTI, Holdco and Merger Sub being true and correct, subject only to de minimis inaccuracies, as of the date of the Master Transaction Agreement and as of the closing date; |
| • | the representations pertaining to existence and good standing, authority and enforceability, preemptive rights and anti-takeover provisions and broker’s fees being true and correct in all material respects as of the date of the Master Transaction Agreement and as of the closing date; |
| • | all other representations and warranties of RTI, Holdco and Merger Sub contained in the Master Transaction Agreement being true and correct (without giving effect to any limitation as to materiality or material adverse effect set forth therein) as of the date of the Master Transaction Agreement and as of the closing date (except to the extent expressly made as of an earlier date, in which case only as of such date), except where the failure of such representations and warranties to be so true and correct has not had an RTI material adverse effect; |
| • | each of RTI, Holdco and Merger Sub having performed and complied in all material respects with all of its covenants and agreements required to be performed by it under the Master Transaction Agreement at or prior to the closing; |
| • | there not having been an RTI material adverse effect since the date of the Master Transaction Agreement; |
| • | RTI shall have delivered to PS Spine a certificate of an officer of RTI stating that the conditions set forth in the five bullet points above have been satisfied; |
| • | PS Spine having obtained the PS Spine written consent; |
| • | the Required Merger Proposal Vote having been obtained; |
| • | the waiting period under the HSR Act having expired or been terminated; |
| • | no governmental entity of competent jurisdiction having enacted, issued, promulgated or entered any order or law that is in effect and that prevents the performance of the Master Transaction Agreement or the consummation of the transactions contemplated thereby, declares unlawful the transactions contemplated by the Master Transaction Agreement or causes such transactions to be rescinded; |
| • | the shares of Holdco common stock to be issued at the closing having been authorized for listing on the Nasdaq Global Market, subject to official notice of issuance; |
140
| • | the registration statement on FormS-4, of which this joint proxy and consent solicitation statement/prospectus forms a part, having become effective under the Securities Act and no stop order suspending the effectiveness of the registration statement on FormS-4 having been issued by the SEC and remaining in effect and no proceedings for that purpose having been initiated or threatened by the SEC unless subsequently withdrawn; |
| • | PS Spine having received certain representations and covenants in certificates of officers of RTI, PS Spine, and Holdco, which representations and covenants are intended to support the ability of each of PS Spine and RTI to take the position that the Merger and/or the Contribution will qualify for the Intended Tax Treatment; and |
| • | RTI not rescinding its obligations under its consulting agreement with Marc Viscogliosi or Francis Magee. |
The condition that the waiting period under the HSR Act expire or terminate has been satisfied, because the termination of the waiting period under the HSR Act was granted by the FTC on November 26, 2018.
The Master Transaction Agreement provides that the representations and warranties made by PS Spine in the Master Transaction Agreement and in the PS Spine Closing Certificate shall survive for 18 months following the closing date, provided that certain representations on anti-corruption laws will survive for five years following the closing date and on existence and good standing, authority and enforceability, title to units and capitalization, subsidiaries, tax matters and broker’s fees shall survive for the applicable statute of limitations plus 60 days.
All representations and warranties made by RTI, Holdco and Merger Sub shall terminate and expire at the closing.
From and after the closing, PS Spine shall indemnify and hold harmless RTI, its affiliates and representatives from and against any damages directly or indirectly suffered by RTI, its affiliates or representatives or that arise from, are connected with or as a result of, directly or indirectly:
| • | any inaccuracy in or breach of any representation or warranty made by PS Spine in the Master Transaction Agreement as of the date of the Master Transaction Agreement or as if such representation or warranty was made as of the closing; |
| • | any inaccuracy in or breach of any representation or warranty made by PS Spine in the PS Spine Closing Certificate; |
| • | any inaccuracy in any information set forth in the certificate executed on behalf of PS Spine by its chief executive officer containing PS Spine’s good faith estimate of certain calculations related to adjustments to Cash Consideration Amount (the “Consideration Certificate”), as more fully described in the Master Transaction Agreement; |
| • | any breach of any covenant or obligation of PS Spine in the Master Transaction Agreement; and |
| • | any liabilities arising from PS Spine’spre-signing reorganization or for any amounts to indemnify or hold harmless any director, manager, officer or employee of Paradigm and its subsidiaries for claims that related to periods prior to the closing in excess of the amount of all insurance proceeds received from the tail policy (the “designated liabilities”). |
PS Spine is not required to make any indemnification payment for any breaches of representations and warranties pursuant to the first two bullet points above until the total amount of all damages that have been suffered or incurred exceeds $850,000 in the aggregate, provided that the deductible shall not apply in cases of fraud or any of the representations on existence and good standing, authority and enforceability, title to units and capitalization, subsidiaries, tax matters, anti-corruption laws and broker’s fees.
141
RTI, together with its affiliates and representatives’, sole and exclusive remedy under the Master Transaction Agreement for any breaches of representations and warranties of PS Spine pursuant to the first two bullet points above is to either (i) withhold and set off against any contingent consideration equal to the amount of damages to which RTI, its affiliates or representatives is entitled to, (ii) permanently reduce the amount of the contingent consideration available to be issued or paid to PS Spine in an amount equal to the amount of damages to which RTI, its affiliates or representatives is entitled to or (iii) reduce the amount of any tax refund attributable to apre-closing tax period and due to PS Spine by the amount of damages to which RTI, its affiliates or representatives is entitled to, in each case up to an aggregate value of $15,000,000. Subject to certain limited exceptions, RTI is also required to withhold, set off or reduce the initial $20,000,000 contingent consideration payment prior to withholding, setting off or reducing any other contingent consideration payment.
The $15,000,000 cap in the immediately preceding paragraph shall not apply, and RTI, its affiliates and representatives shall be entitled to withhold, set off or reduce the contingent consideration in full for (i) cases of fraud, (ii) any breaches or inaccuracies of the representations on existence and good standing, authority and enforceability, title to units and capitalization, subsidiaries, tax matters, anti-corruption laws or broker’s fees or (iii) any claims in respect of inaccuracies in the Consideration Certificate, breaches of any covenant or obligation of PS Spine in the Master Transaction Agreement or any of the designated liabilities.
In the event any shares to be issued as contingent consideration are withheld, set off or reduced, for purposes of such withholding, set off or reduction such shares shall be valued as of when RTI, its affiliates or representatives are determined to be entitled to indemnification pursuant to the Master Transaction Agreement.
Each of RTI and its affiliates and representatives are required to use their commercially reasonable efforts to mitigate the amount of any damages incurred in connection with any matter with respect to which it is entitled to indemnification or reimbursement from PS Spine, including by pursuing recovery under available insurance policies.
Termination of the Master Transaction Agreement
The Master Transaction Agreement may be terminated at any time prior to the closing:
| • | by the mutual written consent of RTI and PS Spine; |
| • | by RTI upon written notice to PS Spine, if the PS Spine written consent is not delivered to RTI within five business days following the effectiveness of the registration statement on FormS-4, of which this joint proxy and consent solicitation statement/prospectus forms a part; |
| • | by RTI upon written notice to PS Spine, if any of PS Spine’s representations and warranties will not be true and correct, or PS Spine has failed to perform any covenant or agreement on the part of PS Spine, such that the conditions to closing set forth above in the section entitled “—Conditions to the Closing” would not be satisfied at or prior to the outside date and the breach or failure to perform is not cured or capable of being cured within thirty (30) days after written notice thereof is delivered to PS Spine (provided that RTI is not permitted to terminate pursuant to this clause if it, Holdco or Merger Sub is then in breach of any of its respective representations, warranties, covenants or agreements such that the conditions to closing set forth above in the section entitled “—Conditions to the Closing” would not be satisfied); |
| • | by PS Spine upon written notice to RTI, if any of RTI’s, Holdco’s or Merger Sub’s representations and warranties will not be true and correct, or RTI, Holdco or Merger Sub has failed to perform any covenant or agreement on the part of RTI, Holdco or Merger Sub, such that the conditions to closing set forth above in in the section entitled “—Conditions to the Closing” would not be satisfied at or prior to the outside date (as defined below) and the breach or failure to perform is not cured or capable of being cured within thirty (30) days after written notice thereof is delivered to RTI (provided that PS Spine is not permitted to terminate pursuant to this clause if it is then in breach of any of its respective representations, warranties, covenants or agreements such that the conditions to closing set forth above in in the section entitled “—Conditions to the Closing” would not be satisfied); |
142
| • | by RTI or PS Spine by written notice to the other if the closing has not occurred on or prior to the outside date, provided that if the closing has not occurred by January 31, 2019, the outside date may be extended by RTI to February 28, 2019 and provided, further, that if the closing has not occurred by February 28, 2019, the outside date may be extended by RTI to March 31, 2019 (which extensions RTI intends to exercise, as necessary), provided, further that the party seeking to terminate pursuant to this clause will not have breached in any material respect the Master Transaction Agreement in any manner that would have proximately caused the failure to consummate the transactions contemplated by the Master Transaction Agreement on or prior to the outside date; |
| • | by RTI or PS Spine if the Required Merger Proposal Vote is not obtained at the RTI special meeting, or any adjournment or postponement thereof; |
| • | by RTI or PS Spine if any law or order is enacted, issued, promulgated or entered by a governmental entity permanently restraining, enjoining or otherwise prohibiting the consummation of the Merger or the Contribution which is final andnon-appealable; provided that the party seeking to terminate shall not have breached in any material respect the Master Transaction Agreement in any manner that will have caused such law or order to be enacted, issued, promulgated or entered; |
| • | PS Spine upon written notice to RTI if the RTI board shall have made a change in recommendation that the RTI stockholders vote to approve the Merger Proposal, Share Issuance Proposal and the Adjournment Proposal, provided that PS Spine may not terminate pursuant to this clause if the Required Merger Proposal Vote has been obtained; and |
| • | by PS Spine upon written notice to RTI if (i) all of the conditions to closing are satisfied (other than conditions that by their terms will be satisfied at closing or conditions that have not been or will not be satisfied as a result of RTI, Holdco or Merger Sub’s breach of, or failure to perform any of its covenants set forth in, the Master Transaction Agreement, (ii) PS Spine has provided notice to RTI that it is prepared, ready, willing and able to consummate the closing and (iii) Holdco, RTI and Merger Sub fail to consummate the closing within three business days after the delivery of such notice and PS Spine stood ready, willing and able to consummate the closing throughout such period. |
Effect of Termination
If the Master Transaction Agreement is terminated, all obligations of the parties will terminate without any liability of any party, provided that certain designated provisions of the Master Transaction Agreement will survive the termination, including those related to indemnification and reimbursement of fees in connection with PS Spine’s financing cooperation obligations and those related to the payment of fees and expenses set forth below and each party will remain liable for any willful and material breach of the Master Transaction Agreement.
Termination Fees
RTI is required to pay a termination fee of $4,500,000 in the event the Master Transaction Agreement is terminated (i) by PS Spine if the RTI board has made a change in recommendation or (ii) by PS Spine or RTI due to the occurrence of the outside date or if the Required RTI Vote shall not have been obtained and, at the time of such termination, PS Spine had the right to terminate the Master Transaction Agreement due to the RTI board having made a change in recommendation. In addition, if such $4,500,000 termination fee becomes payable at a time when RTI, Holdco or Merger Sub is in breach of its obligations described in “—Covenants and Agreements—Financing and Financing Cooperation” above, such that the condition to closing set forth above in the section entitled “—Conditions to the Closing” would not be satisfied, RTI will instead pay PS Spine a termination fee of $9,000,000 (or, if RTI has already paid the $4,500,000 termination fee, it will pay PS Spine an additional $4,500,000).
RTI is required to pay a termination fee of $9,000,000 in the event the Master Transaction Agreement is terminated (i) by PS Spine if (A) all of the conditions to RTI’s, Holdco’s and Merger Sub’s obligation to close are satisfied (other than conditions that by their terms will be satisfied at closing or conditions that have not been or will not be satisfied as a result of RTI, Holdco or Merger Sub’s breach of, or failure to perform any of its covenants set
143
forth in, the Master Transaction Agreement), (B) PS Spine has provided notice to RTI that it is prepared, ready, willing and able to consummate the closing and (C) Holdco, RTI and Merger Sub fail to consummate the closing within 3 business days after the delivery of such notice and PS Spine stood ready, willing and able to consummate the closing throughout such period, or (ii) by PS Spine or RTI due to the occurrence of the outside date and, at the time of such termination, PS Spine had the right to terminate the Master Transaction Agreement pursuant to clause (i) of this paragraph.
If RTI fails to pay any termination fee when due, and in order to obtain such payment, PS Spine commences a suit against RTI that results in a judgment against RTI for such amount, RTI is required to pay PS Spine its reasonable costs and expenses in connection with such suit and interest on the amount payable pursuant to such judgment. For the avoidance of doubt, RTI shall not be required to pay both termination fees to PS Spine (although, in certain circumstances described above, if it has already paid the $4,500,000 termination fee, it will be required to pay PS Spine an additional $4,500,000).
The parties have agreed that if a termination fee becomes due and payable pursuant to the Master Transaction Agreement, the receipt of such termination fee, together with any reimbursement, indemnification or interest owed pursuant to the terms of the Master Transaction Agreement, shall be deemed to be liquidated damages and the sole and exclusive remedy of PS Spine and its affiliates, and following receipt of such termination fee, none of RTI, RTI’s affiliates or RTI’s representatives shall have any other liability to PS Spine in connection with the Master Transaction Agreement.
Amendment
The Master Transaction Agreement may be amended only in writing signed by RTI and PS Spine, provided that after the receipt of the PS Spine written consent no amendment may be made that by law requires further approval of the unitholders of PS Spine without their consent and after the receipt of the Required Merger Proposal Vote, no amendments may be made that by law requires further approval of the RTI stockholders without their approval.
Specific Performance
Each party to the Master Transaction Agreement acknowledged and agreed that a breach by it of the Master Transaction Agreement would cause irreparable damage to the other parties to the Master Transaction Agreement and that no party to the Master Transaction Agreement will have an adequate remedy at law. Therefore, it was agreed that prior to the termination of the Master Transaction Agreement each party will be entitled to specific performance and injunctive relief to prevent breaches of the Master Transaction Agreement and to enforce specifically the terms and provisions of the Master Transaction Agreement.
Notwithstanding the immediately preceding paragraph, PS Spine shall be entitled to specific performance of Holdco’s obligations to pay the Cash Consideration Amount and issue the Stock Consideration Amount to PS Spine and to consummate the closing solely in the event that the following conditions are satisfied: (i) all of the conditions to RTI’s, Holdco’s and Merger Sub’s obligation to close (other than any conditions that by their terms are to be satisfied at the closing, but subject to such conditions being satisfied if the closing would have occurred) have been satisfied or validly waived by RTI, Holdco and Merger Sub and the Master Transaction Agreement has not been terminated, (ii) PS Spine has notified Holdco, RTI and Merger Sub in writing that (A) PS Spine is prepared to consummate the closing and (B) if specific performance is granted and the debt financing is funded, then it would take such actions required of it under the Master Transaction Agreement to cause the closing to occur, (iii) Holdco, RTI and Merger Sub have not consummated the closing by 12:00 p.m. New York City time on the second business day following the date on which the closing was required to occur pursuant to the Master Transaction Agreement and (iv) the debt financing has been funded or will be funded at the closing.
144
On November 1, 2018, in connection with its entry into the Master Transaction Agreement, RTI entered into (i) a Consent Under Credit Agreement (“First Lien Consent”) with JPM pursuant to which JPM agreed to amend the Existing Revolving Credit Agreement in order to, among other things, (a) reduce the aggregate revolving credit commitments under the Existing Revolving Credit Agreement from $100 million to $75 million (the “First Lien ABL Credit Facility”) and (b) consent to the incurrence of the Second Lien Facility (as defined below) and (ii) a Commitment Letter (the “Second Lien Commitment Letter”) with Ares Capital Corporation (“Ares”) pursuant to which Ares agreed to provide the Second Lien Facility in a single draw on the closing date in connection with the Transaction.
First Lien ABL Credit Facility
The amendments to the Existing Revolving Credit Agreement to be made pursuant to the First Lien Consent are subject to customary conditions, including, among others, (i) the accuracy of certain specified representations and warranties, (ii) the absence of certain specified events of default under the Existing Revolving Credit Agreement, (iii) the simultaneous funding of the Second Lien Facility in an amount sufficient to pay RTI’s closing cash purchase price obligations under the Master Transaction Agreement and (iv) the Transaction having been consummated (or substantially simultaneously with the initial borrowing under the Second Lien Facility, having been consummated) substantially in accordance with the terms of the Master Transaction Agreement.
The terms of the First Lien ABL Credit Facility will remain substantially similar as those set forth in the Existing Revolving Credit Agreement, with changes thereto as are outlined in the First Lien Consent to permit (i) the reduction of the aggregate revolving credit commitments under the Existing Revolving Credit Agreement from $100 million to $75 million, (ii) the consummation of the Transaction and (iii) the incurrence of the Second Lien Facility. The First Lien Consent requires that the Transaction be consummated on or prior to March 31, 2019.
Second Lien Facility
The obligations of Ares under the Second Lien Commitment Letter are subject to customary conditions, including, among others, (i) the accuracy of certain specified representations and warranties, (ii) the Transaction having been consummated (or substantially simultaneously with the initial borrowing under the Second Lien Facility, having been consummated) substantially in accordance with the terms of the Master Transaction Agreement, (iii) the absence of any PS Spine material adverse effect, and (iv) the execution and delivery of definitive loan documentation prepared in accordance with documentation principles agreed to by RTI and Ares.
The Second Lien Commitment Letter expires upon the earlier of (i) 5:00 p.m. New York time on March 31, 2019, (ii) the consummation of the Transaction and (iii) the funding of the Second Lien Facility on the closing date.
The proceeds of the Second Lien Facility may be used solely to pay the cash portion of the purchase price under the Master Transaction Agreement, and amounts borrowed under the Second Lien Facility and repaid may not bere-borrowed. The Second Lien Commitment Letter provides that the terms of the Second Lien Facility will be finalized in a credit agreement (the “Second Lien Credit Agreement”) and related documentation to be entered into prior to the closing of the Transaction.
The Second Lien Facility will mature six months after the maturity date of the First Lien ABL Credit Facility. RTI will pay interest on the unpaid principal amount of the Second Lien Facility at a rate per annum, at RTI’s election, equal to either the prime rateplus an applicable margin to be set forth in the Second Lien Credit Agreement or the LIBOR rateplusan applicable margin to be set forth in the Second Lien Credit Agreement. The principal amount of the loans outstanding under the Second Lien Facility will be subject to certain customary mandatory prepayments, including, without limitation, an excess cash flow sweep, upon the issuance of additional indebtedness and the consummation of certain asset dispositions.
The Second Lien Credit Agreement will contain customary (i) representations and warranties, including (but not limited to) certain corporate and organization matters; financial condition; properties; litigation; compliance with laws; certain regulatory matters; taxes; employee benefits matters; material agreements; solvency; and insurance, (ii) affirmative covenants, including (but not limited to) certain reporting and notice obligations; maintenance of
145
corporate existence; conduct of business; compliance with laws; use of proceeds; insurance and further assurances, (iii) negative covenants, including (but not limited to) the incurrence certain additional indebtedness; creation of certain liens; sale and leaseback transactions; consolidations or mergers with, or conveyances, transfers or leases of all or substantially all of its assets to another person; investments, loans, advances, guarantees and acquisitions; payment of dividends and distributions; transactions with affiliates and amendments to material documents and (iv) events of default, including (but not limited to) failure to make required payments; inaccuracy of representations and warranties; failure to comply with covenants; cross-default to other material indebtedness; failure to stay execution of judgments; bankruptcy or insolvency; actual or asserted invalidity or impairment of the credit documentation; invalidity of subordination provisions; and a change of control, in each case substantially similar to those in the Existing Revolving Credit Agreement, with changes as are necessary to account for the Second Lien Facility, the consummation of the Transaction, and as otherwise agreed between RTI and Ares. Under the Second Lien Facility, RTI will also be subject to a total net leverage maintenance covenant and minimum fixed charge coverage covenant to be set forth in the Second Lien Credit Agreement.
The obligations under the Second Lien Facility will be guaranteed on a joint and several basis by each direct or indirect wholly owned subsidiary of RTI, subject to customary exceptions. The obligations under the Second Lien Facility will be secured on a second-lien priority basis (behind the First Lien ABL Credit Facility) by substantially all of the assets of RTI and each guarantor party thereto, subject to customary exceptions.
146
INFORMATION ABOUT RTI, HOLDCO AND MERGER SUB
RTI Surgical, Inc.
11621 Research Circle
Alachua, Florida 32615
Telephone: (386)418-8888
RTI is a global surgical implant company that designs, develops, manufactures and distributes biologic, metal and synthetic implants. RTI’s implants are used in orthopedic, spine, sports medicine, plastic surgery, trauma and other surgical procedures to repair and promote the natural healing of human bone and other human tissues and improve surgical outcomes. RTI manufactures metal and synthetic implants and processes donated human musculoskeletal and other tissue and bovine and porcine animal tissue in producing allograft and xenograft implants using its proprietary BIOCLEANSE®, TUTOPLAST® and CANCELLE® SP sterilization processes. RTI processes tissue at its facilities in Alachua, Florida and Neunkirchen, Germany and manufactures metal and synthetic implants in Marquette, Michigan and Greenville, North Carolina, respectively. RTI is accredited in the U.S. by the American Association of Tissue Banks and is a member of AdvaMed. RTI’s implants are distributed directly to hospitals and free-standing surgery centers throughout the U.S. and in more than 40 countries worldwide with the support of both its and third-party representatives as well as through larger purchasing companies.
RTI common stock is listed on Nasdaq under the symbol “RTIX”.
Additional information about RTI and its subsidiaries is included in documents incorporated by reference in this joint proxy and consent solicitation statement/prospectus. See the section entitled “Where You Can Find More Information”, beginning on page 187.
Bears Holding Sub, Inc.
c/o RTI Surgical, Inc.
520 Lake Cook Road, Suite 315
Deerfield, Illinois 60015
Telephone: (386)418-8888
Bears Holding Sub, Inc., a wholly owned subsidiary of RTI, is a Delaware corporation that was incorporated on October 26, 2018 for the purpose of effecting the matters set forth in the Master Transaction Agreement. To date, Holdco has not conducted any activities other than those incidental to its incorporation and the matters contemplated by the Master Transaction Agreement. Pursuant to the Master Transaction Agreement, at the effective time, the certificate of incorporation of Holdco will be amended and restated, and the name of Holdco will be changed to “RTI Surgical Holdings, Inc.” After the consummation of the Transaction, Holdco will own both RTI and Paradigm as wholly owned subsidiaries. It is expected that the Holdco common stock will be listed on the Nasdaq Global Market under the symbol “RTIX”. The business of Holdco will be the combined businesses currently conducted by RTI and Paradigm.
Bears Merger Sub, Inc.
c/o RTI Surgical, Inc.
11621 Research Circle
Alachua, Florida 32615
Telephone: (386)418-8888
147
Bears Merger Sub, Inc., a wholly owned subsidiary of Holdco, is a Delaware corporation that was incorporated on October 26, 2018 for the purpose of effecting the matters set forth in the Master Transaction Agreement. To date, Merger Sub has not conducted any activities other than those incidental to its formation and the matters contemplated by the Master Transaction Agreement. Pursuant to the Master Transaction Agreement, Merger Sub will be merged with and into RTI, with RTI surviving as a wholly owned subsidiary of Holdco.
148
INFORMATION ABOUT PS SPINE AND PARADIGM
PS Spine Holdco, LLC
505 Park Avenue, 14th Floor
New York, New York 10022
Telephone: (212)367-7274
PS Spine is a Delaware limited liability company that was originally formed on January 28, 2008 under the name Fourth Dimension Holding, LLC. PS Spine changed its name to Paradigm Spine Holdings, LLC on October 6, 2010, to PS 2016, LLC on April 5, 2016 and to PS Spine Holdco, LLC on August 9, 2018.
PS Spine is not engaged in any business and has no material assets, other than the ownership of all of the outstanding equity interests in Paradigm.
Paradigm Spine, LLC
505 Park Avenue, 14th Floor
New York, New York 10022
Telephone: (212)367-7274
Paradigm is a Delaware limited liability company. Paradigm was originally formed as a Delaware corporation on September 27, 2002 under the name Spine Motion, Inc. Paradigm converted from a Delaware corporation to a Delaware limited liability company on April 5, 2005, at which time it changed its name to Spine Motion, LLC. Paradigm then changed its name to Paradigm Spine, LLC on July 14, 2005.
Paradigm is focused on the design and development of solutions for the disease management of spinal stenosis. Paradigm’s signature product is the coflex® Interlaminar Stabilization® device, which is currently used in over 40 countries worldwide. Coflex® is the only lumbar spinal device that has produced Level I evidence in two separate prospective, randomized, controlled studies against two different surgical control groups, changing the standard of care for lumbar spinal stenosis treatment.
149
Overview
Paradigm is focused on the design and development of solutions for the disease management of spinal stenosis. Paradigm’s signature product is the coflex® Interlaminar Stabilization® device, which is currently used in over 40 countries worldwide. Coflex® is the only lumbar spinal device that has produced Level I evidence in two separate prospective, randomized, controlled studies against two different surgical control groups, changing the standard of care for lumbar spinal stenosis treatment.
The controlled launch of coflex® in October 2012 following FDA PMA was focused on maintaining superior clinical outcomes in select markets while continuing to develop further clinical evidence for eventual regional and national coverage policy decisions by private payors. Effective January 1, 2017, the procedure in which coflex® is implanted became covered under its own Common Procedural TerminologyLevel-1 code determined by the American Medical Association. Paradigm believes coverage will increase with the recent release of the European Study of Coflex Against Decompression Alone (“ESCADA”). ESCADA was a Level 1, randomized, controlled study of coflex® and decompression versus decompression-alone, with atwo-yearfollow-up.
In December of 2016, the International Society for the Advancement of Spine Surgery issued its coverage recommendation for Decompression with Interlaminar Stabilization, citing coflex® as the only product that has achieved FDA PMA approval for up to a grade I spondylolisthesis. Further, in May of 2018, the North American Spine Society (“NASS”), the largest spine society in North America, issued a coverage policy recommendation for lumbar interspinous devices without fusion and with decompression for lumbar spinal stenosis patients, as an alternative to lumbar fusion for degenerative lumbar stenosis with or without low-grade spondylolisthesis. Coflex® is an example of such a device that falls within the coverage policy recommendation from NASS. These guidelines will be a mechanism for payors to be more restrictive in their approvals of fusion by substituting coflex®. Paradigm believes this recommendation will be an important element in building a broad base of surgeon support for coflex® and will also provide Paradigm with additional supporting documentation to obtain additional commercial payor coverage for the procedure.
On March 10, 2018, two neurosurgical societies, the Congress of Neurological Surgeons and The American Association of Neurological Surgeons, issued a public statement commenting on the NASS coverage guidelines for the category of lumbar interspinous devices without fusion and decompression (of which coflex® is the only device currently available on the commercial market). Paradigm believes that this unsolicited commentary will be beneficial for obtaining commercial payor coverage policies for coflex®.
Presently, Paradigm has four principal technologies:
| 1. | coflex®:Paradigm’s signature product is the coflex® interlaminar functionally dynamic stabilization device. The initial core market for coflex® is lumbar spinal stenosis patients. Through September 30, 2018, approximately 85% of Paradigm’s worldwide revenues are from the sale of coflex® and the coflex F® product described below. |
| 2. | coflex F®:The coflex F® is an interspinous stabilization device that offers an alternative to pedicle screw fixation as an adjunct to intervetebral fusion in cases of degenerative disc disease with or without mild instabilities in the lumbar spine. The coflex F® implant allows segmental stabilization in combination with interbody fusion cages and is intended to bridge the gap between stand-alone anterior solutions and 360-degree fusions using pedicle screw fixation. Coflex F® received FDA 510(k) clearance in October 2010. |
| 3. | DSS®:The DSS™ Stabilization System provides semi-rigid and rigid stabilization for customized spine stabilization. It is intended to treat patients suffering from degenerative disc disease, spondylolisthesis, kyphosis, stenosis, pseudarthrosis and traumatic injuries of the spine. DSS™ was Paradigm’s first marketable device in the United States, which received its FDA 510(k) approval in January2008. |
150
| 4. | DCI™:DCI™ is a tissue-sparing, motion-preserving and minimally invasive cervical implant. It provides stable, controlled motion in the cervical spine allowing the spine to be functionally dynamic. After insertion, the implant works as a shock absorber to effectively prevent accelerated degeneration in the segments above and below. It is a single piece of titanium alloy implant that is indicated for cervical degenerative disc disease. |
Non-Fusion Scoliosis Franchise
Paradigm’s Growing Spine Profiler is a 3Dre-adjustable system for the correction of the thoracic/cervical spine. The mechanism of the implant develops all the necessary strengths to overcome the resistance to the correction and stabilizes the spine of the growing child without causing fusion. It also allows for lung growth. The sales of Paradigm’s Growing Spine Profiler for the nine months ended September 30, 2018 are inconsequential.
Paradigm’s business strategy is: Surgeon Centric; Indication Specific, and Data Driven.
| • | Surgeon Centric: Every day surgeons are faced with life changing surgeries. Paradigm focuses on developing long-term relationships with the key opinion leaders within the industry and thus often partners and collaborates on the development of new devices that address the unmet needs of surgeons. This gives Paradigm a competitive advantage over the larger companies in the industry. |
| • | Indication Specific: Developing indication-specific devices has many advantages. Paradigm devices are designed to meet an unmet need in the industry. Training is developed specifically for the indication. From a regulatory perspective, having a device cleared for specific indications gives a competitive advantage overoff-label use of a device. An indication-specific approach provides the best opportunity to be reimbursed properly at the highest levels. |
| • | Data Driven: In Paradigm’s industry, data is of the utmost importance. Paradigm focuses on data collection through intensive clinical trials and encourages surgeons to conduct studies with all its products. |
Paradigm currently has a sales organization comprised of independent distributors throughout the United States that focus on coflex®, which represents over 97% of Paradigm’s U.S. 2018 sales, with coflex F® comprising 3% of Paradigm’s U.S. sales in 2018. coflex F® accounts for approximately 130 cases per year and is not a focus of Paradigm’s sales and marketing efforts in the United States. Paradigm reorganized its U.S. sales force in January 2018 and September 2018, moving to a regionally aligned sales structure. Since September 2018, Paradigm has had four Area Vice Presidents (“AVPs”). Each AVP has at least three Regional Sales Managers (“RSMs”) reporting to them. RSMs manage the independent distributors within their territory. Additionally, each AVP has Clinical Specialists who support patient coverage access, Technical Support Specialists who manage special case coverage requests, and Ambulatory Surgery Center Specialists who work with ambulatory surgery centers.
Paradigm has contained its marketing efforts to evidence-based publications and sampleddirect-to-consumer (“DTC”) efforts in select markets pending commercial payor approval. In 2018, Paradigm engaged third-party marketing firms to prepare and execute its DTC strategy throughout 2018. Paradigm currently has two dedicated marketing persons and one consultant.
Outside the United States, Paradigm has a direct sales organization of 7 representatives throughout Germany and Switzerland and sells through stocking distributors in 40 other countries, mainly in Europe and also in the Asia Pacific region, Latin America, the Middle East, India, Africa and Canada. The coflex® family of products represents approximately 40% of international sales, with an additional 48% of international sales represented by dynamic cervical and dynamic pedicle screw stabilization systems. Paradigm is also a distributor of Emerging Implant Technologies cages in Germany and Switzerland and a distributor of Simplify Medical’s cervical artificial disc in Germany, which make up the remaining 12% of international sales.
151
Outside the United States, coflex® was first sold in 2005, followed bycoflex-F® in 2007 andcoflex-F+ in 2016. Specifically, the international business was designed to validate the clinical and commercial efficacy of the coflex® technology, while the U.S. organization completed the FDA IDE clinical trial requirements for coflex®. With the reduced market entry barriers outside the United States, competition and price pressure continue to adversely impact longer term international market opportunities. In direct markets, coflex® implant pricing is approximately $750 per implant, while sales to stocking distributors are approximately 55% of the direct pricing.
Since receiving PMA approval for coflex®, Paradigm has trained over 2,100 surgeons on the coflex® procedure. The increase in trained surgeons is driven primarily by the restructuring of Paradigm’s sales organization that took place in 2018. In the new sales structure, each RSM has been tasked with contracting with five to eight new independent distributors. These distributors are then tasked with training five to six new surgeon users each over the course of the next 18 months. This would result in approximately 500 to 960 trained surgeons. Based on Paradigm’s current conversion rate of 29%, Paradigm would expect these trained surgeons to result in approximately 150 to 300 new users. Paradigm expects this volume of surgeons to be trained by the existing salesforce within the coming 18 to 20 months.
Product manufacturing and sterilization for global distribution are performed by contracted vendors located throughout Germany. Product is manufactured under Paradigm’s specifications, then received and inspected by Paradigm’s wholly owned German subsidiary before release to the United States and all other parties.
Paradigm has three sources of implant manufacturing: Zrinski and Diener in Germany and Orchid in the United States.
Paradigm’s intellectual property portfolio comprises numerous patents, patent applications, trademarks and trademark applications that are licensed in or owned by Paradigm. As of the date of this joint proxy and consent solicitation statement/prospectus, Paradigm’s patent portfolio includes 29 U.S. patents, 70 foreign patents and 10 design patents. Furthermore, Paradigm has filed additional provisional andnon-provisional U.S. and Patent Cooperation Treaty patent applications, and is aggressively prosecuting these applications to strengthen its patent rights to its core technologies.
Paradigm has approximately 94 full-time employees, including 61 employees dedicated to sales and marketing functions, five employees dedicated to research and development functions and the remaining employees dedicated to finance, operations, quality assurance, regulatory and administrative functions.
Paradigm’s U.S. headquarters are located in New York, New York, which supports executive and corporate finance functions. Paradigm’s international headquarters are located in Wurmlingen, Germany, which houses international sales management, distribution, accounting, finance and executive functions.
152
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS OF PARADIGM
You should read this discussion of Paradigm’s financial condition and results of operations in conjunction with the sections entitled “Summary Selected Historical Consolidated Financial Data for Paradigm” and “Risk Factors—Risks Relating to Paradigm” in this joint proxy and consent solicitation statement/prospectus, as well as Paradigm’s consolidated financial statements and related notes included elsewhere in this joint proxy and consent solicitation statement/prospectus. This discussion and analysis contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements involve risks, uncertainties and assumptions. Paradigm’s actual results may differ materially from those anticipated in these forward-looking statements as a result of many factors.
Paradigm is focused on the design and development of solutions for the disease management of spinal stenosis. Paradigm’s signature product is the coflex® Interlaminar Stabilization® device, which is currently used in over 40 countries worldwide. Coflex® is the only lumbar spinal device that has produced Level I evidence in two separate prospective, randomized, controlled studies against two different surgical control groups, changing the standard of care for lumbar spinal stenosis treatment.
The controlled launch of coflex® in October 2012 following FDA PMA was focused on maintaining superior clinical outcomes in select markets while continuing to develop further clinical evidence for eventual regional and national coverage policy decisions by private payors. Paradigm believes coverage will increase with the recent release of the European Study of Coflex Against Decompression Alone, or ESCADA. ESCADA was a Level 1, randomized, controlled study of coflex® and decompression versus decompression-alone, with atwo-yearfollow-up.
In December of 2016, the International Society for the Advancement of Spine Surgery issued its coverage recommendation for Decompression with Interlaminar Stabilization, citing coflex® as the only product that has achieved FDA PMA approval for up to a grade I spondylolisthesis. Further, in May of 2018, the North American Spine Society, or NASS, the largest spine society in North America, issued a coverage policy recommendation for lumbar interspinous devices without fusion and with decompression for lumbar spinal stenosis patients, as an alternative to lumbar fusion for degenerative lumbar stenosis with or withoutlow-grade spondylolisthesis. Coflex® is an example of such a device that falls within the coverage policy recommendation from NASS. These guidelines will be a mechanism for payors to be more restrictive in their approvals of fusion by substituting coflex®. Paradigm believes this recommendation will be an important element in building a broad base of surgeon support for coflex® and will also provide Paradigm with additional supporting documentation to obtain additional commercial payor coverage for the procedure.
On March 10, 2018, two neurosurgical societies, the Congress of Neurological Surgeons and The American Association of Neurological Surgeons, issued a public statement commenting on the NASS coverage guidelines for the category of lumbar interspinous devices without fusion and decompression (of which coflex® is the only device currently available on the commercial market). Paradigm believes that this unsolicited commentary will be beneficial for obtaining commercial payor coverage policies for coflex®.
Paradigm currently has a sales organization comprised of independent distributors throughout the United States that focus on coflex®, which represents over 97% of Paradigm’s U.S. 2018 sales, with coflex F® (a 510(k) product) indicated for single level spinal fusion) comprising 3% of Paradigm’s U.S. sales in 2018. coflex F® accounts for approximately 130 cases per year and is not a focus of Paradigm’s sales and marketing efforts in the United States. Paradigm reorganized its U.S. sales force in January 2018 and September 2018, moving to a regionally aligned sales structure. Since September 2018, Paradigm has had four Area Vice Presidents, or AVPs. Each AVP has at least three Regional Sales Managers, or RSMs, reporting to them. RSMs manage the independent distributors within their territory. Additionally each AVP has Clinical Specialists who support patient coverage access, Technical Support
153
Specialists who manage special case coverage requests, and Ambulatory Surgery Center Specialists who work with ambulatory surgery centers.
Paradigm has contained its marketing efforts to evidence-based publications and sampleddirect-to-consumer, or DTC, efforts in select markets pending commercial payor approval. In 2018, Paradigm engaged third-party marketing firms to prepare and execute its DTC strategy throughout 2018. Paradigm currently has two dedicated marketing persons and one consultant.
Outside the United States, Paradigm has a direct sales organization of 7 representatives throughout Germany and Switzerland and sells through stocking distributors in 40 other countries, mainly in Europe and also in the Asia Pacific region, Latin America, the Middle East, India, Africa and Canada. The coflex® family of products represents approximately 40% of international sales, with an additional 48% of international sales represented by dynamic cervical and dynamic pedicle screw stabilization systems. Paradigm is also a distributor of Emerging Implant Technologies cages in Germany and Switzerland and a distributor of Simplify Medical’s cervical artificial disc in Germany, which make up the remaining 12% of international sales.
Outside the United States, coflex® was first sold in 2005, followed bycoflex-F® in 2007 andcoflex-F+ in 2016. Specifically, the international business was designed to validate the clinical and commercial efficacy of the coflex® technology, while the U.S. organization completed the FDA IDE clinical trial requirements for coflex®. With the reduced market entry barriers outside the United States, competition and price pressure continue to adversely impact longer term international market opportunities. In direct markets, coflex® implant pricing is approximately $750 per implant, while sales to stocking distributors are approximately 55% of the direct pricing.
Since receiving PMA approval for coflex®, Paradigm has trained over 2,100 surgeons on the coflex® procedure. The increase in trained surgeons is driven primarily by the restructuring of Paradigm’s sales organization that took place in 2018. In the new sales structure, each RSM has been tasked with contracting with five to eight new independent distributors. These distributors are then tasked with training five to six new surgeon users each over the course of the next 18 months. This would result in approximately 500 to 960 trained surgeons. Based on Paradigm’s current conversion rate of 29%, Paradigm would expect these trained surgeons to result in approximately 150 to 300 new users. Paradigm expects this volume of surgeons to be trained by its existing salesforce within the coming 18 to 20 months.
Critical Accounting Policies and Significant Judgments and Estimates
This management’s discussion and analysis of Paradigm’s financial condition and results of operations is based on Paradigm’s consolidated financial statements, which Paradigm has prepared in accordance with existing U.S. generally accepted accounting principles. The preparation of those financial statements requires Paradigm to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of those financial statements, as well as the reported revenue and expenses during the reporting periods. Paradigm evaluates its estimates and judgments on an ongoing basis. Paradigm bases its estimates on historical experience and on various other factors that it believes are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Paradigm’s actual results may differ from these estimates under different assumptions or conditions.
Paradigm believes that, of its significant accounting policies, the following policies can be characterized as “critical accounting policies” and are particularly important to the portrayal of its results of operations and financial position. These critical accounting policies may require the application of a higher level of judgment by Paradigm and as a result are subject to an inherent degree of uncertainty.
Revenue Recognition
Paradigm recognizes revenue from sales of implants principally to hospitals and to distributors. For sales to hospitals, revenue is recognized on the date of surgery when the device is implanted. For sales to distributors, revenue
154
is recognized when title and risk of ownership have been transferred, provided that persuasive evidence of an arrangement exists, delivery has occurred, the price is fixed and determinable, the buyer is obligated to pay, the obligation is not contingent on resale of the product, the buyer has economic substance apart from Paradigm, Paradigm has no obligation to bring about the resale of the product, the amount of returns and discounts can be reasonably estimated, the remaining obligations are insignificant and collectability is reasonably assured. Revenue is recorded net of customer discounts and rebates.
Allowance for Doubtful Accounts
Paradigm evaluates the collectability of accounts receivable using a combination of factors. Provisions to the allowance for doubtful accounts receivable are recorded based on a number of factors, including the length of time the receivables are past due, the current business environment and Paradigm’s historical experience. Changes to the provision are recorded in selling, general and administrative expenses.
The following discussion summarizes the key factors Paradigm’s management believes are necessary for an understanding of Paradigm’s financial statements.
Comparison of the Nine Months Ended September 30, 2018 and 2017
Net Sales. During the nine months ended September 30, 2018, Paradigm’s net sales of $30.5 million represented a $3.4 million, or 10.0%, decrease from net sales of $33.9 million for the nine months ended September 30, 2017. The decline in sales was due to the restructuring of Paradigm’s U.S. sales organization in January 2018 and September 2018, as well as current challenges with national private pay reimbursement coverage.
Gross Margin. Paradigm’s gross margin was $26.5 million, or 86.7% of net sales, for the nine months ended September 30, 2018, and $30.0 million, or 88.6% of net sales, for the nine months ended September 30, 2017. A steady product mix and stable cost of products sold provided for consistent gross margin as a percent of sales. The decrease in gross margin as a percent of net sales is due to an increase in the percent of international sales versus U.S. sales in the nine months ended September 30, 2018, as products sold outside the United States have lower margins due to lower selling prices.
Selling, General and Administrative (“SG&A”) Expenses. Paradigm’s SG&A expenses of $39.7 million during the nine months ended September 30, 2018 increased $2.7 million from $37.0 million during the nine months ended September 30, 2017. This is mainly attributable to an increase of $5.6 million in salary and benefits due to the restructuring of Paradigm’s sales organization in 2018. This was offset by a reduction of $3.4 million in clinical and regulatory expenses due to cost savings initiatives.
Research and Development (“R&D”) Expenses. Paradigm’s R&D expenses of $236,000 during the nine months ended September 30, 2018 increased $133,000 from $103,000 during the nine months ended September 30, 2017. The increase is attributed primarily to a $161,000 increase in prototype and manufacturing costs for the development of new instruments.
Other Income (Expense). Other income (expense) includes interest income, preferred unit warrant gains based on changes in fair market value, interest expense, foreign currency exchange and othernon-operating costs when incurred. Net other expense was $17.1 million for the nine months ended September 30, 2018 compared to net other expense of $2.3 million in the quarter ended September 30, 2017. The increase in expense in the nine months ended September 30, 2018 was due to a $5.3 million increase in accrued paid in kind interest on debt and a $1.9 million increase in amortization of financing fees. In addition, a fair market value adjustment of $7.5 million to unit warrants in the nine months ended September 30, 2017 offset expense for that period.
155
Comparison of the Years Ended December 31, 2017 and 2016
Net Sales. For the year ended December 31, 2017, Paradigm’s net sales of $44.8 million represented a $10.8 million, or 19.4%, decrease from net sales of $55.6 million for the year ended December 31, 2016. Paradigm believes that when the procedure covering coflex® received its own Common Procedural Terminology (“CPT”)
Level-1 code in January 2017, this exclusive CPT code led to an increase in coverage denials from commercial payors, resulting in a decrease in sales. In addition, in the second half of 2016, Paradigm reduced sales headcount from 22 to 10 and initiated a Medical Science Liaison model. Accordingly, 22 Medical Science Liaisons were hired to provide surgeon practice-level support with patient selection, billing, coding andpre-authorization submission and denial appeals. The decrease in the sales force and investment into medical affairs also resulted in a decrease in net sales.
Gross Margin. Paradigm’s gross margin was $39.6 million, or 88.4% of net sales for the year ended December 31, 2017, and $49.1 million, or 88.2% of net sales for the year ended December 31, 2016.
SG&A Expenses. Paradigm’s SG&A expenses of $50.7 million for the year ended December 31, 2017 increased $5.6 million from $45.1 million for the year ended December 31, 2016. SG&A expense increased in the year ended December 31, 2017 due to a $5.8 million increase in medical affairs related to the implementation of a Medical Science Liaison team, as well as expenses incurred for services related to reimbursement and coverage support for the coflex® product. In addition, Paradigm incurred $0.9 million of expense related to the restructuring of its executive team in 2017. These increases in expenses were offset by reductions in clinical and regulatory affairs expenses of $1.9 million due to cost containment efforts.
R&D Expenses. Paradigm’s R&D expenses of $256,000 for the year ended December 31, 2017 decreased $177,000 from $433,000 for the year ended December 31, 2016.
Other Income (Expense). Net other expense was $30.8 million and $6.9 million for the year ended December 31, 2017 and 2016, respectively. The increase in other expense in 2017 compared to 2016 relates to accrued paid in kind interest, as well as fees associated with a refinancing of Paradigm’s debt.
Liquidity and Capital Resources
Cash Flows
As of September 30, 2018, Paradigm’s cash and cash equivalents totaled $1.1 million, and it had working capital of approximately $6.0 million.
For the nine months ended September 30, 2018, Paradigm used $10.6 million of cash in operating activities, compared to $12.8 million of cash used during the nine months ended September 30, 2017. Paradigm used this cash primarily to fund its operating loss, net ofnon-cash charges, of $13.0 million during the nine months ended September 30, 2018 and $12.5 million during the nine months ended September 30, 2017.
For the nine months ended September 30, 2018, Paradigm used $284,000 to purchase equipment and software, as well as other investing activities, compared with approximately $851,000 for the nine months ended September 30, 2017. The decrease is due to a reduction in capital equipment purchases of instruments driven by the lower sales in the nine months ended September 30, 2018 compared with the same period of 2017.
For the nine months ended September 30, 2018, Paradigm generated proceeds from financing activities of $1.5 million, compared with $0.6 million used for the nine months ended September 30, 2017. In August 2018, Paradigm borrowed an additional $1.5 million from the lenders under its existing senior secured credit.
Cash used in operating activities was $16.0 million in 2017 and $1.8 million in 2016. Paradigm used this cash primarily to fund its operating loss, net ofnon-cash, of $16.4 million and $0.6 million in the respective years. Paradigm has continued to generate an operating loss because of continued investments in the development, regulatory approvals, clinical studies and commercialization of its coflex® product.
156
In 2017, Paradigm used $904,000 to purchase equipment and software, as well as other investing activities compared with $1.2 million in 2016. The decrease in 2017 is due to a reduction in capital equipment purchases driven by the lower in sales in 2017 compared with 2016.
Sources of Liquidity
From inception to September 30, 2018, Paradigm has incurred an accumulated deficit of $245.0 million, primarily as a result of expenses incurred through a combination of research and development activities and commercialization expenses related to its coflex® product. Paradigm has financed its operations since inception primarily through the private sale of preferred units and credit facilities. Paradigm’s total cash and cash equivalents balance as of September 30, 2018 was $1.1 million.
Paradigm believes that its existing resources will be sufficient to fund its operations through the consummation of the Transaction. In the event the planned Transaction with RTI does not occur, Paradigm will need to raise additional financing immediately to support its operations and planned growth activities. Paradigm’s liquidity and capital requirements will depend on numerous factors, including:
| • | the revenues generated by the sale of products; |
| • | the timing and cost required to expand its sales, marketing and distribution capabilities; |
| • | the cost and effectiveness of its marketing and sales efforts; |
| • | the effect of competing technologies; |
| • | market, reimbursement commercial payor coverage and regulatory developments; |
| • | the cost of research and development programs; and |
| • | the cost involved in protecting its proprietary rights. |
Any equity financing will dilute the equity interests of PS Spine unitholders, and any debt financing could impose significant new financial and operational restrictions on Paradigm. Paradigm cannot assure you that it would be able to obtain additional financing on acceptable terms, or at all.
Contractual Obligations
The following table summarizes Paradigm’s significant contractual commitments as of September 30, 2018.
Contractual Obligation | Total | Less than 1 Year | 1–3 Years | 3–5 Years | More Than 5 Years | |||||||||||||||
Long-term debt obligations | $ | 106,376,000 | $ | 106,376,000 | $ | — | $ | — | $ | — | ||||||||||
Operating lease obligations | 132,000 | 106,000 | 26,000 | — | — | |||||||||||||||
Purchase obligations | 2,199,000 | 1,119,000 | 1,080,000 | — | — | |||||||||||||||
Other long-term liabilities | 24,425,000 | 24,425,000 | — | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total contractual obligations | $ | 133,132,000 | $ | 132,026,000 | $ | 1,106,000 | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Off-Balance Sheet Arrangements
As of September 30, 2018, Paradigm had nooff-balance sheet arrangements.
Quantitative and Qualitative Disclosures about Market Risk
Paradigm’s cash and cash equivalents as of September 30, 2017 and 2018 consisted primarily of cash and money market accounts. Paradigm’s primary exposure to market risk is interest income sensitivity, which is affected by
157
changes in the general level of United States interest rates. Because of the short-term nature of the instruments in Paradigm’s portfolio, however, a sudden change in market interest rates would not be expected to have a material impact on the fair market value of its investment portfolio. Accordingly, Paradigm would not expect its operating results or cash flows to be affected to any significant degree by the effect of a sudden change in market interest rates on its investment portfolio.
Paradigm is subject to exposures resulting from foreign currency exchange fluctuations in the normal course of business because of the global nature of its operations. The direct financial impact of foreign currency exchange includes the impact of currency fluctuations on the transfer of goods between Paradigm’s operations in the United States and abroad and transaction gains and losses. A stronger dollar generally has a negative impact on results from operations outside the United States, while a weaker dollar generally has a positive effect because Paradigm’s products are currently manufactured in or sourced from the United States.
Inflation generally affects Paradigm by increasing its cost of labor. Paradigm does not believe that inflation has had a material effect on its results of operations in 2018 and 2017.
158
INFORMATION ABOUT THE PS SPINE DESIGNATED DIRECTOR
The Master Transaction Agreement provides that, following the closing, until the later of December 31, 2022 and the time at which PS Spine and its unitholders as of immediately prior to the Contribution own less than 10% of Holdco’s outstanding common stock (assuming the conversion of all of Holdco’s outstanding convertible preferred stock into Holdco common stock), PS Spine will be entitled to nominate a Designated Director, which Designated Director will be Jeffrey C. Lightcap, Anthony G. Viscogliosi or any other person mutually agreed by Holdco and PS Spine. The Master Transaction Agreement also provides that, at each meeting of Holdco’s stockholders at which the election of directors is to be considered, Holdco will nominate the Designated Director designated by PS Spine for election to the board of directors of Holdco, solicit proxies from Holdco’s stockholders in favor of the election of such Designated Director and use its reasonable best efforts to cause such Designated Director to be elected to the board of directors of Holdco, including voting all unrestricted proxies in favor of the election of such Designated Director, recommending approval of such Designated Director’s election to the Holdco board and not taking any action designed to diminish the prospects of such Designated Director being elected to the Holdco board.
Jeffrey C. Lightcap, currently a manager of PS Spine and Paradigm, will be the initial Designated Director and, accordingly, will be elected to the Holdco board upon the effective time of the Merger, to hold such office until the next annual meeting of stockholders of Holdco or until his successor has been duly elected and qualified. Mr. Lightcap will be an “independent director” of Holdco, as that term is defined in Rule 5605(a)(2) of the Nasdaq Stock Market LLC Listing Rules.
Mr. Lightcap, age 59, is Senior Managing Director at HealthCor Partners, a private equity firm that invests primarily in growth equity and later stage developmental companies across all sectors of the healthcare and life sciences industry, which he founded in 2008. Prior to joining HealthCor Partners, Mr. Lightcap was a Senior Managing Director at JLL Partners, a leading middle-market private equity fund, from 1997 tomid-2006. Prior to JLL Partners, Mr. Lightcap was a Managing Director at Merrill Lynch & Co., Inc., in charge of leveraged buyout coverage for Merrill Lynch’s mergers and acquisitions group. Prior to joining Merrill Lynch, Mr. Lightcap was a Senior Vice President in the mergers and acquisitions group at Kidder, Peabody & Co. and briefly at Salomon Brothers. Mr. Lightcap serves on the board of a number of companies other than PS Spine and Paradigm, including Iasis Healthcare Corporation, Practice Partners in Healthcare, Corindus, CareView Communications and HeartFlow. Previously, Mr. Lightcap served on the board of directors of AccessClosure and Sadra Medical, in addition to service on other private company boards. Mr. Lightcap received a B.E. in Mechanical Engineering from the State University of New York in 1981 and an M.B.A. from the University of Chicago in 1985. Mr. Lightcap’s investment banking experience, as well as his private equity experience as an investor in healthcare companies, qualifies him to provide valuable insight to the Holdco board.
There are no family relationships between Mr. Lightcap and any of the directors, managers or executive officers of RTI, Holdco, Merger Sub, PS Spine or Paradigm.
Mr. Lightcap does not receive, and during PS Spine and Paradigm’s most recently completed fiscal year did not receive, any compensation for his service as a manager of PS Spine or Paradigm.
Transactions with PS Spine or Paradigm
Neither PS Spine nor Paradigm has entered into any transactions since January 1, 2018 in which Mr. Lightcap, or any member of his immediate family, was or is contemplated to be a party or had or will have a direct or indirect material interest (other than in his capacity as a direct or indirect unitholder or warrantholder of PS Spine).
159
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS
AND MANAGEMENT OF RTI
The following table sets forth information as of January 15, 2019 regarding the beneficial ownership of RTI common stock by: (1) each person known by RTI to own beneficially more than 5% of RTI’s outstanding common stock; (2) each of RTI’s directors; (3) each of RTI’s named executive officers; and (4) all of RTI’s directors and executive officers as a group. Except as otherwise specified, the named beneficial owner has the sole voting and investment power over the shares listed. Unless otherwise indicated, the address of the beneficial owner is: c/o RTI Surgical, Inc., 11621 Research Circle, Alachua, Florida 32615.
| Amount and Nature of Beneficial Ownership(1) | ||||||||||
Title of Class | Name and Address of Beneficial Owner | Number | Percent | |||||||
| Common | Camille I. Farhat(2) | 1,110,619 | 1.8 | |||||||
| Common | Jonathon M. Singer(3) | 264,844 | * | |||||||
| Common | Ryan M. Bartolucci(4) | 10,152 | * | |||||||
| Common | John N. Varela(5) | 127,074 | * | |||||||
| Common | Olivier M. Visa(6) | 122,900 | * | |||||||
| Common | Johannes W. Louw(7) | 95,462 | * | |||||||
| Common | Peter F. Gearen(8) | 185,884 | * | |||||||
| Common | Thomas A. McEachin(9) | 64,424 | * | |||||||
| Common | Curt M. Selquist(10) | 94,050 | * | |||||||
| Common | Mark D. Stolper(11) | 45,462 | * | |||||||
| Common | Christopher R. Sweeney(12) | 54,401 | * | |||||||
| Common | Paul G. Thomas(13) | 54,401 | * | |||||||
| Common | Nicholas J. Valeriani(14) | 65,401 | * | |||||||
| Common | Shirley A. Weis(15) | 75,517 | * | |||||||
| Preferred | WSHP Biologics Holdings, LLC(16) 333 West Wacker Drive, Suite 2800 Chicago, Illinois 60606 | 15,152,761 | 19.6 | |||||||
| Common | Paradigm Capital Management Inc.(17) Nine Elk Street Albany, New York 12207 | 5,930,217 | 9.5 | |||||||
| Common | BlackRock Inc.(18) 55 East 52nd Street New York, New York 10022 | 4,914,369 | 7.9 | |||||||
| Common | Glen Capital Partners LLC(19) 800 South Street, Suite 160 Waltham, Massachusetts 02453 | 4,852,060 | 7.8 | |||||||
| Common | Dimensional Fund Advisors, LP(20) Building One, 6300 Bee Cave Road | |||||||||
| Austin, Texas 78746 | 4,267,312 | 6.9 | ||||||||
| Common | Krensavage Asset Management, LLC(21) 130 E. 59th Street, 11th Floor New York, New York 10022 | 4,438,950 | 7.1 | |||||||
| Common | All current executive officers and directors (14 persons)(22) | 2,370,591 | 3.8 | |||||||
160
| * | Represents beneficial ownership of less than 1%. |
| (1) | Beneficial ownership is determined in accordance with the rules of the SEC, which generally attribute beneficial ownership of securities to persons who possess sole or shared voting power and/or investment power with respect to those securities. Shares of RTI common stock issuable pursuant to restricted stock awards and options, to the extent such options are exercisable or convertible within 60 days after February 1, 2019 (the record date), are treated as outstanding for purposes of computing the percentage of the person holding such securities, but are not treated as outstanding for purposes of computing the percentage of any other person. |
| (2) | Includes 85,000 shares of unvested restricted RTI stock, which will vest on the last day of each calendar quarter at a rate of 42,500 shares per calendar quarter, which commenced on March 31, 2018, continuing until all restricted shares have vested. |
| (3) | Includes 131,460 shares of unvested restricted RTI stock, which will vest as to one third of the underlying shares each year over a three-year period commencing on the first anniversary of the date of grant. |
| (4) | Includes 8,152 shares of unvested restricted RTI stock, which will vest as to one third of the underlying shares each year over a three-year period commencing on the first anniversary of the date of grant. |
| (5) | Includes currently-exercisable options to purchase 59,176 shares of RTI common stock and 39,870 shares of unvested restricted stock, which will vest as to one third of the underlying shares each year over a three-year period commencing on the first anniversary of the date of grant. |
| (6) | Includes 75,900 shares of unvested restricted RTI stock, which will vest as to one third of the underlying shares each year over a three-year period commencing on the first anniversary of the date of grant. |
| (7) | Includes currently-exercisable options to purchase 64,029 shares of RTI common stock and 24,871 shares of unvested restricted RTI stock, which will vest as to one third of the underlying shares each year over a three-year period commencing on the first anniversary of the date of grant. |
| (8) | Includes currently-exercisable options to purchase 45,000 shares of RTI common stock and 17,647 shares of unvested restricted stock, which will vest on February 28, 2019. |
| (9) | Includes 17,647 shares of unvested restricted RTI stock, which will vest on February 28, 2019. |
| (10) | Includes 17,647 shares of unvested restricted RTI stock, which will vest on February 28, 2019. |
| (11) | Includes 17,647 shares of unvested restricted RTI stock, which will vest on February 28, 2019. |
| (12) | Includes 17,647 shares of unvested restricted RTI stock, which will vest on February 28, 2019. |
| (13) | Includes 17,647 shares of unvested restricted RTI stock, which will vest on February 28, 2019. |
| (14) | Includes 17,647 shares of unvested restricted RTI stock, which will vest on February 28, 2019. |
| (15) | Includes 17,647 shares of unvested restricted RTI stock, which will vest on February 28, 2019. |
| (16) | WSHP Biologics Holdings, LLC is the record owner of 50,000 shares of RTI preferred stock, which is convertible at the current conversion price of $4.39 per share into approximately 15,152,761 shares of RTI common stock. The managing member of WSHP Biologics Holdings, LLC is the Water Street Healthcare Partners II, L.P., of which the sole general partner is Water Street Healthcare Management II, L.P (“Management”). The sole general partner of Management is the Water Street Healthcare partners LLC. Due to their relationship with WSHP Biologics Holdings, LLC, each of these entities may be deemed to have shared voting power with respect to the RTI preferred stock beneficially owned by the WSHP Biologics Holdings, LLC, and as a result, each of these entities may be deemed to have shared beneficial ownership of such shares of RTI preferred stock. The individuals who serve on the Investment Committee of the Water Street Partners LLC are Timothy Dugan, James Connelly, Ned Villers, Kevin Swan, Robert Womsley, Peter Strothman and Christopher Sweeney. |
| (17) | Information is derived from Amendment No. 5 to Schedule 13G, filed with the SEC on February 9, 2018, by Paradigm Capital Management Inc. Paradigm Capital Management Inc. is not affiliated with PS Spine or Paradigm. |
| (18) | Information is derived from Amendment No. 9 to Schedule 13G, filed with the SEC on January 29, 2018, by BlackRock, Inc. |
| (19) | Information is derived from Schedule 13G, filed with the SEC on January 19, 2016, by Glen Capital Partners Focus Fund, LP (the “Fund”); Glen Capital Partners LLC (the “Manager”); Glen Capital Partners GP LLC (the “General Partner”); and Gregory L. Summe (“Mr. Summe”). As the sole member of the Manager and General Partner, Mr. Summe may be deemed to share voting and dispositive power with respect to the shares of RTI common stock held by the Fund. Mr. Summe disclaims beneficial ownership of the shares of RTI common stock held by the Fund, except to the extent of his pecuniary interest therein. |
161
| (20) | Information is derived from Amendment No. 5 to Schedule 13G, filed with the SEC on February 9, 2018, by Dimensional Fund Advisors, LP. |
| (21) | Information is derived from Amendment No. 6 to Schedule 13D, filed with the SEC on March 16, 2017, by Krensavage Asset Management, LLC. |
| (22) | Includes options to purchase 168,205 shares of RTI common stock and 506,429 shares of unvested restricted RTI stock, which will vest as to one third of the underlying shares each year over a three-year period commencing on the first anniversary of the date of grant. |
162
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND
MANAGEMENT OF PS SPINE
The following table sets forth information as of January 15, 2019 regarding the beneficial ownership of membership units in PS Spine by: (1) each person known by PS Spine to beneficially own more than 5% of any class of voting membership units in PS Spine; (2) each of PS Spine’s managers, one of which, Mr. Lightcap, is expected to be a director of Holdco following the closing; (3) each of the executive officers of PS Spine; and (4) all of PS Spine’s managers and executive officers as a group. Except as otherwise specified or pursuant to applicable community property laws, each person named in the following table has sole voting and dispositive power over the membership units set forth opposite that person’s name. Unless otherwise indicated, the address of each person named in the following table is: c/o PS Spine Holdco, LLC, 505 Park Avenue, 14th Floor, New York, New York 10022.
| Amount and Nature of Beneficial Ownership(1) | ||||||||||
Name and Address of Beneficial Owner | Title of Class | Number | Percent | |||||||
5% Unitholders | ||||||||||
Entities affiliated with Viscogliosi Brothers, LLC 505 Park Avenue, 14th Floor New York, New York 10022 | Common Units Class A Common Units Class A Preferred Units Class B Preferred Units Class C Preferred Units Class E-1 Preferred Units Class F Preferred Units | | 117,219 126,297 1,805,474 |
| | 3.8 11.1 66.4 5.1 |
| |||
Entities affiliated with HealthCor Partners Management, L.P. 152 West 57th Street, 47th Floor New York, New York 10019 | Common Units Class A Common Units Class E-1 Preferred Units Class F Preferred Units | | 100,000 150,708 9,501,866 9,501,866 | (3)
(4) (2) | | 3.3 13.2 57.2 100.0 |
| |||
Entities affiliated with Trevi Health Capital LLC 660 Madison Avenue, 15th Floor New York, New York 10065 | Common Units Class A Common Units Class C Preferred Units Class E-1 Preferred Units Class F Preferred Units | | 100,000 24,686 16,657 1,516,568 | (3)
| | 3.3 2.2 * 9.1 |
| |||
Praefinium 20, Boulevard Emmanuel Servais L-2535, Luxembourg Grand-Duchy of Luxembourg | Class E-1 Preferred Units | 1,316,656 | 7.9 | |||||||
Medical Strategies Management | Common Units Class D Preferred Units Class E-1 Preferred Units Class E-2 Preferred Units Class F Preferred Units Common Units | | 210,000 77,778 109,006 11,711 109,006 54,745 |
| | 6.9 2.7 * * 100.0 1..8 |
| |||
Entities affiliated with Gary Lowery | Class A Common Units Class A Preferred Units | | 170,000 150,000 |
| | 14.9 5.5 |
| |||
Michael Schulman | Class A Preferred Units Class D Preferred Units | | 150,000 55,556 |
| | 5.5 1.9 |
| |||
163
| Amount and Nature of Beneficial Ownership(1) | ||||||||||
Name and Address of Beneficial Owner | Title of Class | Number | Percent | |||||||
Douglas E. Fechter | Common Units Class A Common Units Class E-1 Preferred Units Class E-2 Preferred Units | | 6,387 175,000 1,098 13,628 |
| | * 15.3 * * |
| |||
Guntmar Eisen | Common Units Class A Common Units Class A Preferred Units | | 292,987 1,392 106,574 |
| | 9.6 * 3.9 |
| |||
Hayfin Services LLP(5) One Eagle Place London, SW1Y 6AF England | Common Units Class A Common Units Class A Preferred Units Class B Preferred Units Class C Preferred Units Class D Preferred Units Class E-1 Preferred Units Class F Preferred Units | | 507,840 701,691 1,805,474 7,018,394 766,594 431,280 11,862,157 12,283,595 |
| | 16.6 61.5 66.4 88.9 13.9 14.8 71.4 100.0 |
| |||
Managers and Executive Officers | ||||||||||
Marc R. Viscogliosi(6) | Common Units Class A Common Units Class A Preferred Units Class B Preferred Units Class C Preferred UnitsClass E-1 Preferred Units Class F Preferred Units | | 175,340 526,297 1,805,474 843,724 | (7) | | 5.7 46.1 66.4 5.1 |
| |||
Steven D. Ward | — | — | — | |||||||
Francis Magee | Common Units | 686,588 | (8) | 22.4 | ||||||
William L. Pfost | Common Units | 30,833 | 1.0 | |||||||
Jeffrey C. Lightcap(9) | Common Units Class A Common Units Class D Preferred Units Class E-1 Preferred Units Class F Preferred Units | | 100,000 150,708 331,204 9,501,866 9,833,070 | (3)
(4) (2) | | 3.3 13.2 11.4 57.2 100.0 |
| |||
Michael Y. Mashaal | Class D Preferred Units Class F Preferred Units | | 100,076 100,076 | (2) | | 3.4 100.0 |
| |||
John C. Moran | Common Units Class A Preferred Units Class B Preferred Units | | 128,992 75,000 106,256 | (10)
| | 4.2 2.8 1.4 |
| |||
David Robbins(11) | Common Units Class A Common Units Class C Preferred Units Class E-1 Preferred Units Class F Preferred Units | | 100,000 24,686 16,657 1,516,568 | (3)
| | 3.3 2.2 * 9.1 |
| |||
Howard Rowe | — | — | — | |||||||
164
| Amount and Nature of Beneficial Ownership(1) | ||||||||||
Name and Address of Beneficial Owner | Title of Class | Number | Percent | |||||||
Anthony G. Viscogliosi(5) | Common Units Class A Common Units Class A Preferred Units Class B Preferred Units Class C Preferred Units Class E-1 Preferred Units Class F Preferred Units | | 249,719 126,297 1,805,474 | (10)
| | 8.2 11.1 66.4 |
| |||
Managers and executive officers as a group (ten (10) persons) | Common Units Class A Common Units Class A Preferred Units Class B Preferred Units Class C Preferred Units Class D Preferred Units Class E-1 Preferred Units Class F Preferred Units | | 1,354,253 701,691 1,880,474 7,124,650 766,594 431,280 11,862,157 12,183,519 | (12)
(4) (2) | | 44.2 61.2 69.2 90.2 13.9 14.8 71.4 100.0 |
| |||
| * | Represents beneficial ownership of less than 1%. |
| (1) | Beneficial ownership is determined in accordance with the rules of the SEC, which generally attribute beneficial ownership of securities to persons who possess sole or shared voting power and/or investment power with respect to those securities. Membership units issuable pursuant to options or warrants, to the extent such options or warrants are exercisable within 60 days of January 15, 2019, are treated as outstanding for purposes of computing the percentage of the person holding such membership units but are not treated as outstanding for purposes of computing the percentage of any other person. |
| (2) | Represents Class F Preferred Units that may be received upon the conversion of certain Class D Preferred Units and/or certainClass E-1 Preferred Units. |
| (3) | Represents currently-exercisable options to purchase 100,000 Common Units. |
| (4) | Includes currently-exercisable warrants to purchase 1,821,374Class E-1 Preferred Units. |
| (5) | Represents membership units with respect to which entities affiliated with Viscogliosi Brothers, LLC, entities affiliated with HealthCor Partners Management, L.P., entities affiliated with Trevi Health Capital LLC, Marc R. Viscogliosi, the Marc R. Viscogliosi GRAT Trust U/A 8/20/07, Jeffrey C. Lightcap, Michael Y. Mashaal and Anthony G. Viscogliosi have granted proxies to Hayfin Services LLP. Such proxies provide that Hayfin Services LLP may vote the membership units subject to the proxy in connection with certain specified matters relating to agreements that PS Spine previously entered into with the Hayfin Entities. Except with respect to those certain specified matters, the applicable unitholder generally retains all voting rights with respect to such unitholder’s membership units. Hayfin Services LLP does not currently expect to exercise any rights that it may have under these proxies in connection with the Transaction. |
| (6) | Includes membership units beneficially owned by entities affiliated with Viscogliosi Brothers, LLC, of which Mr. Viscogliosi is a member, and over which he may exercise shared voting power. |
| (7) | Includes 400,000 Class A Common Units owned by the Marc R. Viscogliosi GRAT Trust U/A 8/20/07. |
| (8) | Represents options to purchase 686,588 Common Units, 400,510 of which are not yet exercisable, but will become exercisable upon the approval of the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution, by the unitholders of PS Spine. |
| (9) | Includes membership units beneficially owned by entities affiliated with HealthCor Partners Management, L.P., of which Mr. Lightcap is Senior Managing Director, and over which he may exercise shared voting power. |
| (10) | Includes currently-exercisable options to purchase 50,000 Common Units. |
| (11) | Includes membership units beneficially owned by entities affiliated with Trevi Health Capital LLC, of which Mr. Robbins is an Advisor andCo-Founder, and over which he may exercise shared voting power. |
| (12) | Includes currently-exercisable options to purchase 986,588 Common Units. |
165
DESCRIPTION OF HOLDCO CAPITAL STOCK
The following summary is a description of the material terms of Holdco’s capital stock, is not complete and is qualified entirely by, and you should refer to the Amended and Restated Certificate of Incorporation of Holdco(the “Holdco charter”), the Amended and Restated Bylaws of Holdco(the “Holdco bylaws”) and the Certificate of Designation of Series A Convertible Preferred Stock of Holdco (“the “Holdco Certificate of Designation”), which are included as exhibits to the registration statement on FormS-4, of which this joint proxy and consent solicitation statement/prospectus forms a part, and the applicable provisions of the DGCL. See also the section entitled “Where You Can Find More Information”, beginning on page187. The description set forth below should be read in conjunction with the section entitled “Comparison of Rights of Holdco Stockholders, RTI Stockholders and the Paradigm Member” beginning on page172.
The following description of Holdco’s capital stock does not purport to be complete and is subject to, and qualified in its entirety by reference to, the complete text of the Holdco charter, the Holdco bylaws, and the Holdco Certificate of Designation.
Immediately following the effective time, Holdco’s authorized capital stock will consist of 155,000,000 shares of capital stock, $0.001 par value, of which 150,000,000 shares will be common stock and 5,000,000 shares will be preferred stock, $0.001 par value, issuable in one or more series.
As of January 15, 2019, there were 62,245,112 shares of RTI common stock issued and outstanding and 50,000 shares of RTI preferred stock issued and outstanding. If the Transaction is completed, (a) each share of RTI common stock issued and outstanding immediately prior to the effective time of the Merger (other than shares held by RTI as treasury shares or by Holdco or Merger Sub immediately prior to the effective time, which will be automatically cancelled and cease to exist) will be converted automatically into one fully paid andnon-assessable share of common stock, par value $0.001 per share, of Holdco, (b) each share of RTI preferred stock issued and outstanding immediately prior to the effective time of the Merger (other than shares held by RTI as treasury shares or by Holdco or Merger Sub immediately prior to the effective time which will be automatically cancelled and cease to exist) will be converted automatically into one fully paid and non-assessable share of Series A preferred stock, par value $0.001 per share, of Holdco and (c) each stock option and restricted stock award granted by RTI will be converted into a stock option or restricted stock award, as applicable, of Holdco with respect to an equivalent number of shares of Holdco common stock on the same terms and conditions as were applicable prior to the closing. Additionally, if the Transaction is completed, Holdco will issue an aggregate of 10,729,614 fully paid andnon-assessable shares of Holdco common stock to PS Spine and the lenders under Paradigm’s existing senior secured credit agreement and their affiliates. We expect that, immediately following the closing there will be outstanding a total of 72,974,726 shares of Holdco common stock and 50,000 shares of Holdco preferred stock (based on 62,245,112 shares of RTI common stock outstanding as of January 15, 2019).
Board of Directors
The directors of RTI at the effective time will be, from and after the effective time, directors of Holdco. Subject to the closing of the Transaction, Holdco expects to increase the number of directors on the Holdco board to appoint Jeffrey C. Lightcap, Senior Managing Director at HealthCor Partners and a current member of the board of managers of PS Spine and Paradigm, to fill the vacancy established thereby, to serve until his successor has been duly elected or appointed and qualified or until his earlier death, resignation or removal. The number of directors may be increased to any number or decreased to any number, not less than one director, by resolution of the Holdco board, with consent of the holders of Holdco preferred stock.
Directors may be removed with or without cause at any special meeting of stockholders duly called and held for such purpose, and vacancies on the Holdco board, including any vacancy created by an increase in the number of
166
directors, may be filled solely by an affirmative vote of a majority of the directors remaining in office, even though less than a quorum. If the Holdco board fills a vacancy, the director’s term expires at the next stockholders meeting at which directors are elected. The Master Transaction Agreement provides that following the closing, until the later of (a) December 31, 2022, and (b) the time at which PS Spine and its unitholders as of immediately prior to the Contribution own less than 10% of Holdco’s outstanding common stock (assuming the conversion of all of Holdco’s outstanding convertible preferred stock into Holdco common stock), PS Spine will be entitled to nominate a Designated Director. The initial Designated Director will be Jeffrey C. Lightcap. Holdco will use its reasonable best efforts to cause such Designated Director to be elected to Holdco board. Holdco will also use its reasonable best efforts to ensure that any Designated Director is removed only if so directed in writing by PS Spine, unless otherwise required by the Master Transaction Agreement or applicable law. In the event of a vacancy resulting from the death, disqualification, resignation, retirement or termination of the term of office of a Designated Director, Holdco will use its reasonable best efforts to cause the Holdco board to fill such vacancy or new directorship with a new Designated Director until the next stockholders meeting at which directors are elected.
Listing
We expect that Holdco common stock will be listed on the Nasdaq Global Market under the symbol “RTIX”.
Anti-takeover
A number of provisions in the Holdco charter, the Holdco bylaws, the Holdco Certificate of Designation and the DGCL may make it more difficult to acquire control of Holdco or remove its management.
Delaware Anti-Takeover Law and Certain Holdco Charter Provisions.As a Delaware corporation that will have a class of voting stock listed on a national securities exchange, Holdco will be subject to the provisions of Section 203 of the DGCL. In general, Section 203 prohibits a public Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years after the time at which such person became an interested stockholder unless: (i) prior to such time, the corporation’s board of directors approved either the business combination or transaction in which the stockholder became an interested stockholder, (ii) upon becoming an interested stockholder, the stockholder owned at least 85% of the corporation’s outstanding voting stock other than shares held by directors who are also officers and certain employee benefit plans or (iii) the business combination is approved by both the corporation’s board of directors and by holders of at leastsixty-six andtwo-thirds percent (662⁄3%) of the corporation’s outstanding voting stock (at a meeting and not by written consent), excluding shares owned by the interested stockholder. For these purposes, a “business combination” includes mergers, asset sales and other similar transactions with an “interested stockholder,” and “interested stockholder” means a stockholder that, together with its affiliates and associates, owns (or, under certain circumstances, has owned within the prior three years) more than 15% of the outstanding voting stock of the corporation. Although Section 203 permits a corporation to elect not to be governed by its provisions, it is expected that Holdco will not make this election.
Stockholder Nominations and Proposals. The Holdco bylaws will require that advance notice of nominees for election as directors be made by a stockholder or stockholder proposals be given to Holdco’s Corporate Secretary, together with certain specified information, no more than 120 calendar days and not less than 90 calendar days before the first anniversary of the date of the proxy statement for the prior annual meeting of stockholders. However, in the event that the date of the annual meeting of stockholders for the current year is more than 30 days following the first anniversary date of the annual meeting of stockholders for the prior year, the nomination of a director or the submission of a proposal will be considered timely if its submitted a reasonable time in advance of the mailing of Holdco’s proxy statement for the annual meeting of stockholders for the current year.
Stockholder Action; Special Meeting of Stockholders. The Holdco charter will provide that Holdco’s stockholders may not take action by written consent. The Holdco bylaws will further provide that special meetings of Holdco’s stockholders may be called by the chairman of the Holdco board, the President of Holdco, a majority of the Holdco board, or the President or Secretary of Holdco at the written request of Holdco stockholders owning a majority of the outstanding capital stock entitled to vote.
167
Supermajority Voting Provisions. The Holdco charter will provide that the affirmative vote of the holders of at leastsixty-six andtwo-thirds percent (662⁄3%) of the outstanding shares of all classes and series of Holdco stock entitled to vote is required to amend the provisions of the Holdco charter and the Holdco bylaws relating to the indemnification of officers, employees, agents, or members of the Holdco board, personal liability of members of the Holdco board, stockholder voting and the calling of special meetings.
Preferred Stock. Holdco will have the ability to issue up to an additional 4,950,000 shares of preferred stock with such rights, privileges, and preferences as the Holdco board may determine. This may have the effect of delaying or preventing a takeover or other change of control of Holdco.
Limitation of Liability and Indemnification Matters
Section 145 of the DGCL permits a corporation to indemnify its directors, officers and employees under certain conditions and subject to certain limitations. The Holdco charter and Holdco bylaws will contain provisions for the indemnification of directors, officers and employees within the limitations permitted by Section 145 of the DGCL. RTI has entered into indemnification agreements with its current directors and executive officers and insures its directors and officers against losses arising from any claim against them as such for wrongful acts or omissions, subject to certain limitations. As of the effective time, such indemnification agreements will be assigned from RTI to Holdco and Holdco is expected to enter into an indemnification agreement with Jeffrey C. Lightcap, the initial Designated Director.
The general effect of such provisions may be to reduce the circumstances in which an officer or director may be required to bear the economic burden of certain liabilities and expense.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling Holdco under such provisions, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Transfer Agent and Registrar
The transfer agent and registrar for Holdco common stock will be Broadridge Financial Solutions, Inc.
Subject to preferences that may be applicable to any preferred stock outstanding at the time, the holders of outstanding shares of Holdco common stock will be entitled to receive dividends out of assets legally available therefor at such times and in such amounts as the Holdco board from time to time may determine. Holders of Holdco common stock will be entitled to one vote for each share held on all matters submitted to a vote of stockholders. Cumulative voting for the election of directors will not be authorized by the Holdco charter, which means that the holders of a majority of the shares voted can elect all of the directors then standing for election. Holders of Holdco common stock will not be entitled to preemptive rights and Holdco common stock will not be subject to conversion or redemption. Upon Holdco’s liquidation, dissolution orwinding-up, the assets legally available for distribution to stockholders will be distributed ratably among the holders of its common stock after payment of liquidation preferences, if any, on any outstanding shares of preferred stock and payment of other claims of creditors.
Under the Holdco charter, the Holdco board will be authorized, without further stockholder action, to provide for the issuance from time to time of up to 5,000,000 shares of preferred stock in one or more series, and to fix for each such series such voting powers, full or limited, and such designations, preferences and relative, participating, optional or other special rights and such qualifications, limitations or restrictions thereof as shall be stated and expressed in the resolution or resolutions adopted by the Holdco board providing for the issue of such series and as may be permitted by the DGCL. The number of authorized shares of preferred stock may be increased or decreased (but not below the
168
number of shares thereof then outstanding) by the affirmative vote of the holders of a majority of the voting power of all the then outstanding shares of the capital stock of Holdco entitled to vote generally in the election of directors, voting together as a single class, without a separate vote of the holders of the preferred stock, or any series thereof, unless a vote of any such holders is required pursuant to any preferred stock designation.
Immediately following the closing, Holdco will have issued 50,000 shares of preferred stock designated as Series A Convertible Preferred Stock with the rights, preferences, and designations set forth in the Holdco Certificate of Designation.
Series A Convertible Preferred Stock
Ranking. Holdco preferred stock will rank senior to Holdco common stock with respect to certain matters, including for rights on liquidation, winding up, and dissolution.
Dividends. The holders of RTI preferred stock were entitled to dividends at an annual rate of 6.00% on the sum of the then applicable liquidation value (the “Liquidation Value”), plus all accrued and unpaid dividends on the RTI preferred stock, from and including the date of issuance until the fifth anniversary of the date of issuance. Dividends stopped accruing for the RTI preferred stock on July 16, 2018 and will not accrue on the Holdco preferred stock, unless there is an event of a default by Holdco under the Holdco Certificate of Designation, in which case dividends will begin accruing and will continue to accrue until the default is cured.
Liquidation. Upon any liquidation, dissolution or winding up of the affairs of Holdco, whether voluntary or involuntary, holders of Holdco preferred stock shall be entitled to receive for each share of the Holdco preferred stock, out of the assets of Holdco available for distribution to Holdco’s stockholders, before any distribution of such assets is made to the holders of junior stock, including Holdco common stock, an amount equal to the greater of: (1) the sum of the Liquidation Value, plus all accrued and unpaid dividends on such shares of Holdco preferred stock and (2) the per share amount of all cash and other property to be distributed in respect of Holdco common stock such holder would have been entitled to had it converted such Holdco preferred stock (without regard to any limits on conversion) immediately prior to the date fixed for such liquidation, dissolution or winding up of Holdco.
Conversion Rights. Ending on the earlier of: (a) July 16, 2021; (b) receipt of written notice of Holdco’s intent to redeem in full or in part the Holdco preferred stock; or (c) immediately prior to a change of control, the Holdco preferred stock may be converted in full or in part, from time to time and at any time, by the holder of such Holdco preferred stock into a number of shares of Holdco common stock equal to the quotient determined by dividing (i) the sum of the Liquidation Value, plus all accrued and unpaid dividends on such shares of Holdco preferred stock by (ii) the conversion price then in effect (the “Conversion Formula”). Immediately following the closing, the conversion price of the Holdco preferred stock is expected to be $4.39 per share.
The Holdco preferred stock will be convertible at Holdco’s election at any date after the earliest to occur of: (i) the date, if any, the average closing price of Holdco common stock for a 20 consecutive day period exceeds $10.25 and (ii) July 16, 2021, into a number of shares of Holdco common stock equal to the quotient determined by the Conversion Formula.
Conversion Price and Price Adjustment Provisions. The conversion price of the Holdco preferred stock will be subject to customary volume weighted price-adjustment provisions which, if triggered, could result in the then-current conversion price per share being reduced. For example, this would occur in the event of certain issuances of Holdco common stock or other convertible securities at prices lower than the then-current conversion price for the Holdco preferred stock or certain other transactions described in the Holdco Certificate of Designation.
Redemption Rights. Holdco may, upon 30 days’ notice, redeem the Holdco preferred stock, in whole or in part, at the Liquidation Value, plus all accrued and unpaid dividends on the shares of Holdco preferred stock being redeemed. At the earlier of a change of control of Holdco and July 16, 2020, the holders of the Holdco preferred stock may require Holdco to redeem their Holdco preferred stock, in whole or in part, at the Liquidation Value, plus all accrued and unpaid dividends on the shares of Holdco preferred stock being redeemed.
169
Director Designation Rights. So long as shares of Holdco preferred stock are outstanding and the Preferred Percentage (defined below) equals or exceeds 5%, the holders of Holdco preferred stock, voting as a separate class, will be entitled to elect a number of directors (the “Preferred Directors”) to the Holdco board equal tothe product of: (i) the Preferred Percentage, and (ii) the total number of directors on the Holdco board, including the number of Preferred Directors appointed or appointable to the Holdco board; provided, that if such product is not a whole number, then the number of Preferred Directors will be the next whole number larger than such product. In no event will the number of directors appointable to the Holdco board by the holders of the Holdco preferred stock exceed two at any time. The “Preferred Percentage” will be defined as (i) the number of shares of Holdco common stock issuable upon the conversion of all of the outstanding Holdco preferred stock (without regard to any restrictions on conversion),plus the number of shares of Holdco common stock that were issued pursuant to the conversion of any Holdco preferred stock,divided by (ii) the number of issued and outstanding shares of Holdco common stock,plus the number of shares of Holdco common stock issuable upon conversion of the Holdco preferred stock (without regard to any restrictions on conversion).
Voting Rights. The Holdco preferred stock will vote together with the Holdco common stock on anas-converted basis upon all matters submitted to a vote of the stockholders of Holdco, such votes to be counted together with the Holdco common stock and not separately as a class. In no event will the holders of shares of Holdco preferred stock be entitled to cast votes for the number of shares of Holdco common stock issuable upon conversion of the Holdco preferred stock that exceedsthe quotient of: (i) the aggregate purchase price paid for such shares of Holdco preferred stock,divided by (ii) $4.17 (the trading price of the RTI common stock on the day immediately prior to the date of issuance of the RTI preferred stock).
Consent Rights. So long as shares of the Holdco preferred stock are outstanding and the Preferred Percentage equals or exceeds 10%, the holders of Holdco preferred stock will have certain consent rights, which such rights, among others, will require Holdco to seek written consent from the holders of a majority of the shares of Holdco preferred stock before taking certain actions, including: (i) liquidating, dissolving or winding up Holdco (whether voluntary or involuntary); (ii) amending, modifying or supplementing any provision of the Holdco charter or Holdco bylaws that would have a material adverse effect on any right, preference, privilege or voting power of the Holdco preferred stock or the holders thereof; (iii) changing the size of the Holdco board; (iv) entering into, amending, modifying or supplementing any agreement, transaction, commitment or arrangement with any of Holdco’s affiliates, except for customary employment arrangements and benefit programs; and (v) agreeing to take any of the foregoing actions. In addition, so long as shares of the Holdco preferred stock are outstanding, without the prior written consent of the holders of a majority of the shares of Holdco preferred stock, Holdco will not, nor will Holdco permit any subsidiary to, redeem, purchase or otherwise acquire directly or indirectly any junior securities (including Holdco common stock), nor will Holdco directly or indirectly declare or pay any dividend or make any distribution upon any junior securities (including Holdco common stock).
In connection with the closing of the sale of RTI preferred stock, RTI entered into an Investor Rights Agreement, dated July 16, 2013, with the current holder of RTI preferred stock, WSHP Biologics Holdings, LLC (the “Investor Rights Agreement”). As of the effective time, the Investor Rights Agreement will be assigned from RTI to Holdco, and all of RTI’s (i) right, title and interest in and (i) liabilities and obligations under the Investor Rights Agreement will be transferred to and assumed by Holdco.
Registration Rights. RTI has agreed to file a registration statement covering the resale of its common stock issuable to the holder of RTI preferred stock upon conversion of its shares of RTI preferred stock, and the holder of RTI preferred stock has demand registration rights and piggyback registration rights under certain circumstances. The registration statement on FormS-3, filed with the SEC on November 1, 2013, fulfills that obligation and will permit the holder of RTI preferred stock to offer the shares acquired by full or partial conversion of the RTI preferred stock for resale from time to time. RTI agreed to pay all expenses resulting from RTI’s obligation to register the shares issuable upon conversion of RTI preferred stock. If the Transaction is completed, Holdco will succeed to such registration statement.
Director Nomination Rights. If the holder of RTI preferred stock is no longer entitled to designate an RTI board member pursuant to the RTI Certificate of Designation, then, for so long as the former holder of RTI preferred stock
170
continues to beneficially own 5% or more of RTI common stock directly or on anas-converted basis, the former holder of RTI preferred stock shall be entitled to nominate up to two directors to the RTI board, proportionate with the ownership percentage of the former holder of RTI preferred stock.
Preemptive Rights. Subject to various exceptions, the holder of RTI preferred stock has customary preemptive rights in the event RTI offers securities to any person or entity, entitling the holder of RTI preferred stock to participate in any such offering in proportion to the percentage of RTI common stock on anas-converted basis held by the holder of RTI preferred stock at the time of the offering.
Information Rights. So long as the holder of RTI preferred stock continues to beneficially own 5% or more of RTI common stock directly or on anas-converted basis, RTI will provide the holder of RTI preferred stock with customary information rights, including providing the holder of RTI preferred stock with: (i) unaudited monthly and unaudited quarterly financial statements; (ii) audited annual financial statements; (iii) a copy of RTI’s financial plan prior to the beginning of each fiscal year and any RTI board-approved revisions thereof; and (iv) other information as the holder of RTI preferred stock reasonably requests that is consistent with materials otherwise provided to members of the RTI board.
171
COMPARISON OF RIGHTS OF HOLDCO STOCKHOLDERS, RTI STOCKHOLDERS
AND THE PARADIGM MEMBER
The rights of RTI stockholders are currently governed by the Amended and Restated Certificate of Incorporation of RTI (the “RTI charter”), the Amended and Restated Bylaws of RTI (the “RTI bylaws”), the Amended and Restated Certificate of Designation of Series A Preferred Stock of RTI (the “RTI Certificate of Designation”) and the DGCL. The rights of the sole member of Paradigm, PS Spine (in such capacity, the “Paradigm Member”), are governed by the Delaware Limited Liability Company Act (the “DLLCA”) and the Amended and Restated Limited Liability Company Agreement of Paradigm (the “Paradigm LLC Agreement”). Upon closing of the Transaction, RTI stockholders and PS Spine, as well as any PS Spine unitholders and lenders and their affiliates to whom Holdco shares are distributed, will become stockholders of Holdco and their rights as stockholders of Holdco will be governed by the Holdco charter, the Holdco bylaws, the Holdco Certificate of Designation and the DGCL.
The following is a summary discussion of the material differences, as of the date of this joint proxy and consent solicitation statement/prospectus, between the current rights of RTI stockholders and the Paradigm Member and their rights as stockholders of Holdco upon closing of the Transaction.
The following description does not purport to be a complete statement of all the differences, or a complete description of the specific provisions referred to in this summary. The identification of specific differences is not intended to indicate that other equally or more significant differences do not exist. RTI stockholders and PS Spine unitholders should read carefully the relevant provisions of the DGCL, the RTI charter, the RTI bylaws, the RTI Certificate of Designation, the DLLCA, the Paradigm LLC Agreement, and the forms of the Holdco charter, the Holdco bylaws and the Holdco Certificate of Designation. The form of the Holdco charter, the form of the Holdco bylaws and the form of the Holdco Certificate of Designation, are filed as exhibits to the registration statement on FormS-4, of which this joint proxy and consent solicitation statement/prospectus forms a part, and are incorporated by reference into this joint proxy and consent solicitation statement/prospectus.
Capitalization
Holdco
The authorized shares of capital stock of Holdco will consist of:
155,000,000 shares of capital stock, $0.001 par value, of which 150,000,000 shares will be common stock and 5,000,000 shares will be preferred stock.
We expect that, immediately after the completion of the Transaction, there will be issued and outstanding a total of 72,979,808 shares of Holdco common stock and 50,000 shares of Holdco preferred stock.
RTI
The current authorized shares of capital stock of RTI is the same as the expected authorized shares of capital stock of Holdco.
As of the close of business on January 15, 2019, there were issued and outstanding a total of 62,245,112 shares of RTI common stock and 50,000 shares of RTI preferred stock.
Paradigm
Authorized and issued ownership units of Paradigm total 100 units.
172
Voting
Holdco
Each holder of Holdco common stock will be entitled to one vote for each share held. Each share of Holdco preferred stock will be entitled to a number of votes equal to the number of whole shares of Holdco common stock such Holdco preferred stock is convertible into under the terms of the Holdco Certificate of Designation. There is a cap on the number of shares of Holdco common stock issuable upon the conversion of the Holdco preferred stock for which holders of Holdco preferred stock may be entitled to vote. Holders of Holdco preferred stock will also be entitled to certain consent rights. For more information about the cap mentioned above and the consent rights mentioned in the immediately preceding sentence, see “Description of Holdco Capital Stock”, beginning on page 166.
RTI
Voting rights are the same as provided above in the Holdco description.
Paradigm
Each member of the board of managers of Paradigm is entitled to one vote on all matters submitted to a vote of the board of managers, including delegation of authority to a manager or managers of the business of Paradigm. The sole member of Paradigm has the right to elect all members of the Paradigm board of managers.
Supermajority Vote Requirements
Holdco
The Holdco charter will provide that the affirmative vote of the holders of at leastsixty-six andtwo-thirds percent (662⁄3%) percent of the outstanding shares of all classes and series of Holdco stock entitled to vote is required to amend the provisions of the Holdco charter and the Holdco bylaws relating to the indemnification of officers, employees, agents, or members of the Holdco board, personal liability of members of the Holdco board, stockholder voting and the calling of special meetings.
RTI
Supermajority vote rights are the as provided above in the Holdco description.
Paradigm
The Paradigm LLC Agreement does not contain any supermajority voting requirements.
Action by Written Consent
Holdco
The Holdco charter will provide that Holdco’s stockholders may not take action by written consent.
RTI
Action by written consent provisions are the same as provided above in the Holdco description.
Paradigm
The sole member of Paradigm may take action by written consent. Pursuant toSection 18-302(d) of the DLLCA, unless otherwise provided in a limited liability company agreement, members of a Delaware limited liability company
173
may take action without a meeting, without prior notice, and without a vote and instead by written consent of the members having the minimum number of votes that would have been required to take the action at a meeting at which all members entitled to vote on the action were present and voted on the issue. The Paradigm LLC Agreement does not restrict the ability of its sole member to act via written consent.
Notice of Stockholder and Member Meetings
Holdco
The Holdco bylaws will provide that written notice of stockholder meetings must state the time, place, and purpose or purposes of the meeting, and must be given to each stockholder entitled to vote at the meeting, not less than 10 and not more than 60 days before the meeting.
RTI
Notice provisions are the same as provided above in the Holdco description.
Paradigm
The Paradigm LLC Agreement does not contain any specific notice requirements for member meetings.
Special Meetings of Stockholders and Members
Holdco
The Holdco bylaws will provide that special meetings of Holdco’s stockholders may be called by the chairman of the Holdco board, the President of Holdco, a majority of the Holdco board, or the President or Secretary of Holdco at the written request of stockholders owning a majority of the outstanding capital stock entitled to vote.
RTI
Special meeting provisions are the same as provided above in the Holdco description.
Paradigm
The Paradigm LLC Agreement does not contain any requirements for calling a special meeting of the member of Paradigm.
Stockholder Proposals
Holdco
The Holdco bylaws will require that advance notice of nominees for election as directors be made by a stockholder or stockholder proposals be given to Holdco’s Corporate Secretary, together with certain specified information, no more than 120 calendar days and not less than 90 calendar days before the first anniversary of the date of the proxy statement for the prior annual meeting of stockholders. However, in the event that the date of the annual meeting of stockholders for the current year is more than 30 days following the first anniversary date of the annual meeting of stockholders for the prior year, the nomination of a director or the submission of a proposal will be considered timely if its submitted a reasonable time in advance of the mailing of Holdco’s proxy statement for the annual meeting of stockholders for the current year.
RTI
Stockholder proposals provisions are the same as provided above in the Holdco description.
174
Paradigm
The Paradigm LLC Agreement does not contain any requirements for including any member proposal on the agenda for a meeting of the member.
Dividends
Holdco
Subject to preferences that may be applicable to any Holdco preferred stock outstanding at the time, the holders of outstanding shares of Holdco common stock will be entitled to receive dividends out of assets legally available therefor at such times and in such amounts as the Holdco board from time to time may determine. The Holdco Certificate of Designation will provide that so long as any shares of Holdco preferred stock remain outstanding, Holdco may not pay any dividend or make any distribution upon any junior securities (including the Holdco common stock) until all accrued and accumulated but unpaid dividends on Holdco preferred stock have been paid or declared with funds irrevocably set apart for payment. In addition, the Holdco Certificate of Designation will provide that so long as any shares of Holdco preferred stock remain outstanding, Holdco may not pay any dividend or make any distribution upon any junior securities (including Holdco common stock) without the prior written consent of the holders of a majority of Holdco preferred stock.
RTI
Dividend provisions are the same as provided above in the Holdco description.
Paradigm
The board of managers of Paradigm may make distributions to the sole member of Paradigm at the times and in the amounts determined by the board of managers, subject to any applicable contractual restrictions.
Number and Election of Directors
Holdco
The directors of RTI at the effective time will be, from and after the effective time, directors of Holdco. Subject to the closing of the Transaction, Holdco expects to increase the number of directors on the Holdco board to appoint Jeffrey C. Lightcap, Senior Managing Director at HealthCor Partners and a current member of the board of managers of PS Spine and Paradigm, to serve until his successor has been duly elected or appointed and qualified or until his earlier death, resignation or removal. The number of directors may be increased to any number or decreased to any number, not less than one director, by resolution of the Holdco board, with consent of the holders of Holdco preferred stock. Directors of Holdco will be elected each year by the stockholders at the annual meeting of stockholders and will hold their offices until the next annual meeting of the stockholders and until their successors are elected and qualified or until there is a decrease in the number of directors.
So long as shares of Holdco preferred stock are outstanding and the Preferred Percentage equals or exceeds 5%, the holders of Holdco preferred stock, voting as a separate class, will be entitled to elect a number of directors to the Holdco board equal to the product of (i) the Preferred Percentage, and (ii) the total number of directors on the Holdco board, including the number of Preferred Directors appointed or appointable to the Holdco board; provided, that if such product is not a whole number, then the number of Preferred Directors will be the next whole number larger than such product. In no event will the number of directors appointable to the Holdco board by the holders of the Holdco preferred stock exceed two at any time.
RTI
The number and election of director provisions are the same as provided above in the Holdco description, except that there is no Designated Director at RTI.
175
Paradigm
The Paradigm LLC Agreement provides for a board of managers. Each member of the board of managers is to be appointed by the sole member of Paradigm to serve until a successor is appointed. The number of members of the board of managers is initially seven, and the sole member of Paradigm may increase the number of managers at any time or fromtime-to-time. The sole member of Paradigm has the right to elect all members of the board of managers.
Removal of Directors and Managers
Holdco
The Holdco bylaws will provide that directors may be removed with or without cause at any special meeting of stockholders duly called and held for such purpose, and vacancies on the Holdco board, including any vacancy created by an increase in the number of directors, may be filled solely by an affirmative vote of a majority of the directors remaining in office, even though less than a quorum. Holdco will also use its reasonable best efforts to ensure that any Designated Director is removed only if so directed in writing by PS Spine, unless otherwise required by the Master Transaction Agreement or applicable law.
RTI
Removal of director provisions are the same as provided above in the Holdco description, except with respect to the Designated Director, which is not contemplated by RTI.
Paradigm
The sole member of Paradigm may replace a member of the Paradigm board of managers at any time.
Vacancies on the Board
Holdco
Any vacancy occurring on the Holdco board, other than a vacancy of a Holdco Preferred Director or a Designated Director, may be filled solely by the affirmative vote of a majority of the remaining directors though less than a quorum of the Holdco board, and a director so chosen shall hold office until the next annual meeting of stockholders. A vacancy of a Holdco Preferred Director will be filled by the affirmative vote of a majority of the holders of Holdco preferred stock. In the event that the holders of Holdco preferred stock fail to elect anyone to fill a vacancy of a Holdco Preferred Director position, such position will remain vacant until the majority of the holders of Holdco preferred stock fill the vacancy. In the event of a vacancy resulting from the death, disqualification, resignation, retirement or termination of the term of office of a Designated Director, Holdco will use its reasonable best efforts to cause the Holdco board to fill such vacancy or new directorship with a new Designated Director until the next stockholders meeting at which directors are elected.
RTI
Voting provisions are the same as provided above in the Holdco description, except with respect to the Designated Director, which is not contemplated by RTI.
Paradigm
The Paradigm LLC Agreement provides that the number of members of the Paradigm board of managers will be seven and that all members of the Paradigm board of managers will be elected by Paradigm’s sole member.
176
Amendment of Organizational Documents
Holdco
Any amendment to the Holdco charter will require the approval of the Holdco board and the approval of the stockholders. In addition, the Holdco charter will provide that paragraphs Ninth and Twelfth therein may not be repealed or amended in any respect and no provision inconsistent therewith may be adopted unless such action is approved by the affirmative vote of the holders ofsixty-six andtwo-thirds percent (662⁄3%) of the outstanding shares of all classes and series of Holdco stock entitled to vote generally in the election of Holdco’s directors. The Holdco bylaws may be amended or repealed and new bylaws may be adopted by a majority vote of the number of directors then constituting the Holdco board at a regular meeting of the Holdco board without prior notice, or at any special meeting of the Holdco board if notice of such alteration, amendment, or repeal be contained in the notice of such special meeting. In addition, the Holdco bylaws will provide that bylaw sections 2.04 and 2.07 may not be repealed or amended in any respect and no provision inconsistent therewith may be adopted unless such action is approved by the affirmative vote of the holders ofsixty-six andtwo-thirds percent (662⁄3%) of the outstanding shares of all classes and series of Holdco stock entitled to vote generally in the election of Holdco’s directors.
So long as shares of the Holdco preferred stock are outstanding and the Preferred Percentage equals or exceeds 10%, the Holdco preferred stock will have certain consent rights, which rights, among others, will require Holdco to seek written consent from the holders of a majority of the shares of Holdco preferred stock before taking certain actions, including, among other things: (i) amending, modifying or supplementing any provision of the Holdco charter or Holdco bylaws that would have a material adverse effect on any right, preference, privilege or voting power of the Holdco preferred stock or the holders thereof, and (ii) changing the size of the Holdco board.
RTI
Amendment of the charter and bylaws provisions are the same as provided above in the Holdco description.
Paradigm
The provisions of the Paradigm LLC Agreement provide that the certificate of formation of Paradigm may be amended only by Paradigm’s sole member. The Paradigm LLC Agreement may only be amended by Paradigm’s sole member.
Limitation on Director and Manager Liability
Holdco
The Holdco charter will provide that no director of Holdco will be personally liable to the corporation or its stockholders for monetary damages for a breach of fiduciary duty as a director, except (i) for any breach of the director’s duty of loyalty to Holdco or its stockholders, (ii) for acts or omissions not in good faith or that involve intentional misconduct or knowing violation of law, (iii) under Section 174 of the DGCL, as same exists or hereafter be amended, or (iv) for any transaction from which the director derived an improper personal benefit.
RTI
Limitation of director liability provisions are the same as provided above in the Holdco description.
Paradigm
The Paradigm LLC Agreement provides no member of the Paradigm board of managers will be liable to Paradigm or its sole member for any act or omission performed or omitted by such member of the board of managers in its capacity as a member of the board of managers taken in good faith. To the fullest extent permitted by law,
177
notwithstanding any duty (including any fiduciary duty) that otherwise might exist at law or in equity, no member of the Paradigm board of managers has any duties (including fiduciary duties) to Paradigm or any of its members or any other person as a result of the Paradigm LLC Agreement, at law or in equity, other than the implied contractual covenant of good faith and fair dealing.
Indemnification
Holdco
Section 145 of the DGCL permits a corporation to indemnify its directors, officers and employees under certain conditions and subject to certain limitations. The Holdco charter and Holdco bylaws will contain provisions for the indemnification of directors, officers and employees within the limitations permitted by Section 145 of the DGCL. RTI has entered into indemnification agreements with its current directors and executive officers and insures its directors and officers against losses arising from any claim against them as such for wrongful acts or omissions, subject to certain limitations. As of the effective time, such indemnification agreements will be assigned from RTI to Holdco and Holdco is expected to enter into an indemnification agreement with Jeffrey C. Lightcap, the initial Designated Director.
The general effect of such provisions may be to reduce the circumstances in which an officer or director may be required to bear the economic burden of certain liabilities and expense.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling Holdco under such provisions, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
RTI
Indemnification provisions are the same as provided above in the Holdco description.
Paradigm
The Paradigm LLC Agreement does not include any indemnification provisions. However, the Amended and Restated Limited Liability Company Agreement of PS Spine (the “PS Spine LLC Agreement”) provides that PS Spine will indemnify any person, to the fullest extent permitted under the DLLCA, against all expenses, liabilities and losses (including attorney fees, judgments, fines, excise taxes or penalties) reasonably incurred or suffered by such person (or one or more of such person’s affiliates) by reason of the fact that such person is or was serving at the request of PS Spine as an officer or manager of Paradigm. Notwithstanding the foregoing, unless the PS Spine board of managers otherwise consents, no such person will be indemnified for any expenses, liabilities or losses that are attributable to such person’s or his or her affiliates’ bad faith or knowing violation of law or for any breaches of any representations, warranties or covenants by such person or its affiliates contained in the PS Spine LLC Agreement or in any other agreement with PS Spine or any of its subsidiaries, including Paradigm. In addition, the PS Spine LLC Agreement also provides that PS Spine may, to the extent deemed advisable by the PS Spine board of managers, indemnify any person who is or was an employee or agent (other than an officer or manager) of Paradigm, if such person would have been entitled to such indemnity if such person had been a manager of Paradigm.
178
BEARS HOLDING SUB, INC. AND SUBSIDIARIES
UNAUDITED PRO FORMA CONDENSED COMBINED
FINANCIAL INFORMATION
The unaudited pro forma condensed combined balance sheet combines the historical consolidated balance sheets of RTI and Paradigm, giving effect to the Transaction as if it had been consummated on September 30, 2018, and the unaudited pro forma condensed combined statements of operations for the nine months ended September 30, 2018 and for the year ended December 31, 2017 combine the historical consolidated statements of operations of RTI and Paradigm, giving effect to the Transaction as if it had occurred on January 1, 2017. The historical consolidated financial information has been adjusted to give effect to pro forma events that are (i) directly attributable to the Transaction, (ii) factually supportable and (iii) with respect to the statement of operations, expected to have a continuing impact on the combined results.
The unaudited pro forma condensed combined financial information should be read in conjunction with the historical audited consolidated financial statements and unaudited interim condensed consolidated financial statements and accompanying notes of RTI and Paradigm, which have been included in or incorporated by reference into this joint proxy and consent solicitation statement/prospectus. The unaudited pro forma condensed combined financial information are not necessarily indicative of the operating results or financial position that would have occurred if the Transaction had been completed at the dates indicated.
The unaudited pro forma condensed combined financial information and related notes were prepared using the purchase method of accounting with Holdco (i.e., Bears Holding Sub, Inc.) treated as the acquiring entity. Accordingly, the total consideration transferred by Holdco to complete the Transaction with Paradigm will be allocated to assets and liabilities based upon their estimated fair values as of the date of completion of the Transaction. The allocation is dependent upon certain valuations and other studies that have not progressed to a stage where there is sufficient information to make a definitive allocation. Additionally, a final determination of the fair value of Paradigm’s assets and liabilities, which cannot be made prior to the completion of the Transaction, will be based on the actual net tangible and intangible assets of Paradigm that exist as of the date of completion of the Transaction. Accordingly, the pro forma purchase price adjustments are preliminary, subject to further adjustments as additional information becomes available and as additional analyses are performed, and have been made solely for the purpose of providing the unaudited pro forma condensed combined financial information presented below. Holdco estimated the fair value of Paradigm’s assets and liabilities based on discussions with Paradigm’s management, due diligence and information presented in public filings. Upon completion of the Transaction, final valuations will be performed. Increases or decreases in the fair value of relevant balance sheet amounts will result in adjustments to the balance sheet and/or statement of operations. There can be no assurances that the final determination will not result in material changes.
The assumptions and estimates underlying the unaudited adjustments in the unaudited pro forma condensed combined financial information are described in the accompanying notes, which should be read together with the unaudited pro forma condensed combined financial information.
Holdco expects to incur significant costs associated with integrating RTI’s and Paradigm’s businesses. The unaudited pro forma condensed combined financial information do not reflect the cost of any integration activities or benefits that may result from synergies that may be derived from integration activities.
The unaudited pro forma condensed combined financial information have been presented for informational purposes only. The pro forma information is not necessarily indicative of what the combined company’s financial position or results of operations would have been had the Transaction been completed as of the dates indicated. In addition, the unaudited pro forma condensed combined financial information do not purport to project the future financial position or operating results of the combined company.
179
BEARS HOLDING SUB, INC. AND SUBSIDIARIES
Unaudited Pro Forma Condensed Combined Statement of Operations
For the Nine Months Ended September 30, 2018
(In thousands, except share and per share data)
| RTI | Paradigm | Pro Forma Adjustments | Combined | |||||||||||||||||
Revenues | $ | 209,639 | $ | 30,532 | $ | — | $ | 240,171 | ||||||||||||
Costs of processing and distribution | 108,262 | 3,885 | — | 112,147 | ||||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Gross profit | 101,377 | 26,647 | — | 128,024 | ||||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Expenses: | ||||||||||||||||||||
Marketing, general and administrative | 87,326 | 39,621 | 6,500 | 3d | 133,447 | |||||||||||||||
Research and development | 10,297 | 484 | — | 10,781 | ||||||||||||||||
Severance and restructuring costs | 1,708 | — | — | 1,708 | ||||||||||||||||
Asset impairment and abandonments | 4,748 | — | — | 4,748 | ||||||||||||||||
Acquisition and integration expenses | 2,741 | — | — | 2,741 | ||||||||||||||||
Cardiothoracic closure business divestiture contingency consideration | (3,000 | ) | — | — | (3,000 | ) | ||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Total expenses | 103,820 | 40,105 | 6,500 | 150,425 | ||||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Operating loss | (2,443 | ) | (13,458 | ) | (6,500 | ) | (22,401 | ) | ||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Other (expense) income: | ||||||||||||||||||||
Interest expense | (2,223 | ) | (17,243 | ) | 9,156 | 3b | (10,310 | ) | ||||||||||||
Interest income | 31 | 60 | — | 91 | ||||||||||||||||
Other income | — | 47 | — | 47 | ||||||||||||||||
Loss on extinguishment of debt | (309 | ) | — | — | (309 | ) | ||||||||||||||
Foreign exchange loss | (23 | ) | — | — | (23 | ) | ||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Total other expense—net | (2,524 | ) | (17,136 | ) | 9,156 | (10,504 | ) | |||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Loss before income tax benefit (provision) | (4,967 | ) | (30,594 | ) | 2,656 | (32,905 | ) | |||||||||||||
Income tax benefit (provision) | 1,646 | (6 | ) | (850 | ) | 3f | 790 | |||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Net loss | (3,321 | ) | (30,600 | ) | 1,806 | (32,115 | ) | |||||||||||||
Convertible preferred dividend | (2,120 | ) | — | — | (2,120 | ) | ||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Net loss applicable to common shares | $ | (5,441 | ) | $ | (30,600 | ) | $ | 1,806 | $ | (34,235 | ) | |||||||||
|
|
|
|
|
|
|
| |||||||||||||
Other comprehensive loss: | ||||||||||||||||||||
Unrealized foreign currency translation loss | (651 | ) | — | — | (651 | ) | ||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Comprehensive loss | $ | (6,092 | ) | $ | (30,600 | ) | $ | 1,806 | $ | (34,886 | ) | |||||||||
|
|
|
|
|
|
|
| |||||||||||||
Net loss per common share—basic | $ | (0.09 | ) | $ | (0.41 | ) | $ | 0.17 | $ | (0.46 | ) | |||||||||
|
|
|
|
|
|
|
| |||||||||||||
Net loss per common share—diluted | $ | (0.09 | ) | $ | (0.41 | ) | $ | 0.17 | $ | (0.46 | ) | |||||||||
|
|
|
|
|
|
|
| |||||||||||||
Weighted average shares outstanding—basic | 63,517,958 | 74,247,572 | 10,729,614 | 3e | 74,247,572 | |||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Weighted average shares outstanding—diluted | 63,517,958 | 74,247,572 | 10,729,614 | 3e | 74,247,572 | |||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
See accompanying notes to unaudited proforma condensed combined financial information
180
BEARS HOLDING SUB, INC. AND SUBSIDIARIES
Unaudited Pro Forma Condensed Combined Statement of Operations
For the Year Ended December 31, 2017
(In thousands, except share and per share data)
| RTI | Paradigm | Pro Forma Adjustments | Combined | |||||||||||||||||
Revenues | $ | 279,563 | $ | 44,832 | $ | — | $ | 324,395 | ||||||||||||
Costs of processing and distribution | 137,042 | 5,208 | — | 142,250 | ||||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Gross profit | 142,521 | 39,624 | — | 182,145 | ||||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Expenses: | ||||||||||||||||||||
Marketing, general and administrative | 115,103 | 50,653 | 8,667 | 3d | 174,423 | |||||||||||||||
Research and development | 13,375 | 256 | — | 13,631 | ||||||||||||||||
Severance and restructuring costs | 12,173 | — | — | 12,173 | ||||||||||||||||
Executive transition costs | 2,781 | — | — | 2,781 | ||||||||||||||||
Asset impairment and abandonments | 3,739 | — | — | 3,739 | ||||||||||||||||
Acquisition and integration expenses | 630 | — | — | 630 | ||||||||||||||||
Gain on cardiothoracic closure business divestiture | (34,090 | ) | — | — | (34,090 | ) | ||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Total expenses | 113,711 | 50,909 | 8,667 | 173,287 | ||||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Operating income (loss) | 28,810 | (11,285 | ) | (8,667 | ) | 8,858 | ||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Other (expense) income: | ||||||||||||||||||||
Interest expense | (3,180 | ) | (38,655 | ) | 27,875 | 3b | (13,960 | ) | ||||||||||||
Interest income | 8 | 301 | — | 309 | ||||||||||||||||
Other income | — | 7,523 | — | 7,523 | ||||||||||||||||
Foreign exchange gain | 87 | — | — | 87 | ||||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Total other expense—net | (3,085 | ) | (30,831 | ) | 27,875 | (6,041 | ) | |||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Income (loss) before income tax benefit (provision) benefit | 25,725 | (42,116 | ) | 19,208 | 2,817 | |||||||||||||||
Income tax (provision) benefit | (19,453 | ) | (106 | ) | (6,147 | ) | 3f | (25,706 | ) | |||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Net income (loss) | 6,272 | (42,222 | ) | 13,061 | (22,889 | ) | ||||||||||||||
Convertible preferred dividend | (3,723 | ) | — | — | (3,723 | ) | ||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Net income (loss) applicable to common shares | $ | 2,549 | $ | (42,222 | ) | $ | 13,061 | $ | (26,612 | ) | ||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Other comprehensive income (loss): | ||||||||||||||||||||
Unrealized foreign currency translation gain (loss) | 1,987 | 1,073 | — | 3,060 | ||||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Comprehensive income (loss) | $ | 4,536 | $ | (41,149 | ) | $ | 13,061 | $ | (23,552 | ) | ||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Net income (loss) per common share—basic | $ | 0.04 | $ | (0.60 | ) | $ | 1.22 | $ | (0.38 | ) | ||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Net income (loss) per common share—diluted | $ | 0.04 | $ | (0.60 | ) | $ | 1.22 | $ | (0.38 | ) | ||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Weighted average shares outstanding—basic | 59,684,289 | 70,413,903 | 10,729,614 | 3e | 70,413,903 | |||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Weighted average shares outstanding—diluted | 60,599,952 | 70,413,903 | 10,729,614 | 3e | 70,413,903 | |||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
See accompanying notes to unaudited proforma condensed combined financial information
181
BEARS HOLDING SUB, INC. AND SUBSIDIARIES
Unaudited Pro Forma Condensed Combined Balance Sheet
As of September 30, 2018
(In thousands)
| RTI | Paradigm | Pro Forma Adjustments | Combined | |||||||||||||||||
| Assets | ||||||||||||||||||||
Current Assets: | ||||||||||||||||||||
Cash and cash equivalents | $ | 10,022 | $ | 1,075 | $ | (1,075 | ) | 2b(i) | $ | 10,022 | ||||||||||
Accounts receivable—net | 44,141 | 5,459 | — | 49,600 | ||||||||||||||||
Inventories—net | 103,891 | 6,506 | — | 110,397 | ||||||||||||||||
Prepaid and other current assets | 8,613 | 1,819 | — | 10,432 | ||||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Total current assets | 166,667 | 14,859 | (1,075 | ) | 180,451 | |||||||||||||||
Property, plant and equipment—net | 77,344 | 491 | — | 77,835 | ||||||||||||||||
Deferred tax assets—net | 11,875 | 14 | — | 11,889 | ||||||||||||||||
Goodwill | 62,864 | — | 118,574 | 2b(iii) | 181,438 | |||||||||||||||
Other intangible assets—net | 26,197 | — | 130,000 | 2b(ii) | 156,197 | |||||||||||||||
Other assets—net | 5,150 | 839 | — | 5,989 | ||||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Total assets | $ | 350,097 | $ | 16,203 | $ | 247,499 | $ | 613,799 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
| Liabilities and Stockholders’ Equity | ||||||||||||||||||||
Current Liabilities: | ||||||||||||||||||||
Accounts payable | $ | 19,282 | $ | 6,273 | $ | 9,000 | 3g | $ | 34,555 | |||||||||||
Accrued expenses | 22,121 | 2,618 | — | 24,739 | ||||||||||||||||
Current portion of deferred revenue | 4,990 | — | — | 4,990 | ||||||||||||||||
Current portion of long-term obligations | — | 129,944 | (129,944 | ) | 3a | — | ||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Total current liabilities | 46,393 | 138,835 | (120,944 | ) | 64,284 | |||||||||||||||
Long-term obligations—less current portion | 49,021 | — | 94,643 | 2a(i), 3a | 143,664 | |||||||||||||||
Contingent Consideration | — | — | 112,176 | 2a(iii) | 112,176 | |||||||||||||||
Other long-term liabilities | 5,759 | — | — | 5,759 | ||||||||||||||||
Deferred revenue | 1,968 | — | — | 1,968 | ||||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Total liabilities | 103,141 | 138,835 | 85,875 | 327,851 | ||||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Preferred stock Series A | 66,180 | 102,940 | (102,940 | ) | 3c | 66,180 | ||||||||||||||
Stockholders’ equity: | ||||||||||||||||||||
Common stock | 63 | 2,836 | (2,826 | ) | 2a(ii), 3c | 73 | ||||||||||||||
Additionalpaid-in capital | 432,077 | 18,451 | 26,281 | 2a(ii), 3c | 476,809 | |||||||||||||||
Accumulated other comprehensive loss | (6,980 | ) | (1,830 | ) | 1,830 | 3c | (6,980 | ) | ||||||||||||
Accumulated deficit | (239,515 | ) | (245,029 | ) | 239,279 | 3a, 3c, 3g | (245,265 | ) | ||||||||||||
Less treasury stock | (4,869 | ) | — | — | (4,869 | ) | ||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Total stockholders’ equity | 180,776 | (225,572 | ) | 264,564 | 219,768 | |||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Total liabilities and stockholders’ equity | $ | 350,097 | $ | 16,203 | $ | 247,499 | $ | 613,799 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
See accompanying notes to unaudited proforma condensed combined financial information
182
NOTES TO UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
Note 1. Basis of Presentation
The unaudited pro forma condensed combined financial information has been derived from financial statements prepared in accordance with GAAP and reflects the proposed acquisition of Paradigm by Holdco.
The underlying financial information of RTI has been derived from the audited consolidated financial statements of RTI included in RTI’s Annual Report on Form10-K for the year ended December 31, 2017 and the unaudited condensed consolidated financial statements of RTI included in RTI’s Quarterly Report on Form10-Q for the nine months ended September 30, 2018. The underlying financial information of Paradigm has been derived from the audited financial statements of Paradigm for the year ended December 31, 2017 and the unaudited condensed consolidated financial statements of Paradigm for the nine months ended September 30, 2018, which are included in this joint proxy and consent solicitation statement/prospectus.
The Transaction has been treated as an acquisition of a business, with Holdco as the acquirer and Paradigm as the acquiree, assuming that the Transaction had been completed on January 1, 2017, for purposes of the unaudited pro forma condensed combined statements of operations, and assuming the Transaction had been completed on September 30, 2018, for purposes of the unaudited pro forma condensed combined balance sheet.
This unaudited pro forma condensed combined financial information is not intended to reflect the financial position and results of operations which would have actually resulted had the Transaction been effected on the dates indicated. Further, the unaudited pro forma results of operations and balance sheet are not necessarily indicative of the results of operations that may be achieved in the future or what may be reflected in any future balance sheet. No account has been taken of the impact of transactions that have occurred or might occur subsequent to the dates referred to above. No adjustment, therefore, has been made for actions which may be taken once the Transaction is complete, such as any integration plans related to Paradigm.
The unaudited pro forma condensed combined financial information reflects the preliminary assessment of fair values of assets acquired (including lives of assets acquired) and liabilities assumed. Fair value estimates were determined based on preliminary discussions between RTI and Paradigm management, due diligence efforts, and information presented in public filings.. The detailed valuation studies necessary to arrive at the required estimates of the fair values for the Paradigm assets acquired and liabilities assumed have not commenced. Significant assets and liabilities that are subject to preparation of valuation studies to determine appropriate fair value adjustments include identifiable intangible assets. There can be no assurances that the final determination will not result in material changes.
Note 2. Total Consideration Transferred and Allocation
a) Estimated Total Consideration Transferred
Under the terms of the Transaction, Paradigm will be acquired by Holdco, for estimated upfront total cash and stock consideration of $142,635 and contingent cash and stock consideration of $112,176. The estimated total consideration transferred was calculated as follows:
| (in thousands, except share and per share data) | ||||||
Total cash portion of the total consideration transferred | $ | 97,893 | i. | |||
Total value of stock portion of the total consideration transferred | 44,742 | ii. | ||||
RTI share price | 4.17 | ii. | ||||
Total shares of Holdco common stock to be issued | 10,729,614 | ii. | ||||
Fair value of contingent consideration | 112,176 | iii. | ||||
Aggregate total consideration transferred | 254,811 | iv. | ||||
183
| i. | The cash portion of the total consideration transferred will come from the net proceeds from Holdco’s issuance of debt. The value of the cash portion of the total consideration transferred is $100,000 less adjustment of $2,107 to meet minimum defined working capital threshold of $7,000. |
| ii. | The value of the stock portion of the total consideration transferred is $44,742, which is reflected in the pro forma financial statements as an increase of $10 to common stock and an increase of $44,732 to additional paid in capital. For purposes of preparing this unaudited pro forma condensed combined financial information, RTI utilized a per share price equal to $4.17, based on the closing price on November 30, 2018 of RTI’s common stock. Each $0.40 change in the price of the RTI common stock would increase or decrease the value of the stock portion of the consideration transferred and goodwill by $4,292. |
| iii. | For purposes of preparing this unaudited pro forma condensed combined financial information, the preliminary assessment of contingent consideration was determined based on preliminary discussions between RTI and Paradigm management, due diligence efforts, and information presented in public filings. To determine the contingent consideration value, RTI management utilized its advisor’s present value calculation, which included assumptions consisting of revenue and Adjusted EBITDA forecasts for Paradigm by RTI management for the duration of the contingency period and discount rate of 11.5%. The detailed valuation studies necessary to arrive at the required estimate of the fair values of contingent consideration have not commenced. There can be no assurances that the final determination will not result in material changes. |
| iv. | The total consideration transferred to be issued in connection with the Transaction reflected in this unaudited pro forma condensed combined financial information does not purport to represent the total consideration transferred that will actually be issued in connection with the Transaction. For example, as discussed above, the estimated fair value of contingent consideration is subject to change as the detailed valuation studies necessary to arrive at the required estimate of fair values have not commenced. |
b) Preliminary Allocation of Total Consideration Transferred to Assets Acquired and Liabilities Assumed
| (in thousands) | ||||||||
Cash and cash equivalents | $ | — | ||||||
Receivables and other current assets | 7,278 | i. | ||||||
Property and equipment | 491 | i. | ||||||
Inventory | 6,506 | i. | ||||||
Intangible assets | 130,000 | ii. | ||||||
Goodwill | 118,574 | iii. | ||||||
Other noncurrent assets | 853 | i. | ||||||
Other liabilities assumed | (8,891 | ) | i. | |||||
|
| |||||||
| $ | 254,811 | |||||||
|
| |||||||
| i. | The unaudited pro forma condensed combined financial information has been prepared using Paradigm’s available financial statements and disclosures as of September 30, 2018. Therefore, except as noted below, the carrying value of assets and liabilities in Paradigm’s financial statements are considered to be a proxy for fair value of those assets and liabilities. |
| ii. | The unaudited pro forma condensed combined financial information has been prepared using Holdco’s preliminary estimate of the total fair value of intangible assets of $130,000. The preliminary assessment was determined based on preliminary discussions between RTI and Paradigm management, and due diligence efforts. The detailed valuation studies necessary to arrive at the required estimate of the fair values of intangibles have not commenced. Refer to note 3d for the specifics on the nature and useful lives of the intangible assets. |
184
| iii. | For purposes of the pro forma analysis, goodwill of $118,574 was included to reflect the total excess of the total consideration transferred over the fair value of the net assets acquired. |
Note 3. Pro Forma Transaction Adjustments
The unaudited pro forma condensed combined financial information reflects the following adjustments:
a) Debt
Holdco intends to finance the cash portion of the total consideration transferred with cash provided through a term loan to be provided in a single draw on the closing date in connection with the Transaction. For purposes of preparing this unaudited pro forma condensed combined financial information, Holdco has included $97,893 in aggregate principal amount under a term loan and $3,250 of debt issuance costs to be settled at closing of the transaction, which represents the amount for which RTI has entered into a Commitment Letter with Ares Capital Corporation.
An adjustment to eliminate Paradigm’s debt of $129,944 was reflected in the unaudited pro forma condensed combined balance sheet as of September 30, 2018. The debt will be satisfied with a combination of the cash and stock consideration provided for at the closing in connection with the transaction.
b) Interest Expense
As discussed in Note 3(a) above, Holdco will borrow approximately $97,893 at closing. Interest will accrue at an annual rate equal to (i)one-month LIBOR plus (i) 8.00%. For purposes of this unaudited pro forma condensed combined financial information, interest calculations were performed assuming an interest rate of 10.35%. Pro forma adjustments have been made to reflect the addition in interest expense of $10,130 and $7,600 for the year ended December 31, 2017 and for the nine months ended September 30, 2018, respectively, and included in interest expense is the amortization of debt issuance cost of $650 and $487 for the year ended December 31, 2017 and for the nine months ended September 30, 2018, respectively, related to the new debt issued based on the assumptions described above.
The adjustment to interest expense assumes the principal, stated amount, assumed rates on the Debt, and the pro forma weighted average shares outstanding do not change from those assumed as described herein, however, a .125% change in the LIBOR interest rate of the Debt would result in an increase or decrease in pro forma annual interest expense of approximately $93 and would increase or decrease pro forma annual earnings per share (basic and diluted) by less than $0.01 per share.
An adjustment to eliminate Paradigm’s interest expense of $38,655 and $17,243 was reflected in the unaudited pro forma condensed combined statement of operations for the year ended December 31, 2017 and the nine months ended September 30, 2018, respectively.
c) Elimination of Paradigm’s Unitholders’ Equity
An adjustment to eliminate Paradigm’s preferred units of $102,940, common units of $2,836, additionalpaid-in capital of $18,451, accumulated other comprehensive loss of $1,830, and accumulated deficit of $245,029 was reflected in the unaudited pro forma condensed combined balance sheet as of September 30, 2018.
d) Amortization Expense
Adjustments were made to increase selling, general and administrative expenses to reflect estimated amortization of $6,500 and $8,667 for the nine months ended September 30, 2018 and year ended December 31, 2017, respectively. These adjustments were based on the assumption that $130,000 of the recorded intangible assets related to Paradigm would be definite lived, consisting of $15,000 related to tradenames, $30,000 related to customer relationships, and $85,000 related to intellectual property. The estimated useful life of these intangible assets is approximately 15 years for tradenames, 15 years for customer relationships, and 15 years for intellectual property. The detailed valuation studies necessary to arrive at the required estimate of the fair values of intangibles have not commenced. There can be no assurances that the final determination will not result in material changes.
e) Net Income per Common Share
Holdco’s calculations of pro forma net income per share of common stock for the nine months ended September 30, 2018 and year ended December 31, 2017 include the impact of items discussed in this Note 3, including the estimated weighted average number of shares of common stock outstanding on a pro forma basis. The pro forma
185
weighted average number of shares of common stock outstanding for the nine months ended September 30, 2018 has been calculated as if the shares issued in connection with the Transaction had been issued and outstanding as of the beginning of the period.
f) Income Tax (Provision) Benefit
Pro forma adjustments were factored at an effective income tax rate of 32%, which is in line with the historical RTI effective tax rate.
g) Accounts Payable
Accounts Payable and retained earnings were adjusted by $9,000 for estimated transaction related costs incurred post September 30, 2018.
Assuming consummation of the Transaction, Holdco stockholders will be entitled to present proposals for consideration at future Holdco annual stockholder meetings, provided that they comply with the proxy rules promulgated by the SEC and Holdco’s bylaws. The deadline for submission of all Holdco stockholder proposals to be considered for inclusion in Holdco’s proxy statement for its next annual meeting will be disclosed in a subsequent filing with the SEC.
RTI will hold an annual meeting of stockholders in 2019 only if the Transaction has not already been completed by the time at which such meeting would be required. As indicated in RTI’s proxy statement filed on March 26, 2018, all stockholder proposals intended to be present at such meeting to be held in 2019 must have been received by RTI’s Corporate Secretary no later than November 26, 2018 in order to be considered for inclusion in the RTI board’s proxy statement and form of proxy card relating to the RTI 2019 Annual Meeting. The proposal must have also complied with Rule14a-8 under the Exchange Act regarding the inclusion of stockholder proposals in RTI sponsored proxy materials. In addition, the proxy solicited by the RTI board for the RTI 2019 Annual Meeting, if any, will confer discretionary authority to vote on any stockholder proposal presented at that meeting, unless RTI is provided with written notice of such proposal by February 9, 2019.
The validity of the Holdco common stock and preferred stock to be issued in connection with the Transaction will be passed upon by Sidley Austin LLP. Certain U.S. federal income tax consequences relating to the transactions contemplated by the Master Transaction Agreement will also be passed upon by Sidley Austin LLP and Dorsey & Whitney LLP.
The financial statements of RTI Surgical, Inc. incorporated in this joint proxy and consent solicitation statement/prospectus by reference from RTI Surgical Inc.’s Annual Report on Form10-K for the year ended December 31, 2017, and the effectiveness of RTI Surgical, Inc.’s internal control over financial reporting have been audited by Deloitte &
186
Touche LLP, an independent registered public accounting firm, as stated in their report, which is incorporated herein by reference. Such financial statements have been so incorporated in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
The consolidated financial statements of Paradigm Spine, LLC and its subsidiaries as of December 31, 2017 and 2016 and for each of the two years in the period ended December 31, 2017 included in this joint proxy and consent solicitation statement/prospectus have been audited by Deloitte & Touche LLP, independent auditors, as stated in their report appearing herein, and are included in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
RTI files annual, quarterly and current reports, proxy statements and other information with the SEC as required by the Exchange Act. The SEC maintains a website that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC. Accordingly, you can obtain the annual, quarterly and current reports, proxy statements and other information RTI files with the SEC, including the documents incorporated by reference in this joint proxy and consent solicitation statement/prospectus, without charge through the SEC’s website at www.sec.gov. You can also obtain such documents from RTI’s website at www.rtix.com.
Holdco has filed with the SEC a registration statement on FormS-4 to register with the SEC the Holdco shares to be issued in connection with the Merger and the Contribution, including the shares constituting the Stock Consideration Amount and the shares that may be issued as part of the Earnout Consideration. This joint proxy and consent solicitation statement/prospectus is a part of that registration statement on FormS-4 and constitutes a prospectus of Holdco in addition to being a proxy statement of RTI for the RTI special meeting and a consent solicitation of PS Holdco with respect to the written consent of the PS Spine unitholders to approve the Master Transaction Agreement and the transactions contemplated thereby, including the Contribution. As allowed by SEC rules, this document does not contain all the information you can find in the registration statement or the exhibits to the registration statement on FormS-4, of which this joint proxy and consent solicitation statement/prospectus forms a part.
In addition, the SEC allows RTI to disclose important information to you by referring you to other documents filed separately with the SEC. This information is considered to be a part of this joint proxy and consent solicitation statement/prospectus, except for any information that is superseded by information included directly in this joint proxy and consent solicitation statement/prospectus.
This joint proxy and consent solicitation statement/prospectus incorporates by reference the documents listed below that RTI has previously filed or will file with the SEC (other than information furnished pursuant to Item 2.02 or Item 7.01 of a Current Report on Form8-K). They contain important information about RTI, its financial condition and other matters.
| • | Annual Report on Form10-K for the fiscal year ended December 31, 2017. |
| • | Proxy Statement on Schedule 14A filed March 26, 2018. |
| • | Quarterly Reports on Form10-Q for the quarterly periods ended March 31, 2018, June 30, 2018 and September 30, 2018. |
| • | Current Reports on Form8-K filed March 5, 2018, May 1, 2018, June 7, 2018, June 25, 2018, August 2, 2018 (other than with respect to Item 2.02), September 26, 2018, November 2, 2018 and November 7, 2018. |
In addition, RTI incorporates by reference any future filing it makes with the SEC under Section 13(a), 13(c), 14 or 15(d) of the Exchange Act (other than information furnished pursuant to Item 2.02 or Item 7.01 of a Current Report
187
on Form8-K) after the date of this joint proxy and consent solicitation statement/prospectus and prior to the date of the RTI special meeting. Such documents are considered to be a part of this joint proxy and consent solicitation statement/prospectus, effective as of the date such documents are filed. In the event of conflicting information in these documents, the information in the latest filed document should be considered correct.
If you are an RTI stockholder and would like additional copies of this joint proxy and consent solicitation statement/prospectus or if you have questions about the proposals to be presented at the RTI special meeting, you should contact RTI’s proxy solicitation agent at the following address and telephone number:

1290 Avenue of the Americas, 9th Floor
New York, NY 10104
All Stockholders Call Toll-Free: (866)391-6921
If you are an RTI stockholder and would like to request documents, please do so by February 28, 2019, in order to receive them before the RTI special meeting. If you request any documents from RTI, it will mail them to you by first class mail, or another equally prompt means.
If you are a unitholder of PS Spine and would like additional copies of this joint proxy and consent solicitation statement/prospectus or a replacement written consent, or if you have questions about the PS Spine consent solicitation, you should contact PS Spine at:
PS Spine Holdco, LLC
505 Park Avenue, 14th Floor
New York, NY 10022
Attn: Secretary
Telephone: (888)273-9897
Email: InvestorRelations@paradigmspine.com
If you are a unitholder of PS Spine and would like to request documents, please do so by February 6, 2019, in order to receive them before the PS Spine deadline for submitting written consents. If you request any documents from PS Spine, it will mail them to you by first class mail, or another equally prompt means.
All information contained in this joint proxy and consent solicitation statement/prospectus relating to RTI and Holdco has been supplied by RTI and Holdco, and all such information relating to PS Spine and Paradigm has been supplied by PS Spine. Information provided by either sets of parties does not constitute any representation, estimate or projection of the other set of parties.
This document is a proxy statement for RTI for the RTI special meeting, as well as a consent solicitation statement for PS Spine. RTI and PS Spine have not authorized anyone to give any information or make any representation about the Merger Proposal, the Share Issuance Proposal, the Master Transaction Agreement, Holdco, RTI, Merger Sub, PS Spine or Paradigm that is different from, or in addition to, that contained in this joint proxy and consent solicitation statement/prospectus. Therefore, if anyone does give you information of this sort, you should not rely on it. The information contained in this document speaks only as of the date of this document unless the information specifically indicates that another date applies.
188
2010 Health Care Reform Laws | 52 | |||
401(k) Plan | 136 | |||
510(k) | 52 | |||
A&E | 31 | |||
Adjusted EBITDA | 107 | |||
Affected Employees | 136 | |||
Aggregate Consideration | 23 | |||
Antitrust Division | 25 | |||
Ares | 145 | |||
August RTI combined financial projections | 103 | |||
AVPs | 151 | |||
Broadridge | 12 | |||
brokernon-votes | 11 | |||
Cash Consideration Amount | 3 | |||
Cash Earnout Amount | 127 | |||
Cash Election Amount | 127 | |||
change in recommendation | 25 | |||
closing | 3 | |||
closing date | 124 | |||
Closing Debt Cash Amount | 46 | |||
CMS | 52 | |||
Code | 27 | |||
combined company . . . . . . . . . Additional Information |
| |||
Commitment Letters | 135 | |||
Consideration Certificate | 141 | |||
Contribution . . . . . . . . . . . . . . Additional Information |
| |||
Conversion Formula | 169 | |||
Covered Recipients | 52 | |||
Covered Shares | 120 | |||
Covered Units | 119 | |||
CPT | 156 | |||
CT Business | 31 | |||
Designated Director | 115 | |||
designated liabilities | 141 | |||
DGCL | 18 | |||
Divestiture Action | 134 | |||
DLLCA | 172 | |||
Dorsey & Whitney | 24 | |||
DTC | 151 | |||
Earnout Consideration | 4 | |||
effective time | 3 | |||
ESCADA | 150 | |||
EV | 85 | |||
Exchange Act | 7 | |||
Existing Revolving Credit Agreement | 25 | |||
FDA | 52 | |||
Final Earnout Shares | 127 | |||
financial projections | 103 | |||
First Lien ABL Credit Facility | 145 | |||
First Lien Consent | 145 | |||
Free Cash Flow | 107 | |||
FTC | 25 | |||
FTM | 91 | |||
Full Earnout | 85 | |||
GAAP | 28 | |||
Giants Revenue | 127 | |||
Giants/Zyga Revenue | 20 | |||
Hayfin Entities | 117 | |||
HHS | 52 | |||
Holdco . . . . . . . . . . . . . . . .. . . Additional Information |
| |||
Holdco board | 8 | |||
Holdco bylaws | 166 | |||
Holdco Certificate of Designation | 166 | |||
Holdco charter | 166 | |||
| Holdco common stock . . . . . . . Additional Information |
| |||
| Holdco preferred stock . . . . . . . Additional Information |
| |||
HSR Act | 24 | |||
IDE | 58 | |||
Initial Earnout Shares | 127 | |||
Intended Tax Treatment | 27 | |||
Intervening Event | 26 | |||
Investor Rights Agreement | 170 | |||
IRS | 40 | |||
ISO | 55 | |||
JPM | 7 | |||
Liquidation Value | 169 | |||
Lock-Up Agreement | 114 | |||
Lock-Up and Permitted Sale Agreement | 114 | |||
Lock-Up Period | 114 | |||
LTM Period | 127 | |||
M&A | 91 | |||
Master Transaction Agreement . Additional Information |
| |||
MCRA | 60 | |||
Meeting Adjournment Proposal | 68 | |||
Merger . . . . . . . . . . . . . . . .. . . Additional Information |
| |||
Merger Proposal | 65 | |||
Merger Sub . . . . . . . . . . . . . .. Additional Information |
| |||
Nasdaq | 7 | |||
NASS | 150 | |||
Non-Competition Agreements | 120 | |||
NPV Aggregate Consideration | 85 | |||
October RTI combined financial projections | 103 | |||
OEM | 31 | |||
OIG | 52 | |||
outside date | 26 | |||
Paradigm . . . . . . . . . . . . . . .. . Additional Information |
| |||
Paradigm board | 60 | |||
Paradigm financial projections | 102 | |||
Paradigm LLC Agreement | 172 | |||
Paradigm Member | 172 | |||
Paradigm Support Agreements | 119 | |||
Paradigm Support Unitholders | 119 | |||
Piper Jaffray | 23 | |||
PMA | 53 | |||
PPACA | 52 | |||
Pre-Closing Refund | 138 | |||
Preferred Directors | 170 | |||
Preferred Percentage | 170 | |||
189
PS Spine . . . . . . . . . . . . . . . . Additional information |
| |||
PS Spine board | 23 | |||
PS Spine consent solicitation | 3 | |||
PS Spine disclosure letter | 123 | |||
PS Spine LLC Agreement | 178 | |||
PS Spine record date | 4 | |||
PS Spine unitholders . . . . . . . . Additional information |
| |||
PS Spine written consent | 70 | |||
QSR | 54 | |||
Required Merger Proposal Vote | 65 | |||
Required PS Spine Vote | 72 | |||
Required Share Issuance Proposal Vote | 67 | |||
Restricted Products | 120 | |||
RSMs | 151 | |||
RTI . . . . . . . . . . . . . . . .. . . . . Additional information |
| |||
RTI adjusted Paradigm financial projections | 102 | |||
RTI board | 9 | |||
RTI bylaws | 172 | |||
RTI Certificate of Designation | 172 | |||
RTI charter | 172 | |||
RTI common stock . . . . . . . . . . Additional information |
| |||
RTI disclosure letter | 123 | |||
RTI preferred stock . . . . . . . . . Additional information |
| |||
RTI Price | 3 | |||
RTI record date | 4 | |||
RTI Restricted Stock Award | 125 | |||
RTI shares | 4 | |||
RTI special meeting | 3 | |||
RTI stock | 4 | |||
RTI Stock Option | 125 | |||
RTI Support Agreements | 120 | |||
RTI Support Stockholders | 120 | |||
SEC | 1 | |||
Second Lien Commitment Letter | 145 | |||
Second Lien Credit Agreement | 145 | |||
Second Lien Facility | 25 | |||
Securities Act | 24 | |||
Sensitivity Assumptions | 87 | |||
Settlement Agreement | 117 | |||
Share Issuance Proposal | 66 | |||
Sidley Austin | 24 | |||
Stock Consideration Amount | 3 | |||
Stock Price Doubles | 85 | |||
Stock Price Triples | 85 | |||
Straddle Period | 137 | |||
Surviving Corporation | 18 | |||
Synergies | 84 | |||
Transaction | 3 | |||
TTM | 87 | |||
U.S. holder | 110 | |||
Upfront Aggregate Consideration | 85 | |||
VWAP | 127 | |||
Zyga | 84 |
190
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS OF PARADIGM
Unaudited Condensed Consolidated Financial Statements of Paradigm | ||||
Condensed Consolidated Balance Sheets as of September 30, 2018 and December 31, 2017 (unaudited) | F-2 | |||
| F-3 | ||||
| F-4 | ||||
| F-5 | ||||
Notes to Condensed Consolidated Financial Statements (unaudited) | F-6 | |||
| F-21 | ||||
Audited Consolidated Financial Statements of Paradigm | ||||
Consolidated Balance Sheets as of December 31, 2017 and 2016 | F-23 | |||
| F-24 | ||||
| F-25 | ||||
Consolidated Statements of Cash Flows for the Years Ended December 31, 2017 and 2016 | F-26 | |||
| F-27 | ||||
F-1
CONDENSED CONSOLIDATED BALANCE SHEETS
AS OF SEPTEMBER 30, 2018 AND DECEMBER 31, 2017
(Unaudited)
| September 30, | December 31, | |||||||
| 2018 | 2017 | |||||||
ASSETS | ||||||||
CURRENT ASSETS: | ||||||||
Cash and cash equivalents | $ | 1,074,807 | $ | 1,753,974 | ||||
Accounts receivable—net of allowance of $587,817 at September 30, 2018, and $525,407 at December 31, 2017 | 5,579,124 | 6,324,450 | ||||||
Inventory | 6,506,453 | 6,480,105 | ||||||
Prepaid expenses and other current assets | 1,819,030 | 1,874,649 | ||||||
|
|
|
| |||||
Total current assets | 14,979,414 | 16,433,178 | ||||||
RESTRICTED CASH | — | 8,993,291 | ||||||
PROPERTY AND EQUIPMENT—Net | 490,543 | 543,458 | ||||||
OTHER ASSETS—Net | 839,140 | 750,296 | ||||||
DEFERRED TAX ASSET—Net | 13,501 | 13,988 | ||||||
|
|
|
| |||||
TOTAL | $ | 16,322,598 | $ | 26,734,211 | ||||
|
|
|
| |||||
LIABILITIES AND MEMBERS’ (DEFICIT) EQUITY | ||||||||
CURRENT LIABILITIES: | ||||||||
Accounts payable | $ | 6,392,963 | $ | 4,522,844 | ||||
Other current liabilities | 2,618,330 | 2,760,714 | ||||||
Current portion of term loan | 129,943,386 | 111,199,426 | ||||||
|
|
|
| |||||
Total liabilities | 138,954,679 | 118,482,984 | ||||||
|
|
|
| |||||
COMMITMENTS AND CONTINGENCIES | ||||||||
MEMBERS’ (DEFICIT) EQUITY: | ||||||||
Preferred units—authorized, 2018—46,467,021 units and 2017—46,467,021 units; issued and outstanding, 2018—35,324,216 units and 2017—35,324,216 units (aggregate liquidation preference $117,708,004 in 2018 and $117,708,004 in 2017) | 102,940,198 | 102,940,198 | ||||||
Common units—authorized, issued, and outstanding, 2018—3,200,754 units and 2017—3,200,754 units | 2,836,156 | 2,836,156 | ||||||
Additionalpaid-in capital | 18,450,343 | 18,447,804 | ||||||
Accumulated deficit | (245,028,711 | ) | (214,428,667 | ) | ||||
Accumulated other comprehensive loss | (1,830,067 | ) | (1,544,264 | ) | ||||
|
|
|
| |||||
Total members’ deficit | (122,632,081 | ) | (91,748,773 | ) | ||||
|
|
|
| |||||
TOTAL | $ | 16,322,598 | $ | 26,734,211 | ||||
|
|
|
| |||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
F-2
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2018 AND 2017
(Unaudited)
| Nine Months Ended | ||||||||
| September 30, | ||||||||
| 2018 | 2017 | |||||||
NET SALES | $ | 30,531,592 | $ | 33,924,803 | ||||
COST OF GOODS SOLD | 4,051,560 | 3,878,073 | ||||||
|
|
|
| |||||
GROSS PROFIT | 26,480,032 | 30,046,730 | ||||||
SELLING, GENERAL AND ADMINISTRATIVE EXPENSES | 39,702,596 | 37,019,777 | ||||||
RESEARCH AND DEVELOPMENT EXPENSES | 235,508 | 102,556 | ||||||
|
|
|
| |||||
LOSS FROM OPERATIONS | (13,458,072 | ) | (7,075,603 | ) | ||||
INTEREST INCOME | 59,390 | 240,949 | ||||||
INTEREST EXPENSE | (17,243,095 | ) | (10,120,294 | ) | ||||
OTHER INCOME—Net | 47,339 | 7,575,720 | ||||||
|
|
|
| |||||
LOSS BEFORE INCOME TAX EXPENSE | (30,594,438 | ) | (9,379,228 | ) | ||||
INCOME TAX EXPENSE | 5,606 | 82,085 | ||||||
|
|
|
| |||||
NET LOSS | (30,600,044 | ) | (9,461,313 | ) | ||||
OTHER COMPREHENSIVE INCOME (LOSS)—Foreign currency translation | 285,803 | (859,243 | ) | |||||
|
|
|
| |||||
COMPREHENSIVE LOSS | $ | (30,314,241 | ) | $ | (10,320,556 | ) | ||
|
|
|
| |||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
F-3
CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN MEMBERS’ DEFICIT
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2018
(Unaudited)
| Preferred Units | Common Units | Additional Paid-In | Accumulated | Accumulated Comprehensive | ||||||||||||||||||||||||||||
| Number of Units | Stated Value | Number of Units | Stated Value | Total | ||||||||||||||||||||||||||||
BALANCE—December 31, 2017 | 35,324,216 | $ | 102,940,198 | 3,200,754 | $ | 2,836,156 | $ | 18,447,804 | $ | (214,428,667 | ) | $ | (1,544,264 | ) | $ | (91,748,773 | ) | |||||||||||||||
Unit-based compensation expense | — | — | — | — | 2,539 | — | — | 2,539 | ||||||||||||||||||||||||
Net loss | — | — | — | — | — | (30,600,044 | ) | — | (30,600,044 | ) | ||||||||||||||||||||||
Foreign currency translation | — | — | — | — | — | — | (285,803 | ) | (285,803 | ) | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
BALANCE—September 30, 2018 | 35,324,216 | $ | 102,940,198 | 3,200,754 | $ | 2,836,156 | $ | 18,450,343 | $ | (245,028,711 | ) | $ | (1,830,067 | ) | $ | (122,632,081 | ) | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
F-4
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE NINE MONTHS ENDED SEPTEMBER, 2018 AND 2017
(Unaudited)
| Nine Months Ended September 30, | ||||||||
| 2018 | 2017 | |||||||
CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
Net loss | $ | (30,600,044 | ) | $ | (9,461,313 | ) | ||
Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
Depreciation and amortization | 279,476 | 656,186 | ||||||
Change in deferred income tax | 487 | (1,113 | ) | |||||
Unit-based compensation | 2,539 | 105,076 | ||||||
Change in fair value of warrant liability | — | (7,458,487 | ) | |||||
Write-offs for inventory obsolescence | 33,341 | 1,948 | ||||||
Bad debt expense | 87,535 | 115,491 | ||||||
Amortization of deferred financing costs | 2,375,547 | 442,429 | ||||||
Accrued interest income | (31,587 | ) | (23,970 | ) | ||||
Paid-in-kind interest | 14,867,335 | 3,159,440 | ||||||
Net changes in operating assets and liabilities: | ||||||||
Accounts receivable | 657,791 | 1,727,419 | ||||||
Inventory | (59,689 | ) | (1,106,627 | ) | ||||
Prepaid expenses and other current assets | 55,619 | (141,317 | ) | |||||
Accounts payable | 1,870,119 | (2,591,223 | ) | |||||
Other current liabilities | (142,385 | ) | 1,799,504 | |||||
|
|
|
| |||||
Net cash used in operating activities | (10,603,916 | ) | (12,776,557 | ) | ||||
|
|
|
| |||||
CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
Capital expenditures | (226,562 | ) | (457,781 | ) | ||||
Investor loan | — | (280,000 | ) | |||||
Security deposit | (3,453 | ) | 22,518 | |||||
Investment in joint venture | (53,804 | ) | (135,621 | ) | ||||
|
|
|
| |||||
Net cash used in investing activities | (283,819 | ) | (850,884 | ) | ||||
|
|
|
| |||||
CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
Unit repurchase | — | (603,500 | ) | |||||
Proceeds from secured debt financing | 1,500,000 | — | ||||||
Issuance costs for term loan | 1,080 | (5,374 | ) | |||||
|
|
|
| |||||
Net cash provided by (used in) financing activities | 1,501,080 | (608,874 | ) | |||||
|
|
|
| |||||
EFFECT OF EXCHANGE RATE ON CASH, CASH EQUIVALENTS, AND | (285,803 | ) | 859,243 | |||||
|
|
|
| |||||
NET DECREASE IN CASH, CASH EQUIVALENTS, AND | (9,672,458 | ) | (13,377,072 | ) | ||||
CASH, CASH EQUIVALENTS, AND RESTRICTED CASH—Beginning of period | 10,747,265 | 49,712,444 | ||||||
|
|
|
| |||||
CASH, CASH EQUIVALENTS, AND RESTRICTED CASH—End of period | $ | 1,074,807 | $ | 36,335,372 | ||||
|
|
|
| |||||
SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION: | ||||||||
Cash paid for interest | $ | — | $ | 6,518,415 | ||||
|
|
|
| |||||
Cash paid for taxes | $ | 108,426 | $ | 69,725 | ||||
|
|
|
| |||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
F-5
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
AS OF SEPTEMBER 30, 2018, AND DECEMBER 31, 2017 AND
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2018 AND 2017
(Unaudited)
| 1. | BUSINESS |
Paradigm Spine, LLC, a Delaware limited liability company, formed on April 5, 2005, through its subsidiaries (collectively, the “Company”) designs, develops, manufactures, and markets nonfusion and fusion spinal implant solutions. Spinal implants are utilized by orthopedic surgeons and neurosurgeons in the treatment of degenerative diseases, deformities, and trauma in all regions of the spine.
The Company sells its products through direct sales in Germany, Switzerland, and United States and through distributors in 46 other countries, mainly in Europe and in Asia Pacific, Latin America, the Middle East, India, Africa, and Canada.
On August 8, 2016, Paradigm Spine, LLC formed a wholly owned subsidiary, Andi’s Belmarall, LLC, a Delaware limited liability company.
Paradigm Spine GmbH, formed on August 5, 2005, is a wholly owned German subsidiary of Andi’s Belmarall, LLC. Paradigm Spine GmbH formed wholly owned subsidiaries, Paradigm Spine Austria GmbH, on March 12, 2007, and Paradigm Spine Switzerland AG, on August 21, 2007.
Paradigm Spine, LLC formed a wholly owned subsidiary, Fourth Dimension Spine, LLC, a Delaware limited liability company, on June 12, 2007. Fourth Dimension Spine, LLC formed a wholly owned subsidiary, Fourth Dimension Spine GmbH, a German limited liability company, on August 31, 2007.
On October 17, 2012, the Company announced US Food and Drug Administration premarket approval of its landmark coflex Interlaminar Technology: the first comparative effectiveness study for the treatment of spinal stenosis. coflex® is a minimally invasive, motion-preserving Interlaminar Stabilization™ device for the treatment ofmoderate-to-severe stenosis, with or without back pain.
Effective January 1, 2017, the procedure in which coflex® is implanted is covered under its own Common Procedural Terminology (CPT)Level-1 code determined by the American Medical Association.
Nature of Business—Paradigm Spine, LLC was formed to be a leader in the field of nonfusion spinal implant technology. The Company is committed to improving the quality of life of patients with spinal diseases through its mission to provide products that are surgeon centric, indication specific, and data driven. Paradigm Spine, LLC is now an innovative leader in the global spine market and believes there is a significant opportunity to improve treatment options for patients suffering from lumbar spinal stenosis andage-related spinal deformities.
| 2. | SIGNIFICANT ACCOUNTING POLICIES |
Basis of Presentation—The condensed consolidated financial statements include the accounts of Paradigm Spine, LLC, its wholly owned subsidiaries—Andi’s Belmarall, LLC and Fourth Dimension Spine, LLC, and their wholly owned subsidiaries. All intercompany balances and transactions are eliminated upon consolidation.
Use of Estimates—The condensed consolidated financial statements are prepared in conformity with accounting principles generally accepted in the United States of America (US GAAP) and include amounts that are based on management’s best estimates and judgments that affect the amounts reported in the condensed consolidated financial statements and accompanying notes. These estimates are based on historical experience and on various other assumptions that are believed to be reasonable under the current circumstances. Actual results could differ from those estimates. Significant estimates include the variables
F-6
and methods used to calculate unit-based compensation, preferred unit warrant liability, detachable warrants, deferred tax allowances, allowance for doubtful accounts receivable, and write-offs for inventory obsolescence.
Foreign Currency Translation—Assets and liabilities of the foreign subsidiaries are translated at rates of exchange in effect at the close of the period, and equity amounts are translated at historical exchange rates. Revenues and expenses are translated at the weighted average of exchange rates in effect during each month. The effect of exchange rate fluctuations on translating foreign currency assets and liabilities into US dollars is included as the currency translation adjustment component of accumulated other comprehensive loss within the condensed consolidated statements of changes in members’ deficit. Realized and unrealized foreign exchange transaction gains and losses are included within the condensed consolidated statements of operations and comprehensive loss.
Cash and Cash Equivalents—The Company considers all highly liquid investments with a maturity of three months or less when purchased to be cash equivalents. Cash and cash equivalents may include demand deposits held in banks and interest-bearing money market funds. The carrying amounts reported in the condensed consolidated balance sheets for cash and cash equivalents are recorded at fair value.
The Company had $9 million of restricted cash as of December 31, 2017, in accordance with the terms of its amended credit agreement, further described in Note 6. The Company early adopted the provisions of Accounting Standards Update (ASU)No. 2016-18,Statement of Cash Flows: Restricted Cash, issued by Financial Accounting Standards Board (FASB) in November 2016. The impact of the early adoption is that the change in restricted cash is not shown as an investing activity, but is included in cash and cash equivalents in the condensed consolidated statements of cash flows.
Concentration of Credit Risk—Financial instruments that potentially subject the Company to significant concentrations of credit risk consist primarily of cash equivalents. The Company invests its excess cash in US government money market funds, and its deposits, at times, exceed federally insured limits. The Company has not experienced any losses from credit risks.
Allowance for Doubtful Accounts Receivable—The Company evaluates the collectability of accounts receivable using a combination of factors. Provisions to the allowance for doubtful accounts receivable are recorded based on a number of factors, including the length of time the receivables are past due, the current business environment, and the Company’s historical experience. Changes to the provision are recorded in selling, general, and administrative expenses.
Inventory—Inventory, which consists exclusively of finished goods manufactured by third parties, is stated at the lower of cost or market, with cost determined on afirst-in,first-out basis or average cost method, depending on the product. The Company reviews inventory for shrinkage or obsolete items based on expected revenues and product life cycles. Write-offs for shrinkage or obsolescence of inventory are recorded in cost of goods sold.
Property and Equipment—Property and equipment are carried at cost, less accumulated depreciation. Depreciation is computed using the straight-line method, based on estimated useful lives of three to seven years for software, computer equipment, and furniture; and is recorded in selling, general, and administrative expenses. Depreciation of instruments is estimated on a useful life of one year, and is recorded in cost of goods sold. Leasehold improvements are amortized over the term of the lease or useful life of the improvements, whichever is shorter. Maintenance and repairs are expensed as incurred. Instruments are hand-held devices used by orthopedic spine surgeons and neurosurgeons during surgical procedures. Instruments are recognized as long-lived assets and included in property and equipment. Instruments in the field are carried at cost, less accumulated depreciation. Property and equipment are reviewed for impairment in accordance with the FASB Accounting Standards Codification (ASC) 360,Property, Plant, and Equipment.
Accumulated Other Comprehensive Loss—The component of accumulated other comprehensive loss includes foreign currency translation adjustments and is included in the condensed consolidated statements of operations and comprehensive loss in accordance with ASC 220,Comprehensive Income.
F-7
Revenue Recognition—The Company recognizes revenue from sales of implants principally to hospitals and to distributors. For sales to hospitals, revenue is recognized on the date of surgery when the device is implanted. For sales to distributors, revenue is recognized when title and risk of ownership have been transferred, provided that persuasive evidence of an arrangement exists, delivery has occurred, the price is fixed and determinable, the buyer is obligated to pay, the obligation is not contingent on resale of the product, the buyer has economic substance apart from the Company, the Company has no obligation to bring about the resale of the product, the amount of returns and discounts can be reasonably estimated, the remaining obligations are insignificant, and collectability is reasonably assured. Revenue is recorded net of customer discounts and rebates.
Income Taxes—Deferred tax liabilities (DTLs) and deferred tax assets (DTAs) are related to the operations of Paradigm Spine GmbH and are recognized for the expected future tax consequences of events that have been included in the condensed consolidated financial statements. The Company accounts for income taxes under the asset and liability method, whereby DTAs and DTLs are determined based on the difference between the condensed consolidated financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce DTAs to the amounts expected to be realized.
The Company is classified as a partnership for domestic federal income tax purposes, and is generally not subject to federal income taxes, but is subject to certain state and local taxes. Each member of the Company bears the economic burden of the income tax liability, if any, related to its proportionate share of the Company’s taxable income. All subsidiaries of the Company have elected to be treated as disregarded entities for domestic tax purposes and, accordingly, all business and assets are reported on Paradigm Spine, LLC’s income tax returns. However, certain subsidiary entities are subject to local country income taxes, which the Company accounts for under the liability method.
Unit-Based Compensation—For the nine months ended September 30, 2018 and 2017, the Company has one unit-based employee compensation plan, which is described more fully in Note 8.
For unit options granted as consideration for services rendered by nonemployees, the Company recognizes expense in accordance with the requirements of ASC 505,Equity Based Payments to Nonemployees. Nonemployee option grants that do not vest immediately upon grant are recorded as an expense over the vesting period of the underlying stock options. At the end of each financial reporting period prior to vesting, the value of these options, as calculated using the Black-Scholes option-pricing model, will be remeasured using the fair value of the Company’s common stock, and the noncash expense recognized during the period will be adjusted accordingly. Since the fair market value of options granted to nonemployees is subject to change in the future, the amount of the future expense will include fair value remeasurements until the stock options are fully vested.
The Company accounts for its unit-based compensation in accordance with ASC 718,Compensation—Stock Compensation. Compensation expense is recognized in the condensed consolidated financial statements on a prospective basis for all unit-based payments granted based upon the grant-date fair value estimated at that time.
The grant-date fair value of awards expected to vest is expensed on a straight-line basis over the vesting period of the related awards.
The Company selected the Black-Scholes option-pricing model as the most appropriate model for determining the estimated fair value for unit-based awards. The fair value is then amortized on a straight-line basis over the requisite service periods of the entire awards, which is generally the vesting period. Use of a valuation model requires management to make certain assumptions regarding a number of complex and subjective variables. Expected volatility was calculated based on a blended weighted average of similar public entities for which historical information was available. The Company will continue to use a weighted-average approach using similar public entity volatility information until historical volatility of the Company is relevant to measure expected volatility for future option grants. The average expected life was
F-8
determined in accordance with the “simplified method” as described in Staff Accounting Bulletin No. 110,“Share-Based Payment”. The risk-free interest rate is based on US Treasuryzero-coupon issues with a remaining term equal to the expected life assumed at the date of grant. Forfeitures are estimated based on voluntary termination behavior as well as a historical analysis of actual option forfeitures.
Derivative Financial Instruments—Derivative financial instruments, as defined in ASC 815,Derivatives and Hedging, consist of the preferred unit warrants issued in connection with the Company’s 2011 term loan described in Note 6. These financial instruments are recorded in the condensed consolidated balance sheets as warrant liability, further described in Notes 3 and 7, with changes in fair value recognized in earnings in the period of change.
Fair Value of Financial Instruments—The Company measures its financial assets and liabilities using the fair value method in accordance with ASC 820,Fair Value Measurements and Disclosures. ASC 820 defines fair value, establishes a framework for measuring fair value under US GAAP, and enhances disclosures about fair value measurements. Fair value is defined under ASC 820 as the exchange price that would be received for an asset or paid to transfer a liability (an exit price in the principal or the most advantageous market for an asset or liability in an orderly transaction between participants on the measurement date). Valuation techniques used to measure fair value under ASC 820 must maximize the use of observable inputs and minimize the use of unobservable inputs. The standard describes a fair value hierarchy based on the levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value, which are as follows:
Level 1—Quoted prices in active markets for identical assets or liabilities that the Company has the ability to access at the measurement day.
Level 2—Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities, quoted prices in markets that are not active, or other inputs that are observable or corroborated by observable market data over substantially the full term of the assets or liabilities.
Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the value of the assets or liabilities.
Recently Issued Accounting Standards—In May 2014, the FASB issued ASU2014-09,Revenue from Contracts with Customers (Topic 606). The Company plans to adopt ASU2014-09 effective January 1, 2019. This new accounting standard outlines a single comprehensive model to use in accounting for revenue arising from contracts with customers. This standard supersedes existing revenue recognition requirements and eliminates most industry-specific guidance from US GAAP. The core principle of the new accounting standard is to recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. The Company does not expect the adoption of the new accounting standard to have a material impact on its condensed consolidated financial statements.
In February 2016, the FASB issued ASUNo. 2016-02,Leases (Topic 842), to increase transparency and comparability among organizations by requiring the recognition ofright-to-use assets and liabilities on the balance sheet and disclosing qualitative and quantitative information about leasing arrangements. The Company plans to adopt ASUNo. 2016-02 effective January 1, 2020. The Company is currently in the process of evaluating the impact of adoption of ASUNo. 2016-02 on its condensed consolidated financial statements.
In May 2017, the FASB issuedASU 2017-09, Compensation—Stock Compensation (Topic 718): Scope of Modification Accounting. The ASU provides guidance on determining which changes to the terms and conditions of share-based payment awards require an entity to apply modification accounting under Topic 718. The Company adoptedASU 2017-09 on January 1, 2018 and it did not have a material impact on its condensed consolidated financial statements.
F-9
| 3. | FAIR VALUE |
The information about each major category of the Company’s financial assets and liabilities measured at fair value on a recurring basis as of September 30, 2018, and December 31, 2017, is presented in the following fair value hierarchy table:
| Quoted Prices in Active Markets for Identical Assets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | Total | |||||||||||||
At September 30, 2018 | ||||||||||||||||
Cash | $ | 1,074,807 | $ | — | $ | — | $ | 1,074,807 | ||||||||
|
|
|
|
|
|
|
| |||||||||
At December 31, 2017 | ||||||||||||||||
Cash | $ | 10,747,265 | $ | — | $ | — | $ | 10,747,265 | ||||||||
|
|
|
|
|
|
|
| |||||||||
The following table reflects the changes in fair value of the Company’s Level 3 financial instruments:
| Level 3 | ||||||||
| Warranty Liability | 2018 | 2017 | ||||||
Balance—beginning of period | $ | — | $ | 7,458,487 | ||||
Fair value adjustments | — | (7,458,487 | ) | |||||
|
|
|
| |||||
Balance—end of period | $ | — | $ | — | ||||
|
|
|
| |||||
The assumptions used in computing the fair value adjustments are disclosed in tabular format within the Warrants section of Note 7.
Some of the Company’s financial instruments are not measured at fair value on a recurring basis, but are recorded at amounts that approximate fair value due to their liquid short-term nature, such as cash and cash equivalents, receivables, and payables.
As more fully described in Note 6, the estimated carrying value of the Company’s term loan at September 30, 2018, and December 31, 2017, approximated its fair value at such dates.
| 4. | PROPERTY AND EQUIPMENT |
Property and equipment—net as of September 30, 2018, and December 31, 2017, consist of the following:
| 2018 | 2017 | Useful Lives | ||||||||||
Computer equipment and furniture | $ | 1,604,055 | $ | 1,612,740 | 3–7 years | |||||||
Computer software | 465,105 | 460,815 | 3–7 years | |||||||||
Instruments | 4,664,931 | 4,629,252 | 1–5 years | |||||||||
Leasehold improvements | 105,838 | 73,766 | Life of lease | |||||||||
|
|
|
| |||||||||
Total property and equipment | 6,839,929 | 6,776,573 | ||||||||||
Accumulated depreciation and amortization | (6,349,386 | ) | (6,233,115 | ) | ||||||||
|
|
|
| |||||||||
Property and equipment—net | $ | 490,543 | $ | 543,458 | ||||||||
|
|
|
| |||||||||
Depreciation and amortization expense was $279,476 and $656,185 for the nine months ended September 30, 2018 and 2017, respectively.
F-10
| 5. | OTHER CURRENT LIABILITIES |
Other current liabilities as of September 30, 2018, and December 31, 2017, consist of the following:
| 2018 | 2017 | |||||||
Employee compensation and related expenses | $ | 1,322,525 | $ | 899,913 | ||||
Royalty withholding tax payable | 19,339 | 30,909 | ||||||
Rebate payable | 153,915 | 82,690 | ||||||
Marketing study costs | 66,210 | 68,595 | ||||||
Income taxes payable | 123,021 | 95,494 | ||||||
Sales discounts payable | 193,414 | 229,716 | ||||||
Other | 739,906 | 1,353,397 | ||||||
|
|
|
| |||||
Total | $ | 2,618,330 | $ | 2,760,714 | ||||
|
|
|
| |||||
| 6. | DEBT |
On June 29, 2011, the Company closed on a $37.5 million five-year term loan from a conglomerate of investors. The Company used the proceeds to repay the existing $15 million line of credit to its lender. The Company issued to the lender detachable10-year warrants to purchase2,263,002 Class E-1 Preferred Units at the exercise price of $4.557, with an initial value of $6,945,301 as part of the consideration of the term loan. These warrants were classified as a liability as more fully disclosed in Note 7. The term loan was scheduled to mature on June 29, 2016, but was repaid in full in 2014 with the proceeds of the secured debt financing described below.
On February 14, 2014, the Company entered into a five-year $75 million secured debt financing transaction with a lender. The Company used the proceeds to repay the outstanding balance of $41.5 million on the existing term loan, as well as a prepayment penalty of $5.2 million and interest of $0.8 million. In connection with the term loan, the Company paid a finder’s fee of $750,000 cash and granted 50,471 warrants, classified in equity, to purchase Class E2 Preferred Units to its agent, at an exercise price of $7.43, with a value of $162,521. The carrying value of the term loan was shown net of a discount related to the issuance of these warrants and for deferred financing costs that were amortized over the period of the loan. The term loan was scheduled to mature on February 14, 2019, but was repaid in full in 2016 with the proceeds of the senior secured term loan described below.
On August 26, 2016, the Company closed on a $100 million five-year senior secured term loan with lenders (2016 credit agreement). The Company used the proceeds to repay the outstanding balance of $54.7 million on the existing secured debt, as well as a prepayment penalty of $1.7 million and interest of $1.1 million. The loan bore an annual interest rate of 12%, with an effective interest rate of 13.15%. In connection with the loan, the Company entered into a placement agent agreement with a related party and paid a $1 million fee to the agent under the agreement using proceeds from the loan. The Company had the option to pay all or a portion of the interest, for each interest payment date occurring prior to the first anniversary of the closing date, as“Payment-In-Kind (PIK)” interest. All such PIK interest is added to the aggregate principal balance of the loan. The loan was subject to certain affirmative, negative, and financial covenants as more fully described in the credit agreement that must be certified by the chief financial officer. In April 2017, the Company notified its lenders that it had not satisfied its first quarter 2017 minimum adjusted net sales covenant, which constitutes an event of default per the terms of the 2016 credit agreement. The Company did not receive a waiver from its lenders for the violation and therefore the lenders were entitled, among other things, to exercise various rights and remedies, including declaring the loan due and payable in whole or in part, and the right to receive interest at the default rate and in cash. The lenders waived their right to apply the default interest rate, but reserved all other rights in a notification letter sent to the Company subsequent to the known default event.
On November 1, 2017, the Company entered into an amended credit agreement with the same lenders. Immediately prior to giving effect to this amendment, the Company prepaid an outstanding principal
F-11
amount of $21,000,000. The loan bears at an annual interest rate of 15%, plusone-month London InterBank Offered Rate (LIBOR), at an effective interest rate of 20.1%. Interest on the loan is payable as PIK interest and added to the outstanding principal amount of the loan. Total interest expense for the nine months ended September 30, 2018 and 2017, amounted to $14,867,335 and $9,677,856, respectively. The 2017 interest expense includes $6,518,415 of cash interest. The carrying value of the secured term loan is shown net of a discount related to deferred financing costs that is amortized over the period of the loan. As of September 30, 2018, and December 31, 2017, the deferred financing costs were $857,773 and $3,234,399, respectively. The amended loan is subject to certain affirmative, negative, and financial covenants as more fully described in the amended credit agreement that must be certified by the chief financial officer of the Company on a monthly and quarterly basis. The financial covenants consist of (i) a minimum liquidity amount of $3 million at all times, (ii) minimum adjusted net sales tested on a monthly basis, and (iii) permitted capital expenditures tested at each fiscal reporting period. In the event of a default, as defined in the amended credit agreement, the Company is required to, among other things, accrue interest at the annual interest rate, plusone-month LIBOR, plus the default interest rate of 3%. As of December 31, 2017, and thereafter, including September 30, 2018, the Company has not been in compliance with the financial covenants under the amended credit agreement. Management evaluated the significance of this event of default and concluded that this has cast substantial doubt about the Company’s ability to meet its financial obligations and consequently its ability to continue as a going concern within one year after the date that these condensed consolidated financial statements are available to be issued. Management also determined that in the absence of a waiver of the existing default, it was possible that the Company would need to raise additional capital; borrow additional money or restructure its current debt, including seeking other sources of long-term financing; or seek a purchaser of its business.
In connection with the execution of the amended credit agreement described above, the Company has entered into an Economic Rights Agreement with an affiliate of the lenders. Upon certain payment trigger events, such as the sale or liquidation of the Company, an initial public offering, full repayment of the loan, or making of prohibited distributions, the Company will pay such affiliate of the lenders $24,425,175. This amount is included in the carrying value of the secured term loan and has been recorded as PIK interest under interest expense in the condensed consolidated statement of operations and comprehensive loss for the three months ended December 31, 2017.
In April 2018, the Company notified its lenders that it had not achieved its first quarter 2018 minimum adjusted net sales covenant, which constitutes an event of default per the terms of the 2016 credit agreement, as amended in 2017. The Company did not receive a waiver from its lenders for the violation and therefore the lenders are entitled, among other things, to exercise various rights and remedies, including declaring the loan due and payable in whole or in part, and the right to receive interest at the default rate.
On August 24, 2018, the Company entered into a First Amendment to Credit Agreement providing the Company the ability to draw up to $5,000,000 with a holder payment amount of four times any amounts drawn. On August 24, 2018, the Company borrowed $1,500,000 pursuant to the First Amendment to Credit Agreement.
Maturities of the principal on the term loan at September 30, 2018, are as follows:
| Years Ended | Term Loan | |||
2018 | $ | 106,375,984 | ||
2019 | — | |||
2020 | — | |||
2021 | — | |||
2022 | — | |||
Total | $ | 106,375,984 | ||
|
| |||
As indicated above in this Note 6 and in Note 13, this loan is currently payable on demand and is classified as a current liability in the condensed consolidated balance sheets as of September 30, 2018, and December 31, 2017.
F-12
| 7. | CAPITAL STRUCTURE |
Common Units—Each holder of Common Units is entitled to vote on all matters and is entitled to one vote for each unit held. Distributions on Common Units will be paid when, as, and if declared by the Company’s board of managers (the “Board of Managers”), and only after each holder of Preferred Units then outstanding shall have first received distributions equal to their capital contributions. As of September 30, 2018, no distributions have been declared or paid by the Company.
Class A Common Units—Each holder of Class A Common Units is entitled to vote on all matters and is entitled to one vote for each Class A Common Unit held. Distributions on Class A Common Units will be paid when, as, and if declared by the Board of Managers, and only after each holder of Preferred Units then outstanding shall have first received distributions equal to their capital contributions. As of September 30, 2018, no distributions have been declared or paid by the Company.
Preferred Units—Preferred Units may be issued from time to time in one or more classes.
F-13
The table below presents information on the classes of preferred units:
| Class A | Class B | Class C | Class D | Class E | Total | |||||||||||||||||||||||||||||||||||||||||||
| Number of Units | Amount | Number of Units | Amount | Number of Units | Amount | Number of Units | Amount | Number of Units | Amount | Number of Units | Amount | |||||||||||||||||||||||||||||||||||||
Balance—December 31, 2016 | 2,717,886 | $ | 3,623,846 | 7,895,271 | $ | 2,866,006 | 5,524,729 | $ | 12,856,827 | 3,050,199 | $ | 11,414,601 | 16,270,242 | $ | 72,782,418 | 35,458,327 | $ | 103,543,698 | ||||||||||||||||||||||||||||||
Share cancellation | — | — | — | — | — | — | (134,111 | ) | (603,500 | ) | — | — | (134,111 | ) | (603,500 | ) | ||||||||||||||||||||||||||||||||
Balance—December 31, 2017 | 2,717,886 | 3,623,846 | 7,895,271 | 2,866,006 | 5,524,729 | 12,856,827 | 2,916,088 | 10,811,101 | 16,270,242 | 72,782,418 | 35,324,216 | 102,940,198 | ||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
Balance—September 30, 2018 | 2,717,886 | $ | 3,623,846 | 7,895,271 | $ | 2,866,006 | 5,524,729 | $ | 12,856,827 | 2,916,088 | $ | 10,811,101 | 16,270,242 | $ | 72,782,418 | 35,324,216 | $ | 102,940,198 | ||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
Class A Preferred Units—The holders of Class A Preferred Units are entitled to one vote for each Class A Preferred Unit held. In the event the Company declares or pays a distribution, such distributions will be paid to Common Unit and Class A Common Unit holders only after each holder of Class A Preferred Units then outstanding shall have first received distributions equal to their capital contributions. Upon any liquidation, sale, merger, dissolution, or winding up of the Company, the holders of Class A Preferred Units have rights in preference to Common and Class A Common Units. As of September 30, 2018, the Company had 2,717,886 Class A Preferred Units authorized for issuance.
As of September 30, 2018, and December 31, 2017, Class A Preferred Units are recorded at their stated value of $1.33 per unit. The liquidation preference of the Class A Preferred Units at September 30, 2018, and December 31, 2017, was $3,623,846. One of the officers and unit holders of the Company, who is also affiliated with a related party, holds the proxy for all Class A Preferred Units.
Class B Preferred Units—The holders of Class B Preferred Units are entitled to one vote for each Class B Preferred Unit held. In the event the Company declares or pays a distribution, such distributions will be paid to Common Unit and Class A Common Unit holders only after each holder of Class B Preferred Units then outstanding shall have first received distributions equal to their capital contributions. Upon any liquidation, sale, merger, dissolution, or winding up of the Company, the holders of Class B Preferred Units have rights in preference to Common Units and Class A Common Units. As of September 30, 2018, and December 31, 2017, the Company had 7,895,271 Class B Preferred Units authorized for issuance.
As of September 30, 2018, and December 31, 2017, Class B Preferred Units are recorded at their stated value of $1.48 per unit, with the exception of 6,756,757 units awarded in exchange for the contribution of intellectual property to the Company, which are recorded at $1,181,048, the original cost of the intellectual property. The liquidation preference of the Class B Preferred Units at September 30, 2018, and December 31, 2017, was $11,685,000.
Class C Preferred Units—The holders of Class C Preferred Units do not have voting rights, except as otherwise required by law. In the event the Company declares or pays a distribution, such distributions will be paid to Common Unit and Class A Common Unit holders only after each holder of Class C Preferred Units then outstanding shall have first received distributions equal to their capital contributions. Upon any liquidation, sale, merger, dissolution, or winding up of the Company, the holders of Class C Preferred Units have rights in preference to Common Units and Class A Common Units. As of September 30, 2018, the Company had 5,695,379 Class C Preferred Units authorized for issuance.
As of September 30, 2018, and December 31, 2017, Class C Preferred Units are recorded at their stated value (issue price of $2.78 per unit, less issuance costs). The liquidation preference of the Class C Preferred Units at September 30, 2018, and December 31, 2017, was $15,359,160.
F-14
Class D Preferred Units—The holders of Class D Preferred Units do not have voting rights, except as otherwise required by law. In the event the Company declares or pays a distribution, such distributions will be paid to Common Unit and Class A Common Unit holders only after each holder of Class D Preferred Units then outstanding shall have first received distributions equal to their capital contributions. Upon any liquidation, sale, merger, dissolution, or winding up of the Company, the holders of Class D Preferred Units have rights in preference to Common Units and Class A Common Units. As of September 30, 2018, the Company had 4,071,444 Class D Preferred Units authorized for issuance.
As of September 30, 2018, and December 31, 2017, Class D Preferred Units are recorded at their stated value (issue price of $4.5 per unit, less issuance costs). The liquidation preference of the Class D Preferred Units at September 30, 2018, and December 31, 2017, was $13,122,396 and $13,122,396, respectively.
Class E Preferred Units—The holders ofClass E-1 Preferred Units are entitled to one vote for eachClass E-1 Preferred Unit held. The holders ofClass E-2 Preferred Units do not have voting rights, except as otherwise required by law. In the event the Company declares or pays a distribution, such distributions will be paid to Common Unit and Class A Common Unit holders only after each holder of Class E Preferred Units then outstanding shall have first received distributions equal to their capital contributions. Upon liquidation, sale, merger, dissolution, or winding up of the Company, the holders of Class E Preferred Units have rights in preference to Common Units and Class A Common Units. As of September 30, 2018, the Company had 25,952,930 Class E Preferred Units authorized for issuance.
As of September 30, 2018, and December 31, 2017, Class E Preferred Units are recorded at their stated value (issue price of $4.557 per unit, less issuance costs). The liquidation preference of the Class E Preferred Units at September 30, 2018, and December 31, 2017, was $73,917,601.
Preferred Unit Distributions—The holders of Class A, Class B, Class C, Class D, and Class E Preferred Units are entitled to receive distributions equal to their capital contributions in preference to the Common Units and Class A Common Units. In accordance with the Seventh Amended and Restated Limited Liability Company Agreement, dated as of May 28, 2009, the Class E Preferred Unit holders are entitled to a special allocation allowance in the event of the liquidation of the Company. If the fair market value of the Liquidation Assets is $350 million or less, prior to the allocation of profits under Sections 4.2(a)(iii) through 4.2(a)(v) of the agreement, profits realized in such liquidation shall be allocated first to the Class E Preferred Unit holders, pro rata in accordance with their percentage interests in such amount as will increase the capital accounts balance of each Class E Preferred Unit holder to its percentage interest of the sum of the capital account balances of all unit holders. If the fair market value of the Liquidation Assets is greater than $350 million, prior to the allocation of profits under Sections 4.2(a)(iii) through 4.2(a)(v) of the agreement, profits realized in such liquidation shall be allocated to the Class E Preferred Unit holders, pro rata in accordance with their percentage interests, in such amount, if any, as shall be necessary to prevent each Class E Preferred Unit holder from receiving as a distribution in such liquidation an amount less than the amount such Class E Preferred Unit holder would have received in the event that the fair market value of the Liquidation Assets equaled $350 million. Distributions are payable only when declared by the Board of Managers. No distributions have been declared from inception through September 30, 2018.
F-15
Warrants—The Company accounts for the warrants to purchase 2,263,002 Class E Preferred Units, issued in connection with its term loan described in Note 6, in accordance with ASC 815. For those warrants that have been deemed to be liabilities, the Company measures the fair value of its liability using an option-pricing model, with changes in fair value recognized as an adjustment to other income (expense). Based on the Company’s valuation methodology, there would be no remaining value for warrant holders and the fair value of the warrants has been adjusted to zero. The assumptions used in computing the fair value are illustrated in the following table:
| 2018 | 2017 | |||||||
Estimated unit prices | $ | 1.57 | $ | 1.57 | ||||
Exercise price | $ | 4.557 | $ | 4.557 | ||||
Expected unit price volatility | 35.0 | % | 35.0 | % | ||||
Risk-free interest rate | 2.6 | % | 2.6 | % | ||||
Expected remaining life of warrants (years) | 3.5 | 3.5 | ||||||
Expected annual dividend per unit | $ | — | $ | — | ||||
The remaining 1,871,845 warrants to purchase Class E Preferred Units and 365,775 warrants to purchase Common Units outstanding have been deemed to be equity instruments and are included as a part of permanent equity.
The following table reflects all warrants outstanding at September 30, 2018:
| Number of Warrants | Amount | Range of Exercise Prices | Range of Expiration Dates | |||||||||||||
Common | 197,498 | $ | 900,005 | $4.56 | June 2019 | |||||||||||
Class E Preferred Units | 4,134,847 | 18,987,503 | $4.557–$7.43 | June 2015 to June 2021 | ||||||||||||
|
|
|
| |||||||||||||
Total | 4,332,345 | $ | 19,887,508 | |||||||||||||
|
|
|
| |||||||||||||
| 8. | UNIT OPTION PLAN |
The total number of the Company’s units authorized to be issued under the incentive plan is capped at 6,939,303 as per Section 9.6 of the Company’s Seventh Amended and Restated LLC Agreement. At September 30, 2018, the number of Common Units available for issuance under the Unit Incentive Plan is 2,535,904.
F-16
The following table summarizes information about unit options outstanding:
| Number of Units (In Thousands) | Weighted- Average Exercise Price | Weighted- Average Remaining Contractual Life (In Years) | Weighted- Average Fair Value | Aggregate Intrinsic Value (In Millions) | ||||||||||||||||
Options outstanding—December 31, 2017 | 1,483 | $ | 4.20 | 3.20 | $ | 1.45 | $ | — | ||||||||||||
Granted | 687 | — | — | — | — | |||||||||||||||
Exercised | — | — | — | — | — | |||||||||||||||
Forfeited/canceled | (671 | ) | — | — | — | — | ||||||||||||||
|
| |||||||||||||||||||
Options outstanding—September 30, 2018 | 1,499 | 3.61 | 5.80 | 0.63 | — | |||||||||||||||
|
| |||||||||||||||||||
Unvested, expected to vest in the future | 458 | 3.06 | — | |||||||||||||||||
Vested and exercisable— | 1,041 | 3.86 | 5.70 | 0.64 | — | |||||||||||||||
|
| |||||||||||||||||||
Vested, exercisable, and expected to vest—September 30, 2018 | 1,499 | — | — | — | — | |||||||||||||||
|
| |||||||||||||||||||
Outstanding nonvested units—beginning of year | 3 | 4.56 | — | 1.71 | — | |||||||||||||||
Nonvested units granted | 687 | — | — | — | — | |||||||||||||||
Vested units | (232 | ) | — | — | — | — | ||||||||||||||
Nonvested units forfeited | — | — | — | — | — | |||||||||||||||
|
| |||||||||||||||||||
Outstanding nonvested units—end of period | 458 | 3.06 | — | — | — | |||||||||||||||
|
| |||||||||||||||||||
There were 686,588 units of options granted in 2018. The weighted-average grant-date fair value per unit of options granted for 2018 was $0. As of September 30, 2018, there is no unrecognized compensation cost related to nonvested units.
Unit-based compensation expense for the nine months ended September 30, 2018 and 2017, was $2,539 and $105,076, respectively.
Unit compensation expense is recorded in selling, general, and administrative expenses in the condensed consolidated statements of operations and comprehensive loss.
The weighted-average assumptions used in the Black-Scholes option-pricing model for options granted as of September 30, 2018 and December 31, 2017, are as follows:
| 2018 | 2017 | |||||||
Expected unit price volatility | — | % | — | % | ||||
Risk-free interest rate | — | % | — | % | ||||
Expected life of options (years) | — | — | ||||||
Expected annual dividend per unit | $ | — | $ | — | ||||
| 9. | INCOME TAXES |
For the nine months ended September 30, 2018 and 2017, the Company incurred income taxes of $5,607 and $82,085, respectively, primarily related to income generated by its subsidiary entities in Germany. The Company’s effective tax rate differs from its statutory tax rates primarily due to the valuation allowance recorded against its net DTAs and due to the Company’s legal structure organized as a limited liability company for domestic tax purposes, which is not subject to federal income taxes, but is subject to certain state and local taxes.
F-17
Deferred income taxes reflect the net effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. The Company’s DTAs relate primarily to net operating loss (NOL) carryforwards. At September 30, 2018, and December 31, 2017, a valuation allowance was recorded to offset the DTA related to NOLs. Significant components of the Company’s DTAs as of September 30, 2018, and December 31, 2017, were as follows:
| 2018 | 2017 | |||||||
DTAs: | ||||||||
Net operating loss carryforwards | $ | 1,289,138 | $ | 1,289,138 | ||||
Bad debt | 47,357 | 47,357 | ||||||
Amortization of intangible asset | — | — | ||||||
Depreciation | 81,034 | 81,034 | ||||||
Other | 10,150 | 10,637 | ||||||
|
|
|
| |||||
Total DTAs | 1,427,679 | 1,428,166 | ||||||
|
|
|
| |||||
DTLs: | ||||||||
Property and equipment | 343,788 | 343,788 | ||||||
Unrealized translation gains | 3,883 | 3,883 | ||||||
Other | 108,615 | 108,615 | ||||||
|
|
|
| |||||
Total DTLs | 456,286 | 456,286 | ||||||
|
|
|
| |||||
Valuation allowance for DTAs | 957,892 | 957,892 | ||||||
|
|
|
| |||||
Net DTAs | $ | 13,501 | $ | 13,988 | ||||
|
|
|
| |||||
At September 30, 2018, and December 31, 2017, the Company has NOLs for German, Austrian, and Swiss income tax purposes, in the aggregate, of approximately $4.9 million and $4.9 million, respectively, which can be offset against future taxable income. According to local tax laws, there is no expiration date for these loss carryforwards in Germany and Austria. In Switzerland, the NOL expires after seven years.
The Company’s subsidiary entity in Germany generally remains subject to tax examination for the year ended December 31, 2012, and later. The Company’s subsidiary entities in Switzerland and Austria remain subject to tax examination for the nine months ended September 30, 2018. In accordance with the accounting guidance for uncertainty in income taxes, the Company had recorded approximately $95,000 and $95,000 of unrecognized tax benefits as of September 30, 2018, and December 31, 2017, respectively. The balance of unrecognized tax benefits as of September 30, 2018, would affect the Company’s effective tax rate, if recognized.
| 10. | LEASES |
Future minimum rental commitments under noncancelable operating leases in effect as of September 30, 2018, are as follows:
2018 | $ | 26,475 | ||
2019 | 105,900 | |||
2020 | — | |||
2021 | — | |||
2022 | — |
Total rental expense for the nine months ended September 30, 2018 and 2017, was $484,036 and $431,676, respectively, and included $314,766 and $314,784 in 2018 and 2017, respectively, for related-party expense.
| 11. | RELATED PARTIES |
Two members of the Board of Managers who are also unit holders of the Company are principals of related entities that provide various services for the Company as follows: consulting services expense totaled
F-18
$2,531,620 and $4,563,223 for the nine months ended September 30, 2018 and 2017, respectively, and rent and other management services expense totaled $676,964 and $521,426 for the nine months ended September 30, 2018 and 2017, respectively.
As of September 30, 2018, and December 31, 2017, $2,137,085 and $1,433,679, respectively, was accrued for amounts due to related parties and is included in accounts payable in the Company’s condensed consolidated balance sheets.
As of September 30, 2018, and December 31, 2017, the Company included a receivable of $390,150 and $409,153, respectively, in other current assets associated with royalty withholding tax amounts due to the Company from its members.
Effective January 31, 2017, the Company entered into an agreement to loan a member of the board $180,000. Interest accrues at 13% per annum and is added to the outstanding principal balance on a quarterly basis. The principal balance, including accrued interest, is payable on the fifth anniversary of the effective date or sooner, dependent upon various events. On March 2, 2017, the Company entered into an agreement to loan the same member of the board $100,000, at the same terms as the prior loan. Interest income for the nine months ended September 30, 2018 and 2017, amounted to $31,587 and $23,970, respectively.
| 12. | COMMITMENTS AND CONTINGENCIES |
From time to time, the Company may have certain contingent liabilities that arise in the ordinary course of its business activities. The Company is not currently subject to any material legal proceedings.
On November 30, 2016, the Company entered into an agreement with a supplier to purchase 20,000 coflex® implants. Under the agreement, the Company guarantees to purchase the implants between January 1, 2017, and December 31, 2018, at various prices that reflect volume discounts. On January 25, 2018, the agreement was extended to December 31, 2019.
During March 2017, the Company entered into a first amendment to an existing agreement with a supplier to purchase 45,000 coflex® implants over a three-year term. The term commences upon achievement of certain operational and performance qualifications. The qualifications were satisfied in January 2018 and the Company entered into an amended agreement with the supplier to purchase 22,500 coflex® implants over a three-year term. The aggregate minimum amount of required purchases during the term is $2,250,000.
The aggregate minimum amount of required purchases at September 30, is as follows:
2018 | $ | 81,032 | ||
2019 | 1,038,263 | |||
2020 | 1,080,000 | |||
2021 | — | |||
2022 | — | |||
|
| |||
Total | $ | 2,199,295 | ||
|
|
| 13. | SUBSEQUENT EVENTS |
On October 2, 2018, the Company borrowed an additional $3,500,000 pursuant to a Second Amendment to the Credit Agreement described in Note 6.
Pursuant to a reorganization effective as of October 31, 2018, PS Spine HoldCo, LLC became the sole equity owner of the Company and all previously outstanding equity securities of the Company were converted into identical equity securities of PS Spine HoldCo, LLC of the same classes. PS Spine HoldCo had been an inactive wholly owned subsidiary of the Company and it has no other owners or assets.
F-19
On November 1, 2018, RTI Surgical, Inc. (RTI), a global surgical implant company, and PS Spine HoldCo, LLC announced that they have entered into a definitive agreement whereby RTI will acquire all outstanding equity interest of the Company in a cash and stock transaction valued at up to $300 million, consisting of $150 million at closing, plus potential future milestone payments.
On December 6, 2018, the Company borrowed an additional $3,000,000 pursuant to a Third Amendment to the Credit Agreement described in Note 6 and incurred an obligation for an additional $3,000,000 under an amendment to its Economic Rights Agreement.
Subsequent events have been evaluated by the Company through December 6, 2018, the date these condensed consolidated financial statements were available to be issued.
F-20
To the Board of Managers and Members of
Paradigm Spine, LLC
New York, New York
We have audited the accompanying consolidated financial statements of Paradigm Spine, LLC and its subsidiaries (the “Company”), which comprise the consolidated balance sheets as of December 31, 2017 and 2016, and the related consolidated statements of operations and comprehensive loss, changes in members’ deficit, and cash flows for the years then ended, and the related notes to the consolidated financial statements.
Management’s Responsibility for the Consolidated Financial Statements
Management is responsible for the preparation and fair presentation of these consolidated financial statements in accordance with accounting principles generally accepted in the United States of America; this includes the design, implementation, and maintenance of internal control relevant to the preparation and fair presentation of consolidated financial statements that are free from material misstatement, whether due to fraud or error.
Auditors’ Responsibility
Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We conducted our audits in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free from material misstatement.
An audit involves performing procedures to obtain audit evidence about the amounts and disclosures in the consolidated financial statements. The procedures selected depend on the auditor’s judgment, including the assessment of the risks of material misstatement of the consolidated financial statements, whether due to fraud or error. In making those risk assessments, the auditor considers internal control relevant to the Company’s preparation and fair presentation of the consolidated financial statements in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control. Accordingly, we express no such opinion. An audit also includes evaluating the appropriateness of accounting policies used and the reasonableness of significant accounting estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements.
We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our audit opinion.
Opinion
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Paradigm Spine, LLC and its subsidiaries as of December 31, 2017 and 2016, and the results of their operations and their cash flows for the years then ended in accordance with accounting principles generally accepted in the United States of America.
Emphasis of Matter Regarding Going Concern
The accompanying financial statements for the year ended December 31, 2017, have been prepared assuming that the Company will continue as a going concern. As discussed in Note 6 to the 2017 consolidated financial statements, the Company was not in compliance with a covenant of its 2016 Credit Agreement, as amended in 2017, during fiscal 2017, which has resulted in the Company’s debt becoming due upon demand. The uncertainty associated with the Company’s ability to repay its outstanding debt obligations as they become due raises substantial doubt about its ability to continue as a going concern. Management’s plans concerning these matters
F-21
are also discussed in Note 6 to the 2017 consolidated financial statements. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty. Our opinion is not modified with respect to this matter.
/s/ Deloitte & Touche LLP
Parsippany, New Jersey
April 27, 2018
F-22
CONSOLIDATED BALANCE SHEETS
AS OF DECEMBER 31, 2017 AND 2016
| 2017 | 2016 | |||||||
ASSETS | ||||||||
CURRENT ASSETS: | ||||||||
Cash and cash equivalents | $ | 1,753,974 | $ | 49,712,444 | ||||
Accounts receivable—net of allowance of $525,407 | 6,324,450 | 8,145,906 | ||||||
Inventory | 6,480,105 | 5,337,100 | ||||||
Prepaid expenses and other current assets | 1,874,649 | 1,865,532 | ||||||
|
|
|
| |||||
Total current assets | 16,433,178 | 65,060,982 | ||||||
RESTRICTED CASH | 8,993,291 | — | ||||||
PROPERTY AND EQUIPMENT—Net | 543,458 | 504,681 | ||||||
OTHER ASSETS—Net | 750,296 | 621,019 | ||||||
DEFERRED TAX ASSET—Net | 13,988 | 9,277 | ||||||
|
|
|
| |||||
TOTAL | $ | 26,734,211 | $ | 66,195,959 | ||||
|
|
|
| |||||
LIABILITIES AND MEMBERS’ (DEFICIT) EQUITY | ||||||||
CURRENT LIABILITIES: | ||||||||
Accounts payable | $ | 4,522,844 | $ | 5,410,062 | ||||
Other current liabilities | 2,760,714 | 1,874,849 | ||||||
Current portion of term loan | 111,199,426 | 101,556,397 | ||||||
|
|
|
| |||||
Total current liabilities | 118,482,984 | 108,841,308 | ||||||
WARRANT LIABILITY | — | 7,458,487 | ||||||
|
|
|
| |||||
Total liabilities | 118,482,984 | 116,299,795 | ||||||
|
|
|
| |||||
COMMITMENTS AND CONTINGENCIES | ||||||||
MEMBERS’ (DEFICIT) EQUITY: | ||||||||
Preferred units—authorized, 2017—46,467,021 units and 2016—46,467,021 units; issued and outstanding, 2017—35,324,216 units and 2016—35,458,327 units (aggregate liquidation preference $117,708,004 in 2017 and $118,311,503 in 2016) | 102,940,198 | 103,543,698 | ||||||
Common units—authorized, issued, and outstanding, 2017—3,200,754 units and 2016—3,200,754 units | 2,836,156 | 2,836,156 | ||||||
Additionalpaid-in capital | 18,447,804 | 18,340,222 | ||||||
Accumulated deficit | (214,428,667 | ) | (172,207,033 | ) | ||||
Accumulated other comprehensive loss | (1,544,264 | ) | (2,616,879 | ) | ||||
|
|
|
| |||||
Total members’ deficit | (91,748,773 | ) | (50,103,836 | ) | ||||
|
|
|
| |||||
TOTAL | $ | 26,734,211 | $ | 66,195,959 | ||||
|
|
|
| |||||
The accompanying notes are an integral part of these consolidated financial statements.
F-23
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
FOR THE YEARS ENDED DECEMBER 31, 2017 AND 2016
| 2017 | 2016 | |||||||
NET SALES | $ | 44,831,667 | $ | 55,640,620 | ||||
COST OF GOODS SOLD | 5,207,472 | 6,545,388 | ||||||
|
|
|
| |||||
GROSS PROFIT | 39,624,195 | 49,095,232 | ||||||
SELLING, GENERAL, AND ADMINISTRATIVE EXPENSES | 50,652,685 | 45,082,658 | ||||||
RESEARCH AND DEVELOPMENT EXPENSES | 256,353 | 433,188 | ||||||
|
|
|
| |||||
(LOSS) INCOME FROM OPERATIONS | (11,284,843 | ) | 3,579,386 | |||||
INTEREST INCOME | 301,077 | 46,366 | ||||||
INTEREST EXPENSE | (38,655,327 | ) | (11,902,048 | ) | ||||
OTHER INCOME—Net | 7,523,050 | 4,934,096 | ||||||
|
|
|
| |||||
LOSS BEFORE INCOME TAX EXPENSE | (42,116,043 | ) | (3,342,200 | ) | ||||
INCOME TAX EXPENSE | 105,591 | 284,087 | ||||||
|
|
|
| |||||
NET LOSS | (42,221,634 | ) | (3,626,287 | ) | ||||
OTHER COMPREHENSIVE INCOME (LOSS)—Foreign currency translation | 1,072,615 | (222,671 | ) | |||||
|
|
|
| |||||
COMPREHENSIVE LOSS | $ | (41,149,019 | ) | $ | (3,848,958 | ) | ||
|
|
|
| |||||
The accompanying notes are an integral part of these consolidated financial statements.
F-24
CONSOLIDATED STATEMENTS OF CHANGES IN MEMBERS’ DEFICIT
FOR THE YEARS ENDED DECEMBER 31, 2017 AND 2016
| Preferred Units | Common Units | Accumulated Other Comprehensive Loss | ||||||||||||||||||||||||||||||
Number of Units | Stated Value | Number of Units | Stated Value | Additional Paid-In Capital | Accumulated Deficit | Total | ||||||||||||||||||||||||||
BALANCE—December 31, 2015 | 35,458,327 | $ | 103,543,698 | 2,986,163 | $ | 2,746,346 | $ | 17,669,820 | $ | (168,580,746 | ) | $ | (2,394,208 | ) | $ | (47,015,090 | ) | |||||||||||||||
Unit-based compensation expense | — | — | — | — | 298,930 | — | — | 298,930 | ||||||||||||||||||||||||
Issuance of common units for option and warrant exercises | — | — | 229,591 | 95,810 | — | — | — | 95,810 | ||||||||||||||||||||||||
Unit cancellation | — | — | (15,000 | ) | (6,000 | ) | — | — | — | (6,000 | ) | |||||||||||||||||||||
Net loss | — | — | — | — | — | (3,626,287 | ) | — | (3,626,287 | ) | ||||||||||||||||||||||
Warrant modification expense | — | — | — | — | 371,472 | — | — | 371,472 | ||||||||||||||||||||||||
Foreign currency translation | — | — | — | — | — | — | (222,671 | ) | (222,671 | ) | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
BALANCE—December 31, 2016 | 35,458,327 | 103,543,698 | 3,200,754 | 2,836,156 | 18,340,222 | (172,207,033 | ) | (2,616,879 | ) | (50,103,836 | ) | |||||||||||||||||||||
Unit-based compensation expense | — | — | — | — | 107,582 | — | — | 107,582 | ||||||||||||||||||||||||
Unit cancellation | (134,111 | ) | (603,500 | ) | — | — | — | — | — | (603,500 | ) | |||||||||||||||||||||
Net loss | — | — | — | — | — | (42,221,634 | ) | — | (42,221,634 | ) | ||||||||||||||||||||||
Foreign currency translation | — | — | — | — | — | — | 1,072,615 | 1,072,615 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
BALANCE—December 31, 2017 | 35,324,216 | $ | 102,940,198 | 3,200,754 | $ | 2,836,156 | $ | 18,447,804 | $ | (214,428,667 | ) | $ | (1,544,264 | ) | $ | (91,748,773 | ) | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-25
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE YEARS ENDED DECEMBER 31, 2017 AND 2016
| 2017 | 2016 | |||||||
CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
Net loss | $ | (42,221,634 | ) | $ | (3,626,287 | ) | ||
Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
Depreciation and amortization | 770,045 | 775,733 | ||||||
Change in deferred income tax | (4,710 | ) | 44,036 | |||||
Unit-based compensation | 107,582 | 298,930 | ||||||
Change in fair value of warrant liability | (7,458,487 | ) | (4,870,111 | ) | ||||
Extension of warrants | — | 371,472 | ||||||
Write-offs for inventory obsolescence | 19,034 | 375,772 | ||||||
Bad debt expense | 271,367 | 133,625 | ||||||
Amortization of deferred financing costs | 971,241 | 1,274,001 | ||||||
Amortization of debt discount | — | 86,458 | ||||||
Accrued interest income | (33,674 | ) | — | |||||
Paid-in-kind interest | 31,165,303 | 4,537,874 | ||||||
Net changes in operating assets and liabilities: | ||||||||
Accounts receivable | 1,550,088 | (550,498 | ) | |||||
Inventory | (1,162,039 | ) | (551,054 | ) | ||||
Prepaid expenses and other current assets | (9,118 | ) | (596,068 | ) | ||||
Accounts payable | (887,218 | ) | 1,786,548 | |||||
Other current liabilities | 885,868 | (1,324,659 | ) | |||||
|
|
|
| |||||
Net cash used in operating activities | (16,036,352 | ) | (1,834,228 | ) | ||||
|
|
|
| |||||
CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
Capital expenditures | (528,341 | ) | (943,336 | ) | ||||
Investor loan | (280,000 | ) | (200,000 | ) | ||||
Security deposit | 22,518 | (11,000 | ) | |||||
Investment in joint venture | (118,604 | ) | (81,003 | ) | ||||
|
|
|
| |||||
Net cash used in investing activities | (904,427 | ) | (1,235,339 | ) | ||||
|
|
|
| |||||
CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
Unit repurchase | (603,500 | ) | — | |||||
Return of investment—common units | — | (6,000 | ) | |||||
Proceeds from exercise of options and warrants | — | 95,810 | ||||||
Repayment of term loan | (21,000,000 | ) | (54,652,513 | ) | ||||
Proceeds from secured debt financing | — | 100,000,000 | ||||||
Issuance costs for term loan | (1,493,515 | ) | (2,907,987 | ) | ||||
|
|
|
| |||||
Net cash (used in) provided by financing activities | (23,097,015 | ) | 42,529,310 | |||||
|
|
|
| |||||
EFFECT OF EXCHANGE RATE ON CASH, CASH EQUIVALENTS AND RESTRICTED CASH | 1,072,615 | (222,671 | ) | |||||
|
|
|
| |||||
NET DECREASE/INCREASE IN CASH, CASH EQUIVALENTS AND RESTRICTED CASH | (38,965,179 | ) | 39,237,072 | |||||
CASH, CASH EQUIVALENTS AND RESTRICTED CASH—Beginning of year | 49,712,444 | 10,475,372 | ||||||
|
|
|
| |||||
CASH, CASH EQUIVALENTS AND RESTRICTED CASH—End of year | $ | 10,747,265 | $ | 49,712,444 | ||||
|
|
|
| |||||
SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION: | ||||||||
Cash paid for interest | $ | 6,518,415 | $ | 6,011,665 | ||||
|
|
|
| |||||
Cash paid for taxes | $ | 206,631 | $ | 563,200 | ||||
|
|
|
| |||||
The accompanying notes are an integral part of these consolidated financial statements.
F-26
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2017 AND 2016
| 1. | BUSINESS |
Paradigm Spine, LLC, a Delaware limited liability company, formed on April 5, 2005, through its subsidiaries (collectively, the “Company”) designs, develops, manufactures, and markets nonfusion and fusion spinal implant solutions. Spinal implants are utilized by orthopedic surgeons and neurosurgeons in the treatment of degenerative diseases, deformities, and trauma in all regions of the spine.
The Company sells its products through direct sales in Germany, Switzerland, and United States and through distributors in 46 other countries, mainly in Europe and in Asia Pacific, Latin America, the Middle East, India, Africa, and Canada.
On August 8, 2016, Paradigm Spine, LLC formed a wholly owned subsidiary, Andi’s Belmarall, LLC, a Delaware limited liability company.
Paradigm Spine GmbH, formed on August 5, 2005, is a wholly owned German subsidiary of Andi’s Belmarall, LLC. On August 21, 2007, Paradigm Spine GmbH formed wholly owned subsidiaries, Paradigm Spine Austria GmbH on March 12, 2007, and Paradigm Spine Switzerland AG.
Paradigm Spine, LLC formed a wholly owned subsidiary, Fourth Dimension Spine, LLC, a Delaware limited liability company, on June 12, 2007. Fourth Dimension Spine, LLC formed a wholly owned subsidiary, Fourth Dimension Spine GmbH, a German limited liability company, on August 31, 2007.
On October 17, 2012, the Company announced US FDA PMA approval of its landmark coflex Interlaminar Technology: the first comparative effectiveness study for the treatment of spinal stenosis. coflex® is a minimally invasive, motion-preserving Interlaminar Stabilization™ device for the treatment ofmoderate-to-severe stenosis, with or without back pain.
Effective January 1, 2017, the procedure in which coflex® is implanted is covered under its own Common Procedural Terminology(CPT) Level-1 code determined by the American Medical Association.
Nature of Business—Paradigm Spine, LLC was formed to be a leader in the field ofnon-fusion spinal implant technology. The Company is committed to improving the quality of life of patients with spinal diseases through its mission: to provide products that are surgeon centric, indication specific, and data driven. Paradigm Spine, LLC is now an innovative leader in the global spine market and believes there is a significant opportunity to improve treatment options for patients suffering from lumbar spinal stenosis andage-related spinal deformities.
| 2. | SIGNIFICANT ACCOUNTING POLICIES |
Basis of Presentation—The consolidated financial statements include the accounts of Paradigm Spine, LLC, its wholly owned subsidiaries—Andi’s Belmarall, LLC and Fourth Dimension Spine, LLC and their wholly owned subsidiaries. All intercompany balances and transactions are eliminated upon consolidation.
Use of Estimates—The consolidated financial statements are prepared in conformity with accounting principles generally accepted in the United States of America (US GAAP) and include amounts that are based on management’s best estimates and judgments that affect the amounts reported in the consolidated financial statements and accompanying notes. These estimates are based on historical experience and on various other assumptions that are believed to be reasonable under the current circumstances. Actual results could differ from those estimates. Significant estimates include the variables and methods used to calculate unit-based compensation, preferred unit warrant liability, detachable warrants, deferred tax allowances, allowance for doubtful accounts receivable, and the write-offs for inventory obsolescence.
F-27
Foreign Currency Translation—Assets and liabilities of the foreign subsidiaries are translated at rates of exchange in effect at the close of the period, and equity amounts are translated at historical exchange rates. Revenues and expenses are translated at the weighted average of exchange rates in effect during each month. The effect of exchange rate fluctuations on translating foreign currency assets and liabilities into US dollars is included as the currency translation adjustment component of accumulated other comprehensive loss within the consolidated statements of changes in members’ deficit. Realized and unrealized foreign exchange transaction gains and losses are included within the consolidated statements of operations and comprehensive income.
Cash and Cash Equivalents—The Company considers all highly liquid investments with a maturity of three months or less when purchased to be cash equivalents. Cash and cash equivalents may include demand deposits held in banks andinterest-bearing money market funds. The carrying amounts reported in the consolidated balance sheets for cash and cash equivalents are recorded at fair value.
The Company has $9 million of restricted cash in accordance with the terms of its amended credit agreement, further described in Note 6. The Company early adopted the provisions of Accounting Standards Update(ASU) No. 2016-18,Statement of Cash Flows: Restricted Cash, issued by Financial Accounting Standards Board (FASB) in November 2016. The impact of the early adoption is that the change in restricted cash is not shown as an investing activity but is included in cash and cash equivalents, in the statement of cash flows.
Concentration of Credit Risk—Financial instruments that potentially subject the Company to significant concentrations of credit risk consist primarily of cash equivalents. The Company invests its excess cash in US government money market funds, and its deposits, at times, exceed federally insured limits. The Company has not experienced any losses from credit risks.
Allowance for Doubtful Accounts Receivable—The Company evaluates the collectability of accounts receivable using a combination of factors. Provisions to the allowance for doubtful accounts receivable are recorded based on a number of factors, including the length of time the receivables are past due, the current business environment, and the Company’s historical experience. Changes to the provision are recorded in selling, general, and administrative expenses.
Inventory—Inventory, which consists exclusively of finished goods manufactured by third parties, is stated at the lower of cost or market, with cost determined on afirst-in,first-out basis or average cost method, depending on the product. The Company reviews inventory for shrinkage or obsolete items based on expected revenues and product life cycles. Write-offs for shrinkage or obsolescence of inventory are recorded in cost of goods sold.
Property and Equipment—Property and equipment are carried at cost, less accumulated depreciation. Depreciation is computed using the straight-line method, based on estimated useful lives of three to seven years for software, computer equipment, and furniture; and is recorded in selling, general, and administrative expenses. Depreciation of instruments is estimated on a useful life of one year, and is recorded in cost of goods sold. Leasehold improvements are amortized over the term of the lease or useful life of the improvements, whichever is shorter. Maintenance and repairs are expensed as incurred. Instruments are hand-held devices used by orthopedic spine surgeons and neurosurgeons during surgical procedures. Instruments are recognized as long-lived assets and included in property and equipment. Instruments in the field are carried at cost, less accumulated depreciation. Property and equipment are reviewed for impairment in accordance with the FASB Accounting Standards Codification (ASC) 360,Property, Plant and Equipment.
Accumulated Other Comprehensive Loss—The component of accumulated other comprehensive loss includes foreign currency translation adjustments and is included in the consolidated statements of operations and comprehensive loss in accordance with ASC 220,Comprehensive Income.
Revenue Recognition—The Company recognizes revenue from sales of implants principally to hospitals and to distributors. For sales to hospitals, revenue is recognized on the date of surgery when the device is
F-28
implanted. For sales to distributors, revenue is recognized when title and risk of ownership have been transferred, provided that persuasive evidence of an arrangement exists, delivery has occurred, the price is fixed and determinable, the buyer is obligated to pay, the obligation is not contingent on resale of the product, the buyer has economic substance apart from the Company, the Company has no obligation to bring about the resale of the product, the amount of returns and discounts can be reasonably estimated, the remaining obligations are insignificant, and collectibility is reasonably assured. Revenue is recorded net of customer discounts and rebates.
Income Taxes—Deferred tax liabilities (DTLs) and deferred tax assets (DTAs) are related to the operations of Paradigm Spine GmbH and are recognized for the expected future tax consequences of events that have been included in the consolidated financial statements. The Company accounts for income taxes under the asset and liability method, whereby DTAs and DTLs are determined based on the difference between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce DTAs to the amounts expected to be realized.
The Company is classified as a limited liability company for domestic tax purposes, and is not subject to federal income taxes, but is subject to certain state and local taxes. Each member is responsible for the tax liability, if any, related to its proportionate share of the Company’s taxable income. All subsidiaries of the Company have elected to be treated as disregarded entities for domestic tax purposes and, accordingly, all business and assets are reported on Paradigm Spine, LLC’s income tax returns. However, certain subsidiary entities are subject to local country income taxes, which the Company accounts for under the liability method.
Unit-Based Compensation—At December 31, 2017 and 2016, the Company has one unit-based employee compensation plan, which is described more fully in Note 8.
For unit options granted as consideration for services rendered by nonemployees, the Company recognizes expense in accordance with the requirements of ASC 505,Equity Based Payments toNon-Employees. Nonemployee option grants that do not vest immediately upon grant are recorded as an expense over the vesting period of the underlying stock options. At the end of each financial reporting period prior to vesting, the value of these options, as calculated using the Black-Scholesoption-pricing model, will be remeasured using the fair value of the Company’s common stock, and the noncash expense recognized during the period will be adjusted accordingly. Since the fair market value of options granted to nonemployees is subject to change in the future, the amount of the future expense will include fair value remeasurements until the stock options are fully vested.
The Company accounts for its unit-based compensation in accordance with ASC 718,Compensation—Stock Compensation. Compensation expense is recognized in the consolidated financial statements on a prospective basis for all unit-based payments granted based upon the grant-date fair value estimated at that time.
The grant-date fair value of awards expected to vest is expensed on a straight-line basis over the vesting period of the related awards.
The Company selected the Black-Scholes option-pricing model as the most appropriate model for determining the estimated fair value for unit-based awards. The fair value is then amortized on a straight-line basis over the requisite service periods of the entire awards, which is generally the vesting period. Use of a valuation model requires management to make certain assumptions regarding a number of complex and subjective variables. Expected volatility was calculated based on a blended weighted average of similar public entities for which historical information was available. The Company will continue to use a weighted-average approach using similar public entity volatility information until historical volatility of the Company is relevant to measure expected volatility for future option grants. The average expected life was determined in accordance with the “simplified method” as described in Staff Accounting Bulletin No. 110. The risk-free interest rate is based on US Treasuryzero-coupon issues with a remaining term equal to the
F-29
expected life assumed at the date of grant. Forfeitures are estimated based on voluntary termination behavior as well as an historical analysis of actual option forfeitures.
Derivative Financial Instruments—Derivative financial instruments, as defined in ASC 815,Derivatives and Hedging, consist of the preferred unit warrants issued in connection with the Company’s 2011 term loan described in Note 6. These financial instruments are recorded in the consolidated balance sheets as warrant liability, further described in Notes 3 and 7, with changes in fair value recognized in earnings in the period of change.
Fair Value of Financial Instruments—The Company measures its financial assets and liabilities using the fair value method in accordance with ASC 820,Fair Value Measurements and Disclosures. ASC 820 defines fair value, establishes a framework for measuring fair value under US GAAP, and enhances disclosures about fair value measurements. Fair value is defined under ASC 820 as the exchange price that would be received for an asset or paid to transfer a liability (an exit price in the principal or the most advantageous market for an asset or liability in an orderly transaction between participants on the measurement date). Valuation techniques used to measure fair value under ASC 820 must maximize the use of observable inputs and minimize the use of unobservable inputs. The standard describes a fair value hierarchy based on the levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value, which are as follows:
Level 1—Quoted prices in active markets for identical assets or liabilities that the Company has the ability to access at the measurement day.
Level 2—Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities, quoted prices in markets that are not active, or other inputs that are observable or corroborated by observable market data over substantially the full term of the assets or liabilities.
Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the value of the assets or liabilities.
Recently Issued Accounting Standards—In May 2014, the FASB issued ASU No. 2014-09,Revenue from Contracts with Customers(Topic 606). The Company plans to adopt ASU 2014-09 effective January 1, 2019. This new accounting standard outlines a single comprehensive model to use in accounting for revenue arising from contracts with customers. This standard supersedes existing revenue recognition requirements and eliminates most industry-specific guidance from US GAAP. The core principle of the new accounting standard is to recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. The Company does not expect the adoption of the new accounting standard to have a material impact on its consolidated financial statements.
In July 2015, the FASB issued ASU No. 2015-11,Simplifying the Measurement of Inventory. This standard requires an entity to measure inventory at the lower of cost or net realizable value. Net realizable value is the estimated selling price in the ordinary course of business, less reasonably predictable costs of completion, disposal, and transportation. These amendments do not apply to inventory that is measured using the last-in, first-out or retail inventory method. The amendments apply to all other inventory, which includes inventory that is measured using the first-in, first-out or average cost. The Company adopted the provisions of this ASU on January 1, 2017 and it did not have a material impact on its consolidated financial statements.
In January 2016, the FASB issued ASU No. 2016-01,Financial Instruments—Overall, which amends the guidance in US GAAP on the classification and measurement of financial instruments. Although the ASU retains many current requirements, it significantly revises an entity’s accounting related to (1) the classification and measurement of investments in equity securities and (2) the presentation of certain fair value changes for financial liabilities measured at fair value. The ASU also amends certain disclosure requirements associated with the fair value of financial instruments. For public business entities, the
F-30
amendments in the ASU are effective for fiscal years beginning after December 15, 2017, including interim periods within those fiscal years. The Company is currently in the process of evaluating the impact of adoption of ASU No. 2016-01 on its consolidated financial statements.
In February 2016, the FASB issued ASU No. 2016-02,Leases(Topic 842), to increase transparency and comparability among organizations by requiring the recognition of right-to-use assets and liabilities on the balance sheet and disclosing qualitative and quantitative information about leasing arrangements. The Company plans to adopt ASU No. 2016-02, effective January 1, 2020. The Company is currently in the process of evaluating the impact of adoption of ASU No. 2016-02 on its consolidated financial statements.
In March 2016, the FASB issued ASU No. 2016-09,Compensation—Stock Compensation, that simplifies several aspects of the accounting for employee share-based payment transactions for both public and nonpublic entities, including the accounting for income taxes, classification of awards as either equity or liabilities, forfeitures, and statutory tax withholding requirements and classification in the statement of cash flows. For public business entities, the amendments in this ASU are effective for annual periods beginning after December 15, 2016, and interim periods within those annual periods. The Company adopted ASU No. 2016-09 on January 1, 2017 and it did not have a material impact on its consolidated financial statements.
In August 2016, the FASB issued ASU No. 2016-15,Statement of Cash Flows—Classification of Certain Cash Receipts and Cash Payments, to reduce diversity in practice in how certain transactions are classified in the statement of cash flows. ASU No. 2016-15 will be applied on a retrospective basis and to each prior reporting period presented and it is effective for public business entities for fiscal years beginning after December 15, 2017, and interim periods within those fiscal years. The Company is currently evaluating the impact of adopting this new standard on its consolidated statements of cash flows.
| 3. | FAIR VALUE |
The information about each major category of the Company’s financial assets and liabilities measured at fair value on a recurring basis as of December 31, 2017 and 2016, is presented in the following fair value hierarchy table:
Quoted Prices in Active Markets for Identical Assets | Significant Other Observable Inputs | Significant Unobservable Inputs | ||||||||||||||
| (Level 1) | (Level 2) | (Level 3) | Total | |||||||||||||
At December 31, 2017 | ||||||||||||||||
Cash | $ | 10,474,265 | $ | — | $ | — | $ | 10,474,265 | ||||||||
Warrant liability | — | — | — | — | ||||||||||||
At December 31, 2016 | ||||||||||||||||
Cash | $ | 49,712,444 | $ | — | $ | — | $ | 49,712,444 | ||||||||
Warrant liability | — | — | 7,458,487 | 7,458,487 | ||||||||||||
The following table reflects the changes in fair value of the Company’s Level 3 financial instruments:
| Level 3 | ||||||||
| Warrant Liability | 2017 | 2016 | ||||||
Balance—beginning of year | $ | 7,458,487 | $ | 12,328,598 | ||||
Fair value adjustments | (7,458,487 | ) | (4,870,111 | ) | ||||
|
|
|
| |||||
Balance—end of year | $ | — | $ | 7,458,487 | ||||
|
|
|
| |||||
The assumptions used in computing the fair value adjustments are disclosed in tabular format within the Warrants section of Note 7.
Some of the Company’s financial instruments are not measured at fair value on a recurring basis, but are recorded at amounts that approximate fair value due to their liquid short-term nature, such as cash and cash equivalents, receivables, and payables.
F-31
As more fully described in Note 6, the estimated carrying value of the Company’s term loan at December 31, 2017, approximated its fair value at such date.
| 4. | PROPERTY AND EQUIPMENT |
Property and equipment—net as of December 31, 2017 and 2016, consist of the following:
| 2017 | 2016 | Useful Lives | ||||||||||
Computer equipment and furniture | $ | 1,612,740 | $ | 1,107,027 | 3–7years | |||||||
Computer software | 460,815 | 415,175 | 3–7years | |||||||||
Instruments | 4,629,252 | 4,794,846 | 1–5years | |||||||||
Leasehold improvements | 73,766 | 64,836 | Life of lease | |||||||||
|
|
|
| |||||||||
Total property and | 6,776,573 | 6,381,884 | ||||||||||
Accumulated depreciation and amortization | (6,233,115 | ) | (5,877,203 | ) | ||||||||
|
|
|
| |||||||||
Property and equipment—net | $ | 543,458 | $ | 504,681 | ||||||||
|
|
|
| |||||||||
Depreciation and amortization expense was $770,045 and $775,733 for the years ended December 31, 2017 and 2016, respectively.
| 5. | OTHER CURRENT LIABILITIES |
Other current liabilities as of December 31, 2017 and 2016, consist of the following:
| 2017 | 2016 | |||||||
Employee compensation and related expenses | $ | 899,913 | $ | 481,434 | ||||
Royalty withholding tax payable | 30,909 | 33,812 | ||||||
Rebate payable | 82,690 | 340,765 | ||||||
Marketing study costs | 68,595 | 145,361 | ||||||
Income taxes payable | 95,494 | 79,822 | ||||||
Sales discounts payable | 229,716 | 181,240 | ||||||
Other | 1,353,397 | 612,415 | ||||||
|
|
|
| |||||
Total | $ | 2,760,714 | $ | 1,874,849 | ||||
|
|
|
| |||||
| 6. | DEBT |
On June 29, 2011, the Company closed on a $37.5 million five-year term loan from a conglomerate of investors. The Company used the proceeds to repay the existing $15 million line of credit to its lender. The Company issued to the lender detachable10-year warrants to purchase 2,263,002Class E-1 Preferred Units at the exercise price of $4.557, with an initial value of $6,945,301 as part of the consideration of the term loan. These warrants were classified as a liability as more fully disclosed in Note 7. The term loan was scheduled to mature on June 29, 2016, but was repaid in full with the proceeds of the secured debt financing described below.
On February 14, 2014, the Company entered into a five-year $75 million secured debt financing transaction with a lender. The Company used the proceeds to repay the outstanding balance of $41.5 million on the existing term loan, as well as a prepayment penalty of $5.2 million and interest of $0.8 million. In connection with the term loan, the Company paid a finder’s fee of $750,000 cash and granted 50,471 warrants, classified in equity, to purchase Class E2 Preferred Units to its agent, at an exercise price of $7.43, with a value of $162,521. The carrying value of the term loan was shown net of a discount related
F-32
to the issuance of these warrants and for deferred financing costs that were amortized over the period of the loan. The term loan was scheduled to mature on February 14, 2019, but was repaid in full in 2016 with the proceeds of the senior secured term loan described below.
On August 26, 2016, the Company closed on a $100 million five-year senior secured term loan with a lender (2016 credit agreement). The Company used the proceeds to repay the outstanding balance of $54.7 million on the existing secured debt, as well as a prepayment penalty of $1.7 million and interest of $1.1 million. The loan bore an annual interest rate of 12%, with an effective interest rate of 13.15%. In connection with the loan, the Company entered into a placement agent agreement with a related party and paid a $1 million fee to the agent under the agreement using proceeds from the loan. The Company had the option to pay all or a portion of the interest, for each interest payment date occurring prior to the first anniversary of the closing date, as“Payment-In-Kind (PIK)” interest, and such PIK interest shall be added to the aggregate principal balance of the loan. The loan was subject to certain affirmative, negative and financial covenants as more fully described in the credit agreement that must be certified by the Chief Financial Officer. In April 2017, the Company notified its lender that it had not achieved its first quarter 2017 minimum adjusted net sales covenant, which constitutes an event of default per the terms of the 2016 credit agreement. The Company did not receive a waiver from its lender for the violation and therefore the lender was entitled, among other things, to exercise various rights and remedies, including declaring the loan due and payable in whole or in part and the right to receive interest at the default rate and in cash. The lender waived their right to apply the default interest rate but reserved all other rights in a notification letter sent to the Company subsequent to the known default event.
On November 1, 2017, the Company entered into second amendment to the 2016 credit agreement, an amended credit agreement with the same lender. Immediately prior to giving effect to this amendment, the Company prepaid an outstanding principal amount of $21,000,000. The loan bears an annual interest rate of 15%, plus one month London InterBank Offered Rate (LIBOR), at an effective interest rate of 20.1%. Interest on the loan is payable as PIK interest and added to the outstanding principal amount of the loan. Total interest expense for the years ended December 31, 2017 and 2016, amounted to $13,258,543 and $4,268,522, respectively. The 2017 interest expense includes $6,518,415 of cash interest. The carrying value of the secured term loan is shown net of a discount related to deferred financing costs that is amortized over the period of the loan. As of December 31, 2017, the deferred financing costs were $3,234,399. The amended loan is subject to certain affirmative, negative and financial covenants as more fully described in the amended credit agreement that must be certified by the Chief Financial Officer of the Company on a monthly and quarterly basis. The financial covenants consist of (i) a minimum liquidity amount of $3 million at all times, (ii) minimum adjusted net sales tested on a monthly basis, and (iii) permitted capital expenditures tested at each fiscal reporting period. In the event of a default, as defined in the amended credit agreement, the Company would be required to, among other things, accrue interest at the annual interest rate plusone-month LIBOR plus the default interest rate of 3%. As of December 31, 2017, the Company was not in compliance with the financial covenants. Management has evaluated the significance of the default event and concluded this has cast substantial doubt about the Company’s ability to meet its financial obligations and consequently its ability to continue as a going concern within one year after the date that these consolidated financial statements are available to be issued. In the absence of a waiver of the existing default, it is possible that the Company will need to raise additional capital, borrow additional money or restructure its current debt including seeking other sources of long-term financing, or seek a purchaser of its business. The outcome of each of these alternatives is not known at this time.
In connection with the execution of the amended credit agreement described above, the Company has entered into an Economic Rights Agreement with the lender. Upon certain payment trigger events, such as the sale or liquidation of the Company, an initial public offering, full repayment of the loan, or making of prohibited distributions, the Company will pay the lender $24,425,175. This amount is included in the carrying value of the secured term loan and has been recorded as PIK interest under interest expense in the consolidated statement of operations and comprehensive loss, in 2017.
F-33
Maturities of the principal on the term loan at December 31, 2017, are as follows:
| Years Ending | ||||
| December 31 | Term Loan | |||
2018 | $ | 90,008,649 | ||
2019 | — | |||
2020 | — | |||
2021 | — | |||
2022 | — | |||
|
| |||
Total | $ | 90,008,649 | ||
|
| |||
As indicated herein Note 6 and in Note 13, this loan is currently payable on demand and is classified as a current liability in the consolidated balance sheet as of December 31, 2017.
| 7. | CAPITAL STRUCTURE |
Common Units—Each holder of Common Units is entitled to vote on all matters and is entitled to one vote for each unit held. Distributions on Common Units will be paid when, as and if declared by the Company’s board of managers (the “Board of Managers”), and only after each holder of Preferred Units then outstanding shall have first received distributions equal to their capital contributions. As of December 31, 2017, no distributions have been declared or paid by the Company.
Class A Common Units—Each holder of Class A Common Units is entitled to vote on all matters and is entitled to one vote for each Class A Common Unit held. Distributions on Class A Common Units will be paid when, as and if declared by the Board of Managers, and only after each holder of Preferred Units then outstanding shall have first received distributions equal to their capital contributions. As of December 31, 2017, no distributions have been declared or paid by the Company.
Preferred Units—Preferred Units may be issued from time to time in one or more classes.
F-34
The table below presents information on the classes of preferred units:
| Class A | Class B | Class C | Class D | Class E | Total | |||||||||||||||||||||||||||||||||||||||||||
| Number of Units | Amount | Number of Units | Amount | Number of Units | Amount | Number of Units | Amount | Number of Units | Amount | Number of Units | Amount | |||||||||||||||||||||||||||||||||||||
Balance—December 31, 2015 | 2,717,886 | $ | 3,623,846 | 7,895,271 | $ | 2,866,006 | 5,524,729 | $ | 12,856,827 | 3,050,199 | $ | 11,414,601 | 16,270,242 | $ | 72,782,418 | 35,458,327 | $ | 103,543,698 | ||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
Balance—December 31, 2016 | 2,717,886 | 3,623,846 | 7,895,271 | 2,866,006 | 5,524,729 | 12,856,827 | 3,050,199 | 11,414,601 | 16,270,242 | 72,782,418 | 35,458,327 | 103,543,698 | ||||||||||||||||||||||||||||||||||||
Share Cancellation | — | — | — | — | — | — | (134,111 | ) | (603,500 | ) | — | — | (134,111 | ) | (603,500 | ) | ||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
Balance—December 31, 2017 | 2,717,886 | $ | 3,623,846 | 7,895,271 | $ | 2,866,006 | 5,524,729 | $ | 12,856,827 | 2,916,088 | $ | 10,811,101 | 16,270,242 | $ | 72,782,418 | 35,324,216 | $ | 102,940,198 | ||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
Class A Preferred Units—The holders of Class A Preferred Units are entitled to one vote for each Class A Preferred Unit held. In the event the Company declares or pays a distribution, such distributions will be paid to Common Unit and Class A Common Unit holders only after each holder of Class A Preferred Units then outstanding shall have first received distributions equal to their capital contributions. Upon any liquidation, sale, merger, dissolution, or winding up of the Company, the holders of Class A Preferred Units have rights in preference to Common and Class A Common Units. As of December 31, 2017, the Company had 2,717,886 Class A Preferred Units authorized for issuance.
As of December 31, 2017 and 2016, Class A Preferred Units are recorded at their stated value of $1.33 per unit. The liquidation preference of the Class A Preferred Units at December 31, 2017 and 2016, was $3,623,846. One of the officers and unit holders of the Company, who is also affiliated with a related party, holds the proxy for all Class A Preferred Units.
Class B Preferred Units—The holders of Class B Preferred Units are entitled to one vote for each Class B Preferred Unit held. In the event the Company declares or pays a distribution, such distributions will be paid to Common Unit and Class A Common Unit holders only after each holder of Class B Preferred Units then outstanding shall have first received distributions equal to their capital contributions. Upon any liquidation, sale, merger, dissolution, or winding up of the Company, the holders of Class B Preferred Units have rights in preference to Common Units and Class A Common Units. As of December 31, 2017, the Company had 7,895,271 Class B Preferred Units authorized for issuance.
As of December 31, 2017 and 2016, Class B Preferred Units are recorded at their stated value of $1.48 per unit, with the exception of 6,756,757 units awarded in exchange for the contribution of intellectual property to the Company, which are recorded at $1,181,048, the original cost of the intellectual property. The liquidation preference of the Class B Preferred Units at December 31, 2017 and 2016, was $11,685,000.
Class C Preferred Units—The holders of Class C Preferred Units do not have voting rights, except as otherwise required by law. In the event the Company declares or pays a distribution, such distributions will be paid to Common Unit and Class A Common Unit holders only after each holder of Class C Preferred Units then outstanding shall have first received distributions equal to their capital contributions. Upon any liquidation, sale, merger, dissolution, or winding up of the Company, the holders of Class C Preferred Units have rights in preference to Common Units and Class A Common Units. As of December 31, 2017, the Company had 5,695,379 Class C Preferred Units authorized for issuance.
As of December 31, 2017 and 2016, Class C Preferred Units are recorded at their stated value (issue price of $2.78 per unit, less issuance costs). The liquidation preference of the Class C Preferred Units at December 31, 2017 and 2016, was $15,359,160.
F-35
Class D Preferred Units—The holders of Class D Preferred Units do not have voting rights, except as otherwise required by law. In the event the Company declares or pays a distribution, such distributions will be paid to Common Unit and Class A Common Unit holders only after each holder of Class D Preferred Units then outstanding shall have first received distributions equal to their capital contributions. Upon any liquidation, sale, merger, dissolution, or winding up of the Company, the holders of Class D Preferred Units have rights in preference to Common Units and Class A Common Units. As of December 31, 2017, the Company had 4,071,444 Class D Preferred Units authorized for issuance.
As of December 31, 2017 and 2016, Class D Preferred Units are recorded at their stated value (issue price of $4.50 per unit, less issuance costs). The liquidation preference of the Class D Preferred Units at December 31, 2017 and 2016, was $13,122,396 and $13,725,896, respectively.
Class E Preferred Units—The holders ofClass E-1 Preferred Units are entitled to one vote for eachClass E-1 Preferred Unit held. The holders ofClass E-2 Preferred Units do not have voting rights, except as otherwise required by law. In the event the Company declares or pays a distribution, such distributions will be paid to Common Unit and Class A Common Unit holders only after each holder of Class E Preferred Units then outstanding shall have first received distributions equal to their capital contributions. Upon liquidation, sale, merger, dissolution, or winding up of the Company, the holders of Class E Preferred Units have rights in preference to Common Units and Class A Common Units. As of December 31, 2017, the Company had 25,952,930 Class E Preferred Units authorized for issuance.
As of December 31, 2017 and 2016, Class E Preferred Units are recorded at their stated value (issue price of $4.557 per unit, less issuance costs). The liquidation preference of the Class E Preferred Units at December 31, 2017 and 2016, was $73,917,601.
Preferred Unit Distributions—The holders of Class A, Class B, Class C, Class D, and Class E Preferred Units are entitled to receive distributions equal to their capital contributions in preference to the Common Units and Class A Common Units. In accordance with the Seventh Amended and Restated Limited Liability Company Agreement, dated as of May 28, 2009, the Class E Preferred Unit holders are entitled to a special allocation allowance in the event of the liquidation of the Company. If the fair market value of the Liquidation Assets is $350 million or less, prior to the allocation of profits under Sections 4.2(a)(iii) through 4.2(a)(v) of the agreement, profits realized in such liquidation shall be allocated first to the Class E Preferred Unit holders, pro rata in accordance with their percentage interests in such amount as will increase the capital accounts balance of each Class E Preferred Unit holder to its percentage interest of the sum of the capital account balances of all unit holders. If the fair market value of the Liquidation Assets is greater than $350 million, prior to the allocation of profits under Sections 4.2(a)(iii) through 4.2(a)(v) of the agreement, profits realized in such liquidation shall be allocated to the Class E Preferred Unit holders, pro rata in accordance with their percentage interests, in such amount, if any, as shall be necessary to prevent each Class E Preferred Unit holder from receiving as a distribution in such liquidation an amount less than the amount such Class E Preferred Unit holder would have received in the event that the fair market value of the Liquidation Assets equaled $350 million. Distributions are payable only when declared by the Board of Managers. No distributions have been declared from inception through December 31, 2017.
F-36
Warrants—The Company accounts for the warrants to purchase 2,263,002 Class E Preferred Units, issued in connection with its term loan described in Note 6, in accordance with ASC 815. For those warrants that have been deemed to be liabilities, the Company measures the fair value of its liability using an option-pricing model, with changes in fair value recognized as an adjustment to other income (expense). Based on the Company’s valuation methodology, there would be no remaining value for warrant holders and the fair value of the warrants has been adjusted to zero. The assumptions used in computing the fair value are illustrated in the following table:
| 2017 | 2016 | |||||||
Estimated unit prices | $ | 1.57 | $ | 6.70 | ||||
Exercise price | $ | 4.557 | $ | 4.557 | ||||
Expected unit price volatility | 35.0 | % | 40.0 | % | ||||
Risk-free interest rate | 2.6 | % | 1.9 | % | ||||
Expected remaining life of warrants (years) | 3.5 | 4.5 | ||||||
Expected annual dividend per unit | $ | — | $ | — | ||||
The remaining 1,871,845 warrants to purchase Class E Preferred Units and 365,775 warrants to purchase Common Units outstanding have been deemed to be equity instruments and are included as a part of permanent equity.
The following table reflects all warrants outstanding at December 31, 2017:
Number of Warrants | Amount | Range of Exercise Prices | Range of Expiration Dates | |||||||||||
Common | 365,775 | $ | 1,657,247 | $ | 4.50–$4.557 | January 2018 to June 2019 | ||||||||
Class E Preferred Units | 4,134,847 | 18,987,503 | $ | 4.557–$7.43 | June 2015 to June 2021 | |||||||||
|
|
|
| |||||||||||
Total | 4,500,622 | $ | 20,644,750 | |||||||||||
|
|
|
| |||||||||||
| 8. | UNIT OPTION PLAN |
The total number of the Company’s units authorized to be issued under the incentive plan is capped at 6,939,303 as per Section 9.6 of the Company’s Seventh Amended and Restated LLC Agreement. At December 31, 2017, the number of Common Units available for issuance under the Unit Incentive Plan is 2,551,992.
F-37
The following table summarizes information about unit options outstanding:
Number of Units (In thousands) | Weighted- Exercise Price | Weighted- Remaining Contractual Life (In years) | Weighted- Fair Value | Aggregate Intrinsic Value (In millions) | ||||||||||||||||
Options outstanding—December 31, 2016 | 2,669 | $ | 4.35 | 5.01 | $ | 1.27 | $ | — | ||||||||||||
Granted | — | — | — | — | — | |||||||||||||||
Exercised | — | — | — | — | — | |||||||||||||||
Forfeited/canceled | (1,186 | ) | — | — | — | — | ||||||||||||||
|
| |||||||||||||||||||
Options outstanding—December 31, 2017 | 1,483 | 4.20 | 3.20 | 1.45 | — | |||||||||||||||
|
| |||||||||||||||||||
Unvested, expected to vest in the future | 3 | 4.56 | — | |||||||||||||||||
Vested and exercisable—December 31, 2017 | 1,480 | 4.19 | 3.20 | 1.45 | — | |||||||||||||||
|
| |||||||||||||||||||
Vested, exercisable, and expected to vest—December 31, 2017 | 1,483 | — | — | — | — | |||||||||||||||
|
| |||||||||||||||||||
Outstanding nonvested units—beginning of year | 102 | 4.56 | — | 2.72 | — | |||||||||||||||
Nonvested units granted | — | — | — | — | — | |||||||||||||||
Vested units | (47 | ) | — | — | — | — | ||||||||||||||
Nonvested units forfeited | (52 | ) | — | — | — | — | ||||||||||||||
|
| |||||||||||||||||||
Outstanding nonvested units—end of year | 3 | 4.56 | — | 1.71 | — | |||||||||||||||
|
| |||||||||||||||||||
There were no units of options granted in 2017. The weighted-average grant-date fair value per unit of options granted for 2016 was $2.98. As of December 31, 2017, the total unrecognized compensation cost related to nonvested unit options granted was $4,000 and is expected to be recognized over a weighted-average period of 0.22 years.
Unit-based compensation expense for the years ended December 31, 2017 and 2016, was $107,581 and $298,930, respectively.
Unit compensation expense is recorded in selling, general, and administrative expenses in the consolidated statements of operations and comprehensive loss.
The weighted-average assumptions used in the Black-Scholes option-pricing model for options granted as of December 31, 2017 and 2016, are as follows:
| 2017 | 2016 | |||||||
Expected unit price volatility | — | % | 47.8 | % | ||||
Risk-free interest rate | — | % | 1.38 | % | ||||
Expected life of options (years) | — | 5.8 | ||||||
Expected annual dividend per unit | $ | — | $ | — | ||||
| 9. | INCOME TAXES |
For the years ended December 31, 2017 and 2016, the Company incurred income taxes of $105,591 and $284,087, respectively, primarily related to income generated by its subsidiary entities in Germany. The Company’s effective tax rate differs from its statutory tax rates primarily due to the valuation allowance
F-38
recorded against its net DTAs and due to the Company’s legal structure organized as a limited liability company for domestic tax purposes, which is not subject to federal income taxes, but is subject to certain state and local taxes.
Deferred income taxes reflect the net effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. The Company’s DTAs relate primarily to net operating loss (NOL) carryforwards. At December 31, 2017 and 2016, a valuation allowance was recorded to offset the DTA related to NOLs. Significant components of the Company’s DTAs for the years ended December 31, 2017 and 2016, were as follows:
| 2017 | 2016 | |||||||
Deferred tax assets: | ||||||||
Net operating loss carryforwards | $ | 1,289,138 | $ | 1,092,720 | ||||
Bad debt | 47,357 | 38,848 | ||||||
Amortization of intangible asset | — | 3,561 | ||||||
Depreciation | 81,034 | 53,636 | ||||||
Other | 10,637 | 9,572 | ||||||
|
|
|
| |||||
Total deferred tax assets | 1,428,166 | 1,198,337 | ||||||
|
|
|
| |||||
Deferred tax liabilities: | ||||||||
Property and equipment | 343,788 | 279,289 | ||||||
Unrealized translation gains | 3,883 | 22,990 | ||||||
Other | 108,615 | 68,117 | ||||||
|
|
|
| |||||
Total deferred tax liabilities | 456,286 | 370,396 | ||||||
|
|
|
| |||||
Valuation allowance for deferred tax assets | 957,892 | 818,664 | ||||||
|
|
|
| |||||
Net deferred tax assets | $ | 13,988 | $ | 9,277 | ||||
|
|
|
| |||||
At December 31, 2017 and 2016, the Company has NOLs for German, Austrian, and Swiss income tax purposes, in the aggregate, of approximately $4.9 million and $4.2 million, respectively, which can be offset against future taxable income. According to local tax laws, there is no expiration date for these loss carryforwards in Germany and Austria. In Switzerland, the NOL expires after seven years.
The Company’s subsidiary entity in Germany generally remains subject to tax examination for the year ended December 31, 2012, and later. The Company’s subsidiary entities in Switzerland and Austria remain subject to tax examination for the year ended December 31, 2017. In accordance with the accounting guidance for uncertainty in income taxes, the Company had recorded approximately $95,000 and $80,000 of unrecognized tax benefits as of December 31, 2017 and 2016, respectively. The balance of unrecognized tax benefits as of December 31, 2017, would affect the Company’s effective tax rate, if recognized.
| 10. | LEASES |
Future minimum rental commitments under noncancelable operating leases in effect as of December 31, 2017, are as follows:
2018 | $ | 109,716 | ||
2019 | — | |||
2020 | — | |||
2021 | — | |||
2022 | — |
Total rental expense in 2017 and 2016 was $593,107 and $537,144, respectively, and included $419,712 and $387,600 in 2017 and 2016, respectively, forrelated-party expense.
F-39
| 11. | RELATED PARTIES |
Two members of the Board of Managers who are also unit holders of the Company are principals of related entities that provide various services for the Company as follows: consulting services expense totaled $5,698,132 and $4,248,287 for 2017 and 2016, respectively; and rent and other management services expense totaled $796,670 and $579,617 for 2017 and 2016, respectively.
As of December 31, 2017 and 2016, $1,433,679 and $934,937, respectively, was accrued for amounts due to related parties and is included in accounts payable in the Company’s consolidated balance sheets.
As of December 31, 2017 and 2016, the Company included a receivable of $409,153 and $352,056, respectively, in other current assets associated with royalty withholding tax amounts due to the Company from its members.
In 2014, an officer of the Company made a personal investment in a vendor of the Company. Total payments to the vendor for the years ended December 31, 2017 and 2016, were $0 and $48,000, respectively.
An officer of the Company is a founding member and significant shareholder in a supplier of inventory and capital equipment instruments of the Company. Total payments to the supplier for the purchase of these assets for the years ended December 31, 2017 and 2016, were $1,346,061 and $441,622, respectively.
In 2014, the Company entered into a nonstocking distribution agreement with an entity, in which an officer of the Company is an owner. The Company is exclusively appointed to sell the products of the entity in Germany and Switzerland. For the years ended December 31, 2017 and 2016, the Company recorded sales of $677,090 and $621,174 on purchases of $555,542 and $425,774, respectively. Total payments to the entity for the years ended December 31, 2017 and 2016, were $593,503 and $366,943, respectively.
Effective January 31 2017, the Company entered into an agreement to loan a member of the board $180,000. Interest accrues at 13% per annum and is added to the outstanding principal balance on a quarterly basis. The principal balance, including accrued interest, is payable on the fifth anniversary of the effective date or sooner, dependent upon various events. On March 2, 2017, the Company entered into an agreement to loan the same member of the board $100,000, at the same terms as the prior loan. Interest income for the year ended December 31, 2017, amounted to $33,673.
In connection with the August 26, 2016, $100 million senior secured loan described in Note 6, the Company entered into a placement agency agreement with a related party and paid the agent a $1 million fee using proceeds from the loan.
| 12. | COMMITMENTS AND CONTINGENCIES |
From time to time, the Company may have certain contingent liabilities that arise in the ordinary course of its business activities. The Company is not currently subject to any material legal proceedings.
On November 30, 2016, the Company entered into an agreement with a supplier to purchase 20,000 coflex® implants. Under the agreement, the Company guarantees to purchase the implants between January 1, 2017, and December 31, 2018, at various prices that reflect volume discounts.
The aggregate minimum amount of required purchases at December 31, 2017, is as follows:
2018 | $ | 1,151,604 | ||
2019 | — | |||
2020 | — | |||
2021 | — | |||
2022 | — | |||
|
| |||
Total | $ | 1,151,604 | ||
|
|
F-40
In the first quarter of 2015, the Company received from the Office of the Inspector General of the US Department of Health and Human Services (the “government”) a subpoena requesting the production of documents in connection with a civil investigation. The government’s investigation resulted from a False Claims Act lawsuit filed by an individual, the relator, in the name of the government. The Company engaged outside counsel to assist in responding to the subpoena. The Company has cooperated fully with the government’s requests. In April 2016, the Company signed a written settlement agreement with the government for $585,000 in resolution of the government’s investigation and the False Claims Act lawsuit from which it initiated. The total legal costs incurred by the Company during the year ended December 31, 2016, associated with the investigation including the settlement amount, were approximately $500,000 and are recorded in selling, general, and administrative expenses in the consolidated statement of operations and comprehensive loss. As of December 31, 2016, this matter is completely closed.
| 13. | SUBSEQUENT EVENTS |
During March 2017, the Company entered into a first amendment to an existing agreement with a supplier to purchase 45,000 coflex® implants over a three-year term. The term commences upon achievement of certain operational and performance qualifications. The qualifications were satisfied in January 2018 and the Company entered into an amended agreement with the supplier to purchase 22,500 coflex® implants over a three-year term. The aggregate minimum amount of required purchases during the term is $2,250,000.
In April 2018, the Company notified its lenders that it had not achieved its first quarter 2018 minimum adjusted net sales covenant, which constitutes an event of default per the terms of the 2016 credit agreement, amended in 2017, described in Note 6. The Company did not receive a waiver from its lenders for the violation and therefore the lenders are entitled, among other things, to exercise various rights and remedies, including declaring the loan due and payable in whole or in part and the right to receive interest at the default rate.
Subsequent events have been evaluated by the Company through April 27, 2018, the date these consolidated financial statements were available to be issued.
F-41
Table of Contents
| Page | ||||||
| ARTICLE I |
| |||||
| THE CONTRIBUTION AND MERGER | A-5 | |||||
1.01 | The Contribution; Purchase Price | A-5 | ||||
1.02 | The Merger | A-5 | ||||
1.03 | Effects of the Merger | A-6 | ||||
1.04 | Effect of Merger on Capital Stock and Equity Interests | A-6 | ||||
1.05 | Treatment of Parent Equity Awards | A-7 | ||||
1.06 | Governing Documents | A-7 | ||||
1.07 | Directors and Officers | A-8 | ||||
1.08 | Adjustment to Cash Purchase Price | A-8 | ||||
1.09 | Withholding | A-9 | ||||
1.10 | Plan of Reorganization | A-9 | ||||
1.11 | Necessary Further Actions | A-9 | ||||
| ARTICLE II |
| |||||
| THE CLOSING; EXCHANGE OF CERTIFICATES | A-9 | |||||
2.01 | The Closing | A-9 | ||||
2.02 | Effective Time; Closing Transactions | A-10 | ||||
2.03 | Member Share Issuance; Closing Payments | A-11 | ||||
2.04 | Parent Exchange Procedures | A-11 | ||||
2.05 | No Further Ownership Rights | A-12 | ||||
2.06 | Earnout Shares and Payments | A-12 | ||||
| ARTICLE III |
| |||||
| REPRESENTATIONS AND WARRANTIES OF THE MEMBER | A-16 | |||||
3.01 | Existence and Good Standing | A-16 | ||||
3.02 | Authority; Enforceability | A-16 | ||||
3.03 | No Violations; Required Filings and Consents | A-17 | ||||
3.04 | Title to Units; Capitalization; Subsidiaries | A-17 | ||||
3.05 | Financial Statements and Other Financial Matters; No Undisclosed Liabilities | A-18 | ||||
3.06 | Absence of Certain Changes | A-19 | ||||
3.07 | Real Property | A-19 | ||||
3.08 | Tax Matters | A-20 | ||||
3.09 | Contracts | A-22 | ||||
3.10 | Intellectual Property | A-23 | ||||
3.11 | Legal Proceedings | A-25 | ||||
3.12 | Orders | A-25 | ||||
3.13 | Employee Benefit Plans | A-25 | ||||
3.14 | Insurance | A-26 | ||||
3.15 | Legal Requirements and Permits | A-27 | ||||
3.16 | Environmental Matters | A-27 | ||||
3.17 | Relationships with Related Persons | A-28 | ||||
3.18 | Employees; Employment Matters and Independent Contractors | A-28 | ||||
3.19 | Material Customers and Suppliers | A-29 | ||||
3.20 | Brokers’ Fees | A-29 | ||||
3.21 | Compliance with Anti-Corruption Laws; Absence of Certain Payments | A-29 | ||||
3.22 | Device Regulatory Matters | A-30 | ||||
A-i
| Page | ||||||
3.23 | Healthcare Regulatory Matters | A-31 | ||||
3.24 | Title, Condition and Sufficiency of Assets | A-32 | ||||
3.25 | Data Protection and Privacy | A-32 | ||||
3.26 | Warranty | A-33 | ||||
3.27 | Company Information | A-34 | ||||
3.28 | Vote Required | A-34 | ||||
3.29 | No Other Representations or Warranties | A-34 | ||||
| ARTICLE IV |
| |||||
REPRESENTATIONS AND WARRANTIES OF PARENT, HOLDCO AND THE MERGER SUB | A-34 | |||||
4.01 | Existence and Good Standing | A-34 | ||||
4.02 | Authority; Enforceability | A-35 | ||||
4.03 | No Violations; Required Filings and Consents | A-36 | ||||
4.04 | Capitalization | A-36 | ||||
4.05 | Subsidiaries | A-37 | ||||
4.06 | Legal Proceedings | A-38 | ||||
4.07 | SEC Filings and Financial Statements | A-38 | ||||
4.08 | Internal Controls | A-39 | ||||
4.09 | Absence of Certain Changes | A-39 | ||||
4.10 | No Undisclosed Liabilities | A-39 | ||||
4.11 | Legal Requirements | A-39 | ||||
4.12 | Orders | A-39 | ||||
4.13 | Tax Matters | A-40 | ||||
4.14 | Intellectual Property | A-40 | ||||
4.15 | Healthcare Regulatory Matters | A-40 | ||||
4.16 | Brokerage | A-40 | ||||
4.17 | Financing | A-40 | ||||
4.18 | Purpose | A-41 | ||||
4.19 | Solvency | A-42 | ||||
4.20 | Parent Information | A-42 | ||||
4.21 | State Takeover Laws | A-42 | ||||
4.22 | No Other Representations or Warranties | A-42 | ||||
| ARTICLE V |
| |||||
COVENANTS OF THE MEMBER | A-42 | |||||
5.01 | Conduct of the Business | A-42 | ||||
5.02 | Access to Books and Records | A-44 | ||||
5.03 | Efforts to Consummate | A-45 | ||||
5.04 | Exclusive Dealing | A-45 | ||||
5.05 | Payoff Letters and Lien Releases | A-45 | ||||
5.06 | Member Approval | A-45 | ||||
5.07 | Notification | A-45 | ||||
5.08 | Financing Cooperation | A-46 | ||||
5.09 | Update of Financial Statements | A-47 | ||||
5.10 | Unitholder Support Agreement | A-47 | ||||
5.11 | Commercial Arrangements | A-48 | ||||
5.12 | Termination of Certain Benefit Plans | A-48 | ||||
A-ii
| Page | ||||||
| ARTICLE VI |
| |||||
COVENANTS OF PARENT, HOLDCO AND MERGER SUB | A-48 | |||||
6.01 | Notification | A-48 | ||||
6.02 | Efforts to Consummate | A-48 | ||||
6.03 | Financing | A-49 | ||||
6.04 | Listing | A-50 | ||||
6.05 | Operations of Parent Prior to the Closing | A-50 | ||||
6.06 | Parent Stockholder Support Agreement | A-51 | ||||
6.07 | Approval by Sole Stockholders of Holdco and Merger Sub | A-51 | ||||
6.08 | Employee Benefits | A-51 | ||||
6.09 | Indemnification; Directors’ and Officers’ Insurance | A-52 | ||||
6.10 | Designated Director | A-53 | ||||
6.11 | Parent Change in Recommendation | A-54 | ||||
6.12 | Post-Closing Sales Leadership | A-54 | ||||
| ARTICLE VII |
| |||||
ACTIONS PRIOR TO THE CLOSING | A-55 | |||||
7.01 | The Joint Proxy and Consent Solicitation Statement/Prospectus and Registration Statement | A-55 | ||||
7.02 | Regulatory Filings | A-57 | ||||
7.03 | State Takeover Laws | A-58 | ||||
| ARTICLE VIII |
| |||||
CONDITIONS TO CLOSING | A-58 | |||||
8.01 | Conditions to Parent’s, Holdco’s and the Merger Sub’s Obligations | A-58 | ||||
8.02 | Conditions to the Member’s Obligations | A-59 | ||||
| ARTICLE IX |
| |||||
INDEMNIFICATION | A-60 | |||||
9.01 | Survival | A-60 | ||||
9.02 | Indemnification | A-61 | ||||
9.03 | Limitations | A-62 | ||||
9.04 | Claim Procedures | A-62 | ||||
9.05 | Determination of Amount | A-63 | ||||
9.06 | Third Person Claims | A-63 | ||||
9.07 | Duty to Mitigate | A-64 | ||||
9.08 | Tax Treatment of Indemnification Claims | A-65 | ||||
| ARTICLE X |
| |||||
TERMINATION | A-65 | |||||
10.01 | Termination | A-65 | ||||
10.02 | Effect of Termination | A-66 | ||||
10.03 | Parent Fee | A-66 | ||||
| ARTICLE XI |
| |||||
ADDITIONAL COVENANTS | A-67 | |||||
11.01 | Disclosure Schedules | A-67 | ||||
11.02 | Tax Matters | A-68 | ||||
A-iii
| Page | ||||||
| ARTICLE XII |
| |||||
DEFINITIONS | A-72 | |||||
12.01 | Definitions | A-72 | ||||
12.02 | Other Definitional Provisions | A-84 | ||||
| ARTICLE XIII |
| |||||
MISCELLANEOUS | A-84 | |||||
13.01 | Press Releases and Public Announcements | A-84 | ||||
13.02 | Expenses | A-85 | ||||
13.03 | Transfer Taxes | A-85 | ||||
13.04 | Notices | A-85 | ||||
13.05 | Succession and Assignment | A-85 | ||||
13.06 | Severability | A-86 | ||||
13.07 | References | A-86 | ||||
13.08 | Construction | A-86 | ||||
13.09 | Amendment and Waiver | A-86 | ||||
13.10 | Entire Agreement | A-87 | ||||
13.11 | Parties in Interest | A-87 | ||||
13.12 | WAIVER OF TRIAL BY JURY | A-87 | ||||
13.13 | Delivery by Facsimile or Email | A-87 | ||||
13.14 | Counterparts | A-88 | ||||
13.15 | Governing Law | A-88 | ||||
13.16 | Jurisdiction | A-88 | ||||
13.17 | Remedies Cumulative | A-88 | ||||
13.18 | Specific Performance | A-88 | ||||
13.19 | Waiver of Conflicts Regarding Representation; Privilege | A-89 | ||||
13.20 | No Recourse; Waiver of Claims | A-90 | ||||
A-iv
INDEX OF EXHIBITS
| Exhibit A | Certificate of Incorporation of Holdco | |
| Exhibit B | Bylaws of Holdco | |
| Exhibit C | Certificate of Designation of Holdco | |
| Exhibit D | Form ofLock-Up Agreement | |
| Exhibit E | Form ofLock-Up and Permitted Sale Agreement | |
| Exhibit F | Parent Tax Representations | |
| Exhibit G | Member Tax Representations | |
| Exhibit H | Giants Product List | |
| Exhibit I | Giants/Zyga Product List | |
| Exhibit J | Working Capital Illustration |
A-v
GLOSSARY OF DEFINED TERMS
The location of the definition of each capitalized term used in this Agreement is set forth in this Glossary:
401(k) Plan | A-48 | |||
Accounting Firm | A-8 | |||
Accounts Receivable | A-72 | |||
Acquisition Transaction | A-45 | |||
Affected Employees | A-51 | |||
Affiliate | A-72 | |||
Agent | A-11 | |||
Agreement | A-4 | |||
Alternative Financing | A-49 | |||
Anti-Corruption Laws Expiration Date | A-61 | |||
Antitrust Laws | A-72 | |||
Ares | A-40 | |||
Basket Amount | A-62 | |||
Business Day | A-72 | |||
Cash | A-72 | |||
Cash Election Amount | A-72 | |||
Cash Purchase Price | A-72 | |||
Certificate of Merger | A-10 | |||
Change of Control Transaction | A-72 | |||
Claim Notice | A-62 | |||
Closing | A-9 | |||
Closing Cash Amount | A-73 | |||
Closing Date | A-10 | |||
Closing Date Schedule | A-8 | |||
Closing Indebtedness Amount | A-73 | |||
Closing Payment Shortfall | A-9 | |||
Code | A-73 | |||
Commitment Letters | A-41 | |||
Company | A-4 | |||
Company Closing Certificate | A-59 | |||
Company Employee Benefit Plan | A-73 | |||
Company Financial Statements | A-18 | |||
Company Group | A-73 | |||
Company Patent Rights | A-23 | |||
Company Privacy Policy | A-73 | |||
Company Real Property | A-19 | |||
Company Transaction Expense | A-73 | |||
Company Units | A-4 | |||
Confidentiality Agreement | A-45 | |||
Consideration Certificate | A-10 | |||
Consulting Agreement | A-5 | |||
Contract | A-73 | |||
Contribution | A-5 | |||
Credit Agreement | A-73 | |||
D&O Insurance Policies | A-52 | |||
D&O Tail Policy | A-52 | |||
D&W | A-73 | |||
Damages | A-73 |
Data Breach | A-73 | |||
Data Protection Laws | A-74 | |||
Designated Director | A-53 | |||
Designated Liabilities | A-74 | |||
Device Permits | A-74 | |||
Device Regulatory Laws | A-74 | |||
DGCL | A-4 | |||
Disclosure Schedules | A-67 | |||
Dispute Notice | A-8 | |||
Divestiture Action | A-57 | |||
DLLCA | A-34 | |||
Earnout Calculations | A-15 | |||
Earnout Dispute Notice | A-15 | |||
Earnout Review Period | A-15 | |||
Earnout Shares | A-12 | |||
Effective Time | A-10 | |||
Election Notice | A-48 | |||
Encumbrance | A-74 | |||
Environmental Claim | A-75 | |||
Environmental Laws | A-75 | |||
Environmental Permit | A-75 | |||
ERA | A-75 | |||
ERISA | A-75 | |||
ERISA Affiliate | A-75 | |||
Exchange Act | A-75 | |||
Expiration Date | A-60 | |||
FCPA | A-29 | |||
Federal Health Care Program | A-75 | |||
Final Earnout Shares | A-75 | |||
Financing | A-41 | |||
Financing Source Related Parties | A-90 | |||
Financing Sources | A-49 | |||
Former Real Property | A-76 | |||
Fraud | A-61 | |||
GAAP | A-76 | |||
Giants Revenue | A-76 | |||
Giants/Zyga Revenue | A-76 | |||
Governing Documents | A-76 | |||
Governmental Entity | A-76 | |||
Group Company Indemnified Parties | A-52 | |||
Group Company(ies) | A-76 | |||
GSA | A-32 | |||
Hazardous Materials | A-76 | |||
Health Care Laws | A-76 | |||
HHS | A-32 | |||
HIPAA | A-74 | |||
HITECH | A-74 | |||
Holdco | A-4 |
A-1
Holdco Common Stock | A-5 | |||
Holdco Preferred Stock | A-6 | |||
Holdco Welfare Benefit Plans | A-52 | |||
HSR Act | A-17 | |||
HSR Approval | A-77 | |||
Indebtedness | A-77 | |||
Indemnification Date | A-77 | |||
Indemnification Price | A-77 | |||
Information Technology Systems | A-77 | |||
Initial Earnout Shares | A-77 | |||
Intellectual Property | A-78 | |||
Interim Company Financial Statements | A-18 | |||
Interim Debt Financing | A-78 | |||
Interim Equity Financing | A-78 | |||
Interim Financing | A-78 | |||
Interim Member Financing | A-78 | |||
Intervening Event | A-78 | |||
Joint Proxy and Consent Solicitation Statement/Prospectus | A-55 | |||
Knowledge of the Company | A-79 | |||
Latest Balance Sheet | A-18 | |||
Latest Balance Sheet Date | A-18 | |||
Law | A-79 | |||
Legal Proceeding | A-79 | |||
Legal Requirement | A-79 | |||
Lenders | A-41 | |||
Liability | A-79 | |||
Lock-Up Agreement | A-10 | |||
LTM Period | A-79 | |||
M&A Contract | A-22 | |||
Material Adverse Effect | A-79 | |||
Material Contract | A-22 | |||
Material Customer | A-29 | |||
Material Permits | A-27 | |||
Material Supplier | A-29 | |||
MCRA | A-48 | |||
MCRA Agreement | A-48 | |||
Member | A-4 | |||
Member Change in Law Opinion | A-60 | |||
Member Fundamental Representations | A-80 | |||
Member Recommendation | A-56 | |||
Member Request | A-15 | |||
MemberTax-Free Opinion | A-60 | |||
Member Units | A-80 | |||
Merger | A-4 | |||
Merger Sub | A-4 | |||
Nasdaq | A-80 | |||
Net Working Capital Amount | A-80 | |||
Non-Competition Agreements | A-5 | |||
OIG | A-32 | |||
Order | A-80 | |||
Ordinary Course of Business | A-80 |
Outside Date | A-65 | |||
Parent | A-4 | |||
Parent Cancelled Shares | A-6 | |||
Parent Capitalization Date | A-36 | |||
Parent Certificate | A-6 | |||
Parent Change in Law Opinion | A-59 | |||
Parent Change in Recommendation | A-54 | |||
Parent Common Stock | A-6 | |||
Parent Disclosure Schedule | A-80 | |||
Parent Equity Awards | A-80 | |||
Parent Financing Failure Fee | A-66 | |||
Parent Fundamental Representations | A-80 | |||
Parent Group Member | A-80 | |||
Parent Indemnitees | A-80 | |||
Parent Intervening Event Fee | A-66 | |||
Parent Material Adverse Effect | A-81 | |||
Parent Preferred Stock | A-6 | |||
Parent Recommendation | A-56 | |||
Parent Record Date | A-56 | |||
Parent Related Parties | A-67 | |||
Parent Restricted Stock Award | A-7 | |||
Parent SEC Reports | A-38 | |||
Parent Stock | A-6 | |||
Parent Stock Option | A-7 | |||
Parent Stockholder Meeting | A-56 | |||
Parent Stockholder Support Agreement | A-5 | |||
Parent Stockholders | A-81 | |||
ParentTax-Free Opinion | A-59 | |||
Parent Transaction Expense | A-81 | |||
Parent Voting Matters | A-55 | |||
Parent’s Knowledge | A-81 | |||
Parent’s Representatives | A-44 | |||
Parties | A-4 | |||
Party | A-4 | |||
Payoff Amount | A-45 | |||
Payoff Letter | A-45 | |||
PEO | A-48 | |||
Permit | A-27 | |||
Permitted Liens | A-81 | |||
Person | A-82 | |||
Personal Data | A-82 | |||
Pre-Closing Tax Refund | A-70 | |||
Property Taxes | A-68 | |||
Real Property Leases | A-82 | |||
Registration Statement | A-55 | |||
Regulatory Approvals | A-82 | |||
Release | A-82 | |||
Representatives | A-82 | |||
Required Amount | A-41 | |||
Required Information | A-82 | |||
Required Parent Vote | A-82 | |||
Restricted Cash | A-83 |
A-2
Review Period | A-8 | |||
Sarbanes-Oxley Act | A-83 | |||
Schedule | A-67 | |||
Scheduled Company Intellectual Property | A-23 | |||
SEC | A-83 | |||
Securities Act | A-83 | |||
Standard Inbound IP Agreements | A-83 | |||
Standard Outbound IP Agreements | A-83 | |||
Stock Consideration | A-5 | |||
Straddle Period | A-83 | |||
Subsidiary | A-83 | |||
Surviving Corporation | A-5 | |||
Target Working Capital | A-83 |
Tax | A-83 | |||
Tax Proceeding | A-70 | |||
Tax Returns | A-84 | |||
Tax Sharing Arrangement | A-84 | |||
Taxes | A-83 | |||
Trade Secrets | A-78 | |||
Trademarks | A-78 | |||
Transaction Documents | A-84 | |||
Transaction Expenses | A-84 | |||
Underpayment | A-9 | |||
Unitholder Support Agreement | A-5 | |||
Unitholders | A-84 | |||
Written Consent | A-45 |
A-3
MASTER TRANSACTION AGREEMENT
THIS MASTER TRANSACTION AGREEMENT (this “Agreement”), dated as of November 1, 2018, is made by and among RTI Surgical, Inc. a Delaware corporation (“Parent”), PS Spine Holdco, LLC, a Delaware limited liability company (the “Member”), Bears Holding Sub, Inc., a Delaware corporation and directly wholly owned Subsidiary of Parent (“Holdco”) and Bears Merger Sub, Inc., a Delaware corporation and direct wholly owned Subsidiary of Holdco (“Merger Sub”). Parent, Holdco, Merger Sub and the Member will each be referred to herein from time to time as a “Party” and, collectively, as the “Parties.” Capitalized terms used and not otherwise defined herein have the meanings set forth inArticle XII below.
WHEREAS, the Parties intend that, on the terms and subject to the conditions set forth in this Agreement, (i) Merger Sub be merged with and into Parent (the “Merger”), with Parent as the surviving entity in the Merger and a wholly owned direct subsidiary of Holdco, in accordance with the applicable provisions of the General Corporation Law of the State of Delaware (the “DGCL”), and (ii) the Member shall contribute all 100% of the equity interests (the “Company Units”) in Paradigm Spine, LLC, a Delaware limited liability company and wholly owned subsidiary of the Member (the “Company”) to Holdco.
WHEREAS, the Board of Managers of the Member has unanimously (i) determined that it is in the best interests of the Member, and declared it advisable, to enter into this Agreement, (ii) approved the execution, delivery and performance by the Member of this Agreement and the consummation of the transactions contemplated hereby, and (iii) upon the terms and subject to the conditions of this Agreement, resolved to recommend that the Unitholders adopt this Agreement and to submit this Agreement to its Unitholders for adoption;
WHEREAS, the Board of Directors of Parent has unanimously (i) determined that it is in the best interests of Parent and Parent’s stockholders, and declared it advisable, to enter into this Agreement, (ii) approved the execution, delivery and performance by Parent of this Agreement and the consummation of the transactions contemplated hereby, including the Merger, and (iii) upon the terms and subject to the conditions of this Agreement, resolved to recommend that the stockholders of Parent adopt this Agreement and to submit this Agreement to the stockholders of Parent for adoption;
WHEREAS, the Board of Directors of Holdco has unanimously (i) determined that it is in the best interests of Holdco and its sole stockholder, and declared it advisable, to enter into this Agreement, (ii) approved the execution, delivery and performance by Holdco of this Agreement and the consummation of the transactions contemplated hereby, including the Merger, and the issuance of shares of Holdco Common Stock and Holdco Preferred Stock in connection with the Merger and Contribution, and (iii) resolved to submit this Agreement to such stockholder for adoption;
WHEREAS, the Board of Directors of Merger Sub has unanimously (i) determined that it is in the best interests of Merger Sub and its sole stockholder, and declared it advisable, to enter into this Agreement, (ii) approved the execution, delivery and performance by Merger Sub of this Agreement and the consummation of the transactions contemplated hereby, including the Merger, and (iii) resolved to submit this Agreement to such stockholder for adoption;
WHEREAS, for U.S. federal income Tax purposes, (i) the Merger and the Contribution, taken together, are intended to qualify as a transaction described in Section 351 of the Code, (ii) the Merger is intended to qualify as a “reorganization” within the meaning of Section 368(a) of the Code and (iii) this Agreement, insofar as it relates to the Merger, is intended to constitute a “plan of reorganization” for purposes of Sections 354, 361 and 368 of the Code and the Treasury Regulations promulgated thereunder;
WHEREAS, as inducement and a condition for the Parties to enter into this Agreement and consummate the transactions contemplated hereby, concurrently with the execution and delivery hereof, each of
A-4
Marc Viscogliosi and Francis Magee is entering into a consulting agreement (each, a “Consulting Agreement”) with Parent, which Consulting Agreement shall not become effective until the Effective Time;
WHEREAS, as inducement and a condition for Parent, Holdco and Merger Sub to enter into this Agreement, concurrently with the execution and delivery hereof, Viscogliosi Brothers, LLC, HealthCor Paradigm Blocker Company Two, Inc. and HealthCor AIV, L.P. are each entering into anon-competition agreement (collectively, the “Non-Competition Agreements”) with Holdco, pursuant to which each of Viscogliosi Brothers, LLC, HealthCor Paradigm Blocker Company Two, Inc. and HealthCor AIV, L.P. has agreed to make certain covenants and agreements relating to competition with the business of the Company;
WHEREAS, as inducement and a condition for Parent, Holdco and Merger Sub to enter into this Agreement, concurrently with the execution and delivery hereof, Viscogliosi Brothers, LLC, VB Acquisition Co. I LLC, HealthCor Paradigm Blocker Company Two, Inc., HealthCor AIV, L.P., Trevi Health Ventures LP and Trevi AIV, LP are each entering into a support agreement (collectively, the “Unitholder Support Agreements”) with Parent and Holdco, pursuant to which each such Unitholder has agreed, among other things and subject to the terms thereof, to consent, in writing, to the adoption of this Agreement by executing the Written Consent and delivering such executed Written Consent to the Member;
WHEREAS, as inducement and a condition for the Member to enter into this Agreement, concurrently with the execution and delivery hereof, Camille I. Farhat, Jonathon M. Singer and WSHP Biologics Holdings, LLC are each entering into a support agreement (collectively, the “Parent Stockholder Support Agreements”) with the Member, pursuant to which each such stockholder of Parent has agreed, among other things and subject to the terms thereof, to vote the Parent Stock beneficially held by such stockholder of Parent in favor of the adoption of this Agreement; and
WHEREAS, Parent, Holdco, Member and Merger Sub desire to make certain representations, warranties, covenants and agreements in connection with this Agreement and to set forth certain conditions to the Contribution and Merger.
NOW, THEREFORE, in consideration of the mutual covenants contained herein and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties hereby agree as follows:
THE CONTRIBUTION AND MERGER
1.01The Contribution; Purchase Price.
(a) Upon the terms and subject to the satisfaction or valid waiver of the conditions set forth in this Agreement and contemporaneously with the Effective Time, the Member shall contribute the Company Units (including any Company Units issued pursuant to an Interim Equity Financing) to Holdco, free and clear of all Encumbrances (other than transfer restrictions under federal securities Laws) (the “Contribution”).
(b) The consideration for the Contribution shall be equal to 10,729,614 fully paid and nonassessable shares of common stock, par value $0.001 per share, of Holdco (“Holdco Common Stock”) (the “Stock Consideration”) and the Cash Purchase Price.
1.02The Merger. Upon the terms and subject to the satisfaction or valid waiver of the conditions set forth in this Agreement and in accordance with the applicable provisions of the DGCL:
(a) At the Effective Time, Merger Sub will merge with and into Parent, the separate corporate existence of Merger Sub shall cease and Parent shall continue as the surviving corporation (the “Surviving
A-5
Corporation”). As a result of the Merger, the Surviving Corporation shall become a direct wholly owned Subsidiary of Holdco.
(b) In connection with the Merger and prior to the Effective Time, Holdco shall take all corporate action necessary to reserve for issuance a sufficient number of shares of Holdco Common Stock and Series A convertible preferred stock, par value $0.001 per share, of Holdco (“Holdco Preferred Stock”) to permit the issuance of shares of (i) Holdco Common Stock to (A) the holders of shares of Parent Common Stock and (B) the Member pursuant toSection 1.01(b) and (ii) Holdco Preferred Stock to the holders of shares of Parent Preferred Stock, in each case as of the Effective Time in accordance with the terms of this Agreement.
1.03Effects of the Merger. The effects of the Merger shall be as provided in this Agreement and in the applicable provisions of the DGCL. Without limiting the generality of the foregoing, and subject thereto, at the Effective Time, all of the assets, property, rights, privileges, powers and franchises of Parent and Merger Sub shall vest in the Surviving Corporation, and all debts, liabilities and duties of Parent and Merger Sub shall become the debts, liabilities and duties of the Surviving Corporation, and the separate legal existence of Merger Sub shall cease for all purposes, all as provided under the DGCL.
1.04Effect of Merger on Capital Stock and Equity Interests. At the Effective Time, by virtue of the Merger and without any action on the part of Parent, Holdco, Merger Sub or the holder of any capital stock of Parent, Holdco or Merger Sub:
(a) Each share of common stock, par value $0.001 per share, of Parent (“Parent Common Stock”) issued and outstanding immediately prior to the Effective Time, other than any Parent Cancelled Shares, shall be converted automatically into one fully paid and nonassessable share of Holdco Common Stock.
(b) Each share of Series A convertible preferred stock, par value $0.001 per share, of Parent (“Parent Preferred Stock” and, collectively with the Parent Common Stock, the “Parent Stock”) issued and outstanding immediately prior to the Effective Time, other than any Parent Cancelled Shares, shall be converted automatically into one fully paid and nonassessable share of Holdco Preferred Stock.
(c) All shares of (A) Parent Common Stock converted into Holdco Common Stock pursuant to Section 1.04(a) and (B) Parent Preferred Stock converted into Holdco Preferred Stock pursuant toSection 1.04(b) shall cease to be outstanding and shall be automatically cancelled and shall cease to exist, and each (i) valid certificate or certificates which immediately prior to the Effective Time represented any such shares of Parent Stock (a “Parent Certificate”)or (ii) non-certificated share of Parent Stock held in book entry form shall, upon the Effective Time, automatically represent an equivalent number of shares of Holdco Common Stock or Holdco Preferred Stock, as applicable (without any requirement for the surrender of any such certificatesor non-certificated shares).
(d) All shares of Parent Stock held by Parent as treasury shares or by Holdco or Merger Sub immediately prior to the Effective Time (the “Parent Cancelled Shares”) shall be automatically cancelled and shall cease to exist, and no consideration shall be delivered in exchange therefor.
(e) Each share of common stock, par value $0.01 per share, of Merger Sub issued and outstanding immediately prior to the Effective Time shall be automatically converted into one fully paid and nonassessable share of common stock of the Surviving Corporation.
(f) At the Effective Time, each share of capital stock of Holdco issued and outstanding immediately prior to the Effective Time and owned by Parent shall remain outstanding. At the Effective Time, each share of capital stock of Holdco issued and outstanding immediately prior to the Effective Time and owned by a Person other than Parent shall be automatically cancelled and shall cease to exist, and no consideration shall be delivered in exchange therefor. Immediately following the Effective Time, shares of capital stock of Holdco owned by the Surviving Corporation shall be surrendered to Holdco without payment therefor.
(g) If prior to the Effective Time, Holdco or Parent, as the case may be, should split, subdivide, consolidate, combine or otherwise reclassify the Holdco Common Stock, Holdco Preferred Stock, Parent
A-6
Common Stock or Parent Preferred Stock, or pay a stock dividend or other stock distribution in Holdco Common Stock, Holdco Preferred Stock, Parent Common Stock or Parent Preferred Stock, as applicable, or otherwise change the Holdco Common Stock, Holdco Preferred Stock, Parent Common Stock or Parent Preferred Stock into any other securities, or make any other such stock dividend or distribution in capital stock of Holdco or Parent in respect of the Holdco Common Stock, Holdco Preferred Stock, Parent Common Stock or Parent Preferred Stock, respectively, then any number or amount contained herein which is based upon the price or the number or fraction of shares of Holdco Common Stock, Holdco Preferred Stock, Parent Common Stock or Parent Preferred Stock, as the case may be, will be appropriately adjusted to proportionately reflect such split, combination, dividend or other distribution or change.
1.05Treatment of Parent Equity Awards.
(a)Parent Stock Options. At the Effective Time, each compensatory option to purchase shares of Parent Common Stock (a “Parent Stock Option”) that is outstanding immediately prior to the Effective Time, whether vested or unvested, shall be converted into a stock option in respect of shares of Holdco Common Stock, on the same terms and conditions as were applicable under such Parent Stock Option immediately prior to the Effective Time (including with respect to vesting), relating to the number of shares of Holdco Common Stock equal to the total number of shares of Parent Common Stock subject to such Parent Stock Option immediately prior to the Effective Time and with an exercise price per share of Holdco Common Stock equal to the exercise price per share of Parent Common Stock subject to such Parent Stock Option immediately prior to the Effective Time.
(b)Parent Restricted Stock Awards. At the Effective Time, each compensatory restricted stock award with respect to shares of Parent Common Stock (a “Parent Restricted Stock Award”) that is outstanding immediately prior to the Effective Time shall be converted into a restricted stock award with the same terms and conditions as were applicable under such Parent Restricted Stock Award immediately prior to the Effective Time (including with respect to vesting), and relating to the number of shares of Holdco Common Stock equal to the total number of shares of Parent Common Stock subject to such Parent Restricted Stock Award immediately prior to the Effective Time. Any accrued but unpaid dividend equivalents with respect to any Parent Restricted Stock Award will be assumed and become an obligation with respect to the applicable converted Parent Restricted Stock Award.
(c)Parent and Holdco Actions. Prior to the Effective Time, Parent shall take all actions necessary (including adopting such resolutions of the Board of Directors of Parent or any committee of the Board of Directors of Parent) to effectuate the treatment of Parent Equity Awards contemplated by this Section 1.05. Holdco shall take all corporate action necessary to reserve for issuance a sufficient number of shares of Holdco Common Stock for delivery with respect to the settlement of converted Parent Equity Awards assumed by it in accordance with thisSection 1.05. Holdco shall file with the SEC, as soon as practicable following the Effective Time, a post-effective amendment to the Registration Statement or a registration statement onForm S-8 (or any successor form), to the extent such form is available, relating to such shares of Holdco Common Stock.
(a) At the Effective Time, the certificate of incorporation andthe by-laws of Merger Sub as in effect immediately prior to the Effective Time shall be the certificate of incorporation andthe by-laws of the Surviving Corporation until thereafter amended in accordance with applicable Law, except that the name of the corporation reflected therein shall be as reasonably determined by Parent prior to Closing.
(b) At the Effective Time, the certificate of incorporation of Holdco shall be amended and restated in the form set forth on Exhibit A. The name of Holdco immediately after the Effective Time shall be “RTI Surgical Holdings, Inc.”. At the Effective Time,the by-laws of Holdco shall be amended and restated in the form set forth on Exhibit B. At the Effective Time, Holdco shall adopt the certificate of designation in the form set forth onExhibit C.
A-7
(a) From and after the Effective Time, (i) the directors of Merger Sub in office immediately prior to the Effective Time shall be the directors of the Surviving Corporation and (ii) the officers of Parent in office immediately prior to the Effective Time shall be the officers of the Surviving Corporation and, in each case, such director or officer shall hold office until his or her respective successor is duly elected or appointed and qualified or until his or her earlier death, resignation or removal in accordance with the governing documents of the Surviving Corporation and applicable Law.
(b) From and after the Effective Time, (i) the directors of Parent in office immediately prior to the Effective Time, as well as Jeffrey C. Lightcap, shall be the Board of Directors of Holdco and (ii) the officers of Parent in office immediately prior to the Effective Time shall be the officers of Holdco and, in each case, shall hold office until his or her respective successor is duly elected or appointed and qualified or until his or her earlier death, resignation or removal in accordance with the governing documents of Holdco and applicable Law, subject, in the case of clause (i), toSection 6.10.
1.08Adjustment to Cash Purchase Price.
(a)Post-Closing Adjustment. As promptly as practicable, but in no event later than sixty (60) days following the Closing Date, Holdco shall prepare or cause to be prepared a statement (the “Closing Date Schedule”) setting forth in reasonable detail Holdco’s calculation of (i) the aggregate amount of all Company Transaction Expenses; (ii) the Closing Indebtedness Amount; (iii) the Closing Cash Amount; (iv) the Net Working Capital Amount; and (v) the Cash Purchase Price.
(b)Review/Disputes.
(i) From and after the Closing, the Member shall provide Holdco and Holdco shall provide the Member, and each shall provide any accountants or advisors retained by Holdco or the Member with reasonable access, during normal business hours, to the relevant books and records of the Company used in the preparation of, or otherwise reasonably relevant to the items referenced inSection 1.08(a). If the Member disputes the calculation of any of the items referenced inSection 1.08(a), then the Member shall deliver a written notice (a “Dispute Notice”) to Holdco at any time during the thirty(30)-day period commencing upon delivery to the Member of all of the items referenced inSection 1.08(a) (the “Review Period”). The Dispute Notice shall set forth the basis for the dispute of any such calculation in reasonable detail.
(ii) If the Member does not deliver a Dispute Notice to Holdco prior to the expiration of the Review Period, Holdco’s calculation of the items referenced inSection 1.08(a) shall be deemed final and binding.
(iii) If the Member delivers a Dispute Notice to Holdco prior to the expiration of the Review Period, then Holdco and the Member shall negotiate in good faith to reach agreement on any items disputed in the Dispute Notice within the thirty(30)-day period commencing upon delivery to Holdco of the Dispute Notice. If the Member and Holdco are unable to reach agreement on all items in the Dispute Notice within such thirty(30)-day period, then either Holdco or the Member may submit the unresolved objections to the office of KPMG US LLP, a national accounting firm (such firm, and any successor thereto, being referred to herein as the “Accounting Firm”), and such firm shall be directed by the Member and Holdco to resolve the unresolved objections in accordance with the immediately following sentence. In connection with the resolution of any such dispute by the Accounting Firm (i) each of Holdco and the Member shall have a reasonable opportunity to meet with the Accounting Firm to provide their views as to any disputed issues, (ii) the Accounting Firm shall determine only such items as remain disputed from the Dispute Notice in accordance with the terms of this Agreement as promptly as reasonably practicable (and in any event, Holdco and the Member shall use their reasonable best efforts to cause the Accounting Firm to make such determination within sixty (60) days of such referral) and upon reaching such determination shall deliver a reasonably detailed copy of its
A-8
calculations to the Member and Holdco and (iii) the determination made by the Accounting Firm of disputes arising from the items referenced inSection 1.08(a) shall be final and binding, absent manifest error;provided,however, that the Accounting Firm’s resolution of each disputed item from the Dispute Notice must be within the range of differences between Holdco’s and the Member’s positions with respect to each such disputed item. The expenses and fees of the Accounting Firm shall be allocated equally between Holdco and the Member.
(c)Payment Upon Final Determination of Adjustments.
(i) If (A) the Cash Purchase Price (calculated based on the items set forth on the Closing Date Schedule, as finally determined in accordance withSection 1.08), is less than (B) the Cash Purchase Price (as determined based upon the Consideration Certificate) (the positive amount of such difference, the “Closing Payment Shortfall”), then the Member shall, within three (3) Business Days following the final determination of matters in accordance withSection 1.08, pay the Closing Payment Shortfall to Holdco by wire transfer of immediately available funds.
(ii) If (A) the Cash Purchase Price (calculated based on the items set forth on the Closing Date Schedule, as finally determined in accordance withSection 1.08), is greater than (B) the Cash Purchase Price (as determined based upon the Consideration Certificate), (the positive amount of such difference, the “Underpayment”), then within three (3) Business Days Holdco shall pay to the Member an amount equal to the Underpayment by wire transfer of immediately available funds.
(d) Nothing in thisSection 1.08 shall limit any rights of any Parent Indemnitee to make an indemnification claim as set forth inSection 9.02;provided,however, that notwithstanding anything inArticle IX to the contrary, none of the Parent Indemnitees shall be entitled to indemnification or reimbursement pursuant toSection 9.02 for any Damages to the extent an accrual for a Liability with respect to such Damages is specifically and actually included in the calculation of the Cash Purchase Price.
1.09Withholding. Notwithstanding any provision contained herein to the contrary, each of Parent and Holdco will be entitled to deduct and withhold from the consideration otherwise payable pursuant to this Agreement such amounts as it is required to deduct and withhold with respect to the making of such payment under any provision of Tax Law. If Parent or Holdco so withholds amounts, such amounts will be treated for all purposes of this Agreement as having been paid to the Person in respect of which such deduction and withholding was made.
1.10Plan of Reorganization. The Merger is intended to qualify as a “reorganization” within the meaning of Section 368(a) of the Code and this Agreement, insofar as it relates to the Merger, is intended to constitute, and is hereby adopted as, a “plan of reorganization” for purposes of Sections 354, 361 and 368 of the Code and the Treasury Regulations promulgated thereunder.
1.11Necessary Further Actions. If, at any time after the Effective Time, any further action is necessary or desirable to vest the Surviving Corporation with full right, title and possession to all assets, properties, rights, privileges, powers and franchises of Parent and Merger Sub, Holdco, Parent and Merger Sub shall, and shall be authorized to, cause their respective directors and officers to take all such lawful and necessary or desirable action, so long as such action is not inconsistent with this Agreement.
THE CLOSING; EXCHANGE OF CERTIFICATES
2.01The Closing. The closing of the Merger and the Contribution (the “Closing”) shall occur by electronic exchange of documents at 8:00 a.m. (Chicago time) on the fifth (5th) Business Day after the satisfaction or waiver (to the extent permitted by applicable Law) of the conditions set forth in Article VIII (other than those conditions
A-9
that by their nature are to be satisfied at the Closing but subject to the satisfaction or, to the extent permitted by applicable Law, waiver of all such conditions as of the Closing), or at such other place, time and date as agreed to in writing by the Parties hereto. The date on which the Closing occurs is called the “Closing Date.”
2.02Effective Time; Closing Transactions.
(a) Subject to the provisions of this Agreement, on the Closing Date: (i) Parent shall file a certificate of merger relating to the Merger as contemplated by the DGCL (the “Certificate of Merger”) with the Secretary of State of the State of Delaware, in such form as required by, and executed in accordance with the DGCL, and (ii) the Member shall effect the Contribution. The Merger and the Contribution shall become effective concurrently (such time as the Merger and the Contribution become effective, the “Effective Time”).
(b) At the Closing, the Member shall deliver the following agreements and documents to Parent:
(i) a certificate, in form and substance reasonably satisfactory to Parent, duly executed on behalf of the Member by the chief executive officer of the Member, containing the Member’s good faith estimate (to be set forth on an accompanying spreadsheet) (such spreadsheet and accompanying certificate being referred to hereafter collectively as the “Consideration Certificate”) of: (A) the aggregate amount of all Company Transaction Expenses (including any Company Transaction Expenses that will become payable after the Effective Time), together with a detailed breakdown thereof; (B) the Closing Indebtedness Amount; (C) the Closing Cash Amount; (D) the Net Working Capital Amount; and (E) the Cash Purchase Price;
(ii) documentation, reasonably satisfactory to Parent, in support of the calculation of the amounts set forth in the Consideration Certificate;
(iii) a certificate of the Secretary of the Member, dated as of the Closing Date and in form and substance reasonably satisfactory to Parent, certifying and attaching: (A) the Governing Documents of the Group Companies, (B) the resolutions adopted by the Board of Managers of the Member to authorize and adopt this Agreement, the Contribution and the other transactions contemplated hereby and (C) the Written Consent;
(iv) written resignations of each officer and director of each Group Company, effective as of the later of the Closing and the date Parent causes such director or officer to be replaced, in form and substance satisfactory to Parent;
(v) a certification from the Member of the Member’snon-foreign status, in form and substance reasonably satisfactory to Parent, in accordance with Treasury Regulation §1.1445-2(b) and Code Section 1446(f), with respect to which Parent shall not have actual knowledge that such certification is false and shall not have received a notice that such certification is false pursuant to Treasury Regulation §1.1445-4;
(vi) a unit power, in form and substance reasonably satisfactory to Parent, duly executed in blank by the Member, with respect to all of the Company Units;
(vii) alock-up agreement duly executed by the Member and in the form ofExhibit D (the “Lock-Up Agreement”);
(viii) a duly executed copy of theLock-Up and Permitted Sale Agreement in the form ofExhibit E from the parties to the Settlement Agreement (other than the Member) that will receive shares of Holdco Common Stock at Closing; and
(ix) any documents or other deliveries of the Member set forth inArticle VIII.
(c) At the Closing, Parent and Holdco shall deliver to the Member the following:
(i) the Certificate of Merger, duly executed by Parent;
A-10
(ii) a certificate of the Secretary of Parent, dated as of the Closing Date and in form and substance reasonably satisfactory to the Member, certifying and attaching: (A) the Governing Documents of each of Parent, Holdco and Merger Sub, (B) the resolutions adopted by the Board of Directors of Parent to authorize and adopt this Agreement and the transactions contemplated hereby, including the Merger, (C) the final report of the inspector of elections with respect to the Parent Stockholder Meeting, (D) the resolutions adopted by the Board of Directors of Holdco to authorize and adopt this Agreement and the transactions contemplated hereby, including the Merger and the issuance of shares of Holdco Common Stock and Holdco Preferred Stock in connection with the Merger and Contribution, (E) the resolutions of Parent, as sole stockholder of Holdco, adopting this Agreement and approving the issuance of shares of Holdco Common Stock and Holdco Preferred Stock in connection with the Merger and Contribution, (F) the resolutions adopted by the Board of Directors of Merger Sub to authorize and adopt this Agreement and the transactions contemplated hereby, including the Merger and (G) the resolutions of Holdco, as sole stockholder of Merger Sub, adopting this Agreement and approving the Merger;
(iii) a duly executed copy of theLock-Up and Permitted Sale Agreement in the form ofExhibit E from Parent and Holdco; and
(iv) any documents or other deliveries of Parent set forth inArticle VIII.
2.03Member Share Issuance; Closing Payments. At the Effective Time, (i) Holdco shall, and Parent shall cause Holdco to, issue to the lenders under the Credit Agreement and any applicable Interim Debt Financing, in a registered transaction pursuant to the Registration Statement that has been declared effective under the Securities Act, uncertificated, book-entry shares that shall not contain any restrictive legend regarding the ability to resell such shares, representing the number of shares of Holdco Common Stock to be paid to the lenders under the Credit Agreement and any such Interim Debt Financing at the Closing as part of the Payoff Amounts, as set forth in the Payoff Letters, and shall cause Broadridge Corporate Issuer Solutions, Inc. (or such other Person as may be mutually agreed upon by Holdco and the Member) (the “Agent”) to credit in the stock ledger and other appropriate books and records of Holdco the number of shares of Holdco Common Stock issued to such lenders in respect of the Stock Consideration, (ii) Holdco shall, and Parent shall cause Holdco to, issue to the Member uncertificated, book-entry shares representing the number of shares of Holdco Common Stock sufficient to deliver the aggregate Stock Consideration not issued to the lenders under the Credit Agreement and any applicable Interim Debt Financing pursuant to clause (i) above, and shall cause the Agent to credit in the stock ledger and other appropriate books and records of Holdco the number of shares of Holdco Common Stock issued to the Member in respect of the Stock Consideration and (iii) in the case of the Cash Purchase Price, pay by wire transfer of immediately available funds the amount of the aggregate Cash Purchase Price (as determined based upon the Consideration Certificate).
2.04Parent Exchange Procedures. Each Parent Certificate representing Parent Stock (other than Parent Cancelled Shares) immediately prior to the Effective Time shall, from and after the Effective Time and as a result of the Merger, represent an equivalent number of shares of Holdco Common Stock or Holdco Preferred Stock, as applicable;provided, however, that if an exchange of Parent Certificates for new certificates is required by Law or applicable rule or regulation, or is desired at any time by Holdco, in its sole discretion, Holdco shall arrange for such exchange ona one-for-one-share basis. At the Effective Time, Holdco shall cause the Agent to credit in the stock ledger and other appropriate books and records of Holdco an equivalent number of shares of Holdco Common Stock or Holdco Preferred Stock, as applicable, for any uncertificated shares of Parent Common Stock or Parent Preferred Stock (other than any Parent Cancelled Shares). For the avoidance of doubt, from and after the Effective Time, the former holders of (i) Parent Common Stock, which has been converted into Holdco Common Stock at the Effective Time, shall be entitled to receive any dividends and distributions which may be made with respect to such shares of Holdco Common Stock and (ii) Parent Preferred Stock which has been converted into Holdco Preferred Stock at the Effective Time, shall be entitled to receive any dividends and distributions which may be made with respect to such shares of Holdco Preferred Stock.
A-11
2.05No Further Ownership Rights. The shares of Holdco Common Stock issued upon conversion of Parent Common Stock and the shares of Holdco Preferred Stock issued upon conversion of Parent Preferred Stock, in each case in accordance with the terms of Article I and this Article II, shall be deemed to have been delivered or paid in full satisfaction of all rights pertaining to the shares of Parent Common Stock and Parent Preferred Stock, as applicable. From and after the Effective Time, (a) all holders of certificates formerly representing shares of Parent Stock or of uncertificated shares of Parent Stock shall cease to have any rights as stockholders of Parent other than the shares of Holdco Common Stock or Holdco Preferred Stock into which the shares represented by such certificates or uncertificated shares have been converted pursuant to this Agreement in accordance with Section 1.04, and (b) the stock transfer books of the Surviving Corporation shall be closed with respect to all shares of Parent Stock outstanding immediately prior to the Effective Time, and there shall be no further registration of transfers on the stock transfer books of Holdco or the Surviving Corporation of shares of Parent Stock that were outstanding immediately prior to the Effective Time. If, after the Effective Time, any certificates or book-entry shares formerly representing shares of Parent Stock are presented to Holdco or the Agent for any reason, such certificates or book-entry shares (as applicable) shall be cancelled and their holders shall be credited shares of Holdco Common Stock or Holdco Preferred Stock as provided in thisArticle II.
2.06Earnout Shares and Payments.
(a) In addition to the Stock Consideration and the Cash Purchase Price payable in respect of the Contribution pursuant to Section 2.03, subject to Holdco’s rights set forth inSection 2.06(e), Holdco shall be required to make cash payments to the Member and issue additional shares of Holdco Common Stock (such shares, the “Earnout Shares”) to the Member, in each case, as provided in this Section 2.06:
(i) if the Giants Revenue is equal to or exceeds $65,000,000 in any LTM period ending on or prior to December 31, 2020, then Holdco shall pay the Member, or as directed by the Member, by wire transfer of immediately available funds, $20,000,000, subject to reduction pursuant toSection 2.06(e), and once such cash payment is made to the Member or according to the Member’s direction, pursuant to thisSection 2.06(a)(i), Holdco shall have no further obligations pursuant to thisSection 2.06(a)(i);provided, that the Parties acknowledge that the Member directs Holdco to pay a portion of this payment in accordance with the terms of the Settlement Agreement;
(ii) if the Giants/Zyga Revenue is greater than $85,000,000 in any LTM Period ending on or prior to December 31, 2021, then Holdco shall issue to the Member the number of Initial Earnout Shares payable to the Member with respect to such LTM Period (less the total number of Earnout Shares that have previously been issued to the Member pursuant to thisSection 2.06(a)(ii)), and once the applicable Initial Earnout Shares (subject to reduction as set forth inSection 2.06(e)) are issued to the Member, pursuant to thisSection 2.06(a)(ii), with respect to an LTM Period in which the Giants/Zyga Revenue is $105,000,000 or greater, Holdco shall have no further obligations pursuant to thisSection 2.06(a)(ii);
(iii) if the Giants/Zyga Revenue is greater than $105,000,000 in any LTM Period ending on or prior to December 31, 2022, then Holdco shall, at its option, either (A) issue to the Member the Final Earnout Shares, subject to reduction pursuant toSection 2.06(e), or (B) pay the Member, by wire transfer of immediately available funds, the Cash Election Amount (subject to reduction as set forth inSection 2.06(e)), and once the applicable Final Earnout Shares are issued to the Member or applicable Cash Election Amount is paid to Member, pursuant to thisSection 2.06(a)(iii), with respect to an LTM Period in which the Giants/Zyga Revenue is $125,000,000 or greater, Holdco shall have no further obligations pursuant to thisSection 2.06(a)(iii); and
(iv) immediately prior to, but subject to the consummation of, (A) a Change of Control Transaction prior to December 31, 2020, (I) the $20,000,000 cash payment set forth inSection 2.06(a)(i) shall be paid and (II) the maximum number of Earnout Shares payable to the Member pursuant to this Section 2.06 shall be issued, in each case less the total number of Earnout Shares and cash payments that have been previously issued or made to the Member pursuant to
A-12
Sections 2.06(a)(i),2.06(a)(ii) and2.06(a)(iii), to the Member (provided that, in lieu of any issuance of Final Earnout Shares pursuant toSection 2.06(a)(iii), Holdco may elect at its option to instead pay, the Member, by wire transfer of immediately available funds, $45,000,000, less the total number of Final Earnout Shares and cash payments that have been previously issued or made to the Member pursuant toSection 2.06(a)(iii)) whereupon such issuance and/or payment, Holdco’s obligation to issue additional Earnout Shares or make any cash payments in the future under this Section 2.06 shall terminate, (B) a Change of Control Transaction on or following December 31, 2020 but prior to December 31, 2021, the maximum number of Earnout Shares payable to the Member pursuant toSection 2.06(a)(ii) andSection 2.06(a)(iii) shall be issued, less the total number of Earnout Shares and cash payments that have been previously issued or made to the Member pursuant toSections 2.06(a)(ii) and2.06(a)(iii), to the Member (provided that, in lieu of any issuance of Final Earnout Shares pursuant toSection 2.06(a)(iii), Holdco may elect at its option to instead pay, the Member, by wire transfer of immediately available funds, $45,000,000, less the total number of Final Earnout Shares and cash payments that have been previously issued or made to the Member pursuant toSection 2.06(a)(iii)) whereupon such issuance and/or payment, Holdco’s obligation to issue additional Earnout Shares or make any cash payments in the future under this Section 2.06 shall terminate or (C) a Change of Control Transaction on or following December 31, 2021 but prior to December 31, 2022, at the election of Holdco, either the number of Final Earnout Shares payable to the Member pursuant toSection 2.06(a)(iii) shall be issued to the Member or Holdco shall pay the Member, by wire transfer of immediately available funds, $45,000,000, in each case less the total number of Final Earnout Shares and cash payments that have been previously issued or made to the Member pursuant toSection 2.06(a)(iii), whereupon such issuance or payment Holdco’s obligation to issue additional Earnout Shares or make any cash payments in the future under thisSection 2.06 shall terminate.
(b) Holdco shall:
(i) provide the Member with a statement setting forth the Giants/Zyga Revenue during such quarter and, if the end of such quarter is at least twelve (12) months after the Closing, for the LTM Period ending at the end of such quarter, in each case, within forty-five (45) days following the completion of each calendar quarter that is not a fiscal year end from the Closing Date through December 31, 2022;
(ii) provide the Member with a statement setting forth the Giants Revenue during such quarter within forty-five (45) days following the completion of each calendar quarter that is not a fiscal year end from the Closing Date through December 31, 2020; and
(iii) provide the Member with a statement setting forth the (A) Giants/Zyga Revenue for the applicable fiscal year prepared by Holdco’s then current financial statement auditor within fifteen (15) days following the completion of Holdco’s fiscal year audit for each fiscal year through December 31, 2022 and (B) Giants Revenue for the applicable fiscal year prepared by Holdco’s then current financial statement auditor within fifteen (15) days following the completion of Holdco’s fiscal year audit for each fiscal year through December 31, 2020.
(c) Holdco’s obligation to issue the Earnout Shares or make any cash payment to the Member in accordance with Section 2.06 is an independent obligation of Holdco and is not otherwise conditioned or contingent upon the satisfaction of any conditions precedent to any preceding or subsequent Earnout Shares.
(d) Subject toSection 2.06(e), any Earnout Shares that Holdco is required to issue or cash payments that Holdco is required to make, in each case, pursuant toSection 2.06(a) shall be issued or made to the Member no later than the earlier of (i) five (5) Business Days following the date upon which Holdco determines that the applicable conditions precedent set forth inSection 2.06(a)(i),2.06(a)(ii) or2.06(a)(iii) have been satisfied or, in the case of a Change of Control Transaction, immediately prior to or substantially concurrent with the consummation of such Change of Control Transaction and (ii) five (5) Business Days following the date upon which the Accounting Firm, in accordance withSection 2.06(g), determines that the applicable conditions precedent set forth inSection 2.06(a)(i),2.06(a)(ii),2.06(a)(iii) orSection 2.06(a)(iv)
A-13
have been satisfied;provided,however, that notwithstanding the foregoing, in no event shall Holdco be required to make a cash payment to the Member pursuant toSection 2.06(a)(i) prior to December 31, 2020.
(e) Notwithstanding anything to the contrary set forth herein:
(i) in the event that it is determined in accordance with this Agreement that any Parent Indemnitee is entitled to be indemnified for Damages pursuant toArticle IX orSection 11.02, Holdco shall have the right to, at Holdco’s sole discretion: (A) withhold and set off against a number of Earnout Shares (or, if applicable, cash payment pursuant toSection 2.06(a)(i) or2.06(a)(iii)) otherwise earned by the Member pursuant to Section 2.06(a), equal to the amount of such Damages such Parent Indemnitee is entitled to pursuant toArticle IX orSection 11.02 hereof (assuming for such withholding and set off of the Earnout Shares, a value per Earnout Share equal to the Indemnification Price);provided,however, that prior to withholding or setting off against any cash payment pursuant toSection 2.06(a)(iii) or Earnout Shares otherwise earned by the Member, Holdco must first withhold and set off such Damages against any cash payment earned by the Member pursuant toSection 2.06(a)(i) but not yet paid; (B) permanently reduce the amount of the Earnout Shares (or, if applicable, cash payment pursuant toSection2.06(a)(i) or2.06(a)(iii)) available to be issued (or paid) to the Member pursuant toSection 2.06(a), equal to the amount of such Damages such Parent Indemnitee is entitled to pursuant toArticle IX orSection 11.02 hereof (assuming for such reduction of the Earnout Shares, a value per Earnout Share equal to the Indemnification Price);provided,however, that prior to permanently reducing the amount of any cash payment pursuant toSection 2.06(a)(iii) or Earnout Shares available to be issued (or paid) to the Member, Holdco must first permanently reduce the full amount of the cash payment available to be paid to the Member pursuant toSection 2.06(a)(i) if no cash payment has been made pursuant toSection 2.06(a)(i); or (C) if applicable, reduce the amount of anyPre-Closing Tax Refund due to the Member pursuant toSection 11.02(d) by the amount of such Damages such Parent Indemnitee is entitled to pursuant toArticle IX orSection 11.02 hereof;
(ii) if, at any time Holdco or any of its Subsidiaries is required to issue any Earnout Shares (or, if applicable, make a cash payment pursuant toSection 2.06(a)(i) or2.06(a)(iii)) or pay anyPre-Closing Tax Refund to the Member pursuant to thisSection 2.06 orSection 11.02(d), any Parent Indemnitee has provided the Member with a Claim Notice for indemnification pursuant toArticle IX orSection 11.02 and the amount of indemnification to which the Parent Indemnitees are entitled in respect of such Claim Notice pursuant to this Agreement has not yet been finally determined pursuant toArticle IX orSection 11.02, Holdco shall have the right to withhold a number of Earnout Shares from such issuance (or, if applicable, withhold an amount from the cash payment pursuant toSection 2.06(a)(i) or2.06(a)(iii)), or, if applicable, withhold from anyPre-Closing Tax Refund due to the Member pursuant toSection 11.02(d) an amount, equal to the amount of Damages (assuming for such withholding of the Earnout Shares, a value per Earnout Share equal to Parent’s estimate of the Indemnification Price (which estimate must be reasonably acceptable to the Member)) claimed in such Claim Notice until the final determination of the amount of indemnification to which the Parent Indemnitees are entitled in respect of such Claim Notice pursuant to this Agreement;provided,however, that if multiple issuances and/or payments, as applicable, of Earnout Shares or cash payments pursuant toSection 2.06(a)(i) or2.06(a)(iii) are earned and have not been made at the time of such withholding and one of such payments is the cash payment otherwise required to be made pursuant toSection 2.06(a)(i), Holdco shall first withhold from any cash payment otherwise required to be made pursuant toSection 2.06(a)(i); and
(iii) upon the final determination of any such claim in clause (ii) hereof, Holdco shall promptly issue to the Member any Earnout Shares (or, if applicable, make the cash payment pursuant toSection 2.06(a)(i) or2.06(a)(iii)), or, if applicable, cause its applicable Subsidiary to pay the Member the portion of anyPre-Closing Tax Refund, which Holdco does not have the right to withhold and set off pursuant to clause (i) hereof.
(f) The Parties understand and agree that (i) the contingent rights to receive any Earnout Shares or cash payment pursuant to thisSection 2.06 shall not be represented by any form of certificate or other instrument,
A-14
are not transferable, except as specifically provided herein, and do not constitute an equity or ownership interest in Holdco or any of its Subsidiaries; (ii) the Member shall not have any rights as a securityholder of Holdco in respect of its contingent right to receive any Earnout Shares hereunder; and (iii) no interest is payable with respect to any Earnout Shares or cash payment pursuant to thisSection 2.06. The Member acknowledges and agrees that (A) the payments set forth in this Section 2.06 are speculative and not guaranteed and subject to numerous factors outside the control of the Parties; (B) none of Holdco, Parent, the Surviving Corporation, the Company nor any of their respective Affiliates or Representatives has promised or projected any payments under this Section 2.06 and the Member has not relied on any projections for any period following the Closing; (C) other than the express covenants and agreements contained in this Agreement, none of Holdco, Parent, the Surviving Corporation, the Company nor any of their respective Affiliates or Representatives owe any fiduciary duties or any other duties (express or implied) to maximize the Giants/Zyga Revenue or Giants Revenue in any applicable period; and (D) the Parties solely intend the express provisions of this Agreement to govern their contractual relationship.
(g) At any time prior to the date on which Holdco has no further obligation pursuant to thisSection 2.06 to issue any Earnout Shares or make any cash payment pursuant toSection 2.06(a)(i) or2.06(a)(iii), upon the request of the Member (a “Member Request”), which Member Request may not be made more than once in any twelve (12)-month period, Holdco shall, during the period commencing upon the delivery to Holdco of the Member Request and ending upon the final determination of the applicable Earnout Calculations pursuant to thisSection 2.06(g), provide the Member, and any accountants or advisors retained by the Member, with reasonable access, during normal business hours, to the relevant books and records of Holdco and its Subsidiaries used in the preparation of, or otherwise reasonably relevant to, Holdco’s calculations of (i) the Giants/Zyga Revenue and Giants Revenue for any applicable period and (ii) the Earnout Shares to be issued and/or cash payments to be made to the Member pursuant toSection 2.06(a) (the “Earnout Calculations”). If the Member disputes any such Earnout Calculations, then the Member shall deliver a written notice (an “Earnout Dispute Notice”) to Holdco at any time during the thirty(30)-day period commencing upon the delivery to Holdco of a Member Request (the “Earnout Review Period”). The Earnout Dispute Notice shall set forth the amount of and basis for the dispute of any such Earnout Calculation in reasonable detail. If the Member does not deliver an Earnout Dispute Notice to Holdco prior to the expiration of the Earnout Review Period, Holdco’s calculation of any Earnout Calculations made prior to the date of the Member Request shall be deemed final and binding. If the Member delivers an Earnout Dispute Notice to Holdco prior to the expiration of the Earnout Review Period, then Holdco and the Member shall negotiate in good faith to reach agreement on any items disputed in the Earnout Dispute Notice within the thirty(30)-day period commencing upon the delivery to Holdco of the Earnout Dispute Notice. If the Member and Holdco are unable to reach agreement on all items in the Earnout Dispute Notice within such thirty(30)-day period, then either Holdco or the Member may submit the unresolved objections to the Accounting Firm, and such Accounting Firm shall be directed by the Member and Holdco to resolve the unresolved objections in accordance with the immediately following sentence. In connection with the resolution of any such dispute by the Accounting Firm (1) each of Holdco and the Member shall have a reasonable opportunity to meet with the Accounting Firm to provide their views as to any disputed issues, (2) the Accounting Firm shall determine only such items as remain disputed from the Earnout Dispute Notice in accordance with the terms of this Agreement as promptly as reasonably practicable (and in any event, Holdco and the Member shall use their reasonable best efforts to cause the Accounting Firm to make such determination within sixty (60) days of such referral) and upon reaching such determination shall deliver a reasonably detailed copy of its calculations to the Member and Holdco and (3) the determination made by the Accounting Firm of disputes arising from the Earnout Calculations shall be final and binding, absent manifest error;provided,however, that the Accounting Firm’s resolution of each disputed item from the Earnout Dispute Notice must be within the range of differences between Holdco’s and the Member’s positions with respect to each such disputed item. The expenses and fees of the Accounting Firm shall be allocated between Holdco and the Member in inverse proportion as they may prevail on the disputed amounts resolved by the Accounting Firm, utilizing the values of such disputed
A-15
amounts as initially submitted by the parties to the Accounting Firm. Such proportional allocation shall be determined by the Accounting Firm at the time its determination is rendered on the disputed items.
(h) If, following the Effective Time, Holdco should split, subdivide, consolidate, combine or otherwise reclassify the Holdco Common Stock, or pay a stock dividend or other stock distribution in Holdco Common Stock, or otherwise change the Holdco Common Stock into any other securities, or make any other such stock dividend or distribution in capital stock of Holdco in respect of the Holdco Common Stock, then any number or amount contained herein which is based upon the price or the number or fraction of shares of Holdco Common Stock will be appropriately adjusted to proportionately reflect such split, combination, dividend or other distribution or change.
REPRESENTATIONS AND WARRANTIES OF THE MEMBER
Except as disclosed in the Disclosure Schedules, the Member hereby represents and warrants to Parent as follows in thisArticle III.
3.01Existence and Good Standing. The Member is a limited liability company duly organized, validly existing and in good standing under the laws of the State of Delaware. Each of the Group Companies is duly organized, validly existing and, to the extent applicable in the respective jurisdiction, in good standing under the Laws of the jurisdiction in which it is incorporated or organized. Each of the Group Companies has all requisite organizational power and authority to own, lease and operate the properties and assets it owns, leases and operates and to carry on its business as such business is conducted. Each of the Group Companies is qualified to do business as a foreign entity in each jurisdiction in which its ownership of property or the conduct of business as now conducted requires it to qualify, except where failure to be so duly qualified would not, individually or in the aggregate, have a Material Adverse Effect. The Member has made available to Parent an accurate and complete copy of each Governing Document of each Group Company, in each case, as in effect as of the date of this Agreement. Such Governing Documents are in full force and effect. No Group Company has been dissolved (aufgelöst) or wound up and, to the Knowledge of the Company, there are no reasons that would justify an administrative cancellation (Amtslöschung) of a Group Company.
3.02Authority; Enforceability. The Member has the full requisite organizational power and authority to execute and deliver this Agreement and the other Transaction Documents to which it is a party (or is contemplated to be a party at the Closing), and, subject to receipt of the Written Consent, to perform its obligations under this Agreement and the other Transaction Documents to which it is a party (or is contemplated to be a party at the Closing), and to consummate the transactions contemplated hereby and thereby. Subject to receipt of the Written Consent, the execution, delivery and performance of this Agreement and the other Transaction Documents to which the Member is a party (or is contemplated to be a party at the Closing) by the Member and the consummation of the transactions contemplated hereby and thereby have been (or will be prior to the Closing) duly and validly authorized by all required organizational action on behalf of the Member. Assuming that this Agreement and each of the other Transaction Documents that is a Contract to which the Member is a party (or is contemplated to be a party at the Closing) is a valid and binding obligation of each other party hereto and thereto, this Agreement and each of the other Transaction Documents to which the Member is a party (or is contemplated to be a party at the Closing) constitutes (or, with respect to such Transaction Documents that are not contemplated to be executed and delivered as of the date hereof, will constitute at the Closing) the valid and binding obligation of the Member enforceable against the Member in accordance with its terms, except as enforceability may be limited by bankruptcy, insolvency, reorganization, moratorium or other Legal Requirements relating to or affecting creditors’ rights generally or by equitable principles (regardless of whether enforcement is sought at law or in equity). The Board of Managers of the Member has unanimously (a) determined that it is in the best interests of the Member, and declared it advisable, to enter into this Agreement and (b) upon the terms and subject to the conditions of this Agreement, resolved to recommend that the Unitholders adopt this Agreement and to submit this Agreement to the Unitholders for adoption.
A-16
3.03No Violations; Required Filings and Consents.
(a) Except as set forth onSchedule 3.03, the execution and delivery of this Agreement by the Member and the execution and delivery of the other Transaction Documents to which the Member is or is contemplated to be a party does not and will not, and, subject to receipt of the Written Consent, the performance and compliance with the terms and conditions hereof and thereof by the Member and the consummation of the transactions contemplated hereby and thereby by the Member will not (with or without notice or passage of time, or both) conflict with, result in any breach of, constitute a default under or an event creating rights of acceleration, termination, modification or cancellation or loss of right under, payment of additional fees under, result in a violation of, result in the creation of any Encumbrance under any assets of any Group Company or, assuming that the consents, approvals, authorizations, notices, reports and other filings described inSection 3.03(b) have been made or obtained, as applicable, and any waiting periods thereunder have been terminated or expired, require any authorization, consent, approval, exemption or other action by or notice to any Governmental Entity or other third party, under:
(i) the Governing Documents of the Member or any of the Group Companies;
(ii) any Law or Order applicable to the Member or the Group Companies or by which any property or asset of the Member or such Group Company is bound or affected; or
(iii) any Material Contract;
except, in the case of clauses (ii) and (iii), for any such conflicts, violations, breaches, defaults or other occurrences that would not, individually or in the aggregate, reasonably be expected to adversely affect the Group Companies, taken as a whole, in any material respect.
(b) Except (i) for the applicable requirements of the Hart-Scott-Rodino Antitrust Improvements Act of 1976 (the “HSR Act”), (ii) for compliance with the federal securities Laws and any applicable U.S. state securities or “blue sky” Laws and foreign securities Laws and (iii) as would not reasonably be expected to adversely affect the Group Companies, taken as a whole, in any material respect, neither Member nor any Group Company is required to submit any notice, report or other filing with any Governmental Entity in connection with the execution, delivery or performance by it of this Agreement or any other Transaction Document to which it is or is contemplated to be a party or the consummation of the transactions contemplated hereby or thereby and no consent, approval or authorization of any Governmental Entity is required to be obtained by the Member or any Group Company in connection with the Member’s execution, delivery and performance of this Agreement or any other Transaction Document to which it is or is contemplated to be a party or the consummation of the transactions contemplated hereby or thereby.
3.04Title to Units; Capitalization; Subsidiaries.
(a) The authorized and outstanding equity interests of the Company as of the date hereof are as set forth inSchedule 3.04(a). The Company Units represent all of the issued and outstanding equity interests of the Company. The Company Units were duly and validly issued and are fully paid andnon-assessable. The Member owns all of the issued and outstanding equity interests of the Company, free and clear of all Encumbrances, other than transfer restrictions under federal securities Laws and Encumbrances created under the Credit Agreement or any Interim Debt Financing that will be terminated at Closing upon payoff of the Indebtedness thereunder. There are no outstanding, issued or authorized obligations, options, warrants, convertible securities, stock appreciation rights, profit interests, capital stock or other rights, agreements, arrangements or commitments of any kind relating to the equity interests of the Company or obligating the Company to issue or sell any equity interests, shares of capital stock of, or any other interest in, the Company. There are no outstanding contractual obligations of the Company to repurchase, redeem or otherwise acquire any equity interests of the Company or to provide funds to, or make any investment in, any other Person. There are no agreements or understandings in effect with respect to the voting or transfer of any of the equity interests of the Company.
(b)Schedule 3.04(b) completely and accurately sets forth the name, place of incorporation or formation and authorized and issued and outstanding equity of each Subsidiary of the Company. As of the date hereof,
A-17
each Subsidiary of the Company is directly or indirectly wholly owned by the Company. Each Group Company’s issued and outstanding shares of capital stock, nominal share capital or other equity securities have been, to the extent applicable, duly authorized and validly issued and are fully paid andnon-assessable and free of any additional payment obligations (Nachschusspflichten). Except as set forth inSchedule 3.04(b), there are no agreements requiring any Group Company to issue, transfer, sell or otherwise dispose of any shares of capital stock or other securities of any Group Company, including any options, warrants, subscriptions, rights, calls or other similar commitments or agreements relating thereto. Except as set forth inSchedule 3.04(b), no shares of capital stock or other securities of any Group Company, are subject to any proxies, voting agreements, voting trusts or other similar arrangements which affect the rights of holder(s) to vote such securities, nor are any stockholder agreements,buy-sell agreements, restricted stock purchase agreements, preferred stock purchase agreements, warrant purchase agreements, stock issuance agreements, stock option agreements, rights of first refusal or other similar agreements existing as of the date hereof with respect to such securities which in any manner would affect the title of any holder(s) to such securities or the rights of any holder(s) to sell the same free and clear of all Encumbrances.
(c) As of the date hereof, the lists of shareholders, which have from time to time been held on record with the German commercial register (Handelsregister) of any of the Group Companies (to the extent applicable), have at all times accurately reflected the ownership in all shares in the respective Group Company.
3.05Financial Statements and Other Financial Matters; No Undisclosed Liabilities.
(a) Set forth inSchedule 3.05 are the following financial statements (collectively, the “Company Financial Statements”):
(i) the unaudited, consolidated balance sheet of the Company and its consolidated Subsidiaries as of August 31, 2018 (such balance sheet, the “Latest Balance Sheet” and such date, the “Latest Balance Sheet Date”), and the related consolidated income statement for the eight (8)-month period then ended (collectively, the “Interim CompanyFinancial Statements”); and
(ii) the audited, consolidated balance sheets of the Company and its consolidated Subsidiaries as of December 31, 2017, December 31, 2016 and December 31, 2015 and the related consolidated statements of operations and comprehensive income (loss), member’s equity and cash flows for the years ended December 31, 2017, December 31, 2016 and December 31, 2015.
(b) The Company Financial Statements have been prepared on a consistent basis with the Company’s past practices in accordance with GAAP applied on a consistent basis throughout the periods involved (except as may be expressly set forth in the notes thereto), and fairly present in all material respects the financial condition and results of operations, cash flows and changes in member’s equity of the Group Companies (taken as a whole) at the respective dates and for the respective periods described above, except as set forth onSchedule 3.05(b) and in the case of the Interim Company Financial Statements, subject to the absence of explanatory footnote disclosures andyear-end adjustments required by GAAP, none of which would be material. Since December 31, 2017, no Group Company has changed its accounting policies, principles, methods or practices in any material respect, and all of such policies, principles, methods and practices are in accordance with GAAP.
(c) There are no Liabilities (whether accrued, absolute, asserted or unasserted, known or unknown, primary or secondary, direct or indirect, contingent or otherwise) that would be required by GAAP to be reflected on a consolidated balance sheet of the Company and its Subsidiaries, other than any such Liabilities (i) incurred in the Ordinary Course of Business since the Latest Balance Sheet Date, (ii) reflected or reserved against on the Interim Company Financial Statements, (iii) arising or incurred in connection with a transaction or agreement contemplated by this Agreement (excluding the obligations set forth inSection 5.01) or (iv) that would not, individually or in the aggregate, reasonably be expected to be material to the Group Companies taken as a whole.
A-18
(d) Except as set forth inSchedule 3.05(d), none of the Group Companies has any Indebtedness outstanding as of the date hereof.
(e) The Company maintains internal accounting controls sufficient to provide reasonable assurances that (i) transactions are executed in accordance with management’s general or specific authorizations, (ii) transactions are recorded as necessary to permit preparation of financial statements in conformity with GAAP and to maintain accountability for assets, (iii) access to assets is permitted only in accordance with management’s general or specific authorization and (iv) the recorded accountability for assets is compared with the existing assets at reasonable intervals and appropriate action is taken with respect to any differences.
(f) Except as set forth inSchedule 3.05(f), all of the Accounts Receivable (i) represent legal, valid and binding obligations that arose in bona fide transactions from products sold or services rendered by a Group Company in the ordinary course of business and (ii) are not the subject of any Legal Proceedings as of the date hereof. To the Knowledge of the Company, there are no contests, claims, counterclaims, rights of set off or other defenses with respect to the Accounts Receivable.
3.06Absence of Certain Changes. During the period from the Latest Balance Sheet Date to the date hereof, except as set forth inSchedule 3.06, each Group Company has conducted its business in the Ordinary Course of Business (except with respect to the negotiation of and entry into this Agreement) and:
(a) there has not been a Material Adverse Effect; and
(b) none of the Group Companies has taken any action that would have been prohibited or otherwise restricted underSection 5.01 hereof, had such action been taken during thepre-Closing period, and none of the Group Companies has agreed or committed, whether orally or in writing, to take such an action.
(a) None of the Group Companies owns or has ever owned any real property.
(b)Schedule 3.07(b) is a true and complete list of all real property in which any of the Group Companies owns a leasehold or subleasehold interest or holds a valid right to occupy or use (the “Company Real Property”) and a complete and correct list of the Real Property Leases applicable thereto. A true and complete copy of each of the Real Property Leases has been made available to Parent and none of the Real Property Leases has been modified in any respect, except to the extent that such modifications are disclosed by the copies made available to Parent. The title in and to the leasehold or subleasehold interests in or valid rights to occupy or use the Company Real Property of each of the Group Companies is free and clear of any Encumbrances, except for Permitted Liens. Each of the Real Property Leases is in full force and effect and the Group Companies hold valid and existing leasehold or subleasehold interests thereunder for the term thereof. To the Company’s Knowledge, no event or circumstance has occurred or exists which, if not remedied, would, either with or without notice or the passage of time or both, constitute a material breach or default by a Group Company, or permit the termination, modification or acceleration of rent under, any Real Property Lease.
(c) The Company Real Property constitutes all of the material real property used in the conduct of the business as conducted by the Group Companies. There are no leases, subleases, leases, occupancy agreements or other agreements, (other than the Real Property Leases) granting to any Person the right of use or occupancy of any Company Real Property. True and complete copies of the most recent title policies or commitments (and underlying documents), surveys, appraisals, zoning reports, SNDAs and estoppels with respect to the Company Real Property in any Group Company’s possession have been made available to Parent.
(d) With respect to each parcel of Company Real Property:
(i) to the Knowledge of the Company, except as would not be material to the Group Companies, none of the buildings, structures, improvements or appurtenances thereon are located outside of the
A-19
boundary lines of such land, contravenes any setback requirement, zoning ordinance or other administrative regulation (whether or not permitted because of priornon-conforming use), encroaches on any easement which may burden the land or violates any restrictive covenant or any provision of any Legal Requirement;
(ii) the Company has not received any written notice that there is any pending and, to the Knowledge of the Company, there are not any threatened condemnation proceedings, lawsuits or administrative actions relating thereto, or other matters materially and adversely affecting the use, occupancy, or value thereof; and
(i) such parcel has access, sufficient for the conduct of the business as conducted by the Group Companies, to public roads and to all utilities used in the operation of the business at that location.
3.08Tax Matters. Except as set forth inSchedule 3.08:
(a) all material Taxes (whether or not shown on any Tax Return) for which a Group Company is liable have been timely paid;
(b) all Tax Returns required to have been filed by or with respect to a Group Company have been timely filed, and all such Tax Returns are complete and accurate in all material respects and disclose all material Taxes required to be paid by or with respect to the Group Companies for the periods covered thereby;
(c) no extension of time within which to file any Tax Return required to have been filed by or with respect to a Group Company is in effect;
(d) no waiver of any statute of limitations relating to Taxes for which the Group Companies are liable is in effect, and no written request for such a waiver is outstanding;
(e)Schedule 3.08(e) sets forth a schedule of the Tax Returns referred to inSection 3.08(b) with respect to which neither the appropriate Governmental Entity has completed its examination (with all issues finally resolved) nor the period for assessment of the associated Taxes (taking into account all applicable extensions and waivers) has expired;
(f) there is no action, suit, investigation, audit, claim or assessment pending or proposed or threatened, in writing or otherwise to the Knowledge of the Company, with respect to Taxes for which the Group Companies may be liable;
(g) no Governmental Entity (whether within or without the United States) with respect to which a Group Company has not filed a particular type of Tax Return or paid a particular type of Tax has asserted that such Group Company is required to file such Tax Return or pay such type of Tax in such taxing jurisdiction;
(h) all deficiencies asserted or assessments made as a result of any examination of the Tax Returns referred to inSection 3.08(b) have been paid in full or otherwise finally resolved;
(i) the charges, accruals and reserves for Taxes with respect to the Group Companies reflected on the books of the Group Companies (excluding any provision for deferred income Taxes) are adequate to cover Tax liabilities accruing through the end of the last period for which the Group Companies have recorded items on their respective books, and since the end of the last period for which the Group Companies have recorded items on their respective books, none of the Group Companies has incurred any Tax liability, engaged in any transaction, or taken any other action, other than in the ordinary course of business;
(j) there are no Tax rulings, requests for rulings, or closing agreements relating to Taxes for which a Group Company may be liable that could affect the Group Company’s liability for Taxes for any taxable period ending after the Closing Date;
(k) no Group Company will be required to include or accelerate the recognition of any item in income, or exclude or defer any deduction or other tax benefit, in each case in any taxable period (or portion thereof)
A-20
after Closing, as a result of any change in method of accounting, closing agreement, intercompany transaction, installment sale, the receipt of any prepaid amount, in each case prior to Closing, or as a result of any election under Section 965(h) of the Code;
(l) no election under Section 336(e) of the Code or the Treasury Regulations thereunder will affect any item of income, gain, loss or deduction of a Group Company after the Closing;
(m) all Tax Sharing Arrangements and Tax indemnity arrangements relating to the Group Companies (other than this Agreement) will terminate prior to the Closing Date and none of the Group Companies will have any Liability thereunder on or after the Closing Date;
(n) there are no liens for Taxes upon the assets of the Group Companies except liens relating to current Taxes not yet due;
(o) all Taxes which the Group Companies are required by law to withhold or to collect for payment have been duly withheld and collected and have been paid to the appropriate Governmental Entity;
(p) no Group Company has been a member of any Company Group other than each Company Group of which it is presently a member, and no Group Company currently has or has had any direct or indirect ownership interest in any corporation, partnership, joint venture or other entity (other than Group Companies);
(q) no Group Company has any liability for Taxes of another Person under Treasury Regulation §1.1502-6 (or any similar provision of state, local or foreign law), under any agreement or arrangement, as a transferee or successor, or by contract or otherwise;
(r) no Group Company has participated in any “listed transaction” within the meaning of Treasury Regulation §1.6011-4(b)(2) and, with respect to each transaction in which the Group Company has participated that is a “reportable transaction” within the meaning of Treasury Regulation §1.6011-4(b)(1), such participation has been properly disclosed on IRS Form 8886 (Reportable Transaction Disclosure Statement) and on any corresponding form required under state, local or other Law;
(s) any powers of attorney granted by the Group Companies prior to the Closing relating to Taxes will terminate and be of no effect following the Closing;
(t) the Company is disregarded as an entity separate from the Member for federal income tax purposes;
(u)Schedule 3.08(u) sets forth the federal income tax classification of each Group Company and discloses whether an election under Treasury Regulation§ 301.7701-3 with respect to the federal income tax classification of such Group Company has been made;
(v) during the last three (3) years, no Group Company has been a party to any transaction treated by the parties thereto as one to which Section 355 of the Code (or any similar provision of state, local or foreign law) applied;
(w) no attributes of a Group Company will be reduced or reattributed pursuant to Treasury Regulation §1.1502-36;
(x) no Group Company is or during the past twelve (12)-month period has been, a United States shareholder (within the meaning of Section 951(b) of the Code) of a controlled foreign corporation (within the meaning of Section 957 of the Code);
(y) no Group Company has or has ever had a permanent establishment in any country other than the country of its organization;
(z) there are no Tax credits, grants or similar amounts that are or could be subject to clawback or recapture as a result of (1) the transactions contemplated by this Agreement or (2) a failure by a Group Company to satisfy one or more requirements on which the credit, grant or similar amount is or was conditioned;
A-21
(aa) no transaction contemplated by this Agreement is subject to withholding under Sections 1445 or 1446 of the Code (or similar provision of state, local or foreign law);
(bb) none of the Member, the Company or any other Group Company has established or has been required to establish a Subpart F income recapture account within the meaning of Treasury Regulation §1.952-1(f) with respect to a Group Company; and
(cc) neither the Member nor any Group Company has taken any action and, to the Knowledge of the Company, there is no fact, agreement, plan or other circumstance that is reasonably likely to prevent or impede (i) the Merger and the Contribution, taken together, from qualifying as a transaction described in Section 351 of the Code or (ii) the Merger from qualifying as a “reorganization” within the meaning of Section 368(a) of the Code.
(a)Schedule 3.09(a) sets forth a correct and complete list of the following Contracts to which any of the Group Companies is a party or bound as of the date hereof, other than those that have terminated or have been fully performed in accordance with their terms or that have no material, continuing rights or obligations thereunder (each, as amended to date, a “Material Contract”):
(i) each lease or agreement under which a Group Company is lessee of, or holds or operates any personal property owned by any other party, for which the annual rental exceeds $250,000, which lease or agreement cannot be cancelled by such Group Company upon thirty (30) days or less notice without penalty to any Group Company;
(ii) each Contract or group of related Contracts that involves future payments, performance or services or delivery of goods or materials to or by any of the Group Companies of any amount or value reasonably expected to exceed $250,000 in the 2018 fiscal year or the 2019 fiscal year, which Contract or group of related Contracts cannot be cancelled by the applicable Group Company upon thirty (30) days or less notice without penalty to any Group Company;
(iii) each Contract requiring or providing for any capital expenditure by the Group Companies in excess of $250,000;
(iv) each joint venture, partnership or strategic alliance with a third party;
(v) each Contract that prohibits any Group Company from competing in any line of business, in any field of use or in any geographic area or that restricts any Group Company’s ability to solicit or hire any person as an employee;
(vi) each Contract with any Affiliate or current or former director, officer, employee or equity holder of any Group Company (other than Contracts relating to any person’s employment with a Group Company, Company Employee Benefit Plans and Contracts solely among Group Companies);
(vii) each Contract under which any Group Company has made advances or loans to another Person, other than with respect to employee advances for business expenses in the Ordinary Course of Business;
(viii) each Contract relating to the incurrence, assumption or guarantee of any Indebtedness;
(ix) each Contract for the sale of products by any Group Company that (A) contains “most favored nation” pricing or similar pricing terms or any exclusive or preferential rights to provide, sell or distribute any product of such Group Company to any Person or any other exclusive provisions running in favor or against any of the Group Companies or (B) contains any terms providing for a special or extended warranty;
(x) each Contract relating to an acquisition, sale, merger or divestiture, by any Group Company, of all or substantially all of the equity interests or assets of any Person or business that contains any ongoing covenants or indemnification obligation by or for the benefit of a Group Company (an “M&A Contract”);
A-22
(xi) each Contract with a Governmental Authority;
(xii) each Contract with a physician owned distributorship;
(xiii) each Contract with a Material Customer or Material Supplier (excluding standard confidentiality agreements and purchase orders);
(xiv) each Contract with any labor union or collective bargaining association representing any employee of a Group Company, including any foreign equivalent thereof; and
(xv) each Real Property Lease.
(b) With respect to each Material Contract, and except as set forth inSchedule 3.09(b), (i) such Material Contract is the legal and valid obligation of the Group Company party thereto, and, to the Knowledge of the Company, of each other party thereto, enforceable against each of the Group Companies party thereto and, to the Knowledge of the Company, each other party thereto, in accordance with its terms, except as enforceability may be limited by bankruptcy, insolvency, reorganization, moratorium or other Legal Requirements relating to or affecting creditors’ rights generally or by equitable principles (regardless of whether enforcement is sought at law or in equity), (ii) such Material Contract is in full force and effect, and the Group Company party thereto has performed all material obligations required to have been performed by it under the Material Contracts and no Company is in breach of, or default under, any Material Contract in any material respect, and to the Knowledge of the Company no other party to any Material Contract is in breach or default thereunder in any material respect, and (iii) no Group Company has received or given a written notice of its intent to terminate, modify, amend or otherwise materially alter the terms and conditions of any Material Contract or has received any written claim of default under any Material Contract. The Company has furnished or made available to Parent true and complete copies of all Material Contracts, including any amendments, waivers or other changes to such Material Contracts.
(a) To the Knowledge of the Company, the Group Companies own, or are licensed or otherwise possess legal rights to all patents, design registrations, patent applications, trademarks, trade names, service marks, trade dress, copyrights, domain names, mask works, schematics, technology,know-how, trade secrets, confidential information, customer lists, technical information, technical data, process technology, plans, drawings and blue prints, inventions, algorithms, devices, systems, processes, computer software programs and applications to the extent the same are used in and are material to the respective businesses of the Group Companies.
(b)Schedule 3.10(b) lists all: (i) pending applications and registrations or issued patents for patents and design rights (“Company Patent Rights”), pending applications and registrations for trademarks and service marks and pending applications and registrations for copyrights owned by the Group Companies (collectively “Scheduled Company Intellectual Property”), including whether each such item of Intellectual Property is solely owned orco-owned and, where applicable, the jurisdictions, both domestic and foreign, in which each such item of Intellectual Property has been issued or registered or in which any application for such issuance and registration has been filed; (ii) written licenses, sublicenses and other agreements as to which any Group Company is a party and pursuant to which any Person is authorized to use any Intellectual Property owned by any Group Company, other than Standard Outbound IP Agreements; (iii) written licenses, sublicenses and other agreements to which any Group Company is a party and pursuant to which such Group Company is authorized to use any third party’s Intellectual Property that is incorporated or used in any product of such Group Company or which is material to its respective operations, and royalties owed under any such agreement, other than Standard Inbound IP Agreements; and (iv) all agreements to which any Group Company is a party that provide for an optional or contingent license, sublicense or other agreement as described in clauses (ii) or (iii) above in this paragraph.
(c) No Group Company is obligated to pay any material royalties and/or fees to any third party with respect to any Intellectual Property license, other than under those licenses listed inSchedule 3.10(c). None
A-23
of the Company Patent Rights were developed under a funding agreement with the Government of the United States of America or with any state governments, pursuant to which the government of the United States of America or any state governments has rights relative thereto.
(d) Except as set forth onSchedule 3.10(d), to the Knowledge of the Company, no Group Company has received (i) any notice from, or demand or claim by, any third party that any of the Scheduled Company Intellectual Property, including any Company Patent Rights, are not solely owned by the Group Companies, except as identified inSchedule 3.10(d), or that the Company Patent Rights are subject to a compulsory license, or (ii) any notice challenging the validity or enforceability of any of the Company Patent Rights.
(e) To the Knowledge of the Company, no allowable or allowed subject matter of the Company Patent Rights listed inSchedule 3.10(b) as being solely owned is subject to any competing or interfering claims by any third party. Except as set forth onSchedule 3.10(e), no allowable or allowed subject matter of such Company Patent Rights has been the subject of any interference,re-examination or opposition proceedings.
(f) To the Knowledge of the Company, except as set forth onSchedule 3.10(f), each of the Group Companies has taken all action necessary to maintain the enforceability and registration of all Scheduled Company Intellectual Property material to the operation of the respective businesses of the Group Companies. Except as set forth onSchedule 3.10(f), all annuities, maintenance fees or other fees necessary to maintain the pendency or right to assert the Company Patent Rights due on or before the date hereof have been paid according to proper entity status in the relevant jurisdiction. The registrations to Company Patent Rights are in good standing and none of the Company Patent Rights have lapsed, been disclaimed or been dedicated to the public other than as indicated inSchedule 3.10(f).
(g) Each of the Group Companies have taken commercially reasonable measures to maintain and protect the secrecy, confidentiality and value of the Trade Secrets of such Group Company. To the Knowledge of the Company, no unauthorized disclosure of any such Trade Secret has been made.
(h) Except as set forth inSchedule 3.10(h), to the Knowledge of the Company, within the three (3) years preceding the date of this Agreement, no Group Company has received any written invitation to license or written charge, complaint, claim, demand, or notice that any Group Company has infringed, misappropriated, or acted in conflict with any of the Intellectual Property owned by any third party. There is no actual, pending or, to the Knowledge of the Company, threatened litigation relating to any Intellectual Property that, individually or in the aggregate, has resulted in a Material Adverse Effect, except as set forth inSchedule 3.10(h). To the Knowledge of the Company, no Group Company is in violation or infringement of any Intellectual Property of any third party.
(i) Except as disclosed onSchedule 3.10(i), to the Knowledge of the Company, there is no unauthorized use, disclosure, infringement or misappropriation of any Scheduled Company Intellectual Property, or any third party Intellectual Property rights to the extent licensed to any Group Company, by any third party. Except as disclosed onSchedule 3.10(i), no Group Company has, in the three (3) years preceding the date of this Agreement, sent to any third Person any written charge, complaint, claim, demand or notice asserting that such Person has infringed, misappropriated, or acted in conflict with any of the Intellectual Property owned by such Group Company.
(j) Subject to any necessary notices and consents, to the Knowledge of the Company, the execution and delivery of this Agreement and the consummation of the transactions contemplated hereby and thereby will not result in the forfeiture, cancellation, termination or other material impairment, or breach of, or give rise to any right of any Person to cancel, terminate or otherwise impair the right of the Group Companies to own or use or otherwise exercise any other rights that the Group Companies currently have with respect to any Scheduled Company Intellectual Property that is, individually or in the aggregate, material to the Group Companies, except as identified inSchedule 3.10(j).
(k) Each Group Company has entered into written confidentiality agreements with all employees and third parties to whom such Group Company has disclosed material confidential Intellectual Property, except where a failure to do so would not have a Material Adverse Effect.
A-24
3.11Legal Proceedings. Except as set forth inSchedule 3.11, as of the date hereof, there are no, and since January 1, 2016 there have not been any, Legal Proceedings pending, nor, to the Knowledge of the Company, is there any Legal Proceeding threatened against any of the Group Companies (excluding any negotiations with Contract counterparties in the ordinary course of business).
3.12Orders. Except as set forth inSchedule 3.12, there is no judgment, order, writ, injunction, decree or other similar award outstanding (whether rendered by a court, administrative agency or other Governmental Entity, or by arbitration) against any Group Company or by which any Group Company is bound that involves a material unsatisfied monetary obligation or otherwise materially affects the ongoing business or any material assets or properties of any Group Company, and no Group Company is in breach of any such judgment, order, writ, injunction, decree or similar award in any material respect;provided that the representation in this sentence is not intended to cover Permits (which are covered inSection 3.15).
(a)Schedule 3.13(a) sets forth an accurate, current, and complete list of all Company Employee Benefit Plans.
(b) There has been made available to Parent, with respect to each Company Employee Benefit Plan in effect as of the date hereof, the following (in each case, as applicable): (i) a copy of the current plan document for each Company Employee Benefit Plan (or in the case of an unwritten Company Employee Benefit Plan, a written description thereof) and related agreements (including trusts, insurance contracts and policies) and all amendments thereto; (ii) a copy of each current annual report (Form 5500 series) and actuarial report, with respect to each such plan (including all schedules and attachments); (iii) a copy of each current summary plan description, together with each summary of material modification required under ERISA with respect to such plan; (iv) with respect to each such plan that is intended to be qualified under Section 401(a) of the Code, a copy of the most recent favorable opinion, advisory, or determination letter issued by the Internal Revenue Service with respect to the qualified status of such plan; and (v) all correspondence to and from any Governmental Entity relating to each such plan during the prior six (6) years.
(c) Each Company Employee Benefit Plan has been maintained, operated and administered in all material respects in accordance with its terms and any related documents or agreements, and all applicable Legal Requirements, including ERISA and the Code. Each Company Employee Benefit Plan intended to be qualified under Section 401(a) of the Code is so qualified, and nothing has occurred that could reasonably be expected to adversely affect the qualified status of any such Company Employee Benefit Plan. Except as may be required by applicable Legal Requirements, each Company Employee Benefit Plan can be amended, terminated, or otherwise discontinued, in each case without Liability to any Group Company, other than normal administrative costs. No fiduciary (within the meaning of Section 3(21) of ERISA) has committed a breach of fiduciary duty that could subject any Group Company to any material Liability (including on account of an indemnification obligation). No Group Company nor any ERISA Affiliate thereof has any Liability (i) on account of any violation of the health care requirements of Part 6 or 7 of Title I of ERISA or Section 4980B or 4980D of the Code or the Affordable Care Act or (ii) under Section 502(i), 502(l) or Section 4975 of the Code. No Group Company has incurred any material excise Taxes under Chapter 43 of the Code with respect to any Company Employee Benefit Plan and nothing has occurred with respect to any Company Employee Benefit Plan that could reasonably be expected to subject a Group Company to any such Tax.
(d) Except as would not reasonably be expected to adversely affect the Group Companies in any material respect, all contributions and payments and obligations (including premiums, fees and expenses) in respect of a Company Employee Benefit Plan that are due and required under the terms of such Company Employee Benefit Plan, Contract, or applicable Legal Requirements have been fully and timely made and all contributions and payments or other obligations that have accrued but are not yet due have either been made or have been reflected in full on the Company Financial Statements.
A-25
(e) No Company Employee Benefit Plan is subject to Title IV of ERISA, Section 302 of ERISA or Section 412 of the Code. None of the Group Companies nor any ERISA Affiliate of any Group Company sponsor, maintain, contribute to or have any Liability (contingent or current) with respect to a plan subject to Title IV of ERISA, Section 302 of ERISA or Section 412 of the Code (or done so or had any such Liability at any time within the six (6) years prior to the Closing Date). None of the Group Companies nor any ERISA Affiliate of any Group Company contributes to or has an obligation to contribute to, nor at any time within the six (6) years prior to the Closing Date contributed to, had an obligation to contribute to, or was a participating employer in any “multiemployer plan” as defined in Section 3(37) of ERISA or Section 414(f) of the Code or a “multiple employer plan” within the meaning of Section 210(a) of ERISA or Section 413(c) of the Code.
(f) No Company Employee Benefit Plan provides welfare benefits (including retiree life insurance, retiree health benefits, and other retiree employee welfare benefits) to any Person for any reason after such Person terminates employment with a Group Company, other than as required by Section 4980B of the Code. No Group Company has any Liability to provide, or ever represented, promised, or contracted (whether in oral or written form) to any Person that such Person would be provided with, post-termination welfare benefits, other than as required by Section 4980B of the Code. No Company Employee Benefit Plan provides, or has any obligation to provide, welfare benefits to any Person who is not a current or former employee of a Group Company, or a beneficiary thereof. No Company Employee Benefit Plan is, and no Group Company has any Liability with respect to, a “multiple employer welfare arrangement” (as defined in Section 3(40)(A) of ERISA).
(g) There are no claims or Legal Proceedings pending (other than routine claims for benefits) or, to the Knowledge of the Company, threatened, against any of the Company Employee Benefit Plans, assets of any of the Company Employee Benefit Plans, or any administrator or fiduciary of any Company Employee Benefit Plan with respect to the operation of such Company Employee Benefit Plan, and, to the Knowledge of the Company, there are no facts or circumstances that would be reasonably likely to form the basis for any such claims or Legal Proceedings.
(h) Each Company Employee Benefit Plan that is subject to Section 409A of the Code has been administered, operated and maintained in all material respects according to the requirements of Section 409A of the Code, and no Group Company has been required to withhold or pay any Taxes as a result of a failure to comply with Section 409A of the Code. No Group Company has any obligation to make a“gross-up” or similar payment in respect of any Taxes that may become payable under Section 409A of the Code.
(i) Except as set forth inSchedule 3.13(i), neither the execution, delivery, nor performance of this Agreement nor the consummation of the Contribution or any other transaction contemplated by this Agreement will or can reasonably be expected to (either alone or upon the occurrence of any additional or subsequent events) (i) entitle any current or former employee, officer, director, or consultant or independent contractor of any Group Company, as such, to any payment (including severance pay or similar compensation), cancellation of Indebtedness, or increase in compensation; (ii) result in the acceleration of the time of payment, funding, or vesting under any Company Employee Benefit Plan; or (iii) result in any increase in benefits payable under any Company Employee Benefit Plan. No amount paid or payable (whether in cash, property, or the form of benefits) in connection with the Contribution or any other transaction contemplated by this Agreement to any employee, officer or director of any of the Group Companies or any of their respective Affiliates who is a “disqualified individual” (within the meaning of Section 280G of the Code) will or may (either alone or upon the occurrence of any additional or subsequent events) be an “excess parachute payment” (within the meaning of Section 280G of the Code), or would constitute an “excess parachute payment” if such amount was subject to the provisions of Section 280G of the Code. No Group Company has any obligation to make a“gross-up” or similar payment with respect to any Taxes that may become payable under Section 4999 of the Code.
3.14Insurance.Schedule 3.14 sets forth a list of all policies of insurance maintained by, or for the benefit of, each Group Company (specifying the insurer and type of insurance) and also lists each insurance claim (other
A-26
than a claim that resulted in coverage of less than $50,000) made by a Group Company since January 1, 2016 (including with respect to insurance obtained but not currently maintained). Except as set forth inSchedule 3.14, all insurance coverage maintained with respect to the Group Companies is occurrence-based. With respect to each insurance policy listed inSchedule 3.14, no Group Company or, to the Knowledge of the Company, insurer, is in breach or default (including with respect to the payment of premiums or the giving of notices), under such policy in any material respect. All such policies are in full force and effect (except for policies that have expired under their terms in the ordinary course and have been replaced by insurance policies with substantially similar coverage) and no written or, to the Knowledge of the Company, oral, notice of early cancellation or early termination has been received by any Group Company with respect to any such policy.
3.15Legal Requirements and Permits.
(a) Each of the Group Companies is, and since January 1, 2016 has been, in compliance in all material respects with all applicable Legal Requirements and Orders, including all Health Care Laws. No Group Company has received written or, to the Knowledge of the Company, oral, notice that it is, and to the Knowledge of the Company no Group Company is, under investigation by any Governmental Entity with respect to any alleged material violation of any applicable Legal Requirements. To the Knowledge of the Company, since January 1, 2016, no Group Company has received any subpoena, written demand, inquiry, information request, complaint, allegation or notice ofnon-compliance with or violation of any Legal Requirements, including any Health Care Laws, in any material respect.
(b) The Group Companies have been granted all licenses, permits, Device Permits, consents, approvals, franchises and other authorizations under any Legal Requirement (each a “Permit”) necessary for and material to the conduct of the business as conducted as of the date hereof, taken as a whole (collectively, the “Material Permits”). The Material Permits are valid and in full force and effect. Each Group Company is in compliance in all material respects with all of its Material Permits. There is no lawsuit or similar proceeding pending or, to the Knowledge of the Company, threatened, to revoke, suspend, withdraw or terminate any Material Permit.
(a) Each of the Group Companies is, and at all times since January 1, 2013 has been, in compliance in all material respects with all applicable Environmental Laws.
(b) Each of the Group Companies holds and is, and at all times since January 1, 2013 has been, in compliance in all material respects with all Environmental Permits required to operate at the Company Real Property and to carry on their respective businesses as now conducted, all such Environmental Permits are in full force and effect, and there are no Legal Proceedings pending or, to the Knowledge of the Company, threatened, that seek the revocation, cancellation, suspension or adverse modification of any such Environmental Permit.
(c) As of the date hereof, there is no material Environmental Claim pending or, to the Knowledge of the Company, threatened against any of the Group Companies.
(d) There has been no Release of, or exposure to, any Hazardous Materials at any Company Real Property or any Former Real Property or, to the Knowledge of the Company, at any third Person site to which Hazardous Materials generated by any Group Company were sent for treatment, recycling, storage or disposal.
(e) No Group Company has entered into or is subject to any Order relating to compliance with, or the Release or cleanup of Hazardous Materials under, any applicable Environmental Laws.
(f) Except as set forth inSchedule 3.16, none of the Group Companies has assumed or provided indemnity against any material Liability of any other Person under any Environmental Laws.
(g) The Member has made available to Parent complete and correct copies of all environmental site assessments, compliance audits, notices of violation, consent orders, and other material environmental
A-27
reports possessed or under the control of a Group Company and that relate to any Group Company’s environmental compliance, the environmental condition of any Company Real Property or Former Real Property, the investigation of any Hazardous Materials under applicable Environmental Laws or to comply with any Environmental Law.
3.17Relationships with Related Persons. Except as set forth inSchedule 3.17, the Group Companies are not parties to any Contracts with any Affiliate, shareholder, employee, officer or director of any Group Company other than Contracts governing an individual’s provision of services to the Group Companies and employee benefits and other than Contracts solely among Group Companies. No Group Company has loaned any amounts that remain outstanding to any director, officer, shareholder, member, manager or employee of any Group Company, and no Group Company has borrowed funds from any of the foregoing that remains outstanding. There are no loans, advances or Indebtedness incurred by any Group Company from any director, officer, shareholder, member, manager, employee or Affiliate of any Group Company. Except as set forth onSchedule 3.17, no Affiliate (other than a Group Company) of a Group Company, (i) owns any material property right, tangible or intangible, which is used by a Group Company in the conduct of its business or (ii) owns, directly or, to the Knowledge of the Company, indirectly, any Person that is a material customer, supplier, competitor or lessor of any Group Company.
3.18Employees; Employment Matters and Independent Contractors.
(a) None of the Group Companies domiciled in the United States is bound by or subject to any collective bargaining agreement or other Contract with any labor union. To the Knowledge of the Company, except as set forth inSchedule 3.18(a), as of the date hereof, no labor union has requested or has sought to represent any of the employees of the Group Companies in the United States. As of the date hereof and within the twelve (12) months prior to the date hereof, there is no, nor has there been any strike, lockout, work stoppage, slowdown, picketing or other labor dispute involving the employees of the Group Companies in the United States pending or, to the Knowledge of the Company, threatened against any Group Company. No Group Company has engaged in any plant closing or employee layoff activities since the Latest Balance Sheet Date that would violate or require notice under the Worker Adjustment and Retraining Notification Act of 1988, as amended, or any similar state or local plant closing or mass layoff statute, rule or regulation, and no Group Company has any outstanding Liability under any such Law.
(b) Except as set forth inSchedule 3.18(b), each Group Company is in compliance in all material respects with all applicable Laws respecting labor, employment, fair employment practices (including equal employment opportunity laws), terms and conditions of employment, classification of employees (as exempt ornon-exempt for overtime purposes), workers’ compensation, occupational safety and health, immigration, affirmative action, employee and data privacy, plant closings, and wages and hours. Since January 1, 2016, each Group Company has properly completed and retained a FormI-9 with respect to each of its current and past employees in the United States and has, in good faith, verified and fully recorded on the FormI-9 the information for the documents establishing identity and work authorization for each of its U.S. employees and has provided to Parent complete and accurate copies of all such requested FormI-9s, together with copies of the U.S. employees’ supporting documentation evidencing that such employees have valid work authorization to be employed by the Group Companies. All material payments due from any Group Company on account of wages have been paid or properly accrued as a liability on the books of such Group Company. Except as set forth onSchedule 3.18(b), there is no pending or, to the Knowledge of the Company, threatened charge, complaint, arbitration, audit, investigation or other action brought by or on behalf of, or otherwise involving, any current or former employee, any person alleged to be a current or former employee, any applicant for employment, or any class of the foregoing, or any Governmental Entity, that involves the labor or employment relations and practices of any Group Company that could reasonably be expected to result, individually or in the aggregate, in any material Liability to any Group Company.
(c) To the Knowledge of the Company, no senior executive or other key employee of any Group Company is party to any confidentiality,non-competition,non-solicitation, proprietary rights or other such
A-28
agreement that would materially restrict the performance of such Person’s employment duties with any Group Company or the ability of any Group Company to conduct its business.
(d) Except as set forth inSchedule 3.18(d), no collective labor agreement, works council or employee representative bodies or similar agreement is applicable to the Group Companies domiciled outside the United States or any of their employees, and there are no agreements with any trade union, staff association or staff works council or other organization of employees or workers. As of the date hereof and within the twelve (12) months prior to the date hereof, there is not, nor has there been, any material labor dispute involving the employees of the Group Companies outside the United States pending or, to the Knowledge of the Company, threatened against any Group Company.
(e) The Group Companies have complied with all material information reporting and backup withholding requirements, including the maintenance of required records with respect thereto, in connection with amounts paid to any employee, independent contractor, creditor or other third party.
(f) Except as would not reasonably be expected to result in any material Liability to the Group Companies, the Group Companies have correctly classified those individuals performing services as common law employees, leased employees, independent contractors or agents.
(g) To the Knowledge of the Company, no Group Company has any material Liability under any applicable Law, including under or on account of any Company Employee Benefit Plan, arising out of the misclassification of any person as a consultant, independent contractor or temporary employee, as applicable, and no such person is entitled to any compensation or benefits in any amount from any Group Company under any applicable Law or Company Employee Benefit Plan that he or she has not received and that has not been properly accrued as a Liability on the books of such Group Company.
3.19Material Customers and Suppliers.Schedule 3.19 sets forth a true and complete list of (a) the 20 largest customers of the Group Companies on a consolidated basis (based on aggregate gross revenues) (each, a “Material Customer”) and (b) the 20 largest suppliers of the Group Companies on a consolidated basis (based on dollar volume of purchases from such suppliers) (each, a “Material Supplier”), in each case, for the twelve (12)-month period ended December 31, 2017. To the Knowledge of the Company, there exists no condition or event that, after notice or passage of time or both, would constitute a default by any party to any Material Contract with a Material Customer or Material Supplier. Since January 1, 2017, no Material Customer has notified any Group Company in writing of any complaint concerning the products and services provided to such Material Customer (excluding any negotiations with such Material Customers in the ordinary course of business) and no Material Customer or Material Supplier has notified any Group Company in writing that it intends to terminate, discontinue or materially and adversely change its relationship with any Group Company.
3.20Brokers’ Fees. Except as set forth inSchedule 3.20, no Group Company is liable for any investment banking fee, finder’s fee, brokerage payment or other like payment in connection with the origination, negotiation or consummation of the transactions contemplated herein that will be the obligation of Parent or any of the Group Companies (following the Closing).
3.21Compliance with Anti-Corruption Laws; Absence of Certain Payments.
(a) To the Knowledge of the Company, no employee, agent, or representative of the Group Companies has at any time since January 1, 2013: (i) violated or engaged in any activity, practice or conduct which would violate the Foreign Corrupt Practices Act of 1977, as amended (the “FCPA”), or any other applicable anti-bribery or anti-corruption Law; (ii) used corporate funds or assets for any unlawful contribution, gift, entertainment or other unlawful expense, or made any bribe, rebate, payoff, influence payment, kickback or other unlawful payment of funds or received or retained any funds in violation of any applicable Law; or (iii) directly or indirectly, made, offered, promised or authorized any payment or gift of any money or anything of value to or for the benefit of any “foreign official” (as such term is defined in the FCPA), foreign political party or official thereof or candidate for foreign political office for the purpose of
A-29
(A) influencing any official act or decision of such official, party or candidate, (B) inducing such official, party or candidate to use his, her or its influence to affect any act or decision of a foreign governmental authority, or (C) securing any improper advantage, in the case of (A), (B) and (C) above in order to assist the Group Companies in obtaining or retaining business for or with, or directing business to, any Person.
(b) The Group Companies have maintained systems of internal controls (including accounting systems, purchasing systems and billing systems) to ensure compliance with the FCPA or any other applicable anti-bribery or anti-corruption Law.
(c) To the Knowledge of the Company, no employee, agent, or representative of the Group Companies is the subject of any allegation, voluntary disclosure, investigation, prosecution or other enforcement action related to the FCPA or any other anti-corruption Law.
(d) The Group Companies have since January 1, 2013 complied with all Laws relating to export control and trade sanctions or embargoes.
3.22Device Regulatory Matters.
(a) Except as set forth onSchedule 3.22(a), each of the Group Companies, is, and since January 1, 2016, has been, in compliance, in all material respects, with all applicable Device Regulatory Laws. Except as set forth inSchedule 3.22(a), each of the Group Companies has not, at any time since January 1, 2016, received written notice of any pending action, suit, proceeding, hearing, investigation, claim, demand or notice relating to its business alleging any failure to so comply with applicable Device Regulatory Law. Except as set forth onSchedule 3.22(a), the Group Companies have not received, since January 1, 2016, any written notice of inspectional observations or adverse findings, establishment inspection reports, “warning letters,” “untitled letters,” recalls, field notifications, seizure or similar correspondence or notice from any Governmental Entity alleging or asserting materialnon-compliance with any applicable Device Regulatory Laws or Device Permits, and there is no such action or proceeding pending or, to the Knowledge of the Company, threatened.
(b) All manufacturing operations for the Group Companies conducted by or on behalf of the Group Companies have, at all times since January 1, 2016, been conducted in compliance in all material respects with the Quality System Regulation (21 CFR Part 820) and equivalent requirements applicable in the European Union pursuant to the Device Regulatory Laws. No Group Company’s product that was manufactured or sold since January 1, 2016, is or was adulterated within the meaning of 21 U.S.C. § 351, misbranded within the meaning of 21 U.S.C. § 352, seized, detained, subject to a suspension of manufacturing, distribution, or marketing, was deemed to compromise the health and/or safety of patients, users or, where applicable, other persons within the meaning of Directive 90/385/EEC, Directive 93/42/EEC or Directive 98/79/EC or has been recalled, is subject to a recall, or is subject to imminent plans to issue a recall and to the Knowledge of the Company, there are no facts or circumstances that would be reasonably likely to cause: (i) any such product to become adulterated or misbranded; (ii) the seizure, detention, or suspension of manufacturing, distribution, or marketing of any such product; (iii) a change in the labeling or classification of any such product; (iv) the termination, seizure or suspension of marketing of any such product; or (v) a recall of any such product.
(c) (i) The Group Companies, have, at all times since January 1, 2016, obtained, possessed, and maintained in good standing all material Device Permits from any Governmental Entity necessary for their businesses or operations, (ii) none of the Group Companies is in material violation of or default under any such Device Permits, and (iii) no such Device Permit will be revoked, terminated prior to its normal expiration date or not renewed solely as a result of the consummation of the transactions contemplated by this Agreement. The Group Companies have at all times since January 1, 2016, held and been in material compliance with all Device Permits that are required for the conduct and operation of their businesses and have made all required filings with, or notifications to, all Governmental Entities pursuant to the Device Regulatory Laws. To the Knowledge of the Company, each third party that is a supplier or contractor for the Group Companies holds and is in compliance in all material respects with all Device Permits required by
A-30
Device Regulatory Laws insofar as they reasonably pertain to the products or services the third party provides to the Group Companies in connection with its business. There is no materially false or misleading information or significant omission in any product application or other submission or statement related to any Device Permits submitted by the Group Companies to any Governmental Entity administering Device Regulatory Laws in connection with, or in the conduct or operation of their businesses. Neither the Group Companies nor, to the Knowledge of the Company, any of their employees or agents, have committed any act, made any statement or failed to make any statement that would reasonably be expected to provide a basis for the U.S. Food and Drug Administration to invoke its policy with respect to “Fraud, Untrue Statements of Material Facts, Bribery and Illegal Gratuities” set forth in 56 Fed. Reg. 46191 (September 10, 1991) and any amendments thereto.Schedule 3.22(c) sets forth a true and complete list of each material Device Permit necessary for the conduct of the Group Companies’ businesses as conducted on the date hereof.
(d) Except as would not reasonably be expected to adversely affect the Group Companies in any material respect, (i) the Group Companies have not, with respect to the Group Companies’ businesses, received written notice of any demand, claim, action, suit, inquiry, hearing, proceeding, notice of violation or investigation from, by or before any Governmental Entity or other Person relating to any product of the businesses, or claim or lawsuit involving a product of the Group Companies’ businesses which is currently pending or, to Knowledge of the Company, threatened, by any Person, (ii) no product recall or post-sale warning is ongoing or under consideration by the Group Companies with respect to any of their products, and (iii) all products of the Group Companies comply with applicable Laws, industry standards and warranties and other specifications provided to customers and other third parties. Since January 1, 2016, except as set forth inSchedule 3.22(d), no customer or other Person has made a material claim relating to the repair, rework, replacement or return of, or any claim for breach of warranty in respect of, or refund of the purchase price of, any product of the Group Companies’ businesses.
3.23Healthcare Regulatory Matters.
(a) No Group Company, nor, any of their respective officers, directors, or employees, nor to the Knowledge of the Company, any of the their respective Affiliates or any third parties, in each case, acting on behalf of a Group Company, has (i) knowingly presented or caused to be presented a claim for reimbursement for services to any state or federal Governmental Entity, including any Federal Health Care Program, that is for an item or service that is known or should be known to be: (A) not provided as claimed; (B) not provided in accordance with applicable Health Care Laws; or (C) false or fraudulent; (ii) knowingly offered, paid, solicited, or received any remuneration (including any kickback, bribe, rebate, or fee), overtly or covertly, in cash or in kind: (A) in return for referring any individual to a Person for the furnishing or arranging for the furnishing of any item or service for which payment may be made in whole or in part by a Federal Health Care Program or (B) to secure any improper advantage or to obtain or retain business that would cause the Group Company to be in violation of any Health Care Laws, including the federal Anti-Kickback Statute (42 U.S.C. §1320a-7b); (iii) otherwise given, received, offered to pay to or solicited any remuneration from, in cash or kind, directly or indirectly, any past or present patient, customer, physician, other healthcare provider, supplier, vendor, contractor, Federal Health Care Program, other government program, or other Person in violation of any Health Care Laws, including the federal Anti-Kickback Statute (42 U.S.C. §1320a-7b); or (iv) knowingly made or caused to be made or induced or sought to induce the making of any false statement or representation (or omitted to state a material fact required to be stated therein) in order that any past or present patient, customer, physician, other healthcare provider, supplier, vendor, or contractor may receive reimbursement from a Federal Health Care Program or government program or in order that a Group Company may collect reimbursement from a Governmental Entity or Federal Health Care Program.
(b) The reimbursement support provided through Coflex Connect and any other similar programs, including insurance coverage benefits investigations, coding advice, prior authorization and appeals support provided by each of the Group Companies has complied and currently complies with all Health Care Laws.
A-31
(c) No Group Company, nor, any of their respective officers, directors, or employees, nor to the Knowledge of the Company, any of the their respective Affiliates or any third parties, in each case, acting on behalf of a Group Company, is or in the last five (5) years has been: (i) under or subject to any pending or threatened investigation, subpoena, civil investigative demand, inquiry, corporate integrity agreement, deferred ornon-prosecution agreement, or similar instrument or obligation, or request for documents or information by any Governmental Entity (including the Department of Justice, the U.S. Department of Health and Human Services (“HHS”), the HHS Office of Inspector General (“OIG”), the Centers for Medicare and Medicaid Services, any other HHS agency, any state Medicaid agency, or any state Attorney General); (ii) excluded, suspended or debarred by OIG or the General Services Administration (“GSA”) from participating in any federal healthcare program (as such term is defined at 42 U.S.C. §1320a-7b(f) and its implementing regulations); (iii) debarred under 21 U.S.C. § 335a or excluded pursuant to 42 U.S.C.§ 1320a-7; (iv) convicted of any crime or engaged in any conduct that would reasonably be expected to result in debarment under 21 U.S.C. § 335a or exclusion pursuant to 42 U.S.C.§ 1320a-7; (v) a party to an agreement or settlement with any Governmental Entity with regard to any allegednon-compliance with, or violation of, any Law, or (vi) the subject or target of U.S. or other economic sanctions or export restrictions.
3.24Title, Condition and Sufficiency of Assets.
(a) Except as set forth onSchedule 3.24(a) or as would not reasonably be expected to adversely affect the Group Companies in any material respect, the Company or one of its Subsidiaries owns good title to, or holds pursuant to valid and enforceable leases, all of the items of tangible, personal property shown to be owned or leased by it on the Latest Balance Sheet, free and clear of all Encumbrances, except for Permitted Liens, except for items that have been sold or disposed of subsequent to the date hereof in the Ordinary Course of Business consistent with past practices.
(b) The buildings, plants, structures, and equipment owned or leased by the Group Companies are in all material respects in good operating condition and repair, and adequate for the uses to which they are being put, and, except as described onSchedule 3.24(b), to the Knowledge of the Company, none of such buildings, plants, structures, or equipment is in need of maintenance or repairs other than ordinary, routine maintenance conducted in the Ordinary Course of Business that is not material in nature or cost, individually or in the aggregate.
(c) Except as reflected in the Company Financial Statements or as set forth inSchedule 3.24(c), the assets and properties of the Group Companies constitute in all respects all of the assets and properties reasonably necessary to operate the business as conducted by the Group Companies on the date hereof, other than assets that, individually or in the aggregate, are not material to the business.
3.25Data Protection and Privacy.
(a) Each Group Company has, since January 1, 2016: (i) complied in all material respects with applicable Company Privacy Policies and Data Protection Laws, including through adopting all appropriate technical and organizational security measures to protect Personal Data against a Data Breach; (ii) except as would not reasonably be expected to adversely affect the Group Companies in any material respect, (A) obtained and maintained all registrations and notifications required under applicable Data Protection Laws, and (B) any processing of personal data by or on its behalf has been in accordance with such registrations and notifications; (iii) duly provided data subjects with relevant information notices and acquired any necessary consent of data subjects to the processing of their data, as required under applicable Data Protection Laws, and any processing of Personal Data by or on its behalf has been in accordance with such notices and consents; and (iv) in place written agreements with any third party which it has authorized to have access to Personal Data, including processors, to ensure that the third party respects and maintains the confidentiality and security of the Personal Data and complies at all times with applicable Data Protection Laws and such written agreements with such processors include processing provisions as required under Data Protection Laws.
A-32
(b) As at the date hereof: (i) each Group Company is in compliance in all material respects with the terms of all Contracts to which it is a party relating to data privacy, security, or breach notification (including provisions that impose conditions or restrictions on the collection, use, storage, transfer or disposal of Personal Data); (ii) no Group Company has received a written notice (including any enforcement notice), letter or complaint from a supervisory authority or any data subject alleging breach by it of any Data Protection Laws nor has it been involved in any litigation with respect to its processing of Personal Data; (iii) no data subject has been awarded compensation by a supervisory authority or by a court of law from any Group Company under any Data Protection Laws; (iv) no written request has been made by a data subject to, or order has been made by a supervisory authority or a court of law against, any Group Company for access to, the rectification, restriction, blocking, erasure or destruction of any Personal Data under any Data Protection Laws; and (v) no Group Company has transferred Personal Data outside of the European Economic Area other than in compliance with Data Protection Laws in all material respects.
(c) Following the Closing Date, Holdco and the Group Companies will be entitled to process the Personal Data comprised in the information of the Group Companies created prior to the Closing Date that are in the control or possession of the Holdco and/or the Group Companies after the Closing Date in the manner in which that Personal Data was processed by the Group Companies in the course of business in the three (3) years immediately prior to the Closing Date, including adaptations made due to the introduction ofEU-Regulation 2016/679 (General Data Protection Regulation) in May 2018, in compliance with Data Protection Laws.
(d) Neither the execution, delivery or performance of this Agreement or any of the other agreements contemplated by this Agreement, nor the consummation of any of the transactions contemplated by this Agreement or any such other agreements, nor the Member’s provision to Parent, Holdco and Merger Sub, or Parent’s, Holdco’s or Merger Sub’s possession or use of, Personal Data or any other data or information in the databases of the Group Companies, will result in any violation of the Member’s or any Group Company’s Privacy Policy, contract, or any Data Protection Laws or other Laws pertaining to privacy, Personal Data, data security, or spyware.
(e) Each Group Company has established and is in material compliance with an information security program that: (i) includes administrative, technical and physical safeguards designed to safeguard the security, confidentiality, and integrity of the Information Technology Systems and the data hosted therein; (ii) is designed to protect against unauthorized access to the Information Technology Systems and the systems of any third party service providers that have access to the Information Technology Systems and the data hosted therein; and (iii) complies with applicable Data Protection Laws in all material respects.
(f) Since January 1, 2016, no Group Company has suffered a Data Breach with respect to the Information Technology Systems and no breach or violation of any security program described above has occurred or is threatened, and, to the Knowledge of the Company, there has been no unauthorized or illegal use of or access to the Information Technology Systems and the data hosted therein. No third party service provider working on behalf of a Group Company has notified any Group Company that the third party service provider has had, and, to the Knowledge of the Company, no third party service provider working on behalf of a Group Company has had, a Data Breach with respect to any data collected or used in connection with the operation of the business. No Group Company has notified, nor been required to notify, any data subject or supervisory authority of any Data Breach.
For purposes of thisSection 3.25, the words “data subject”, “processing” and “processor” shall have the meaning given to them under Data Protection Laws.
3.26Warranty. Except as set forth onSchedule 3.26, each product that has been sold or put into trade inventory by the Group Companies since January 1, 2016, conformed and complied in all material respects with all express and implied warranties, except where the failure to conform and comply with such warranties would not reasonably be expected to exceed the reserve for warranty claims set forth on the Latest Balance Sheet. No products manufactured and/or sold by the Group Companies since January 1, 2016, have been the subject of any
A-33
recall by the Group Companies or any Governmental Entity or other similar action.Schedule 3.26 provides a true, accurate and complete list of the standard terms and conditions for each of the products of the Group Companies, complete and correct copies of which have been made available to Parent. Except as set forth inSchedule 3.26, no product manufactured, sold or delivered by the Group Companies is subject to any guarantee, warranty, or other indemnity, express or implied, beyond the listed standard terms and conditions. No Liability exists for any return claim, warranty claim or other obligation to provide parts and service on, or to repair or replace, any finished products sold or delivered by the Group Companies in connection with the operation of their business at any time on or prior to the Closing Date beyond the amounts reserved for warranty expense reflected in the Latest Balance Sheet or expected to be reflected in the consolidated balance sheet of the Group Companies as of the Closing Date.
3.27Company Information. None of the information supplied or to be supplied by the Member or any of the Group Companies or any of their respective Affiliates expressly for inclusion in filings with the SEC, including the Parent SEC Reports, mailings to Parent’s stockholders or the Unitholders with respect to the transactions contemplated by this Agreement, any supplements thereto and/or in any other document filed with any Governmental Entity in connection herewith (including the Joint Proxy and Consent Solicitation Statement/Prospectus and the Registration Statement), will, at the date of filing and/or mailing, as the case may be, or, with respect to the Joint Proxy and Consent Solicitation Statement/Prospectus and the Registration Statement, at the time of any meeting of Parent’s stockholders to be held in connection with the Merger, including the Parent Stockholder Meeting, contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they are made, not misleading (subject to the qualifications and limitations set forth in the materials provided by the Member or any Group Company or that are included in such filings and/or mailings). No representation or warranty is made by the Member or any of its respective Affiliates with respect to statements made or incorporated by reference in any of the foregoing documents based on information supplied or to be supplied by, or on behalf of, Parent or any of its Affiliates.
3.28Vote Required. The approval of the Unitholders holdingtwo-thirds (2/3) of the outstanding Member Units, other than Class C Preferred Member Units, Class D Preferred Member Units andClass E-2 Preferred Member Units, given in writing or by vote at a meeting, consenting or voting as a single class, is the only approval of the Unitholders required under the Delaware Limited Liability Company Act (the “DLLCA”) and the Member’s Governing Documents to approve this Agreement and the transactions contemplated hereby.
3.29No Other Representations or Warranties. Except for the representations and warranties of Parent, Holdco and Merger Sub set forth in this Agreement or in a certificate delivered pursuant to this Agreement, the Member hereby acknowledges and agrees that neither Parent nor any of its Subsidiaries, nor any of their respective Representatives, has made or is making any other express or implied representation or warranty with respect to Parent, any of its Subsidiaries or their respective businesses or operations, including with respect to any information provided or made available to the Member or the Company.
REPRESENTATIONS AND WARRANTIES OF PARENT, HOLDCO AND THE MERGER SUB
Except as disclosed in the Parent SEC Reports filed with or furnished to the SEC after January 1, 2017 and prior to the date of this Agreement (other than any information that is contained solely in the “Risk Factors” section of such Parent SEC Reports and any forward-looking statements or other statements that are similarly predictive or forward-looking in nature), or in the Parent Disclosure Schedule, each of Parent, Holdco and the Merger Sub hereby represents and warrants to the Company as follows:
4.01Existence and Good Standing. Each of Parent, Holdco and Merger Sub is a corporation duly organized, validly existing and in good standing under the Laws of the State of Delaware. Each of Parent, Holdco and
A-34
Merger Sub has all requisite corporate power and authority to own, lease and operate the properties and assets it owns, leases and operates and to carry on its business as such business is conducted, except where failure to have such power and authority would not, individually or in the aggregate, have a Parent Material Adverse Effect. Each of Parent, Holdco and Merger Sub is qualified to do business as a foreign entity in each jurisdiction in which its ownership of property or the conduct of business as now conducted requires it to qualify, except where failure to be so duly qualified would not, individually or in the aggregate, have a Parent Material Adverse Effect. Parent has made available to the Member an accurate and complete copy of each Governing Document of Parent, Holdco and Merger Sub, in each case, as in effect as of the date of this Agreement. Such Governing Documents are in full force and effect. There is no pending, or to Parent’s Knowledge, threatened, action for the dissolution, liquidation or insolvency of Parent, Holdco or the Merger Sub.
4.02Authority; Enforceability. Each of Parent, Holdco and Merger Sub has the full requisite corporate power and authority to execute and deliver this Agreement and the other Transaction Documents to which it is a party (or is contemplated to be a party at the Closing), and, subject to receipt of the Required Parent Vote, the adoption of this Agreement by Holdco in its capacity as the sole stockholder of Merger Sub and the adoption of this Agreement by Parent in its capacity as the sole stockholder of Holdco, to perform its obligations under this Agreement and the other Transaction Documents to which it is a party (or is contemplated to be a party at the Closing), and to consummate the transactions contemplated hereby and thereby. The execution, delivery and performance of this Agreement and the other Transaction Documents to which Parent, Holdco or Merger Sub is a party (or is contemplated to be a party at the Closing), by Parent, Holdco and the Merger Sub, as applicable, and the consummation of the transactions contemplated hereby and thereby have been duly and validly authorized by all requisite corporate action, other than the Required Parent Vote, the adoption of this Agreement by Holdco in its capacity as the sole stockholder of Merger Sub and the adoption of this Agreement by Parent in its capacity as the sole stockholder of Holdco, and no other proceedings on their part are necessary to authorize the execution, delivery or performance of this Agreement and such Transaction Documents. This Agreement and each of the other Transaction Documents to which Parent, Holdco or Merger Sub is a party (or is contemplated to be a party at the Closing) has been (or, with respect to the Transaction Documents that are not contemplated to be executed and delivered as of the date hereof, will be prior to Closing) duly executed and delivered by Parent, Holdco and the Merger Sub, as applicable, and, assuming that this Agreement is a valid and binding obligation of the Member, this Agreement and each of the other Transaction Documents that is a Contract to which Parent, Holdco or Merger Sub is a party (or is contemplated to be a party at the Closing) constitutes (or, with respect to such Transaction Documents that are not contemplated to be executed and delivered as of the date hereof, will constitute at the Closing) a valid and binding obligation of Parent, Holdco and the Merger Sub, as applicable, enforceable in accordance with its terms, except as enforceability may be limited by bankruptcy, insolvency, reorganization, moratorium or other Legal Requirements relating to or affecting creditors’ rights generally or by equitable principles (regardless of whether enforcement is sought at law or in equity). The shares of Holdco Common Stock and Holdco Preferred Stock to be issued as a result of the Merger and the shares of Holdco Stock constituting the Stock Consideration and the Earnout Shares have been duly authorized and, when issued pursuant to this Agreement, will be validly issued, fully paid andnon-assessable and free of preemptive rights, and will be issued in compliance with all applicable U.S. federal and state securities Laws. As of the date hereof, the Board of Directors of Parent has unanimously (a) determined that it is in the best interests of Parent and Parent’s stockholders, and declared it advisable, to enter into this Agreement, (b) approved the Merger and (c) upon the terms and subject to the conditions of this Agreement, resolved to recommend that the stockholders of Parent adopt this Agreement and to submit this Agreement to the stockholders of Parent for adoption. The Board of Directors of Holdco has unanimously (i) determined that it is in the best interests of Holdco and its sole stockholder, and declared it advisable, to enter into this Agreement, (ii) approved the Merger, and the issuance of shares of Holdco Common Stock and Holdco Preferred Stock in connection with the Merger and Contribution and (iii) resolved to submit this Agreement to its sole stockholder for adoption. The Board of Directors of Merger Sub has unanimously (x) determined that it is in the best interests of Merger Sub and its sole stockholder, and declared it advisable, to enter into this Agreement, (y) approved the Merger and (z) resolved to submit this Agreement to its sole stockholder for adoption.
A-35
4.03No Violations; Required Filings and Consents.
(a) Except as set forth onSchedule 4.03, the execution and delivery of this Agreement by Parent, Holdco and Merger Sub and the execution and delivery of the other Transaction Documents to which Parent, Holdco or Merger Sub is or is contemplated to be a party does not and will not, and, subject to the receipt of the Required Parent Vote, the adoption of this Agreement by Holdco in its capacity as the sole stockholder of Merger Sub and the adoption of this Agreement by Parent in its capacity as the sole stockholder of Holdco, the performance and compliance with the terms and conditions hereof and thereof by Parent, Holdco and Merger Sub, as applicable, and the consummation of the transactions contemplated hereby and thereby by Parent, Holdco and Merger Sub, as applicable, will not (with or without notice or passage of time, or both) conflict with, result in any breach of, constitute a default under or an event creating rights of acceleration, termination, modification or cancellation or loss of right under, payment of additional fees under, result in a violation of, result in the creation of any Encumbrance under any assets of Parent, Holdco or Merger Sub or, assuming that the consents, approvals, authorizations, notices, reports and other filings described inSection 4.03(b) have been made or obtained, as applicable, and any waiting periods thereunder have terminated or expired, require any authorization, consent, approval, exemption or other action by or notice to any Governmental Entity or other third party, under:
(i) the Governing Documents of Parent, Holdco or Merger Sub;
(ii) any Law or Order applicable to Parent, Holdco or Merger Sub, or by which any property or asset of Parent, Holdco or Merger Sub is bound or affected; or
(iii) any Contract binding upon Parent or any of its Subsidiaries that is material to the Parent and its Subsidiaries, taken as a whole, including each contract binding upon Parent or any of its Subsidiaries that is required to be filed by Parent as an exhibit to a Parent SEC Report pursuant to Item 601(b)(10) of RegulationS-K promulgated by the SEC;
except, in the case of clauses (ii) and (iii), for any such conflicts, violations, breaches, defaults or other occurrences that would not, individually or in the aggregate, have a Parent Material Adverse Effect.
(b) Except (i) for the applicable requirements of the HSR Act, (ii) for compliance with the federal securities Laws and any applicable U.S. state securities or “blue sky” Laws and foreign securities Laws, (iii) for compliance with the rules and regulations of Nasdaq, (iv) for the filing of the Certificate of Merger with the Secretary of State of the State of Delaware and (v) as would not have a Parent Material Adverse Effect, neither Parent, Holdco nor the Merger Sub is required to submit any notice, report or other filing with any Governmental Entity in connection with the execution, delivery or performance by it of this Agreement or any other Transaction Document to which it is or is contemplated to be a party or the consummation of the transactions contemplated hereby or thereby and no consent, approval or authorization of any Governmental Entity is required to be obtained by Parent, Holdco or the Merger Sub in connection with its execution, delivery and performance of this Agreement or any other Transaction Document to which it is or is contemplated to be a party or the consummation of the transactions contemplated hereby or thereby.
(a) The authorized capital stock of Parent consists of 150,000,000 shares of Parent Common Stock and 5,000,000 shares of Parent Preferred Stock. As of October 29, 2018 (the “Parent Capitalization Date”), 63,461,700 shares of Parent Common Stock were issued and outstanding, and 50,000 shares of Parent Preferred Stock were issued and outstanding. As of the Parent Capitalization Date, there were 4,380,552 shares of Parent Common Stock issuable upon the exercise of outstanding Parent Stock Options, 30,212 shares of Parent Common Stock subject to outstanding Parent Restricted Stock Awards (assuming achievement of the applicable performance goals at the maximum level), 15,152,761 shares of Parent Common Stock issuable upon the conversion of issued and outstanding shares of Parent Preferred Stock and 1,221,180 shares of Parent Common Stock were held in treasury. From the Parent Capitalization Date to the
A-36
date of this Agreement, (i) Parent has not issued any shares of Parent Common Stock or Parent Preferred Stock except pursuant to the exercise of Parent Stock Options and the Settlement of Parent Restricted Stock Awards outstanding as of the Parent Capitalization Date in accordance with their terms and (ii) Parent has not issued any Parent Stock Options or Parent Restricted Stock Awards, except to directors, employees and contractors of Parent and its Subsidiaries in the Ordinary Course of Business.
(b) The authorized capital stock of Holdco consists of 1,000 shares of Holdco Common Stock. As of October 29, 2018, 1,000 shares of Holdco Common Stock were issued and outstanding, all of which have been duly authorized and validly issued and are fully paid andnon-assessable. As of the date hereof, all of the issued and outstanding capital stock of Holdco is, and immediately prior to the Effective Time all of the issued and outstanding capital stock of Holdco will be, owned, beneficially and of record, directly by Parent.
(c) The authorized capital stock of Merger Sub consists of 1,000 shares of common stock, par value $0.01 per share, 1,000 of which are issued and outstanding. All such shares of capital stock of Merger Sub have been duly authorized and validly issued and are fully paid andnon-assessable. All of the issued and outstanding capital stock of Merger Sub is, and immediately prior to the Effective Time all of the issued and outstanding capital stock of Merger Sub will be, owned, beneficially and of record, directly by Holdco.
(d) Except as set forth above in Section 4.04(a), as of the Parent Capitalization Date no shares of capital stock of Parent are issued and outstanding and Parent does not have outstanding, and there are not, any securities convertible into or exchangeable for any shares of capital stock of Parent, any rights to subscribe for or to purchase or any options or warrants for the purchase of, or any agreements providing for the issuance (contingent or otherwise) of, or any calls, commitments or known claims of any other character relating to the issuance of, any capital stock of Parent, or any stock or securities convertible into or exchangeable for any capital stock of Parent; and Parent is not subject to any obligation (contingent or otherwise) to repurchase or otherwise acquire or retire, or to register under the Securities Act, any shares of capital stock of Parent. Parent does not have outstanding any bonds, debentures, notes or other obligations the holders of which have the right to vote (or which are convertible into or exercisable for securities having the right to vote) with the stockholders of Parent on any matter. Except as set forth above inSection 4.04(a), as of the Parent Capitalization Date there are no outstanding stock options, restricted stock units, restricted stock, stock appreciation rights, “phantom” stock rights, performance units, or other compensatory rights or awards (in each case, issued by Parent or any of its Subsidiaries), that are convertible into or exercisable for a share of Parent Common Stock on a deferred basis or otherwise or other rights that are linked to, or based upon, the value of capital stock of Parent. Except as set forth inSchedule 4.04(d), there are no outstanding contractual obligations of Parent (i) affecting the voting rights of, (ii) containing any right of first refusal with respect to or (iii) granting any preemptive or similar antidilutive rights with respect to Parent Common Stock, Parent Preferred Stock or any other equity interests in Parent.
(e) The issued and outstanding shares of Parent Common Stock and Parent Preferred Stock (i) have been duly authorized and validly issued and are fully paid andnon-assessable and free of preemptive rights and (ii) were issued in compliance with all applicable U.S. federal and state securities Laws. Parent has no rights plan, “poison-pill” or other similar agreement or arrangement or any anti-takeover provision in its Governing Documents that is, or at the Effective Time shall be, applicable to Parent, the Parent Common Stock, the Parent Preferred Stock, the Merger, the Contribution or the other transactions contemplated by this Agreement.
4.05Subsidiaries. Parent and its Subsidiaries do not directly or indirectly own, or hold any rights to acquire, any capital stock or any other securities, interests or investments in any other Person other than (a) their Subsidiaries or (b) investments in marketable securities acquired in the Ordinary Course of Business in accordance with Parent’s investment policy or that constitute cash or cash equivalents.Schedule 4.05 sets forth, as of the date of this Agreement, each of Parent’s material Subsidiaries and the ownership interest of Parent in each such material Subsidiary. The outstanding shares of capital stock, or membership interests or other ownership interests of, each material Subsidiary of Parent, as applicable, are validly issued, fully paid and nonassessable and are owned of record and beneficially by Parent, directly or indirectly. Parent owns,
A-37
beneficially and of record, directly or indirectly, all of the shares of capital stock of, or membership interests or other ownership interests in, Holdco, Merger Sub and each material Subsidiary of Parent, free and clear of any Encumbrances other than Permitted Liens. Such outstanding shares of capital stock of, or membership interests or other ownership interests in, Holdco, Merger Sub and each material Subsidiary of Parent, as applicable, are the sole outstanding securities of such Subsidiaries. Except for the Holdco Common Stock, Holdco Preferred Stock, stock options in respect of shares of Holdco Common Stock and restricted stock awards relating to Holdco Common Stock, in each case, to be issued as a result of the Merger and the shares of Holdco Stock constituting the Stock Consideration and the Earnout Shares, Holdco, Merger Sub and the material Subsidiaries of Parent do not have outstanding any securities convertible into or exchangeable for any capital stock of, or membership interests or other ownership interests in, such Subsidiaries, any rights to subscribe for or to purchase or any options or warrants for the purchase of, or any agreements providing for the issuance (contingent or otherwise) of, or any calls, commitments or claims of any other character relating to the issuance of, any capital stock of, or membership interests or other ownership interests in, such Subsidiaries, or any stock or securities convertible into or exchangeable for any capital stock of, or membership interests or other ownership interests in, such Subsidiaries; and none of Parent, Holdco, Merger Sub or any of Parent’s material Subsidiaries is subject to any obligation (contingent or otherwise) to repurchase or otherwise acquire or retire, or to register under the Securities Act, any capital stock of, or membership interests or other ownership interests in, any Subsidiary of Parent, other than the registration under the Securities Act of the securities of Holdco to be issued pursuant toSections 1.01(b),1.04(a),1.04(b),2.06(a).
4.06Legal Proceedings. Except as set forth inSchedule 4.06, there are no material Legal Proceedings pending or, to Parent’s Knowledge, threatened, that (a) challenge the validity or enforceability of Parent’s, Holdco’s or Merger Sub’s obligations under this Agreement or the other Transaction Documents to which Parent, Holdco or the Merger Sub is or is contemplated to be a party, (b) seek to prevent, delay or otherwise would reasonably be expected to materially and adversely affect the consummation by Parent, Holdco or the Merger Sub of the transactions contemplated herein or therein or (c) would have, individually or in the aggregate, a Parent Material Adverse Effect.
4.07SEC Filings and Financial Statements.
(a) Parent and its Subsidiaries have filed or furnished, as applicable, on a timely basis, each registration statement, prospectus, form, statement, certification, report and other document (together with all amendments thereof and supplements thereto) required to be filed or furnished by Parent or any of its Subsidiaries pursuant to the Exchange Act or the Securities Act with the SEC since January 1, 2016 (as such documents have since the time of their filing been amended or supplemented and to the extent publicly available, the “Parent SEC Reports”). As of their respective dates, after giving effect to any amendments or supplements thereto filed prior to the date hereof, the Parent SEC Reports (i) comply in all material respects with the requirements of the Exchange Act or the Securities Act, as the case may be, the Sarbanes-Oxley Act and the applicable rules and regulations promulgated thereunder and (ii) do not contain any untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they were made, not misleading.
(b) As of the date of this Agreement, there are no outstanding or unresolved comments in comment letters received from the staff of the SEC with respect to any of the Parent SEC Reports, and, to Parent’s Knowledge, none of the Parent SEC Reports is subject to ongoing SEC review. The audited consolidated financial statements and unaudited interim consolidated financial statements (including, in each case, the notes and schedules thereto) included or incorporated by reference in the Parent SEC Reports (i) at the time they were filed or furnished (A) complied in all material respects with the applicable accounting requirements and the published rules and regulations of the SEC with respect thereto and (B) were prepared in accordance with GAAP applied on a consistent basis during the periods involved (except as may be expressly set forth in the notes thereto and except with respect to unaudited statements as permitted byForm 10-Q of the SEC) and (ii) fairly present (subject, in the case of the unaudited interim financial statements included therein, tonormal year-end adjustments which would not be material individually or in
A-38
the aggregate and the absence of complete footnotes) in all material respects the consolidated financial position of Parent and its consolidated Subsidiaries as of the respective dates thereof and the consolidated results of their operations, cash flows and changes in stockholders’ equity of Parent and its consolidated Subsidiaries for the respective periods then ended. Parent has heretofore furnished to the Member complete and correct copies of all amendments and modifications that have not been filed by Parent with the SEC to all agreements, documents and other instruments that previously had been filed by Parent with the SEC and are currently in effect. Parent is in compliance in all material respects with the applicable listing and corporate governance rules and regulations of Nasdaq.
4.08Internal Controls. Parent has designed and maintains disclosure controls and procedures required byRule 13a-15 or15d-15 under the Exchange Act. Such disclosure controls and procedures are designed to ensure that information required to be disclosed by Parent in its filings with the SEC under the Exchange Act is recorded, processed, summarized and reported on a timely basis to the individuals responsible for the preparation of Parent’s filings with the SEC under the Exchange Act. Parent maintains internal control over financial reporting (as defined inRule 13a-15 or15d-15, as applicable, under the Exchange Act). Such internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP. Based on its most recent evaluation of internal controls over financial reporting prior to the date hereof, management of Parent has disclosed to Parent’s auditors and the audit committee of the Board of Directors of Parent (a) any significant deficiencies or material weaknesses in the design or operation of Parent’s internal controls over financial reporting or (b) any fraud, whether or not material, that involves management or other employees who have a significant role in Parent’s internal control over financial reporting. Parent has made available to the Member, prior to the date of this Agreement, either materials relating to or a summary of any disclosure of matters described in clauses (a) or (b) in the immediately preceding sentence made by management of Parent to Parent’s auditors or the audit committee of the Board of Directors of Parent.
4.09Absence of Certain Changes. During the period from June 30, 2018 to the date hereof, Parent and its Subsidiaries have conducted their respective businesses in the Ordinary Course of Business (except with respect to the negotiation of and entry into this Agreement) and there has not been a Parent Material Adverse Effect.
4.10No Undisclosed Liabilities. Except as set forth inSchedule 4.10, there are no Liabilities (whether accrued, absolute, asserted or unasserted, known or unknown, primary or secondary, direct or indirect, contingent or otherwise) that would be required by GAAP to be reflected on a consolidated balance sheet of Parent and its Subsidiaries, other than any such Liabilities (i) incurred in the Ordinary Course of Business since June 30, 2018, (ii) reflected or reserved against on the financial statements in the Parent SEC Reports that have been filed with or furnished to the SEC as of the date hereof, (iii) arising or incurred in connection with a transaction or agreement contemplated by this Agreement (excluding the obligations set forth inSection 6.05) or (iv) that would not, individually or in the aggregate, have a Parent Material Adverse Effect.
4.11Legal Requirements. Except as set forth inSchedule 4.11 or as would not, individually or in the aggregate, have a Parent Material Adverse Effect, each of Parent and its Subsidiaries is, and since January 1, 2016 has been, in compliance with all applicable Legal Requirements and Orders. Except as set forth inSchedule 4.11 or as would not, individually or in the aggregate, have a Parent Material Adverse Effect, neither Parent nor any of its Subsidiaries has received written notice that it is, and to Parent’s Knowledge, neither Parent nor any of its Subsidiaries is, under investigation by any Governmental Entity with respect to any alleged violation of any applicable Legal Requirements. To Parent’s Knowledge, except as set forth inSchedule 4.11 or as would not, individually or in the aggregate, have a Parent Material Adverse Effect, neither Parent nor any of its Subsidiaries has received any subpoena, written demand, inquiry, information request, complaint, allegation or notice ofnon-compliance with or violation of any Legal Requirements.
4.12Orders. There is no judgment, order, writ, injunction, decree or other similar award outstanding (whether rendered by a court, administrative agency or other Governmental Entity, or by arbitration) against
A-39
Parent or any of its Subsidiaries or by which Parent or any of its Subsidiaries is bound that would have a Parent Material Adverse Effect.
4.13Tax Matters. Parent has not taken any action and, to Parent’s Knowledge, there is no fact, agreement, plan or other circumstance that is reasonably likely to prevent or impede (i) the Merger and the Contribution, taken together, from qualifying as a transaction described in Section 351 of the Code or (ii) the Merger from qualifying as a “reorganization” within the meaning of Section 368(a) of the Code.
(a) To Parent’s Knowledge, Parent and its Subsidiaries own, or are licensed or otherwise possess legal rights to all patents, design registrations, patent applications, trademarks, trade names, service marks, trade dress, copyrights, domain names, mask works, schematics, technology,know-how, trade secrets, confidential information, customer lists, technical information, technical data, process technology, plans, drawings and blue prints, inventions, algorithms, devices, systems, processes, computer software programs and applications to the extent the same are used in and are material to the respective businesses of Parent and its Subsidiaries.
(b) Parent and its Subsidiaries have taken commercially reasonable measures to maintain and protect the secrecy, confidentiality and value of their Trade Secrets. To Parent’s Knowledge, no unauthorized disclosure of any material Trade Secrets, including Trade Secrets relating to Parent and its Subsidiaries’ BIOCLEANSE and TUTOPLAST processes, has been made.
(c) Except as set forth inSchedule 4.14(c), to Parent’s Knowledge, within the three (3) years preceding the date of this Agreement, neither Parent nor any of its Subsidiaries has received any written invitation to license or written charge, complaint, claim, demand, or notice that Parent or any of its Subsidiaries has infringed, misappropriated, or acted in conflict with any of the Intellectual Property owned by any third party.
(d) There is no actual, pending or, to Parent’s Knowledge, threatened litigation, now or within the three (3) years preceding the date of this Agreement, relating to any Intellectual Property that would, individually or in the aggregate, result in a Parent Material Adverse Effect, except as set forth inSchedule 4.14(d).
4.15Healthcare Regulatory Matters. Neither Parent, nor any of its officers, directors, or employees, in each case, acting on behalf of Parent, is or in the last five (5) years has been: (i) under or subject to any pending or threatened investigation, subpoena, civil investigative demand, inquiry, corporate integrity agreement, deferred ornon-prosecution agreement, or similar instrument or obligation, or request for documents or information by any Governmental Entity (including the Department of Justice, HHS, the OIG, the Centers for Medicare and Medicaid Services, any other HHS agency, any state Medicaid agency, or any state Attorney General); (ii) excluded, suspended or debarred by OIG or the GSA from participating in any federal healthcare program (as such term is defined at 42 U.S.C. §1320a-7b(f) and its implementing regulations); (iii) debarred under 21 U.S.C. § 335a or excluded pursuant to 42 U.S.C.§ 1320a-7; (iv) convicted of any crime or engaged in any conduct that would reasonably be expected to result in debarment under 21 U.S.C. § 335a or exclusion pursuant to 42 U.S.C.§ 1320a-7; or (v) a party to an agreement or settlement with any Governmental Entity with regard to any allegednon-compliance with, or violation of, any Law.
4.16Brokerage. Except for Parent’s obligations to Piper Jaffray & Co., there are no claims for brokerage commissions, finders’ fees or similar compensation in connection with the transactions contemplated by this Agreement based on any agreement made by or on behalf of Parent, Holdco, the Merger Sub or any of Parent’s other Subsidiaries.
(a) Parent has delivered to the Member true, complete and correct copies of (i) the executed debt commitment letter from Ares Capital Management LLC (“Ares”), including all exhibits, schedules, annexes
A-40
and amendments thereto, and (ii) the consent letter from JPMorgan Chase Bank, N.A. (together with Ares, collectively, the “Lenders”), and each fee letter associated therewith (each of which such letters may be redacted as set forth in such commitment letters) (as each may be amended, modified, supplemented, replaced or extended from time to time in accordance withSection 6.03, collectively, the “Commitment Letters”) providing the terms and conditions on which the Lenders have committed to provide or continue to provide, as applicable, to Parent debt financing in the amounts set forth therein for the purposes of financing the transactions contemplated by this Agreement (such debt financing, as may be amended, modified, supplemented or replaced from time to time in accordance withSection 6.03, the “Financing”). As of the date hereof, except for (i) the Commitment Letters and as expressly set forth in the Commitment Letters and (ii) customary engagement letters andnon-disclosure agreements with the Lenders or their respective Affiliates which do not impact the conditionality or amount of the Financing, there are no side letters or Contracts, understandings or arrangements to which Parent or any of its Subsidiaries is party relating to the Financing that would adversely affect the availability of all or any portion of the Financing.
(b) As of the date hereof, (i) the Commitment Letters are in full force and effect and are the legal, valid, binding and enforceable obligation of Parent, and, to Parent’s Knowledge, each of the Lenders party thereto, in each case, subject to applicable bankruptcy, insolvency, reorganization, moratorium and other Legal Requirements relating to or affecting creditors’ rights generally or equitable principles (regardless of whether enforcement is sought at law or in equity); (ii) the Commitment Letters have not been amended or modified in any respect and, to Parent’s Knowledge, no such amendment or modification is contemplated or pending (other than amendments or modifications to the Debt Commitment Letters that are permitted bySection 6.03) as of the date hereof; and (iii) the commitments contained in the Commitment Letters have not been withdrawn, terminated, reduced or rescinded in any respect. Parent has paid in full any and all fees required to be paid under the Commitment Letters that are due and payable on or prior to the date hereof.
(c) There are no conditions precedent or other contingencies (including pursuant to any “flex” provisions in the Commitment Letters or otherwise) related to the funding of the full amount (or any portion) of the Financing nor any contingencies that would allow the Lenders to reduce the total amount of the Financing, except as expressly set forth in the Commitment Letters. As of the date of this Agreement, assuming the accuracy of the representations and warranties set forth in Article III, the satisfaction of the conditions precedent to Parent’s obligations under this Agreement set forth inSection 8.01 and the compliance and performance by the Member of its covenants and agreements set forth in this Agreement, to Parent’s Knowledge, there is no fact or occurrence existing as of the date hereof that makes any of the assumptions or statements set forth in the Commitment Letters inaccurate or that causes the Commitment Letters to be ineffective with respect to Parent or that precludes the satisfaction of the conditions under Parent’s control set forth in the Commitment Letters.
(d) Assuming the Financing is funded in accordance with the Commitment Letters, based on the terms and the compliance and performance by the Member of its covenants and agreements set forth in this Agreement, Holdco and its Subsidiaries will have at and as of the Closing Date funds sufficient to pay any of each of Holdco’s and its Subsidiaries’ respective obligations under this Agreement (other than any obligation to make a cash payment to the Member pursuant toSection 2.06) or the Commitment Letters, including (i) the Cash Purchase Price, (ii) the Closing Indebtedness Amount, (iii) the amount of unpaid Company Transaction Expenses, (iv) all fees and expenses required to be paid by Holdco or any of its Subsidiaries in connection with the Financing, (v) any repayment or refinancing of any outstanding Indebtedness of the Company and its Subsidiaries contemplated by the Commitment Letters and (vi) all fees, expenses and other payments (other than cash payments to the Member pursuant toSection 2.06) required to be paid by Holdco or any of its Subsidiaries pursuant to this Agreement (the amount so required to make such payments, excluding Parent’s other sources of funds, the “Required Amount”).
4.18Purpose. Holdco and Merger Sub are each newly organized corporations, formed solely for the purpose of engaging in the transactions contemplated by this Agreement. Neither Holdco nor Merger Sub has engaged in any business activities or conducted any operations other than in connection with the transactions contemplated by this Agreement. Each of Holdco and Merger Sub is a wholly owned Subsidiary of Parent.
A-41
4.19Solvency. Parent is not entering into this Agreement with the intent to hinder, delay or defraud either present or future creditors of any of the Group Companies.
4.20Parent Information. None of the information supplied or to be supplied by Parent or any of its Affiliates expressly for inclusion in the Parent SEC Reports or any other filings with the SEC, mailings to Parent’s stockholders or the Unitholders with respect to the transactions contemplated by this Agreement, any supplements thereto and/or in any other document filed with any Governmental Entity in connection herewith (including the Joint Proxy and Consent Solicitation Statement/Prospectus and the Registration Statement), will, at the date of filing and/or mailing, as the case may be, or, with respect to the Joint Proxy and Consent Solicitation Statement/Prospectus and the Registration Statement, at the time of any meeting of Parent’s stockholders to be held in connection with the Merger, including the Parent Stockholder Meeting, contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they are made, not misleading (subject to the qualifications and limitations set forth in the materials provided by Parent or that is included in the applicable filings). No representation or warranty is made by Parent, Holdco or Merger Sub with respect to statements made or incorporated by reference in any of the foregoing documents based on information supplied or to be supplied by, the Company or any of its Affiliates.
4.21State Takeover Laws. The Board of Directors of each of Parent and Holdco has taken all necessary actions so that no “fair price,” “moratorium,” “control share acquisition” or other anti-takeover Law, including Section 203 of the DGCL, will apply with respect to or as a result of the execution of this Agreement or the Parent Stockholder Support Agreements or the consummation of the Merger and the Contribution or the other transactions contemplated hereby.
4.22No Other Representations or Warranties. Except for the representations and warranties of the Member set forth in this Agreement or in a certificate delivered pursuant to this Agreement, Parent, Holdco and Merger Sub each hereby acknowledge and agree that neither the Member nor any of its Subsidiaries, nor any of their respective Representatives, has made or is making any other express or implied representation or warranty with respect to the Member, any of its Subsidiaries or their respective businesses or operations, including with respect to any information provided or made available to the Parent, Holdco or Merger Sub.
COVENANTS OF THE MEMBER
(a) From the date hereof until the earlier of the termination of this Agreement and the Closing Date, except (1) as set forth inSchedule 5.01, (2) if Parent will have consented (such consent, solely to the extent set forth inSection 5.01(b), not to be unreasonably withheld, conditioned or delayed), (3) as required by applicable Law, (4) with respect to an Interim Financing or (5) as otherwise expressly contemplated by this Agreement, the Member will cause the Group Companies to conduct their business, in all material respects, in the Ordinary Course of Business, and the Member shall cause the Group Companies to use their respective commercially reasonable efforts to preserve intact the current business organization and ongoing operations of the Group Companies, maintain relations and goodwill with suppliers, customers, landlords, employees and creditors with whom such Group Company has a relationship, and maintain the properties and assets of the Group Companies in their current state of repair and condition (excluding normal wear and tear).
(b) From the date hereof until the earlier of the termination of this Agreement and the Closing Date, except (v) as set forth inSchedule 5.01, (w) if Parent will have consented (such consent not to be unreasonably withheld, conditioned or delayed in respect of clauses (ix), (x), (xi), (xiv), (xvi), (xvii)(A),
A-42
(xx), (xxi) and (xxii) (solely to the extent relating to one of the preceding clauses)), (x) as required by applicable Law, (y) with respect to an Interim Financing or (z) as otherwise expressly contemplated by this Agreement, the Member will not, and will not permit any of the Group Companies to:
(i) issue, sell or deliver any of the Company’s or any other Group Company’s equity securities or issue or sell any securities convertible into, or options with respect to, or warrants to purchase or rights to subscribe for, any of the Company’s or any other Group Company’s equity securities;
(ii) recapitalize, reclassify, combine, split, subdivide or redeem, declare any stock or equity dividend, purchase or otherwise acquire or otherwise make any change in, directly or indirectly, any Group Company’s equity interests or make any other change with respect to any Group Company’s capital structure;
(iii) amend its Governing Documents;
(iv) in the case of any Group Company, make any redemption or purchase of its equity interests;
(v) create any new Subsidiary;
(vi) (A) sell, assign or transfer any material portion of its tangible assets (other than sales of inventory in the Ordinary Course of Business), or (B) mortgage, encumber, pledge, or impose any Encumbrance upon any of its assets, other than Permitted Liens;
(vii) adopt a plan of complete or partial liquidation, dissolution, merger or consolidation of any of the Group Companies;
(viii) sell, assign, transfer or exclusively license any material patents, trademarks, trade names or copyrights;
(ix) enter into any contract, agreement or arrangement that would be a Material Contract if entered into prior to the date hereof (other than a renewal or replacement of any existing Material Contract that is expiring by its terms, so long as the terms and conditions of such renewal or replacement contract, agreement or arrangement, in the aggregate, are not materially less favorable to the Company or its applicable Subsidiary than the existing Material Contract);
(x) terminate, cause the termination of, amend or modify any Material Contract (other than the Credit Agreement, the ERA and the other Contracts contemplated thereby) in any material respect (other than in the Ordinary Course of Business), or waive or release any rights or claims thereunder;
(xi) pay, discharge or satisfy any material claims or liabilities other than in the Ordinary Course of Business, or fail to pay or otherwise satisfy (except if being contested in good faith) any material accounts payable, liabilities, or obligations when due and payable, in each case, other than liabilities or obligations due and payable under the Credit Agreement, the ERA or any other Contract contemplated thereby;
(xii) directly or indirectly, merge with or into, consolidate with or acquire any material asset out of the Ordinary Course of Business;
(xiii) make any capital contributions to, or investments in, or any advance or loan to, or acquire the securities of, any Person other than a wholly owned Subsidiary of the Company;
(xiv) make any capital expenditures or commitments therefor other than those reflected in the Company’s budget as of the date hereof as listed onSchedule 5.01(a)(xiv) hereto or in an amount not to exceed $250,000;
(xv) in the case of any Group Company, enter into any transaction with any of its directors, officers or employees outside the Ordinary Course of Business;
(xvi) except as required under the terms of any written agreement or Company Employee Benefit Plan as in effect on the date hereof, (A) increase salaries, bonuses or other compensation or remuneration and benefits payable by a Group Company to any of its employees, officers, directors or other service providers
A-43
other than increases in base compensation in the Ordinary Course of Business; (B) increase the benefits provided to any Person under any Company Employee Benefit Plan; (C) hire or engage the services of any Person with annual base compensation in excess of $200,000; or (D) terminate or amend any Company Employee Benefit Plan or adopt any new arrangement for the benefit or welfare of any officer or employee, director or other service provider of any Group Company that would be a Company Employee Benefit Plan if it were in existence as of the date hereof (other than in conjunction with agreements or arrangements that are entered into in the Ordinary Course of Business with new hire employees);
(xvii) settle any Legal Proceeding if (A) the amount payable by any Group Company in connection therewith would exceed $200,000 or (B) would be reasonably likely to have a material effect on the post-Closing operations of the business of any Group Company or agree to any injunctive or other equitable relief;
(xviii) cancel any material third-party Indebtedness owed to any Group Company;
(xix) recognize any labor union or enter into, modify, or amend any collective bargaining agreement or engage in any substantive communications with any labor union regarding any Party’s anticipated actions on or after the Closing with respect to such union;
(xx) in the case of any Group Company, prepare or file any Tax Return inconsistent with past practice or, on any such Tax Return, take any position or adopt any method that is inconsistent with positions taken or methods used in preparing or filing similar Tax Returns in prior periods, settle or compromise any claim relating to Taxes, or otherwise settle any dispute relating to Taxes;
(xxi) in the case of any Group Company, make, modify or revoke any income or other material election with respect to Taxes, consent to any waiver or extension of time to assess or collect any Taxes payable by any Group Company, file any amended Tax Returns or fail to file for or otherwise surrender any refund claim on behalf of any Group Company; or
(xxii) agree, whether orally or in writing, to do any of the foregoing, or agree, whether orally or in writing, to any action or omission that would result in any of the foregoing.
(c) From the date hereof until the earlier of the termination of this Agreement and the Closing Date, without the prior written approval of Parent (which approval may be given or denied in Parent’s sole discretion), except as set forth inSchedule 5.01 or with respect to an Interim Financing, the Member shall not permit any Group Company to, directly or indirectly, declare or pay any cash dividend on, or make any cash payment on account of, the purchase, redemption, defeasance, retirement or other acquisition of, any of its capital stock or common shares, as applicable, or make any other cash distribution in respect thereof, either directly or indirectly.
(d) Nothing contained in this Agreement shall give Parent, directly or indirectly, the right to control or direct any Group Company’s operations prior to the Closing. Prior to the Closing, the Group Companies shall exercise, consistent with the terms and conditions of this Agreement, complete control and supervision over their operations.
5.02Access to Books and Records. From the date hereof until the earlier of the termination of this Agreement and the Closing Date, the Member will provide Parent and its authorized representatives (the “Parent’s Representatives”) with reasonable access during normal business hours, and upon reasonable notice, to the offices, properties, personnel, and all financial books and records of the Group Companies in order for Parent to have the opportunity to make such investigation as it will reasonably desire in connection with the consummation of the transactions contemplated hereby;provided,however, that in exercising access rights under thisSection 5.02, Parent and the Parent’s Representatives will not be permitted to interfere unreasonably with the conduct of the business of any Group Company. Notwithstanding anything contained herein to the contrary, the foregoing shall not require any Group Company to permit any Phase I or Phase II environmental site assessments or any other invasive or intrusive sampling activities, and no such access or examination will be permitted to the extent that it would require any Group Company to disclose information subject to attorney-client privilege or
A-44
attorney work-product privilege or violate any applicable Law. Parent acknowledges that Parent is and remains bound by the Confidentiality Agreement between Parent and the Company dated April 24, 2018 (the “Confidentiality Agreement”).
5.03Efforts to Consummate. Subject to the terms and conditions herein provided, from the date hereof until the earlier of the termination of this Agreement and the Closing Date, the Member will use its reasonable best efforts to take, or cause to be taken, all actions and to do, or cause to be done, all things reasonably necessary, proper or advisable to consummate and make effective as promptly as practicable the transactions contemplated by this Agreement (including the satisfaction, but not a waiver, of the closing conditions set forth inSection 8.02), and will not take any action specifically intended to prevent the Closing. The Parties acknowledge and agree that nothing contained in thisSection 5.03 will limit, expand or otherwise modify in any way any efforts standard explicitly applicable to any of the Company’s obligations under this Agreement. During the period prior to the earlier of the termination of this Agreement and the Closing Date, the Member shall, and shall cause the Group Companies to, act diligently and reasonably and shall, use its, and shall cause the Group Companies to use their, respective commercially reasonable efforts to secure any consents, waivers and approvals of any third party (other than with respect to any Regulatory Approvals, which shall be subject toSection 7.02) required to be obtained by the Member or any of the Group Companies to consummate the transactions contemplated by this Agreement.
5.04Exclusive Dealing. During the period from the date hereof through the Closing or the earlier termination of this Agreement, the Member shall not, and shall cause the Group Companies not to, take any action to directly or indirectly initiate, solicit or engage in discussions or negotiations with, or knowingly provide any information to, any Person (other than Parent and the Parent’s Representatives) concerning any purchase of any of the Company Units or any merger, sale of substantially all of the assets of the Group Companies or similar transactions involving the Group Companies (other than (i) assets sold in the Ordinary Course of Business or (ii) an Interim Financing) (each such transaction, an “Acquisition Transaction”). The Member will, and will cause the Group Companies to, cease and cause to be terminated (a) any existing discussions, communications or negotiations with any Person (other than Parent and the Parent’s Representatives) conducted heretofore with respect to any Acquisition Transaction and (b) any such Person’s and its authorized representatives’ access to any electronic data room granted in connection with any Acquisition Transaction.
5.05Payoff Letters and Lien Releases. At least three (3) Business Days prior to the anticipated Closing (or such shorter period of time as the Parent may reasonably agree), the Member will deliver to Parent a customary payoff letter or letters (collectively, the “Payoff Letters”) executed by or on behalf of the lenders of the Indebtedness described in clause (a) herein, which letter(s) will set forth (a) the total amount of cash and shares of Holdco Common Stock required to be paid at the Effective Time (i) pursuant to the Settlement Agreement and (ii) to satisfy in full the repayment of all Indebtedness under the Credit Agreement (including any applicable Interim Debt Financing (other than Interim Debt Financing covered by the Settlement Agreement)) and, in each case, if any, all prepayment penalties, premiums and breakage costs that become payable upon such repayment in accordance with the Credit Agreement and the Settlement Agreement, as applicable (the “Payoff Amounts”), (b) upon receipt of the Payoff Amounts, the lenders’ obligation to release all liens and other security securing the Indebtedness described in clause (a) (including, in respect of the Indebtedness covered by the Settlement Agreement, prior to the Closing), and to terminate the other agreements contemplated thereby with respect to the Company and its Subsidiaries, and (c) wire transfer and stock issuance instructions for paying the applicable Payoff Amount.
5.06Member Approval. The Member shall use its reasonable best efforts to, as soon as reasonably practicable after the Registration Statement is declared effective under the Securities Act, deliver to Parent a certified copy of the written consent of the Unitholders adopting this Agreement in accordance with the DLLCA and the Governing Documents of the Member (the “Written Consent”).
5.07Notification. From the date hereof until the earlier of the termination of this Agreement and the Closing Date, if after the date hereof the Member becomes aware of any fact or condition arising after the date hereof that
A-45
constitutes a breach of any representation or warranty made by the Member inArticle III or of any covenant that would cause the conditions set forth inSection 8.01(a) orSection 8.01(b), as applicable, not to be satisfied as of the Closing Date, the Member will disclose in writing to Parent such breach. If the Member fails to notify Parent under thisSection 5.07, (a) Parent shall only be entitled to seek indemnification for breach of thisSection 5.07 if and to the extent Parent is otherwise entitled to indemnification pursuant toSection 9.02, for breach of a representation and warranty or covenant and (b) a failure to comply with thisSection 5.07 shall not cause the failure of any condition set forth inSection 8.01(a) orSection 8.01(b) to be satisfied unless the underlying change, event or development would independently result in the failure of a condition set forth inSection 8.01(a) orSection 8.01(b) to be satisfied. All information obtained by Parent pursuant to thisSection 5.07 shall be governed by the terms of the Confidentiality Agreement.
(a) Prior to the Closing, at Parent’s sole expense, the Member shall, and shall cause each of its Subsidiaries to, use its and their reasonable best efforts to provide, and cause their respective Representatives to provide, all cooperation reasonably requested by Parent in connection with the arrangement of the Financing and that is customary in connection with the arrangement of financings similar to those contemplated by the Commitment Letters, in a manner that does not unreasonably interfere with the business or ongoing operations of the Member and its Subsidiaries, including:
(i) participating and making the appropriate senior officers of the Company available to participate in a reasonable number of meetings (including customaryone-on-one meetings with Financing Sources and potential financing sources and senior management and other Representatives of the Company), due diligence sessions, drafting sessions, presentations, “road shows” and sessions with prospective rating agencies in connection with the Financing;
(ii) cooperating with the marketing efforts and due diligence efforts of Parent and its financing sources, including assisting with the preparation of customary offering memoranda, private placement memoranda, bank offering memoranda (including a bank information memorandum that does not include materialnon-public information), syndication memoranda, lender presentations and similar documents and other customary marketing materials (including causing the Company and its Subsidiaries to deliver customary representation letters, authorization letters, confirmations and undertakings, in each case as contemplated by the Commitment Letters) required in connection with the Financing;
(iii) providing Parent and its financing sources with the Required Information in a timely fashion such that Parent is able to comply with its obligations under the Commitment Letters with respect thereto and other pertinent information regarding the business of the Company and its Subsidiaries as may be reasonably requested in writing by Parent in order to consummate the Financing and supplementing the same to the extent any such Required Information or other information, to the knowledge of the Member, contains any material misstatement of fact or omits to state any material fact necessary to make such information not misleading, as soon as reasonably practicable after obtaining knowledge thereof;
(iv) executing and delivering any credit agreements, notes, guarantees, pledge and security documents, hedging arrangements and other definitive financing documents or other requested certificates or documents (including a certificate with respect to solvency matters);
(v) taking such actions as are reasonably requested by Parent to assist with collateral audits and due diligence examination, including collateral eligibility criteria and other related criteria described in or contemplated by the Commitment Letters and granting any valuation agent acting on behalf of the Financing Sources, at reasonable times and on reasonable advance notice, access to the books and records of the Group Companies for the preparation of a valuation report with respect to the assets of the Group Companies to be included in the collateral contemplated by the Financing;
(vi) assisting with the preparation of the borrowing base certificate contemplated by the Commitment Letters and facilitating the granting of a security interest (and perfection thereof) in the collateral contemplated by the Financing; and
A-46
(vii) at least five (5) Business Days prior to Closing, providing all documentation and other information about the Group Companies that the Financing Sources have reasonably determined is required to comply with applicable Law to the extent required under applicable “know your customer” and anti-money laundering rules and regulations, including the USA PATRIOT Act (provided, that if the Member does not provide such documentation at least five (5) Business Days prior to Closing, the Member shall not be deemed to be in breach of thisSection 5.08(a)(vii) if such documentation or information was not requested, in writing, from the Member by Parent or a Financing Source at least five (5) Business Days prior to the Closing);
provided, that nothing in this Agreement, (including thisSection 5.08) shall (x) require any such cooperation to the extent that it would (A) require the Member or any of its Subsidiaries to enter into or approve any agreement or other documentation that would be effective at any time before the time that will be immediately prior to the Closing or the effectiveness of which is not conditioned on the Closing (other than the representation letters, authorization letters, confirmations and undertakings referred to in clause (ii) above), (B) require the Member, or any of its Subsidiaries or any of their respective boards of directors (or equivalent bodies) to approve or authorize the Financing (provided, that the Member and its Subsidiaries shall reasonably cooperate with Parent to appoint Parent’s designees to the respective boards of directors (or equivalent bodies) of the Group Companies as of immediately prior to Closing for purposes of approving resolutions, the effectiveness of which is to be conditioned upon the Closing, related to the Financing), (C) cause any condition to the Closing set forth inSection 8.01 orSection 8.02 to not be satisfied or otherwise cause any breach of this Agreement that would provide Parent the right to terminate this Agreement or seek indemnity under the terms hereof (unless, in each case, waived by Parent) or (D) conflict with or violate the Member’s or its Subsidiaries’ respective Governing Document or any applicable Law or (y) require the Member, any of its Subsidiaries or any Representative thereof to cause the delivery of legal opinions, reliance letters or any certificates that would be effective at any time before the time that will be immediately prior to the Closing or that the effectiveness of which is not conditioned on the Closing (other than the representation letters, authorization letters, confirmations and undertakings referred to in clause (ii) above).
(b) Parent shall, promptly upon the request of the Member, reimburse the Member for all reasonable and documentedout-of-pocket costs and expenses (including reasonable attorneys’ fees) incurred by the Member and its Subsidiaries in connection with the cooperation contemplated bySection 5.08(a) and Parent and Holdco shall, on a joint and several basis, indemnify and hold harmless the Member and its Subsidiaries and their respective Representatives from and against any and all liabilities, losses, damages, claims, interest, awards, judgments, penalties and reasonable and documentedout-of-pocket costs or expenses suffered or incurred by any of them in connection with the Financing (including any actions under thisSection 5.08) and any information utilized in connection therewith (other than any information prepared or provided by the Member or any of its Subsidiaries or any of their respective Representatives) except, in each case with respect to the foregoing indemnity, to the extent resulting from bad faith, gross negligence or willful misconduct of the Member or any of its Subsidiaries or any of their respective Representatives.
5.09Update of Financial Statements. During the period from the date of this Agreement through the Closing Date or the earlier termination of this Agreement pursuant toArticle X, the Member shall cause the Company to prepare in the ordinary course of business consistent with past practice, and deliver to Parent promptly upon completion, but in any event no later than fifteen (15) days after the end of the applicable fiscal month, consolidated financial statements for the Company and its Subsidiaries for each fiscal month ending after September 30, 2018, consisting of a balance sheet as of the end of such month and statements of operations for that month and for the portion of the year then ended.
5.10Unitholder Support Agreements. The Member shall not register the transfer of any Covered Units (as defined in the Unitholder Support Agreements) made or attempted to be made in violation of the Unitholder Support Agreements.
A-47
5.11Commercial Arrangements. Prior to Closing, the Member shall use its reasonable best efforts to cause the Company to negotiate and enter into a written master consulting agreement, including statements of work, with Musculoskeletal Clinical Regulatory Advisors, LLC (“MCRA”), in substantially the same form, and for substantially the same terms and conditions, and, in each case, in a form and substance reasonably satisfactory to Parent, as set forth in that certain Amended and Restated Consulting Agreement between MCRA and the Company or its applicable Affiliate, dated as of November 1, 2013, including any amendments or addenda thereto (the “MCRA Agreement”);provided that the consideration to be paid by the Company for any services rendered under the new master consulting agreement with MCRA shall be equal to in all material respects the consideration paid for the same or similar services set forth in the MCRA Agreement.
5.12Termination of Certain Benefit Plans. The Member shall cause the Group Companies to take (or cause to be taken) all actions necessary or appropriate, effective no later than the day immediately preceding the Closing Date, to (i) terminate each Company Employee Benefits Plan that contains a cash or deferred arrangement intended to qualify under Section 401(a) of the Code (a “401(k) Plan”) and (ii) terminate each Group Company’s participation in all 401(k) Plans sponsored by a professional employer organization (“PEO”), unless Parent, in its sole and absolute discretion, agrees to sponsor and maintain any such 401(k) Plan by providing the Member with written notice of such election (an “Election Notice”) at least five (5) Business Days prior to the Closing Date. Unless Parent provides an Election Notice to the Member, the Member shall cause the applicable Group Company to deliver to Parent, at least three (3) Business Days prior to the Closing Date, evidence that the applicable Group Company’s board of directors has validly adopted resolutions to (a) terminate each Company Employee Benefit Plan that is a 401(k) Plan and (b) terminate the applicable Group Company’s participation in any 401(k) Plan sponsored by a PEO (with the form and substance of such resolutions to be subject to review and approval of Parent), in each case effective no later than the date immediately preceding the Closing Date. In the event that the distributions of assets from the trust of a terminated 401(k) Plan (or a 401(k) Plan sponsored by a PEO in which a Group Company has terminated participation) is reasonably anticipated to trigger liquidation charges, surrender charges, or other fees to be imposed upon the account of any participant or beneficiary of such terminated 401(k) Plan or upon any Group Company, then the Member shall cause the Group Companies to take such actions as are necessary to reasonably estimate the amount of such charges or fees and provide such estimate in writing to Parent prior to the Effective Time.
COVENANTS OF PARENT, HOLDCO AND MERGER SUB
6.01Notification. From the date hereof until the earlier of the termination of this Agreement and the Closing Date, if after the date hereof Parent has knowledge of any fact or condition that constitutes a breach of any representation or warranty made inArticle IV or any covenant that would cause the conditions set forth inSection 8.02(a) orSection 8.02(b), as applicable, not to be satisfied as of the Closing Date, Parent will disclose in writing to the Member such breach. If Parent fails to notify the Member under thisSection 6.01, a failure to comply with thisSection 6.01 shall not cause the failure of any condition set forth inSection 8.02(a) orSection 8.02(b) to be satisfied unless the underlying change, event or development would independently result in the failure of a condition set forth inSection 8.02(a) orSection 8.02(b) to be satisfied.
6.02Efforts to Consummate. Subject to the terms and conditions herein provided, from the date hereof until the earlier of the termination of this Agreement and the Closing Date, Parent, Holdco and the Merger Sub will each use its reasonable best efforts to take, or cause to be taken, all actions and to do, or cause to be done, all things reasonably necessary, proper or advisable to consummate and make effective as promptly as practicable the transactions contemplated by this Agreement (including the satisfaction, but not waiver, of the Closing conditions set forth inArticle VIII), and will not take any action specifically intended to prevent the Closing. The Parties acknowledge and agree that nothing contained in thisSection 6.02 will limit, expand or otherwise modify in any way any efforts standard explicitly applicable to any of Parent’s, Holdco’s and/or the Merger Sub’s
A-48
respective obligations under this Agreement. During the period prior to the earlier of the termination of this Agreement and the Closing Date, Parent shall use its, and shall cause its Subsidiaries to use their respective, commercially reasonable efforts to secure any consents, waivers and approvals of any third party (other than with respect to any Regulatory Approvals, which shall be subject toSection 7.02) required to be obtained by Parent or any of its Subsidiaries to consummate the transactions contemplated by this Agreement.
(a) Parent shall use reasonable best efforts to consummate the Financing on a timely basis to facilitate the Closing when required bySection 2.01 on the terms and conditions described in the Commitment Letters, including using reasonable best efforts to (i) maintain in effect the Commitment Letters, (ii) ensure the accuracy of all representations and warranties of Parent set forth in the Commitment Letters, (iii) comply with all covenants of Parent set forth in the Commitment Letters, (iv) satisfy (or obtain waivers to satisfy) on, or prior to, the Closing Date (and, in any event, prior to the Outside Date), all conditions to funding in the Commitment Letters and such definitive agreements to be entered into pursuant thereto applicable to Parent that are within Parent’s control, (v) negotiate and enter into definitive agreements with respect to the Financing on terms and conditions described in the Commitment Letters in all material respects and (vi) enforce its rights under the Commitment Letters. In the event that all conditions precedent in the Financing Commitments have been satisfied, or upon funding will be satisfied, Parent will use its reasonable best efforts to cause the Financing Sources party to such Commitment Letters to fund on the Closing Date the full amount of the Financing.
(b) If all or any portion of the Financing becomes unavailable on the terms and conditions contemplated in the applicable Commitment Letter (other than as a result of (i) a breach by Member of this Agreement which prevents or renders impracticable the consummation of the Financing or (ii) the termination of the Commitment Letters pursuant to the terms thereof): (x) Parent shall notify the Member in writing as soon as reasonably practicable; and (y) Parent shall use reasonable best efforts to arrange to obtain any such unavailable portion from alternative sources (the “Alternative Financing”) (such alternative sources, together with the Lenders and any other parties to any joinder agreements, credit agreements or other definitive agreements with respect to the Commitment Letters, the “Financing Sources”), which Alternative Financing (A) shall be on terms that are not materially less favorable, taken as a whole, to Parent than those in the applicable Commitment Letter (including flex provisions) as in effect on the date hereof and (B) shall not contain any terms that would not be permitted to be effected by an amendment, modification or waiver of the applicable Commitment Letter pursuant toSection 6.03(c). Notwithstanding anything to the contrary in this Agreement, in no event shall the “reasonable best efforts” of Parent be deemed or be construed to require Parent to, and Parent shall not be required to (i) pay any fees in the aggregate in excess of those contemplated by the Commitment Letters as in effect on the date hereof (including any flex provisions contained therein) or (ii) agree to conditionality or economic terms of the Financing (or any Alternative Financing) that are materially less favorable than those contemplated by the Commitment Letters as in effect on the date hereof (including any flex provisions contained therein).
(c) Parent shall keep the Member informed on a reasonably current basis in reasonable detail with respect to the Financing (and any Alternative Financing), and shall provide the Member with prompt notice if it becomes aware of any material adverse change with respect to the availability of the Financing (or any Alternative Financing). Without limiting the generality of the foregoing, Parent shall notify the Member as promptly as reasonably practicable, and in any event within five (5) Business Days, (i) if there exists any material breach, material default, repudiation, cancellation or termination by any party to a Commitment Letter or any definitive document related to the Financing, in each case, of which Parent obtains knowledge, (ii) of the receipt by Parent of any written notice or other written communication from any Person with respect to (x) any material breach, material default, repudiation, cancellation or termination by any party to a Commitment Letter or any definitive document related to the Financing or (y) a material dispute or disagreement between or among Parent, on the one hand, and any parties to any Commitment Letter, or any definitive document related to the Financing, on the other hand, in each case, with respect to the obligation to fund the Financing at Closing in an amount, together with Parent’s other sources of funds, at least equal to the Required Amount on the terms and conditions
A-49
set forth in the applicable Commitment Letters;provided that in no event will Parent be under any obligation to disclose any information pursuant to thisSection 6.03 that is subject to (I) attorney-client or similar privilege or (II) a binding confidentiality obligation that would prohibit the disclosure of such information to the Member pursuant to its terms. Parent may amend, replace, supplement, modify or waive any provision of any Commitment Letter or definitive agreements relating to any Financing to (A) add and/or replace lenders, arrangers, bookrunners, agents or similar entities, as applicable, if the addition and/or replacement of such Persons would not, individually or in the aggregate, prevent, significantly delay or materially impair the availability of the Financing or the consummation of the transactions contemplated by this Agreement, (B) modify pricing and/or implement or exercise any of the flex provisions contained in the Commitment Letters or (C) reallocate commitments or assign or reassign titles or roles to, or between or among, any parties to the Commitment Letters. Parent shall not permit, and shall not permit Holdco or Merger Sub to make, any amendment, replacement, supplement, modification or waiver of any provision of any Commitment Letter or any definitive agreement relating to the Financing, without the prior written consent of the Member, if such amendment, replacement, supplement, modification or waiver would (x) decrease the aggregate amount of the Financing below an amount that, when combined with Parent’s other sources of funds, is less than the Required Amount or (y) impose new or additional conditions or otherwise expand any of the conditions to the receipt of any of the Financing in a manner that would reasonably be expected to prevent or materially impede or delay the consummation of the transactions contemplated by this Agreement as and when required bySection 2.01. Parent shall request that each of the Financing Sources provide, at least thirty (30) days prior to the Closing Date, a list of each document and other item of information about the Group Companies that the Financing Sources have reasonably determined (as of such date) is required to comply with applicable Law to the extent required under applicable “know your customer” and anti-money laundering rules and regulations, including the USA PATRIOT Act.
(d) Notwithstanding anything to the contrary in this Agreement, a violation ofSection 6.03(a),(b) and/or(c) shall not give rise to a termination right in favor of the Member or otherwise permit the Member to decline to consummate the Closing on the terms and conditions set forth in this Agreement if Parent, Holdco and Merger Sub each satisfy its obligations to consummate the Closing as and when required pursuant to this Agreement.
6.04Listing. Holdco and Parent shall use their reasonable best efforts to cause the shares of Holdco Common Stock to be issued in connection with the Merger, the shares of Holdco Common Stock to be issued in respect of the Stock Consideration and the shares of Holdco Common Stock to be reserved upon settlement or exercise of equity awards in respect of Holdco Common Stock to be approved for listing on Nasdaq, subject to official notice of issuance, prior to the Effective Time.
6.05Operations of Parent Prior to the Closing. Between the date hereof and the earlier of the termination of this Agreement in accordance with its terms and the Closing, except (a) as otherwise expressly contemplated by this Agreement, (b) required by applicable Law or (c) with the prior approval of the Member, Parent shall, and shall cause its Subsidiaries, to conduct their business, in all material respects, in the Ordinary Course of Business and in compliance with applicable Law. Without limiting the generality of the foregoing, except as (w) as otherwise expressly contemplated by this Agreement, (x) set forth on the Parent Disclosure Schedule or in the Parent SEC Reports filed with or furnished to the SEC after January 1, 2017 and prior to the date of this Agreement (other than any information that is contained solely in the “Risk Factors” section of such Parent SEC Reports and any forward-looking statements or other statements that are similarly predictive or forward-looking in nature), (y) required by applicable Law or (z) with the prior approval of the Member, neither Parent nor any of its Subsidiaries shall take any of the following actions between the date hereof and the earlier of the termination of this Agreement in accordance with its terms and the Closing:
(a) make any amendment or modification to any of the Parent Governing Documents;
(b) issue any additional shares of capital stock, membership interests or partnership interests or other equity securities or grant any option, warrant or right to acquire any capital stock, membership interests or partnership interests or other equity securities or issue any security convertible into or exchangeable for
A-50
such securities or alter in any way any of its outstanding securities or make any change in outstanding shares of capital stock, membership interests or partnership interests or other ownership interests or its capitalization, whether by reason of a reclassification, recapitalization, stock split or combination, exchange or readjustment of shares, stock dividend or otherwise, except, in each case, for (A) grants of Parent Equity Awards in the Ordinary Course of Business, or (B) shares of Parent Common Stock issuable upon settlement or exercise, as applicable, of outstanding Parent Equity Awards in accordance with their terms;
(c) declare, set aside or pay dividends or other distributions (whether payable in cash, stock, property or a combination thereof) in respect of the capital stock of Parent;
(d) redeem, retire, purchase or otherwise acquire, directly or indirectly, any shares of the capital stock, membership interests or partnership interests or other ownership interests of Parent or any of its Subsidiaries, other than (A) in connection with (x) required Tax withholding in connection with the vesting and/or exercise of Parent Equity Awards and (y) forfeitures of Parent Equity Awards pursuant to their terms as in effect on the date of this Agreement or (B) purchases or acquisitions of Parent Common Stock to offset dilution from Parent Stock Options and other Parent Equity Awards;
(e) adopt a plan of complete or partial liquidation or dissolution, or acquire another business, in each case, to the extent such action would, or would reasonably be expected to, prevent, materially delay or materially impair the consummation of the transactions contemplated by this Agreement;
(f) adopt a plan of merger or consolidation, enter into any binding share exchange, business combination or similar transaction with another Person or restructure or reorganize, in each case, to the extent (i) such action is not an action contemplated bySection 6.05(e), (ii) such action would, or would reasonably be expected to, prevent, materially delay or materially impair the consummation of the transactions contemplated by this Agreement and (iii) the Board of Directors of Parent has not effected a Parent Change in Recommendation pursuant toSection 6.11 in connection with such action; or
(g) agree, whether orally or in writing, to do any of the foregoing, or agree, whether orally or in writing, to any action or omission that would result in any of the foregoing.
6.06Parent Stockholder Support Agreements. Parent shall instruct its transfer agent not to register the transfer of any Covered Shares (as defined in the Parent Stockholder Support Agreements) made or attempted to be made in violation of the Parent Stockholder Support Agreements.
6.07Approval by Sole Stockholders of Holdco and Merger Sub. Immediately following the execution and delivery of this Agreement by the Parties, (a) Parent, as sole stockholder of Holdco, shall adopt this Agreement and approve the issuance of shares of Holdco Common Stock and Holdco Preferred Stock in connection with the Merger and Contribution, in accordance with Section 228 of the DGCL, by written consent, and (b) Holdco, as sole stockholder of Merger Sub, shall adopt this Agreement and approve the Merger, in accordance with Section 228 of the DGCL, by written consent.
(a) Holdco agrees that, for a period of not less than one (1) year following the Closing, it shall, or it shall cause its applicable Subsidiary to, provide all individuals who are employees of any Group Company (including employees who are not actively at work on account of illness, disability or leave of absence) as of the Closing (the “Affected Employees”), while employed by Holdco, or any of its Subsidiaries, with salaries, annual bonus opportunities (excluding equity and long-term incentive awards) and employee benefits that are substantially comparable in the aggregate to those provided to such Affected Employees immediately prior to the Closing. From and after the Closing, Holdco shall cause each Group Company to comply with the terms (including terms which provide for or permit amendment or termination) of all contracts, agreements, plans and commitments of such Group Company as in effect immediately prior to the Closing that are applicable to any employees or current or former directors or managers of such Group Company.
A-51
(b) Holdco shall cause each Affected Employee to receive full credit for service accrued, or deemed accrued, as of immediately prior to the Closing with the applicable Group Company for all purposes (including for purposes of eligibility, vesting and benefit accrual, but excluding benefit accrual under any defined benefit pension plan), under any employee benefit plan, program or arrangement established or maintained by Holdco or any of its Subsidiaries under which such Affected Employee may be eligible to participate from or after the Closing to the same extent recognized by the applicable Group Company immediately prior to the Closing (except to the extent that it would result in a duplication of benefits).
(c) With respect to the welfare benefit plans, programs and arrangements maintained, sponsored or contributed to by Holdco or any of its Subsidiaries (the “Holdco Welfare Benefit Plans”) in which an Affected Employee may become eligible to participate from or after the Closing, Holdco shall use commercially reasonable efforts to (i) cause to be waived all limitations as toat-work conditions, if any, with respect to participation and coverage requirements applicable to each such Affected Employee and his or her eligible dependents under any Holdco Welfare Benefit Plan to the same extent waived under an analogous Company Employee Benefit Plan, (ii) cause any eligible expenses incurred by any Affected Employee and his or her eligible dependents under a Company Employee Benefit Plan during the plan year in which such individuals transition their coverage to an analogous Holdco Welfare Benefit Plan to be taken into account under such Holdco Welfare Benefit Plan for purposes of satisfying all deductible,co-insurance and maximumout-of-pocket requirements applicable to such Affected Employee and his or her eligible dependents as if such amounts had been paid in accordance with such Holdco Welfare Benefit Plan and (iii) waive any waiting period limitation or evidence of insurability requirement that would otherwise be applicable to an Affected Employee and his or her eligible dependents from or after the Closing during the plan year in which such individuals transition their coverage to an analogous Holdco Welfare Benefit Plan.
(d) Without limiting the generality of the foregoing, nothing contained in thisSection 6.08 will create any third party beneficiary rights in any Person not a Party hereto, including any employee of a Group Company or beneficiary or dependent thereof. Nothing contained in thisSection 6.08, express or implied, (i) shall be construed to establish, amend or modify any Company Employee Benefit Plan or other benefit plan, program or arrangement, (ii) require Holdco or any of its Subsidiaries to continue any Company Employee Benefit Plan or other benefit plan, program or arrangement, or prevent the amendment, modification or termination thereof, in accordance with the terms thereof and applicable Law, following the Closing or (iii) guarantee employment for any period of time or preclude the ability of Holdco or any of its Subsidiaries to terminate any employee for any reason.
6.09Indemnification; Directors’ and Officers’ Insurance.
(a) To the fullest extent permitted by Law, from and after the Closing, all rights to indemnification, as provided in the Governing Documents of the Group Companies as of the date hereof, in favor of the current or former employees, directors, managers and/or officers of the Group Companies (the “Group Company Indemnified Parties”) with respect to their activities on behalf of the Group Companies prior to the Closing, shall survive the Closing and shall continue in full force and effect (without amendment adverse to such Group Company Indemnified Parties) for a period of not less than six (6) years following the Closing, and Holdco shall not, and shall cause each of the Group Companies not to, derogate such rights.
(b) Prior to the Closing, the Member shall cause the Company to purchase, at Parent’s sole cost and expense as a Parent Transaction Expense, a six (6) year prepaid “tail policy,” with terms, conditions, retentions and limits of liability that are at least as favorable to the beneficiaries thereof as provided in the Company’s existing directors’ and officers’ insurance policies and the Company’s existing fiduciary liability insurance policies (collectively, the “D&O Insurance Policies”) as of the date hereof, with respect to matters existing or occurring at or prior to the Closing, covering, without limitation, the transactions contemplated hereby (the “D&O Tail Policy”);provided,however, that the Company may not purchase a D&O Tail Policy with an aggregate annual premium in excess of 200% of the aggregate annual premium most recently paid by the Company prior to the date hereof to maintain the D&O Insurance Policies (and if
A-52
the aggregate annual premium for such D&O Tail Policy exceeds such amount, the Company shall purchase a D&O Tail Policy with the greatest coverage available for a cost not exceeding such amount). Following the Closing, Holdco shall not, and shall cause each of the Group Companies not to, terminate or amend the D&O Tail Policy. Following the Closing, Holdco shall cause the Group Companies to use commercially reasonable efforts to recover amounts under the D&O Tail Policy.
(c) If Holdco or any of its successors or assigns shall (i) consolidate with or merge into any other Person and shall not be the continuing or surviving corporation or entity of such consolidation or merger, (ii) transfer all or substantially all of its properties and assets to any Person or (iii) cease to exist for any reason, then, and in each such case, proper provisions shall be made so that the successors and assigns of Holdco shall assume all of the obligations of Holdco set forth in thisSection 6.09.
(d) The provisions of thisSection 6.09 are intended to be for the benefit of, and shall be enforceable by, each of the Group Company Indemnified Parties and their respective heirs and legal representatives, who are third party beneficiaries of thisSection 6.09, with full rights of enforcement against Holdco as if a party hereto. The rights of each Group Company Indemnified Party under this Section 6.09 shall be in addition to any rights such Group Company Indemnified Party may have under any applicable indemnification agreement to which such Group Company Indemnified Party is a party. The obligations set forth in thisSection 6.09 shall not be terminated, amended or otherwise modified in any manner that adversely affects any Group Company Indemnified Party, or an heir or legal representative thereof, without the prior written consent of such affected Group Company Indemnified Party or other Person.
6.10Designated Director. Following the Closing, until the later of (a) December 31, 2022, and (b) the time at which the Ownership Percentage is less than ten percent (10%), the Member shall be entitled to nominate one director to the Board of Directors of Holdco (the “Designated Director”), which Designated Director shall be Jeffrey C. Lightcap, Anthony G. Viscogliosi or any other person mutually agreed by Holdco and the Member. In accordance with the provisions of this Section 6.10, at each meeting of Holdco’s stockholders at which the election of directors is to be considered, Holdco shall nominate the Designated Director designated by the Member for election to the Board of Directors of Holdco by the holders of Holdco voting stock and solicit proxies from Holdco’s stockholders in favor of the election of such Designated Director. Holdco shall use its reasonable best efforts to cause such Designated Director to be elected to the Board of Directors of Holdco (including voting all unrestricted proxies in favor of the election of such Designated Director and including recommending approval of such Designated Director’s election to the Board of Directors of Holdco) and shall not take any action designed to diminish the prospects of such Designated Director being elected to the Board of Directors of Holdco. Each Designated Director elected pursuant to this Section 6.10 shall continue to hold office until the next annual meeting of the stockholders of Holdco and until his or her successor is elected and qualified in accordance with thisSection 6.10 and the Governing Documents of Holdco, unless such Designated Director is earlier removed from office by the Member or at such time as such Designated Director’s death, resignation, retirement or disqualification. Holdco shall use its reasonable best efforts to ensure that any Designated Director is removed only if so directed in writing by the Member, unless otherwise required by this Section 6.10 or applicable Law. In the event of a vacancy on the Board of Directors of Holdco resulting from the death, disqualification, resignation, retirement or termination of the term of office of a Designated Director, Holdco shall use its reasonable best efforts to cause the Board of Directors of Holdco to fill such vacancy or new directorship with a new Designated Director, as provided hereunder, to serve until the next annual meeting of the stockholders of Holdco (and at such meeting, such new Designated Director, or another Designated Director designated by the Member, will be nominated to be elected to the Board in the manner set forth in this Section 6.10). If the Member fails or declines to fill the vacancy, then the directorship shall remain open until such time as the Member elects to fill it with a Designated Director hereunder. Each Designated Director shall be entitled to compensation (including committee and committee chair compensation), reimbursement of expenses incurred in such capacity and indemnification and advancement of expenses on the same basis as Holdco provides such compensation, reimbursement, indemnification and advancement to the othernon-management members of its Board of Directors. From the Closing Date until the date on which Holdco has no further obligation pursuant toSection 2.06 to issue any Earnout Shares or make any cash payment pursuant to
A-53
Section 2.06(a)(i) or2.06(a)(iii), Holdco shall (i) provide the Designated Director with access to the business of Holdco and its Subsidiaries sufficient to provide information with respect to the strategic direction and performance of Holdco’s spine business and (ii) keep the Designated Director informed and keep the Designated Director involved with respect to the search and interview process for any business unit leader of Holdco’s spine business unit following the Closing.
6.11Parent Change in Recommendation.
(a) Except as permitted by Section 6.11(b), the Board of Directors of Parent and each committee of the Board of Directors of Parent shall not change, withhold, withdraw, qualify or modify (or publicly propose or resolve to change, withhold, withdraw, qualify or modify), in a manner adverse to the Member, the Parent Recommendation.
(b) Notwithstanding anything in this Agreement to the contrary, at any time prior to the time the Required Parent Vote is obtained, the Board of Directors of Parent may change, withhold, withdraw, qualify or modify (or publicly propose or resolve to change, withhold, withdraw, qualify or modify) the Parent Recommendation in a manner adverse to the Member (a “Parent Change in Recommendation”) if, and only if, Parent’s Board of Directors determines in good faith, after consultation with outside counsel and, if Parent’s Board of Directors reasonably determines, after consultation with outside counsel, it to be necessary in connection with its fulfillment of its fiduciary duties under applicable Law, a financial advisor of nationally recognized reputation, that the failure to take such action would be inconsistent with its fiduciary duties under applicable Law as a result of an Intervening Event;provided that no Parent Change in Recommendation may be made until after 5:00 p.m., New York City time, on the fifth (5th) Business Day following the Member’s receipt of written notice from Parent advising that Parent’s Board of Directors intends to take such action and specifying the reasons therefor, which notice shall include a reasonably detailed description of the applicable Intervening Event. After providing such notice and prior to effecting such Parent Change in Recommendation, (i) Parent shall, during such five (5) Business Day period, negotiate in good faith with the Member and its Representatives, to the extent the Member wishes to negotiate, with respect to any revisions to the terms of the transactions contemplated by this Agreement proposed by the Member, and (ii) in determining whether it may still under the terms of this Agreement make a Parent Change in Recommendation, the Board of Directors of Parent shall take into account any changes to the terms of this Agreement proposed by the Member and any other information provided by the Member in response to such notice during such five (5) Business Day period.
6.12Post-Closing Sales Leadership. Except as may be otherwise provided for herein, from the Closing Date until December 31, 2022, Holdco shall use its reasonable best efforts to cause the Sales Leader to be Bill Pfost. Holdco shall use its reasonable best efforts to cause progress meetings to be conducted, and progress reports to be prepared, on a monthly basis (or on such other frequency as agreed by Holdco’s Chief Executive Officer, Marc Viscogliosi and the leader of the sales force for coflex® and SImmetry products (the “Sales Leader”)). Holdco’s Chief Executive Officer, the Sales Leader, and Marc Viscogliosi shall each be entitled to attend such meetings (which, for the avoidance of doubt, may include additional individuals) and Holdco shall use its commercially reasonable efforts to cause such meetings to be scheduled so that such individuals may participate in person or remotely. In the event of a vacancy in the Sales Leader position, Holdco shall use its reasonable best efforts to fill such vacancy within a reasonable period of time.
A-54
ACTIONS PRIOR TO THE CLOSING
The respective Parties hereto covenant and agree to take the following actions:
7.01The Joint Proxy and Consent Solicitation Statement/Prospectus and Registration Statement.
(a) As promptly as reasonably practicable after the execution of this Agreement, Parent, Holdco and the Member shall jointly prepare and Parent shall file with the SEC a joint proxy and consent solicitation statement/prospectus (the “Joint Proxy and Consent Solicitation Statement/Prospectus”), and Holdco shall prepare and cause to be filed with the SEC a registration statement on FormS-4 (the “Registration Statement”), in which the Joint Proxy and Consent Solicitation Statement/Prospectus will be included as a prospectus, in each case, in connection with the transactions contemplated by this Agreement, which Joint Proxy and Consent Solicitation Statement/Prospectus and Registration Statement shall comply as to form in all material respects with the applicable requirements of the Securities Act, Exchange Act, the DGCL, the DLLCA and the Nasdaq rules, for the purpose of (i) soliciting proxies from Parent Stockholders for the purpose of obtaining the Required Parent Vote at the Parent Stockholder Meeting to be called and held for such purpose, (ii) soliciting written consents from the Unitholders for the purpose of obtaining the Written Consent and (iii) registering the issuance of the securities of Holdco to be issued pursuant toSections 1.01(b),1.04(a),1.04(b),2.03 and2.06(a). Parent, Holdco and the Member will use their respective reasonable best efforts to (x) respond as promptly as reasonably practicable to and resolve all comments received from the SEC or its staff concerning the Joint Proxy and Consent Solicitation Statement/Prospectus or the Registration Statement, (y) have the Registration Statement declared effective under the Securities Act as promptly as practicable after such filing and (z) keep the Registration Statement effective for so long as is necessary under applicable federal securities Laws to complete the transactions contemplated by this Agreement.
(b) As soon as reasonably practicable after the Registration Statement is declared effective under the Securities Act (but in no event earlier than the Parent Record Date), Parent shall cause a copy of the Joint Proxy and Consent Solicitation Statement/Prospectus to be delivered to each Parent Stockholder who was a Parent Stockholder as of the Parent Record Date. As soon as reasonably practicable after the Registration Statement is declared effective under the Securities Act, Parent shall use its reasonable best efforts to duly call, give notice of and hold the Parent Stockholder Meeting for the purpose of voting on (i) the adoption of this Agreement and (ii) the other proposals submitted to the vote of the Parent Stockholders in the Joint Proxy and Consent Solicitation Statement/Prospectus (collectively, the “Parent Voting Matters”), and, except to the extent that the Board of Directors of Parent shall have effected a Parent Change in Recommendation pursuant toSection 6.11, shall use its reasonable best efforts to solicit from each Parent Stockholder a proxy or vote in favor of the Parent Voting Matters. Without the prior written consent of the Member, the adoption of this Agreement, matters of procedure and matters required by applicable Law to be voted on by the Parent Stockholders in connection therewith shall be the only matters that Parent shall propose to be acted upon by the Parent Stockholders at the Parent Stockholder Meeting. Parent shall not, without the consent of the Member, adjourn or postpone, cancel, recess or reschedule the Parent Stockholder Meeting;provided, that Parent may, without the consent of (but after consultation with) the Member, adjourn or postpone the Parent Stockholder Meeting (A) if, as of the time for which the Parent Stockholder Meeting is originally scheduled (as set forth in the Joint Proxy and Consent Solicitation Statement/Prospectus), there are insufficient shares of Parent Stock represented (either in person or by proxy) to constitute a quorum necessary to conduct the business of the Parent Stockholder Meeting, (B) if the failure to adjourn or postpone the Parent Stockholder Meeting would reasonably be expected to be a violation of applicable Law or for the distribution of any required supplement or amendment to the Joint Proxy and Consent Solicitation Statement/Prospectus or (C) to solicit additional proxies if Parent reasonably determines that it is necessary to do so in order to obtain the Required Parent Vote;provided,further, that the date of the Parent Stockholder Meeting may not be postponed or adjourned for more than an aggregate
A-55
of thirty (30) days in connection with any postponements or adjournments in reliance on the immediately preceding proviso.Except to the extent that the Board of Directors of Parent shall have effected a Parent Change in Recommendation pursuant toSection 6.11, Parent shall, through Parent’s Board of Directors, recommend to its stockholders that they vote in favor of the Parent Voting Matters (the “Parent Recommendation”) and shall include such recommendation in the Joint Proxy and Consent Solicitation Statement/Prospectus. Parent shall, as soon as reasonably practicable after the Registration Statement is declared effective under the Securities Act, set a record date (the “Parent Record Date”) for determining the Parent Stockholders entitled to attend a meeting of the Parent Stockholders to vote on the Parent Voting Matters (the “Parent Stockholder Meeting”).
(c) As soon as reasonably practicable after the Registration Statement is declared effective under the Securities Act, the Member shall cause a copy of the Joint Proxy and Consent Solicitation Statement/Prospectus to be delivered to each of the Unitholders. Subject to the other provisions of this Agreement, as soon as reasonably practicable after the Registration Statement is declared effective under the Securities Act, the Member shall use its reasonable best efforts to solicit approval by written consent from the Unitholders for the purpose of obtaining the Written Consent. The Member shall, through the Member’s Board of Managers, recommend to the Unitholders that they provide their written consent to the adoption of this Agreement (the “Member Recommendation”) and shall include such recommendation in the Joint Proxy and Consent Solicitation Statement/Prospectus and the Member Recommendation shall not be withdrawn or modified in a manner adverse to Parent, and no resolutions by the Board of Managers of the Member or any committee thereof to withdraw such recommendation or modify such recommendation in a manner adverse to Parent shall be adopted.
(d) No filing of, or amendment or supplement to, the Joint Proxy and Consent Solicitation Statement/Prospectus or the Registration Statement, or response to SEC comments with respect thereto, will be made by Parent or Holdco without the approval of the Member (such approval not to be unreasonably withheld, conditioned or delayed) or without providing the Member a reasonable opportunity to review and comment thereon. If at any time there shall be discovered by any Party any information that should be set forth in an amendment or supplement to the Joint Proxy and Consent Solicitation Statement/Prospectus or the Registration Statement, so that the Joint Proxy and Consent Solicitation Statement/Prospectus or the Registration Statement would not include a misstatement of material fact or omit to state any material fact necessary to make the statements therein, in light of the circumstances under which they were made, not misleading, such Party shall promptly notify Parent, Holdco and the Member, and each of Parent, Holdco and the Member shall use its reasonable best efforts to cause an appropriate amendment or supplement describing such information to be promptly filed with the SEC and, to the extent required by applicable Law, disseminated to the Parent Stockholders or the Unitholders. Parent and Holdco will promptly notify the Member upon the receipt of any comments from the SEC or its staff or any request from the SEC or its staff for amendments or supplements to the Joint Proxy and Consent Solicitation Statement/Prospectus or the Registration Statement, and will, as promptly as practicable after receipt thereof, provide the Member with copies of all material correspondence between them and their Representatives, on the one hand, and the SEC and its staff, on the other hand, and all written comments with respect to the Joint Proxy and Consent Solicitation Statement/Prospectus or the Registration Statement received from the SEC or its staff and advise the Member on any oral comments with respect to the Joint Proxy and Consent Solicitation Statement/Prospectus or the Registration Statement received from the SEC or its staff. Holdco will advise the Member, promptly after it receives notice thereof, of the time of effectiveness of the Registration Statement and the issuance of any stop order relating thereto or the suspension of the qualification of the shares of Holdco Common Stock or Holdco Preferred Stock issuable in connection with the transactions contemplated hereby for offering or sale in any jurisdiction. Parent and Holdco will also take any other reasonable actions required to be taken (if any) under state securities or “blue sky” laws in connection with the transactions contemplated hereby, and the Member will use its reasonable best efforts to furnish all information concerning the Member and the Group Companies as Parent or Holdco may reasonably request in connection with any such actions.
A-56
(a) Within ten (10) Business Days after the date hereof, with respect to the Merger, the Contribution and the other transactions contemplated by this Agreement, the Parties shall make, or cause to be made, the filing required (if any) of each of them or any of their respective Subsidiaries or Affiliates under the HSR Act. The Parties hereto shall make, or cause to be made, as promptly as practicable, all filings necessary to obtain all Regulatory Approvals other than the HSR Approval or other filings under Antitrust Laws as set forth inSchedule 3.03(b). The Parties hereto shall: (i) cooperate and coordinate with the other Parties in the making of any filings or submissions that are required to be made under any applicable Laws or requested to be made by any Governmental Entity in connection with the transactions contemplated by this Agreement; (ii) respond promptly to any requests for additional information made by any Governmental Entity; (iii) upon the terms and subject to the conditions set forth in this Agreement, use their reasonable best efforts to cause the expiration or termination of any applicable waiting periods under any applicable Laws as soon as reasonably practicable; (iv) provide the other Parties with a reasonable opportunity to review and comment on any filing, submission, response to an information request or other (verbal or written) communication to be submitted or made to any Governmental Entity, and such receiving Party shall consider any such received comments in good faith; (v) advise the other Parties (and, where applicable, provide a copy) of any written or oral communications that it receives from any Governmental Entity regarding such filings (including in respect of any supplementary filings or submissions) and otherwise in connection with satisfying the Regulatory Approvals; (vi) provide the other Party with a reasonable opportunity to participate in any meetings with any Governmental Entity (subject to any opposition by a Governmental Entity to a particular Party’s participation in such meeting) and participate in, or review, any material communication before it is made to any Governmental Entity; and (vii) furnish to the other Parties or their outside counsel all information reasonably required or requested in connection with any application or other filing under the rules and regulations of any applicable Law in connection with the transactions contemplated by this Agreement. Notwithstanding the foregoing, (A) each Party has the right to redact or otherwise exclude a Party from receiving any confidential competitively sensitive information required to be shared under thisSection 7.02, in which event disclosure of such material may be limited to the other Party’s external counsel, and (B) no Party shall be required to share with any other Party any documents or information to the extent that such material reveals that Party’s negotiating objectives, strategies, or consideration expectations. The Parties shall not agree to an extension of any waiting period or review being undertaken by a Governmental Entity without the other Party’s prior written consent.
(b) Without limiting the generality of the foregoing, in connection with the efforts referenced inSection 7.02(a) to obtain all necessary consents, approvals, waivers and authorizations of any Governmental Entity required, each Party to this Agreement shall use reasonable best efforts to lift any restraint, injunction or other legal bar to the transactions contemplated by this Agreement, including if necessary to obtain clearance by any Governmental Entity before the Outside Date, but subject to the remainder of thisSection 7.02(b) (including the limitations set forth below), committing, agreeing or submitting (or offering to commit, agree, or submit) to any consent decree, hold separate order, sale, divestiture, lease, license, transfer, disposal, Encumbrance, other change or restructuring of, or operating restriction with respect to the businesses, properties, product lines, assets, permits, operations, rights, or interest therein of Parent or its Subsidiaries, or the Company or its Subsidiaries, and any other actions that limit the freedom of action with respect to, or the ability to retain, any business or assets of Parent or its Subsidiaries or the Company or its Subsidiaries (all of the foregoing, a “Divestiture Action”);provided, that, notwithstanding the foregoing, neither Parent nor any of its Subsidiaries shall be required to, and the Company and its Subsidiaries shall not, without the prior written consent of Parent, (x) commit, agree, submit (or offer to commit, agree or submit) to any Divestiture Action, if doing so would, individually or in the aggregate, reasonably be expected to be material to (1) Parent and its Subsidiaries or (2) the Company and its Subsidiaries (in each case of (1) and (2), as measured on a scale relative to the Company and its Subsidiaries, taken as a whole) or (y) commit, agree, or submit (or offer to commit, agree, or submit) to any Divestiture Action not conditioned on the consummation of the Merger and the Contribution. In addition, each Party shall use its commercially reasonable efforts to defend through litigation on the merits any claim asserted in court by any
A-57
Person in order to avoid entry of, or to have vacated or terminated, any Order (whether temporary, preliminary or permanent) that would prevent the Closing.
7.03State Takeover Laws. In the event that any “fair price,” “moratorium,” “control share acquisition” or other anti-takeover Law is or becomes applicable to any of the transactions contemplated by this Agreement, any Unitholder Support Agreement or any Parent Stockholder Support Agreement, the Parties shall use their reasonable best efforts to ensure that the transactions contemplated by this Agreement, the Unitholder Support Agreements and the Parent Stockholder Support Agreements may be consummated as promptly as reasonably practicable on the terms and subject to the conditions set forth in this Agreement and otherwise eliminate or minimize the effects of such Law on this Agreement, the Unitholder Support Agreements and the Parent Stockholder Support Agreements and the transactions contemplated hereby and thereby.
CONDITIONS TO CLOSING
8.01Conditions to Parent’s, Holdco’s and the Merger Sub’s Obligations. The obligations of Parent, Holdco and the Merger Sub to consummate the transactions contemplated by this Agreement are subject to the satisfaction (or, if permitted by applicable Law, waiver by Parent, Holdco and the Merger Sub in writing) of the following conditions as of the Closing Date:
(a) (i) The representations and warranties set forth inSection 3.04(a) shall be true and correct, subject only tode minimis inaccuracies, at and as of the date hereof and the Closing Date as though made at and as of the Closing Date, (ii) the other Member Fundamental Representations shall be true and correct in all material respects at and as of the date hereof and the Closing Date as though made at and as of the Closing Date (except to the extent expressly made as of an earlier date, in which case only as of such date) and (iii) all other representations and warranties of the Company contained inArticle III of this Agreement shall be true and correct (without giving effect to any limitation as to “materiality” or “Material Adverse Effect” set forth therein, other than with respect toSection 3.06(a)) at and as of the date hereof and the Closing Date as though made at and as of the Closing Date (except to the extent expressly made as of an earlier date, in which case only as of such date), except, in the case of this clause (iii), where the failure of such representations and warranties to be so true and correct (without giving effect to any limitation as to “materiality” or “Material Adverse Effect” set forth therein (other than with respect toSection 3.06(a))) has not had a Material Adverse Effect;
(b) The Member will have performed and complied with in all material respects all of the covenants and agreements required to be performed by it under this Agreement at or prior to the Closing;
(c) The Member shall have obtained the Written Consent;
(d) The Required Parent Vote shall have been obtained;
(e) The applicable waiting periods, if any, under the HSR Act will have expired or been terminated;
(f) No Governmental Entity of competent jurisdiction shall have enacted, issued, promulgated or entered any Order or Law that is in effect and that prevents the performance of this Agreement or the consummation of any of the transactions contemplated hereby, declares unlawful the transactions contemplated by this Agreement or causes such transactions to be rescinded;
(g) There will not have been a Material Adverse Effect pursuant to clause (b) of the definition thereof since the date hereof;
(h) The amount of the Cash Purchase Price set forth in the Consideration Certificate shall be equal to or greater than zero dollars ($0);
A-58
(i) The shares of Holdco Common Stock issuable pursuant to the Merger and the shares of Holdco Common Stock representing the Stock Consideration shall have been approved for listing on Nasdaq, subject to official notice of issuance;
(j) The Registration Statement shall have become effective under the Securities Act. No stop order suspending the effectiveness of the Registration Statement shall have been issued by the SEC and remain in effect and no proceedings for that purpose shall have been initiated or threatened by the SEC unless subsequently withdrawn;
(k) Parent shall have received representations of an officer of Parent, Holdco, and the Member made substantially in the form attached hereto asExhibit F;provided that this condition shall be deemed not to be satisfied if (i) Sidley Austin LLP, tax counsel to Parent, has delivered an opinion (the “Parent Change in Law Opinion”) that, as a result of a change in law occurring after the date of this Agreement, and based on the representations of officers of Parent, Holdco, Merger Sub, and the Member made substantially in the form attached hereto asExhibit F, it is or would be unable to provide an opinion to the effect that (A) the Merger and the Contribution, taken together, will qualify as a transaction described in Section 351 of the Code or (B) the Merger will qualify as a “reorganization” within the meaning of Section 368(a) of the Code (the “ParentTax-Free Opinion”) and (ii) Parent is unable to obtain the ParentTax-Free Opinion from an alternative tax counsel pursuant toSection 11.02(g)(ii);
(l) Neither Marc Viscogliosi nor Francis Magee shall have rescinded his obligations under his applicable Consulting Agreement;
(m) None of Viscogliosi Brothers, LLC, HealthCor Paradigm Blocker Company Two, Inc. and HealthCor AIV, L.P. shall have rescinded its obligations under its applicableNon-Competition Agreement;
(n) None of the parties to the Settlement Agreement shall have rescinded their obligations under the Settlement Agreement, the Settlement Agreement shall be in full force and effect and shall not have been amended in any manner adverse to Holdco, Parent or Merger Sub and the transactions contemplated by the Settlement Agreement shall have been performed in accordance with the terms of the Settlement Agreement; and
(o) The Member will have delivered to Parent a certificate of an authorized officer of the Member in his or her capacity as such, dated as of the Closing Date, stating that the conditions specified inSection 8.01(a),Section 8.01(b) andSection 8.01(g) have been satisfied (the “Company Closing Certificate”).
8.02Conditions to the Member’s Obligations. The obligation of the Member to consummate the transactions contemplated by this Agreement is subject to the satisfaction (or, if permitted by applicable Law, waiver by the Member in writing) of the following conditions as of the Closing Date:
(a) (i) The representations and warranties set forth inSections 4.04(a),(b),(c) and(d) shall be true and correct, subject only tode minimis inaccuracies, at and as of the date hereof and the Closing Date as though made at and as of the Closing Date (except to the extent expressly made as of an earlier date, in which case only as of such date), (ii) the other Parent Fundamental Representations will be true and correct in all material respects at and as of the date hereof and the Closing Date as though made at and as of the Closing Date (except to the extent expressly made as of an earlier date, in which case only as of such date) and (iii) all other representations and warranties contained inArticle IV of this Agreement will be true and correct (without giving effect to any limitation as to “materiality” or “Parent Material Adverse Effect” set forth therein, other than with respect toSection 4.09) at and as of the date hereof and the Closing Date as though made at and as of the Closing Date (except to the extent expressly made as of an earlier date, in which case only as of such date), except, in the case of this clause (iii), where the failure of such representations and warranties to be so true and correct (without giving effect to any limitation as to “materiality” or “Parent Material Adverse Effect” set forth therein (other than with respect toSection 4.09)) has not had a Parent Material Adverse Effect;
A-59
(b) Parent, Holdco and Merger Sub will have performed and complied with in all material respects all the covenants and agreements required to be performed by them under this Agreement at or prior to the Closing;
(c) The Member shall have obtained the Written Consent;
(d) The Required Parent Vote shall have been obtained;
(e) The applicable waiting periods, if any, under the HSR Act will have expired or been terminated;
(f) No Governmental Entity of competent jurisdiction shall have enacted, issued, promulgated or entered any Order or Law that is in effect and that prevents the performance of this Agreement or the consummation of any of the transactions contemplated hereby, declares unlawful the transactions contemplated by this Agreement or causes such transactions to be rescinded;
(g) There will not have been a Parent Material Adverse Effect pursuant to clause (b) of the definition thereof since the date hereof;
(h) The shares of Holdco Common Stock issuable pursuant to the Merger and the shares of Holdco Common Stock representing the Stock Consideration shall have been approved for listing on Nasdaq, subject to official notice of issuance;
(i) The Registration Statement shall have become effective under the Securities Act. No stop order suspending the effectiveness of the Registration Statement shall have been issued by the SEC and remain in effect and no proceedings for that purpose shall have been initiated or threatened by the SEC unless subsequently withdrawn;
(j) The Member shall have received representations of an officer of the Member and Holdco made substantially in the form attached hereto asExhibit G;provided that this condition shall be deemed not to be satisfied if (i) D&W, tax counsel to the Member, has delivered an opinion (the “Member Change in Law Opinion”) that, as a result of a change in law occurring after the date of this Agreement, and based on the representations of officers of the Member and Holdco made substantially in the form attached hereto asExhibit G, it is or would be unable to provide an opinion to the effect that the Merger and the Contribution, taken together, will qualify as a transaction described in Section 351 of the Code (the “MemberTax-Free Opinion”) and (ii) the Member is unable to obtain the MemberTax-Free Opinion from an alternative tax counsel pursuant toSection 11.02(g)(ii); and
(k) Parent shall not have rescinded its obligations under its Consulting Agreement with Marc Viscogliosi or Francis Magee;
(l) Parent will have delivered to the Member a certificate of an authorized officer of Parent in his or her capacity as such, dated as of the Closing Date, stating that the conditions specified inSection 8.02(a),Section 8.02(b) andSection 8.02(g), have been satisfied.
INDEMNIFICATION
(a)General Survival. Subject toSection 9.01(a) throughSection 9.01(c), the representations and warranties made by the Member in this Agreement and the representations and warranties set forth in the Company Closing Certificate (in each case other than the Member Fundamental Representations and the representations and warranties made by the Member inSection 3.21) shall survive the Effective Time until 11:59 pm (Central time) on the date that is eighteen (18) months following the Closing Date (the “Expiration Date”);provided,however,that if, at any time on or prior to the Expiration Date, any Parent
A-60
Indemnitee delivers to the Member a Claim Notice alleging the existence of an inaccuracy in or a breach of any of such representations and warranties and asserting a claim for recovery underSection 9.02 based on such alleged inaccuracy or breach, then the claim asserted in such Claim Notice shall survive the Expiration Date until such time as such claim is fully and finally resolved.
(b)Specified Representations.
(i) Notwithstanding anything to the contrary contained inSection 9.01(a), the representations and warranties made by the Member inSection 3.21 shall survive the Effective Time until 11:59 pm (Central time) on the date that is five (5) years following the Closing Date (the “Anti-Corruption Laws Expiration Date”);provided,however,that if, at any time on or prior to the Anti-Corruption Laws Expiration Date, any Parent Indemnitee delivers to the Member a Claim Notice alleging the existence of an inaccuracy in or a breach of any of the representations and warranties made by the Member inSection 3.21 and asserting a claim for recovery underSection 9.02 based on such alleged inaccuracy or breach, then the claim asserted in such Claim Notice shall survive the Anti-Corruption Laws Expiration Date until such time as such claim is fully and finally resolved.
(ii) Notwithstanding anything to the contrary contained inSection 9.01(a), the Member Fundamental Representations shall survive the Effective Time until sixty (60) days after the expiration of all applicable statutes of limitation (taking into account extensions thereof);provided, that, to the extent any of the foregoing survival periods is limited by Section 8106(c) of Title 10 of the Delaware Code, such survival period will be limited to the maximum period permitted by such section.
(c)Fraud. Notwithstanding anything to the contrary contained in this Agreement, the limitations set forth inSection 9.01(a) andSection 9.01(b) shall not apply with respect to any Fraud on the part of the Member or any Group Company. For purposes of thisArticle IX, “Fraud” means an actual and intentional fraud with respect to the making of the representations and warranties pursuant toArticle III or pursuant to the Company Closing Certificate.
(d)Parent Representations. All representations and warranties made by Parent, Holdco and Merger Sub shall terminate and expire as of the Effective Time, and any Liability of Parent, Holdco and Merger Sub with respect to such representations and warranties shall thereupon cease.
(a)Indemnification. From and after the Closing (but subject toSection 9.01, the other limitations set forth in thisArticle IX andSection 1.08(d)), the Member shall hold harmless and indemnify each of the Parent Indemnitees from and against, and shall compensate and reimburse each of the Parent Indemnitees for, any Damages that are directly or indirectly suffered or incurred at any time by any of the Parent Indemnitees or to which any of the Parent Indemnitees may otherwise directly or indirectly become subject at any time (regardless of whether or not such Damages relate to any third party claim) and that arise directly or indirectly from or as a result of, or are directly or indirectly connected with:
(i) any inaccuracy in or breach of any representation or warranty made by the Member in this Agreement as of the date of this Agreement;
(ii) any inaccuracy in or breach of any representation or warranty made by the Member: (A) in this Agreement as if such representation or warranty was made on and as of the Closing; or (B) in the Company Closing Certificate;
(iii) regardless of the disclosure of any matter set forth in the Disclosure Schedule, any inaccuracy in any information set forth in the Consideration Certificate, including any failure to properly calculate the Company Transaction Expenses, the Closing Indebtedness Amount, and the Cash Purchase Price;
(iv) any breach of any covenant or obligation of the Member in this Agreement required to be performed prior to the Closing, or any breach of any covenant or obligation of the Member in this Agreement required to be performed at or after the Closing; and
A-61
(v) regardless of the disclosure of any matter set forth in the Disclosure Schedule, the Designated Liabilities.
(b)Damage to Holdco. The Parties acknowledge and agree that, if any Group Company suffers, incurs or otherwise becomes subject to any Damages as a result of or in connection with any inaccuracy in or breach of any representation or warranty made by the Member or breach of any covenant or obligation of the Member, then (without limiting any of the rights of Group Companies as Parent Indemnitees (except as otherwise set forth in thisSection 9.02(b))) Holdco shall also be deemed, by virtue of its ownership of the Group Companies, to have incurred Damages as a result of and in connection with such inaccuracy or breach;provided, however, that the Parent Indemnitees shall only be entitled to recover any Damages in connection with an inaccuracy or breach once and, in the event a Parent Indemnitee has recovered for such Damages, or such Damages are specifically and actually included in the calculation of the Cash Purchase Price, no Parent Indemnitee shall be entitled to recover again for such Damages already recovered.
(a)Basket. Subject toSection 9.03(b), the Member shall not be required to make any indemnification payment pursuant toSection 9.02(a)(i) orSection 9.02(a)(ii) for any inaccuracy in or breach of any representation or warranty in this Agreement or in the Company Closing Certificate until such time as the total amount of all Damages that have been directly or indirectly suffered or incurred by any one or more of the Parent Indemnitees, or to which any one or more of the Parent Indemnitees has or have otherwise directly or indirectly become subject, in each case, arising from such inaccuracy or breach and all other Damages arising from any other inaccuracies or breaches of any representations or warranties, exceeds $850,000 in the aggregate (the “Basket Amount”). If the total amount of such Damages exceeds the Basket Amount, then the Parent Indemnitees shall only be entitled to be indemnified against and compensated and reimbursed for the portion of such Damages exceeding the Basket Amount.
(b)Applicability of Basket. The limitations set forth inSection 9.03(a) shall not apply: (i) to any Fraud; (ii) to inaccuracies in or breaches of any of the Member Fundamental Representations or any of the representations or warranties set forth inSection 3.21; or (iii) to any of the matters referred to inSections 9.02(a)(iii) throughSection 9.02(a)(v), inclusive.
(c)Cap. Subject toSection 9.03(d), the Parent Indemnitees sole and exclusive remedy under this Agreement for monetary Damages resulting from the matters referred to inSections 9.02(a)(i) and9.02(a)(ii) shall be to take the actions set forth inSection 2.06(e), up to an aggregate value of $15,000,000 worth of Earnout Shares (or, if applicable, cash payment pursuant toSection 2.06(a)(i) or2.06(a)(iii)), assuming, for purposes of any withholding, set off or reduction of the Earnout Shares, that each Earnout Share is equal in value to the Indemnification Price.
(d)Applicability of Cap. The $15,000,000 limitation set forth inSection 9.03(c) shall not apply, and the Parent Indemnitees shall be entitled to reduce the amount of any issuance of Earnout Shares (or, if applicable, cash payment pursuant toSection 2.06(a)(i) or2.06(a)(iii)) by the amount of applicable Damages, up to the total value of the Earnout Shares (or, if applicable, cash payment pursuant toSection 2.06(a)(i) or2.06(a)(iii)) to be issued to the Member (assuming, for purposes of any reduction of the Earnout Shares, that each Earnout Share is equal in value to the Indemnification Price): (i) to any Fraud; (ii) to inaccuracies in or breaches of any of the Member Fundamental Representations; or (iii) to any of the matters referred to inSections 9.02(a)(iii) throughSection 9.02(a)(v), inclusive;provided,however, that prior to reducing the amount of any cash payment pursuant toSection 2.06(a)(iii) or Earnout Shares available to be issued (or paid) to the Member, Holdco must first reduce the full amount of the cash payment earned but not yet paid to the Member pursuant toSection 2.06(a)(i). The Parent Indemnitees’ sole and exclusive remedy under this Agreement for monetary Damages resulting from the matters referred to in clauses (i), (ii) and (iii) above shall be to take the actions set forth in thisSection 9.03(d).
9.04Claim Procedures. Any Parent Indemnitee seeking indemnification hereunder shall promptly give to the Member a notice (a “Claim Notice”) describing in reasonable detail the facts giving rise to the claim for
A-62
indemnification hereunder and shall include in such Claim Notice (if then known) the amount or the method of computation of the amount of such claim, and a reference to the provision of this Agreement or any other agreement, document or instrument executed hereunder or in connection herewith upon which such claim is based;provided,however, that a Claim Notice in respect of any Legal Proceeding by or against a third Person as to which indemnification will be sought shall be given promptly after the Legal Proceeding is commenced. The failure to give notice as provided in thisSection 9.04 shall not relieve the Member of its obligations hereunder except to the extent it shall have been materially prejudiced by such failure.
(a) In calculating any Damage, such amounts shall be net of any third-party insurance, indemnification or other proceeds which have actually been recovered by the Parent Indemnitee, net of reasonable expenses incurred by the Parent Indemnitee in obtaining such recovery (including any applicable increases in premiums or deductibles in respect of any third-party insurance). If a Parent Indemnitee receives a payment required by thisArticle IX from the Member in respect of any Damages and subsequently receives insurance, indemnification or other proceeds from a third Person in respect of such Damages for which a payment has been received from the Member, Holdco shall, subject to the other terms and conditions of this Agreement, make a cash payment to the Member equal in value to such insurance, indemnification or other proceeds (such amount not to exceed the amount of Damages paid by the Member), net of the actual expenses incurred by the Parent Indemnitee in obtaining such recovery and any other applicable increases in premiums or deductibles in respect of any third party insurance.
(b) For the sole purpose of determining Damages (and not for determining whether any inaccuracies in or breaches of representations or warranties have occurred), the representations and warranties made by the Member in this Agreement and the representations and warranties set forth in the Company Closing Certificate shall not be deemed qualified by any references to materiality, Material Adverse Effect or any other similar qualification limiting the scope of such representation or warranty.
(c) After the giving of any Claim Notice pursuant toSection 9.04, the amount of indemnification to which a Parent Indemnitee shall be entitled under thisArticle IX shall be determined: (i) by the written agreement between the Parent Indemnitee and the Member; (ii) by a final judgment or decree of any court of competent jurisdiction; or (iii) by any other means to which the Parent Indemnitee and the Member shall agree. The judgment or decree of a court shall be deemed final when the time for appeal, if any, shall have expired and no appeal shall have been taken or when all appeals taken shall have been finally determined.
(a) Any Parent Indemnitee seeking indemnification provided for under this Agreement in respect of, arising out of or involving a claim or demand made by any third Person against the Parent Indemnitee shall notify the Member in writing, and in reasonable detail, of the third Person claim promptly after receipt by such Parent Indemnitee of written notice of the third Person claim (including personal service of process). Thereafter, the Parent Indemnitee shall deliver to the Member, promptly after the Parent Indemnitee’s receipt thereof, copies of all notices and documents (including court papers) received by the Parent Indemnitee relating to the third Person claim. The failure to give notice as provided in thisSection 9.06 shall not relieve the Member of its obligations hereunder except to the extent it shall have been materially prejudiced by such failure.
(b) In the event of the initiation of any Legal Proceeding against the Parent Indemnitee by a third Person for which Parent Indemnitee is seeking indemnification provided for under this Agreement prior to the time at which (i) the Member has earned any Earnout Shares or cash payment pursuant toSection 2.06 or (ii) the Company and its Subsidiaries have achieved 85% satisfaction of the revenue target set forth in the Company’s then-current annual operating plan as reviewed by the board of directors of Holdco, Parent Indemnitee shall then have the right, using counsel reasonably satisfactory to the Member, to investigate, contest, or settle such third Person claim. The Member may thereafter participate in (but not control) the
A-63
defense of any such third Person claim with its own counsel at its own expense. Parent Indemnitee may not settle any such third Person claim without the consent of the Member, such consent not to be unreasonably withheld, conditioned or delayed;provided,however, that the Parent Indemnitee may settle any such third Person claim without such consent if such settlement contains an unconditional release by the Person or Persons asserting such third Person claim to the Member from all Liability with respect to such third Person claim without requiring payment of any amounts in connection therewith or imposing any future restrictions on the conduct of the Member.
(c) In the event of the initiation of any Legal Proceeding against a Parent Indemnitee by a third Person for which such Parent Indemnitee is seeking indemnification provided for under this Agreement after the earlier of (i) the time at which the Member has earned any Earnout Shares or cash payment pursuant toSection 2.06 or (ii) the time at which the Company and its Subsidiaries have achieved 85% satisfaction of the revenue target set forth in the Company’s then-current annual operating plan as reviewed by the board of directors of Holdco, and (A) that relates solely to the payment of money damages, (B) does not contemplate any injunctive or othernon-monetary relief, (C) does not involve criminal allegations, and (D) is a Legal Proceeding in which the Member does not have conflicting or adverse interests to the Parent Indemnitee, the Member shall have twenty (20) days following receipt of a Claim Notice to deliver to the Parent Indemnitee a written acknowledgement that it will undertake, conduct and control (in accordance with the terms hereof), through counsel of its own choosing (provided that such counsel must be reasonably acceptable to the Parent Indemnitee) and at its own expense, the investigation, contestation or settlement thereof;provided, that the Parent Indemnitee may participate in such investigation, contestation or settlement through counsel chosen by the Parent Indemnitee and paid at its own expense. The Member shall not, except with the consent of the Parent Indemnitee (which consent shall not be unreasonably withheld, conditioned or delayed), enter into any settlement that (I) is not exclusively monetary, (II) shall not be paid entirely by the Member and (III) does not include as an unconditional term thereof the giving by the Person or Persons asserting such third Person claim to all Parent Indemnitees of an unconditional release from all Liability with respect to such third Person claim. If the Member does not notify the Parent Indemnitee that it will undertake, conduct and control (in accordance with the terms hereof) the investigation, contestation or settlement of such third Person claim within the applicable period set forth in thisSection 9.06(c), expressly declines in writing to undertake, conduct and control the investigation, contestation or settlement of such claim or is not eligible pursuant to thisSection 9.06(c) to conduct and control the investigation, contestation or settlement of such claim, the Parent Indemnitee shall then have the right, using counsel reasonably satisfactory to the Member, to investigate, contest, or settle such third Person claim. The Member may thereafter participate in (but not control) the investigation, contestation and settlement of any such third Person claim with its own counsel at its own expense. Parent Indemnitee may not settle any such third Person claim without the consent of the Member, such consent not to be unreasonably withheld, conditioned or delayed;provided,however, that the Parent Indemnitee may settle any such third Person claim without such consent if such settlement contains an unconditional release by the Person or Persons asserting such third Person claim to the Member from all Liability with respect to such third Person claim without requiring payment of any amounts in connection therewith or imposing any future restrictions on the conduct of the Member.
(d) Whether the Member or the Parent Indemnitee undertakes, conducts or controls the investigation, contestation or settlement of any such third Person claim, the Member and the Parent Indemnitee shall each cooperate fully (and shall each cause its Affiliates to cooperate fully) with the other in the investigation, contestation and settlement of such third Person claim pursuant to thisSection 9.06.
9.07Duty to Mitigate. Each Parent Indemnitee shall use its commercially reasonable efforts to mitigate the amount of any Damages that it incurs in connection with any matter with respect to which it is entitled to indemnification or reimbursement pursuant to thisArticle IX, including by pursuing recovery under available insurance policies of the Group Companies (or, if the Group Companies do not have any insurance policies of their own following the Closing, under available insurance policies of Holdco and its Subsidiaries);provided, that that the foregoing shall not be deemed to impose any obligation or duty on such Parent Indemnitee to initiate
A-64
any legal proceeding or incur any out of pocket costs or expenses (other thande minimis costs or expenses);provided,further, that the failure of such Parent Indemnitee to successfully mitigate or otherwise reduce such Damages shall not affect such Parent Indemnitee’s right to seek indemnification with respect to such Damages so long as such Parent Indemnitee shall have used its commercially reasonable efforts to mitigate.
9.08Tax Treatment of Indemnification Claims. Parent and the Member agree to report each indemnification payment made in respect of Damages as an adjustment to the Stock Consideration for federal income Tax purposes unless the indemnified party determines in good faith that such reporting position is incorrect (it being understood that if any reporting position is later disallowed in any administrative or court proceedings, the indemnifying party shall indemnify the indemnified party for the effects of such disallowance).
TERMINATION
10.01Termination. This Agreement may be terminated at any time prior to the Closing:
(a) by the mutual written consent of Parent and the Member;
(b) by Parent by written notice to the Member, if the Written Consent shall not have been provided to Parent within five (5) Business Days following the effectiveness of the Registration Statement;
(c) by Parent by written notice to the Member, if any of the representations or warranties of the Member set forth inArticle III will not be true and correct, or if the Member has failed to perform any covenant or agreement on the part of the Member set forth in this Agreement (including an obligation to consummate the Closing), such that the conditions to the Closing set forth in eitherSection 8.01(a) orSection 8.01(b) would not be satisfied at or prior to the Outside Date and the breach or breaches causing such representations or warranties not to be true and correct, or the failure to perform any covenant or agreement, as applicable, are not cured (if capable of being cured) within thirty (30) days after written notice thereof is delivered to the Member;provided,however, that Parent will not be permitted to terminate this Agreement pursuant to thisSection 10.01(c) if Parent, Holdco or Merger Sub is then in breach of any of their respective representations, warranties, covenants or agreements set forth in this Agreement in a manner such that the conditions to the Closing set forth in eitherSection 8.02(a) orSection 8.02(b) would not be satisfied;
(d) by the Member by written notice to Parent, if any of the representations or warranties of Parent, Holdco or Merger Sub set forth inArticle IV will not be true and correct, or if Parent, Holdco or Merger Sub has failed to perform any covenant or agreement on the part of Parent, Holdco or Merger Sub, respectively, set forth in this Agreement (including an obligation to consummate the Closing), such that the conditions to the Closing set forth in eitherSection 8.02(a) orSection 8.02(b) would not be satisfied at or prior to the Outside Date and the breach or breaches causing such representations or warranties not to be true and correct, or the failures to perform any covenant or agreement, as applicable, are not cured (if capable of being cured) within thirty (30) days after written notice thereof is delivered to Parent;provided,however, that the Member will not be permitted to terminate this Agreement pursuant to thisSection 10.01(d) if the Member is then in breach of any of its representations, warranties, covenants or agreements set forth in this Agreement in a manner such that the conditions to the Closing set forth in eitherSection 8.01(a) orSection 8.01(b) would not be satisfied;
(e) by Parent or the Member by written notice to the Member or Parent, as applicable, if the Closing has not occurred on or prior to January 31, 2019 (such date, as may be extended pursuant to this clause (e), the “Outside Date”);provided,however, that if the Closing has not occurred on or prior to January 31, 2019, the Outside Date may be extended by Parent until February 28, 2019 by written notice delivered to the Member on or after January 22, 2019, but on or before January 31, 2019;provided,further, that if the
A-65
Closing has not occurred on or prior to February 28, 2019, the Outside Date may be extended by Parent until March 31, 2019 by written notice delivered to the Member on or after February 19, 2019, but on or before February 28, 2019;provided,further, that the Party seeking to terminate this Agreement pursuant to this Section 10.01(e) will not have (provided that if such Party is Parent, neither Parent nor Holdco nor Merger Sub will have) breached in any material respect this Agreement in any manner that will have proximately caused the failure to consummate the transactions contemplated by this Agreement on or prior to the Outside Date;
(f) by Parent or the Member if the Required Parent Vote shall not have been obtained at the Parent Stockholders Meeting duly convened therefor (as such meeting may have been adjourned or postponed in accordance with this Agreement);
(g) by Parent or the Member if any Law or Order enacted, issued, promulgated or entered by a Governmental Entity of competent jurisdiction permanently restraining, enjoining or otherwise prohibiting consummation of the Merger or the Contribution shall become final andnon-appealable and the Party seeking to terminate this Agreement pursuant to thisSection 10.01(g) shall not have (provided that if such Party is Parent, neither Parent nor Holdco nor Merger Sub shall have) breached in any material respect this Agreement in any manner that will have proximately caused such Law or Order to be enacted, issued, promulgated or entered;
(h) by the Member by written notice to Parent, if the Board of Directors of Parent shall have made a Parent Change in Recommendation;provided,however, that the Member shall not have a right to terminate this Agreement pursuant to thisSection 10.01(h) if the Required Parent Vote has been obtained; and
(i) by the Member by written notice to Parent, if (a) all of the conditions set forth inSection 8.01 (other than any conditions that by their terms are to be satisfied at the Closing (but subject to such conditions being satisfied if the Closing would have occurred) and other than any conditions that have not been satisfied (or would not have been satisfied at the Closing) as a result of Parent, Holdco or Merger Sub’s breach of, or failure to perform any of their respective covenants or agreements contained in, this Agreement) have been satisfied or validly waived by Holdco, Parent and Merger Sub, (b) the Member has provided notice to Parent in writing that the Member is prepared, ready, willing and able to consummate the Closing and (c) Holdco, Parent and Merger Sub fail to consummate the Closing within three (3) Business Days after delivery of such notice and Member stood ready, willing and able to consummate the Closing throughout such period.
10.02Effect of Termination. In the event of the termination of this Agreement pursuant toSection 10.01, all obligations of the Parties hereunder will terminate without any Liability of any Party to any other Party;provided, that, notwithstanding the foregoing (a) the indemnification and reimbursement provisions ofSection 5.08(b), thisSection 10.02,Section 10.03,Section 11.01,Article XII andArticle XIII hereof shall survive the termination of this Agreement and (b) no termination of this Agreement will relieve a Party from any Liability arising from or relating to any willful and material breach of a representation or a covenant by such Party prior to such termination;provided, that a failure by Parent to consummate the Closing due to a Financing Failure shall not be a willful and material breach.
(a) In the event that this Agreement is terminated pursuant to (a) Section 10.01(h) or (b) Section 10.01(e) orSection 10.01(f) at a time when the Member had a right to terminate this Agreement pursuant toSection 10.01(h), Parent will pay to the Member, prior to or concurrent with such termination, in the case of a termination by Parent, or within three (3) Business Days thereafter, in the case of a termination by the Member, $4,500,000 (the “Parent Intervening Event Fee”).
(b) In the event that this Agreement is terminated pursuant to (a) Section 10.01(i) or (b) Section 10.01(e) at a time when the Member had a right to terminate this Agreement pursuant toSection 10.01(i), Parent will pay to the Member, prior to or concurrent with such termination, in the case of a termination by Parent, or within three (3) Business Days thereafter, in the case of a termination by the Member, $9,000,000 (the “Parent Financing Failure Fee”).
A-66
(c) Notwithstanding anything to the contrary in thisSection 10.03, if the Parent Intervening Event Fee becomes payable at a time when Parent, Holdco or Merger Sub is in breach of its obligations pursuant toSection 6.03 such that the Member would have the right to terminate this Agreement pursuant toSection 10.01(d), Parent shall instead pay the Member the Parent Financing Failure Fee (or, if Parent has already paid the Parent Intervening Event Fee, an amount equal to the Parent Financing Failure Fee minus the Parent Intervening Event Fee).
(d) All payments under thisSection 10.03 shall be made by wire transfer of immediately available funds to the account designated by the recipient thereof. The Parties acknowledge that the agreements contained in this Section 10.03 are an integral part of the transactions contemplated by this Agreement, and that, without these agreements, the Parties would not enter into this Agreement. Accordingly, if Parent fails to promptly pay any amount due pursuant to this Section 10.03, and, in order to obtain such payment, the Member commences a suit that results in a judgment against Parent for such amount, Parent shall pay to the Member (i) its reasonable costs and expenses (including reasonable attorneys fees) in connection with such suit and (ii) interest on the amount payable pursuant to such judgment, at the prime rate as published in the Wall Street Journal in effect on the date such payment was originally required to be made pursuant to this Section 10.03, with such interest being payable in respect of the period from the date that payment was originally required to be made pursuant to thisSection 10.03 through the date of payment. In the event that the Member shall receive payment of the Parent Intervening Event Fee or Parent Financing Failure Fee, the receipt of such fee, together with any reimbursement, indemnification or interest owed pursuant toSection 5.08(b) or thisSection 10.03, shall be deemed to be liquidated damages and the sole and exclusive remedy of the Member and its Subsidiaries and Unitholders against Parent, Holdco, Merger Sub, the Financing Sources, any other financing source of Parent and any of their respective former, current or future equity holders, controlling Persons, directors, managers, officers, employees, agents, Affiliates or assignees and any and all former, current or future equity holders, controlling Persons, directors, officers, employees, agents, general or limited partners, managers, management companies, members, Affiliates or assignees of any of the foregoing, and any and all former, current or future heirs, executors, administrators, trustees, successors or assigns of any of the foregoing (collectively, “Parent Related Parties”), and no Parent Related Party shall have any other Liability for any or all losses or damages suffered or incurred by the Member or any other Person in connection with this Agreement (and the termination hereof), the transactions contemplated hereby (and the abandonment thereof), the Financing, the Commitment Letters or any matter forming the basis for the termination of this Agreement, and none of the Member, any of its Affiliates or any other Person shall be entitled to bring or maintain any other claim, action or proceeding against Parent, Holdco, Merger Sub or any other Parent Related Party arising out of this Agreement, the Financing, the Commitment Letters or any of the transactions contemplated hereby, or any matters forming the basis for the termination of this Agreement. Under no circumstances shall Parent be required to pay both the Parent Intervening Event Fee and the Parent Financing Failure Fee, it being understood and agreed that, except as set forth inSection 10.03(c), only one payment of either the Parent Intervening Event Fee and the Parent Financing Failure Fee shall ever be payable hereunder.
ADDITIONAL COVENANTS
11.01Disclosure Schedules. All schedules attached hereto (each, a “Schedule” and, collectively, the “Disclosure Schedules”) are incorporated herein and expressly made a part of this Agreement as though completely set forth herein. All references to this Agreement herein or in any of the Schedules will be deemed to refer to this entire Agreement, including all Schedules. The Schedules have been arranged for purposes of convenience in separately numbered sections corresponding to the sections of this Agreement; however, any item disclosed in any part, subpart, section or subsection of the Schedule referenced by a particular section or subsection in this Agreement will be deemed to have been disclosed with respect to every other part, subpart,
A-67
section and subsection in another Schedule if the relevance of such disclosure to such other part, subpart, section or subsection is reasonably apparent on its face, notwithstanding the omission of an appropriate cross-reference. Any item of information, matter or document disclosed or referenced in, or attached to, the Disclosure Schedules will not (a) be used as a basis for interpreting the terms “material,” “Material Adverse Effect” or other similar terms in this Agreement or to establish a standard of materiality, (b) be deemed or interpreted to expand the scope of the Member’s, Parent’s, Holdco’s or Merger Sub’s respective representations and warranties, obligations, covenants, conditions or agreements contained herein or (c) constitute, or be deemed to constitute, an admission to any third Person concerning such item or matter. No disclosure in the Disclosure Schedules relating to any possible breach or violation of any Contract or Law will be construed as an admission or indication that any such breach or violation exists or has actually occurred. Capitalized terms used in the Disclosure Schedules and not otherwise defined therein have the meanings given to them in this Agreement.
(a)Liability for Taxes.
(i) The Member shall hold harmless and indemnify each of the Parent Indemnitees from and against, and shall compensate and reimburse each of the Parent Indemnitees for, any Damages that are directly or indirectly suffered or incurred at any time by any of the Parent Indemnitees or to which any of the Parent Indemnitees may otherwise directly or indirectly become subject at any time (regardless of whether or not such Damages relate to any third party claim) and that arise directly or indirectly from or as a result of or are directly or indirectly connected with (A) Taxes imposed on any Group Company, or for which any Group Company is otherwise liable, as a result of having been a member of a Company Group during any taxable year or period that ends on or before the Closing Date and, with respect to any Straddle Period, the portion of such Straddle Period ending on and including the Closing Date, (B) Taxes imposed on a Group Company, or for which a Group Company is otherwise liable, for any taxable year or period that ends on or before the Closing Date and, with respect to any Straddle Period, the portion of such Straddle Period ending on and including the Closing Date, (C) Taxes imposed on a Parent Group Member as a result of such Parent Group Member being a United States shareholder (within the meaning of Section 951(b) of the Code) of any Group Company, to the extent such amounts are attributable to Subpart F income (within the meaning of Section 952(a) of the Code) of such Group Company arising in (or that, but for the limitation under 952(c) of the Code, would have arisen in) a taxable year of the Group Company ending on or prior to the Closing Date or, with respect to any Straddle Period, the portion of such Straddle Period ending on and including the Closing Date and (D) Taxes imposed on any cancellation of indebtedness income arising as a result of the transactions contemplated by this Agreement;provided,however, that the Member shall not be liable for any Tax liability to the extent such Tax liability is taken into account in computing the Net Working Capital Amount, as finally determined pursuant toSection 1.08.
(ii) For purposes ofSection 11.02(a)(i), whenever it is necessary to determine the Liability for Taxes of a Group Company for a Straddle Period, the determination of the Taxes of the Group Company for the portion of the Straddle Period ending on and including the Closing Date shall be determined by assuming that the Straddle Period consisted of two (2) taxable years or periods, one which ended at the close of the Closing Date and the other which began at the beginning of the day following the Closing Date and items of income, gain, deduction, loss or credit of the Group Company for the Straddle Period shall be allocated between such two (2) taxable years or periods on a “closing of the books basis” by assuming that the books of the Group Company were closed at the close of the Closing Date;provided,however, that exemptions, allowances, deductions or Taxes that are calculated on an annual basis, such as ad valorem and other similar Taxes imposed on property (“Property Taxes”), franchise based solely on capital, and depreciation deductions, shall be apportioned between such two (2) taxable years or periods on a daily basis. In determining whether a Property Tax is attributable to a Tax period ending on or before the Closing Date or a Straddle Tax Period (or portion thereof), any Property Tax shall be deemed a Property Tax attributable to the taxable period specified on the relevant Property Tax bill . For purposes ofSection 11.02(a)(i), whenever it is necessary to determine the liability for Taxes of a United States shareholder (within the meaning of
A-68
Section 951(b) of the Code) of a controlled foreign corporation (within the meaning of Section 957 of the Code) attributable to amounts included in the income of such United States shareholder under Section 951 of the Code for the taxable year or period of such controlled foreign corporation that begins on or before and ends after the Closing Date, the determination of liability for any such Taxes shall be made by assuming that the taxable year or period of the controlled foreign corporation consisted of two (2) taxable years or periods, one which ended at the close of the Closing Date and the other of which began at the beginning of the day following the Closing Date and relevant items of income, gain, deduction, loss or credit of the controlled foreign corporation shall be allocated between such two (2) taxable years or periods on a “closing of the books basis” by assuming that the books of the controlled foreign corporation were closed at the close of the Closing Date;provided,however, that Subpart F income (within the meaning of Section 952 of the Code) of the controlled foreign corporation shall be determined without regard to Section 952(c) of the Code.
(b)Tax Returns.
(i) Member shall prepare or cause to be prepared and timely file or cause to be timely filed when due (taking into account all extensions properly obtained) (x) all Tax Returns that are required to be filed by or with respect to the Group Companies on a combined, consolidated or unitary basis with the Member, (y) all income Tax Returns of or with respect to the Group Companies for any taxable year or period that ends on or before the Closing Date and (z) all other Tax Returns that are required to be filed by or with respect to the Group Companies on or prior to the Closing Date. In each case the Member shall remit or cause to be remitted any Taxes due in respect of such Tax Returns. To the extent that any such Taxes due in respect to such Tax Returns are taken into account in computing the Net Working Capital amount as finally determined pursuant toSection 1.08, Parent shall remit the amount of such Taxes to the Member no later than five (5) days prior to the due date (including extensions) for filing such Tax Returns. To the extent permitted by Law, all Tax Returns required to be filed by the Member shall be signed by the Member (or its designee that is reasonably acceptable to Parent);provided that Parent and Holdco shall reasonably cooperate with the Member to provide appropriate authorization to the Member to sign such Tax Returns, or to make available an officer or other authorized person of the relevant Group Company to sign such Tax Returns, if and to the extent required by Law. Parent shall timely file or cause to be timely filed when due (taking into account all extensions properly obtained) all other Tax Returns that are required to be filed by or with respect to the Group Companies after the Closing Date and Parent shall remit or cause to be remitted any Taxes due in respect of such Tax Returns.
(ii) All Tax Returns of or with respect to the Group Companies for any taxable year or period that ends on or before the Closing Date, that Member is required to file or cause to be filed in accordance with thisSection 11.02(b) shall be prepared and filed in a manner consistent with past practice and, on such Tax Returns, no position shall be taken, election made or method adopted that is inconsistent with positions taken, elections made or methods used in preparing and filing similar Tax Returns in prior periods. With respect to any Tax Return to be filed by the Member pursuant toclause (y) of the first sentence ofSection 11.02(b)(i), not less than thirty (30) days prior to the due date for such Tax Return, taking into account extensions (or, if such due date is within thirty (30) days following the Closing Date, as promptly as practicable following the Closing Date), the Member shall provide Parent with a draft copy of such Tax Return for Parent’s review and approval (such approval not to be unreasonably withheld, conditioned or delayed).
(iii) All Tax Returns of or with respect to the Group Companies for any taxable year or period that ends on or before the Closing Date and for any Straddle Period that Parent is required to file or cause to be filed in accordance with thisSection 11.02(b) shall be prepared and filed in a manner consistent with past practice and, on such Tax Returns, no position shall be taken, election made or method adopted that is inconsistent with positions taken, elections made or methods used in preparing and filing similar Tax Returns in prior periods, in each case except as required by applicable Law.
A-69
(c)Contest Provisions.
(i) Parent shall notify the Member in writing upon receipt by Parent, any of its Affiliates or, after the Closing Date, any of the Group Companies of notice of any pending or threatened federal, state, local or foreign Tax audits or assessments of any of the Group Companies relating to any taxable period ending on or before the Closing Date or to any Straddle Period.
(ii) The conduct of any Tax audit or administrative or court proceeding relating to a Tax liability for which the Member would be required to indemnify Parent Group Members pursuant toSection 11.02(a) and that relates solely to a taxable year or period ending on or before the Closing Date (a “Tax Proceeding”) shall be governed as follows:
A) Prior to the time at which (i) the Member has earned any Earnout Shares or cash payment pursuant toSection 2.06 or (ii) the Company and its Subsidiaries have achieved 85% satisfaction of the revenue target set forth in the Company’s then-current annual operating plan as reviewed by the board of directors of Holdco, Parent shall have the sole right to represent the Group Companies’ interests in any Tax Proceeding, and to employ counsel of Parent’s choice;provided,however, that the Member and its Representatives shall be permitted, at the Member’s expense, to be present at, and participate in, any such audit or proceeding; and
B) After the earlier of (i) the time at which the Member has earned any Earnout Shares or cash payment pursuant toSection 2.06 and (ii) the time at which the Company and its Subsidiaries have achieved 85% satisfaction of the revenue targets set forth in the Company’s then-current annual operating plan as reviewed by the board of directors of Holdco, the Member shall have the right, upon written notice to Parent, to elect to represent the Group Companies’ interests in any Tax Proceeding, and to employ counsel of the Member’s choice, at the Member’s expense;provided,however, that (y) Parent and its representatives shall be permitted to be present at, and participate in, any such audit or proceeding and (z) the Member shall not be entitled to settle, either administratively or after the commencement of litigation, any claim for Taxes for which the Group Companies may be liable without the prior written consent of Parent.
(iii) Parent shall have the sole right to represent any Group Company’s interests in any Tax audit or administrative or court proceeding relating to Tax liabilities other than those for which the Member has exercised such right pursuant to paragraph (c)(ii)(B) of thisSection 11.02 and to employ counsel of Parent’s choice. Parent shall have the sole right to defend any Group Company with respect to any issue, and settle or compromise any issue, arising in connection with any Tax audit or administrative or court proceeding to the extent Parent shall have agreed in writing to forego any indemnification under this Agreement with respect to such issue.
(d)Tax Refunds. Subject toSection 2.06(e), the Member shall be entitled to receive from any Group Company all refunds (or credits for overpayments) of Taxes of any Group Company (including any interest thereon) for any taxable year or period that ends on or before the Closing Date and, with respect to any Straddle Period, the portion of such Straddle Period ending on and including the Closing Date, to the extent such refund (or credit for overpayment), plus interest, is not taken into account in computing the Net Working Capital Amount, as finally determined pursuant toSection 1.08 (a “Pre-Closing Tax Refund”). Subject toSection 2.06(e), upon receipt by Parent or any of its Affiliates (including any Group Company) of anyPre-Closing Tax Refund, Parent will, and will cause its applicable Affiliate to, deliver and pay over, by wire transfer of immediately available funds, the amount of suchPre-Closing Tax Refund (less any amounts taken into account in computing the Net Working Capital Amount) to the Member. Such payment shall be made to the Member no later than thirty (30) days following the actual receipt by Parent or its applicable Affiliate (including any Group Company) of suchPre-Closing Tax Refund or, in the case of a Tax credit, no later than thirty (30) days after such Tax credit is applied to actually reduce Taxes of Parent or its Affiliates (including any Group Company).
A-70
(e)Assistance and Cooperation. After the Closing Date, each of the Member and Parent shall (and shall cause their respective Affiliates to):
(i) timely sign and deliver such certificates or forms as may be necessary or appropriate to establish an exemption from (or otherwise reduce), or file Tax Returns or other reports with respect to, Taxes described inSection 13.03 (relating to sales, transfer and similar Taxes);
(ii) assist the other Party in preparing any Tax Returns which such other Party is responsible for preparing and filing in accordance withSection 11.02(b), and in connection therewith, provide the other Party with any necessary powers of attorney;
(iii) cooperate fully in preparing for and defending any audits of, or disputes with taxing authorities regarding, any Tax Returns of any Group Company;
(iv) make available to the other and to any taxing authority as reasonably requested all information, records, and documents relating to Taxes of the Group Companies; and
(v) furnish the other with copies of all correspondence received from any taxing authority in connection with any Tax audit or information request with respect to any Taxes or Tax Returns of the Group Companies;provided, that Parent shall only be obligated to furnish copies of such correspondence to the Member to the extent such audit or information request relates to Taxes for which the Member may be liable under the terms of this Agreement.
(f)Termination of Tax Allocation Arrangements. Any Tax Sharing Arrangement entered into by the Member or any Affiliate of the Member, on the one hand, and a Group Company, on the other hand, shall be terminated as to the Group Companies on or prior to the Closing, and after the Closing the Group Companies shall not have any Liability thereunder.
(g)Transaction Tax Treatment.
(i) None of Parent, Holdco, Merger Sub, the Member, or any Group Company shall take, or omit to take, any action that would, or would reasonably be expected to, prevent or impede (i) the Merger and the Contribution, taken together, from qualifying as a transaction described in Section 351 of the Code or (ii) the Merger from qualifying as a “reorganization” within the meaning of Section 368(a) of the Code.
(ii) If either (i) Parent obtains the Parent Change in Law Opinion from Sidley Austin LLP, it shall use reasonable best efforts to obtain a ParentTax-Free Opinion from an alternative tax counsel of similar standing; or (ii) the Member receives the Member Change in Law Opinion from D&W, it shall use reasonable best efforts to obtain a MemberTax-Free Opinion from an alternative tax counsel of similar standing.
(iii) Parent and the Member acknowledge and agree that the payment of cash pursuant toSection 2.06 shall be treated as property described in Section 351(b) of the Code and the issuance of Earnout Shares pursuant toSection 2.06 shall be treated as transfer of stock described in Section 351(a) of the Code.
(iv) Parent and the Member acknowledge and agree that for federal and any applicable state, local or foreign Tax purposes, any Indebtedness outstanding under the Credit Agreement or any applicable Interim Debt Financing shall be treated as Indebtedness of the Member which was not assumed by Holdco in connection with the Contribution, and any cancellation of indebtedness income resulting from the repayment of such Indebtedness shall be treated as income of the Member for such Tax purposes.
(h)Survival of Obligations. Notwithstanding anything to the contrary in this Agreement, the obligations of the Parties set forth in thisSection 11.02 shall survive until sixty (60) days after the expiration of all applicable statutes of limitation (taking into account extensions thereof).
(i)Sole and Exclusive Remedy. Notwithstanding anything in thisSection 11.02 to the contrary, Parent Indemnitees’ and Parent Group Members’ sole and exclusive remedy under this Agreement for monetary Damages (including, for the avoidance of doubt, Taxes) resulting from the matters referred to in thisSection 11.02 shall be to take the actions set forth inSection 2.06(e).
A-71
DEFINITIONS
12.01Definitions. For purposes hereof, the following terms when used herein will have the respective meanings set forth below:
“Accounts Receivable” means all accounts and notes receivable of the Group Companies included in the Net Working Capital Amount.
“Affiliate” of any particular Person means any other Person controlling, controlled by, or under common control with, such particular Person, where “control” means the possession, directly or indirectly, of the power to direct the management and policies of a Person whether through the ownership of voting securities, contract or otherwise.
“Antitrust Laws” means any federal, state or foreign Law, regulation or decree designed to prohibit, restrict or regulate actions for the purpose or effect of monopolization or restraint of trade or the significant impediment of effective competition.
“Business Day” means a day that is neither a Saturday nor a Sunday nor any other day on which banking institutions in New York, New York are authorized or obligated by Law to close.
“Cash” means, as of any time of determination, all cash and cash equivalents held by any Group Company and marketable securities with a maturity of ninety (90) days or less when purchased by the Group Companies held by any Group Company at such time and determined in accordance with GAAP. For avoidance of doubt, Cash will (a) be calculated net of issued but uncleared checks and drafts written or issued by any Group Company and (b) exclude any Restricted Cash.
“Cash Election Amount” means the amount in cash (rounded to the nearest whole U.S. Dollar) equal to (i) $45,000,000,multiplied by (ii) the quotient of(A) (1) the Giants/Zyga Revenue for the applicable LTM Period,minus (2) $105,000,000,divided by (B) $20,000,000, which amount shall be subject to reduction as set forth inSection 2.06(e);provided,however, that if the Giants/Zyga Revenue for the applicable LTM Period is greater than $125,000,000, for purposes of this calculation the Giants/Zyga Revenue shall be deemed to equal $125,000,000, it being understood that under no circumstances shall the Cash Election Amount exceed $45,000,000.
“Cash Purchase Price” means: (A) $100,000,000;plus(B) the Closing Cash Amount;minus(C) the Closing Indebtedness Amount;minus(D) the aggregate amount of all Company Transaction Expenses;minus (E) the amount, if any, by which the Net Working Capital Amount is less than the Target Working Capital.
“Change of Control Transaction” means any of the following: (a) consummation of a merger or consolidation of Holdco with or into any other corporation or other entity in which holders of Holdco’s voting securities immediately prior to such merger or consolidation will not, directly or indirectly, continue to (i) hold at least a majority of the outstanding voting securities of Holdco and (ii) have the right to appoint or designate a majority of the board of directors of Holdco; (b) the acquisition by any unrelated person or any group of unrelated persons, acting together in any transaction or related series of transactions, of such quantity of Holdco’s voting securities as causes such person, or group of persons, to (i) own beneficially, directly or indirectly, as of the time immediately after such transaction or related series of transactions, 50 percent or more of the combined voting power of the voting securities of Holdco and (ii) have the right to appoint or designate a majority of the board of directors of Holdco; or (c) consummation of the sale, transfer, conveyance or other disposition (by merger, consolidation, sale of equity interests or assets or otherwise), in one or a series of related transactions, of all or substantially all the assets of the Coflex Business to any third Person.
A-72
“Closing Cash Amount” means the aggregate amount of outstanding Cash as of the Effective Time.
“Closing Indebtedness Amount” means: (A) the aggregate amount of outstanding Indebtedness as of the Effective Time;minus (B) any outstanding Indebtedness as of the Effective Time to be paid to the lenders under the Credit Agreement or any applicable Interim Debt Financing at the Closing by the issuance of Holdco Common Stock, as set forth in the Payoff Letters.
“Code” means the Internal Revenue Code of 1986, as amended.
“Coflex Business” means the business of developing, manufacturing, marketing and selling coflex® spinal fixation products.
“Company Employee Benefit Plan” means each “employee benefit plan” within the meaning of Section 3(3) of ERISA and all other retirement, welfare, equity and equity-based, severance, retention, employment, individual consulting,change-of-control, bonus, incentive, deferred compensation, pension, employee loan, fringe benefit, and other benefit or compensation plan, agreement, program, practice, arrangement, or policy, whether written or unwritten, sponsored, maintained, contributed to, or required to be contributed to, by any of the Group Companies for the benefit of any current or former officer, employee, director, consultant, other service provider, or beneficiary or dependent thereof, of the Group Companies, to which a Group Company is a party or for which any of the Group Companies have or could reasonably be expected to have any Liability.
“Company Group” means any group of entities filing Tax Returns on a combined, consolidated, unitary or similar basis that, at any time on or before the Closing Date, includes or has included a Group Company or any direct or indirect predecessor of a Group Company.
“Company Privacy Policy” means each external or internal privacy policy, as of the date hereof, of the Company or any of its Subsidiaries, including any policy relating to: (a) the privacy of individuals in connection with any Company website or product of the Group Companies; (b) the collection, storage, disclosure, and transfer of any Personal Data; and (c) any employee information.
“Company Transaction Expense” means a Transaction Expense which the Company is obligated to pay and which remains unpaid as of the Closing;provided,however, that, notwithstanding anything in this Agreement to the contrary, any Transaction Expense included in the Closing Indebtedness Amount shall not be a Company Transaction Expense.
“Contract” means any agreement, contract, arrangement, lease, loan agreement, security agreement, license, indenture or other similar instrument or obligation to which the party in question is a party.
“Credit Agreement” means that certain Amended and Restated Credit Agreement and Guaranty, dated as of November 1, 2017, among the Company, the Subsidiaries of the Company party thereto, the lenders party thereto and Hayfin Services LLP, as administrative agent for the lenders, as amended.
“D&W” means Dorsey & Whitney LLP.
“Damages” means any loss, damage, injury, decline in value, Liability, claim, demand, settlement, judgment, award, fine, penalty, Tax, fee (including reasonable attorneys’ fees), charge, cost (including costs of investigation) or expense of any nature;provided,however, notwithstanding anything in this Agreement to the contrary, Damages shall not include any punitive damages, except to the extent payable to any third Person pursuant to a claim by a third Person described inSection 9.06.
“Data Breach” means the unauthorized access, use, disclosure, acquisition, or modification of Personal Data or any other data security incident requiring notification to impacted Persons or regulators under applicable Data Protection Laws.
A-73
“Data Protection Laws” means any and all applicable data protection and privacy laws in force from time to time in those parts of the world in which a Group Company is established, operates or processes Personal Data (either directly or via a third party), including the provisions of the following that set forth privacy or data security requirements that apply to Personal Data: the Federal Trade Commission Act, 15 U.S.C. § 45; theCAN-SPAM Act of 2003, 15 U.S.C. §§ 7701 et seq.; the Telephone Consumer Protection Act, 47 U.S.C. § 227; California Online Privacy Protection Act, Cal. Bus. & Prof. Code § 22575, et seq.; laws governing the privacy or security of health or medical information, including biometric information, including the Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), the Health Information Technology for Economic and Clinical Health Act (“HITECH”), and HITECH implementing regulations, including the Standards for Privacy of Individually Identifiable Health Information, codified at 45 C.F.R. Parts 160 and 164, Subparts A and E, the Security Standards for the Protection of Electronic Protected Health Information, codified at 45 C.F.R. Parts 160 and 164, Subpart A and C, Notification in the Case of Breach of Unsecured Protected Health Information, codified at 45 C.F.R. Parts 160 and 164, Subpart D, and the Standards for Electronic Transactions and Code Sets, codified at 45 C.F.R. Parts 160 and 162; laws governing notification to consumers, employees or other individuals and regulatory authorities following Data Breaches, including Cal. Civ. Code § 1798.82, N.Y. Gen. Bus. Law §899-aa, and Mass. Gen. Law 93H; federal, state, and local laws governing data security, including Massachusetts Gen. Law Ch. 93H, 201 C.M.R. 17.00, and Nev. Rev. Stat. 603A; Cal Civ. Code § 1798.83; local, state, and federal, and privacy, data protection, information security, or related laws relating to the collection, processing, storage, disclosure, disposal, or other handling of Personal Data; international laws, including the European Union’s Directive on Privacy and Electronic Communications (2002/58/EC), General Data Protection Regulation (2016/679), as implemented by countries in the European Economic Area and the United Kingdom.
“Designated Liabilities” means any of the following: (a) the amount, if any, by which the amount of all Liabilities of the Group Companies to indemnify or hold harmless any director, manager, officer or employee of the Group Companies for claims that relate to periods prior to the Closing exceeds the amount of all insurance proceeds received from the D&O Tail Policy by the Group Companies on account of such Liabilities; and (b) any Liability arising from the transactions contemplated by that certain Agreement and Plan of Merger, dated as of October 26, 2018, among the Company, the Member and PS Merger, LLC, including the merger of the Company with and into PS Merger, LLC, with the Company being the surviving entity in such merger.
“Device Permits” means all applications, permits, approvals, clearances, authorizations, licenses, registrations, certificates, concessions, variances, permissions and exemptions issued or recognized by the U.S. Food and Drug Administration or any other equivalent Governmental Entity to the Group Companies (including investigational device exemptions, device premarket approvals, devicepre-market clearances, establishment registrations and product listings, manufacturing, distribution or wholesaling approvals and authorizations, labeling approvals, or their foreign equivalents), that are required for the research, development, testing, manufacture, distribution, importation, exportation, licensing, marketing, processing, storage, transportation, use, promoting or sale of the Group Companies’ products or components thereof, including any authorizations, applications, approvals, clearances, licenses, permits, certificates or exemptions held by a third party contract manufacturer or development partner that are intended to be transferred to the Group Companies pursuant to a Contract between any third party and the Group Companies.
“Device Regulatory Laws” means the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 301 et seq. and all other applicable Laws (domestic or foreign) relating to the procurement, design, research, development, testing, studying, manufacture, production, processing or handling, storage, distribution, importation, exportation, licensing, labeling or packaging, advertising, use, quality, sale, marketing or promotion of medical device products (including any component of the foregoing products) of the Group Companies, including any implementing regulations of each such Law promulgated under Title 21, Code of Federal Regulations, and analogous state laws and regulations, consumer product safety laws or analogous Laws of other Governmental Entities.
“Encumbrance” means any lease, pledge, option, easement, deed of trust, right of way, encroachment, conditional sales agreement, security interest, mortgage, adverse claim, encumbrance, covenant, condition,
A-74
restriction of record, title defect, charge or restriction of any kind (except for restrictions on transfer under the Securities Act and other applicable securities laws), including any restriction on the use, voting, transfer, receipt of income or other exercise of any attributes of ownership, whether voluntarily incurred or arising by operation of Law, and includes any agreement to give any of the foregoing in the future.
“Environmental Claim” means any claim, action, Legal Proceeding, cause of action, investigation or written notice by any Person alleging potential Liability (including potential Liability for investigatory costs, cleanup costs, governmental response costs, natural resources damages, property damages, personal injuries, or penalties) arising out of, based on or resulting from (a) the actual or alleged presence, Release or threatened Release of any Hazardous Materials at any location, whether or not owned or operated by the Company, or (b) any actual or alleged violation of any Environmental Law.
“Environmental Laws” means all federal, state, local and foreign Legal Requirements (including common law) or Orders of any Governmental Entity relating to pollution or protection of human health and safety as it relates to exposure to any Hazardous Substance or the protection, investigation or restoration of the environment, including all those relating to the generation, handling, transportation, treatment, storage, disposal, distribution, labeling, discharge, Release, threatened Release, control, or cleanup of any Hazardous Materials.
“Environmental Permit” means any permit, license, authorization, registration, exemption, exception, certification or other governmental consent required by or from a Governmental Entity under Environmental Laws.
“ERA” means that certain Amended and Restated Economic Rights Agreement, dated as of November 1, 2018, by and between the Company, the Member, Key Unitholders (as defined therein), SOF II Paradigm Cayco Limited, and Hayfin Services LLP, as representative for the Holders (as defined therein).
“ERISA” means the Employee Retirement Income Security Act of 1974, as amended.
“ERISA Affiliate” means any entity, trade or business that is a member of a group described in Section 414(b), (c), (m) or (o) of the Code or Section 4001(b)(l) of ERISA that includes the Group Companies.
“Exchange Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder.
“Federal Health Care Program” means any “federal health care program” as such term is defined in 42 U.S.C. §1320a-7b(f), including Medicare, Medicaid, the Children’s Health Insurance Program (CHIP), the U.S. Department of Veterans Affairs, TRICARE and similar or successor programs that are funded, in whole or in part, by the government of the United States of America.
“Final Earnout Shares” means the (a) number of shares (rounded to the nearest whole share) of Holdco Common Stock with an aggregate value of $45,000,000 based on the volume weighted average closing trading price of the Holdco Common Stock on Nasdaq (or any other national stock exchange or quotation system on which Holdco Common Stock is then listed or quoted) for (i) the last five (5) trading days in the applicable fiscal period in which such shares are earned, in the case of Final Earnout Shares to be issued pursuant toSection 2.06(a)(iii), or (ii) the five (5) trading day period ending three (3) Business Days prior to a Change of Control Transaction, in the case of Final Earnout Shares to be issued pursuant toSection 2.06(a)(iv), in each case, as reported by the Wall Street Journal or, if not reported therein, in another authoritative source mutually selected by Parent and the Member,multiplied by (b) the quotient of (i) (A) the Giants/Zyga Revenue for the applicable LTM Period,minus (B) $105,000,000,divided by (ii) $20,000,000, which number of shares shall be subject to reduction as set forth inSection 2.06(e);provided,however, that if the Giants/Zyga Revenue for the applicable LTM Period is greater than $125,000,000, for purposes of this calculation the Giants/Zyga Revenue shall be deemed to equal $125,000,000.
A-75
“Financing Failure” means a failure by Holdco, Parent and/or Merger Sub to receive the proceeds from the Financing or any Alternative Financing.
“Former Real Property” means all real property formerly owned, leased or operated by any Group Company.
“GAAP” means United States generally accepted accounting principles consistently applied.
“Giants Revenue” means, for the applicable calculation period, the net revenue of Holdco and its Subsidiaries in respect of the products set forth onExhibit H, calculated in accordance with GAAP as consistently applied by Holdco.
“Giants/Zyga Revenue” means, for the applicable calculation period, the net revenue of Holdco and its Subsidiaries in respect of the products set forth onExhibit I, calculated in accordance with GAAP as consistently applied by Holdco.
“Governing Documents” means the legal document(s) by which any Person (other than an individual) establishes its legal existence or which govern its internal affairs. For example, the “Governing Documents” of a Delaware corporation are its certificate of incorporation andby-laws, the “Governing Documents” of a Delaware limited partnership are its partnership agreement and certificate of limited partnership and the “Governing Documents” of a Delaware limited liability company are its limited liability company agreement and certificate of formation.
“Governmental Entity” means any federal, state, local or foreign government or political subdivision thereof, or any agency or instrumentality of such government or political subdivision, or any self-regulated organization or othernon-governmental regulatory authority or quasi-governmental authority (to the extent that the rules, regulations or orders of such organization or authority have the force of Law), or any arbitrator, court or tribunal of competent jurisdiction.
“Group Companies” means the Company and each of its direct and indirect Subsidiaries.
“Hazardous Materials” means: (i) any chemical, constituent, material, pollutant, contaminant, substance or waste that is regulated by any Governmental Entity or for which Liability or standards of care are imposed, in each case, under any Environmental Law; (ii) petroleum or petroleum products; and (iii) radioactive materials, explosives, asbestos or asbestos containing materials, polychlorinated biphenyls, radon, and infectious or medical wastes.
“Health Care Laws” means all applicable Legal Requirements relating to the regulation, provision, or administration of, sale or marketing of, or payment for health care items or services, including: (i) the federal Anti-Kickback Statute (42 U.S.C. §1320a-7b(b)), the federal Physician Self-Referral Prohibition (commonly referred to as the “Stark Law”) (42 U.S.C. §§ 1395nn and 1396b(s)), the federal False Claims Act (31 U.S.C. § 3729et seq.), the Federal Criminal False Claims Act (18 U.S.C. § 287), the False Statements Relating to Health Care Matters law (18 U.S.C. § 1035), the federal Civil Monetary Penalties Law (42 U.S.C. §§1320a-7a and1320a-8), the federal Beneficiary Anti-Inducement Statute (42 U.S.C. §1320a-7a(a)(5)), the HIPAA All Payor Fraud Statute (18 U.S.C. § 1347), the federal Exclusion Laws (42 U.S.C.§1320a-7), the Program Fraud Civil Remedies Act (31 U.S.C. §§ 3801-3812), or any regulations promulgated pursuant to each of the foregoing statutes, or similar state or local statutes or regulations; (ii) HIPAA, as amended by HITECH, any regulations promulgated thereunder, and similar state or local statutes or regulations governing the privacy or security of patient information; (iii) the Medicare statute (Title XVIII of the Social Security Act) and the regulations promulgated thereunder; (iv) the Medicaid statute (Title XIX of the Social Security Act) and the regulations promulgated thereunder; (v) the Children’s Health Insurance Program (CHIP) statute (Title XXI of the Social Security Act) and the regulations promulgated thereunder; (vi) TRICARE (10 U.S.C. §1071et seq.) and the
A-76
regulations promulgated thereunder; (vii) the U.S. Department of Veterans Affairs (including 38 U.S.C. § 1701et seq.), and the regulations promulgated thereunder; (viii) federal or state Legal Requirements relating to billing, or claims for, reimbursement of items or services submitted to any third party payor (including a Federal Healthcare Program), employer or similar entity, or patient; (ix) any other federal or state Legal Requirements relating to fraudulent, abusive or unlawful practices connected in any way with the provision or marketing of health care items or services; (x) the federal Physician Payment Sunshine Act (42 U.S.C. §1320a-7h), its implementing regulations, and similar state transparency reporting Legal Requirements; (xii) the Medicare Secondary Payer Laws (42 U.S.C. § 1395yet seq.) and implementing regulations; (xiii) each of (i) through (xii) as amended from time to time; and (xiv) the Advanced Medical Technology Association Code on Interactions with Health Care Professionals. “Health Care Laws” further includes all directives, regulations, decisions, guidelines and other regulatory framework with relevance for the business issued by the European Union, and any relevant national legal framework in European Union Member States, or any other international or national legal framework that is applicable to the business.
“HSR Approval” means the filing of a Notification and Report Form with the United States Federal Trade Commission and the United States Department of Justice under the HSR Act and the expiration or termination of any applicable waiting period thereunder, if required.
“Indebtedness” means, as of any time of determination, without duplication, the aggregate consolidated indebtedness of the Group Companies, including, without duplication, (i) any obligations under any indebtedness for borrowed money, (ii) any obligations evidenced by any note, bond, debenture or other debt security, (iii) any payment obligations in respect of banker’s acceptances or letters of credit or any other commitment by which a Person assures a financial institution against loss, (iv) anyoff-balance sheet financing, including synthetic leases and project financing, (v) all obligations under leases that have been, in accordance with GAAP, recorded as capital leases, (vi) any obligations with respect to interest rate swaps, collars, caps and similar hedging obligations (including any applicable breakage costs), (vii) in respect of“earn-out” obligations and all other obligations for the deferred and unpaid purchase price of property or services (other than trade payables and accrued expenses incurred in the ordinary course of business), (viii) any obligations referred to in the foregoing clauses (i) through (vii) of any Person which are either guaranteed or secured by any Encumbrance upon any of the Group Companies or any of their respective assets or properties and (ix) accrued and unpaid or declared and unpaid interest of any such foregoing obligation and all premiums, penalties, charges, fees, expenses and other amounts that are or would be due (including with respect to early termination) in connection with the payment and satisfaction in full of such obligations. Notwithstanding the foregoing, “Indebtedness” does not include any intercompany obligations exclusively between or among the Group Companies.
“Indemnification Date” means, with respect to a claim for indemnification by a Parent Indemnitee pursuant toArticle IX orSection 11.02, the date on which it is determined in accordance with this Agreement that such Parent Indemnitee is entitled to be indemnified for Damages pursuant to Article IX orSection 11.02, as applicable.
“Indemnification Price” means, with respect to an applicable Indemnification Date, the volume weighted average closing trading price of the Holdco Common Stock on Nasdaq (or any other national stock exchange or quotation system on which Holdco Common Stock is then listed or quoted) for the five (5) trading day period ending one (1) Business Day prior to such Indemnification Date, as reported by the Wall Street Journal or, if not reported therein, in another authoritative source mutually selected by Parent and the Member.
“Information Technology Systems” means all communications systems and computer systems used by a Group Company, including all hardware, software and websites (but excluding networks generally available to the public).
“Initial Earnout Shares” means the number of shares of Holdco Common Stock (rounded to the nearest whole share) equal to the sum of (i) (A) 10,729,614 shares of Holdco Common Stock,multiplied by (B) the
A-77
quotient of(1) (I) the Giants/Zyga Revenue for the applicable LTM Period,minus (II) $85,000,000,divided by (2) $11,764,705.88 (provided,however, that if the Giants/Zyga Revenue for the applicable LTM Period is greater than $96,764,705.88, for purposes of the calculation set forth in this clause (i) only, the Giants/Zyga Revenue shall be deemed to equal $96,764,705.88) and (ii) the greater of (A) zero (0) shares of Holdco Common Stock and (B) (1) the number of shares (rounded to the nearest whole share) of Holdco Common Stock with an aggregate value of $35,000,000 based on the volume weighted average closing trading price of the Holdco Common Stock on Nasdaq (or any other national stock exchange or quotation system on which Holdco Common Stock is then listed or quoted) for (y) the last five (5) trading days in the applicable fiscal period in which such shares are earned, in the case of Initial Earnout Shares to be issued pursuant toSection 2.06(a)(ii), or (z) the five (5) trading day period ending three (3) Business Days prior to a Change of Control Transaction, in the case of Initial Earnout Shares to be issued pursuant toSection 2.06(a)(iv), in each case, as reported by the Wall Street Journal or, if not reported therein, in another authoritative source mutually selected by Parent and the Member,multiplied by (2) the quotient of(I) (a) the Giants/Zyga Revenue for the applicable LTM Period,minus (b) $96,764,705.88,divided by (II) $8,235,294.12 (provided,however, that if the Giants/Zyga Revenue for the applicable LTM Period is greater than $105,000,000, for purposes of this clause (ii) (B) only, the Giants/Zyga Revenue shall be deemed to equal $105,000,000), which number of shares shall be subject to reduction as set forth inSection 2.06(e).
“Intellectual Property” means all intellectual property, regardless of form, including: (i) patents and patent applications, inventions and discoveries, including articles of manufacture, business methods, compositions of matter, improvements, machines, methods, and processes and new uses for any of the preceding items; (ii) published and unpublished works of authorship, including audiovisual works, collective works, computer programs, compilations, databases, derivative works, literary works, mask works, and sound recordings; (iii) words, names, symbols, devices, designs, and other designations, and combinations of the preceding items, used to identify or distinguish a business, good, group, product, or service or to indicate a form of certification, including logos, product designs, and product features (“Trademarks”); and (iv) confidential and proprietary information, andknow-how, including confidential processes, schematics, databases, formulae, drawings, prototypes, models, designs,know-how, concepts, methods, devices, technology, research and development results and records, inventions, compositions, reports, data, mailing lists, business plans, and customer lists, in each case, to the extent protectable under applicable Law as a trade secret (“Trade Secrets”).
“Interim Debt Financing” means any Indebtedness incurred by any of the Group Companies between the date hereof and the earlier of the termination of this Agreement and the Closing Date;provided, that such Indebtedness (including all applicable prepayment penalties, premiums and breakage costs) is repaid in full at or prior to the Closing and any Encumbrance created thereunder is terminated upon such repayment.
“Interim Equity Financing” means any sale of Company Units, by the Company, to the Member for cash between the date hereof and the earlier of the termination of this Agreement and the Closing Date;provided, the cash used by the Member to purchase such Company Units must be equal to or less than the net cash proceeds received by the Member pursuant to an Interim Member Financing.
“Interim Financing” means an Interim Debt Financing or Interim Equity Financing.
“Interim Member Financing” means any sale of Member Units or other equity interests in the Member, by the Member, for cash between the date hereof and the earlier of the termination of this Agreement and the Closing Date;provided, that in connection with any Interim Member Financing, the purchaser of Member Units or other equity interests in the Member, in each case, with voting rights, must irrevocably consent to and agree to vote such Member Units or other equity interests in favor of the transactions contemplated by this Agreement (provided, that such irrevocable consent and agreement may terminate upon the earlier of (i) the termination of this Agreement and (ii) the Closing).
“Intervening Event” means any event, fact, circumstances, development or occurrence relating to or affecting Parent or its Subsidiaries, including the business, operations, assets or liabilities of Parent or its
A-78
Subsidiaries, and which was not known or reasonably foreseeable to the Board of Directors of Parent as of the date of this Agreement;provided,however, that neither an actual or anticipated Financing Failure nor the actual or anticipated consequences thereof shall be an Intervening Event.
“Knowledge of the Company” means the actual knowledge of Marc Viscogliosi, Francis Magee, Steve Ward, Alberto Jurado, René Pröll and Colleen Nelson as of the date hereof, after reasonable inquiry of their direct reports and, with respect to the references to “Knowledge of the Company” inSections 3.15 (to the extent such references relate to Health Care Laws),3.22 and3.23 only, in addition to the direct reports referenced above, representatives of MCRA who support the Group Companies.
“Law” means any foreign, federal, state or local statute, law, ordinance, regulation, rule, code, injunction, judgment, decree or order enacted, adopted, issued, promulgated or enforced by any Governmental Entity.
“Legal Proceeding” means any claim, action, audit, hearing, investigation, litigation, suit, arbitration or proceeding (whether civil, criminal, administrative, judicial or investigative, whether formal or informal, whether public or private) commenced, brought, conducted or heard by or before, or otherwise involving, any Governmental Entity or arbitrator.
“Legal Requirement” means all applicable Laws, agency guidance, Permits, bylaws, variances, conditions and licenses of a Governmental Entity having jurisdiction over the assets or the properties of any Party or any Group Company, as applicable, and the operations thereof.
“Liability” means any direct or indirect liability, Indebtedness, obligation, commitment, expense, claim, deficiency or guaranty of or by any Person of any type, known or unknown, and whether accrued, absolute, contingent, matured, unmatured or otherwise.
“LTM Period” means any consecutive twelve (12)-month period ending (a) on March 31, June 30, September 30 or December 31 of a particular year and (b) at least twelve (12) months after the Closing Date.
“Material Adverse Effect” means any event, change, occurrence, fact, development or effect that (a) would reasonably be expected to, individually or in the aggregate, prevent, materially delay or materially impair the ability of the Member or the Group Companies to consummate the transactions contemplated hereby or (b) has had or would reasonably be expected to have, individually or in the aggregate, a material adverse effect on the business, condition (financial or otherwise) or results of operations of the Group Companies, taken as a whole, other than any event, change, occurrence, fact, development or effect arising out of, attributable to or resulting from (i) general business or economic changes or developments in any of the industries in which the Group Companies operate, (ii) changes in regional, national or international political conditions (including any outbreak or escalation of hostilities, any acts of war (whether or not declared) or terrorism or any other national or international calamity, crisis or emergency) or in general economic, business, regulatory, political or market conditions or in national or international financial markets, (iii) epidemics, pandemics, natural disasters, calamities or other force majeure events, (iv) changes in any applicable Laws or applicable accounting regulations or principles or interpretations thereof, (v) any change or effect primarily caused by the announcement or pendency of the transactions contemplated hereby, including the impact thereof on any relationship of any Group Company with any of its customers, employees, financing sources, distributors or suppliers, (vi) any failure by the Group Companies to meet any projections or forecasts or estimates of revenues, earnings or other financial performance or results of operations for any period (it being understood that the exception in this clause shall not prevent or otherwise affect a determination that any event, change, occurrence, fact, development or effect underlying such failure has resulted in, or contributed to, a Material Adverse Effect), (vii) any actions expressly required by this Agreement, or the failure to take any specific action expressly prohibited by this Agreement (excluding any action prohibited underSection 5.01, unless the Member has requested, but has not received within a reasonable period of time after such request, Parent’s consent to such
A-79
action) or (viii) any change resulting or arising from the identity of, or any facts or circumstances relating to, Parent or any of its Affiliates;provided, that in the case of each of the clauses (i) through (iv) set forth above, only to the extent that any such event, change, occurrence, fact, development or effect has not had, and would not reasonably be expected to have, individually or in the aggregate, a disproportionate effect on the Group Companies relative to other companies in the Group Companies’ industry.
“Member Fundamental Representations” means the representations and warranties of the Member set forth inSection 3.01,Section 3.02,Section 3.04(a),Section 3.04(b),Section 3.08 andSection 3.20.
“Member Units” means units of the Member, which units represent interests in the profits, losses and distributions of the Member.
“Nasdaq” means The Nasdaq Global Market of The Nasdaq Stock Market LLC.
“Net Working Capital Amount” (which can be positive or negative) means (A) the consolidated current assets of the Group Companies (other than any asset in respect of Cash or Restricted Cash)minus (B) the consolidated current liabilities (other than any such liabilities to the extent included in the Closing Indebtedness Amount or Company Transaction Expenses), in each case, as of the Effective Time and as calculated in accordance with GAAP and the methodology illustrated onExhibit J.
“Order” means any order, injunction, judgment, decree, ruling, writ, assessment or arbitration award of a Governmental Entity.
“Ordinary Course of Business” means, with respect to any Person, actions that are consistent in all material respects with the past practices of such Person, taken in the ordinary course of the normalday-to-day operations of such Person.
“Ownership Percentage” means, at any time of determination, the percentage equal to (a) the number of shares of Holdco Common Stock issued and outstanding and issuable upon conversion of the Holdco Preferred Stock (with each share of Holdco Preferred Stock deemed to represent the number of shares of Holdco Common Stock issuable upon conversion of such share of Holdco Preferred Stock at such time of determination (without regard to any restrictions on conversion)) that the Member and the Unitholders that were Unitholders as of immediately prior to the Contribution beneficially own (as determined pursuant to Rule13d-3 under the Exchange Act, including the sixty(60)-day provision in paragraph (d)(1)(i) thereof),divided by (b) the total number of shares of Holdco Common Stock issued and outstanding and issuable upon conversion of the Holdco Preferred Stock (with each share of Holdco Preferred Stock deemed to represent the number of shares of Holdco Common Stock issuable upon conversion of such share of Holdco Preferred Stock at such time of determination (without regard to any restrictions on conversion)).
“Parent Disclosure Schedule” means a Schedule referencing the appropriate section or clause of this Agreement and delivered by Parent, Holdco and Merger Sub to the Member on or prior to the date hereof.
“Parent Equity Awards ” means the Parent Stock Options and Parent Restricted Stock Awards.
“Parent Fundamental Representations” means the representations and warranties of Parent set forth inSections 4.01,4.02,4.04 and4.16.
“Parent Group Member” means Parent and each of its direct and indirect Subsidiaries.
“Parent Indemnitees” means the following Persons: (a) Parent; (b) Parent’s current and future Affiliates (including Merger Sub, Holdco and, following the Contribution, the Group Companies); (c) the respective Representatives of the Persons referred to in clauses “(a)” and “(b)” above; and (d) the respective successors and assigns of the Persons referred to in clauses “(a),” “(b)” and “(c)” above;provided,however, that the Member shall not be deemed to be a “Parent Indemnitee.”
A-80
“Parent Material Adverse Effect” means any change, effect, event, occurrence, state of facts or development that (a) would reasonably be expected to, individually or in the aggregate, prevent, materially delay or materially impair the ability of Parent, Holdco or the Merger Sub to consummate the transactions contemplated hereby or (b) has had or would reasonably be expected to have, individually or in the aggregate, a material adverse effect on the business, condition (financial or otherwise) or results of operations of Parent and its Subsidiaries, taken as a whole, other than any event, change, occurrence, fact, development or effect arising out of, attributable to or resulting from (i) general business or economic changes or developments in any of the industries in which Parent and its Subsidiaries operate, (ii) changes in regional, national or international political conditions (including any outbreak or escalation of hostilities, any acts of war (whether or not declared) or terrorism or any other national or international calamity, crisis or emergency) or in general economic, business, regulatory, political or market conditions or in national or international financial markets, (iii) epidemics, pandemics, natural disasters, calamities or other force majeure events, (iv) changes in any applicable Laws or applicable accounting regulations or principles or interpretations thereof, (v) any change or effect primarily caused by the announcement or pendency of the transactions contemplated hereby, including the impact thereof on any relationship of Parent or any of its Subsidiaries with any of its customers, employees, financing sources, distributors or suppliers, (vi) any failure by Parent and its Subsidiaries to meet any projections or forecasts or estimates of revenues, earnings or other financial performance or results of operations for any period (it being understood that the exception in this clause shall not prevent or otherwise affect a determination that any change, effect, event, occurrence, state of facts or development underlying such failure has resulted in, or contributed to, a Parent Material Adverse Effect), (vii) any decline in the price or trading volume of Parent Common Stock on Nasdaq (it being understood that the exception in this clause shall not prevent or otherwise affect a determination that any change, effect, event, occurrence, state of facts or development underlying such failure has resulted in, or contributed to, a Parent Material Adverse Effect), (viii) any actions expressly required by this Agreement, or the failure to take any specific action expressly prohibited by this Agreement (excluding any action prohibited bySection 6.05, unless Parent has requested, but has not received within a reasonable period of time after such request, the Member’s consent to such action) or (ix) any change resulting or arising from the identity of, or any facts or circumstances relating to, the Member or any of its Affiliates;provided, that in the case of each of the clauses (i) through (iv) set forth above, only to the extent that any such change, effect, event, occurrence, state of facts or development has not had, and would not reasonably be expected to have, individually or in the aggregate, a disproportionate effect on Parent and its Subsidiaries relative to other companies in Parent and its Subsidiaries’ industry.
“Parent Stockholders” means the holders of Parent Common Stock and Parent Preferred Stock.
“Parent Transaction Expense” means a Transaction Expense which Parent, Holdco or Merger Sub is obligated to pay.
“Parent’s Knowledge” or any similar phrase, with respect to Parent, means the actual knowledge of Camille I. Farhat, Jonathon M. Singer, Julius Avisa, Paul A. Montague, Enrico Sangiorgio, John N. Varela and Olivier M. Visa, after reasonable inquiry of their direct reports.
“Permitted Liens” means: (a) statutory liens for current Taxes or other governmental charges not yet due and payable or the amount or validity of which is being contested in good faith by appropriate proceedings by the Group Companies and for which appropriate reserves have been established in accordance with GAAP; (b) mechanics’, carriers’, workers’, repairers’ and similar statutory liens arising or incurred in the Ordinary Course of Business for amounts that are not delinquent, unless being contested in good faith by appropriate proceedings and for which adequate accruals or reserves have been established; (c) zoning, entitlement, building and other land use regulations or ordinances imposed by Governmental Entities having jurisdiction over the Company Real Property that are not violated by the use and operation as of the date hereof or the proposed use of the Company Real Property; (d) covenants, conditions, restrictions, easements and other similar matters of record affecting title to the Company Real Property that do not detract from the value or materially impair the occupancy or use of the Company Real Property for the purposes for which it is used as of the date hereof or
A-81
proposed to be used in connection with the Group Companies’ businesses; (e) an Encumbrance created pursuant to the Credit Agreement that will be terminated at Closing upon payoff of the applicable Payoff Amount; (f) an Encumbrance created pursuant to an Interim Debt Financing that will be terminated at or prior to Closing; and (g) such imperfections of title and Encumbrances, if any, which are not material, individually or in the aggregate, in character, amount or extent, and which do not materially detract from the value, or materially interfere with the present or intended use, of the property subject thereto or affected thereby.
“Person” means an individual, a partnership, a corporation, a limited liability company, an association, a joint stock company, a trust, a joint venture, an unincorporated organization or a Governmental Entity or any department, agency or political subdivision thereof.
“Personal Data” means a natural person’s name, street address or specific geolocation information, date of birth, telephone number,e-mail address, online contact information, photograph, biometric data, social security number, driver’s license number, passport number, tax identification number, any government-issued identification number, financial account number, credit card number, any information that would permit access to a financial account, a user name and password that would permit access to an online account, any persistent identifier such as customer number held in a cookie, an Internet Protocol address, a processor or device serial number, or a unique device identifier, any data that, if it were subject to a Data Breach, would require notification under Data Protection Laws, or any other piece of information that allows the identification of a natural person.
“Real Property Leases” means all leases, subleases, licenses, concessions and other Contracts applicable to the Company Real Property, and any ancillary documents pertaining thereto, including, for example, amendments, modifications, supplements, exhibits, schedules, addenda and restatements thereto and thereof.
“Regulatory Approvals” means any consent, approval, authorization or permit of, or filing with or notification to, any Governmental Entity.
“Release” means any release, spill, emission, discharge, leak, pumping, injection, deposit, disposal, dispersal, leaching or migration into the environment (including ambient air, surface water, groundwater and surface or subsurface strata) or into or out of any property, including the movement of Hazardous Materials through or in the air, soil, surface water, groundwater or property.
“Representatives” means the officers, directors, managers, employees, attorneys, accountants, advisors, representatives, consultants and agents of a Person.
“Required Information” shall mean (a) unaudited consolidated balance sheets and related statements of income and cash flows of the Company for each fiscal month ended after September 30, 2018 and at least thirty (30) days prior to the Closing Date, (b) unaudited consolidated balance sheets and related statements of income and cash flows of the Company for each fiscal quarter ended after June 30, 2018 and at least forty-five (45) days prior to the Closing Date, and (c) information reasonably required by Parent in order to prepare apro forma consolidated balance sheet and related statements of income and cash flow of Parent as of the last day of the most recent fiscal month ended at least thirty (30) days prior to the Closing Date, prepared after giving effect to the transactions contemplated by this Agreement as if the transactions contemplated by this Agreement have occurred as of such date.
“Required Parent Vote” means (a) the affirmative vote of the holders of a majority of the outstanding shares of Parent Common Stock and Parent Preferred Stock (on a fully converted basis) entitled to vote thereon in favor of the adoption of this Agreement and (b) the written consent or affirmative vote of the holders of a majority of the outstanding shares of Parent Preferred Stock entitled to vote thereon, voting separately as a class, in favor of the adoption of this Agreement.
A-82
“Restricted Cash” means any Cash that is not freely accessible by the Group Companies, including any Cash posted to support letters of credit, performance bonds or other similar obligations or deposited with third parties as a security deposit.
“Sarbanes-Oxley Act” means the Sarbanes-Oxley Act of 2002, as amended.
“SEC” means the U.S. Securities and Exchange Commission.
“Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder.
“Settlement Agreement” means that certain letter agreement, dated as of November 1, 2018, among the Member, the Company, Hayfin Services LLP, SOFII Paradigm Cayco Limited, Hayfin Special Opportunities Fund II (AIV I) LP, Hayfin Special Opportunities Fund II (AIV IB) LP, Hayfin Opal LuxCo 3 SARL, Hayfin SOF IICo-Invest LuxCo 2 SARL and Hayfin Topaz LuxCo 3 SCA.
“Standard Inbound IP Agreements” means(i) non-disclosure agreements granting to any Group Company a limited right to use a third party’s confidential information entered into by the Group Company in the Ordinary Course of Business, (ii) “shrink wrap” and similar generally available commercialend-user licenses to software that are not redistributed with the Company products, (iii) employment agreements and consulting agreements pursuant to which any Group Company obtains rights to use Intellectual Property created in the scope of such employment or provision of services for the Group Company, and (iv) agreements granting any Group Company a right to use third party Trademarks in connection with the Group Company’s marketing or advertising of Company products or such third party’s products.
“Standard Outbound IP Agreements” means(i) non-disclosure agreements granting to a third party a limited right to use any Group Company’s confidential information entered into by the Group Company in the Ordinary Course of Business, and (ii) agreements granting a third party a right to use any Group Company’s Trademarks in connection with such third party’s marketing or advertising of Company products.
“Straddle Period” means any taxable period that includes (but does not end on) the Closing Date.
“Subsidiary” means, with respect to any Person, any corporation of which a majority of the total voting power of shares of stock entitled (without regard to the occurrence of any contingency) to vote in the election of directors, managers or trustees thereof is at the time owned or controlled, directly or indirectly, by such Person or one or more of the other Subsidiaries of such Person or a combination thereof, or any partnership, limited liability company, association or other business entity of which a majority of the partnership, limited liability company or other similar ownership interest is at the time owned or controlled, directly or indirectly, by such Person or one or more Subsidiaries of such Person or a combination thereof. For purposes of this definition, a Person is deemed to have a majority ownership interest in a partnership, limited liability company, association or other business entity if such Person is allocated a majority of the gains or losses of such partnership, limited liability company, association or other business entity or is or controls the managing member or general partner or similar position of such partnership, limited liability company, association or other business entity.
“Target Working Capital” means $7,000,000.
“Tax” or “Taxes” means: (i) any federal, state, local or foreign net income, gross income, gross receipts, windfall profit, severance, property, production, sales, use, license, excise, franchise, employment, payroll, withholding on amounts paid to or by any Person, alternative oradd-on minimum, ad valorem, value-added, transfer, stamp, or environmental tax (including taxes under Code Section 59A), escheat payments or any other tax, custom, duty, governmental fee or other like assessment or charge of any kind whatsoever, together with any interest or penalty, addition to tax or additional amount imposed by any Governmental Entity and
A-83
(ii) any liability for the payment of amounts determined by reference to amounts described in clause (i) as a result of being or having been a member of any group of corporations that files, will file, or has filed Tax Returns on a combined, consolidated, unitary or similar basis, as a result of any obligation under any agreement or arrangement (including any Tax Sharing Arrangement), as a result of being a transferee or successor, or by contract or otherwise.
“Tax Returns” means any return, report or similar statement required to be filed with respect to any Tax (including any attached schedules), including any information return, claim for refund, amended return or declaration of estimated Tax.
“Tax Sharing Arrangement” means any written or unwritten agreement or arrangement providing for the allocation or payment of Tax liabilities or for Tax benefits between or among members of any group of corporations that files, will file, or has filed Tax Returns on a combined, consolidated or unitary basis.
“Transaction Documents” means, collectively, this Agreement, theLock-Up Agreement, the Confidentiality Agreement, the Consulting Agreements, theNon-Competition Agreements and all of the certificates, instruments and agreements required to be delivered by any of the Parties at the Closing pursuant to this Agreement, theLock-Up Agreement, the Confidentiality Agreement, any Consulting Agreement or anyNon-Competition Agreement.
“Transaction Expenses” means, with respect to each of the Company, on the one hand, and Parent, Holdco or Merger Sub, on the other hand, all of its costs and expenses incident to the negotiation and preparation of this Agreement and the other documents and agreements contemplated hereby and the performance and compliance with all agreements and conditions contained herein to be performed or complied with, including the fees, expenses and disbursements of its counsel and accountants, due diligence expenses, advisory and consulting fees, underwriting and other third-party fees required to consummate the Merger or the Contribution, the fees and expenses charged by a lender pursuant to the Payoff Letters, any severance payments, transaction bonuses, change in control payments and any other similar payments triggered as a result thereof (including the employer’s share of any payroll Taxes related thereto) and other costs and expenses associated with any of the foregoing.
“Unitholders” means the owners of Member Units.
12.02Other Definitional Provisions.
(a)Accounting Terms. Accounting terms that are not otherwise defined in this Agreement have the meanings given to them under GAAP. To the extent that the definition of an accounting term defined in this Agreement is inconsistent with the meaning of such term under GAAP, the definition set forth in this Agreement will control.
(b)Successor Laws. Any reference to any particular Code section or Law will be interpreted to include any revision of or successor to that section regardless of how it is numbered or classified.
MISCELLANEOUS
13.01Press Releases and Public Announcements. No Party will issue any press release or make any similar public announcement relating to the subject matter of this Agreement without the prior written approval of Parent and the Member (which approval will not be unreasonably withheld, conditioned or delayed);provided,however, that any Party may make any public disclosure it believes in good faith is required by applicable Law or the rules of a securities exchange upon which a Party’s securities are traded (in which case the disclosing Party will use its reasonable best efforts to allow the other Parties reasonable time to review and comment on such public disclosure in advance of its issuance (and will consider in good faith all such comments reasonably proposed)).
A-84
13.02Expenses. Except as expressly provided herein, at the Closing, (a) the Member or the Company shall pay the Company Transaction Expenses and (b) Parent shall pay the Parent Transaction Expenses. Except as expressly provided herein, if this Agreement is terminated in accordance herewith, then the Member or the Company shall pay the Company Transaction Expenses and Parent shall pay the Parent Transaction Expenses.
13.03Transfer Taxes. All transfer Taxes, recording fees and other similar Taxes that are imposed on any of the Parties hereto by any Governmental Entity incurred in connection with the consummation of the transactions contemplated by this Agreement, shall be paid 50% by Parent as a Parent Transaction Expense and 50% by the Member or the Company as a Company Transaction Expense.
13.04Notices. Unless otherwise provided herein, all notices, requests, demands, claims, consents, approvals and other communications hereunder will be in writing. Any notice, request, demand, claim, consent, approval or other communication hereunder will be deemed duly given (a) when delivered personally to the recipient, (b) one (1) Business Day after being sent to the recipient by reputable overnight courier service (charges prepaid), (c) three (3) Business Days after being mailed to the recipient by certified or registered mail, return receipt requested and postage prepaid, or (d) immediately upon facsimile transmission (with a written or electronic confirmation of delivery) if sent during normal business hours of the recipient or, if not sent during normal business hours of the recipient, then on the next Business Day, and in each case addressed to the intended recipient as set forth below:
Notices to Parent, Holdco, the Surviving Corporation and/or Merger Sub:
RTI Surgical, Inc.
520 Lake Cook Road, Suite 680
Deerfield, Illinois 60015
Attn: Jonathon Singer
with a copy to (which will not constitute notice):
Sidley Austin LLP
One South Dearborn
Chicago, Illinois 60603
Attn: Larry Barden and Seth Katz
Facsimile No.: (312)853-7036
Notices to the Member:
PS Spine HoldCo, LLC
505 Park Avenue, 14th Floor
New York, New York 10022
Attn: Marc R. Viscogliosi
Facsimile No.: (212)826-9509
with a copy to (which will not constitute notice):
Dorsey & Whitney LLP
51 West 52nd Street
New York, New York 10019
Attn: John B. Wade
Facsimile No.: (212)953-7201
Any Party may change the address to which notices, requests, demands, claims and other communications hereunder are to be delivered by giving the other Parties notice in the manner herein set forth.
13.05Succession and Assignment. This Agreement will inure to the benefit of, and be binding upon, the successors and permitted assigns of the Parties. Neither this Agreement nor any of the rights, interests or obligations hereunder will be assignable by Parent, Holdco, the Merger Sub or the Member, without the prior
A-85
written consent of the other Parties;provided,however, that Holdco or Parent may (a) assign its rights under this Agreement to any Affiliate of Holdco or Parent, if such assignment occurs prior to the mailing of the Joint Proxy and Consent Solicitation Statement/Prospectus to the Parent Stockholders or the Unitholders, (b) assign its rights under this Agreement to any Affiliate of Holdco or Parent or to any future purchaser of Holdco or Parent, if such assignment occurs after the Closing, or (c) collaterally assign any or all of their rights and interests hereunder to one or more lenders of Parent or the Surviving Company, if such assignment occurs after the Closing;provided,further, that no such assignment shall release Holdco or Parent from its obligations hereunder. Any assignment in contravention of the preceding sentence shall be null and void.
13.06Severability. Whenever possible, each provision or portion of any provision of this Agreement shall be interpreted in such manner as to be effective and valid under applicable Law, but if any provision or portion of any provision of this Agreement is held to be invalid, illegal or unenforceable in any respect under any applicable Law or rule in any jurisdiction, such invalidity, illegality or unenforceability shall not affect any other provision or portion of any provision in such jurisdiction, and this Agreement shall be reformed, construed and enforced in such jurisdiction as if such invalid, illegal or unenforceable provision or portion of any provision had never been contained herein.
13.07References. The table of contents and the section and other headings and subheadings contained in this Agreement and the exhibits hereto are solely for the purpose of reference, are not part of the agreement of the Parties, and will not in any way affect the meaning or interpretation of this Agreement or any exhibit hereto. All references to days (excluding Business Days) or months will be deemed references to calendar days or months. All references to “$” will be deemed references to United States dollars. Unless the context otherwise requires, any reference to a “Section,” “Exhibit,” “Disclosure Schedule” or “Schedule” will be deemed to refer to a section of this Agreement, an exhibit to this Agreement or a schedule to this Agreement, as applicable. Unless the context otherwise requires, any reference to a “clause” in any Section of this Agreement will be deemed to refer to a clause in such Section. The words “hereof,” “herein” and “hereunder” and words of similar import referring to this Agreement refer to this Agreement as a whole and not to any particular provision of this Agreement. The word “including” or any variation thereof means “including, without limitation” and will not be construed to limit any general statement that it follows to the specific or similar items or matters immediately following it. Unless otherwise specifically provided for herein, the word “or” will not be deemed to be exclusive. References to “written” or “in writing” include in electronic form. Any reference to any federal, state, local or foreign statute or law will be deemed also to refer to all rules and regulations promulgated thereunder, unless the context requires otherwise. All terms defined in this Agreement will have the defined meanings when used in any certificate or other document made or delivered pursuant hereto unless otherwise defined therein. The definitions contained in this Agreement are applicable to the singular as well as the plural forms of such terms and to the masculine as well as to the feminine and neuter genders of such term. Where a word or phrase is defined herein, each of its other grammatical forms shall have a corresponding meaning. No summary of this Agreement prepared by a Party, in a Parent SEC Report or otherwise, shall affect the meaning or interpretation of this Agreement. A reference to any party to this Agreement or any other agreement shall include such party’s predecessors, successors and permitted assigns.
13.08Construction. The Parties have participated jointly in the negotiation and drafting of this Agreement. In the event an ambiguity or question of intent or interpretation arises, this Agreement will be construed as if drafted jointly by the Parties and no presumption or burden of proof will arise favoring or disfavoring any Party by virtue of the authorship of any of the provisions of this Agreement.
13.09Amendment and Waiver. Except as otherwise set forth in this Agreement, any provision of this Agreement or the Disclosure Schedules hereto may be amended or waived only in a writing signed (a) in the case of any amendment, by Parent and the Member and (b) in the case of a waiver, by the Party or Parties waiving rights hereunder;provided,however, that (i) after the receipt of the Written Consent, no amendment to this Agreement will be made that by Law requires further approval by the Unitholders without the consent of the Unitholders and (ii) after the receipt of the Required Parent Vote, no amendment to this Agreement will be made
A-86
that by Law requires further approval by the Parent Stockholders without such approval. No waiver of any provision hereunder or any breach or default thereof will extend to or affect in any way any other provision or prior or subsequent breach or default.
13.10Entire Agreement. This Agreement (together with the documents and agreements to be delivered pursuant hereto), the Confidentiality Agreement, the Consulting Agreements, theNon-Competition Agreements, theLock-Up Agreement, the Unitholder Support Agreements and the Parent Stockholder Support Agreements constitute the entire agreement among the Parties and supersedes any prior understandings, agreements or representations by or among the Parties, written or oral, to the extent they relate in any way to the subject matter hereof. The exhibits and schedules identified in this Agreement are incorporated herein by reference and made a part hereof as if set forth in full herein.
13.11Parties in Interest. This Agreement shall be binding upon and inure solely to the benefit of each Party, and, except as provided in (i) Section 5.08(b) (with respect to which the Representatives described in suchSection 5.08(b) shall be third party beneficiaries), (ii)Section 6.09 (with respect to which the Group Company Indemnified Parties and their respective heirs and legal representatives shall be third party beneficiaries, but only following the Closing), (iii)Article IX (with respect to which the Parent Indemnitees shall be third party beneficiaries, but only following the Closing), (iv)Section 10.03(d) (with respect to which the Parent Related Parties shall be third party beneficiaries), (v)Section 13.19 (with respect to which D&W shall be a third party beneficiary) and (vi)Section 13.20 (with respect to which the Financing Source Related Parties shall be third party beneficiaries), nothing in this Agreement, express or implied, is intended to or shall confer upon any Person other than the Parties and their respective successors and permitted assigns any legal or equitable right, benefit or remedy of any nature whatsoever under or by reason of this Agreement. Notwithstanding the immediately preceding sentence, following the Effective Time, (a) the provisions ofArticle I relating to issuance of Holdco Common Stock upon the conversion of Parent Common Stock shall be enforceable by the holders of Parent Common Stock that does not constitute Parent Cancelled Shares, (b) the provisions ofArticle I relating to issuance of Holdco Preferred Stock upon the conversion of Parent Preferred Stock shall be enforceable by the holders of Parent Preferred Stock that does not constitute Parent Cancelled Shares, (c) the provisions ofArticle I relating to issuance of stock options in respect of Holdco Common Stock upon the conversion of Parent Stock Options shall be enforceable by the holders of Parent Stock Options and (d) the provisions ofArticle I relating to issuance of restricted stock awards relating to Holdco Common Stock upon the conversion of Parent Restricted Stock Awards shall be enforceable by the holders of Parent Restricted Stock Awards.
13.12WAIVER OF TRIAL BY JURY. EACH OF THE PARTIES TO THIS AGREEMENT HEREBY WAIVES, TO THE FULLEST EXTENT PERMITTED BY LAW, ANY RIGHT TO TRIAL BY JURY OF ANY CLAIM, DEMAND, ACTION OR CAUSE OF ACTION (A) ARISING UNDER THIS AGREEMENT OR (B) IN ANY WAY CONNECTED WITH OR RELATED OR INCIDENTAL TO THE DEALINGS OF THE PARTIES HERETO IN RESPECT OF THIS AGREEMENT OR ANY OF THE TRANSACTIONS RELATED HERETO, IN EACH CASE WHETHER NOW EXISTING OR HEREAFTER ARISING, AND WHETHER IN CONTRACT, TORT, EQUITY OR OTHERWISE. EACH OF THE PARTIES TO THIS AGREEMENT HEREBY AGREES AND CONSENTS THAT ANY SUCH CLAIM, DEMAND, ACTION OR CAUSE OF ACTION WILL BE DECIDED BY COURT TRIAL WITHOUT A JURY, AND THAT THE PARTIES TO THIS AGREEMENT MAY FILE A COPY OF THIS AGREEMENT WITH ANY COURT AS WRITTEN EVIDENCE OF THE CONSENT OF THE PARTIES HERETO TO THE WAIVER OF THEIR RIGHT TO TRIAL BY JURY.
13.13Delivery by Facsimile or Email. This Agreement and any signed agreement entered into in connection herewith or contemplated hereby, and any amendments hereto or thereto, to the extent signed and delivered by means of a facsimile machine or scanned pages via electronic mail, will be treated in all manner and respect as an original contract and will be considered to have the same binding legal effects as if it were the original signed version thereof delivered in person.
A-87
13.14Counterparts. This Agreement may be executed simultaneously in two or more counterparts, each of which will be deemed an original, but all of which will constitute one agreement.
13.15Governing Law. All issues and questions concerning the construction, validity, interpretation and enforceability of this Agreement and the exhibits and schedules hereto will be governed by, and construed in accordance with, the Laws of the State of Delaware, without giving effect to any choice of Law or conflict of Law rules or provisions (whether of the State of Delaware or any other jurisdiction) that would cause the application of the Laws of any jurisdiction other than the State of Delaware.
13.16Jurisdiction. Any Legal Proceeding seeking to enforce any provision of, or based on any matter arising out of or in connection with, this Agreement or the transactions contemplated hereby will be brought and determined exclusively in the Delaware Court of Chancery of the State of Delaware;provided that if the Delaware Court of Chancery does not have subject matter jurisdiction, any such Legal Proceeding will be brought exclusively in the United States District Court for the District of Delaware or any other court of the State of Delaware, and each of the Parties hereby consents to the exclusive jurisdiction of such courts (and of the appropriate appellate courts therefrom) in any such Legal Proceeding and irrevocably waives, to the fullest extent permitted by Law, any objection that it may now or hereafter have to the laying of the venue of any such Legal Proceeding in any such court or that any such Legal Proceeding that is brought in any such court has been brought in an inconvenient forum. Process in any such Legal Proceeding may be served on any Party anywhere in the world, whether within or without the jurisdiction of any such court. Without limiting the foregoing, each Party agrees that service of process on such Party as provided inSection 13.04 will be deemed effective service of process on such Party.
13.17Remedies Cumulative. Except as otherwise provided herein, any and all remedies herein expressly conferred upon a Party will be deemed cumulative with, and not exclusive of, any other remedy conferred hereby, or by law or equity upon such Party, and the exercise by a Party of any one remedy will not preclude the exercise of any other remedy.
(a) Each Party agrees that irreparable damage would occur and that the Parties would not have any adequate remedy at law in the event that any of the provisions of this Agreement were not performed in accordance with their specific terms or were otherwise breached, and that money damages or other legal remedies would not be an adequate remedy therefor. Accordingly, in addition to any other remedies available under this Agreement, the Parties agree that, prior to the termination of this Agreement, each Party will be entitled to an injunction or injunctions, specific performance and other equitable relief to prevent any other Party’s breach of this Agreement and to enforce specifically the terms and provisions of this Agreement (including any Party’s obligation to consummate the transactions contemplated by this Agreement if required to do so hereunder). Each Party agrees that it will not oppose the granting of an injunction, specific performance and other equitable relief when expressly available pursuant to the terms of this Agreement, and hereby waives (i) any defenses in any Legal Proceeding for an injunction, specific performance or other equitable relief, including the defense that the other Parties have an adequate remedy at law or an award of specific performance is not an appropriate remedy for any reason at law or equity and (ii) any requirement under Law to post a bond, undertaking or other security as a prerequisite to obtaining equitable relief.
(b) NotwithstandingSection 13.18(a), the Member shall be entitled to specific performance of Holdco’s obligations to pay the Cash Purchase Price and issue the Stock Consideration to the Member and to consummate the Closing solely in the event that each of the following conditions is satisfied: (i) all of the conditions set forth inSection 8.01 (other than any conditions that by their terms are to be satisfied at the Closing, but subject to such conditions being satisfied if the Closing would have occurred) have been satisfied or validly waived by Parent, Holdco and Merger Sub and this Agreement has not been terminated in accordance withSection 10.01, (ii) the Member has notified Holdco, Parent and Merger Sub in writing
A-88
that (A) the Member is prepared to consummate the Closing and (B) if specific performance is granted and the Financing is funded, then it would take such actions required of it under this Agreement to cause the Closing to occur, (iii) Holdco, Parent and Merger Sub have not consummated the Closing by 12:00 p.m. New York City time on the second (2nd) Business Day following the date on which the Closing was required to occur pursuant toSection 2.01 and (iv) the Financing has been funded or will be funded at the Closing. For the avoidance of doubt, in no event shall the Member be entitled to enforce the obligation of, or seek to force, Holdco, Parent and/or Merger Sub to cause the Closing to occur if the Financing has not been funded (or will not be funded at the Closing).
13.19Waiver of Conflicts Regarding Representation; Privilege.
(a) Recognizing that D&W has acted as legal counsel to the Member and the Group Companies, and that D&W intends to act as legal counsel to the Member and one or more of the Member’s Affiliates after the consummation of the Closing, each of the Parties hereby consents to and waives, on behalf of the Group Companies, and agrees to cause each of the Group Companies to consent to and waive, any conflicts that may arise in connection with D&W representing the Member or any of the Member’s Affiliates after the consummation of the Closing, whether in connection with this Agreement or otherwise. The Parties also agree that the fact that D&W may be deemed from time to time to have acted as legal counsel to the Member and the Group Companies prior to the consummation of the Closing shall not prevent D&W from representing the Member or the Unitholders or any of their Affiliates in connection with any matters, including any matter involving this Agreement or the transactions contemplated hereby or any disputes between any of the Parties hereto that may arise after the consummation of the Closing. Each of the Parties acknowledges that such consent and waiver is voluntary, that it has been carefully considered and that it has consulted with counsel or has been advised they should do so. Any communications prior to the Closing with respect to this Agreement or the transactions contemplated hereby between any Group Company and D&W will become the property of the Member following the Closing and will not be disclosed to Holdco or any of its Subsidiaries without the consent of the Member.
(b) Notwithstanding the Closing, the Parties agree that neither Holdco nor any of its Subsidiaries, including the Group Companies, shall have the right to assert attorney-client privilege as topre-Closing and post-Closing communications between the Member or the Group Companies (for the Group Companies, only with respect topre-Closing communications), on one hand, and D&W, on the other hand, to the extent that the privileged communications relate to this Agreement or to the transactions contemplated hereby, other than to the extent necessary to protect against disclosure to any third party. The Parties agree that only the Member shall be entitled to assert or waive such attorney-client privilege in connection with such communications following the Closing. As of the consummation of the Closing and thereafter, (i) the client communications, work product and files generated and maintained by D&W as a result of D&W’s representation of the Member and the Group Companies in connection with this Agreement or any of the transactions contemplated hereby shall be and become the exclusive property of the Member, and any attorney-client privilege with respect thereto may be waived on behalf of the Member only by the Member, (ii) D&W shall have no duty whatsoever to reveal or disclose any such communications, work product or files to Holdco or any of its Subsidiaries (including, after the Closing, the Group Companies) by reason of any attorney-client relationship or otherwise and (iii) in the event any dispute arises between any of the Parties and/or any of the Group Companies, whether involving this Agreement or the transactions contemplated hereby or otherwise, each of Parent, Holdco and Merger Sub agrees, on behalf of itself and the Group Companies, that it and the Group Companies shall not offer into evidence or otherwise attempt to use or assert any attorney-client communications, files or work product in connection with such dispute and/or any suit, action or proceeding with respect thereto. The foregoing shall not extend to (A) any communication unrelated to this Agreement or the transactions contemplated, (B) communications among any of the Member or the Group Companies, on the one hand, and any Person other than D&W, on the other hand, or (C) any post-Closing communications between any Group Company and D&W or any other legal counsel. Notwithstanding the foregoing, in the event that a dispute arises between Parent, Holdco, Merger Sub and/or any of the Group Companies, on the one hand, and a third party, on the other hand, Parent,
A-89
Holdco, Merger Sub and/or any of the Group Companies, may, with consent of the Member (which consent shall not be unreasonably withheld) access and usepre- or post-Closing communications to the extent it or they believes reasonably necessary to defend the dispute. Nothing in thisSection 13.19 shall limit the professional obligation of confidentiality of D&W to the Member and the Group Companies or shall be deemed or construed as a waiver of any applicable privileges or protections that may be asserted as against any person other than the Parties.
13.20No Recourse; Waiver of Claims. Notwithstanding anything to the contrary in this Agreement, the Member (on behalf of itself and each of its current or future direct or indirect equity holders, unitholders, managers, assignees, Affiliates and Representatives) hereby waives (i) any rights or claims against any Financing Source or any of their respective Affiliates and any of their respective former, current and future direct or indirect equity holders, controlling persons, stockholders, agents, Affiliates, members, managers, general or limited partners, assignees or Representatives (collectively, the “Financing Source Related Parties”) in connection with this Agreement, the Financing or the Commitment Letters, whether at law or equity, in contract, in tort or otherwise, and (ii) any right such party may have to a trial by jury in respect of any litigation directly or indirectly arising out of or relating in any way to the Financing or the Commitment Letters, and the Member (on behalf of itself and each of its current or future direct or indirect equity holders, unitholders, managers, assignees, Affiliates and Representatives) agrees not to commence or support (and if commenced, agrees to dismiss or otherwise terminate) any dispute, suit, claim, litigation, investigation, proceeding or other action against any Financing Source Related Party in connection with this Agreement, the Financing or the Commitment Letters. In furtherance and not in limitation of the foregoing waiver, it is agreed that no Financing Source Related Party shall have any liability for any claims, losses, settlements, damages, costs, expenses, fines or penalties to the Member (or any of its current or future direct or indirect equity holders, unitholders, managers, assignees, Affiliates and Representatives) in connection with this Agreement or the transactions contemplated hereby, including any dispute arising out of or relating in any way to the Financing or the Commitment Letters. Each party hereto agrees that, except as specifically set forth in the Commitment Letters, all claims or causes of action against any of the Financing Source Related Parties in any way relating to the Financing or the Commitment Letters, shall be exclusively governed by, and construed in accordance with, the internal laws of the State of New York, without giving effect to principles or rules or conflict of laws to the extent such principles or rules would require or permit the application of laws of another jurisdiction. Notwithstanding the foregoing, nothing in thisSection 13.20 shall in any way limit, qualify or modify the rights and obligations of the parties to the Commitment Letters to each other thereunder or in connection therewith. Notwithstanding anything to the contrary contained in this Agreement, the Financing Source Related Parties are intended third-party beneficiaries of, and shall be entitled to the protections of, this provision.
* * * * *
A-90
IN WITNESS WHEREOF, the Parties have executed this Master Transaction Agreement on the day and year first above written.
| RTI SURGICAL, INC. | ||
| By: | /s/ Jonathon M. Singer | |
| Name: Jonathon M. Singer | ||
| Its: Chief Financial and Administrative Officer, Corporate Secretary | ||
[Master Transaction Agreement]
A-91
IN WITNESS WHEREOF, the Parties have executed this Master Transaction Agreement on the day and year first above written.
| BEARS HOLDING SUB, INC. | ||
| By: | /s/ Jonathon M. Singer | |
| Name: Jonathon M. Singer | ||
| Its: Chief Financial and Administrative Officer, Corporate Secretary | ||
[Master Transaction Agreement]
A-92
IN WITNESS WHEREOF, the Parties have executed this Master Transaction Agreement on the day and year first above written.
| BEARS MERGER SUB, INC. | ||
| By: | /s/ Jonathon M. Singer | |
| Name: Jonathon M. Singer | ||
| Its: President | ||
[Master Transaction Agreement]
A-93
IN WITNESS WHEREOF, the Parties have executed this Master Transaction Agreement on the day and year first above written.
| PS SPINE HOLDCO, LLC | ||
| By: | /s/ Marc R. Viscogliosi | |
| Name: Marc R. Viscogliosi | ||
| Its: Chairman & Chief Executive Officer | ||
[Master Transaction Agreement]
A-94
Exhibit A
AMENDED AND RESTATED
CERTIFICATE OF INCORPORATION
OF
[ ]
* * *
FIRST: The name of the corporation is [ ].
SECOND: The address of the registered office of the corporation in the State of Delaware is United Corporate Services, Inc., 874 Walker Road, Suite C, Dover, Kent County, DE 19904. The name of corporation’s registered agent at such address is United Corporate Services, Inc.
THIRD: The purpose of the corporation is to engage in any lawful act or activity for which a corporation may be organized under the General Corporation Law of Delaware as set forth in Title 8 of the Delaware Code 1953, as amended (the “GCL”).
FOURTH:Capital Stock:
This corporation is authorized to issue 155,000,000 shares of capital stock, $0.001 par value, of which 150,000,000 shares shall be Common Stock, $0.001 par value and 5,000,000 shares shall be Preferred Stock, $0.001 par value.
| (A) | Preferred Stock. The Board of Directors is expressly authorized to provide for the issue of all or any shares of the Preferred Stock, in one or more series, and to fix for each such series such voting powers, full or limited, and such designations, preferences and relative, participating, optional or other special rights and such qualifications, limitations or restrictions thereof as shall be stated and expressed in the resolution or resolutions adopted by the Board of Directors providing for the issue of such series (a “Preferred Stock Designation”) and as may be permitted by the GCL. The number of authorized shares of Preferred Stock may be increased or decreased (but not below the number of shares thereof then outstanding) by the affirmative vote of the holders of a majority of the voting power of all the then outstanding shares of the capital stock of the corporation entitled to vote generally in the election of directors, voting together as a single class, without a separate vote of the holders of the Preferred Stock, or any series thereof, unless a vote of any such holders is required pursuant to any Preferred Stock Designation. |
| (B) | Common Stock. Except as otherwise required by law or as otherwise provided in any Preferred Stock Designation, the holders of the Common Stock shall exclusively possess all voting power and each share of Common Stock shall have one vote. |
FIFTH: The corporation is to have perpetual existence.
SIXTH: In furtherance and not in limitation of the powers conferred by statute, the Board of Directors is expressly authorized to make, alter or repeal the by-laws of the corporation. Notwithstanding anything in this Amended and Restated Certificate of Incorporation to the contrary, Bylaw Sections 2.04, 2.07 and paragraphs Ninth and Twelfth of this Amended and Restated Certificate of Incorporation may not be repealed or amended in any respect, and no provision inconsistent therewith may be adopted by the stockholders unless such action is approved by the affirmative vote of the holders of sixty-six and two-thirds percent (66 2/3%) of the outstanding shares of all classes and series of the corporation entitled to vote generally in the election of the corporation’s directors.
A-95
SEVENTH: Directors shall be elected for a one-year term expiring at the next annual meeting of stockholders and until his or her successor shall be duly elected and qualified, subject to his or her earlier death, resignation, retirement or removal from service as a director. Any vacancy on the Board of Directors for any reason, and any directorships resulting from any increase in the number of directors of the Board of Directors, may be filled by a majority of the Board of Directors then in office, although less than a quorum, or a sole remaining director and any directors so chosen shall hold office until the next annual meeting of the stockholders and until their successors shall be duly elected and qualified, subject to their earlier death, resignation, retirement or removal from service as a director. Notwithstanding the foregoing, whenever the holders of any one or more classes or series of stock issued by the corporation shall have the right, voting separately by class or series, to elect directors at an annual or special meeting of stockholders, the election, term of office, filling of vacancies and other features of such directorships shall be governed by the terms of this Amended and Restated Certificate of Incorporation applicable thereto, and the number of such directors shall not be counted in determining the maximum number of directors permitted under the foregoing provision of this paragraph Seventh in each case unless expressly provided by such terms.
EIGHTH: Meetings of stockholders may be held within or without the State of Delaware, as the by-laws may provide. Upon consummation of the corporation’s initial public offering of its Common Stock, stockholder action may not be taken by written consent in lieu of a meeting. The books of the corporation may be kept (subject to any provision of the GCL) outside the State of Delaware at such place or places as may be designated from time to time by the board of directors or in the by-laws of the corporation. Election of directors need not be by written ballot unless the by-laws of the corporation shall so provide.
NINTH: The corporation shall indemnify each person who is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, or is or was a director, officer, employee or agent of a foreign or domestic corporation that was a predecessor corporation of this corporation or another enterprise at the request of the predecessor corporation to the fullest extent permitted by Section 145 of the GCL, as amended. The indemnification provided for herein shall not be deemed exclusive of any other rights to which those indemnified may be entitled under any by-law, agreement, vote of stockholders or disinterested directors or otherwise, both as to action in an official capacity and as to action in another capacity while holding such office, and such indemnification shall continue as to a person who has ceased to be such a person and shall inure to the benefit of the heirs, executors and administrators of such a person.
Any amendment, repeal or modification of the foregoing provisions of this paragraph Ninth shall not adversely affect any right or protection of a director, officer, agent, or other person existing at the time of, or increase the liability of any director of this corporation with respect to any acts or omissions of such director, officer or agent occurring prior to, such amendment, repeal or modification.
TENTH: The corporation reserves the right to amend, alter, change or repeal any provision contained in this Amended and Restated Certificate of Incorporation, in the manner now or thereafter prescribed by statute, and all rights conferred on the stockholders herein are granted subject to this reservation.
ELEVENTH: Whenever a compromise or arrangement is proposed between this corporation and its creditors or any class of them and/or between this corporation and its stockholders or any class of them, any court of equitable jurisdiction within the State of Delaware may, on the application in a summary way of this corporation or of any creditor or stockholder thereof or on the application of any receiver or receivers appointed for this corporation under the provisions of Section 291 of the GCL or on the application of trustees in dissolution or of any receiver or receivers appointed for this corporation under the provisions of Section 279 of the GCL, order a meeting of the creditors or class of creditors, and/or of the stockholders or class of stockholders of this corporation, as the case may be, to be summoned in such manner as the said court directs. If a majority in number representing three-fourths in value of the creditors or class of creditors, and/or of the stockholders or class of stockholders of this corporation, as the case may be, agree to any compromise or arrangement and to any
A-96
reorganization of this corporation as consequence of such compromise or arrangement, the said compromise or arrangement and the said reorganization shall, if sanctioned by the court to which the said application has been made, be binding on all the creditors or class of creditors, and/or on all the stockholders or class of stockholders, of this corporation, as the case may be, and also on this corporation.
TWELFTH: A director of this corporation shall not be personally liable to the corporation or its stockholders for monetary damages for the breach of any fiduciary duty as a director, except (i) for any breach of the director’s duty of loyalty to the corporation or its stockholders, (ii) for acts or omissions not in good faith or that involve intentional misconduct or knowing violation of law, (iii) under Section 174 of GCL, as the same exists or hereafter may be amended, or (iv) for any transaction from which the director derived an improper personal benefit. If the GCL is amended after the date of incorporation of the corporation to authorize corporate action further eliminating or limiting the personal liability of directors, then the liability of a director of the corporation shall be eliminated or limited to the fullest extent permitted by the GCL, as so amended.
Any repeal or modification of the foregoing paragraph by the stockholders of the corporation shall be prospective only, and shall not adversely affect any limitation on the personal liability of a director of the corporation existing at the time of such repeal or modification.
A-97
Exhibit B
AMENDED AND RESTATED
BYLAWS
OF
[ �� ]
Effective [●], 2018
ARTICLE I
OFFICES
SECTION 1.01 REGISTERED OFFICE. The registered office of the corporation in the State of Delaware shall be in the City of Dover, County of Kent, and the name of its registered agent shall be United Corporate Services, Inc.
SECTION 1.02 OTHER OFFICES. The corporation may also have offices at such other places both within and without the State of Delaware as the Board of Directors may from time to time determine or the business of the corporation may require.
ARTICLE II
MEETINGS OF STOCKHOLDERS
SECTION 2.01 PLACE OF MEETING. All meetings of stockholders for the election of directors shall be held at such place, either within or without the State of Delaware, as shall be designated from time to time by the Board of Directors and stated in the notice of the meeting.
SECTION 2.02 ANNUAL MEETING. The annual meeting of stockholders shall be held at such date and time as shall be designated from time to time by the Board of Directors and stated in the notice of the meeting.
SECTION 2.03 VOTING LIST. The officer who has charge of the stock ledger of the corporation shall prepare and make, at least 10 days before every meeting of stockholders, a complete list of the stockholders entitled to vote at the meeting, arranged in alphabetical order, and showing the address of each stockholder and the number of shares registered in the name of each stockholder. Such list shall be open to the examination of any stockholder, for any purpose germane to the meeting, during ordinary business hours, for a period of at least 10 days prior to the meeting, either at a place within the city where the meeting is to be held, which place shall be specified in the notice, or if not so specified, at the place where the meeting is to be held. The list shall also be produced and kept at the time and place of the meeting during the whole time thereof, and may be inspected by any stockholder who is present.
SECTION 2.04 SPECIAL MEETING. Special meetings of the stockholders, for any purpose or purposes, unless otherwise prescribed by statute or by the Certificate of Incorporation, may be called by the Chairman of the Board or by the President of the corporation or by the Board of Directors or by written order of a majority of the directors and shall be called by the President or the Secretary at the request in writing of stockholders owning a majority in amount of the entire capital stock of the corporation issued and outstanding and entitled to vote. Such request shall state the purposes of the proposed meeting. The Chairman of the Board or the President of the corporation or directors so calling, or the stockholders so requesting, any such meeting shall fix the time and any place, either within or without the State of Delaware, as the place for holding such meeting.
A-98
SECTION 2.05 NOTICE OF MEETING. Written notice of the annual, and each special meeting of stockholders, stating the time, place, and purpose or purposes thereof, shall be given to each stockholder entitled to vote thereat, not less than 10 nor more than 60 days before the meeting.
SECTION 2.06 QUORUM. The holders of a majority of the shares of the corporation’s capital stock issued and outstanding and entitled to vote thereat, present in person or represented by proxy, shall constitute a quorum at any meeting of stockholders for the transaction of business, except as otherwise provided by statute or by the Certificate of Incorporation. Notwithstanding the other provisions of the Certificate of Incorporation or these bylaws, the holders of a majority of the shares of the corporation’s capital stock entitled to vote thereat, present in person or represented by proxy, whether or not a quorum is present, shall have power to adjourn the meeting from time to time, without notice other than announcement at the meeting, until a quorum shall be present or represented. If the adjournment is for more than 30 days, or if after the adjournment a new record date is fixed for the adjourned meeting, a notice of the adjourned meeting shall be given to each stockholder of record entitled to vote at the meeting. At such adjourned meeting at which a quorum shall be present or represented, any business may be transacted which might have been transacted at the meeting as originally notified.
SECTION 2.07 VOTING. When a quorum is present at any meeting of the stockholders, the vote of the holders of a majority of the shares of the corporation’s capital stock having voting power present in person or represented by proxy shall decide any question brought before such meeting, unless the question is one upon which, by express provision of the statutes, of the Certificate of Incorporation or of these bylaws, a different vote is required, in which case such express provision shall govern and control the decision of such question. Every stockholder having the right to vote shall be entitled to vote in person, or by proxy appointed by an instrument in writing subscribed by such stockholder, bearing a date not more than three years prior to voting, unless such instrument provides for a longer period, and filed with the Secretary of the corporation before, or at the time of, the meeting. If such instrument shall designate two or more persons to act as proxies, unless such instrument shall provide the contrary, a majority of such persons present at any meeting at which their powers thereunder are to be exercised shall have and may exercise all the powers of voting or giving consents thereby conferred, or if only one be present, then such powers may be exercised by that one; or, if an even number attend and a majority do not agree on any particular issue, each proxy so attending shall be entitled to exercise such powers in respect of the same portion of the shares as he is of the proxies representing such shares.
SECTION 2.08 VOTING OF STOCK OF CERTAIN HOLDERS. Shares of the corporation’s capital stock standing in the name of another corporation, domestic or foreign, may be voted by such officer, agent, or proxy as the bylaws of such corporation may prescribe, or in the absence of such provision, as the Board of Directors of such corporation may determine. Shares standing in the name of a deceased person may be voted by the executor or administrator of such deceased person, either in person or by proxy. Shares standing in the name of a guardian, conservator, or trustee may be voted by such fiduciary, either in person or by proxy, but no such fiduciary shall be entitled to vote shares held in such fiduciary capacity without a transfer of such shares into the name of such fiduciary. Shares standing in the name of a receiver may be voted by such receiver. A stockholder whose shares are pledged shall be entitled to vote such shares, unless in the transfer by the pledgor on the books of the corporation, he has expressly empowered the pledgee to vote thereon, in which case only the pledgee, or his proxy, may represent the stock and vote thereon.
SECTION 2.09 TREASURY STOCK. The corporation shall not vote, directly or indirectly, shares of its own capital stock owned by it; and such shares shall not be counted in determining the total number of outstanding shares of the corporation’s capital stock.
SECTION 2.10 FIXING RECORD DATE. The Board of Directors may fix in advance a date, which shall not be more than 60 days nor less than 10 days preceding the date of any meeting of stockholders, nor more than 60 days preceding the date for payment of any dividend or distribution, or the date for the allotment of rights, or the date when any change, or conversion or exchange of capital stock shall go into effect, or a date in connection with obtaining a consent, as a record date for the determination of the stockholders entitled to notice of, and to
A-99
vote at, any such meeting and any adjournment thereof, or entitled to receive payment of any such dividend or distribution, or to receive any such allotment of rights, or to exercise the rights in respect of any such change, conversion or exchange of capital stock, or to give such consent, and in such case such stockholders and only such stockholders as shall be stockholders of record on the date so fixed, shall be entitled to such notice of, and to vote at, any such meeting and any adjournment thereof, or to receive payment of such dividend or distribution, or to receive such allotment of rights, or to exercise such rights, or to give such consent, as the case may be, notwithstanding any transfer of any stock on the books of the corporation after any such record date fixed as aforesaid.
SECTION 2.11 NOTICE OF STOCKHOLDER BUSINESS AND NOMINATIONS
(a) To be properly brought before the annual meeting or special meeting, nominations of persons for election to the board of directors or other business must be either: (i) specified in the notice of meeting (or any supplement thereto) given by or at the direction of the board of directors; (ii) otherwise properly brought before the meeting by or at the direction of the board of directors or (iii) otherwise properly brought before the meeting by a stockholder of record.
(b) For nominations or other business to be properly brought before an annual meeting by a stockholder pursuant to clause (iii) of Section 2.11(a) of these bylaws, (i) the subject matter thereof must be a matter which is a proper subject matter for stockholder action at such meeting; (ii) the stockholder must have been a stockholder of record of the corporation at the time the notice required by this Section 2.11 is delivered to the corporation and must be entitled to vote at the meeting; and (iii) the stockholder must have given timely written notice thereof by mail, courier or personal delivery to (A) the Nominating and Corporate Governance Committee of the board of directors, care of the corporate secretary of the corporation, for nominations, or (B) the corporate secretary of the corporation, for other business. To be considered timely, a stockholder’s notice must be delivered to or mailed and received by the secretary of the corporation at the principal executive offices of the corporation not more than 120 days and not less than 90 calendar days prior to the first anniversary of the date of the proxy statement for the prior annual meeting of stockholders. Notwithstanding the above, in the event that the date of the annual meeting of stockholders for the current year is more than 30 days following the first anniversary date of the annual meeting of stockholders for the prior year, the submission of a recommendation will be considered timely if it is submitted a reasonable time in advance of the mailing of the corporation’s proxy statement for the annual meeting of stockholders for the current year.
A stockholder’s notice shall set forth: (i) as to each person whom the stockholder proposes to nominate for election as a director, (A) all information relating to such person that is required by Item 401 of the Securities and Exchange Commission’s (“SEC”) Regulation S-K; (B) the information required by Item 403 of SEC Regulation S-K; (C) the information required by Item 404 of SEC Regulation S-K; (D) a description of all relationships between the proposed nominee and the recommending stockholder and any agreements or understandings between the recommending stockholder and the nominee regarding the nomination; (E) a description of all relationships between the proposed nominee and any of the corporation’s competitors, customers, suppliers, labor unions or other persons or entities with special interests regarding the corporation known to the recommending stockholder or the nominee; (F) a statement by the stockholder supporting his, her or its view that the proposed nominee possesses the minimum qualifications prescribed by the Nominating and Corporate Governance Committee of the board of directors for nominees or directors from time to time, including those that may be set forth in the corporation’s Corporate Governance Guidelines, and a brief description of the contributions that the nominee would be expected to make to the board of directors and to the governance of the corporation; (G) a statement whether, in the view of the stockholder, the nominee, if elected, would represent all stockholders and not serve for the purpose of advancing or favoring any particular stockholder or other constituency of the corporation; and (H) such nominee’s written consent to being interviewed by the Nominating and Corporate Governance Committee of the board of directors (including the nominee’s contact information for this purpose), and, if nominated and elected, to serve as a director of the corporation; (ii) as to any other business the stockholder proposes to bring before the annual meeting, (A) a brief
A-100
description of such business and the reasons for conducting such business at the annual meeting and (B) any material interest in such business of the stockholder and any beneficial owner on whose behalf the proposal or nomination is made; and (iii) as to the stockholder giving the notice (or, if submitted by a group of two or more stockholders, as to each stockholder in the group), (A) the name and address, including telephone number, of such stockholder; (B) the number of shares of the corporation that are beneficially owned and held of record by such stockholder and the time period for which such shares have been held; (C) if the stockholder is not a stockholder of record, a statement from the record holder of the shares verifying the holdings of the stockholder and a statement from the stockholder of the length of time that the shares have been held (alternatively, the stockholder may furnish a current Schedule 13D, Schedule 13G, Form 3, Form 4, or Form 5 filed with the SEC reflecting the holdings of the stockholder, together with a statement of the length of time that the shares have been held); and (D) a statement from the stockholder as to whether the stockholder has a good faith intention to continue to hold the reported shares through the date of the corporation’s next annual meeting of stockholders.
(c) Notwithstanding anything in Section 2.11(b) of these bylaws to the contrary, in the event that the number of directors to be elected to the board of directors at an annual meeting is increased and there is no public announcement by the corporation naming the nominees for the additional directorships at least 100 days prior to the first anniversary of the preceding year’s annual meeting, a stockholder’s notice required by this Section 2.11 shall also be considered timely, but only with respect to nominees for the additional directorships, if it shall be delivered to the secretary at the principal executive offices of the corporation not later than the close of business on the 10th day following the day on which such public announcement is first made by the corporation.
(d) Notwithstanding the foregoing provisions of this Section 2.11, a stockholder who seeks to have any proposal included in the corporation’s proxy materials must provide notice as required by and otherwise comply with the applicable requirements of the rules and regulations under the Securities Exchange Act of 1934. Nothing in this Section 2.11 shall be deemed to affect any rights of stockholders to request inclusion of proposals or nominations in the corporation’s proxy statement pursuant to applicable rules and regulations promulgated under the Securities Exchange Act of 1934.
Only persons nominated in accordance with the procedures set forth in this Section 2.11 shall be eligible to serve as directors and only such business shall be conducted at an annual meeting of stockholders as shall have been brought before the meeting in accordance with the procedures set forth in this Section 2.11. The chairman of the meeting shall have the power and the duty to determine whether a nomination or any business proposed to be brought before the meeting has been made in accordance with the procedures set forth in these bylaws and, if any proposed nomination or business is not in compliance with these bylaws, to declare that such business shall not be transacted at such meeting and such nomination shall be disregarded.
ARTICLE III
BOARD OF DIRECTORS
SECTION 3.01 POWERS. The business and affairs of the corporation shall be managed by its Board of Directors, which may exercise all such powers of the corporation and do all such lawful acts and things as are not by statute or by the Certificate of Incorporation or by these bylaws directed or required to be exercised or done by the stockholders.
SECTION 3.02 NUMBER, ELECTION AND TERM. The number of directors that shall constitute the whole Board of Directors shall be not less than one. Such number of directors shall from time to time be fixed and determined by resolution of the Board of Directors and shall be set forth in the notice of any meeting of stockholders held for the purpose of electing directors. No reduction of the authorized number of directors shall have the effect of removing any director before that director’s term of office expires. The directors shall be appointed or elected as set forth in the Seventh Article of the Certificate of Incorporation and each director
A-101
elected shall hold office for the period set forth in the Seventh Article of the Certificate of Incorporation. Directors need not be residents of Delaware or stockholders of the corporation.
SECTION 3.03 VACANCIES, ADDITIONAL DIRECTORS, AND REMOVAL FROM OFFICE. If any vacancy occurs in the Board of Directors caused by death, resignation, retirement, disqualification, or removal from office of any director, or otherwise, or if any new directorship is created by an increase in the authorized number of directors, a majority of the directors then in office, though less than a quorum, or a sole remaining director, may choose a successor or fill the newly created directorship; and a director so chosen shall hold office until the next annual meeting of stockholders and until his successor shall be duly elected and shall qualify, unless sooner displaced. Any director may be removed either for or without cause at any special meeting of stockholders duly called and held for such purpose.
SECTION 3.04 REGULAR MEETING. A regular meeting of the Board of Directors shall be held each year, without other notice than this bylaw, at the place of, and immediately following, the annual meeting of stockholders; and other regular meetings of the Board of Directors shall be held each year, at such time and place as the Board of Directors may provide, by resolution, either within or without the State of Delaware, without other notice than such resolution.
SECTION 3.05 SPECIAL MEETING. A special meeting of the Board of Directors may be called by the Chairman of the Board of Directors or by the President of the corporation and shall be called by the Secretary on the written request of any two directors. The Chairman or President so calling, or the directors so requesting, any such meeting shall fix the time and any place, either within or without the State of Delaware, as the place for holding such meeting.
SECTION 3.06 NOTICE OF SPECIAL MEETING. Written notice of special meetings of the Board of Directors shall be given to each director at least 48 hours prior to the time of such meeting. Any director may waive notice of any meeting. The attendance of a director at any meeting shall constitute a waiver of notice of such meeting, except where a director attends a meeting for the purpose of objecting to the transaction of any business because the meeting is not lawfully called or convened. Neither the business to be transacted at, nor the purpose of, any special meeting of the Board of Directors need be specified in the notice or waiver of notice of such meeting, except that notice shall be given of any proposed amendment to the bylaws if it is to be adopted at any special meeting or with respect to any other matter where notice is required by statute.
SECTION 3.07 QUORUM. A majority of the Board of Directors shall constitute a quorum for the transaction of business at any meeting of the Board of Directors, and the act of a majority of the directors present at any meeting at which there is a quorum shall be the act of the Board of Directors, except as may be otherwise specifically provided by statute, by the Certificate of Incorporation or by these bylaws. If a quorum shall not be present at any meeting of the Board of Directors, the directors present thereat may adjourn the meeting from time to time, without notice other than announcement at the meeting, until a quorum shall be present.
SECTION 3.08 ACTION WITHOUT MEETING. Unless otherwise restricted by the Certificate of Incorporation or these bylaws, any action required or permitted to be taken at any meeting of the Board of Directors, or of any committee thereof as provided in Article IV of these bylaws, may be taken without a meeting, if a written consent thereto is signed by all members of the Board of Directors or of such committee, as the case may be, and such written consent is filed with the minutes of proceedings of the Board of Directors or such committee.
SECTION 3.09 COMPENSATION. Directors, as such, shall not be entitled to any stated salary for their services unless voted by the stockholders or the Board of Directors; but by resolution of the Board of Directors, a fixed sum and expenses of attendance, if any, may be allowed for attendance at each regular or special meeting of the Board of Directors or any meeting of a committee of directors. No provision of these bylaws shall be construed to preclude any director from serving the corporation in any other capacity and receiving compensation therefor.
A-102
ARTICLE IV
COMMITTEES OF DIRECTORS
SECTION 4.01 DESIGNATION, POWERS AND NAME. The Board of Directors may, by resolution passed by a majority of the whole Board of Directors, designate one or more committees, including, if they shall so determine, an Executive Committee, each such committee to consist of two or more of the directors of the corporation. The committee shall have and may exercise such of the powers of the Board of Directors in the management of the business and affairs of the corporation as may be provided in such resolution. The committee may authorize the seal of the corporation to be affixed to all papers that may require it. The Board of Directors may designate one or more directors as alternate members of any committee, who may replace any absent or disqualified member at any meeting of such committee. In the absence or disqualification of any member of such committee or committees, the member or members thereof present at any meeting and not disqualified from voting, whether or not he or they constitute a quorum, may unanimously appoint another member of the Board of Directors to act at the meeting in the place of any such absent or disqualified member. Such committee or committees shall have such name or names and such limitations of authority as may be determined from time to time by resolution adopted by the Board of Directors.
SECTION 4.02 MINUTES. Each committee of directors shall keep regular minutes of its proceedings and report the same to the Board of Directors when required.
SECTION 4.03 COMPENSATION. Members of special or standing committees may be allowed compensation for attending committee meetings, if the Board of Directors shall so determine.
ARTICLE V
NOTICE
SECTION 5.01 METHODS OF GIVING NOTICE. Whenever under the provisions of applicable statutes, the Certificate of Incorporation or these bylaws, notice is required to be given to any director, member of any committee, or stockholder, such notice shall be in writing and delivered personally or mailed to such director, member, or stockholder; provided that in the case of a director or a member of any committee such notice may be given orally or by telephone or facsimile or other secure electronic means, such as encrypted e-mail. If mailed, notice to a director, member of a committee, or stockholder shall be deemed to be given when deposited in the United States mail first class in a sealed envelope, with postage thereon prepaid, or when delivered through reputable, recognized overnight courier service, addressed, in the case of a stockholder, to the stockholder at the stockholder’s address as it appears on the records of the corporation or, in the case of a director or a member of a committee, to such person at his business address. If sent by telegraph, notice to a director or member of a committee shall be deemed to be given when the telegram, so addressed, is delivered to the telegraph company. If sent by other secure electronic means, such as encrypted e-mail, notice to a director or member of a committee shall be deemed to be given upon verification of receipt.
SECTION 5.02 WRITTEN WAIVER. Whenever any notice is required to be given under the provisions of an applicable statute, the Certificate of Incorporation, or these bylaws, a waiver thereof in writing, signed by the person or persons entitled to said notice, whether before or after the time stated therein, shall be deemed equivalent thereto.
A-103
ARTICLE VI
OFFICERS
SECTION 6.01 OFFICERS. The officers of the corporation shall be a Chairman of the Board and a Vice Chairman of the Board (if such offices are created by the Board), a Chief Executive Officer, a President, one or more Vice Presidents, any one or more of which may be designated Executive Vice President or Senior Vice President, a Secretary and a Treasurer. The Board of Directors may appoint such other officers and agents, including Assistant Vice Presidents, Assistant Secretaries, and Assistant Treasurers, in each case as the Board of Directors shall deem necessary, who shall hold their offices for such terms and shall exercise such powers and perform such duties as shall be determined by the Board. Any two or more offices may be held by the same person. No officer shall execute, acknowledge, verify or countersign any instrument on behalf of the corporation in more than one capacity, if such instrument is required by law, by these bylaws or by any act of the corporation to be executed, acknowledged, verified, or countersigned by two or more officers. The Chairman and Vice Chairman of the Board shall be elected from among the directors. With the foregoing exceptions, none of the other officers need be a director, and none of the officers need be a stockholder of the corporation.
SECTION 6.02 ELECTION AND TERM OF OFFICE. The officers of the corporation shall be elected annually by the Board of Directors at its first regular meeting held after the annual meeting of stockholders or as soon thereafter as conveniently possible. Each officer shall hold office until his successor shall have been chosen and shall have qualified or until his death or the effective date of his resignation or removal, or until he shall cease to be a director in the case of the Chairman and the Vice Chairman.
SECTION 6.03 REMOVAL AND RESIGNATION. Any officer or agent elected or appointed by the Board of Directors may be removed without cause by the affirmative vote of a majority of the Board of Directors whenever, in its judgment, the best interests of the corporation shall be served thereby, but such removal shall be without prejudice to the contractual rights, if any, of the person so removed. Any officer may resign at any time by giving written notice to the corporation. Any such resignation shall take effect at the date of the receipt of such notice or at any later time specified therein, and unless otherwise specified therein, the acceptance of such resignation shall not be necessary to make it effective.
SECTION 6.04 VACANCIES. Any vacancy occurring in any office of the corporation by death, resignation, removal, or otherwise, may be filled by the Board of Directors for the unexpired portion of the term.
SECTION 6.05 SALARIES. The salaries of all officers and agents of the corporation shall be fixed by the Board of Directors or pursuant to its direction; and no officer shall be prevented from receiving such salary by reason of his also being a director.
SECTION 6.06 CHAIRMAN OF THE BOARD. The Chairman of the Board (if such office is created by the Board) shall preside at all meetings of the Board of Directors or of the stockholders of the corporation. The Chairman shall formulate and submit to the Board of Directors or the Executive Committee matters of general policy for the corporation and shall perform such other duties as usually appertain to the office or as may be prescribed by the Board of Directors or the Executive Committee.
SECTION 6.07 VICE CHAIRMAN OF THE BOARD. The Vice Chairman of the Board (if such office is created by the Board) shall, in the absence or disability of the Chairman of the Board, perform the duties and exercise the powers of the Chairman of the Board. The Vice Chairman shall perform such other duties as from time to time may be prescribed by the Board of Directors or the Executive Committee or assigned by the Chairman of the Board.
SECTION 6.08 CHIEF EXECUTIVE OFFICER. Subject to the control of the Board of Directors, the Chief Executive Officer shall in general supervise and control the business and affairs of the corporation. He shall have
A-104
the power to appoint and remove subordinate officers, agents and employees, except those elected or appointed by the Board of Directors. The Chief Executive Officer shall keep the Board of Directors and the Executive Committee fully informed and shall consult them concerning the business of the corporation. He may sign with the Secretary or any other officer of the corporation thereunto authorized by the Board of Directors, any deeds, bonds, mortgages, contracts, checks, notes, drafts, or other instruments that the Board of Directors has authorized to be executed, except in cases where the signing and execution thereof has been expressly delegated by these bylaws or by the Board of Directors to some other officer or agent of the corporation, or shall be required by law to be otherwise executed. He shall vote, or give a proxy to any other officer of the corporation to vote, all shares of stock of any other corporation standing in the name of the corporation and in general he shall perform all other duties normally performed by a Chief Executive Officer and such other duties as may be prescribed by the stockholders, the Board of Directors, or the Executive Committee. In the absence of the Chairman of the Board or the Vice Chairman of the Board (if such offices are created by the Board), the Chief Executive Officer shall preside at all meetings of the Board of Directors and of the stockholders. He may also preside at any such meeting attended by the Chairman or Vice Chairman of the Board if he is so designated by the Chairman, or in the Chairman’s absence by the Vice Chairman.
SECTION 6.09 PRESIDENT. The President shall report to the Chief Executive Officer and shall implement the general directives, plans and policies formulated by the Chief Executive Officer. The President shall have authority to exercise all the powers delegated to him by the Chief Executive Officer. He may sign with the Secretary or any other officer of the corporation thereunto authorized by the Board of Directors, certificates for shares of the corporation and any deeds, bonds, mortgages, contracts, checks, notes, drafts, or other instruments that the Board of Directors has authorized to be executed, except in cases where the signing and execution thereof has been expressly delegated by these bylaws or by the Board of Directors to some other officer or agent of the corporation, or shall be required by law to be otherwise executed and shall perform such other duties as may be prescribed by the Chief Executive Officer, the Board of Directors, or the Executive Committee.
SECTION 6.10 VICE PRESIDENTS. In the absence of the President, or in the event of his inability or refusal to act, the Executive Vice President (or in the event there shall be no Vice President designated Executive Vice President, any Vice President designated by the Board) shall perform the duties and exercise the powers of the President. Any Vice President may sign, with the Secretary or Assistant Secretary, certificates for shares of the corporation. The Vice Presidents shall perform such other duties as from time to time may be assigned to them by the President, the Board of Directors or the Executive Committee.
SECTION 6.11 SECRETARY. The Secretary shall (a) keep the minutes of the meetings of the stockholders, the Board of Directors and committees of directors; (b) see that all notices are duly given in accordance with the provisions of these bylaws and as required by law; (c) be custodian of the corporate records and of the seal of the corporation, and see that the seal of the corporation or a facsimile thereof is affixed to all certificates for shares prior to the issue thereof and to all documents, the execution of which on behalf of the corporation under its seal is duly authorized in accordance with the provisions of these bylaws; (d) keep or cause to be kept a register of the post office address of each stockholder which shall be furnished by such stockholder; (e) sign with the President, or an Executive Vice President or Vice President, certificates for shares of the corporation, the issue of which shall have been authorized by resolution of the Board of Directors; (f) have general charge of the stock transfer books of the corporation; and (g) in general, perform all duties normally incident to the office of Secretary and such other duties as from time to time may be assigned to him by the President, the Board of Directors or the Executive Committee.
SECTION 6.12 TREASURER. If required by the Board of Directors, the Treasurer shall give a bond for the faithful discharge of his duties in such sum and with such surety or sureties as the Board of Directors shall determine. He shall (a) have charge and custody of and be responsible for all funds and securities of the corporation; (b) receive and give receipts for moneys due and payable to the corporation from any source whatsoever and deposit all such moneys in the name of the corporation in such banks, trust companies, or other depositories as shall be selected in accordance with the provisions of Section 7.03 of these bylaws; (c) prepare, or
A-105
cause to be prepared, for submission at each regular meeting of the Board of Directors, at each annual meeting of the stockholders, and at such other times as may be required by the Board of Directors, the President or the Executive Committee, a statement of financial condition of the corporation in such detail as may be required; and (d) in general, perform all the duties incident to the office of Treasurer and such other duties as from time to time may be assigned to him by the President, the Board of Directors or the Executive Committee.
SECTION 6.13 ASSISTANT SECRETARY AND TREASURER. The Assistant Secretaries and Assistant Treasurers shall, in general, perform such duties as shall be assigned to them by the Secretary or the Treasurer, respectively, or by the President, the Board of Directors, or the Executive Committee. The Assistant Secretaries and Assistant Treasurers shall, in the absence of the Secretary or Treasurer, respectively, perform all functions and duties which such absent officers may delegate, but such delegation shall not relieve the absent officer from the responsibilities and liabilities of his office. The Assistant Secretaries may sign, with the President or a Vice President, certificates for shares of the corporation, the issue of which shall have been authorized by a resolution of the Board of Directors. The Assistant Treasurers shall respectively, if required by the Board of Directors, give bonds for the faithful discharge of their duties in such sums and with such sureties as the Board of Directors shall determine.
ARTICLE VII
CONTRACTS, CHECKS AND DEPOSITS
SECTION 7.01 CONTRACTS. Subject to the provisions of Section 6.01, the Board of Directors may authorize any officer, officers, agent, or agents, to enter into any contract or execute and deliver any instrument in the name of and on behalf of the corporation, and such authority may be general or confined to specific instances.
SECTION 7.02 CHECKS. All checks, demands, drafts, or other orders for the payment of money, notes, or other evidences of indebtedness issued in the name of the corporation, shall be signed by such officer or officers or such agent or agents of the corporation, and in such manner, as shall be determined by the Board of Directors.
SECTION 7.03 DEPOSITS. All funds of the corporation not otherwise employed shall be deposited from time to time to the credit of the corporation in such banks, trust companies, or other depositories as the Board of Directors may select.
ARTICLE VIII
CERTIFICATES OF STOCK
SECTION 8.01 ISSUANCE. Each stockholder of this corporation shall be entitled to a certificate or certificates showing the number of shares of capital stock registered in his name on the books of the corporation. The certificates shall be in such form as may be determined by the Board of Directors, shall be issued in numerical order and shall be entered in the books of the corporation as they are issued. They shall exhibit the holder’s name and number of shares and shall be signed by the President or a Vice President and by the Secretary or an Assistant Secretary. If any certificate is countersigned (1) by a transfer agent other than the corporation or any employee of the corporation, or (2) by a registrar other than the corporation or any employee of the corporation, any other signature on the certificate may be a facsimile. If the corporation shall be authorized to issue more than one class of stock or more than one series of any class, the designations, preferences, and relative participating, optional, or other special rights of each class of stock or series thereof and the qualifications, limitations, or restrictions of such preferences and rights shall be set forth in full or summarized on the face or back of the certificate which the corporation shall issue to represent such class of stock; provided that, except as
A-106
otherwise provided by statute, in lieu of the foregoing requirements there may be set forth on the face or back of the certificate which the corporation shall issue to represent such class or series of stock, a statement that the corporation will furnish to each stockholder who so requests the designations, preferences and relative, participating, optional or other special rights of each class of stock or series thereof and the qualifications, limitations, or restrictions of such preferences and rights. All certificates surrendered to the corporation for transfer shall be canceled and no new certificate shall be issued until the former certificate for a like number of shares shall have been surrendered and canceled, except that in the case of a lost, stolen, destroyed, or mutilated certificate a new one may be issued therefor upon such terms and with such indemnity, if any, to the corporation as the Board of Directors may prescribe. Certificates shall not be issued representing fractional shares of stock.
SECTION 8.02 LOST CERTIFICATES. The Board of Directors may direct a new certificate or certificates to be issued in place of any certificate or certificates theretofore issued by the corporation alleged to have been lost, stolen, or destroyed, upon the making of an affidavit of that fact by the person claiming the certificate of stock to be lost, stolen or destroyed. When authorizing such issue of a new certificate or certificates, the Board of Directors may, in its discretion and as a condition precedent to the issuance thereof, require (1) the owner of such lost, stolen, or destroyed certificate or certificates, or his legal representative, to advertise the same in such manner as it shall require, (2) such owner to give the corporation a bond in such sum as it may direct as indemnity against any claim that may be made against the corporation with respect to the certificate or certificates alleged to have been lost, stolen, or destroyed, or (3) both.
SECTION 8.03 TRANSFERS. Upon surrender to the corporation or the transfer agent of the corporation of a certificate for shares duly endorsed or accompanied by proper evidence of succession, assignment, or authority to transfer, it shall be the duty of the corporation to issue a new certificate to the person entitled thereto, cancel the old certificate, and record the transaction upon its books. Transfers of shares shall be made only on the books of the corporation by the registered holder thereof, or by his attorney thereunto authorized by power of attorney and filed with the Secretary of the corporation or the Transfer Agent.
SECTION 8.04 REGISTERED STOCKHOLDERS. The corporation shall be entitled to treat the holder of record of any share or shares of the corporation’s capital stock as the holder in fact thereof and, accordingly, shall not be bound to recognize any equitable or other claim to or interest in such share or shares on the part of any other person, whether or not it shall have express or other notice thereof, except as otherwise provided by the laws of the State of Delaware.
ARTICLE IX
DIVIDENDS
SECTION 9.01 DECLARATION. Dividends with respect to the shares of the corporation’s capital stock, subject to the provisions of the Certificate of Incorporation, if any, may be declared by the Board of Directors at any regular or special meeting, pursuant to applicable law. Dividends may be paid in cash, in property, or in shares of capital stock, subject to the provisions of the Certificate of Incorporation.
SECTION 9.02 RESERVE. Before payment of any dividend, there may be set aside out of any funds of the corporation available for dividends such sum or sums as the Board of Directors from time to time, in their absolute discretion, think proper as a reserve or reserves to meet contingencies, or for equalizing dividends, or for repairing or maintaining any property of the corporation, or for such other purpose as the Board of Directors shall think conducive to the interest of the corporation, and the Board of Directors may modify or abolish any such reserve in the manner in which it was created.
A-107
ARTICLE X
INDEMNIFICATION
SECTION 10.01 THIRD PARTY ACTIONS. The corporation shall indemnify any director or officer of the corporation, and may indemnify any other person, who was or is a party or is threatened to be made a party to any threatened, pending, or completed action, suit, or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of the corporation) by reason of the fact that he is or was a director, officer, employee, or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee, or agent of another corporation, partnership, joint venture, trust, or other enterprise, against expenses (including attorneys’ fees), judgments, fines, and amounts paid in settlement actually and reasonably incurred by him in connection with such action, suit, or proceeding if he acted in good faith and in a manner he reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful. The termination of any action, suit, or proceeding by judgment, order, settlement, or conviction, or upon a plea of NOLO CONTENDERE or its equivalent, shall not, of itself, create a presumption that the person did not act in good faith and in a manner which he reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had reasonable cause to believe that his conduct was unlawful.
SECTION 10.02 ACTIONS BY OR IN THE RIGHT OF THE CORPORATION. The corporation shall indemnify any director or officer and may indemnify any other person who was or is a party or is threatened to be made a party to any threatened, pending, or completed action or suit by or in the right of the corporation to procure a judgment in its favor by reason of the fact that he is or was a director, officer, employee, or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee, or agent of another corporation, partnership, joint venture, trust, or other enterprise against expenses (including attorneys’ fees) actually and reasonably incurred by him in connection with the defense or settlement of such action or suit if he acted in good faith and in a manner he reasonably believed to be in or not opposed to the best interests of the corporation and except that no indemnification shall be made in respect of any claim, issue, or matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery or the court in which such action or suit was brought shall determine upon application that, despite the adjudication of liability but in view of all the circumstances of the case, such person is fairly and reasonably entitled to indemnity for such expenses as the Court of Chancery or such other court shall deem proper.
SECTION 10.03 MANDATORY INDEMNIFICATION. To the extent that a director, officer, employee, or agent of the corporation has been successful on the merits or otherwise in defense of any action, suit, or proceeding referred to in Sections 10.01 and 10.02, or in defense of any claim, issue, or matter therein, he shall be indemnified against expenses (including attorneys’ fees) actually and reasonably incurred by him in connection therewith.
SECTION 10.04 DETERMINATION OF CONDUCT. Any indemnification under Section 10.01 or 10.02 of this Article X (unless ordered by a court) shall be made by the corporation only as authorized in the specific case upon a determination that indemnification of the director, officer, employee or agent is proper in the circumstances because he has met the applicable standard of conduct set forth in Section 10.01 or 10.02 of this Article X. Such determination shall be made (a) by a majority vote of directors who were not parties to such action, suit or proceeding, even though less than a quorum, or (b) if there are no such directors, or if such directors so direct, by independent legal counsel in a written opinion, or (c) by the stockholders.
SECTION 10.05 PAYMENT OF EXPENSES IN ADVANCE. Expenses incurred in defending a civil or criminal action, suit, or proceeding shall be paid by the corporation in advance of the final disposition of such action, suit, or proceeding upon receipt of an undertaking by or on behalf of the director, officer, employee, or
A-108
agent to repay such amount if it shall ultimately be determined that he is not entitled to be indemnified by the corporation as authorized in this Article X.
SECTION 10.06 INDEMNITY NOT EXCLUSIVE. The indemnification and advancement of expenses provided or granted hereunder shall not be deemed exclusive of any other rights to which those seeking indemnification or advancement of expenses may be entitled under the Certificate of Incorporation, any other bylaw, agreement, vote of stockholders, or disinterested directors or otherwise, both as to action in his official capacity and as to action in another capacity while holding such office.
SECTION 10.07 DEFINITIONS. For purposes of this Article X:
(a) “the corporation” shall include, in addition to the resulting corporation, any constituent corporation (including any constituent of a constituent) absorbed in a consolidation or merger that, if its separate existence had continued, would have had power and authority to indemnify its directors, officers, and employees or agents, so that any person who is or was a director, officer, employee, or agent of such constituent corporation, or is or was serving at the request of such constituent corporation as a director, officer, employee, or agent of another corporation, partnership, joint venture, trust, or other enterprise, shall stand in the same position under this Article X with respect to the resulting or surviving corporation as he would have with respect to such constituent corporation if its separate existence had continued;
(b) “other enterprises” shall include employee benefit plans;
(c) “fines” shall include any excise taxes assessed on a person with respect to any employee benefit plan;
(d) “serving at the request of the corporation” shall include any service as a director, officer, employee, or agent of the corporation that imposes duties on, or involves services by, such director, officer, employee, or agent with respect to an employee benefit plan, its participants or beneficiaries; and
(e) a person who acted in good faith and in a manner he reasonably believed to be in the interest of the participants and beneficiaries of an employee benefit plan shall be deemed to have acted in a manner “not opposed to the best interests of the corporation” as referred to in this Article X.
SECTION 10.08 CONTINUATION OF INDEMNITY. The indemnification and advancement of expenses provided or granted hereunder shall, unless otherwise provided when authorized or ratified, continue as to a person who has ceased to be a director, officer, employee, or agent and shall inure to the benefit of the heirs, executors, and administrators of such a person.
ARTICLE XI
MISCELLANEOUS
SECTION 11.01 SEAL. The corporate seal, if one is authorized by the Board of Directors, shall have inscribed thereon the name of the corporation, and the words “Corporate Seal, Delaware.” The seal may be used by causing it or a facsimile thereof to be impressed or affixed or otherwise reproduced.
SECTION 11.02 BOOKS. The books of the corporation may be kept (subject to any provision contained in the statutes) outside the State of Delaware at the offices of the corporation, or at such other place or places as may be designated from time to time by the Board of Directors.
A-109
ARTICLE XII
AMENDMENT
These bylaws may be altered, amended, or repealed by a majority of the number of directors then constituting the Board of Directors at any regular meeting of the Board of Directors without prior notice, or at any special meeting of the Board of Directors if notice of such alteration, amendment, or repeal be contained in the notice of such special meeting.
A-110
Exhibit C
CERTIFICATE OF DESIGNATION OF
SERIES A CONVERTIBLE PREFERRED STOCK OF
[ ]
Pursuant to Section 151 of the
General Corporation Law of the State of Delaware
[ ] (the “Corporation”), a corporation organized and existing under the General Corporation Law of the State of Delaware (the “DGCL”), certifies that, pursuant to authority conferred upon the board of directors of the Corporation (the “Board”) by the FOURTH Article of the Amended and Restated Certificate of Incorporation of the Corporation, as amended from time to time (the “Certificate of Incorporation”), and pursuant to the provisions of DGCL Section 151, the Board adopted and approved the following resolution providing for the designations, preferences and relative, participating, optional and other rights, and the qualifications, limitations and restrictions of the Series A Convertible Preferred Stock:
WHEREAS, the Certificate of Incorporation provides for two classes of shares of capital stock known as common stock, par value $0.001 per share (the “Common Stock”), and preferred stock, par value $0.001 per share (the “Preferred Stock”);
WHEREAS, the Certificate of Incorporation authorizes the issuance of 5,000,000 shares of Preferred Stock;
WHEREAS, the Board is authorized by the Certificate of Incorporation as permitted by the DGCL to provide for the issuance of the shares of Preferred Stock in one or more series and to establish from time to time the number of shares to be included in each such series and to fix the voting powers, designations, preferences and relative, participating, optional and other rights of the shares of each such series and the qualifications, limitations and restrictions thereof;
NOW, THEREFORE, BE IT RESOLVED, that the Board does hereby fix the number of shares to be included in such series of Preferred Stock and the voting powers, designations, preferences, rights, qualifications, limitations and restrictions of the shares of such series of Preferred Stock as follows:
Section 1. Designation. The designation of this series of Preferred Stock is “Series A Convertible Preferred Stock,” par value $0.001 per share (the “Series A Preferred”).
Section 2. Number of Series A Preferred Shares. The authorized number of shares of Series A Preferred is 50,000.
Section 3. Defined Terms and Rules of Construction.
(a) Definitions. As used herein with respect to the Series A Preferred:
“Affiliate” of any Person shall mean any other Person directly or indirectly controlling or controlled by or under direct or indirect common control with such Person. For purposes of this definition, “control” when used with respect to any Person has the meaning specified in Rule12b-2 under the Exchange Act; and the terms “controlling” and “controlled” have meanings correlative to the foregoing.
“Board” shall have the meaning set forth in the preamble hereto.
A-111
“Business Day” means any day except Saturday, Sunday and any day on which banking institutions in New York, New York generally are closed as a result of federal, state or local holiday.
“Bylaws” shall mean the Amended and Restated Bylaws of the Corporation in effect on the date hereof and as amended from time to time in accordance with the terms therein and herein.
“Capital Stock” shall mean any and all shares, interests, rights to purchase, warrants, options, participations or other equivalents of or interests in (in each case however designated) stock issued by the Corporation.
“Certificate of Designation” shall mean this Certificate of Designation relating to the Series A Preferred, as it may be amended from time to time in accordance with the terms hereof.
“Certificate of Incorporation” shall have the meaning set forth in the preamble hereto.
“Change of Control” shall mean (a) any sale or other disposition of all or substantially all of the assets of the Corporation and its Subsidiaries on a consolidated basis in any transaction or series of related transactions, (b) any sale, transfer or issuance or series of related sales, transfers and/or issuances of shares of the Capital Stock by the Corporation or any holder thereof which results in any single Person or group (as defined in Rule13d-5 of the Exchange Act) becoming the beneficial owners of Capital Stock representing (x) 50% or more of the voting power of all outstanding voting Capital Stock or (y) the power to elect a majority of the Board (under ordinary circumstances, by contract or otherwise), or (c) any merger or consolidation to which the Corporation is a party unless after giving effect to such merger no single Person or group (as defined in Rule13d-5 of the Exchange Act) is the beneficial owner of Capital Stock possessing the voting power to elect a majority of the Board or the surviving Person’s board of directors (or similar governing body) or becomes the beneficial owner of greater than 50% of the Corporation’s or such surviving Person’s issued and outstanding common stock or securities convertible into common stock of such Person.
“Closing Price” shall mean the price per share of the final trade of the Common Stock, other Capital Stock or similar equity interest, as applicable, on the applicable Trading Day (or the last trade of the Capital Stock or similar equity interest preceding the applicable Trading Day if no trades of such securities were made on the applicable Trading Day) on the principal national securities exchange or securities market on which the Common Stock, other Capital Stock or similar equity interest is listed or admitted to trading;provided that if the Capital Stock is not so listed or traded, the Closing Price shall be equal to the fair market value, as reasonably determined in good faith by the Board.
“Commission” shall mean the U.S. Securities and Exchange Commission, including the staff thereof.
“Common Stock” shall have the meaning set forth in the recitals hereto.
“Common Stock Deemed Outstanding” shall mean, at any given time, the sum of (a) the number of shares of Common Stock actually outstanding at such time, plus (b) the number of shares of Common Stock issuable upon exercise of Options actually outstanding at such time, plus (c) the number of shares of Common Stock issuable upon conversion or exchange of Convertible Securities actually outstanding at such time (treating as actually outstanding any Convertible Securities issuable upon exercise of Options actually outstanding at such time), in each case, regardless of whether or not the Options or Convertible Securities are actually exercisable at such time.
“Conversion Cap” shall have the meaning ascribed to it inSection 8(a).
“Conversion Price” shall mean $4.39, but as it may be adjusted from time to time in accordance withSection 9.
“Conversion Restriction” shall have the meaning set forth inSection 8(a).
A-112
“Conversion Standstill Period” shall have the meaning ascribed to it inSection 8(a).
“Conversion Stock” shall mean Common Stock or other capital stock of the Corporation then issuable upon conversion of the Series A Preferred in accordance with the terms ofSection 8.
“Convertible Securities” shall mean any stock , securities (other than Options) or obligations of indebtedness directly or indirectly convertible into or exchangeable for Common Stock.
“Corporation” shall have the meaning set forth in the preamble hereto.
“Date of Issuance” shall mean, for any Series A Preferred Share, the date on which the Corporation initially issues such Series A Preferred Share (without regard to any subsequent transfer of such Series A Preferred Share or reissuance of the certificate(s) representing such Series A Preferred Share).
“Deemed Liquidation” shall have the meaning ascribed to it inSection 5(b).
“Dividend Reference Date” shall have the meaning set forth inSection 4(a).
“DGCL” shall have the meaning set forth in the preamble hereto.
“Event of Noncompliance” shall have the meaning set forth inSection 12.
“Exchange Act” shall mean the Securities Exchange Act of 1934, as amended.
“Excluded Issuances” means any issuance or sale (or deemed issuance or sale in accordance withSection 9(d)) by the Corporation after the Date of Issuance of: (a) shares of Common Stock issued on the conversion of the Series A Preferred; (b) up to an aggregate of 5,700,000 shares of Common Stock (as such number of shares is equitably adjusted for subsequent stock splits, stock combinations, stock dividends and recapitalizations) issued directly or upon the exercise of Options to directors, officers, employees, or consultants of the Corporation in connection with their service as directors of the Corporation, their employment by the Corporation or their retention as consultants by the Corporation, in each case authorized by the Board and issued pursuant to the Incentive Plans (including all such shares of Common Stock and Options outstanding prior to the Date of Issuance); or (c) shares of Common Stock issued upon the conversion or exercise of Options (other than Options covered by clause (b) above) issued prior to the Date of Issuance, provided that such securities are not amended after the date hereof to increase the number of shares of Common Stock issuable thereunder or to lower the exercise or conversion price thereof.
“Forced Conversion” shall have the meaning set forth inSection 8(b).
“Forced Conversion Noncompliance Event” shall mean (i) the failure of the Corporation to make any redemption payment with respect to any Series A Preferred Share which it is required to make hereunder, whether or not such payment is legally permissible or is prohibited by any debt financing agreement of the Corporation or any of its Subsidiaries or any other agreement to which the Corporation is subject or (ii) the breach by the Corporation of its obligations underSection 8(e). The foregoing shall constitute Forced Conversion Noncompliance Events whatever the reason or cause for any such Forced Conversion Noncompliance Event and whether it is voluntary or involuntary or is effected by operation of law or pursuant to any judgment, decree or order of any court or any order, rule or regulation of any administrative or governmental body and regardless of the effects of any subordination provisions.
“Incentive Plans” means the Corporation’s 1998 Stock Option Plan, 2004 Equity Incentive Plan, 2010 Equity Incentive Plan, 2015 Incentive Compensation Plan, 2018 Incentive Compensation Plan, 1996 TMI Stock Option Plan, TMI 2006 Incentive andNon-Statutory Stock Option Plan, and any other equity incentive plan of the Corporation adopted and approved by the Board after the date of this Certificate, in each case as may be amended, supplemented or modified from time to time as approved by the Board.
A-113
“Investment Agreement” shall mean that certain Investment Agreement by and between the Corporation and Investor, dated as of June 12, 2013.
“Investor Rights Agreement” shall mean that certain Investor Rights Agreement by and between the Corporation and Investor, dated on or about the Date of Issuance.
“Investor” shall mean WSHP Biologics Holdings, LLC.
“Junior Securities” shall mean any class or series of Capital Stock other than (i) the Series A Preferred or (ii) any class or series of Capital Stock that is specifically designated as senior orpari passu to the Series A Preferred in any amendment, modification or supplement to the Certificate of Incorporation (including as a result of a new certificate of designation), which the holders of Series A Preferred have consented to in accordance withSection 11 hereof and, if applicable, Section 3.5 of the Investor Rights Agreement.
“Liquidation” shall have the meaning ascribed to it inSection 5(a).
“Liquidation Preference” shall have the meaning ascribed to it inSection 5(a).
“Liquidation Value” of any Series A Preferred Share as of any particular date shall mean $1,000.
“Noncompliance Period” shall have the meaning ascribed to it inSection 12(c).
“Optional Conversion Eligibility Time” shall mean the earlier of the following: (i) immediately prior to a Change of Control and (ii) the eighth anniversary of the Date of Issuance.
“Optional Redemption Eligibility Time” shall mean the earlier of the following: (i) immediately prior to a Change of Control and (ii) the seventh anniversary of the Date of Issuance.
“Options” shall mean any rights, warrants or options to subscribe for or purchase Common Stock or Convertible Securities.
“Organic Change” shall have the meaning ascribed to it inSection 9(e).
“Person” or “person” shall mean an individual, corporation, limited liability company, association, partnership, group (as such term is used in Section 13(d)(3) of the Exchange Act), trust, joint venture, business trust or unincorporated organization, or a government or any agency or political subdivision thereof.
“Preferred Director” shall have the meaning ascribed to it inSection 10(b).
“Preferred Percentage” shall mean, at any time of determination, the percentage equal to (i) the number of shares of Common Stock issuable upon the conversion of all of the Series A Preferred Shares outstanding at such time of determination (without regard to any restrictions on conversion (including the Conversion Standstill Period, the Conversion Cap and the Conversion Restriction)), plus the number of shares of Common Stock outstanding at such time of determination that were issued pursuant to the conversion of any Series A Preferred Shares,divided by (ii) the number of shares of Common Stock issued and outstanding at such time of determination, plus the number of shares of Common Stock issuable upon conversion of the Series A Preferred outstanding at such time (without regard to any restrictions on conversion (including the Conversion Standstill Period, the Conversion Cap and the Conversion Restriction)).
“Preferred Stock” shall have the meaning set forth in the recitals hereto.
“Purchase Rights” shall have the meaning ascribed to it inSection 11.
A-114
“Redemption Date” shall mean, as to any Series A Preferred Share, the date specified in the notice of any redemption at the Corporation’s option or at the holder’s option;provided that no such date shall be a Redemption Date unless the amount payable to such Series A Preferred Share hereunder is actually paid in full on such date, and if not so paid in full, the Redemption Date shall be the date on which such amount is fully paid.
“Registrable Securities” shall have the meaning set forth in the Investor Rights Agreement.
“Related Party” shall mean (i) any officer or director of the Corporation or other Person that owns at least 5% of the Common Stock on anas-converted fully diluted basis (which for such calculation shall aggregate stockholdings of Affiliates and of immediate family members sharing the same household with such Persons), (ii) any officer or director of any of the Corporation’s Subsidiaries, or (iii) any member of any such Person’s immediate family sharing the same household or any of their respective Affiliates.
“Series A Dividend Reference Dates” shall have the meaning ascribed to it inSection 4(b).
“Series A Preferred Majority Holders” shall mean, as of any time of determination, the holders of a majority of the Series A Preferred Shares outstanding as of such time of determination.
“Series A Preferred” shall have the meaning ascribed to it inSection 1.
“Series A Preferred Share” shall have the meaning ascribed to it inSection 4(a).
“Series A Unpaid Dividends” shall have the meaning ascribed to it inSection 4(a).
“Shareholder Approval” shall mean all approvals of the stockholders of the Corporation necessary to approve the transactions contemplated under the Investment Agreement and the issuance of the Series A Shares contemplated thereby, including any approvals by the holders of Common Stock for the issuance of twenty percent (20%) or more of the number of shares of Common Stock outstanding before such issuance, and the issuance of twenty percent (20%) or more of the voting power of the Company to any one holder of Series A Shares as may be required under law or the listing standards of NASDAQ (or any successor thereto or other trading market on which the Common Stock is listed), including NASDAQ Market Place Rule 5635(b) and (d) or NASDAQ Market Place Rule 5640, and Interpretative Material (IM)5635-2, as applicable.
“Shareholder Approval Deadline” shall have the meaning ascribed to it inSection 4(e).
“Shelf Registration Statement” shall have the meaning set forth in Investor Rights Agreement.
“Stock Price Forced Conversion Event” shall have the meaning ascribed to it inSection 8(b).
“Subsidiary” means any Person of which at least (i) a majority of the equity and (ii) a majority of the voting interests, are owned or controlled, directly or indirectly, by the Corporation, by any one or more of its Subsidiaries, or by any combination of the Corporation and one or more of its Subsidiaries. For the avoidance of doubt, any reference to any Subsidiary of the Company shall include RTI Donor Services, Inc.
“Taxes” shall mean any federal, state, local or foreign income, gross receipts, branch profits, license, payroll, employment, excise, severance, stamp, occupation, premium, windfall profits, escheat, environmental, customs duties, capital stock, franchise, profits, withholding, social security, unemployment, disability, real property, personal property, sales, use, transfer, registration, ad valorem, value added, alternative oradd-on minimum or estimated tax or other tax of any kind whatsoever, including any interest, penalty or addition thereto, whether disputed or not and including any obligation to indemnify or otherwise assume or succeed to the Tax liability of any other Person by law, by contract or otherwise.
A-115
“Trading Day” shall mean any Business Day on which the Common Stock is traded, or able to be traded, on the principal national securities exchange on which the Common Stock is listed or admitted to trading.
(b) Rules of Construction. Unless the context otherwise requires: (i) words in the singular include the plural, and in the plural include the singular; (ii) “including” means including without limitation; (iii) references to any Section or clause refer to the corresponding Section or clause, respectively, of this Certificate of Designation; (iv) any reference to a day or number of days, unless expressly referred to as a Business Day or Trading Day, shall mean the respective calendar day or number of calendar days; (v) references to Sections of or Rules under the Exchange Act shall be deemed to include substitute, replacement or successor Sections or Rules, and any term defined by reference to a Section of or Rule under the Exchange Act shall include Commission and judicial interpretations of such Section or Rule; and (vi) headings are for convenience of reference only.
Section 4. Dividends.
(a) General Obligations. When, as and if declared by the Board and to the extent permitted under the DGCL, the Corporation shall pay preferential dividends in cash to the holders of the Series A Preferred as provided in thisSection 4(a). Dividends on each share of the Series A Preferred (each a “Series A Preferred Share,” and collectively, the “Series A Preferred Shares”) shall accrue on a daily basis at the rate of six percent (6.0%) per annum (or twelve percent (12.0%) per annum if, and for so long as, required pursuant toSection 4(e)) on the sum of (x) the Liquidation Value thereof, plus (y) all accrued and accumulated but unpaid dividends on such Series A Preferred Share (such amount in clause (y), the “Series A Unpaid Dividends”), from and including the Date of Issuance and until the fifth anniversary of the Date of Issuance. For the avoidance of doubt (but subject toSection 12(c)), dividends shall not accrue for Series A Preferred Shares after the fifth anniversary of the Date of Issuance. Series A Unpaid Dividends shall be fully paid or declared with funds irrevocably set apart for payment before any dividends, distributions, redemptions or other payments may be made with respect to any Junior Securities, other than to (A) declare or pay any dividend or distribution payable on the Common Stock in shares of Common Stock or (B) repurchase Common Stock held by employees or consultants of the Corporation for not more than $0.001 per share or other de minimis amounts per share upon termination of their employment or services in accordance with agreements providing for such repurchase existing as of the date of this Certificate or in accordance with any Incentive Plan or any other equity incentive plan of the Corporation adopted and approved by the Board after the date of this Certificate.
(b) Dividend Reference Dates. To the extent not paid in cash on March 31, June 30, September 30, and December 31 of each year, beginning September 30, 2013 (the “Series A Dividend Reference Dates”), all dividends which have accrued on each Series A Preferred Share during the three- month period (or other period, if any, in the case of the initial Series A Dividend Reference Date) ending upon each such Series A Dividend Reference Date shall be accumulated and shall remain accumulated dividends with respect to such Series A Preferred Share until paid in cash to the holder thereof.
(c) Distribution of Partial Dividend Payments. Except as otherwise provided herein, if at any time the Corporation pays less than the total amount of dividends then accrued and accumulated with respect to the Series A Preferred Shares, such payment shall be made pro rata among the holders thereof based upon the aggregate Series A Unpaid Dividends in respect of the Series A Preferred Shares held by each such holder.
(d) Participating Dividends. In addition to any other dividends accruing, accumulating or declared hereunder, in the event that the Corporation declares or pays any dividends upon the Common Stock (whether payable in cash, securities or other property), other than (i) dividends payable on the Common Stock solely in shares of Common Stock and (ii) to the extent constituting a dividend, any repurchases of Common Stock held by employees or consultants of the Corporation for not more than $0.001 per share or other de minimis amounts per share upon termination of their employment or services in accordance with agreements providing for such repurchase existing as of the date of this Certificate or in accordance with any Incentive Plan or any other equity incentive plan of the Corporation adopted and approved by the Board after the date of this Certificate, the
A-116
Corporation shall also declare and pay to the holders of the Series A Preferred at the same time that it declares and pays such dividends to the holders of the Common Stock the dividends which would have been declared and paid with respect to the Common Stock issuable upon conversion of the Series A Preferred Shares had all (i.e., without regard to any restrictions on conversion (including the Conversion Standstill Period, the Conversion Cap and the Conversion Restriction) at such time) of such outstanding Series A Preferred Shares been converted immediately prior to the record date for such dividend, or if no record date is fixed, the date as of which the record holders of Common Stock entitled to such dividends are to be determined.
(e) Dividend Rate Adjustment. In the event that Shareholder Approval has not been obtained within 180 days after the Date of Issuance (the “Shareholder Approval Deadline”), then the dividend rate on each Series A Preferred Share shall automatically (without any further action) increase to the rate of twelve percent (12.0%) per annum, commencing on the day after the Shareholder Approval Deadline and ending on (and including) the date on which Shareholder Approval has been obtained.
Section 5. Liquidation
(a) Normal Liquidation. Upon any liquidation, dissolution or winding up of the Corporation (whether voluntary or involuntary) (collectively with a Deemed Liquidation, a “Liquidation”), each holder of Series A Preferred then outstanding shall be entitled to be paid, out of the assets of the Corporation available for distribution to its stockholders, before any distribution or payment is made upon any Junior Securities by reason of their ownership thereof, an amount in cash equal to the greater of (i) the sum of (x) the aggregate Liquidation Value of all Series A Preferred Shares held by such holder, plus (y) all Series A Unpaid Dividends thereon and (ii) the amount to which such holder would be entitled to receive upon such Liquidation if all (without regard to any restrictions on conversion (including the Conversion Standstill Period, the Conversion Cap and the Conversion Restriction) at such time) of such holder’s Series A Preferred was converted into Conversion Stock immediately prior to such event (such greater amount, the “Liquidation Preference”), and the holders of Series A Preferred shall not be entitled to any further payment with respect to their Series A Preferred Shares. If, upon any Liquidation, the Corporation’s assets available to be distributed among the holders of the Series A Preferred are insufficient to permit payment to such holders of the aggregate amount which they are entitled to be paid under thisSection 5(a), then the entire assets available to be distributed to the Corporation’s stockholders shall be distributed pro rata among such holders of Series A Preferred Shares based upon the aggregate Liquidation Value plus all Series A Unpaid Dividends of the Series A Preferred held by each such holder. Not less than 30 days prior to the payment date stated therein (or such lesser period as may be agreed by the Series A Preferred Majority Holders), the Corporation shall deliver written notice of any Liquidation to each record holder of Series A Preferred, setting forth in reasonable detail the amount of proceeds to be paid with respect to each Series A Preferred Share and each Junior Security in connection with such Liquidation.
(b) Deemed Liquidation. The occurrence of a Change of Control shall be deemed to be a liquidation, dissolution and winding up of the Corporation for purposes of thisSection 5(b) (a “Deemed Liquidation”), and the holders of the Series A Preferred shall be entitled to receive from the Corporation the Liquidation Preference with respect to the Series A Preferred upon such occurrence. The Corporation shall mail written notice of any Change of Control to each record holder of Series A Preferred Shares not less than 30 nor more than 60 days prior to the date on which such Change of Control is consummated.
Section 6. Redemption.
(a) Redemptions at the Option of the Holder. Beginning at the Optional Redemption Eligibility Time, any holder of Series A Preferred may, at any time and from time to time, request redemption, out of funds legally available therefor, of all or any portion of the Series A Preferred Shares held by such holder by delivering written notice of such request to the Corporation specifying the number of Series A Preferred Shares to be so redeemed and the date of such redemption (which may not be earlier than 60 days after delivery of such redemption notice). The Corporation shall be required to redeem on the date so specified in such holder’s written notice delivered to
A-117
the Corporation all or any portion of their Series A Preferred Shares with respect to which such redemption requests have been made at a price per Series A Preferred Share in cash equal to the Series A Liquidation Value thereof, plus all Series A Unpaid Dividends thereon.
(b) Redemptions at the Option of the Corporation. Beginning on the fifth anniversary of the Date of Issuance, the Corporation may at any time and from time to time, redeem, out of funds legally available therefor, all or any portion of the Series A Preferred Shares held by such holder by delivering written notice of such request to such holder in accordance withSection 6(d). Upon any such redemption, the Corporation shall pay a price per Series A Preferred Share with respect to which such redemption requests have been made in cash equal to the Series A Liquidation Value thereof, plus all Series A Unpaid Dividends thereon. Notwithstanding anything to the contrary herein, each holder of Series A Preferred Shares to be redeemed by the Corporation may elect to convert all or any portion of the Series A Preferred Shares held by such holder into Conversion Stock pursuant toSection 8 at any time prior to the applicable Redemption Date.
(c) Redemption Payments. For each Series A Preferred Share to be redeemed hereunder, the Corporation shall be obligated on the date specified in the notice of redemption delivered by the holder(s) of Series A Preferred Shares pursuant toSection 6(a) or by the Corporation pursuant toSection 6(b), as the case may be, to pay to the holder thereof (upon surrender by such holder at the Corporation’s principal office of the certificate representing such Series A Preferred Share) in immediately available funds the amount required pursuant toSection 6(a) orSection 6(b), as applicable. If the funds of the Corporation legally available for redemption of Series A Preferred Shares pursuant toSection 6(a) on any Redemption Date are insufficient to redeem the total number of Series A Preferred Shares to be redeemed on such date, then without limiting any rights or remedies herein or otherwise, those funds which are legally available shall be used to redeem the maximum possible number of Series A Preferred Shares pro rata among the holders of the Series A Preferred Shares to be redeemed pursuant toSection 6(a) based upon the aggregate Liquidation Value, plus Series A Unpaid Dividends of such Series A Preferred Shares held by each such holder. At any time thereafter when additional funds of the Corporation are legally available for the redemption of Series A Preferred Shares pursuant toSection 6(a) such funds shall immediately be used to redeem the balance of the Series A Preferred Shares which the Corporation has become obligated to redeem on any Redemption Date but which it has not redeemed. For the avoidance of doubt, (x) references to “legally available” funds herein shall mean the amount of assets of the Corporation that may be used for a redemption of shares under Section 160 of the DGCL, and (y) the Corporation shall be required to take all actions as are necessary to obtain available funds to satisfy its redemption obligations, including selling assets and borrowing funds. For the avoidance of doubt, the Corporation shall be in breach of its obligations under this Certificate of Designation if it fails to pay in cash all amounts required to be paid by the Corporation pursuant toSection 6(a) on the redemption date specified in any redemption notice delivered by Investor in accordance withSection 6(a).
(d) Notice of Redemption by Corporation. The Corporation shall mail written notice of each redemption of Series A Preferred (other than a redemption at the request of a holder or holders of Series A Preferred) to each record holder thereof not more than 60 nor less than 30 days prior to the date on which such redemption is to be made. Upon mailing any notice of redemption which relates to a redemption at the Corporation’s option, the Corporation shall become obligated to redeem the total number of Series A Preferred Shares specified in such notice at the time of redemption specified therein except to the extent such holder converts such Series A Preferred Shares into Conversion Stock prior to such redemption.
(e) Reissuances of Certificates. In case fewer than the total number of Series A Preferred Shares represented by any certificate are redeemed, a new certificate representing the number of unredeemed Series A Preferred Shares shall be issued to the holder thereof without cost to such holder within five business days after surrender of the certificate representing the redeemed Series A Preferred Shares.
(f) Determination of the Number of Each Holder’s Series A Preferred Shares to be Redeemed. Except as otherwise provided inSection 6(c), the number of Series A Preferred Shares to be redeemed from each holder
A-118
thereof in redemptions hereunder shall be the number of Series A Preferred Shares determined by multiplying the total number of Series A Preferred Shares to be redeemed times a fraction, the numerator of which shall be the total number of Series A Preferred Shares then held by such holder and the denominator of which shall be the total number of Series A Preferred Shares then outstanding.
(g) Redeemed or Otherwise Acquired Series A Preferred Shares. Any Series A Preferred Shares which are redeemed or otherwise acquired by the Corporation shall be canceled and retired to authorized but unissued shares and shall not be reissued, sold or transferred.
(h) Other Redemptions or Acquisitions. The Corporation shall not, nor shall it permit any Subsidiary to, redeem or otherwise acquire any Series A Preferred Shares, except as expressly authorized herein.
Section 7. Priority of Series A Preferred Shares. Except as specifically provided herein, so long as any Series A Preferred Shares remain outstanding, without the prior written consent of the Series A Preferred Majority Holders, the Corporation shall not, nor shall it permit any Subsidiary to, redeem, purchase or otherwise acquire directly or indirectly any Junior Securities, nor shall the Corporation directly or indirectly declare or pay any dividend or make any distribution upon any Junior Securities.
Section 8. Conversion.
(a) Conversion at the Option of the Holder. From and after the end of the period beginning on the Date of Issuance and ending at the Optional Conversion Eligibility Time (such period, the “Conversion Standstill Period”), each Series A Preferred Share may be converted, at any time and from time to time, at the option of the holder thereof into a number of shares of Common Stock equal to the quotient determined by dividing (i) the sum of the Liquidation Value, plus the Series A Unpaid Dividends thereon at such time, by (ii) the Conversion Price then in effect;provided, that (i) prior to the receipt of the Shareholder Approval, the Series A Preferred shall not be convertible pursuant to thisSection 8 into more than 19.99% of the number of shares of Common Stock outstanding immediately prior to the Date of Issuance (subject to a proportionate adjustment in the event of a stock split, stock dividend, combination or other proportionate reduction or increase for the Common Stock) such conversion (such limitation, the “Conversion Cap”); and (ii) prior to the first vote of the shareholders of the Corporation with respect to the Shareholder Approval, no Series A Preferred Shares may be converted (the “Conversion Restriction”). Series A Preferred Shares shall immediately and permanently cease to be subject to the Conversion Cap upon receipt of Shareholder Approval. The Conversion Restriction shall no longer apply after the first vote of the shareholders of the Corporation with respect to the Shareholder Approval, whether or not Shareholder Approval is obtained. Notwithstanding anything herein to the contrary, (x) Section 6(b) andSection 6(d) shall govern the timing of any conversion if the Company exercises its rights to redeem Series A Preferred Shares pursuant toSection 6(b); and (y) each holder of Series A Preferred Shares may elect to convert all or any portion of the Series A Preferred Shares held by such holder into Conversion Stock pursuant toSection 8 immediately prior to or in connection with a Change of Control.
(b) Conversion at the Option of the Corporation. On any date following the earlier to occur of (i) the date, if any, that the average Closing Price during any 20 consecutive Trading Day period is greater than $10.25 (subject to a proportionate adjustment in the event of a stock split, stock dividend, combination or other proportionate reduction or increase to the Common Stock) (a “Stock Price Forced Conversion Event”), and (ii) the eighth anniversary of the Date of Issuance, if (A) no Forced Conversion Noncompliance Event has occurred and is continuing, (B) there is an effective Shelf Registration Statement covering the resale of all of the Registrable Securities and (C) in the case of a Stock Price Forced Conversion Event, the Closing Price on the Trading Day immediately preceding the date on which the Corporation delivers notice of conversion pursuant toSection 8(c) is greater than $10.25 (subject to a proportionate adjustment in the event of a stock split, stock dividend, combination or other proportionate reduction or increase to the Common Stock), then the Corporation may, cause the conversion of all, but ( subject to the Conversion Cap and the Conversion Restriction) not less than all, of the Series A Preferred Shares into a number of shares of Common Stock per Series A Preferred Share
A-119
equal to the quotient determined by dividing (x) the sum of the Liquidation Value, plus the Series A Unpaid Dividends thereon at such time, by (y) the Conversion Price then in effect. Any Series A Preferred Shares not converted due to the Conversion Cap and/or the Conversion Restriction shall continue outstanding on the terms set forth herein after such conversion. In connection with any such conversion, any Unpaid Series A Dividends shall be deemed to convert into Conversion Stock prior to any Series A Preferred Shares. The exercise by the Corporation of its rights under thisSection 8(b) shall be referred to as the “Forced Conversion”. Notwithstanding the foregoing, no Forced Conversion shall be permitted until all governmental body filings, consents, authorizations and approvals (including under the Hart-Scott Rodino Antitrust Improvements Act of 1976, as amended) that are required for such Forced Conversion shall have been made and obtained by the Corporation; accordingly, the holders of the Series A Preferred Shares and the Corporation will promptly take all actions necessary to make any such required filings and cooperate in connection with any such required filings.
(c) Conversion Procedure. In the case of a conversion pursuant toSection 8(a), the conversion date shall be the date on which the certificate(s) representing such Series A Preferred Shares and a duly signed and completed notice of conversion of such Series A Preferred Share is received by the Corporation. In the case of a conversion pursuant toSection 8(b), the conversion date shall be a date specified in the notice from the Corporation to the holder(s) of Series A Preferred, which may not be less than ten (10) days after the holder of such Series A Preferred Shares has received written notice from the Corporation of its election to convert the Series A Preferred Shares; provided, that, for the avoidance of doubt, at any time after delivery of the Corporation’s conversion notice pursuant toSection 8(b), any holder of Series A Preferred may cause all or any portion of such holder’s Series A Preferred Shares to be redeemed by the Corporation if permitted by, and in accordance with,Section 6(a). As soon as possible (but in any event within five (5) business days) after a conversion of Series A Preferred Shares has been effected, the Corporation shall deliver to the converting holder, a certificate or certificates representing the number of shares of Common Stock issuable by reason of such conversion in such names or names and such denominations as the converting holder has specified. In case fewer than the total number of Series A Preferred Shares represented by any certificate are converted, a new certificate representing the number of Series A Preferred Shares not converted shall be issued to the holder thereof without cost to such holder within five business days after surrender of the certificate representing the redeemed Series A Preferred Shares.
(d) Cooperation. The Corporation shall not close its books against the transfer of Series A Preferred Shares or of Common Stock issued or issuable upon conversion of Series A Preferred Shares in any manner which interferes with the timely conversion of the Series A Preferred Shares. Without limiting Section 6.6 of the Investment Agreement orSection 8(b) above, the Corporation shall assist and cooperate (at its expense) with any holder of Series A Preferred Shares required to make any governmental filings or obtain any governmental approval prior to or in connection with any conversion of Series A Preferred Shares hereunder (including, without limitation, making any governmental filings required to be made by the Corporation).
(e) Common Stock Reserved for Issuance. The Corporation shall at all times when any Series A Preferred Shares are outstanding reserve and keep available out of its authorized and unissued shares of Common Stock, solely for the purpose of issuance upon the conversion of the Series A Preferred Shares, the number of shares of Common Stock that would be issuable upon the conversion of all outstanding Series A Preferred Shares, assuming for the purposes of this calculation that at all times (i) the Shareholder Approval has been obtained, (ii) the Conversion Restriction does not apply, and (iii) the Conversion Standstill Period does not apply. All shares of Common Stock which are so issuable shall, when issued, be duly and validly issued, fully paid and nonassessable and free from all taxes, liens, charges and encumbrances. The Corporation shall take all such actions as may be necessary to assure that all such shares of Common Stock may be so issued without violation of any applicable law or governmental regulation or any requirements of any domestic securities exchange or market upon which shares of Common Stock may be listed (except for official notice of issuance which shall be immediately delivered by the Corporation upon each such issuance and except for any such law, regulation or requirement applicable because of the business or nature of the holder). The Corporation shall not take any action which would cause the number of authorized but unissued shares of Common Stock to be less than the number of
A-120
such shares required to be reserved hereunder for issuance upon conversion of the Series A Preferred Shares in accordance with thisSection 8(d).
(f) Taxes. The Corporation shall pay any and all transfer Taxes that may be payable in respect of the issue or delivery of shares of Common Stock on conversion of the Series A Preferred Shares; provided that the Corporation shall not be required to pay transfer Taxes in respect of shares of Conversion Stock issued in the name of, or delivered to, a person other than Investor.
Section 9. Adjustments to Conversion Price. In order to prevent dilution of the conversion rights granted underSection 8, the Conversion Price and the number of shares of Conversion Stock issuable on conversion of the Shares of Series A Preferred shall be adjusted from time to time pursuant to thisSection 9.
(a) Adjustment to Conversion Price upon Issuance of Common Stock. Except as provided inSection 9(b) and except in the case of an event described in eitherSection 9(d) orSection 9(e), if the Corporation, at any time or from time to time after the Date of Issuance, issues or sells, or in accordance withSection 9(c) is deemed to have issued or sold, any shares of Common Stock without consideration or for consideration per share less than the Conversion Price in effect immediately prior to such issuance or sale (or deemed issuance or sale), then immediately upon such issuance or sale (or deemed issuance or sale) the Conversion Price shall be reduced (and in no event increased) to a Conversion Price equal to the quotient determined by dividing: (i) the sum of (1) the product derived by multiplying the Conversion Price in effect immediately prior to such issuance or sale (or deemed issuance or sale) by the number of shares of Common Stock Deemed Outstanding immediately prior to such issuance or sale (or deemed issuance or sale), plus (2) the aggregate consideration, if any, received by the Corporation upon such issuance or sale (or deemed issuance or sale); by (ii) the sum of (1) the number of shares of Common Stock Deemed Outstanding immediately prior to such issuance or sale (or deemed issuance or sale) plus (2) the aggregate number of shares of Common Stock issued or sold (or deemed issued or sold) by the Corporation in such issuance or sale (or deemed issuance or sale).
(b) Exceptions To Adjustment Upon Issuance of Common Stock. Anything herein to the contrary notwithstanding, there shall be no adjustment to the Conversion Price with respect to any Excluded Issuance.
(c) Effect on Conversion Price of Certain Events. For purposes of determining the adjusted Conversion Price underSection 9(a), the following shall be applicable:
(1) Issuance of Rights or Options. If the Corporation, at any time or from time to time after the Date of Issuance, in any manner grants or sells (whether directly or by assumption in a merger or otherwise) any Options, whether or not such Options or the right to convert or exchange any Convertible Securities issuable upon the exercise of such Options are immediately exercisable, and the price per share (determined as provided in this paragraph and inSection 9(c)(5)) for which Common Stock is issuable upon the exercise of such Options, or upon the conversion or exchange of Convertible Securities issuable upon the exercise of such Options, is less than the Conversion Price in effect immediately prior to the time of the granting or sale of such Options, then the total maximum number of shares of Common Stock issuable upon the exercise of such Options or upon conversion or exchange of the total maximum amount of Convertible Securities issuable upon the exercise of such Options shall be deemed to have been issued by the Corporation at the time of the granting or sale of such Options (and thereafter shall be deemed to be outstanding for purposes of adjusting the Conversion Price underSection 9(a)), at a price per share equal to the quotient determined by dividing (i) the sum (which sum shall constitute the applicable consideration received for purposes ofSection 9(a)) of (x) the total amount, if any, received or receivable by the Corporation as consideration for the granting or sale of all such Options, plus (y) the minimum aggregate amount of additional consideration payable to the Corporation upon the exercise of all such Options, plus (z), in the case of such Options which relate to Convertible Securities, the minimum aggregate amount of additional consideration, if any, payable to the Corporation upon the issuance or sale of all such Convertible Securities and the conversion or exchange of all such Convertible Securities, by (ii) the total maximum number of shares of Common Stock
A-121
issuable upon the exercise of all such Options or upon the conversion or exchange of all Convertible Securities issuable upon the exercise of all such Options. Except as otherwise provided inSection 9(c)(3), no further adjustment of the Conversion Price shall be made when Convertible Securities are actually issued upon the exercise of such Options or when Common Stock is actually issued upon the exercise of such Options or the conversion or exchange of such Convertible Securities.
(2) Issuance of Convertible Securities. If the Corporation, at any time or from time to time after the Date of Issuance, in any manner issues or sells (whether directly or by assumption in a merger or otherwise) any Convertible Securities, whether or not the right to convert or exchange any such Convertible Securities is immediately exercisable, and the price per share (determined as provided in this paragraph and inSection 9(c)(5)) for which Common Stock is issuable upon conversion or exchange of such Convertible Securities is less than the Conversion Price in effect immediately prior to the time of such issue or sale, then the total maximum number of shares of Common Stock issuable upon conversion or exchange of the total maximum amount of such Convertible Securities shall be deemed to have been issued by the Corporation at the time of the issuance or sale of such Convertible Securities (and thereafter shall be deemed to be outstanding for purposes of adjusting the Conversion Price pursuant toSection 9(a)), at a price per share equal to the quotient determined by dividing (i) the sum (which sum shall constitute the applicable consideration received for purposes ofSection 9(a)) of (x) the total amount, if any, received or receivable by the Corporation as consideration for the issue or sale of such Convertible Securities, plus (y) the minimum aggregate amount of additional consideration, if any, payable to the Corporation upon the conversion or exchange of all such Convertible Securities, by (ii) the total maximum number of shares of Common Stock issuable upon the conversion or exchange of all such Convertible Securities. Except as otherwise provided inSection 9(c)(3), no further adjustment of the Conversion Price shall be made when Common Stock is actually issued upon the conversion or exchange of such Convertible Securities, and if any such issue or sale of such Convertible Securities is made upon exercise of any Options for which adjustments of the Conversion Price had been or are to be made pursuant to other provisions of thisSection 9(c), no further adjustment of the Conversion Price shall be made by reason of such issue or sale.
(3) Change in Option Price or Conversion Rate. Upon any change in any of (A) the total amount received or receivable by the Corporation as consideration for the granting or sale of any Options or Convertible Securities referred to inSection 9(c)(1) orSection 9(c)(2), (B) the minimum aggregate amount of additional consideration, if any, payable to the Corporation upon the exercise of any Options or upon the issuance, conversion or exchange of any Convertible Securities referred to inSection 9(d)(1) orSection 9(c)(2), (C) the rate at which Convertible Securities referred to inSection 9(c)(1) orSection 9(c)(2) are convertible into or exchangeable for Common Stock, or (D) the maximum number of shares of Common Stock issuable in connection with any Options referred to inSection 9(c)(1) or any Convertible Securities referred to inSection 9(c)(2) (in each case, other than in connection with an Excluded Issuance), then (whether or not the original issuance or sale of such Options or Convertible Securities resulted in an adjustment to the Conversion Price pursuant to thisSection 9) the Conversion Price in effect at the time of such change shall be adjusted or readjusted, as applicable, to the Conversion Price which would have been in effect at such time pursuant to the provisions of thisSection 9 had such Options or Convertible Securities still outstanding provided for such changed consideration or conversion rate, as the case may be, at the time initially granted, issued or sold, but only if as a result of such adjustment or readjustment the Conversion Price then in effect is reduced.
(4) Treatment of Expired Options and Unexercised Convertible Securities. Upon the expiration of any Option or the termination of any right to convert or exchange any Convertible Security without the exercise of any such Option or right, the Conversion Price then in effect hereunder shall be adjusted immediately pursuant to the provisions of thisSection 9 to the Conversion Price which would have been in effect at the time of such expiration or termination had such Option or Convertible Security, to the extent outstanding immediately prior to such expiration or termination, never been issued.
A-122
(5) Calculation of Consideration Received. If any Common Stock, Option or Convertible Security is, at any time or from time to time after the Date of Issuance, issued or sold or deemed to have been issued or sold in accordance withSection 9(c) (A) for cash, the consideration received therefor shall be deemed to be the net amount received by the Corporation therefor; (B) for consideration other than cash, the amount of the consideration other than cash received by the Corporation shall be the fair value of such consideration, except where such consideration consists of marketable securities, in which case the amount of consideration received by the Corporation shall be the market price (as reflected on any securities exchange, quotation system or association or similar pricing system covering such security) for such securities as of the end of business on the date of receipt of such securities; (C) for no specifically allocated consideration in connection with an issuance or sale of other securities of the Corporation, together comprising one integrated transaction, the amount of the consideration therefor shall be deemed to be the fair value of such portion of the aggregate consideration received by the Corporation in such transaction as is attributable to such shares of Common Stock, Options or Convertible Securities, as the case may be, issued in such transaction; or (D) to the owners of thenon-surviving entity in connection with any merger in which the Corporation is the surviving corporation, the amount of consideration therefor shall be deemed to be the fair value of the portion of the net assets and business of thenon-surviving entity that is attributable to such Common Stock, Options or Convertible Securities, as the case may be, issued to such owners. The fair value of any consideration or net assets other than cash and marketable securities shall be determined jointly by the Corporation and the Series A Preferred Majority Holders. If such parties are unable to reach agreement within a reasonable period of time, the fair value of such consideration shall be determined by an independent appraiser experienced in valuing such type of consideration jointly selected by the Corporation and the Series A Preferred Majority Holders. The determination of such appraiser shall be final and binding upon the parties, and the fees and expenses of such appraiser shall be borne by the Corporation.
(6) Integrated Transactions. In case any Option is issued in connection with the issue or sale of other securities of the Corporation, together comprising one integrated transaction in which no specific consideration is allocated to such Option by the parties thereto, the Option shall be deemed to have been issued for a consideration of $.001.
(7) Treasury Series A Preferred Shares. The number of shares of Common Stock outstanding at any given time shall not include shares owned or held by or for the account of the Corporation or any Subsidiary, and the disposition of any shares so owned or held shall be considered an issue or sale of Common Stock for the purpose of thisSection 9.
(8) Record Date. For purposes of any adjustment to the Conversion Price in accordance with thisSection 9, if the Corporation takes a record of the holders of Common Stock for the purpose of entitling them (a) to receive a dividend or other distribution payable in Common Stock, Options or Convertible Securities or (b) to subscribe for or purchase Common Stock, Options or Convertible Securities, then such record date shall be deemed to be the date of the issue or sale of the shares of Common Stock deemed to have been issued or sold upon the declaration of such dividend or the making of such other distribution or the date of the granting of such right of subscription or purchase, as the case may be.
(d) Adjustment to Conversion Price Upon Dividend, Subdivision or Combination of Common Stock. If the Corporation, at any time or from time to time after the Date of Issuance, (i) pays a dividend or make any other distribution upon the Common Stock or any other capital stock of the Corporation payable in shares of Common Stock or in Options or Convertible Securities, or (ii) subdivides (by any stock split, recapitalization or otherwise) its outstanding shares of Common Stock into a greater number of shares, the Conversion Price in effect immediately prior to such dividend, distribution or subdivision shall be proportionately reduced, and if the Corporation at any time combines (by reverse stock split or otherwise) its outstanding shares of Common Stock into a smaller number of shares, the Conversion Price in effect immediately prior to such combination shall be proportionately increased. Any adjustment under thisSection 9(d) shall become effective at the close of business on the date the dividend, subdivision or combination becomes effective.
A-123
(e) Adjustment to Conversion Price Upon Reorganizations, Mergers, Consolidation, Merger or Sale. Any recapitalization, reorganization, reclassification, consolidation, merger, sale of all or substantially all of the Corporation’s assets to another Person or other similar transaction (other than any such transaction covered bySection 9(d)), in each case which entitles the holders of Common Stock to receive (either directly or upon subsequent Liquidation) stock, securities or assets with respect to or in exchange for Common Stock, is referred to herein as an “Organic Change”. Prior to the consummation of any Organic Change, the Corporation shall make appropriate provisions (in form and substance satisfactory to the Series A Preferred Majority Holders) to insure that each of the holders of the Series A Preferred shall thereafter have the right to acquire and receive, in lieu of or in addition to (as the case may be) the shares of Common Stock immediately theretofore acquirable and receivable upon the conversion of such holder’s Series A Preferred Shares, such shares of stock, securities or assets as such holder would have received in connection with such Organic Change if such holder had converted its Series A Preferred Shares immediately prior to such Organic Change. In each such case, the Corporation shall also make appropriate provisions (in form and substance satisfactory to the Series A Preferred Majority Holders) to insure that the provisions of thisSection 9 shall thereafter be applicable to the Series A Preferred Shares (including, in the case of any such consolidation, merger or sale in which the successor or purchasing Person is other than the Corporation, an immediate adjustment of the Conversion Price to the value per share for the Common Stock reflected by the terms of such consolidation, merger or, sale or similar transaction, and a corresponding immediate adjustment in the number of shares of Conversion Stock acquirable upon conversion of Series A Preferred Shares, if the value so reflected is less than the Conversion Price in effect immediately prior to such consolidation, merger or, sale or similar transaction). The Corporation shall not effect any such consolidation, merger or sale, unless prior to the consummation thereof, the successor Person (if other than the Corporation) resulting from consolidation or merger or the Person purchasing such assets assumes by written instrument (in form and substance satisfactory to the Series A Preferred Majority Holders), the obligation to deliver to each such holder such shares of stock, securities or assets as, in accordance with the foregoing provisions, such holder may be entitled to acquire.
(f) Certain Events. If any event occurs of the type contemplated by the provisions of thisSection 9 but not expressly provided for by such provisions (including, without limitation, the granting of stock appreciation rights, phantom stock rights or other rights with equity features), then the Board shall make an appropriate adjustment in the Conversion Price so as to protect the rights of the holders of Series A Preferred Shares in a manner consistent with the provisions of thisSection 9;provided that no such adjustment pursuant to thisSection 9 shall increase the Conversion Price or decrease the number of shares of Conversion Stock issuable as otherwise determined pursuant to thisSection 9.
(g) Notices. Immediately upon any adjustment of the Conversion Price, the Corporation shall give written notice thereof to all holders of Series A Preferred Shares, setting forth in reasonable detail and certifying the calculation of such adjustment. The Corporation shall give written notice to all holders of Series A Preferred Shares at least 20 days prior to the date on which the Corporation closes its books or takes a record (i) with respect to any dividend or distribution upon Common Stock, (ii) with respect to any pro rata subscription offer to holders of Common Stock or (iii) for determining rights to vote with respect to any Organic Change or Liquidation. The Corporation shall also give written notice to the holders of Series A Preferred Shares at least 20 days prior to the date on which any Organic Change shall take place.
Section 10. Voting Rights; Election of Directors
(a) Voting Generally. Without limiting any rights provided to the holders of shares of Series A Preferred under the DGCL, the holders of shares of Series A Preferred shall be entitled to vote as a single class with the holders of the Common Stock on all matters submitted to a vote of stockholders of the Corporation, except with respect to the Shareholder Approval;provided that, prior to the first vote of the shareholders of the Corporation with respect to the Shareholder Approval, the Series A Preferred shall have no voting rights, except as otherwise required by applicable law. Each holder of shares of the Series A Preferred shall be entitled to the number of votes equal to the largest number of full shares of Common Stock into which all shares of Preferred Stock held of
A-124
record by such holder could then be converted (taking into account, for the avoidance of doubt, all Unpaid Series A Dividends thereon convertible into shares of Common Stock, any Conversion Price adjustments made pursuant toSection 9 and the Conversion Cap and without regard to (i.e., ignoring) the Conversion Standstill Period) as of the record date for the determination of the stockholders entitled to vote on such matters or, if no such record date is established, at the date such vote is taken or any written consent of stockholders is first executed;provided, however, that no holder of Series A Preferred shall be entitled to cast votes for the number of shares of Common Stock issuable upon conversion of such Series A Preferred Shares held by such holder that exceeds (subject to a proportionate adjustment for any stock split, stock dividend, combination, recapitalization or other proportionate reduction or increase in the Common Stock) the quotient of (x) the aggregate purchase price paid by such holder of Series A Preferred for its Series A Preferred Shares, divided by (y) the lesser of (i) $4.40, and (ii) the Closing Price of the Common Stock on the Trading Day immediately prior to the Date of Issuance of such holder’s Series A Preferred. The holders of Series A Preferred Shares shall be entitled to notice of any meeting of stockholders in accordance with the Bylaws of the Corporation.
(b) Election of Directors. In the election of directors of the Board, for so long as any Series A Preferred Shares are outstanding and the Preferred Percentage equals or exceeds 5%, the holders of the Series A Preferred Shares, in addition to the other voting rights set forth herein, shall be entitled to elect that number of directors to the Board (each, a“Preferred Director”) equal to the product of: (i) the Preferred Percentage, and (ii) the total number of directors on the Board, including the number of Preferred Directors appointed or appointable to the Board;provided, that if such product is not a whole number, then the number of Preferred Directors shall be the next whole number larger than such product (e.g., if such product is 1.11 or 1.51, then the number of Preferred Directors shall be two);provided, further that the number of Preferred Directors shall not exceed two at any time.
(c) Term. Each Preferred Director appointed pursuant toSection 10(b) shall continue to hold office until the earliest to occur of (i) the first annual meeting of the stockholders of the Corporation following such time that the holders of the Series A Preferred Shares do not have the requisite Preferred Percentage to appoint such Preferred Director pursuant toSection 10(b), (ii) such Preferred Director is removed from office by the affirmative vote of the Series A Preferred Majority Holders, and (iii) such time as such Preferred Director’s death, resignation, retirement or disqualification. Any vacancy created by the removal, death, resignation, retirement or disqualification of a Preferred Director shall be filled by the affirmative vote of the Series A Preferred Majority Holders. If the holders of the Series A Preferred Shares for any reason fail to elect anyone to fill any such directorship or vacancy, such position shall remain vacant until such time as such holders elect a director to fill such position and shall not be filled by resolution or vote of the Board or the Corporation’s other stockholders. The Corporation shall take all such action as may be reasonably requested by such holders to effectSection 10(b) and thisSection 10(c) (including nominating and recommending the designees of the holders of the Series A Preferred Shares for election).
Section 11. Consent Rights. In addition to any rights that the holders of Series A Preferred Shares may have pursuant to the DGCL, for so long as (x) any Series A Preferred Shares are outstanding and (y) the Preferred Percentage is at least ten percent (10%), the Corporation will not, without first obtaining the written consent or affirmative vote of the Series A Preferred Majority Holders, voting separately as a class, take any of the following actions: (i) liquidate, dissolve orwind-up the Corporation (whether voluntary or involuntary), (ii) amend, modify, supplement or repeal any provision of the Certificate of Incorporation or Bylaws that would have a material adverse effect on any right, preference, privilege or voting power of the Series A Preferred Shares or the holders thereof (it being understood that, for the avoidance of doubt, any amendment, modification or supplement to the Certificate of Incorporation (including as a result of new certificate of designation) to create, authorize, designate or issue any equity securities of the Corporation senior to orpari passuwith the Series A Preferred Shares would have a material adverse effect on the rights, preferences, privileges and/or voting power of the Series A Preferred Shares or the holders thereof), (iii) change the size of the Board; (iv) enter into, amend, modify or supplement any agreement, transaction, commitment or arrangement with any Related Party, except for customary employment arrangements and benefit programs; or (v) agree to take any of the foregoing actions.
A-125
Section 12. Events of Noncompliance.
(a) Definition. An Event of Noncompliance shall have occurred if:
(1) the Corporation fails to make any redemption payment with respect to the Series A Preferred which it is required to make hereunder, whether or not such payment is legally permissible or is prohibited by any agreement to which the Corporation is subject;
(2) the Corporation breaches or otherwise fails to perform or observe (i) any other covenant or agreement set forth herein or in the Investor Rights Agreement or (ii) any covenant or agreement set forth in the Investment Agreement required to be performed or observed by the Corporation after the closing of the transactions contemplated by the Investment Agreement;
(3) the Corporation or any material Subsidiary makes an assignment for the benefit of creditors or admits in writing its inability to pay its debts generally as they become due; or an order, judgment or decree is entered adjudicating the Corporation or any material Subsidiary bankrupt or insolvent; or any order for relief with respect to the Corporation or any material Subsidiary is entered under the Federal Bankruptcy Code; or the Corporation or any material Subsidiary petitions or applies to any tribunal for the appointment of a custodian, trustee, receiver or liquidator of the Corporation or any material Subsidiary or of any substantial part of the assets of the Corporation or any material Subsidiary, or commences any proceeding (other than a proceeding for the voluntary liquidation and dissolution of a Subsidiary) relating to the Corporation or any material Subsidiary under any bankruptcy, reorganization, arrangement, insolvency, readjustment of debt, dissolution or liquidation law of any jurisdiction; or any such petition or application is filed, or any such proceeding is commenced, against the Corporation or any material Subsidiary and either (a) the Corporation or any such Subsidiary by any act indicates its approval thereof, consent thereto or acquiescence therein or (b) such petition, application or proceeding is not dismissed within 60 days.
(b) If an Event of Noncompliance occurs, then, from and after the occurrence of the Event of Noncompliance and until such time as no Event of Noncompliance exists (such period, the “Noncompliance Period”), the Series A Preferred Shares shall accrue dividends at the dividend rate described inSection 4(a) (as if the entire Noncompliance Period were after the Date of Issuance and prior to the fifth anniversary of the Date of Issuance) plus an increment of one (1) percentage point. Thereafter, until such time as no Event of Noncompliance exists, the dividend rate shall increase automatically at the end of each succeeding90-day period by an additional increment of 1 percentage point (but in no event shall the dividend rate exceed fifteen percent (15%)). Any increase of the dividend rate resulting from the operation of this paragraph shall terminate as of the close of business on the date on which no Event of Noncompliance exists, subject to subsequent increases pursuant to this paragraph.
Section 13. Other Rights. If any Event of Noncompliance exists, each holder of Series A Preferred shall also have any other rights which such holder is entitled to under any contract or agreement at any time and any other rights which such holder may have pursuant to applicable law.
Section 14. Corporate Opportunities. To the fullest extent permitted by DGCL Section 122, the Corporation renounces any interest or expectancy of the Corporation in, or in being offered an opportunity to participate in, business opportunities that are presented to any Preferred Director.
Section 15. Registration of Transfer. The Corporation shall keep at its principal office a register for the registration of Series A Preferred Shares. Upon the surrender of any certificate representing Series A Preferred Shares at such place, the Corporation shall, at the request of the record holder of such certificate, execute and deliver (at the Corporation’s expense) a new certificate or certificates in exchange therefor representing in the aggregate the number of Series A Preferred Shares represented by the surrendered certificate. Each such new certificate shall be registered in such name and shall represent such number of Series A Preferred Shares as is requested by the holder of the surrendered certificate and shall be substantially identical in form to the
A-126
surrendered certificate, and dividends shall accrue on the Series A Preferred Shares represented by such new certificate from the date to which dividends have been fully paid on such Series A Preferred Shares represented by the surrendered certificate.
Section 16. Replacement. Upon receipt of evidence reasonably satisfactory to the Corporation (it being understood that an affidavit of the registered holder shall be satisfactory) of the ownership and the loss, theft, destruction or mutilation of any certificate evidencing Series A Preferred Shares, and in the case of any such loss, theft or destruction, upon receipt of indemnity reasonably satisfactory to the Corporation (provided that if the holder is a financial institution or other institutional investor its own agreement shall be satisfactory), or, in the case of any such mutilation upon surrender of such certificate, the Corporation shall (at its expense) execute and deliver in lieu of such certificate a new certificate of like kind representing the number of Series A Preferred Shares of such class represented by such lost, stolen, destroyed or mutilated certificate and dated the date of such lost, stolen, destroyed or mutilated certificate, and dividends shall accrue on the Series A Preferred Shares represented by such new certificate from the date to which dividends have been fully paid on such lost, stolen, destroyed or mutilated certificate.
Section 17. Amendment and Waiver. No amendment, modification, alteration, repeal or waiver of any provision of this Certificate of Designation shall be binding or effective without the prior written consent of the Board and the Series A Preferred Majority Holders, voting separately as a class; provided that no amendment, modification, alteration, repeal or waiver of the terms or relative priorities of the Series A Preferred may be accomplished by the merger, consolidation or other transaction of the Corporation with another Person unless the Corporation has obtained the prior written consent of the Series A Preferred Majority Holders.
Section 18. Notices. Except as otherwise expressly provided hereunder, all notices referred to herein shall be given in writing and shall be deemed effectively given (a) if given by personal delivery, upon actual delivery, (b) if given by facsimile, upon receipt of confirmation of a completed transmittal, (c) if given by mail, upon the earlier of (i) actual receipt of such notice by the intended recipient or (ii) five (5) Business Days after such notice is deposited in first class mail, postage prepaid, and (d) if by an internationally recognized overnight courier for overnight delivery, one (1) Business Day after delivery to such courier for overnight delivery, in each case, (i) to the Corporation, at its principal executive offices and (ii) to any stockholder, at such holder’s address as it appears in the stock records of the Corporation (unless otherwise indicated by any such holder).
A-127
IN WITNESS WHEREOF, the Corporation has caused this Certificate of Designation to be duly executed and acknowledged by its undersigned duly authorized officer this of , 2018.
| [ ] | ||
| By: |
| |
| Name: |
| |
| Title: |
| |
Signature Page to Certificate of Designation
A-128
Exhibit D
LOCK-UP AGREEMENT
[●], 201[8/9]
Reference is made to that certain Master Transaction Agreement, dated as of [●], 2018 (the “Master Transaction Agreement”), by and among [●], a Delaware limited liability company, [●], a Delaware corporation (“Parent”), [●], a Delaware corporation (“Holdco”), and [●], a Delaware corporation (“Merger Sub”). Capitalized terms used but not otherwise defined herein shall have the respective meanings ascribed to such terms in the Master Transaction Agreement.
As a material inducement to each of Parent, Holdco and Merger Sub to enter into the Master Transaction Agreement and to consummate the transaction contemplated thereby, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the undersigned hereby agrees as follows:
The undersigned hereby agrees not to, during the period commencing from the Closing and ending on the earlier of (x) the date that is one hundred eighty (180) days after the Closing Date and (y) the date on which Holdco consummates a liquidation, merger, share exchange or other similar transaction with an unaffiliated third party that results in all of Holdco’s stockholders having the right to exchange their equity holdings in Holdco for cash, securities or other property (the “Lock-Up Period”): (i) lend, offer, pledge, hypothecate, encumber, donate, assign, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, any shares of Holdco Common Stock issued in respect of the Stock Consideration (the “Restricted Securities”) or (ii) enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of the Restricted Securities, whether any such swap or other arrangement is to be settled by delivery of Restricted Securities or other securities, in cash or otherwise (any of the foregoing described in clauses (i) or (ii), a “Prohibited Transfer”). The foregoing sentence shall not apply to the transfer of any or all of the Restricted Securities owned by the undersigned (A) as abona fide gift or gifts, (B) by will or intestate succession upon the death of the undersigned, (C) to any Permitted Transferee (as defined below), (D) pursuant to a court order or settlement agreement related to the distribution of assets in connection with the dissolution of marriage or civil union or (E) as a distribution to limited partners, stockholders, unitholders, members of or owners of similar equity interests in the undersigned; provided, however, that in any of cases (A), (B), (C), (D) or (E) it shall be a condition to such transfer that the transferee executes and delivers to Holdco an agreement stating that the transferee is receiving and holding the Restricted Securities subject to the provisions of this Agreement applicable to the undersigned, and there shall be no further transfer of such Restricted Securities except in accordance with this Agreement. As used in this Agreement, the term “Permitted Transferee” shall mean: (I) the members of the undersigned’s immediate family (for purposes of this Agreement, “immediate family” shall mean with respect to any natural person, any of the following: such person’s spouse, the siblings of such person and his or her spouse, and the direct descendants and ascendants (including adopted and step children and parents) of such person and his or her spouse and siblings), (II) any trust for the direct or indirect benefit of the undersigned or the immediate family of the undersigned, (III) if the undersigned is a trust, the trustor or beneficiary of such trust or the estate of a beneficiary of such trust or (IV) any affiliate of the undersigned. The undersigned further agrees to execute such agreements as may be reasonably requested by Holdco that are consistent with the foregoing or that are necessary to give further effect thereto.
If any Prohibited Transfer is made or attempted contrary to the provisions of this Agreement, the undersigned acknowledges and agrees that such purported Prohibited Transfer shall be null and voidab initio, and that Holdco is entitled to refuse to recognize any such purported transferee of the Restricted Securities as one of its stockholders for any purpose. The undersigned further acknowledges and agrees that, in order to enforce the immediately preceding sentence, Holdco may impose stop-transfer instructions with respect to the Restricted Securities of the undersigned (and permitted transferees and assigns thereof) until the end of theLock-Up Period.
A-129
During theLock-Up Period, the undersigned acknowledges and agrees that each certificate evidencing any Restricted Securities shall be stamped or otherwise imprinted with a legend in substantially the following form, in addition to any other applicable legends:
THE SHARES REPRESENTED BY THIS CERTIFICATE ARE SUBJECT TO AND MAY ONLY BE TRANSFERRED IN COMPLIANCE WITH ALOCK-UP AGREEMENT, A COPY OF WHICH IS ON FILE AT THE PRINCIPAL OFFICE OF THE COMPANY.
For the avoidance of any doubt, the undersigned shall retain all rights as a stockholder of Holdco with respect to the Restricted Securities during theLock-Up Period, including the right to vote any Restricted Securities that are entitled to vote.
Notwithstanding anything to the contrary contained herein, in the event that the Master Transaction Agreement is terminated in accordance with its terms prior to the Closing, this Agreement and all rights and obligations of the undersigned hereunder shall automatically terminate and be of no further force or effect.
The undersigned hereby represents and warrants that the undersigned has full power and authority to enter into this Agreement. All authority herein conferred or agreed to be conferred and any obligations of the undersigned shall be binding upon the successors, permitted assigns, heirs or personal representatives of the undersigned.
All issues and questions concerning the construction, validity, interpretation and enforceability of this Agreement will be governed by, and construed in accordance with, the Laws of the State of Delaware, without giving effect to any choice of Law or conflict of Law rules or provisions (whether of the State of Delaware or any other jurisdiction) that would cause the application of the Laws of any jurisdiction other than the State of Delaware.
Any and all remedies herein expressly conferred upon Holdco will be deemed cumulative with, and not exclusive of, any other remedy conferred hereby, or by law or equity upon Holdco, and the exercise by Holdco of any one remedy will not preclude the exercise of any other remedy. The undersigned agrees that irreparable damage would occur and that Holdco would not have any adequate remedy at law in the event that any of the provisions of this Agreement were not performed in accordance with their specific terms or were otherwise breached, and that money damages or other legal remedies would not be an adequate remedy therefor. Accordingly, in addition to any other remedies available under this Agreement, the undersigned agrees that, prior to the termination of theLock-Up Period, Holdco will be entitled to an injunction or injunctions, specific performance and other equitable relief to prevent the undersigned’s breach of this Agreement and to enforce specifically the terms and provisions of this Agreement. The undersigned agrees that the undersigned will not oppose the granting of an injunction, specific performance and other equitable relief when expressly available pursuant to the terms of this Agreement, and hereby waives (i) any defenses in any Legal Proceeding for an injunction, specific performance or other equitable relief, including the defense that Holdco has an adequate remedy at law or an award of specific performance is not an appropriate remedy for any reason at law or equity and (ii) any requirement under Law to post a bond, undertaking or other security as a prerequisite to obtaining equitable relief.
This Agreement, to the extent signed and delivered by means of a facsimile machine or scanned pages via electronic mail, will be treated in all manner and respect as an original contract and will be considered to have the same binding legal effects as if it were the original signed version thereof delivered in person.
[SIGNATURE PAGE FOLLOWS]
A-130
| Very truly yours, | ||||
| Print Name of Stockholder: | ||||
| Signature (for individuals): | ||||
| Signature (for entities): | ||||
| By: | ||||
| Name: | ||||
| Title: | ||||
[Signature Page toLock-Up Agreement]
A-131
Exhibit E
LOCK-UP AND PERMITTED SALE AGREEMENT
[●], 201[8/9]
Reference is made to that certain Master Transaction Agreement, dated as of [●], 2018 (the “Master Transaction Agreement”), by and among [●], a Delaware limited liability company, [●], a Delaware corporation (“Parent”), [●], a Delaware corporation (“Holdco”), and [●], a Delaware corporation (“Merger Sub”). Capitalized terms used but not otherwise defined herein shall have the respective meanings ascribed to such terms in the Master Transaction Agreement. As a material inducement to each of Parent, Holdco and Merger Sub to enter into the Master Transaction Agreement and to consummate the transaction contemplated thereby, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, each of Holdco and [Hayfin Topaz Luxco 3 SCA, Hayfin Opal II Luxco 3 Sarl, Hayfin SOF IICo-Invest LuxCo 2 Sarl, Hayfin Special Opportunities Fund II (AIV I) LP and Hayfin Special Opportunities Fund II (AIV IB) LP] (collectively, “Hayfin”) hereby agree as set forth below.
The undersigned hereby agrees not to, during the period commencing from the Closing and ending on the earlier of (x) the date that is one hundred eighty (180) days after the Closing Date and (y) the date on which Holdco consummates a liquidation, merger, share exchange or other similar transaction with an unaffiliated third party that results in all of Holdco’s stockholders having the right to exchange their equity holdings in Holdco for cash, securities or other property (the “Lock-Up Period”), without the prior written consent of Holdco: (i) lend, offer, pledge, hypothecate, encumber, donate, assign, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, any shares of Holdco Common Stock issued in respect of the Stock Consideration (the “Lock-Up Securities”) or (ii) enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of theLock-Up Securities, whether any such swap or other arrangement is to be settled by delivery ofLock-Up Securities or other securities, in cash or otherwise (any of the foregoing described in clauses (i) or (ii), but excluding any Permitted Transfer (as defined below), a “Prohibited Transfer”).
Notwithstanding the foregoing, and subject to the conditions below, the undersigned may transfer theLock-Up Securities during theLock-Up Period (a “Permitted Transfer”):
| i. | subject to the prior written approval of Holdco, which shall not be unreasonably withheld, conditioned or delayed, pursuant to an arrangement with any nationally recognized financial institution; |
| ii. | as a bona fide gift or gifts, provided that the donee or donees, beneficiary or beneficiaries, heir or heirs or legal representatives thereof agree to be bound in writing by the restrictions set forth herein; |
| iii. | to any trust, partnership, limited liability company or other entity for the direct or indirect benefit of the undersigned, provided that the trustee of the trust or the partnership, limited liability company or other entity agrees to be bound in writing by the restrictions set forth herein; |
| iv. | as a distribution to any wholly-owned subsidiary, partners, members or stockholders of the undersigned, provided that the transferee agrees to be bound in writing by the restrictions set forth herein; |
| v. | to the undersigned’s affiliates, shareholders, members, partners, subsidiaries, affiliates or to any investment fund or other entity controlled, advised or managed by (or under common control with), the undersigned, provided that the transferee agrees to be bound in writing by the restrictions set forth herein; |
A-132
| vi. | to a nominee or custodian of a person or entity to whom a disposition or transfer would be permissible under clauses (ii) through (v) above; provided, that the transferee agrees to be bound in writing by the restrictions set forth herein; |
| vii. | pursuant to an order of a court or regulatory agency or to comply with any regulations related to the undersigned’s ownership of theLock-Up Securities; |
| viii. | for the avoidance of doubt, any other shares of Holdco Common Stock that were acquired in open market transactions following the date of the Master Transaction Agreement; |
| ix. | in connection with collateral, hypothecation, or other pledge arrangements to support a credit facility entered into in the ordinary course; and |
| x. | pursuant to a bona fide third-party tender offer for all or substantially all of the outstanding shares of Holdco’s common stock, merger, consolidation or other similar transaction involving a change of control (as defined below) of Holdco; provided, that in the event that such tender offer, merger, consolidation or other such transaction is not completed, theLock-Up Securities shall remain subject to the restrictions contained in thisLock-Up and Permitted Sale Agreement. |
For the purposes of clause (x) above, “change of control” shall mean the consummation of any bona fide third party tender offer, merger, purchase, consolidation or other similar transaction the result of which is that any “person” (as defined in Section 13(d)(3) of the Exchange Act), or group of persons, becomes the beneficial owner (as defined in Rules13d-3 and13d-5 of the Exchange Act) of a majority of the total voting power of the voting stock of the Company
If any Prohibited Transfer is made or attempted contrary to the provisions of thisLock-Up and Permitted Sale Agreement, the undersigned acknowledges and agrees that such purported Prohibited Transfer shall be null and voidab initio, and that Holdco is entitled to refuse to recognize any such purported transferee of theLock-Up Securities as one of its stockholders for any purpose. Hayfin further acknowledges and agrees that, in order to enforce the immediately preceding sentence, Holdco may impose stop-transfer instructions with respect to theLock-Up Securities of the undersigned (and permitted transferees and assigns thereof) until the end of theLock-Up Period.
Notwithstanding the foregoing, until 2 years after the Closing Date, if Holdco (i) proposes to file a registration statement (whether or not for sale for its own account) (“Registration Statement”) that registers the transfer of any shares of Holdco’s common stock (the “Registrable Securities”) under the Securities Act (except with respect to registration statements on FormS-4 or any comparable or successor form in connection with any acquisition of any entity or business by Holdco, FormS-8 or any comparable or successor form in connection with shares issuable pursuant to option or employee benefit plans or any other form not available for registering securities of Holdco for sale to the public), (ii) proposes to sell such Registrable Securities pursuant to an effective FormS-3 or any similar short-form Registration Statement (a “Shelf Takedown”) or (iii) receives a demand request by any holder of Registrable Securities pursuant to which Holdco is required to effect a registration for resale of the Registrable Securities under the Securities Act (a “Demand Request”), Holdco shall provide prompt written notice to the undersigned of such proposal or Demand Request at least 15 business days prior to the anticipated filing date of such Registration Statement pursuant to such proposal or Demand Request (or, in the case of a Shelf Takedown not covered by clause (i) above, prior to the agreement to sell such Registrable Securities). The undersigned shall have the right to include in such Registration Statement such number of Registerable Securities as the undersigned requests, provided that Holdco shall have the right to postpone or withdraw the filing of any such Registration Statement or Shelf Takedown. The undersigned can make such a request by giving written notice to Holdco within 10 business days after the receipt of Holdco’s notice of the proposed registration. Notwithstanding the foregoing, and subject to the immediately following sentence, if the managing underwriter of such registration advises Holdco in writing that, in its opinion, the number of Registrable Securities requested to be included in the registration exceeds the largest number of shares that can be sold without having an adverse effect on such offering, including the price at which such shares can
A-133
be sold, then Holdco will include in such registration only the number of Registrable Securities recommended by the managing underwriter, with each of Holdco, other potential selling shareholders and the undersigned including shares in such Registration Statement in proportion to the percentage of the shares proposed to be sold prior to Holdco receiving such managing underwriter’s advice. The parties agree that nothing in this paragraph shall require Holdco to violate any of its obligations under the Investor Rights Agreement, dated as of July 16, 2013, by and between Parent and WSHP Biologics Holdings, LLC (“WHSP”), as amended (the “IRA”) to fulfill its demand registration obligations to WSHP under Section 2.2 of the IRA, and that, if necessary in response to a demand by WSHP pursuant to the IRA, Holdco may reduce the number of Registrable Securities of the undersigned included in such registration in anon-proportionate manner that is at least as favorable to the undersigned as to Holdco.
Holdco shall pay all customary fees and expenses incident to its performance of or compliance with the preceding paragraph, including all registration and filing fees, fees and expenses of compliance with securities or blue sky laws, Financial Industry Regulatory Authority fees, exchange listing fees, printing expenses, transfer agent’s and registrar’ s fees, cost of distributing prospectuses in preliminary and final form as well as any supplements thereto, and all reasonable fees and disbursements of one counsel for the undersigned, counsel for Holdco and all independent certified public accountants and other persons retained by Holdco (collectively, “Registration Expenses”); provided that Registration Expenses shall not include any underwriting discounts or commissions attributable to the sale of Registerable Securities by the undersigned.
For the avoidance of any doubt, subject to the terms of thisLock-Up and Permitted Sale Agreement, Hayfin shall retain all rights as a stockholder of Holdco with respect to theLock-Up Securities during theLock-Up Period, including the right to vote anyLock-Up Securities that are entitled to vote and the right to receive any dividends or distributions with respect to theLock-Up Securities.
Notwithstanding anything to the contrary contained herein, in the event that the Master Transaction Agreement is terminated in accordance with its terms prior to the Closing, thisLock-Up and Permitted Sale Agreement and all rights and obligations of the undersigned hereunder shall automatically terminate and be of no further force or effect.
Each of the parties hereto hereby represents and warrants that the undersigned has full power and authority to enter into thisLock-Up and Permitted Sale Agreement. All authority herein conferred or agreed to be conferred and any obligations of each of the undersigned shall be binding upon the successors, permitted assigns, heirs or personal representatives of the undersigned.
Any right to trial by jury with respect to any action, suit or proceeding or claim arising in connection with or as a result of any matter referred to in thisLock-Up and Permitted Sale Agreement is hereby irrevocably waived by the parties hereto. The parties hereby agree and consent that any such action, suit or proceeding or claim will be decided by a court trial without a jury, and that the parties may file a copy of thisLock-Up and Permitted Sale Agreement with any court as written evidence of the consent of the parties hereto to the waiver of their right to trial by jury.
All issues and questions concerning the construction, validity, interpretation and enforceability of thisLock-Up and Permitted Sale Agreement will be governed by, and construed in accordance with, the Laws of the State of Delaware, without giving effect to any choice of Law or conflict of Law rules or provisions (whether of the State of Delaware or any other jurisdiction) that would cause the application of the Laws of any jurisdiction other than the State of Delaware.
Any Legal Proceeding seeking to enforce any provision of, or based on any matter arising out of or in connection with, thisLock-Up and Permitted Sale Agreement or the transactions contemplated hereby will be brought and determined exclusively in the Delaware Court of Chancery of the State of Delaware;provided that if the Delaware Court of Chancery does not have subject matter jurisdiction, any such Legal Proceeding will be
A-134
brought exclusively in the United States District Court for the District of Delaware or any other court of the State of Delaware, and each of the parties hereby consents to the exclusive jurisdiction of such courts (and of the appropriate appellate courts therefrom) in any such Legal Proceeding and irrevocably waives, to the fullest extent permitted by Law, any objection that it may now or hereafter have to the laying of the venue of any such Legal Proceeding in any such court or that any such Legal Proceeding that is brought in any such court has been brought in an inconvenient forum. Process in any such Legal Proceeding may be served on any party anywhere in the world, whether within or without the jurisdiction of any such court.
Any and all remedies herein expressly conferred upon the parties hereto will be deemed cumulative with, and not exclusive of, any other remedy conferred hereby, or by law or equity upon such party, and the exercise by such party of any one remedy will not preclude the exercise of any other remedy. Each party agrees that irreparable damage would occur and that the counter-party would not have any adequate remedy at law in the event that any of the provisions of thisLock-Up and Permitted Sale Agreement were not performed in accordance with their specific terms or were otherwise breached, and that money damages or other legal remedies would not be an adequate remedy therefor. Accordingly, in addition to any other remedies available under thisLock-Up and Permitted Sale Agreement, the parties agrees that, prior to the termination of theLock-Up Period, each party will be entitled to an injunction or injunctions, specific performance and other equitable relief to prevent the counter-parties breach of thisLock-Up and Permitted Sale Agreement and to enforce specifically the terms and provisions of thisLock-Up and Permitted Sale Agreement. The parties agrees that the neither party will oppose the granting of an injunction, specific performance and other equitable relief when expressly available pursuant to the terms of thisLock-Up and Permitted Sale Agreement, and each party hereby waives (i) any defenses in any Legal Proceeding for an injunction, specific performance or other equitable relief, including the defense that such party has an adequate remedy at law or an award of specific performance is not an appropriate remedy for any reason at law or equity and (ii) any requirement under Law to post a bond, undertaking or other security as a prerequisite to obtaining equitable relief.
ThisLock-Up and Permitted Sale Agreement, to the extent signed and delivered by means of a facsimile machine or scanned pages via electronic mail, will be treated in all manner and respect as an original contract and will be considered to have the same binding legal effects as if it were the original signed version thereof delivered in person.
The parties may sign any number of copies of thisLock-Up and Permitted Sale Agreement. Each signed copy shall be an original, but all of them together represent the same agreement.
[SIGNATURE PAGE FOLLOWS]
A-135
IN WITNESS WHEREOF, the parties have executed thisLock-Up and Permitted Sale Agreement on the day and year first above written.
| Hayfin1: | [●] | |||
| By: |
| |||
| Its: |
| |||
| Holdco: | [●] | |||
| By: |
| |||
| Its: |
| |||
| 1 | NTD: Update for specific Hayfin entities once confirmed. |
[Signature Page toLock-Up and Permitted Sale Agreement]
A-136
Exhibit F
[LETTERHEAD OF THE MEMBER]
[●], 2018
Sidley Austin LLP
1 South Dearborn Street
Chicago, IL 60603
Dorsey & Whitney LLP
51 West 52nd Street
New York, New York 10019
Ladies and Gentlemen:
We are delivering this letter to you in connection with the Master Transaction Agreement dated as of [●], 2018 (the “MTA”), among [BEARS], a Delaware corporation (“Parent”), [●], a Delaware corporation and directly wholly owned Subsidiary of Parent (“Holdco”), [●], a Delaware corporation and direct wholly owned Subsidiary of Holdco (“Merger Sub”), [●], a Delaware limited liability company (the “Member”), as amended or supplemented through the date hereof, pursuant to which Merger Sub will merge with and into Parent with Parent surviving as a wholly owned subsidiary of Holdco (the “Merger”) and the Member will contribute 100% of the equity interests in [GIANTS], a Delaware limited liability company and wholly owned subsidiary of the Member (the “Company”) to Holdco (the “Contribution”).
Pursuant to Sections 8.01(k) and 8.02(j) of the MTA, it is a condition to each of Parent and the Member’s respective obligations to consummate the transactions contemplated by the MTA that Parent and the Member, respectively, receive a copy of this letter. We acknowledge that in connection with the delivery of this letter, Sidley Austin LLP and Dorsey & Whitney LLP may rely on the statements made in this letter in rendering tax opinions to their respective clients. We certify that the statements made in this letter are true, correct and complete in all material respects as of the date hereof. We acknowledge that (i) in rendering its opinion each firm may rely, without further inquiry, on the accuracy of the statements made in this letter and on the accuracy of the representations and warranties and the satisfaction of the covenants and obligations contained in the MTA, and (ii) each firm’s opinion may be subject to certain limitations and qualifications, including that it may not be relied upon if any such statements, representations or warranties are not accurate or if any of such covenants or obligations are not satisfied in all material respects. We acknowledge and agree that, for purposes of rendering each firm’s opinion, each firm may consider each of the statements made in this letter to be true, correct and complete as of the date hereof without regard to any qualification as to knowledge or belief.
Unless otherwise specified, capitalized terms used but not defined in this letter shall have the meaning ascribed to them in the MTA. References to the MTA include all exhibits, schedules thereto. All Section references are to the Code, unless otherwise specified.
Representations
| 1. | The facts, representations and covenants relating to the Merger and the Contribution as described in the MTA, the Joint Proxy and Consent Solicitation Statement/Prospectus and the other documents included or described in the Joint Proxy and Consent Solicitation Statement/Prospectus, each as amended or supplemented through the date hereof, are true, correct and complete in all material respects to the extent they relate to the Member or the Company or were provided by the Member or the Company. The Merger and the Contribution will be consummated in accordance with the MTA (without waiver or modification of |
A-137
| any provisions thereof) and as described in the Joint Proxy and Consent Solicitation Statement/Prospectus. Other than those described or referred to in the MTA and the Joint Proxy and Consent Solicitation Statement/Prospectus, there are no material agreements, arrangements or understandings, either written or oral, between or among (a) Holdco or any of its subsidiaries (or any of its affiliates or stockholders), (b) Parent or any of its subsidiaries (or any of its affiliates or stockholders), (c) the Company or any of its subsidiaries (or any of its affiliates or members), or (d) the Member or any of its subsidiaries (or any of its affiliates or members) concerning the transactions contemplated by the MTA. |
| 2. | The Merger and the Contribution are being undertaken for the valid business purposes described in the Joint Proxy and Consent Solicitation Statement/Prospectus, and not for the purpose of tax avoidance. |
| 3. | The Merger and the Contribution will occur under and in compliance with the MTA, which is a single, integrated plan agreed upon before the Merger and the Contribution in which the rights of the parties are defined, and, in the case of the Merger, in accordance with the Delaware General Corporation Law. |
| 4. | None of the Member, the Company, their affiliates or their successors or agents shall take any position on any Federal, state or local income or franchise tax return, or take any other tax reporting position, that is inconsistent with (i) the qualification of (A) the Merger and the Contribution, taken together, as a transaction described in Section 351 of the Code or (B) the Merger as a reorganization within the meaning of Section 368(a) of the Code or (ii) the representations set forth herein, in each case except to the extent otherwise required by a “determination” as defined in Section 1313(a) of the Code or the execution of an Internal Revenue Service Form870-AD, or to the extent otherwise required by applicable state or local law. |
| 5. | The terms and conditions of the MTA, including the Stock Consideration and the Cash Purchase Price, and all of the agreements described under the headings [ ]1 in the Joint Proxy and Consent Solicitation Statement/Prospectus (to the extent relating to the Contribution and any ancillary agreements relating thereto), were determined byarm’s-length negotiations between Parent, the Company and the Member. |
| 6. | The undersigned is authorized to make all the representations and certifications set forth herein on behalf of the Member. |
| 7. | Immediately before the Merger and the Contribution, the only outstanding equity units in the Company will be a single class of units (the “Company Units ”) and, to the knowledge of the Member, the only outstanding stock of Parent will be (i) a single class of common stock and (ii) a single class of convertible preferred stock (collectively, the “Parent Stock”). |
| 8. | As a result of the Contribution, the Company will be a direct wholly owned subsidiary of Holdco. |
| 9. | At the time of the Contribution, the Company will be disregarded as an entity separate from the Member for U.S. federal income tax purposes. |
| 10. | To the knowledge of the Member, immediately after the Merger and the Contribution, Holdco will have only (i) a single class of outstanding voting common stock and (ii) a single class of convertible preferred stock (collectively, the “Holdco Stock”), and the former stockholders of Parent, taken together with the Member, the members of the Member, and the holders of certain indebtedness of the Member, will hold 100% of the Holdco Stock. |
| 11. | In connection with the Contribution, (a) the Member will not receive in exchange for Company Units, directly or indirectly, any consideration other than the Stock Consideration, the Cash Purchase Price and contingent rights to receive, at later dates, the Earnout Shares and cash payments pursuant to Section 2.06 of the MTA, (b) no shares of Holdco Stock will be redeemed for cash or other property provided, directly or indirectly, by Holdco, (c) no liabilities of the Member will be assumed by Holdco, and (d) no Company Units will be acquired subject to any liabilities. |
| 1 | Note to Draft: This provision is intended to refer to a description of the MTA in the Joint Proxy and Consent Solicitation Statement/Prospectus. |
A-138
| 12. | Each of the Company and the Member has, at all relevant times, used the accrual method of accounting for federal income tax purposes. |
| 13. | No Holdco Stock that will be received in connection with the Contribution represents separatelybargained-for consideration that is allocable to any employment, consulting or similar agreement or arrangement with Holdco. |
| 14. | Neither the Member nor the Company has made, and neither the Member nor the Company has any plan or intention to make, any distributions prior to, in contemplation of or otherwise in connection with, the Merger and the Contribution to holders of Company Units, except that the Member intends to transfer, to its members and to the holder of the ERA, any Holdco Stock remaining after the transfer of Holdco Stock in satisfaction of certain obligations of the Member. |
| 15. | The Member does not have apre-arranged plan to transfer, sell or otherwise dispose of Holdco Stock received in the Contribution, except that (i) the Member intends to transfer no more than 60% of the Holdco Stock to be received on the date of the Contribution to holders of certain indebtedness of the Member and (ii) the Member intends to transfer the remaining amount of Holdco Stock received on the date of the Contribution to its members in proportion to their membership interests and to the holder of the ERA. |
| 16. | The economic rights arising from the ERA are treated as an equity interest in the Member for U.S. federal income tax purposes. |
| 17. | Neither the Member nor the Company is under the jurisdiction of a court in a title 11 or similar case within the meaning of Section 368(a)(3)(A) of the Code. |
| 18. | No Holdco Stock is being received by the Member as part of a plan of liquidation of a corporation. |
| 19. | There will be no indebtedness between Holdco and the Member or its members, and there will be no indebtedness created in favor of the Member or its members, except to the extent of installment obligations of Holdco set forth in Sections 2.06 and 11.02(d) of the MTA, in each case as a result of the Contribution. |
| 20. | In connection with the Merger and the Contribution: |
| (a) | No services will be rendered to or for the benefit of Holdco by the Member. |
| (b) | To the knowledge of Member, Holdco will not acquire any of its own indebtedness or any stock in another corporation (other than Parent Stock or Company Units). |
| (c) | To the knowledge of Member, Holdco will not transfer an interest in Parent or the Company, neither Parent nor the Company will be liquidated, neither Parent nor the Company will be merged or consolidated with any other corporation (other than pursuant to the Merger and the Contribution) or converted into a partnership, and neither Parent nor the Company will transfer any assets (except in the ordinary course of business), except for dispositions made in the ordinary course of business or transfers permitted by Treasury RegulationsSection 1.368-2(k) (provided the requirements of Treasury RegulationsSection 1.368-1(d) are satisfied). |
| (d) | To the knowledge of Member, Parent will not be converted into a “disregarded entity” (within the meaning of Treasury RegulationSection 301.7701-3). |
| (e) | To the knowledge of Member, Holdco has no plan or intention (and Parent has no plan or intention to cause Holdco) to alter the terms of the Holdco Stock or any other equity interest in Holdco or to issue additional shares of Holdco Stock. |
| (f) | To the knowledge of the Member, no licenses, leases or similar rights will be granted, nor will any property be leased back to any person, by Holdco, Parent or Company in connection with or as a result of the Merger or the Contribution. |
| (g) | None of the Holdco Stock to be received in connection with the Contribution (including Earnout Shares, if any) will be placed in escrow. |
A-139
| (h) | With respect to any Holdco Stock to be received in connection with the Contribution that will be issued after the date of the Contribution under a contingent stock arrangement: (i) such Holdco Stock will be issued within six (6) years from the date of the Contribution; (ii) there is a valid business reason for not issuing all of such Holdco Stock immediately; (iii) the issuance of such Holdco Stock will not be triggered by an event the occurrence or nonoccurrence of which is within the control of the Member; and (iv) the mechanism for the calculation of the additional Holdco Stock to be issued is objective and readily ascertainable. |
| (i) | No Holdco Stock will be issued after the Closing under a contingent stock arrangement, other than the Earnout Shares. |
| 21. | To the knowledge of the Member, after the Merger and Contribution, Holdco will remain in existence. |
| 22. | With regard to any patents, copyrights, franchises, trademarks, trade names or technical“know-how” owned by the Company: |
| (a) | Such patents or patent applications qualify as “property” within the meaning of section 351 of the Code, and the Company possess all substantial rights in such patents or patent applications within the meaning of section 1235 of the Code. |
| (b) | The Company possesses all rights, title and interests for each such copyright, in each medium of exploitation. |
| (c) | Following the Contribution, the Member will not retain any power, right, or continuing interest in the franchises, trademarks or trade names possessed by the Company. |
| (d) | Any technicalknow-how possessed by the Company is “property” within the meaning of Revenue Ruling64-56, C.B.1964-1 (Part I), and as such is afforded substantial legal protection against unauthorized disclosure and use under the laws of the country from which it is being transferred. |
| 23. | Holdco and Parent, on the one hand, and the Company and the Member, on the other hand, will pay their respective expenses, if any, incurred in connection with the Contribution. None of Holdco, Merger Sub, or Parent has agreed to assume, or will directly or indirectly assume, any expense or other liability, whether fixed or contingent, of the Member (other than liabilities of the Company addressed in Paragraph 23, below). |
| 24. | With regard to any direct or indirect liabilities of the Company as of the time of the Contribution: |
| (a) | such liabilities were incurred in the ordinary course of business and are associated with the direct and indirect assets of the Company; and |
| (b) | the adjusted basis and the fair market value (in each case, respectively) of the direct and indirect assets of the Company will be equal to or exceed the sum of the liabilities to which such assets are subject. |
| 25. | The Member will not retain any rights in Company Units exchanged in the Contribution (other than rights inherent in the ownership of Holdco Stock). |
[Signature Page Follows]
A-140
| Very truly yours, | ||
| [MEMBER] | ||
| by | ||
| ||
| Name: | ||
| Title: | ||
[Signature Page to Tax Representation Letter]
A-141
[LETTERHEAD OF BEARS]
[●], 2018
Sidley Austin LLP
1 South Dearborn Street
Chicago, IL 60603
Dorsey & Whitney LLP
51 West 52nd Street
New York, New York 10019
Ladies and Gentlemen:
We are delivering this letter to you in connection with the Master Transaction Agreement dated as of [●], 2018 (the “MTA”), among [BEARS], a Delaware corporation (“Parent”), [●], a Delaware corporation and directly wholly owned Subsidiary of Parent (“Holdco”), [●], a Delaware corporation and direct wholly owned Subsidiary of Holdco (“Merger Sub”), [●], a Delaware limited liability company (the “Member”), as amended or supplemented through the date hereof, pursuant to which Merger Sub will merge with and into Parent with Parent surviving as a wholly owned subsidiary of Holdco (the “Merger”) and the Member will contribute 100% of the equity interests in [GIANTS], a Delaware limited liability company and wholly owned subsidiary of the Member (the “Company”) to Holdco (the “Contribution”).
Pursuant to Sections 8.01(k) and 8.02(j) of the MTA, it is a condition to each of Parent and the Member’s respective obligations to consummate the transactions contemplated by the MTA that Parent and the Member, respectively, receive a copy of this letter. We acknowledge that in connection with the delivery of this letter, Sidley Austin LLP and Dorsey & Whitney LLP may rely on the statements made in this letter in rendering tax opinions to their respective clients. We certify that the statements made in this letter are true, correct and complete in all material respects as of the date hereof. We acknowledge that (i) in rendering its opinion each firm may rely, without further inquiry, on the accuracy of the statements made in this letter and on the accuracy of the representations and warranties and the satisfaction of the covenants and obligations contained in the MTA, and (ii) each firm’s opinion may be subject to certain limitations and qualifications, including that it may not be relied upon if any such statements, representations or warranties are not accurate or if any of such covenants or obligations are not satisfied in all material respects. We acknowledge and agree that, for purposes of rendering each firm’s opinion, each firm may consider each of the statements made in this letter to be true, correct and complete as of the date hereof without regard to any qualification as to knowledge or belief.
Unless otherwise specified, capitalized terms used but not defined in this letter shall have the meaning ascribed to them in the MTA. References to the MTA include all exhibits, schedules thereto. All Section references are to the Code, unless otherwise specified.
General Representations
| 1. | The facts, representations and covenants relating to the Merger and the Contribution as described in the MTA, the Joint Proxy and Consent Solicitation Statement/Prospectus and the other documents included or described in the Joint Proxy and Consent Solicitation Statement/Prospectus, each as amended or supplemented through the date hereof, are true, correct and complete in all material respects to the extent they relate to Parent, Holdco or the Merger Sub or were provided by Parent, Holdco or the Merger Sub. The Merger and the Contribution will be consummated in accordance with the MTA (without waiver or modification of any provisions thereof) and as described in the Joint Proxy and Consent Solicitation Statement/Prospectus. Other than those described or referred to in the MTA and the Joint Proxy and Consent Solicitation Statement/Prospectus, there are no material agreements, arrangements or understandings, either |
A-142
| written or oral, between or among (a) Holdco or any of its subsidiaries (or any of its affiliates or stockholders), (b) Parent or any of its subsidiaries (or any of its affiliates or stockholders), (c) the Company or any of its subsidiaries (or any of its affiliates or members), or (d) the Member or any of its subsidiaries (or any of its affiliates or members) concerning the transactions contemplated by the MTA. |
| 2. | The Merger and the Contribution are being undertaken for the valid business purposes described in the Joint Proxy and Consent Solicitation Statement/Prospectus, and not for the purpose of tax avoidance. |
| 3. | The Merger and the Contribution will occur under and in compliance with the MTA, which is a single, integrated plan agreed upon before the Merger and the Contribution in which the rights of the parties are defined, and, in the case of the Merger, in accordance with the Delaware General Corporation Law. |
| 4. | None of Holdco, Parent, Merger Sub or their affiliates (including, after the Closing, the Company) or their successors or agents shall take any position on any Federal, state or local income or franchise tax return, or take any other tax reporting position, that is inconsistent with (i) the qualification of (A) the Merger and the Contribution, taken together, as a transaction described in Section 351 of the Code or (B) the Merger as a reorganization within the meaning of Section 368(a) of the Code or (ii) the representations set forth herein, in each case except to the extent otherwise required by a “determination” as defined in Section 1313(a) of the Code or the execution of an Internal Revenue Service Form870-AD, or to the extent otherwise required by applicable state or local law. |
| 5. | The terms and conditions of the MTA, including the Stock Consideration and the Cash Purchase Price, and all of the agreements described under the headings [ ]1 in the Joint Proxy and Consent Solicitation Statement/Prospectus (to the extent relating to the Contribution and any ancillary agreements relating thereto), were determined byarm’s-length negotiations between Parent, Company and the Member. |
| 6. | The undersigned is authorized to make all the representations and certifications set forth herein on behalf of Holdco, Merger Sub, and Parent. |
Section 351 and Reorganization Representations
| 7. | Immediately before the Merger and the Contribution, the only outstanding stock of Parent will be (i) a single class of common stock and (ii) a single class of convertible preferred stock (collectively, the “Parent Stock”), and, to the knowledge of Parent, immediately before the Contribution, the only outstanding equity interests in the Company will be a single class of equity units (the “Company Units”). |
| 8. | Holdco is a newly formed corporation. At all times during Holdco’s corporate existence until the Merger and the Contribution (a) Holdco has had and will have only (i) a single class of outstanding voting common stock and (ii) a single class of convertible preferred stock (collectively, the “Holdco Stock”), (b) Parent has owned and will own 100% of the Holdco Stock and (c) Holdco has not conducted, and will not conduct, any business activities or other operations of any kind other than the issuance of its stock to Parent and other activities required in connection with the Merger and the Contribution. |
| 9. | Merger Sub is a newly formed corporation. At all times during Merger Sub’s corporate existence until the Merger (a) Merger Sub has had and will have only a single class of outstanding stock, (b) Holdco has owned and will own 100% of the outstanding stock of Merger Sub and (c) Merger Sub has not conducted, and will not conduct, any business activities or other operations of any kind other than the issuance of its stock to Holdco and other activities required in connection with the Merger and the Contribution. |
| 10. | At the time of the Merger, Merger Sub will have no liabilities, and in the Merger, Parent will not assume any liabilities of Merger Sub or acquire any assets from Merger Sub subject to any liabilities. |
| 11. | In the Merger, Merger Sub will merge with and into Parent, with Parent surviving the Merger. In the Merger, (a) each share of outstanding Parent Stock will be converted into one share of Holdco Stock, (b) the |
| 1 | Note to Draft: This provision is intended to refer to a description of the MTA in the Joint Proxy and Consent Solicitation Statement/Prospectus. |
A-143
| outstanding stock of Merger Sub will be converted into Parent Stock and (c) the shares of Holdco Stock held by Parent will be canceled. As a result of the Merger, Parent will be a direct wholly owned subsidiary of Holdco. |
| 12. | As a result of the Contribution, the Company will be a direct wholly owned subsidiary of Holdco. Holdco does not have any plan or intention to take any action that is inconsistent with the Company being treated as disregarded as an entity separate from Holdco for U.S. federal income tax purposes. |
| 13. | In the Merger, Holdco will acquire Parent Stock solely in exchange for voting stock of Holdco. Parent Stock is the only asset, property or right that will be transferred to Holdco in the Merger. |
| 14. | The fair market value of the Holdco Shares issued to the Parent stockholders pursuant to the Merger will be approximately equal to the fair market value of the Parent Stock exchanged by the Parent stockholders pursuant to the Merger. |
| 15. | Immediately after the Merger and the Contribution, (a) Parent Stock will be the only outstanding classes of stock of Parent, and Holdco will hold 100% of the Parent Stock and (b) Holdco Stock will be the only outstanding class of stock of Holdco and the former stockholders of Parent, taken together with the Member, the members of the Member, and the holders of certain indebtedness of the Member, will hold 100% of the Holdco Stock. |
| 16. | In connection with the Merger, (a) no holder of Parent Stock will receive in exchange for Parent Stock, directly or indirectly, any consideration other than the Holdco Stock, (b) no shares of Holdco Stock will be redeemed for cash or other property provided, directly or indirectly, by Holdco, (c) no liabilities of Parent will be assumed by Holdco and (d) no Parent Stock will be acquired subject to any liabilities. |
| 17. | In connection with the Contribution, (a) the Member will not receive in exchange for Company Units, directly or indirectly, any consideration other than the Stock Consideration, the Cash Purchase Price and contingent rights to receive, at later dates, the Earnout Shares and cash payments pursuant to the terms of Section 2.06 of the MTA, (b) no shares of Holdco Stock will be redeemed for cash or other property provided, directly or indirectly, by Holdco, (c) no liabilities of the Member will be assumed by Holdco and (d) no Company Units will be acquired subject to any liabilities. |
| 18. | None of Holdco, Merger Sub or Parent is or will be an investment company as defined in Section 368(a)(2)(F)(iii) and (iv) of the Code. Holdco is not, and will not be, an investment company within the meaning of Section 351(e)(1) of the Code and Treasury RegulationsSection 1.351-1(c)(1)(ii). Holdco is not, and will not be, a “personal service corporation” within the meaning of Section 269A of the Code. |
| 19. | None of Holdco, Merger Sub or Parent is under the jurisdiction of a court in a title 11 or similar case within the meaning of Section 368(a)(3)(A) of the Code or is the subject of any other receivership, insolvency, bankruptcy or similar proceeding. |
| 20. | None of the compensation received or to be received by any stockholder-employee or stockholder-independent contractor of Parent (including pursuant to any consulting agreement) from Holdco or Parent represents separate consideration for, or is allocable to, any Parent Stock held by such person. No Holdco Stock that will be received by any stockholder-employee or stockholder-independent contractor of Parent in the Merger represents separatelybargained-for consideration that is allocable to any employment, consulting or similar agreement or arrangement with Holdco or Parent. |
| 21. | No Holdco Stock that will be received in connection with the Contribution represents separatelybargained-for consideration that is allocable to any employment, consulting or similar agreement or arrangement with Holdco. |
| 22. | Parent has not made, and does not have any plan or intention to make, any distributions prior to, in contemplation of or otherwise in connection with, the Merger to holders of Parent Stock. |
| 23. | Neither Holdco nor any person related to Holdco within the meaning of Treasury RegulationsSection 1.368-1(e)(4) has any plan or intention to reacquire or redeem any Holdco Stock issued in the |
A-144
| Merger, either directly or indirectly through any transaction, agreement or arrangement with any other person (including by derivative transactions, such as equity swaps, that would have the economic effect of an acquisition), except in connection with any plan or intention to purchase stock on the open market as part of a repurchase program that satisfies the requirements of Revenue Ruling99-58,1999-2 C.B. 701. Neither Parent nor any person related to Parent within the meaning of Treasury RegulationsSection 1.368-1(e)(4) has, either directly or indirectly through any transaction, agreement or arrangement with any other person (including by derivative transactions, such as an equity swap, that would have the economic effect of an acquisition), redeemed or acquired, or has any plan or intention to redeem or acquire, any Parent Stock in contemplation of the Merger, or otherwise as part of a plan of which the Merger is a part. |
Additional Section 351 Representations
| 24. | It is expected, and you may assume for purposes of your opinion, that (i) stockholders of Parent that have apre-arranged plan to transfer, sell or otherwise dispose of Holdco Stock received in the Merger and (ii) shares issued as equity compensation to employees of Holdco or its subsidiaries will, taken together with the transfer of Holdco Stock described in Paragraph 25, below, represent less than 15% of the outstanding shares of Holdco Stock immediately after the Merger. |
| 25. | To the knowledge of Holdco and Parent, the Member does not have apre-arranged plan to transfer, sell or otherwise dispose of Holdco Stock received in the Contribution, except that to the knowledge of Holdco and Parent, (i) the Member intends to transfer up to 60% of the Holdco Stock to be received on the date of the Contribution to holders of certain indebtedness of the Member and (ii) Member intends to transfer the remaining amount of Holdco Stock received on the date of the Contribution to its members in proportion to their membership interests and to the holder of the ERA. |
| 26. | To the knowledge of Holdco and Parent, the stock or securities received in the Merger by a stockholder of Parent that is under the jurisdiction of a court in a title 11 or similar case within the meaning of Section 368(a)(3)(A) of the Code will not be used to satisfy the indebtedness of such stockholder. |
| 27. | No Holdco Stock is being received by the stockholders of Parent as part of a plan of liquidation of a corporation. |
| 28. | None of the Holdco Stock to be received by Parent stockholders in the Merger is “Section 306 stock” within the meaning of section 306(c)(3) of the Code. |
| 29. | There will be no indebtedness between Holdco and Parent stockholders, and there will be no indebtedness created in favor of Parent stockholders, in each case as a result of the Merger. |
| 30. | There will be no indebtedness between Holdco and the Member or its members, and there will be no indebtedness created in favor of the Member or its members, except to the extent of installment obligations of Holdco set forth in Sections 2.06 and 11.02(d) of the MTA, in each case as a result of the Contribution. |
| 31. | In connection with the Merger and the Contribution: |
| (a) | No services will be rendered to or for the benefit of Holdco by stockholders of Parent (other than stockholders of Parent who are also employees of Parent, in which case, no services shall be performed by such stockholders other than in their capacity as employees of Parent). |
| (b) | Holdco will not acquire any of its own indebtedness or any stock in another corporation (other than Parent Stock or Company Units). |
| (c) | Holdco will not transfer an interest in Parent or the Company, neither Parent nor the Company will be liquidated, neither Parent nor the Company will be merged or consolidated with any other entity (other than pursuant to the Merger and the Contribution) or converted into a partnership, and neither Parent nor the Company will transfer any assets (except in the ordinary course of business), except for dispositions made in the ordinary course of business or transfers permitted by Treasury RegulationsSection 1.368-2(k) (provided the requirements of Treasury RegulationsSection 1.368-1(d) are satisfied). |
A-145
| (d) | Parent will not be converted into a “disregarded entity” (within the meaning of Treasury RegulationSection 301.7701-3). |
| (e) | Holdco has no plan or intention (and Parent has no plan or intention to cause Holdco) to alter the terms of the Holdco Stock or any other equity interest in Holdco or to issue additional shares of Holdco Stock. |
| (f) | No licenses, leases or similar rights will be granted, nor will any property be leased back to any person, by Holdco, Parent or Company in connection with or as a result of the Merger or the Contribution. |
| (g) | None of the Holdco Stock to be received in connection with the Contribution (including Earnout Shares, if any) will be placed in escrow. |
| (h) | With respect to any Holdco Stock to be received in connection with the Contribution that will be issued after the date of the Contribution under a contingent stock arrangement: (i) such Holdco Stock will be issued within six (6) years from the date of the Contribution; (ii) there is a valid business reason for not issuing all of such Holdco Stock immediately; (iii) the issuance of such Holdco Stock will not be triggered by an event the occurrence or nonoccurrence of which is within the control of the Member; and (iv) the mechanism for the calculation of the additional Holdco Stock to be issued is objective and readily ascertainable. |
| (i) | No Holdco Stock will be issued after the Closing under a contingent stock arrangement, other than the Earnout Shares. |
| 32. | After the Merger and Contribution, Holdco will remain in existence. |
| 33. | Following the Contribution, the Member will not retain any power, right, or continuing interest in the franchises, trademarks or trade names possessed by the Company. |
| 34. | With regard to any accounts receivable or commissions due owned by the Company, following the Contribution, Holdco will report items which, but for the Contribution, would have resulted in income or deduction to the Member in a period subsequent to the Contribution and such items will constitute income or deductions to Holdco when received or paid by it, and the proceeds received in the collection of the income items will be included as ordinary income in computing the taxable income of Holdco. |
| 35. | The stockholders of Parent will not retain any rights in Parent Stock exchanged in the Merger (other than rights inherent in the ownership of Holdco Stock). |
| 36. | The Member will not retain any rights in the Company Units transferred in the Contribution (other than rights inherent in the ownership of Holdco Stock). |
| 37. | Except for expenses solely and directly related to the Merger within the guidelines set forth in Revenue Ruling73-54,1973-1 C.B. 187, (i) Holdco, Merger Sub, Parent, on the one hand, and Parent stockholders, on the other hand, will pay their respective expenses, if any, incurred in connection with the Merger, (ii) Holdco and Parent, on the one hand, and the Company and the Member, on the other hand, will pay their respective expenses, if any, incurred in connection with the Contribution; and (iii) none of Holdco, Merger Sub, or Parent has agreed to assume, or will directly or indirectly assume, any expense or other liability, whether fixed or contingent, of any Parent stockholder or the Member (other than liabilities of the Company addressed in Paragraph 39, below). |
| 38. | Following the Merger and the Contribution, Holdco and, to the knowledge of Holdco, every “significant transferor” (within the meaning of Treasury RegulationSection 1.351-3(d)(1)) (if any), will comply with the record-keeping and information filing requirements of Treasury RegulationSection 1.351-3. |
| 39. | To knowledge of Holdco and Parent, with regard to any direct or indirect liabilities of the Company as of the time of the Contribution, such liabilities were incurred in the ordinary course of business and are associated with the direct and indirect assets of the Company. |
A-146
Additional Reorganization Representations
| 40. | At the time of and immediately after the Merger, there will be no outstanding options, convertible securities, or any other type of right pursuant to which any person could acquire stock in Parent, other than employee stock-based compensation that will be converted in the Merger into stock-based compensation with respect to Holdco Stock. |
| 41. | Holdco has no plan or intention to cause or allow Parent to issue additional shares of stock or take any other action that could result in Holdco owning less than 100% of the stock of Parent. |
| 42. | At all times during the five years prior to the Merger, none of Holdco, Merger Sub or any of their respective subsidiaries have owned or will own, directly or indirectly, any stock or securities of Parent or any instrument giving the holder the right to acquire any such stock or securities. |
| 43. | Parent conducts a “historic business” within the meaning of Treasury RegulationsSection 1.368-1(d) and Holdco will continue the “historic business” of Parent or use a significant portion of Parent’s “historic business assets” in a business following the Merger (as such terms are defined in Treasury RegulationsSection 1.368-1(d)), either directly or through one or more members of its qualified group of corporations (as defined in Treasury RegulationsSection 1.368-1(d)(4)(ii)). |
| 44. | On the date of the Merger, the adjusted basis and fair market value of the assets of Holdco will exceed the sum of its liabilities, plus the amount of liabilities, if any, to which such assets are subject and the adjusted basis and fair market value of the assets of Parent will exceed the sum of its liabilities, plus the amount of liabilities, if any, to which such assets are subject. |
| 45. | Immediately after the Merger, Parent will hold (i) at least 90 percent of the fair market value of the net assets and at least 70 percent of the fair market value of the gross assets held by Parent immediately prior to the Merger and (ii) at least 90 percent of the fair market value of the net assets and at least 70 percent of the fair market value of the gross assets held by Merger Sub immediately prior to the Merger. For purposes of this representation, amounts (i) used (or to be used) by Parent or Merger Sub to pay Merger expenses, (ii) paid (or to be paid) by Parent or Merger Sub to redeem stock, securities, warrants, or options of Parent as part of any overall plan of which the Merger are a part, or (iii) distributed (or to be distributed) by Parent or Merger Sub to the holders of Parent Stock as part of any overall plan of which the Merger are a part, in each case will be treated as assets of Parent or Merger Sub, as applicable, held immediately prior to the Merger. Any dispositions of assets that were held by Parent or Merger Sub prior to the Merger that were or are made in contemplation of, or as part of any overall plan that includes, the Merger have been or will be made for fair market value, and the proceeds thereof will be retained by Parent. |
| 46. | Prior to the Merger, there will be no intercorporate indebtedness existing between Holdco or any of its subsidiaries, including Merger Sub, on the one hand, and Parent or any of its subsidiaries, on the other hand. |
[Signature Page Follows]
A-147
| Very truly yours, | ||
| [BEARS] | ||
| by | ||
| ||
| Name: | ||
| Title: | ||
| [HOLDCO] | ||
| by | ||
| ||
| Name: | ||
| Title: | ||
[Signature Page to Tax Representation Letter]
A-148
Exhibit G
[LETTERHEAD OF THE MEMBER]
[●], 2018
Sidley Austin LLP
1 South Dearborn Street
Chicago, IL 60603
Dorsey & Whitney LLP
51 West 52nd Street
New York, New York 10019
Ladies and Gentlemen:
We are delivering this letter to you in connection with the Master Transaction Agreement dated as of [●], 2018 (the “MTA”), among [BEARS], a Delaware corporation (“Parent”), [●], a Delaware corporation and directly wholly owned Subsidiary of Parent (“Holdco”), [●], a Delaware corporation and direct wholly owned Subsidiary of Holdco (“Merger Sub”), [●], a Delaware limited liability company (the “Member”), as amended or supplemented through the date hereof, pursuant to which Merger Sub will merge with and into Parent with Parent surviving as a wholly owned subsidiary of Holdco (the “Merger”) and the Member will contribute 100% of the equity interests in [GIANTS], a Delaware limited liability company and wholly owned subsidiary of the Member (the “Company”) to Holdco (the “Contribution”).
Pursuant to Sections 8.01(k) and 8.02(j) of the MTA, it is a condition to each of Parent and the Member’s respective obligations to consummate the transactions contemplated by the MTA that Parent and the Member, respectively, receive a copy of this letter. We acknowledge that in connection with the delivery of this letter, Sidley Austin LLP and Dorsey & Whitney LLP may rely on the statements made in this letter in rendering tax opinions to their respective clients. We certify that the statements made in this letter are true, correct and complete in all material respects as of the date hereof. We acknowledge that (i) in rendering its opinion each firm may rely, without further inquiry, on the accuracy of the statements made in this letter and on the accuracy of the representations and warranties and the satisfaction of the covenants and obligations contained in the MTA, and (ii) each firm’s opinion may be subject to certain limitations and qualifications, including that it may not be relied upon if any such statements, representations or warranties are not accurate or if any of such covenants or obligations are not satisfied in all material respects. We acknowledge and agree that, for purposes of rendering each firm’s opinion, each firm may consider each of the statements made in this letter to be true, correct and complete as of the date hereof without regard to any qualification as to knowledge or belief.
Unless otherwise specified, capitalized terms used but not defined in this letter shall have the meaning ascribed to them in the MTA. References to the MTA include all exhibits, schedules thereto. All Section references are to the Code, unless otherwise specified.
Representations
| 1. | The facts, representations and covenants relating to the Merger and the Contribution as described in the MTA, the Joint Proxy and Consent Solicitation Statement/Prospectus and the other documents included or described in the Joint Proxy and Consent Solicitation Statement/Prospectus, each as amended or supplemented through the date hereof, are true, correct and complete in all material respects to the extent they relate to the Member or the Company or were provided by the Member or the Company. The Merger and the Contribution will be consummated in accordance with the MTA (without waiver or modification of any provisions thereof) and as described in the Joint Proxy and Consent Solicitation Statement/Prospectus. |
A-149
| Other than those described or referred to in the MTA and the Joint Proxy and Consent Solicitation Statement/Prospectus, there are no material agreements, arrangements or understandings, either written or oral, between or among (a) Holdco or any of its subsidiaries (or any of its affiliates or stockholders), (b) Parent or any of its subsidiaries (or any of its affiliates or stockholders), (c) the Company or any of its subsidiaries (or any of its affiliates or members), or (d) the Member or any of its subsidiaries (or any of its affiliates or members) concerning the transactions contemplated by the MTA. |
| 2. | The Merger and the Contribution are being undertaken for the valid business purposes described in the Joint Proxy and Consent Solicitation Statement/Prospectus, and not for the purpose of tax avoidance. |
| 3. | The Merger and the Contribution will occur under and in compliance with the MTA, which is a single, integrated plan agreed upon before the Merger and the Contribution in which the rights of the parties are defined, and, in the case of the Merger, in accordance with the Delaware General Corporation Law. |
| 4. | None of the Member, the Company, their affiliates or their successors or agents shall take any position on any Federal, state or local income or franchise tax return, or take any other tax reporting position, that is inconsistent with (i) the qualification of (A) the Merger and the Contribution, taken together, as a transaction described in Section 351 of the Code or (B) the Merger as a reorganization within the meaning of Section 368(a) of the Code or (ii) the representations set forth herein, in each case except to the extent otherwise required by a “determination” as defined in Section 1313(a) of the Code or the execution of an Internal Revenue Service Form870-AD, or to the extent otherwise required by applicable state or local law. |
| 5. | The terms and conditions of the MTA, including the Stock Consideration and the Cash Purchase Price, and all of the agreements described under the headings [ ]1 in the Joint Proxy and Consent Solicitation Statement/Prospectus (to the extent relating to the Contribution and any ancillary agreements relating thereto), were determined byarm’s-length negotiations between Parent, the Company and the Member. |
| 6. | The undersigned is authorized to make all the representations and certifications set forth herein on behalf of the Member. |
| 7. | Immediately before the Merger and the Contribution, the only outstanding equity units in the Company will be a single class of units (the “Company Units ”) and, to the knowledge of the Member, the only outstanding stock of Parent will be (i) a single class of common stock and (ii) a single class of convertible preferred stock (collectively, the “Parent Stock”). |
| 8. | As a result of the Contribution, the Company will be a direct wholly owned subsidiary of Holdco. |
| 9. | At the time of the Contribution, the Company will be disregarded as an entity separate from the Member for U.S. federal income tax purposes. |
| 10. | To the knowledge of the Member, immediately after the Merger and the Contribution, Holdco will have only (i) a single class of outstanding voting common stock and (ii) a single class of convertible preferred stock (collectively, the “Holdco Stock”), and the former stockholders of Parent, taken together with the Member, the members of the Member, and the holders of certain indebtedness of the Member, will hold 100% of the Holdco Stock. |
| 11. | In connection with the Contribution, (a) the Member will not receive in exchange for Company Units, directly or indirectly, any consideration other than the Stock Consideration, the Cash Purchase Price and contingent rights to receive, at later dates, the Earnout Shares and cash payments pursuant to Section 2.06 of the MTA, (b) no shares of Holdco Stock will be redeemed for cash or other property provided, directly or indirectly, by Holdco, (c) no liabilities of the Member will be assumed by Holdco, and (d) no Company Units will be acquired subject to any liabilities. |
| 12. | Each of the Company and the Member has, at all relevant times, used the accrual method of accounting for federal income tax purposes. |
| 1 | Note to Draft: This provision is intended to refer to a description of the MTA in the Joint Proxy and Consent Solicitation Statement/Prospectus. |
A-150
| 13. | No Holdco Stock that will be received in connection with the Contribution represents separatelybargained-for consideration that is allocable to any employment, consulting or similar agreement or arrangement with Holdco. |
| 14. | Neither the Member nor the Company has made, and neither the Member nor the Company has any plan or intention to make, any distributions prior to, in contemplation of or otherwise in connection with, the Merger and the Contribution to holders of Company Units, except that the Member intends to transfer, to its members and to the holder of the ERA, any Holdco Stock remaining after the transfer of Holdco Stock in satisfaction of certain obligations of the Member. |
| 15. | The Member does not have apre-arranged plan to transfer, sell or otherwise dispose of Holdco Stock received in the Contribution, except that (i) the Member intends to transfer no more than 60% of the Holdco Stock to be received on the date of the Contribution to holders of certain indebtedness of the Member and (ii) the Member intends to transfer the remaining amount of Holdco Stock received on the date of the Contribution to its members in proportion to their membership interests and to the holder of the ERA. |
| 16. | The economic rights arising from the ERA are treated as an equity interest in the Member for U.S. federal income tax purposes. |
| 17. | Neither the Member nor the Company is under the jurisdiction of a court in a title 11 or similar case within the meaning of Section 368(a)(3)(A) of the Code. |
| 18. | No Holdco Stock is being received by the Member as part of a plan of liquidation of a corporation. |
| 19. | There will be no indebtedness between Holdco and the Member or its members, and there will be no indebtedness created in favor of the Member or its members, except to the extent of installment obligations of Holdco set forth in Sections 2.06 and 11.02(d) of the MTA, in each case as a result of the Contribution. |
| 20. | In connection with the Merger and the Contribution: |
| (a) | No services will be rendered to or for the benefit of Holdco by the Member. |
| (b) | To the knowledge of Member, Holdco will not acquire any of its own indebtedness or any stock in another corporation (other than Parent Stock or Company Units). |
| (c) | To the knowledge of Member, Holdco will not transfer an interest in Parent or the Company, neither Parent nor the Company will be liquidated, neither Parent nor the Company will be merged or consolidated with any other corporation (other than pursuant to the Merger and the Contribution) or converted into a partnership, and neither Parent nor the Company will transfer any assets (except in the ordinary course of business), except for dispositions made in the ordinary course of business or transfers permitted by Treasury RegulationsSection 1.368-2(k) (provided the requirements of Treasury RegulationsSection 1.368-1(d) are satisfied). |
| (d) | To the knowledge of Member, Parent will not be converted into a “disregarded entity” (within the meaning of Treasury RegulationSection 301.7701-3). |
| (e) | To the knowledge of Member, Holdco has no plan or intention (and Parent has no plan or intention to cause Holdco) to alter the terms of the Holdco Stock or any other equity interest in Holdco or to issue additional shares of Holdco Stock. |
| (f) | To the knowledge of the Member, no licenses, leases or similar rights will be granted, nor will any property be leased back to any person, by Holdco, Parent or Company in connection with or as a result of the Merger or the Contribution. |
| (g) | None of the Holdco Stock to be received in connection with the Contribution (including Earnout Shares, if any) will be placed in escrow. |
| (h) | With respect to any Holdco Stock to be received in connection with the Contribution that will be issued after the date of the Contribution under a contingent stock arrangement: (i) such Holdco Stock will be issued within six (6) years from the date of the Contribution; (ii) there is a valid business reason for not |
A-151
| issuing all of such Holdco Stock immediately; (iii) the issuance of such Holdco Stock will not be triggered by an event the occurrence or nonoccurrence of which is within the control of the Member; and (iv) the mechanism for the calculation of the additional Holdco Stock to be issued is objective and readily ascertainable. |
| (i) | No Holdco Stock will be issued after the Closing under a contingent stock arrangement, other than the Earnout Shares. |
| 21. | To the knowledge of the Member, after the Merger and Contribution, Holdco will remain in existence. |
| 22. | With regard to any patents, copyrights, franchises, trademarks, trade names or technical“know-how” owned by the Company: |
| (a) | Such patents or patent applications qualify as “property” within the meaning of section 351 of the Code, and the Company possess all substantial rights in such patents or patent applications within the meaning of section 1235 of the Code. |
| (b) | The Company possesses all rights, title and interests for each such copyright, in each medium of exploitation. |
| (c) | Following the Contribution, the Member will not retain any power, right, or continuing interest in the franchises, trademarks or trade names possessed by the Company. |
| (d) | Any technicalknow-how possessed by the Company is “property” within the meaning of Revenue Ruling64-56, C.B.1964-1 (Part I), and as such is afforded substantial legal protection against unauthorized disclosure and use under the laws of the country from which it is being transferred. |
| 23. | Holdco and Parent, on the one hand, and the Company and the Member, on the other hand, will pay their respective expenses, if any, incurred in connection with the Contribution. None of Holdco, Merger Sub, or Parent has agreed to assume, or will directly or indirectly assume, any expense or other liability, whether fixed or contingent, of the Member (other than liabilities of the Company addressed in Paragraph 23, below). |
| 24. | With regard to any direct or indirect liabilities of the Company as of the time of the Contribution: |
| (a) | such liabilities were incurred in the ordinary course of business and are associated with the direct and indirect assets of the Company; and |
| (b) | the adjusted basis and the fair market value (in each case, respectively) of the direct and indirect assets of the Company will be equal to or exceed the sum of the liabilities to which such assets are subject. |
| 25. | The Member will not retain any rights in Company Units exchanged in the Contribution (other than rights inherent in the ownership of Holdco Stock). |
[Signature Page Follows]
A-152
| Very truly yours, | ||
| [MEMBER] | ||
| by | ||
| ||
| Name: | ||
| Title: | ||
[Signature Page to Tax Representation Letter]
A-153
[LETTERHEAD OF BEARS]
[●], 2018
Sidley Austin LLP
1 South Dearborn Street
Chicago, IL 60603
Dorsey & Whitney LLP
51 West 52nd Street
New York, New York 10019
Ladies and Gentlemen:
We are delivering this letter to you in connection with the Master Transaction Agreement dated as of [●], 2018 (the “MTA”), among [BEARS], a Delaware corporation (“Parent”), [●], a Delaware corporation and directly wholly owned Subsidiary of Parent (“Holdco”), [●], a Delaware corporation and direct wholly owned Subsidiary of Holdco (“Merger Sub”), [●], a Delaware limited liability company (the “Member”), as amended or supplemented through the date hereof, pursuant to which Merger Sub will merge with and into Parent with Parent surviving as a wholly owned subsidiary of Holdco (the “Merger”) and the Member will contribute 100% of the equity interests in [GIANTS], a Delaware limited liability company and wholly owned subsidiary of the Member (the “Company”) to Holdco (the “Contribution”).
Pursuant to Sections 8.01(k) and 8.02(j) of the MTA, it is a condition to each of Parent and the Member’s respective obligations to consummate the transactions contemplated by the MTA that Parent and the Member, respectively, receive a copy of this letter. We acknowledge that in connection with the delivery of this letter, Sidley Austin LLP and Dorsey & Whitney LLP may rely on the statements made in this letter in rendering tax opinions to their respective clients. We certify that the statements made in this letter are true, correct and complete in all material respects as of the date hereof. We acknowledge that (i) in rendering its opinion each firm may rely, without further inquiry, on the accuracy of the statements made in this letter and on the accuracy of the representations and warranties and the satisfaction of the covenants and obligations contained in the MTA, and (ii) each firm’s opinion may be subject to certain limitations and qualifications, including that it may not be relied upon if any such statements, representations or warranties are not accurate or if any of such covenants or obligations are not satisfied in all material respects. We acknowledge and agree that, for purposes of rendering each firm’s opinion, each firm may consider each of the statements made in this letter to be true, correct and complete as of the date hereof without regard to any qualification as to knowledge or belief.
Unless otherwise specified, capitalized terms used but not defined in this letter shall have the meaning ascribed to them in the MTA. References to the MTA include all exhibits, schedules thereto. All Section references are to the Code, unless otherwise specified.
A-154
General Representations
| 1. | The facts, representations and covenants relating to the Merger and the Contribution as described in the MTA, the Joint Proxy and Consent Solicitation Statement/Prospectus and the other documents included or described in the Joint Proxy and Consent Solicitation Statement/Prospectus, each as amended or supplemented through the date hereof, are true, correct and complete in all material respects to the extent they relate to Parent, Holdco or the Merger Sub or were provided by Parent, Holdco or the Merger Sub. The Merger and the Contribution will be consummated in accordance with the MTA (without waiver or modification of any provisions thereof) and as described in the Joint Proxy and Consent Solicitation Statement/Prospectus. Other than those described or referred to in the MTA and the Joint Proxy and Consent Solicitation Statement/Prospectus, there are no material agreements, arrangements or understandings, either written or oral, between or among (a) Holdco or any of its subsidiaries (or any of its affiliates or stockholders), (b) Parent or any of its subsidiaries (or any of its affiliates or stockholders), (c) the Company or any of its subsidiaries (or any of its affiliates or members), or (d) the Member or any of its subsidiaries (or any of its affiliates or members) concerning the transactions contemplated by the MTA. |
| 2. | The Merger and the Contribution are being undertaken for the valid business purposes described in the Joint Proxy and Consent Solicitation Statement/Prospectus, and not for the purpose of tax avoidance. |
| 3. | The Merger and the Contribution will occur under and in compliance with the MTA, which is a single, integrated plan agreed upon before the Merger and the Contribution in which the rights of the parties are defined, and, in the case of the Merger, in accordance with the Delaware General Corporation Law. |
| 4. | None of Holdco, Parent, Merger Sub or their affiliates (including, after the Closing, the Company) or their successors or agents shall take any position on any Federal, state or local income or franchise tax return, or take any other tax reporting position, that is inconsistent with (i) the qualification of (A) the Merger and the Contribution, taken together, as a transaction described in Section 351 of the Code or (B) the Merger as a reorganization within the meaning of Section 368(a) of the Code or (ii) the representations set forth herein, in each case except to the extent otherwise required by a “determination” as defined in Section 1313(a) of the Code or the execution of an Internal Revenue Service Form870-AD, or to the extent otherwise required by applicable state or local law. |
| 5. | The terms and conditions of the MTA, including the Stock Consideration and the Cash Purchase Price, and all of the agreements described under the headings [ ]1 in the Joint Proxy and Consent Solicitation Statement/Prospectus (to the extent relating to the Contribution and any ancillary agreements relating thereto), were determined byarm’s-length negotiations between Parent, Company and the Member. |
| 6. | The undersigned is authorized to make all the representations and certifications set forth herein on behalf of Holdco, Merger Sub, and Parent. |
Section 351 and Reorganization Representations
| 7. | Immediately before the Merger and the Contribution, the only outstanding stock of Parent will be (i) a single class of common stock and (ii) a single class of convertible preferred stock (collectively, the “Parent Stock”), and, to the knowledge of Parent, immediately before the Contribution, the only outstanding equity interests in the Company will be a single class of equity units (the “Company Units”). |
| 8. | Holdco is a newly formed corporation. At all times during Holdco’s corporate existence until the Merger and the Contribution (a) Holdco has had and will have only (i) a single class of outstanding voting common stock and (ii) a single class of convertible preferred stock (collectively, the “Holdco Stock”), (b) Parent has owned and will own 100% of the Holdco Stock and (c) Holdco has not conducted, and will not conduct, any business activities or other operations of any kind other than the issuance of its stock to Parent and other activities required in connection with the Merger and the Contribution. |
| 1 | Note to Draft: This provision is intended to refer to a description of the MTA in the Joint Proxy and Consent Solicitation Statement/Prospectus. |
A-155
| 9. | Merger Sub is a newly formed corporation. At all times during Merger Sub’s corporate existence until the Merger (a) Merger Sub has had and will have only a single class of outstanding stock, (b) Holdco has owned and will own 100% of the outstanding stock of Merger Sub and (c) Merger Sub has not conducted, and will not conduct, any business activities or other operations of any kind other than the issuance of its stock to Holdco and other activities required in connection with the Merger and the Contribution. |
| 10. | At the time of the Merger, Merger Sub will have no liabilities, and in the Merger, Parent will not assume any liabilities of Merger Sub or acquire any assets from Merger Sub subject to any liabilities. |
| 11. | In the Merger, Merger Sub will merge with and into Parent, with Parent surviving the Merger. In the Merger, (a) each share of outstanding Parent Stock will be converted into one share of Holdco Stock, (b) the outstanding stock of Merger Sub will be converted into Parent Stock and (c) the shares of Holdco Stock held by Parent will be canceled. As a result of the Merger, Parent will be a direct wholly owned subsidiary of Holdco. |
| 12. | As a result of the Contribution, the Company will be a direct wholly owned subsidiary of Holdco. Holdco does not have any plan or intention to take any action that is inconsistent with the Company being treated as disregarded as an entity separate from Holdco for U.S. federal income tax purposes. |
| 13. | In the Merger, Holdco will acquire Parent Stock solely in exchange for voting stock of Holdco. Parent Stock is the only asset, property or right that will be transferred to Holdco in the Merger. |
| 14. | The fair market value of the Holdco Shares issued to the Parent stockholders pursuant to the Merger will be approximately equal to the fair market value of the Parent Stock exchanged by the Parent stockholders pursuant to the Merger. |
| 15. | Immediately after the Merger and the Contribution, (a) Parent Stock will be the only outstanding classes of stock of Parent, and Holdco will hold 100% of the Parent Stock and (b) Holdco Stock will be the only outstanding class of stock of Holdco and the former stockholders of Parent, taken together with the Member, the members of the Member, and the holders of certain indebtedness of the Member, will hold 100% of the Holdco Stock. |
| 16. | In connection with the Merger, (a) no holder of Parent Stock will receive in exchange for Parent Stock, directly or indirectly, any consideration other than the Holdco Stock, (b) no shares of Holdco Stock will be redeemed for cash or other property provided, directly or indirectly, by Holdco, (c) no liabilities of Parent will be assumed by Holdco and (d) no Parent Stock will be acquired subject to any liabilities. |
| 17. | In connection with the Contribution, (a) the Member will not receive in exchange for Company Units, directly or indirectly, any consideration other than the Stock Consideration, the Cash Purchase Price and contingent rights to receive, at later dates, the Earnout Shares and cash payments pursuant to the terms of Section 2.06 of the MTA, (b) no shares of Holdco Stock will be redeemed for cash or other property provided, directly or indirectly, by Holdco, (c) no liabilities of the Member will be assumed by Holdco and (d) no Company Units will be acquired subject to any liabilities. |
| 18. | None of Holdco, Merger Sub or Parent is or will be an investment company as defined in Section 368(a)(2)(F)(iii) and (iv) of the Code. Holdco is not, and will not be, an investment company within the meaning of Section 351(e)(1) of the Code and Treasury RegulationsSection 1.351-1(c)(1)(ii). Holdco is not, and will not be, a “personal service corporation” within the meaning of Section 269A of the Code. |
| 19. | None of Holdco, Merger Sub or Parent is under the jurisdiction of a court in a title 11 or similar case within the meaning of Section 368(a)(3)(A) of the Code or is the subject of any other receivership, insolvency, bankruptcy or similar proceeding. |
| 20. | None of the compensation received or to be received by any stockholder-employee or stockholder-independent contractor of Parent (including pursuant to any consulting agreement) from Holdco or Parent represents separate consideration for, or is allocable to, any Parent Stock held by such person. No Holdco Stock that will be received by any stockholder-employee or stockholder-independent contractor of Parent in |
A-156
| the Merger represents separatelybargained-for consideration that is allocable to any employment, consulting or similar agreement or arrangement with Holdco or Parent. |
| 21. | No Holdco Stock that will be received in connection with the Contribution represents separatelybargained-for consideration that is allocable to any employment, consulting or similar agreement or arrangement with Holdco. |
| 22. | Parent has not made, and does not have any plan or intention to make, any distributions prior to, in contemplation of or otherwise in connection with, the Merger to holders of Parent Stock. |
| 23. | Neither Holdco nor any person related to Holdco within the meaning of Treasury RegulationsSection 1.368-1(e)(4) has any plan or intention to reacquire or redeem any Holdco Stock issued in the Merger, either directly or indirectly through any transaction, agreement or arrangement with any other person (including by derivative transactions, such as equity swaps, that would have the economic effect of an acquisition), except in connection with any plan or intention to purchase stock on the open market as part of a repurchase program that satisfies the requirements of Revenue Ruling99-58,1999-2 C.B. 701. Neither Parent nor any person related to Parent within the meaning of Treasury RegulationsSection 1.368-1(e)(4) has, either directly or indirectly through any transaction, agreement or arrangement with any other person (including by derivative transactions, such as an equity swap, that would have the economic effect of an acquisition), redeemed or acquired, or has any plan or intention to redeem or acquire, any Parent Stock in contemplation of the Merger, or otherwise as part of a plan of which the Merger is a part. |
Additional Section 351 Representations
| 24. | It is expected, and you may assume for purposes of your opinion, that (i) stockholders of Parent that have apre-arranged plan to transfer, sell or otherwise dispose of Holdco Stock received in the Merger and (ii) shares issued as equity compensation to employees of Holdco or its subsidiaries will, taken together with the transfer of Holdco Stock described in Paragraph 25, below, represent less than 15% of the outstanding shares of Holdco Stock immediately after the Merger. |
| 25. | To the knowledge of Holdco and Parent, the Member does not have apre-arranged plan to transfer, sell or otherwise dispose of Holdco Stock received in the Contribution, except that to the knowledge of Holdco and Parent, (i) the Member intends to transfer up to 60% of the Holdco Stock to be received on the date of the Contribution to holders of certain indebtedness of the Member and (ii) Member intends to transfer the remaining amount of Holdco Stock received on the date of the Contribution to its members in proportion to their membership interests and to the holder of the ERA. |
| 26. | To the knowledge of Holdco and Parent, the stock or securities received in the Merger by a stockholder of Parent that is under the jurisdiction of a court in a title 11 or similar case within the meaning of Section 368(a)(3)(A) of the Code will not be used to satisfy the indebtedness of such stockholder. |
| 27. | No Holdco Stock is being received by the stockholders of Parent as part of a plan of liquidation of a corporation. |
| 28. | None of the Holdco Stock to be received by Parent stockholders in the Merger is “Section 306 stock” within the meaning of section 306(c)(3) of the Code. |
| 29. | There will be no indebtedness between Holdco and Parent stockholders, and there will be no indebtedness created in favor of Parent stockholders, in each case as a result of the Merger. |
| 30. | There will be no indebtedness between Holdco and the Member or its members, and there will be no indebtedness created in favor of the Member or its members, except to the extent of installment obligations of Holdco set forth in Sections 2.06 and 11.02(d) of the MTA, in each case as a result of the Contribution. |
| 31. | In connection with the Merger and the Contribution: |
| (a) | No services will be rendered to or for the benefit of Holdco by stockholders of Parent (other than stockholders of Parent who are also employees of Parent, in which case, no services shall be performed by such stockholders other than in their capacity as employees of Parent). |
A-157
| (b) | Holdco will not acquire any of its own indebtedness or any stock in another corporation (other than Parent Stock or Company Units). |
| (c) | Holdco will not transfer an interest in Parent or the Company, neither Parent nor the Company will be liquidated, neither Parent nor the Company will be merged or consolidated with any other entity (other than pursuant to the Merger and the Contribution) or converted into a partnership, and neither Parent nor the Company will transfer any assets (except in the ordinary course of business), except for dispositions made in the ordinary course of business or transfers permitted by Treasury RegulationsSection 1.368-2(k) (provided the requirements of Treasury RegulationsSection 1.368-1(d) are satisfied). |
| (d) | Parent will not be converted into a “disregarded entity” (within the meaning of Treasury RegulationSection 301.7701-3). |
| (e) | Holdco has no plan or intention (and Parent has no plan or intention to cause Holdco) to alter the terms of the Holdco Stock or any other equity interest in Holdco or to issue additional shares of Holdco Stock. |
| (f) | No licenses, leases or similar rights will be granted, nor will any property be leased back to any person, by Holdco, Parent or Company in connection with or as a result of the Merger or the Contribution. |
| (g) | None of the Holdco Stock to be received in connection with the Contribution (including Earnout Shares, if any) will be placed in escrow. |
| (h) | With respect to any Holdco Stock to be received in connection with the Contribution that will be issued after the date of the Contribution under a contingent stock arrangement: (i) such Holdco Stock will be issued within six (6) years from the date of the Contribution; (ii) there is a valid business reason for not issuing all of such Holdco Stock immediately; (iii) the issuance of such Holdco Stock will not be triggered by an event the occurrence or nonoccurrence of which is within the control of the Member; and (iv) the mechanism for the calculation of the additional Holdco Stock to be issued is objective and readily ascertainable. |
| (i) | No Holdco Stock will be issued after the Closing under a contingent stock arrangement, other than the Earnout Shares. |
| 32. | After the Merger and Contribution, Holdco will remain in existence. |
| 33. | Following the Contribution, the Member will not retain any power, right, or continuing interest in the franchises, trademarks or trade names possessed by the Company. |
| 34. | With regard to any accounts receivable or commissions due owned by the Company, following the Contribution, Holdco will report items which, but for the Contribution, would have resulted in income or deduction to the Member in a period subsequent to the Contribution and such items will constitute income or deductions to Holdco when received or paid by it, and the proceeds received in the collection of the income items will be included as ordinary income in computing the taxable income of Holdco. |
| 35. | The stockholders of Parent will not retain any rights in Parent Stock exchanged in the Merger (other than rights inherent in the ownership of Holdco Stock). |
| 36. | The Member will not retain any rights in the Company Units transferred in the Contribution (other than rights inherent in the ownership of Holdco Stock). |
| 37. | Except for expenses solely and directly related to the Merger within the guidelines set forth in Revenue Ruling73-54,1973-1 C.B. 187, (i) Holdco, Merger Sub, Parent, on the one hand, and Parent stockholders, on the other hand, will pay their respective expenses, if any, incurred in connection with the Merger, (ii) Holdco and Parent, on the one hand, and the Company and the Member, on the other hand, will pay their respective expenses, if any, incurred in connection with the Contribution; and (iii) none of Holdco, Merger Sub, or Parent has agreed to assume, or will directly or indirectly assume, any expense or other liability, whether fixed or contingent, of any Parent stockholder or the Member (other than liabilities of the Company addressed in Paragraph 39, below). |
A-158
| 38. | Following the Merger and the Contribution, Holdco and, to the knowledge of Holdco, every “significant transferor” (within the meaning of Treasury RegulationSection 1.351-3(d)(1)) (if any), will comply with the record-keeping and information filing requirements of Treasury RegulationSection 1.351-3. |
| 39. | To knowledge of Holdco and Parent, with regard to any direct or indirect liabilities of the Company as of the time of the Contribution, such liabilities were incurred in the ordinary course of business and are associated with the direct and indirect assets of the Company. |
Additional Reorganization Representations
| 40. | At the time of and immediately after the Merger, there will be no outstanding options, convertible securities, or any other type of right pursuant to which any person could acquire stock in Parent, other than employee stock-based compensation that will be converted in the Merger into stock-based compensation with respect to Holdco Stock. |
| 41. | Holdco has no plan or intention to cause or allow Parent to issue additional shares of stock or take any other action that could result in Holdco owning less than 100% of the stock of Parent. |
| 42. | At all times during the five years prior to the Merger, none of Holdco, Merger Sub or any of their respective subsidiaries have owned or will own, directly or indirectly, any stock or securities of Parent or any instrument giving the holder the right to acquire any such stock or securities. |
| 43. | Parent conducts a “historic business” within the meaning of Treasury RegulationsSection 1.368-1(d) and Holdco will continue the “historic business” of Parent or use a significant portion of Parent’s “historic business assets” in a business following the Merger (as such terms are defined in Treasury RegulationsSection 1.368-1(d)), either directly or through one or more members of its qualified group of corporations (as defined in Treasury RegulationsSection 1.368-1(d)(4)(ii)). |
| 44. | On the date of the Merger, the adjusted basis and fair market value of the assets of Holdco will exceed the sum of its liabilities, plus the amount of liabilities, if any, to which such assets are subject and the adjusted basis and fair market value of the assets of Parent will exceed the sum of its liabilities, plus the amount of liabilities, if any, to which such assets are subject. |
| 45. | Immediately after the Merger, Parent will hold (i) at least 90 percent of the fair market value of the net assets and at least 70 percent of the fair market value of the gross assets held by Parent immediately prior to the Merger and (ii) at least 90 percent of the fair market value of the net assets and at least 70 percent of the fair market value of the gross assets held by Merger Sub immediately prior to the Merger. For purposes of this representation, amounts (i) used (or to be used) by Parent or Merger Sub to pay Merger expenses, (ii) paid (or to be paid) by Parent or Merger Sub to redeem stock, securities, warrants, or options of Parent as part of any overall plan of which the Merger are a part, or (iii) distributed (or to be distributed) by Parent or Merger Sub to the holders of Parent Stock as part of any overall plan of which the Merger are a part, in each case will be treated as assets of Parent or Merger Sub, as applicable, held immediately prior to the Merger. Any dispositions of assets that were held by Parent or Merger Sub prior to the Merger that were or are made in contemplation of, or as part of any overall plan that includes, the Merger have been or will be made for fair market value, and the proceeds thereof will be retained by Parent. |
| 46. | Prior to the Merger, there will be no intercorporate indebtedness existing between Holdco or any of its subsidiaries, including Merger Sub, on the one hand, and Parent or any of its subsidiaries, on the other hand. |
[Signature Page Follows]
A-159
| Very truly yours, | ||
| [BEARS] | ||
| by |
| |
| Name: | ||
| Title: | ||
| [HOLDCO] | ||
| by |
| |
| Name: | ||
| Title: | ||
[Signature Page to Tax Representation Letter]
A-160
Exhibit H
Giants Product List
Omitted.
A-161
Exhibit I
Giants/Zyga Product List
Omitted.
A-162
Exhibit J
Working Capital Illustration
Omitted.
A-163
 | 800 Nicollet Mall, Minneapolis, MN 55402 | |||||
| Tel: 612-303-6000 | Tel: 800-333-6000 | Fax: 612-303-1036 | ||||
| Piper Jaffray & Co. Since 1895. Member SIPC and NYSE | ||||||
November 1, 2018
Board of Directors
RTI Surgical, Inc.
11621 Research Circle
Alachua, FL 32615
Members of the Board:
You have requested our opinion as to the fairness, from a financial point of view, to Bears Holding Sub, Inc. (“Holdco”) of the Aggregate Consideration (as defined below) to be paid by Holdco, a directly wholly owned subsidiary of RTI Surgical, Inc. (the “Company”), for all 100% of the equity interests of Paradigm Spine, LLC (“Target”), pursuant to the Master Transaction Agreement to be entered into by and among the Company, PS Spine Holdco, LLC (the “Member”), Holdco and Bears Merger Sub, Inc., a direct wholly owned subsidiary of Holdco (“Merger Sub”), to be dated as of November 1, 2018 (the “Agreement”).
The Agreement provides for, among other things, (1) Merger Sub to be merged with and into the Company (the “Merger”), with the Company surviving as a wholly owned direct subsidiary of Holdco and (2) the Member to contribute all 100% of the equity interests in Target to Holdco (the “Contribution” and, collectively with the Merger, the “Transaction”), pursuant to which:
| a) | each share of common stock, par value $0.001 per share, of the Company (“Company Common Stock”) issued and outstanding immediately prior to such time as the Merger and the Contribution become effective (the “Effective Time”), other than any shares of Company Stock (as defined below) held by the Company as treasury shares or by Holdco or Merger Sub immediately prior to the Effective Time (the “Company Cancelled Shares”), will be converted into one share of common stock, par value $0.001 per share, of Holdco (“Holdco Common Stock”); |
| b) | each share of Series A convertible preferred stock, par value $0.001 per share, of the Company (“Company Preferred Stock” and, collectively with the Company Common Stock, the “Company Stock”) issued and outstanding immediately prior to the Effective Time, other than any Company Cancelled Shares, will be converted into one share of Series A convertible preferred stock, par value $0.001 per share, of Holdco; and |
| c) | as consideration for the Contribution, Holdco will issue and/or deliver to the Member (i) 10,729,614 shares of Holdco Common Stock (the “Stock Consideration”), (ii) $100,000,000 in cash (the “Cash Purchase Price”) (subject to certain adjustments for net debt, working capital and transaction expenses, as to which adjustments we express no opinion), and (iii) additional shares of Holdco Common Stock (such shares, the “Earnout Shares”) (or, at Holdco’s option, make a cash payment (the aggregate of any such cash payment, together with the Earnout Shares, the “Earnout Payment”)), in each case as provided below: |
| (A) | If the net revenue for Holdco and its subsidiaries in respect of products of Target is equal to or exceeds $65,000,000 in any LTM Period (as defined in the Agreement) ending on or prior to December 31, 2020, then Holdco shall pay the Member $20,000,000 in cash, subject to certain reductions; |
| (B) | If the net revenue of Holdco and its subsidiaries in respect of products of both Target and Zyga Technology, Inc. (“Zyga”), a subsidiary of the Company (“Target/Zyga Revenue”), is greater than $85,000,000 in any LTM Period ending on or prior to December 31, 2021, then Holdco shall issue to the Member the number of shares of Holdco Common Stock equal to the sum of (i) (A) |
B-1
| 10,729,614 shares of Holdco Common Stock, multiplied by(B) the quotient of (1) (I) the Target/Zyga Revenue (but in no event greater than $96,764,705.88) for the applicable LTM Period, minus (II) $85,000,000, divided by (2) $11,764,705.88 and (ii) the greater of (A) zero shares of Holdco Common Stock and (B) (1) the number of shares of Holdco Common Stock with an aggregate value of $35,000,000 based on the volume weighted average closing trading price (“VWAP”) of the Holdco Common Stock as described in the Agreement, multiplied by (2) the quotient of (I) (a) the Target/Zyga Revenue (but in no event greater than $105,000,000) for the applicable LTM Period, minus (b) $96,764,705.88, divided by(II) $8,235,294.12, subject to certain reductions (less the total number of Earnout Shares that have previously been issued to the Member pursuant to this paragraph (B)); |
| (C) | If the Target/Zyga Revenue in any LTM Period ending on or prior to December 31, 2022 is equal to or greater than $105,000,000, then Holdco shall, at its option, either (i) issue to the Member the (A) number of shares of Holdco Common Stock with an aggregate value of $45,000,000 based on the VWAP of the Holdco Common Stock as described in the Agreement, multiplied by (B) the quotient of (1) (I) the Target/Zyga Revenue (but in no event greater than $125,000,000) for the applicable LTM Period, minus (II) $105,000,000, divided by (2) $20,000,000, subject to certain reductions, or (ii) pay the Member the amount in cash equal to (A) $45,000,000, multiplied by (B) the quotient of (1) (I) the Target/Zyga Revenue (but in no event greater than $125,000,000) for the applicable LTM Period, minus (II) $105,000,000, divided by (2) $20,000,000, subject to certain reductions; and |
| (D) | In the event of a Change of Control Transaction (as defined in the Agreement), Holdco shall pay the Member the maximum Earnout Payment payable to the Member, based on the applicable time of consummation of such Change of Control Transaction in respect of the Earnout Payments described above. |
The aggregate of the Stock Consideration, the Cash Purchase Price and the Earnout Payment, all as more fully set forth in the Agreement, is referred to herein as “Aggregate Consideration”. For purposes of our analyses, we have (i) analyzed the estimated present value of the Aggregate Consideration based on the estimated present value of Earnout Payments as reflected in the financial forecasts furnished to us by the Company, which forecasts contemplate payment of the maximum possible amount under the Earnout Payments payment calculation mechanics, but which do not reflect the earliest possible payment of such Earnout Payments, and (ii) assumed that there is no Change of Control Transaction that affects the timing of any such Earnout Payments.
In connection with our review of the Transaction, and in arriving at our opinion, we have: (i) reviewed and analyzed the financial terms of a draft received on November 1, 2018 of the Agreement; (ii) reviewed and analyzed certain information, including financial forecasts, relating to the business, earnings, cash flow, assets, liabilities and prospects of each of the Company (including revenue projections relating to Zyga) and Target that were furnished to us by the Company, which included the analyses and forecasts of certain cost savings and operating efficiencies expected by management of the Company and Target to result from the Transaction (the “Synergies”); (iii) conducted discussions with members of senior management and representatives of the Company and Target concerning the matters described in clause (ii) above, as well as the business of Target, Zyga and their respective prospects before and after giving effect to the Transaction and the Synergies; (iv) compared the financial performance of Target with that of certainpublicly-traded companies that we deemed relevant; and (v) reviewed the financial terms, of certain business combination transactions that we deemed relevant. In addition, we have conducted such other analyses, examinations and inquiries and considered such other financial, economic and market criteria as we have deemed necessary in arriving at our opinion.
We have relied upon and assumed, without assuming liability or responsibility for independent verification, the accuracy and completeness of all information that was publicly available or was furnished, or otherwise made available, to us or discussed with or reviewed by us. We have further relied upon the assurances of the management of the Company that the financial information provided has been prepared on a reasonable basis in
B-2
accordance with industry practice, and that they are not aware of any information or facts that would make any information provided to us incomplete or misleading. Without limiting the generality of the foregoing, for the purpose of this opinion, we have assumed that with respect to financial forecasts, estimates and other forward-looking information (including the Synergies) reviewed by us, that such information has been reasonably prepared based on assumptions reflecting the best currently available estimates and judgments of the management of the Company as to the expected future revenue of Zyga and future results of operations and financial condition of Target. We express no opinion as to any such financial forecasts, estimates or forward-looking information (including the Synergies) or the assumptions on which they were based. We have relied, with your consent, on advice of the outside counsel and the independent accountants to the Company and on the assumptions of the management of the Company as to all accounting, legal, tax and financial reporting matters with respect to each of Holdco, the Company, Target and the Agreement.
In arriving at our opinion, we have assumed that the executed Agreement will be in all material respects identical to the last draft reviewed by us. We have relied upon and assumed, without independent verification, that (i) the representations and warranties of all parties to the Agreement and all other related documents and instruments that are referred to therein are true and correct, (ii) each party to such agreements will fully and timely perform all of the covenants and agreements required to be performed by such party, (iii) the Transaction will be consummated pursuant to the terms of the Agreement without amendments thereto and (iv) all conditions to the consummation of the Transaction will be satisfied without waiver by any party of any conditions or obligations thereunder and (v) that any adjustments to any of the components of the Aggregate Consideration will not result in any adjustment to the Aggregate Consideration that is material to our analysis. Additionally, we have assumed that all the necessary regulatory approvals and consents required for the Transaction will be obtained in a manner that will not adversely affect any of Holdco, the Company, Target or the contemplated benefits of the Transaction.
In arriving at our opinion, we have not performed any appraisals or valuations of any specific assets or liabilities (fixed, contingent or other) of any of Holdco, the Company or Target, and have not been furnished or provided with any such appraisals or valuations, nor have we evaluated the solvency of any of Holdco, the Company or Target under any state or federal law relating to bankruptcy, insolvency or similar matters. The analyses performed by us in connection with this opinion were going concern analyses. We express no opinion regarding the liquidation value of any of Holdco, the Company, Target or any other entity. Without limiting the generality of the foregoing, we have undertaken no independent analysis of any pending or threatened litigation, regulatory action, possible unasserted claims or other contingent liabilities, to which any of Holdco, the Company, Target or any of their affiliates is a party or may be subject, and at the direction of the Company and with its consent, our opinion makes no assumption concerning, and therefore does not consider, the possible assertion of claims, outcomes or damages arising out of any such matters. We have also assumed that none of Holdco, the Company nor Target is party to any material pending transaction, including without limitation any financing, recapitalization, acquisition or merger, divestiture orspin-off, other than the Transaction.
No company or transaction used in any analysis for purposes of comparison is identical to any of Holdco, the Company or Target or the Transaction. Accordingly, an analysis of the results of the comparisons is not mathematical; rather, it involves complex considerations and judgments about differences in the companies and transactions to which each of Holdco, the Company and Target and the Transaction were compared and other factors that could affect the public trading value or transaction value of the companies.
This opinion is necessarily based upon the information available to us and facts and circumstances as they exist and are subject to evaluation on the date hereof; events occurring after the date hereof could materially affect the assumptions used in preparing this opinion. We are not expressing any opinion herein as to the price at which shares of Company Common Stock or Holdco Common Stock may trade following announcement of the Transaction or at any future time. We have not undertaken to reaffirm or revise this opinion or otherwise comment upon any events occurring after the date hereof and do not have any obligation to update, revise or reaffirm this opinion.
B-3
We have been engaged by the Company to act as its financial advisor in connection with the Transaction and we will receive a fee from the Company for providing our services, a significant portion of which is contingent upon the consummation of the Transaction. We will also receive a fee for rendering this opinion. Our opinion fee is not contingent upon the consummation of the Transaction or the conclusions reached in our opinion. The Company has also agreed to indemnify us against certain liabilities and reimburse us for certain expenses in connection with our services. We are currently engaged as debt placement agent for a portfolio company of Viscogliosi Brothers, LLC, a stockholder of Target, and have, in the past three years, provided financial advisory and financing services to other portfolio companies of such stockholder of Target, including serving as sole and exclusive arranger and placement agent for a debt and equity financing of Centinel Spine, Inc. completed in 2017, and may continue to do so and have received, and may receive, fees for the rendering of such services. In addition, in the ordinary course of our business, we and our affiliates may actively trade securities of the Company for our own account or the account of our customers and, accordingly, may at any time hold a long or short position in such securities. We may also, in the future, provide investment banking and financial advisory services to Holdco, the Company, Target or entities that are affiliated with Holdco, the Company or Target, for which we would expect to receive compensation.
This opinion is provided solely to the Board of Directors of the Company in connection with its consideration of the Transaction and is not intended to be and does not constitute a recommendation to any stockholder of the Company as to how such stockholder should act or vote with respect to the Transaction or any other matter. Except with respect to the use of this opinion in connection with the joint proxy and consent solicitation statement/prospectus relating to the Transaction in accordance with our engagement letter with the Company, this opinion shall not be disclosed, referred to, published or otherwise used (in whole or in part), nor shall any public references to us be made, without our prior written approval. This opinion has been approved for issuance by the Piper Jaffray Opinion Committee.
This opinion addresses solely the fairness, from a financial point of view, to Holdco of the proposed Aggregate Consideration set forth in the Agreement and does not address any other terms or agreement relating to the Transaction or any other terms of the Agreement. We were not requested to opine as to, and this opinion does not address: (i) the basic business decision to proceed with or effect the Transaction; (ii) the merits of the Transaction relative to any alternative transaction or business strategy that may be available to the Company; (iii) any other terms contemplated by the Agreement or the fairness of the Transaction to, or any consideration received in connection therewith by, any creditor or other constituency of the Company: or (iv) the solvency or financial viability of any of Holdco, the Company or Target at the date hereof, upon consummation of the Transaction, or at any future time. Furthermore, we express no opinion with respect to the amount or nature of compensation to any officer, director or employee of any party to the Transaction, or any class of such persons, relative to the Aggregate Consideration to be paid by Holdco in the Transaction or with respect to the fairness of any such compensation.
Based upon and subject to the foregoing and based upon such other factors as we consider relevant, it is our opinion that the Aggregate Consideration to be paid by Holdco is fair, from a financial point of view, to Holdco as of the date hereof.
Sincerely,

PIPER JAFFRAY & CO.
B-4