Portugal, Romania, Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland/LI, The Netherlands, Turkey and the United Kingdom), Australia, Canada, Hong Kong, India and Japan. The European patent in this family (European Patent No. 3218504) was opposed by a third party in April 2021. The opposition was dismissed, and the patent was maintained as granted by the European Patent Office (“EPO”) in an oral proceeding on May 9, 2023. This patent family also includes pending applications in the United States, Europe, Australia, Japan, China and Hong Kong. The granted United States patent from the first patent family related to our replicating technology is expected to expire in April 2037 due to patent term adjustment. The granted patents in Europe, Australia, Canada, Hong Kong, India and Japan, and the pending applications are expected to expire in 2035, not giving effect to any potential patent term extensions or patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees. The second patent family in our replicating platform portfolio is jointly owned by us and the University of Basel. The rights of the University of Basel under this patent family are exclusively licensed to us. This second patent family includes patents granted in Australia, China, Hong Kong, Japan, Macao, Eurasia, India, Israel, Japan, Korea, Mexico, and Singapore. This patent family also includes pending applications in various countries, including in the United States, Europe, Eurasia, Hong Kong, Korea, China, Australia, New Zealand, Mexico, Japan, Brazil, Singapore, India, and Israel. The granted patents and the pending applications are expected to expire in 2037, not giving effect to any potential patent term extensions or patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees. Our replicating technology is being employed or may be employed in one or more of the product candidates or programs described herein.
Oncology Technology Portfolio
For the application of our non-replicating and replicating technologies in oncology, we own three patent families relating to potential clinical uses of our product candidates, such as combination treatments. One of the patent families includes a patent granted in the United States and patents granted in Europe (validated in Albania, Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Macedonia, Malta, Monaco, Norway, Poland, Portugal, Romania, Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland/LI, The Netherlands, Turkey and the United Kingdom), Hong Kong, Australia, China, Macao, India and Japan. This patent family also includes pending applications in the United States, Europe, Canada, India and Hong Kong. A second patent family includes a patent granted in China and pending applications in the United States, Europe, Australia, and Hong Kong. The third patent family includes pending applications in the United States and Europe. The granted patents and pending applications within the three patent families are expected to expire between 2036 and 2043, not giving effect to any potential patent term extensions or patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
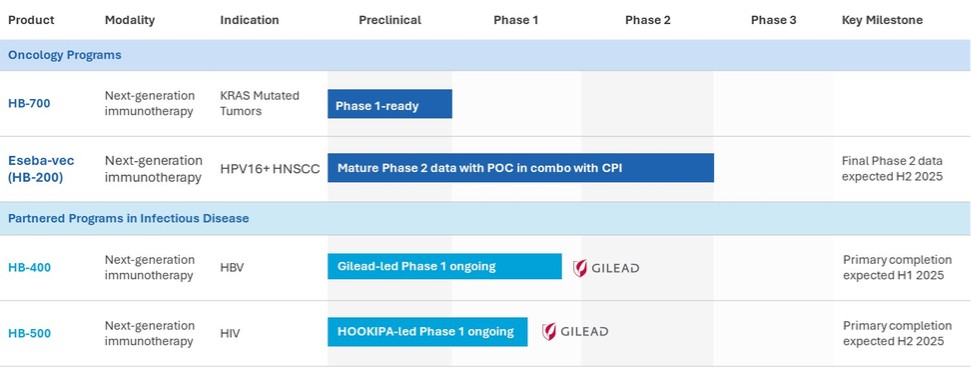
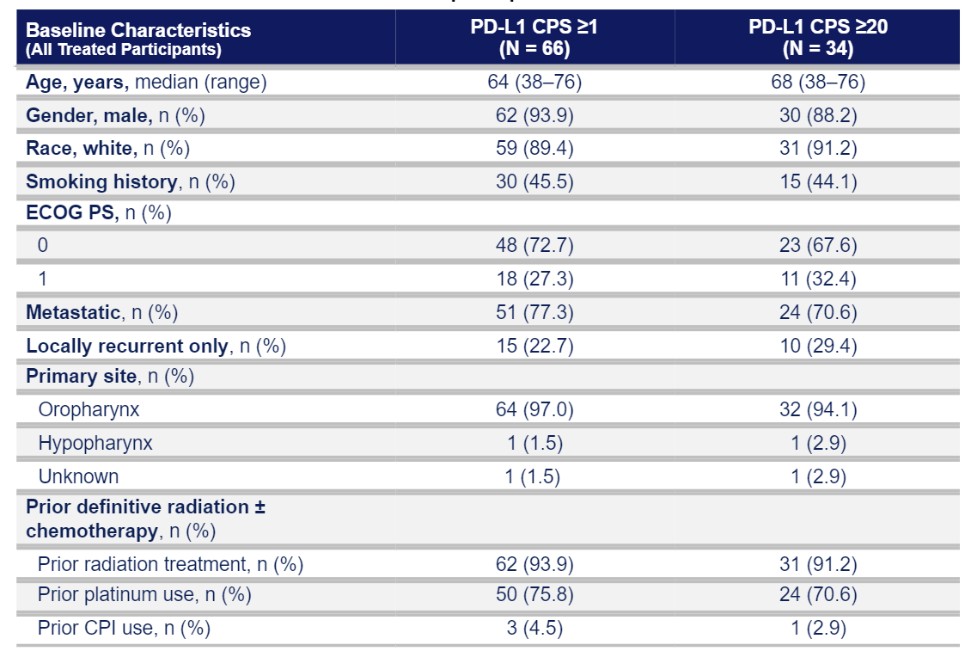
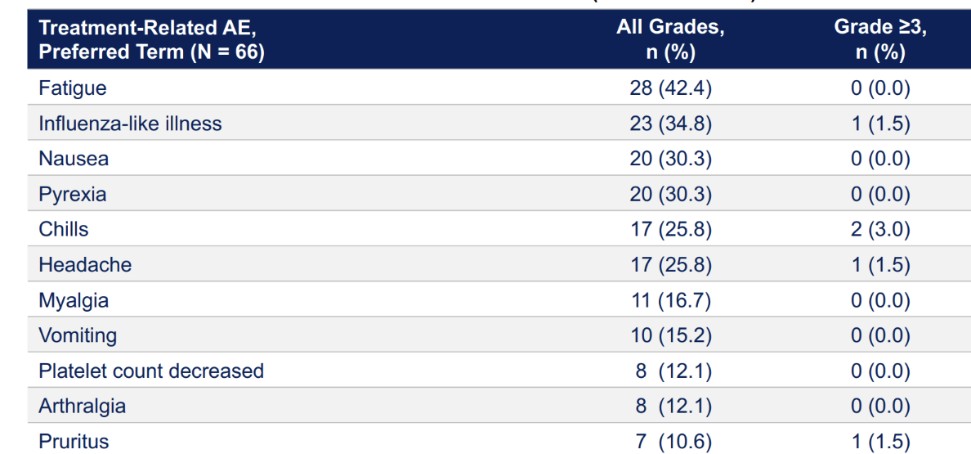
Eseba-vec (HPV16+ Head and Neck Squamous Cell Carcinoma)
HB-201 and HB-202, the two components of our eseba-vec product candidate, rely on our replicating technology and, depending on their clinical implementation, may relate to one or more applications in our oncology patent portfolio. In addition to the replicating and oncology patent portfolios, we own two patent families that relate more specifically to our HB-200 product candidate. The first patent family includes two patents granted in the United States and patents granted in Australia, China, Macao, India and Japan with claims directed to compositions of matter. This patent family also includes pending applications in the United States, Europe, Australia, Canada, China, India, Japan and Hong Kong. The second patent family includes pending applications in the United States, Europe, Eurasia, Australia, Brazil, Canada, China, Costa Rica, Hong Kong, India, Israel, Japan, Korea, Mexico, New Zealand, Peru, Singapore, South Africa relating to HB-200 treatment regimens. Excluding the replicating and oncology patent portfolios, the granted patents and pending applications specifically related to our HB-200 product candidate are expected to expire in 2036 and 2041, respectively, not giving effect to any potential patent term extensions or patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
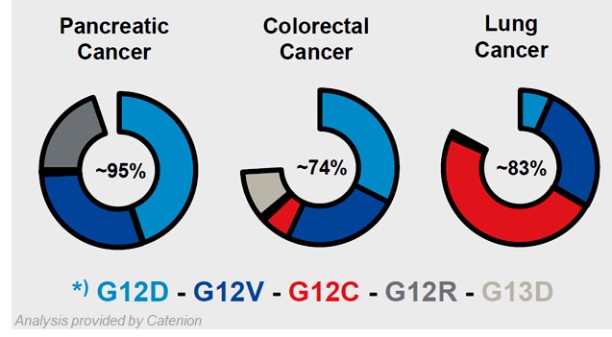
HB-700 (KRAS Mutated Tumors)
Our HB-700 product candidate relies on our replicating technology and, depending on its clinical implementation, may relate to one or more applications in our oncology patent portfolio. In addition to the replicating