- ERAS Dashboard
- Financials
- Filings
-
Holdings
-
Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
8-K Filing
Erasca (ERAS) 8-KRegulation FD Disclosure
Filed: 13 Jan 25, 9:10am

On a Journey to Erase Cancer Erasca Corporate Presentation January 2025 Eric N., naporafenib clinical trial participant and cancer survivor, and his wife Margaret Exhibit 99.1

We caution you that this presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements regarding our future results of operations and financial position, business strategy, research and development plans, the anticipated timing (including the timing of initiation and the timing of data readouts), costs, design and conduct of our ongoing and planned preclinical studies and clinical trials for our product candidates, the potential therapeutic benefits of our product candidates, our intellectual property protection, the timing and likelihood of success of our plans and objectives, the impact of the deprioritization of certain programs, and future results of anticipated product development efforts, are forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar expressions. The inclusion of forward-looking statements should not be regarded as a representation by us that any of our plans will be achieved. Actual results may differ from those set forth in this presentation due to the risks and uncertainties inherent in our business, including, without limitation: our approach to the discovery and development of product candidates based on our singular focus on shutting down the RAS/MAPK pathway, a novel and unproven approach; we only have one product candidate in clinical development and all of our other development efforts are in the preclinical stage; our assumptions about ERAS-0015's or ERAS-4001's development potential are based in large part on the preclinical data generated by the licensors and we may observe materially and adversely different results as we conduct our planned studies and trials; our assumptions around which programs may have a higher probability of success may not be accurate, and we may expend our limited resources to pursue a particular product candidate and/or indication and fail to capitalize on product candidates or indications with greater development or commercial potential; the analysis of pooled Phase 1 and Phase 2 naporafenib plus trametinib data covers two clinical trials with different designs and inclusion criteria, which cannot be directly compared, and therefore may not be a reliable indicator of ORR, mPFS, or mOS data; due to differences between trial designs and subject characteristics, comparing data across different trials may not be a reliable indicator of data; preliminary results of clinical trials are not necessarily indicative of final results and one or more of the clinical outcomes may materially change as patient enrollment continues, following more comprehensive reviews of the data and more patient data become available, including the risk that an uPR to treatment may not ultimately result in a cPR to treatment after follow-up evaluations; our SEACRAFT trials may not support the registration of naporafenib; later developments with the FDA or EU health authorities may be inconsistent with the feedback received to date regarding our development plans and trial designs; Fast Track Designation may not lead to a faster development or regulatory review or approval process, and does not increase the likelihood that our product candidates will receive marketing approval; potential delays in the commencement, enrollment, data readout, and completion of clinical trials and preclinical studies; our dependence on third parties in connection with manufacturing, research, and preclinical and clinical testing; unexpected adverse side effects or inadequate efficacy of our product candidates that may limit their development, regulatory approval, and/or commercialization, or may result in recalls or product liability claims; unfavorable results from preclinical studies or clinical trials; results from preclinical studies or early clinical trials not necessarily being predictive of future results; the inability to realize any benefits from our current licenses, acquisitions, or collaborations, and any future licenses, acquisitions, or collaborations, and our ability to fulfill our obligations under such arrangements; regulatory developments in the United States and foreign countries; our ability to obtain and maintain intellectual property protection for our product candidates and maintain our rights under intellectual property licenses; our ability to fund our operating plans with our current cash, cash equivalents, and marketable securities into the second half of 2027; and other risks described in our prior filings with the Securities and Exchange Commission (SEC), including under the heading “Risk Factors” in our annual report on Form 10-K for the year ended December 31, 2023, and any subsequent filings with the SEC. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and we undertake no obligation to update such statements to reflect events that occur or circumstances that exist after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, which is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us. Disclaimer: Forward Looking Statements & Market Data
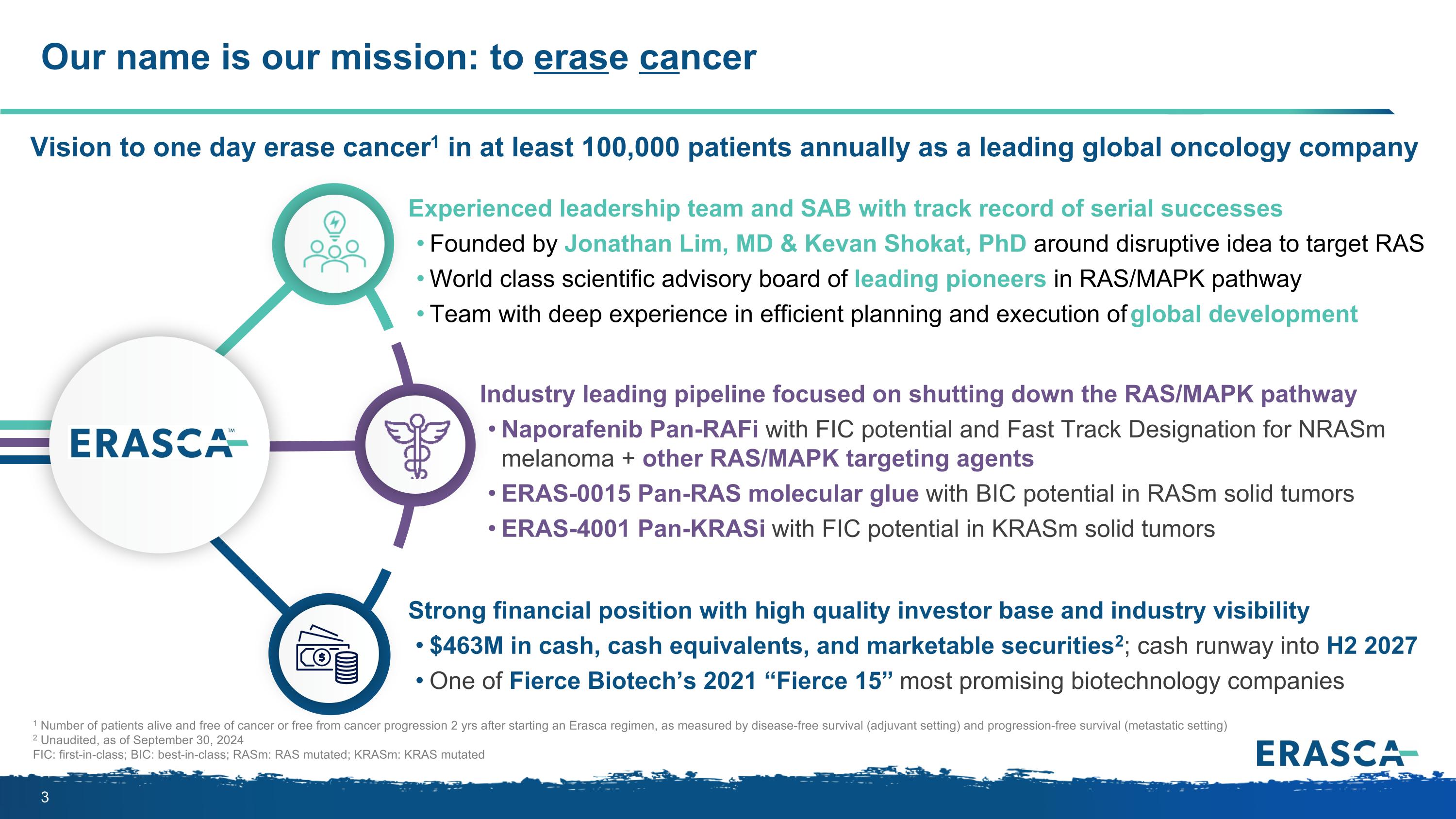
Our name is our mission: to erase cancer 1 Number of patients alive and free of cancer or free from cancer progression 2 yrs after starting an Erasca regimen, as measured by disease-free survival (adjuvant setting) and progression-free survival (metastatic setting) 2 Unaudited, as of September 30, 2024 FIC: first-in-class; BIC: best-in-class; RASm: RAS mutated; KRASm: KRAS mutated Vision to one day erase cancer1 in at least 100,000 patients annually as a leading global oncology company Industry leading pipeline focused on shutting down the RAS/MAPK pathway Naporafenib Pan-RAFi with FIC potential and Fast Track Designation for NRASm melanoma + other RAS/MAPK targeting agents ERAS-0015 Pan-RAS molecular glue with BIC potential in RASm solid tumors ERAS-4001 Pan-KRASi with FIC potential in KRASm solid tumors Experienced leadership team and SAB with track record of serial successes Founded by Jonathan Lim, MD & Kevan Shokat, PhD around disruptive idea to target RAS World class scientific advisory board of leading pioneers in RAS/MAPK pathway Team with deep experience in efficient planning and execution of global development Strong financial position with high quality investor base and industry visibility $463M in cash, cash equivalents, and marketable securities2; cash runway into H2 2027 One of Fierce Biotech’s 2021 “Fierce 15” most promising biotechnology companies
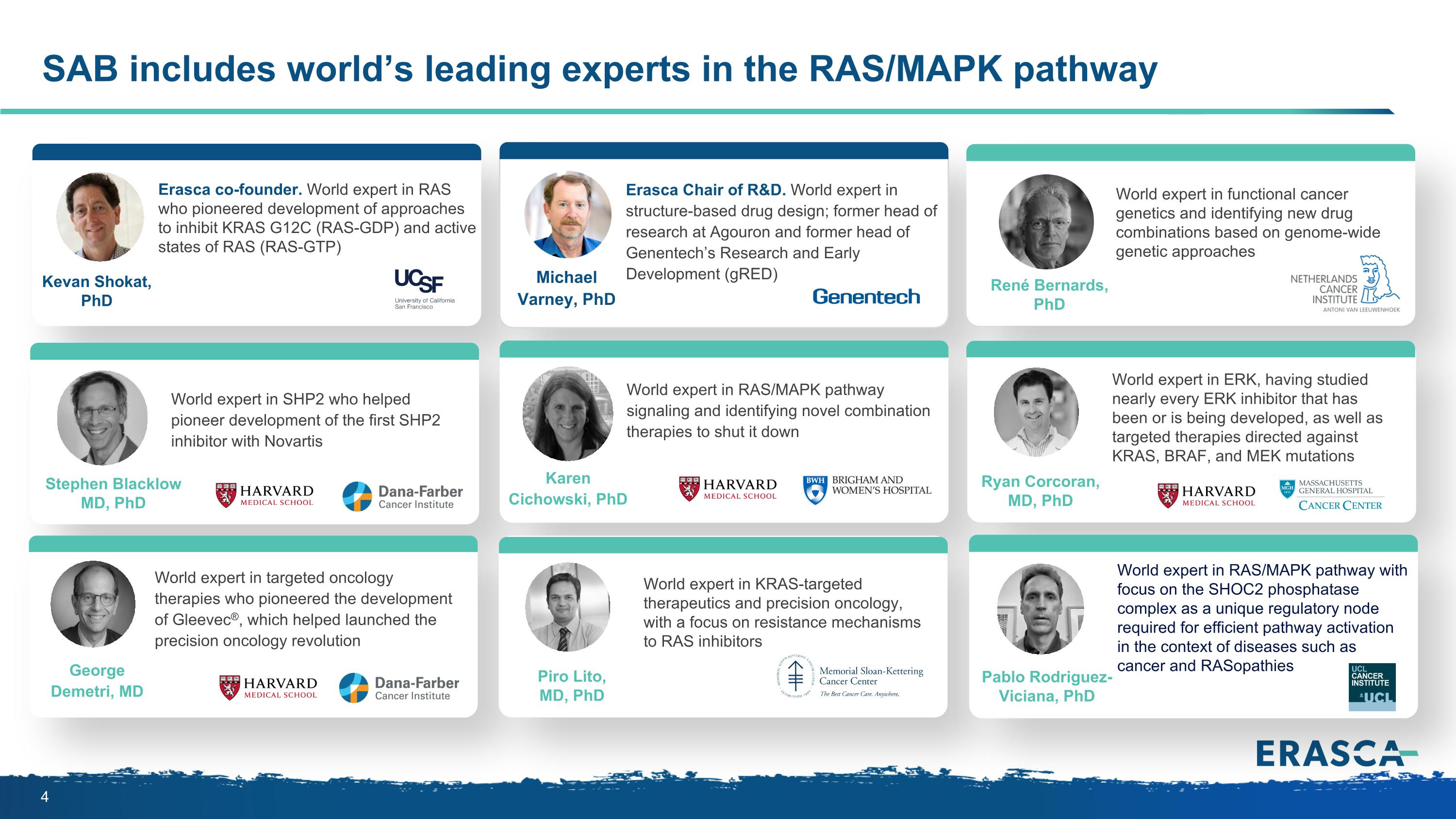
SAB includes world’s leading experts in the RAS/MAPK pathway Erasca co-founder. World expert in RAS who pioneered development of approaches to inhibit KRAS G12C (RAS-GDP) and active states of RAS (RAS-GTP) Kevan Shokat, PhD Erasca Chair of R&D. World expert in structure-based drug design; former head of research at Agouron and former head of Genentech’s Research and Early Development (gRED) Michael Varney, PhD Stephen Blacklow MD, PhD World expert in SHP2 who helped pioneer development of the first SHP2 inhibitor with Novartis René Bernards, PhD World expert in functional cancer genetics and identifying new drug combinations based on genome-wide genetic approaches World expert in RAS/MAPK pathway signaling and identifying novel combination therapies to shut it down Karen Cichowski, PhD World expert in ERK, having studied nearly every ERK inhibitor that has been or is being developed, as well as targeted therapies directed against KRAS, BRAF, and MEK mutations Ryan Corcoran, MD, PhD World expert in targeted oncology therapies who pioneered the development of Gleevec®, which helped launched the precision oncology revolution George Demetri, MD World expert in KRAS-targeted therapeutics and precision oncology, with a focus on resistance mechanisms to RAS inhibitors Piro Lito, MD, PhD World expert in RAS/MAPK pathway with focus on the SHOC2 phosphatase complex as a unique regulatory node required for efficient pathway activation in the context of diseases such as cancer and RASopathies Pablo Rodriguez-Viciana, PhD
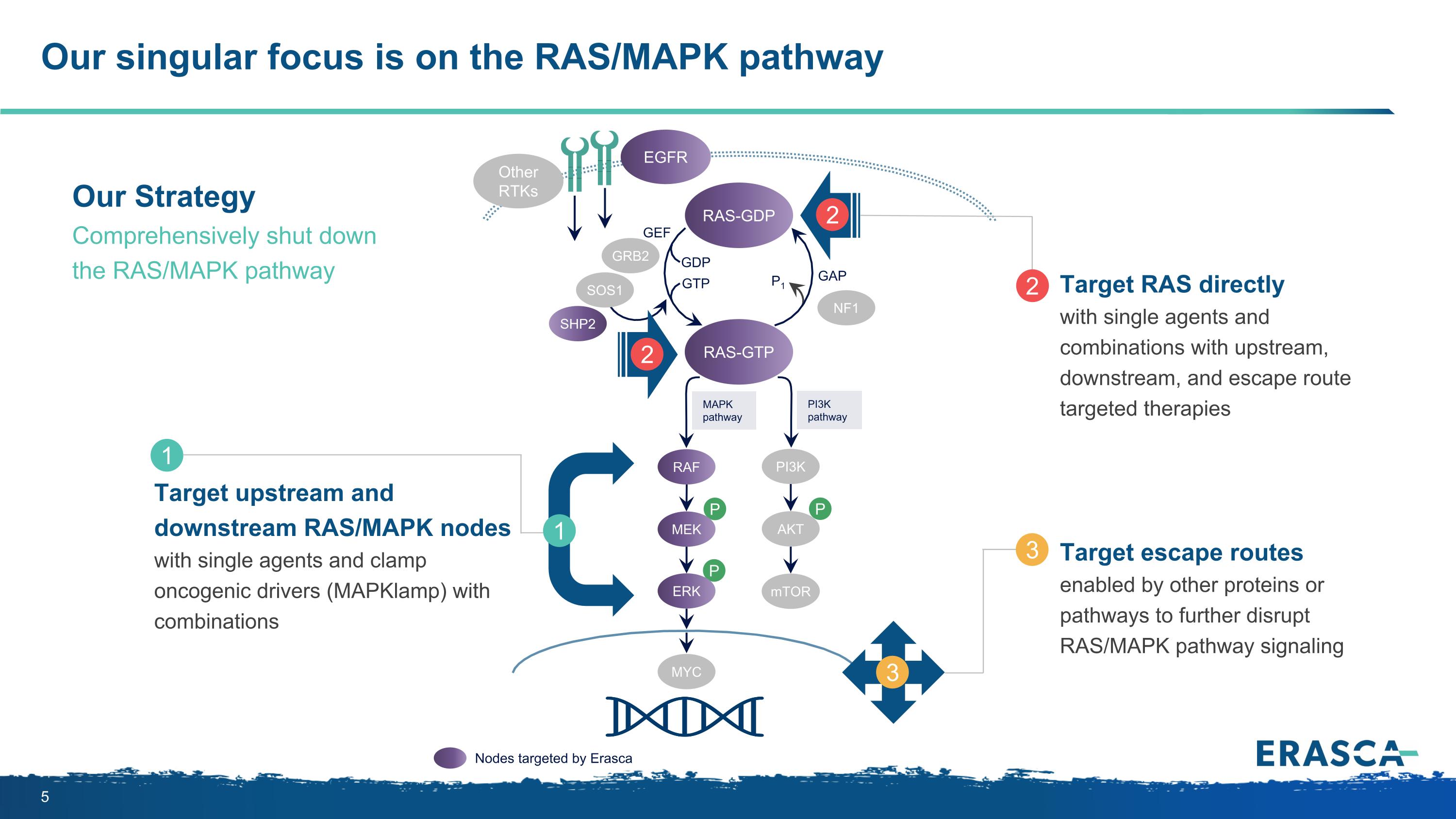
Our singular focus is on the RAS/MAPK pathway P P P MAPK pathway PI3K pathway RAS-GTP GRB2 SHP2 GAP GDP GTP P1 NF1 SOS1 RAF MEK PI3K AKT mTOR ERK RAS-GDP EGFR Other RTKs MYC GEF Nodes targeted by Erasca Target upstream and downstream RAS/MAPK nodes with single agents and clamp oncogenic drivers (MAPKlamp) with combinations 1 1 Target RAS directly with single agents and combinations with upstream, downstream, and escape route targeted therapies 2 2 2 Target escape routes enabled by other proteins or pathways to further disrupt RAS/MAPK pathway signaling 3 3 Our Strategy Comprehensively shut down the RAS/MAPK pathway
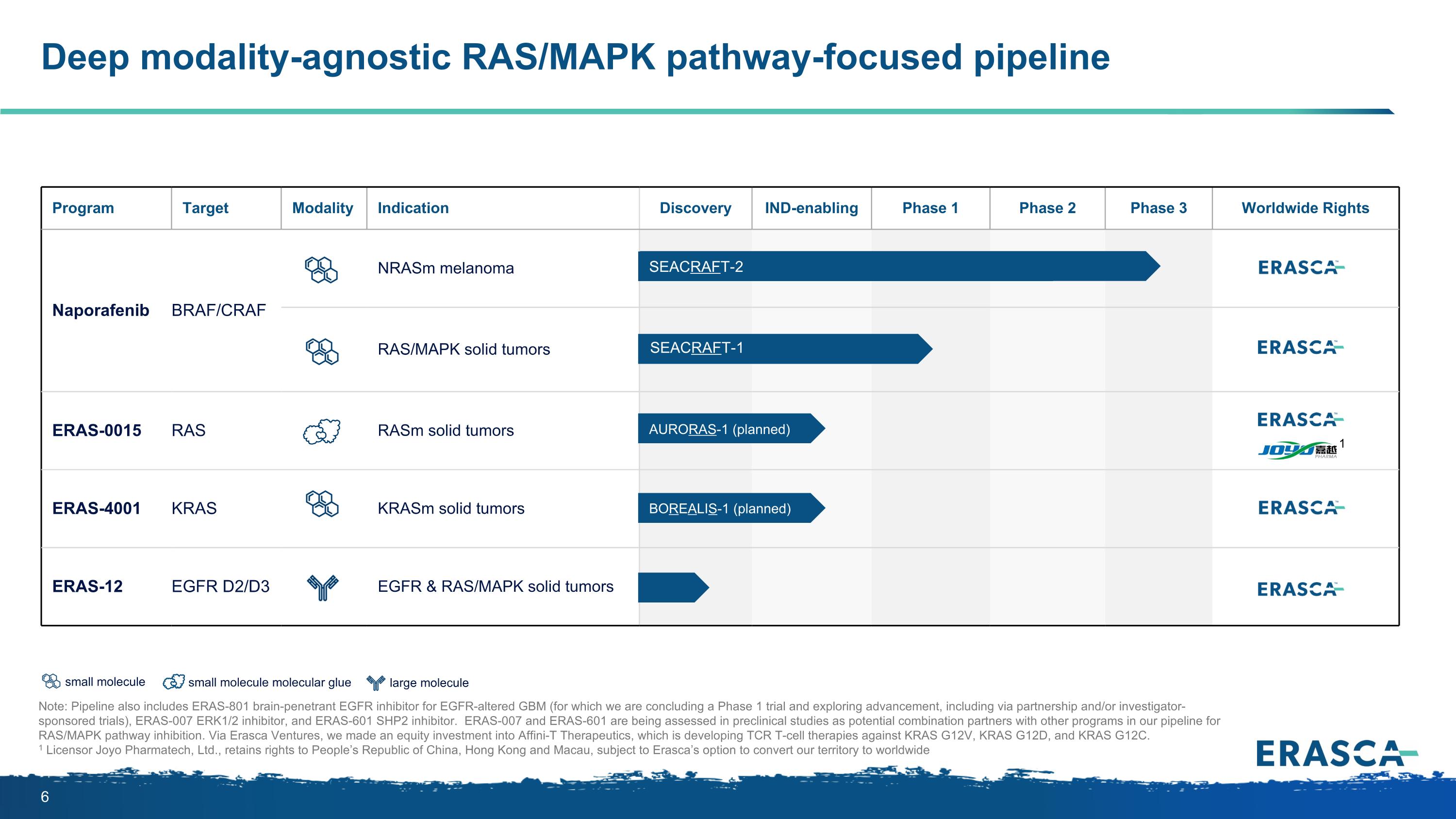
large molecule small molecule Deep modality-agnostic RAS/MAPK pathway-focused pipeline Note: Pipeline also includes ERAS-801 brain-penetrant EGFR inhibitor for EGFR-altered GBM (for which we are concluding a Phase 1 trial and exploring advancement, including via partnership and/or investigator-sponsored trials), ERAS-007 ERK1/2 inhibitor, and ERAS-601 SHP2 inhibitor. ERAS-007 and ERAS-601 are being assessed in preclinical studies as potential combination partners with other programs in our pipeline for RAS/MAPK pathway inhibition. Via Erasca Ventures, we made an equity investment into Affini-T Therapeutics, which is developing TCR T-cell therapies against KRAS G12V, KRAS G12D, and KRAS G12C. 1 Licensor Joyo Pharmatech, Ltd., retains rights to People’s Republic of China, Hong Kong and Macau, subject to Erasca’s option to convert our territory to worldwide Program Target Modality Indication Discovery IND-enabling Phase 1 Phase 2 Phase 3 Worldwide Rights Naporafenib BRAF/CRAF NRASm melanoma RAS/MAPK solid tumors ERAS-0015 RAS RASm solid tumors ERAS-4001 KRAS KRASm solid tumors ERAS-12 EGFR D2/D3 EGFR & RAS/MAPK solid tumors SEACRAFT-1 SEACRAFT-2 AURORAS-1 (planned) BOREALIS-1 (planned) 1 small molecule molecular glue
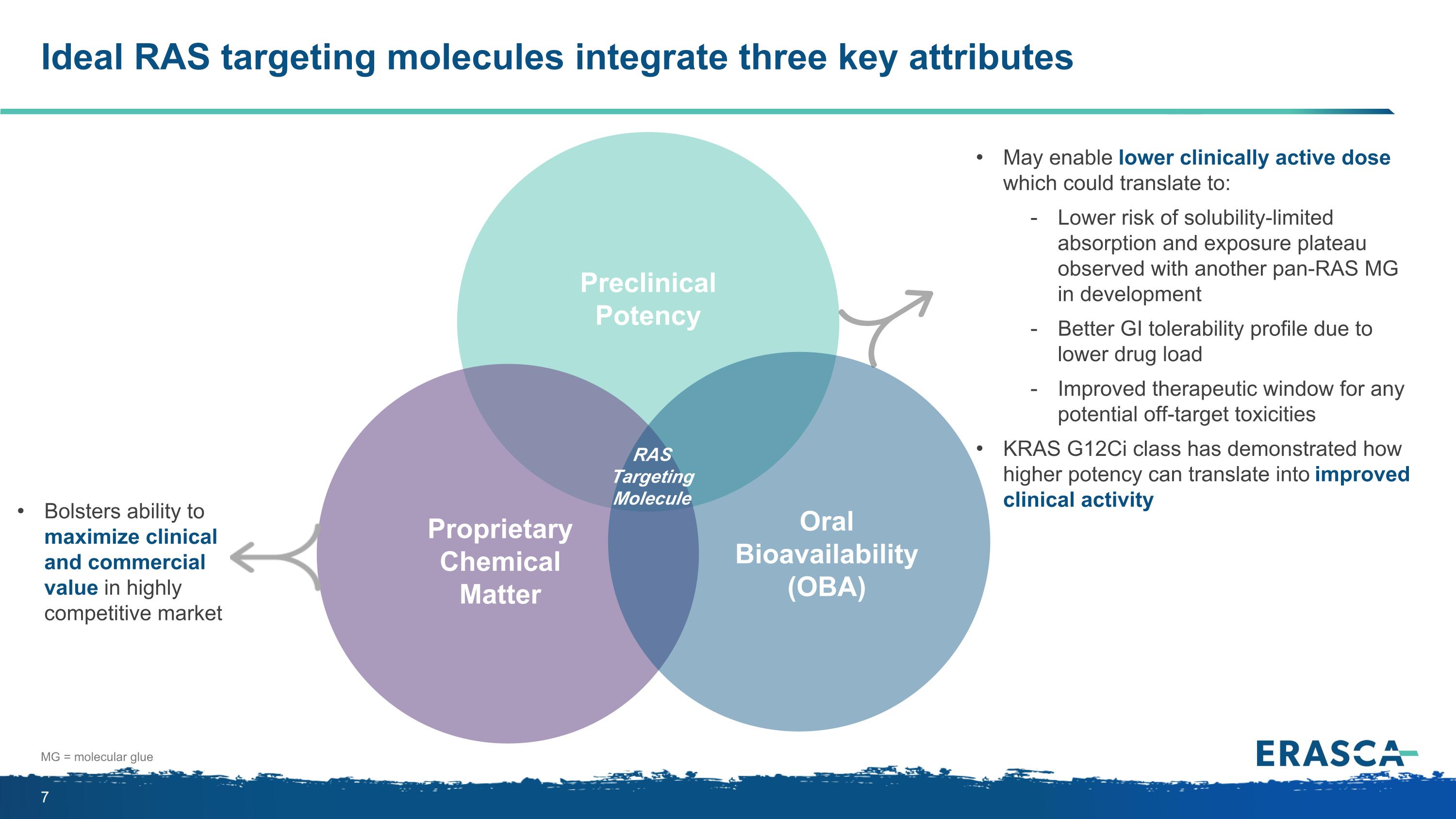
Ideal RAS targeting molecules integrate three key attributes Proprietary Chemical Matter Oral Bioavailability (OBA) Preclinical Potency RAS Targeting Molecule Bolsters ability to maximize clinical and commercial value in highly competitive market May enable lower clinically active dose which could translate to: Lower risk of solubility-limited absorption and exposure plateau observed with another pan-RAS MG in development Better GI tolerability profile due to lower drug load Improved therapeutic window for any potential off-target toxicities KRAS G12Ci class has demonstrated how higher potency can translate into improved clinical activity MG = molecular glue
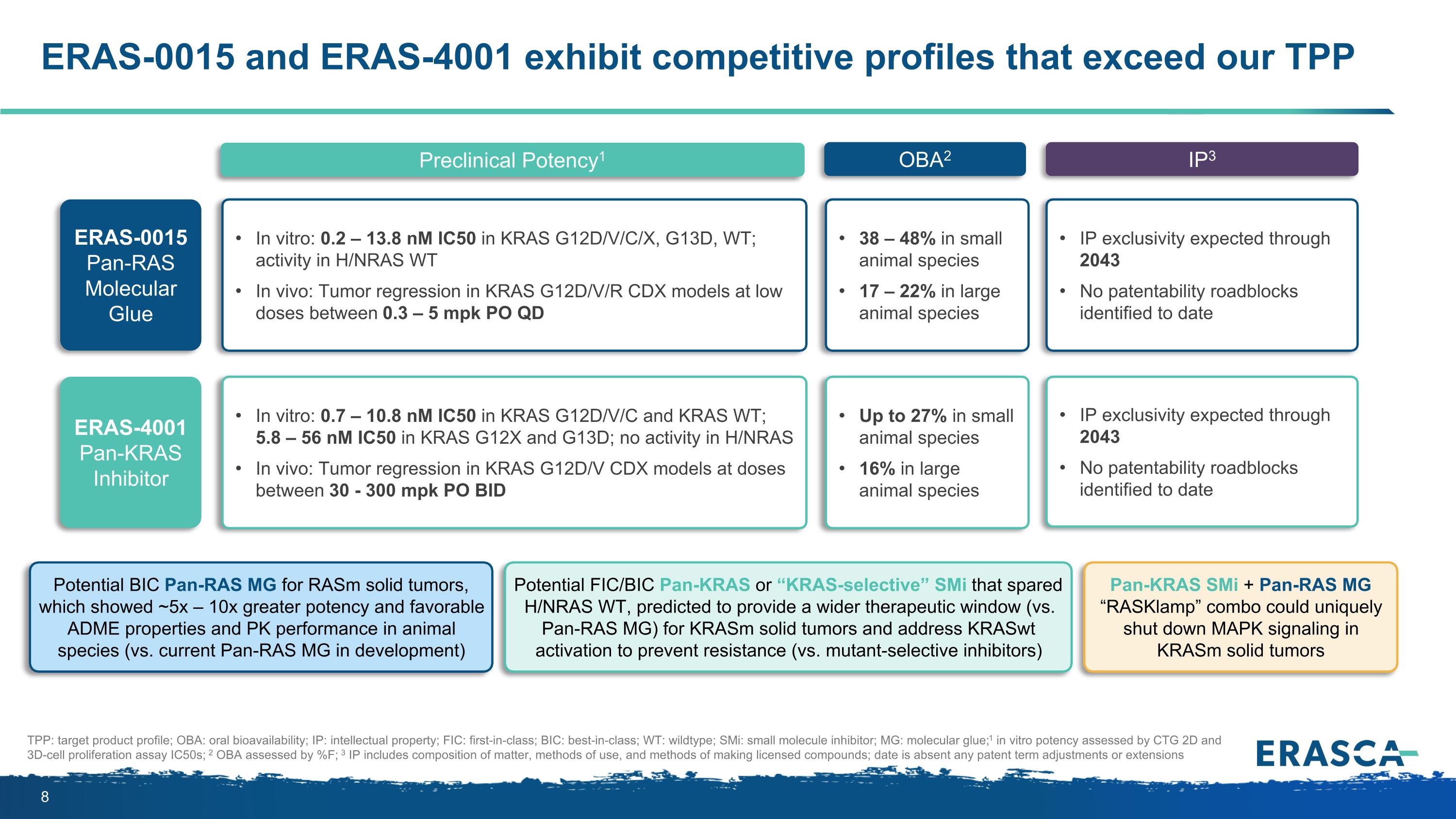
ERAS-0015 and ERAS-4001 exhibit competitive profiles that exceed our TPP Preclinical Potency1 OBA2 IP3 TPP: target product profile; OBA: oral bioavailability; IP: intellectual property; FIC: first-in-class; BIC: best-in-class; WT: wildtype; SMi: small molecule inhibitor; MG: molecular glue; 1 in vitro potency assessed by CTG 2D and 3D-cell proliferation assay IC50s; 2 OBA assessed by %F; 3 IP includes composition of matter, methods of use, and methods of making licensed compounds; date is absent any patent term adjustments or extensions ERAS-0015 Pan-RAS Molecular Glue In vitro: 0.2 – 13.8 nM IC50 in KRAS G12D/V/C/X, G13D, WT; activity in H/NRAS WT In vivo: Tumor regression in KRAS G12D/V/R CDX models at low doses between 0.3 – 5 mpk PO QD 38 – 48% in small animal species 17 – 22% in large animal species IP exclusivity expected through 2043 No patentability roadblocks identified to date Potential BIC Pan-RAS MG for RASm solid tumors, which showed ~5x – 10x greater potency and favorable ADME properties and PK performance in animal species (vs. current Pan-RAS MG in development) ERAS-4001 Pan-KRAS Inhibitor Up to 27% in small animal species 16% in large animal species IP exclusivity expected through 2043 No patentability roadblocks identified to date Pan-KRAS SMi + Pan-RAS MG “RASKlamp” combo could uniquely shut down MAPK signaling in KRASm solid tumors Potential FIC/BIC Pan-KRAS or “KRAS-selective” SMi that spared H/NRAS WT, predicted to provide a wider therapeutic window (vs. Pan-RAS MG) for KRASm solid tumors and address KRASwt activation to prevent resistance (vs. mutant-selective inhibitors) In vitro: 0.7 – 10.8 nM IC50 in KRAS G12D/V/C and KRAS WT; 5.8 – 56 nM IC50 in KRAS G12X and G13D; no activity in H/NRAS In vivo: Tumor regression in KRAS G12D/V CDX models at doses between 30 - 300 mpk PO BID
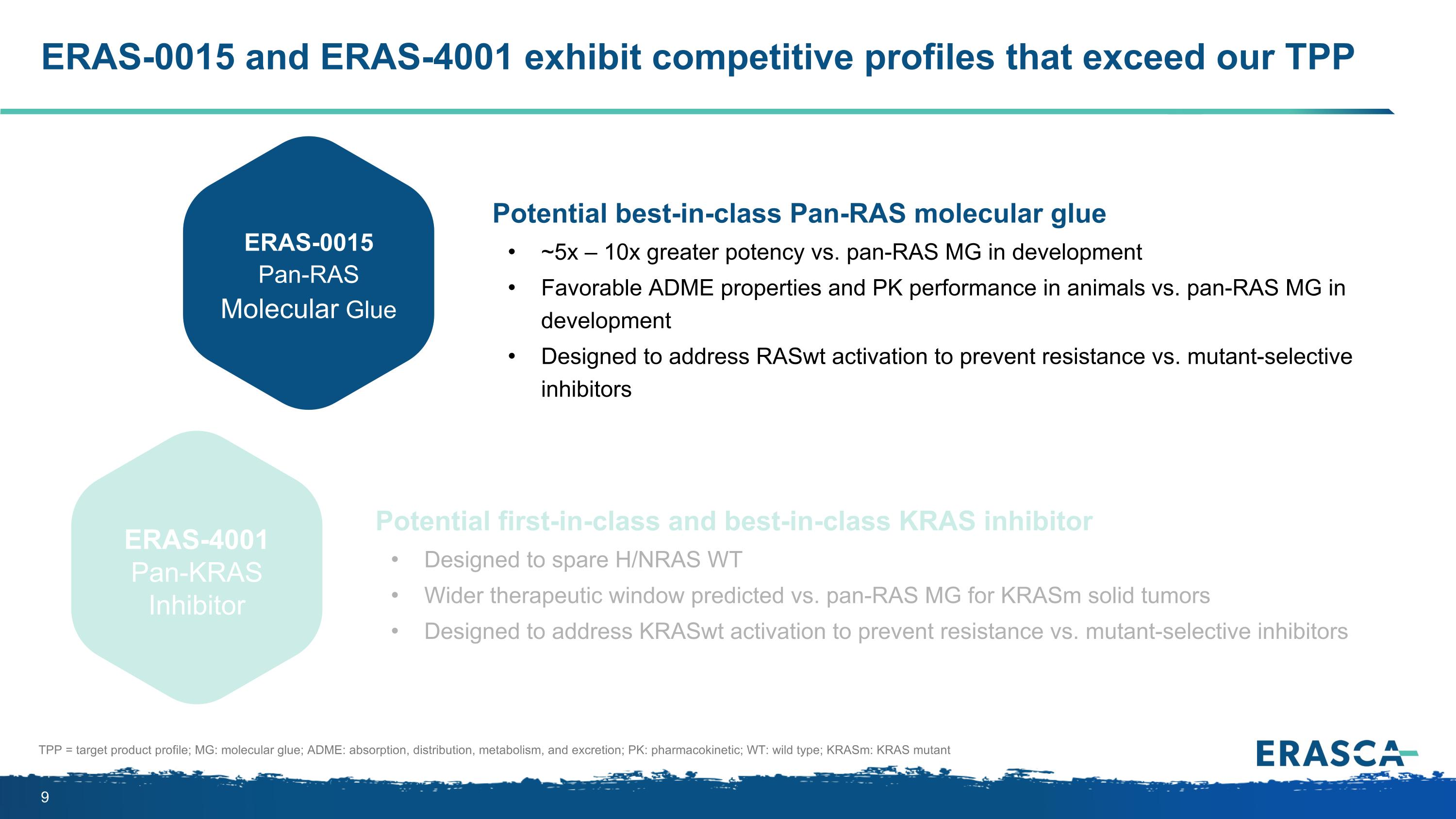
ERAS-0015 and ERAS-4001 exhibit competitive profiles that exceed our TPP Potential best-in-class Pan-RAS molecular glue ~5x – 10x greater potency vs. pan-RAS MG in development Favorable ADME properties and PK performance in animals vs. pan-RAS MG in development Designed to address RASwt activation to prevent resistance vs. mutant-selective inhibitors Potential first-in-class and best-in-class KRAS inhibitor Designed to spare H/NRAS WT Wider therapeutic window predicted vs. pan-RAS MG for KRASm solid tumors Designed to address KRASwt activation to prevent resistance vs. mutant-selective inhibitors ERAS-0015 Pan-RAS Molecular Glue ERAS-4001 Pan-KRAS Inhibitor TPP = target product profile; MG: molecular glue; ADME: absorption, distribution, metabolism, and excretion; PK: pharmacokinetic; WT: wild type; KRASm: KRAS mutant
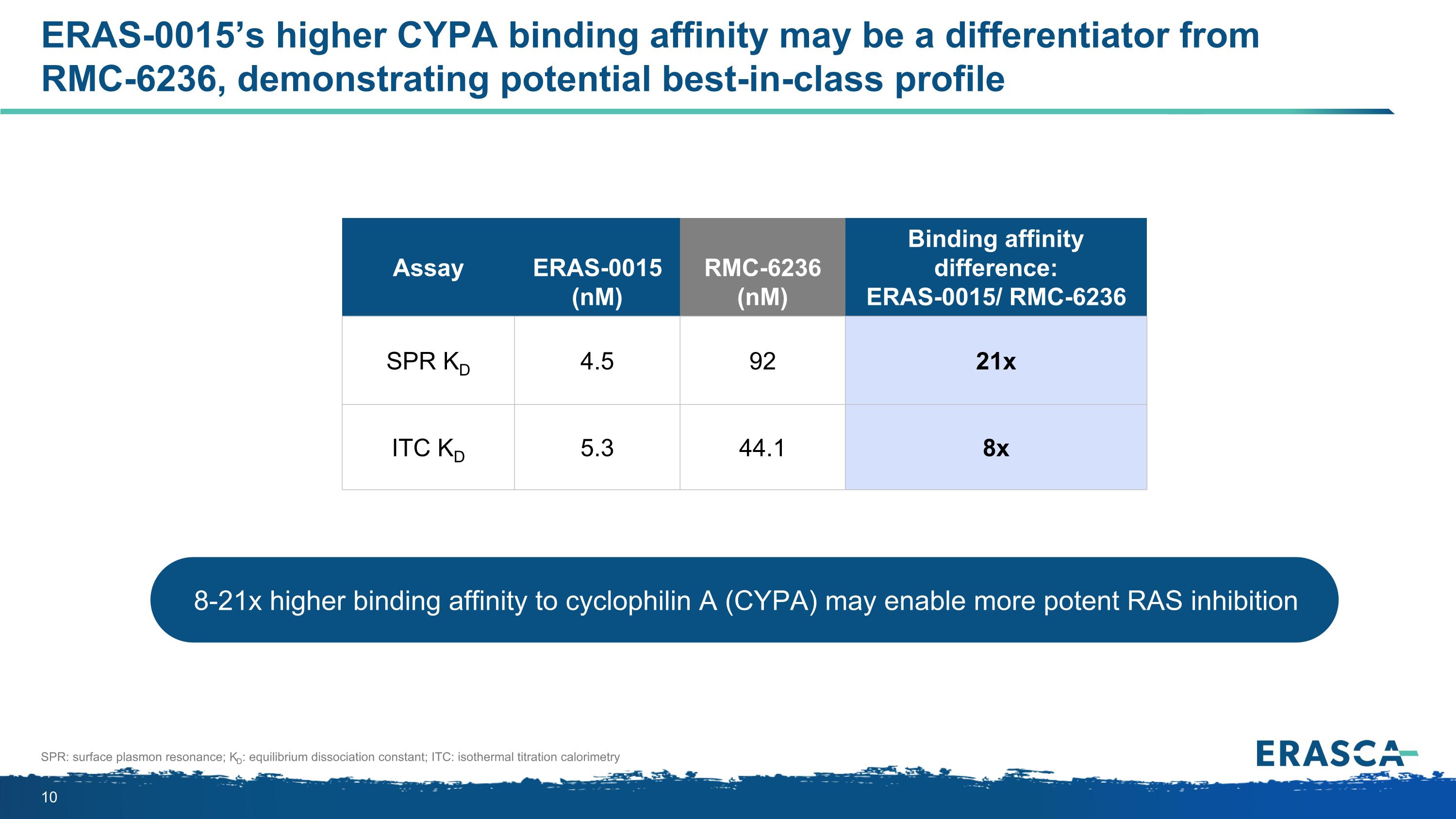
ERAS-0015’s higher CYPA binding affinity may be a differentiator from RMC-6236, demonstrating potential best-in-class profile SPR: surface plasmon resonance; KD: equilibrium dissociation constant; ITC: isothermal titration calorimetry 8-21x higher binding affinity to cyclophilin A (CYPA) may enable more potent RAS inhibition Assay ERAS-0015 (nM) RMC-6236 (nM) Binding affinity difference: ERAS-0015/ RMC-6236 SPR KD 4.5 92 21x ITC KD 5.3 44.1 8x
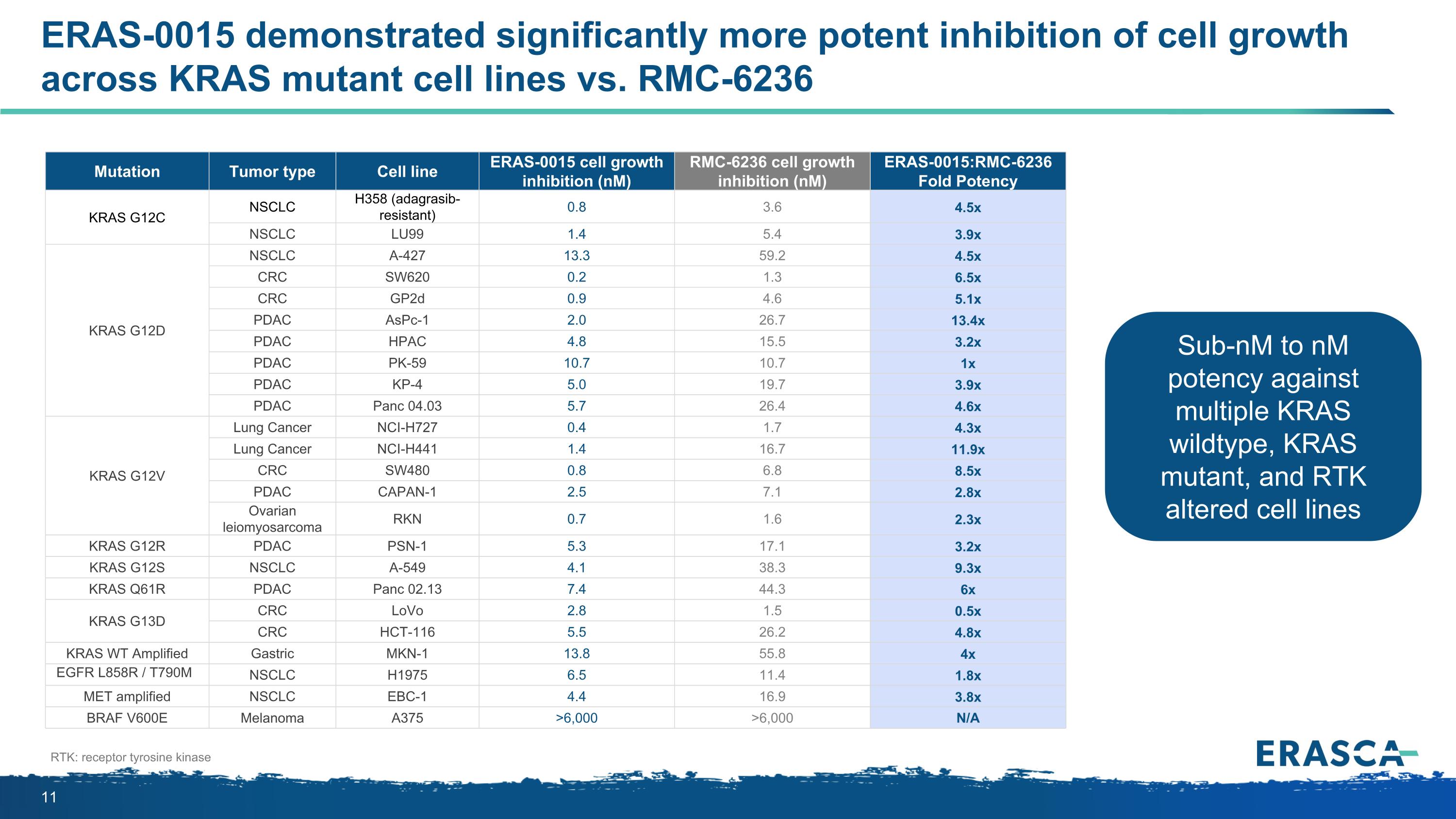
Mutation Tumor type Cell line ERAS-0015 cell growth inhibition (nM) RMC-6236 cell growth inhibition (nM) ERAS-0015:RMC-6236 Fold Potency KRAS G12C NSCLC H358 (adagrasib-resistant) 0.8 3.6 4.5x NSCLC LU99 1.4 5.4 3.9x KRAS G12D NSCLC A-427 13.3 59.2 4.5x CRC SW620 0.2 1.3 6.5x CRC GP2d 0.9 4.6 5.1x PDAC AsPc-1 2.0 26.7 13.4x PDAC HPAC 4.8 15.5 3.2x PDAC PK-59 10.7 10.7 1x PDAC KP-4 5.0 19.7 3.9x PDAC Panc 04.03 5.7 26.4 4.6x KRAS G12V Lung Cancer NCI-H727 0.4 1.7 4.3x Lung Cancer NCI-H441 1.4 16.7 11.9x CRC SW480 0.8 6.8 8.5x PDAC CAPAN-1 2.5 7.1 2.8x Ovarian leiomyosarcoma RKN 0.7 1.6 2.3x KRAS G12R PDAC PSN-1 5.3 17.1 3.2x KRAS G12S NSCLC A-549 4.1 38.3 9.3x KRAS Q61R PDAC Panc 02.13 7.4 44.3 6x KRAS G13D CRC LoVo 2.8 1.5 0.5x CRC HCT-116 5.5 26.2 4.8x KRAS WT Amplified Gastric MKN-1 13.8 55.8 4x EGFR L858R / T790M NSCLC H1975 6.5 11.4 1.8x MET amplified NSCLC EBC-1 4.4 16.9 3.8x BRAF V600E Melanoma A375 >6,000 >6,000 N/A ERAS-0015 demonstrated significantly more potent inhibition of cell growth across KRAS mutant cell lines vs. RMC-6236 RTK: receptor tyrosine kinase Sub-nM to nM potency against multiple KRAS wildtype, KRAS mutant, and RTK altered cell lines
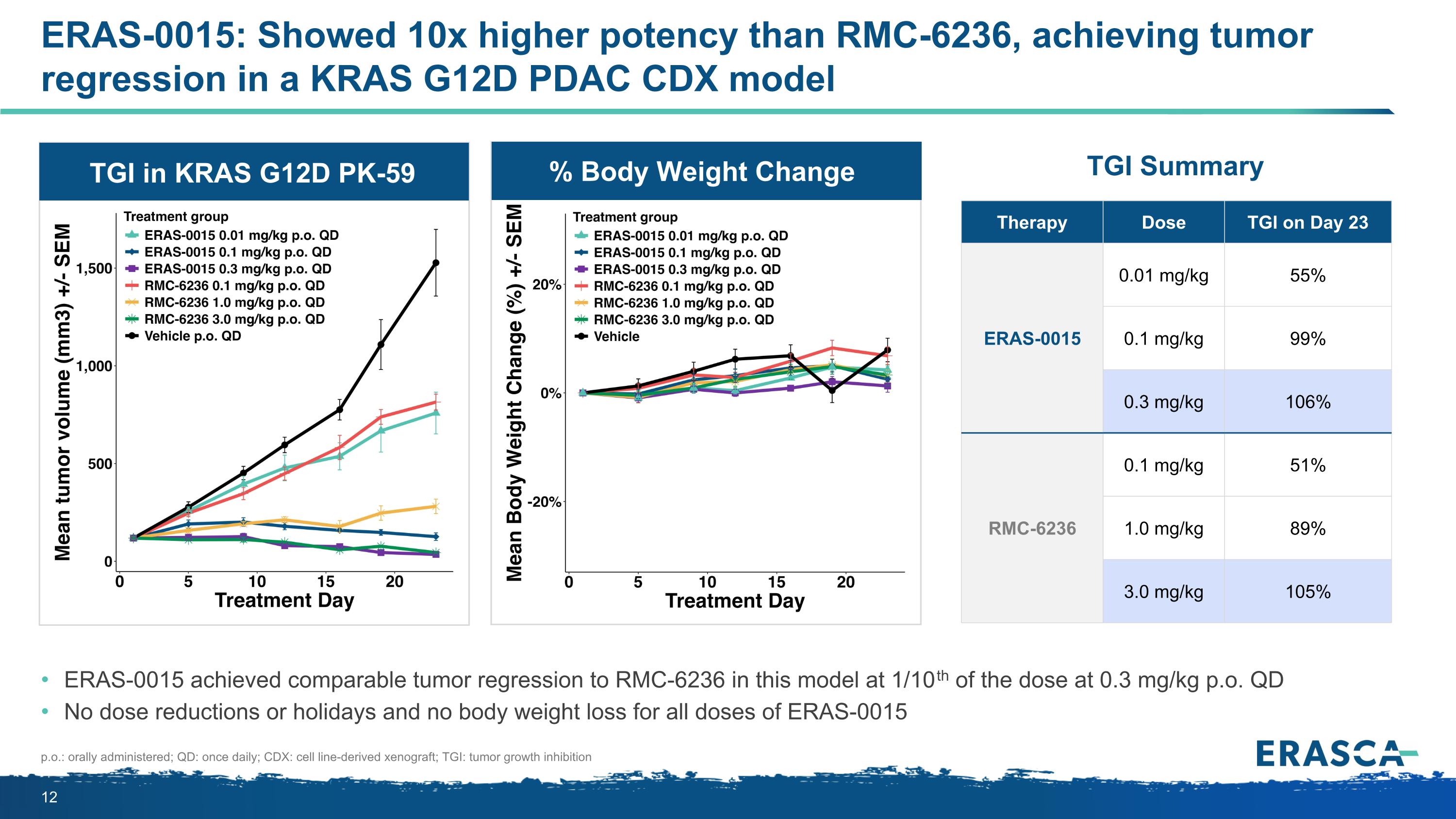
ERAS-0015: Showed 10x higher potency than RMC-6236, achieving tumor regression in a KRAS G12D PDAC CDX model ERAS-0015 achieved comparable tumor regression to RMC-6236 in this model at 1/10th of the dose at 0.3 mg/kg p.o. QD No dose reductions or holidays and no body weight loss for all doses of ERAS-0015 p.o.: orally administered; QD: once daily; CDX: cell line-derived xenograft; TGI: tumor growth inhibition Therapy Dose TGI on Day 23 ERAS-0015 0.01 mg/kg 55% 0.1 mg/kg 99% 0.3 mg/kg 106% RMC-6236 0.1 mg/kg 51% 1.0 mg/kg 89% 3.0 mg/kg 105% TGI Summary TGI in KRAS G12D PK-59 % Body Weight Change
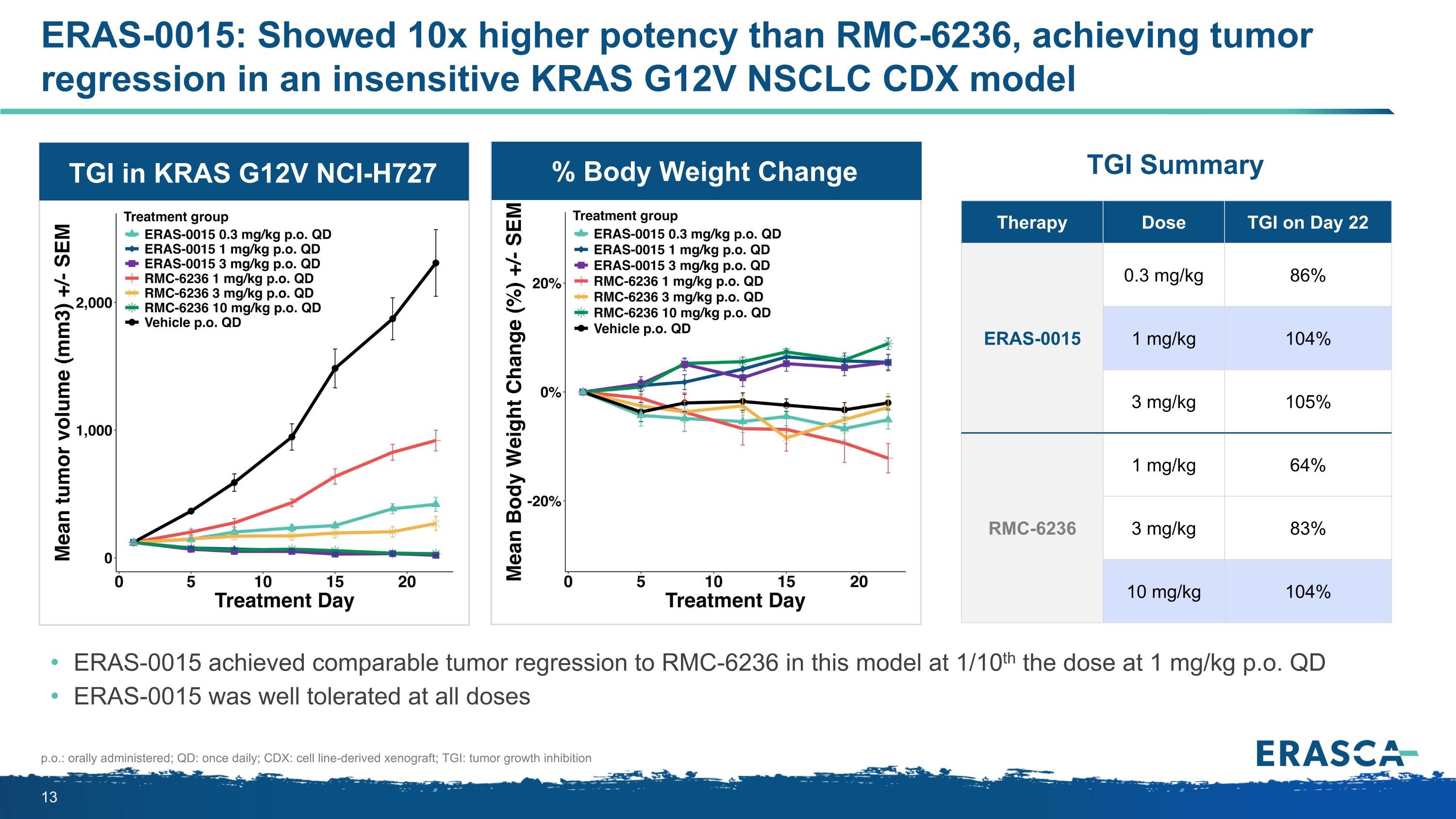
ERAS-0015: Showed 10x higher potency than RMC-6236, achieving tumor regression in an insensitive KRAS G12V NSCLC CDX model ERAS-0015 achieved comparable tumor regression to RMC-6236 in this model at 1/10th the dose at 1 mg/kg p.o. QD ERAS-0015 was well tolerated at all doses Therapy Dose TGI on Day 22 ERAS-0015 0.3 mg/kg 86% 1 mg/kg 104% 3 mg/kg 105% RMC-6236 1 mg/kg 64% 3 mg/kg 83% 10 mg/kg 104% TGI Summary p.o.: orally administered; QD: once daily; CDX: cell line-derived xenograft; TGI: tumor growth inhibition TGI in KRAS G12V NCI-H727 % Body Weight Change
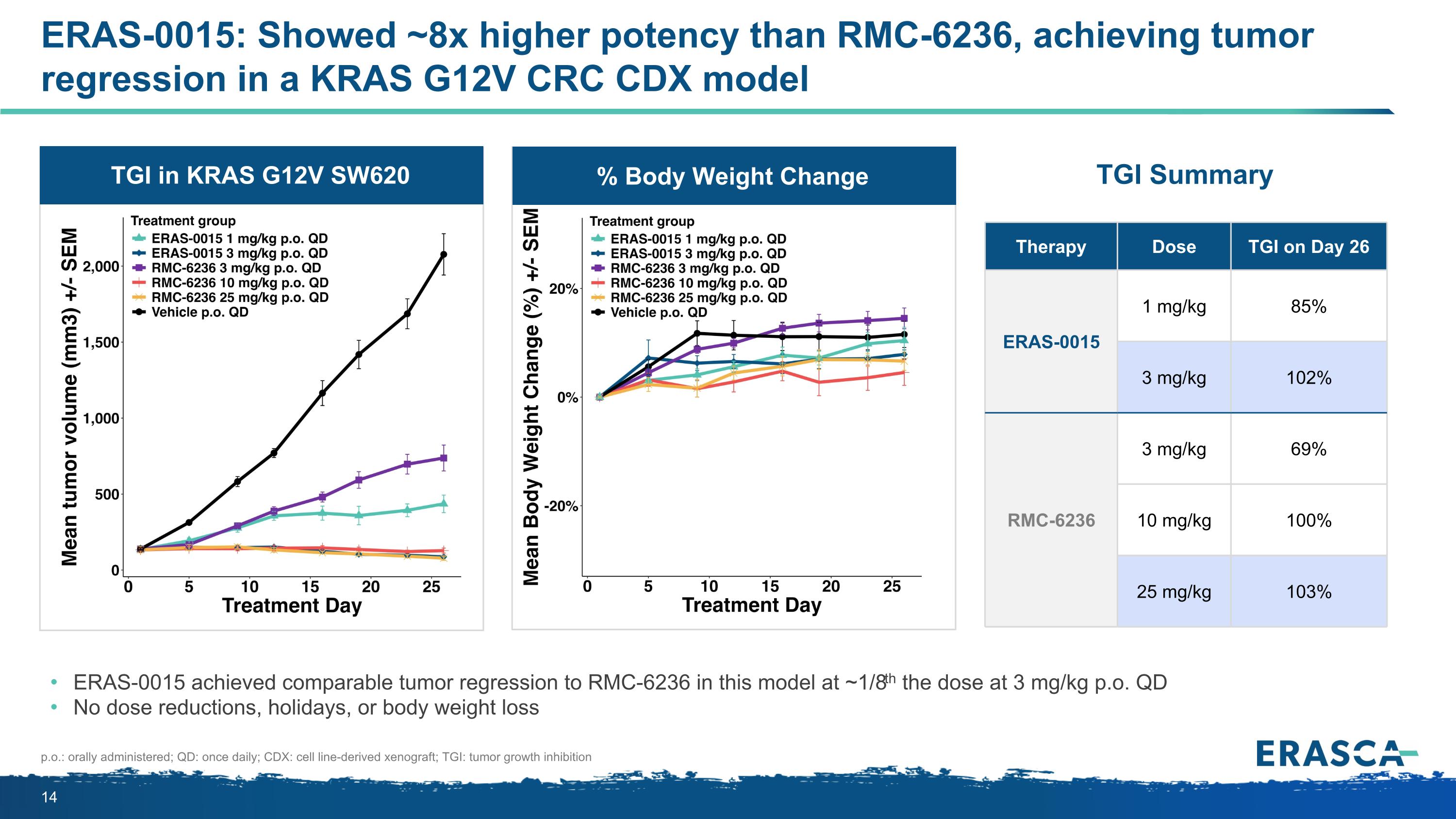
ERAS-0015: Showed ~8x higher potency than RMC-6236, achieving tumor regression in a KRAS G12V CRC CDX model ERAS-0015 achieved comparable tumor regression to RMC-6236 in this model at ~1/8th the dose at 3 mg/kg p.o. QD No dose reductions, holidays, or body weight loss Therapy Dose TGI on Day 26 ERAS-0015 1 mg/kg 85% 3 mg/kg 102% RMC-6236 3 mg/kg 69% 10 mg/kg 100% 25 mg/kg 103% p.o.: orally administered; QD: once daily; CDX: cell line-derived xenograft; TGI: tumor growth inhibition % Body Weight Change TGI in KRAS G12V SW620 TGI Summary
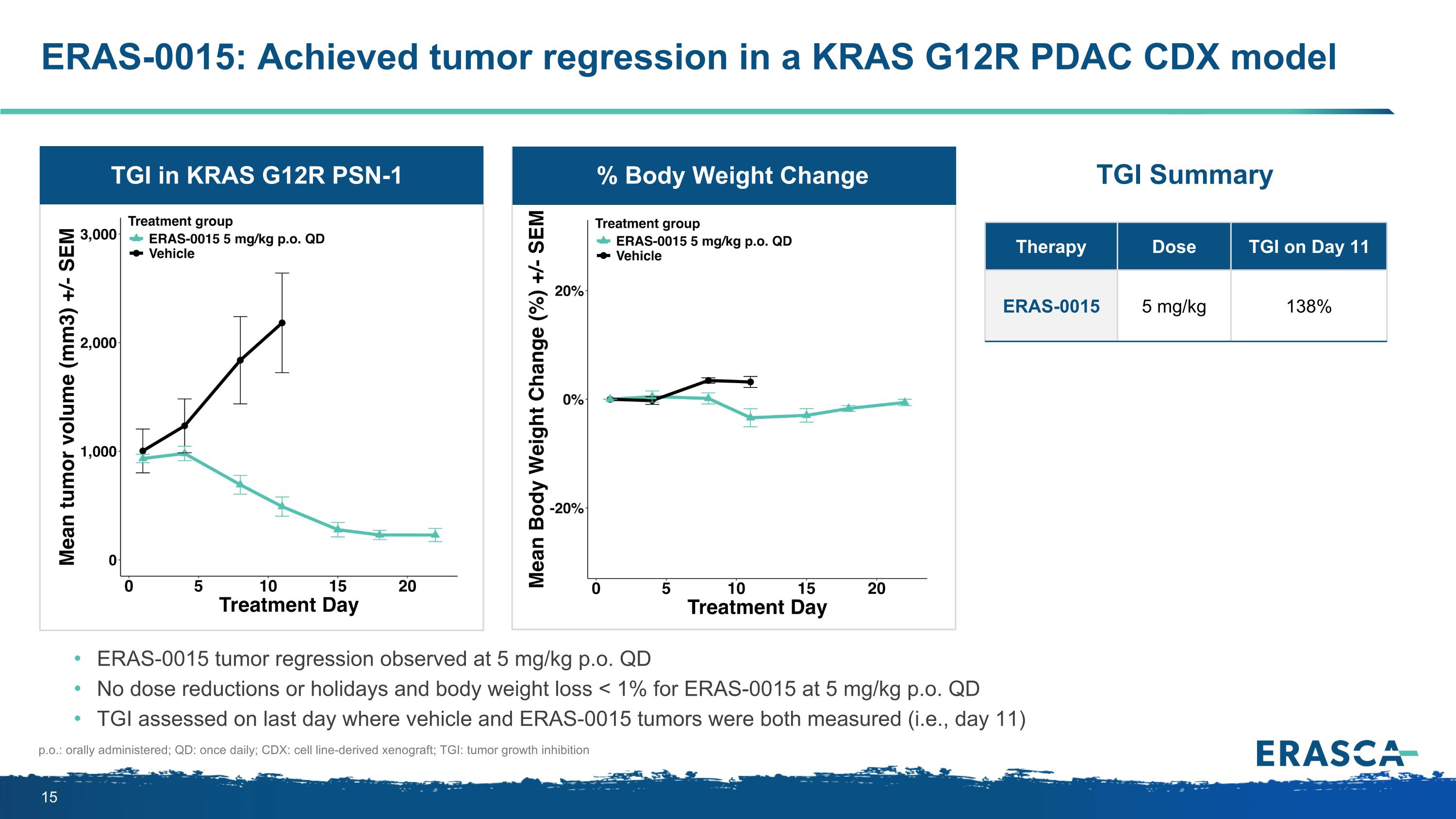
ERAS-0015: Achieved tumor regression in a KRAS G12R PDAC CDX model ERAS-0015 tumor regression observed at 5 mg/kg p.o. QD No dose reductions or holidays and body weight loss < 1% for ERAS-0015 at 5 mg/kg p.o. QD TGI assessed on last day where vehicle and ERAS-0015 tumors were both measured (i.e., day 11) p.o.: orally administered; QD: once daily; CDX: cell line-derived xenograft; TGI: tumor growth inhibition Therapy Dose TGI on Day 11 ERAS-0015 5 mg/kg 138% % Body Weight Change TGI in KRAS G12R PSN-1 TGI Summary
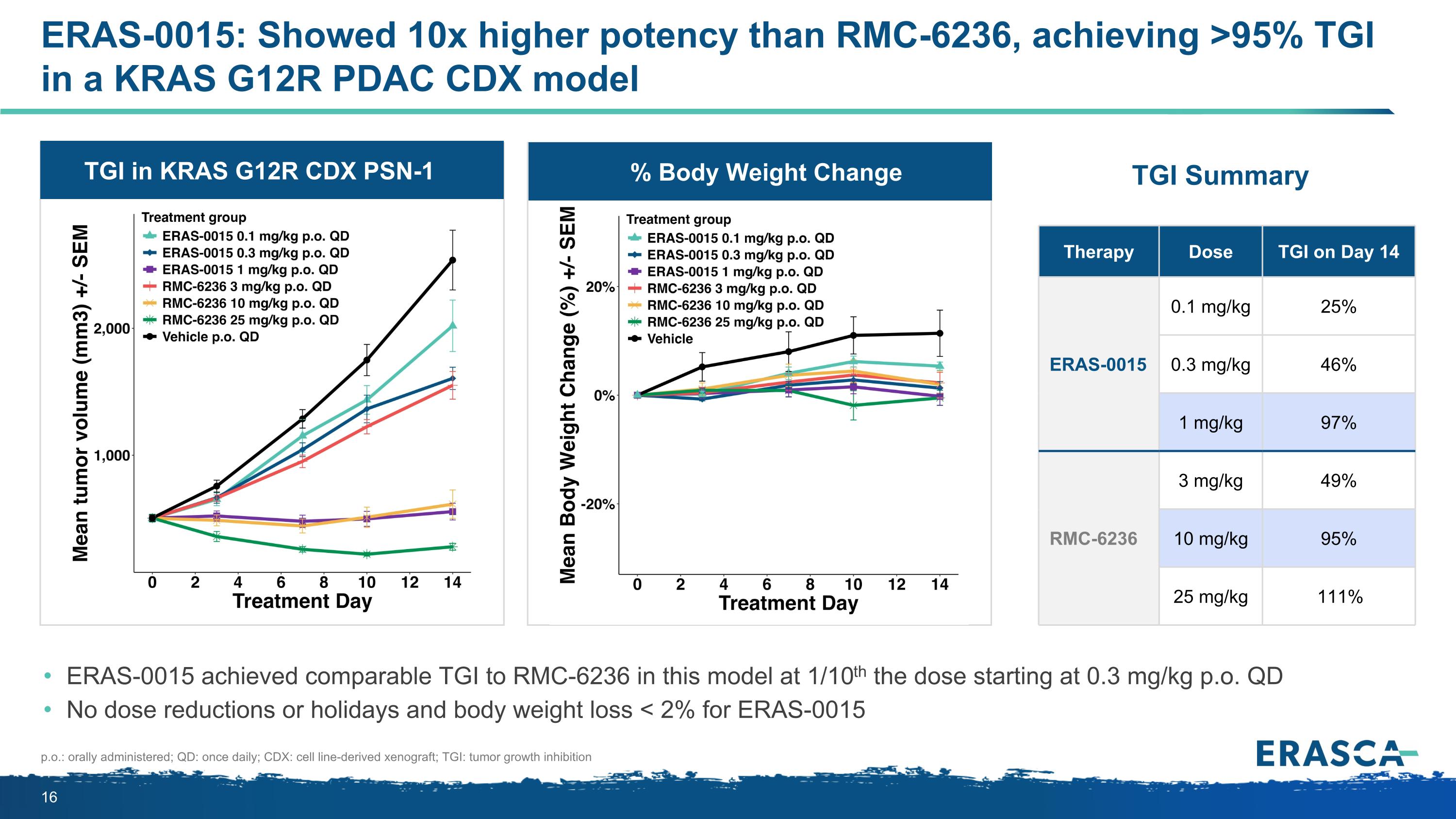
TGI in KRAS G12R CDX PSN-1 % Body Weight Change ERAS-0015: Showed 10x higher potency than RMC-6236, achieving >95% TGI in a KRAS G12R PDAC CDX model ERAS-0015 achieved comparable TGI to RMC-6236 in this model at 1/10th the dose starting at 0.3 mg/kg p.o. QD No dose reductions or holidays and body weight loss < 2% for ERAS-0015 Therapy Dose TGI on Day 14 ERAS-0015 0.1 mg/kg 25% 0.3 mg/kg 46% 1 mg/kg 97% RMC-6236 3 mg/kg 49% 10 mg/kg 95% 25 mg/kg 111% p.o.: orally administered; QD: once daily; CDX: cell line-derived xenograft; TGI: tumor growth inhibition TGI Summary
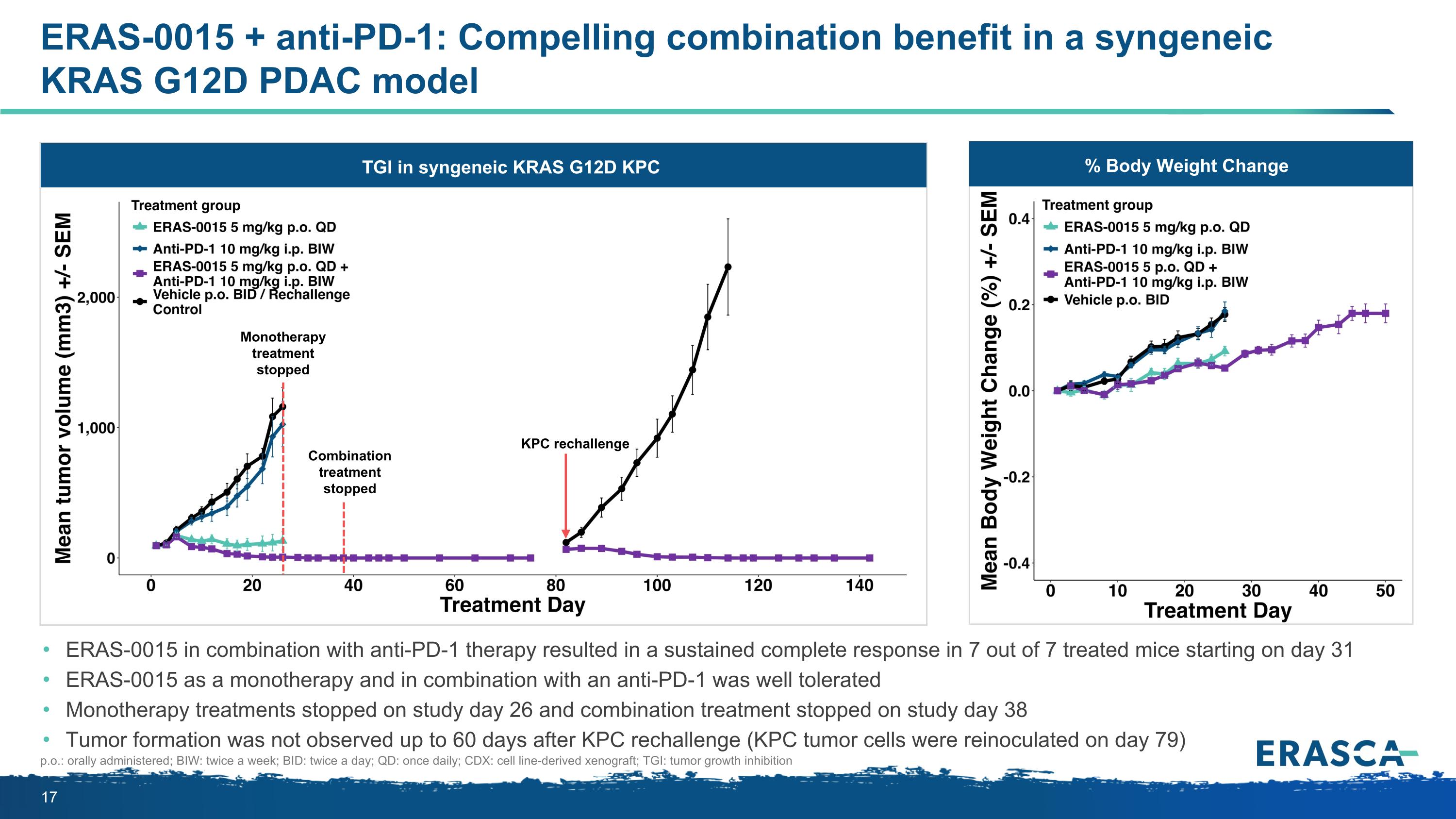
ERAS-0015 + anti-PD-1: Compelling combination benefit in a syngeneic KRAS G12D PDAC model ERAS-0015 in combination with anti-PD-1 therapy resulted in a sustained complete response in 7 out of 7 treated mice starting on day 31 ERAS-0015 as a monotherapy and in combination with an anti-PD-1 was well tolerated Monotherapy treatments stopped on study day 26 and combination treatment stopped on study day 38 Tumor formation was not observed up to 60 days after KPC rechallenge (KPC tumor cells were reinoculated on day 79) p.o.: orally administered; BIW: twice a week; BID: twice a day; QD: once daily; CDX: cell line-derived xenograft; TGI: tumor growth inhibition % Body Weight Change Body Weight Change TGI in syngeneic KRAS G12D KPC KPC rechallenge Monotherapy treatment stopped Combination treatment stopped
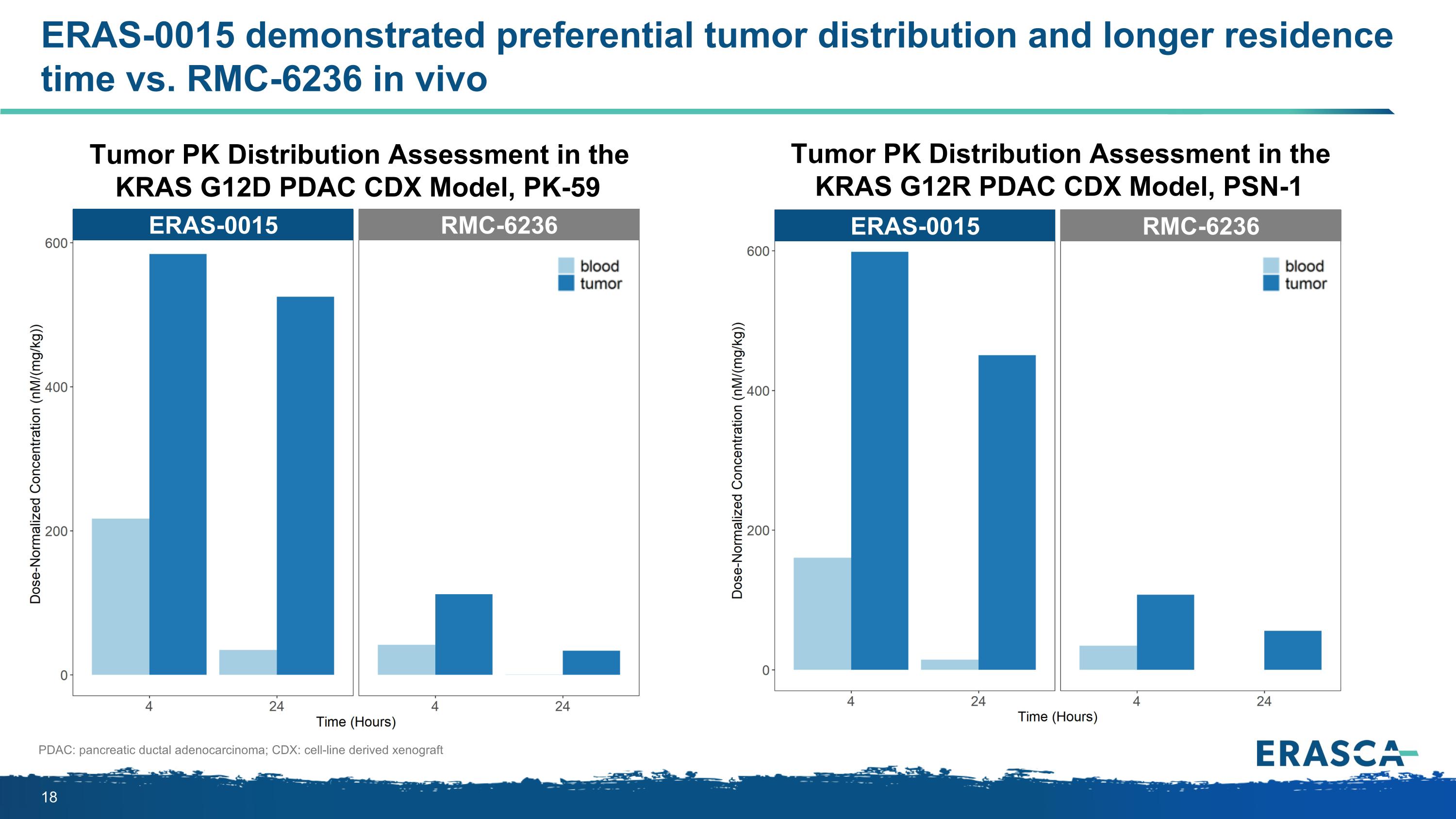
ERAS-0015 demonstrated preferential tumor distribution and longer residence time vs. RMC-6236 in vivo PDAC: pancreatic ductal adenocarcinoma; CDX: cell-line derived xenograft Tumor PK Distribution Assessment in the KRAS G12D PDAC CDX Model, PK-59 Tumor PK Distribution Assessment in the KRAS G12R PDAC CDX Model, PSN-1 ERAS-0015 RMC-6236 ERAS-0015 RMC-6236
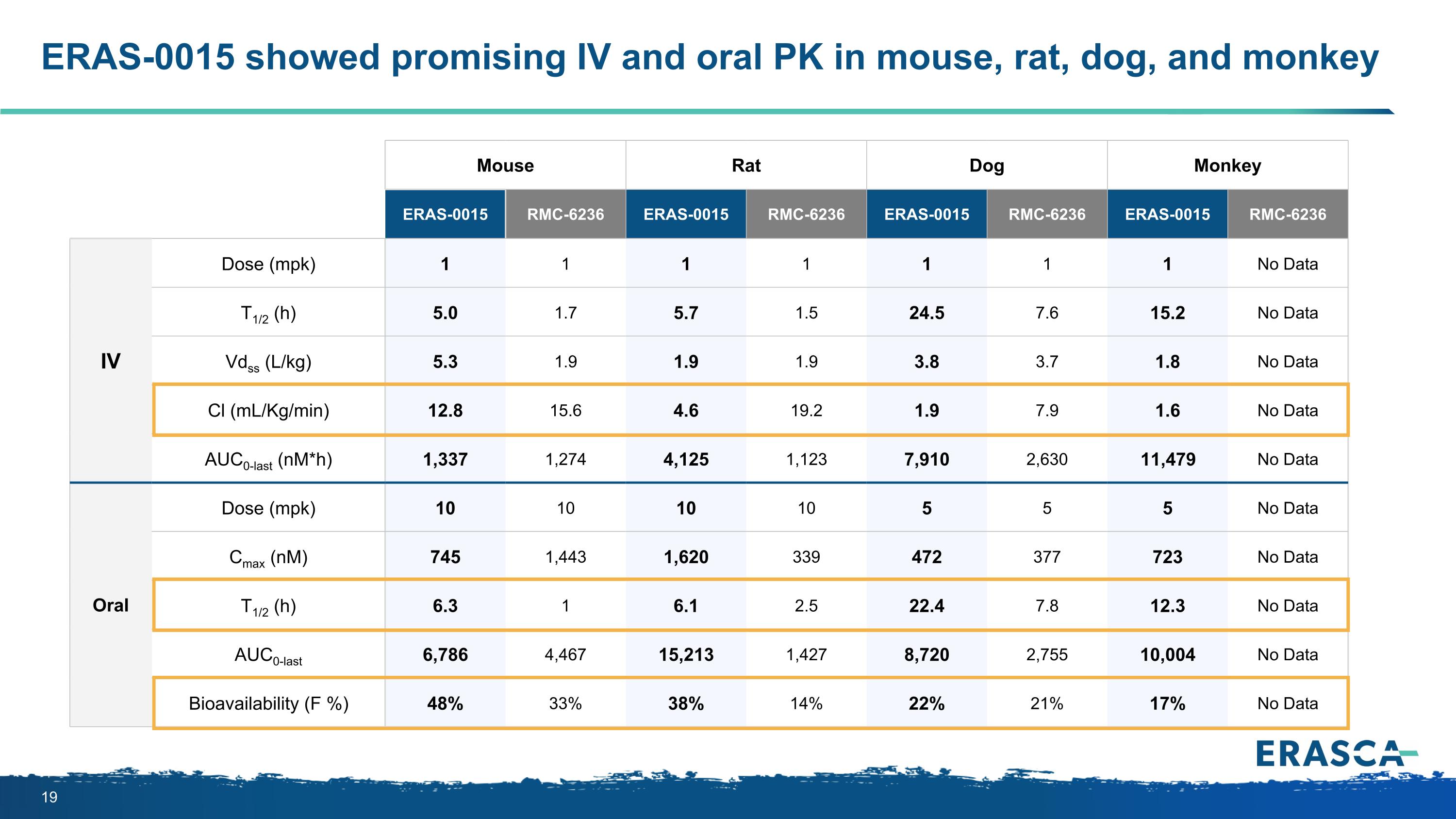
ERAS-0015 showed promising IV and oral PK in mouse, rat, dog, and monkey Mouse Rat Dog Monkey ERAS-0015 RMC-6236 ERAS-0015 RMC-6236 ERAS-0015 RMC-6236 ERAS-0015 RMC-6236 IV Dose (mpk) 1 1 1 1 1 1 1 No Data T1/2 (h) 5.0 1.7 5.7 1.5 24.5 7.6 15.2 No Data Vdss (L/kg) 5.3 1.9 1.9 1.9 3.8 3.7 1.8 No Data Cl (mL/Kg/min) 12.8 15.6 4.6 19.2 1.9 7.9 1.6 No Data AUC0-last (nM*h) 1,337 1,274 4,125 1,123 7,910 2,630 11,479 No Data Oral Dose (mpk) 10 10 10 10 5 5 5 No Data Cmax (nM) 745 1,443 1,620 339 472 377 723 No Data T1/2 (h) 6.3 1 6.1 2.5 22.4 7.8 12.3 No Data AUC0-last 6,786 4,467 15,213 1,427 8,720 2,755 10,004 No Data Bioavailability (F %) 48% 33% 38% 14% 22% 21% 17% No Data
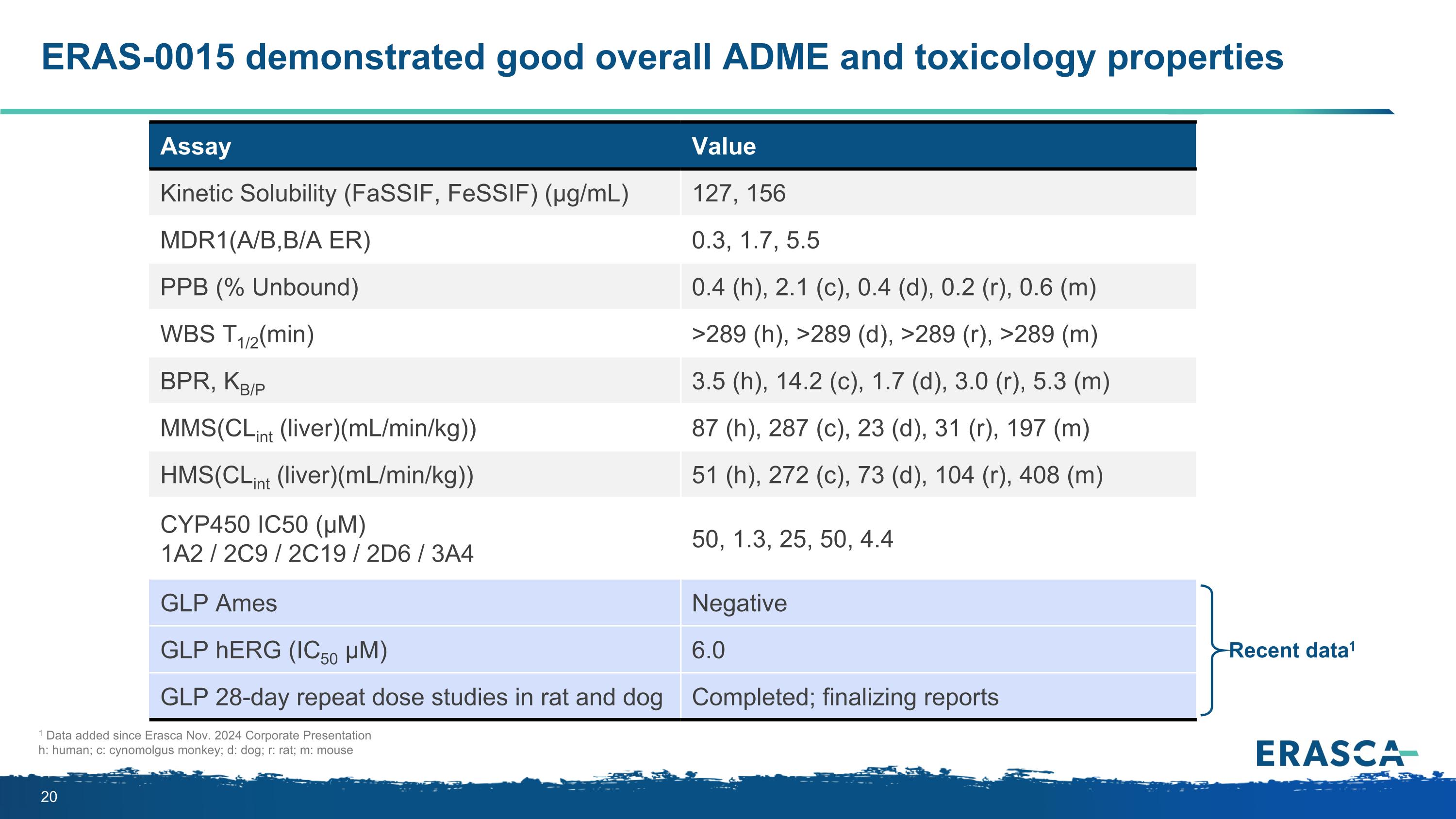
ERAS-0015 demonstrated good overall ADME and toxicology properties Assay Value Kinetic Solubility (FaSSIF, FeSSIF) (μg/mL) 127, 156 MDR1(A/B,B/A ER) 0.3, 1.7, 5.5 PPB (% Unbound) 0.4 (h), 2.1 (c), 0.4 (d), 0.2 (r), 0.6 (m) WBS T1/2(min) >289 (h), >289 (d), >289 (r), >289 (m) BPR, KB/P 3.5 (h), 14.2 (c), 1.7 (d), 3.0 (r), 5.3 (m) MMS(CLint (liver)(mL/min/kg)) 87 (h), 287 (c), 23 (d), 31 (r), 197 (m) HMS(CLint (liver)(mL/min/kg)) 51 (h), 272 (c), 73 (d), 104 (r), 408 (m) CYP450 IC50 (µM) 1A2 / 2C9 / 2C19 / 2D6 / 3A4 50, 1.3, 25, 50, 4.4 GLP Ames Negative GLP hERG (IC50 μM) 6.0 GLP 28-day repeat dose studies in rat and dog Completed; finalizing reports Recent data1 1 Data added since Erasca Nov. 2024 Corporate Presentation h: human; c: cynomolgus monkey; d: dog; r: rat; m: mouse
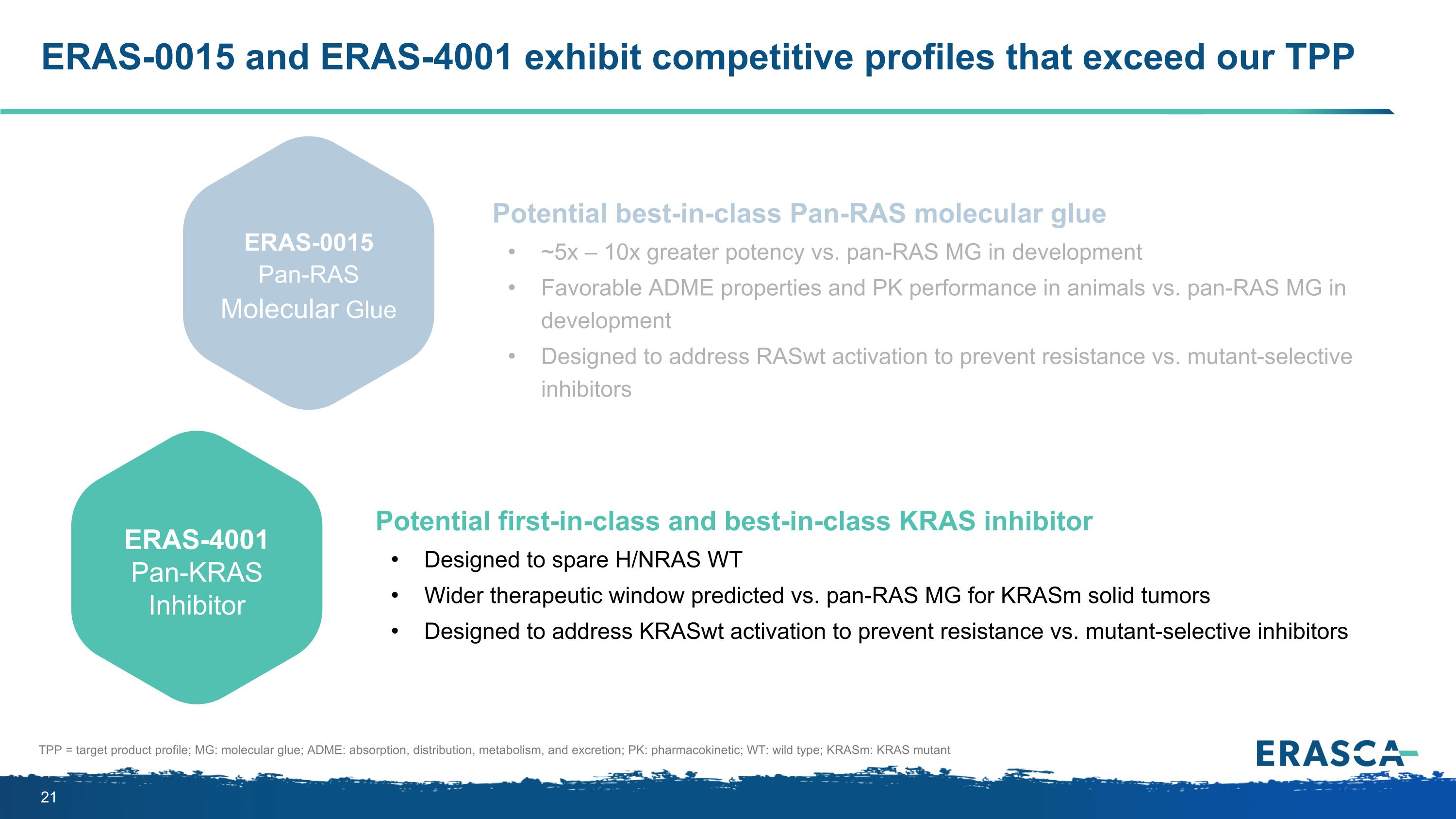
ERAS-0015 and ERAS-4001 exhibit competitive profiles that exceed our TPP Potential best-in-class Pan-RAS molecular glue ~5x – 10x greater potency vs. pan-RAS MG in development Favorable ADME properties and PK performance in animals vs. pan-RAS MG in development Designed to address RASwt activation to prevent resistance vs. mutant-selective inhibitors Potential first-in-class and best-in-class KRAS inhibitor Designed to spare H/NRAS WT Wider therapeutic window predicted vs. pan-RAS MG for KRASm solid tumors Designed to address KRASwt activation to prevent resistance vs. mutant-selective inhibitors ERAS-0015 Pan-RAS Molecular Glue ERAS-4001 Pan-KRAS Inhibitor TPP = target product profile; MG: molecular glue; ADME: absorption, distribution, metabolism, and excretion; PK: pharmacokinetic; WT: wild type; KRASm: KRAS mutant
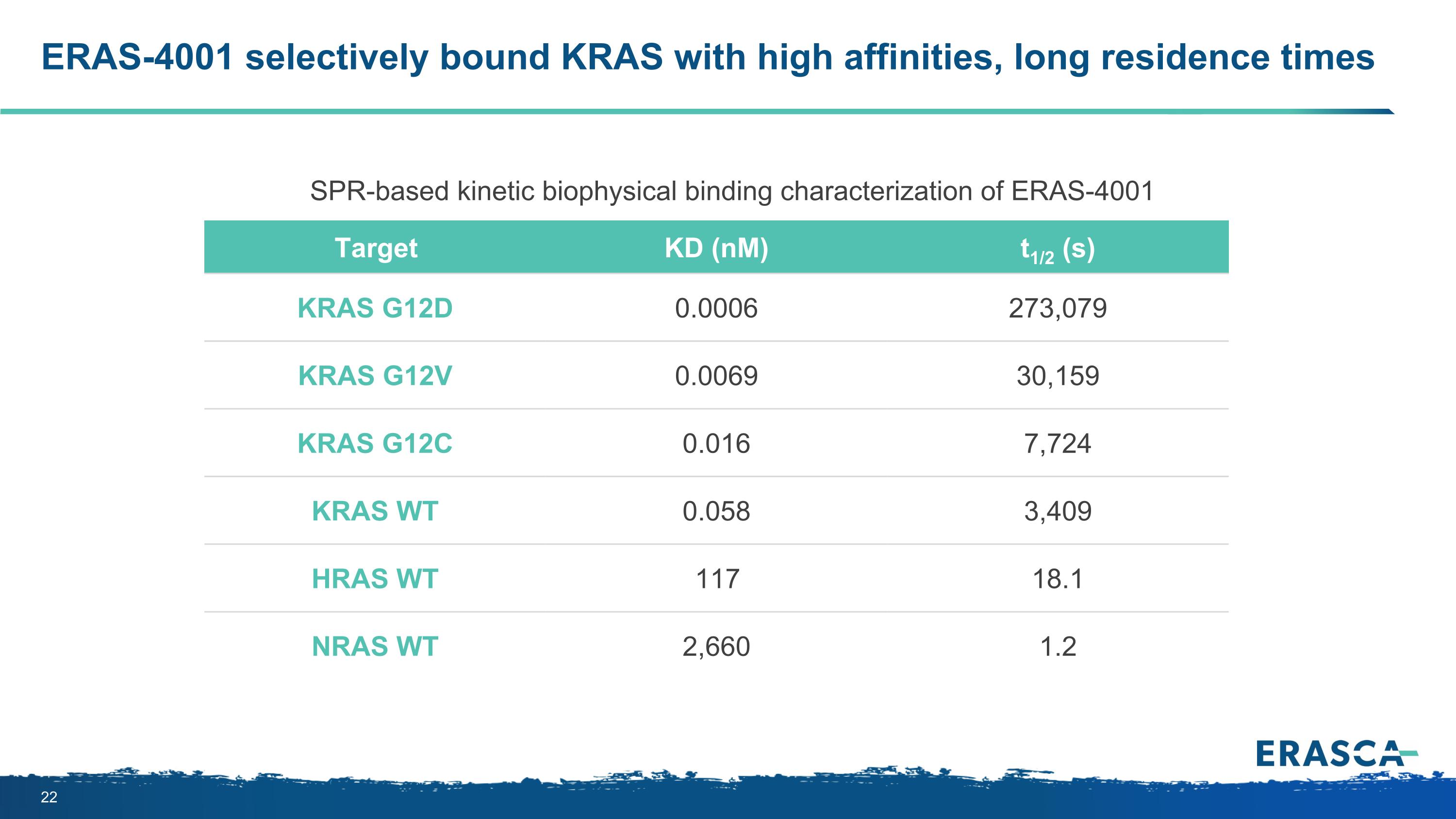
ERAS-4001 selectively bound KRAS with high affinities, long residence times Target KD (nM) t1/2 (s) KRAS G12D 0.0006 273,079 KRAS G12V 0.0069 30,159 KRAS G12C 0.016 7,724 KRAS WT 0.058 3,409 HRAS WT 117 18.1 NRAS WT 2,660 1.2 SPR-based kinetic biophysical binding characterization of ERAS-4001

ERAS-4001 showed potent activity against both GTP- and GDP-bound KRAS Assay Class Assay Target ERAS-4001 IC50 (nM) Biochemical Functional RAS-RAF Binding Assay (RBD) RBD KRAS G12D GDP 1.6 RBD KRAS G12D GMPPNP* 6.8 * GMPPNP is a nonhydrolyzable GTP analogue intended to approximate GTP-bound KRAS
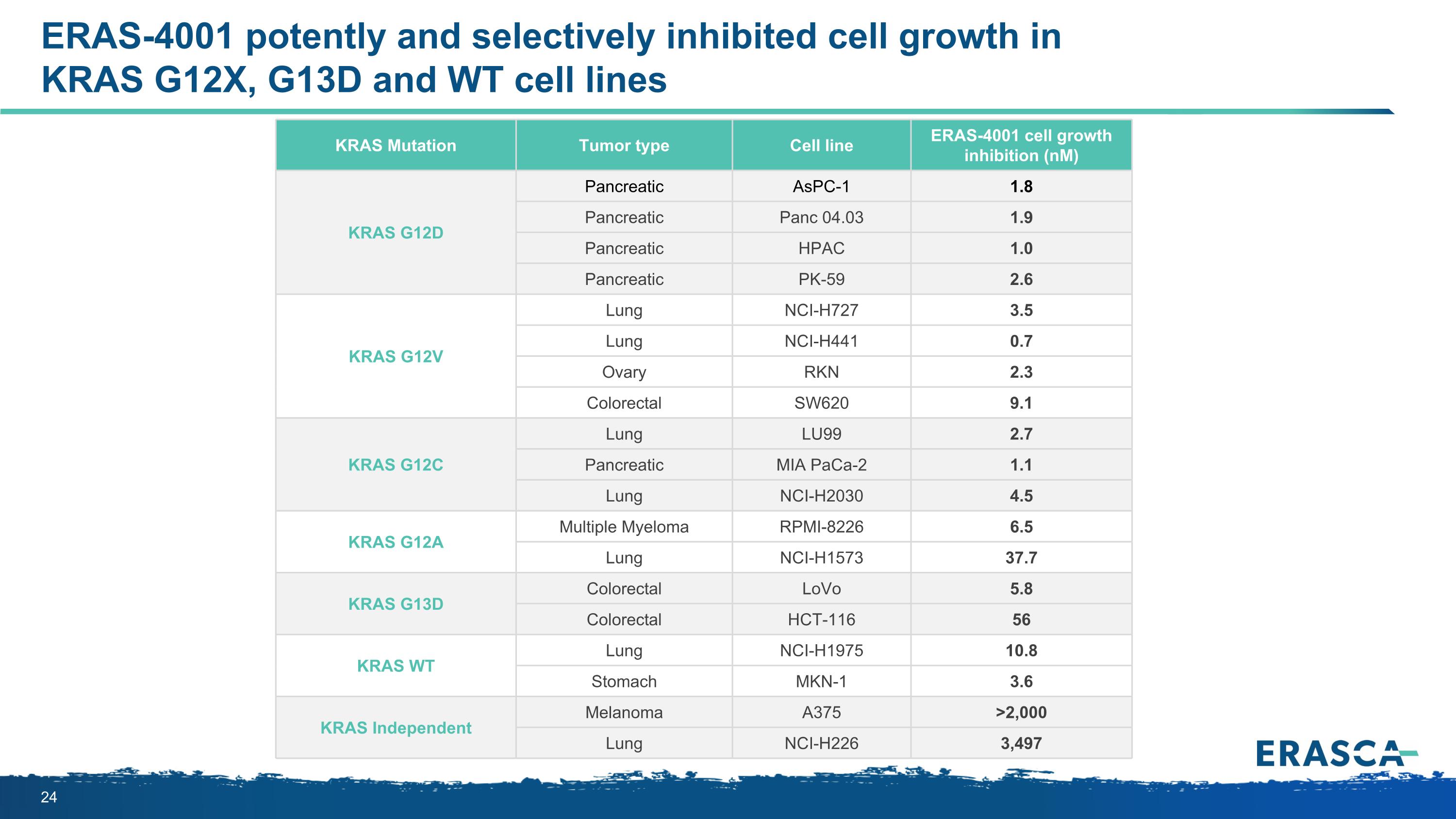
ERAS-4001 potently and selectively inhibited cell growth in KRAS G12X, G13D and WT cell lines KRAS Mutation Tumor type Cell line ERAS-4001 cell growth inhibition (nM) KRAS G12D Pancreatic AsPC-1 1.8 Pancreatic Panc 04.03 1.9 Pancreatic HPAC 1.0 Pancreatic PK-59 2.6 KRAS G12V Lung NCI-H727 3.5 Lung NCI-H441 0.7 Ovary RKN 2.3 Colorectal SW620 9.1 KRAS G12C Lung LU99 2.7 Pancreatic MIA PaCa-2 1.1 Lung NCI-H2030 4.5 KRAS G12A Multiple Myeloma RPMI-8226 6.5 Lung NCI-H1573 37.7 KRAS G13D Colorectal LoVo 5.8 Colorectal HCT-116 56 KRAS WT Lung NCI-H1975 10.8 Stomach MKN-1 3.6 KRAS Independent Melanoma A375 >2,000 Lung NCI-H226 3,497
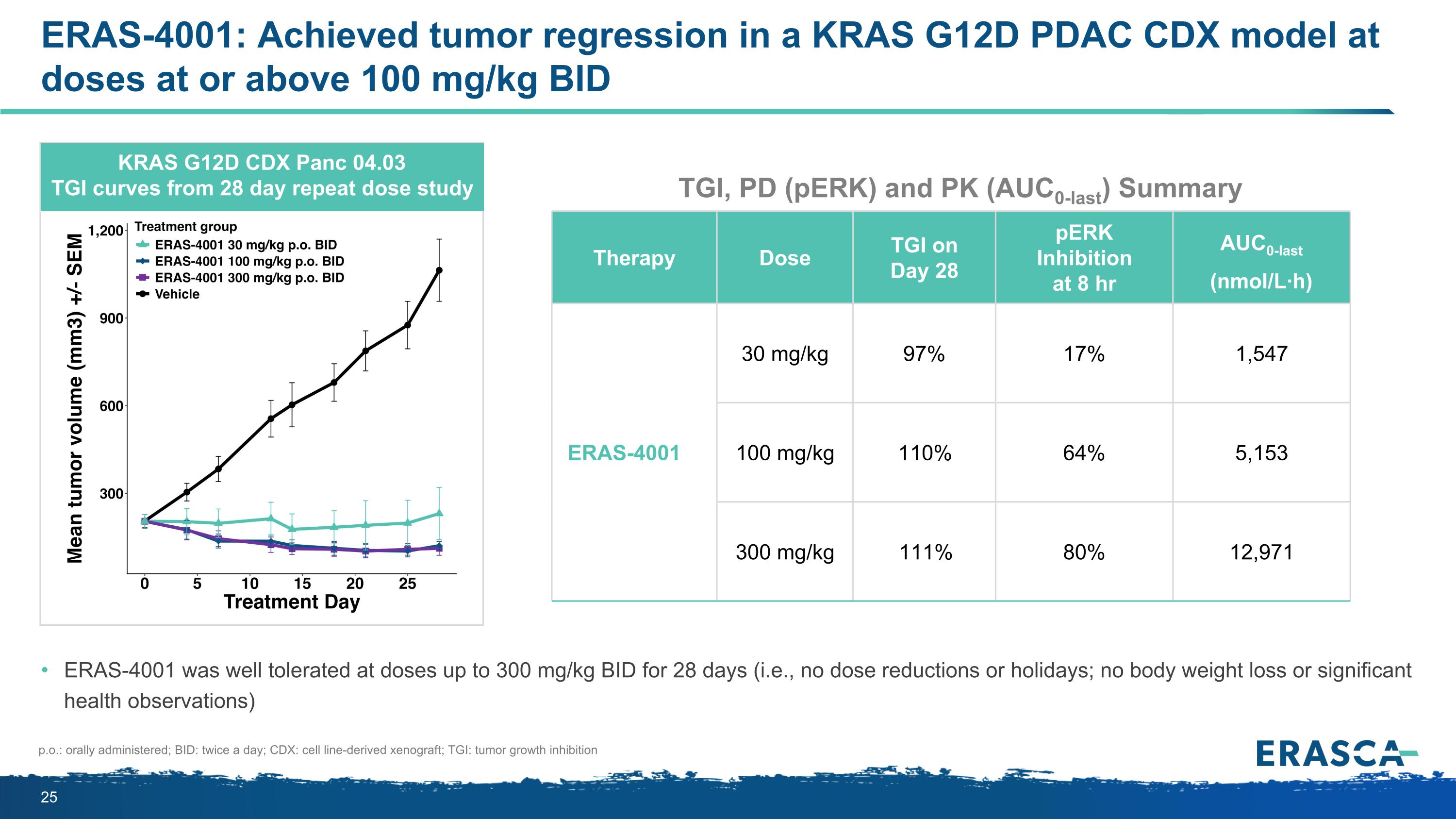
ERAS-4001: Achieved tumor regression in a KRAS G12D PDAC CDX model at doses at or above 100 mg/kg BID KRAS G12D CDX Panc 04.03 TGI curves from 28 day repeat dose study ERAS-4001 was well tolerated at doses up to 300 mg/kg BID for 28 days (i.e., no dose reductions or holidays; no body weight loss or significant health observations) p.o.: orally administered; BID: twice a day; CDX: cell line-derived xenograft; TGI: tumor growth inhibition Therapy Dose TGI on Day 28 pERK Inhibition at 8 hr AUC0-last (nmol/L·h) ERAS-4001 30 mg/kg 97% 17% 1,547 100 mg/kg 110% 64% 5,153 300 mg/kg 111% 80% 12,971 TGI, PD (pERK) and PK (AUC0-last) Summary
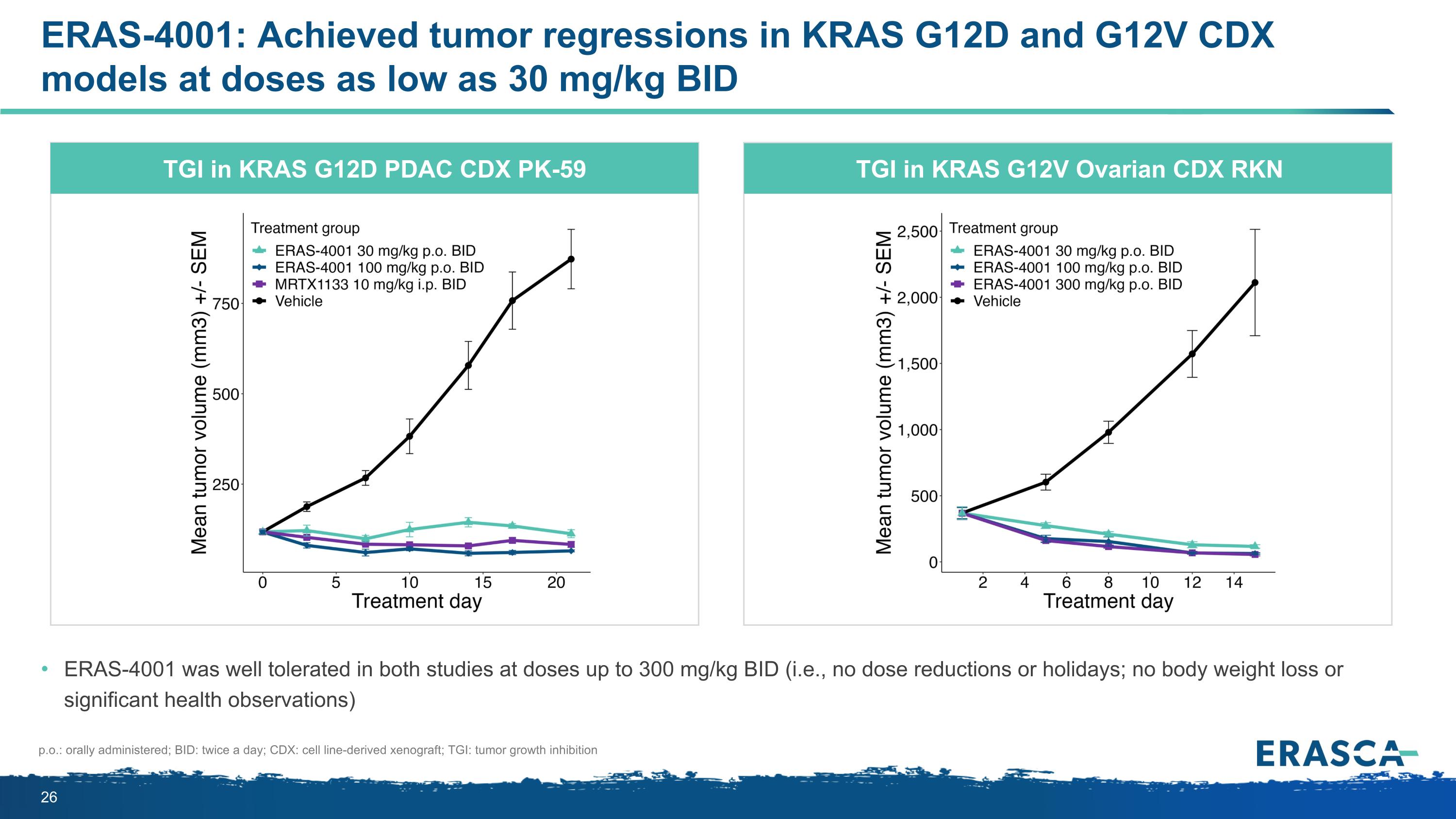
ERAS-4001: Achieved tumor regressions in KRAS G12D and G12V CDX models at doses as low as 30 mg/kg BID ERAS-4001 was well tolerated in both studies at doses up to 300 mg/kg BID (i.e., no dose reductions or holidays; no body weight loss or significant health observations) TGI in KRAS G12D PDAC CDX PK-59 TGI in KRAS G12V Ovarian CDX RKN p.o.: orally administered; BID: twice a day; CDX: cell line-derived xenograft; TGI: tumor growth inhibition
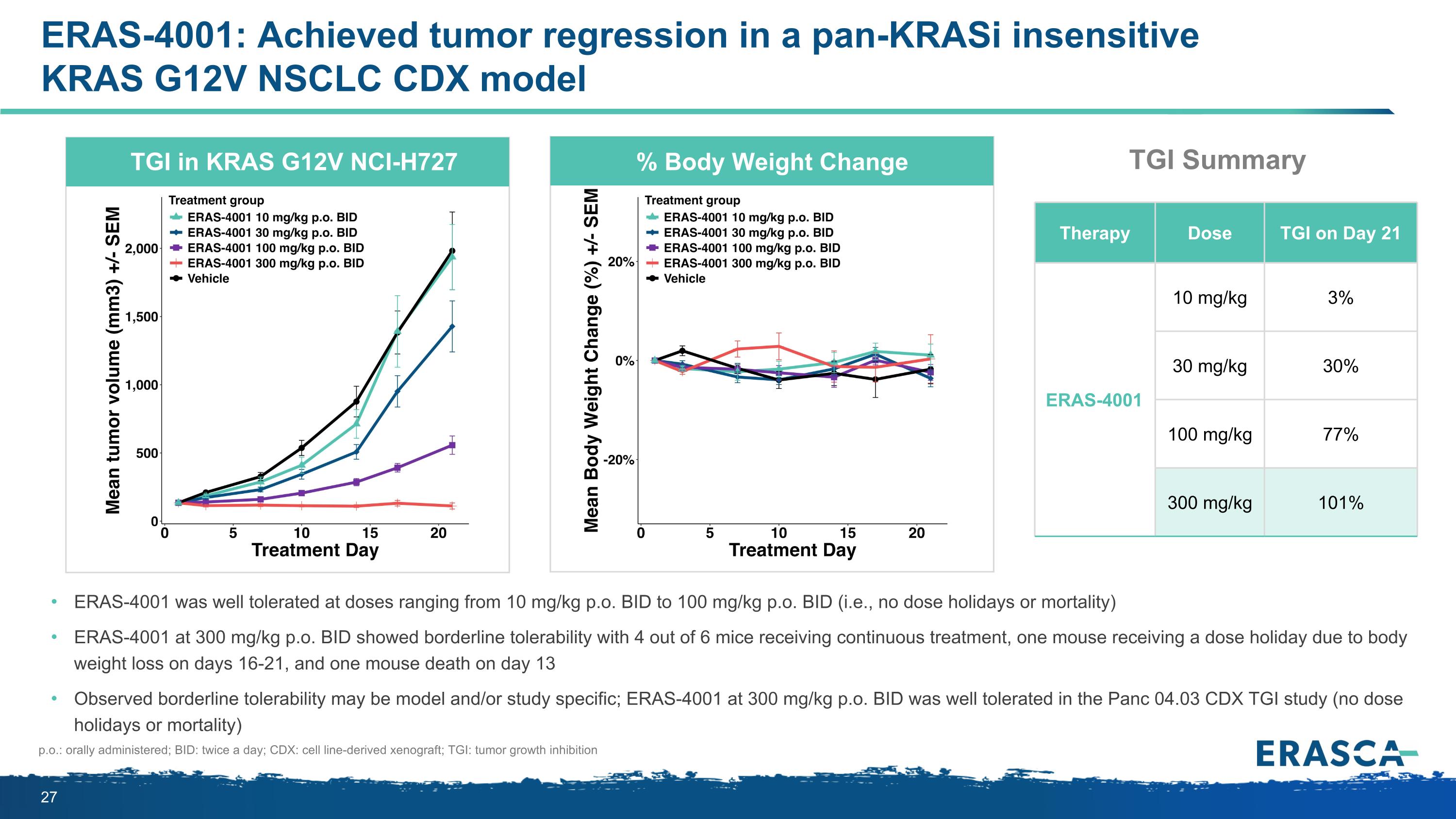
ERAS-4001: Achieved tumor regression in a pan-KRASi insensitive KRAS G12V NSCLC CDX model ERAS-4001 was well tolerated at doses ranging from 10 mg/kg p.o. BID to 100 mg/kg p.o. BID (i.e., no dose holidays or mortality) ERAS-4001 at 300 mg/kg p.o. BID showed borderline tolerability with 4 out of 6 mice receiving continuous treatment, one mouse receiving a dose holiday due to body weight loss on days 16-21, and one mouse death on day 13 Observed borderline tolerability may be model and/or study specific; ERAS-4001 at 300 mg/kg p.o. BID was well tolerated in the Panc 04.03 CDX TGI study (no dose holidays or mortality) p.o.: orally administered; BID: twice a day; CDX: cell line-derived xenograft; TGI: tumor growth inhibition TGI in KRAS G12V NCI-H727 % Body Weight Change Therapy Dose TGI on Day 21 ERAS-4001 10 mg/kg 3% 30 mg/kg 30% 100 mg/kg 77% 300 mg/kg 101% TGI Summary
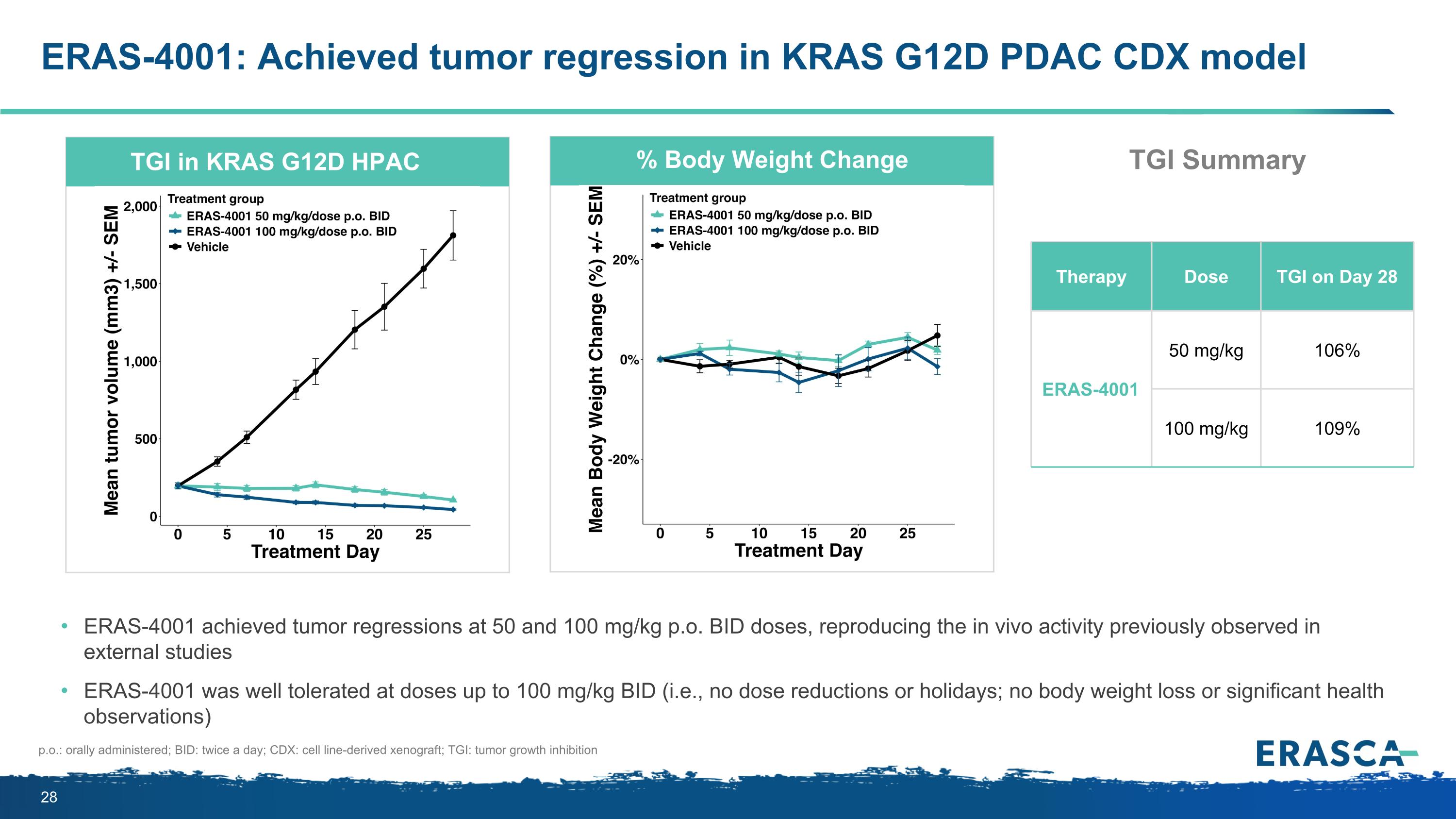
ERAS-4001: Achieved tumor regression in KRAS G12D PDAC CDX model p.o.: orally administered; BID: twice a day; CDX: cell line-derived xenograft; TGI: tumor growth inhibition TGI in KRAS G12D HPAC % Body Weight Change Therapy Dose TGI on Day 28 ERAS-4001 50 mg/kg 106% 100 mg/kg 109% ERAS-4001 achieved tumor regressions at 50 and 100 mg/kg p.o. BID doses, reproducing the in vivo activity previously observed in external studies ERAS-4001 was well tolerated at doses up to 100 mg/kg BID (i.e., no dose reductions or holidays; no body weight loss or significant health observations) TGI Summary
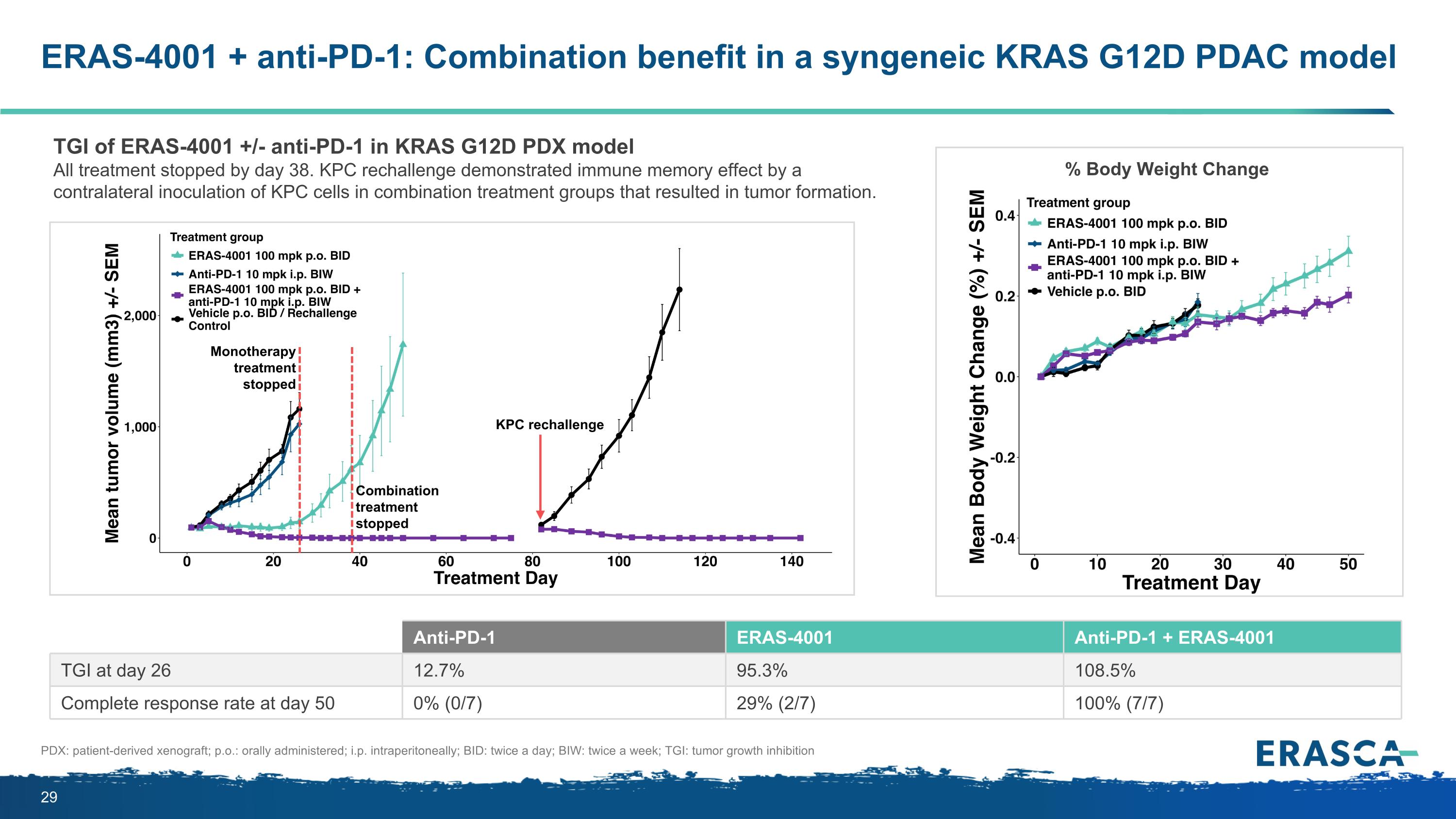
ERAS-4001 + anti-PD-1: Combination benefit in a syngeneic KRAS G12D PDAC model Anti-PD-1 ERAS-4001 Anti-PD-1 + ERAS-4001 TGI at day 26 12.7% 95.3% 108.5% Complete response rate at day 50 0% (0/7) 29% (2/7) 100% (7/7) TGI of ERAS-4001 +/- anti-PD-1 in KRAS G12D PDX model All treatment stopped by day 38. KPC rechallenge demonstrated immune memory effect by a contralateral inoculation of KPC cells in combination treatment groups that resulted in tumor formation. % Body Weight Change PDX: patient-derived xenograft; p.o.: orally administered; i.p. intraperitoneally; BID: twice a day; BIW: twice a week; TGI: tumor growth inhibition Monotherapy treatment stopped Combination treatment stopped KPC rechallenge
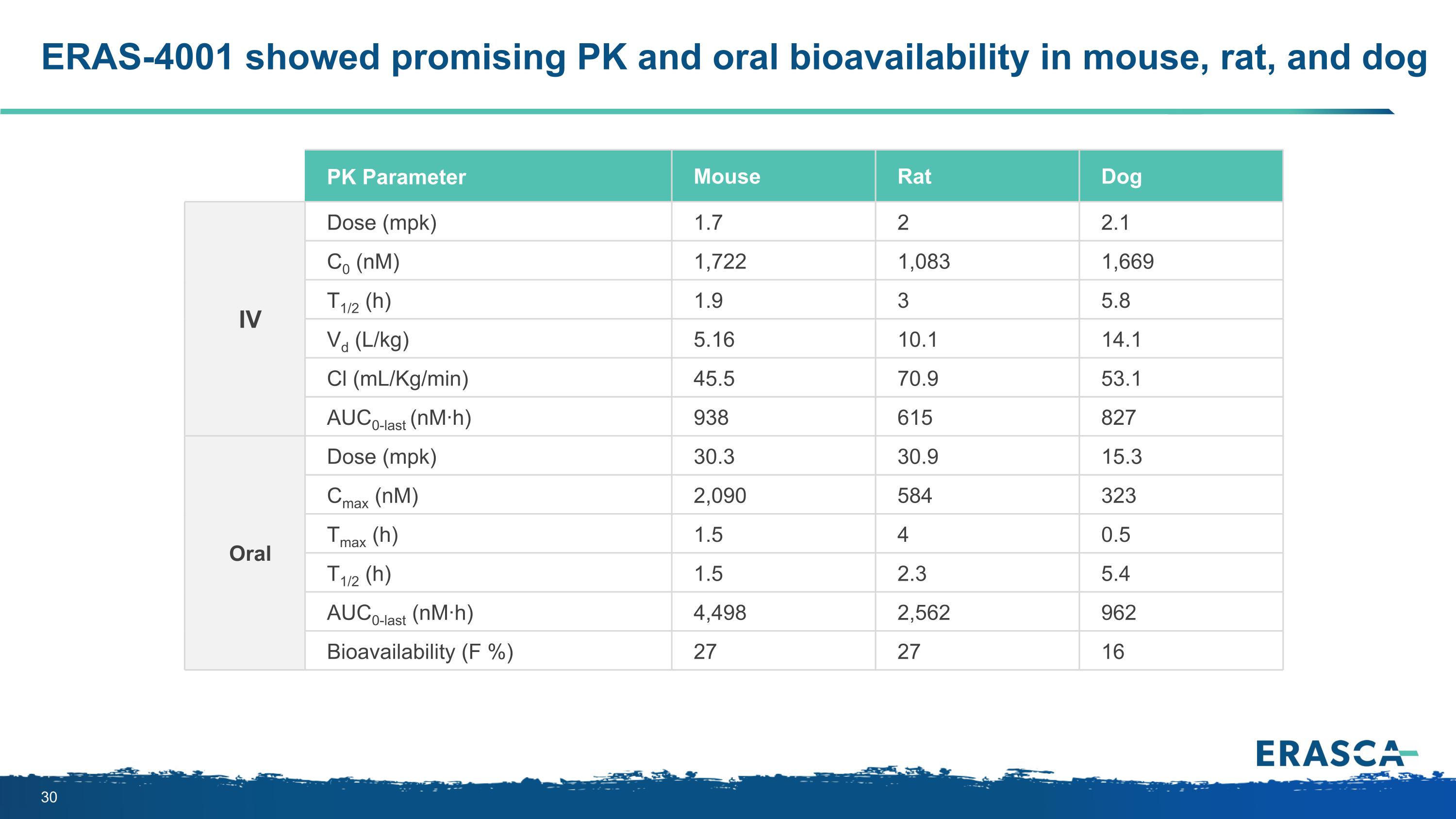
ERAS-4001 showed promising PK and oral bioavailability in mouse, rat, and dog PK Parameter Mouse Rat Dog IV Dose (mpk) 1.7 2 2.1 C0 (nM) 1,722 1,083 1,669 T1/2 (h) 1.9 3 5.8 Vd (L/kg) 5.16 10.1 14.1 Cl (mL/Kg/min) 45.5 70.9 53.1 AUC0-last (nM·h) 938 615 827 Oral Dose (mpk) 30.3 30.9 15.3 Cmax (nM) 2,090 584 323 Tmax (h) 1.5 4 0.5 T1/2 (h) 1.5 2.3 5.4 AUC0-last (nM·h) 4,498 2,562 962 Bioavailability (F %) 27 27 16
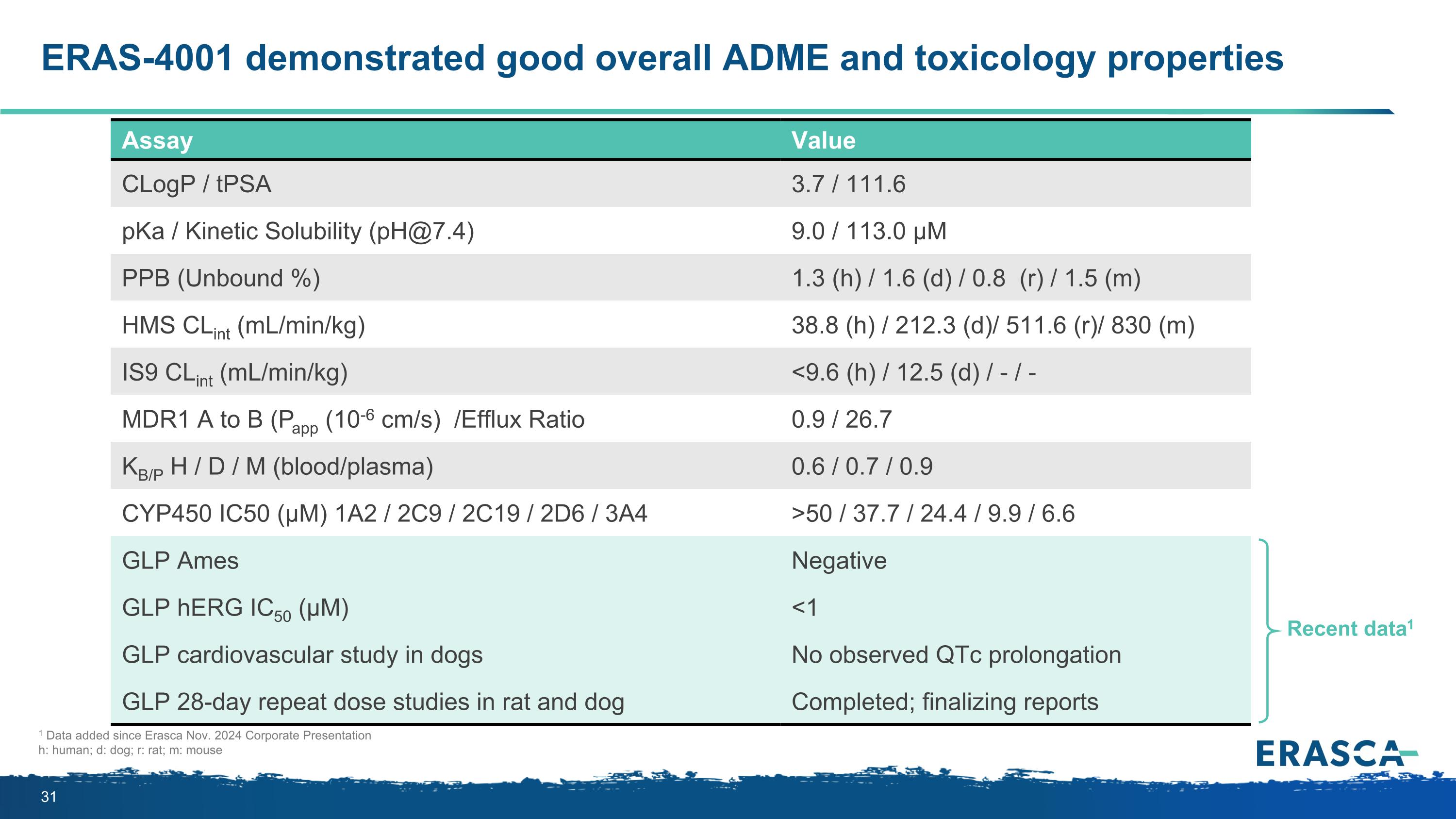
ERAS-4001 demonstrated good overall ADME and toxicology properties Assay Value CLogP / tPSA 3.7 / 111.6 pKa / Kinetic Solubility (pH@7.4) 9.0 / 113.0 µM PPB (Unbound %) 1.3 (h) / 1.6 (d) / 0.8 (r) / 1.5 (m) HMS CLint (mL/min/kg) 38.8 (h) / 212.3 (d)/ 511.6 (r)/ 830 (m) IS9 CLint (mL/min/kg) <9.6 (h) / 12.5 (d) / - / - MDR1 A to B (Papp (10-6 cm/s) /Efflux Ratio 0.9 / 26.7 KB/P H / D / M (blood/plasma) 0.6 / 0.7 / 0.9 CYP450 IC50 (µM) 1A2 / 2C9 / 2C19 / 2D6 / 3A4 >50 / 37.7 / 24.4 / 9.9 / 6.6 GLP Ames Negative GLP hERG IC50 (µM) <1 GLP cardiovascular study in dogs No observed QTc prolongation GLP 28-day repeat dose studies in rat and dog Completed; finalizing reports Recent data1 1 Data added since Erasca Nov. 2024 Corporate Presentation h: human; d: dog; r: rat; m: mouse
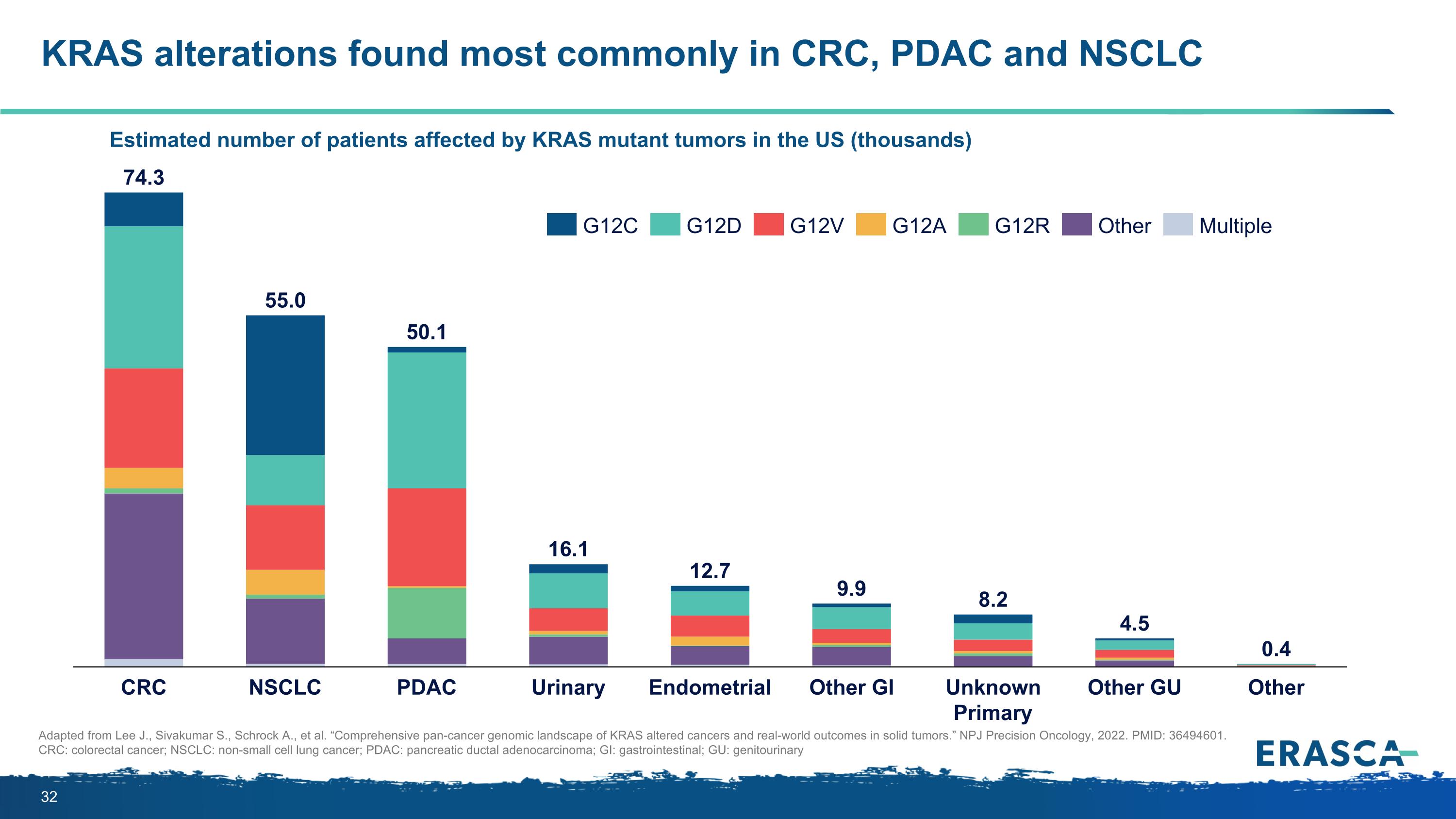
KRAS alterations found most commonly in CRC, PDAC and NSCLC Estimated number of patients affected by KRAS mutant tumors in the US (thousands) Adapted from Lee J., Sivakumar S., Schrock A., et al. “Comprehensive pan-cancer genomic landscape of KRAS altered cancers and real-world outcomes in solid tumors.” NPJ Precision Oncology, 2022. PMID: 36494601. CRC: colorectal cancer; NSCLC: non-small cell lung cancer; PDAC: pancreatic ductal adenocarcinoma; GI: gastrointestinal; GU: genitourinary CRC NSCLC PDAC Urinary Endometrial Other GI Unknown Primary Other GU Other 74.3 55.0 50.1 16.1 12.7 9.9 8.2 4.5 0.4 G12C G12D G12V G12A G12R Other Multiple
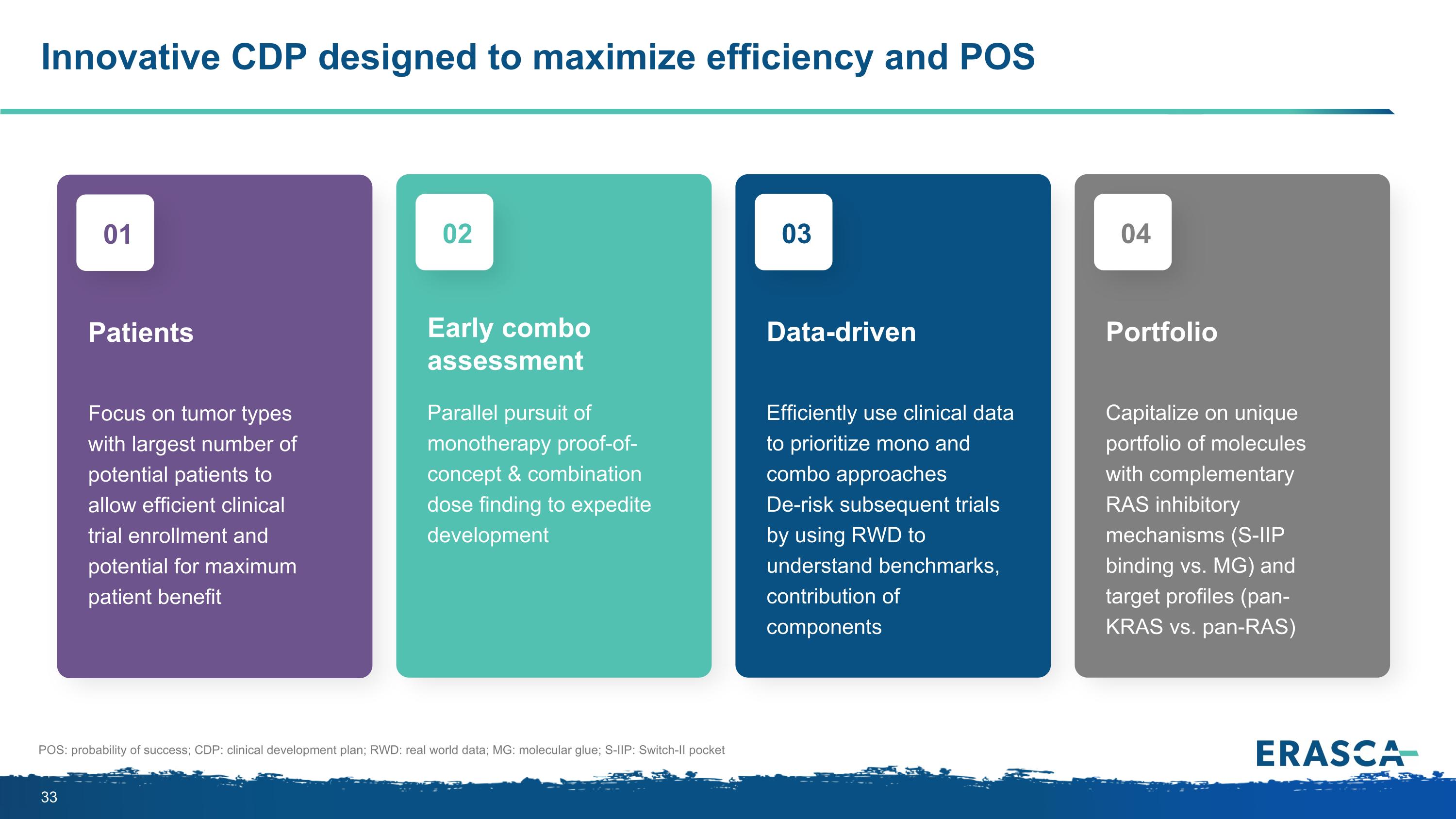
Focus on tumor types with largest number of potential patients to allow efficient clinical trial enrollment and potential for maximum patient benefit Patients 01 Parallel pursuit of monotherapy proof-of-concept & combination dose finding to expedite development Early combo assessment 02 Efficiently use clinical data to prioritize mono and combo approaches De-risk subsequent trials by using RWD to understand benchmarks, contribution of components Data-driven 03 Capitalize on unique portfolio of molecules with complementary RAS inhibitory mechanisms (S-IIP binding vs. MG) and target profiles (pan-KRAS vs. pan-RAS) Portfolio 04 Innovative CDP designed to maximize efficiency and POS POS: probability of success; CDP: clinical development plan; RWD: real world data; MG: molecular glue; S-IIP: Switch-II pocket
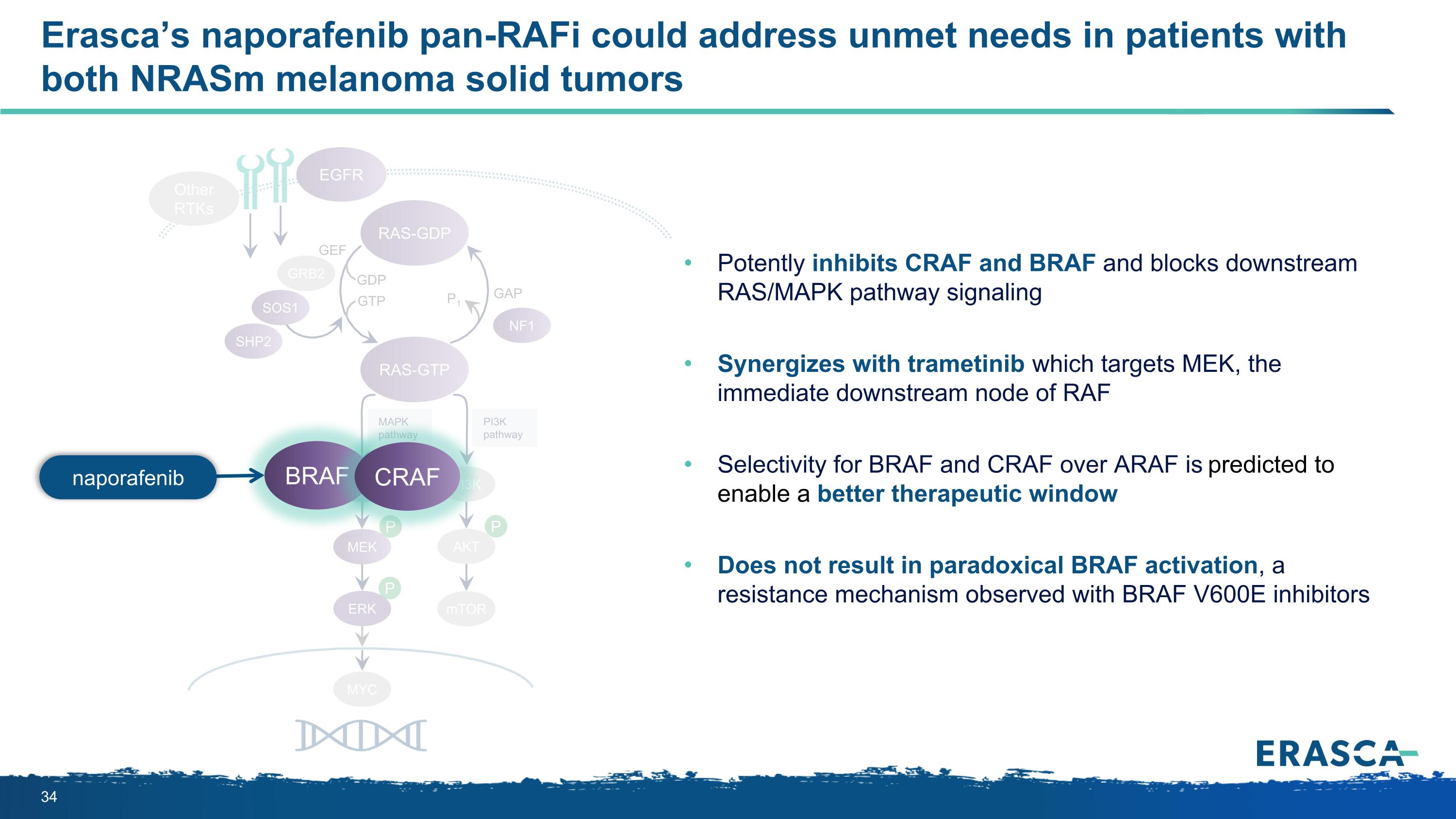
P P P MAPK pathway PI3K pathway RAS-GTP GRB2 SHP2 GAP GDP GTP P1 NF1 SOS1 RAF MEK PI3K AKT mTOR RAS-GDP EGFR Other RTKs MYC GEF Erasca’s naporafenib pan-RAFi could address unmet needs in patients with both NRASm melanoma solid tumors ERK BRAF CRAF Potently inhibits CRAF and BRAF and blocks downstream RAS/MAPK pathway signaling Synergizes with trametinib which targets MEK, the immediate downstream node of RAF Selectivity for BRAF and CRAF over ARAF is predicted to enable a better therapeutic window Does not result in paradoxical BRAF activation, a resistance mechanism observed with BRAF V600E inhibitors naporafenib
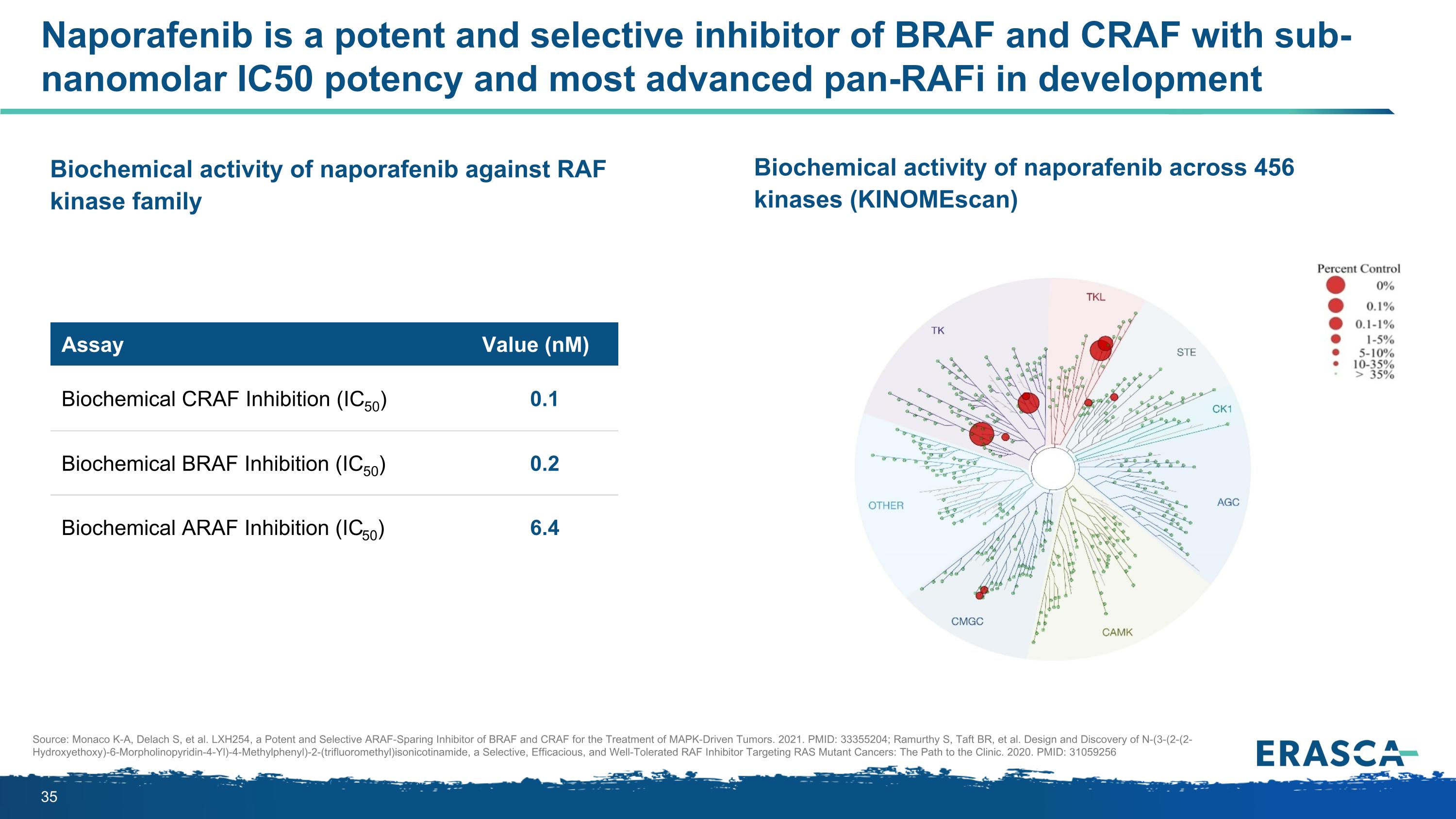
Naporafenib is a potent and selective inhibitor of BRAF and CRAF with sub-nanomolar IC50 potency and most advanced pan-RAFi in development Assay Value (nM) Biochemical CRAF Inhibition (IC50) 0.1 Biochemical BRAF Inhibition (IC50) 0.2 Biochemical ARAF Inhibition (IC50) 6.4 Biochemical activity of naporafenib against RAF kinase family Biochemical activity of naporafenib across 456 kinases (KINOMEscan) Source: Monaco K-A, Delach S, et al. LXH254, a Potent and Selective ARAF-Sparing Inhibitor of BRAF and CRAF for the Treatment of MAPK-Driven Tumors. 2021. PMID: 33355204; Ramurthy S, Taft BR, et al. Design and Discovery of N-(3-(2-(2-Hydroxyethoxy)-6-Morpholinopyridin-4-Yl)-4-Methylphenyl)-2-(trifluoromethyl)isonicotinamide, a Selective, Efficacious, and Well-Tolerated RAF Inhibitor Targeting RAS Mutant Cancers: The Path to the Clinic. 2020. PMID: 31059256
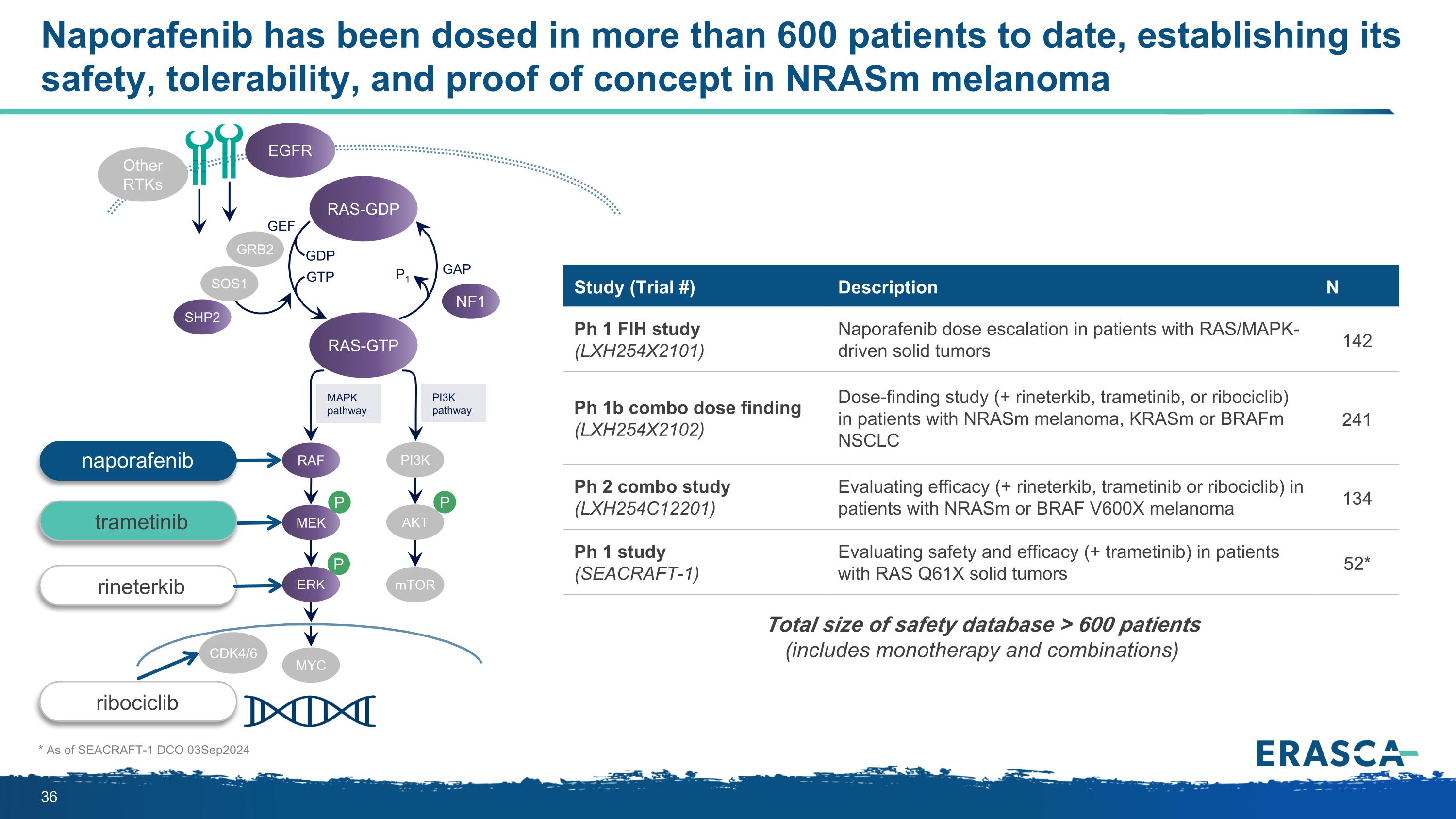
Naporafenib has been dosed in more than 600 patients to date, establishing its safety, tolerability, and proof of concept in NRASm melanoma * As of SEACRAFT-1 DCO 03Sep2024 P P P MAPK pathway PI3K pathway RAS-GTP GRB2 SHP2 GAP GDP GTP P1 NF1 SOS1 RAF MEK PI3K AKT mTOR ERK RAS-GDP EGFR Other RTKs MYC GEF CDK4/6 Study (Trial #) Description N Ph 1 FIH study (LXH254X2101) Naporafenib dose escalation in patients with RAS/MAPK-driven solid tumors 142 Ph 1b combo dose finding (LXH254X2102) Dose-finding study (+ rineterkib, trametinib, or ribociclib) in patients with NRASm melanoma, KRASm or BRAFm NSCLC 241 Ph 2 combo study (LXH254C12201) Evaluating efficacy (+ rineterkib, trametinib or ribociclib) in patients with NRASm or BRAF V600X melanoma 134 Ph 1 study (SEACRAFT-1) Evaluating safety and efficacy (+ trametinib) in patients with RAS Q61X solid tumors 52* Total size of safety database > 600 patients (includes monotherapy and combinations) rineterkib ribociclib naporafenib trametinib
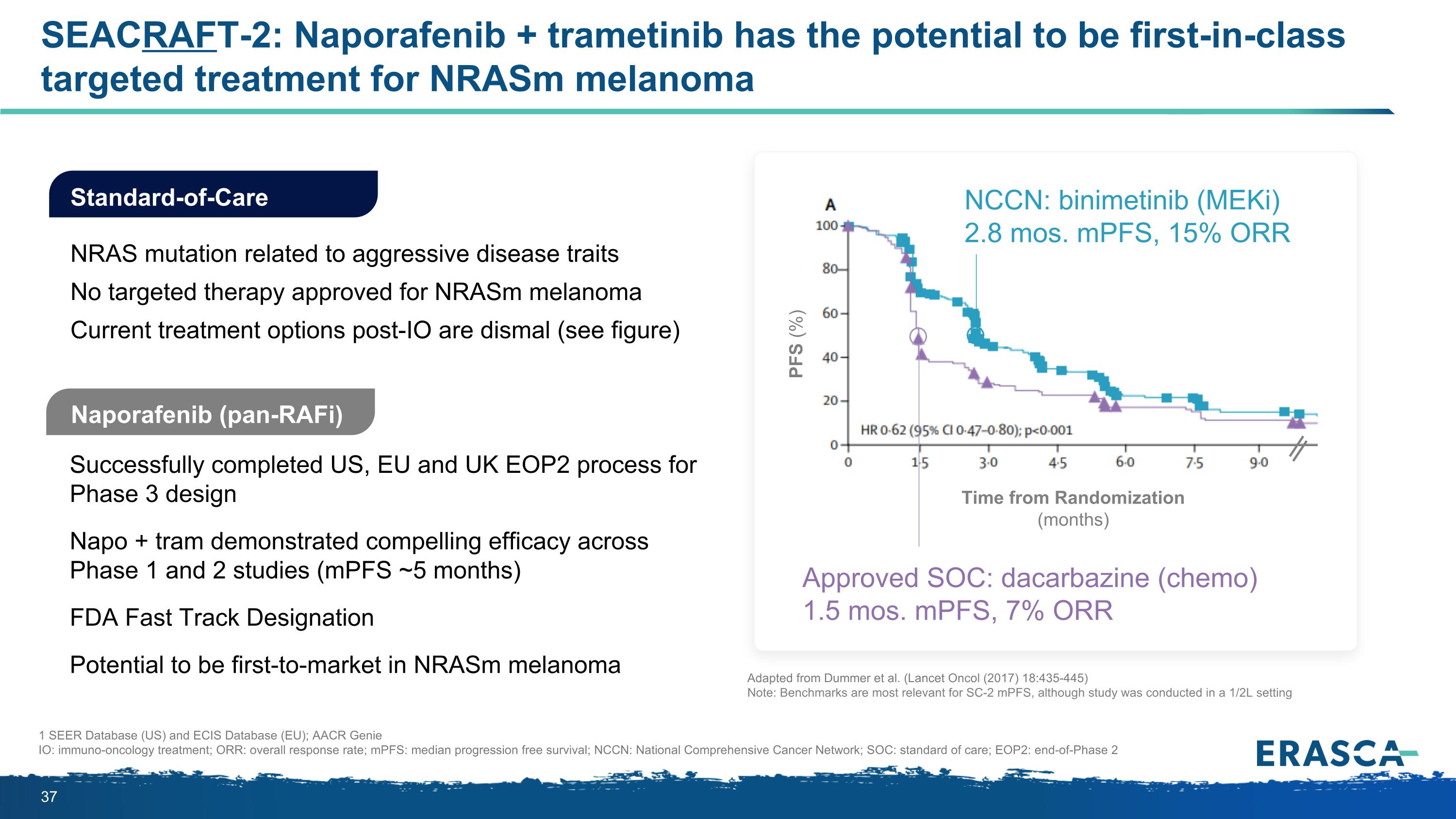
SEACRAFT-2: Naporafenib + trametinib has the potential to be first-in-class targeted treatment for NRASm melanoma Naporafenib (pan-RAFi) Successfully completed US, EU and UK EOP2 process for Phase 3 design Napo + tram demonstrated compelling efficacy across Phase 1 and 2 studies (mPFS ~5 months) FDA Fast Track Designation Potential to be first-to-market in NRASm melanoma Standard-of-Care NRAS mutation related to aggressive disease traits No targeted therapy approved for NRASm melanoma Current treatment options post-IO are dismal (see figure) 1 SEER Database (US) and ECIS Database (EU); AACR Genie IO: immuno-oncology treatment; ORR: overall response rate; mPFS: median progression free survival; NCCN: National Comprehensive Cancer Network; SOC: standard of care; EOP2: end-of-Phase 2 Time from Randomization (months) PFS (%) Approved SOC: dacarbazine (chemo) 1.5 mos. mPFS, 7% ORR NCCN: binimetinib (MEKi) 2.8 mos. mPFS, 15% ORR Adapted from Dummer et al. (Lancet Oncol (2017) 18:435-445) Note: Benchmarks are most relevant for SC-2 mPFS, although study was conducted in a 1/2L setting
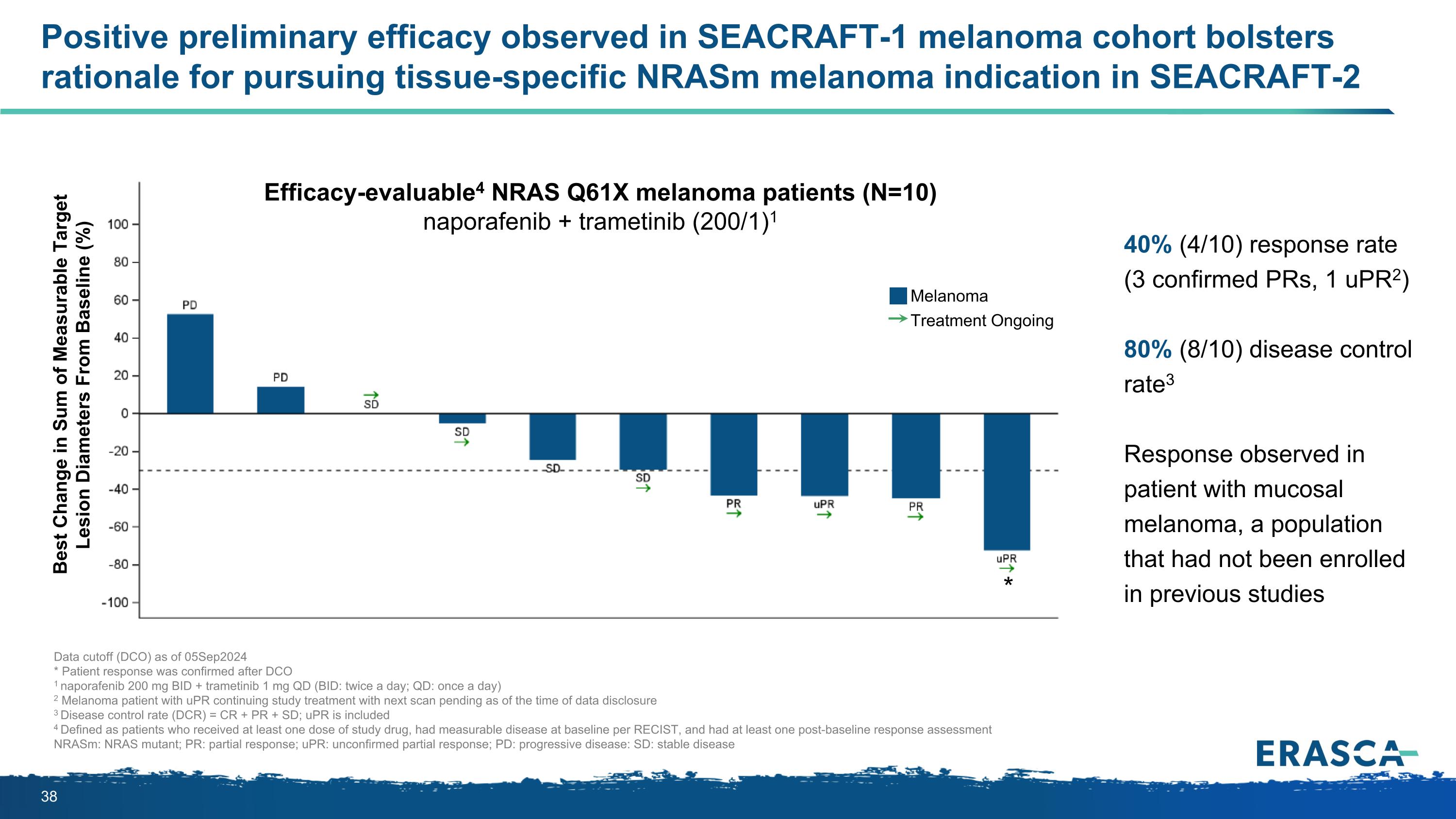
Positive preliminary efficacy observed in SEACRAFT-1 melanoma cohort bolsters rationale for pursuing tissue-specific NRASm melanoma indication in SEACRAFT-2 Data cutoff (DCO) as of 05Sep2024 * Patient response was confirmed after DCO 1 naporafenib 200 mg BID + trametinib 1 mg QD (BID: twice a day; QD: once a day) 2 Melanoma patient with uPR continuing study treatment with next scan pending as of the time of data disclosure 3 Disease control rate (DCR) = CR + PR + SD; uPR is included 4 Defined as patients who received at least one dose of study drug, had measurable disease at baseline per RECIST, and had at least one post-baseline response assessment NRASm: NRAS mutant; PR: partial response; uPR: unconfirmed partial response; PD: progressive disease: SD: stable disease 40% (4/10) response rate (3 confirmed PRs, 1 uPR2) 80% (8/10) disease control rate3 Response observed in patient with mucosal melanoma, a population that had not been enrolled in previous studies Best Change in Sum of Measurable Target Lesion Diameters From Baseline (%) Efficacy-evaluable4 NRAS Q61X melanoma patients (N=10) naporafenib + trametinib (200/1)1 Melanoma Treatment Ongoing *
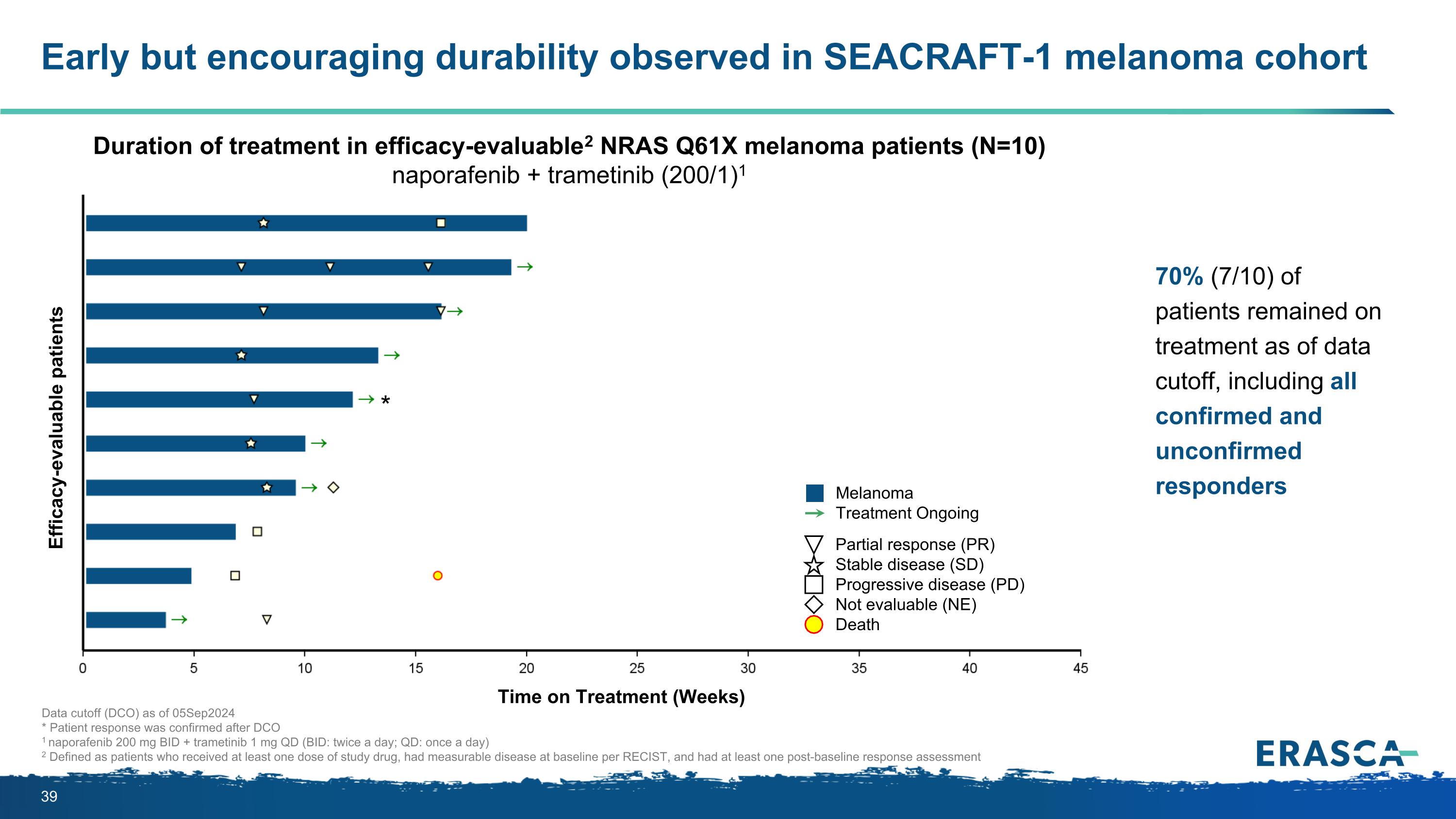
Early but encouraging durability observed in SEACRAFT-1 melanoma cohort Data cutoff (DCO) as of 05Sep2024 * Patient response was confirmed after DCO 1 naporafenib 200 mg BID + trametinib 1 mg QD (BID: twice a day; QD: once a day) 2 Defined as patients who received at least one dose of study drug, had measurable disease at baseline per RECIST, and had at least one post-baseline response assessment 70% (7/10) of patients remained on treatment as of data cutoff, including all confirmed and unconfirmed responders Efficacy-evaluable patients Duration of treatment in efficacy-evaluable2 NRAS Q61X melanoma patients (N=10) naporafenib + trametinib (200/1)1 Partial response (PR) Stable disease (SD) Progressive disease (PD) Not evaluable (NE) Death Melanoma Treatment Ongoing Time on Treatment (Weeks) *
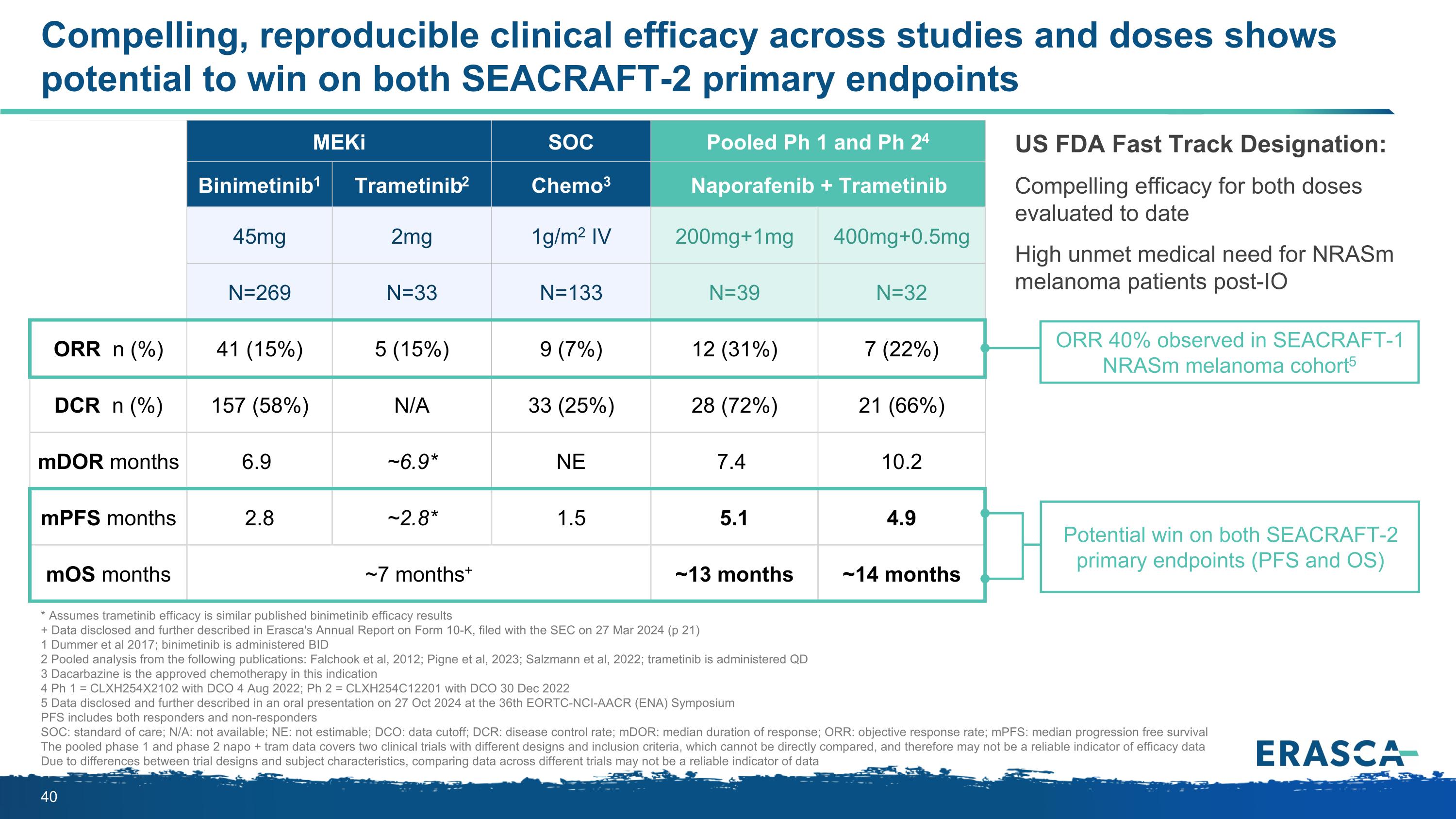
Compelling, reproducible clinical efficacy across studies and doses shows potential to win on both SEACRAFT-2 primary endpoints MEKi Published literature SOC Pooled Ph 1 and Ph 24 Binimetinib1 Trametinib2 Chemo3 Naporafenib + Trametinib 45mg 2mg 1g/m2 IV 200mg+1mg 400mg+0.5mg N=269 N=33 N=133 N=39 N=32 ORR n (%) 41 (15%) 5 (15%) 9 (7%) 12 (31%) 7 (22%) DCR n (%) 157 (58%) N/A 33 (25%) 28 (72%) 21 (66%) mDOR months 6.9 ~6.9* NE 7.4 10.2 mPFS months 2.8 ~2.8* 1.5 5.1 4.9 mOS months ~7 months+ ~13 months ~14 months US FDA Fast Track Designation: Compelling efficacy for both doses evaluated to date High unmet medical need for NRASm melanoma patients post-IO * Assumes trametinib efficacy is similar published binimetinib efficacy results + Data disclosed and further described in Erasca's Annual Report on Form 10-K, filed with the SEC on 27 Mar 2024 (p 21) 1 Dummer et al 2017; binimetinib is administered BID 2 Pooled analysis from the following publications: Falchook et al, 2012; Pigne et al, 2023; Salzmann et al, 2022; trametinib is administered QD 3 Dacarbazine is the approved chemotherapy in this indication 4 Ph 1 = CLXH254X2102 with DCO 4 Aug 2022; Ph 2 = CLXH254C12201 with DCO 30 Dec 2022 5 Data disclosed and further described in an oral presentation on 27 Oct 2024 at the 36th EORTC-NCI-AACR (ENA) Symposium PFS includes both responders and non-responders SOC: standard of care; N/A: not available; NE: not estimable; DCO: data cutoff; DCR: disease control rate; mDOR: median duration of response; ORR: objective response rate; mPFS: median progression free survival The pooled phase 1 and phase 2 napo + tram data covers two clinical trials with different designs and inclusion criteria, which cannot be directly compared, and therefore may not be a reliable indicator of efficacy data Due to differences between trial designs and subject characteristics, comparing data across different trials may not be a reliable indicator of data Potential win on both SEACRAFT-2 primary endpoints (PFS and OS) ORR 40% observed in SEACRAFT-1 NRASm melanoma cohort5
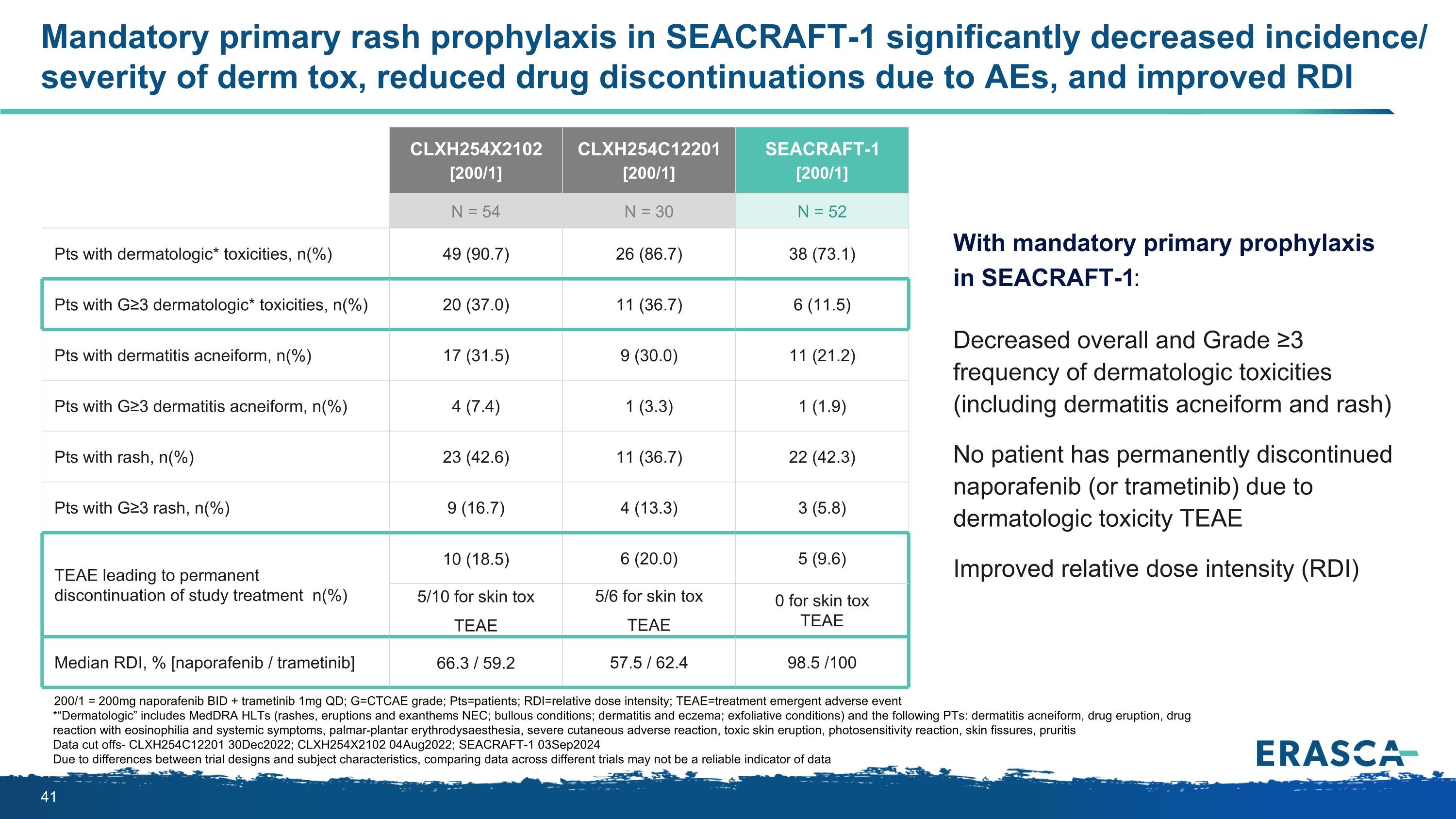
Mandatory primary rash prophylaxis in SEACRAFT-1 significantly decreased incidence/ severity of derm tox, reduced drug discontinuations due to AEs, and improved RDI 200/1 = 200mg naporafenib BID + trametinib 1mg QD; G=CTCAE grade; Pts=patients; RDI=relative dose intensity; TEAE=treatment emergent adverse event *“Dermatologic” includes MedDRA HLTs (rashes, eruptions and exanthems NEC; bullous conditions; dermatitis and eczema; exfoliative conditions) and the following PTs: dermatitis acneiform, drug eruption, drug reaction with eosinophilia and systemic symptoms, palmar-plantar erythrodysaesthesia, severe cutaneous adverse reaction, toxic skin eruption, photosensitivity reaction, skin fissures, pruritis Data cut offs- CLXH254C12201 30Dec2022; CLXH254X2102 04Aug2022; SEACRAFT-1 03Sep2024 Due to differences between trial designs and subject characteristics, comparing data across different trials may not be a reliable indicator of data With mandatory primary prophylaxis in SEACRAFT-1: Decreased overall and Grade ≥3 frequency of dermatologic toxicities (including dermatitis acneiform and rash) No patient has permanently discontinued naporafenib (or trametinib) due to dermatologic toxicity TEAE Improved relative dose intensity (RDI) CLXH254X2102 [200/1] CLXH254C12201 [200/1] SEACRAFT-1 [200/1] N = 54 N = 30 N = 52 Pts with dermatologic* toxicities, n(%) 49 (90.7) 26 (86.7) 38 (73.1) Pts with G≥3 dermatologic* toxicities, n(%) 20 (37.0) 11 (36.7) 6 (11.5) Pts with dermatitis acneiform, n(%) 17 (31.5) 9 (30.0) 11 (21.2) Pts with G≥3 dermatitis acneiform, n(%) 4 (7.4) 1 (3.3) 1 (1.9) Pts with rash, n(%) 23 (42.6) 11 (36.7) 22 (42.3) Pts with G≥3 rash, n(%) 9 (16.7) 4 (13.3) 3 (5.8) TEAE leading to permanent discontinuation of study treatment n(%) 10 (18.5) 6 (20.0) 5 (9.6) 5/10 for skin tox TEAE 5/6 for skin tox TEAE 0 for skin tox TEAE Median RDI, % [naporafenib / trametinib] 66.3 / 59.2 57.5 / 62.4 98.5 /100
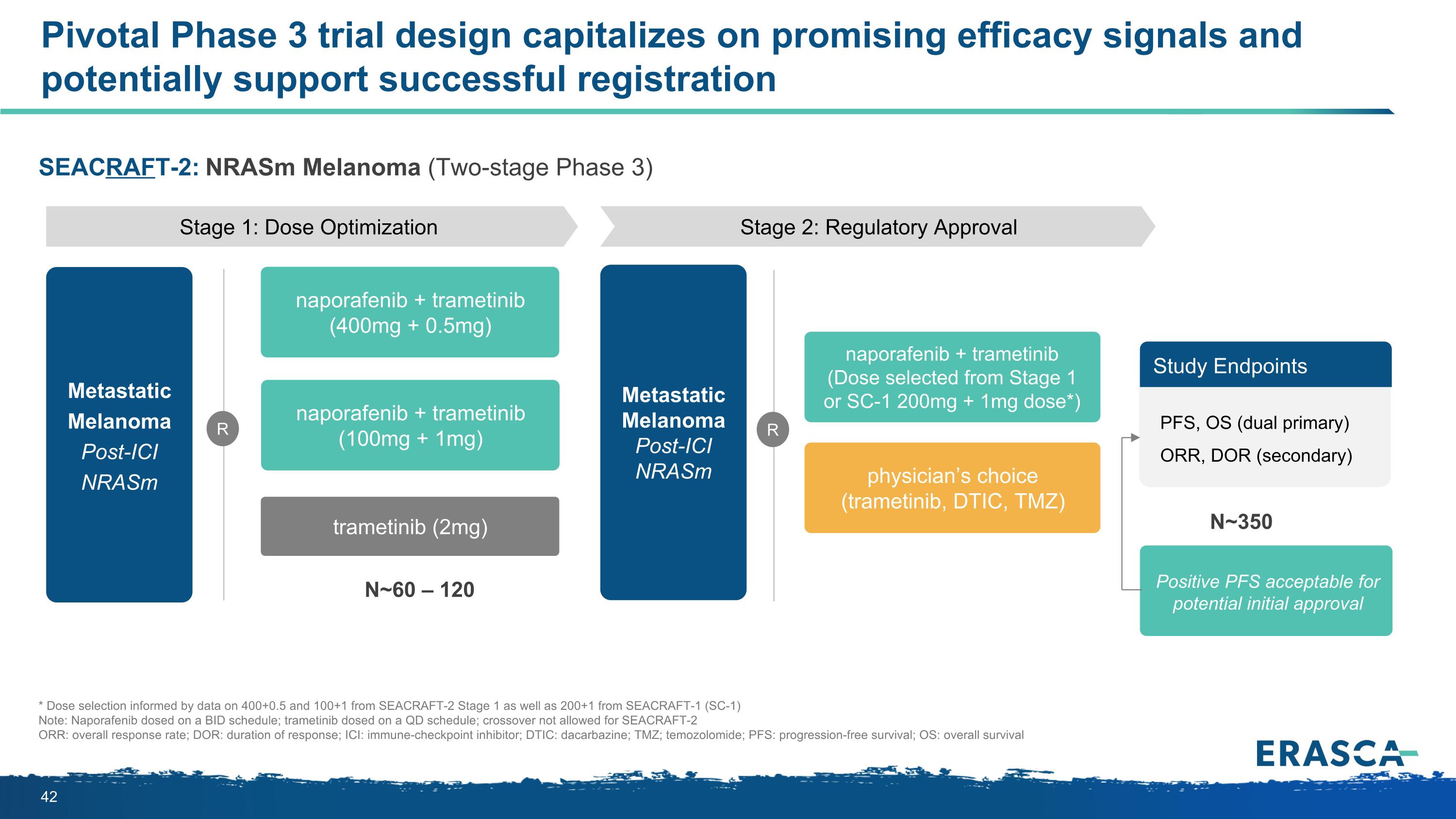
SEACRAFT-2: NRASm Melanoma (Two-stage Phase 3) Pivotal Phase 3 trial design capitalizes on promising efficacy signals and potentially support successful registration N~350 Positive PFS acceptable for potential initial approval * Dose selection informed by data on 400+0.5 and 100+1 from SEACRAFT-2 Stage 1 as well as 200+1 from SEACRAFT-1 (SC-1) Note: Naporafenib dosed on a BID schedule; trametinib dosed on a QD schedule; crossover not allowed for SEACRAFT-2 ORR: overall response rate; DOR: duration of response; ICI: immune-checkpoint inhibitor; DTIC: dacarbazine; TMZ; temozolomide; PFS: progression-free survival; OS: overall survival Stage 1: Dose Optimization Metastatic Melanoma Post-ICI NRASm R Stage 2: Regulatory Approval N~60 – 120 naporafenib + trametinib (400mg + 0.5mg) naporafenib + trametinib (100mg + 1mg) trametinib (2mg) Metastatic Melanoma Post-ICI NRASm R naporafenib + trametinib (Dose selected from Stage 1 or SC-1 200mg + 1mg dose*) physician’s choice (trametinib, DTIC, TMZ) Study Endpoints PFS, OS (dual primary) ORR, DOR (secondary)
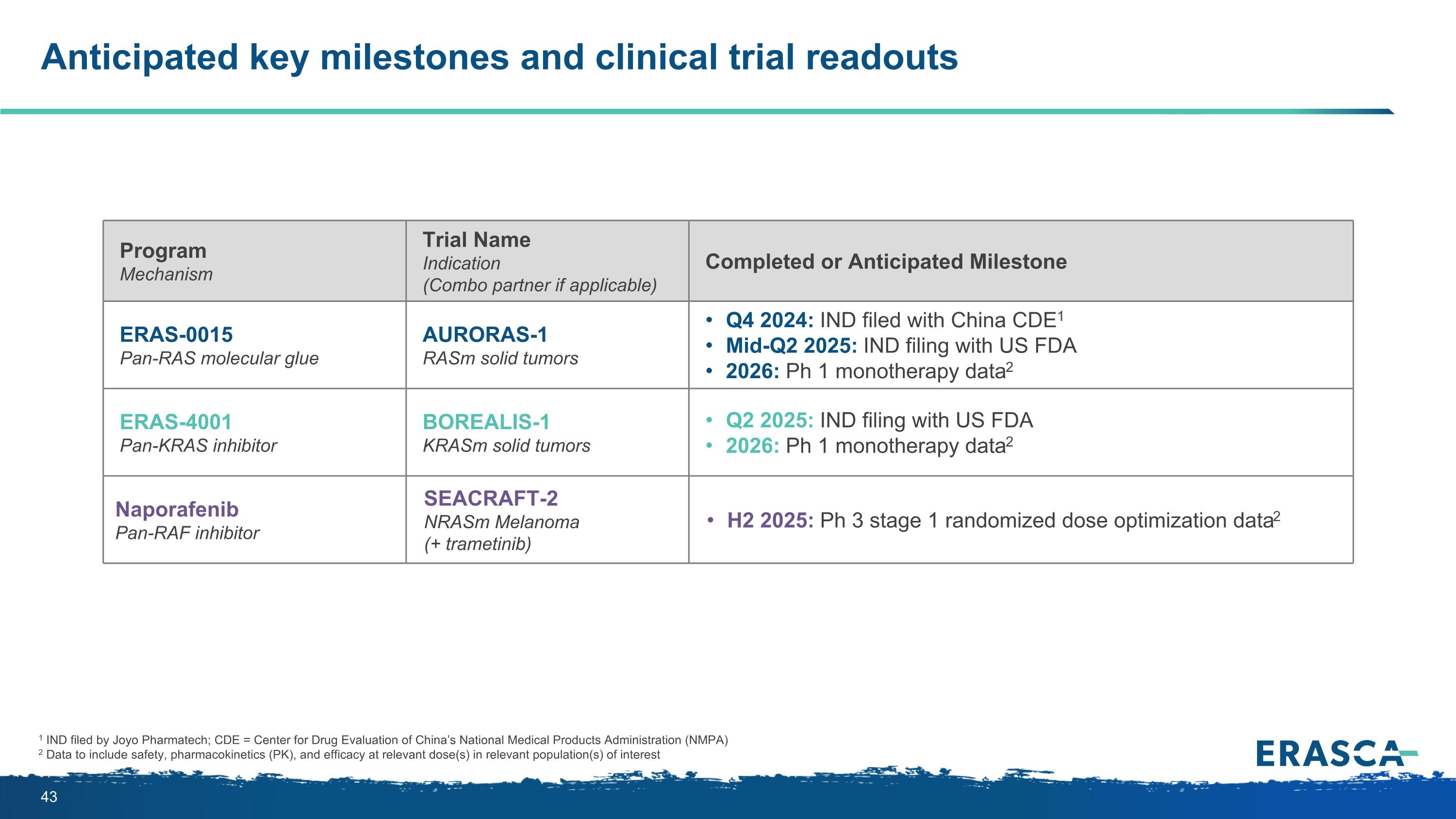
Anticipated key milestones and clinical trial readouts 1 IND filed by Joyo Pharmatech; CDE = Center for Drug Evaluation of China’s National Medical Products Administration (NMPA) 2 Data to include safety, pharmacokinetics (PK), and efficacy at relevant dose(s) in relevant population(s) of interest Program Mechanism Trial Name Indication (Combo partner if applicable) Completed or Anticipated Milestone ERAS-0015 Pan-RAS molecular glue AURORAS-1 RASm solid tumors Q4 2024: IND filed with China CDE1 Mid-Q2 2025: IND filing with US FDA 2026: Ph 1 monotherapy data2 ERAS-4001 Pan-KRAS inhibitor BOREALIS-1 KRASm solid tumors Q2 2025: IND filing with US FDA 2026: Ph 1 monotherapy data2 Naporafenib Pan-RAF inhibitor SEACRAFT-2 NRASm Melanoma (+ trametinib) H2 2025: Ph 3 stage 1 randomized dose optimization data2

Compelling investment thesis Experienced team with track record of serial successes Seasoned drug developers who have advanced multiple programs from discovery to IND to global approvals World-class Scientific Advisory Board Includes leading pioneers in the RAS/MAPK pathway (Shokat, UCSF; Lito, MSKCC; Rodriguez-Viciana, UCL; Cichowski, HMS; Blacklow, HMS; Corcoran, MGH), precision oncology (Demetri, DFCI; Bernards, NKI), and biopharma (Varney, Genentech) Broad portfolio to erase cancer We have built one of the deepest pipelines in the industry to comprehensively shut down the RAS/MAPK pathway, with the potential to address unmet needs in over 5 million patients globally Phase 3 company with two additional programs entering clinic in 2025 Differentiated programs including naporafenib, a Phase 3 pan-RAF inhibitor for NRASm melanoma, and a potential best-in-class RAS franchise composed of a pan-RAS molecular glue and pan-KRASi Multiple potential near-term and long-term value drivers Focused clinical development plan with multiple clinical readouts

THANK YOU!
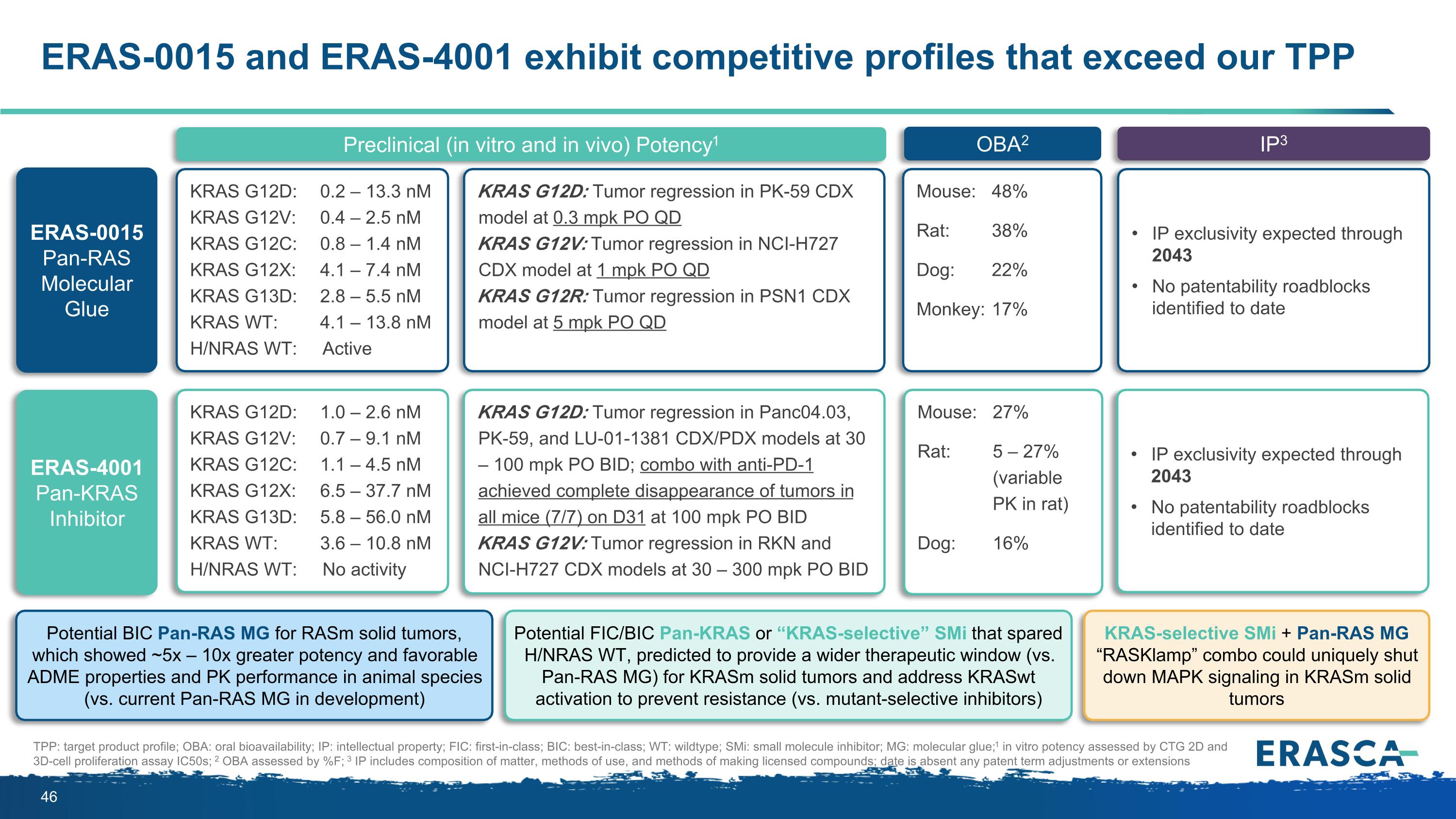
ERAS-0015 and ERAS-4001 exhibit competitive profiles that exceed our TPP Preclinical (in vitro and in vivo) Potency1 OBA2 IP3 TPP: target product profile; OBA: oral bioavailability; IP: intellectual property; FIC: first-in-class; BIC: best-in-class; WT: wildtype; SMi: small molecule inhibitor; MG: molecular glue; 1 in vitro potency assessed by CTG 2D and 3D-cell proliferation assay IC50s; 2 OBA assessed by %F; 3 IP includes composition of matter, methods of use, and methods of making licensed compounds; date is absent any patent term adjustments or extensions ERAS-0015 Pan-RAS Molecular Glue KRAS G12D: 0.2 – 13.3 nM KRAS G12V: 0.4 – 2.5 nM KRAS G12C: 0.8 – 1.4 nM KRAS G12X: 4.1 – 7.4 nM KRAS G13D: 2.8 – 5.5 nM KRAS WT: 4.1 – 13.8 nM H/NRAS WT: Active KRAS G12D: Tumor regression in PK-59 CDX model at 0.3 mpk PO QD KRAS G12V: Tumor regression in NCI-H727 CDX model at 1 mpk PO QD KRAS G12R: Tumor regression in PSN1 CDX model at 5 mpk PO QD Mouse: 48% Rat: 38% Dog: 22% Monkey: 17% IP exclusivity expected through 2043 No patentability roadblocks identified to date Potential BIC Pan-RAS MG for RASm solid tumors, which showed ~5x – 10x greater potency and favorable ADME properties and PK performance in animal species (vs. current Pan-RAS MG in development) ERAS-4001 Pan-KRAS Inhibitor KRAS G12D: 1.0 – 2.6 nM KRAS G12V: 0.7 – 9.1 nM KRAS G12C: 1.1 – 4.5 nM KRAS G12X: 6.5 – 37.7 nM KRAS G13D: 5.8 – 56.0 nM KRAS WT: 3.6 – 10.8 nM H/NRAS WT: No activity KRAS G12D: Tumor regression in Panc04.03, PK-59, and LU-01-1381 CDX/PDX models at 30 – 100 mpk PO BID; combo with anti-PD-1 achieved complete disappearance of tumors in all mice (7/7) on D31 at 100 mpk PO BID KRAS G12V: Tumor regression in RKN and NCI-H727 CDX models at 30 – 300 mpk PO BID Mouse: 27% Rat: 5 – 27% (variable PK in rat) Dog: 16% IP exclusivity expected through 2043 No patentability roadblocks identified to date KRAS-selective SMi + Pan-RAS MG “RASKlamp” combo could uniquely shut down MAPK signaling in KRASm solid tumors Potential FIC/BIC Pan-KRAS or “KRAS-selective” SMi that spared H/NRAS WT, predicted to provide a wider therapeutic window (vs. Pan-RAS MG) for KRASm solid tumors and address KRASwt activation to prevent resistance (vs. mutant-selective inhibitors)
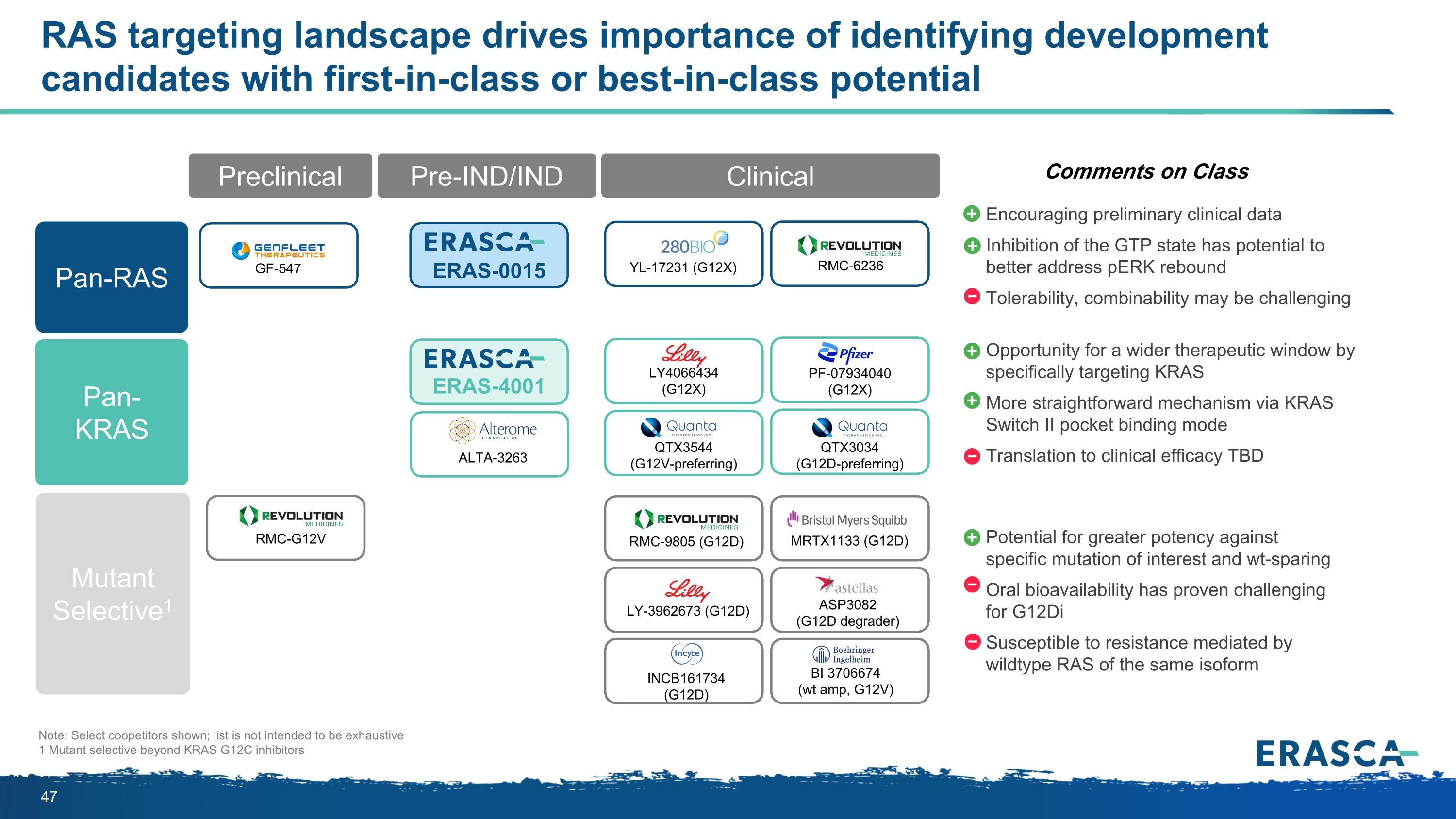
Comments on Class Opportunity for a wider therapeutic window by specifically targeting KRAS More straightforward mechanism via KRAS Switch II pocket binding mode Translation to clinical efficacy TBD Potential for greater potency against specific mutation of interest and wt-sparing Oral bioavailability has proven challenging for G12Di Susceptible to resistance mediated by wildtype RAS of the same isoform Note: Select coopetitors shown; list is not intended to be exhaustive 1 Mutant selective beyond KRAS G12C inhibitors Encouraging preliminary clinical data Inhibition of the GTP state has potential to better address pERK rebound Tolerability, combinability may be challenging RAS targeting landscape drives importance of identifying development candidates with first-in-class or best-in-class potential Clinical Preclinical Pre-IND/IND Clinical YL-17231 (G12X) RMC-G12V GF-547 QTX3034 (G12D-preferring) RMC-6236 ERAS-0015 ERAS-4001 PF-07934040 (G12X) LY4066434 (G12X) ALTA-3263 QTX3544 (G12V-preferring) MRTX1133 (G12D) ASP3082 (G12D degrader) BI 3706674 (wt amp, G12V) LY-3962673 (G12D) INCB161734 (G12D) RMC-9805 (G12D) Pan-RAS Pan-KRAS Mutant Selective1
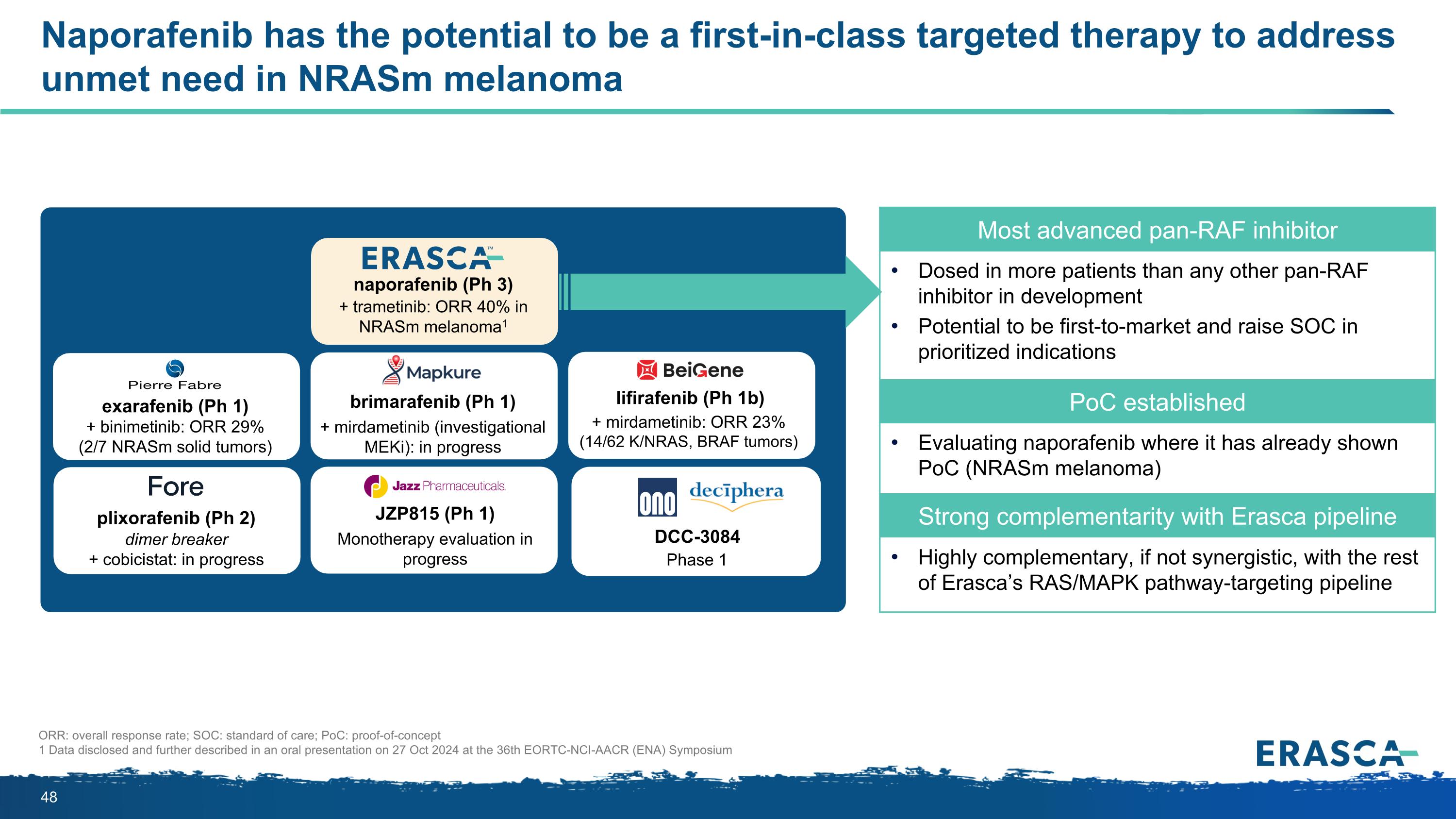
Naporafenib has the potential to be a first-in-class targeted therapy to address unmet need in NRASm melanoma Most advanced pan-RAF inhibitor Dosed in more patients than any other pan-RAF inhibitor in development Potential to be first-to-market and raise SOC in prioritized indications PoC established Evaluating naporafenib where it has already shown PoC (NRASm melanoma) Strong complementarity with Erasca pipeline Highly complementary, if not synergistic, with the rest of Erasca’s RAS/MAPK pathway-targeting pipeline naporafenib (Ph 3) + trametinib: ORR 40% in NRASm melanoma1 lifirafenib (Ph 1b) + mirdametinib: ORR 23% (14/62 K/NRAS, BRAF tumors) plixorafenib (Ph 2) dimer breaker + cobicistat: in progress JZP815 (Ph 1) Monotherapy evaluation in progress brimarafenib (Ph 1) + mirdametinib (investigational MEKi): in progress exarafenib (Ph 1) + binimetinib: ORR 29% (2/7 NRASm solid tumors) DCC-3084 Phase 1 ORR: overall response rate; SOC: standard of care; PoC: proof-of-concept 1 Data disclosed and further described in an oral presentation on 27 Oct 2024 at the 36th EORTC-NCI-AACR (ENA) Symposium
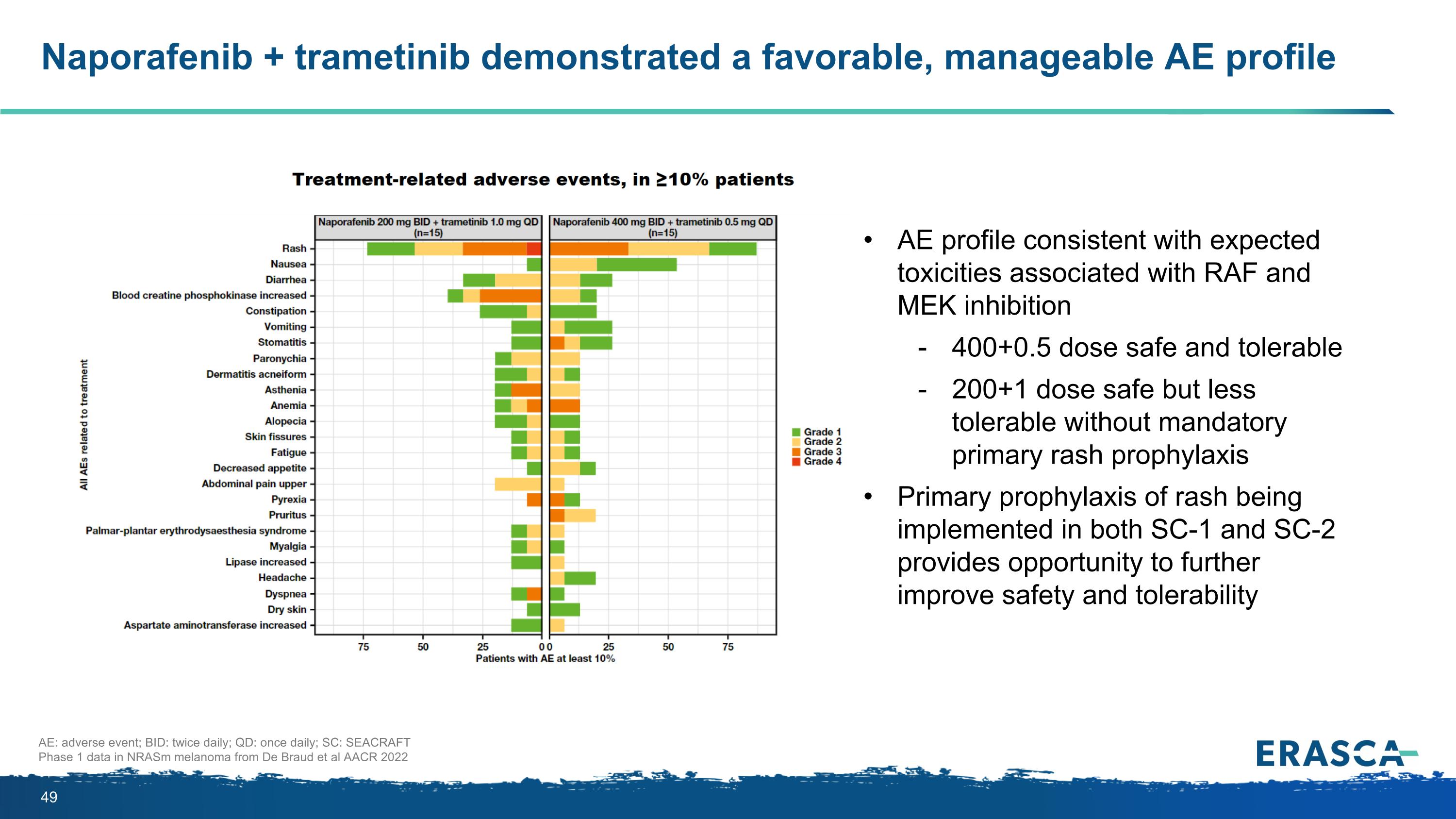
Naporafenib + trametinib demonstrated a favorable, manageable AE profile AE: adverse event; BID: twice daily; QD: once daily; SC: SEACRAFT Phase 1 data in NRASm melanoma from De Braud et al AACR 2022 AE profile consistent with expected toxicities associated with RAF and MEK inhibition 400+0.5 dose safe and tolerable 200+1 dose safe but less tolerable without mandatory primary rash prophylaxis Primary prophylaxis of rash being implemented in both SC-1 and SC-2 provides opportunity to further improve safety and tolerability
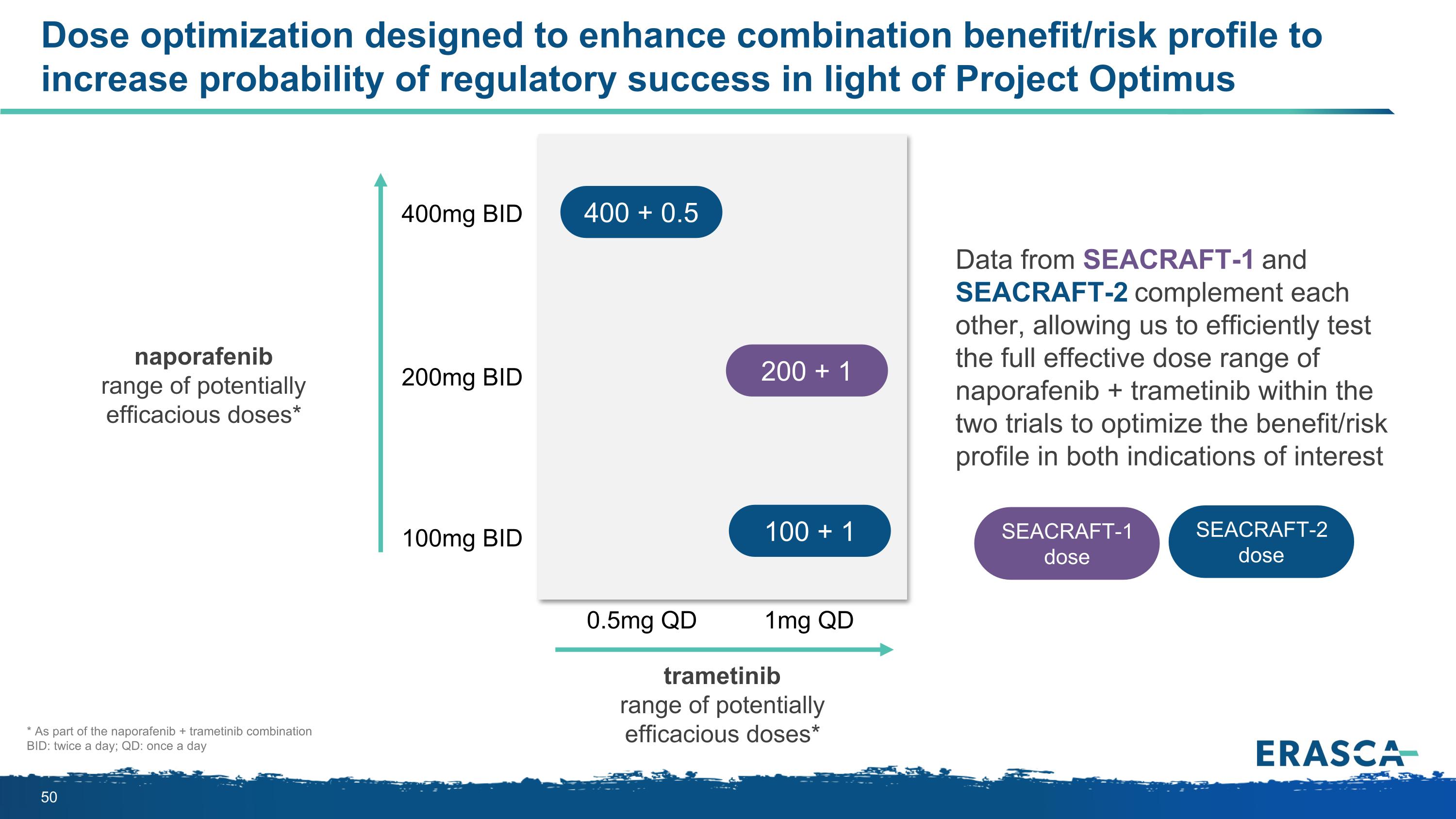
Dose optimization designed to enhance combination benefit/risk profile to increase probability of regulatory success in light of Project Optimus naporafenib range of potentially efficacious doses* 400mg BID 100mg BID 200mg BID 0.5mg QD 1mg QD Data from SEACRAFT-1 and SEACRAFT-2 complement each other, allowing us to efficiently test the full effective dose range of naporafenib + trametinib within the two trials to optimize the benefit/risk profile in both indications of interest * As part of the naporafenib + trametinib combination BID: twice a day; QD: once a day trametinib range of potentially efficacious doses* 400 + 0.5 100 + 1 SEACRAFT-2 dose SEACRAFT-1 dose 200 + 1
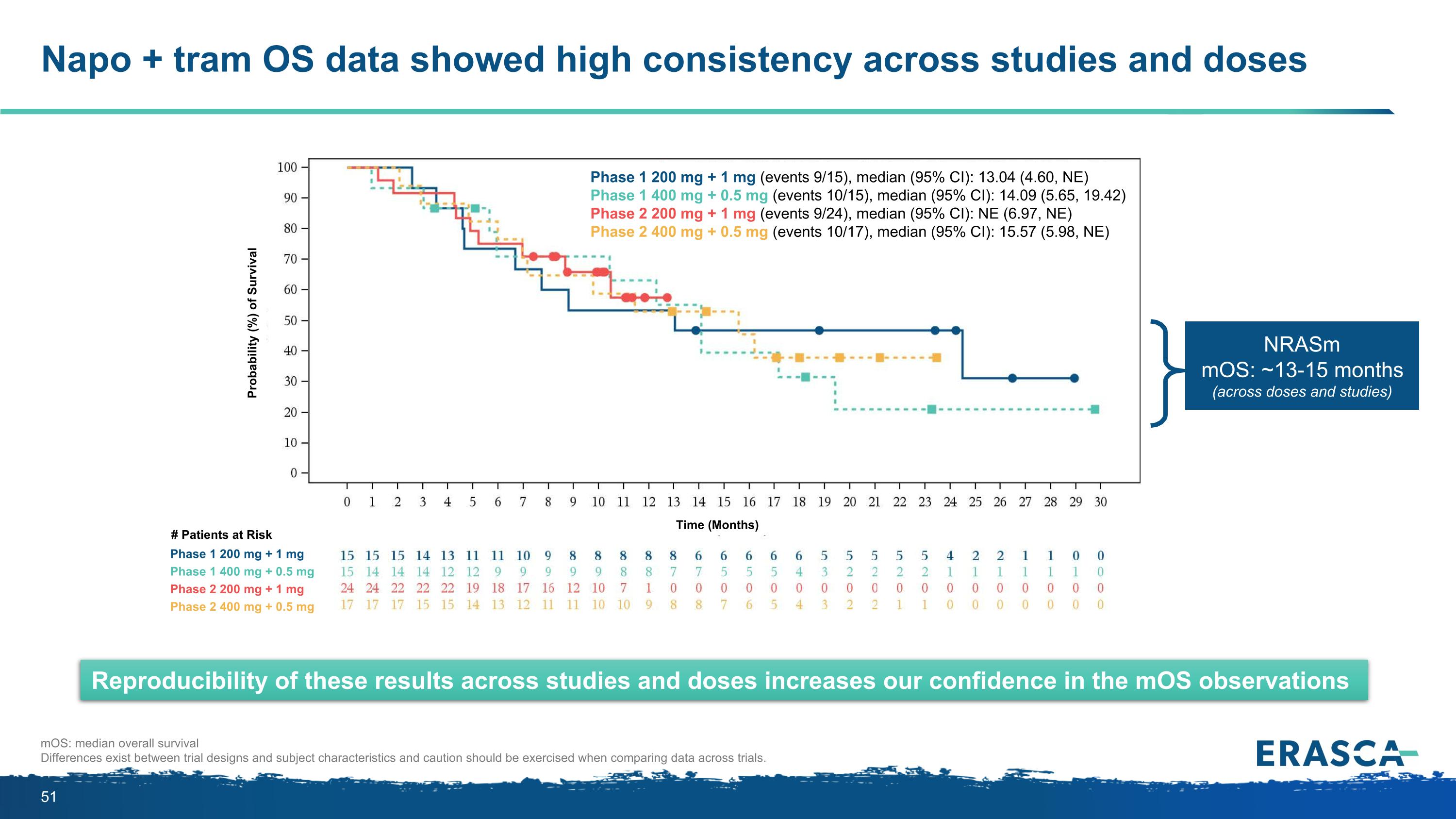
Napo + tram OS data showed high consistency across studies and doses mOS: median overall survival Differences exist between trial designs and subject characteristics and caution should be exercised when comparing data across trials. Reproducibility of these results across studies and doses increases our confidence in the mOS observations NRASm mOS: ~13-15 months (across doses and studies) Phase 1 200 mg + 1 mg (events 9/15), median (95% CI): 13.04 (4.60, NE) Phase 1 400 mg + 0.5 mg (events 10/15), median (95% CI): 14.09 (5.65, 19.42) Phase 2 200 mg + 1 mg (events 9/24), median (95% CI): NE (6.97, NE) Phase 2 400 mg + 0.5 mg (events 10/17), median (95% CI): 15.57 (5.98, NE) Phase 1 200 mg + 1 mg Phase 1 400 mg + 0.5 mg Phase 2 200 mg + 1 mg Phase 2 400 mg + 0.5 mg # Patients at Risk Time (Months) Probability (%) of Survival