
Erasca R&D Day September 2022 Exhibit 99.1

We caution you that this presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements regarding our future results of operations and financial position, business strategy, research and development plans, the anticipated timing, costs, design and conduct of our ongoing and planned preclinical studies and clinical trials for our product candidates, the potential benefits from our current or future arrangements with third parties, the timing and likelihood of success of our plans and objectives, and future results of anticipated product development efforts, are forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar expressions. The inclusion of forward-looking statements should not be regarded as a representation by us that any of our plans will be achieved. Actual results may differ from those set forth in this presentation due to the risks and uncertainties inherent in our business, including, without limitation: our approach to the discovery and development of product candidates based on our singular focus on shutting down the RAS/MAPK pathway, a novel and unproven approach; we are early in our development efforts and have only three product candidates in early clinical development and all of our other development efforts are in the preclinical or development stage; the retrospective analysis of pooled data covers multiple clinical trials with different designs, inclusion criteria, and dosing regimens, which cannot be directly compared, and therefore may not be a reliable indicator of efficacy and safety data; interim results of clinical trials are not necessarily indicative of final results and one or more of the clinical outcomes may materially change as patient enrollment continues, following more comprehensive reviews of the data and more patient data become available; potential delays in the commencement, enrollment, and completion of clinical trials and preclinical studies; our dependence on third parties in connection with manufacturing, research, and preclinical and clinical testing; unexpected adverse side effects or inadequate efficacy of our product candidates that may limit their development, regulatory approval, and/or commercialization, or may result in recalls or product liability claims; unfavorable results from preclinical studies or clinical trials; results from preclinical studies or early clinical trials not necessarily being predictive of future results; the inability to realize any benefits from our current licenses and acquisitions and any future licenses, acquisitions, or collaborations, and our ability to fulfill our obligations under such arrangements; regulatory developments in the United States and foreign countries; our ability to obtain and maintain intellectual property protection for our product candidates; our ability to fund our operating plans with our current cash, cash equivalents, and investments; our ability to maintain undisrupted business operations due to the COVID-19 pandemic, including delaying or disrupting our clinical trials, manufacturing, and supply chain; unstable market and economic conditions having serious adverse consequences on our business, financial condition and stock price; and other risks described in our prior filings with the Securities and Exchange Commission (SEC), including under the heading “Risk Factors” in our annual report on Form 10-K for the year ended December 31, 2021, and any subsequent filings with the SEC. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and we undertake no obligation to update such statements to reflect events that occur or circumstances that exist after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, which is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us. Disclaimer: Forward looking statements and market data

Company Overview Erasca pipeline and drug development strategy Key scientific hypotheses and supportive preclinical data Dr. David Hong, MD Anderson Cancer Center Discussant Future directions Key milestones and clinical trial readouts Closing Remarks Q&A Session ERAS-007 / ERAS-601 Preliminary Results Retrospective pooled efficacy analysis Preliminary safety and pharmacokinetic assessment Clinical development plan for combinations Erasca R&D Day Agenda
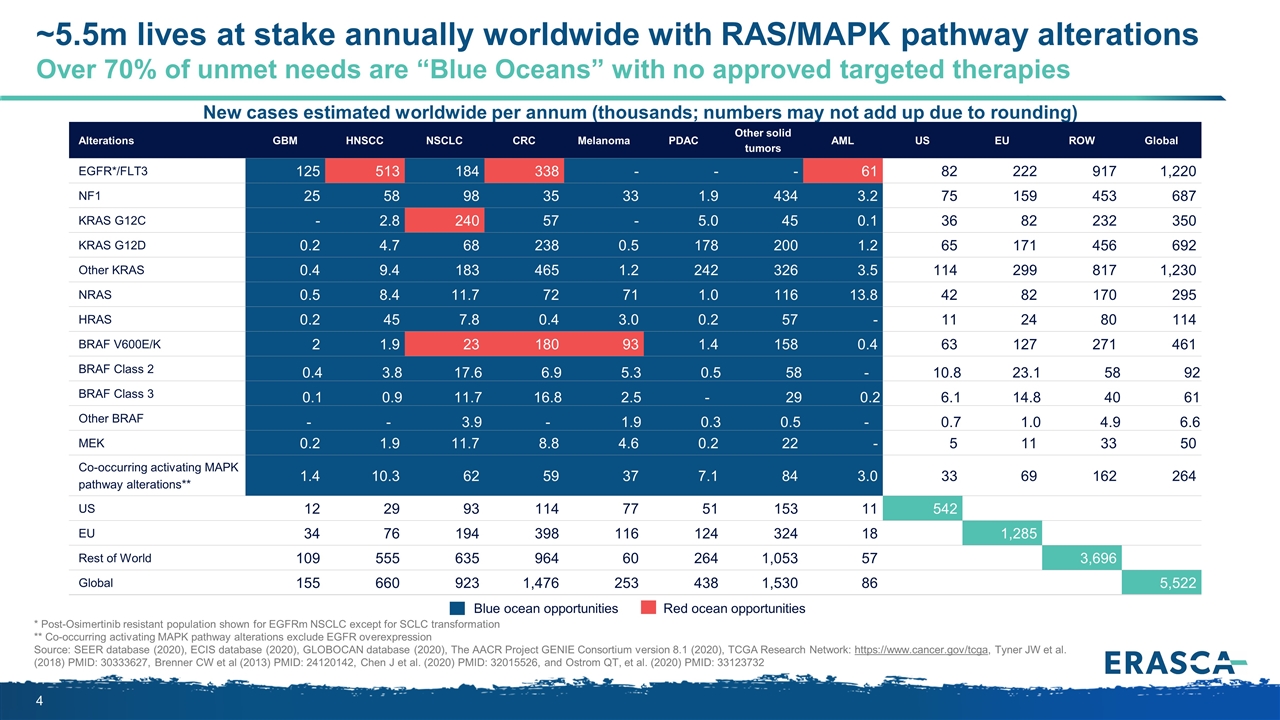
* Post-Osimertinib resistant population shown for EGFRm NSCLC except for SCLC transformation ** Co-occurring activating MAPK pathway alterations exclude EGFR overexpression Source: SEER database (2020), ECIS database (2020), GLOBOCAN database (2020), The AACR Project GENIE Consortium version 8.1 (2020), TCGA Research Network: https://www.cancer.gov/tcga, Tyner JW et al. (2018) PMID: 30333627, Brenner CW et al (2013) PMID: 24120142, Chen J et al. (2020) PMID: 32015526, and Ostrom QT, et al. (2020) PMID: 33123732 New cases estimated worldwide per annum (thousands; numbers may not add up due to rounding) Blue ocean opportunities Red ocean opportunities Alterations GBM HNSCC NSCLC CRC Melanoma PDAC Other solid tumors AML US EU ROW Global EGFR*/FLT3 125 513 184 338 - - - 61 82 222 917 1,220 NF1 25 58 98 35 33 1.9 434 3.2 75 159 453 687 KRAS G12C - 2.8 240 57 - 5.0 45 0.1 36 82 232 350 KRAS G12D 0.2 4.7 68 238 0.5 178 200 1.2 65 171 456 692 Other KRAS 0.4 9.4 183 465 1.2 242 326 3.5 114 299 817 1,230 NRAS 0.5 8.4 11.7 72 71 1.0 116 13.8 42 82 170 295 HRAS 0.2 45 7.8 0.4 3.0 0.2 57 - 11 24 80 114 BRAF V600E/K 2 1.9 23 180 93 1.4 158 0.4 63 127 271 461 BRAF Class 2 0.4 3.8 17.6 6.9 5.3 0.5 58 - 10.8 23.1 58 92 BRAF Class 3 0.1 0.9 11.7 16.8 2.5 - 29 0.2 6.1 14.8 40 61 Other BRAF - - 3.9 - 1.9 0.3 0.5 - 0.7 1.0 4.9 6.6 MEK 0.2 1.9 11.7 8.8 4.6 0.2 22 - 5 11 33 50 Co-occurring activating MAPK pathway alterations** 1.4 10.3 62 59 37 7.1 84 3.0 33 69 162 264 US 12 29 93 114 77 51 153 11 542 EU 34 76 194 398 116 124 324 18 1,285 Rest of World 109 555 635 964 60 264 1,053 57 3,696 Global 155 660 923 1,476 253 438 1,530 86 5,522 ~5.5m lives at stake annually worldwide with RAS/MAPK pathway alterations Over 70% of unmet needs are “Blue Oceans” with no approved targeted therapies
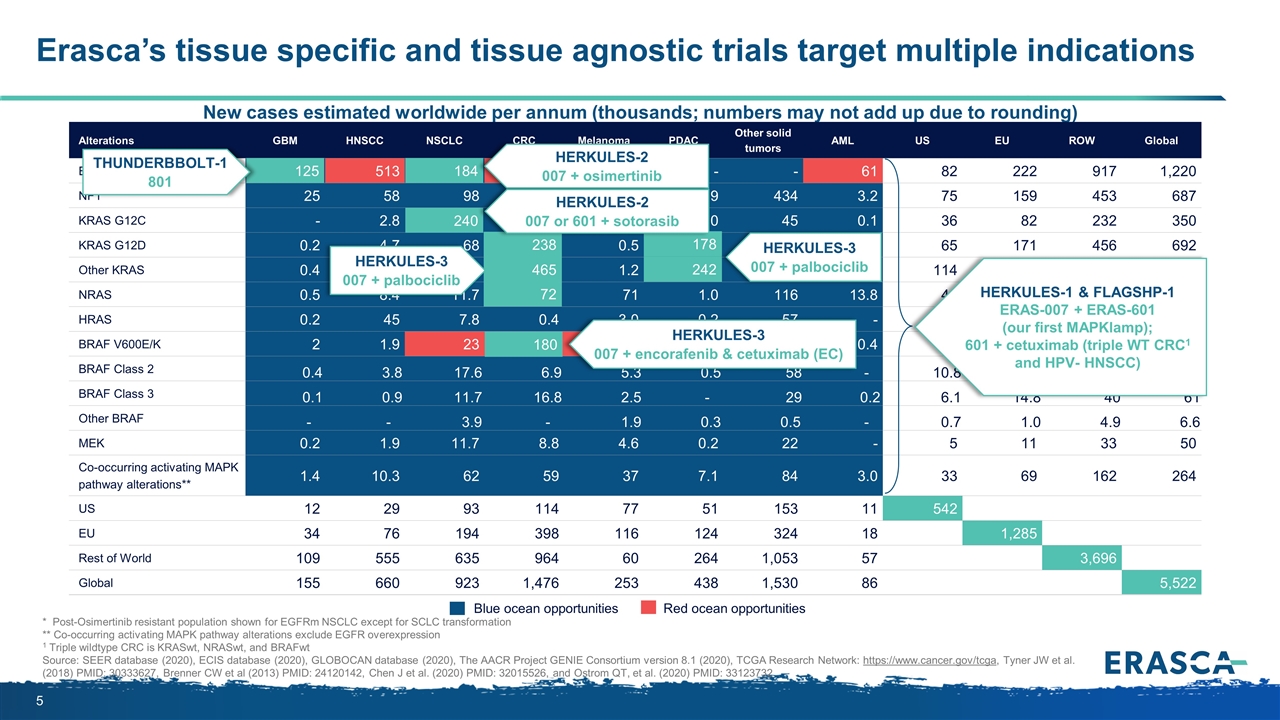
Erasca’s tissue specific and tissue agnostic trials target multiple indications New cases estimated worldwide per annum (thousands; numbers may not add up due to rounding) Blue ocean opportunities Red ocean opportunities Alterations GBM HNSCC NSCLC CRC Melanoma PDAC Other solid tumors AML US EU ROW Global EGFR*/FLT3 125 513 184 338 - - - 61 82 222 917 1,220 NF1 25 58 98 35 33 1.9 434 3.2 75 159 453 687 KRAS G12C - 2.8 240 57 - 5.0 45 0.1 36 82 232 350 KRAS G12D 0.2 4.7 68 238 0.5 178 200 1.2 65 171 456 692 Other KRAS 0.4 9.4 183 465 1.2 242 326 3.5 114 299 817 1,230 NRAS 0.5 8.4 11.7 72 71 1.0 116 13.8 42 82 170 295 HRAS 0.2 45 7.8 0.4 3.0 0.2 57 - 11 24 80 114 BRAF V600E/K 2 1.9 23 180 93 1.4 158 0.4 63 127 271 461 BRAF Class 2 0.4 3.8 17.6 6.9 5.3 0.5 58 - 10.8 23.1 58 92 BRAF Class 3 0.1 0.9 11.7 16.8 2.5 - 29 0.2 6.1 14.8 40 61 Other BRAF - - 3.9 - 1.9 0.3 0.5 - 0.7 1.0 4.9 6.6 MEK 0.2 1.9 11.7 8.8 4.6 0.2 22 - 5 11 33 50 Co-occurring activating MAPK pathway alterations** 1.4 10.3 62 59 37 7.1 84 3.0 33 69 162 264 US 12 29 93 114 77 51 153 11 542 EU 34 76 194 398 116 124 324 18 1,285 Rest of World 109 555 635 964 60 264 1,053 57 3,696 Global 155 660 923 1,476 253 438 1,530 86 5,522 * Post-Osimertinib resistant population shown for EGFRm NSCLC except for SCLC transformation ** Co-occurring activating MAPK pathway alterations exclude EGFR overexpression 1 Triple wildtype CRC is KRASwt, NRASwt, and BRAFwt Source: SEER database (2020), ECIS database (2020), GLOBOCAN database (2020), The AACR Project GENIE Consortium version 8.1 (2020), TCGA Research Network: https://www.cancer.gov/tcga, Tyner JW et al. (2018) PMID: 30333627, Brenner CW et al (2013) PMID: 24120142, Chen J et al. (2020) PMID: 32015526, and Ostrom QT, et al. (2020) PMID: 33123732 184 240 72 180 465 125 238 242 178 HERKULES-3 007 + palbociclib HERKULES-3 007 + encorafenib & cetuximab (EC) HERKULES-1 & FLAGSHP-1 ERAS-007 + ERAS-601 (our first MAPKlamp); 601 + cetuximab (triple WT CRC1 and HPV- HNSCC) HERKULES-2 007 + osimertinib THUNDERBBOLT-1 801 HERKULES-3 007 + palbociclib HERKULES-2 007 or 601 + sotorasib
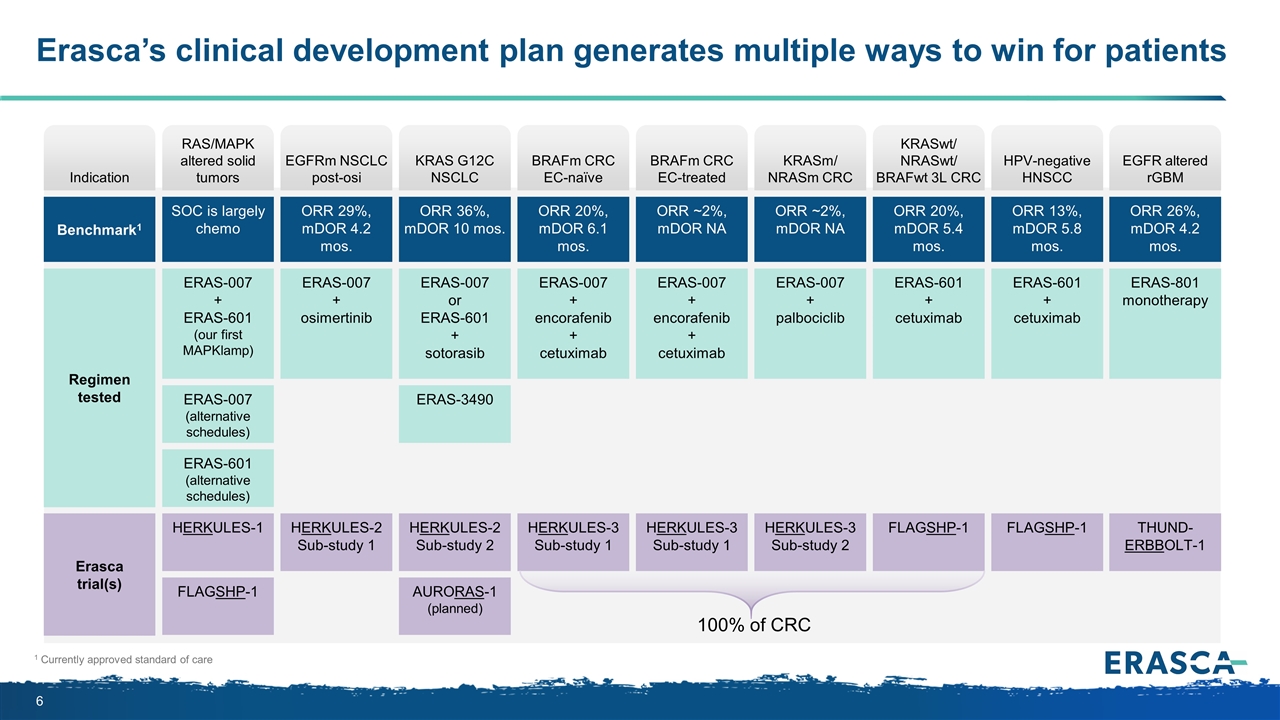
Erasca’s clinical development plan generates multiple ways to win for patients 1 Currently approved standard of care Indication Benchmark1 Regimen tested Erasca trial(s) HERKULES-2 Sub-study 1 EGFRm NSCLC post-osi ERAS-007 + osimertinib ORR 29%, mDOR 4.2 mos. HERKULES-3 Sub-study 1 BRAFm CRC EC-naïve ERAS-007 + encorafenib + cetuximab ORR 20%, mDOR 6.1 mos. HERKULES-3 Sub-study 1 BRAFm CRC EC-treated ERAS-007 + encorafenib + cetuximab ORR ~2%, mDOR NA HERKULES-3 Sub-study 2 KRASm/ NRASm CRC ERAS-007 + palbociclib ORR ~2%, mDOR NA FLAGSHP-1 KRASwt/ NRASwt/ BRAFwt 3L CRC ERAS-601 + cetuximab ORR 20%, mDOR 5.4 mos. THUND- ERBBOLT-1 EGFR altered rGBM ERAS-801 monotherapy ORR 26%, mDOR 4.2 mos. HERKULES-2 Sub-study 2 KRAS G12C NSCLC ERAS-007 or ERAS-601 + sotorasib ORR 36%, mDOR 10 mos. ERAS-3490 AURORAS-1 (planned) HERKULES-1 RAS/MAPK altered solid tumors ERAS-007 + ERAS-601 (our first MAPKlamp) SOC is largely chemo ERAS-007 (alternative schedules) ERAS-601 (alternative schedules) FLAGSHP-1 100% of CRC FLAGSHP-1 HPV-negative HNSCC ERAS-601 + cetuximab ORR 13%, mDOR 5.8 mos.

Are there more responsive subsets within the Blue Ocean where promising combination approaches can be particularly effective? ? Key scientific hypotheses and supportive preclinical data
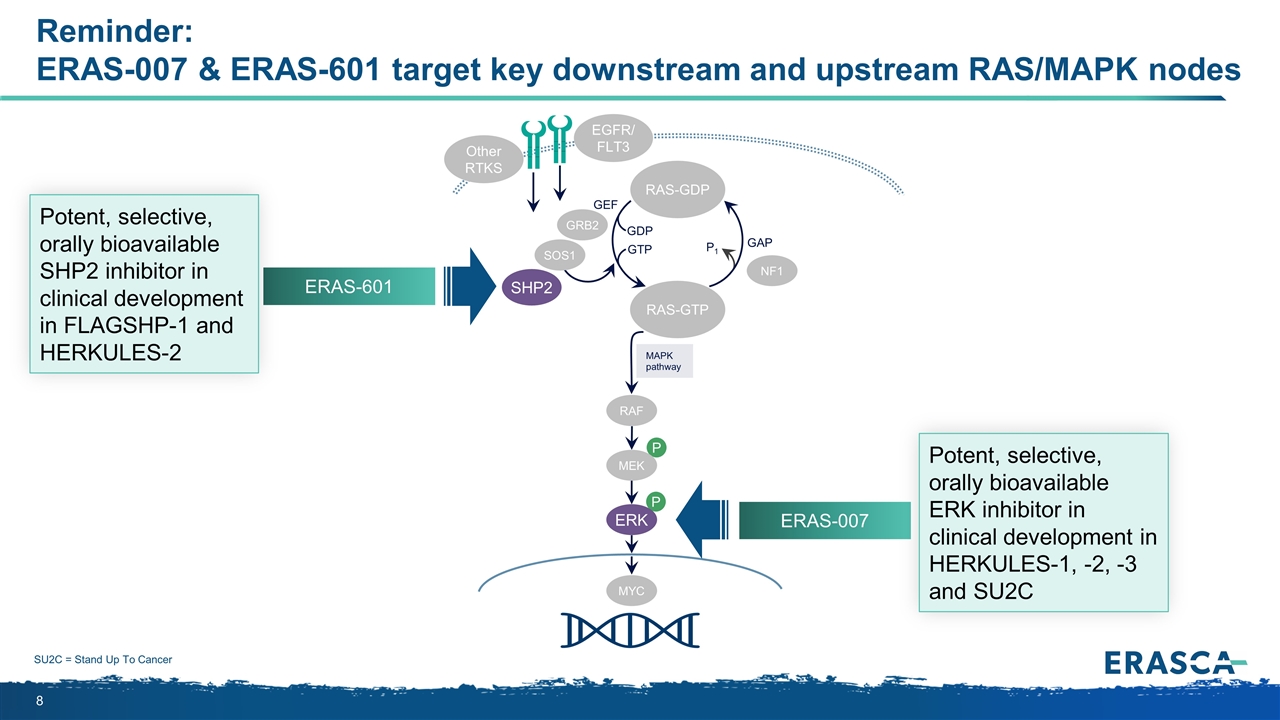
Reminder: ERAS-007 & ERAS-601 target key downstream and upstream RAS/MAPK nodes P P MAPK pathway RAS-GTP GRB2 SHP2 GAP GDP GTP P1 NF1 SOS1 RAF MEK ERK RAS-GDP EGFR/ FLT3 Other RTKS MYC GEF ERAS-601 ERAS-007 SU2C = Stand Up To Cancer Potent, selective, orally bioavailable SHP2 inhibitor in clinical development in FLAGSHP-1 and HERKULES-2 Potent, selective, orally bioavailable ERK inhibitor in clinical development in HERKULES-1, -2, -3 and SU2C
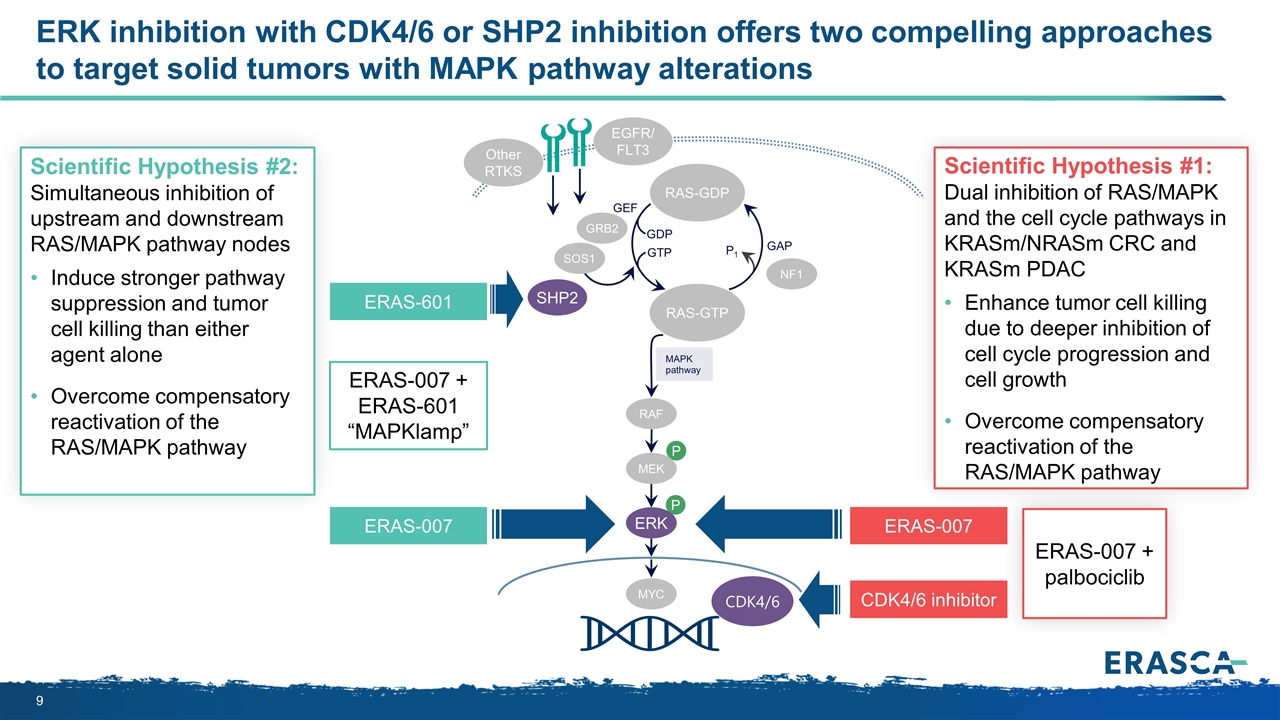
ERK inhibition with CDK4/6 or SHP2 inhibition offers two compelling approaches to target solid tumors with MAPK pathway alterations P P MAPK pathway RAS-GTP GRB2 SHP2 GAP GDP GTP P1 NF1 SOS1 RAF MEK ERK RAS-GDP EGFR/ FLT3 Other RTKS MYC GEF Scientific Hypothesis #1: Dual inhibition of RAS/MAPK and the cell cycle pathways in KRASm/NRASm CRC and KRASm PDAC Enhance tumor cell killing due to deeper inhibition of cell cycle progression and cell growth Overcome compensatory reactivation of the RAS/MAPK pathway ERAS-601 ERAS-007 CDK4/6 CDK4/6 inhibitor ERAS-007 + ERAS-601 “MAPKlamp” Scientific Hypothesis #2: Simultaneous inhibition of upstream and downstream RAS/MAPK pathway nodes Induce stronger pathway suppression and tumor cell killing than either agent alone Overcome compensatory reactivation of the RAS/MAPK pathway ERAS-007 ERAS-007 + palbociclib
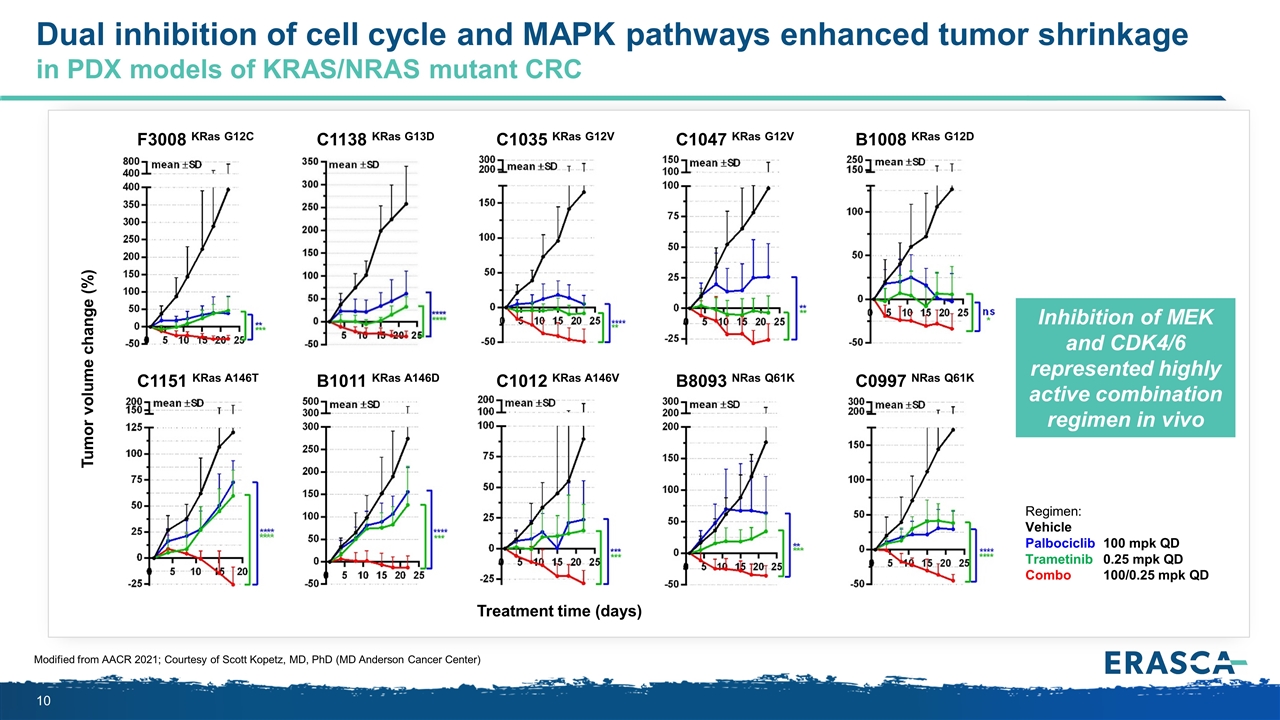
Dual inhibition of cell cycle and MAPK pathways enhanced tumor shrinkage in PDX models of KRAS/NRAS mutant CRC Modified from AACR 2021; Courtesy of Scott Kopetz, MD, PhD (MD Anderson Cancer Center) Regimen: Vehicle Palbociclib100 mpk QD Trametinib0.25 mpk QD Combo100/0.25 mpk QD C1047 KRas G12V B8093 NRas Q61K C1035 KRas G12V C1012 KRas A146V B1008 KRas G12D C0997 NRas Q61K F3008 KRas G12C C1151 KRas A146T C1138 KRas G13D B1011 KRas A146D Tumor volume change (%) Treatment time (days) Inhibition of MEK and CDK4/6 represented highly active combination regimen in vivo
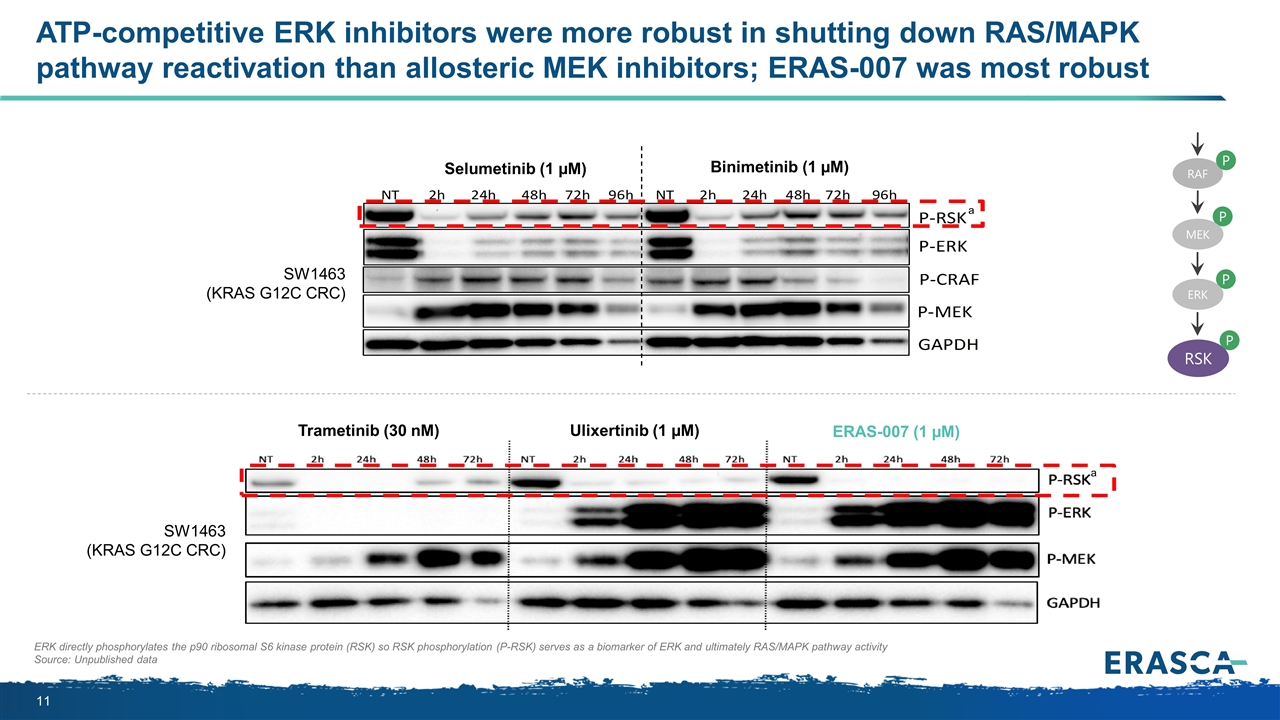
ERK directly phosphorylates the p90 ribosomal S6 kinase protein (RSK) so RSK phosphorylation (P-RSK) serves as a biomarker of ERK and ultimately RAS/MAPK pathway activity Source: Unpublished data ATP-competitive ERK inhibitors were more robust in shutting down RAS/MAPK pathway reactivation than allosteric MEK inhibitors; ERAS-007 was most robust SW1463 (KRAS G12C CRC) Selumetinib (1 µM) Binimetinib (1 µM) SW1463 (KRAS G12C CRC) Trametinib (30 nM) Ulixertinib (1 µM) ERAS-007 (1 µM) a a P MEK P ERK RSK P RAF P
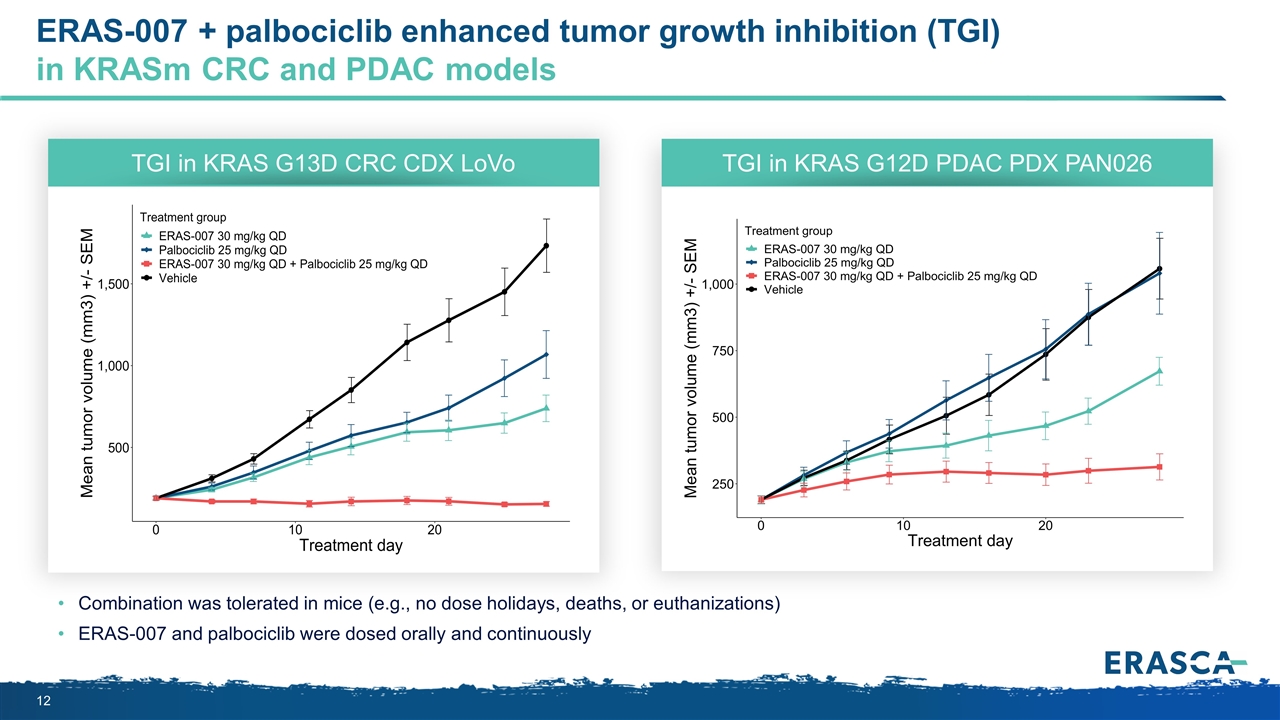
ERAS-007 + palbociclib enhanced tumor growth inhibition (TGI) in KRASm CRC and PDAC models Combination was tolerated in mice (e.g., no dose holidays, deaths, or euthanizations) ERAS-007 and palbociclib were dosed orally and continuously TGI in KRAS G13D CRC CDX LoVo TGI in KRAS G12D PDAC PDX PAN026
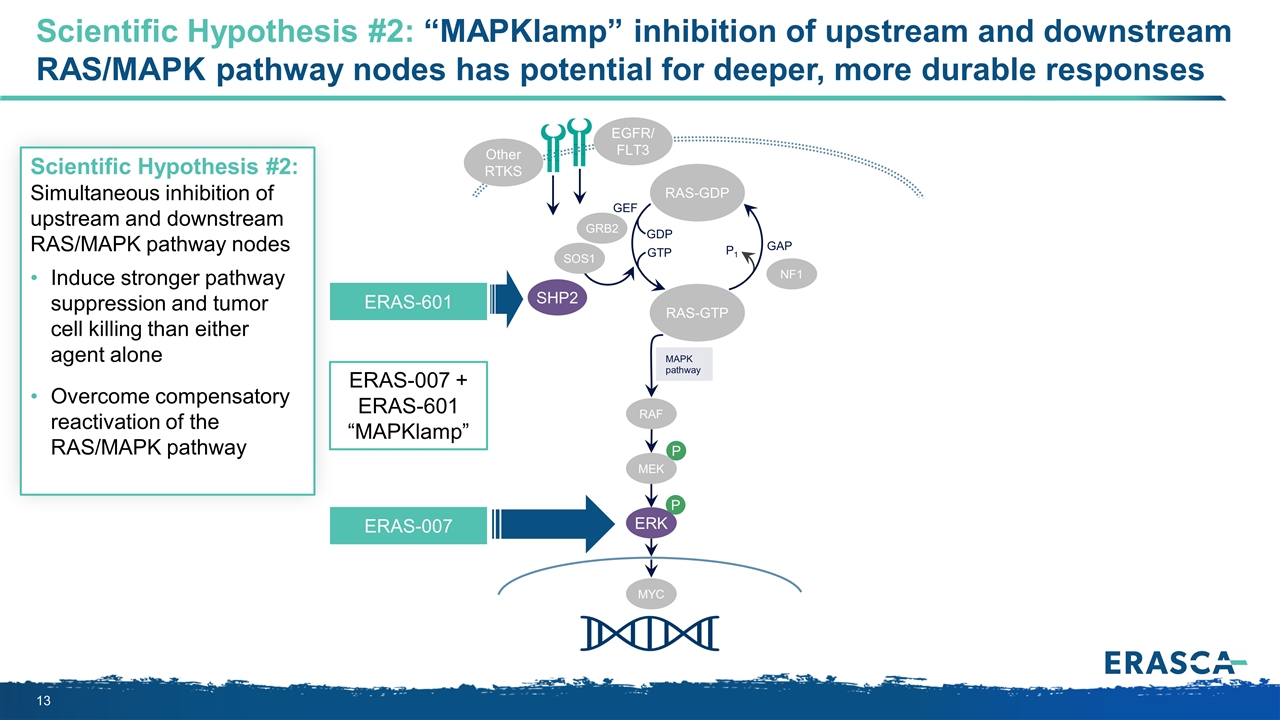
Scientific Hypothesis #2: “MAPKlamp” inhibition of upstream and downstream RAS/MAPK pathway nodes has potential for deeper, more durable responses P P MAPK pathway RAS-GTP GRB2 SHP2 GAP GDP GTP P1 NF1 SOS1 RAF MEK ERK RAS-GDP EGFR/ FLT3 Other RTKS MYC GEF ERAS-601 ERAS-007 + ERAS-601 “MAPKlamp” Scientific Hypothesis #2: Simultaneous inhibition of upstream and downstream RAS/MAPK pathway nodes Induce stronger pathway suppression and tumor cell killing than either agent alone Overcome compensatory reactivation of the RAS/MAPK pathway ERAS-007
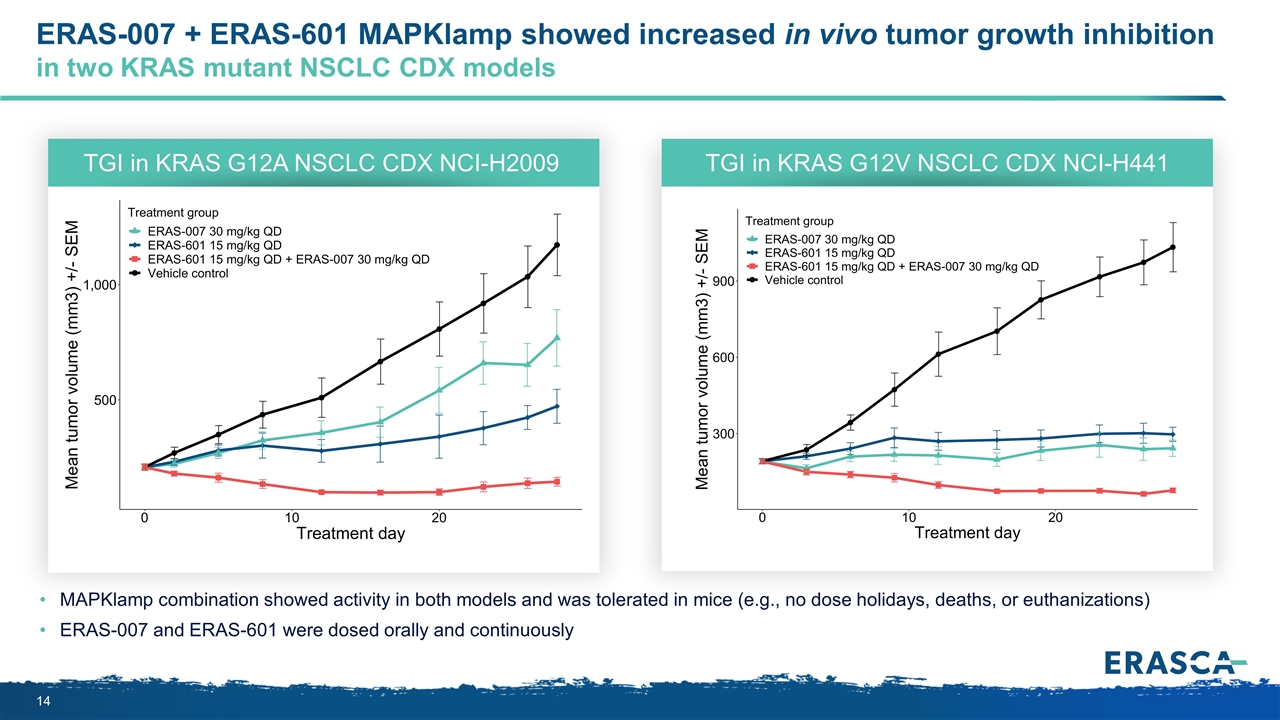
ERAS-007 + ERAS-601 MAPKlamp showed increased in vivo tumor growth inhibition in two KRAS mutant NSCLC CDX models MAPKlamp combination showed activity in both models and was tolerated in mice (e.g., no dose holidays, deaths, or euthanizations) ERAS-007 and ERAS-601 were dosed orally and continuously TGI in KRAS G12A NSCLC CDX NCI-H2009 TGI in KRAS G12V NSCLC CDX NCI-H441
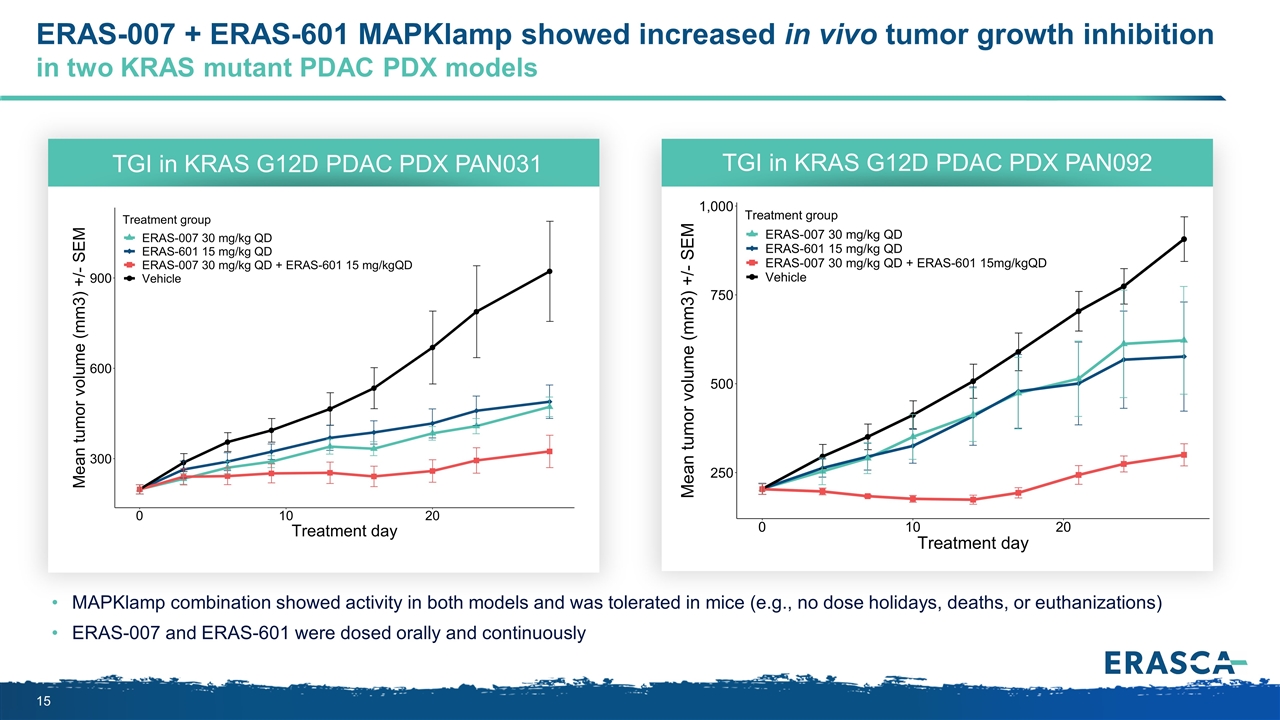
ERAS-007 + ERAS-601 MAPKlamp showed increased in vivo tumor growth inhibition in two KRAS mutant PDAC PDX models MAPKlamp combination showed activity in both models and was tolerated in mice (e.g., no dose holidays, deaths, or euthanizations) ERAS-007 and ERAS-601 were dosed orally and continuously TGI in KRAS G12D PDAC PDX PAN031 TGI in KRAS G12D PDAC PDX PAN092
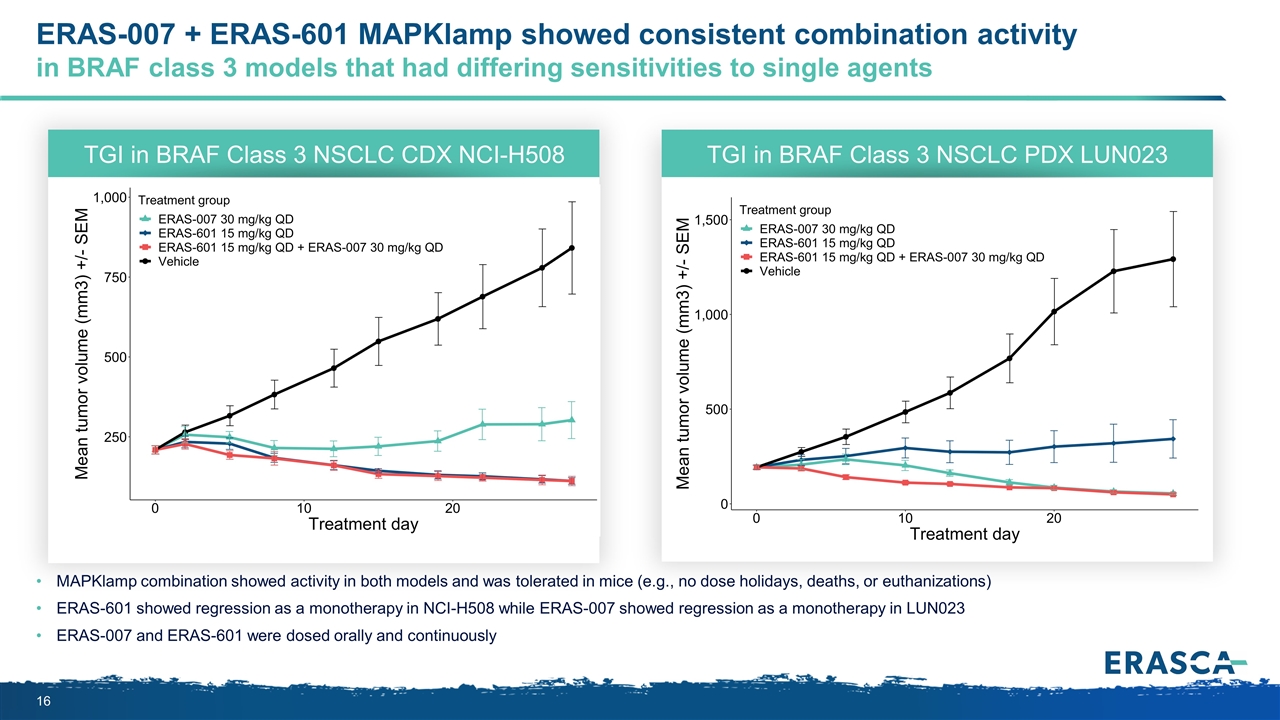
ERAS-007 + ERAS-601 MAPKlamp showed consistent combination activity in BRAF class 3 models that had differing sensitivities to single agents MAPKlamp combination showed activity in both models and was tolerated in mice (e.g., no dose holidays, deaths, or euthanizations) ERAS-601 showed regression as a monotherapy in NCI-H508 while ERAS-007 showed regression as a monotherapy in LUN023 ERAS-007 and ERAS-601 were dosed orally and continuously TGI in BRAF Class 3 NSCLC CDX NCI-H508 TGI in BRAF Class 3 NSCLC PDX LUN023
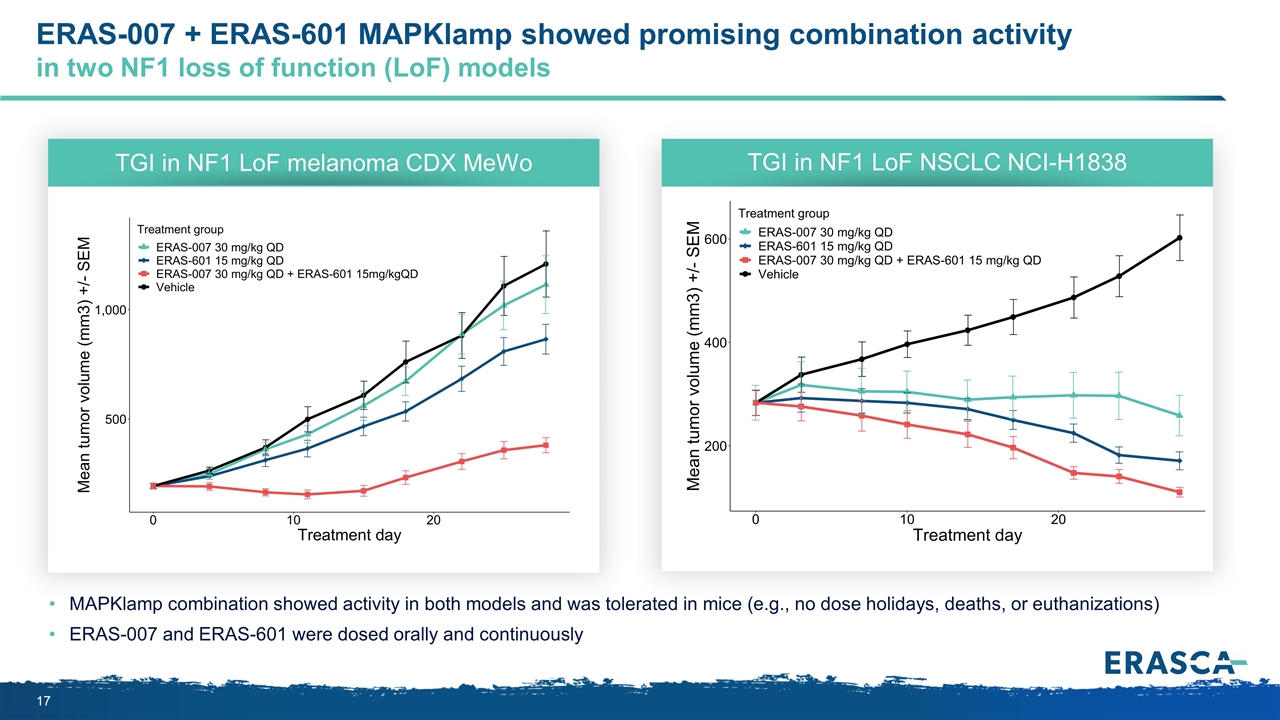
ERAS-007 + ERAS-601 MAPKlamp showed promising combination activity in two NF1 loss of function (LoF) models MAPKlamp combination showed activity in both models and was tolerated in mice (e.g., no dose holidays, deaths, or euthanizations) ERAS-007 and ERAS-601 were dosed orally and continuously TGI in NF1 LoF melanoma CDX MeWo TGI in NF1 LoF NSCLC NCI-H1838

Are there more responsive subsets within the Blue Ocean where ERK + cell cycle or ERK + SHP2 inhibition can be particularly effective? ? Preliminary clinical data
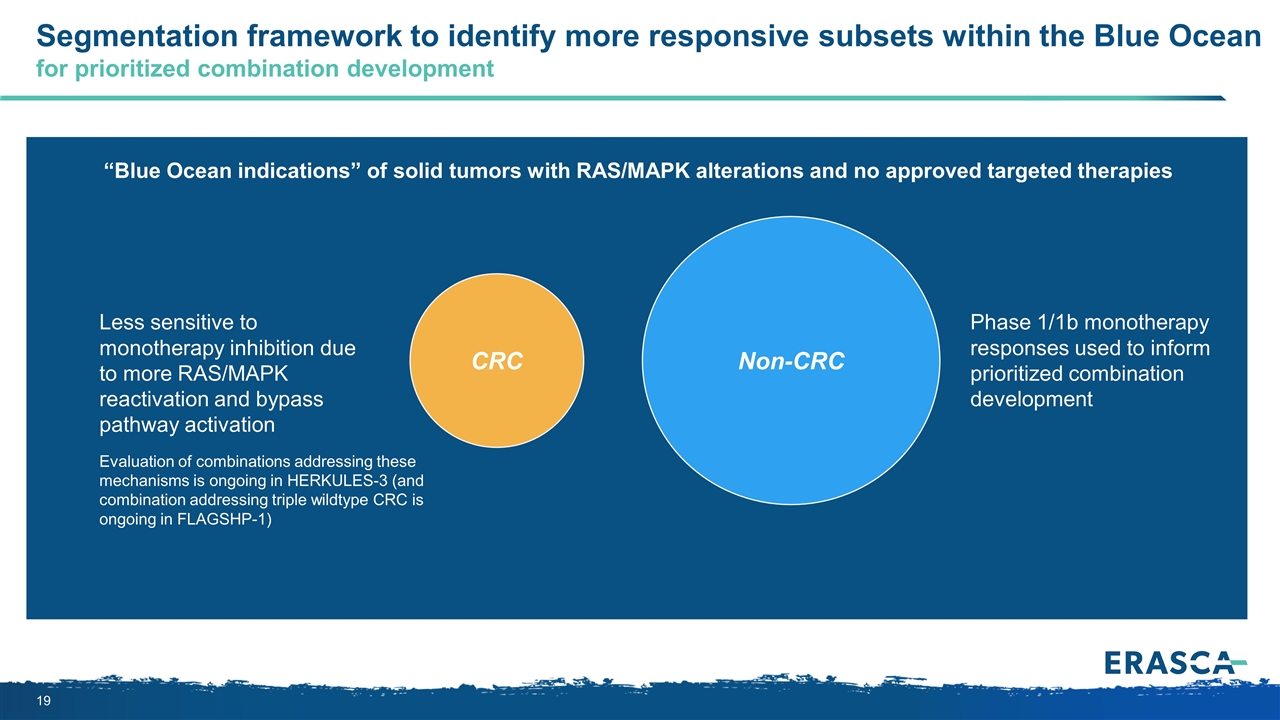
CRC Segmentation framework to identify more responsive subsets within the Blue Ocean for prioritized combination development Non-CRC Evaluation of combinations addressing these mechanisms is ongoing in HERKULES-3 (and combination addressing triple wildtype CRC is ongoing in FLAGSHP-1) Phase 1/1b monotherapy responses used to inform prioritized combination development “Blue Ocean indications” of solid tumors with RAS/MAPK alterations and no approved targeted therapies Less sensitive to monotherapy inhibition due to more RAS/MAPK reactivation and bypass pathway activation
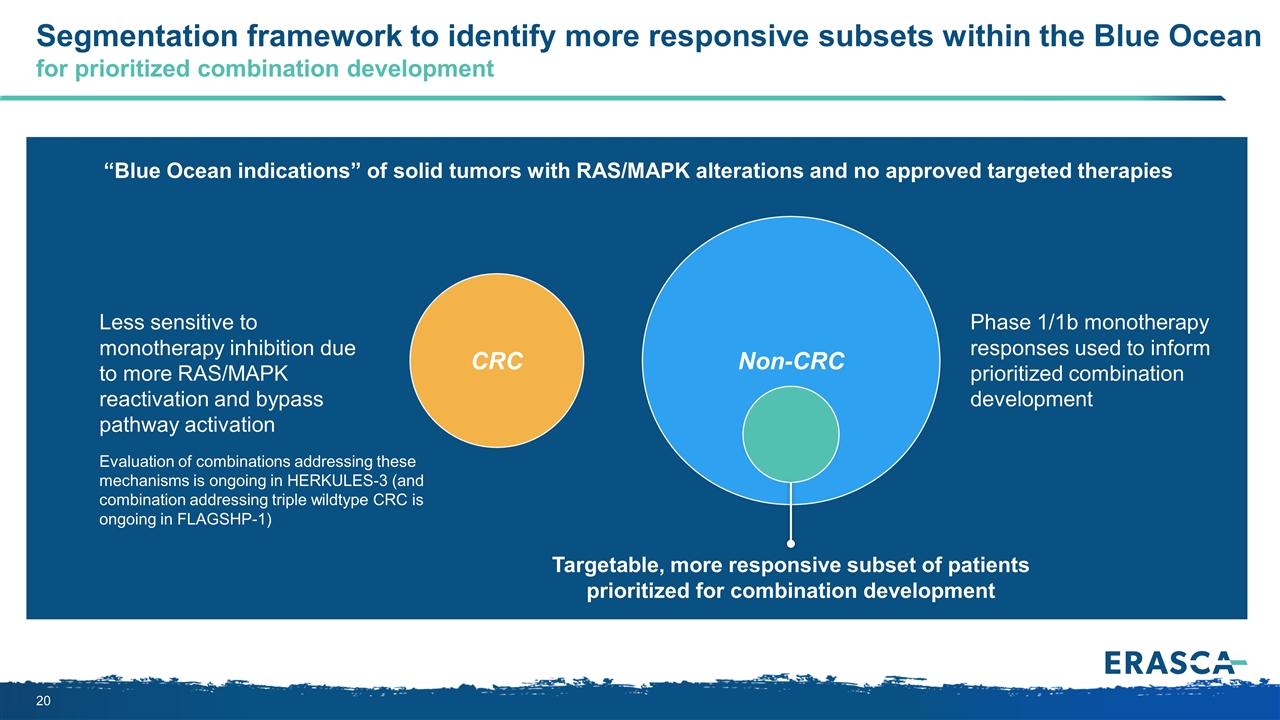
CRC Segmentation framework to identify more responsive subsets within the Blue Ocean for prioritized combination development Non-CRC Evaluation of combinations addressing these mechanisms is ongoing in HERKULES-3 (and combination addressing triple wildtype CRC is ongoing in FLAGSHP-1) Targetable, more responsive subset of patients prioritized for combination development “Blue Ocean indications” of solid tumors with RAS/MAPK alterations and no approved targeted therapies Less sensitive to monotherapy inhibition due to more RAS/MAPK reactivation and bypass pathway activation Phase 1/1b monotherapy responses used to inform prioritized combination development
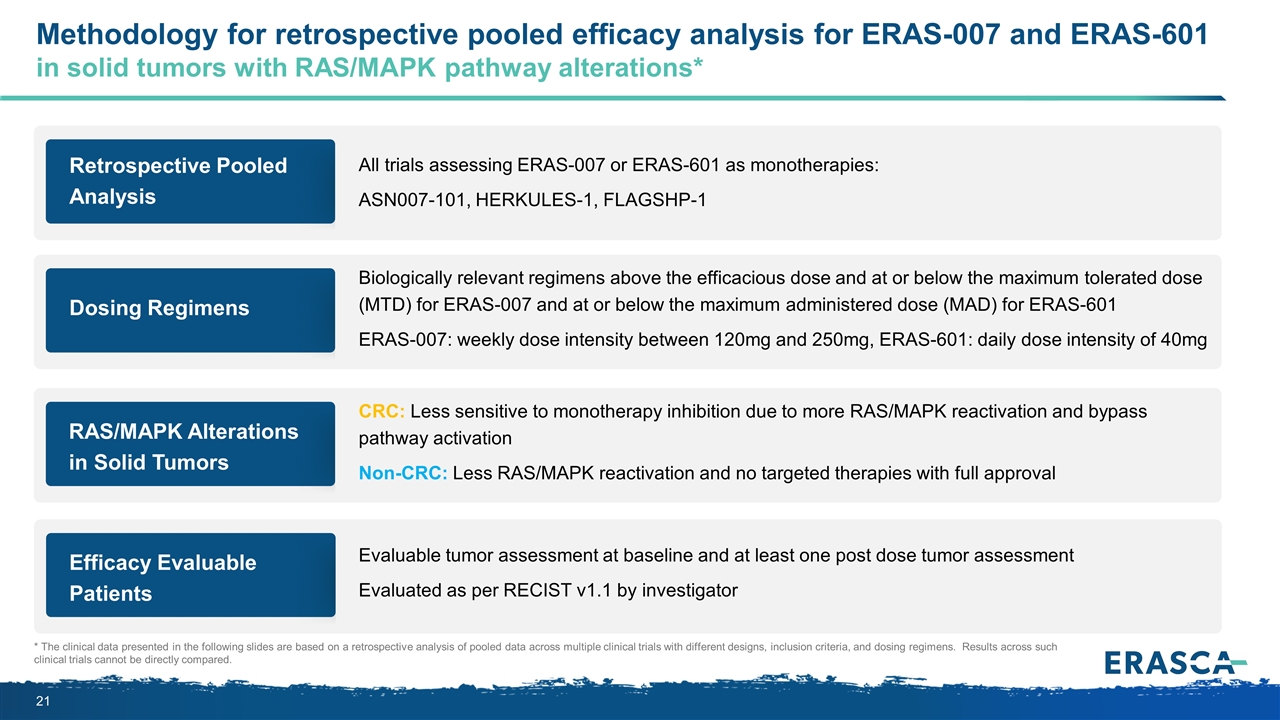
Retrospective Pooled Analysis All trials assessing ERAS-007 or ERAS-601 as monotherapies: ASN007-101, HERKULES-1, FLAGSHP-1 Methodology for retrospective pooled efficacy analysis for ERAS-007 and ERAS-601 in solid tumors with RAS/MAPK pathway alterations* * The clinical data presented in the following slides are based on a retrospective analysis of pooled data across multiple clinical trials with different designs, inclusion criteria, and dosing regimens. Results across such clinical trials cannot be directly compared. Dosing Regimens Biologically relevant regimens above the efficacious dose and at or below the maximum tolerated dose (MTD) for ERAS-007 and at or below the maximum administered dose (MAD) for ERAS-601 ERAS-007: weekly dose intensity between 120mg and 250mg, ERAS-601: daily dose intensity of 40mg RAS/MAPK Alterations in Solid Tumors CRC: Less sensitive to monotherapy inhibition due to more RAS/MAPK reactivation and bypass pathway activation Non-CRC: Less RAS/MAPK reactivation and no targeted therapies with full approval Efficacy Evaluable Patients Evaluable tumor assessment at baseline and at least one post dose tumor assessment Evaluated as per RECIST v1.1 by investigator
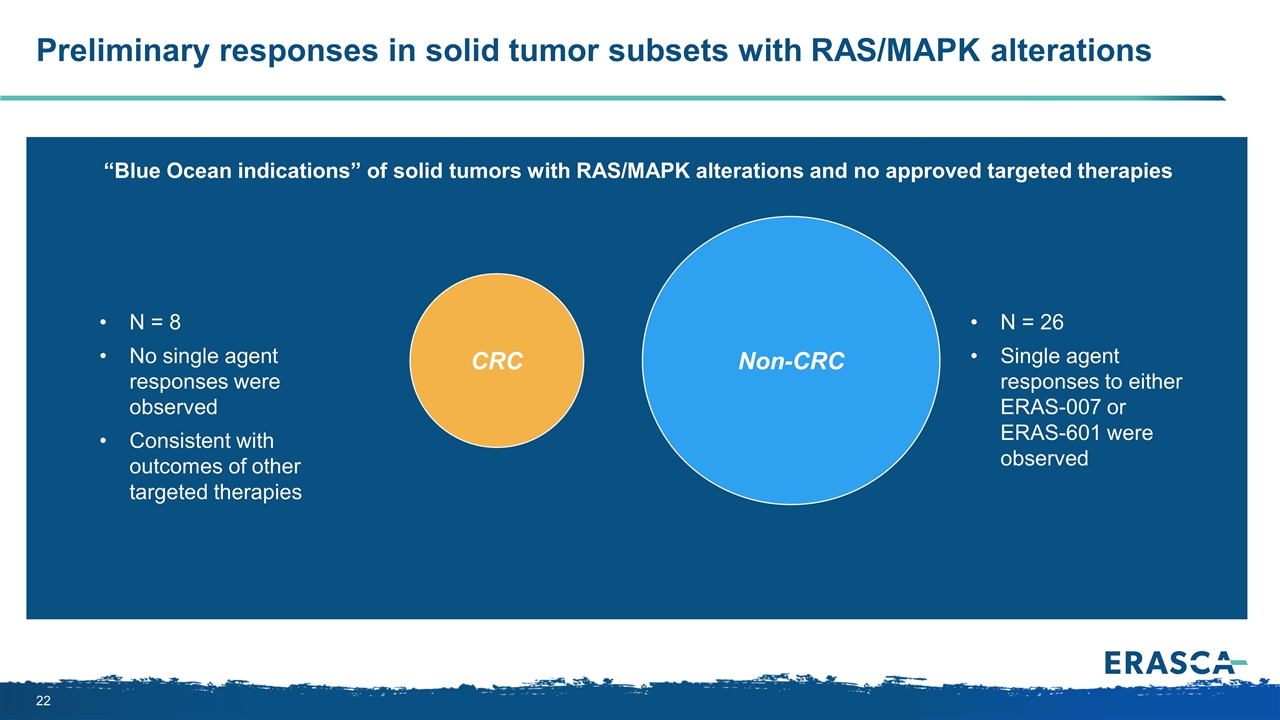
CRC Preliminary responses in solid tumor subsets with RAS/MAPK alterations Non-CRC N = 26 Single agent responses to either ERAS-007 or ERAS-601 were observed “Blue Ocean indications” of solid tumors with RAS/MAPK alterations and no approved targeted therapies N = 8 No single agent responses were observed Consistent with outcomes of other targeted therapies
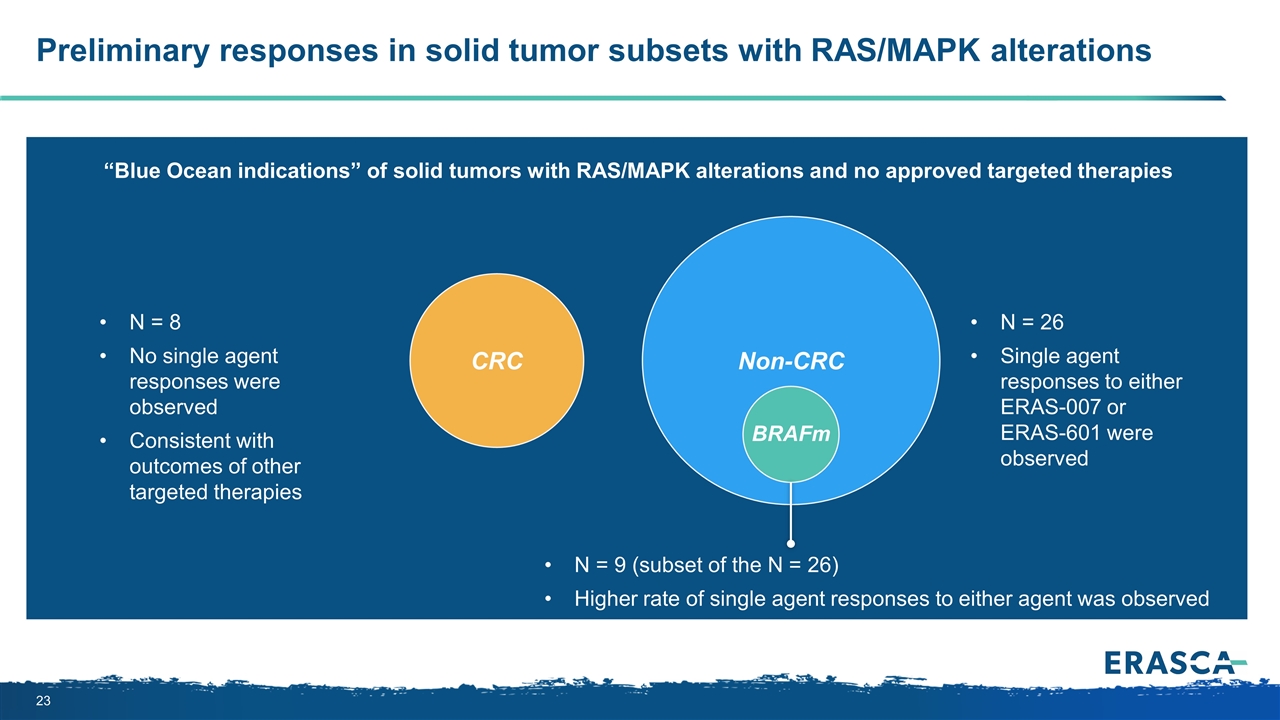
CRC Preliminary responses in solid tumor subsets with RAS/MAPK alterations Non-CRC N = 26 Single agent responses to either ERAS-007 or ERAS-601 were observed N = 9 (subset of the N = 26) Higher rate of single agent responses to either agent was observed “Blue Ocean indications” of solid tumors with RAS/MAPK alterations and no approved targeted therapies N = 8 No single agent responses were observed Consistent with outcomes of other targeted therapies BRAFm
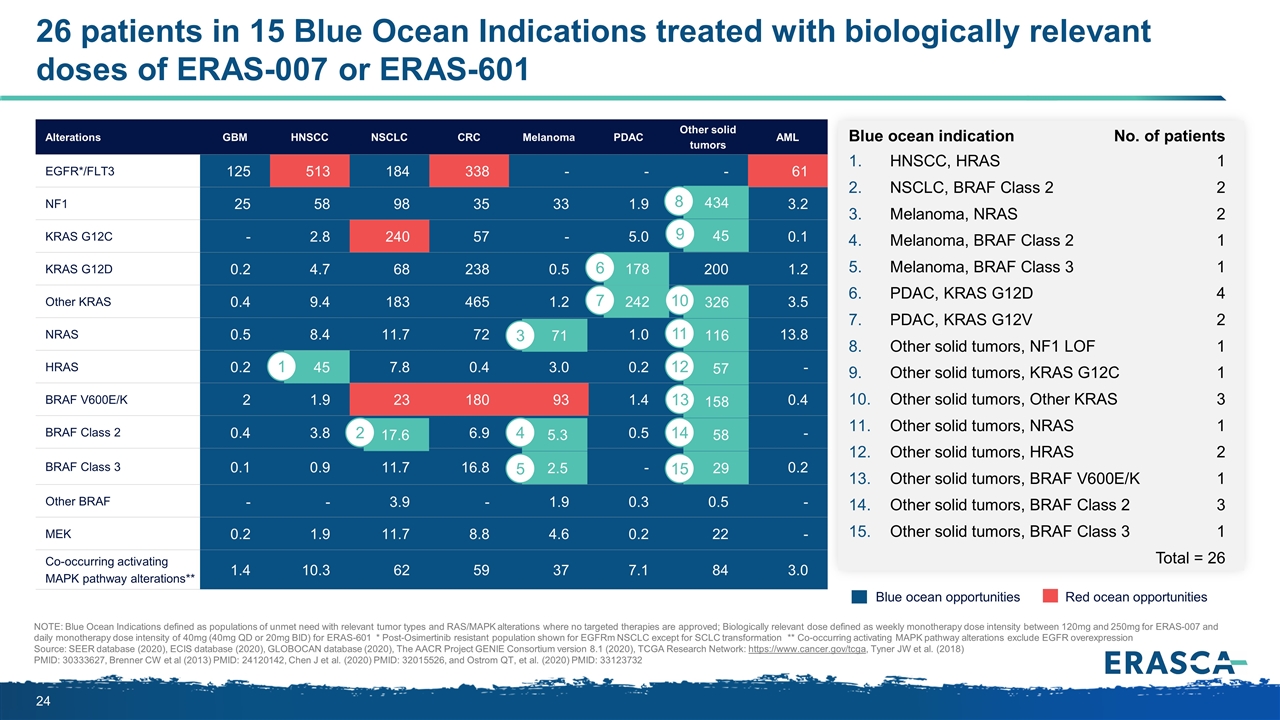
Alterations GBM HNSCC NSCLC CRC Melanoma PDAC Other solid tumors AML EGFR*/FLT3 125 513 184 338 - - - 61 NF1 25 58 98 35 33 1.9 434 3.2 KRAS G12C - 2.8 240 57 - 5.0 45 0.1 KRAS G12D 0.2 4.7 68 238 0.5 178 200 1.2 Other KRAS 0.4 9.4 183 465 1.2 242 326 3.5 NRAS 0.5 8.4 11.7 72 71 1.0 116 13.8 HRAS 0.2 45 7.8 0.4 3.0 0.2 57 - BRAF V600E/K 2 1.9 23 180 93 1.4 158 0.4 BRAF Class 2 0.4 3.8 17.6 6.9 5.3 0.5 57.5 - BRAF Class 3 0.1 0.9 11.7 16.8 2.5 - 28.7 0.2 Other BRAF - - 3.9 - 1.9 0.3 0.5 - MEK 0.2 1.9 11.7 8.8 4.6 0.2 22 - Co-occurring activating MAPK pathway alterations** 1.4 10.3 62 59 37 7.1 84 3.0 158 17.6 45 5.3 58 2.5 242 178 29 45 326 71 116 57 434 Blue ocean indication No. of patients HNSCC, HRAS1 NSCLC, BRAF Class 22 Melanoma, NRAS2 Melanoma, BRAF Class 21 Melanoma, BRAF Class 31 PDAC, KRAS G12D4 PDAC, KRAS G12V2 Other solid tumors, NF1 LOF1 Other solid tumors, KRAS G12C1 Other solid tumors, Other KRAS3 Other solid tumors, NRAS1 Other solid tumors, HRAS2 Other solid tumors, BRAF V600E/K1 Other solid tumors, BRAF Class 23 Other solid tumors, BRAF Class 31 Total = 26 26 patients in 15 Blue Ocean Indications treated with biologically relevant doses of ERAS-007 or ERAS-601 NOTE: Blue Ocean Indications defined as populations of unmet need with relevant tumor types and RAS/MAPK alterations where no targeted therapies are approved; Biologically relevant dose defined as weekly monotherapy dose intensity between 120mg and 250mg for ERAS-007 and daily monotherapy dose intensity of 40mg (40mg QD or 20mg BID) for ERAS-601 * Post-Osimertinib resistant population shown for EGFRm NSCLC except for SCLC transformation ** Co-occurring activating MAPK pathway alterations exclude EGFR overexpression Source: SEER database (2020), ECIS database (2020), GLOBOCAN database (2020), The AACR Project GENIE Consortium version 8.1 (2020), TCGA Research Network: https://www.cancer.gov/tcga, Tyner JW et al. (2018) PMID: 30333627, Brenner CW et al (2013) PMID: 24120142, Chen J et al. (2020) PMID: 32015526, and Ostrom QT, et al. (2020) PMID: 33123732 2 1 4 5 7 6 10 13 14 15 Blue ocean opportunities Red ocean opportunities 3 11 12 8 9
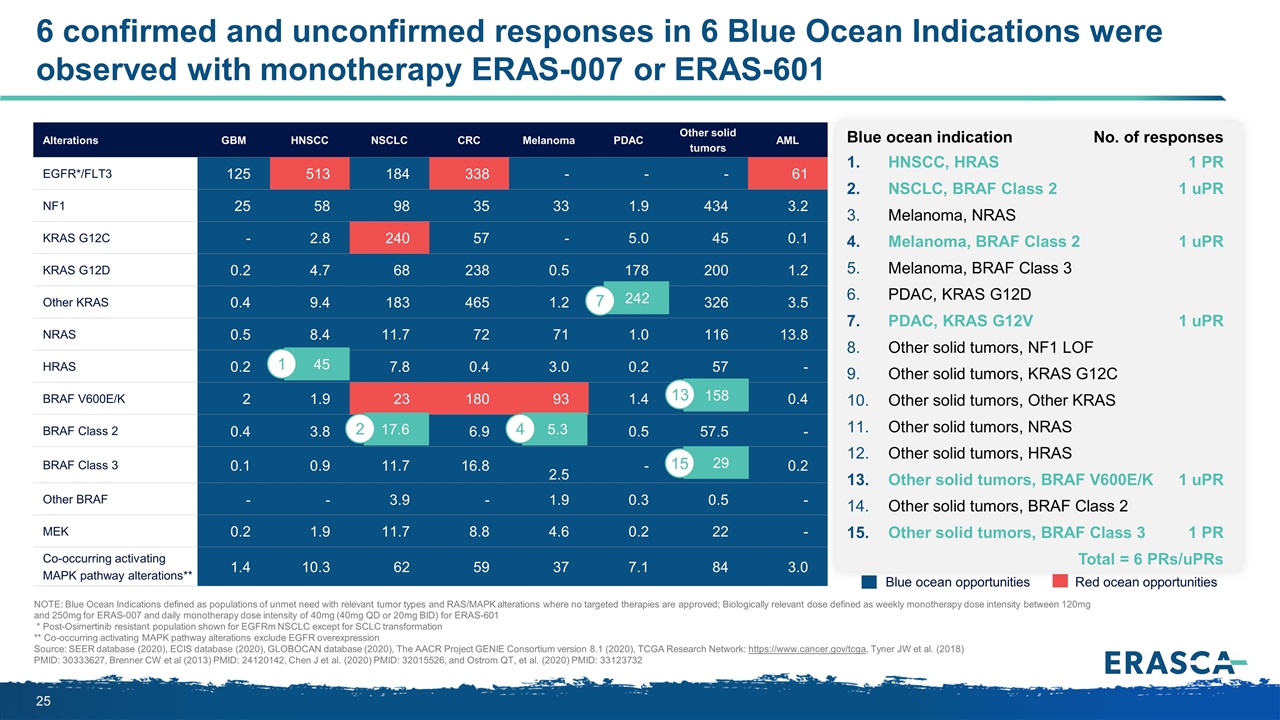
Alterations GBM HNSCC NSCLC CRC Melanoma PDAC Other solid tumors AML EGFR*/FLT3 125 513 184 338 - - - 61 NF1 25 58 98 35 33 1.9 434 3.2 KRAS G12C - 2.8 240 57 - 5.0 45 0.1 KRAS G12D 0.2 4.7 68 238 0.5 178 200 1.2 Other KRAS 0.4 9.4 183 465 1.2 242 326 3.5 NRAS 0.5 8.4 11.7 72 71 1.0 116 13.8 HRAS 0.2 45 7.8 0.4 3.0 0.2 57 - BRAF V600E/K 2 1.9 23 180 93 1.4 158 0.4 BRAF Class 2 0.4 3.8 17.6 6.9 5.3 0.5 57.5 - BRAF Class 3 0.1 0.9 11.7 16.8 2.5 - 28.7 0.2 Other BRAF - - 3.9 - 1.9 0.3 0.5 - MEK 0.2 1.9 11.7 8.8 4.6 0.2 22 - Co-occurring activating MAPK pathway alterations** 1.4 10.3 62 59 37 7.1 84 3.0 6 confirmed and unconfirmed responses in 6 Blue Ocean Indications were observed with monotherapy ERAS-007 or ERAS-601 NOTE: Blue Ocean Indications defined as populations of unmet need with relevant tumor types and RAS/MAPK alterations where no targeted therapies are approved; Biologically relevant dose defined as weekly monotherapy dose intensity between 120mg and 250mg for ERAS-007 and daily monotherapy dose intensity of 40mg (40mg QD or 20mg BID) for ERAS-601 * Post-Osimertinib resistant population shown for EGFRm NSCLC except for SCLC transformation ** Co-occurring activating MAPK pathway alterations exclude EGFR overexpression Source: SEER database (2020), ECIS database (2020), GLOBOCAN database (2020), The AACR Project GENIE Consortium version 8.1 (2020), TCGA Research Network: https://www.cancer.gov/tcga, Tyner JW et al. (2018) PMID: 30333627, Brenner CW et al (2013) PMID: 24120142, Chen J et al. (2020) PMID: 32015526, and Ostrom QT, et al. (2020) PMID: 33123732 Blue ocean opportunities Red ocean opportunities 158 17.6 2 45 1 5.3 4 242 7 29 13 15 Blue ocean indication No. of responses HNSCC, HRAS1 PR NSCLC, BRAF Class 21 uPR Melanoma, NRAS Melanoma, BRAF Class 21 uPR Melanoma, BRAF Class 3 PDAC, KRAS G12D PDAC, KRAS G12V1 uPR Other solid tumors, NF1 LOF Other solid tumors, KRAS G12C Other solid tumors, Other KRAS Other solid tumors, NRAS Other solid tumors, HRAS Other solid tumors, BRAF V600E/K1 uPR Other solid tumors, BRAF Class 2 Other solid tumors, BRAF Class 31 PR Total = 6 PRs/uPRs
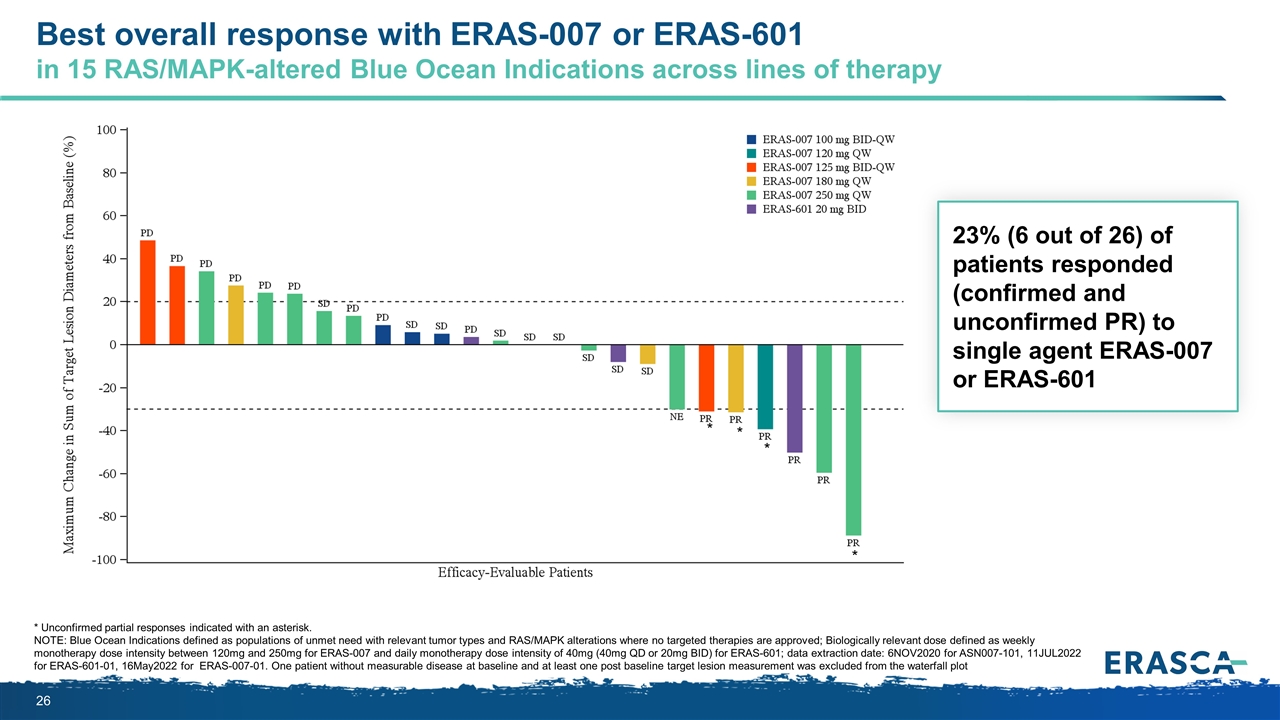
Best overall response with ERAS-007 or ERAS-601 in 15 RAS/MAPK-altered Blue Ocean Indications across lines of therapy * Unconfirmed partial responses indicated with an asterisk. NOTE: Blue Ocean Indications defined as populations of unmet need with relevant tumor types and RAS/MAPK alterations where no targeted therapies are approved; Biologically relevant dose defined as weekly monotherapy dose intensity between 120mg and 250mg for ERAS-007 and daily monotherapy dose intensity of 40mg (40mg QD or 20mg BID) for ERAS-601; data extraction date: 6NOV2020 for ASN007-101, 11JUL2022 for ERAS-601-01, 16May2022 for ERAS-007-01. One patient without measurable disease at baseline and at least one post baseline target lesion measurement was excluded from the waterfall plot 23% (6 out of 26) of patients responded (confirmed and unconfirmed PR) to single agent ERAS-007 or ERAS-601 * * * *
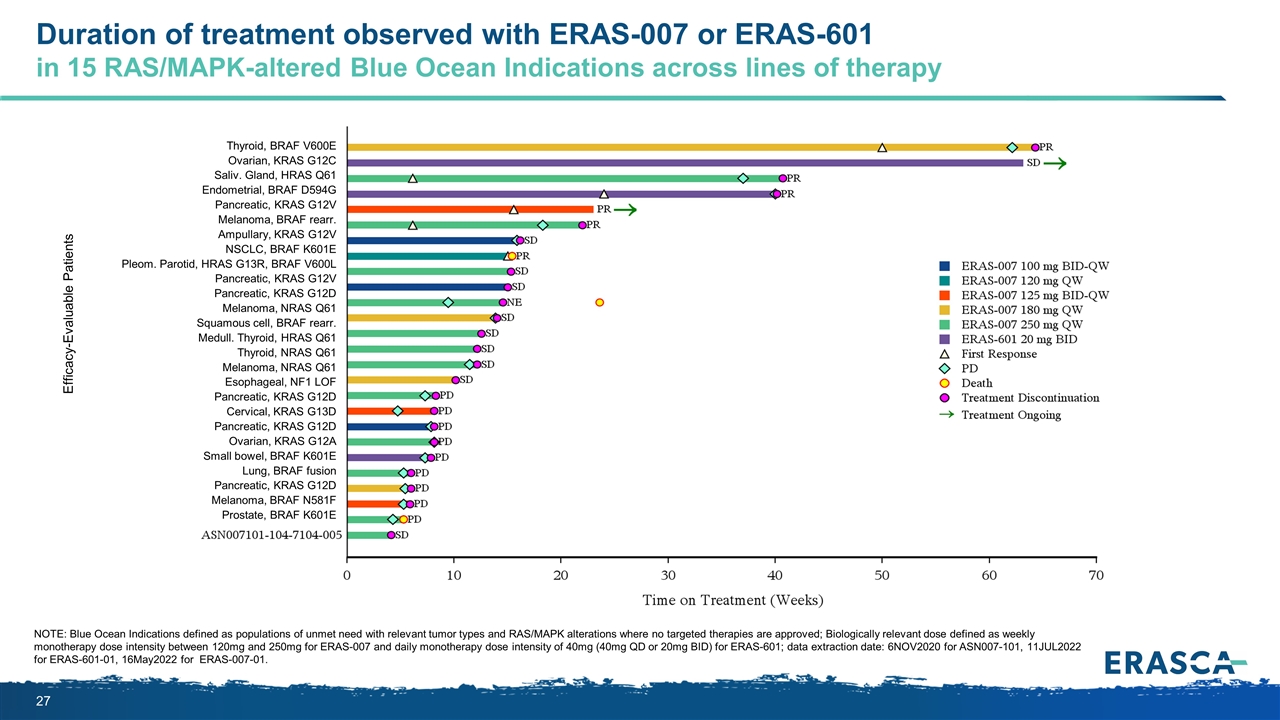
Duration of treatment observed with ERAS-007 or ERAS-601 in 15 RAS/MAPK-altered Blue Ocean Indications across lines of therapy Efficacy-Evaluable Patients Thyroid, BRAF V600E Ovarian, KRAS G12C Saliv. Gland, HRAS Q61 Endometrial, BRAF D594G Pancreatic, KRAS G12V Melanoma, BRAF rearr. Ampullary, KRAS G12V NSCLC, BRAF K601E Pleom. Parotid, HRAS G13R, BRAF V600L Pancreatic, KRAS G12V Pancreatic, KRAS G12D Melanoma, NRAS Q61 Squamous cell, BRAF rearr. Medull. Thyroid, HRAS Q61 Thyroid, NRAS Q61 Melanoma, NRAS Q61 Esophageal, NF1 LOF Pancreatic, KRAS G12D Cervical, KRAS G13D Pancreatic, KRAS G12D Ovarian, KRAS G12A Small bowel, BRAF K601E Lung, BRAF fusion Pancreatic, KRAS G12D Melanoma, BRAF N581F Prostate, BRAF K601E NOTE: Blue Ocean Indications defined as populations of unmet need with relevant tumor types and RAS/MAPK alterations where no targeted therapies are approved; Biologically relevant dose defined as weekly monotherapy dose intensity between 120mg and 250mg for ERAS-007 and daily monotherapy dose intensity of 40mg (40mg QD or 20mg BID) for ERAS-601; data extraction date: 6NOV2020 for ASN007-101, 11JUL2022 for ERAS-601-01, 16May2022 for ERAS-007-01.
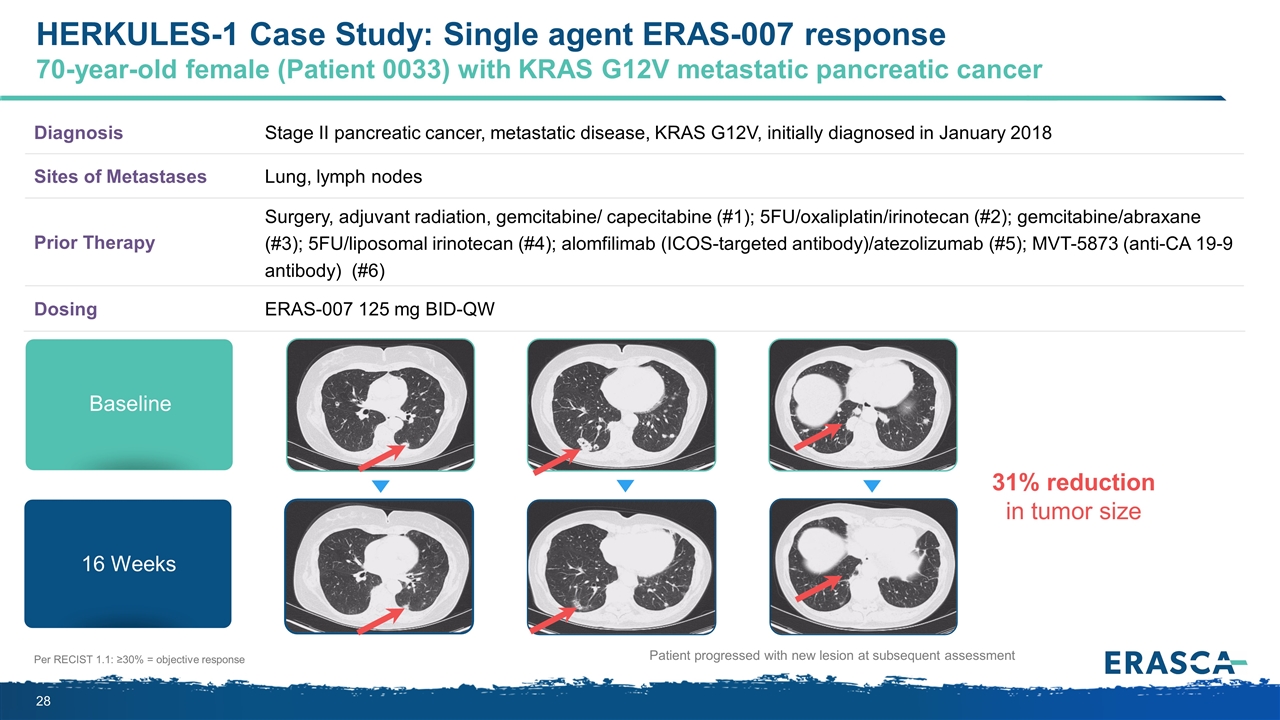
Per RECIST 1.1: ≥30% = objective response Diagnosis Stage II pancreatic cancer, metastatic disease, KRAS G12V, initially diagnosed in January 2018 Sites of Metastases Lung, lymph nodes Prior Therapy Surgery, adjuvant radiation, gemcitabine/ capecitabine (#1); 5FU/oxaliplatin/irinotecan (#2); gemcitabine/abraxane (#3); 5FU/liposomal irinotecan (#4); alomfilimab (ICOS-targeted antibody)/atezolizumab (#5); MVT-5873 (anti-CA 19-9 antibody) (#6) Dosing ERAS-007 125 mg BID-QW Patient progressed with new lesion at subsequent assessment Baseline 16 Weeks 31% reduction in tumor size HERKULES-1 Case Study: Single agent ERAS-007 response 70-year-old female (Patient 0033) with KRAS G12V metastatic pancreatic cancer
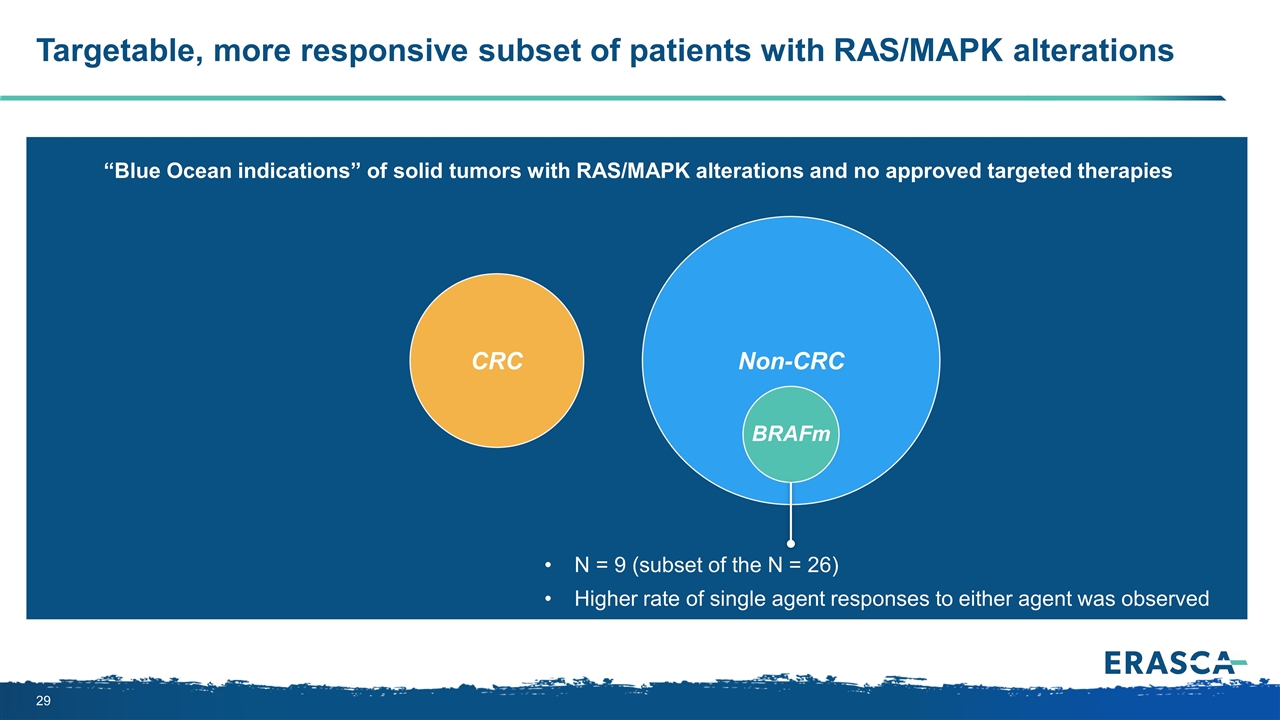
CRC Targetable, more responsive subset of patients with RAS/MAPK alterations Non-CRC N = 9 (subset of the N = 26) Higher rate of single agent responses to either agent was observed “Blue Ocean indications” of solid tumors with RAS/MAPK alterations and no approved targeted therapies BRAFm
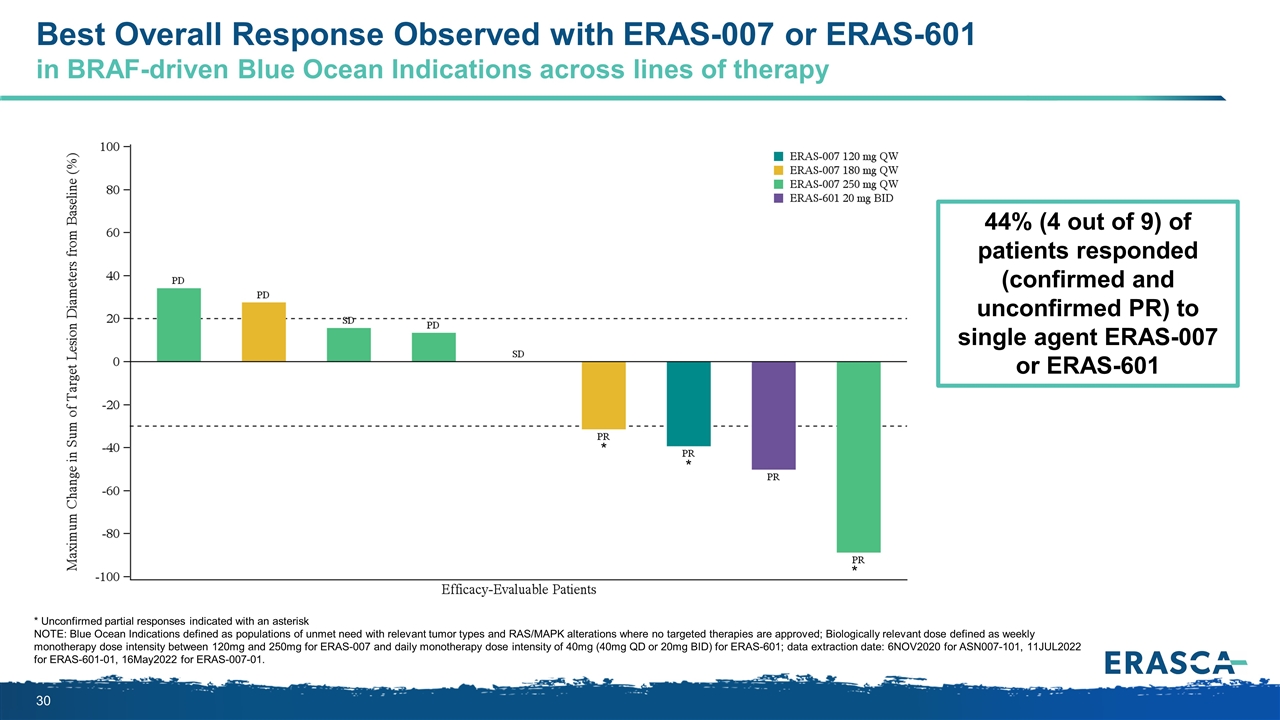
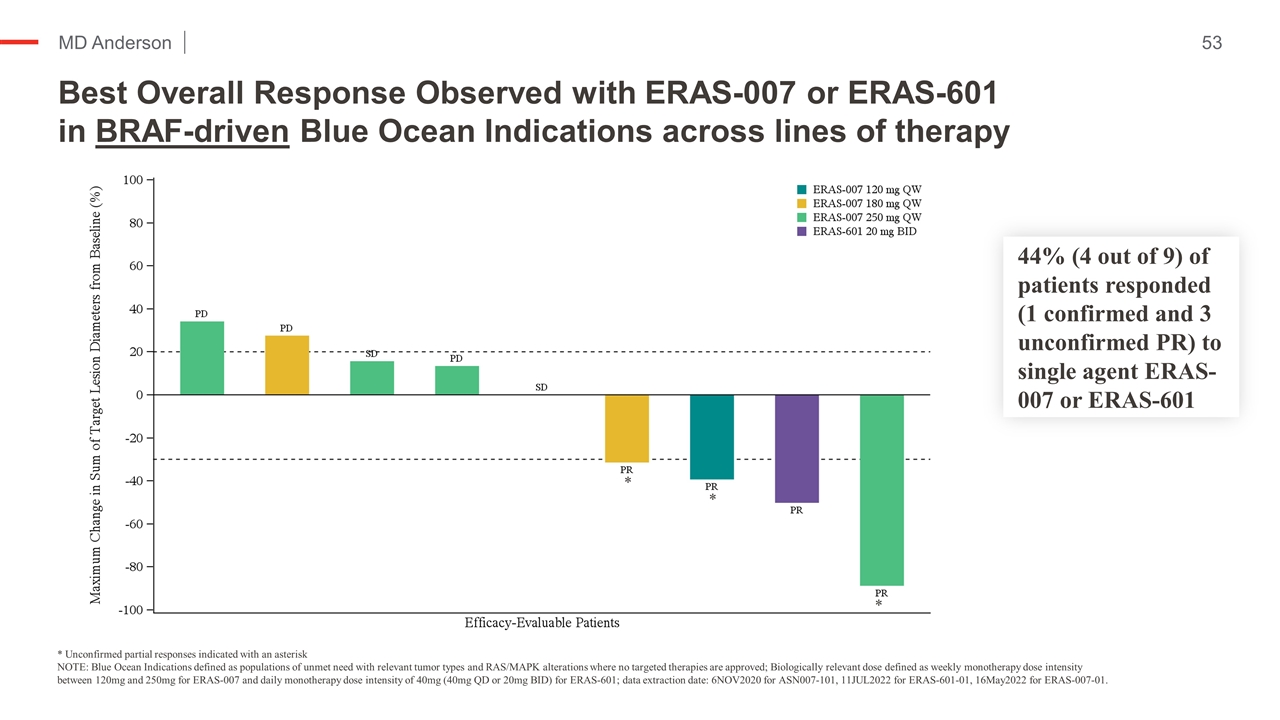
* Unconfirmed partial responses indicated with an asterisk NOTE: Blue Ocean Indications defined as populations of unmet need with relevant tumor types and RAS/MAPK alterations where no targeted therapies are approved; Biologically relevant dose defined as weekly monotherapy dose intensity between 120mg and 250mg for ERAS-007 and daily monotherapy dose intensity of 40mg (40mg QD or 20mg BID) for ERAS-601; data extraction date: 6NOV2020 for ASN007-101, 11JUL2022 for ERAS-601-01, 16May2022 for ERAS-007-01. * * * Best Overall Response Observed with ERAS-007 or ERAS-601 in BRAF-driven Blue Ocean Indications across lines of therapy 44% (4 out of 9) of patients responded (confirmed and unconfirmed PR) to single agent ERAS-007 or ERAS-601
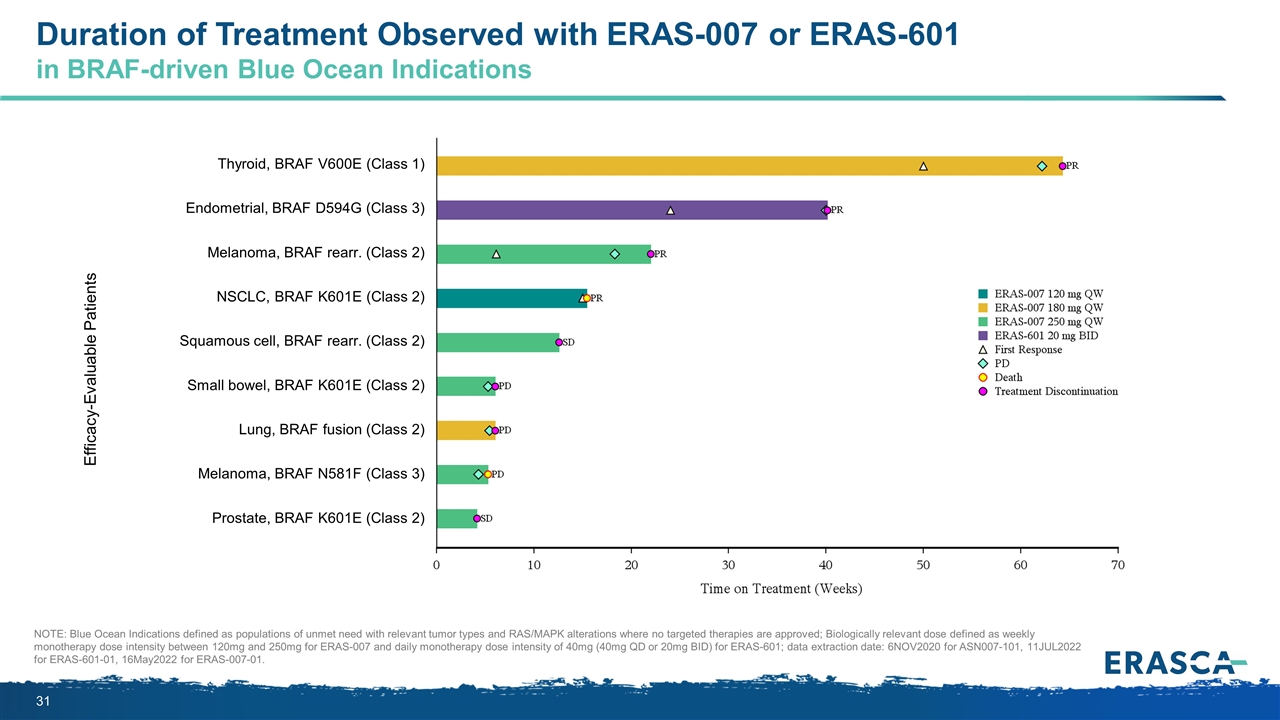
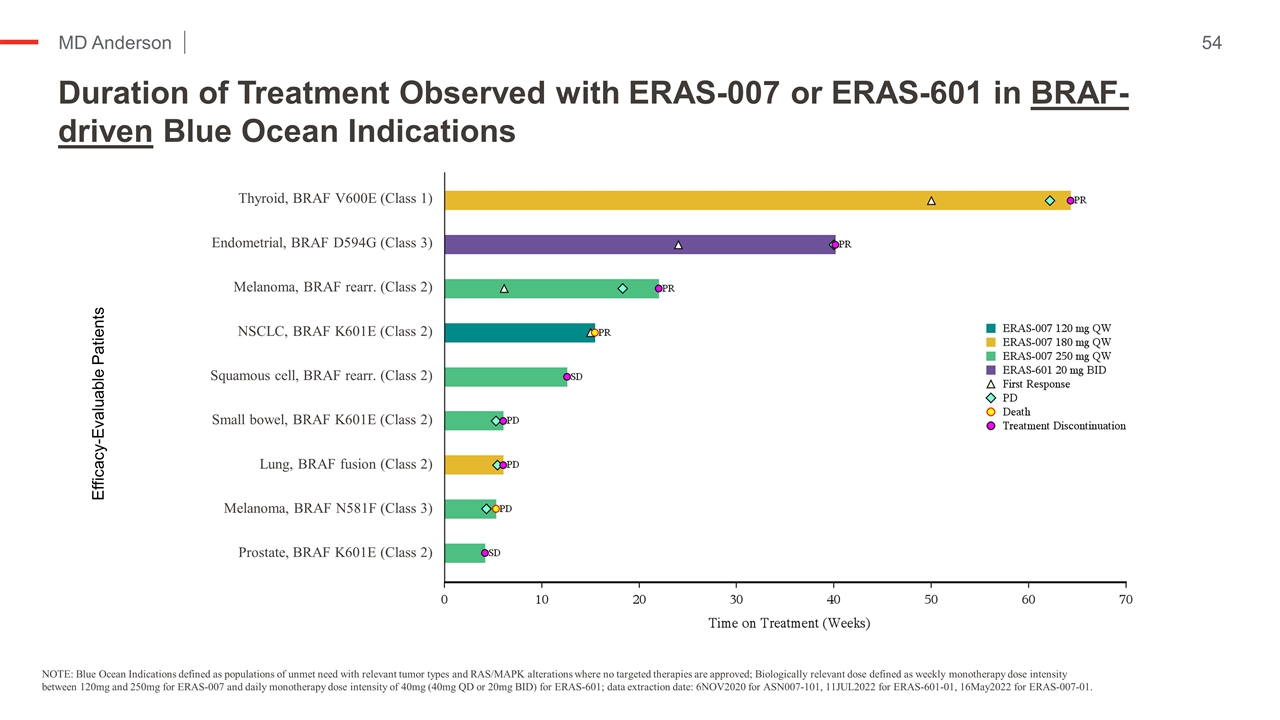
Duration of Treatment Observed with ERAS-007 or ERAS-601 in BRAF-driven Blue Ocean Indications NOTE: Blue Ocean Indications defined as populations of unmet need with relevant tumor types and RAS/MAPK alterations where no targeted therapies are approved; Biologically relevant dose defined as weekly monotherapy dose intensity between 120mg and 250mg for ERAS-007 and daily monotherapy dose intensity of 40mg (40mg QD or 20mg BID) for ERAS-601; data extraction date: 6NOV2020 for ASN007-101, 11JUL2022 for ERAS-601-01, 16May2022 for ERAS-007-01. Efficacy-Evaluable Patients Thyroid, BRAF V600E (Class 1) Endometrial, BRAF D594G (Class 3) Melanoma, BRAF rearr. (Class 2) NSCLC, BRAF K601E (Class 2) Squamous cell, BRAF rearr. (Class 2) Small bowel, BRAF K601E (Class 2) Lung, BRAF fusion (Class 2) Melanoma, BRAF N581F (Class 3) Prostate, BRAF K601E (Class 2)
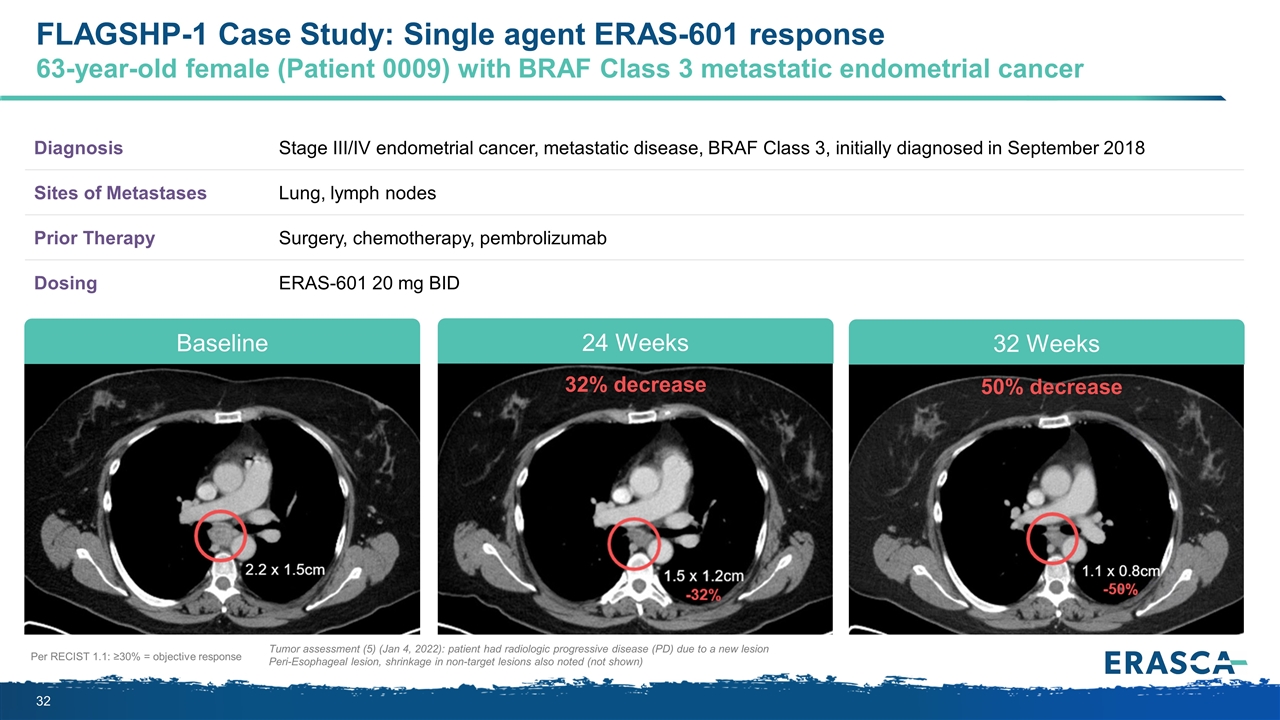
Per RECIST 1.1: ≥30% = objective response Tumor assessment (5) (Jan 4, 2022): patient had radiologic progressive disease (PD) due to a new lesion Peri-Esophageal lesion, shrinkage in non-target lesions also noted (not shown) Diagnosis Stage III/IV endometrial cancer, metastatic disease, BRAF Class 3, initially diagnosed in September 2018 Sites of Metastases Lung, lymph nodes Prior Therapy Surgery, chemotherapy, pembrolizumab Dosing ERAS-601 20 mg BID 50% decrease 32% decrease Baseline 24 Weeks 32 Weeks FLAGSHP-1 Case Study: Single agent ERAS-601 response 63-year-old female (Patient 0009) with BRAF Class 3 metastatic endometrial cancer
Are there more responsive subsets within the Blue Ocean where ERK + cell cycle or ERK + SHP2 inhibition can be particularly effective? Single agent activity of ERAS-007 and ERAS-601 indicates responsiveness in certain tumor types and molecular alterations Combination approaches have potential to deepen responses and improve durability Non-CRC Solid Tumors RAS/MAPK alterations BRAF-driven RAS/MAPK alterations
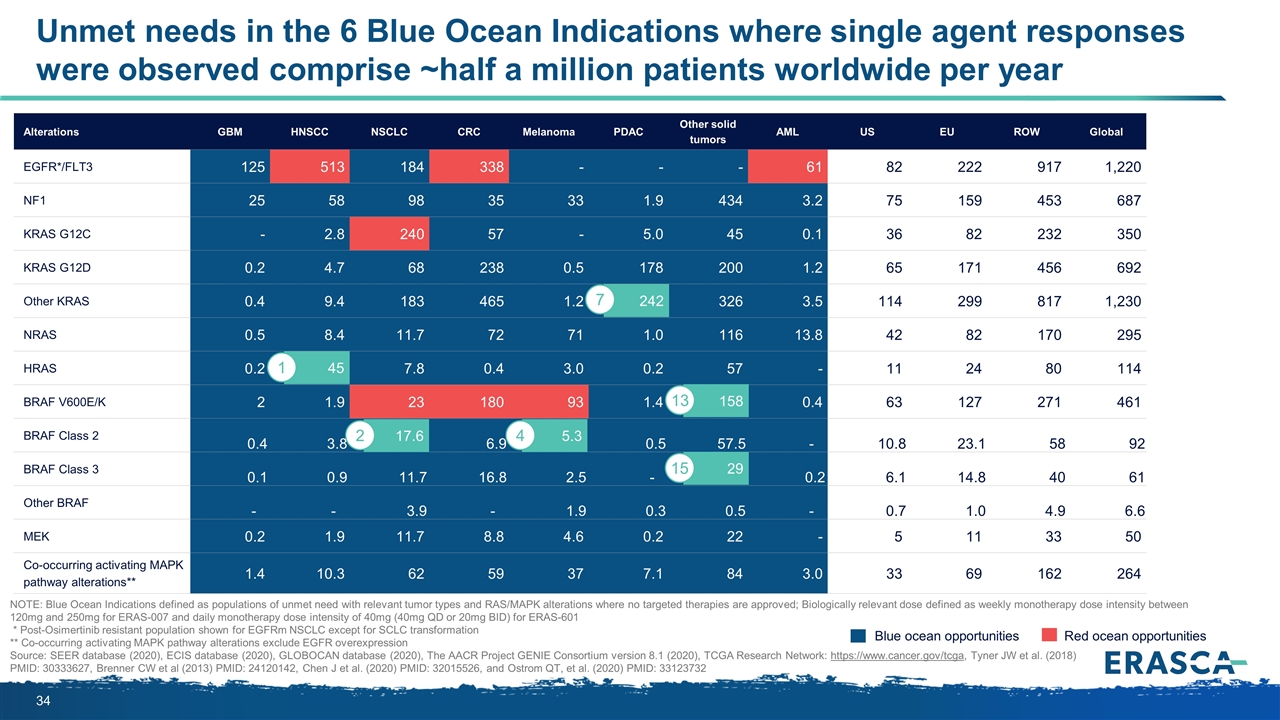
Unmet needs in the 6 Blue Ocean Indications where single agent responses were observed comprise ~half a million patients worldwide per year Alterations GBM HNSCC NSCLC CRC Melanoma PDAC Other solid tumors AML US EU ROW Global EGFR*/FLT3 125 513 184 338 - - - 61 82 222 917 1,220 NF1 25 58 98 35 33 1.9 434 3.2 75 159 453 687 KRAS G12C - 2.8 240 57 - 5.0 45 0.1 36 82 232 350 KRAS G12D 0.2 4.7 68 238 0.5 178 200 1.2 65 171 456 692 Other KRAS 0.4 9.4 183 465 1.2 242 326 3.5 114 299 817 1,230 NRAS 0.5 8.4 11.7 72 71 1.0 116 13.8 42 82 170 295 HRAS 0.2 45 7.8 0.4 3.0 0.2 57 - 11 24 80 114 BRAF V600E/K 2 1.9 23 180 93 1.4 158 0.4 63 127 271 461 BRAF Class 2 0.4 3.8 17.6 6.9 5.3 0.5 57.5 - 10.8 23.1 58 92 BRAF Class 3 0.1 0.9 11.7 16.8 2.5 - 28.7 0.2 6.1 14.8 40 61 Other BRAF - - 3.9 - 1.9 0.3 0.5 - 0.7 1.0 4.9 6.6 MEK 0.2 1.9 11.7 8.8 4.6 0.2 22 - 5 11 33 50 Co-occurring activating MAPK pathway alterations** 1.4 10.3 62 59 37 7.1 84 3.0 33 69 162 264 Blue ocean opportunities Red ocean opportunities NOTE: Blue Ocean Indications defined as populations of unmet need with relevant tumor types and RAS/MAPK alterations where no targeted therapies are approved; Biologically relevant dose defined as weekly monotherapy dose intensity between 120mg and 250mg for ERAS-007 and daily monotherapy dose intensity of 40mg (40mg QD or 20mg BID) for ERAS-601 * Post-Osimertinib resistant population shown for EGFRm NSCLC except for SCLC transformation ** Co-occurring activating MAPK pathway alterations exclude EGFR overexpression Source: SEER database (2020), ECIS database (2020), GLOBOCAN database (2020), The AACR Project GENIE Consortium version 8.1 (2020), TCGA Research Network: https://www.cancer.gov/tcga, Tyner JW et al. (2018) PMID: 30333627, Brenner CW et al (2013) PMID: 24120142, Chen J et al. (2020) PMID: 32015526, and Ostrom QT, et al. (2020) PMID: 33123732 158 17.6 2 45 1 5.3 4 242 7 29 13 15
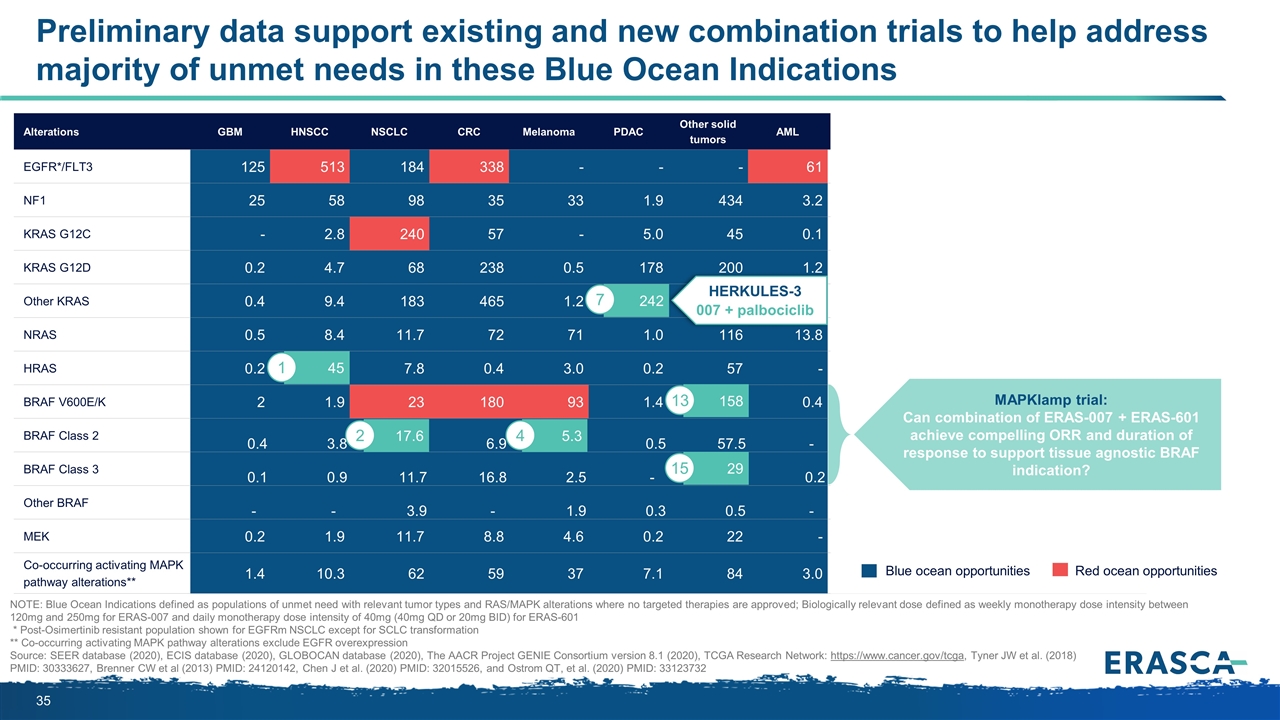
Preliminary data support existing and new combination trials to help address majority of unmet needs in these Blue Ocean Indications Alterations GBM HNSCC NSCLC CRC Melanoma PDAC Other solid tumors AML US EU ROW Global EGFR*/FLT3 125 513 184 338 - - - 61 82 222 917 1,220 NF1 25 58 98 35 33 1.9 434 3.2 75 159 453 687 KRAS G12C - 2.8 240 57 - 5.0 45 0.1 36 82 232 350 KRAS G12D 0.2 4.7 68 238 0.5 178 200 1.2 65 171 456 692 Other KRAS 0.4 9.4 183 465 1.2 242 326 3.5 114 299 817 1,230 NRAS 0.5 8.4 11.7 72 71 1.0 116 13.8 42 82 170 295 HRAS 0.2 45 7.8 0.4 3.0 0.2 57 - 11 24 80 114 BRAF V600E/K 2 1.9 23 180 93 1.4 158 0.4 63 127 271 461 BRAF Class 2 0.4 3.8 17.6 6.9 5.3 0.5 57.5 - 10.8 23.1 58 92 BRAF Class 3 0.1 0.9 11.7 16.8 2.5 - 28.7 0.2 6.1 14.8 40 61 Other BRAF - - 3.9 - 1.9 0.3 0.5 - 0.7 1.0 4.9 6.6 MEK 0.2 1.9 11.7 8.8 4.6 0.2 22 - 5 11 33 50 Co-occurring activating MAPK pathway alterations** 1.4 10.3 62 59 37 7.1 84 3.0 33 69 162 264 Blue ocean opportunities Red ocean opportunities NOTE: Blue Ocean Indications defined as populations of unmet need with relevant tumor types and RAS/MAPK alterations where no targeted therapies are approved; Biologically relevant dose defined as weekly monotherapy dose intensity between 120mg and 250mg for ERAS-007 and daily monotherapy dose intensity of 40mg (40mg QD or 20mg BID) for ERAS-601 * Post-Osimertinib resistant population shown for EGFRm NSCLC except for SCLC transformation ** Co-occurring activating MAPK pathway alterations exclude EGFR overexpression Source: SEER database (2020), ECIS database (2020), GLOBOCAN database (2020), The AACR Project GENIE Consortium version 8.1 (2020), TCGA Research Network: https://www.cancer.gov/tcga, Tyner JW et al. (2018) PMID: 30333627, Brenner CW et al (2013) PMID: 24120142, Chen J et al. (2020) PMID: 32015526, and Ostrom QT, et al. (2020) PMID: 33123732 MAPKlamp trial: Can combination of ERAS-007 + ERAS-601 achieve compelling ORR and duration of response to support tissue agnostic BRAF indication? HERKULES-3 007 + palbociclib 158 17.6 2 45 1 5.3 4 242 7 29 13 15
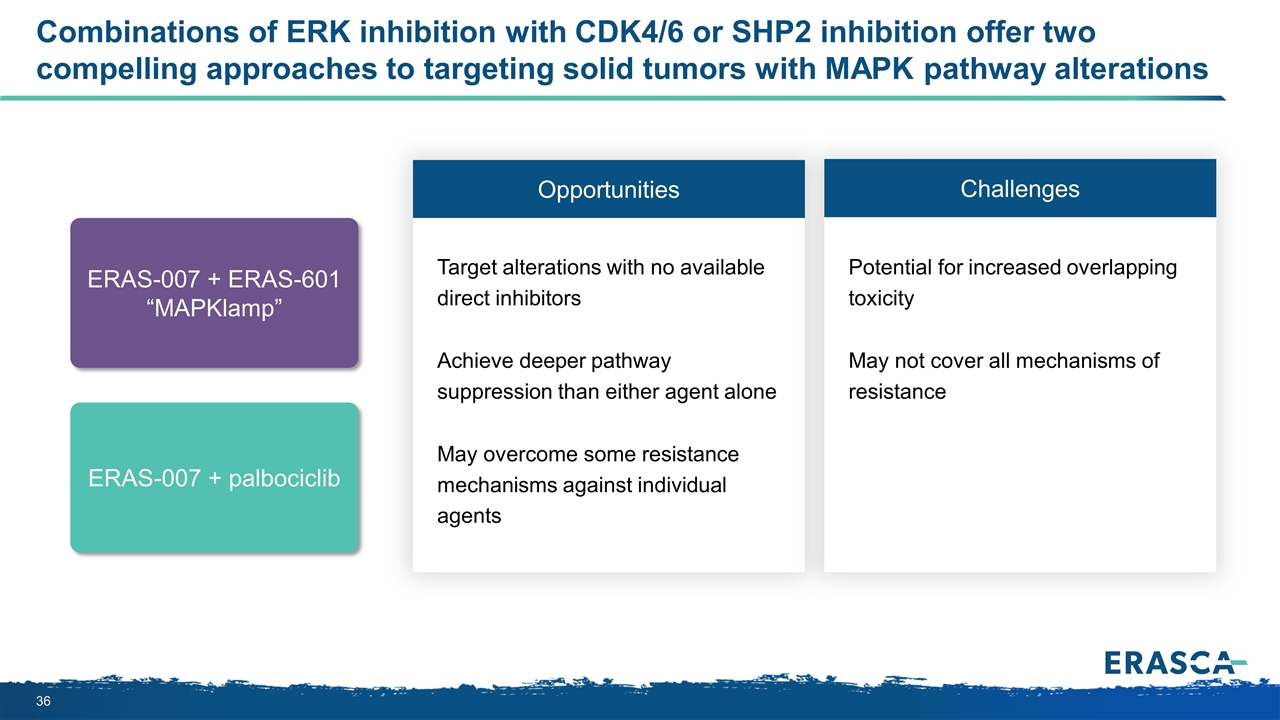
Combinations of ERK inhibition with CDK4/6 or SHP2 inhibition offer two compelling approaches to targeting solid tumors with MAPK pathway alterations Opportunities Challenges Target alterations with no available direct inhibitors Achieve deeper pathway suppression than either agent alone May overcome some resistance mechanisms against individual agents Potential for increased overlapping toxicity May not cover all mechanisms of resistance ERAS-007 + palbociclib ERAS-007 + ERAS-601 “MAPKlamp”

Can ERAS-007 be combined safely with palbociclib or ERAS-601 in patients? ?
Expected TRAEs associated with ERK and CDK4/6 inhibitors have been limited Common ERKi TRAEs Common CDK4/6i TRAEs TRAE: treatment‐related adverse event Skin Gastrointestinal Eye General (fatigue) Hematologic Gastrointestinal Infections General (fatigue)
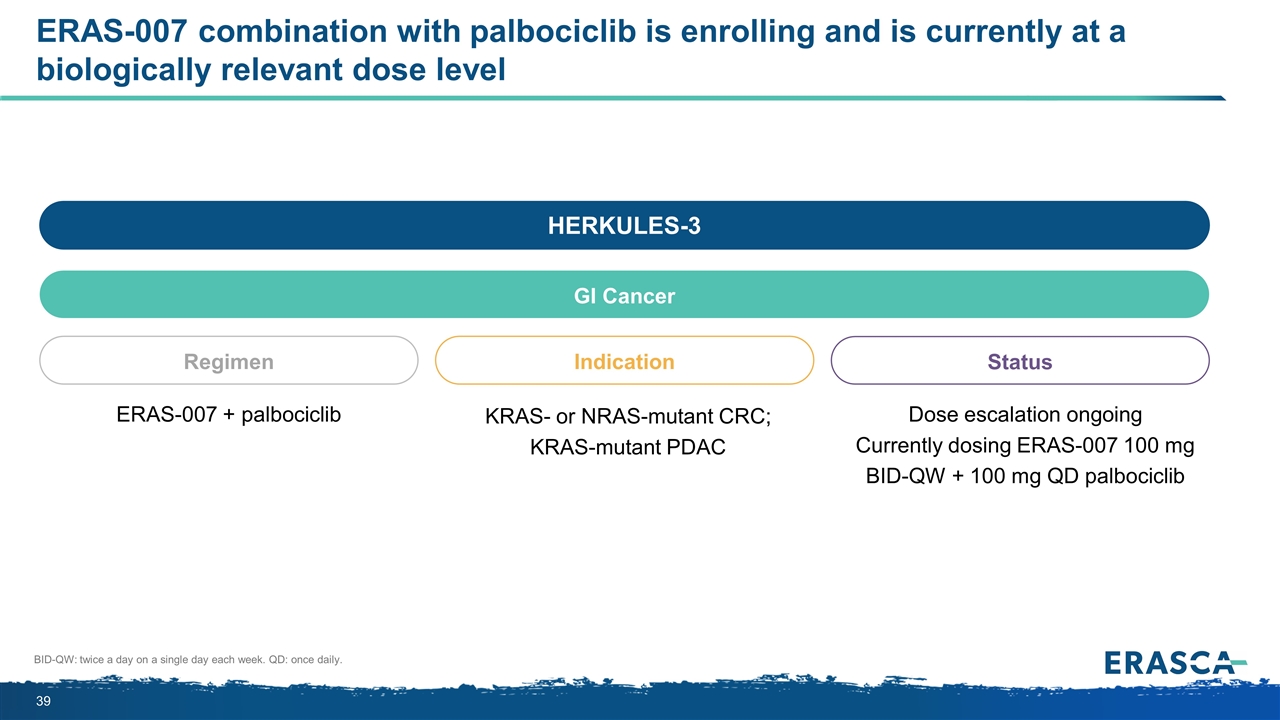
ERAS-007 combination with palbociclib is enrolling and is currently at a biologically relevant dose level BID-QW: twice a day on a single day each week. QD: once daily. HERKULES-3 GI Cancer Regimen ERAS-007 + palbociclib Indication Status KRAS- or NRAS-mutant CRC; KRAS-mutant PDAC Dose escalation ongoing Currently dosing ERAS-007 100 mg BID-QW + 100 mg QD palbociclib
Expected TRAEs associated with SHP2 and ERK inhibitors have been limited TRAE: treatment‐related adverse event Common ERKi TRAEs Skin Gastrointestinal Eye General (fatigue) Common SHP2i TRAEs Gastrointestinal General (peripheral edema) Hematologic Cardiovascular Hepatic
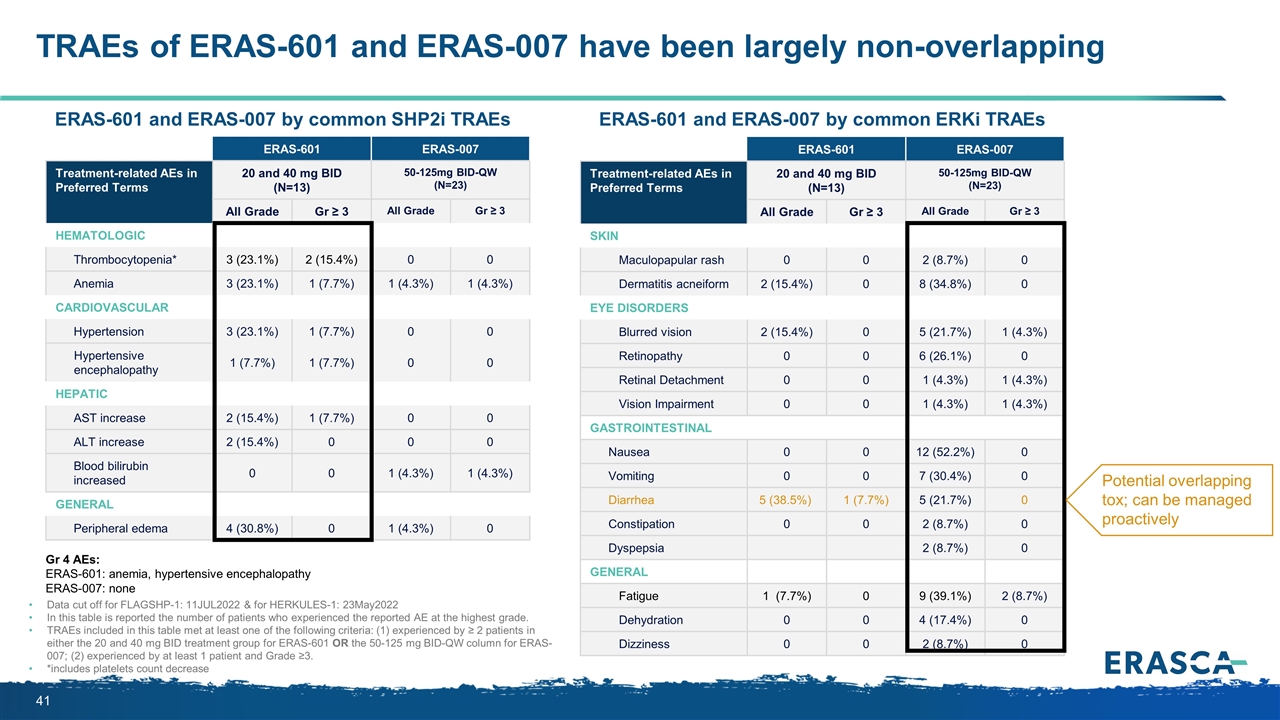
ERAS-601 ERAS-007 Treatment-related AEs in Preferred Terms 20 and 40 mg BID (N=13) 50-125mg BID-QW (N=23) System Organ Class / Preferred Term All Grade Gr ≥ 3 All Grade Gr ≥ 3 Hematologic Thrombocytopenia* 3 (23.1%) 2 (15.4%) 0 0 Anemia 3 (23.1%) 1 (7.7%) 1 (4.3%) 1 (4.3%) Cardiovascular Hypertension 3 (23.1%) 1 (7.7%) 0 0 Hypertensive encephalopathy 1 (7.7%) 1 (7.7%) 0 0 Hepatic AST increase 2 (15.4%) 1 (7.7%) 0 0 ALT increase 2 (15.4%) 0 0 0 Blood bilirubin increased 0 0 1 (4.3%) 1 (4.3%) General Peripheral edema 4 (30.8%) 0 1 (4.3%) 0 TRAEs of ERAS-601 and ERAS-007 have been largely non-overlapping Data cut off for FLAGSHP-1: 11JUL2022 & for HERKULES-1: 23May2022 In this table is reported the number of patients who experienced the reported AE at the highest grade. TRAEs included in this table met at least one of the following criteria: (1) experienced by ≥ 2 patients in either the 20 and 40 mg BID treatment group for ERAS-601 OR the 50-125 mg BID-QW column for ERAS-007; (2) experienced by at least 1 patient and Grade ≥3. *includes platelets count decrease ERAS-601 ERAS-007 Treatment-related AEs in Preferred Terms 20 and 40 mg BID (N=13) 50-125mg BID-QW (N=23) System Organ Class / Preferred Term All Grade Gr ≥ 3 All Grade Gr ≥ 3 Skin Maculopapular rash 0 0 2 (8.7%) 0 Dermatitis acneiform 2 (15.4%) 0 8 (34.8%) 0 EYE DISORDERS Blurred vision 2 (15.4%) 0 5 (21.7%) 1 (4.3%) Retinopathy 0 0 6 (26.1%) 0 Retinal Detachment 0 0 1 (4.3%) 1 (4.3%) Vision Impairment 0 0 1 (4.3%) 1 (4.3%) Gastrointestinal Nausea 0 0 12 (52.2%) 0 Vomiting 0 0 7 (30.4%) 0 Diarrhea 5 (38.5%) 1 (7.7%) 5 (21.7%) 0 Constipation 0 0 2 (8.7%) 0 Dyspepsia 2 (8.7%) 0 General Fatigue 1 (7.7%) 0 9 (39.1%) 2 (8.7%) Dehydration 0 0 4 (17.4%) 0 Dizziness 0 0 2 (8.7%) 0 ERAS-601 and ERAS-007 by common SHP2i TRAEs ERAS-601 and ERAS-007 by common ERKi TRAEs Potential overlapping tox; can be managed proactively Gr 4 AEs: ERAS-601: anemia, hypertensive encephalopathy ERAS-007: none
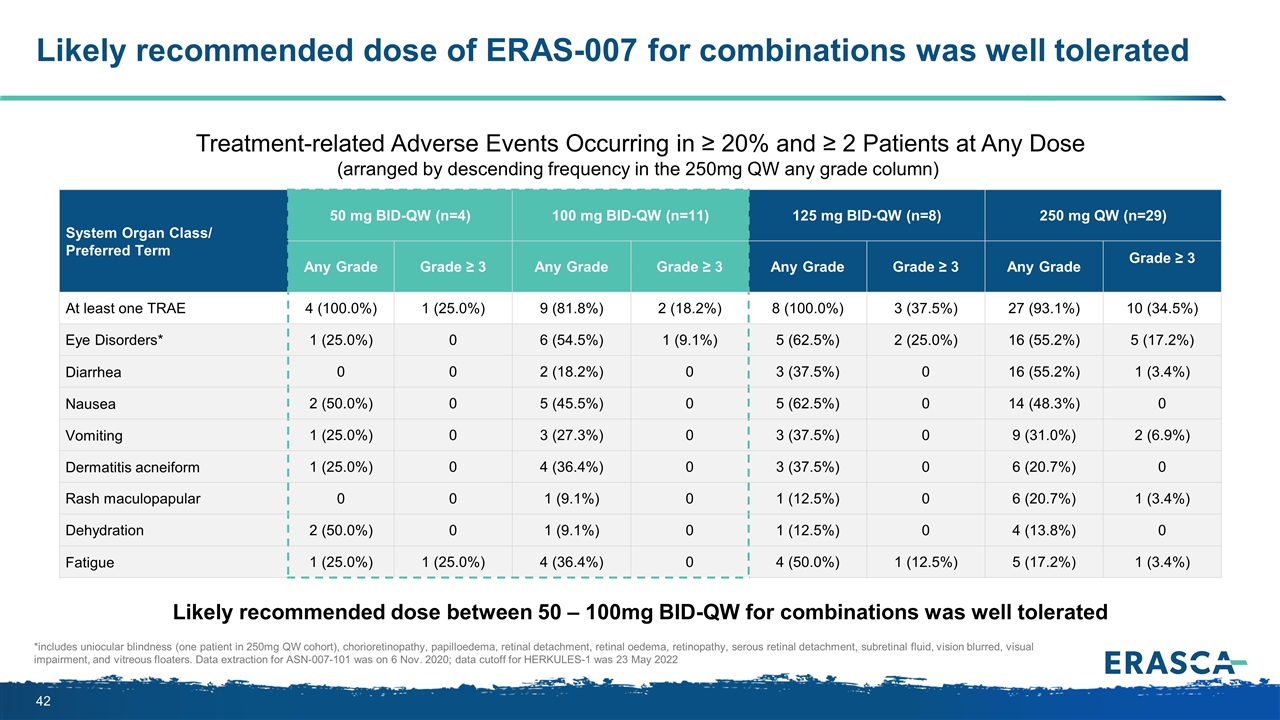
Likely recommended dose of ERAS-007 for combinations was well tolerated *includes uniocular blindness (one patient in 250mg QW cohort), chorioretinopathy, papilloedema, retinal detachment, retinal oedema, retinopathy, serous retinal detachment, subretinal fluid, vision blurred, visual impairment, and vitreous floaters. Data extraction for ASN-007-101 was on 6 Nov. 2020; data cutoff for HERKULES-1 was 23 May 2022 System Organ Class/ Preferred Term 50 mg BID-QW (n=4) 100 mg BID-QW (n=11) 125 mg BID-QW (n=8) 250 mg QW (n=29) Any Grade Grade ≥ 3 Any Grade Grade ≥ 3 Any Grade Grade ≥ 3 Any Grade Grade ≥ 3 At least one TRAE 4 (100.0%) 1 (25.0%) 9 (81.8%) 2 (18.2%) 8 (100.0%) 3 (37.5%) 27 (93.1%) 10 (34.5%) Eye Disorders* 1 (25.0%) 0 6 (54.5%) 1 (9.1%) 5 (62.5%) 2 (25.0%) 16 (55.2%) 5 (17.2%) Diarrhea 0 0 2 (18.2%) 0 3 (37.5%) 0 16 (55.2%) 1 (3.4%) Nausea 2 (50.0%) 0 5 (45.5%) 0 5 (62.5%) 0 14 (48.3%) 0 Vomiting 1 (25.0%) 0 3 (27.3%) 0 3 (37.5%) 0 9 (31.0%) 2 (6.9%) Dermatitis acneiform 1 (25.0%) 0 4 (36.4%) 0 3 (37.5%) 0 6 (20.7%) 0 Rash maculopapular 0 0 1 (9.1%) 0 1 (12.5%) 0 6 (20.7%) 1 (3.4%) Dehydration 2 (50.0%) 0 1 (9.1%) 0 1 (12.5%) 0 4 (13.8%) 0 Fatigue 1 (25.0%) 1 (25.0%) 4 (36.4%) 0 4 (50.0%) 1 (12.5%) 5 (17.2%) 1 (3.4%) Treatment-related Adverse Events Occurring in ≥ 20% and ≥ 2 Patients at Any Dose (arranged by descending frequency in the 250mg QW any grade column) Likely recommended dose between 50 – 100mg BID-QW for combinations was well tolerated
Will ERAS-601 and ERAS-007 provide adequate target inhibition at the combination dose and schedule? ?
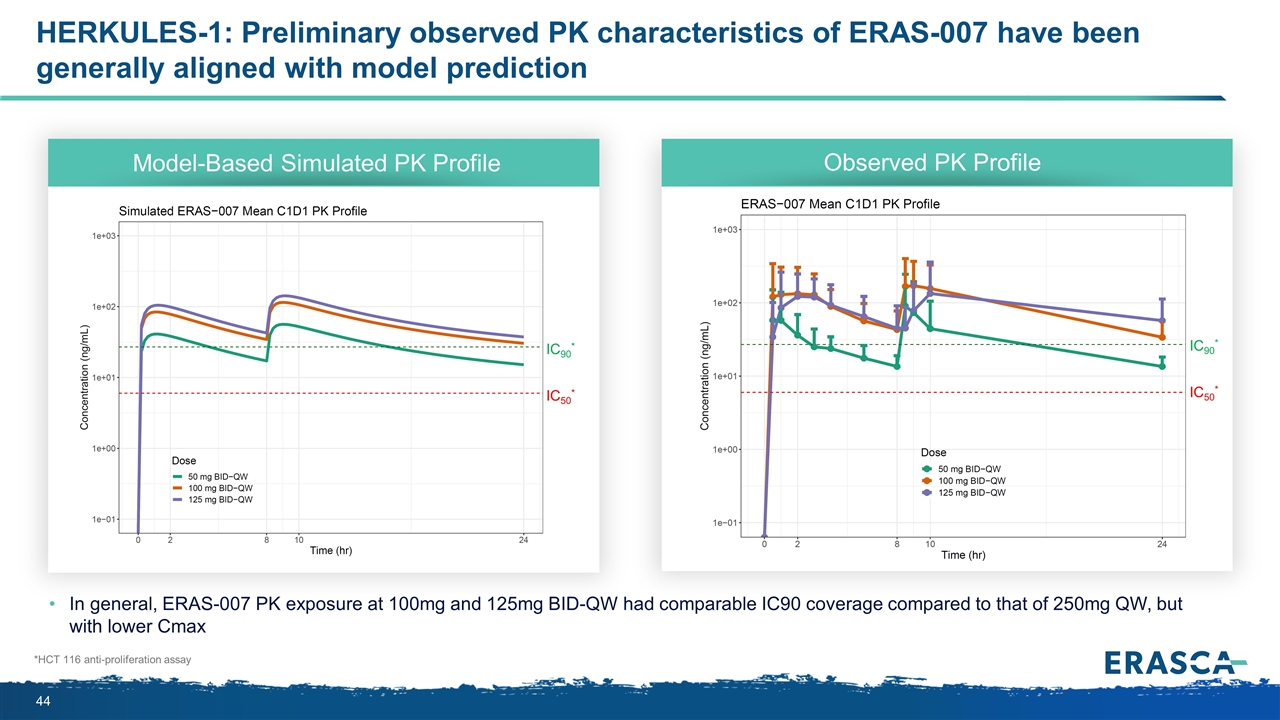
HERKULES-1: Preliminary observed PK characteristics of ERAS-007 have been generally aligned with model prediction IC90* IC50* Model-Based Simulated PK Profile Observed PK Profile In general, ERAS-007 PK exposure at 100mg and 125mg BID-QW had comparable IC90 coverage compared to that of 250mg QW, but with lower Cmax *HCT 116 anti-proliferation assay IC90* IC50*
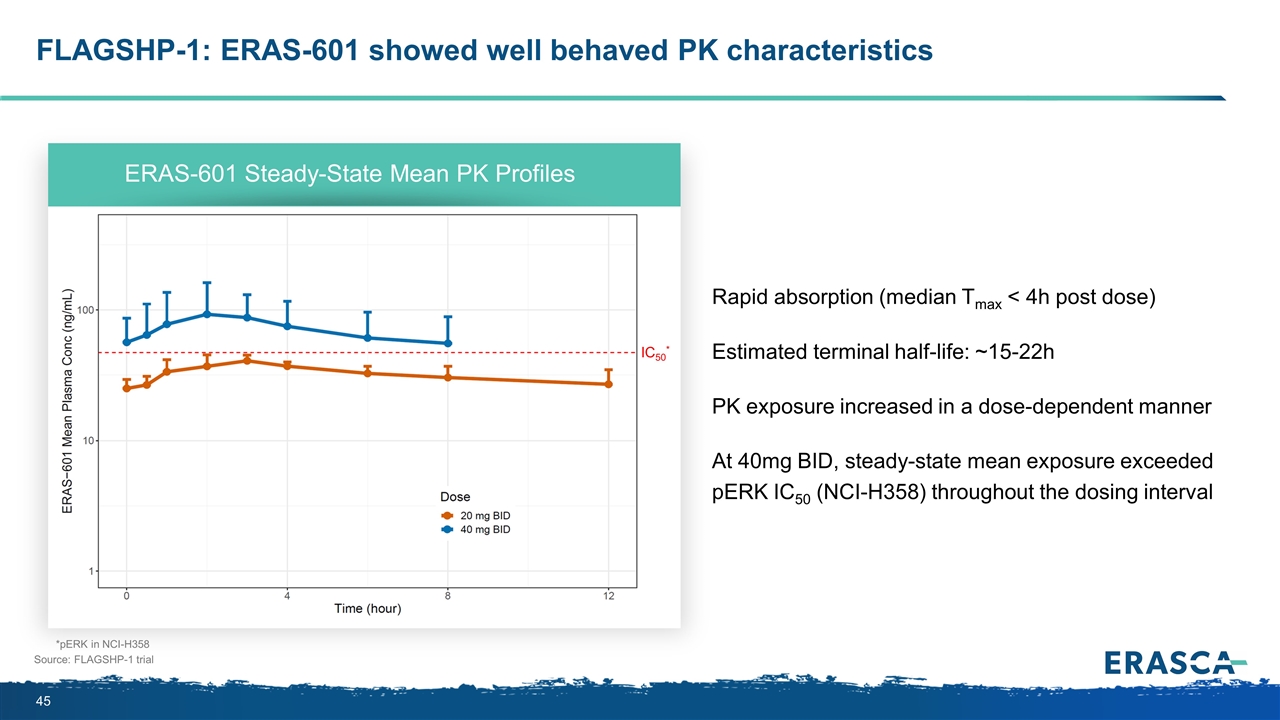
FLAGSHP-1: ERAS-601 showed well behaved PK characteristics Source: FLAGSHP-1 trial Rapid absorption (median Tmax < 4h post dose) Estimated terminal half-life: ~15-22h PK exposure increased in a dose-dependent manner At 40mg BID, steady-state mean exposure exceeded pERK IC50 (NCI-H358) throughout the dosing interval ERAS-601 Steady-State Mean PK Profiles *pERK in NCI-H358 IC50*
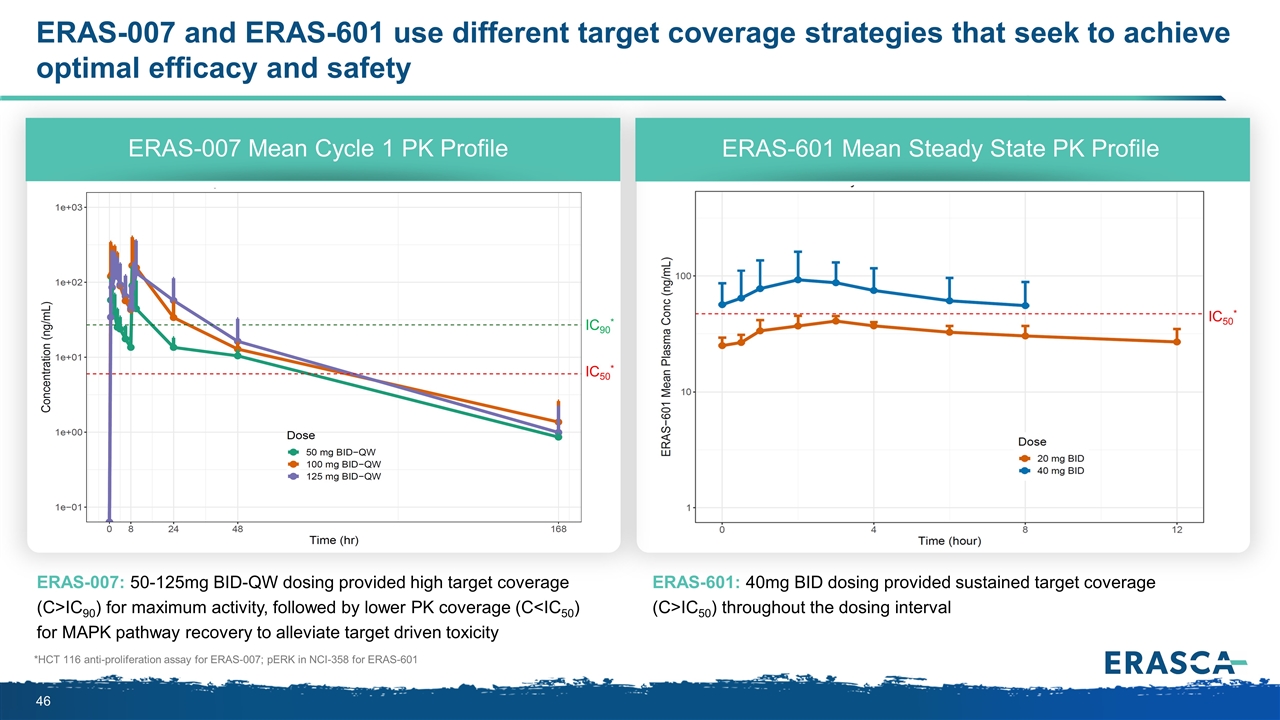
ERAS-007 and ERAS-601 use different target coverage strategies that seek to achieve optimal efficacy and safety ERAS-007: 50-125mg BID-QW dosing provided high target coverage (C>IC90) for maximum activity, followed by lower PK coverage (C<IC50) for MAPK pathway recovery to alleviate target driven toxicity ERAS-601: 40mg BID dosing provided sustained target coverage (C>IC50) throughout the dosing interval ERAS-007 Mean Cycle 1 PK Profile ERAS-601 Mean Steady State PK Profile *HCT 116 anti-proliferation assay for ERAS-007; pERK in NCI-358 for ERAS-601 IC90* IC50* IC50*
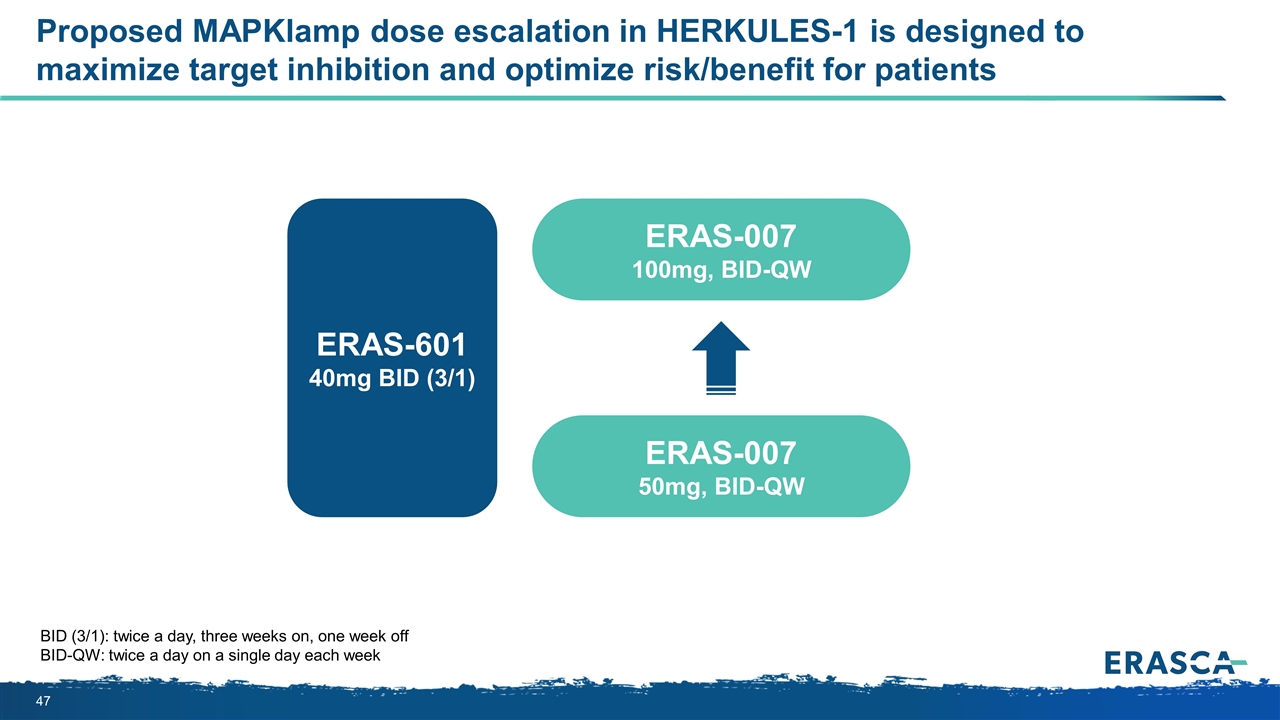
Proposed MAPKlamp dose escalation in HERKULES-1 is designed to maximize target inhibition and optimize risk/benefit for patients BID (3/1): twice a day, three weeks on, one week off BID-QW: twice a day on a single day each week ERAS-007 100mg, BID-QW ERAS-601 40mg BID (3/1) ERAS-007 50mg, BID-QW
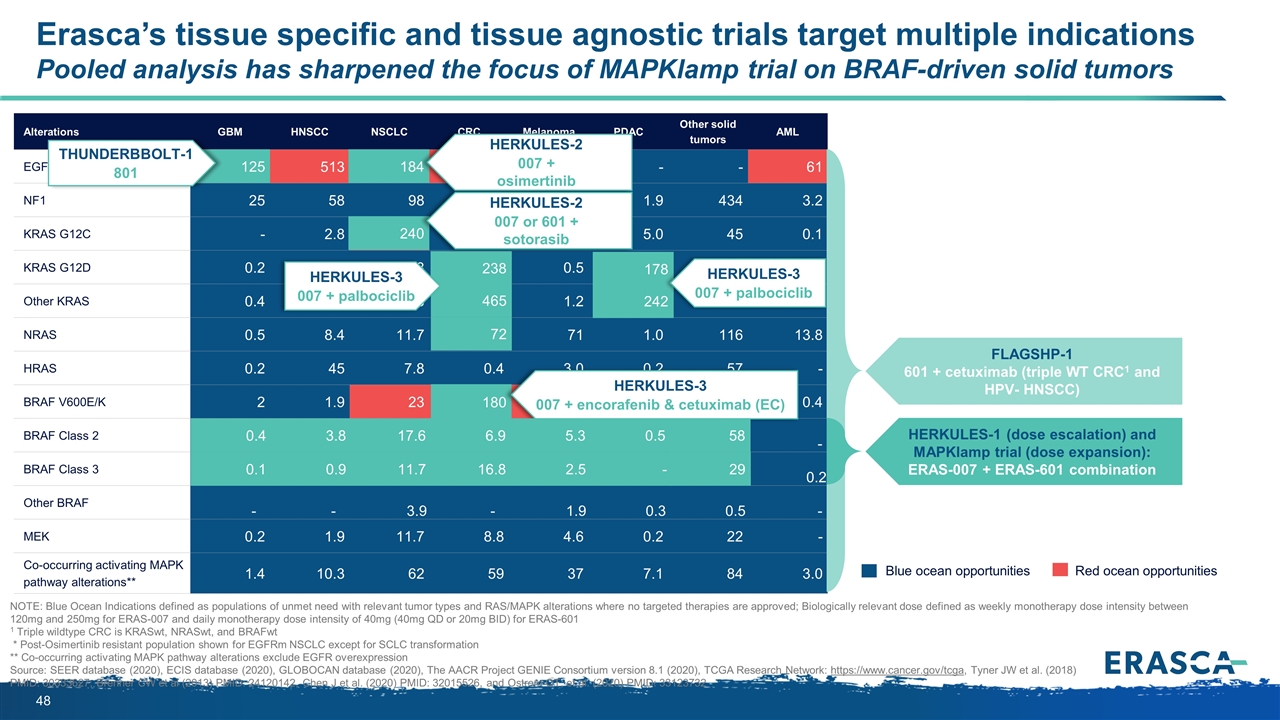
Erasca’s tissue specific and tissue agnostic trials target multiple indications Pooled analysis has sharpened the focus of MAPKlamp trial on BRAF-driven solid tumors Alterations GBM HNSCC NSCLC CRC Melanoma PDAC Other solid tumors AML EGFR*/FLT3 125 513 184 338 - - - 61 NF1 25 58 98 35 33 1.9 434 3.2 KRAS G12C - 2.8 240 57 - 5.0 45 0.1 KRAS G12D 0.2 4.7 68 238 0.5 178 200 1.2 Other KRAS 0.4 9.4 183 465 1.2 242 326 3.5 NRAS 0.5 8.4 11.7 72 71 1.0 116 13.8 HRAS 0.2 45 7.8 0.4 3.0 0.2 57 - BRAF V600E/K 2 1.9 23 180 93 1.4 158 0.4 BRAF Class 2 0.4 3.8 17.6 6.9 5.3 0.5 57.5 - BRAF Class 3 0.1 0.9 11.7 16.8 2.5 - 28.7 0.2 Other BRAF - - 3.9 - 1.9 0.3 0.5 - MEK 0.2 1.9 11.7 8.8 4.6 0.2 22 - Co-occurring activating MAPK pathway alterations** 1.4 10.3 62 59 37 7.1 84 3.0 17.6 242 Blue ocean opportunities Red ocean opportunities NOTE: Blue Ocean Indications defined as populations of unmet need with relevant tumor types and RAS/MAPK alterations where no targeted therapies are approved; Biologically relevant dose defined as weekly monotherapy dose intensity between 120mg and 250mg for ERAS-007 and daily monotherapy dose intensity of 40mg (40mg QD or 20mg BID) for ERAS-601 1 Triple wildtype CRC is KRASwt, NRASwt, and BRAFwt * Post-Osimertinib resistant population shown for EGFRm NSCLC except for SCLC transformation ** Co-occurring activating MAPK pathway alterations exclude EGFR overexpression Source: SEER database (2020), ECIS database (2020), GLOBOCAN database (2020), The AACR Project GENIE Consortium version 8.1 (2020), TCGA Research Network: https://www.cancer.gov/tcga, Tyner JW et al. (2018) PMID: 30333627, Brenner CW et al (2013) PMID: 24120142, Chen J et al. (2020) PMID: 32015526, and Ostrom QT, et al. (2020) PMID: 33123732 184 HERKULES-2 007 + osimertinib 125 THUNDERBBOLT-1 801 240 72 465 238 HERKULES-3 007 + palbociclib HERKULES-2 007 or 601 + sotorasib 3.8 0.4 0.1 0.9 180 HERKULES-3 007 + encorafenib & cetuximab (EC) 178 HERKULES-3 007 + palbociclib FLAGSHP-1 601 + cetuximab (triple WT CRC1 and HPV- HNSCC) HERKULES-1 (dose escalation) and MAPKlamp trial (dose expansion): ERAS-007 + ERAS-601 combination 11.7 6.9 16.8 5.3 2.5 0.5 58 29 -

Discussant: Dr. David Hong Deputy Chair in the Department of Investigational Cancer Therapeutics MD Anderson Cancer Center

David Hong, MD Professor, Deputy Chair Department of Investigational Cancer Therapeutics
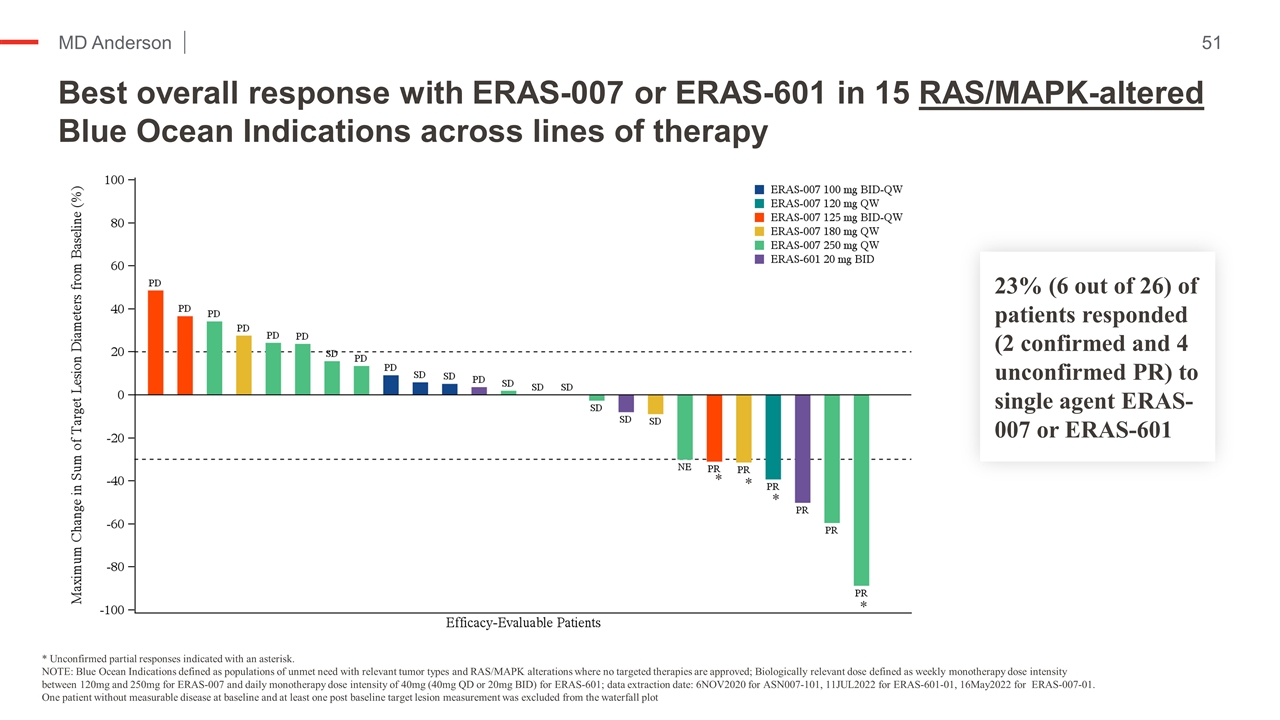
Best overall response with ERAS-007 or ERAS-601 in 15 RAS/MAPK-altered Blue Ocean Indications across lines of therapy Kopetz et al NEJM ‘19 * Unconfirmed partial responses indicated with an asterisk. NOTE: Blue Ocean Indications defined as populations of unmet need with relevant tumor types and RAS/MAPK alterations where no targeted therapies are approved; Biologically relevant dose defined as weekly monotherapy dose intensity between 120mg and 250mg for ERAS-007 and daily monotherapy dose intensity of 40mg (40mg QD or 20mg BID) for ERAS-601; data extraction date: 6NOV2020 for ASN007-101, 11JUL2022 for ERAS-601-01, 16May2022 for ERAS-007-01. One patient without measurable disease at baseline and at least one post baseline target lesion measurement was excluded from the waterfall plot 23% (6 out of 26) of patients responded (2 confirmed and 4 unconfirmed PR) to single agent ERAS-007 or ERAS-601 * * * *
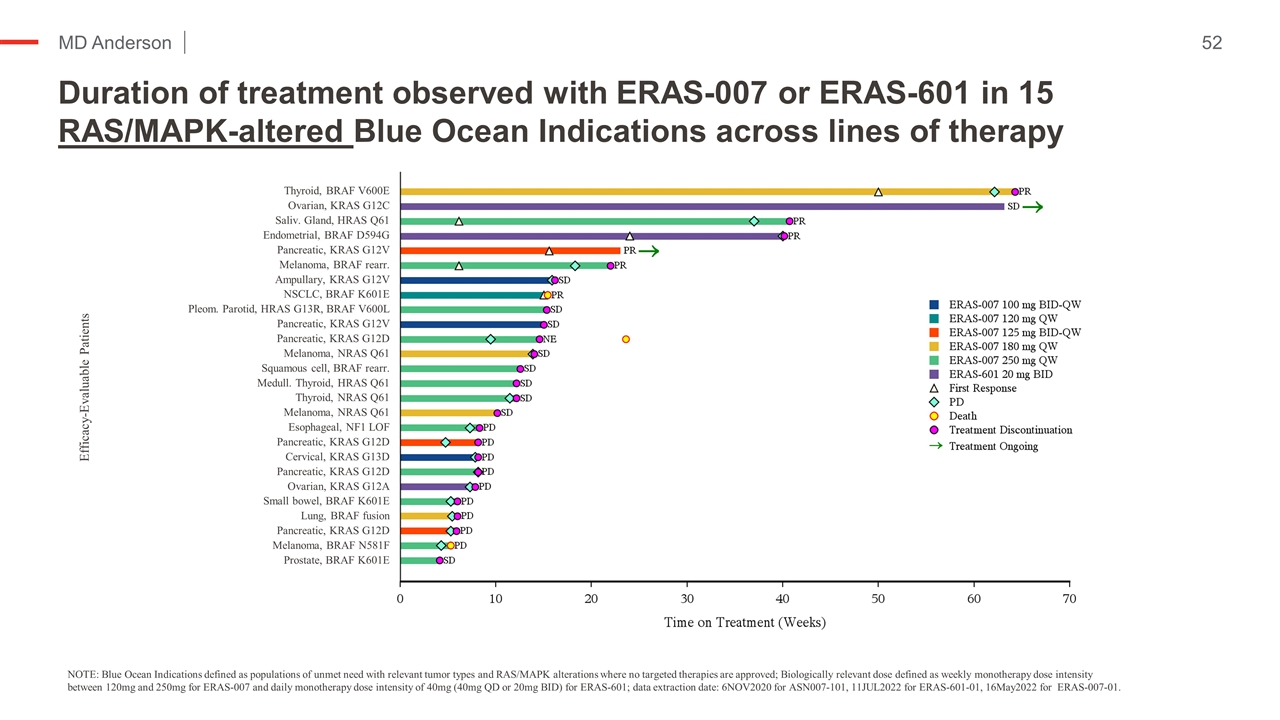
Duration of treatment observed with ERAS-007 or ERAS-601 in 15 RAS/MAPK-altered Blue Ocean Indications across lines of therapy Kopetz et al NEJM ‘19 NOTE: Blue Ocean Indications defined as populations of unmet need with relevant tumor types and RAS/MAPK alterations where no targeted therapies are approved; Biologically relevant dose defined as weekly monotherapy dose intensity between 120mg and 250mg for ERAS-007 and daily monotherapy dose intensity of 40mg (40mg QD or 20mg BID) for ERAS-601; data extraction date: 6NOV2020 for ASN007-101, 11JUL2022 for ERAS-601-01, 16May2022 for ERAS-007-01. Efficacy-Evaluable Patients Thyroid, BRAF V600E Ovarian, KRAS G12C Saliv. Gland, HRAS Q61 Endometrial, BRAF D594G Pancreatic, KRAS G12V Melanoma, BRAF rearr. Ampullary, KRAS G12V NSCLC, BRAF K601E Pleom. Parotid, HRAS G13R, BRAF V600L Pancreatic, KRAS G12V Pancreatic, KRAS G12D Melanoma, NRAS Q61 Squamous cell, BRAF rearr. Medull. Thyroid, HRAS Q61 Thyroid, NRAS Q61 Melanoma, NRAS Q61 Esophageal, NF1 LOF Pancreatic, KRAS G12D Cervical, KRAS G13D Pancreatic, KRAS G12D Ovarian, KRAS G12A Small bowel, BRAF K601E Lung, BRAF fusion Pancreatic, KRAS G12D Melanoma, BRAF N581F Prostate, BRAF K601E
Best Overall Response Observed with ERAS-007 or ERAS-601 in BRAF-driven Blue Ocean Indications across lines of therapy Kopetz et al NEJM ‘19 * Unconfirmed partial responses indicated with an asterisk NOTE: Blue Ocean Indications defined as populations of unmet need with relevant tumor types and RAS/MAPK alterations where no targeted therapies are approved; Biologically relevant dose defined as weekly monotherapy dose intensity between 120mg and 250mg for ERAS-007 and daily monotherapy dose intensity of 40mg (40mg QD or 20mg BID) for ERAS-601; data extraction date: 6NOV2020 for ASN007-101, 11JUL2022 for ERAS-601-01, 16May2022 for ERAS-007-01. * * * 44% (4 out of 9) of patients responded (1 confirmed and 3 unconfirmed PR) to single agent ERAS-007 or ERAS-601
Duration of Treatment Observed with ERAS-007 or ERAS-601 in BRAF-driven Blue Ocean Indications Kopetz et al NEJM ‘19 NOTE: Blue Ocean Indications defined as populations of unmet need with relevant tumor types and RAS/MAPK alterations where no targeted therapies are approved; Biologically relevant dose defined as weekly monotherapy dose intensity between 120mg and 250mg for ERAS-007 and daily monotherapy dose intensity of 40mg (40mg QD or 20mg BID) for ERAS-601; data extraction date: 6NOV2020 for ASN007-101, 11JUL2022 for ERAS-601-01, 16May2022 for ERAS-007-01. Efficacy-Evaluable Patients Thyroid, BRAF V600E (Class 1) Endometrial, BRAF D594G (Class 3) Melanoma, BRAF rearr. (Class 2) NSCLC, BRAF K601E (Class 2) Squamous cell, BRAF rearr. (Class 2) Small bowel, BRAF K601E (Class 2) Lung, BRAF fusion (Class 2) Melanoma, BRAF N581F (Class 3) Prostate, BRAF K601E (Class 2)
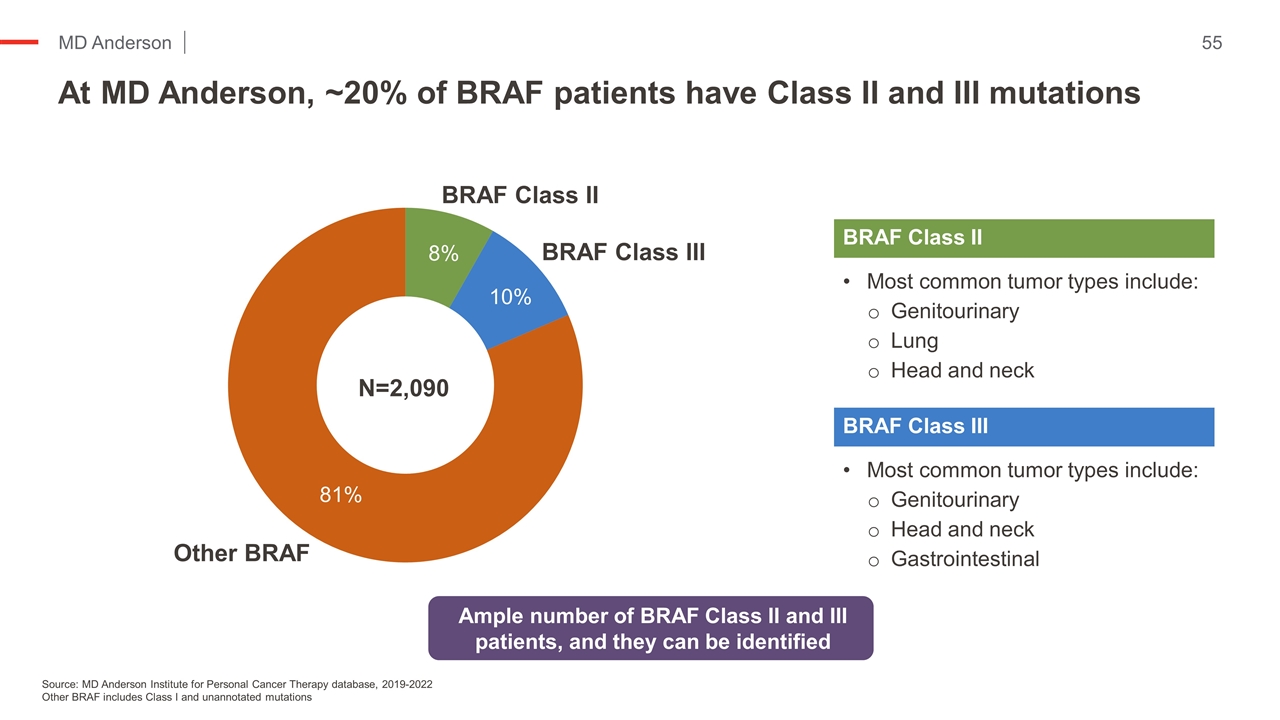
At MD Anderson, ~20% of BRAF patients have Class II and III mutations Kopetz et al NEJM ‘19 Source: MD Anderson Institute for Personal Cancer Therapy database, 2019-2022 Other BRAF includes Class I and unannotated mutations Most common tumor types include: Genitourinary Lung Head and neck BRAF Class II Most common tumor types include: Genitourinary Head and neck Gastrointestinal BRAF Class III Ample number of BRAF Class II and III patients, and they can be identified N=2,090 8% 10% 81%

Conclusions Kopetz et al NEJM ‘19 While Erasca data set is small, encouraging monotherapy activity has been observed with both ERAS-007 and ERAS-601 in patients with tumors driven by an activated RAS/MAPK pathway. 6 of 26 patients with RAS/MAPK alterations experienced a confirmed or unconfirmed PR independent of tumor type. Half of the patients that responded were heavily pre-treated. 4 of 9 patients with BRAF-driven solid tumors experienced a confirmed or unconfirmed PR. Three of the 4 responding patients with BRAF mutations had BRAF Class II/III alterations, for which there are no approved targeted therapies. Since ERAS-007 and ERAS-601 target two critical convergent nodes in the RAS/MAPK, complete pathway inhibition may be easier to obtain using the combination versus each drug in isolation. The clinical data Erasca has generated to date on both of their investigational molecules (ERAS-007 and ERAS-601) support further exploration of the combination and indicate that the team has good insight into the potential overlapping toxicities. The initial data suggest that the AEs are largely non-overlapping, monitorable, and manageable for ERAS-007 and ERAS-601. MD Anderson plans to participate in Erasca’s MAPKlamp (ERAS-007 + ERAS-601) trial.

Future Directions and Key Milestones
Future directions ERAS-007 and ERAS-601 are being developed as foundational combination agents for targeting solid tumors with RAS/MAPK alterations. These agents are currently being explored: In combination with multiple other agents, including inhibitors of BRAF (e.g., encorafenib), EGFR (e.g., cetuximab), and CDK4/6 (e.g., palbociclib) Across several tumor types (e.g., BRAF V600E CRC, KRASm/NRASm CRC, KRASm PDAC, KRAS G12C NSCLC, EGFRm NSCLC) Promising monotherapy antitumor activities of ERAS-007 and ERAS-601 heighten our conviction for combos in the HERKULES and FLAGSHP trials, including the new MAPKlamp (ERAS-007 + ERAS-601) combo The MAPKlamp dose escalation is expected to begin by H1 2023
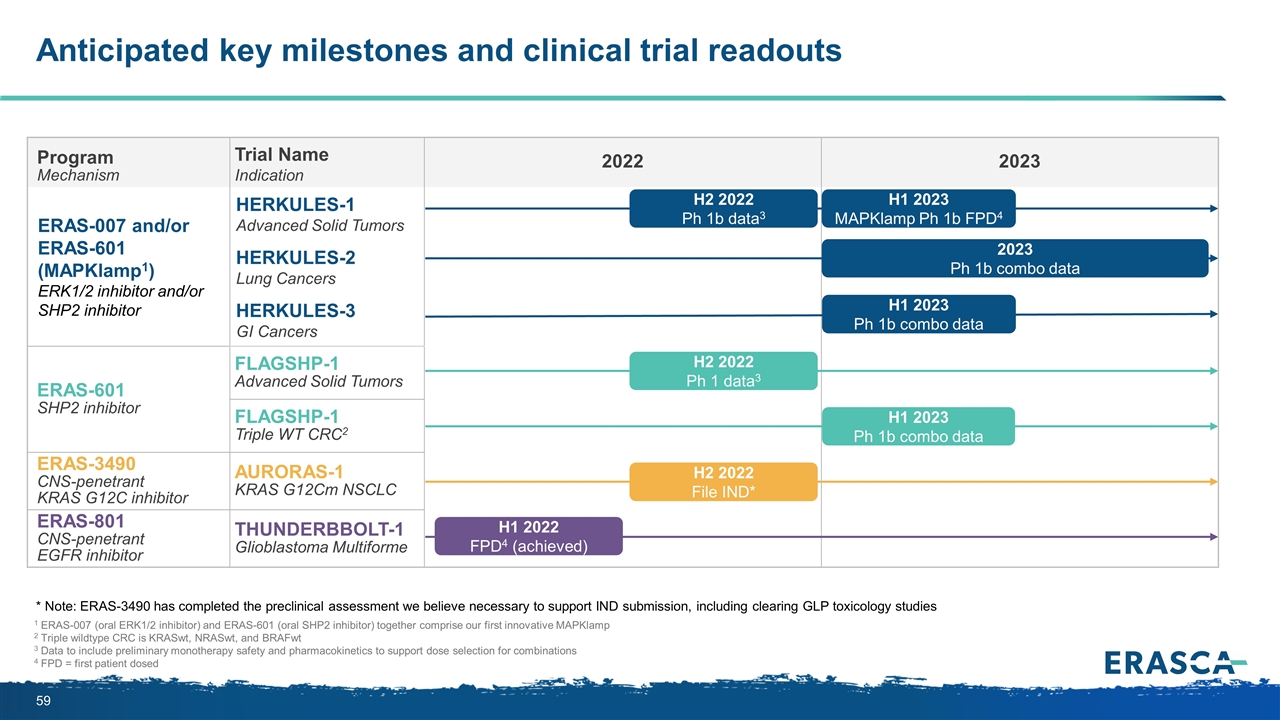
Anticipated key milestones and clinical trial readouts 1 ERAS-007 (oral ERK1/2 inhibitor) and ERAS-601 (oral SHP2 inhibitor) together comprise our first innovative MAPKlamp 2 Triple wildtype CRC is KRASwt, NRASwt, and BRAFwt 3 Data to include preliminary monotherapy safety and pharmacokinetics to support dose selection for combinations 4 FPD = first patient dosed Program Mechanism Trial Name Indication 2022 2023 ERAS-007 and/or ERAS-601 (MAPKlamp1) ERK1/2 inhibitor and/or SHP2 inhibitor HERKULES-1 Advanced Solid Tumors HERKULES-2 Lung Cancers HERKULES-3 GI Cancers ERAS-601 SHP2 inhibitor FLAGSHP-1 Advanced Solid Tumors FLAGSHP-1 Triple WT CRC2 ERAS-3490 CNS-penetrant KRAS G12C inhibitor AURORAS-1 KRAS G12Cm NSCLC ERAS-801 CNS-penetrant EGFR inhibitor THUNDERBBOLT-1 Glioblastoma Multiforme H2 2022 File IND* H2 2022 Ph 1 data3 H1 2023 Ph 1b combo data 2023 Ph 1b combo data H2 2022 Ph 1b data3 H1 2023 Ph 1b combo data H1 2023 MAPKlamp Ph 1b FPD4 * Note: ERAS-3490 has completed the preclinical assessment we believe necessary to support IND submission, including clearing GLP toxicology studies H1 2022 FPD4 (achieved)

Q&A
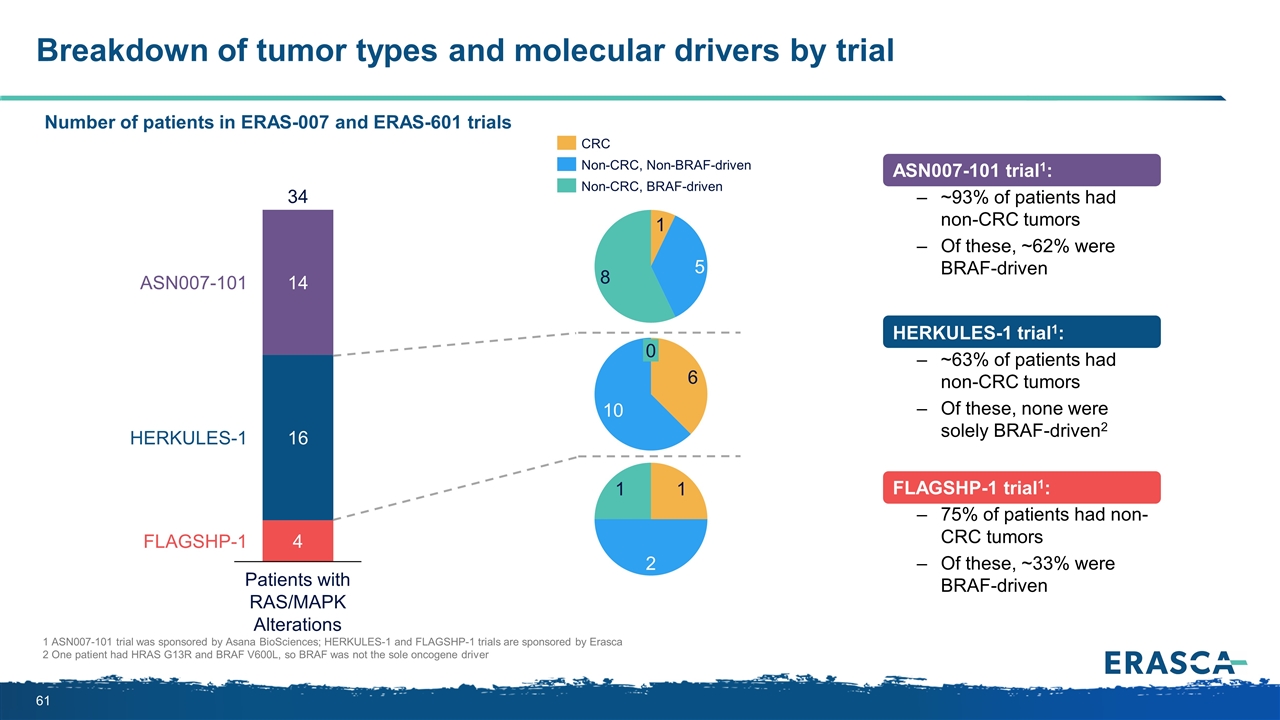
Breakdown of tumor types and molecular drivers by trial 1 ASN007-101 trial was sponsored by Asana BioSciences; HERKULES-1 and FLAGSHP-1 trials are sponsored by Erasca 2 One patient had HRAS G13R and BRAF V600L, so BRAF was not the sole oncogene driver ASN007-101 trial1: ~93% of patients had non-CRC tumors Of these, ~62% were BRAF-driven HERKULES-1 trial1: ~63% of patients had non-CRC tumors Of these, none were solely BRAF-driven2 FLAGSHP-1 trial1: 75% of patients had non-CRC tumors Of these, ~33% were BRAF-driven Number of patients in ERAS-007 and ERAS-601 trials
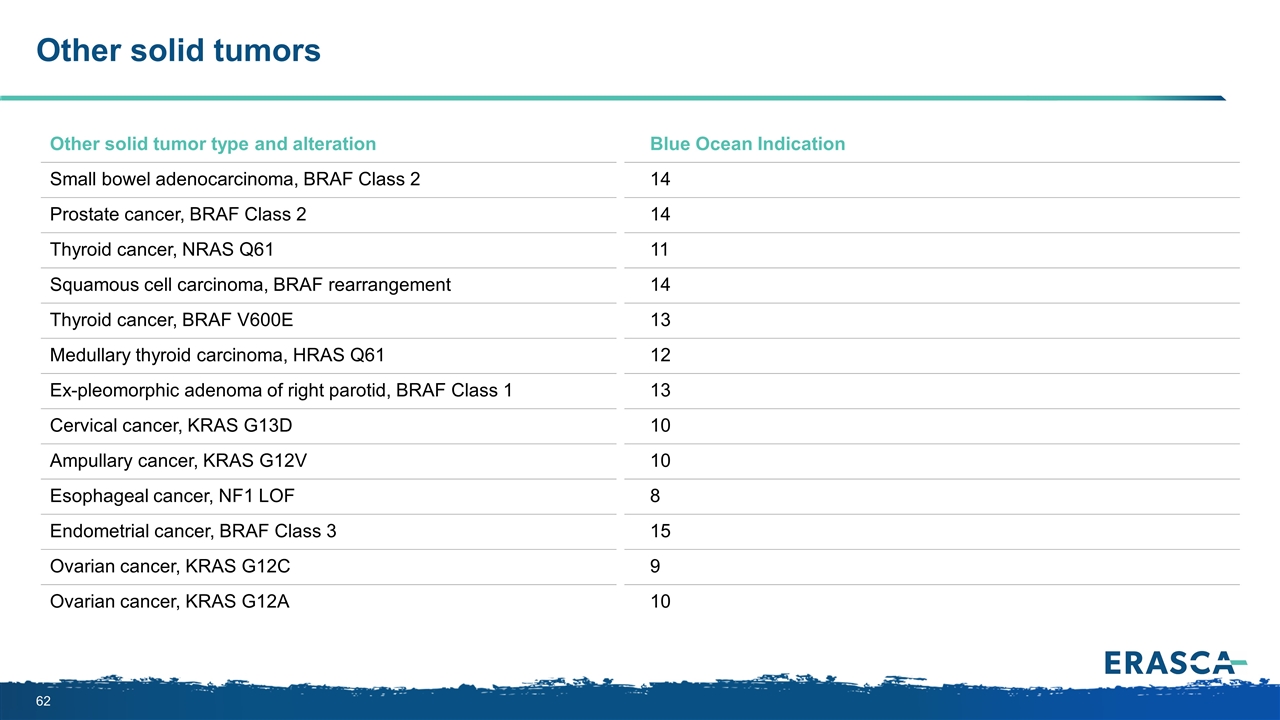
Other solid tumors Other solid tumor type and alteration Blue Ocean Indication Small bowel adenocarcinoma, BRAF Class 2 14 Prostate cancer, BRAF Class 2 14 Thyroid cancer, NRAS Q61 11 Squamous cell carcinoma, BRAF rearrangement 14 Thyroid cancer, BRAF V600E 13 Medullary thyroid carcinoma, HRAS Q61 12 Ex-pleomorphic adenoma of right parotid, BRAF Class 1 13 Cervical cancer, KRAS G13D 10 Ampullary cancer, KRAS G12V 10 Esophageal cancer, NF1 LOF 8 Endometrial cancer, BRAF Class 3 15 Ovarian cancer, KRAS G12C 9 Ovarian cancer, KRAS G12A 10





























































