
Company Update Dr. Michael Perry December 14, 2021 Exhibit 99.1

Certain statements in this presentation and the accompanying oral commentary are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical facts contained in this presentation, including statements regarding our future financial condition, technology platform, development strategy, prospective products, pipeline and milestones, regulatory objectives, expected payments from and outcomes of collaborations, and likelihood of success, are forward-looking statements. Such statements are predictions only and involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. These risks and uncertainties include, among others, the costs, timing and results of clinical trials and other development activities; the uncertainties inherent in the initiation and enrollment of clinical trials; the uncertainties associated with the COVD-19 pandemic; the unpredictability of the timing and results of regulatory submissions and reviews; market acceptance for approved products and innovative therapeutic treatments; competition; the possible impairment of, inability to obtain and costs of obtaining intellectual property rights; and possible safety or efficacy concerns, general business, financial and accounting risks and litigation. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. More information concerning us and such risks and uncertainties is available in our public filings with the U.S. Securities and Exchange Commission, including our most recent Quarterly Report on Form 10-Q for the quarter ended June 30, 2021 and our most recent Annual Report on Form 10-K for the year ended June 30, 2020. We are providing this information as of its date and do not undertake any obligation to update or revise it, whether as a result of new information, future events or circumstances or otherwise, except as required by law. Additional information may be available in press releases or other public announcements and public filings made after the date of this presentation. AVITA Medical’s products are Rx only. Please reference the Instructions for Use for more information on indications, contraindications, warnings, precautions and adverse events. In the United States, RECELL® is approved for use in patients suffering acute thermal burns. Use of RECELL in other patient populations is either prohibited by United States law or may be made available pursuant to a relevant investigational device exemption granted by the FDA (and likewise limited by United States law to investigational use only). Legal Disclaimers

Fiscal 2022 RECELL® total net revenue growth of 105% vs prior year Cumulative U.S. commercial sales since September 2018 FDA approval exceeding $46M Soft Tissue Pivotal Trial: 94% Enrolled Vitiligo Pivotal Trial: 83% enrolled and the remaining 17% scheduled before year-end Transitional Pass-Through Payment Application Approved by CMS for Reimbursement in the Outpatient Setting Effective January 1st, 2022 FDA Approval of Pediatric Label Expansion New Ease of Use RECELL Device Submitted to FDA for Review Completion of RECELL Systems delivery to Biomedical Advanced Research and Development Authority (BARDA) under Vendor Managed Inventory Plan for emergency preparedness ($7.6M revenue) AVITA completed $69.1M Public Offering of Stock on NASDAQ Key Additions of Executives: Michael Holder, CFO & Kathy McGee, COO Key Accomplishments Since Last Shareholder Meeting Quarters referenced in calendar year. As of January 1, 2022 Avita Medical will report on a calendar year basis. Accomplishments

AVITA Leadership Team Dr. Michael S. Perry CEO >30 years experience Affiliations: Michael Holder CFO >30 years experience Affiliations: Donna Shiroma General Counsel >20 years experience Affiliations: Andrew Quick CTO >25 years experience Affiliations: Kathy McGee COO >25 years experience Affiliations: Erin Liberto CCO >20 years experience Affiliations:
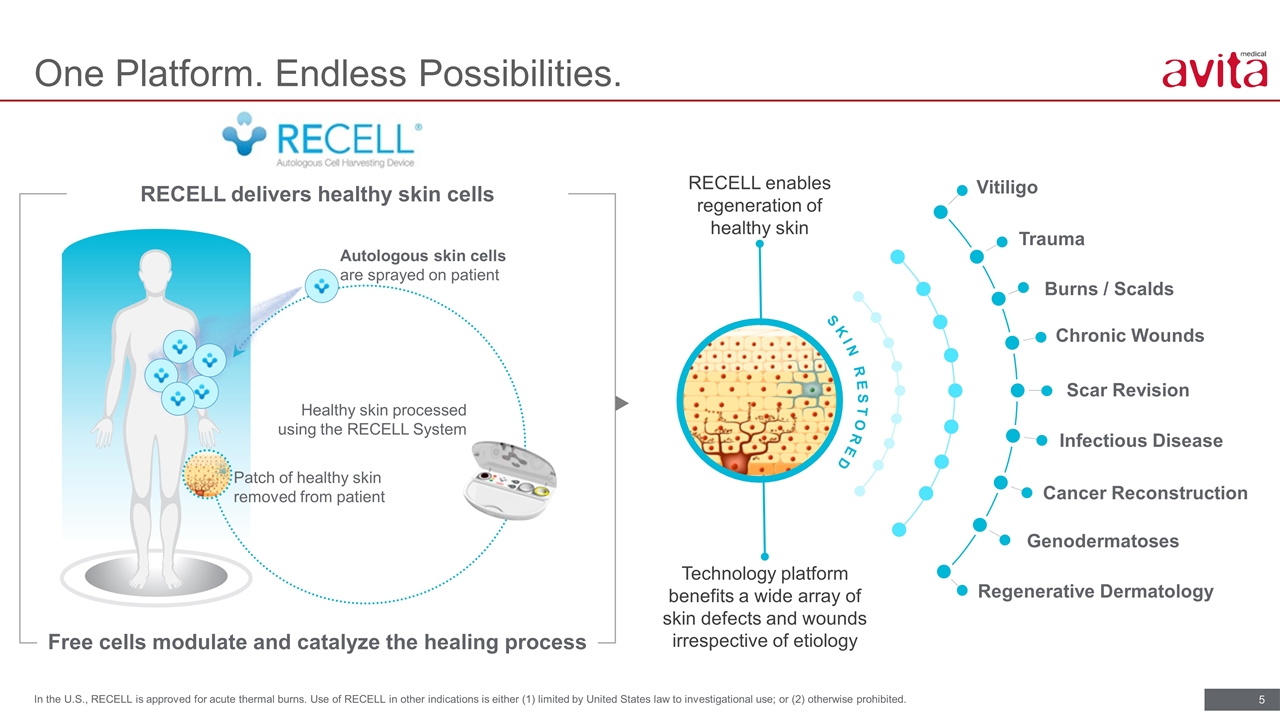
One Platform. Endless Possibilities. In the U.S., RECELL is approved for acute thermal burns. Use of RECELL in other indications is either (1) limited by United States law to investigational use; or (2) otherwise prohibited. RECELL enables regeneration of healthy skin Patch of healthy skin removed from patient Healthy skin processed using the RECELL System Autologous skin cells are sprayed on patient RECELL delivers healthy skin cells Free cells modulate and catalyze the healing process Technology platform benefits a wide array of skin defects and wounds irrespective of etiology Vitiligo Burns / Scalds Scar Revision Infectious Disease Regenerative Dermatology Trauma Chronic Wounds Cancer Reconstruction Genodermatoses SKIN RESTORED

Development Pipeline and Growth Potential
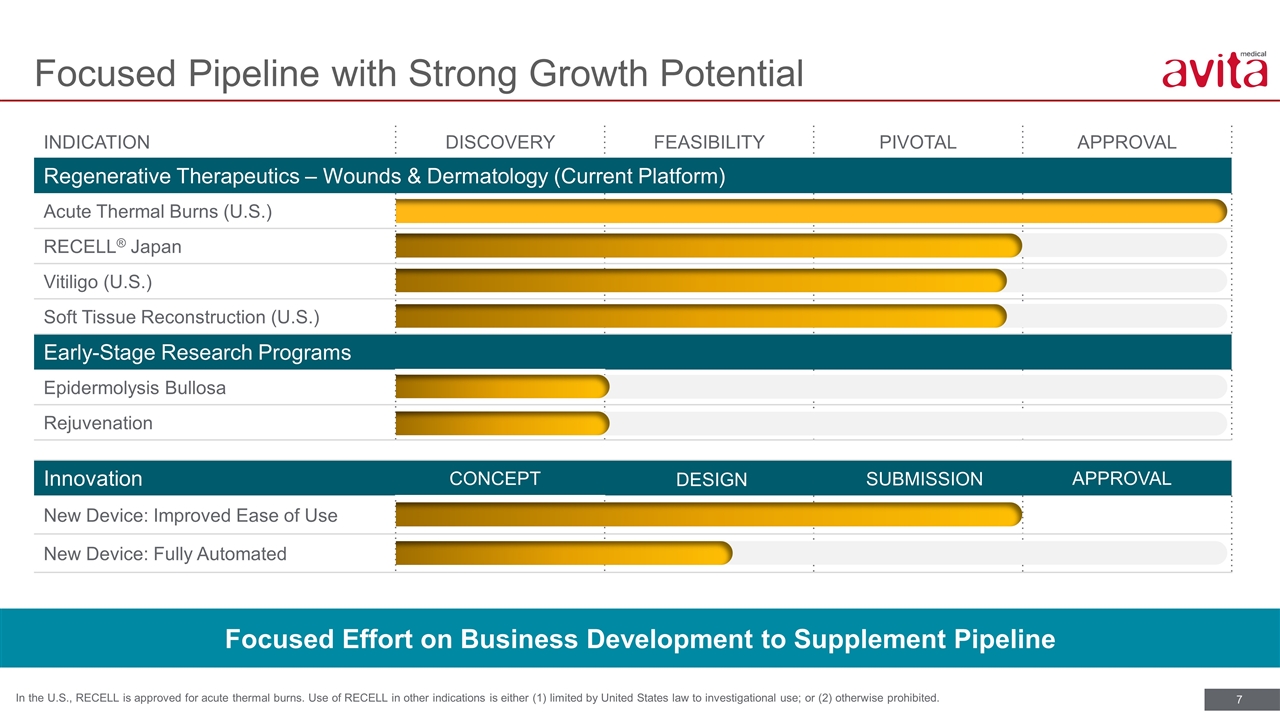
Innovation New Device: Improved Ease of Use New Device: Fully Automated INDICATION DISCOVERY FEASIBILITY PIVOTAL APPROVAL Regenerative Therapeutics – Wounds & Dermatology (Current Platform) Acute Thermal Burns (U.S.) RECELL® Japan Vitiligo (U.S.) Soft Tissue Reconstruction (U.S.) Early-Stage Research Programs Epidermolysis Bullosa Rejuvenation Focused Pipeline with Strong Growth Potential In the U.S., RECELL is approved for acute thermal burns. Use of RECELL in other indications is either (1) limited by United States law to investigational use; or (2) otherwise prohibited. CONCEPT DESIGN APPROVAL SUBMISSION Focused Effort on Business Development to Supplement Pipeline
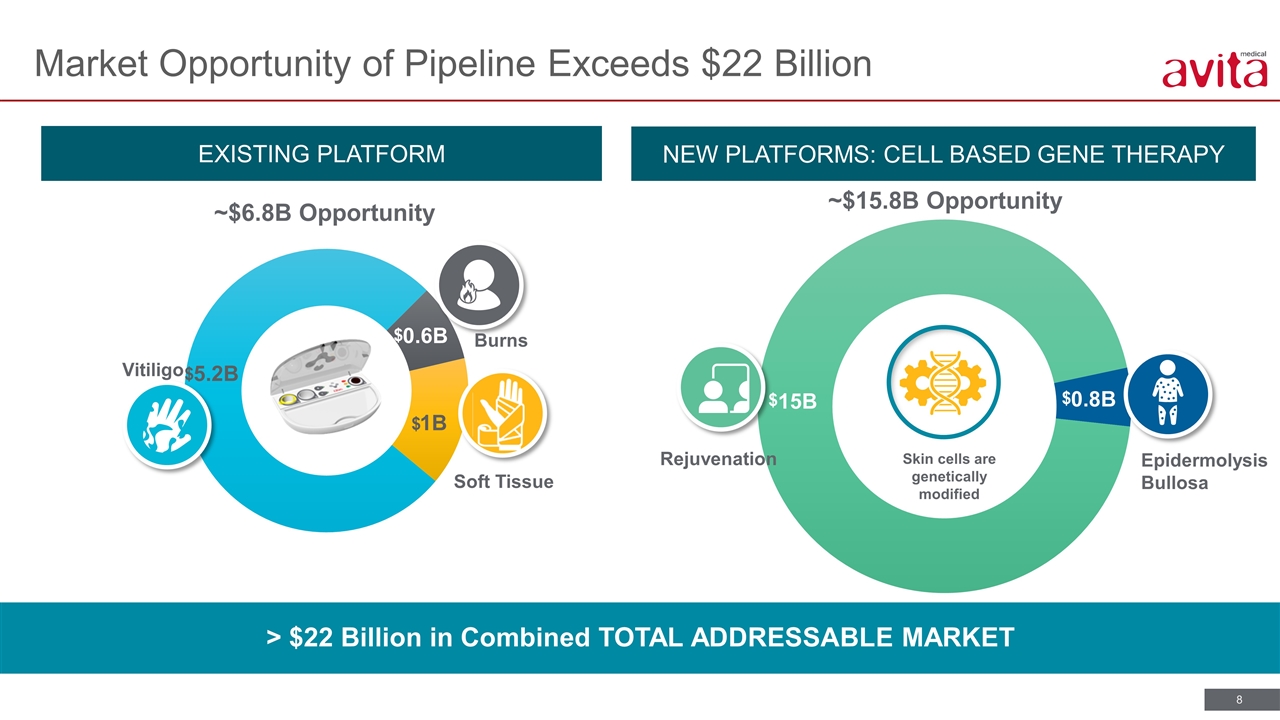
EXISTING PLATFORM NEW PLATFORMS: CELL BASED GENE THERAPY > $22 Billion in Combined TOTAL ADDRESSABLE MARKET Market Opportunity of Pipeline Exceeds $22 Billion Skin cells are genetically modified ~$6.8B Opportunity ~$15.8B Opportunity Burns Soft Tissue Vitiligo Epidermolysis Bullosa $ $ $ $ $
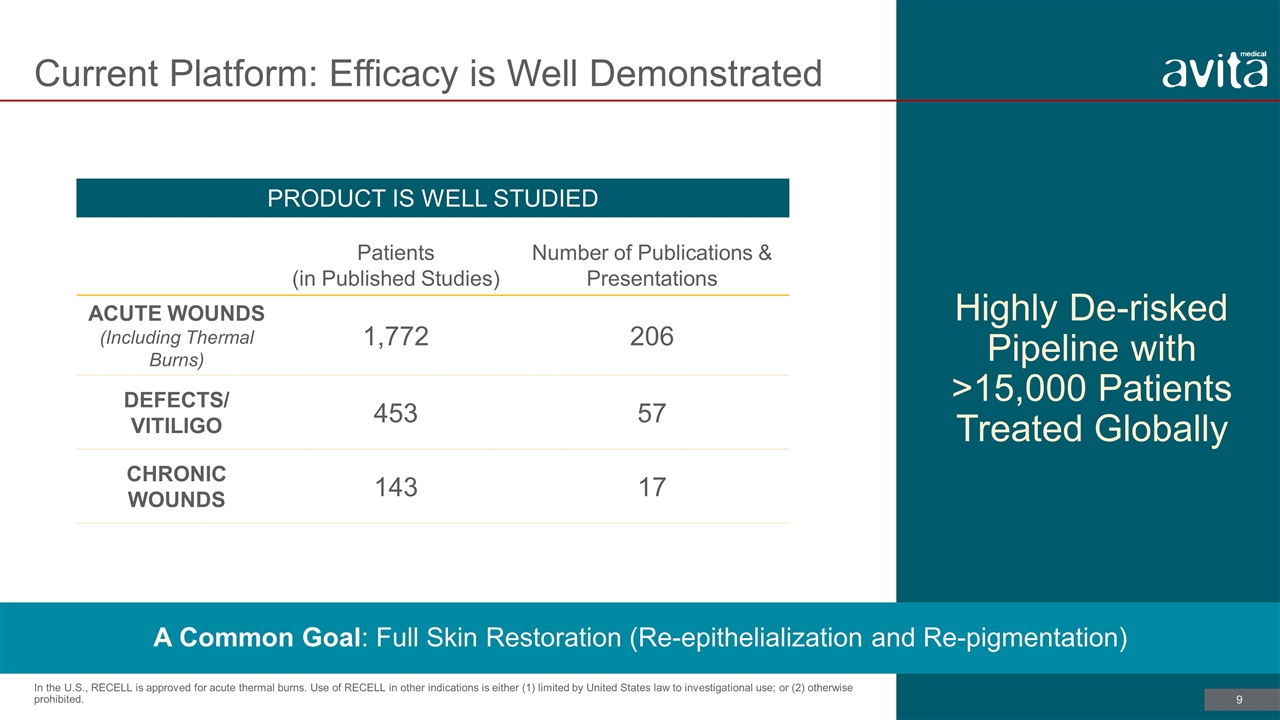
Current Platform: Efficacy is Well Demonstrated Highly De-risked Pipeline with >15,000 Patients Treated Globally In the U.S., RECELL is approved for acute thermal burns. Use of RECELL in other indications is either (1) limited by United States law to investigational use; or (2) otherwise prohibited. Patients (in Published Studies) Number of Publications & Presentations ACUTE WOUNDS (Including Thermal Burns) 1,772 206 DEFECTS/ VITILIGO 453 57 CHRONIC WOUNDS 143 17 A Common Goal: Full Skin Restoration (Re-epithelialization and Re-pigmentation) PRODUCT IS WELL STUDIED
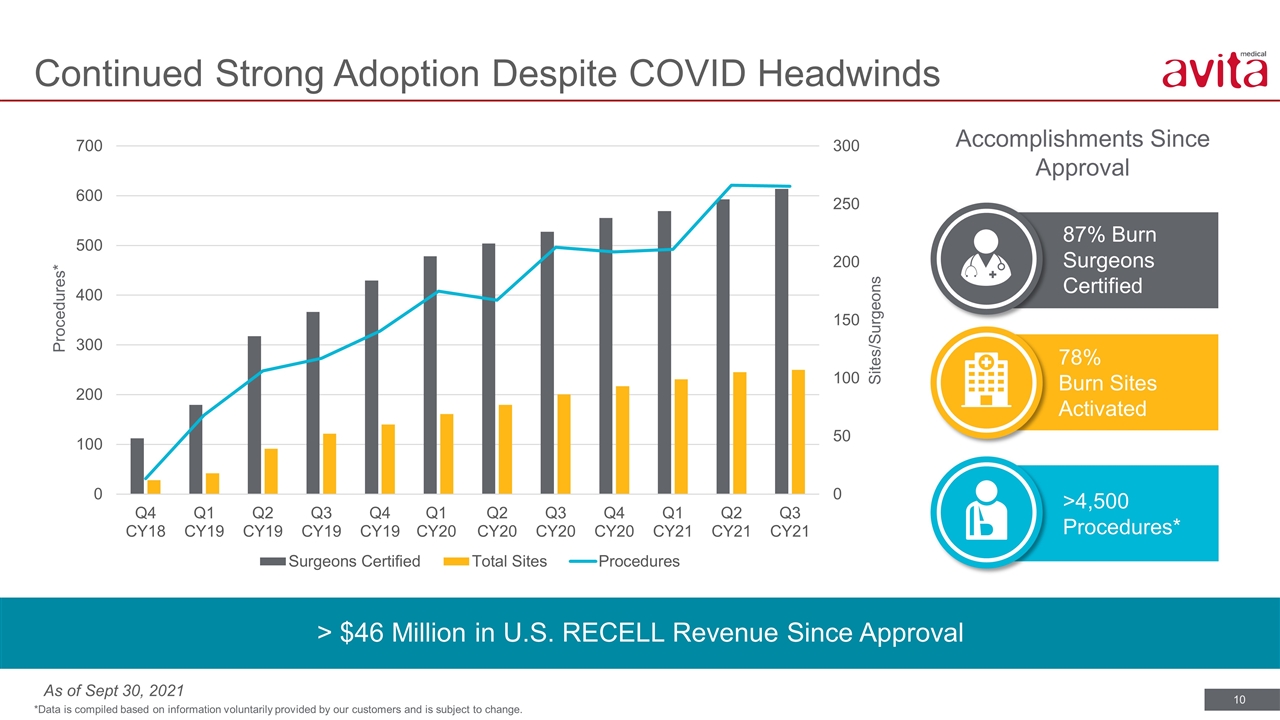
> $46 Million in U.S. RECELL Revenue Since Approval 78% Burn Sites Activated 87% Burn Surgeons Certified Accomplishments Since Approval Continued Strong Adoption Despite COVID Headwinds *Data is compiled based on information voluntarily provided by our customers and is subject to change. Procedures* Sites/Surgeons >4,500 Procedures* As of Sept 30, 2021
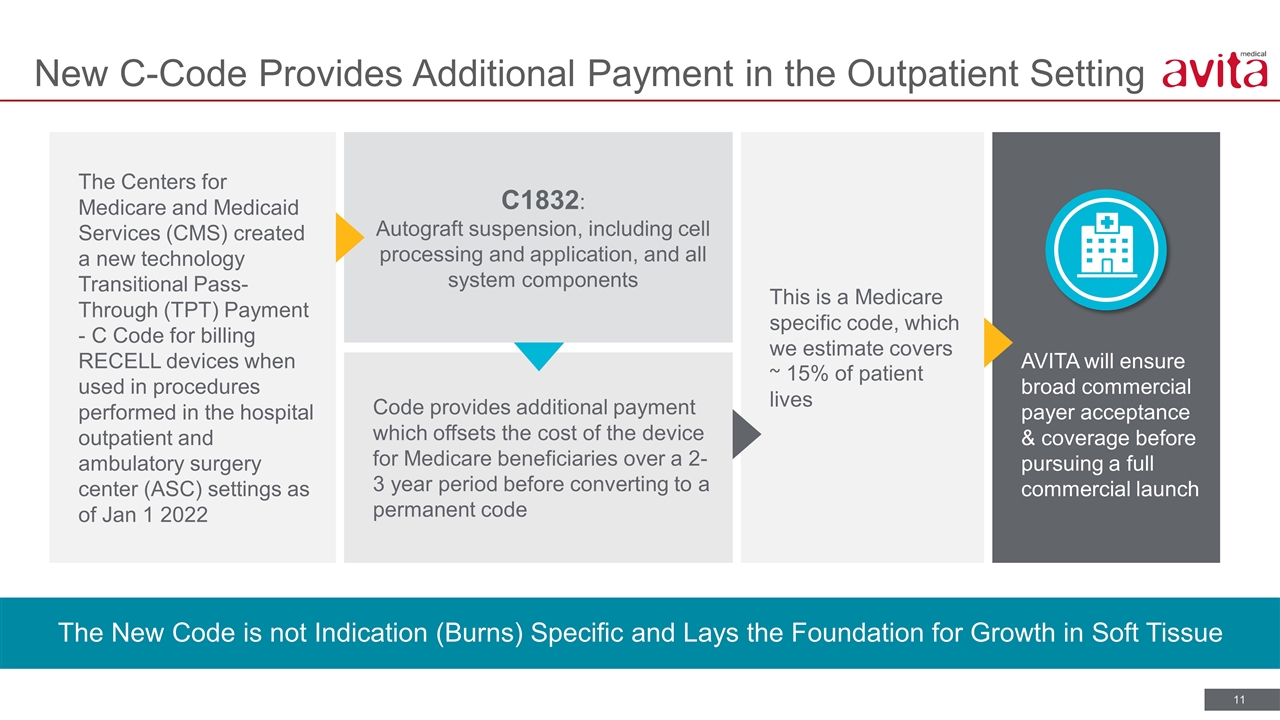
AVITA will ensure broad commercial payer acceptance & coverage before pursuing a full commercial launch This is a Medicare specific code, which we estimate covers ~ 15% of patient lives C1832: Autograft suspension, including cell processing and application, and all system components Code provides additional payment which offsets the cost of the device for Medicare beneficiaries over a 2-3 year period before converting to a permanent code New C-Code Provides Additional Payment in the Outpatient Setting The New Code is not Indication (Burns) Specific and Lays the Foundation for Growth in Soft Tissue The Centers for Medicare and Medicaid Services (CMS) created a new technology Transitional Pass-Through (TPT) Payment - C Code for billing RECELL devices when used in procedures performed in the hospital outpatient and ambulatory surgery center (ASC) settings as of Jan 1 2022

New Ease of Use Device Submitted for FDA Approval * Market Research March 2020 HCPs N=15 Only 1 Set of Hands Required in the Sterile Field; Steps Reduced By 1/3rd Reduced number of steps New Device Simplified Process Improved Usability 94% of users of the RECELL System believe it will reduce their workload/allow them to perform other duties*

FDA Approval in Pediatric Full-Thickness & Larger Burns ~25% of all burns occur in children Instructions for Use. RECELL® Autologous Cell Harvesting Device NBR – National Burns Repository * N = 41, “will significantly or somewhat impact RECELL usage” 80% of RECELL Customers Stated that these New Label Enhancements Will Positively Impact Their Usage of RECELL NUMBER OF TREATMENTS 3.6 1.6 NUMBER OF TREATMENTS 5.9 2.4 NBR Control RECELL 56% fewer mean procedures with RECELL (N=284) p < 0.0001 Adults with >50% TBSA 60% fewer mean procedures with RECELL (N=318) p < 0.0001 FEWER PROCEDURES REQUIRED FOR DEFINITIVE CLOSURE VS CONVENTIONAL AUTOGRAFT1 Pediatric Patients
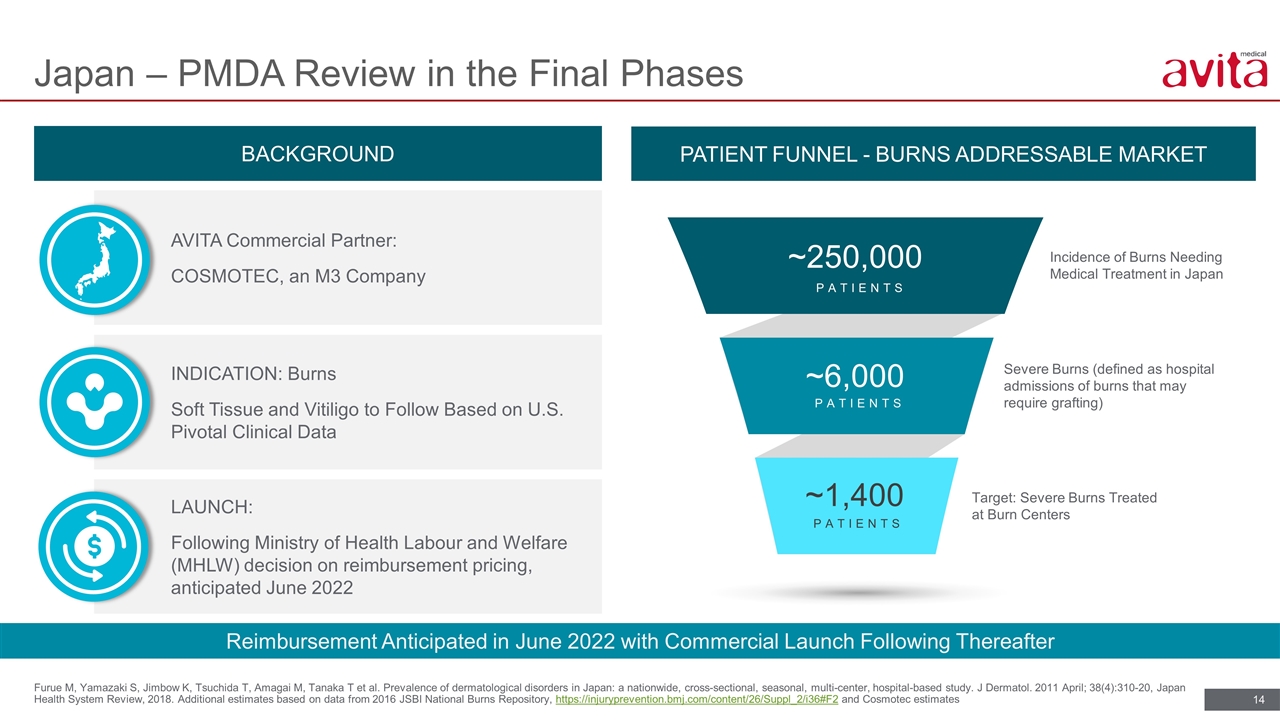
Furue M, Yamazaki S, Jimbow K, Tsuchida T, Amagai M, Tanaka T et al. Prevalence of dermatological disorders in Japan: a nationwide, cross-sectional, seasonal, multi-center, hospital-based study. J Dermatol. 2011 April; 38(4):310-20, Japan Health System Review, 2018. Additional estimates based on data from 2016 JSBI National Burns Repository, https://injuryprevention.bmj.com/content/26/Suppl_2/i36#F2 and Cosmotec estimates Japan – PMDA Review in the Final Phases Incidence of Burns Needing Medical Treatment in Japan Severe Burns (defined as hospital admissions of burns that may require grafting) Target: Severe Burns Treated at Burn Centers ~1,400 ~6,000 ~250,000 PATIENTS PATIENTS PATIENTS BACKGROUND Reimbursement Anticipated in June 2022 with Commercial Launch Following Thereafter PATIENT FUNNEL - BURNS ADDRESSABLE MARKET INDICATION: Burns Soft Tissue and Vitiligo to Follow Based on U.S. Pivotal Clinical Data LAUNCH: Following Ministry of Health Labour and Welfare (MHLW) decision on reimbursement pricing, anticipated June 2022 AVITA Commercial Partner: COSMOTEC, an M3 Company

Vitiligo: Unmet Need, No FDA-Approved Products Advances in Vitiligo: An Update on Medical and Surgical Treatments. A. Dillon, et al. J Clin Aesth Derm. 2017. Willingness-to-Pay and Quality of Life in Patients with Vitiligo. Radtke, et al. BJD. 2009. SIGNIFICANT UNMENT NEED Up to 2% of the population affected (~6.5M in the US) Vitiligo impacts quality of life (QoL) – 25% of patients with vitiligo reported a DLQI >10, which indicates severe QoL reductions, compared with 34% in psoriasis patients No FDA-approved medical treatments; extremely low patient and physician satisfaction with existing products LIMITED TREATMENT OPTIONS In the U.S., RECELL is approved for acute thermal burns. Use of RECELL in other indications is either (1) limited by United States law to investigational use; or (2) otherwise prohibited. Phototherapy 2-3 treatments / week for a few months to over a year Typically combined with a topical drug Not Durable Melanocyte-Keratinocyte Transplantation For repigmentation of stable lesions Requires substantial laboratory equipment Performed rarely and only at 3 highly specialized academic centers in the United States
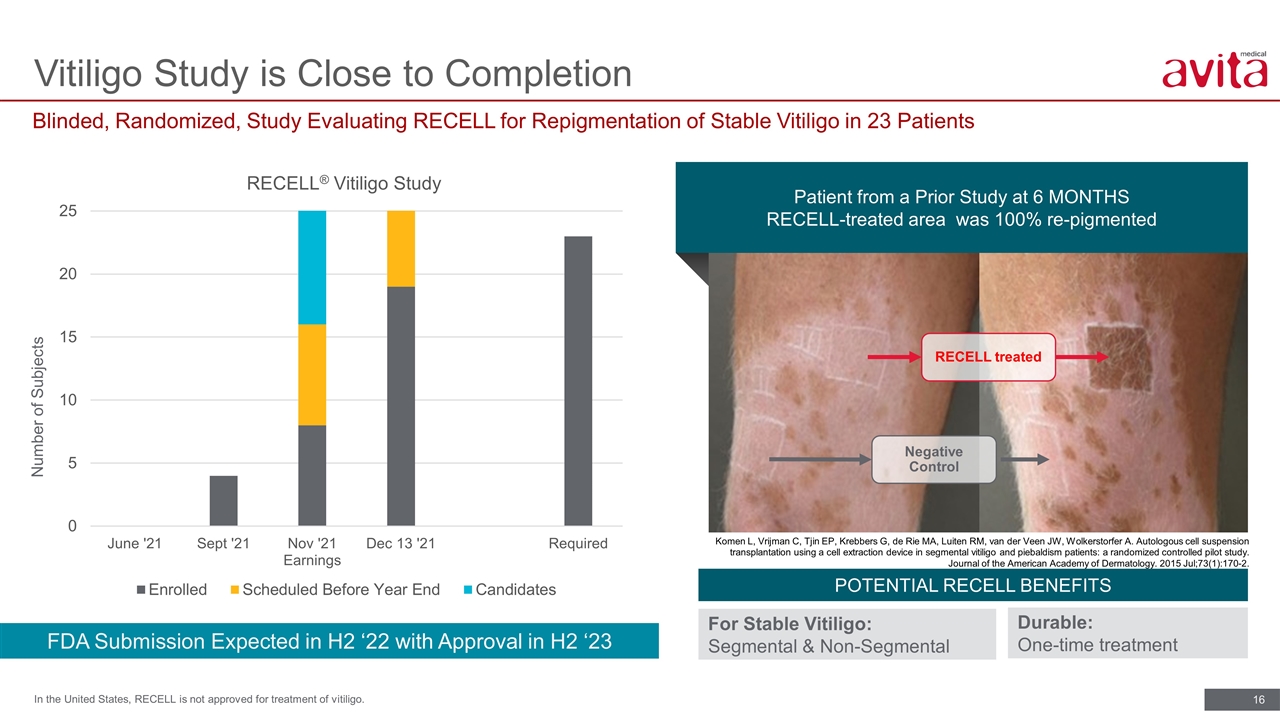
Blinded, Randomized, Study Evaluating RECELL for Repigmentation of Stable Vitiligo in 23 Patients Vitiligo Study is Close to Completion In the United States, RECELL is not approved for treatment of vitiligo. Patient from a Prior Study at 6 MONTHS RECELL-treated area was 100% re-pigmented RECELL treated Negative Control Komen L, Vrijman C, Tjin EP, Krebbers G, de Rie MA, Luiten RM, van der Veen JW, Wolkerstorfer A. Autologous cell suspension transplantation using a cell extraction device in segmental vitiligo and piebaldism patients: a randomized controlled pilot study. Journal of the American Academy of Dermatology. 2015 Jul;73(1):170-2. FDA Submission Expected in H2 ‘22 with Approval in H2 ‘23 Number of Subjects POTENTIAL RECELL BENEFITS For Stable Vitiligo: Segmental & Non-Segmental Durable: One-time treatment N=23
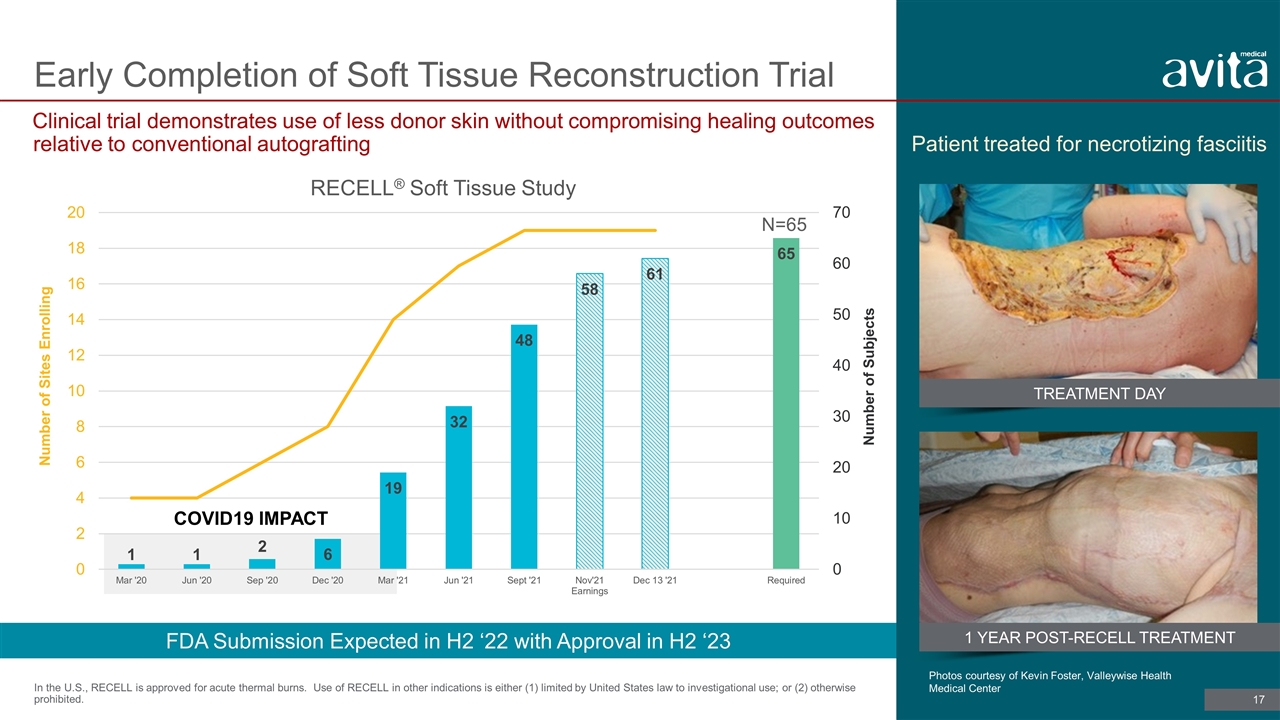
Early Completion of Soft Tissue Reconstruction Trial Clinical trial demonstrates use of less donor skin without compromising healing outcomes relative to conventional autografting In the U.S., RECELL is approved for acute thermal burns. Use of RECELL in other indications is either (1) limited by United States law to investigational use; or (2) otherwise prohibited. COVID19 IMPACT N=65 Patient treated for necrotizing fasciitis Photos courtesy of Kevin Foster, Valleywise Health Medical Center FDA Submission Expected in H2 ‘22 with Approval in H2 ‘23 TREATMENT DAY 1 YEAR POST-RECELL TREATMENT
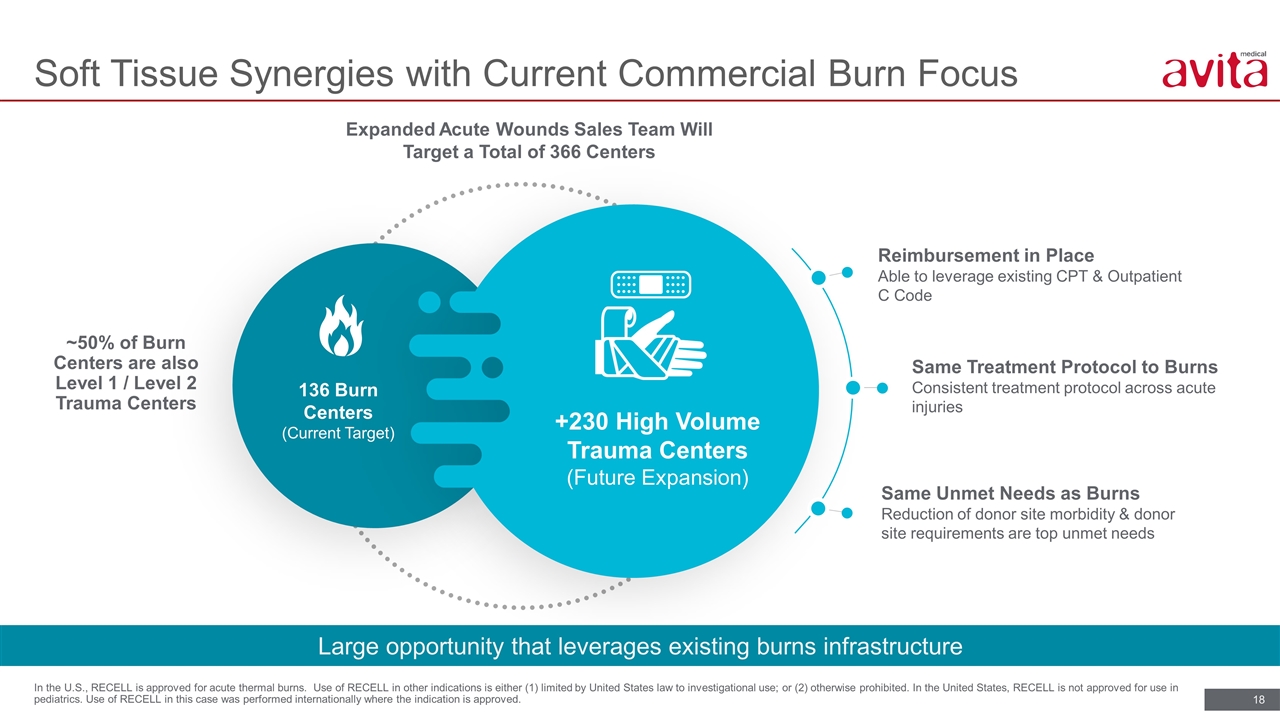
Soft Tissue Synergies with Current Commercial Burn Focus In the U.S., RECELL is approved for acute thermal burns. Use of RECELL in other indications is either (1) limited by United States law to investigational use; or (2) otherwise prohibited. In the United States, RECELL is not approved for use in pediatrics. Use of RECELL in this case was performed internationally where the indication is approved. ~50% of Burn Centers are also Level 1 / Level 2 Trauma Centers Expanded Acute Wounds Sales Team Will Target a Total of 366 Centers Reimbursement in Place Able to leverage existing CPT & Outpatient C Code 136 Burn Centers (Current Target) +230 High Volume Trauma Centers (Future Expansion) Same Treatment Protocol to Burns Consistent treatment protocol across acute injuries Same Unmet Needs as Burns Reduction of donor site morbidity & donor site requirements are top unmet needs Large opportunity that leverages existing burns infrastructure
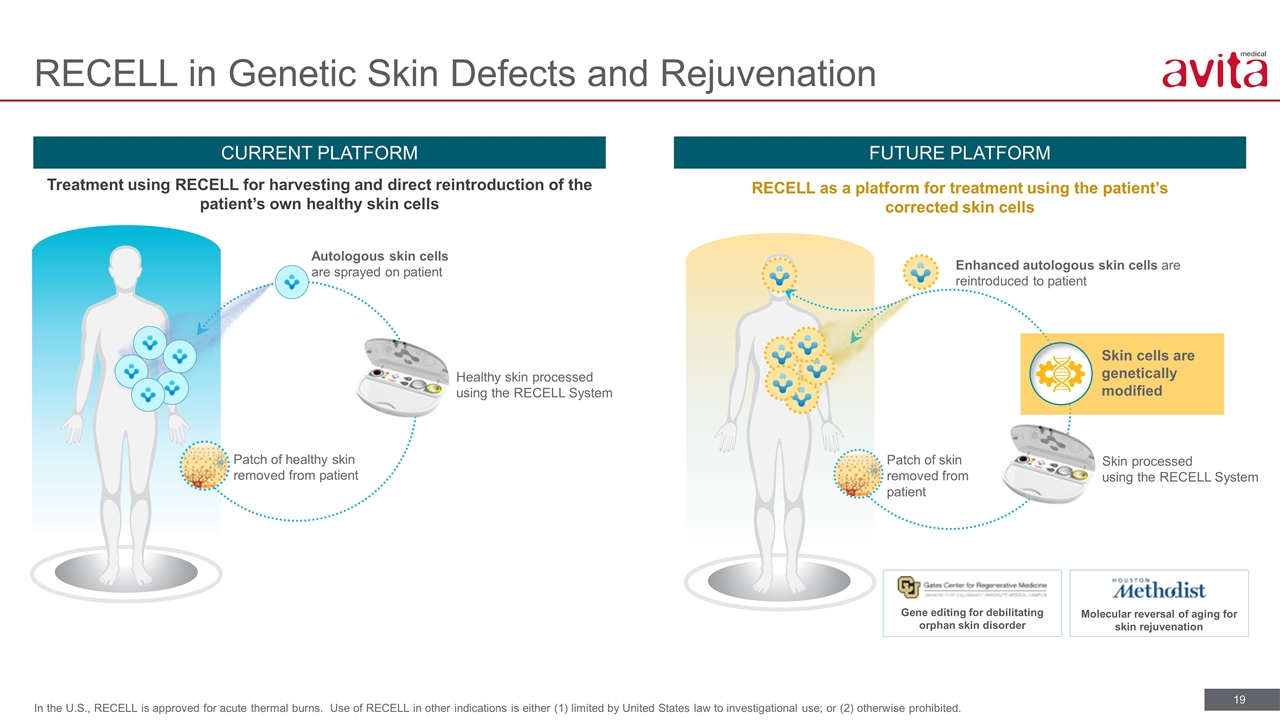
RECELL in Genetic Skin Defects and Rejuvenation CURRENT PLATFORM FUTURE PLATFORM Treatment using RECELL for harvesting and direct reintroduction of the patient’s own healthy skin cells RECELL as a platform for treatment using the patient’s corrected skin cells Patch of healthy skin removed from patient Healthy skin processed using the RECELL System Autologous skin cells are sprayed on patient Skin processed using the RECELL System Patch of skin removed from patient Enhanced autologous skin cells are reintroduced to patient Gene editing for debilitating orphan skin disorder Molecular reversal of aging for skin rejuvenation Skin cells are genetically modified In the U.S., RECELL is approved for acute thermal burns. Use of RECELL in other indications is either (1) limited by United States law to investigational use; or (2) otherwise prohibited.
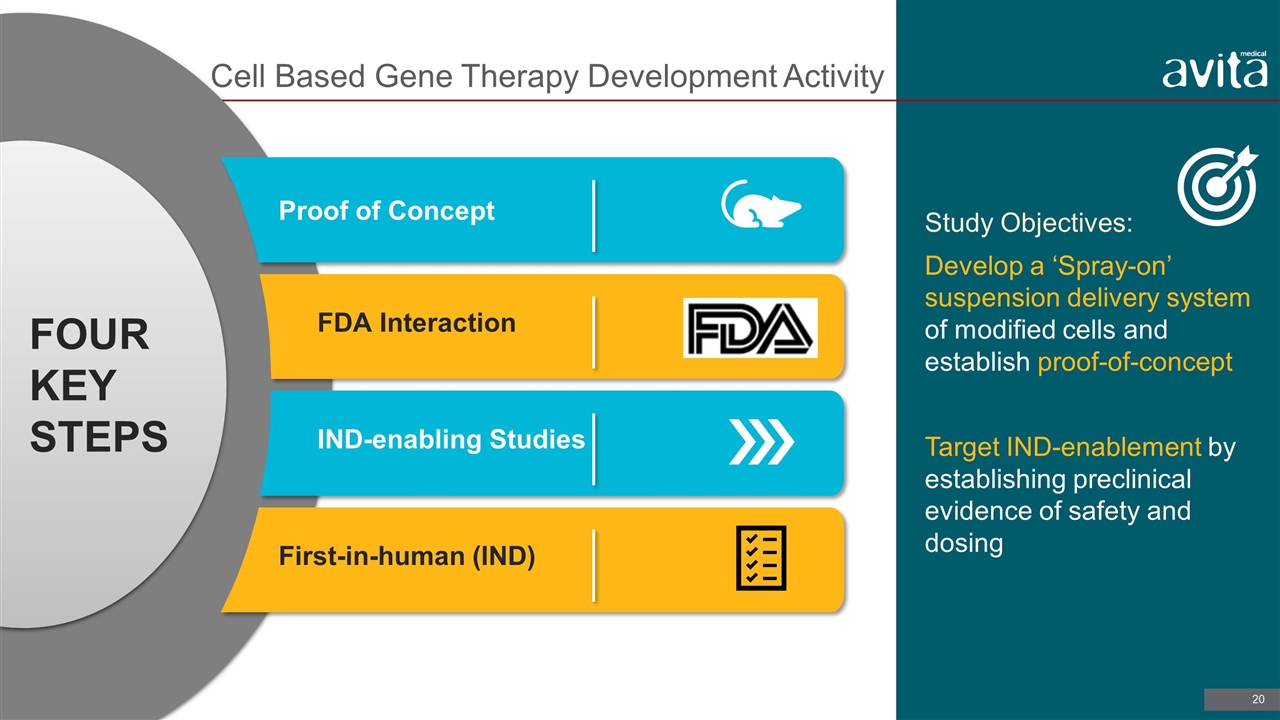
Cell Based Gene Therapy Development Activity Study Objectives: Develop a ‘Spray-on’ suspension delivery system of modified cells and establish proof-of-concept Target IND-enablement by establishing preclinical evidence of safety and dosing Proof of Concept FDA Interaction IND-enabling Studies First-in-human (IND) Four key steps
Exploring Cell-Based Gene Therapy for Epidermolysis Bullosa In the U.S., RECELL is approved for acute thermal burns. Use of RECELL in other indications is either (1) limited by United States law to investigational use; or (2) otherwise prohibited. THE CHALLENGE THE OPPORTUNITY DEBILITATING Skin fragility, disability, cancer HIGH UNMET NEED No FDA-approved treatment, only palliative measures COST BURDEN Care of $200K-$500K per year per patient CURATIVE: Technology for precise correction of genetic defect & banking for future use (vs ameliorating symptoms) EFFICIENT: Suspension-based approach eliminates growth & transport of fragile skin sheets CONVENIENT: Suspension-based product simplifies application onto patient wounds (vs surgical anchoring of epidermal sheets which can result in issues with “take rates”
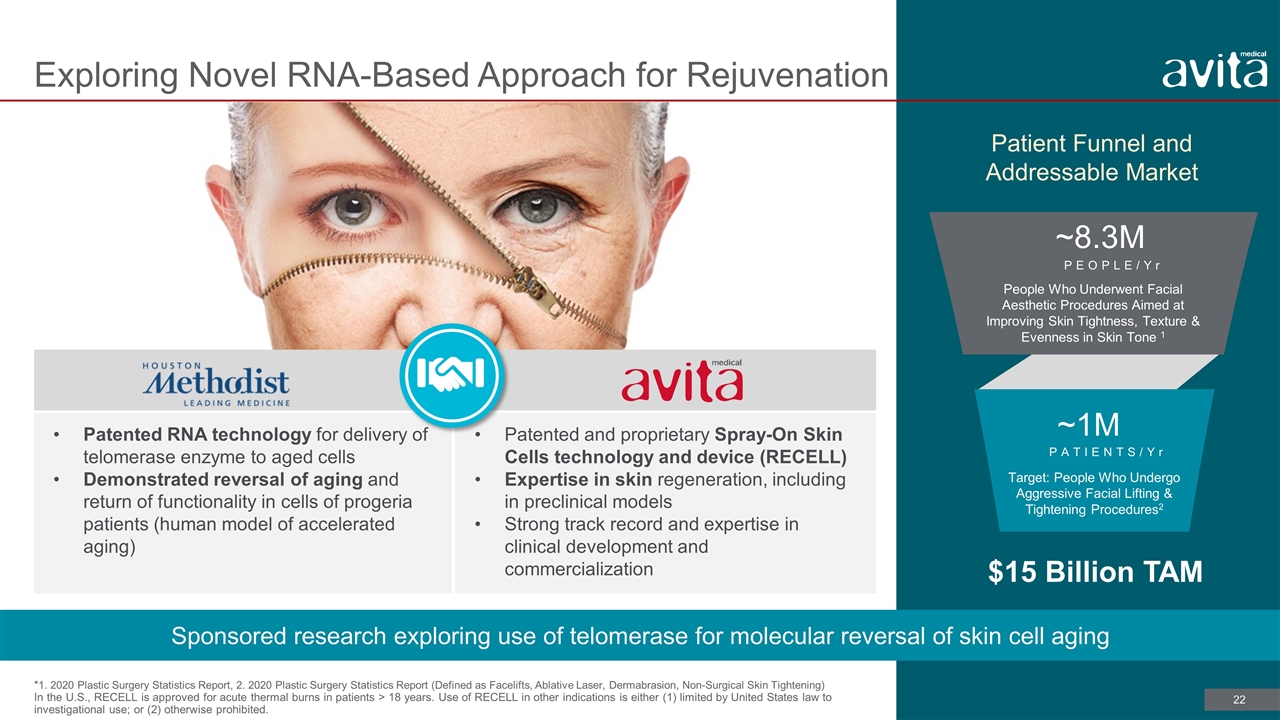
Patented RNA technology for delivery of telomerase enzyme to aged cells Demonstrated reversal of aging and return of functionality in cells of progeria patients (human model of accelerated aging) Patented and proprietary Spray-On Skin Cells technology and device (RECELL) Expertise in skin regeneration, including in preclinical models Strong track record and expertise in clinical development and commercialization Exploring Novel RNA-Based Approach for Rejuvenation *1. 2020 Plastic Surgery Statistics Report, 2. 2020 Plastic Surgery Statistics Report (Defined as Facelifts, Ablative Laser, Dermabrasion, Non-Surgical Skin Tightening) In the U.S., RECELL is approved for acute thermal burns in patients > 18 years. Use of RECELL in other indications is either (1) limited by United States law to investigational use; or (2) otherwise prohibited. Sponsored research exploring use of telomerase for molecular reversal of skin cell aging Patient Funnel and Addressable Market ~1M ~8.3M PEOPLE/Yr PATIENTS/Yr $15 Billion TAM People Who Underwent Facial Aesthetic Procedures Aimed at Improving Skin Tightness, Texture & Evenness in Skin Tone 1 Target: People Who Undergo Aggressive Facial Lifting & Tightening Procedures2

Corporate
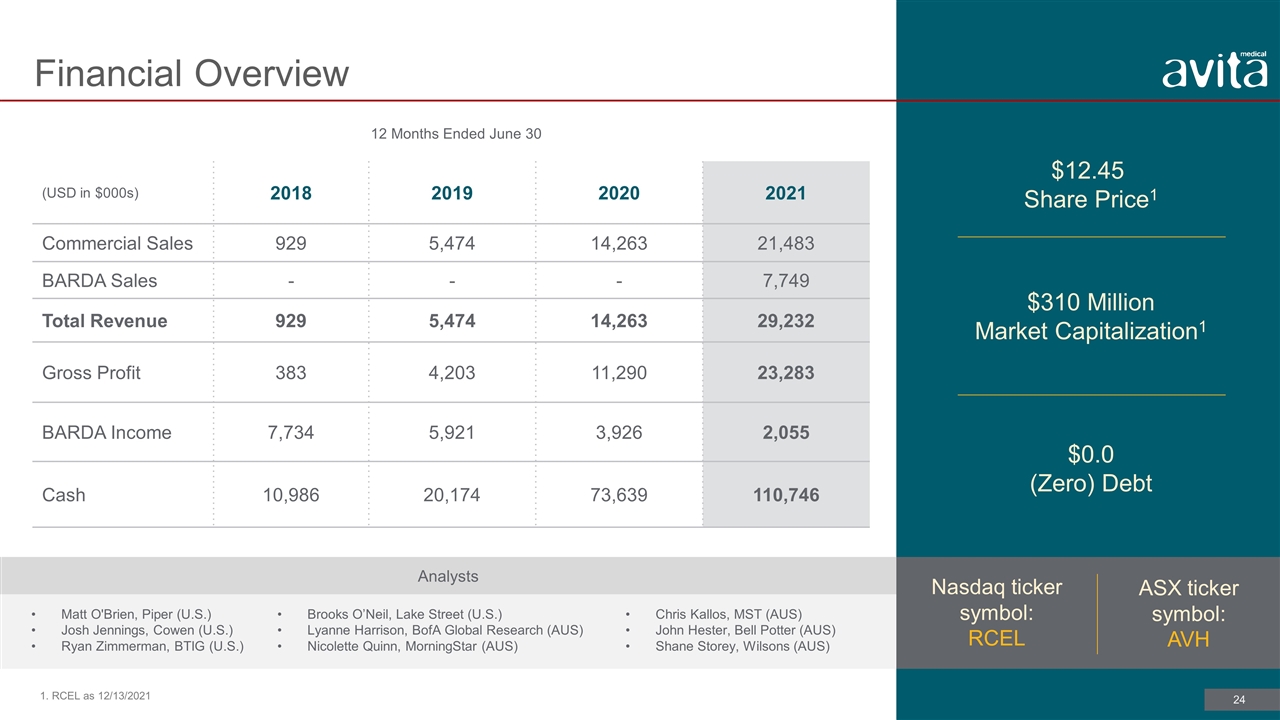
12 Months Ended June 30 (USD in $000s) 2018 2019 2020 2021 Commercial Sales 929 5,474 14,263 21,483 BARDA Sales - - - 7,749 Total Revenue 929 5,474 14,263 29,232 Gross Profit 383 4,203 11,290 23,283 BARDA Income 7,734 5,921 3,926 2,055 Cash 10,986 20,174 73,639 110,746 $12.45 Share Price1 Financial Overview 1. RCEL as 12/13/2021 $310 Million Market Capitalization1 $0.0 (Zero) Debt Nasdaq ticker symbol: RCEL ASX ticker symbol: AVH Analysts Matt O'Brien, Piper (U.S.) Josh Jennings, Cowen (U.S.) Ryan Zimmerman, BTIG (U.S.) Chris Kallos, MST (AUS) John Hester, Bell Potter (AUS) Shane Storey, Wilsons (AUS) Brooks O’Neil, Lake Street (U.S.) Lyanne Harrison, BofA Global Research (AUS) Nicolette Quinn, MorningStar (AUS)

A Global Total of 56 Granted Patents & 26 Pending Applications Note: AVITA Medical owns granted patents in Austria, Australia, Belgium, Brazil, France, Germany, Hong Kong, Italy, Japan, Netherlands, Portugal, Spain, Sweden, Turkey, United Kingdom and USA. AVITA Medical owns pending patent applications in Brazil, Canada, China, Europe, Hong Kong and USA. Patent count as of 6/30/2021 ROBUST PROTECTION ACROSS PATENT FAMILIES EXPANDING PORTFOLIO TO SUPPORT CURRENT AND FUTURE INDICATIONS Next Generation RECELL devices to improve ease of use in burns and pipeline indications Potential to license patented technology for telomerase mRNA that has the potential to reverse aging of skin cells Potential to license technologies for suspension-based delivery of genetically modified cells, with applications to genetic skin disorders Commercial RECELL device, composition of matter, and associated methods of use Cell Suspension Preparation Technique and Use Method of preparing cell suspension with exogenous agent to promote wound healing Cell Suspension And Use Thereof Automated system for preparing cell suspension and method of production Systems and Methods for Tissue Processing and Preparation of Cell Suspension Therefrom Robust and Expanding Patent Estate: Expiration from 2022 to 2040 All-in-one RECELL kit, system, and associated method of use Devices, Methods, and Kits for Preparing a Cell Suspension Method and system for validating the use of a cell suspension for administration to a patient Methods for Identifying Cell Suspensions with Therapeutic Potential for Skin Regeneration Bioactive suspension derived from freshly disaggregated tissue, and associated methods of preparation and use Bioactive Therapeutic Suspensions with Cellular-Based Supernatant

Quarters referenced in calendar year. As of January 1, 2022 Avita Medical will report on a calendar year basis. Value Creation Events: Looking Forward Projected Key Milestones Vitiligo Pivotal Trial Last Patient Enrolled / Vitiligo Commercial launch Last patient enrolled in Soft Tissue Trial / Soft Tissue Commercial Launch Outpatient Launch PMDA Approval of Burns in Japan FDA Approval of New ‘Ease of Use’ RECELL Device EB: Initial proof of concept for delivery of genetically modified skin cells in suspension Telomerase/Rejuvenation: Initial proof of concept on impact of telomerase on human skin in a mouse model Q4 21 / H2 23 Q1 22 / H2 23 H1 22 Q4 21

There are numerous risk factors involved with the Company’s business. Some of these risks can be mitigated by the use of safeguards and appropriate systems and controls, but some are outside the control of the Company and cannot be mitigated. Accordingly, an investment in the Company carries no guarantee with respect to the payment of dividends, return of capital or price at which securities will trade. The following is a summary of the more material matters to be considered. However, this summary is not exhaustive. Potential investor should consult their professional advisors before deciding whether to invest. Technological Change: Technological change presents the Company with significant opportunities for growth. However, the risk remains that any competitor may introduce new technology enabling it to gain a significant competitive advantage over the Company. Reliance on key personnel: The Company's success depends to a significant extent upon its key management personnel, as well as other management and technical personnel including sub-contractors. The loss of the services of any such personnel could have an adverse effect on the Company. Competition: The Company competes with other companies in the United States as well as in Australia and internationally. Some of these companies have greater financial and other resources than the Company and, as a result, may be in a better position to compete for future business opportunities. There can be no assurance that the Company can compete effectively with these companies. Patent Protection: The patent protection that the Company may obtain varies from product to product and country to country and may not be sufficient, including to maintain product exclusivity. Patent rights are also limited in time and do not always provide effective protection for products and services: competitors may successfully avoid patents through design innovation, the Company may not hold sufficient evidence of infringement to bring suit, or the infringement claim may not result in a decision that the rights are valid, enforceable or infringed. Legislation or regulatory actions subsequent to the filing date of a patent application may affect what an applicant is entitled to claim in a pending application and may also affect whether a granted patent can be enforced in certain circumstances. Laws relating to biotechnology remain the subject of ongoing political controversy in some countries. The risk of changed laws affecting patent rights is generally considered greater for the biotechnology field than in other longer established fields. Change in government policy and legislation: Any material adverse changes in relevant government policies or legislation of Australia / United States may affect the viability and profitability of the Company, and consequent returns to investors. The activities of the Company are subject to various federal, state and local laws governing prospecting, development, production, taxes, labor standards and occupational health and safety, and other matters. Risk Factors and Disclosures

INDICATIONS FOR USE: The RECELL® Autologous Cell Harvesting Device is indicated for the treatment of acute thermal burn wounds. The RECELL device is used by an appropriately-licensed healthcare professional at the patient’s point of care to prepare autologous RES® Regenerative Epidermal Suspension for direct application to acute partial-thickness thermal burn wounds in patients 18 years of age and older or application in combination with meshed autografting for acute full-thickness thermal burn wounds in pediatric and adult patients. . CONTRAINDICATIONS: RECELL is contraindicated for: the treatment of wounds clinically diagnosed as infected or with necrotic tissue, the treatment of patients with a known hypersensitivity to trypsin or compound sodium lactate (Hartmann’s) solution, patients having a known hypersensitivity to anesthetics, adrenaline/epinephrine, povidone-iodine, or chlorhexidine solutions. WARNINGS: Autologous use only. Wound beds treated with a cytotoxic agent (e.g., silver sulfadiazine) should be rinsed prior to application of the cell suspension. RECELL is provided sterile and is intended for single-use. Do not use if packaging is damaged or expired. Choose a donor site with no evidence of cellulitis or infection and process skin immediately. A skin sample should require between 15 and 30 minutes contact with Enzyme. Contact in excess of 60 minutes is not recommended. RECELL Enzyme is animal derived and freedom from infectious agents cannot be guaranteed. PRECAUTIONS: RECELL is not intended for use without meshed autograft for treatment of full-thickness burn wounds. The safety and effectiveness of RECELL without meshed autograft have not been established for treatment of partial-thickness burn wounds: on the hands and articulating joints, >320 cm2, in patients with wounds totaling >20% total body surface area (TBSA). The safety and effectiveness of RECELL with autografting have not been established for treatment of full-thickness burn wounds: on the hands and articulated joints, and in patients younger than 28 days of age (neonates). SPECIAL PATIENT POPULATIONS: The safety and effectiveness of RECELL have not been established for treatment of acute thermal partial-thickness burn wounds in pediatric patients younger than 18 years of age. Important Safety Information
Revolutionary treatment using a patient’s own skin for life-changing outcomes In the U.S., RECELL is approved for acute thermal burns. Use of RECELL in other indications is either (1) limited by United States law to investigational use; or (2) otherwise prohibited.




























