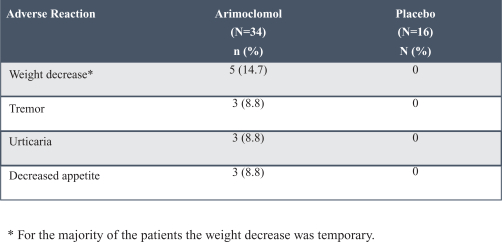
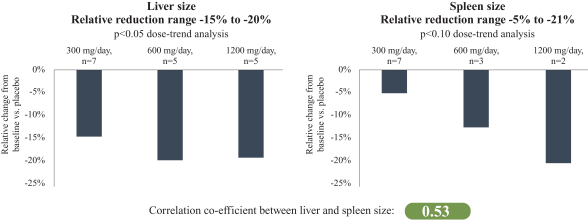
Further, the ACA, among other things, amended the intent requirement of the federal Anti-Kickback Statute and certain criminal statutes governing healthcare fraud. A person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it. In addition, the ACA provided that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act.
Because of the breadth of these laws and the narrowness of their exceptions and safe harbors, it is possible that our business activities can be subject to challenge under one or more of such laws. The full scope and enforcement of each of these laws is uncertain and subject to rapid change in the current environment of healthcare reform. Federal and state enforcement bodies have continued to increase their scrutiny of interactions between healthcare companies and healthcare providers, which has led to a number of investigations, prosecutions, convictions and settlements in the healthcare industry.
Efforts to ensure that our internal operations and future business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, including, without limitation, damages, monetary fines, imprisonment, disgorgement of profits, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, additional reporting or oversight obligations if we become subject to a corporate integrity agreement or other agreement to resolve allegations of noncompliance with the law and curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and pursue our strategy. If any of the physicians or other healthcare providers or entities with whom we expect to do business, including future collaborators, are found not to be in compliance with applicable laws, they may be subject to significant criminal, civil or administrative sanctions, including exclusions from participation in government healthcare programs, which could also affect our business.
Nearly all aspects of our activities are subject to substantial regulation and compliance and staying up-to-date with such regulation is time-consuming and expensive.
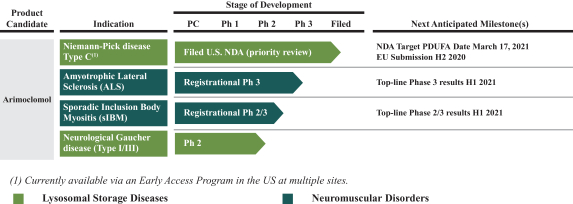
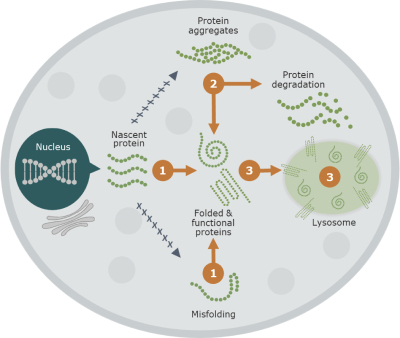
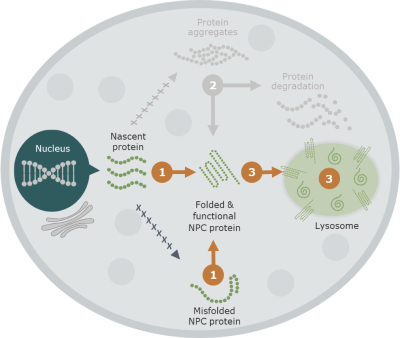
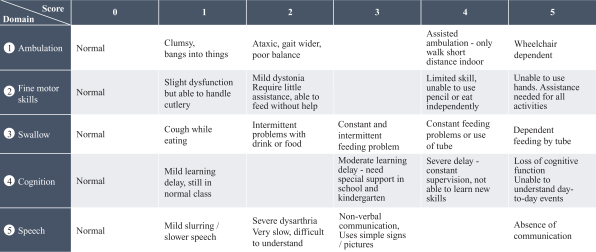
Our business activities are subject to a wide range of laws as well as regulations, including those promulgated by the FDA and EMA, and other regulatory authorities, regulating matters such as orphan drug designations, clinical trials, use of data, animal testing, approval processes, requirements for production, marketing, sales, pricing, pharmacovigilance and intellectual property rights. Compliance with such laws is time-consuming and expensive. In addition, the FDA, the EMA or comparable regulatory authorities may change their policies, adopt additional regulations or revise existing regulations or take other actions, which may prevent or delay approval of arimoclomol. Changes to the prevailing legal or regulatory regime, may cause us to incur significant costs, revise, delay or discontinue all or part of our development program or adopt new processes and procedures in order to comply with new laws or regulations, which may negatively impact how we develop, attest, produce, market or sell our products, if approved, for instance, by making it more costly and demanding to develop or obtain approval for arimoclomol and this may materially and adversely affect our business, results of operations, cash flows, financial condition and/or prospects.
Even if we obtain regulatory approval for arimoclomol, it will remain subject to ongoing regulatory oversight.
Even if we obtain regulatory approvals for arimoclomol, such approvals will be subject to ongoing regulatory requirements for manufacturing, labeling, packaging, storage, advertising, promotion, record-keeping and submission of safety and other post-market information. Any regulatory approvals that we receive for arimoclomol may also be subject to a risk evaluation and mitigation strategy limitations on the approved indicated uses for which the drug may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing, including Phase 4 trials, and surveillance to monitor the quality, safety and efficacy of the drug. Such regulatory requirements may differ from country to country depending on where we have received regulatory approval.
40