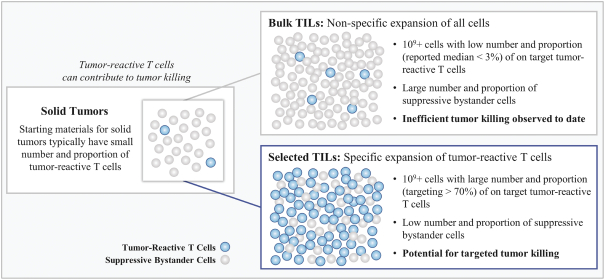
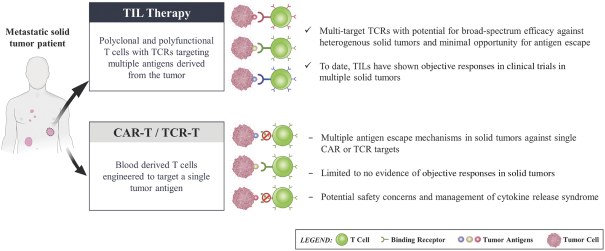
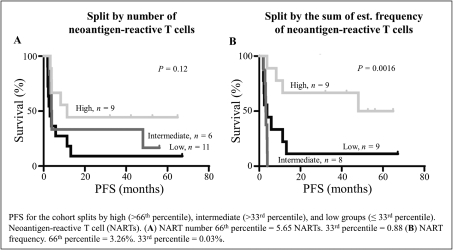
with respect to any patent applications filed by us in the future, nor can we be sure that any of our existing patents or any patents that may be granted to us in the future will be commercially useful in protecting our commercial products and methods of manufacturing the same. We may rely, in some circumstances, on trade secrets to protect our technology. However, trade secrets can be difficult to protect. See “Risk Factors—Risks Related to Our Intellectual Property.”
We actively seek to protect our proprietary technology, inventions, and other intellectual property that is commercially important to the development of our business by a variety of means, such as seeking, maintaining, and defending patent rights, whether developed internally or licensed from third parties. We also may rely on trade secrets and know-how relating to our proprietary technology platform, on continuing technological innovation and on in-licensing opportunities to develop, strengthen and maintain the strength of our position in the field of cell therapy that may be important for the development of our business. We may also seek patent protection or rely upon trade secret rights to protect other technologies that may be used to discover and validate targets, as well as to manufacture and develop novel cell or viral therapy products. Additional regulatory protection may also be afforded through data exclusivity, market exclusivity and patent term extensions where available.
As of May 2, 2023, we own or exclusively license 14 issued U.S. patents and 96 issued foreign patents in Australia, Austria, Belgium, Brazil, Canada, China, France, Germany, Great Britain, Hong Kong, India, Ireland, Israel, Italy, Japan, Lebanon, Luxembourg, Mexico, Netherlands, Russia, and Spain. We currently own or exclusively license 13 pending U.S. patent applications, eight U.S. provisional applications, and 121 pending foreign patent applications in Algeria, Argentina, Australia, Brazil, Canada, Chile, China, Colombia, Egypt, Europe, Gulf Coast Cooperation, Hong Kong, India, Israel, Japan, Korea, Malaysia, Mexico, New Zealand, Peru, Philippines, Singapore, South Africa, Thailand, Ukraine and Vietnam.
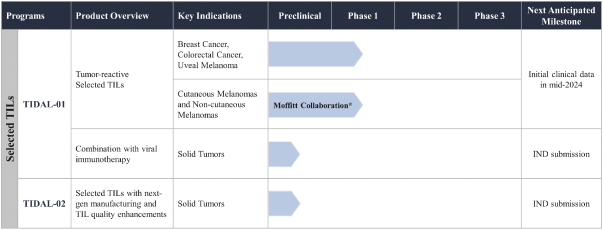
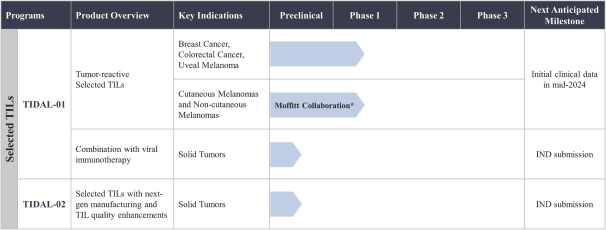
TIL Therapy, Including TIDAL-01
We own four patent families related to TIL therapy that are filed worldwide. The first TIL-001, includes 12 patent applications pending in Australia, Brazil, Canada, China, Europe, Hong Kong, India, Israel, Korea, Mexico, New Zealand and the United States. The TIL-001 patent applications are directed to a processing method for producing autologous T cells for the treatment of cancer and resulting cell therapy compositions, which, if issued, are expected to expire in 2040, without taking into account any possible patent term adjustment or extension and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
The second family, TIL-002, includes 13 patent applications pending in Australia, Brazil, Canada, China, Europe, Hong Kong, India, Israel, Japan, Korea, Mexico, New Zealand and the United States. The TIL-002 patent applications are related to further aspects of processes for producing a TIL therapy and related compositions and methods, and patents that issue from this family, if any, are expected to expire in 2040, without taking into account any possible patent term adjustment or extension and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
The third family, TIL-003, includes 10 patent applications pending in Australia, Canada, China, Europe, Hong Kong, Israel, Japan, Korea, New Zealand and the United States. The TIL-003 patent applications are directed to methods of producing tumor-reactive T cell compositions using modulatory agents, and patents that issue from this family are expected to expire in 2040, without taking into account any possible patent term adjustment or extension and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
The fourth family, TIL-004, includes 12 patent applications pending in Australia, Brazil, Canada, China, Europe, Israel, India, Japan, Korea, Mexico, New Zealand and the United States. The TIL-004 patent applications are directed to methods for ex vivo enrichment and expansion of tumor-reactive T cells and related compositions, and any patents that issue from this family are expected to expire in 2041, without taking into
157