Filed Pursuant to General Instruction II.L of Form F-10
File No. 333-229744
PROSPECTUS SUPPLEMENT
(To Base Shelf Prospectus dated March 13, 2019)

EMERALD HEALTH THERAPEUTICS, INC.
Up to $39,000,000 of Common Shares
This prospectus supplement (the "Prospectus Supplement") of Emerald Health Therapeutics, Inc. (the "Company"), together with the short form base shelf prospectus dated March 13, 2019 to which it relates (the "Prospectus"), qualifies the distribution of common shares (the "Common Shares" and each Common Share being qualified hereunder, an "Offered Share") of the Company having an aggregate offering price of up to $39,000,000 (the "Offering") in each of the Provinces of Canada. The Company has entered into an equity distribution agreement dated March 27, 2019 (the "Equity Distribution Agreement") with GMP Securities L.P. (the "Agent") pursuant to which the Company may distribute Offered Shares from time to time through the Agent, as agent for the distribution of the Offered Shares, in accordance with the terms of the Equity Distribution Agreement. See "Plan of Distribution".
The Common Shares are listed and posted for trading on the TSX Venture Exchange (the "TSXV") under the symbol "EMH". The closing price of the Common Shares on the TSXV on March 26, 2019, the last trading day completed prior to this Prospectus Supplement, was $4.19.The Company has applied to list on the TSXV the Offered Shares qualified hereunder and has received conditional approval for such listing. The listing of the Offered Shares will be subject to the Company fulfilling the listing requirements of the TSXV.
An investment in the Offered Shares is speculative and involves a high degree of risk. The risk factors identified in this Prospectus Supplement, the Prospectus and the documents incorporated by reference herein and therein should be carefully reviewed and evaluated by prospective purchasers before purchasing the Offered Shares. See "Risk Factors" in this Prospectus Supplement, the Prospectus and the documents incorporated by reference herein and therein.
This Offering is made by a Canadian issuer that is permitted under a multi-jurisdictional disclosure system adopted by the United States and Canada to prepare this Prospectus Supplement and the accompanying Prospectus in accordance with Canadian disclosure requirements. Prospective investors should be aware that such requirements are different from those applicable to issuers in the United States. Financial statements incorporated herein by reference have been prepared in accordance with International Financial Reporting Standards ("IFRS") as issued by the International Accounting Standards Board ("IASB"), and may not be comparable to financial statements of United States companies.
Prospective investors should be aware that the acquisition, holding or disposition of the Common Shares may have tax consequences. Such consequences for investors are not discussed herein. Prospective investors are encouraged to consult their own tax advisor with respect to their own particular circumstances.
The enforcement by investors of civil liabilities under the U.S. federal securities laws may be affected adversely by the fact that the Company is existing under and governed by the laws of the province of British Columbia and the federal laws of Canada, that some or all of the Company’s officers and directors are residents of Canada, and that a substantial portion of the Company’s assets and the assets of the officers and directors of the Company are located outside the United States.
NEITHER THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION (THE "SEC"), ANY CANADIAN SECURITIES REGULATOR NOR ANY STATE SECURITIES REGULATOR HAS APPROVED OR DISAPPROVED OF THE SECURITIES OFFERED HEREBY OR DETERMINED IF THIS PROSPECTUS SUPPLEMENT OR THE ACCOMPANYING PROSPECTUS IS TRUTHFUL OR COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENCE.
Prospective purchasers should rely only on the information contained in or incorporated by reference in this Prospectus Supplement and the Prospectus. The Company has not authorized any other person to provide prospective purchasers with different information. The Company is not offering the securities in any jurisdiction in which the Offering is not permitted.
Sales of Offered Shares, if any, under this Prospectus Supplement are anticipated to be made in transactions that are deemed to be "at-the-market distributions" as defined in National Instrument 44-102 –Shelf Distributions ("NI 44-102"), including sales made directly on the TSXV or any other recognized “marketplace” within the meaning of National Instrument 21-101 –Marketplace Operation (each, a "Marketplace") in Canada. The Offered Shares will be distributed at market prices prevailing at the time of the sale or at prices to be negotiated with purchasers of such Offered Shares at the time of sale. As a result, prices may vary as between purchasers and during the period of distribution.There is no minimum amount of funds that must be raised under the Offering. This means that the Offering may terminate after only raising a small portion of the offering amount set out above, or none at all.See "Plan of Distribution".
The Company will pay the Agent compensation for its services in acting as agent in the sale of Offered Shares under the terms of the Equity Distribution Agreement. The Company will pay the Agent compensation equal to 2.5% of the gross proceeds from the sale of Offered Shares under the Equity Distribution Agreement. The Company estimates that the total expenses that it will incur for the Offering (including fees payable to stock exchanges, securities regulatory authorities and its counsel and its auditors, but excluding compensation payable to the Agent under the terms of the Equity Distribution Agreement) will be approximately $430,000. See "Plan of Distribution".
No underwriter or dealer involved in the Offering, no affiliate of such an underwriter or dealer, and no person or company acting jointly or in concert with such an underwriter or dealer may over-allot Common Shares in connection with the Offering or effect any other transactions that are intended to stabilize or maintain the market price of the Common Shares.
Dr. Avtar Dhillon and Mr. Punit Dhillon, each of whom is a director of the Company, reside outside of Canada and have appointed Bennett Jones LLP at 2600 - 1066 West Hastings Street, Vancouver, British Columbia V6E 3X1, as their agent for service of process in Canada. Prospective purchasers are advised that it may not be possible for a purchaser to enforce judgements obtained in Canada against any person or company that is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction or resides outside of Canada, even if the party has appointed an agent for service of process.
The registered and records office of the Company is located at 2600 - 1066 West Hastings Street, Vancouver, British Columbia V6E 3X1. The head office of the Company is located at210 – 800 West Pender Street, Vancouver, British Columbia V6C 1J8.
TABLE OF CONTENTS - PROSPECTUS SUPPLEMENT
TABLE OF CONTENTS – PROSPECTUS
IMPOrtant notice about information in this prospectus supplement
This document is in two parts. The first part is this Prospectus Supplement, which describes the terms of the Offering and also adds to and updates information contained in the Prospectus and the documents incorporated by reference therein. The second part, the Prospectus, gives more general information, some of which may not apply to the Offering.
No person is authorized by the Company to provide any information or to make any representation other than as contained in this Prospectus Supplement or the Prospectus in connection with the issue and sale of the Offered Shares. Prospective purchasers should rely only on the information contained or incorporated by reference in this Prospectus Supplement and the Prospectus in connection with the purchase of the Offered Shares. Information in this Prospectus Supplement updates and modifies the information in the Prospectus and information incorporated by reference therein. Prospective purchasers should assume that the information appearing in this Prospectus Supplement and the Prospectus is accurate only as of the date on the front of such documents and that information contained in any document incorporated by reference is accurate only as of the date of that document unless specified otherwise. The Company's business, financial condition, financial performance and prospects may have changed since those dates.
The address of the Company's website iswww.emeraldhealth.ca. Information contained on the Company's website does not form part of this Prospectus Supplement or the Prospectus nor is it incorporated by reference herein or therein. Prospective purchasers should rely only on information contained or incorporated by reference in this Prospectus Supplement and the Prospectus.
Market data and certain industry forecasts used in this Prospectus Supplement and the Prospectus and the documents incorporated by reference herein or therein were obtained from market research, publicly available information and industry publications. The Company believes that these sources are generally reliable, but the accuracy and completeness of the information is not guaranteed. The Company has not independently verified this information and does not make any representation as to the accuracy of this information.
The Company's annual consolidated financial statements that are incorporated by reference into this Prospectus Supplement and the Prospectus have been prepared in accordance with IFRSas issued by the IASB.
CAUTIONARY STATEMENTS REGARDING FORWARD-LOOKING INFORMATION
Certain statements contained in this Prospectus Supplement, the Prospectus and the documents incorporated by reference herein and therein constitute forward-looking information or forward-looking statements under applicable securities laws (collectively, "forward-looking statements"). These statements relate to future events or future performance, business prospects or opportunities of the Company that are based on forecasts of future results, estimates of amounts not yet determined and assumptions of management made in light of management's experience and perception of historical trends, current conditions and expected future developments. All statements other than statements of historical fact may be forward-looking statements. Any statements that express or involve discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, assumptions or future events or performance (often, but not always, using words or phrases such as "seek", "anticipate", "plan", "continue", "estimate", "expect", "may", "will", "project", "predict", "forecast", "potential", "targeting", "intend", "could", "might", "should", "believe" and similar expressions) are not statements of historical fact and may be "forward-looking statements".
Examples of forward-looking statements in this Prospectus Supplement, the Prospectus and the documents incorporated by reference herein and therein include, but are not limited to, statements in respect of: termination of the Offering upon the maximum amount of sales of Offered Shares being completed thereunder; sales of Common Shares under the Offering; the use of net proceeds of the Offering; the Company's intention to complete the Offering on the terms and conditions described herein; the listing of the Offered Shares on the TSXV; the anticipated effect of the Offering on the performance of the Company; the approval of Licence (as defined below) applications submitted to Health Canada; the Company's intention to significantly increase its production of cannabis and cannabis oils through a multi-phase expansion plan; the building of a Health Canada licenced production facility to expand growing capability; the development of the Joint Venture (as defined below) as a standalone entity; the complete 1.1 million square foot space at the1.1-million square footDelta 3 greenhouse (the "JV Facility") in Delta, British Columbia being in full production in 2019; the rapid and cost effective acceleration of cannabis production by the Joint Venture; the exercise by the Joint Venture of options to lease or purchase additional greenhouses from Village Farms (as defined below); the potential aggregate production capacity of the Delta 1 and Delta 2 greenhouses and the JV Facility, all of which are located in Delta, British Columbia; the use of the credit facility established by the Joint Venture; the purchase by the Company of 40% of the Joint Venture's production of cannabis cultivation during 2019; the expansion of the Company's operations in Richmond, British Columbia and the costs associated with such expansion; the Company's expectation of requirements for quantities of CBD (as defined below); payment of an additional $22.5 million in cash in respect of the acquisition of Verdélite and Verdélite Holdings (each as defined below); the anticipated date that Verdélite's indoor facility will begin production, the expansion cost, the size of the facility and its production of cannabis; the Company's expectation that the acquisition of Verdélite will strengthen its ability to market products in Quebec and Eastern Canada; the high-yield production of the Verdélite facility; the expansion of Avalite Sciences Inc.'s (formerly, Northern Vine Canada Inc.) ("Avalite") operations; the receipt of excise duty licences from the Canada Revenue Agency; the Company's use of proceeds of financings; the quality, suitability for sale and cannabinoid concentration in the hemp harvested through the supply agreement with EHH (as defined below); the suitability of infrastructure at the facility of Factors R&D Technology, Inc. ("FTI") and FTI's extraction of hemp biomass into CBD oil on behalf of the Company; the services to be provided by FTI to the Company; the entering into of an exclusive agreement between EHN (as defined below) and FTI; the entering into of definitive agreements with the Company's business partners; the approval of patent applications that have been submitted by the Company; potential proceeds from the exercise of the Company's outstanding common share purchase warrants; the acceleration by the Company of the expiry date of the Company's outstanding warrants; actions taken by the Company to maintain or adjust its capital structure; increases to the Company's plant diversity and product offering; improvements to the Company's cultivation, manufacturing and standardization processes; partnerships with professional organizations in connection with educating medical doctors and other healthcare professionals about cannabis products; the development of distribution channels for non-medical cannabis products in Canada; anticipated long-term future profitability of the Company; potential effects of regulations under the Cannabis Act (as defined below) and related legislation introduced by provincial governments; the expected implementation of changes to the Cannabis Act to allow the sale of edible cannabis products; the undertaking of clinical research to study the effects of the Company's products on client health; the Company's longer term strategy of becoming a leading provider of quality products for the broader adult recreational cannabis market; the ability of the Company to take advantage of the legalization of adult use recreational cannabis; the Company's intentions to acquire and/or construct additional cannabis production and manufacturing facilities and to expand the Company's marketing initiatives both in Canada and internationally; the Company building valuable intellectual property in Canada which could lead to accelerated sales growth and profit margins; and future sales opportunities in other emerging medical markets and the effect that each risk factor will have on the Company.
Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause actual results or events to differ materially from those anticipated in such forward-looking statements. Prospective purchasers are cautioned that any such statements are not guarantees of future performance and actual results or developments may differ materially from those projected in the forward-looking statements. These forward-looking statements involve risks and uncertainties relating to, among others: the market price of cannabis; ability of the Company to secure cannabis supply; continued availability of capital financing and general economic, market or business conditions; reliance on licences to produce and sell cannabis and cannabis oils issued to the Company under the Cannabis Act and its ability to maintain these licences; regulatory risks relating to the Company's compliance with the Cannabis Act; failure to obtain regulatory approvals for expansion of the Company's current production facilities, development of new production facilities and greenhouse retrofits by the Company and the Joint Venture; Avalite's reliance on its dealer licence issued to it under the Controlled Drugs and Substances Act (Canada) to provide analytical testing of cannabis and importation of CBD and its ability to maintain this licence; the Company's ability to execute its multi-phase expansion plan and its plans with the Joint Venture; failure to execute a definitive agreement with FTI; changes in laws, regulations and guidelines; changes in government; changes in government policy; increased competition in the cannabis market in Canada and internationally; the limited operating history of the Company; the Company's reliance on key persons; failure of counterparties to perform contractual obligations; failure to obtain additional financing; unfavourable publicity or consumer perception of the Company and the cannabis industry; the impact of any negative scientific studies on the effects of cannabis; demand for labour; difficulties in construction or in obtaining qualified contractors to complete expansion projects and greenhouse retrofits; impact of any recall of the Company's products; actual operating and financial performance of facilities, equipment and processes relative to specifications and expectations; results of litigation; the Company's ability to develop and commercialize pharmaceutical or nutraceutical products; failure to obtain regulatory approval for pharmaceutical or nutraceutical products; changes in the Company's over-all business strategy; stock exchange rules or policies which may restrict the Company's business; and the Company's assumptions stated herein being correct. Additional factors that could cause actual results to differ materially include, but are not limited to, the risk factors described herein and in the Prospectus and as discussed in the Company's financial statements and other filings, under the heading "Risk Factors" in the Company's most recent annual information form and under the section "Risks and Uncertainties" in the Company's annual and interim management's discussion and analysis incorporated by reference herein.
The Company believes that the expectations reflected in any forward-looking statements are reasonable, but no assurance can be given that these expectations will prove to be correct and such forward-looking statements included in, or incorporated by reference into, this Prospectus Supplement or the Prospectus should not be unduly relied upon. These statements speak only as of the date of this Prospectus Supplement or the Prospectus, as applicable. The Company does not intend, and does not assume any obligation, to update these forward-looking statements, except as required by applicable laws. Actual results may differ materially from those expressed or implied by such forward-looking statements.
MEANING OF CERTAIN REFERENCES
For simplicity, the Company uses terms in this Prospectus Supplement to refer to the investments and operations of the Company and its direct and indirect subsidiaries as a whole. Accordingly, in this Prospectus Supplement, unless the context otherwise requires, the "Company" is referring to Emerald Health Therapeutics, Inc. and its direct and indirect subsidiaries andPure Sunfarms Corp. (the "Joint Venture") (to the extent applicable).
DOCUMENTS INCORPORATED BY REFERENCE
This Prospectus Supplement is deemed to be incorporated by reference in the Prospectus solely for the purpose of the Offering. Other documents are also incorporated or deemed to be incorporated by reference in the Prospectus and reference should be made to the Prospectus for full particulars thereof.
Information has been incorporated by reference in this Prospectus Supplement and the Prospectus from documents filed with securities commissions or similar authorities in each of the provinces of Canada (the "Qualifying Jurisdictions"). Copies of the documents incorporated herein or therein by reference or a copy of the Company's permanent information record may be obtained on request without charge from the Chief Financial Officer of the Company at 210 – 800 West Pender Street, Vancouver, British Columbia V6C 1J8 or by accessing the disclosure documents available through the Internet on the System for Electronic Document Analysis and Retrieval (SEDAR), which can be accessed atwww.sedar.com.
As at the date hereof, the following documents of the Company, filed with the securities commissions or similar authorities in the Qualifying Jurisdictions, are specifically incorporated by reference into and form an integral part of this Prospectus Supplement, provided that such documents are not incorporated by reference to the extent that their contents are modified or superseded by a statement contained in this Prospectus Supplement, the Prospectus or in any other subsequently filed document that is also incorporated by reference in this Prospectus Supplement, as further described below:
| (a) | the annual information form of the Company dated March 29, 2018 for the year ended December 31, 2017 (the "Annual Information Form"); |
| (b) | the audited consolidated annual financial statements of the Company as at and for the years ended December 31, 2017 and December 31, 2016, together with the notes thereto and the independent auditor's report thereon (as amended); |
| (c) | the management's discussion and analysis of the Company for the year ended December 31, 2017; |
| (d) | the unaudited condensed interim consolidated financial statements of the Company for the three and nine months ended September 30, 2018 and September 30, 2017, together with the notes thereto (the "Interim Financial Statements"); |
| (e) | the management's discussion and analysis of the Company for the three and nine months ended September 30, 2018 (the "Interim MD&A"); |
| (f) | the management information circular of the Company dated May 9, 2018 regarding the annual general meeting of shareholders of the Company held on May 31, 2018; |
| (g) | the material change report of the Company dated January 4, 2018 announcing the entering into of a binding term sheet with a single Canadian institutional investor (the "Investor") pursuant to which the Investor agreed to purchase 3,000,000 units of the Company at a price of $5.00 per unit and purchase 2,000,000 Common Shares of the Company at a price of $5.00 per share from Emerald Health Sciences, Inc. (the "Major Shareholder") (the "January 2018 Offering"); |
| (h) | the material change report of the Company dated January 15, 2018 announcing the closing of the January 2018 Offering; |
| (i) | the material change report of the Company dated February 26, 2018 announcing the entering into of a binding term sheet with the Investor pursuant to which the Investor agreed to purchase 3,000,000 units of the Company at a price of $6.00 per unit and purchase 2,000,000 Common Shares of the Company at a price of $6.00 per share from the Major Shareholder (the "February 2018 Offering") and the closing of the February 2018 Offering; |
| (j) | the material change report of the Company dated May 11, 2018 announcing the closing of the acquisition ofVerdélite Sciences, Inc. (formerly, Agro-Biotech Sciences Inc.) ("Verdélite") and Verdélite Property Holdings, Inc. (formerly, Agro-Biotech Property Holdings Inc.) ("Verdélite Holdings"); |
| (k) | the material change report of the Company dated May 15, 2018 announcing the entering into of a binding term sheet with the Investor pursuant to which the Investor agreed to purchase 4,000,000 units of the Company at a price of $4.20 per unit and purchase 2,000,000 Common Shares of the Company at a price of $4.20 per share from the Major Shareholder (the "May 2018 Offering"); |
| (l) | the material change report of the Company dated May 31, 2018 announcing the closing of the May 2018 Offering; |
| (m) | the material change report of the Company dated October 3, 2018 announcing the Company's agreement to purchase from Emerald Health Hemp Inc. ("EHH") CBD-containing hemp biomass for extraction into CBD oil; |
| (n) | the material change report of the Company dated December 4, 2018 announcing the resignation of Chris Wagner as Chief Executive Officer and the appointment of Avtar Dhillon as President; and |
| (o) | the material change report of the Company dated December 13, 2018 announcing the entering into of a binding term sheet with the Investor pursuant to which the Investor agreed to purchase 4,000,000 common shares of the Company at a price of $2.70 per Common Share (the "December 2018 Offering") and the closing of the December 2018 Offering. |
Any document of the type referred to in the preceding paragraph (excluding confidential material change reports), and all other documents of the type required to be incorporated by reference in a short form prospectus by National Instrument 44-101 -Short Form Prospectus Distributions of the Canadian Securities Administrators, filed by the Company with a securities commission or similar regulatory authority in Canada after the date of this Prospectus Supplement and prior to the termination of any offering of securities hereunder shall be deemed to be incorporated by reference into this Prospectus Supplement.
In addition, to the extent that any document or information incorporated by reference into this Prospectus Supplement is included in any report on Form 6-K, Form 40-F, Form 20-F, Form 10-K, Form 10-Q or Form 8-K (or any respective successor form) that is filed with or furnished to the SEC after the date of this Prospectus Supplement and prior to the date that all Offered Shares offered hereunder are sold or the Offering is otherwise terminated, such document or information shall be deemed to be incorporated by reference as an exhibit to the registration statement of which this Prospectus Supplement forms a part (in the case of documents or information deemed furnished on Form 6-K or Form 8-K, only to the extent specifically stated therein).
Any statement contained in this Prospectus Supplement, the Prospectus or in a document incorporated or deemed to be incorporated by reference herein or therein shall be deemed to be modified or superseded, for purposes of this Prospectus Supplement and the Prospectus, to the extent that a statement contained herein or therein or in any other subsequently filed document that also is or is deemed to be incorporated by reference in this Prospectus Supplement or the Prospectus modifies or supersedes that statement. Any such modifying or superseding statement need not state that it has modified or superseded a prior statement or include any other information set forth in the document that it modifies or supersedes. The making of a modifying or superseding statement shall not be deemed an admission for any purposes that the modified or superseded statement, when made, constituted a misrepresentation, an untrue statement of a material fact or an omission to state a material fact that is required to be stated or that is necessary to make a statement not misleading in light of the circumstances in which it was made. Any statement so modified or superseded shall not be considered in its unmodified or superseded form to constitute part of this Prospectus Supplement or the Prospectus; rather only such statement as so modified or superseded shall be considered to constitute part of this Prospectus Supplement and the Prospectus.
The Company has not provided or otherwise authorized any other person to provide the prospective purchasers with information other than as contained or incorporated by reference in this Prospectus Supplement or the Prospectus. If prospective purchasers are provided with different or inconsistent information, they should not rely on it.
DOCUMENTS FILED AS PART OF THE REGISTRATION STATEMENT
In addition to the documents specified in this Prospectus Supplement and in the accompanying Prospectus under "Documents Incorporated by Reference", the Equity Distribution Agreement described in this Prospectus Supplement will be filed with the SEC and will form part of the registration statement of which this Prospectus Supplement forms a part.
ELIGIBILITY FOR INVESTMENT
In the opinion of Bennett Jones LLP, counsel to the Company, and Blake, Cassels & Graydon LLP, counsel to the Agent, based on the provisions of theIncome Tax Act (Canada) and the regulations thereunder (collectively, the “Tax Act”) in force as of the date hereof, the Offered Shares will be qualified investments at the time of acquisition by a trust governed by a registered retirement savings plan (“RRSP”), a registered retirement income fund (“RRIF”), a deferred profit sharing plan (“DPSP”), a registered education savings plan (“RESP”), a registered disability savings plan (“RDSP”) or a tax free savings account (“TFSA”), each as defined in the Tax Act (collectively, “Plans”), provided that, at the time of acquisition by the Plan, the Offered Shares are listed on a “designated stock exchange” as defined in the Tax Act (which currently includes Tiers 1 and 2 of the TSXV) or Emerald Health Therapeutics, Inc. is a “public corporation” as defined in the Tax Act.
Notwithstanding the foregoing, if the Offered Shares are “prohibited investments” for the purposes of a TFSA, RDSP, RRSP, RRIF or RESP, the holder, annuitant or subscriber of such Plan, as the case may be, will be subject to a penalty tax in respect of the Offered Shares as set out in the Tax Act. The Offered Shares will not be a “prohibited investment” provided the holder, annuitant or subscriber of a TFSA, RDSP, RRSP, RRIF or RESP, as the case may be, deals at arm’s length with the Company for purposes of the Tax Act and does not have a “significant interest” (within the meaning of the Tax Act for purposes of the prohibited investment rules) in the Company. In addition, the Offered Shares will not be a “prohibited investment” if the Offered Shares are “excluded property” (within the meaning of the Tax Act for purposes of the prohibited investment rules) for trusts governed by a TFSA, RDSP, RRSP, RRIF or RESP. Prospective purchasers should consult their own tax advisors to ensure that the Offered Shares will not be a prohibited investment for a trust governed by a TFSA, RDSP, RRSP, RRIF or RESP in their particular circumstances.
SUMMARY OF THE BUSINESS
The principal business of the Company is the production and sale of cannabis pursuant to theCannabis Act (Canada) (the "Cannabis Act"). The Company currently offers a variety of dried cannabis strains and cannabis oils each with varying levels of Tetrahydrocannabinol ("THC"), cannabidiol ("CBD") and other cannabinoids.
The Company does not engage in any U.S. marijuana-related activities as defined in Canadian Securities Administrators Staff Notice 51-352 (Revised) dated February 8, 2018. To the extent that the Company pursues international expansion, it will only conduct business in jurisdictions outside of Canada where such operations are legally permissible in accordance with the laws of the jurisdiction and applicable Canadian regulatory and stock exchange requirements, including the Company's undertaking with the TSXV, as applicable.
More information regarding the business of the Company can be found in the Company's most recent annual information form and other documents which are incorporated herein by reference. See "Documents Incorporated by Reference".
Recent Developments
On March 14, 2019, the Company announced that it had fulfilled its first purchase order of cannabis products from the Ontario Cannabis Retail Corporation (operating as the Ontario Cannabis Store).
On March 18, 2019, the Company announced that it had appointed Allan Rewak as the Vice-President of Communications and Stakeholder Relations. Mr. Rewak's appointment will be effective on April 15, 2019.
RISK FACTORS
Investing in the Offered Shares involves a high degree of risk. The Offered Shares should be considered a speculative investment due to the high-risk nature of the Company's business. In addition to the other information contained in this Prospectus Supplement, the Prospectus and the documents incorporated by reference herein and therein, prospective purchasers should carefully consider the risks described under the "Risk Factors" section of this Prospectus Supplement, the Prospectus and the documents incorporated herein and therein by reference before purchasing any Offered Shares. If any such risks actually occur, the Company's business, financial condition, financial performance and prospects could materially suffer. As a result, the trading price of the Company's securities, including the Common Shares, could decline, and purchasers might lose all or part of their investment. The risks set out in this Prospectus Supplement and the Prospectus are not the only risks that the Company faces; risks and uncertainties not currently known to it or that it currently deems to be immaterial may also materially and adversely affect its business, financial condition, financial performance and prospects. Prospective purchasers should also refer to the other information set forth or incorporated by reference in this Prospectus Supplement and the Prospectus, including in the Company's most recent annual information form.
Net Proceeds to the Company from the Offering
There is no certainty that $39,000,000 will be raised under the Offering. The Agent has agreed to use its commercially reasonable efforts to sell the Offered Shares when and to the extent requested by the Company, but the Company is not required to request the sale of any minimum amount of Offered Shares qualified under this Prospectus Supplement and, if it requests a sale, the Agent is not obligated to purchase any Offered Shares that are not sold. As a result, the Company may raise substantially less than the maximum total Offering amount or none at all.
Number of Offered Shares to be Offered
The Offered Shares will be sold by the Agent at the market price prevailing at the time of sale and, therefore, there is no certainty as to the number of Offered Shares that may be sold under the Offering. If the prevailing market price for the Common Shares declines, then the Company will be able to issue more Offered Shares under the Offering and investors may suffer greater dilution.
Reliance on Licences
The Company's ability to grow, store and sell cannabis in Canada is dependent on the Company's cultivation and sales licences ("Licences"). Failure to comply with the requirements of the Licences, or any failure to maintain the Licences would have a material adverse impact on the business, financial condition and financial performance of the Company. The Company believes it will meet the requirements of the Cannabis Act for further extensions or renewals of the Licences. However, should Health Canada not extend or renew one or more of the Licences, or should it renew a Licence on different terms, the business, financial condition and results of the operation of the Company would be materially adversely affected.
Risks Relating to the Joint Venture
Although the Company has certain rights pursuant to the shareholders' agreement governingthe Joint Venture, the Company does not directly control the management of the Joint Venture and the Joint Venture has its own management. Success of the Joint Venture will depend, in part, on the expertise of such management. The business of the Joint Venture is itself subject to the operational and business risks inherent in the large-scale production of cannabis and to that extent, the business of the Joint Venture will be subject to many of the same business risks applicable to the Company and which are set out elsewhere in this Prospectus Supplement. In particular, the production and sale of cannabis at the Joint Venture's facilities in Delta, British Columbia is subject to obtaining and maintaining all necessary permits and licences. There can be no assurance that the Company and the Joint Venture will be successful in obtaining and maintaining all such permits and licences. In the event that all such licences and permits are not obtained or maintained then the Joint Venture will not be permitted to produce or sell cannabis which would have a material adverse effect on the Company's business, results of operations and financial performance.
Pursuant to the shareholders' agreement governing the Joint Venture (the "JV SHA"), the Company has advanced $20.0 million in full satisfaction of its initial obligation in respect of the Company's equity contributions to the Joint Venture. The Company has advanced an additional $13.0 million as a loan to the Joint Venture. The Joint Venture may require additional capital. To the extent the Joint Venture is unable to internally fund its operating requirements or expansion plans it may make additional capital calls on its shareholders. Failure by the Company to meet such a capital call would not constitute a default under the JV SHA, but in the event that its joint venture partner,Village Farms International Inc. ("Village Farms"), elects to make its capital contributions the Company's interest in the Joint Venture may, in certain circumstances, be diluted. If the Company elects to fund a capital call but Village Farms fails to do so then the Company may need to advance additional capital in order to meet the Joint Venture's needs. There can be no assurance that the Company or Village Farms will have the necessary capital resources to meet a capital call when and if made by the Joint Venture. In the event that the Joint Venture cannot raise the necessary funds from its shareholders, including the Company, it may need to raise additional funds through debt or equity financings that may be dilutive to the Company's interest in the Joint Venture. If the Joint Venture cannot obtain adequate capital to the extent required on favorable terms or at all, it may be required to scale back or halt entirely its operating or expansion plans and its business, financial condition and results of operations could be adversely affected. Disputes may arise between the Company and its joint venture partner, Village Farms, that may adversely affect the success of the Joint Venture and which would have a material adverse effect on the Company's business, results of operations and financial performance. Failure by the Company to otherwise comply with its obligations under the JV SHA may result in the Company being in default under the shareholders' agreement and could result in the Company losing some or all of its interest in the Joint Venture.
Competition
The Company faces intense competition from other companies, some of which have longer operating histories and greater financial resources and manufacturing and marketing experience than the Company. Increased competition by larger and better financed competitors could materially and adversely affect the business, financial condition and financial performance of the Company.
Because of the early stage of the industry in which the Company operates, the Company expects to face additional competition from new entrants. If the number of users of legal cannabis in Canada increases, the demand for products is expected to increase and the Company expects that competition will become more intense, as current and future competitors begin to offer an increasing number of diversified products. To remain competitive, the Company will require a continued high level of investment in research and development, marketing, sales and customer support. The Company may not have sufficient resources to maintain research and development, marketing, sales and customer support efforts on a competitive basis which could materially and adversely affect the business, financial condition and financial performance of the Company.
The Company's success depends in part on its ability to attract and retain customers. There are many factors which could impact the Company's ability to attract and retain customers, including but not limited to competition from other companies in the industry, the Company's ability to continually produce desirable and effective product, the successful implementation of the Company's customer-acquisition plan and the continued growth in the aggregate number of customers selecting cannabis. The Company's continued success depends in part on its ability to anticipate and respond to these changes. The success of the Company's product offering depends on a number of factors, including the Company's ability to:
| (a) | accurately anticipate customer needs; |
| (b) | innovate and develop new products or product enhancements that meet these needs; |
| (c) | successfully commercialize new products or product enhancements in a timely manner; |
| (d) | price products competitively; |
| (e) | manufacture and deliver products in sufficient volumes and in a timely manner; and |
| (f) | differentiate products from those of competitors. |
The Company also faces competition from unlicensed and unregulated market participants, including individuals or groups that produce cannabis without a license similar to that under which the Company currently produces and illegal dispensaries and black market participants selling cannabis and cannabis-based products in Canada. These competitors may be able to offer products at lower prices than the Company's products and with higher concentrations of active ingredients than the Company is authorized to produce and sell and using delivery methods, including edibles, concentrates and extract vaporizers, that the Company is currently prohibited from offering in Canada. The competition presented by these participants, and any unwillingness by consumers currently utilizing these unlicensed distribution channels to begin purchasing from licensed producers for any reason, or any inability of law enforcement authorities to enforce existing laws prohibiting the unlicensed cultivation and sale of cannabis and cannabis-based products, could adversely affect the Company's revenue, market share, result in increased competition through the black market for cannabis or have an adverse impact on the public perception of cannabis use and licensed cannabis producers and dealers.
Expansion Risks
There is no guarantee that the Company's plans to acquire and/or construct additional cannabis production and manufacturing facilities and to expand the Company's marketing and sales initiatives will be successful. Any such activities will require, among other things, various regulatory approvals, licences and permits (such as additional site licences from Health Canada under the Cannabis Act, as applicable) and there is no guarantee that all required approvals, licences and permits will be obtained in a timely fashion or at all. The Company is currently constructing a second production facility in Richmond, British Columbia which will require local government approval, licencing by Health Canada and significant investment of capital. Neither local government approval, Health Canada licencing nor the availability of capital are assured.
The Company's expansion plans will also require significant amounts of capital, including the following expenditures forecast to be made in the current fiscal year:
| (a) | the Company is required to make a payment on May 1, 2019 of $22.5 million in cash to the vendors in connection with the acquisition of Verdélite and Verdélite Holdings; |
| (b) | the Company has budgeted approximately $5.5 million to be spent on fully completing the build-out of the Verdélite facility; |
| (c) | the Company expects to incur additional costs of approximately $8 million in connection with the completion of construction at its Richmond facility; and |
| (d) | the Company has budgeted approximately $1.5 million to be spent on completing the Avalite analytical facility. |
There is no guarantee that the Company will be able to obtain the necessary capital, which could result in significant delays or could prevent the Company from completing any of the foregoing activities as anticipated or at all. The failure of the Company to successfully execute its expansion strategy (including receiving required regulatory approvals and permits) could adversely affect the Company's business, financial condition and financial performance and may result in the Company failing to meet anticipated or future demand for its products, when and if it arises. See also "Additional Financing" and "Factors which may Prevent Realization of Growth Targets".
Additional Financing
The building and operation of the Company's facilities and business are capital intensive. In order to execute its anticipated growth strategy, the Company will require additional equity and/or debt financing to support on-going operations, to satisfy capital expenditures or to undertake acquisitions or other business combination transactions. There can be no assurance that additional financing will be available to the Company when needed or on terms which are acceptable. The Company's inability to raise financing to support on-going operations or to fund capital expenditures or acquisitions could limit the Company's growth and may have a material adverse effect upon future profitability. The Company may require additional financing to fund its operations to the point where it is generating positive cash flows.
If additional funds are raised through further issuances of equity or convertible debt securities, existing shareholders could suffer significant dilution. The Prospectus allows for, subject to securities regulatory requirements and limitations, the potential offering of up to an aggregate of $150 million (of which, following termination of the Offering upon completion of the sale of the maximum number of Offered Shares permitted thereunder, the Company may still issue $111 million) of Common Shares, preferred shares, warrants, subscription receipts, units or debt securities, or any combination thereof, from time to time in one or more offerings, and is intended to give the Company the flexibility to take advantage of financing opportunities when, and if, market conditions are favorable to the Company. The specific terms of such future offerings, if any, would be established, subject to the approval of the board of directors of the Company, at the time of such offering and will be described in detail in a prospectus supplement filed at the time of any such offering. Any new equity securities issued by the Company could have rights, preferences and privileges superior to those of holders of Common Shares.
The perceived risk of dilution may negatively impact the price of the Common Shares and may cause shareholders to sell their Common Shares, which would contribute to a decline in the price of the Common Shares. Moreover, the perceived risk of dilution and the resulting downward pressure on the Company's share price could encourage investors to engage in short sales of the Common Shares, which could further contribute to progressive price declines in the Common Shares.
Any debt financing secured in the future could involve restrictive covenants relating to capital raising activities and other financial and operational matters, which may make it more difficult for the Company to obtain additional capital and to pursue business opportunities, including potential acquisitions.
In addition, the Company has in the past received a substantial amount of its debt financing from the Major Shareholder pursuant to the terms of a loan agreement between the Company and the Major Shareholder. There is no guarantee that the Major Shareholder will continue to provide funds when needed by the Company or that the terms of such loan agreement will remain the same or acceptable to the Company.
Change in Laws, Regulations and Guidelines
The Company's operations are subject to a variety of laws, regulations and guidelines relating to the manufacture, management, transportation, storage and disposal of cannabis but also including laws and regulations relating to health and safety, privacy, the conduct of operations and the protection of the environment. While, to the knowledge of the Company's management, the Company is currently in material compliance with all such laws, changes to such laws, regulations and guidelines due to matters beyond the control of the Company may cause adverse effects to the Company's operations and the financial condition of the Company.
The Cannabis Act was enacted on October 17, 2018 and as a result, the full impact of the regulatory regime thereunder is uncertain at this time. It is likely the regulatory regime under the Cannabis Act will evolve over time and it is uncertain how Health Canada and other applicable government agencies will interpret and enforce the Cannabis Act and related federal and provincial legislation (collectively, "Cannabis Legislation"). Cannabis Legislation may be revised in the future and additional legislation may be adopted. If Cannabis Legislation is interpreted or enforced more strictly or is amended to be more restrictive than under the previous regulatory regime, such regulatory changes could adversely affect the Company's operations, business, financial condition and financial performance.
Future changes to Cannabis Legislation may have a materially adverse impact on the Company including, but not limited to:
| (a) | restrictions on the Company's ability to run its business as it currently operates or the imposition of restrictions on licence holders under the Cannabis Act, including restrictions on the products that may be produced or made available by licence holders, restrictions on strains (including restrictions on potency) and types of products (oil, resin, concentrates, edible products containing cannabis extracts), and additional restrictions on advertising of the Company's products; |
| (b) | reduction of barriers to entry for new entrants to the industry, some of whom may have more financial resources and marketing expertise than the Company; |
| (c) | the imposition of restrictions on distribution which would impact the Company's ability to sell its products; |
| (d) | limitations on the types of customers the Company can sell to (for example, age restrictions) or the amount of product that purchasers may buy, any of which may reduce the number of the Company's possible customers or the average amount of purchased product; |
| (e) | the implementation of additional taxes on the Company's products, which may reduce the demand for the Company's products and reduce the quantity of products sold by the Company; and |
| (f) | the imposition of requirements on licence holders, including in relation to labeling requirements for the Company's products and the manner in which the products are required to be tested or approved for sale, which could increase the cost of producing the Company's products and could reduce the Company's earnings and margins. |
While the impact of any such changes are uncertain, it is not expected that any such changes will have an effect on the Company's operations that are materially different than the effect on similar-sized companies in the same business as the Company.
Regulatory Risks
The activities of the Company are subject to regulation by governmental authorities, particularly Health Canada. Achievement of the Company's business objectives are contingent, in part, upon compliance with regulatory requirements enacted by these governmental authorities and obtaining all regulatory approvals, where necessary, for the sale of its products. The Company will also require Health Canada and other regulatory approval in order to proceed with construction of new growing facilities and will be required to apply for and obtain additional licences under the Cannabis Act before it begins growing cannabis at any such facilities. The Company cannot predict the time required to secure all appropriate regulatory approvals for its new facilities or products, or the extent of testing and documentation that may be required by Health Canada or other governmental authorities. Any delays in obtaining, or failure to obtain regulatory approvals would significantly delay the development of facilities, markets and/or products and could have a material adverse effect on the business, financial performance and financial condition of the Company.
Failure of Facility Infrastructure
The Company's facilities require regular maintenance on both the heating and cooling systems and regular power component maintenance on the generator and delivery systems. Any failure of the heating and cooling systems or electrical delivery systems could have a material and adverse effect on the Company's business, financial condition and financial prospects.
Shelf Life of Inventory
The Company holds finished goods in inventory and its inventory has a shelf life. Finished goods in the Company's inventory include dried cannabis and cannabis oil products. The Company follows Health Canada's testing requirements for product release and re-tests its inventory for information purposes. Based on such testing results and management's experience, the Company believes that there is no significant change in product composition during a twelve-month storage under its current vault conditions. The Company's typical turnover rate for inventory varies between two weeks and six months from final production, however this turnover rate may change and its inventory may reach its expiration and may not be sold. Even though management of the Company on a regular basis reviews the amount of inventory on hand, reviews the remaining shelf life and estimates the time required to manufacture and sell such inventory, write-down of inventory may still be required. Any such write-down of inventory could have a material adverse effect on the Company's business, financial condition, and financial performance.
Product Liability
As a manufacturer and distributor of products designed to be ingested by humans, the Company faces an inherent risk of exposure to product liability claims, regulatory action and litigation if any of its products are alleged to have caused significant loss or injury. In addition, the manufacture and sale of the Company's products involve the risk of injury to consumers due to tampering by unauthorized third parties or product contamination. Previously unknown adverse reactions resulting from human consumption of the Company's products alone or in combination with other medications or substances could occur. The Company may be subject to various product liability claims, including, among others, that the Company's products caused injury or illness, include inadequate instructions for use or include inadequate warnings concerning possible side effects or interactions with other substances. A product liability claim or regulatory action against the Company could result in increased costs, could adversely affect the Company's reputation with its consumers generally, and could have a material adverse effect on the financial performance and the financial condition of the Company. There can be no assurances that the Company will be able to obtain or maintain product liability insurance on acceptable terms or with adequate coverage against potential liabilities. Such insurance is expensive and may not be available in the future on acceptable terms, or at all. The inability to obtain sufficient insurance coverage on reasonable terms or to otherwise protect against potential product liability claims could result in the Company incurring significant losses in the event of a successful claim and could prevent or inhibit the commercialization of the Company's potential products.
Product Recalls
Manufacturers and distributors of products are sometimes subject to orders for the recall or return of their products for a variety of reasons, including product defects, such as contamination, unintended harmful side effects or interactions with other substances, packaging safety and inadequate or inaccurate labeling disclosure. All such recalls must adhere to the relevant provisions in Cannabis Legislation. If any of the Company's products are recalled due to an alleged product defect or for any other reason, the Company could be required to incur the unexpected expense of the recall and any legal proceedings that might arise in connection with the recall. The Company may lose a significant amount of sales and may not be able to replace those sales at an acceptable margin or at all. In addition, a product recall may require significant management attention. Although the Company has detailed procedures in place for testing finished products, there can be no assurance that any quality, potency or contamination problems will be detected in time to avoid unforeseen product recalls, regulatory action or lawsuits. A recall for any of the foregoing reasons could lead to decreased demand for the Company's products and could have a material adverse effect on the financial performance and financial condition of the Company. Additionally, product recalls may lead to increased scrutiny of the Company's operations by Health Canada or other regulatory agencies, requiring further management attention and potential legal fees and other expenses.
Risks Inherent in an Agricultural Business
The Company's business involves the growing of cannabis and hemp, both of which are agricultural products. As such, the business is subject to the risks inherent in the agricultural business, such as weather, insects, plant diseases and similar agricultural risks (some of which may be caused by climate change). Although the Company grows its cannabis products indoors under climate controlled conditions and carefully monitors the growing conditions with trained personnel, there can be no assurance that natural elements will not have a material adverse effect on the production of its products.
Vulnerability to Rising Energy Costs
The Company's growing operations consume considerable energy, making the Company vulnerable to rising energy costs (which may be caused by climate change). Rising or volatile energy costs may adversely impact the business of the Company and its ability to operate profitably.
Transportation Disruptions
Due to the perishable and premium nature of the Company's products, the Company will depend on fast and efficient delivery services to distribute its product. Any prolonged disruption of delivery services could have an adverse effect on the financial condition and financial performance of the Company. Rising costs associated with delivery services used by the Company to ship its products may also adversely impact the business of the Company and its ability to operate profitably.
Reliance on Key Inputs
The Company's business is dependent on a number of key inputs and their related costs including raw materials and supplies related to its growing operations, as well as electricity, water and other local utilities. Any significant interruption, increase in costs or other negative change in the availability or economics of the supply chain for key inputs(some of which may be caused by climate change)could materially impact the business, financial condition and financial performance of the Company. Some of these inputs may only be available from a single supplier or a limited group of suppliers. If a sole source supplier was to go out of business, the Company might be unable to find a replacement for such source in a timely manner or at all. If a sole source supplier were to be acquired by a competitor, that competitor may elect not to sell to the Company in the future. Any inability to secure required supplies and services or to do so on appropriate terms could have a materially adverse impact on the business, financial condition and financial performance of the Company.
Dependence on Suppliers and Skilled Labour
The ability of the Company to compete and grow will be dependent on it having access, at a reasonable cost and in a timely manner, to skilled labour, equipment, parts and components. No assurances can be given that the Company will be successful in maintaining its required supply of skilled labour, equipment, parts and components. It is also possible that the final costs of the major equipment contemplated by the Company's capital expenditure program may be significantly greater than anticipated by the Company's management and may be greater than the funds available to the Company, in which circumstance the Company may curtail, or extend the time frames for completing its capital expenditure plans. This could have an adverse effect on the financial results of the Company.
Intellectual Property
The Company has limited protection under law against potential infringements of its product names or marks, or against other misappropriation of its other intellectual property including its trade name and plant strains. For example, the status of intellectual property in plant strain may be uncertain at law and may be difficult to prove in practice. Monitoring infringement or misappropriation of intellectual property can be difficult and expensive, and the Company may not be able to detect every infringement or misappropriation of its proprietary rights. Even if the Company does detect infringement or misappropriation of its proprietary rights, litigation to enforce these rights could cause the Company to divert financial and other resources away from its business operations.
Additionally, third parties may claim that products or marks that the Company has independently developed or which bear certain of the Company's trademarks infringe upon their intellectual property rights and there can be no assurance that one or more of the Company's products or marks will not be found to infringe upon third-party intellectual property rights in the future.
Risks Related to Nutraceutical Products
The Company's subsidiary, Emerald Health Naturals Inc. ("EHN"), is a company focused on the distribution, sale and development of natural health products designed to stimulate the human endocannabinoid system, known as the Endo-line. This unique line of products has never been sold in Canada, and therefore sales of such products will be subject to all of the risks commonly associated with the sale of newly developed products, including those set forth above under "Competition". The benefits of such products have not been proven in clinical trials and may not materialize. If the benefits of such products are not as expected by the Company then sales of such products may be adversely impacted. In addition, demand for such products in Canada is unknown and sales of these products may not meet the Company's expected targets.
The Endo-line of products was licensed from Emerald Health Bioceuticals, Inc. ("EHB"), an American company which is a related party that currently manufactures and sells these products in the United States. As a part of the license, EHB will supply the products to EHN for distribution and sale. EHN does not have control over the manufacturing process of the products, and thus, cannot guarantee a consistent supply of the product for the Canadian market.
The Endo-line of products are generally not patented domestically or abroad, and the legal protections afforded by common law and contractual proprietary rights in such products provide only limited protection and may be time-consuming and expensive to enforce or maintain. Further, despite the Company's efforts, it may be unable to prevent third parties from infringing upon or misappropriating its proprietary rights or from independently developing non-infringing products that are competitive with, equivalent to or superior to the Endo-line products.
Failure of Counterparties to Fulfill Obligations
The Company is party to various agreements with third parties related to supply, research and other matters. Parties to contracts do not always honour contractual terms and contracts themselves may be subject to interpretation or technical defects. To the extent counterparties do not abide by their contractual obligations, the Company would be forced to take legal action to enforce its contractual rights. Such litigation may be time consuming and costly and there is no guarantee of success. Any proceedings or actions or any decisions determined adversely to the Company, may have a material and adverse effect on the Company's profitability, results of operations and financial condition.
Difficulty to Forecast
The Company must rely largely on its own market research to forecast sales as detailed forecasts are not generally obtainable from other sources at this early stage of the legal cannabis industry in Canada. A failure in the demand for its products to materialize as a result of competition, technological change, regulatory burden or other factors could have a material adverse effect on the business, financial performance and financial condition of the Company.
Management of Growth
The Company may be subject to growth-related risks including capacity constraints and pressure on its internal systems and controls. The ability of the Company to manage growth effectively will require it to continue to implement and improve its operational and financial systems and to expand, train and manage its employee base. The inability of the Company to deal with this growth may have a material adverse effect on the Company's business, financial condition, financial performance and prospects.
Factors which may Prevent Realization of Growth Targets
The Company is currently in the early development stage and its growth strategy contemplates expanding its production facilities with additional production resources and constructing new growing facilities. There is a risk that such construction and expansion will not be achieved on time, on budget, or at all, as they can be adversely affected by a variety of factors, including some that are discussed elsewhere in these risk factors and the following:
| (a) | delays in obtaining, or conditions imposed by, regulatory approvals and licences including approvals from Health Canada and under local by-laws; |
| (c) | environmental pollution; |
| (d) | non-performance by third party contractors; |
| (e) | increases in materials or labour costs; |
| (f) | production falling below expected levels of output or efficiency; |
| (g) | breakdown, aging or failure of equipment or processes; |
| (h) | contractor or operator errors; |
| (i) | labour disputes, disruptions or declines in productivity; |
| (j) | inability to attract sufficient numbers of qualified workers; |
| (k) | disruption in the supply of energy and utilities; and |
| (l) | major incidents and/or catastrophic events such as fires, explosions, earthquakes or storms. |
As a result, there is a risk that the Company may not have product or sufficient product available for shipment to meet future demand when it arises. Failure to satisfy such future demand may have a material adverse effect on the Company's revenue and financial performance and may result in the loss of future customers and market share.
Unfavourable Publicity or Consumer Perception
The Company believes the cannabis industry is highly dependent upon consumer perception regarding the safety, efficacy and quality of the cannabis produced. Consumer perception of the Company's products can be significantly influenced by scientific research or findings, regulatory investigations, litigation, media attention and other publicity regarding the consumption of cannabis products. There can be no assurance that future scientific research, findings, regulatory proceedings, litigation, media attention or other research findings or publicity will be favourable to the cannabis market or any particular product, or consistent with earlier publicity. Future research reports, findings, regulatory proceedings, litigation, media attention or other publicity that are perceived as less favourable than, or that question, earlier research reports, findings or publicity could have a material adverse effect on the demand for the Company's products and the business, financial performance, financial condition and cash flows of the Company. The Company's dependence upon consumer perceptions means that adverse scientific research reports, findings, regulatory proceedings, litigation, media attention or other publicity, whether or not accurate or with merit, could have a material adverse effect on the Company, the demand for the Company's products, and the business, financial performance, financial condition and cash flows of the Company. Further, adverse publicity reports or other media attention regarding the safety, efficacy and quality of cannabis in general, or the Company's products specifically, or associating the consumption of cannabis with illness or other negative effects or events, could have such a material adverse effect. Such adverse publicity reports or other media attention could arise even if the adverse effects associated with such products resulted from consumers' failure to consume such products appropriately or as directed.
Damage to the Company's Reputation
Damage to the Company's reputation could be the result of the actual or perceived occurrence of any number of events, and could include any negative publicity, whether true or not. The increased usage of social media and other web-based tools used to generate, publish and discuss user-generated content and to connect with other users has made it increasingly easier for individuals and groups to communicate and share opinions and views in regard to the Company and its activities, whether true or not. Although the Company believes that it operates in a manner that is respectful to all stakeholders and that it takes care in protecting its image and reputation, the Company does not ultimately have direct control over how it is perceived by others. Reputational loss may result in decreased investor confidence, increased challenges in developing and maintaining community relations and an impediment to the Company's overall ability to advance its projects, thereby having a material adverse impact on financial performance, financial condition, cash flows, growth prospects and market price of the Company's securities.
Third Party Reputational Risk
The parties with which the Company does business may perceive that they are exposed to reputational risk as a result of the Company's cannabis business activities. This may impact the Company's ability to retain current partners, such as its banking relationship, or source future partners as required for growth or future expansion. Failure to establish or maintain such business relationships could have a material adverse effect on the Company.
Financial Losses
The Company has incurred losses in recent periods. The Company may not be able to achieve or maintain profitability and will continue to experience negative cash flow from operations in the foreseeable future. In addition, the Company expects to continue to increase operating expenses as it implements initiatives to continue to grow its business. If the Company's revenues do not increase to offset these expected increases in costs and operating expenses, the Company will not be profitable and this may jeopardize the Company’s ability to continue as a going concern.
Limited Operating History
The Company was incorporated in 2013 and has yet to generate significant revenue. The Company is therefore subject to many of the risks common to early-stage enterprises, including under-capitalization, cash shortages, limitations with respect to personnel, financial, and other resources and lack of revenues. There is no assurance that the Company will be successful in achieving a return on shareholders' investment and the likelihood of success must be considered in light of the early stage of the Company's operations.
Conflicts of Interest
The officers and directors of the Company may be engaged in a range of business activities and accordingly, the Company may be subject to potential conflicts of interest. Dr. Avtar Dhillon, Mr. Jim Heppell and Mr. Punit Dhillon, each of whom is a director of the Company, are also directors and/or officers of the Major Shareholder, a control person of the Company. In addition, Dr. Avtar Dhillon is also the chief executive officer and a principal securityholder of the Major Shareholder. The Company's executive officers and directors may also devote time to their outside business interests, so long as such activities do not materially or adversely interfere with their duties to the Company. In some cases, the Company's executive officers and directors may have fiduciary obligations associated with these business interests that interfere with their ability to devote time to the Company's business and affairs and that could adversely affect the Company's operations. These business interests could require significant time and attention of the Company's executive officers and directors and could adversely affect the Company's operations, business, financial condition and financial performance.
The Company has entered into various agreements with related parties of the Company, each of whom has some of the same directors and officers of the Company, including: a management agreement with the Major Shareholder pursuant to which the Major Shareholder provides certain management services to the Company in exchange for a monthly fee of $350,000 (the "Independent Contractor Agreement"); a hemp supply agreement with EHH, a subsidiary of the Major Shareholder; a research agreement with Emerald Health Biotechnology Espana S.L.U. (formerly, VivaCell Biotechnologies Spain S.L.U.) ("EH Spain"), a wholly owned subsidiary of the Major Shareholder; a lease agreement for the Richmond facility with a company owned by the Company's President, Dr. Avtar Dhillon; a supply and distribution agreement with Avricore Health Inc. (formerly, VANC Pharmaceuticals Inc.), a company whose Chief Executive Officer is a director of the Company; and the supply agreement with the Joint Venture. Additional information regarding these arrangements can be found in thedocuments which are incorporated herein by reference. See "Documents Incorporated by Reference". All such transactions were exempt from valuation and minority approval requirements.
In addition, the Company may become involved in other transactions which conflict with the interests of its directors and the officers who may from time to time deal with persons, firms, institutions or corporations with which the Company may be dealing, or which may be seeking investments similar to those desired by it. The interests of these persons could conflict with those of the Company. In addition, from time to time, these persons may be competing with the Company for available investment opportunities. Such conflicts could adversely affect the Company's operations, business, financial condition and financial performance.
Conflicts of interest, if any, will be subject to the procedures and remedies provided under applicable laws. In particular, in the event that such a conflict of interest arises at a meeting of the Company's directors, a director who has such a conflict is required to disclose his/her conflict and interest in the transaction and abstain from voting (where applicable) for or against the approval of such participation or such terms. In accordance with applicable laws, the directors of the Company are required to act honestly, in good faith and in the best interests of the Company.
Reliance on Management
The success of the Company is primarily dependent upon the ability, expertise, judgment, discretion and good faith of its senior management. While employment agreements are customarily used as a primary method of retaining the services of key employees, these agreements cannot assure the continued services of such employees indefinitely. Any loss of the services of any such individuals could have a material adverse effect on the Company's business, financial performance or financial condition. In addition, the Company has entered into the Independent Contractor Agreement pursuant to which the Major Shareholder provides certain management services to the Company in exchange for a monthly fee of $350,000. If such agreement were terminated, it may have a material adverse impact on the Company's business, financial performance or financial condition.
General Business Risk and Liability
Given the nature of Company's business, it may from time to time be subject to claims or complaints from investors or others in the normal course of business. The legal risks facing the Company and its directors, officers, employees or agents in this respect include potential liability for violations of securities laws, breach of fiduciary duty and misuse of investors' funds. Violations of securities laws and breaches of fiduciary duty could result in civil liability, fines, sanctions, or the suspension or revocation of the Company's right to carry on its existing business. The Company may incur significant costs in connection with such potential liabilities.
Operating Risk and Insurance Coverage
The Company has insurance to protect its assets, operations and employees. While the Company believes its insurance coverage addresses all material risks to which it is exposed and is adequate and customary in its current state of operations, such insurance is subject to coverage limits and exclusions and may not be available for all of the risks and hazards to which the Company is exposed. In addition, no assurance can be given that such insurance will be adequate to cover the Company's liabilities or will be generally available in the future or, if available, that premiums will be commercially justifiable. If the Company were to incur substantial liability and such damages were not covered by insurance or were in excess of policy limits, or if the Company were to incur such liability at a time when it is not able to obtain liability insurance, the business, financial performance and financial condition of the Company could be materially adversely affected.
Integration of Acquired Businesses
The Company has in the past acquired, directly or indirectly, interests in businesses complementary to the business of the Company and the Company may in the future make additional acquisitions. The success of such acquisitions will depend in part on successfully consolidating functions and integrating operations, procedures and personnel in a timely and efficient manner, as well as on the Company's ability to realize the anticipated growth opportunities and synergies, efficiencies and cost savings from integrating such businesses. This integration may require the dedication of substantial management effort, time and resources which may divert management's focus and resources from other strategic opportunities of the Company and from operational matters during this process. Any such integration processes may result in the loss of key employees and the disruption of ongoing business and employee relationships that may adversely affect the Company's ability to achieve the anticipated benefits of any such acquisitions.
Future Litigation
The Company may become party to litigation (including arbitration or mediation) from time to time, which could adversely affect its business. Should any litigation in which the Company becomes involved be determined against the Company or should the Company enter into a settlement, the amount of the award or settlement could adversely affect the Company's resources and its ability to continue operating and the market price for the Common Shares. Monitoring and defending litigation, whether or not meritorious, can be time consuming and may result in significant expenses, including legal fees and other costs. Even if the Company is involved in litigation and is successful, litigation can redirect significant Company resources and attention away from the business of the Company and may have a material adverse effect on the Company's business, reputation, financial condition, financial performance and financial prospects.
Securities class action litigation often has been brought against companies following periods of volatility in the market price of their securities. The Company may in the future be the target of similar litigation. Securities litigation could result in substantial costs and damages and divert management's attention and resources.
Information Systems Security Threats
The Company has entered into agreements with third parties for hardware, software, telecommunications and other information technology ("IT") services in connection with its operations. The Company's operations depend, in part, on how well it and its suppliers protect networks, equipment, IT systems and software against damage from a number of threats, including, but not limited to, cable cuts, damage to physical plants, natural disasters, terrorism, fire, power loss, hacking, computer viruses, vandalism and theft. The Company's operations also depend on the timely maintenance, upgrade and replacement of networks, equipment, IT systems and software, as well as pre-emptive expenses to mitigate the risks of failures. Any of these and other events could result in information system failures, delays and/or increase in capital expenses. The failure of information systems or a component of information systems could, depending on the nature of any such failure, adversely impact the Company's reputation and financial performance.
The Company has not experienced any material losses to date relating to cyber-attacks or other information security breaches, but there can be no assurance that the Company will not incur such losses in the future. The Company's risk and exposure to these matters cannot be fully mitigated because of, among other things, the evolving nature of these threats. As a result, cyber security and the continued development and enhancement of controls, processes and practices designed to protect systems, computers, software, data and networks from attack, damage or unauthorized access is a priority. As cyber threats continue to evolve, the Company may be required to expend additional resources to continue to modify or enhance protective measures or to investigate and remediate any security vulnerabilities.
Furthermore, there are a number of federal and provincial laws protecting the confidentiality of certain patient health information, including patient records, and restricting the use and disclosure of that protected information. In particular, the privacy rules under thePersonal Information Protection and Electronics Documents Act (Canada) ("PIPEDA"), protect medical records and other personal health information by limiting their use and disclosure to the minimum level reasonably necessary to accomplish the intended purpose. If the Company was found to be in violation of the privacy or security rules under PIPEDA or other laws protecting the confidentiality of patient health information, it could be subject to sanctions and civil or criminal penalties, which could increase its liabilities, harm its reputation and have a material adverse effect on the business, results of operations and financial condition of the Company.
Environmental and Employee Health and Safety Regulations
The Company's operations are subject to environmental and safety laws and regulations concerning, among other things, emissions and discharges to water, air and land, the handling and disposal of hazardous and non-hazardous materials and wastes, and employee health and safety. The Company will incur ongoing costs and obligations related to compliance with environmental and employee health and safety matters. Governmental approvals and permits are currently, and may in the future be, required in connection with the Company's operations. Failure to comply with environmental and safety laws and regulations may result in additional costs for corrective measures, penalties or in restrictions on the Company's manufacturing operations. In addition, changes in environmental, employee health and safety or other laws, more vigorous enforcement thereof or other unanticipated events could require extensive changes to the Company's operations or give rise to material liabilities, which could have a material adverse effect on the business, financial performance and financial condition of the Company.
TSXV Restrictions on Business
The Company has delivered an undertaking to the TSXV confirming that, while listed on the TSXV, the Company will only conduct the business of the direct and indirect production, sale, extraction and distribution of cannabis and its extracts and derivatives in Canada, pursuant to one or more licences issued by Health Canada in accordance with applicable Canadian law, unless prior approval is obtained from the TSXV. This undertaking could have an adverse effect on the Company's ability to expand its business into other areas when the Company's competitors have no such restrictions. All such restrictions could materially and adversely affect the growth, business, financial condition and financial performance of the Company.
Restrictions on Sales Activities
The legal cannabis industry in Canada is in its early development state and restrictions on sales and marketing activities imposed by Health Canada, various medical associations, other governmental or quasi-governmental bodies or voluntary industry associations may adversely affect the Company's ability to conduct sales and marketing activities and could have a material adverse effect on the Company's business, financial performance or financial condition.
The Market Price of the Common Shares May be Subject to Wide Price Fluctuations
The market price of the Common Shares may be subject to wide fluctuations in response to many factors, including variations in the financial performance of the Company, divergence in financial results from analysts' expectations, changes in earnings estimates by stock market analysts, changes in the business prospects for the Company, general economic conditions, legislative changes, possible efforts by short sellers to drive down the market price of the Common Shares (see "Effect of Short Sales" below) and other events and factors outside of the Company's control. In addition, stock markets have from time to time experienced extreme price and volume fluctuations, which, as well as general economic and political conditions, could adversely affect the market price for the Common Shares.
The market price of the Common Shares and the common shares of other companies that investors may consider to be comparable to the Company have experienced significant price and volume fluctuations recently. In particular, the market price of such shares are impacted by news reports relating to competitive developments, market prices of competitor stocks, regulatory changes and other related issues in the legal cannabis industry, including the Cannabis Act.
Limited Market for Common Shares
The Company is listed on the TSXV, however, there can be no assurance that an active and liquid market for the Common Shares will develop or be maintained and an investor may find it difficult to resell Common Shares.
Effect of Short Sales
Any downward pressure on the price of Common Shares could encourage short sales by third parties. In a short sale, a prospective seller borrows shares from a shareholder or broker and sells the borrowed shares. The prospective seller anticipates that the share price will decline, at which time the seller can purchase shares at a lower price for delivery back to a lender. The seller profits when the share price declines because it is purchasing shares at a price lower than the sale price of the borrowed shares. For the Company, short sales of Common Shares could place downward pressure on the market price of the Common Shares by increasing the number of Common Shares being sold, which could lead to a decline in the market price of the Common Shares.
As it is in the short seller's interest for the market price to decline, some short sellers publish, or arrange for the publication of, opinions or characterizations regarding the relevant issuer, its business practices and prospects and similar matters calculated to or which may create negative market momentum, which may permit them to obtain profits for themselves as a result of selling the shares short. Issuers whose securities have historically had limited trading volumes and/or have been susceptible to relatively high volatility levels can be particularly vulnerable to such short seller attacks. In such a case, the issuer may have very little recourse against the short seller. The publication of any such commentary regarding the Company in the future may bring about a temporary, or possibly long term, decline in the market price of the Common Shares. No assurances can be made that declines in the market price of the Common Shares will not occur in the future, in connection with such commentary by short sellers or otherwise. When the market price of a company's stock drops significantly, it is not unusual for shareholder lawsuits to be filed or threatened against the company and its board of directors and for the company to suffer reputational damage. Such lawsuits could cause the Company to incur substantial costs and divert the time and attention of the Company's board of directors and management. Reputational damage may also affect the Company's ability to maintain and develop business relationships, which could likewise adversely affect the Company's earnings. Negative reports issued by short sellers could also negatively impact the Company's ability to attract and retain employees.
A Substantial Number of Common Shares are Owned by the Major Shareholder
A significant percentage of the Company's outstanding Common Shares are owned by a single shareholder, the Major Shareholder, who is a "control person" under applicable Canadian securities laws. For more information, see "Conflicts of Interest" above. As such, the Major Shareholder is in a position to exercise influence over matters requiring shareholder approval, including: the determination of significant corporate actions that could otherwise be beneficial to the Company's other shareholders; the election and removal of directors; amendments to the Company's corporate governing documents; andbusiness combinations. The Company's interests and those of the Major Shareholder may at times conflict. The concentration of control by a single shareholder may practically preclude an unsolicited take-over bid for the Common Shares, and this may adversely impact the value and trading price of the Common Shares.
Dividends
The Company has no earnings or dividend record and does not anticipate paying any dividends on the Common Shares in the foreseeable future. The declaration and payment of dividends is subject to approval by the Company's board of directors in accordance with, among other things, applicable corporate law. Any dividends paid by the Company would be subject to applicable tax and, potentially, withholdings.
Actions against the Company and its Directors and Officers
The Company and its subsidiaries are corporations organized under the laws of the Province of British Columbia or other Canadian jurisdictions. Certain of the Company's directors and officers reside principally in Canada. Because all or a substantial portion of the Company's assets and the assets of these persons are located in Canada, it may not be possible for foreign investors to effect service of process from outside of Canada upon the Company or those persons. Furthermore, it may not be possible to enforce against the Company foreign judgments obtained in courts outside of Canada based upon the civil liability provisions of the securities laws or other laws in those jurisdictions.
Holding Company
The Company is a holding company and essentially all of its assets are shares of its subsidiaries and the Joint Venture. As a result, investors in the Company are subject to the risks attributable to its subsidiaries and the Joint Venture. As a holding company, the Company conducts substantially all of its active business through its subsidiaries and the Joint Venture, which together generate substantially all of its revenues. Consequently, the Company's cash flows and ability to complete current or desirable future enhancement opportunities are dependent on the earnings of its subsidiaries and the Joint Venture and the distribution of those earnings to the Company. The ability of its subsidiaries and the Joint Venture to pay dividends and other distributions will depend on their financial performance and will be subject to applicable laws and regulations which require that solvency and capital standards be maintained and contractual restrictions contained in the instruments governing its debt. In the event of a bankruptcy, liquidation or reorganization of a subsidiary or the Joint Venture, holders of indebtedness and trade creditors may be entitled to payment of their claims from the assets of such entity before the Company.
CONSOLIDATED CAPITALIZATION
The following table sets forth the Company's cash, indebtedness and shareholders' equity as of September 30, 2018 on an actual basis. This table should be read in conjunction with the Interim Financial Statements and Interim MD&A.
| Description of Capital | | As at September 30, 2018 | |
| Cash | | | 52,905,302 | |
| Indebtedness | | | 2,536,758 | |
| Number Common shares outstanding | | | 136,850,193 | |
| Number of Warrants outstanding | | | 8,411,764 | |
| Shareholders' Equity | | | | |
| Share Capital | | | 193,262,959 | |
| Warrants | | | 4,360,000 | |
| Contributed Surplus | | | 13,235,310 | |
| Accumulated other comprehensive loss | | | - | |
| Accumulated Deficit | | | (38,955,587 | ) |
| Total Shareholders' Equity | | | 171,902,682 | |
There have been no material changes in the share and loan capital of the Company on a consolidated basis, since September 30, 2018, except that, subsequent to such date, the Company issued 4,000,000 Common Shares to the Investor pursuant to the December 2018 Offering for gross aggregate proceeds of $10.8 million and a total of 2,006,243 share options previously issued to employees, directors and consultants were exercised by the holders thereof for aggregate proceeds of $1,358,249. In addition, subsequent to September 30, 2018, the Company’s cash and cash equivalents have changed to approximately $25 million as at March 1, 2019 as a result of the Company’s operating, investment and other growth-related activities, including payments for costs for the completion of its capital projects, for research and development, working capital and general corporate purposes.
USE OF PROCEEDS
The net proceeds from the Offering are not determinable in light of the nature of the distribution. The net proceeds of any given distribution of Offered Shares through the Agent in an "at-the-market distribution" will represent the gross proceeds after deducting the applicable compensation payable to the Agent under the Equity Distribution Agreement and the expenses of the distribution.
The Company currently intends to use the net proceeds from the Offering, if any, to fund a portion of the remaining payments to the vendors in connection with the acquisition of Verdélite and Verdélite Holdings; the costs for the completion of its capital projects; and for research and development; and working capital and general corporate purposes.
Although the Company intends to use the net proceeds from the Offering as set forth above, the actual allocation of the net proceeds may vary from those allocations set out above, depending on future developments in the Company's business operations or unforeseen events, including those listed under the "Risk Factors" section of this Prospectus Supplement and the Prospectus. For example, the Company had negative operating cash flow for the fiscal year ended December 31, 2017 and the nine month period ended September 30, 2018. The Company’s cash and cash equivalents total approximately $25 million as at March 1, 2019. To the extent the Company has negative cash flows in future periods, the Company may use a portion of its general working capital to fund such negative cash flow. Prospective purchasers are cautioned that, notwithstanding the Company's current intentions regarding the use of the net proceeds of the Offering, there may be circumstances where a reallocation of the net proceeds may be advisable for reasons that management believes, in its discretion, are in the Company's best interests.
PRIOR SALES
Common Shares
The following table summarizes details of the Common Shares issued by the Company during the 12-month period prior to the date of this Prospectus Supplement:
| Date of Issuance | | Price Per Common Share | | Number of Common Shares |
| May 1, 2018 | | $4.54(3) | | 9,911,894 |
| May 22, 2018 | | $4.20 | | 4,000,000 |
| May 31, 2018 | | $0.55(1) | | 40,000 |
| June 21, 2018 | | $0.55(1) | | 40,000 |
| August 2, 2018 | | $0.41(1) | | 10,000 |
| August 2, 2018 | | $0.55(1) | | 30,000 |
| August 2-3, 2018 | | $0.72(1) | | 60,000 |
| August 14, 2018 | | $3.66(4) | | 1,093,938 |
| August 16, 2018 | | $1.22(1) | | 5,555 |
| August 30, 2018 | | $0.72(1) | | 50,000 |
| September 7, 2018 | | $0.72(1) | | 15,000 |
| September 11, 2018 | | $0.72(1) | | 50,000 |
| September 14, 2018 | | $0.72(1) | | 2,500 |
| September 20, 2018 | | $1.42(1) | | 694 |
| September 21, 2018 | | $0.40(1) | | 50,000 |
| September 21, 2018 | | $1.22(1) | | 5,000 |
| October 3, 2018 | | $1.16(1) | | 7,780 |
| October 10, 2018 | | $0.40(1) | | 50,000 |
| October 10, 2018 | | $1.22(1) | | 7,500 |
| October 16, 2018 | | $4.25(1) | | 3,750 |
| October 17, 2018 | | $1.47(1) | | 25,000 |
| October 17, 2018 | | $0.72(1) | | 50,000 |
| October 18, 2018 | | $1.42(1) | | 3,470 |
| October 22, 2018 | | $1.19(1) | | 37,500 |
| October 24, 2018 | | $0.40(1) | | 75,000 |
| October 24, 2018 | | $1.22(1) | | 4,444 |
| October 24, 2018 | | $0.72(1) | | 6,951 |
| November 1, 2018 | | $1.22(1) | | 5,000 |
| November 1, 2018 | | $0.72(1) | | 26,528 |
| Date of Issuance | | Price Per Common Share | | Number of Common Shares |
| November 9, 2018 | | $0.40(1) | | 40,000 |
| December 5, 2018 | | $1.29(1) | | 250,000 |
| December 10, 2018 | | $2.70 | | 4,000,000 |
| January 3, 2019 | | $0.72(1) | | 25,000 |
| January 10, 2019 | | $1.51(1) | | 50,000 |
| January 17, 2019 | | $0.18(1) | | 49,992 |
| January 17, 2019 | | $0.72(1) | | 24,996 |
| January 17, 2019 | | $0.00(5) | | 355,000 |
| January 24, 2019 | | $1.38(1) | | 9,033 |
| January 28, 2019 | | $0.40(1) | | 50,000 |
| January 28, 2019 | | $0.72(1) | | 130,000 |
| January 28, 2019 | | $1.22(1) | | 80,000 |
| January 29, 2019 | | $1.22(1) | | 30,000 |
| January 30, 2019 | | $0.40(1) | | 60,000 |
| January 30, 2019 | | $0.72(1) | | 62,509 |
| February 6, 2019 | | $0.40(1) | | 50,000 |
| February 7, 2019 | | $0.18(1) | | 75,003 |
| February 11, 2019 | | $1.22(1) | | 8,333 |
| February 21, 2019 | | $0.72(1) | | 37,513 |
| February 21, 2019 | | $0.40(1) | | 50,000 |
| February 21, 2019 | | $1.27(1) | | 50,000 |
| February 25, 2019 | | $1.22(1) | | 5,000 |
| March 1, 2019 | | $1.22(1) | | 5,000 |
| March 5, 2019 | | $0.72(1) | | 13,330 |
| March 11, 2019 | | $1.22(1) | | 47,222 |
| March 13, 2019 | | $0.40(1) | | 50,000 |
| March 18, 2019 | | $0.72(1) | | 5,389 |
| March 18, 2019 | | $1.38(1) | | 20,000 |
| March 20, 2019 | | $0.72(1) | | 5,000 |
| March 20, 2019 | | $1.38(1) | | 10,000 |
| March 20, 2019 | | $1.22(1) | | 5,000 |
| March 20, 2019 | | $0.40(1) | | 50,000 |
___________
Notes:
| (1) | Issued for cash pursuant to the exercise of stock options. |
| (2) | Issued for cash pursuant to the exercise of common share purchase warrants. |
| (3) | Issued to satisfy 50% of the purchase price of Verdélite, of which 4,955,947 will be held in escrow until May 1, 2019. |
| (4) | Issued as partial payment for the purchase price for the remaining shares of Avalite. |
| (5) | Issued upon vesting of restricted share units of the Company. |
Warrants
The following table summarizes details of the common share purchase warrants issued by the Company during the 12-month period prior to the date of this Prospectus Supplement:
| Date of Issuance | | Number of Warrants Issued | | Exercise Price | | Expiry Date |
| May 22, 2018 | | 4,000,000 | | $5.20 | | November 22, 2019 |
As of the date hereof, there are 8,411,761 warrants of the Company outstanding.
Options
The following table summarizes details of the stock options issued by the Company during the 12-month period prior to the date of this Prospectus Supplement:
| Date of Grant | | Number of Options Granted | | Exercise Price | | Expiry Date |
| March 19, 2018 | | 25,000 | | $5.85 | | March 19, 2023 |
| March 26, 2018 | | 150,000 | | $5.69 | | March 26, 2023 |
| April 6, 2018 | | 50,000 | | $4.55 | | April 6, 2023 |
| May 22, 2018 | | 15,000 | | $4.70 | | May 22, 2023 |
| May 28, 2018 | | 15,000 | | $4.21 | | May 28, 2023 |
| June 11, 2018 | | 190,000 | | $4.27 | | June 11, 2023 |
| June 20, 2018 | | 50,000 | | $4.08 | | June 20, 2023 |
| June 28, 2018 | | 10,000 | | $3.69 | | June 28, 2023 |
| July 3, 2018 | | 15,000 | | $3.70 | | July 3, 2023 |
| July 9, 2018 | | 30,000 | | $3.58 | | July 9, 2023 |
| July 18, 2018 | | 15,000 | | $2.49 | | July 18, 2023 |
| July 23, 2018 | | 12,500 | | $2.59 | | July 23, 2023 |
| August 7, 2018 | | 60,000 | | $2.72 | | August 7, 2023 |
| August 20, 2018 | | 625,000 | | $3.21 | | August 20, 2023 |
| August 23, 2018 | | 25,000 | | $4.30 | | August 23, 2023 |
| September 1, 2018 | | 185,000 | | $4.58 | | September 1, 2023 |
| September 10, 2018 | | 25,000 | | $5.20 | | September 10, 2023 |
| October 1, 2018 | | 150,000 | | $4.60 | | October 1, 2023 |
| October 29, 2018 | | 10,000 | | $3.40 | | October 29, 2023 |
| November 1, 2018 | | 161,000 | | $3.88 | | November 1, 2023 |
| December 1, 2018 | | 472,500 | | $3.07 | | December 1, 2023 |
| January 1, 2019 | | 88,000 | | $2.83 | | January 1, 2024 |
| February 1, 2019 | | 160,000 | | $3.48 | | February 1, 2024 |
| March 1, 2019 | | 5,000 | | $3.88 | | March 1, 2024 |
| March 4, 2019 | | 200,000 | | $3.84 | | March 4, 2024 |
As of the date hereof, there are 8,837,213 stock options of the Company outstanding.
Restricted Share Units
Restricted share units of the Company ("RSUs") will be settled by the issuance of Common Shares on the vesting date. No RSUs have been issuedby the Company during the 12-month period prior to the date of this Prospectus.
As of the date hereof, there are 475,000 RSUsoutstanding.
TRADING PRICE AND VOLUME
The Common Shares trade on the TSXV under the symbol "EMH". On March 26, 2019, the last trading day completed prior to the filing of this Prospectus Supplement, the closing price of the Common Shares on the TSXV was $4.19. The price range and trading volume of the Common Shares for each month from March 2018 to March 2019, as reported by the TSXV, are set out below:
| Month | | High | | Low | | Total Volume |
| March 1 - 26, 2019 | | $4.66 | | $3.42 | | 15,875,809 |
| February 2019 | | $4.27 | | $3.20 | | 24,435,249 |
| January 2019 | | $3.75 | | $2.68 | | 18,004,745 |
| December 2018 | | $3.10 | | $2.55 | | 3,316,455 |
| November 2018 | | $5.25 | | $3.00 | | 16,887,393 |
| October 2018 | | $5.24 | | $2.87 | | 20,496,651 |
| September 2018 | | $5.92 | | $4.24 | | 31,678,367 |
| August 2018 | | $4.96 | | $2.55 | | 32,355,196 |
| July 2018 | | $3.73 | | $2.44 | | 21,607,262 |
| June 2018 | | $4.50 | | $3.52 | | 13,609,070 |
| May 2018 | | $4.95 | | $4.02 | | 11,040,707 |
| April 2018 | | $5.28 | | $3.69 | | 17,499,500 |
| March 2018 | | $6.35 | | $4.81 | | 20,884,300 |
DESCRIPTION OF SECURITIES BEING DISTRIBUTED
The authorized capital of the Company consists of an unlimited number of Common Shares. As at September 30, 2018, there were 136,850,193 Common Shares issued and outstanding. As at the date of this Prospectus Supplement, there were 142,856,436 Common Shares issued and outstanding.
The Offered Shares have all of the characteristics, rights and restrictions of the Common Shares. The holders of Common Shares are entitled to receive notice of and to attend all annual and special meetings of the Company's shareholders and to one vote in respect of each Common Share held at the record date for each such meeting. The holders of Common Shares are entitled, at the discretion of the board of directors of the Company, to receive out of any or all of the Company's profits or surplus properly available for the payment of dividends, any dividend declared by the board of directors of the Company and payable by the Company on the Common Shares. The holders of Common Shares will participate pro rata in any distribution of the assets of the Company upon liquidation, dissolution or winding-up or other distribution of the assets of the Company. Such participation will be subject to the rights, privileges, restrictions and conditions attached to any of the Company's securities issued and outstanding at such time ranking in priority to the Common Shares upon the liquidation, dissolution or winding-up of the Company. Common Shares are issued only as fully paid and are non-assessable.
PLAN OF DISTRIBUTION
Sale of Offered Shares, if any, will be made in transactions that are deemed to be "at-the-market" distributions as defined in NI 44-102, involving sales made directly on the TSXV or any other Marketplace. Subject to the terms and conditions of the Equity Distribution Agreement and upon instructions from the Company, the Agent will use its commercially reasonable efforts, consistent with its normal trading and sales practices, applicable laws, the terms of the ATM Decision (as defined below) and the applicable rules of the TSXV and any other Marketplace, to sell Offered Shares in accordance with the parameters specified by the Company. No offers or sales will be made pursuant to this Prospectus Supplement in the United States or on any United States securities exchange or inter-dealer quotation system. The Offered Shares will be distributed at market prices prevailing at the time of the sale or at prices to be negotiated with purchasers of the Offered Shares at the time of sale. As a result, prices may vary as between purchasers and during the period of distribution.
The Company will instruct the Agent as to the number of Offered Shares to be sold by the Agent from time to time by sending the Agent a notice (a "Placement Notice") that requests that the Agent sell up to a specified dollar amount or a specified number of Offered Shares and specifies any parameters in accordance with which the Company requires that the Offered Shares be sold. The parameters set forth in the Placement Notice may not conflict with the provisions of the Equity Distribution Agreement. Under the ATM Decision, the number of Offered Shares sold pursuant to an at-the-market distribution on any trading day will not exceed 25% of the total trading volume of the Common Shares on the TSXV and on any other Marketplace on that day. The aggregate market value of all Offered Shares sold in at-the-market distributions under the Equity Distribution Agreement will not exceed 10% of the aggregate market value of the then outstanding Common Shares calculated in accordance with section 9.2 of NI 44-102 as at the last trading day of the month before the month in which the first trade under the at-the-market distribution is made. See "Exemptions". The Company or the Agent may suspend the offering of Offered Shares upon proper notice and subject to the terms and conditions set forth in the Equity Distribution Agreement. A copy of the Equity Distribution Agreement is available electronically under the Company's profile at www.sedar.com.
The Company has applied to list on the TSXV the Offered Shares qualified hereunder and has received conditional approval for such listing. The listing of the Offered Shares will be subject to the Company fulfilling the listing requirements of the TSXV.
The Company will pay the Agent for its services in acting as agent in the sale of Offered Shares, under the terms of the Equity Distribution Agreement, compensation equal to 2.5% of the gross proceeds from the sales of Offered Shares made under the Equity Distribution Agreement. The Company estimates that the total expenses that it will incur for the Offering (including fees payable to stock exchanges, securities regulatory authorities, its counsel and its auditors, but excluding compensation payable to the Agent under the terms of the Equity Distribution Agreement) will be approximately $430,000. Settlement for sales of Offered Shares will occur on the second business day following the date on which any sales are made, or on such earlier date as is agreed by the Company and the Agent to be industry practice for regular-way trading, in return for payment of the net proceeds to the Company.
No underwriter or dealer involved in the Offering, no affiliate of such an underwriter or dealer, and no person or company acting jointly or in concert with such an underwriter or dealer may over-allot Common Shares in connection with the Offering or effect any other transactions that are intended to stabilize or maintain the market price of the Common Shares.
In connection with the sale of the Offered Shares on behalf of the Company, the Agent will be an underwriter as defined in applicable securities legislation in Canada, and the compensation of the Agent will be deemed to be underwriting commissions or discounts. The Company has agreed to provide indemnification and contribution to the Agent against, among other things, certain civil liabilities, including liabilities under applicable securities legislation in Canada.
Exemptions
Pursuant to a decision document dated March 19, 2019 issued by the British Columbia Securities Commission (as principal regulator on behalf of the securities regulatory authorities in each of the Provinces of Canada) and the Ontario Securities Commission (the "ATM Decision"): (a) the Agent and any other registered investment dealer acting as selling agent for the Agent are exempt from the requirement under securities legislation in each of the provinces of Canada to send or deliver to a purchaser of Offered Shares under the Offering the latest prospectus (including any prospectus supplement) and any amendment thereto and, as a result, the withdrawal right and the right of action under applicable securities laws for non-delivery of the Prospectus, as supplemented, will not apply to the Offering; and (b) the Corporation is exempt from: (i) the requirement to include in this Prospectus Supplement the form of issuer and underwriter certificate prescribed by sections 2.1 and 2.2 of Appendix A of NI 44-102 provided that, among other things, the certificates in the form set out in the decision referred to above is included in this Prospectus Supplement; (ii) the requirement to include in the Prospectus the statements specified by items 2 and 3 of section 5.5 of NI 44-102, provided that modified statements are included in the Prospectus; and (iii) the requirement to include in this Prospectus Supplement the statement respecting purchasers' statutory rights of withdrawal and remedies for rescission and damages prescribed by Item 20 of Form 44-101F1 -Short Form Prospectus, provided that, among other things, the disclosure set out under the heading "Statutory Rights of Withdrawal and Rescission" is included herein.
The ATM Decision also requires, among other things set out therein, that the Company disclose the number and average selling price of Offered Shares sold, as well as the gross proceeds, commission and net proceeds from sales hereunder in: (i) a report to be filed on SEDAR within seven calendar days after the end of each calendar month during which Offered Shares are sold pursuant to the Offering; and (ii) its annual and interim financial statements and management's discussion and analysis filed on SEDAR.
LEGALMATTERS
Certain legal matters in connection with the Offering will be passed upon on behalf of the Company by Bennett Jones LLP, counsel to the Company, and on behalf of the Agent by Blake, Cassels & Graydon LLP, counsel to the Agent.
As of the date of this Prospectus Supplement, the partners and associates of Bennett Jones LLP and Blake, Cassels & Graydon LLP beneficially own, directly or indirectly, in the aggregate less than 1% of the issued and outstanding Common Shares.
AUDITOR, TRANSFER AGENT AND REGISTRAR
Deloitte LLP is the auditor of the Company. Deloitte LLP is independent within the meaning of the Rules of Professional Conduct of the Chartered Professional Accountants of British Columbia.
The transfer agent and registrar for the Common Shares is Computershare Trust Company of Canada at its principal transfer offices in Vancouver, British Columbia.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
The Company has filed with the SEC a registration statement on Form F-10 relating to certain of its securities, including the Common Shares. This Prospectus Supplement and the accompanying Prospectus, which constitute a part of the registration statement, do not contain all of the information contained in the registration statement, certain items of which are contained in the exhibits to the registration statement as permitted by the rules and regulations of the SEC. Statements included or incorporated by reference in this Prospectus Supplement and the accompanying Prospectus about the contents of any contract, agreement or other documents referred to are not necessarily complete, and in each instance you should refer to the exhibits for a more complete description of the matter involved. Each such statement is qualified in its entirety by such reference.
The Company is subject to the information requirements of the U.S. Exchange Act and applicable Canadian securities legislation and, in accordance therewith, files reports and other information with the SEC and with the securities regulators in Canada. Under the multi-jurisdictional disclosure system adopted by the United States and Canada, documents and other information that the Company files with the SEC may be prepared in accordance with the disclosure requirements of Canada, which are different from those of the United States. As a foreign private issuer within the meaning of rules under the U.S. Exchange Act, the Company is exempt from the rules under the U.S. Exchange Act prescribing the furnishing and content of proxy statements, and the Company’s officers, directors and principal shareholders are exempt from the reporting and short swing profit recovery provisions contained in Section 16 of the U.S. Exchange Act. In addition, the Company is not required to publish financial statements as promptly as United States companies.
You may read any document that the Company has filed with the SEC at the SEC's public reference room in Washington, D.C. You may also obtain copies of those documents from the public reference room of the SEC at 100 F Street, N.E., Washington, D.C. 20549 by paying a fee. You should call the SEC at 1 800 SEC 0330 or access its website at www.sec.gov for further information about the public reference rooms. You may read and download some of the documents that the Company has filed with the SEC's EDGAR system at www.sec.gov. You may read and download any public document that the Company has filed with the Canadian securities regulatory authorities under the Company’s profile on the SEDAR website at www.sedar.com.
Base Shelf Prospectus
Prospectus Dated March 13, 2019

EMERALD HEALTH THERAPEUTICS, INC.
$150,000,000
Common Shares
Preferred Shares
Warrants
Subscription Receipts
Units
Debt Securities
This short form base shelf prospectus (the "Prospectus") relates to the offering for sale by Emerald Health Therapeutics, Inc. (the "Company") from time to time, during the 25-month period that this Prospectus, including any amendments hereto, remains valid, of up to $150,000,000 (or the equivalent in other currencies based on the applicable exchange rate at the time of the offering) in the aggregate of: (i) common shares ("Common Shares") in the capital of the Company; (ii) preferred shares ("Preferred Shares") in the capital of the Company, issuable in series; (iii) warrants ("Warrants") of the Company to purchase other Securities (as defined below); (iv) subscription receipts ("Subscription Receipts") of the Company convertible into other Securities; (v) units ("Units") of the Company comprised of one or more of any of the other Securities, or any combination of such Securities; and (vi) debt securities ("Debt Securities") of the Company, issuable in series (the Common Shares, Preferred Shares, Warrants, Subscription Receipts, Units and Debt Securities are collectively referred to herein as the "Securities"). The Securities may be offered in amounts, at prices and on terms to be determined based on market conditions at the time of sale and set forth in an accompanying prospectus supplement (each, a "Prospectus Supplement"). In addition, the Securities may be offered and issued in consideration for the acquisition of other businesses, assets or securities by the Company or one of its subsidiaries. The consideration for any such acquisition may consist of Securities separately, a combination of Securities or any combination of, among other things, Securities, cash and assumption of liabilities. One or more securityholders (each, a "Selling Securityholder") of the Company may also offer and sell Securities under this Prospectus. See "Selling Securityholders".
The Company is filing this Prospectus in connection with the concurrent filing of a Registration Statement (as defined below) in the United States.
The specific terms of any Securities offered will be described in the applicable Prospectus Supplement including, where applicable: (i) in the case of Common Shares, the number of Common Shares offered, the offering price, whether the Common Shares are being offered for cash, the person offering the Common Shares (the Company and/or one or more Selling Securityholders) and any other terms specific to the Common Shares; (ii) in the case of Preferred Shares, the designation of the particular series, the number of Preferred Shares offered, the offering price, any voting rights, any rights to receive dividends, any terms of redemption, any conversion or exchange rights, the person offering the Preferred Shares (the Company and/or one or more Selling Securityholders) and any other specific terms; (iii)in the case of Warrants, the number of Warrants being offered, the offering price, whether the Warrants are being offered for cash, the designation, number and terms of the other Securities purchasable upon exercise of the Warrants, and any procedures that will result in the adjustment of those numbers, the exercise price, the dates and periods of exercise, the person offering the Warrants (the Company and/or one or more Selling Securityholders) and any other terms specific to the Warrants being offered; (iv) in the case of Subscription Receipts, the number of Subscription Receipts being offered, the offering price, whether the Subscription Receipts are being offered for cash, the terms, conditions and procedures for the conversion of the Subscription Receipts into other Securities, the designation, number and terms of such other Securities, the person offering the Subscription Receipts (the Company and/or one or more Selling Securityholders), and any other terms specific to the Subscription Receipts; (v) in the case of Units, the number of Units being offered, the offering price, the number and terms of the Securities comprising the Units, the person offering the Units (the Company and/or one or more Selling Securityholders), and any other term specific to the Units; and (vi) in the case of Debt Securities, the specific designation of the particular series, aggregate principal amount, the currency or the currency unit for which the Debt Securities may be purchased, the maturity, interest provisions, authorized denominations, offering price covenants, events of default, any terms for redemption or retraction, any exchange or conversion terms, whether the debt is senior or subordinated and any other terms specific to the Debt Securities being offered. A Prospectus Supplement relating to a particular offering of Securities may include terms pertaining to the Securities being offered thereunder that are not within the terms and parameters described in this Prospectus. Where required by statute, regulation or policy, and where the Securities are offered in currencies other than Canadian dollars, appropriate disclosure of foreign exchange rates applicable to the Securities will be included in the Prospectus Supplement describing the Securities.
All shelf information permitted under applicable laws to be omitted from this Prospectus will be contained in one or more Prospectus Supplements that will be delivered to purchasers together with this Prospectus except in cases where an exemption from such delivery has been obtained. Each Prospectus Supplement will be incorporated by reference into this Prospectus for the purposes of securities legislation as of the date of the Prospectus Supplement and only for the purposes of the distribution of the Securities to which the Prospectus Supplement pertains.
The Company and the Selling Securityholder(s) may offer and sell the Securities to or through underwriters or dealers purchasing as principals and may also sell directly to one or more purchasers or through agents or pursuant to applicable statutory exemptions. See "Plan of Distribution". The Prospectus Supplement relating to a particular offering of Securities will identify, if applicable, each underwriter, dealer or agent, as the case may be, engaged by the Company or the Selling Securityholder(s) in connection with the offering and sale of the Securities, and will set forth the terms of the offering of such Securities, including, to the extent applicable, any fees, discounts or any other compensation payable to underwriters, dealers or agents in connection with the offering, the method of distribution of the Securities, the identity of the Selling Securityholder(s), if any, the initial issue price (in the event that the offering is a fixed price distribution), the proceeds that the Company or the Selling Securityholder(s) will, or expects to receive and any other material terms of the plan of distribution.
The Securities may be sold from time to time in one or more transactions at a fixed price or prices or at non-fixed prices, including sales in transactions that are deemed to be "at-the-market distributions" as defined in National Instrument 44-102 -Shelf Distributions, including sales made directly on the TSX Venture Exchange (the "Exchange") or other existing trading markets for the Common Shares. If offered on a non-fixed price basis, the Securities may be offered at market prices prevailing at the time of sale, at prices determined by reference to the prevailing price of a specified Security in a specified market or at prices to be negotiated with purchasers. If offered on a non-fixed price basis, the compensation payable to an underwriter, dealer or agent in connection, if applicable, with any such sale will be decreased by the amount, if any, by which the aggregate price paid for Securities by the purchasers is less than the gross proceeds paid by the underwriter, dealer or agent to the Company. The price at which the Securities will be offered and sold may vary from purchaser to purchaser and during the period of distribution.
In connection with any offering of Securities, other than an "at-the-market distribution" (as defined under applicable Canadian securities legislation), unless otherwise specified in a Prospectus Supplement, the underwriters, dealers or agents, as the case may be, may over-allot or effect transactions which stabilize, maintain or otherwise affect the market price of the Securities at a level other than those which otherwise might prevail on the open market. Such transactions may be commenced, interrupted or discontinued at any time. A purchaser who acquires Securities forming part of the underwriters', dealers' or agents' over-allocation position acquires those Securities under this Prospectus and the Prospectus Supplement relating to the particular offering of Securities, regardless of whether the over-allocation position is ultimately filled through the exercise of the over-allotment option or secondary market purchases. See "Plan of Distribution". No underwriter or dealer involved in an "at-the-market distribution" under this Prospectus, no affiliate of such an underwriter or dealer and no person or company acting jointly or in concert with such underwriter or dealer will over-allot Securities in connection with such distribution or effect any other transactions that are intended to stabilize or maintain the market price of the Securities.
The issued and outstanding Common Shares of the Company trade on the Exchange under the symbol "EMH" and are quoted for trading on the OTCQX Best Market under the symbol "EMHTF". On March 12, 2019, the last trading day prior to the date of this Prospectus, the closing price of the Common Shares on the Exchange was $3.82.
Unless otherwise specified in the applicable Prospectus Supplement, each series or issue of Securities (other than Common Shares) will not be listed on any securities exchange. Accordingly, there is currently no market through which the Securities (other than Common Shares) may be sold and purchasers may not be able to resell such Securities purchased under this Prospectus. This may affect the pricing of such Securities in the secondary market, the transparency and availability of trading prices, the liquidity of such Securities and the extent of issuer regulation relating to such Securities. See "Risk Factors".
The Company is permitted, under a multijurisdictional disclosure system adopted by the securities regulatory authorities in Canada and the United States ("MJDS"), to prepare this Prospectus in accordance with the disclosure requirements of Canada. Prospective purchasers in the United States should be aware that such requirements are different from those of the United States. The financial statements included or incorporated by reference herein have been prepared in accordance with International Financial Reporting Standards ("IFRS")as issued by the International Accounting Standards Board ("IASB") and may not be comparable to financial statements of United States companies. The audit of such financial statements are subject to Canadian generally accepted auditing standards and auditor independence standards.
The enforcement by purchasers of civil liabilities under the United States federal securities laws may be affected adversely by the fact that the Company is governed by the laws of British Columbia, Canada, that some or all of its officers and directors are residents of a foreign country, that some or all of the experts named in this Prospectus are, and the underwriters, dealers or agents named in any Prospectus Supplement may be, residents of a foreign country, and a substantial portion of the assets of the Company and said persons may be located outside of the United States.
THESE SECURITIES HAVE NOT BEEN APPROVED OR DISAPPROVED BY THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION (THE "SEC") NOR ANY STATE OR CANADIAN SECURITIES COMMISSION OR REGULATORY AUTHORITY NOR HAS THE SEC OR ANY STATE OR CANADIAN SECURITIES COMMISSION PASSED UPON THE ACCURACY OR ADEQUACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENCE.
Prospective purchasers should be aware that the acquisition of the Securities may have tax consequences in Canada and the United States. Such consequencesfor purchasers who are resident in, or citizens of, the United States may not be described fully herein or in any applicable Prospectus Supplement. Prospective purchasers should read the tax discussion contained in this Prospectus under the heading "Certain Income Tax Considerations" as well as the tax discussion, if any, contained in the applicable Prospectus Supplement with respect to a particular offering of Securities.
An investment in the Securities is subject to a number of risks. See "Risk Factors" for a more complete discussion of these risks.
No underwriter has been involved in the preparation of this Prospectus or performed any review of the contents hereof.
The Company is not making an offer of the Securities in any jurisdiction where such offer is not permitted.
All dollar amounts in this Prospectus are in Canadian dollars, unless otherwise indicated. See "Currency Presentation and Exchange Rate Information".
The Company's head office address is210 – 800 West Pender Street, Vancouver, British Columbia V6C 1J8 and its registered office is located at 2600 – 1066 West Hastings Street, Vancouver, British Columbia, V6E 3X1.
Dr. Avtar Dhillon and Mr. Punit Dhillon, each of whom is a director of the Company, reside outside of Canada and have appointed Bennett Jones LLP at 2600 - 1066 West Hastings Street, Vancouver, British Columbia V6E 3X1, as their agent for service of process in Canada. Prospective purchasers are advised that it may not be possible for purchasers to enforce judgements obtained in Canada against any person or company that is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction or who resides outside of Canada, even if the party has appointed an agent for service of process.
No person is authorized by the Company to provide any information or to make any representation other than as contained in this Prospectus in connection with the issue and sale of the Securities offered hereunder. Prospective purchasers should rely only on the information contained or incorporated by reference in this Prospectus and any applicable Prospectus Supplement in connection with an investment in Securities. Prospective purchasers should assume that the information appearing in this Prospectus or any Prospectus Supplement is accurate only as of the date on the front of those documents and that information contained in any document incorporated by reference is accurate only as of the date of that document unless otherwise specified as the Company's business, financial condition, results of operations and prospects may have changed since those dates.
Market data and certain industry forecasts used in this Prospectus or any applicable Prospectus Supplement and the documents incorporated by reference herein or therein were obtained from market research, publicly available information and industry publications. The Company believes that these sources are generally reliable, but the accuracy and completeness of the information is not guaranteed. The Company has not independently verified this information and does not make any representation as to the accuracy of this information.
TABLE OF CONTENTS
CAUTIONARY STATEMENTS REGARDING FORWARD-LOOKING INFORMATION
Certain statements contained in this Prospectus and the documents incorporated by reference herein constitute forward-looking information or forward-looking statements under applicable securities laws (collectively, "forward-looking statements"). These statements relate to future events or future performance, business prospects or opportunities of the Company that are based on forecasts of future results, estimates of amounts not yet determined and assumptions of management made in light of management's experience and perception of historical trends, current conditions and expected future developments. All statements other than statements of historical fact may be forward-looking statements. Any statements that express or involve discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, assumptions or future events or performance (often, but not always, using words or phrases such as "seek", "anticipate", "plan", "continue", "estimate", "expect", "may", "will", "project", "predict", "forecast", "potential", "targeting", "intend", "could", "might", "should", "believe" and similar expressions) are not statements of historical fact and may be "forward-looking statements".
Examples of forward-looking statements in this Prospectus and the documents incorporated by reference herein include, but are not limited to, statements in respect of: the approval of Licence (as defined below) applications submitted to Health Canada; the Company's intention to significantly increase its production of cannabis and cannabis oils through a multi-phase expansion plan; the building of a Health Canada licenced production facility to expand growing capability; the development of the Joint Venture (as defined below) as a standalone entity; the complete 1.1 million square foot space at the JV Facility (as defined below) in Delta, British Columbia being in full production in 2019; the rapid and cost effective acceleration of cannabis production by the Joint Venture; the exercise by the Joint Venture of options to lease or purchase additional greenhouses from Village Farms (as defined below); the potential aggregate production capacity of the Delta 1 and Delta 2 greenhouses and the JV Facility, all of which are located in Delta, British Columbia; the use of the Credit Facility (as defined below) by the Joint Venture; the purchase by the Company of 40% of the Joint Venture's production of cannabis cultivation during 2019; the expansion of the Company's operations in Richmond, British Columbia and the costs associated with such expansion; the Company's expectation of requirements for quantities of CBD (as defined below); payment of an additional $22.5 million in cash in respect of the acquisition of Verdélite and Verdélite Holdings (each as defined below); the anticipated date that Verdélite's indoor facility will begin production, the expansion cost, the size of the facility and its production of cannabis; the Company's expectation that the acquisition of Verdélite will strengthen its ability to market products in Quebec and Eastern Canada; the high-yield production of the Verdélite facility; the expansion of Avalite's (as defined below) operations; the receipt of excise duty licences from the Canada Revenue Agency; the Company's use of proceeds of financings; the quality, suitability for sale and cannabinoid concentration in the hemp harvested through the supply agreement with EHH (as defined below); the suitability of infrastructure at the facility of FTI (as defined below) and FTI's extraction of hemp biomass into CBD oil on behalf of the Company; the services to be provided by FTI to the Company; the entering into of an exclusive agreement between EHN (as defined below) and FTI; the entering into of definitive agreements with the Company’s business partners; the approval of patent applications that have been submitted by the Company; potential proceeds from the exercise of the Company's outstanding common share purchase warrants; the acceleration by the Company of the expiry date of the Company's outstanding warrants; actions taken by the Company to maintain or adjust its capital structure; increases to the Company's plant diversity and product offering; improvements to the Company's cultivation, manufacturing and standardization processes; partnerships with professional organizations in connection with educating medical doctors and other healthcare professionals about cannabis products; the development of distribution channels for non-medical cannabis products in Canada; anticipated long-term future profitability of the Company; potential effects of regulations under the Cannabis Act (as defined below) and related legislation introduced by provincial governments; the expected implementation of changes to the Cannabis Act to allow the sale of edible cannabis products; the undertaking of clinical research to study the effects of the Company's products on client health; the Company's longer term strategy of becoming a leading provider of quality products for the broader adult recreational cannabis market; the ability of the Company to take advantage of the legalization of adult use recreational cannabis; the Company's intentions to acquire and/or construct additional cannabis production and manufacturing facilities and to expand the Company's marketing initiatives both in Canada and internationally; the Company building valuable intellectual property in Canada which could lead to accelerated sales growth and profit margins; and future sales opportunities in other emerging medical markets and the effect that each risk factor will have on the Company.
Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause actual results or events to differ materially from those anticipated in such forward-looking statements. Prospective purchasers are cautioned that any such statements are not guarantees of future performance and actual results or developments may differ materially from those projected in the forward-looking statements. These forward-looking statements involve risks and uncertainties relating to, among others: the market price of cannabis; ability of the Company to secure cannabis supply; continued availability of capital financing and general economic, market or business conditions; reliance on licences to produce and sell cannabis and cannabis oils issued to the Company under the Cannabis Act and its ability to maintain these licences; regulatory risks relating to the Company's compliance with the Cannabis Act; failure to obtain regulatory approvals for expansion of the Company's current production facilities, development of new production facilities and greenhouse retrofits by the Company and the Joint Venture; Avalite's reliance on its dealer licence issued to it under the Controlled Drugs and Substances Act (Canada) to provide analytical testing of cannabis and importation of CBD and its ability to maintain this licence; the Company's ability to execute its multi-phase expansion plan and its plans with the Joint Venture; failure to execute a definitive agreement with FTI; changes in laws, regulations and guidelines; changes in government; changes in government policy; increased competition in the cannabis market in Canada and internationally; the limited operating history of the Company; the Company's reliance on key persons; failure of counterparties to perform contractual obligations; failure to obtain additional financing; unfavourable publicity or consumer perception of the Company and the cannabis industry; the impact of any negative scientific studies on the effects of cannabis; demand for labour; difficulties in construction or in obtaining qualified contractors to complete expansion projects and greenhouse retrofits; impact of any recall of the Company's products; actual operating and financial performance of facilities, equipment and processes relative to specifications and expectations; results of litigation; the Company's ability to develop and commercialize pharmaceutical or nutraceutical products; failure to obtain regulatory approval for pharmaceutical or nutraceutical products; changes in the Company's over-all business strategy; stock exchange rules or policies which may restrict the Company's business; and the Company's assumptions stated herein being correct. Additional factors that could cause actual results to differ materially include, but are not limited to, the risk factors described herein and as discussed in the Company's financial statements and other filings, under the heading "Risk Factors" in the Company's Annual Information Form (as defined herein) and under the section "Risks and Uncertainties" in the Company's Annual MD&A and Interim MD&A (each as defined herein).
The Company believes that the expectations reflected in any forward-looking statements are reasonable, but no assurance can be given that these expectations will prove to be correct and such forward-looking statements included in, or incorporated by reference into, this Prospectus should not be unduly relied upon. These statements speak only as of the date of this Prospectus or as of the date specified in the documents incorporated by reference into this Prospectus, as the case may be. The Company does not intend, and does not assume any obligation, to update these forward-looking statements, except as required by applicable laws. Actual results may differ materially from those expressed or implied by such forward-looking statements.
FINANCIAL INFORMATION
Unless otherwise indicated, all financial information included and incorporated by reference in this Prospectus is determined using IFRS as issued by IASB, which differs from United States generally accepted accounting principles.
CURRENCY PRESENTATION AND EXCHANGE RATE INFORMATION
The financial statements of the Company incorporated by reference in this Prospectus are reported in Canadian dollars. In this Prospectus, all dollar amounts referenced, unless otherwise indicated, are expressed in Canadian dollars and are referred to as "$". United States dollars are referred to as "US$". The high, low and closing exchange rates for Canadian dollars in terms of the United States dollar for each of the indicated periods, as quoted by the Bank of Canada, were as follows:
| | | Year ended December 31
(C$) | |
| | | 2018 | | | 2017 | | | 2016 | |
| High | | | 1.3642 | | | | 1.3743 | | | | 1.4589 | |
| Low | | | 1.2288 | | | | 1.2128 | | | | 1.2544 | |
| Closing | | | 1.3642 | | | | 1.2545 | | | | 1.3427 | |
On March 12, 2019, the last trading day prior to the date of this Prospectus, the exchange rate for Canadian dollars in terms of the United States dollar, as quoted by the Bank of Canada, was US$1.00 = C$1.3378.
DOCUMENTS INCORPORATED BY REFERENCE
Information has been incorporated by reference in this Prospectus from documents filed with securities commissions or similar authorities in each of the provinces of Canada (the"Qualifying Provinces"). Copies of the documents incorporated herein by reference or a copy of the Company's permanent information record may be obtained on request without charge from the Chief Financial Officer of the Company at 210 – 800 West Pender Street, Vancouver, British Columbia V6C 1J8 or by accessing the disclosure documents available through the Internet on the System for Electronic Document Analysis and Retrieval (SEDAR), which can be accessed at www.sedar.com. Documents filed with, or furnished to, the SEC are available through the SEC's Electronic Data Gathering and Retrieval System, or EDGAR, at www.sec.gov.
As at the date hereof, the following documents of the Company, filed with the securities commissions or similar authorities ineach of the Qualifying Provinces and filed with, or furnished to, the SEC, are specifically incorporated by reference into and form an integral part of this Prospectus, provided that such documents are not incorporated by reference to the extent that their contents are modified or superseded by a statement contained in this Prospectus or in any other subsequently filed document that is also incorporated by reference in the Prospectus, as further described below:
| (a) | the annual information form of the Company dated March 29, 2018 for the year ended December 31, 2017 (the "Annual Information Form"); |
| (b) | the audited consolidated annual financial statements of the Company as at and for the years ended December 31, 2017 and December 31, 2016, together with the notes thereto and the independent auditor's report thereon (as amended); |
| (c) | the management's discussion and analysis of the Company for the year ended December 31, 2017; |
| (d) | the unaudited consolidated interim financial statements of the Company as at and for the three and nine months ended September 30, 2018 and September 30, 2017, together with the notes thereto (the "Interim Financial Statements") |
| (e) | the management's discussion and analysis of the Company for the three and nine months ended September 30, 2018 (the "Interim MD&A"); |
| (f) | the management information circular of the Company dated May 9, 2018 regarding the annual general meeting of shareholders of the Company held on May 31, 2018; |
| (g) | the material change report of the Company dated January 4, 2018 announcing the entering into of a binding term sheet with a single Canadian institutional investor (the "Investor") pursuant to which the Investor agreed to purchase 3,000,000 units of the Company at a price of $5.00 per unit and purchase 2,000,000 Common Shares of the Company at a price of $5.00 per share from Sciences (as defined below) (the "January Offering"); |
| (h) | the material change report of the Company dated January 15, 2018 announcing the closing of the January Offering; |
| (i) | the material change report of the Company dated February 26, 2018 announcing the entering into of a binding term sheet with the Investor pursuant to which the Investor agreed to purchase 3,000,000 units of the Company at a price of $6.00 per unit and purchase 2,000,000 Common Shares of the Company at a price of $6.00 per share from Sciences (the "February Offering") and the closing of the February Offering; |
| (j) | the material change report of the Company dated May 11, 2018 announcing the closing of the acquisition of Verdélite Sciences, Inc. ("Verdélite"); |
| (k) | the material change report of the Company dated May 15, 2018 announcing the entering into of a binding term sheet with the Investor pursuant to which the Investor agreed to purchase 4,000,000 units of the Company at a price of $4.20 per unit and purchase 2,000,000 Common Shares of the Company at a price of $4.20 per share from Sciences (the "May Offering"); |
| (l) | the material change report of the Company dated May 31, 2018 announcing the closing of the May Offering; |
| (m) | the material change report of the Company dated October 3, 2018 announcing the Company's agreement to purchase from Emerald Health Hemp Inc. ("EHH") CBD-containing hemp biomass for extraction into CBD oil; |
| (n) | the material change report of the Company dated December 4, 2018 announcing the resignation of Chris Wagner as Chief Executive Officer and the appointment of Dr. Avtar Dhillon as President; and |
| (o) | the material change report of the Company dated December 13, 2018 announcing the entering into of a binding term sheet with the Investor pursuant to which the Investor agreed to purchase 4,000,000 common shares of the Company at a price of $2.70 per Common Share (the "December Offering") and the closing of the December Offering. |
Any document of the type referred to in the preceding paragraph (excluding confidential material change reports), and all other documents of the type required to be incorporated by reference in a short form prospectus by National Instrument 44-101 -Short Form Prospectus Distributions of the Canadian Securities Administrators, filed by the Company with a securities commission or similar regulatory authority in Canada after the date of this Prospectus and prior to the termination of any offering of Securities hereunder shall be deemed to be incorporated by reference into this Prospectus.
In addition, to the extent that any document or information incorporated by reference into this Prospectus is included in any report on Form 6-K, Form 40-F, Form 20-F, Form 10-K, Form 10-Q or Form 8-K (or any respective successor form) that is filed with or furnished to the SEC pursuant to the United States Securities Exchange Act of 1934, as amended (the "U.S. Exchange Act") after the date of this Prospectus, that document or information shall be deemed to be incorporated by reference as an exhibit to the Registration Statement (as defined below) of which this Prospectus forms a part (in the case of Form 6-K and Form 8-K, if and to the extent set forth therein).
Any statement contained in this Prospectus or in a document incorporated or deemed to be incorporated by reference in this Prospectus shall be deemed to be modified or superseded, for purposes of this Prospectus, to the extent that a statement contained herein or in any other subsequently filed document that also is or is deemed to be incorporated by reference in this Prospectus modifies or supersedes that statement. Any such modifying or superseding statement need not state that it has modified or superseded a prior statement or include any other information set forth in the document that it modifies or supersedes. The making of a modifying or superseding statement shall not be deemed an admission for any purposes that the modified or superseded statement, when made, constituted a misrepresentation, an untrue statement of a material fact or an omission to state a material fact that is required to be stated or that is necessary to make a statement not misleading in light of the circumstances in which it was made. Any statement so modified or superseded shall not be considered in its unmodified or superseded form to constitute part of this Prospectus; rather only such statement as so modified or superseded shall be considered to constitute part of this Prospectus.
Upon a new annual information form and related annual audited consolidated financial statements and management's discussion and analysis being filed by the Company with, and where required, accepted by, the applicable securities regulatory authorities during the term of this Prospectus: (i) the previous annual information form, the previous annual audited consolidated financial statements and related management's discussion and analysis; (ii) all interim financial statements and related management's discussion and analysis, all material change reports and all business acquisition reports filed by the Company prior to the commencement of the Company's financial year in respect of which the new annual information form is filed; and (iii) any business acquisition report for acquisitions completed since the beginning of the financial year in respect of which the new annual information form is filed (only if such report is incorporated by reference into the current annual information form or at least nine months of the acquired business or related businesses operations are incorporated into the Company's current annual audited consolidated financial statements) shall be deemed no longer to be incorporated by reference into this Prospectus for purposes of future offers and sales of Securities hereunder.
Upon new interim financial statements and related management's discussion and analysis being filed by the Company with the applicable securities regulatory authorities in Canada during the term of this Prospectus, all interim financial statements and related management's discussion and analysis filed prior to the new interim consolidated financial statements shall be deemed no longer to be incorporated by reference into this Prospectus for purposes of future offers and sales of Securities hereunder.
Upon a new information circular relating to an annual meeting of shareholders being filed by the Company with applicable securities regulatory authorities in Canada subsequent to the date of this Prospectus and prior to the date on which this Prospectus ceases to be effective, the information circular for the preceding annual meeting of shareholders and any other information circular filed by the Company prior to the commencement of the Company's financial year in respect of which the new annual information form is filed shall be deemed no longer to be incorporated by reference into this Prospectus for purposes of offers and sales of Securities under this Prospectus.
A Prospectus Supplement containing the specific terms of any Securities offered thereunder will be delivered to purchasers of such Securities together with this Prospectus to the extent required under applicable securities laws except in cases where an exemption from such delivery has been obtained and will be deemed to be incorporated by reference into this Prospectus as of the date of such Prospectus Supplement solely for the purposes of the Securities offered thereunder.
In addition, certain marketing materials (as that term is defined in applicable Canadian securities legislation) may be used in connection with a distribution of Securities under this Prospectus and the applicable Prospectus Supplement(s). Any "template version" of "marketing materials" (as those terms are defined in applicable Canadian securities legislation) pertaining to a distribution of Securities, and filed by the Company after the date of the Prospectus Supplement for the distribution and before termination of the distribution of such Securities, will be deemed to be incorporated by reference in that Prospectus Supplement for the purposes of the distribution of Securities to which the Prospectus Supplement pertains.
The Company has not provided or otherwise authorized any other person to provide purchasers with information other than as contained or incorporated by reference in this Prospectus or any Prospectus Supplement. If a purchaser is provided with different or inconsistent information, he or she should not rely on it.
DOCUMENTS FILED AS PART OF THE REGISTRATION STATEMENT
The following documents have been, or will be, filed with the SEC as part of the Registration Statement (as defined below) of which this Prospectus forms a part: (1) the documents listed under "Documents Incorporated by Reference"; (2) the consent of Deloitte LLP; (3) powers of attorney from certain of the Company's directors and officers; and (4) the form of indenture for Debt Securities. A copy of the formof warrant indenture, subscription receipt agreement, or statement of eligibility of trustee on Form T-1, as applicable, will be filed by post-effective amendment or by incorporation by reference to documents filed or furnished with the SEC under the U.S. Exchange Act.
AVAILABLE INFORMATION
The Company has concurrently filed with the SEC an amended registration statement on Form F-10 (amending the registration statement on Form F-10 filed with the SEC on February 20, 2019, as amended on February 26, 2019) (the "Registration Statement") under theUnited States Securities Act of 1933, as amended (the "U.S. Securities Act"), with respect to the Securities. This Prospectus which constitutes a part of the Registration Statement, does not contain all of the information contained in the Registration Statement, certain items of which are contained in the exhibits to the Registration Statement as permitted by the rules and regulations of the SEC. Statements included or incorporated by reference in this Prospectus about the contents of any contract, agreement or other documents referred to are not necessarily complete, and in each instance the prospective purchasers should refer to the exhibits for a more complete description of the matter involved. Each such statement is qualified in its entirety by such reference.
The Company is subject to the information requirements of applicable Canadian securities legislation and, in accordance therewith, files reports and other information with the applicable securities regulators in Canada. Upon effectiveness of the Registration Statement, the Company will be subject to the information requirements of the U.S. Exchange Act and will file reports and information with the SEC. Under MJDS adopted by the United States and Canada, documents and other information that the Company files with the SEC may be prepared in accordance with the disclosure requirements of Canada, which are different from those of the United States. As a foreign private issuer within the meaning of rules made under the U.S. Exchange Act, the Company will be exempt from the rules under the U.S. Exchange Act prescribing the furnishing and content of proxy statements, and the Company's officers, directors and principal shareholders are exempt from the reporting and short swing profit recovery provisions contained in Section 16 of the U.S. Exchange Act. In addition, the Company will not be required to publish financial statements as promptly as United States companies.
A prospective purchaser may read any document that the Company has filed with the SEC at the SEC's public reference room in Washington, D.C. A prospective purchaser may also obtain copies of those documents from the public reference room of the SEC at 100 F Street, N.E., Washington, D.C. 20549 by paying a fee. A prospective purchaser should call the SEC at 1-800-SEC-0330 or access its website at www.sec.gov for further information about the public reference rooms. A prospective purchaser may read and download some of the documents that the Company has filed with the SEC's EDGAR system at www.sec.gov. A prospective purchaser may read and download any public document that the Company has filed with the Canadian securities regulatory authorities under the Company's profile on the SEDAR website at www.sedar.com.
MEANING OF CERTAIN REFERENCES
For simplicity, the Company uses terms in this Prospectus to refer to the investments and operations of the Company and its direct and indirect subsidiaries as a whole. Accordingly, in this Prospectus, unless the context otherwise requires, the "Company" is referring to Emerald Health Therapeutics, Inc. and itsdirect and indirect subsidiaries.
SUMMARY OF THE BUSINESS
The principal business of the Company is the production and sale of cannabis pursuant to theCannabis Act (Canada) (the "Cannabis Act"). The Company currently offers a variety of dried cannabis strains and cannabis oils each with varying levels of Tetrahydrocannabinol ("THC"), cannabidiol ("CBD") and other cannabinoids.
The Company does not engage in any U.S. marijuana-related activities as defined in Canadian Securities Administrators Staff Notice 51-352 (Revised) dated February 8, 2018. To the extent that the Company pursues international expansion, it will only conduct business in jurisdictions outside of Canada where such operations are legally permissible in accordance with the laws of the jurisdiction and applicable Canadian regulatory and stock exchange requirements.
Licences
The Company holds licences from Health Canada under the Cannabis Act to produce and sell cannabis products in accordance with applicable laws in Canada. When the Cannabis Act came into force on October 17, 2018, the Company's cultivation and sale licences ("Licences"), which were issued under theAccess to Cannabis for Medical Purposes Regulations, were deemed to be their functionally equivalent licences under the Cannabis Act. The Company currently indirectly holds a number of Licences through its wholly-owned direct and indirect subsidiaries, Emerald Health Therapeutics Canada, Inc. (the "Operating Subsidiary"), Verdélite and Avalite Sciences Inc. (formerly Northern Vine Canada Inc.) ("Avalite"), as well as others which are held by its joint venture Pure Sunfarms Corp. (the "Joint Venture"). The Licences held by the Operating Subsidiary permit it to cultivate cannabis and produce and sell dried cannabis, cannabis oils, cannabis plants and cannabis seeds; the Licence held by Verdélite permits it to cultivate cannabis; the Licence held by Avalite permits it to process cannabis and produce cannabis oil; and the Licences held by the Joint Venture permit it to cultivate cannabis and produce and sell dried cannabis, cannabis oils, cannabis plants and cannabis seeds, all in accordance with the terms and conditions specified in the applicable Licence and the Cannabis Act. Particulars of the Licences are set out in the table below.
| Licence Holder | Jurisdiction | Licence Held | Authorized Products for Sale in Some Capacity | Applications Submitted | Original Date of Licensing | Date of Amendment of Licence | Expiration Date of Licence | Application Status |
| Emerald Health Therapeutics Canada, Inc. | Victoria, BC | Standard Cultivation | Plants / Seeds, Dried / Fresh Cannabis, and Cannabis Oil | -- | February 5, 2014 | November 9, 2018 | November 8, 2019 | -- |
| Standard Processing | -- |
| Sale for Medical Purposes | -- |
| -- | -- | To amend licence (to expand licensed facility) | -- | -- | -- | Submitted January 18, 2019; pending approval |
| Emerald Health Therapeutics Canada, Inc. (2nd Site) | Victoria, BC | Sale for Medical Purposes | Cannabis products | -- | October 6, 2017 | November 10, 2018 | October 6, 2020 | -- |
| -- | -- | Standard Cultivation | -- | -- | -- | Submitted March 1, 2019; pending approval |
| -- | -- | Standard Processing | -- | -- | -- | Submitted March 1, 2019; pending approval |
| Emerald Health Therapeutics Canada, Inc. (3rd Site) | Richmond, BC | -- | -- | Standard Cultivation | -- | -- | -- | Submitted January 16, 2019; pending approval |
| Verdélite Sciences, Inc. (formerly Agro-Biotech Inc.) | Saint-Eustache, QC | Standard Cultivation | Plants / Seeds to provinces and territories | -- | January 12, 2018 | November 8, 2018 | January 12, 2021 | -- |
| -- | -- | | -- | -- | -- | -- |
| -- | -- | Standard Processing | -- | -- | -- | Submitted December 14, 2018; pending approval |
Avalite Sciences Inc. (formerly Northern Vine Canada Inc.) | Langley, BC | Standard Processing | -- | -- | January 17, 2019 | January 17, 2019 | January 17, 2020 | -- |
| Dealer's Licence+ | -- | -- | September 22, 2016 | N/A | December 31, 2019 | -- |
| Analytical Testing | -- | -- | -- | -- | December 31, 2019 | -- |
| Research | -- | -- | -- | -- | December 31, 2019 | -- |
| Pure Sunfarms Corp. | Delta, BC | Standard Cultivation | Plants / Seeds to provinces/territories | -- | March 2, 2018 | March 11, 2019 | March 2, 2021 | -- |
| | | -- | -- | Standard Processing | -- | -- | | Submitted December 18, 2018; pending approval |
| Emerald Health Naturals Inc. | Kelowna, BC1 | -- | -- | Standard Processing | -- | -- | -- | Submitted October 16, 2018; pending approval |
| -- | -- | Sale for Medical Purposes | -- | -- | -- | Submitted October 16, 2018; pending approval |
Notes:
| + | Indicates a licenced dealer under the Controlled Drugs and Substances Act (Canada). |
| 1 | The Company is working with FTI to obtain this licence. |
Facilities
| · | Verdélite. Verdélite Holdings owns and Verdélite operates an 88,000 square foot facility in Saint –Eustache, Quebec, which is expected to be in full production by mid-2019. The Company budgeted $13 million for the completion of the facility, of which $7.4 million has been spent to date installing partitioning walls, HVAC, electrical, plumbing, environmental control and security systems, processing areas, to prepare it for cultivating and processing cannabis in compliance with the requirements of the Cannabis Act. |
| · | The Joint Venture. The Joint Venture owns and operates a 1.1-million square foot Delta 3 greenhouse (the "JV Facility") in Delta, British Columbia of which the Joint Venture's cultivation licence currently permits it to produce cannabis in approximately 1.03 million square feet. The Joint Venture has incurred approximately $58 million upgrading and retrofitting the JV Facility, removing tomato growing equipment and fixtures and installing partitioning walls, HVAC, electrical, plumbing, environmental control and security systems, nursery structures, processing areas, a vault, and shade systems to prepare it for cultivating and processing cannabis in compliance with the requirements of the Cannabis Act. The facility is substantially complete now. |
| · | Avalite.Avalite owns and operates a 3,742 square foot facility located in Langley, British Columbia at which chemical analyses and testing of cannabis products is conducted. |
| · | Victoria. The Company leases 16,000 square feet of mixed-use space in Victoria, British Columbia with approximately 500 square feet of cultivation area with the balance dedicated to laboratory and packaging activities and the Company's national call centre for customer service. |
| · | Richmond. The Company is constructing a 150,000 square foot facility in Richmond, British Columbia. Construction originally commenced in May 2017. The Company estimates that it will incur a total of $20 million installing partitioning walls, HVAC, electrical, plumbing, environmental control and security systems, nursery structures, processing areas, a vault, and shade systems to prepare it for cultivating and processing cannabis in compliance with the requirements of the Cannabis Act. The Company has incurred expenses related to this facility of approximately $13 million and approximately $7 million of additional construction costs are expected to be incurred to complete the facility. The facility is expected to be substantially complete by June 30, 2019. |
Inter-Corporate Relationships
The Company owns:
| (a) | 100% of the shares of the Operating Subsidiary, a British Columbia-based licence holder under the Cannabis Act; |
| (b) | 100% of the shares of Verdélite, a Quebec-based licence holder under the Cannabis Act; |
| (c) | 100% of the shares of Verdélite Property Holdings, Inc. (formerly, Agro-Biotech Property Holdings Inc.) ("Verdélite Holdings"), a Quebec-based holding corporation that owns the real property used by Verdélite; and |
| (d) | 51% of the shares of EHN, a joint venture between the Company and EHB. |
The Company, through the Operating Subsidiary, also holds:
| (a) | a 50% equity interest in the Joint Venture, a joint venture between the Company and Village Farms International Inc. ("Village Farms") incorporated for the purpose of producing, cultivating and distributing wholesale cannabis and cannabis extracts; and |
| (b) | 100% of the shares of Avalite, a British Columbia-based licenced dealer under the provisions of theControlled Drugs and Substances Act (Canada) and a licence holder under the Cannabis Act. |
The Company also owns 51% of the shares of Emerald Health Naturals Inc. ("EHN"), a joint venture between the Company and Emerald Health Bioceuticals, Inc. ("EHB"). EHN holds the exclusive Canadian distribution rights for EHB's endocannabinoid-supporting nutritional products which consist of nutritional supplements that use non-cannabis, non-psychoactive plant-based bioactive compounds to support the body's endocannabinoid system.
The following chart illustrates the Company's corporate structure.
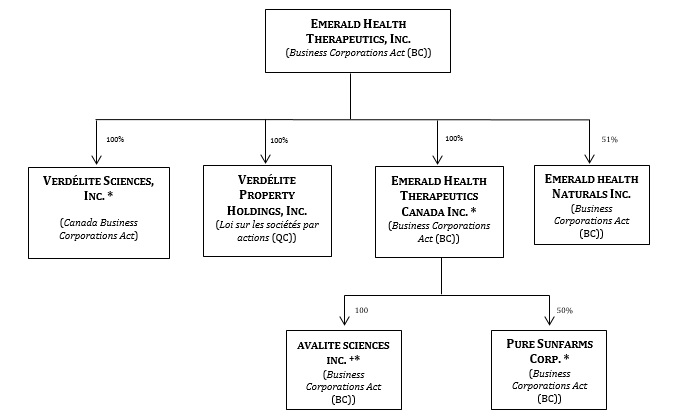
Notes:
| * | Indicates a licence holder under the Cannabis Act. |
| + | Indicates a licenced dealer under the Controlled Drugs and Substances Act (Canada). |
More detailed information regarding the business of the Company, its operations and its assets can be found in the Annual Information Form and other documents which are incorporated in this Prospectus by reference. See "Documents Incorporated by Reference."
Recent Developments
Between March 2018 and August 2018 the Company filed 17 provisional US patent applications covering, among other things, the Company's unique Defined Dose cannabis dosage forms and formulations. The applications have not yet been approved and the Company has no indication as to when or if they will be approved.
On April 17, 2018 the Company entered into a binding agreement with EHB (a partially owned subsidiary of Sciences), EHN, GAB Innovations, Inc. and Dr. Gaetano Morello, a director of Emerald Health Sciences Inc. ("Sciences"), with respect to the formation of the business and operations of EHN. On January 10, 2019 the Company closed its acquisition of 51% of EHN and EHB granted EHN the exclusive Canadian distribution rights to EHB's product line of endocannabinoid-supporting nutritional products in exchange for 49% ownership of EHN. Sciences is a control person of the Company.
On April 30, 2018 the Company entered into a supply agreement with the Joint Venture whereby the Company will purchase 40% of the Joint Venture's production in 2018 and 2019.
On May 2, 2018 the Company acquired 100% of the issued and outstanding shares of Verdélite and its affiliate Verdélite Holdings for consideration of $90 million, subject to adjustment, payable 50% in cash and 50% in Common Shares. The Company paid $22.5 million in cash upon closing and $45 million of the purchase price was satisfied by the issuance of 9,911,894 Common Shares, of which 4,955,947 Common Shares will be held in escrow until May 1, 2019, pursuant to an escrow agreement. An additional $22.5 million in cash is payable by the Company to the vendors on May 1, 2019.
On April 24, 2018, a statement of claim (the "Claim") was served on Verdélite and its former shareholders by Pivot Pharmaceuticals Inc. ("Pivot"), a party with whom Verdélite and its former shareholders had previously entered into a non-binding letter of intent with respect to a potential sale of Verdélite. The Claim sought to recover $72.4 million in damages for loss of profits allegedly suffered as a result of the bad faith and lack of cooperation of Verdélite and its shareholders in the negotiations surrounding the contemplated acquisition by Pivot of Verdélite. On January 30, 2019, the Company announced that Verdélite had entered into a release, discharge and transaction agreement (the "Settlement Agreement") settling the claims made by Pivot against Verdélite and its former shareholders. Pursuant to the Settlement Agreement, the Claim against Verdélite has been discharged without Verdélite making any payment or providing any compensation to Pivot.
On May 15, 2018, the Company exercised its right to purchase additional common shares of Avalite for $2.75 million, increasing its ownership stake of Avalite from 53% to 65%.
On July 24, 2018, the Company signed a non-binding memorandum of understanding (the "MOU") with the British Columbia Liquor Distribution Branch ("BCLDB") to supply cannabis products to the BCLDB to serve the non-medical market throughout the province from the date the Cannabis Act comes into force. The MOU is in BCLDB's standard form. Pursuant to the MOU, the Company has agreed to make 1,086 kg of cannabis products available for purchase by the BCLDB if the BCLDB elects to purchase cannabis products from the Company.
As of August 2018, the Company determined that it will no longer pursue the formation of CannaChain Technologies Inc., a proposed joint venture with DMG Blockchain Solutions Inc.
On August 8, 2018, the Company signed a non-binding term sheet to form a strategic alliance with Factors R&D Technology, Inc. ("FTI"), a division of Factors Group of Nutritional Companies Inc. In this exclusive arrangement, FTI has agreed to provide to the Company pharmaceutical-grade, industrial-scale manufacturing capacity as well as expertise in GMP-level extraction, softgel production, and packaging focused on the emerging market opportunities for medicinal cannabis in Canada and internationally. Further, the term sheet provides that FTI will be issued shares of EHN representing 25% of EHN's issued share capital. The parties agreed to use their best efforts to enter into a definitive agreement within 60 days of the execution of the term sheet. However, the definitive agreement is quite complex, and the parties are currently deep into negotiations regarding a draft of the definitive agreement and are working towards finalizing the agreement.
On August 15, 2018, the Company acquired the remaining shares of Avalite for a purchase price of $2,000,000 in cash and 1,093,938 Common Shares. The transaction increased the Company's ownership of Avalite from 65% to 100%.
On September 26, 2018, the Company announced that it has agreed to purchase from EHH CBD-containing hemp biomass for extraction into CBD oil. The supply agreement is for five years with an option to extend for an additional two years. Five hundred acres of hemp was harvested in October 2018 from farms located in Manitoba and Prince Edward Island and one thousand acres is expected to be harvested in each subsequent year of the agreement. CBD yield from the 2018 harvest has yet to be determined, pending analysis and extraction. EHH is a wholly-owned subsidiary of Sciences, a control person of the Company, and EHH is therefore a related party of the Company.
In October 2018 the Company entered into a research agreement with Emerald Health Biotechnology Espana S.L.U. (formerly, VivaCell Biotechnologies Spain S.L.U.) ("EH Spain"), an institute focused on cannabis research, which will provide contract research organization services to the Company to elucidate the mechanism of action of proprietary formulations and dosage forms that the Company is developing. EH Spain is a wholly-owned subsidiary of Sciences, a control person of the Company, and EH Spain is therefore a related party of the Company.
As of October 2018, the Company determined that it will no longer pursue the development of the e-commerce platform in partnership with Namaste Technologies Inc. described in its press release of January 30, 2018.
On November 28, 2018, the Company announced that Dr. Avtar Dhillon, the Company's Executive Chairman, was appointed President of the Company and the Company's Chief Executive Officer, Chris Wagner, had stepped down.
On December 3, 2018, the Company announced the December Offering and on December 7, 2018 the Company closed the December Offering by issuing 4,000,000 Common Shares to the Investor at a price of $2.70 per share for aggregate gross proceeds of $10,800,000.
On December 4, 2018, the Company announced that EHN had received product licences and natural product numbers from Health Canada to sell its product line of endocannabinoid-supporting nutritional products in Canada.
On January 10, 2019, the Company announced the resignation of Chris Wagner as a director of the Company.
On January 15, 2019, the Company announced a secondary offering of 2,800,000 Common Shares by Sciences, which closed on January 16, 2019. Sciences is a control person of the Company. After completion of the secondary offering, Sciences held approximately 28.6% of the Common Shares on a fully-diluted basis. Each of MFDI, LLC and Dr. Avtar Dhillon (a director of the Company) is a principal shareholder of Sciences.
On January 29, 2019, the Company announced that EHB, its joint venture partner in EHN, had launched its Endo products in Whole Foods Markets across the United States of America. EHN has secured Canadian distribution rights for this product line that was developed to support the body's endocannabinoid system. Canadian retailers have not yet been announced.
On February 5, 2019, the Company announced that it had entered into a binding licence agreement with Indena S.p.A. ("Indena"), an arm's length party, pursuant to which Indena granted the Company a perpetual exclusive licence for the use in Canada of Indena's CBD-extraction technology, and contract manufacturing services for CBD extraction. The Company has agreed to pay Indena a license fee of €450,000 (payable in two tranches of €250,000 and €200,000, respectively). The first payment is not payable until a definitive agreement has been entered into and the second payment is not payable until certain technological information has been transferred to the Company. The parties expect to enter into a definitive agreement upon completion of ongoing negotiations.
On February 8, 2019, the Company announced that the Joint Venture had been informed by the Ontario Cannabis Retail Corporation, operating as the Ontario Cannabis Store (the "OCS"), that it had been selected to supply the OCS with Pure Sunfarms-branded cannabis products for the non-medical market in the Province of Ontario. Sales of cannabis by the Joint Venture to the OCS cannot commence until the Joint Venture has obtained the requisite packaging and processing licences from Health Canada.
On February 13, 2019, the Company announced that the Joint Venture had entered into a credit agreement with Bank of Montreal, as agent and lead lender, and Farm Credit Canada, as lender, in respect of a $20 million secured non-revolving term loan (the "Credit Facility"). The Joint Venture intends to use the funds available under the Credit Facility to finance the final costs of converting the JV Facility for cannabis production, the vast majority of which was completed in January of this year. The funds available under the Credit Facility may also be used for general corporate purposes. The Credit Facility, which matures on February 7, 2022, is secured by the Joint Venture's greenhouse facility, and contains customary financial and restrictive covenants. The Company is not a party to the Credit Facility, but has provided a limited guarantee in the amount of $10 million in connection with the Credit Facility. The Joint Venture has drawn down approximately $12 million of the Credit Facility to date.
USE OF PROCEEDS
The use of proceeds from the sale of Securities will be described in the applicable Prospectus Supplement relating to a specific offering and sale of Securities. Among other potential uses, the Company may use the net proceeds from the sale of Securities for general corporate purposes, including funding ongoing operations and/or working capital requirements, to repay indebtedness outstanding from time to time, capital projects and potential future acquisitions.
Management of the Company will retain broad discretion in allocating the net proceeds of any offering of Securities under this Prospectus and the Company's actual use of the net proceeds will vary depending on the availability and suitability of investment and development opportunities and its operating and capital needs from time to time. All expenses relating to an offering of Securities and any compensation paid to underwriting dealers or agents as the case may be, will be paid out of the proceeds from the sale of Securities, unless otherwise stated in the applicable Prospectus Supplement. The Company will not receive any proceeds from the sale of Securities by a Selling Securityholder.
The Company may, from time to time, issue securities (including Securities) other than pursuant to this Prospectus.
Past Offerings
The table below summarizes the use of proceeds from past offerings that have been conducted by the Company.
| Offering / Proceeds Raised | | Use of Proceeds – as disclosed prospectus supplement | | Subsequent Use of Proceeds |
January 9, 2018 Treasury Offering ($15,000,000) | | Completion of capital projects and potential future expansion and acquisitions; for research and development; expanding the Company’s existing extraction capabilities; and working capital over the next 12 months | | $2.3 million for development of Richmond facility; $4 million equity investments in the Joint Venture; $2.75 million to increase ownership of Avalite from 53% to 65%; $5 million for loans subsequently made to the Joint Venture to fund development its production facility; balance for working capital. |
February 14, 2018 Treasury Offering ($18,000,000) | | Completion of capital projects and potential future expansion and acquisitions; for research and development; expanding the Company’s existing extraction capabilities; and working capital over the next 12 months. | | $8 million in loans to the Joint Venture to fund development its production facility; $2 million to increase ownership of Avalite from 65% to 100%; $2 million for development of Richmond facility; and $6 million of the acquisition cost of Verdélite. |
May 23, 2018 Treasury Offering ($16,800,000) | | Completion of recently acquired Agro-Biotech facility in Quebec, working capital and general corporate purposes. | | Funding of the acquisition cost of Verdélite. |
December 7, 2018 Treasury Offering ($10,800,000) | | Completion of capital projects, research and development, working capital and general corporate purposes. | | $10.8 million to substantially complete Richmond and Verdélite facilities. |
EARNINGS COVERAGE RATIO
Earnings coverage ratios will be provided as required in the applicable Prospectus Supplement with respect to the issuance of Preferred Shares and/or Debt Securities pursuant to this Prospectus.
CONSOLIDATED CAPITALIZATION OF THE COMPANY
The following table sets forth the Company's cash, indebtedness and shareholders' equity as of September 30, 2018 on an actual basis. This table should be read in conjunction with the Interim Financial Statements and Interim MD&A.
| Description of Capital | | As at September 30, 2018 | |
| Cash | | | 52,905,302 | |
| Indebtedness | | | 2,536,758 | |
| Number Common shares outstanding | | | 136,850,193 | |
| Number of Warrants outstanding | | | 8,411,764 | |
| Shareholders' Equity | | | | |
| Share Capital | | | 193,262,959 | |
| Warrants | | | 4,360,000 | |
| Contributed Surplus | | | 13,235,310 | |
| Accumulated other comprehensive loss | | | - | |
| Accumulated Deficit | | | (38,955,587 | ) |
| Total Shareholders' Equity | | | 171,902,682 | |
There have been no material changes in the share and loan capital of the Company on a consolidated basis, since September 30, 2018, except that, subsequent to such date, the Company issued 4,000,000 common shares to the Investor pursuant to the December Offering for gross aggregate proceeds of $10.8 million and a total of 1,910,854 share options previously issued to employees, directors and consultants were exercised for aggregate proceeds of $1,283,269.
SELLING SECURITYHOLDERs
This Prospectus may also, from time to time, relate to the offering of the Securities by way of a secondary offering (each, a "Secondary Offering") by one or more Selling Securityholders.
The terms under which Securities may be offered by Selling Securityholders will be described in the applicable Prospectus Supplement. The Prospectus Supplement for or including any offering of Securities by Selling Securityholders will include, without limitation, where applicable: (i) the names of the Selling Securityholders; (ii) the number and type of Securities owned, controlled or directed by each Selling Securityholder; (iii) the number of Securities being distributed for the accounts of each Selling Securityholder; (iv) the number of Securities to be owned, controlled or directed by each Selling Securityholder after the distribution and the percentage that number or amount represents out of the total number of outstanding Securities; (v) whether the Securities are owned by the Selling Securityholders, both of record and beneficially, of record only or beneficially only; (vi) if a Selling Securityholder purchased any of the Securities held by him, her or it in the 12 months preceding the date of the Prospectus Supplement, the date or dates the Selling Securityholder acquired the Securities; and (vii) if a Selling Securityholder acquired the Securities held by him, her or it in the 12 months preceding the date of the Prospectus Supplement, the cost thereof to the Selling Securityholder in the aggregate and on a per security basis
PLAN OF DISTRIBUTION
The Company may from time to time during the 25-month period that this Prospectus, including any amendments hereto, remains valid, offer for sale and issue up to an aggregate of $150,000,000 in Securities hereunder.
The Company may offer and sell the Securities to or through underwriters or dealers purchasing as principals and may also sell directly to one or more purchasers or through agents or pursuant to applicable statutory exemptions. The Prospectus Supplement relating to a particular offering of Securities will identify each underwriter, dealer or agent, as the case may be, engaged by the Company in connection with the offering and sale of the Securities, the Selling Securityholders, if any, and will set forth the terms of the offering of such Securities, including, to the extent applicable, any fees, discounts or any other compensation payable to underwriters, dealers or agents in connection with the offering, the method of distribution of the Securities, the initial issue price, the proceeds that the Company will receive and any other material terms of the plan of distribution. Any initial offering price and discounts, concessions or commissions allowed or reallowed or paid to dealers may be changed from time to time.
Similarly, one or more Selling Securityholders of the Company may sell Securities to or through underwriters or dealers purchasing as principals and may also sell the Securities to one or more purchasers directly, through statutory exemptions, or through agents designated from time to time. See "Selling Securityholders".
In addition, Securities may be offered and issued in consideration for the acquisition of other businesses, assets or securities by the Company or one of its subsidiaries. The consideration for any such acquisition may consist of the Securities separately, a combination of Securities or any combination of, among other things, Securities, cash and assumption of liabilities.
Securities may be sold from time to time in one or more transactions at a fixed price or prices or at prices which may be changed or at market prices prevailing at the time of sale, at prices related to such prevailing prices or at negotiated prices, including sales in transactions that are deemed to be "at-the-market distributions" as defined in National Instrument 44-102 -Shelf Distributions, including sales made directly on the Exchange or other existing trading markets for the Common Shares. The price at which the Securities will be offered and sold may vary from purchaser to purchaser and during the period of distribution.
In connection with the sale of the Securities, underwriters, dealers or agents may receive compensation from the Company or from other parties, including in the form of underwriters', dealers' or agents' fees, commissions or concessions. Underwriters, dealers and agents that participate in the distribution of the Securities may be deemed to be underwriters for the purposes of applicable Canadian securities legislation and any such compensation received by them from the Company and any profit on the resale of the Securities by them may be deemed to be underwriting commissions.
In connection with any offering of Securities, except as otherwise set out in a Prospectus Supplement relating to a particular offering of Securities and other than in relation to an "at-the-market" distribution, the underwriters, dealers or agents, as the case may be, may over-allot or effect transactions intended to fix, stabilize, maintain or otherwise affect the market price of the Securities at a level other than those which otherwise might prevail on the open market. Such transactions may be commenced, interrupted or discontinued at any time.
Underwriters, dealers or agents who participate in the distribution of the Securities may be entitled, under agreements to be entered into with the Company, to indemnification by the Company against certain liabilities, including liabilities under Canadian securities legislation and the U.S. Securities Act, or to contribution with respect to payments which such underwriters, dealers or agents may be required to make in respect thereof. Such underwriters, dealers and agents may be customers of, engage in transactions with, or perform services for, the Company in the ordinary course of business.
Unless otherwise specified in the applicable Prospectus Supplement, each series or issue of Securities (other than Common Shares) will be a new issue of Securities with no established trading market. Accordingly, there is currently no market through which the Securities (other than Common Shares) may be sold and purchasers may not be able to resell such Securities purchased under this Prospectus. This may affect the pricing of such Securities in the secondary market, the transparency and availability of trading prices, the liquidity of such Securities and the extent of issuer regulation. See "Risk Factors".
This Prospectus constitutes a public offering of these Securities only in those jurisdictions where they may be lawfully offered for sale and therein only by persons permitted to sell such Securities.
DESCRIPTION OF SECURITIES
The following is a brief summary of certain general terms and provisions of the Securities as at the date of this Prospectus. The summary does not purport to be complete and is indicative only. The specific terms of any Securities to be offered under this Prospectus, and the extent to which the general terms described in this Prospectus apply to such Securities, will be set forth in the applicable Prospectus Supplement. Moreover, a Prospectus Supplement relating to a particular offering of Securities may include terms pertaining to the Securities being offered thereunder that are not within the terms and parameters described in this Prospectus.
Description of Common Shares
The following is a brief summary of the material attributes of the Common Shares. This summary does not purport to be complete.
The holders of Common Shares are entitled to one vote per share at all meetings of shareholders of the Company except separate meetings of the holders of another class or series of shares of the Company. Subject to the preferences accorded to holders of any class of shares of the Company ranking senior to or concurrent with the Common Shares, the Common Shares are entitled to dividends, if and when declared by the Company's board of directors, and to the distribution of the residual assets of the Company in the event of the liquidation, dissolution or winding-up of the Company.
Description of Preferred Shares
The following is a brief summary of certain general terms and provisions of the Preferred Shares that may be offered pursuant to this Prospectus. This summary does not purport to be complete. The particular terms and provisions of the Preferred Shares as may be offered pursuant to this Prospectus will be set forth in the applicable Prospectus Supplement pertaining to such offering of Preferred Shares, and the extent to which the general terms and provisions described below may apply to such Preferred Shares will be described in the applicable Prospectus Supplement.
The Preferred Shares are issuable in series. The Preferred Shares of each series rank in parity with the Preferred Shares of every other series with respect to dividends and return of capital and are entitled to a preference over the Common Shares and any other shares ranking junior to the Preferred Shares with respect to priority in the payment of dividends and the distribution of assets in the event of the liquidation, dissolution or winding-up of the Company.
The Company's board of directors is empowered to fix the number of shares and the rights to be attached to the Preferred Shares of each series, including the amount of dividends and any conversion, voting and redemption rights. Subject to the articles of incorporation for the Company and to applicable law, the Preferred Shares as a class are not entitled to receive notice of or attend or vote at meetings of the Company's shareholders.
Each applicable Prospectus Supplement will set forth the terms and other information with respect to the Preferred Shares being offered thereby, which may include, without limitation, the following (where applicable):
| · | the maximum number of Preferred Shares; |
| · | the designation of the series; |
| · | the annual dividend rate, if any, and whether the dividend rate is fixed or variable, the date from which dividends will accrue, and the dividend payment dates; |
| · | the priority of the Preferred Shares in respect to the payment of dividends and the distribution of assets in the event of the liquidation, dissolution or winding-up of the Company; |
| · | the price and the terms and conditions for redemption, if any, including whether redeemable at the Company's option or at the option of the holder, the time period for redemption, and payment of any accumulated dividends; |
| · | the terms and conditions, if any, for conversion or exchange for shares of any other class of the Company or any other series of Preferred Shares, or any other securities or assets, including the price or the rate of conversion or exchange and the method, if any, of adjustment; |
| · | whether such Preferred Shares will be listed on any securities exchange; |
| · | the voting rights, if any; |
| · | any other rights, privileges, restrictions, or conditions; |
| · | certain material Canadian and United States tax consequences of owning the Preferred Shares; and |
| · | any other material terms and conditions of the Preferred Shares. |
Description of Warrants
The following is a brief summary of certain general terms and provisions of the Warrants that may be offered pursuant to this Prospectus. This summary does not purport to be complete. The particular terms and provisions of the Warrants as may be offered pursuant to this Prospectus will be set forth in the applicable Prospectus Supplement pertaining to such offering of Warrants, and the extent to which the general terms and provisions described below may apply to such Warrants will be described in the applicable Prospectus Supplement.
Warrants may be offered separately or together with other Securities, as the case may be. Each series of Warrants may be issued under a separate warrant indenture or warrant agency agreement to be entered into between the Company and one or more banks or trust companies acting as warrant agent or may be issued as stand-alone contracts. The applicable Prospectus Supplement will include details of the warrant indenture or agreements, if any, governing the Warrants being offered. The warrant agent, if any, will be expected to act solely as the agent of the Company and will not assume a relationship of agency with any holders of warrant certificates or beneficial owners of Warrants. A copy of any warrant indenture or any warrant agency agreement relating to an offering of Warrants will be filed by the Company with the relevant securities regulatory authorities in Canada after it has been entered into by the Company.If applicable, the Company will file with the SEC as exhibits to the Registration Statement of which this Prospectus is a part, or will incorporate by reference from a Report of Foreign Private Issuer on Form 6-K that the Company files with the SEC, any warrant indenture or any warrant agency agreement describing the terms and conditions of such Warrants that the Company is offering before the issuance of such Warrants.
Each applicable Prospectus Supplement will set forth the terms and other information with respect to the Warrants being offered thereby, which may include, without limitation, the following (where applicable):
| · | the designation of the Warrants; |
| · | the aggregate number of Warrants offered and the offering price; |
| · | the designation, number and terms of the other Securities purchasable upon exercise of the Warrants, and procedures that will result in the adjustment of those numbers; |
| · | the exercise price of the Warrants; |
| · | the dates or periods during which the Warrants are exercisable including any "early termination" provisions; |
| · | the designation, number and terms of any Securities with which the Warrants are issued; |
| · | if the Warrants are issued as a unit with another Security, the date on and after which the Warrants and the other Security will be separately transferable; |
| · | whether such Warrants are to be issued in registered form, "book-entry only" form, bearer form or in the form of temporary or permanent global securities and the basis of exchange, transfer and ownership thereof; |
| · | any minimum or maximum amount of Warrants that may be exercised at any one time; |
| · | whether such Warrants will be listed on any securities exchange; |
| · | any terms, procedures and limitations relating to the transferability, exchange or exercise of the Warrants; |
| · | certain material Canadian and United States tax consequences of owning the Warrants; and |
| · | any other material terms and conditions of the Warrants. |
Description of Subscription Receipts
The following is a brief summary of certain general terms and provisions of the Subscription Receipts that may be offered pursuant to this Prospectus. This summary does not purport to be complete. The particular terms and provisions of the Subscription Receipts as may be offered pursuant to this Prospectus will be set forth in the applicable Prospectus Supplement pertaining to such offering of Subscription Receipts, and the extent to which the general terms and provisions described below may apply to such Subscription Receipts will be described in the applicable Prospectus Supplement.
Subscription Receipts may be offered separately or together with other Securities, as the case may be. The Subscription Receipts may be issued under a subscription receipt agreement. The applicable Prospectus Supplement will include details of the subscription receipt agreement, if any, governing the Subscription Receipts being offered. The Company will file a copy of any subscription receipt agreement relating to an offering of Subscription Receipts with the relevant securities regulatory authorities in Canada after it has been entered into by the Company.If applicable, the Company will file with the SEC as exhibits to the Registration Statement of which this Prospectus is a part, or will incorporate by reference from a Report of Foreign Private Issuer on Form 6-K that the Company files with the SEC, any subscription receipt agreement describing the terms and conditions of such Subscription Receipts that the Company is offering before the issuance of such Subscription Receipts.
Each applicable Prospectus Supplement will set forth the terms and other information with respect to the Subscription Receipts being offered thereby, which may include, without limitation, the following (where applicable):
| · | the number of Subscription Receipts; |
| · | the price at which the Subscription Receipts will be offered; |
| · | the terms, conditions and procedures for the conversion of the Subscription Receipts into other Securities; |
| · | the dates or periods during which the Subscription Receipts are convertible into other Securities; |
| · | the designation, number and terms of the other Securities that may be exchanged upon conversion of each Subscription Receipt; |
| · | the designation, number and terms of any other Securities with which the Subscription Receipts will be offered, if any, and the number of Subscription Receipts that will be offered with each Security; |
| · | whether such Subscription Receipts are to be issued in registered form, "book-entry only" form, bearer form or in the form of temporary or permanent global securities and the basis of exchange, transfer and ownership thereof; |
| · | terms applicable to the gross or net proceeds from the sale of the Subscription Receipts plus any interest earned thereon; |
| · | certain material Canadian and United States tax consequences of owning the Subscription Receipts; and |
| · | any other material terms and conditions of the Subscription Receipts. |
Description of Units
The following is a brief summary of certain general terms and provisions of the Units that may be offered pursuant to this Prospectus. This summary does not purport to be complete. The particular terms and provisions of the Units as may be offered pursuant to this Prospectus will be set forth in the applicable Prospectus Supplement pertaining to such offering of Units, and the extent to which the general terms and provisions described below may apply to such Units will be described in the applicable Prospectus Supplement.
Units may be offered separately or together with other Securities, as the case may be.
The Company will also add to disclosure in any subsequent Prospectus Supplement whereby Units are offered the form of any unit agreement between the Company and a unit agent that describes the terms and conditions of the issue of Units being offered, and any supplemental agreements. If applicable, the Company will file with the SEC as exhibits to the registration statement of which this Prospectus is a part or will incorporate by reference from a current report on Form 6-K that the Company files with the SEC, any unit agreement describing the terms and conditions of such Units that the Company is offering before the issuance of such Units.
Each applicable Prospectus Supplement will set forth the terms and other information with respect to the Units being offered thereby, which may include, without limitation, the following (where applicable):
| · | the price at which the Units will be offered; |
| · | the designation, number and terms of the Securities comprising the Units; |
| · | whether the Units will be issued with any other Securities and, if so, the amount and terms of these Securities; |
| · | terms applicable to the gross or net proceeds from the sale of the Units plus any interest earned thereon; |
| · | the date on and after which the Securities comprising the Units will be separately transferable; |
| · | whether the Securities comprising the Units will be listed on any securities exchange; |
| · | whether such Units or the Securities comprising the Units are to be issued in registered form, "book-entry only" form, bearer form or in the form of temporary or permanent global securities and the basis of exchange, transfer and ownership thereof; |
| · | any terms, procedures and limitations relating to the transferability, exchange or exercise of the Units; |
| · | certain material Canadian and United States tax consequences of owning the Units; and |
| · | any other material terms and conditions of the Units. |
Description of Debt Securities
The following is a brief summary of certain general terms and provisions of the Debt Securities that may be offered pursuant to this Prospectus. This summary does not purport to be complete. The particular terms and provisions of the Debt Securities as may be offered pursuant to this Prospectus will be set forth in the applicable Prospectus Supplement pertaining to such offering of Debt Securities, and the extent to which the general terms and provisions described below may apply to such Debt Securities will be described in the applicable Prospectus Supplement.
The Debt Securities may be offered separately, in one or more series, or together with other Securities, as the case may be. The Debt Securities are expected to be governed by, one or more indentures (each, an "Indenture"), in each case between the Company and a financial institution authorized to carry on business as a trustee (each, a "Trustee"). To the extent such Debt Securities are issued into the United States, each such Indenture is expected to begoverned by and construed in accordance with the laws of the State of New York and certain provisions of theUnited States Trust Indenture Act of 1939, as amended. Otherwise, such Indenture is expected to be governed by laws of the Province of British Columbia or such other laws as set forth in the applicable Prospectus Supplement.
Each applicable Prospectus Supplement will set forth the terms and other information with respect to the Debt Securities of the series being offered thereby, which may include, without limitation, the following (where applicable):
| · | the designation of the series, aggregate principal amount and authorized denominations of such Debt Securities and the denominations in which bearer Debt Securities will be issuable; |
| · | the currency or currency units for which the Debt Securities may be purchased and the currency or currency unit in which the principal and any interest is payable; |
| · | the percentage of the principal amount or the price at which such Debt Securities will be issued or whether such Debt Securities will be issued on a non-fixed price basis; |
| · | the date or dates on which such Debt Securities will mature; |
| · | the rate or rates per annum at which such Debt Securities will bear interest (if any), or the method of determination of such rates (if any); |
| · | the dates on which such interest will be payable and the record dates for such payments; |
| · | the general terms or provisions pursuant to which the Debt Securities are to be issued; |
| · | the name of the Trustee under the Indenture pursuant to which the Debt Securities are to be issued (if applicable); |
| · | any redemption, retraction or call terms or terms under which such Debt Securities may be defeased; |
| · | whether such Debt Securities are to be issued in registered form, "book-entry only" form, bearer form or in the form of temporary or permanent global securities ("Global Debt Securities") and the basis of exchange, transfer and ownership thereof; |
| · | any exchange or conversion terms; |
| · | the general terms or provisions, if any, pursuant to which such Debt Securities are to be guaranteed or secured; |
| · | whether such Debt Securities will be subordinated to other liabilities of the Company; |
| · | any material covenants included for the benefit of holders of Debt Securities; |
| · | whether such Debt Securities will be issuable in the form of one or more registered global securities, and if so, the identity of the depository for such registered global Debt Securities; |
| · | each office or agency where payments on the Debt Securities will be made (if other than the offices or agencies described under the heading "Payment" below) and each office or agency where the Debt Securities may be presented for registration of transfer or exchange; and |
| · | any other material terms and conditions of the Debt Securities, including events of default and amendment provisions. |
The Company may issue Debt Securities under an Indenture bearing no interest or interest at a rate below the prevailing market rate at the time of issuance and, in such circumstances, the Company may offer and sell those Debt Securities at a discount below their stated principal amount. The Company will describe in the applicable Prospectus Supplement any Canadian federal income tax consequences and may describe United States federal income tax consequences and other special considerations applicable to any discounted Debt Securities or other Debt Securities offered and sold at par which are treated as having been issued at a discount for applicable federal income tax purposes.
Any Debt Securities issued by the Company will be direct, unconditional and, unless otherwise specified, will be unsecured obligations of the Company and will rank equally among themselves and with all of the Company's other unsecured, unsubordinated obligations, except to the extent prescribed by law. Unless otherwise described in the applicable Prospectus Supplement, Debt Securities issued by the Company will be structurally subordinated to all existing and future liabilities, including trade payables and other indebtedness, of the Company's subsidiaries. The Company will agree to provide to the Trustee (i) annual reports containing audited financial statements and (ii) quarterly reports for the first three quarters of each fiscal year containing unaudited financial information.
Form, Denomination, Exchange and Transfer
Unless otherwise indicated in the applicable Prospectus Supplement relating to such series, the Company will issue Debt Securities of a particular series only in fully registered form without coupons, and in denominations of US$1,000 and integral multiples of US$1,000. Debt Securities may be presented for exchange and registered Debt Securities may be presented for registration of transfer in the manner to be set forth in the Indenture and in the applicable Prospectus Supplement, without service charges. The Company may, however, require payment sufficient to cover any taxes or other governmental charges due in connection with the exchange or transfer. The Company will appoint the Trustee as security registrar. Bearer Debt Securities and the coupons applicable to bearer Debt Securities thereto will be transferable by delivery.
Payment
Unless otherwise indicated in the applicable Prospectus Supplement, the Company will make payments on registered Debt Securities (other than Global Debt Securities) at the office or agency of the Trustee, except that the Company may choose to pay interest (a) by cheque mailed to the address of the person entitled to such payment as specified in the security register, or (b) by wire transfer to an account maintained by the person entitled to such payment as specified in the security register. Unless otherwise indicated in the applicable Prospectus Supplement, the Company will pay any interest due on registered Debt Securities to the persons in whose name such registered Securities are registered on the day or days specified in the applicable Prospectus Supplement.
Registered Global Debt Securities
Unless otherwise indicated in the applicable Prospectus Supplement, registered Debt Securities of a particular series will be issued in global form that will be deposited with, or on behalf of, a depositary (the "Depositary") identified in the Prospectus Supplement relating to such series. Global Debt Securities will be registered in the name of the Depositary, and the Debt Securities included in the Global Debt Securities may not be transferred to the name of any other direct holder unless the special circumstances described below occur. Any person wishing to own Debt Securities issued in the form of Global Debt Securities must do so indirectly through an account with a broker, bank or other financial institution that, in turn, has an account with the Depositary.
Special Purchaser Considerations for Global Debt Securities
The Company's obligations under the Indenture, as well as the obligations of the Trustee and those of any third parties employed by the Company or the Trustee, run only to persons who are registered as holders of Debt Securities. For example, once the Company makes payment to the registered holder, the Company has no further responsibility for the payment even if that holder is legally required to pass the payment along to a purchaser but does not do so. As an indirect holder, a purchaser's rights relating to a Global Debt Security will be governed by the account rules of the purchaser's financial institution and of the Depositary, as well as general laws relating to debt securities transfers.
A purchaser should be aware that when Debt Securities are issued in the form of Global Debt Securities:
| (a) | the purchaser cannot have Debt Securities registered in the purchaser's own name, |
| (b) | the purchaser cannot receive physical certificates for the purchaser's interest in the Debt Securities, |
| (c) | the purchaser must look to his or her own bank, brokerage firm or other financial institution for payments on the Debt Securities and protection of his or her legal rights relating to the Debt Securities, |
| (d) | the purchaser may not be able to sell interests in the Debt Securities to some insurance companies and other institutions that are required by law to hold the physical certificates of Debt Securities that they own, |
| (e) | the Depositary's policies will govern payments, transfers, exchange and other matters relating to the purchaser's interest in the Global Debt Security; the Company and the Trustee will have no responsibility for any aspect of the Depositary's actions or for its records of ownership interests in the Global Debt Security; the Company and the Trustee also do not supervise the Depositary in any way, and |
| (f) | the Depositary will usually require that interests in a Global Debt Security be purchased or sold within its system using same-day funds. |
Special Situations When Global Debt Security Will be Terminated
In a few special situations described below, a Global Debt Security will terminate and interests in it will be exchanged for physical certificates representing Debt Securities. After that exchange, a purchaser may choose whether to hold Debt Securities directly or indirectly through an account at its bank, brokerage firm or other financial institution. Purchasers must consult their own banks, brokers or other financial institutions to find out how to have their interests in Debt Securities transferred into their own names, so that they will become registered holders of the Debt Securities previously represented by each Global Debt Security.
The special situations for termination of a Global Debt Security are:
| (a) | when the Depositary notifies the Company that it is unwilling, unable or no longer qualified to continue as Depositary (unless a replacement Depositary is named); and |
| (b) | when and if the Company decides to terminate a Global Debt Security. |
When a Global Debt Security terminates, the Depositary (and not the Company or the Trustee) will be responsible for deciding the names of the institutions that will be the initial direct holders.
Events of Default
Unless otherwise indicated in the applicable Prospectus Supplement relating to such series, the term "Event of Default" with respect to Debt Securities of a particular series issued under the Indenture means any of the following:
| (a) | default in the payment of the principal amount, redemption price or fundamental change purchase price on any Debt Security of such series when it becomes due and payable; |
| (b) | default in the payment of interest, additional interest amounts or other additional amounts, if any, upon any Debt Security of such series, when such amounts become due and payable, and continuance of such default for a period of 30 days; |
| (c) | default in the performance of any covenant, agreement or condition of the Company in the Indenture or the Debt Securities of such series and continuance of such default for a period of 60 days after there has been given, by registered or certified mail, to the Company by the Trustee or to the Company and the Trustee by the holders of at least 25% in aggregate principal amount of all outstanding Debt Securities a written notice specifying such default and requiring it to be remedied and stating that such notice is a "Notice of Default" under the Indenture; |
| (d) | failure by the Company to convert any Debt Securities into Common Shares and/or for cash at the Company's election upon exercise of a holder's conversion right (if any) and such failure continues for five business days or more; |
| (e) | default in the payment of any indebtedness (other than indebtedness that is non-recourse to the Company or its subsidiaries) for borrowed money by the Company or any of its subsidiaries (all or substantially all of the outstanding voting securities of which are owned, directly or indirectly, by the Company) in an outstanding principal amount in excess of US$10,000,000 (or such other amount set forth in the applicable Indenture and described in the applicable Prospectus Supplement) when such amounts become due at final maturity or upon acceleration, and such indebtedness is not discharged, or such default in payment or acceleration is not cured or rescinded within the period specified in such instrument; |
| (f) | the rendering of a final judgment or judgments (not subject to appeal and not covered by insurance) against the Company or any of its subsidiaries in excess of US$10,000,000 (or such other amount set forth in the applicable Indenture and described in the applicable Prospectus Supplement) which remains unstayed, undischarged or unbonded for a period of 60 days; |
| (g) | failure by the Company to give notice of a "fundamental change" as set forth in the Indenture; |
| (h) | failure by the Company to comply with its obligations under the Indenture regarding consolidations, mergers and the sale, conveyance or leasing of substantially all of its assets; |
| (i) | the entry by a court having jurisdiction in the premises of (i) a decree or order for relief in respect of the Company or any of its subsidiaries of a voluntary case or proceeding under any applicable United States or Canadian federal, state or provincial bankruptcy, insolvency, reorganization or other similar law or (ii) a decree or order adjudging the Company as bankrupt or insolvent, or approving as properly filed a petition seeking reorganization, arrangement, adjustment or composition of or in respect of the Company under any applicable United States or Canadian federal, state or provincial law or (iii) appointing a custodian, receiver, liquidator, assignee, trustee, sequestrator or other similar official of the Company or of any substantial part of its property, or ordering the winding up or liquidation of its affairs, and the continuance of any such decree or order for relief or any such other appointment, decree or order unstayed and in effect for a period of 60 consecutive days; or |
| (j) | the commencement by the Company or any of its subsidiaries of a voluntary case or proceeding under any applicable United States or Canadian federal, state or provincial bankruptcy, insolvency, reorganization or other similar law or of any other case or proceeding to be adjudicated a bankrupt or insolvent, or the consent by it to the entry of a decree or order for relief in respect of the Company in an involuntary case or proceeding under any applicable United States or Canadian federal, state or provincial bankruptcy, insolvency, reorganization or other similar law or to the commencement of any bankruptcy or insolvency case or proceeding against it, or the filing by it of a petition or answer or consent seeking reorganization or relief under any applicable federal or state law, or the consent by it to the filing of such petition or to the appointment of or taking possession by a custodian, receiver, liquidator, assignee, trustee, sequestrator or other similar official of the Company or of any substantial part of its property, or the making by it of an assignment for the benefit of creditors. |
If an Event of Default occurs and is continuing with respect to Debt Securities of any series, then the Trustee or the holders of not less than 25% in principal amount of the outstanding Debt Securities of a particular series (or such other amount set forth in the applicable Indenture and described in the applicable Prospectus Supplement) may require the principal amount of all the outstanding Debt Securities of such series and any accrued but unpaid interest on such Debt Securities be paid immediately. However, at any time after a declaration of acceleration with respect to Debt Securities of any series has been made and before a judgment or decree for payment of the money due has been obtained, the holders of a majority in principal amount of the outstanding Debt Securities of a particular series, by written notice to the Company and the Trustee, may, under certain circumstances, rescind and annul such acceleration.
Other than its duties in the case of an Event of Default, the Trustee will not be obligated to exercise any of its rights and powers under the Indenture at the request or direction of any of the holders, unless the holders have offered to the Trustee reasonable indemnity. If the holders provide indemnity satisfactory to the Trustee, the holders of a majority in principal amount of the outstanding Debt Securities of a particular series may, subject to certain limitations, direct the time, method and place of conducting any proceeding for any remedy available to the Trustee, or exercising any trust or power conferred on the Trustee, with respect to the Debt Securities of such series.
No holder of a Debt Security will have any right to institute any proceedings, unless:
| (a) | such holder has previously given to the Trustee written notice of a continuing Event of Default with respect to the Debt Securities, |
| (b) | the holders of at least 25% in principal amount of the outstanding Debt Securities of such series have made written request and have offered reasonable indemnity to the Trustee to institute such proceedings as trustee, and |
| (c) | the Trustee has failed to institute such proceeding and has not received from the holders of a majority in the aggregate principal amount of outstanding Debt Securities of such series a direction inconsistent with such request, within 60 days after such notice, request and offer. |
These limitations do not apply, however, to a suit instituted by the holder of a Debt Security for the enforcement of payment of principal of or interest on such Debt Security on or after the applicable due date of such payment.
So long as any Debt Securities are issued and outstanding, the Company will be required to furnish to the Trustee annually an officers' certificate as to the performance of certain of its obligations under the Indenture and as to any default in such performance.
Modifications and Waivers
The Company may modify or amend the Indenture with the consent of the holders of a majority in aggregate principal amount of all outstanding Debt Securities; provided, however, unless otherwise stated in the applicable Prospectus Supplement, that the Company will be required to receive consent from the holder of each outstanding Debt Security of a particular series to:
| (a) | extend the fixed maturity of any Debt Security of such series, |
| (b) | reduce the principal amount of or reduce the interest rate on or extend the stated time for payment of interest, including additional interest amounts and additional amounts, if any, on any Debt Security of such series, |
| (c) | reduce the redemption price or fundamental change purchase price of any Debt Security of such series, |
| (d) | after the occurrence of a fundamental change, make any change that adversely affects the right of holders of the Debt Securities of such series to require the Company to purchase such Debt Securities in accordance with the terms thereof and the Indenture, |
| (e) | change the currency of any payment amount or conversion ratio of any Debt Security of such series from the original currency or class of conversion shares as provided in the Indenture, |
| (f) | make any change that impairs the right of holders of Debt Securities of such series to convert any Debt Security, |
| (g) | make any change that impairs the right of holders to institute suit for payment of the Debt Securities of such series, |
| (h) | reduce the percentage in principal amount of outstanding Debt Securities of such series, the consent of whose holders is required for any such supplemental indenture, or the consent of whose holders is required for any waiver (of compliance with certain provisions of the Indenture or certain defaults thereunder and their consequences) provided for in the Indenture, |
| (i) | modify the obligation of the Company to maintain an agency in the city of New York as required under the Indenture, |
| (j) | change the ranking of the Debt Securities of such series in any manner that adversely affects the rights of holders of Debt Securities of such series under the Indenture, or |
| (k) | modify any of the provisions relating to the foregoing modifications or waivers of past defaults, except to increase any such percentage or to provide that certain other provisions of the Indenture cannot be modified or waived without the consent of the holder of each outstanding Debt Security affected thereby. |
The holders of a majority in principal amount of Debt Securities may, on behalf of the holders of all Debt Securities, waive the Company's compliance with certain restrictive provisions of the Indenture. The holders of a majority in principal amount of outstanding Debt Securities may waive any past default under the Indenture, except a default in the payment of the principal of or interest on any Debt Security or in respect of any item listed immediately before this paragraph.
The Indenture or the Debt Securities may be amended or supplemented, without the consent of any holder of such Debt Securities, in certain circumstances as set out in the Indenture, including, among other things, to cure any ambiguity or inconsistency, comply with applicable law or to make any change, in any case, that does not have a materially adverse effect on the rights of any holder of such Debt Securities.
Consent to Jurisdiction and Service
For Debt Securities issued into the United States, under the Indenture, the Company will irrevocably appoint an authorized agent upon which process may be served in any suit, action or proceeding arising out of or relating to the Debt Securities or the Indenture that may be instituted in any United States federal or New York state court located in the city of New York and will submit to such non-exclusive jurisdiction.
Governing Law
To the extent Debt Securities are issued into the United States, each such Indenture is expected to be governed by and construed in accordance with the laws of the State of New York but without giving effect to applicable principles of conflicts of law to the extent that the application of the law of another jurisdiction would be required thereby and certain provisions of theUnited States Trust Indenture Act of 1939, as amended. Otherwise,such Indenture is expected to be governed by laws of the Province of British Columbia or such other laws as set forth in the applicable Prospectus Supplement.
The Trustee
The Trustee under the Indenture or its affiliates may provide other services to the Company in the ordinary course of their business.
The Indenture will contain certain limitations on the rights of the Trustee, as long as it or any of its affiliates remains the Company's creditor, to obtain payment of claims in certain cases or to realize on certain property received on any claim as security or otherwise. The Trustee and its affiliates will be permitted to engage in other transactions with the Company. If the Trustee or any affiliate acquires any conflicting interest and a default occurs with respect to the Debt Securities, the Trustee must eliminate the conflict or resign.
PRIOR SALES
Common Shares
The following table summarizes details of the Common Shares issued by the Company during the 12-month period prior to the date of this Prospectus:
| Date of Issuance | | Price Per Common Share | | | Number of Common Shares | |
| March 13, 2018 | | $ | 0.40 | (1) | | | 100,000 | |
| March 16, 2018 | | $ | 0.72 | (1) | | | 20,000 | |
| May 1, 2018 | | $ | 4.54 | (4) | | | 9,911,894 | |
| May 22, 2018 | | $ | 4.20 | | | | 4,000,000 | |
| May 31, 2018 | | $ | 0.55 | (1) | | | 40,000 | |
| June 21, 2018 | | $ | 0.55 | (1) | | | 40,000 | |
| August 2, 2018 | | $ | 0.41 | (1) | | | 10,000 | |
| August 2, 2018 | | $ | 0.55 | (1) | | | 30,000 | |
| August 2-3, 2018 | | $ | 0.72 | (1) | | | 60,000 | |
| August 14, 2018 | | $ | 3.66 | (5) | | | 1,093,938 | |
| August 16, 2018 | | $ | 1.22 | (1) | | | 5,555 | |
| August 30, 2018 | | $ | 0.72 | (1) | | | 50,000 | |
| September 7, 2018 | | $ | 0.72 | (1) | | | 15,000 | |
| September 11, 2018 | | $ | 0.72 | (1) | | | 50,000 | |
| September 14, 2018 | | $ | 0.72 | (1) | | | 2,500 | |
| September 20, 2018 | | $ | 1.42 | (1) | | | 694 | |
| September 21, 2018 | | $ | 0.40 | (1) | | | 50,000 | |
| September 21, 2018 | | $ | 1.22 | (1) | | | 5,000 | |
| October 3, 2018 | | $ | 1.16 | (1) | | | 7,780 | |
| October 10, 2018 | | $ | 0.40 | (1) | | | 50,000 | |
| October 10, 2018 | | $ | 1.22 | (1) | | | 7,500 | |
| October 16, 2018 | | $ | 4.25 | (1) | | | 3,750 | |
| October 17, 2018 | | $ | 1.47 | (1) | | | 25,000 | |
| October 17, 2018 | | $ | 0.72 | (1) | | | 50,000 | |
| October 18, 2018 | | $ | 1.42 | (1) | | | 3,470 | |
| October 22, 2018 | | $ | $1.19 | (1) | | | 37,500 | |
| October 24, 2018 | | $ | $0.40 | (1) | | | 75,000 | |
| October 24, 2018 | | $ | $1.22 | (1) | | | 4,444 | |
| October 24, 2018 | | $ | $0.72 | (1) | | | 6,951 | |
| November 1, 2018 | | $ | 1.22 | (1) | | | 5,000 | |
| November 1, 2018 | | $ | $0.72 | (1) | | | 26,528 | |
| Date of Issuance | | Price Per Common Share | | | Number of Common Shares | |
| November 9, 2018 | | $ | 0.40 | (1) | | | 40,000 | |
| December 5, 2018 | | $ | 1.29 | (1) | | | 250,000 | |
| December 10, 2018 | | $ | 2.70 | | | | 4,000,000 | |
| January 3, 2019 | | $ | 0.72 | (1) | | | 25,000 | |
| January 10, 2019 | | $ | 1.51 | (1) | | | 50,000 | |
| January 17, 2019 | | $ | 0.18 | (1) | | | 49,992 | |
| January 17, 2019 | | $ | 0.72 | (1) | | | 24,996 | |
| January 17, 2019 | | $ | 0.00 | (6) | | | 355,000 | |
| January 24, 2019 | | $ | 1.38 | (1) | | | 9,033 | |
| January 28, 2019 | | $ | 0.40 | (1) | | | 50,000 | |
| January 28, 2019 | | $ | 0.72 | (1) | | | 130,000 | |
| January 28, 2019 | | $ | 1.22 | (1) | | | 80,000 | |
| January 29, 2019 | | $ | 1.22 | (1) | | | 30,000 | |
| January 30, 2019 | | $ | 0.40 | (1) | | | 60,000 | |
| January 30, 2019 | | $ | 0.72 | (1) | | | 62,509 | |
| February 6, 2019 | | $ | 0.40 | (1) | | | 50,000 | |
| February 7, 2019 | | $ | 0.18 | (1) | | | 75,003 | |
| February 11, 2019 | | $ | 1.22 | (1) | | | 8,333 | |
| February 21, 2019 | | $ | 0.72 | (1) | | | 37,513 | |
| February 21, 2019 | | $ | 0.40 | (1) | | | 50,000 | |
| February 21, 2019 | | $ | 1.27 | (1) | | | 50,000 | |
| February 25, 2019 | | $ | 1.22 | (1) | | | 5,000 | |
| March 1, 2019 | | $ | 1.22 | | | | 5,000 | |
| March 5, 2019 | | $ | 0.72 | | | | 5,000 | |
| March 11, 2019 | | $ | 1.22 | (1) | | | 47,222 | |
| March 15, 2019 | | $ | 0.40 | (1) | | | 50,000 | |
Notes:
| 1. | Issued for cash pursuant to the exercise of stock options. |
| 2. | Issued for cash pursuant to the exercise of common share purchase warrants. |
| 3. | Issued to satisfy 50% of the purchase price of Verdélite, of which 4,955,947 will be held in escrow until May 1, 2019. |
| 4. | Issued as partial payment for the purchase price for the remaining shares of Avalite. |
| 5. | Issued upon vesting of restricted share units of the Company. |
Warrants
The following table summarizes details of the common share purchase warrants issued by the Company during the 12-month period prior to the date of this Prospectus:
| Date of Issuance | | Number of Warrants Issued | | | Exercise Price | | | Expiry Date |
| May 22, 2018 | | | 4,000,000 | | | $ | 5.20 | | | November 22, 2019 |
As of the date hereof, there are 8,411,761 warrants of the Company outstanding.
Stock Options
The following table summarizes details of the stock options issued by the Company during the 12-month period prior to the date of this Prospectus:
| Date of Grant | | Number of Options Granted | | | Exercise Price | | | Expiry Date |
| March 8, 2018 | | | 50,000 | | | $ | 5.67 | | | March 8, 2023 |
| March 19, 2018 | | | 25,000 | | | $ | 5.85 | | | March 19, 2023 |
| March 26, 2018 | | | 150,000 | | | $ | 5.69 | | | March 26, 2023 |
| April 6, 2018 | | | 50,000 | | | $ | 4.55 | | | April 6, 2023 |
| May 22, 2018 | | | 15,000 | | | $ | 4.70 | | | May 22, 2023 |
| May 28, 2018 | | | 15,000 | | | $ | 4.21 | | | May 28, 2023 |
| June 11, 2018 | | | 190,000 | | | $ | 4.27 | | | June 11, 2023 |
| June 20, 2018 | | | 50,000 | | | $ | 4.08 | | | June 20, 2023 |
| June 28, 2018 | | | 10,000 | | | $ | 3.69 | | | June 28, 2023 |
| July 3, 2018 | | | 15,000 | | | $ | 3.70 | | | July 3, 2023 |
| July 9, 2018 | | | 30,000 | | | $ | 3.58 | | | July 9, 2023 |
| July 18, 2018 | | | 15,000 | | | $ | 2.49 | | | July 18, 2023 |
| July 23, 2018 | | | 12,500 | | | $ | 2.59 | | | July 23, 2023 |
| August 7, 2018 | | | 60,000 | | | $ | 2.72 | | | August 7, 2023 |
| August 20, 2018 | | | 625,000 | | | $ | 3.21 | | | August 20, 2023 |
| August 23, 2018 | | | 25,000 | | | $ | 4.30 | | | August 23, 2023 |
| September 1, 2018 | | | 185,000 | | | $ | 4.58 | | | September 1, 2023 |
| September 10, 2018 | | | 25,000 | | | $ | 5.20 | | | September 10, 2023 |
| October 1, 2018 | | | 150,000 | | | $ | 4.60 | | | October 1, 2023 |
| October 29, 2018 | | | 10,000 | | | $ | 3.40 | | | October 29, 2023 |
| November 1, 2018 | | | 161,000 | | | $ | 3.88 | | | November 1, 2023 |
| December 1, 2018 | | | 472,500 | | | $ | 3.07 | | | December 1, 2023 |
| January 1, 2019 | | | 88,000 | | | $ | 2.83 | | | January 1, 2024 |
| February 1, 2019 | | | 160,000 | | | $ | 3.48 | | | February 1, 2024 |
| March 1, 2019 | | | 5,000 | | | $ | 3.88 | | | March 1, 2024 |
| March 4, 2019 | | | 200,000 | | | $ | 3.84 | | | March 4, 2024 |
As of the date hereof, there are 8,932,602 stock options of the Company outstanding.
Restricted Stock Units
Restricted share units of the Company ("RSUs") will be settled by the issuance of Common Shares on the vesting date. No RSUs have been issuedby the Company during the 12-month period prior to the date of this Prospectus.
As of the date hereof, there are 475,000 RSUsoutstanding.
TRADING PRICE AND VOLUME
The Common Shares trade on the Exchange under the symbol "EMH". On March 12, 2019, the last trading day completed prior to the filing of this Prospectus, the closing price of the Common Shares on the Exchange was $3.82. The price range and trading volume of the Common Shares for each month from March 2018 to March 12, 2019, as reported by the Exchange, are set out below:
| Month | | High | | | Low | | | Total Volume | |
| March 1 - March 12, 2019 | | $ | 4.04 | | | $ | 3.42 | | | | 5,449,595 | |
| February , 2019 | | $ | 4.27 | | | $ | 3.20 | | | | 24,435,249 | |
| January 2019 | | $ | 3.75 | | | $ | 2.68 | | | | 18,004,745 | |
| December 2018 | | $ | 3.10 | | | $ | 2.04 | | | | 11,534,652 | |
| November 2018 | | $ | 5.25 | | | $ | 3.00 | | | | 16,909,293 | |
| October 2018 | | $ | 5.24 | | | $ | 2.87 | | | | 20,496,651 | |
| September 2018 | | $ | 5.92 | | | $ | 4.24 | | | | 31,678,367 | |
| August 2018 | | $ | 4.96 | | | $ | 2.55 | | | | 32,355,196 | |
| July 2018 | | $ | 3.73 | | | $ | 2.44 | | | | 21,607,262 | |
| June 2018 | | $ | 4.50 | | | $ | 3.52 | | | | 13,609,070 | |
| May 2018 | | $ | 4.95 | | | $ | 4.02 | | | | 11,040,707 | |
| April 2018 | | $ | 5.28 | | | $ | 3.69 | | | | 17,499,500 | |
| March 2018 | | $ | 6.35 | | | $ | 4.81 | | | | 20,884,300 | |
CERTAIN INCOME TAX CONSIDERATIONS
The applicable Prospectus Supplement may describe certain Canadian federal income tax consequences to a purchaser who is a non-resident of Canada or to a purchaser who is a resident of Canada of acquiring, owning and disposing of any of the Securities offered thereunder.
The applicable Prospectus Supplement may also describe certain United States federal income tax consequences of the acquisition, ownership and disposition of Securities by an initial purchaser who is a "U.S. person" (within the meaning of the United States Internal Revenue Code), if applicable, including, to the extent applicable, any such consequences relating to Securities payable in a currency other than the United States dollar, issued at an original issue discount for United States federal income tax purposes or containing early redemption provisions or other special terms.
RISK FACTORS
Before deciding to invest in any Securities, prospective purchaser in the Securities should consider carefully the risk factors and the other information contained and incorporated by reference in this Prospectus and the applicable Prospectus Supplement relating to a specific offering of Securities before purchasing the Securities. An investment in the Securities offered hereunder is speculative and involves a high degree of risk. Information regarding the risks affecting the Company and its business is provided in the documents incorporated by reference in this Prospectus, including in the Company's most recent annual information form under the heading "Risk Factors". Additional risks and uncertainties not known to the Company or that management currently deems immaterial may also impair the Company's business, financial condition, results of operations or prospects. See "Documents Incorporated by Reference".
Negative Cash Flow from Operating Activities
The Company has not yet achieved positive operating cash flow, and the Company will continue to experience negative cash flow from operations in the foreseeable future. The Company has incurred net losses in the past and may incur losses in the future unless it can derive sufficient revenues from its business. Such future losses could have an adverse effect on the market price of the Company's securities, which could cause investors to lose part or all of their investment.
Financial Statements contain a Going Concern Disclosure
The Company's interim and annual financial statements contain a going concern disclosure. The Company has a limited operating history and a history of negative cash flow from operating activities. The Company's ability to continue as a going concern is dependent upon its ability to: raise additional capital; achieve sustainable revenues and profitable operations; and obtain the necessary financing to meet obligations and repay liabilities when they become due. No assurances can be given that the Company will be successful in achieving these goals. If the Company is unable to achieve these goals, its ability to carry out and implement planned business objectives and strategies will be significantly delayed, limited or may not occur. These material circumstances cast substantial doubt on the Company's ability to continue as a going concern and ultimately on the appropriateness of the use of the accounting principles applicable to a going concern. The Company's financial statements do not include adjustments to amounts and classifications of assets and liabilities that might be necessary should the Company be unable to continue as a going concern. The Company continues to have access to equity and debt capital from public and private markets in Canada but there are no guarantees that such capital will be available.
No Assurance of Active or Liquid Market
No assurance can be given that an active or liquid trading market for the Common Shares will be sustained. If an active or liquid market for the Common Shares fails to be sustained, the prices at which such shares trade may be adversely affected. Whether or not the Common Shares will trade at lower prices depends on many factors, including the liquidity of the Common Shares, prevailing interest rates and the markets for similar securities, general economic conditions and the Company's financial condition, historic financial performance and future prospects.
There is no public market for the Preferred Shares, Warrants, Subscription Receipts, Units or Debt Securities and, unless otherwise specified in the applicable Prospectus Supplement, the Company does not intend to apply for listing of such Securities on any securities exchanges. If the Preferred Shares, Warrants, Subscription Receipts, Units or Debt Securities are traded after their initial issue, they may trade at a discount from their initial offering prices depending on prevailing interest rates (as applicable), the market for similar securities and other factors including general economic conditions and the Company's financial condition. There can be no assurance as to the liquidity of the trading market for the Preferred Shares, Warrants, Subscription Receipts, Units or Debt Securities or that a trading market for these securities will develop.
Public Markets and Share Prices
The market price of the Common Shares and any other Securities offered hereunder that become listed and posted for trading on the Exchange or any other stock exchange could be subject to significant fluctuations in response to variations in the Company's financial results or other factors. In addition, fluctuations in the stock market may adversely affect the market price of the Common Shares and any other Securities offered hereunder that become listed and posted for trading on the Exchange or any other stock exchange regardless of the financial performance of the Company. Securities markets have also experienced significant price and volume fluctuations from time to time. In some instances, these fluctuations have been unrelated or disproportionate to the financial performance of issuers. Market fluctuations may adversely impact the market price of the Common Shares and any other Securities offered hereunder that become listed and posted for trading on the Exchange or any other stock exchange. There can be no assurance of the price at which the Common Shares or any other Securities offered hereunder that become listed and posted for trading on the Exchange or any other stock exchange will trade.
Additional Issuances and Dilution
The Company may issue and sell additional securities of the Company to finance its operations or future acquisitions. Shareholders of the Company, including Sciences, may also sell Securities they hold or may hold in the future, including pursuant to a prospectus supplement to this Prospectus. The Company cannot predict the size of future issuances of securities of the Company or the effect, if any, that future issuances and sales of securities will have on the market price of any securities issued and outstanding from time to time. Sales or issuances of substantial amounts of securities of the Company, or the perception that such sales could occur, may adversely affect prevailing market prices for Securities issued and outstanding from time to time. With any additional sale or issuance of securities of the Company, holders will suffer dilution with respect to voting power and may experience dilution in the Company's earnings per share. Moreover, this Prospectus may create a perceived risk of dilution resulting in downward pressure on the price of the Common Shares, which could contribute to progressive declines in the prices of such securities.
Changes in Interest Rates
Prevailing interest rates will affect the market price or value of the Debt Securities. The market price or value of the Debt Securities may decline as prevailing interest rates for comparable debt instruments rise, and increase as prevailing interest rates for comparable debt instruments decline.
Fluctuations in Foreign Currency Markets
Debt Securities denominated or payable in foreign currencies may entail significant risk. These risks include, without limitation, the possibility of significant fluctuations in the foreign currency markets, the imposition or modification of foreign exchange controls and potential liquidity in the secondary market. These risks will vary depending upon the currency or currencies involved and will be more fully described in the applicable Prospectus Supplement.
Broad Discretion in Use of Net Proceeds
Management of the Company will have broad discretion with respect to the application of net proceeds received by the Company from the sale of Securities under this Prospectus or a future Prospectus Supplement and may spend such proceeds in ways that do not improve the Company's results of operations or enhance the value of the Common Shares or its other securities issued and outstanding from time to time. Any failure by management to apply these funds effectively could result in financial losses that could have a material adverse effect on the Company's business or cause the price of the Securities issued and outstanding from time to time to decline. The Company will not receive any proceeds from any sale of Securities by any Selling Securityholder.
Enforceability of Actions
The Company is a company incorporated under the laws of the province of British Columbia. Certain of the Company's directors and officers, as well as the experts named in this Prospectus, reside principally in Canada. Because substantially all of the Company's assets and the assets of these persons are located outside of the United States, it may not be possible for a purchaser to effect service of process within the United States upon the Company or those persons. Furthermore, it may not be possible for a purchaser to enforce against the Company or those persons in the United States, judgments obtained in United States courts based upon the civil liability provisions of the United States federal securities laws or other laws of the United States. There is doubt as to the enforceability, in original actions in Canadian courts, of liabilities based upon United States federal securities laws and as to the enforceability in Canadian courts of judgments of United States courts obtained in actions based upon the civil liability provisions of the United States federal securities laws. Therefore, it may not be possible to enforce those actions against the Company, certain of the Company's directors and officers or the experts named in this prospectus. Additionally, some of the directors and officers of the Company reside outside of Canada. Some or all of the assets of such persons may be located outside of Canada. Therefore, it may not be possible forpurchaser to collect or to enforce judgments obtained in Canadian courts predicated upon the civil liability provisions of applicable Canadian securities laws against such persons.
Treatment as Passive Foreign Investment Company
United States purchasers should be aware that they could be subject to certain adverse United States federal income tax consequences in the event that the Company is classified as a passive foreign investment company ("PFIC") for United States federal income tax purposes. The determination of whether the Company is a PFIC for a taxable year depends, in part, on the application of complex United States federal income tax rules, which are subject to differing interpretations, and the determination will depend on the composition of the Company's income, expenses and assets from time to time and the nature of the activities performed by the Company's officers and employees. Prospective purchasers should carefully read the tax discussion in any applicable Prospectus Supplement for more information and consult their own tax advisers regarding the likelihood and consequences of the Company being treated as a PFIC for United States federal income tax purposes, including the advisability of making certain elections that may mitigate certain possible adverse U.S. federal income tax consequences but may result in an inclusion in gross income without receipt of such income.
Foreign Private Issuer Status and Emerging Growth Company Status
As a foreign private issuer, as defined in Rule 3b-4 under the U.S. Exchange Act, the Company is exempt from certain of the provisions of the United States federal securities laws. For example, the United States proxy rules and the Section 16 reporting and "short swing" profit rules do not apply to foreign private issuers.
However, if the Company were to lose its status as a foreign private issuer, these regulations would immediately apply and the Company would also be required to commence reporting on forms required of United States companies, such as Forms 10-K, 10-Q and 8-K, rather than the forms currently available to the Company, such as Forms 40-F and 6-K. Compliance with these additional disclosure and timing requirements under these securities laws would likely result in increased expenses and would require the Company's management to devote substantial time and resources to comply with new regulatory requirements.
Further, to the extent that the Company were to offer or sell its securities outside of the United States, the Company would have to comply with the more restrictive Regulation S requirements that apply to United States companies, and the Company would no longer be able to utilize the multijurisdictional disclosure system forms for registered offerings by Canadian companies in the United States, which could limit the Company's ability to access United States' capital markets in the future.
The Company also qualifies as an "emerging growth company" as defined in theUnited States Jumpstart Our Business Startups Act of 2012. An emerging growth company may take advantage of specified reduced reporting requirements that are otherwise generally applicable to public companies that are not emerging growth companies. These reduced reporting requirements include an exemption from compliance with the auditor attestation requirement on the effectiveness of our internal control over financial reporting. The Company may take advantage of some of these exemptions until it is no longer an emerging growth company. The Company could remain an emerging growth company for up to five years, although circumstances could cause the Company to lose that status earlier, including if the market value of the Common Shares held by non-affiliates exceeds US$700.0 million as of the end of our most recently completed second fiscal quarter, if we have total annual gross revenue of US$1.07 billion or more during any fiscal year, or if we issue more than US$1.0 billion in non-convertible debt during any three-year period.
EXEMPTION FROM NATIONAL INSTRUMENT 44-102
Pursuant to a decision of the Autorité des marchés financiers dated February 25, 2019, the Company was granted a permanent exemption from the requirement to translate into French this Prospectus as well as the documents incorporated by reference therein and any Prospectus Supplement to be filed in relation to an "at-the-market" distribution. This exemption is granted on the condition that this Prospectus and any Prospectus Supplement (other than in relation to an "at-the-market" distribution) be translated into French if the Company offers Securities to Quebec purchasers in connection with an offering other than in relation to an "at-the-market" distribution.
LEGAL MATTERS
Unless otherwise specified in a Prospectus Supplement relating to any Securities offered, certain legal matters in connection with the offering of Securities will be passed upon on behalf of the Company by Bennett Jones LLP, as to Canadian legal matters and Dorsey & Whitney LLP, Denver, Colorado, as to United States legal matters. In addition, certain legal matters in connection with any offering of Securities will be passed upon for any underwriters, dealers or agents by counsel to be designated at the time of the offering by such underwriters, dealers or agents, as the case may be.
AUDITOR, TRANSFER AGENT AND REGISTRAR
Deloitte LLP is the auditor of the Company. Deloitte LLP is independent within the meaning of the Rules of Professional Conduct of the Chartered Professional Accountants of British Columbia.
The transfer agent and registrar for the Common Shares is Computershare Trust Company of Canada at its principal transfer offices in Vancouver, British Columbia.
ENFORCEABILITY OF CIVIL LIABILITIES
The Company is governed by the laws of British Columbia and its principal place of business is outside the United States. The majority of the directors and officers of the Company and the experts named herein are resident outside of the United States and a substantial portion of the Company's assets and the assets of such persons are located outside of the United States. Consequently, it may be difficult for United States purchasers to effect service of process within the United States on the Company, its directors or officers or such experts, or to realize in the United States on judgments of courts of the United States predicated on civil liabilities under the U.S. Securities Act. Purchasers should not assume that Canadian courts would enforce judgments of United States courts obtained in actions against the Company or such persons predicated on the civil liability provisions of the United States federal securities laws or the securities or "blue sky" laws of any state within the United States or would enforce, in original actions, liabilities against the Company or such persons predicated on the United States federal securities or any such state securities or "blue sky" laws.
The Company filed with the SEC, concurrently with the Registration Statement, an appointment of agent for service of process on Form F-X. Under the Form F-X, the Company appointed DL Services Inc., as its agent for service of process in the United States in connection with any investigation or administrative proceeding conducted by the SEC, and any civil suit or action brought against or involving the Company in a United States court, arising out of or related to or concerning the offering of Securities under this Prospectus.


