Exhibit 99.1

Public limited company with a Board of Directors with share capital of 32,764,700.60 euros
Registered office: 14 Avenue de l'Opéra – 75001 PARIS
Paris Commercial Register (RCS) 492 002 225
HALF-YEAR FINANCIAL REPORT
AS OF JUNE 30, 2022
Half-year financial report as of June 30, 2022
CONTENTS
| 1. | Statement by the person responsible for the half-year financial report | 4 | |
| 1.1 | Person responsible for the half-year financial report | 4 | |
| 1.2 | Statement by the person responsible | 4 | |
| 2. | Activity report as of June 30, 2022 | 5 | |
| 2.1 | Summary presentation of the financial statements | 5 | |
| 2.2 | Company activity and results | 6 | |
| 2.3 | Situation in Ukraine and its impact on the company’s business | 9 | |
| 2.4 | Subsequent events | 10 | |
| 2.5 | Evolution and outlook | 11 | |
| 2.6 | Risk factors and related party transactions | 11 | |
| 3. | UNAUDITED INTERIM CONDENSED consolidated financial statements under IFRS for the six-month period ended June 30, 2022 | 12 | |
| Statement of consolidated financial position | 12 | ||
| Statement of consolidated operations | 13 | ||
| Statement of consolidated comprehensive loss | 14 | ||
| Statement of changes in consolidated shareholders’ equity | 15 | ||
| Statement of consolidated cash flows | 16 | ||
| Notes to the unaudited interim condensed consolidated financial statements | 17 | ||
| 4. | Statutory Auditors' limited review report on the half-year condensed consolidated financial statements prepared in accordance with IFRS standards as adopted in the European Union | 49 | |
Page 2 of 49
Half-year financial report as of June 30, 2022
GENERAL NOTES
Definitions
In this half-year financial report, and unless otherwise indicated:
| • | The terms “Company” or “Biophytis” refer to Biophytis SA whose registered office is located at 14 Avenue de l'Opéra – 75001 PARIS, France, registered with the Paris Trade and Companies Registry under number 492 002 225 and its subsidiaries Instituto Biophytis do Brasil (Brazil) and Biophytis Inc (United States); |
| • | “Financial Report” means this half-year financial report as of June 30, 2022. |
About Biophytis
Biophytis SA is a clinical-stage biotechnology company focused on the development of therapeutics that slow the degenerative processes associated with aging and improve functional outcomes for patients suffering from age-related diseases, including severe respiratory failure in patients suffering from COVID-19.
Sarconeos (BIO101), our leading drug candidate, is a small molecule, administered orally, under development for the treatment of sarcopenia, as part of a Phase 2 clinical trial in the United States and Europe (SARA-INT). It is also being investigated in a two-part Phase 2-3 clinical trial (COVA) for the treatment of severe respiratory symptoms of COVID-19 in Europe, Latin America and the United States. A pediatric formulation of Sarconeos (BIO101) is being developed for the treatment of Duchenne muscular dystrophy.
The Company is based in Paris, France, and Cambridge, Massachusetts. The Company’s ordinary shares are listed on Euronext Growth Paris (Ticker: ALBPS - ISIN: FR0012816825) and its ADSs (American Depositary Shares) are listed on the Nasdaq (Ticker BPTS - ISIN: US09076G1040).
For more information: www.biophytis.com
Page 3 of 49
Half-year financial report as of June 30, 2022
1. Statement by the person responsible for the half-year financial report
1.1 Person responsible for the half-year financial report
Stanislas Veillet, Chairman and Chief Executive Officer
1.2 Statement by the person responsible
(Art. 222-3 - 4° of the General Regulations of the French financial markets authority (Autorité des marchés financiers – AMF))
“I certify, to the best of my knowledge, that the condensed financial statements for the past half-year have been prepared in accordance with applicable accounting standards and give a true and fair view of the assets, financial position and results of the Company and of all the companies included in the consolidation, and that this Financial Report presents an accurate picture of the significant events that occurred during the first six months of the financial year, their impact on the financial statements, the main transactions between related parties and that it describes the main risks and uncertainties for the remaining six months of the financial year”.
Paris, January 30, 2022
Stanislas Veillet, Chairman and Chief Executive Officer
Page 4 of 49
Half-year financial report as of June 30, 2022
2. Activity report as of June 30, 2022
2.1 Summary presentation of the financial statements
Statement of consolidated financial position
| (Amounts in thousands of euros) | December 31, 2021 (as restated) (1) | June 30, 2022 | ||||||
| NON-CURRENT ASSETS | 3,506 | 3,552 | ||||||
| Patents and software | 2,757 | 2,660 | ||||||
| Property, plant and equipment | 563 | 717 | ||||||
| Other non-current financial assets | 186 | 175 | ||||||
| CURRENT ASSETS | 31,366 | 30,334 | ||||||
| Other receivables | 6,536 | 10,181 | ||||||
| Other current financial assets | 904 | 407 | ||||||
| Cash and cash equivalents | 23,926 | 19,745 | ||||||
| TOTAL ASSETS | 34,872 | 33,886 | ||||||
| EQUITY | 5,803 | 2,961 | ||||||
| Share capital | 27,191 | 32,765 | ||||||
| Share premium | 27,781 | 8,410 | ||||||
| Other reserves | (124 | ) | (81 | ) | ||||
| Retained earnings | (49,013 | ) | (38,101 | ) | ||||
| Non-controlling interests | (32 | ) | (32 | ) | ||||
| NON-CURRENT LIABILITIES | 6,259 | 5,210 | ||||||
| Employee benefit obligations | 205 | 195 | ||||||
| Non-current financial liabilities | 5,518 | 5,015 | ||||||
| Non-current derivative financial instruments | 536 | 0 | ||||||
| CURRENT LIABILITIES | 22,810 | 25,716 | ||||||
| Current financial liabilities | 12,037 | 13,913 | ||||||
| Provisions | - | 75 | ||||||
| Trade payables | 7,606 | 10,089 | ||||||
| Accrued taxes and employee benefits payable | 1,998 | 1,288 | ||||||
| Current derivative financial instruments | 788 | 62 | ||||||
| Other creditors and miscellaneous liabilities | 381 | 289 | ||||||
| TOTAL EQUITY AND LIABILITIES | 34,872 | 33,886 | ||||||
(1) Refer to Note 2.7 “Restatement of Previously Issued Financial Statements” of the notes to the unaudited interim condensed financial statements for the six month period ended June 30, 2022 in part 3.
Page 5 of 49
Half-year financial report as of June 30, 2022
Statement of consolidated operations and comprehensive loss
| (Amounts in thousands of euros) | First half 2021 | First half 2022 | ||||||
| Revenue | - | - | ||||||
| Cost of sales | - | - | ||||||
| Gross margin | - | - | ||||||
| Research and development expenses, net | (7,594 | ) | (6,867 | ) | ||||
| General and administrative expenses | (2,919 | ) | (5,053 | ) | ||||
| Operating loss | (10,513 | ) | (11,920 | ) | ||||
| Net financial expense | (2,732 | ) | (478 | ) | ||||
| Income tax benefit (expense) | - | - | ||||||
| Net loss for the period | (13,245 | ) | (12,398 | ) | ||||
| Remeasurements of the defined benefit liability (asset) | 7 | 40 | ||||||
| Foreign currency translation adjustment | 3 | 46 | ||||||
| Other compehensive income | 10 | 86 | ||||||
| Total comprehensive loss for the period | (13,235 | ) | (12,312 | ) | ||||
| Total comprehensive loss for the period - attribuable to owners of the parent | (13,235 | ) | (12,312 | ) | ||||
Statement of consolidated cash flows
| (Amounts in thousands of euros) | First half 2021 | First half 2022 | ||||||
| Net cash (used in) provided by: | ||||||||
| Operating activities | (13,492 | ) | (10,261 | ) | ||||
| Investing activities | 12,474 | (10 | ) | |||||
| Financing activities | 18,156 | 6,032 | ||||||
| Effect of exchange rate changes on cash and cash equivalents | 2 | 58 | ||||||
| Net increase (decrease) in cash and cash equivalents | 17,140 | (4,181 | ) | |||||
2.2 Company activity and results
2.2.1 Activity
First-half 2022 saw the continuation of the development strategy pursued by the Company over several years.
| A) | In operational terms, the Company continued to develop its main clinical programs while strengthening its pre-clinical assets: |
Development of Sarconeos (BIO101) for Sarcopenia – SARA program. Following the completion of the Phase 2b SARA-INT study in 2021, the Company has been working with experts and regulatory authorities to design Phase 3. Several meetings were held with these experts and the European (EMA) and US (FDA) regulatory authorities in the first half of the year, in relation to the design of the Phase 3 study, an overview of which was presented at the SCWD conference in June 2022.
Development of Sarconeos (BIO101) for the prevention of respiratory deterioration in COVID-19 patients – COVA program. The Company announced in early April 2022 that, in light of the evolving epidemic, it was halting patient enrollment in its Phase 2-3 study after the enrollment of the 237th patient and would work with CROs to report the study's results in the third quarter of 2022.
Page 6 of 49
Half-year financial report as of June 30, 2022
In June 2022, the Company announced that it was presenting new pre-clinical efficacy data for its product Sarconeos (BIO101) in SMA (Spinal Muscular Atrophy) at the SMA Cure 2022 conference in Anaheim, California, USA, from June 16 to 19, 2022. This strengthens the pre-clinical data available for Sarconeos (BIO101) as a treatment for orphan neuromuscular disorders.
| B) | Financially, the first half of the year was marked by the drawdown of two 4 million euros tranches of ORNANE bonds each under the new 32 million euros agreement signed with Atlas in 2021. |
2.2.2 Governance
The Combined General Meeting of Shareholders of the Company was unable to validly deliberate due to the lack of a quorum on June 3, 2022. The Combined General Meeting was held on the second call on June 21, 2022.
The shareholders approved all the resolutions presented by the Board of Directors to the Combined Shareholders' Meeting, and in particular those ratifying the delegations of powers to the Board of Directors to decide on the issue of shares and/or securities.
2.2.3 Operating expenses
The Company’s operating loss was -11,920 thousand euros as of June 30, 2022 compared to -10,513 thousand euros as of June 30, 2021.
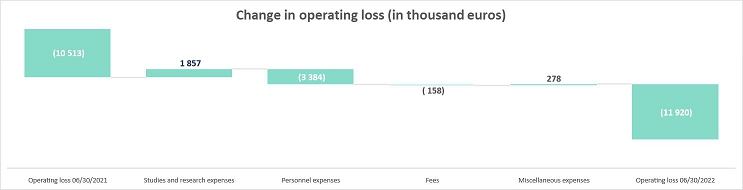
This change is mainly due to an increase of the personnel expenses for an amount of 3,384 thousand euros offset by a decrease of the studies and research expenses.
Page 7 of 49
Half-year financial report as of June 30, 2022
2.2.4 Net financial expense
| (Amounts in thousands of euros) | June 30, 2021 | June 30, 2022 | ||||||
| Interest and amortized cost of bonds (1) | (252 | ) | (1,024 | ) | ||||
| Changes in fair value of convertible bonds (1) | (691 | ) | 1,028 | |||||
| Negma financial indemnity | (187 | ) | - | |||||
| Provision related to Negma litigation | (1,508 | ) | (75 | ) | ||||
| Financial income related to the return of penalties by Negma | 20 | - | ||||||
| Other financial expenses | (80 | ) | (33 | ) | ||||
| Transaction costs related to the issuance of convertible bonds | (38 | ) | (380 | ) | ||||
| Other financial income | 3 | (14 | ) | |||||
| Foreign exchange gains (losses) | 1 | - | ||||||
| Total net financial expense | (2,732 | ) | (478 | ) | ||||
(1) see Note 12.2 “Bonds” of Notes to the consolidated half-year financial statements for the period ended June 30, 2022 in part 3.
The net financial expense amounted to -478 thousand euros as of June 30, 2022, compared to -2,732 thousand euros as of June 30, 2021. This change is mainly due to the incomes related to the changes in fair value of convertible bonds. This is partially offset by an increase of the financial interests.
Page 8 of 49
Half-year financial report as of June 30, 2022
2.2.5 Cash and liquid assets
The Company’s cash and cash equivalents amounted to 19,7 million euros as of June 30, 2022, compared to 23,9 million euros as of December 31, 2021. This change is mainly due to an improvement in the working capital requirement and the drawdown of the first two tranches of the ORNANE financing line set up with Atlas for 4 million euros in April 2022 and 4 million euros in June 2022.
| Selected items of the condensed consolidated half-year financial statements | ||||||||
| (Amounts in thousands of euros) | First half 2021 | First half 2022 | ||||||
| Cash flows (used in) from operating activities | (13,492 | ) | (10,261 | ) | ||||
| Operating cash flows before change in working capital requirements | (9,449 | ) | (8,296 | ) | ||||
| (-) Change in working capital requirements (net of depreciation of trade receivables and inventories) | 4,043 | (1,965 | ) | |||||
| Cash flows (used in) from investing activities | 12,474 | (10 | ) | |||||
| Acquisition of intangible assets and property, plant and equipment | (29 | ) | (22 | ) | ||||
| Interest on investment accounts | 3 | - | ||||||
| Sale of term deposit classified as other non-current financial assets | 12,500 | 12 | ||||||
| Cash flows (used in) from financing activities | 18,155 | 6,032 | ||||||
| Proceeds from share capital increases, net of bond conversions | 16,584 | - | ||||||
| Costs paid in relation to equity transactions | (2,253 | ) | - | |||||
| Negma indemnities paid | - | - | ||||||
| Negma compensation received (net) | (1,077 | ) | - | |||||
| Subscription of warrants (BSA) | - | - | ||||||
| Exercise of warrants (BSA) & founders’ warrants (BSPCE) | 738 | 7 | ||||||
| Sale of term deposit classified as other non-current financial assets | - | 344 | ||||||
| Reimbursement of prefinanced CIR receivables, net of guarantee deposit | - | - | ||||||
| Proceeds from conditional advances, net of repayments | 400 | 4 | ||||||
| Repayment of repayable advances | (136 | ) | (149 | ) | ||||
| Proceeds from subsidies | 153 | |||||||
| Repayment of borrowings | - | (1,259 | ) | |||||
| Issue of non-convertible bonds, net of repayments | 4,089 | 8,000 | ||||||
| financial interest paid | (190 | ) | (687 | ) | ||||
| Cost incurred in relation to the issuance of convertible and non-convertible bonds | - | (380 | ) | |||||
| Net effect of exchange rate changes on cash and cash equivalents | 2 | 58 | ||||||
| Increase (decrease) in cash | 17,140 | (4,181 | ) | |||||
2.3 Situation in Ukraine and its impact on the company’s business
As of February 24, 2022, Russia significantly intensified its military operations in Ukraine. In response, the European Union, the United States, and certain other countries have imposed significant sanctions and export controls on Russia, Belarus, and certain individuals and entities linked to Russian or Belarusian political, commercial, and financial organizations, and the European Union, the United States, and some other countries could impose additional sanctions, trade restrictions, and other retaliatory measures if the conflict continues or escalates.
Page 9 of 49
Half-year financial report as of June 30, 2022
During the first half of 2022, the situation in Ukraine did not have a significant impact on the Company's operations.
2.4 Subsequent events
COVA study results
On September 7, 2022, the company published the very promising results of the Phase 2-3 study of the COVA program to treat COVID-19-related respiratory failure. In the primary analysis, Sarconeos (BIO101) was shown to reduce the risk of respiratory failure or early death at 28 days (primary endpoint) by 39% compared to the placebo (15.8% versus 26.0%, adjusted difference of 11.8% in favor of treatment, p=0.07). Sarconeos (BIO101) reduced both the proportion of patients with respiratory failure (12.7% versus 21.5%) and early death (0.8% versus 2.8%). Sarconeos (BIO101) also significantly delayed (p=0.03) adverse progression to respiratory failure or early death over a maximum treatment period of 28 days.
ORNANE conversions - ATLAS contracts
Since June 30, 2022, the Company has converted 4 convertible bonds under Tranche 8 of the ATLAS 2020 Contract, the totality of the 160 convertible bonds of the Tranche 1 and 12 convertible bonds of the Tranche 2, both related to the ATLAS 2021 Contract, for a total amount of 4.7 million euros (100 thousand of euros for Tranche 8, 4 million euros for Tranche 1, and 600 thousand of euros for Tranche 2). The operations gave rise to the creation of 65,341,520 new Company shares (1,331,948 shares for Tranche 8, 57,506,246 shares for Tranche 1 and 13,721,104 shares for Tranche 2).
The Company has signed on October 20, 2022, a notification of exercise of Tranche for 160 new convertible bonds with a principal amount equal to 25,000€ in two instalments :
| • | first installment for 80 convertible bonds of tranche 3 with a principal amount equal to € 25,000 and received on November 10, 2022; and |
| • | second installment for 80 convertible bonds of tranche 3 with a principal amount equal to € 25,000 and received on January 11, 2023. |
Negma Group Ltd litigation
The Paris Court of Appeal has:
- confirmed the judgment of the Paris Commercial Court of March 16, 2021 (The Company has already executed in 2021 the entire judment);
- ordered Biophytis to pay Negma 75,000 euros under article 700 of the Code of Procedure civil as well as costs.
Page 10 of 49
Half-year financial report as of June 30, 2022
2.5 Evolution and outlook
Sarconeos (BIO101)
Given the progress of its clinical programs for its drug candidate, Sarconeos (BIO101), Biophytis intends to pursue the following activities:
Sarcopenia (SARA program)
The Company intends to finalize the design of the Phase 3 clinical trial and obtain the necessary approvals from the European and American regulatory authorities in the first half of 2023 in order to be able to initiate this trial. Given the size of the study and the potential market, Biophytis intends to conduct this Phase 3 study in partnership with pharmaceutical companies, either as part of a co-development project or as part of a transfer of commercial exploitation rights.
Severe respiratory failure related to COVID-19 (COVA program)
Equipped with the results of the Phase 2-3 trial, Biophytis can now finalize the analysis of the data. The next step is to present the results in the coming months to the regulatory agencies, health authorities and Biophytis' partners, particularly in Europe, the United States and Brazil, to determine under what conditions the Company can pursue the development of Sarconeos (BIO101) for COVID-19. At the same time, it is considering the possibility of amending and continuing the Early Access Program (EAP) authorized in Brazil and expanding it to other territories.
Duchenne muscular dystrophy (MYODA program)
Following IND (Investigational New Drug) Application approval from the FDA and clearance from the Belgian authorities, the study, which had been delayed due to the COVID-19 pandemic, has been resumed. Biophytis is proceeding with preparations for the launch of the MYODA Phase 1-2 study of Sarconeos (BIO101) for Duchenne muscular dystrophy (DMD), with the objective of enrolling the first patient in this study in the first half of 2023.
MACA program
The Company is continuing its pre-clinical development work on Macuneos (BIO201) and its backup, to develop its drug candidate for dry AMD. Depending on the results and available funding, the Phase 1 clinical trial for this indication could begin in 2023.
2.6 Risk factors and related party transactions
2.6.1 Risk factors
The risk factors are of those presented in the 2021 Annual Financial Report in Appendix 2 “Risks and uncertainties” to faced by the Company”.
2.6.2 Transactions between related parties
Transactions with related parties are as those presented in the 2021 Annual Financial Report in Note 20 “Related parties” of section 3: “Consolidated financial statements prepared in accordance with IFRS as of and for the year ended December 31, 2021” and in Note 19 “Related parties” of section 4: “Annual financial statements of BIOPHYTIS SA for the financial year ended December 31, 2021”.
Page 11 of 49
Half-year financial report as of June 30, 2022
| 3. | UNAUDITED INTERIM CONDENSED consolidated financial statements under IFRS for the six-month period ended June 30, 2022 |
Statement of consolidated financial position
| AS OF | ||||||||||||
| (amounts in thousands of euros) | NOTES | DECEMBER 31, 2021 (as restated) (1) | JUNE 30, 2022 | |||||||||
| ASSETS | ||||||||||||
| Patents and software | 3 | 2,757 | 2,660 | |||||||||
| Property, plant and equipment | 4 | 563 | 717 | |||||||||
| Other non-current financial assets | 5, 9 | 186 | 175 | |||||||||
| Total non-current assets | 3,506 | 3,552 | ||||||||||
| Other receivables | 7, 9 | 6,536 | 10,181 | |||||||||
| Other current financial assets | 6, 9 | 904 | 407 | |||||||||
| Cash and cash equivalents | 8, 9 | 23,926 | 19,745 | |||||||||
| Total current assets | 31,366 | 30,334 | ||||||||||
| TOTAL ASSETS | 34,872 | 33,886 | ||||||||||
| LIABILITIES AND SHAREHOLDERS' EQUITY | ||||||||||||
| Shareholders' equity | ||||||||||||
| Share capital | 10 | 27,191 | 32,765 | |||||||||
| Premiums related to the share capital | 27,781 | 8,410 | ||||||||||
| Treasury shares | (51 | ) | (54 | ) | ||||||||
| Foreign currency translation adjustment | (73 | ) | (27 | ) | ||||||||
| Retained earnings - attributable to owners of the parent | (17,851 | ) | (25,704 | ) | ||||||||
| Net loss - attributable to owners of the parent | (31,163 | ) | (12,398 | ) | ||||||||
| Shareholders' equity - attributable to owners of the parent | 5,835 | 2,993 | ||||||||||
| Non-controlling interests | (32 | ) | (32 | ) | ||||||||
| Total shareholders' equity | 5,803 | 2,961 | ||||||||||
| Liabilities | ||||||||||||
| Employee benefit obligations | 13 | 205 | 195 | |||||||||
| Non-current financial liabilities | 9, 12 | 5,743 | 5,015 | |||||||||
| Non-current derivative financial instruments | 12 | 412 | 0 | |||||||||
| Total non-current liabilities | 6,361 | 5,210 | ||||||||||
| Current financial liabilities | 9, 12 | 12,037 | 13,913 | |||||||||
| Provisions | - | 75 | ||||||||||
| Trade payables | 9, 14.1 | 7,606 | 10,089 | |||||||||
| Accrued taxes and employee benefits payable | 14.2 | 1,998 | 1,288 | |||||||||
| Current derivative financial instruments | 788 | 62 | ||||||||||
| Other creditors and miscellaneous liabilities | 14.3 | 381 | 289 | |||||||||
| Total current liabilities | 22,810 | 25,716 | ||||||||||
| TOTAL LIABILITIES AND SHAREHOLDERS' EQUITY | 34,872 | 33,886 | ||||||||||
(1) Refer to note 2.7 “Restatement of Previously Issued Financial Statements”
Page 12 of 49
Half-year financial report as of June 30, 2022
Statement of consolidated operations
FOR THE SIX-MONTH PERIOD ENDED JUNE 30, | ||||||||||||
| (amounts in thousands of euros, except share and per share data) | NOTES | 2021 | 2022 | |||||||||
| Revenue | - | - | ||||||||||
| Cost of sales | - | - | ||||||||||
| Gross margin | - | - | ||||||||||
| Research and development expenses, net | 15.1 | (7,594 | ) | (6,867 | ) | |||||||
| General and administrative expenses | 15.2 | (2,919 | ) | (5,053 | ) | |||||||
| Operating loss | (10,513 | ) | (11,920 | ) | ||||||||
| Financial expenses | (2,064 | ) | (1,492 | ) | ||||||||
| Financial income | 23 | (14 | ) | |||||||||
| Change in fair value of convertible notes | (691 | ) | 1,028 | |||||||||
| Net financial expense | 16 | (2,732 | ) | (478 | ) | |||||||
| Loss before taxes | (13,245 | ) | (12,398 | ) | ||||||||
| Income taxes | - | - | ||||||||||
| Net loss for the period | (13,245 | ) | (12,398 | ) | ||||||||
| Attributable to owners of the company | (13,245 | ) | (12,398 | ) | ||||||||
| Non-controlling interests | - | - | ||||||||||
| Basic and diluted weighted average number of shares outstanding | 110,680,727 | 147,803, 141 | ||||||||||
| Basic loss per share (€/share) | 17 | (0.12 | ) | (0,08 | ) | |||||||
| Diluted loss per share (€/share) | 17 | (0.12 | ) | (0,08 | ) | |||||||
Page 13 of 49
Half-year financial report as of June 30, 2022
Statement of consolidated comprehensive loss
| FOR THE SIX-MONTH PERIOD ENDED JUNE 30, | ||||||||
| (amounts in thousands of euros) | 2021 | 2022 | ||||||
| Net loss for the period | (13,245 | ) | (12,398 | ) | ||||
| Items that will not be reclassified to profit or loss | ||||||||
| Remeasurements of the defined benefit liability (asset) | 7 | 40 | ||||||
| Items that will be reclassified to profit or loss | ||||||||
| Foreign currency translation adjustment | 3 | 46 | ||||||
| Other comprehensive income | 10 | 86 | ||||||
| Total comprehensive loss | (13,235 | ) | (12,312 | ) | ||||
| Attributable to owners of the company | (13,235 | ) | (12,312 | ) | ||||
| Non-controlling interests | - | |||||||
Page 14 of 49
Half-year financial report as of June 30, 2022
Statement of changes in consolidated shareholders’ equity
(amounts in thousands of euros, except share data) | Notes | Share capital – number of shares | Share capital | Premiums related to the share capital | Accumulated deficit and net loss | Foreign currency translation adjustment | Share based payment | Split accounting impact related to convertible notes and warrants attached to non- convertible bonds | Treasury Shares | Shareholders' equity - Attributable to owners of the company | Non- controlling interests | Shareholders' equity | ||||||||||||||||||||||||||||||||||||
| As of January 1, 2021 | 100,757,097 | 20,151 | 22,538 | (46,739 | ) | (72 | ) | 5,521 | 944 | (42 | ) | 2,299 | (31 | ) | 2,268 | |||||||||||||||||||||||||||||||||
| Net loss for the period | - | - | - | (13,245 | ) | - | - | - | - | (13,245 | ) | - | (13,245 | ) | ||||||||||||||||||||||||||||||||||
| Other comprehensive income | - | - | - | 7 | 3 | - | - | - | 10 | - | 10 | |||||||||||||||||||||||||||||||||||||
| Total comprehensive income (loss) | - | - | - | (13,238 | ) | 3 | - | - | - | (13,235 | ) | - | (13,235 | ) | ||||||||||||||||||||||||||||||||||
| Conversion of convertible notes | 12 | 1,199,868 | 240 | 860 | - | - | - | - | - | 1,100 | - | 1,100 | ||||||||||||||||||||||||||||||||||||
| Share capital increase | 10 | 12,000,000 | 2,400 | 14,184 | - | - | - | - | - | 16,584 | - | 16,584 | ||||||||||||||||||||||||||||||||||||
| Exercise of warrants | 10 | 1,852,276 | 370 | 368 | - | - | - | - | - | 738 | - | 738 | ||||||||||||||||||||||||||||||||||||
| Treasury shares movements, net | 10 | - | - | - | - | - | - | - | (6 | ) | (6 | ) | - | (6 | ) | |||||||||||||||||||||||||||||||||
| Allocation of premiums to retained earnings | - | - | (17,505 | ) | 17,505 | - | - | - | - | - | - | - | ||||||||||||||||||||||||||||||||||||
| Gains and losses, net related to treasury shares | - | - | - | 20 | - | - | - | - | 20 | - | 20 | |||||||||||||||||||||||||||||||||||||
| Equity settled share-based payments | 11 | - | - | �� | - | - | - | 754 | - | - | 754 | - | 754 | |||||||||||||||||||||||||||||||||||
| Costs incurred in relation to equity transactions | 10 | - | - | (2,253 | ) | - | - | - | - | - | (2,253 | ) | - | (2,253 | ) | |||||||||||||||||||||||||||||||||
| As of June 30, 2021 | 115,809,241 | 23,162 | 18,191 | (42,453 | ) | (69 | ) | 6,275 | 944 | (48 | ) | 6,001 | (31 | ) | 5,970 | |||||||||||||||||||||||||||||||||
| As of January 1, 2022 (as restated) (1) | 135,953,657 | 27 191 | 27,781 | (58,852 | ) | (72 | ) | 8,943 | 896 | (51 | ) | 5,835 | (32 | ) | 5,803 | |||||||||||||||||||||||||||||||||
| Net loss for the period | - | - | (12,398 | ) | - | - | - | - | (12,398 | ) | (0 | ) | (12,398 | ) | ||||||||||||||||||||||||||||||||||
| Other comprehensive income | - | - | 40 | 46 | - | - | - | 86 | - | 86 | ||||||||||||||||||||||||||||||||||||||
| Total comprehensive income (loss) | - | - | (12,358 | ) | 46 | - | - | - | (12,312 | ) | (0 | ) | (12,312 | ) | ||||||||||||||||||||||||||||||||||
| Conversion of convertible notes | 12 | 27,847,526 | 5,570 | 374 | - | - | - | - | 5,943 | - | 5,943 | |||||||||||||||||||||||||||||||||||||
| Share capital increase | 10 | |||||||||||||||||||||||||||||||||||||||||||||||
| Exercise of warrants | 10 | 22,320 | 4 | 2 | - | - | - | - | 7 | - | 7 | |||||||||||||||||||||||||||||||||||||
| Treasury shares movements, net | 10 | - | - | - | - | - | (3 | ) | (3 | ) | - | (3 | ) | |||||||||||||||||||||||||||||||||||
| Allocation of premiums to retained earnings | - | (19,748 | ) | 19,748 | - | - | - | - | - | - | - | |||||||||||||||||||||||||||||||||||||
| Gains and losses, net related to treasury shares | (29 | ) | (29 | ) | (29 | ) | ||||||||||||||||||||||||||||||||||||||||||
| Equity settled share-based payments | 11 | - | - | - | - | 3,553 | - | - | 3,533 | - | 3,533 | |||||||||||||||||||||||||||||||||||||
| Costs incurred in relation to equity transactions | 10 | |||||||||||||||||||||||||||||||||||||||||||||||
| IFRS 16 impact | - | - | 18 | - | - | - | - | 18 | - | 18 | ||||||||||||||||||||||||||||||||||||||
| As of June 30, 2022 | 163 823 503 | 32,765 | 8,410 | (51,474 | ) | (27 | ) | 12,477 | 896 | (54 | ) | 2,993 | (32 | ) | 2,961 | |||||||||||||||||||||||||||||||||
(1) Refer to note 2.7 “Restatement of Previously Issued Financial Statements
Page 15 of 49
Half-year financial report at June 30, 2022
Statement of consolidated cash flows
| FOR THE SIX-MONTH PERIOD ENDED JUNE 30, | ||||||||||||
| (amounts in thousands of euros) | NOTES | 2021 | 2022 | |||||||||
| Cash flows from operating activities | ||||||||||||
| Net loss for the period | (13,245 | ) | (12,398 | ) | ||||||||
| Adjustments to reconcile net loss to cash flows used in operating activities | ||||||||||||
| Amortization and depreciation of intangible and tangible assets | 3, 4 | 130 | 150 | |||||||||
| Additions of provisions, net of reversals | 1,529 | 43 | ||||||||||
| Expenses associated with share-based payments | 11 | 754 | 3,533 | |||||||||
| Gross financial interest paid | 17 | 190 | 1,219 | |||||||||
| Changes in fair value of convertible notes | 17 | 691 | (1,028 | ) | ||||||||
| Financial indemnity, net, Negma | 17 | 167 | - | |||||||||
| Proceeds from grants | 260 | - | ||||||||||
| Interests on investment accounts | (3 | ) | - | |||||||||
| Unwinding of conditional advances and other financial expenses | 16 | 15 | ||||||||||
| Amortized cost of convertible notes and non-convertible bonds | 17 | 62 | 169 | |||||||||
| Operating cash flows before change in working capital requirements | (9,449 | ) | (8,296 | ) | ||||||||
| (+) Change in working capital requirements (net of depreciation of trade receivables and inventories) | (4,041 | ) | (1,965 | ) | ||||||||
| (Increase) decrease in other non-current financial assets | - | - | ||||||||||
| (Increase) decrease in other receivables | (3,971 | ) | (3,646 | ) | ||||||||
| Increase (decrease) in trade payables | (48 | ) | 2,483 | |||||||||
| Increase (decrease) in tax and social security liabilities | 9 | (711 | ) | |||||||||
| Increase (decrease) in other creditors and miscellaneous liabilities | (32 | ) | (92 | ) | ||||||||
| Cash flows (used in) from operating activities | (13,491 | ) | (10,261 | ) | ||||||||
| Cash flows used in investing activities | ||||||||||||
| Acquisition of intangible and tangible assets | 3, 4 | (29 | ) | (22 | ) | |||||||
| Interests on investment accounts | 3 | |||||||||||
| Sale of term deposit classified as other current financial assets | 6,7 | 344 | ||||||||||
| Sale of term deposit classified as other non-current financial assets | 5 | 12,500 | 12 | |||||||||
| Cash flows (used in) from investing activities | 12,474 | 333 | ||||||||||
| Cash flows from financing activities | ||||||||||||
| Proceeds from share capital increase, net of Negma indemnity (1) | 10 | 16,584 | - | |||||||||
| Costs paid in relation to equity transactions | (2,253 | ) | - | |||||||||
| Net contractual penalties received from (paid to) Negma | (1,077 | ) | - | |||||||||
| Subscription of warrants (BSA) | - | - | ||||||||||
| Exercise of warrants (BSA) and founders’ warrants (BSPCE) | 10 | 738 | 7 | |||||||||
| Reimbursement of the prefinanced CIR receivables, net of guarantee deposit | 12 | - | 153 | |||||||||
| Proceeds from conditional advances | 12.1 | 400 | 4 | |||||||||
| Repayment of conditional advances | 12.1 | (136 | ) | (149 | ) | |||||||
| Financial interest paid | (190 | ) | (687 | ) | ||||||||
| Proceeds from the issuance of non-convertible bonds and convertible notes | 12.2 | 5,820 | 8,000 | |||||||||
| Repayment of non-convertible bonds | 12.2 | (1,731 | ) | (1,259 | ) | |||||||
| Repayment of lease liabilities | - | |||||||||||
| Cost incurred in relation to the issuance of convertible notes and non-convertible bonds | 17 | - | (380 | ) | ||||||||
| Change in short-term bank overdrafts | - | - | ||||||||||
| Cash flows (used in) from financing activities | 18,155 | 5,689 | ||||||||||
| Net effect of exchange rate changes on cash and cash equivalents | 2 | 58 | ||||||||||
| Decrease in cash and cash equivalents | 17,140 | (4,181 | ) | |||||||||
| Cash and cash equivalents at the beginning of the period | 8 | 5,847 | 23,926 | |||||||||
| Cash and cash equivalents at the end of the period | 8 | 22,987 | 19,745 | |||||||||
Page 16 of 49
Half-year financial report at June 30, 2022
Notes to the unaudited interim condensed consolidated financial statements
(Unless otherwise noted, the condensed consolidated half-year financial statements are presented in thousands of euros. Some amounts may be rounded for the calculation of financial information contained in the Financial Statements. Accordingly, the totals in some tables may not be the exact sum of the preceding figures.)
Note 1: General information about the Company
Incorporated in September 2006, Biophytis is a clinical-stage biotechnology company focused on the development of therapeutics that slow the degenerative processes associated with aging and improve functional outcomes for patients suffering from age-related diseases, including severe respiratory failure in patients suffering from COVID - 19.
Sarconeos (BIO101), the Company’s leading drug candidate, is a small molecule, administered orally, currently in clinical Phase 2b in sarcopenia (SARA-INT) in the United States and Europe. A pediatric formulation of Sarconeos (BIO101) is being developed for the treatment of Duchenne Muscular Dystrophy (DMD).
Since April 2020, Sarconeos (BIO101) is also being developed as a treatment for patients with COVID-19 related respiratory failure in a Phase 2/3 clinical study (COVA) in the United States, Europe and Latin America.
Biophytis is a French joint stock company (société anonyme) and has its registered office located at 14, avenue de l’Opéra, 75001 Paris, France (register Number at the Company’s house: 492 002 225 RCS PARIS). The Company's ordinary shares are listed on the Euronext Growth Paris market (Ticker: ALBPS -ISIN: FR0012816825) and ADSs (American Depositary Shares) are listed on the Nasdaq (Ticker BPTS - ISIN: US09076G1040).
Biophytis and its subsidiaries are referred to hereinafter as “Biophytis,” or the “Company.”
The following information constitutes the Notes to the condensed interim financial statements for the six-month period ended June 30, 2022 with comparative information required under IAS 34 Interim Financial Reporting.
The unaudited condensed consolidated interim financial statements of Biophytis, or the “Financial Statements”, have been prepared under the responsibility of management of the Company and were approved and authorized for issuance by the Company’s Board of Directors on January 26, 2023.
Note 2: Accounting principles, rules and methods
2.1 Statement of compliance
The Company has prepared its Financial Statements for the six-month periods ended June 30, 2022 and June 30, 2021 in accordance with International Financial Reporting Standards, or IFRS, as issued by the International Accounting Standards Boards, or IASB. The term “IFRS” refers collectively to international accounting and financial reporting standards (IASs and IFRSs) and to interpretations of the interpretations committees (IFRS Interpretations Committee, or IFRS IC, and Standing Interpretations Committee, or SIC), whose application is mandatory for the periods presented.
The Financial Statements for the six-month period ended June 30, 2022 have been prepared in accordance with the international accounting standard IAS 34 Interim financial reporting. The financial information may not be indicative of other periods or results to be expected for the full year.
Page 17 of 49
Half-year financial report at June 30, 2022
Due to the listing of ordinary shares of the Company on Euronext Growth Paris (formerly known as Alternext Paris) and in accordance with the European Union’s regulation No. 1606/2002 of July 19, 2002, the Financial Statements of the Company are also prepared in accordance with IFRS as adopted by the European Union, or EU, whose application is mandatory for the periods presented.
As of June 30, 2022, all IFRS that the IASB has published and that are mandatory are the same as those endorsed by the EU and mandatory in the EU. As a result, the Financial Statements comply with IFRS as issued by the IASB and as adopted by the EU.
2.2 Going concern
The Board of Directors approved the Financial Statements on a going concern basis despite the loss of 12 million euros for the six-month period ended June 30, 2022. This analysis takes into account:
Cash and cash equivalents as of June 30, 2022 amounted to 19,7 million euros; and
The potential use of a funding line of convertible notes established in June 2021 with Atlas that could generate up to 24 million euros of additional funding (8 tranches with a nominal value of 4 million euros each, the first and the second tranches have been issued in April and June 2022, respectively).
2.3 Accounting methods
The accounting principles adopted for the Financial Statements as of and for the six-month period ended June 30, 2022 are the same as for the year ended December 31, 2021 with the exception of the following new standards, amendments and interpretations whose application was mandatory for the Company as of January 1, 2022:
| • | Amendments to IAS 37 - Provisions, contingent liabilities and contingent assets – Onerous contracts, concept of costs directly linked to the contract Amendments applicable from January 1, 2022 |
| • | Amendment to IAS 16 Property, plant and equipment – income generated before intended use |
| • | Amendment to IFRS 3 reference conceptual framework. |
| • | IFRIC interpretation relating to the recognition of configuration or customization costs in the context of a SaaS-type contract (IAS 38 Intangible assets) |
Adoptions of these standards have not had a material impact on the Financial Statements.
Recently issued accounting pronouncements that may be relevant to the Company’s operations but have not yet been adopted are as follows:
| • | Amendments to IAS 1 Presentation of Financial Statements: Classification of Liabilities as Current or Non-current and Classification of Liabilities as Current or Non-current – Deferral of Effective Date issued on January 23, 2020 and July 15, 2020 respectively and whose application is for annual reporting periods beginning on or after January 1, 2023; |
| • | Amendments to IAS 1 Presentation of Financial Statements and IFRS Practice Statement 2: Disclosure of Accounting policies issued on February 12, 2021 and whose application is for annual reporting periods beginning on or after January 1, 2023; |
Page 18 of 49
Half-year financial report at June 30, 2022
| • | Amendments to IAS 8 Accounting policies, Changes in Accounting Estimates and Errors: Definition of Accounting Estimates issued on February 12, 2021 and whose application is for annual reporting periods beginning on or after January 1, 2023; and |
| • | Amendments to IAS 12 Income Taxes: Deferred Tax related to Assets and Liabilities arising from a Single Transaction issued on May 7, 2021 and whose application is for annual reporting periods beginning on or after January 1, 2023. |
The Company is currently assessing whether the adoption of these standards, amendments to standards and interpretations will have a material impact on its financial statements at the date of adoption.
Moreover, the accounting treatment for the Kreos 2021 contracts as of and for the six-month period ended June 30, 2022 is not the same as for the year ended December 31, 2021 previously issued (see Note 2.7).
Page 19 of 49
Half-year financial report at June 30, 2022
2.4 Translation of the financial statements of foreign subsidiaries
The financial statements of entities whose functional currency is not the euro are translated as follows:
| • | assets and liabilities are translated using the closing rate of the period; |
| • | income statement items are translated using the average rate of the period as long as it is not called into question by significant changes in rate; and |
| • | equity items are translated using the historical rate. |
The exchange differences arising on translation are recognized in Other Comprehensive Income.
The exchange rates used for the preparation of the Financial Statements are as follows:
Closing rate AS OF | Average rate for the Six-month period ended | |||||||||||||||
| EXCHANGE RATE | DECEMBER 31, 2021 | JUNE 30, 2022 | JUNE 30, 2021 | JUNE 30, 2022 | ||||||||||||
| BRL | 6.3101 | 5.4229 | 6.4902 | 5.5565 | ||||||||||||
| USD | 1.1326 | 1.0387 | 1.2053 | 1.0934 | ||||||||||||
2.5 Use of judgments and estimates
To prepare the financial statements in accordance with the IFRS, the main judgements and estimates made by the Company’s management as well as the main assumptions are consistent with those applied in preparing the annual financial statements as of December 31, 2021 previously issued, with the exception of the judgments and estimates related to Kreos 2021 contracts (see Note 2.7).
Such estimates are based on the assumption of going concern and are based on the information available at the time of their preparation.
The pandemic linked to COVID-19 and the situation in Ukraine did not lead to the use of new estimates or significant new judgments in the first half of 2022.
2.6 Impact of the Covid-19 health crisis on the June 30, 2022 accounts
Neither the Covid-19 health crisis nor the situation in Ukraine had a significant impact on the Company's operations.
2.7 Restatement of previously issued financial statements
In connection with the preparation of those unaudited interim condensed consolidated financial statements, the Company identified an error in the accounting for the venture loan agreement and bonds issue agreement signed with Kreos on November 19, 2021 in the consolidated financial statements for the year ended December 31, 2021. Pursuant to the provisions of IAS 8, the Company has decided that it is necessary to restate the Company’s previously issued consolidated financial statements for the 2021 financial year in order to make certain restatements required to reflect the proper accounting treatment of these agreements.
Page 20 of 49
Half-year financial report at June 30, 2022
At the initial recognition of this transaction, a “Day One Loss” of €1,444 million was calculated and reported as a financial asset on the Company’s balance sheet. The Day One Loss was deferred and amortised over the term of the contract in financial expenses for the difference between the fair value of the convertible bonds plus the fair value of the attached warrants as estimated by the Company on the one hand, and the corresponding transaction prices (i.e., proceeds received) on the other hand, assuming that the contractual interest of the straight bonds was a market rate for Biophytis at the transaction date.
The Company reconsidered all the characteristics listed in the Kreos financing contract, which includes various instruments (straight bonds, convertible bonds, and warrants) in view of the way negotiations conducted with Kreos took place.
Initially the Company considered that the interest rate applied to the straight bonds was a market rate, leading to a Day One Loss on convertible bonds and warrants which was not offset by a Day One Gain on straight bonds. Reconsidering this assessment, the Company concluded that all instruments negotiated at the same time with Kreos should have been analysed together and the amount of cash received on November 19, 2021 (before transaction costs) was deemed to correspond to the fair value of all the instruments contracted with Kreos. As a result, the Company revised the credit spread used at issuance date so that Day One Losses and Day One Gains identified on each financial instrument that was part of the transaction with Kreos offset each other at the inception of the agreement, except for the third tranche (C) of the straight bonds which was subject to specific negotiation with Kreos and issued in December 2021.
In the course of the restatement, the various instruments (straight bonds, convertible bonds and warrants) were recognized at inception on the balance sheet for the fair value estimated by the Company which corresponds as a whole to the consideration received (before transaction costs). At the level of each instrument, value differences are identified between i) the nominal value of the instruments entered in the contract and ii) their fair value estimated by the Company based on unobservable inputs: these value differences are individually analyzed as Day One Losses and Day One Gains, which are required to be deferred under IFRS 9. Since the instruments are linked to each other, traded as a whole, the Company considered that the recognition of all of these gains and losses by the end of the reporting period is a reflection of the economics of the transaction.
The following table summarizes the impact of the restatement on the various instruments of the Kreos financing contract.
| As of November 19, 2021 | As of December 31, 2021 | |||||||||||||||||||||||
| (amounts in thousands of euros) | As filed | Restatements | As restated | As filed | Restatements | As restated | ||||||||||||||||||
| Convertible bonds | 2,198 | (566 | ) | 1,632 | 2,215 | (568 | ) | 1,647 | ||||||||||||||||
| Straight bonds | 3,153 | (538 | ) | 2,615 | 3,865 | (540 | ) | 3,325 | ||||||||||||||||
| Conversion options | 819 | (355 | ) | 464 | 916 | (380 | ) | 536 | ||||||||||||||||
| Warrants | 708 | 2 | 710 | 788 | - | 788 | ||||||||||||||||||
| Day one loss | (1,444 | ) | 1,444 | - | (1,390 | ) | 1,390 | - | ||||||||||||||||
Page 21 of 49
Half-year financial report at June 30, 2022
The following presents a reconciliation of the impacted financial statement line items as previously filed to the restated amounts as of and for the year ended December 31, 2021. The previously reported amounts reflect those included in the annual report as of and for the year ended December 31, 2021. These amounts are labelled as “As filed” in the tables below. The amounts labelled “As previously issued” represent the effects of this restatement due to the corrections required to reflect the proper accounting treatment of these agreements.
See note 12.
Year ended December 31, 2021
Statement of Consolidated Financial Position
| AS OF DECEMBER 31, 2021 | ||||||||||||
| (amounts in thousands of euros) | As previously issued | Restatements | As restated | |||||||||
| ASSETS | ||||||||||||
| Patents and software | 2,757 | 2,757 | ||||||||||
| Property, plant and equipment | 563 | 563 | ||||||||||
| Other non-current financial assets | 1,251 | (1,065 | ) | 186 | ||||||||
| Total non-current assets | 4,571 | (1,065 | ) | 3,506 | ||||||||
| Other receivables | 6,536 | 6,536 | ||||||||||
| Other current financial assets | 1,229 | (325 | ) | 904 | ||||||||
| Cash and cash equivalents | 23,926 | 23,926 | ||||||||||
| Total current assets | 31,691 | (325 | ) | 31,366 | ||||||||
| TOTAL ASSETS | 36,262 | (1,390 | ) | 34,872 | ||||||||
| LIABILITIES AND SHAREHOLDERS' EQUITY | ||||||||||||
| Shareholders' equity | ||||||||||||
| Share capital | 27,191 | - | 27,191 | |||||||||
| Premiums related to the share capital | 27,781 | - | 27,781 | |||||||||
| Treasury shares | (51 | ) | - | (51 | ) | |||||||
| Foreign currency translation adjustment | (73 | ) | - | (73 | ) | |||||||
| Accumulated deficit - attributable to shareholders of Biophytis | (17,865 | ) | 15 | (17,850 | ) | |||||||
| Net income (loss) - attributable to shareholders of Biophytis | (31,246 | ) | 83 | (31,163 | ) | |||||||
| Shareholders' equity - attribuable to shareholders of Biophytis | 5,737 | 98 | 5,835 | |||||||||
| Non-controlling interests | (32 | ) | - | (32 | ) | |||||||
| Total shareholders' equity | 5,705 | 98 | 5,803 | |||||||||
| Employee benefit obligations | 205 | - | 205 | |||||||||
| Non-current financial liabilities | 6,293 | (775 | ) | 5,518 | ||||||||
| Derivative liabilities | 916 | (380 | ) | 536 | ||||||||
| Total non-current liabilities | 7,414 | (1,153 | ) | 6,259 | ||||||||
| Current financial liabilities | 12,370 | (333 | ) | 12,036 | ||||||||
| Provisions | - | - | - | |||||||||
| Trade payables | 7,606 | - | 7,606 | |||||||||
| Tax and social liabilities | 1,998 | - | 1,998 | |||||||||
| Current derivative financial instruments | 788 | 788 | ||||||||||
| Other creditors and miscellaneous liabilities | 381 | - | 381 | |||||||||
| Total current liabilities | 23,143 | (333 | ) | 22,810 | ||||||||
| TOTAL LIABILITIES AND SHAREHOLDERS' EQUITY | 36,262 | (1,390 | ) | 34,872 | ||||||||
Page 22 of 49
Half-year financial report at June 30, 2022
STATEMENT OF CONSOLIDATED OPERATIONS
| FOR THE YEAR ENDED DECEMBER 31, 2021 | ||||||||||||||||
| (amounts in thousands of euros) | Note | As previously issued | Restatements | As restated | ||||||||||||
| Gross margin | - | - | - | |||||||||||||
| Research and development expenses, net | (19,665 | ) | - | (19,665 | ) | |||||||||||
| General and administrative expenses | (7,150 | ) | - | (7,150 | ) | |||||||||||
| Operating loss | (26,815 | ) | - | (26,815 | ) | |||||||||||
| Financial expenses | (2,581 | ) | 64 | (2,517 | ) | |||||||||||
| Financial income | 24 | - | 24 | |||||||||||||
| Change in fair value of convertible notes | (1,875 | ) | 19 | (1,856 | ) | |||||||||||
| Net Financial expense | (4,432 | ) | 83 | (4,349 | ) | |||||||||||
| Loss before taxes | (31,247 | ) | 83 | (31,164 | ) | |||||||||||
| Income taxes benefit | - | - | - | |||||||||||||
| Net loss | (31,247 | ) | 83 | (31,164 | ) | |||||||||||
| Attributable to shareholders of Biophytis | (31,247 | ) | 83 | (31,163 | ) | |||||||||||
| Non-controlling interests | (1 | ) | - | (1 | ) | |||||||||||
| Basic and diluted weighted average number of shares outstanding | 118,282,679 | - | 118,282,679 | |||||||||||||
| Basic loss per share (€/share) | (0,26 | ) | - | (0,26 | ) | |||||||||||
| Diluted loss per share (€/share) | (0,26 | ) | - | (0,26 | ) | |||||||||||
STATEMENT OF CONSOLIDATED COMPREHENSIVE LOSS
| FOR THE YEAR ENDED DECEMBER 31, 2021 | ||||||||||||
| (amounts in thousands of euros) | As previously issued | Restatements | As restated | |||||||||
| Net loss for the year | (31,247 | ) | 83 | (31,164 | ) | |||||||
| Items that will not be reclassified to profit or loss | ||||||||||||
| Actuarial gains and losses | 23 | - | 23 | |||||||||
| Items that will be reclassified to profit or loss | ||||||||||||
| Foreign currency translation adjustment | - | |||||||||||
| Other comprehensive income (loss) | 23 | - | 23 | |||||||||
| Total comprehensive loss | (31,224 | ) | 83 | (31,141 | ) | |||||||
| Attributable to shareholders of Biophytis | (31,223 | ) | 83 | (31,140 | ) | |||||||
| Non-controlling interests | (1 | ) | - | (1 | ) | |||||||
Page 23 of 49
Half-year financial report at June 30, 2022
STATEMENT OF CHANGES IN CONSOLIDATED SHAREHOLDERS’ EQUITY
| FOR THE YEAR ENDED DECEMBER 31, 2021 | ||||||||||||
As previously issued | Restatements | As restated | ||||||||||
| (amounts in thousands of euros) | Shareholders’ equity - Attributable to shareholders of Biophytis | |||||||||||
| As of January 1, 2021 | 2,266 | 2,266 | ||||||||||
| Net loss for the period | (31,247 | ) | 83 | (31,164 | ) | |||||||
| Other comprehensive income (loss) | 23 | - | 23 | |||||||||
| Total comprehensive income (loss) | (31,224 | ) | 83 | (31,141 | ) | |||||||
| Conversion of convertible notes | 10,940 | - | 10,940 | |||||||||
| Share capital increase | 20,204 | - | 20,204 | |||||||||
| Exercise of warrants (BSA) and founders' warrants (BSPCE) | 742 | - | 742 | |||||||||
| Subscription of warrants (BSA) | (62 | ) | 15 | (47 | ) | |||||||
| Allocation of premiums to retained earnings | 1,521 | - | 1,521 | |||||||||
| Treasury shares movements, net | - | - | - | |||||||||
| Gains and losses, net related to treasury shares | (9 | ) | - | (9 | ) | |||||||
| Equity settled share-based payments | 2 | - | 2 | |||||||||
| Biophytis shares to be received from Negma | 3,422 | - | 3,422 | |||||||||
| Cost incurred in relation to public offering on the NASDAQ | (2,099 | ) | - | (2,099 | ) | |||||||
| As of December 31, 2021 | 5,705 | 98 | 5,803 | |||||||||
Page 24 of 49
Half-year financial report at June 30, 2022
Statement of Consolidated Cash flows
| FOR THE YEAR ENDED DECEMBER 31, 2021 | ||||||||||||
| (amounts in thousands of euros) | As previously issued | Restatements | As restated | |||||||||
| Net loss for the period | (31,247 | ) | 83 | (31,164 | ) | |||||||
| Amortization and depreciation of intangible and tangible assets | 311 | - | 311 | |||||||||
| Additions of provisions, net of reversals | 39 | - | 39 | |||||||||
| Expenses associated with share-based payments | 3,422 | - | 3,422 | |||||||||
| Financial interests and conversion settled with cash payment | 562 | - | 562 | |||||||||
| Spread of the deferred loss | 54 | - | 54 | |||||||||
| Changes in fair value of convertible notes | 1,875 | (19 | ) | 1,856 | ||||||||
| Financial indemnity, net, NEGMA | 1,675 | - | 1,675 | |||||||||
| Interest on investment accounts | (4 | ) | - | (4 | ) | |||||||
| Unwinding of conditional advances and other financial expenses | 397 | - | 397 | |||||||||
| Amortized cost of non-convertible bonds | 132 | (64 | ) | 68 | ||||||||
| Operating cash flows before change in working capital requirements | (22,785 | ) | - | (22,785 | ) | |||||||
| (-) Change in working capital requirements (net of depreciation of trade receivables and inventories) | (1,010 | ) | - | (1,010 | ) | |||||||
| Cash flows from operating activities | (23,795 | ) | - | (23,795 | ) | |||||||
| Cash flows used in investing activities | 12,160 | - | 12,160 | |||||||||
| Cash flows from financing activities | 29,715 | - | 29,715 | |||||||||
| - | ||||||||||||
| Net effect of exchange rate changes on cash and cash equivalents | (1 | ) | - | (1 | ) | |||||||
| Increase (decrease) in cash and cash equivalents | 18,079 | - | 18,079 | |||||||||
| - | ||||||||||||
| Cash and cash equivalents at the beginning of the period (including bank overdrafts) | 5,847 | - | 5,847 | |||||||||
| Cash and cash equivalents at the end of the period (including bank overdrafts) | 23,926 | - | 23,926 | |||||||||
The adjustments presented above have been reported to the notes to the financial statements. Therefore, in the tables of the Notes, the amounts that are presented as of December 31, 2021 are the amounts that are labelled “As restated” in the tables above.
Page 25 of 49
Half-year financial report at June 30, 2022
Note 3: Patents and software
| (amounts in thousands of euros) | Patents | Software | Total | |||||||||
| GROSS AMOUNT | ||||||||||||
| As of January 1, 2022 | 3,652 | 32 | 3,684 | |||||||||
| Addition | - | - | - | |||||||||
| Disposal | (2 | ) | - | (2 | ) | |||||||
| As of June 30, 2022 | 3,650 | 32 | 3,682 | |||||||||
| AMORTIZATION | ||||||||||||
| As of January 1, 2022 | 895 | 32 | 927 | |||||||||
| Increase | 95 | - | 95 | |||||||||
| Decrease | - | - | - | |||||||||
| As of June 30, 2022 | 990 | 32 | 1,022 | |||||||||
| NET BOOK VALUE | ||||||||||||
| As of January 1, 2022 | 2,757 | - | 2,757 | |||||||||
| As of June 30, 2022 | 2,660 | - | 2,660 | |||||||||
No impairment was recognized on the intangible assets of the Company during the six-month periods ended June 30, 2022 and 2021, respectively. The Company determined that there was limited impact of the COVID-19 pandemic on the Company’s assets.
The Company co-owns certain patents with state-owned partners.
As part of the Intellectual Property agreement signed with the Company's CEO (see Note 19.2) and its amendment, the total patent rights acquired from the Company's CEO as of June 30, 2022 amounted to 1,350 thousand euros and are amortized over a 19-year period.
During the six-months period ended June 30, 2022, the change in intangible assets is mainly due to the amortization of the patents.
Page 26 of 49
Half-year financial report at June 30, 2022
Note 4: Property, plant and equipment
| (Amounts in thousands of euros) | Equipment and tooling | Equipment and tooling (right of use) | Fixture and fittings | Office, IT equipment, furniture | Buildings | Total | ||||||||||||||||||
| GROSS AMOUNT | ||||||||||||||||||||||||
| As of January 1, 2022 | 340 | 181 | 114 | 96 | 500 | 1,232 | ||||||||||||||||||
| Addition | 1 | 271 | - | 21 | - | 293 | ||||||||||||||||||
| Disposal | - | - | - | - | - | - | ||||||||||||||||||
| Exchange effect | (0 | ) | - | 8 | 1 | - | 8 | |||||||||||||||||
| As of June 30, 2022 | 341 | 452 | 123 | 117 | 500 | 1 533 | ||||||||||||||||||
| DEPRECIATION | ||||||||||||||||||||||||
| As of January 1, 2022 | 250 | 181 | 106 | 75 | 56 | 669 | ||||||||||||||||||
| Increase | 22 | 3 | 4 | 4 | 111 | 143 | ||||||||||||||||||
| Decrease | - | - | - | - | - | - | ||||||||||||||||||
| Exchange effect | (0 | ) | - | 4 | 1 | - | 5 | |||||||||||||||||
| As of June 30, 2022 | 273 | 184 | 178 | 79 | 167 | 817 | ||||||||||||||||||
| NET BOOK VALUE | ||||||||||||||||||||||||
| As of January 1, 2022 | 90 | - | 8 | 21 | 444 | 563 | ||||||||||||||||||
| As of June 30, 2022 | 68 | 268 | 9 | 39 | 333 | 716 | ||||||||||||||||||
No impairment was recognized on property, plant and equipment of the Company during the six-month periods ended June 30, 2022, and 2021, respectively.
Note 5: Other non-current financial assets
| (amounts in thousands of euros) | AS OF DECEMBER 31, 2021 (As restated) (1) | AS OF JUNE 30, 2022 | ||||||
| Cash reserve related to the liquidity agreement | 72 | 40 | ||||||
| Guarantee deposit related to the 2021 Kreos loan contract (see note 12.2.3) | 104 | 126 | ||||||
| Miscellaneous | 10 | 9 | ||||||
| Total other non-current financial assets | 186 | 175 | ||||||
(1) Refer to note 2.7 “Restatement of Previously Issued Financial Statements”
Page 27 of 49
Half-year financial report at June 30, 2022
Note 6: Other current financial assets
| (amounts in thousands of euros) | AS OF DECEMBER 31, 2021 (As restated) (1) | AS OF JUNE 30, 2022 | ||||||
| Guarantee deposit as part of the research tax credit prefinancing from NEFTYS (see Note 12) | 584 | 407 | ||||||
| Guarantee deposit related to the non-convertible bonds (Kreos 2018 contract) | 320 | - | ||||||
| Short term deposits | - | - | ||||||
| Total other current financial assets | 904 | 407 | ||||||
(1) Refer to note 2.7 “Restatement of Previously Issued Financial Statements”
Note 7: Other receivables
| (amounts in thousands of euros) | AS OF DECEMBER 31, 2021 | AS OF JUNE 30, 2022 | ||||||
| Research tax credit (1) | 3,941 | 6,555 | ||||||
| Value added tax | 1,008 | 845 | ||||||
| Prepaid expenses (2) | 1,418 | 1,818 | ||||||
| Suppliers - advances payment and debit balance | 125 | 947 | ||||||
| Receivable from CACEIS in relation with the exercises of BSA/BSPCE | 2 | 4 | ||||||
| Miscellaneous | 42 | 12 | ||||||
| Total other receivables | 6,536 | 10,181 | ||||||
(1) French Research Tax Credit (“CIR”)
CIR recorded as of June 30, 2022 includes the CIR estimated for the six-month period ended June 30, 2022 (2,475 thousand euros) and the CIR for the year ended December 31, 2021 (4,080 thousand euros). The CIR is estimated on the basis of the expenses that meet the eligibility criteria.
In December 2021, a portion of the CIR receivables for 2020 was prefinanced by Neftys (see note 12).
(2) Prepaid expenses mainly relate to annual insurance premium paid in February 2021 following the Company’s US IPO and listing on Nasdaq and research and studies expenses.
Note 8: Cash and cash equivalents
Cash and cash equivalents are broken down as follows:
| (amounts in thousands of euros) | AS OF DECEMBER 31, 2021 | AS OF JUNE 30, 2022 | ||||||
| Bank accounts | 16,926 | 13,745 | ||||||
| Short-term deposits | 7,000 | 6,000 | ||||||
| Total cash and cash equivalents | 23,926 | 19,745 | ||||||
As of December 31, 2021, the Company owned two short-term deposits with an initial maturity of one month:
| • | A short-term deposit of 2,000 thousand euros with a maturity on January 1, 2022, and an interest rate of 0.03%. |
| • | A short-term deposit of 5,000 thousand euros with a maturity on January 26, 2022, and an interest rate of 0.03%. |
Page 28 of 49
Half-year financial report at June 30, 2022
As of June 30, 2022, the Company owned two short-term deposits:
| • | A short-term deposit of 5,000 thousand euros with a maturity on July 26, 2022, and an interest rate of 0.03%; and |
| • | A short-term deposit of 1,000 thousand euros with a maturity on July 8, 2022, and an interest rate of 0.03%. |
In accordance with IAS 7, these short-term deposits were recorded under cash and cash equivalents.
Note 9: Financial assets and liabilities and impacts on consolidated statement of profit or loss
The Company’s financial assets and liabilities are measured as follows as of December 31, 2021:
| AS OF DECEMBER 31, 2021 (As restated) (1) | ||||||||||||||||
| Value - Statement of financial position (IFRS 9) | ||||||||||||||||
| (amounts in thousands of euros) |
Value - | Fair value | Fair value through profit or loss | Amortized cost | ||||||||||||
| Non-current financial assets | 186 | 186 | - | 186 | ||||||||||||
| Other receivables | 5,119 | 5,119 | - | 5,119 | ||||||||||||
| Other current financial assets | 904 | 904 | - | 904 | ||||||||||||
| Cash and cash equivalents | 23,926 | 23,926 | 23,926 | - | ||||||||||||
| Total financial assets | 30,134 | 30,134 | 23,926 | 6,208 | ||||||||||||
| Non-current financial liabilities | 5,518 | 5,618 | 5,518 | |||||||||||||
| Non-current derivative financial instruments | 536 | 536 | 536 | - | ||||||||||||
| Current financial liabilities | 12,037 | 12,037 | 6,627 | 5,409 | ||||||||||||
| Current derivative financial instruments | 788 | 788 | 788 | - | ||||||||||||
| Trade payables | 7,606 | 7,606 | - | 7,606 | ||||||||||||
| Tax and social liabilities | 1,998 | 1,998 | - | 1,998 | ||||||||||||
| Miscellaneous liabilities | 381 | 381 | - | 381 | ||||||||||||
| Total financial liabilities | 28,863 | 28,963 | 7,951 | 20,912 | ||||||||||||
(1) Refer to note 2.7 “Restatement of Previously Issued Financial Statements”
The Company’s financial assets and liabilities are measured as follows as of June 30, 2022:
| AS OF JUNE 30, 2022 | ||||||||||||||||
| Value - Statement of financial position (IFRS 9) | ||||||||||||||||
| (amounts in thousands of euros) |
Value - | Fair value | Fair value through profit or loss | Amortized cost | ||||||||||||
| Non-current financial assets | 175 | 175 | - | 175 | ||||||||||||
| Other receivables | 10,181 | 10,181 | - | 10,181 | ||||||||||||
| Other current financial assets | 407 | 407 | - | 407 | ||||||||||||
| Cash and cash equivalents | 19,745 | 19,745 | 19,745 | - | ||||||||||||
| Total financial assets | 30,509 | 30,509 | 19,745 | 10,764 | ||||||||||||
| Non-current financial liabilities | 5,015 | 4,906 | 5,015 | |||||||||||||
| Non-current derivative financial instruments | - | - | - | - | ||||||||||||
| Current financial liabilities | 13,913 | 13,913 | 8,917 | 4,996 | ||||||||||||
| Current derivative financial instruments | 62 | 62 | 62 | - | ||||||||||||
| Trade payables | 10,089 | 10,089 | - | 10,089 | ||||||||||||
| Tax and social liabilities | 1,288 | 1,288 | - | 1,288 | ||||||||||||
| Miscellaneous liabilities | 289 | 289 | - | 289 | ||||||||||||
| Total financial liabilities | 30,656 | 30,547 | 8,979 | 21,568 | ||||||||||||
| Page 29 of 49 |
Half-year financial report at June 30, 2022
The impact of the Company’s financial assets and liabilities on the consolidated statement of profit or loss are as follows for the six-month period ended June 30, 2021 and 2022:
| AS OF JUNE 30, 2021 | AS OF JUNE 30, 2022 | |||||||||||||||
| (amounts in thousands of euros) | Interest | Change in fair value | Interest | Change in fair value | ||||||||||||
| Profit or loss impact of liabilities | ||||||||||||||||
| Derivative financial instruments | 1,262 | |||||||||||||||
| Liabilities at amortized cost: non-convertible bonds | (289 | ) | (1,004 | ) | ||||||||||||
| Liabilities at fair value: convertible notes | (691 | ) | 1,028 | |||||||||||||
| Liabilities at amortized cost: advances | (16 | ) | (15 | ) | ||||||||||||
Note 10: Share capital
| AS OF DECEMBER 31, 2021 | AS OF | |||||||
| Share capital (in thousands of euros) | 27,191 | 32,765 | ||||||
| Number of outstanding shares | 135,953,657 | 163,823,503 | ||||||
| Nominal value per share (in euros) | 0.20 | € | 0,20 | € | ||||
Share capital
As of June 30, 2022, the share capital of the Company was 32 764 700,60 euros, divided into 163 823 503 fully subscribed ordinary shares with a nominal value of 0.20 euros per share.
Outstanding shares exclude warrants (“BSA”) granted to certain investors, free shares and founders’ warrants (“BSPCE”) granted to certain employees and members of the Board of Directors that have not yet vested (free shares) or been exercised (BSA and BSPCE).
As of June 30, 2022, the premiums of the Company were 9,671 thousand euros. This included the allocation of the premiums to retained earnings for an amount of 19,748 thousand euros decided at the Shareholders Meeting of April 29, 2022.
Changes in share capital
During the six months ended June 30, 2022, 220 bonds held by Atlas were converted into new shares generating the issue of 27,847,526 shares with a par value of 0.20 euros, representing a capital increase of 5,570 thousand euros and an issue premium of -70 thousand euros (based on the fair value of the shares issued on the date of conversion).
Following the exercise of warrants during the period, the share capital was increased by 4 thousand euros through the issue of 22,320 new shares, with an issue premium of a total amount of 2 thousand euros.
| Page 30 of 49 |
Half-year financial report at June 30, 2022
Note 11: Warrants and founders’ warrants
BSA warrants issued to Kreos Group
See Note 12.2
BSA warrants issued to investors
On April 3, 2020, the Company launched a public offering of share subscription warrants. The main objective of the transaction was to allow existing shareholders the ability to participate in the COVA program and the future development of the Company, and eventually to consolidate its equity.
Upon completion of the public offering, the Company issued 7,475,708 share subscription warrants, after full exercise of the extension clause.
The subscription price was 0.06 euros per warrant. The warrants can be exercised for a period of 5 years from April 30, 2020, at an exercise price of 0.27 euros per new share.
Each warrant gives its holder the right to subscribe for one new Biophytis share.
Activity for BSA warrants issued to investors that were outstanding during the six-month period ended June 30, 2022 is summarized in the table below:
| Number of outstanding warrants | |||||||||||||||||||||||||||
| Type | Grant date | As of | Granted | Exercised | Lapsed | As of June 30, 2022 | Number of shares which can be subscribed | ||||||||||||||||||||
| Warrants2020 | 04/07/2020 | 2,492,871 | - | (15,865 | ) | 2,477,006 | 2,477,006 | ||||||||||||||||||||
| Total | 2,492,871 | - | (15,865 | ) | 2,477,006 | 2,477,006 | |||||||||||||||||||||
BSA warrants issued pursuant to equity-compensation plan
The following table summarizes the data related to the warrants issued pursuant to equity-compensation plans as well as the assumptions adopted for valuation in accordance with IFRS 2:
| Characteristics | Assumptions | |||||||||||||||||||||||||
| Type | Grant date | Number of warrants granted | Maturity date | Exercise price | Volatility | Risk-free rate | IFRS2 Initial valuation (Black- Scholes) in thousands of euros | |||||||||||||||||||
| Warrants2021 | 17/06/2022 | 398,476 | 17/06/2026 | € | 0.097 | 63 | % | 0.62 | % | 17 | ||||||||||||||||
On June 17, 2022, the Company allocated 398,476 warrants giving the right to subscribe to one new ordinary share with a nominal value of twenty-euro cents (€0.20). The issue price is €0.0048 and the strike price is €0.0967.
| Page 31 of 49 |
Half-year financial report at June 30, 2022
The exercise of BSA 2020 is possible from the 1st anniversary date of the subscription and up to 4 years later (i.e. between June 17, 2023 and June 17, 2026).
Activity for BSA warrants issued pursuant to equity-compensation plans that were outstanding during the six-month period ended June 30, 2022 are summarized in the table below:
| Number of outstanding warrants | |||||||||||||||||||||
| Type | Grant date | As of | Granted | Exercised | Lapsed | As of | Number of shares which can be subscribed | ||||||||||||||
| BSA 2020 | 07/04/2020 | 2 492 871 | (15 865 | ) | 2 477 006 | 2 477 006 | |||||||||||||||
| BSA 2021 | 17/06/2022 | - | 398 476 | 398 476 | 398 476 | ||||||||||||||||
| Total | 2 492 871 | 398 476 | (15 865 | ) | 2 875 482 | 2 875 482 | |||||||||||||||
Founders’ warrants (“BSPCE”)
The following table summarizes the data related to BSPCE founder’s warrants issued as well as the assumptions used for valuation in accordance with IFRS 2:
| Characteristics | Assumptions | |||||||||||||||||||||
| Type | Grant date | Number of warrants granted | Maturity date | Exercise price | Volatility | Risk-free rate | IFRS 2 Initial valuation (Black-Scholes) in thousands of euros | |||||||||||||||
| Founders' warrants2019 | 03/04/2020 | 2 000 000 | 03/04/2026 | 0,27 | € | 48,36 | % | -0,62 | % | 674 | ||||||||||||
| Founders' warrants2020 | 22/12/2020 | 1 499 089 | 22/12/2026 | 0,47 | € | 57,80 | % | -0,77 | % | 508 | ||||||||||||
| Founders' warrants2021 | 15/09/2021 | 4 379 122 | 15/09/2027 | 0,73 | € | 79,11 | % | -0,73 | % | 677 | ||||||||||||
Activity for BSPCE founder’s warrants that were outstanding during the six-month period ended June 30, 2022 are summarized in the table below:
| Number of outstanding warrants | ||||||||||||||||||||
| Type | Grant date | As of January 1, 2022 | Granted | Exercised | Lapsed | As of | Number of shares which can be subscribed | |||||||||||||
| Founders' warrants2019 | 03/04/2020 | 1 470 218 | (40 071) | 1 432 299 | 837 754 | |||||||||||||||
| Founders' warrants2020 | 22/12/2020 | 1 087 875 | (80 143) | 1 049 955 | 287 532 | |||||||||||||||
| Founders' warrants2021 | 15/09/2021 | 4 310 654 | (370 096) | 4 187 289 | 1 313 520 | |||||||||||||||
| Total | 6 868 747 | (490 309) | 6 669 543 | 2 438 806 | ||||||||||||||||
The vesting period of these BSPCE founder’s warrants are summarized in the table below:
| Type | Vesting period | ||||||||||||
| Founders' warrants2019 | 1/3 as of 04/10/2020 | 1/3 as of 04/10/2022 | 1/3 as of 04/10/2024 | ||||||||||
| Founders' warrants2020 | 1/3 as of 12/22/2020 | 1/3 as of 12/22/2022 | 1/3 as of 12/22/2024 | ||||||||||
| Founders' warrants2021 | 1/3 as of 09/15/2021 | 1/3 as of 09/15/2022 | 1/3 as of 09/15/2023 | ||||||||||
| Page 32 of 49 |
Half-year financial report at June 30, 2022
Free shares
| Characteristics | Assumptions | ||||||||||||||||||
| Type | Grant date | Number of free shares granted | Maturity date | Exercise price | Volatility | Risk-free rate | IFRS 2 Initial valuation (Black- Scholes) in thousands of euros | ||||||||||||
| Free shares2020 | 12/22/2020 | 2,500,911 | N/A | N/A | N/A | N/A | 2,311 | ||||||||||||
| Free shares2021-1 | 09/15/2021 | 6,631,068 | N/A | N/A | N/A | N/A | 4,936 | ||||||||||||
| Free shares2021-2 | 04/25/2022 | 1,591,334 | N/A | N/A | N/A | N/A | 271 | ||||||||||||
Activity for the unvested free shares that were outstanding during the six-month period ended June 30, 2022 are summarized in the table below:
| Number of unvested free shares | |||||||||||||||
| Type | Grant date | Unvested free | Granted | Vested | Cancelled | Unvested free | |||||||||
| Free shares2020 | 12/22/2020 | 2,500,911 | 2,500,911 | ||||||||||||
| Free shares2021-1 | 09/15/2021 | 6,631,068 | 6,631,068 | ||||||||||||
| Free shares2021-2 | 04/25/2022 | 1 591 334 | 1 591 334 | 1,591,334 | |||||||||||
| Total | 9,131,979 | 10,723,313 | |||||||||||||
The vesting period of these free shares are summarized in the table below:
| Type | Vesting period | ||
| Free shares2020 | Vesting period of 2 years followed by a holding period of 2 years | ||
| Free shares2021 | Vesting period of 1 year followed by a holding period of 1 year |
Stock-based compensation expense recognized for the periods presented
(amounts in thousands of euros)
| SIX-MONTH PERIOD ENDED JUNE 30, 2021 | SIX-MONTH PERIOD ENDED JUNE 30, 2022 | ||||||||||||||||||||||||||||||||
| Type | Probabilistic cost of the plan | Cumulative expenses - beginning of period | Expense for the period | Cumulative expense to date | Probabilistic cost of the plan | Cumulative expenses - beginning of period | Expense for the period | Cumulative expense to date | |||||||||||||||||||||||||
| BSA 2020-2021 | 17 | ||||||||||||||||||||||||||||||||
| Founders’ warrants2019 | 1,008 | 498 | 103 | 601 | 961 | 684 | 130 | 814 | |||||||||||||||||||||||||
| Founders’ warrants2020 | 751 | 258 | 78 | 336 | 653 | 384 | 81 | 465 | |||||||||||||||||||||||||
| Founders’ warrants2021 | - | - | - | - | 1,257 | 508 | 266 | 774 | |||||||||||||||||||||||||
| Free shares2020 | 2,311 | 28 | 573 | 601 | 2,311 | 1,184 | 565 | 1,749 | |||||||||||||||||||||||||
| Free shares2021-1 | 4,936 | 1,447 | 2,425 | 3,872 | |||||||||||||||||||||||||||||
| Free shares2021-2 | 271 | - | 49 | 49 | |||||||||||||||||||||||||||||
| Total | 4,070 | 784 | 754 | 1,538 | 10,389 | 4,207 | 3,533 | 7,723 | |||||||||||||||||||||||||
| Page 33 of 49 |
Half-year financial report at June 30, 2022
Note 12: Borrowings and financial liabilities
| (amounts in thousands of euros) | AS OF DECEMBER 31, 2021 (As restated) (1) | AS OF JUNE 30, 2022 | ||||||
| Conditional advances | 906 | 711 | ||||||
| Non-convertible bonds | 2,740 | 4,670 | ||||||
| Convertible bonds | 1,647 | (695 | ) | |||||
| Non-current lease obligations | 225 | 329 | ||||||
| Non-current financial liabilities | 5,518 | 5,015 | ||||||
| Non-current derivative financial instruments | 536 | - | ||||||
| Conditional advances | 377 | 439 | ||||||
| Non-convertible bonds | 1,524 | 202 | ||||||
| Convertible notes | 6,627 | 9,631 | ||||||
| Financial liabilities related to the prefinancing of a portion of the research tax credit receivables (2) | 3,287 | 3,367 | ||||||
| Current lease obligations | 221 | 275 | ||||||
| Current financial liabilities | 12,036 | 13,913 | ||||||
| Current derivative financial instruments | 788 | 62 | ||||||
(1) Refer to note 2.7 “Restatement of Previously Issued Financial Statements”
(1) Financial liabilities related to the prefinancing of a portion of the research tax credit (CIR) receivables
In December 2020, a portion of the CIR receivables for 2020 were prefinanced by FONDS COMMUN DE TITRISATION PREDIREC INNOVATION 2020 with NEFTYS CONSEIL SARL as arranger, or NEFTYS. Consequently, the Company recorded:
| • | a liability for the amount due to NEFTYS at the time of CIR collection; |
| • | a financial asset for the amounts deducted by NEFTYS on the receivables sold (considered as a guarantee deposit, see Note 6); and |
| • | a current asset for the CIR research tax credit payable by the French State. |
In accordance with IFRS 9, the financial liability due to NEFTYS was determined using the amortized cost method for 3,287 thousand euros and 3,367 thousand euros as of December 31, 2021 and June 30, 2022, respectively.
Breakdown of financial liabilities by maturity
The maturity of financial liabilities of the Company is broken down as follows:
| Current | Non-current | |||||||||||||||
| (amounts in thousands of euros) | AS OF DECEMBER 31, 2021 (As restated) (1) | < 1 year | 1 to 5 years | >5 years | ||||||||||||
| Conditional advances | 1,284 | 377 | 746 | 160 | ||||||||||||
| Non-convertible bonds | 4,264 | 1,524 | 2,740 | - | ||||||||||||
| Convertible notes | 8,273 | 6,627 | 1,647 | - | ||||||||||||
| Lease liabilities | 445 | 221 | 225 | - | ||||||||||||
| Financial liabilities related to the prefinancing of a portion of the research tax credit receivables | 3,287 | 3,287 | - | - | ||||||||||||
| Total financial liabilities | 17,553 | 12,036 | 5,358 | 160 | ||||||||||||
| Derivative financial instruments | 1,324 | 788 | 536 | |||||||||||||
(1) Refer to note 2.7 “Restatement of Previously Issued Financial Statements”
| Current | Non-current | |||||||||||||||
| (amounts in thousands of euros) | AS OF JUNE 30, 2022 | < 1 year | 1 to 5 years | >5 years | ||||||||||||
| Conditional advances | 1,150 | 439 | 618 | 93 | ||||||||||||
| Non-convertible bonds | 4,872 | 202 | 4,670 | - | ||||||||||||
| Convertible notes | 8,936 | 9,631 | (695 | ) | - | |||||||||||
| Lease liabilities | 604 | 275 | 329 | - | ||||||||||||
| Financial liabilities related to the prefinancing of a portion of the research tax credit receivables | 3,367 | 3,367 | - | - | ||||||||||||
| Total financial liabilities | 18,928 | 13,913 | 4,922 | 93 | ||||||||||||
| Derivative financial instruments | 62 | 62 | - | |||||||||||||
| Page 34 of 49 |
Half-year financial report at June 30, 2022
12.1 Conditional advances
| (amounts in thousands of euros) | BPI -Sarcob | BPI – BIO101 | AFM - Téléthon | BPI – BIO201 | Total | |||||||||||||||
| As of January 1, 2022 | 56 | 474 | 386 | 367 | 1,283 | |||||||||||||||
| (+) Proceeds from conditional advances | - | - | - | - | - | |||||||||||||||
| (-) Repayment | (39 | ) | (110 | ) | - | - | (149 | ) | ||||||||||||
| Subsidies | - | - | - | - | - | |||||||||||||||
| Financial expenses | 1 | 7 | 4 | 3 | 15 | |||||||||||||||
| As of June 30, 2022 | 19 | 371 | 389 | 370 | 1,150 | |||||||||||||||
Breakdown of conditional advances by maturity in repayment value
| (amounts in thousands of euros) | BPI -Sarcob | BPI – BIO101 | AFM - Téléthon | BPI – BIO201 | Total | |||||||||||||||
| As of June 30, 2022 | 20 | 385 | 400 | 400 | 1,205 | |||||||||||||||
| <1 year | 20 | 220 | 200 | - | 440 | |||||||||||||||
| 1 year to 5 year | - | 165 | 200 | 308 | 673 | |||||||||||||||
| >5 years | - | - | - | 93 | 93 | |||||||||||||||
12.2 Convertible notes and non-convertible bonds
Issuance of convertible notes to ATLAS – 2020 Atlas Contract
| (amounts in thousands of euros) | ATLAS ORNANE - 2020 Atlas Contract | |||
| As of January 1, 2022 | 6,627 | |||
| (+) Gross proceeds | - | |||
| (+/-) Change in fair value of financial liabilities | 565 | |||
| (-) Conversion | 5,943 | |||
| As of June 30, 2022 | 119 | |||
In April 2020, the Company signed a convertible bond financing of up to 24 million euros from Atlas to continue the development of Sarconeos (BIO 101) through the issuance of multiple convertible notes over a 3-year period (the “960 3-year note warrants”). This contract replaced the Negma Group contract.
The Company issued a first tranche of 3 million euros on April 29, 2020, a second tranche of 3 million euros on June 19, 2020 and a third tranche of 3 million euros on August 28, 2020. On May 27, 2021, the Company issued a fourth and fifth tranche of 3 million euros each. Those bonds were issued with a discount of 3% of nominal value (i.e., 180 thousand euros as of the fourth and fifth tranches)
| Page 35 of 49 |
Half-year financial report at June 30, 2022
Other issuance costs were incurred by the Company for approximately 37 thousand euros for the fourth and fifth tranches.
Main characteristics of the ORNANE note warrants
The 960 3-year note warrants require their holder to exercise them, at the Company’s request, in tranches of 120 warrants each. Each warrant grants its holder the right to one ORNANE. Note warrants may not be transferred and are not subject to a request for admission to trading on the Euronext Growth market.
Main characteristics of the ORNANE ATLAS 2020
The ORNANE (Bonds) have a par value of 25,000 euros and are issued at a subscription price of 97% of the nominal value. They do not bear interest and have a 24-month maturity from issuance. Holders of ORNANE may request at any time the Company to convert them into shares during their maturity period, and at that time, the Company has to redeem the ORNANE in cash. At the end of the term, and if the ORNANE have not yet been converted, the ORNANE are automatically converted.
The holder may ask to convert the ORNANE at any time at the conversion parity determined by the following formula: N = CA /CP, where
| · | “N” is the number of shares yielded by the conversion, | |
| · | “CA” is the par value of the ORNANEs (i.e., 25,000 euros), | |
| · | “CP” is the conversion price (i.e., 97% of the lowest volume weighted average price over the 10 trading days preceding the date on which conversion is requested). |
On the day of the conversion request, the Company may redeem the ORNANE in cash using the following formula: V = CA/CP x CPr, where
| · | “V” is the amount redeemed to the holder, | |
| · | “CPr” is the lowest price between (i) the weighted average closing price prior to the conversion and (ii) the lowest weighted average prices of the previous 10 trading days x 1.15 |
ORNANE may be transferred by their holders only to Affiliates and will not be subject to a request for admission to trading on the Euronext Growth market.
Accounting treatment
The Company determined that it could not reliably estimate separately the fair value of the conversion option embedded in the convertible bonds and therefore concluded that the entire hybrid contract should be measured at fair value through profit or loss until settlement.
The fair value is measured using a Longstaff-Schwartz valuation model.
Page 36 of 49
Half-year financial report at June 30, 2022
The tables below summarizes the key inputs to measure the fair value of the convertible notes:
| Tranche 7 | ||||||||
| ATLAS | At issuance date | June 30, 2022 | ||||||
| Number of outstanding convertible notes | 120 | - | ||||||
| Conversion price | 0,74 | € | - | |||||
| Volatility | 70 | % | - | |||||
| Risk-free rate | -0,45 | % | - | |||||
| Value of the convertible notes (in thousands of €) | 3,518 | - | ||||||
| Tranche 8 | ||||||||
| ATLAS | At issuance date | June 30, 2022 | ||||||
| Number of outstanding convertible notes | 120 | 4 | ||||||
| Conversion price | 0,42 | € | 0,10 | € | ||||
| Volatility | 70 | % | 58,14 | % | ||||
| Risk-free rate | -0,38 | % | 0,78 | % | ||||
| Value of the convertible notes (in thousands of €) | 3,646 | 119 | ||||||
As of December 31, 2021, all of the convertible bonds issued to Atlas (tranches 1, 2, 3, 4, 5 and 6) have been converted.
As of June 30, 2022, 220 convertible bonds have been converted generating the issuance of 27,847,526 new ordinary shares under tranches 7 and 8. As of the closing date, 4 bonds remain to be converted, for a total nominal of 100,000 euros.
Issuance of convertible notes to ATLAS – 2021 Atlas Contract
| (amounts in thousands of euros) | ATLAS ORNANE - 2020 Atlas Contract | |||
| As of January 1, 2022 | - | |||
| (+) Gross proceeds | 8,000 | |||
| (+/-) Change in fair value of financial liabilities | 799 | |||
| (-) Conversion | 0 | |||
| As of June 30, 2022 | 8,799 | |||
In June 2021, the Company signed a new convertible bond financing of up to 32 million euros (8 tranches with a nominal value of 4 million euros each) with Atlas (the “2021 Atlas Contract”) to continue the development of Sarconeos (BIO 101) through the issuance of multiple convertible notes.
The new financing instrument provides for the issuance of up to 1,280 bonds with an option for exchange in cash and/or conversion into new or existing shares (ORNANE). Subject to the issue of the eighth and final tranche under the 2020 Atlas Contract, the 32 million euros total financing can be drawn by Biophytis over the next three years, without obligation, through eight successive tranches of 4 million euros each. This facility is intended to secure the Company cash position in order to continue the development of its clinical activities in particular further development of Sarconeos (BIO 101).
The bond tranches are issued with a discount of 4% of the nominal. A commitment commission of 500,000 euros will be paid over the various draws, at the rate of 12% per draw.
As of June 30, 2022, the Company issued 360 ORNANE bonds (first and second tranches) for a total amount of 8 million euros under the ATLAS 2021 Contract. Issue premiums were paid for 320 thousand euros, and transaction costs for 60 thousand euros.
Page 37 of 49
Half-year financial report at June 30, 2022
Main characteristics of the ORNANE ATLAS 2021
The ORNANE will have a par value of 25 000 euros. They will not bear interest and will have a 24-month maturity from issuance.
The holder of ORNANE may request at any time to convert them into shares during their maturity period, and the Company shall have the right to redeem the ORNANE in cash. In case of cash redemption, the amount reimbursed will be limited to 110% of the principal.
At the end of the maturity period, and in the case where the ORNANE would not have been redeemed either in cash or in new or existing shares, the holder will have the obligation to convert the ORNANE.
The holder can ask to convert the ORNANE at any time at the conversion parity determined by the following formula:
N = CA / CP, where
| · | “N” is the number of shares yielded by the conversion, | |
| · | “CA” is the par value of the ORNANEs (i.e., 25,000 euros each), | |
| · | “CP” is the conversion price (i.e., the lowest stock market price observed over the 10 days preceding the conversion request). |
On the day of the conversion request, the Company may redeem the ORNANE in cash using the following formula: V = (CA/CP) * CPr, where
| · | “V” is the amount to be redeemed to the holder. | |
| · | “CPr” is the revised price. |
The revised price is the lowest price between (i) the volume weighted average price over the 10 trading days preceding the date on which conversion is requested and (ii) P*1.10
The revised price is the lower of (i) the market price preceding the conversion date and (ii) the conversion price*1.10
ORNANE may be transferred by their holders only to Affiliates and will not be subject to a request for trading admission on the Euronext Growth market.
Accounting treatment:
The Company determined that it could not reliably estimate separately the fair value of the conversion option embedded in the convertible bonds and therefore concluded that the entire hybrid contract should be measured at fair value through profit or loss until settlement.
The fair value is measured using a Longstaff-Schwartz valuation model.
Page 38 of 49
Half-year financial report at June 30, 2022
The tables below summarizes the key inputs to measure the fair value of the convertible notes:
| Tranche 1 | ||||||||
| ATLAS | At issuance date | June 30, 2022 | ||||||
| Number of outstanding convertible notes | 160 | 160 | ||||||
| Conversion price | 0,23 | € | 0,10 | € | ||||
| Volatility | 70 | % | 70 | % | ||||
| Risk-free rate | 0,54 | % | 1,36 | % | ||||
| Value of the convertible notes (in thousands of €) | 4,000 | 4,400 | ||||||
| Tranche 2 | ||||||||
| ATLAS | At issuance date | June 30, 2022 | ||||||
| Number of outstanding convertible notes | 160 | 160 | ||||||
| Conversion price | 0,10 | € | 0,10 | € | ||||
| Volatility | 70 | % | 70 | % | ||||
| Risk-free rate | 1,82 | % | 1,36 | % | ||||
| Value of the convertible notes (in thousands of €) | 4,000 | 4,400 | ||||||
As of June 30, 2022, none of the 320 convertible bonds issued have been converted.
Issuance of convertible and non-convertible bonds to Kreos
| (financial liabilities in thousand euros) (1) | KREOS 2018 | KREOS 2021 Non- Convert. Tranches | KREOS 2021 Convert. Tranches | KREOS 2021 Bifurcated derivatives | KREOS 2021 BSA 2018 Buyback | Total | ||||||||||||||||||
| As of December 31, 2020 | 4 392 | 0 | 0 | 0 | 0 | 4 392 | ||||||||||||||||||
| (+) Gross proceed Tr A & B | 2 811 | 1 771 | 943 | (26 | ) | 5 500 | ||||||||||||||||||
| (+)Gross proceed Tr C | 677 | 677 | ||||||||||||||||||||||
| (-) Fees charged on the bond loan | (97 | ) | (28 | ) | (125 | ) | ||||||||||||||||||
| (+/-) Fair value of derivative instruments | 150 | 150 | ||||||||||||||||||||||
| (+/-) Amortized cost | 96 | 29 | 13 | 138 | ||||||||||||||||||||
| (-) Repayment | (3 550 | ) | (3 550 | ) | ||||||||||||||||||||
| As of December 31, 2021 (As restated) (2) | 938 | 3,325 | 1,647 | 1,324 | (48 | ) | 7 186 | |||||||||||||||||
| (+/-) Fair value of derivative instruments | (1 262 | ) | (1 262 | ) | ||||||||||||||||||||
| (+/-) Amortized cost | 6 | 163 | 69 | 238 | ||||||||||||||||||||
| (-) Repayment | (944 | ) | (314 | ) | (1 258 | ) | ||||||||||||||||||
| As of June 30, 2022 | 0 | 3,174 | 1 716 | 62 | (48 | ) | 4 904 | |||||||||||||||||
| (1) | Financial liabilities for all columns, except for the impact of the buyback of the 2018 BSA for 26 thousand which impacts equity under IAS 32.22 |
| (2) | Refer to note 2.7 “Restatement of Previously Issued Financial Statements” |
Issuance of non-convertible bonds for the benefit of Kreos – Contract 2018
On September 10, 2018, the Company signed a venture loan agreement and bonds issue agreement with Kreos, which provides for up to 10 million euros in funding to the Company through the issuance of non-convertible bonds in four separate tranches of 2.5 million euros each, plus the issuance of attached warrants in connection with the first tranche. As required under the terms of the venture loan agreement, the Company pledged a security interest in the Company’s assets to Kreos. The Company also granted a security interest in the business as a going concern, including a portion of the Company’s patents, to Kreos.
Page 39 of 49
Half-year financial report at June 30, 2022
Each tranche of non-convertible bonds bears a 10% annual interest rate and must be repaid in cash in 36 monthly installments commencing in April 2019.
Pursuant to the terms of the agreements, the Company has the right, at any time but with no less than 30 days’ prior notice to Kreos, to prepay or purchase the non-convertible bonds, exclusively in full. The prepayment will be equal to (i) the principal amount outstanding, plus (ii) the sum of all interest repayments, which would have been paid throughout the remainder of the term of the relevant tranche discounted by 10% per annum.
The first and second tranches of non-convertible bonds were issued on September 10, 2018, the third tranche of non-convertible bonds was issued on December 17, 2018 and the final tranche was issued on March 1, 2019, for total gross proceeds to the Company of €10 million. Guarantee deposits totaling 320 thousand euros (80 thousand euros per tranche) were withheld by Kreos from the proceeds received by the Company. The amount withheld will be deducted from the last installment to be repaid by the Company. It is presented under “Other non-current financial assets” (see Note 5).
The BSA warrants issued to Kreos as part of the first tranche give the holder the right to subscribe for 442,477 ordinary shares at an exercise price of €2.67 per share for a term of 7 years.
These BSA warrants were valued at 319 thousand euros and were recorded as an equity instrument and as a reduction in the value of the debt
Accounting treatment of KREOS 2018 hybrid financing
In accordance with IFRS 9, the non-convertible debt component was initially recognized at fair value and subsequently measured at amortized cost. The effective interest rate post recognition of warrants as a reduction of the debt was 13.59%.
The non-convertible debt component amounted to €0.9 million as of December 31, 2021. As of June 30, 2022, the financing is fully repaid.
Issuance of convertible and non-convertible bonds for the benefit of Kreos – Contract 2021
On November 19, 2021, the Company entered into a "venture loan agreement" with KREOS in lieu of a framework agreement organizing the issue of a bond loan for an amount of up to €10 million through the issue of 7,75 million euros in non-convertible bonds ("Straight bonds"), the issue of 2.25 million euros in convertible bonds ("Convertible bonds"), and the issue of Biophytis share subscription warrants. The issuance of the first tranche is conditioned to the subscription of the warrants previously mentioned.
The loan agreement comprises four tranches of respectively 2.5 million euros (A), 3.0 million euros (B), 2.5 million euros (C) and 2.0 million euros ( D). The first two tranches (A and B) were drawn upon signature of the contract on November 19, 2021, the third tranche (C) limited to 677 thousand euros was drawn on December 31, 2021 [and the last tranche (D ) was not drawn by the Company. Tranches A and B include a non-convertible portion (“straight bonds”) for a nominal amount of 3,259 thousand euros, and a convertible portion for 2,250 thousand euros.
The non-convertible bonds bear interest at the annual rate of 10% and must be repaid in cash in 36 monthly installments beginning April 1, 2022. The convertible bonds bear interest at the annual rate of 9.5%. The Company will redeem the convertible bonds for their principal amount no later than March 31, 2025, unless they are previously converted into shares, at the option of Kreos Capital, at a fixed conversion price of €0.648 (unless the Company has paid dividends). The conversion options can be exercised at any time, until December 31, 2024.
Page 40 of 49
Half-year financial report at June 30, 2022
Under the terms of the contract, the Company has the possibility at any time, provided it complies with prior notification to Kreos of at least 30 days, to reimburse or repurchase the non-convertible and convertible bond loans. The reimbursement will be equal to (1) the amount of the principal remaining due, increased by (2) the sum accounting for 90% of the interest that the Company should have paid over the remaining duration of the tranche concerned.
According to the contractual terms of the convertible bonds, in the event of conversion into shares of the convertible bond, Kreos will have to pay the Company an amount equal to 10% of the total interest paid by the Company. In the event of partial conversion on this date, the amount will be reduced accordingly.
In accordance with the financing contract entered into with KREOS, the Company issued to Kreos Capital 2,218,293 BSA giving the right to subscribe to new ordinary shares of the Company, on the basis of one share for one BSA. The warrants can be exercised over a period of 7 years after their issuance. The exercise price of the BSAs was set at €0.56.
By subscribing to the BSAs, Kreos Capital has expressly waived the right to exercise the 2018 BSAs as they were held following their detachment from the non-convertible bonds subscribed on September 10, 2018 as part of the 2018 loan structure. The redemption value of the BSA 2018 by Biophytis is estimated at (26) thousand euros, paid when drawing the first tranches of the 2021 contract.
In the event of the exercise of the BSA 2021, Kreos has the option of limiting the amount of cash paid to Biophytis for the shares subscribed, in return for the waiver of a portion of the BSA it holds (“waiver option”), The waiver premium is calculated as the difference between the 30-day VWAP (« Volume-weighted average price ») of the shares and the exercise price of the BSAs.
The “venture loan agreement” provides for the pledge of the Company's goodwill, the pledge of bank account balances as well as the pledge of intellectual property rights for the benefit of Kreos.
The venture loan agreement with KREOS imposes certain operational and financial restrictions. These commitments may limit the ability of the company and its subsidiaries, in certain circumstances, to, among other things:
· incur additional debt;
· create or incur liens;
· sell or transfer assets; and
· pay dividends.
This contract also contains certain customary restrictive covenants and events of default, including in the event of a change of control.
Accounting treatment of KREOS 2021 hybrid financing
The analysis of the characteristics of the hybrid contract according to the IFRS9 and IAS32 criteria led to the need to recognize the conversion options, as well as the BSAs, as derivative instruments separate from the host contract (no equity component insofar as these options do not in all circumstances lead to the delivery of a fixed number of shares, for a fixed price).
Page 41 of 49
Half-year financial report at June 30, 2022
The amount of cash of €5 million, received on November 19, 2021 (excluding transaction costs) corresponds to the estimated fair value of the instruments put in place on the date the funds were drawn: financial debt for tranches A and B for €(4,3) million (convertible and non-convertible), liability derivatives for premiums received on options sold for €(1,2) million (€464 thousand for conversion options and €710 thousand for BSAs issued), and financial compensation of €48 thousand for the 2018 BSAs bought back by Biophytis from KREOS.
On the date of implementation of the contract (November 19, 2021), on the basis of the contractual conditions to be respected to release the drawings, a nil fair value was retained for the drawing rights on tranches C and D.
Regarding the third tranche (C) of the straight bond issued in December 2021 for €677 thousand (excluding transaction costs), as the drawdown conditions were fulfilled outside the framework of the contract, the company analyzed the drawdown of the third tranche (C) as a new loan contract, with Kreos Capital VI UK. As such, the third tranche (C) is recognized for its fair value on the balance sheet, estimated on the basis of the financing rate deducted from the Kreos VI financing. The entry value of the liabilities of the Tranche C leads to the recognition of a day one Gain of €98 thousand1. Given the unobservable nature of the market rate, the day one gain is deferred on the Company’s balance sheet and recorded as financial liabilities.
In accordance with IAS 32, the redemption value of the 2018 BSAs was recognized for €48 thousand1 as a reduction in equity, consistent with the treatment applied to the BSAs issued in 2018.
The financial debt components are accounted for according to the principles of amortized cost, based on an average effective interest rate of 26.37% for the non-convertible tranches, and 22.85% for the convertible tranches.
Derivative instruments are valued at their Fair Value on the balance sheet, with changes in fair value recorded in the income statement. Fair value is estimated using a binomial valuation model for convertible bonds, and a Black & Scholes valuation model for BSAs.
The table below presents the valuations of the conversion options:
| Fair value of bifurcated conversion options of tranches A and B (maturing March 2025) | At issuance date (11/19/2021) | 12/31/21 (as restated) (1) | 06/30/22 | |||||||||
| Number of obligations in circulation | 2 250 000 | 2 250 000 | 2 250 000 | |||||||||
| Number of shares that can be subscribed | 2 250 000 | 2 250 000 | 2 250 000 | |||||||||
| Share price | 0,451 | € | 0,4949 | € | 0,094 | € | ||||||
| Exercise price | 0,648 | € | 0,648 | € | 0,648 | € | ||||||
| Volatility over a 12 months period | 85 | % | 85 | % | 70 | % | ||||||
| Risk-free rate | - | % | - | % | 1,55 | % | ||||||
| Credit spread | 23,14 | % | 23,14 | % | 23,14 | % | ||||||
| Fair value of the derivative instrument (in K€) | (464 | ) | (536 | ) | (0 | ) | ||||||
| Change in the fair value of the derivative instrument (in K€) | (72 | ) | 536 | |||||||||
(1) Refer to note 2.7 “Restatement of Previously Issued Financial Statements”
Page 42 of 49
Half-year financial report at June 30, 2022
The table below shows the valuations of the BSA 2021 attached to the KREOS contract:
BSA – KREOS 2021
Derivative instruments | At issuance date (19/11/2021) | 12/31/2021 (as restated) (1) | 06/30/2022 | |||||||||
| Number of BSAs in circulation | 2 218 293 | 2 218 293 | 2 218 293 | |||||||||
| Exercise price per share | 0,56 | € | 0,56 | € | 0,56 | € | ||||||
| Maturity | 7 years | 6,88 years | 6,5 years | |||||||||
| Volatility | 85 | % | 85 | % | 70 | % | ||||||
| Risk-free rate | - | - | 1,97 | % | ||||||||
| Fair value of BSA 2021 issued in favor of KREOS (in K€) | (710 | ) | (788 | ) | (62 | ) | ||||||
| Change in the fair value of the derivative instrument (in K€) | (78 | ) | 727 | |||||||||
(1) Refer to note 2.7 “Restatement of Previously Issued Financial Statements”
Note 13: Employee benefit obligation
The employee benefit obligation consists of the provision for retirement indemnity, assessed in accordance with the applicable collective bargaining agreement (i.e., the Collective Agreement of the “Pharmaceutical industry”).
This commitment only applies to employees under French law. The main actuarial assumptions used for the valuation of the retirement indemnity are as follows:
| AS OF DECEMBER 31, 2021 | AS OF JUNE 30, 2022 | |||
| Retirement age | Voluntary retirement between 65 and 67 years old | |||
| Collective agreement | Pharmaceutical industry | Pharmaceutical industry | ||
| Discount rate (IBOXX Corporates AA) | 0.98% | 3,22% | ||
| Mortality table | INSEE 2017 | INSEE 2017 | ||
| Salary increases | 2.00% | 3,00% | ||
| Turn-over | Medium | Moyen | ||
| Social security contributions | 43% | 44% | ||
The provision for the retirement indemnity has evolved as follows:
| (amounts in thousands of euros) | Employee benefit obligations | |||
| As of January 1, 2022 | 205 | |||
| Service cost | 28 | |||
| Interests cost | 1 | |||
| Remeasurements of the defined benefit liability (asset) | (40 | ) | ||
| As of June 30, 2022 | 195 | |||
Note 14: Current liabilities
14.1 Trade payables
| AS OF | ||||||||
| (amounts in thousands of euros) | DECEMBER 31, 2021 | JUNE 30, 2022 | ||||||
| Research and development suppliers | 6,669 | 8,761 | ||||||
| General and administrative suppliers | 937 | 1,328 | ||||||
| Total trade payables | 7,606 | 10,089 | ||||||
Page 43 of 49
Half-year financial report at June 30, 2022
The change in trade payables to research and development suppliers is primarily due to the increase in expenses associated with the Company’s ongoing clinical trials and research costs (refer to 15.1) and in particular, expenses related to the SARA clinical program and the launch of the COVA program.
14.2 Tax and social liabilities
| AS OF | ||||||||
| (amounts in thousands of euros) | DECEMBER 31, 2021 | JUNE 30, 2022 | ||||||
| Personnel expenses | 658 | 583 | ||||||
| Social security expenses | 1,202 | 645 | ||||||
| Other taxes | 138 | 59 | ||||||
| Total tax and social liabilities | 1,998 | 1,288 | ||||||
14.3 Other creditors and miscellaneous liabilities
| AS OF | ||||||||
| (amounts in thousands of euros) | DECEMBER 31, 2021 | JUNE 30, 2022 | ||||||
| Attendance fees | 202 | 107 | ||||||
| Deferred income (1) | 175 | 178 | ||||||
| Other | 4 | 4 | ||||||
| Total other creditors and miscellaneous liabilities | 381 | 289 | ||||||
(1) as part of the BPI France conditional advance “BIO 201” project, the Company was entitled to receive a grant of 380 thousand euros (see Note 12.1), which has been recorded as deferred income as of June 30, 2021, for 307 thousand euros (73 thousand euros recognized as a grant).
Note 15 : Provisions
| (amounts in thousands of euros) | 31/12/2021 | Additions | Reversals | Release of surplus provision | 30/06/2022 | |||||||||||||||
| Provisions for litigation | - | - | - | - | - | |||||||||||||||
| Provisions for risks | - | 75 | - | - | 75 | |||||||||||||||
| Total provisions | - | 75 | - | - | 75 | |||||||||||||||
Page 44 of 49
Half-year financial report at June 30, 2022
Note 16: Details of expenses and products by function
16.1 Research and development expenses
| FOR THE SIX-MONTH PERIOD ENDED JUNE 30, | ||||||||
| (amounts in thousands of euros) | 2021 | 2022 | ||||||
| Personnel expenses | (1,340 | ) | (2,950 | ) | ||||
| Purchases and external expenses | (8,292 | ) | (6,435 | ) | ||||
| Other | (141 | ) | (99 | ) | ||||
| Research and development expenses | (9,773 | ) | (9,485 | ) | ||||
| Research tax credit | 2,146 | 2,614 | ||||||
| Subsidies | 33 | 4 | ||||||
| Research tax credit and subsidies | 2,179 | 2,618 | ||||||
| Research and development expenses, net | (7,594 | ) | (6,867 | ) | ||||
Research and development expenses relate to activities in connection with conducting clinical trials, non-clinical studies of the drug candidates for the treatment of age-related diseases and the treatment of severe respiratory failure in patients suffering from COVID-19.
16.2 General and administrative expenses
| FOR THE SIX-MONTH PERIOD ENDED JUNE 30, | ||||||||
| (amounts in thousands of euros) | 2021 | 2022 | ||||||
| Personnel expenses | (908 | ) | (2,682 | ) | ||||
| Purchases and external expenses | (1,992 | ) | (2,235 | ) | ||||
| Other | (19 | ) | (136 | ) | ||||
| General and administrative expenses | (2,919 | ) | (5,053 | ) | ||||
16.3 Personnel expenses
| FOR THE SIX-MONTH PERIOD ENDED JUNE 30, | ||||||||
| (amounts in thousands of euros) | 2021 | 2022 | ||||||
| Wages and salaries | (1,494 | ) | (2,100 | ) | ||||
| Share-based payments | (754 | ) | (3,533 | ) | ||||
| Personnel expenses | (2,248 | ) | (5,633 | ) | ||||
The Company’s average headcount is 24 during the six-month period ended June 30, 2022 compared to 24 during the six-month period ended June 30, 2021.
Note 17: Net financial income and expenses
| FOR THE SIX-MONTH PERIOD ENDED JUNE 30, | ||||||||
| (amounts in thousands of euros) | 2021 | 2022 | ||||||
| Financial interest and amortized cost of the non-convertible bonds (1) | (252 | ) | (1,004 | ) | ||||
| Changes in fair value of convertible notes (1) | (691 | ) | 1,028 | |||||
| Negma financial indemnity | (187 | ) | - | |||||
| Accrual of provision in relation with Negma litigation | (1,508 | ) | (75 | ) | ||||
| Other financial expenses | (80 | ) | (33 | ) | ||||
| Transaction costs related to the issuance of convertible notes | (38 | ) | (380 | ) | ||||
| Net financial income related to Negma returning to Biophytis damages paid (2) | 20 | - | ||||||
| Other financial income | 3 | (14 | ) | |||||
| Foreign exchange gains (losses) | 1 | - | ||||||
| Total net financial expense | (2,732 | ) | (478 | ) | ||||
(1) Refer to Note 12.2 Convertible notes and non-convertible bonds
Page 45 of 49
Half-year financial report at June 30, 2022
Note 18: Earnings per share
Basic earnings per share are calculated by dividing the net earnings attributable to the Company’s shareholders by the weighted average number of ordinary shares outstanding during the year. Instruments giving rights to capital on a deferred basis (BSA, BSPCE, etc.) are considered anti-dilutive because they lead to an increase in earnings per share. As a result, diluted earnings per share are identical to basic earnings per share.
| FOR THE SIX-MONTH PERIOD ENDED JUNE 30, | ||||||||
| 2021 | 2022 | |||||||
| Weighted average number of outstanding shares | 110,732,068 | 147,857,451 | ||||||
| Treasury shares | 51,341 | 54,310 | ||||||
| Weighted average number of outstanding shares (without Treasury shares) | 110,680,727 | 147,803,141 | ||||||
| Net loss (in thousands of euros) | (13,245 | ) | (12,398 | ) | ||||
| Basic loss per share (€/share) | (0.12 | ) | (0,08 | ) | ||||
| Diluted loss per share (€/share) | (0.12 | ) | (0,08 | ) | ||||
Note 19: Related Parties
19.1 Compensation due to executive officers
| FOR THE SIX-MONTH PERIOD ENDED JUNE 30, | ||||||||
| (amounts in thousands of euros) | 2021 | 2022 | ||||||
| Fixed compensation | 514 | 617 | ||||||
| Variable compensation | 144 | 230 | ||||||
| Exceptional compensation | - | 36 | ||||||
| Benefits in kind | 15 | 13 | ||||||
| Directors fees | 175 | 70 | ||||||
| Share-based payments | 736 | 3,426 | ||||||
| Consulting fees | - | 81 | ||||||
| Total compensation of executive officers | 1,584 | 4,474 | ||||||
Other than the legal retirement indemnities, no post-employment benefits have been granted to our Chief Executive Officer or members of the Board of Directors.
19.2 Other related parties
No new significant transactions were concluded with the Company's related parties during the first six months of the 2022 financial year.
Note 20: Off-balance-sheet commitments
The off-balance-sheet commitments have not changed significantly since December 31, 2021.
Page 46 of 49
Half-year financial report at June 30, 2022
Note 21: Subsequent events
Results COVA study
On September 7, 2022, the company published the very promising results of the Phase 2-3 study of the COVA program to treat COVID-19-related respiratory failure. In the primary analysis, Sarconeos (BIO101) was shown to reduce the risk of respiratory failure or early death at 28 days (primary endpoint) by 39% compared to the placebo (15.8% versus 26.0%, adjusted difference of 11.8% in favor of treatment, p=0.07). Sarconeos (BIO101) reduced both the proportion of patients with respiratory failure (12.7% versus 21.5%) and early death (0.8% versus 2.8%). Sarconeos (BIO101) also significantly delayed (p=0.03) adverse progression to respiratory failure or early death over a maximum treatment period of 28 days.
Negma Group Ltd litigation
The Paris Court of Appeal has:
- confirmed the judgment of the Paris Commercial Court of March 16, 2021 (The Company has already executed in 2021 the entire judment);
- ordered Biophytis to pay Negma 75,000 euros under article 700 of the Code of Procedure civil as well as costs.
ORNANEs conversions - ATLAS contracts
| Atlas conversions post 06/30/2022 | Date | Bonds | Shares | Amount (€) | ||||||||||
| As of July 31, 2022 | 0 | 0 | 0 | |||||||||||
| ATLAS tranche 8 | 08/03/2022 | 4 | 1,331,948 | 100,000 | ||||||||||
| ATLAS tranche 1 | 08/03/2022 | 4 | 1,291,989 | 100,000 | ||||||||||
| - 8 ORNANEs ATLAS tr 1 | 08/05/2022 | 8 | 2,583,979 | 200,000 | ||||||||||
| - 8 ORNANEs ATLAS tr 1 | 08/11/2022 | 8 | 2,493,765 | 200,000 | ||||||||||
| - 8 ORNANEs ATLAS tr 1 | 08/12/2022 | 8 | 2,463,054 | 200,000 | ||||||||||
| - 16 ORNANEs ATLAS tr 1 | 08/16/2022 | 16 | 4,761,904 | 400,000 | ||||||||||
| - 8 ORNANEs ATLAS tr 1 | 08/25/2022 | 8 | 2,164,502 | 200,000 | ||||||||||
| - 8 ORNANEs ATLAS tr 1 | 08/30/2022 | 8 | 2,164,502 | 200,000 | ||||||||||
| As of August 31, 2022 | 64 | 19,255,643 | 1,600,000 | |||||||||||
| - 20 ORNANEs ATLAS tr 1 | 09/07/2022 | 20 | 5,482,456 | 500,000 | ||||||||||
| - 20 ORNANEs ATLAS tr 1 | 09/07/2022 | 20 | 5,482,456 | 500,000 | ||||||||||
| As of September 30, 2022 | 104 | 30,220,555 | 2,600,000 | |||||||||||
| - 4 ORNANEs ATLAS tr 1 | 10/11/2022 | 4 | 1,623,376 | 100,000 | ||||||||||
| - 4 ORNANEs ATLAS tr 1 | 10/20/2022 | 4 | 1,666,666 | 100,000 | ||||||||||
| As of October 31, 2022 | 112 | 33,510,597 | 2,800,000 | |||||||||||
| - 12 ORNANEs ATLAS tr 1 | 11/03/2022 | 12 | 5,281,690 | 300,000 | ||||||||||
| - 12 ORNANEs ATLAS tr 1 | 11/03/2022 | 12 | 5,281,690 | 300,000 | ||||||||||
| - 4 ORNANEs ATLAS tr 1 | 11/15/2022 | 4 | 1,976,284 | 100,000 | ||||||||||
| - 4 ORNANEs ATLAS tr 1 | 11/16/2022 | 4 | 2,012,072 | 100,000 | ||||||||||
| - 6 ORNANEs ATLAS tr 1 | 11/28/2022 | 6 | 3,232,758 | 150,000 | ||||||||||
| - 10 ORNANEs ATLAS tr 1 | 11/30/2022 | 10 | 5,387,931 | 250,000 | ||||||||||
| As of November 30, 2022 | 160 | 56,683,022 | 4,000,000 | |||||||||||
| - 4 ORNANEs ATLAS tr 1 | 12/01/2022 | 4 | 2,155,172 | 100,000 | ||||||||||
| - 2 ORNANEs ATLAS tr 2 | 12/01/2022 | 2 | 1,077,586 | 50,000 | ||||||||||
| - 6 ORNANEs ATLAS tr 2 | 12/01/2022 | 6 | 3,232,758 | 150,000 | ||||||||||
| - 4 ORNANEs ATLAS tr 2 | 12/01/2022 | 4 | 2,192,982 | 100,000 | ||||||||||
| As of December 31, 2022 | 176 | 65,341,520 | 4,400,000 | |||||||||||
| - 4 ORNANEs ATLAS tr 2 | 01/03/2023 | 4 | 2 392 344 | 100 000 | ||||||||||
| - 4 ORNANEs ATLAS tr 2 | 01/05/2023 | 4 | 2 392 344 | 100 000 | ||||||||||
| - 4 ORNANEs ATLAS tr 2 | 01/12/2023 | 4 | 2 433 090 | 100 000 | ||||||||||
| Au 12 janvier 2023 | 188 | 72 559 298 | 4 700 000 | |||||||||||
Page 47 of 49
Half-year financial report at June 30, 2022
Since June 30, 2022, the Company has converted 4 convertible bonds under Tranche 8 of the ATLAS 2020 Contract, the totality of the 160 convertible bonds of the Tranche 1 and 12 convertible bonds of the Tranche 2, both related to the ATLAS 2021 Contract, for a total amount of 4.7 million euros (100 thousand of euros for Tranche 8,4 million euros for Tranche 1, and 600 thousand of euros for Tranche 2). The operations gave rise to the creation of 65,341,520 new Company shares (1,331,948 shares for Tranche 8, and 57,506,246 shares for Tranche 1 and 13,721,104 shares for Tranche 2).
The Company has signed on October 20, 2022, a notification of exercise of Tranche for 160 new convertible bonds with a principal amount equal to 25,000€ in two instalments:
| · | first installment for 80 convertible bonds of tranche 3 with a principal amount equal to € 25,000 and received on November 10, 2022; and |
| · | second installment for 80 convertible bonds of tranche 3 with a principal amount equal to € 25,000 and received on January 11, 2023. |
Page 48 of 49
Half-year financial report at June 30, 2022
| 4. | Statutory Auditors' limited review report on the half-year condensed consolidated financial statements prepared in accordance with IFRS standards as adopted in the European Union |
This is a free translation into English of the statutory auditor’s review report issued in French and is provided solely for the convenience of English-speaking readers. This report should be read in conjunction with, and is construed in accordance with, French law and professional auditing standards applicable in France.
Biophytis S.A.
| Statutory Auditors Review Report on interim condensed consolidated financial statements Error! Unknown document property name.Error! Unknown document property name. |
| Error! Unknown document property name. |
| To the Chief Executive Officer, |
In our quality of statutory auditors of Biophytis S.A. and in answer to your request, we conducted a review of the interim condensed consolidated financial statements for the period from January 1, 2022 to June 30, 2022 ("the financial statements"), which are attached to this report.
These financial statements are the responsibility of the Board of directors. Our responsibility is to express a conclusion on these financial statements based on our review.
We conducted our review in accordance with professional standards applicable in France and the professional doctrine of the French national auditing body (Compagnie nationale des commissaires aux comptes) related to this engagement. A review consists primarily of making inquiries of persons responsible for financial and accounting matters and applying analytical procedures. A review is substantially less in scope than an audit conducted in accordance with professional standards applicable in France and consequently does not enable us to obtain assurance that we would become aware of all significant matters that might be identified in an audit. Accordingly, we do not express an audit opinion.
Based on our review, nothing has come to our attention that causes us to believe that the accompanying condensed interim financial statements as of September 30,2022 are not prepared in conformity with IAS 34 - standard of the IFRSs as adopted by the European Union applicable to interim financial information.
Without qualifying our conclusion, we draw your attention to the following matters set out in Notes 2.2 “Going Concern” to the consolidated interim financial statements regarding the assumptions underlying the application of the going concern principle, and 2.7 “Restatement of previously issued financial statements” relating to the restatement of the previously published financial statements for the year ended December 31, 2021.
The Statutory Auditors
French original signed by Cédric Adens and Olivier Bochet
Page 49 of 49