AN OFFERING STATEMENT PURSUANT TO REGULATION A RELATING TO THESE SECURITIES HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION. INFORMATION CONTAINED IN THIS PRELIMINARY OFFERING CIRCULAR IS SUBJECT TO COMPLETION OR AMENDMENT. THESE SECURITIES MAY NOT BE SOLD NOR MAY OFFERS TO BUY BE ACCEPTED BEFORE THE OFFERING STATEMENT FILED WITH THE COMMISSION IS QUALIFIED. THIS PRELIMINARY OFFERING CIRCULAR SHALL NOT CONSTITUTE AN OFFER TO SELL OR THE SOLICITATION OF AN OFFER TO BUY NOR MAY THERE BE ANY SALES OF THESE SECURITIES IN ANY STATE IN WHICH SUCH OFFER, SOLICITATION OR SALE WOULD BE UNLAWFUL BEFORE REGISTRATION OR QUALIFICATION UNDER THE LAWS OF SUCH STATE. THE COMPANY MAY ELECT TO SATISFY ITS OBLIGATION TO DELIVER A FINAL OFFERING CIRCULAR BY SENDING YOU A NOTICE WITHIN TWO BUSINESS DAYS AFTER THE COMPLETION OF THE COMPANY'S SALE TO YOU THAT CONTAINS THE URL WHERE THE FINAL OFFERING CIRCULAR OR THE OFFERING STATEMENT IN WHICH SUCH FINAL OFFERING CIRCULAR WAS FILED MAY BE OBTAINED.
PRELIMINARY OFFERING CIRCULAR DATED AUGUST 28, 2020
MONOGRAM ORTHOPAEDICS, INC.

3913 Todd Lane, Austin, TX 78744
(512) 399-2656
www.monogramorthopedics.com
UP TO 4,784,689 SHARES OF SERIES B PREFERRED STOCK, PLUS UP TO 478,468 BONUS SHARES (1)
UP TO 4,784,689 SHARES OF COMMON STOCK INTO WHICH THE SERIES B PREFERRED STOCK MAY CONVERT(2)
PRICE: $6.27 PER SHARE
Holders of our Series B Preferred Stock have limited voting rights compared to holders of our Common Stock. For instance, holders of our Common Stock will have the right to elect two directors as a single class, while holders of our Series B Preferred Stock will only vote for a single director together with the holders of our Common Stock. See "Securities Being Offered" at Page 38 for more information on the rights of our Series B Preferred Stock.
| Price to Public (3) | Underwriting discount and commissions (4) | Proceeds to issuer (5) | ||||||||||
| Per share | $ | 6.27 | $ | 0.44 | $ | 5.83 | ||||||
| Total Maximum | $ | 30,000,000 | $ | 2,100,000 | $ | 27,900,000 | ||||||
| (1) | The company is offering up to 4,784,689 shares of Series B Preferred Stock, plus up to 478,468 additional shares of Series B Preferred Stock eligible to be issued as Bonus Shares to investors based upon investment level. See “Plan of Distribution and Selling Securityholders” for further details. |
| (2) | The Series B Preferred Stock is convertible into Common Stock either at the discretion of the investor or automatically upon the occurrence of certain events, like effectiveness of registration of the Common Stock in an initial public offering. The total number of shares of the Common Stock into which the Series B Preferred Stock may be converted will be determined by dividing the original issue price per share by the conversion price per share. See "Securities Being Offered" at page 38 for additional details. |
| (3) | Does not include effective discount that would result from the issuance of Bonus Shares. For details of the effective discount. See “Plan of Distribution and Selling Securityholders.” |
| (4) | The company has engaged StartEngine Primary, LLC, member FINRA/SIPC (“StartEngine Primary”), to act as a placement agent in this offering as set forth in “Plan of Distribution and Selling Securityholder” and its affiliate StartEngine Crowdfunding, Inc. to perform administrative and technology-related functions in connection with this offering. The company will pay a cash commission of 7% to StartEngine Primary on sales of the Series B Preferred Stock, and the company will issue Series B Preferred Stock to StartEngine Primary in an amount equal to 2% of the shares issued to investors in this offering (excluding bonus shares). Shares issued to StartEngine Primary will be subject to a lock-up provision in accordance with FINRA requirements. The company will also pay a $15,000 advance fee for reasonable accountable out of pocket expenses actually anticipated to be incurred by StartEngine Primary. Any unused portion of this fee not actually incurred by StartEngine Primary will be returned to the company. FINRA fees will be paid by the company. See “Plan of Distribution and Selling Securityholders” for details of compensation payable to third parties in connection with this offering. The maximum amount the company would pay StartEngine Primary in commissions is $2,100,000, and the maximum amount of Series B Preferred shares the company would issue StartEngine is 95,693. This does not include transaction fees paid directly to StartEngine Primary by investors. |
| (5) | Investors will be required to pay directly to StartEngine Primary a processing fee equal to 3.5% of the investment amount at the time of the investors’ subscription. This fee will be refunded in the event the company does not raise any funds in this offering. See “Plan of Distribution and Selling Securityholders” for additional discussion of this processing fee. |
The company expects that the amount of expenses of the offering that it will pay will be approximately $75,000, not including commissions or state filing fees.
The company has engaged Prime Trust, LLC (the "Escrow Agent") to hold funds tendered by investors. We may hold a series of closings at which we receive the funds from the Escrow Agent and issue the shares to investors. The offering will terminate at the earlier of: (1) the date at which the maximum offering amount has been sold, (2) up to three years from the date upon which the Securities and Exchange Commission qualifies the Offering Statement of which this Offering Circular forms a part, or (3) the date at which the offering is earlier terminated by the company in its sole discretion. In the event we have not sold the minimum amount of shares by [___], or sooner terminated by the company, any money tendered by potential investors will be promptly returned by the Escrow Agent. The offering is being conducted on a best-efforts basis.
1
INVESTING IN THE SERIES B PREFERRED STOCK OF MONOGRAM ORTHOPAEDICS, INC. IS SPECULATIVE AND INVOLVES SUBSTANTIAL RISKS. YOU SHOULD PURCHASE THESE SECURITIES ONLY IF YOU CAN AFFORD A COMPLETE LOSS OF YOUR INVESTMENT. SEE “RISK FACTORS” BEGINNING ON PAGE 6 TO READ ABOUT THE MORE SIGNIFICANT RISKS YOU SHOULD CONSIDER BEFORE BUYING THE SERIES B PREFERRED STOCK OF THE COMPANY.
THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION DOES NOT PASS UPON THE MERITS OR GIVE ITS APPROVAL OF ANY SECURITIES OFFERED OR THE TERMS OF THE OFFERING, NOR DOES IT PASS UPON THE ACCURACY OR COMPLETENESS OF ANY OFFERING CIRCULAR OR OTHER SOLICITATION MATERIALS. THESE SECURITIES ARE OFFERED PURSUANT TO AN EXEMPTION FROM REGISTRATION WITH THE COMMISSION; HOWEVER THE COMMISSION HAS NOT MADE AN INDEPENDENT DETERMINATION THAT THE SECURITIES OFFERED ARE EXEMPT FROM REGISTRATION
GENERALLY NO SALE MAY BE MADE TO YOU IN THIS OFFERING IF THE AGGREGATE PURCHASE PRICE YOU PAY IS MORE THAN 10% OF THE GREATER OF YOUR ANNUAL INCOME OR NET WORTH. DIFFERENT RULES APPLY TO ACCREDITED INVESTORS AND NON-NATURAL PERSONS. BEFORE MAKING ANY REPRESENTATION THAT YOUR INVESTMENT DOES NOT EXCEED APPLICABLE THRESHOLDS, WE ENCOURAGE YOU TO REVIEW RULE 251(d)(2)(i)(C) OF REGULATION A. FOR GENERAL INFORMATION ON INVESTING, WE ENCOURAGE YOU TO REFER TO www.investor.gov.
Sales of these securities will commence on approximately [_].
The company is following the "Offering Circular" format of disclosure under Regulation A.
In the event that we become a reporting company under the Securities Exchange Act of 1934, we intend to take advantage of the provisions that relate to “Emerging Growth Companies” under the JOBS Act of 2012. See “Implications of Being an Emerging Growth Company.”
2
TABLE OF CONTENTS
In this Offering Circular, the term “Monogram Orthopaedics” “Monogram”, “we”, “us”, “our” or “the company” refers to Monogram Orthopaedics, Inc.
THIS OFFERING CIRCULAR MAY CONTAIN FORWARD-LOOKING STATEMENTS AND INFORMATION RELATING TO, AMONG OTHER THINGS, THE COMPANY, ITS BUSINESS PLAN AND STRATEGY, AND ITS INDUSTRY. THESE FORWARD-LOOKING STATEMENTS ARE BASED ON THE BELIEFS OF, ASSUMPTIONS MADE BY, AND INFORMATION CURRENTLY AVAILABLE TO THE COMPANY’S MANAGEMENT. WHEN USED IN THE OFFERING MATERIALS, THE WORDS “ESTIMATE,” “PROJECT,” “BELIEVE,” “ANTICIPATE,” “INTEND,” “EXPECT” AND SIMILAR EXPRESSIONS ARE INTENDED TO IDENTIFY FORWARD-LOOKING STATEMENTS, WHICH CONSTITUTE FORWARD LOOKING STATEMENTS. THESE STATEMENTS REFLECT MANAGEMENT’S CURRENT VIEWS WITH RESPECT TO FUTURE EVENTS AND ARE SUBJECT TO RISKS AND UNCERTAINTIES THAT COULD CAUSE THE COMPANY’S ACTUAL RESULTS TO DIFFER MATERIALLY FROM THOSE CONTAINED IN THE FORWARD-LOOKING STATEMENTS. INVESTORS ARE CAUTIONED NOT TO PLACE UNDUE RELIANCE ON THESE FORWARD-LOOKING STATEMENTS, WHICH SPEAK ONLY AS OF THE DATE ON WHICH THEY ARE MADE. THE COMPANY DOES NOT UNDERTAKE ANY OBLIGATION TO REVISE OR UPDATE THESE FORWARD-LOOKING STATEMENTS TO REFLECT EVENTS OR CIRCUMSTANCES AFTER SUCH DATE OR TO REFLECT THE OCCURRENCE OF UNANTICIPATED EVENTS.
3
Overview
Monogram Orthopaedics, Inc was incorporated under the laws of the State of Delaware on April 21, 2016, as “Monogram Arthroplasty Inc.” On March 27, 2017, the company changed its name to “Monogram Orthopaedics, Inc.” Monogram Orthopaedics is developing a product solution architecture with the intention to enable mass personalization of orthopedic implants by linking 3D printing and robotics via automated digital image analysis algorithms. The company has a robot prototype that can execute optimized paths for high precision insertion of optimized implants in synthetic bone specimens. These implants and cut-paths are prepared based on proprietary Monogram implant designs. Monogram intends to produce and market robotic surgical equipment and related software, orthopaedic implants, tissue ablation tools, navigation consumables, and other miscellaneous instrumentation necessary for the execution of reconstructive joint replacement procedures.
The Offering
| Securities offered: | Maximum of 4,784,689 shares of Series B Preferred Stock, plus up to 478,468 additional shares of Series B Preferred Stock eligible to be issued as Bonus Shares |
| Securities outstanding before the Offering (as of August 12, 2020) | |
| Common Stock | 4,836,935 shares |
| Series A Preferred Stock | 4,897,559 shares |
| Series B Preferred Stock | 0 shares |
| Securities outstanding after the Offering: | |
| Series A Preferred Stock | 4,897,559 (1) (2) |
| Series B Preferred Stock | 4,784,689 shares (1) (2) |
| Common Stock | 4,836,935 shares (1) |
| (1) | Does not reflect the amount of potential shares issuable to Pro-Dex, Inc. (“Pro-Dex”) under the terms of its warrant agreement (filed herewith as Exhibit 6.11) as of the date of this offering circular. Pro-Dex, Inc. has the right to purchase up to 5% of the outstanding Common Stock and Preferred Stock of the company as of the date of the exercise, calculated on a post-exercise basis. After the offering, assuming a maximum raise, Pro-Dex will have the right to acquire up to 241,846 shares of Common Stock, 244,877 shares of Series A Preferred Stock, and 239,234 shares of Series B Preferred Stock. | |
| (2) | Does not reflect the amount of potential shares issuable to ZB Capital Partners, LLC under the terms of its warrant agreement (filed herewith as Exhibit 6.13), which, if exercised, would result in the issuance of 273,972 shares of Series A Preferred Stock, or 171,527 shares of Series B Preferred Stock in this offering. |
Implications of Being an Emerging Growth Company
As an issuer with less than $1 billion in total annual gross revenues during our last fiscal year, we will qualify as an “emerging growth company” under the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”) and this status will be significant if and when we become subject to the ongoing reporting requirements of the Exchange Act upon filing a Form 8-A. An emerging growth company may take advantage of certain reduced reporting requirements and is relieved of certain other significant requirements that are otherwise generally applicable to public companies. In particular, as an emerging growth company we:
| · | will not be required to obtain an auditor attestation on our internal controls over financial reporting pursuant to the Sarbanes-Oxley Act of 2002; |
| · | will not be required to provide a detailed narrative disclosure discussing our compensation principles, objectives and elements and analyzing how those elements fit with our principles and objectives (commonly referred to as “compensation discussion and analysis”); |
| · | will not be required to obtain a non-binding advisory vote from our shareholders on executive compensation or golden parachute arrangements (commonly referred to as the “say-on-pay,” “say-on-frequency” and “say-on-golden-parachute” votes); |
| · | will be exempt from certain executive compensation disclosure provisions requiring a pay-for-performance graph and CEO pay ratio disclosure; |
| · | may present only two years of audited financial statements and only two years of related Management’s Discussion and Analysis of Financial Condition and Results of Operations, or MD&A; and |
| · | will be eligible to claim longer phase-in periods for the adoption of new or revised financial accounting standards. |
4
We intend to take advantage of all of these reduced reporting requirements and exemptions, including the longer phase-in periods for the adoption of new or revised financial accounting standards, and hereby elect to do so. Our election to use the phase-in periods may make it difficult to compare our financial statements to those of non-emerging growth companies and other emerging growth companies that have opted out of the phase-in periods under Section 107 of the JOBS Act.
Under the JOBS Act, we may take advantage of the above-described reduced reporting requirements and exemptions for up to five years after our initial sale of common equity pursuant to a registration statement declared effective under the Securities Act of 1933, as amended, or such earlier time that we no longer meet the definition of an emerging growth company. Note that this offering, while a public offering, is not a sale of common equity pursuant to a registration statement, since the offering is conducted pursuant to an exemption from the registration requirements. In this regard, the JOBS Act provides that we would cease to be an “emerging growth company” if we have more than $1 billion in annual revenues, have more than $700 million in market value of our Common Stock held by non-affiliates, or issue more than $1 billion in principal amount of non-convertible debt over a three-year period.
Certain of these reduced reporting requirements and exemptions are also available to us due to the fact that we may also qualify, once listed, as a “smaller reporting company” under the Commission’s rules. For instance, smaller reporting companies are not required to obtain an auditor attestation on their assessment of internal control over financial reporting; are not required to provide a compensation discussion and analysis; are not required to provide a pay-for-performance graph or CEO pay ratio disclosure; and may present only two years of audited financial statements and related MD&A disclosure.
Selected Risks Associated with Our Business
Our business is subject to a number of risks and uncertainties, including those highlighted in the section titled “Risk Factors” immediately following this summary. These risks include, but are not limited to, the following:
| · | We are a comparatively early-stage company that has incurred operating losses in the past, expect to incur operating losses in the future, and may never achieve or maintain profitability. |
| · | Monogram depends on a licensing agreement for its intellectual property, which, if terminated, would significantly impair its ability to continue its operations. Significant delays in the development of Monogram’s technology may result in a default on the terms of this agreement, which increases the risk of this licensing agreement being terminated. |
| · | Our technology is not yet fully developed, and there is no guarantee that we will ever successfully develop the technology that is essential to our business. Furthermore, the end products would have an extremely high technical sophistication level that makes it difficult to estimate the costs required to develop those technologies accurately. |
| · | Our business plan is predicated on obtaining market clearance from the Food and Drug Administration (“FDA”) under Section 510(k) of the Federal Food, Drug, and Cosmetic Act, or the FDCA. If we are unable to obtain Section 510(k) clearance, it is unlikely that we will be able to continue to operate as a going concern. |
| · | We could be adversely affected by product liability, product recall, personal injury or other health and safety issues. |
| · | Reductions in third party reimbursement levels, from private or government agency plans, and potential changes in industry pricing benchmarks for joint replacements could materially and adversely affect our results of operations. |
| · | We may be subject to patient data protection requirements. |
| · | We operate in a highly competitive industry that is dominated by several very large, well-capitalized market leaders, and the size and resources of some of our competitors may allow them to compete more effectively than we can. |
| · | We rely on third parties to provide services essential to the success of our business. If the third parties we rely on to provide services necessary to our business become insolvent, it would be materially disruptive to our business, and we may incur high costs and time to secure alternative supply. |
| · | We expect to raise additional capital through equity and/or debt offerings to support our working capital requirements and operating losses. |
| · | All of our assets are pledged as collateral to a lender. |
| · | The company is controlled by its officers and directors. |
| · | In certain circumstances, investors will not have dissenters’ rights |
| · | Investors in this offering must vote their shares to approve of certain future events, including our sale. |
| · | This investment is illiquid. |
| · | The auditor included a “going concern” note in its audit report. |
| · | Investors in this offering may not be entitled to a jury trial with respect to claims arising under the subscription agreement and investors’ rights agreement, which could result in less favorable outcomes to the plaintiff(s) in any action under these agreements. |
| · | We are offering bonus shares to some investors in this offering, which effectively gives them a discount on their investment. |
5
The SEC requires the company to identify risks that are specific to its business and its financial condition. The company is still subject to all the same risks that all companies in its industry, and all companies in the economy, are exposed to. These include risks relating to economic downturns, political and economic events and technological developments (such as cyber-attacks and the ability to prevent such attacks). Additionally, early-stage companies are inherently more risky than more developed companies, and the risk of business failure and complete loss of your investment capital is present. You should consider general risks as well as specific risks when deciding whether to invest.
Risks Related to Our Company
We have a limited operating history upon which you can evaluate our performance and have not yet generated profits. Accordingly, our prospects must be considered in light of the risks that any new company encounters. Our company was incorporated under the laws of the State of Delaware on April 21, 2016, and we have not yet generated revenues or profits. The likelihood of our creation of a viable business must be considered in light of the problems, expenses, difficulties, complications, and delays frequently encountered in connection with the growth of a business, operation in a competitive industry, and the continued development of our technology and products. We anticipate that our operating expenses will increase for the near future, and there is no assurance that we will be profitable in the near future. You should consider our business, operations, and prospects in light of the risks, expenses, and challenges faced as an emerging growth company.
The auditor included a “going concern” note in its audit report. We may not have enough funds to sustain the business until it becomes profitable. Even if we raise funds through this offering, we may not accurately anticipate how quickly we may use the funds and whether these funds are sufficient to bring the business to profitability.
Our technology is not yet fully developed, and there is no guarantee that we will successfully develop our technology. Monogram is developing sophisticated technology that will require significant technical and regulatory expertise to develop and commercialize. If we are unable to develop and commercialize our technology and products successfully, it will significantly affect our viability as a company.
We are subject to substantial governmental regulation relating to the manufacturing, labeling, and marketing of our products, and will continue to be for the lifetime of our company. The FDA and other governmental authorities in the United States regulate the manufacturing, labeling, and marketing of our products. The process of obtaining regulatory approvals to market a medical device can be expensive and lengthy, and applications may take a long time to be approved if they are approved at all. Our compliance with the quality system, medical device reporting regulations, and other laws and regulations applicable to the manufacturing of products within our facilities and those contracted by third parties is subject to periodic inspections by the FDA and other governmental authorities. Complying with regulations, and, if necessary, remedial actions can be significantly expensive. Failure to comply with applicable regulatory requirements may subject us to a range of sanctions, including substantial fines, warning letters that require corrective action, product seizures, recalls, halting product manufacturing, revocation of approvals, exclusion from future participation in government healthcare programs, substantial fines, and criminal prosecution.
We are subject to federal and state healthcare regulations and laws relating to anti-bribery and anti-corruption, and non-compliance with such laws could lead to significant penalties. State and Federal anti-bribery laws, healthcare fraud and abuse laws dictate how we conduct the relationships that we and our distributors and others that market our products have with healthcare professionals, such as physicians and hospitals. We also must comply with a variety of other laws that protect the privacy of individually identifiable healthcare information. These laws and regulations are broad in scope and are subject to evolving interpretation, and we could be required to incur substantial costs to monitor compliance or to alter our practices if we are found not to be in compliance. In addition, violations of these laws may be punishable by criminal or civil sanctions, including substantial fines, imprisonment of current or former employees, and exclusion from participation in governmental healthcare programs.
Government regulations and other legal requirements affecting our company are subject to change. Such change could have a material adverse effect on our business. We operate in a complex, highly regulated environment. The numerous federal, state and local regulations that our business is subject to include, but are not limited to: federal and state registration and regulation of medical devices; applicable governmental payor regulations including Medicare and Medicaid; data privacy and security laws and regulations including those under the Health Insurance Portability and Accountability Act of 1996 (“HIPAA”); the Affordable Care Act (“ACA”) or any successor to that act; laws and regulations relating to the protection of the environment and health and safety matters, including those governing exposure to, and the management and disposal of, hazardous substances; regulations regarding food and drug safety including those of the Food and Drug Administration (“FDA”), and consumer protection and safety regulations including those of the Consumer Product Safety Commission, as well as state regulatory authorities, governing the availability, sale, advertisement and promotion of products we sell; federal and state laws governing health care fraud and abuse; anti-kickback laws; false claims laws; and laws against the corporate practice of medicine. The FDA and state regulatory authorities have broad enforcement powers, including the ability to seize or recall products and impose significant criminal, civil and administrative sanctions for violations of these laws and regulations.
6
Changes in laws, regulations, and policies and the related interpretations and enforcement practices may significantly affect our cost of doing business as we endeavor to maintain compliance with such new policies and laws. Changes in laws, regulations, and policies and the related interpretations and enforcement practices generally cannot be predicted may require extensive system and operational changes. Noncompliance with applicable laws and regulations could result in civil and criminal penalties that could adversely affect our business, including suspension of payments from government programs; loss of required government certifications; loss of authorizations to participate in or exclusion from government programs, including the Medicare and Medicaid programs; loss of licenses; and significant fines or monetary penalties. Any failure to comply with applicable regulatory requirements could result in significant legal and financial exposure, damage our reputation, and have a material adverse effect on our business operations, financial condition, and results of operations.
We have not yet obtained clearance of our products by the U. S. Food and Drug Administration, or FDA, which is critical to our business plan. In order to sell our products, we must obtain market clearance from the Food and Drug Administration (“FDA”) under Section 510(k) of the Federal Food, Drug, and Cosmetic Act, or the FDCA (see “The Company’s Business – Regulation.”). If Monogram is unable to obtain Section 510(k) clearance, we will not be able to sell our products, and it is unlikely that we will be able to continue to operate as a going concern. In addition, the FDA may request clinical data with our 510(k) submission. The FDA has indicated an increased focus on robotic technologies that perform automated operations and may request clinical data for our robot and/or implants. If the FDA requires such information, it will materially and adversely impact our development timeline and increase the cost to obtain market clearance. In addition, our development timeline is currently dependent on the amount of funds we raise in this offering. If we only raise the minimum offering amount, we estimate it will take substantially longer for us to obtain FDA clearance, and would require us to raise additional capital from outside sources, which the company may not be able to achieve successfully. These factors combined may impact our ability to continue to operate as a going concern.
We anticipate initially sustaining operating losses. It is expected that we will initially sustain operating losses in seeking Section 510(k) clearance. Our ability to become profitable depends on obtaining 510(k) clearance, and subsequent success in licensing and selling of products. There can be no assurance that this will occur. Unanticipated problems and expenses are often encountered in offering new products, which may impact whether the company is successful. Furthermore, we may encounter substantial delays and unexpected costs related to development, technological changes, marketing, regulatory requirements, and changes to such requirements or other unforeseen difficulties. There can be no assurance that we will ever become profitable. If the company sustains losses over an extended period of time, it may be unable to continue in business.
Our products may not gain market acceptance among hospitals, surgeons, physicians, patients, healthcare payors, and the medical community. A critical element in our commercialization strategy is to persuade the medical community on the efficacy of our products and to educate then on their safe and effective use. Surgeons, physicians, and hospitals may not perceive the benefits of our products and could be unwilling to change from the devices they are currently using. A number of factors may limit the market acceptance of our products, including the following:
| · | rate of adoption by healthcare practitioners; |
| · | rate of a product’s acceptance by the target population; |
| · | timing of market entry relative to competitive products; |
| · | availability of third-party reimbursement; |
| · | government review and approval requirements; |
| · | the extent of marketing efforts by us and third-party distributors or agents retained by us; and |
| · | side effects or unfavorable publicity concerning our products or similar products. |
Notably, in our simulations, our current methods of robotic execution take longer than conventional methods of insertion. If we are unable to reduce the time of our surgical procedure, it may adversely impact market reception of our products. Our inability to successfully commercialize our products will have a material adverse effect on the value of your investment.
We could be adversely affected by product liability, personal injury or other health and safety issues. We could be adversely impacted by the supply of defective products. We are also exposed to risks relating to the surgical robotic technology services and products we provide. Defective products or errors in our technology could lead to serious injury or death. Product liability or personal injury claims may be asserted against us with respect to any of the products we supply or the services we provide. Monogram is also liable for harms caused by any faults in raw materials or products supplied by third-party manufacturers and suppliers that our company utilizes. It is our responsibility to have a quality management system in place and to audit our suppliers to ensure that products supplied to our company meet proper standards. Should a product or other liability issues arise, the coverage limits under insurance programs and the indemnification amounts available to us may not be adequate to protect us against claims and judgments. We also may not be able to maintain such insurance on acceptable terms in the future. We could suffer significant reputational damage and financial liability if we experience any of the foregoing health and safety issues or incidents, which could have a material adverse effect on our business operations, financial condition and results of operations.
7
If third-party payors fail to provide appropriate levels of reimbursement for the use of our products, our revenues could be adversely affected. Sales of our products depend on the availability of adequate reimbursement from third-party payors. In each market in which we do business, our inability to obtain reimbursement approval or the failure of third-party payors to reimburse health care providers at a level that justifies the use of our products instead of cheaper alternatives will hurt our business.
Moreover, we are unable to predict what changes will be made to the reimbursement methodologies used by third-party payors in the future. Changes in political, economic, and regulatory influences may significantly affect healthcare financing and reimbursement practices. For example, there have been multiple attempts through legislative action and legal challenges to repeal or amend the ACA. We cannot predict whether current or future efforts to repeal or amend these laws will be successful, nor can we predict the impact that such a repeal or amendment and any subsequent legislation would have on our business and reimbursement levels. There have also been a number of other proposals and enactments by the federal government and various states to reduce Medicaid reimbursement levels in response to budget deficits, and we expect additional proposals in the future. We cannot assure you that recent or future changes reimbursement policies and practices will not materially and adversely affect our results of operations. Efforts to control healthcare costs, including costs of reconstructive joint replacement, are continuous, and reductions in third party reimbursement levels could materially and adversely affect our results of operations.
We rely on a licensing agreement with the Icahn School of Medicine at Mount Sinai. We are party to a licensing agreement (and related option agreement) with Icahn School of Medicine at Mount Sinai (“Mount Sinai”) pursuant to which Mount Sinai has granted Monogram an exclusive license to patents related to customizable bone implants, surgical planning software, and surgical robots (see “The Company’s Business – Intellectual Property”). The patent, software, technical information, know-how, etc. licensed under this agreement is integral to our company’s core products and technology. As such, we are reliant on the licensing agreement with Mount Sinai to operate our business. Under the terms of our licensing agreement, Mount Sinai has the right to terminate our license for the patent if we materially breach any of our obligations under the licensing agreement. Further, the licensing agreement expires upon the later of (i) 12 years from the first commercial sale of such any product that we sell using the intellectual property covered in the licensed patent or (ii) expiration of the licensed patent. If our arrangement with Mount Sinai were to end, we would no longer be able to use the intellectual property covered by the patent, which could significantly affect our business.
We may default on our obligations under the licensing agreement with the Icahn School of Medicine at Mount Sinai, which could result in termination of the agreement. Pursuant to the terms of the licensing agreement with Mount Sinai (and amendment thereto filed as Exhibits 6.8 and 6.14), we must have a first commercial sale our products within seven (7) years of the Effective Date of the agreement, or by October 10, 2024. Failure to meet this deadline would constitute a breach of our agreement, and Mount Sinai would have the right to give us a notice of default, and could ultimately terminate the licensing agreement if we fail to cure this default within sixty (60) days. A termination of this licensing agreement would also terminate our related option agreement with Mount Sinai, as the option agreement is governed by the terms of the licensing agreement. Currently, we expect to achieve a commercial sale within this timeframe. If we are unsuccessful in doing so, however, we would be in default, and would be exposed to the risk of Mount Sinai terminating the agreement, along with our right to license its intellectual property. Such a result would materially impact our ability to operate as a going concern.
We operate in a highly competitive industry that is dominated by several very large, well-capitalized market leaders and is continuously evolving. New entrants to the market, existing competitor actions, or other changes in market dynamics could adversely impact us. The level of competition in the orthopaedic market is high, with several very large, well-capitalized competitors holding a majority share of the market. Changes in market dynamics or actions of competitors or manufacturers, including industry consolidation and the emergence of new competitors and strategic alliances, could materially and adversely impact our business. Disruptive innovation by existing or new competitors could alter the competitive landscape in the future and require us to accurately identify and assess such changes and make timely and effective changes to our strategies and business model to compete effectively.
Currently, we are not aware of any well-known orthopaedic companies that broadly offer robotic technology in combination with surgical navigation for the insertion of patient-specific press-fit orthopaedic implants. Nonetheless, many of our competitors in this market have significant financial resources. They may seek to extend their robotics and orthopaedic implant technology to accommodate the robotic insertion of patient-specific press-fit implants. Further, several companies offer surgical navigation systems for use in arthroplasty procedures that provide a minimally invasive means of viewing the anatomical site. As such, other companies may create similar technology and/or products to that which we are trying to develop, which would increase competition in our industry. As competition increases, a significant increase in general pricing pressures could occur, which could require us to reevaluate our pricing structures to remain competitive. For example, if we are not able to anticipate and successfully respond to changes in market conditions, it could result in a loss of customers or renewal of contracts or arrangements on less favorable terms.
8
Successful infringement claims against us could result in significant monetary liability or prevent us from selling some of our products. If successfully developed, our products and technology may be highly disruptive to a very large and growing market. Our competitors are well-capitalized with significant intellectual property protection and resources and may initiate infringement lawsuits against our company. Such litigation could be expensive and could also prevent us from selling our products, which would significantly harm our ability to grow our business as planned.
Our failure to attract and retain highly qualified personnel in the future could harm our business. As the company grows, it will be required to hire and attract additional qualified professionals such as software engineers, robotics engineers, machine vision and machine learning experts, biomechanical engineers, project managers, regulatory professionals, sales and marketing professionals, accounting, legal, and finance experts. The company may not be able to locate or attract qualified individuals for such positions, which will affect the company’s ability to grow and expand its business.
We rely on third-party manufacturers and service providers. Our third-party partners provide a variety of essential business functions, including distribution, manufacturing, and many others. It is possible that some of these third parties will fail to perform their services or will perform them in an unacceptable manner. If we encounter problems with one or more of these parties, and they fail to perform to expectations, it would be materially disruptive to our business, and we may incur high costs and time to secure alternative supply, or be unable to secure an alternative supply altogether. Such an occurrence could have a material adverse impact on the company.
Our future success is dependent on the continued service of our small management team. Monogram is managed by four directors and one executive officer. Our success is dependent on their ability to manage all aspects of our business effectively. Because we are relying on our small management team, we lack certain business resources that may hurt our ability to efficiently operate or grow our business. Any loss of key members of our executive team could have a negative impact on our ability to manage and grow our business effectively. We do not maintain a key person life insurance policy on any of the members of our senior management team. As a result, we would have no way to cover the financial loss if we were to lose the services of our directors or officers.
We expect to raise additional capital through equity and/or debt offerings to support our working capital requirements and operating losses. To fund future growth and development, the company will likely need to raise additional funds in the future by offering shares of its Common or Preferred Stock and/or other classes of equity, or debt that convert into shares of common or Preferred Stock, any of which offerings would dilute the ownership percentage of investors in this offering. See “Dilution.” In order to issue sufficient shares in this regard, we may be required to amend our certificate of incorporation to increase our authorized capital stock, which would require us to obtain the consent of a majority of our shareholders. Furthermore, if the company raises capital through debt, the holders of our debt would have priority over holders of common and Preferred Stock, and the company may be required to accept terms that restrict its ability to incur more debt. We cannot assure you that the necessary funds will be available on a timely basis, on favorable terms, or at all, or that such funds, if raised, would be sufficient. The level and timing of future expenditure will depend on a number of factors, many of which are outside our control. If we are not able to obtain additional capital on acceptable terms, or at all, we may be forced to curtail or abandon our growth plans, which could adversely impact the company, its business, development, financial condition, operating results or prospects.
Any valuation at this stage is difficult to assess. The valuation for this Offering was established by the company. Unlike listed companies that are valued publicly through market-driven stock prices, the valuation of private companies, especially early-stage companies, is challenging to assess, and you may risk overpaying for your investment.
If we cannot raise sufficient funds, we will not succeed. We are offering shares of our Series B Preferred Stock in the amount of up to $30,000,000 in this Offering on a best-efforts basis and may not raise the entire amount. Even if the maximum amount is raised, we are likely to need additional funds in the future to grow. The technology and products we are developing are highly sophisticated, and we may also encounter technical challenges that require more capital than anticipated by the management team to overcome. If we cannot raise those funds for whatever reason, including reasons relating to the company itself or to the broader economy, the company may not survive. If we raise a substantially lesser amount than the Maximum Raise, we will have to find other sources of funding for some of the plans outlined in “Use of Proceeds To Issuer.”.
Our technologies are highly complex, and development budget estimates may not be accurately or sufficiently forecasted. While management makes every effort to predict anticipated development costs accurately, the project and technology complexity of the products makes it difficult to forecast these required development costs accurately. It is not uncommon to encounter unforeseen technical challenges that introduce unanticipated development costs. The actual development costs may not be the same as the anticipated development costs. If the actual development costs are materially above those anticipated by management, it could materially adversely impact our business.
Our products may require more technical complexity than anticipated and our engineers may not be able to overcome these technical challenges. While management makes every effort to anticipate the technical challenges of product development, we may encounter unforeseen complexity that we cannot overcome, or that may be difficult to overcome without incurring significant time or cost that was not anticipated or budgeted. For example, we have found it challenging to revise our first-generation tibial design. To facilitate more efficient removal, we may need to make design changes to features like the locking mechanism that were not anticipated and introduce additional cost, time and complexity. Additional unforeseen challenges as this could hinder our plan of operations, slowing our progress and increasing our costs, which may harm your investment in our company.
9
We may not gain acceptance by Group Purchasing Organizations or other purchasing entities. Many hospital systems and Ambulatory Surgery Centers use group purchasing organizations to negotiate pricing and supply from vendors. Many of these organizations are large and risk-averse, and gaining adoption at reasonable terms can be challenging. If we are unable to secure contracts with widely used Group Purchasing Organizations, we may struggle to gain market adoption, which would materially adversely affect our business.
We may use independent distributors to represent our products. Monogram may use contracted employees and independent distributors to represent our products to surgeons, hospitals, and Ambulatory Surgery Centers. Such independent distributors and contractors are not employees of the company and may conduct business in a manner that is unethical or even illegal. Monogram could incur liability for unlawful business practices conducted by such independent distributors or contractors. If a distributor violates the terms of our agreements, it could materially adversely affect our business.
Our products require a level of accuracy that we may never be able to achieve. To obtain FDA approval on our system we will need to demonstrate that we can accurately position implants in robotically prepared bone specimens. The KUKA LBR Med robot that we are using has never before been used or validated for this application, and it may not be able to perform to the accuracies required. Preparing bone to the accuracies required is a highly challenging task with numerous sources of error that we may never be able to overcome. We have not yet achieved high accuracy cuts in a cadaveric bone specimen. If we cannot execute a robotic surgical plan with sufficient accuracy, it will materially adversely impact our business and market reputation.
Our assets may become pledged as collateral to a lender. We may enter into financing arrangements with lenders that contain covenants that limit our ability to engage in specified types of transactions. These covenants may limit our ability to, among other things:
| · | petition for bankruptcy; |
| · | assignment of the notes to other creditors; |
| · | appointment of a receiver of any property of the company; and |
| · | consolidate, merge, sell, or otherwise dispose of all or substantially all of our assets. |
10
A breach of any of these covenants could result in a default under the terms of such a financing in which the lender could elect to declare all amounts outstanding thereunder to be immediately due and payable. We may need to pledge all of our assets as collateral to secure additional financing.
Acquisition opportunities may present themselves that in hindsight did not achieve the positive results anticipated by our management. From time to time, acquisition opportunities may become available to the company. Those opportunities may involve the acquisition of specific assets, like intellectual property or inventory, or may involve the assumption of the business operations of another entity. Our goal with any future acquisition is that any acquisition should be able to contribute neutral to positive EBITDA to the company after integration. To effect these acquisitions, we will likely be required to obtain lender financing or issue additional shares of stock in exchange for the shares of the target entity. If the performance of the acquired assets or entity does not produce positive results for the company, the terms of the acquisition, whether it is interest rate on debt, or additional dilution of stockholders, may prove detrimental to the financial results of the company, or the performance of your particular shares.
The novel coronavirus (COVID-19) pandemic may have an impact on our business, financial condition and results of operations. The COVID-19 pandemic has rapidly escalated in the United States, creating significant uncertainty and economic disruption, and leading to record levels of unemployment nationally. Numerous state and local jurisdictions have imposed, and others in the future may impose, shelter-in-place orders, quarantines, shut-downs of non-essential businesses, and similar government orders and restrictions on their residents to control the spread of COVID-19. The extent to which COVID-19 ultimately impacts our business, financial condition and results of operations will depend on future developments, which are highly uncertain and unpredictable, including new information which may emerge concerning the severity and duration of the COVID-19 outbreak and the effectiveness of actions taken to contain the COVID-19 outbreak or treat its impact, among others. In addition to the COVID-19 disruptions possibility adversely impacting our business and financial results, they may also have the effect of heightening many of the other risks described here under “Risk Factors,” including risks relating to changes due to our limited operating history; our ability to generate sufficient revenue, to generate positive cash flow; our relationships with third parties, and many other factors. We will endeavor to minimize these impacts, but there can be no assurance relative to the potential impacts that may be incurred
Risks Related to the Securities in this Offering
In certain circumstances, investors will not have dissenters' rights. The investors’ rights agreement that investors will execute in connection with the offering contains a “drag-along” provision whereby investors agree to vote any shares they own in the same manner as the majority holders of our other classes of stock. Specifically, and without limitation, if the majority holders of our other classes of stock determine to sell the company, depending on the nature of the transaction, investors will be forced to sell their stock in that transaction regardless of whether they believe the transaction is the best or highest value for their shares, and regardless of whether they believe the transaction is in their best interests.
We have previously granted anti-dilution rights in the form of preemptive rights to certain holders of our Common Stock. The effect of those rights is that at any time we intend to issue additional shares of our stock that would dilute those holders, they would first have the right to acquire additional shares to maintain their pro rata ownership. While investors in this offering will be granted certain participation rights in future offerings of securities by the company, investors who are not accredited investors may not be able to participate in all of those offerings if such offering relies upon Rule 506(b) or (c) of Regulation D. As a result, upon future issuances of stock by the company, investors in this offering may experience more substantial dilution than other stockholders. See Exhibit 6.8 for further information about the preemptive rights granted to certain holders of our Common Stock.
We will be required to enter into a stock purchase agreement with Mount Sinai for the shares that have already been issued by the company. Pursuant to the Licensing Agreement with Mount Sinai, the company will provide Mount Sinai with “customary agreements reasonably required by any future institutional equity investors with respect to the voting of its common stock and regarding subjecting the common stock held by [Mount Sinai] to rights of first refusal and co-sale…” for the Common Stock that has already been issued to Mount Sinai. We will be required to finalize such agreements before September 22, 2019. We have not begun negotiations on this terms of these agreements, however, once finalized, investors in this offering may believe such terms to be superior to those receive by investors in this offering.
Investors in this offering may not be entitled to a jury trial with respect to claims arising under the subscription agreement, the investors’ rights agreement, which could result in less favorable outcomes to the plaintiff(s) in any action under these agreements. Investors in this offering will be bound by the subscription agreement and investors’ rights agreement, both of which include a provision under which investors waive the right to a jury trial of any claim they may have against the company arising out of or relating to these agreements. By signing these agreements, the investor warrants that the investor has reviewed this waiver with his or her legal counsel, and knowingly and voluntarily waives the investor’s jury trial rights following consultation with the investor’s legal counsel.
11
If we opposed a jury trial demand based on the waiver, a court would determine whether the waiver was enforceable based on the facts and circumstances of that case in accordance with the applicable state and federal law. To our knowledge, the enforceability of a contractual pre-dispute jury trial waiver in connection with claims arising under the federal securities laws has not been finally adjudicated by a federal court. However, we believe that a contractual pre-dispute jury trial waiver provision is generally enforceable, including under the laws of the State of Texas, which governs the subscription agreement and investors’ rights agreement, and in the Court of Chancery in the State of Delaware. In determining whether to enforce a contractual pre-dispute jury trial waiver provision, courts will generally consider whether the visibility of the jury trial waiver provision within the agreement is sufficiently prominent such that a party knowingly, intelligently, and voluntarily waived the right to a jury trial. We believe that this is the case with respect to the subscription agreement and investors’ rights agreement. You should consult legal counsel regarding the jury waiver provision before entering into the subscription agreement and investors’ rights agreement.
If you bring a claim against the company in connection with matters arising under either the investors’ rights agreement or the subscription agreement, including claims under federal securities laws, you may not be entitled to a jury trial with respect to those claims, which may have the effect of limiting and discouraging lawsuits against the company. If a lawsuit is brought against the company under the either of these agreements, it may be heard only by a judge or justice of the applicable trial court, which would be conducted according to different civil procedures and may result in different outcomes than a trial by jury would have had, including results that could be less favorable to the plaintiff(s) in such an action.
Nevertheless, if this jury trial waiver provision is not permitted by applicable law, an action could proceed under the terms of the subscription agreement or investors’ rights agreement with a jury trial. No condition, stipulation, or provision of the subscription agreement or investors’ rights agreement serves as a waiver by any holder of common shares or by us of compliance with any substantive provision of the federal securities laws and the rules and regulations promulgated under those laws.
In addition, when the shares are transferred, the transferee is required to agree to all the same conditions, obligations and restrictions applicable to the shares or to the transferor with regard to ownership of the shares, that were in effect immediately prior to the transfer of the Shares, including but not limited to the investors’ rights agreement or subscription agreement.
We are offering bonus shares to some investors in this offering, which effectively gives them a discount on their investment. Certain investors in this offering who invest more than $5,000 or more than $10,000, are entitled to receive bonus shares based on the amount of their investment, which effectively gives them a discount on their investment; see “Plan of Distribution and Selling Securityholders.” Therefore, the value of shares of investors who invest less than $5,000 or $10,000 and pay the full price in this offering will be immediately diluted by investments made by investors entitled to receive the bonus shares, who will effectively pay less per share.
Our Amended and Restated Certificate of Incorporation also includes a forum selection provision, which could result in less favorable outcomes to the plaintiff(s) in any action against our company. Our Amended and Restated Certificate of Incorporation includes a forum selection provision that requires any claims against the company by stockholders not arising under the federal securities laws to be brought in the Court of Chancery State in the state of Delaware. This forum selection provision may limit investors’ ability to bring claims in judicial forums that they find favorable to such disputes and may discourage lawsuits with respect to such claims.
This investment is illiquid. There is no currently established market for reselling these securities. If you decide that you want to resell these securities in the future, you may not be able to find a buyer. Although the company intends to apply in the future for quotation of its Common Stock on an over-the-counter market, or similar, exchange, there are several requirements that the company may or may not be able to satisfy in a timely manner. Even if we obtain that quotation, we do not know the extent to which investor interest will lead to the development and maintenance of a liquid trading market. You should assume that you may not be able to liquidate your investment for some time or be able to pledge these shares as collateral.
You will need to keep records of your investment for tax purposes. As with all investments in securities, if you sell our Series B Preferred Stock at a profit or loss, you will probably need to pay tax on the long- or short-term capital gains that you realize or apply the loss to other taxable income. If you do not have a regular brokerage account or your regular broker will not hold our Series B Preferred Stock for you (and many brokers refuse to hold securities issued under Regulation A) there will be nobody keeping records for you for tax purposes. You will have to keep your own records and calculate the gain or loss on any sales of the Series B Preferred Stock.
12
The value of your investment may be diluted if the company issues additional options. A pool of unallocated options is typically reserved for future employees, which affects the fully-diluted pre-money valuation for this offering. The price per share of the Series B Preferred Stock has been calculated assuming a 2% post-money unallocated option pool, which may not account for all additional options the company will issue after the offering and may not provide adequate protection against the dilution investors may face due to such additional issuances. Any option issuances by the company over the 2% pool will lower the value of your shares.
Investors in this offering will receive our Series B Preferred Stock, which has limited voting rights compared to our Common Stock. Investors in this offering that purchase our Series B Preferred Stock will have limited voting rights compared to those of the holders of our Common Stock. Our Amended and Restated Certificate of Incorporation states that the holders of our Common Stock are entitled to elect two (2) directors of the corporation to our Board of Directors alone as a class. Our Preferred Stockholders, therefore, will have no choice as to the election of two members of the Board of Directors of the company. The Preferred Stockholders also do not have the right to vote for any directors of the corporation as a standalone class, which is a right granted to our Common Stockholders. The holders of our Preferred Stock are entitled to vote together with the holders of the Common Stock for the election of one (1) independent director, and may vote together with the holders of the Common Stock on any additional directors to be elected to our Board of Directors after the initial (3) directors are elected. Therefore, investors in this offering will very likely not be able to exert the same amount of control over the management of the company as the holders of the Common Stock. See “Securities Being Offered” for more information on the voting rights of our Series B Preferred Stock.
13
Dilution means a reduction in value, control, or earnings of the shares the investor owns.
Immediate dilution
An early-stage company typically sells its shares (or grants options over its shares) to its founders and early employees at a very low cash cost, because they are, in effect, putting their “sweat equity” into the company. When the company seeks cash investments from outside investors, like you, the new investors typically pay a much more significant sum for their shares than the founders or earlier investors, which means that the cash value of your stake is diluted because all the shares are worth the same amount, and you paid more than earlier investors for your shares.
The following table compares the price that new investors are paying for their shares with the effective cash price paid by existing shareholders, giving effect to full conversion of all outstanding convertible notes and assuming that the shares are sold at $6.27 per share. The schedule presents shares and pricing as issued and reflects all transactions since inception, which gives investors a better picture of what they will pay for their investment compared to the company’s insiders than just including such transactions for the last 12 months, which is what the SEC requires.
The following table presents the approximate effective cash price paid for all share and potential shares issuable by the company as of August 28, 2020.
| Date Issued | Issued Shares | Potential Shares | Total Issued & Potential Shares | Effective Cash Price per Share at Issuance or Potential Conversion | ||||||||||||||||
| Common Shares: | ||||||||||||||||||||
| Common Shares | 2020 | 519,831 | (3) | — | 519,831 | $ | 0.0001376 | |||||||||||||
| Common Shares | 2019 | 4,227,722 | (4) | — | 4,227,722 | $ | 0.0001542 | |||||||||||||
| Common Shares | 2017 and 2018 | 85,819 | — | 85,819 | $ | 0.0025000 | ||||||||||||||
| Preferred Shares: | ||||||||||||||||||||
| Series-A Preferred Shares | 2020 | 2,940,121 | 2,940,121 | $ | 4.0000000 | |||||||||||||||
| Series-A Preferred Shares | 2019 | 702,021 | — | 702,021 | $ | 4.0000000 | ||||||||||||||
| Convertible Notes: | ||||||||||||||||||||
| Convertible Notes | 2020 | 1,255,417 | (5) | 1,255,417 | $ | 1.1070475 | ||||||||||||||
| Options: | ||||||||||||||||||||
| 2019 Stock Option and Grant Plan | 2020 | — | 1,350,857 | 1,350,857 | $ | 3.0628377 | ||||||||||||||
| 2019 Stock Option and Grant Plan | 2019 | — | 453,200 | 453,200 | $ | 0.6868564 | ||||||||||||||
| Warrants: | ||||||||||||||||||||
| Zimmerman Warrant (1) | 2019 | — | 273,973 | 273,973 | $ | 3.6500000 | ||||||||||||||
| Pro-Dex, Inc. Warrant (2) | 2018 | — | 597,859 | 597,859 | $ | 2.0907937 | ||||||||||||||
| Total Common Share Equivalents | 9,734,493 | 2,222,688 | 11,957,182 | $ | 1.8688763 | |||||||||||||||
| Investors in this offering, assuming $30 Million raised | 2020 | 4,784,689 | (6) | -- | 4,784,689 | $ | 6.27 | |||||||||||||
| Total After Inclusion of this Offering | 14,519,182 | 2,222,688 | 16,741,870 | $ | 3.126681 | |||||||||||||||
14
| (1) | We have entered into a warrant to purchase capital stock with ZB Capital Partners LLC (the “ZB Capital”) (filed herewith as Exhibit 6.13), Under the terms of the warrant, the ZB Capital has the right to acquire $1,000,000 worth of shares of the company’s stock upon the occurrence of the company raising $5,000,000 in an equity financing. We believe it is reasonable to assume that ZB Capital would exercise its right to purchase 273,972 shares of Series A Preferred Stock at a price of $3.65 per share. However, if they so elected and assuming the company raised $5,000,000 in this offering, ZB Capital alternatively has the right to purchase the shares being sold in the equity financing at the same per share price paid by other investors in this offering, but reduced by the 7.0% fee to the Placement Agent in this offering. ZB Capital has represented to the company that they do not intend to exercise their warrant in this offering. As such, we have excluded the potential shares issuable to ZB Capital from the dilution calculation as we are not able to make a reasonable determination of the effective cash price to be paid. For reference, were ZB Capital to exercise its warrant as part of this offering, it would be able to acquire up to 171,527 shares of our Series B Preferred Stock in exchange for cash consideration of $1,000,000. |
| (2) | This entry reflects the amount of potential shares issuable to Pro-Dex, Inc. (“Pro-Dex”) under the terms of its warrant agreement (filed herewith as Exhibit 6.11) as of the date of this offering circular. As of the date of this offering circular, Pro-Dex has warrants to acquire 597,859 shares of Monogram’s capital stock. However, the warrant agreement includes automatic, non-dilution provisions, which would increase the number of shares acquirable in the event of capital raising by the company. |
| (3) | The shares of Common Stock issued in 2020 reflects the number of shares of Common Stock issued to Mount Sinai pursuant to the Licensing Agreement included as Exhibit 6.8 to this offering circular. This issuance was triggered by the company’s previous Series A Offering, in which it raised $14,435,668 in gross proceeds. |
| (4) | The shares of Common Stock issued in 2019 were issued to Benjamin Sexson and Douglas Unis in consideration for past and future services to the company and under the terms of the Licensing Agreement included as Exhibit 6.8, respectively. In addition, this number includes the amount of shares of Common Stock issued to Mount Sinai pursuant to the Licensing Agreement. |
| (5) | In 2020, all previous outstanding convertible notes of the company issued between 2017 and 2020 either converted into Series A Preferred Stock of the Company or were repaid. |
| (6) | Does not include up to 478,468 bonus shares that may be issued to investors in this offering. |
The following table illustrates the dilution that new investors will experience upon investment in the company relative to existing holders of our securities. Because this calculation is based on the net tangible assets of the company, we are calculating based our net tangible book value of $(274,632) as of December 31, 2019, as included in our audited financial statements. As such, this table does not include shares issued in 2019. However, the values have been adjusted to reflect the reverse split effected on May 28, 2019, as noted above.
The offering costs assumed in the following table includes up to $2,100,000 in commissions to StartEngine Primary, as well as legal and accounting fees incurred for this Offering.
15
Future dilution
Another important way of looking at dilution is the dilution that happens due to future actions by the company. The investor’s stake in a company could be diluted due to the company issuing additional shares. In other words, when the company issues more shares, the percentage of the company that you own will go down, even though the value of the company may go up. You will own a smaller piece of a larger company. This increase in the number of shares outstanding could result from a stock offering (such as an initial public offering, another crowdfunding round, a venture capital round, angel investment), employees exercising stock options, or by conversion of certain instruments (e.g., convertible bonds, preferred shares or warrants) into stock.
If the company decides to issue more shares, an investor could experience value dilution, with each share being worth less than before, and control dilution, with the total percentage an investor owns being less than before. There may also be earnings dilution, with a reduction in the amount earned per share (though this typically occurs only if the company offers dividends, and most early-stage companies are unlikely to offer dividends, preferring to invest any earnings into the company).
The type of dilution that hurts early-stage investors most occurs when the company sells more shares in a “down round,” meaning at a lower valuation than in earlier offerings. An example of how this might occur is as follows (numbers are for illustrative purposes only):
| · | In June 2017 Jane invests $20,000 for shares that represent 2% of a company valued at $1 million. |
| · | In December, the company is doing very well and sells $5 million in shares to venture capitalists on a valuation (before the new investment) of $10 million. Jane now owns only 1.3% of the company but her stake is worth $200,000. |
| · | In June 2018, the company has run into serious problems, and in order to stay afloat, it raises $1 million at a valuation of only $2 million (the “down round”). Jane now owns only 0.89% of the company, and her stake is worth only $26,660. |
This type of dilution might also happen upon the conversion of convertible notes into shares. Typically, the terms of convertible notes issued by early-stage companies provide that in the event of another round of financing, the holders of the convertible notes get to convert their notes into equity at a “discount” to the price paid by the new investors, i.e., they get more shares than the new investors would for the same price. Additionally, convertible notes may have a “price cap” on the conversion price, which effectively acts as a share price ceiling. Either way, the holders of the convertible notes get more shares for their money than new investors. In the event that the financing is a “down round,” the holders of the convertible notes will dilute existing equity holders, and even more, than the new investors do because they get more shares for their money. Investors should pay careful attention to the amount of convertible notes that the company has issued (and may issue in the future and the terms of those notes.
If you are making an investment expecting to own a certain percentage of the company or expecting each share to hold a certain amount of value, it’s important to realize how the value of those shares can decrease by actions taken by the company. Dilution can make drastic changes to the value of each share, ownership percentage, voting control, and earnings per share.
16
PLAN OF DISTRIBUTION AND SELLING SECURITYHOLDERS
Plan of Distribution
The company is offering up to 4,784,689 shares of Series B Preferred Stock (not including bonus shares) (the “Shares”) on a “best efforts” basis at a price of $6.27 per share. The minimum subscription is $250.80, or 40 shares.
The company has engaged StartEngine Primary as its sole and exclusive placement agent to assist in the placement of its securities. StartEngine Primary is under no obligation to purchase any securities or arrange for the sale of any specific number or dollar amount of securities.
Commissions and Discounts
The following table shows the total discounts and commissions payable to the placement agents in connection with this offering, assuming we raise the maximum amount of offering proceeds:
| Per Share | ||||
| Public offering price | $ | 6.27 | ||
| Placement Agent commissions | $ | 2,100,000 | (1) | |
| Proceeds, before expenses, to us | $ | 27,900,000 | ||
| (1) | StartEngine Primary, LLC will receive commissions of 7.0% of the offering proceeds. |
The company will also be required to issue to StartEngine Primary shares of our Series B Preferred Stock equal to 2.0% of the total amount of shares sold in this offering (excluding bonus shares). Shares issued to StartEngine Primary will be subject to a lock-up provision in accordance with FINRA requirements. If we raise the maximum amount in this offering, we would issue 95,693 shares to StartEngine Primary.
Other Terms
StartEngine Primary has also agreed to perform the following services in exchange for the compensation discussed above:
| · | design, build, and create the company’s campaign page, |
| · | provide the company with a dedicated account manager and marketing consulting services, |
| · | provide a standard purchase agreement to execute between the company and investors, which may be used at the company’s option and |
| · | coordinate money transfers to the company. |
In addition to the commission described above, the company agrees to pay StartEngine Primary a fee of $15,000 for out of pocket accountable expenses paid prior to commencing. Any portion of this amount not expended and accounted for will be returned to the company. Assuming the full amount of the offering is raised, we estimate that the total fees and expenses of the offering payable by the company to StartEngine Primary will be approximately $2,115,000.
StartEngine Primary will charge you a non-refundable processing fee equal to 3.5% of the amount you invest at the time you subscribe for our securities, equivalent to $0.2195 per share. This fee will be refunded in the event the company does not raise any funds in this offering.
StartEngine Primary intends to use an online platform provided by StartEngine Crowdfunding, Inc. (“StartEngine”), an affiliate of StartEngine Primary, at the domain name www.startengine.com (the “Online Platform”) to provide technology tools to allow for the sales of securities in this offering. In addition, StartEngine will assist with the facilitation of credit and debit card payments through the Online Platform. Fees for credit and debit card payments will be passed onto investors at cost, and the company will reimburse StartEngine for transaction fees and return fees that it incurs for returns and chargebacks, pursuant to a Credit Card Services Agreement.
17
Bonus Shares for Certain Investors
Certain investors in this offering are eligible to receive bonus shares of Series B Preferred Stock, which effectively gives them a discount on their investment. Those investors will receive, as part of their investment, additional shares for their shares purchased (“Bonus Shares”) equal to 5% or 10% of the shares they purchase, depending upon the investment level of such investors. Investors that invest at least $5,000 in this offering will receive a 5% bonus over $5,000 on their investment. Investors that invest at least $10,000 will receive a 10% bonus over $10,000. Investors receiving the 5% bonus will pay an effective price of approximately $5.95 per share before the StartEngine processing fee, while investors receiving the 10% bonus will pay an effective price of approximately $5.64 per share before the StartEngine processing fee. The StartEngine processing fee will be assessed on the full share price of $6.27, and not the effective, post bonus, price.
Subscription Procedure
After the Commission has qualified the Offering Statement, we will accept tenders of funds to purchase the Series B Preferred Stock. The company may close on investments on a “rolling” basis (so not all investors will receive their shares on the same date). Investors may subscribe by tendering funds by wire, credit, or debit card or ACH transfer to the escrow account to be set up by the Escrow Agent. Tendered funds will remain in escrow until closing has occurred. Upon closing, funds tendered by investors will be made available to the company for its use.
The minimum investment in this offering is $250.80, or 40 shares of Series B Preferred Stock.
Investors will be required to complete a subscription agreement in order to invest. The subscription agreement includes a representation by the investor to the effect that, if the investor is not an “accredited investor” as defined under securities law, the investor is investing an amount that does not exceed the greater of 10% of his or her annual income or 10% of your net worth (excluding the investor’s principal residence).
The subscription procedure is summarized as follows:
| 1. | Go to the company’s page on www.startengine.com/monogram and click on the “Invest Now” button; | |
| 2. | Complete the online investment form; | |
| 3. | Deliver funds directly by wire, debit card, credit card or electronic funds transfer via ACH to the specified account; | |
| 4. | Once funds or documentation are received an automated AML check will be performed to verify the identity and status of the investor; | |
| 5. | Once AML is verified, investor will electronically receive, review, execute and deliver to us a Subscription Agreement. |
The company has entered into an Escrow Services Agreement with Prime Trust LLC (the “Escrow Agent”) and StartEngine Primary. Investor funds will be held by the Escrow Agent pending closing or termination of the offering. All subscribers will be instructed by the company or its agents to transfer funds by wire, credit or debit card, or ACH transfer directly to the escrow account established for this offering. The company may terminate the offering at any time for any reason at its sole discretion. Investors should understand that acceptance of their funds into escrow does not necessarily result in their receiving shares; escrowed funds may be returned.
Prime Trust is not participating as an underwriter or placement agent or sales agent of this offering and will not solicit any investment in the company, recommend the company’s securities or provide investment advice to any prospective investor, and no communication through any medium, including any website, should be construed as such, or distribute this Offering Circular or other offering materials to investors. The use of Prime Trust’s technology should not be interpreted and is not intended as an endorsement or recommendation by it of the company or this offering. All inquiries regarding this offering or escrow should be made directly to the company.
In the event that the company terminates the offering while investor funds are held in escrow, those funds will promptly be refunded to each investor without deduction or interest and in accordance with Rule 10b-9 under the Exchange Act.
Pursuant to our agreement with StartEngine Primary, the company agrees that 6% of the total funds received into escrow will be held back as a deposit hold in case of any ACH refunds or credit card chargebacks. The hold will remain in effect for 180 days following the close of the offering. 60 days after the close of the offering, 4% of the deposit hold will be released to the company. The remaining 2% will be held for the final 120 days of the deposit hold. After such further 120 days, the remaining 2% will be released to the company. Based on the assumed maximum amount that we might owe StartEngine Primary, we estimate the deposit hold could be for up to $1,800,000.
18
No Minimum Offering Amount
There is no minimum offering amount in this offering and the company may close on any funds that it receives. Potential investors should be aware that there can be no assurance that any other funds will be invested in this offering other than their own funds.
No Selling Security holders
No securities are being sold for the account of security holders; all net proceeds of this offering will go to the company.
Transfer Agent and Registrar
The company has engaged StartEngine Secure LLC, a registered transfer agent with the SEC, who will serve as transfer agent to maintain shareholder information on a book-entry basis.
Provisions of Note in Our Subscription Agreement and Investors’ Rights Agreement
Our subscription agreement and investors’ rights agreement include forum selection provisions that require any claims against the company based on the subscription agreement and/or investors’ rights agreement not arising under the federal securities laws to be brought in a court of competent jurisdiction in the State of Texas. These forum selection provisions may limit investors’ ability to bring claims in judicial forums that they find favorable to such disputes and may discourage lawsuits with respect to such claims. The company has adopted these provisions to limit the time and expense incurred by its management to challenge any such claims. As a company with a small management team, this provision allows its officers not to lose a significant amount of time traveling to any particular forum so they may continue to focus on operations of the company.
Jury Trial Waiver
The subscription agreement and investors’ rights agreement provides that subscribers waive the right to a jury trial of any claim they may have against us arising out of or relating to the subscription agreement. By signing the subscription agreement and investors’ rights agreement, the investor warrants that the investor has reviewed this waiver with the investor’s legal counsel, and knowingly and voluntarily waives his or her jury trial rights following consultation with the investor’s legal counsel. If we opposed a jury trial demand based on the waiver, a court would determine whether the waiver was enforceable given the facts and circumstances of that case in accordance with applicable case law.
19
Assuming a maximum raise of $30,000,000, the net proceeds of this offering would be approximately $27,822,000 after subtracting estimated offering costs of $2,100,000 to StartEngine Primary in commissions, $15,000 in audit fees, $3,000 in Edgarization fees, $10,000 in blue sky related fees, and $50,000 in legal fees. If Monogram successfully raises the maximum amount under this raise, the company intends to try and fund the development of the Monogram total knee and robotic system. While the company believes it has a differentiated press-fit hip implant design, management believes that given the complexity of these products it will be most efficient to focus exclusively on the development and launch of the total knee before undertaking the development of additional implant applications.
Assuming a raise of $15,000,000, representing 50% of the maximum offering amount, the net proceeds would be approximately $13,872,000 after subtracting estimated offering costs of $1,050,000 to StartEngine Primary in commissions, $15,000 in audit fees, $3,000 in Edgarization fees, $10,000 in blue sky related fees, and $50,000 in legal fees. In such an event, Monogram would adjust its use of proceeds by limiting the speed of growth and by limiting the amount of additional recruiting of new employees. Monogram would intend to try and fund the development of the Monogram total knee and robotic system. If Monogram were able to develop the Monogram total knee and robotic system successfully, we would have limited working capital with which to support the market launch. We would very likely need additional capital to facilitate sales.
Assuming a raise of $5,000,000 representing 16.6% of the maximum offering amount, net proceeds would be approximately 4,572,000 after subtracting estimated offering costs of $350,000 to StartEngine Primary in commissions, $15,000 in audit fees, $3,000 in Edgarization fees, $10,000 in blue sky related fees, and $50,000 in legal fees. In such an event, Monogram would adjust its use of proceeds by limiting the scope of development considerably and would focus on advancing both the Monogram total knee and robotic system simultaneously, but it is unlikely that it would commercialize either product. Management believes it has assembled a high-caliber engineering team for both the total knee and robotic system developments that would be difficult to reassemble and believes that it is not in the best interest of the company to prioritize one product (either the robot or the implant) at the expense of the other product, which, given the complexity and development costs of both products would likely be required to commercialize. Furthermore, the company believes that advancing the Monogram total knee and robotic system in parallel is in the best interests of the company, given management’s view of the growing market demand for both navigated robotics and press-fit total knee implants. The company would likely require additional capital within a shorter time-frame, and it is unlikely that the company would obtain an FDA approval on any products if the company pursues the development of both products in parallel, which management believes to be in the best interest of the company. The company would also limit its speed of growth and limit the amount of additional recruiting of new employees.
| Percent | $5,000,000 Raise | $15,000,000 Raise | Maximum Offering $30,000,000 Raise | |||||||||||||
| Allocation | Use Category | % | Use Category | % | Use Category | |||||||||||
| 48.67 | % | Product Development | 42.0 | % | Product Development | 28.42 | % | Product Development | ||||||||
| 39.07 | % | Payroll | 37.9 | % | Payroll | 29.98 | % | Payroll | ||||||||
| 6.12 | % | General Administrative | 5.4 | % | General Administrative | 7.12 | % | Working Capital | ||||||||
| 3.47 | % | Marketing | 8.4 | % | Marketing | 8.70 | % | Marketing | ||||||||
| 2.19 | % | Commissions | 5.7 | % | Commissions | 5.96 | % | Commissions | ||||||||
| 0.00 | % | Working Capital | 0.2 | % | Working Capital | 19.54 | % | General Administrative | ||||||||
| 0.50 | % | Offering Expenses | 0.4 | % | Offering Expenses | 0.27 | % | Offering Expenses | ||||||||
| (1) | The company’s CEO, Benjamin Sexson, has contracted with the company for an annual salary of $250,000 per year (see Exhibits 6.2, 6.3, and 6.4). In addition, Mr. Sexson is eligible to earn an annual bonus in an amount of 50% of his aggregate base salary earned in such year, subject to the achievement of certain performance goals, milestones and objectives, as established from time to time by an appropriate committee of the company’s Board. A portion of the proceeds from this offering will go towards paying Mr. Sexson’s current salary payments, and may go towards a bonus for Mr. Sexson, if such bonus is granted as described above. Additionally, Mr. Sexson is entitled to severance payments by the company equal to six months' of his base salary at the time of his termination if he is terminated without cause. The company could use the proceeds of this offering to pay Mr. Sexson’s severance payments in the event of such a termination. Doug Unis, a director of the company, also receives a consulting fee of $35,000 for consulting services unrelated to his position as director. This agreement is included as Exhibit 6.1. A portion of the proceeds of this offering will go towards paying Mr. Unis’s consulting fee. |
Because the offering is a “best efforts,” we may close the offering without sufficient funds for all the intended purposes set out above, or even to cover the costs of this offering. The above use of proceeds assumes a 510(k) submission without a request for clinical data from the FDA. Please see our “Risk Factors” for additional disclosures related to a clinical data request from the FDA.
The company reserves the right to change the above use of proceeds if management believes it is in the best interests of the company.
20
Overview
Monogram Orthopaedics, Inc was incorporated under the laws of the State of Delaware on April 21, 2016, as “Monogram Arthroplasty Inc.” On March 27, 2017, the company changed its name to “Monogram Orthopaedics Inc.” Monogram Orthopaedics is working to develop a product solution architecture with the goal to enable mass personalization of orthopedic implants by linking 3D printing and robotics with advanced pre-operative imaging. The company has a robot prototype that can execute optimized paths for high precision insertion of optimized implants in synthetic bone specimens. These implants and cut-paths are prepared based on proprietary Monogram implant designs. Monogram intends to produce and market robotic surgical equipment and related software, orthopaedic implants, tissue ablation tools, navigation consumables, and other miscellaneous instrumentation necessary for the execution of reconstructive joint replacement procedures.
On February 24, 2020, the company executed an industrial net lease at 3913 Todd Lane in Austin, Texas, which serves as its new headquarters.
Our Background
Our company’s business is based on ideas formulated by Dr. Douglas Unis, an Associate Professor of Orthopaedic Surgery, and technology developed by Dr. Unis, Professor Anthony Costa, the head of the Neurosurgery Simulation Core, and Sulaiman Somani, a medical student at the Mount Sinai School of Medicine (“MSSM”).
Our founding philosophy is that advances in technology will usher in new way of thinking about reconstructive joint procedures and orthopedic implants. We believe that the future of orthopedic joint replacements lies in build-to-order, press-fit patient optimized implants that are inserted into bone cavities prepared by high precision robotic tools and that rely on natural biologic fixation rather than cement. We believe that to facilitate the cost-efficient delivery of anatomy restoring patient optimized implants it is necessary to develop efficient processes for designing and fabricating implants and surgical plans. We also believe that to prepare the surgical plans and execute the robotic procedures advanced imaging such as a CT scan or MRI are required. Patient optimized implants require high-precision bone preparation that moves beyond two dimensional planar cuts or alignment, for example. It is our assessment that for these processes to be truly scalable they require a high degree of optimization and a high functioning navigated surgical robot capable of executing complex cutpaths; i.e. a product solution architecture with image processing, scalable customized implant design, pre-operative planning, and robotic execution.
It is our view that press-fit 3D printed patient optimized implants that rely on biologic fixation will prove to be clinically superior over the long term while also alleviating the tremendous inventory burden and capital inefficiencies of generic implant distribution. We believe that implants should be designed and optimized to fit and restore a patient anatomy and that the ability of a robot to execute irregular cuts could exceed the capabilities of even the most skilled surgeons. Monogram believes that the use of patient specific implants and robotic surgery will, over time, reduce complication, failure rates and reduce costs considerably.
Principal Products and Services
Monogram’s primary business will be to design and manufacture optimized total knee replacements, and eventually, if we are successful at commercializing our total knee replacement and have sufficient capital and market interest, hip, knee, shoulder and extremities implants using primarily 3D printed manufacturing as well as the equipment required for insertion, including:
| · | Navigated surgical robots with optical tracking equipment and a cutting end-effector, | |
| · | Pre-operative and intra-operative software guidance application, | |
| · | Consumable Tissue ablation tools, and | |
| · | Navigation consumables (fiducial markers, tracked retractors, etc.). |
The Monogram robotic system and related hardware (optical tracking equipment, end-effector, etc.) are multi-use capital equipment. To properly use the robotic system, Monogram’s pre-operative planning software, robotic controls and intra-operative software are needed. This software will be subject to an annual license, fees for which will be based on the scope of use (for example total knee arthroplasty). Each clinical application will be billed separately. During the procedure, a mix of re-usable and single use instrumentation is needed. The elements of our system are sold separately but generally must be used with the system to properly perform its intended clinical function.
21
A significant percentage of orthopaedic medical devices are outsourced to original equipment manufacturers (OEMs). Monogram intends to outsource the manufacture of its products (including implants and instrumentation needed to execute reconstructive joint replacements) to established suppliers that may already be approved suppliers for the most significant market participants and may have decades of product-specific manufacturing expertise. On or around March 31st, 2020, Monogram entered into a supply and license agreement with a medical technology company with manufacturing capabilities that markets their own branded orthopaedic products. Monogram intents to secure the supply of certain components from this company. Monogram intends to work with FDA registered ISO 13485 approved suppliers with the proper Quality Management Systems and product specific expertise.
According to analysis conducted on orthopaedic procedures, as of 2019 the average cost of implant components for total hip procedures was approximately $5,004 and for primary total knee procedures $4,325. Monogram expects to price our products consistent with the market.
Market
According to sources and analysis trusted by management, the orthopaedic devices market is considered to be highly concentrated, with the top seven market participants accounting for almost 65% of total sales as of 2019. The joint reconstruction devices market, which will be Monogram’s primary target market, reconstructive joint replacements, is even more concentrated with the top four market participants accounting for approximately 73% of sales for the total market. The knee market segment is even more consolidated with the four most significant players controlling 82% of the market and no other company controlling more than 2.2%. The total joint replacement devices market as of 2019 was approximately $19.6 billion globally. In the United States, the number of hip replacement procedures was estimated to be to 669,100 and the number of knee replacements was estimated to be 1,059,200 in 2019.
Most patients who undergo reconstructive joint replacement surgeries are aged between 50 and 80 years old, with the average patient age for hip and knee replacements around approximately 65 years of age. Many of these patients rely on third-party payors, principally federal Medicare, state Medicaid, and private health insurance plans, to pay for all or a portion of the costs and fees associated with joint replacement surgeries.
According to the orthopaedic industry statistical analysis and research, the reconstructive joint replacement market is expected to grow at an annual rate of between 3 and 4 percent with growth driven primarily by an aging population, the obesity epidemic and developments in advanced materials that have improved the longevity of implants and their efficacy for younger patients which grows the patient candidate pool. The fastest-growing patient demographic are patients aged 45 to 54 years of age. It should be noted that COVID-19 has had a significant and material adverse impact on the orthopedic market that will likely result in permanent demand destruction. These market growth estimates may not properly factor in the effects of the COVID-19 crisis, and management expects that the market for orthopaedic procedures could shrink and that the adverse impacts could last for an extended period.
Management believes that the market for robotics and surgically prepared press-fit implants will outpace broader market growth primarily because of the limited market penetration and observed growth of the Stryker Corporation, which utilizes navigated robotics and press-fit implants. In particular, management has paid close attention to Stryker’s performance in the CT based robotically prepared press-fit knee market. Stryker’s $241.6M year-over-year revenue growth in joint replacement sales from 2018 to 2019 was approximately 2.5 times as much as Zimmer Biomet, Depuy Synthes and Smith & Nephew year-over-year revenue growth combined. The Stryker Corporation markets the MAKO, a robotic-arm assisted technology that uses a CT-based preoperative plan to help surgeons provide patients with a personalized surgical experience. From 2018 to 2019, the Styker Corporation increased sales in its knee segment by 7.7%. Monogram believes this growth indicates low penetration of press-fit knees as a percentage of Total Knee Replacements.
According to sources and analysis trusted by management, less than 10% of knees are uncemented. The Stryker Corporation has indicated in a Company Conference Presentation on February 27, 2019 at the SVB Leerink Global Healthcare Conference, that there are 5,000 orthopaedic hospitals in the US, the majority of which they think would be a candidate for at least one robot. As of Q4 2019, the Stryker Corporation had approximately 700 robots installed in the US. According to another source trusted by the robotic assisted procedure growth rate in knees may be as high as 29.2% compounded annually over the next 7 years. Monogram management believes that robot penetration and the use of surgical robots for bone preparation of press-fit implants remains low. This is, in part, why management believes it is in the best interest of the company to simultaneously pursue the development of a novel press-fit knee that can be inserted with a robotic system.
Management believes that optimized press-fit (also “uncemented”) implants combined with navigated robotic insertion will grow, driven by an industry focus on normalizing patient outcomes and efforts to mitigate clinical risk and improve productivity (one of the potential benefits of not using bone cement). At the same conference, the Stryker Corporation described the limitations of cement; handling time, set-up time, odor related to it, and most significantly, leaving behind another foreign body that can degrade over time and cause implant loosening. Monogram’s implants will not utilize bone cement, which we believe provides an opportunity for us to disrupt this market, especially when combined with a surgical robotic system. With the technology and product infrastructure we are developing, we believe we may be in a prime position to capitalize on this growing market. Because press-fit implants rely on natural biologic fixation rather than cement, the initial stability of the implants may be important to facilitate proper osteointegration and long-term stability. Management believes that these types of implants are well suited for a surgical robotic system capable of executing high accuracy cuts.
22
Competition
We face competition from large, well-known companies in the medical device industry as a whole, as well as specifically in the orthopaedic medical device industry. Currently, the top five market participants in the joint replacement devices market are Zimmer Biomet Holdings, Inc., DePuy Orthopaedics, Inc., a Johnson & Johnson company, Stryker Corporation, and Smith & Nephew, Inc. These companies dominate the market for orthopaedic products. These companies, as well as other companies like ConforMIS, Inc., offer implant solutions, including (depending on the competitor) a combination of conventional instruments and generic implants, robotics and generic implants, or patient-specific instruments (“PSI”) and cemented patient-specific implants for use in conventional total and partial orthopaedic replacement surgeries.
Relevant technical considerations for the evaluation of orthopaedic surgical robotics include:
| · | The use of advanced imaging for pre-operative planning; for example, the Mako Robot, which is owned by the Stryker Corporation, uses a CT scan to develop the pre-operative plan; |
| · | The degrees of freedom of the robotic system; for example, Monogram is trying to commercialize a seven degree-of-freedom robotic arm; |
| · | The use of a cutting end-effector; some robotic systems do not utilize cutting end effectors but robotically position jigs that constrain the manual instrumentation used to execute the cutting; |
| · | The execution of the surgical plan; some robotic systems require the user to initiate the cutting and constrain the tool within a virtual cutting boundary while in other robotic systems, the robot is “active,” i.e., the robot executes preplanned cut paths. |
| · | The use of navigation for real-time object tracking (usually optically); some robotic systems do not actively track objects in the surgical field. |
Currently, we are not aware of any widely commercialized orthopaedic companies that broadly offer robotic technology in combination with surgical navigation for the insertion of patient-specific press-fit orthopaedic implants. To our knowledge the only use of robotic technology in combination with surgical navigation is for the insertion of generic orthopaedic implants. These competitors and other medical device companies have significant financial resources. They may seek to extend their robotics and orthopaedic implant technology to accommodate the robotic insertion of patient-specific implants. A number of these and other companies also offer surgical navigation systems for use in arthroplasty procedures that provide a minimally invasive means of viewing the anatomical site.
Our Innovative Approach
Monogram’s principal innovation over our competition will be our ability to produce robotically inserted press-fit orthopaedic implants rapidly and at scale. The product solution architecture that we are developing may enable the rapid fabrication of optimized robotically inserted orthopaedic implants.
The Monogram technology platform consists of a workflow to designate optimized press-fit implants from patient-specific CT scans, and integrate into a workflow for robotic execution. We seek to optimize the design and insertion of press-fit implants using data from raw CT images. The navigated robot is designed to execute cut paths that may be optimized for time to surgically prepare the corresponding cavities to facilitate high-precision insertion of the implants.
We believe that Monogram’s navigated robot features several enhancements that may enhance the user experience as compared to the current robots in use. The robot features seven degrees-of-freedom with control algorithms that leverage the kinematic redundancy of the arm to avoid interoperative boundaries and optimize the surgical execution. The company is exploring methods of tracking objects that may be of clinical significance that may not be visible from CT images. Monogram is also working on trying to reduce surgical time and improve the accuracy of execution to the greatest extent possible. For example, the company is researching the accuracy of various cutting instruments to determine if, for instance, a mill can prepare cuts with greater precision than a saw in a time-efficient manner. The management team believes that a highly dependable robot that reduces surgical time while executing high accuracy cuts is the highest priority for successful market adoption.
Press-fit orthopaedic implants are generally understood to perform better when high initial stability is achieved. Stability may depend on design features and a tight fit. It is not always straightforward to design implants that can be both easily inserted and removed while remaining highly stable. The Monogram press-fit implants are designed such that cortical contact, and therefore stability, are maximized while remaining insertable. The Monogram implants are designed to reconstruct the native patient anatomy as closely as possible. A challenge with press-fit orthopaedic implants is also removal. Implants that become infected may need to be removed. Monogram is working on trying to develop highly stable implants that can also be easily removed without causing significant damage to the remaining bone.
23
With generic implants in hips, for example, manual bone preparation can contribute to periprosthetic fracture, dislocation, leg length inequality, subsidence and early loosening, and suboptimal function outcomes. With generic knee implants, aseptic loosening of the tibial component and malalignment can be reasons for failure. Current hip stems, for example, can have limited options to restore anatomy. For instance, most implants are available in only two widths despite wide human anatomic variations. Generic implants can be geometric as opposed to organic in shape, which limits the amount of direct bone contact required for initial stability and long-term biological fixation. There is currently no commercially viable way to produce implants matching both the internal bone cavity and the external biomechanics of the joint. The challenges of designing implants that restore anatomy, are highly stable and easily revisable are significant. There are currently limited methods for precisely sculpting an implant’s exact complement in the bone.
Our surgical approach will attempt to use additively manufactured (AM) press-fit tibial knee implants that will require robotically milled complementary cavities to be insertable. For our first-generation products, we will be combining a novel Monogram tibial design with a licensed generic femoral implant, inserts, and locking mechanism to try to reduce the initial complexity of the development. To try and reduce the regulatory risk, we will be making the first-generation implant insertable with manual instrumentation as well as robotically so that the implant and robot can be submitted to the FDA as separate submissions. Monogram is a pre-commercialization company that has not yet validated our manufacturing method or the clinical efficacy of our products. The scope of development and capabilities may be affected by our ability to commercialize certain aspects of our technology. The commercial implementations of our designs may differ considerably from the initial design concepts. For example, cutting titanium is challenging and may require design adjustments. The goal of our implants is to more accurately restore patient anatomy and mitigate some of the potential causes of failure described above. We have conducted preliminary testing that we interpret to be supportive of our hypothesis that more accurate restoration of patient anatomy and robotic insertion of patient-specific implants improves initial stability, and we believe to warrant further research. We will continue to focus our development efforts with a focus on high accuracy robotic execution, and implant design. Our testing will likely include comparisons with implants that may represent the existing standard of care as a benchmark to try and demonstrate that the initial stability of our implants shows less micromotion than their generic counterparts.
Furthermore, validation of the mechanical strength of our products is critically important to our success. In addition to stability testing, our R&D efforts will also test the mechanical strength requirements mandated by the FDA. Considerable work remains to validate our implant design. Robotic bone preparation for the insertion of implants is challenging and requires many technical steps; for example, the robot must be properly calibrated, the patient bone must be accurately correlated to the pre-operative plan, the robotic arm control must efficiently execute the plan, etc. There are numerous sources of error that make it challenging to prepare cavities with sufficient accuracy. Our robot, the KUKA LBR Med, has never been used for this application. We have found that to make cavities in simulated bone specimens for the insertion of our implants is highly challenging. We have not yet achieved high accuracy cuts in a cadaveric bone specimen. Also, our robotic execution is currently more time consuming than manual insertion methods. The added time to register the bone and to robotically execute the cuts is a disadvantage of our system. While our system has performed well in synthetic bone specimens in the past, we need to prove commercial feasibility in a range of human bones to test our ability to produce corresponding implants and cavities.
Management believes that the Monogram equipment may be cheaper and more capital efficient than traditional knee and hip replacement systems. For example, the Mako robot produced by Stryker Corporation (Ticker: SYK) is the dominant leader in navigated surgical robotics with 700 robots installed globally, based on public information from a Q4 2019 Stryker Corp Earnings Call. Further, public information from a Q3 2018 Stryker Corp Earnings Call, Stryker established that it was selling its Mako robots for $1,000,000 while reporting gross profit margins on its robot sales of 62%. Our management believes that this could imply a production cost of approximately $380,000 per robot. We estimate the cost to produce our robotic system will be below this cost. Investors should note, our assumptions about the production costs of Stryker may be inaccurate, or may not be current. Furthermore, management would expect that any competitors in the market with competitive offerings would be in a better position to discount their products than Monogram because they are larger and more established companies. We do not anticipate that reducing the capital required to distribute our products will have a material impact on demand for our products.
Sales & Orders
The specific sales process for each of our product categories is as follows:
Surgical Robot with End-Effector
Generally, the company must identify a surgeon within the organization willing to advocate for the purchase of the capital equipment to the hospital. Orders are placed by hospital finance and buying departments in advance of any surgical procedures. Cost is often a significant objection to purchase. Monogram intends to address this objection by offering high performing equipment at a price that is competitive with other market participants. Some of Monogram’s competitors offer hospitals financing options for large equipment purchases. Monogram will explore offering financing options. Investors should note that Monogram may incur losses from the initial placement of robotic systems at discounted prices.
24
Monogram intends to distribute its products initially through independent distributors and contractors. We will be making efforts to secure contracts with national Group Purchasing Organizations, although we cannot guarantee favorable agreements will be secured. Monogram will also likely sell service contracts and extended warranties.
Cutting Tools and Navigation Consumables
Consumable equipment is generally billed on a per-use basis and associated with the specific surgical case for which they were used. The hospital takes stock of consumed materials which are billed by Monogram.
Technology Platform
Monogram will license its technology platform to hospitals, which will provide those hospitals with access to Monogram’s surgeon planning portal. The motion control and intra-operative control algorithms are embedded as part of the robotic surgical system.
Implants
Monogram will receive orders for implants through the Monogram technology platform. The hospital will generally aggregate the materials used in a given surgical case, which are billed by Monogram.
Design
The Monogram press-fit implant designs seeks to try to optimize for initial stability. Monogram uses raw CT images to guide this process. Monogram utilizes technology to determine the implant designs that are sent to a manufacturer to produce. Monogram may combine specific existing generic implant components with specific proprietary monogram components. For example, for knees, we may combine our tibial component with a generic locking mechanism, insert, and femoral component. For hips, we may combine a Monogram hip stem with other generic components of the total hip implant system, such as the head, liner, and acetabular cup. Monogram will be producing a proprietary tibia, but the other components of the total knee replacement (femoral implant and plastic insert) may be standard. For the first generation Monogram knee, we will not develop a custom femur or inserts. Monogram is focusing its development efforts only where management believes there is a clear potential to drive clinical benefit from technology advances.
Monogram’s other products are pre-designed, and therefore will only require manufacture and distribution to reach the end-customer.
Manufacturing
The implant designs generated with the Monogram technology will be 3D printed out of titanium. Our titanium implants will be a biocompatible medical-grade titanium alloy with a chemical composition corresponding to ISO 5832-3, ASTM F1472, and ASTM B348. Our implants will either be manufactured by an established ISO13485 contract manufacturer or the medical technology partner from which we have licensed certain implant components. The company is in discussions with development and manufacturing companies for these services.
Manufacturing of our surgical robots, navigation consumables, and cutting tools will be outsourced to well-established FDA registered ISO13485 approved manufacturers with proven quality management systems. Our robot arm is the LBR Med, which is manufacturing by the KUKA Robotics Corporation.
Quality Control and Dispatch
Our proposed distribution model contemplates using a distribution facility to ship our products to customers. Such facilities will receive final products from our suppliers that have been approved by their respective quality management systems. Our distribution facility will then conduct a final inspection of the products, and, once approved, ship them to our customers. Our distribution facility may assemble or repackage certain of these components for shipment.
Our Market
We intend to market our products to orthopaedic surgeons, hospitals (or other medical facilities), and patients. Our ideal customers are hospitals in high population metropolitan regions that tend to employ high-volume technology-focused surgeons.
Through the use of direct sales representatives, independent sales representatives, and distributors, we intend to market and sell our products in the United States and over time in other markets if we are able to scale operations in the United States successfully. We intend to try and enter contractual arrangements with national Group Purchasing Organizations that may contract with hospitals to source products.
25
Research and Development
The company currently has several Research and development (“R&D”) initiatives underway. These initiatives include micromotion studies of press-fit knee implants, testing of a hybrid knee that combines generic femoral components and locking mechanisms, cadaveric testing and performance testing of a robot mounted navigation system. In addition Monogram is testing novel methods of registration and tracking. Much of our current research also relates to improving the accuracy of robotic execution with a focus on arm calibration and control as well as navigation. R&D amounted to $194,287 and $31,700 for the years ended December 31, 2019 and 2018, respectively. In 2018, the majority of our R&D expenses were related to costs incurred developing and testing our patient-specific hip implant for initial stability with the UCLA Orthopaedic Biomechanics Laboratory. In 2019, the majority of our R&D related expenses were related to research and testing of our patient optimized tibial component with the Orthopaedic Biomechanics and Advanced Surgical Technologies Laboratory at the University of Nebraska Medical Center and the development of our navigated robotic system. We expect to continue spending at elevated levels on R&D as we continue our development. We intend to continue our research such as cadaveric studies of our knee implants for both robotic and manual placement, development of our surgical navigation systems, development of our guidance applications and continued development and testing of our implants.
The company has installed a 352 square foot cadaver lab in its Austin facility to support the research and development initiatives of the company. The cadaver lab has a dedicated surgical robot and navigation system that can be used to support testing and product development. Monogram currently has 3 surgeons under contract to support our engineers with subject matter expertise, design input, and testing services.
Employees
The company currently has 12 full-time employees that are expected to work out of our headquarters at 3913 Todd Lane, Suite 307, Austin, TX 78744. Due to the COVID-19 pandemic, 3 of our employees that were expected to relocate have been unable to relocate to Austin, TX.
Regulation
Medical products and devices are regulated by the Food and Drug Administration (the “FDA”) in the United States and can be regulated by foreign governments for devices sold internationally. The Federal Food, Drug, and Cosmetic Act and regulations issued by the FDA regulate testing, manufacturing, packaging, and marketing of medical devices. Under the current regulations and standards, we believe that our products and devices are subject to general controls, including compliance with labeling and record-keeping rules. In addition, our medical devices require pre-market clearance, which for our products and devices will require a 510(k) premarket notification submission.
Further, our manufacturing processes and facilities are also subject to regulations, including the FDA’s QSR requirements (formerly Good Manufacturing Practices). These regulations govern the way we manufacture our products and maintain documentation for our manufacturing, testing, and control activities. In addition, to the extent we manufacture and sell products abroad, those products are subject to the relevant laws and regulations of those countries.
Finally, the labeling of our products and devices, our promotional activities and marketing materials are regulated by the FDA and various state agencies. Violations of regulations promulgated by these agencies may result in administrative, civil, or criminal actions against our manufacturers or us by the FDA or governing state agencies.
As of the date of this Offering Circular, Monogram has not yet received clearance to market its products in the United States (FDA) or internationally, and as such is not currently selling and distributing any products. Monogram has engaged a regulatory consulting group, “Musculoskeletal Clinical Regulatory Advisers, LLC” to assist with its 510(k) premarket notification submission for our system of products and technology. On March 31, 2020, Monogram executed a binding term sheet that outlined a license, supply, and distribution agreement with a medical device company with orthopedic implants. Management believes it is in the best interest of the company to pursue separate regulatory submissions for the robot and implants. Our first generation total knee implants will be insertable with both manual instrumentation and robotically. We hope to submit to the FDA our 510(k) premarket notification submission for the robot within three years of the closing of the Offering, which occurred in April 2020, and expect approval by the FDA within 12 months of submission. Monogram believes it is in the best strategic interests of the company to pursue the development of the total knee implants and surgical robot simultaneously. Management believes we may require considerably more capital beyond what has already been raised to pursue a separate implant approval and robot approval. We anticipate that we may be able to submit to the FDA our 510(k) premarket notification for the implants in 2021. However, we may need to make adjustments to the design based on surgeon feedback and other considerations that could extend this timeline from previous expectations. The design is not finalized and management is currently evaluating the design and the impact of design changes on the costs and the timeline. With sufficient funding and no clinical data request, we do not anticipate that the knee implant 510(k) would FDA approval later than 2022. The implants would be designed for insertion with both manual instruments and with a surgical robot, but would not be approved for insertion with the surgical robot until such time as the FDA approved the surgical robot. For the robot submission, the company expects FDA approval of its 510(k) premarket notification by 2024. If we do not raise sufficient capital in this offering it is likely additional capital will be required to complete the submission and obtain the FDA approval (see “USE OF PROCEEDS TO ISSUER” section). Notably, these timelines for both the implants and the robot assume no requests for clinical data from the FDA. The company does not anticipate it will be able to accelerate the timeline for the robot submission at this time.
26
Intellectual Property
The majority of our patents or trademarks are licensed from Mount Sinai. The following patent applications are currently under review:
| ID Type | Patent Name | Application | Filing Date | |||
| Patent Application Number | APPARATUS, METHOD AND SYSTEM FOR PROVIDING CUSTOMIZABLE BONE IMPLANTS | 16/153,334 | 5-Oct-18 | |||
| Patent Application Number | CUSTOMIZED TIBIAL TRAYS, METHODS, AND SYSTEMS FOR KNEE REPLACEMENT | PCT/US2020/020279 | 28-Feb-20 | |||
| U.S. Provisional Patent Application Number | REGISTRATION AND/OR TRACKING OF A PATIENT'S BONE EMPLOYING A PATIENT SPECIFIC BONE JIG | 62/990,827 | 17-Mar-20 | |||
| Patent Application Number | CUSTOM HIP DESIGN AND INSERTABILITY ANALYSIS | PCT/US2020/028499 | 16-Apr-20 | |||
| U.S. Provisional Patent Application Number | CUSTOMIZED TIBIAL TRAYS, METHODS, AND SYSTEMS FOR KNEE REPLACEMENT (with screws) | 63/027,098 | 19-May-20 | |||
| Patent Application Number | A SYSTEM AND METHOD FOR INTERACTION AND DEFINITION OF TOOL PATHWAYS FOR A ROBOTIC CUTTING TOOL | PCT/US20/33810 | 20-May-20 | |||
| Patent Application Number | ROBOT MOUNTED CAMERA REGISTRATION AND TRACKING SYSTEM FOR ORTHOPEDIC AND NEUROLOGICAL SURGERY | PCT/US2020/035408 | 29-May-20 |
Additionally, the company has developed its own intellectual property, which would not require a license from Mount Sinai. Information on patent filings by the company are included below:
| ID Type | Patent Name | Application | Filing Date | |||
| Provisional Patent Application | NAVIGATION AND/OR ROBOTIC TRACKING METHODS AND SYSTEMS | 63/037,699 | 11-Jun-20 |
Acquisition Opportunities
We do not have any current plans to acquire the assets or operation of other entities, but we believe that opportunities may become available. Should there be an opportunity to make an acquisition, our goal would be to ensure that the assets or operations to be acquired are a good fit, and the acquisition terms are in line with the benefits to the company. Acquisitions would likely be in the form of cash and equity. The cash portion of any acquisition would likely come from obtaining financing from lenders or future equity financing rounds, neither of which have been identified. Such financing would require that the company take on new expenses related to either the servicing of new debt or broker commission fees. Any equity used for an acquisition would come from issuing additional shares of the company’s stock in exchange for the stock of the acquired entity. The issuance of stock would likely occur in a transaction that is not registered with the Securities and Exchange Commission and could result in the dilution of the investors in the Offering. Additionally, investor consent would not be sought if the company had sufficient authorized shares available.
Litigation
From time to time, the company may be involved in a variety of legal matters that arise in the normal course of business. The company is not currently involved in any litigation, and its management is not aware of any pending or threatened legal actions relating to its intellectual property, conduct of its business activities, or otherwise.
The company leases office space at 3913 Todd Lane, Suite 307, Austin, TX 78744, which serves as its headquarters. Monogram intends to lease distribution facilities in the future.
27
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion of our financial condition and results of operations for the fiscal years ended December 31, 2019 and December 31, 2018 should be read in conjunction with our financial statements and the related notes included in this Offering Circular. The following discussion contains forward-looking statements that reflect our plans, estimates, and beliefs. Our actual results could differ materially from those discussed in the forward-looking statements.
Overview
Monogram Orthopaedics, Inc was incorporated under the laws of the State of Delaware on April 21, 2016 as “Monogram Arthroplasty Inc.” On March 27, 2017, the company changed its name to “Monogram Orthopaedics, Inc.” Monogram Orthopaedics is developing a product solution architecture intending to enable mass personalization of press-fit orthopedic implants by linking 3D printing and robotics via automated digital image analysis algorithms. The company has a robot prototype that can execute optimized paths for high precision insertion of optimized press-fit implants in synthetic bone specimens. These implants and cut-paths are prepared based on proprietary Monogram designs. Monogram intends to produce and market robotic surgical equipment and related software, orthopaedic implants, tissue ablation tools, navigation consumables, and other miscellaneous instrumentation necessary for the execution of reconstructive joint replacement procedures.
Results of Operations
Year ended December 31, 2019 Compared to Year ended December 31, 2018
The company is in an early stage of development. The company did not generate revenues for the years ended December 31, 2019 and 2018.
Our costs and expenses currently consist of general and administrative expenses primarily composed of salaries, travel, and office expenses of administrative employees and contractors, software license fees, and other overhead expenses. Costs and expenses totaled $1,676,695 for the year ended December 31, 2019 compared to $650,127for the year ended December 31, 2018, an increase of 158%, primarily due to:
| · | General and administrative expenses increased to $179,671 for the year ended December 31, 2019 from $101,679 for the year ended December 31, 2018, a 77% increase, due primarily to increases in fees incurred in connection with the Offering, as well as increases in salaries and payroll taxes, reductions in insurance premiums, and small medical equipment purchases that were not made in 2018; | |
| · | Marketing and advertising expenses increased to $205,207 for the year ended December 31, 2019 from $3,154 for the year ended December 31, 2018 in connection with the Offering that was conducted by the company in 2019. Marketing and advertising efforts by the company prior to commencing the Offering were minimal; |
| · | Wages and payroll related expenses decreased to $344,757 for the year ended December 31, 2019 from $378,523 for the year ended December 31, 2018, a 8.9% decrease primarily due to reductions in company personnel in 2019. However, the company incurred $614,870 in stock-based compensation expenses during the year ended December 31, 2019, of which $0 was incurred during the year ended December 31, 2018, in conjunction with the issuance of Common Stock and options to purchase Common Stock; |
| · | Legal and professional services expenses increased to $106,140 for the year ended December 31, 2019 from $104,114 for the year ended December 31, 2018, an increase of 1.9% due to fees incurred in connection with the issuances of convertible notes, intellectual property filings, contract negotiations, and this Offering; |
| · | Research and development costs increased to $194,287 for the year ended December 31, 2019 from $31,700 for the year ended December 31, 2018, a 512.9% increase, due to research related to testing of our patient optimized press-fit implant components with the Orthopaedic Biomechanics and Advanced Surgical Technologies Laboratory at the University of Nebraska Medical Center and the development of our navigated robotic system; and |
Other expenses increased to $119,570 for the year ended December 31, 2019 from $62,141 for the year ended December 31, 2018, an increase of 92.4%. This increase was primarily due to:
| · | Interest expenses increasing to $124,470 for 2019 from $62,184 for the year ended December 31, 2018, an increase of 100.2% primarily due to the issuance of additional convertible notes see “—Liquidity and Capital Resources” below; and |
| · | Depreciation expense increasing to $31,763 for 2019 from $30,957 for 2018, an increase of 2.6%, due to depreciation related to equipment purchased in 2018 and 2019 that were not subject to a full year of depreciation in 2018. |
28
As a result of the foregoing, the company generated a net loss of $1,796,265 for the year ended December 31, 2019 compared to a net loss of $712,268 for the year ended December 31, 2018, an 152.2% increase in net loss.
Since the end of the period covered by our financial statements, our legal and professional, research and development, payments to contractors, and marketing and advertising expenses are expected to increase in connection with the Offering. Our expenses related to wages and payroll taxes have temporarily decreased due to employees voluntarily deferring wages and temporary reductions in headcount. We expect wages and payroll tax expenses to increase following the Offering. We expect rent to decrease following a move to a less expensive facility.
Liquidity and Capital Resources
At December 31, 2019 the company’s cash on hand was $2,319,393. The company is not generating revenues and requires the continued infusion of new capital to continue business operations. The company has recorded losses since inception, and as of December 31, 2019, had negative working capital of $462,380 and a stockholders’ deficit of $274,632. The company has historically been capitalized by contributions from related parties and its officers and directors. The company plans to continue to try to raise additional capital through crowdfunding offerings, equity issuances, or any other method available to the company. Absent additional capital, the company may be forced to significantly reduce expenses and could become insolvent.
On September 20, 2019, the company commenced an offering under Regulation A under the Securities Act of 1933 pursuant to which it offered shares of its Series A Preferred Stock (the “Series A Offering”). As of December 31, 2019, the company had sold 702,021 shares of its Series A Preferred Stock in the Series A Offering for total gross proceeds of $2,808,084. On March 17, 2020, the company filed a 253G2 supplement in connection with the Series A Offering, indicating that the company intended to terminate the Series A Offering on April 24, 2020 (the “Termination Date”). The company terminated the Series A Offering on the Termination Date. The company received $14,568,568 of gross proceeds from sale of 3,642,142 shares of Series A Preferred Stock in the Series A Offering.
The company estimates that the proceeds raised from the Series A Offering can continue to fund the company’s current rate of operations through 2021 without raising additional capital. However, the company has determined that additional capital raising activity will be beneficial for enhancing the operations of the company.
Issuances of Equity and Convertible Notes
Since its inception, the company has funded operations through the issuance of equity securities and convertible notes. During the year ended December 31, 2018, the company issued convertible for total proceeds of $1,148,000. During the year ended December 31, 2019, the company did not have any convertible promissory note issuances that resulted in proceeds to the company. In February 2019, the company issued a convertible promissory note to Benjamin Sexson, the company’s CEO, in consideration for deferred compensation in the amount of $48,000 that were converted into convertible notes. The company did not receive any proceeds from issuances of Common Stock in 2018. The company received cash proceeds of $489 from the issuance of shares of Common Stock to its Chief Executive Officer in 2019. As described above, the company sold shares of Series A Preferred Stock in the Offering in 2019 and 2020 – see “Liquidity and Capital Resources”.
Indebtedness
Since its inception, the company has entered into convertible notes in the combined principal amount of $2,124,000. Convertible notes comprising $1,830,000 of the $2,124,000 bear interest at 6% per year. Convertible notes comprising $294,000 of $2,124,000 bear interest at 4% per year. The combined principal amount and the accrued interest of these notes were $2,329,719 as of December 31, 2019. On April 29th, 2020, the company repaid a note payable to Pro-Dex, Inc. in the amount of $934,866.67, reducing this total balance. On July 10th, 2020, the company repaid a note (issued on July 12, 2018) payable to another holder in the amount of $44,000, also reducing its total indebtedness. The remaining balance of these notes was represented by notes that were subject to mandatory conversion upon closing of an equity financing in which the company issues and sells shares of its Preferred Stock for gross proceeds equal to or exceeding $5,000,000. These notes were previously filed as exhibits to the company’s Form 1-A filed on September 10, 2019. On April 23rd, 2020, the company triggered this conversion from sales of its Series A Preferred Stock in the Offering, resulting in aggregate gross proceeds to the company of $5,057,772 as of the same date. As such, as of the date of this Offering Circular, these notes are no longer outstanding. A total of 255,417 shares of its Series A Preferred Stock have been issued to the noteholders for the conversion of their notes.
The company currently has no material commitments for capital expenditures.
29
Trend Information
Our primary addressable market is for knee procedures, specifically primary Total Knee Arthroplasty (“TKA”) procedures (Monogram has a novel Total Hip Arthroplasty (“THA”) design but we will not be pursuing commercialization until after the knee has been successful approved). The intention of reconstructive joint replacement procedures is to replace diseased or damaged bone with fabricated implants designed to restore patient function. Management of the company has reviewed third party reports trusted by management which identify that approximately 1,059,200 TKA procedures where were conducted in the United States in 2019, compared to 1,011,300 TKA procedures in 2018. This increase in TKA procedures represents a year-over-year increase in surgical volume from 2018 to 2019 of 4.7% for TKA procedures. Generally, the fabricated implants are surgically inserted, and fixation is achieved via cement or osseointegration (“press-fit,” “cementless,” “uncemented”). Monogram is focusing its developments on cementless knee fixation.
Joint reconstruction and musculoskeletal care are widely recognized as highly effective treatments as measured by the rates of long-term survivability. As such, we expect the procedure volumes to continue to grow with strong demographic tailwinds. Industry publications identify the global market for Knee Joint Reconstruction Sales in 2019 was estimated to be $9. Those same publications projected the market for Knee Joint Reconstruction Sales to increase to $10.7bn by 2023. While insurers and other healthcare providers such as Centers for Medicare & Medicaid Services ("CMS") seem to recognize that these procedures are generally effective at returning patients to productivity, pressures persist in improving quality and reducing cost. We believe these pressures are a potential tailwind for technologies that help surgeons achieve reproducibly, total “episode of care” positive outcomes (reducing the length of stay, reducing revision surgeries, supporting better patient outcomes, etc.). Notably, growth estimates do not factor in the adverse impacts of the COVID-19 pandemic and are likely overstated.
The push for reproducible positive outcomes has been positive for the adoption of computer assisted surgical robotics. Despite this robot adoption is still early. We believe that robotic adoption and the penetration of computer assistive tools remains in the earlier stages of adoption. For instance, in public information available from its Q4 2019 earnings call, Stryker Corporation indicated that it had performed 24,000 Total Knee procedures in the quarter. At the Evercore HEALTHCONx Healthcare Conference on November 28, 2018, Styker executives indicated that there were approximately 600 robots installed globally but 5,000 hospitals in the US alone that are a target for a least one robot. According to research sources trusted by management, the robotic procedure growth rate may be as high as 29.2% compounded annually over the next 7 years.
It should be noted that the emergence of 3D printing technologies allow manufacturers to print porous structures directly into implants. As identified in the above industry studies, it is our view that the growth and demand for press-fit uncemented implants are increasing and that the penetration of press-fit implants for knee replacements remains low. Further, we believe that the combination of robotics and 3D printing appears to be highly synergistic because of the benefits of precision bone preparation for press-fit implants. Moreover, we believe that advances in 3D printing will continue to improve the mechanical properties and viability of 3D printed implants in a range of applications.
In conclusion, it is our view that computer-assisted robotic procedures will continue to increase market penetration and improve. Advances in image processing, navigation, robotics, and advanced manufacturing are favorable developments.
Relaxed Ongoing Reporting Requirements
If we become a public reporting company in the future, we will be required to publicly report on an ongoing basis as an “emerging growth company” (as defined in the Jumpstart Our Business Startups Act of 2012, which we refer to as the JOBS Act) under the reporting rules set forth under the Exchange Act. For so long as we remain an “emerging growth company”, we may take advantage of certain exemptions from various reporting requirements that are applicable to other Exchange Act reporting companies that are not “emerging growth companies”, including but not limited to:
| · | not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act; |
| · | taking advantage of extensions of time to comply with certain new or revised financial accounting standards; |
| · | being permitted to comply with reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements; and |
| · | being exempt from the requirement to hold a non-binding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. |
30
If we become a public reporting company in the future, we expect to take advantage of these reporting exemptions until we are no longer an emerging growth company. We would remain an “emerging growth company” for up to five years, although if the market value of our Common Stock that is held by non-affiliates exceeds $700 million as of any June 30 before that time, we would cease to be an “emerging growth company” as of the following December 31.
If we do not become a public reporting company under the Exchange Act for any reason, we will be required to publicly report on an ongoing basis under the reporting rules set forth in Regulation A for Tier 2 issuers. The ongoing reporting requirements under Regulation A are more relaxed than for “emerging growth companies” under the Exchange Act. The differences include, but are not limited to, being required to file only annual and semiannual reports, rather than annual and quarterly reports. Annual reports are due within 120 calendar days after the end of the issuer’s fiscal year, and semiannual reports are due within 90 calendar days after the end of the first six months of the issuer’s fiscal year.
In either case, we will be subject to ongoing public reporting requirements that are less rigorous than Exchange Act rules for companies that are not “emerging growth companies,” and our shareholders could receive less information than they might expect to receive from more mature public companies.
Plan of Operations and Milestones
We are not yet operational and have yet to generate any revenues. We have established the following milestones in our plan of operations for the next 12 months:
| · | The company has not established a minimum raise requirement. The proceeds from this offering are intended to advance the company farther towards FDA Approval of its 510(k) premarket notification (which we refer to herein as “FDA Approval”). Nonetheless, if we raise the lowest amount set out in “Use of Proceeds” ($5,000,000), the company does not believe it would be able to obtain FDA Approval of its products and technology. The company anticipates the total combined engineering development cost to develop and commercialize both the total knee implant and the robotic surgical systems in parallel are approximately $13 million without consideration for the general and administrative costs, sales costs and other capital requirements to inventory, market and promote the finished products. This is an increase over previously anticipated costs disclosed by the company in its offering circular related to its Series A Offering. The increase is primarily related to the need to remediate technical features of both the robot and implants to incorporate findings from testing, expert input, and regulatory input. For example, surgeon feedback and testing of our implant indicates we may need to make significant revisions to the locking mechanism and fixation features. We may have to make significant changes to the arm control and guidance application as part of our regulatory strategy. With sufficient funding, we anticipate we may be able to submit to the FDA our 510(k) premarket notification for the knee implant in 2021. However, we may need to make adjustments to the design based on surgeon feedback and other considerations that could extend this timeline from previous expectations. The knee implant design is still not finalized, and management is currently evaluating the design feedback and the impact of design changes on the costs and timeline. With sufficient funding and no clinical data request, we do anticipate that the knee implant would obtain FDA Approval no later than 2022. We anticipate that we will file the robotic system with the FDA by 2023 and the company expects FDA Approval by 2024. These timeline estimates assume no clinical data requests from the FDA or unforeseen technical challenges. If we raise less than $15 million in this offering, we will seek additional capital through private offerings to accredited or institutional investors to secure the additional capital necessary to reach FDA Approval. |
| · | In 2020, Monogram’s management has successfully recruited several key engineering positions for the company; specifically, we have hired a Vice President of Engineering, a Director of Implants and Instruments, and a Director of Software. We have grown the team to 12 full time employees. Our Vice President of Engineering has significant experience with and will be responsible for supporting the implementation of the company’s Quality Management System. We anticipate that over the next 12 months we will increasing our headcount to approximately 26 full-time employees to support the growth. |
| · | On July 1st, 2020, the company entered into a supply and distribution agreement with the same established orthopedic technology company from which on March 31st, 2020, it previously entered into a licensing agreement. As part of this distribution agreement, Monogram hired the Senior Director of US Sales of that company to join Monogram as the Sales Manager for the South East Region. Monogram intends to engage surgeons and coordinate with independent distributors to establish active distribution channels. Monogram intends to sell future products, in part, through any distribution channels we can establish for these existing products. As part of these sales efforts, Monogram would share our product pipeline strategy, and on a selective basis under non-disclosure agreements, would bring individual surgeons and distributors to our cadaver lab to demonstrate our products in development. Monogram management believes that a strategy of building out our distribution and sales channels now with purchased products may be helpful to build interest and accelerate the distribution of our future products when they obtain FDA Approval. |
31
| · | If our distribution and sales strategy described above is successful, we anticipate we will begin selling our knee implants and robot shortly after they receive FDA Approval. Notably, we will require additional capital to promote our new products. Furthermore, we will be submitting the knee implant and the robotic system as separate submissions to the FDA. As such, the first generation knee implant will be insertable with manual instrumentation. Our objective will be to cultivate surgeon interest in our implants with manual instruments so that when the robot is approved, the transition would be smoother. |
| · | In summary, we estimate that a minimum of approximately $15 million will be needed to obtain FDA Approval for the implants and surgical robot, assuming no clinical data requests. If we raise less than $15,000,000 in this offering, we will seek to raise additional capital from other sources. If we raise $15 million, we anticipate we may be able to submit to the FDA our 510(k) premarket notification for the knee implant in 2021. However, we may need to make adjustments to the design based on surgeon feedback and other considerations that could extend this timeline from previous expectations. The knee implant design is still not finalized, and management is currently evaluating the design feedback and the impact of design changes on the costs and timeline. With sufficient funding and no clinical data request, we do not anticipate that the knee implant would obtain FDA Approval later than 2022. We anticipate that we will file the robotic system to the FDA by 2023, and the company expects FDA Approval by 2024. These timeline estimates assume no clinical data requests from the FDA or unforeseen technical challenges. We do not expect to accelerate the timeline as previously anticipated even with additional capital at this time. As identified in our “Risk Factors,” our timeline to commercialization is dependent on the FDA review process. We expect to generate revenues within a year of FDA Approval. |
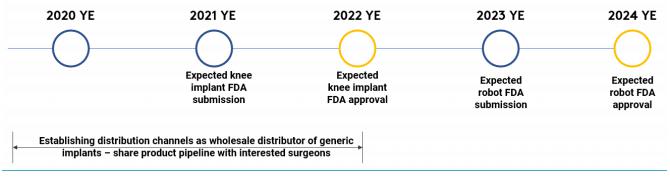
32
DIRECTORS, EXECUTIVE OFFICERS AND SIGNIFICANT EMPLOYEES
| Name | Position | Age | Date Appointed to Current Position | Approximate hours per week for part-time employees | ||||
| Executive Officers | ||||||||
| Benjamin Sexson | Chief Executive Officer, President | 37 | April 2018 | N/A | ||||
| Directors | ||||||||
| Benjamin Sexson | Director | 37 | April 2018 | N/A | ||||
| Dr. Douglas Unis | Director | 52 | April 2016 | 4 | ||||
| Rick Van Kirk | Director | 60 | April 2016 | 0 (Approx. 8 hours a year) | ||||
| Noel Goddard | Director | 46 | July 2020 | 0 (Approx. 8 hours a year) |
| (1) | Mr. Van Kirk was elected by Pro-Dex, Inc. pursuant to rights granted to Pro-Dex. Inc. via a secured promissory note agreement. The agreement provides that Pro-Dex, Inc. shall have the right to appoint one director of the company so long as Pro-Dex, Inc. holds the note or any of the securities issuable upon conversion of the note. As of the date of this Offering Circular, this note has been repaid, and is no longer outstanding. No portion of the note converted into any securities of the Company. |
Benjamin Sexson, CFA – CEO and Director
Benjamin Sexson is the Chief Executive Officer and a Director of Monogram Orthopedics, and has served in such capacities since he joined the company in April 2018. and has Prior to joining Monogram, Mr. Sexson served as the Director of Business Development at Pro-Dex, Inc., one of the largest OEM manufacturer of Orthopedic Robotic End-Effectors in the world from October 2015 to April 2018. In his tenure at Pro-Dex, Mr. Sexson was responsible for helping support the development, management and launch of the company’s first ever custom proprietary product solution and successfully negotiating the highest margin distribution agreements with a major strategic partner. In addition, Mr. Sexson helped secure and negotiate two additional major development agreements and has helped expand the company’s addressable markets from powered surgical tools in CMF to Thoracic, Trauma, Spine and Extremities as well as other product applications. Mr. Sexson is a named inventor on multiple patent applications at Pro-Dex. Prior to joining Pro-Dex, Mr. Sexson started Brides & Hairpins, a successful B2B retail brand that currently supplies Nordstrom, Bloomingdales, Urban Outfitters. Prior to that, Mr. Sexson worked in various finance positions and is a CFA Charterholder. Mr. Sexson graduated with honors from Caltech with a Bachelor’s Degree in Mechanical Engineering in 2006.
Dr. Douglas Unis – Founder and Director
Dr. Douglas Unis is a board certified orthopedic surgeon specializing in adult reconstructive surgery and is the founder and Chief Medical Officer of Monogram Orthopedics, Inc. Dr. Unis founded Monogram Orthopedics in 2015, and has served as a Director of the company since its inception. Dr. Unis has served as an Associate Professor at the Icahn School of Medicine since November 2015 and has been a practicing surgeon since 2004. He began serving as an Assistant Professor at Icahn School of Medicine at Mount Sinai in March 2014, until becoming an Associate Professor in November 2015. Dr. Unis has consulted with many leading orthopedic companies including Zimmer Biomet and Think Surgical. Prior to founding Monogram Orthopaedics, Dr. Unis was a consultant with Think Surgical, working with them for over 4 years to help with the development of their robotic total hip and knee arthroplasty system. Dr. Unis is widely recognized as a leader and innovator in the NYC area having performed the regions’ first muscle sparing anterior total hip replacement in 2005. Dr. Unis earned his BA from Duke University and Doctor of Medicine from Case Western Reserve University and later completing his residency at Northwestern University and a fellowship from Rush University in Adult Reconstruction.
Rick Van Kirk – Director
Mr. Richard L. Van Kirk is a Director of Monogram, and has served in this capacity since our inception. He is the Chief Executive Officer of Pro-Dex, Inc. (“Pro-Dex”), the largest OEM manufacturer of Orthopedic Robotic End-Effectors on the market. Mr. Van Kirk also serves on Pro-Dex’s Board of Directors. Mr. Van Kirk was appointed to the Board of Directors of Pro-Dex concurrent with his appointment as it’s CEO in January 2015. He joined Pro-Dex in January 2006 and was named Pro-Dex’s Vice President of Manufacturing in December 2006. In April 2013 he was appointed as the Chief Operating Officer of Pro-Dex. Mr. Van Kirk’s career includes over 13 years of management experience in manufacturing. Mr. Van Kirk previously served as Manufacturing Manager and Manager of Product Development at Comarco Wireless Technologies, ChargeSource Division, which provides power and charging functionality for popular electronic devices and wireless accessories. Prior to Comarco, Mr. Van Kirk was General Manager at Dynacast, a leader in precision die casting. Mr. Van Kirk earned a BA in Business Administration at California State University, Fullerton and an MBA from Claremont Graduate School.
Noel Goddard – Director
Ms. Noel Goddard is a seed investor with the Accelerate NY Seed Fund, where she has served as a principal from November 2017 and helped build a portfolio of 24 companies across deep technology and life science sectors. She is a serial entrepreneur, having founded/led two life science startup companies and most recently, a deep tech company. Since April 2020 she has served as the CEO at Qunnect, which builds hardware for scalable quantum networking. From July 2015 to August 2017. Ms. Goddard was the CTO of Symbiotic Health which focused on oral delivery of cellular and biologic therapeutics to the lower GI tract. In January 2013, Ms. Goddard founded a food safety diagnostics company, Goddard Labs, in Calverton, NY, and worked with Sapling Learning, a STEM educational software startup acquired by Macmillan Learning. Ms. Goddard obtained her PhD from Rockefeller University, performed postdoctoral research at Harvard Medical School as a fellow in the Society of Fellows, and served as an Assistant Professor of Physics at Hunter College, CUNY, before joining the NY entrepreneurial community.
33
COMPENSATION OF DIRECTORS AND EXECUTIVE OFFICERS
For the fiscal year ended December 31, 2019, we compensated our three highest-paid directors and executive officers as follows:
| Name and Position | Capacities in which compensation was received | Cash compensation ($) | Other compensation ($) | Total compensation ($) | ||||||||||
| Benjamin Sexson, CEO, Director | - | 31,875 | (1) | 186,975 | (3) | $ | 221,975 | |||||||
| Dr. Douglas Unis, Director | Consultant | 35,000 | (2) | 175,313 | (4) | $ | 207,188 | |||||||
| Rick Van Kirk, Director | - | - | - | |||||||||||
| Noel Goddard, Director | - | - | - | - | ||||||||||
| (1) | The company has an Employment Agreement with its CEO, Benjamin Sexson. The employment agreement and its amendments are filed as Exhibits 6.2, 6.3, and 6.4 to this offering circular. The agreement provides for an annual base salary of $70,000, which is subject to increase upon the occurrence of two events. The first of these events is the receipt by the company of $500,000 in financing from Mount Sinai. As of the date of this Offering Circular, this event has already occurred, and Mr. Sexson’s annual base salary increased to $120,000. The second of these events is the company’s close of a round of equity financing of at least $5,000,0000 on or prior to April 23, 2020, which would increase Mr. Sexson’s base salary to $250,000 per year, which occurred, and therefore has increased Mr. Sexson’s base salary to $250,000 per year as of the date of this Offering Circular. In addition, Mr. Sexson is eligible to earn an annual bonus in an amount of 50% of his aggregate base salary earned in such year, subject to the achievement of certain performance goals, milestones and objectives, as established from time to time by an appropriate committee of the company’s Board. Additionally, Mr. Sexson is entitled to severance payments by the company equal to six months' of his base salary at the time of his termination if he is terminated without cause. Mr. Sexson earned an annual salary of $31,875.00 from January 01, 2019 – December 31, 2019. Mr. Sexson was entitled to be compensated $120,000, however, Mr. Sexson opted to defer payment of his full salary in 2019. As of December 31, 2019, Mr. Sexson had deferred an amount totaling $88,125. As of the date of this Offering Circular, this deferred amount is has been repaid. | |
| (2) | Mr. Unis earned consulting fee of $35,000 in 2019 in consideration for his services as a consultant to the company, pursuant to the Consulting Agreement between Mr. Unis and the company (See Exhibit 6.1). Mr. Unis receives no compensation for his services as a director. | |
| (3) | Consists of a stock option grant to purchase 160,000 shares of common stock at a price of $0.61 per share valued at $10,838 and a share grant of 1,957,080, valued at $176,137. | |
| (4) | Consists of a stock option grant to purchase 180,000 shares of common stock at a price of $0.61 per share valued at $12,193 and a share grant of 1,812,447, valued at $163,120. |
For the fiscal year ended December 31, 2019, we paid our directors as a group (3) for their services as directors. There are four directors as of the date of this Offering Circular.
Other than cash and stock-based compensation set out above, no other compensation was provided to the executive officers or directors in their capacities as officers and directors of the company.
On May 7, 2019, the company adopted an incentive plan, which reserved 2,000,000 shares of common stock for issuance under the plan and 600,000 shares allowed for issuance pursuant to Incentive Stock Options. As of August 12, 2020, (i) Benjamin Sexson has been granted options for 535,000 shares under the plan, of which 40,000 have vested; (ii) Doug Unis has been granted options for 555,000 under the plan, of which 45,000 have vested; (iii) Rick Van Kirk has been granted options for 4,000 shares under the plan, of which none have vested; and (iv) Noel Goddard has been granted options for 1,000 shares under the plan, of which none have vested. The Company amended its 2019 Stock Option Plan on August 15, 2020, and the Amended and Restated 2019 Stock Option Plan now reserves 2,600,000 shares of common stock for issuance under the Plan, with up to 780,000 shares allowed for issuance pursuant to Incentive Stock Options.
34
SECURITY OWNERSHIP OF MANAGEMENT AND CERTAIN SECURITYHOLDERS
The following table sets out, as of August 12, 2020 the voting securities of the company that are owned by executive officers and directors, and other persons holding more than 10% of any class of the company’s voting securities, or having the right to acquire those securities. The table assumes that all options and warrants have vested. The company’s voting securities include all shares of the company’s Common Stock.
| Name and Address of Beneficial Owner | Title of class | Amount and nature of beneficial ownership | Amount and nature of beneficial ownership acquirable | Percent of class | ||||||||||
| Benjamin Sexson, 3913 Todd Lane, Austin, TX 78744 (1) | Common Stock | 1,997,080 | 0 | 41.29 | % | |||||||||
| Dr. Douglas Unis, 3913 Todd Lane, Austin, TX 78744 (3) | Common Stock | 1,767,723 | 0 | 36.55 | % | |||||||||
| ZB Capital Partners LLC, 1000 4th Street, Suite 795, San Rafael, CA 94901 | Preferred Stock (Series A) | 1,040,251 | 273,973 | (2) | 26.83 | % | ||||||||
| The Icahn School of Medicine at Mount Sinai, 1 Gustave L. Levy Pl, New York, NY 10029 (3) | Common Stock | 1,124,594 | 0 | 23.25 | % | |||||||||
| (1) | Pursuant to Mr. Sexson’s employment agreement, Mr. Sexson is entitled to pre-emptive rights permitting him preserve his vested equity position in the company in the event of any additional issuances of company common stock (or securities convertible into common stock), at a per-share price equal to the then current fair market value, as reasonably determined by the Board. Mr. Sexson does not intend to exercise this pre-emptive right. | |
| (2) | The acquirable shares for ZB Capital Partners LLC are comprised of shares issuable to ZB Capital Partners via the exercise of Warrants, and assumes they will be exercised for Series A Preferred Stock of the company. (See the Warrant Agreement filed as Exhibit 6.11 to this Offering Circular.) | |
| (3) | Pursuant to the Icahn School of Medicine at Mount Sinai Policies on Intellectual Property, Dr. Unis is entitled to 65% of the 33.3% equity distribution to inventors. Dr. Unis has elected to defer receiving this distribution. These shares, which approximate to 243,418 shares, have not been included in this consideration of beneficial ownership. |
35
INTEREST OF MANAGEMENT AND OTHERS IN CERTAIN TRANSACTIONS
On October 10, 2017, the company entered into an Exclusive Licensing Agreement (the “Licensing Agreement”) with Icahn School of Medicine at Mount Sinai (“Mount Sinai”), an entity which is affiliated with one of our Directors, Doug Unis, who is employed as an associate professor at Mount Sinai. The Licensing Agreement grants Monogram a revenue-bearing, world-wide right and (a) exclusive license, with the right to grant sublicenses (on certain conditions) to certain intellectual property relating to customizable bone implants and surgical planning software and (b) non-exclusive license, with the right to grant sublicenses on certain conditions, to certain technical information for the exploitation of the intellectual property in its field of use and (c) royalty-free, irrevocable license for certain derivative works to be used either commercially outside the field of use or teaching, patient care or non-commercial academic research purposes. Mount Sinai was granted equity in the company pursuant to the Licensing Agreement, along with the right to maintain 12% of the fully-diluted outstanding Common Stock of the company until the company receives an aggregate of $10,000,000 in cash in exchange for its equity securities. As stated above under “Security Ownership of Management and Certain Securities Holders”, Dr. Unis, Mr. Costa, Mr. Somani and the Icahn School of Medicine at Mount Sinai have each agreed, pursuant to a separate agreement to which the company is not a party, to split that 12% as follows: 7.4% to Mount Sinai, 1.6% to Dr. Unis, 1%, to Mr. Somani, and 0.4% to Mr. Costa, with the remaining 0.6% going to the laboratory in which the intellectual property that is the subject of the Licensing Agreement was generated. As of the date of this Offering Circular, all shares issuable to Mount Sinai pursuant to the terms of the Licensing Agreement have been issued. Pursuant to the terms the Licensing Agreement (and the Amendment thereto filed as Exhibit 6.14), we must have a first commercial sale our products within seven (7) years of the Effective Date of the agreement, or by October 10, 2024. Failure to meet this deadline would constitute a breach of our agreement, and Mount Sinai would have the right to give us a notice of default, and could ultimately terminate the Licensing Agreement if we fail to cure this default within sixty (60) days.
In addition, as part of the Licensing Agreement, we are obligated to enter into a stock purchase agreement with Mount Sinai for the shares of Common Stock already issued to Mount Sinai. We have not begun negotiating that stock purchase agreement with Mount Sinai, but are required to enter into such an agreement before September 22, 2019. The stock purchase agreement will include “reasonable or customary agreements reasonably required by any future institutional equity investors with respect to the voting of its common stock, and regarding subjecting the common stock held by [Mount Sinai] to rights of first refusal and co-sale…”.
On March 18, 2019, the company entered into an option agreement (the “Option Agreement”) with Mount Sinai pursuant to which the company was granted an option to license additional intellectual property rights under the terms and conditions as set forth in the aforementioned Licensing Agreement. The company exercised this option on March 26th, 2019 for an exercise fee of $1,000. (See Exhibit 6.9 for further information.) The intellectual property licensed pursuant to this Option Agreement is detailed under “Description of Business – Intellectual Property”. Since this Option Agreement is governed by the terms of the Licensing Agreement, any termination of the Licensing Agreement would automatically terminate this Option Agreement.
Payments under the agreement include: annual license maintenance fees, milestone payments (upon completion of certain events, such as FDA Clearance of Monogram’s custom implants), running royalties (subject to certain adjustments) and sublicense fees.
On December 20, 2018, the company entered into a development and supply agreement with Pro-Dex, Inc., whereby Pro-Dex, Inc. and the company agreed, subject to certain conditions, to negotiate and endeavor to enter into a future agreements through which Pro-Dex, Inc. would develop and supply end-effectors, gearing, and saws, and other surgical products to Monogram. Richard L. Van Kirk is the Chief Executive Officer of Pro-Dex, Inc. and is a Director of Monogram. The conditions to enter into the future development and supply agreements are (i) the raise of at least $5,000,000 in equity capital through the issuance of Common Stock or Preferred Stock by Monogram on or before October 19, 2019, and (ii) the entry into a separate modification agreement regarding the terms of the convertible note issued by Monogram to Pro-Dex, Inc. Monogram and Pro-Dex, Inc. have entered into that modification agreement, dated December 20, 2018, as detailed further below. Richard L. Van Kirk is the Chief Executive Officer of Pro-Dex, Inc. and is a Director of Monogram.
On June 23, 2017, the company issued a convertible promissory note to Doug Unis, a Director of Monogram, in the principal amount of $50,000. The note consisted of two proposed tranches, one for $28,000 and a second tranche for $22,000, the release of which was subject to the achievement of certain milestones. The second $22,000 tranche was never contributed under this note. The note bears interest at 4% per year with balance due and payable on June 23, 2019. On January 19, 2018, the company issued a convertible promissory note to American IRA, LLC FBO Julia Jordan IRA, of which Doug Unis is an assign, in the principal amount of $28,000. The note bears interest at 4% per year with balance due and payable on January 19, 2020. Julia Jordan is Doug Unis’s wife. On May 30, 2018, the company issued a convertible promissory note to Doug Unis, a Director of Monogram, in the principal amount of $15,000. The note bears interest at 4% per year with balance due and payable on May 30, 2020. The company considers all three of these notes to be payable to Doug Unis, with $71,000 in principal balance and $5,969 at December 31, 2019. On April 23, 2020, all three of these notes converted into shares of the company’s Series A Preferred Stock as a result of the company’s receipt of gross proceeds of $5,000,000 or more from the Offering, and are no longer outstanding.
36
On April 19, 2017, the company issued a convertible promissory note to Pro-Dex, Inc., in the principal amount of $800,000. Richard L. Van Kirk is the Chief Executive Officer of Pro-Dex, Inc. and is a Director of Monogram. The company and Pro-Dex, Inc. subsequently amended the terms of the note via an agreement dated December 20, 2018. Pursuant the agreement as amended, the company granted Pro-Dex, Inc. the right to appoint one (1) director to the Board of Monogram so long as the note is outstanding. That director is Rick Van Kirk, who currently serves as a director of our company. As of December 31, 2019 this note was still outstanding, and had accrued interest of $117,295. On April 29, 2020, the company repaid all outstanding principal and interest due on this note. As a result, as of the date of this Offering Circular, this note is no longer outstanding.
On December 20, 2018, the company issued warrants to Pro-Dex, Inc. to purchase up to 5% of the outstanding Common Stock and Preferred Stock of the company as of the date of the exercise, calculated on a post-exercise basis. The warrants have an exercise price of $1,250,000, and may be exercised at any time prior to (i) December 20, 2025, (ii) the closing of an initial public offering of the company’s securities, or (iii) a liquidation event by the company. Richard L. Van Kirk is the Chief Executive Officer of Pro-Dex, Inc. and is a Director of Monogram.
On February 11, 2019, the company issued a convertible promissory note to Benjamin Sexson, Director and CEO of Monogram, in the principal amount of $48,000. The note bears interest at 4% per year with balance due and payable on February 11, 2020. On April 23, 2020, this note automatically converted into shares of the company’s Series A Preferred Stock as a result of the company’s receipt of gross proceeds of $5,000,000 or more from the Offering. As a result, it is no longer outstanding as of the date of this Offering Circular.
37
General
The company is offering shares of Series B Preferred Stock in this offering. The Series B Preferred Stock may be converted into shares of the Common Stock of the company at the discretion of each investor, or automatically upon the occurrence of certain events, like an initial public offering. The company is therefore qualifying up to 4,784,689 shares of Series B Preferred Stock, convertible into 4,784,689 shares of Common Stock, under the Offering Statement of which this Offering Circular is a part.
The following description summarizes the most important terms of the company’s capital stock. This summary does not purport to be complete and is qualified in its entirety by the provisions of Monogram’s Amended and Restated Certificate of Incorporation and bylaws, copies of which have been filed as exhibits to the Offering Statement of which this Offering Circular is a part. For a complete description of Monogram’s capital stock, you should refer to the Amended and Restated Certificate of Incorporation and bylaws of the company and to the applicable provisions of Delaware law.
The authorized capital stock of the company consists of Common Stock, par value $0.001 per share, and Preferred Stock, par value $0.001 per share. The total number of authorized shares of Common Stock of Monogram is 22,000,000 and the total number of authorized shares of Preferred Stock is 14,000,000, of which 5,500,000 is designated as Series A Preferred Stock, and 8,000,000is designated as Series B Preferred Stock
As of August 12, 2020, the outstanding shares of the company included:
| Class | Authorized | Issued and Outstanding | ||||||
| Series A Preferred Stock | 5,500,000 | 4,897,559 | ||||||
| Series B Preferred Stock | 8,000,000 | 0 | ||||||
| Common Stock | 22,000,000 | 4,836,935 | ||||||
On May 7, 2019, the company adopted an incentive plan, which the company subsequently amended on August 15, 2020 (the “Amended and Restated 2019 Stock Option and Grant Plan” or “Plan”. The Plan reserves 2,500,000 shares of common stock for issuance under the Plan and 840,000 shares allowed for issuance pursuant to Incentive Stock Options.
Provisions of Note in Our Amended and Restated Certificate of Incorporation
Our Amended and Restated Certificate of Incorporation includes a forum selection provision that requires any claims against the company by stockholders not arising under the federal securities laws to be brought in the Court of Chancery State in the state of Delaware. This forum selection provision may limit investors’ ability to bring claims in judicial forums that they find favorable to such disputes and may discourage lawsuits with respect to such claims. The company has adopted this provision to limit the time and expense incurred by its management to challenge any such claims. As a company with a small management team, this provision allows its officers to not lose a significant amount of time travelling to any particular forum so they may continue to focus on operations of the company.
Common Stock
Voting Rights
Each holder of the company’s Common Stock is entitled to one vote for each share on all matters submitted to a vote of the shareholders, including the election of directors. In addition, holders of our Common Stock are entitled to vote as a separate class for the election of two (2) directors of the company’s Board of Directors. Holders of our Preferred Stock may not vote on the election of these directors.
Dividend Rights
Holders of Common Stock are entitled to receive dividends, as may be declared from time to time by the Board of Directors out of legally available funds as detailed in the company’s Restated Articles. The company has never declared or paid cash dividends on any of its capital stock and currently does not anticipate paying any cash dividends after this offering or in the foreseeable future.
38
Liquidation Rights
In the event of a voluntary or involuntary liquidation, dissolution, or winding up of the company, the holders of the Common Stock are entitled to share ratably in the net assets legally available for distribution to shareholders after the payment of all debts and other liabilities of the company. Holders of our Preferred Stock are entitled to a liquidation preference that is senior to holders of the Common Stock, and therefore would receive dividends and liquidation assets prior to the holders of the Common Stock.
Preferred Stock
The terms of the Series A Preferred Stock and Series B Preferred Stock are substantially the same. As such, the following description of the Series B Preferred Stock is applicable to the Series A Preferred Stock, unless otherwise noted below.
Series B Preferred Stock
Voting Rights
Each holder of the company’s Series B Preferred Stock is entitled to one vote for each share on all matters submitted to a vote of the shareholders, including the election of directors, subject to the following restrictions:
| · | The holders of our Common Stock are entitled to elect two (2) directors to the company’s Board of Directors as a standalone class. The Series B Preferred Stockholders may not exercise any voting rights in the election of these directors. |
Holders of our Series B Preferred Stock specifically have the right to vote with the holders of the Common Stock to elect:
| · | one (1) independent director to the company’s Board of Directors; and |
| · | any additional directors to the company’s Board of Directors after the elections outlined above. |
Each holder of Series B Preferred Stock will be entitled to one vote for each share of Common Stock into which such share of Preferred Stock could be converted. Fractional votes will not be permitted and if the conversion results in a fractional share, it will be disregarded.
Additionally, the holders of the Series B Preferred Stock are entitled to certain protective provisions that require the company to obtain the written consent or affirmative vote of a majority of the outstanding shares of Preferred Stock prior to effecting certain corporate actions, comprised of the following:
| (a) | alter the rights, powers or privileges of the Preferred Stock in a way that adversely affects the Preferred Stock; |
| (b) | increase or decrease the authorized number of shares of any class or series of capital stock; |
| (c) | authorize or create (by reclassification or otherwise) any new class or series of capital stock having rights, powers, or privileges set forth in the Amended and Restated Certificate of Incorporation of the company, as then in effect, that are senior to or on a parity with any series of Preferred Stock; |
| (d) | redeem or repurchase any shares of Common Stock or Preferred Stock (other than pursuant to employee or consultant agreements giving the company the right to repurchase shares upon the termination of services pursuant to the terms of the applicable agreement); |
| (e) | declare or pay any dividend or otherwise make a distribution to holders of Preferred Stock or Common Stock; |
| (f) | increase or decrease the number of directors of the company; |
| (g) | liquidate, dissolve, or wind-up the business and affairs of the company |
39
Dividend Rights
Holders of Series B Preferred Stock will be entitled to receive dividends as may be declared from time to time by the Board of Directors out of legally available funds and on a pari passu basis with holders of the Common Stock. The company has never declared or paid cash dividends on any of its capital stock and currently does not anticipate paying any cash dividends after this offering or in the foreseeable future.
Conversion Rights
Shares of Series B Preferred Stock will be convertible, at the option of the holder, at any time, into fully paid and nonassessable shares of the company’s Common Stock at the then-applicable conversion rate. Initially, the conversion rate will be one share of Common Stock per share of Series B Preferred Stock. The conversion rate is subject to adjustment in the event of stock splits, reverse stock splits or the issuance of a dividend or other distribution payable in additional shares of Common Stock.
Additionally, each share of Series B Preferred Stock will automatically convert into Common Stock:
| i) | immediately prior to the closing of a firm commitment underwritten public offering of the company’s Common Stock on Form S-1, registered under the Securities Act, at a per share price not less than the Original Issue Price (as defined below) adjusted for any stock dividends, combinations, splits, recapitalizations and the like, for a total offering proceeds $5,000,000 or more (before deduction of underwriters commissions and expenses); or |
| ii) | upon the affirmative election of the holders of a majority of the outstanding shares of Preferred Stock, voting as a single class and on an as-converted basis. |
In either of these events, the shares will convert in the same manner as a voluntary conversion.
Right to Receive Liquidation Distributions
In the event of a liquidation, dissolution or winding up of the company, whether voluntary or involuntary, or certain other events (each a “Deemed Liquidation Event”) such as the sale or merger of the company, all holders of Series B Preferred Stock will be entitled to a liquidation preference that is senior to holders of the Common Stock. Holders of Series B Preferred Stock will receive a liquidation preference equal to the greater of (a) an amount for each share equal to the Original Issue Price for such share, adjusted for any stock dividends, combinations, splits, recapitalizations and the like (the “liquidation preference”) plus any declared but unpaid dividends with respect to such shares or (b) such amount per share as would have been payable had all shares of Series B Preferred Stock been converted into Common Stock immediately prior to such liquidation, dissolution or winding up or Deemed Liquidation Event. Initially, the liquidation preferences for the shares of Series B Preferred Stock will be $6.27 per share (the “Original Issue Price”).
If, upon such liquidation, dissolution, or winding up or Deemed Liquidation Event, the assets (or the consideration received in a transaction) that are distributable to the holders of Preferred Stock are insufficient to permit the payment to such holders of the full amount of their respective liquidation preference, then all of such funds will be distributed ratably among the holders of the Preferred Stock in proportion to the full amounts to which they would otherwise be entitled to receive.
After the payment of the full liquidation preference of the Series B Preferred Stock, the remaining assets of the company legally available for distribution (or the consideration received in a transaction), if any, will be distributed ratably to the holders of the Common Stock in proportion to the number of shares of Common Stock held by each such holder.
(Note: The “Original Issue Price” for the Series A Preferred Stock is $4.00. All other terms are the same.)
Drag Along Right
The investors’ rights agreement that investors will execute in connection with the offering contains a “drag-along provision” related to the certain events, such as the sale, merger or dissolution of the company (a “Liquidating Event”). Investors who purchase Series B Preferred Stock agree that, if the board of directors, the majority of the holders of the company’s Common Stock, and the majority of the holders of the company’s Series B Preferred Stock vote in favor of such a Liquidating Event, then such holders of Series B Preferred Stock will vote in favor of the transaction if such vote is solicited, refrain from exercising dissenters’ rights with respect to Liquidating Event, and deliver any documentation or take other actions reasonably requested by the company or the other holders in connection with the Liquidating Event.
40
Information Rights
The company also agrees in the investors’ rights agreement to grant certain information rights to investors in this offering that invest $50,000 or more in this offering (“Major Purchasers”). The information rights provided to Major Purchasers include: (1) annual unaudited financial statements for each fiscal year of the company, including an unaudited balance sheet as of the end of such fiscal year, an unaudited income statement, and an unaudited statement of cash flows, all prepared in accordance with generally accepted accounting principles and practices; and (2) quarterly unaudited financial statements for each fiscal quarter of the company (except the last quarter of the company’s fiscal year), including an unaudited balance sheet as of the end of such fiscal quarter, an unaudited income statement, and an unaudited statement of cash flows, all prepared in accordance with generally accepted accounting principles and practices, subject to changes resulting from normal year-end audit adjustments. If the company has audited records of any of the foregoing, it will provide those in lieu of the unaudited versions.
Additional Rights and Participation Rights
The investors’ rights agreement that investors will execute in connection with the offering grants investors and their transferees certain rights in connection with the company’s next equity offering. If in its next equity offering after the date that an investor executes the investors’ rights agreement (the “Next Financing”) the company issues securities that (a) have rights, preferences or privileges that are more favorable than the terms of the Series B Preferred Stock or (b) provide all such future investors in the Next Financing contractual terms such as registration rights, the company agrees to provide substantially equivalent rights to the investor with respect to the Series B Preferred Stock (with appropriate adjustment for economic terms or other contractual rights), including the amount of the Series B preferred stock liquidating distributions, through the investor’s proxy, if applicable, subject to the investor’s execution of any documents, including, if applicable, investor rights, co-sale, voting, and other agreements, executed by the investors purchasing securities in the Next Financing (the “Next Financing Documents”), provided that certain rights may be reserved for investors with a minimum amount of investment in the Next Financing. Upon the execution and delivery of the Next Financing Documents, the investors’ rights agreement (excluding any then-existing and outstanding obligations) will be automatically amended and restated by and into the Next Financing Documents and will be terminated and of no further force or effect. As a result, the rights of investors who participate in any Next Financing will instead be governed by the Next Financing Documents.
In the investors’ rights agreement, the company also grants investors in this offering participation rights. Investors will have the right of first refusal to purchase the investor’s Pro Rata Share of any New Securities (each as defined below) that the company may issue in the Next Financing. The investor will have no right to purchase any New Securities if the investor cannot demonstrate to the company’s reasonable satisfaction that the investor is at the time of the proposed issuance of New Securities eligible to purchase such New Securities under applicable securities laws. An investor’s “Pro Rata Share” means the ratio of (i) the number of shares of the company’s Common Stock issued or issuable upon conversion of the Series B Preferred Stock owned by the investor, to (ii) that number of shares of the company’s capital stock equal to the sum of (A) all shares of the company’s capital stock (on an as-converted basis) issued and outstanding, assuming exercise or conversion of all options, warrants and other convertible securities and promissory notes, and (B) all shares of the company’s capital stock reserved and available for future grant under any equity incentive or similar plan.
“New Securities” means any shares of the company’s capital stock to be issued in the Next Financing, including Common Stock or Preferred Stock, whether now authorized or not, and rights, options or warrants to purchase Common Stock or Preferred Stock, and securities of any type whatsoever that are, or may become, convertible or exchangeable into Common Stock or Preferred Stock “New Securities” does not include: (i) shares of Common Stock issued or issuable upon conversion of any outstanding shares of Preferred Stock; (ii) Common Stock or Series B Preferred Stock issued upon conversion of any outstanding convertible notes; a(iii) shares of Common Stock or Preferred Stock issuable upon exercise of any options, warrants, or rights to purchase any securities of the company outstanding as of the date the Offering Statement is qualified by the Commission and any securities issuable upon the conversion thereof; (iv) shares of Common Stock or Preferred Stock issued in connection with any stock split or stock dividend or recapitalization; (v) shares of Common Stock (or options, warrants or rights therefor) granted or issued after the date the Offering Statement is qualified by the Commission to employees, officers, directors, contractors, consultants or advisers to, the company or any subsidiary of the company pursuant to incentive agreements, stock purchase or stock option plans, stock bonuses or awards, warrants, contracts or other arrangements that are approved by the board of directors; (vi) shares of the company’s Series B Preferred Stock issued in this offering; (vii) any other shares of Common Stock or Preferred Stock (and/or options or warrants therefor) issued or issuable primarily for other than equity financing purposes and approved by the board of directors; (vii) shares of Common Stock issued or issuable by the company to the public pursuant to a registration statement filed under the Securities Act; and (ix) any other shares of the company’s capital stock, the issuance of which is specifically excluded by approval of the board of directors.
The company will send investors, or investors’ proxies, if applicable, a notice describing the type of New Securities and the price and the general terms upon which the it proposes to issue the New Securities. An investor will have fourteen (14) days from the date of notice, to agree to purchase a quantity of New Securities, up to their Pro Rata Share. If an investor fails to exercise in full the right of first refusal within the 14-day period, then the company will have one hundred twenty (120) days after that to sell the New Securities with respect to which the investor’s right of first refusal was not exercised. If the company has not issued and sold the minimum amount of New Securities to be sold in the Next Financing within the 120-day period, then the company will not issue or sell any New Securities without again first offering those New Securities to investors in accordance with the terms of the investors’ rights agreement.
41
Benjamin Sexson, our CEO, is entitled to pre-emptive rights permitting him preserve his vested equity position in the company in the event of any additional issuances of company common stock (or securities convertible into common stock), at a per-share price equal to the then current fair market value, as reasonably determined by the Board. Mr. Sexson does not intend to exercise this pre-emptive right.
(Note: Investors in the Company’s Series A Offering entered into an investors’ rights agreement with identical terms to the investors’ rights agreement in this offering. A copy of this agreement is filed as Exhibit 3.1 to this Offering Statement, of which this Offering Circular forms a part.)
42
| TABLE OF CONTENTS |
| Page | ||
| Independent Auditor’s Report | F-1 | |
| Financial Statements as of December 31, 2019 and 2018 and for the years then ended: | ||
| Balance Sheets | F-2 | |
| Statements of Operations | F-3 | |
| Statements of Changes in Stockholders’ Equity | F-4 | |
| Statements of Cash Flows | F-5 | |
| Notes to Financial Statements | F-6–F-26 |
43
 | |
| INDEPENDENT AUDITOR’S REPORT | |
| To the Board of Directors and Stockholders | |
 | of Monogram Orthopaedics, Inc. |
| We have audited the accompanying financial statements of Monogram Orthopaedics, Inc., which comprise the balance sheets as of December 31, 2019 and 2018, and the related statements of operations, stockholders’ equity (deficit), and cash flows for the years then ended, and the related notes to the financial statements. | |
| Management’s Responsibility for the Financial Statements | |
Members of: | Management is responsible for the preparation and fair presentation of these financial statements in accordance with accounting principles generally accepted in the United States of America; this includes the design, implementation, and maintenance of internal control relevant to the preparation and fair presentation of financial statements that are free from material misstatement, whether due to fraud or error.
|
| Auditor’s Responsibility | |
802 N. Washington PO Box 2163 Spokane, Washington 99210-2163 | Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free from material misstatement. |
| P 509-624-9223 TF 1-877-264-0485 mail@fruci.com www.fruci.com | An audit involves performing procedures to obtain audit evidence about the amounts and disclosures in the financial statements. The procedures selected depend on the auditor’s judgment, including the assessment of the risks of material misstatement of the financial statements, whether due to fraud or error. In making those risk assessments, the auditor considers internal control relevant to the entity’s preparation and fair presentation of the financial statements in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the entity’s internal control. Accordingly, we express no such opinion. An audit also includes evaluating the appropriateness of accounting policies used and the reasonableness of significant accounting estimates made by management, as well as evaluating the overall presentation of the financial statements. |
| We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our audit opinion. | |
| Going Concern | |
| The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has sustained recurring losses from operations and has significant accumulated and working capital deficits. These factors raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty. | |
| Opinion | |
| In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Monogram Orthopaedics, Inc. as of December 31, 2019 and 2018, and the results of its operations and its cash flows for the years then ended in accordance with accounting principles generally accepted in the United States of America. | |
 | |
| Spokane, Washington | |
| May 1, 2020 |
| F-1 |
MONOGRAM ORTHOPAEDICS INC.
| December 31, 2019 | December 31, 2018 | |||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 2,319,393 | $ | 922,108 | ||||
| Prepaid expenses | 65,000 | - | ||||||
| Total current assets | 2,384,393 | 922,108 | ||||||
| Equipment, net of accumulated depreciation | 235,748 | 262,146 | ||||||
| Total assets | $ | 2,620,141 | $ | 1,184,254 | ||||
| Liabilities and Stockholders’ Deficit | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | ||||||||
| Trade | $ | 241,850 | $ | 257,480 | ||||
| Related party | 8,518 | 31,128 | ||||||
| Accrued interest payable | 78,072 | 78,568 | ||||||
| Accrued interest payable – related parties | 124,964 | 7,227 | ||||||
| Accrued officer salary payable | 88,125 | 48,877 | ||||||
| Warrant liability | 229,244 | - | ||||||
| Current portion of long-term debt | 1,205,000 | 1,025,000 | ||||||
| Current portion of long-term debt – related parties | 871,000 | 904,000 | ||||||
| Total current liabilities | 2,846,773 | 2,352,280 | ||||||
| Long-term debt | - | 180,000 | ||||||
| Long-term debt – related parties | 48,000 | 43,000 | ||||||
| Total liabilities | 2,894,773 | 2,575,280 | ||||||
| Commitments and Contingencies | - | - | ||||||
| Stockholders’ deficit: | ||||||||
| Preferred stock, $.001 par value; 7,850,000,shares authorized, 702,021 and 0 shares issued and outstanding in 2019 and 2018, respectively | 702 | - | ||||||
| Common stock, $.001 par value; 13,225,000 shares authorized, 4,317,104 and 89,382 shares issued and outstanding in 2019 and 2018, respectively | 4,317 | 89 | ||||||
| Capital in excess of par value | 2,909,875 | 2,146 | ||||||
| Accumulated deficit | (3,189,526 | ) | (1,393,261 | ) | ||||
| Total stockholders’ deficit | (274,632 | ) | (1,391,026 | ) | ||||
| Total liabilities and stockholders’ deficit | $ | 2,620,141 | $ | 1,184,254 | ||||
Accompanying notes are an integral part of these financial statements
| F-2 |
MONOGRAM ORTHOPAEDICS INC.
| Years Ended December 31, | ||||||||
| 2019 | 2018 | |||||||
| Revenues | $ | - | $ | - | ||||
| Cost and expenses: | ||||||||
| Wages and payroll related expenses | 344,757 | 378,523 | ||||||
| General and administrative | 179,671 | 101,679 | ||||||
| Marketing and advertising | 205,207 | 3,154 | ||||||
| Legal and professional services | 106,140 | 104,114 | ||||||
| Depreciation | 31,763 | 30,957 | ||||||
| Stock-based compensation | 614,870 | - | ||||||
| Research and development | 194,287 | 31,700 | ||||||
| Total costs and expenses | 1,676,695 | 650,127 | ||||||
| Loss from operations | (1,676,695 | ) | (650,127 | ) | ||||
| Other income (expense) | ||||||||
| Interest expense | (124,470 | ) | (62,184 | ) | ||||
| Interest income | 4,900 | 43 | ||||||
| Total other income (expense) | (119,570 | ) | (62,141 | ) | ||||
| Net income (loss) before taxes | (1,796,265 | ) | (712,268 | ) | ||||
| Income tax | - | - | ||||||
| Net income (loss) | $ | (1,796,265 | ) | $ | (712,268 | ) | ||
| Basic and diluted earnings (loss) per share | $ | (0.62 | ) | $ | (6.89 | ) | ||
| Weighted average number of basic and diluted shares outstanding | 2,896,015 | 103,333 | ||||||
Accompanying notes are an integral part of these financial statements
| F-3 |
MONOGRAM ORTHOPAEDICS INC.
Statements of Stockholders’ Equity (Deficit)
For the Years Ended December 31, 2019 and 2018
| Preferred Stock | Common Stock | Capital in Excess of | Accumulated | Total Stockholders' Equity | ||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Par Value | Deficit | (Deficit) | ||||||||||||||||||||||
| Balance, December 31, 2017 | - | $ | - | 127,382 | $ | 127 | $ | 3,058 | $ | (680,993 | ) | $ | (677,808 | ) | ||||||||||||||
| Net loss, December 31, 2018 | - | - | - | - | - | (712,268 | ) | (712,268 | ) | |||||||||||||||||||
| Stock repurchase | - | - | (38,000 | ) | (38 | ) | (912 | ) | - | (950 | ) | |||||||||||||||||
| Balance, December 31, 2018 | - | - | 89,382 | 89 | 2,146 | (1,393,261 | ) | (1,391,026 | ) | |||||||||||||||||||
| Net loss, December 31, 2019 | - | - | - | - | - | (1,796,265 | ) | (1,796,265 | ) | |||||||||||||||||||
| Stock option expense | 5,303 | 5,303 | ||||||||||||||||||||||||||
| Common stock issuances | - | - | 4,227,722 | 4,228 | 376,584 | - | 380,812 | |||||||||||||||||||||
| Sale of preferred shares | 702,021 | 702 | - | - | 2,525,842 | - | 2,526,544 | |||||||||||||||||||||
| Balance, December 31, 2019 | 702,021 | $ | 702 | 4,317,104 | $ | 4,317 | $ | 2,909,875 | $ | (3,189,526 | ) | $ | (274,632 | ) | ||||||||||||||
Accompanying notes are an integral part of these financial statements
| F-4 |
MONOGRAM ORTHOPAEDICS INC.
| Year | Year | |||||||
| Ended | Ended | |||||||
| December 31, | December 31, | |||||||
| 2019 | 2018 | |||||||
| Operating activities | ||||||||
| Net loss | $ | (1,796,265 | ) | $ | (712,268 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Stock-based compensation | 614,870 | — | ||||||
| Depreciation | 31,763 | 30,957 | ||||||
| Changes in non-cash working capital balances: | ||||||||
| Prepaid expenses | (65,000 | ) | - | |||||
| Accounts payable | (15,630 | ) | 233,207 | |||||
| Accrued officer salary payable | 87,248 | 48,877 | ||||||
| Accrued payable – related parties | (22,610 | ) | 30,178 | |||||
| Accrued interest payable | 117,241 | 62,184 | ||||||
| Cash used in operating activities | (1,048,383 | ) | (306,865 | ) | ||||
| Investing activities | ||||||||
| Purchase of equipment | (5,365 | ) | (154,915 | ) | ||||
| Cash used in investing activities | (5,365 | ) | (154,915 | ) | ||||
| Financing activities | ||||||||
| Proceeds from notes payable | - | 1,105,000 | ||||||
| Proceeds from sale of preferred stock | 2,526,544 | - | ||||||
| Proceeds from note payable – related parties | - | 43,000 | ||||||
| Proceeds from sale of common stock | 489 | - | ||||||
| Payment of related party loans | (76,000 | ) | - | |||||
| Cash provided by financing activities | 2,451,033 | 1,148,000 | ||||||
| Increase in cash and cash equivalents during the period | 1,397,285 | 686,220 | ||||||
| Cash and cash equivalents, beginning of the period | 922,108 | 235,888 | ||||||
| Cash and cash equivalents, end of the period | $ | 2,319,393 | $ | 922,108 | ||||
| Cash paid for: | ||||||||
| Interest | $ | 7,229 | $ | - | ||||
| Income taxes | $ | - | $ | - | ||||
| Non-cash financing activities | ||||||||
| Note issued for officer's salary | $ | 48,000 | $ | - | ||||
| Repurchase of common stock by related party | $ | - | $ | 950 | ||||
Accompanying notes are an integral part of these financial statements
| F-5 |
MONOGRAM ORTHOPAEDICS INC.
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2019 AND 2018
1. Description of Business and Summary of Accounting Principles
Description of the Organization
Monogram Orthopaedics Inc. (“Monogram,” “we,” “our,” or the “Company”), incorporated in the state of Delaware on April 21, 2016, is developing a product solution architecture for enabling mass personalization of orthopedic implants by linking 3D printing and robotics via automated digital image analysis algorithms.
The company has a working navigated robot prototype that can optically track a simulated surgical target and execute optimized auto-generated cut paths for high precision insertion of patient specific implants in synthetic bone specimens. These implants and cut-paths are generated with proprietary Monogram software algorithms.
The financial statements are presented in United States dollars and have been prepared in accordance with generally accepted accounting principles in the United States of America. The Company’s fiscal year end is December 31. The Company operated from its headquarters in Brooklyn, New York until January 2020 when it moved to Austin, Texas.
Income Taxes
The Company accounts for income taxes under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income and the reversal of deferred tax liabilities during the period in which related temporary differences become deductible. A valuation allowance has been established to eliminate the Company’s deferred tax assets as it is more likely than not that none of the deferred tax assets will be realized.
The Company recognizes the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position are measured based on the largest benefit that has a greater than fifty percent likelihood of being realized upon settlement with the tax authorities. Changes in recognition or measurement are reflected in the period in which the change in judgment occurs. The Company records interest related to unrecognized tax benefits in interest expense and penalties in income tax expense. The Company has determined that it had no significant uncertain tax positions requiring recognition or disclosure.
| F-6 |
The Company records uncertain tax positions in accordance with ASC 740 on the basis of a two-step process whereby (1) we determine whether it is more likely than not that the tax positions will be sustained on the basis of the technical merits of the position and (2) for those tax positions that meet the more-likely-than-not recognition threshold, we recognize the largest amount of tax benefit that is more than 50 percent likely to be realized upon ultimate settlement with the related tax authority.
Revenue Recognition
In May 2014, the FASB issued ASU 2014-09, "Revenue from Contracts with Customers (Topic 606)." The Company adopted the new standard as of January 1, 2018, utilizing a full retrospective transition method. Adoption of the new standard resulted in no changes for revenue recognition related as the Company has not yet generated revenue.
Earnings (Loss) Per Share
Earnings (loss) per share is computed by dividing net income or loss by the weighted-average number of shares outstanding. To the extent that stock options, warrants and convertible preferred stock are anti-dilutive, they are excluded from the calculation of diluted earnings (loss) per share. See Note 9 for details of potentially dilutive securities.
Cash and Cash Equivalents
The Company considers all highly liquid investments purchased with original maturities of three months or less to be cash equivalents. The Company did not have any cash equivalents during fiscal 2019 and 2018. The Company uses two financial institutions for its cash balances, and occasionally maintains cash balances that exceed federally insured limits.
Equipment
Equipment expenditures are recorded at cost. Costs which extend the useful lives or increase the productivity of the assets are capitalized, while normal repairs and maintenance that do not extend the useful life or increase the productivity of the asset are expensed as incurred. Equipment, including the Company’s robot, are depreciated on the straight-line method over the estimated useful lives of the assets. Equipment will be depreciated over a five-year useful life. Any construction in progress is stated at cost and depreciation will commence once the project is constructed and placed in service.
| F-7 |
Asset Impairment
Long-lived assets, such as property, plant, and equipment are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. If circumstances require a long-lived asset or asset group be tested for possible impairment, the Company first compares undiscounted cash flows expected to be generated by that asset or asset group to its carrying amount. If the carrying amount of the long-lived asset or asset group is not recoverable on an undiscounted cash flow basis, an impairment is recognized to the extent that the carrying amount exceeds its fair value. Fair value is determined through various valuation techniques including discounted cash flow models, quoted market values and third-party independent appraisals, as considered necessary. The Company recorded no asset impairment in 2019 or 2018.
General and Administrative Expenses
General and administrative expenses include salaries, travel and office expenses of administrative employees and contractors; software license fees; and other overhead expenses.
Stock-Based Compensation
The Company applies the fair value method of ASC 718, Share Based Payment, formerly Statement of Financial Accounting Standards No. l23R "Accounting for Stock Based Compensation," in accounting for its stock-based compensation. This accounting standard states that compensation cost is measured at the grant date based on the value of the award and is recognized over the service period, which is usually the vesting period, if any. As the Company does not have sufficient, reliable, and readily determinable values relating to its common stock, the Company has used (i) the stock value pursuant to an independent valuation dated February 27, 2019 to record stock-based compensation at a price of $0.09 per share and (ii) its most recent sale of stock of $4.00 per share in December 2019 for valuing stock-based compensation at December 31, 2019.
Research and Development Costs
Research and development (“R&D”) costs are expensed as incurred and amounted to $194,287 and $31,700 for the years ended December 31, 2019 and 2018, respectively. The company currently has several R&D initiatives underway including mechanical testing of a patient specific hip, micromotion studies of a patient specific press-fit knee implant, and performance testing of a robot mounted navigation system.
| F-8 |
Advertising Costs
Advertising and marketing costs are expensed as incurred and amounted to $205,207and $3,154 for the years ended December 31, 2019 and 2018, respectively.
Use of Estimates
In preparing financial statements in conformity with generally accepted accounting principles, management is required to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. The most significant estimates relate to the warrant liability, valuations of stock-based compensation and the income tax valuation allowance. On a continual basis, management reviews its estimates, utilizing currently available information, changes in facts and circumstances, historical experience and reasonable assumptions. After such reviews, and if deemed appropriate, those estimates are adjusted accordingly. Actual results could differ from those estimates.
Recent Accounting Pronouncements
In June 2016, the FASB issued ASU No. 2016-13, Financial Instruments-Credit Losses. The new guidance provides better representation about expected credit losses on financial instruments. This Update requires the use of a methodology that reflects expected losses and requires consideration of a broader range of reasonable and supportive information to inform credit loss estimates. This ASU is effective for reporting periods beginning after December 15, 2022, with early adoption permitted. The company is studying the impact of adopting the ASU in 2023, and what effect it could have. The Company believes the accounting change would not have a material effect on the financial statements.
In March 2017 the FASB issued ASU 2017-04, Intangibles—Goodwill and Other (Topic 350) Simplifying the Test for Goodwill Impairment. This amendment simplifies the measurement of goodwill by eliminating Step 2 from the goodwill impairment test. This update is effective for fiscal years beginning after December 15, 2021. The adoption of ASU No. 2017-04 is not expected to have a material impact on the Company’s financial statements.
In June 2018, the FASB issued ASU 2018-07, Improvement to Nonemployee Share-based Payment Accounting, which simplifies the accounting for share-based payments. The company elected early adoption of this ASU, using the modified retrospective approach, so that all stock compensation to employees and nonemployees is treated under the same guidance as in ASC 718. No outstanding stock option grants were required to be revalued upon the adoption of this ASU.
| F-9 |
Management does not believe that any other recently issued, but not yet effective, accounting standards could have a material effect on the accompanying financial statements. As new accounting pronouncements are issued, we will adopt those that are applicable under the circumstances.
2. Going Concern Matters and Realization of Assets
The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the ordinary course of business. However, the Company has sustained recurring losses from its continuing operations and as of December 31, 2019, had negative working capital of $462,380 and a stockholders’ deficit of $274,632. In addition, the Company is unable to meet its obligations as they become due and sustain its operations. The Company believes that its existing cash resources are not sufficient to fund its continuing operating losses, capital expenditures, lease and debt payments and working capital requirements.
The Company may not be able to raise sufficient additional debt, equity or other cash on acceptable terms, if at all. Failure to generate sufficient revenues, achieve certain other business plan objectives or raise additional funds could have a material adverse effect on the Company’s results of operations, cash flows and financial position, including its ability to continue as a going concern, and may require it to significantly reduce, reorganize, discontinue or shut down its operations.
In view of the matters described above, recoverability of a major portion of the recorded asset amounts shown in the accompanying balance sheet is dependent upon continued operations of the Company which, in turn, is dependent upon the Company’s ability to meet its financing requirements on a continuing basis, and to succeed in its future operations. The financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or amounts and classification of liabilities that might be necessary should the Company be unable to continue in its existence. Management’s plans include:
| 1. | Continue to raise cash for research, product development and working capital purposes by selling equity under an offering statement that has been qualified by the Securities and Exchange Commission under Tier II of Regulation A. The Company is offering up to 5,000,000 shares of Series A preferred stock, at a price of $4.00 per share, which may convert into shares of common stock on a one-for-one basis. With sufficient cash available to the Company, it can make the additional development expenditures necessary to produce a commercially viable product and generate revenues, and consequently cut monthly operating losses. |
| 2. | Continue to develop its technology and intellectual property and look for industry partners to use or sell its product. |
| F-10 |
Management has determined, based on its recent history and its liquidity issues that it is not probable that management’s plan will sufficiently alleviate or mitigate, to a sufficient level, the relevant conditions or events noted above. Accordingly, the management of the Company has concluded that there is substantial doubt about the Company’s ability to continue as a going concern within one year after the issuance date of these financial statements.
There can be no assurance that the Company will be able to achieve or maintain cash-flow-positive operating results. If the Company is unable to generate adequate funds from operations or raise sufficient additional funds, the Company may not be able to repay its existing debt, continue to develop its product, respond to competitive pressures or fund its operations. As a result, the Company may be required to significantly reduce, reorganize, discontinue or shut down its operations. The financial statements do not include any adjustments that might result from this uncertainty.
3. Equipment
Equipment, net consists of the following as of December 31, 2019 and 2018
| 2019 | 2018 | |||||||
| Computer equipment | $ | 32,430 | $ | 27,065 | ||||
| Medical equipment | 6,142 | 6,142 | ||||||
| Robot | 123,250 | 123,250 | ||||||
| Work-in-process equipment | 150,000 | 150,000 | ||||||
| 311,822 | 306,457 | |||||||
| Accumulated depreciation | (76,074 | ) | (44,311 | ) | ||||
| Equipment, net | $ | 235,748 | $ | 262,146 | ||||
For the years ended December 31, 2019 and 2018, depreciation expense amounted to $31,763 and $30,957, respectively.
4. Debt
The following table summarizes components debt as of December 31, 2019 and 2018:
| 2019 | 2018 | |||||||
| Convertible term notes | $ | 1,125,000 | $ | 1,125,000 | ||||
| Secured convertible term notes | 80,000 | 80,000 | ||||||
| Convertible term notes – related parties | 919,000 | 947,000 | ||||||
| Total Debt | $ | 2,124,000 | $ | 2,152,000 | ||||
| F-11 |
All of the notes are convertible and they mature at various times from April 30, 2020 through February 11, 2021, as noted in the table below:
| Description | Principal | Maturity Date | Interest Rate | Valuation Cap | ||||||||
| Related party note | $ | 450,000 | 4/30/2020 | 6% | $ | 6,000,000 | ||||||
| Related party note | 350,000 | 4/30/2020 | 6% | $ | 6,000,000 | |||||||
| Related party note | 28,000 | 5/31/2020 | 4% | $ | 6,000,000 | |||||||
| Unsecured term note | 50,000 | 5/31/2020 | 4% | $ | 6,000,000 | |||||||
| Unsecured term note | 50,000 | 5/31/2020 | 4% | $ | 6,000,000 | |||||||
| Related party note | 28,000 | 5/31/2020 | 4% | $ | 6,000,000 | |||||||
| Related party note | 15,000 | 5/31/2020 | 4% | $ | 6,000,000 | |||||||
| Secured term note | 40,000 | 7/30/2020 | 4% | $ | 6,000,000 | |||||||
| Secured term note | 40,000 | 7/12/2020 | 6% | $ | 6,000,000 | |||||||
| Unsecured term note | 25,000 | 9/18/2020 | 6% | $ | 10,000,000 | |||||||
| Unsecured term note | 50,000 | 11/9/2020 | 6% | $ | 8,000,000 | |||||||
| Unsecured term note | 700,000 | 4/30/2020 | 4% | $ | 6,000,000 | |||||||
| Unsecured term note | 225,000 | 4/30/2020 | 6% | $ | 8,000,000 | |||||||
| Unsecured term note | 25,000 | 12/24/2020 | 6% | $ | 10,000,000 | |||||||
| Related party note | 48,000 | 2/11/2021 | 4% | $ | 6,000,000 | |||||||
| Total | $ | 2,124,000 | ||||||||||
Interest expense amounted to $124,470 and $62,184 for the years ended December 31, 2019 and 2018, respectively. Accrued interest payable amounted to $78,072 and $78,568 at December 31, 2019 and 2018, respectively. Accrued interest payable to related parties amounted to $124,964 and $7,227 at December 31, 2019 and 2018, respectively.
Ten of the above notes contained original maturity dates in 2019. Those notes were renegotiated to extend the maturity dates to April 30, 2020 and May 31, 2020. In conjunction with the extension of the maturity dates, the Company paid aggregate fees to the note holders of $26,250, which are recorded as a component of general and administrative expenses.
The notes payable are convertible into equity upon the closing of a Financing (as hereinafter defined). The term "Equity Securities" means the class of the Company's preferred stock issued in the Financing. The Equity Securities issued upon conversion of the notes shall be of the same class of Equity Securities purchased by investors in the Financing but shall be designated as a separate series of Equity Securities that shall have the same rights and preferences of the Equity Securities purchased by new purchasers in the Financing, except that the "Original Issue Price" of the series Equity Securities issued to holders of notes, as set forth in the Company's then-current Certificate of Incorporation for the purposes of calculating liquidation preferences, conversion ratios, anti-dilution adjustments, dividends and the like, will be the Conversion Price (as hereinafter defined). Additionally, the note holders shall receive pro rata participation rights with respect to all future equity issuances, subject to customary exceptions, such that each note holder shall have the right to participate in future equity issuances in an amount that permits it to maintain its fully-diluted ownership in the Company after each equity issuance. At that time, all of the principal amount outstanding under the notes and any accrued and unpaid interest thereon shall be converted automatically at the Conversion Price without further action of the note holders into shares of equity securities issued at such Financing. The term "Conversion Price" means an amount equal to the lesser of (i) eighty percent (80%) of the per share price paid in the Financing or (ii) the price equal to the quotient of the amount in the “Valuation Cap” column in the table above, divided by the aggregate number of fully diluted outstanding shares of the Company's common stock, as defined, immediately prior to the initial closing of the Financing. The term "Financing" means any equity financing for the account of the Company involving the issuance and sale of shares of Equity Securities which occurs on or before the notes mature and at which time the aggregate gross proceeds received by the Company (excluding any amounts from the conversion of any of the notes and any other convertible notes previously issued by the Company) equals or exceeds $5,000,000.
| F-12 |
Until the payment or conversion of the entire principal amount of the notes and the payment or conversion of the entire accrued interest thereon, the Company shall not take any of the following actions without the prior written consent of the note holders (which may be granted or withheld in the note holders' discretion):
consummate any sale of the Company or consent to the consummation of any sale of the Company;
increase or decrease the total number of authorized shares of common stock of the Company, except in connection with any capital raising securities issuance (including, without limitation, any Financing);
pay compensation to any employee of the Company in excess of $180,000 per year;
declare or pay any dividends or make any other distributions to the holders of common stock of the Company;
change the authorized number of directors of the Company to more than five or less than three;
incur any future indebtedness in excess of $20,000 in the aggregate other than deferred expenses that the Company and payee thereof agree can be converted into convertible debt, however any additional indebtedness of any kind shall be expressly made subordinate to this Note; or
change the principal business of the Company or enter into a new line of business.
The secured convertible term notes grant a security interest in and to all of the Company’s right, title and interest the Company’s assets, tangible and intangible, wherever located, whether now existing or acquired in the future, including, but not limited to (i) all fixtures and personal property of every kind; and (ii) all proceeds and products derived from the Company’s assets; and all books and records.
5. Fair Value Measurements
The Company uses fair value measurements to record fair value adjustments to certain assets and liabilities and to determine fair value disclosures of financial instruments on a recurring basis.
| F-13 |
Fair Value Hierarchy
The Fair Value Measurements Topic of FASB’s ASC 820 establishes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to measurements involving significant unobservable inputs (Level 3 measurements). The three levels of the fair value hierarchy are as follows:
Level 1 inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities that the Company has the ability to access at the measurement date.
Level 2 inputs are inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly or indirectly.
Level 3 inputs are unobservable inputs for the asset or liability.
Determination of Fair Value
Under the Fair Value Measurements Topic of the FASB Accounting Standards Codification, the Company bases its fair value on the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. It is the Company’s policy to maximize the use of observable inputs and minimize the use of unobservable inputs when developing fair value measurements, in accordance with the fair value hierarchy. Fair value measurements for assets and liabilities where there exists limited or no observable market data and, therefore, are based primarily upon management’s own estimates, are often calculated based on current pricing policy, the economic and competitive environment, the characteristics of the asset or liability and other such factors. Therefore, the results cannot be determined with precision and may not be realized in an actual sale or immediate settlement of the asset or liability. Additionally, there may be inherent weaknesses in any calculation technique, and changes in the underlying assumptions used, including discount rates and estimates of future cash flows, that could significantly affect the results of current or future value.
Valuation methodologies used for assets and liabilities recorded at fair value and for estimating fair value where it is practicable to do so for financial instruments not recorded at fair value are as follows:
Cash and cash equivalents, accounts receivable, and accounts payable
The Company considers all highly liquid investments with maturities of three months or less to be cash equivalents. In general, carrying amounts approximate fair value because of the short maturity of these instruments.
| F-14 |
Long-lived Assets
Long-lived assets are measured at fair value on a non-recurring basis and are classified in Level 3 of the fair value hierarchy. The fair value is estimated utilizing unobservable inputs, including appraisals on real estate as well as evaluations of the marketability and potential relocation of other assets in similar condition and similar market areas.
Debt
At December 31, 2019 and 2018, the Company’s convertible debt was carried at its face value plus accrued interest. Based on the financial condition of the Company, it is impracticable for the Company to estimate the fair value of its short and long-term debt.
Stock-Based Compensation
The Company records stock-based compensation based upon (i) the stock value pursuant to an independent valuation prepared in compliance with Internal Revenue Code Section 409A and Accounting Standards Codification 718 dated February 27, 2019 that assigned a fair market value to the Company's common stock at a price of $0.09 per share or (ii) its most recent sale of stock of $4.00 per share in December 2019.
Warrant Liability Measured and Recognized at Fair Value on a Recurring Basis
The following table presents the amounts of warrant liability measured at fair value on a recurring basis as of December 31, 2019 and 2018
The fair value of the warrant liability is generally measured using pricing models with no observable inputs. These measurements are classified as Level 3 within the fair value of hierarchy.
| Total | (Level 1) | (Level 2) | (Level 3) | |||||||||||||
| December 31, 2019 | ||||||||||||||||
| Warrant liability | $ | 229,244 | - | - | $ | 229,244 | ||||||||||
| December 31, 2018 | ||||||||||||||||
| Warrant liability | $ | - | - | - | - | |||||||||||
The Company has no instruments with significant off-balance sheet risk.
| F-15 |
6. Income Taxes
At December 31, 2019, the Company had net operating loss carryforwards for Federal income tax purposes of approximately $2,800,000 expiring in the years of 2020 through 2035. Utilization of the net operating losses may be subject to annual limitations provided by Section 382 of the Internal Revenue Code and similar State provisions.
The Tax Cuts and Jobs Act ("Tax Act") was enacted on December 22, 2017. Among numerous provisions, the Tax Act reduces the U.S. federal corporate tax rate from 35% to 21%, requires companies to pay a one-time transition tax on earnings of certain foreign subsidiaries that were previously tax deferred, and creates new taxes on certain foreign sourced earnings. As a result of the Tax Act, the Company remeasured certain deferred tax assets and liabilities based on the rates at which they are expected to reverse in the future, which is generally 21%.
Due to the net losses incurred, the Company recorded no income tax expense for the years ended December 31, 2019 and 2018.
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s deferred tax assets and liabilities as of December 31, 2019 and 2018 were as follows:
| 2019 | 2018 | |||||||
| Deferred tax assets, net: | ||||||||
| Net operating loss carryforwards | $ | 590,000 | $ | 300,000 | ||||
| Valuation allowance | 590,000 | 300,000 | ||||||
| Net deferred assets | $ | — | $ | — | ||||
The valuation allowance increased to approximately $590,000 at December 31, 2019, from $300,000 at December 31, 2018.
The following is a reconciliation of the tax provisions for the years ended December 31, 2019 and 2018 with the statutory Federal income tax rates:
| Percentage of Pre-Tax Income | ||||||||
| 2019 | 2018 | |||||||
| Statutory Federal income tax rate | 21.0 | % | 21.0 | % | ||||
| Loss generating no tax benefit | (21.0 | ) | (21.0 | ) | ||||
| Effective tax rate | — | — | ||||||
The Company did not have any material unrecognized tax benefits as of December 31, 2019 and 2018. The Company does not expect the unrecognized tax benefits to significantly increase or decrease within the next twelve months. The Company recorded no interest and penalties relating to unrecognized tax benefits as of and during the years ended December 31, 2019 and 2018. The Company is subject to U.S. federal income tax, as well as taxes by various state jurisdictions. The Company is currently open to audit under the statute of limitations by the federal and state jurisdictions for the years ending December 31, 2017 through 2019.
| F-16 |
7. Commitments and Contingencies
Litigation
The Company accrues for loss contingencies associated with outstanding litigation, claims and assessments for which management has determined it is probable that a loss contingency exists and the amount of loss can be reasonably estimated. Costs for professional services associated with litigation claims are expensed as incurred. As of December 31, 2019, the Company has not accrued or incurred any amounts for litigation matters.
Leases
The Company leased its headquarters on a month-to-month basis under a lease agreement that expired on August 31, 2019. For the years ended December 31, 2019 and 2018, rent expense amounted to $28,465 and $35,674, respectively.
Effective January 1, 2019, the Company adopted ASU No. 2016-02 (“ASU 2016-02”), Leases using the modified retrospective transition approach utilizing the effective date as the date of initial application. The adoption of the ASU had no impact on the Company’s financial statements due to the short-term nature of the lease agreement.
Non-Dilutive Warrant
On December 20, 2018, the Company issued a non-dilutive warrant that expires on December 20, 2025. The warrant is convertible into 5% of the outstanding equity of the Company at a purchase price of $1,250,000, and is valued at $229,244 and $0 at December 31, 2019 and 2018 respectively. As the company issues shares of common or preferred stock, this warrant liability is subject to increasing in value.
8. Stockholders’ Deficit
The Company is authorized to issue 13,225,000 shares of its common stock, par value $0.001 per share (the "Common Stock”), and 7,850,000 shares of preferred stock, par value $0.001 per share. The Company has designated 5,000,000 shares of preferred stock as Series A Preferred Stock.
The board of directors authorized a reverse split of the Common Stock on a 1-for-25 basis, whereby the Company issued to each of its stockholders one share of Common Stock for every 25 shares of Common Stock held by such stockholder. The reverse split was effective on May 29, 2019. The financial statements as of and for the year ended December 31, 2018 have been presented to give effect to the reverse split.
| F-17 |
In May 2018, the company purchased all of the 38,000 shares issued to one of the founding shareholders for a payment of $970, which were immediately retired and recorded as unissued stock. As a result of this transaction, the common stock account decreased by $38 and capital in excess of par value decreased by $912.
In the year ended December 31, 2019, the Company recorded $614,870 in stock-based compensation expense in conjunction with the issuance of Common Stock and options to purchase Common Stock. The Company also received cash proceeds of $489 from the issuance of shares of Common Stock to its Chief Executive Officer. A total of 4,227,722 common shares were issued in 2019.
The Company filed a preliminary offering circular dated September 10, 2019 to raise up to $20,000,000 through the use of an offering statement that has been qualified by the Securities and Exchange Commission under Tier II of Regulation A. The Company is offering up to 5,000,000 shares of Series A Preferred Stock, at a price of $4.00 per share, which may convert into shares of Common Stock on a one-for-one basis. On December 27, 2019, the Company executed a rolling close of its offering and issued 702,021 shares of Series A Preferred Stock. Gross proceeds amounted to $2,808,084 and offering costs, which were recorded as a reduction in capital in excess of par value, totaled $282,242, resulting in net proceeds of $2,526,544
The Series A Preferred Stock is convertible into Common Stock either at the discretion of the investor or automatically upon the occurrence of certain events, like effectiveness of registration of the Common Stock in an initial public offering. The rights and preferences associated with the Common Stock and Series A Preferred Stock are as follows:
Common Stock
Voting Rights
Each holder of the company’s Common Stock is entitled to one vote for each share on all matters submitted to a vote of the shareholders, including the election of directors. In addition, holders of our Common Stock are entitled to vote as a separate class for the election of two (2) directors of the company’s Board of Directors. Holders of our Preferred Stock may not vote on the election of these directors.
Dividend Rights
Holders of Common Stock are entitled to receive dividends, as may be declared from time to time by the Board of Directors out of legally available funds as detailed in the company’s Restated Articles. The company has never declared or paid cash dividends on any of its capital stock.
| F-18 |
Liquidation Rights
In the event of a voluntary or involuntary liquidation, dissolution, or winding up of the company, the holders of the Common Stock are entitled to share ratably in the net assets legally available for distribution to shareholders after the payment of all debts and other liabilities of the company. Holders of the Series A Preferred Stock are entitled to a liquidation preference that is senior to holders of the Common Stock, and therefore would receive dividends and liquidation assets prior to the holders of the Common Stock.
Series A Preferred Stock
Voting Rights
Each holder of the company’s Series A Preferred Stock is entitled to one vote for each share on all matters submitted to a vote of the shareholders, including the election of directors, subject to the following restrictions:
| The holders of our Common Stock are entitled to elect two (2) directors to the company’s Board of Directors as a standalone class. The Preferred Stockholders may not exercise any voting rights in the election of these directors. |
Holders of our Preferred Stock specifically have the right to vote with the holders of the Common Stock to elect:
| one (1) independent director to the company’s Board of Directors; and |
| any additional directors to the company’s Board of Directors after the elections outlined above. |
Each holder of Series A Preferred Stock will be entitled to one vote for each share of Common Stock into which such share of Preferred Stock could be converted. Fractional votes will not be permitted and if the conversion results in a fractional share, it will be disregarded.
Additionally, the holders of the Series A Preferred Stock are entitled to certain protective provisions that require the company to obtain the written consent or affirmative vote of a majority of the outstanding shares of Preferred Stock prior to effecting certain corporate actions, comprised of the following:
| a) | alter the rights, powers or privileges of the Preferred Stock in a way that adversely affects the Preferred Stock; |
| F-19 |
| b) | increase or decrease the authorized number of shares of any class or series of capital stock; |
| c) | authorize or create (by reclassification or otherwise) any new class or series of capital stock having rights, powers, or privileges set forth in the Certificate of Incorporation of the company, as then in effect, that are senior to or on a parity with any series of Preferred Stock; |
| d) | redeem or repurchase any shares of Common Stock or Preferred Stock (other than pursuant to employee or consultant agreements giving the company the right to repurchase shares upon the termination of services pursuant to the terms of the applicable agreement); |
| e) | declare or pay any dividend or otherwise make a distribution to holders of Preferred Stock or Common Stock; |
| f) | increase or decrease the number of directors of the company; | |
| g) | liquidate, dissolve, or wind-up the business and affairs of the company |
Dividend Rights
Holders of Series A Preferred Stock will be entitled to receive dividends as may be declared from time to time by the Board of Directors out of legally available funds and on a pari passu basis with holders of the Common Stock. The company has never declared or paid cash dividends on any of its capital stock.
Conversion Rights
Shares of Series A Preferred Stock will be convertible, at the option of the holder, at any time, into fully paid and nonassessable shares of the company’s Common Stock at the then-applicable conversion rate. Initially, the conversion rate will be one share of Common Stock per share of Series A Preferred Stock. The conversion rate is subject to adjustment in the event of stock splits, reverse stock splits or the issuance of a dividend or other distribution payable in additional shares of Common Stock.
Additionally, each share of Series A Preferred Stock will automatically convert into Common Stock:
| i | a) | immediately prior to the closing of a firm commitment underwritten public offering of the company’s Common Stock on Form S-1, registered under the Securities Act, at a per share price not less than the Original Issue Price (as defined below) adjusted for any stock dividends, combinations, splits, recapitalizations and the like, for a total offering proceeds $5,000,000 or more (before deduction of underwriters commissions and expenses); or | |
| b) | upon the affirmative election of the holders of a majority of the outstanding shares of Preferred Stock, voting as a single class and on an as-converted basis. |
| F-20 |
In either of these events, the shares will convert in the same manner as a voluntary conversion.
Right to Receive Liquidation Distributions
In the event of a liquidation, dissolution or winding up of the company, whether voluntary or involuntary, or certain other events (each a “Deemed Liquidation Event”) such as the sale or merger of the company, all holders of Series A Preferred Stock will be entitled to a liquidation preference that is senior to holders of the Common Stock. Holders of Series A Preferred Stock will receive a liquidation preference equal to the greater of (a) an amount for each share equal to the Original Issue Price for such share, adjusted for any stock dividends, combinations, splits, recapitalizations and the like (the “liquidation preference”) plus any declared but unpaid dividends with respect to such shares or (b) such amount per share as would have been payable had all shares of Series A Preferred Stock been converted into Common Stock immediately prior to such liquidation, dissolution or winding up or Deemed Liquidation Event. Initially, the liquidation preferences for the shares of Series A Preferred Stock will be $4.00 per share (the “Original Issue Price”).
If, upon such liquidation, dissolution, or winding up or Deemed Liquidation Event, the assets (or the consideration received in a transaction) that are distributable to the holders of Preferred Stock are insufficient to permit the payment to such holders of the full amount of their respective liquidation preference, then all of such funds will be distributed ratably among the holders of the Preferred Stock in proportion to the full amounts to which they would otherwise be entitled to receive.
After the payment of the full liquidation preference of the Series A Preferred Stock, the remaining assets of the company legally available for distribution (or the consideration received in a transaction), if any, will be distributed ratably to the holders of the Common Stock in proportion to the number of shares of Common Stock held by each such holder.
Drag Along Right
The investors’ rights agreement contains a “drag-along provision” related to the certain events, such as the sale, merger or dissolution of the company (a “Liquidating Event”). Investors who purchase Series A Preferred Stock agree that, if the board of directors, the majority of the holders of the company’s Common Stock, and the majority of the holders of the company’s Series A Preferred Stock vote in favor of such a Liquidating Event, then such holders of Series A Preferred Stock will vote in favor of the transaction if such vote is solicited, refrain from exercising dissenters’ rights with respect to Liquidating Event, and deliver any documentation or take other actions reasonably requested by the company or the other holders in connection with the Liquidating Event.
| F-21 |
Information Rights
The Company also agreed in the investors’ rights agreement to grant certain information rights to Series A Preferred Stockholders that invested $50,000 or more (“Major Purchasers”). The information rights provided to Major Purchasers include: (1) annual unaudited financial statements for each fiscal year of the company, including an unaudited balance sheet as of the end of such fiscal year, an unaudited income statement, and an unaudited statement of cash flows, all prepared in accordance with generally accepted accounting principles and practices; and (2) quarterly unaudited financial statements for each fiscal quarter of the company (except the last quarter of the company’s fiscal year), including an unaudited balance sheet as of the end of such fiscal quarter, an unaudited income statement, and an unaudited statement of cash flows, all prepared in accordance with generally accepted accounting principles and practices, subject to changes resulting from normal year-end audit adjustments. If the company has audited records of any of the foregoing, it will provide those in lieu of the unaudited versions.
Additional Rights and Participation Rights
The investors’ rights agreement grants Series A Preferred Stockholders and their transferees certain rights in connection with the Company’s next equity offering. If in its next equity offering after the date that an investor executes the investors’ rights agreement (the “Next Financing”) the company issues securities that (a) have rights, preferences or privileges that are more favorable than the terms of the Series A Preferred Stock or (b) provide all such future investors in the Next Financing contractual terms such as registration rights, the company agrees to provide substantially equivalent rights to the investor with respect to the Series A Preferred Stock (with appropriate adjustment for economic terms or other contractual rights), including the amount of the Series A preferred stock liquidating distributions, through the investor’s proxy, if applicable, subject to the investor’s execution of any documents, including, if applicable, investor rights, co-sale, voting, and other agreements, executed by the investors purchasing securities in the Next Financing (the “Next Financing Documents”), provided that certain rights may be reserved for investors with a minimum amount of investment in the Next Financing. Upon the execution and delivery of the Next Financing Documents, the investors’ rights agreement (excluding any then-existing and outstanding obligations) will be automatically amended and restated by and into the Next Financing Documents and will be terminated and of no further force or effect. As a result, the rights of investors who participate in any Next Financing will instead be governed by the Next Financing Documents.
| F-22 |
In the investors’ rights agreement, the Company also grants investors participation rights. Investors will have the right of first refusal to purchase the investor’s Pro Rata Share of any New Securities (each as defined below) that the company may issue in the Next Financing. The investor will have no right to purchase any New Securities if the investor cannot demonstrate to the Company’s reasonable satisfaction that the investor is at the time of the proposed issuance of New Securities eligible to purchase such New Securities under applicable securities laws. An investor’s “Pro Rata Share” means the ratio of (i) the number of shares of the company’s Common Stock issued or issuable upon conversion of the Series A Preferred Stock owned by the investor, to (ii) that number of shares of the company’s capital stock equal to the sum of (A) all shares of the company’s capital stock (on an as-converted basis) issued and outstanding, assuming exercise or conversion of all options, warrants and other convertible securities and promissory notes, and (B) all shares of the Company’s capital stock reserved and available for future grant under any equity incentive or similar plan.
“New Securities” means any shares of the Company’s capital stock to be issued in the Next Financing, including Common Stock or Preferred Stock, whether now authorized or not, and rights, options or warrants to purchase Common Stock or Preferred Stock, and securities of any type whatsoever that are, or may become, convertible or exchangeable into Common Stock or Preferred Stock.
Warrants
On December 20, 2018 the Company issued a 7-year non-dilutive cashless warrant to purchase (i) shares of common stock equal to five percent (5%), calculated on a post-exercise basis, of the fully diluted capitalization of the Company, as of the date or dates of exercise, plus (ii) shares of preferred stock of each class or series of preferred stock of the Company equal to five percent (5%), calculated on a post-exercise basis, of the total issued and outstanding number of preferred shares of the Company, as of the date or dates of exercise. At December 31, 2019, based upon the Black-Scholes valuation model, with assumptions including: (1) a term of 5.975 years; (2) a volatility rate of 19.8% (3) a discount rate of 1.69% and (4) zero dividends, the warrant had a nominal value. At December 31, 2018, based upon the Black-Scholes valuation model, with assumptions including: (1) a term of 7 years; (2) a volatility rate of 100% (3) a discount rate of 1.00% and (4) zero dividends, the warrant had a nominal value.
Stock Options
The Company adopted a stock option plan and granted stock options to nine qualified individuals. Based upon a February 27, 2019 valuation performed by an independent and qualified financial consultant, all stock option grants were valued at $0.09 cents per share, which represents the estimated market value of a share of common stock at a date that was close to the date of the grants. Each grant vest in approximately four years, and expires in ten years from the date of the grant. The weighted average remaining life of the grants is 9.5 years at December 31, 2019.
| F-23 |
Stock-based compensation expense from the option grants amounted to $5,303 for the year ended December 31, 2019. The remaining unrecognized option expense that will be recognized as the option grants vest is $32,625.
The following table summarizes the option grant activity for the years ended December 31, 2019 and 2018.
| Option Number of Shares | Option Exercise Price Per Share | $ | Average Exercise Price | |||||||||
| Options outstanding December 31, 2018 | - | - | $ | - | ||||||||
| Issued during year ended December 31, 2019 | 573,200 | $ 0.09 - $4.00 | $ | 1.29 | ||||||||
| Exercised/canceled during year ended December 31, 2019 | - | - | $ | - | ||||||||
| Options outstanding December 31, 2019 | 573,200 | $ 0.09 - $4.00 | $ | 1.29 | ||||||||
| Options exercisable, December 31, 2019 | 76,918 | $ 0.09 - $4.00 | $ | 0.84 | ||||||||
9. Loss Per Common Share
Loss per common share data was computed as follows:
| 2019 | 2018 | |||||||
| Net loss | $ | (1,796,265 | ) | $ | (712,268 | ) | ||
| Weighted average common shares outstanding | 2,896,015 | 103,333 | ||||||
| Effect of dilutive securities | — | — | ||||||
| Weighted average dilutive common shares outstanding | 2,896,015 | 103,333 | ||||||
| Earnings (loss) per common share – basic | $ | (0.62 | ) | $ | (6.89 | ) | ||
| Earnings (loss) per common share – diluted | $ | (0.62 | ) | $ | (6.89 | ) | ||
| F-24 |
For the year ended December 31, 2019, the Company excluded 702,021 shares of Common Stock issuable upon conversion of Series A Preferred Stock and 704,268 shares of Common Stock issuable upon the exercise of outstanding options and warrants to purchase Common Stock from the calculation of net loss per share because the effect would be anti-dilutive. For the year ended December 31, 2018, the Company excluded 4,469 shares of common stock issuable upon the exercise of outstanding warrants to purchase common stock from the calculation of net loss per share because the effect would be anti-dilutive. All of the Company’s debt is convertible into shares of common stock, however, the debt cannot be converted until certain contingencies are met. Consequently, any potentially issuable shares of common stock resulting from a debt conversion have not been considered.
10. Related Party Transactions
The Company has transactions with officers and directors or entities related to the officers and directors that are classified as related party transactions. Such transactions and balances as of and for the years ended December 31, 2019 and 2018 are as follows:
| 2019 | 2018 | |||||||
| Accounts payable | $ | 8,518 | $ | 31,128 | ||||
| Accrued interest payable | $ | 124,964 | $ | 7,227 | ||||
| Officer salary payable | $ | 88,125 | $ | 48,877 | ||||
| Current portion of long-term debt | $ | 1,205,000 | $ | 1,025,000 | ||||
| Long-term debt | $ | 48,000 | $ | 43,000 | ||||
The Company owes a board member $71,000 in notes payable at December 31, 2019 and 2018. The same board member is also owed $5,969 and $3,129 in accrued interest payable, and $4,000 and $26,488 in accounts payable at December 31, 2019 and 2018, respectively. This board member also received a stock option grant to purchase 180,000 shares of common stock at a price of $0.61 per share. The grant vests over a four-year period ending on May 27, 2023 and expires on May 27, 2029. The grant was valued at $12,193; $1,821 in expense was recorded in the year ended December 31, 2019 and $10,372 is the remaining unrecognized option expense at December 31, 2019.
The Company owes its Chief Executive Officer $4,518 and $4,611 in accounts payable and $88,125 and $48,877 in salary payable at December 31, 2019 and 2018, respectively. On May 27, 2019, the Chief Executive Officer received a stock option grant to purchase 160,000 shares of common stock at a price of $0.61 per share. The grant vests over a four-year period ending on May 27, 2023 and expires on May 27, 2029. The grant was valued at $10,838; $1,618 in expense was recorded in the year ended December 31, 2019 and $9,220 is the remaining unrecognized option expense at December 31, 2019.
| F-25 |
The Company owed its former Chief Executive Officer $76,000 in notes payable at December 31, 2018 and $4,098 in accrued interest payable. The $76,000 in debt and a total of $7,229 in accrued interest payable was paid in full on December 31, 2019.
As of December 31, 2019 and 2018, the Company owes a related-party lender notes payable of $800,000 and $800,000 and accrued interest payable of $117,295 and $69,295, respectively. This lender also holds the anti-dilutive warrant for which the Company has recorded a warrant liability of $229,244. See Notes 5 and 8.
Stock-based compensation to officers in the year ended December 31, 2019 amounted to $339,257. 1,957,080 shares of Common Stock issued to the Company's Chief Executive Officer were recorded as $176,137 in stock-based compensation and 1,812,447 shares of Common Stock issued to the Company's Chief Medical Officer were recorded as $163,120 in stock-based compensation. The Common Stock issuances were valued at a price of $0.09 per share based upon an independent valuation prepared in compliance with Internal Revenue Code Section 409A and Accounting Standards Codification 718.
11. Subsequent Events
On January 20, 2020, the Company hired a Vice President of Engineering. In addition to his base salary, he received a stock option (“Option”) to purchase 100,000 shares of Common Stock at an exercise price of $4.00 per share. 25% of the Option shall vest and become exercisable on his first anniversary if he continues to have a service relationship with the Company at such time. Thereafter, the remaining 75% of the Option shall vest and become exercisable in twelve equal 6-month installments over the six-year period following the first anniversary date, provided he continues to have a service relationship with the Company on each vesting date.
On February 24, 2020, the Company executed an industrial net lease at 3913 Todd Lane Austin, Texas, and moved its corporate headquarters to this location. The premises is approximately 4,100 square feet and the lease term is from March 1, 2020 to March 31, 2024. In the first year of the lease, the monthly base rent is $5,408, and it increases to $5,909 in the fourth year.
On April 24, 2020, the Company completed a raise of approximately $13,600,000 by selling its Series A Preferred Stock at $4.00 a share under an offering statement that was qualified by the Securities and Exchange Commission under Tier II of Regulation A. This amount represents approximately $10,800,000 additional gross proceeds since its first rolling close in December 2019.
The Company evaluated subsequent events through May 1, 2020, the date these original financial statements were available to be issued. There were no other material subsequent events that required recognition or additional disclosure in these financial statements.
| F-26 |
PART III
INDEX TO EXHIBITS
The documents listed in the Exhibit Index of this report are incorporated by reference or are filed with this report, in each case as indicated below.
44
*To be filed by amendment.
45
SIGNATURES
Pursuant to the requirements of Regulation A, the issuer certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form 1-A and has duly caused this Offering Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in Austin, State of Texas, on, August 28, 2020.
| MONOGRAM ORTHOPAEDICS, INC. | ||
| By | /s/ Benjamin Sexson | |
| Benjamin Sexson, Chief Executive Officer | ||
| Monogram Orthopaedics, Inc. | ||
| The following persons in the capacities and on the dates indicated have signed this Offering Statement. | ||
| /s/ Benjamin Sexson | ||
| Benjamin Sexson, Chief Executive Officer, Principal Financial Officer, Principal Accounting Officer, Director | ||
Date: August 28, 2020 | ||
| /s/ Doug Unis | ||
| Doug Unis, Director | ||
Date: August 28, 2020 | ||
| /s/ Noel Goddard | |
Noel Goddard, Director | |
Date: August 28, 2020 |
46