- ADCT Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
6-K Filing
ADC Therapeutics (ADCT) 6-KCurrent report (foreign)
Filed: 12 Jun 20, 8:32am
EXHIBIT 99.2

Presented at the Virtual Edition of the 25th European Hematology Association Congress (EHA25 Virtual), June 11 – 21, 2020 ADCT Investor Call European Hematology Association June 12, 2020

Presented at the Virtual Edition of the 25th European Hematology Association Congress (EHA25 Virtual), June 11 – 21, 2020 This presentation and the accompanying oral presentation have been prepared by ADC Therapeutics SA ("ADC Therapeutics“, “we” or “us”) for informational purposes only and not for any other purpose. Nothing contained in this presentation is, or should be constr ued as, a recommendation, promise or representation by the presenter or ADC Therapeutics or any officer, director, employee, agent or a dvi sor of ADC Therapeutics. This presentation does not purport to be all-inclusive or to contain all of the information you may desire. In formation provided in this presentation and the accompanying oral presentation speak only as of the date hereof. This presentation includes forward - looking statements, beliefs or opinions, including statements with respect to our business st rategies and plans, competitive position and our objectives for future operations and our financial performance. These forward - looking statem ents involve known and unknown risks and uncertainties, including those described in our filings with the U.S. Securities and Exch ang e Commission, many of which are beyond our control and all of which are based on our management's current beliefs and expectati ons about future events. These forward - looking statements include all matters that are not historical facts. Forward - looking statements ma y and often do differ materially from actual results. No assurance can be given that such future results will be achieved. Such forward - look ing statements contained in this document speak only as of the date of this document. We expressly disclaim any obligation or undertaking to up date these forward - looking statements contained in this document to reflect any change in our expectations or any change in events, conditi ons, or circumstances on which such statements are based unless required to do so by applicable law. No representations or warranties (e xpressed or implied) are made about the accuracy of any such forward - looking statements. Certain information contained in this presentation relates to or is based on studies, publications, surveys, and other data d eri ved from third - party sources and our own internal estimates and research. While we believe that these third - party sources to be reliable as of the date of this presentation, we have not independently verified, and we make no representation as to the adequacy, fairness, accuracy o r completeness of, any information obtained from third - party sources. In addition, all of the market data included in this present ation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Fi nally, although we believe our own internal research is reliable, such research has not been verified by any independent source. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy securities, nor shall there be an y sale of securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification und er the securities laws of any such state or jurisdiction. 2 2

Presented at the Virtual Edition of the 25th European Hematology Association Congress (EHA25 Virtual), June 11 – 21, 2020 ADCT Making Progress in 2020 • Successful initial public offering upsized to $267M • Initial $65M tranche of $115M Deerfield convertible debenture Raised Significant Capital to Fund Our Programs • Lonca oral presentation of Phase II pivotal data in RR DLBCL at virtual EHA • Lonca plus ibrutinib Phase I/II combination data e - poster at EHA • Additional clinical and early pipeline assets moving forward Progressing Our Clinical Trials and Pipeline • BLA submission on track for H2 2020 • Commercial buildout underway Preparing for Commercial Launch 3

Presented at the Virtual Edition of the 25th European Hematology Association Congress (EHA25 Virtual), June 11 – 21, 2020 Initial Results of a Phase 2 Study of Loncastuximab Tesirine, a Novel Pyrrolobenzodiazepine - Based Antibody - Drug Conjugate, in Patients With Relapsed or Refractory Diffuse Large B - Cell Lymphoma Carmelo Carlo - Stella, MD 1 , Pier Luigi Zinzani, MD 2 , Brad Kahl, MD 3 , Paolo F. Caimi, MD 4 , Melhem Solh, MD 5 , Kirit Ardeshna , MD 6 , Anastasios Stathis, MD 7 , Mehdi Hamadani, MD 8 , Weiyun Ai, MD, PhD 9 , Brian Hess, MD 10 , Juan Pablo Alderuccio, MD 11 , Jay Feingold, MD, PhD 12 , David Ungar, MD 12 , Shui He, PhD 12 , Yajuan Qin, MD, PhD 12 , John Radford, MD, FMedSci 13 1 Department of Oncology and Hematology, Humanitas Cancer Center, Humanitas University, Milan, Italy; 2 Institute of Hematology “ Seràgnoli ” University of Bologna, Bologna, Italy; 3 Department of Medicine, Oncology Division, Washington University, St. Louis, MO, USA; 4 University Hospitals Cleveland Medical Center/Case Western Reserve University, Cleveland, OH, USA; 5 Blood and Marrow Transplant Program at Northside Hospital, Atlanta, GA, USA; 6 Department of Hematology, University College London Hospitals NHS Foundation Trust, London, UK; 7 Oncology Institute of Southern Switzerland, Bellinzona , Switzerland; 8 Division of Hematology and Oncology, Medical College of Wisconsin, Milwaukee, WI, USA; 9 Division of Hematology and Oncology, Department of Medicine, University of California, San Francisco, CA, USA; 10 Division of Hematology and Medical Oncology, Department of Medicine, Medical University of South Carolina, Charleston, SC, USA; 11 Sylvester Comprehensive Cancer Center, University of Miami, Miami, FL, USA; 12 ADC Therapeutics America Inc., Murray Hill, NJ, USA; 13 University of Manchester and The Christie NHS Foundation Trust, Manchester Academic Health Centre, Manchester, UK

Presented at the Virtual Edition of the 25th European Hematology Association Congress (EHA25 Virtual), June 11 – 21, 2020 75 µg/kg 150 µg/kg 30 - min infusion Lonca Q3W for up to 1 year After 2 cycles First 2 cycles Q12W for up to 3 years Follow - up Total enrolment: 145 patients Futility requirements met: ORR for first 52 patients 1 LOTIS 2 Study Design: Single - arm, Open - label Phase 2 Study Primary objective: Evaluate efficacy, using ORR (central review), and safety of the full Phase 2 study population Patient population: Patients with R/R DLBCL following ≥2 lines of prior systemic therapy 5
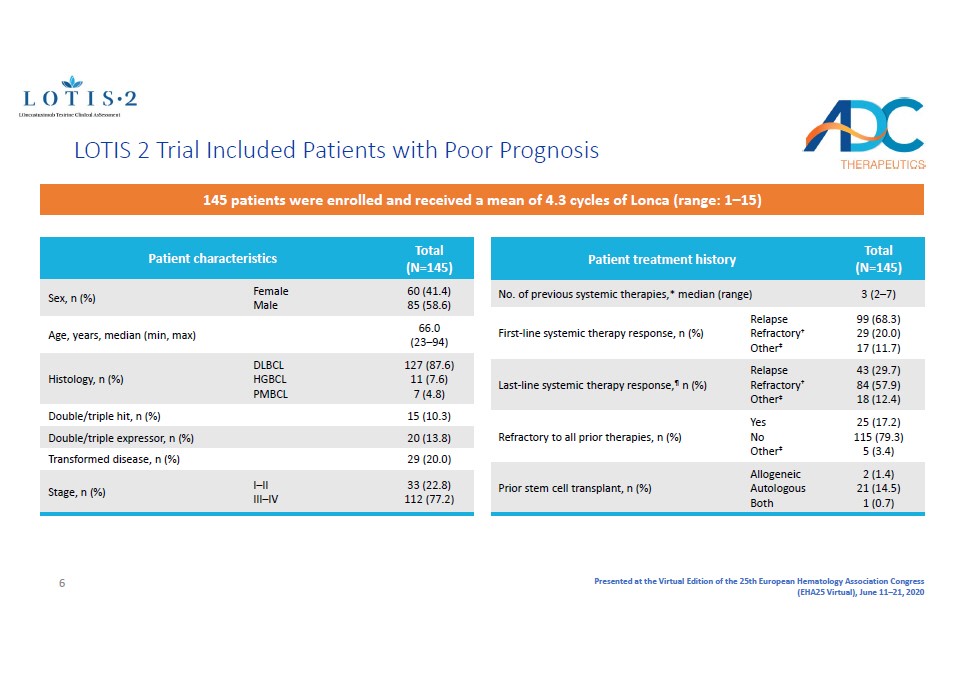
Presented at the Virtual Edition of the 25th European Hematology Association Congress (EHA25 Virtual), June 11 – 21, 2020 Patient characteristics Total (N=145) Sex, n (%) Female Male 60 (41.4) 85 (58.6) Age, years, median (min, max) 66.0 (23 – 94) Histology, n (%) DLBCL HGBCL PMBCL 127 (87.6) 11 (7.6) 7 (4.8) Double/triple hit, n (%) 15 (10.3) Double/triple expressor , n (%) 20 (13.8) Transformed disease , n (%) 29 (20.0) Stage, n (%) I – II III – IV 33 (22.8) 112 (77.2) Patient treatment history Total (N=145) No. of previous systemic therapies,* median (range) 3 (2 – 7) First - line systemic therapy response, n (%) Relapse Refractory † Other ‡ 99 (68.3) 29 (20.0) 17 (11.7) Last - line systemic therapy response, ¶ n (%) Relapse Refractory † Other ‡ 43 (29.7) 84 (57.9) 18 (12.4) Refractory to all prior therapies, n (%) Yes No Other ‡ 25 (17.2) 115 (79.3) 5 (3.4) Prior s tem cell transplant, n (%) Allogeneic Autologous Both 2 (1.4) 21 (14.5) 1 (0.7) 145 patients were enrolled and received a mean of 4.3 cycles of Lonca (range: 1 – 15) LOTIS 2 Trial Included Patients with Poor Prognosis 6

Presented at the Virtual Edition of the 25th European Hematology Association Congress (EHA25 Virtual), June 11 – 21, 2020 24.1 26.8 14.3 24.1 23.6 45.5 0 10 20 30 40 50 60 70 80 90 100 All patients (N=145) DLBCL-NOS (n=127) HGBCL (n=11) PMBCL (n=7) Response (%) Complete Response Partial Response 48.3 ( 70 /145) 50.4 (64/127) 45.5 (5/ 11 ) 14.3 (1/7) Total Population ORR 48.3% and CRR 24.1% ORR in the total population was 48.3% ( 95% CI: 39.9, 56.7) and an additional 15.2% (22 pts) had stable disease 7
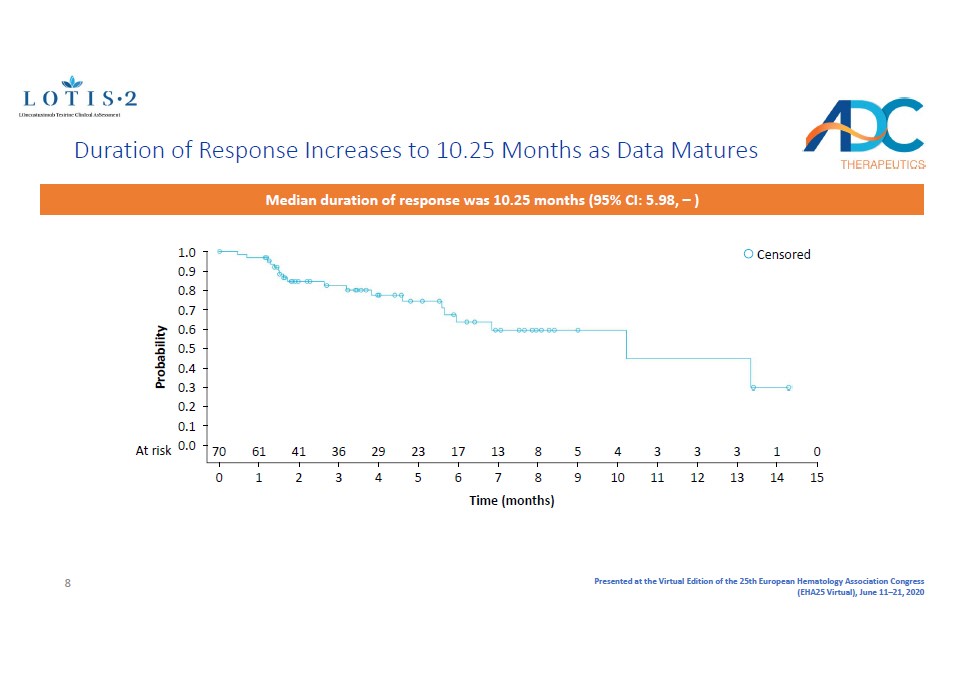
Presented at the Virtual Edition of the 25th European Hematology Association Congress (EHA25 Virtual), June 11 – 21, 2020 1.0 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0.0 At risk Probability 0 70 1 61 2 41 3 36 4 29 5 23 6 17 7 13 8 8 9 5 10 4 11 3 12 3 13 3 14 1 15 0 Time (months) Censored Duration of Response Increases to 10.25 Months as Data Matures Median duration of response was 10.25 months ( 95% CI: 5.98, – ) 8
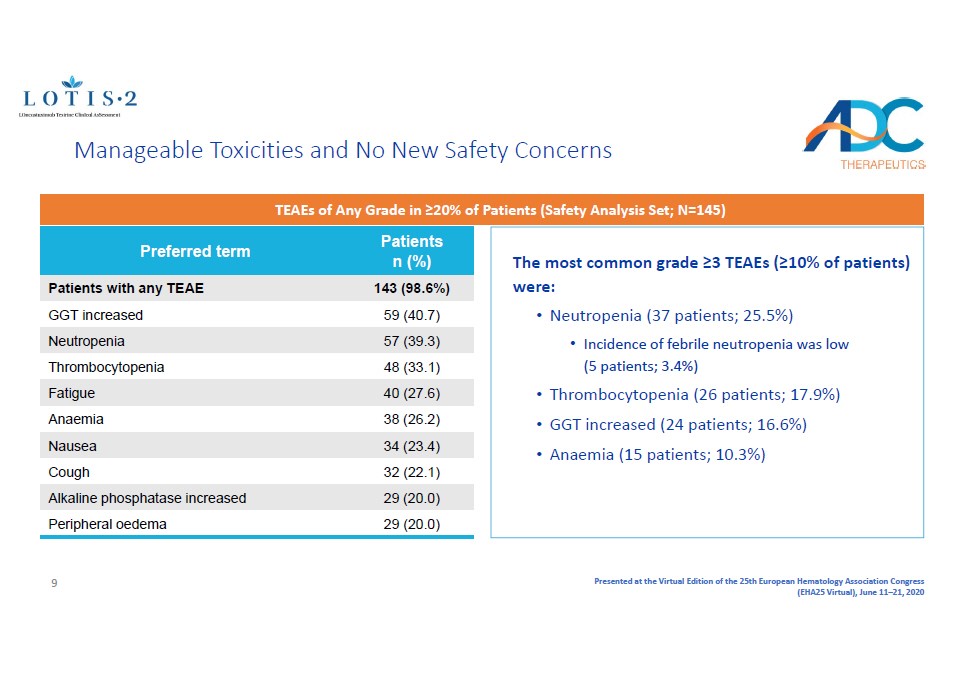
Presented at the Virtual Edition of the 25th European Hematology Association Congress (EHA25 Virtual), June 11 – 21, 2020 Manageable Toxicities and No New Safety Concerns Preferred term Patients n (%) Patients with any TEAE 143 (98.6%) GGT increased 59 (40.7) Neutropenia 57 (39.3) Thrombocytopenia 48 (33.1) Fatigue 40 (27.6) Anaemia 38 (26.2) Nausea 34 (23.4) Cough 32 (22.1) Alkaline phosphatase increased 29 (20.0) Peripheral oedema 29 (20.0) The most common grade ≥3 TEAEs (≥10% of patients) were: • Neutropenia (37 patients; 25.5%) • Incidence of febrile neutropenia was low (5 patients; 3.4%) • Thrombocytopenia (26 patients; 17.9%) • GGT increased (24 patients; 16.6%) • Anaemia (15 patients; 10.3%) TEAEs of Any Grade in ≥20% of Patients (Safety Analysis Set; N=145) 9
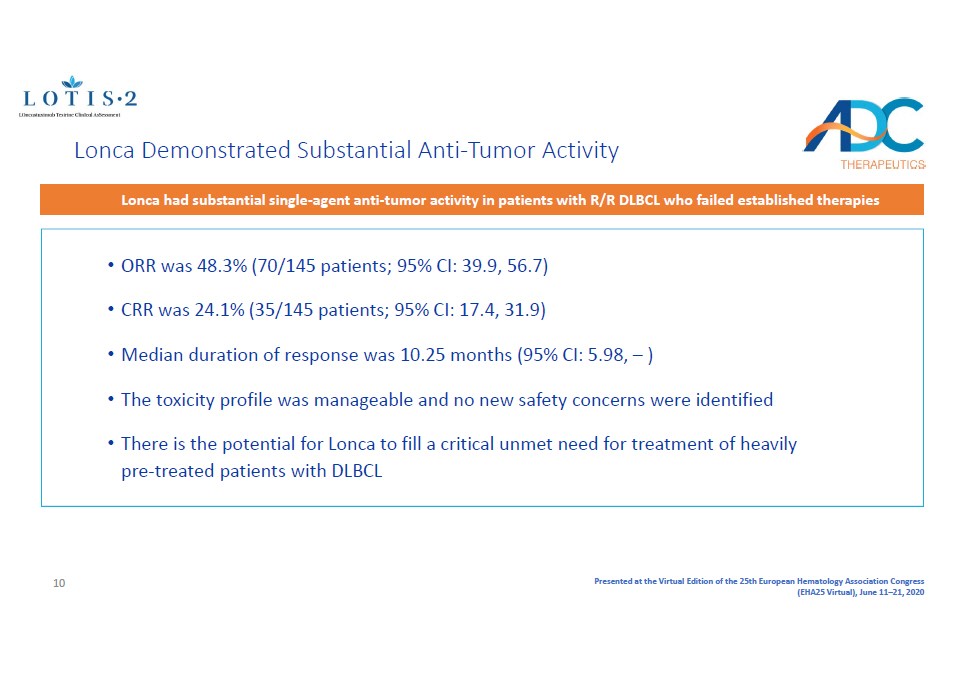
Presented at the Virtual Edition of the 25th European Hematology Association Congress (EHA25 Virtual), June 11 – 21, 2020 • ORR was 48.3% (70/145 patients; 95% CI: 39.9, 56.7 ) • CRR was 24.1% (35/145 patients; 95% CI: 17.4, 31.9) • Median duration of response was 10.25 months ( 95% CI: 5.98, – ) • The toxicity profile was manageable and no new safety concerns were identified • There is the potential for Lonca to fill a critical unmet need for treatment of heavily pre - treated patients with DLBCL Lonca Demonstrated Substantial Anti - Tumor Activity Lonca had substantial single - agent anti - tumor activity in patients with R/R DLBCL who failed established therapies 10

Presented at the Virtual Edition of the 25th European Hematology Association Congress (EHA25 Virtual), June 11 – 21, 2020 Interim results of a phase 1/2 study of loncastuximab tesirine ( Lonca ) combined with ibrutinib in advanced diffuse large B - cell lymphoma (DLBCL) or mantle cell lymphoma (MCL) Julien Depaus , MD 1 , Annette Ervin - Haynes, DO, MPA 2 , Carmelo Carlo - Stella, MD 3 , Pier Luigi Zinzani, MD 4 , Alessandro Rambaldi, MD 5,6 , Locke J. Bryan, MD 7 , Massimo Magagnoli , MD 3 , Giuseppe Gritti , MD, PhD 5 , Grace Chao, MS 2 , Joseph Boni, PhD 2 , Ilva Dautaj, MS, MBA 2 , Jennifer Adeleye, PhD 2 , Joseph Maly, MD 8 , Gilles Salles, MD 9 , Tycel Phillips, MD 10 , Nina Wagner - Johnston, MD 11 1 Department of Hematology , CHU UCL Namur site Godinne , Yvoir , Belgium; 2 Clinical Development, ADC Therapeutics, Murray Hill, NJ, USA; 3 Department of Oncology and Hematology, Humanitas Cancer Center, Humanitas University, Milan, Italy; 4 Institute of Hematology “ Seràgnoli ” University of Bologna, Bologna, Italy; 5 Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo, Italy; 6 Department of Oncology - Hematology, University of Milan, Milan, Italy; 7 Department of Medicine, Division of Hematology /Oncology, Georgia Cancer Center at Augusta University, Augusta, GA, USA; 8 Norton Cancer Institute, Louisville, KY, USA; 9 Department of Hematology , Hôpital Lyon Sud, Pierre - Bénite , France; 10 University of Michigan Comprehensive Cancer Center , Ann Arbor, MI, USA; 11 Department of Oncology, The Sidney Kimmel Comprehensive Cancer Center , Johns Hopkins University School of Medicine, Baltimore, MD, USA
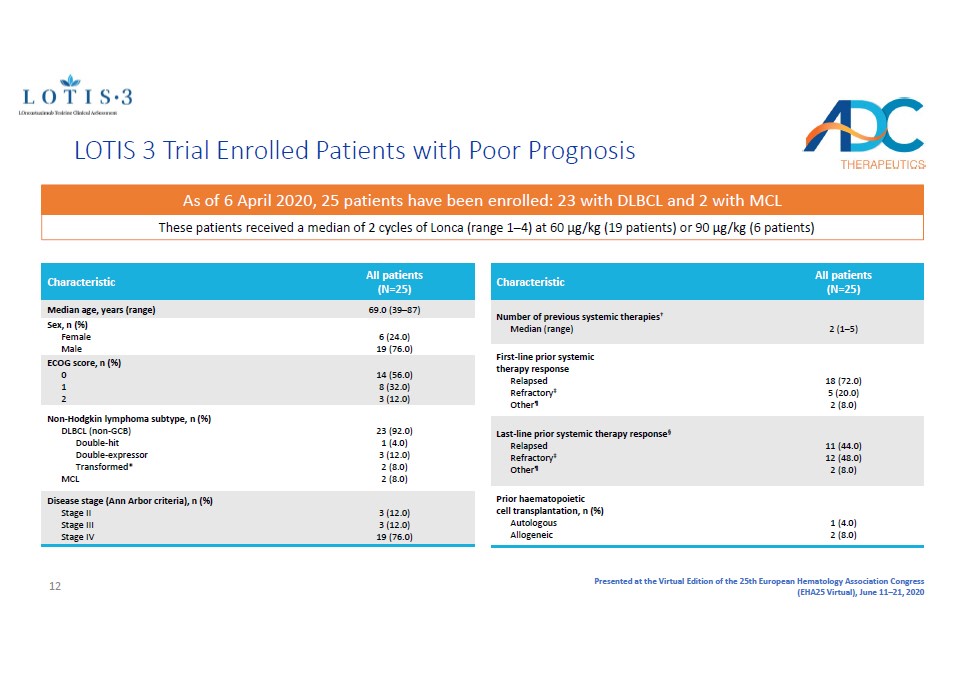
Presented at the Virtual Edition of the 25th European Hematology Association Congress (EHA25 Virtual), June 11 – 21, 2020 LOTIS 3 Trial Enrolled Patients with Poor Prognosis Characteristic All patients (N=25) Median age, years (range) 69.0 (39 – 87) Sex, n (%) Female Male 6 (24.0) 19 (76.0) ECOG score, n (%) 0 1 2 14 (56.0) 8 (32.0) 3 (12.0) Non - Hodgkin lymphoma subtype, n (%) DLBCL (non - GCB) Double - hit Double - expressor Transformed* MCL 23 (92.0) 1 (4.0) 3 (12.0) 2 (8.0) 2 (8.0) Disease stage (Ann Arbor criteria), n (%) Stage II Stage III Stage IV 3 (12.0) 3 (12.0) 19 (76.0) Characteristic All patients (N=25) Number of previous systemic therapies † Median (range) 2 (1 – 5) First - line prior systemic therapy response Relapsed Refractory ‡ Other ¶ 18 (72.0) 5 (20.0) 2 (8.0) Last - line prior systemic therapy response § Relapsed Refractory ‡ Other ¶ 11 (44.0) 12 (48.0) 2 (8.0) Prior haematopoietic cell transplantation, n (%) Autologous Allogeneic 1 (4.0) 2 (8.0) These patients received a median of 2 cycles of Lonca (range 1 – 4) at 60 µg/kg (19 patients) or 90 µg/kg (6 patients) As of 6 April 2020, 25 patients have been enrolled: 23 with DLBCL and 2 with MCL 12
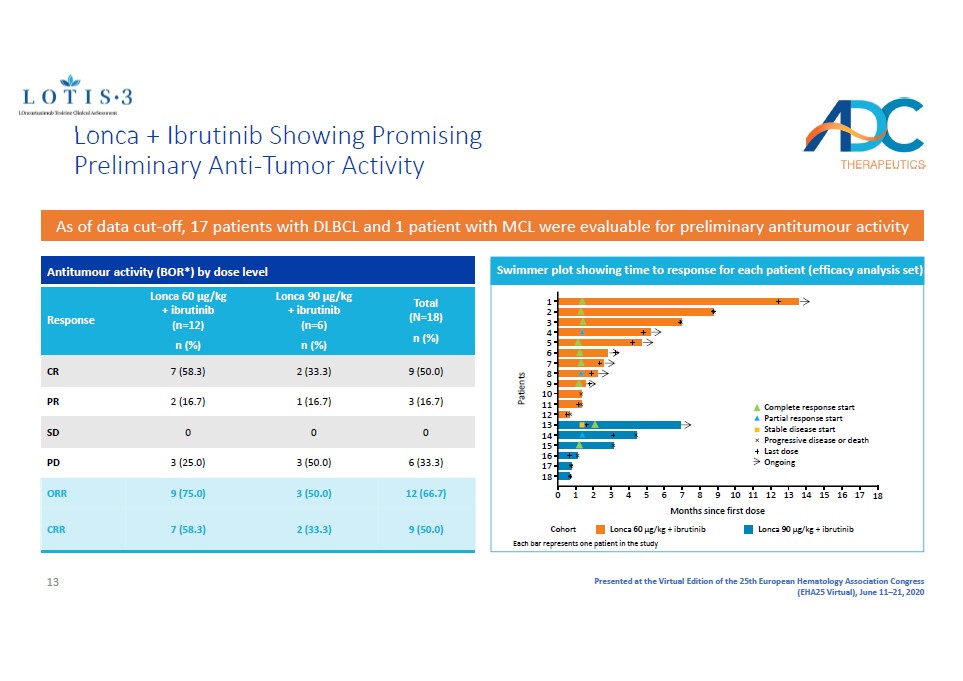
Presented at the Virtual Edition of the 25th European Hematology Association Congress (EHA25 Virtual), June 11 – 21, 2020 Lonca + Ibrutinib Showing Promising Preliminary Anti - Tumor Activity As of data cut - off, 17 patients with DLBCL and 1 patient with MCL were evaluable for preliminary antitumour activity Antitumour activity (BOR*) by dose level Response Lonca 60 µg/kg + ibrutinib (n=12) n (%) Lonca 90 µg/kg + ibrutinib (n=6) n (%) Total (N=18) n (%) CR 7 (58.3) 2 (33.3) 9 (50.0) PR 2 (16.7) 1 (16.7) 3 (16.7) SD 0 0 0 PD 3 (25.0) 3 (50.0) 6 (33.3) ORR 9 (75.0) 3 (50.0) 12 (66.7) CRR 7 (58.3) 2 (33.3) 9 (50.0) Lonca 60 µg/kg + ibrutinib Cohort Lonca 90 µg/kg + ibrutinib 0 1 3 6 9 12 18 17 16 15 14 13 11 10 8 7 5 4 2 18 14 9 5 1 17 16 15 13 12 11 10 8 7 6 4 3 2 Patients Complete response start Partial response start Stable disease start Progressive disease or death Last dose Ongoing Months since first dose Each bar represents one patient in the study Swimmer plot showing time to response for each patient (efficacy analysis set) 13
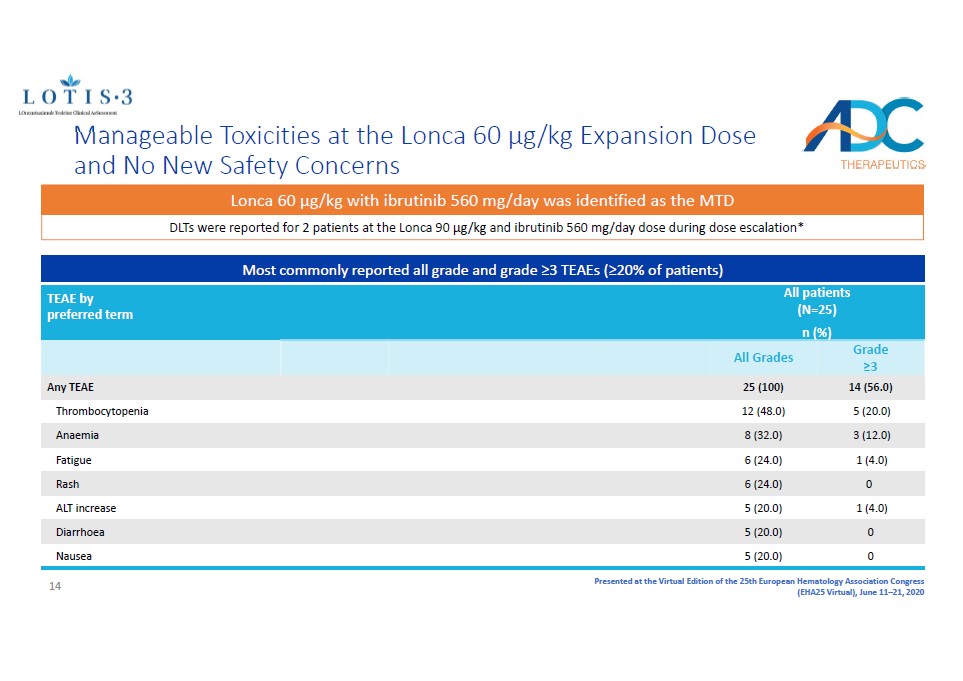
Presented at the Virtual Edition of the 25th European Hematology Association Congress (EHA25 Virtual), June 11 – 21, 2020 Manageable Toxicities at the Lonca 60 µg/kg Expansion Dose and No New Safety Concerns Most commonly reported all grade and grade ≥3 TEAEs (≥20% of patients) TEAE by preferred term All patients (N=25) n (%) All Grades Grade ≥3 Any TEAE 25 (100) 14 (56.0) Thrombocytopenia 12 (48.0) 5 (20.0) Anaemia 8 (32.0) 3 (12.0) Fatigue 6 (24.0) 1 (4.0) Rash 6 (24.0) 0 ALT increase 5 (20.0) 1 (4.0) Diarrhoea 5 (20.0) 0 Nausea 5 (20.0) 0 DLTs were reported for 2 patients at the Lonca 90 µg/kg and ibrutinib 560 mg/day dose during dose escalation* Lonca 60 µg/kg with ibrutinib 560 mg/day was identified as the MTD 14
Presented at the Virtual Edition of the 25th European Hematology Association Congress (EHA25 Virtual), June 11 – 21, 2020 • The combination has a manageable TEAE and toxicity profile at the MTD of Lonca 60 µg/kg with ibrutinib 560 mg/day • ORR at this dose level is 75%, with a CRR of 58.3% • In patients with DLBCL treated with Lonca 60 µg/kg, ORR is 72.7% with a CRR of 63.6% • PK profiles demonstrate good exposure throughout the dosing interval • This study is continuing to enroll patients Encouraging Anti - Tumor Activity for Lonca + Ibrutinib Interim results show encouraging anti - tumor activity for Lonca in combination with ibrutinib in patients with R/R DLBCL or MCL, with an overall ORR of 66.7% and a CRR of 50% 15

Presented at the Virtual Edition of the 25th European Hematology Association Congress (EHA25 Virtual), June 11 – 21, 2020 Thank you 16