
Phase 3 EMERGENT-3 Trial of KarXT in Schizophrenia �Topline Data Results March 20, 2023 Exhibit 99.2

Forward looking statements This presentation and other related material may contain a number of “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including statements regarding Karuna’s expectation about any or all of the following: (i) the timing, progress and results of preclinical studies and clinical trials for KarXT and other product candidates it may develop, including statements regarding the timing of initiation and completion of studies or trials and related preparatory work and the period during which the results of the trials will become available; (ii) Karuna’s research and development plans, including its plans to explore the therapeutic potential of KarXT in additional indications; (iii) Karuna’s plans to develop and commercialize KarXT, KAR-2618 and other product candidates; and (iv) the timing of and Karuna’s ability to submit for, obtain and maintain marketing approvals for its product candidates. Forward-looking statements can be identified by terms such as “could,” “expects,” “intends,” “may,” “plans,” “potential,” “should,” “will,” “would,” or similar expressions and the negative of those terms. Karuna has based these forward-looking statements largely on its current expectations and projections about future events and financial trends that it believes may affect its business, financial condition and results of operations. Although Karuna believes that such statements are based on reasonable assumptions, forward-looking statements are neither promises nor guarantees and they are necessarily subject to a high degree of uncertainty and risk. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond Karuna’s control, you should not rely on these forward-looking statements as predictions of future events. These risks and uncertainties include, among others: outcomes of Karuna’s planned and ongoing clinical trials and studies may not be favorable; one or more of Karuna’s product candidate programs may not proceed as planned for technical, scientific or commercial reasons; availability and timing of results from preclinical studies and clinical trials; uncertainty about regulatory approval to conduct clinical trials or to market products; uncertainties regarding intellectual property protection; risks relating to business interruptions resulting from the coronavirus (COVID-19) pandemic; and those risk and uncertainties described under the heading “Risk Factors” in Karuna’s Annual Report on Form 10-K for the year ended December 31, 2022, and filed with the Securities and Exchange Commission on February 23, 2023, and in any other subsequent filings made by Karuna with the U.S. Securities and Exchange Commission, which are available at www.sec.gov. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date they are made. Karuna disclaims any obligation or undertaking to update or revise any forward-looking statements contained in this presentation, other than to the extent required by law.

Introduction Bill Meury �President and Chief Executive Officer Phase 3 EMERGENT-3 trial results Steve Brannan, M.D. �Chief Medical Officer Building a leading neuroscience company Bill Meury Q&A Agenda 3 Bill Meury Steve Brannan, M.D. Steve Paul, M.D. President of R&D and Chief Scientific Officer Andrew Miller, Ph.D. Founder and Chief Operating Officer Troy Ignelzi�Chief Financial Officer Will Kane�Chief Commercial Officer


5 Sources: GBD 2017, Patel et al. 2014, Remington et al. 2016, Goff et al. 2011, Liebermann et al. 2005 One of the leading causes of disability worldwide, with onset in late-teens / early-adulthood 21M+ people living with schizophrenia globally Estimated potential life lost is 10-20 years compared to general population – partly attributed to cardiovascular & metabolic comorbidities and increased suicide rate Chronic psychiatric syndrome affecting how one thinks, feels, and behaves Schizophrenia
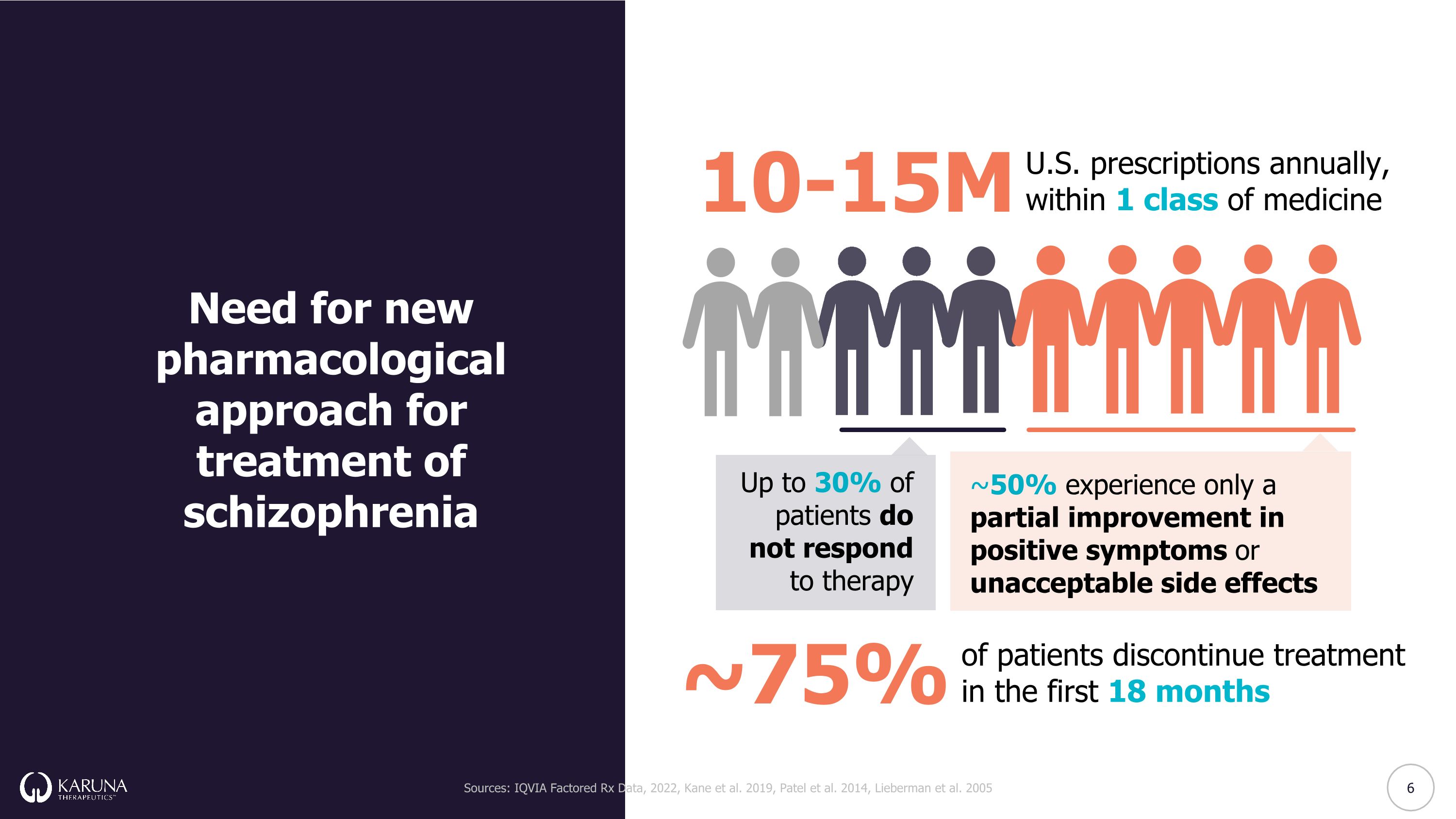
Up to 30% of patients do not respond to therapy ~50% experience only a partial improvement in positive symptoms or unacceptable side effects ~75% of patients discontinue treatment in the first 18 months 10-15M U.S. prescriptions annually, within 1 class of medicine Need for new pharmacological approach for treatment of schizophrenia Sources: IQVIA Factored Rx Data, 2022, Kane et al. 2019, Patel et al. 2014, Lieberman et al. 2005

Phase 3 �EMERGENT-3 �trial topline results Steve Brannan, M.D. Chief Medical Officer 7
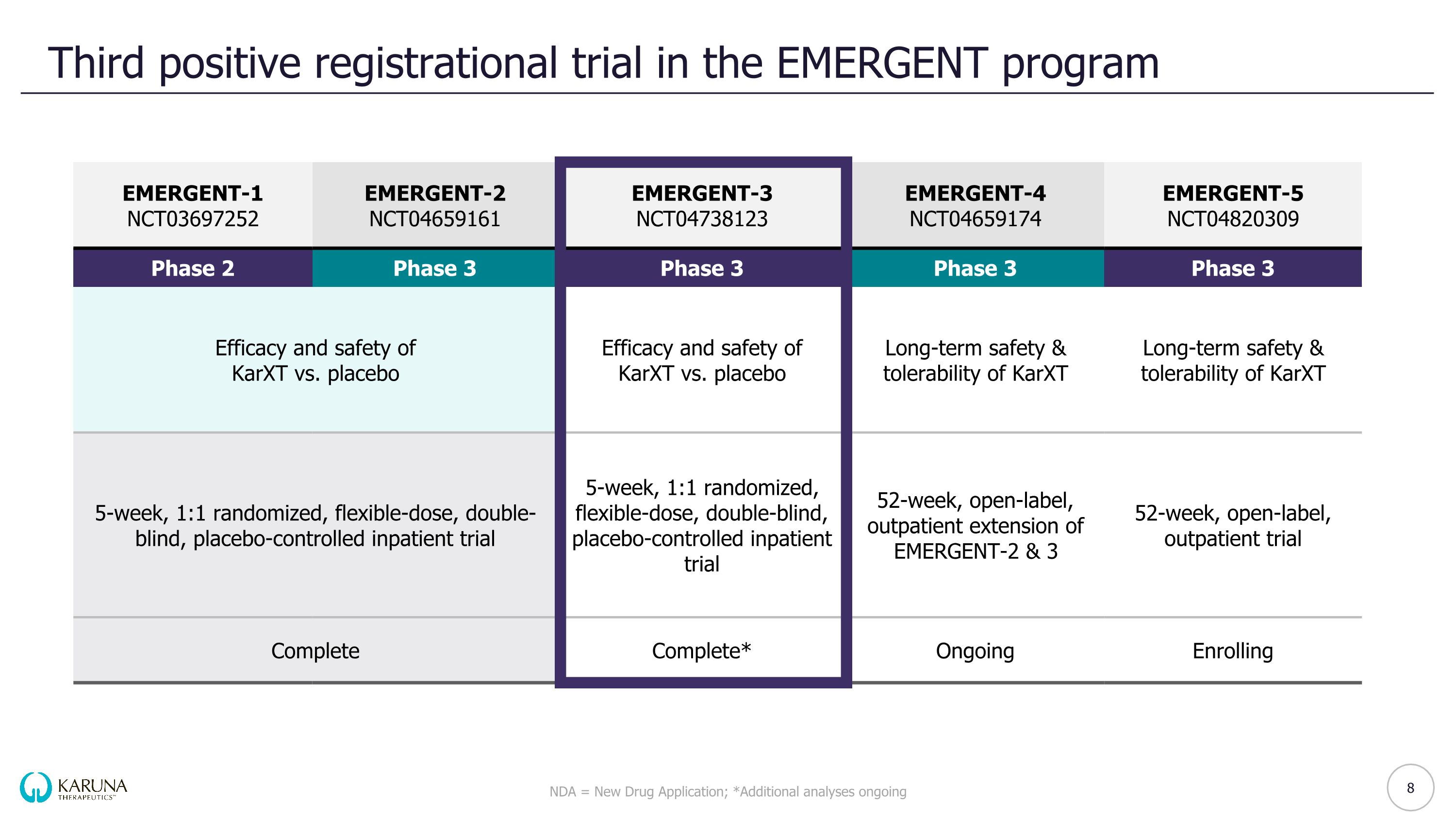
Third positive registrational trial in the EMERGENT program NDA = New Drug Application; *Additional analyses ongoing EMERGENT-1 NCT03697252 EMERGENT-2 NCT04659161 EMERGENT-3 NCT04738123 EMERGENT-4 NCT04659174 EMERGENT-5 NCT04820309 Phase 2 Phase 3 Phase 3 Phase 3 Phase 3 Efficacy and safety of�KarXT vs. placebo Efficacy and safety of�KarXT vs. placebo Efficacy and safety of�KarXT vs. placebo Long-term safety & tolerability of KarXT Long-term safety & tolerability of KarXT 5-week, 1:1 randomized, flexible-dose, double-blind, placebo-controlled inpatient trial 5-week, 1:1 randomized, flexible-dose, double-blind, placebo-controlled inpatient trial 5-week, 1:1 randomized, flexible-dose, double-blind, placebo-controlled inpatient trial 52-week, open-label, outpatient extension of EMERGENT-2 & 3 52-week, open-label, outpatient trial Complete Complete Complete* Ongoing Enrolling
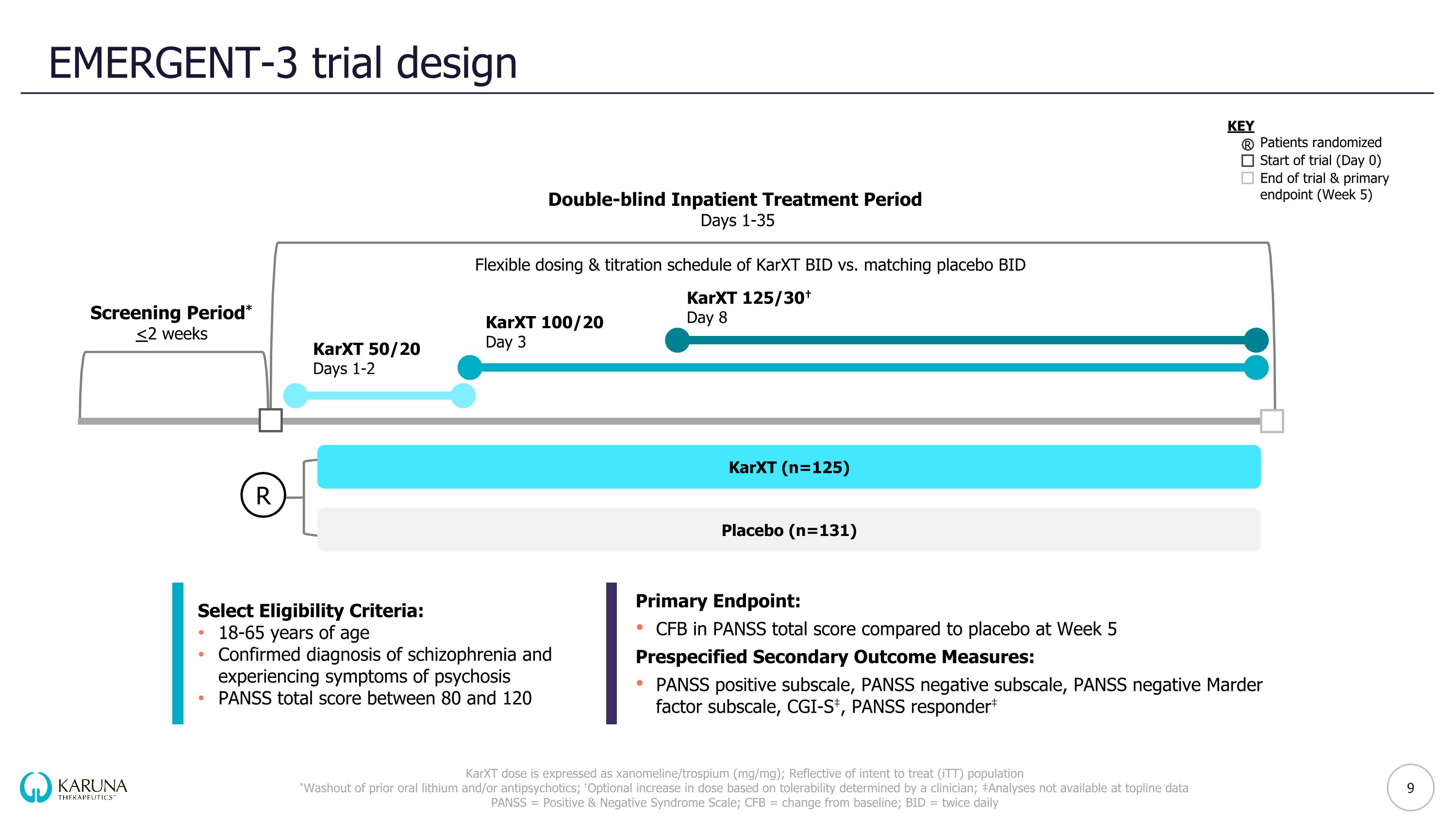
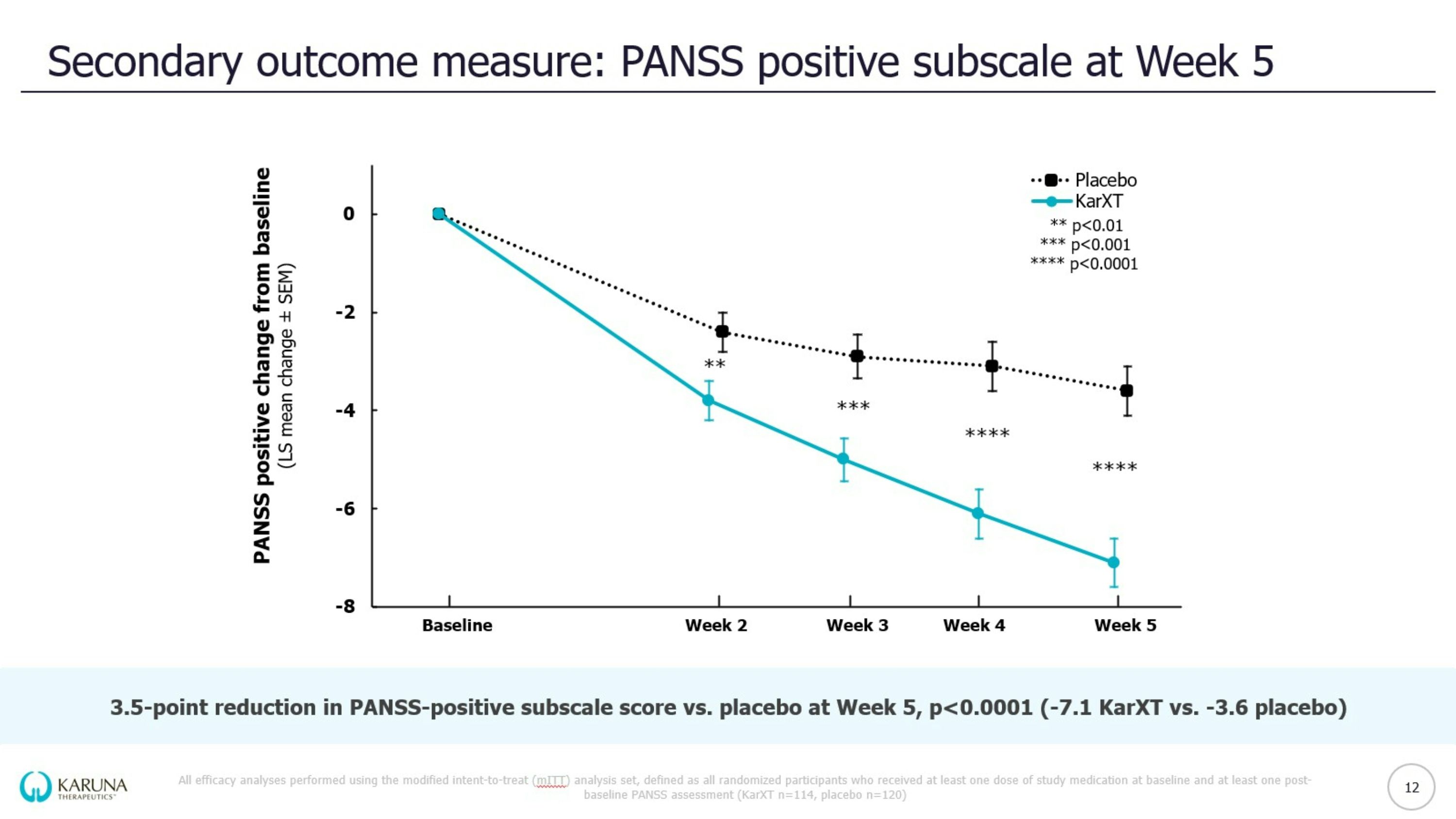
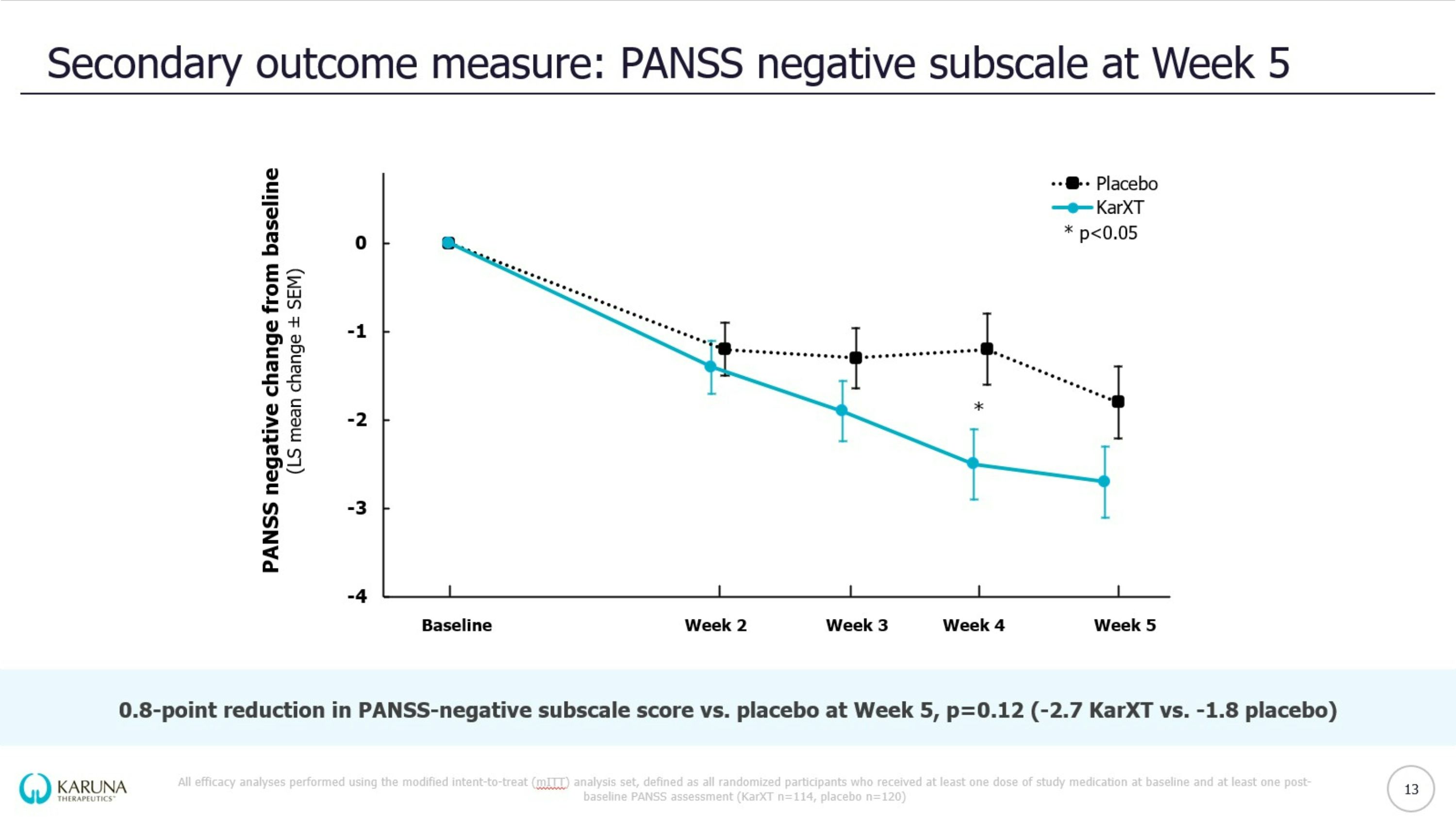
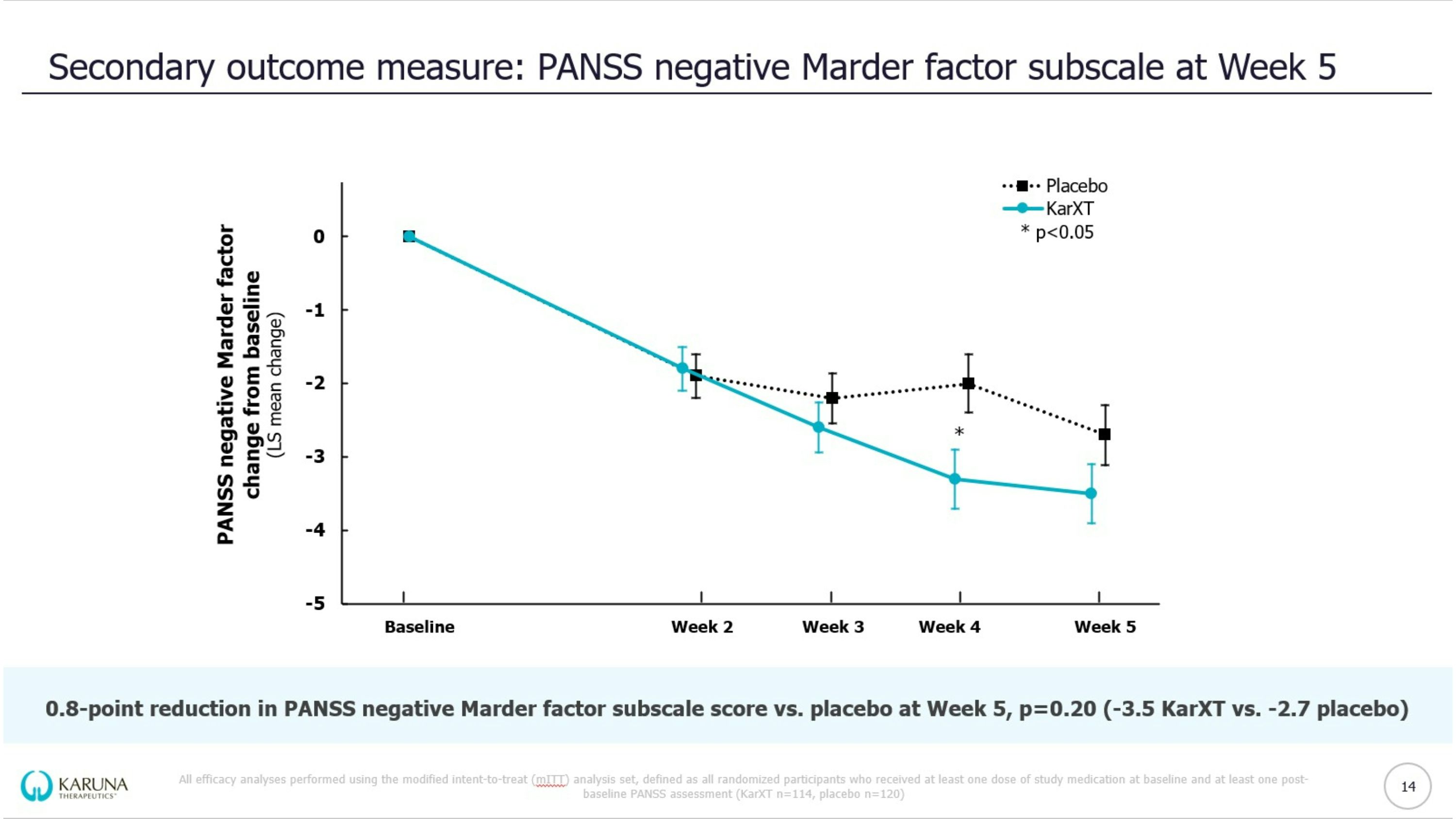
EMERGENT-3 trial design KarXT dose is expressed as xanomeline/trospium (mg/mg); Reflective of intent to treat (iTT) population *Washout of prior oral lithium and/or antipsychotics; †Optional increase in dose based on tolerability determined by a clinician; ‡Analyses not available at topline data PANSS = Positive & Negative Syndrome Scale; CFB = change from baseline; BID = twice daily Double-blind Inpatient Treatment Period Days 1-35 Screening Period* <2 weeks KarXT (n=125) Placebo (n=131) KEY Patients randomized Start of trial (Day 0) End of trial & primary endpoint (Week 5) Flexible dosing & titration schedule of KarXT BID vs. matching placebo BID R KarXT 50/20 Days 1-2 KarXT 100/20 Day 3 KarXT 125/30† Day 8 R 9 Select Eligibility Criteria: 18-65 years of age Confirmed diagnosis of schizophrenia and experiencing symptoms of psychosis PANSS total score between 80 and 120 Primary Endpoint: CFB in PANSS total score compared to placebo at Week 5 Prespecified Secondary Outcome Measures: PANSS positive subscale, PANSS negative subscale, PANSS negative Marder factor subscale, CGI-S‡, PANSS responder‡
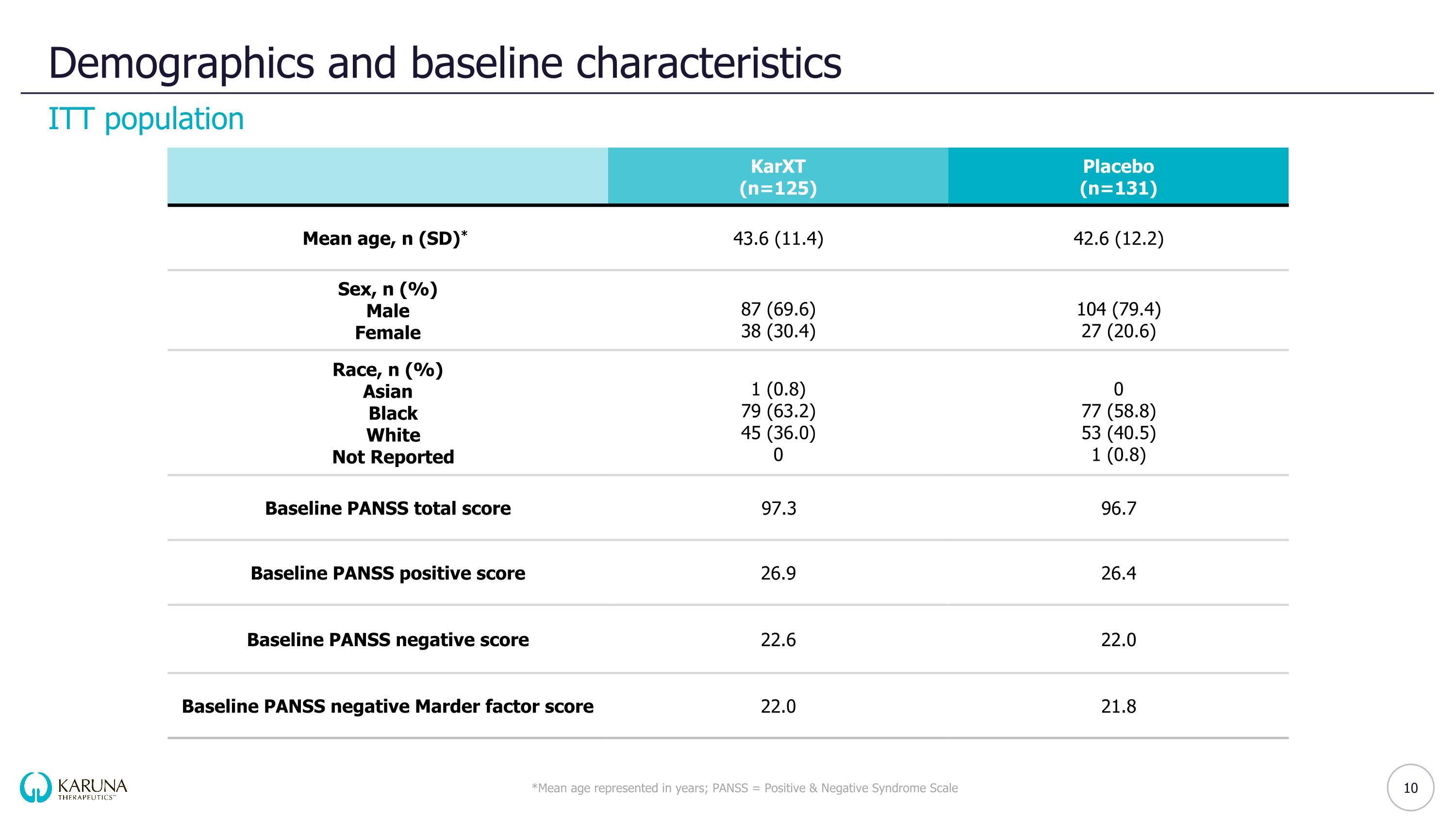
Demographics and baseline characteristics *Mean age represented in years; PANSS = Positive & Negative Syndrome Scale ITT population 10 KarXT (n=125) Placebo (n=131) Mean age, n (SD)* 43.6 (11.4) 42.6 (12.2) Sex, n (%) Male Female 87 (69.6) 38 (30.4) 104 (79.4) 27 (20.6) Race, n (%) Asian Black White Not Reported 1 (0.8) 79 (63.2) 45 (36.0) 0 0 77 (58.8) 53 (40.5) 1 (0.8) Baseline PANSS total score 97.3 96.7 Baseline PANSS positive score 26.9 26.4 Baseline PANSS negative score 22.6 22.0 Baseline PANSS negative Marder factor score 22.0 21.8
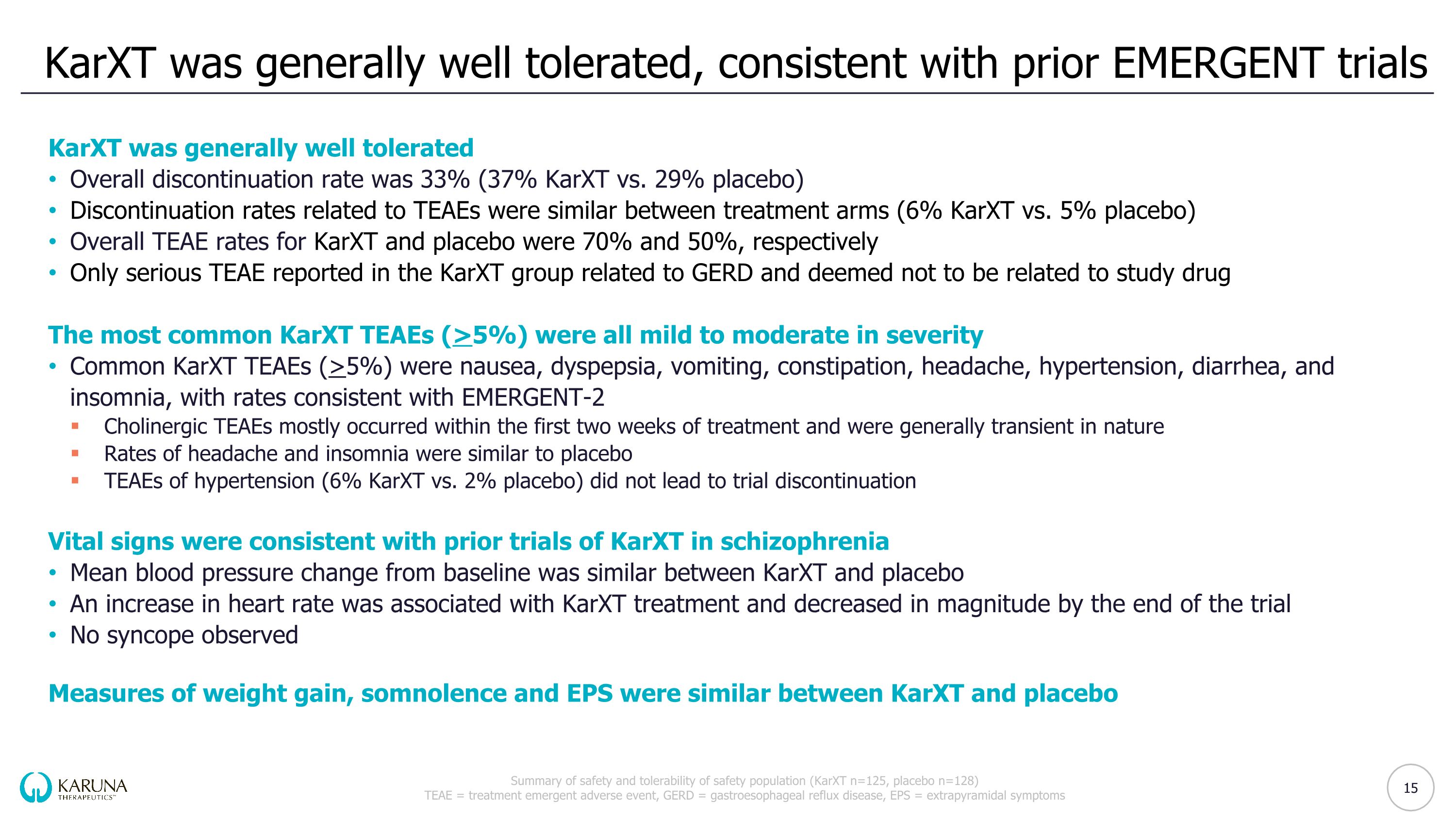
KarXT was generally well tolerated, consistent with prior EMERGENT trials Summary of safety and tolerability of safety population (KarXT n=125, placebo n=128) TEAE = treatment emergent adverse event, GERD = gastroesophageal reflux disease, EPS = extrapyramidal symptoms KarXT was generally well tolerated Overall discontinuation rate was 33% (37% KarXT vs. 29% placebo) Discontinuation rates related to TEAEs were similar between treatment arms (6% KarXT vs. 5% placebo) Overall TEAE rates for KarXT and placebo were 70% and 50%, respectively Only serious TEAE reported in the KarXT group related to GERD and deemed not to be related to study drug The most common KarXT TEAEs (>5%) were all mild to moderate in severity Common KarXT TEAEs (>5%) were nausea, dyspepsia, vomiting, constipation, headache, hypertension, diarrhea, and insomnia, with rates consistent with EMERGENT-2 Cholinergic TEAEs mostly occurred within the first two weeks of treatment and were generally transient in nature Rates of headache and insomnia were similar to placebo TEAEs of hypertension (6% KarXT vs. 2% placebo) did not lead to trial discontinuation Vital signs were consistent with prior trials of KarXT in schizophrenia Mean blood pressure change from baseline was similar between KarXT and placebo An increase in heart rate was associated with KarXT treatment and decreased in magnitude by the end of the trial No syncope observed Measures of weight gain, somnolence and EPS were similar between KarXT and placebo 15

Building a leading neuroscience company Bill Meury 16
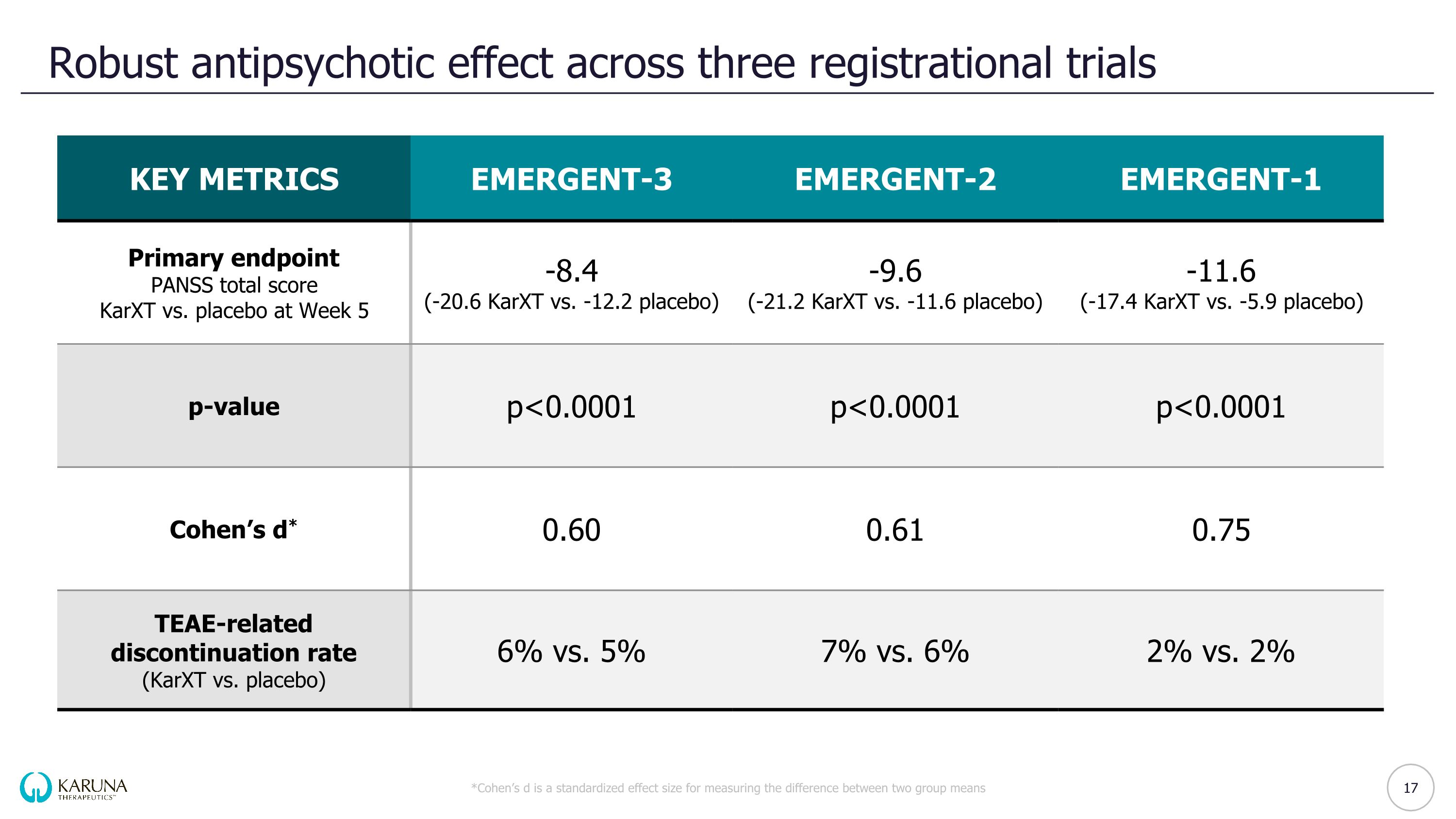
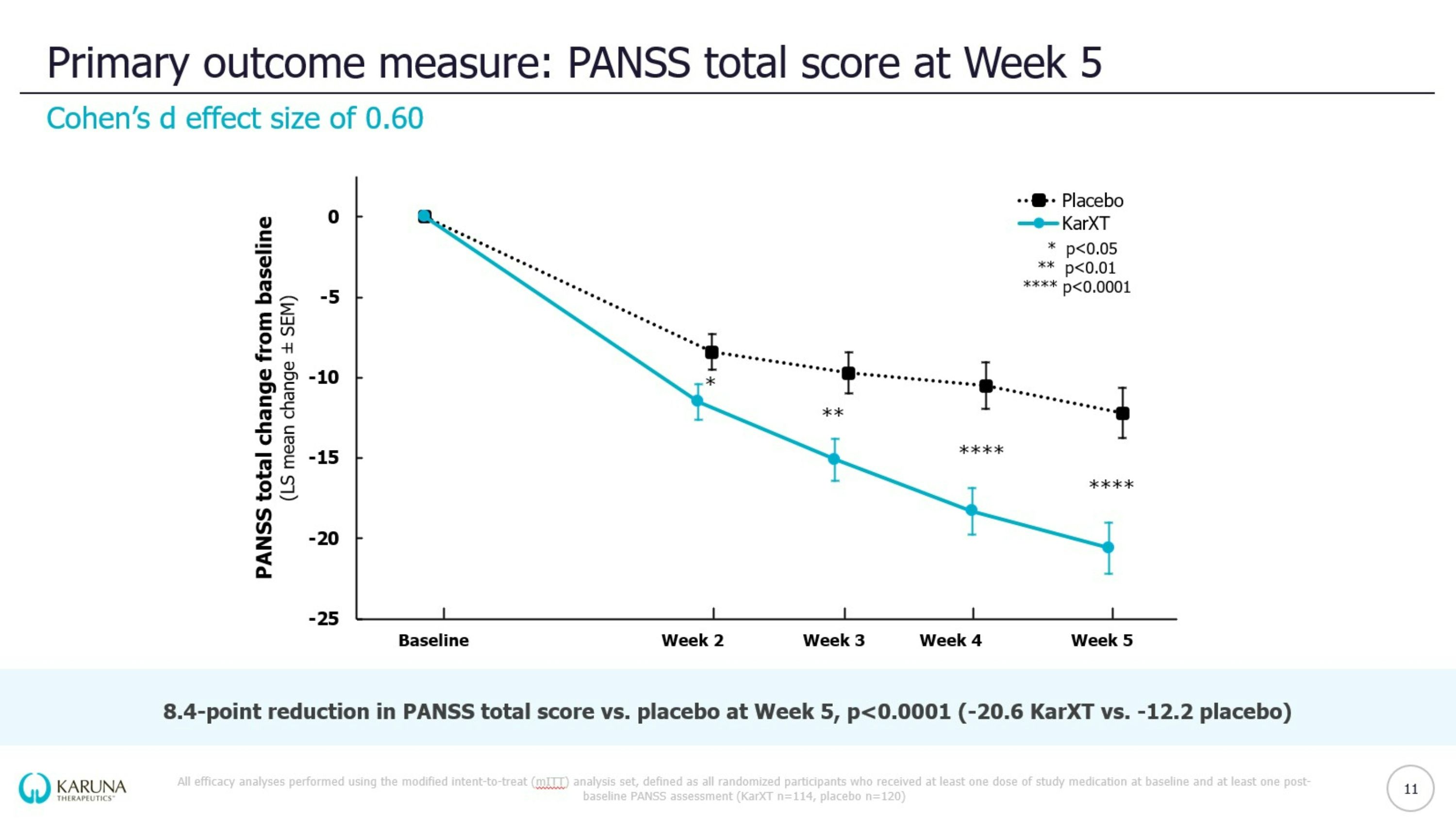
Robust antipsychotic effect across three registrational trials *Cohen’s d is a standardized effect size for measuring the difference between two group means KEY METRICS EMERGENT-3 EMERGENT-2 EMERGENT-1 Primary endpoint PANSS total score KarXT vs. placebo at Week 5 -8.4 (-20.6 KarXT vs. -12.2 placebo) -9.6 (-21.2 KarXT vs. -11.6 placebo) -11.6 (-17.4 KarXT vs. -5.9 placebo) p-value p<0.0001 p<0.0001 p<0.0001 Cohen’s d* 0.60 0.61 0.75 TEAE-related discontinuation rate (KarXT vs. placebo) 6% vs. 5% 7% vs. 6% 2% vs. 2% 17

18 KarXT has the potential to be a completely new and advanced treatment for people with schizophrenia Novel mechanism of action Early and sustained reduction of positive and negative symptoms of schizophrenia Generally well tolerated, with consistent side effect profile May not be associated with common AEs of current medications
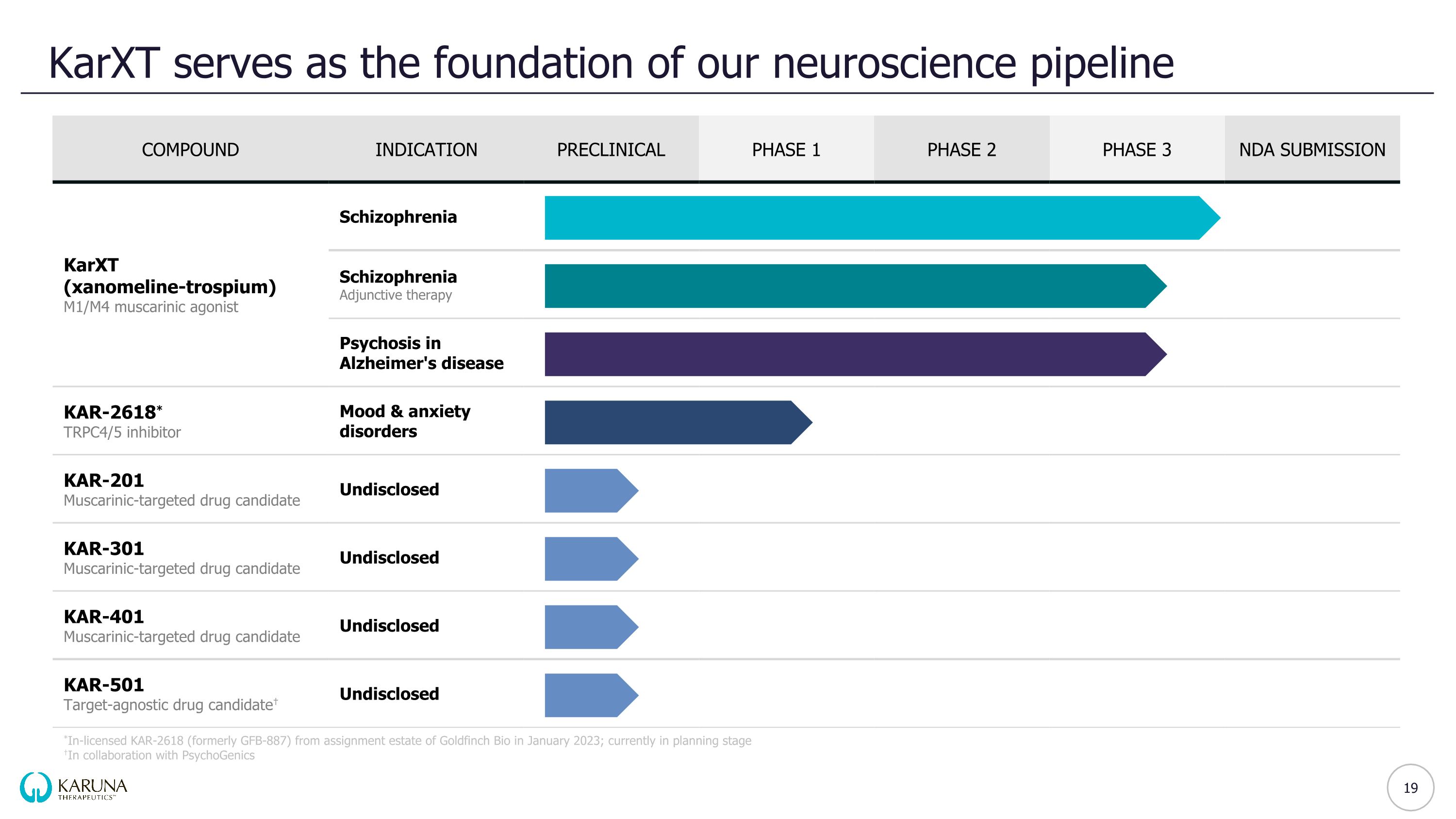
KarXT serves as the foundation of our neuroscience pipeline *In-licensed KAR-2618 (formerly GFB-887) from assignment estate of Goldfinch Bio in January 2023; currently in planning stage †In collaboration with PsychoGenics COMPOUND INDICATION PRECLINICAL PHASE 1 PHASE 2 PHASE 3 NDA SUBMISSION KarXT (xanomeline-trospium) M1/M4 muscarinic agonist Schizophrenia Schizophrenia Adjunctive therapy Psychosis in Alzheimer's disease KAR-2618* TRPC4/5 inhibitor Mood & anxiety disorders KAR-201 Muscarinic-targeted drug candidate Undisclosed KAR-301 Muscarinic-targeted drug candidate Undisclosed KAR-401 Muscarinic-targeted drug candidate Undisclosed KAR-501 Target-agnostic drug candidate† Undisclosed
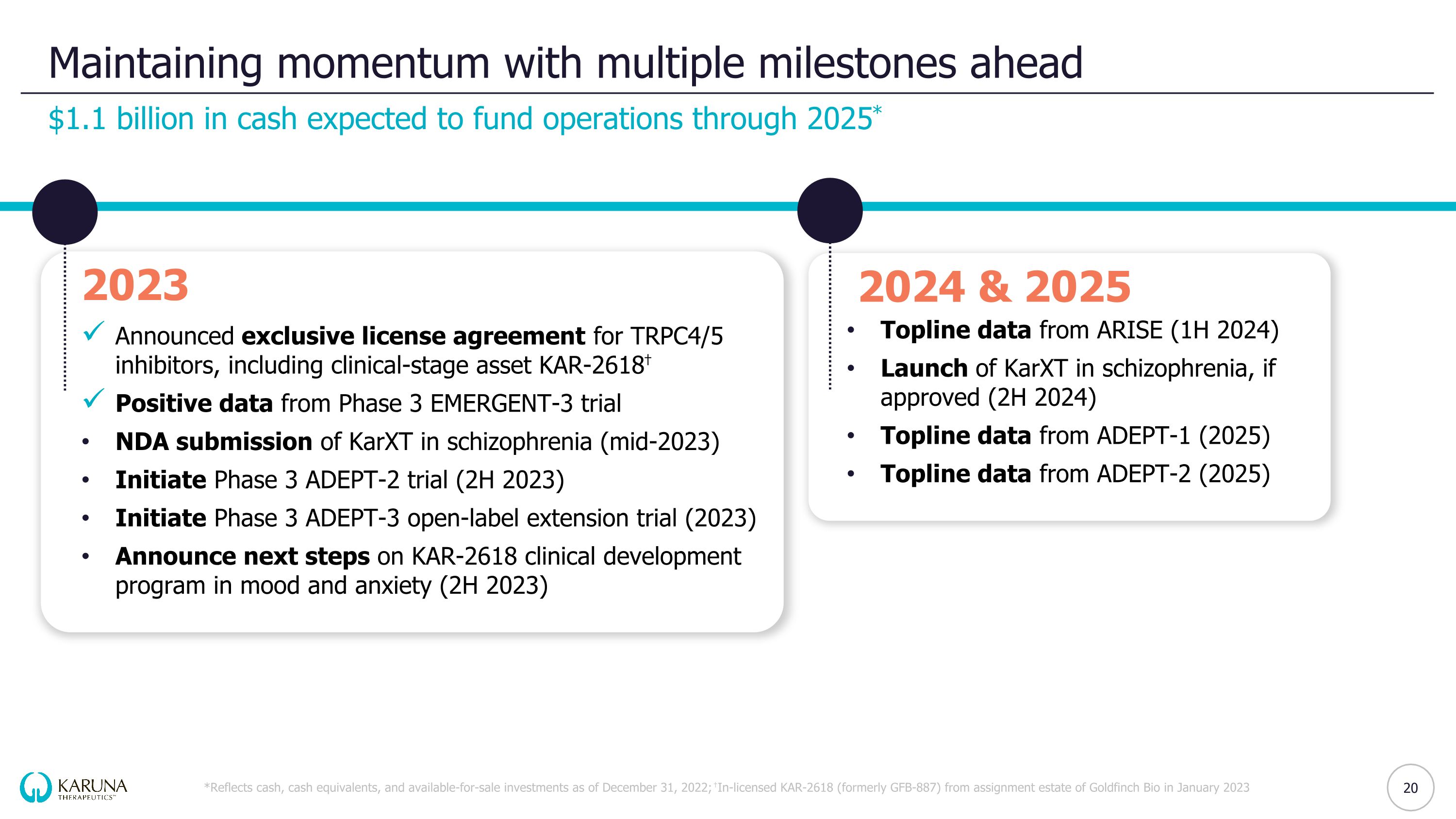
Maintaining momentum with multiple milestones ahead $1.1 billion in cash expected to fund operations through 2025* *Reflects cash, cash equivalents, and available-for-sale investments as of December 31, 2022; †In-licensed KAR-2618 (formerly GFB-887) from assignment estate of Goldfinch Bio in January 2023 2023 Announced exclusive license agreement for TRPC4/5 inhibitors, including clinical-stage asset KAR-2618† Positive data from Phase 3 EMERGENT-3 trial NDA submission of KarXT in schizophrenia (mid-2023) Initiate Phase 3 ADEPT-2 trial (2H 2023) Initiate Phase 3 ADEPT-3 open-label extension trial (2023) Announce next steps on KAR-2618 clinical development program in mood and anxiety (2H 2023) 2024 & 2025 Topline data from ARISE (1H 2024) Launch of KarXT in schizophrenia, if approved (2H 2024) Topline data from ADEPT-1 (2025) Topline data from ADEPT-2 (2025)
Q&A 21




















