r
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Amendment No. 1)
(Mark One)
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2023
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission file number 001-38958
Karuna Therapeutics, Inc.
(Exact name of registrant as specified in its charter)
| |
Delaware | 27-0605902 |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification Number) |
| |
99 High Street, 26th Floor Boston, Massachusetts | 02110 (Zip Code) |
(Address of principal executive offices) |
(857) 449-2244
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| | | | |
Title of each class | | Trading Symbol(s) | | Name of exchange on which registered |
Common Stock, $0.0001 Par Value | | KRTX | | The Nasdaq Global Market |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | |
Large accelerated filer | ☒ | Accelerated filer | ☐ |
Non-accelerated filer | ☐ | Smaller reporting company | ☐ |
|
| Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of June 30, 2023, the aggregate market value of the registrant’s Common Stock, $0.0001 par value per share, held by non-affiliates of the registrant, based on the last sale price of the Common Stock at the close of business on that date, was $5,872.6 million. For purposes of foregoing calculation only, all directors and executive officers of the registrant are assumed to be affiliates of the registrant.
As of March 12, 2024, there were 38,199,272 shares of the registrant’s Common Stock, $0.0001 par value per share, outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
EXPLANATORY NOTE
Karuna Therapeutics, Inc., is filing this Amendment No. 1 (this “Amendment No. 1”) to the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023 (the “2023 Annual Report”), filed with the Securities and Exchange Commission (the “SEC”) on February 22, 2024, only for the purpose of including the Part III information required under the instructions to Form 10-K and the general rules and regulations under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), which information was previously omitted from our 2023 Annual Report in reliance on General Instruction G(3) to Form 10-K, which permits the omitted information to be incorporated by reference in the 2023 Annual Report from our definitive proxy statement so long as such proxy statement is filed no later than 120 days after the end of the Company’s fiscal year. Except where the context otherwise requires or where otherwise indicated, the terms “Karuna,” “we,” “us,” “our,” “our company,” “the Company,” and “our business” refer to Karuna Therapeutics, Inc., a Delaware corporation, and its consolidated subsidiary.
This Amendment No. 1 amends and restates only Part III, Items 10 (Directors, Executive Officers and Corporate Governance), 11 (Executive Compensation), 12 (Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters), 13 (Certain Relationships and Related Transactions, and Director Independence) and 14 (Principal Accountant Fees and Services) of our 2023 Annual Report filed with the SEC on February 22, 2024, in their entirety as set forth herein. In addition, this Amendment No. 1 deletes the reference on the cover page of our 2023 Annual Report to the incorporation by reference of portions of our proxy statement into Part III of such 2023 Annual Report, as well as updates the outstanding share number referenced thereon. No other Items of our 2023 Annual Report filed with the SEC on February 22, 2024, have been amended or revised in this Amendment No. 1, and all such other Items shall be as set forth in such 2023 Annual Report.
In addition, pursuant to SEC rules, Item 15 of Part IV of our 2023 Annual Report filed with the SEC on February 22, 2024, is hereby amended solely to include, as Exhibits 31.3 and 31.4, new certifications of our principal executive officer and principal financial officer pursuant to Rule 13a-14(a) under the Exchange Act. Because no financial statements have been included in this Amendment No. 1, and because this Amendment No. 1 does not contain or amend any disclosure with respect to paragraphs 3, 4 and 5 of Items 307 and 308 of Regulation S-K, the corresponding certifications have been omitted. We are not including the certifications under Section 906 of the Sarbanes-Oxley Act of 2002 as no financial statements are being filed with this Amendment No. 1.
No other information has been updated for any subsequent events occurring after we filed our 2023 Annual Report with the SEC on February 22, 2024. Accordingly, this Amendment No. 1 should be read in conjunction with the 2023 Annual Report and our other filings made with the SEC subsequent to the filing of such 2023 Annual Report.
Karuna Therapeutics, Inc.
Index
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
DIRECTOR BIOGRAPHIES
The following table sets forth information concerning our directors as of March 14, 2024. The biographical description of each director includes the specific experience, qualifications, attributes and skills that the board of directors would expect to consider if it were making a conclusion currently as to whether such person should serve as a director.
| | |
CLASS I DIRECTORS – TERM EXPIRING AT THE 2026 ANNUAL MEETING OF STOCKHOLDERS | AGE | DIRECTOR SINCE |
| | |
Bill Meury has served as our President and Chief Executive Officer, and as member of our board of directors, since January 2023. Mr. Meury is currently Crown Chairman and Senior Operating Advisor at Hildred Capital Management, a private equity firm focusing on the healthcare industry, where he was a partner from May 2020 to December 2022. He previously served as Executive Vice President and Chief Commercial Officer of Allergan from May 2016 until Allergan’s acquisition by AbbVie in May 2020. Prior to this role, he served as Allergan’s President, Branded Pharma from March 2015 to May 2016, and Executive Vice President, Commercial, North American Brands from July 2014 to March 2015. Mr. Meury served as Executive Vice President, Sales and Marketing at Forest Laboratories prior to its acquisition by Allergan (then known as Actavis). He joined Forest in 1993 and held multiple roles of increasing responsibility in Marketing, New Products, Business Development, and Sales. Before joining Forest, Mr. Meury worked in public accounting for Reznick Fedder & Silverman and in financial reporting for MCI Communications. Mr. Meury serves on the board of directors of Syndax Pharmaceuticals, Inc. (Nasdaq: SNDX) and The Jed Foundation. He received a B.S. in Economics from the University of Maryland. Our board of directors believes that Mr. Meury is qualified to serve on our board of directors due to his extensive experience in the pharmaceutical industry as well as his expertise launching and commercializing medicines for psychiatric and neurological conditions. | 56 | January 2023 |
|
|
|
Laurie Olson has served as a member of our board of directors since August 2020. Ms. Olson worked at Pfizer Inc. from 1987 until 2018, serving in various roles of increasing authority during her tenure there, including Executive Vice President–Strategy and Commercial Operations from 2012 to 2018, Senior Vice President–Portfolio Management and Analysis from 2008 to 2012, Vice President–Commercial Development from 2006 to 2008 and Vice President–Worldwide Marketing from 2002 to 2006. Ms. Olson currently serves on the board of directors of Quanterix Corporation (Nasdaq: QTRX) and as a member of the Board of Trustees of the Mystic Seaport Museum in Mystic, Connecticut. Ms. Olson received a B.A. in Economics from State University of New York at Stony Brook and a Master of Business Administration in Marketing from Hofstra University. Our board of directors believes that Ms. Olson is qualified to serve on our board of directors due to her extensive experience as an executive in the pharmaceutical industry. | 61 | August 2020 |
|
|
|
David Wheadon, M.D., has served as a member of our board of directors since December 2020. Dr. Wheadon served as Senior Vice President, Global Regulatory Affairs, Patient Safety and Quality Assurance for AstraZeneca Pharmaceuticals from 2014 to 2019 and as Executive Vice President, Research and Advocacy at Juvenile Diabetes Research Foundation (JDRF) from 2013 to 2014. From 2009 to 2013, Dr. Wheadon served as Senior Vice President, Scientific and Regulatory Affairs and as a member of the Management Committee of the Pharmaceutical Research and Manufacturers of America (PhRMA). Prior to his joining PhRMA, Dr. Wheadon held senior regulatory and clinical development leader roles at Abbott Laboratories and GlaxoSmithKline plc. Dr. Wheadon began his career as a clinical research physician in neuroscience at Eli Lilly. Dr. Wheadon currently serves on the board of directors of PSKW, LLC (d/b/a ConnectiveRx), Vaxart, Inc. (Nasdaq: VXRT) and Sotera Health Company (Nasdaq: SHC), and formerly served on the board of directors of ChemoCentryx, Inc. and Assertio Holdings, Inc. (formerly Assertio Therapeutics, Inc.). Dr. Wheadon holds an A.B. from Harvard College and an M.D. from Johns Hopkins University School of Medicine. He completed his postgraduate training in Psychiatry at the Tufts, New England Medical Center. Our board of directors believes Dr. Wheadon is qualified to serve as a member of the Board due to his extensive experience as an executive in the pharmaceutical industry and expertise in regulatory affairs, government policy and clinical strategy. | 66 | December 2020 |
| | |
| | |
CLASS II DIRECTORS – TERM EXPIRING AT THE 2024 ANNUAL MEETING OF STOCKHOLDERS | AGE | DIRECTOR SINCE |
|
|
|
Christopher J. Coughlin has served as a member of our board of directors since April 2020 and as our chairman since January 2023. Previously, Mr. Coughlin served as our lead independent director from March 2022 to January 2023. Mr. Coughlin served as Senior Advisor to the Chief Executive Officer and Board of Directors of Tyco until September 2012. Prior to that, he was Executive Vice President and Chief Financial Officer of Tyco International from 2005 to 2010. During his tenure, he played a central role in the separation of Tyco into five independent, public companies. Prior to joining Tyco, he worked as the Chief Operating Officer of the Interpublic Group of Companies from June 2003 to December 2004 and as Chief Financial Officer from August 2003 to June 2004. Previously, Mr. Coughlin was Executive Vice President and Chief Financial Officer of Pharmacia Corporation from 1998 until its acquisition by Pfizer in 2003. Prior to that, he was Executive Vice President of Nabisco Holdings and President of Nabisco International. From 1981 to 1996 he held various positions, including Chief Financial Officer, at Sterling Winthrop. Mr. Coughlin currently serves on the board of directors of Centene Corporation (NYSE: CNC). Mr. Coughlin also previously served on the board of directors of Allergan plc, Alexion Pharmaceuticals, Dun & Bradstreet Corp., Hologic Inc., and Prestige Consumer Healthcare Inc. Mr. Coughlin received a B.S. in accounting from Boston College. Our board of directors believes that Mr. Coughlin is qualified to serve on our board of directors due to his extensive experience in complex financial and accounting matters, including public accounting and reporting, and his broad experience as a public company director. | 71 | April 2020 |
|
|
|
James Healy, M.D., Ph.D., has served on our board of directors since June 2019. Dr. Healy has been a General Partner of Sofinnova Investments (formerly Sofinnova Ventures), a biotech investment firm, since June 2000. Prior to June 2000, Dr. Healy held various positions at Sanderling Ventures, Bayer Healthcare Pharmaceuticals (as successor to Miles Laboratories) and ISTA Pharmaceuticals, Inc. Dr. Healy is currently on the board of directors of of ArriVent BioPharma, Inc. (Nasdaq: AVBP), Bolt Biotherapeutics, Inc. (Nasdaq: BOLT), Natera, Inc. (Nasdaq: NTRA), Y- mAbs Therapeutics, Inc. (Nasdaq: YMAB) and three private companies. Previously, he served as a board member of Ascendis Pharma A/S, Amarin Corporation, Auris Medical Holding AG, CinCor Pharma Inc., Coherus BioSciences, Inc., Edge Therapeutics, Inc., Hyperion Therapeutics, Inc., InterMune, Inc., Iterum Therapeutics plc, Anthera Pharmaceuticals, Inc., Durata Therapeutics, Inc., CoTherix, Inc., Movetis NV, NuCana plc, ObsEva SA and several private companies. In 2011, Dr. Healy won the IBF Risk Innovator Award and was named as one of the industry’s top leading Life Science investors in 2013 by Forbes Magazine. Dr. Healy holds an M.D. and a Ph.D. in Immunology from Stanford University School of Medicine and holds a B.A. in Molecular Biology and a B.A. in Scandinavian Studies from the University of California, Berkeley. He was previously a Director on the Board of the National Venture Capital Association (NVCA) and the Board of the Biotechnology Industry Organization (BIO). Our board of directors believes that Dr. Healy is qualified to serve as a director due to his significant medical background, extensive experience investing and working in the life science industry and his extensive service on the boards of directors of other life sciences companies. | 59 | June 2019 |
|
|
|
Jeffrey Jonas, M.D., has served as a member of our board of directors since October 2018. Dr. Jonas has been a Partner at Cure Ventures, a life sciences venture capital firm, since January 2024. Prior to that, Dr. Jonas served as the Chief Executive Officer of ABio-X from November 2022 to September 2023. From December 2020 to November 2022, he was the Chief Innovation Officer of Sage Therapeutics, Inc., or Sage, and from August 2013 to December 2020 he served as Sage's Chief Executive Officer and President. From November 2012 to August 2013, Dr. Jonas served as the President of the Regenerative Medicine Division of Shire plc, or Shire, and from July 2008 to November 2012 as Senior Vice President of Research and Development, Pharmaceuticals at Shire. From February 2007 to July 2008, Dr. Jonas served as the Executive Vice President of Ionis Pharmaceuticals, Inc., formerly known as ISIS Pharmaceuticals, Inc., from January 2002 to January 2007 as Chief Medical Officer and Executive Vice President of Forest Laboratories, Inc., and from 1991 to 1996 in senior-level positions at Upjohn Laboratories. Dr. Jonas also founded AVAX Technologies, Inc. and SCEPTOR Industries, Inc., where he served as the Chief Executive Officer, President and a Director. Dr. Jonas is currently on the board of directors of Generation Bio Co. (Nasdaq: GBIO), Sage (Nasdaq: SAGE), Kenai Therapeutics and Noema Pharma. Dr. Jonas received his B.A. from Amherst College and M.D. from Harvard Medical School. He completed a residency in psychiatry at Harvard Medical School, and he served as Chief Resident in psychopharmacology at McLean Hospital, Harvard Medical School. Our board of directors believes that Dr. Jonas is qualified to serve on our board of directors due to his more than 30 years of experience on both the scientific and business sides of the pharmaceutical and healthcare industries, particularly in the Central Nervous System field. | 71 | October 2018 |
| | |
CLASS III DIRECTORS – TERM EXPIRING AT THE 2025 ANNUAL MEETING OF STOCKHOLDERS | AGE | DIRECTOR SINCE |
Steven Paul, M.D., has served as a member of our board of directors since March 2018. From January 2023 to January 2024, he served as our Chief Scientific Officer and President of Research and Development. Prior to that, he served as our President, Chief Executive Officer and Chairman from August 2018 to January 2023. Previously, Dr. Paul was the President and Chief Executive Officer of Voyager Therapeutics, Inc. from September 2014 to August 2018. Dr. Paul also serves as a venture partner at Third Rock Ventures, LLC, a life sciences venture capital firm. Together with Third Rock, Dr. Paul co-founded Sage Therapeutics, Inc. and Voyager Therapeutics, Inc. From August 2010 to September 2014, Dr. Paul was a professor of neuroscience, psychiatry and pharmacology at Weill Cornell Medical College. Prior to that, from 1993 to 2010, Dr. Paul held several key positions at Eli Lilly, including Executive Vice President for Science and Technology, President of the Lilly Research Laboratories, Vice President of Neuroscience (CNS) Research, and Group Vice President of Discovery Research. Prior to Eli Lilly, from 1988 to 1993, Dr. Paul served as the Scientific Director of the National Institute of Mental Health, or NIMH. From 1982 to 1988 Dr. Paul served as a laboratory branch chief and tenured investigator at NIMH. Dr. Paul also served as Medical Director in the Commissioned Corps of the United States Public Health Service. Dr. Paul is an elected fellow of the American Association for the Advancement of Science and a member of the National Academy of Medicine. Dr. Paul is currently on the board of directors of Sage Therapeutics, Inc. (Nasdaq: SAGE) and serves as chairman of the board of Rapport Therapeutics, Inc. Dr. Paul is also on the board of the Foundation for the National Institutes of Health, or NIH. Dr. Paul previously served on the board of Alnylam Pharmaceuticals, Inc., Voyager Therapeutics, Inc. and Sigma Aldrich Corporation. Dr. Paul was appointed by the Secretary of the Department of Health and Human Services as a member of the advisory committee to the Director of the NIH from 2001 to 2006. Dr. Paul was also a member of the National Advisory Mental Health Council (2008-2012) and is board certified by the American Board of Psychiatry and Neurology. Dr. Paul received his B.A. in Biology and Psychology from Tulane University, and his M.S. and M.D. degrees from the Tulane University School of Medicine. Our board of directors believes that Dr. Paul is qualified to serve on our board of directors due to his extensive career in neuroscience and his leadership and managerial experiences at various pharmaceutical and biotechnology companies and healthcare organizations. | 73 | August 2018 |
| | |
Atul Pande, M.D., has served on our board of directors since June 2019. Dr. Pande served as Chief Medical Advisor of PureTech Health plc from 2018 to 2020, and previously served as its Chief Medical Officer from February 2017 to 2018 and as a Senior Advisor from July 2016 through February 2017. Dr. Pande has also served as President and Chief Executive Officer of Verity BioConsulting LLC, a drug development consulting firm, since 2014. He previously served as Chief Medical Officer of Tal Medical, Inc., a clinical-stage medical device company, from December 2014 to December 2017. From 2007 to April 2014, Dr. Pande was Senior Vice President and Senior Advisor, Pharmaceutical R&D at GlaxoSmithKline plc, a pharmaceutical company. He has also held senior roles at Pfizer Inc., Parke-Davis/Warner-Lambert, a subsidiary of Pfizer Inc., and Lilly Research Laboratories, a division of Eli Lilly, all of which are pharmaceutical companies. Dr. Pande is also a non-executive board member of Autifony Therapeutics Limited, Immunovant Sciences Ltd. (Nasdaq: IMVT) and Pangea Bio. He was previously a non-executive board member of Sio Gene Therapies (Nasdaq: SIOX) and Perception Neurosciences. He serves on the Scientific Advisory Board of MMS Holdings, a private CRO, and previously served as an advisor to Centrexion Corporation. Dr. Pande received his MBBS (Bachelor of Medicine, Bachelor of Surgery) and his M.D. from the University of Lucknow, India. He completed his research fellowship training in psychiatry at the University of Michigan Medical School and his postgraduate specialty training and psychiatry residency program at Western University. Our board of directors believes that Dr. Pande is qualified to serve on our board of directors due to his significant medical background and extensive experience in the life science industry. | 69 | June 2019 |
| | |
Denice Torres has served on our board of directors since December 2020. Ms. Torres is the founder and Chief Executive Officer of The Ignited Company, which she founded in November 2017. Ms. Torres is also the founder of The Mentoring Place, which she founded in 2017. From December 2004 to December 2017, Ms. Torres served in roles of increasing authority at Johnson & Johnson, ultimately serving as chief strategy and business transactions officer, medical device. Prior to that, from 1990 to 2004, Ms. Torres held various senior commercial leadership roles at Eli Lilly, including executive director of global neuroscience and director of U.S. women’s health. Ms. Torres currently serves on the board of directors of 2seventy bio, Inc. (Nasdaq: TSVT), Glaukos Corp. (Nasdaq: GKOS), Thirty Madison, and National Resilience Inc. Ms. Torres previously served on the boards of bluebird bio, Inc. and Surface Oncology, Inc. Ms. Torres holds an M.B.A. from the University of Michigan, a J.D. from Indiana University School of Law, and a B.S. in Psychology from Ball State. Our board of directors believes Ms. Torres is qualified to serve on our board of directors due to her extensive experience as an executive in the pharmaceutical industry. | 64 | December 2020 |
EXECUTIVE OFFICERS
The following table sets forth information regarding our executive officers, as of March 14, 2024:
| | |
Name | Age | Position(s) |
|
|
|
Bill Meury (1) | 56 | President and Chief Executive Officer |
Andrew Miller, Ph.D. | 42 | President of Research and Development |
Jason Brown | 36 | Chief Financial Officer |
Stephen Brannan, M.D. | 66 | Chief Medical Officer |
William Kane | 61 | Chief Commercial Officer |
_______________
(1) Mr. Meury is also a director of the Company and his biographical information appears on page 4.
Andrew Miller, Ph.D., has served as our President of Research and Development since January 2024. Prior to that, he served as our Chief Operating Officer from August 2018 to January 2024 and served as a member of our board of directors from April 2012 to March 2019. Dr. Miller was our founder and prior to serving as our Chief Operating Officer, he was our President and Chief Executive Officer from July 2016 to August 2018. From August 2008 to July 2016, Dr. Miller held several positions at PureTech Health plc, last serving as a Vice President, Venture Partner, and in such capacity served as Chief Operating Officer of Tal Medical and acting Chief Operating Officer of Entrega, Inc. He is currently a member of the board of directors of Entrega, Inc. Dr. Miller received a B.S. in Chemical Engineering from the University of Illinois with highest honors and completed his Ph.D. in Chemical Engineering at the Massachusetts Institute of Technology.
Jason Brown has served as our Chief Financial Officer since September 2023. Prior to that, he served as our Senior Vice President, Finance, from January 2022 to September 2023, and as our Vice President, Finance, from August 2018 to January 2022. Mr. Brown worked at PureTech Health plc from 2016 to 2018 in corporate finance, and, prior to that, held multiple roles of increasing responsibility in financial planning and analysis at Novartis AG. Mr. Brown received a bachelor’s degree in Economics from Hamilton College and an MBA from Boston College.
Stephen Brannan, M.D., has served as our Chief Medical Officer since March 2017. From July 2016 to February 2017, Dr. Brannan was an independent consultant. Prior to that, he served as the Vice President Clinical Research and Medical Affairs at Forum Pharmaceuticals Inc. from August 2015 to June 2016. From May 2011 to August 2015, Dr. Brannan served as the Therapeutic Head of Neuroscience at Takeda Pharmaceutical Company. Dr. Brannan has been active in the development of multiple important central nervous system treatments, including Cymbalta, Exelon Patch, Trintellix, and VNS for Treatment Resistant Depression, while holding various roles at Forum, Takeda, Novartis, Cyberonics and Eli Lilly. Prior to joining the pharmaceutical industry, Dr. Brannan worked on the faculty at the University of Texas Health Science Center at San Antonio, or UTHSCSA. Dr. Brannan trained in psychiatry at UTHSCSA, received his A.B. from Harvard College and holds a M.D. degree from the University of Texas Health Science Center at Dallas (Southwestern Medical School).
William Kane has served as our Chief Commercial Officer since February 2023. From November 2021 to December 2022, Mr. Kane was an independent consultant. From June 2020 to October 2021, Mr. Kane was Executive Vice President and Chief Commercial Officer of BioXcel Therapeutics, Inc. Prior to that, he served in multiple leadership roles at Allergan plc, most recently as Senior Vice President, U.S. General Medicine, from January 2017 to May 2020, and prior to that, as Vice President, Internal Medicine Brands, from July 2014 to December 2016 and Vice President, CNS Marketing, from October 2013 to June 2014. Mr. Kane has also held senior level commercial positions at leading biopharma companies, including Pfizer and Sepracor (now Sumitomo Pharma). Mr. Kane holds a B.A. in Government from Connecticut College and an M.B.A. from the Wharton School at the University of Pennsylvania.
CORPORATE GOVERNANCE
Board Composition
We currently have nine directors and the terms of office of the directors are divided into three classes:
•Class I, whose term will expire at the Annual Meeting of Stockholders to be held in 2026;
•Class II, whose term will expire at the Annual Meeting of Stockholders to be held in 2024; and
•Class III, whose term will expire at the Annual Meeting of Stockholders to be held in 2025.
Class I consists of Bill Meury, Laurie Olson and David Wheadon, M.D., Class II consists of Christopher Coughlin, James Healy, M.D., Ph.D. and Jeffrey Jonas, M.D., and Class III consists of Atul Pande, M.D., Steven Paul, M.D. and Denice Torres. At each Annual Meeting of Stockholders, the successors to directors whose terms will then expire shall serve from the time of election and qualification until the third Annual Meeting following election and until their successors are duly elected and qualified. A resolution of the board of directors may change the authorized number of directors. Any additional directorships resulting from an increase in the number of directors will be distributed among the three classes so that, as nearly as possible, each class will consist of one-third of the directors. This classification of the board of directors may have the effect of delaying or preventing changes in control or management of our company.
Board Independence
Our board of directors has determined that each of our directors, except for Bill Meury, who serves as our President and Chief Executive Officer, Steven Paul, M.D., who served as our Chief Scientific Officer and President of Research and Development from January 2023 to January 2024, and Jeffrey Jonas, M.D., who formerly served as the Chief Innovation Officer of Sage Therapeutics, Inc., or Sage, where Dr. Paul formerly served as a member of the compensation committee, has no relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and is independent within the meaning of the director independence standards of the Nasdaq Stock Market, or Nasdaq, rules and the SEC.
At least annually, our board of directors will evaluate all relationships between us and each director in light of relevant facts and circumstances for the purposes of determining whether a material relationship exists that might signal a potential conflict of interest or otherwise interfere with such director’s ability to satisfy his or her responsibilities as an independent director. Based on this evaluation, our board of directors will make an annual determination of whether each director is independent within the meaning of Nasdaq and SEC independence standards.
Board Meetings and Attendance
Our board of directors held fourteen meetings during the fiscal year ended December 31, 2023. Each of the directors attended at least 75% of the meetings of the board of directors and the committees of the board of directors on which he or she served during the fiscal year ended December 31, 2023 (in each case, which were held during the period for which he or she was a director and/or a member of the applicable committee). The Company encourages its directors to attend the Annual Meeting of Stockholders. Each of our directors then in office attended our 2023 annual meeting of stockholders.
Board Committees
Our board of directors has established four standing committees: the audit committee, the compensation committee, the nominating and corporate governance committee, and the science and technology committee, each of which is described more fully below. Each of the audit committee, compensation committee and nominating and corporate governance committee is comprised solely of independent directors. Each of the audit committee, the compensation committee, the nominating and corporate governance committee, and the science and technology committee operates pursuant to a written charter and, on an annual basis, each committee reviews and assesses the adequacy of its charter and submits any proposed changes to its charter to the board of directors for approval. The charters for the audit committee, compensation committee, nominating and corporate governance committee, and science and technology committee are all available on our website at www.karunatx.com under “Investors & Media” at “Corporate Governance” and “Charters and Governance.”
Audit Committee
Our audit committee is currently composed of Christopher Coughlin, James Healy, M.D., Ph.D. and Laurie Olson, with Mr. Coughlin serving as chair of the committee. Our board of directors has determined that each member of the audit committee meets the independence requirements of Rule 10A-3 under the Securities Exchange Act of 1934, as amended, or the Exchange Act, and the applicable listing standards of Nasdaq. Our board of directors has determined that each of Mr. Coughlin and Dr. Healy is an “audit committee financial expert” within the meaning of the SEC regulations and applicable listing standards of Nasdaq. During the fiscal year ended December 31, 2023, the audit committee met five times. The audit committee’s responsibilities include:
•appointing, approving the compensation of, and assessing the independence of our independent registered public accounting firm;
•pre-approving auditing and permissible non-audit services, and the terms of such services, to be provided by our independent registered public accounting firm;
•reviewing the overall audit plan with our independent registered public accounting firm and members of management responsible for preparing our financial statements;
•reviewing and discussing with management and our independent registered public accounting firm our annual and quarterly financial statements and related disclosures as well as critical accounting policies and practices used by us;
•coordinating the oversight and reviewing the adequacy of our internal control over financial reporting;
•establishing policies and procedures for the receipt and retention of accounting-related complaints and concerns;
•recommending based upon the audit committee’s review and discussions with management and our independent registered public accounting firm whether our audited financial statements should be included in our Annual Report on Form 10-K;
•monitoring the integrity of our financial statements and our compliance with legal and regulatory requirements as they relate to our financial statements and accounting matters;
•preparing the audit committee report required by SEC rules to be included in our annual proxy statement;
•reviewing all related person transactions for potential conflict of interest situations and approving all such transactions;
•reviewing quarterly earnings releases; and
•reviewing the Company’s risk assessment and risk management processes, including with respect to information security risks.
Compensation Committee
Our compensation committee is currently composed of Atul Pande, M.D., Christopher Coughlin and Denice Torres, with Dr. Pande serving as chair of the committee. Our board of directors has determined that each current member of the compensation committee is “independent” as defined under the applicable listing standards of Nasdaq. During the fiscal year ended December 31, 2023, the compensation committee met four times. The compensation committee’s responsibilities include:
•annually reviewing and recommending for approval by the board the corporate goals and objectives relevant to the compensation of our chief executive officer;
•evaluating the performance of our chief executive officer in light of such corporate goals and objectives and recommending the compensation of our chief executive officer for approval by the board;
•reviewing and approving the compensation of our other executive officers;
•reviewing and establishing our overall management compensation structure, philosophy and policy;
•overseeing and administering our compensation and similar plans;
•reviewing and making recommendations to our board of directors about our policies and procedures for the grant of equity-based awards;
•evaluating and assessing potential and current compensation advisors in accordance with the independence standards identified in the applicable Nasdaq rules;
•retaining or obtaining the advice of compensation consultants, legal counsel and other advisors;
•appointing, compensating and overseeing the work of any compensation consultant, legal counsel or other adviser it retains;
•evaluating and making recommendations to the board of directors about director compensation;
•preparing the compensation committee report required by SEC rules to be included in this Amendment No. 1;
•reviewing and discussing with our board of directors corporate succession plans for our CEO and executive officers;
•overseeing and monitoring our strategies, programs, initiatives and actions related to human capital management within our workforce; and
•administering our compensation recovery policy.
Historically, our compensation committee has made most of the adjustments to annual cash compensation for the executive team, determined executive bonus and equity awards, and established new performance objectives at the chief executive officer and Company levels. Additionally, our compensation committee considers matters related to the overall effectiveness of the Company’s compensation strategy, and potential modifications to that strategy in relation to market trends, plans or approaches to compensation. Generally, the compensation committee’s process comprises two related elements: the determination of compensation levels and the establishment of performance objectives for the current year. For executives other than the chief executive officer, our compensation committee solicits and considers evaluations and recommendations submitted to the compensation committee by the chief executive officer. In the case of the chief executive officer, the compensation committee is responsible for recommending his compensation for approval by the board.
For all executives and directors, as part of its deliberations, the compensation committee may review and consider, as appropriate, materials such as financial reports and projections, operational data, tax and accounting information, tally sheets that set forth the total compensation that may become payable to executives in various hypothetical scenarios, the total mix of executive and director compensation, executive and director stock ownership information, company stock performance data, analyses of historical executive compensation levels and current Company-wide compensation levels and analyses of executive and director compensation paid at a peer group of other companies approved by our compensation committee. The compensation committee also retains Aon’s Human Capital Solutions practice, a division of Aon plc (formerly known as Radford), or Aon, as its external compensation consultant and considers Aon’s input on certain compensation matters as it deems appropriate. No other fees were paid to Aon except fees related to determining or recommending compensation.
The compensation committee may establish and delegate authority to one or more subcommittees consisting of one or more of its members when the committee deems it appropriate. Additionally, the compensation committee may delegate its authority to one or more officers of the Company, including our chief executive officer, to grant certain equity awards to employees that are not executive officers, and in 2023 it delegated such authority to our chief executive officer, our chief financial officer, and our then vice president of legal affairs and secretary.
Compensation Committee Interlocks and Insider Participation
For the 2023 fiscal year, Atul Pande, M.D., Christopher Coughlin and Denice Torres served as members of our compensation committee. None of the members of our compensation committee is, or has ever been, an officer or employee of our company. During the 2023 fiscal year, no executive officer of our company served as: (i) a member of the compensation committee (or other committee of the board of directors performing equivalent functions or, in the absence of any such committee, the entire board of directors) of another entity, one of whose executive officers served on our compensation committee; (ii) a director of another entity, one of whose executive officers served on our compensation committee; or (iii) a member of the compensation committee (or other committee of the board of directors performing equivalent functions or, in the absence of any such committee, the entire board of directors) of another entity, one of whose executive officers served as a director of our company.
Nominating and Corporate Governance Committee
Our nominating and corporate governance committee is composed of Laurie Olson, Atul Pande, M.D. and David Wheadon, M.D., with Ms. Olson serving as chair of the committee. Our board of directors has determined that each current member of our nominating and corporate governance committee is “independent” as defined under the applicable listing standards of Nasdaq. During the fiscal year ended December 31, 2023, the nominating and corporate governance committee met four times. The nominating and corporate governance committee’s responsibilities include:
•developing and recommending to the board of directors criteria for board and committee membership;
•establishing procedures for identifying and evaluating board of director candidates, including nominees recommended by stockholders;
•reviewing the size and composition of the board of directors to ensure that it is composed of members containing the appropriate skills and expertise to advise us;
•identifying individuals qualified to become members of the board of directors;
•recommending to the board of directors the persons to be nominated for election as directors and to each of the board’s committees;
•developing, recommending to the board of directors for approval, and annually reviewing and reassessing the adequacy of, the Company's code of business conduct and ethics and corporate governance guidelines;
•overseeing and making recommendations to the Board regarding the Company’s environmental, social and governance, or ESG, initiatives; and
•overseeing the evaluation of our board of directors.
Science and Technology Committee
In August 2023, our board of directors established a science and technology committee as a standing committee of the board of directors. Our science and technology committee is composed of Jeffrey Jonas, M.D., Atul Pande, M.D., Steven Paul, M.D. and David Wheadon, M.D., with Dr. Paul serving as chair of the committee. During the fiscal year ended December 31, 2023, the science and technology committee met one time. The science and technology committee’s responsibilities include:
•reviewing, evaluating, and advising the board of directors and management regarding the Company’s research and development plans and strategy;
•reviewing, evaluating, and advising the board of directors and management regarding the quality, direction and competitiveness of the Company’s research and development programs;
•reviewing, evaluating, and advising the board of directors and management regarding the scientific and medical aspects of proposed transactions, including significant business development transactions, that require approval by the board of directors;
•reviewing and considering management’s decisions regarding the allocation, deployment and utilization of, and investment in, the Company’s scientific assets;
•monitoring progress of the Company’s research and development pipeline;
•reviewing and assessing the Company’s intellectual property portfolio and strategy; and
•identifying and discussing emerging science and technology trends and issues with potential relevance to the Company’s current or future research and development programs.
Our board of directors may from time to time establish other committees.
Identifying and Evaluating Director Nominees
Our board of directors is responsible for selecting its own members. The board of directors delegates the selection and nomination process to the nominating and corporate governance committee, with the expectation that other members of the board of directors, and of management, will be requested to take part in the process as appropriate.
Generally, our nominating and corporate governance committee identifies candidates for director nominees in consultation with management, through the use of search firms or other advisors, through the recommendations submitted by stockholders or through such other methods as the nominating and corporate governance committee deems to be helpful to identify candidates. Once candidates have been identified, our nominating and corporate governance committee confirms that the candidates meet all of the minimum qualifications for director nominees established by the nominating and corporate governance committee. The nominating and corporate governance committee may gather information about the candidates through interviews, detailed questionnaires, background checks or any other means that the nominating and corporate governance committee deems to be appropriate in the evaluation process. The nominating and corporate governance committee then meets as a group to discuss and evaluate the qualities and skills of each candidate, both on an individual basis and taking into account the overall composition and needs of our board of directors. Based on the results of the evaluation process, the nominating and corporate governance committee recommends candidates for the board of directors’ approval as director nominees for election to the board of directors.
Director Qualifications and Diversity
Our nominating and corporate governance committee’s priority in selecting board members is the identification of persons who will further the interests of our company through their established record of professional accomplishment, the ability to contribute positively to the collaborative culture among board members, and professional and personal experiences and expertise relevant to our growth strategy. They will consider, among other things, the following qualifications, skills and attributes when recommending candidates for the Board’s selection as director nominees for the Board and as candidates for appointment to the Board’s committees: a nominee shall have experience at a strategic or policymaking level in a business, government, non-profit or academic organization of high standing; a nominee shall be highly accomplished in his or her respective field, with superior credentials and recognition; a nominee shall be well regarded in the community and shall have a long-term reputation for high ethical and moral standards; a nominee shall have sufficient time and availability to devote to our affairs, particularly in light of the number of boards of directors on which such nominee may serve; and, to the extent a nominee serves or has previously served on other boards, the nominee shall have a demonstrated history of actively contributing at board meetings.
Our board of directors also believes that diversity of viewpoints, background, experience and other characteristics, such as gender, race, ethnicity, culture, nationality and sexual orientation, are an important part of its makeup. When evaluating candidates for nomination as new directors, our board of directors will:
(1) Consider candidates with diverse backgrounds in terms of knowledge, experience, skills and other characteristics in the context of the needs of the Company at that point in time with a view to creating a Board with a diversity of experience and perspectives; and
(2) Include in the pool from which new director nominees are chosen candidates with a diversity of gender, race, ethnicity, culture, nationality or sexual orientation (and any third-party engaged to identify candidates for such pool will be asked to do the same).
The nominating and corporate governance committee will consider candidates recommended by stockholders. The policy adopted by the nominating and corporate governance committee provides that candidates recommended by stockholders are given appropriate consideration in the same manner as other candidates.
| | | | |
Board Diversity Matrix (As of March 14, 2024) |
Board Size: | | | | |
Total Number of Directors | 9 |
| Female | Male | Non-Binary | Did not Disclose
Gender |
Gender: | | | | |
Directors | 2 | 7 | — | — |
Number of Directors who identify in Any of the Categories Below: | | | | |
African American or Black | — | 1 | — | — |
Alaskan Native or Native American | — | — | — | — |
Asian | — | 1 | — | — |
Hispanic or Latinx | — | — | — | — |
Native Hawaiian or Pacific Islander | — | — | — | — |
White | 1 | 5 | — | — |
Two or More Races or Ethnicities | 1 | — | — | — |
LGBTQ+ | 3 |
Did not Disclose Demographic Background | — |
Persons with Disabilities | — |
Non-Management Director Meetings
In addition to the meetings of the committees of the board of directors described above, in connection with the board of directors’ meetings, the non-management directors met six times in executive session during the fiscal year ended December 31, 2023.
Communication with the Directors of Karuna Therapeutics
Any interested party with concerns about our company may report such concerns to the board of directors or the chair of our board of directors or nominating and corporate governance committee, by submitting a written communication to the attention of such director at the following address:
c/o Karuna Therapeutics, Inc.
99 High Street, 26th Floor
Boston, Massachusetts 02110
United States
You may submit your concern anonymously or confidentially by postal mail. You may also indicate whether you are a stockholder, supplier, or other interested party.
A copy of any such written communication may also be forwarded to the Company’s legal counsel and a copy of such communication may be retained for a reasonable period of time. The director may discuss the matter with the Company’s legal counsel, with independent advisors, with non-management directors, or with the Company’s management, or may take other action or no action as the director determines in good faith, using reasonable judgment, and applying his or her own discretion.
Communications may be forwarded to other directors if they relate to important substantive matters and include suggestions or comments that may be important for other directors to know. In general, communications relating to corporate governance and long-term corporate strategy are more likely to be forwarded than communications relating to ordinary business affairs, personal grievances, and matters as to which we receive repetitive or duplicative communications.
The audit committee oversees the procedures for the receipt, retention, and treatment of complaints received by the Company regarding accounting, internal accounting controls, or audit matters, and the confidential, anonymous submission by employees of concerns regarding questionable accounting, internal accounting controls or auditing matters.
Leadership Structure and Risk Oversight
The leadership structure of our board of directors currently separates the positions of chief executive officer and chair of the board of directors, although we do not have a corporate policy requiring that structure. Separating these positions allows our chief executive officer, who is also a member of our board of directors, to focus on our day-to-day business operations and strategic direction, while allowing our board chair, who is an independent member of the board of directors, to focus on matters related to corporate governance, including management oversight and strategic guidance. While the board of directors believes that this is the most appropriate structure at this time, the nominating and corporate governance committee evaluates the board leadership structure from time to time, and may recommend alterations to this structure in the future.
Risk is inherent with every business, and how well a business manages risk can ultimately determine its success. We face a number of risks, including risks relating to our financial condition, development and commercialization activities, operations, strategic direction and intellectual property as more fully discussed under “Risk Factors” in our 2023 Annual Report and in our subsequent filings with the SEC. Management is responsible for the day-to-day management of risks we face, while our board of directors, as a whole and through its committees, has responsibility for the oversight of risk management. In its risk oversight role, our board of directors has the responsibility to satisfy itself that the risk management processes designed and implemented by management are adequate and functioning as designed.
The role of the board of directors in overseeing the management of our risks is conducted primarily through committees of the board of directors, as disclosed in the descriptions of each of the committees above and in the charters of each of the committees. The full board of directors (or the appropriate board committee in the case of risks that are under the purview of a particular committee) discusses with management our major risk exposures, their potential impact on us, and the steps we take to manage them. When a board committee is responsible for evaluating and overseeing the management of a particular risk or risks, the chairman of the relevant committee reports on the discussion to the full board of directors during the committee reports portion of the next board meeting. This enables the board of directors and its committees to coordinate the risk oversight role, particularly with respect to risk interrelationships.
Environmental, Social, and Governance Efforts
Our board of directors and management believe that ESG matters are central to the success of the Company and have made the furtherance of ESG initiatives a priority. Our board of directors exercises oversight over ESG issues primarily through the nominating and corporate governance committee, which is actively involved in ESG initiatives across the Company and makes recommendations to our board of directors regarding the Company’s ESG initiatives. Our board of directors oversees ESG issues more broadly to ensure that ESG risks and opportunities are being appropriately addressed by management. In order to appropriately incentivize our executive officers to prioritize ESG matters, we include an ESG metric tied to employee satisfaction, diversity and inclusion in our corporate goals.
Our ESG program is centered around patients, our employees, and our communities.
Patients
At Karuna, our goal is to discover and develop medicines with the potential to make a difference for people living with psychiatric and neurological conditions. As such, we invest in innovation on behalf of such patients, including those living with schizophrenia and psychosis in Alzheimer's disease, or AD. In furtherance of this goal, in 2023 and 2022 we incurred research and development expenses of $364.1 million and $224.2 million, respectively. We also engage with and support patients through our work with patient advocacy organizations, key opinion leaders, and other patient stakeholders to improve the understanding of, and reduce stigma surrounding, mental illnesses such as schizophrenia and AD-related psychosis.
Our Employees
We believe that our employees are critical to the success of our mission, and that our future success will depend in large part on our continued ability to attract, hire and retain qualified personnel. We continuously strive to ensure that employee morale remains strong, and conduct employee engagement satisfaction surveys and monitor employee turnover rates as part of this process.
Compensation and Benefits
We offer robust compensation packages, including competitive base pay, incentive compensation and equity programs, and provide a broad range of benefits, including a 401(k) plan, healthcare and insurance benefits, a health savings account, paid time off, paid family and medical leave, and various health and wellness programs. Through our equity incentive plan, we aim to attract, retain and reward personnel through the granting of equity-based compensation awards
in order to increase shareholder value and the success of our Company by motivating such individuals to perform to the best of their abilities and achieve our objectives.
Growth and Wellness
We are committed to the professional development of our employees, who can take advantage of various learning opportunities and training programs. As part of our development program, we provide our employees with reimbursement for certain external training programs that are related to their current work or career development. In addition, aligned with our mission and to support our employees’ mental health, we offer employees online resources to assist with professional and financial coaching, wellness courses, and therapy services. Employee-led committees focused on learning and development as well as recognition and wellness play a critical role in improving our offerings in these areas.
Diversity, Equity and Inclusion
We are committed to cultivating and preserving a culture of diversity, equity and inclusion, and aim to foster an inclusive environment through respect, collaboration, and open communication. All employees receive non-discrimination training, and those involved in the talent recruitment process receive behavioral interviewing training to avoid bias in hiring. We also embrace and encourage the differences among our employees that make them unique, and believe that these differences, as well as corresponding diversity of opinions and thought, contribute to our success as a company. Our efforts are driven by an employee-led diversity, equity and inclusion committee with support and guidance from the executive team.
Our Communities
We take our responsibility as a member of our local community seriously. Our employee-led community, outreach and giving committee helps to guide our strategy and approach for community engagement and partnerships with various non-profit organizations. For example, we sponsor certain non-profit organizations whose missions align closely with our own, such as This Is My Brave, which aims to reduce stigma in serious mental illness, and Clubhouse International, which provides support for those living with mental illness. We are also involved with other non-profit organizations, such Life Science Cares and Catie's Closet, that seek to improve the lives of disadvantaged individuals in the Boston area and surrounding communities.
We are committed to continued improvement with respect to ESG matters across the organization, and will continue to assess and implement changes as we examine our policies and practices and identify areas for improvement.
Code of Business Conduct and Ethics
We are committed to the highest standards of integrity and ethics in the way we conduct our business. Our board of directors adopted a Code of Business Conduct and Ethics, which applies to our directors, officers and employees, including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. Our Code of Business Conduct and Ethics establishes our policies and expectations with respect to a wide range of business conduct, including the preparation and maintenance of our financial and accounting information, our compliance with laws, and possible conflicts of interest.
Under our Code of Business Conduct and Ethics, each of our directors and employees is required to report suspected or actual violations to the extent permitted by law. We maintain a third-party whistleblower hotline, available 24 hours a day, seven days a week, that can be used to anonymously report suspected violations of our Code of Business Conduct and Ethics or illegal activities in good faith without fear of retaliation. Any whistleblower reports received would be reviewed with our audit committee. In addition, we have adopted separate procedures concerning the receipt and investigations of complaints relating to accounting or audit matters. These procedures have been adopted by the board of directors and are administered by our audit committee.
A current copy of our Code of Business Conduct and Ethics is posted on our website at www.karunatx.com. If we make any substantive amendments to, or grant any waivers from, the Code of Business Conduct and Ethics for any officer or director, we will disclose the nature of such amendment or waiver on our website or in a current report on Form 8-K.
ITEM 11. EXECUTIVE COMPENSATION
COMPENSATION DISCUSSION AND ANALYSIS
Overview
Our compensation committee is responsible for reviewing and approving, or recommending for approval by the board of directors, the compensation of our named executive officers, including base salary, cash and equity incentive compensation levels, severance arrangements, change in control benefits and other forms of executive compensation. The compensation committee is also responsible for evaluating our company’s performance against its goals and making related recommendations to our board of directors, assessing the performance of our named executive officers, and ensuring our compensation program is aligned with the objectives described below and competitive with those of other companies in our industry that compete with us for talent. This section discusses the principles underlying our compensation committee’s policies and decisions with respect to the compensation of our named executive officers.
For 2023, our named executive officers were as follows:
•Bill Meury, our President and Chief Executive Officer;
•Steven Paul, M.D., our former President and Chief Executive Officer, and former Chief Scientific Officer and President of Research and Development(1);
•Jason Brown, our Chief Financial Officer;
•Troy Ignelzi, our former Chief Financial Officer(2);
•Andrew Miller, Ph.D., our President of Research and Development(3);
•Stephen Brannan, M.D., our Chief Medical Officer; and
•William Kane, our Chief Commercial Officer.
_______________
(1)Effective January 3, 2023, Dr. Paul resigned as President and Chief Executive Officer and was appointed Chief Scientific Officer and President of Research and Development. Effective January 16, 2024, Dr. Paul resigned as Chief Scientific Officer and President of Research and Development but remains a member of the Company's board.
(2)Mr. Ignelzi departed from his position as Chief Financial Officer effective September 29, 2023.
(3)Dr. Miller served as Chief Operating Officer from August 2018 until his appointment as President of Research and Development effective upon Dr. Paul's departure from that position on January 16, 2024.
Executive Summary and Company Background
We are a clinical-stage biopharmaceutical company driven to create and deliver transformative medicines for people living with psychiatric and neurological conditions. Our pipeline is primarily built on the broad therapeutic potential of our proprietary lead product candidate, KarXT (xanomeline-trospium), an oral modulator of muscarinic receptors that are located both in the central nervous system, or CNS, and various peripheral tissues. KarXT combines xanomeline, a novel muscarinic agonist, with trospium, an approved muscarinic antagonist, to preferentially stimulate muscarinic receptors in the CNS. We are initially developing KarXT for the treatment of acute psychosis in adults with schizophrenia, as well as for the treatment of psychosis in Alzheimer's disease, or AD.
On December 22, 2023, we entered into an agreement and plan of merger, or the Merger Agreement, with Bristol-Myers Squibb Company, or Bristol Myers Squibb, pursuant to which Bristol Myers Squibb has agreed to acquire the Company for $330 per share in cash, for a total equity value of approximately $14.0 billion, on the terms and subject to the conditions set forth therein. The transaction is expected to close in the first half of 2024, subject to customary closing conditions, including approval of our stockholders and receipt of required regulatory approvals. See Part I, Item 1A, “Risk Factors,” Part II, Item 7, “Management's Discussion and Analysis of Financial Condition and Results of Operations,” and Note 1 of the Notes to Consolidated Financial Statements included in our 2023 Annual Report for additional information regarding the transaction.
Key 2023 Achievements
Research and Development
•Submitted our New Drug Application, or NDA, for KarXT for the treatment of schizophrenia in adults to the U.S. Food and Drug Administration, or FDA, which accepted the NDA for review with a Prescription Drug User Fee Act (PDUFA) action date of September 26, 2024;
•Announced positive topline results from our Phase 3 EMERGENT-3 trial evaluating the efficacy, safety and tolerability of KarXT compared to placebo for the treatment of acute psychosis in adults with schizophrenia;
•Announced positive results from our Phase 1b Ambulatory Blood Pressure Monitoring trial of KarXT in schizophrenia;
•Completed enrollment in EMERGENT-5, our second open-label extension study evaluating the long-term safety and tolerability of KarXT in schizophrenia in support of the KarXT NDA;
•Initiated our Phase 3 ADEPT-2 trial evaluating the efficacy and safety of KarXT compared to placebo in up to 400 adults with moderate to severe psychosis related to AD, and our related open-label extension study, ADEPT-3; and
•Completed a Phase 1 multiple ascending dose study of an advanced oral formulation of KarXT.
Commercialization
•Hired William Kane as Chief Commercial Officer, and hired our Head of Sales; and
•Continued to grow and scale our commercial organization and advance preparations for a potential commercial launch.
Corporate
•Acquired exclusive license to Goldfinch Bio, Inc.'s investigational transient receptor potential canonical 4 and 5 (TRPC4/5) channel candidates, including the lead clinical-stage TRPC4/5 candidate, KAR-2618;
•Completed a follow-on public offering with aggregate net proceeds to the Company of $436.7 million;
•Operated within 10% of our approved budget to support research, development, commercial and long-term corporate objectives;
•Grew headcount from 194 to 317 to support clinical, commercial and other development goals; and
•Maintained high employee satisfaction, low turnover and positive diversity, equity and inclusion outcomes and ESG initiatives.
Chief Executive Officer Transition
In December 2022, as a result of a thoughtful succession plan initiated by Dr. Paul, our former President, Chief Executive Officer, and chairman of the board, we announced the appointment of Mr. Meury as our President and Chief Executive Officer, and as a member of the Company's board of directors, effective January 3, 2023. Dr. Paul transitioned to the role of Chief Scientific Officer and President of Research and Development, and Christopher Coughlin, our former lead independent director, assumed the role of chairman of the board.
New Chief Executive Officer Compensation Package
To determine Mr. Meury's compensation package in connection with his appointment, the compensation committee, with input from Aon, its compensation consultant, considered the compensation paid to chief executive officers, including
recent new hire chief executive officers, at our peer companies described below. Mr. Meury's on-hire compensation consisted of the following elements:
Annual Compensation
•Annual Base Salary: $750,000 per year
•Bonus Target: 75% of annual base salary
•Equity Award: Mr. Meury did not receive an annual equity award for 2023. He was first eligible for, and received, an annual equity award in 2024.
On-Hire Compensation
oIncentive stock options with a value of $8,000,000 and an exercise price equal to the closing price of our common stock as reported on the Nasdaq Global Market on the date of grant
oRestricted stock units, or RSUs, with a value of $8,000,000
In determining the value of Mr. Meury's annual cash compensation, the compensation committee targeted the 50th percentile of the market, in line with the compensation committee's general philosophy of targeting the 50th percentile of our peer group with respect to base salary and annual cash incentive award opportunities. The number of shares subject to Mr. Meury’s on-hire equity awards were calculated using the average closing price of the Company’s common stock during the 30-day period up to and including the date of grant. Mr. Meury's on-hire equity awards are greater in value than he would typically receive as part of his annual compensation because the award is intended to provide Mr. Meury with an immediate and meaningful ownership stake in the Company that will align his interests with those of our stockholders.
2023 “Say-on-Pay” Vote
At our 2023 annual meeting of stockholders, our stockholders were provided with the opportunity to cast a non-binding advisory vote on the compensation of our named executive officers for the 2022 fiscal year (a “say-on-pay” proposal). The stockholders approved, on an advisory basis, the compensation of our named executive officers, as disclosed in our 2023 proxy statement, with support from 65.1% of the votes cast. In the second half of 2023, we undertook an extensive shareholder engagement campaign to better understand our shareholders' perspectives regarding our executive compensation and corporate governance programs.
2023 Shareholder Engagement
Our Engagement Process
We believe that engaging with stockholders is fundamental to the Company's success and our commitment to good governance. We have historically reached out to a majority of our largest shareholders in advance of our annual meeting of stockholders to answer any questions and obtain shareholder feedback regarding our governance and compensation practices as disclosed in that year’s proxy statement, and any related topics that they wish to discuss. In 2023, we also contacted our shareholders in the second half of the year after the annual meeting of stockholders had taken place to understand their views of our business, corporate governance and compensation practices, and related matters.
Since our 2023 annual meeting of stockholders, we have contacted holders of approximately 70% of our outstanding shares for potential engagement. Such shareholders comprised the majority of our top 25 shareholders, and included shareholders who voted against our most recent "say on pay" proposal. Seven shareholders representing approximately 55% of our outstanding shares, accepted our offer to engage, and we participated in a discussion with four of these shareholders, representing approximately 40% of our outstanding shares. Calls with the remaining three of these seven shareholders were postponed before they took place in light of the pending merger transaction with Bristol Myers Squibb. The shareholders with which we held calls comprised four of our top five shareholders, and included a shareholder who voted against our most recent "say on pay" proposal.
Our engagement team consisted of members of management from our legal, investor relations, corporate affairs and finance functions. As part of our outreach, we communicated that our independent Board Chair would be available to participate in the discussion upon request; no shareholders that we ultimately spoke with requested his participation. Topics discussed included executive compensation, corporate governance and our environmental, social and
governance, or ESG, program. Management relayed stockholder feedback to the Compensation Committee in the fourth quarter of 2023.
Shareholder Feedback and our Response
Our shareholders provided feedback on several topics, including our long-term incentive equity compensation program, certain corporate governance matters and additional information that we could disclose to shareholders in our filings to better enable them to make informed voting decisions. Management spoke with our compensation committee to discuss potential changes to our compensation and governance practices based on this feedback, and certain shareholder input regarding our disclosure has been reflected in this filing.
Unless and until the proposed merger with Bristol Myer Squibb closes, the board of directors and management intend to continue improving upon our engagement with our shareholders through a year-round regular engagement process to better understand their views of our business, corporate governance practices and compensation program.
Key Aspects of 2023 Executive Compensation: Strong Performance Orientation
Delivered Very Strong Stock Price Performance and Total Shareholder Return
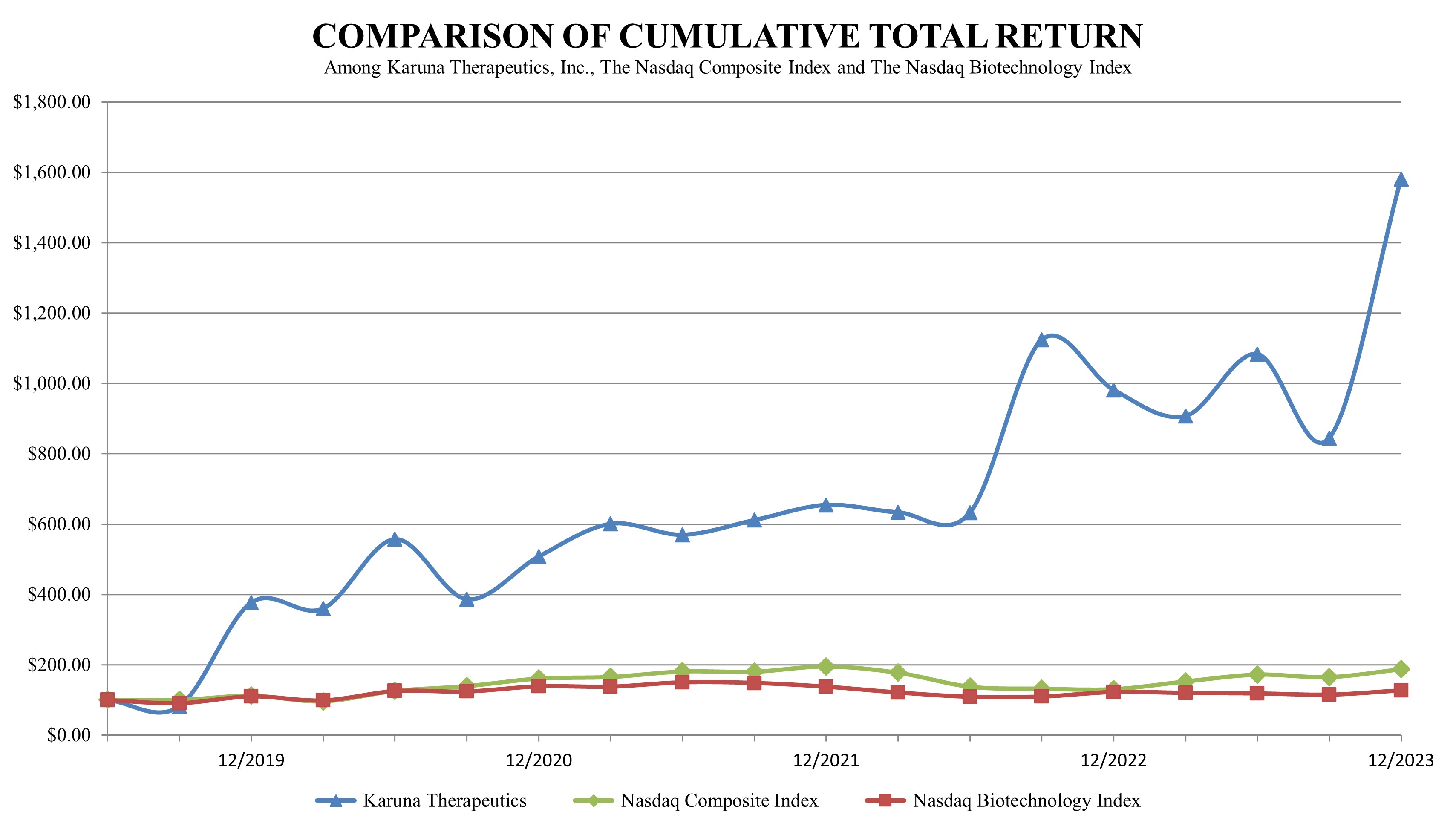
We completed our initial public offering in June 2019. Our Total Shareholder Return, or TSR, for 2023 was 61%, and our cumulative TSR since our June 2019 IPO through the end of 2023 was 1,481%, outperforming relevant indices.
Substantial Majority of CEO and other NEO Compensation, Total and Equity, is Variable and At-Risk
Approximately 95% of our chief executive officer’s 2023 total compensation, and 100% of the long-term incentive equity component, were variable and at risk. Our compensation philosophy is performance-based and focuses on aligning the financial interests of our executive officers with those of our shareholders. Generally, this is accomplished by placing a substantial portion of our executive officers’ total compensation “at risk.” We consider compensation to be “at-risk” if it is subject to achievement of meaningful pre-set, objective financial or operating goals, such as in our annual cash incentive program, or if it depends on stock price appreciation or value, as in our long-term incentive program.
Until December 31, 2022, consistent with the market practice of similar, recently-public companies in our industry, and in order to focus executives on growth and increasing shareholder value at this early stage of our development, equity
grants consisted solely of options, which are appreciation awards that only have value if the stock price increases. In our view, stock options are inherently performance-based, requiring stock price appreciation before there is any real value earned, and are simple. No amount of time will make a stock option deliver any value unless the company’s stock price increases. In addition, stock options reward our named executive officers for increasing shareholder value over the lengthier term of the option, relative to other equity compensation, which we believe is consistent with the longer pharmaceutical development cycle.
In January 2023, our board of directors began utilizing grants of RSUs in addition to stock options for the purpose of rewarding our executive officers. Grants of RSUs encourage executive ownership of Karuna shares, provide balance to stock option grants, which only have value if the stock appreciates, and align the incentives of our executives with the interests of our stockholders. These awards also provide executives with some certainty regarding the value of the compensation they are receiving during periods of stock market volatility, which furthers our fundamental goal of awarding compensation that helps attract and retain highly skilled employees. In addition, awarding full-value shares in the form of RSUs in the long-term portion of total compensation is less dilutive than stock option grants and consistent with the compensation practices of a majority of our peer group.
The graphics below illustrate the mix of fixed base salary, annual incentive and long-term target incentive compensation we provided in 2023 to our chief executive officer and, on an average basis, to our other named executive officers, and the high proportion that is variable and at-risk.
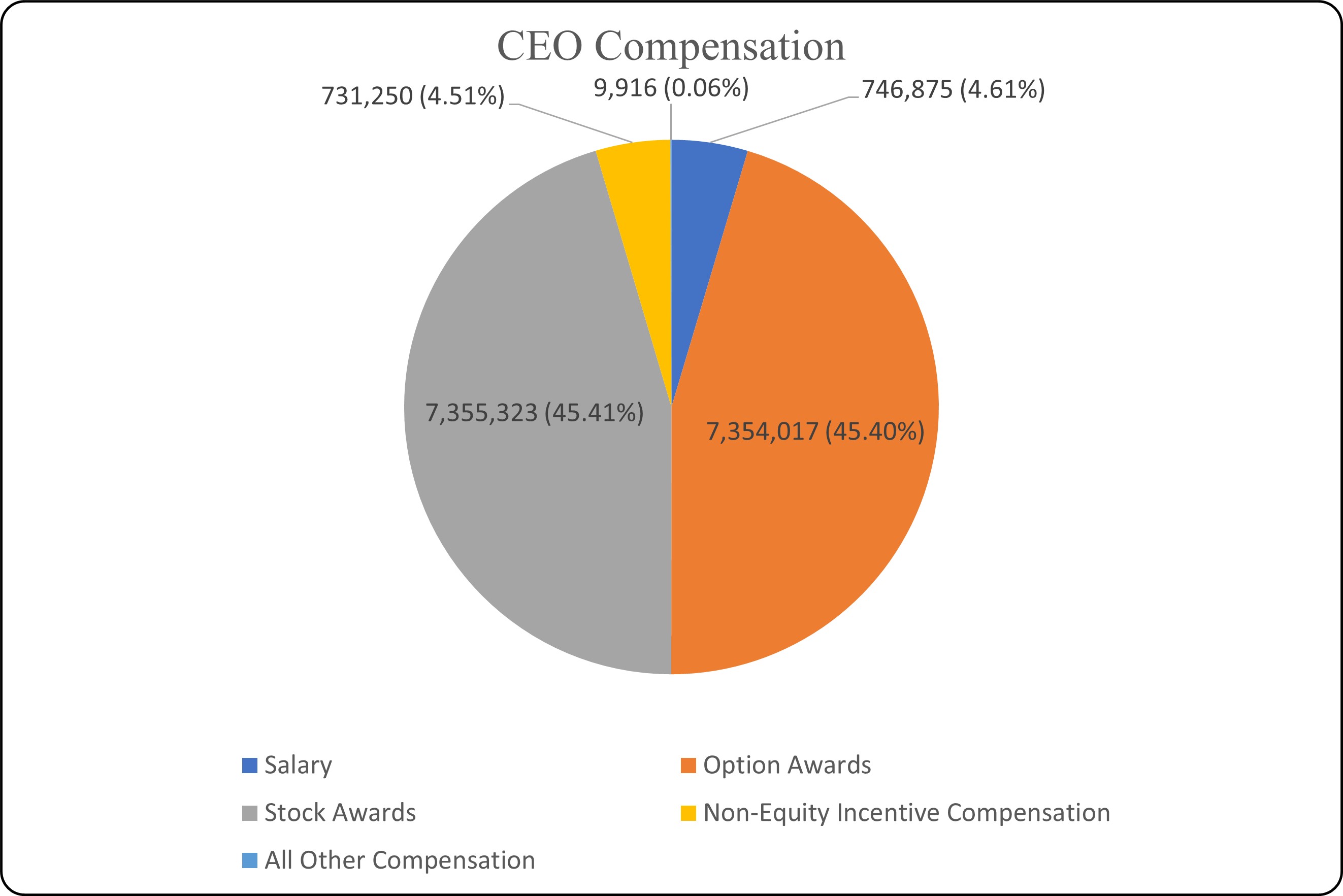
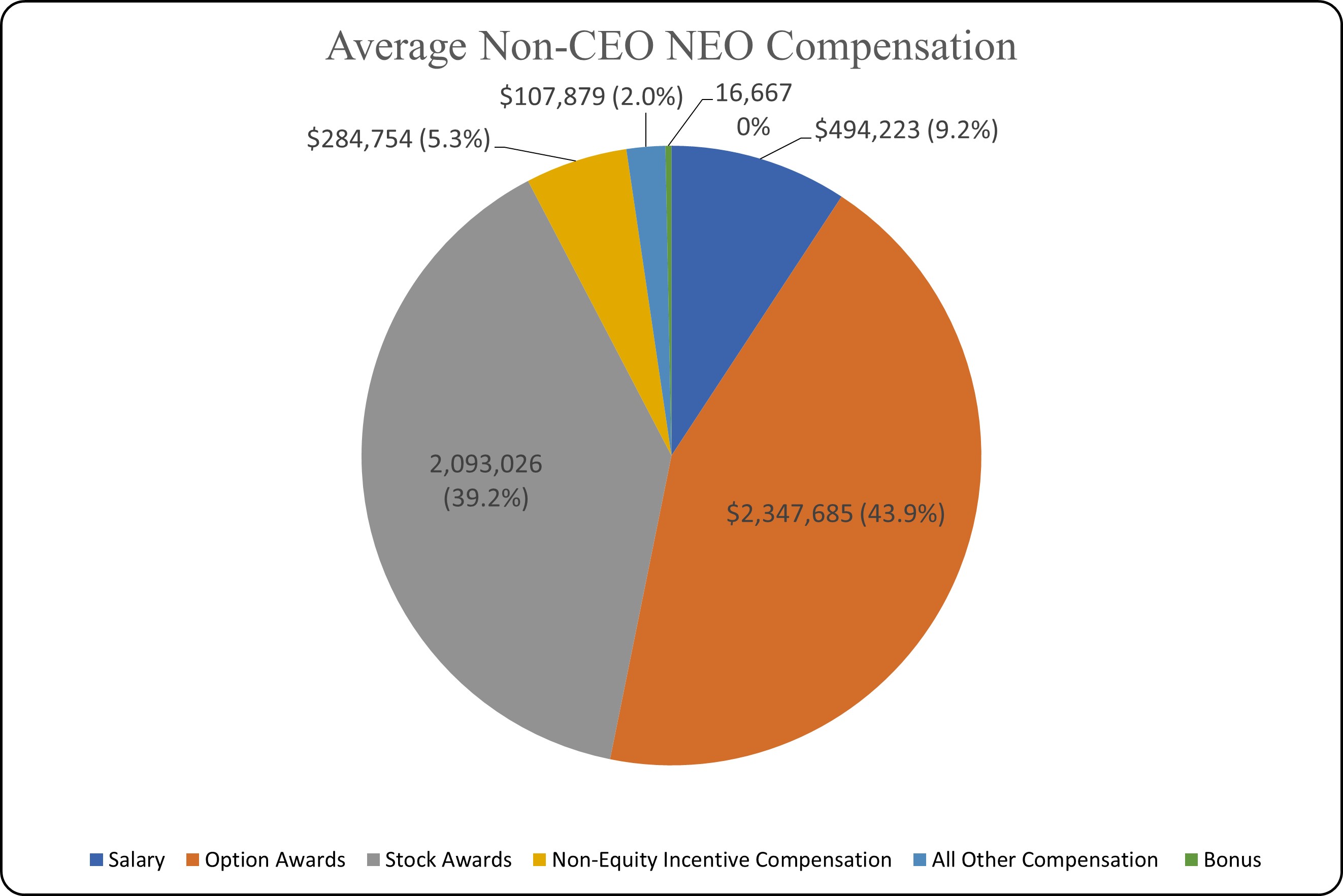
The performance-based metrics and the proportion of total compensation that was variable and at-risk further enhanced the link between pay and performance for the former chief executive officer and our other named executive officers and strengthened the alignment of the interests of the executive officers with those of our shareholders.
Short-Term Annual Cash Incentive: Rigorous, Pre-Set Clinical and Other Development and Corporate Goals
At the outset of 2023, we established research and development, regulatory, operational and financial objectives under our annual cash incentive program. These objectives were rigorous, aggressive and challenging, attainable only with strong performance, and took into account the relevant opportunities and risks. The compensation committee evaluated performance achievement relative to the goals as described in the section of this Amendment No.1 titled "Elements of our Compensation Program - Annual Cash Incentive Bonuses".
Peer Group: Assessed and Updated Peer Group to Reflect Current Market Capitalization
Consistent with best practices for corporate governance, the compensation committee annually reassesses the group of peer companies used as a reference point for evaluating executive compensation. In connection with determining the compensation of the chief executive officer and other executive officers, in the second half of 2022, the compensation committee conducted a review of our peer group to ensure its continued appropriateness, and updated the peer group utilized by our compensation committee with respect to compensation decisions for 2023.
In light of the increase in our organizational size and market capitalization, the compensation committee revised the peer group selection criteria. The peer group selection criteria were updated to reference a market capitalization range of approximately $2.0 billion to $17.6 billion, which resulted in the removal of ten companies and the addition of seven new companies, as compared to our 2022 peer group. See the section titled “Compensation Peer Groups and Peer Selection Process” for more information regarding our 2023 peer group.
Philosophy and Objectives of our Compensation Program
The core elements of the compensation committee’s executive compensation philosophy are as follows:
•Link pay to performance and achievement of our corporate and strategic goals;
•Align executive officers’ interests with those of the Company and our stockholders, generally through the use of equity as a significant component of compensation;
•Provide market competitive compensation to attract, motivate and retain qualified talent at executive levels; and
•Design programs that we believe are simple and transparent.
Reward achievement of corporate and strategic goals (pay for performance): Our overarching mission is to create and deliver transformative medicines for people living with psychiatric and neurological conditions. We have developed a set of clearly defined corporate and strategic goals to accomplish this mission, with the majority of the total compensation of our executive officers tied to the achievement of such goals. Our executive officers are incentivized to achieve these goals, which include measurable research and development, regulatory, operational and financial objectives, and awarded for doing so, all of which, the compensation committee believes, will help build long-term stockholder value. Performance measures are reviewed annually to ensure that we continue to align our pay programs with our business strategy, create sustainable value and motivate the right behaviors.
Align the interests of our executive officers and employees with those of our stockholders by promoting an ownership culture: Equity-based compensation constitutes a significant portion of our executive officers’ overall compensation. The compensation committee uses equity, when appropriate, as a long-term incentive to reward executive officers for (i) achieving multi-year strategic goals and (ii) delivering sustained long-term value to stockholders. The compensation committee believes using equity in this manner aligns the interests of executive officers with those of our stockholders by ensuring that they share a common interest in our stock price performance. We value an ownership mindset and believe that granting equity bolsters this culture among executive officers by giving them a personal stake in Karuna’s growth and success.
Offer competitive compensation to attract and retain talent: The biopharmaceutical industry is fiercely competitive, particularly in the metropolitan Boston area, and we must compete for executive talent. To manage our business and
carry out our strategy, we seek high-caliber executive officers and managers who have diverse experience, expertise, capabilities, and backgrounds. In recruiting our executive officers and determining competitive pay levels, the compensation committee references the amounts and compensation structures of executive officers in the companies in our compensation peer group and in industry surveys.
Design straightforward compensation programs and plans and administer them transparently: In order for incentive compensation to serve its purpose of motivating participants to achieve results, the participants must have a clear understanding of the goals and targets by which they will be measured, and the rewards that they will receive for various levels of achievement of those goals, including the value of those rewards. The compensation committee seeks to design programs that give participants a clear line of sight to understandable metrics and sufficient control over performance in furtherance of the goals, to motivate them effectively to achieve our business objectives and to reward them appropriately, as a means of executing our strategy.
Components of our Compensation Program
In order to achieve its executive compensation program objectives, the compensation committee utilizes the components of compensation set forth below. The compensation committee regularly reviews all components of the program in order to verify that each executive officer’s total compensation is consistent with our compensation philosophy and objectives and that each component is serving a purpose in supporting the execution of our strategy.
| | |
Element | Description | Purpose |
|
|
|
Base Salary | Fixed cash compensation
Determined based on each executive officer’s role, individual skills, experience, performance and positioning relative to our peer group. | Base salaries are intended to provide stable compensation to executive officers, allow us to attract and retain skilled executive talent and maintain a consistent, stable leadership team. |
|
|
|
Short-Term Incentives: Annual Cash Incentive Opportunities | Variable cash compensation based on the level of achievement of certain annual performance objectives that are pre-determined, consisting of research and development, regulatory, operational and financial-based milestone objectives | Annual cash incentive opportunities are designed to motivate our executive officers to achieve our short-term goals; payout levels are generally determined based on the degree of achievement of performance milestones. |
|
|
|
Long-Term Incentives: Equity-Based Compensation | Variable equity-based compensation subject to multi-year vesting.
Stock Options: Right to purchase shares at a price equal to the closing price of our common stock on the grant date. Stock options only have value if our stock price appreciates. RSUs: Right to receive shares upon the applicable vesting date. RSUs provide balance to stock options, which only have value if our stock price appreciates. | Equity-based compensation is designed to motivate and reward executive officers to achieve multi-year strategic goals and to deliver sustained long-term value to stockholders, as well as to attract and retain executive officers for the long term, and to align the interests of our executive officers and employees with those of our stockholders. |
Compensation Program Governance
We assess the effectiveness of our executive compensation program from time to time and review risk mitigation and governance matters, which include maintaining the following best practices:
| | |
What We Do |
|
| | |

| Pay for Performance | The majority of total executive compensation opportunity is variable and at-risk as it is dependent on the performance of the Company’s stock price. |
| | |

| Balance short-term and long-term incentive compensation | We use a mix of short-term and long-term incentive compensation, with an emphasis on long-term incentive compensation consisting of equity awards with multi-year vesting periods. |
| | |

| Peer Group Benchmarking | We compare compensation program design and levels against competitive market data based on our compensation peer group, which we review and adjust annually. |
| | |

| Independent Compensation Consultant | We have engaged an independent compensation consultant to provide information and advice for use in compensation committee decision-making. |
| | |

| Double Trigger Change-in-Control Severance | We have entered into agreements with our named executive officers that provide certain financial benefits if there is both a change in control and termination of employment (a “double trigger”). A change in control alone will not trigger severance pay. |
| | |

| Shareholder Engagement | Engage with shareholders on executive compensation and corporate governance matters. |
|
What We Don’t Do |
|
| | |

| No Excessive Perks | We do not provide large perquisites to executive officers. |
| | |

| No Excise Tax Gross-Ups | We do not provide excise tax gross-ups on change-in-control or severance payments. |
| | |

| No Hedging or Pledging of Company Shares | We do not permit our executive officers and directors to pledge or hedge their shares. |
| | |

| No Special Health, Welfare or Retirement Plans | Our executive officers participate in our retirement, health and welfare benefits programs on the same basis as our other employees. |
| |
|

| No Guaranteed Annual Bonus or Salary Increase | We do not provide our executive officers with guaranteed annual salary increases or annual or multi-year guaranteed bonuses. |
| | |

| No Re-Pricing of Stock Options | We do not re-price stock options without stockholder approval, and we do not grant discounted stock options. |
Role of the Compensation Committee
The compensation committee is responsible for establishing our compensation philosophy and objectives; determining the structure, components and other elements of our compensation program; and reviewing and approving the compensation of the named executive officers, other than the chief executive officer. The compensation committee is responsible for recommending for approval by the board the compensation of our chief executive officer.
To verify the alignment of the program with our business strategy and with the items that we believe drive the creation of stockholder value, the compensation committee reviews the elements of our executive compensation program throughout the year and determines whether it would be appropriate to make any changes to program components.
Our board of directors and its compensation committee annually review the compensation for each of our executive officers in relation to our peers (see “Compensation Peer Groups and Peer Selection Process” section below). In setting executive cash compensation (base salaries and incentive cash bonuses) and granting equity incentive awards, the compensation committee and the board of directors consider compensation for comparable positions in the market; the qualifications, experience and historical compensation levels of our executives; each officer’s individual performance as compared to our expectations and objectives; our desire to motivate the executive to achieve short- and long-term results that are in the best interests of our stockholders; and the desire and need to ensure the executive’s long-term commitment to our Company. The compensation committee and the board of directors generally target compensation for each executive officer to be competitive with the compensation provided by our peers for the same or a similar position. The compensation committee uses independent third-party benchmark analytics as a reference point to inform compensation decisions on each executive, including the mix of base salary, bonus and long-term incentives.
Our board of directors, with respect to our chief executive officer, and our compensation committee, with respect to our other executive officers, have historically determined the compensation of each of our executives. Our compensation committee typically reviews and discusses management’s proposed compensation with the chief executive officer for all executives other than the chief executive officer, with management absent for such discussions. Based on those
discussions and its discretion, taking into account the factors noted above, the compensation committee then approves, or recommends to the board of directors for approval, the compensation for each executive officer, with the chief executive officer absent for any discussions by the compensation committee or the board of directors with respect to chief executive officer compensation.
Role of the Compensation Consultant
In connection with fulfilling its duties, the compensation committee recognizes that there is value in procuring independent, objective expertise and counsel, and has the authority to retain an independent compensation consultant to assist it in carrying out its responsibilities and duties. Our compensation committee engaged Aon's Human Capital Solutions practice, a division of Aon, plc (formerly known as Radford), or Aon, as its independent compensation consultant to advise on executive compensation matters for 2023, including overall compensation program design, peer group development and updates, and director and executive compensation benchmarking surveys.
We develop our compensation programs after reviewing publicly available compensation data and subscription survey data for our peer group, as provided by Aon. The board of directors and the compensation committee consider Aon’s data, analysis and recommendations on certain compensation matters as they deem appropriate.
Aon reports directly to our compensation committee and the committee has the sole authority to retain, terminate and obtain the advice of Aon at the Company’s expense. Our compensation committee has assessed the independence of Aon consistent with Nasdaq listing standards and has concluded that the engagement of Aon does not raise any conflicts of interest.
Compensation Peer Groups and Peer Selection Process
In evaluating the total compensation of our named executive officers, our compensation committee, using information provided by Aon, annually reassesses a peer group of publicly traded, national and regional companies in the biopharmaceutical and biotechnology industries that is selected based on a balance of the following criteria:
•companies whose number of employees, stage of development and market capitalization are similar, though not necessarily identical, to ours;
•companies with similar executive positions to ours;
•companies against which we believe we compete for executive talent; and
•public companies based in the United States whose compensation and financial data are available in proxy statements or through widely available compensation surveys.
In light of the increase in our organizational size and market capitalization, the compensation committee revised our previous peer group selection criteria to reference a market capitalization range of approximately $2.0 billion to $17.6 billion. As a result, based on the criteria set forth above, our peer group for 2023 compensation decisions, referred to as our 2023 peer group, was approved by our compensation committee in September 2022 and was comprised of the companies listed below. As of August 2022, Karuna’s market cap was in the 46th percentile as compared to the market cap of our peer group (using a 30-day average).
| | |
Acadia Pharmaceuticals Inc. | Cytokinetics, Inc.* | Legend Biotech Corporation* |
|
|
|
Apellis Pharmaceuticals, Inc. | Denali Therapeutics Inc. | Mirati Therapeutics, Inc. |
|
|
|
Biohaven Pharmaceutical Holding Company Ltd. | Exelixis, Inc.* | Neurocrine Biosciences, Inc.* |
| | |
BioMarin Pharmaceutical Inc.* | Intra-Cellular Therapies, Inc. | Sage Therapeutics, Inc. |
| |
|
Cerevel Therapeutics Holdings, Inc. | Jazz Pharmaceuticals plc* | Sarepta Therapeutics, Inc.* |
| | |
| | |
The seven companies marked with an asterisk were added to the 2023 peer group; and ten companies that no longer met the criteria were removed (Alector, Inc., Allakos Inc., Arvinas, Inc., Axsome Therapeutics, Inc., BioXcel Therapeutics, Inc., Cortexyme, Inc., Kodiak Sciences Inc., Prothena Corporation plc, Rhythm Pharmaceuticals, Inc. and TG Therapeutics, Inc.). We believe that the compensation practices of our 2023 peer group provided us with appropriate executive
compensation comparisons for evaluating the compensation of our named executive officers for 2023. Notwithstanding the similarities of our 2023 peer group, due to the nature of our business, we compete for executive talent with many public companies that are larger and more established than we are or that possess greater resources than we do, or with smaller private companies that may be able to offer greater equity compensation potential, as well as with prestigious academic and non-profit institutions.
Although our compensation committee and board generally target compensation for each executive officer to be competitive with the compensation provided by our peers for the same or a similar position, they may consider other criteria, including market factors, the experience level of the executive and the executive’s performance against established corporate goals, in determining variations to this general target range.
Role of the Chief Executive Officer
The compensation committee works with our chief executive officer to set the target total direct compensation of each of our named executive officers other than our chief executive officer. As part of this process, our chief executive officer evaluates each named executive officer, determines his recommendations for the target compensation of each named executive officer, and delivers his evaluations and compensation recommendations to the compensation committee.
Elements of our Compensation Program
Annual Base Salary
We provide base salaries to our named executive officers to compensate them with a fair and competitive base level of compensation for services rendered during the year. Prior to making its decision, our compensation committee typically reviews and discusses with the chief executive officer his recommendations for the compensation of our named executive officers other than the chief executive officer. For 2023, we targeted the 50th percentile of our 2023 peer group for base salaries. Except as otherwise noted in the table below, the annual base salary of each of our named executive officers was increased in 2023 in order to move the amounts more closely into alignment with the market medians.
Except as noted below, the table below presents the base salaries for each of our named executive officers for the years 2022 and 2023, as approved by the board of directors and compensation committee, as applicable. Except as noted below, the 2023 base salaries became effective on January 1, 2023.
| | | | | | | | | | | | |
Named Executive Officer | | 2022 Annualized Base Salary | | | January 2023 Change (Approximate) % | | | 2023 Annualized Base Salary | |
Bill Meury(1) | | $ | - | | | | - | | | $ | 750,000 | |
Steven Paul, M.D.(2) | | $ | 700,000 | | | -11% | | | $ | 625,000 | |
Jason Brown(3) | | $ | 350,000 | | | 44% | | | $ | 505,000 | |
Troy Ignelzi | | $ | 460,000 | | | 12% | | | $ | 515,000 | |
Andrew Miller, Ph.D. | | $ | 500,000 | | | 12% | | | $ | 560,000 | |
Stephen Brannan, M.D. | | $ | 470,000 | | | 9% | | | $ | 510,000 | |
William Kane(4) | | $ | - | | | | - | | | $ | 530,000 | |
__________________
(1)Mr. Meury was appointed as President and Chief Executive Officer effective as of January 3, 2023, and his 2023 base salary became effective on this date. As such, there is no 2022 base salary to report.
(2)Dr. Paul resigned as President and Chief Executive Officer and was appointed as Chief Scientific Officer and President of Research and Development effective as of January 3, 2023. His 2023 base salary in this position became effective on this date. Dr. Paul's decrease in base salary corresponds to this change in position.
(3)Mr. Brown was promoted to Chief Financial Officer effective as of September 29, 2023, and his 2023 base salary in this position became effective on this date. Mr. Brown's increase in base salary corresponds to this change in position.
(4)Mr. Kane was appointed as Chief Commercial Officer effective as of February 6, 2023, and his 2023 base salary became effective on this date. As such, there is no 2022 base salary to report.
Annual Cash Incentive Bonuses
Our annual bonus program is intended to reward our named executive officers for meeting individual and/or corporate performance goals for a fiscal year. For 2023, we targeted the 50th percentile of our 2023 peer group for annual cash incentive award opportunities. In February 2023, our compensation committee approved the annual performance-based cash incentive program for 2023 and our board of directors established the corporate goals for 2023.
Our corporate goals are comprised of key strategic, operational and corporate performance goals relating to clinical development, research, commercial, business development and corporate advancement objectives. As outlined in the table below, each of our corporate goals has a designated weighting and the board of directors assesses the achievement of each corporate goal. The corporate goals consist of pre-established “base” goals, the achievement of which would result in up to 100% of target bonus opportunities being earned based on corporate performance, and pre-established “stretch” goals, the achievement of which would reflect exceptional performance above and beyond the expected level of performance and that would result in up to an additional 55% of target bonus opportunities being earned based on corporate performance. In no event may the Company achieve greater than 155% of corporate goals when considering the base and stretch goals.
| | | | | | | | | | | | | | | | | | |
Category | | Goal | | Timing | | Assessment | | Weighting | | | Achievement | | | Payout | |
KarXT NDA | | NDA submitted and filed; FDA Advisory Committee preparation initiated, and pre-filing inspection readiness achieved | | Q3-Q4 | | Achieved: KarXT NDA submitted Q3 2023 and filing accepted by FDA in Q4 2023; FDA Advisory Committee preparation initiated; Pre-filing inspection readiness achieved | | | 50 | % | | | 100 | % | | | 50 | % |
| | Complete Phase 1B Ambulatory Blood Pressure Monitoring study | | Q4 | | Achieved: Announced positive topline data from ABPM study in Q4 2023 | | | 5 | % | | | 100 | % | | | 5 | % |
| | Stretch goal: Complete EMERGENT-5 enrollment | | Q2 | | Achieved: Completed EMERGENT-5 enrollment in Q2 2023 | | | 10 | % | | | 100 | % | | | 10 | % |
| | Stretch goal: Positive EMERGENT-3 topline data | | Q1 | | Achieved: Announced positive topline data from EMERGENT-3 study in Q1 2023 | | | 15 | % | | | 100 | % | | | 15 | % |
Commercial Readiness | | Execute 2023 Medical Affairs plan | | Q4 | | Achieved: Executed 2023 Medical Affairs plan, including all applicable components | | | 5 | % | | | 100 | % | | | 5 | % |
| | Finalize sales projections and production forecast, as well as trade and sample packaging design | | Q1 | | Achieved: Finalized sales projections and production forecast, and trade and sample packaging design | | | 5 | % | | | 100 | % | | | 5 | % |
| | Hire Chief Commercial Officer and Head of Sales; complete sizing for sales force and managed care account organization | | Q3 | | Achieved: Hired CCO in Q1 2023; hired Head of Sales in Q2 2023; completed sizing for sales force and managed care account organization | | | 5 | % | | | 100 | % | | | 5 | % |
Maximizing KarXT | | ARISE enrollment ahead of internal projections | | Q4 | | Not Achieved | | | 5 | % | | | — | % | | | — | % |
| | ADEPT-1: Activate targeted number of clinical sites; Initiate ADEPT-2 and ADEPT-3 | | Q2-Q3 | | Achieved: Activated targeted number of sites for ADEPT-1
Achieved: Initiated ADEPT-2 and ADEPT-3 | | | 5 | % | | | 100 | % | | | 5 | % |
| | Stretch goal: Complete MAD with KarXT second generation formulation | | Q4 | | Achieved: Completed MAD with KarXT second generation | | | 5 | % | | | 100 | % | | | 5 | % |
| | Stretch goal: Decision on fourth indication and development of a protocol synopsis | | Q4 | | Not Achieved | | | 5 | % | | | — | % | | | — | % |
| | Stretch goal: Execute regional licensing deal for KarXT | | Q4 | | Not Achieved | | | 5 | % | | | — | % | | | — | % |
Expanding the Pipeline | | Complete in-life of IND enabling studies for KAR-401 | | Q4 | | Not Achieved | | | 5 | % | | | — | % | | | — | % |
| | Stretch goal: Identify and approve NCE development candidate | | Q4 | | Not Achieved | | | 5 | % | | | — | % | | | — | % |
| | Stretch goal: Execute in-license for pipeline expansion | | Q4 | | Achieved: Executed in-license agreement for Goldfinch Bio's TRPC4/5 candidates, including KAR-2618, in Q1 2023 | | | 10 | % | | | 100 | % | | | 10 | % |
People, Culture and Capabilities | | Scale organization within 10% of target to support R&D, commercial and long-term corporate objectives | | Q4 | | Achieved: Added new hires to scale organization within 10% of target | | | 5 | % | | | 100 | % | | | 5 | % |
| | Maintain high employee satisfaction above internal goal and low turnover (<biotech benchmark), and positive diversity, equity and inclusion outcomes and ESG initiatives | | Q4 | | Achieved: Maintained employee satisfaction above internal goal as measured in employee engagement survey; maintained turnover rate below biotech benchmark; maintained positive DEI outcomes as measured in employee engagement survey; progressed ESG initiatives | | | 5 | % | | | 100 | % | | | 5 | % |
| | Maintain strong balance sheet; operate within 10% of approved budget | | Q4 | | Achieved: Maintained strong balance sheet, bolstered by March 2023 offering; operated within 10% of approved budget | | | 5 | % | | | 100 | % | | | 5 | % |
TOTAL | | | | | | | | | 155 | % | | | | | | 130 | % |
In December 2023, the board of directors evaluated our performance against the 2023 corporate goals. As described in detail in the table above, the board of directors measured each base and stretch goal that was achieved or not achieved. The board of directors considered the achievement of the clear and objective pre-established base and stretch corporate goals, offset by the base and stretch corporate goals that were not achieved in 2023. Accordingly, the board of directors determined that 130% of the corporate goals were achieved by the Company in 2023.
The table below shows the target award under the annual performance-based cash incentive program as a percentage of each named executive officer’s annual base salary in 2023, the target cash award opportunity in dollars for 2023 and the actual cash bonus payments to our named executive officers for 2023 performance, as well as the actual bonus payment as a percentage of the target award opportunity. Such 2023 cash bonus payments were made to our named executive officers in December 2023, rather than in early 2024, in order to mitigate the potential adverse tax consequences of Section 280G and Section 4999 of the Internal Revenue Code of 1986, as amended, that could arise in connection with
the transactions contemplated by the Merger Agreement. Our compensation committee considers it appropriate that each named executive officer’s annual cash bonus is based entirely upon achievement of our corporate performance goals.
| | | | | | | | | | | | | | | | |
Named Executive Officer | | 2023 Target Cash Incentive Award (% of Base Salary) | | | 2023 Target Cash Incentive Award Opportunity ($) | | | 2023 Actual Cash Incentive Award Payment (% of 2023 Target Cash Incentive Award Opportunity) | | | 2023 Cash Incentive Award Payment ($) | |
Bill Meury | | | 75 | % | | | 562,500 | | | | 130 | % | | | 731,250 | |
Steven Paul, M.D. | | | 50 | % | | | 312,500 | | | | 130 | % | | | 406,250 | |
Jason Brown | | | 45 | % | | | 227,250 | | | | 130 | % | | | 295,425 | |
Troy Ignelzi(1) | | | 45 | % | | | 231,750 | | | | — | % | | | — | |
Andrew Miller, Ph.D. | | | 50 | % | | | 280,000 | | | | 130 | % | | | 364,000 | |
Stephen Brannan, M.D. | | | 45 | % | | | 229,500 | | | | 130 | % | | | 298,350 | |
William Kane | | | 50 | % | | | 265,000 | | | | 130 | % | | | 344,500 | |
__________________
(1)Mr. Ignelzi departed from his position as Chief Financial Officer effective as of September 29, 2023. As such, he did not receive a 2023 cash incentive award payment. See "Potential Payments Upon a Change in Control" in this Amendment No. 1 for a description of Mr. Ignelzi's severance arrangement in connection with his departure.
Long-Term Incentives; Equity-based Compensation
Although we do not have a formal policy with respect to the grant of equity incentive awards to our named executive officers, we believe that equity grants provide our named executive officers with a strong link to our long-term performance, create an ownership culture and help to align the interests of our named executive officers and our stockholders. In addition, we believe that equity grants with a time-based vesting feature promote executive retention because this feature incentivizes our named executive officers to remain in our employment during the vesting period.
The compensation committee typically recommends for approval by the board of directors long-term incentive grants for named executive officers at the start of their employment, and annually thereafter in connection with their annual performance review. Additionally, the board of directors may periodically, on a limited and judicious basis, grant additional equity awards based on individual role, performance and contribution, as well as competitive market data and information.
None of our named executive officers are currently party to an employment agreement that provides for an automatic award of stock options or other equity. In 2023, we granted equity awards to our named executive officers with time-based vesting, in the form of stock options and RSUs. The number of shares subject to these equity awards was determined by reviewing the equity grants made to the executive officers of our 2023 peer group, both evaluating the Black-Scholes option fair value of the stock option grants and the fair value of the restricted stock unit grants, as well as the value such awards represented compared to the value of the company overall. For 2023, we targeted the 75th percentile of our 2023 peer group with respect to annual equity incentive awards. Our compensation committee made the decision to target the 75th percentile to account for the fact that we compete for executive talent with public companies that are in many cases outside of our peer group, including many public companies that are larger and more established than we are or that possess greater resources than we do, and with smaller private companies that may be able to offer greater equity compensation potential, as well as with prestigious academic and non-profit institutions. Our compensation committee determined that setting long-term incentive grants at the 75th percentile would help us attract and retain top caliber executive talent while ensuring that such compensation was tied to the creation of shareholder value. Although our compensation committee and board generally target compensation for each executive officer to be competitive with the compensation provided by our peers for the same or a similar position, they may consider other criteria, including market factors, the experience level of the executive and the executive’s performance against established corporate goals, in determining variations to this general target range.
The stock options that we grant to our named executive officers typically become exercisable as to 25% of the shares underlying the option on the first anniversary of the grant date, and as to an additional 1/12th of the shares underlying the option quarterly thereafter, subject to the named executive officer’s continued service through each vesting date. The time-based vesting schedules of awards granted in 2023 were consistent with our previous time-based vesting awards. The exercise price of all stock options equals the closing price of our common stock as reported on the Nasdaq Global Market on the grant date. The RSUs that we grant to our named executive officers typically vest as to each 25% of the RSUs on each anniversary of the grant date over four years, subject to the named executive officer’s continued service
through each vesting date. We granted RSUs to our named executive officers for the first time in 2023. On December 21, 2023, the board of directors approved certain tax-planning actions to mitigate the adverse tax consequences of Section 280G and Section 4999 of the Internal Revenue Code of 1986, as amended, that could arise in connection with proposed merger transaction with Bristol Myers Squibb. Specifically, the board of directors approved, among other things, the accelerated vesting and settlement of 50% of the RSU awards granted to Mr. Meury in January 2023 and to Mr. Kane in February 2023, which accelerated awards vested on December 28, 2023.
The following table sets forth the number of shares of common stock issuable upon exercise of time-based stock options and the number of RSUs granted to our named executive officers in 2023:
| | | | | | | | | | |
Named Executive Officer | | Grant Date | | Option Award
Shares
(# Shares) | | | RSU Award Shares
(# Shares) | |
Bill Meury(1) | | 1/3/2023 | | | 63,065 | | | | 38,073 | |
Steven Paul, M.D. | | 2/9/2023 | | | 22,800 | | | | 11,400 | |
Jason Brown(2) | | 2/13/2023 | | | 7,100 | | | | 3,550 | |
| | 9/29/2023 | | | 7,696 | | | | 4,496 | |
Troy Ignelzi | | 2/9/2023 | | | 17,350 | | | | 8,650 | |
Andrew Miller, Ph.D. | | 2/9/2023 | | | 25,100 | | | | 12,550 | |
Stephen Brannan, M.D. | | 2/9/2023 | | | 17,350 | | | | 8,650 | |
William Kane(3) | | 2/6/2023 | | | 29,937 | | | | 17,656 | |
__________________
(1)The grant of stock options and RSUs to Mr. Meury in 2023 consists of his on-hire award in connection with his appointment as President and Chief Executive Officer. For more information regarding this on-hire award, see the Section above titled "New Chief Executive Officer Compensation Package".
(2)Mr. Brown was promoted to Chief Financial Officer effective as of September 29, 2023. As such, he received two grants of stock options and RSUs in 2023: one in connection with the annual grant of awards to employees in February 2023, and one in connection with his promotion in September 2023.
(3)The grant of stock options and RSUs to Mr. Kane in 2023 consists of his on-hire award in connection with his appointment as Chief Commercial Officer.
401(k) Plan
We administer a 401(k) retirement plan which is intended to be a tax-qualified defined contribution plan under Section 401(k) of the Internal Revenue Code. In general, all of our employees are eligible to participate. The 401(k) plan includes a salary deferral arrangement pursuant to which participants may elect to reduce their current compensation by up to the statutorily prescribed limit and have the amount of the reduction contributed to the 401(k) plan. We contribute to each employee’s 401(k) account, in the first quarter of each year, 3% of his or her eligible earnings from the prior year. Such contributions are not subject to vesting.
Health and Welfare Benefits
All of our full-time employees, including our named executive officers, are eligible to participate in certain medical, disability and life insurance benefit programs as offered by us. We pay the full premiums for dental, vision, term life insurance and disability for all of our employees, including our executive officers. We do not sponsor any qualified or non-qualified defined benefit plans for any of our employees or executives.
Severance and Change of Control Benefits
Each named executive officer is also eligible for severance benefits in specified circumstances, as set forth in each such officer’s employment agreement. Under the terms of these agreements, upon execution and effectiveness of a severance agreement and release of claims, each named executive officer will be entitled to severance payments if at any time, including within 12 months following (or, with respect to our Chief Executive Officer, within 3 months before) a change in control:
•we terminate his employment without cause; or
•he terminates employment with us for good reason.
We provide these severance arrangements because we believe that, in a competitive market for talent, severance arrangements are necessary to attract and retain high quality executives. In addition, the change in control benefit allows and incentivizes executives to maintain their focus on our business during a period when they otherwise might be distracted. Please refer to “ — Employment Arrangements with our Named Executive Officers” below for a more detailed discussion of severance benefits for our named executive officers.
Tax and Accounting Considerations
Deductibility of Executive Compensation
Generally, Section 162(m) of the Code, or Section 162(m), disallows a federal income tax deduction for public corporations of remuneration in excess of $1 million paid in any fiscal year to certain specified executive officers. In designing our executive compensation program and determining the compensation of our executive officers, including our named executive officers, the compensation committee considers a variety of factors, including the potential impact of the Section 162(m) deduction limit. The deductibility of some types of compensation depends upon the timing of an executive officer’s vesting or exercise of previously granted rights. Further, interpretations of and changes in the tax laws, and other factors beyond the compensation committee’s control also affect the deductibility of compensation. The compensation committee will consider various alternatives to preserving the deductibility of compensation payments and benefits to the extent consistent with its compensation goals. However, to maintain flexibility to compensate our executive officers in a manner designed to promote our short-term and long-term corporate goals, our compensation committee has not adopted a policy that all compensation must be deductible. The compensation committee believes that our stockholders’ interests are best served if its discretion and flexibility in awarding compensation is not restricted in order to allow such compensation to be consistent with the goals of our executive compensation program, even though some compensation awards may result in non-deductible compensation expense.
Accounting for Stock-Based Compensation
We follow the Financial Accounting Standard Board’s Accounting Standards Codification Topic 718, or FASB ASC Topic 718, for our stock-based compensation awards. FASB ASC Topic 718 requires us to measure the compensation expense for all share-based payment awards made to our employees and non-employee members of our board of directors, including stock options to purchase shares of our common stock, RSUs, and other stock awards, based on the grant date “fair value” of these awards. This calculation is performed for accounting purposes and reported in the executive compensation tables required by the federal securities laws, even though the recipient of the awards may never realize any value from their awards.
Compensation risk assessment
We believe that our executive compensation program does not encourage excessive or unnecessary risk taking. This is primarily due to the fact that our compensation programs are designed to encourage our executive officers and other employees to remain focused on both short-term and long-term strategic goals, in particular in connection with our pay-for-performance compensation philosophy. As a result, we do not believe that our compensation programs are reasonably likely to have a material adverse effect on us.
Hedging and Pledging Policy
We have an insider trading policy that is applicable to all of our employees and the non-employee members of our board of directors. The policy prohibits those individuals and their related persons from engaging in any hedging transactions involving our securities at any time, including buying or selling puts, calls, other derivative securities of the Company, or any derivative securities that provide the economic equivalent of ownership of any of the Company’s securities or an opportunity, whether direct or indirect, to profit from any change in the value of the Company’s securities. In addition, the policy prohibits those individuals and their related persons from pledging any Company securities as collateral for a loan or using Company securities as collateral in a margin account.
Compensation Recovery (Clawback) Policy
In 2023, we adopted a compensation recovery, or clawback, policy consistent with Nasdaq’s applicable listing standards, setting forth the circumstances in which our Section 16 officers may be obligated to repay or forfeit "excess compensation" in connection with an accounting restatement. Each named executive officer identified herein and currently employed by the Company is also a Section 16 officer and is subject to the terms and conditions of this policy. A copy of the clawback policy is filed as Exhibit 97.1 to the 2023 Annual Report.
Compensation Committee Report
The compensation committee has reviewed and discussed the Compensation Discussion and Analysis with the management of the Company. Based on this review and these discussions, we have recommended to the board of directors that the Compensation Discussion and Analysis be included in this Amendment No. 1 to the Company's Annual Report on Form 10-K for the year ended December 31, 2023, which was filed with the SEC on February 22, 2024.
The preceding report has been furnished by the following members of the Committee:
Atul Pande, M.D.
Christopher Coughlin
Denice Torres
EXECUTIVE AND DIRECTOR COMPENSATION
Executive Compensation
Summary Compensation Table
The following table sets forth the total compensation awarded to, earned by and paid to our named executive officers during the fiscal years set forth below.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Name and Principal Position | | Year | | Salary ($) | | | Bonus ($) | | | Stock Awards ($) (1) | | | Option Awards ($) (2) | | | Non-Equity Incentive Compensation ($) (3) | | | All Other Compensation ($) (4) | | | Total ($) | |
Bill Meury (5) | | 2023 | | | 746,875 | | | - | | | | 7,355,323 | | | | 7,354,017 | | | | 731,250 | | | | 9,916 | | | | 16,197,381 | |
President and Chief | | | | | | | | | | | | | | | | | | | | | | | |
Executive Officer | | | | | | | | | | | | | | | | | | | | | | | |
Steven Paul, M.D. (6) | | 2023 | | | 625,000 | | | - | | | | 2,134,764 | | | | 2,520,105 | | | | 406,250 | | | - | | | | 5,686,119 | |
Former President and | | 2022 | | | 700,000 | | | - | | | - | | | | 9,905,953 | | | | 609,000 | | | | 9,150 | | | | 11,224,103 | |
Chief Executive Officer | | 2021 | | | 645,000 | | | - | | | - | | | | 11,933,397 | | | | 549,863 | | | | 8,700 | | | | 13,136,960 | |
Jason Brown (7) | | 2023 | | | 404,275 | | | - | | | | 1,441,083 | | | | 1,564,241 | | | | 295,425 | | | | 9,210 | | | | 3,714,235 | |
Chief Financial Officer | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
Troy Ignelzi (8) | | 2023 | | | 386,250 | | | - | | | | 1,619,799 | | | | 1,917,711 | | | - | | | | 612,206 | | (9) | | 4,535,966 | |
Former Chief Financial | | 2022 | | | 460,000 | | | - | | | - | | | | 3,270,515 | | | | 266,800 | | | | 9,150 | | | | 4,006,465 | |
Officer | | 2021 | | | 430,000 | | | - | | | - | | | | 3,808,217 | | | | 266,600 | | | | 8,700 | | | | 4,513,517 | |
Andrew Miller, Ph.D. (10) | | 2023 | | | 560,000 | | | - | | | | 2,350,113 | | | | 2,774,326 | | | | 364,000 | | | | 13,161 | | | | 6,061,600 | |
President, Research and | | 2022 | | | 500,000 | | | - | | | - | | | | 4,939,490 | | | | 326,250 | | | | 9,150 | | | | 5,774,890 | |
Development | | 2021 | | | 460,000 | | | - | | | - | | | | 4,932,618 | | | | 320,850 | | | | 8,700 | | | | 5,722,168 | |
Stephen Brannan, M.D. | | 2023 | | | 510,000 | | | - | | | | 1,619,799 | | | | 1,917,711 | | | | 298,350 | | | | 2,867 | | | | 4,348,727 | |
Chief Medical Officer | | 2022 | | | 470,000 | | | - | | | - | | | | 3,661,629 | | | | 272,600 | | | | 9,150 | | | | 4,413,379 | |
| | 2021 | | | 450,000 | | | - | | | - | | | | 3,786,098 | | | | 279,000 | | | | 8,700 | | | | 4,523,798 | |
William Kane (11) | | 2023 | | | 479,811 | | | | 100,000 | | (12) | | 3,392,600 | | | | 3,392,013 | | | | 344,500 | | | | 9,831 | | | | 7,718,756 | |
Chief Commercial Officer | | | | | | | | | | | | | | | | | | | | | | | |
__________________
(1)Amounts reported represent the aggregate grant date fair value of the RSUs granted to such named executive officers during 2023 as computed in accordance with FASB ASC Topic 718, not including any estimates of forfeitures related to service-based vesting conditions.
(2)Amounts reported represent the aggregate grant date fair value of the stock options granted to such named executive officers during 2023, 2022, and 2021, as computed in accordance with FASB ASC Topic 718, not including any estimates of forfeitures related to service-based vesting conditions. See Note 8 of “Notes to Consolidated Financial Statements” in our 2023 Annual Report filed with the SEC on February 22, 2024 for a discussion of assumptions made by the Company in determining the aggregate grant date fair value of our option awards.
(3)Amounts reported reflect the annual cash incentive bonus paid based upon achievement of certain corporate performance objectives described above under “Annual Cash Incentive Bonuses.”
(4)Amounts reported reflect matching contributions to our 401(k) plan, in addition to other amounts denoted in the footnotes to this column.
(5)Mr. Meury commenced employment with us on January 3, 2023 and, accordingly, his 2023 base salary has been prorated to reflect his partial year of service. Mr. Meury also serves as a member of our board of directors but does not receive any additional compensation for his service as a director.
(6)Effective January 3, 2023, Dr. Paul resigned as President and Chief Executive Officer and was appointed Chief Scientific Officer and President of Research and Development. Effective January 16, 2024, Dr. Paul resigned as Chief Scientific Officer and President of Research and Development. Dr. Paul continues to serve as a member of our board of directors but did not receive any additional compensation for his service as a director while he was an employee of the Company.
(7)Mr. Brown was promoted to Chief Financial Officer effective September 29, 2023. Accordingly, his 2023 base salary reflects his partial year of service as Chief Financial Officer and his partial year of service in his prior role as SVP, Finance. Mr. Brown was not a named executive officer for 2022 or 2021. As a result, no compensation for Mr. Brown for 2022 or 2021 is included in this table.
(8)Mr. Ignelzi departed from his position as Chief Financial Officer as of September 29, 2023.
(9)Amount reported represents severance benefits payable to Mr. Ignelzi pursuant to his separation agreement, consisting of $386,250, representing an amount equal to nine months of his base salary, payable in substantially equal installments over nine months following his termination, and $225,956, representing his pro-rated target bonus for 2023.
(10)Dr. Miller served as Chief Operating Officer from August 2018 until his appointment as President of Research and Development effective upon Dr. Paul's departure from that position on January 16, 2024.
(11)Mr. Kane commenced employment with us on February 6, 2023 and, accordingly, his 2023 base salary has been prorated to reflect his partial year of service.
(12)Amount reported represents a sign-on bonus that Mr. Kane is obligated to repay to the Company in the event he resigns or his employment is terminated by the Company for "cause" (as defined in his employment agreement) within two years following February 6, 2023.
Grants of Plan-Based Awards for Fiscal Year 2023
The following table sets forth information concerning each grant of an award made to a named executive officer during the fiscal year ended December 31, 2023 under any plan, contract, authorization or arrangement pursuant to which cash, securities, similar instruments or other property may be received.
| | | | | | | | | | | | | | | | | | | | |
Name | | Date of
Grant | | Estimated Future
Payouts Under Non-
Equity Incentive
Plan Awards: Target
($)(1) | | | All Other Stock
Awards: Number of
Shares of Stock or Units (#)(2) | | | All Other Option
Awards: Number of
Securities Underlying
Options (#)(3) | | | Exercise or
Base Price of
Option Awards
($)(4) | | Grant Date Fair
Value of Stock and
Option Awards
($)(5) | |
Bill Meury | | — | | | 562,500 | | | — | | | — | | | — | | — | |
| | 1/3/2023 | (6) | — | | | | 38,073 | | | | 63,065 | | | 193.19 | | | 14,709,340 | |
Steven Paul, M.D. | | — | | | 312,500 | | | | | | — | | | — | | — | |
| | 2/9/2023 | | — | | | | 11,400 | | | | 22,800 | | | 187.26 | | | 4,654,869 | |
Jason Brown | | — | | | 227,250 | | | — | | | — | | | — | | — | |
| | 2/13/2023 | | — | | | | 3,550 | | | | 7,100 | | | 191.79 | | | 1,485,620 | |
| | 9/29/2023 | (7) | — | | | | 4,496 | | | | 7,696 | | | 169.09 | | | 1,519,704 | |
Troy Ignelzi | | — | | | 231,750 | | | — | | | — | | | — | | — | |
| | 2/9/2023 | | — | | | | 8,650 | | | | 17,350 | | | 187.26 | | | 3,537,510 | |
Andrew Miller, Ph.D. | | — | | | 280,000 | | | — | | | — | | | — | | — | |
| | 2/9/2023 | | — | | | | 12,550 | | | | 25,100 | | | 187.26 | | | 5,124,439 | |
Stephen Brannan, M.D. | | — | | | 229,500 | | | | | | — | | | — | | — | |
| | 2/9/2023 | | — | | | | 8,650 | | | | 17,350 | | | 187.26 | | | 3,537,510 | |
William Kane | | — | | | 265,000 | | | — | | | — | | | — | | — | |
| | 2/6/2023 | (8) | — | | | | 17,656 | | | | 29,937 | | | 192.15 | | | 6,784,614 | |
__________________
(1)Represents the target amount of each executive’s cash payments under our 2023 annual performance-based cash incentive program as described in “Compensation Discussion and Analysis” above. Actual payments made for 2023 are provided in the “Summary Compensation Table.” As there are no threshold or maximum amounts with respect to these performance-based cash payments, the columns “Threshold ($)” and “Maximum ($)” are inapplicable and therefore have been omitted from this table.
(2)Represents RSUs awarded with time-based vesting criteria established by the compensation committee and described in Compensation Discussion and Analysis above. To mitigate potential adverse tax consequences under Section 280G of the Internal Revenue Code of 1986, as amended, in connection with the proposed merger transaction with Bristol Myers Squibb, the Company accelerated vesting for 50% of the RSU awards granted to Mr. Meury in January 2023 and to Mr. Kane in February 2023, which accelerated awards vested on December 28, 2023.
(3)Represents options awarded with time-based vesting criteria established by the compensation committee and described in Compensation Discussion and Analysis above.
(4)The exercise price of these stock options is equal to the closing price of our common stock as reported on the Nasdaq Global Market on the grant date.
(5)These amounts represent the aggregate grant date fair value of awards granted to our named executive officers in 2023, computed in accordance with FASB ASC Topic 718, not including any estimates of forfeitures related to service-based vesting conditions. See Note 8 of “Notes to Consolidated Financial Statements” in our 2023 Annual Report filed with the SEC on February 22, 2024 for a discussion of assumptions made by the Company in determining the aggregate grant date fair value of our option awards.
(6)The grant of stock options and RSUs to Mr. Meury in January 2023 consists of his on-hire award in connection with his appointment as President and Chief Executive Officer.
(7)The grant of stock options and RSUs to Mr. Brown in September 2023 consists of the equity award in connection with his promotion to Chief Financial Officer.
(8)The grant of stock options and RSUs to Mr. Kane in February 2023 consists of his on-hire award in connection with his appointment as Chief Commercial Officer.
Equity Compensation
Outstanding equity awards at December 31, 2023
The following table sets forth information concerning the outstanding equity awards held by each of the named executive officers as of December 31, 2023.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Option Awards | | | Stock Awards | |
Name | | Vesting
commencement
date | | | Number of
securities
underlying
unexercised
options
(#) exercisable | | | Number of
securities
underlying
unexercised
options
(#) unexercisable | | | Option
exercise
price
($/share) | | | Option
expiration
date | | | Number of Shares or Units of Stock That Have Not Vested (#) | | | Market Value of Shares or Units of Stock That Have Not Vested
($)(1) | |
Bill Meury | | 1/3/2023 | | | | - | | | | 63,065 | | (2) | | 193.19 | | | 1/2/2033 | | | | - | | | | - | |
| | 1/3/2023 | | | | - | | | | - | | | | - | | | | - | | | | 19,037 | | (3) | | 6,025,401 | |
Steven Paul, M.D. | | 2/28/2018 | | | | 71,628 | | | | - | | | | 7.04 | | | 4/29/2028 | | | | - | | | | - | |
| | 3/15/2019 | | | | 64,466 | | | | - | | | | 7.04 | | | 4/29/2028 | | | | - | | | | - | |
| | 6/15/2018 | | | | 784,555 | | | | - | | | | 7.27 | | | 8/8/2028 | | | | - | | | | - | |
| | 3/15/2019 | | | | 449,463 | | | | - | | | | 9.20 | | | 3/20/2029 | | | | - | | | | - | |
| | 3/15/2019 | | | | 87,494 | | | | - | | | | 9.20 | | | 3/28/2029 | | | | - | | | | - | |
| | 3/15/2019 | | | | 15,205 | | | | - | | | | 9.20 | | | 4/7/2029 | | | | - | | | | - | |
| | 6/15/2019 | | | | 616,703 | | | | - | | | | 16.00 | | | 6/26/2029 | | | | - | | | | - | |
| | 6/15/2019 | | | | 71,121 | | | | - | | | | 20.02 | | | 6/27/2029 | | | | - | | | | - | |
| | 2/14/2020 | | | | 131,250 | | | | 8,750 | | (2) | | 99.72 | | | 2/13/2030 | | | | - | | | | - | |
| | 2/23/2021 | | | | 111,272 | | | | 50,578 | | (2) | | 131.64 | | | 2/22/2031 | | | | - | | | | - | |
| | 2/16/2022 | | | | 64,269 | | | | 82,631 | | (2) | | 111.97 | | | 2/16/2032 | | | | - | | | | - | |
| | 2/9/2023 | | | | - | | | | 22,800 | | (2) | | 187.26 | | | 2/8/2033 | | | | - | | | | - | |
| | 2/9/2023 | | | | - | | | | - | | | | - | | | | - | | | | 11,400 | | (4) | | 3,608,214 | |
Jason Brown | | 2/14/2020 | | | | 2,188 | | | | 1,093 | | (2) | | 99.72 | | | 2/13/2030 | | | | - | | | | - | |
| | 2/23/2021 | | | | 1,875 | | | | 4,687 | | (2) | | 131.64 | | | 2/22/2031 | | | | - | | | | - | |
| | 2/16/2022 | | | | 1,875 | | | | 8,437 | | (2) | | 111.97 | | | 2/16/2032 | | | | - | | | | - | |
| | 2/13/2023 | | | | - | | | | 7,100 | | (2) | | 191.79 | | | 2/12/2033 | | | | - | | | | - | |
| | 9/29/2023 | | | | - | | | | 7,696 | | (2) | | 169.09 | | | 9/28/2033 | | | | - | | | | - | |
| | 2/13/2023 | | | | - | | | | - | | | | - | | | | - | | | | 3,550 | | (4) | | 1,123,611 | |
| | 9/29/2023 | | | | - | | | | - | | | | - | | | | - | | | | 4,496 | | (4) | | 1,423,029 | |
Troy Ignelzi | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
Andrew Miller, Ph.D. | | 7/18/2016 | | | | 47,817 | | | | - | | | | 2.92 | | | 10/11/2026 | | | | - | | | | - | |
| | 8/15/2018 | | | | 11,578 | | | | - | | | | 7.27 | | | 8/8/2028 | | | | - | | | | - | |
| | 3/21/2019 | | | | 231,992 | | | | - | | | | 9.20 | | | 3/20/2029 | | | | - | | | | - | |
| | 2/14/2020 | | | | 65,625 | | | | 4,375 | | (2) | | 99.72 | | | 2/13/2030 | | | | - | | | | - | |
| | 2/23/2021 | | | | 45,994 | | | | 20,906 | | (2) | | 131.64 | | | 2/22/2031 | | | | - | | | | - | |
| | 2/16/2022 | | | | 32,047 | | | | 41,203 | | (2) | | 111.97 | | | 2/16/2032 | | | | - | | | | - | |
| | 2/9/2023 | | | | - | | | | 25,100 | | (2) | | 187.26 | | | 2/8/2033 | | | | - | | | | - | |
| | 2/9/2023 | | | | - | | | | - | | | | - | | | | - | | | | 12,550 | | (4) | | 3,972,201 | |
Stephen Brannan, M.D. | | 3/1/2017 | | | | 12,177 | | | | - | | | | 5.45 | | | 6/1/2027 | | | | - | | | | - | |
| | 3/21/2019 | | | | 6,362 | | | | - | | | | 9.20 | | | 3/20/2029 | | | | - | | | | - | |
| | 2/14/2020 | | | | 46,875 | | | | 3,125 | | (2) | | 99.72 | | | 2/13/2030 | | | | - | | | | - | |
| | 2/23/2021 | | | | 5,304 | | | | 16,046 | | (2) | | 131.64 | | | 2/22/2031 | | | | - | | | | - | |
| | 2/16/2022 | | | | 23,757 | | | | 30,543 | | (2) | | 111.97 | | | 2/16/2032 | | | | - | | | | - | |
| | 2/9/2023 | | | | - | | | | 17,350 | | (2) | | 187.26 | | | 2/8/2033 | | | | - | | | | - | |
| | 2/9/2023 | | | | - | | | | - | | | | - | | | | - | | | | 8,650 | | (4) | | 2,737,812 | |
William Kane | | 2/6/2023 | | | | - | | | | 29,937 | | (2) | | 192.15 | | | 2/5/2033 | | | | - | | | | - | |
| | 2/6/2023 | | | | - | | | | - | | | | - | | | | - | | | | 8,828 | | (3) | | 2,794,150 | |
__________________
(1)The market value of the stock awards is determined by multiplying the number of shares subject to the award by $316.51, which was the closing price of our common stock on December 29, 2023, the last trading day of the fiscal year.
(2)This option vests as to 25% of the shares on the first anniversary of the vesting commencement date, with additional vesting as to 6.25% of the shares underlying the option award on each three month anniversary thereafter.
(3)Represents RSUs that vest in equal 25% installments over four years on each anniversary of the vesting commencement date. To mitigate potential adverse tax consequences under Section 280G of the Internal Revenue Code of 1986, as amended, in connection with the proposed merger transaction with Bristol Myers Squibb, the Company accelerated vesting for 50% of these RSUs, which occurred on December 28, 2023.
(4)Represents RSUs that vest in equal 25% installments over four years on each anniversary of the vesting commencement date.
Option Exercises and Stock Vested in Fiscal Year 2023
The following table sets forth information concerning option exercises and stock vested for each of our named executive officers during the fiscal year ended December 31, 2023:
| | | | | | | | | | | | | | | | |
| | Option Awards | | | Stock Awards | |
Name | | Number of
shares acquired
on exercise (#) | | | Value realized on
exercise ($) (1) | | | Number of shares acquired on vesting (#) | | | Value realized on vesting ($) (2) | |
Bill Meury | | | — | | | | — | | | | 19,036 | | | | 6,021,087 | |
Steven Paul, M.D. |
| | — | | | | — | | | | — | | | | — | |
Jason Brown | | | 44,214 | | | | 6,381,784 | | | | — | | | | — | |
Troy Ignelzi |
| | 218,523 | | | | 46,847,041 | | | | — | | | | — | |
Andrew Miller, Ph.D. |
| | 38,500 | | | | 7,345,695 | | | | — | | | | — | |
Stephen Brannan, M.D. | | | 62,500 | | | | 7,475,479 | | | | — | | | | — | |
William Kane | | | — | | | | — | | | | 8,828 | | | | 2,792,296 | |
__________________
(1)Value realized on exercise of stock option awards does not represent proceeds from any sale of any common stock acquired upon exercise, but is determined by multiplying the number of shares acquired upon exercise by the difference between the exercise price of the option and the closing price of our common stock on the Nasdaq Global Market at each time of exercise.
(2)To mitigate adverse tax consequences under Section 280G of the Internal Revenue Code of 1986, as amended, in connection with the proposed merger transaction with Bristol Myers Squibb, the Company accelerated vesting for 50% of the RSUs granted to Mr. Meury and Mr. Kane on January 3, 2023 and February 6, 2023, respectively. The value realized upon vesting represents the number of vested RSUs multiplied by the closing price of our common stock on the Nasdaq Global Market at the time of vesting, which occurred on December 28, 2023.
Employment Arrangements with our Named Executive Officers
We have entered into employment agreements with each of our named executive officers, which, unless otherwise noted below, were amended and restated effective December 22, 2023. Except as noted below, these employment agreements provide for “at will” employment.
Employment Agreement with Bill Meury
Under the amended and restated employment agreement with Bill Meury, Mr. Meury's base salary was set at $750,000, which will be reviewed annually by our compensation committee, and he will be eligible to earn annual incentive compensation with a target amount equal to 75% of his base salary. Mr. Meury is also eligible to participate in the employee benefit plans available to our employees, including our stock option plan, subject to the terms of those plans.
Mr. Meury's employment agreement provides that, in the event that his employment is terminated by us without “cause” or Mr. Meury resigns for “good reason” (each as defined in his employment agreement), subject to the execution and effectiveness of a separation agreement, including a general release of claims in our favor, he will be entitled to receive (i) an amount equal to 18 months of his base salary, payable in substantially equal installments over 18 months following his termination, (ii) his pro-rated target bonus, (iii) acceleration of vesting of all time-based stock options and other stock-based awards held by Mr. Meury that would have vested in the 18 months following his termination, and (iv) if Mr. Meury elects continuation of health coverage under COBRA, continued health coverage at the active employees’ rate until the earlier of 18 months following his termination, the date he becomes eligible for group medical benefits with another employer or the end of his COBRA health continuation period.
In lieu of the payments and benefits described in the preceding sentence, in the event that Mr. Meury’s employment is terminated by us without cause or Mr. Meury resigns for good reason, in either case within 3 months prior to or 12 months following a “change in control” (as defined in his employment agreement), subject to the execution and effectiveness of a separation agreement, including a general release of claims in our favor, he will be entitled to receive (i) an amount equal to 24 months of his base salary plus two times his target bonus for the year, payable within 60 days of his date of termination, (ii) full acceleration of vesting of all time-based stock options and other stock-based awards held by Mr. Meury on the termination date, and (iii) if Mr. Meury elects continuation of health coverage under COBRA, continued health coverage at the active employees’ rate until the earlier of 24 months following his termination, the date he becomes eligible for group medical benefits with another employer or the end of Mr. Meury’s COBRA health continuation period.
The payments and benefits provided to Mr. Meury under his employment agreement in connection with a change in control may not be eligible for a federal income tax deduction for the Company pursuant to Section 280G of the Code. These payments and benefits also may be subject to an excise tax under Section 4999 of the Code. If the payments or benefits payable to Mr. Meury in connection with a change in control would be subject to the excise tax on golden parachutes imposed under Section 4999 of the Code, then those payments or benefits will be reduced if such reduction would result in a higher net after-tax benefit to such officer.
In addition, Mr. Meury has executed an Employee Invention and Non-Disclosure Agreement and a Non-Competition and Non-Solicitation Agreement which contain certain restrictive covenants, including, among other things, non-competition and non-solicitation provisions that apply during the term of Mr. Meury’s employment and for 12 months thereafter.
Employment Agreement with Steven Paul, M.D.
Dr. Paul resigned from his position as our Chief Scientific Officer and President of Research and Development effective as of January 16, 2024. Prior to such resignation, Dr. Paul was party to a second amended and restated employment agreement with us as described below.
Under the second amended and restated employment agreement, Dr. Paul’s base salary was set at $625,000, subject to annual review by our compensation committee, and he was eligible to earn annual incentive compensation with a target amount equal to 50% of his base salary. Dr. Paul was also eligible to participate in the employee benefit plans available to our employees, including our stock option plan, subject to the terms of those plans.
Dr. Paul’s employment agreement provided that, in the event that his employment was terminated by us without “cause” or Dr. Paul resigns for “good reason” (each as defined in his employment agreement), subject to the execution and effectiveness of a separation agreement, including a general release of claims in our favor, he would be entitled to receive (i) an amount equal to 12 months of his base salary, payable in substantially equal installments over 12 months following his termination, (ii) his pro-rated target bonus, (iii) acceleration of vesting of all time-based stock options and other stock-based awards held by Dr. Paul that would have vested in the 12 months following his termination, and (iv) if Dr. Paul elected continuation of health coverage under COBRA, continued health coverage at the active employees’ rate until the earlier of 12 months following his termination, the date he became eligible for group medical benefits with another employer or the end of his COBRA health continuation period.
In lieu of the payments and benefits described in the preceding sentence, in the event that Dr. Paul’s employment was terminated by us without cause or Dr. Paul resigned for good reason, in either case within 12 months following a “change in control” (as defined in his employment agreement), subject to the execution and effectiveness of a separation agreement, including a general release of claims in our favor, he would be entitled to receive (i) an amount equal to 18 months of his base salary plus his 1.5 times his target bonus for the year, payable within 60 days of his date of termination, (ii) full acceleration of vesting of all time-based stock options and other stock-based awards held by Dr. Paul on the termination date, and (iii) if Dr. Paul elected continuation of health coverage under COBRA, continued health coverage at the active employees’ rate until the earlier of 18 months following his termination, the date he became eligible for group medical benefits with another employer or the end of Dr. Paul’s COBRA health continuation period.
The payments and benefits provided to Dr. Paul under his employment agreement in connection with a change in control may not be eligible for a federal income tax deduction for the Company pursuant to Section 280G of the Code. These payments and benefits also may be subject to an excise tax under Section 4999 of the Code. If the payments or benefits payable to Dr. Paul in connection with a change in control would be subject to the excise tax on golden parachutes imposed under Section 4999 of the Code, then those payments or benefits would be reduced if such reduction would result in a higher net after-tax benefit to such officer.
In addition, Dr. Paul has executed an Employee Invention and Non-Disclosure Agreement and a Non-Competition and Non-Solicitation Agreement which contain certain restrictive covenants, including, among other things, non-competition and non-solicitation provisions that apply during the term of Dr. Paul’s employment and for 12 months thereafter.
Employment Agreement with Jason Brown
Under the second amended and restated employment agreement with Jason Brown, Mr. Brown’s base salary was set at $505,000, which will be reviewed annually by our compensation committee, and he will be eligible to earn annual incentive compensation with a target amount equal to 45% of his base salary. Mr. Brown is also eligible to participate in
the employee benefit plans available to our employees, including our stock option plan, subject to the terms of those plans.
Mr. Brown’s employment agreement provides that, in the event that his employment is terminated by us without “cause” or Mr. Brown resigns for “good reason” (each as defined in his employment agreement), subject to the execution and effectiveness of a separation agreement, including a general release of claims in our favor, he will be entitled to receive (i) an amount equal to twelve months of his base salary, payable in substantially equal installments over twelve months following his termination, (ii) his pro-rated target bonus, and (iii) if Mr. Brown elects continuation of health coverage under COBRA, continued health coverage at the active employees’ rate until the earlier of twelve months following his termination, the date he becomes eligible for group medical benefits with another employer or the end of Mr. Brown’s COBRA health continuation period.
In lieu of the payments and benefits described in the preceding sentence, in the event that Mr. Brown’s employment is terminated by us without cause or Mr. Brown resigns for good reason, in either case within 12 months following a “change in control” (as defined in his employment agreement), subject to the execution and effectiveness of a separation agreement, including a general release of claims in our favor, he will be entitled to receive (i) an amount equal to 18 months of his base salary, plus 1.5 times his annual target bonus, payable within 60 days of his date of termination, (ii) full acceleration of vesting of all time-based stock options and other stock-based awards held by Mr. Brown on the termination date, and (iii) if Mr. Brown elects continuation of health coverage under COBRA, continued health coverage at the active employees’ rate until the earlier of 18 months following his termination, the date he becomes eligible for group medical benefits with another employer or the end of Mr. Brown’s COBRA health continuation period.
The payments and benefits provided to Mr. Brown under his employment agreement in connection with a change in control may not be eligible for a federal income tax deduction for the Company pursuant to Section 280G of the Code. These payments and benefits also may be subject to an excise tax under Section 4999 of the Code. If the payments or benefits payable to Mr. Brown in connection with a change in control would be subject to the excise tax on golden parachutes imposed under Section 4999 of the Code, then those payments or benefits will be reduced if such reduction would result in a higher net after-tax benefit to such officer.
In addition, Mr. Brown has executed an Employee Invention and Non-Disclosure Agreement and a Non-Competition and Non-Solicitation Agreement which contain certain restrictive covenants, including, among other things, non-competition and non-solicitation provisions that apply during the term of Mr. Brown’s employment and for 12 months thereafter.
Employment Agreement with Troy Ignelzi
Mr. Ignelzi departed from his position as our Chief Financial Officer effective as of September 29, 2023. In connection with his departure, we entered into a separation agreement with Mr. Ignelzi that provides for a general release and waiver of claims against us, and pursuant to which Mr. Ignelzi received the severance benefits that are provided for under his amended and restated employment agreement as described below.
Under the amended and restated employment agreement with Troy Ignelzi that was effective upon the closing of our initial public offering in 2019, Mr. Ignelzi’s base salary was initially set at $400,000, which was subject to annual review by our compensation committee, and he was eligible to earn annual incentive compensation with a target amount equal to 40% of his base salary. Mr. Ignelzi was also eligible to participate in the employee benefit plans available to our employees, including our stock option plan, subject to the terms of those plans.
Mr. Ignelzi’s employment agreement provided that, in the event that his employment was terminated by us without “cause” (as defined in his employment agreement) or Mr. Ignelzi resigned for “good reason” (as defined in his employment agreement), subject to the execution and effectiveness of a separation agreement, including a general release of claims in our favor, he would be entitled to receive (i) an amount equal to nine months of his base salary, payable in substantially equal installments over nine months following his termination, (ii) his pro-rated target bonus, and (iii) if Mr. Ignelzi elected continuation of health coverage under COBRA, continued health coverage at the active employees’ rate until the earlier of nine months following his termination, the date he became eligible for group medical benefits with another employer or the end of Mr. Ignelzi’s COBRA health continuation period.
In lieu of the payments and benefits described in the preceding sentence, in the event that Mr. Ignelzi’s employment was terminated by us without cause or Mr. Ignelzi resigned for good reason, in either case within 12 months following a “change in control” (as defined in his employment agreement), subject to the execution and effectiveness of a separation agreement, including a general release of claims in our favor, he would be entitled to receive (i) an amount equal to 12
months of his base salary, plus his annual target bonus, payable within 60 days of his date of termination, (ii) full acceleration of vesting of all time-based stock options and other stock-based awards held by Mr. Ignelzi on the termination date, and (iii) if Mr. Ignelzi elected continuation of health coverage under COBRA, continued health coverage at the active employees’ rate until the earlier of 12 months following his termination, the date he became eligible for group medical benefits with another employer or the end of Mr. Ignelzi’s COBRA health continuation period.
In addition, Mr. Ignelzi executed an Employee Invention and Non-Disclosure Agreement and a Non-Competition and Non-Solicitation Agreement which contained certain restrictive covenants, including, among other things, non-competition and non-solicitation provisions that applied during the term of Mr. Ignelzi’s employment and for 12 months thereafter.
Employment Agreement with Andrew Miller, Ph.D.
Under the second amended and restated employment agreement with Andrew Miller, Ph.D., Dr. Miller’s base salary was set at $560,000, which will be reviewed annually by our compensation committee, and he will be eligible to earn annual incentive compensation with a target amount equal to 50% of his base salary. Dr. Miller is also eligible to participate in the employee benefit plans available to our employees, including our stock option plan, subject to the terms of those plans.
Dr. Miller’s employment agreement provides that, in the event that his employment is terminated by us without “cause” or Dr. Miller resigns for “good reason” (each as defined in his employment agreement), subject to the execution and effectiveness of a separation agreement, including a general release of claims in our favor, he will be entitled to receive (i) an amount equal to twelve months of his base salary, payable in substantially equal installments over twelve months following his termination, (ii) his pro-rated target bonus, and (iii) if Dr. Miller elects continuation of health coverage under COBRA, continued health coverage at the active employees’ rate until the earlier of twelve months following his termination, the date he becomes eligible for group medical benefits with another employer or the end of Dr. Miller’s COBRA health continuation period.
In lieu of the payments and benefits described in the preceding sentence, in the event that Dr. Miller’s employment is terminated by us without cause or Dr. Miller resigns for good reason, in either case within 12 months following a “change in control” (as defined in his employment agreement), subject to the execution and effectiveness of a separation agreement, including a general release of claims in our favor, he will be entitled to receive (i) an amount equal to 18 months of his base salary, plus 1.5 times his annual target bonus, payable within 60 days of his date of termination, (ii) full acceleration of vesting of all time-based stock options and other stock-based awards held by Dr. Miller on the termination date, and (iii) if Dr. Miller elects continuation of health coverage under COBRA, continued health coverage at the active employees’ rate until the earlier of 18 months following his termination, the date he becomes eligible for group medical benefits with another employer or the end of Dr. Miller’s COBRA health continuation period.
The payments and benefits provided to Dr. Miller under his employment agreement in connection with a change in control may not be eligible for a federal income tax deduction for the Company pursuant to Section 280G of the Code. These payments and benefits also may be subject to an excise tax under Section 4999 of the Code. If the payments or benefits payable to Dr. Miller in connection with a change in control would be subject to the excise tax on golden parachutes imposed under Section 4999 of the Code, then those payments or benefits will be reduced if such reduction would result in a higher net after-tax benefit to such officer.
In addition, Dr. Miller has executed an Employee Invention and Non-Disclosure Agreement and a Non-Competition and Non-Solicitation Agreement which contain certain restrictive covenants, including, among other things, non-competition and non-solicitation provisions that apply during the term of Dr. Miller’s employment and for 12 months thereafter.
Employment Agreement with Stephen Brannan, M.D.
Under the second amended and restated employment agreement with Stephen Brannan, Dr. Brannan’s base salary was set at $510,000, which will be reviewed annually by our compensation committee, and he will be eligible to earn annual incentive compensation with a target amount equal to 45% of his base salary. Dr. Brannan is also eligible to participate in the employee benefit plans available to our employees, including our stock option plan, subject to the terms of those plans.
Dr. Brannan’s employment agreement provides that, in the event that his employment is terminated by us without “cause” or Dr. Brannan resigns for “good reason” (each as defined in his employment agreement), subject to the execution and effectiveness of a separation agreement, including a general release of claims in our favor, he will be entitled to receive (i) an amount equal to twelve months of his base salary, payable in substantially equal installments over twelve months following his termination, (ii) his pro-rated target bonus, and (iii) if Dr. Brannan elects continuation of health coverage under COBRA, continued health coverage at the active employees’ rate until the earlier of twelve months following his termination, the date he becomes eligible for group medical benefits with another employer or the end of Dr. Brannan’s COBRA health continuation period.
In lieu of the payments and benefits described in the preceding sentence, in the event that Dr. Brannan’s employment is terminated by us without cause or Dr. Brannan resigns for good reason, in either case within 12 months following a “change in control” (as defined in his employment agreement), subject to the execution and effectiveness of a separation agreement, including a general release of claims in our favor, he will be entitled to receive (i) an amount equal to 18 months of his base salary, plus 1.5 times his annual target bonus, payable within 60 days of his date of termination, (ii) full acceleration of vesting of all time-based stock options and other stock-based awards held by Dr. Brannan on the termination date, and (iii) if Dr. Brannan elects continuation of health coverage under COBRA, continued health coverage at the active employees’ rate until the earlier of 18 months following his termination, the date he becomes eligible for group medical benefits with another employer or the end of Dr. Brannan’s COBRA health continuation period.
The payments and benefits provided to Dr. Brannan under his employment agreement in connection with a change in control may not be eligible for a federal income tax deduction for the Company pursuant to Section 280G of the Code. These payments and benefits also may be subject to an excise tax under Section 4999 of the Code. If the payments or benefits payable to Dr. Brannan in connection with a change in control would be subject to the excise tax on golden parachutes imposed under Section 4999 of the Code, then those payments or benefits will be reduced if such reduction would result in a higher net after-tax benefit to such officer.
In addition, Dr. Brannan has executed an Employee Invention and Non-Disclosure Agreement and a Non-Competition and Non-Solicitation Agreement which contain certain restrictive covenants, including, among other things, non-competition and non-solicitation provisions that apply during the term of Dr. Brannan’s employment and for 12 months thereafter.
Employment Agreement with William Kane
Under the amended and restated employment agreement with William Kane, Mr. Kane's base salary was set at $530,000, which will be reviewed annually by our compensation committee, and he will be eligible to earn annual incentive compensation with a target amount equal to 50% of his base salary. Mr. Kane also received a sign-on bonus in the amount of $100,000, which he is obligated to repay to the Company in the event he resigns or his employment is terminated by the Company for "cause" (as defined in his employment agreement) within two years following February 6, 2023. Mr. Kane is also eligible to participate in the employee benefit plans available to our employees, including our stock option plan, subject to the terms of those plans.
Mr. Kane’s employment agreement provides that, in the event that his employment is terminated by us without “cause” or Mr. Kane resigns for “good reason” (each as defined in his employment agreement), subject to the execution and effectiveness of a separation agreement, including a general release of claims in our favor, he will be entitled to receive (i) an amount equal to twelve months of his base salary, payable in substantially equal installments over twelve months following his termination, (ii) his pro-rated target bonus, and (iii) if Mr. Kane elects continuation of health coverage under COBRA, continued health coverage at the active employees’ rate until the earlier of twelve months following his termination, the date he becomes eligible for group medical benefits with another employer or the end of Mr. Kane’s COBRA health continuation period.
In lieu of the payments and benefits described in the preceding sentence, in the event that Mr. Kane’s employment is terminated by us without cause or Mr. Kane resigns for good reason, in either case within 12 months following a “change in control” (as defined in his employment agreement), subject to the execution and effectiveness of a separation agreement, including a general release of claims in our favor, he will be entitled to receive (i) an amount equal to 18 months of his base salary, plus 1.5 times his annual target bonus, payable within 60 days of his date of termination, (ii) full acceleration of vesting of all time-based stock options and other stock-based awards held by Mr. Kane on the termination date, and (iii) if Mr. Kane elects continuation of health coverage under COBRA, continued health coverage at the active employees’ rate until the earlier of 18 months following his termination, the date he becomes eligible for group medical benefits with another employer or the end of Mr. Kane’s COBRA health continuation period.
The payments and benefits provided to Mr. Kane under his employment agreement in connection with a change in control may not be eligible for a federal income tax deduction for the Company pursuant to Section 280G of the Code. These payments and benefits also may be subject to an excise tax under Section 4999 of the Code. If the payments or benefits payable to Mr. Kane in connection with a change in control would be subject to the excise tax on golden parachutes imposed under Section 4999 of the Code, then those payments or benefits will be reduced if such reduction would result in a higher net after-tax benefit to such officer.
In addition, Mr. Kane has executed an Employee Invention and Non-Disclosure Agreement and a Non-Competition and Non-Solicitation Agreement which contain certain restrictive covenants, including, among other things, non-competition and non-solicitation provisions that apply during the term of Mr. Kane’s employment and for 12 months thereafter.
Potential Payments upon Termination or Change in Control
The amount of compensation and benefits payable to each named executive officer under their employment agreements in various termination and/or change-in-control situations has been estimated in the tables below, which assumes that such termination and/or change-in-control occurred on December 31, 2023. For purposes of the following tables, we have used $316.51 per share, which was the closing price of our common stock as reported on the Nasdaq Global Market on December 29, 2023, the last trading day of the year 2023, to estimate the value of our common stock upon acceleration. The value of the option vesting acceleration was calculated by multiplying the number of unvested shares underlying stock options subject to vesting acceleration as of December 31, 2023 by the difference between the closing price of our common stock as reported on the Nasdaq Global Market on December 29, 2023 and the exercise price for such unvested stock options.
For the avoidance of doubt, the amounts in the tables below represent estimated potential payments. No named executive officer's employment was terminated on December 31, 2023 and a change in control did not occur on that date. Moreover, there can be no assurance that a termination of employment, a change in control, or both would produce the same or similar results as those set forth below if either or both of them occurred on any other date.
As Mr. Ignelzi terminated employment with the Company prior to December 31, 2023, he is not included in the tables below. The severance payments and benefits that Mr. Ignelzi is entitled to receive in connection with his termination of employment are described separately below. Although Dr. Paul terminated his employment with the Company after December 31, 2023, no severance payments or benefits were triggered in connection with such termination, and therefore he is also not included in the tables below.
| | | | | | | | | | | | | | | |
| | | | Triggering Event | | |
Name | | Benefit | | Sale Event Without
Termination of
Employment ($) | | | Resignation for Good
Reason or Termination
Without Cause More Than 3 Months Before a
Sale Event
or More Than 12
Months Following a
Sale Event ($) | | | Resignation for
Good Reason or
Termination
Without Cause
Within 3 Months Before or 12 Months
Following a
Sale Event ($) | | |
Bill Meury | | Severance Payments | | | — | | | | 1,125,000 | | (1) | | 1,500,000 | | (2) |
| | Cash incentive payments | | | — | | | | 562,500 | | (3) | | 1,125,000 | | (4) |
| | Health care continuation | | | — | | | | 31,660 | | (5) | | 42,214 | | (6) |
| | Acceleration of equity
award vesting | | | — | | | | 4,374,777 | | (7) | | 13,802,577 | | (8) |
| | Total | | | — | | | | 6,093,937 | | | | 16,469,790 | | |
__________________
(1)Represents an amount equal to 18 months of the primary executive officer’s base salary in effect as of December 31, 2023, payable in substantially equal installments over 18 months following his termination.
(2)Represents a lump sum in cash in an amount equal to 24 months of the primary executive officer’s base salary in effect as of December 31, 2023.
(3)Represents a lump sum payment equal to the executive’s target annual cash incentive bonus.
(4)Represents a lump sum payment equal to two times the executive’s target annual cash incentive bonus.
(5)Represents a monthly cash payment for 18 months for continued medical and dental benefits for the primary executive officer, based upon the premium rate in effect as of December 31, 2023.
(6)Represents a monthly cash payment for 24 months for continued medical and dental benefits for the primary executive officer, based upon the premium rate in effect as of December 31, 2023.
(7)Represents the acceleration of vesting as to 100% of the unvested equity awards held by the primary executive officer that would have vested in the 18 months following his termination.
(8)Represents the acceleration of vesting as to 100% of the unvested equity awards held by the primary executive officer held on the date of termination.
| | | | | | | | | | | | | | | |
| | | | Triggering Event | | |
Name | | Benefit | | Sale Event Without
Termination of
Employment ($) | | | Resignation for Good
Reason or Termination
Without Cause Before a
Sale Event
or More Than 12
Months Following a
Sale Event ($) | | | Resignation for
Good Reason or
Termination
Without Cause
Within 12 Months
Following a
Sale Event ($) | | |
Jason Brown | | Severance Payments | | | — | | | | 505,000 | | (1) | | 757,500 | | (2) |
| | Cash incentive payments | | | — | | | | 227,250 | | (3) | | 340,875 | | (4) |
| | Health care continuation | | | — | | | | 21,107 | | (5) | | 31,660 | | (6) |
| | Acceleration of equity
award vesting | | | — | | | | — | | | | 7,395,837 | | (7) |
| | Total | | | — | | | | 753,357 | | | | 8,525,872 | | |
Andrew Miller, Ph.D. | | Severance Payments | | | — | | | | 560,000 | | (1) | | 840,000 | | (2) |
| | Cash incentive payments | | | — | | | | 280,000 | | (3) | | 420,000 | | (4) |
| | Health care continuation | | | — | | | | 21,107 | | (5) | | 31,660 | | (6) |
| | Acceleration of equity
award vesting | | | — | | | | — | | | | 20,457,386 | | (7) |
| | Total | | | — | | | | 861,107 | | | | 21,749,046 | | |
Stephen Brannan, M.D. | | Severance Payments | | | — | | | | 510,000 | | (1) | | 765,000 | | (2) |
| | Cash incentive payments | | | — | | | | 229,500 | | (3) | | 344,250 | | (4) |
| | Health care continuation | | | — | | | | 15,036 | | (5) | | 22,554 | | (6) |
| | Acceleration of equity
award vesting | | | — | | | | — | | | | 14,871,457 | | (7) |
| | Total | | | — | | | | 754,536 | | | | 16,003,261 | | |
William Kane | | Severance Payments | | | — | | | | 530,000 | | (1) | | 795,000 | | (2) |
| | Cash incentive payments | | | — | | | | 265,000 | | (3) | | 397,500 | | (4) |
| | Health care continuation | | | — | | | | 21,107 | | (5) | | 31,660 | | (6) |
| | Acceleration of equity
award vesting | | | — | | | | — | | | | 6,517,116 | | (7) |
| | Total | | | — | | | | 816,107 | | | | 7,741,276 | | |
__________________
(1)Represents an amount equal to 12 months of the named executive officer’s base salary in effect as of December 31, 2023, payable in substantially equal installments over 12 months following his termination
(2)Represents a lump sum in cash in an amount equal to 18 months of the named executive officer’s base salary in effect as of December 31, 2023
(3)Represents a lump sum payment equal to each executive’s target annual cash incentive bonus.
(4)Represents a lump sum payment equal to 1.5 times each executive’s target annual cash incentive bonus.
(5)Represents a monthly cash payment for 12 months for continued medical and dental benefits for the named executive officer, based upon the premium rate in effect as of December 31, 2023.
(6)Represents a monthly cash payment for 18 months for continued medical and dental benefits for the named executive officer, based upon the premium rate in effect as of December 31, 2023.
(7)Represents the acceleration of vesting as to 100% of the unvested equity awards held by the named executive officer.
Separation Arrangement with Mr. Ignelzi
As described in more detail above under the heading "Employment Arrangements with our Named Executive Officers", Mr. Ignelzi departed from his position as our Chief Financial Officer effective as of September 29, 2023. Pursuant to his separation agreement, subject to compliance with non-solicitation and confidentiality obligations, Mr. Ignelzi is entitled to the following severance benefits: (i) $386,250, representing an amount equal to nine months of his base salary, payable in substantially equal installments over nine months following his termination, (ii) $225,956, representing his pro-rated target bonus for 2023, and (iii) continued health coverage at the active employees’ rate until the earlier of nine months following his termination, the date he becomes eligible for group medical benefits with another employer or the end of Mr. Ignelzi's COBRA health continuation period.
CEO Pay Ratio Disclosure
Under the Dodd-Frank Wall Street Reform and Consumer Protection Act, or the Dodd-Frank Act, we are required to disclose the median of the annual total compensation of our employees, the annual total compensation of our chief executive officer during 2023, Bill Meury, and the ratio of these two amounts. The pay ratio included in this information is a reasonable estimate calculated in a manner consistent with Item 402(u) of Regulation S-K. The pay ratio reported by other companies may not be comparable to the pay ratio reported below, as other companies have different employee populations and compensation practices and may use different methodologies, measurement dates, exclusions, estimates and assumptions in calculating their own pay ratios.
We have estimated the median of the 2023 annual total compensation of our employees, excluding Mr. Meury, to be $455,691. The 2023 annual total compensation of Mr. Meury is $16,200,506. Based on this information, the ratio of the 2023 annualized total compensation of Mr. Meury to the estimated median of the 2023 annual total compensation of our employees was 36 to 1. We believe this pay ratio is a reasonable estimate calculated in a manner consistent with SEC rules.
Methodology
To identify the median employee, as well as to determine the total annual compensation of our median employee and our chief executive officer, we took the following steps:
•We determined that, as of December 31, 2023, our employee population consisted of 316 individuals, excluding Mr. Meury.
•To identify the “median employee” from our employee population, for each employee employed for all of 2023 we included the amount of salary paid as of December 31, 2023, the bonus earned for 2023, employer contributions under our 401(k) plan, and the grant date fair value of stock options and restricted stock units awarded in 2023, each as reflected in our human resources, payroll and equity award systems. For employees who were employed on December 31, 2023 but were not employed for all of 2023, we combined their annualized 2023 base salary, their target bonus for 2023 multiplied by our corporate bonus multiplier, employer contributions under our 401(k) plan, and the grant date fair value of stock options and restricted stock units awarded in 2023, each as reflected in our human resources, payroll and equity award systems. We did not make any cost-of-living adjustments.
•Once we identified our median employee, we calculated such employee’s compensation for 2023 using the same methodology as used to calculate Mr. Meury's total compensation in the “Summary Compensation Table”.
•Mr. Meury commenced employment with us effective January 3, 2023. Therefore, as he was not employed for all of 2023, we subtracted the amount reported in his 2023 "Salary" column in the Summary Compensation Table included elsewhere in this Amendment No. 1 from the corresponding "Total" column and added in its place his annualized 2023 base salary to determine his total 2023 annual compensation for this pay ratio disclosure.
Director Compensation in 2023
The following table sets forth the compensation we paid to our non-employee directors during the year ended December 31, 2023. Neither Bill Meury, our president and chief executive officer during fiscal year 2023, nor Steven Paul, M.D., our former president and chief executive officer, and chief scientific officer and president of research and development during fiscal year 2023, received any compensation for their service as a director during fiscal year 2023, and, consequently, are not included in this table. The compensation received by each of Mr. Meury and Dr. Paul as an employee during the year ended December 31, 2023 is presented in the “Summary Compensation Table” above.
| | | | | | | | | | | | | | | | | | | | |
Name | | Fees Earned or Paid in Cash ($) | | | Stock Awards ($)(1) | | | Option Awards
($) (2) | | | All Other
Compensation ($) | | | Total ($) | |
Christopher J. Coughlin | | | 103,703 | | (3) | | 235,323 | | (4) | | 235,322 | | (5) | | — | | | | 574,348 | |
James Healy, M.D., Ph.D. | | | 58,000 | | | | 235,323 | | (4) | | 235,322 | | (6) | | — | | | | 528,645 | |
Jeffrey Jonas, M.D. | | | 50,598 | | | | 235,323 | | (4) | | 235,322 | | (7) | | — | | | | 521,243 | |
Laurie Olson | | | 69,000 | | | | 235,323 | | (4) | | 235,322 | | (8) | | — | | | | 539,645 | |
Atul Pande, M.D. | | | 73,598 | | | | 235,323 | | (4) | | 235,322 | | (9) | | — | | | | 544,243 | |
Denice Torres | | | 56,250 | | | | 235,323 | | (4) | | 235,322 | | (10) | | — | | | | 526,895 | |
David Wheadon, M.D. | | | 56,098 | | | | 235,323 | | (4) | | 235,322 | | (11) | | — | | | | 526,743 | |
__________________
(1)The amounts reported represent the aggregate grant date fair value of RSUs granted during 2023 as computed in accordance with FASB ASC Topic 718, not including any estimates of forfeitures related to service-based vesting conditions..
(2)The amounts reported represent the aggregate grant date fair value of stock options granted during 2023 as computed in accordance with FASB ASC Topic 718, not including any estimates of forfeitures related to service-based vesting conditions. See Note 8 of “Notes to Consolidated Financial Statements” in our 2023 Annual Report filed with the SEC on February 22, 2024 for a discussion of assumptions made by the Company in determining the aggregate grant date fair value of our equity awards. Each director was awarded an annual award in the form of a stock option to purchase 1,904 shares of common stock, which had an estimated grant date fair value of $123.59 per share.
(3)Pursuant to the option in the Company's amended and restated non-employee director compensation policy for non-employee directors to receive equity in lieu of their cash retainer (as described below), Mr. Coughlin received 976 options to purchase common stock in lieu of a portion of his cash retainer during 2023, which had a grant date fair value equal to the amount of the cash retainer foregone, using our then-current Black-Scholes valuation model.
(4)As of December 31, 2023, each of Mr. Coughlin, Dr. Healy, Dr. Jonas, Ms. Olson, Dr. Pande, Ms. Torres and Dr. Wheadon held an aggregate of 990 unvested RSUs.
(5)As of December 31, 2023, Mr. Coughlin held options to purchase an aggregate of 41,380 shares of our common stock.
(6)As of December 31, 2023, Dr. Healy held options to purchase an aggregate of 27,904 shares of our common stock.
(7)As of December 31, 2023, Dr. Jonas held options to purchase an aggregate of 64,047 shares of our common stock.
(8)As of December 31, 2023, Ms. Olson held options to purchase an aggregate of 28,404 shares of our common stock.
(9)As of December 31, 2023, Dr. Pande held options to purchase an aggregate of 27,904 shares of our common stock.
(10)As of December 31, 2023, Ms. Torres held options to purchase an aggregate of 40,404 shares of our common stock.
(11)As of December 31, 2023, Dr. Wheadon held options to purchase an aggregate of 40,404 shares of our common stock.
Our board of directors adopted an amended and restated non-employee director compensation policy in June 2023 that is designed to provide a total compensation package that enables us to attract and retain, on a long-term basis, high caliber non-employee directors. Under the policy as amended, all non-employee directors are paid cash compensation as set forth below:
| | | | |
| | Annual
Retainer | |
Board of Directors: | | | |
All non-employee members | | $ | 50,000 | |
Chairman | | $ | 35,500 | |
Audit Committee: | | | |
Chairman | | $ | 22,000 | |
Non-Chairman members | | $ | 11,000 | |
Compensation Committee: | | | |
Chairman | | $ | 20,000 | |
Non-Chairman members | | $ | 10,000 | |
Nominating and Corporate Governance Committee: | | | |
Chairman | | $ | 12,000 | |
Non-Chairman members | | $ | 6,000 | |
Science and Technology Committee: | | | |
Chairman | | $ | 15,000 | |
Non-Chairman members | | $ | 7,500 | |
Under the policy, upon initial election or appointment to the board of directors, new non-employee directors receive a one-time stock option grant to purchase such number of shares of our common stock with an aggregate grant date fair value equal to approximately $353,000 using our then-current Black-Scholes valuation model, or the Initial Option Award, and an initial, one-time award of such number of RSUs equal to approximately $353,000 divided by the fair market value of a share of our common stock on the grant date, or the Initial RSU Award and, together with the Initial Option Award, the Initial Awards. The Initial Option Award shall vest in equal monthly installments over three years, provided, however, that all vesting shall cease if the director resigns from the board of directors or otherwise ceases to serve as a director of the Company, unless the board of directors determines that the circumstances warrant continuation of vesting. The Initial Option Award shall expire ten years from the date of grant, and shall have a per share exercise price equal to the Fair Market Value (as defined in the Company’s 2019 Stock Option and Incentive Plan) of the Company’s common stock on the date of grant. The Initial RSU Award shall vest in equal monthly installments over three years, provided, however, that all vesting shall cease if the director resigns from the board of directors or otherwise ceases to serve as a director of the Company, unless the board of directors determines that the circumstances warrant continuation of vesting.
In each subsequent year of a non-employee director’s tenure, each continuing non-employee member of the board of directors, other than a director receiving the Initial Awards during the same calendar year, will receive an annual award of options to purchase such number of shares of our common stock with an aggregate grant date fair value equal to approximately $235,500 using our then-current Black-Scholes valuation model, or the Annual Option Award, and an annual award of such number of restricted stock units equal to approximately $235,500 divided by the fair market value of a share of our common stock on the grant date, or the Annual RSU Award. The Annual Option Award and the Annual RSU Award shall each vest in full upon the earlier to occur of the first anniversary of the date of grant or the date of the next annual meeting of stockholders; provided, however, that all vesting shall cease if the director resigns from the board of directors or otherwise ceases to serve as a director, unless the board of directors determines that the circumstances warrant continuation of vesting. The Annual Option Award shall expire ten years from the date of grant, and shall have a per share exercise price equal to the Fair Market Value (as defined in the Company’s 2019 Stock Option and Incentive Plan) of the Company’s common stock on the date of grant.
As set forth in the table above, each non-employee director is paid an annual retainer of $50,000 for their services. Our chairman receives an additional annual retainer of $35,500 for his services. Non-employee directors serving on committees of our board of directors are entitled to an additional annual payment, as set forth in the table above. Such cash retainers are paid quarterly in arrears, and may be pro-rated based on the number of actual days served by the director during such calendar quarter. Each non-employee director may elect to receive all or a portion of her or his cash compensation in the form of an equity award of stock options to purchase common stock based on the Black-Scholes option-pricing model incorporating the 30 day average of the stock price through and including the date of grant and with a per share exercise price equal to the fair market value as of the date of grant. Any such election shall be made (i) for any continuing non-employee director, before the start of the calendar year with respect to any cash compensation for such calendar year and (ii) for any new non-employee director, within 30 days of her or his election to the board of directors. 25% of such stock option shall vest at the end of each three month period following the date of grant, and will be fully vested on the first anniversary of the date of grant.
Equity Compensation Plan Information
The following table presents aggregate summary information as of December 31, 2023, regarding the common stock that may be issued upon the exercise of options and rights under all of our existing equity compensation plans:
| | | | | | | | | | | | |
| | Column (A) | | | Column (B) | | | Column (C) | |
Plan Category | | Number of Securities to be Issued Upon Exercise of Outstanding Options, Restricted Stock Units and Other Rights | | | Weighted Average Exercise Price of Outstanding Options | | | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column A) | |
Equity Compensation Plans Approved by Stockholders (1) | | | 5,555,130 | | | $ | 75.70 | | | 3,039,617 (2) | |
Equity Compensation Plans Not Approved by Stockholders | | | — | | | $ | — | | | | — | |
Total | | | 5,555,130 | | | $ | 75.70 | | | 3,039,617 (3) | |
__________________
(1)These plans consist of our 2009 Stock Incentive Plan, or 2009 Plan, 2019 Stock Option and Incentive Plan, or 2019 Plan, and 2019 Employee Stock Purchase Plan, or ESPP.
(2)As of December 31, 2023, (i) 1,653,433 shares remained available for future issuance under our 2019 Plan and (ii) 1,386,184 shares remained available for future issuance under our ESPP. No shares remained available for future issuance under the 2009 Plan as of December 31, 2023. Our 2019 Plan has an evergreen provision that allows for an annual increase in the number of shares available for issuance under the 2019 Plan to be added on the first day of each fiscal year, starting with fiscal year 2020, in an amount equal to 4% of the number of shares of our common stock outstanding on the immediately preceding December 31 or such lesser amount determined by our board of directors or the compensation committee of our board of directors. Our ESPP has an evergreen provision that allows for an annual increase in the number of shares available for issuance under the ESPP to be added on the first day of each fiscal year, starting in fiscal year 2020, in an amount equal to 1% of the total number of shares of our common stock outstanding on the immediately preceding December 31 or such lesser amount determined by our board of directors or the compensation committee of our board of directors.
(3)This amount excludes 1,523,101 shares of common stock that became issuable under the 2019 Plan on January 1, 2024 and 380,775 shares of common stock that became issuable under the ESPP on January 1, 2024, in each case pursuant to the evergreen provisions of the 2019 Plan and ESPP.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets forth the amount of common stock of the Company beneficially owned, directly or indirectly, as of March 12, 2024, by (i) each current director of the Company, (ii) each named executive officer of the Company, (iii) all directors and executive officers of the Company as a group, and (iv) each person who is known to the Company to beneficially own more than five percent (5%) of the outstanding shares of common stock of the Company, as determined through SEC filings, and the percentage of the common stock outstanding represented by each such amount. All shares of common stock shown in the table reflect sole voting and investment power except as otherwise noted.
Beneficial ownership is determined by the rules of the SEC and includes voting or investment power of the securities. As of March 12, 2024, the Company had 38,199,272 shares of common stock outstanding. Shares of common stock subject to options that are currently exercisable or are exercisable within 60 days after March 12, 2024 are considered to be outstanding for purposes of computing the percentage ownership of the persons holding these options but are not to be considered outstanding for the purpose of computing the percentage ownership of any other person. Unless otherwise indicated, the address for each person listed below is c/o Karuna Therapeutics, Inc., 99 High Street, 26th Floor, Boston, Massachusetts 02110.
| | | | | | | | |
Name and Address of Beneficial Owner | | Number of
Shares
Beneficially
Owned | | | Percentage of
Shares
Beneficially
Owned | |
5% Stockholders | | | | | | |
The Vanguard Group(1) | | | 3,224,786 | | | | 8.4 | % |
T. Rowe Price Investment Management, Inc.(2) | | | 2,709,629 | | | | 7.1 | % |
Blackrock, Inc. and subsidiaries (3) | | | 2,015,374 | | | | 5.3 | % |
T. Rowe Price Associates, Inc.(4) | | | 1,963,957 | | | | 5.1 | % |
Directors and Named Executive Officers | | | | | | |
Bill Meury(5) | | | 30,050 | | | * | |
Steven Paul, M.D.(6) | | | 2,613,493 | | | | 6.4 | % |
Jason Brown(7) | | | 24,689 | | | * | |
Troy Ignelzi | | | 77,855 | | | * | |
Andrew Miller, Ph.D.(8) | | | 470,008 | | | | 1.2 | % |
Stephen Brannan, M.D.(9) | | | 113,241 | | | * | |
William Kane(10) | | | 14,356 | | | * | |
Christopher J. Coughlin(11) | | | 39,476 | | | * | |
James Healy, M.D., Ph.D.(12) | | | 1,439,649 | | | | 3.8 | % |
Jeffrey Jonas, M.D.(13) | | | 62,143 | | | * | |
Laurie Olson(14) | | | 26,500 | | | * | |
Atul Pande, M.D.(15) | | | 26,000 | | | * | |
Denice Torres(16) | | | 38,500 | | | * | |
David Wheadon, M.D.(17) | | | 38,500 | | | * | |
All executive officers and directors as a group (13 persons)(18) | | | 4,936,605 | | | | 11.9 | % |
__________________
* Represents holdings of less than 1%.
(1)This information is based on disclosure contained in Schedule 13G/A filed with the SEC by The Vanguard Group, on February 13, 2024, reporting ownership as of December 29, 2023. The Vanguard Group has shared voting power with respect to 14,369 shares of Karuna’s common stock, sole dispositive power with respect to 3,174,100 shares of Karuna’s common stock and shared dispositive power with respect to 50,686 shares of Karuna’s common stock. The address of The Vanguard Group is 100 Vanguard Boulevard, Malvern, PA 19355.
(2)This information is based on disclosure contained in Schedule 13G filed with the SEC by T. Rowe Price Investment Management, Inc. on February 14, 2024, reporting ownership as of December 31, 2023. T. Rowe Price Investment Management, Inc. has sole voting power with respect to 1,019,305 shares of Karuna’s common stock and sole dispositive power with respect to 2,709,629 shares of Karuna’s common stock. The address of T. Rowe Price Investment Management, Inc. is 100 E. Pratt Street, Baltimore, MD 21202.
(3)This information is based on disclosure contained in Schedule 13G filed with the SEC by Blackrock, Inc. on January 31, 2024, reporting beneficial ownership as of December 31, 2023 for Blackrock, Inc. and its subsidiaries. Blackrock, Inc. has sole voting power with respect to 1,917,599 shares of Karuna’s common stock, and sole dispositive power with respect to 2,015,374 shares of Karuna’s common stock. The address of Blackrock, Inc. is 50 Hudson Yards, New York, NY 10001.
(4)This information is based on disclosure contained in Schedule 13G/A filed with the SEC by T. Rowe Price Associates, Inc. on February 14, 2024, reporting ownership as of December 31, 2023. T. Rowe Price Associates, Inc. has sole voting power with respect to 305,100 shares of Karuna’s common stock and sole dispositive power with respect to 1,961,587 shares of Karuna’s common stock. The address of T. Rowe Price Associates, Inc. is 100 E. Pratt Street, Baltimore, MD 21202.
(5)Consists of (a) 10,342 shares of common stock held by Mr. Meury, and (b) 19,708 shares of common stock underlying options exercisable within 60 days of March 12, 2024.
(6)Consists of (a) 2,046 shares of common stock held by Dr. Paul, (b) 33,882 shares of common stock held by The Steven M Paul Revocable Trust, a family trust of which Dr. Paul is the trustee, (b) 10,000 shares of common stock held by the Jann E Paul Revocable Trust, a family trust of which Dr. Paul is the trustee, (c) 35,877 shares of common stock held by the Jann E. Paul GRAT III, a grantor retained annuity trust established for the benefit of Dr. Paul and his descendants, (d) 30,304 shares of common stock held by the Steven M. Paul Family 2021 Delaware Trust, of which the trustee is an independent institution and for which Dr. Paul acts as investment adviser, (e) 30,304 shares of common stock held by the Steven M. Paul Family 2024 Trust, a family trust established for the benefit of Dr. Paul's spouse and her descendents, and (f) 2,471,080 shares of common stock underlying options exercisable within 60 days of March 12, 2024.
(7)Consists of (a) 14,009 shares of common stock held by Mr. Brown, and (b) 10,680 shares of common stock underlying options exercisable within 60 days of March 12, 2024.
(8)Consists of (a) 13,977 shares of common stock held by Dr. Miller, and (b) 456,031 shares of common stock underlying options exercisable within 60 days of March 12, 2024.
(9)Consists of (a) 33,617 shares of common stock held by Dr. Brannan, and (b) 77,124 shares of common stock underlying options exercisable within 60 days of March 12, 2024.
(10)Consists of (a) 5,000 shares of common stock held by Mr. Kane, and (b) 9,356 shares of common stock underlying options exercisable within 60 days of March 12, 2024.
(11)Consists of 39,476 shares of common stock underlying options exercisable within 60 days of March 12, 2024.
(12)Consists of (a) 32,500 shares of common stock held by Dr. Healy, (b) 26,000 shares of common stock underlying options exercisable within 60 days of March 12, 2024, (c) 1,372,441 shares of Karuna’s common stock held by Sofinnova Venture Partners X, L.P. (“SVP X”), (d) 8,025 of Karuna’s common stock held by Sofinnova Management X, L.P. (“SM X LP”), and (e) 683 shares of Karuna’s common stock held by Sofinnova Synergy Master Fund, L.P. (the “Fund”). Dr. Healy is a managing member of Sofinnova Management X-A, L.L.C., (“SM X LLC”), the general partner of SM X LP, which is the general partner of SVP X. SM X LLC may be deemed to have sole voting and dispositive power over the shares held by SM X LP, each of SM X LLC and SM X LP may be deemed to have sole voting and dispositive power over the shares held by SVP X, and, together with the other managing member of SM X LLC, Dr. Healy may be deemed to have shared power to vote and dispose of the shares held by SM X LP and SVP X. Dr. Healy is a managing member of Sofinnova Synergy Fund GP, LLC, or the GP, the general partner of the Fund. The GP may be deemed to have sole voting and dispositive power over the shares held by the Fund, and, together with the other managing member of the GP, Dr. Healy may be deemed to have shared power to vote and dispose of the shares held by the Fund. Dr. Healy disclaims beneficial ownership of the shares held by SVP X, SM X LP and the Fund except to the extent of his pecuniary interest therein. The address of Dr. Healy and Sofinnova Investments, Inc. is 3000 Sand Hill Road, Bldg 4, Suite 250, Menlo Park, CA 94025.
(13)Consists of 62,143 shares of common stock underlying options exercisable within 60 days of March 12, 2024.
(14)Consists of 26,500 shares of common stock underlying options exercisable within 60 days of March 12, 2024.
(15)Consists of 26,000 shares of common stock underlying options exercisable within 60 days of March 12, 2024.
(16)Consists of 38,500 shares of common stock underlying options exercisable within 60 days of March 12, 2024.
(17)Consists of 38,500 shares of common stock underlying options exercisable within 60 days of March 12, 2024.
(18)Consists of (a) 1,635,507 shares of common stock, and (b) 3,301,098 shares of common stock underlying options exercisable within 60 days of March 12, 2024, in each case held or beneficially owned by all of Karuna’s current executive officers and directors serving as of March 12, 2024 as a group. The beneficial ownership of Mr. Ignelzi was not included in this calculation as he was not an executive officer as of March 12, 2024.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Related Person Transactions
Other than the compensation agreements and other arrangements described in “Executive Compensation” and elsewhere in this Amendment No. 1 and the relationships and transactions described below, since January 1, 2023, there was no transaction or series of transactions to which we were or will be a party in which the amount involved exceeded or will exceed $120,000 and in which any director, executive officer, holder of more than five percent of our capital stock or any member of their immediate families had or will have a direct or indirect material interest.
Investors’ Rights Agreement
We are a party to an amended and restated investors’ rights agreement, dated as of March 15, 2019, with holders of our preferred stock, including some of our former 5% stockholders and entities affiliated with our directors. Such holders consisted of entities affiliated with PureTech Health, ARCH Ventures, Wellcome Trust and Sofinnova Investments, each a former 5% stockholder. Sofinnova Investments has a representative on our board of directors. The representatives designated by PureTech Health each resigned from the board in 2019, and the representative designated by ARCH Ventures did not stand for reelection to the Board when his term expired at the 2021 Annual Meeting. The investor rights agreement provides these holders the right to demand that we file a registration statement or request that their shares be covered by a registration statement that we are otherwise filing. See “Description of Capital Stock—Registration Rights” for additional information regarding these registration rights.
Executive Officer and Director Compensation
See the section entitled “Executive Compensation” for information regarding compensation of our executive officers and directors.
Indemnification Agreements
We have entered into agreements to indemnify our directors and executive officers. These agreements will, among other things, require us to indemnify these individuals for certain expenses (including attorneys’ fees), judgments, fines and settlement amounts reasonably incurred by such person in any action or proceeding, including any action by or in our right, on account of any services undertaken by such person on behalf of our Company or that person’s status as a member of our board of directors, to the maximum extent allowed under Delaware law.
Policies and Procedures for Related Person Transactions
Our board of directors reviews and approves transactions with directors, officers and holders of 5% or more of our voting securities and their affiliates, each a related party. We have a written related party transactions policy that such transactions must be approved by our audit committee. Pursuant to this policy, the audit committee has the primary responsibility for reviewing and approving or disapproving “related party transactions,” which are transactions between us and related persons in which the aggregate amount involved exceeds or may be expected to exceed $120,000 and in which a related person has or will have a direct or indirect material interest. For purposes of this policy, a related person will be defined as a director, executive officer, nominee for director, or greater than 5% beneficial owner of our common stock, in each case since the beginning of the most recently completed year, and their immediate family members. Our audit committee charter provides that the audit committee shall review and approve or disapprove any related party transactions.
Director Independence
Our board of directors has determined that each of our directors, except for Bill Meury, who serves as our President and Chief Executive Officer, Steven Paul, M.D., who served as our Chief Scientific Officer and President of Research and Development from January 2023 to January 2024, and Jeffrey Jonas, M.D., who formerly served as the Chief Innovation Officer of Sage, where Dr. Paul formerly served as a member of the compensation committee, has no relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and is independent within the meaning of the director independence standards of the Nasdaq rules and the SEC.
At least annually, our board of directors will evaluate all relationships between us and each director in light of relevant facts and circumstances for the purposes of determining whether a material relationship exists that might signal a potential conflict of interest or otherwise interfere with such director’s ability to satisfy his or her responsibilities as an independent director. Based on this evaluation, our board of directors will make an annual determination of whether each director is independent within the meaning of Nasdaq and SEC independence standards.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
Our independent public accounting firm is KPMG LLP, Boston, Massachusetts, PCAOB Auditor ID 185.
Independent Registered Public Accounting Firm Fees
The following is a summary and description of fees incurred by KPMG LLP for the fiscal years ended December 31, 2023, 2022 and 2021.
| | | | | | | | | | | | |
Fee Category | | Year ended
December 31,
2023 | | | Year ended
December 31,
2022 | | | Year ended
December 31,
2021 | |
Audit Fees(1) | | $ | 1,128,855 | | | $ | 1,031,876 | | | $ | 946,010 | |
Audit-Related Fees(2) | | | 32,310 | | | | — | | | | — | |
Tax Fees | | | — | | | | — | | | | — | |
All Other Fees(3) | | | — | | | | — | | | | — | |
Total | | $ | 1,161,165 | | | $ | 1,031,876 | | | $ | 946,010 | |
(1)“Audit Fees” consist of fees for the audit of our annual consolidated financial statements, the review of the interim consolidated financial statements, the filing of our Form S-3 Registration Statement and the accompanying prospectus filed with the S-3 Registration Statement to provide for “at the market” offerings under the Registration Statement in June 2023, the filing of prospectus supplements in March 2021 and August 2022 in connection with follow-on public offerings under our S-3 Registration Statement, Section 404 attestation services, and other professional services provided in connection with regulatory filings.
(2)"Audit-Related Fees" consist of fees paid to KPMG LLP for due diligence and accounting consultation related to the proposed merger transaction with Bristol Myers Squibb.
(3)“All other fees” consist of non-audit fees paid to KPMG LLP for access to its proprietary accounting disclosure checklist.
Pre-Approval Policies and Procedures
The Company’s audit committee has adopted procedures requiring the pre-approval of all non-audit services performed by the Company’s independent registered public accounting firm in order to assure that these services do not impair the auditor’s independence. These procedures generally approve the performance of specific services subject to a cost limit for all such services. This general approval is to be reviewed, and if necessary modified, at least annually. Management must obtain the specific prior approval of the audit committee for each engagement of the independent registered public accounting firm to perform other audit-related or other non-audit services. The audit committee does not delegate its responsibility to approve services performed by the independent registered public accounting firm to any member of management.
The standard applied by the audit committee in determining whether to grant approval of any type of non-audit service, or of any specific engagement to perform a non-audit service, is whether the services to be performed, the compensation to be paid for such services and other related factors are consistent with the independent registered public accounting firm’s independence under guidelines of the SEC and applicable professional standards. Relevant considerations include whether the work product is likely to be subject to, or implicated in, audit procedures during the audit of our financial statements, whether the independent registered public accounting firm would be functioning in the role of management or in an advocacy role, whether the independent registered public accounting firm’s performance of the service would enhance our ability to manage or control risk or improve audit quality, whether such performance would increase efficiency because of the independent registered public accounting firm’s familiarity with our business, personnel, culture, systems, risk profile and other factors, and whether the amount of fees involved, or the non-audit services portion of the total fees payable to the independent registered public accounting firm in the period would tend to reduce the independent registered public accounting firm’s ability to exercise independent judgment in performing the audit.
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
Exhibits
The exhibits required by Item 601 of Regulation S-K and Item 15(b) of this Annual Report on Form 10-K are listed in the Exhibit Index immediately preceding the signature page of this Annual Report on Form 10-K. The exhibits listed in the Exhibit Index are incorporated by reference herein.
EXHIBIT INDEX
* Filed herewith
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | |
| KARUNA THERAPEUTICS, INC. |
| |
Date: March 14, 2024 | By: | /s/ William Meury |
| | William Meury President and Chief Executive Officer (Principal Executive Officer) |




