
Developing novel therapies to dramatically improve the lives of people with psychiatric and neurological disorders Topline Data from Phase 2 Trial of KarXT in Acute Psychosis in Patients with Schizophrenia Exhibit 99.2

Legal Disclaimer OVERVIEW These slides and the accompanying oral presentation contain forward-looking statements and information. You should not place undue reliance on forward-looking statements, as these statements are based upon our current expectations, forecasts, and assumptions and are subject to significant risks and uncertainties. These statements may be identified by words such as “may,” “will,” “should,” “could,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “forecast,” “continue,” or the negative of these terms or other words or terms of similar meaning. Statements, including forward-looking statements, speak only to the date they are provided (unless an earlier date is indicated), and we do not undertake any obligation to publicly update any statements, including forward-looking statements, whether as a result of new information, future events, or otherwise, except as required by law. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance, achievements or events and circumstances reflected in the forward-looking statements will occur. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Neither we nor any other person makes any representation as to the accuracy or completeness of such data or undertakes any obligation to update such data after the date of this presentation. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk.

IntroductionTroy Ignelzi / Chief Financial Officer Summary of Phase 2 ResultsSteve Paul, M.D. / Chairman, CEO and President Phase 2 Results AnalysisStephen Brannan, M.D. / Chief Medical Officer KarXT Development PlanAndrew Miller, Ph.D. / Founder & Chief Operating Officer Summary RemarksSteve Paul, M.D. / President & Chief Executive Officer Questions & AnswersSteve Paul, M.D. / President & Chief Executive Officer Andrew Miller, Ph.D. / Founder & Chief Operating Officer Stephen Brannan, M.D. / Chief Medical Officer Agenda Overview

KarXT Phase 2 Trial Results in Acute Psychosis in Patients with Schizophrenia

Chronic, disabling disorder typically diagnosed in late teenage years or early adulthood Characterized by recurring episodes of psychosis requiring long-term treatment with antipsychotic drugs in most patients Affects over 21 million people worldwide 2.7 million Americans (0.5% - 1.0% of U.S. population) had schizophrenia in 2017 Today’s standard of care rely on same mechanism as drugs of the 1950s (first antipsychotic drug, chlorpromazine, discovered in 1952) In many patients, approved antipsychotics offer modest efficacy and significant side effects Schizophrenia: The “cancer of psychiatry” Summary of Phase 2 Results
Summary of Topline Phase 2 Results Summary of Phase 2 Results KarXT is a novel mechanism of action therapeutic targeting central nervous system (CNS) indications representing large and underserved patient populations characterized by psychosis and cognitive impairment Met the primary endpoint with a statistically significant (P<0.0001) and clinically meaningful 11.6 point improvement on the PANSS total score from baseline vs. placebo. The PANSS total improvement over placebo was significant at all assessed time points. Statistically significant reduction in the secondary endpoints of PANSS-positive and PANSS-negative subscales at all assessed time points. KarXT was well tolerated: The overall discontinuation rate and the discontinuation rate due to treatment emergent adverse events on KarXT was similar to placebo 91% of patients escalated to the high dose of KarXT as part of the flexible dose design No evidence of somnolence, extrapyramidal side effects or weight gain Data supports entering Phase 3 development with a completely unique new mechanism to treat psychosis in schizophrenia

KarXT Phase 2 Trial Results Analysis
Phase 2 Trial Design Overview Same fundamental trial design and primary endpoint used in pivotal studies to support registration of other antipsychotic drugs Randomized, double-blind, placebo-controlled, five week, inpatient trial Enrolled 182 schizophrenia patients with acute psychosis (N=90 on KarXT, N=92 on Placebo) Patients were washed out of any antipsychotic drugs prior to randomization Flexible dose, two-arm trial with 1:1 randomization to KarXT or placebo with a five-week treatment period Days 1-2: 50/20 KarXT BID (50 mg xanomeline/20 mg trospium) Days 3-7: 100/20 KarXT BID Days 8-35: 100/20 KarXT BID with optional increase to 125/30 KarXT BID; titration based only on tolerability Primary endpoint of change in total PANSS from baseline vs. placebo at week 5 in the modified intent to treat population (mITT) Other endpoints: PANSS-positive and –negative subscales, CGI, PANSS Marder factor, cognitive battery, and others Phase 2 Results Analysis
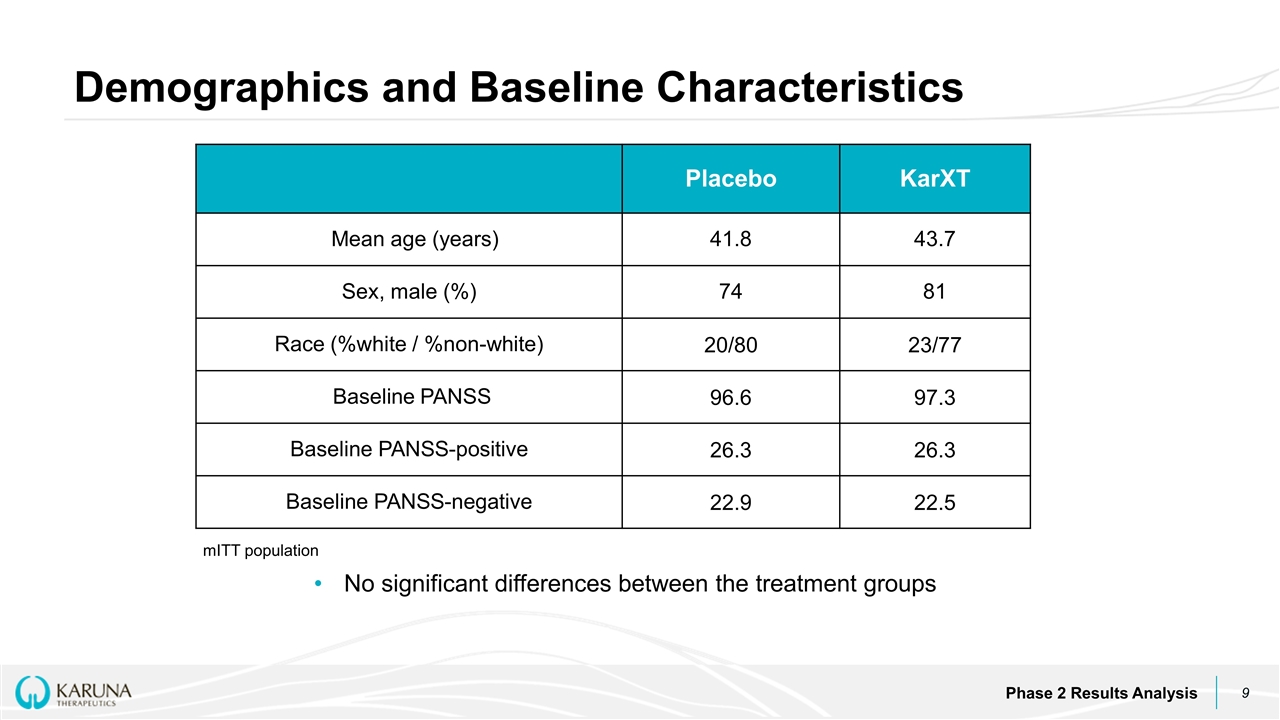
Demographics and Baseline Characteristics Phase 2 Results Analysis Placebo KarXT Mean age (years) 41.8 43.7 Sex, male (%) 74 81 Race (%white / %non-white) 20/80 23/77 Baseline PANSS 96.6 97.3 Baseline PANSS-positive 26.3 26.3 Baseline PANSS-negative 22.9 22.5 mITT population No significant differences between the treatment groups
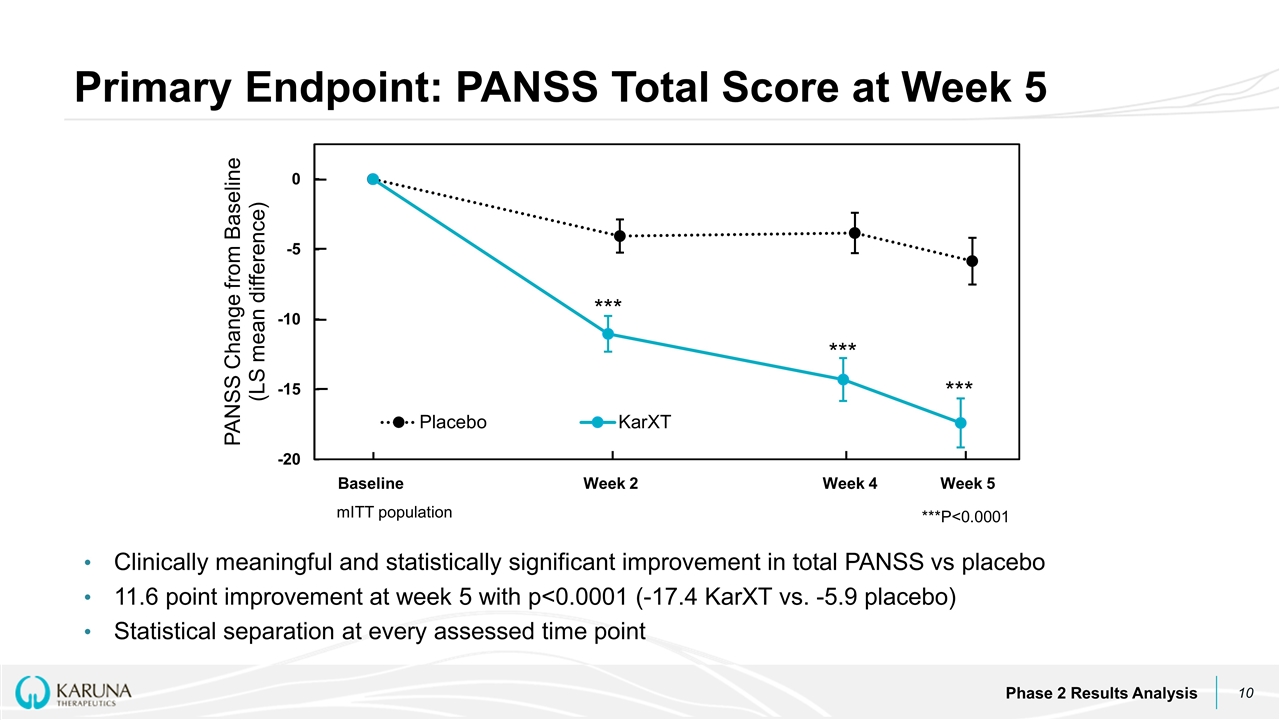
Baseline Week 2 Week 4 Week 5 *** *** *** Clinically meaningful and statistically significant improvement in total PANSS vs placebo 11.6 point improvement at week 5 with p<0.0001 (-17.4 KarXT vs. -5.9 placebo) Statistical separation at every assessed time point Primary Endpoint: PANSS Total Score at Week 5 Phase 2 Results Analysis ***P<0.0001 mITT population
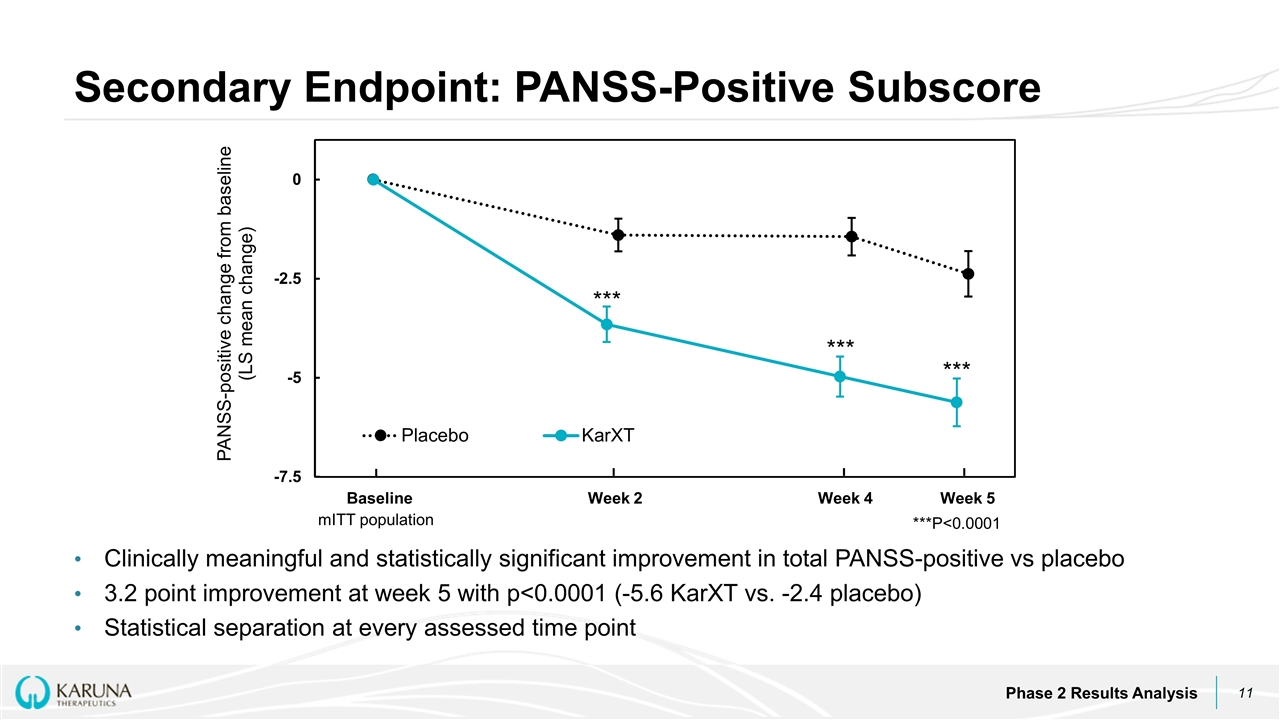
Clinically meaningful and statistically significant improvement in total PANSS-positive vs placebo 3.2 point improvement at week 5 with p<0.0001 (-5.6 KarXT vs. -2.4 placebo) Statistical separation at every assessed time point Secondary Endpoint: PANSS-Positive Subscore Phase 2 Results Analysis Baseline Week 2 Week 4 Week 5 *** *** *** ***P<0.0001 mITT population
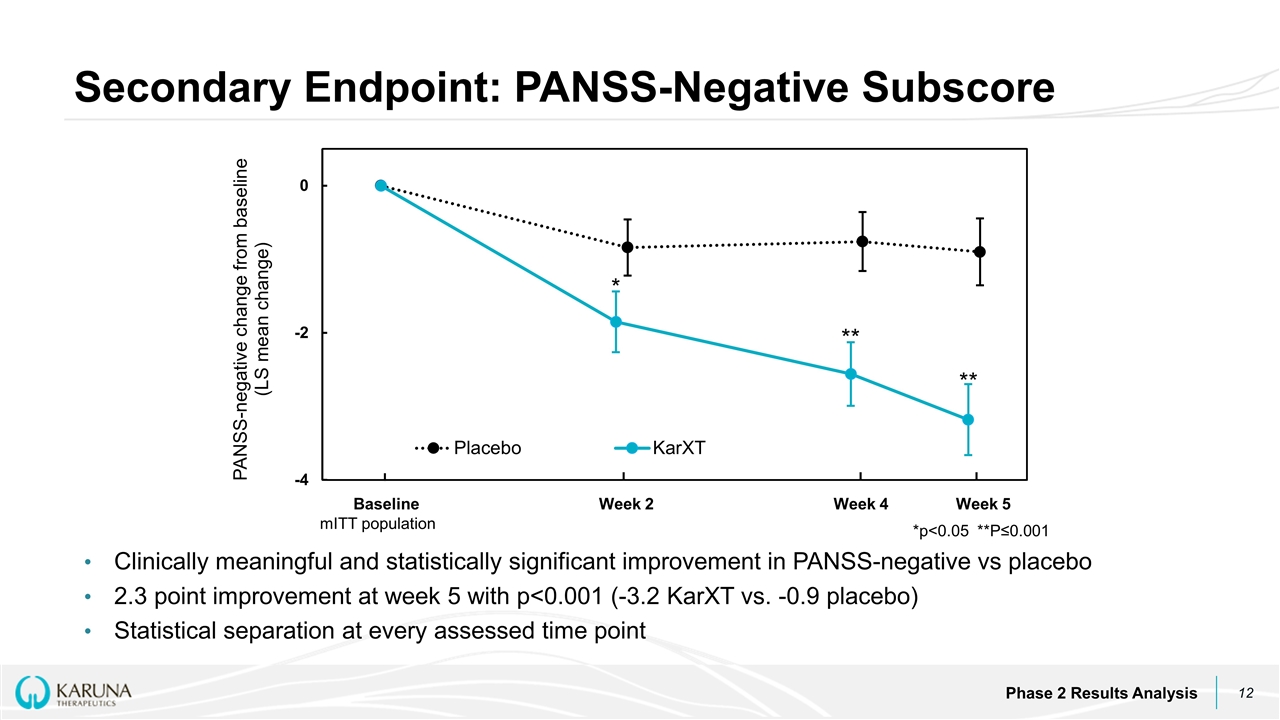
Clinically meaningful and statistically significant improvement in PANSS-negative vs placebo 2.3 point improvement at week 5 with p<0.001 (-3.2 KarXT vs. -0.9 placebo) Statistical separation at every assessed time point Secondary Endpoint: PANSS-Negative Subscore Phase 2 Results Analysis Baseline Week 2 Week 4 Week 5 ** ** * *p<0.05 **P≤0.001 mITT population
KarXT was well tolerated Overall discontinuation rate on KarXT (20%) similar to placebo (21%) The number of discontinuations due to treatment emergent adverse events was equal in the KarXT and placebo arms (N=2 in each group) Dose escalation rate on KarXT was high and similar to placebo 91% of KarXT subjects escalated to 125/30 KarXT (vs. 97% on placebo); 4% percent de-escalated back to 100/20 KarXT dose (vs. 1% on placebo) Overall treatment emergent adverse event rate on KarXT was 54% vs. 43% on placebo Most common adverse events were constipation, nausea, dry mouth, dyspepsia, and vomiting, which were all mild or moderate in severity and did not lead to discontinuations Somnolence, weight gain, and extrapyramidal symptoms/akathisia similar to placebo No syncope, no mean change in BP, 5.5 bpm peak mean placebo adjusted resting HR increase with downward trend after week 2, and one discontinuation due to elevated GGT One serious adverse event on KarXT: patient discontinued treatment and subsequently sought hospital care for worsening psychosis, meeting the regulatory definition of an SAE. All other TEAEs were mild or moderate Summary of Safety and Tolerability Phase 2 Results Analysis data from safety population

KarXT Development Plan

Achieved the initial goals of KarXT development: Improve tolerability of xanomeline Maintain and confirm previous therapeutic benefits of xanomeline Three double-blind, placebo-controlled studies supporting therapeutic benefit of xanomeline/KarXT KarXT Phase 2 study in patients with schizophrenia Small Phase 2 study in patients with schizophrenia with xanomeline-alone Phase 2 study in patients with Alzheimer’s disease with xanomeline-alone Large existing safety database with xanomeline and KarXT: >1000 patients enrolled in studies with KarXT or xanomeline 68 Alzheimer’s disease patients treated with xanomeline for at least one year Robust data including 3 efficacy studies and long-term safety going into Phase 3 Multiple efficacy and safety studies support Phase 3 Development of KarXT KarXT Development Plan
Complete final analysis of all data and endpoints to further inform path forward Plan an end of Phase 2 meeting with FDA to discuss data and development path including Phase 3 trial design Meeting anticipated in Q2 2020 Pending FDA meeting, we anticipate advancing to Phase 3 using a similar trial design as our Phase 2 trial with trial initiation anticipated by the end of 2020 Topline Phase 1b data in pain expected in mid 2020 Topline Phase 1b data in healthy elderly volunteers expected in 2H 2020 KarXT Development Moving Forward KarXT Development Plan
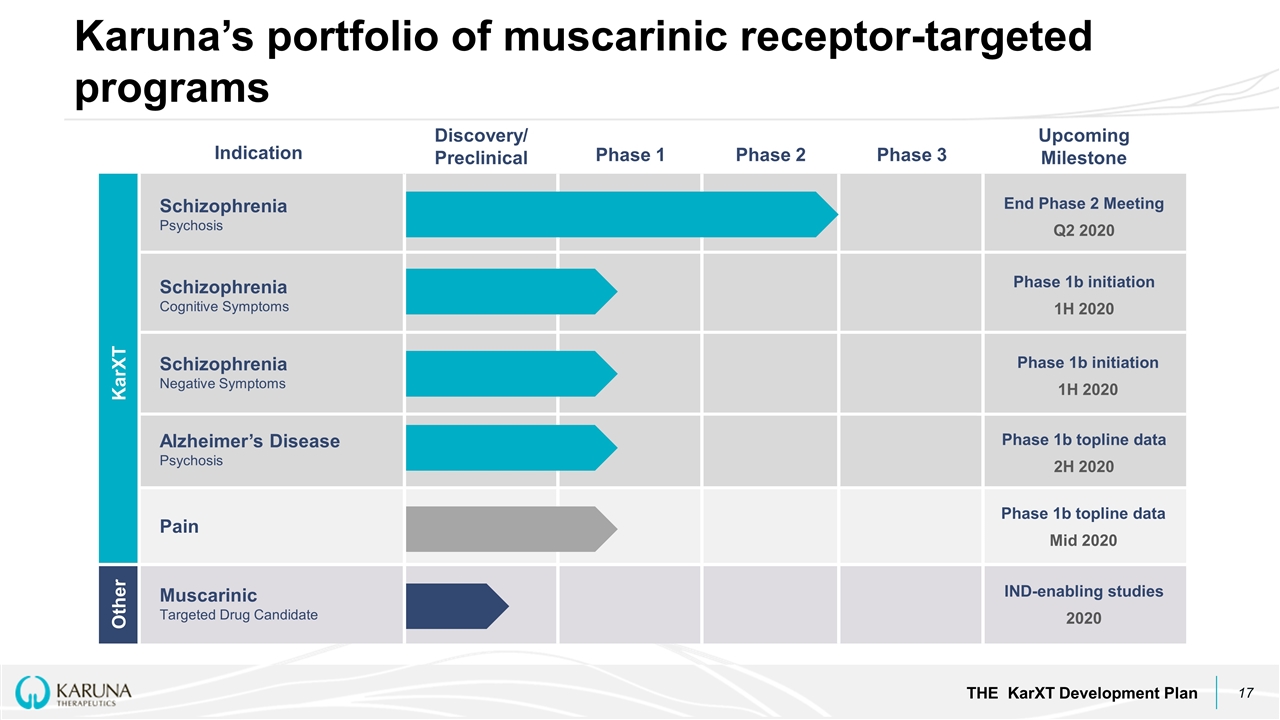
Karuna’s portfolio of muscarinic receptor-targeted programs THE KarXT Development Plan Indication Discovery/ Preclinical Phase 1 Phase 2 Phase 3 Upcoming Milestone Schizophrenia Psychosis Schizophrenia Cognitive Symptoms Schizophrenia Negative Symptoms Alzheimer’s Disease Psychosis Pain Muscarinic Targeted Drug Candidate KarXT Other End Phase 2 Meeting Q2 2020 Phase 1b initiation 1H 2020 Phase 1b initiation 1H 2020 Phase 1b topline data 2H 2020 Phase 1b topline data Mid 2020 IND-enabling studies 2020

Summary Comments

Developing novel therapies to dramatically improve the lives of people with psychiatric and neurological disorders


















