Free signup for more
- Track your favorite companies
- Receive email alerts for new filings
- Personalized dashboard of news and more
- Access all data and search results
Filing tables
Filing exhibits
BNTX similar filings
- 17 Jun 22 Pfizer-BioNTech COVID-19 Vaccine Receives FDA Emergency Use Authorization for Children 6 Months through 4 Years of Age
- 15 Jun 22 Pfizer and BioNTech Provide Update on Rolling Submission to European Medicines Agency for a Potential Variant-Adapted Vaccine
- 6 Jun 22 Positive Phase 1 Data from mRNA-based Individualized Neoantigen Specific Immunotherapy in Patients with Resected Pancreatic Cancer presented at ASCO
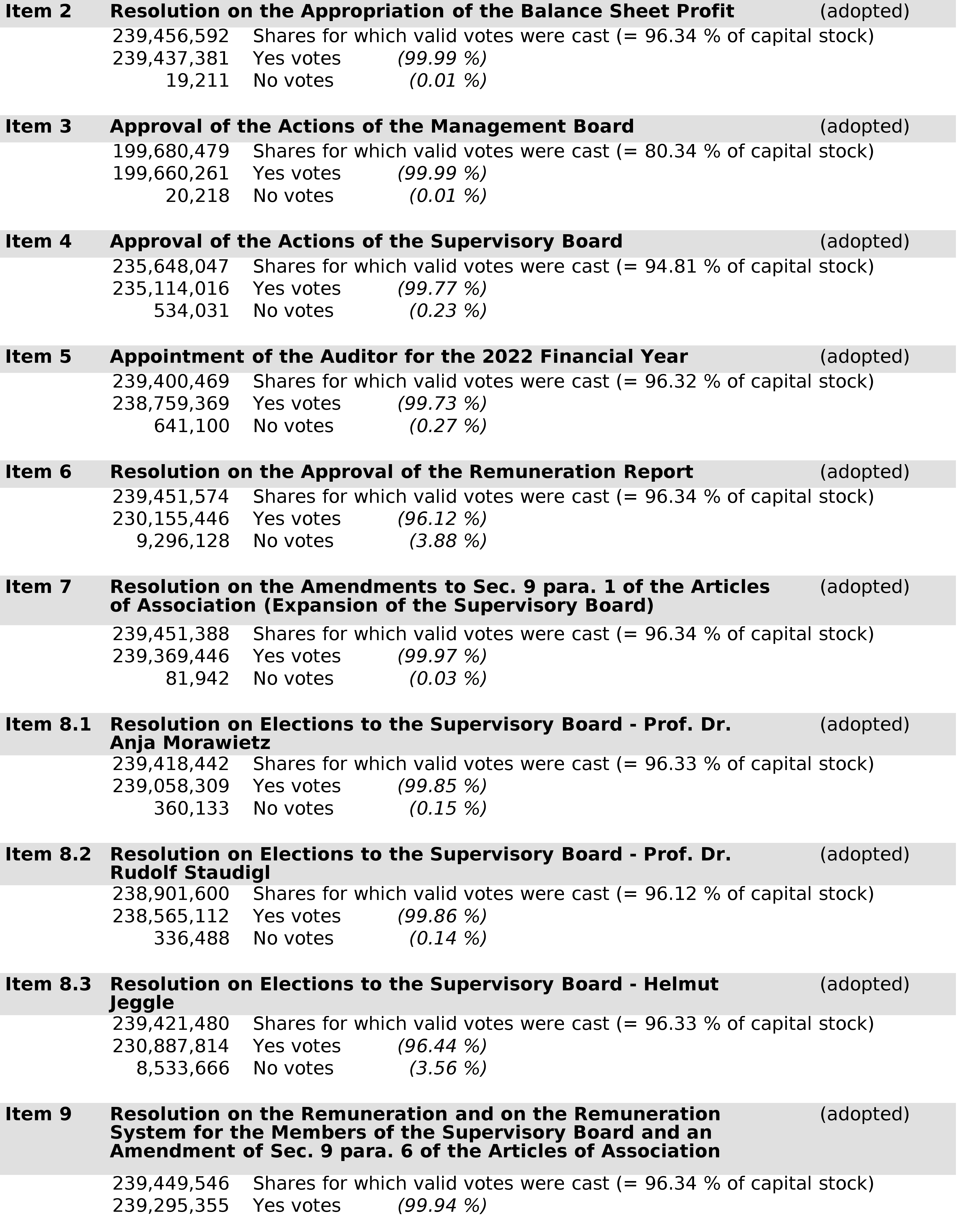
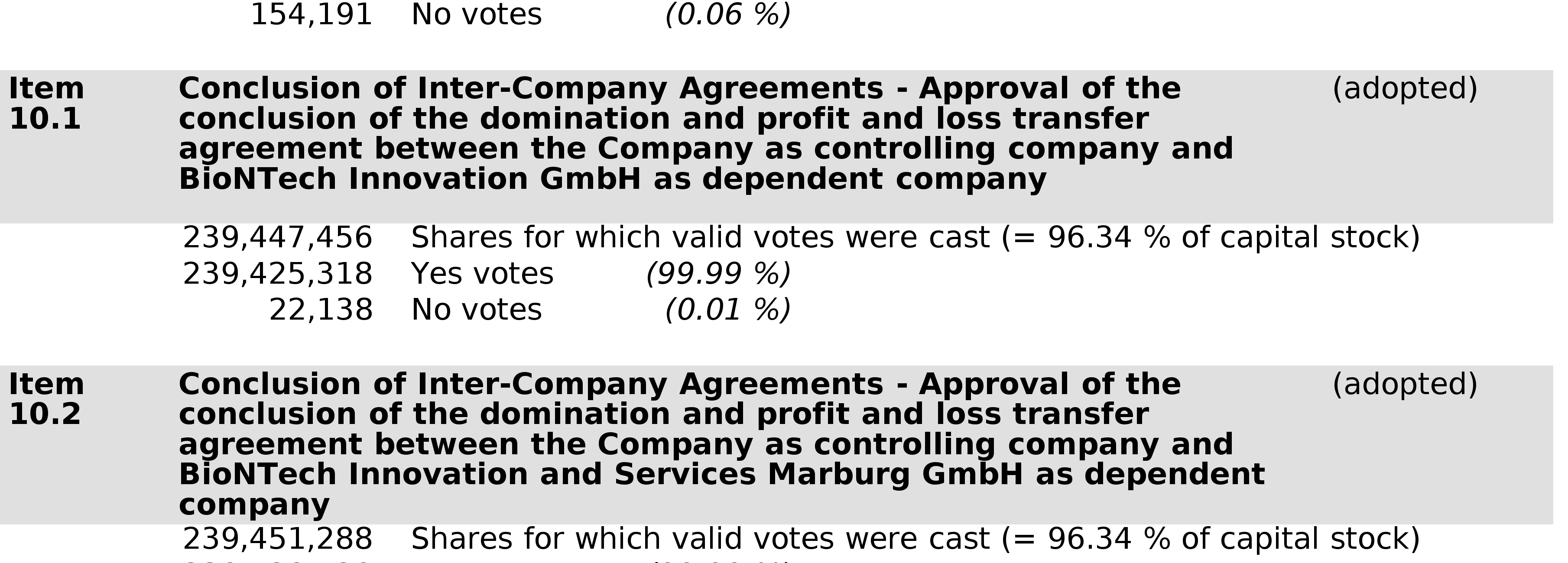
- 1 Jun 22 Voting Results from BioNTech’s Annual General Meeting 2022
- 23 May 22 Current report (foreign)
- 17 May 22 Pfizer and BioNTech Granted U.S. Emergency Use Authorization for Booster Dose of Their COVID-19 Vaccine in Children 5 Through 11 Years of Age
- 16 May 22 Pfizer and BioNTech Provide Update on COVID-19 Vaccine Supply Agreement with European Commission
Filing view
External links