
43rd J.P. Morgan Healthcare Conference Prof. Ugur Sahin, M.D. CEO & Co-founder 14 January 2025 9:00 – 9:40 AM PST Exhibit 99.2

This Slide Presentation Includes Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, but not limited to, statements concerning: BioNTech’s expected revenues and net profit/(loss), particularly for those figures that are derived from preliminary estimates provided by BioNTech’s partners; the rate and degree of market acceptance of BioNTech’s investigational medicines, if approved; the initiation, timing, progress, results, and cost of BioNTech’s research and development programs, including BioNTech’s current and future preclinical studies and clinical trials, including statements regarding the expected timing of initiation, enrollment, and completion of studies or trials and related preparatory work and the availability of results, and the timing and outcome of applications for regulatory approvals and marketing authorizations; BioNTech’s expectations regarding potential future commercialization in oncology, including goals regarding timing and indications, potential combination approaches, and estimated addressable patient populations; the targeted timing and number of additional potentially registrational trials, and the registrational potential of any trial BioNTech may initiate; discussions with regulatory agencies; BioNTech’s expectations with respect to intellectual property; BioNTech’s acquisition of Biotheus, which is subject to customary closing conditions, including regulatory approvals; the impact of BioNTech’s acquisition of Biotheus upon closing; collaboration and licensing agreements; and BioNTech’s estimated cash balance. In some cases, forward-looking statements can be identified by terminology such as “will,” “may,” “should,” “expects,” “intends,” “plans,” “aims,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue,” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. The forward-looking statements in this presentation are based on BioNTech’s current expectations and beliefs of future events, and are neither promises nor guarantees. You should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond BioNTech’s control and which could cause actual results to differ materially and adversely from those expressed or implied by these forward-looking statements. These risks and uncertainties include, but are not limited to: the uncertainties inherent in research and development, including the ability to meet anticipated clinical endpoints, commencement and/or completion dates for clinical trials, projected data release timelines, regulatory submission dates, regulatory approval dates and/or launch dates, as well as risks associated with preclinical and clinical data, including the data discussed in this release, and including the possibility of unfavorable new preclinical, clinical or safety data and further analyses of existing preclinical, clinical or safety data; the nature of the clinical data, which is subject to ongoing peer review, regulatory review and market interpretation; products and/or product candidates that may compete with BioNTech’s product candidates, including those with different mechanisms of action and different manufacturing and distribution constraints, on the basis of, among other things, efficacy, cost, convenience of storage and distribution, breadth of approved use, side-effect profile and durability of immune response; the timing of and BioNTech’s ability to obtain and maintain regulatory approval for its product candidates; BioNTech’s and its counterparties’ ability to manage and source necessary energy resources; BioNTech’s ability to identify research opportunities and discover and develop investigational medicines; the ability and willingness of BioNTech’s third-party collaborators to continue research and development activities relating to BioNTech's development candidates and investigational medicines; unforeseen safety issues and potential claims that are alleged to arise from the use of products and product candidates developed or manufactured by BioNTech; BioNTech’s and its collaborators’ ability to commercialize and market its product candidates, if approved; BioNTech’s ability to manage its development and related expenses; regulatory developments in the United States and other countries; BioNTech’s ability to effectively scale its production capabilities and manufacture its products and product candidates; risks relating to the global financial system and markets; and other factors not known to BioNTech at this time. You should review the risks and uncertainties described under the heading “Risk Factors” in BioNTech’s Report on Form 6-K for the period ended September 30, 2024 and in subsequent filings made by BioNTech with the SEC, which are available on the SEC’s website at www.sec.gov. These forward-looking statements speak only as of the date hereof. Except as required by law, BioNTech disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this presentation in the event of new information, future developments or otherwise. Furthermore, certain statements contained in this presentation relate to or are based on studies, publications, surveys and other data obtained from third-party sources and BioNTech’s own internal estimates and research. While BioNTech believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, any market data included in this presentation involves assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. While BioNTech believes its own internal research is reliable, such research has not been verified by any independent source. In addition, BioNTech is the owner of various trademarks, trade names and service marks that may appear in this presentation. Certain other trademarks, trade names and service marks appearing in this presentation are the property of third parties. Solely for convenience, the trademarks and trade names in this presentation may be referred to without the ® and TM symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. 2 A glossary of defined terms can be found at the end of the presentation.

3 Building a Global Immunotherapy Powerhouse Translating Science into Survival
Strengthened position by planned Biotheus acquisition2: Securing global control of BNT3271 and expanded pipeline and in-house immunotherapy capabilities 2024 Accomplishments Position Us for Success in 2025 4 1. BNT327/PM8002 partnered with Biotheus. In this presentation, BNT327/PM8002 will further be referred to as “BNT327”; 2. Expected to close in Q1 2025, subject to satisfaction of customary closing conditions, including regulatory approvals; 3. Partnered with Pfizer; 4. Preliminary, unaudited figure; consists of cash, cash equivalents and security investments, as of December 31, 2024. Oncology Portfolio BNT327/PM80021 Corporate Development COVID-193 and Infectious Disease Vaccines Cash Balance4 Advanced oncology portfolio into late stage with 15 ongoing Phase 2 and Phase 3 trials Presented multiple datasets for BNT3271 and announced pivotal trials targeting unmet needs in SCLC, TNBC, and NSCLC Maintained leading COVID-193 market share globally (>50%) underscoring competitive strength and progressed early-stage infectious disease pipeline Strengthened balance sheet through strong financial performance, reinforcing long-term growth potential: ~€ 17.4 bn total cash and cash equivalents plus security investments4
We Have Unique Capabilities to Build Tomorrow’s Personalized Precision Medicines 5 . Deep genomics & immunology expertise to analyze patient data Individualized treatment platforms to address inter-individual variability AI-infused, digitally-integrated target & drug discovery and development Automated in-house manufacturing to serve patients on time and globally Tailored on-demand immunotherapies Off-the-shelf drugs Targeted therapies Immunomodulators mRNA cancer immunotherapies Clinical samples Therapeutic modalities Inter- individual variability Personalized Omics

Our Leading Scientific Capabilities are Fueled by AI to Pioneer Personalized Immunotherapies 6 1. Partnered with Genentech, a member of the Roche Group. 2. From trials BNT122-01, GO39733, GO40558 and ML41081; 3. Castle et al. 2011 Cancer Res; 4.“Top 500, The List”, June 2023. Semi-automated manufacturing capabilities for iNeST1 iNeST1: Personalized immunotherapy platform utilizing AI to create therapies unique to each patients’ tumor ➢ 4 ongoing trials ➢ >450 patients treated2 ➢ 18,000 neoantigens selected2 Development of novel DeepChain platform combining cutting-edge AI and bio-engineering Automated dry-wet lab to enhance discovery capabilities In-house supercomputing cluster is among worldwide top 1004 AI empowered bio-engineeringPersonalized immunotherapy Optimization of mRNA design & structure Computational extension of immunotherapy target space3 AI

Space for curative approaches Immunomodulators Novel checkpoint inhibitors cytokines, immune agonists mRNA cancer immunotherapies Targeted therapies ADCs, CAR-T, Ribomabs SynergySynergy Synergy • Focus on the critical IO pathways • Targeting different complementary pathways in cancer immunity cycle may promote a durable anti-tumor effect • Eliminate polyclonal residual disease with multi-antigen and individualized approaches • Polyspecific activity by targeting multiple antigens at once • Establish long-lasting immunological memory to prevent relapses • Precise and potent modalities for fast onset tumor reduction • ADC as potential “augmenters” of immunomodulators and mRNA cancer vaccines • Focus on HER2, HER3, TROP2, B7H3 ADCs as combination partners 7 Immunomodulators Targeted therapies mRNA cancer immunotherapies We are Uniquely Positioned to Combine Approaches to Transform Cancer Care

8 Targeted therapies Targeted therapies Immunomodulators Space for curative approaches mRNA cancer immunotherapies SynergySynergy Synergy Immunomodulators BNT312/ GEN10425 BNT315/ GEN10535 Anti-OX40 BNT316/ ONC-3926 Anti-CTLA4 Anti-4-1BB Anti- CD40 / BNT314/ GEN10595 Anti-4-1BB Anti-EpCAM / BNT3273 Anti-PD-L1 / Anti-VEGF BNT151 BNT152 BNT153 Ribo- cytokines IL2, IL7 iNeST4 Fully Individualized FixVac Off-the-shelf mRNA BNT324/ DB-13111 BNT323/ DB-13031 Anti-HER2 ADC Anti-B7H3 ADC BNT325/ DB-13051 BNT326/ YL2022 Anti-TROP2 ADC Anti-HER3 ADC BNT211 CAR-T Anti-CLDN6 CAR-T BNT141 BNT142 Ribomabs Anti-CLDN6 Anti-CLDN18.2 Partnered with 1. DualityBio; 2. MediLink; 3. BNT327/PM8002 partnered with Biotheus; 4. Genentech, a member of the Roche Group; 5. Genmab; 6. OncoC4 Our Unique Pipeline Has the Potential for a Curative Approach to Cancer mRNA cancer immunotherapies

PD-L1/VEGF-A antibody BNT3272 Novel mRNA cancer immunotherapy Potential to become the next-generation IO-backboneNext-generation of personalized cancer therapy targeting tumor associated antigens and mutations 9 1. Partnered with Genentech, a member of the Roche Group; 2. BNT327/PM8002 partnered with Biotheus. FixVac & iNeST1 Our next-generation IO-backbone Our Priorities are Novel mRNA Cancer Immunotherapy and Next-Generation IO-Backbone Resected cancers (adjuvant, ctDNA+) Neoadjuvant, 1L advanced/metastatic Late stage, refractory cancers Clinical stage candidates for combination therapy ADCs Cell and gene therapiesIO molecules Addressing the full continuum of cancer across different stages Priority Pan-tumor Programs mRNA immunotherapies

PD-L1/VEGF-A antibody BNT3272 Novel mRNA cancer immunotherapy Potential to become the next-generation IO-backboneNext-generation of personalized cancer therapy targeting tumor associated antigens and mutations 10 FixVac & iNeST1 Our next-generation IO-backbone BNT327 as Potential Next-Generation IO-Backbone Resected cancers (adjuvant, ctDNA+) Neoadjuvant, 1L advanced/metastatic Late stage, refractory cancers Clinical stage candidates for combination therapy ADCs Cell and gene therapiesIO molecules Addressing the full continuum of cancer across different stages Priority Pan-tumor Programs mRNA immunotherapies 1. Partnered with Genentech, a member of the Roche Group; 2. BNT327/PM8002 partnered with Biotheus.

BNT3271: Data from 750 Patients Across Multiple Indications Highlight the Potential to Establish a New Standard of Care 11 10+ indications studied2 >750 patients enrolled 20 clinical trials ongoing or planned 3 global potentially registrational trials Clinical activity across indications Including SCLC, NSCLC, TNBC, HCC, MPM and others Including studies in 1L or 2L with SoC CTx and novel combinations Focus on 1L TNBC, SCLC, and NSCLC 1.BNT327/PM8002 partnered with Biotheus; 2. Indications included in Ph2a: NSCLC, mucosal melanoma, renal cell carcinoma, endometrial cancer, cervical cancer, platinum resistant ovarian cancer.
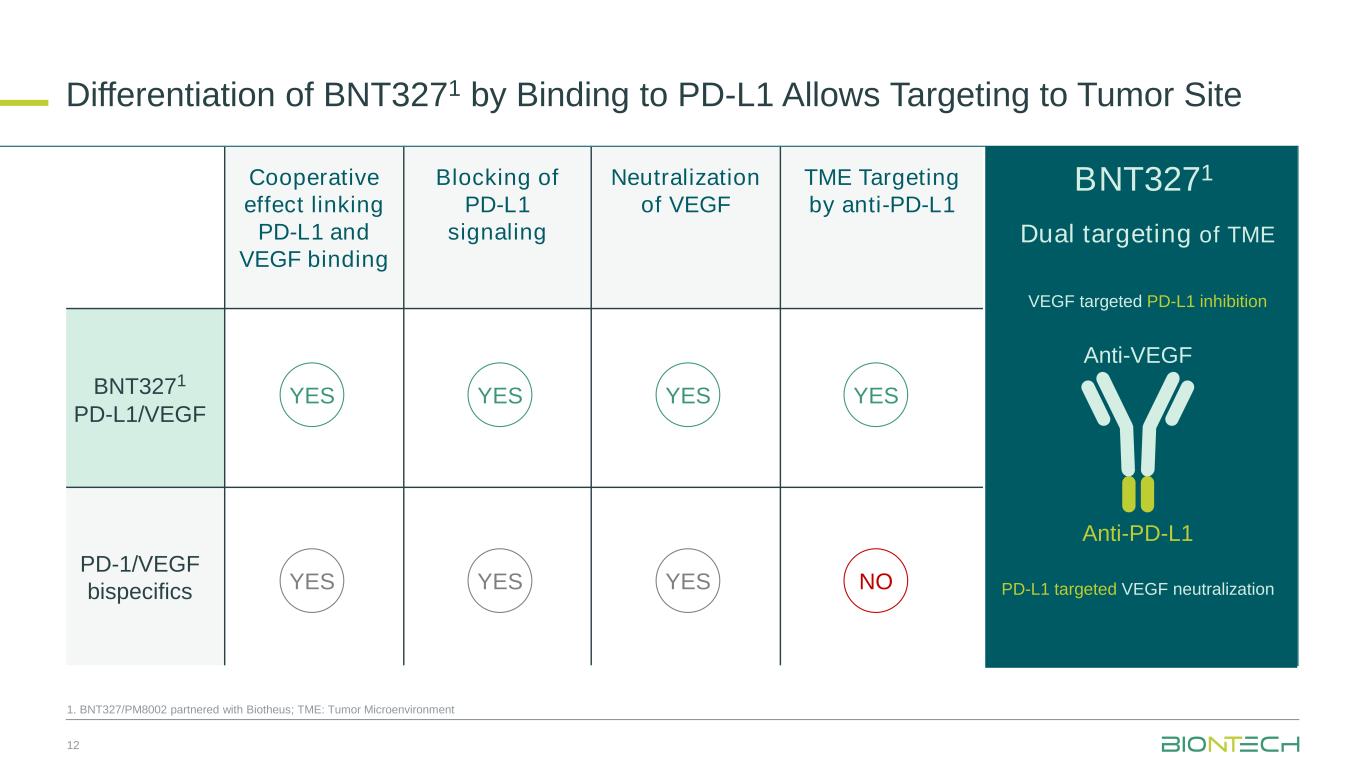
Cooperative effect linking PD-L1 and VEGF binding Blocking of PD-L1 signaling Neutralization of VEGF TME Targeting by anti-PD-L1 BNT3271 PD-L1/VEGF PD-1/VEGF bispecifics Dual targeting of TME Anti-PD-L1 Anti-VEGF YES YES YES YES NO YESYES YES 1. BNT327/PM8002 partnered with Biotheus; TME: Tumor Microenvironment Differentiation of BNT3271 by Binding to PD-L1 Allows Targeting to Tumor Site 12 BNT3271 VEGF targeted PD-L1 inhibition PD-L1 targeted VEGF neutralization

BNT3271 + chemo in 1L TNBC, Y. Meng et al. Presented at ESMO 2024. Presentation 384MO BNT3271 May Drive Clinical Benefit Irrespective of PD-L1 Status 13 76.9% 56.3% 100.0% ORR Objective response rate BNT3271 has the potential to become a backbone IO therapy for significant patient populations currently not addressed by existing IO therapies PD-L1 negative2 PD-L1 low2 PD-L1 high2 -100 -80 -60 -40 -20 0 20 -100 -80 -60 -40 -20 0 20 -100 -80 -60 -40 -20 0 20 1.BNT327/PM8002 partnered with Biotheus; 2. PD-L1 status in TNBC: negative= CPS<1; low= 1≤CPS<10; high= CPS10 Waterfall plotsPD-L1 status
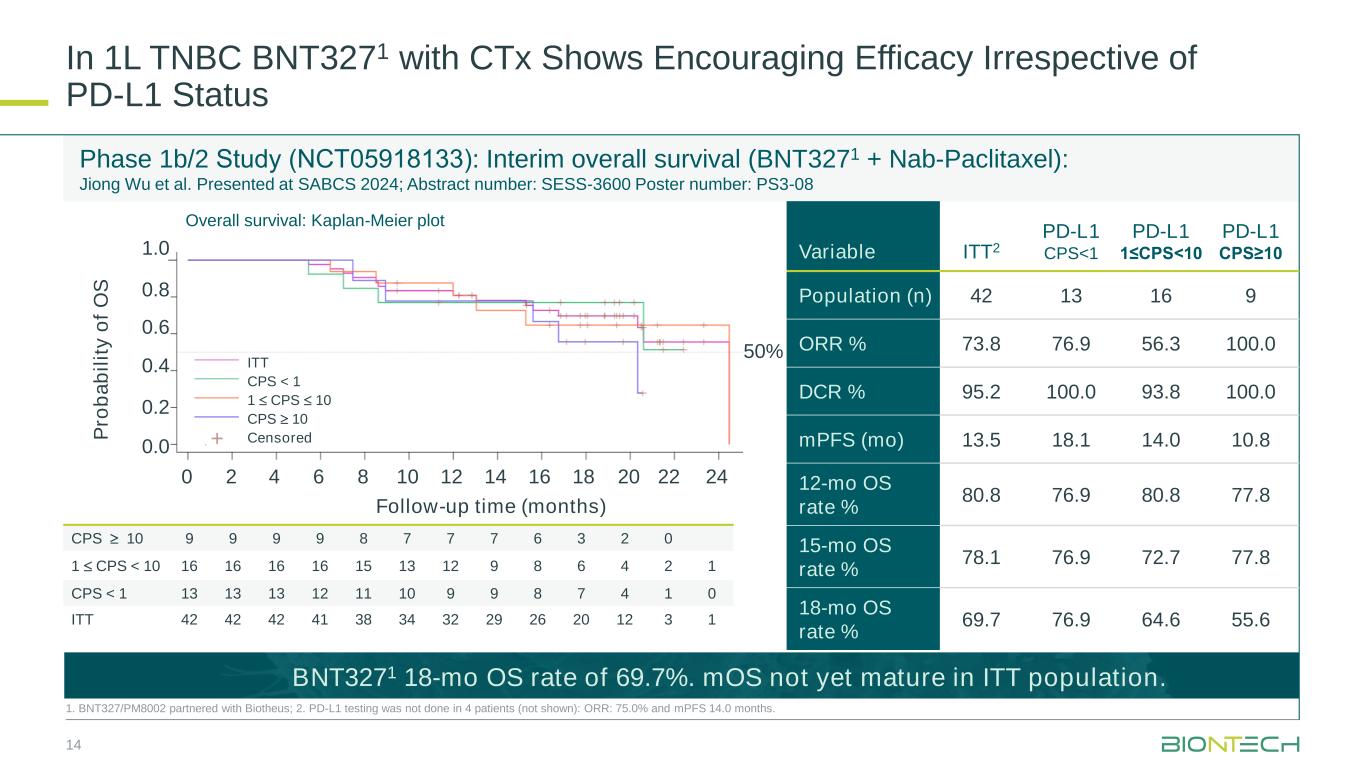
In 1L TNBC BNT3271 with CTx Shows Encouraging Efficacy Irrespective of PD-L1 Status 14 1. BNT327/PM8002 partnered with Biotheus; 2. PD-L1 testing was not done in 4 patients (not shown): ORR: 75.0% and mPFS 14.0 months. CPS 10 9 9 9 9 8 7 7 7 6 3 2 0 1 CPS < 10 16 16 16 16 15 13 12 9 8 6 4 2 1 CPS < 1 13 13 13 12 11 10 9 9 8 7 4 1 0 ITT 42 42 42 41 38 34 32 29 26 20 12 3 1 Phase 1b/2 Study (NCT05918133): Interim overall survival (BNT3271 + Nab-Paclitaxel): Jiong Wu et al. Presented at SABCS 2024; Abstract number: SESS-3600 Poster number: PS3-08 0 2 4 6 8 10 12 14 16 18 20 22 24 Follow-up time (months) P ro b a b il it y o f O S 1.0 0.8 0.6 0.4 0.2 0.0 50% + ITT CPS < 1 1 CPS 10 CPS 10 Censored Overall survival: Kaplan-Meier plot Variable ITT2 PD-L1 CPS<1 PD-L1 1≤CPS<10 PD-L1 CPS≥10 Population (n) 42 13 16 9 ORR % 73.8 76.9 56.3 100.0 DCR % 95.2 100.0 93.8 100.0 mPFS (mo) 13.5 18.1 14.0 10.8 12-mo OS rate % 80.8 76.9 80.8 77.8 15-mo OS rate % 78.1 76.9 72.7 77.8 18-mo OS rate % 69.7 76.9 64.6 55.6 BNT3271 18-mo OS rate of 69.7%. mOS not yet mature in ITT population.

Broad Combination Strategy Across Indications Aiming to Establish Next-Generation IO-Backbone 15 1. NCI SEER https://training.seer.cancer.gov/index.html. 2.US incidence source: NIH and American Cancer Society data EU incidence source: European Cancer Information System Anti-PD-L1 Anti-VEGF Anti-PD-(L)1 therapy addresses ~1.5 M new cancer cases in the US and EU annually with medical need remaining high (5-year survival < 50%)1 >1.4 M estimated new cancer cases in the US and EU annually that cannot be addressed by current IO therapies Anti-PD-(L)1 approved Anti-PD-(L)1 not approved US and EU Cancer Incidence2 Breast (non TNBC) TNBC PD-L1 <10% CRC (MSS) EGFRmut NSCLC Pancreatic Ovarian GBM Lung Melanoma RCC Endometrial HNSCC/nasopharyngeal TNBC PD-L1 >10% HCC Gastric CRC (MSI-H)
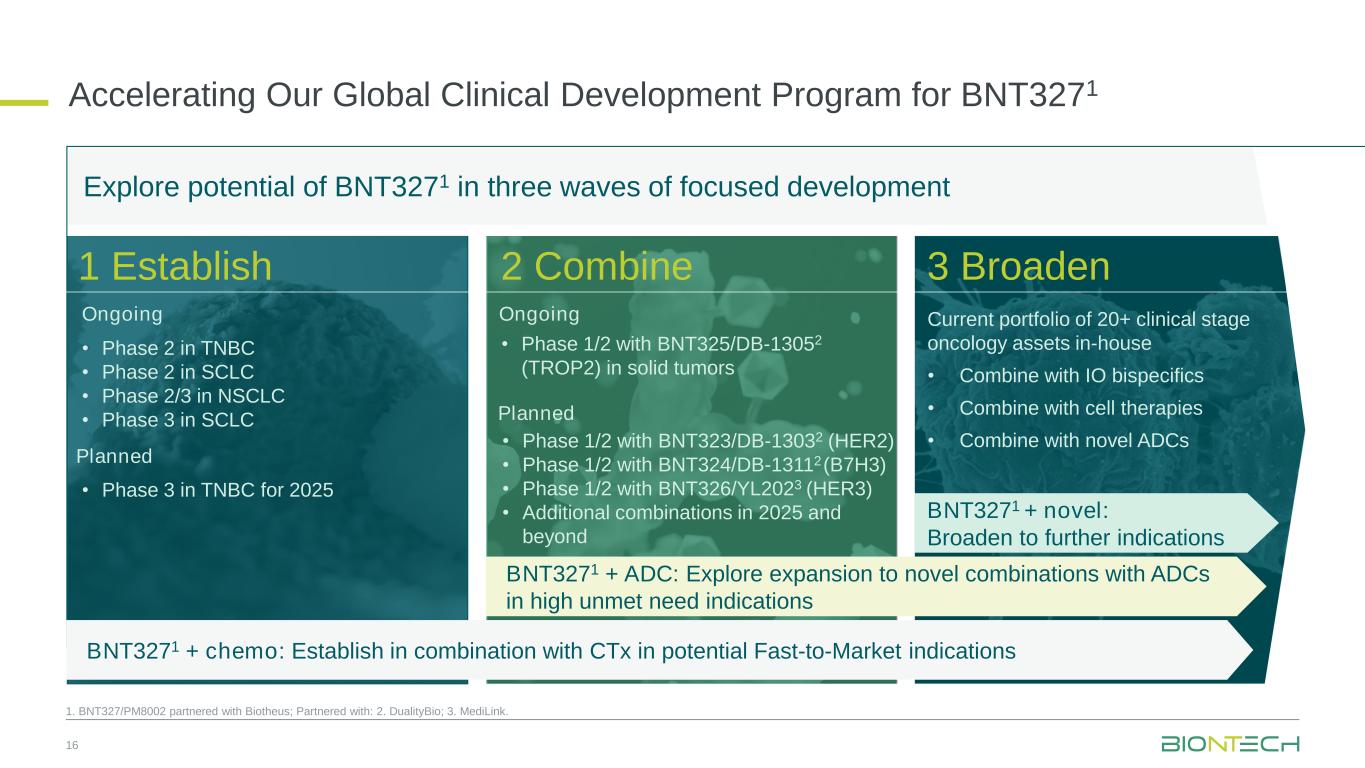
Accelerating Our Global Clinical Development Program for BNT3271 1. BNT327/PM8002 partnered with Biotheus; Partnered with: 2. DualityBio; 3. MediLink. Explore potential of BNT3271 in three waves of focused development • Phase 2 in TNBC • Phase 2 in SCLC • Phase 2/3 in NSCLC • Phase 3 in SCLC Ongoing Planned • Phase 3 in TNBC for 2025 3 Broaden2 Combine1 Establish Ongoing Planned 16 BNT3271 + chemo: Establish in combination with CTx in potential Fast-to-Market indications BNT3271 + ADC: Explore expansion to novel combinations with ADCs in high unmet need indications BNT3271 + novel: Broaden to further indications • Phase 1/2 with BNT323/DB-13032 (HER2) • Phase 1/2 with BNT324/DB-13112(B7H3) • Phase 1/2 with BNT326/YL2023 (HER3) • Additional combinations in 2025 and beyond • Phase 1/2 with BNT325/DB-13052 (TROP2) in solid tumors Current portfolio of 20+ clinical stage oncology assets in-house • Combine with IO bispecifics • Combine with cell therapies • Combine with novel ADCs

BNT3271: Data Readouts Expected in 2025 17 1. BNT327/PM8002 partnered with Biotheus; 2. Partnered with DualityBio. Indication Target Population Regimen Phase Region SCLC 1L or 2L + chemo 2 Global TNBC 1L or 2L + chemo 2 Global Multiple solid tumors Multiple lines + BNT325/DB-13052 1/2 Global SCLC 1L + chemo 2 China SCLC 2L + chemo 2 China MPM 1L + chemo 2 China
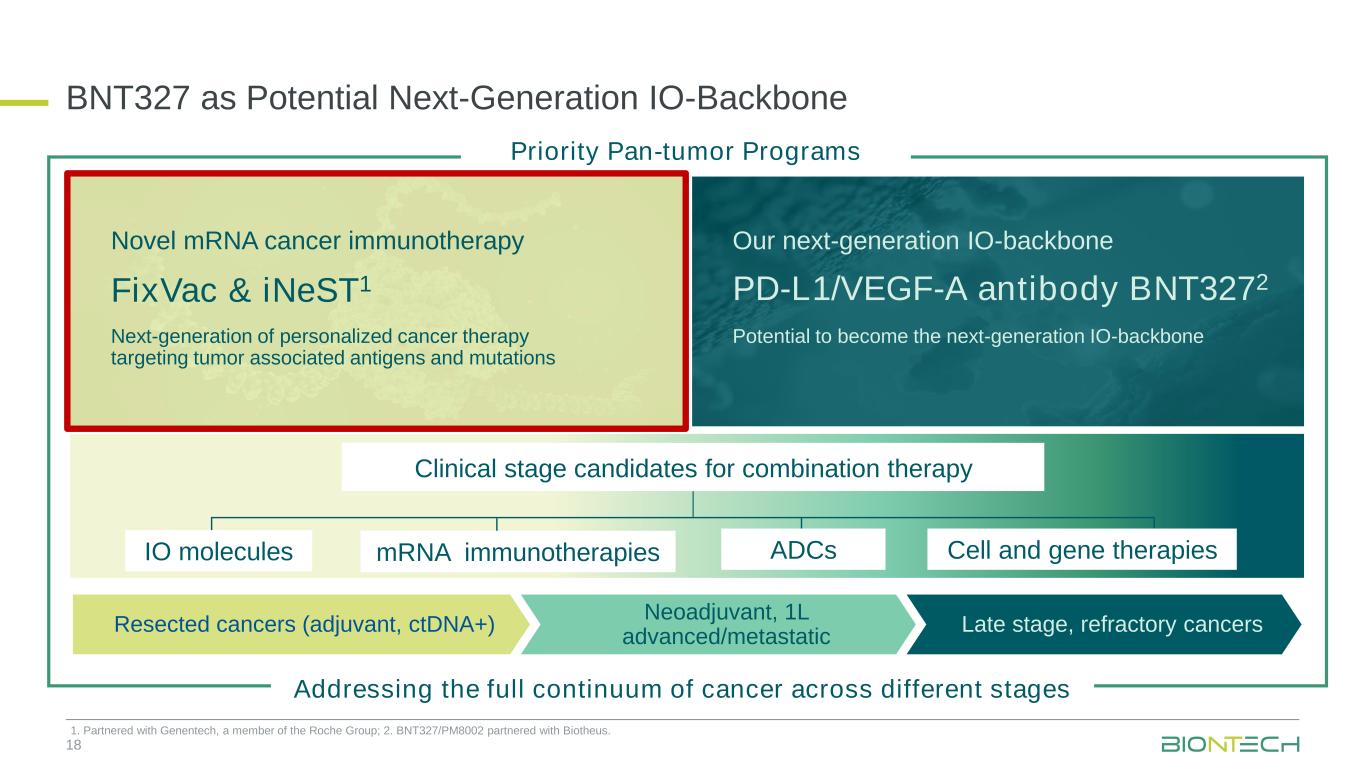
PD-L1/VEGF-A antibody BNT3272 Novel mRNA cancer immunotherapy Potential to become the next-generation IO-backboneNext-generation of personalized cancer therapy targeting tumor associated antigens and mutations 18 FixVac & iNeST1 Our next-generation IO-backbone BNT327 as Potential Next-Generation IO-Backbone Resected cancers (adjuvant, ctDNA+) Neoadjuvant, 1L advanced/metastatic Late stage, refractory cancers Clinical stage candidates for combination therapy ADCs Cell and gene therapiesIO molecules Addressing the full continuum of cancer across different stages Priority Pan-tumor Programs mRNA immunotherapies 1. Partnered with Genentech, a member of the Roche Group; 2. BNT327/PM8002 partnered with Biotheus.
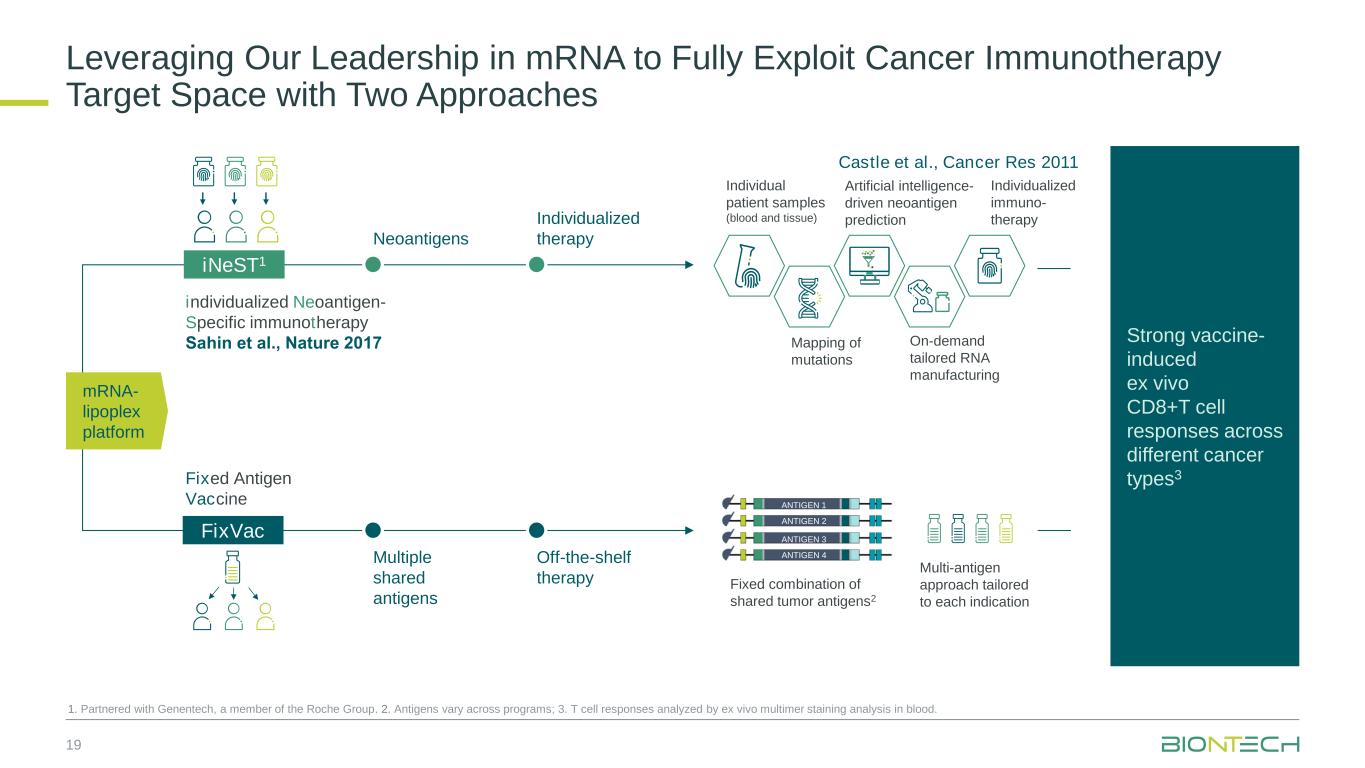
Leveraging Our Leadership in mRNA to Fully Exploit Cancer Immunotherapy Target Space with Two Approaches 19 Fixed combination of shared tumor antigens2 Multi-antigen approach tailored to each indication Neoantigens Individualized therapy Multiple shared antigens Off-the-shelf therapy iNeST1 FixVac individualized Neoantigen- Specific immunotherapy Sahin et al., Nature 2017 Fixed Antigen Vaccine ANTIGEN 1 ANTIGEN 2 ANTIGEN 3 ANTIGEN 4 Strong vaccine- induced ex vivo CD8+T cell responses across different cancer types3 mRNA- lipoplex platform 1. Partnered with Genentech, a member of the Roche Group. 2. Antigens vary across programs; 3. T cell responses analyzed by ex vivo multimer staining analysis in blood. Individual patient samples (blood and tissue) Artificial intelligence- driven neoantigen prediction On-demand tailored RNA manufacturing Individualized immuno- therapy Mapping of mutations Castle et al., Cancer Res 2011
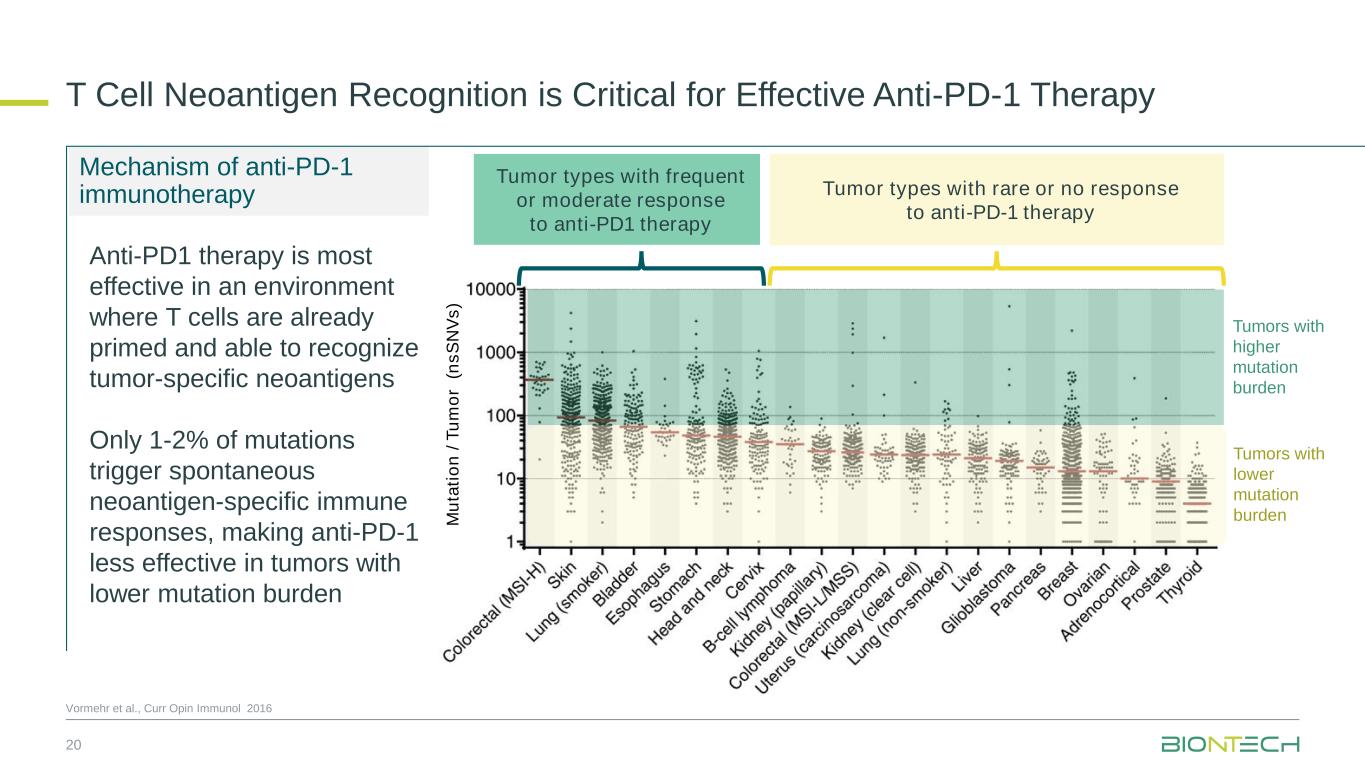
T Cell Neoantigen Recognition is Critical for Effective Anti-PD-1 Therapy 20 Vormehr et al., Curr Opin Immunol 2016 Tumors with lower mutation burdenM u ta ti o n / T u m o r ( n s S N V s ) Mechanism of anti-PD-1 immunotherapy Anti-PD1 therapy is most effective in an environment where T cells are already primed and able to recognize tumor-specific neoantigens Only 1-2% of mutations trigger spontaneous neoantigen-specific immune responses, making anti-PD-1 less effective in tumors with lower mutation burden Tumors with higher mutation burden Tumor types with frequent or moderate response to anti-PD1 therapy Tumor types with rare or no response to anti-PD-1 therapy
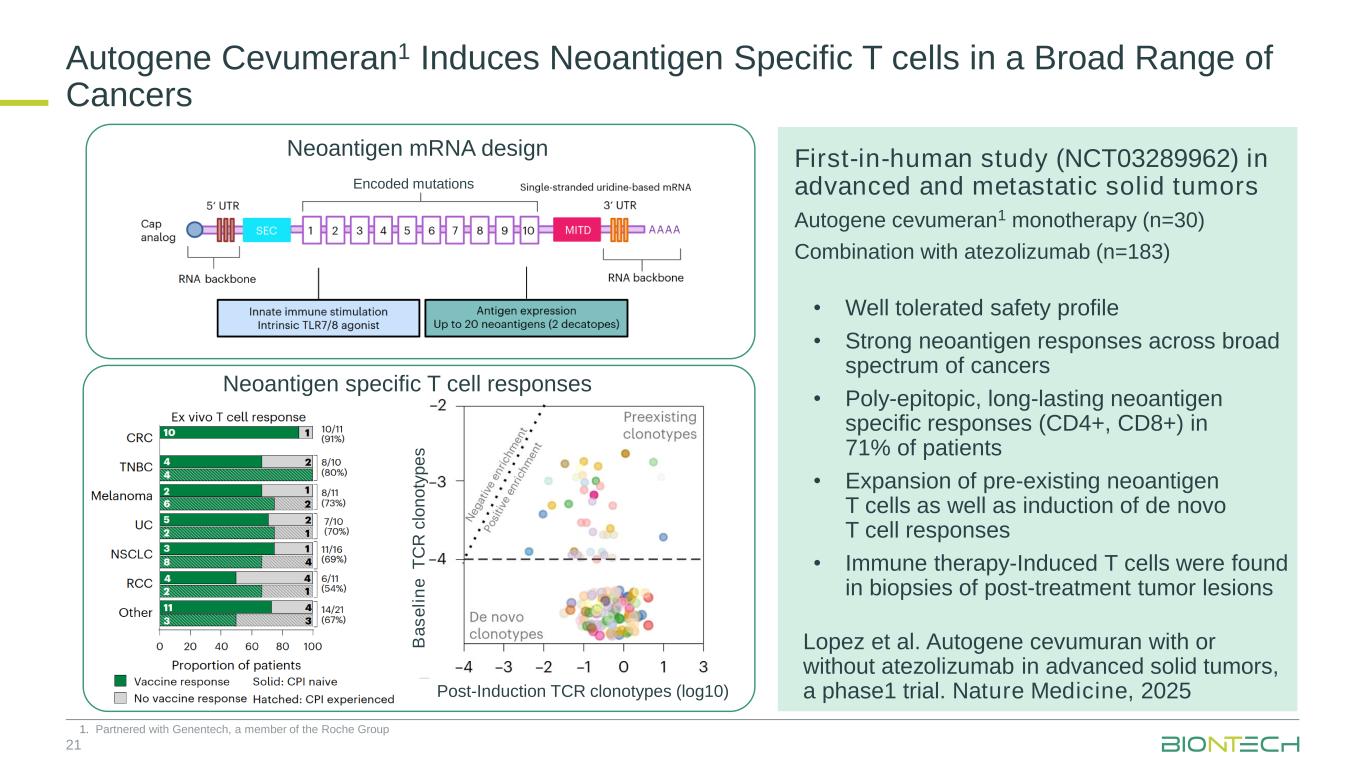
Encoded mutations 21 First-in-human study (NCT03289962) in advanced and metastatic solid tumors Autogene cevumeran1 monotherapy (n=30) Combination with atezolizumab (n=183) Neoantigen mRNA design B a s e li n e T C R c lo n o ty p e s Post-Induction TCR clonotypes (log10) Neoantigen specific T cell responses Autogene Cevumeran1 Induces Neoantigen Specific T cells in a Broad Range of Cancers 1. Partnered with Genentech, a member of the Roche Group • Well tolerated safety profile • Strong neoantigen responses across broad spectrum of cancers • Poly-epitopic, long-lasting neoantigen specific responses (CD4+, CD8+) in 71% of patients • Expansion of pre-existing neoantigen T cells as well as induction of de novo T cell responses • Immune therapy-Induced T cells were found in biopsies of post-treatment tumor lesions Lopez et al. Autogene cevumuran with or without atezolizumab in advanced solid tumors, a phase1 trial. Nature Medicine, 2025
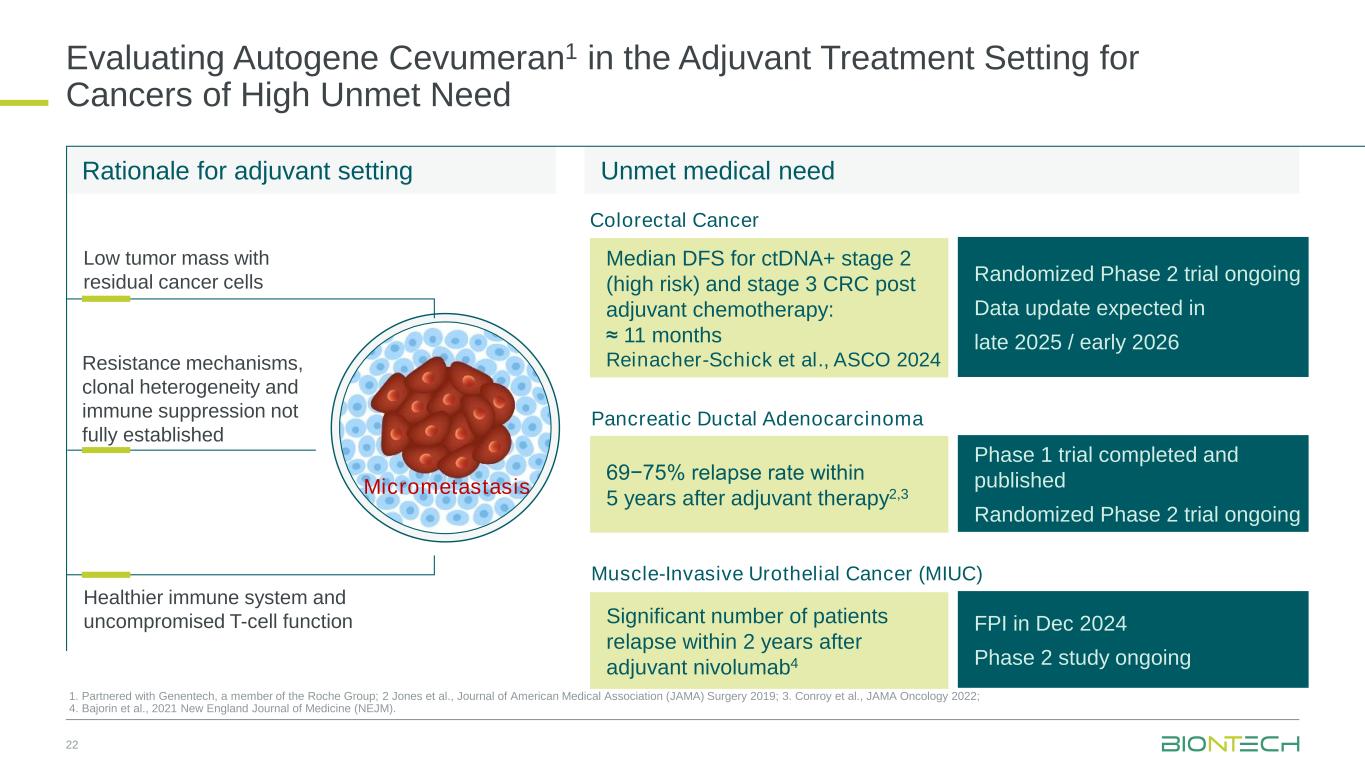
Evaluating Autogene Cevumeran1 in the Adjuvant Treatment Setting for Cancers of High Unmet Need 22 1. Partnered with Genentech, a member of the Roche Group; 2 Jones et al., Journal of American Medical Association (JAMA) Surgery 2019; 3. Conroy et al., JAMA Oncology 2022; 4. Bajorin et al., 2021 New England Journal of Medicine (NEJM). Rationale for adjuvant setting Low tumor mass with residual cancer cells Healthier immune system and uncompromised T-cell function Resistance mechanisms, clonal heterogeneity and immune suppression not fully established Pancreatic Ductal Adenocarcinoma 69−75% relapse rate within 5 years after adjuvant therapy2,3 Phase 1 trial completed and published Randomized Phase 2 trial ongoing Median DFS for ctDNA+ stage 2 (high risk) and stage 3 CRC post adjuvant chemotherapy: ≈ 11 months Reinacher-Schick et al., ASCO 2024 Randomized Phase 2 trial ongoing Data update expected in late 2025 / early 2026 Colorectal Cancer Unmet medical need Significant number of patients relapse within 2 years after adjuvant nivolumab4 Muscle-Invasive Urothelial Cancer (MIUC) FPI in Dec 2024 Phase 2 study ongoing Micrometastasis

Response to Autogene Cevumeran1 Correlates with Delayed PDAC Recurrence 23 1. Partnered with Genentech, a member of the Roche Group. Phase 1, investigator-initiated trial in resectable PDAC: 3-year follow-up data Balachandran et al., AACR 2024. #CT025 & Rojas et al., Nature 2023 Years At risk Responder 8 8 7 5 0 Non-responder 8 5 1 1 0 3-year median follow-up P = 0.007, HR: 0.14 (0.03-0.59) 0 20 40 60 80 100 0 1 2 3 4 Median RFS: Not reached Median RFS: 13.4 months R F S ( % ) Years Immune responder (n = 8) Immune non-responder (n = 8) Immunogenic Non-immunogenic No data N u m b e r o f n e o a n ti g e n s in a u to g e n e c e v u m e ra n 20 15 10 5 0 Patient Responders (n=8) Non-responders (n=8) 10 29 25 25 5 6 14 1 3 19 4 20 9 18 23 28 R0/R1 R0 R0 R0 R1 R0 R0 R0 R1 R0 R1 R0 R0 R0 R0 R0 R0 Half of all patients mounted neoantigen-specific de novo T cell responses against at least one vaccine neoantigen
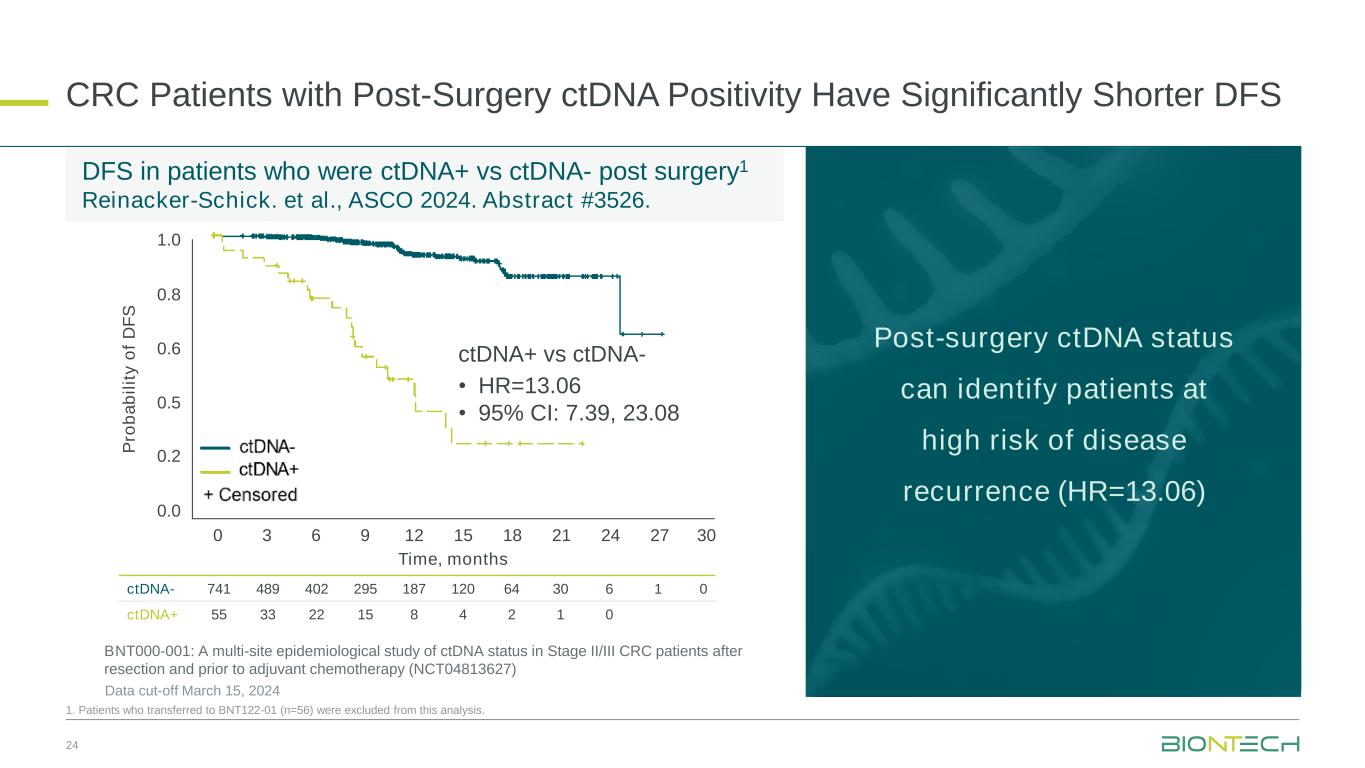
DFS in patients who were ctDNA+ vs ctDNA- post surgery1 Reinacker-Schick. et al., ASCO 2024. Abstract #3526. 24 1. Patients who transferred to BNT122-01 (n=56) were excluded from this analysis. ctDNA- 741 489 402 295 187 120 64 30 6 1 0 ctDNA+ 55 33 22 15 8 4 2 1 0 P ro b a b il it y o f D F S Time, months 0.0 0.2 0.5 0.6 0.8 1.0 0 3 6 9 12 15 18 21 24 27 30 CRC Patients with Post-Surgery ctDNA Positivity Have Significantly Shorter DFS ctDNA+ vs ctDNA- • HR=13.06 • 95% CI: 7.39, 23.08 BNT000-001: A multi-site epidemiological study of ctDNA status in Stage II/III CRC patients after resection and prior to adjuvant chemotherapy (NCT04813627) Data cut-off March 15, 2024 Post-surgery ctDNA status can identify patients at high risk of disease recurrence (HR=13.06)
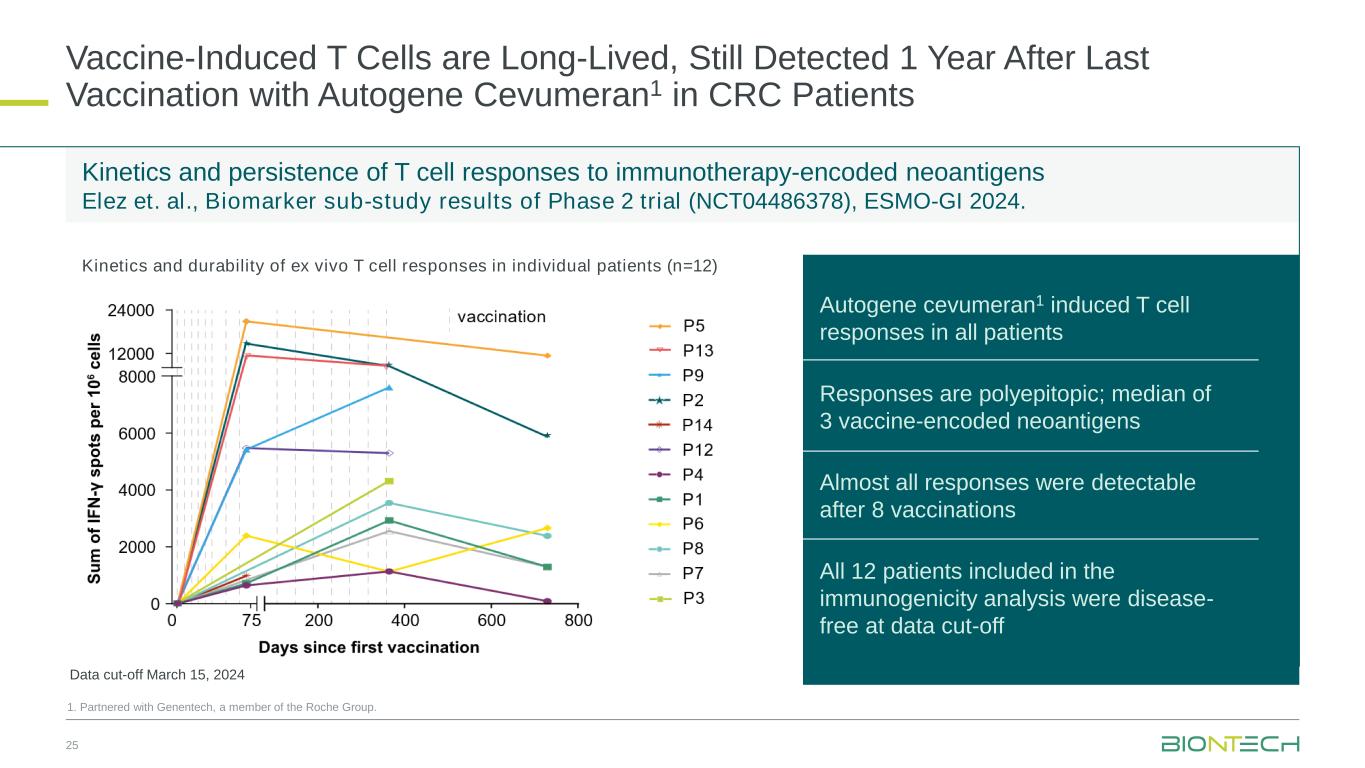
Kinetics and persistence of T cell responses to immunotherapy-encoded neoantigens Elez et. al., Biomarker sub-study results of Phase 2 trial (NCT04486378), ESMO-GI 2024. Vaccine-Induced T Cells are Long-Lived, Still Detected 1 Year After Last Vaccination with Autogene Cevumeran1 in CRC Patients 25 1. Partnered with Genentech, a member of the Roche Group. Autogene cevumeran1 induced T cell responses in all patients Responses are polyepitopic; median of 3 vaccine-encoded neoantigens Almost all responses were detectable after 8 vaccinations All 12 patients included in the immunogenicity analysis were disease- free at data cut-off Kinetics and durability of ex vivo T cell responses in individual patients (n=12) Data cut-off March 15, 2024

Adjuvant SoC chemotherapy for 12–24 weeks Ongoing Randomized Phase 2 Trial Evaluating Autogene Cevumeran in ctDNA+ CRC Patients 26 1. Partnered with Genentech, a member of the Roche Group. Autogene cevumeran1 15 doses: 6×Q1W, 2×Q2W, 7×Q6W Observational watchful waiting Key inclusion criteria • Patients with surgically-resected Stage II (high-risk) or Stage III CRC • Post surgery ctDNA+ N=327 R 1:1 BNT122-01: Phase 2 multi-site, open-label, randomized, controlled trial (NCT04486378) vs. watchful waiting in adjuvant colorectal cancer First data expected in late 2025 / early 2026 Key endpoints DFS; Efficacy: RFS, TTR, TTF, OS; Change in ctDNA status

Multiple Clinical Trials Demonstrate Execution Across iNeST and FixVac Portfolios 27 1. Partnered with Genentech, a member of the Roche Group; 2. In collaboration with Regeneron. Individualized immunotherapy: iNeST FixVac Autogene cevumeran (BNT122/RO7198457)1 BNT1112 BNT113 BNT116 Adjuvant 1L R/R R/R 1L Multiple settings MIUC Phase 2 CRC Phase 2 PDAC Phase 2 Melanoma Phase 2 Solid tumors Phase 1 Melanoma Phase 2 HPV16+ HNSCC Phase 2 NSCLC Phase 1 & 2 + Nivolumab Monotherapy + Atezolizumab + mFOLFIRINOX + Pembrolizumab + Atezolizumab + Cemiplimab + Pembrolizumab Monotherapy, + Cemiplimab or CTx or aCTLA4 Recruitment ongoing Recruitment ongoing Data presented from epi sub-study at ASCO 2024 and from biomarker sub-study at ESMO-GI 2024. Recruitment ongoing Data presented from investigator-initiated Ph 1 trial at ASCO 2022 & AACR 2024 and published (Rojas et al., Nature 2023). Enrollment completed Ph 1 data on prototype vaccine published (Sahin et al., Nature 2017). Analysis of Ph 2 PFS as primary endpoint will be based on events and defined when reporting results. Enrollment completed Data presented at AACR 2020. Data published (Lopez et al., Nature Medicine 2025) Enrollment completed Positive topline data announced July 2024 Data presented from Ph 1 at multiple conferences incl. SITC 2021 and published (Sahin et al., Nature 2020). Recruitment ongoing Ph 2 data presented at multiple conferences incl. ESMO-IO 2022 Data from safety run-in of Ph 2 trial and Ph 1/2 IIT presented at ESMO 2024. Recruitment ongoing in Ph 2 in 1L NSCLC2 Ph 1 trial ongoing. Data presented at SITC 2023, AACR 2024, and SITC 2024. Data expected in 2025 or 2026
Our Priorities for 2025 1. Partnered with Pfizer; 2. BNT327/PM8002 partnered with Biotheus; 3. Partnered with DualityBio. mRNA Cancer Immunotherapy BNT3272 Commercial Readiness in OncologyCOVID-19 Vaccine1 & ID » Expect multiple randomized Phase 2 data readouts » Execute 7 ongoing Phase 2 trials and first novel combination trials » Advance 3 global registration- enabling trials in potential fast-to-market indications » Generate first BNT3272+ ADC combination data sets » Advance BNT323/DB-13033 towards BLA submission » Build targeted AI-enabled commercialization team in key markets » Maintain global COVID-19 vaccine1 market leadership » Advance next-gen and combination offerings 2025 28 » Multiple updates expected on ID pipeline

Advancing Our Vision for Oncology: A Once In a Generation Opportunity to Transform Medicine for Cancer Patients Execute on late-stage trials for BNT3271 and our mRNA cancer immunotherapy portfolio Continuation of our novel combination strategy Prepare and execute launches of multiple oncology products across the world A diversified multi- product global immunotherapy powerhouse 2025 2026-2029 2030 Turning Science into Survival 29 1. BNT327/PM8002 partnered with Biotheus

Thank you

BNT3271 1L SCLC Phase 2 data BNT3271 2L SCLC Phase 1/2 data BNT3271 1L ES-SCLC and 2L SCLC Phase 2 DO data BNT3271 1L and 2L TNBC Phase 2 DO data BNT3271 + BNT325 / DB-13052 Multiple solid tumors Phase 1 data BNT323 / DB-13032 2L+ HER2 EC Phase 2 data Autogene cevumeran (BNT122 / RO7198457)3 ctDNA+ adj. CRC Phase 2 topline data BNT1114 2L+ melanoma Phase 2 data BNT1164 + cemiplimab PD-L1 > 1% NSCLC Phase 1 data BNT323 / DB-13032 2L+ HER2 EC Regulatory submission Expected Potential Value Creating Milestones and Trials Partnered with: 1. Biotheus; 2. DualityBio; 3. Genentech, a member of Roche Group; 4. In collaboration with Regeneron. Catalyst-rich period for later-stage pipeline to support company goal to achieve a diversified, cashflow-generating multi-product oncology portfolio by 2030 2025+ Data update Regulatory event 31

Glossary n L nth line HPV Human papilloma virus PDAC Pancreatic ductal adenocarcinoma AACR American Association for Cancer Research HR Hazard ratio / hormone receptor PD-(L)1 Programmed cell death protein (ligand) 1 ADC Antibody-drug conjugate ID Infectious disease PFS Progression-free survival AI Artificial intelligence IFN Interferon QxW Every x week(s) ASCO American Society of Clinical Oncology IIT Investigator initiated trial RCC Renal cell carcinoma BLA Biologics License Applications IL-x Interleukin x RFS Recurrence-free survival CAR-T Chimeric antigen receptor T cell iNeST Individualized NeoAntigen-Specific Therapy R/R Relapsed/refractory CD-x Cluster of differentiation IO Immuno-oncology SABCS San Antonio Breast Cancer Symposium CLDN6 Claudin 6 ITT Intention to treat (ES)SCLC (Extensive stage) small cell lung cancer CPS Combined positive score MITD Microtubule interacting and trafficking domain SEC Selenocysteinyl-tRNA CPI Checkpoint inhibitor MIUC Muscle-invasive urothelial carcinoma SITC Society of Immunotherapy of Cancer CRC Colorectal cancer m Median SoC Standard of care ctDNA Circulating tumor DNA mo Months TCR T-cell receptor CTx Chemotherapy MPM Malignant pleural mesothelioma TLR7/8 Toll-like receptor 7/8 DCR Disease control rate mRNA Messenger ribonucleic acid TME Tumor microenvironment DFS Disease-free survival MSI-H(L) High(low)-frequence microsatellite instability TNBC Triple-negative breast cancer DO Dose optimization MSS Microsatellite stability TROP2 Trophoblast cell-surface antigen 2 EC Endometrial cancer NCT National clinical trial TTF Time to treatment failure EpCAM Epithelial cell adhesion molecule NIH National Institutes of Health TTR Time to response ESMO European Society for Medical Oncology NSCLC Non-small cell lung cancer UC Urothelial cancer GI Gastrointestinal nsSNV Nonsynonymous somatic variants UTR Untranslated region HCC Hepatocellular carcinoma ORR Objective response rate VEGF(R) Vascular endothelial growth factor (receptor) HER2 (or 3) Human epidermal growth factor receptor 2 (or 3) OS Overall survival HNSCC Head and neck squamous cell carcinoma OX40 CD134 32































